Note: Descriptions are shown in the official language in which they were submitted.
CA 02660814 2009-02-13
WO 2008/020270 PCT/1B2006/052815
1
Description
POSITIVELY CHARGED WATER-SOLUBLE PRODRUGS OF
ARYL- AND HETEROARYLPROPIONIC ACIDS WITH VERY
FAST SKIN PENETRATION RATE
Technical Field
[1] The present invention relates to the preparations of positively charged
and water-
soluble pro-drugs of aryl- and heteroarylpropionic acids and related compounds
and
their medicinal use in treating any nonsteroidal anti-inflammatory drugs
(NSAIAs)-treatable conditions in humans or animals. More specifically, the
present
invention is to overcome the side effects that are associated with the use of
NSAIAs.
These pro-drugs can be administered orally or transdermally.
Background Art
[2] There are 2-aryl- and heteroarylpropionic acids, 3-aryl- and
heteroarylpropionic
acids and cyclized aryl- and heteroarylpropionic acids. 2-(6-methoxy-2-
naphthyl)
propionic acid (naproxen), a-methyl-4-(2-thienylcarbonyl)benzeneacetic acid
(suprofen), a-methyl-(p-chlorobenzoy1)-5-methoxy-2-methylindole-3-acetic acid,
2-(2-fluoro-4-biphenylyl)propionic acid (flurbiprofen),
6-chloro-a-methyl-9H-carbazole-2-acetic acid (carprofen), a-methyl-5H-[1]
benzopyrano[2,3-131pyridine-7-acetic acid (pranoprofen),
2-(4-chloropheny1)-a-methyl-5-benzoxazoleacetic acid (benoxaprofen), a-
methy1-4-[(2-methyl-2-propenypaminolbenzeneacetic acid (alminoprofen),
5-benzoyl-a-methyl-2-thiopheneacetic acid (tiaprofenic acid),
3-chloro-4-(2,5-dihydro-1H-pyrrol-1-y1)-a-methylbenzeneacetic acid
(pirprofen),
2-(10, 11-dihydro-10-oxodibenzo(b,f)thiepin-2-yl)propionic acid (zaltoprofen),
2-(8-methy1-10, 11-dihydro-11-oxodibenz(b,Doxepin-2-yl)propionic acid
(bermoprofen), 214-(2-oxocyclopentyl-methyl)phenyllpropionic acid
(loxoprofen),
4-(1,3-dihydro-1-oxo-2H-isoindo1-2-y1)-a-methylbenzeneacetic acid
(indoprofen),
a,3-dichloro-4-cyclohexylbenzeneacetic acid (fenclorac), and related compounds
are
members of 2-aryl and heteroarylpropionic acid group of nonsteroidal anti-
inflammatory drugs. 4,5-Dipheny1-2-oxazole propionic acid (oxaprozin),
3-(4-biphenylylcarbonyl)propionic acid (fenbufen),
5-(4-chloropheny1)-beta-hydroxy-2-furanpropionic acid (orpanoxin), and related
compounds are members of 3-aryl and heteroarylpropionic acid group of
nonsteroidal
anti-inflammatory drugs. 5-benzoy1-2,3-dihydro-1H-pyrrolizine-1-carboxylic
acid
(ketorolac), 6-chloro-5-cyclohexy1-2,3-dihydro-1H-indene-1-carboxylic acid
(clidanac), and related compounds are members of cyclized aryl- and heteroaryl-
CA 02660814 2009-02-13
WO 2008/020270 PCT/1B2006/052815
2
propionic acid group of nonsteroidal anti-inflammatory drugs. They are used
for the
relief of signs and symptoms of rheumatoid arthritis and osteoartluitis and
for the
treatment of dysmenorrhea. They are also used for the treatment of acute gouty
arthritis
and ankylosing spondylitis. The may be used for the treatment of dementia
(McGeer;
Patrick L. et al. U.S. Pat. No. 5,192,753).
[3] Unfortunately, a number of side effects are associated with the use of
naproxen,
suprofen, a-methyl-(p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetic acid,
flurbiprofen, carprofen, pranoprofen, benoxaprofen, alminoprofen, tiaprofenic
acid,
pirprofen, zaltoprofen, bermoprofen, loxoprofen, indoprofen, fenclorac,
oxaprozin,
fenbufen, orpanoxin, ketorolac, clidanac, and related compounds, most notably
GI dis-
turbances such as dyspepsia, gastroduodenal bleeding, gastric ulcerations, and
gastritis.
Fishman (Fishman; Robert, U.S. Pat. No. 7,052,715) indicated that an
additional
problem associated with oral medications, is that the concentration levels
which must
be achieved in the bloodstream must be significant in order to effectively
treat distal
areas of pain or inflammation. These levels are often much higher than would
be
necessary if it were possible to accurately target the particular site of pain
or injury.
Fishman and many others (Van Engelen et al. U.S. Pat. No. 6,416,772; Macrides
et al.
U.S. Pat. No. 6,346,278; Kirby et al. U.S. Pat. No. 6,444,234, Roentsch, et
al., U.S.
Pat. No. 5,654,337, Park, et al., U.S. Pat. No. 6,190,690, Pearson et al. U.S.
Pat. No.
6,528,040 and Botknecht et al. U.S. Pat. No. 5,885,597) have tried to develop
a
delivery system for transdermal application by formulation. It is very
difficult,
however, to deliver therapeutically effective plasma levels of these kind
drugs into the
host by formulation, due to the slow skin penetration rate. Susan Milosovich,
et al.
designed and prepared testosterony1-4-dimethylaminobutyrate.HC1 (TSBH), which
has
a lipophilic portion and a tertiary amine groups that exists in the protonated
form at
physiological pH. They found that the prodrug (TSBH) diffuses through human
skin
-60 times faster than does the drug (TS) itself [Susan Milosovich, et al., J.
Pharm. Sci.,
82, 227(1993).
Disclosure of Invention
Technical Problem
[4] Naproxen, suprofen, a-methyl-(p-chlorobenzoy1)-5-methoxy-2-methylindole
3-acetic acid, flurbiprofen, carprofen, pranoprofen, benoxaprofen,
alminoprofen,
tiaprofenic acid, pitprofen, zaltoprofen, bermoprofen, loxoprofen, indoprofen,
fenclorac, oxaprozin, fenbufen, orpanoxin, ketorolac, clidanac, and related
compounds,
have been used medicinally for many years. They are used for the relief of
signs and
symptoms of rheumatoid arthritis and osteoarthritis, for the treatment of
dysmenorrhea.
[5] Unfortunately, a number of side effects are associated with the use of
NSAIAs,
CA 02660814 2009-02-13
WO 2008/020270 PCT/1B2006/052815
3
most notably GI disturbances such as dyspepsia, gastroduodenal bleeding,
gastric ul-
cerations, and gastritis. They are not soluble in aqueous solution and gastric
juice.
They stay in the GI tract for a long time and thus, may cause gastric mucosal
cell
damage.
Technical Solution
[6] This invention relates to the preparation of novel positively charged
pro-drugs of
aryl- and heteroarylpropionic acids and related compounds and their use
medicinally.
2-(6-methoxy-2-naphthyl)propionic acid (naproxen), a-
methy1-4-(2-thienylcarbonyl)benzeneacetic acid (suprofen), cc-
methyl-(p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetic acid,
2-(2-fluoro-4-biphenylyl)propionic acid (flurbiprofen),
6-chloro-a-methyl-9H-carbazole-2-acetic acid (carprofen), a-methyl-5H-[1]
benzopyrano[2,3-b]pyridine-7-acetic acid (pranoprofen),
2-(4-chloropheny1)-a-methyl-5-benzoxazoleacetic acid (benoxaprofen), cc-
methy1-4-[(2-methyl-2-propenypaminolbenzeneacetic acid (alminoprofen),
5-benzoyl-a-methyl-2-thiopheneacetic acid (tiaprofenic acid),
3-chloro-4-(2,5-dihydro-1H-pyrrol-1-y1)-a-methyl benzeneacetic acid
(piiprofen),
2-(10, 11-dihydro-10-oxodibenzo(b,f)thiepin-2-yl)propionic acid (zaltoprofen),
2-(8-methy1-10, 11-dihydro-11-oxodibenz(b,Doxepin-2-yl)propionic acid
(bermoprofen), 214-(2-oxocyclopentyl-methyl)phenyl]propionic acid
(loxoprofen),
4-(1,3-dihydro-1-oxo-2H-isoindo1-2-y1)-a-methylbenzeneacetic acid
(indoprofen),
a,3-dichloro-4-cyclohexylbenzeneacetic acid (fenclorac), and related compounds
are
member s of 2-aryl and heteroarylpropionic acid group of nonsteroidal anti-
inflammatory drugs. The pro-drugs of 2-aryl- and heteroarylpropionic acids
have the
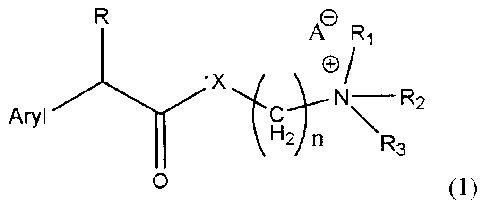
general formula (1) "Structure 1",
A Ri
'X
Aryl cNR2
\R3
0
Structure 1
In structure 1, R represents CH, OH, Cl, F, or Br; Ri represents H, one of any
alkyl,
alkyloxyl, alkenyl or alkynyl residues having 1 to 12 carbon atoms, or aryl
residues; R2
represents H, one of any alkyl, alkyloxy, alkenyl or alkynyl residues having 1
to 12
carbon atoms, or aryl residues; R3 represents H, one of any alkyl, alkyloxy,
alkenyl or
alkynyl residues having 1 to 12 carbon atoms, or aryl residues; X represents
0, S, NH,
CA 02660814 2009-02-13
WO 2008/020270 PCT/1B2006/052815
4
OCH COO, OCH COS, or OCH CONH; A- represents Cl-, Br, F, r, Ac0-, citrate, or
2 2 2
any negative ions; and n=0,1, 2, 3, 4, 5, 6, 7, 8, 9, 10 ; Aryl represents:
14001 s
0
In which, X represents CH30, Cl, F, CH3S,
CHF20
Y
R
C=0
14111
X
In which, X represents F, Cl. H
In which, Y represents CH30, F, CH3CO,
(CH3)2N, CH3, or CH2¨CH-CH2, X represents
Cl, F, CF3, CH3S0,or CI-13S; R represents CH3,
C2Hs, C3H7
In which, X represents Cl, Br, F, CH3
CA 02660814 2009-02-13
WO 2008/020270 PCT/1B2006/052815
X 10
\o
H2
In which_ X represents Cl, Br, F, CH3
0
N
X
In which, X represents Cl, F, Br
X
N
o In
which, X represents Cl, Br, F, or CH30
In which, X represents CO or
Nit Z
R x 110
=
In which, X represents 0 or S, Y represents
CH, and CO, and Z represents, CO and CH,,
R represents H, CH3, C4-15
All R groups may include C, H, 0, S, or N atoms and may have single, double,
and
treble bonds. Any CH2 groups may be replaced with 0, S, or NH.
[7] 4,5-Dipheny1-2-oxazole propionic acid (oxaprozin),
3-(4-biphenylylcarbonyl)propionic acid (fenbufen),
5-(4-chloropheny1)-beta-hydroxy-2-furanpropanoic acid (orpanoxin), and related
CA 02660814 2009-02-13
WO 2008/020270
PCT/1B2006/052815
6
compounds are members of 3-aryl and heteroarylpropionic acid group of
nonsteroidal
anti-inflammatory drugs. The pro-drugs of 3-aryl- and heteroarylpropionic
acids have
the general formula (2) 'Structure 2'.
A Ri
N ¨ R2
\ R3
0
Structure 2
In structure 2, W represents H, OH, Cl, F, or Br; Ri represents H, one of any
alkyl,
alkyloxyl, alkenyl or alkynyl residues having 1 to 12 carbon atoms, or aryl
residues; R2
represents H, one of any alkyl, alkyloxy, alkenyl or alkynyl residues having 1
to 12
carbon atoms, or aryl residues; R3 represents H, one of any alkyl, alkyloxy,
alkenyl or
alkynyl residues having 1 to 12 carbon atoms, or aryl residues; X represents
0, S, NH,
OCH COO, OCH COS, or OCH CONH; A- represents Cl-, Br-, F, r, Ac0-, citrate, or
2 2 2
any negative ions; and n=0,1,2,3,4,5,6,7,8,9,10 ................... ; W
represents OH, Cl, or F; Y
represents H, Cl, OH, or CH; and Z represents:
0
x ___________________________________________________________ oN
In which, X represents Cl, F, or Br
411
All R, R, R, R' and R groups may include C, H, 0, S, N atoms and may have
single,
1 2 3 4
double, and treble bonds. Any CH2 groups may be replaced with 0, S, or NH.
[8] In the general formula (2) 'Structure 2', when W represents H, Y and
Z together
represent:
CA 02660814 2009-02-13
WO 2008/020270
PCT/1B2006/052815
7
X (Y) (Y)
1110 (Z)
(Z)
0
In which, X represents Cl, F, or Br
The cyclized aryl- and heteroarylpropionic acid are formed. They are
5-benzoy1-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid (ketorolac),
6-chloro-5-cyclohexy1-2,3-dihydro-1H-indene-1-carboxylic acid (clidanac) and
related
compounds.
[9] Drug absorption, whether from the gastrointestinal tract or other
sites, requires the
passage of the drug in a molecular form across the barrier membrane. The drug
must
first dissolve, and if the drug possesses the desirable biopharmaceutical
properties, it
will pass from a region of high concentration to a region of low concentration
across
the membrane into the blood or general circulation. All biological membranes
contain
lipids as major constituents. The molecules that play the dominant roles in
membrane
formation all have phosphate-containing highly polar head groups, and, in most
cases,
two highly hydrophobic hydrocarbon tails. Membranes are bilayers, with the hy-
drophilic head groups facing outward into the aqueous regions on either side.
Very hy-
drophilic drugs cannot pass the hydrophobic layer of membrane and very
hydrophobic
drugs will stay in the hydrophobic layer as part of the membrane due to their
sim-
ilarities and cannot enter the cytosol on the inside efficiently.
[10] The goal of this invention is to avoid the side effects of naproxen,
suprofen, a-
methyl-(p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetic acid, flurbiprofen,
carprofen, pranoprofen, benoxaprofen, alminoprofen, tiaprofenic acid,
pirprofen,
zaltoprofen, bermoprofen, loxoprofen, indoprofen, fenclorac, oxaprozin,
fenbufen,
orpanoxin, ketorolac, clidanac, and related compounds by increasing the their
solubility in gastric juice and their penetration rate through the membrane
and skin
barrier which will make it administrable transdermally (topical application).
These
novel pro-drugs have two structural features in common: they have a lipophilic
portion
and a primary, secondary, or tertiary amine group that exists in the
protonated form
(hydrophilic part) at physiological pH. Such a hydrophilic-lipophilic balance
is
required for efficient passage through the membrane barrier [Susan Milosovich,
et al.,
J. Phann. Sci., 82, 227(1993)1. The positively charged amino groups largely
increase
CA 02660814 2009-02-13
WO 2008/020270 PCT/1B2006/052815
8
the solubility of the drugs. The solubility of diethylaminoethyl
2-(6-methoxy-2-naphthyl)propionate.AcOH, diethylaminoethyl a-
methy1-442-thienylcarbonyl)benzeneacetate.AcOH, diethylaminoethyl a-
methyl4p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetate.AcOH, diethy-
laminoethyl 2-(2-fluoro-4-biphenylyl)propionate.AcOH, diethylaminoethyl
6-chloro-a-methyl-9H-carbazole-2-acetate.AcOH, diethylaminoethyl a-methy1-5H-
[1]
benzopyrano[2,3-blpyridine-7-acetate.AcOH, diethylaminoethyl
2(4-chloropheny1)-a-methy1-5-benzoxazoleacetate.AcOH, diethylaminoethyl a-
methy1-44(2-methyl-2-propenypaminolbenzeneacetate.AcOH, diethylaminoethyl
5-benzoyl-a-methyl-2-thiopheneacetate.AcOH, diethylaminoethyl
3-chloro-4-(2,5-dihydro-1H-pyrrol-1-y1)-a-methyl benzeneacetate.AcOH, diethy-
laminoethyl 2-(10, 11-dihydro-10-oxodibenzo(b,f)thiepin-2-yl)propionate.AcOH,
di-
ethylaminoethyl 248-methy1-10,
11-dihydro-11-oxodibenz(b,f)oxepin-2-yl)propionate.AcOH, diethylaminoethyl
2[4(2-oxocyclopentyl-methyl)phenyllpropionate.AcOH, diethylaminoethyl
4-(1,3-dihydro-1-oxo-2H-isoindo1-2-y1)-a-methylbenzeneacetate.AcOH, diethy-
laminoethyl a,3-dichloro-4-cyclohexylbenzeneacetate.AcOH, diethylaminoethyl
4,5-Dipheny1-2-oxazole propionate.AcOH, diethylaminoethyl
3(4-biphenylylcarbonyl)propionate.AcOH, diethylaminoethyl
5-(4-chloropheny1)-beta-hydroxy-2-furanpropionate.AcOH, diethylaminoethyl
5-benzoy1-2,3-dihydro-1H-pyrrolizine-1-carboxylate.AcOH, diethylaminoethyl
6-chloro-5-cyclohexy1-2,3-dihydro-1H-indene-1-carboxylate.AcOH, naproxen,
suprofen, a-methyl(p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetic acid,
flurbiprofen, carprofen, pranoprofen, benoxaprofen, alminoprofen, tiaprofenic
acid,
pirprofen, zaltoprofen, bermoprofen, loxoprofen, indoprofen, fenclorac,
oxaprozin,
fenbufen, orpanoxin, ketorolac, clidanac in water are >450 mg, >400 mg, >450
mg, >
450 mg, >350 mg, >450 mg, >400 mg, >450 mg, >400 mg, >450 mg, >350 mg, >400
mg, >350 mg, >400 mg, >350 mg, >400 mg, >400 mg, >350 mg, >450 mg, >350 mg,
<0.1 mg, <0.1 mg, <0.1 mg, <0.1 mg, <0.1 mg, <0.1 mg, <0.1 mg, <0.1 mg, <0.1
mg,
<0.1 mg, <0.1 mg, <0.1 mg, <0.1 mg, <0.1 mg, <0.1 mg, <0.1 mg, <0.1 mg, <0.1
mg,
<0.1 mg, and <0.1 mg/ml, In many instances, the lowest or rate-limiting step
in the
sequence is the dissolution of the drug. Naproxen, suprofen, a-
methyl4p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetic acid, flurbiprofen,
carprofen, pranoprofen, benoxaprofen, alminoprofen, tiaprofenic acid,
pirprofen,
zaltoprofen, bermoprofen, loxoprofen, indoprofen, fenclorac, oxaprozin,
fenbufen,
orpanoxin, ketorolac, clidanac, and related compounds have a very low
solubility in
gastric juice. They stay in the GI tract for a long time and thus, may cause
gastric
mucosal cell damage. When these new pro-drugs are administered orally in a
dosage
CA 02660814 2009-02-13
WO 2008/020270
PCT/1B2006/052815
9
form such as a tablet, capsule, solution, or suspension, they will dissolve in
the gastric
juice immediately. The positive charge on the amino groups of these pro-drugs
will
bond to the negative charge on the phosphate head group of membrane. Thus, the
local
concentration of the outside of the membrane will be very high and will
facilitate the
passage of these pro-drugs from a region of high concentration to a region of
low con-
centration. When these pro-drugs enter the membrane, the hydrophilic part will
push
the pro-drug into the cytosol, a semi-liquid concentrated aqueous solution or
suspension. Due to the short stay in GI tract, the pro-drugs will not cause
gastric
mucosal cell damage. The pH of the stomach is 1-3, so the negative charge on
the
phosphate head group of membrane is bonded with proton (M. The positive
charges
of prodrugs cannot bond to the negative charge on the phosphate head group of
the
gastric mucosa. These prodrugs will be free both of primary insult (direct
acid damage)
and secondary insult (prostaglandin inhibition) to the stomach.
1111 The penetration rates of aryl- and heteroarylpropionic acids and
their positively
charged prodrugs and related compounds through human skin were measured in
vitro
by using modified Franz cells, which were isolated from human skin tissue (360-
400
pm thick) of the anterior and posterior thigh areas. The receiving fluid
consisted of 10
ml of 2% bovine serum albumin in normal saline and was stirred at 600 rpm. The
cumulative amounts of these prodrugs and their parent drugs penetrating the
skin
versus time were determined by a specific high-performance liquid
chromatography
method. The results using a donor consisting of either a 30% solution of these
prodrugs
or a 30% suspension of naproxen, suprofen, a-
methyl-(p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetic acid, flurbiprofen,
carprofen, pranoprofen, benoxaprofen, alminoprofen, tiaprofenic acid,
pirprofen,
zaltoprofen, bermoprofen, loxoprofen, indoprofen, fenclorac, oxaprozin,
fenbufen,
orpanoxin, ketorolac, clidanac in 2mL of pH 7.4 phosphate buffer (0.2M) are
shown in
Figure 1, Figure 2, Figure 3, or Figure 4. Apparent flux values of 3.5 mg, 3.0
mg, 4.0
mg, 3.5 mg, 4.0 mg, 3.8 mg, 4.0 mg, 3.5 mg, 4.2 mg, 3.5 mg, 3.7 mg, 4.1 mg,
3.4 mg,
4.2 mg, 3.8 mg, 4.0 mg, 3.6 mg, 4.1 mg, 3.8 mg, 4.0 mg, 0.03 mg, 0.03 mg, 0.03
mg,
0.03 mg, 0.04 mg, 0.03 mg, 0.04 mg, 0.03 mg, 0.03 mg, 0.03 mg, 0.03 mg, 0.04
mg,
0.03 mg, 0.03 mg, 0.04 mg, 0.03 mg, 0.04 mg, 0.03 mg, 0.03 mg, and 0.04
mg/cm2/h
were calculated for diethylaminoethyl 2-(6-methoxy-2-naphthyl)propionate.AcOH,
di-
ethylaminoethyl a-methyl-4-(2-thienylcarbonyl)benzeneacetate.AcOH, diethy-
laminoethyl a-methyl-(p-chlorobenzoy1)-5-methoxy-2-methylindole 3-
acetate.AcOH,
diethylaminoethyl 2-(2-fluoro-4-biphenylyl)propionate.AcOH, diethylaminoethyl
6-chloro-a-methyl-9H-carbazole-2-acetate.AcOH, diethylaminoethyl a-methy1-5H-
[1]
benzopyrano[2,3-blpyridine-7-acetate.AcOH, diethylaminoethyl
2-(4-chloropheny1)-a-methyl-5-benzoxazoleacetate.AcOH, diethylaminoethyl a-
CA 02660814 2009-02-13
WO 2008/020270 PCT/1B2006/052815
methyl-4-[(2-methyl-2-propenypaminolbenzeneacetate.AcOH, diethylaminoethyl
5-benzoyl-a-methyl-2-thiopheneacetate.AcOH, diethylaminoethyl
3-chloro-4-(2,5-dihydro-1H-pyrrol-1-y1)-a-methyl benzeneacetate.AcOH, diethy-
laminoethyl 2-(10, 11-dihydro-10-oxodibenzo(b,f)thiepin-2-yl)propionate.AcOH,
di-
ethylaminoethyl 248-methy1-10,
11-dihydro-11-oxodibenz(b,f)oxepin-2-yl)propionate.AcOH, diethylaminoethyl
2[4(2-oxocyclopentyl-methyl)phenyllpropionate.AcOH, diethylaminoethyl
4-(1,3-dihydro-1-oxo-2H-isoindo1-2-y1)-a-methylbenzeneacetate.AcOH, diethy-
laminoethyl a,3-dichloro-4-cyclohexylbenzeneacetate.AcOH, diethylaminoethyl
4,5-Dipheny1-2-oxazole propionate.AcOH, diethylaminoethyl
3(4-biphenylylcarbonyl)propionate.AcOH, diethylaminoethyl
5(4-chloropheny1)-beta-hydroxy-2-furanpropionate.AcOH, diethylaminoethyl
5-benzoy1-2,3-dihydro-1H-pyrrolizine-1-carboxylate.AcOH, diethylaminoethyl
6-chloro-5-cyclohexy1-2,3-dihydro-1H-indene-1-carboxylate.AcOH, naproxen,
suprofen, a-methyl(p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetic acid,
flurbiprofen, carprofen, pranoprofen, benoxaprofen, alminoprofen, tiaprofenic
acid,
pirprofen, zaltoprofen, bermoprofen, loxoprofen, indoprofen, fenclorac,
oxaprozin,
fenbufen, orpanoxin, ketorolac, and clidanac diffuses through human skin. The
results
suggest that the pro-drug, diethylaminoethyl
2-(6-methoxy-2-naphthyl)propionate.AcOH, diethylaminoethyl a-
methy1-442-thienylcarbonyl)benzeneacetate.AcOH, diethylaminoethyl a-
methyl4p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetate.AcOH, diethy-
laminoethyl 2-(2-fluoro-4-biphenylyl)propionate.AcOH, diethylaminoethyl
6-chloro-a-methyl-9H-carbazole-2-acetate.AcOH, diethylaminoethyl a-methy1-5H-
[1]
benzopyrano[2,3-blpyridine-7-acetate.AcOH, diethylaminoethyl
2(4-chloropheny1)-a-methy1-5-benzoxazoleacetate.AcOH, diethylaminoethyl a-
methy1-4-[(2-methyl-2-propenypaminolbenzeneacetate.AcOH, diethylaminoethyl
5-benzoyl-a-methyl-2-thiopheneacetate.AcOH, diethylaminoethyl
3-chloro-4-(2,5-dihydro-1H-pyrrol-1-y1)-a-methyl benzeneacetate.AcOH, diethy-
laminoethyl 2-(10, 11-dihydro-10-oxodibenzo(b,f)thiepin-2-yl)propionate.AcOH,
di-
ethylaminoethyl 248-methy1-10,
11-dihydro-11-oxodibenz(b,f)oxepin-2-yl)propionate.AcOH, diethylaminoethyl
2[4(2-oxocyclopentyl-methyl)phenylipropionate.AcOH, diethylaminoethyl
4-(1,3-dihydro-1-oxo-2H-isoindo1-2-y1)-a-methylbenzeneacetate.AcOH, diethy-
laminoethyl a,3-dichloro-4-cyclohexylbenzeneacetate.AcOH, diethylaminoethyl
4,5-Dipheny1-2-oxazole propionate.AcOH, diethylaminoethyl
3(4-biphenylylcarbonyl)propionate.AcOH, diethylaminoethyl
5(4-chloropheny1)-beta-hydroxy-2-furanpropionate.AcOH, diethylaminoethyl
CA 02660814 2009-02-13
WO 2008/020270
PCT/1B2006/052815
11
5-benzoy1-2,3-dihydro-1H-pyrrolizine-1-carboxylate.AcOH, or diethylaminoethyl
6-chloro-5-cyclohexy1-2,3-dihydro-1H-indene-1-carboxylate.AcOH diffuses
through
human skin -100-130 times faster than does naproxen, suprofen, a-
methyl4p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetic acid, flurbiprofen,
carprofen, pranoprofen, benoxaprofen, alminoprofen, tiaprofenic acid,
pirprofen,
zaltoprofen, bermoprofen, loxoprofen, indoprofen, fenclorac, oxaprozin,
fenbufen,
orpanoxin, ketorolac, or clidanac . The results suggest that the positive
charge on the
dialkyaminoethyl group has a very important role in the passage of the drug
across the
membrane and skin barrier. Other prodrugs of the general 'Structure 1' or
general 'St
ructure 2' have very high penetration rates and are very close to that of
diethy-
laminoethyl 2-(6-methoxy-2-naphthyl)propionate.AcOH.
[12] The in vivo rates of penetration of aryl- and heteroarylpropionic
acids and their
positively charged prodrugs and related compounds through the skin of intact
hairless
mice were compared. The donor consisted of a 20% solution of these compounds
in 1
mL of isopropanol applied to a 10 cm2 on the backs of the hairless mice.
Plasma levels
of naproxen, suprofen, a-methyl(p-chlorobenzoy1)-5-methoxy-2-methylindole
3-acetic acid, flurbiprofen, carprofen, pranoprofen, benoxaprofen,
alminoprofen,
tiaprofenic acid, pliprofen, zaltoprofen, bermoprofen, loxoprofen, indoprofen,
fenclorac, oxaprozin, fenbufen, orpanoxin, ketorolac, clidanac were determined
by a
specific high-performance liquid chromatography method. The results (Figure 5,
Figure 6, Figure 7, Figure 8) show that the peak levels of diethylaminoethyl
2-(6-methoxy-2-naphthyl)propionate.AcOH, diethylaminoethyl a-methyl-4(2-thien
ylcarbonyl)benzeneacetate.AcOH, diethylaminoethyl a-
methyl4p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetate.AcOH, diethy-
laminoethyl 2(2-fluoro-4-biphenylyppropionate.AcOH, diethylaminoethyl
6-chloro-a-methyl-9H-carbazole-2-acetate.AcOH, diethylaminoethyl a-methy1-5H-
[1]
benzopyrano[2,3-blpyridine-7-acetate.AcOH, diethylaminoethyl
2(4-chloropheny1)-a-methy1-5-benzoxazoleacetate.AcOH, diethylaminoethyl a-
methy1-41(2-methyl-2-propenypaminolbenzeneacetate.AcOH, diethylaminoethyl
5-benzoyl-a-methyl-2-thiopheneacetate.AcOH, diethylaminoethyl
3-chloro-4-(2,5-dihydro-1H-pyrrol-1-y1)-a-methyl benzeneacetate.AcOH, diethy-
laminoethyl 2-(10, 11-dihydro-10-oxodibenzo(b,f)thiepin-2-yl)propionate.AcOH,
di-
ethylaminoethyl 248-methy1-10,
11-dihydro-11-oxodibenz(b,f)oxepin-2-yl)propionate.AcOH, diethylaminoethyl
2[4(2-oxocyclopentyl-methyl)phenylipropionate.AcOH, diethylaminoethyl
4-(1,3-dihydro-1-oxo-2H-isoindo1-2-y1)-a-methylbenzeneacetate.AcOH, diethy-
laminoethyl a,3-dichloro-4-cyclohexylbenzeneacetate.AcOH, diethylaminoethyl
4,5-Dipheny1-2-oxazole propionate.AcOH, diethylaminoethyl
CA 02660814 2009-02-13
WO 2008/020270 PCT/1B2006/052815
12
3(4-biphenylylcarbonyl)propionate.AcOH, diethylaminoethyl
5(4-chloropheny1)-beta-hydroxy-2-furanpropionate.AcOH, diethylaminoethyl
5-benzoy1-2,3-dihydro-1H-pyrrolizine-1-carboxylate.AcOH, diethylaminoethyl 6-
ch
loro-5-cyclohexy1-2,3-dihydro-1H-indene-1-carboxylate.AcOH were reached -50
minutes after application of the donor systems. It takes 2-4 hours for
naproxen,
suprofen, a-methyl(p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetic acid,
flurbiprofen, carprofen, pranoprofen, benoxaprofen, alminoprofen, tiaprofenic
acid,
pirprofen, zaltoprofen, bermoprofen, loxoprofen, indoprofen, fenclorac,
oxaprozin,
fenbufen, orpanoxin, ketorolac, clidanac to reach their peak plasma level when
they are
taken orally. The peak plasma levels were -0.01 mg/ml for naproxen, suprofen,
a-
methyl4p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetic acid, flurbiprofen,
carprofen, pranoprofen, benoxaprofen, alminoprofen, tiaprofenic acid,
pirprofen,
zaltoprofen, bermoprofen, loxoprofen, indoprofen, fenclorac, oxaprozin,
fenbufen,
orpanoxin, ketorolac, clidanac and -2 mg/ml for diethylaminoethyl
2-(6-methoxy-2-naphthyl)propionate.AcOH, diethylaminoethyl a-
methy1-442-thienylcarbonyl)benzeneacetate.AcOH, diethylaminoethyl a-
methyl4p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetate.AcOH, diethy-
laminoethyl 2-(2-fluoro-4-biphenylyl)propionate.AcOH, diethylaminoethyl
6-chloro-a-methyl-9H-carbazole-2-acetate.AcOH, diethylaminoethyl a-methy1-5H-
[1]
benzopyrano[2,3-blpyridine-7-acetate.AcOH, diethylaminoethyl
2(4-chloropheny1)-a-methy1-5-benzoxazoleacetate.AcOH, diethylaminoethyl a-
methy1-4-[(2-methyl-2-propenypaminolbenzeneacetate.AcOH, diethylaminoethyl
5-benzoyl-a-methyl-2-thiopheneacetate.AcOH, diethylaminoethyl
3-chloro-4-(2,5-dihydro-1H-pyrrol-1-y1)-a-methyl benzeneacetate.AcOH, diethy-
laminoethyl 2-(10, 11-dihydro-10-oxodibenzo(b,f)thiepin-2-yl)propionate.AcOH,
di-
ethylaminoethyl 248-methy1-10,
11-dihydro-11-oxodibenz(b,f)oxepin-2-yl)propionate.AcOH, diethylaminoethyl
2[4(2-oxocyclopentyl-methyl)phenylipropionate.AcOH, diethylaminoethyl
4-(1,3-dihydro-1-oxo-2H-isoindo1-2-y1)-a-methylbenzeneacetate.AcOH, diethy-
laminoethyl a,3-dichloro-4-cyclohexylbenzeneacetate.AcOH, diethylaminoethyl
4,5-Dipheny1-2-oxazole propionate.AcOH, diethylaminoethyl
3-(4-biphenylylcarbonyl)propionate.AcOH, diethylaminoethyl
5(4-chloropheny1)-beta-hydroxy-2-furanpropionate.AcOH, diethylaminoethyl
5-benzoy1-2,3-dihydro-1H-pyrrolizine-1-carboxylate.AcOH, or diethylaminoethyl
6-chloro-5-cyclohexy1-2,3-dihydro-1H-indene-1-carboxylate.AcOH (approximately
200 times difference). -2 mg,/m1 of naproxen, suprofen, a-
methyl4p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetic acid, flurbiprofen,
carprofen, pranoprofen, benoxaprofen, alminoprofen, tiaprofenic acid,
pirprofen,
CA 02660814 2009-02-13
WO 2008/020270 PCT/1B2006/052815
13
zaltoprofen, bermoprofen, loxoprofen, indoprofen, fenclorac, oxaprozin,
fenbufen,
orpanoxin, ketorolac, clidanac in plasma is more than 20-100 times higher than
plasma
level for effective analgesia and effective anti-inflammatory activity. This
is a very
exciting result. It will be very easy and fast to deliver therapeutically
effective plasma
level of naproxen, suprofen, a-methyl(p-chlorobenzoy1)-5-methoxy-2-
methylindole
3-acetic acid, flurbiprofen, carprofen, pranoprofen, benoxaprofen,
alminoprofen,
tiaprofenic acid, pirprofen, zaltoprofen, bermoprofen, loxoprofen, indoprofen,
fenclorac, oxaprozin, fenbufen, orpanoxin, ketorolac, clidanac into the host
by admin-
istration of these prodrugs transdennally. These results suggest that the pro-
drugs can
be administered not only orally, but also transdermally for any kind of
medical
treatments. The in vivo rates of penetration of other Pro-drugs of the general
'Structure
1' or general 'Structure 2' are close to that of diethylaminoethyl
2-(6-methoxy-2-naphthyl)propionate.AcOH.
1-131 To check the gastroduodenal bleeding caused by these prodrugs, rats
were orally
administered with 50 mg/kg of diethylaminoethyl 2-(6-methoxy-2-naphthyl
)propionate.AcOH, diethylaminoethyl a-
methy1-442-thienylcarbonyl)benzeneacetate.AcOH, diethylaminoethyl a-
methyl-(p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetate.AcOH, diethy-
laminoethyl 2-(2-fluoro-4-biphenylyl)propionate.AcOH, diethylaminoethyl
6-chloro-a-methyl-91-1-carbazole-2-acetate.AcOH, diethylaminoethyl a-methyl-
51141]
benzopyrano[2,3-blpyridine-7-acetate.AcOH, diethylaminoethyl
2(4-chloropheny1)-a-methy1-5-benzoxazoleacetate.AcOH, diethylaminoethyl a-
methy1-4-[(2-methyl-2-propenyDaminolbenzeneacetate.AcOH, diethylaminoethyl
5-benzoyl-a-methyl-2-thiopheneacetate.AcOH, diethylaminoethyl
3-chloro-4-(2,5-dihydro-1H-pyrrol-1-y1)-a-methyl benzeneacetate.AcOH, diethy-
laminoethyl 2-(10, 11-dihydro-10-oxodibenzo(b,f)thiepin-2-yl)propionate.AcOH,
di-
ethylaminoethyl 248-methy1-10,
11-dihydro-11-oxodibenz(b,f)oxepin-2-yl)propionate.AcOH, diethylaminoethyl
2[4(2-oxocyclopentyl-methyl)phenyllpropionate.AcOH, diethylaminoethyl
441 ,3-dihydro-l-oxo-2H-i soindo1-2-y1)-a-methylbenzeneacetate.AcOH, diethy-
laminoethyl a,3-dichloro-4-cyclohexylbenzeneacetate.AcOH, diethylaminoethyl
4,5-Dipheny1-2-oxazole propionate.AcOH, diethylaminoethyl
3(4-biphenylylcarbonyl)propionate.AcOH, diethylaminoethyl
5(4-chloropheny1)-beta-hydroxy-2-furanpropionate.AcOH, diethylaminoethyl
5-benzoy1-2,3-dihydro-1H-pyrrolizine-1-carboxylate.AcOH, diethylaminoethyl
6-chloro-5-cyclohexy1-2,3-dihydro-1H-indene-1-carboxylate.AcOH, naproxen,
suprofen, a-methyl(p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetic acid,
flurbiprofen, carprofen, pranoprofen, benoxaprofen, ahninoprofen, tiaprofenic
acid,
CA 02660814 2009-02-13
WO 2008/020270 PCT/1B2006/052815
14
pirprofen, zaltoprofen, bermoprofen, loxoprofen, indoprofen, fenclorac,
oxaprozin,
fenbufen, orpanoxin, ketorolac, clidanac per day for 21 days. We found an
average of
1-4 mg of fecal blood per gram of feces in the naproxen, suprofen, a-
methyl4p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetic acid, flurbiprofen,
carprofen, pranoprofen, benoxaprofen, alminoprofen, tiaprofenic acid,
pirprofen,
zaltoprofen, bermoprofen, loxoprofen, indoprofen, fenclorac, oxaprozin,
fenbufen,
orpanoxin, ketorolac, clidanac groups and none in diethylaminoethyl
2-(6-methoxy-2-naphthyl)propionate.AcOH, diethylaminoethyl a-
methy1-442-thienylcarbonyl)benzeneacetate.AcOH, diethylaminoethyl a-
methyl4p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetate.AcOH, diethy-
laminoethyl 2-(2-fluoro-4-biphenylyl)propionate.AcOH, diethylaminoethyl
6-chloro-a-methyl-9H-carbazole-2-acetate.AcOH, diethylaminoethyl a-methyl-
511411
benzopyrano[2,3-blpyridine-7-acetate.AcOH, diethylaminoethyl
2(4-chloropheny1)-a-methy1-5-benzoxazoleacetate.AcOH, diethylaminoethyl a-
methy1-44(2-methy1-2-propenyflaminoThenzeneacetate.AcOH, diethylaminoethyl
5-benzoyl-a-methyl-2-thiopheneacetate.AcOH, diethylaminoethyl
3-chloro-4-(2,5-dihydro-1H-pyrrol-1-y1)-a-methyl benzeneacetate.AcOH, diethy-
laminoethyl 2-(10, 11-dihydro-10-oxodibenzo(b,f)thiepin-2-yl)propionate.AcOH,
di-
ethylaminoethyl 248-methy1-10,
11-dihydro-11-oxodibenz(b,f)oxepin-2-yl)propionate.AcOH, diethylaminoethyl
2[4(2-oxocyclopentyl-methyl)phenylipropionate.AcOH, diethylaminoethyl
4-(1,3-dihydro-1-oxo-2H-isoindo1-2-y1)-a-methylbenzeneacetate.AcOH, diethy-
laminoethyl a,3-dichloro-4-cyclohexylbenzeneacetate.AcOH, diethylaminoethyl
4,5-Dipheny1-2-oxazole propionate.AcOH, diethylaminoethyl
3(4-biphenylylcarbonyl)propionate.AcOH, diethylaminoethyl
5(4-chloropheny1)-beta-hydroxy-2-furanpropionate.AcOH, diethylaminoethyl
5-benzoy1-2,3-dihydro-1H-pyrrolizine-1-carboxylate.AcOH, diethylaminoethyl
6-chloro-5-cyclohexy1-2,3-dihydro-1H-indene-1-carboxylate.AcOH groups.
[141 The acute toxicity of the prodrugs was investigated. The LD50 orally
in mice are:
2.2 g/kg, 0.8 g/kg, 0.7g/kg , 0.75 g/kg , 1.3 g/kg , 3.5 g/kg, 1.1 g/kg, 0.6
g/kg, 0.2 g/kg
for diethylaminoethyl 2(6-methoxy-2-naphthyppropionate.AcOH, diethylaminoethyl
a-methyl-4(2-thienylcarbonyl)benzeneacetate.AcOH, diethylaminoethyl
6-chloro-a-methyl-9H-carbazole-2-acetate.AcOH, diethylaminoethyl a-methyl-
511411
benzopyrano[2,3-bipyridine-7-acetate.AcOH, diethylaminoethyl
2(4-chloropheny1)-a-methy1-5-benzoxazoleacetate.AcOH, diethylaminoethyl
4-(1,3-dihydro-1-oxo-2H-isoindo1-2-y1)-a-methylbenzeneacetate.AcOH, diethy-
laminoethyl a-methy1-44(2-methyl-2-propenyl)aminolbenzeneacetate.AcOH, diethy-
laminoethyl a,3-dichloro-4-cyclohexylbenzeneacetate.AcOH, diethylaminoethyl
CA 02660814 2009-02-13
WO 2008/020270 PCT/1B2006/052815
6-chloro-5-cyclohexy1-2,3-dihydro-1H-indene-1-carboxylate.AcOH. The results
show
that the prodrugs are less toxic than their parent drugs, naproxen (LD50=1.234
g/kg),
suprofen (LD50=0.59 g/kg), carprofen (400 mg/kg), pranoprofen (447 mg/kg),
benoxaprofen (LD5o=0.8 g/kg), alminoprofen (LD50=2400 mg/kg), indoprofen (LD50
=0.7 mg/kg), fenclorac (LD50=0.43 g/kg), clidanac (LD50=0.035 g/kg).
[15] Naproxen, suprofen, a-methyl(p-chlorobenzoy1)-5-methoxy-2-methylindole
3-acetic acid, flurbiprofen, carprofen, pranoprofen, benoxaprofen,
alminoprofen,
tiaprofenic acid, piiprofen, zaltoprofen, bermoprofen, loxoprofen, indoprofen,
fenclorac, oxaprozin, fenbufen, orpanoxin, ketorolac, or clidanac have
demonstrated
anti-inflammatory, analgesic, antipyretic, and antirheumatic activity. A good
prodrug
should go back to the parent drug in plasma. Diethylaminoethyl ester group of
these
prodrugs can be rapidly cleaved by the enzymes in human plasma in vitro and
more
than 90% of the pro-drugs are changed back to their parent drugs. Due to the
pro-drugs
having a much better absorption rate, the prodrugs will have more strength
than their
parent drugs at the same dosage. The analgetic, antipyretic, and anti-
inflammatory
activities of these prodrugs were tested using naproxen, suprofen, a-
methyl4p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetic acid, flurbiprofen,
carprofen, pranoprofen, benoxaprofen, alminoprofen, tiaprofenic acid,
pirprofen,
zaltoprofen, bermoprofen, loxoprofen, indoprofen, fenclorac, oxaprozin,
fenbufen,
orpanoxin, ketorolac, or clidanac as a comparison.
[16] Analgetic activity: The prolongation time of the pain threshold of a
mouse tail was
determined in accordance with the D'Amour-Smith Method (J. Pharmacol. Exp.
Ther.,
72, 74(1941)). After 50mg/kg of these prodrugs were administered
transdennally, the
tails of mice were exposed to heat and the prolongation time of pain threshold
was
determined. The results obtained are shown in Figure 9, Figure 10, Figure 11,
and
Figure 12. Diethylaminoethyl 2-(6-methoxy-2-naphthyl)propionate.AcOH, diethy-
laminoethyl a-methyl-4(2-thienylcarbonyl)benzeneacetate.AcOH,
diethylaminoethyl
a-methyl(p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetate.AcOH, diethy-
laminoethyl 2-(2-fluoro-4-biphenylyl)propionate.AcOH, diethylaminoethyl
6-chloro-a-methy1-91-I-carbazole-2-acetate.AcOH, diethylaminoethyl a-methy1-5H-
[1]
benzopyrano[2,3-blpyridine-7-acetate.AcOH, diethylaminoethyl
2(4-chloropheny1)-a-methy1-5-benzoxazoleacetate.AcOH, diethylaminoethyl a-
methy1-4-[(2-methyl-2-propenypaminolbenzeneacetate.AcOH, diethylaminoethyl
5-benzoyl-a-methyl-2-thiopheneacetate.AcOH, diethylaminoethyl
3-chloro-4-(2,5-dihydro-1H-pyrrol- 1-y1)-a-methyl benzeneacetate.AcOH, diethy-
laminoethyl 2-(10, 11-dihydro-10-oxodibenzo(b,f)thiepin-2-yl)propionate.AcOH,
di-
ethylaminoethyl 248-methy1-10,
11-dihydro-11-oxodibenz(b,f)oxepin-2-yl)propionate.AcOH, diethylaminoethyl
CA 02660814 2009-02-13
WO 2008/020270 PCT/1B2006/052815
16
2[4(2-oxocyclopentyl-methyl)phenylipropionate.AcOH, diethylaminoethyl
4-(1,3-dihydro-1-oxo-2H-isoindo1-2-y1)-a-methylbenzeneacetate.AcOH, diethy-
laminoethyl a,3-dichloro-4-cyclohexylbenzeneacetate.AcOH, diethylaminoethyl
4,5-Dipheny1-2-oxazole propionate.AcOH, diethylaminoethyl
3(4-biphenylylcarbonyl)propionate.AcOH, diethylaminoethyl
5(4-chloropheny1)-beta-hydroxy-2-furanpropionate.AcOH, diethylaminoethyl
5-benzoy1-2,3-dihydro-1H-pyrrolizine-1-carboxylate.AcOH, diethylaminoethyl
6-chloro-5-cyclohexy1-2,3-dihydro-1H-indene-1-carboxylate.AcOH have shown
analgesic activity nicely.
[17] The number of writhings that occurred when mice were administered an
acetic acid
solution intraperitoneally were counted, and the rate of inhibition based on
the control
group was calculated. diethylaminoethyl 2-(6-methoxy-2-
naphthyl)propionate.AcOH
(100 mg/kg, B), diethylaminoethyl a-
methy1-442-thienylcarbonyl)benzeneacetate.AcOH (100 mg/kg, C),
diethylaminoethyl
a-methyl(p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetate.AcOH (100 mg/kg,
D), diethylaminoethyl 2-(2-fluoro-4-biphenylyl)propionate.AcOH (100 mg/kg, E),
di-
ethylaminoethyl 6-chloro-a-methyl-9H-carbazole-2-acetate.AcOH (100 mg/kg, F),
di-
ethylaminoethyl a-methyl-5H-[11benzopyrano[2,3-b]pyridine-7-acetate.AcOH (100
mg/kg, G), diethylaminoethyl
2(4-chloropheny1)-a-methy1-5-benzoxazoleacetate.AcOH (100 mg/kg, H), diethy-
laminoethyl a-methyl-4-[(2-methyl-2-propenyl)aminolbenzeneacetate.AcOH (100
mg/
kg, I), diethylaminoethyl 5-benzoyl-a-methyl-2-thiopheneacetate.AcOH (100
mg/kg,
J), diethylaminoethyl 3-chloro-4-(2,5-dihydro-1H-pyrrol-1-y1)-a-methyl benze-
neacetate.AcOH (100 mg/kg, K), diethylaminoethyl 2-(10,
11-dihydro-10-oxodibenzo(b,f)thiepin-2-yl)propionate.AcOH (100 mg/kg, L),
diethy-
laminoethyl 248-methy1-10,
11-dihydro-11-oxodibenz(b,f)oxepin-2-yl)propionate.AcOH (100 mg/kg, M), diethy-
laminoethyl 2[4(2-oxocyclopentyl-methyl)phenyllpropionate.AcOH (100 mg/kg, N),
diethylaminoethyl
441,3-dihydro-l-oxo-2H-isoindo1-2-y1)-a-methylbenzeneacetate.AcOH (100 mg/kg,
0), diethylaminoethyl a,3-dichloro-4-cyclohexylbenzeneacetate.AcOH (100 mg/kg,
P), diethylaminoethyl 4,5-Dipheny1-2-oxazole propionate.AcOH (100 mg/kg, Q),
di-
ethylaminoethyl 3(4-biphenylylcarbonyl)propionate.AcOH (100 mg,/kg, R), diethy-
laminoethyl 5(4-chloropheny1)-beta-hydroxy-2-furanpropionate.AcOH (100 mg/kg,
S), diethylaminoethyl 5-benzoy1-2,3-dihydro-1H-pyrrolizine-1-carboxylate.AcOH
(100
mg/kg, T), diethylaminoethyl
6-chloro-5-cyclohexy1-2,3-dihydro-1H-indene-1-carboxylate.AcOH (100 mg/kg, U)
were administered transdermally the mice 30 minutes before the acetic acid
solution
CA 02660814 2009-02-13
WO 2008/020270 PCT/1B2006/052815
17
was administered. The A group is the control group. The results are shown in
Table 1.
Table 1. The rate of writhings inhibition by prodrugs of aryl- and het-
eroarylpropionic acids
[18]
Group Dose (mg/kg) No. of Writhings %
A 0 35.0 -
B 100 17.1 51
C 100 15.7 55
D 100 13.8 61
E 100 15.6 55
F 100 14.2 59
G 100 16.1 54
H 100 17.1 51
I 100 15.6 55
J 100 13.2 62
K 100 14.0 60
L 100 14.2 59
M 100 13.8 61
N 100 15.7 55
O 100 13.2 62
P 100 15.2 57
Q 100 15.7 55
R 100 14.2 59
S 100 15.6 55
T 100 16.1 54
U 100 15.2 57
The results show that the prodrugs demonstrate exceptional analgetic activity.
Other
compounds of the general 'Structure l' or general 'Structure 2' show similar
analgetic
activity.
[19] Antipyretic activity: Rats received a sterilized E. coli suspension
as a pyrogen. The
control group is group A. 2 hours later, diethylaminoethyl
2-(6-methoxy-2-naphthyl)propionate.AcOH (100 mg/kg, B), diethylaminoethyl a-
CA 02660814 2009-02-13
WO 2008/020270 PCT/1B2006/052815
18
methyl-4-(2-thienylcarbonyl)benzeneacetate.AcOH (100 mg/kg, C),
diethylaminoethyl
a-methyl-(p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetate.AcOH (100 mg/kg,
D), diethylaminoethyl 2-(2-fluoro-4-biphenylyl)propionate.AcOH (100 mg/kg, E),
di-
ethylaminoethyl 6-chloro-a-methyl-9H-carbazole-2-acetate.AcOH (100 mg/kg, F),
di-
ethylaminoethyl a-methyl-5H-[ llbenzopyrano[2,3-b]pyridine-7-acetate.AcOH (100
mg/kg, G), diethylaminoethyl
2-(4-chloropheny1)-a-methyl-5-benzoxazoleacetate.AcOH (100 mg/kg, H), diethy-
laminoethyl a-methyl-4-[(2-methyl-2-propenyl)aminolbenzeneacetate.AcOH (100
mg/
kg, I), diethylaminoethyl 5-benzoyl-a-methyl-2-thiopheneacetate.AcOH (100
mg/kg,
J), diethylaminoethyl 3-chloro-4-(2,5-dihydro-1H-pyrrol-1-y1)-a-methyl benze-
neacetate.AcOH (100 mg/kg, K), diethylaminoethyl 2-(10,
11-dihydro-10-oxodibenzo(b,f)thiepin-2-yl)propionate.AcOH (100 mg/kg, L),
diethy-
laminoethyl 2-(8-methy1-10,
11-dihydro-11-oxodibenz(b,f)oxepin-2-yl)propionate.AcOH (100 mg/kg, M), diethy-
laminoethyl 244-(2-oxocyclopentyl-methyl)phenyllpropionate.AcOH (100 mg/kg,
N),
diethylaminoethyl
4-(1,3-dihydro-1-oxo-2H-isoindo1-2-y1)-a-methylbenzeneacetate.AcOH (100 mg/kg,
0), diethylaminoethyl a,3-dichloro-4-cyclohexylbenzeneacetate.AcOH (100 mg/kg,
P), diethylaminoethyl 4,5-Dipheny1-2-oxazole propionate.AcOH (100 mg/kg, Q),
di-
ethylaminoethyl 3-(4-biphenylylcarbonyl)propionate.AcOH (100 mg/kg, R), diethy-
laminoethyl 5-(4-chloropheny1)-beta-hydroxy-2-furanpropionate.AcOH (100 mg/kg,
S), diethylaminoethyl 5-benzoy1-2,3-dihydro-1H-pyffolizine-1-carboxylate.AcOH
(100
mg/kg, T), diethylaminoethyl
6-chloro-5-cyclohexy1-2,3-dihydro-1H-indene-1-carboxylate.AcOH (100 mg/kg, U)
were administered transdermally. The body temperature of rats was taken at 90
min.
intervals before and after the administration of the test compounds. The
results are
shown in Table 2.
Table 2. Antipyretic Activity of prodrugs of aryl- and heteroarylpropionic
acids.
[20]
Compound t=0 min. t=90 min. t=180 min. t=270 min.
A (Control 37.34 0.05 37.36 0.07 37.37 0.05 37.44 0.08
group)
E (100mg/kg) 37.33 0.07 36.80 0.06 36.72 0.05 36.50 0.08
F (100mg/kg) 37.28 0.06 36.65 0.06 36.58 0.08 36.45 0.07
B (100mg/kg) 37.35 0.06 36.71 0.05 36.60 0.08 36.59 0.07
CA 02660814 2009-02-13
WO 2008/020270
PCT/1B2006/052815
19
M (100mg/kg) 37.29 0.07 36.82 0.06 36.70 0.05 36.67 0.08
C (100mg/kg) 37.28 0.06 36.68 0.05 36.62 0.08 36.58 0.07
D (100mg/kg) 37.27 0.06 36.76 0.05 36.65 0.08 36.49 0.07
E (100mg/kg) 37.25 0.07 36.82 0.06 36.70 0.05 36.50 0.08
F (100mg/kg) 37.23 0.06 36.69 0.06 36.52 0.08 36.40 0.07
J (100mg/kg) 37.26 0.06 36.65 0.06 36.58 0.08 36.36 0.07
G (100mg/kg) 37.27 0.06 36.68 0.05 36.62 0.08 36.58 0.07
H (100mg/kg) 37.25 0.06 36.71 0.05 36.65 0.08 36.64 0.07
I (100mg/kg) 37.26 0.07 36.80 0.06 36.70 0.05 36.57 0.08
II (100mg/kg) 37.25 0.06 36.71 0.05 36.65 0.08 36.64 0.07
J (100mg/kg) 37.28 0.06 36.65 0.06 36.58 0.08 36.56 0.07
K (100mg/kg) 37.25 0.06 36.75 0.05 36.62 0.08 36.58 0.07
M (100mg/kg) 37.24 0.07 36.82 0.06 36.70 0.05 36.67 0.08
L (100mg/kg) 37.23 0.06 36.81 0.05 36.65 0.08 36.61 0.07
M (100mg/kg) 37.29 0.07 36.82 0.06 36.60 0.05 36.67 0.08
J (100mg/kg) 37.22 0.06 36.65 0.06 36.58 0.08 36.51 0.07
The results shown that the prodrugs demonstrated strong antipyretic activity
at 100
mg/kg dose. Other compounds of the general 'Structure l' and general
'Structure 2'
show similar antipyretic activity.
[21] Anti-inflammatory activity: diethylaminoethyl
2-(6-methoxy-2-naphthyl)propionate.AcOH (100 mg/kg, B), diethylaminoethyl a-
methy1-442-thienylcarbonyl)benzeneacetate.AcOH (100 mg/kg, C),
diethylaminoethyl
a-methyl(p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetate.AcOH (100 mg/kg,
D), diethylaminoethyl 2(2-fluoro-4-biphenylyl)propionate.AcOH (100 mg/kg, E),
di-
ethylaminoethyl 6-chloro-a-methyl-9H-carbazole-2-acetate.AcOH (100 mg/kg, F),
di-
ethylaminoethyl a-methyl-5H-[11benzopyrano[2,3-b]pyridine-7-acetate.AcOH (100
mg/kg, G), diethylaminoethyl
2(4-chloropheny1)-a-methy1-5-benzoxazoleacetate.AcOH (100 mg/kg, H), diethy-
laminoethyl a-methyl-4-[(2-methyl-2-propenyl)aminolbenzeneacetate.AcOH (100
mg/
kg, I), diethylaminoethyl 5-benzoyl-a-methyl-2-thiopheneacetate.AcOH (100
mg/kg,
J), diethylaminoethyl 3-chloro-4(2,5-dihydro-1H-pyrrol-1-y1)-a-methyl benze-
neacetate.AcOH (100 mg/kg, K), diethylaminoethyl 2-(10,
11-dihydro-10-oxodibenzo(b,f)thiepin-2-yl)propionate.AcOH (100 mg,/kg, L),
diethy-
laminoethyl 248-methy1-10,
CA 02660814 2009-02-13
WO 2008/020270 PCT/1B2006/052815
11-dihydro-11-oxodibenz(b,f)oxepin-2-yl)propionate.AcOH (100 mg/kg, M), diethy-
laminoethyl 214-(2-oxocyclopentyl-methyl)phenyllpropionate.AcOH (100 mg/kg,
N),
diethylaminoethyl
4-(1,3-dihydro-1-oxo-2H-isoindo1-2-y1)-a-methylbenzeneacetate.AcOH (100 mg/kg,
0), diethylaminoethyl a,3-dichloro-4-cyclohexylbenzeneacetate.AcOH (100 mg/kg,
P), diethylaminoethyl 4,5-Dipheny1-2-oxazole propionate.AcOH (100 mg/kg, Q),
di-
ethylaminoethyl 3-(4-biphenylylcarbonyl)propionate.AcOH (100 mg/kg, R), diethy-
laminoethyl 5-(4-chloropheny1)-beta-hydroxy-2-furanpropionate.AcOH (100 mg/kg,
S), diethylaminoethyl 5-benzoy1-2,3-dihydro-1H-pyffolizine-1-carboxylate.AcOH
(100
mg/kg, T), diethylaminoethyl
6-chloro-5-cyclohexy1-2,3-dihydro-1H-indene-1-carboxylate.AcOH (100 mg/kg, U)
were administered transdermally. Group A is the controlled group. 60 minutes
later, a
carrageenin solution was administered subcutaneously to the foot pads of the
rats. The
volume of the hind paw was measured at every hour after the administration of
the
carrageenin, and the rate of increase in the volume of the paw was calculated
and
designated as the rate of swelling (%). The results obtained are shown in
Figure 13,
Figure 14, Figure 15, and Figure 16. The results show that these prodrugs by
transdermal administration demonstrated good anti-inflammatory activity. Other
compounds of the general 'Structure l' or general 'Structure 2' show similar
anti-
inflammatory activity.
[22] It is also known that a high oral dose of some of NSAIAs shows an anti-
reactive-antiasthmatic activity by inhibition of the cyclooxygenase activity.
Due to
their very high membrane penetration rate, these prodrugs can be used in
treating
asthma by spraying into the mouth or nose of the host.
[23] These prodrugs can also be used to treat psoriasis, acne, sunburn or
other skin
disorders due to their anti-inflammatory properties and very high skin
penetration rate.
[24] The present invention relates to pharmaceutical preparations
comprising of
prodrugs of the general 'Structure l' or general 'Structure 2' in addition to
customary
auxiliaries and excipients, e.g. in the form of tablets, capsules or solutions
for admin-
istration orally and in the form of solutions, lotion, ointment, emulsion or
gel for
transdermal administration. The new active compounds of the general 'Structure
1' or
general 'Structure 2'can be combined with vitamins such as A, B, C or E or
beta-
carotene, or other pharmaceuticals, such as folic acid, etc., for treating any
NSAIAs-
treatable conditions in humans or animals.
[25] Transdermal therapeutic application systems of compounds of the
general'
Structure l' or general 'Structure 2' or a composition comprising of at least
one
compound of the general 'Structure l' or general 'Structure 2' as an active
ingredient,
can be used for treating any NSAIAs-treatable conditions in humans or animals.
These
CA 02660814 2009-02-13
WO 2008/020270 PCT/1B2006/052815
21
systems can be a bandage or a patch comprising of one active substance-
containing
matrix layer and an impermeable backing layer. The most preferable system is
an
active substance reservoir, which has a permeable bottom facing the skin. By
controlling the rate of release, this system enables NSAIAs to reach
constantly optimal
therapeutic blood levels to increase effectiveness and reduce the side effects
of
NSAIAs. These systems can be worn on the wrist, ankle, arm, leg, or any part
of body.
[26] The compounds of the general formula (1) 'Structure l' or general
formula (2)
'Structure 2' indicated above can be prepared from functional derivatives of
aryl- and
heteroarylpropionic acids, for example, acid halides or mixed anhydrides of
the general
formula (3) 'Structure 3' and general formula (4) 'Structure 4'.
Aryl
0 w 0
Structure 4
Structure 3
In structure 3 & 4, X represents halogen, alkoxycarbonyl or substituted
aryloxy-
carbonyloxy, Aryl, R, Y, Z, or W represent same groups as that are listed in
'structure
l' or 'structure 2', by reaction with compounds of the general formula (5)
'Structure 5',
R3
H-X
I91) \pp
\ 2 n
Structure 5
In structure 5, R represents H, one of any alkyl, alkyloxy, alkenyl, or
alkynyl residues
3
having 1 to 12 carbon atoms, or aryl residues; R4 represents H, one of any
alkyl,
alkyloxy, alkenyl, or alkynyl residues having 1 to 12 carbon atoms, or aryl
residues; X
represents 0, S or NH; and n=0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10
[27] The compounds of the general formula (1) 'Structure l' or general
formula (2)
'Structure 2' indicated above can be prepared from naproxen, suprofen, a-
methyl-(p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetic acid, flurbiprofen,
carprofen, pranoprofen, benoxaprofen, alminoprofen, tiaprofenic acid,
pirprofen,
zaltoprofen, bermoprofen, loxoprofen, indoprofen, fenclorac, oxaprozin,
fenbufen,
orpanoxin, ketorolac, clidanac, and related compounds, by reaction with
compounds of
CA 02660814 2009-02-13
WO 2008/020270 PCT/1B2006/052815
22
the general formula (5) 'Structure 5' by using coupling reagents, such as
N,N'-Dicyclohexylcarbodiimide, N, N'-Diisopropylcarbodiimide, 0-
(Benzotriazol-1-y1)-N,N,N',N'-tetramethyluronium tetrafluoroborate, 0-
(Benzotriazol-1-y1)-N,N,N,N'-tetramethyluronium hexafluorophosphate,
Benzotriazol-
1-yl-oxy-tris (dimethylamino)phosphonium hexafluorophosphate, et al.
[28] When X represents 0, the compounds of the general formula (1)
'Structure 1' or
general formula (2) 'Structure 2' indicated above can be prepared from metal
salts or
organic base salts of naproxen, suprofen, a-
methyl-(p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetic acid, flurbiprofen,
carprofen, pranoprofen, benoxaprofen, alminoprofen, tiaprofenic acid,
pirprofen,
zaltoprofen, bermoprofen, loxoprofen, indoprofen, fenclorac, oxaprozin,
fenbufen,
orpanoxin, ketorolac, clidanac, and related compounds, by reaction with
compounds of
the general formula (6) 'Structure 6'.
e
A R2
= Z ,,4 6/
3
\-R :
Structure 6
In structure 6, R represents H, one of any alkyl, alkyloxy, alkenyl, or
alkynyl residues
2
having 1 to 12 carbon atoms, or aryl residues; R3 represents H, one of any
alkyl,
alkyloxy, alkenyl, or alkynyl residues having 1 to 12 carbon atoms, or aryl
residues; R4
represents H, one of any alkyl, alkyloxy, alkenyl, or alkynyl residues having
1 to 12
carbon atoms, or aryl residues; Z represents halogen, or p-toluenesulphonyl, A-
represents Cr, Br-, F, I-, Ac0-, citrate, or any negative ions; and
n=0,1,2,3,4,5
[29] When X represents 0, the compounds of the general formula (1)
'Structure 1' or
general formula (2) 'Structure 2' indicated above can be prepared from
immobilized
base salts of naproxen, suprofen, a-
methyl-(p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetic acid, flurbiprofen,
carprofen, pranoprofen, benoxaprofen, alminoprofen, tiaprofenic acid,
pirprofen,
zaltoprofen, bermoprofen, loxoprofen, indoprofen, fenclorac, oxaprozin,
fenbufen,
orpanoxin, ketorolac, clidanac, and related compounds of the general formula
(7)
'Structure 7',
CA 02660814 2009-02-13
WO 2008/020270 PCT/1B2006/052815
23
0
HB
Aryl
0
Structure 7
In structure 7, P represents cross-linked resin; Aryl represents aryl- and
heteroaryl
groups that are listed in 'structure l' and 'structure 2', B represents any
base groups,
such as pyridine, piperidine, triethylamine, or other base groups, by reaction
with
compounds of the general formula (6) 'Structure 6'.
Advantageous Effects
[301 These pro-drugs of naproxen, suprofen, a-
methyl-(p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetic acid, flurbiprofen,
carprofen, pranoprofen, benoxaprofen, alminoprofen, tiaprofenic acid,
pirprofen,
zaltoprofen, bermoprofen, loxoprofen, indoprofen, fenclorac, oxaprozin,
fenbufen,
orpanoxin, ketorolac, clidanac, and related compounds have a lipophilic
portion and a
hydrophilic portion (the amine groups that exist in the protonated form at
physiological
pH). The positively charged amino groups of these pro-drugs have two major
advantages. First, it largely increases the solubility of the drugs; when
these new pro-
drugs are administered orally in a dosage form such as a tablet, capsule,
solution, or
suspension, they will dissolve in gastric juice immediately. Second, the
positive charge
on the amino group of these pro-drugs will bond to the negative charge on the
phosphate head group of membrane. Thus, the local concentration outside of the
membrane will be very high and will facilitate the passage of these pro-drugs
from a
region of high concentration to a region of low concentration. When these pro-
drugs
enter the membrane, the hydrophilic part will push the pro-drugs into the
cytosol, a
semi-liquid concentrated aqueous solution or suspension. Due to the short stay
in the
GI tract, the pro-drugs will not cause gastric mucosal cell damage. Experiment
results
show that more than 90% of the pro-drugs were changed back to the drugs
itself. The
pro-drugs have a much better absorption rate, and thus the pro-drugs will have
better
strength than naproxen, suprofen, a-
methyl-(p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetic acid, flurbiprofen,
carprofen, pranoprofen, benoxaprofen, alminoprofen, tiaprofenic acid,
pirprofen,
zaltoprofen, bermoprofen, loxoprofen, indoprofen, fenclorac, oxaprozin,
fenbufen,
orpanoxin, ketorolac, clidanac, and related compounds at the same dosage. The
CA 02660814 2009-02-13
WO 2008/020270 PCT/1B2006/052815
24
experiment results suggest that the pro-drugs, diethylaminoethyl
2-(6-methoxy-2-naphthyl)propionate.AcOH, diethylaminoethyl a-
methy1-442-thienylcarbonyl)benzeneacetate.AcOH, diethylaminoethyl a-
methyl4p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetate.AcOH, diethy-
laminoethyl 2-(2-fluoro-4-biphenylyl)propionate.AcOH, diethylaminoethyl
6-chloro-a-methyl-9H-carbazole-2-acetate.AcOH, diethylaminoethyl a-methy1-5H-
[1]
benzopyrano[2,3-blpyridine-7-acetate.AcOH, diethylaminoethyl
2(4-chloropheny1)-a-methy1-5-benzoxazoleacetate.AcOH, diethylaminoethyl a-
methy1-44(2-methyl-2-propenypaminolbenzeneacetate.AcOH, diethylaminoethyl
5-benzoyl-a-methyl-2-thiopheneacetate.AcOH, diethylaminoethyl
3-chloro-4-(2,5-dihydro-1H-pyrrol-1-y1)-a-methyl benzeneacetate.AcOH, diethy-
laminoethyl 2-(10, 11-dihydro-10-oxodibenzo(b,f)thiepin-2-yl)propionate.AcOH,
di-
ethylaminoethyl 248-methy1-10,
11-dihydro-11-oxodibenz(b,f)oxepin-2-yl)propionate.AcOH, diethylaminoethyl
2[4(2-oxocyclopentyl-methyl)phenyllpropionate.AcOH, diethylaminoethyl
4-(1,3-dihydro-1-oxo-2H-isoindo1-2-y1)-a-methylbenzeneacetate.AcOH, diethy-
laminoethyl a,3-dichloro-4-cyclohexylbenzeneacetate.AcOH, diethylaminoethyl
4,5-Dipheny1-2-oxazole propionate.AcOH, diethylaminoethyl
3(4-biphenylylcarbonyl)propionate.AcOH, diethylaminoethyl
5-(4-chloropheny1)-beta-hydroxy-2-furanpropionate.AcOH, diethylaminoethyl
5-benzoy1-2,3-dihydro-1H-pyrrolizine-1-carboxylate.AcOH, or diethylaminoethyl
6-chloro-5-cyclohexy1-2,3-dihydro-1H-indene-1-carboxylate.AcOH diffuses
through
human skin -100-130 times faster than does naproxen, suprofen, a-
methyl4p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetic acid, flurbiprofen,
carprofen, pranoprofen, benoxaprofen, alminoprofen, tiaprofenic acid,
pirprofen,
zaltoprofen, bermoprofen, loxoprofen, indoprofen, fenclorac, oxaprozin,
fenbufen,
orpanoxin, ketorolac, or clidanac, and related compounds. It takes 2-4 hours
for
naproxen, suprofen, a-methyl(p-chlorobenzoy1)-5-methoxy-2-methylindole 3-
acetic
acid, flurbiprofen, carprofen, pranoprofen, benoxaprofen, alminoprofen,
tiaprofenic
acid, pirprofen, zaltoprofen, bermoprofen, loxoprofen, indoprofen, fenclorac,
oxaprozin, fenbufen, orpanoxin, ketorolac, or clidanac, and related compounds
to reach
the peak plasma level when they are taken orally, but these prodrugs only took
about
40-50 minutes to reach the peak plasma level. The most exciting result is that
the pro-
drugs can be administered not only orally, but also transdermally for any type
of
medical treatment and should avoid most of the side effects of naproxen,
suprofen, a-
methyl4p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetic acid, flurbiprofen,
carprofen, pranoprofen, benoxaprofen, alminoprofen, tiaprofenic acid,
pirprofen,
zaltoprofen, bermoprofen, loxoprofen, indoprofen, fenclorac, oxaprozin,
fenbufen,
CA 02660814 2009-02-13
WO 2008/020270 PCT/1B2006/052815
orpanoxin, ketorolac, or clidanac, and related compounds, most notably GI dis-
turbances such as dyspepsia, gastroduodenal bleeding, gastric ulcerations,
gastritis, and
renal toxicity. Another great benefit of transdermal administration of these
pro-drugs is
that administering medication, especially to children, will be much easier.
Description of Drawings
[31] Figure 1: Cumulative amounts of diethylaminoethyl
2-(6-methoxy-2-naphthyl)propionate.AcOH (A, 30% solution), diethylaminoethyl a-
methy1-442-thienylcarbonyl)benzeneacetate.AcOH (B, 30% solution), diethy-
laminoethyl a-methyl(p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetate.AcOH
(C, 30% solution), diethylaminoethyl 2-(2-fluoro-4-biphenylyl)propionate.AcOH
(D,
30% solution), diethylaminoethyl 6-chloro-a-methyl-9H-carbazole-2-acetate.AcOH
(E, 30% solution), naproxen (F, 30% suspension), suprofen (G, 30% suspension),
a-
methyl4p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetic acid (H, 30%
suspension), flurbiprofen (I, 30% suspension), or carprofen (J, 30%
suspension)
crossing isolated human skin tissue in Franz cells (n=5). In each case, the
vehicle was
pH 7.4 phosphate buffer (0.2 M).
[32] Figure 2: Cumulative amounts of diethylaminoethyl a-methyl-5H-[1]
benzopyrano[2,3-b]pyridine-7-acetate.AcOH (A, 30% solution), diethylaminoethyl
2(4-chloropheny1)-a-methy1-5-benzoxazoleacetate.AcOH (B, 30% solution), diethy-
laminoethyl a-methyl-4-[(2-methyl-2-propenyl)aminolbenzeneacetate.AcOH (C, 30%
solution), diethylaminoethyl 5-benzoyl-a-methyl-2-thiopheneacetate.AcOH (D,
30%
solution), diethylaminoethyl 3-chloro-4(2,5-dihydro-1H-pyrrol-1-y1)-a-methyl
benze-
neacetate.AcOH (E, 30% solution), pranoprofen (F, 30% suspension),
benoxaprofen
(G, 30% suspension), alminoprofen (H, 30% suspension), tiaprofenic acid (I,
30%
suspension), or pirprofen (J, 30% suspension) crossing isolated human skin
tissue in
Franz cells (n=5). In each case, the vehicle was pH 7.4 phosphate buffer (0.2
M).
[33] Figure 3: Cumulative amounts of diethylaminoethyl 2-(10,
11-dihydro-10-oxodibenzo(b,f)thiepin-2-yl)propionate.AcOH (A, 30% solution),
di-
ethylaminoethyl 248-methy1-10,
11-dihydro-11-oxodibenz(b,f)oxepin-2-yl)propionate.AcOH (B, 30% solution),
diethy-
laminoethyl 2[4(2-oxocyclopentyl-methyl)phenyllpropionate.AcOH (C, 30%
solution), diethylaminoethyl
4-(1,3-dihydro-1-oxo-2H-isoindo1-2-y1)-a-methylbenzeneacetate.AcOH (D, 30%
solution), diethylaminoethyla,3-dichloro-4-cyclohexylbenzeneacetate.AcOH (E,
30%
solution), zaltoprofen (F, 30% suspension), bermoprofen (G, 30% suspension),
loxoprofen (II, 30% suspension), indoprofen (I, 30% suspension), or fenclorac
(J, 30%
suspension) crossing isolated human skin tissue in Franz cells (n=5). In each
case, the
vehicle was pH 7.4 phosphate buffer (0.2 M).
CA 02660814 2009-02-13
WO 2008/020270 PCT/1B2006/052815
26
[34] Figure 4: Cumulative amounts of diethylaminoethyl 4,5-Dipheny1-2-
oxazole
propionate.AcOH (A, 30% solution), diethylaminoethyl
3(4-biphenylylcarbonyl)propionate.AcOH (B, 30% solution), diethylaminoethyl
5(4-chloropheny1)-beta-hydroxy-2-furanpropionate.AcOH (C, 30% solution),
diethy-
laminoethyl 5-benzoy1-2,3-dihydro-1H-pyrrolizine-1-carboxylate.AcOH (D, 30%
solution), diethylaminoethyl
6-chloro-5-cyclohexy1-2,3-dihydro-1H-indene-1-carboxylate.AcOH (E, 30%
solution),
oxaprozin (F, 30% suspension), fenbufen (G, 30% suspension), orparioxin (11,
30%
suspension), ketorolac (I, 30% suspension), or clidanac (J, 30% suspension)
crossing
isolated human skin tissue in Franz cells (n=5). In each case, the vehicle was
pH 7.4
phosphate buffer (0.2 M).
[35] Figure 5: Total plasma levels of naproxen, suprofen, a-
methyl4p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetic acid, flurbiprofen,
carprofen after topical application of 1 ml of a 20% solution of
diethylaminoethyl
2-(6-methoxy-2-naphthyl)propionate.AcOH (A), diethylaminoethyl a-
methy1-442-thienylcarbonyl)benzeneacetate.AcOH (B), diethylaminoethyl a-
methyl4p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetate.AcOH (C), diethy-
laminoethyl 2-(2-fluoro-4-biphenylyl)propionate.AcOH (D), diethylaminoethyl
6-chloro-a-methyl-9H-carbazole-2-acetate.AcOH (E), naproxen (F) , suprofen
(G), a-
methyl4p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetic acid (H),
flurbiprofen
(I), or caiprofen (J) in isopropanol to the backs of hairless mice (n=5).
[36] Figure 6: Total plasma levels of pranoprofen, benoxaprofen,
alminoprofen,
tiaprofenic acid, or pliprofen after topical application of diethylaminoethyl
a-
methy1-5H-[1]benzopyrano[2,3-blpyridine-7-acetate.AcOH (A), diethylaminoethyl
2(4-chloropheny1)-a-methy1-5-benzoxazoleacetate.AcOH (B), diethylaminoethyl a-
methy1-4-[(2-methyl-2-propenypaminolbenzeneacetate.AcOH (C), diethylaminoethyl
5-benzoyl-a-methyl-2-thiopheneacetate.AcOH (D), diethylaminoethyl
3-chloro-4-(2,5-dihydro-1H-pyrrol-1-y1)-a-methyl benzeneacetate.AcOH (E),
pranoprofen (F), benoxaprofen (G), alminoprofen (H), tiaprofenic acid (I), or
pirprofen
(J) 1 ml of a 20% solution of in isopropanol to the backs of hairless mice
(n=5).
[37] Figure 7: Total plasma levels of zaltoprofen, bermoprofen, loxoprofen,
indoprofen,
fenclorac after topical application of diethylaminoethyl 2-(10,
11-dihydro-10-oxodibenzo(b,f)thiepin-2-yl)propionate.AcOH (A),
diethylaminoethyl
248-methy1-10, 11-dihydro-11-oxodibenz(b,floxepin-2-yl)propionate.AcOH (B), di-
ethylaminoethyl 2[4(2-oxocyclopentyl-methyl)phenyllpropionate.AcOH (C), diethy-
laminoethyl 441,3-dihydro-1-oxo-211-isoindo1-2-y1)-a-methylbenzeneacetate.AcOH
(D), diethylaminoethyla,3-dichloro-4-cyclohexylbenzeneacetate.AcOH (E),
zahoprofen (F), bermoprofen (G), loxoprofen (H), indoprofen (I), fenclorac (J)
1 ml of
CA 02660814 2009-02-13
WO 2008/020270 PCT/1B2006/052815
27
a 20% solution of in isopropanol to the backs of hairless mice (n=5).
[381 Figure 8: Total plasma levels of oxaprozin, fenbufen, oipanoxin,
ketorolac, and
clidanac after topical application of diethylaminoethyl 4,5-Dipheny1-2-oxazole
propionate.AcOH (A), diethylaminoethyl 3(4-biphenylylcarbonyl)propionate.AcOH
(B), diethylaminoethyl 5(4-chloropheny1)-beta-hydroxy-2-furanpropionate.AcOH
(C),
diethylaminoethyl 5-benzoy1-2,3-dihydro-1H-pyrrolizine-1-carboxylate.AcOH (D),
di-
ethylaminoethyl 6-chloro-5-cyclohexy1-2,3-dihydro-1H-indene-1-carboxylate.AcOH
(E), oxaprozin (F), fenbufen (G), orpanoxin (H), ketorolac (I), or clidariac
(J)1 ml of a
20% solution of in isopropanol to the backs of hairless mice (n=5).
[391 Figure 9: The prolongation time of the pain threshold of mice tails
after 50mg/kg of
diethylaminoethyl 2-(6-methoxy-2-naphthyl)propionate.AcOH (B),
diethylaminoethyl
a-methyl-4(2-thienylcarbonyl)benzeneacetate.AcOH (C), diethylaminoethyl a-
methyl4p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetate.AcOH (D), diethy-
laminoethyl 2-(2-fluoro-4-biphenylyl)propionate.AcOH (E), diethylaminoethyl
6-chloro-a-methyl-9H-carbazole-2-acetate.AcOH (F) were administered
transdermally.
Group A is the control group.
[401 Figure 10: The prolongation time of the pain threshold of mice tails
after 50mg/kg
of diethylaminoethyl a-methyl-5H-[11benzopyrano[2,3-blpyridine-7-acetate.AcOH
(G), diethylaminoethyl 2(4-chloropheny1)-a-methy1-5-benzoxazoleacetate.AcOH
(H),
diethylaminoethyl a-methyl-4-[(2-methyl-2-propenypamino]benzeneacetate.AcOH
(I),
diethylaminoethyl 5-benzoyl-a-methyl-2-thiopheneacetate.AcOH (J), diethy-
laminoethyl 3-chloro-4(2,5-dihydro-1H-pyrrol-1-y1)-a-methyl
benzeneacetate.AcOH
(K) were administered transdermally. Group A is the control group.
[411 Figure 11: The prolongation time of the pain threshold of mice tails
after 50mg/kg
of diethylaminoethyl 2-(10,
11-dihydro-10-oxodibenzo(b,f)thiepin-2-yl)propionate.AcOH (L),
diethylaminoethyl
248-methyl- 10, 11-dihydro-11-oxodibenz(b,floxepin-2-y1)propionate.AcOH (M),
di-
ethylaminoethyl 2[4(2-oxocyclopentyl-methyl)phenyllpropionate.AcOH (N), diethy-
laminoethyl 441,3-dihydro-1-oxo-211-isoindo1-2-y1)-a-methylbenzeneacetate.AcOH
(0), diethylaminoethyl a,3-dichloro-4-cyclohexylbenzeneacetate.AcOH (P), were
ad-
ministered transdermally. Group A is the control group.
[421 Figure 12: The prolongation time of the pain threshold of mice tails
after 50mg/kg
of diethylaminoethyl 4,5-Dipheny1-2-oxazole propionate.AcOH (Q),
diethylaminoethyl
3(4-biphenylylcarbonyl)propionate.AcOH (R), diethylaminoethyl
5(4-chloropheny1)-beta-hydroxy-2-furanpropionate.Ac011 (S), diethylaminoethyl
5-benzoy1-2,3-dihydro-1H-pyrrolizine-1-carboxylate.AcOH (T), diethylaminoethyl
6-chloro-5-cyclohexy1-2,3-dihydro-1H-indene-1-carboxylate.AcOH (U) were ad-
ministered transdermally. Group A is the control group.
CA 02660814 2009-02-13
WO 2008/020270 PCT/1B2006/052815
28
[43] Figure 13. The rate of swelling (%) after a carrageenin injection. 1
Hour before the
carrageenin injection, diethylaminoethyl 2-(6-methoxy-2-
naphthyl)propionate.AcOH
(100 mg/kg, B), diethylaminoethyl a-
methy1-4-(2-thienylcarbonyl)benzeneacetate.AcOH (100 mg/kg, C),
diethylaminoethyl
a-methyl-(p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetate.AcOH (100 mg/kg,
D), diethylaminoethyl 2-(2-fluoro-4-biphenylyl)propionate.AcOH (100 mg/kg, E),
di-
ethylaminoethyl 6-chloro-a-methyl-9H-carbazole-2-acetate.AcOH (100 mg/kg, F)
were administered transdermally. A group is the control group.
[44] Figure 14. The rate of swelling (%) after a carrageenin injection. 1
Hour before the
carrageenin injection, diethylaminoethyl a-methyl-5H-Mbenzopyrano[2,3-b]
pyfidine-
7-acetate.AcOH (100 mg/kg, G), diethylaminoethyl
2-(4-chloropheny1)-a-methyl-5-benzoxazoleacetate.AcOH (100 mg/kg, H), diethy-
laminoethyl a-methyl-4-[(2-methyl-2-propenyl)aminolbenzeneacetate.AcOH (100
mg,/
kg, I), diethylaminoethyl 5-benzoyl-a-methyl-2-thiopheneacetate.AcOH (100
mg/kg,
J), diethylaminoethyl 3-chloro-4-(2,5-dihydro-1H-pyrrol-1-y1)-a-methyl benze-
neacetate.AcOH (100 mg/kg, K) were administered transdermally. Group A is the
control group.
[45] Figure 15. The rate of swelling (%) after a carrageenin injection. 1
Hour before the
carrageenin injection, diethylaminoethyl 2-(10,
11-dihydro-10-oxodibenzo(b,f)thiepin-2-yl)propionate.AcOH (100 mg/kg, L),
diethy-
laminoethyl 2-(8-methy1-10,
11-dihydro-11-oxodibenz(b,f)oxepin-2-yl)propionate.AcOH (100 mg/kg, M), diethy-
laminoethyl 214-(2-oxocyclopentyl-methyl)phenyllpropionate.AcOH (100 mg/kg,
N),
diethylaminoethyl 4-(1,3-dihydro-1-oxo-2H-isoindo1-2-y1)-a-methylbenz
eneacetate.AcOH (100 mg/kg, 0), diethylaminoethyl
a,3-dichloro-4-cyclohexylbenzeneacetate.AcOH (100 mg/kg, P) were administered
transdermally. Group A is the control group.
[46] Figure 16. The rate of swelling (%) after a carrageenin injection. 1
Hour before the
carrageenin injection, diethylaminoethyl 4,5-Dipheny1-2-oxazole
propionate.AcOH
(100 mg/kg, Q), diethylaminoethyl 3-(4-biphenylylcarbonyl)propionate.Ac0H (100
mg/kg, R), diethylaminoethyl
5-(4-chloropheny1)-beta-hydroxy-2-furanpropionate.AcOH (100 mg/kg, S), diethy-
laminoethyl 5-benzoy1-2,3-dihydro-1H-pyrrolizine-1-carboxylate.AcOH (100
mg/kg,
T), diethylaminoethyl
6-chloro-5-cyclohexy1-2,3-dihydro-1H-indene-1-carboxylate.AcOH (100 mg/kg, U)
were administered transdermally. Group A is the control group.
[47] Figure 17. In structure 1, R represents CU, OH, Cl, F, or Br; Ri
represents H, one
of any alkyl, alkyloxyl, alkenyl or alkynyl residues having 1 to 12 carbon
atoms, or
CA 02660814 2009-02-13
WO 2008/020270 PCT/1B2006/052815
29
aryl residues; R2 represents H, one of any alkyl, alkyloxy, alkenyl or alkynyl
residues
having 1 to 12 carbon atoms, or aryl residues; R3 represents H, one of any
alkyl,
alkyloxy, alkenyl or alkynyl residues having 1 to 12 carbon atoms, or aryl
residues; X
represents 0, S, NH, OCH2C00, OCH2COS, or OCH2CONH; A- represents Cl, Br, F
, I, Ac0-, citrate, or any negative ions; and n=0,1,2,3,4,5,6,7,8,9,10 ;
Aryl
represents aryl- and heteroaryl groups
[48] Figure 18. In structure 2, W represents H, OH, Cl, F, or Br; Ri
represents H, one of
any alkyl, alkyloxyl, alkenyl or alkynyl residues having 1 to 12 carbon atoms,
or aryl
residues; R2 represents H, one of any alkyl, alkyloxy, alkenyl or alkynyl
residues
having 1 to 12 carbon atoms, or aryl residues; R3 represents H, one of any
alkyl,
alkyloxy, alkenyl or alkynyl residues having 1 to 12 carbon atoms, or aryl
residues; X
represents 0, S, NH, OCH2C00, OCH2COS, or OCH2CONH; A- represents Cl, Br, F
, I, Ac0-, citrate, or any negative ions; and n=0,1,2,3,4,5,6,7,8,9,10 ; Y
or Y and Z
together represent aryl- and heteroaryl groups.
Best Mode
Preparation of diethylaminoethyl
2-(6-methoxy-2-naphthyl)propionate.AcOH
[49] 11.7 g (0.1 mol) of diethylaminoethanol was dissolved in 10% sodium
bicarbonate
(200 ml) and acetone (100 ml). 24.9 g (0.1 mol) of 2-(6-methoxy-2-naphthyl)
propionyl chloride was added into the reaction mixture. The mixture is stirred
for 3
hours at RT. The solvents are evaporated off. The residue is suspended in
ethyl acetate
(500m1). 5% sodium bicarbonate (200 ml) is added into the reaction mixture
with
stiffing. Ethyl acetate layer is collected and washed with water (3 x 500 ml).
The ethyl
acetate solution was dried over anhydrous sodium sulfate. Sodium sulfate is
removed
by filtration. 6 g of acetic acid is added into the reaction mixture with
stirring. The
organic solution was evaporated off. After drying, it yielded 36 g of the
desired
product (89.9 %). Hygroscopic product; Solubility in water: 350 mg/ml;
Elementary
analysis: C221131N05; MW: 389.49. Calculated % C: 67.84; H: 8.02; N: 3.60; 0:
20.54;
Found % C: 67.82; H: 8.04; N: 3.58; 0: 20.56. 1H-NMR (400 MHz, DO): 6: 1.36
(t,
6H), 1.50 (d, 3H), 2.11 (s, 3H), 3.20 (m, 4H), 3.47(m, 2H), 3.70 (s, 3H), 3.78
(m, 1H),
4.48 (t, 2H), 6.88 (b, 1H), 6.98 (s, 1H), 7.03 (d, 1H), 7.18 (d, 111), 7.43
(s, 1H), 7.50 (d,
1H), 7.54 (d, 1H).
Mode for Invention
Preparation of diethylaminoethyl a-methy1-4-(2-thieny1carbony1) benze-
neacetate.AcOH.
[50] 28.1 g (0.1 mol) of a-methyl-4-(2-thienylcarbonyl) benzeneacetyl
chloride was
dissolved in 100 ml of chloroform. The mixture was cooled to 0 C. 15 ml of tri-
CA 02660814 2009-02-13
WO 2008/020270 PCT/1B2006/052815
ethylamine and 11.7 g (0.1 mol) of diethylaminoethanol were added into the
reaction
mixture. The mixture is stirred for 3 hours at RT. The solvents are evaporated
off. The
residue is dissolved in methanol (300m1), 5% sodium bicarbonate (200 ml) is
added
into the reaction mixture. The mixture is stiffed for 3 hr. The mixture is
evaporated to
dryness. Methanol (300 ml) is added into the residue with stirring. Solid is
removed by
filtration and washed with methanol. The solution is evaporated to dryness and
the
residue is dissolved in chloroform (200 ml). 6 g of acetic acid is added into
the reaction
mixture with stirring. Some solid is removed by filtration. Another 6 g of
acetic acid is
added into the reaction mixture with stirring. The organic solution was
evaporated off.
After drying, it yielded 35 g of the desired product (83.2%). Hygroscopic
product;
Solubility in water: 400 mg/ml; Elementary analysis: C22H3iNO5S; MW: 419.53.
Calculated % C: 62.68; H: 7.41; N: 3.32; 0: 18.98; S: 7.61; Found % C: 62.63;
H:
7.45; N: 3.31; 0: 19.01; S: 7.60. 1H-N4R (400 MHz, DO): 8: 1.36 (t, 6H), 1.45
(d,
3H), 2.11 (s, 3H), 3.20 (m, 411), 3.47(m, 211), 3.78 (m, 1H), 4.48 (t, 2H),
6.88 (b, 1H),
6.98 (s, 111), 7.31 (d, 2H), 7.05 (m, 1H), 7.43 (m, 211), 7.70 (d, 211).
Preparation of S-diethylarninoethyl
2-(2-fluoro-4-biphenylyl)propionate.AcOH
[51] 13.2 g (0.1 mol) of diethylaminoethyl mercaptan was dissolved in 10%
sodium bi-
carbonate (200 ml) and acetone (100 ml). 26.3 g (0.1 mol) of 2-(2-fluoro-4-
biphenyly1)
propionyl chloride was added into the reaction mixture. The mixture is stirred
for 3
hours at RT. The solvents are evaporated off. The residue is suspended in
ethyl acetate
(500m1). 5% sodium bicarbonate (200 ml) is added into the reaction mixture
with
stirring. Ethyl acetate layer is collected and washed with water (3 x 500 ml).
The ethyl
acetate solution was dried over anhydrous sodium sulfate. Sodium sulfate is
removed
by filtration. 6 g of acetic acid is added into the reaction mixture with
stirring. The
organic solution was evaporated off. After drying, it yielded 36 g of the
desired
product (85.8%). Hygroscopic product; Solubility in water: 400 mg/ml;
Elementary
analysis: C231130FNO3S; MW: 419.55. Calculated % C: 65.84; H: 7.21; F: 4.53;
N:
3.34; 0: 11.44; S: 7.64. Found % C: 65.80; H: 7.23; F: 4.55; N: 3.32, 0:
11.47; S:
7.63. 111-NMR (400 MHz, D20): 8: 1.35 (t, 611), 1.44 (d, 311), 2.11 (s, 311),
3.20 (m,
4H), 3.30(t, 2H), 3.80 (m, 111), 3.88 (t, 2H), 6.88 (b, 111), 6.88 (m, 1H),
6.95 (m, 1H),
7.22 (m, 111), 7.32 (m, 211), 7.41 (m, 111), 7.48 (m, 211).
Preparation of N-diethylaminoethyl 5-benzoy1-2,
3-dihydro-1H-pyrrolizine-1-carboxylarnide.AcOH.
[521 11.6 g (0.1 mol) of diethylaminoethylamine was dissolved in 10%
sodium bi-
carbonate (200 ml) and acetone (100 ml). 27.4 g (0.1 mol) of 5-benzoy1-2,
3-dihydro-1H-pyrrolizine-1-carboxyly1 chloride was added into the reaction
mixture.
CA 2660814 2017-05-17
81621270
31
The mixture is stirred for 3 hours at RT. The solvents are evaporated off. The
residue is
suspended in ethyl acetate (500m1). 5% sodium bicarbonate (200 ml) is added
into the
reaction mixture with stirring. Ethyl acetate layer is collected and washed
with water (3
x 500 m1). The ethyl acetate solution was dried over anhydrous sodium sulfate.
Sodium
sulfate is removed by filtration. 6 g of acetic acid is added into the
reaction mixture
with stirring. The organic solution was evaporated off. After drying, it
yielded 35 g of
the desired product (84.8 %). Hygroscopic product; Solubility in water: 400
metril;
Elementary analysis: C.H31N304; MW: 412.50. Calculated % C: 66.81; H: 7.56; N:
10.16; 0: 15.48; Found % C: 66.90; H: 7.38; N: 10.18; 0: 15.54. 'H-NIV1R (400
MHz,
DO): 8: 1.39 (t, 611), 2.10 (s, 311), 2.27 (m, 211), 3.22 (in, 411), 3.50(t,
211), 3.60 (t,
2
211), 3.80 (m, 211), 3.71 (in, 111), 5.85 (m, 111), 6.70 (m, 1H), 6.85 (b,
1H), 7.32 (b,
1H), 7.40 (m, 1H), 7.45 (in, 211), 7.78 (m, 211).
Preparation of N-diethylairninoethyl 4, 5-Dipheny1-2-oxazole pro-
pionamideAcOH
[53] 29.3 g (0.1 mol) of 4, 5-Dipheny1-2-oxazole propionic acid was
dissolved in 100 ml
of acetonitrile. 32.1 g of 0-(Benzotriazol-1-371)-N,N,N,N-tetramethyluronium
tetraflu-
oroborate and 30 ml of triethylamine were added into the reaction mixture.
11.6 g of
diethylaminoethylamine was added into the reaction mixture. The mixture was
stirred
for 3 hours at RT. The solvents were evaporated off. 250 ml of ethyl acetate
was added
into the reaction mixture and the mixture was washed with water (3 x 100 ml).
The
organic solution was dried over anhydrous sodium sulfate. Sodium sulfate was
removed by filtration. 6 g of acetic acid was added into the reaction mixture
with
stirring. Hexane (200 ml) was added. The solid product was collected by
filtration.
After drying, it yielded 40 g of the desired product (88.6%). Hygroscopic
product;
Solubility in water: 400 mg/ml; Elementary analysis: C26H33N304; MW: 451.56.
Calculated % C: 69.16; 11: 7.37; N: 9.31; 0: 14.17; Found % C: 69.11; H: 7.40;
N:
9.30; 0: 14.19. 'H-NMR (400 MHz, DO): 8: 1.41 (t, 61), 2.10 (s, 311), 2.45 (t,
211),
2.76 (t, 211), 3.22 (m, 411), 3.49(t, 211), 3.60 (t, 211), 6.87 (b, 111), 7.22
(b, 111), 7.22
(in, 211), 7.32 (m, 4H), 7.47 (m, 411).
Preparation of diethylarninoethyl
6-ehloro-a-methyl-911-earbazo1e-2-acetate.AcOH.
[54] 60 g of Polymer-bound triethylamine (3 mol/g, 100-200 mesh) was
suspended in
180 ml of chloroform. 27.4 g (0.1 mol) of 6-chloro-a-methy1-9H-carbazole-2-
acetic
acid, was added into the mixture with stirring. 43 g (0.15niol) of
diethylaminoethyl
bromide.HBr was added into the mixture and the mixture was stirred for 5 hours
at RT.
The polymer was removed by filtration and washed with tetrahydrofuran (3 x 50
m1).
8.2 g (0.1 mol) of sodium acetate was added into the reaction mixture with
stirring,
CA 02660814 2013-10-18
=
51915-44
32
The mixture was stirred for 2 h. The solid was removed by filtration and
washed with
chloroform (3 x 50 ml). The solution was concentrated in vacuo to 100 ml. Then
300
ml of hexane was added into the solution. The solid product was collected by
filtration
and washed with hexane (3 x 100 ml). After drying, it yielded 38 g of the
desired
product (87.8%). Hygroscopic product; Solubility in water: 400 mg/m1;
Elementary
analysis: C231129C1N204; MW: 432.94. Calculated % C: 63.81; 11: 6.75; Cl:
8.19, N:
6.47; 0: 14.78; Found % C: 63.85; H: 6.78; Cl: 8.17; N: 6.44; 0: 14.76. 'H-NMR
(400
MHz, DO): 6: 1.39 (t, 611), 1.47 (d, 3H), 2.11 (s, 311), 3.21 (m, 4H), 3.49(m,
2H), 3.77
(m, 111), 4.48 (t, 211), 6.80 (b, 111), 6.85 (m, 111), 7.10 (m, 111), 7.05 (m,
11-1), 7.26 (m,
11-1), 7.34 (m, 111), 7.50 (m, 111), 7.52 (m, 1H).
Industrial Applicability
[55] The pro-drugs of the general formula (1) 'Structure l' and
general formula (2)
'Structure 2' are superior to naproxen, suprofen, a-
methyl-(p-chlorobenzoy1)-5-methoxy-2-methylindole 3-acetic acid, flurbiprofen,
carprofen, pranoprofen, benoxaprofen, alminoprofen, tiaprofenic acid,
pirprofen,
zaltoprofen, bermoprofen, loxoprofen, indoprofen, fenclorac, oxaprozin,
fenbufen,
orpanoxin, ketorolac, or clidanac, and related compounds. They may be used
medicinally in treating any naproxen, suprofen, a-
methyl-(p-chlorobenzoyI)-5-methoxy-2-methylindole 3-acetic acid, flurbiprofen,
carprofen, pranoprofen, benoxaprofen, ahninoprofen, tiaprofenic acid,
pirprofen,
zahoprofen, bermoprofen, loxoprofen, indoprofen, fenclorac, oxaprozin,
fenbufen,
orpanoxin, ketorolac, clidanac, and related compounds-treatable conditions in
humans
or animals. They may be used for the relief of signs and symptoms of
rheumatoid
arthritis and osteoarthritis, the reduction of fever, and the treatment of
dysinenorrhea.
They may be also prescribed for diabetic neuropathy and acute migraine
headache.
Due to their very high membrane penetration rate, these pro-drugs can be used
in
treating asthma by inhalation to a host. They can be used to treat psoriasis,
acne,
sunburn or other skin disorders due to their anti-inflammatory properties.
[561