Note: Descriptions are shown in the official language in which they were submitted.
CA 02662811 2009-04-27
HYDROCARBON-BASED FRACTURING FLUID COMPOSITIONS,
METHODS OF PREPARATION AND METHODS OF USE
FIELD OF THE INVENTION
[0001] The invention describes improved fracturing compositions, methods of
preparing fracturing compositions and methods of use. Importantly, the subject
invention overcomes problems in the use of mists and/or slugs as an effective
fracturing composition particularly having regard to the ability of a mist to
transport an effective volume of proppant into a formation. As a result, the
subject technologies provide an effective economic solution to using high
ratio
gas fracturing compositions that can be produced in a continuous (i.e. non-
batch)
process without the attendant capital and operating costs of current pure gas
fracturing equipment.
BACKGROUND OF THE INVENTION
[0002] As is well known in the hydrocarbon industry, many wells require
"stimulation" in order to promote the recovery of hydrocarbons from the
production zone of the well.
[0003] One of these stimulation techniques is known as "fracturing" in which a
fracturing fluid composition is pumped under high pressure into the well
together
with a proppant such that new fractures are created and passageways within the
production zone are held open with the proppant. Upon relaxation of pressure,
the combination of the new fractures and proppant having been forced into
those
fractures increases the ability of hydrocarbons to flow to the wellbore from
the
production zone.
-1-
CA 02662811 2009-04-27
[0004] There are a significant number of fracturing techniques and
fluid/proppant
compositions that promote the formation of fractures in the production zone
and
the delivery of proppants within those fractures. The most commonly employed
methodologies seek to create and utilize fracturing fluid compositions having
a
high viscosity that can support proppant materials so that the proppant
materials
can be effectively carried within the fracturing fluid. In other words, a
viscous fluid
will support a proppant within the fluid in order that the proppant can be
carried a
greater distance within the fracture or in some circumstances carried at all.
In
addition, fracturing fluids are commonly designed such that upon relaxation of
viscosity (or other techniques) and over time (typically 90 minutes or so),
the fluid
viscosity drops and the proppant is "dropped" in the formation, and the
supporting fluid flows back to the wellbore. The proppant, when positioned in
the
fracture seeks to improve the permeability of the production zone in order
that
hydrocarbons will more readily flow to the well. An effective fracturing
operation
can increase the flow rate of hydrocarbons to the well by at least one order
of
magnitude by reducing well to formation communication impairment. Many wells
won't produce long term in an economic manner without being stimulated by
methods such as fracturing.
[0005] Fracturing fluid compositions are generally characterized by the
primary
constituents within the composition. The most commonly used fracturing fluids
are water-based or hydrocarbon-based fluids, defined on the basis of either
water or a hydrocarbon being the primary constituent of the specific
composition.
Each fracturing fluid composition is generally chosen on the basis of the
subterranean formation characteristics and the economics of conducting a
fracturing operation at a particular well or group of wells.
[0006] In the case of hydrocarbon-based fluids, in order to increase the
viscosity
of liquid hydrocarbon, various "viscosifying" additives may be added to the
hydrocarbon-based fluid at the surface such that the viscosity of the
hydrocarbon-based fluid is substantially increased thereby enabling it to
support
-2-
CA 02662811 2009-04-27
proppant. As is known, these hydrocarbon-based fluids may include other
additives such as breakers and/or other additives to impart various properties
to
the fluid as known to those skilled in the art. The most commonly used
viscosifying additives are phosphate esters and metal complexors that are used
to create fluids having moderate to high viscosities.
[0007] During a fracturing operation, the fracturing composition (without any
proppant) is initially pumped into the well at a sufficiently high pressure
and flow
rate to fracture the formation. After fracturing has been initiated, proppant
is
added to the fracturing fluid, and the combined fracturing fluid and proppant
is
forced into the fractures in the production zone. When pressure is released
and
over time (typically 90 minutes), the viscosity of the fracturing fluid drops
so that
the proppant separates or drops out of the fracturing fluid within the
formation
and the "de-viscosified" fracturing fluid flows back to the well where it is
removed
up the well back to the wellhead at surface.
[0008] Problems in this type of fracturing are the volumes and cost of liquid
hydrocarbon required and the attendant issues relating to the disposal of the
liquid hydrocarbon that has been pumped downhole and ultimately recovered
from the well. As a result, in some cases the industry has moved away from
pure
hydrocarbon-based fracturing fluids in favor of those technologies that
utilize a
high proportion of gas (usually nitrogen) as the fracturing fluid, or cheaper
fluids
such as aqueous fluids.
[0009] The use of a high proportion of gas has several advantages including
minimizing formation damage, reducing fluid supply costs as well as a
reduction
in the fluid disposal costs of fluid that is recovered from the well. For
example,
whereas liquid hydrocarbon may reduce the ability of a production zone to flow
by adherence to pore throats in the matrix rock of the formation and/or by
hydrostatically holding back the formation with a column of flow back fluid in
the
well, high gas compositions will often minimize such damage and/or effects and
will otherwise migrate from the formation more readily. In addition, gas
injected
-3-
CA 02662811 2009-04-27
and thus recovered from a well can simply be released to the atmosphere
thereby obviating the need for decontamination and disposal of a substantial
volume of non-gaseous materials recovered from the well.
[0010] With high ratio gas fracturing compositions, the characteristics of the
compositions can be similarly controlled or affected by the use of additives.
Generally, gas fracturing compositions can be characterized as a pure gas
fracturing composition (typically a fluid comprising around 100% C02 or
nitrogen)
or energized and foamed fluids (typically a fracturing composition comprising
less
than about 75% nitrogen by volume when dealing with hydrocarbon based
fluids).
[0011]A pure 100% gas fracturing composition will have minimal viscosity and
instead will rely on high turbulence to transport proppant as it is pumped
into the
production zone. Unfortunately, while such techniques are effective in limited
batch operations, the need for expensive, highly specialized, pressurized
pumping, mixing and containment equipment substantially increases the cost of
an effective fracturing operation. For example, a fracturing operation that
can
only utilize a batch process is generally limited in size to the volumetric
capacity
of a single pumping and containment unit. As it is economically impractical to
employ multiple units at a single fracturing operation, the result is that
very high
volume gas fracturing operations can only be effectively employed in
relatively
limited circumstances. For example, a pure gas fracturing operation would
typically be limited to pumping 300-32,000 kg of sand (proppant) into a well
and
may also be limited to the type of proppant that can be used in some
circumstances.
[0012] The use of non-energized, energized and foamed fluids as fracturing
fluids
are generally not limited to batch operations as fluid mixing and pumping
equipment for such fluids is generally not at the same scale in terms of the
complexity/cost of equipment that is required for pure gas operations. In
other
words, the mixing and pumping equipment for a non-energized/energized/
-4-
CA 02662811 2009-04-27
foamed fluid fracturing operation is substantially less expensive and
importantly,
can produce effectively large and continuous volumes of fracturing fluid mixed
with most types of available proppant. That is, while a 100% gas fracturing
operation may be able to deliver up to 32,000 kg of proppant to a formation, a
non-energized/energized/foamed fluid fracturing operation may be able to
deliver
in excess of 10 times that amount.
[0013] The characteristics of energized and foamed fluids are briefly outlined
below as known to those skilled in the art.
[0014] An energized fluid will generally have less than about 53% (volume % at
down hole pressure and temperature) gas together with a liquid phase typically
either water or hydrocarbon based. An energized fluid is further characterized
by
a continuous fluid phase with gas bubbles that are not concentrated enough to
interact with each other to increase viscosity. For example, the overall
viscosity
of an energized fluid comprised of a fluid phase and nitrogen gas may be in
the
range of 200 cP which is a "mid-point" between the viscosity of a typical
hydrocarbon-based phase (300 cP) and a nitrogen gas phase (0.01 cP). As is
known, and in the context of this description, viscosity values measured in
centipoise (cP) are dependent on shear rate and temperature. In this
specification, all viscosity values are referenced to a shear rate of 170 sec'
and
293 K.
[0015] Foams will generally have greater than about 53 vol% gas but less than
about an upper limit of 75 vol% gas with the remainder being a gelled liquid
hydrocarbon phase. Stable hydrocarbon foams generally have an upper limit
that is lower than that of water foams, which for water is about 85 vol%.
Foams
are characterized as having a continuous fluid film between adjacent gas
bubbles
where the gas bubbles are concentrated enough to interact with each other to
increase viscosity. Foams require the addition of foaming agents that promote
stability of the gas bubbles. For example, the viscosity of a hydrocarbon foam
will
typically be in the range of 200-1000 cP which may be 2 to 10 times greater
than
-5-
CA 02662811 2009-04-27
the viscosity of the hydrocarbon liquid phase (20-800 cP) and many times
greater
than the viscosity of the gas phase (0.01-0.1 cP).
[0016] Hydrocarbon based fluids behave differently than water based fluids in
terms of the solubility preferences between nitrogen and carbon dioxide, the
two
most commonly used fracturing gases as well as other factors as discussed
below. Water based fluids have similar solubility properties with either gas
under
a large range of pressures and temperatures, wherein nearly all the added gas
forms a second and distinct gas phase when creating a foam or emulsion. In
comparison, hydrocarbon based fluids have a tendency to combine with carbon
dioxide to form a single miscible phase under some temperatures and pressures
whereas nitrogen has a very small solubility in hydrocarbon fluids. As such,
carbon dioxide miscibility with the hydrocarbon based fluid, depending on the
pressure and temperature, can range in effect from completely involving all
mixed gas to leave a single miscible liquid phase without a gas phase to
having
nearly a liquid hydrocarbon phase with a gas phase and no miscibility effects.
[0017] In addition, when a hydrocarbon based fluid includes chemical additives
at
sufficient concentrations to cause various effects, and with carbon dioxide
forming a single miscible phase with the hydrocarbon, a hydrocarbon/carbon
dioxide system may have the effect of diluting the active chemicals and
changing
the fluid properties.
[0018] Further still, the amount of carbon dioxide that will form a single
miscible
phase with hydrocarbon based fluids is highly variable depending on the
pressure, temperature and specific blend of components of the hydrocarbon
fluid
which may be affected by pressure and temperature in the wells during a
fracturing operation.
[0019] Furthermore, hydrocarbon based fluids have a greater chemical
sensitivity
to carbon dioxide gas compared to nitrogen gas. The most commonly used
breaker technology for hydrocarbon fluids is a high pH breaker such as
-6-
CA 02662811 2009-04-27
magnesium oxide as the active ingredient. Carbon dioxide creates a low pH in
trace water which can counteract the high pH breaker to affect the designed
fluid
chemistry to form viscosity and reduce it again over an intended quantity of
time.
[0020] There are also differences in safety implications regarding hydrocarbon
based fluids and water based fluids. For example, the normal injection methods
of water based fracturing fluids into a well and ultimately the production
formation
will utilize either of or a combination of tubing, casing or coiled tubing.
For
hydrocarbon based fracturing fluids, these fluids are normally restricted from
being injected via coiled tubing due to the safety risk in the event of a
coiled
tubing leak or burst, and accordingly would normally be restricted to
injection via
casing, tubing or manifolded casing and tubing. Moreover, the safety risk is
intensified when compressed gases are combined with the hydrocarbon based
fluid.
Mists
[0021 ] As is known, when the gas concentration is increased above about 75%
in
a hydrocarbon based fluid or above about 85% for water based fluids,
(typically
90-97%), the stability of a typical foam will decrease, such that the foam
will "flip"
such that the gas phase becomes continuous and the liquid hydrocarbon phase
is dispersed with the gas phase as small droplets or in larger slugs. This is
commonly referred to as a "mist". The viscosity of a mist will generally
revert to a
"mid-point" of viscosity close to that of the gas (i.e. approximately 1-3
orders of
magnitude lower than that of a foam) with the result being that the ability to
support proppant based on viscosity is substantially reduced.
[0022] As a result, fracturing compositions generally avoid the formation of
mists
and instead favor stabilizing foams and otherwise maximizing viscosities.
[0023] A review of the prior art shows that the active promotion and use of a
mist
as a fracturing composition within hydrocarbon based fracturing fluids has not
been considered.
-7-
CA 02662811 2009-04-27
[0024] For example, US Patent 7,261,158 discloses a high concentration gas
fracturing composition that is a "coarse foam"; US Patent 6,844,297 discloses
fracturing compositions including an amphoteric glycinate surfactant that
increases viscosity and enables viscosity control of the compositions through
pH
adjustment; US Patent 6,838,418 discloses fracturing fluid including a polar
base,
a polyacrylate and an "activator" that ionizes the polyacrylate to a
hydroscopic
state; US Patent 4,627,495 discloses methods using carbon dioxide and nitrogen
to create high gas concentration foams; US Patent 7,306,041 discloses acid
fracturing compositions that contain a gas component; US Publication
2007/0204991 describes a method and apparatus for fracturing utilizing a
combined liquid propane/nitrogen mixture; US Publication 2006/0065400
describes a method for stimulating a formation using liquefied natural gas;
and,
US Publication 2007/0023184 describes a well product recovery process using a
gas and a proppant.
SUMMARY OF THE INVENTION
[0025] In accordance with the invention, there is provided fracturing fluid
compositions and methods of preparing and using such compositions for
fracturing a well.
[0026] In its broadest form, the fracturing fluid compositions comprise: a
liquid
component for temporarily supporting a proppant within the liquid component at
surface, the liquid component including: a viscosified liquid hydrocarbon
component having an initial viscosity sufficient to temporarily support
proppant
admixed within the viscosified liquid hydrocarbon component; and, a breaker
for
relaxing the viscosity of the viscosified liquid hydrocarbon component within
a
pre-determined period; wherein the concentration of breaker within the liquid
component is sufficient to relax the initial viscosity of the liquid component
to less
than 10 cP at 170 sec' at 293K within a pre-determined time period of 30
minutes.
-8-
CA 02662811 2009-04-27
[0027] In further embodiments, the fracturing fluid composition further
includes a
proppant admixed within the viscosified liquid hydrocarbon component. Still
further, the composition may include a gas component admixed with the liquid
hydrocarbon under high turbulence conditions sufficient to support the
proppant
within a combined liquid hydrocarbon/gas component mixture wherein the
combined liquid hydrocarbon/gas component mixture is characterized as a mist
or liquid slug. The gas component may be nitrogen.
[0028] In further embodiments, the combined liquid hydrocarbon/gas component
mixture is 3-25 vol% liquid component and 75-97 vol% gas component exclusive
of the proppant.
[0029] In another embodiment, the pre-determined period is less than 10
minutes.
[0030] In one embodiment, the initial viscosity of the liquid component is 15-
1000
centipoise (cP) at 170 sec' at 293K prior to mixing with proppant or gas
component.
[0031] In other embodiments, the mass of proppant is 0.25-5.0 times the mass
of
the liquid component or 1.0-2.5 times the mass of the liquid component.
[0032] In other embodiments, the viscosified liquid hydrocarbon component
includes 0.4-3.0 wt% gelling and complexor agents. In yet further embodiments,
the gelling agent may be a phosphate ester and the complexor agents may be
any one of or a combination of iron sulphate and an amine complexing agent. In
one embodiment, the breaker is magnesium oxide or calcium oxide.
[0033] In one embodiment, the liquid component includes less than 0.1 vol% non-
foaming surfactant.
[0034] In another aspect of the invention, a method of fracturing a formation
within a well is provided comprising the steps of: preparing a liquid
component at
-9-
CA 02662811 2009-04-27
surface in a blender, the liquid component including: a viscosified liquid
hydrocarbon component having an initial viscosity sufficient to temporarily
support proppant admixed within the viscosified liquid hydrocarbon component;
and, a breaker for relaxing the viscosity of the viscosified liquid
hydrocarbon
component within a pre-determined period wherein the concentration of breaker
within the viscosified liquid hydrocarbon component is sufficient to relax the
viscosity of the liquid hydrocarbon component to less than 10 cP at 170 sec -1
at
293K within 30 minutes; mixing the proppant into the liquid component in the
blender; introducing the proppant/liquid component into a high pressure pump
and increasing the pressure to well pressure; introducing a gas component the
high pressure pump and increasing the pressure to well pressure; mixing the
gas
component with the proppant/liquid component under high turbulence conditions;
and, pumping the combined gas and fluid at a high rate down the well.
[0035] In one embodiment, the combined gas and fluid is characterized as a
mist
or slug at the formation.
[0036] In further embodiments, the combined gas and fluid in step f) is 3-25
vol%
liquid component and 75-97 vol% gas component exclusive of the proppant.
[0037] In another embodiment, the initial viscosity of the viscosified liquid
hydrocarbon component is 15-1000 centipoise (cP) at 170 sec' at 293K prior to
mixing with proppant or gas component.
[0038] In further embodiments the mass of proppant mixed in is 0.25-5.0 times
the mass of the liquid component.
[0039] In other embodiments, the viscosified liquid component includes 0.4 to
3.0
wt% gelling and complexor agents that may be selected from phosphate esters,
magnesium oxide and calcium oxide.
[0040] Non-foaming surfactant may be mixed with the viscosified liquid
component.
-10-
CA 02662811 2009-04-27
[0041] In one embodiment, the process is continuous (i.e. non-batch) and may
be
preceded by a 100% gas pad.
BRIEF DESCRIPTION OF THE FIGURES
[0042] The invention is described with reference to the accompanying figures
in
which:
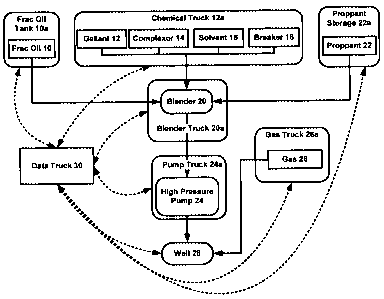
Figure 1 is an overview of a typical equipment configuration for a
fracturing operation in accordance with the invention;
Figure 2 is a graph showing liquid component viscosity vs. time for
different concentrations of breaker;
Figure 3 is a graph showing foam stability vs. time for liquid component
compositions having different concentrations of foaming or non-foaming
surfactant agents; and
Figure 4 is a graph showing proppant support characteristics from sand
sample settling rates falling through liquid component compositions having
different concentrations of breaker.
DETAILED DESCRIPTION
[0043] With reference to the accompanying figures, novel fracturing
compositions, methods of preparation and methods of use are described.
Importantly, the subject technologies overcome problems in the use of mists as
an effective fracturing composition particularly having regard to the ability
of a
mist to transport an effective volume of proppant into the formation. As a
result,
the subject technologies provide an effective economic solution to using high
-11-
CA 02662811 2009-12-18
ratio gas fracturing compositions that can be produced in a continuous (i.e.
non-
batch) process without the attendant capital and operating costs of current
pure
gas fracturing equipment.
[0044] Generally, compositions prepared in accordance with the invention
include
a liquid component (hydrocarbon-based component) and a gas component in
proportions that promote the formation of a mist. In the context of this
description
reference to a gas component refers to a compound that is a gas at standard
temperature and pressure (288 K and 1 atm) such as nitrogen that is used in
fracturing.
[0045] More specifically, the present compositions include a 3-25% liquid
component (typically about 5%) and a 75-97% gas component (typically about
95%).
[0046] With reference to Figure 1, fracturing fluid compositions are generally
prepared and utilized in accordance with the following methodology:
a. A liquid component (e.g. frac oil 10) having desired properties is
prepared at surface in a blender 20 with chemical additives (e.g.
gellant 12, complexor 14, surfactant 16, breaker 18) from chemical
truck 12a and frac oil tank 10a.
b. Proppant 22 from proppant storage 22a is added to the liquid
component;
c. The combined liquid/proppant mixture is introduced into a high
pressure pump 24 and pressurized to well pressure by the high
pressure pump in pump truck 24a;
d. A gas component 26 (typically, nitrogen) is introduced into a high
pressure line leading to the well 28 where it mixes with the
combined liquid/proppant mixture;
-12-
CA 02662811 2009-04-27
e. The pressurized combined liquid/proppant/gas is pumped at a high
rate down the well 28;
f. The fracturing operation proceeds with the above fracturing fluid
compositions being continuously prepared at the surface with
varying ratios;
g. Upon completion, surface mixing and pressurization are ceased
and the surface equipment is detached and removed from the well;
h. The well is flowed to remove as much fracturing gas and liquid
component as possible and turned over to production of
hydrocarbons from the production zone;
i. Control of the system and data from the system is provided by or
received by data truck 30.
[0047] It is understood that in normal fracturing operations using methods
described herein, proppant stages would be preceded by a 100% gas pad stage.
[0048] As shown in Figure 1, and as will be explained in greater detail below,
the
preparation and blending of the liquid and gas components is achieved at a
well
site utilizing portable equipment.
[0049] Importantly, in comparison to past non-energized, energized or foamed
fluid technologies, the subject technology does not require the supply of as
high
volume of fluids for injection nor the disposal of as high volumes of fluids
recovered from the well as the relative proportion of liquid hydrocarbon in
the
overall fracturing fluid composition is substantially lower than that of a non-
energized, energized or foamed fluid. In comparison to past 100% pure gas
technologies, the subject technology, by virtue of the liquid component
supporting proppant prior to mixing, the need for specialized, pressurized
batch
mixing equipment is eliminated.
-13-
CA 02662811 2009-04-27
Fluid Compositions
Liquid Component
[0050] The liquid component generally comprises (A) a gelled liquid
hydrocarbon,
(B) a breaker, and (C) a non-foaming surfactant(s). The liquid component is
designed to impart adequate but short-lived viscosity to the liquid component
such that proppant can be temporarily supported within the liquid component at
surface without settling and plugging surface pumping equipment. It is further
designed such that the viscosity of the liquid component promptly relaxes
during
and after fracturing to promote mist or liquid slug formation and ensure flow
back
to the well. In the context of this description, viscosity is measured at 170
sec -1
and referenced to 293K.
A-Gelled Liquid hydrocarbon
[0051]The gelled liquid hydrocarbon is formed from about 98 wt% liquid
hydrocarbon, 0.51 wt% alkyl phosphate ester, 0.09 wt% aryl ether phosphate
ester (gelling agents), 0.29 wt% ferric sulphate (complexor), and 0.12 wt% C2-
C18 tertiary alkoxylated amines (complexor). The complexors act as
crosslinkers
to increase viscosity by chemically linking polymer chains together. Suitable
gelling agents are known to those skilled in the art. Preferred gelling agents
are
phosphate esters. Gelling agents are typically liquids so as to promote easy
operational mixing and continuous mixing with liquid hydrocarbon. The
viscosity
range generated can be from 50 to 1000 cP but, as noted, are shear and
temperature sensitive.
B-Breaker
[0052] The breaker is typically a pH shifting agent added to the liquid
component
for relaxing viscosity in a controlled manner. Suitable breakers include those
known to those skilled in the art such as magnesium oxide. Typically, a
breaker
in the present invention is selected that reduces liquid component viscosity
over
-14-
CA 02662811 2009-04-27
a maximum 30 minute time period and preferably 10 minutes or less. For
example, liquid component viscosity may initially be in the range of 50 to
1000 cP
at a shear rate of 170 sec-' and be effectively reduced to 1-10 cP over a 90
minute period. The amount of magnesium oxide and temperature are measured
and/or controlled to provide the designed relaxation in viscosity.
[0053] In one embodiment, breaker activity is controlled to relax viscosity
within
about 10 minutes so as to more readily promote the formation of a mist or
liquid
slugs.
C-Surfactant
[0054] Surfactant is a further additive that is intended to prevent the
formation of
emulsions if the hydrocarbon comes into contact with in situ formation water,
if
present. More specifically, the surfactant is designed to promote the return
of the
liquid component back to the well after pressure release by allowing less
fluid to
be trapped in the reservoir matrix pores due to interaction with formation
water
and rock as known to those skilled in the art.
[0055] With reference to Table 1, various liquid component compositions are
described. In accordance with the invention, it is understood that the primary
functions of the liquid component is to temporarily support proppant for a
short
time at surface prior to mixing with the gas component but not promote the
formation of stable foams on mixing. As such, various additives including
surfactants are not essential to the invention in that in specific
applications,
surfactants may not be added to the fluid composition.
-15-
CA 02662811 2009-04-27
Table 1-Liquid Component Additives
Additive Amount (% of Examples and/or Composition (% of
total liquid unmixed component)
component)
A-Gelled Liquid 98 wt% One of many frac oil of many brands from
Liquid hydrocarbon many public suppliers. For example, FO 200
Hydrocarbon from the supplier ICTC, an ECL Company, is
to 10 wt% aromatics and 90 to 95 wt%
aliphatics.
Gelling Agent 0.2-1.5 wt% Phosphate ester (Century Oilfield Services
Inc., Calgary, Alberta)
Complexor 0.2-1.5 wt% Iron sulphate and amine blend (Century
Oilfield Services Inc., Calgary, Alberta)
B-Breaker Breaker 0.1-10 vol% Magnesium oxide 20 to 40 wt% diluted in
mineral oil 60 to 80 wt% and 1 wt%
suspension package (Century Oilfield
Services Inc., Calgary, Alberta)
C-Surfactant Surfactant <0.1 vol% Non-foaming Surfactant / Demulsifier eg.
Alkyl Alkoxylate, Organic Polyol (Century
Oilfield Services Inc., Calgary, Alberta)
Field Methodology and Equipment
[0056] As noted above, Figure 1 shows an overview of the equipment and
method of fracturing a well in accordance with the invention. Base fluids
including
liquid hydrocarbon 10 (from liquid hydrocarbon tank 10a), gelling agent 12,
complexor 14, surfactant 16 and breaker 18 (from a chemical truck 12a) are
selectively introduced into a blender 20 (on blender truck 20a) at desired
concentrations in accordance with the desired properties of the fluid
composition.
Upon establishment of the desired viscosity of the fluid composition, proppant
22
(from proppant storage 22a) is added to the composition and blended prior to
introduction into a high pressure pump 24 (on pump truck 24a). Gas 26 (from
gas
truck 26a) is introduced to a high pressure line between the high pressure
pump
24 and a well 28 prior to introduction into the well 28. A data truck 30 is
-16-
CA 02662811 2009-04-27
configured to the equipment to collect and display real time data for
controlling
the equipment and to generate reports relating to the fracturing operation.
[0057] The blender blends the base fluids and proppant and chemical and
includes appropriate inlets and valves for the introduction of the base fluids
from
the liquid hydrocarbon tanks and chemical truck and proppant storage. The
blender preferably includes a high shear tub capable of blending in the range
of
1000-5000 kg (preferably about 2200 kg) of proppant per m3 of fluid.
[0058] The base liquid components including gelling agent, complexor, -non-
foaming surfactant and breaker are delivered to a field site in a chemical
truck
12a. The chemical truck includes all appropriate chemical totes, pumps, piping
and computer control systems to deliver appropriate volumes of each base
liquid
component to the blender 20.
[0059] Liquid hydrocarbon tanks 10a include valves to deliver liquid
hydrocarbon
to the blender via the blender hoses.
[0060] The high pressure pump(s) typically each have a nominal power rating in
the range of 1500 kW and be capable of pumping up to 2 m3/minute of liquid
fracturing fluid and proppant through 4.5-5" pump heads in order to produce
surface operating well pressures up to 103.5 psi. Depending on the size of the
fracturing operation, 1-6 liquid high pressure pumps may be required.
[0061] Nitrogen is the gas predominantly used in field applications to dilute
the
slurry of fluid and proppant from the high pressure pump. For clarity in
describing
the fracturing fluid composition, in the industry and in the context of this
description, it is known that nitrogen is bought and sold and measured in
terms of
its volume with reference to standard conditions (1 atm and 15 C or
thereabouts
and referred to in units of "scm" (standard cubic meters or cubic meters under
standard conditions as noted above). The physical state of nitrogen received
at
a well site is in a refrigerated liquid form stored at about 1 atm gauge
pressure (2
-17-
CA 02662811 2009-04-27
atm absolute pressure) and about -145 C to -190 C. The ratio of 1 m3 of liquid
nitrogen as delivered is equivalent to about 682 scm at standard atmospheric
conditions. Nitrogen is pumped in its cryogenic liquid state taking it from
storage
pressure to well pressure, then gasified by heating it to 20 C, whereupon it
enters
the high pressure line where it mixes with the fracturing liquid composition
and
proppant.
[0062] This turbulent mixture is then pumped down the well where it warms up
to
as much as the formation temperature and reaches the pressures used to
fracture the production zone. The estimated temperature and pressure under
pumping conditions of the production zone is used to estimate the compression
of nitrogen in the form of the number of standard cubic meters per cubic meter
of
actual space at the production zone.
[0063] For example, 1 m3/min of cryogenic liquid from the nitrogen truck may
be
pressurized to 20 MPa surface pressure, heated to 20 C, mixed with the fluid
and
proppant at the desired volume % ratios and pumped in the well to the
production
zone. If the pumping pressure and temperature of fracturing into the
production
zone is 18 MPa and 30 C, the compression at these conditions is about 160 scm
occupying 1 m3 of actual space. The 682 scm/min of nitrogen rate as it would
be
referred to in the field operations relates to an actual flow rate into the
production
zone during fracturing of 4.26 m3/min (682 scm/min divided by the compression
ratio of 160 scm/m). When the frac is flowed back, as pressure and temperature
changes the nitrogen gas expands as it flows with fluid to flow back tanks at
surface for separation and disposal.
[0064] Generally, the fracturing composition is formulated for a desired
composition input to the formation at formation conditions. As such, the ratio
between the fluid component and gas component as measured in volume % at
the surface will likely be different to what is delivered at the formation. As
known
to those skilled in the art, the difference between surface pressure and
bottom
hole pressure may have either a positive or negative variance depending on
-18-
CA 02662811 2009-04-27
parameters including the hydrostatic pressure and friction pressures between
the
surface and the formation. For example, for a typical fracturing composition
in
accordance with the invention, where a 10/90 volume % liquid/gas composition
is
to be injected at the formation, may depending on the depth of the formation
and
the friction pressures of the specific composition conveyance equipment
require
either higher or lower ratio of liquid to gas mixing at surface at a given
surface
pressure.
Lab Examples
[0065] Test samples of the fluid composition were prepared in accordance with
the following general methodology. A volume of a base fluid (for example FO-
200 liquid hydrocarbon from Innovative Chemical Technologies Canada Ltd.
(ICTC, an ECL Company) was measured in a beaker from a bulk source and
added to a variable speed Waring blender. The fracturing liquid component
additives were measured in disposal plastic syringes from bulk sources. The
Waring blender was turned on to an appropriate speed and the additives were
added to the base fluid sequentially. The samples were blended for about 0.5
minutes (or slightly longer as required). To foam a sample, the Waring blender
was turned to a higher speed setting for at least 10 seconds. The fracturing
fluid
test sample was then ready to be used in the various experiments.
[0066] Test samples of the proppant (sand) were prepared in accordance with
the following general methodology. All proppant was taken from a bulk source
as
common products available to industry.
[0067] Test samples of the fluid were measured for proppant (sand) support
under static conditions using the following general methodology. A fracturing
fluid composition was prepared and a sand sample was obtained according the
previous methodologies described. 90% of the volume of a fluid sample was
blended without sand in one Waring blender. The remaining 10% of the volume
of a fluid sample was blended with sand in a second Waring blender. The fluid
-19-
CA 02662811 2009-04-27
sample without proppant was quickly placed in a graduated cylinder with the
sand laden fluid sample placed on top. The sand volume accumulation was
observed at the bottom of the graduated cylinder and compared to the initial
proppant sample used. A longer accumulation time (i.e. a lower fall rate for
the
particles) indicated a greater tendency of the fracturing fluid to support
proppant.
[0068] Test samples of the fluid were measured for viscosity with the
following
general methodology. A Brookfield PVS rheometer (Brookfield Engineering
Laboratories, Middleboro, MA) was utilized to measure the viscosity of the
liquid
fracturing fluid compositions. The oil bath temperature was set to a specific
temperature according to each experiment. 250 mL of liquid fracturing fluid
composition was blended in a Waring blender. A 50 mL plastic syringe was used
to transfer a 35 mL sample from the prepared liquid fracturing fluid
composition in
the Waring blender to the rheometer cup. The cup was screwed on the
rheometers such that the bob was appropriately immersed in the fluid, the
sealed
cup was exposed to 400 psi nitrogen gas above the fluid, and the cup immersed
in the oil bath for temperature control according to the general procedures as
known to those skilled in the art.
Experiments
Viscosity vs. Time
[0069] Figure 2 shows the effect of varying breaker concentration on viscosity
of
a liquid fracturing fluid composition as a function of time. The fluid
composition
was a blend of FO 200 Frac Oil with 0.51 wt% alkyl phosphate ester, 0.09 wt%
aryl ether phosphate ester, 0.29 wt% ferric sulphate, 0.12 wt% C2-C18 tertiary
alkoxylated amines, 0.03 wt% surfactant and varying wt% of magnesium oxide.
The viscosity was measured at 20 C and a shear rate of 170 sec'. As shown,
as the breaker concentration is varied from 0.53 to 1.58 wt%, the viscosity of
the
fluid composition relaxes in approximately one tenth of the time to 10 cP at a
shear rate of 170 sec -1 (6 to 12 minutes compared to 78 to 84 minutes).
-20-
CA 02662811 2009-04-27
[0070] Past fracturing stimulation operations involving hydrocarbon base
fluids
finish in significantly more time than 6 to 12 minutes. The standard, as known
to
those skilled in the art, is to have higher viscosity values until the design
time
planned for the fracturing stimulation plus contingency time is reached which
is
usually, or by default, to be about 90 minutes. This invention demonstrates
that
the temporary viscosity of the fracturing fluid is brought below 10 cP
(considered
a "broken" or relaxed fluid) before the fracturing stimulation operation is
finished
(or before the proppant is substantively delivered to the formation).
Foam Stability
[0071] Figure 3 shows the effect of introducing additives that are known
foaming
agents as compared to other additives with a null effect on foaming by
measuring
foam stability as a function of time. A blend of liquid hydrocarbon base fluid
with
additive concentrations of 0.34 wt% alkyl phosphate ester, 0.06 wt% aryl ether
phosphate ester, 0.19 wt% ferric sulphate, 0.08 wt% C2-C18 tertiary
alkoxylated
amines, 0.91 wt% magnesium oxide, 0.06 wt% surfactant, and various additives
and loadings of foaming surfactant agents and non-foaming surfactant agents
are shown in Figure 3. In these experiments, the liquid fracturing fluid
composition was agitated in a Waring blender at the 100% (maximum) speed
setting to produce foam. After cessation of agitation, the height of the foam
was
measured immediately and at time intervals thereafter.
[0072] As shown, the amount of foaming agents of 0.4450 wt% isopariffinic
hydrocarbon and 0.0550 wt% fluoroacrylate copolymer resin resulted in
reasonable foam stability that shows the reference case of normal operation
used in foam injections into wells. Reasonable foam stability was also
observed
with 0.03 wt% non-foaming surfactant with the foaming agents of 0.4450 wt%
isopariffinic hydrocarbon and 0.0550 wt% fluoroacrylate copolymer resin which
shows that non-foaming surfactant agent neither significantly encourages or
discourages the generation of a stable foam.
-21 -
CA 02662811 2009-04-27
[0073] A standard gelled hydrocarbon blend as a pure liquid component is given
for reference (specifically, 0.34 wt% alkyl phosphate ester, 0.06 wt% aryl
ether
phosphate ester, 0.19 wt% ferric sulphate, 0.08 wt% C2-C18 tertiary
alkoxylated
amines, 0.91 wt% magnesium oxide and 0.06 wt% surfactant).
[0074] However, a fluid containing 0.03 wt% of a non-foaming surfactant agent
and the absence of foaming agents showed an almost instant collapse of foam
stability after cessation of agitation.
[0075] This experiment shows that the gelled hydrocarbon component of the
fracturing fluid can be created without foam quality.
Proppant Support
[0076] Figure 4 shows the effect of proppant support in various fracturing
fluid
compositions that have varying breaker loadings and foam agent use. 500 mL of
a common fracturing fluid composition for a foamed well injection was created
using a FO-200 liquid hydrocarbon base fluid with additive concentrations of
0.34
wt% alkyl phosphate ester, 0.06 wt% aryl ether phosphate ester, 0.19 wt%
ferric
sulphate, 0.08 wt% C2-C18 tertiary alkoxylated amines, 0.91 wt% magnesium
oxide, 0.03 wt% surfactant, 0.4450 wt% isopariffinic hydrocarbon (foaming
agent), 0.0550 wt% fluoroacrylate copolymer resin (foaming agent). The
stirring /
foaming method created a total foam height of 650 mL at the start of the
experiment. This common fracturing fluid composition is represented by the
curve labeled "normal break time with foamer". A new composition was created
as a preferred embodiment of this invention was created using 1000 mL FO-200
liquid hydrocarbon base fluid with additive concentrations of 0.34 wt% alkyl
phosphate ester, 0.06 wt% aryl ether phosphate ester, 0.19 wt% ferric
sulphate,
0.08 wt% C2-C18 tertiary alkoxylated amines, 1.58 wt% magnesium oxide, 0.05
wt% surfactant. This new composition is represented by the curve labeled
"extremely short break time" which had no foaming agents added and had highly
elevated breaker additive concentrations. The stirring / foaming method
created
a total volume of 1000 mL (no added volume due to foam) which is a larger
-22-
CA 02662811 2009-04-27
sample than the common fracturing composition to increase the accuracy of the
extremely high sand fall rates. The fracturing compositions were mixed for 5
minutes prior to being used for the experiment to allow for the varying
breaker
amounts to cause a varying viscosity for the samples. 30/60 mesh Canadian
sand was used (SG of 2.61) for this experiment. Sand sample settling rates
were
measured for each of the common and new fracturing compositions. Figure 4
shows the sand sample accumulation times for the 2 trials. For 30/60 mesh
Canadian sand, the fall rate of the common fracturing composition was 0.47
cm/minute, and the new fracturing composition in this invention is 6.03
cm/minute
with the absence of foaming agents and increased breaker loadings. The new
fracturing composition supports the sand much less effectively as it has 12.8
times the fall rate compared to the compositions commonly used by those
skilled
in the art.
Field Examples
[0077] The following are representative examples of field trials of the
subject
technology.
Field Example 1: 50-08W4
[0078] The well was characterized by having perforations from 490 to 493.5 m
in
the Viking formation production zone. The stimulation was pumped down 139.7
mm, 20.8 kg/m, J-55 casing to attempt to place 10,000 kg of 20/40 sand into
the
production zone.
[0079] Prior to the fracture, the well was 1.82 E3M3 / operating day.
[0080] At the job site, all truck-mounted equipment was positioned and
connected
in accordance with standard operating practice. All fluid tanks were filled
with
liquid hydrocarbon PWC-150 supplied by ICTC. Liquid hydrocarbon was heated
to 15-25 C prior to the fracturing operation.
-23-
CA 02662811 2009-04-27
[0081] The high pressure surface line pipe was pressure tested to 30.0 MPa,
and
the well had a maximum working pressure of 25.0 MPa.
[0082] At the perforation zone, an initial 100% nitrogen pad of 1500 scm
(standard cubic meters) was injected into the producing zone to create at
least
one fracture at the rate of 500 scm/minute. After the initial 100% nitrogen
pad, a
fluid composition having a base fluid of liquid hydrocarbon with the additives
of
0.51 wt% alkyl phosphate ester, 0.09 wt% aryl ether phosphate ester, 0.29 wt%
ferric sulphate, 0.12 wt% C2-C18 tertiary alkoxylated amines, 1.58 wt%
magnesium oxide, and 0.08 wt% surfactant was prepared in the blender.
[0083] Proppant (20/40 mesh sand) was admixed to the fluid composition at a
ratio of 2000 kg of sand per m3 of fluid. As known to those skilled in the art
there
may be several stages and fluid and proppant ratios developed before the well
is
flushed.
[0084] The rate of fluid / sand slurry mixture started at 0.64 m3/min and was
increased to 0.79 m3/min during the proppant pumping. The overall perforation
equivalent rate of gas, fluid and proppant in the formation was estimated to
start
at 5.10 m3/min and was decreased to 4.61 m3/min during the proppant stages.
[0085] Nitrogen gas was introduced to the high pressure line between the high
pressure pump and well head. The nitrogen gas rate was varied to result in 3
different rates ranging from 304 scm/min down to 262 scm/min which diluted the
fluid and sand composition pumped down the well to the formation. The gas
quality (gas volume at the perforations divided by the gas and fluid volume at
the
perforations) was 100% in the pad and ranged between 86% and 82% in the
proppant/fluid stages to result in an overall inject gas quality placed in the
formation of 95.6%. The intended volume of proppant was not injected into the
well, and this did not include the flush of the well of proppant, and only the
material that passed the perforations into the production zone. The overall
-24-
CA 02662811 2009-04-27
concentration of sand started at 100 kg of sand/m3 of combined fluid and gas
and
increased to 200 kg/m3 of combined fluid and gas.
[0086] Overall, the surface pressure during fracturing varied from about a
lowest
value of 11.0 MPa to 25.0 MPa (maximum surface pumping pressure limitation
was reached) with an initial surface breakdown pressure to initiate the frac
at
12.2 MPa. In total, 3,850 kg of proppant was delivered to the formation in 7
minutes from the time that the fracture operations started pumping until
pumping
was stopped.
[0087] Upon completion, the well was vacated and an estimated 1.8 m3 of fluid
was recovered from the well for disposal. In comparison to an energized fluid
frac, this represented a 3 fold decrease in the amount of liquid hydrocarbon
requiring disposal.
[0088] Gas flow rates from the well after fracturing averaged 3.64 E3M3/day
flowing following the frac which represents a 200% increase in production.
Field Example 2: 51-08W4
[0089] The well was characterized by having perforations from 529 to 537 m in
the Medicine Hat formation production zone. The stimulation was pumped down
114.4 mm, 14.14 kg/m, J-55 casing to attempt to place 10,000 kg of 20/40 sand
into the production zone.
[0090] Prior to the fracture, the well was 2.41 E3M3 / operating day.
[0091] At the job site, all truck-mounted equipment was positioned and
connected
in accordance with standard operating practice. All fluid tanks were filled
with
liquid hydrocarbon PWC-150 supplied by ICTC. Liquid hydrocarbon was heated
to 15-25 C prior to the fracturing operation.
[0092] The high pressure surface line pipe was pressure tested to 30 MPa, and
the well had a maximum working pressure of 24.0 MPa.
-25-
CA 02662811 2009-04-27
[0093] At the perforation zone, an initial 100% nitrogen pad of 3500 scm was
injected into the producing zone to create at least one fracture at the rate
of 500
scm/minute. After the initial 100% nitrogen pad, a fluid composition having a
base fluid of liquid hydrocarbon with the additives of 0.51 wt% alkyl
phosphate
ester, 0.09 wt% aryl ether phosphate ester, 0.29 wt% ferric sulphate, 0.12 wt%
C2-C18 tertiary alkoxylated amines, 1.58 wt% magnesium oxide, 0.08 wt%
surfactant was prepared in the blender.
[0094] Proppant (20/40 mesh sand) was admixed to the fluid composition at a
ratio of 2000 kg of sand per m3 of fluid.
[0095] The rate of fluid / sand slurry mixture started at 0.63 m3/min and
increased
to 2.44 m3/min during the proppant pumping. The overall perforation equivalent
rate of gas, fluid and proppant in the formation was estimated to start at 6.0
m3/min and remain approximately constant during the proppant stages.
[0096] Nitrogen gas was introduced to the high pressure line between the high
pressure pump and well head. The nitrogen gas rate was varied to result in 5
different rates ranging from 495 scm/min down to 329 scm/min which diluted the
fluid and sand composition pumped down the well head to the formation. The
gas quality (gas volume at the perforations divided by the gas and fluid
volume at
the perforations) was 100% in the pad and ranged between 94% and 79% in the
proppant/fluid stages to result in an overall inject gas quality placed in the
formation of 94%. This did not include the flush of the well of proppant, and
only
the material that passed the perforations into the production zone. The
overall
concentration of sand started at 1250 kg of sand/m3 of combined fluid and gas
and increased to 425 kg/m3 of combined fluid and gas.
[0097] Overall, the surface pressure during fracturing varied from about a
lowest
value of 11 MPa to 12.9 MPa with an initial surface breakdown pressure to
initiate the frac at 13.8 MPa. In total, 9,630 kg of proppant was delivered to
the
-26-
CA 02662811 2009-04-27
formation in 16 minutes from the time that the fracture operations started
pumping until the well was flushed of proppant.
[0098] Upon completion, the well was vacated and an estimated 4.0 m3 of fluid
was recovered from the well for disposal. In comparison to an energized fluid
frac, this represented a 3 fold decrease in the amount of liquid hydrocarbon
requiring disposal.
[0099] Gas flow rates from the well after fracturing were 3.93 E3M3/day the
following full calendar month that the well was produced which was a 63%
increase.
Summary/Conclusions
[0100] In summary, the lab and field test data showed that substantially lower
quantities of liquid hydrocarbon can be used to create fracturing compositions
that in combination with novel mixing and pumping methods are effective in
providing high mass proppant fractures. Importantly, the subject technologies
demonstrate that the use of mists and/or slugs can be used as an effective
fracturing composition particularly having regard to the ability of a mist
and/or
slug to transport an effective volume of proppant into the formation using
conventional fracturing equipment. As a result, the subject technologies
provide
an effective economic solution to using high concentration gas fracturing
compositions 'that can be produced in a continuous (i.e. non-batch) process
without the attendant capital and operating costs of current pure gas
fracturing
equipment.
-27-
_ _.. .z- w w 'Taewswsma ++w Mmn+H. xn ~an.w,pynam{y