Note: Descriptions are shown in the official language in which they were submitted.
CA 02663036 2015-07-16
A METHOD OF SIMULTANEOUSLY ABRASIVELY BLASTING
AND DOPING SURFACES
FIELD OF THE INVENTION
[02] The present invention relates to methods of bombarding
surfaces of articles, such medical devices, with dopants.
BACKGROUND OF THE INVENTION
[03] The bombardment of metal surfaces with so-called abrasive
materials is finding an increasing number of technical applications in recent
years. Techniques such as grit blasting, shot blasting, sand blasting, shot
peening and micro abrasion fall under this category of surface treatment
technique. In each of these techniques, generally, an abrasive material, shot
or grit, is mixed with a fluid and delivered at high velocity to impinge the
surface to be treated. The technique used to deliver the abrasive material can
be classified as wet or dry depending on the choice of fluid medium used to
deliver the abrasive to the surface, usually water and air respectively. The
generic term "abrasive bombardment" is used to refer to all such techniques in
this specification.
[04] Applications of these technologies include metal cutting, cold
working metallic surfaces to induce desirable strain characteristics and the
pre-treatment of surfaces to induce desirable texture (surface roughness) for
the purposes of enhanced adhesion of further coating materials. (See
Solomon et al., Welding research, 2003. October: p. 278-287; Momber et al.,
Tribology International, 2002. 35: p. 271-281; Arola et al., J. Biomed. Mat.
Res., 2000. 53(5): p. 536-546; and Arola and Hall, Machining science and
technology, 2004. 8(2): p. 171-192.). An example of the latter is to be found
-.1-
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
in the biomedical sector where titanium implants are grit blasted with alumina
or silica to achieve an optimum level of surface roughness that will maximize
the adhesion of plasma sprayed hydroxyapatite (HA) coatings on the surface
of the implants. HA coated implants are desirable because of the biomimetic
properties of the apatite layer but an optimum bonding strength between the
titanium surface and the apatite layer is also necessary.
[05] It has been known for some time that during the bombardment
of these surfaces some of the abrasive material becomes impregnated in the
surface of the metal itself, which has generated some interest in these
techniques as possible candidates for modifying surface chemistry in general.
(See Arola et al. and Arola and Hall, supra). Again with reference to the
biomedical sector one study has looked at shot blasting as a means of putting
a hydroxyapatite layer directly on to a titanium surface in an effort to
bypass
the costly plasma spray process. Ishikawa, K., et al., Blast coating method:
new method of coating titanium surface with Hydroxyapatite at room
temperature. J. Biomed. Mat. Res., 1997. 38: p. 129-134. In this study, HA of
an unspecified particle size distribution was used as the abrasive. However,
given that the deposited layer of apatite could be removed with a benign
washing regime it seems that a strong bond with the surface of the metal was
not achieved.
[06] Choi et al. (KR20030078480) refer to the use of a single calcium
phosphate particle as a grit blasting media for the purposes of embedding the
grit in the surface of dental implants but particle in excess of 190 pm are
disclosed.
[07] U.S. Patent No. 6,502,442 ([6]) refers to the use of sintered HA
as the abrasive using water as the fluid medium. Some impregnation of the
HA was achieved in this instance as the HA was thermally processed.
[08] Muller et al. (US2004158330) disclosed blasting particles
comprising calcium phosphate contained in a glassy matrix. Other
disclosures (e.g., U.S. Patent Nos. 4,752,457 and 6,210,715) describe
methods for the manufacture of calcium phosphate micro-spheres usually
- 2
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
comprising a polymer component and complex methods of manufacturing the
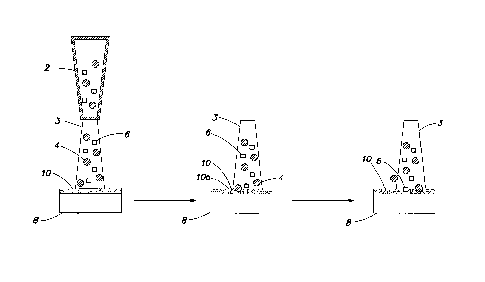
same, but their effectiveness as blasting media was not elucidated.
[09] The RocatecTM system for the silicization of metallic and other
surfaces also uses individual particles having multiple components. This
technology is used extensively in the dental arena. In this instance an
alumina particle having an outer adherent layer of silica is propelled at a
pre-
roughened surface and upon impact the local heat generated in the vicinity of
the impact causes the shattered silica outer layer to become fused to the
surface a process referred to as ceramicization.
[10] Bru-Magniez et al. (U.S. Patent No. 6,431,958) have disclosed
hard abrasive materials with multiple stratified layers for use in blasting
abrasive bombardment techniques to modify surfaces. In this instance the
purpose of the process was to embed or otherwise attach the stratified layer
around the abrasive particles to the surface being treated. The outer layer
comprises at least one polymer while the core ceramic material of choice is an
oxide, carbide, nitride, or carbonitride.
[11] The use of multiple stratified polymeric layers has been
proposed. Lange et al. (U.S. Patent No. 6,468,658) have disclosed a particle
composed of a core base material and an outer adherent layer of titanium
dioxide for blasting purposes
[12] Further applications of abrasive bombardment for the purposes
of surface modification are to be found in the biomedical sector such as for
example the use of micro abrasion to clean the oxide slag from the struts of
laser machined coronary stents and the impregnation of the surfaces of
pacemakers and defibrillators with silica to increase the adhesion of further
polymer coatings to the device.
[13] A commonality among these examples is the use of a single
type of solid particle in the fluid stream.
[14] The recent significant interest in surface modification technology
as it relates to biomedical devices is fueled by the success of the Drug
Eluting
Stent (DES). Since the introduction of endovascular techniques in the 1990's
revascularisation strategies have changed dramatically over the last number
- 3 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
of years. However, in-stent restenosis (ISR) remains a problem wherein
rupture of the vessel lining at the stent site can cause platelet activation,
the
secretion of inflammation mediators and eventually smooth muscle cell (SMC)
formation, a process analogous to scar formation around a wound site.
Furthermore as the stent also contacts the blood it should not induce a
foreign
body reaction (FBR) in the tissue or blood cells, i.e., it should be
biocompatible. The DES uses surface modification technology to combat
these problems wherein the surface of the stent is used to deliver active
agents (anti-restenosis and anti-thrombosis agents) usually in a polymer
matrix locally to the device site where they are most needed. This technology
was pioneered by Cordis with there Cypher stent which received FDA
approval in 2003. Since then a number of other DES have appeared on the
market all aimed at reducing ISR and thrombosis in patients that have
percutaneous coronary intervention (PCI) procedures. All of these active
devices use a polymer matrix to carry the drug on the surface of the stent and
control its elution characteristics in vivo.
[15] However problems have arisen with the DES attributed to a
number of factors, among them, achieving proper control of the elution
characteristics of the drug(s). The polymer matrix (which degrades with time
to release the drug and the polymer degradation products) has been identified
as a possible culprit in patients with hypersensitivity. Thus, there are
continuing efforts to develop new methods to control the delivery and elution
of the drugs.
[16] A large body of prior art in the stent arena has been directed
towards achieving passive coatings on the stent surface to mediate ISR.
These include such processes as nitriding and carbon-nitriding, the use of
carbon and silicon carbide coatings as well as processes to thicken or
augment the native oxide layer on the surface of the stent materials including
oxidation, ion implantation and electrochemical treatments such as
electropolishing or electroplating with inert metals. All such processes
however have a number of disadvantages and no one treatment technique as
such provides the ideal surface for optimal clinical results.
- 4 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
[17] Another arena of relevance is the area of biofilm formation at the
surfaces of implantable devices wherein bacteria at the surface of implant
surfaces arrange themselves into films with three dimensional macroscopic
structure. In this instance the film itself can represent a barrier to
standard
antimicrobial treatments such as for example the systemic use of antibiotics.
It is reported that the systemic dose of antibiotic required to kill bacterial
biofilm infections can be up to 1000 times the systemic dose required to kill
their planktonic counterparts in suspension often inducing unwanted and
serious side effects in patients. Localized drug delivery at the surfaces of
implantable devices has been mentioned as one method to target
antimicrobial agents at the implant surface where they are most needed,
preventing biofilm formation with the added advantage of using much lower
dose rates than systemic treatments.
[18] Currently most bactericidal strategies for localized drug delivery
use polymer coatings or polymer micro spheres embedded in other suitable
carrier matrices as carriers for antibacterial agents. In addition calcium
phosphate salts including hydroxyapatite have been proposed as suitable
carriers for antibiotics. Biomimetic deposition has been used to deposit nano
crystalline apatite layers on the surfaces of orthopedic metallic implants
that
can then be loaded with drugs precipitated onto the inorganic coating from
solution in a separate step (US20040131754). Such strategies can have dual
advantage as for example in the arena of orthopedic implants where the
calcium phosphate salt provides an osteoconductive benefit at the surface
inducing bone in-growth in vivo while the antibiotic reduces the risk of
biofilm
formation, both factors contributing heavily to the need for revision
procedures. However this approach is limited by the available surface area at
the surface of the implant as this determines the amount of antibiotic that
can
be loaded. Furthermore the approach is multi-step as often the attachment of
the ceramic layer involves high temperature (as for example in the case of
plasma sprayed calcium phosphate coatings) or the attachment of the drug
requires precise control of the pH and other process parameters precluding
the simultaneous attachment of the inorganic salt and the antibacterial agent.
- 5 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
Among the antibiotics that have been attached to metal surfaces via such
methods are gentamycin, tobramycin, vancomycin, ampicillin, and others.
[19] The range of therapeutic agents that could provide benefit for
patients if present at the surface of implants is not limited to antibiotics
or
immuno-suppressants. Several studies have focused on placing other
therapeutic agents at the surface of implantable devices to induce desirable
in
vivo responses. For example, some studies have focused on placing the
functional molecules involved in these cascades at the surfaces of the
implants. These include for example proteins among them hormones, growth
factors, structural proteins, immunogens and antigens. As a corollary of this
much work has focused on the design of peptides and proteins that have
structural similarity to the active sites of the proteins involved in
biological
pathways. For example the use of RGD peptides in orthopedic applications,
or bactericidal peptides have been proposed as strategies for combating
bacterial infection in instances, e.g., where the bacteria have high
resistance
to conventional antibiotics.
[20] As medical implants are increasingly tailored to the needs of the
patient they can also be viewed as a means to deliver therapeutic agents for
the treatment of other more patient specific diseases for example diabetes,
cancers and other diseases not directly related to the primary function of the
implant. An in vivo device lends itself to multiple functions wherein the
surface of the device becomes a vehicle to deliver therapeutic agents that
might be required to treat other diseases the patient may have.
[21] The limiting factors in achieving therapeutic agent delivery
capacity at the surfaces of implants generally surround the engineering and
processing aspects. Methods to put these agents on the surface are required
that are commensurate with maintaining the activity and structural integrity
of
the agents themselves and controlling the surface chemistry particularly there
elution kinetics in vivo. As many of the agents desired are biological in
nature, temperature and solution parameters such as pH etc can present
barriers to realizing the benefit of the above mentioned surface modification
strategies.
- 6 -
CA 02663036 2014-12-19
[22] Surface modification of implant surfaces is not limited
to the field of therapeutic agent delivery alone. In many cases surface
modification of the implantable device may be required for the purposes of
tailoring the physical properties of the surface such as, for example, in
titanium based devices used in coronary intervention procedures, an dint
he treatment of pathological calcifications such as kidney stones. It would,
however, be desirable to have devices with higher radio-opacity than that
currently associated with these devices in vitro. This would facilitate their
radiographic or even magnetic resonance imaging externally and dispense
with the need for invasive procedures or endoscopes currently used with
minimally invasive procedures. Examples include the doping of nitinol
alloys with tertiary heavy elements such as platinum, palladium or tungsten
among others to increase the radio opacity of the resulting alloy for
biomedical and other applications (US Patent Nos. 7,128,757, 6,776,795,
and 6,569,194).
SUMMARY OF THE INVENTION
[22a] Certain exemplary embodiments provide a method of
treating a metal substrate, comprising: removing a metal oxide from a
surface of the metal substrate by abrasively blasting the metal oxide
surface with an abrasive material to expose a metal surface; and
delivering particles comprising a dopant from at least one fluid jet to the
metal surface to impregnate the surface of the substrate with the dopant
such that no laminate layer of dopant results; wherein the removing is
performed substantially simultaneously with the delivering and wherein
the particles comprising dopant are different from the abrasive material.
[22b] Other exemplary embodiments provide a method of treating
an article surface, the method comprising: delivering substantially
simultaneously a first set of particles comprising a dopant and a second
- 7 -
CA 02663036 2014-12-19
0
set of particles comprising an abrasive from at least one fluid jet to a
surface of an article to impregnate the surface of the article with the
dopant such that no laminate layer of dopant results, the first set of
particles comprising a different material from the second set of particles.
[23] The present invention is directed towards providing an
improved treatment process for the purposes of modifying the surfaces of
articles, such as metallic articles with desirable materials so as to induce
at
least one of desirable chemical, physical and/or biological characteristics
in those surfaces.
[24] One embodiment provides a method of treating a metal
substrate, comprising:
removing a metal oxide from a surface of the metal substrate
to expose a metal surface; and
delivering particles comprising a dopant from at least one
fluid jet to the metal surface to impregnate the surface of the substrate with
the dopant.
[25] One embodiment provides a method of treating an article
surface, the method comprising:
delivering substantially simultaneously a first set of particles
comprising a dopant and a second set of particles comprising an abrasive
- 7a -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
from at least one fluid jet to a surface of an article to impregnate the
surface of
the article with the dopant.
[26] In other embodiments, the dopant can be polymers, metals,
ceramics, therapeutic agents, and combinations thereof. The article can be a
medical device, such as an implantable medical device.
BRIEF DESCRIPTION OF THE DRAWINGS
[27] Various embodiments of the invention will be understood from
the following description, the appended claims and the accompanying
drawings, in which:
[28] FIG. 1 is a schematic representation of a treatment process of
the invention;
[29] FIG. 2A is an XPS spectrum of cp titanium surfaces grit blasted
with HA only;
[30] FIG. 2B is an XPS spectrum of cp titanium surfaces grit blasted
with HA:Alumina mix;
[31] FIGS. 3A and 3B show comparative XPS spectra of Ca 2p
(FIG. 3A) and P 2p (FIG. 3B) core levels of HA only blasted cp titanium (fine
line) and 50:50 HA:alumina blasted cp titanium (coarse line);
[32] FIG. 4 shows XPS spectra of the Ti 2p core level on the sample
grit blasted with 100% HA (top) and the sample grit blasted with a 50:50
HA:alumina mix (bottom);
[33] FIG. 5 shows XPS maps of a 0.2 x 0.2 mm square on cp
titanium surfaces showing (a) concentration and distribution of Ca on 50:50
grit blasted sample, (b) concentration and distribution of Ti on 50:50 grit
blasted sample; (c) concentration and distribution of Ca on the 100% HA grit
blasted sample; (d) concentration and distribution of Ti on the 100% HA grit
blasted sample;
[34] FIGS. 6A and 6B show comparative XPS spectra of the Ca 2p
and P 2p core levels in the case of HA only blasted Cp titanium (fine line)
and
50:50 HA:silica bead blasted cp titanium (coarse line);
- 8 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
[35] FIG. 7 is a pair of XPS survey scans of two different samples
blasted with a 50:50 HA/silica bead mix, showing the reproducibility of the
results;
[36] FIG. 8 shows bacterial assays of gentamycin/HA treated
surfaces for (1) Staphylococcus aureus, (2) Escherichia coli, and (3)
Pseudomonas aeruginosa where the left sample for each assay is a negative
control, and "IZ" indicates the growth inhibition zone;
[37] FIGS. 9A, 9B, and 9C are schematic diagrams of three different
nozzle configurations to deliver the dopants and abrasive to a surface;
[38] FIG. 10, shows three photographs of the inhibition zone (IZ) on
an agar plate inoculated with S. aureus and exposed to vancomycin coupon
(Plate 1) and inoculated with E. Coll and exposed to Tobramycin (Plates 2
and 3);
[39] FIG. 11A shows FTIR spectra of duplicate 100 pm alumina bead
samples (a) and (b);
[40] FIG. 11B shows FTIR spectra of duplicate 150 pm alumina bead
samples (a) and (b);
[41] FIG. 12A is an XRD pattern of surface HA (alumina; 50 !Am);
[42] FIG. 12 B is an XRD pattern of surface HA (alumina; 100 m);
[43] FIG. 13 show XPS survey spectra for duplicate HA controls;
[44] FIG. 14 is an SEM (scanning electron microscopy) image of an
HA adlayer on a stainless steel (ASTM F1586) surface;
[45] FIG. 15 is an energy dispersive x-ray (EDX) spectrum for HA on
a stainless steel (ASTM F1586) surface;
[46] FIG. 16 is SEM image of HA adlayer on the surface of CP
titanium (ASTM F67);
[47] FIG. 17 is EDX spectrum for HA on CP titanium (ASTM F67)
surface;
[48] FIG. 18 is an AFM (Atomic Force Microscopy) analysis of the
thickness of the HA adlayer on the CP titanium surface, where FIG. 18A is an
AFM image and FIG. 18B is the corresponding AFM plot;
- 9 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
[49] FIG. 19 is an SEM image of Si02 nanoporous micro-particles on
the surface of Grade 5 Titanium (Ti6AL-4V to ASTM F136);
[50] FIGS. 20A and 20B are SEM images of nanoporous HA adlayer
on the surface of aluminium at a magnification of x 50 (20A) and x 650 (20B);
and
[51] FIG. 21 an SEM image of nanoporous HA adlayer on the
surface of nitinol.
DETAILED DESCRIPTION
[52] One embodiment provides a treatment process of impregnating
a surface, such as a metal surface, with a dopant. The strength of the bond
between the dopant and the surface and the concentration of dopant achieved
in or on the surface can be improved over conventional methods of surface
impregnation techniques. The invention relates to dopants that induce
desirable chemical, physical and biological properties in the surface of
biomedical implants.
[53] Generally the dopant is a material that is incorporated in the
bombarded surface but does not extensively impregnate the surface if used
as the sole solid component in such a bombardment technique. If the
material is delivered to the surface within a high velocity fluid jet on its
own, no
or minimal surface impregnation will occur. Such circumstances can arise for
a number of reasons; the material may not have sufficient particle size or be
of sufficient density and hardness to breech the metal surface and
impregnate. It may also be a consequence of the nature of the surface itself.
[54] In most metallic materials an oxide layer forms at the surface,
which will be harder than the bulk metal or alloy. Metal surfaces (especially
those of titanium and titanium derived alloy) are naturally contaminated in
air
by a variety of contaminants. The detailed physical and chemical properties of
any metal surface depend on the conditions under which they are formed.
The inherent reactivity of the metal can also attract various environmental
chemicals /contaminants that oxidize on the surface. For example, titanium is
a highly reactive metal, which is readily oxidized by several different media.
- 10 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
This results in titanium always being covered in an oxide layer. This oxide
layer is chemically stable but not always chemically inert, as the oxide layer
can continue to react with various reactants in its environment, e.g., organic
molecules. Traditionally, modification of the titanium surface/oxide layer
whereby any new materials in the oxide layer occurred as a by-product of that
process. In some cases the new material in the oxide layer can be
advantageous to the eventual functionality of the surfaces affected; however,
in some cases the new material can constitute an unwanted intrusion.
("Titanium in Medicine," D.M. Brunette; P.Tengvall, M.Textor; P.Thompson,
Springer, New York; ISBN 3-540-66936-1.)
[55] The present invention is directed to the intentional addition of a
material of choice to the surface. One embodiment takes advantage of the
inherent reactivity of metals by the temporary removal of the oxide layer
overlying the metal substrate, and treating the newly exposed metal beneath
to add a new material (a dopant). Depending on the nature of that added
material, the surface properties of the metal article can be tailored
according
to its intended functional requirements.
[56] Titanium and its alloys always form an oxide layer at the
surface. This oxide layer is typically inert and unreactive, while titanium
itself
is highly reactive and will instantaneously form an oxide layer on exposure to
atmospheric environment. Formation of an oxide layer is often a desired
property of an implant device.
[57] Examples of dopants in the biomedical device sector includes
e.g., hydroxyapatite, drug eluting polymers and other drug delivery systems,
and the article to be impregnated comprises a metal such as, e.g., titanium,
steel, cobalt chrome and alloys thereof.
[58] Accordingly, one embodiment of the present invention provides
a method of treating a metal substrate, comprising:
removing a metal oxide from a surface of the metal substrate to
expose a metal surface; and
- 11 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
delivering particles comprising a dopant from at least one fluid
jet to the metal surface to impregnate the surface of the substrate with the
dopant.
[59] In one embodiment, the metal surface is sufficiently reactive in
the presence of air that a new oxide layer can form, thus preventing addition
of dopant to a metal surface layer. In one embodiment, the present invention
involves adding the dopant prior to reoxidation of the newly formed metal
surface. In one embodiment, the step of removing the metal oxide surface is
performed under an inert atmosphere. In another embodiment, the removing
is performed substantially simultaneously with the delivering such that the
metal surface is not substantially oxidized prior to the delivering.
[60] The metal oxide layer can be removed by a variety of
techniques. In one embodiment, the removing comprises abrasively blasting
the metal oxide surface. the step of abrasively blasting in itself can be
performed by a number of methods, e.g., grit blasting, micro blasting, water
jet blasting, and shot peening, as discussed in further detail below, as well
as
any other means of abrasive bombardment as known in the art. In one
embodiment, the step of abrasively blasting is performed substantially
simultaneously with the step of delivering the particles comprising the
dopant,
e.g., two streams of particles can be aimed at the metal oxide surface where
one stream abrasively blasts the oxide surface to expose the new metal
surface and the other stream bombards the new metal surface with dopant.
[61] In another embodiment, the removing is selected from at least
one step of drilling, cutting, forming, milling, micromachining, scratching,
grinding, polishing, and abrading. In another embodiment, the removing is
selected from at least one step of acid etching, alkaline etching, and
treating
with hydrogen peroxide. In yet another embodiment, the removing comprises
a laser treatment selected from ablation, marking/etching, welding, cutting,
and cladding. In another embodiment, the removing comprises a plasma
treatment selected from etching and cleaning.
[62] As stated above, in certain of the embodiments described
herein, the process of the oxide removal may be performed in an inert
- 12 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
environment to expose the new metal surface for a sufficient time to conduct
the treatment process e.g., the addition of a new material to the surface
before re-exposing the surface to an oxygen rich environment. At that time,
the oxide layer can regenerate, but influenced/ modified by the entrapped
added dopant(s).
[63] In one embodiment, equipment for removing the oxide layer
prior to or substantially simultaneously with bombarding the surface can be
incorporated with the fluid jet as a stand alone unit or can be incorporated
into
a manufacturing line. The equipment can be used in a point of use setting
whereby it would constitute an aseptic surgery based machine that a surgeon
could use in an operating room for custom/prescriptive surface modification
prior to implantation of the device in the patient. Disposable dopant
carrier/filter cartridges can be used to avoid therapeutic cross contamination
and ease of cleaning.
[64] If the dopant is delivered simultaneously to the surface with an
abrasive impacting with sufficient energy (a material with sufficient particle
size, density and hardness) to breech the oxide layer a window of opportunity
can be created where the dopant material may be taken up by the surface
before the oxide layer reforms around it. The dopant material can become
strongly bound within the oxide layer of the surface. Thus, the surface can be
impregnated with materials that impart desirable properties to the surface in
a
cost effective manner at ordinary temperatures. Furthermore the energy
dissipated at the impact site of the abrasive may be sufficient for the dopant
to
become ceramicised or otherwise bonded to the surface. Accordingly, one
embodiment provides a method of treating an article surface, the method
comprising delivering substantially simultaneously a first set of particles
comprising a dopant and a second set of particles comprising an abrasive
from at least one fluid jet to a surface of an article to impregnate the
surface of
the article with the dopant.
[65] One embodiment of the present invention relates to the
impregnation of metallic surfaces with a material of choice (here after
dopant)
using conventional abrasive bombardment techniques by mixing the dopants
- 13 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
with an abrasive (shot or grit) material of choice at the surface. The
abrasive,
impinging the surface with sufficient force to breech the oxide layer or
otherwise deform the surface to be treated, creates a window of opportunity
wherein the dopant(s) may be taken up by the surface or otherwise
incorporated into or onto the surface.
[66] The embodiments of the invention are encompassed in but not
limited to the schematic representation of the invention in FIG. 1. FIG. 1
(left)
schematically shows a fluid jet (nozzle) 2 that simultaneously delivers a
stream 3 comprising a set of abrasive particles 4 and a set of dopant
particles
6. Particle sets 4 and 6 bombard a surface 10 of a substrate 8. In one
embodiment, the substrate 8 is a metal substrate and the surface 10 is an
oxide layer. As a result of bombardment by the abrasive particles 4, the
surface oxide layer is disrupted, and breaches in the oxide layer 10 result to
expose a new surface 10a of substrate 8 (center). In the case of a metal
substrate, the newly exposed surface is a metal surface. As the particle
stream 3 continues to impinge substrate 8, the dopant particles 6 (right) are
integrated into the surface 10 of substrate 8. Where the substrate is a metal
substrate, a new oxide layer 10 reforms around the dopant particles 6.
[67] In certain embodiments, the dopant materials include but are not
limited to materials desired at an implant surface for the purposes of
steering
and improving the body tissue-implant interaction. The dopant can comprise
materials such as polymers, metals, ceramics (e.g., metal oxides, metal
nitrides), and combinations thereof, e.g., blends of two or more thereof.
[68] Exemplary dopants include, modified calcium phosphates,
including Ca5(PO4)30H, CaHP0.4.2H20, CaHPO4, Ca8H2(PO4)6.5H20, 0C-
Ca3(PO4)2, f3-Ca3(PO4)2 or any modified calcium phosphate containing
carbonate, chloride, fluoride, silicate or aluminate anions, protons,
potassium,
sodium, magnesium, barium or strontium cations.
[69] Other exemplary dopants include titania (Ti02), zirconia,
hydroxyapatite, silica, carbon, and chitosan/chitin.
[70] In one embodiment, the dopant is a combination of an agent-
carrying media and at least one therapeutic agent (including biomolecules and
- 14 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
biologics). Potential carriers for therapeutic agents Including antibiotics,
immuno suppressants, antigenic peptides, bactericidal peptides, structural
and functional proteins have been disclosed in US Patent No. 6,702,850).
Calcium phosphate coatings as the drug carrier can also be used (see U.S.
Patent Nos. 6,426,114, 6,730,324, and U.S. Provisional Application No.
60/410,307, the disclosures of which are incorporated herein by reference).
Dopants that can act as agent-carrying media include nanoporous,
mesoporpous, nanotubes, micro-particles of various materials including
hydroxyapatite, silica, carbon, and titania (T102) capable of carrying
therapeutic agents, biomolecules and biologics. Particulates and powders
(e.g. titania powder) can be either adhesively bonded or covalently attached
(tethered) to the therapeutic agents, biomolecules and biologics.
[71] Composites of media and carriers (e.g. sintered together), and
combinations of carriers can convey drugs and biologics and can control
elution profiles.
[72] Other exemplary dopants include barium titanate, zeolites
(aluminosilicates), including siliceacous zeolite and zeolites containing at
least
one component selected from phosphorous, silica, alumina, zirconia, calcium
carbonate, biocompatible glass, calcium phosphate glass. The dopant can
also be a growth factor consisting of epidermal growth factors, transforming
growth factor a, transforming growth factor p, vaccinia growth factors,
fibroblast growth factors, insulin-like growth factors, platelet derived
growth
factors, cartilage derived growth factors, interlukin-2, nerve cell growth
factors,
hemopoietic cell growth factors, lymphocyte growth factors, bone
morphogenic proteins, osteogenic factors or chondrogenic factors.
[73] In one embodiment, the dopant is hydroxyapatite deposited on a
titanium surface. Both HA and TiO2 constitute excellent biocompatible
biointerfaces, both being biostable and safe in the body. Both can be termed
bioreactive in that they can induce specific responses in certain tissues
particularly bone tissue. The surface resulting from the deposition of HA on
titanium as delivered by the micro-blasting technique combines the benefits of
both materials. The TiO2 is not fully covered by the dopant (HA) and therefore
. -15-
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
still presents to the biological tissue, while the HA affixed on and in the
surface is not denatured by the deposition process and therefore conveys its
full benefit to the surrounding tissue. In this manner the different benefits
of
both biomaterials can brought to bear in the biointerface and when further
combined with the surface texture/morphology best suited to intended
functionality of the implant, and moreover the availability of a drug delivery
mechanism, can provide various methods for tailoring the therapeutic,
compositional and morphological profile available to the patient end user.
[74] In one embodiment, the dopant is a therapeutic agent. The
therapeutic agent can be delivered as a particle itself, or immobilized on a
carrier material. Exemplary carrier materials include any of the other dopants
listed herein (those dopants that are not a therapeutic agent) such as
polymers, calcium phosphate, titanium dioxide, silica, biopolymers,
biocompatible glasses, zeolite, demineralized bone, de-proteinated bone,
allograft bone, and composite combinations thereof.
[75] Exemplary classes of therapeutic agents include anti-cancer
drugs, anti-inflammatory drugs, immunosuppressants, an antibiotic, heparin, a
functional protein, a regulatory protein, structural proteins, oligo-peptides,
antigenic peptides, nucleic acids, immunogens, and combinations thereof.
[76] In one embodiment, the therapeutic agent is chosen from
antithrombotics, anticoagulants, antiplatelet agents, thrombolytics,
anti proliferatives, anti-inflammatories, antimitotic, antimicrobial, agents
that
inhibit restenosis, smooth muscle cell inhibitors, antibiotics, fibrinolytic,
immunosuppressive, and anti-antigenic agents.
[77] Exemplary anticancer drugs include acivicin, aclarubicin,
acodazole, acronycine, adozelesin, alanosine, aldesleukin, allopurinol sodium,
altretamine, aminoglutethimide, amonafide, ampligen, amsacrine, androgens,
anguidine, aphidicolin glycinate, asaley, asparaginase, 5-azacitidine,
azathioprine, Bacillus calmette-guerin (BCG), Baker's Antifol (soluble), beta-
2'-deoxythioguanosine, bisantrene HCI, bleomycin sulfate, busulfan,
buthionine sulfoximine, BWA 773U82, BW 502U83.HCI , BW 7U85 mesylate,
ceracemide, carbetimer, carboplatin, carmustine, chlorambucil,
- 16 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
chloroquinoxaline-sulfonamide, chlorozotocin, chromomycin A3, cisplatin,
cladribine, corticosteroids, Corynebacterium parvum, CPT-11, crisnatol,
cyclocytidine, cyclophosphamide, cytarabine, cytembena, dabis maleate,
dacarbazine, dactinomycin, daunorubicin HCI, deazauridine, dexrazoxane,
dianhydrogalactitol, diaziquone, dibromodulcitol, didemnin B,
diethyldithiocarbamate, diglycoaldehyde, dihydro-5-azacytidine, doxorubicin,
echinomycin, edatrexate, edelfosine, eflornithine, Elliott's solution,
elsamitrucin, epirubicin, esorubicin, estramustine phosphate, estrogens,
etanidazole, ethiofos, etoposide, fadrazole, fazarabine, fenretinide,
filgrastim,
finasteride, flavone acetic acid, floxuridine, fludarabine phosphate, 5-
fluorouracil, Fluosol®, flutamide, gallium nitrate, gemcitabine, goserelin
acetate, hepsulfam, hexamethylene bisacetamide, homoharringtonine,
hydrazine sulfate, 4-hydroxyandrostenedione, hydrozyurea, idarubicin HCI,
ifosfamide, interferon alfa, interferon beta, interferon gamma, interleukin-1
alpha and beta, interleukin-3, interleukin-4, interleukin-6, 4-ipomeanol,
iproplatin, isotretinoin, leucovorin calcium, leuprolide acetate, levamisole,
liposomal daunorubicin, liposome encapsulated doxorubicin, lomustine,
lonidamine, maytansine, mechlorethamine hydrochloride, melphalan,
menogaril, merbarone, 6-mercaptopurine, mesna, methanol extraction residue
of Bacillus calmette-guerin, methotrexate, N-methylformamide, mifepristone,
mitoguazone, mitomycin-C, mitotane, mitoxantrone hydrochloride,
monocyte/macrophage colony-stimulating factor, nabilone, nafoxidine,
neocarzinostatin, octreotide acetate, ormaplatin, oxaliplatin, paclitaxel,
pala,
pentostatin, piperazinedione, pipobroman, pirarubicin, piritrexim,
piroxantrone
hydrochloride, PIXY-321, plicamycin, porfimer sodium, prednimustine,
procarbazine, progestins, pyrazofurin, razoxane, sargramostim, semustine,
spirogermanium, spiromustine, streptonigrin, streptozocin, sulofenur, suramin
sodium, tamoxifen, taxotere, tegafur, teniposide, terephthalamidine,
teroxirone, thioguanine, thiotepa, thymidine injection, tiazofurin, topotecan,
toremifene, tretinoin, trifluoperazine hydrochloride, trifluridine,
trimetrexate,
tumor necrosis factor, uracil mustard, vinblastine sulfate, vincristine
sulfate,
vindesine, vinorelbine, vinzolidine, Yoshi 864, zorubicin, and mixtures
thereof.
-17-
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
[78] Exemplary therapeutic agents include immunogens such as a
viral antigen, a bacterial antigen, a fungal antigen, a parasitic antigen,
tumor
antigens, a peptide fragment of a tumor antigen, meta static specific
antigens,
a passive or active vaccine, a synthetic vaccine or a subunit vaccine.
[79] The dopant may be a protein such as an enzyme, antigen,
growth factor, hormone, cytokine or cell surface protein.
[80] The dopant may be a pharmaceutical compound such as an
anti-neoplastic agent, an anti-bacterial agent, an anti parasitic agent, an
anti-
fungal agent, an analgesic agent, an anti-inflammatory agent, a
chemotherapeutic agent, an antibiotic or combinations thereof.
[81] The dopant could also be growth factors, hormones,
immunogens, proteins or pharmaceutical compounds that are part of a drug
delivery system such as those immobilized on zeolite or polymeric matrices,
biocompatible glass or natural porous apitic templates such as coralline HA,
demineralised bone, deproteinated bone, allograft bone, collagen or chitin.
[82] In one embodiment, the dopant is an anti-inflammatory drugs
selected from non-steroidal anti-inflammatory drugs, COX-2 inhibitors,
glucocorticoids, and mixtures thereof. Exemplary non-steroidal anti-
inflammatory drugs include aspirin, diclofenac, indomethacin, sulindac,
ketoprofen, flurbiprofen, ibuprofen, naproxen, piroxicam, tenoxicam, tolmetin,
ketorolac, oxaprosin, mefenamic acid, fenoprofen, nambumetone,
acetaminophen, and mixtures thereof. Exemplary COX-2 inhibitors include
nimesulide, NS-398, flosulid, L-745337, celecoxib, rofecoxib, SC-57666, DuP-
697, parecoxib sodium, JTE-522, valdecoxib, SC-58125, etoricoxib, RS-
57067, L-748780, L-761066, APHS, etodolac, meloxicam, S-2474, and
mixtures thereof. Exemplary glucocorticoids are include hydrocortisone,
cortisone, prednisone, prednisolone, methylprednisolone, meprednisone,
triamcinolone, paramethasone, fluprednisolone, betamethasone,
dexamethasone, fludrocortisone, desoxycorticosterone, and mixtures thereof.
[83] Other exemplary therapeutic agents include cell cycle inhibitors
in general, apoptosis-inducing agents, antiproliferative/antimitotic agents
including natural products such as vinca alkaloids (e.g., vinblastine,
- 18 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
vincristine, and vinorelbine), paclitaxel, colchicine, epidipodophyllotoxins
(e.g.,
etoposide, teniposide), enzymes (e.g., L-asparaginase, which systemically
metabolizes L-asparagine and deprives cells that do not have the capacity to
synthesize their own asparagine); antiplatelet agents such as G(GP) I lb/Illa
inhibitors, GP-1Ia inhibitors and vitronectin receptor antagonists;
antiproliferative/antimitotic alkylating agents such as nitrogen mustards
(mechlorethamine, cyclophosphamide and analogs, melphalan, chlorambucil),
ethylenimines and methylmelamines (hexamethylmelamine and thiotepa),
alkyl sulfonates-busulfan, nitrosoureas (carmustine (BCNU) and analogs,
streptozocin), triazenes--dacarbazine (DTIC); antiproliferative/antimitotic
antimetabolites such as folic acid analogs (methotrexate), pyrimidine analogs
(fluorouracil, floxuridine, and cytarabine), purine analogs and related
inhibitors
(mercaptopurine, thioguanine, pentostatin and 2-chlorodeoxyadenosine
(cladribine)); platinum coordination complexes (cisplatin, carboplatin),
procarbazine, hydroxyurea, mitotane, aminoglutethimide; hormones (e.g.,
estrogen); anticoagulants (heparin, synthetic heparin salts and other
inhibitors
of thrombin); fibrinolytic agents (such as tissue plasminogen activator,
streptokinase and urokinase), aspirin, dipyridamole, ticlopidine, clopidogrel,
abciximab; antimigratory; antisecretory (breveldin); anti-inflammatory: such
as
adrenocortical steroids (cortisol, cortisone, fluorocortisone, prednisone,
prednisolone, 6a-methylprednisolone, triamcinolone, betamethasone, and
dexamethasone), non-steroidal agents (salicylic acid derivatives e.g.,
aspirin;
para-aminophenol derivatives e.g., acetaminophen; indole and indene acetic
acids (indomethacin, sulindac, and etodalac), heteroaryl acetic acids
(tolmetin, diclofenac, and ketorolac), arylpropionic acids (ibuprofen and
derivatives), anthranilic acids (mefenamic acid, and meclofenamic acid),
enolic acids (piroxicam, tenoxicam, phenylbutazone, and oxyphenthatrazone),
nabumetone, gold compounds (auranofin, aurothioglucose, gold sodium
thiomalate); immunosuppressives: (cyclosporine, tacrolimus (FK-506),
sirolimus (rapamycin), azathioprine, mycophenolate mofetil); antigenic agents:
vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF);
angiotensin receptor blockers; nitric oxide donors; anti-sense
oligionucleotides
- 19 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
and combinations thereof; cell cycle inhibitors, mTOR inhibitors, and growth
factor receptor signal transduction kinase inhibitors; retinoid; cyclin/CDK
inhibitors; HMG co-enzyme reductase inhibitors (statins); and protease
inhibitors (matrix protease inhibitors).
[84] In one embodiment, the dopant is an antibiotic chosen from
tobramycin, vancomycin, gentamicin, ampicillin, penicillin, cephalosporin C,
cephalexin, cefaclor, cefamandole and ciprofloxacin, dactinomycin,
actinomycin D, daunorubicin, doxorubicin, idarubicin, penicillins,
cephalosporins, and quinolones, anthracyclines, mitoxantrone, bleomycins,
plicamycin (mithramycin), mitomycin, and mixtures thereof.
[85] In one embodiment, the dopant is a protein chosen from
albumin, casein, gelatin, lysosime, fibronectin, fibrin, chitosan, polylysine,
polyalanine, polycysteine, Bone Morphogenetic Protein (BMP), Epidermal
Growth Factor (EGF), Fibroblast Growth Factor (bFGF), Nerve Growth Factor
(NGF), Bone Derived Growth Factor (BDGF), Transforming Growth Factor-
.beta.1 (TGF-.beta.1), Transforming Growth Factor-.beta. (TGF-.beta.), the tri-
peptide arginine-glycine-aspartic acid (RGD), vitamin D3, dexamethasone,
and human Growth Hormone (hGH), epidermal growth factors, transforming
growth factor a, transforming growth factor 13, vaccinia growth factors,
fibroblast growth factors, insulin-like growth factors, platelet derived
growth
factors, cartilage derived growth factors, interlukin-2, nerve cell growth
factors,
hemopoietic cell growth factors, lymphocyte growth factors, bone
morphogenic proteins, osteogenic factors, chondrogenic factors, or and
mixtures thereof.
[86] In one embodiment, the dopant is a heparin selected from
recombinant heparin, heparin derivatives, and heparin analogues or
combinations thereof.
[87] In one embodiment, the dopant is an oligo-peptide, such as a
bactericidal oligo-peptide.
[88] In one embodiment, the dopant is an osteoconductive or
osteointegrative agent.
-20-
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
[89] In one embodiment, the dopant is an immunosuppressant, such
as cyclosporine, rapamycin and tacrolimus (FK-506), ZoMaxx, everolimus,
etoposide, mitoxantrone, azathioprine, basiliximab, daclizumab, leflunomide,
lymphocyte immune globulin, methotrexate, muromonab-CD3,
mycophenolate, and thalidomide.
[90] In one embodiment, the carrier material is a polymer such as
polyurethanes, polyethylene terephthalate, PLLA-poly-glycolic acid (PGA)
copolymer (PLGA), polycaprolactone, poly-(hydroxybutyrate/hydroxyvalerate)
copolymer, poly(vinylpyrrolidone), polytetrafluoroethylene, poly(2-
hydroxyethylmethacrylate), poly(etherurethane urea), silicones, acrylics,
epoxides, polyesters, urethanes, parlenes, polyphosphazene polymers,
fluoropolymers, polyamides, polyolefins, and blends and copolymers thereof.
[91] In one embodiment, the dopant is a radio opaque material, such
as those chosen from alkalis earth metals, transition metals, rare earth
metals, and oxides, sulphates, phosphates, polymers and combinations
thereof.
[92] In one embodiment, the carrier material is a biopolymer selected
from polysaccharides, gelatin, collagen, alginate, hyaluronic acid, alginic
acid,
carrageenan, chondroitin, pectin, chitosan, and derivatives, blends and
copolymers thereof.
[93] In one embodiment, the dopant is delivered in a gaseous carrier
fluid, such as nitrogen, hydrogen, argon, helium, air, ethylene oxide, and
combinations thereof. In another embodiment, the dopant is delivered in a
liquid carrier fluid. In one embodiment, the liquid is also an etching liquid
(basic or acidic) In one embodiment, the dopant is delivered in an inert
environment.
[94] Another embodiment relates to the chemical treatment of metal
surfaces for the purposes of adhesion. Good adhesion of paints and
polymeric coatings to metal surfaces is an area of increasing technical
importance. This technology can be used to pre-treat a surface by
impregnating it with compounds having desired chemical functionality. These
-21 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
include but are not limited to polymers or silica materials having siloxane
groups.
[95] The pretreatment can be used to lay down a very strongly bound
layer of seed polymer material on the surface. Further polymer coatings could
then be attached to this seed layer rather than trying to attaching it
directly to
the surface of the metal.
[96] The dopant is not limited to one compound but could be any
combination of any of the materials listed or even any material(s) that do(es)
not have the necessary mechanical properties to impregnate the surface if
delivered singularly at high velocity to the surface.
[97] In one embodiment, the dopant can be any material so long as it
is passive, i.e., unreactive with the surface. It simply has to be at the
surface
when the oxide layer is breeched by the abrasive so that the oxide reforms
around it.
[98] In one embodiment, the dopant is nanocrystalline.
[99] In one embodiment, the dopant is nanocrystalline
hydroxyapatite.
[100] In one embodiment the abrasive has a suitable property chosen
from at least one of size, shape, hardness, and density to break the oxide
layer. In one embodiment, the abrasive has a modus hardness ranging from
0.1 to 10, such as a modus hardness ranging from 1 to 10, or a modus
hardness ranging from 5 to 10. In another embodiment, the abrasive has a
particle size ranging from 0.1 pm to 10000 pm, such as a particle size ranging
from 1 pm to 5000 pm, or a particle size ranging from 10 pm to 1000 pm.
[101] Abrasive materials to be used in this invention include but are
not limited to shot or grit made from silica, alumina, zirconia, barium
titanate,
calcium titanate, sodium titanate, titanium oxide, glass, biocompatible glass,
diamond, silicon carbide, calcium phosphate, calcium carbonate, metallic
powders, carbon fiber composites, polymeric composites, titanium, stainless
steel, hardened steel, carbon steel chromium alloys or any combination
thereof.
- 22 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
[102] The pressure of the fluid jet will also be a factor in determining
the impact energy of the abrasive. The abrasive and dopant(s) do not have to
be delivered to the surface through the same jet. They could be in any
number of separate jets as long as they deliver the solid components to the
surface at the substantially the same time, e.g., prior to reformation of the
oxide layer if the surface is a metal. This allows a large amount of
flexibility in
optimizing the invention towards a specific need. In one embodiment, the
fluid jet is selected from wet blasters, abrasive water jet peening machines,
and wet shot peening machines. In one embodiment, the at least one fluid jet
operates at a pressure ranging from 0.5 to 100 bar, such as a pressure
ranging from 1 to 30 bar, or a pressure ranging from 1 to 10 bar.
[103] In another embodiment, the at least one fluid jet is selected
from dry shot peening machines, dry blasters, wheel abraders, grit blasters),
sand blasters(s), and micro-blasters. In one embodiment, the at least one
fluid jet operates at a pressure ranging from 0.5 to 100 bar, such as a
pressure ranging from 1 to 30 bar, or a pressure ranging from 3 to 10 bar.
[104] In other embodiments, blasting equipment can be used in
conjunction with controlled motion such as CNC or robotic control. The
blasting can be performed in an inert environment.
[105] In one embodiment, the dopants and abrasives are contained in
the same reservoir and are delivered to a surface from the same jet (nozzle).
In another embodiment, the dopant is contained in one reservoir and abrasive
contained in a separate reservoir, and multiple nozzles deliver the dopants
and abrasives. The multiple nozzles can take the form of a jet within a jet,
i.e., the particles from each jet bombard the surface at the same incident
angle. In another embodiment, the multiple are spatially separated so as to
bombard the surface at different incident angles yet hit the same spot on the
surface simultaneously.
[106] FIGS. 9A, 9B, and 9C are schematic diagrams of three different
nozzle configurations to deliver the dopants and abrasive to a surface: single
nozzle (9A), multiple nozzles with dopants and abrasives delivered from
separate reservoirs where one nozzle is situated within another nozzle (96);
- 23 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
and multiple, separate nozzles with dopants and abrasives delivered from
separate reservoirs (9C). More specifically, FIG. 9A shows a single nozzle 20
for delivering a single stream 23 of abrasive particles 24 and dopant
particles
26 to a substrate 28. FIG. 9B shows that multiple nozzles with dopants and
abrasives delivered from separate reservoirs can be used, where FIG. 9B
illustrates one nozzle 30 for delivering a stream 33 of abrasive particles 24
situated within another nozzle 40 for delivering a stream 43 of dopant
particles 26, where streams 33 and 43 are coaxial. Multiple, separate nozzles
with dopants and abrasives delivered from separate reservoirs can also be
used, as indicated in FIG. 9C, which shows nozzles 30 and 40, for delivering
streams 33 and 43 of abrasive particles 24 and dopant particles 26,
respectively.
[107] It can be readily appreciated that where more than one type of
dopant is used, dopants can be delivered from a single nozzle, or from
separate nozzles. For example, where the dopant combination is a
therapeutic agent combined with another particle (e.g., hydroxyapatite), a two
nozzle design can be used for delivering the dopant combination from one
nozzle and the abrasive from the second nozzle. In another embodiment, a
three nozzle configuration can be used where the therapeutic agent is
delivered from a first nozzle, the second set of dopant particles is delivered
from a second nozzle, and the abrasive is delivered from a third nozzle.
[108] In one embodiment, the article is an implantable medical device.
Exemplary medical devices include catheters, guide wires, and baskets used
in the removal of pathological calcifications. In the case of biomedical
devices
it is desirable that the level of impregnation of the abrasive itself in the
surface
is minimal. The abrasive should further be biocompatible as it is likely that
some impregnation will occur.
[109] In one embodiment, the article is a metal, such as those metals
chosen from pure metals, metal alloys, intermetals comprising single or
multiple phases, intermetals comprising amorphous phases, intermetals
comprising single crystal phases, and intermetals comprising polycrystalline
phases. Exemplary metals include titanium, titanium alloys (e.g., NiTi or
- 24 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
nitinol), ferrous alloys, stainless steel and stainless steel alloys, carbon
steel,
carbon steel alloys, aluminum, aluminum alloys, nickel, nickel alloys, nickel
titanium alloys, tantalum, tantalum alloys, niobium, niobium alloys, chromium,
chromium alloys, cobalt, cobalt alloys, precious metals, and precious metal
alloys. In one embodiment, the metal is titanium.
[110] In one embodiment the abrasive material is alumina (10 Mesh)
while the dopant is HA with a particle size range of 0.1 to 3 pm. The mixed
media is achieved by mixing the dopant and abrasive between the ratio of
5:95 and 95:5 HA to Silica volume % but more preferably between the ratio of
80:20 to 20:80 and most preferably in the ratio range 60:40 to 40:60. The
silica bead has a Mohs hardness in the range of 0.1 to 10 but most preferably
in the range of 2 to 10 and most preferably in the range 5 to 10. This mixed
media is delivered to a titanium surface using a standard grit blasting
machine
operating in the pressure range of 0.5 Bar to 20 Bar, such as a pressure
range of 2 to 10 bar, or a pressure range of 4 Bar to 6 Bar. The distance
between the nozzle and the surface can be in the range of 0.1 mm to 100
mm, such as a range of 0.1 mm to 50 mm, or a range of 0.1 mm to 20mm.
The angle of the nozzle to the surface can range from 10 degrees to 90
degrees, such as a range of 30 degrees to 90 degrees, or a range of 70 to 90
degrees.
[111] In another embodiment the abrasive material is silica (10 Mesh)
while the dopant is HA with a particle size range of 0.1 to 3 pm. The mixed
media is achieved by mixing the dopant and abrasive between the ratio of
5:95 and 95:5 HA to alumina weight % but more preferably between the ratio
of 80:20 to 20:80 and most preferably in the ratio range 60:40 to 40:60. The
Alumina grit has a Mohs hardness in the range of 0.1 to 10, such as a range
of 2 to 10, or a range of 5 to 10. This mixed media can be delivered to a
titanium surface using a standard grit blasting machine operating in the
pressure range 0.5 Bar to 20 Bar, such as a pressure range of 2 to 10 bar, a
range of 4 Bar to 6 Bar. The distance between the nozzle and the surface
can range from 0.1 mm to 100 mm, such as a range of 0.1 mm to 50 mm, or a
range of 0.1 mm to 20mm. The angle of the nozzle to the surface can range
- 25 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
from 10 degrees to 90 degrees, such as a range of 30 degrees to 90 degrees,
or a range of 70 to 90 degrees.
[112] One of ordinary skill in the art can appreciate the influence of
machine parameters including jet velocity, operating pressure, venturi
configuration, angle of incidence and surface to nozzle distances on the
extent of impregnation of the dopant in the surface using these mixed media.
[113] One of ordinary skill in the art can appreciate the effect of the
size, shape, density and hardness of the abrasive material used on the extent
of impregnation of the dopant in the surface using these mixed media.
[114] One of ordinary skill in the art can appreciate the effect of the
fluid stream itself, the blasting equipment using a gas medium (typically air)
the effects of using inert gases as a carrier fluid e.g. N2 or noble gases
such
as Ar and He on the extent of impregnation of the dopant in the surface using
these mixed media.
[115] In the case of wet blasting equipment using a liquid as a carrier
fluid (normally water), One of ordinary skill in the art can appreciate the
effect
of acidity and basicity on the extent of impregnation of the dopant in the
surface using these mixed media.
[116] As disclosed herein, the disclosed methods can be useful for
modifying the surfaces of medical devices. In the context of medical device
applications, dopants can be active (eliciting a biological response) or
passive
(not eliciting a biological response). Passive dopants can be conveyed to
enhance lubricity or render a substrate radio-opaque, of enhance wear
characteristics or enhance adhesion of an ad-layer, etc. Active agents can
evoke a response from the host tissue in vivo, enhancing the functionality of
the device or the surgery, or delivering a benefit as a secondary function to
the device.
[117] The process is a deposition process allowing for the addition of
material(s) to a surface by a methodology typically used to remove material
from a surface. In one embodiment, the method allows for the impregnation
of the surface using:
- 26 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
1. abrasive bombardment to convey an additional material onto
and/or into a surface;
2. the removal of oxide layers from a surface in an inert environment
and the subsequent deposition of additional material onto or into
the surface prior to allowing the surface to oxidise over again; or
3. a combination of 1 and 2 above
[1 1 8] The process can be used to modify, augment or treat surfaces
such as to change surface characteristics/properties including one or more of:
= morphology/topography/form/texture/roughness/microstructure
= surface area
= surface porosity
= structure ¨ order/disorder of molecular assemblies, inclusions,
vacancies, and organisation
= crystallinity, size, distribution and orientation of crystals
= chemistry,
= chemical composition,
= elemental composition
= chemical state of elements
= molecular composition
= functional groups
= molecular adlayers
= adventitious contaminants and impurities
= oxide layer porosity, thickness and composition,
= biochemistry
= biological performance
= surface energy - lipophilic /lipophobic properties
= wetabillity ¨ hydrophilic and hydrophobic properties,
= adsorption ¨ physisorption and chemisorption
= electric properties ¨ surface potentials and surface charges,
dielectric constant
= magnetic properties
- 27 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
= optical properties ¨ optical reflection/absorption
= surface mechanical properties ¨ Elastic/plastic nature of surface
layers, tensile/compressive forces in the surface
= surface dynamic properties ¨ mobility of atoms and molecules
[119] The effect on the surface is such as to modify the chemistry and
topography of the surface material resulting in an infinite range of
manifestations. The desired outcome resulting from the treatment is
influenced by:
= the substrate material and its surface characteristics
= the treatment process parameters and the environmental
conditions
= the abrasive(s) and its mechanical and chemical properties, size,
hardness, morphology etc
= the dopant material(s) and its chemical and mechanical
properties, whether it is a carrier medium for additional agents
(e.g. therapies), or an active or passive agent, or a composite or a
cocktail mix.
[120] In one embodiment, the methods described herein can provide
one or more of the following feature
= a room temperature process
= no degradation of the dopant material(s) due to temperature or
process
= ability to convey temperature sensitive agents to the surface
intact.
= one step process that is manufacturing friendly
= no conformal polymer film required to convey therapeutic agents
= no laminate layer results ¨ cannot be chipped or peeled off
= adaptable to allowing implants to be custom treated for specific
applications
= has application in industrial sectors outside the Medical Device
sector, e.g., industries that use titanium, e.g., the aerospace sector,
- 28 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
the food sector (use of titanium pipes), and the semiconductor
sector, etc.
EXAMPLES
Example 1
[121] This example describes the modification of a titanium substrate
using hydroxyapatite (HA) as the dopant and alumina bead as the abrasive.
[122] A mixed media was prepared consisting of 50 weight percent
alumina (White Saftigrit: Mesh size 150, 88 micron particle size, Mohs
hardness 9, Guyson international Ltd) and 50 weight percent HA (Fluka
Synthetic hydroxyapatite (Fluka production GmbH, Buchs, Switzerland, part of
the Sigma-Aldrich family). A Rocatec TM grit blaster operating at a pressure
of
bar was used to grit blast a 2cm x 2cm CP titanium coupon (Titanium Sheet
Grade 2 Medical to ASTM F67 Spec.). The nozzle to surface distance was 1
cm and the nozzle was held at 90 to the surface. The silicon carbide nozzle
had an orifice diameter of lmm and traversed the surface at 2cm per sec.
The surface was subjected to three passes.
[123] Two further samples of Titanium (Titanium Sheet Grade 2
Medical to ASTM F67 Spec.) were subjected to the same treatment but with
the media consisting of HA only.
[124] The samples were then subjected to a cleaning treatment
involving 20 minutes ultrasonic washing in deionized water to remove any
material that was not intimately affixed to the surface. After the ultrasonic
cleaning the samples were rinsed with deionized water and air-dried in an
oven at 95 C for one hour.
[125] Samples were submitted for XPS (X-Ray photoelectron
spectroscopy) analysis to determine the relative concentration of Ca, P, Ti
and Al at the surfaces. FIG. 2 shows the wide scans of both samples, where
FIG. 2A is an XPS survey scan of titanium treated with hydroxyapatite, and
FIG. 2B is an XPS survey scan of titanium treated with the mixed media of
50:50 HA/alumina. As can be seen the concentration of Ca and P (indicative
- 29 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
of HA) in the sample grit blasted using the mixed media technique was
significantly higher than those seen in the sample grit blasted with HA only.
This is further confirmed by the higher resolution scans of the narrow
regions.
FIGS. 3 and 4 show the Ca 2p, P 2p and Ti 2p core levels on the 50% HA:
50% Alumina and 100% HA samples. Specifically, FIGS. 3A and 3B show
comparative XPS spectra of Ca 2p (FIG. 3A) and P 2p (FIG. 3B) core levels
of HA only blasted cp titanium (fine line) and 50:50 HA:alumina blasted cp
titanium (coarse line), and FIG. 4 shows XPS spectra of the Ti 2p core level
on the sample grit blasted with 100% HA (top) and the sample grit blasted
with a 50:50 HA:alumina mix (bottom), indicating that the titanium is
substantially covered by HA. In the case of the mixed media grit blasted
sample a significant increase in the concentration of both Calcium and
Phosphorous was observed in comparison with the sample blasted with HA
only. Furthermore the Ca:P ratio was found to be 1.65 confirming that the
material on the surface was indeed HA.
[1 26] A further indication of the presence of a significant surface layer
of HA was the greatly reduced Ti concentration observed at the mixed media
blasted surface in comparison with that observed at the 100 % HA blasted
surface indicating a layer of HA of substantial thickness (>10 nrn). XPS can
be used to calculate the relative concentrations of species at a surface to
within an error of 10%) by normalizing the areas under the core level curves
with the RSF (Relative Scattering Factor) for each element. The calculated
atomic ratio of Ca/Ti at the surface is given in table 1. This value best
represents the level of coverage at the surfaces. In the case of the
Alumina/HA grit blasted sample the relative concentration of Ca to Ti is
approximately 30 times that observed on the 100% HA blasted sample.
- 30 -
CA 02663036 2009-03-06
WO 2008/033867
PCT/US2007/078197
Table 1. The atomic ratio of Ca/Ti as determined from the narrow XPS
scans at the surface of the grit blasted Cp Ti surfaces
BLASTING MEDIA (WEIGHT %/ WEIGHT CA/TI RELATIVE
%) RATIO RATIO
100% HA 0.45 0.98
100% HA 0.47 1.02
50% HA: 50% Alumina 13.43 29.20
50% HA: 50% Silica bead 1.96 4.26
50% HA: 50% Silica bead 2.01 4.37
[127] In order to asses the uniformity of the HA concentration coating
on the surface XPS surface maps (0.2 x 0.2mm) were run on both samples
sitting on the Ti 2P and Ca 2P peaks, the right and left panels of FIG. 5
respectively. The uniformity of color observed is indicative of the uniformity
of
distribution of the HA on the substrate material.
[128] These results indicate that simultaneous bombardment allows
the HA to become impregnated in the titanium surface. Further more given
that both samples were subjected to a rigorous ultrasonic cleaning cycle, it
is
likely that the HA that remains on the surface was strongly bound on the
substrate.
Example 2
[129] This Example describes the modification of a titanium substrate
using hydroxyapatite as the dopant and silica bead as the abrasive.
[130] A mixed media was prepared consisting of 50 weight percent
silica bead (Honite 14: 75 -150 micron particle size range, Mohs hardness 5
Guyson international Ltd) and 50 weight percent HA (Fluka Synthetic
hydroxyapatite). A Rocatec TM grit blaster operating at a pressure of 5 bar
was
used to grit blast two 2cm x 2cm CP Titanium coupon (Titanium Sheet Grade
2 Medical to ASTM F67 Spec). The nozzle to surface distance was 1 cm and
the nozzle was held at 900 to the surface. The silicon carbide nozzle had an
- 31 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
orifice diameter of lmm and traversed the surface at 2cm sec-1. The surface
was subjected to three passes.
[131] The samples were then subjected to a cleaning treatment
involving 20 minutes ultrasonic washing in deionized water to remove any
material that was not intimately affixed to the surface. After the ultrasonic
cleaning the samples were rinsed with deionized water and air-dried in an
oven at 95 C for one hour.
[132] Samples were submitted for XPS (X-Ray photoelectron
spectroscopy) analysis to determine the relative concentration of Ca, P, Ti
and Si at the surfaces. A comparison of are Ca 2p core level in one of the
samples and the 100% HA grit blasted sample is shown in the right panel of
FIG. 6. The P 2p core levels on both samples are shown in the left panel of
FIG. 6. In the case of the mixed media grit blasted sample a significant
increase in the concentration of both calcium and phosphorous was observed
in comparison with the sample blasted with HA only although not as high as
was the case with alumina.
[133] The calculated atomic ratio of Ca/Ti at the surfaces is given in
Table 1. In the case of the silica bead/HA grit blasted sample the relative
concentration of Ca to Ti is approximately 4 times that observed on the 100%
HA blasted samples. Table 1 also demonstrates the reproducibility of the
results achievable with this technique given that the Ca/Ti ratio measured on
the samples treated with the same mixed media are approximately the same.
This is further demonstrated in FIG. 7 which shows the similarity in the
survey
scans of the two samples.
Example 3
[134] This Example describes the modification of a titanium substrate
using hydroxyapatite/gentamycin as the dopant and alumina bead as the
abrasive.
[135] A mixed media was prepared consisting of 50 weight percent
alumina (White Saftigrit: Mesh size 150, 88 micron particle size, Mohs
hardness 9, Guyson international Ltd), 40 weight percent HA (Fluka Synthetic
- 32 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
hydroxyapatite) and 10 weight percent Gentamycin. A Rocatec TM grit blaster
operating at a pressure of 5 bar was used to grit blast three 0.5cm x 0.5cm
CP titanium coupons (Titanium Sheet Grade 2 Medical to ASTM F67 Spec).
Control coupons were blasted with HA and alumina only. The nozzle to
surface distance was 1 cm and the nozzle was held at 90 to the surface. The
silicon carbide nozzle had an orifice diameter of lmm and traversed the
surface at 2cm sec-1. The surface was subjected to three passes.
[136] The samples were then subjected to a cleaning treatment
involving 20 minutes ultrasonic washing in deionized water to remove any
material that was not intimately affixed to the surface. After the ultrasonic
cleaning the samples were rinsed with deionized water and air-dried in an
oven at 40 C for one hour.
[137] The release and antibacterial activity of the antibiotic loaded
surfaces was evaluated against three bacterial species [Staphylococcus
aureus (FIG. 8.1), Escherichia coli (FIG. 8.2) and Pseudomonas aeruginosa
(FIG. 8.3)], identified as opportunistic pathogens colonizing peri-prosthetic
tissue post operation and a major cause of the corrosion of implants, using an
agar disc-diffusion method.
[138] In brief, the bacteria were grown from stock cultures on brain
heart infusion (BHI) agar at 37 C for 16 h and isolated colonies were used to
seed fresh cultures in 10 ml Luria Broth (LB). After incubation at 37 C for 12-
16 h with shaking (200 rpm), the cultures were diluted in Mueller Hinton (MH)
broth to give an OD 600 of 0.05. A 350-pl volume of each bacterial
suspension was streaked using clinical swabs on MH agar plates containing
agar to a depth of 4 mm. Following this the coupons of material were placed
on the agar. The plates were inverted and incubated under aerobic conditions
(36 h, 37 C).
[139] The possibility that the implant material was inhibitory with
respect to microbial growth independent of the activity of the released
Gentamycin was eliminated by using the control samples not having the
antibiotic loaded on the surface (negative control) labeled 1 in FIGS. 8.1,
8.2,
- 33 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
and 8.3 respectively. The antibiotic loaded samples are labeled 2 in FIGS.
8.1, 8.2, and 8.3 respectively.
[140] The results are shown in FIG. 8. In the case of each of the three
bacterial species tested, an inhibition zone where bacterial growth is
inhibited
(labeled IZ in FIGS. 8.1, 8.2 and 8.3 respectively) was seen around the
HA/Gentamycin treated samples. This indicates that the Gentamycin was
incorporated into the surface by the process and furthermore that the
antibiotic remains active through the blasting process.
Example 4
[141] This Example describes the modification of a titanium substrate
using hydroxyapatite/vancomycin as the dopant and alumina bead as the
abrasive.
[142] A mixed media was prepared consisting of 67 weight percent
alumina (White Saftigrit: Mesh size 150, 88 micron particle size, Mohs
hardness 9, Guyson international Ltd), 30 weight percent HA (Fluka Synthetic
hydroxyapatite) and 3 weight percent Vancomycin. A RocatecTM grit blaster
operating at a pressure of 5 bar was used to grit blast eighteen 10mm
diameter Grade 5 titanium discs (Titanium 6AL-4V Sheet Medical to ASTM
F136 Spec). Control discs were blasted with HA and alumina only. The
nozzle to surface distance was 0.5 cm and the nozzle was held at 90 to the
surface. The silicon carbide nozzle had an orifice diameter of lmm and
traversed the surface at 2cm sec-1. The surface was subjected to three
passes.
[143] A number of the samples were then subjected to a cleaning
treatment involving 20 minutes ultrasonic washing in deionized water to
remove any material that was not intimately affixed to the surface. After the
ultrasonic cleaning the samples were rinsed with deionized water and allowed
to air-dry in an oven at 40 C for one hour.
[144] The release and antibacterial activity of the antibiotic loaded
surfaces was evaluated against the bacterial species Staphylococcus aureus
(NCIMB 9518), identified as an opportunistic pathogen colonizing peri-
- 34 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
prosthetic tissue post operation, using an agar disc-diffusion method. FIG.
10, Plate 1 is a photograph of the inhibition zone (IZ) on an agar plate
inoculated with S. aureus and exposed to the vancomycin-doped coupon.
[145] Tests were carried out according to BSAC (British Society for
Antimicrobial Chemotherapy) Disc Diffusion method for Antimicrobial
Susceptibility testing (Version 2.1.1, January 2002). A bacterial suspension
containing 107 CFU/ml of Staphylococcus aureus NCIMB 9518 was prepared
from fresh overnight cultures, and 0.5 ml of this suspension was
homogeneously spread onto isosensitest agar plates. Following this the
coupons of material were placed on the agar. The plates were incubated
under aerobic conditions (20 hours @ 37 C).
[146] The possibility that the implant material was inhibitory with
respect to microbial growth was eliminated by using control samples not
having the antibiotic loaded on the surface (negative control).
[147] The results are shown in FIG. 10 plate 1, as demonstrated by an
inhibition zone pointing to inhibited bacterial growth around the
HA/vancomycin treated samples. This indicates that the vancomycin was
incorporated into the surface by the process and furthermore that the
antibiotic remains active through the blasting process.
Example 5
[148] This Example describes the modification of a titanium substrate
using hydroxyapatite/ tobramycin as the dopant and alumina bead as the
abrasive.
[149] A mixed media was prepared consisting of 67 weight percent
alumina (White Saftigrit: Mesh size 150, 88 micron particle size, Mohs
hardness 9, Guyson international Ltd), 30 weight percent HA (Fluka Synthetic
hydroxyapatite) and 3 weight percent Tobramycin. A Rocatec TM grit blaster
operating at a pressure of 5 bar was used to grit blast eighteen 10mm
diameter Grade 5 titanium discs (Titanium 6AL-4V Sheet Medical to ASTM
F136 Spec). Control discs were blasted with HA and alumina only. The
nozzle to surface distance was 0.5 cm and the nozzle was held at 90 to the
- 35 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
surface. The silicon carbide nozzle had an orifice diameter of lmm and
traversed the surface at 2cm sec-1. The surface was subjected to three
passes.
[150] A number of the samples were then subjected to a cleaning
treatment involving 20 minutes ultrasonic washing in deionized water to
remove any material that was not intimately affixed to the surface. After the
ultrasonic cleaning the samples were rinsed with deionized water and allowed
to air-dry in an oven at 40 C for one hour.
[151] The release and antibacterial activity of the antibiotic loaded
surfaces was evaluated against the bacterial species Escherichia coli (NCIMB
12210), identified as an opportunistic pathogen colonizing peri-prosthetic
tissue post operation, using an agar disc-diffusion method. FIG. 10, Plates 2
and 3 are photographs of the inhibition zone (IZ) on an agar plate inoculated
with E. Coli and exposed to the tobramycin doped coupon.
[152] Tests were carried out according to BSAC (British Society for
Antimicrobial Chemotherapy) Disc Diffusion method for Antimicrobial
Susceptibility testing (Version 2.1.1, January 2002). A bacterial suspension
containing 107 CFU/ml of E. coli NCIMB 12210 was prepared from fresh
overnight cultures, and 0.5 ml of this suspension was homogeneously spread
onto isosensitest agar plates. Following this the coupons of material were
placed on the agar. The plates were incubated under aerobic conditions (20
hours @ 37 C).
[153] The possibility that the implant material was inhibitory with
respect to microbial growth was eliminated by using control samples not
having the antibiotic loaded on the surface (negative control).
[154] The results are shown in FIG. 10, Plates 2 and 3, as
demonstrated by an inhibition zone pointing to inhibited bacterial growth
around the HA/Tobramycin treated samples. This indicates that the
Tobramycin was incorporated into the surface by the process and furthermore
that the antibiotic remains active through the blasting process.
- 36 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
Example 6
[155] This example describes the modification of a titanium substrate
using hydroxyapatite as the dopant and abrasives of varying size/hardness.
[156] A mixed media was prepared consisting of 80 weight percent
abrasive (50, 100 micron particle size Silica bead, Mohs hardness 6, Comco
Inc.; 50, 100, 150 micron particle size Alumina bead, Mohs hardness 9,
Comco Inc.) and 20 weight percent HA (Fluke Synthetic hydroxyapatite). A
Comco MB1000 Micro-blaster operating at a blast pressure of 80psi was used
to grit blast nine lOmm diameter Grade 5 titanium discs (Titanium 6AL-4V
Sheet Medical to ASTM F136 Spec) for each abrasive type. The nozzle to
surface distance was 15 mm and the nozzle was held at 90 to the surface.
The HP (high performance) nozzle used had an orifice diameter of 0.060 inch
and traversed the surface at 3.175mmsec-1. The surface was subjected to
one pass through the centre of each metal disc.
[157] The samples were then subjected to a cleaning treatment
involving 20 minutes ultrasonic washing in deionized water to remove any
material that was not intimately affixed to the surface. After the ultrasonic
cleaning the samples were rinsed with deionized water and air-dried in an
oven at 40 C for one hour.
[158] Samples were submitted for XPS (X-Ray photoelectron
spectroscopy); FTIR (Fourier Transform Infrared Spectroscopy); Surface
Roughness analysis ¨ Stylus Profilometry; (XRD) X-Ray Diffraction, to
determine the relative concentration of Ca, P, and Ti at the surface of each
sample in conjunction with the morphological characteristics of each sample.
[159] Table 2 indicates the results shown for titanium blasted with
abrasives of varying particle size and hardness, as indicated by XPS. Figs.
11A and 11B show FTIR spectra plots for duplicate 100pm and 150pm
alumina bead respectively.
- 37 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
Table 2: XPS atomic concentrations of surface elements (and Ca:P ratio)
as a function of blast particle size and hardness
100pM 50pM Alu 100pM Alu 150pM Alu
Elements Control Glass Oxide Oxide Oxide
Bead Bead Bead Bead
0 1s 37.78 54.80 53.38 54.71 54.12
C 1s * 44.33 20.73 24.95 23.08 24.02
N 1s 3.37 0.25 0.57 0.84 0.55
Ti 2p 5.00 0.23 1.18 1.32 0.86
Ca 2p 0.28** 14.58 12.23 12.36 11.99
P 2p 0.29 ** 9.40 7.69 7.68 8.47
Al 2p 8.94 ***
Ca/P Ratio n/a 1.55 1.61 1.61 1.42
* Normal adventitious Carbon level on Titanium & its alloys - can be
higher depending on
the forming / manufacturing processes undergone.
** Adventitious HA due to cross contamination from treated samples.
*** Aluminium in the TiAl4V6 alloy (Grade 5 Titanium).
[160] FIG. 11A shows FTIR spectra of duplicate 100 pm alumina bead
samples (a) and (b),and FIG. 11B shows FTIR spectra of 150 duplicate pm
alumina bead samples (a) and (b).
[161] Table 3 indicates the results shown for abrasives of varying
particle size and hardness, as indicated by stylus profilometry.
Table 3: Stylus profilometry of surface topography showing roughness
as a function of blast particle size and hardness
loopm 50pM Alu 100pM Alu 150pM Alu
Glass Oxide Oxide Oxide
Bead Bead Bead Bead
Avg. Surface
Roughness
(PM) 0.35 0.37 0.62 0.61
Std Dev 0.06 0.03 0.05 0.02
- 38 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
[162] Figs. 12A and 12B show XRD patterns for 50pm and 100pm
alumina bead samples, respectively.
[163] The data indicates that varying the size and hardness of the
abrading media will result in varying surface morphology as expected, but
also in differences in the quantity and coverage of Hydroxyapatite in the
adlayer.
Example 7
[164] This Example describes the modification of a titanium substrate
by delivering hydroxyapatite as the dopant in one particle stream and alumina
bead as the abrasive in a separate particle stream using a twin nozzle, while
varying blast parameters and the abrasive to dopant ratio.
[165] An experiment was conducted to control the uniformity of the
flow of abrasive and dopant materials to the surface being treated by loading
the materials into the reservoirs of two separate Comco MB 1000 Micro-
blaster units feeding separate nozzles aimed at the same point on the
surface, as schematically depicted in FIG. 9C. The following parameters were
varied; nozzle diameters, distance of nozzles from the surface, blast
pressure,
incident angle and the ratio of abrasive to dopant at the point of contact
with
the substrate (See Table 4: Test Parameters variations to study effect on HA
deposition and surface topography).100 micron particle size Alumina bead,
(Mohs hardness 9, Comco Inc.) was used in all test runs. The Synthetic HA
(Glantreo Ltd, Cork, Ireland) used had a particle size range of 20 to 60
microns. Nine lOmm diameter Grade 5 titanium discs (Titanium 6AL-4V Sheet
Medical to ASTM F136 Spec) were treated for each run. The surface was
subjected to one pass through the centre of each metal disc at a feed rate of
3.175mmsec-1.
- 39 -
CA 02663036 2009-03-06
WO 2008/033867
PCT/US2007/078197
Table 4: Test Parameter variations to study effect on HA deposition and
surface topography
Run A: Nozzle B: Nozzle C: Blast D: Incident E:
Abrasive to
Diameter Distance Pressure Angle Dopant
Ratio
1 30 12 95 90 70:30
2 30 18 95 45 90:10
3 60 18 60 90 90:10
4 30 18 95 90 90:10
46 15 80 67.5 80:20
6 30 12 95 45 70:30
7 60 12 60 90 70:30
8 30 12 60 45 90:10
9 30 18 60 90 70:30
60 18 95 45 70:30
11 60 12 60 45 70:30
12 60 18 60 45 90:10
13 30 12 60 90 90:10
14 60 12 95 90 90:10
60 12 95 45 90:10
16 30 18 60 45 70:30
17 60 18 95 90 70:30
[166] The samples were then subjected to a cleaning treatment
involving 20 minutes ultrasonic washing in deionized water to remove any
material that was not intimately affixed to the surface. After the ultrasonic
cleaning the samples were rinsed with deionized water and air-dried in an
oven at 40 C for one hour.
[167] Samples were submitted for XPS (X-Ray photoelectron
spectroscopy); FTIR (Fourier Transform Infrared Spectroscopy); Surface
Roughness analysis ¨ Stylus Profilometry; to determine the relative
concentration of Ca, P, and Ti at the surface of each sample in conjunction
with the morphological characteristics of each sample. Results for XPS
analysis are shown in Table 5 and results for stylus profilometry are shown in
Table 6.
- 40 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
Table 5: XPS atomic concentrations of surface elements (and CA:P ratio)
as a function of varying blast parameters and abrasive to dopant ratio.
Element 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17
0 is 45.7
48.7 49.3 43.9 44.8 44.6 51.1 39.8 48.0 42.9 51.0 50.0 50.8 46.2 48.8 47.8
53.2
C is * 32.6
32.9 32.6 36.2 34.2 42.7 30.1 44.7 30.4 40.3 36.5 34.2 27.1 37.3 31.3 31.0
30.1
Na is 0.6 0.3 0.0 0.4 0.3 1.1 1.9 0.7 0.2 0.7
1.9 1.8 0.4 3.6 1.3 0.4 2.1
Ti 2p 0.4 2.9 2.0 2.0 1.8 5.7 3.7 6.7 0.2 3.3
6.0 5.2 0.7 2.8 3.7 0.7 4.7
Ca 2p 13.3 10.1 10.3 11.2 12.3 4.0 8.2 5.6
13.5 7.9 3.1 5.7 13.9 6.8 9.3 12.9 6.4
P 2p 7.4 5.0 5.8 6.2 6.5 1.9 4.4 2.6 7.8 4.9
1.4 3.1 7.1 3.3 5.5 7.2 3.5
Ca/P 1.81
2.00 1.77 1.80 1.89 2.01 1.99 2.17 1.73 1.61 2.3 1.83 1.94 2.04 1.72 1.80 1.84
Ratio
*Normal adventitious Carbon level on Titanium & its alloys - can be higher
depending on the
forming / manufacturing processes undergone.
Table 6: XPS atomic concentrations of duplicate HA controls
HA powder control Atomic
Concentration (%)
Peak Position BE Point A Point B
(eV)
0 1s 532.5 38.02 40.29
Ca 2p 346.5 13.08 13.72
C 1s 285 41.58 38.65
P 2p 133.5 7.31 7.34
Ca:P ratio 1.79 1.87
Table 7: Stylus profilometry of surface roughness as a function of
varying blast parameters and abrasive to dopant ratio
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17
Average
Surface
0.50 0.46 0.59 0.40 0.55 0.41 0.51 0.46 0.43 0.57 0.53 0.55 0.51 0.53 0.55
0.48 0.54
Roughness
(PM)
Std Dev 0.02
0.03 0.05 0.03 0.02 0.03 0.03 0.02 0.03 0.04 0.01 0.04 0.03 0.02 0.05 0.04
0.04
-41 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
[168] The data indicates that varying the blasting parameters and
abrasive:dopant ratio, as outlined in the experiment, results in varying
surface
morphology as expected, but also in differences in the quantity and coverage
of hydroxyapatite in the adlayer. The HA controls data indicates that the
process does not have a detrimental effect on the HA quality as exemplified
by the Ca:P (calcium to phosphate) ratio data, as shown in FIG. 13, XPS
Survey Spectra for duplicate HA controls.
Example 8
[169] This Example describes the modification of a stainless steel
substrate and a Grade 2 titanium substrate using hydroxyapatite as the
dopant and alumina bead as the abrasive.
[170] A mixed media was prepared consisting of 80 weight percent
alumina (White Saftigrit: Mesh size 150, 88 micron particle size, Mohs
hardness 9, Guyson international Ltd) and 20 weight percent HA (Synthetic
HA, particle size 20 ¨ 60 microns, Glantreo Ltd, Cork, Ireland). A RocatecTM
grit blaster operating at a pressure of 5 bar was used to grit blast a
Stainless
Steel tube (Medical grade Stainless Steel to ASTM 1586 spec) used to
manufacture cardiac stents) and Grade 2 Titanium sheet (Titanium Sheet
Grade 2 Medical to ASTM F67 Spec). The nozzle to surface distance was 0.5
cm and the nozzle was held at 90 to the surface. The silicon carbide nozzle
had an orifice diameter of lmm and traversed the surface at 2cm sec-1. The
surface was subjected to three passes.
[171] The sample was then subjected to a cleaning treatment
involving 20 minutes ultrasonic washing in deionized water to remove any
material that was not intimately affixed to the surface. After the ultrasonic
cleaning the samples were rinsed with deionized water and air-dried in an
oven at 40 C for one hour.
[172] Samples were submitted for SEM/ EDX (Scanning Electron
Microscopy/Energy Dispersive X-Ray) analysis and AFM analysis to
determine if HA was affixed to the surface of both materials. Figures 14 (SEM)
and 15 (EDX) show a well affixed layer of HA on the surface of the stainless
- 42 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
steel sample that gives good coverage as indicated in Table 8, with a
thickness up to 6.5 microns (see Fig 14). As expected the CP Titanium
displayed an adherent layer of HA, see FIG. 16 (SEM) and FIG. 17 (EDX),
while Table 9 shows the elemental analysis of the surface. FIG. 18 (AFM)
shows that the affixed HA layer has a thickness of 7 microns.
Table 8: Elemental Analysis of the HA-Stainless Steel interface
Element Weight % Atomic %
33.19 43.85
o 47.78 47.39
Al 2.12 1.25
Si 0.58 0.33
6.14 3.14
Ca 10.19 4.04
Ca/P = 1.28
Table 9: Elemental Analysis of the HA-Titanium interface
Element Weight % Atomic %
0 33.76 58.38
6.41 5.73
Ca 11.81 8.15
Ti 48.02 27.73
Ca/P = 1.42
Example 9
[173] This Example describes the modification of a titanium substrate
using Alumina bead as the abrasive and a nano-porous silica as the dopant.
Nanoporous silica is known as a suitable drug elution carrier.
[174] A mixed media was prepared consisting of 50 volume percent
alumina (100 micron particle size, Mohs hardness 9, Comco Inc.) and 50
volume percent Mesoporous Silica (particle size is approx. 1 microns; pore
size 10 nanometers, Glantreo Ltd, Cork, Ireland). A Comco MB1000 Micro-
- 43 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
blaster operating at a blast pressure of 80psi was used to grit blast nine
lOmm diameter Grade 5 titanium discs (Titanium 6AL-4V Sheet Medical to
ASTM F136 Spec). The nozzle to surface distance was 15 mm and the
nozzle was held at 900 to the surface. The HP (high performance) nozzle
used had an orifice diameter of 0.060 inch and traversed the surface at
3.175mm sec-1. The surface was subjected to one pass through the centre of
each metal disc.
[175] The samples were then subjected to a cleaning treatment
involving 20 minutes ultrasonic washing in deionized water to remove any
material that was not intimately affixed to the surface. After the ultrasonic
cleaning the samples were rinsed with deionized water and air-dried in an
oven at 40 C for one hour.
[176] Samples were submitted for SEM (Scanning Electron
Microscopy) analysis to determine the presence of the Silica micro-particles
on the surface of the Grade 5 Titanium. Fig 19 displays the Silica particles
affixed to the surface.
Example 10
[177] This Example describes the modification of aluminum and nitinol
substrates with nanoporous HA (a drug elution carrier) as a dopant and
alumina bead as an abrasive.
[178] A mixed media was prepared consisting of 90 weight percent
alumina (White Saftigrit: Mesh size 150, 88 micron particle size, Mohs
hardness 9, Guyson international Ltd) and 10 weight percent nanoporous HA
(particle size average 50 Microns; irregular non-spherical particles; pore
size
3 - 4 nanometers, Glantreo Ltd, Cork, Ireland). A RocatecTM grit blaster
operating at a pressure of 5 bar was used to grit blast Aluminum and Nitinol.
The nozzle to surface distance was 0.5 cm and the nozzle was held at 90 to
the surface. The silicon carbide nozzle had an orifice diameter of lmm and
traversed the surface at 2cm sec-1. The surfaces were subjected to three
passes.
- 44 -
CA 02663036 2009-03-06
WO 2008/033867 PCT/US2007/078197
[179] The samples were then subjected to a cleaning treatment
involving 20 minutes ultrasonic washing in deionized water to remove any
material that was not intimately affixed to the surface. After the ultrasonic
cleaning the samples were rinsed with deionized water and air-dried in an
oven at 40 C for one hour.
[180] Samples were submitted for SEM (Scanning Electron
Microscopy) analysis to determine the presence of the Nanoporous HA on the
aluminum and nitinol surfaces. FIGS. 20A and 206 are SEM images of the
nanoporous HA adlayer on the aluminum surface, and FIG. 21 is a SEM
image of the nanoporous HA adlayer on the nitinol surface.
- 45 -