Note: Descriptions are shown in the official language in which they were submitted.
CA 02663781 2009-04-22
-1-
SYSTEM AND METHOD FOR ADDING AND TRACKING PRODUCT
INFORMATION TO A PATIENT RECORD
FIELD OF THE INVENTION
[0001] The present system and method relate to a system and method for
adding product information to a patient record. More particularly, the system
and
method further relates to product tracking and replenishing purposes.
BACKGROUND OF THE INVENTION
[0002] Today's health care facilities include a wide range of
establishments, from small and relatively simple medical clinics to large and
complex hospitals. All together, health care facilities use a large amount of
products for treating patients having various health conditions. In some
instances, it is required to keep track of products that have been used for
treatment, or installed within patients, to ensure compatibility or to readily
be able
to contact patients for product recalls. More particularly, in large
hospitals,
keeping track of such products that have been used on or installed within
patients is a challenge, as it is performed manually, requires filling in
multiple
forms and is thus time consuming and requires a certain discipline from the
part
of both practitioners and personnel.
[0003] Efficient ways of monitoring product usage in health care facilities
have been disclosed for product replenishment purposes. In Canadian
application 2,587,186, there is disclosed a system and method for
automatically
alerting hospital supply personnel when the amount left of a given
individually
packed product is below a threshold. According to an aspect, each individually
packed product has an identification label affixed to its package, as the
product is
used, the package is discarded into a garbage bin that is equipped with a
label
scanner. When the package of the product is scanned, the bin is adapted to
transmit to a central server data indicative of the product's identification
label.
The central server then, according to the transmitted data, calculates and
updates a database with the number of packed products that are left over. When
CA 02663781 2009-04-22
- 2-
the number of the particular packed product drops below a product specific
threshold, the central server transmits an alert to a designated supply
personnel
for him to take action at replenishing the supply of the particular product
that is
about to run out.
[0004] However, prior art systems for monitoring products in health care
facilities do not allow the automatic tracking of products that have been used
for
treatment or inserted within a patient in particular.
SUMMARY OF THE INVENTION
[0005] In accordance with an aspect of the present invention, there is
provided a system for adding product information to a patient record. The
system comprises a product database, a patient database, a product detector, a
patient detector and an adding module. The product database stores the product
information corresponding to a product. The patient database stores the
patient
record corresponding to a patient or a link to a patient record. The product
detector detects a product identifier corresponding to the product information
and
generates a product identifier message. The patient detector detects a patient
identifier corresponding to the patient record and generates a patient
identifier
message. Upon receipt of the patient identifier message and the product
identifier message, the adding module adds the product information to the
patient
record.
[0006] In accordance with another aspect of the present invention, there is
provided a method for adding product information to a patient record. The
method inserts into a product database the product information corresponding
to
a product, and inserts into a patient database the patient record
corresponding to
a patient. Then, a product identifier corresponding to the product information
is
detected and a product identifier message is generated. A patient identifier
corresponding to the patient record is also detected, and a patient identifier
message is generated. Upon receipt of the product identifier message ahd the
patient identifier message, the product information is added to the patient
record.
CA 02663781 2009-04-22
- 3-
BRIEF DESCRIPTION OF THE DRAWINGS
[0007] For a better understanding of aspects of the system and method
described herein, and to show more clearly how they may be carried into
effect,
reference will be made to the accompanying drawings in which:
[0008] Figure 1 is a block diagram of the system for adding a product
information to a patient record;
[0009] Figure 2 is an exemplary database diagram;
[0010] Figure 3 is a block diagram of a product detector affixed to a
receptacle according to an aspect of the system;
[0011] Figure 4 is a block diagram of the product detector and a patient
detector that are affixed to the receptacle according to an aspect of the
system;
[0012] Figure 5 is a block diagram of the product detector and an adding
module affixed to the receptacle according to an aspect of the system;
[0013] Figure 6 is a block diagram of the product detector, the patient
detector and the adding module affixed to the receptacle according to an
aspect
of the system; and
[0014] Figure 7 is a flow diagram of a method for adding product
information to a patient record.
DETAILED DESCRIPTION OF THE INVENTION
[0015] The present relates to a system and method for adding product
information to a patient record. More particularly, the present relates to
adding
product information to a patient record for tracking purposes in a medical
CA 02663781 2009-04-22
- 4-
environment. Although the singular form is used throughout the present
specification and claims, it should be clear to those skilled in the art that
in the
medical environment, multiple products and patient records are concurrently
respectively used and maintained, and that the present system and method are
not limited to the adding of one product information to one patient record,
but
rather the adding of product information to patient record in general.
[0016] Presented in Fig. 1 is a system 100 for adding product information
to a patient record, the system 100 comprises a product database 102 for
storing
product information 104 for a product. To be specific, the product may
correspond to one or many of a wide range of medical products such as medical
implants, medical prostheses, medical substances or any other type of medical
products used for treating, reconstructing or preventing a medical condition
from
occurring. The product information 104 may be stored in the product database
102 for example before the product is shelved for use, or when the product is
received by a hospital. The system 100 further comprises a patient database
106
for storing a patient record 108 or a link to a hospital database. The patient
record 108 corresponds to a description of an individual patient that is
registered
at a hospital. Usually, the patient record 108 holds entries that are given by
the
patient at patient registration. The product database 102 and the patient
database 106 may be separate databases as depicted on Figure 1, or may be
incorporated into one single database holding both the product information 104
the patient record 108.
[0017] As further presented in Fig. 1, the system 100 comprises a product
detector 110 for detecting a product identifier 112. More precisely, the
product
identifier 112 corresponds to the product information 104 stored in the
product
database 102. As the product identifier 112 is placed in proximity with the
product
detector 110, the product detector 110 detects the product identifier 112 and
generates a corresponding product identifier message 114. The product
identifier
112 may be any type of electronically, optically or electromagnetically
detectable
CA 02663781 2009-04-22
- 5-
identifier, such as for example a bar code label, a Radio Frequency
Identifier, a
microchip, etc. The product identifier 112 may be affixed to a product, or to
a
product container or on a product package.
[0018] Similarly, the system 100 comprises a patient detector 116 for
detecting a patient identifier 118. More precisely, the patient identifier 118
corresponds to the patient record 108. The patient detector 116 detects the
patient identifier 118 when the patient identifier is placed in proximity with
the
patient detector 116. Once the patient identifier 118 is detected, the patient
detector 116 generates a corresponding patient identifier message 120. The
patient identifier 118 may be any type of electronically or optically
detectable
identifier, such as for example a bar code label, a Radio Frequency tag, a
microchip, etc. The patient identifier may be affixed to the patient, affixed
to a
patient board, to a patient's bed, to a patient's bracelet, or to any other
surface
corresponding to or on the patient.
[0019] The system 100 of Fig. 1 further comprises an adding module 122.
The adding module 122 is adapted to receive either directly or ultimately the
product identifier message 114 and the patient identifier message 120. Upon
receipt of the product identifier message 114 and the patient identifier
message
120, the adding module 122 adds the product information 104 to the patient
record 108. According to one aspect, the adding module 122 is electronically
or
wirelessly connected to both the product detector 110 and the patient detector
116, and receives from them, respectively, the product identifier message 114
and the patient identifier message 120. Based on the received messages 114,
120, the adding module 122 is adapted to add the corresponding product
information 104 to the corresponding patient record 108. For doing this, in
one
aspect, the adding module 122 is adapted to store the product information 104
in
the patient record 108. In another aspect, the adding module 122 is adapted to
store the patient record 108 in the product information 104. In yet another
aspect,
the adding module 122 is adapted to store in a linking database 124 a link 126
CA 02663781 2009-04-22
- 6-
that corresponds to the product information 104 and the patient record 108. In
yet another aspect, instead of storing the product information 104 in the
patient
record 108 or vice versa, a software pointer may be used to link directly the
product information 104 and the patient record 108.
[0020] According to one aspect of the system 100, the databases (102,106
and 124) are located in a central server that is adapted to connect to a local
network of the hospital. When the adding module 122 is also connected to the
local network, it is possible for the adding module 122 to access the
databases
(102,106 and 124) by connecting to the central server. Consequently, it is
possible for multiple adding modules 122 to access the same databases
(102,106 and 124) simultaneously, when multiple patients receive medical care
at the same time. Moreover, there is no need for the adding module 122 to make
a database selection according to the product information 104 or to the
patient
record 108. Since all databases (102,106 and 124) are centralized, each
product
information 104 is stored in the same product database 102 and each patient
record 108 is stored in the same patient database 106.
[0021] It will be understood by a skilled reader that it is possible for the
linking database 124 and the patient database 106 to be implemented as one
single database and that it is also possible for the linking database 124 and
the
product database 102 to be implemented as one single database.
[0022] Presented in Fig.2, according to another aspect, the product
database 102, the patient database 106 and the linking database 124 are a same
database 200. The database 200 comprises a product table 202, a patient table
204 and a linking table 206. In both the product table 202 and the patient
table
204 there are various attribute fields, such as a product attribute field 208
and a
patient attribute field 210, for respectively defining the product information
104
and the patient record 108. Moreover, in both the product table 202 and the
patient table 204 there are a reference field, such as a product reference
field
212 and a patient reference field 214, for respectively referencing the
product
CA 02663781 2009-04-22
- 7-
information 104 and the patient record 108. Although the number and the type
of
attribute fields 208, 220 is variable, in one exemplary aspect, the product
information 104 comprises the following attribute fields 208: product code,
product serial number, product name, product purchase date, product expiry
date, product supplier contact information and product manufacturer's number.
In
contrast, the patient record 108 comprises at least one of the following
attribute
fields 210: a patient number, an event number, patient hospital card number,
patient medical insurance number, patient name, patient date of birth, patient
sex, patient contact information, patient emergency contact information.
Alternately, the product information 104 and the patient record 108 could
include
one or several of the listed attribute fields.
[0023] Further presented in Fig.2 is the linking table 206 that comprises
the product reference field 212 and the patient reference field 214. These
reference fields together form the link 126 which corresponds to adding the
referenced product information 104 to the referenced patient record 108. Thus,
in the event of a product recall, where a particular product having a given
serial
number needs to be retraced, it is possible with the present system 100, to
find
the patient on whom the particular product was used or implanted.
[0024] Returning to Fig. 1, according to an aspect, the system 100 further
comprises a user interface module 130 for allowing a user to retrieve data
stored
in at least one of the databases (102,106 and124). It is possible for the user
to
retrieve various types of data corresponding to either the product information
104, the patient record 108 or the link 126. According to one aspect, the user
interface module 130 allows a user to retrieve "patient contact information"
based
on a "product serial number". With this user interface module 130, it is
possible
for the user to trace the patient(s) in which or on which a specific product
has
been used. According to another case, the user interface module 130 allows the
user to retrieve a "product serial number" based on a "patient hospital card
number". Furthermore, it is possible for the user to list the products that
have
CA 02663781 2009-04-22
- 8-
been used in or on a specific patient. Depending on the various attribute
fields
that define either the product information 104 or the patient record 108, the
user
interface module 130 allows the user to perform correlations and extract
corresponding resulting data.
[0025] Moreover, according to yet another aspect, the system 100 further
comprises a reporting module 132. The reporting module 132 is adapted to
produce a report according to data corresponding to either the product
information 104, the patient record 108 or the link 126. The reporting module
132
is adapted to produce a report based on a request of the user through the user
interface module 130. In one aspect the reporting module 132 is adapted to
produce a report for tracking purposes. In another aspect the report module
132
is adapted to produce a report for managing the product expiry date. When an
expired product is identified, the report module 132 is adapted to
automatically
send the report to a user or a supplier for having the expired product
replaced.
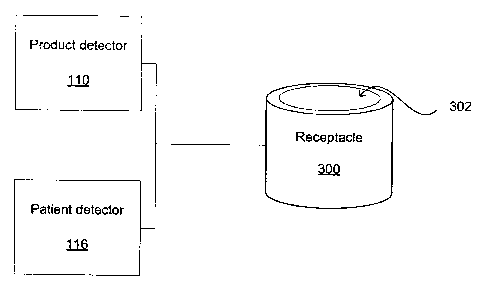
[0026] Presented in Fig. 3, the product detector 110 is affixed to a
receptacle 300. In one aspect of the present invention, the receptacle 300 is
a
bin for containing product packages to be discarded. Once the product package
is opened, and it's contained product is withdrawn from the product package
for
being used on or within a patient, the product package is thrown into the
receptacle 300. According to one aspect, the product detector 110 is adapted
to
detect the product identifier 112 when the product package enters the
receptacle
300. In this aspect, the product detector 110 is directed towards an opening
302
of the receptacle 300 and, as the product package passes through the opening
302, the product detector 110 is adapted to detect the product identifier 112
that
is affixed to the product package. Furthermore, the receptacle 300 is adapted
to
meet hospital regulations in terms of infection control and safety issue.
[0027] According to another aspect, the product detector 110 is adapted to
detect the product identifier 112 once the product package is within the
receptacle 300. In this aspect, the product detector 110 is directed towards
an
CA 02663781 2009-04-22
-9-
inner side of the receptacle 300 and, once the product package is placed in
the
receptacle 300, the product detector 110 is adapted to detect the product
identifier 112 that is affixed to the product package.
[0028] According to yet another aspect, the product detector 110 is
adapted to generate the product identifier message 114 only once, after the
product package is placed in the receptacle 300. In this aspect, the product
detector 110 comprises a memory for storing a list of detected product
identifiers.
As the product identifier 112 is detected, the product detector 110 compares
the
product identifier 112 to the list, and if there is a match, the product
detector 110
does not generate the product identifier message 114. However, if there is no
match, the product detector 110 generates the product identification message
114 and then adds the product identifier 112 to the list. Consequently, this
reduces the number of product identification messages 114 that are generated
and liberates the adding module 122 from having to make the verification of
whether there is duplication in the received product identifier messages 114.
[0029] Presented in Fig. 4, both the product detector 110 and the patient
detector 116 are affixed to the receptacle 300. In general, the receptacle 300
may be provided with wheels and be located for allowing easy access thereto,
such as in proximity to where medical care is given. According to one aspect,
the
patient detector 116 is adapted to detect the patient identifier 118 when the
patient or the patient identifier 118 becomes in proximity with the receptacle
300,
such as for example when the patient enters a room where the receptacle 300 is
located. The room may be any type of area that is enclosed, semi-enclosed or
open. In one aspect, the room is a hospital room where medical care is
normally
given to the patient. In another aspect, the location is a mobile hospital bed
where the patient is normally laid down for receiving medical care, etc. As
the
patient or the patient identifier 118 becomes in proximity with the patient
detector
116, the patient identifier 118 is detected. Thereafter, when medical care is
given
to the patient and products are used on the patient, for each product used the
CA 02663781 2009-04-22
- 10-
product identifier 112 is detected, once the product package is thrown into
the
receptacle 300. The corresponding product information 104 is then added to the
patient record 108 corresponding to the last detected patient identifier 118.
[0030] According to another aspect, the patient detector 116 is adapted to
detect the patient identifier 118 when the patient identifier 118 has been in
proximity therewith for a certain time. In this aspect, the patient detector
116 may
be directed toward a specific location where the patient is usually placed for
receiving care. Furthermore, the patient detector 116 may be adapted to
generate the patient identifier message 120 only once, thus reducing the
number
of patient identification messages 120 generated and liberating the adding
module 122 from having to make a verification of whether there is duplication
in
the received patient identifier messages 120. The patient detector 116 may
also
be a card that is brought in proximity or that is inserted into the receptacle
300. A
keypad may also be used to enter the patient identifier 118 into the
receptacle
300.
[0031] Presented in Fig.5, according to an aspect of the system 100, the
product detector 110 is affixed to the receptacle 300 along with the adding
module 122. This aspect may enable the addition of the product information 104
to the patient record 108 from the receptacle 300. Consequently, risk of
loosing
the product identifier message 114 is lowered. Also, the product identifier
message 114 is timely received by the adding module 122 since there is no need
for the message 114 to travel through a communication network for reaching the
adding module 122. Furthermore, it makes it possible for a user to
instantaneously track the products that have been used on the patient.
[0032] According to another aspect, a logging module 500 is connected to
the adding module 122. Once the product information 104 is added to the
patient record 108, the adding module 122 generates a description of the
addition process. To do so, the adding module 122 queries the database that
holds the product attribute fields 208 and the patient attribute fields 210,
and from
CA 02663781 2009-04-22
- 11-
this, the adding module 122 generates the description of the addition process.
The logging module 500 then uses the description of the addition process and
produces a log. The description of the addition process may comprise one or
several of the following attributes: a patient hospital card number, a patient
name, a product serial number, a product name, a product expiry date and a
time
and date at which the addition process took place.
[0033] According to yet another aspect, an alert module 502 may be
connected to the adding module 122. Before adding the product information 104
to the patient record 108, the adding module 122 verifies if there are any
existent
errors that may render the addition process impossible or undesirable. When
there exists an error or a counter indication, the adding module 122 generates
an
error message and the alert module 502 then produces an alert for informing
the
medical personal of the error or counter indication. Various causes of error
are
possible such as a product expiry date.
[0034] Presented in Fig. 6, according to an aspect of the system 100, the
product detector 110, the patient detector 116 and the adding module 122 are
all
affixed to the receptacle 300, and are in direct connection there between. In
this
particular aspect, the addition process of the product information 104 to the
patient record 108 takes place at the receptacle 300. As both the product
detector 110 and the patient detector 116 are affixed to the receptacle 300,
the
risk of loosing either of the product identifier message 114 or the patient
identifier
message 120 before the adding module 122 receives the messages 114,120 is
lower.
[0035] A skilled reader will recognize that it is possible for the databases
(102, 106, 124, 200), the product detector 110, the patient detector 116 or
the
adding module 122 to either be independently connected, connected to one
another or to be connected to any other part of the system 100 without
affecting
the workings of this system 100. The connection there between may be done
through a wired or a wireless network, may be direct or indirect. The product
CA 02663781 2009-04-22
- 12-
detector 110 and the patient detector 116 thus generate respectively the
product
identifier message 114 and the patient identifier message 120 and send the
product identifier message 114 and the patient identifier message 120 to the
adding module 122 which is either co-located, or separate there from. Any type
of communication means and standards may be used to perform the sending of
the patient identifier message 120 and product identifier message 114 to the
adding module 122.
[0036] Presented in Fig. 7 is a method 700 for adding product information
to a patient record. The method 700 comprises inserting 702 into the product
database 102 the product information 104, and inserting 704 into the patient
database 106 the patient record 108. Moreover, the method 700 comprises
detecting 706 the product identifier 112 corresponding to the product
information
104, and detecting 708 the patient identifier 118 corresponding to the patient
record 108. Also, the method 700 comprises adding 710 the product information
104 to the patient record 108. According to one aspect, the method 700 further
comprises alerting 712 a user when an incompatibility is detected with the
product information 104. According to another aspect, the method 700 further
comprises managing 714 a product expiry date for identifying an expired
product
and generating 716 a report for notifying the user or the supplier that the
identified expired product must be replaced.
[0037] While the present system 100 and method 700 have been shown and
described with reference to different aspects thereof, it will be recognized
by
those skilled in the art that various changes in form and detail may be made
herein without departing from the spirit and scope of the invention as defined
by
the appended claims.