Note: Descriptions are shown in the official language in which they were submitted.
CA 02665792 2009-04-07
WO 2008/043785 PCT/EP2007/060766
TWO-STAGE PROCESS FOR THE CONVERSIOIsI OF TAR SAND TO
LIQUID FUELS AND SPECIALTY CHEMICALS
BACKGROUND OF THE INVENTION
1. Field of the Invention
[oaoif The present invention relates to the conversion of hydrogen-poor fluid
hydrocarbons to liquid fuels and specialty chemicals.
lt has long been recognized that hydrogen-poor fluid hydrocarbons such as
tar sands, oil shales, and heavy crudes, in particular tar sands, are
abundantly available and form a potential source of liquid fuels and valuable
chemicals.
[0002J lt is known to distill the lighter fractions off these hydrogen-poor
fluid
hydrocarbons to obtain a material similar to crude ail. This distillate is
then
further processed as one would a regular crude. This is an inefficient
approach, as it leaves a significant portion of the starting material in an
unconverted state.
[0003] It is far more desirable to find a way for converting virtually all of
the
hydrogen-poor fluid hydrocarbon to liquid fuel and valuable chemicals.
[0004] There is a need for a low-cost process that is able to convert a large
proportion of the hydrocarbons present in hydrogen-poor fluid hydrocarbons
under conditions that are mild enough to avoid high equipment and energy
costs and/or substantial degradation of the conversion products.
[00051 Hydrogen-poor fluid hydrocarbons are characterized by a high viscosity,
which makes them difficult to process. It is desirable to develop processes
that are able to process these hydrocarbons without requiring expensive
equipment or demanding processing conditions in terms of temperature and
pressure.
CA 02665792 2009-04-07
WO 2008/043785 PCT/EP2007/060766
-2-
SUMMARY OF THE INVENTION
[0006] As used herein, the term "Tar Sand" means hydrogen-poor fluid
hydrocarbon
materials, and encompasses tar sand per se, oil sand, oil shale, heavy crude
oil, bottoms from refinery processes, and the like. In general, these
materials
contain less than 15% hydrogen, often only about 10% hydrogen.
[00071 The present invention relates to a process for converting Tar Sand to a
liquid
fuel comprising the steps of:
a) activating the Tar Sand to make it more susceptible to conversion;
b) optionally, adding a solvent;
c) partially converting the activated Tar Sand to form solubilized
material;
d) subjecting unconverted Tar Sand from step c) to a conversion process.
[ooos] Due to the activation taking place in step a), optionaily aided by the
addition
of a solvent (step b), step c) can be carried out under mild conditions. As a
result the product obtained in step c) is not substantially degraded.
Unconverted Tar Sand from step c) is subsequently subjected to a second
conversion in step d). Optionally, and preferably, converted Tar Sand
obtained in step c) is removed from the unconverted Tar Sand before the
latter is subjected to a second.. conversion in step d). If conversion
products
from step c) are first removed, step d) may be carried out under more severe
conditions than step c). In the alternative, step d) may be preceded by a
second activation step so that the unconverted Tar Sand is more susceptible
to the conversion process of step d).
BRIEF DESCRIPTION OF THE DRAWINGS
Cooo9l Figure 1 is a schematic diagram of an embodiment of the process of the
present invention.
[ooio] Figure 2 is a schematic diagram of an alternate embodiment of the
process of
the present invention.
looizj DESCRIPTION OF ILLUSTRATIVE EMBODIMENTS
CA 02665792 2009-04-07
WO 2008/043785 PCT/EP2007/060766
-3-
[0012] The following is a description of certain embodiments of the invention,
given
by way of example only.
[0013] The present invention relates to a process for converting Tar Sand to a
liquid
fuel comprising the steps of:
a) activating the Tar Sand to make it more susceptible to conversion;
b) optionally, adding a solvent;
c) partially converting the activated Tar Sand to form solubilized material;
d) subjecting unconverted Tar Sand from step c) to a conversion process.
[00141 The process provides the general advantage of requiring less severe
process
conditions than, for example, traditional HTU or pyrolysis processes.
Accordingly, the process is more cost-effective and requires simpler less
expensive equipment. The process is also environmentally more acceptable,
and produces product of higher quality, and more suitable for conversion to
fuels and chemicals.
[oojs] Particular embodiments of the process provide homogenous, intimate
mixtures of Tar Sand material with a solvent and/or a solid and/or a liquid
additive, which provides advantages for subsequent conversion.
[0016] One important aspect of the process of the present invention is that it
removes easily converted components of the Tar Sand material from the
reaction mixture after a first conversion, allowing other, more difficultly
converted components to be subjected to somewhat more severe conversion
conditions without exposing the products already obtained to undesired
degradation.
[0017) Overall, the process of the present invention results in better yields
of
products of a better quality than has heretofore been possible, and does so at
a lower consumption of energy and lower capital equipment costs,
foozs1 Although the process is described herein in the context of the
production of
liquid fuels, it will be understood that the process may be used for
converting
CA 02665792 2009-04-07
WO 2008/043785 PCT/EP2007/060766
-4-
Tar Sand to feedstock chemicals, specialty chemicais, nano-composites,
construction materials, cardboard and paper products, and the like.
[ooi9t Step a) of the process generally involves providing an intimate mixture
of the
Tar Sand with a solvent, a particulate solid and/or liquid material, or both a
solvent and a particulate solid material. If the material is an oil shale it
is
desirable to reduce the particle size of the Tar Sand by processes such as
milling, grinding, and the like.
C00201 Processes for providing intimate mixtures of Tar Sand material are
described
in Patent Applications EP 061135638 and EP 061135810, the disclosures of
which are incorporated herein by reference. An alternate process involves the
use of an extruder and/or a kneader. Kneading is very suitable to provide
homogeneous and intimate mixing and allows for reactions to take place,
while extrusion provides high shear mechanical treatment of the materials. In
particular the use of a screw extruder is preferred for use herein, because it
allows for operation at high pressures without requiring expensive equipment.
[oo2zj It will be understood that the mixing step may be combined with the
process
of reducing the particle size of the Tar Sand material. For example, ball
milling or grinding of the Tar Sand in the presence of a particulate solid
material will result in an intimate mixture of the Tar Sand and the
particulate
solid material.
100221 Focusing now on the use of an extruder and/or kneader for the purpose
of
activating the Tar Sand, it is possible to operate the process at increased
temperature. Many screw extruders are provided with a heating mantle
through which steam or heated oil may be circulated. It is also possible to
inject steam into nozzles provided in predetermined locations of the barrel.
Steam injection provides a combined effect of heating the Tar Sand and
adding a solvent (water),
[0023] The pressure inside the extruder is determined by the viscosity of the
mass
within the extruder, the design of the screw within the extruder (for example,
a
tapered pitch screw provides a higher pressure than a constant pitch screw),
CA 02665792 2009-04-07
WO 2008/043785 PCT/EP2007/060766
..5_
and the design of the perforated plate at the outlet of the extruder. The back
pressure provided by this plate is a function of the amount of open area in
relation to the amount of closed area, with lower open area/closed area ratios
providing greater back pressure.
f00241 If a single pass through an extruder does not provide sufficient
mixing, two or
more extruders may be provided in series, or the material may be subjected
to two or more passes through one extruder. Similarly, the capacity of a plant
may be readily increased by operating two or more extruders in parallel.
[00251 Suitable solvents for use in step b) include water, alcohols (in
particular
ethanol and glycerol), bio-oil or other products from the subsequent
conversion of the Tar Sand, liquid acids, aqueous solutions of acids and
bases, liquid C02, and the like. Water is the preferred solvent in most
applications, because of its availability, low cost, and ease of handling.
Liquids that are produced during the subsequent conversion of the Tar Sand
are also readily available and may be preferred for that reason.
[00261 Suitable solid materials for use in step a) include solid acids and
bases, salts,
minerals, clays, layered materials, and the like. Solid materials having
catalytic properties are preferred. Examples include metal oxides, metal
hydroxides, alkaline and alkaline earth oxides, hydroxides, carbonates,
hydroxyl carbonates, hydrotalcite-like materials, etc. As has been noted
earlier, it may be desirable to add several solid materials to the Tar Sand,
or a
combination of one or more solid materials and one or more solvents.
[0027] It is possible to add precursors of inorganic solids, and causing them
to
solidify or even crystallize during the mixing process. For example, certain
inorganic solids may precipitate from solution in response to an increase in
temperature or a change in pH. An increase in temperature may be effected
in the kneader by heating the barrel, or by injecting steam. A pH change may
be effected by injecting a solution of an acid or a base. Similarly, amorphous
materials may be caused to crystallize by increasing the temperature of the
mixture.
CA 02665792 2009-04-07
WO 2008/043785 PCT/EP2007/060766
6
[00281 As mentioned above, the addition of a solvent is optional. For example,
in
many cases water is the solvent of choice. Many forms of Tar Sand contain
sufficient quantities of water for the present purpose, obviating the need for
adding additional solvent. It may even be desirable to remove water during
the activation step. This may for example be accomplished by heating the Tar
Sand to a temperature above 100 C, and letting off steam via pressure
valves located along the barrel of the extrude, if an extruder is used in the
process.
[00291 In many cases conversion step c) commences while the activated Tar Sand
is
still being processed in the kneader or extruder or in both. If this process
is
not completed in the kneader, the activated Tar Sand may be processed
further in a second kneader, or it may be subjected to a second pass through
the first kneader. Alternatively, the Tar Sand may be transferred to a
different
processor to complete step c). A suitable example of such a processor is a
filter press, which can be operated at desirable conditions of temperature and
pressure.
[OO3o] It is highly preferred that liquid products resulting from conversion
step c) be
separated from unconverted Tar Sand. The purpose of this separation is two-
fold. Firstly, it reduces the mass of material that needs to be subjected to
further conversion in step d), which makes the operation of step d) more
efficient. Secondly, it avoids subjecting liquid conversion products from step
c) to the subsequent conversion process, avoiding a degradation of this liquid
product by such further processing.
[00311 In a specific embodiment, part of the first conversion takes place in a
filter
press under conditions of increased temperature and pressure. This may be
accomplished by loading the activated Tar Sand into a filter press, and
injecting steam to increase both the temperature and the pressure. After this
first conversion step is completed the filter press is de-pressurized over a
filter
medium, such as a filter cloth or screen, and the reaction product is
separated into a liquid filtrate stream and a filter cake. The liquid stream
comprises solvent and liquid conversion product, as well as fine particles of
CA 02665792 2009-04-07
WO 2008/043785 PCT/EP2007/060766
-7-
unconverted Tar Sand. The filter cake comprises unconverted Tar Sand,
retained solvent, and liquid reaction product.
C00321 As used herein, the term "unconverted Tar Sand" refers to Tar Sand that
has
not been converted to a liquid product in step c). The term includes Tar Sand
material that has not undergone any chemical conversion. The term also
includes Tar Sand that has undergone some conversion, but insufficient to
form a liquid. For example, hydrocarbons may have been converted to
hydrocarbons of a lower average molecular weight, but still be semi-solid.
This would be considered "unconverted Tar Sand" within the meaning of this
term as used herein. Such a material may well be an "activated unconverted
Tar Sand" if its molecular weight is reduced andbr its macro and/or micro
structure has changed, in a way that makes it more susceptible for conversion
to a liquid product in step d).
[0033] in the alternative the liquid may be separated from remaining solids by
nano-
filtration or membrane separation. Instead of a filtration technique an
extractive separation may be used.
[0034] The unconverted Tar Sand is subjected to a second conversion process in
step d). If the liquid conversion product of step c) is removed from the
unconverted Tar Sand prior to step d), this second conversion may be carried
out under more severe conditions than the first conversion, without risking
degradation of reaction products already formed. For example, the
unconverted Tar Sand may be subjected to a conventional HTU or pyrolysis
process.
[0035] In a preferred embodiment of the process of the present invention, the
unconverted Tar Sand is activated prior to step d) so that step d) may be
carried out under less severe conditions than the prior art HTU and pyrolysis
processes. In many cases the unconverted Tar Sand from step c) is already
activated, for example because inorganic particulate materials added in step
a) are carried over with the unconverted Tar Sand into step d). The
unconverted Tar Sand may also be activated as a result of a partial
CA 02665792 2009-04-07
WO 2008/043785 PCT/EP2007/060766
-8-
conversion in step c), insufficient to render the Tar Sand liquid, but
sufficient
to make it more susceptible to further conversion.
[0036] Any conversion process is suitable for use in step d). HTU and
pyrolysis have
already been mentioned; desirably, these processes are conducted under
conditions as mild as the activation of the Tar Sand will permit. Gasification
may be a desirable option, for example to create gaseous fuel for meeting the
heat requireme.nts of the overall process.
[0037) In most cases, both steps c) and d) produce a mixture of liquid
hydrocarbons,
which may be converted to suitable liquid transportation fuels in modified
refinery processes such as fluid catalytic cracking, hydroconversion, thermal
conversion, and the like. In these processes the Tar Sand derived liquid
hydrocarbons may be the sole feedstock, or they may be blended with
conventional, crude oil-based feedstocks.
[oo38] In another embodiment the activation step a) is conducted in a
kneader/extruder assembly in the presence of an inorganic solid, for example
an alkaline or alkaline earth metal oxide or hydroxide, and the product of
step
c) is hydrothermally treated in step d). The inorganic material which is
homogenously mixed in step a) / step b) is thus most effectively dispersed
and present in steps c) and/or d) in intimate contact with the unconverted Tar
Sand, resulting in an efficient conversion. Said solids may possess catalytic
properties that further enhance the conversion process.
j00391 In another embodiment the inorganic additive introduced in step a) may
be
simply a heat transferring medium, like for example sand, clay or a mineral,
ore or soil, which may have also catalytic properties. In this case the
product
of step c) can be subjected to a pyrolysis conversion process. The advantage
of this process is that here the heat transfer medium is in close and intimate
contact with the Tar Sand in a dispersed form.
[0040] In another embodiment the activation in step a) may involve the
addition of an
acid or a base, which, aided by the application of heat and/or steam, will
break down the compact structure of the Tar Sand composite, rendering it
CA 02665792 2009-04-07
WO 2008/043785 PCT/EP2007/060766
-9-
more susceptible to a subsequent conversion, for example by acid hydrolysis
and/or enzymatic conversion.
[0041] In another embodiment the Tar Sand in step a) containing water and
optionally an additive is heated above 100 C, while being mechanically
treated, so that the water is allowed to evaporate.
[0042] In another embodiment, the Tar Sand is mechanically processed in the
presence of other carbonaceous materials such as coal, lignite, and biomass
in step a) and step b) optionally with the addition of additives, followed by
gasification of the unconverted materiais.
[00431 In another embodiment the Tar Sand is intimately mixed with an additive
in a
ball mill, grinding the components together to form the activated Tar Sand.
Optionally a liquid solvent can be added.
[0044] In another embodiment the Tar Sand is grinded with an additive in a
fluidized
and/or spouted bed, as disclosed in US6O/831,220, the disclosures of which
are incorporated herein by reference. Optionally a liquid solvent can be
added.
[0045] In another embodiment the unconverted Tar Sand of step c) is converted
to
paraffins suitable for diesel fuels.
[0046] In another embodiment the unconverted Tar Sand of step c), which often
appears to be the fibrous crystalline cellulose coated with an additive, is
converted to materials suitable for paper, board or construction materials
[00471 In another embodiment the unconverted material (mainly crystalline
cellulose) is converted to a transportation fuel by aqueous phase reforming as
suggested by Huber et al., see: G.W. Huber, J.N. Chheda, C.J. Barrett, J.A.
Dumesic, Science 308 (2005) 1446.
[0048] In another embodiment the solubilized material is converted to a
transportation fue[ by aqueous phase reforming as suggested by Huber et al.,
see: G.W. Huber, J.N. Chheda, C.J. Barrett, J.A. Dumesic, Science 308
(2005)1446.
CA 02665792 2009-04-07
WO 2008/043785 PCT/EP2007/060766
-10-
(o04s) In another embodiment the unconverted material of step b and/or c) is
first
submitted to eiectromagnetic and/or ultrasound energy, optionally in the
presence of a polar solvent such as ethanol. Following this treatment the so
activated material is converted by any of the above means.
[oo5oa In another embodiment the unconverted material of step b and/or c),
which
comprises a material susceptible to the absorption of electro-magnetic
radiation is first submitted to electromagnetic optionally in the presence of
a
polar solvent such as ethanol. Following this treatment the so activated
material is converted by any of the above means.
[om i I In another embodiment the unconverted material of step b and/or c) is
first
submitted to intimate mixing with an additive, optionally in the presence of a
solvent such as ethanol. Following this treatment the so activated material is
converted by any of the above means.
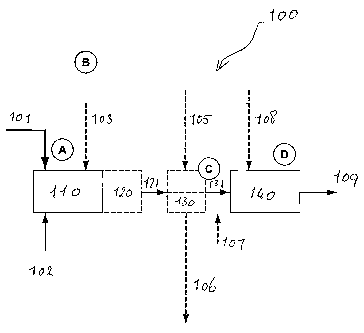
[00521 Figure 1 shows one particular embodiment 100 of the process of the
present
invention. Tar Sand 101 and catalyst 102 are mixed in mechanical mixer 110,
with the optional addition of solvent 103. After mixing the mixture is
transferred to a first reactor 120, for a first, partial, conversion step.
This
conversion step is carried out under mild conditions.
[ao53j The partially converted mixture 121 is transferred to a first product
recovery
means 130. Optionally solvent 105 is added at this stage. Reaction product
106 is separated from the mixture, and removed for further processing. The
removal of reaction product 106 ensures that reaction product 106 is not
subjected to the subsequent, more severe conversion in reactor 140.
[0054] The unconverted portion 131 of the mixture is transferred to a second
conversion reactor 140, where it is subjected to a more severe conversion
reaction. Optionally, additional catalyst 107 is added at this stage.
Optionally
also, solvent 108 may be added.
[00551 Finally, reaction product 109 is recovered from reactor 140.
[0056) Figure 2 shows an alternate embodiment 200 of the process of the
present
invention.
CA 02665792 2009-04-07
WO 2008/043785 PCT/EP2007/060766
-tl-
[oo57] Tar Sand 201 and catalyst 202 are mixed in mechanical mixer 210, with
the
optional addition of solvent 203. After mixing the mixture is transferred to a
first reactor 220, for a first, partial, conversion step. This conversion step
is
carried out under mild conditions.
[00581 The partially converted mixture 221 is transferred to a first product
recovery
means 230. Optionally solvent 205 is added at this stage. Reaction product
206 is separated from the mixture, and removed for further processing. The
removal of reaction product 206 ensures that reaction product 206 is not
subjected to the subsequent, more severe conversion in reactor 240.
[0059] The unconverted portion 231 of the mixture is transferred to a second
conversion reactor 240, where it is subjected to a more severe conversion
reaction. Optionally, additional catalyst 207 is added at this stage.
Optionally
also, solvent 208 may be added.
[006o] Reaction product 209 is recovered from reactor 240, and combined with
reaction product 206 for further processing in refinery process 250. Process
250 may comprise any number of conventional refinery processes, such as
fluid catalytic cracking (FCC), hyrotreatment processing (HTP), thermal
cracking (TC), and the like.
[0061j Thus, the invention has been described by reference to certain
embodiments
discussed above. It will be recognized that these embodiments are
susceptible to various modifications and alternative forms well known to those
of skill in the art.