Note: Descriptions are shown in the official language in which they were submitted.
CA 02666390 2009-04-14
WO 2008/055152 PCT/US2007/082992
REACTOR AND PROCESS FOR UPGRADING HEAVY HYDROCARBqN
OILS
FIELD OF THE INVENTION
The present invention relates to upgrading of hydrocarbons, especially heavy
hydrocarbons such as whole heavy oil, bitumen, and the like using
supercritical water.
BACKGROUND OF THE INVENTION
Oil produced from a significant number of oil resenres around the world is
simply too heavy to flow under ambient conditions. This makes it challenging
to bring remote, heavy oil resources closer to the markets. One typical
example is the Hamaca field in Venezuela. In order to render such heavy oils
flowable, one of the most common methods known in the art is to reducethe
viscosity and density by mixing the.heavyoil with a.sufficient diluent. The
diluent may be naphtha, or any other stream with a.significantly higher API
gravity (i.e., much lower density) than the heavy oil.
For a case such as Hamaca, diluted crude oil is sent from the production
wellhead via pipeline to an upgrading facility. Two key operations occur at
the
upgrading facility: (1) the diluent stream is recovered and recycled back to
the
production wellhead in a separate pipeline,-and (2) the heavy oil is upgraded
with suitable technology known:in the art (coking, hydrocracking,
hydrotreating, etc.) to produce higher-value products for market. Some typical
characteristics of these higher-value products include: lower sulfur content,
lower metals content, lower total acid number (TAN), lower residuum content,
higher API gravity, and lower-:viscosity. Most of these desirable
characteristics are achieved by reacting the heavy oil with hydrogen gas at
high temperatures.and pressures in the presence of a catalyst. In the case of
Hamaca, the upgraded crude is sent further to the end-users via tankers.
l
CA 02666390 2009-04-14
WO 2008/055152 PCT/US2007/082992
These diluent.addition / removal processes and hydrogen-addition or other
upgrading processes have a number of disadvantages:
1. The infrastructure required for the handling, recovery, and recycle of
diluent could be expensive, especially over long distances: Diluent
availability
is another potential issue.
2. Hydrogen-addition processes-such as hydrotreating or hydrocracking
require significant investments in capital and.infrastructure..
3. Hydrogen-addition processes also have high operating costs, since
hydrogen production.costs::are highly sensitiveto natural gas prices. Some
remote heavy oil reserves may not even have access to sufficient quantities of
low-cost natural gas to support a hydrogen plant. These hydrogen-addition
processes also generally require expensive catalysts and resource intensive
catalyst handling techniques, including catalyst regeneration.
4. In some cases, the refineries and/or upgrading facilities that are
located closest to the production site may have neither the capacity nor the
facilities to accept the heavy oil.
5. Coking is often used at refineries or upgrading.facilities: Significant
amounts of by-product solid coke are rejected during the coking process,
leading to lower liquid hydrocarbon yield. In addition, the liquid products
from
a coking plant often need further hydrotreating. Further, the volume of the
product from the coking process is significantly less than the volume of the
feed crude oil.
A process according to the present invention overcomes these: disadvantages
by using supercritical water to upgrade a heavy hydrocarbon feedstock, into an
upgraded hydrocarbon product or syncrude with highly desirable properties
(low sulfur content, low metals content, lower density (higher API), lower
viscosity, lower residuum content, etc.). The process neither requires
external
supply of hydrogen nor must it use catalysts. Further, the process in the
present invention does not produce an appreciable coke by-product.
In comparison with the traditional processes for syncrude production,
advantages that may be obtained bythe.practice of the present invention
2
CA 02666390 2009-04-14
WO 2008/055152 PCT/US2007/082992
include a high liquid hydrocarbon yield; no need for externally-supplied
hydrogen; no need to provide catalyst; significant increases:in API gravity in
the upgraded hydrocarbon product; significant viscosity reduction in the
upgraded hydrocarbon product; and significant reduction in sulfur, metals,
nitrogen; TAN, and MCR (micro-carbon residue) in the upgraded hydrocarbon
product.
Various methods of treating heavy hydrocarbons using supercritical water are
disclosed in the patent literature. Examples include U.S. Patent Nos.
3,948,754,
3,948,755, 3,960,706, 3,983,027, 3,988,238; 3,989,618, 4,005;005, 4,151,068,
4,557,820, 4,559,127, 4,594,141; 4,840,725, 5t611;915, 5,914,031 and
6,887,369 and EP671454.
U.S. Patent No 4,840,725 discloses a process for conversiorr of high boiling
liquid organic materials to lower boiling materials using supercritical water
in a
tubular continuous reactor. The water and hydrocarbon are separately
preheated and mixed in a high-pressure feed pump just before being fed to
the reactor.
U.S. Patent No..5,914,031 discloses a three zone reactor design so thafthe
reactant activity, reactant solubility and phase separation of products can be
optimized separately by controlling temperature and pressure. However, all
the examples given in the patent were obtained using batch operation.
U.S. Patent No. 6,887,369 discloses. a supercritical water pretreatment
process using hydrogen or carbon monoxide preferably carried out in a deep
well reactor to hydrotreat and hydrocrack carbonaceous material. The deep
well reactor is adapted from underground oil wells, and consists of multiple,
concentric tubes. The deep well reactor described in the patent is operated
by introducing feed streams in the core tubes and:returning reactor effluent
in
the outer annular section.
3
CA 02666390 2009-04-14
WO 2008/055152 PCT/US2007/082992
Although the above-mentioned patents disclosed and claimed various
methods and processes for heavy oil upgrading using supercritical water,
such as operating range of temperature and pressure; water to oil ratio, etc,
none. has disclosed the design of the reactor ordesign related process
controls for heavy oil upgrading using supercritical water. In fact, most of
the
examples disclosed in the patents were obtained through batch tests using an
autoclave. Although there are numerous references to reactor design for
processes involving supercritical water, most of them are for the application
of
waste treatment and none of those references has addressed the design of a
reactor for both heavy oil and supercritical water, which is fundamentally
different from processes of waste treatment using supercritical water, as
discussed below.
It has long been known :in the art that supercritical water can be used for
waste treatment, especially for treating wastewater containing organic
contaminants. Therefore, there are numerous.disclosures in the literature on
reactor design for waste treatment using supercritical water, tended to
address the following issues:
(1) Solid handling. Waste streams typically contain both organic
and inorganic materials. Although organic: materials can be destroyed
quickly through supercritical water oxidation, inorganic materials are
insoluble in supercritical water. Several patents address this concern.
For example, U.S. Patent Nos. 5;560,823 and 5,567,698 incorporated
by reference herein disclose a reversible flow reactor having two
reaction zones which are alternately used for supercritical water
oxidation while the remaining reaction zone is flushed with subcritical
effluent from the active reaction zone. U.S. Patent No. 6,264,844,
incorporated by reference herein, discloses a#ubular reactor for
supercritical water oxidation. The velocity of the reaction mixture is
sufficient to prevent settling of solid. Inorganic salts in the effluent
mixture, which are insoluble. at conditions of:supercritical temperature
and pressure for water, are dissolved in a liquid water phase during
cooling down of the effluent mixture at an outlet end of the reactor.
.4
CA 02666390 2009-04-14
WO 2008/055152 PCT/US2007/082992
5' (2) Oxidizer management. U.S. Patent Nos. 5,384,051 and
5,558,783, incorporated by reference herein, disclose a reactor design
for supercritical wastewater oxidation: It contains a reaction zone inside
the. containment vessel and a permeable liner around the reaction
zone. An oxidizer is mixed with a carrier fluid such as water. The
mixture is heated and pressurized to supercritical conditions, and then
introduced to.the reaction zone gradually and uniformly by forcing it
radially inward through the permeable liner and toward the reaction
zone. The permeable liner-permits the continuous, gradual, uniform
dispersion of a reactant and therefore promotes an even and efficient
reaction. The liner also isolates the pressure vessel from high
temperature and oxidizing conditions found in the reaction zone,
allowing a reduction in cost of the pressure vessel. EP 1489046
discloses a double=vessel design with a reaction vessel placed inside a
pressure vessel. Reaction takes place inside. the reactor vessel at:high
temperature, pressure and corrosive environments. The outer pressure
vessel will only.see water.
(3) Containment of toxic material. Some waste stream contains
contaminants that are extremely harmful to. humans and the
environment, therefore the possibility of releasing of such harmful
material has to be addressed in the reactor design. U.S. Patent No.
6,168,771, incorporated by reference herein, discloses a reactor design
including an autoclave inside a pressure vessel. The pressure between
autoclave and pressure.vessel is essentially equal to that inside the
autoclave, therefore. eliminating possible.leaking oftoxic material inside
the autoclave.
Although heavy oil upgrading using supercritical water may be considered
similar.in some respects to waste treatment using supercritical water, and can
be implemented using various elements.of reactors designed for waste
treatment, there are significant differences in requirement for reactor design
for heavy hydrocarbon upgrading from that.for waste treatment. Specifically,
the following are among the many issues to be addressed in designing a
5
CA 02666390 2009-04-14
WO 2008/055152 PCT/US2007/082992
reactor in which to conduct an effective. process for heavy oil upgrading
using
supercritical water:
(1) Importance of selectivity. For waste treatment, the only
performance target is conversion. In other words,the reaction is non-
selective total oxidation and there is no need.to worry about selectivity,
which makes the reactor design much easier. For heavy oil upgrading,
the feed is a mixture containing broad range of materials, and the
reactions involved are much more complex. We need not only to
consider conversion, but also more importantly to pursue high
selectivity, since non=selectiv.e reactions will lead to low-value
byproducts such as solid coke or gases. Obviously, reactor design for
selective reactions in a complex system is: very different and much
more challenging than that for non-selective total oxidation.
(2) High concentration of feed. Typically the organic component
concentration in the waste stream is low, and in many.situations the
concentration is only in the ppm range. For:oil upgrading, it is
preferable to run the reaction using the lowest possible water to oil ratio
to reduce capital and operating cost. The oil concentration is typically
several orders of magnitude higher in upgrading as opposed to waste
treatment.
(3) High density and viscosity. One distinguishing feature of heavy
oil is high density and viscosity. In :fact, this is one of the primary
reasons that the oil has to be upgraded. The density of heavy oil is very
close to liquid water, and viscosity can.be as high as 10,000 cp. High
density and viscosity, together with high concentration make the
dispersion of heavy oil into supercritical water an important
consideration.
6
CA 02666390 2009-04-14
WO 2008/055152 PCT/US2007/082992
SUMMARY OF THE INVENTION
The present invention relates to an apparatus for upgrading a hydrocarbon by
reaction with a fluidcomprising water under supercritical water conditions
comprising: means for dispersing and mixing the fluid comprising water and
the hydrocarbon under conditions which disfavor thermal cracking and
formation of coke; meansfor injecting a dispersed water-hydrocarbon mixture
into a reaction zone under supercritical waterco.nditions; a reaction zone
having means for.maintaining a. uniform temperature within said reaction
zone; means for controlling the residence time in the reaction zone within
determined limits and means for avoiding the settling of inorganic solids
within
the reaction zone; and means for recovering an upgraded hydrocarbon.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a process flow diagram of one. embodiment of a process
employing an apparatus of the present invention.
Figure 2 is a process flow diagram of another embodiment of a process
employing an apparatus of the present invention.
Figure 3 is a process flow diagram of another embodiment of a process.
employing an apparatus of the present invention..
Figure 4 is a process flow diagram of another embodiment of a process
employing an apparatus of the;presentinvention:
Figure 5 is a process flow diagram of another embodimenfiof a process
employing an apparatus of the present invention.
7
CA 02666390 2009-04-14
WO 2008/055152 PCT/US2007/082992
DESCRIPTION OF THE PREFERRED EMBODIMENTS
Reactants
Water and hydrocarbons, preferably heavy hydrocarbons are the two
reactants employed in a process according to the present invention.
Any hydrocarbon can be suitably upgraded by a process according to.the
present invention. Preferred are heavy hydrocarbons having an API gravity of
less than 20 . Among the preferred heavy hydrocarbons are heavy crude oil,
heavyhydrocarbons extracted from tar sands, commonly called tar sand
bitumen, such as Athabasca tar sand bitumen obtained:from Canada, heavy
petroleum crude oils such as Venezuelan Orinoco heavy oil belt crudes
Boscan heavy oil, heavy hydrocarbon fractions obtained from crude petroleum
oils patticularly heavy vacuum gas oils, vacuum residuum as well as
petroleum tar, tar sands and coal tar. Other examples of heavy hydrocarbon
feedstocks which can be used are oil shale,:shale oil, and. asphaltenes.
Water
Any source of water may be used in the fluid comprising water in practicing
the present invention. Sources of water include but are not limited to
drinking
water, treated or untreated wastewater, riverwater, lake water, seawater,
produced water or the like.
Mixing
In accordance with the invention, the heavy hydrocarbon feed and a fluid
comprising water that has been heated to a temperature higher than its
critical
temperature are contacted in a mixing zoneprior to entering the reaction
zone. In accordance with the invention, mixing may be accomplished in many
ways and is preferably accomplished by a technique that.does not employ
mechanical moving parts. Such means of mixing may include, but are not
limited to, use of static rnixers, spray nozzles, sonic or ultrasonic
agitation, or
thermal siphons.
8
CA 02666390 2009-04-14
WO 2008/055152 PCT/US2007/082992
The oil and water should be heated and mixed so that the combined stream
will reach supercritical conditions in the reaction zone.
Itwas found that by avoiding excessive heating of the feed oil, the formation
of byproduct such as solid residues is reduced significantly. One aspect of
this
invention is to employ a heating sequence so that the temperature and
pressure of the hydrocarbons and water will.reach supercritical reaction
conditions in a controlled manner. This will avoid excessive local heating of
oil, which will lead to solid formation and lower quality product. In order to
achieve better performance; the oil should only be heated up with sufficient
amount of water present and around the hydrocarbon molecules. This
requirement can be met by mixing. oil with water before heating.
In one embodiment of the present invention, water is heated to a temperature
higher than its critical temperature, and then mixed with oil. The temperature
of heavy oil feed should be kept in the range of about 100 C to 200 C to avoid
thermal cracking but still high enough to..maintain a reasonable pressure
drop.
The water stream temperature should be highlenough to make sure that after
mixing with oil, the temperature of the oil=water mixture is still higher than
the
water supercritical temperature. In this embodiment, the oil is actually
heated
up by water. An abundance of water molecules surrounding the hydrocarbon
molecules will significantly suppress condensation reactions and therefore
reduce formation of coke and solid product.
The required temperature of the supercritical water stream, Tscw, can be
estimated based on reaction temperature, TR, and water to. oil ratio. Since
the
heat capacity of water changes significantly in the range near its critical
conditions, for a given reaction temperature, the required temperature for the
supercritical water stream increases almost exponentially with decreasing
water-to-oil ratio. The lower the water-to-oil ratio, the higher the TSCVV.
The
relationship, however, is very nonlinear since.higher Tscw leads to a lower
heat capacity (far away from the critical point).
9
CA 02666390 2009-04-14
WO 2008/055152 PCT/US2007/082992
In another embodiment, water is heated up to supercritical conditions. Then
the supercritical water mixed with heavy oil feedin a mixer. The temperature
of heavy oil feed should be kept in the range of about 100 C to 200 C to avoid
thermal cracking but still high enough.to maintain reasonable pressure drop.
After mixing with heavy oil, the temperature of the water-oil mixture would be
lower than critical temperature of water; therefore a second heater is needed
to raise the temperature of the mixturestream to above the critical
temperature of water. In this embodiment, the heavy oil is first partially
heated
up by water, and then the water-oil mixture is heated to supercritical
conditions by the second heater.
Other methods of mixing and heating sequences.based on the above
teachings may be used to accomplish these objectives as will be recognized
by those skilled in the art.
Reaction conditions
After the reactants have been.mixed, they are passed into a reaction zone.in
which they are allowed to react under temperature and pressure conditions of
supercritical water, i.e. supercritical water conditions, in the absence of
externally added hydrogen, for a residence time sufficient to allow upgrading
reactions to occur. Thereaction is preferably allowed to occur in the absence
of externally added catalysts or promoters, atthough the.use of such catalysts
and promoters is permissible in accordance.with the present invention.
"Hydrogen" as used herein in the phrase, "in the absence of externally added
hydrogen" means hydrogen gas. This phraseis. not intended to exclude all
sources of hydrogen that are available as: reactants. Other molecules such as
saturated hydrocarbons may act as a hydrogen source during the reaction by
donating hydrogen to other unsaturated hydrocarbons.. In addition, H2 may be
formed in-situ during the reaction through steam reforming of hydrocarbons
.and water-gas-shift reaction.
CA 02666390 2009-04-14
WO 2008/055152 PCT/US2007/082992
The reaction zone preferably comprises a reactor, which is equipped with a
means for collecting the reaction products (syncrude, water, and gases), and
a section, preferably at the bottom, where any metals or solids (the "dreg
stream") may accumulate.
Supercritical water conditions include a temperature from 374 C (the critical
temperature of water) to 1000 C, preferably from 374 C to 600 C and most
preferably from 374 C to 400 C, a pressure from 3,205 (the critical pressure
of water) to 10,000 psia, preferably from 3,205 psia to 7,200 psia and most
preferably from 3,205 to 4,000 psia, an. oil/water volume ratio from 1:0.1 to
1:10, preferably from 1: 0.5 to 1:3 and most preferably.about 1:1 to 1:2.
The reactants are allowed to react under these conditions for a sufficient
time
to allow upgrading reactions to occur. Preferably,.the residence time will be
selected to allow the upgrading reactions.to occur selectively and to
the.fullest
extent without having undesirable side reactions of coking or residue
formation, Reactor residence times may be from 1 minute to 6 hours,
preferably from 8 minutes to 2 hours and most preferably from20 to 40
minutes.
The Reactor
A reactor designed for heavy oil upgrading using supercritical water in
accordance with the present invention will preferably include the following
features:
The reactor will have means for adequate oil-water mixing and dispersion.
Contrary to the conventional thermal cracking in an uncontrolled fashion that
will lead to excessive formation of light hydrocarbon and therefore lower
liquid
hydrocarbon yield atthe temperature and pressure under supercritical water
conditions, heavy hydrocarbons will hydrothermally crack into lighter
components. Furthermore, hydrocarbon radicals formed from thermal
cracking will also recombine and polymerize and eventually become coke.
Water molecules, especially under supercritical conditions, can quench and
1.1
CA 02666390 2009-04-14
WO 2008/055152 PCT/US2007/082992
stabilize hydrocarbon radicals and therefore_preventthem from over cracking
and polymerization. To avoid over.cracking into light hydrocarbons and coke
formation, the heavy hydrocarbon molecules are: preferably surrounded by
water molecules to the greatest practical extent. Therefore, the reactor
includes means to assure adequate mixing of oil with water for the purpose of
achieving a high yield ofiiquid hydrocarbons. Such means should be chosen
so as to be able to. handle heavy oil feed which has low API gravity and high
viscosity at high oil to Water ratio. Depending on specific applications such
means can include, among others,.(a) nozzles; (b) static mixer; (c) stirring
vessel; (d) micro-channel device; and sonic and ultrasonic.device.
The reaction zone In accordance with the present invention will preferably:
(1) Provide an appropriate residence time to achieve high
conversion and liquid yield. Controlling the residence time narrowly
within determined limits is a very important factor for heavy oil
upgrading using supercritical water. The desired products of heavy oil
upgrading areliquid hydrocarbons. Insufficient residence time will lead
to low conversion and hence low liquid hydrocarbon yield. On the.other
hand, excess conversion will lead to low value by products such as
light hydrocarbon:gas and coke: In order to achieve highly selective
conversion to liquid hydrocarbons, it;is critical to..maintain adequate
residence time.
(2) Provide sufficient heat transfer rate to maintain uniform
temperature distribution. In comparing other supercritical water
applications, heavy oil is:a much more complicated feed and heavy oil
upgrading is a very complex, process: In addition, as indicated above,
the desired liquid hydrocarbon is an intermediate product from
selective, partial reaction. Therefore, it is extremely important to control
reaction temperature to achieve high liqL,id hydrocarbon yield.
Adequate control of reaction temperature can be achieved by providing
enough heat transfer area, uniform feed distribution; or by quenching.
(3) Be able to handle solid formed during the reaction. During the
reaction, small amounts of solid byproducts, primarily inorganic
12
CA 02666390 2009-04-14
WO 2008/055152 PCT/US2007/082992
materials (metals, sulfur; coke etc), will be formed, and the reaction
zone mustbe able to handle such solids so they will not cause
operating problems and will not contaminate the liquid hydrocarbon
product.
The present invention also employs a separation zone for product recovery.
The effluent stream from the reaction zone contains liquid hydrocarbon
product, gas, water under supercritical conditions and solids. The liquid
hydrocarbons are generally separated from other components to achieve high
yield. The preferred way is to.remove the solid first,-and then bring the
fluid
phase containing hydrocarbon products,.supercritical water and gas
byproducts out of supercritical condition by lowing temperature, pressure or
both so that hydrocarbon product and water will condense into liquid phase.
The solids are primarily inorganic materials formed during the reactions and
can be separated. from the supercritical fluid phase using separation
techniques known in the art, which could be-a disengaging:zone in the reactor
or a separate device such as settling vessel, filter, cyclone etc.
Another option for separating;the solids is to bring the product stream out.of
supercritical regime by lowing temperature or pressure.or both. Then the solid
will precipitate. A potential disadvantage of this option is that some.of the
inorganic components in the solid may dissolve in water, which may
contaminate the liquid hydrocarbon product. It should be noted that
depending on the specific applications, a reactor for heavy oil upgrading
using
supercritical water in accordance with the present invention may have more
than one of each of the three components listed above:
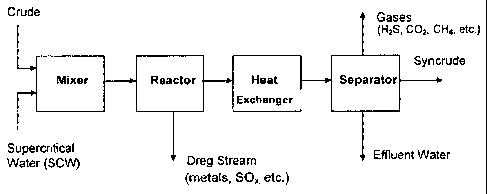
Figure 1 shows an embodiment of the present invention, which has been used
in a laboratory. An inline mixer is used for mixing heavy oil with water. For
this specific embodiment it is a static.mixer. The reaction zone comprises a
spiral tube reactor with large length to diameter ratio to attainhigh velocity
inside the reactor, whic-h is helpful to maintain oil-water dispersion. This
design also makes the fluid flow inside the reactor close to plUg flow and
13
CA 02666390 2009-04-14
WO 2008/055152 PCT/US2007/082992
therefore achieves narrow residence time distribution for selective conversion
to desired liquid hydrocarbons. Inorganic solids in the feed and formed during
the reaction will not dissolve in supercritical water. High velocity inside
the
reactor also prevents settling of those inorganic solids. The small diameter
of
the reactor body also provides large specificsurface area.for heat transfer to
maintain uniform temperature distribution inside the reactor. The length of
the
reactor can be designed based on residence time needed for specific
conversion. A second vessel is added to settle the solids. The. temperature
and pressure is maintained at the same values as those in the spiral tube so
that the fluid in the second vessel is still at supercritical water
conditions. Due
to the larger cross-sectional area of the second vessel the fluid velocity is
much lower. As a result, inorganic materials separated from. the fluid wi11
settle
down in. the vessel, and can be removed from the system. The fluid containing
hydrocarbon products, supercritical water and gas byproducts is cooled while
maintaining at the same pressure as in the reactor, and hydrocarbon products
and water are condensed in the high pressure separator.
A spiral tube with a high length to diameter ratio, which may be from 50 to
10,000, preferably from 100 to 4,000 may be. used as reactor body. Use of
such a reactor has the advantages of high velocity, narrow residence time
distribution, and large surface for.heat transfe.r. The length to diameter
ratio is
a useful parameter to determine preferred reactor configurations. The
diameter may be determined by velocity needed to avoid solids precipitation
and then the length can be selected to provide the desired residence time.
Other reactor configurations known to those in the art can be used to achieve.
similar effects, such as a serpentine reactor.
In the embodiments shown in Figure 1 the separation zone for removing solid
and recovering hydrocarbon products is a vessel with a dip tube. Other fluid -
solid separation devices known in the art can be used to achieve the
separation effect, which includes, but not limited to, -cyclone, filter,
ceramic
membrane, settling tank, etc.
14
CA 02666390 2009-04-14
WO 2008/055152 PCT/US2007/082992
ln the embodiment shown in Figure 1, as well as in other embodiments
described herein, the mixer; reaction and separation zones are separated.
Such arrangement is convenient for laboratory research, and is used as an
illustrative example. It is within the scope of the present invention and in
some
applications will be beneficial to integrate these three functions into one
vessel.
As mentioned above, the reactor may include rriore than one piece of each
function devices. Figure 2 shows an example. In order to avoid over cracking
of the feed to form undesired byproducts such as light hydrocarbon gases.and
coke, heavy hydrocarbon molecules are preferably surrounded. by sufficient
water molecules. Generally speaking, a higher water to oil ratio will be
helpful
to maintain the desired environment. However, high water to oil ratio also
means high equipment and operating cost. The embodiment shown in Figure
2 can achieve high water to oil ratio locally without increasing overall water
to
feed ratio. Instead of mixing all the feed oil with water at reactor inlet,
this
embodiment uses multiple injections of oil to maintain a desired water to oil
ratio. Such a design is also helpful to control reaction temperature. By
distributing feed oil: more uniformly through the reactor length, reaction
temperature will not increase too much due to the exothermic nature of the
reactions.
Only two injections were shown in Figure.2. This is not intended as a
Iimitation..A reactor with multiple injections may also be used. In addition,
one
or more settling vessels can be added to a reactor with a multiple injection
configuration to achieve solid separation under supercritical conditions.
Figure 3 shows yet another embodiment with more than one mixing and
reaction zones. A second mixer, which may or may not be the same as. the
first mixer, is added between reaction zone to enhance the oil/supercritical
water mixing. Again, multiple mixers and reaction zones can be used.
The upgrading reaction is exothermic. A reactor with a large surface area
helps to maintain uniform temperature distribution inside the reactor.
CA 02666390 2009-04-14
WO 2008/055152 PCT/US2007/082992
Depending on feed properties, heat exchange through the surface area
provided by the reactor may or may not be enough. Water can be used to
quench the reaction stream and thereby control the reaction temperature.
Figure 4 shows an embodiment.of using water to quench the reaction stream
between two reaction zones. The amount:of water used for quenching should
be enough to bring down the reaction temperature whilethe reaction stream
after quenching still maintain supercritical conditions. Multiple
reaction.zones
and water quenching may be necessary for some feeds.
The quenching water can also be used to for product recovery, as shown in
Figure 5. After reaction the product stream.is quenched by liquid water. The
solid will be washed out by the water, and due to the temperature reduction
caused by quenching water and the hydrocarbons will condense as liquid.
Reaction Product Separation
After the reaction has progressed sufhciently, a single phase reaction product
is withdrawn from the reaction zone, cooled, and separated into gas, effluent
water, and upgraded hydrocarbon phases. This separation is preferably done
by cooling the stream and using one or more two-phase separators, three-
phase separators, or other gas-oil-water separatiori device known in the art.
However, any method of separation can be used in accordance with the
invention.
The composition of gaseous produ.ct obtained by treatment of the heavy
hydrocarbons in accordance with the process of the present, invention will
depend on feed properties and typically comprises light hydrocarbons, water
vapor, acid gas (.CO2 and H2S), methane and hydrogen. The effluent water
may be used, reused or discarded. It may be recycled to e.g.. the feed water
tank, the feed water treatment system or to the reaction zone.
The upgraded hydrocarbon product, which issometimes referred to as
"syncrude" herein may be upgraded. further or processed into other
16
CA 02666390 2009-04-14
WO 2008/055152 PCT/US2007/082992
hydrocarbon products using methods that are known in the hydrocarbon
processing art.
The process of the present invention may be carried out either asa
continuous or semi-continuous process or a batch process or as a continuous
process. In the continuous process the entire system operates with a feed
stream of oil and a separate feed stream of supercritical water and reaches a
steady state; whereby all the flow rates, temperatures, pressures, and
composition of the inlet, outlet, and recycle streams do not vary appreciably
with time.
While not being bound:to any theory of operation, it is believed that a number
of upgrading reactions are occurring simultaneously at the supercritical water
conditions used in the present process.: In a preferred embodiment of the
invention the major chemical/upgrading reactions are believed to be:
Thermal Cracking: CxHy -~ lighter hydrocarbons
Steam Reforming: CXHy + 2xH20 = .xC02 + (2x+y/2)H2
Water-Gas-Shift: CO + H20 = C02 + H2
Demetalization: CXHyNiw+ H20/H2 -> NiO/Ni(OH) 2 + lighter hydrocarbons
Desulfurization: CxHySZ + H20/H2 = H2S + lighter hydrocarbons
The exact pathway may depend. on the reactor operating conditions
(temperature, pressure, O/W volume ratio), reactor design (mode of
contactlmixing, sequence of heating), and the hydrocarbon feedstock.
The following Examples are illustrative of the present invention, but are. not
intended to limit the invention in any waybeyond what is contained in the
claims which follow.
EXAMPLE 1 - Process Conditions.
Oil and supercritical water arecontacted.in a mixer prior to entering the
reactor. The reactor is equipped with an inner tube for collecting the
products
17
CA 02666390 2009-04-14
WO 2008/055152 PCT/US2007/082992
(syncrude,. excess water, and gas), and a bottom section where any metals or
solids comprising a "dreg stream" of indeterminate properties or composition
may accumuiate. The shell-side of the reactor is* kept isothermal during the
reaction with a clamshell furnace and temperature controller. Preferred
reactor residence times are 20-40 minutes, with preferred oii/water volume
ratios on the order of 1:3. Preferred temperatures are around 374 - 400 C,
with the pressure at 3200-4000 psig. The reactor product stream leaves as a
single phase, and is cooled and separated into gas, syncrude, and effluent
water. The effluent water is recycled back to the reactor. Sulfur from the
original feedstock accumulates in the dreg stream for the most part, with
lesser amounts primarily in the form of H2S found in the gas. phase and water
phase.
As the next examples will show, very littie gas is produced in most cases.
With suitable choice of operating conditions, it is also possible to reduce or
nearly eliminate the "dreg stream." Elimination of the dreg stream means that
a greater degree ofhydrocarbon is recovered as syncrude, but it also means
that metals and sulfur will accumulate elsewhere, such as in the water and
gas streams.
EXAMPLE 2 -Properties of the Product Syncrude.
A Hamaca crude oil was diluted with a diluent hydrocarbon at a ratio of 5:1
(20 vol% of diluent). The. diiuted Hamaca crude oil properties were measured
before reacting it with the supercritical water process as referred to in
Example 1 and Fig. 2. The properties of the crude were as foiiows: 12.8 API
gravity at 60/60; 1329 CST viscosity @400C; 7.66 wt%o C/H ratio; 13.04 wt%
MCRT;. 3.54 wt% sulfur; 0:56 wt% nitrogen; 3.05 mg KOH/gm acid number;
1.41 wt lo water; 371 ppm Vanadium; and.86 ppm: Nickel. The diluted Hamaca
crude: oii after the super critical water treatment was converted into a
:syncrude with the following properties: 24.1 API gravity at 60/60; 5.75 CST
viscosity @40 C:; 7:40 wt% C/H ratio;. 2:25 wt%.MCRT; 2.83 wt% sulfur; 0.28
wt lo nitrogen; 1.54 mg KOH/gm'acid number; 0.96 wt% water; 24 ppm
18
CA 02666390 2009-04-14
WO 2008/055152 PCT/US2007/082992
Vanadium; and 3 ppm Nickel. Substantial reductions in metals and residues
were observed, with simultaneous increase.in the API gravity and a significant
decrease in the viscosity of the original crude oil feedstock. There were
modest reductions in the Total Acid number, sulfur concentration, and
nitrogen concentration which could be improved with further optimization of
the reaction conditions.
When the diluted Hamaca crude was sent directly to the reactor without being
first heated with supercritical water, the product syncrude had the following
properties: 14.0 API gravity at 60/60; 188 CST viscosity @40 C; 8.7 wt%
MCRT; 3.11 wt% sulfur; 267 ppm Vanadium; and 59 ppm Nickel. This
comparison demonstrates the importance of the heating sequence of the
present invention.
Apart from the occasional, small accumulation of a dreg stream, there is very
little coking or solid byproducts formed in the supercritical water reaction.
The
material balance was performed for two separate experimental runs.
In the experimental run.with no dreg stream formed, the starting feedstock of
diluted Hamaca crude at 60 grams produced a syncrude product of 59.25
grams which corresponds to a high overall recovery of 99 percent. It was
thought that due tothe absence of a dreg stream, the experimental mass
balance was impacted. in the determination of the sulfur and metals. The gas
phase did not contain metals species-and had little sulfur compounds. It was
hypothesized that a portion of the metal and sulfur may have accumulated on
the walls of the reactor or downstream plumbing..
In the experimental run with a dreg stream formed, the starting.feedstock of
diluted Hamaca crude at 30 grams produced a syncrude product of 22.73
grams. The dreg stream that was formed accounted for 5.5 grams: The
overall recovery with the dreg stream was 96.7 percent. In the dreg stream,
sulfur,accounted for 31 !o of the total sulfur with the remaining sulfur in
the oil
product, water phase, and gas phase. The. nietals content of the dreg stream
19
CA 02666390 2009-04-14
WO 2008/055152 PCT/US2007/082992
accounted for 82% of the total metals with the remaining metals in the oil
product. For commercial operations, it may be preferable. to minimize the
formation of a dreg stream, since it represents a 18%.reduction in syncrude
product, and generates a lower value product stream that impacts the process
in terms of economics and disposal concerns.
Undiluted Boscan crude oil properties were measured before :reacting it with
the supercritical water process of the present invention. The.properties of
the
crude were as follows: 9 API gravity at 60/60; 1,140 CST viscosity @40 C;
8.0 wt% C/H ratio; 16 wt lo MCRT; 5.8 wt% Sulfur; and 1,280 ppm Vanadium;.
The undiluted Boscan crude oil after the super critical water treatment was
converted into a syncrude with the following properties: 22 API gravity at
60/60; 9 CST viscosity @40 C; 7.6 wt Io C/H ratio; 2.5 wt% MCRT; 4.6%
sulfur; and 130 ppm Vanadium;
A simulated distillation analysis of the original crude oil vs. the syncrude
products from different experimental runs shows that the syncrude prepared
in accordance with the present invention clearly, has superior properties than
the original crude. Specifically,the syncrudes contain a higher fraction of
lower-boiling fractions. 51 % of the diluted Hamaca crude boils across a range
of temperatures of less than 1000 F, while employing a process according to
the present invention using supercritical water depending on process
configurations, between.79 to 94% of the syncrude boils across a range of
temperatures of less than 1000 F. 40% of the undiluted Boscan crude boils
across a range of temperatures of less than 1.000 F, while employing a
process according to the present invention using supercritical water, 93% of
the syncrude boils across a range of.temperatures`of less than 1000 F.
There are numerous variations on the present invention which are possible in
light of the teachings and supporting examples described herein. It is
therefore understood that within the scope of the'following claims, the
invention may be practiced otherwise than. as specifically described or
exemplified herein.