Note: Descriptions are shown in the official language in which they were submitted.
CA 02666767 2009-04-17
METHOD FOR TREATING WASTE CONTAINING PRECIOUS METALS AND DEVICE
FOR IMPLEMENTING SAID METHOD
FIELD OF THE INVENTION
The present invention relates to a method for treating waste containing
precious metals as
well as a device for implementing this method.
TECHNICAL BACKGROUND
The increase in the use of calculating machines, mobile phones, electronic
apparatuses and
other short life high-tech apparatuses generates an increasing amount of waste
containing rare
and precious metals. This situation poses the problem of recovering and
treating the metals
contained in this waste. Such waste is thus a real source of non-ferrous, rare
and precious metals.
Electronic waste is presently collected, exported and treated in large
industrial complexes
for non-ferrous metals often requiring several plants in succession for
extracting lead, copper and
zinc where these high value materials are diluted in flows of raw materials of
mining or
secondary origin. Present methods are therefore optimized in order to produce
lead, copper or
pure zinc in large amounts, but they are poorly adapted for producing rare and
precious metals
present in small amounts.
It is therefore desirable to design a method for treating waste with which a
large proportion
of the precious metals contained in the waste may be recovered.
SUMMARY OF THE INVENTION
A first object of the invention is a method for treating waste containing
precious metals,
comprising the following successive steps:
- contacting the waste with a molten lead-based composition;
- skimming the mixture obtained; and
- refining the skimmed mixture by electrolysis so as to recover the precious
metals.
According to a particular embodiment, in the skimming step, residues are
recovered, which
are treated by:
- contacting the residues with a second molten lead-based composition;
- skimming the mixture obtained; and
- recovering the skimmed mixture in order to provide at least one portion of
the portion of
the aforementioned molten lead-based composition.
According to a particular embodiment, the step of refining the skimmed mixture
comprises
the following sub-steps:
CA 02666767 2009-04-17
2
- casting the skimmed mixture into anodes;
- electrolysis of a fluorosilicic acid solution by using said anodes; and
- recovering anodic sludges containing the precious metals.
According to a particular embodiment, the aforementioned method comprises
before,
simultaneously or after the step of recovering anodic sludges, the step of:
- recovering lead and possibly tin cathode deposits of in order to provide at
least one
portion of the molten lead-based composition and/or of the second molten lead-
based
composition.
According to a particular embodiment, the step of refining the skimmed mixture
comprises, after the sub-step of recovering anodic sludges, the following
additional sub-steps:
- melting the recovered anodic sludges in the presence of oxygen,
- skimming the molten anodic sludges; and
- casting the molten and skimmed anodic sludges into ingots.
According to a particular embodiment, each molten lead-based composition
comprises 0-
50% of tin, preferably 0-20% of tin.
According to a particular embodiment, the aforementioned method comprises,
prior to the
step of contacting the waste with a molten lead-based composition, the step
of:
- extracting copper from the waste by selective dissolution.
According to a particular embodiment, the copper extraction step comprises the
following
sub-steps:
- selectively dissolving the waste in the presence of sulfuric acid, iron
sulfate and oxygen;
- treating the obtained solution by filtration and/or electrolysis and/or
precipitation;
- recovering copper on the one hand and other metal impurities on the other
hand.
According to a particular embodiment, the aforementioned method comprises,
prior to the
copper extraction, the following step:
- combustion of the waste by pyrolysis, producing carbonaceous gases; and
optionally
- post-combustion of the carbonaceous gases.
According to a particular embodiment, the aforementioned method further
comprises a
preliminary step of milling the waste and/or analyzing milled waste.
According to a particular embodiment, the precious metals comprise one or more
metals
selected from gold, silver, platinum, palladium, rhodium, ruthenium, iridium,
osmium and
mixtures thereof.
According to a particular embodiment, the waste is selected from catalytic
exhaust
mufflers and electronic waste such as electronic cards.
= CA 02666767 2009-04-17
3
According to a particular embodiment, more than 90% by mass, preferably more
than 99%
by mass of the precious metals contained in the waste are recovered according
to the
aforementioned method.
According to a particular embodiment, the supernatant materials comprise
ceramics, glass
fibers and/or ferrites.
Another object of the invention is an installation for treating waste
containing precious
metals, comprising:
- at least one container for covering with lead;
- a molten lead-based composition feed line, connected to the inlet of the
lead covering
container;
- a pretreated materials feed line, connected to the inlet of the lead
covering container;
- skimming means associated with the lead covering container;
- a skimmed mixture withdrawal line, connected to the outlet of the lead
covering
container;
- means for refining the skimmed mixture by electrolysis, fed by the skimmed
mixture
withdrawal line; and
= - a precious metals withdrawal line, connected to the outlet of the means
for refining the
skimmed mixture.
According to a particular embodiment, the aforementioned treatment
installation further
comprises:
- a skimming residues withdrawal line, connected to the outlet of the skimming
means;
- at least one additional lead covering container, fed by skimming residues
withdrawal
line on the one hand, and by an additional molten lead-based composition feed
line on
the other hand;
- additional skimming means associated with the additional lead covering
container; and
- an additional skimmed mixture withdrawal line, connected to the outlet of
the additional
lead covering container and feeding the molten lead-based composition feed
line.
According to a particular embodiment, the means for refining the skimmed
mixture by
electrolysis comprise:
- means for casting anodes;
- means for Betts electrolysis; and
- means for recovering anodic sludges.
According to a particular embodiment, the means for refining the skimmed
mixture by
electrolysis comprise:
CA 02666767 2009-04-17
4
- means for recovering lead-tin, feeding the additional molten lead-based
composition
feed line.
According to a particular embodiment, the aforementioned treatment
installation further
comprises:
- copper extraction means, one outlet of which is connected to the pretreated
materials
feed line; and
- a primary materials feed line, connected to the inlet of the copper
extraction means.
According to a particular embodiment, the copper extraction means comprise:
- at least one selective dissolution container fed by the raw materials feed
line;
- a depleted electrolyte feed line, connected to the inlet of the selective
dissolution
container;
- electrolysis means,
- means for transferring rich electrolyte, connecting the selective
dissolution container to
the electrolysis means;
- means for stripping the cathodes; and
- means for recycling depleted electrolyte, connected to the outlet of the
electrolysis
means.
According to a particular embodiment, the aforementioned treatment
installation further
comprises:
- means for pyrolysis of the waste, connected to the outlet of the raw
materials feed line;
- a waste feed line feeding the pyrolysis means; and optionally
- a gas exhaust line at the outlet of the pyrolysis means and feeding post-
combustion
means.
According to a particular embodiment, the aforementioned treatment
installation further
comprises:
- milling and analyzing means fed by a raw waste feed line and feeding the
waste feed
line.
According to a particular embodiment, the aforementioned method is applied in
the
aforementioned installation.
According to a particular embodiment, the aforementioned installation is
intended for
implementing the aforementioned method.
The present invention makes it is possible to overcome the drawbacks of the
state of the
art. It more particularly provides a specific method for treating and
recovering waste containing
precious metals, which harmoniously combines metallurgical sequences and
avoids dilution of
the contained metals in a production flow of primary metals. The invention
also provides a single
CA 02666767 2009-04-17
installation for separating the constituents from the waste and in particular
for recovering
precious metals.
According to certain particular embodiments, the invention also has the
advantageous
features listed below.
5 - The method according to the invention is very flexible and it may be
adapted to
foreseeable changes in the composition of electronic cards.
- The method according to the invention does not have the drawback of using
the customary
technique of extracting precious metals by oxidization of lead, the so-called
cupellation
operation, followed by the operation for reducing lead oxide.
- With the invention, it is possible to retain metals in the metal form as
much as possible:
precious metals are kept in the metal form throughout the method, and the lead
and tin
are kept in the metal form right up to step (d) included. This makes it
possible to
minimize the carrying away of the precious metals by metal oxides.
- With the invention, the precious metals may be collected at a single outlet.
- The invention may be applied with a controlled environmental impact.
SHORT DESCRIPTION OF THE DRAWINGS
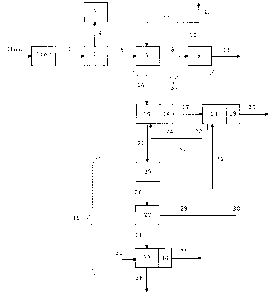
Fig. 1 schematically illustrates an exemplary installation for treating waste
according to the
invention.
Fig. 2 illustrates exemplary milling and analyzing means which may be used
within the
framework of the waste treatment installation according to the invention. The
dotted arrows
designate the gas flows. The arrows with a double line designate solid flows.
Fig. 3 illustrates exemplary pyrolysis and postcombustion means which may be
used
within the framework of the waste treatment installation according to the
invention. The dotted
arrows designate gas flows. The arrows with a simple black line designate
liquid flows. The
arrows with a double line designate solid flows.
Fig. 4 illustrates exemplary copper extraction means which may be used within
the
framework of the waste treatment installation according to the invention. The
dotted arrows
designate gas flows. The arrows with a simple black line designate liquid
flows. The arrows with
a double line designate solid flows.
Fig. 5 illustrates a particular example of a lead covering container which may
be used
within the framework of the invention.
Fig. 6 illustrates exemplary refining means which may be used within the
framework of the
waste treatment installation according to the invention. The dotted arrows
designate the gas
flows. The arrows with a simple black line designate liquid flows. The arrows
with a double line
designate solid flows.
CA 02666767 2009-04-17
6
DESCRIPTION OF EMBODIMENTS OF THE INVENTION
The invention is now described in more detail and in a non-limiting way in the
following
description.
Waste treatment installation
Referring to Fig. 1, an installation for treating waste according to the
invention
schematically comprises the following components.
Provision is made for a waste feed line 1 at the entry of the treatment
installation. This
waste feed line 1 may optionally be connected to the outlet of the milling and
analysis means
lter fed by a raw waste feed line lbis.
The waste feed line 1 like all the other feed, transfer or withdrawal lines
mentioned in the
present description may include a single route or several routes (branches) in
parallel.
According to an embodiment, the waste feed line 1 feeds pyrolysis means 2. At
the outlet
of the pyrolysis means 2, provision is made for means for feeding primary
materials 6, which
feed copper extraction means 37. At the outlet of the copper extraction means
37, provision is
made for a pre-treated materials feed line 14, which feeds a lead covering
container 15. This
embodiment is particularly well adapted to treating used electronic cards.
According to an alternative, the pyrolysis means 2 are absent, and the waste
feed line 1
directly feeds the copper extraction means 37 (in this case it is considered
that the waste feed line
1 coincides with the primary materials feed line 6).
According to another alternative, the copper extraction means 37 are absent,
and the
primary materials feed line 6 directly feeds the lead covering container 15
(in this case it is
considered that the primary materials feed line 6 coincides with the pre-
treated materials feed
line 14).
According to still another alternative, both the pyrolysis means 2 and the
copper extraction
means 37 are absent, and the waste feed line 1 directly feeds the lead
covering container 15 (in this
case it is considered that the waste feed line 1 and the pre-treated materials
feed line 14 coincide).
This alternative is particularly adapted to the treatment of used catalytic
exhaust mufflers, since the
latter practically contain no copper.
When they are present, the outlet of the pyrolysis means 2 may be connected to
a gas
exhaust line 4 which may feed postcombustion means 5.
When they are present, the copper extraction means 37 may include a selective
dissolution
container 7 fed at the inlet by the pre-treated materials feed line 6 and fed
by a depleted
electrolyte feed line 11 on the other hand. The pre-treated materials feed
line 14 is then
connected to the outlet of the selective dissolution container 7, while a rich
electrolyte transfer
CA 02666767 2009-04-17
7
line 8 feeds electrolysis means 9. Means for stripping the cathodes 13 are
provided in association
with electrolysis means 9, and a depleted electrolyte recycling line 10 is
provided at the outlet of
the electrolysis means 9. This depleted electrolyte recycling line 10 may feed
the depleted
electrolyte feed line 11 and/or a depleted electrolyte treating line 12.
The lead covering container 15, which is fed by the pre-treated materials feed
line 14, is
also fed by a molten lead-based composition feed line 24. Skimming means 16
are associated
with the lead covering container 15. At the outlet of the lead covering
container 15, provision is
made for a skimmed mixture withdrawal line 21, which feeds means for refining
the skimmed
mixture 36. A precious metals withdrawal line 38 is provided at the outlet of
the means for
refining the skimmed mixture 36.
Provision may also be made for a skimming residues withdrawal line 17 at the
outlet of the
skimming means 16, which may feed an additional lead covering container 18.
This additional
lead covering container 18 is then also fed by an additional molten lead-based
composition feed
line 31. Additional skimming means 19 are provided in association with the
additional lead
covering container 18 and an additional skimmed mixture withdrawal line 22 is
provided at the
outlet of the additional lead covering container 18. This additional skimmed
mixture withdrawal
line 22 may, just like the skimmed mixture withdrawal line 21, feed the means
for refining the
skimmed mixture 36. But according to a preferred alternative, the additional
skimmed mixture
withdrawal line 22 feeds the molten lead-based composition feed line 24. A
complementary
source of molten lead-based composition 23 may optionally be provided in order
to complete this
feed. An additional skimming residues withdrawal line 20 may be provided at
the outlet of the
additional skimming means 19.
The means for refining the skimmed mixture 36 more specifically provide means
for
casting anodes 25, a system for transferring anodes 26, Betts electrolysis
means 27. At the Betts
electrolysis means 27, provision is made for means for recovering lead-tin 29
and means for
recovering anodic sludges 28. The means for recovering lead-tin 29 feed
(optionally together
with a fresh lead feed line 30 which may be provided as an option) the
additional molten lead-
based composition feed line 31.
The means for recovering anodic sludges 28 feed melting means 33, which are
further
provided with an oxygen feed 32. Final skimming means 34 are provided in
association with the
melting means 32. The precious metals withdrawal line 38 is connected to the
outlet of the
melting means 32, which also include a residues discharging line 35.
Now referring to Fig. 2, a possible example is described in more detail
hereinbelow for the
first portion of the waste treatment installation, dedicated to receiving,
milling and analyzing the
incoming waste (cf references lbis, lter of Fig. 1).
CA 02666767 2009-04-17
8
According to this example, the installation comprises means for receiving
waste 101,
which may notably comprise an unloading hall, and which are for example
suitable for receiving
trucks. Weighing means 102 are provided at these waste receiving means 101, as
well as storage
means 103. At the outlet of the storage means 103, provision is made for
dosing means 104
adapted for dumping waste on a main conveyor 105 (belt or the like). The main
conveyor 105
distributively feeds a first secondary conveyor 106, a second secondary
conveyor 107 and a third
secondary conveyor 108.
The first secondary conveyor 106 feeds a coarse mill 109. A fine mill 111 is
also provided,
fed by the second secondary conveyor 107 on the one hand and by a transfer
line 110 on the
other hand stemming from the outlet of the coarse mill 109. The mills 109, 111
may each have a
typical capacity of 5-10 t/h. A collector conveyor 112 is provided at the
outlet of the fine mill
111 and joins the third secondary conveyor 108. On the path of the third
secondary conveyor
108, provision is also made for sampling means 113 (for example a ladle), with
which analysis
means 114 may be fed.
Moreover, the third secondary conveyor 108 distributively feeds a first
tertiary conveyor
115 and a second tertiary conveyor 117. The first tertiary conveyor 115 feeds
a silo for storing
waste 116. As for the second tertiary conveyor 117, it feeds a container 118,
at the outlet of
which a return conveyor 119 feeds the storage means 103. An air
decontamination system 120 is
set up at the coarse mill 109 and the fine mill 111 and feeds a sleeve filter
121, which may have a
typical capacity of 5,000 Nm3/h. The sleeve filter 121 is connected to a
chimney 123 as well as
to a fines recovery line 122, which feeds the silo for storing waste 116.
It is obvious that one skilled in the art will be able to adapt the thereby
described means to
the needs of the installation, for example by varying the number or the type
of mills or the
capacity of the different mills used.
Referring now to Fig. 3, a possible example is described in more detail herein
below for the
portion of the treatment installation which is comprised between the waste
feed line 1(hereafter
201) and the primary materials feed line 6 (hereafter 205a, 205b).
According to this example, the waste feed line 201 is provided at the outlet
of the
aforementioned silo for storing waste 116 and feeds via hoppers three
pyrolysis ovens 202a, 202b,
202c arranged in parallel. The pyrolysis ovens 202a, 202b, 202c may be tubular
screw ovens,
electrically heated from the outside. As an example, ovens with a length of 5
m and a diameter of
cm, with a power of 100 kW, with a variable screw velocity, may be used. The
number of ovens
may be varied depending on the needs of each installation.
A calcinated waste recovery line 203 is provided at the outlet of the
pyrolysis ovens 202a,
35 202b, 202c and feeds two silos for storing calcinated waste 204a, 204b. The
number of these
storage silos may be varied depending on the needs of each installation. The
calcinated waste
CA 02666767 2009-04-17
9
recovery line 203 may be a jacketed conveyor provided with water cooling
means. At the outlet
of each silo for storing calcinated waste 204a, 204b a respective primary
materials feed conduit
205a, 205b is provided (both of these conduits forming together the primary
materials feed line
6).
Moreover, at the outlet of each pyrolysis oven 202a, 202b, 202c, provision is
made for a
respective gas exhaust conduit 206a, 206b, 206c (the whole of these conduits
corresponding to
the aforementioned gas exhaust line 4). Each gas exhaust gas conduit 206a,
206b, 206c feeds a
respective post-combustion chamber 207a, 207b, 207c. A typical example of the
volume of the
post-combustion chamber 207a, 207b, 207c is 15 m3. Each post-combustion
chamber 207a,
207b, 207c is further fed by a respective air intake conduit 208a, 208b, 208c.
A burnt gases collecting conduit 209 connects the outlet of the post-
combustion chambers
207a, 207b, 207c to the inlet of a vertical cooling chamber 210. A water
coolant feed line 211 is
also provided at the inlet of the cooling chamber 210. For example, spraying
ramps located in the
high portion of the chamber may be provided. A pre-cooled gases recovery
conduit 212 is
provided at the outlet of the cooling chamber 210, and feeds a sleeve filter
214. An air intake
conduit 213 is connected to the pre-cooled gases recovery conduit 212. The
sleeve filter 214 may
for example have a capacity of 4,000 Nm3/h. A fines withdrawal conduit 215 on
the one hand
and a stripped gases withdrawal conduit 216 are connected to the outlet of the
sleeve filter 214.
The stripped gases withdrawal conduit 216 feeds a chimney 217.
Now referring to Fig. 4, a possible example for the portion of the treatment
installation
which essentially comprises the copper extraction means 37, is described in
more detail
hereinbelow.
Each primary materials feed conduit 205a, 205b feeds a respective selective
dissolution
container 301a, 301b, which may for example be a closed 20 m3 reactor in epoxy
resin/fiber
glass with a large thickness, provided with a lid and a stirrer with variable
speed. It is possible to
provide such a single container or on the contrary a larger number of them
depending on the
production needs. Each selective dissolution container 301a, 301b is also fed
by a depleted
electrolyte feed conduit 303. An oxygen supply 304 is moreover provided at the
bottom of each
selective dissolution container 301a, 301b.
A respective selective post-dissolution emptying line 305a, 305b is provided
at the outlet
of each selective dissolution container 301a, 301b which feeds a respective
press filter 306a,
306b. A system for collecting solids 307 is placed at the outlet of the press
filters 306a, 306b and
feeds a drying oven 308, at the outlet of which is found the pre-treated
material conduit 309
(corresponding to reference 14 in Fig. 1). The drying oven 308 may be a screw
oven similar to
those used for pyrolysis.
CA 02666767 2009-04-17
Moreover, each press filter 306a, 306b is provided at the outlet with a
respective filtered
liquid withdrawal conduit 310a, 310b which feeds a single vat 302. The latter
in turn feeds via a
transfer line 311, a tank for storing rich electrolyte 312 which may for
example have a capacity
of 60 m3.
5 The other major component of this portion of the installation is the
electrodeposition unit
314. The electrodeposition unit 314 comprises a certain number of electrolysis
tanks 315a, 315b,
315c, 315d, 315e, the number of tanks (five in this example) being adaptable
to the production
needs. Each electrolysis tank 315a, 315b, 315c, 315d, 315e comprises a certain
number of
electrolysis cells depending on the production needs, for example eight in the
present example.
10 As an example, each electrolysis cell may have a useful volume of 4 m3 and
contain 31 stainless
steel cathodes and 30 lead/calcium anodes with a useful surface area of 1 mz
per face. The
electrolysis tanks 315a, 315b, 315c, 315d, 315e are fed in parallel by a rich
electrolyte transfer
conduit 313 connected to the outlet of the tank for storing rich electrolyte
312. The
electrodeposition unit 314 is completed by a system for stripping the cathodes
316.
At the outlet of the electrodeposition unit 314, a depleted electrolyte
recycling line 317 is
provided, which feeds a first tank for storing depleted electrolyte 318 (for
example with a
capacity of 60 m3) and a second tank for storing depleted electrolyte 319 (for
example with a
capacity of 25 m3). The first tank for storing depleted electrolyte 318 is the
source for feeding the
depleted electrolyte feed conduit 303. The second tank for storing depleted
electrolyte 319 feeds
a first stripping reactor 320 (for example with a capacity of 15 m3.). This
first stripping reactor
320 is also fed by a lime feed line 321. At the outlet of the first stripping
reactor 320 a first pulp
withdrawal line 323 is connected, which feeds an additional press filter 324.
Moreover, the fines withdrawal conduit 215 described in connection with Fig. 3
feeds a
second stripping reactor 325 (for example with a capacity of 5 m3) provided
with a water and
lime supply (not shown). At the outlet of the latter is found a second pulp
withdrawal line 326,
which also feeds the additional press filter 324. A washed fines withdrawal
line 327, a lime
sulfate withdrawal line 328 and an acid juice withdrawal line 329 are
connected at the outlet of
the additional press filter 324. The washed fines withdrawal line 327 may feed
one of the
selective dissolution containers 301a, 301b or both. The acid juice withdrawal
line 329 may feed
a tank body, not shown, with additional devices downstream for treating
halides.
A gas decontamination system 330 passes through the whole of the storage tanks
312, 318,
319 of the selective dissolution containers 301a, 301b and of the stripping
reactors 320, 325 and
feeds a washing tower 331. The storage tanks 312, 318, 319, the stripping
reactors 320, 325 and
the washing tower 331 may be in epoxy resin/fiber glass with a standard
thickness. The washing
tower 331 comprises at the outlet an additional acid juices withdrawal line
332, which may in
CA 02666767 2009-04-17
11
return feed the tank for storing depleted electrolyte 318. The washing tower
331 may have a
capacity of 5 m3, be provided with standard lining and operate with water.
Again referring generally to Fig. 1, the lead covering container 15 and the
additional lead
covering container 18 may be as illustrated in Fig. 5.
Each container then comprises a kettle 401 (with a capacity of 50 tons for
example)
surrounded by a heating chamber 402 provided with burners 403. A stirrer 404
(for example a
stirrer with a vertical axis propeller) is immersed into the kettle 401. The
kettle 401 is fed by a
feeder 407, which, depending on the case, is connected to the inlet of the pre-
treated materials
feed line 14 or to the skimming residues withdrawal line 17. On the side of
the kettle 401, a
skimming machine 405 is provided, consisting in a scraper with a stainless
steel jointed arm
attached to a tilted plane. An enclosing cover 406 allows the surface of the
contents of the kettle
to be isolated and is adapted so as to provide inertization with nitrogen.
Suction means 408 are
provided above the kettle 401 and are connected to a sleeve filter not shown.
Means for
discharging combustion gases 409 adapted to collecting gases emitted by the
burners are
connected to the heating chamber 402. The stirrer 404 may advantageously be
disassembled in
order to allow transfer of the contents of the kettle 401.
Downstream from the lead covering containers 15, 18, means are found for
casting anodes
25, which notably comprise a kettle of the type described in Fig. 5, but
without necessarily the
skimming and stirring devices. This kettle may comprise an enclosing cover and
suction.
The last major portion of the present installation relates to refining and
notably
encompasses references 27, 33, 34 of Fig. 1. The latter is described with
reference to Fig. 6
hereinbelow.
This portion of the installation comprises a Betts electrolysis unit 501,
which contains a
plurality of rows 502a, 502b of Betts electrolysis cells (two in this
example). Each row 502a,
502b may for example comprise five cells, each cell including 30 anodes and 31
cathodes with a
useful surface area of 1 m2 per face, for a useful cell volume of 4 m3. The
rows 502a, 502b are
fed in parallel with electrolyte from a Betts reactor 503. A return pumping
system may be
provided for facilitating circulation of the electrolyte. The Betts reactor
503 is fed by a
fluorosilicic acid feed line 504 on the one hand and by a litharge feed line
505 on the other hand.
At the outlet of the Betts electrolysis unit 501, a used Betts electrolyte
collecting line 506
provides a return to the second tank for storing depleted electrolyte 319 of
Fig. 4.
An electrolysis decontamination system 507 provides collection of the gases at
the Betts
electrolysis 501 and at the Betts reactor 503, and their transfer towards a
washing tower 508,
with a typical volume of 5 m3. A washing juice collecting line 509 provides a
return towards the
second tank for storing depleted electrolyte 319 of Fig. 4.
CA 02666767 2009-04-17
12
The Betts electrolysis unit 501 is moreover provided with means for stripping
cathodes
510. The means for stripping cathodes 510 provide a cathode feed line 511
which itself feeds a
kettle 512 providing melting of the cathodes. The kettle 512 is of the type
described in Fig. 5, but
without necessarily skimming and stirring devices. This kettle 512 may
comprise an enclosing
cover and suction. It feeds via a lead-tin feed line 513, possibly together
with a fresh lead feed
line 30, the additional molten lead-based composition feed line 31 (see Fig.
1). The whole of the
references 510-513 correspond to means for recovering lead-tin 29.
The Betts electrolysis unit 501 is moreover provided with means for scraping
anode stubs
514 which feed an anodic sludges collecting line 516 (the whole forming an
example of anodic
sludge recovery means 28). This anodic sludges collecting line 516 feeds a
unit for treating anodic
sludges 517 which may comprise washing means, weighing means and/or means for
storing in a
safe. A washed sludges transfer line 518 connects the unit for treating anodic
sludges 517 to an
oxidation oven (with a power of 800 kW, 1 ton capacity, for example), which
also receives at the
entry an air or oxygen intake line 519. At the outlet of the oxidation oven
520, an ingot collecting
line 521 may ensure return to the safe storing means of the anodic sludge
treatment unit 516. The
litharge feed line 505 is also connected to the outlet of the oxidation oven
520. A fumes collecting
line 522 is also provided at the oxidation oven 520, it may be connected
towards the same filtration
system as the one provided at the lead covering containers.
Method for treatiniz waste
An exemplary method for treating waste containing precious metals is described
hereinbelow, in the case when the waste is used electronic cards. In this
example, the method
comprises 5 main phases:
- milling;
- pyrolysis;
- copper extraction by selective dissolution (leaching);
- lead covering; and
- refining.
This example corresponds to the use of the treatment installation described
above in
connection with Figs. 1-6. Production capacity is of the order of 25,000 tons
per year or of the
order of 72 tons of waste per day. In the case when the waste is catalytic
mufflers, it is possible
to do without the pyrolysis and copper extraction steps.
The electronic cards are received at the means for receiving waste 101. The
electronic
cards arrive at the entry of the installation by batches (containers, big
bags, barrels), which are
weighed at the weighing means 102, labelled, recorded and stored at the
storage means 103.
The cards may arrive in three main forms:
CA 02666767 2009-04-17
13
1) entire cards requiring double milling before any treatment;
2) pre-milled cards requiring simple milling before any treatment, and
3) properly milled cards with a size less than 5 mm, not requiring any
additional milling
before treatment.
This is why with the transport system described above, the cards are directed
according to
their nature: either successively towards the coarse mill 109 and then the
fine mill 111 (case 1
above); or directly to the fine mill 111 (case 2 above); or directly to the
silo for storing waste 116
(case 3 above). The coarse mill 109 performs milling or grinding of the waste
reducing them to a
size of less than 25 mm, while the fine mill 111 performs milling or grinding
of the waste
reducing them to the required size of less than 5 mm. Moreover, before
entering the properly
milled cards into the silo for storing waste 116, the latter undergo automated
sampling at the
sampling means 113, which periodically interrupt the flow of cards. For
example, 300 kg of
sample per 24 t batch may be sampled. The sample is then analyzed by the
analyzing means 114
after quartering in the laboratory in order to achieve a final sample mass of
4-5 kg. It is preferred
to only treat a given batch of waste when the result of the analysis is known,
in order to adapt the
treatment parameters. This is why, before the sample analysis is carried out,
the cards return via
the return conveyor 119 to the storage means 103.
The premises of the mills 109, 111 are decontaminated, and the fines suspended
in air are
recovered and re-injected into the silo for storing waste 116.
The milled electronic cards are then extracted at the base of the silo for
storing waste 116
and feed the hoppers located above the entry of each of the three pyrolysis
ovens 202a, 202b,
202c. The bulk density of the product is 0.7 at the entry of the ovens.
Pyrolysis is useful for
degrading and removing the organic materials contained in the cards. This is a
controlled
combustion of carbonaceous chains, which is carried out while maintaining the
metals of the
waste in the metallic state.
The dwelling time in the ovens may be comprised between 20 and 90 min and is
preferentially 30 min. The operating temperature may be comprised between 350
and 550 C and
preferably have the value of about 400 C. By controlling the temperatures, the
negative pressure
and the screw velocity, it is possible to keep the operation under control.
Each oven typically has
a treatment capacity of 1 t/h. Pyrolysis gases rich in phenolic compounds
emerge at 400 C from
each oven.
The calcinated cards exiting each oven are cooled on the calcinated waste
recovery line
203 (jacketed conveyor) and are then stored in the silos 204a, 204b feeding
the copper leaching.
The product appears with a black appearance due to residual carbon from
pyrolysis of the
plastics. It has a density of about 0.5.
CA 02666767 2009-04-17
14
The pyrolysis gases from each oven are burnt at a high temperature in the post-
combustion
chamber 207a, 207b, 207c (dwelling time of 2 s) in order to destroy all the
carbonaceous
molecules and possible dioxins and furans. Thus, quasi the whole of the carbon
chains is
recovered in the form of energy which may be recovered and used in the actual
process. The
oxidizing air is preheated to 400 C in order to provide proper inflammation of
the gases. A
controlled air supplement is required for regulating the chamber exit
temperature to 1,100 C and
avoiding formation of NOX. A supplemental 800 kW burner ensures that the
temperature is
sufficient for the combustion to occur, notably during the transient phases.
Continuous control of
the entry and exit post-combustion temperatures is achieved and the incoming
dilution air may
be regulated.
The gases at 1,100 C arrive in the cooling chamber 210 in order to be
subjected therein to
water quenching. The coolant water is fed at a flow rate of 10 m3/hour. The
water is entirely
transformed into steam by absorbing a considerable portion of the energy of
the gases. The
cooled gases exit the chamber at about 200 C. By controlling the exit
temperature, it is possible
to regulate the injected water flow rate.
These gases are then finely cooled with air to about 150 C before entering the
sleeve filter
214. The sleeves retain the fine solid particles, notably containing halides.
Their purge is carried
out in the following sector of copper leaching. The totally stripped gases are
rejected into the
chimney 217. Continuous monitoring is carried out therein (analysis of the
gases, dust levels...).
As regards extraction of copper, the latter is carried out by means of a
sequence of
hydrometallurgical operations: leaching in an oxidizing acid medium,
filtration of the residue,
electrodeposition of the copper requiring the use of several reactors, storage
tanks, filters, pumps
and a set of electrolysis cells and of current generators.
The daily throughput of calcinated cards coming from the silos 204a, 204b
containing 12 t
of copper is treated in selective dissolution containers 301a, 301b (closed
reactors) in 11
leachings, each lasting 4 hours. The operation is as follows: transfer to the
pump of 15 m3 of
copper-depleted and acid-rich electrolyte (at 85 C) from the tank for storing
depleted electrolyte
318. One then proceeds by introducing 4.8 t of calcinated cards with fine
oxygen bubbling at the
bottom of the reactor. The depleted electrolyte is a solution containing
sulphuric acid (50-200
g/L, preferably about 100 g/L) and soluble iron as iron sulphate (5-20 g/L,
preferably about 10
g/L) which has to be maintained in the form of Fe3+ (with oxygen) for
efficiently etching the
copper.
Maintaining the temperature is provided by injecting fresh steam. Finally, the
contents of
the reactor are filtered on the press filter 306a or 306b or both. The copper-
rich juices are
transferred towards the tank for storing rich electrolyte 312 feeding the
electrolysis cells.
CA 02666767 2009-04-17
The electrolyte is enriched with iron and nickel which are dissolved at the
same time as
copper. A daily purge has to be carried out on the depleted electrolyte
exiting the electrolysis
cells. It is sent to the second tank for storing depleted electrolyte 319 and
its treatment is carried
out in the first stripping reactor 320 twice daily. These very acid juices
containing iron, nickel
5 and a little copper are treated with lime up to a pH of 8.5. Calcium
sulphate precipitates carrying
along metal hydroxides. This pulp is filtered on the additional press filter
324. The obtained
residue (10-15 t/d) is placed in a landfill site. The juices are recycled to
the first tank for storing
depleted electrolyte 318.
The fines of the filter of the pyrolysis are treated in the second stripping
reactor 325 every
10 2 days in the presence of water and some lime at pH 9. The halides (mainly
chlorides and
bromides) pass into the solution. The pulp is filtered on the additional press
filter 324: the
residue (500 kg) is recycled to the selective dissolution containers 301a,
301b and the juices (3
m3) enriched in halides are stored in a tank body for subsequent treatment.
The whole of the reactors, storage tanks, filters, are decontaminated and the
vapors and
15 droplets are absorbed by the washing tower 331. The obtained acid juices
are regularly purged
and recycled to the tank for storing depleted electrolyte 318.
In the process, the depleted electrolyte is raised to 85 C and maintained at
this temperature
by means of a coil fed with steam. The rich electrolyte is cooled to 50 C by a
coil fed with cold
water. This cold water may then be used in the chamber for vaporizing the hot
gases from the
post-combustion of the pyrolysis.
The wet solid residues (40 t/d) stemming from the press filter 306a and 306b
are rich in
precious metals. They are dried in the drying oven 308. They are powdery and
have a black
color, the glass fibers which are the main compound thereof being broken
during the stirring in
the etching tank. The solid and liquid flows are regularly sampled and
analyzed.
As regards the electrodeposition step, the flow stemming from the rectifiers,
passes in series
from electrolysis cell to electrolysis cell and in parallel at the electrodes
of each cell. Current
density may be from 50 to 400 A/mz, preferably about 200 A/m2 and the
temperature of the
electrolyte may be from 20 to 80 C, preferably from 45 to 50 C. The
concentration of ferric ions is
maintained as low as possible, and in any case at a level less than 10 g/L.
When the total iron
concentration reaches a value of 10-30 g/L, a portion of the electrolyte is
purified by precipitation
of iron and filtration of the precipitate.
The rich electrolyte coming from the tank for storing rich electrolyte 312 is
sent into the 1 st
row of eight cells. The cells are positioned as a cascade so as to allow
circulation of the electrolyte
and a pump sends back the juices from the last cell to the first. The
circulating flow rate is of the
order of 15 m3/h. The electrolyte takes 24 hrs for being depleted in copper
which is deposited on
the cathodes. By adding a surfactant, a fine and regular copper deposit may be
obtained. The
CA 02666767 2009-04-17
16
depleted electrolyte is pumped towards the tank for storing depleted
electrolyte 318. The cells are
then again filled with rich electrolyte. Each row may be emptied and then
filled with electrolyte
every 4.5-5 hrs.
Every 5 days, a row of cells is stripped. The recovered copper cathodes (12 t)
are rinsed
with water and the copper is separated from its stainless steel support by a
suitable mechanical
tool. The obtained copper is sampled and stored.
It should further be noted that the whole of the solid and liquid flows are
advantageously
regularly sampled and analyzed.
The step of selective copper extraction is important when the starting
material contains a
large copper proportion. Indeed, copper is able to form stable compounds which
are insoluble in
liquid lead, said compounds often containing precious metals. This is why it
is necessary to get
rid of the largest amount possible of copper before starting with the
following lead covering and
refining steps, otherwise a large amount of precious metals would be lost in
said stable
compounds.
Moreover, the selective copper extraction step enables quasi the whole of the
copper to be
selectively extracted as a commercial product (pure copper cathodes), which
may be re-melted as
ingots.
The metals dissolved in the electrolyte (iron, aluminium, nickel) may
advantageously be
removed during periodic operations for regenerating the electrolyte.
It should be noted that electrodeposition of copper may be replaced with an
operation of
copper sulphate crystallisation, which is a commercial product.
According to the type of waste, it is possible to do without either of the
pyrolysis and
copper extraction steps by selective dissolution or both. This is the case for
example when the
waste consists in catalytic mufflers. In this case, the carbon chain and
copper contents do not
justify the presence of both of these steps, and the milled used catalytic
mufflers are directly
subjected to the lead covering step.
The lead covering step comprises contacting the pre-treated materials (i.e.
after milling,
optional pyrolysis, optional copper extraction) with a molten lead-based
composition in the lead
covering container 15. The molten lead-based composition comprises lead in
majority and may
comprise from 0 to 50% tin, preferably less than 20% tin. This composition is
in the liquid state.
It is used as a collector and extractor of precious metals, which are found
solubilized in a
non-oxidized form. The lead and optionally the tin of this composition partly
stem from added
metals contained in electronic cards, and partly from metals recovered
subsequently.
Dissolution is carried out in the following way: stirring is started and it
generates a vortex
of molten lead in the kettle. The feeder 407 pours the materials to be covered
with lead into the
CA 02666767 2009-04-17
17
core of the vortex. The operation lasts for about 15 minutes. The temperature
may then be
comprised between 350 and 550 C and preferably is about 500 C.
A phase of skimming or phase of separating the elements without any affinity
for lead is
started subsequently. In this phase, stirring is stopped, and the inert
portions with their precious
metals (ceramics, glass fibers, ferrites...) having been washed away move up
to the surface
where they float. The skimming machine 405 is then started and with it the
supematant materials
may be recovered. When these supernatant materials have been removed from the
lead bath, the
operation is repeated. Skimming may be carried out at a temperature comprised
between 250 and
450 C, for example of about 270 C for a lead-tin alloy with 30% by weight of
tin.
The supernatant materials may again be treated in the same way in the
additional lead
covering container 18. Indeed, a small amount of lead (and of precious metals)
is carried away
with the supernatant materials during skimming, and it is therefore useful to
repeat the operation
in a second kettle in order to avoid loss of precious metals. The inert
materials collected upon
skimming at the additional lead covering material 18 are sent to a landfill as
ultimate waste after
having been optionally sampled and analyzed. The molten lead-based composition
contained in
the additional lead covering container 18 has a small concentration of
precious metals (less than
100 g per ton) and it is sent back by pumping towards the lead covering
container 15.
The lead covering phase may last for several days. It is considered that it is
completed
when the precious metal content in the molten lead-based composition reaches a
threshold value,
for example located between 2 and 4 kg per ton of lead.
One may then proceed with an optional decoppering operation, consisting of
adding sulphur
in the lead-based composition vortex, in order to form copper mattes which are
sent back into a
selective dissolution container 301a, 301b for extracting copper.
Next, the molten lead-based composition with the solubilized precious metals
is sent to a
storage kettle from which this composition is cast into anodes (this forms the
means for casting
anodes 25).
With the following Betts refining step, the precious metals contained in the
thereby cast
anodes may be released. During this step, lead and tin are removed from the
anodes by
electrolysis in a fluorosilicic medium, which is known as the Betts process,
at the Betts
electrolysis unit 501. The electrolysis cells are fed with an electrolyte for
example containing
about 90 g/L of lead and 80 g/L of free acid. This electrolyte is prepared by
solubilizing litharge
(PbO) in fluorosilicic acid at the Betts reactor 503. Electrolysis may be
carried out for example at
a current density of 350 A/m2 and at an electrolyte temperature of 40 C.
During the electrolysis,
the solid lead and tin of the anodes are dissolved in the electrolyte, while
the deposits of lead and
tin build up on the cathodes. By adding surfactant in the electrolyte, it is
possible to make this
deposit fine and smooth. The precious metals themselves remain at the anodes.
CA 02666767 2009-04-17
18
In this configuration, the electrolyte does not concentrate impurities or only
very little. A
total purge may only be carried out once or twice a year. In this case, the
electrolyte is sent to the
level of the second tank for storing depleted electrolyte 319 for a lime
treatment in the first
stripping reactor 320, and a new electrolyte is prepared. A partial purge may
be required for
lowering the lead content with sulphuric acid in the Betts reactor 503.
The reactor 503 and the electrolysis cells are decontaminated and the gas
effluents are sent
to the washing tower 508. The washing juices are treated in the first
stripping reactor 320 via the
second tank for storing depleted electrolyte 319.
In the example described herein, the two rows of five cells contain 60x5=300
anodes each
of 450 kg. Six days are required for consuming 80% of the anodes which
therefore release their
precious metals (432 kg) as anodic sludges, i.e. dissolved anode residues. The
anodic sludges
may, depending on the cases, fall into dust in baskets or have an adhering
cell structure.
Preferably electrolysis is interrupted before complete dissolution of the
anodes. Anodic sludges
may thereby be recovered by scraping.
The produced Ph/Sn cathodes represent 108 t for these six days.
Two strippings of cathodes are advantageously performed every three days and
two
scrapings of the anodes at the same time. The cathodes are recycled towards
the kettle 512 in
order to produce commercial lead-tin in ingots and/or for feeding the
additional lead covering
container 18 with a molten lead-based composition.
The anodic sludges are washed, weighed and stored in a safe. Advantageously,
the anodic
sludges are melted once to twice a week in the oxidation oven 520 at a
temperature of 1,000 C.
This step of melting anodic sludges, in the presence of oxygen gas (or air)
enables at least a
portion of the lead and of the tin to be oxidized, which were still contained
in the anodic sludges.
Litharge (PbO) forms at the surface: it is cast into plates and it may dope
the electrolyte with lead
when necessary. The fumes from the oven are channelled towards the lead
covering filter. The
liquid precious alloy may be cast into ingots (25 kg) which are stored
batchwise in a safe. All the
ingots are sampled and then weighed before being marketed.
The method described above is designed in order to limit as much as possible
the losses of
precious metals at all stages of the method. Namely:
- with the fines recovery line 122, the fine fragments of milled waste which
pass into
ambient air at the milling, may again be introduced into the system;
- with the fines withdrawal conduit 215, the metal fraction carried away with
the
carbonaceous gas during pyrolysis may be recovered;
- with the washed fines withdrawal line 327, it is possible to re-inject into
the main
circuit the fragments of materials to be treated carried away with the
electrolyte(s);
CA 02666767 2009-04-17
19
- by using an additional lead covering container 18 in addition to the lead
covering
container 15, precious metals inadvertently carried away with the inert
materials
during the main lead covering may be recovered;
- besides, the copper which was not extracted during the selective extraction
step by
dissolution is recovered as copper mattes at the lead covering container 15
and is re-
injected into the main circuit.
Thus, with the method, it is possible to recover in fine more than 90 or even
95% by
weight, preferably more than 99% by weight, advantageously more than 99.9% by
weight of the
precious metals initially contained in the waste.
Tables 1 and 2 below give an estimation of the chemical composition of the
products
during the different steps of the treatment method, in the case when the waste
is typical used
electronic cards.
Table 1- Chemical composition duriny, the method (lst part)
After lead
Raw After pyrolysis After Cu covering: liquid
waste extraction phase
Carbon chains 45% 6% 9.6% Traces
Glass fibers 23% 41.4% 66.2% Traces
Copper 17% 30.6% 0.2% 400 g/t
Lead 2% 3.6% 5.8% Phase
Tin 3% 5.4% 8.6% Phase
Iron, nickel 5% 9% 8% Traces
Aluminium 0.8% 1.4% Traces Traces
Silver 700 g/t 1.26 kg/t 2.6 kg/t 2.6%
Gold 200 g/t 360 g/t 580 g/t 5.8 kg/t
Palladium 100 g/t 180 g/t 290 g/t 2.9 kg/t
Table 2 - Chemical composition during the method (2 d part~
After lead After refining: After refining:
covering: residues anodic sludges cathode
Carbon chains 29% - -
Glass fibers 76.6% - -
Copper 0.2% 0.2% 400 g/t
Lead 2% 50% Phase
Tin 0.5% 10% Phase
Iron, nickel 8.8% - -
Silver 4 g/t 26% 20 g/t
Gold 1 g/t 5.8% 2 g/t
CA 02666767 2009-04-17
Palladium 0.5 g/t 2.9% 1 g/t