Note: Descriptions are shown in the official language in which they were submitted.
RECU 11/03/2009 20:21 00223388995 PT4
03/11/2009 15:14 FAX 819 953 9538 CIPO / PCT OFFICE Q003/024
PCT/CA2007/001932
02 September 2008 02-09-2008
COMPOSITION FOR THE TREATMENT OF SKIN CONDITIONS
F:ELD OF THE INVENTION
The present invention relates generally to a composition for topical
application that
comprises an extraction solution mixed with a pharmaceutically acceptable
carrier, wherein the
solution is produced by repeatedly filtering a liquid through a
filter/extraction colunrnn housing
crushed mollusk shell particles. This composition can be used for the
treatment of a variety of
skin conditions.
BACKGROUND OF THE INVENTION
The present invention is an improvement over Canadian Patent Application No.
2,566,562, wherein a process is disclosed for the preparation of a solution
for use in the treatment
o' skin diseases. Further developments have been revealed that outline the use
of the
w,'orementioned solution for the production of a new composition. For a ready
understanding of
the solution itself, and the process of manufacture therefor, the reader is
directed to Canadian
Patent Application No. 2,556,562.
Shells derived from mollusks are generated as industrial waste from fisheries
around the
world. It is common practice to dump the shell waste into the ocean. However,
shells have been
e:Y"iciently used as a source of calcium and for obtaining antibacterial
agents, as well as for
purifying water. It has been shown that the powder obtained from shells of
scallops, oysters,
c:ams, and other mollusks, or a solution containing the powder, has
antibacterial and antiviral
p.roperties, as well as a use as a water purifying agent. It has also been
found that the aforesaid
powder has demonstrated useful properties when applied as a deodorant for
sterilization, as a
preservative agent and for selected medicinal use.
Medicinal properties of mollusk shell powder and extract are well proven and
tested. For
example, European Patent Application No. 1676583 describes a therapeutic agent
for periodontal
disease that is prepared by utilizing a scallop shell material, and which has
antimicrobial activity
against periodontal disease-inducing bacteria. In particular, the shells are
crushed and are then
subjected to calcination, to convert the calcium carbonate in the original
shells to calcium oxide,
by firing at a high temperature. An aqueous solution is then formed with the
newly formed
calcium oxide powder and the original calcium carbonate powder.
I
A1ZlIDED 8E~8T
CA 02667903 2009-04-28
CA 02667903 2009-04-28
WO 2008/052326 PCT/CA2007/001932
U.S. Patent Application No. 2004/0028748 describes a remedy for
dermatophytosis,
obtained from a product formed by grinding shells having a crystalline
structure and forming an
aqueous dispersion of the product. In particular, the ground shells are
calcinated by heating the
particles in excess of 1000 C, and the aqueous solution is obtained by the use
of both the
originally ground shells and calcinated shells.
Japanese Patent Application No. 2004/256785 describes a soap which has an
auxiliary
therapeutic effect for relieving itching associated with atopic derrnatitis
and psoriasis. The
primary active ingredient in the soap is a fine powder prepared by high
temperature calcination
of scallop shells, oyster shells, clam shells and the like.
United States Patent No. 6,627,229 describes an antiviral agent produced by
applying a
heat treatment of between 700 C to 1200 C to the pulverized powder of a
calcium-containing
substance originating from animals, such as clamshell, crustacean shell, bone,
coral and pearl.
U.S. Patent Nos. 6,365,193 and 6,488,978 to Sasaki et al. disclose that burned
shells can
be used as antibacterial agents and water purifying agents. Sasaki et al.
disclose heating a shell
in an atmosphere of inactive gas and burning the shell. In particular, the
antibacterial agent is
obtained by burning a powder from the shell of a surf clam in an atmosphere of
inactive gas. The
powder can be easily dissolved into water and used as an antibacterial
solution.
The above uses of shells demonstrate the vast area of applications in which
shell
properties can be exploited. Further developments directed to new uses of
shell material are
desired and needed to manage and reduce the great quantity of shell waste
produced every year.
Most of the above-identified applications require the use of crushed shells or
a powder
obtained from the shells of mollusks, where in all instances, heat treatment
under high
temperatures are employed, in order to effect calcination upon the shell
particles. All of these
procedures consequently require expensive equipment and complex set-up
procedures to achieve
these high temperatures, when in fact, calcination may not be a necessary step
in order to reveal
the inherent medicinal properties of the shell particles.
Furthermore, a large proportion of therapeutic agents that are prescribed
and/or
recommended by physicians for skin conditions contain harsh chemicals, such as
lower
monohydric alcohols i.e. ethanol and isopropanol. However, the abundance of
these alcohols in
the therapeutic agents often contributes to drying and irritation of the skin
where the agent is
applied. The lack of these and other additives in the present invention will
minimize the adverse
2
RECU 11/03/2009 20:21 00223388995 PT4
03/11/2009 15:14 FAX 819 953 9538 CIPO / PCT OFFICE C7J004/024
PCT/CA2007/001932
30 Dece ber 2008 30-12-2008
effects associated with these chemicals.
SUMMARY OF THE INVENTION
It is an object of the present invention to provide a composition, comprising
an extract
from mollusk shells, containing mainly calcium, that is safe for the human
body and
environmentally friendly. Further, this composition inherits the
antibacterial, antiviral and
ancillary healing properties of the shells and as indicated heretofore may
effectively be
utilized for the treatment of sldn diseases. Additionalty, it is desired that
the composition
lOes not cause any significant drying or irritation of the skin.
Moreover, the composition and process of manafaotme thereof of the present
invention are simple, and as such, are economically advantageous. Therefore,
the
,:,omposition of the present invention can be available to the general public
at an affordable
-3rice.
According to an aspect of the present invention, there is provided a
composition for
topical application, comprising an exttaction solution mixed with a
pharmaceutically
acceptable catrier, wherein the solution is produced by repeatedly filtering a
liquid through a
i3lter/extrac6on column housing crushed mollusk shell particles.
According to another aspect of the present invention, there is provided a
composition
for topical application, comprising fine cnished mollusk shell particles mixed
with a
pharmaceutically acceptable carrier, the crushed molIusk shell particles being
boiled and/or
baked at a temperatire in the range of 100 C to about 300 C prior to placement
in the
column.
According to a further aspect of the present invention, there is provided a
composition
for topical application comprising dissolving fine crushed mollusk shell
particles into a
solution, the crushed mollusk shell particles being boiled and/or baked at a
temperature in the
range of 100 C to about 300 C prior to placement in the column.
BRIEF DESCRIPTZON OF T'1-IE DRAWINGS
The above and other features of the present invention will become more
apparent from
the appended drawings, wherein:
Figure 1 shows a cross-sectional view of the extraction column comprising an
inlet, an
outlet, and a housing member,
3
AIGUIDSD 8H&aT
CA 02667903 2009-04-28
RECU 11/03/2009 20:21 00223388995 PT4
03/11/2009 15:15 FAX 819 953 9538 CIPO / PCT OFFICE 1A005/024
BCT/CA2007/001932
30 Dsce ber 2008 30-12-2008
Figure 2a is an exploded cross-sectional view of the upper half of the
extraction
column and illustrates the empty column set-up;
Figare 2b is an exploded cross-sectional view of the upper half of the
extraction
column and illustiates the column filled with the packing member;
3a
Abff.tIDED SHEET
CA 02667903 2009-04-28
RECU 11/03/2009 20:21 00223388995 PT4
03/11/2009 15:15 FAX 819 953 9538 CIPO / PCT OFFICE lM006/024
~ _.. . ----- -------.-.~.-- --- ...... . . .... .._ . . -------- --_..... ..
.. - . .
PCT/CA2007/001932
02 9epte ber 2008 02-09-2006
= =
Figure 3 is an exploded cross-sectional view of the lower half of the
extraction column
and illustrates the empty column set-up;
Figure 4 illustrates a cross-sectional view of the apparatus comprising two
extraction
columns in a tandem arrangement;
Figure 5 illustrates the measured turbidity of the extraction solution with
exposure time;
Figure 6 illustrates the increase of the amount of calcium det.ermined in the
extraction
solution with exposure time;
Figure 7 illustrates the changes that occur in pH of the extraction solution
with exposure
time and also compares the effect utilizing different ratios of shell
particles to solvent has on pH;
Figure 8 illustrates the changes that occur in the conductivity of the
extraction solution
with exposure time and also compares the effect utilizing different ratios of
shell particles to
solvent has on conductivity;
Figure 9 illustrates the changes that occur of the amount of calcium recovered
in the
extraction solution with exposure time and also compares the effect utilizing
different ratios of
shell particles to solvent has on calcium.
DETAILED DESCRIPTION OF THE INVENTION
One component of an embodiment of the present invention is an extraction
solution that
is mixed with a pharmaceutically acceptable carrier. The process for
production of the extraction
solution is similar to that which is disclosed in the priority document of the
present application,
that being Canadian Patent Application No. 2,556,562, and is herein discussed
with reference to
the Figures.
Fig. 1 illustrates a filter/extraction column (10) comprising an inlet (11),
an outlet (13),
two screens (14), and a housing member (12). The housing member (12) defines a
passageway
(1 2a) that can be filled with crushed mollusk shells.
Fig. 2a illustrates an exploded view ofthe upper half ofthe filter/extraction
column (10),
comprising a top coupling (16), a screw-in stopper (15) and an outlet (13)
placed in the stopper
(15). A screen (14) may be placed at the top of the housing member (12) in
order to contain the
cnsshed mollusk shells in the filter/extraction column (10). The top coupling
(16) is fitted with
a coupling inside lip (17) in order to attach the screen (14) to the housing
member. An
interchangeable seal (18), such as silicon can be used to inhibit leaking of
the column.
4
ANMBD SHEET
CA 02667903 2009-04-28
RECU 11/03/2009 20:21 00223388995 PT4
03/11/2009 15:15 FAX 819 953 9538 CIPO / PCT OFFICE Q007/024
-:- _._..__.. ......._._._._.. _ ._ -.. . . .. .. _.._ .. _
PCT/CA2007/001932
02 8eptmober 2008 02-09-2008
Fig. 2b illustrates an exploded view ofthe lowerhalfofthe extraction column,
comprising
tue bottom coupling (16), stopper (15) and an inlet (11) placed in the stopper
(15). The bottom
coupling can also be fitted with the coupling inside lip (17) in order to
attach the screen (14) to
the housing member (12) so as to contain the crushed mollusk shells into the
filter/extraction
column. The inlet (11) is designed for connection to a plastic valve and a
pump used to feed the
column with a solvent. An interchangeable silicon seal (18) can be used to
avoid leaking of the
column.
Fig. 3 illustrates the filter/extraction column (10), comprising an inlet
(11), an outlet (13),
top and bottom identical screens (14), and a housing member (12). The housing
member (12)
contains crushed mollusk shell particles which may be of equal or different
sizes. In one
embodiment shown in Fig. 3 the housing member contains at the bottom portion
crushed shell
particles of sizes between 0.5 and 1 mm in diameter (20) and crushed shell
particles of sizes
between 3 and 4 mm in diameter (21) in the upper portion. However, the crushed
mollusk shells
can be comprised of particles of about equal size distributed evenly along the
passageway
according to the filter/extraction needs in one or a plurality of zones. A
zone is defined as
c.ontaining crushed shell particles of approximately the same diameter. The
packing of the
column with crushed shell particles can follow a regular distribution
according to a desired
gradient or an irregular distribution.
Fig. 4 illustrates a cross-sectional view of the apparatus (1) according to
the present
invention and comprises at least two filter/extraction columns (2,3), in a
tandem arrangement.
In the two-column apparatus (1) the,filter/extraction columns are arranged
linearly so that the
outlet (13) of the first column (2) is connected to the inlet (11) of the
second column (3) by a
connecting tube (23). The housing member (12) of the first column (2) can
contain at the bottom
thereof crushed shell particles of sizes between 0.5 and i mm in diameter
(20), and crushed shell
particles of sizes between 3 and 4 mm in diameter (21) thereafter. The housing
member (12) of
the second colunm (3) may contain crushed shell particles coated with iron
oxide or hematite
(24). It is believed that coating the crushed shell particles with iron oxide
or hematite may
substantially improve the purifying properties of the filter/extraction
columns by reducing the
metal content in the solution passed through the apparatus (1). Analysis of
the water passed
through the filter/extraction columns apparatus (1) showed nearly zero content
of aluminum and
arsenic in the extraction solution.
S
ALUlIDED 8A8ET
CA 02667903 2009-04-28
RECU 11/03/2009 20:21 00223388995 PT4
03/11/2009 15:15 FAX 819 953 9538 CIPO / PCT OFFICE R008/024
PCT/CA2007/001932
02 9ept ber 2008 02-09-2008
In the practice of the process of the present invention for production of an
extraction
solution, shells are collected directly off the fishing boat and put in tote
boxes for transportation
to the manufacturing plant. The sheils are then cleaned by electric drills and
wire brushes and
throughly washed with water under high pressure to ensure effective cleaning.
The cleaning and
washing steps may be followed by a cooling step in which the shells are left
on a wire rack for
an amount of time necessary to cool the shells to the ambient temperature. In
the next.step the
saells are then subjected to a heat treatment, by any means known to one
sldlled in the art, e.g.
boiling or baking, such that the shells reach a temperature in the range of
about 100 C to about
300 C. It is also contemplated within an aspect of the present invention that
the shells are
subjected to multiple heat treatments, such as boiling and baking, as long as
the shells reach the
aforesaid temperature range. The main purpose of the beat treatment is to
remove any remaining
mollusk tissue, to coerce the opening of the intrinsic pores of the shells,
and to further expose the
inner layer of the shells by eradicating the protective layer. This heat
treatment makes the inner
layer, which contains the majority of the active ingredients, more susceptible
to leaching when
used in the above-described apparatus.
At this point, the whole of the shells are crushed by any means known to one
skilled in
the art. The resulting particles are then separated and sorted according to
size by the use of
sieves, filters or the like. Scallop shell particles of a size about 1 mm to
about 6 mm in diameter
are preferably selected, more preferably is a size of about 2 mm to 5 mm in
diameter, and most
preferably is a size of about 3 mm to 4 mm in diameter. Although particles of
a size ranging from
aoout 10 m to about 25 mm may be utilized while still remaining within the
scope of the present
invention. These particles are then introduced into the filter/extraction
column. Alternatively,
particles of about the same diameter or a mixture of particles with a range of
different diameters
may be used in the filter/extraction column according to the present
invention.
The effectiveness of the extraction solution, and hence, the composition
itself, in the
tneatment of a variety of sldn conditions will be determined by several
factors including the pH
o-Fthe solvent used and the particle size of the crushed shells that form the
paclcing members of
the extraction column. Preferably, the solvent is water. More preferably, the
solvent is distilled
water or reverse osmosis purified water. By passing the water through the
filter/extraction
column (10) one can control, inter alia, the turbidity, the pH, the calcium
content, the
conductivity and the suspended solids ofthe extraction solution. The number
ofpassesi the water
flowrate and the ratio of water to shells in the column determine the
properties of the extraction
solution, and hence the composition itself. The particle diameter of the
crushed shells has been
6
AMEMED SHEET
CA 02667903 2009-04-28
RECU 11/03/2009 20:21 00223388995 PT4
03/11/2009 15:16 FAX 819 953 9538 CIPO / PCT OFFICE 1A009/024
-c-._... .-- ----- .. . -._...-------_- . -------.. -.- ... _ .. ---...... -
PCT/CA2007/001932
I T 02 eeptember 2008 02-09-2008
= . .
shown to be relatively inversely proportionate to the effectiveness of the
composition. For
example, it is preferred that the particle diameter of the crushed shell be
between 1 mm and 6
rnm, more preferably between 2 mm and 5 mm, and most preferably between 3 mm
and 4 nun,
in order to produce an extraction solution that is effective as a topical
composition when
combined with an acceptable carrier.
Fig. 5 illustrates the Turbidity vs. Exposure time as determined in Example 1
detailed
below. It will be noted that the measured turbidity of the extraction solution
decreases as it is
subject to exposure time to the filter/extraction column. Tbe turbidity was
measured after each
pass of solvent tiirough the extraction column. After an exposure time of 12
hours the measured
turbidity of the extraction solution was about 2.2 NTU. The above measurements
of turbidity are
correlated with the measurements of the amount of suspended solids in the
extraction solution
&fler each pass. Accordingly, as is shown in Table I of Exanzple 1 as time
passes the amount of
suspended solids decreases from 6 mg/L after the first pass of solvent through
the filter/extraction
column to about 0 mg/L after 12 hours.
Fig. 6 illustrates the amount of calcium measured after eacb pass of water
through the
extraction column versus the exposure time. The final extaction solution
contains approximately
121 times more calcium than the starting solvent. It is preferred that the
calcium content of the
extraction solution is higher than 14 mg/L, and more preferably is higher than
20 mg/L. Other
factors that can impact upon the effectiveness of the extraction solution, and
hence the
composition, are the pH and the turbidity of the extraction solution. In order
to optimize the
effectiveness of the extraction solution, while still making it tolerable to
topical application, the
pH of the extraction solution is preferably between 6.0 and 10.0, more
preferably between 6.5
and 9.0 and most preferably between 7.0 and 8Ø The turbidity should be less
than 6 NTU
iJnits.
According to an embodiment of the present invention, a composition is formed
when the
extraction solution, wherein the extraction solution is pmduced by repeatedly
filtering a liquid
through a filter/extraction column housing crushed mollusk shell particles,
after having attained
all of its desired properties through this filtration process, is in admixture
with a pharmaceutically
acceptable carrier. Specific methods or processes that are used to combine the
extraction solution
with the pharmaceutically acceptable carrier are not limited, and any sort of
mixing method
utilizing any sort of known mixing apparatus is contemplated within the scope
of the present
invention.
A preferred process of mixing the extraction solution with an acceptable
carrier comprises
7
A14MIDSD SHEET
CA 02667903 2009-04-28
RECU 11/03/2009 20:21 00223388995 PT4
03/11/2009 15:16 FAX 819 953 9538 CIPO / PCT OFFICE Z010/024
,.-.... .--.. --... -..- -------- -- ----- ---..---- -..--_-- _ ... .._....-..-
...
PCT/CA2007/001932
02 9epte ber 2008 02-09-2008
Ltilizing a common kitchen mixing apparatus. The extraction solution, in it's
entirety, is added
incrementally to half of the total weight of the acceptable carrier at the
beginning of the process.
After thorough mixing has occurred, the remaining carrier is added and
incorporated into the
composition. The benefit to this process is that the development of air
bubbles within the
composition is significantly reduced and it also helps to ensure constant
consistency of the
product.
The pharmaceutically acceptable carrier of the present invention can be, but
is not limited
to, a cream, a lotion, a gel, an ointment and a paste. Preferably, the carrier
is petrolatum, a base
emulsifying ointment or Eucerin. More preferably, the carrier is a base
emulsifying ointment
cr Eucerin"`''. In an alternate embodiment of the present invention, the
carrier may include any
combinations of the above-mentioned compounds.
According to an embodiment of the present invention, when the acceptable
carrier is
Eucerin~', the extraction solution is mixed with the Eucerin" at a ratio of
about 1:1 to about 7:1,
ty weight. Preferably, the ratio of extraction solution to Eucerin" is 7:1, by
weight. The upper
1 mit of these ratios is defined by the ability of the extraction solution to
be incorporated into the
F?ucerin. At the upper limit of the stated ratios, that being 7:1, the
Eucerin"" becomes
saturated, and subsequent addition of extraction solution is no longer
incorporated. Accordingly,
any ratio of extraction solution to Eucerin' that allows for supersaturation
of Eucerin" with
tie extraction solution resides within the scope of the present invention.
These ratios produce
a cream that is easy to apply onto the skin.
According to an embodiment of the present invention when the acceptable
carrier is a
tase emulsifying ointment, the extraction solution is mixed with the base
emulsifying ointment
at a ratio of about 0.5:1 to about 7:1, by weight. Preferably, the ratio of
extraction solution to
base emulsifying ointment is 5:1, by weight. The upper limit of these ratios
is defined by the
ability of the extraction solution to be incorporated into the base
emulsifying ointment. At the
upper limit of the stated ratios, that being 7:1, the base emulsifying
ointment becomes sahuated,
and subsequent addition ofextraction solution is no longer incorporated.
Accordingly, any ratio
of extraction solution to base emulsifying ointment that allows for the
supersaturation of base
emuisifying ointment with the extraction solution resides within the scope of
the present
invention. These ratios produce a cream that is smooth, easy to apply onto the
skin and appears
tt) be absorbed with ease through the pores of the skin.
8
AtMIDSD SHEET
CA 02667903 2009-04-28
RECU 11/03/2009 20:21 00223388995 PT4
03/11/2009 15:16 FAX 819 953 9538 CIPO / PCT OFFICE Q011/024
PCT/CA2007/001932
02 September 2008 02-09-2008,
According to another embodiment of the present invention, a composition is
formed when
fine crushed mollusk shell particles are added to a ph.arniaceutically
acceptable carrier. In the
present invention, "fine cnished mollusk shell particle" is defined as a
crushed mollusk shell
particle that is less than or equal to 250 m in diameter. More preferably, a
fine crushed mollusk
chell particle is less than or equal to 150 m in diameter. The
pharmaceutically acceptable
carrier is as defined above.
The fine crushed mollusk shell particles are prepared according to the crushed
mollusk
shell particles of the present invention. That is, after the shells have been
adequately cleaned,
the shells are then subjected to at least one heat treatfnent, by any means
known to one skilled
in the art, e.g. boiling and/or baking, such that the shells reach a
temperature in the range of about
11.00 C to about 300 C. Preferably, the shells are baked at about 300 C for 15
minutes and/or
are boiled at about 100 C for 15 minutes prior to placement in the column. The
shells are then
arushed by any means known to one skilled in the art. The resulting particles
are then separated
and sorted according to size by the use of sieves, filters or the like. It is
during this sorting step
that the fine crushed mollusk shell particles are identified. These fine
crushed mollusk shell
particles are then incorporated into a composition comprising a
pharmaceutically acceptable
c=.arrier. Preferably, the fine particles are incorporated into the
composition at about 0.01% to
0.5%, by weight. More preferably, the fine particles are incorporated into the
composition at
about 0.03%, by weight. The limitation in regard to the. addition of the fine
particles to the
composition is that even though the particles are fine, they are still quite
granular in nature.
Accordingly, if the fine particles are incorporated into the composition at a
higher percentage by
weight than the above-mentioned range, it has been found that the use of the
composition is
limited as it will become excessively abrasive and has an overwhelming
tendency to severely
dehydrate the sldn when applied.
According to a preferred embodiment of the present invention, a composition is
formed
when the extraction solution of the present invention is in admixture with a
phatmaceutically
acceptable carrier, and wherein fine crushed mollusk shell particles are
subsequently added to
this composition. Similar to the parameters listed above, when the carrier is
Eucerin", the ratio
of extraction solution to carrier is about 1:1 to about 7:1, by weight.
Preferably, the ratio of
extraction solution to EucerinT' is 7:1, by weight. When the carrier is the
base emulsifying
ointment, the ratio of extraction solution to carrier is about 0.5:1 to about
7:1, by weight.
Preferably, the ratio of extraction solution to base emulsifying ointment is
5:1, by weight. The
fine particles are identical to those defined above. Preferably, the fine
particles are incorporated
9
AIMIIDSD SBBET
CA 02667903 2009-04-28
RECU 11/03/2009 20:21 00223388995 PT4
03/11/2009 15:17 FAX 819 953 9538 CIPO / PCT OFFICE Q012/024
PCT/CA2007/001932
02 Septe¾ober 2008 02-09-2008
into the composition of the present embodiment at about 0.01% to 0.5%, by
weight. More
preferably, the fine particles are incorporated into the composition at about
0.03%, by weight.
The limitation in regard to the addition of the fine particles to the
composition is that even though
the particles are fine, they are still quite granular in nature. Accordingly,
if the fine particles are
incorporated into the composition at a higher percentage by weight than the
above-mentioned
range, it has been found that the use of the composition is limited as it will
become excessively
abrasive and has an overwhelming tendency to severely dehydrate the skin when
applied.
A further embodiment of the present invention is the use of any of the above-
described
compositions for topical application. Preferably, the use of any of the
compositions according
to the present invention is for the treatment of at least one skin condition.
More preferably, the
use of the compositions according to the present invention are for the
treatment of at least one
skin condition, where the at least one skin condition is selected from the
group consisting of
psoriasis, acne, herpes-zoster, skin diseases associated with varicella-zoster
virus, insect bites,
insect stings, blisters, xerodeima, burns, sunburns, rashes, athlete's foot,
eczema and dermatitis,
including contact dermatitis and atopic dermatitis.
According to yet another embodiment ofthe present invention, fine
crushedmollusk shell
particles are dissolved into an aqueous solution. Preferably, the fine
particles are incorporated
into the solution at about 5% to 20%, by weight. The aqueous solution may be
any solution that
is capable of dissolving the above-mentioned amount of fine crushed mollusk
shell particles,
provided that it is also safe for topical application. Preferably, the aqueous
solution is water or
the extraction solution. It is contemplated within an embodiment of the
present invention that
rhis solution that contains dissolved fine crushed mollusk shell particles can
be used for the
ireatment of at least one skin condition. Preferably, the at least one skin
condition is selected
:'rom the group consisting of hyperhidrosis and pitted keratolysis.
According to yet another embodiment of the present invention, the extraction
solution is
ireated with sodium hydroxide (NaOH). Preferably, the NaOH is introduced into
the extraction
solution of about 0.1% to 4%, by weight. Preferably, the extraction solution
has a calcium
concentration of about 30 mg/L to > 1000 mg/L. The addition of the NaOH, upon
being
=horoughly dispersed through the solution, will cause an increase in the pH of
the solution,
:wulting in the concomitant precipitation of some of the calcium. This
extraction solution,
:ncluding the precipitated calcium, may be used interchangeably, with any of
the extraction
,iolutions as described according to the compositions of the present
invention.
AtOIDBD 9HS&T
CA 02667903 2009-04-28
RECU 11/03/2009 20:21 00223388995 PT4
03/11/2009 15:17 FAX 819 953 9538 CIPO / PCT OFFICE IA013/024
PCT/CA2007/001932
02 Septe ber 2008 02-09-2008
. .
Example 1
An extraction column (10) was constructed with a 6 inch diameter 4 feet long
PVC pipe
with two couplings (16) closing each end as described in Figure 1. The top
coupling (16) had a
90 elbow screw-in with a 3/4 inch plastic tube connected thereto. The top
coupling (16) and
the plastic tube connected to it represent the outlet (13) according to the
present invention.
The bottom coupling (16) as constructed had a straight adapter screwed in the
coupling
stopper and connected to a plastic tube representing the inlet (11) according
to the present
invention. A 12 V, 360 gallon/hour pump was connected to the inlet of the
column (10) and was
used to pump distilled water through the column (10). The top and the bottom
of the column (10)
were designed in the same way except that the bottom coupling (16) had a 3/4
nipple with a 3/4
plastic tube connected to a plastic valve and the 12 V pump. Two screens (14)
as shown in Fig.
1 were used to keep the crushed scallop shells inside the passageway (12a).
The composition of the crushed shells was comprised of a mixture of 0.5 and 1
mm
e:iameter scallop shell particles. The mass of the smaller particles was
approximately 10 kg and
be column (10) was filled to within two inches of the screen (14) of the upper
coupling. 'ilie rest
of the column (10) was filled with larger diameter particles of 3 and 4 mm.
After the first pass of distilled water, the extraction solution had a high
suspended solids
concentration. The suspended solids concentrations were found to diminish with
exposure time.
Table I shows the suspended solids concentration in the solution as measured
by the Hach
Company DR- 2400 Spectrometer. This method of determining suspended solids is
a simple,
direct measurement which does not require the filtration or ignition/weighing
steps as do
gravimetric procedures. While the USEPA specifies the gravimetric method for
solids
determinations, this method is often used for checking in-plant processes.
Test results are
measured at 810 nm. This method is documented in the Hach Water Analysis
Handbook, method
8006 page 963.
The accuracy of the spectrometric method of measuring the suspended solids
concentration was compared against the gravimetric method as described in the
Hach Water
Analysis Handbook, method 8271 page 947. The mass of the aluminum dish was
measured with
E. Scientech 120 analytical balance to the nearest 1 mg. A 100 ml sample from
the solution was
taken in situ and placed into the aluminum dish. The dish with sample was
placed in a preheated
crven and evaporated at 103-105 C for approximately six hours. The disb was
then taken out of
the oven and allowed to cool at room temperature in a desiccator. The dish
with sample was then
taken out of the desiccator and mass measurements were effected to the nearest
0.1 mg with the
11
I-lGlIDBD SHEBT
CA 02667903 2009-04-28
RECU 11/03/2009 20:21 00223388995 PT4
03/11/2009 15:18 FAX 819 953 9538 CIPO / PCT OFFICE 1N14/024
PCT/CA2007/001932
02 eepte ber 2008 02-09-2008
Scientech 120 analytical balance. This was the first mass measurement of the
sample. The dish
and sample were put into the preheated oven again for a period of one hour and
mass
measurements were effected until the results did not differ by more than 0.4
mg. A second
lla
ANMOED 88E8T
CA 02667903 2009-04-28
CA 02667903 2009-04-28
WO 2008/052326 PCT/CA2007/001932
measurement of the mass was done in the same manner as above. Table 2 below
shows the
suspended solids concentration in the solution as measured by the gravimetric
method.
Total Solids Analysis
Table 2
Initial tray lst dried d dried weight Weight diff otal Solids
eight eight (g) g) 1 S` and 2na weight g/L
B (A) 0.4 m
ra 1 8.1982 8.2109 8.2106 0.0003 0.124
ra 2 8.2245 8.2395 8.2393 0.0002 0.147
Tray 3 8.31 8.3253 8.3250 0.0003 0.15
Tray 4 8.2486 8.2620 8.2620 0.0000 0.144
ra 5 8.2659 8.2805 8.2806 0.0001 0.147
Total Solids Calculations
Equation: mg/L Total solids= ( A - B) X 1000
Sample volume ml
Where:
A = Weight (mg) of sample + tray
B = Weight (mg) of dish
% Error of Results
% of error =(DhDl) x 100 0.15 mg - 0.124mg) x 100 = 0.5 % error
# of data points 5
Where:
Dh = Highest numerical data results obtained
D1= Lowest numerical data results obtained
Example 2
Two extraction columns (10) were constructed as indicated above in Example 1
(see Fig.
4). The two columns (10) were arranged in tandem with the outlet of the first
column (10)
directly connected to the inlet of the second column (10). A 12 V, 360
gallon/hour pump was
connected to the inlet of the first column (10) and was used to pump distilled
water through the
first and second column (10).
The packing member of the first column (10) was made of a mix of smaller 0.5
and 1 mm
in diameter scallop shell particles. The mass of the smaller particles was
about 10 kg while the
column should be filled two inches below the screen (14) of the upper coupling
(16). The rest
of the column (10) was filled with larger diameter particles of 3 and 4 mm as
described in
Example 1.
The packing member of the second column (10) was made of crushed scallop shell
12
RECU 11/03/2009 20:21 00223388995 PT4
03/11/2009 15:18 FAX 819 953 9538 CIPO / PCT OFFICE 9015/024
PCT/CA2007/001932
02 9epte ber 2008 02-09-2008
particles coated with iron oxide or hematite (Fe2O3). The coating of the
crushed shell particles
with iron oxide can be effected by any process Imown to a person skilled in
the art. In the present
invention, the coating of the scallop shells is effected by soaking the shells
in iron oxide or
hematite for 4 hours, then baldng the shells and solution for 4 hours at 200
C. The shells should
then be washed with distilled water and dried in an oven at 200 C for three
hours.
Contaminated water with a high content of aluminum and arsenic was passed
through the
apparatus comprising the two extraction columns arranged in tandem. It has
been found in,
practice that the aluminum and arsenic content of the resulting extraction
solution was reduced
to 0 m
Example 3
An extraction column (10) was constructed with a 5.08 cm diameter, 79 cm long
clear
acrylic pipe with two couplings (16) closing each end as descn'bed in Figure
1. The top coupling
(16) had a 90 elbow screw-in with a 3/4 inch plastic tube connected thereto.
The top coppling
(16) and the plastic tube connected to it represent the outlet (13) according
to the present
ilvention.
The bottom coupling (16) as constructed had a straight adapter screwed in the
coupling
(16) stopper and connected to a plastic tube representing the inlet (11)
according to the present
civention. A 12 V, 360 gallon/hour pump was connected to the inlet of the
column (10) and was
used to pump distilled water or reverse osmosis-derived water, through the
column (10). The top
and the bottom of the column (10) were designed in the same way except that
the bottom
coupling (I6) had a 3/4 nipple with a 3/4 plastic tube connected to a plastic
valve and the 12 V
pump. Two screens (14) as shown in Fig. 1 were used to keep the crushed
scallop shells inside
tae passageway (12a).
Scallop shells were washed and cleaned with distilled water and then dried
such that the
shells reached a temperature of 100 T. The shells were then crushed, and after
sorting based
i:pon size, the composition utilized was comprised of a mixture of 3 and 4 mm
diameter scallop
shell particles. The volume and type of the solvent loaded onto the column
(10) was 1 L ofwater.
Two distinct scenarios were tested in regard to the amount of crushed shells
in order to
optintize running conditions. The amount of shells that was utilized was
either A) about 0.35 lb,
providing for a ratio of water to scallop shells of about 1 to 0.35 (w/w), or
B) about 1 lb,
providing for a ratio of water to scallop shells of about I to 1(w/w). The
column was filled to
within two inches of the screen (14) ofthe upper coupling (16). The water was
then successively
13
A1UtIDED SHEET
CA 02667903 2009-04-28
RECU 11/03/2009 20:21 00223388995 PT4
03/11/2009 15:18 FAX 819 953 9538 CIPO / PCT OFFICE Q016/024
PCT/CA2007/001932
02 9epte ber 2008 02-09-2008
rin through the column. In both scenarios, the solvent outflow was about 2.16
L/minute.
When the resulting extraction solution was analyzed, there was some variation
in the
measured parameters according to which ratio of scallop shells to water was
utilized. As can be
seen from Fig. 7, pH of the solutions are fairly close in value.
Referring now to Fig. 8, the conductivity, measured in micro siemens, is
presented for
both of the above-mentioned scenarios. The latter scenario (B) where a greater
proportion of
shells are utilized produced a higher conductivity. The maximum conductivity
attained over the
observed time frame for both scenarios was (A) 58.9 lr,s/cm and (B) 95.8
s/cm.
In order to understand the relationship between the two different scenarios
and
conductivity, calcium concentration was measured. As can be seen from Fig. 9,
the latter
scenario (B) contained a considerably higher calcium concentration than the
former scenario (A).
After the final pass of the solution from scenario A through the column, the
solution contained
2Ø83 times more calcium than the initial solution, whereas after the final
pass of the solution
from scenario B through the column (10), the solution contained 32.5 times
more calcium than
the initial solution.
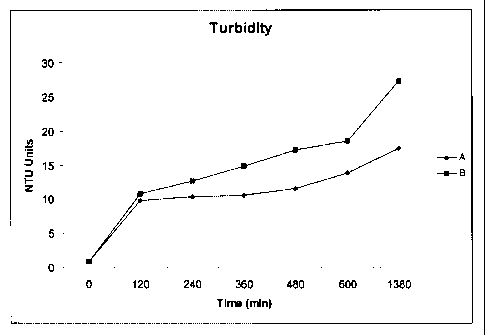
Referring now to Figure 10, the turbidity, measured in NTU Units, is presented
for both
af the above-mentioned scenarios. There is a general overall decrease in
measured turbidity over
time, however, there is very little difference, if any; when a comparison is
made between the
solutions that were made with different ratios of shell particles to solvent.
Both scenarios were
able to achieve values of less than one NTU Unit.
This series of Examples illustrates that the extraction of calcium from the
crushed shell
particles continues to be more efficient in accordance with an increase in the
ratio of crushed
shell particles to solvent. It should be noted that ratios of crushed shell
particles to solvent other
than those listed here are all contemplated within the scope ofthe invention,
and should be based
upon the desired properties of the resulting extraction solution.
The embodiments of the invention described herein are exemplary and numerous
modifications, variations and rearrangements can be readily envisioned to
achieve substantially
equivalent results, all of which are intended to be embraced within the spirit
and scope of the
invention.
14
AIffiID8D SSBET
CA 02667903 2009-04-28
RECU 11/03/2009 20:21 00223388995 PT4
03/11/2009 15:18 FAX 819 953 9538 CIPO / PCT OFFICE Q017/024
PCT/CA2007/001932
02 8epte ber 2008 02-09-2008
INDUSTRIAL APPLICABILITY
The invention provides a topically applied composition for the treatment of
various skin
conditions, where an extraction solution is prepared by repeatedly filtering a
liquid through a
filter/extraction column housing crushed mollusk shell particles and is then
in admixture with a
pAatmaceutically acceptable carrier. The resulting composition has numerous
properties. In
particular, this composition can be effectively implemented as treatment for a
plethora of skin-
associated maladies. Further, the present invention provides an application
for an industrial
w-aste product, mollusk shells, that are generated in large quantities from
fisheries around the
world, and it is believed that it would be fiscally and environmentally sound
to implement uses
of this waste product that is beneficial to the public.
{
AMMIDIM SHEET
CA 02667903 2009-04-28