Note: Descriptions are shown in the official language in which they were submitted.
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
1
EMISSION TREATMENT SYSTEMS AND METHODS
CROSS REFERENCE TO RELATED APPLICATION
[0001] This application claims priority to U.S. Provisional
Patent Application Ser. No. 60/868,289, filed on December 1,
2006, U.S. Provisional Patent Application Ser. No. 60/868,293,
filed December 1, 2006, U.S. Provisional Patent Application
Ser. No. 60/870,323, filed December 15, 2006, and U.S. Patent
Application Ser. No. 11/947,409 filed November 29, 2007, the
disclosures of which are hereby incorporated by reference in
their entirety.
FIELD OF THE INVENTION
[0002] Embodiments of the invention relate generally to diesel
exhaust treatment filters, systems, and methods. More
particularly, embodiments of the present invention pertain to
diesel exhaust treatment systems and methods that include
coated particulate filters coated with an oxidation catalyst.
BACKGROUND
[0003] Compression ignition diesel engines have great utility
and advantage as vehicle power trains because of their inherent
fuel economy and high torque at low speed. Diesel engines run
at a high air to fuel (A/F) ratio under very fuel lean
conditions. Because of this, they have very low emissions of
gas phase hydrocarbons and carbon monoxide. However, diesel
exhaust is characterized by relatively high emissions of
nitrogen oxides (NOX) and particulates. Diesel engine exhaust
is a heterogeneous mixture which contains not only gaseous
emissions such as carbon monoxide ("CO"), unburned hydrocarbons
("HC") and nitrogen oxides ("NOX"), but also condensed phase
materials (liquids and solids) which constitute the so-called
particulates or particulate matter. Emissions treatment
systems for diesel engines must treat all of the components of
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
2
the exhaust to meet emissions standards set by various
regulatory agencies throughout the world.
[0004] The total particulate matter emissions of diesel exhaust
contain three main components. One component is the solid, dry,
carbonaceous fraction or soot fraction. This dry carbonaceous
fraction contributes to the visible soot emissions commonly
associated with diesel exhaust. A second component of the
particulate matter is the soluble organic fraction ("SOF").
The SOF can exist in diesel exhaust either as a vapor or as an
aerosol (fine droplets of liquid condensate) depending on the
temperature of the diesel exhaust. It is generally present as
condensed liquids at the standard particulate collection
temperature of 52 C in diluted exhaust, as prescribed by a
standard measurement test, such as the U.S. Heavy Duty
Transient Federal Test Procedure. These liquids arise from two
sources: (1) lubricating oil swept from the cylinder walls of
the engine each time the pistons go up and down; and (2)
unburned or partially burned diesel fuel. The third component
of the particulate matter is the so-called sulfate fraction,
which is formed from small quantities of sulfur components
present in the diesel fuel.
[0005] Catalyst compositions and substrates on which the
compositions are disposed are typically provided in diesel
engine exhaust systems to convert certain or all of these
exhaust components to innocuous components. For instance,
oxidation catalysts, which may be referred to as diesel
oxidation catalysts (DOCs), containing platinum group metals,
base metals and combinations thereof, facilitate the treatment
of diesel engine exhaust by promoting the conversion of both
unburned hydrocarbons (HC) and carbon monoxide (CO) gaseous
pollutants, and some proportion of the particulate matter
through oxidation of these pollutants to carbon dioxide and
water. Such catalysts have generally been disposed on various
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
3
substrates (e.g., honeycomb flow through monolith substrates),
which are placed in the exhaust of diesel engines to treat the
exhaust before it vents to the atmosphere. Certain oxidation
catalysts also promote the oxidation of NO to N02.
[0006] In addition to the use of oxidation catalysts, diesel
particulate filters are used to achieve high particulate matter
reduction in diesel emissions treatment systems. Typical
ceramic wall flow filter substrates are composed of refractory
materials such as cordierite or silicon-carbide. Wall flow
substrates are particularly useful to filter particulate matter
from diesel engine exhaust gases. A common construction is a
multi-passage honeycomb structure having the ends of alternate
passages on the inlet and outlet sides of the honeycomb
structure plugged. This construction results in a
checkerboard-type pattern on either end. Passages plugged on
the inlet axial end are open on the outlet axial end. This
permits the exhaust gas with the entrained particulate matter
to enter the open inlet passages, flow through the porous
internal walls and exit through the channels having open outlet
axial ends. The particulate matter is thereby filtered on to
the internal walls of the substrate. The gas pressure forces
the exhaust gas through the porous structural walls into the
channels closed at the upstream axial end and open at the
downstream axial end. The accumulating particles will increase
the back pressure from the filter on the engine. Thus, the
accumulating particles have to be continuously or periodically
burned out of the filter to maintain an acceptable back
pressure.
[0007] Catalyst compositions deposited along the internal walls
of the wall flow substrate assist in the regeneration of the
filter substrates by promoting the combustion of the
accumulated particulate matter. The combustion of the
accumulated particulate matter restores acceptable back
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
4
pressures within the exhaust system. Soot combustion can be
passive (e.g., with catalyst on the wall flow filter and
adequately high exhaust temperatures), though for many
applications active soot combustion is also required (e.g.,
production of a high temperature exotherm in the exhaust up-
stream of the filter). Both processes utilize an oxidant such
as 02 or N02 to combust the particulate matter.
[0008] Passive regeneration processes combust the particulate
matter at temperatures within the normal operating range of the
diesel exhaust system. Preferably, the oxidant used in the
regeneration process is N02 since the soot fraction combusts at
much lower temperatures than those needed when 02 serves as the
oxidant. While 02 is readily available from the atmosphere, N02
can be generated through the use of upstream oxidation
catalysts to oxidize NO in the exhaust stream. An example of a
passive regeneration process is disclosed in United States
Patent Nos. 6,753,294 and 7,097,817.
[0009] Active regeneration processes are generally needed to
clear out the accumulated particulate matter, and restore
acceptable back pressures within the filter. The soot fraction
of the particulate matter generally requires temperatures in
excess of 500 C to burn under oxygen rich (lean) conditions,
which are higher temperatures than those typically present in
diesel exhaust. Active regeneration processes are normally
initiated by altering the engine management to raise
temperatures in front of the filter up to 500-630 C.
Depending on driving mode, high exotherms can occur inside the
filter when the cooling during regeneration is not sufficient
(low speed/low load or idle driving mode). Such exotherms may
exceed 800 C or more within the filter. One common way that
has been developed to accomplish active regeneration is the
introduction of a combustible material (e.g., diesel fuel) into
the exhaust and burning it across a flow-thru diesel oxidation
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
catalyst (DOC) mounted up-stream of the filter. The exotherm
from this auxiliary combustion provides the sensible heat (e.g.
about 500-700 C) needed to burn soot from the filter in a
short period of time (e.g. about 2-20 min.).
5 [0010] An example of a system is shown in United States
patent no. 6,928,806. The DOC functions during the active
regeneration mode to light-off and burn fuel injected into the
low temperature (e.g., about 250-300 C) exhaust (directly or
via the engine) and thereby produce an exotherm to heat the
exhaust entering the particulate filter to the temperatures
required (about 500-650 C) to combust accumulated soot from
the filter, thereby regenerating the filter to reduce the
operating pressure drop across the filter associated with the
soot accumulation.
[0011] High material costs associated with platinum group
metal-containing compositions augment the need to slow or
prevent the degradation of catalyst coatings due to active
regeneration events. Catalyst coatings disposed on wall flow
filters often contain platinum group metal components as active
catalyst components to ensure acceptable conversions of the
gaseous emissions (HC, CO) of the diesel exhaust to innocuous
components (e.g., C02r H20) The loadings of such components
are generally adjusted so that the catalyst substrate meets
emissions regulations even after catalyst aging. Consequently,
coating designs that maximize the efficiency and durability of
platinum group metal usage along the substrate are desirable.
[0012] Certain conventional coating designs for wall flow
substrates have a homogeneous distribution of catalyst coating
along the entire axial length of the internal walls. In such
designs the platinum group metal concentrations are typically
adjusted to meet the emissions requirements under the most
stringent conditions. Most often such conditions refer to the
catalyst's performance after the catalyst has aged. The cost
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
6
associated with the required platinum group metal concentration
is often higher than is desired.
[0013] As can be appreciated from the above, current
particulate filter systems pose a number of issues concerning
precious metals material costs and degradation of the catalyst
on the particulate filter due to exposure to high temperatures.
Accordingly, it would be desirable to provide alternatives
diesel engine in exhaust treatment systems and methods that
alleviate one or more of these issues.
SUMMARY
[0014] According to an embodiment of the invention, an
emission treatment system for treatment of an exhaust stream
comprising NOx and particulate matter is provided, which
comprises a particulate filter having an axial length and
elements for trapping particulate matter contained in an
exhaust stream flowing through the filter and a light-off
oxidation catalyst composition extending from the inlet end
towards the outlet end to a length that is less than the axial
length of the walls to provide an inlet zone in an amount
sufficient to light-off at a temperature less than about 300 C
and generate an exotherm to burn soot trapped in the filter.;
and a NOx reducing catalyst located upstream of the wall flow
monolith. In certain embodiments, the system may include an NH3
destruction catalyst located downstream from the NOx reducing
catalyst. In one embodiment, the particulate filter may
comprise a wall flow monolith disposed within the exhaust
stream and having a plurality of longitudinally extending
passages bounded by longitudinally extending walls, the
passages comprising inlet passages having an open inlet end and
a closed outlet end, and outlet passages having a closed inlet
end and an open outlet end, the walls having a porosity of at
least 40% with an average pore size of at least 5 microns and
the wall flow monolith comprising a light-off oxidation
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
7
catalyst composition permeating the walls and extending from
the inlet end towards the outlet end to a length that is less
than the axial length of the walls to provide an inlet zone.
[0015] In one or more embodiments, the NOx reducing catalyst
comprises a lean NOx catalyst. In embodiments including a lean
NOx catalyst, the system may further comprise a reductant
introduction port in fluid communication with a hydrocarbon
reductant, the reductant introduction port located upstream
from the lean NOX catalyst. The lean NOx catalyst can also be
incorporated into the oxidation catalyst/particulate filter by
zoning or layering. In other embodiments, the NOx reducing
catalyst comprises a lean NOX trap.
[0016] In one or more embodiments, the NOx reducing catalyst
comprises an SCR catalyst. In one or more embodiments
including an SCR catalyst, the system may include an optional
introduction port located upstream from the SCR catalyst, the
introduction port is in fluid communication with an ammonia or
ammonia precursor. The system may further include an injector
in fluid communication with the introduction port, the injector
configured to periodically meter the ammonia or an ammonia
precursor into the exhaust stream.
[0017] In one or more embodiments, the system may further
include an NH3 destruction catalyst located downstream from the
SCR catalyst. In certain embodiments, the NH3 destruction
catalyst may be coated on the particulate filter. The system
may also include an exotherm-producing agent introduction port
located upstream of the wall flow monolith, the exotherm-
producing agent introduction port in fluid communication with
an exotherm-producing agent capable of generating a temperature
sufficient to periodically burn particulate accumulated in the
wall-flow monolith. The exotherm-producing agent may comprise
a fuel such as diesel fuel.
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
8
[0018] Another embodiment of the invention pertains to a
method of treating an exhaust stream from a diesel engine. In
one embodiment, the method comprises disposing within the
exhaust stream containing particulate matter a wall flow
monolith and having a plurality of longitudinally extending
passages bounded by longitudinally extending walls, the
passages comprising inlet passages having an open inlet end and
a closed outlet end, and outlet passages having a closed inlet
end and an open outlet end, the walls having a porosity of at
least 40% with an average pore size of at least 5 microns and
the wall flow monolith comprising a light-off oxidation
catalyst composition permeating the walls and extending from
the inlet end towards the outlet end to a length that is less
than the axial length of the walls to provide an inlet zone.
The method may further comprise disposing a NOx reducing
catalyst upstream of the wall flow monolith and periodically
introducing an exotherm-producing agent upstream of the wall
flow monolith to generate an exotherm in the wall flow monolith
sufficient to combust particulate matter trapped within the
wall flow monolith.
BRIEF DESCRIPTION OF THE DRAWINGS
[0019] Figure 1A is a schematic illustration of an emission
treatment system in accordance with an embodiment of the
invention;
[0020] Figure 1B is a schematic illustration of an emission
treatment system in accordance with another embodiment of the
invention;
[0021] Figure 1C is a schematic illustration of an emission
treatment system in accordance with another embodiment of the
invention;
[0022] Figure 2 is a perspective view of a wall flow filter
substrate;
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
9
[0023] Figure 3 is a section view of a wall flow filter
substrate;
[0024] Figure 4 shows an embodiment of a system including
urea reservoir and injector;
[0025] Figure 5 is a graph showing particulate filter out
exhaust gas temperature as a function of test run time with
supplemental diesel fuel injected into the exhaust up stream of
the particulate filter;
[0026] Figure 6 is a graph showing particulate filter out
exhaust gas temperature as a function of supplemental diesel
fuel injected into the exhaust up stream of the particulate
filter;
[0027] Figure 7 is a diagram showing the location of
thermocouples installed within the particulate filter substrate
for measurement of internal temperatures;
[0028] Figure 8 is a graph showing internal particulate
filter temperatures as a function of location in the
particulate filter, plus inlet and outlet exhaust gas
temperatures, during a fuel light-off test;
[0029] Figure 9 is a graph showing exhaust temperatures and
Delta P across a particulate filter during an active
regeneration test with soot loaded in the particulate filter;
and
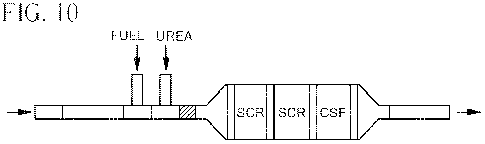
[0030] Figure 10 is a schematic view of a system used in
Example 5.
DETAILED DESCRIPTION
DEFINITIONS
[0031] The following terms shall have the meanings set for
below according to embodiments of the invention:
[0032] "Activated alumina" has its usual meaning of a high
BET surface area alumina, comprising one or more of gamma-,
theta- and delta aluminas.
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
[0033] "BET surface area" has its usual meaning of referring
to the Brunauer, Emmett, Teller method for determining surface
area by N2 absorption. Unless otherwise specifically stated,
all references herein to the surface area of the catalyst
5 support components or other catalyst components means the BET
surface area.
[0034] "Bulk form," when used to describe the physical form
of a material (e.g., ceria), means the material is present as
discrete particles that can be as small as 1 to 15 microns in
10 diameter or smaller, as opposed to having been dispersed in
solution onto another material such as gamma alumina. By way
of example, in some embodiments of the invention, particles of
ceria are admixed with particles of gamma alumina so that ceria
is present in bulk form, as opposed to, for example,
impregnating alumina particles with aqueous solutions of ceria
precursors which upon calcination are converted to ceria
disposed on the alumina particles.
[0035] "Cerium component" means one or more oxides of cerium
(e.g., CeO2) .
[0036] "Downstream" and "Upstream," when used to describe an
article, catalyst substrate or zone, refer to the relative
positions in the exhaust system as sensed in the direction of
the flow of the exhaust gas stream.
[0037] "High surface area support" means support materials
with a BET surface area that is approximately greater than 10
m2/g, preferably greater than 150 m2/g.
[0038] "Platinum group metal component" or "PGM" refers to
the platinum group metals or oxides thereof. Preferred platinum
group metal components are platinum, palladium, rhodium iridium
components, and combinations thereof.
[0039] "Diesel oxidation catalyst" or "DOC" refers to a
catalyst promoting oxidation processes in diesel exhaust, to
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
11
reduce emissions of the organic fraction of diesel
particulates, gas-phase hydrocarbons, and/or carbon monoxide.
[0040] "Active regeneration" refers to the introduction of a
combustible material (e.g., diesel fuel) into the exhaust and
burning it across an oxidation catalyst to generate an exotherm
from that provides heat (e.g. about 500-700 C) needed to burn
particulate matter such as soot from the filter.
[0041] An ammonia destruction catalyst or AMOX refers to a
catalyst that promotes the oxidation of NH3 to ideally nitrogen
but in general to a mixture of nitrogen NOx and N20.
[0042] "Particulate filter" is a filter designed to remove
particulate matter from an exhaust gas stream such as soot, and
particulate filters include, but are not limited to honeycomb
wall flow filters, partial filtration filter, a wire mesh
filter, wound fiber filters, sintered metal filters; and foam
filters.
[0043] Before describing several exemplary embodiments of
the invention, it is to be understood that the invention is not
limited to the details of construction or process steps set
forth in the following description. The invention is capable
of other embodiments and of being practiced or being carried
out in various ways.
[0044] According to one or more embodiments of the
invention, a separate, upstream light-off oxidation catalyst is
eliminated from a diesel engine emission treatment system and
incorporated directly onto a particulate filter itself by
placing light-off oxidation catalyst components at the inlet
end of the filter channels extending an adequate length from
the inlet end towards the outlet end of the filter. In this
way, during active regeneration, the introduced combustible
fuel is lit-off and burnt on the inlet end of the filter, thus
producing the necessary exotherm at a temperature of about 500-
700 C within the filter to combust accumulated soot in the
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
12
filter. In one or more embodiments, it may be desirable to
provide an auxiliary oxidation catalyst to oxidize a portion of
the NOx to N02.
[0045] According to embodiments of the invention, active
regeneration can be accomplished by or on the filter alone, and
the need for a separate light-off oxidation catalyst in the
system is eliminated. Eliminating a component from the system
provides a benefit of eliminating a substrate and associated
canning of the system. In turn, this elimination of a separate
component reduces overall system volume, and potentially
reduces the amount of expensive precious group metal (PGM)
loading required for the system. Furthermore, providing an
integrated light off oxidation catalyst on the particulate
filter reduces the overall system back-pressure on the engine,
which is associated with fuel consumption. In addition, in
systems that include a NOX reducing catalyst, for example an SCR
catalyst or lean NOx catalyst downstream from the integrated
light-off oxidation catalyst/particulate filter provides a
greater amount heat for these downstream devices compared to
systems in which the light-off oxidation catalyst and
particulate soot filter are provided as separate components.
The integrated light-off oxidation catalyst/particulate filter
can be moved closer to the engine. Reducing the size of the
system by integrating the oxidation catalyst and soot filter
reduces the heat loss from the particulate filtering sub-
system, thereby allowing any downstream component to operate at
higher temperature. Higher temperatures generally result in
higher catalytic activity, and therefore, integrating the
oxidation catalyst in the particulate filter will likely result
in better performance of the NOx removal components downstream
of the particulate filtering sub-system.
[0046] Furthermore, by disposing a NOx reducing catalyst
upstream of the oxidation catalyst/particulate filter,
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
13
hydrocarbons can be supplied to the NOx reducing catalyst,
eliminating the need to supply a separate reductant source. In
addition, a single introduction port or injector can be used to
supply an auxiliary reductant, for example, urea, and the fuel
to perform the burning of the soot in the particulate filter.
In addition, an ammonia destruction catalyst can be integrated
onto the outlet end of the substrate having the SCR catalyst,
which would eliminate the need for a separate ammonia
destruction catalyst. A further benefit of placing the
nitrogen reducing catalyst upstream of the particulate filter
is that the nitrogen reducing catalyst is not exposed to the
extreme temperatures associated with the active regeneration of
the particulate filter. Furthermore, when an SCR is used as
the NOx reducing catalyst, this enables a broader range of
materials to be used for the SCR catalyst composition. For
example, vanadium materials can be used in place of or together
with zeolites, to reduce the cost of the SCR catalyst.
[0047] When the NOx reducing catalyst uses NH3 or an NH3
precursor as the reducing agent, a separate injector can be
provided upstream of the SCR catalyst. With the fuel addition
point (for filter regeneration) provided downstream of the SCR
catalyst, the nitrogen reducing catalyst is not exposed to the
extreme temperatures associated with the active regeneration of
the particulate filter. The absence of high temperature
exposure caused by forced filter regenerations allows a smaller
SCR catalyst volume with corresponding cost saving and
packaging advantages. Further, the absence of the high
temperature exposure enables a broader range of materials to be
used for the SCR catalyst composition. For example, vanadium
materials can be used in place of or together with zeolites, to
reduce the cost of the SCR catalyst and improve its
effectiveness. In addition, an ammonia destruction catalyst can
be integrated onto the outlet end of the substrate having the
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
14
SCR catalyst, which would eliminate the need for a separate
ammonia destruction catalyst. In a further system optimization,
the NH3 destruction catalyst can be integrated into the soot
filter either as zone or a uniform coating. By doing so, the
overall system volume is reduced with a corresponding cost and
packaging advantages.
[0048] Integration of the light-off/oxidation catalyst
function and particulate removal functions into a single
catalyst article is accomplished using a wall flow substrate
coated with a light-off oxidation catalyst composition. The
light-off/oxidation catalyst composition contains a sufficient
loading of precious group metal composition to achieve light-
off at a temperature less than about 300 C (e.g, from about
2200 C to 300 C).to generate an exotherm to burn soot
collected in the filter. Temperatures generated by the
exotherm typically are between about 500 C and 700 C.
Although there may be a number of ways to incorporate the
light-off/burning function onto the particulate filter itself,
one method would be to apply this function to the particulate
filter as a zone of catalyst on the up-stream, inlet end of the
particulate filter substrate (e.g. honeycomb, wall-flow filter
substrate). This inlet catalytic zone which will be exposed to
relatively low exhaust temperatures 220-300 C) and will have
to have a high enough catalytic activity to accomplish the
light-off, plus reasonably complete combustion of the injected
fuel to produce the high temperatures, for example, about 500-
700 C required for filter regeneration. Although there are a
variety of catalyst compositions that can accomplish this, an
exemplary composition would be comprised of precious group
metals (PGM's) dispersed on a suitable support and at a loading
level suitable to light-off and burn the injected fuel., and is
described in more detail below. The inlet zone will typically
extend at least 10% of the axial length of the filter, and in
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
various embodiments, the inlet zone extends at least about 20%,
25%, 30%, 35%, 40%, 45%, 50% or up to about 75% of the axial
length of the filter. The inlet zone may be directly on the
walls of the filter, or the inlet zone may be formed over a
5 catalytic coating that extends the entire axial length of the
filter. The underlying catalytic coating may be a base metal
oxide such as an oxide of copper, cobalt, chromium, cerium,
etc. or a precious group metal composition. In embodiments in
which the underlying coating is a precious group metal
10 composition, the loading is typically less than or equal to 10
g/ft3. Thus, the particulate filter may have a catalytic
coating of platinum or other suitable precious group metal at a
loading of 10 g/ft3 covering the entire axial length of the
walls of the filter and a second coating extending from the
15 inlet end for only a portion of the axial length of the filter
at a higher loading sufficient to light-off and produce the
exotherm to burn off soot collected in the filter.
[0049] An embodiment of the inventive emission treatment
system is shown in FIG. 1A. As can be seen in FIG. 1A, the
exhaust containing gaseous pollutants (including unburned
hydrocarbons, carbon monoxide and NOx) and particulate matter
is conveyed from the engine 15 to a NOx reducing catalyst 11.
[0050] Upstream of the NOx reducing catalyst 11, a
reductant, for example, ammonia, is injected as a spray via a
nozzle (not shown) into the exhaust stream. Aqueous urea shown
on one line 18 can serve as the ammonia precursor which can be
mixed with air on another line 19 in an optional mixing station
16. An introduction port or valve 14 can be used to meter
precise amounts of aqueous urea which are converted in the
exhaust stream to ammonia. The exhaust stream with the added
ammonia is conveyed to a NOx-reducing catalyst 11, for example,
an SCR catalyst, which may be coated on an appropriate
substrate such as a honeycomb filter. On passing through the
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
16
reducing catalyst 11, the NOx component of the exhaust gas
stream is converted through the selective catalytic reduction
of NOx with ammonia to nitrogen. As noted above, if the NOx
reducing catalyst is a lean NOx catalyst, a separate reductant
does not necessarily need to be injected into the system, and
the reductant can be a hydrocarbon reductant supplied from the
engine.
[0051] Depending on the desired level of NOx removal, one or
more NOx reducing catalysts can be disposed downstream of the
NOx reducing catalyst 11. For example, the additional SCR
catalyst may be disposed on a monolithic, honeycomb flow
through substrate, a ceramic foam substrate or metallic
substrate downstream of the NOx reducing catalyst 11.
[0052] Downstream from the NOx reducing catalyst 11 is a
particulate filter 12, for example, a wall flow monolith
comprising wall elements having a light-off oxidation catalyst
composition permeating at least an inlet zone of the walls, as
will described further below. In the light-off oxidation
catalyst permeating the walls of the particulate filter 12,
unburned gaseous and non-volatile hydrocarbons (i.e., the SOF)
and carbon monoxide are largely combusted to form carbon
dioxide and water. Removal of substantial proportions of the
SOF using the oxidation catalyst, in particular, helps prevent
deposition of excessive particulate matter on the particulate
filter 12, which could become clogged by excessive particulate
matter. In addition, a substantial proportion of the NO of the
NOx component is oxidized to N02 in the oxidation catalyst
portion of the particulate filter 12. The particulate matter
including the soot fraction and the VOF are also largely
removed (greater than 80%) by the particulate filter. The
particulate matter deposited on the particulate filter is
combusted through active regeneration of the filter, which
process is aided by the presence of the integrated light-off
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
17
oxidation catalyst composition. Active regeneration is
initiated according to a predetermined event, for example,
after a predetermined mileage or time period, or upon sensing a
predetermined back pressure in the particulate filter 12.
Active regeneration occurs when a hydrocarbon, for example,
diesel fuel is injected into a hydrocarbon introduction port 9
located upstream from the filter 12 and in communication with a
hydrocarbon source (not shown). Of course, it will be
appreciated that diesel fuel is a convenient hydrocarbon source
due to the fact that it is on board of the vehicle. A
sufficient amount of hydrocarbon is introduced into
introduction port 9 to generate an exotherm to combust the soot
trapped in filter 12. As those skilled in the art will
appreciate, the exotherm generated will depend on the inlet
exhaust temperature into the particulate filter, the amount of
fuel injected and the precious metal loading of the particulate
filter. Different precious metals will require different
loading to generate the exotherm to burn off soot trapped in
the filter.
[0053] An optional configuration is shown in FIG. 1B, where
the emission treatment system is provided with an NH3-
destruction catalyst, such as a slip oxidation catalyst 13
downstream of the NOx reducing catalyst 11 and particulate
filter 12. The slip oxidation catalyst can be coated, for
example, with a composition containing base metals and less
than 0.5 wt% of platinum. This provision can be used to
oxidize any excess NH3 before it is vented to the atmosphere.
According to one or more embodiments, the NH3-destruction
catalyst may be disposed on the particulate filter 12. Similar
to the embodiment described above, a hydrocarbon introduction
port 9 is located upstream of the filter to introduce a
suitable hydrocarbon such as diesel fuel to generate an
exotherm to burn soot trapped in the filter 12.
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
18
[0054] In an important alternative embodiment shown in FIG.
1C, a system is shown in which a reductant, for example,
ammonia, is injected as a spray via a nozzle (not shown) into
the exhaust stream downstream from the engine 15. Similar to
the embodiment shown in FIG. 1A, aqueous urea shown on one line
18 can serve as the ammonia precursor which can be mixed with
air on another line 19 in an optional mixing station 16. An
introduction port or valve 14 can be used to meter precise
amounts of aqueous urea which are converted in the exhaust
stream to ammonia downstream from the engine 15. A NOx-
reducing catalyst, for example, an SCR catalyst contained on an
appropriate substrate, is disposed downstream from the
reductant introduction port or valve 14. A light-off diesel
oxidation catalyst 17 is provided downstream from the NOx
reducing catalyst, and a particulate filter 20 is provided
downstream from the diesel oxidation catalyst 17. According to
this embodiment, the diesel oxidation catalyst 20 may be a
separate component provided on an appropriate substrate such as
a honeycomb filter. The particulate filter may be a bare
particulate filter or a catalyzed particulate filter, for
example, a catalyzed soot filter (CSF) containing precious
metal along the entire axial length of the filter.
Alternatively, the particulate filter can be a zoned
particulate filter, for example, a wall flow filter including a
zone on the inlet end containing a light off/diesel oxidation
catalyst. Like in the embodiments described in Figures 1A and
1B, a hydrocarbon introduction port 9 is located upstream of
the filter to introduce a suitable hydrocarbon such as diesel
fuel to generate an exotherm to burn soot trapped in the filter
12. An optional ammonia destruction catalyst 13 may be
provided downstream from the particulate filter 20.
WALL FLOW SUBSTRATES
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
19
[0055] The particulate filter may be embodied in many forms.
For example, the particulate filters may be in the form of a
honeycomb wall flow filter, a partial filtration filter, a wire
mesh filter, a wound fiber filter, a sintered metal filters and
a foam filter. In specific embodiments, the particulate filter
is a wall flow filter. Wall flow substrates useful for
supporting the oxidation catalyst compositions have a plurality
of fine, substantially parallel gas flow passages extending
along the longitudinal axis of the substrate. Typically, each
passage is blocked at one end of the substrate body, with
alternate passages blocked at opposite end-faces. Such
monolithic carriers may contain up to about 700 or more flow
passages (or "cells") per square inch of cross section,
although far fewer may be used. For example, the carrier may
have from about 7 to 600, more usually from about 100 to 400,
cells per square inch ("cpsi"). The cells can have cross
sections that are rectangular, square, circular, oval,
triangular, hexagonal, or are of other polygonal shapes. Wall
flow substrates typically have a wall thickness between 0.002
and 0.1 inches. Examples of suitable wall flow substrates have
a wall thickness of between 0.002 and 0.015 inches.
[0056] FIGS. 2 and 3 illustrate a wall flow filter substrate
which has a plurality of passages 52. The passages are
bounded or enclosed by the internal walls 53 of the filter
25 substrate. The substrate has an inlet end 54 and an outlet end
56. Alternate passages are plugged at the inlet end with inlet
plugs 58, and at the outlet end with outlet plugs 60 to form
opposing checkerboard patterns at the inlet 54 and outlet 56.
A gas stream 62 enters through the unplugged channel inlet 64,
30 is stopped by outlet plug 60 and diffuses through channel walls
53 (which are porous) to the outlet side 66. The gas cannot
pass back to the inlet side of walls because of inlet plugs 58.
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
[0057] Suitable wall flow filter substrates are composed of
ceramic-like materials such as cordierite, cx-alumina, silicon
carbide, silicon nitride, zirconia, mullite, spodumene,
alumina-silica-magnesia, aluminum titanate or zirconium
5 silicate, or of porous, refractory metal. Wall flow substrates
may also be formed of ceramic fiber composite materials.
Examples of suitable wall flow substrates are formed from
cordierite and silicon carbide. Such materials are able to
withstand the environment, particularly high temperatures,
10 encountered in treating the exhaust streams.
[0058] Suitable wall flow substrates for use in the
inventive system include thin porous walled honeycombs
(monolith)s through which the fluid stream passes without
causing too great an increase in back pressure or pressure
15 across the article. According to embodiments of the invention,
ceramic wall flow substrates used in the system are formed of a
material having a porosity of at least 40% (e.g., from 50 to
75%) having a mean pore size of at least 5 microns (e.g., from
5 to 30 microns). In certain embodiments, the substrates have
20 a porosity of at least 55% and have a mean pore size of at
least 10 microns. When substrates with these porosities and
these mean pore sizes are coated with the techniques described
below, adequate levels of SCR catalyst compositions can be
loaded onto the substrates to achieve excellent NOx conversion
efficiency. These substrates are still able retain adequate
exhaust flow characteristics, i.e., acceptable back pressures,
despite the light-off oxidation catalyst loading. U.S. Pat.
No. 4,329,162 is herein incorporated by reference with respect
to the disclosure of suitable wall flow substrates. Wall flow
substrates can also be metallic, i.e. have no porosity, and the
pore size is typically lower than of a wall flow filter.
[0059] The porous wall flow filter used according to
embodiments of the invention is catalyzed in that the wall of
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
21
said element has thereon or contained therein one or more
catalytic materials. Catalytic materials may be present on the
inlet side of the element wall alone, the outlet side alone,
both the inlet and outlet sides, or the wall itself may consist
all, or in part, of the catalytic material. To coat the wall
flow substrates with the light-off oxidation catalyst
composition, the substrates are immersed vertically in a
portion of the catalyst slurry such that the top of the
substrate is located just above the surface of the slurry. In
this manner, slurry contacts the inlet face of each honeycomb
wall, but is prevented from contacting the outlet face of each
wall. This results in a portion of the walls on the inlet end
of the substrate being coated, forming an inlet zone. The
substrate is removed from the slurry, and excess slurry is
removed from the wall flow substrate first by allowing it to
drain from the channels, then by blowing with compressed air
(against the direction of slurry penetration), and then by
pulling a vacuum from the direction of slurry penetration. By
using this technique, the catalyst slurry permeates the walls
of the substrate, yet the pores are not occluded to the extent
that undue back pressure will build up in the finished
substrate. As used herein, the term "permeate" when used to
describe the dispersion of the catalyst slurry on the
substrate, means that the catalyst composition is dispersed
throughout the wall of the substrate, and not just on an outer
surface of the wall as a coating layer. The coating can be
applied by any suitable technique, such as by immersing the
substrate into the coating a using a vacuum to draw the coating
up into the channels of the substrate, as described in United
States patent nos. 6,478,874; 5,866,210 and 5,963,832, the
entire content of each patent incorporated herein by reference.
[0060] After coating with catalyst, the substrates are dried
typically at least about 100 C and calcined at a higher
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
22
temperature (e.g., 300 to 450 C). After calcining, the
catalyst loading can determined be through calculation of the
coated and uncoated weights of the substrate. As will be
apparent to those of skill in the art, the catalyst, loading
can be modified by altering the solids content of the coating
slurry. Alternatively, repeated immersions of the substrate in
the coating slurry can be conducted, followed by removal of the
excess slurry as described above.
Oxidation Catalyst Compositions
[0061] The oxidation catalyst formed on the particulate
filter can be formed from any composition that provides
effective combustion of unburned gaseous and non-volatile
hydrocarbons (i.e., the SOF) and carbon monoxide. In addition,
the oxidation catalyst should be effective to convert a
substantial proportion of the NO of the NOx component to N02.
As used herein, the term "substantial conversion of NO of the
NOx component to NO2" means at least 20%, and preferably between
30 and 60%. Catalyst compositions having these properties are
known in the art, and include platinum group metal- and base
metal-based compositions. An example of oxidation catalyst
composition that may be used in the emission treatment system
contains a platinum group component (e.g., platinum, palladium
or rhodium components) dispersed on a high surface area,
refractory oxide support (e.g., y-alumina. A suitable platinum
group metal component is platinum.
[0062] Platinum group metal-based compositions suitable for
use in forming the oxidation catalyst are also described in
U.S. Pat. No. 5,100,632 (the '632 patent) hereby incorporated
by reference. The '632 patent describes compositions that have
a mixture of platinum, palladium, rhodium, and ruthenium and an
alkaline earth metal oxide such as magnesium oxide, calcium
oxide, strontium oxide, or barium oxide with an atomic ratio
between the platinum group metal and the alkaline earth metal
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
23
of about 1:250 to about 1:1, and preferably about 1:60 to about
1:6.
[0063] Catalyst compositions suitable for the oxidation
catalyst may also be formed using base metals as catalytic
agents. For example, U.S. Pat. No. 5,491,120 (the disclosure
of which is hereby incorporated by reference) discloses
oxidation catalyst compositions that include a catalytic
material having a BET surface area of at least about 10 m2/g and
consist essentially of a bulk second metal oxide which may be
one or more of titania, zirconia, ceria-zirconia, silica,
alumina-silica, and cx-alumina.
[0064] Also useful are the catalyst compositions disclosed
in U.S. Pat. No. 5,462,907 (the '907 patent, the disclosure of
which is hereby incorporated by reference). The '907 patent
teaches compositions that include a catalytic material
containing ceria and alumina each having a surface area of at
least about 10 m2/g, for example, ceria and activated alumina in
a weight ratio of from about 1.5:1 to 1:1.5. Alternatively,
palladium in any desired amount may be included in the
catalytic material. Additional useful compositions are
disclosed in U.S. Pat. No.7,078,074, the entire content of
which is incorporated herein by reference.
[0065] The PGM loading on the inlet zone can be varied to
between about 20 g/ft3 and 200 g/ft3, more specifically between
about 30 g/ft3 and 150 g/ft3, and in a specific embodiment
between about 40 g/ft3 and 100 g/ft3. These amounts can be
incrementally varied in amounts of 5 g/ft3 between these ranges.
In specific embodiments, the PGM is can be chosen from Pt
and/or Pd, both of which are good oxidation catalysts for
hydrocarbons. The current price of platinum is much higher
than for palladium, thus the latter offers the advantage of
reduced cost; however, this may change in the future depending
on PGM demand. Platinum is very active for hydrocarbon
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
24
oxidation reactions and is rather resistant to poisoning.
Palladium can be less active and is susceptible to poisoning,
e.g. by sulfur. However, under lean exhaust conditions and
temperatures that might exceed 800 C, platinum can experience
thermal sintering and thereby reduction in oxidation activity.
Addition of palladium and its interaction with the platinum
results in a substantial reduction in the high temperature
sintering of the platinum and thereby maintenance of its
oxidation activity. If the temperatures of exposure are kept
low, Pt-only may be a good option to obtain the highest
possible oxidation activity. However, in configurations in
which high temperatures (e.g. 800 C) are anticipated,
especially internal to the filter, inclusion of some Pd is
desired. Pt:Pd ratios to obtain acceptable Pt stability with
the highest oxidation activity are between about 10:1 and 4:1;
however, ratios as low as 2:1 and 1:1 are also within the scope
of the invention. Higher Pd contents (e.g., 1:2) are also
within the scope of the present invention. In certain
embodiments, Pd with no platinum may be used.
[0066] The PGM is dispersed on a suitable support material
such as a refractory oxide with high surface area and good
thermal stability such as a high surface area aluminum oxide.
High surface area aluminas are suitable supports for PGM and
SBa-150 (Sasol North America) with surface area of 138-158 m2/g
and pore volume of 0.44-0.55 cm3/g (N2) is an example of a
suitable alumina support. Also aluminas stabilized with a
second oxide are suitable supports. Lanthana stabilization of
alumina provides a suitable support for PGM. For example GA-
200L (4 wt. % La203) stabilized alumina (Engelhard, Port Allen,
LA) with surface area of 190-250 m2/g and pore volume of 0.5
cm3/g (N2) is a suitable stabilized alumina. Also mixtures of
aluminas are suitable supports, for example 50:50 wt. SBa-150
plus GA-200L. Other aluminas that are doped or treated with
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
oxides such as Si02, Zr02, Ti02, etc.) to provide stabilization
or improved surface chemistries can also be utilized. Other
suitable support materials, include, but are not limited to Zr02
and Ti02 can be used. In addition to the PGM support oxides
5 discussed above it might prove useful to include other
catalytically functional oxides to incorporate into the
catalytic zone. Examples of these include Ce02r Pr6011, V205,
and Mn02 and combinations thereof and solid solution oxide
mixtures, etc. These oxides can contribute to burning of
10 hydrocarbons, especially heavy fuel derived hydrocarbons, and
deposited coke/soot derived from disproportination (i.e.,
dehydrogenation or oxidative dehydrogenation) of the injected
fuel and in this way give additional combustion activity to the
catalytic zone, plus prevent deactivation of the PGM by the
15 deposition hydrocarbon derived coke.
[0067] The loading of the oxidation catalyst in the zone on
the filter substrate is typically is limited to control the
contribution of the physical volume of the catalyst coating
filling the pore volume of the filter substrate and thereby
20 adversely affecting the flow resistance through the filter wall
and thus the back-pressure. On the other hand, with high
loadings of PGM on the support oxide we have to provide
sufficient surface area for good PGM dispersion. As an
example, a PGM loading on the inlet zone of about 60 g/ft3, a
25 dry gain (DG) of 0.5 g/in3 in the zone is acceptable. The DG
can be adjusted taking into consideration the optimum PGM
loading, alumina to other (denser) oxide weight ratio, and
other factors.
[0068] The ratio of the zone length/volume to total filter
length/volume can vary between about 0.20 to 0.9, for example,
this value can be 0.25, 0.5 or 0.75. Thus for example, an
11.25" diameter x 14.0" long filter substrate a zone
length/depth of ca. 3.0" could be used or a ratio of 0.21 of
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
26
total length/volume of the filter. However, determination of
the most effective zone length/volume ratio will be part of
catalyzed filter optimization for a particular exhaust system
design.
[0069] The inlet catalytic zone (length/volume ratio can
vary) for light-off and combustion of the injected fuel. The
non-zoned portion of the filter can be blank and uncatalyzed or
catalyzed. This is accomplished by application of a coating to
the full length of the filter substrate. This can be done
prior to the application of oxidation catalyst zone coating,
but this is not necessary and the main body coat can be applied
after the zone coat. The main body coat will typically (but
not necessarily) have a lower PGM loading and slurry washcoat
DG than the inlet zone coat. The lower PGM affords lower cost
and the lower DG affords lower pressure-drop across the filter.
It is possible to apply this coating as a separate, outlet zone
coat. This can be accomplished by applying the inlet fuel
combustion zone coat to the desired length/depth to one end of
the filter substrate and then applying the outlet coat to the
opposite end of the substrate to the desired length/depth.
This catalyst coating is applied into the pore structure of the
filter wall and does not occur as a discrete coating on the
filter wall. The composition of the main body or outlet zone
coating can be varied. Typically, the catalytic coating is
comprised of PGM on alumina(s). An example catalyst has a
coating comprised of 10g/ft3 Pt-Pd (10:1 ratio) supported on
[SBa-150 + GA-200L aluminas (50:50 wt ratio) and applied to the
full length of the filter support at a DG = 0.25g/in3. The main
body coating contributes to further combustion of any injected
fuel that is not completely combusted on the inlet zone coat.
This ensures that all the hydrocarbon and any possible partial
oxidation products such as carbon monoxide are fully oxidized
before they exit the filter.
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
27
NOx Reducing Catalysts
[0070] For most US heavy duty diesel applications starting
in 2007 engine design and calibration will be sufficient to
meet the NOx standard. For most US heavy duty diesel
applications starting in 2007 the after treatment system
utilizes engine design and calibration. However, in the United
States, particularly starting in 2010, stricter NOx emissions
standards are not expected to be met by engine design and
calibration measures alone and a NOx reduction after treatment
catalyst will be required. The NOx reducing catalyst according
to one or more embodiments of the invention can comprise a
selective catalytic reduction (SCR) catalyst, a lean NOx
catalyst, a lean NOx trap (LNT), or a combination of these.
This could also be applied to light duty diesel applications.
[0071] It should be noted that the engine-out NOx is mainly
in the form of NO with low levels of N02 and that the PGM
loadings and ratios employed in the zone and body of the zoned
particulate filter can be tailored to control the level of
filter-out NO2 versus NO. The oxidation reaction, represented
by NO + 1-2 02 --> N02r can be controlled by the PGM function.
The effectiveness of the down-stream SCR or LNT can be enhanced
by control of the NO2/NO ratio.
[0072] For an SCR reaction, there are three reaction
conditions can be considered depending on the N02/NO ratio:
(1) Standard :
4 NH3 + 4 NO + 02 --> 4 N2 + 6 H20
(2) "Fast":
4 NH3 + 2 NO + 2 N02 --> 4 N2 + 6 H20
(3) "Slow":
4 NH3 + 3 NO2 --> 3.5 N2 + 6 H20.
[0073] From the above three conditions, it can be seen that
the desired "fast" or more efficient SCR reaction occurs if the
NO2 to NO ratio is 1:1 and relative to engine-out it is expected
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
28
to require an oxidation function to increase the relative
amount of N02. According to embodiments of the invention, the
PGM on the zoned particulate filter can contribute to this
function and tailoring the PGM loading and ratio can be used to
achieve this. It is believed that the 1:1 ratio will give the
best down-stream SCR reaction. Higher levels of N02 are
detrimental in that it gives a slower SCR reaction. For the
LNT operation, it is necessary to oxidize engine-out NO as
fully as possible to N02 as LNT's absorb NOx principally as
nitrates. Tailoring the zoned-CSF's PGM loading and ratio
would achieve this. LNT operation would be expected to require
higher loading levels of PGM with most if not all the PGM in
the form of Pt.
[0074] Suitable SCR catalyst compositions for use in the
system are able to effectively catalyze the reduction of the
NOx component at temperatures below 600 C., so that adequate
NOx levels can be treated even under conditions of low load
which typically are associated with lower exhaust temperatures.
Preferably, the catalyst article is capable of converting at
least 50% of the NOx component to N2, depending on the amount of
reductant added to the system. Another desirable attribute for
the composition is that it possesses the ability to catalyze
the reaction of 02 with any excess NH3 to N2 and H20, so that NH3
is not emitted to the atmosphere. Useful SCR catalyst
compositions used in the inventive system also have should
resist degradation upon exposure to sulfur components, which
are often present in diesel exhaust gas compositions. Another
suitable SCR catalyst composition comprises vanadia-titania.
[0075] Suitable SCR catalyst compositions are described, for
instance, in U.S. Pat. Nos. 4,961,917 (the '917 patent) and
5,516,497, which are both hereby incorporated by reference in
their entirety. Compositions disclosed in the '917 patent
include one or both of an iron and a copper promoter present in
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
29
a zeolite in an amount of from about 0.1 to 30 percent by
weight, preferably from about 1 to 5 percent by weight, of the
total weight of promoter plus zeolite. In addition to their
ability to catalyze the reduction of NOx with NH3 to N2, the
disclosed compositions can also promote the oxidation of excess
NH3 with 02, especially for those compositions having higher
promoter concentrations.
[0076] Zeolites used in such compositions are resistant to
sulfur poisoning, sustain a high level of activity for the SCR
process. These zeolites have a pore size large enough to
permit adequate movement of the reactant molecules NO and NH3 in
to, and the product molecules N2 and H20 out of, the pore system
in the presence of sulfur oxide molecules resulting from short
term sulfur poisoning, and/or sulfate deposits resulting from
long term sulfur poisoning. The pore system of suitable size
is interconnected in all three crystallographic dimensions. As
is well known to the those skilled in the zeolite art, the
crystalline structure of zeolites exhibits a complex pore
structure having more or less regularly recurring connections,
intersections and the like. Pores having a particular
characteristic, such as a given dimension diameter or cross-
sectional configuration, are said to be one dimensional if
those pores do not intersect with other like pores. If the
pores intersect only within a given plane with other like
pores, the pores of that characteristic are said to be
interconnected in two (crystallographic) dimensions. If the
pores intersect with other like pores lying both in the same
plane and in other planes, such like pores are said to be
interconnected in three dimensions, i.e., to be "three
dimensional". It has been found that zeolites which are highly
resistant to sulfate poisoning and provide good activity for
both the SCR process and the oxidation of ammonia with oxygen,
and which retain good activity even when subject to high
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
temperatures, hydrothermal conditions and sulfate poisons, are
zeolites which have pores which exhibit a pore diameter of at
least about 7 Angstroms and are interconnected in three
dimensions. While embodiments of the present invention are not
5 to be bound by any specific theory, it is believed that the
interconnection of pores of at least 7 Angstroms diameter in
three dimensions provides for good mobility of sulfate
molecules throughout the zeolite structure, thereby permitting
the sulfate molecules to be released from the catalyst to free
10 a large number of the available adsorbent sites for reactant
NOx and NH3 molecules and reactant NH3 and 02 molecules. Any
zeolites meeting the foregoing criteria are suitable for use in
the practices of the present invention; specific zeolites which
meet these criteria are USY, Beta and ZSM-20. Other zeolites
15 may also satisfy the aforementioned criteria.
[0077] The NOx reducing catalyst may comprise a lean NOx
catalyst. Lean NOx catalysts are typically classified as
either a low temperature NOx catalyst or a high temperature NOx
catalyst. The low temperature lean NOx catalyst is platinum
20 based (Pt-based) and does not have to have a zeolite present to
be active, but Pt/zeolite catalysts appear to have better
selectivity against formation of N20 as a by-product than other
catalysts, such as Pt/alumina catalysts. Generally, a low
temperature lean NOx catalyst has catalytically active
25 temperature ranges of about 180 to 350 C with highest
efficiencies at a temperature of about 250 C. High
temperature lean NOx catalysts have base metal/zeolite
compositions, for example Cu/ZSM-5. High temperature NOx
catalysts have a lower temperature range of about 300-350 C
30 with highest efficiency occurring around 400 C. Different
embodiments of the present invention use either high or low
temperature lean NOx catalysts with an HC reductant.
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
31
[0078] The NOx reducing catalyst may comprise a lean NOx
trap. Lean NOx traps are disclosed in U.S. Patent Nos.
5,875,057 and 6,471,924, the entire content of each patent
incorporated herein by reference. In general, a lean NOx trap
containing a combination of a NOx sorbent and an oxidation
catalyst, which sorbs NOx onto the trap member during selected
periods of time, e.g., when the temperature of the gaseous
stream is not suited for catalytic lean NOx abatement. During
other periods of time, e.g., when the temperature of the
gaseous stream being treated is suitable for catalytic lean NOx
abatement, the combustible component on the trap is oxidized to
thermally desorb the NOx from the trap member. A lean NOx trap
typically comprises a catalytic metal component such as one or
more platinum group metals and/or a base metal catalytic metal
component such as oxides of one or more of copper, cobalt,
vanadium, iron, manganese, etc.
[0079] The NOx reducing catalyst compositions can be coated
onto honeycomb flow-through monolith substrates formed of
refractory metallic or ceramic (e.g., cordierite) materials.
Alternatively, oxidation catalysts may be formed on to metallic
or ceramic foam substrates which are well-known in the art.
These oxidation catalysts, by virtue of the substrate on which
they are coated (e.g., open cell ceramic foam), and/or by
virtue of their intrinsic oxidation catalytic activity provide
some level of particulate removal.
[0080] According to one or more embodiments of the
invention, a reductant dosing system is provided upstream of
the NOx reducing catalyst and downstream of the particulate to
inject a NOx reductant into the exhaust stream. As disclosed
in U.S. Pat. No. 4,963,332, NOx upstream and downstream of the
catalytic converter can be sensed, and a pulsed dosing valve
can be controlled by the upstream and downstream signals. In
alternative configurations, the systems disclosed in U.S. Pat.
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
32
No. 5,522,218, where the pulse width of the reductant injector
is controlled from maps of exhaust gas temperature and engine
operating conditions such as engine rpm, transmission gear and
engine speed. Reference is also made to the discussion of
reductant pulse metering systems in U.S. Pat. No. 6,415,602,
the discussion of which is hereby incorporated by reference.
[0081] In the embodiment of FIG. 4, an aqueous urea
reservoir 22 stores a urea/water solution aboard the vehicle
which is pumped through a pump 21 including a filter and
pressure regulator to a urea injector 16. Urea injector 16 is
a mixing chamber which receives pressure regulated air on line
19 which is pulsed by a control valve to urea injector 16. An
atomized urea/water/air solution results, which is pulsed
injected through a nozzle 23 into exhaust pipe 24 upstream of
the NOx reducing catalyst 11, which is upstream of the
particulate filter 12, and an optional NH3 destruction catalyst
(not shown).
[0082] This invention is not limited to the aqueous urea
metering arrangement shown in FIG. 4. It is contemplated that a
gaseous nitrogen based reagent will be utilized. For example, a
urea or cyanuric acid prill injector can meter solid pellets of
urea to a chamber heated by the exhaust gas to gasify the solid
reductant (sublimation temperature range of about 300 to 400
C). Cyanuric acid will gasify to isocyanic acid (HNCO) and
urea will gasify to ammonia and HNCO. With either reductant, a
hydrolysis catalyst can be provided in the chamber and a slip
stream of the exhaust gas metered into the chamber (the exhaust
gas contains sufficient water vapor) to hydrolyze (temperatures
of about 150 to 350 C) HNCO to produce ammonia. In addition,
for lean NOx catalysts, hydrocarbons generated from the engine
may provide a suitable amount of reductant for the system.
[0083] In addition to urea and cyanuric acid, other nitrogen
based reducing reagents or reductants especially suitable for
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
33
use in the control system of the present invention includes
ammelide, ammeline, ammonium cyanate, biuret, cyanuric acid,
ammonium carbamate, melamine, tricyanourea, and mixtures of any
number of these. However, the invention in a broader sense is
not limited to nitrogen based reductants but can include any
reductant containing hydrocarbons such as distillate fuels
including alcohols, ethers, organo-nitro compounds and the like
(e.g., methanol, ethanol, diethyl ether, etc.) and various
amines and their salts (especially their carbonates), including
guanidine, methyl amine carbonate, hexamethylamine, etc.
NH3-Destruction Catalyst Compositions
[0084] In one or more embodiments, the NH3 destruction
catalyst is composed of a platinum group metal component
dispersed on a refractory inorganic oxide support. When the NH3
destruction catalyst is deposited on the monolith carrier, the
platinum group metal component is typically present at from 0.1
to 40 g/ft3, and preferably, from 0.5 to 10 g/ft3. At these
concentrations the platinum group metal component is effective
for the oxidation of ammonia to form N2, but has a diminished
propensity to cause oxidation of ammonia to form NOx. As
described above, higher concentrations of platinum in the
composition are liable to promote the conversion of excess
ammonia to NOx and not to N2. Moreover, lower levels of
platinum group metal components are desired to minimize the
formation of sulfates that contribute to the mass of the
particulate matter that is discharged to the atmosphere.
[0085] Suitable platinum group metal components include
platinum, palladium, rhodium and iridium components. Platinum
is especially suitable. In embodiments of the invention, where
platinum is used in the NH3 destruction catalyst, the platinum
component can be sulfated to further moderate the catalytic
activity of the platinum component and control NOx formation.
The sulfation can be performed by treatment of the composition
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
34
with sulfuric acid, or alternatively, by subjecting the final
coated composition to an exhaust stream derived from an
internal combustion engine that uses fuel that contains higher
levels of sulfur component (e.g.,>350 ppm).A
[0086] An exemplary NH3 destruction catalyst material is
composed of platinum dispersed on one or both of bulk ceria and
activated alumina. Such compositions are similar to those
described in U.S. Pat. No. 5,462,907, the disclosure of which
is hereby incorporated by reference. The catalytic material
can be prepared in the form of an aqueous slurry of ceria and
alumina particles, the particles being impregnated with the a
water-dispersible or water-soluble platinum precursor. The
slurry can then applied to the carrier, dried and calcined to
form a catalytic material coating ("washcoat") thereon.
Typically, the ceria and alumina particles are mixed with water
and an acidifier such as acetic acid, nitric acid or sulfuric
acid, and ball milled to a desired particle size. Alternatively
the slurry can be dried and calcined before being coated on the
carrier.
[0087] The platinum catalytic metal component is preferably
incorporated into the ceria particles or into the ceria and
alumina particles. The ceria-alumina acts not only as a
catalyst but also as a support for the platinum catalytic metal
component. Such incorporation with the platinum precursor can
also be conducted after the ceria-alumina catalytic material is
coated as a washcoat onto a suitable carrier, by impregnating
the coated carrier with a solution of a suitable platinum
precursor, followed by drying and calcination. However,
preferably, the ceria particles or both the ceria and alumina
particles are impregnated with a suitable platinum precursor
before a coating of the ceria-alumina catalytic material is
applied to the carrier. In either case, the platinum metal is
added to the ceria-alumina catalytic material as, e.g., a
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
solution of a soluble platinum compound, the solution serving
to impregnate the ceria and alumina particles (or the ceria-
alumina coating on the carrier), which may then be dried and
the platinum fixed thereon. Fixing can be carried out by
5 calcination or by treatment with hydrogen sulfide or by other
known means, to render the metal in water-insoluble form.
[0088] Generally, the slurry of ceria and activated alumina
particles, with the platinum solution, will be deposited upon
the carrier substrate and dried and calcined to adhere the
10 catalytic material to the carrier and, to revert the platinum
compound to the elemental metal or its oxide. Suitable
platinum precursors for use in the foregoing process include
potassium platinum chloride, ammonium platinum thiocyanate,
amine-solubilized platinum hydroxide and chloroplatinic acid,
15 as is well-known in the art. During calcination, or at least
during the initial phase of use of the catalyst, such
compounds, if present, are converted into the catalytically
active elemental platinum metal or its oxide.
[0089] When the catalytic material is applied as a thin
20 coating to a suitable carrier, such as described above, the
proportions of ingredients are conventionally expressed as
weight of material per gross unit volume of catalyst, as this
measure accommodates the presence of different cell densities,
wall thicknesses, gas flow passages, etc. Grams per cubic inch
25 ("g/in3") units are used to express the quantity of relatively
plentiful components such as the ceria-alumina catalytic
material, and grams per cubic foot ("g/ft3" ) units are used to
express the quantity of the sparsely used ingredients, such as
the platinum metal. For typical diesel exhaust applications,
30 the ceria-alumina catalytic material generally may comprise
from about 0.25 to about 4.0 g/in3, preferably from about 0.25
to about 3.0 g/in3 of the coated carrier substrate, and from
about 0.1 to 10 g/ft3 of platinum.
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
36
Optional Components
[0090] Generally, other ingredients may be added to the
catalyst composition such as conventional thermal stabilizers
for the alumina, e.g., rare earth metal oxides such as ceria.
Thermal stabilization of high surface area ceria and alumina to
prevent phase conversion to less catalytically effective low
surface area forms is well-known in the art. Such thermal
stabilizers may be incorporated into the bulk ceria or into the
bulk activated alumina, by impregnating the ceria (or alumina)
particles with, e.g., a solution of a soluble compound of the
stabilizer metal, for example, an aluminum nitrate solution in
the case of stabilizing bulk ceria. Such impregnation is then
followed by drying and calcining the impregnated ceria
particles to convert the aluminum nitrate impregnated therein
into alumina.
[0091] In addition, the catalyst compositions may contain
other catalytic ingredients such as other base metal promoters
or the like. However, in one embodiment, the catalyst
composition of the present invention consists essentially only
of the high surface area ceria and high surface area alumina,
preferably present in a weight proportion of 1.5:1 to 1:1.5,
with or without thermal stabilizers impregnated therein, and,
from 0.1 to 10 g/ft3 of platinum.
EXAMPLES
[0092] The following examples further illustrate the present
invention, but of course, should not be construed as in any way
limiting its scope.
EXAMPLE 1 - PREPARATION OF ZONE COATED CATALYZED PARTICULATE
FILTER SAMPLE
[0093] A zoned catalyzed soot filter (CSF) consistent with
this invention was prepared as follows:
[0094] A cordierite wall-flow filter substrate (Corning CO)
having a round cross section with dimensions of 10.5" dia. X
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
37
12.0" long and having a cell spacing of 200 cpsi with a filter
wall thickness of 0.012" was used. The coating of this
substrate consisted of:
a. An optional first coating of fugitive water soluble
polymer, Rhoplex P-376 (Rohm & Haas) applied to the entire
substrate that after drying resulted in a DG = 0.25g/in3. One
purpose of this polymer coating is to fill the smallest of
pores in the cordierite filter porosity, thereby allowing
better distribution of the subsequent catalytic coating in the
wall of the filter substrate.
b. A first catalytic coating applied to the full length of
the wall flow filter substrate. This coating was comprised of
platinum and palladium impregnated onto a 50:50 wt mixture of
lanthanum stabilized alumina, GA-200L (Engelhard), containing
4% La203 and alumina, SBa-150 (Sasol North America). Platinum
was first impregnated onto the mixture of aluminas as an
aqueous solution of monoethanol-amine stabilized Pt (IV)
hydroxide and then with palladium as an aqueous solution of Pd
(II) nitrate. The resulting PGM impregnated alumina mixture
with Pt to Pd ratio of 10:1 was milled in water to achieve a
particle size distribution with 90% less than 7 microns,
following which the resultant slurry was adjusted for pH = 4
and solids for coating. The first catalytic coating was
applied to the full length of the wall flow filter substrate in
one pass to achieve a DG = 0.26g/in3 and having a total Pt + Pd
loading of 10g/ft3 with a Pt to Pd ratio of 10:1.
c. A second, zone catalytic coat was then applied to the
inlet end of the wall flow filter substrate to a length (depth)
of 3". This coating was comprised of platinum and palladium
impregnated onto a 50:50 wt. mixture of lanthanum stabilized
alumina, GA-200L (Engelhard), containing 4% La203 and alumina,
SBa-150 (Sasol North America). Platinum was first impregnated
onto the mixture of aluminas as an aqueous solution of
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
38
monoethanol-amine stabilized Pt (IV) hydroxide and then with
palladium as an aqueous solution of Pd (II) nitrate. The
resulting PGM impregnated alumina mixture with Pt to Pd ratio
of 10:1 was milled in water to achieve a particle size
distribution with 90% less than 7 microns, following which the
resultant slurry was adjusted for pH = 4 and solids for
coating. The second, zone catalytic coating was applied to the
inlet 3" of the wall flow filter substrate in one pass to
achieve a DG = 0.53g/in3 within the zone and having a total Pt +
Pd loading of 60g/ft3 with a Pt to Pd ratio of 10:1.
[0095] This resulted in a zoned catalyzed soot filter (CSF)
having an overall total Pt + Pd loading level of 25.Og/ft3 with
overall Pt to Pd ratio of 10:1.
EXAMPLE 2 - Fuel Light-Off Over Zoned CSF
[0096] In order to demonstrate active regeneration
capability of the zoned CSF a fuel light-off test in the engine
test cell was conducted. This testing was run using a
turbocharged 7.6 liter, 225 HP diesel engine installed in an
engine test cell and connected to a dynamometer. The testing
was conducted using the zoned catalyzed soot filter (CSF)
described in Example 1, above.
[0097] For the light-off testing the zoned catalyzed soot
filter (CSF) was mounted in the exhaust line of the engine in a
position 10 ft. downstream of the engine's turbocharger. The
exhaust line was equipped with a fuel injector through which
supplemental diesel fuel could be introduced into the exhaust
stream. This fuel injector was a standard type used for
gasoline engines and it was mounted just downstream of the
engine's turbocharger. Between the diesel fuel injector and
the zoned catalyzed soot filter (CSF) was mounted an inline
mixer to assist mixing of the atomized, injected fuel with the
exhaust stream. All tests were conducted using ultra low
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
39
sulfur (< 15 ppm S) diesel fuel both for engine operation and
supplemental fuel injected into the exhaust.
[0098] For the test the engine was operated at a speed of
1570 rpm and a torque of 745 Nm which resulted in a total
exhaust flow of 740 std. m3/hr with an exhaust temperature at
the inlet of the zoned catalyzed soot filter (CSF) of 300 C as
measured by a thermocouple mounted just upstream of the face of
the CSF. A thermocouple was also mounted just downstream of
the CSF outlet face to measure the exhaust temperature at that
location.
[0099] Starting with a relatively clean, soot free zoned
catalyzed soot filter (CSF) the system was allowed to
equilibrate and stabilize for temperature. Following this (ca.
122 minutes runtime), diesel fuel was introduced at varying
levels into the exhaust via the fuel injector described above
and the exhaust temperatures at the inlet and outlet of the CSF
were monitored. The results are shown in Figure 5. Initially
the CSF in and CSF out exhaust temperatures were the same
(300 C), but as increasing amounts of fuel were injected into
the upstream exhaust the CSF outlet temperature increased. For
one segment (ca. 130-135 minutes runtime) with 1.2 g/sec diesel
fuel injected into the exhaust the CSF outlet exhaust gas
temperature was 545 C which was an increase of 245 C above the
inlet exhaust gas temperature. This exhaust temperature is in
the range sufficient to give soot combustion in the filter
under active regeneration conditions. Measurement of the
exhaust gas total hydrocarbon content during this segment
showed ca. 13,000 ppm Cl at the CSF inlet location, but only
2.7 ppm Cl at the CSF outlet indicating essentially complete
combustion of the supplemental injected diesel fuel in the CSF.
[0100] Figure 6 shows the inlet vs. outlet exhaust
temperature data for the light-off test above as a function of
the rate of injection of diesel fuel into the exhaust up stream
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
of the zoned catalyzed soot filter (CSF). This shows a regular
increase in CSF out exhaust temperature with increase in
injected diesel fuel and that temperatures of 600 C can be
attained for injection rates of 1.5 g/sec. At some injection
5 rate levels the exhaust temperature data appears as bar or
range which reflects the temperature-time heat up response on
changing from one injection rate to the next higher one.
EXAMPLE 3 - Fuel Light-Off Testing with Temperature
Measurements in the Zoned Catalyzed Soot Filter (CSF) Bed
10 [0101] The test in EXAMPLE 2 above measured the effect of
light-off of injected fuel on exhaust gas temperatures under
one engine speed and load condition. CSF out exhaust
temperatures as high as 600 C were attained which are in a good
range for achieving reasonably rapid combustion of soot in the
15 CSF for active regeneration.
[0102] The test of EXAMPLE 3 extended investigation to
include measurement of temperatures within the zoned catalyzed
filter. These measurements allowed characterization of both
axial and radial distribution of temperatures within the CSF to
20 demonstrate how the light-off of injected fuel developed and
its uniformity. Further, the temperatures within the CSF were
more representative of local temperatures in the same regions
where the soot combustion was taking place during active
regeneration.
25 [0103] In addition the tests of EXAMPLE 3 were conducted at
different engine speeds that gave different exhaust flows, plus
different torque levels were employed at these speeds to give
lower CSF inlet exhaust temperatures than were run for EXAMPLE
2.
30 [0104] For these tests the same engine and test set up were
used as in EXAMPLE 2, except that the zoned catalyzed soot
filter (CSF) was fitted with internal thermocouples to measure
the internal filter temperatures. Ten (10) thermocouples were
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
41
installed in the filter in a configuration shown in Figure 7.
This configuration consisted of five (5) thermocouples
installed down the centerline of the filter to measure the
temperatures in the very middle of the filter body. These
thermocouples were positioned at 1" from the inlet face (TC1),
3" from the inlet face and at the rear of the inlet zone (TC2),
6" from the inlet face and at the filter axial mid-point (TC3),
9" from the inlet face (TC4) and 11" from the inlet face (TC5).
In addition five (5) thermocouples were installed in a line
that was located 1" radially from the outer edge of the zoned
catalyzed soot filter and were at corresponding positions from
the inlet face of the filter of 1" (TC11), 3" (TC12), 6"
(TC13), 9" (TC14) and 11" (TC15) .
[0105] The thermocouples used in EXAMPLE 2 to measure
exhaust gas temperatures near the inlet and outlet faces of the
CSF were also in place for exhaust gas temperature measurement
in this test.
[0106] The testing consisted of running light-off tests with
injected fuel at three characteristic engine speeds: A-speed =
1580 rpm, B-speed = 1940 rpm and C-speed = 2680 rpm. The
injected fuel rate was held constant at each speed condition
and the engine torque was varied to give different inlet
exhaust gas temperatures between 350 C and 250 C. Stabilized
temperatures were recorded for inlet and outlet exhaust gas and
for the internal thermocouples installed in the CSF.
[0107] The results for the A-speed tests are given in TABLE
I, below:
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
42
TABLE I - A-Speed Data for Fuel Light-Off
A-Speed = 1580 rpm
Fuel Injection Rate = 1.66g/sec
Test Point 1 2 3 4
Exhaust Flow (std. m3/hr) 739 720 700 682
Zoned CSF-In Gas Temp.(C) 305 285 268 256
Zoned CSF-Out Gas Temp.(C) 658 629 604 607
Filter Internal Temperatures:
TC1 Centerline 1" in Temp.(C) 498 454 433 419
TC2 Centerline 3" in Temp.(C) 537 499 470 465
TC3 Centerline 6" in Temp.(C) 599 565 533 532
TC4 Centerline 9" in Temp.(C) 663 634 604 608
TC5 Centerline 11" in Temp.(C) 700 669 635 641
TC11 Edge 1" in Temp.(C) 489 446 408 351
TC12 Edge 3" in Temp.(C) 528 488 465 455
TC13 Edge 6" in Temp.(C) 598 565 533 531
TC14 Edge 9" in Temp.(C) 653 615 589 592
TC15 Edge 11" in Temp. C 700 669 635 641
[0108] With a diesel fuel injection rate of 1.66 g/sec and
for inlet exhaust temperatures in the range of 305 C to 256 C
and exhaust flows in the range of 739 - 682 std. m3/hr it was
possible to achieve CSF outlet exhaust temperatures in the
range of 658 C to 607 C which give a good range for reasonably
rapid soot combustion in a filter.
[0109] Furthermore, high internal temperatures > 500 C within
the filter could be attained over much of the length of the
filter which are sufficient to give reasonably rapid soot
combustion from the filter. The internal temperatures down the
centerline of the filter and 1" from the outer edge of the
filter showed good radial uniformity of temperature in the
filter during the light-off test. The internal temperatures
measured at the position 1" in from the inlet face of the
filter were lower than those measured further in from the inlet
face, but this is understandable in that light-off of the
injected fuel was being initiated in this region. Still the
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
43
internal temperatures 1" in from the inlet face of the CSF were
163 C to 193 C higher than the inlet exhaust gas temperature.
[0110] The exhaust gas and internal substrate temperatures
for A-speed test point 1(305 C inlet gas temperature) are shown
graphically in Figure 8. It can be seen that there was
essentially a linear increase in internal substrate temperature
down the length of the CSF with temperatures > 500 C over most
of the length of the CSF to facilitate reasonably rapid soot
combustion in the filter for active regeneration. Furthermore,
the CSF internal temperatures at the centerline of the filter
and 1" from the outer edge of the filter were nearly identical
which showed good uniformity of light-off and active fuel
burning.
[0111] The results for the B-speed tests are given in TABLE
II, below:
TABLE II - B-Speed Data for Fuel Light-Off
B-Speed = 1940 rpm
Fuel Injection Rate = 2.25g/sec
Test Point 1 2 3 4
Exhaust Flow (std. m3/hr) 979 968 935 928
Zoned CSF-In Gas Temp.(C) 294 281 271 261
Zoned CSF-Out Gas Temp.(C) 646 652 657 656
Filter Internal Temperatures:
TC1 Centerline 1" in Temp.(C) 463 458 447 431
TC2 Centerline 3" in Temp.(C) 498 497 489 482
TC3 Centerline 6" in Temp.(C) 551 560 556 556
TC4 Centerline 9" in Temp.(C) 631 645 645 638
TC5 Centerline 11" in Temp.(C) 679 696 698 698
TC11 Edge 1" in Temp.(C) 447 423 375 326
TC12 Edge 3" in Temp.(C) 491 490 480 467
TC13 Edge 6" in Temp.(C) 550 557 549 543
TC14 Edge 9" in Temp.(C) 610 621 621 616
TC15 Edge 11" in Temp. C 673 691 691 691
[0112] These results were similar but for a higher exhaust
volumetric flow condition and thus shorter contact time than
for the A-speed tests. CSF outlet exhaust gas temperatures and
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
44
internal filter temperatures in the range of 500 C to ca. 700 C
should were attained which would give reasonably rapid
combustion of soot from the filter under these active
regeneration conditions.
[0113] The results from the C-speed tests are given in TABLE
III,below:
TABLE III - C-Speed Data for Fuel Light-Off
C-Speed = 2680 rpm
Fuel Injection Rate = 1.80g/sec
Test Point 1 2 3 4 5 6 7
Exhaust Flow (std. m3/hr) 969 917 874 830 770 723 713
Zoned CSF-In Gas Temp.(C) 351 330 317 301 281 262 251
Zoned CSF-Out Gas Temp.(C) 645 650 651 651 659 668 678
Filter Internal Temperatures:
TC1 Centerline 1" in Temp.(C) 515 503 499 492 479 460 448
TC2 Centerline 3" in Temp.(C) 546 535 531 527 524 505 511
TC3 Centerline 6" in Temp.(C) 590 584 582 581 585 586 587
TC4 Centerline 9" in Temp.(C) 649 650 651 653 665 670 685
TC5 Centerline 11" in Temp.(C) 679 685 686 689 702 712 730
TC11 Edge 1" in Temp.(C) 513 503 498 491 478 457 446
TC12 Edge 3" in Temp.(C) 540 532 527 525 519 504 503
TC13 Edge 6" in Temp.(C) 587 582 579 578 584 586 587
TC14 Edge 9" in Temp.(C) 634 633 633 634 648 653 665
TC15 Edge 11" in Temp.(C) 673 678 682 682 697 712 730
[0114] The results for these tests were similar and showed
good light-off of injected fuel that gave high enough CSF
outlet gas temperatures and internal filter temperatures to
give reasonably rapid soot combustion from the filter under
active regeneration.
EXAMPLE 4 - Active Regeneration of Zoned Catalyzed Soot
Filter with Soot Loading in the Filter
[0115] The same zoned catalyzed soot filter (CSF) used for
testing in EXAMPLES 1-3 was loaded with soot on a 6.6 liter 330
HP engine at a speed of 3200 rpm and torque of 125 Nm. The
soot loaded filter (2.8 g/liter soot) was placed in the exhaust
line of the 7.6 liter 225 HP engine employed in EXAMPLES 2-3
and which was equipped with the same supplemental diesel fuel
injector used for active regeneration. The engine was adjusted
to a speed of 1566 rpm and torque of 680 Nm to achieve a CSF
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
inlet exhaust gas temperature of 303 C with exhaust flow of 705
std. m3/hr. Once stabilized the pressure drop (Delta P) across
the filter under these conditions was measured as 8.57 KPa.
Supplemental diesel fuel injection into the exhaust (1.62
5 g/sec) was established to initiate an active regeneration which
was continued for ca. 25 min. The results of this active
regeneration are shown in Figure 9. It can be seen that the
CSF out exhaust gas temperature increased with supplemental
fuel injection to a level of 656 C suitable for active soot
10 combustion. The level of Delta P also increased with increase
in exhaust temperature but reached a peak of ca. 11.5 KPa after
ca. 2 min. of runtime, following which Delta P was reduced and
at the end of the run was ca. 9.3 KPa with outlet exhaust
temperature at 656 C for a reduction of 2.2 KPa from peak Delta
15 P. The supplemental fuel injection was then terminated and the
CSF out exhaust gas temperature returned to the same level as
the CSF in exhaust gas temperature (303 C). The level of Delta
P across the filter at this point was measured as 5.87 KPa for
a reduction of 2.70 KPa relative to the level before the active
20 regeneration. Weighing of the filter after the active
regeneration showed a 60% reduction in the level of soot in the
filter. This was not considered to be an optimized process or
test, but it clearly demonstrated active regeneration with the
zoned catalyzed filter (CSF) for reduction of filter Delta P
25 and soot loading.
EXAMPLE 5
[0116] A system consisting of a urea/fuel injector, a BASF
REX-1848 V/Ti SCR catalyst, and a zoned CSF with a length of 15
inches and a diameter of 12 inches prepared according to the
30 method described in Example 1 was put in the exhaust stream of
a turbocharged diesel engine. A schematic drawing of the system
is shown in Figure 10. The engine was operated at several
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
46
ce " #
egi s # P#
# #
EXAMPLE 6
[0117] The system described in Example 5 was actively
regenerated by injecting diesel fuel through the injector
upstream of the SCR catalysts. The amount of fuel was
controlled to sequentially reach 550 C, 600 C, and 650 C for
20, 10 and 10 min respectively.
EXAMPLE 7
[0118] The SCR activity of the system described in Example 5
was measured after the active regeneration of Example 6.
Results are shown in the table and are identical within the
accuracy of the test to the results obtained in Example 5. The
data shows that the injection of diesel fuel did not negatively
impact the performance of the SCR catalyst.
#
#
#~r # S
EXAMPLE 8
[0119] A zeolitic SCR catalyst was prepared by ion-
exchanging zeolite R to a Cu content of 2 wt% and coating the
resulting catalyst on a substrate at a loading of 2.9 g in-3. An
NH3 oxidation (AMOX) catalyst was prepared by impregnation a
Fe/R catalyst sequentially with Pt (0.17 wt%) and Cu (9.6 wt%
as CuO) . The SCR and the AMOX catalyst and a zoned CSF with a
CA 02671452 2009-05-29
WO 2008/070551 PCT/US2007/086069
47
length of 15 inch and a diameter of 12 inch prepared according
to the method described in Example 1 was put in the exhaust
stream of a turbocharged diesel engine with the CSF downstream
of the SCR catalyst. The engine was operated at several steady
state conditions. The table shows the NOx conversion over the
SCR catalyst at various conditions.
~ ~ ~~ i e~ ~ e
# ~ # # s
# ~ # #/
EXAMPLE 9
[0120] The system described in Example 7 was actively
regenerated by injecting diesel fuel through the injector
upstream of the SCR catalysts. The amount of fuel was
controlled to sequentially reach 550 C, 600 C, and 650 C for
20, 10 and 10 min respectively.
EXAMPLE 10
[0121] The SCR activity of the system described in Example 7
was measured after the active regeneration of Example 8.
Results are shown in the table and are identical within the
accuracy of the test to the results obtained in Example 7.
#t $ 1 S~
[0122] While the foregoing is directed to embodiments of the
present invention, other and further embodiments of the
invention may be devised without departing from the basic scope
thereof, and the scope thereof is determined by the claims that
follow.