Note: Descriptions are shown in the official language in which they were submitted.
CA 02673837 2014-10-23
WO 2008/085764
PCT/US2007/089017
STORAGE AND DISPENSING DEVICES FOR ADMINISTRATION
OF ORAL TRANSMUCOSAL DOSAGE FORMS
Field of the Invention
[0001] The invention relates to drug dispensing devices, methods and systems
for
oral transmucosal administration of small volume drug dosage forms to a
subject,
wherein the drug dosage forms comprise sufentanil for treatment of pain.
15 Background Of The Invention.
[0002] Oral dosage forms account for approximately eighty percent of all the
drug
dosage forms on the market. Oral dosage forms are non-invasive, easily
administered and have high patient compliance.
[0003] Orally administered therapeutic agents are rapidly transported to the
20 stomach and small intestine for absorption across the gastrointestinal
(GI) mucosal
membranes into the blood. The efficiency of absorption of a drug following
oral
administration can be low because of metabolism within the GI tract and first-
pass
metabolism within the liver resulting in relatively lengthy onset times or
erratic
absorption characteristics that are not well suited to control acute
disorders. The
25 majority of oral dosage forms on the market are designed for GI
delivery. Relatively
few oral dosage forms are designed for delivery through the oral muc,osa.
[0004] However, oral transmucosal delivery offers a number of advantages in
that
it can provide a shorter onset time to maximal plasma concentration (C) than
oral
delivery, in particular for lipophilic drugs. This is because the drug rapidly
passes
30 directly and efficiently through the epithelium of the highly
vascularized mucosal
tissue to the plasma, thus rapidly reaching the circulation while avoiding the
slower,
often inefficient and variable GI uptake. It is therefore advantageous for a
drug to be
1
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
delivered through the mucus membranes of the oral cavity, (e.g., via the
sublingual
route), when rapid onset, consistent Tmax and Cmõ are advantageous.
[0005] In carrying out oral transmucosal drug delivery, the drug is absorbed
through the epithelial membranes of the oral cavity. However, frequently the
key
risk associated with oral transmucosal delivery is the enhanced potential for
swallowing the medication owing to the continuous generation, backward flow
and
swallowing of the saliva. This becomes a particular risk when the used dosage
forms
are large enough to produce a significant saliva response, which, in turn,
leads to
swallowing or drug and/or removal of the dosage form from the oral mucosa.
[0006] Various solid dosage forms, such as sublingual tablets, troches,
lozenges,
lozenges-on-a-stick, chewing gums, and buccal patches have been used to
deliver
drugs via the oral mucosal tissue. Solid dosage forms such as lozenges and
tablets
have been used for oral transmucosal delivery of drugs, e.g., nitroglycerin
sublingual
tablets.
[0007] The relevant art does not describe a dispensing device for delivery of
a
drug dosage form to the oral mucosa, such as the sublingual space, where the
device facilitates proper placement of the drug dosage form.
[0008] Reproducible and effective drug delivery technology represents an
area of
active research, in particular, as it applies to controlled substances such as
opioids.
Controlled access oral transmucosal drug dispensing systems offer numerous
advantages over conventional means of drug administration such as oral and
intravenous routes, the most important of which is enhanced safety, with
additional
advantages being rapid and consistent onset of action, more consistent and
predictable plasma concentrations and higher and more consistent
bioavailability
than currently available dosage forms.
[0009] This is particularly relevant to the treatment of pain, more
specifically,
acute, intermittent and breakthrough pain.
[0010] Therefore, a need exists for a device and system that can be used to
administer a controlled substance, such as an opioid (e.g., by patient-
controlled
administration), for treatment of pain, wherein the device provides for safe
and
controlled delivery via the oral mucosa, while minimizing the potential for
drug abuse
and/or diversion.
[0011] The present invention addresses these needs.
2
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
Brief Summary Of The Invention.
[0012] The invention provides drug dispensing devices, methods, systems and
kits
for oral transmucosal, e.g., sublingual administration of a bioadhesive small
volume
sufentanil-containing drug dosage form to a subject using a device.
[0013] The device may be hand-held and portable and comprises a cartridge
containing one or more drug dosage forms (typically from 1 to about 200)
dosage
forms.
[0014] Each dosage form comprises 5 mcg, 10mcg, 15 mcg, 20mcg, 30mcg,
40mcg, 50mcg, 60mcg, 70mcg, 80mcg or 100mcg of sufentanil and has a volume of
less than 100 microliters or a mass of less than 100mg.
[0015] In using the device, the dispensing end of the device is inserted into
the
mouth of the subject and a dosage form is dispensed through the dispensing end
of
the device such that it is placed on an oral mucosal membrane (e.g., in the
sublingual space) of the subject.
[0016] The dispensing end of the device has a proboscis comprising a shroud
for
placing the dosage form and the shroud includes a means to prevent or retard
saliva
and other moisture ingress into the device, such that the dosage forms remain
dry
prior to placement on the oral mucosal membrane.
[0017] The device further comprises a lock-out feature for setting a lock-out
time
wherein a dosage form cannot be dispensed from the device during the lock-out
time. The lock-out time may be a fixed time lock-out interval, a predetermined
lock-
out interval, a predetermined variable lock-out interval, a lock-out interval
determined by an algorithm or a variable lock-out interval communicated to the
device from a remote computer, docking station or other device.
[0018] The cartridge may comprise one or more shipping tablets wherein at
least
one shipping tablet is dispensed prior to dispensing of a dosage form.
[0019] The cartridge may include a smart cartridge recognition system
comprising
a physical keyed feature on the cartridge, an optically detected feature or
pattern, a
bar code on the cartridge, a magnetic tag on the cartridge, an RFID tag on the
cartridge, an electronic microchip on the cartridge, or a combination thereof.
[0020] The dispensing device further comprises a user identification means
wherein the user identification means is a radio frequency identification
(RFID)
3
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
reader configured to couple with a matching RFID tag on a patient to be
identified
and the dispensing device is unlocked or enabled when the RFID reader on the
dispensing device detects a matching RFID tag on a patient.
[0021] The dispensing device may also comprise a means for recording dosing,
use
history, or both, alone or in combination with a means to view or download the
dosing and/or use history.
[0022] Following placement of a dosage from on an oral mucosal membrane of the
subject, erosion of the dosage form is complete in from about 30 seconds to
about
30 minutes.
[0023] In carrying out the method, a single sublingual administration of a
dosage
form to a subject results in a bioavailability of at least 50%, an AUC with a
coefficient
of variation of less than 40%, a Tmax with a coefficient of variation of less
than 40%;
repeated sublingual administrations of a dosage form to a subject results in a
bioavailability that is greater than the bioavailability following a single
sublingual
administration to the subject and the Tmax following repeated sublingual
administration and the time of the previous sublingual administration is
shorter than
the T,T,õ following a single sublingual administration to the subject.
[0024] When a bioadhesive small volume sufentanil-containing drug dosage form
is
administered to the sublingual cavity of subject using a device, an amount of
drug
selected from the group consisting of at least 55%, at least 60%, at least
65%, at
least 70%, at least 75%, at least 80%, at least 85%, at least 90%, at least
95%, at
least 98% and at least 99% of the total amount of drug in the dosage form is
absorbed via the sublingual route.
[0025] Administration of a sufentanil-containing drug dosage form using a drug
dispensing deice may be patient controlled and may be used for treating pain
in a
subject, wherein following administration of the dosage form, pain relief is
evident.
Brief Description Of The Drawings.
[0026] Figs. 1A ¨ E provide a schematic depiction of an exemplary dispensing
device wherein the device is designed to deliver drug dosage forms to oral
mucosa of
a patient under treatment.
[0027] Fig. 2 is a schematic depiction of an exemplary dispensing device
showing
features designed to block or retard saliva and moisture ingress.
4
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
[0028] Figs. 3A and 3B are schematic depictions of an exemplary geometry for a
dispensing tip.
[0029] Figs. 4A ¨ D are a schematic depiction of an exemplary proboscis of a
dispensing device.
[0030] Figs. 5A ¨ D provide a series of flow diagrams for use of an exemplary
device showing the stages of push rod/tablet interaction during device use,
wherein
Fig. 5A shows the LOAD feature; Fig. 5B shows the CALIBRATE feature; Fig. 5C
shows the DISPENSE feature; and Fig. 5D shows the DISASSEMBLE feature.
[0031] Fig. 6 is a schematic depiction of an exemplary device showing
the stages
of push rod/tablet interaction during device use.
[0032] Fig 7 is a schematic architecture connection diagram illustrating
the
various components that may be included in a drug dispensing device or system.
[0033] Fig. 8A is a block diagram illustrating one aspect of
communication in the
drug dispensing system, including an RFID tag, a drug dispensing device, a
base
station/dock and a healthcare provider personal computer.
[0034] Fig. 8B is a block diagram illustrating another aspect of
communication in
a drug dispensing system, including an RFID tag, a drug dispensing device, a
portable docking FOB, a base station and a healthcare provider personal
computer.
[0035] Figure 9 is a graphic depiction of sufentanil plasma
concentrations
following intravenous dosing or sublingual single dose administration of three
different strengths of sufentanil dosage forms in healthy human volunteers (n
= 12).
[0036] Figure 10 is a graphic depiction of sufentanil plasma
concentrations
following sublingual administration of a sufentanil formulation #44
(equivalent to
human #47 formulation; n=3) compared to intravenous sufentanil administration
(n=3) in a healthy, conscious Beagle dog model. Error bars represents standard
errors around the mean (SEM).
[0037] Figure 11 is a graphic depiction of sufentanil plasma
concentrations
following sublingual administration of a sufentanil solution (n=6) or
following oral
ingestion of a sufentanil (n=6) compared to intravenous administration of
sufentanil
(n=3) in a healthy, conscious Beagle dog model. Error bars represents
standard
error around the mean (SEM).
[0038] Figure 12 is a graphic depiction of alfentanil plasma
concentrations
following sublingual administration of an alfentanil NanoTabTm (n=2) compared
to
intravenous alfentanil administration (n=3) in a healthy, conscious Beagle dog
model. Error bars represents standard error around the mean (SEM).
5
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
[0039] Figure 13 is a graphic depiction of the effect of pH on
dissolution kinetics
of sufentanil from exemplary oral transmucosal formulation.
[0040] Figures 14A and 14B are schematic depictions of an exemplary single
dose
applicator.
[0041] Figures 15A ¨ C provide an illustration of one type of single dose
applicator and use thereof in delivering a dosage form to a subject.
[0042] Figures 16A ¨ F provide an illustration of six additional single
dose
applicators.
[0043] Figure 17 provides an illustration of a multiple dose applicator
where a
plurality of single dose applicators are stored prior to use.
[0044] Figures 18A ¨ C provide an illustration of additional single dose
applicator
and multiple dose applicator embodiments.
[0045] Figures 19A ¨ B provide an illustration of two stages of use of
one
embodiment of a single dose applicator.
[0046] Figs. 20A ¨ 20D are schematic depictions of additional examples of
single
dose applicators (SDAs).
[0047] Figs. 21A ¨ 21D provide a schematic depiction of a multiple dose
applicator or container which provides for storage of a plurality of SDAs
prior to use,
and the use of the SDAs for sublingual administration of a drug dosage form.
Detailed Description.
I. Introduction
[0048] Provided herein are compositions, methods, systems and kits for
oral
transmucosal administration of opioid-containing small volume dosage forms
using a
device. Oral transmucosal delivery of the dosage forms minimizes the saliva
response
and therefore minimizes delivery of the drug to the GI tract, such that the
majority of
drug is delivered across the oral mucosa. The small volume dosage forms have
bioadhesive properties which facilitate adherence to the oral mucosa, thus
minimizing the risk of ingestion and inefficient delivery due to swallowing.
[0049] The following disclosure describes the dosage forms, devices, methods,
systems and kits which constitute the invention. The invention is not limited
to the
specific dosage forms, devices, methodology, systems, kits or medical
conditions
described herein, as such may, of course, vary. It is also to be understood
that the
terminology used herein is for the purpose of describing particular
embodiments
only, and is not intended to limit the scope of the present invention.
6
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
[0050] It must be noted that as used herein and in the appended claims, the
singular forms "a", "and", and "the" include plural references unless the
context
clearly dictates otherwise. Thus, for example, reference to "a drug
formulation"
includes a plurality of such formulations and reference to "a drug delivery
device"
includes systems comprising drug dosage forms and delivery device devices for
containment, storage and delivery of such dosage forms.
[0051] Unless defined otherwise, all technical and scientific terms used
herein
generally have the same meaning as commonly understood to one of ordinary
skill in
the art to which this invention belongs. Although any methods, devices and
materials
similar or equivalent to those described herein can be used in the practice or
testing
of the invention, the preferred methods, devices and materials are now
described.
[0052] The publications discussed herein are provided solely for their
disclosure
prior to the filing date of the present application. Nothing herein is to be
construed
as an admission that the invention is not entitled to antedate such a
disclosure by
virtue of prior invention.
II. Definitions
[0053] The term "active agent" or "active" may be used interchangeably herein
with the term "drug" and is meant to refer to any therapeutically active
agent.
[0054] The term "adhere" is used herein with reference to a drug dosage form
or
formulation that is in contact with a surface such as a mucosal surface and is
retained on the surface without the application of an external force. The term
"adhere" is not meant to imply any particular degree of sticking or bonding,
nor is it
meant to imply any degree of permanency.
[0055] The term "analgesic drug" as used herein includes sufentanil or a
sufentanil congener, such as alfentanil, fentanyl, lofentanil, carfentanil,
remifentanil,
trefentanil, or mirfentanil, as well as formulations comprising one or more
therapeutic compounds. Use of the phrase "sufentanil or a congener" is not
meant
to be limiting to use of, or formulations comprising, only one of these
selected opioid
compounds. Furthermore, reference to sufentanil alone or to a selected
sufentanil
congener alone, e.g., reference to "alfentanil", is understood to be only
exemplary of
the drugs suitable for delivery according to the methods of the invention, and
is not
meant to be limiting in any way.
[0056] The term "AUC" as used herein means "area under the curve" in a
plot of
concentration of drug in plasma versus time. AUC is usually given for the time
7
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
interval zero to infinity, however, clearly plasma drug concentrations cannot
be
measured 'to infinity' for a patient so mathematical approaches are used to
estimate
the AUC from a limited number of concentration measurements. In a practical
sense,
the AUC (from zero to infinity) represents the total amount of drug absorbed
by the
body, irrespective of the rate of absorption. This is useful when trying to
determine
whether two formulations of the same dose release the same dose of drug to the
body. The AUC of a transmucosal dosage form compared to that of the same
dosage
administered intravenously serves as the basis for a measurement of
bioavailability.
[0057] The term "bioadhesion" as used herein refers to adhesion to a
biological
surface including mucosal membranes.
[0058] The term "bioavailability" or "F" as used herein means "percent
bioavailability" and represents the fraction of drug absorbed from a test
article as
compared to the same drug when administered intravenously. It is calculated
from
the AUC. of the test article following delivery via the intended route versus
the AUC.
for the same drug after intravenous administration. It is calculated from the
equation: Bioavailability (%) = AUC. (test article)/ AUC. (intravenous
route/article).
[0059] The term "breakthrough pain" as used herein, is a transitory flare of
pain
of moderate to severe intensity occurring on a background of otherwise
controlled
pain. "Breakthrough pain" can be intense for short periods of time, as short
as 1 or 2
minutes or as long as 30 minutes or more.
[0060] The term "cartridge" is used herein with reference to a replaceable,
single
use disposable cartridge configured to hold one or more drug dosage forms,
typically, up to 200 drug dosage forms. The cartridge typically comprises a
smart
cartridge recognition system with a physical keyed feature on the cartridge, a
bar
code on the cartridge, a magnetic tag on the cartridge, an RFID tag on the
cartridge,
an electronic microchip on the cartridge, or a combination thereof. The
cartridge may
comprise one or more shipping tablets wherein at least one shipping tablet is
dispensed prior to dispensing of a dosage form.
[0061] The term "Cmax" as used herein means the maximum observed plasma
concentration following administration of a drug.
[0062] The term "congener" as used herein refers to one of many variants or
configurations of a common chemical structure.
[0063] The term "disintegration" is used interchangeably herein with "erosion"
and means the physical process by which a dosage form breaks down and pertains
to the physical integrity of the dosage form alone. This can occur in a number
of
8
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
different ways including breaking into smaller pieces and ultimately, fine and
large
particulates or, alternatively, eroding from the outside in, until the dosage
form has
disappeared.
[0064] The term "dissolution" as used herein means the process by which the
active ingredient is dissolved from the tablet in the presence of a solvent,
in vitro, or
physiological fluids in vivo, e.g., saliva, irrespective of the mechanism of
release,
diffusion, erosion or combined erosion and diffusion.
[0065] The term "dispensing device", "drug dispensing device", "dispenser",
"drug
dispenser", "drug dosage dispenser," device" and" drug delivery device" are
used
interchangeably herein and refer to a device that dispenses a drug dosage
form. The
dispensing device provides for controlled and safe delivery of a
pharmaceutically
active substance (e.g., an opioid such as sufentanil) formulated in the dosage
form.
The device may be adapted for storage and/or delivery of a dosage form such as
a
lozenge, pill, tablet, capsule, membrane, strip, liquid, patch, film, gel,
spray or other
form.
[0066] The term "dispensing end" as used herein with reference to a device
means the portion of the device comprising the proboscis and shroud which
serves
to deliver a drug dosage form to the oral mucosa of a subject.
[0067] The term "drug", "medication", "pharmacologically active agent",
"therapeutic agent" and the like are used interchangeably herein and generally
refer
to any substance that alters the physiology of an animal and can be
effectively
administered by the oral transmucosal route.
[0068] The term "erosion time" means the time required for a solid dosage form
to break down until the dosage form has disappeared.
[0069] The term "FOB" refers to a small, portable handheld, powered electronic
docking device that can be used in conjunction with the drug dispensing device
to
upload data, download data, control access to the drug dispensing device,
control
access to the drug dosage forms, or enhance or otherwise alter the user
interface of
the drug dispensing device. A FOB may communicate and dock with a drug
dispensing device either in a wired or wireless fashion. A FOB may be adapted
to
attach to a cord so as to allow the FOB to hang from the neck of a healthcare
professional such as a physician or caregiver, particularly in the hospital
setting. A
drug dispensing device may communicate with the physician or care giver via
the
FOB.
9
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
[0070] The terms "formulation" and "drug formulation" as used herein refer to
a
physical composition containing at least one pharmaceutically active
substance,
which may be provided in any of a number of dosage forms for delivery to a
subject.
The dosage form may be provided to the patient as a lozenge, pill, capsule,
membrane, strip, liquid, patch, film, gum, gel, spray or other form.
[0071] The term "hydrogel-forming preparation", means a solid formulation
largely devoid of water which upon contact with an aqueous solution, e.g., a
bodily
fluid, and in particular that of the oral mucosa, absorbs water in such a way
that it
forms a hydrated gel in situ. The formation of the gel follows unique
disintegration
(or erosion) kinetics while allowing for release of the therapeutic agent over
time.
Additionally, the term "hydrogel-forming preparation' describes a solid
formulation
largely devoid of water which upon contact with bodily fluids, and in
particular those
in the oral cavity, transforms into a film that releases the drug. Such films
increase
the surface area available for drug release and absorption thus enabling
faster
absorption of the drug.
[0072] The term "lock-out feature" is used herein with reference to a
feature of
the device which provides for a "lock-out time".
[0073] The term "lock-out time" is used herein with reference to the period of
time during which the device does not allow drug accessibility, i.e., a dosage
form
cannot be dispensed during the "lock-out time". "Lock-out time" may be
programmable, a fixed time interval, a predetermined interval, a predetermined
variable interval, an interval determined by an algorithm or a variable
interval
communicated to the device from a remote computer or docking station.
[0074] The term "LogP" as used herein means logarithm of the ratio of
equilibrium concentrations of un-ionized compound between octanol and water. P
also called the "octanol-water partition coefficient" and serves as a means to
quantify
the hydrophiobicity or lipophilicity of, a chemical characteristic of a given
drug.
[0075] The term "mucoadhesion" is used herein in to refer to the adhesion to
mucosal membranes which are covered by mucus, such as those in the oral cavity
and may be used interchangeably herein with the term "bioadhesion" which
refers to
adhesion to any biological surface.
[0076] The term "mucosal membrane" refers generally to any of the mucus-
coated biological membranes in the body. Absorption through the mucosal
membranes of the oral cavity is of particular interest. Thus, oral mucosal
absorption,
i.e., buccal, sublingual, gingival and palatal absorption are specifically
contemplated.
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
[0077] The term "mucosal-depot" is used herein in its broadest sense to refer
to a
reservoir or deposit of a pharmaceutically active substance within or just
beneath the
mucosal membrane.
[0078] The term "non-ordered particulate mixture" or "non-ordered mixture" is
used herein with reference to a formulation where the mixture is not ordered
with
respect to the pharmaceutically active agent and the bioadhesive material or
bioadhesion promoting agent, or other formulation components. In addition, it
is
used herein with reference to any formulation prepared by a process that
involves
dry mixing wherein drug particles are not uniformly distributed over the
surface of
larger carrier particles. Such 'non-ordered' mixing may involve dry mixing of
particles
in a non-ordered fashion, where there is no requirement with respect to the
order of
addition/mixing of specific excipients with the drug, bioadhesive material or
bioadhesion promoting agent and/or disintegrants. Further in the non-ordered
mixing
process, there is no limitation on the size of the drug particles. The drug
particles may be larger than 25pm. In addition, a "non-ordered mixture"
includes any
mixing processes in which the primary carrier particles do not incorporate a
disintegrant within. Finally the "non-ordered mixture" may be prepared by any
'wet
mixing' processes, i.e. processes in which a solvent or non-solvent is added
during
the mixing process or any mixing process in which the drug is added in a
solution or
suspension form.
[0079] The term "operatively connected" as used herein means the components
are provided in a device so as to function as intended to achieve an aim. For
example, a memory device operatively connected to a CPU which is further
operatively connected to a release mechanism may be meant to indicate that,
upon
actuation, the CPU communicates with the memory device to check the status or
history of drug delivery, and then further communicates with the release
mechanism
(e.g., via a solenoid and a switch) to release and dispense a drug.
[0080] The terms "oral transmucosal dosage form" and "drug dosage form" may
be used interchangeably herein and refer to a dosage form which comprises a
pharmaceutically active substance, e.g., a drug such as sufentanil. The oral
dosage
form is used to deliver the pharmaceutically active substance to the
circulation by
way of the oral mucosa and is typically a "sublingual dosage form", but in
some
cases other oral transmucosal routes may be employed. The dosage form provides
for delivery of the pharmaceutically active substance across the oral mucosa
and not
via swallowing followed by GI absorption, by controlling the formulation the
timing
11
CA 02673837 2014-10-23
WO 2008/085764
PCT/US2007/089017
for release of the pharmaceutically active substance can be achieved. The
dosage
form comprises pharmaceutically acceptable excipients and may be referred to
as a
NanoîabTM, as detailed in U.S. Patent No. 8,202,535. The dosage form
comprises a formulation that is neither effervescent nor does it comprise an
essentially water-free, ordered mixture of microparticles of drug adhered to
the
surface of carrier particles, where the carrier particles are substantially
larger than
the microparticles of drug.
[0081] The terms "oral transmucosal drug delivery" and "oral
transmucosal
administration" as used herein refer to drug delivery that occurs
substantially via the
oral transmucosal route and not via swallowing followed by GI absorption. This
includes delivery via buccal, sublingual and gum transmucosal areas.
[0082] The term "proboscis" is used interchangeably with the terms "dispensing
tip" a "delivery tip", and refers to a dispensing and/or positioning tip of a
drug
dosage form dispenser that delivers a dosage form to the oral mucosa (e.g.,
the
=
sublingual space).
[0083] The term "radio frequency identification device" or "RFID" is used with
reference to an automatic identification method, which relies on storing and
remotely
retrieving data using devices called RFID tags, wherein the RFID tag is
applied to, or
incorporated into a product, or person for the purpose of identification using
radiowaves. Some tags can be read from several meters away and beyond the line
of
sight of the reader.
[0084] The term "replaceable cartridge" or "disposable cartridge" is used with
reference to a cartridge for housing drug dosage forms which is typically
configured
to hold up to 200 drug dosage forms, wherein the cartridge is designed to be
used
and discarded.
[0085] The term "shipping tablet" is used herein with reference to an
"initialization", or "shipping" tablet which is the same size and shape as a
drug-
containing dosage form but does not contain a pharmaceutically active
substance.
The "shipping tablet" may comprise a placebo dosage form that does not contain
a
pharmaceutically active substance or may be made of plastic or other material.
It is
the first thing dispensed from a new cartridge after insertion into a
dispensing
device. The device has a means for differentiating between the shipping tablet
and a
dosage form containing a pharmaceutically active substance.
12
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
[0086] The term "shroud" is used to describe a partial or complete covering of
the
dispensing end of the device which protects the delivery port from contact
with saliva
or other moisture in the oral cavity and forms a barrier between the device,
the oral
mucosa and tongue, has a relief for dosage form delivery, and an interior that
is
hydrophobic or hydrophilic which serves to minimize or eliminate saliva
ingress or
moisture ingress. The "shroud" creates a barrier from the oral mucosa
contacting the
valve area and dosage form, aiding in dosage form dispensing and discouraging
dosage form adherence to the shroud. The shroud may have a rounded interior
surface or other geometry to stop the dosage form adhering to the shroud. The
shroud limits the ability of the tongue or oral mucosa to contact the dosage
form
dispensing area, thereby controlling saliva contact and ingress. The shroud
shields
the valve from moisture and saliva ingress from the tongue and other mucosa
and
provides an area for the dosage form to exit the device without "sticking" to
the
wetted distal valve or shroud area. The shroud also comprises a cut-out/
relief in
order to mitigate the dragging of dosage forms when the device is removed from
the
oral space. The valve functions with the shroud to control saliva and moisture
ingress, as well as aid in delivery of the dosage form.
[0087] The term "subject" includes any subject, generally a mammal
(e.g.,
human, canine, feline, equine, bovine, ungulate etc.), adult or child, in
which
treatment for a disorder is desired. The terms "subject" and "patient" may be
used
interchangeably herein.
[0088] The term "systems that include a drug dosage form and a dispensing
device" as used herein refers to a drug dispensing system for delivery and/or
monitoring of drug administration. The system may be used to monitor and
deliver a
pharmaceutically active substance, e.g., an opioid such as sufentanil, wherein
the
amount of drug delivered, corresponding efficacy and safety are enhanced over
currently available systems. The system may have one or more features that
provide
for improved safety and ease of use over currently available systems including
a
security feature that prevents unauthorized access to the stored drugs, a
dosing
lock-out feature, a means for identifying an individual user for controlled
drug
access, a dose counting feature, a memory means for retaining information
about
dose delivery, and an interface for bidirectional exchange of information with
a user,
a drug cartridge, or another device such as a computer.
[0089] The term "small volume drug dosage form" or "small volume dosage form"
is used herein with reference to a small volume dosage form that has a volume
of
13
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
less than 100p1 and a mass of less than 100mg. More specifically, the dosage
form
has a mass of less than 100mg, 90mg, 80mg,170mg, 60mg, 50mg, 40mg, 30mg,
29mg,28mg, 27mg, 26mg, 25mg, 24mg, 23mg, 22mg, 21mg, 20mg, 19mg,18mg,
17mg, 16mg, 15mg, 14mg, 13mg, 12mg, 11mg, 10mg, 9mg, 8mg, 7mg, 6mg or
5mg or a volume of less than 100p1, 90p1, 80p1, 70p1, 60 pl, 50p1, 40p1, 30p1,
29mg,
28mg, 27p1, 26p1, 25p1, 24p1, 23p1, 22p1, 21p1, 20p1, 19p1, 18p1, 17p1, 16p1,
15p1,
14p1, 13p1, 12p1, 11p1, 10p1, 9p1, 8p1, 7p1, 6p1 or 5p1. The "dosage form" may
or may
not have bioadhesive characteristics and may form a hydrogel upon contact with
an
aqueous solution.
[0090] The "dosage form" may be used to deliver any drug that can be
administered by the oral transmucosal route in an amount amenable to
administration via the small size of the dosage form, i.e. 0.25pg to 99.9mg,
1pg to
50mg or 1pg to 10mg.
[0091] The term "small volume sufentanil-containing drug dosage form" is used
herein with reference to a small volume dosage form that contains a dose of
sufentanil selected from about 2 micrograms (mcg) to about 200 mcg of
sufentanil,
e.g., 5 mcg, 10 mcg, 15 mcg, 20mcg, 30mcg, 40mcg, 50mcg, 60mcg, 70mcg, 80mcg
or 100mcg of sufentanil.
[0092] The term "solid dosage form" or "solid drug dosage form" is used herein
with reference to a small volume dosage form that is a solid, e.g., a lozenge,
a pill, a
tablet, a membrane or a strip.
[0093] The term "sublingual", means literally "under the tongue" and refers to
administering a drug dosage form via the mouth in such a way that the
pharmaceutically active substance is rapidly absorbed via the blood vessels
under the
tongue rather than via the digestive tract. Absorption occurs via the highly
vascularized sublingual mucosa and allows the pharmaceutically active
substance
more direct access to the blood circulation, providing for direct systemic
administration independent of GI influences.
[0094] The term "Therapeutic Time Ratio" or "TTR" presents the average time
that the drug is present at therapeutic levels, defined as time within which
the drug
plasma concentration is maintained above 50% of Cmax normalized by the drug's
elimination half-life and it is calculated by the formula: TTR= (Time above
50% of
Cmax)/(Terminal intravenous elimination half-life of the drug). The last term
is
obtained from literature data for the drug of interest in the appropriate
species.
14
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
[0095] The term "Tmõ" as used herein means the time point of maximum
observed plasma concentration.
[0096] The term "Tonset" as used herein means the observed "time of onset" and
represents the time required for the plasma drug concentration to reach 50% of
the
maximum observed plasma concentration, Cmax.
[0097] The term "therapeutically effective amount" means an amount of a
therapeutic agent, or a rate of delivery of a therapeutic agent (e.g., amount
over
time), effective to facilitate a desired therapeutic effect, such as pain
relief. The
precise desired therapeutic effect (e.g., the degree of pain relief, and
source of the
pain relieved, etc.) will vary according to the condition to be treated, the
tolerance of
the subject, the drug and/or drug formulation to be administered (e.g., the
potency
of the therapeutic agent (drug), the concentration of drug in the formulation,
and
the like), and a variety of other factors that are appreciated by those of
ordinary skill
in the art.
III. Drug Dosage Forms.
[0098] The claimed small volume oral transmucosal drug dosage forms produce a
reduced saliva response as compared with conventional, larger dosage forms
that
are intended to deliver a drug in the oral cavity. The dosage forms contain a
pharmaceutically active substance and provide for high absorption rates of the
pharmaceutically active substance across the oral mucosa and reduced uptake
via
the gastrointestinal tract, thereby offering a more consistent and
reproducible
pharmacokinetic and corresponding pharmacodynamic profile.
[0099] The dosage forms are typically a "sublingual dosage form", but in some
cases other oral transmucosal routes may be employed. The dosage form is a
substantially homogeneous composition which comprises one or more active drugs
together with pharmaceutically acceptable excipients.
[00100] The preferred site for oral transmucosal drug delivery is the
sublingual
space, although in certain embodiments it may be advantageous for the dosage
form
to be placed inside the cheek, or to adhere to the roof of the mouth or the
gum.
[00101] The dosage forms provide for the delivery of a greater percentage (and
amount) of the drug via the oral mucosa and a corresponding decrease in
delivery
via the gastrointestinal (GI) tract as compared to traditional oral dosage
forms and
other oral transmucosal dosage forms.
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
[00102] Typically, the dosage forms are generally adapted to adhere to the
oral
mucosa (i.e. are bioadhesive) during the period of drug delivery, and until
most or all
of the drug has been delivered from the dosage form to the oral mucosa.
[00103] The claimed dosage forms have a mass of less than 100mg and a volume
of less than 100u1. More specifically, the dosage forms have a mass of less
than
100mg, 90mg, 80mg,170mg, 60mg, 50mg, 40mg, 30mg, 29mg,28mg, 27mg, 26mg,
25mg, 24mg, 23mg, 22mg, 21mg, 20mg, 19mg,18mg, 17mg, 16mg, 15mg, 14mg,
13mg, 12mg, 11mg, 10mg, 9mg, 8mg, 7mg, 6mg or 5mg or a volume of less than
100p1, 90p1, 80p1, 70p1, 60 pl, 50p1, 40p1, 30p1, 29mg, 28mg, 27p1, 26p1,
25p1, 24p1,
23p1, 22p1, 21p1, 20p1, 19p1, 18p1, 17p1, 16p1, 15p1, 14p1, 13p1, 12p1, 11p1,
10p1, 9p1,
8p1, 7p1, 6p1 or 5p1. The dosage forms typically have bioadhesive
characteristics and
may form a hydrogel upon contact with an aqueous solution.
[00104] The dosage forms typically have an erosion time of from 30 seconds up
to
5 minutes, up to 10 minutes, up to 15 minutes or up to 30 minutes.
[00105] In general, at least 55%, at least 60%, at least 65%, at least 70%, at
least 75%, at least 80%, at least 85%, at least 90%, at least 95%, at least
98% or
at least 99% of the total amount of pharmaceutically active substance in a
dosage
form administered to the oral mucosa of a subject is absorbed via the oral
transmucosal route.
[0106] The dosage forms may have essentially any shape, examples of which
include a round disc with a flat, concave, or convex face, an ellipsoid shape,
a
spherical shape, a polygon with three or more edges and flat, concave, or
convex
faces. The dosage forms may be symmetrical or asymmetrical, and may have
features or geometries that allow for controlled, convenient, and easy
storage,
handling, packaging or dosing.
[0107] Oral transmucosal drug delivery is simple, non-invasive, and can
be
administered by a caregiver or patient with minimal discomfort. A dosage form
for
oral transmucosal delivery may be solid or non-solid. In one preferred
embodiment,
the dosage from is a solid that turns into a hydrogel following contact with
saliva. In
another preferred embodiment, the dosage from is a solid that erodes without
forming a hydrogel following contact with saliva.
[0108] Generally, oral transmucosal delivery of pharmaceutically active
substances is achieved using solid dosage forms such as lozenges or tablets,
however, liquids, sprays, gels, gums, powders, and films and the like may also
be
used.
16
CA 02673837 2014-10-23
WO 2008/085764
PCT/US2007/089017
[0109] For certain drugs, such as those with poor bioavailability via the GI
tract,
e.g., lipophilic opioids such as sufentanil and alfentanil, oral transmucosal
delivery is
a more effective delivery route than GI delivery. For such lipophilic drugs,
oral
transmucosal delivery has a shorter onset time (i.e., the time from
administration to
therapeutic effect) than does oral GI delivery and provides better
bioavailability and
more consistent pharmacokinetics.
[0110] The claimed drug dosage forms are designed and adapted to reduce the
saliva response, thus reducing the amount of drug swallowed, and thereby
delivering
a substantial amount of drug to a subject via the oral mucosa. The claimed
drug
dosage forms also provide efficacious delivery of drug via the oral mucosa and
a
consistent plasma level within the therapeutic window.
[0111] The claimed dosage forms comprise substantially homogeneous
formulations which include at least 0.0010/0 percent by weight of the
pharmaceutically active substance in combination with pharmaceutically
acceptable
excipients. Typically the claimed dosage forms comprise from 0.01-99% or from
about 0.254 to 99.9mg, from about 1pg to 50mg or from about 1pg to 10mg w/w
of the pharmaceutically active substance.
[0112] Formulations for preparation of the claimed dosage forms and methods of
making them are described in US Patents 8,252,3 29 and 8,252,328.
An exemplary formulation is bioadhesive and comprises from about 0.0004% to
about 0.04% sufentanil, e.g., 0.0005%, 0.0010/0, 0.002%, 0.003%, 0.004%,
0.006%, 0.008%, 0.010/0, 0.012%, 0.014% or 0.016% sufentanil. In general, the
formulation comprises(a) a non-ordered mixture of a pharmaceutically active
amount
of a drug; (b) a bioadhesive material which provides for adherence to the oral
mucosa of the subject; and (c) stearic acid, wherein dissolution of a dosage
form
comprising the formulation is independent of pH, e.g., over a pH range of
about 4 to
8.
[0113] Pharmaceutically active agents for use in a formulation of the
invention are
characterized by logarithm of the octanol-water partition coefficient (LogP)
between
0.006 and 3.382.
[0114] A pharmaceutical dosage form of the invention for oral transmucosal
delivery may be solid or non-solid. In one preferred embodiment, the dosage
from is
a solid that transforms into a hydrogel following contact with saliva. In
another
preferred embodiment, the dosage form is a solid that transforms into a
bioadhesive
film upon contact with saliva.
17
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
[0115] In a preferred embodiment, such formulations are designed to form a
film
which is visible following tablet disintegration. Upon placement on the oral
mucosa,
the dosage form absorbs water such that upon full hydration, it spreads across
the
mucosal surface, thus transforming into a bioadhesive film containing the
active
drug. This transformation results in a significant increase of the surface
area
available for drug release, thus accelerating drug diffusion and release from
the
dosage form. Owing to the higher contact surface area, drug absorption occurs
fast,
resulting in fast onset of action.
[0116] Numerous
suitable nontoxic pharmaceutically acceptable carriers for use in
oral dosage forms can be found in Remington's Pharmaceutical Sciences, 17th
Edition, 1985.
[0117] It will be understood that the formulation is converted into a dosage
form
for delivery to a subject using procedures routinely employed by those of
skill in the
art, such as direct compression, wet granulation, etc. The process for
preparation of
the dosage form is optimized for each formulation in order to achieve high
dose
content uniformity.
[0118] While not wishing to be bound by theory, in one exemplary embodiment,
when a drug dosage form is placed in the sublingual cavity, preferably under
the
tongue on either side of the frenulum linguae, it adheres upon contact. As the
dosage form is exposed to the moisture of the sublingual space the dosage form
absorbs water, resulting in erosion of the dosage form and release of the
active drug
to the circulation of the subject.
IV. Sufentanil.
[0119] Opioids are widely used for the treatment of pain, and are generally
delivered intravenously, orally, epidurally, transdermally, rectally and
intramuscularly.
Morphine and its analogues are commonly delivered intravenously and are
effective
against severe, chronic and acute pain. However, they can also have severe
respiratory depressive effects if not used appropriately and also suffer from
a high
abuse potential. The predominant cause of morbidity and mortality from pure
opioid
overdoses is due to respiratory complications.
[0120] One exemplary use of the claimed drug dosage forms is with application
to
pain-relief. When the claimed drug dosage forms are used for treatment of
pain,
they comprise a drug such as an opioid or opioid agonist and are utilized to
treat
both acute and chronic pain of moderate to severe intensity.
18
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
[0121] The active agent in such drug dosage forms is sufentanil or a
sufentanil
congener such as alfentanil, fentanyl, lofentanil, carfentanil, remifentanil,
trefentanil,
or mirfentanil. In a preferred embodiment, sufentanil is the active agent.
Sufentanil
may be provided as sufentanil citrate, sufentanil base, or a combination
thereof.
[0122] Another preferred embodiment relies on a sufentanil congener as the
active agent. Yet another preferred embodiment relies on a combination of
sufentanil
and at least one additional agent for treatment of analgesia as the active
agent, e.g.,
a combination of sufentanil and alfentanil. Various opioid drugs have
different
pharmacokinetic profiles and different interactions with mu opioid receptor
splice
variants and, therefore, may be used in combination to enhance the therapeutic
effect.
[0123] Sufentanil (N-[(4-(Methoxymethy1-1-(2-(2-thienypethyl)-4-
piperidiny1)]-N-
phenylpropanamide), is used as a primary anesthetic, to produce balanced
general
anesthesia in cardiac surgery, for epidural administration during labor and
delivery
and has been administered experimentally in both intranasal and liquid oral
formulations. A commercial form of sufentanil used for IV delivery is the
SUFENTA
FORTE formulation. This liquid formulation contains 0.075 mg/ml sufentanil
citrate
(equivalent to 0.05 mg of sufentanil base) and 9.0 mg/ml sodium chloride in
water.
It has a plasma elimination half-life of 148 minutes, and 80% of the
administered
dose is excreted in 24 hours.
[0124] Following transbuccal administration of fentanyl using a lozenge
(e.g.,
Actiq ), the bioavailability is 50%, although the Tmõ for the 200 mcg dosage
of
Actiq ranges from 20 ¨ 120 minutes resulting from erratic GI uptake due to
the fact
that 75% of the fentanyl is swallowed (Actiq package insert). More recent
publications on the Tmax of Actiq indicate that these original times were
skewed
towards more rapid onset (Fentora package insert indicates a range of Tmõ for
Actiq
extending up to 240 minutes). Fentora (a fentanyl buccal tablet) exhibits a
bioavailability of 65%, with reported swallowing of 50% of the drug. In
contrast to
the claimed dosage forms, both Actiq and Fentora suffer from the disadvantage
that
substantial amounts of lozenge-administered fentanyl are swallowed by the
patient.
[0125] Although sufentanil and fentanyl have many similarities as potent mu-
opioid receptor agonists, they have been shown to differ in many key ways.
Multiple
studies have demonstrated sufentanil to be in the range of 7 ¨ 24 times more
potent
than fentanyl (SUFENTA package insert; Paix A, et al. Pain, 63:263-69, 1995;
Reynolds L, et al., Pain, 110:182-188, 2004). Therefore, sufentanil may be
19
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
administered using a smaller dosage form, avoiding the increased saliva
response of
a larger dosage form and thereby minimizing the amount of drug that is
swallowed.
This leads to minimal GI uptake.
[0126] In addition, fentanyl and other opiate agonists, have the
potential for
deleterious side effects including respiratory depression, nausea, vomiting
and
constipation. Since fentanyl has a 30% bioavailability from the GI route, this
swallowed drug can contribute to the Cmax plasma levels to a significant
degree and
results in the erratic Cmax and Tmax observed with these products.
[0127] Further, the lipid solubility (octanol-water partition
coefficient) of
sufentanil (1778:1) is greater than fentanyl (816:1). Sufentanil also displays
increased protein binding (91 - 93%) compared with fentanyl (80 - 85%)
(SUFENTA
and Actiq package inserts, respectively). Sufentanil has a pKa of 8.01,
whereas the
pKa of fentanyl is 8.43 (Paradis et al., Therapeutic Drug Monitoring, 24:768-
74,
2002). These differences can affect various pharmacokinetic parameters, for
example, sufentanil has been shown to have a faster onset of action and faster
recovery times than fentanyl (Sanford et al., Anesthesia and Analgesia, 65:259-
66,
1986). As compared to fentanyl, use of sufentanil can result in more rapid
pain relief
with the ability to titrate the effect and avoid overdosing.
[0128] Importantly, sufentanil has been shown to produce endocytosis of the mu-
opioid receptor 80,000 times more potently than fentanyl (Koch et al.,
Molecular
Pharmacology, 67:280-87, 2005). The result of this receptor internalization is
that
neurons continue to respond to sufentanil more robustly over time than with
fentanyl, suggesting that clinically less tolerance would develop to
sufentanil
compared to fentanyl with repeated dosing.
[0129] The use of sufentanil clinically has predominantly been limited to
IV
administration in operating rooms or intensive care units. There have been a
few
studies on the use of liquid sufentanil preparations for low-dose intranasal
administration (Helmers et al., 1989; Jackson K, et al., J Pain Symptom
Management
2002: 23(6): 450-452) and case reports of sublingual delivery of a liquid
sufentanil
preparation (Gardner-Nix J., J Pain Symptom Management. 2001 Aug; 22(2):627-
30; Kunz KM, Theisen JA, Schroeder ME, Journal of Pain and Symptom Management,
8:189-190, 1993). In most of these studies, the smallest dosing of sufentanil
in
adults was 5 mcg in opioid naIve patients. Liquid administered to the oral or
nasal
mucosa suffers from lower bioavailability and possibly a shorter duration of
action as
demonstrated by the animal studies (sublingual liquid) described herein, as
well as
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
the literature (nasal liquid drops ¨ Helmers et al., 1989). Gardner-Nix
provides
analgesic data (not pharmacokinetic data) produced by liquid sublingual
sufentanil
and describes the analgesic onset of liquid sublingual sufentanil occurring
within 6
minutes but the duration of pain relief lasted only approximately 30 minutes.
[0130] Prior to the work of the current inventors, no pharmacokinetic data
had
been published on sublingual sufentanil in any form.
[0131] The claimed drug dosage forms contain from about 0.25 to about 200mcg
of sufentanil per dosage form for oral transmucosal delivery. In one exemplary
embodiment, each dosage form contains from about 0.25 to about 200mcg of
sufentanil, alone or combination with one or more other therapeutic agents or
drugs.
[0132] Exemplary drug dosage forms for administration to children
(pediatric
patients) contain from about 0.25 to about 120mcg of sufentanil per dosage
form.
For example, a drug dosage form for administration to children may contain
about
0.25, 0.5, 1, 2.5, 4, 5, 6, 8, 10, 15, 20, 40, 60 or 120mcg of sufentanil for
oral
transmucosal delivery. It follows that for pediatric patients, an exemplary
dose range
is from at least about 0.02 mcg/kg to about 0.5 mcg/kg with a preferable range
of
from about 0.05 to about 0.3 mcg/kg.
[0133] Exemplary drug dosage forms for administration to adults contain
from
about 2.5 to about 200mcg of sufentanil per dosage form. For example, a drug
dosage form for administration to adults may contain about 2.5, 3, 5, 7.5, 10,
15, 20,
40, 60, 80, 100, 120, 140, 180 or 200mcg or more of sufentanil for oral
transmucosal
delivery.
[0134] Preferably, a sufentanil-containing dosage form comprises from
about 2 to
about 200 micrograms (mcg) of sufentanil, e.g., 5 mcg, 10 mcg, 15 mcg, 20mcg,
30mcg, 40mcg, 50mcg, 60mcg, 70mcg, 80mcg or 100 mcg of sufentanil.
[0135] As will be understood by those of skill in the art, the dose will be on
the
low end of the range for children and the high end of the range for adults
dependent
upon body mass, in particular when administered long term to opioid-tolerant
adults.
Prior to the work of the current inventors, small-volume oral transmucosal
drug
delivery dosage forms of sufentanil have not been described.
[0136] In various embodiments, the claimed dosage forms provide effective pain
relief in all types of patients including children, adults of all ages who are
opioid
tolerant or naIve and non-human mammals. The invention finds utility in both
the
inpatient and outpatient setting and in the field.
21
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
V. Congeners of Sufentanil
[0137] Congeners of sufentanil find use in the compositions, methods and
systems described herein, examples of which include remifentanil and
alfentanil.
[0138] In certain embodiments, the dosage form comprises at least 0.005% to as
much as 99.9% by weight of alfentanil, lofentanil, carfentanil, remifentanil,
trefentanil or mirfentanil. The percentage of active ingredient(s) will vary
dependent upon the size of the dosage form and nature of the active
ingredient(s),
optimized to obtain maximal delivery via the oral mucosal route. In some
aspects
more than one active ingredient may be included in a single dosage form.
[0139] Remifentanil is a potent sufentanil congener that is metabolized
much
more rapidly than fentanyl or sufentanil, but may be suitable for treatment of
acute
pain when delivered via a sustained-release formulation. A remifentanil-
containing
dosage form typically comprises from about 0.25 mcg to 99.9 mg of
remifentanil.
The dose ranges for the remifentanil formulation may include 0.1 mcg/kg ¨ 50
mcg/kg over a time period of 20 minutes, for example, for both adult and
pediatric
patients. These dosages may be repeated at appropriate time intervals, which
may
be shorter than the time intervals for fentanyl or sufentanil.
[0140] Alfentanil is also a potent sufentanil congener that is rapidly
metabolized
but may be suitable for use in a sustained-release formulation. The dosage
forms
may contain from about 10 to about 10000 mcg of alfentanil per dosage form for
oral transmucosal delivery. As will be understood by those of skill in the
art, the dose
will be on the low end of the range for children and the high end of the range
for
adults dependent upon body mass, in particular when administered long term to
opioid-tolerant adults.
[0141] Exemplary dosage forms for administration to children (pediatric
patients)
contain from about 10 to about 6300 mcg of alfentanil per dosage form. For
example, a dosage form for administration to children may contain about 10,
25, 50,
130, 210, 280, 310, 420, 600, 780, 1050, 2100, 3000 or 6300 mcg of alfentanil
for
oral transmucosal delivery.
[0142] Exemplary dosage forms for administration to adults contain from about
70 to about 10000 mcg of alfentanil per dosage form. For example, a dosage
form
for administration to adults may contain about 70, 140, 160, 210, 280, 310,
420,
600, 780, 1050, 2100, 3000, 6300 or 10000 mcg or more of alfentanil for oral
transmucosal delivery.
22
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
[0143] Following delivery of a single dose of a sufentanil-, alfentanil-
, or
remifentanil-containing dosage form to a human subject, the plasma level of
sufentanil, alfentanil or remifentanil may reach a maximum level within 60
minutes,
e.g., between 5 and 50 minutes or between 10 and 40 minutes following
administration.
VI. Treatment Of Pain.
[0144] Patients suffering from chronic painful conditions can also have
intermittent exacerbations of their pain, requiring acute use of fast-acting
breakthrough opioids in addition to their use of slow-onset time-release
opioids for
their baseline chronic pain.
[0145] Breakthrough pain or procedural pain can be intense for short
periods of
time, as short as 1 or 2 minutes or as long as 30 minutes or more, therefore
there
would be a significant advantage in providing an opioid formulation that
produced
more rapid clinically effective plasma levels with a more consistent and
predictable
period of effect, but also had a limited half-life to avoid excessive opioid
dosing for
short duration pain events.
[0146] Opioids remain the most powerful from of analgesics, however, improved
forms are needed that have minimal side effects, and can be provided in a
manner in
which patient use can be easily tracked by the physician.
[0147] Using current treatment methods, pain control is attempted using a
number of interventions, which generally include: patient-controlled analgesia
(PCA),
continuous epidural infusion (CEI), other types of acute pain control,
palliative care
pain control, and home health patient pain control. These methods meet with
varying degrees of success with respect to duration of control, ease of
treatment and
safety versus side effects.
[0148] The need for rapid treatment of acute pain occurs in many different
clinical
situations, including post-operative recuperation, rheumatoid arthritis,
failed back,
end-stage cancer (i.e., breakthrough pain), etc. Post-operatively, for
example,
patients suffer from severe pain for the first few days followed by days of
mild to
moderate levels of pain.
[0149] The most common analgesic used to treat moderate to severe post-
operative pain is IV morphine. This is either delivered on an as needed" basis
by a
nurse to the patient by an IV injection or commonly a morphine syringe is
placed in a
PCA pump and the patient self-administers the opioid by pressing a button
which has
23
CA 02673837 2014-10-23
WO 2008/085764
PCT/US2007/089017
a lock-out feature. Other opioids, such as hydromorphone and fentanyl may also
be
used in this manner.
[0150] Treatment of acute pain is also necessary for patients in an outpatient
setting. For example, many patients suffer from chronic pain and require the
use of
opioids on a weekly or daily basis to treat their pain. While they may have a
long-
acting oral or transdermal opioid preparations to treat their chronic
underlying pain
levels, they often need short-acting potent opioids to treat their severe
breakthrough
pain levels.
[0151] Treatment of acute pain is also necessary in the field" under highly
sub-
optimal conditions. Paramedics or military medics often are required to treat
severe
acute pain in un-sterile situations, where needles used for IV or IM
administration
can result in unintended needle sticks, risk of infection, etc. Oral opioid
tablets often
take 60 minutes to provide relief which is too long for someone in severe
pain.
[0152] In a number of clinical settings, there is clearly a need for improved
means
to administer a drug that produces effective pain relief in a manner that is
titratable,
safe and convenient, and non-invasive that provides relief from acute, severe
breakthrough or intermittent pain over an appropriate period of time.
[0153] The claimed methods and systems rely on administration of dosage forms
comprising a pharmaceutically active substance such as sufentanil which is
effective
for the treatment of pain (acute, intermittent or breakthrough pain) using a
dispensing device that includes features such as lock-out, a means for user
identification prior to drug administration and a means to protect the dosage
forms
stored therein. The claimed methods and systems thereby provide significant
advantages over currently available treatment modalities in terms of both
safety and
efficacy.
VI. Dispensing Devices.
[0154] Dispensing devices, methods and systems for oral transmucosal
administration of small volume drug dosage forms are provided. The dispensing
devices are typically handheld and portable and comprise a housing having a
dispensing end which typically has a proboscis with a shroud that provide a
means
for blocking or retarding saliva ingress and/or moisture control. The
dispensing
devices further provide safety features such as a means for lock-out and a
means for
user identification.
[0155] The claimed dispensing devices, methods and systems comprise delivery
of small volume dosage forms to the oral mucosa.
24
CA 02673837 2014-10-23
WO 2008/085764
PCT/US2007/089017
Blockina/Retardina Saliva and Moisture Inaress
[0156] In some embodiments, the claimed dispensing devices comprise a means
for minimizing or eliminating saliva ingress and moisture ingress into the
dispensing
device: (1) to avoid wetting the dosage forms therein; (2) to isolate any
saliva that
enters the dispensing device in such a manner that the dosage forms therein
remain
dry; (3) to absorb or adsorb any saliva that enters the dispensing device in
such a
manner that the dosage forms remain dry; (4) to block saliva and moisture from
entering the device to protect the dosage forms from vapor and liquid phase
moisture, or (5) any combination thereof.
[0157] The dispensing device may have a means for preventing and/or
controlling
humidity ingress due to ambient conditions outside of the device.
[0158] The means for minimizing or eliminating saliva ingress or preventing
other
moisture from entering the dispensing device includes, but is not limited to,
one or
more flexible or rigid seals, one or more flexible or rigid wipers, use of one
or more
absorbent material components such as a desiccant or pad, a door or latch that
is
manually or automatically opened and closed, multiple stage delivery systems,
a
positive air pressure and airflow, or an air gap or prescribed distance or
barrier/shroud maintained between the dosage form delivery orifice and the
mucous
membrane tissues within the mouth that may transport the saliva. The shroud
limits
the ability of the tongue or oral mucosa to contact the dosage form dispensing
area,
thereby controlling saliva contact and ingress. By inhibiting or eliminating
the
"wetness" inside the shroud and on the surface of the valve/seal, the dosage
form is
dispensed without adhesion occurring between the dosage form and the shroud or
valve/seal.
[0159] To protect the drug dosage forms from exposure to moisture either from
humidity, saliva ingress, or accidental exposure to other water based liquids,
the
dispensing device and the container or cartridge which houses the dosage forms
within the device contains a desiccant.
[0160] Means for trapping or otherwise isolating saliva or moisture if it
enters the
device include, but are not limited to, a hydrophilic wicking material or
component,
an absorbent or adsorbent material or component, a desiccant material or
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
component, a separate track or channel for moisture to collect, a separate
channel to
communicate moisture to the absorbents or adsorbents, or any combination of
these
materials or components.
[0161] A desiccant is a sorbant, in the form of a solid, liquid, or gel
that has an
affinity for water, and absorbs or adsorbs moisture from it's surrounding,
thus
controlling the moisture in the immediate environment. Any commercial
desiccant
may be used. Commercial desiccants typically take the form of pellets,
canisters,
packets, capsules, powders, solid materials, papers, boards, tablets, adhesive
patches, and films, and can be formed for specific applications, including
injection
moldable plastics. There are many types of solid desiccants, including silica
gel
(sodium silicate, which is a solid, not a gel), alumino-silicate, activated
alumina,
zeolite, molecular sieves, montmorillonite clay, calcium oxide and calcium
sulfate, or
others, any of which may be used in the claimed dispensing devices. Different
desiccants have different affinities to moisture or other substances, as well
as
different capacities, and rates of absorption or adsorption. Also, different
types of
desiccants will come to equilibrium at different relative humidities in their
immediate
surroundings. As a means for protecting the dosage forms and the internal
portions
of the dispensing device from moisture, one or more desiccants may be employed
at
the proboscis; in or adjacent to the dosage form; in or adjacent the delivery
pathway; in or adjacent the dosage form, tablet magazine or cartridge; in or
adjacent to other components of the dispensing device; formed as an injection
molded component of the dispensing device; a compressed desiccant that is
pressed
into location; or desiccant in any other location within or without the
device.
[0162] In one preferred embodiment, the desiccant snaps into a cavity in the
side
of the cartridge. There are holes in the desiccant cavity that connect it to
the dosage
form stack, exposing the dosage forms to desiccant and keeping them dry.
[0163] The claimed dispensing devices rely on valves, pads, seals, the rest
position of the push rod, proboscis design and a shroud to minimize or
eliminate
saliva ingress or moisture into the dispensing device during administration of
the
dosage form.
[0164] Valves for use in the claimed devices are typically dome/trocar type
valves
that provide enough sealing force to keep saliva and/or moisture from entering
the
device and serve to minimize or eliminate saliva ingress or moisture by
closing the
distal orifice during dispensing and after a dosage form has been dispensed.
26
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
[0165] Pads for use in the claimed devices have various geometries that
aid in
contacting or communicating with the pushrod in order to removed liquid from
the
push rod surface. Such pads typically contain hydrophilic properties and serve
to
minimize or eliminate saliva ingress or moisture ingress by transporting the
liquid
away from the track and push rod.
[0166] Seals and wipers for use in the claimed devices are designed to
maintain a
uniform seal around a drug dosage form and a pushrod during delivery and are
characterized by flexible materials that impart a seal around the dosage form
and
pushrod and serve to minimize or eliminate saliva ingress or moisture by
sealing and
wiping the orifice and pushrod before, during, and after dispensing.
[0167] The rest position of the push rod in the claimed devices is
characterized by
positioning the pushrod in an intermediate location distal to the cartridge
exit, and
proximal to the distal dispensing orifice and serves to minimize or eliminate
saliva
ingress and moisture by allowing the pushrod to reside in a location that
contains a
desiccant, absorbents, or channel that dries the pushrod while at rest between
dosage dispenses.
[0168] The proboscis design for use in the claimed devices is characterized by
a
distal device shape, typically an S-shape, that aids in use of the device
and/or
placement of the tip on the oral mucosa of the subject. The shape typically
has
curves, angles, and geometries such that it enables proper use of the device
and
placement of the dosage form on the oral mucosa of the subject, e.g., in the
sublingual space.
[0169] The shroud of the claimed devices has a geometry that forms a barrier
between the device and the oral mucosa and tongue, a relief for dosage form
delivery, and an interior that is hydrophobic or hydrophilic and serves to
minimize or
eliminate saliva ingress or moisture ingress by creating a barrier from the
oral
mucosa contacting the valve area and dosage form, aiding in dosage form
dispensing
and discouraging dosage form adherence to the shroud. The shroud may have a
rounded interior surface or other geometries to mitigate the dosage form
adhering to
the shroud. The shroud limits the ability of the tongue or oral mucosa to
contact the
dosage form dispensing area, thereby controlling saliva contact and ingress.
[0170] Figs. 1A-E provide schematic depictions of a variety of aspects
of one
embodiment of a drug dispensing device constructed to hold a plurality of
dosage
forms for oral transmucosal delivery. Fig. 1A is a schematic depiction of a
fully
assembled or single piece dispensing device 11 of the invention. In Fig. 1B,
the
27
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
dispensing device 11 includes a reusable head 13 and a disposable body 15; in
Fig.
1C the dispensing device 11 further includes a cartridge 17 in Fig. 1D the
dispensing
device 11 includes a valve 33, a proboscis 31, a latch button 19, a power
train
coupling 25, a hub lock 21 and a dispense button 23; and Fig. 1E is a
schematic
depiction of a reassembled and complete dispensing device 11.
[0171] Fig. 2 provides a schematic depiction of an exemplary dispensing
device
wherein the dispensing tip comprises a shroud 29 having a one or more of: a
wiping/sealing valve 37, an absorbent pad 39, a drug drying chamber/moisture
communication channel 43, desiccant in the channel 45, a cartridge 17
containing
dosage forms 67 and desiccant in the cartridge 47.
[0172] Figs. 3A and 3B are schematic depictions of an exemplary geometry for
the dispensing tip that prevents contact of one or more seals 33, 35 with the
moist
or wet surface of the oral mucosa via a shroud 29.
[0173] Figs. 4A ¨ D are a schematic depiction of an exemplary proboscis 31 of
a
dispensing device 11 wherein the proboscis 31 comprises a shroud 29, a valve
33
for dispensing a dosage form 67 and a cut-out/relief 55 for the dosage form 67
to
be placed against the oral mucosa and not moved when the device 11 is
withdrawn
following dispensing.
[0174] A means for minimizing saliva ingress and moisture into the claimed
devices is important for preservation of the integrity of dosage forms during
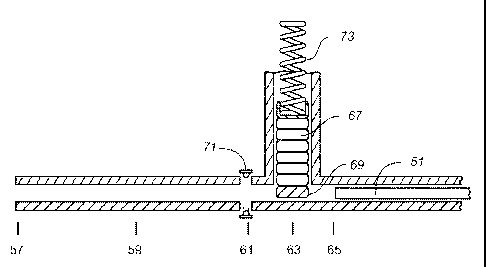
storage,
e.g., prior to an between oral transmucosal administrations.
[0175] The claimed dispensing devices may be used to administer a drug dosage
form that is sensitive to moisture and/or humidity. In such cases, a drug
dosage
form cartridge serves to protect the drug dosage form from liquid and vapor
phase
moisture, including humidity, liquid moisture, saliva, mucus, etc. The
cartridge may
be cylindrical, disk-shaped, helical, rectilinear, non-ordered, or may take
the form of
any assemblage of drug dosage forms that allows the drug dispensing device to
dispense them in a controlled manner. To prevent the unused drug dosage forms
from absorbing moisture or otherwise becoming exposed to moisture prior to
use,
the cartridge may provide a means of sealing the drug dosage forms from
exposure
to moisture. This may accomplished by use of a cartridge that contains
individually
packaged drug dosage forms separated by a thin impermeable foil or impermeable
material such that when one drug dosage form is dispensed from the cartridge,
the
seal protecting the remaining dosage forms remains unbroken. Alternatively,
the
dosage forms may be packaged in such a manner within the cartridge that two or
28
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
more dosage forms are packaged together in each separate sealed compartment.
In
some embodiments, all of the dosage forms in a cartridge may be packaged
together
in a foil sealed compartment.
[0176] A drug cartridge that houses small volume drug dosage forms within the
dispensing device may afford a seal against moisture by means of a septum, an
elastomeric seal or valve, a sliding, translating, hinged door or valve, or by
means of
sealing against another component of the drug dispensing device when loaded.
In
this manner, a single re-sealable seal may be opened either independently or
by
means of the passage of a dosage out of the cartridge. Once the dosage form is
delivered from the cartridge, the re-sealable seal on the cartridge may be re-
sealed
to prevent moisture or other contaminants from damaging the remaining drug
dosage forms within the cartridge. The cartridge may further have a non-re-
sealable
seal that is broken when it is loaded into the drug dispensing device or upon
delivery
of the first dosage form from the cartridge.
[0177] In other embodiments, the cartridge contains a desiccant or other
absorbent or adsorbent material to absorb or adsorb moisture that penetrates
the
cartridge either prior to use or during normal use. A cartridge for use in a
claimed
dispensing device may contain any combination of individually sealed dosage
forms,
multiply sealed dosage forms, re-sealable seals, non-re-sealable seals,
desiccants,
absorbents, or adsorbents. In one embodiment, a cartridge for use in the
dispensing
device in holds sufficient drug dosage forms for 1-5 days of treatment, e.g.
40
dosage forms or sufficient drug dosage forms to provide 48 to 72 hours of
treatment.
Pushrod Design
[0178] Figs. 5A ¨ D provide a series of flow diagrams for use of an exemplary
dispensing device showing pusher logic, wherein Fig. 5A shows the LOAD
feature;
Fig. 5B shows the device calibration logic flow. Referring to Fig. 6, the
pushrod 51 is
advanced from position 65, picks up the shipping tablet 69 at position 63, and
is
further advanced to position 61. At position 61, the device senses the
presence of
the shipping tablet 69 and/or push rod 51. In doing so, the device is
calibrated and
knows the location of the shipping tablet 69 and/or end of the push rod 51
regardless of assembly tolerances, variations in push rod length and push rod
end
conditions. Following this calibration, the push rod 51 advances the shipping
tablet
69 from position 61 to position 57 where the shipping tablet 69 is dispensed
from
the device. During this operation, the device is able to distinguish between a
shipping tablet 69, a push rod 51, and a drug dosage form 67. This
differentiation
29
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
enables the device to confirm that a cartridge is unused because a shipping
tablets is
the first thing dispensed from a new cartridge during device setup. The
feature that
provides the means for differentiating between the shipping tablet, push rod,
and
dosage form 67 may be optical, physical, RF, electronic (resistive,
capacitive, or
other) or magnetic. The push rod 51 advance from position 65 and position 57
described above, could be continuous or intermittent and a physical stop at
position
61 is not required. The push rod 51 then retracts from position 57 to position
59,
placing the device 11 in the ready position with the push rod 51 under the
remaining dosage forms 67. In this position, the push rod 51 keeps dosage
forms
67 from inadvertently falling out of the device 11.
[0179] Fig. 5C shows the device dispense logic flow. Referencing Fig. 6,
following
a dose command, the push rod 51 retracts from position 59 to position 65,
allowing
the dosage forms 67 to advance into the push rod track. The push rod 51 then
advances from position 65, picks up a dosage form at position 63, and then
dispenses the dosage forms 67 from the device at position 57. Between
positions
63 and 57, the presence of a dosage form 67 is sensed/confirmed at position 61
by
the position sensor. The push rod then retracts from position 57 to position
59,
placing it in the ready position with the push rod 51 is under the remaining
dosage
forms 67. In this position, the push rod 51 is allowed to dry before the next
dosage
form 67 dispense, as well as keeps dosage forms 67 from inadvertently falling
out of
the device 11.
[0180] Fig. 5D shows the device disassemble logic flow. Following a
"disassemble"
command, the push rod 51 is moved to position 65. This allows for the removal
of
any remaining dosage forms 67 without push rod interference.
[0181] Fig. 6 is a schematic depiction of an exemplary dispensing device
showing
the stages of push rod/ dosage form interaction during device use. In Fig 6,
the push
rod 51, dosage forms 67, shipping tablet 69, spring 73 and position sensor 71
are
shown. During use, the push rod 51 moves between positions 57, 59, 61, 63 and
65, also shown in Fig. 6 and further detailed in Figs. 5A-D.
Dosing History/Feedback
[0182] Further embodiments of the device include the ability to store
historical
use information and the ability to transmit such information. The device may
be
capable of unidirectional (downloading) or bidirectional information transfer.
For
example, an exchange of information may be accomplished by downloading stored
information to a computer through a physically wired interface, such as a USB
or any
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
other communication connection. Alternatively, information may be communicated
via a wireless system.
[0183] In another embodiment, the dispensing device has a dose counting
feature that monitors and stores the history of drug usage. Such information
may
include historical use information, for example the number of dosages stored
and
dispensed, and the times of dispensing.
Calibration
[0184] The dispensing device may be capable of self-calibration of the
dispense
mechanism, or the device may be calibrated manually. This process may employ a
shipping tablet with a feature or features that physically differentiate it
from a drug
dosage form or the push rod. These features may be designed so that device
calibration precision is higher that that attainable using a dosage form or
push rod.
The differentiating feature may be physical, optical, radio frequency (RF),
electronic
or magnetic.
User Identification Feature
[0185] In one aspect, the dispensing device comprises a detecting means for
user
identification such as a fingerprint reader, an optical retinal reader, a
voice
recognition system, a face recognition system, a dental imprint recognition
system, a
visual recognition system, or a DNA reader. The dispensing device may employ
one
or more means to identify the user, enabling the system to determine if a
dispensing
request is being made in an authorized or unauthorized manner. It is important
for
effective delivery of many potential drugs and drug dosage forms to ensure
that the
dispensing device is not accidentally or intentionally used by an unauthorized
individual to prevent accidental or intentional diversion of the drug. Such
user
identification systems may recognize one or more users, for example, in an
inpatient
hospital setting the dispensing device could be programmed to recognize the
patient
to whom it is prescribed, as well as authorized healthcare providers such as
nurses
and physicians. In an outpatient home setting, for example, the dispensing
device
may only respond to the patient to whom it is prescribed.
[0186] The dispensing device may employ any means of user identification,
including fingerprint identification, RFID detection with the use of an active
or
passive RFID tag on bracelet, necklace, clip, belt, strap, adhesive patch,
implant, or
means of locating and affixing a tag, retina identification, DNA
identification, voice
recognition, password or code entry, physical key, electronic or magnetic key,
personal area network identification using the human body or clothing as a
data or
31
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
signal conduit, optical scanner or face recognition, sonic, subsonic or
ultrasonic
identification, or any other means of identifying an individual and verifying
their
identity.
[0187] One method of user identification is the use of a short distance ("near
field") passive RFID tag attached to a bracelet, necklace, adhesive patch,
clothing
tag, orally mounted device, like an orthodontic retainer, belt, strap, some
combination of these, or another location. When an RFID tag is used in the
"near
field", roughly defined as about 16% of the wavelength of the received signal,
the
tag behaves in the inductive mode of operation, coupling between the reader
and
tag antenna magnetically. The near field is characterized by at least two
features:
first is a rapid decline in field strength with distance, and second is a
strong
directionality of the signal. In the near field, the signal strength falls off
very rapidly,
with a signal strength loss of approximately 60dB per decade in distance. For
good
inductive coupling between the transmitter antenna and the RFID tag antenna,
the
two antennas are oriented in parallel planes with the axes through the center
of each
antenna in close proximity. Strong signal strength (robust user
identification) is
provided when the device is very close to the RFID tag. At the same time, a
very
poor signal is provided when the device is further away from the tag, which
helps
prevent unauthorized use by someone other than the patient who attempts to use
the device. It is preferable to operate in this near field region with good
antenna
alignment. Furthermore, it is preferable to operate with a very short distance
of
adequate signal strength for a positive identification, so that it is very
difficult to
receive a signal if the device is not in the proper orientation and proximity
to the
RFID tag. To attain a short distance and a proper alignment between antennas,
the
dispensing device may be designed so as to properly locate the RFID reader
antenna, mounted in the dispensing device, adjacent to an RFID tag antenna,
mounted, for example, on a wrist band or bracelet, or a clothing tag on the
collar, or
an adhesive patch on the hand, arm, cheek, neck, or elsewhere. Furthermore, an
RFID tag antenna on a wrist band or bracelet may be held in proper alignment
and
location by means of a small adhesive patch that prevents the bracelet from
moving
or rotation on the wrist.
[0188] In another embodiment, the dispensing device employs a high frequency
RFID reader for use in the inpatient (hospital, clinic, etc.) setting,
operating on or
near the 13.56MHz frequency band, and the patient is be fitted with a matching
RFID tag and antenna on a disposable bracelet or wrist band, designed in such
a
32
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
way that if the bracelet or wrist band is removed the RFID tag, the antenna,
or
another component of the associated circuit will be damaged or destroyed,
rendering
the bracelet or wrist band non-functional. In one example, the range of the
RFID
communication is short, between 0 inches and 10 inches preferably, more
preferably
between 0 and 5 inches, and most preferably between 0 and 3 inches, and may
additionally be directional, allowing proper use by the intended patient to be
easy
and reliable, while at the same time making unauthorized use by another
individual
difficult, very difficult, or impossible.
Lock Out
[0189] The dispensing device provides for lock out, requiring the patient to
communicate with the physician or other authorized care giver to unlock the
device
for the next fixed period. In this way the device and dock provide for safe
drug
administration due to greater physician oversight and care management.
[0190] The dispensing device provides a means for adjusting both the initial
dose
and subsequent doses, as well as the lock-out time. The initial dose and lock
out
time may subsequently be adjusted dependent upon patient response, duration of
treatment and the like.
[0191] The
initial timed lock-out period for a claimed dispensing device is typically
from about 1 minute to about 60 minutes, from 3 minutes to 40 minutes or from
5
minutes to 30 minutes, and in particular cases is set at any one minute
interval from
1 to 60 minutes, e.g., 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 ,16, 17, 18, 19,
20, 21, 22,
23 ,24, 25, 26, 27, 28, 29, 30, 31, 32, 33 ,34, 35, 36, 37, 38, 39,40, 41, 42,
43 144,
45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59 or 60 minutes.
[0192] In some cases, a dispensing device has a fixed lockout between doses
and
may exhibit a shutdown after a fixed period of time. In other cases, the lock-
out time
is a programmable lock-out time. The lock-out time may also be a fixed time
lock-out
interval, a predetermined lock-out interval, a predetermined variable lock-out
interval, a lock-out interval determined by an algorithm or a variable lock-
out interval
communicated to the device from a remote computer or docking station.
Additional Features
[0193] A dispensing device may provide the ability to recognize a specific
cartridge by a mechanical, optical (e.g. bar code), electronic (e.g.
microchip),
magnetic, radio frequency, chemical, or other means of detecting and
identifying the
cartridge. In one exemplary embodiment, the drug-containing cartridge contains
a
33
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
physical keying detail on the cartridge that is physically detected by a
sensor or
switch or a series of sensors or switches in the dispensing device.
Furthermore, the
dispensing device may communicate uni-directionally or bi-directionally with
the
cartridge to exchange information. Such information may include drug name,
dosage
strength, usage information, lockout period, manufacturing lot number,
indications
for use, side effects, drug interactions, date of manufacture, date of
expiration, serial
number, number of doses in the cartridge, or any other relevant information.
The
dispensing device may be able to write, in addition to read, information to
the
cartridge, like date used, health care provider or other user identification,
number of
doses used, etc.
[0194] The dispensing device may provide mechanical protection for the dosage
forms contained therein, preventing breakage, chipping, hydration etc.,
thereby
allowing for dispensing of the undamaged dosage forms contained therein. This
is of
particular importance for small fragile and friable dosage forms.
[0195] The drug dispensing device may be powered by a battery, capacitor, fuel
cell, or other power supply source, or may require no electrical power, but be
manually activated.
[0196] In some embodiments, the dispensing device is capable of issuing alarms
or other notifications when functional or safety issues arise. The alarm or
other
notification may trigger an alert on the dispensing device, on a dock or other
peripheral device; on a computer or by means of a wired or wireless network,
or may
alert other remote devices. The alarm or notification may be audible, tactile,
visual,
or may employ other means of notifying one or more individuals.
Docking Station
[0197] In certain embodiments, the device includes a portable or fixed docking
station that may query the device, reset it between dosing, lock it when not
properly
accessed, and control the dosing regimen. The drug dispensing device may
communicate with a physician or care giver, via the dock, or by a wired or
wireless
communication means.
[0198] The dispensing device may employ one or more levels of interface for
different types of authorized users, for example the patient, the nurse, the
physician,
pharmacist or other authorized medical or healthcare personnel. These
different
interfaces may include components such as keypads, buttons, graphical icons
and
instructions, lights, LED's, monochrome or color graphical or text displays,
touch-
screens, LCD's, sounds, tactile feedback, voice recognition interfaces, and
other input
34
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
and output devices and means. The activity, or mode, of the user interface may
be
determined by the mode of operation of the dispensing device, by a login or
access
activity by a user such as a password or code entry, by the connection or
disconnection of the dispensing device from a dock, computer, or network, or
by the
detection of an authorized access key, such as a key, and/or RFID tag, or
similar
combination. Upon changing the interface mode, the functionality of the device
may
be changed, either activating, inactivating or changing the functionality of
the
various interface components described above. By allowing the device to have
one or
more interface modes, with differing functionality associated with each one,
the
device can be optimized for various uses.
Base Station
[0199] In some embodiments the drug dispensing system includes a base station
for recharging the drug dispensing device and the portable docking FOB between
uses. This base station allows for recharging the batteries or fuel cells in
multiple
dispensing devices and/or FOBS simultaneously. In addition to recharging the
drug
dispensing devices and FOBs, the base station may provide one or more of the
following functionality: wireless or wired connectivity to a peripheral
device,
computer or network; feedback on the charging state for the devices being
recharges; an interface for viewing, adding, deleting, or modifying the data
on a
drug dispensing device or FOB; a means for synchronizing data between multiple
drug dispensing devices and/or FOBS; and a means for conducting a diagnostic
test
on drug dispensing devices and/or FOBs.
Single and Multiple Dose applicators
[0200] The invention provides disposable applicators for delivering
dosage forms
comprising sufentanil to the oral mucosa of a patient such that application to
a pre-
determined location for drug delivery (e.g. the mouth, sublingual space, etc.)
is
effected.
[0201] In one approach to the invention, a dosage form is delivered to
the oral
mucosa, using a single dose applicator (SDA). The dosage form is provided in a
child-resistant drug dispensing device or packaging and delivered for example,
to the
sublingual cavity. The dosage form may be self-administered or alternatively,
the
dosage form is administered with assistance with or without a device.
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
[0202] In one embodiment, a SDA is used to a drug dosage forms, provided as a
solid tablet, a liquid capsule, a gel capsule, a liquid, a gel, a powder, a
film, a strip, a
ribbon, a spray, a mist, a patch, or any other suitable drug dosage form.
[0203] The SDA may contain the dosage form within, may have the drug dosage
form attached or affixed to it, may have the dosage form dissolved in it, and
may
afford a seal against moisture, humidity, and light. The single dose
applicator may be
manually manipulated by a patient, healthcare provider, or other user to place
the
dosage form in the proper location for drug delivery.
[0204] In practicing the invention, a single- or multiple-dose applicator or
drug
dispensing device may be used to deliver tablets or other dosage forms into
the
hand, mouth, under the tongue, or to other locations appropriate for specific
drug
delivery needs.
[0205] In one embodiment, a single- or multiple-dose applicator or drug
dispensing
device is used to deliver a dosage form to the oral mucosa, e.g., the
sublingual
space.
[0206] The dosage forms inside the dispensing device remain dry prior to
dispensing, at which point a single dosage form is dispensed from the device
into the
mouth, e.g., the sublingual space, wherein a patient's saliva will wet the
tablet and
allow for tablet disintegration/erosion and drug delivery.
[0207] The SDA may be provided as a pair of forceps, a syringe, a stick or
rod, a
straw, a pad, a capsule, a cup, a spoon, a strip, a tube, an applicator, a
dropper, a
patch, an adhesive pad, an adhesive film, a sprayer, an atomizer, or any other
form
suitable for the application of a single drug dosage form to the oral mucosa
of a
subject, e.g., the oral mucosa in the sublingual space. As will be understood
by one
of skill in the art, the SDA design may vary, so long as it is effective to
place a drug
dosage form, such as a tablet, in the desired location on an oral mucosal
membrane,
e.g., in the sublingual space, in a manner that preserves integrity of the
drug dosage
form in the dispensing process. After use, the SDA is disposed of, so as to
eliminate
the risk of contaminating the drug dispensing device with saliva, or other
contaminants.
[0208] For sublingual administration, a small volume dosage form is
administered
sublingually by placement under the tongue, using an SDA, generally adjacent
the
frenulum.
36
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
[0209] The dosage form may be provided in a package that consists of molded
plastic or laminate that has indentations Cblisters") into which a dosage form
is
placed, referred to herein as a "blister pack". A cover, typically a laminated
material
or foil, is used to seal to the molded part. A blister pack may or may not
have pre-
formed or molded parts and may be used to package an SDA of any type.
[0210] Such blister packs may be provided in a child resistant multiple drug
dispenser (MDA), which may serve to dispense the dosage forms housed therein
or
may be used for storage of a plurality of SDAs.
[0211] Figs. 15A-C, Figs. 16A-F, Figs. 18A-C and Figs. 19A and B are schematic
depictions of exemplary embodiments of a SDA of the invention.
[0212] In one approach, the present invention provides disposable single dose
applicators comprising a blister pack 151, which contains drug dosage forms 67
inside a housing and a handle 131, wherein a backing, such as a foil seal 135
covers the dosage form 67 and the handle 131, as shown for example in Fig. 16B
and 16D.
[0213] In one embodiment, the disposable single dose applicator, the
combination
of housing or tube 129 and handle 131 has the shape of a spoon.
[0214] The housing or tube 129 for the dosage form 67 is a blister pack 151
that
accommodates a unit dose of a dosage form 67 for administration to a subject.
The
dosage form 67 is sealed in the blister pack 151 by a foil or other type of
seal 135.
[0215] In some embodiments, the foil or other type of seal 135 is removed
prior
to administration of the dosage form 67 and the handle 131 is used to place
the
dosage form 67 in the appropriate location against the oral mucosa of the
subject
such that the dosage form 67 adheres to the oral mucosa. See, e.g., Figs. 16B,
16D,
16E and 16F. In other embodiments, the foil or other type of seal 135 is
perforated
and removed prior to administration of the dosage form 67 by folding the
applicator
123 at the perforation 149 prior to administration where the handle 131 is
used to
place the dosage form 67 in the appropriate location against the oral mucosa
of a
subject. See, e.g., Figs. 19A and B. This permits the handling of only a
single drug
dosage form 67at a time and prevents the other individually sealed drug dosage
forms 67 from becoming exposed to saliva, humidity and the like.
[0216] The foil or other type of seal 135 of a disposable applicator 123
including
handle 131 is typically made of a single piece of foil laminate, paper,
plastic or other
37
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
covering, i.e. an applicator tab 147 that spans the back of the housing or
tube 129
alone or both the housing or tube 129 and the handle 131, effectively seals
the
dosage form 67 in a blister pack 151 or other container.
[0217] The handle 131 enables proper placement of the dosage form 67 without
touching the dosage form 67.
[0218] A plurality of single dose applicators may be provided as a series of
individual single dose applicators attached by the backing or housed in
multiple dose
dispenser 137.
[0219] Figs. 14A and 146 show one embodiment of a single dose applicator 123
a dispensing device for delivering drug dosage forms. The dispensing device
shown
in Fig. 14A depicts the single dose applicator 123 that is ready to dispense a
drug
dosage form 67. In one aspect of this embodiment, a user pinches the single
dose
applicator 125 which opens the applicator and a drug dosage form 67 is
dispensed
as shown in Fig. 146.
[0220] Figs. 15A ¨ C show an embodiment of a single dose applicator 123 that
is
comprised of a applicator shaped as a tube 129, a stopper seal 127, a handle
131
(e.g., an ergonomic handle), and a single dosage form 67. Fig 15A shows the
single
dose applicator 123 in its sealed configuration, prior to use. Fig 156 shows
the single
dose applicator 123 with its stopper seal 127 removed, forming an opening 133,
and ready for use. Fig 15C shows the single dose applicator 123 tilted so as
to
dispense the dosage form 67 on the oral mucosa, e.g., in the sublingual space.
[0221] Figs. 16A ¨ F show several alternate embodiments of the single dose
applicator 123. In all of these figures the applicator seal 127 is broken and
the
applicator is tilted so as to drop the drug dosage form 67 adjacent an oral
mucosal
membrane in the mouth of a subject, e.g., under the tongue for sublingual
dosage
form placement. Fig. 16A shows a tube like applicator 129 with a handle 131
located axially under the tube 129. Fig. 166 shows an applicator formed as a
thermoform or blister package 151 with a foil seal 135 that is peeled so as to
open
the applicator package 141 prior to placing the dosage form 67. Fig. 16C shows
an
applicator that is a tube 129 which is broken to break the seal prior to
dosage form
67 placement. Fig. 16D shows a blister pack tube 151 type dosage form package
141 with a handle 131 such that after the seal 135 is peeled back the blister
pack
151 can be held and tilted to place the drug dosage form 67, on an oral
mucosal
38
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
membrane. Figs. 16E and 16F show blister pack 151 type packaging with a handle
131 shaped like a flower or an animal, respectively, to be used for single
dose
applicator 123 designed for pediatric use. Other single dose applicator shapes
could
include cartoon characters, animals, super-heroes or other appropriate shapes
for
pediatric applications.
[0222] Figure 18A shows a flat rigid applicator 123 with a dosage form 67
adhered to one end, for example, by means of a rapidly dissolving ingestible
adhesive material such that when the applicator end with the dosage form is
placed
under the tongue, the adhesive dissolves, the dosage form 67 is placed on an
oral
mucosal membrane, such as in the sublingual space, and the applicator can be
removed. Fig 18B shows an applicator 123 made from a water permeable material,
impregnated with drug, forming a material and dosage from matrix. When the
impregnated end of this applicator 123 is placed under in the mouth on an oral
mucosal membrane, the moisture in the saliva dissolves the drug and delivers
it
transmucosally. Figure 18C shows dissolving film dosage forms 145 and a dosage
form package with a plurality of dissolving film dosage forms 143 within it.
The
dissolving film dosage form 143 is removed from the package 141 and placed on
an
oral mucosal membrane, e.g., in the sublingual space where it dissolves and
delivers
the drug transmucosally.
[0223] Figs. 19A ¨ B provides an illustrations of two stages of use of one
embodiment of a single dose applicator 123. Figure 19A shows the applicator
123 in
its configuration prior to use, with two applicator tabs 147, two perforations
149,
and a blister pack 151 containing a dosage form 67. In order to administer the
dosage form 67, the two applicator tabs 147 are bent downward at the
perforations
149, forming a handle 131, and the seal 135 is peeled back to reveal the
blister
pack 151 and allow the dosage form 67 to be dropped on an oral mucosal
membrane, e.g., in the sublingual space.
[0224] In another embodiment, a drug dispensing device of the invention may
contain a plurality of SDA's, in a cartridge or individually packaged, and may
dispense a single SDA containing a single drug dosage form for use by the
patient,
healthcare provider, or user. The drug dispensing device may dispense single
SDA's
in the same way and with the same features as would be advantageous for the
dispensing of single drug dosage forms described in the invention.
39
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
[0225] In yet another embodiment the multiple dose applicator 137 is a device
which comprises one or more drug dosage forms 67 or single dose applicators
123,
a portable power means, like a battery, a printed circuit board, a data
connectivity
means, and a user interface. In this embodiment the drug dispensing device may
include the ability to perform one or more of the following functions: record
drug
dosage dispensing history, check user identification by means of fingerprint
identification, RFID, voice recognition, etc., allow the dosage history to be
transferred to another device, computer or network, and/or provide a lockout
period
between dose dispenses.
[0226] Fig. 17 is a schematic depiction of an exemplary multiple dose
applicator
137 for delivering dispensing drug dosage forms 67, each individually packaged
in a
single dose applicator 123.
[0227] Figs. 20A ¨ 20D are schematic depictions of additional examples
of SDAs,
including a tweezer or reverse scissor-type SDA (20A), where a drug dosage
form 67
is held between the two sides 153 of the SDA 123 such that when the latch 19
is
released, the drug dosage form 67 is no longer held by the SDA and can be
placed
on an oral mucosal membrane by the user; a syringe-type SDA (20B) with a
circular
channel, where a drug dosage form 67 is pushed out of the end of the channel
when
a user pushes 155, the slider or plunger 159; a pusher-type SDA (20C) with a
rectangular channel where a drug dosage form 67 is pushed out of the end of
the
channel when a user pushes 155, the slider 159; or a slider-type SDA (20D)
where
drug dosage form 67 is held in a pocket 161 and the drug dosage form 67
becomes
accessible when a user pulls 157 a slider 159.
[0228] Figs. 21A ¨ 21D provide a schematic depiction of a multiple dose
applicator (MDA) 137 or container for storage of a plurality of SDAs 123 prior
to use
(21A); where in the exemplified embodiment, there is a slot in the upper cover
of the
MDA 137 for removal of individual SDAs 123 (21B); such that each individual
SDA
123 comprises a drug dosage form 67 (21C); and the SDA 123 facilitates
placement
of the drug dosage form 67 under the tongue in the sublingual space (21D).
VII. Methods and Systems for Delivering Small Volume Dosage Forms
Using a Device.
[0229] Methods and systems for delivering small volume dosage forms, e.g.
sufentanil-containing dosage forms using a device are provided. Fig 7 provides
a
schematic architecture connection diagram illustrating the various components
that
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
may be included in a dispensing device or system for dispensing small volume
drug
dosage forms, including a device with a separate head 13, body 15 and
cartridge
17, a portable docking fob 113, Patient RFID 115 and a base station 117.
[0230] A block diagram illustrating one aspect of communication in a drug
dispensing system, including an RFID tag, a drug dispensing device, a base
station/dock and a healthcare provider personal computer system wherein a drug
dispensing device can communicate with the physician or care giver, via the
dock, or
by means of a wired or wireless communication method is provided in Fig. 8A.
[0231] A block diagram illustrating another aspect of communication in a drug
dispensing system, including an RFID tag, a drug dispensing device, a portable
docking fob, a base station and a healthcare provider personal computer is
provided
in Fig. 83. The drug dispensing device may communicate with the physician or
care
giver, via the FOB, by means of a wired or wireless communication method to
provide usage information and information regarding the respiratory status or
blood
pressure of the patient to the physician at regular intervals. The FOB can be
adapted
to attach to a cord so as to allow the fob to hang from the neck of the
physician or
caregiver.
[0232] Exemplary features of the dispensing device include the
following:
In one embodiment, the head, body, and cartridge comprise the handheld portion
of
the device. This device assembly has a latch to disconnect the head and body,
and a
dispense button for patient use. The device also has lights to show lock-out
status,
errors, and power. In this embodiment, the cartridge which contains the drug
dosage
forms and the body are used a single time only.
[0233] The system may comprise a portable dock which is handheld, independent
of the patient device and solely for healthcare professional use. The dock
enables
higher level feature use such as deeper queries into patient device use, the
ability to
upload device data, unlocking of the head/body and the tether, lockout
override for
dosing the patient, and a larger reading display. The dock is also used to
setup and
take down the patient device.
[0234] The system may also comprise an RFID bracelet that is activated via the
dock and is worn by the patient to establish and control dosing to correct
patient and
to that patient alone. This feature prohibits use of the device by others.
41
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
[0235] The system may further comprise a recharging base used to charge the
dock and heads and is also used to update the heads and docks when new
software
becomes available or when new users are programmed into the system.
[0236] The drug dosage forms are typically provided in single use disposable
cartridges which are loaded into the device prior to administration.
[0237] Exemplary set-up instructions for the device include the
following steps:
The device head and dock are charged on the recharging station.
The device body and wristband are removed from the packaging.
The device head and dock are removed from the charging station.
The cartridge is loaded into the body by inserting a cartridge into the device
body as indicated ensuring that the cartridge "clicks' and is locked in place.
The device body (with cartridge) is assembled onto the head.
The power button on the assembled device is pushed to power-up the
system.
The power button on the dock is pushed to power-up the dock.
The assembled device is plugged into the dock.
A healthcare professional scans their fingerprint or inputs a unique password
in order to unlock the dock.
The device reads the label on the cartridge and the dock displays setup
information, for example, the drug name, the quantity of tablets, the drug
concentration, the preset lockout time, the duration of use (72 hours), and
the
battery status of the head.
After the information is read from the cartridge and displayed on the dock,
the healthcare professional will be requested to confirm that all information
is correct
and will require a witness to verify the information.
The dock will require that the patient wristband be paired to the device by
bringing the wristband close to the device.
The device will read the band and request confirmation of the band number;
selection and confirmation of the number
The patient ID is entered into the dock. i.e. patient medical record number
The wristband is placed on the patient's hand that will be used to operate
device.
Then, the dock will indicate that it is ready to dispense a plastic
initialization
tablet or "shipping tablet".
42
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
Upon confirmation, the device will dispense a plastic initialization tablet or
"shipping tablet". This step is used by the device to calibrate the dispensing
mechanism, initiate the cartridge for use, and allows the healthcare
professional to
verify proper use and to train the patient with a "shipping" or placebo-type
tablet.
Once the plastic initialization tablet or "shipping tablet" is dispensed, the
dock
will require the healthcare professional to confirm that the plastic tablet
was
dispensed.
After confirmation, the display will indicate that the device is ready for
use.
[0238] In some cases, a tether can be connected to the device via the dock.
The
dock will allow the healthcare professional to lock and unlock the tether as
required.
[0239] If a patient will self administer a drug dosage form using the
device, the
patient will be trained prior to use.
[0240] Exemplary use of the claimed devices and systems is provided in
Examples
6-8.
VIII. In Vivo Human studies
[0241] Provided herein is pharmacokinetic data obtained in animals and
humans
based on studies where sufentanil and alfentanil were administered via the
sublingual route using the claimed small volume dosage forms.
[0242] A human clinical study was performed using healthy volunteers. The
study
which is detailed in Example 1 below was performed with 12 subjects (6 men and
6
women) using sublingual sufentanil dosage forms containing either 2.5mcg, 5mcg
or
10mcg of sufentanil base corresponding to 3.7mcg, 7.5mcg or 15 mcg of
sufentanil
citrate, respectively (see Table 1). All excipients were "pharmaceutically
acceptable"
(inactive and have GRAS or "generally recognized as safe" status.
[0243] Sufentanil dosage forms designed for sublingual use were compared to IV
sufentanil, administered through an IV catheter as a continuous infusion over
10
minutes. Plasma samples were drawn from a different IV catheter at a remote
location. The assay demonstrated good inter-day precision and accuracy at the
high,
medium and low quality control sample concentrations.
[0244] The dosage forms for this study eroded over a period of 10-30 minutes
in
all subjects. After placement of each sufentanil dosage form in the sublingual
cavity
of the 12 healthy volunteers, a remarkably consistent pharmacokinetic profile
was
obtained (see Figure 9 and Table 2). The bioavailability compared to IV
administration for single administration of all three dosages averaged 91%,
which is
far superior to that measured for commercially available fentanyl transmucosal
43
CA 02673837 2014-10-23
WO 2008/085764
PCT/US2007/089017
preparations, Actiq and Fentora (47% and 65%, respectively ¨ Fentora package
insert). Although this high bioavailability could be due to a number of
factors
including but not limited to erosion time, it is likely that the lack of
saliva produced
by the small size of the dosage forms limits the swallowing of the drug and
avoids
the low bioavailability typical of drug absorption via the GI route. Both
Fentora and
Actiq package inserts claim at least 50% and 75% of the drug dose,
respectively, is
swallowed via the saliva, and both exhibit lower bioavailability than the
claimed
dosage forms.
[0245] The dosage forms used in this clinical trial had a volume of
approximately
5 microliters (mass of 5.5-5.85 mg), a small fraction of the size of Actiq or
Fentora
lozenges. The dog studies described in Example 4 demonstrate that sufentanil
has
very poor GI bioavailability (12%), therefore, given the high bioavailability
of the
sufentanil dosage forms, wherein drug is administered by the oral transmucosal
route, the data supports the conclusion that greater than 75% of the drug is
absorbed transmucosally. Therefore, less than 25% of the drug is swallowed,
which
is a much lower percentage than is swallowed with Fentora or Actiq.
[0246] Importantly, this bioavailability is also linked to the
consistency of total
drug delivered to the patient. For example, the total plasma drug area under
the
curve (AUC 0-infinity) for sufentanil dosage forms 10mcg was 0.0705 + 0.0194
hrx
ng/ml (mean + standard deviation (SD)).This SD is only 27.5% of the total AUC.
Coefficient of variation (CV) is a term to describe the percent SD of the
mean. The
coefficient of variation for the fentanyl products, Fentora (AUC is 45%) and
Actiq
(AUC is 41%; Fentora*package insert), while the coefficient of variation for
the
claimed sublingual sufentanil dosage forms is less than 40%. Therefore, the
total
dose delivered to the subject is not only more bioavailable for the sufentanil
dosage
forms but it is more consistent.
[0247] The sufentanil sublingual dosage forms are also superior in terms of
consistent drug plasma levels early after administration. The CrT,õ obtained
with the
10 mcg sufentanil dosage form was 27.5 + 7.7 pg/ml. The coefficient of
variation of
the Cõ,,, is therefore only 28%. The Cmax for Fentoreand Actiq suffer from
variability
of GI uptake of drug. Fentora reports a Ciõ,, of 1.02 + 0.42 ng/ml, therefore
the
coefficient of variation of the Cõ,,, is 41%. The range of coefficients of
variation for
the various doses of Fentora is from 41% to 56% (package insert). Actiq
coefficient
of variation of Cmax is reported as 33% (Fentora package insert).
*Trademark
44
CA 02673837 2014-10-23
WO 2008/085764
PCT/US2007/089017
[0248] In addition to superior bioavailability and consistency in plasma
concentrations, the time to C,õ,õ, also referred to as T,õ is important since
quick and
consistent onset of pain relief is important in the treatment of acute pain.
The Tim,
for 10 mcg sufentanil dosage forms was 40.8 + 13.2 minutes (range 19.8 ¨ 60
minutes). The reported average Tmax for Fentora is 46.8 with a range of 20
¨240
minutes. The T,õ, for Actiq is 90.8 minutes, range 35 ¨ 240 minutes (Fentora
package insert). Therefore, the consistency in onset of analgesia for
sufentanil
dosage forms is markedly improved over Fentora and ACciq, with a 400% decrease
in
the slowest onset of T.
[0249] Important in the treatment of acute pain, especially acute breakthrough
pain, is a consistent and relatively short half-life of the drug. The plasma
elimination
half-life of the 10 mcg sufentanil dosage form was 1.71 + 0.4 hours, which
allows
the drug to be titratable for various levels of pain. If the breakthrough pain
event
lasts longer than 1.5 hours then the patient can dose with another dosage
form. The
plasma elimination half-life of Actiq and Fentora are 3.2 hours and 2.63
hours,
respectively, for the lowest doses. The half-lives for the higher doses
increase
substantially for these drugs, thereby limiting the titratability of these
drugs.
[0250] Although still in development, published data allows comparison of the
sufentanil pharmacokinetic data provided herein to that of Rapinyl, a fentanyl
sublingual fast-dissolve lozenge. As previously mentioned, the observed
bioavailability for the claimed sufentanil dosage averaged 91% as compared to
the
published bioavailability for Rapinyl which is approximately 70% (Bredenberg,
New
Concepts in Administration of Drugs in Tablet Form, Acta Universitatis
Upsaliensis,
Uppsala, 2003). The coefficient of variation of the AUC (0-infinity) for
Rapinyl ranges
from 25-42% depending on dose, whereas the coefficient of variation for the
claimed
10 mcg sufentanil dosage forms is 27.5%. This high bioavailability would
suggest
that regardless of dose, the sufentanil dosage forms have a consistently low
coefficient of variation of AUC, whereas this is not true for Rapinyl. In
fact, the
coefficient of variation around the AUC for all three doses of sufentanil
exemplified
herein (2, 5, and 10 mcg) averaged 28.6%, demonstrating that the observed low
coefficient of variation is not dependent on dose.
[0251] The coefficient of variation of the C,õ,, for Rapinyi varies from 34 ¨
58%
depending on dose. As shown by the data presented herein, administration of
the
10 mcg sufentanil dosage form resulted in a C,õ,, with a coefficient of
variation of
only 28%, and the average coefficient of variation of for the 2, 5, and 10
mcg
*Trademark 45
CA 02673837 2014-10-23
WO 2008/085764
PCT/US2007/089017
doses was 29.4%, indicating minimal variability depending on dose. Similarly,
the
coefficient of variation for Tm, with Rapinyl ranges from 43 ¨ 54% depending
on
dose, whereas for our sufentanil dosage forms, this coefficient of variation
for T,õ
averages only 29% over all three dosage strengths. This consistent onset of
action
achieved with sublingual sufentanil dosage forms allows a safer redosing
window
when compared to any of the three comparator drugs, since rising plasma levels
are
contained to a shorter period.
[0252] Additionally, as with Fentora and Actiq, Rapinyl demonstrates a longer
plasma elimination half-life (5.4 ¨ 6.3 hours, depending on dose) than the
claimed
sufentanil dosage forms. The plasma elimination half-life of sufentanil dosage
forms
ranged from 1.5 ¨ 2 hours following a single oral transmucosal administration
in
humans (Table 2), which allows for more titratability and avoids overdosing.
As will
be understood by those of skill in the art, the half-life described herein for
the
exemplified dosage forms may be adjusted by modification of the component and
relative amounts of the excipients in the formulation used to make a given
dosage
form. The ability to titrate to higher plasma levels by administering
repetitive doses
of the sublingual sufentanil dosage forms was also tested in this human study.
[0253] Repeat dosing of 5 mcg dosage forms every 10 minutes for four dosings
resulted in a bioavailability of 96%, indicating that repetitive dosing to
achieve higher
plasma levels while still maintaining high bioavailability is possible.
Whether treating
post-operative pain or cancer break-through pain, being able to efficiently
titrate to
an individual's own level of pain relief is important.
[0254] Another aspect of the PK curves generated by sublingual sufentanil
dosage
forms is the plateau phase, which allows for a period of consistent plasma
levels,
which is important for both safety and efficacy. Compared to either IV bolus
administration (see Animal Studies Examples 2 - 5) or the 10 minute IV
infusion in
our human study (Example 1 and Figure 9), the PK profile for the sufentanil
dosage
forms is clearly safer. Rapid, high Cmõ plasma levels are avoided. Given the
ability of
opioids to produce respiratory depression, avoiding these high peaks in the PK
profile
is advantageous.
[0255] An important mathematical ratio that demonstrates the prolonged plateau
phase of the measured blood plasma levels of sufentanil following
administration of a
dosage form is the time spent above 50% of C,õõ divided by the known IV
terminal
elimination half-life of the drug:
46
*Trademark
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
Therapeutic Time Ratio = Time of offset of Cmõ/2 ¨ Time of onset of Cmõ2
IV Elimination Half-Life of the Drug
[0256] The elimination half-life is an intrinsic property of the molecule
and is
measured most reliably using the IV route to avoid contamination from
continued
uptake of drug from the sublingual route. The IV elimination half-life for 5
mcg of
sufentanil in our human study was 71.4 minutes due to the detection limits of
the
assay at these low doses. The published IV elimination half-life for
sufentanil at
much higher doses is 148 minutes, due to detection of both the rapid alpha-
elimination mechanism of redistribution and the longer beta phase of
elimination via
metabolism and excretion. This published elimination half-life is more
accurate and
more appropriate to use in the above equation. The time spent above 50% of
Cmax on
average for the 12 volunteers for the 2.5, 5 and 10 mcg dosage strengths was
110
minutes, 111 minutes and 106 minutes, respectively. Therefore, the Therapeutic
Time Ratio for these specific sufentanil dosage forms ranged from 0.72 ¨ 0.75.
As
the formulation of the dosage forms is varied, erosion time of the dosage form
will
be either decreased or increased, and one might see a range of Therapeutic
Time
Ratios from approximately 0.2 ¨ 2.0 for sufentanil.
[0257] The Therapeutic Time Ratio is a measure of how successfully short-
acting
drugs are formulated to produce an increase in therapeutic time and increase
safety
by avoiding high peak plasma Cmõ concentrations. For example, as a comparison,
the sufentanil IV arm of the human study demonstrated a Therapeutic Time Ratio
of
10min/148min = 0.067. This low ratio value for the IV arm, therefore, is a
measure
of the high peak produced by IV infusion of sufentanil and demonstrates that
this
formulation does not produce a significant plateau phase. There is a 10-fold
higher
Therapeutic Time Ratio for the sufentanil formulations listed in Table 1 (the
dosages
used in the human study) versus IV sufentanil, indicating a prolonged
therapeutic
plateau profile for these formulations.
[0258] The uptake of transmucosal medications via small volume drug dosage
forms results in a more consistent drug delivery between individual dosages
and
individual patients as compared to that of currently available oral
transmucosal
dosage forms for which a large fraction of drug uptake occurs via the GI
route.
[0259] The methods and systems described herein are designed to work
effectively in the unique environment of the oral cavity, providing for higher
levels of
drug absorption and pain relief than currently available systems. The claimed
47
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
methods and systems are designed to avoid the high peak plasma levels of
intravenous administration by entry into the circulation via the sublingual
mucosa.
[0260] The claimed methods and systems further provide for independent control
of bioadhesion, dosage form disintegration (erosion) and drug dissolution and
release over time, together with administration using a device to provide a
safe
delivery profile. The device-administered oral transmucosal dosage forms
provide
individual, repetitive doses that include a defined amount of the active agent
(e.g.,
sufentanil), thereby allowing the patient or care giver to accurately titrate
the
amount of drug delivered and to adjust the amount as appropriate in a safe and
effective manner. The lock-out feature of the dispensing device adds to the
safety of
the drug delivery profile.
[0261] An advantage of the oral transmucosal dosage forms herein is that they
exhibit high consistent bioavailability and can maintain the plasma drug
concentration within a targeted therapeutic window with significantly lower
variability
for a longer duration than currently available medications or systems for
treatment of
pain. The high peak plasma levels typically observed for IV dosage forms are
blunted
following administration of sufentanil-counting dosage forms. In addition, a
rapid
decline in plasma levels is avoided since the drug is continually crossing
from the oral
cavity into the bloodstream during the length of time of erosion of the dosage
form
or longer, thus providing plasma pharmacokinetics with an extended plateau
phase
as compared to the IV route of administration. Further, treatment with the
claimed
methods and systems provides for improved safety by minimizing the potentially
deleterious side effects of the peaks and troughs in the plasma drug
pharmacokinetics, which are typical of currently available medications or
systems for
treatment of pain.
[0262] Advantages of the claimed sublingual dosage forms over various liquid
forms for either sublingual or intranasal administration include local release
of drug
from the dosage form over time with minimal swallowing of liquid drug via
either the
nasal or oral/GI route. Published pharmacokinetic data following
administration of
intranasal sufentanil liquid (15 mcg) in humans demonstrates a bioavailability
of 78%
(Helmers et al., Canadian Journal of Anaesthesia 36:494-497, 1989). Sublingual
liquid sufentanil administration (5 mcg) in Beagle dogs (see Example 4 below)
resulted in a bioavailability of 40%. The aforementioned bioavailability data
are less
than the 9 1 % average bioavailability that was obtained in human volunteers
using
48
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
sufentanil administered sublingually in the form of a small volume dosage form
(see
Example 1 below).
[0263] Due to the small size of the oral transmucosal dosage forms, repeated
placement in the sublingual cavity over time is possible. Minimal saliva
production
and minimal physical discomfort occurs due to the small size, which allows for
repetitive dosing over days to weeks to months. Given the lipid profile of the
sublingual cavity, the sublingual route, also allows for slower release into
the plasma
for certain drugs, such as sufentanil, which may be due to utilization of a
"depot"
effect that further stabilizes plasma levels compared to buccal delivery.
[0264] The oral transmucosal dosage forms are designed to fit comfortably
under
the tongue such that the drug form erodes sufficiently slowly to avoid the
immediate
peak plasma levels followed by significant drop off seen in prior art
formulations such
as described in US. Patent No. 6,759,059 (Rapinyl), wherein fentanyl was
administered via tablets containing 400mcg of fentanyl which resulted in a
peak
plasma level of 2.5ng/mlfollowed by an immediate drop in plasma level. Fentora
(fentanyl buccal tablet) also suffers from a lack of a plateau phase but
rather has a
steep incline up to the Cmax followed by a significant drop-off in plasma
levels
(Fentora package insert).
Animal studies
[0265] A series of studies in awake, alert Beagle dogs was performed to more
fully elucidate the properties of small volume oral transmucosal dosage forms
using
various drugs and formulations. A comparison of the claimed system for oral
transmucosal administration of sufentanil relative to administration of liquid
sublingual sufentanil or swallowed sufentanil dosage forms was made to
evaluate
various attributes of the drug dosage forms. The results support the claim
that the
small, volume drug dosage forms of the invention are well tolerated
sublingually (as
demonstrated by use in awake dogs) and result in higher bioavailability and
more
consistent pharmacokinetic data than other oral transmucosal dosage forms,
including instilled liquids.
[0266] The first Beagle dog study was carried out to compare a sublingual 5
mcg
sufentanil dosage form to IV sufentanil as described more fully in Example 2
below.
A total of three Beagle dogs were studied and the results are graphed in
Figure 10
and tabulated in Table 3. The bioavailability of the sublingual sufentanil
dosage
forms was 75% compared to IV. Therefore, similar to the human data, this
49
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
bioavailability data in dogs confirms the superior attributes of the dosage
forms over
larger dosage forms. Furthermore, similar to the human data, the coefficient
of
variation for the AUC was low, 14%, compared to the variation of other
commercial
transmucosal dosage forms. The Therapeutic Time Ratio of the sublingual
sufentanil
dosage forms is 0.28 whereas the Ratio for IV sufentanil is 0.05 (using the
published
IV elimination half-life of sufentanil in dogs of 139 minutes). Therefore,
similar to
humans, the 5 mcg dosage form in Table 1 resulted in a much higher Therapeutic
Time Ratio (5.6-fold) compared to IV sufentanil in dogs.
[0267] Additional studies determined the effect of varying the formulation on
the
pharmacokinetic profile. This study is explained more fully in Example 3
below. By
prolonging the erosion time of the dosage form, the plasma half-life was
extended
from 33 minutes for the medium disintegrating formulation (in Example 2) to
205
minutes. The Therapeutic Time Ratio was increased from 0.28 to 1.13 for the
slow
disintegrating dosage forms. This study illustrates the flexibility of the
small volume
dosage forms, and the ability based on excipient selection, to alter the PK of
the
drug. This flexibility is possible due to the small size of the dosage forms,
which
allows either short or prolonged contact time with the sublingual mucosa
without
dislodging or creating excess saliva which would prematurely wash the drug
into the
GI tract.
[0268] Another study in Beagle dogs was performed to evaluate the advantages
of the sublingual dosage form over liquid administration sublingually. This
study is
described more fully in Example 4 below. The results indicate that although
delivery
of sufentanil (5 mcg) in an instilled liquid form to the sublingual cavity
results in
rapid Tmax, this method of drug administration results in very low
bioavailability
(40%) compared to sublingual sufentanil dosage forms (75%). This is probably
due
to swallowing of the liquid drug. Moreover, the AUC is extremely variable, as
shown
by the high coefficient of variation (82%). The Cmax is also highly variable
with this
method of drug administration, demonstrating a coefficient of variation of
72%. The
Therapeutic Time Ratio for instilled liquid sufentanil sublingually was
calculated as
0.06, very similar to the IV sufentanil arm for this study which demonstrated
a Ratio
of 0.03. Therefore, this instilled sublingual liquid profile does not provide
the
advantageous therapeutic plateau observed with the sublingual dosage forms.
These
findings support that the high sublingual bioavailability observed from
different
formulations is not intrinsic to the molecule but rather it is a direct result
of the
unique design of the dosage form and its formulation. The strong adherence of
the
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
small dosage forms s to the oral mucosa in the sublingual cavity minimizes the
variability in the surface area available for absorption, as is the case of a
liquid
solution, thus improving delivery of the molecule to the systemic circulation.
In
addition, owing to its unique design and small dimensions, the dosage forms
does
not elicit significant saliva production, thus reducing the potential for
ingestion of the
released drug. Both factors contribute to the higher and more uniform drug
absorption from the sublingual cavity.
[0269] An additional part of this study in Example 4 was the determination of
the
bioavailability of swallowed sufentanil dosage forms. Since there is little to
no data
on sufentanil GI bioavailability in the literature, it was important to
further evaluate
the low bioavailability of this route of administration to further support the
observation that drug from the sublingual dosage forms could not be swallowed
and
maintain a high bioavailability. As indicated by the PK analysis data in Table
7, oral
bioavailability of sufentanil from the swallowed dosage forms is very low,
approximately 12%. In addition, as predicted from the known erratic GI uptake
of
fentanyl, the swallowed dosage forms demonstrated extremely high variability
both
in the amount of drug absorbed (AUC) and the pharmacokinetics of absorption
(Cmax,
Tmax) as shown in Table 7. These data support the conclusion that bioadhesive
sublingual dosage forms strongly adhere in the sublingual cavity in such a
manner
that they don't dislodge, thus avoiding oral ingestion and avoiding the high
variability
of plasma levels which is typical when drug is absorbed via the GI route.
[0270] Additional studies evaluating alfentanil, formulated into small
volume
dosage forms were performed in Beagle dogs and are more fully described in
Example 5 below.
[0271] Alfentanil dosage forms resulted in a bioavailability of 94% compared
to IV
alfentanil and a coefficient of variation of 5% for the AUC, 7% for Cmax and
28% for
Tmax. The Therapeutic Time Ratio was calculated as 0.33, compared to 0.04 for
the
IV alfentanil arm of this study (calculated using a published IV elimination
half-life of
104 min for alfentanil in dogs). Therefore, the alfentanil formulation (as
described in
Example 5) produces an 8-fold improved Therapeutic Time Ratio over the IV
alfentanil arm. The high bioavailability of this formulation again supports
the claim
that minimal swallowing of drug occurs with use of the dosage forms.
51
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
VIII. Utility Of Small-Volume Oral Transmucosal Dosage Forms.
[0272] The claimed dosage forms find utility in delivery of any drug that can
be
administered by the oral transmucosal route. The small volume oral
transmucosal
dosage forms provide for high bioavailability, low variability in Tmax, low
variability in
Cmax and low variability in AUC. The dosage forms also provide for prolonged
plasma
levels within the therapeutic window.
[0273] In one exemplary embodiment described in detail herein, the dosage
forms find utility in treating a subject suffering from pain that may be
associated
with any of a variety of identifiable or unidentifiable etiologies. In this
embodiment,
the dosage forms find utility in suppression or mitigation of pain. The term
"treatment" or "management" of pain is used here to generally describe
regression,
suppression, or mitigation of pain so as to make the subject more comfortable,
as
determined for example by pain score.
[0274] The invention finds utility in the treatment of both opioid naive
patients
and opioid tolerant patients.
[0275] The term "opioid naive patient" is used herein with reference to a
patient
who has not received repeated administration of an opioid substance over a
period
of weeks to months.
[0276] The term "opioid tolerant patient" as used herein means a physiological
state characterized by a decrease in the effects of an opioid substance (e.g.,
analgesia, nausea or sedation) with chronic administration. An opioid
substance is a
drug, hormone, or other chemical substance that has analgesic, sedative and/or
narcotic effects similar to those containing opium or its derivatives. If
analgesic
tolerance develops, the dose of opioid substance is increased to result in the
same
level of analgesia. This tolerance may not extend to side effects and side
effects may
not be well tolerated as the dose is increased.
[0277] The dosage forms find particular utility in the treatment of acute pain
or
other conditions "in the field", i.e., under highly sub-optimal conditions.
Paramedics
or military medics often are required to treat severe acute pain or other
injuries or
conditions in non-sterile situations, where needles used for IV or IM
administration
can result in unintended needle sticks, risk of infection, etc. Oral opioid
tablets often
take 60 minutes to provide relief which is too long for someone in severe
pain. The
claimed dosage forms find utility in addressing this need.
[0278] When the dosage forms are used for the treatment of pain, the claimed
methods and systems find utility in administration of drugs to pediatric and
adult
52
CA 02673837 2009-06-23
WO 2008/085764 PCT/US2007/089017
populations and in treatment of human and non-human mammals, as well as in
opioid tolerant and opioid naive patient populations.
[0279] Application of the claimed methods and systems is not limited to any
particular therapeutic indication. As such, the claimed dosage forms find
utility in
administration of drugs to pediatric and adult populations and in the
treatment of
human and non-human mammals.
[0280] The dosage forms find utility in pediatric applications, since the
comfortable and secure nature of the dosage form allows children to readily
accept
this mode of therapy and will reliably deliver drug transmucosally. Specific
examples
include, but are not limited to, treatment of pediatric acute pain when IV
access is
not available or inconvenient, treatment of pediatric asthma when the child is
not
able to use an inhaled route of administration effectively, treatment of
nausea when
a child can not or will not swallow a pill, pre-procedural sedation when a
child is NPO
(no oral intake allowed) or a more rapid onset is required.
[0281] The dosage forms find further utility in veterinary applications.
Specific
examples include, but are not limited to, any treatment of an acute condition
for
which IV administration is not readily available or inconvenient, such as pain
relief,
anxiety/stress relief, pre-procedural sedation, etc.
[0282] The following examples are provided to illustrate the invention and are
not
intended to limit any aspect of the invention as set forth above or in the
claims
below.
[0283] The dosage forms for the dosage forms described above can be tested for
in vivo drug pharmacokinetics in both humans and a suitable animal model
following
sublingual administration.
[0284] The following examples demonstrate the ability of the dosage forms to
allow a consistent absorption profile of sufentanil citrate following
sublingual
administration in human volunteers and awake, alert Beagle dog model.
Example 1. Sublingual Sufentanil dosage forms Administered Sublingually in
Adult
Human Volunteers.
Table 1. Sufentanil Formulations Used in the Human Clinical Study
#46 #47 #48
2.5 ug Sufentanil Base 5.0 ug Sufentanil Base 10.0 ug
Sufentanil Base
Ingredient Mass in Mass in % Mass in Mass in Mass in %
Mass in Mass Mass % Mass in
Batch (g) tablet, tablet, w/w Batch tablet, mg
tablet, in in tablet,
mg (9) w/w Batch table w/w
(g) t mg
Sufentanil 0.3750 0.00375 0.068 0.75 0.0075 0.136
1.50 0.01 0.273
53
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
#46 #47 #48
2.5 ug Sufentanil Base 5.0 ug Sufentanil Base 10.0 ug
Sufentanil Base
Ingredient Mass in Mass in % Mass in Mass in Mass in %
Mass in Mass Mass % Mass in
Batch (g) tablet, tablet, w/w Batch tablet, mg
tablet, in in tablet,
mg (9) w/w Batch table w/w
(g) t mg
Citrate 50
Mannitol 200SD 406.60 4.066 73.931 406.3 4.063 73.866
405.50 4.05 73.727
Poloxamer 11 0.110 2.000 11 0.110 2.000 11 0.11
2.00
(Lutrol F68) 0
Polyox WSR 303 16.5 0.165 3.000 16.5 0.165 3.000
16.5 0.16 3.00
5
PEG-8000 82.5 0.825 15.001 82.5 0.825 14.999 82.5
0.82 15.00
5
Stearic Acid 27.5 0.275 5.000 27.5 0.275 5.000
27.5 0.27 5.00
5
Mg Stearate 5.5 0.055 1.000 5.5 0.055 1.000 5.5 0.05
1.00
5
Total 549.975 5.49975 100 550.050 5.5005 100 550.00 5.5
100
Calculated Strength (Sufentanil base) 0.002506159 0.005012
0.010025
[0285] A human clinical study was performed using healthy volunteers. The
study
was performed with 12 subjects (6 men and 6 women) using sufentanil-containing
dosage forms (formulations #46 - #48 shown in Table 1) manufactured to have a
5 volume of 5 pL, a mass of approximately 5.5 mg, and determined to have a
uniform
size for all dosage strengths with dimensions of approximately 3 mm in
diameter and
0.8 mm in thickness. Sufentanil dosage forms contained either 2.5mcg, 5mcg or
10mcg of sufentanil base corresponding to 3.7mcg, 7.5mcg or 15mcg of
sufentanil
citrate, respectively. All excipients were inactive and have GRAS ("generally
recognized as safe") status. The sufentanil dosage forms were tested for
sublingual
use. Study staff administered individual dosage forms to a subject by placing
them
directly at the base of the frenulum using blunt-tipped forceps.
[0286] For
bioavailability calculations, intravenous sufentanil, 5 mcg was diluted in
0.9% saline to a total volume of 20 mL, and was administered through an Iv
catheter as a continuous infusion over 10 minutes. Plasma samples were drawn
from a different Iv catheter at a remote location. This human trial was a
cross-over
design with wash-out periods between transitions from higher to lower doses.
Subjects were blocked with the opioid antagonist naltrexone daily to avoid
opioid-
induced side-effects.
54
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
= Day 0: IV sufentanil Infusion:
O Seventeen samples were collected:
-5.0 (before the start of infusion), 2.5, 5, 7.5, 10, 12.5, 15, 20, 30, 45,
60, 90, 120,
160, 320, 480 and 640 minutes
= Day 2: sublingual 2.5 mcg sufentanil dosage forms;
O Seventeen samples:
-5.0 (before dosage form administration), 2.5, 5, 7.5, 10, 12.5, 15, 20, 30,
45, 60,
90, 120, 160, 320, 480 and 640 minutes
= Day 3: sublingual 5.0 mcg sufentanil dosage forms;
o Seventeen samples:
-5.0 (before dosage form administration), 2.5, 5, 7.5, 10, 12.5, 15, 20, 30,
45, 60,
90, 120, 160, 320, 480 and 640 minutes
= Day 4: sublingual 10.0 mcg sufentanil dosage forms;
O Seventeen samples:
-5.0 (before dosage form administration), 2.5, 5, 7.5, 10, 12.5, 15, 20, 30,
45, 60,
90, 120, 160, 320, 480 and 640 minutes
= Day 7: sublingual 5.0 mcg sufentanil dosage forms repeated 4 times at 10
minute intervals;
O Twenty three samples:
-5.0 (before the first dosage form administration), 5, 7.5 minutes
10 (immediately prior to the second dosage form administration), 15, 17.5
minutes
20 (immediately prior to the third dosage form administration), 25, 27.5
minutes
(immediately prior to the fourth dosage form administration), 35, 40, 45, 50,
55,
60, 90, 120, 150, 190, 350, 510 and 670 minutes
25 [0287] The total volume of blood required for pharmacokinetic sampling
was
approximately 455 mL.
[0288] Sufentanil concentrations in plasma samples were determined using a
validated LC-MS/MS sufentanil human plasma assay. The assay demonstrated good
inter-day precision and accuracy at the high, medium and low quality control
sample
30 concentrations.
CA 02 673 83 7 2 0 0 9-0 6-2 3
WO 2008/085764 PCT/US2007/089017
[0289] The dosage forms for this study eroded over a period of 10-30 minutes
in
all subjects. After placement of each sufentanil sublingual dosage forms in
the
sublingual cavity of the 12 healthy volunteers, a remarkably consistent
pharmacokinetic profile was obtained for the three dosages (Figure 9).
Table 2. PK Analyses of the IV (5 mcg) and Sublingual Sufentanil Dosing Arms
in the
Human Clinical Study using Three Dosage Strengths (2.5 mcg = #46, 5 mcg = #47,
10
mcg = #48)
Plasma
AUC Absorption
ANK
(hr*ng/m1) (00 Elmnat
on Therapeuti
Variability (pgCym..L) (min) Half
O F.
O
Half-life
Time Ratio':':
(mean+SD) (0/0 CV)
(hr) ........
0813 9.6
Intravenous Sufentanil 0.0368 0.0076 - 20.7 0. 8 1.19
0.18 0.067
0.0281 1.
Sublingual Sufentanil
0.0068 43.7.8 8
dosage form 0.0178 0.0044 97.8 24.7 0 0021 1.65
0.43 0.74
(Formulation #46) .
Sublingual Sufentanil
0.0109 46.2
dosage form 0.0273 0.0093 76.7 34.1 0 0035 17.4
1.54 0.57 0.75
(Formulation #47) .
Sublingual Sufentanil
0.0275 40.8
dosage form 0.0705 0.0194 98.2 27.5 0 0077 13.2
1.71 0.40 0.72
(Formulation #48) .
Repeat Dosing of #47
0.0464 62.4
Sufentanil dosage 0.1403 0.0361 96.4 25.7 0 0124 13.8
1.97 0.30 NA
form every 10 min. x 4 .
Represents the relative time that the drug achieves therapeutic levels (above
50% of Cmax) and it is calculated by the formula: TTR= (Time spent above 50%
of
Cmõ) / (IV Terminal elimination half-life). The denominator is obtained from
literature
and is 148 min in humans for sufentanil.
Example 2. In vivo Evaluation of Sublingual Sufentanil dosage forms in a Dog
Model.
[0290] The following Examples 2-5 are using the Beagle dog model and the
formulations for the dosage forms all are using a dosage form with a total
mass of
5.5 mg. The in vivo pharmacokinetics (PK) of sufentanil following sublingual
administration of the 5 mcg dosage forms (formulation #44 for dogs, which is
the
same as the human formulation #47) described above were evaluated in a healthy
Beagle dog model. Briefly, single 5mcg dosage forms described above were
administered sublingually in fully awake healthy dogs by direct placement in
the
sublingual cavity. A total of three dogs were evaluated. Following
administration, the
position of the dosage form in the sublingual cavity was observed visually at
5 - 15
minute intervals following administration. The sublingual sufentanil PK was
compared
with that of IV administered sufentanil at the same dose level.
[0291] All dogs were catheterized via the cephalic vein for blood collections
up to
2 hours post-dosing. Through the 2-hour post-dose blood collection, all dogs
were
fitted with an Elizabethan collar to prevent removal of the catheter. The
catheter
was removed following the 2-hour blood collection. The 4-, 8-, and 24-hour
post-
56
CA 02673837 2009-06-23
WO 2008/085764 PCT/US2007/089017
dose blood collection were collected from the cephalic or other suitable vein.
Approximately 2 ml of blood were collected into pre-chilled tubes containing
potassium EDTA at the following time points: prior to dosing and approximately
1, 3,
5, 10, 15, 30 min, 1, 2, 4, 8 and 24 hours post-dose. The samples were
analyzed
with the appropriately validated LC/MS/MS method for the determination of
sufentanil citrate in dog plasma. The sufentanil plasma concentrations and the
pharmacokinetic results are shown in Figure 10 and Table 3.
Table 3. PK Analysis of Sufentanil Sublingual Dosage Forms Compared to
Intravenous Sufentanil in Beagle Dogs.
Absorption ::: T - Plasma
AUC F
Therapeutic
.G1. 11
Variability (min) (pg/m4 m.ax
Half-life =
1111 P (Pleari SDyll =.:-. Time
Ratio'
(13/0 CV) (min)
Intravenous
211.5 48.2 22.8 536.7 186.1 1.6 0.6
10.3 4.5 0.05 0.02
Sufentanil
Sublingual
Sufentanil dosage
74.8
form 161.2 23.1 14.3 222.7
25.9 11.7 2.5 33.3 5.8 0.28 0.16
10.7
(Formulation
#44)
-max,
Represents the relative time that the drug achieves therapeutic levels (above
50% of C land
it is calculated by the formula: TTR= (Time spent above 50% of Cmax) / (IV
Terminal elimination half-
life). The denominator is obtained from literature and is 139 min in beagle
dogs for sufentanil.
Example 3: Exemplary Sufentanil Dosage Forms to Control Drug Release and In
Vivo
Pharmacokinetics.
[0292] For purposes of illustration, a longer duration dosage form
(formulation
#58) was prepared with sufentanil citrate in order to evaluate a slower rate
of drug
release and in vivo pharmacokinetics of a longer-acting dosage form. This
slower
disintegrating sufentanil dosage form, as described in Table 4 was prepared by
direct
compression and tested as described above. The range of erosion times in dogs
was
35 ¨ 120 minutes and the bioadhesion of the placebo formulation was measured
as
described above and determined to be 0.18 + 0.08 N/cm2.
[0293] Sample analysis was performed using a validated LC/MS/MS method for
analysis of sufentanil in dog plasma. Pharmacokinetic analysis was performed
using a
non-compartmental model of absorption. The results of a limited PK analysis
are
shown in Table 5.
57
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
Table 4. Slow Disintegrating Sufentanil Formulation.
Formulation # 58
Composition
Sufentanil citrate 0.5456
Mannitol 40.3
Carbopol 971 20.00
PEG 8000 25.60
HPMC 10.00
Polyox 303 2.60
Mg Stearate 1.00
Total 100.00
Table 5. PK Analyses for the Slow-Disintegrating Sublingual Sufentanil Dosage
Form
in Beagle Dogs.
Plasma
Therapeutic
Group Half-life Time Ratio'
Sublingual
formulation 205 93.1 1.13 0.69
#58
1 Represents
the relative time that the drug achieves therapeutic levels (above 50% of
Cma.) and
it is calculated by the formula: TTR= (Time spent above 50% of Cmax) / (IV
Terminal elimination half-
life). The denominator is obtained from the literature and is 139 min in
beagle dogs for sufentanil.
Example 4. In Vivo Study of Sublingual Sufentanil Solution and Swallowing of
Sufentanil dosage forms in a Dog Model.
A. Evaluation of Bioavailability of Sufentanil Following Sublingual
Administration of a
Solution Dosage Form
[0294] The bioavailability of sufentanil following sublingual
administration from a
solution as compared to that intravenously was evaluated in a healthy,
conscious
Beagle dog animal model, as indicated in Table 6. In both arms of the study
the
commercially available formulation of sufentanil citrate (Sufenta 50 pg/mL)
was
used and was dosed at the same total dose of 5 mcg of sufentanil base.
Intravenous
administrations (Group 1) were performed by single administration (n=3) of
Sufenta 50 pg/mL by bolus injection to the cephalic vein via a sterile needle
and
syringe of appropriate size. For the sublingual administrations (Group 2) the
test
article was prepared by appropriately diluting Sufenta 50 pg/mL with 0.9% w/w
to
the same final dose of 5 mcg of sufentanil base and was administered twice
sublingually (n=6 total), with each dose separated by a minimum of a 2-day
58
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
washout. Doses were slowly applied under the tongue, adjacent to the frenulum
via
a sterile syringe. Blood samples were collected from a jugular or other
suitable vein
prior to dosing and approximately 1, 3, 5, 10, 15, 30 min, 1, 2, 4, 8 and 24
hours
post-dose. Approximately 2 mL of blood were collected per time-point into pre-
chilled
tubes containing K2 EDTA. The samples were centrifuged at 3,000 x g for
approximately 10 minutes in a refrigerated centrifuge. Plasma was collected
and
frozen within 20 minutes of centrifugation at approximately -70 C and was
maintained at the same temperature until analysis. Sample analysis was
performed
using a validated LC/MS/MS method for analysis of sufentanil in dog plasma.
[0295] Pharmacokinetic analysis was performed using a non-compartmental
model of absorption. The sufentanil plasma concentrations are graphed in
Figure 11.
The results of the PK analysis are shown in Table 7.
B. Evaluation of Bioavailability of Sufentanil Following Oral Ingestion of a
Dosage
Form
[0296] The bioavailability of sufentanil following ingestion of a 5 mcg
sufentanil
dosage form (formulation #44, which is the same formulation as #47 used in the
human study above) as compared to intravenous sufentanil administration was
evaluated in a healthy, conscious Beagle dog animal model, as described in the
previous example. A single 5 mcg dosage form was administered twice orally,
with
each dose separated by a minimum of a 2-day washout for a total of n=6 (Table
6).
The dosage forms were placed manually as far back as possible in the throat
and
flushed with water to promote the swallow response in the animal.
Pharmacokinetic
analysis was performed using a non-compartmental model of absorption. The
sufentanil plasma concentrations are shown graphed in Figure 11. The results
of the
PK analysis are shown in Table 7.
Table 6. Organization of Test Groups
Dose Route of Number of Total
Treatme
Group Level
Administratio Animalsb Number of
nt
(pg)a (Males) Animals, n
Sufentani
1 5.0 IV 3 3
I solution
Sufentani
2 5.0 Sublingual 3 c 6
I solution
Ingested
Sufentani
3 5.0 Oral 3C 6
I dosage
form
59
CA 02673837 2009-06-23
WO 2008/085764 PCT/US2007/089017
a =Expressed as a free base.
b =Same animals will be used for Groups 1 through 3 with a minimum 2-day
washout period between dosing.
c =Group 2 & 3 animals were dosed twice with a minimum 2-day washout
period for a total of n=6
d =Normal (0.9% w/w) saline was used to dilute the test article (Sufenta 50
pg/mL) to the desired concentration.
Table 7. PK Analyses of Intravenously Administered Sufentanil Compared to a
Sublingually Instilled Sufentanil Solution and an Ingested Sufentanil Dosage
Form in
Beagle Dogs.
Absorption Plasma TherapeutiC
Group
AUC Fl Tma. Cma.
Variability Half-life
Plateau
(Mean SD) (0/0) (min) (pg/mL)
(0/0 CV) (min)
Ratiol
Intravenous
123.3 49.3 21.8 1.0 0.0 536.7 186.1 2.8 0.4 0.02 0.0
Sufentanil
Sublingual
Sufentanil 58.3 36.4 40.0 32.7 81.8 4.3 1.0 236.4 170.0 8.3 4.5 0.04 0.02
solution
Ingested
15.9 22.4 12.2 15.3 134.2 14.6 9.9 33.8 33.2 22.5 16.8
0.13 0.09
dosage form
Represents the relative time that the drug achieves therapeutic levels (above
50% of Cmõ) and it is calculated by the formula: TTR= (Time spent above 50% of
Cmax) / (IV Terminal elimination half-life). The denominator is obtained from
the
literature and is 139 min. in beagle dogs for sufentanil.
Example 5: In vivo Evaluation of Sublingual Alfentanil HCI Dosage Forms in a
Dog
Model.
[0297] For purposes of illustration of another drug use for the dosage
form, an
additional dosage form was prepared with alfentanil HCI in order to
demonstrate the
ability of the dosage forms described in this application to effectively
improve the PK
of alfentanil compared to that of the IV route of administration. The
formulation
composition, a medium disintegrating dosage form, is described in Table 11.
The
erosion time in dogs of formulation #63 was 20 minutes and the bioadhesion was
measured at 0.056 + 0.01 N/cm2for the placebo formulation.
[0298] The dosing parameters for this study are shown in Table 12. The
alfentanil
plasma concentrations are graphed in Figure 12. PK analysis was performed
using a
CA 02 673 83 7 2 0 0 9-0 6-2 3
WO 2008/085764 PCT/US2007/089017
non-compartmental absorption model. The results of the PK analysis are shown
in
Table 13. Blood sampling and storage mirrored the conditions described
earlier;
sample analysis was performed using a validated LC/MS/MS method for analysis
of
alfentanil in dog plasma.
Table 11. Exemplary Alfentanil Dosage Form
Formulation #63 wo
composition
Alfentanil HCI 5.00
Mannitol 52.00
Carbopol 974 7.00
PEG 8000 35.00
Mg Stearate 1.00
Total 100.00
Table 12. Dosing Parameters for Administration of Sublingual Alfentanil Dosage
Forms and an Intravenous Alfentanil Solution in Beagle Dogs.
Dose Levet' houte Of Number of Animali
11 Group Treatment .
(pg)a Administration (Males)
Alfentanil
1 253 IV 3
solution
Alfentanil
2 239.0 16.2 Sublingual 2
dosage form
a = Expressed as a free base.
b = Same animals were used for Groups 1 and 2 with a minimum 2-day
washout
period between dosing.
Table 13. PK Analyses of Alfentanil Sublingual Dosage Forms compared to
Intravenous Alfentanil in Beagle Dogs.
Absorption Plasma
AUCTRIM
Therapeutic
Group Variability (min) Crnax (ng/mL) Half-
life
(Mean SD) (0/0) Time
Ratio' .
Intravenous
15.3 1.6 10.5 1 0 139.1 76.4 4.4 2.4 0.04
0.02
Alfentanil
Sublingual
Alfentanil dosage 14.4 0.7 94.1 4.6 4.9 15.0 4.2 35.5
2.6 40.8 8.5 0.33 0.07
form
[0299] Represents
the relative time that the drug achieves therapeutic levels
(above 50% of Cmax) and it is calculated by the formula: TTR= (Time spent
above
61
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
50% of Cmõ) / (IV Terminal elimination half-life). The denominator is obtained
from
literature and is 104 min. in beagle dogs.
Example 6: Acute Pain Management in the Outpatient Setting by Administering a
Sufentanil-Containing Dosage Form Using a Device.
[0300] A pharmacist loads a drug dispensing device with a drug cartridge which
includes 40 sufentanil dosage forms. Each cartridge has two colored
initialization
tablets (called "shipping tablets") arranged to be the first two tablets
dispensed. The
device has a means for loading the cartridge, which is either a port, hatch,
or door
that is secure and inaccessible to unauthorized users. Once the pharmacist has
loaded the cartridge into the device, he locks the device access port, hatch
or door.
The pharmacist then docks the dispensing device for the first time to a dock
that is
connected to a personal or other computer, using the docking connector, and
then
programs the device. Programming involves uploading the dosage strength of the
dosage forms, the number of dosage forms loaded in the device, the prescribed
frequency of dosage form usage, the number of dosage forms to be used per day,
the current date and time, the preferred language, a valid thumbprint or other
identification for identifying the patient, and the physician's identification
information,
in case the device is lost and found.
[0301] Once the dispensing device is programmed, the pharmacist demonstrates
proper usage and tests the device by dispensing a single shipping tablet. The
pharmacist then gives the dispensing device to the patient and observes the
patient
dispense a shipping tablet to ensure proper usage and functionality. Along
with the
dispensing device, the pharmacist provides the patient with a radio frequency
identification (RFID) tag that must be within approximately 5 inches of the
device to
allow the dispensing device to operate.
[0302] When the patient wants to administer a dose of the drug, he or she will
hold the dispensing device, and push any button to wake the device up from its
sleep mode. The device will query the user for either a thumbprint reading or
a
personal identification number (PIN). The device will then search for a
validated
RFID key within range. Once these conditions are met, the dispensing device
will
query its internal memory and clock to make sure that the dosage regimen
programmed by the pharmacist is not being violated by the current usage
request.
62
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
At this point the device displays status information, such as the date and
time, the
number of doses left, the last time a dosage was used, the patient's name,
etc., and
the pharmacist informs the patient that the device is ready to dispense the
dosage
forms by a visual and/or audible signal.
[0303] The patient will hold the dispensing end of the device under his or her
tongue and press the dispensing lever. When the dosage form is dispensed a
tone
will sound to inform the patient that the dosage form was properly delivered.
At this
point the device will lock down to prevent further dispensing until the
preprogrammed lock-out time has passed, at which time the device will be ready
to
use again.
Example 7: Acute Pain Management in the Inpatient Setting by administering a
Sufentanil-Containing Dosage Form Using A Device.
[0304] A post operative patient requires acute pain treatment following
surgery.
The surgeon prescribes oral transmucosal sufentanil to be administered using
the
drug dispensing device. The attending nurse takes the prescription order to
the
pharmacist or automated pharmaceutical inventory management system (e.g.
Pyxis)
and obtains a sufentanil-containing drug cartridge for sublingual delivery.
The
cartridge is labeled and equipped with an RFID electronic tag containing drug
label
information. The cartridge is labeled and equipped with an RFID electronic tag
containing drug label information.
[0305] The nurse then takes a disposable dispensing portion of the drug
dispensing device from inventory, and proceeds to a base station to obtain a
reusable controller portion of the drug dispensing device that has completed
its
recharge cycle and is ready for use. The nurse inserts the drug cartridge into
the
disposable dispensing portion, and then affixes this to the reusable
controller portion
of the drug dispensing device and locks the disposable portion into the
reusable
portion of the drug dispensing device. At this point the device reads the RFID
tag on
the drug cartridge and uploads the appropriate drug information, including the
type
of drug, the dosage strength, the programmed lockout period between doses,
etc.
The nurse confirms the proper drug cartridge information has been read by the
drug
dispensing device and gives the drug dispensing device to the patient for
patient
controlled dispensing of the pain medication.
63
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
[0306] When the patient requires pain medication, she takes the drug
dispensing
device in her hand, and places the dispensing tip in her mouth, under her
tongue
and presses the dispense button. The drug dispensing device then does an
internal
check to ensure that the proper lockout period has elapsed since the last
dosage
dispense. At this point the drug dispensing device dispenses a dosage form
under
the patient's tongue and provides feedback that dosing was successful. The
patient
removes the drug dispensing device from her mouth and allows the sublingual
dosage form to dissolve under her tongue. The patient may attempt to dispense
as
frequently as she desires, but the drug dispensing device will only allow
successful
dosing after the appropriate lockout period has elapsed. The drug dispensing
device
electronically logs the dispensing attempts and successful dispenses in its
dosing
history.
[0307] Periodically the nurse checks on the patient and drug dispensing
device.
During such checks, the nurse inspects the drug dispensing device to see that
there
are no errors and to check the number of remaining dosage forms in the drug
dispensing device, and returns it to the patient.
[0308] When the patient is discharged, the nurse takes the drug dispensing
device and unlocks the reusable portion from the disposable portion, disposes
of the
cartridge and disposable portion of the drug dispensing device. The nurse then
connects the reusable portion of the device to a computer and uploads the
patient
use information from the drug dispensing device to the computer for input into
the
patient's medical records. The nurse cleans the reusable controller portion
and
returns it to the base station for recharging.
Example 8: Acute Pain Management in the Inpatient Setting by administering a
Sufentanil-Containing Dosage Form Using A Device and a Portable Dock.
[0309] A post operative patient requires acute pain treatment following
surgery.
The surgeon prescribes oral transmucosal sufentanil to be administered using
the
drug dispensing device. The attending nurse takes the prescription order to
the
pharmacist or automated pharmaceutical inventory management system (e.g.
Pyxis)
and obtains a sufentanil-containing drug cartridge for sublingual delivery.
The
cartridge is labeled and equipped with an RFID electronic tag containing drug
label
information. The cartridge is labeled and equipped with an RFID electronic tag
64
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
containing drug label information. The cartridge includes a shipping tablet or
initialization tablet in the first to be dispensed location of the dosage form
stack.
[0310] The nurse then takes a disposable dispensing portion of the drug
dispensing device from inventory, and proceeds to a base station to obtain a
reusable controller portion of the drug dispensing device that has completed
its
recharge cycle and is ready for use. The nurse inserts the drug cartridge into
the
disposable dispensing portion, and then affixes this to the reusable
controller portion
of the drug dispensing device. Next, the nurse takes a portable dock (or
docking fob)
from the base station where it has been recharging, and docks the assembled
drug
dispensing device to the portable dock. The portable dock and the assembled
drug
dispensing device communicate electronically and a setup menu comes up on the
portable dock for setting up the drug dispensing device.
[0311] At this point the device locks the reusable and disposable portions
together, reads the RFID tag on the drug cartridge and uploads the appropriate
drug
information, including the type of drug, the dosage strength, the lockout
period
between doses, etc. The dispensing device writes a code to the RFID tag on the
cartridge identifying it as a used cartridge. The nurse enters her fingerprint
in the
fingerprint reader on the portable dock to gain secured access and proceeds to
set
up the drug dispensing device for use. The set up procedure includes entering
user
identification, the nurse's identification, confirming the proper time on the
device,
and confirming the proper drug cartridge information. The nurse then takes a
disposable RFID bracelet and places this adjacent to the drug dispensing
device at
which point the drug dispensing device reads the tag and the nurse confirms
that the
proper bracelet tag has been read.
[0312] The nurse then confirms proper setup of the drug dispensing device by
pressing the dispensing button once. The drug dispensing device actuates,
dispensing the shipping tablet facsimile into the nurses hand, confirming
proper
operation. The drug dispensing device detects the dispensing of the shipping
tablet,
allowing for an internal system check of proper operation and internal
calibration of
the newly assembled system. If the internal dispensing check is successful,
the
portable dock queries the nurse to confirm that the shipping table was
properly
dispensed, and the nurse confirms the proper setup. The nurse then disengages
the
CA 02673837 2009-06-23
WO 2008/085764
PCT/US2007/089017
drug dispensing device from the portable dock, and proceeds to the patient's
bedside
for the final steps of setup.
[0313] The nurse places the RFID bracelet on the patient's wrist and affixes a
theft resistant tether to the patient's bed and the other end to the drug
dispensing
device. The nurse then instructs the patient on proper use of the sublingual
drug
dispensing device, and gives the drug dispensing device to the patient for
patient
controlled dispensing of sufentanil.
[0314] When the patient requires pain medication, she takes the drug
dispensing
device in her hand, and places the dispensing tip in her mouth, under her
tongue
and presses the dispensing button. The drug dispensing device then does an
internal
check to ensure that the proper lockout period has elapsed since the last
dosage
dispense, and that the patient's RFID bracelet is present and readable. At
this point
the drug dispensing device dispenses a dosage form under the patient's tongue
and
provides a feedback that dosing was successful. The patient removes the drug
dispensing device from her mouth and allows the sublingual dosage form to
dissolve
under her tongue. The patient may attempt to dispense as frequently as she
desires,
but the drug dispensing device will only allow successful dosing after the
appropriate
lockout period has elapsed. The drug dispensing device electronically logs the
dispensing attempts and successful dispenses in its dosing history.
[0315] Periodically the nurse checks on the patient and device. During such
a
patient check in the nurse brings a portable docking FOB and docks the device
to the
FOB. The electronic connection enables the nurse to download the information
from
the drug dispensing device to the fob. This information includes the use
history, drug
information, number of remaining dosage forms and duration of use since
initial set
up. The nurse then enters her fingerprint in the finger print scanner to gain
access to
the information and to drug dispensing device. Because the patient is
requiring an
additional dose of drug prior to the lockout period expiring, the nurse
overrides the
lockout period and then returns the drug dispensing device to the patient at
which
point the patient is able to take another dose.
[0316] The nurse leaves the patient's room with the portable docking FOB and
returns to the nurse's station to record the dosing history in the patient's
records.
When finished the nurse returns the FOB to the base station for recharging.
66
CA 02673837 2014-10-23
WO 2008/085764
PCT/US2007/089017
[0317] When the patient has used all of the dosage forms in the drug
dispensing
device, the nurse brings the portable docking fob into the patient's room and
docks
the drug dispensing device to the FOB. The nurse then enters her fingerprint
in the
fingerprint scanner on the fob to gain secured access to the drug dispensing
device.
Next, the nurse unlocks the security tether and disconnects the drug
dispensing
device from the bed. She then unlocks the drug dispensing device and removes
it
from the fob for disassembly. The nurse disconnects the disposable portion
from the
reusable portion, and removes the cartridge from the disposable portion. The
nurse
disposes of the disposable portion and the cartridge, and wipes the reusable
controller portion with an antiseptic wipe to clean it before returning it to
the base
station. The reusable controller portion requires that the nurse return it to
the base
station where it recharges and runs an internal diagnostic test before being
ready for
use again.
[0318] The nurse then proceeds to set up a new drug dispensing device as
described above and provides this to the patient.
67