Note: Descriptions are shown in the official language in which they were submitted.
CA 02673938 2009-06-26
1
Method for desulphurizing nitrogen oxide storage catalysts in the exhaust gas
system of a lean burn engine
Description
The invention relates to a method for desulphurizing nitrogen oxide storage
catalysts in
the exhaust gas system of a lean burn engine with two or more cylinders.
Lean burn engines refer to diesel engines, and also to petrol engines with
direct petrol
injection and CNG engines (compressed natural gas = methane) which can be
operated
under lean conditions. To remove nitrogen oxides from the exhaust gas of lean
burn
engines, what are known as nitrogen oxygen storage catalysts can be used.
During its storage phase, a nitrogen oxide storage catalyst oxidizes the
nitrogen
monoxide present in the lean exhaust gas to nitrogen dioxide and then stores
it in the
form of nitrates. The mode of operation of nitrogen oxide storage catalysts is
described
in detail in the SAE publication SAE 950809. For oxidation of nitrogen
monoxide, a
storage catalyst contains, as catalytically active components, usually
platinum with or
without palladium. For storage of the nitrogen oxides as nitrates, basic
oxides,
carbonates or hydroxides of alkali metals, alkaline earth metals and rare
earth metals are
used; preference is given to using basic compounds of barium and of strontium.
After exhaustion of its storage capacity, a storage catalyst has to be
regenerated during a
regeneration phase. To this end, the exhaust gas is briefly enriched, for
example by
operating the engine with a rich air/fuel mixture. In the rich exhaust gas,
the nitrogen
oxides are desorbed again and reduced to nitrogen over the catalytically
active
components with the aid of the rich exhaust gas constituents. For this
purpose, the
storage catalyst usually contains rhodium in addition to the platinum.
Storage phase and regeneration phase alternate regularly. The alternation of
storage
phase and regeneration phase is referred to as alternating rich/lean
operation. The
storage phase usually lasts between 60 and 200 seconds, whereas the duration
of the
regeneration phase is only between 1 and 10% of the storage phase and thus
comprises
CA 02673938 2009-06-26
2
only a few seconds.
The function of nitrogen oxide storage catalysts is impaired by sulphur
compounds
which are present in the fuel and motor oil and get into the exhaust gas
essentially in the
form of sulphur dioxide in the course of combustion, and are bound by the
nitrogen
oxide storage catalysts in the form of very stable sulphates. This is at the
expense of the
nitrogen oxide storage capacity. At high sulphur contents in the fuel (> 10
ppm),
nitrogen oxide storage catalysts therefore frequently have to be
desulphurized. To this
end, the exhaust gas has to be brought to desulphurizing conditions, i.e. it
has to be
enriched and its temperature has to be raised. The air/fuel ratio lambda k of
the exhaust
gas should be lowered to a value below 0.98, preferably to below 0.95, and the
exhaust
gas temperature should be brought to a value between 600 and 750 C. Under
these
conditions, the sulphates formed are decomposed and emitted as hydrogen
sulphide or
preferably as sulphur dioxide.
When a nitrogen oxide storage catalyst is contacted with a sulphur-containing
exhaust
gas, the storage catalyst thus, as well as the regular regeneration to remove
the nitrogen
oxides stored, also has to be desulphurized from time to time in order to
reverse a
continuous deterioration in the nitrogen oxide storage capacity as a result of
sulphates
formed. The interval between two desulphurizations depends on the sulphur
content of
the fuel, but even at high sulphur contents is generally still several
operating hours of
the engine and is thus significantly greater than the interval between two
regenerations
to remove the nitrogen oxides stored. For the desulphurization, usually 2 to
10 minutes
are required. It thus likewise lasts longer than the nitrogen oxide
regeneration of the
storage catalyst.
The frequent desulphurization is at the expense of fuel consumption and leads,
owing to
the necessary high exhaust gas temperatures, to rapid ageing of the catalysts.
Therefore,
motor vehicles with lean burn petrol engines have to date been sold only on
the
European market, since fuels with a sulphur content of less than 10 ppm are
supplied
here. In the USA, the emissions legislation is particularly strict, but the
sulphur content
in the fuel for petrol engines here is at present still up to 30 ppm. In other
regions, the
sulphur content in the fuel is still significantly higher.
CA 02673938 2009-06-26
3
The development of motor vehicles with lean bum petrol engines for markets
with a
high sulphur content in the fuel thus has to take into account that, in this
case, the
nitrogen oxide storage catalysts have to be desulphurized frequently. In
addition to the
disadvantages of frequent desulphurization which have already been mentioned,
namely
the increased fuel consumption and the high thermal stress on the catalysts, a
further
disadvantage which occurs is a high emission of hydrocarbons and nitrogen
oxides
during the desulphurization, since the rich exhaust gas during the
desulphurization
contains high concentrations of uncombusted hydrocarbons, carbon monoxide and
nitrogen oxides, and also ammonia formed from the nitrogen oxides over the
catalysts,
but barely any oxygen to convert these exhaust gas components over the
catalysts. They
are therefore released to the environment in uncleaned form as pollutants.
However, American emissions legislation stipulates that the limits for
hydrocarbons,
carbon monoxide and nitrogen oxides, which were already very low in any case,
have to
be complied with even taking account of the desulphurization of the nitrogen
oxide
storage catalysts. For this purpose, the emissions during the desulphurization
of nitrogen
oxide storage catalysts are applied to the entire driving cycle envisaged for
the
emissions measurements. It has been found that even the emissions during a
single
desulphurization of nitrogen oxide storage catalysts can exceed the stipulated
limits for
so-called SULEVs (SULEV = Super Ultra Low Emission Vehicle).
It is an object of the present invention to specify a method for
desulphurizing nitrogen
oxide storage catalysts which largely suppresses the increased pollutant
emissions
during the desulphurization and thus makes it possible to comply with limits
for lean
burn internal combustion engines even in the case of sulphur-containing fuels.
This object is achieved by the method described by Claim 1. The method
requires a lean
burn engine with two or more cylinders, which are divided into a first group
and a
second group. The exhaust gases of the two cylinder groups are released into
exhaust
legs assigned to each. Each exhaust leg contains at least one nitrogen oxide
storage
catalyst for removal of the nitrogen oxides in the exhaust gas. The two
exhaust legs
open downstream of the storage catalysts into a common exhaust leg at a
confluence.
For further aftertreatment of the exhaust gas, the common exhaust leg contains
a
catalyst which, under stoichiometric conditions, has a three-way function,
which allows
CA 02673938 2009-06-26
4
it to simultaneously remove hydrocarbons, ammonia, nitrogen oxides and carbon
monoxide from the stoichiometric exhaust gas. This catalyst is referred to
hereinafter as
three-way catalyst for short.
The lean burn engine can be configured as an in-line engine in which all
cylinders are
arranged in succession in a single cylinder bank. Alternatively, each group of
cylinders
can be combined in a separate cylinder bank.
According to the invention, the nitrogen oxide storage catalysts in the two
exhaust legs
are desulphurized offset in time with respect to one another. The
desulphurization
conditions needed for this purpose can be established by engine measures or by
external
measures. The engine measures include the operation of the group of cylinders
assigned
in each case with a rich air/fuel mixture, the postinjection of fuel, a late
combustion
position or a multistage combustion. These measures can also be combined with
one
another. For external establishment of the desulphurization conditions, the
exhaust gas
can be enriched by injecting fuel into the particular exhaust leg upstream of
the nitrogen
oxide storage catalyst, and its temperature can be raised to desulphurization
temperature, for example, by external heating. The external heating can also
be
undertaken by means of oxidation catalysts arranged upstream of the nitrogen
oxide
storage catalysts and combustion of the fuel injected on these catalysts.
During the desulphurization of one nitrogen oxide storage catalyst, the other
nitrogen
oxide storage catalyst is operated under lean exhaust gas conditions of the
lean burn
engine. The air/fuel ratios of the exhaust gases in the two exhaust legs are
adjusted with
respect to one another such that the exhaust gas in the common exhaust leg
ideally has
an air/fuel ratio lambda of 1 over the entire desulphurization time, i.e. is
of
stoichiometric composition. In the real case, the air/fuel ratio in the common
exhaust leg
will deviate downward or upward from the ideal value, to a greater or lesser
degree,
variably with time, owing to the dynamic operating conditions of the engines.
The other nitrogen oxide storage catalyst is desulphurized in a corresponding
manner
offset in time with respect to the first nitrogen oxide storage catalyst.
Between two
desulphurizations, the two nitrogen oxide storage catalysts are operated in
the known
alternating operation between storage phase and regeneration phase.
CA 02673938 2009-06-26
For the desulphurization of one nitrogen oxide storage catalyst, the exhaust
gas is
enriched to an air/fuel ratio lambda of < 1, preferably to < 0.98 and
particularly to
< 0.95. At the same time, the second storage catalyst is operated at an
air/fuel ratio of
the exhaust gas lambda of > 1, preferably > I.I. After the combination of the
two
5 exhaust gas streams, the combined exhaust gas has an air/fuel ratio of about
lambda = 1.
Under these conditions, the three-way catalyst in the common exhaust leg can
virtually
completely eliminate the pollutant components from the rich exhaust gas of one
exhaust
leg with the pollutant components from the lean exhaust gas of the other
exhaust leg.
A further advantage of the invention is that the desulphurization can be
performed under
constantly rich exhaust gas conditions. This is because the hydrogen sulphide
formed is
converted to sulphur dioxide over the three-way catalyst in the common exhaust
leg. In
contrast, in the desulphurization methods known from the prior art, the
exhaust gas is
usually switched back and forth between lean and rich in rapid alternation, in
order to
suppress the formation of hydrogen sulphide. However, this alternating
operation
requires high exhaust gas temperatures for the desulphurization and leads to
higher fuel
consumption and longer desulphurization times compared to the method described
here.
During the desulphurization of one storage catalyst, the second storage
catalyst can be
operated with constantly lean exhaust gas. The regular nitrogen oxide
regeneration of
the second storage catalyst during the desulphurization of the first storage
catalyst is
unnecessary. Although the storage capacity of the second storage catalyst for
nitrogen
oxides is already exhausted about 1 to 2 minutes after the start of
desulphurization of
the first storage catalyst, the nitrogen oxides which therefore break through
the nitrogen
oxide storage catalyst are completely converted by the downstream three-way
catalyst.
The catalyst in the common exhaust leg must be able to fulfil the function of
a three-
way catalyst under stoichiometric exhaust gas conditions. For this purpose, it
contains at
least one noble metal from the group of platinum, palladium and rhodium. The
catalyst
preferably contains palladium and/or rhodium. In addition, the catalyst may
contain so-
called oxygen storage materials, particularly cerium oxide or a mixed oxide
containing
cerium oxide. Preference is given to using a catalyst configured specially as
a three-way
catalyst. Alternatively, however, instead of a three-way catalyst, a nitrogen
oxide
storage catalyst can be used in the common exhaust leg. This fulfils the same
purpose as
CA 02673938 2009-06-26
6
a three-way catalyst when the exhaust gas is of stoichiometric composition,
i.e.
possesses an air/fuel ratio of lambda = 1. In the normal operation of the
emission
control system, the storage catalyst may contribute additionally to the
conversion of
nitrogen oxides by storing them during the lean phase and converting them to
nitrogen
by means of short rich pulses.
The stipulation of the air/fuel ratio lambda = 1 for the exhaust gas in the
common
exhaust leg during the desulphurization should of course be understood only
within the
context of the tolerances customary for this purpose of 0.04, preferably
0.02. In
particular cases, it may even be advantageous to set the air/fuel ratio in the
common
exhaust leg at a slightly rich or slightly lean level within the tolerance
range specified.
A slightly lean exhaust gas in the common exhaust leg may be advantageous when
the
hydrogen sulphide formed in the desulphurization is to be oxidized to sulphur
dioxide
over the three-way catalyst. When a nitrogen oxide storage catalyst is used
instead of a
true three-way catalyst, a slightly rich exhaust gas can prevent the sulphur
dioxide or
hydrogen sulphide formed in the desulphurization from being absorbed by the
nitrogen
oxide storage catalyst in the conunon exhaust leg to form sulphates.
For exact regulation of the required exhaust gas composition in the common
exhaust
leg, it is advisable to arrange an oxygen probe upstream and/or downstream of
the three-
way catalyst. This probe passes its lambda signal on to an engine control
system. When
the desulphurization conditions are established through engine measures, the
combustion in the two cylinder groups is conducted such that a very
substantially
stoichiometric exhaust gas mixture is present in the common exhaust leg.
Suitable
oxygen probes are linear lambda probes or so-called jump probes. Nitrogen
oxide
probes can also be used to measure the oxygen content.
When engine measures to establish the desulphurization conditions are
undesirable or
impossible, as may be the case, for example, in diesel engines, rich or lean
exhaust gas
mixtures in the particular exhaust legs can also be established during the
desulphurization by direct injection of fuel into the particular exhaust legs.
The invention is illustrated in detail with reference to Figures 1 and 2. The
figures
show:
CA 02673938 2009-06-26
7
Fi$!ure 1: emission control system for performing the method for
desulphurization with
reduced emission of pollutants
Figure 2: a further embodiment of the emission control system for performance
of the
method for desulphurization with reduced emission of pollutants
Fi2ure 3: schematic diagram of the offset operation of the two cylinder banks
of the
emission control systems according to Figures 1 and 2
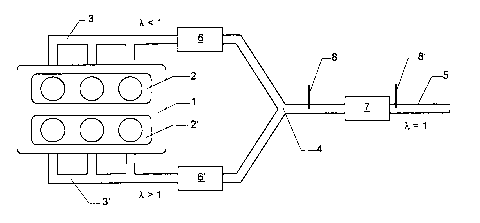
Figure 1 shows an emission control system for performing the desulphurization
method
with reduced pollutant emission. Reference numeral (1) denotes a lean burn
engine with
two cylinder banks (2) and (2'). The exhaust gases of these cylinder banks are
released
into the two exhaust legs (3) and (3'). At the confluence (4), the two exhaust
gas lines
(3) and (3') are combined to form a common exhaust leg (5). For storage and
conversion of the nitrogen oxides emitted by the lean burn engine (1), the
nitrogen
oxide storage catalysts (6) and (6') are arranged in the exhaust legs (3) and
(3'). The
three-way catalyst or nitrogen oxide storage catalyst (7) is present in the
common
exhaust leg. Reference numerals (8) and (8') denote the possible positions of
an oxygen
probe (lambda probe).
To desulphurize the nitrogen oxide storage catalyst (6), the cylinders of the
cylinder
bank (2) are operated with a rich air/fuel mixture by an engine control system
which is
not shown. This leads to an exhaust gas with an air/fuel ratio less than 1,
whose
temperature is raised to the necessary desulphurization temperature of about
700 C, for
example by postinjection. Over the entire desulphurization period of about 2
to
10 minutes, the cylinders of the second cylinder bank (2') are operated with a
lean
air/fuel mixture. The correspondingly lean exhaust gas with lambda greater
than 1 has a
temperature of 300 to 400 C which is optimal for the nitrogen oxide storage
catalyst. At
the confluence of the two exhaust legs, the two exhaust gas streams are mixed
and lead
to a combined exhaust gas with a temperature between the desulphurization
temperature
and the normal exhaust gas temperature. The oxygen content of the combined
exhaust
gas is measured with the oxygen probes (8) and/or (8') and regulated with the
aid of the
engine control system to give a value of the air/fuel ratio as close as
possible to 1.
CA 02673938 2009-06-26
8
Figure 2 shows a variant of the emission control system for performing the
method.
Upstream of the nitrogen oxide storage catalysts (6) and (6'), a further
catalyst (9) and
(9') is inserted into each exhaust leg. This may be a further nitrogen oxide
storage
catalyst, a three-way catalyst or an oxidation catalyst. All three catalyst
types can
further reduce the pollutant emission of the emission control system. In the
case of
diesel engines, it may be advantageous to arrange a diesel particulate filter,
with or
without a catalytic coating, between the catalysts (9) and (6), and between
(9') and (6'),
or beyond each of catalysts (6) and (6').
Figure 3 is a schematic diagram of the offset operation of the two cylinder
banks (2) and
(2') of the emission control systems of Figures 1 and 2 as a function of the
operating
time t. The brief desulphurization of catalyst (6) is always undertaken during
the normal
rich/lean alternating operation of catalyst (6'), and vice versa. For this
purpose, the
mode of operation of the two cylinder banks (2) and (2') is correspondingly
switched as
described above.