Note: Descriptions are shown in the official language in which they were submitted.
CA 02676695 2016-01-22
Ultraviolet Sanitization In Pharmacy Environments
TECHNICAL FIELD
[0001/0002] Various embodiments relate to handling medical containers
such as syringes, vials, and IV bags.
BACKGROUND
[0003] Many medications are delivered to a patient from an intravenous (IV)
bag into which a quantity of a medication is introduced. Sometimes, the
medication
may be an admixture with a diluent. In some cases, the IV bag contains only
the
medication and diluent. In other cases, the IV bag may also contain a carrier
or other
material to be infused into the patient simultaneously with the medication.
Medication can also be delivered to a patient using a syringe.
[0004] Medication is often supplied, for example, in powder form in a
medication container or in a vial. A diluent liquid may be supplied for making
an
admixture with the medication in a separate or diluent container or vial. A
pharmacist
may mix a certain amount of medication (e.g., which may be in dry form such as
a
powder) with a particular amount of a diluent according to a prescription. The
admixture may then be delivered to a patient.
[0005] One function of the pharmacist is to prepare a dispensing container,
such as an IV bag or a syringe, that contains a proper amount of diluent and
= CA 02676695 2009-07-24
WO 2008/101353
PCT/CA2008/000348
medication according to the prescription for that patient. Some prescriptions
(e.g.,
insulin) may be prepared to suit a large number of certain types of patients
(e.g.,
diabetics). In such cases, a number of similar IV bags containing similar
medication
can be prepared in a batch, although volumes of each dose may vary, for
example.
Other prescriptions, such as those involving chemotherapy drugs, may require
very
accurate and careful control of diluent and medication to satisfy a
prescription that is
tailored to the needs of an individual patient.
[0006] The preparation of a prescription in a syringe or an IV bag may
involve, for example, transferring fluids, such as medication or diluent,
among vials,
syringes, and/or IV bags. IV bags are typically flexible, and may readily
change
shape as the volume of fluid they contain changes. IV bags, vials, and
syringes are
commercially available in a range of sizes, shapes, and designs.
SUMMARY
[0007] Systems and methods to reduce bioburden on at least a portion of a
fluid transfer port include supplying a dose of radiation to the fluid
transfer port that is
in optical communication with at least one source of radiation. In an
illustrative
example, a medical container, such as a vial or IV bag, receives a dose of
ultraviolet
(UV) energy substantially at a predetermined region of a fluid transfer site.
In some
examples, such a sanitization process may precede a fluid transfer operation
in which
a fluid is transferred into or out of the medical container by passing through
the
sanitized region. Such fluid transfers may be used in automated or semi-
automated
pharmaceutical processes, such as drug reconstitution. Various embodiments may
further include one or more seal assemblies, each seal assembly having an
aperture
through which the radiation dose is supplied from the source to a controlled
region on
the fluid transfer port.
[0008] In one embodiment, an Automated Pharmacy Admixture System
(APAS) may include an automated system to transport medical containers such as
bags, vials, or syringes in a compounding chamber that may be regulated to a
pressure
above or below atmospheric pressure. In one implementation, the automated
transportation system is configured to grasp and convey syringes, IV bags, and
vials
of varying shapes and sizes from a storage system in an adjacent chamber that
may be
regulated at a pressure above or below atmospheric pressure. Various
embodiments
may include a controller adapted to actuate the automated transportation
system to
2
CA 02676695 2009-07-24
WO 2008/101353
PCT/CA2008/000348
bring a fill port of an IV bag, vial, or syringe into register with a filling
port at a fluid
transfer station in the chamber. One implementation includes a sanitization
system
that can substantially sanitize a bung on a fill port of a vial or IV bag in
preparation
for transport to the fluid transfer station. A port sanitization system (PSS)
may be
used in the sanitization of vial and bag ports in an IV admixture compounding
application. The PSS system may be a stand-alone or table top system, or may
be
adapted for integration into an APAS cell. The PSS may include one or more
radiation (e.g., UV) sources; one or more mechanisms for holding a medical
container
(e.g., drug vial, IV bag and syringe); one or more mechanisms for radiation
sealing or
containment; one or more cooling, purging and/or venting systems; a control
and
monitoring system; and interlocks and/or safety mechanisms.
[0009] The PSS may utilize a single centralized UV source or multiple
distributed UV sources. The UV source(s) can deliver UV radiation in a pulsed
and/or
constant wave form and by continuous emission, intermittent emission or pulsed
emission. The UV source(s) can deliver a predetermined dose in a fixed or
variable
profile based on the target biocontaminant(s). To reduce transmission loss, at
least
one optical conduit (e.g., light pipe, optical fiber, and optical waveguide)
may be used
to transmit the UV radiation from the UV source(s) to the object(s) to be
sanitized.
[0010] The PSS may include one or more aperture assembly for sealing or
containing the UV radiation. The sealing assembly can be designed such that in
operation the sealing assembly does not touch the area(s) to be sanitized. In
some
embodiments, the sealing aperture assembly includes at least one baffle that
is
configured to form one or more apertures. In some embodiments, the sealing
aperture
assembly includes a gasket that is formed around an aperture. A pressure
chamber
may be used to engage a medical container with the sealing assembly by
substantially
forming a light seal between them. In some embodiments, the sealing aperture
assembly includes a concave receptacle with an aperture. In some embodiments,
multiple sealing aperture assemblies are used to cover medical containers with
different shapes and sizes.
[0011] The PSS may incorporate a controller that can determine which
radiation seal assembly should be used based on the size and/or shape of the
medical
container to be sanitized.
3
CA 02676695 2014-08-25
[0012] The PSS may also include an actuator that can move various
components (e.g., the medical container, the apparatus for holding the medical
container, the radiation sealing assembly, and the UV source) either
individually or in
concert to bring the portion of the fluid transfer port to be sanitized into
optical
communication with the UV source through the aperture of the radiation seal
assembly.
[0013] Various embodiments may provide one or more of the following
advantages. The APAS may compound toxic and/or volatile substances, such as
those
used for chemotherapy, in a substantially aseptic chamber at pressure below
ambient
pressure to substantially avoid unintentional escape of the substances outside
of the
chamber. Also, the APAS may be programmed to select medical containers, such
as
IV bags, syringes, and/or vials, according to site-specific (e.g., hospital)
protocols for
containers for particular drug orders. Additionally, medical items, including
IV bag
and vial bung ports, may be positioned to receive a sanitizing dose of
ultraviolet,
which can effectively decrease bioburden (e.g., viruses, bacteria, mold,
etc.). Further
advantages may include reduction or elimination of sanitizing consumables, and
a
significantly reduced risk of explosive fumes (in the enclosed cell context)
associated
with some consumable sanitizers.
[0013a] In accordance with an aspect of the present invention, there
is
provided a system to automate pharmaceutical compounding, the system
comprising:
a chamber; an automated system to transport medical containers within the
chamber; a
compounding system disposed in the chamber to transfer medicaments between
medical containers; and a sanitization system to substantially reduce
bioactivity on at
least a portion of a fluid transfer port of a medical container, the
sanitization system
comprising: at least one radiation source to supply a dose of radiation; a
plurality of
radiation seal assemblies, each radiation seal assembly having an aperture and
configured to engage a fluid transfer port of a medical container having a
particular
shape; a controller to determine which radiation seal assembly corresponds to
the
fluid transfer port to be sanitized; and an actuator to bring the portion of
the fluid
transfer port to be sanitized into optical communication with the radiation
source
through the aperture of the determined radiation seal assembly.
[0013b] In accordance with another aspect of the present invention, there
is
provided a sanitization system to substantially reduce bioactivity on at least
portion of
4
CA 02676695 2014-08-25
a fluid transfer port of a medical container, the system comprises: at least
one
radiation source to supply a dose of radiation; a plurality of radiation seal
assemblies,
each radiation seal assembly having an aperture and configured to engage a
fluid
transfer port of a medical container having a particular shape; and an
actuator to bring
the portion of the fluid transfer port to be sanitized into optical
communication with
the radiation source through the aperture of the radiation seal assembly
determined to
correspond to the fluid transport to be sanitized.
[0013c] In accordance with another aspect of the present invention,
there is provided a method of substantially sanitizing at least a portion of a
fluid
transfer port of a medical container, the method comprising the steps of:
providing a
sanitization system having at least one radiation source and a plurality of
radiation
seal assemblies, each radiation seal assembly having an aperture and
configured to
engage a fluid transfer port of a medical container having a particular shape;
retrieving the medical container to be sanitized; determining which radiation
seal
assembly corresponds to the medical container; engaging the medical container
with
the determined radiation seal assembly; and supplying a predetermined dose of
radiation from the radiation source to the portion of the fluid transfer port
to be
sanitized to substantially reduce bioactivity thereon.
[0013d] In accordance with another aspect of the present invention,
there is provided a system to automate pharmaceutical compounding, the system
comprising: a chamber; an automated system to transport medical containers
within
the chamber; a compounding system disposed in the chamber to transfer
medicaments
between medical containers; and a sanitization system to substantially reduce
bioactivity on at least a portion of a fluid transfer port of a medical
container, the
sanitization system comprising: at least one radiation source to supply a dose
of
radiation; a first radiation seal assembly having a first aperture and
configured to
engage a first fluid transfer port of a first type of medical container having
a particular
shape; at least a second radiation assembly having a second aperture and
configured
to engage a second fluid transfer port of a second type of medical container
having a
particular shape substantially different from the first type of medical
container; a
controller to determine which radiation seal assembly corresponds to the fluid
transfer
port to be sanitized; and an actuator to bring the portion of the fluid
transfer port to be
4a
= CA 02676695 2014-08-25
sanitized into optical communication with the radiation source through the
aperture
of the determined radiation seal assembly.
[0013e] In accordance with another aspect of the present
invention,
there is provided a sanitization system to substantially reduce bioactivity on
at least a
portion of a fluid transfer port of a medical container, the system comprises:
at least
one radiation source to supply a dose of radiation; a first radiation seal
assembly
having a first aperture and configured to engage a first fluid transfer port
of a first
type of medical container having a particular shape; at least a second
radiation
assembly having a second aperture and configured to engage a second fluid
transfer
port of a second type of medical container having a particular shape
substantially
different from the first type of medical container; and an actuator to bring
the portion
of the fluid transfer port to be sanitized into optical communication with the
radiation
source through the aperture of the radiation seal assembly determined to
correspond to
the fluid transfer port to be sanitized.
[0013f] In accordance with another aspect of the present
invention,
there is provided a method of substantially sanitizing at least a portion of a
fluid
transfer port of a medical container, the method comprising the steps of:
providing a
sanitization system having at least one radiation source, a first radiation
seal assembly
having a first aperture and configured to engage a first fluid transfer port
of a first
type of medical container having a particular shape, and at least a second
radiation
assembly having a second aperture and configured to engage a second fluid
transfer
port of a second type of medical container having a particular shape
substantially
different from the first type of medical container; retrieving the medical
container to
be sanitized; determining which radiation seal assembly corresponds to the
medical
container; engaging the medical container with the determined radiation seal
assembly; and supplying a predetermined dose of radiation from the radiation
source
to the portion of the fluid transfer port to be sanitized to substantially
reduce
bioactivity thereon.
[0013g] In accordance with another aspect of the present
invention,
there is provided a system to automate pharmaceutical compounding, the system
comprising: a chamber; an automated system to transport medical containers
within
the chamber; a compounding system disposed in the chamber to transfer
medicaments
between medical containers; and a sanitization system to substantially reduce
4b
CA 02676695 2014-08-25
bioactivity on at least a portion of a first fluid transfer port of a first
form of medical
container and a second fluid transfer port of a second form of medical
container, the
sanitization system comprising: at least one radiation source to supply a dose
of
radiation; a first radiation seal assembly having a first aperture and being
configured
to engage the first fluid transfer port of the first form of medical
container; at least a
second radiation seal assembly substantially different from the first
radiation seal
assembly, wherein the second radiation seal assembly has a second aperture and
is
configured to engage the second fluid transfer port of the second form of
medical
container, the second form medical container being substantially different
from the
first form of medical container; a controller to determine which radiation
seal
assembly corresponds to a third fluid transfer port to be sanitized, wherein
the third
fluid transfer port is part of a third medical container, the third medical
container
being one of the first form and the second form; and an actuator to bring the
portion
of the third fluid transfer port to be sanitized into optical communication
with the
radiation source through the aperture of the determined radiation seal
assembly.
[0013h] In accordance with another aspect of the present invention,
there is provided a sanitization system to substantially reduce bioactivity on
at least a
portion of a first fluid transfer port of a first form of medical container
and a second
fluid transfer port of a second form of medical container, the system
comprising: at
least one radiation source to supply a dose of radiation; a first radiation
seal assembly
having a first aperture and being configured to engage the first fluid
transfer port of
the first form of medical container; at least a second radiation seal assembly
substantially different from the first radiation seal assembly, wherein the
second
radiation seal assembly has a second aperture and is configured to engage the
second
fluid transfer port of the second form of medical container, wherein the
second form
of medical container has particular shape substantially different from the
first form of
medical container; a controller to determine which radiation seal assembly
corresponds to a third fluid transfer port to be sanitized, wherein the third
fluid
transfer port is part of a third medical container, the third medical
container being one
of the first form and the second form; and an actuator to bring the portion of
the third
fluid transfer port to be sanitized into optical communication with the
radiation source
through the aperture of the radiation seal assembly determined to correspond
to the
third fluid transfer port.
4c
. CA 02676695 2014-08-25
. ,
[0013i] In accordance with another aspect of the present
invention,
there is provided a method of substantially sanitizing at least a portion of a
first fluid
transfer port of a first form of medical container and at least a portion of a
second
fluid transfer port of a second form of medical container, the method
comprising the
steps of: providing a sanitization system having at least one radiation
source, a first
radiation seal assembly having a first aperture and being configured to engage
the first
fluid transfer port of the first form of medical container, and a second
radiation seal
assembly substantially different from the first radiation seal assembly,
wherein the
second radiation seal assembly has a second aperture and is configured to
engage the
second fluid transfer port of the second form of medical container, wherein
the second
form of medical container has a particular shape substantially different from
the first
form of medical container; retrieving a third medical container to be
sanitized,
wherein the third medical container has one of the first form and the second
form;
determining, using a controller, which radiation seal assembly corresponds to
the third
medical container; engaging the third medical container with the determined
radiation
seal assembly; and supplying a predetermined dose of radiation from the
radiation
source to a portion of a third fluid transfer port of the third medical
container to be
sanitized to substantially reduce bioactivity thereon.
[0013j] In accordance with another aspect of the present
invention,
there is provided a system to automate medical compounding, the system
comprising:
a compounding chamber; an air handling system arranged to provide air flow
through
the compounding chamber, the air handling system being arranged to produce a
substantially uniform airflow from a ceiling of the compounding chamber toward
a
floor of the compounding chamber to inhibit contamination of medical
containers; an
automated system to transport medical containers amongst a plurality of
positions
within the chamber; a recognition system to identify medical containers using
one or
more verification operations the recognition system identifying a first
medical
container as a first form of medical container and a second medical container
as a
second form of medical container, wherein the first form and the second form
differ in
at least one of size, shape and type; a compounding system disposed in the
chamber to
transfer medicaments between medical containers; and a sanitization system to
substantially reduce bioactivity on at least a first portion of a first fluid
transfer port of
the first form of medical container and a second portion of a second fluid
transfer port
4d
CA 02676695 2014-08-25
of the second form of medical container, the sanitization system comprising: a
first
radiation source, and an actuator to bring at least the first portion of the
first fluid
transfer port into optical communication with the first radiation source; and
a
controller to determine a sanitizing profile corresponding to a third fluid
transfer port
to be sanitized, wherein the third fluid transfer port is part of a third
medical
container, the third medical container having a third form corresponding to
one of the
first form and the second form, the sanitizing profile is based in part on at
least one of
a size, a type or a shape of the third medical container, and the sanitizing
profile
includes at least one of a waveform, and amplitude, a time, and intensity, and
a pulse
repetition characteristic; wherein the controller changes a configuration of
the
sanitization system according to the sanitizing profile.
[0013k] In accordance with another aspect of the present invention,
there is provided a sanitization system in an automated pharmaceutical
compounding
system to substantially sanitize at least a portion of a first fluid transfer
port of a first
form of medical container and at least a portion of a second fluid transfer
port of a
second form of medical container, wherein the second form of medical container
has a
particular size, type, or shape substantially different from the first form of
medical
container, the sanitization system comprising: a first UV source to supply a
dose of
radiation; a processor to determine a profile of UV output corresponding to a
third
fluid transfer port to be sanitized, wherein the third fluid transfer port is
part of a third
medical container, the third medical container having one of the first form
and the
second form, the profile of UV output is based in part on at least one of a
size, type, or
shape of the third medical container, and the profile of UV output includes at
least one
of a waveform, an amplitude, a time an intensity, and a pulse repetition
characteristic;
and a first exposure orifice to supply radiation to the third fluid transfer
port, wherein
the processor configures the sanitization system to apply radiation through
the first
exposure orifice according to the profile of UV output.
[00131] In accordance with another aspect of the present invention,
there is provided a robotic automated pharmaceutical processing system
comprising: a
processor-based interface configured to receive requests to prepare one or
more
pharmaceutical prescriptions; and a controller coupled to the interface and
configured
to operate an automated prescription preparation device in response to the
received
requests, the automated prescription preparation device comprising: a scale
station to
4e
= CA 02676695 2014-08-25
verify a weight of a filled medical container, a fluid transfer station for
performing a
fluid transfer operation, and a sanitization station including a first
radiation source to
supply a dose of radiation, wherein the controller changes the configuration
of the
sanitization station to apply radiation to a first fluid transfer port of a
first medical
container based upon one or more parameters associated with the first medical
container, and a robotic manipulator configured to convey the first medical
container
to the sanitization station for sanitizing, and convey the first medical
container to the
fluid transfer station for filling.
[0014] The details of one or more embodiments are set forth in the
accompanying drawings and the description below. Other features, objects, and
advantages will be apparent from the description and drawings, and from the
claims.
DESCRIPTION OF DRAWINGS
[0015] FIG. 1 shows an illustrative Automated Pharmacy Admixture System
(APAS) cell.
[0016] FIG. 2 shows a top cut-away view of the APAS cell of FIG. 1.
[0017] FIGS. 3A-3C show cross-sectional views of an illustrative port
sanitization system (PSS).
[0018] FIGS. 4A-4C show cross-sectional views of an illustrative PSS that
accepts variously sized objects to be sanitized in an APAS cell.
[0019] FIG. 5 shows an illustrative enclosed PSS.
[0020] FIG. 6 shows an exemplary PSS without surrounding walls.
[0021] FIG. 7 shows an illustrative PSS with gripper mechanism.
4f
CA 02676695 2009-07-24
WO 2008/101353
PCT/CA2008/000348
[0022] FIG. 8A and 8B shows an exemplary IV bag and drug vial sanitization,
respectively.
[0023] FIGS. 9A and 9B show a top view and an isoparametric view of an
exemplary cleaner carousel, respectively.
[0024] FIG. 10 is a block diagram of an exemplary control module for the PSS
of FIGS. 3A-3C.
[0025] FIGS. 11A-11F show cross-sectional views of an illustrative P SS in an
APAS cell.
[0026] FIG. 12 shows an exemplary apparatus for performing a fluid transfer
operation.
DETAILED DESCRIPTION OF ILLUSTRATIVE EMBODIMENTS
[0027] An Automated Pharmacy Admixture System (APAS) may include a
manipulator that transports medical containers such as bags, vials, or
syringes about a
substantially aseptic admixing chamber. In some examples, the chamber includes
a
number of processing stations at which the medical containers can be processed
to
perform reconstitution for prescription medication doses. In particular
examples, such
processing stations may include apparatus to substantially sanitize,
disinfect, and/or
sterilize portions of the medical containers prior to performing a fluid
transfer
operation.
In an example implementation, a gripper assembly is configured to
substantially universally grasp and retain syringes, IV bags, and vials of
varying
shapes and sizes. In an illustrative embodiment, a gripping device may include
claws
configured to grasp a plurality of different types of IV bags, each type
having a
different fill port configuration. Embodiments may include a controller
adapted to
actuate a transport assembly to place a fill port of the bag, vial or syringe
into register
with a filling port such as a cannula located at a filling station, or be
equipped with
carousel transport systems that are adapted to convey bags, vials, and
syringes to the
admixture system and deliver constituted medications in bags, vials or
syringes to an
egress area.
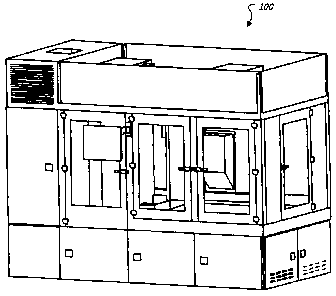
[0028] FIG. 1 shows an illustrative Automated Pharmacy Admixture System
(APAS) cell device 100 for use within a hospital pharmacy environment. The
APAS
cell 100 may autonomously admix contents of syringes and IV bags using
automation
technologies. For example, embodiments of the APAS cell 100 may perform one or
CA 02676695 2009-07-24
,
,
WO 2008/101353
PCT/CA2008/000348
more operations that might otherwise be performed by pharmacy staff within a
laminar airflow hood. The APAS cell 100 includes a robotic cell that automates
the
compounding and dispensing of drug doses into IV bags and/or syringes, such as
those that may be prepared in hospital pharmacies. The robotic cell may use a
syringe-based fluid transfer process, and may employ a robotic manipulator
(e.g., a
multiple degree of freedom arm) for moving drug vials, syringes, and IV bags
through
the cell as the medications are processed.
[0029] FIG. 2 shows an illustrative top cut-away view of the APAS cell of
FIG. I. The APAS cell 100 includes two chambers. An inventory chamber 202 is
used as an inventory loading area, which can be accessed by an operator to
load the
APAS cell 100 through a loading door (not shown). In some embodiments, the
inventory chamber 202 provides a substantially aseptic environment, which may
be an
ISO Class 5 environment that complies with clean room standards. A processing
chamber 204 includes the compounding area in which the admixture and/or
compounding processes may occur. In some embodiments, the processing chamber
204 provides a substantially aseptic environment, which may be an ISO Class 5
environment that complies with clean room standards. Mounted on the exterior
of the
APAS cell 100 are two of the monitors 102, which may serve as input/output
devices.
[0030] The inventory chamber 202 includes two inventory rack carousels 210
and 212 and a temporary inventory rack 214. The temporary inventory rack 214
may
be used to locate in-process drug vials that contain enough material to
provide
multiple doses. Each inventory rack carousel 210 or 212 may support multiple
inventory racks (not shown). In some applications, an operator may remove one
or
more racks from the carousels 210, 212 and replace them with racks loaded with
inventory. The racks may be loaded onto the carousels 210, 212 according to a
load
map, which may be generated by the operator for submission to the APAS cell
100, or
generated by the APAS cell 100 and communicated to the operator. The chambers
202, 204 are substantially separated by a dividing wall 216.
[0031] The processing chamber 204 includes a multiple degree of freedom
robotic arm 218, and the robotic arm 218 further includes a gripper that can
be used,
for example, to pick items from a pocket on a rack or to grasp items within
the APAS
cell 100 for manipulation. The robotic arm 218 may respond to command signals
from a controller (not shown) to pick up, manipulate, or reposition inventory
items
6
CA 02676695 2009-07-24
WO 2008/101353
PCT/CA2008/000348
within the processing chamber 204, and in or around the carousels 210, 212.
The
robotic arm 218 may manipulate inventory items, for example, by picking a
vial, IV
bag, or syringe from a rack of the carousels 210, 212 in the inventory chamber
202,
and moving the item to a station in the processing chamber 204 for use in
compound
preparation. In some examples, the robotic arm 218 may manipulate inventory
items
on the carousels 210, 212 through access port (not shown) in the dividing wall
216.
The dividing wall 216 may be substantially sealed so that a substantially
aseptic
environment may be maintained for compounding processes in the processing
chamber 204.
[0032] According to an illustrative example, an incoming drug order from a
remote user station (not shown) involves a batch production order for syringes
to be
charged with individual doses of a drug that is reconstituted from a drug
provided in
one or more vials. The operator, for example, may preload the drug into the
APAS
cell 100 during a loading process by loading the carousel 210 with inventory
racks of
the drug vials, and by interfacing with the APAS cell 100 using the
input/output
device 102 to initiate, monitor, and/or control the loading process. As the
APAS cell
100 is processing a previous order, the operator may load the carousel 212
with
inventory racks of syringes, drug vials, and IV bags for the next batch
production
order while the APAS cell 100 is operating the carousel 210. Once the loading
process is complete, the operator may submit the batch production process,
which
may begin immediately, or after other processing is completed.
[0033] To execute the batch production, in this example, the robotic arm 218
may pick a syringe from a pocket in a rack in carousel 210. The syringe in the
carousel may have a needle and a needle cap. The needle cap is removed for
processing in the APAS cell 100. The robotic arm 218 may convey the syringe to
a
decapper/deneedler station 220 where the needle cap is removed from the
syringe/needle assembly to expose the needle. The robotic arm 218 moves the
syringe to a scale station 226 where the syringe is weighed to determine its
empty
weight. The robotic arm 218 may transfer the syringe to a needle-up syringe
manipulator 222 where a dose of the drug is drawn from a vial, which was
previously
placed there by the robotic arm 218 after one or more verification operations
(e.g.
weighing, bar code scanning, and/or machine vision recognition techniques).
The
robotic arm 218 moves the syringe to the decapper/deneedler station 220 where
the
7
CA 02676695 2009-07-24
WO 2008/101353 PC
T/CA2008/000348
needle is removed from the syringe and disposed of into a sharps container
(not
shown). The robotic arm 218 then moves the syringe to a syringe capper station
224,
where the needleless syringe is capped. The robotic arm 218 moves the syringe
to a
scale station 226 where the syringe is weighed to confirm the predetermined
dose
programmed into the APAS cell. The robotic arm 218 then moves the syringe to a
printer and labeling station 228 to receive a computer readable identification
(ID)
label that is printed and applied to the syringe. This label may have a bar
code or
other computer readable code printed on it which may contain, for example,
patient
information, the name of the drug in the syringe, the amount of the dose, as
well as
date and/or lot code information for the inputs. The robotic arm 218 then
moves the
syringe to an output scanner station 230 where the information on the ID label
is read
by the scanner to verify that the label is readable. The APAS cell 100 may
report
back to the remote user station using a local communication network, for use
in
operations planning. The syringe is then taken by the robotic arm 218 and
dropped
into the syringe discharge chute 232 where it is available to the pharmacy
technician,
for example, to be placed in inventory within the hospital pharmacy. As the
process
continues, there may be times during the drug order process where the robotic
arm
218 removes an empty vial from the needle-up syringe manipulator 222 and
places it
into a waste chute 233.
[0034] In another illustrative example, a syringe may be used both as an input
containing a fluid (e.g., diluent or known drug compound) to be admixed in a
compounding process, and as an output containing a prepared dose suitable for
delivery to a patient. Such a syringe may be needed to fulfill a special
reconstitution
order programmed into the APAS cell 100 via the input/output capabilities of
the
monitor 102, for example. In another example, the order may be a stat order,
which
may be received from a hospital interface. In this example, the operator
performs in
situ loading by placing the syringes to be used for both reconstitution and
dosing in
pockets on a rack already located on the carousel 210. The operator enters the
reconstitution order into the APAS cell 100. The robotic arm 218 picks the
selected
syringe from a pocket in the rack in the carousel 210 and moves it to the
decapper/deneedler station 220, where the needle cap is removed from the
syringe/needle combination, thereby exposing the needle. The syringe is then
transferred by the robotic arm 218 to a needle-down syringe manipulator 234.
At the
8
CA 02676695 2009-07-24
WO 2008/101353 PC
T/CA2008/000348
station 234, diluent is drawn into the syringe from a diluent supply IV bag
236
previously placed there by the robotic arm 218. The diluent supply 236 may be
contained in an IV bag which is hung on the needle-down syringe manipulator
234 by
a clip (not shown). An air extraction process may be performed to prime the IV
bag,
if needed. The syringe then punctures the membrane of the diluent port 238 in
a
needle-down orientation. The syringe is actuated to remove, for example, a
predetermined amount of the diluent from the IV bag. The needle-down syringe
manipulator 234 then moves a reconstitution vial 250, placed there previously
by the
robotic arm 218, under the syringe. The diluent in the syringe is transferred
to the vial
for reconstitution with the vial contents. The robotic arm 218 then moves the
vial to a
mixer 248 for shaking according to a mixing profile. The robotic arm 218 then
moves
the vial to the needle-up syringe manipulator 222 where the appropriate amount
of the
reconstituted drug is drawn from the vial into an "output" syringe that was
previously
conveyed there by the robotic arm 218.
[0035] In another embodiment, the APAS cell 100 may receive a production
order to prepare compounds that may involve IV bags as input inventory items
or as
outputs. In some examples, an IV bag may be selected as a diluent source for
reconstitution in a drug order to be output into another medical container. In
other
examples, the selected IV bag may be used for output after preparation of the
drug
order is completed. Some IV bags may be placed on the carousels 210, 212 and
used
as an input that may be at least partially filled with a diluent that may be
used to
reconstitute drugs. The reconstituted drugs may be output in the form of
charged
syringes or IV bags. The operator loads racks of syringes and IV bags into the
carousel 210 for use in the production order. During the production order, the
robotic
arm 218 picks an IV bag from a rack on the carousel 210 and moves it to the
scale and
bag ID station 226. At this station, the IV bag is identified by bar code or
pattern
matching and its weight is recorded. This may be done, for example, as an
error
check, and/or to positively identify the type and/or volume of diluent being
used for
reconstitution. If the IV bag is selected as a diluent source, then the bag
may be
weighed before use to confirm the presence of the diluent in the IV bag. If
the IV bag
is selected for output, it may be weighed multiple times, such as before,
during, and/or
after each fluid transfer step, for example. As a post-transfer verification
step, the
weight may be re-checked after fluid transfer operations have occurred to
determine if
9
CA 02676695 2009-07-24
. .
WO 2008/101353
PC T/CA2008/000348
the change in weight is within an expected range. Such checks may detect, for
example, leaks, spills, overfills, or material input errors. In this example,
the robotic
arm 218 moves the IV bag to a port cleaner station 240 where a ultraviolet
(UV) light
or other sanitizing process may be used to substantially sterilize, disinfect
or sanitize
at least a portion of the IV bag port. The robotic arm 218 moves the IV bag to
the
needle-up syringe manipulator 222 where a pre-filled syringe has been loaded.
The
IV bag may be inverted so that the fill port is oriented downwardly for the
fill process.
The contents of the syringe may then be injected into the IV bag. The robotic
arm
218 then conveys the IV bag to the scale station 226 where the IV bag is
weighed to
confirm the predetermined dose programmed into the APAS cell 100. The robotic
arm 218 then moves the IV bag to a bag labeler tray station 242 where a label
printed
by the printer and labeling station 228 is applied to the IV bag. The robotic
arm 218
may move the IV bag to the output scanner station 230, where the information
on the
ID label is read by the scanner to verify that the label is readable. One or
more further
verification checks may be performed. The IV bag is then taken by the robotic
arm
218 and dropped into the IV bag discharge chute 244 where it is available to
the
pharmacy technician, for example, to be placed in inventory within the
hospital
pharmacy.
[0036] In another embodiment, a vial (or other medical item or container) may
be prepared for reconstitution. During the performing of this process by the
APAS
cell 100, the vial may be identified at a vial ID station where, for example,
a bar
coded label on the vial may be read by a scanner and/or image hardware in
combination with image processing software. The captured information may be
processed to identify the contents of the vial and correlate it to what is
expected. In
some implementations, as an alternative to or in combination with bar code
scanning,
the APAS cell 100 may employ pattern matching on the vial using optical
scanning
techniques. Also, in the reconstitution process, vial mixers 248 may be used
to mix
the vial contents with the diluent before using it for dosing.
[0037] In some embodiments, the robotic manipulator may include apparatus
for reading machine readable indicia in the APAS, including the compounding
chamber and/or the storage chamber. For example, the manipulator may include a
fiber optic camera for taking images that can be processed to compare to
stored image
information (e.g., bitmaps). In other examples, the reading apparatus may
include
CA 02676695 2014-08-25
optical scanning (e.g., bar code) or RFID (radio frequency identification).
Some
embodiments may transmit image information wirelessly (e.g., using infrared or
RF
(radio frequency) transmissions) to a receiver coupled to the APAS. Such a
receiver
may be located inside or outside the chamber with the robotic manipulator.
Such a
reader may be used to read machine readable indicia at various locations in
and
around the compounding chamber, including through windows and on portions of
the
storage carousels that are exposed to the compounding chamber.
[0038] In the embodiments described here, a UV port sanitization system
(PSS) is used in the sanitization of vial and bag ports in an IV admixture
compounding application. Variants of the system described here may also
include
sanitization of syringe bodies. The system may be part of an APAS cell or used
as a
stand alone device. Examples of an APAS system are described in further
detail, for
example, in U.S. Patent Application Serial No. 11/316,795, entitled "Automated
Pharmacy Admixture System," and filed on December 22, 2005, and U.S. Patent
Application Serial No. 11/389,995, entitled "Automated Pharmacy Admixture
System," and filed on March 27, 2006.
[0039] In general, operations to sanitize an object may refer to operations to
reduce the bioburden on the object to be sanitized. In some applications, a
sanitizing
operation may be intended to reduce active (e.g., living) bioburden to some
degree. In
some embodiments, the disclosed sanitization of an object may substantially
disinfect
at least a portion of the object. In some other embodiments, the disclosed
sanitization
of an object may substantially sterilize at least a portion of the object.
Exemplary
desired bioburden inactivation is greater than or equal to a 6 log reduction,
but could
vary slightly from this, depending on the target organism. In some
embodiments, at
least 99.9999%, 99.99%, 99%, 95%, 90%, 80%, 75%, 70%, 60%, or at least about
50% of a particular biocontaminant may be killed or incapacitated. In some
embodiments, between about 1 and 100% of a particular biocontaminant may be
inactivated.
[0040] In an exemplary embodiment, the mechanism of sanitizing the target
object may be through the exposure of ultraviolet radiation. This exposure may
be
delivered in, among other methods, a pulsed and/or constant wave form. In some
embodiments, the dose of ultraviolet radiation may include one or more pulses.
In
11
CA 02676695 2009-07-24
WO 2008/101353
PCT/CA2008/000348
other embodiments, the dose may include a timed exposure at a controlled
intensity.
For example, the intensity may be controlled by modulation of current and/or
voltage
applied to the radiation source to substantially achieve the controlled
radiation level,
which may increase, decrease, and/or remain substantially constant during the
dose
time period. In some embodiments, a controller may achieve time-varying or
constant
radiation level by modulation of optical path's transmission characteristics,
such as by
selecting which of a number of optical paths to use to couple the radiation
from the
source to the target region on the fill port, and/or by modulating
characteristics of the
optical coupling (e.g., filtering) to couple more or less radiation from the
source to the
target. The radiation subjected to the target is known as the delivered dose.
The dose
includes an accumulated exposure value. In an illustrative example, a desired
dose is
one that is predetermined based on, for example, a desired accumulated
exposure
value at a specific energy density selected to be sufficient to inactivate one
or more
types of biocontaminants to a selected degree. In general, sanitization may
involve,
for example, reducing the number of viable microorganisms present in a sample.
[0041] Biocontaminants, known as the bioburden, may include, but are not
limited to, viruses, bacteria, molds, protozoa and yeasts, for example. In a
range of
examples, ultraviolet radiation may be used to kill one or more types of the
biocontaminants on, around, or within portions of an I.V. bag, syringe and/or
vial,
such as around the fill port of such I.V. bag, syringe and/or vial. In some
cases for
example, such bioburden may be found in environments such as medical clinics,
hospitals, hospital pharmacies, research laboratories, or other facilities in
which
pharmaceuticals may be packaged, prepared, stored, transported, or otherwise
handled. Some embodiments may be beneficially applied to provide or enhance
sanitization of vials, syringes, packaging (e.g., I.V. bags), tubing, access
ports, and/or
associated equipment (e.g., handling equipment, including robotic
manipulators),
fluids (e.g., water), or other materials that may come into proximity and/or
contact
with objects for which sanitization may be a concern. Some applications may
relate
to the preparation of pharmaceutical and/or medical devices, such as delivery
systems
for providing parental nutrition or insulin to patients, for example.
[0042] In various embodiments, the UV port sanitization system (PSS) may
include one or more of the following components: one or more UV sources; one
or
more vial, syringe, and/or bag port holding systems or methods; one or more
systems
12
CA 02676695 2009-07-24
WO 2008/101353
PCT/CA2008/000348
or methods for appropriate sealing or containment of UV for both drug/fluid
and/or
user protection; one or more cooling, purging and/or venting systems; a
control and
monitoring system; and interlocks and/or safety mechanisms.
[0043] In various examples, some embodiments of the PSS may include a
single centralized UV source with selectable masks or apertures for the
variety of
vials and bags. Some embodiments may also utilize multiple distributed sources
that
can be conveniently located (e.g., for replacement, maintenance), or combined
with
other subsystems or functions in the APAS cell context. In the example
described
here, the amount of UV time exposure required for sanitization is a function
of the
energy level received by the target at the required frequency spectrum.
However, a
predetermined exposure time for a dose may be based on other criteria. Both
fixed
and variable profiles may be executed at various levels of intensity, number,
spacing,
and timing. The outputs of UV sources may decay over time. Calibration and/or
closed-loop control may be implemented by a processor, such as on a
programmable
logic controller or an embedded controller, to compensate such decay to
maintain
desired profiles (e.g., a predetermined accumulated dose of radiation).
[0044] In some embodiments that have multiple UV sources, the PSS may
include apparatus to focus or direct radiation supplied from each of the UV
sources
onto one or more selected regions or spots, or combine their output patterns
using
offsets to provide the desired illumination pattern at the fill port of a
target. UV
sources may have non-uniform output patterns. By changing pattern centerlines,
an
aggregate output energy pattern that meets desired requirements can be
generated.
One example is to have 3 UV sources combined in such a manner as to provide a
nearly uniform energy output over a much wider range than could be achieved
with
focusing the 3 UV sources onto a single spot.
[0045] A UV source may include, for example, flash bulbs to produce very
high peak energy levels, on the order Of 1 J/cm2, or 10 J/cm2, or 30 J/cm2 in
the UV-C
band, which may include, but are not limited to, between 100 nm and 280 nm. In
some examples, these are provided in very short bursts ranging from less than
1 ns to
100 ms at frequencies from about 0.01 Hz to about 1 kHz. Some pulsed bulbs may
generate a wide band spectrum. In some embodiments, the UV light output may
include a wider spectrum of radiation. For example, the pulsed UV light may
include
energy content in the UV-A, UV-B, and UV-C ranges, and may include some energy
13
CA 02676695 2009-07-24
WO 2008/101353
PCT/CA2008/000348
content at wavelengths shorter and/or longer than UV wavelengths, e.g., IR or
visible
light.
[0046] UV sources such as mercury vapor lamps, metal halide lamps and
other constant wave sources generally provide energies in the range of about 1
mJ/cm2 to 400 mJ/cm2 in the UV-C band, or more. Packaged either singly or with
multiple source packages to increase total power, such UV sources can provide
suitable energy levels for sanitization in a specified time. Lower or higher
energy
levels may also be used depending on the sanitization time constraints.
[0047] UV sources such as LEDs can be tailored to provide energy in very
narrow bands including, for example, UV-C. Output spectrums can be tailored to
provide total spectrums within, for example, 500 nm, 100 nm, 10 nm or 1 nm
or
less of the center band frequency of about 250-290 nm or 265-275 nm. This may
advantageously affect heating, ozone production, and/or operator safety of the
broader
spectrum bulbs. LEDs and/or LED arrays in the power range of 1 mJ/cm2 to 400
mJ/cm2, or more, packaged either singly or with multiple source packages to
increase
total power, provide suitable energy levels for sanitization at high
throughput for
automated applications. Lower or higher energy levels may also be used
depending,
for example, on the sanitization time constraints. In various embodiments, one
or
more UV LED sources may be placed at various locations distributed and
directed to
illuminate at least one surface to be sanitized. UV LEDs may be distributed in
rectangular, linear, curvilinear, circular, spherical, or other patterns to
expose one or
more regions and/or surfaces to UV radiation. In various applications,
predefined
LEDs may be selected to operate at selected times to provide a dose of UV
radiation.
The dose and selection of which LEDs to activate and the timing of their
activation
may be determined according to the type and/or size of the object (e.g. vial,
IV bag, or
the like) to be exposed. The LEDs can be activated in series, in parallel,
overlapping
or the like, and the timing of the activation often depends on the purposes to
be
achieved, such as high power, long duration with lower power, preserving
source
lifetime and more. Examples of UV LEDs that may be used in some embodiments
are described in, for example, US Published Patent Application 2004/0099869
filed
on Oct. 22, 2003, the contents of which are incorporated by reference.
[0048] In some embodiments, the UV lamp in the port sanitization system
may be cooled and/or cleaned by a flow of clean air. Such air flow may cool
and/or
14
CA 02676695 2009-07-24
WO 2008/101353
PCT/CA2008/000348
substantially reduce particulate or organic solvents from depositing on the
lamp
surfaces. Connecting it to a low-pressure peripheral duct can force the air to
be drawn
into the UV lamp housing from just below the fan filter unit outlet (where it
is
cleanest) and to flow over the UV lamp to provide cooling. In some
embodiments,
such cooling may be performed without additional air moving elements that may
generate air currents that may disrupt controlled laminar air flow patterns in
a
compounding area.
[0049] Example methods to deliver a required dose of ultraviolet radiation to
a
target may include continuous emission, intermittent emission and pulsed
emission.
For continuous emission, suitable sources may require warm-up time and
typically do
not suit repetitive and/or frequent on-off cycles. Examples of such sources
include,
but are not limited to, mercury vapor lamps, fluorescent backlights, and metal
halide
lamps, and combinations of these and other sources. For intermittent emission,
suitable sources can operate continuously and also have the capability of
repetitive
and/or frequent on-off cycles (e.g., LEDs, and lasers). For pulsed emission,
suitable
sources include the sources that are designed to flash at specified
frequencies with
specified pulse widths, such as flash bulbs using Xenon or other appropriate
gases.
[0050] In one example of UV sanitization, an optical conduit (e.g., light
pipe,
optical fiber, optical waveguide) can be used, for example, to reduce
transmission
losses between at least one UV source and the sanitization target. In some
implementations, the optical conduit allows transmission of a particular
wavelength
range (e.g., a UV wavelength range used for sanitization). The conduit can be
placed
in close proximity to the UV source such that substantially most or all of the
UV light
emitted by the UV source (e.g., a diffuse source) impinges on the entry plane
of the
conduit. In some implementations, once the UV light enters the conduit, losses
within
the conduit can be a function of the conduit material and construction. For
example,
an optical conduit may include one or more optical fibers, or one or more
formed
structures (e.g., glass or plastic structures). Light exiting the optical
conduit may pass
through one or more optical lenses. One or more convex and/or concave lenses
may
be selectively applied (e.g., on a rotating mechanism) to provide selective
control of
the beam width incident on the surface(s) to be sanitized.
[0051] In some implementations, one or more optical conduits can be
arranged to gather and/or combine UV light from one or more UV sources and
CA 02676695 2014-08-25
transmit the UV light to one or more sanitization targets concurrently or
simultaneously. For example, multiple UV sources can be combined using an
optical
conduit to focus the UV light onto a single sanitization target. In another
example, a
single UV source can be split using multiple optical conduits to direct UV
light at
multiple sanitization targets. In another example, UV light incident on the
target
surface(s) from a first optical conduit can substantially overlap or combine
with UV
light emitted from a second optical conduit. In some implementations, one or
more
UV sources can include a light emitting diode (LED) or a Xenon flash UV
source.
Examples of flash UV sources are described with reference to FIGS. 26A through
29C of U.S. Patent Application Serial No. 11/389,995, entitled "Automated
Pharmacy
Admixture System," and filed by Eliuk, et al. on March 27, 2006.
[0052] In some implementations, the optical conduit may include an exit plane
arranged in close proximity to the target such that diffusion losses between
the
conduit exit plane and the sanitization target are substantially minimized. In
some
implementations, the conduit allows the UV source to be located substantially
remotely from a sanitization target (e.g., due to packaging or mounting
constraints,
and/or to simplify maintenance of the UV source). In some implementations, a
remotely located UV source allows maintenance to be performed on the UV source
(e.g., replacing a bulb) without contaminating the surfaces to be sanitized
(e.g., fluid
ports and needles). In some implementations, a remotely located UV source
protects
users from, for example, a flash from an LED or Xenon flash UV source. In some
implementations, the amount of benefit from the conduit can vary depending on
factors such as light conduit losses (e.g., coupling or transmission losses),
sanitization
target size, number of UV sources, conduit geometry, etc. In some
implementations,
the conduit provides about a 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%,
100%, 150%, 200%, 300%, 500%, 1000% or more increase in energy striking the
target for UV sources illuminating vial bungs or IV bag fluid ports through a
light
conduit as compared to the same sanitization target at the same distance from
the
same UV source without a light conduit.
[0053] The PSS as described incorporates one or more systems or methods for
holding medical containers (e.g., drug vials, syringes, or IV bags) whose
ports are to
be sanitized (i.e., receive a sanitizing dose of radiation). Examples of drug
vials
16
CA 02676695 2009-07-24
WO 2008/101353
PCT/CA2008/000348
include, but are not limited to, 1 ml to 100 ml with the associated full range
of vial
seal/stopper sizes and types. Example bags and/or I.V. containers include, but
are not
limited to, all sizes of IV fluid bags of solution (e.g., saline solution,
dextrose, sterile
water and combinations thereof) including, but not limited to, sizes up to 3
liters. In
one APAS cell application, the item to be sanitized may be held by the cell
robot, or
handed off to another holding mechanism. One holder embodiment achieves the
holding through the use of a gripper or clip. The gripper or clip in this case
may
contact the vial on the vial top, neck or body. Another holder embodiment
could use
a platen on which a vial is placed. Yet another holder embodiment could use a
cradle
on which the vial is placed. Still another holder embodiment involves the use
of
vacuum, in combination with the sealing/containment function, to hold vials in
place
through suction on the top (area to be sanitized). Another embodiment may
incorporate a movable platen that is used to firmly engage the vial into the
exposure
orifice when properly installed by the user, manipulator and/or robot. The
movable
platen can be driven by a spring, motor, pneumatics or hydraulics. The robot
holder/holding mechanism used may be stationary, or may involve moving
continuously or in steps through a variety of positions. Additionally, the
holding
mechanism or method may be combined with another subsystem function to improve
cell efficiency. In the case where the object to be manipulated is a vial, the
PSS
holder may be combined with the vial ID operational function. In the case of
IV bags,
the PSS holder may be combined with the bag scale/ID station.
[0054] In various embodiments, the holding mechanisms/methods may also be
generally applicable to syringes. Some options may also apply to the stand
alone
PSS. For the stand alone PSS system, the operator may hold the item to be
sanitized
manually in the required position. The holding options described above include
embodiments with a stationary holder, or with a mobile holder including 1 or 2
additional axes of motion to position the item for sanitization. In additional
embodiments, an automated transfer mechanism may be used to remove the object
from the chamber after exposure to the ultraviolet radiation. The automated
transfer
mechanism may include a robotic manipulator and/or a rotating platen and may
manipulate or move the object in response to a sequence of commands
automatically
generated by a processor executing a program of instructions. Location
features may
17
CA 02676695 2009-07-24
WO 2008/101353
PCT/CA2008/000348
be included to aid in the positioning of the object by an operator (e.g.,
pharmacy staff)
in the correct location in the PSS chamber.
[0055] FIGS. 3A-3C show cross-sectional views of an illustrative PSS 300. In
an illustrative embodiment, a PSS 300 may be used to sanitize items within an
automated pharmacy compounding device, such as an APAS cell 100, an example of
which was described with reference to FIGS. 1 and 2. In this example, the PSS
300
can be used to sanitize items that include, but are not limited to, drug vial
ports, IV
bag ports, and syringes. The sanitizing process performed by the PSS 300 may
be
used alone or in combination with one or more other cleaning processes, such
as an
alcohol wipe.
[0056] The PSS 300 may be used to perform operations to sanitize objects
placed within a PSS chamber. In this example, the PSS 300 includes an
ultraviolet
(UV) lamp 305, a lamp housing 310, a baffle 315, and a chamber wall 320. The
chamber wall 320 may substantially reflect and/or absorb radiation so as to
substantially contain UV radiation 325 from the UV lamp 305 within the PSS
chamber. The UV radiation 325 from the UV lamp 305 may illuminate an object
330
placed within the PSS chamber. In this example, the object 330 is a drug vial
that is
positioned to be exposed to the UV radiation 325 by a manipulator 335. The
manipulator 335 may be a robotic gripper.
[0057] FIGS. 3A-3C show a single chamber embodiment where the lamp
housing 310 and the UV lamp 305 are mounted above the object 330 with the UV
radiation 325 directed downward. In other embodiments, one or more UV
radiation
sources may be directed upward and/or from the sides, either alone or in
combination
with the downward directed UV lamp 305. An illustrative UV radiation source
for
the lamp 305 is a Xenon lamp. The size of the light aperture in the baffles
315 may
be suitable for the objects being sanitized. The object 330 may be presented
to the
UV radiation source by mechanical or robotic mechanisms.
[0058] The PSS 300 of FIG. 3A includes a baffle 315 arranged to form an
opening to pass the light onto an object 330. The PSS 300 of FIG. 3B includes
baffles
315 arranged to provide a substantially cylindrical or tubular, vertically
oriented
lumen through which to illuminate an object 330. The baffles 315 may have
reflective surfaces. The PSS 300 of FIG. 3C includes baffles 315 arranged to
form a
partial conical surface with an aperture to direct substantially all light to
an object 330
18
CA 02676695 2009-07-24
WO 2008/101353
PCT/CA2008/000348
disposed near the aperture. Other similar arrangements of baffle
configurations may
be used to direct a substantial fraction of the light that enters the PSS
chamber to an
object placed near one or more apertures like those of FIGS. 3A-3C. In some
embodiments, the baffling may be automatically or manually reconfigurable to
provide suitable illumination of the object. For example, the baffles may be
on a
rotatable carousel that can be repositioned (e.g., by an actuator motor), to
position the
most effective baffle to illuminate the size, type, and/or shape of the
object.
[0059] The PSS 300 may be adapted for integration into an APAS cell 100, or
configured for stand-alone (e.g. table-top, free-standing) operation for use
in a
hospital pharmacy or similar environment. Information to identify a medical
container (e.g., content, shape and/or size) may be received by a controller
from a
pharmaceutical prescription database. In the hospital pharmacy type of
environment,
pharmacy staff may prepare prescriptions by using an extension tool (e.g.,
tongs) to
grasp the object to be sanitized and place it into the PSS chamber for
sanitization.
Location features (not shown) may be included to aid in the positioning of the
object
by the pharmacy staff in the correct location in the PSS chamber.
[0060] FIGS. 4A-4C show cross-sectional views of an illustrative PSS 400
that accepts variously sized objects to be sanitized. In FIG. 4A, an object
405 is a
large vial, in FIG. 4B, an object 405 is a small vial, and in FIG. 4C, an
object 405 is
an IV bag. In each example, a manipulator 410 may move along a trajectory
suitable
for positioning an object in a suitable location to be sanitized in the PSS
chamber 400.
In FIG. 4C, the IV bag 405, for example, may be flexed (e.g., if empty) to be
positioned in the PSS chamber 400 so that an IV bag port 415 can be sanitized
before
making physical contact with a syringe (e.g., to perform a manual or automated
fluid
transfer operation).
[0061] Accordingly, the object to be sanitized need not provide a primary
light
seal. Chamber walls 420, in combination with the manipulator 410, may provide
effective light containment. The chamber walls 420 may include features such
as
baffles 425, reflective surfaces, and/or absorptive surfaces to further
minimize escape
of UV radiation from the PSS chamber 400.
[0062] In one example of the PSS, walls may substantially enclose the
chamber, with at least one wall having an opening for receiving a medical
container
and a portion of the transfer mechanism. FIG. 5 illustrates one exemplary
19
CA 02676695 2009-07-24
WO 2008/101353
PCT/CA2008/000348
embodiment of an enclosed PSS 500. A vial 560 to be sanitized can be placed on
a
spring loaded platen 570 by either a robotic or manual method for the case of
a stand
alone system. A seal assembly 540 is pushed up by the spring platen 570
through the
vial 560 to form a seal with a pressure chamber 530. The pressure chamber 530
can
substantially form a pressure seal between the vial port and the seal assembly
by
reducing the pressure of the chamber to provide a substantial vacuum. In some
examples, the pressure chamber 530 is evacuated to promote a light tight seal.
In
various embodiments the pressure chamber may operate as an interlock that
disables
radiation output unless a measurement signals from a pressure sensor in the
chamber
confirms that pressure in the chamber meets predetermined criteria (e.g.,
maintains at
least a minimum threshold vacuum or positive applied pressure level), thus
confirming that the vial port is properly seated in the seal assembly 540 and
that the
seal assembly 540 is properly seated against the pressure chamber 530. The
depicted
example shows a hinged seal assembly. Other variations may include a rotating
carousel of seal assemblies or simply a single fixed seal assembly. The
pressure
chamber 530 may be at least partially enclosed on the top by UV transparent
glass
520 or equivalent. The pressure/vacuum may be monitored in the chamber 530 to
determine when to enable the UV source 510. Other criteria may be used to
enable
the UV source such as a signal indicating a door 550 is closed to
substantially contain
radiation within the PSS 500.
[0063] FIG. 6 illustrates an exemplary PSS 600 without walls surrounding the
item to be sanitized 660. The seal assembly 640 is shown as a hinged seal
assembly;
other embodiments may include a rotating carousel of different seal assemblies
or a
single fixed seal assembly. Spring loaded platen 670 can be used to push the
vial 660
and seal assembly 640 upward to seat with the pressure chamber 630.
Alternatively,
spring loaded platen 670 can be omitted and the manipulator or operator can
hold the
vial 660 in the position to seat the seal assembly 640. In some other
embodiments, at
least one seal assembly is operable to provide an adjustable aperture (e.g.,
iris) or
masking profile for controlling the size, shape, and/or location of the
predetermined
region to be exposed to the dose of radiation. If vacuum is used in the
pressure
chamber 630, the manipulator or operator may release its grip of the vial 660
and rely
on the vacuum to hold the vial 660 in place while sanitization process takes
place.
CA 02676695 2009-07-24
WO 2008/101353
PCT/CA2008/000348
When vacuum is generated indicating a proper seal, the UV source 610 is
enabled and
the dose is delivered though the UV transparent glass 620 to the port of the
vial 660.
[0064] FIG. 7 shows an exemplary PSS 700 that includes a gripper and an axis
of motion. In this example the operator (in the manual case) or robot places
the vial
760 onto the platen 770. The gripper 790 is lowered by the motorized slide 780
and
the vial 760 is picked up by the grip fingers 795. The vial 760 is then
elevated to the
seal assembly 740 which is seated against the pressure chamber 730. A
secondary
light barrier such as a baffle 750 may be used. When pressure/vacuum is
generated
indicating a proper seal, the UV source 710 is enabled and the dose is
delivered
though the UV transparent glass 720 to the port of the vial 760.
[0065] Depending on the safety environment, containment of the UV energy
may not be needed. For example, some products (e.g., sterile water bags) may
not be
affected by UV light exposure. If personnel safety in terms of sufficient UV
containment is provided by, for example, the cell walls and doors,
sanitization in an
enclosed setting may not be required. Where operational circumstances permit
reduced light containment specifications, processing and/or transport times
may be
reduced by simplified motion trajectories, thereby enhancing throughput for
manual
or automated sanitization processes. In some embodiments, one or more optical
sensors may be located in and around the PSS to detect the presence and/or
intensity
of "leaked" radiation that may escape from around the light seal, through the
medical
container, or otherwise, from the primary optical path between the radiation
source
and the predetermined target region. A controller may monitor such sensors,
and take
some corrective action should the detected leakage exceed a predetermined
level.
Examples of corrective actions may include, but are not limited to, generating
a
notification signal (e.g., electronic message to an operator, warning light,
or the like),
disabling the radiation source, or attempting to reconfigure the light seal
assembly by,
for example, selecting a different light seal that may provide improved
sealable
engagement with the current medical container. In this way, the optimal
available
light seal for any (perhaps unrecognized) medical container may be determined
and
recorded in a data store for use in future operations based on leakage sensor
feedback.
[0066] FIG. 8A and 8B illustrates an exemplary IV bag and drug vial
sanitization, respectively. In FIG. 8A, a robot (not shown) gabs an IV bag 810
from
a prior station (e.g., an IV bag scale or rack) using robot fingers 805 and
transports the
21
CA 02676695 2009-07-24
WO 2008/101353
PCT/CA2008/000348
bag 810 to a PSS. The robot then places the bag port 815 into close proximity
with a
UV source 820. A light or laser emitter 830 and detector 832 are used to
detect the
presence or absence of a bag port. The emitter 830 emits a light or laser beam
that
can be detected by the detector 832. When a bag port is placed between the
emitter
830 and detector 832, the beam is broken which then sends a signal to the
controller
(not shown) to enable the UV source 820. When the robot places the bag port
815 in
the correct position, the port 815 breaks the emitted beam, thereby enabling
the UV
source 820 to illuminate the portion of the port to be sanitized. After the
required
dose is provided, the robot moves the bag 810 to the next station, which could
be any
a scale, a temporary holding station, or a syringe manipulator.
[0067] A shield/mask may be used to prevent UV from hitting the bag
contents or escaping into the cell. For bags, UV exposure may not be an issue
unless
there are drugs in the bags. The escape of UV into the surrounding chamber or
environment may be controlled by small clearances and/or the shape of the
robot
fingers 805 that can cover most of the opening. Surfaces of the robot or
actuator that
may be exposed to UV radiation may be treated to promote controlled
reflection,
absorption, diffusion, or a combination of these or other
[0068] In one implementation, a flexible mask with a slit is used. The robot
pushes the bag port through the slit so that the mask sits between the upper
and lower
protrusions of the robot fingers 805. This effectively seals the entire lower
portion of
the light path, while leaving open the path between the robot fingers and the
assembly
surface just above it (where the emitter 830 sits).
[0069] In FIG. 8B, an appropriate movable aperture seal assembly 840 is
moved into alignment, based on the identifying information about the drug vial
to be
sanitized 812. The movable seal assembly 840 has some vertical compliance and
rests just below two mating surfaces 850 when aligned. The two mating surfaces
850
may be two machined surfaces. A robot (not shown) grabs the vial 812 from a
prior
station (e.g., vial weighing station or rack, container cover/seal removal
station, or the
like) using robot fingers 805 and takes the vial 812 directly underneath the
seal
assembly 840. The robot then moves the vial 812 upward, deforming a flexible
mask
860 and bringing the movable seal assembly 840 into contact with the mating
surfaces
850. A vacuum port (not shown) is used to draw air from the chamber created as
a
result of the seal assembly 840 contacting the mating surfaces 850. A pressure
sensor
22
CA 02676695 2009-07-24
WO 2008/101353
PCT/CA2008/000348
(not shown) is used to measure the pressure inside the chamber. If the
pressure
decreases to a defined level, the vial 812 is in the correct position and a
substantial
light seal has been created. An o-ring or other gasket type material may be
used with
the mating surfaces to improve light seal. A UV source 820 is then enabled,
thereby
illuminating the portion of the vial port 817 to be sanitized. After the
required dose is
provided, the robot moves the vial 812 to the next station, which could be a
scale, a
temporary holding station, or a syringe manipulator. In one implementation,
light seal
may be provided by placing a cover over the drug vial to be sanitized.
[0070] Some embodiments of the PSS chamber may be customized for the
specific range of objects to be sanitized, taking into consideration
requirements such
as: object access requirements to the light source, object size, light
containment, and
distance of the object from the light source.
[0071] In various embodiments, the sealing systems or methods may be
designed not to touch the areas of the stopper or fluid transfer port to be
sanitized.
This may help to protect the areas to be sanitized from both microbial and
drug cross-
contamination. Referring to FIGS. 9A and 9B, aperture seal assemblies 910a-f
are
designed to incorporate chamfered guides 920a-f to aid engagement. To engage
an
item to be sanitized (e.g., a vial), the operator (in the manual case) or
robot centers the
item in the aperture 930. If the item position on insertion is too far out, it
will not
engage. The size of the aperture 930 is configured to be larger than the size
of the
areas to be sanitized, so if engaged the areas to be sanitized will not be
touched and
full exposure of the areas to be sanitized will be assured. Proper engagement
provides
sealing between the pressure chamber (not shown) and the seal assembly 910.
The
seal assemblies 910a-f can be removed and/or interchanged from the rotating or
fixed
carousel 900.
In some embodiments, a rigid, semi-rigid, or flexible gasket 940 (e.g.,
rubber,
foam, plastic, or flexible UV blocking or reflective material) may be formed
around
an aperture 930. When a fluid port of a vial or IV bag is to be sanitized, an
operator
in a pharmacy or a robot arm in an APAS cell may place the fluid port to be
sanitized
in proximity to the aperture 930 such that the gasket 940 forms a substantial
light seal
interface with a body of the vial or IV bag. The aperture 930 may provide a
substantially UV-transparent window through which one or more surfaces on the
fluid
port may be exposed to ultraviolet radiation through the window.
23
CA 02676695 2009-07-24
WO 2008/101353
PCT/CA2008/000348
[0072] The aperture gaskets 940a-f, may generally include, but are not limited
to, materials that are compliant to form a seal (e.g., silicone rubbers). Such
materials
may also be selected and screened to provide suitable resistance to heat and
UV
exposure for the applicable embodiments of the PSS. One embodiment comprises
several gasket apertures 930a-f (see FIGS. 9A and 9B) that combine to cover a
wide
range of vial seal/stopper diameters. Each aperture 930 may handle a sub range
of
vial top sizes. Sealing is achieved on the outer edge of the metal part of the
vial top,
removed from the stopper or port puncture area in the center. These apertures
930a-f
may be in fixed locations, or indexed via a variety of means to a fixed
interface
location. Guiding features may also be incorporated to guide the travel of the
item to
be sanitized into the exposure aperture. The quality of the seal is verified
prior to and
during exposure by monitoring the pressure/vacuum in the pressure chamber (not
shown).
[0073] Instead of, or in combination with, a flexible boot, some embodiments
may provide a receptacle 910f to receive a fluid port in proximity of the UV
exposure
port. The receptacle 910f may be sized to receive one or more sizes and styles
of
fluid ports for IV bags, and one or more sizes and styles of fluid ports for
vials. A
concave opening receptacle 910f may be adapted to receive a range of sizes.
One or
more differently sized and/or shaped receptacles may be provided. In some
embodiments, receptacles may be interchanged to accommodate a wide range of
items
to be sanitized. Different receptacles may have locating pins, rotating and/or
sliding
features to retain a receptacle being used. Interlock features may be
integrated into
each receptacle. For example, proximity or pressure sensors may be used to
determine when a receptacle is properly installed and a properly sized vial or
IV bag
fluid port is being inserted or pressed into the receptacle to be exposed to
the
ultraviolet radiation.
[0074] In some embodiments, the automated transfer mechanism may provide
at least a partial light seal around at least a portion of the opening on the
PSS chamber
wall. For example, a manipulator may be adapted to provide a thin (e.g.,
pencil-like)
extension apparatus to extend the reach of the manipulator through a reduced
width
(e.g., narrower) slot in the narrow portion of the opening in the PSS chamber
housing.
Such extension apparatus, or the external portion of the manipulator itself,
may be
provided with baffling to provide either an internal or an external light seal
around
24
CA 02676695 2009-07-24
WO 2008/101353 PC
T/CA2008/000348
some or all of the openings in the PSS chamber housing. For example, a
flexible
rectangular baffling (e.g., plastic, rubber, or foam with reflective or
absorptive
coating) may be used to provide a substantial UV light seal over some or all
of the
narrow and/or wide openings in the PSS chamber housing when an object is
positioned to receive UV radiation.
[0075] In some embodiments, the object to be sanitized may provide an
effective light seal. The design of the baffles shown in FIGS. 3B-3C may be
such that
the object effectively seals the opening in the baffle when the object is
brought into
substantial contact with the baffle. Also, the baffle design may be compliant
(e.g.
flexible baffle material, spring mounted baffle assembly, or bellows) such
that some
tolerance in the positioning of the object against the baffle is afforded. The
opening
in the baffle may be sized to maximize the amount of UV radiation on the
targeted
area to be sanitized.
[0076] In some embodiments, cooling and venting systems may be included
to, for example, cool the UV source, cool the sealing materials and their
mounting
structures, cool the object(s) to be sanitized, and/or to remove ozone gas
that may be
generated by some UV sources.
[0077] A typical implementation for cooling and venting may utilize the
suction piped from the APAS exhaust fan plenum to draw cooling air through the
PSS
as required, and at the same time could be used to vent ozone if the
applicable APAS
cell has a vented exhaust. Another embodiment may utilize local fans to
provide
cooling air, drawing air from the clean cell air to provide cooling. This air
could flow
back into the cell or be routed to the local exhaust air duct. In still
another
embodiment, both exhaust suction and local fans may be combined to provide
increased air flow, and/or to capture ozone. In yet another embodiment,
cooling air
can be obtained directly from HEPA filtered fan filter units. In one
implementation, a
combination of conductive and convective heat transfer mechanisms are used to
manage the thermal load of the UV source. For any of these implementations, an
ozone catalyst may be placed in the air flow to reduce the amount of ozone
that is
generated and re-circulated. The catalyst, in one example, may be local to the
PSS
housing to reduce its size and immediately reduce ozone levels. The catalyst
may
also be placed in line with the exhaust filter to scrub ozone repeatedly
and/or when
the cell operates in a recirculation mode. In some embodiments, the input air
may be
CA 02676695 2014-08-25
filtered to prevent particles from getting to the object(s) to be sanitized.
The filtered
air may also prevent particles from contacting the UV lamp, thereby increasing
bulb
life and efficiency. In some cases, the PSS may be designed for application
within the
APAS cell ISO class 5 clean air environment.
[0078] FIG. 10 is a block diagram of a control module 1000 for the
illustrative
PSS 300 of FIGS. 3A-3C. In an illustrative embodiment, the PSS 300 discussed
herein may include a PSS chamber, a UV lamp assembly, and the control module
1000. The control module 1000 may include a processing unit 1005, a COM port
1010, one or more sensors 1015, an apparatus for operating an air handling
system
1020, an input/output (I/0) port 1025, and a power supply 1030. The processing
unit
1005 can be used to supervise, monitor, and control operations according to
programmed instructions and/or hardware configurations (e.g., analog, digital,
PAL,
and/or ASIC circuits). The sensors 1015 may include, but are not limited to,
temperature, smoke, contaminant, vibration, position, and light intensity
sensors. The
I/O port 1025 can be used to receive and send signals to the sensors 1015
and/or
actuators (e.g., motors, UV lamp) in the PSS 300. In some embodiments, the
control
module 1000 may send and/or receive status and control information to or from
a host
computer or controller via the COM port 1010. The COM port 1010 may be serial
or
parallel, and may use packet or non-packet based communication protocols
(e.g., RS-
232, USB, Firewire) to receive and/or send signals to a master controller. An
example
of the apparatus for operating an air handling system 1020 was described with
respect
to FIG. 22 of U.S. Patent Application Serial No. 11/389,995, entitled
"Automated
Pharmacy Admixture System," and filed by Eliuk, et al. on March 27, 2006. The
elements in the control module 1000 can combine to operate the PSS 300 to
sanitize
objects in pharmaceutical applications. In some embodiments a user interface
1035
may be included. A stand alone device would be one example where a user
interface
1035 would be included.
[0079] A PSS may use system information available to an APAS controller,
for example, to optimize the UV sanitizing process. For example, the APAS cell
100
may contain the control module 1000, as shown in reference to FIG. 10, to
control its
operations that may transfer control information (e.g., indicative of the next
object to
be sanitized) to the PSS 300 via the COM port 1010. Such control information
may
26
CA 02676695 2009-07-24
WO 2008/101353
PCT/CA2008/000348
include optimal waveform, amplitude, pulse repetition number and rate, object
size,
type, and/or shape-related information. The controller in the PSS 300 may
respond by
configuring the power supply 1030 and trigger controls to generate a
sanitizing profile
tailored to sanitize the next object. Such optimization promotes effective
sanitizing
without generating unnecessary heat, consuming unnecessary energy, prematurely
aging the flash element, or introducing unnecessary delay in the sanitizing
process. In
some embodiments, the robotic arm may be unable to perform other tasks during
the
UV sanitizing process. In other embodiments, the robotic arm may release the
object,
perform one or more other actions, and return to grasp and convey the object
after
sanitization is complete.
[0080] In response to a start signal, a dose of ultraviolet radiation may be
delivered. The dose may be according to a pre-programmed set of instructions,
at a
specified intensity, duty cycle, repetition rate (e.g., fixed or variable),
and number of
pulses, or total energy. The start signal may be generated by a switch that is
pressed
when the body of the object is pressed into the boot, or a proximity sensor
(e.g.,
optical sensor, Hall effect sensor to detect robot arm, or the like) detects
the fluid port
in position or other relevant features, a signal generated by a controller or
another
switch (which may be manually pressed), or a combination of such these or
other
detection techniques.
[0081] In some implementations, a UV light sensor may be provided to
measure the UV light intensity to monitor the sufficiency of a light pulse.
Sensors
may be used to monitor the condition of the bulb(s) and the intensity of the
emission
and/or flash. This monitoring may take place during normal usage and/or as
part of a
regular maintenance schedule. The sensors may also be monitored to confirm
that the
appropriate light dose has been delivered. If, for example, the processing
unit
determines that a UV waveform fails to meet an average minimum threshold over
multiple pulses, then the processing unit may generate a fault signal over the
COM
port 1010. In some embodiments, a sensor may measure the approximate total
energy
delivered, and send feedback information to a controller. The controller may
enable
UV output until a predetermined threshold of energy is delivered. Additionally
the
sensors could be used as part of a regular (e.g., daily) self-diagnostic
routine that
would warn operators of diminished emission from the UV source, thus allowing
for
replacement of said source prior to failure. In some embodiments, a fraction
of the
27
CA 02676695 2009-07-24
WO 2008/101353
PCT/CA2008/000348
UV energy is tapped using mirrors or other reflective, or partly reflective,
media.
This allows the use of sensors having lower energy handling capacity to
monitor the
total output from a UV source.
[0082] In some embodiments, a sensor (e.g., light beam, proximity, contact, or
vacuum/pressure) may be included in the PSS chamber to monitor the position or
proximity of the object to be subjected to the UV radiation. The sensor may
also be
used to monitor the position or proximity of an item displaced by the presence
of the
object (e.g. switch) with respect to the bulb. The sensor may provide an
interlock
such that the bulb power cannot be enabled if the object is not in the correct
position.
Sensors may also be used to monitor airflow and shut down the system if
inadequate
airflow is detected. The bulb or array of bulbs/lamps/LEDs may have
temperature
and air flow monitoring.
[0083] In various examples, interlocks may advantageously provide enhanced
operator safety in APAS cells and stand alone embodiments of the PSS, proper
and
reliable operation of the PSS, and/or protection of PSS equipment from damage
or
misuse. For example, an interlock may be provided to disable the light source
until a
portion of the object is in the PSS chamber such that a substantially complete
light
seal is formed to prevent substantial light from escaping. Suitable interlocks
may
include, but are not limited to, temperature monitoring of light source(s),
door(s) on
the PSS or the APAS cell or both, light leakage sensing, vacuum seals, air
flow,
position sensors, ozone level monitoring, and laser.
[0084] For manual operation, some embodiments may include a feedback
signal to indicate to an operator that the UV profile has completed, or that
the item to
be sanitized has been exposed to the selected dose of UV. In some embodiments,
a
display may indicate an exposure level, such as based on time, number of
pulses
delivered, or total energy delivered. In some embodiments, the operator may
control
the exposure level based on how long the item is pressed into the boot.
[0085] In an exemplary embodiment, the PSS operates as follows. An
automated transfer mechanism, such as a robotic arm, retrieves a medical
container
(e.g., drug vial or IV bag) from an inventory. From the multiple radiation
seal
assemblies that cover medical containers with different sizes and shapes, the
controller of the PSS system determines which radiation seal assembly
corresponds to
the medical container retrieved, based on the size and/or shape or the like of
the
28
CA 02676695 2009-07-24
WO 2008/101353
PCT/CA2008/000348
medical container. The robot arm can then present the medical container to the
ultraviolet light source of the PSS by engaging the medical container with the
corresponding radiation seal assembly. Or the robot arm can place the medical
container on a holding apparatus, which can then be actuated to couple the
medical
container with the corresponding radiation assembly proximate the UV source.
The
controller then instructs the UV light source to emit UV light at the correct
intensity
and for the needed duration to achieve the desired effect of sanitization of
the exposed
fluid transfer port (e.g., drug vial seal/stopper or port or IV bag injection
port) either
in a pulsed or continuous wave form. If the item being sanitized cannot be
exposed to
the emitted spectrum (for example, UV light can affect the drug contained in a
vial),
the sealing/containment system or method may ensure that the drug or IV fluid
container and/or contents is exposed to substantially reduced or no UV light
as
required. If the item being sanitized is not affected by exposure to UV light,
the
sealing/containment system or method may be designed to only limit exposure to
the
operator, or not be included at all if the potential outcome of exposure is
acceptable.
The cooling, purging and or venting system keeps the PSS and item being
sanitized
cool and vents or otherwise controls the buildup of ozone gas if any. The
control
system controls all aspects of the PSS operation. Monitoring on the system
confirms
that the correct UV exposure was produced, and that the target was in the
correct
location to receive the dose. Interlocks and safety mechanisms ensure that the
UV
source will not operate without appropriate safeguards or conditions in place.
After
the PSS sanitizes selected surfaces (e.g., drug vial ports and IV bag ports)
using an
ultraviolet (UV) light, a fluid transfer operation may be performed via the
sanitized
fluid transfer port.
[0086] Some embodiments may provide one or more further features. For
example, in cooperation with the features such as interlocks, sensors etc. the
sanitization process may be initiated by user input (e.g., by the touch of a
button or
other trigger device). There may be audible and or visual indications to cue
the
operator or inform of progress. The operator may have settings available for
such
things as exposure time, size of port, and height of vial for example.
[0087] FIGS. 11A-11F show cross-sectional views of an illustrative PSS 1100
in the APAS cell 100 of FIG. 1. The PSS 1100 can use a rotating platen 1105
with a
perpendicular vertical wall 1110 to position an object 1115 to be sanitized.
Except for
29
CA 02676695 2009-07-24
WO 2008/101353 PC
T/CA2008/000348
differences as noted or where not applicable, the discussion above regarding
embodiments of the PSS 300 are generally applicable to embodiments of the PSS
1100. For example, the PSS 300 may operate using a control module, an example
of
which is described above with reference to FIG. 10.
[0088] The object 1115 to be sanitized is loaded on the platen 1105 external
to
the PSS chamber. The platen 1105 is rotated using an appropriate drive
mechanism,
(e.g., stepper motor, servo motor, mechanical linkage coupled to a solenoid)
to
position the object 1115 inside the PSS chamber where it can be exposed to the
UV
radiation 1120. The vertical wall 1110 serves as a baffle to substantially
provide a
light seal for the chamber that may keep most of the UV radiation 1120 from
escaping. In some embodiments, sensors (e.g., encoder on platen shaft, index
mark
using Hall effect sensor, opto-interrupter, etc.) may be used to detect when
the platen
1105 is in position for loading or pulsing, or when the walls 1110 are in a
sealing
position. While positioned in the chamber, the object 1115 may receive a dose
of UV
radiation, as has been described. The platen 1105 then rotates to position the
object
1115 (portions of which may be substantially sanitized) outside of the PSS
chamber,
where it may be retrieved for further processing.
[0089] The PSS 1100 may be adapted for integration into an APAS cell 100,
or configured for stand-alone (e.g., table-top) operation for use in a
hospital pharmacy
or similar environment. In the hospital pharmacy type of environment, pharmacy
staff may prepare prescriptions by loading one or more objects to be sanitized
on the
platen 1105, perform the sanitizing, and retrieve the sanitized object for
further
processing after the platen 1105 rotates the object out of the chamber.
Information
indicating the form of medical container (e.g., size, shape, type) may be
requested
and/or obtained from a pharmacy computer system, for example, via a direct or
networked data channel, which may be wired and/or wireless. As is known in the
art,
various data transfers may involve packets of data, and/or error detection and
correction to ensure data integrity.
[0090] In some embodiments, the wall 1110 may further include multiple
compartments (e.g., three, four, five, six, seven, eight or more) on the
platen 1105.
The walls may be uniformly distributed such that when any of the compartments
is
exposed to the UV radiation 1120, a portion of the wall 1110 is positioned to
form a
light seal.
CA 02676695 2009-07-24
WO 2008/101353
PCT/CA2008/000348
[0091] In other embodiments, the platen 1105 may be a circular or non-
circular track. It may advance substantially continuously, or in segments
according to
chambers. In some embodiments, the platen 1105 may advance in response to a
user
command, such as from a keypad or "start" button. In other embodiments, the
platen
1105 may advance upon detecting the weight of one or more objects to be
processed.
[0092] Similar to the discussion with reference to FIGS. 3A-3C, the PSS 1100
may be configured to include other arrangements of a baffle 1125, examples of
which
can be shown in FIGS. 11C, 11D, 11E, and 11F.
[0093] Other modifications may be made to the PSS 1000. For example, an
illustrative embodiment of the PSS 1100 that includes a larger (or
distributed) lamp
system 1150 in combination with an illustrative embodiment of the baffle 1125
is
shown in FIG. 12F. In this example, the UV radiation 1120 can be distributed
over a
broader area. The baffling 1125 and reflective surface on the platen 1105 can
provide
a broadly distributed UV radiation pattern over top and side surfaces of an
object
1115b. Moreover, the platen 1105 is carrying two objects 1115a and 1115b. The
object 1115b can be in the PSS chamber, and the object 1115a can be external
to the
PSS chamber. This multi-object carrying capability of the PSS system 1100 can
promote efficient handling, for example, in a hospital pharmacy environment in
which
UV sanitizing processing time may affect productivity and throughput.
[0094] In another embodiment, the platen 1105 may be adapted to receive a
tray of objects that are to be sanitized. For example, a tray of two or more
vials to be
sanitized may be placed on portion of the platen 1105 that is external to the
PSS
chamber. The trays may include carrying handles for convenient placement
and/or
stacking of vials. Such trays may be prepared in advance, and can later be
efficiently
batch processed, thereby saving time and labor for processing pharmaceutical
admixtures.
[0095] To aid in aseptic processing, the entire PSS 1100 may be designed for
use within an ISO class 5 clean air environment. Such an environment may be
present, for example, within a containment cabinet in an APAS cell, or present
in a
hospital pharmacy laminar airflow hood. An air cooling system may be used, if
needed, to dissipate the heat in the lamp housing 1170 or chamber 1175.
31
CA 02676695 2009-07-24
WO 2008/101353
PCT/CA2008/000348
[0096] In addition to the above-described examples, UV sanitizing systems
may be implemented using systems, methods, or computer program products other
than the examples described above.
[0097] In various embodiments, a PSS system may communicate using
suitable communication methods, equipment, and techniques. For example, the
PSS
control module may communicate with the APAS control unit and/or a hospital
pharmacy network using point-to-point communication in which a message is
transported directly from the source to the receiver over a dedicated physical
link
(e.g., fiber optic link, point-to-point wiring, daisy-chain). Other
embodiments may
transport messages by broadcasting to all or substantially all devices that
are coupled
together by a communication network, for example.
[0098] In some embodiments, each PSS system may be programmed with the
same information and be initialized with substantially identical information
stored in
non-volatile memory. In other embodiments, one or more PSS systems may be
custom configured to perform specific functions. For example, one PSS system
may
be configured to perform both custom and batch processing functions by
responding
to information about the objects to be sanitized.
[0099] In one aspect, an automated sanitizing system for a pharmacy
environment for killing or incapacitating biocontaminants may present one or
more
objects to be sanitized. The system can include a chamber with a pulsed or
constant
wave form ultraviolet source. The system further can include an automated
transport
mechanism to place an object to be sanitized into the chamber for exposure to
ultraviolet radiation from the ultraviolet source.
[0100] In various embodiments, the automated transport mechanism may
further be to remove the object from the chamber after exposure to the
ultraviolet
radiation. The automated transport mechanism may include a robotic manipulator
and/or a rotating platen. The automated transport mechanism may manipulate or
move the object in response to a sequence of commands automatically generated
by a
processor executing a program of instructions.
[0101] Walls may substantially enclose the chamber, at least one wall having
an opening for receiving the object and a portion of the transport mechanism.
In some
embodiments, the automated transport mechanism may provide at least a partial
light
seal around at least a portion of the opening.
32
CA 02676695 2009-07-24
WO 2008/101353
PCT/CA2008/000348
[0102] The ultraviolet source may provide ultraviolet radiation in response to
a trigger signal. The controller may generate one or more pulses or timed
constant
wave of a controlled waveform. The waveform may be controlled to provide a
desired amplitude, shape, and/or intensity. The controller may generate a
plurality of
controlled pulses or constant wave according to a selected sanitizing routine.
The
selected sanitizing routine may correspond to characteristics, such as type,
size, or
manufacturer, of the object to be sanitized. The controller may receive
messages over
a communication link, and the messages may contain information about the
characteristics of the object to be sanitized.
[0103] The object to be sanitized may include a portion of a vial, an IV bag,
or
a syringe. The biocontaminants to be killed or incapacitated may include one
or more
viruses, bacteria, and/or fungi. The ultraviolet radiation may include UV-A,
UV-B,
and/or UV-C wavelengths. Some embodiments may expose a fluid transfer port to
be
sanitized to a combined dose of both continuous and pulsed radiation over a
predetermined period of time.
[0104] Some systems may be stand-alone or table top systems; other systems
may be adapted for integration into an APAS.
[0105] In another aspect, a method of sanitizing at least one object surface
may include generating a motion trajectory command to cause a transport
mechanism
to place an object within a chamber. The method may also include exposing at
least a
portion of the object to a dose of ultraviolet radiation.
[0106] In some embodiments, the dose of ultraviolet radiation may include
one or more pulses or timed constant wave. The method may further include
identifying a number of pulses or constant wave of ultraviolet radiation that
is
sufficient to kill or incapacitate one or more types of biocontaminants to a
selected
degree. The selected degree may be substantially all biocontaminants, such as
at least
99.9999%, 99.99%, 99%, 95%, 90%, 80%, 75%, 70%, 60%, or at least about 50%. In
some embodiments, between 1 and 100% of a particular biocontaminant may be
killed or substantially incapacitated by the dose of ultraviolet radiation.
[0107] After the PSS sanitizes selected surfaces (e.g., drug vial ports, IV
bag
ports and syringes) using an ultraviolet (UV) light, a fluid transfer
operation may be
performed. FIG. 12 shows an example of an apparatus 1200 for performing a
fluid
transfer operation. Exemplary aspects of a similar syringe manipulator
apparatus are
33
CA 02676695 2014-08-25
described, for example, with reference to FIG. 7 in U.S. Patent Application
Serial No.
11/937,846, entitled "Control of Fluid Transfer Operations," and filed by
Doherty, et
al. on November 9, 2007. In some implementations, care should be taken during
the
fluid transfer operation to prevent a protective cover 1202 in the
uncontrolled position
from contaminating a selected surface (e.g., a needle 1204), or obstructing
the
insertion of the needle 1204 into a desired fluid port. The protective cover
1202
covers a first fluid transfer port 1206 of a container 1208. The container
1208 also
includes a second fluid transfer port 1210. In one example, the fluid transfer
operation is performed using the second fluid transfer port 1210 of the
container 1208.
[0108] The container 1208 is held by a container manipulator 1212. The
container manipulator 1212 can move in a horizontal and vertical direction to
align a
particular container and fluid transfer port with the needle 1204.
[0109] In an illustrative example, a draw from a vial may be performed as
follows. First, the syringe plunger may be positioned to draw in a pre-
determined
amount of air into the syringe barrel. This amount may be determined based on
the
required fluid volume of the prescription (first pull). The predetermined
amount of air
can replace the volume of fluid that is drawn with an approximately equal
volume of
air. So if 10 ml of fluid is being drawn, 10 ml of air can be pushed in to
replace it.
During this process, the system may estimate or monitor the `headspace' in the
vial.
In a preferred embodiment, the method may maintain a slight negative pressure
in the
vial.
[0110] Second, the syringe plunger can be actuated to draw a predetermined
amount of fluid from the vial. In this case it can generate a negative
pressure. This
can be limited so that pull does not exceed a force limit (e.g., by limiting
motor
current to a threshold level.) Third, the syringe plunger can be actuated to
push a
volume of air into the vial to replace the volume of fluid removed. Fourth,
the syringe
plunger can be retracted again to an amount approximately equal to the amount
of air
pumped into the vial. Fifth, the cycle can continue until the required amount
of fluid
is drawn into the syringe from the vial. Sixth, at the end of the cycling, the
volume in
the syringe can substantially match the required draw amount, and there can be
a
slight negative pressure in the vial.
34
CA 02676695 2014-08-25
[0111] In an illustrative example, a draw from an IV bag may be performed as
follows. The IV bag may hang by its fill port on the indexer of a needle down
syringe
manipulator station. The indexer then moves the IV bag to a position under a
syringe
needle. The IV bag port then engages the syringe needle. A syringe plunger may
be
withdrawn so that air is drawn out of the IV bag and into the syringe. The
syringe
plunger may be withdrawn until the change in torque, for example, is detected
and, in
some embodiments, for some additional time to give margin on the draw
resulting in a
small amount of fluid draw and/or an IV bag that is negatively pressurized
relative to
ambient pressure. The indexer then lowers the IV bag.
[0112] Similar to the draw from a vial or IV bag as described above, one
skilled in the art would readily appreciate that a dispense into a vial or IV
bag may
also be performed.
[0113] A number of implementations have been described. Nevertheless, it
will be understood that various modifications may be made without departing
from
the scope. For example, advantageous results may be achieved if the steps of
the
disclosed techniques were performed in a different sequence, if components in
the
disclosed systems were combined in a different manner, or if the components
were
replaced or supplemented by other components. The functions and processes
(including algorithms) may be performed in hardware, software, or a
combination
thereof, and some implementations may be performed on modules or hardware not
identical to those described. Accordingly, other implementations are within
the scope
of the following disclosure.