Note: Descriptions are shown in the official language in which they were submitted.
CA 02676730 2009-09-09
System and Process for Producing Fresh Water
The present invention relates to the production of fresh water.
BACKGROUND
Water is one of the most vital natural resources for a!l life on Earth. The
availability and quality of water has always played an important part in
determining not
only where people can live, but also their quality of life. Domestic use
includes water
that is used in the home every day such as for drinking, food preparation,
bathing,
washing clothes and dishes, flushing toilets, and watering lawns and gardens.
Commercial water use includes fresh water for motels, hotels, restaurants,
office
buildings, other commercial facilities, and civilian and military
institutions. Industrial
water use is a valuable resource to a nation's industries for such purposes as
processing, cleaning, transportation, dilution, and cooling in manufacturing
facilities.
Major water-using industries include steel, chemical, paper, and petroleum
refining.
Water is used in the production of electricity in thermoelectric power plants
that are
fueled by fossil fuels, nuclear fission, or geothermal. Irrigation water use
is water
artificially applied to farm, orchard, pasture, and horticultural crops, as
well as water
used to irrigate pastures, for frost and freeze protection, chemical
application, crop
cooling, and harvesting. Livestock water use includes water for stock animals,
feed
lots, dairies, fish farms, and other nonfarm needs. Water is needed for the
production
of red meat, poultry, eggs, milk, and wool, and for horses, rabbits, and pets.
The planet's water reserves are estimated at 1,304,100 teratons (1 teraton is
1012 tons) of which freshwater reserves only account for 2.82% of this figure.
Page l
CA 02676730 2009-09-09
Agriculture consumes 70% of the world's freshwater, industry 20% and
households
10%. Between 1900 and 1995, drinking water demand grew twice as fast as the
world
population. By 2025, this demand should grow another 40%. In fifty years, the
Canadian Agency for International Development has predicted that some forty
countries could lack adequate drinking water. This will inevitably lead to
conflict, even
wars, as local areas, provinces and countries will go to any length to defend
their fresh
water resources.
Almost all conventional power plants, including coal, oil, natural gas, and
nuclear facilities, employ water cycles in the generation of electricity.
Recently
available data from the U.S. Geologic Survey shows that thermoelectric power
plants,
in the U.S.A., use more than 195 billion gallons of water per day. Such
immense
water needs produce equally immense concerns given the likelihood of future
droughts
and shortages, especially during the summer months. The addition of new
conventional power plants therefore, has inherent water-related risks that may
result in
electric utilities no longer able to construct them.
In Canada, there are vast oil sand resources estimate at 1.7 trillion barrels
(270x109 m) of bitumen. Water is required to convert bitumen into synthetic
crude oil.
A recent report by the Pembina Institute shows that it requires about 2-4.5 m3
of water
to produce one cubic metre (m) of synthetic crude. The need for industrial
water use
will increase with population growth and global warming as the demand for fuel
and
electricity increases.
Page 2
CA 02676730 2009-09-09
DESCRIPTION OF PRIOR ART
Hydrogen is commonly produced by extraction from hydrocarbon fossil fuels via
a chemical path. Hydrogen may also be extracted from water via biological
production
in an algae bioreactor, or using electricity (by electrolysis), chemicals (by
chemical
reduction) or heat (by thermolysis). Commercial bulk hydrogen is usually
produced
by the steam reforming of fossil fuels such as natural gas, gasoline. At high
temperatures (700-1100 C), steam (H20) reacts with methane (CH4) to yield
syngas.
The heat required to drive the process is generally supplied by burning some
portion of
the methane. There are other processes that can be used to recover hydrogen
and
these are well known and established processes.
Oxygen is present in air and there are two main methods to extract oxygen from
air. The most common method is to fractionally distill liquefied air into its
various
components with nitrogen distilling as a vapor while oxygen is left as a
liquid. The
other major method of producing oxygen gas involves passing a stream of clean,
dry
air through one bed of a pair of identical zeolite molecular sieves, which
absorbs the
nitrogen and delivers a gas stream that is 90% to 93% oxygen. Simultaneously,
nitrogen gas is released from the other nitrogen-saturated zeolite bed, by
reducing the
chamber operating pressure and diverting part of the oxygen gas from the
producer
bed through it, in the reverse direction of flow. After a set cycle time the
operation of
the two beds is interchanged, thereby allowing for a continuous supply of
gaseous
oxygen to be pumped through a pipeline. This is known as pressure swing
adsorption.
There are other processes that can be used to recover hydrogen and these are
well
known and established processes.
Page 3
CA 02676730 2009-09-09
The combustion of hydrogen and oxygen is well known. The combustion of
hydrogen and oxygen yields extreme heat and water vapour in the form of steam.
The combustion temperature of hydrogen and oxygen is around 3200 C.
Conventional industrial boilers or gas turbines are not designed to handle
such
extreme temperatures and they would experience metal fatigue and melting if
exposed
to such temperature.
At ambient temperatures, the oxygen and nitrogen gases in air will not react
with each other. In an internal combustion engine, combustion of a mixture of
air and
fuel produces combustion temperatures high enough to drive endothermic
reactions
between atmospheric nitrogen and oxygen in the flame, yielding various oxides
of
nitrogen (NOX). Nox can penetrate deeply into sensitive lung tissue and damage
it
causing premature death in extreme cases. Inhalation of such particies may
cause or
worsen respiratory diseases such as emphysema, bronchitis it may also
aggravate
existing heart disease.
As NOX moves to the atmosphere it eventually forms nitric acid which
contributes to acid rain consequently NOX emissions are regulated by the
various
Environmental Protection Agencies. Consequently, it is extremely critical to
ensure
that the no air is present in the combustor that is combusting hydrogen and
oxygen as
the extreme combustion temperature will result in NOXs that will exceed the
environmental standards.
Page 4
CA 02676730 2009-09-09
Today the only place where pure hydrogen is combusted with pure oxygen is in
the fueling of rockets. Hydrogen, which is the propellant, is used because it
is the
lightest in weight and oxygen is required for the combustion.
SUMMARY OF THE INVENTION
In one aspect, the present invention relates to combusting hydrogen and
oxygen under high pressure to produce superheated steam and heat. The heat
generated through the combustion of hydrogen and oxygen is then extracted and
can
be used as an energy input in another process, such as in the generation of
electricity.
The extraction of the heat condenses the superheated steam to produce fresh
water.
The generated electricity can be used internally in a plant using a process
embodying
the principles of the invention (thereby reducing the amount of external
electricity that
needs to be purchased), or be sold to an external source resulting in a
revenue
stream.
BRIEF DESCRIPTION OF THE DRAWINGS
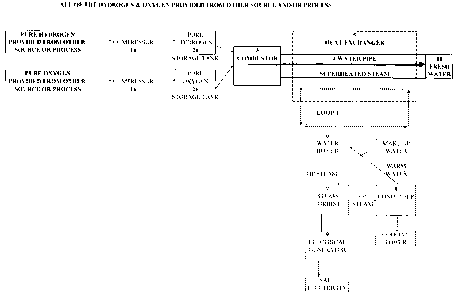
FIG. 1 illustrates processes according to an embodiment of the present
invention where the hydrogen and oxygen are provided from other source(s)
and/or
process(es) to be combusted under high pressure to produce fresh water. The
heat
extracted from the superheated steam is used to generate electricity according
to one
embodiment of the present invention.
FIG. 2 illustrates one embodiment of a hydrogen and oxygen combustor
according to the present invention.
Page 5
CA 02676730 2009-09-09
FIG. 3 illustrates one embodiment of a heat exchanger used for extracting heat
from the combustion of hydrogen and oxygen to produce superheated steam
according to the present invention.
FIG. 4 illustrates one embodiment of the present process where part of the
heat
extracted from the superheated steam is used to generate electricity and the
balance
of the heat extracted is used in a industrial/chemical process according to
the present
invention.
DESCRIPTION OF THE PREFERRED EMBODIMENT
Referring initially to FIG. 1, in one embodiment, of the present invention a
process where all of the hydrogen and oxygen are provided from external
sources
and/or process. Hydrogen can be produced by extraction from hydrocarbon fossil
fuels via a chemical path. Hydrogen may also be extracted from water via
biological
production in an algae bio-reactor, Similarly, oxygen can be obtained by
fractional
distillation of liquid air. The imported hydrogen and oxygen are then
combusted under
high pressure to produce superheated steam and heat. The heat generated
through
the combustion of hydrogen and oxygen is then extracted by the heat exchanger
system is used to generate electricity. The extraction of the heat by the heat
exchanger system condenses the superheated steam to produce fresh water. The
generated electricity can be used internally (thereby reducing the plant's
external
electrical purchase) or be sold to an external source resulting in a revenue
stream.
Once hydrogen and oxygen are obtained, they are separated into different
storage tanks under high pressure. Pressure is used so as to minimize the
amount of
the required storage. In addition, high pressure gas is required for the
combustion is
the combustor in a later stage of the process. A compression pressure of 2
Page 6
CA 02676730 2009-09-09
atmospheres can be used for example. A compressor 1a is used to compress
hydrogen and store it in a storage tank 2a, and a compressor 1b is used to
compress
oxygen and store it in a storage tank 2b. . The hydrogen and oxygen gases will
be
cooled by their respective compressor la, lb operating at elevated pressure
(i.e
greater than 1 atmosphere). A compression pressure of 2 atmospheres can be
used
for example.
As shown in FIG. 2, pressurized hydrogen 31 and pressurized oxygen 32 are
then injected into a combustor 3 to generate high pressure high temperature
superheated steam 33. The pressurized hydrogen and oxygen ensures that the
combustion will occur under high pressure thus preventing air from entering
the
combustor thereby preventing the creation of nitrous oxide ("NOX"). The
combustion
pressure will exceed 1 atmosphere so as to exclude the air from entering the
combustor. A combustion pressure of 2 atmospheres can be used for example. The
combustion chamber is designed to withstand high combustion temperatures
without
significant heat loss. The combustion chamber is preferably constructed of
refractory
materials or has high temperature ceramic surface coatings 34. Another means
for
carrying out high temperature combustion is described in U.S. Patent No.
7,128,005,
details of which are incorporated herein by reference. The combustion process
produces superheated steam at high temperatures. The heat from the superheated
steam is extracted through a heat exchanger S. The material in the system is
chosen
from material that is suitable for high temperature operation. Current
technology has
the capacity to deal with heat in excess of 3200 C. For example, there are
ceramics
that can withstand the heat and thus could line the surface of the combustor,
the
appropriate selection of which is within the knowledge of a person of ordinary
skill in
the art.
Page 7
CA 02676730 2009-09-09
As shown in FIG 3, the superheated steam 41 so produced is at a combustion
temperature of about 3200 C. This high temperature superheated steam then
flows
through a water pipe 4, transferring heat to a high temperature heat exchanger
system
5. The returned heat exchanger fluid from loop 1 enters the heat exchanger
system at
43. The heat energy extracted by the heat exchanger system from the high
temperature superheated steam is then returned to water boiler 42 to heat the
water
used in the electrical generating process through loop 1. The superheated
steam
produced by the combustion process is cooled by the extraction of the heat by
the
heat exchanger system to produce fresh water 10. The water pipe 44 serves the
purpose of containing the superheated steam isolated so that no impurities are
introduced into the process of fresh water creation. The water pipe and the
combustor
are hermetically sealed thereby ensuring that no air or contaminants will
enter the
process. The superheated steam exiting from the combustor to the water pipe is
also
under pressure thus ensuring that no air will enter the water pipe. It will be
understood
by those skilled in the art that any number of suitable types of collection
vessels
(referred to generally as a "collector") can be used in place of a water pipe
for
condensing steam and the present invention is not limited to the use of a
water pipe.
The wall thickness of the water pipe can be tapered as the temperature
gradient
reduces along the water pipe due to heat extraction. The tapered wall reduces
the
cost of the water pipe. Heat is extracted from the water pipe by way of
suitable heat
exchangers. The combustor and the water pipe containing high temperature
superheated steam and are made of material that can stand high temperatures,
such
as refractory material. The heat exchanger fluid is not in direct contact with
the super
Page 8
CA 02676730 2009-09-09
saturated steam. Many known industries such as nuclear plants, foundries,
rockets
etc. operate at very high temperatures and consequently, the selection of
appropriate
heat exchanger and heat exchanger fluids suitable for the process is within
the
knowledge of a person of ordinary skill in the art.
Preferably, the combustor 3 and the high temperature heat exchanger 5 are
insulated so as to minimize heat loss and maximize their efficiencies. The
selection of
insulating materials is within the knowledge of a person of ordinary skill in
the art.
Another embodiment of the present invention as shown in FIG. 4 illustrates a
process where part of the heat extracted from the superheated steam is used to
generate electricity and the balance is used in an industrial/chemical
process. The
generated electricity can be used internally (thereby reducing the plant's
external
electrical purchase) or be sold to an external source resulting in a revenue
stream.
It will be further understood by those skilled in the art that the system of
the
present invention can be configured in a number of ways. For example, in
certain
embodiments, multiple units can be used such as two combustors, and four heat
exchangers.
While preferred processes are described, various modifications, alterations,
and changes may be made without departing from the spirit and scope of the
process
according to the present invention as defined in the appended claims. Many
other
configurations of the described processes may be useable by one skilled in the
art.
Page 9