Note: Descriptions are shown in the official language in which they were submitted.
CA 02678276 2009-09-09
RECOVERING METAL VALUES FROM A METALLIFERROUS MATERIAL
FIELD OF THE INVENTION
[0001] This invention relates to the recovery of one or more metal values
from a
metalliferrous material and, more particularly, to improving the recovery of
one or more
metal values from a metalliferrous material by neutralizing acid being
produced during
the process.
BACKGROUND OF THE INVENTION
[0002] Nickel and cobalt are recovered from laterite ores using sulphide
precipitation. An example of this process is described in U.S. Patent No.
7,387,767.
[0003] The sulphide precipitation step produces acid which has a tendency
to
build up in the reaction zone, thereby limiting the recoveries of nickel and
cobalt by
precipitation of their sulphides.
SUMMARY OF THE INVENTION
[0004] In one aspect, there is provided a method of treating a
metalliferrous
material, comprising: providing a metalliferrous material including at least
one target
metallic element; solubilising the metalliferrous material so as to effect
production of an
intermediate product including an operative solution, wherein the operative
solution
includes a solvent component and a solute component, and wherein the solute
component
includes at least one solute component-based target metallic element and each
one of the
at least one solute component¨based target metallic element corresponds to a
one of the
at least one target metallic element of the metalliferrous material such that
the operative
solution includes at least one target metallic element; contacting the
operative solution
with an operative reagent in an operative reaction zone so as to effect
production of a
product mixture including an operative reaction product and hydronium ion,
wherein the
operative reaction product includes at least one operative reaction product-
based target
metallic element and each one of the at least one operative reaction product-
based target
metallic element corresponds to a one of the at least one solute component-
based target
- 1 -
CA 02678276 2009-09-09
metallic element of the operative solution, wherein the operative solution
being contacted
in the operative reaction zone includes a total molar quantity of target
metallic element in
the operative reaction zone, wherein each one of the at least one solute
component-based
target metallic element includes a respective target metallic element molar
quantity in the
operative reaction zone, such that at least one respective target metallic
element molar
quantity is provided in the operative reaction zone, and wherein the total
moles of target
metallic element in the operative reaction zone is the sum of the at least one
respective
target metallic element molar quantity in the operative reaction zone; and
providing at
least one hydronium ion depletion agent in the operative reaction zone such
that
contacting between any one of the at least one hydronium ion depletion agent
and the
hydronium ion in the operative reaction zone effects a reactive process which
consumes
the hydronium ion, wherein each one of the at least one hydronium ion
depletion agent
includes at least one dissolved aluminium complex material and each one of the
at least
one dissolved aluminium complex material includes a respective molar quantity
of
operative aluminium in the operative reaction zone such that each one of the
at least one
hydronium ion depletion agent includes at least one respective molar quantity
of
operative aluminium in the operative reaction zone, and such that each one of
the at least
one hydronium ion depletion agent includes a subtotal operative aluminium
molar
quantity in the operative reaction zone defined by the sum of the respective
at least one
respective molar quantity of operative aluminium in the operative reaction
zone such that
at least one subtotal operative aluminium molar quantity in the operative
reaction zone is
provided, and wherein a total moles of operative aluminium in the operative
reaction
zone is the sum of the at least one subtotal operative aluminium molar
quantity in the
operative reaction zone; wherein the total moles of operative aluminium in the
operative
reaction zone, relative to the total moles of target metallic element in the
operative
reaction zone, is pre-determined.
[0005] In one
aspect, there is provided a method of treating a metalliferrous
material, comprising: providing a metalliferrous material including at least
one target
metallic element; solubilising the metalliferrous material so as to effect
production of an
intermediate product including an operative solution, wherein the operative
solution
includes a solvent component and a solute component, and wherein the solute
component
- 2 -
CA 02678276 2009-09-09
includes at least one solute-based target metallic element and each one of the
at least one
solute-based target metallic element corresponds to a one of the at least one
target
metallic element of the metalliferrous material, such that the operative
solution includes
at least one target metallic element; contacting the operative solution with
an operative
reagent in an operative reaction zone so as to effect production of a product
mixture
including an operative reaction product and hydronium ion, wherein the
operative
reaction product includes at least one operative reaction product-based target
metallic
element and each one of the at least one operative reaction product-based
target metallic
element corresponds to a one of the at least one solute-based target metallic
element of
the operative solution; providing at least one hydronium ion depletion agent
in the
operative reaction zone for effecting contacting between the hydronium ion in
the
reaction zone and at least one of the at least one hydronium ion depletion
agent, wherein
the contacting between any one of the at least one hydronium ion depletion
agent and the
hydronium ion in the operative reaction zone effects a reactive process which
consumes
the hydronium ion, wherein at least one of the at least one hydronium ion
depletion agent
includes at least one dissolved aluminium complex material; recovering
aluminium-
comprising residue from at least one of the solubilising or contacting steps
such that
recovered aluminium-comprising residue is provided and the recovered aluminium-
comprising residue includes recovered aluminium; subjecting at least a
fraction of the
recovered aluminium-comprising residue to a reactive process so as to effect
production
of at least one aluminium-comprising residue-derived hydronium ion depletion
agent; and
providing at least one of the at least one aluminium-comprising residue-
derived
hydronium ion depletion agent to the operative reaction zone such that at
least a fraction
of the at least one hydronium ion depletion agent provided in the operative
reaction zone
includes at least one of the at least one aluminium-comprising residue-derived
hydronium
ion depletion agent, such that at least a fraction of the recovered aluminium
is recycled.
- 3 -
CA 02678276 2009-09-09
BRIEF DESCRIPTION OF DRAWINGS
[0006] The system and method of the preferred embodiments of the
invention
will now be described with the following accompanying drawings:
[0007] Figure 1 is a process flow diagram of an embodiment of the
invention.
DETAILED DESCRIPTION
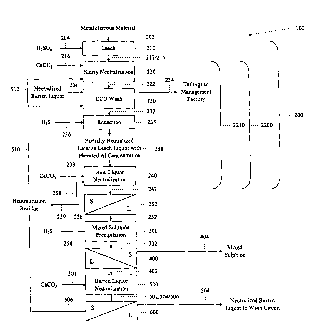
[0008] Referring to Figure 1, there is provided a method 100 of treating
a
metalliferrous material 202.
[0009] The method includes providing a metalliferrous material 202. For
example, the metalliferrous material 202 is an ore, a concentrate, or any
other metal-
containing material. For example, the metalliferrous material 202 is a
laterite ore. For
example, the metalliferrous material 202 is a laterite ore which consists
primarily of
limonite and, in some embodiments, includes minor amounts of saprolite
minerals. As a
further example, the metalliferrous material includes 0.01 to 2.5% Ni, 0.01 to
2.5% Co, 1
to 20% Al, 0.1 to 5% Cr, 5 to 60% Fe, 0.01 to 25% Mg, 0.01 to 5% Mn and 0.1 to
40%
Si. As a further example, the metalliferrous material 202 includes 0.5 to 1.5%
Ni, 0.05 to
0.2% Co, 3 to 8% Al, 1 to 3% Cr, 45 to 55% Fe, 0.1 to 2% Mg, 0.5 to 2% Mn and
1 to
5% Si.
[0010] For example, the metalliferrous material 202 is conditioned such
that any
one of several characteristics of the metalliferrous material 202 is modified
to improve
the suitability of the metalliferrous material 202 for leaching. An
exemplary
characteristic which could be modified is particle size. Another exemplary
characteristic
which could be modified is composition.
[0011] For example, the metalliferrous material 202 is metalliferrous
particulate
material.
[0012] The metalliferrous material 202 includes at least one target
metallic
element. For example, the target metallic element is nickel (Ni). As a further
example,
the target metallic element is cobalt (Co). As a further example, the at least
one target
- 4 -
CA 02678276 2009-09-09
metallic element includes nickel and cobalt. As a further example, the at
least one target
metallic element is nickel and cobalt.
[0013] The metalliferrous material 202 is solubilised by a solubilization
process
200 so as to effect production of an intermediate product 242 including an
operative
solution 252. The operative solution 252 is an aqueous solution and includes a
solvent
component and a solute component. The solute component includes at least one
solute
component-based target metallic element. Each one of the at least one solute
component-
based target metallic element corresponds to a one of the at least one target
metallic
element of the metalliferrous material such that the operative solution
includes at least
one target metallic element.
[0014] The operative solution 252 is contacted with an operative reagent
254 in
an operative reaction zone 300 so as to effect production of a product mixture
302
including an operative reaction product and hydronium ion. The operative
reaction
product includes at least one operative reaction product-based target metallic
element.
Each one of the at least one operative reaction product target metallic
element component
corresponds to a one of the at least one solute component-based target
metallic element.
[0015] At least one hydronium ion depletion agent is also provided in the
operative reaction zone 300. Each one of the at least one hydronium ion
depletion agent
includes at least one dissolved aluminium complex material. Contacting between
any
one of the at least one hydronium ion depletion agent and the hydronium ion in
the
operative reaction zone 300 effects a reactive process which consumes the
hydronium
ion. For example, a suitable dissolved aluminium complex material includes a
polynuclear aluminium species. As a further example, a suitable dissolved
aluminium
complex material is a compound of Al2(OH)2(H20)84+.
[0016] In some embodiments, the operative solution 252 being contacted
with the
operative reagent 254 during the contacting of the operative solution 252 with
the
operative reagent 254 in the operative reaction zone 300 is separated from the
intermediate product 242 prior to the contacting with the operative reagent
254.
- 5 -
CA 02678276 2009-09-09
[0017] In some embodiments, at least a fraction of the at least one
operative
reaction product is a solid reaction product.
[0018] In some embodiments, the operative reagent 254 includes an acid
producing sulphide reagent, such as gaseous hydrogen sulphide. Other suitable
examples
of acid producing sulphide reagents include sodium hydrosulphide, ammonium
hydrosulphide, and potassium hydrosulphide.
[0019] In some embodiments, the method further includes separating at
least a
fraction of the at least one operative reaction product from the product
mixture 302. For
example, the separation is a solid-liquid separation process.
[0020] In some embodiments, the method further includes: (i) separating a
target
metallic element depleted solution product 402 from the product mixture in a
solid-liquid
separator 400, and (ii) contacting the target metallic element depleted
solution product
402 with a neutralizing agent 501 in a residual product treatment zone 500 to
effect
production of a residual slurry 502 including a residual operative solution
504 and target
metallic element depleted solution product-derived solid aluminium comprising
residue
506. Relative to the target metallic element depleted solution product 402,
the residual
operative solution 504 is characterized by a higher pH.
[0021] In some embodiments, the aluminium of at least a fraction of the
at least
one hydronium ion depletion agent provided in the operative reaction zone 300
is derived
from the metalliferrous material 202. In this respect, for example, the
metalliferrous
material 202 further includes at least one metalliferrous material-based
aluminium-
comprising component, wherein the metalliferrous material-based aluminium-
comprising
component includes metalliferrous material-based aluminium, such that the
metalliferrous material 202 further includes metalliferrous material-based
aluminium.
For example, a one of the at least one metalliferrous material-based aluminium
component is gibbsite [A 1 (OH)3]. In some embodiments, at least a fraction of
the at least
one metalliferrous material-originating aluminium-comprising solid material
includes
aluminium hydroxide, and the aluminium of at least a fraction of the at least
one
- 6 -
CA 02678276 2009-09-09
hydronium ion depletion agent provided in the operative reaction zone is
derived from
the aluminium hydroxide.
[0022] When aluminium of at least a fraction of the at least one
hydronium ion
depletion agent is derived from the metalliferrous material 202, in some
embodiments,
the metalliferrous material 202 also includes aluminium, and the solubilising
of the
metalliferrous material 202 includes: (i) leaching the metalliferrous material
202 with a
leachant 204 in a leaching zone 210 so as to effect production of a leachate
212 including
the at least one solute component-based target metallic element and aluminium,
and (ii)
contacting a neutralizing agent with the leachate 212 in a neutralization
process 2200 so
as to effect production of at least one leachate-derived hydronium ion
depletion agent,
wherein each one of the at least one leachate-derived hydronium ion depletion
agent
includes aluminium derived from the metalliferrous material. In some
embodiments, the
leachant 204 includes an acidic solution. For example, a suitable leachant 204
includes
concentrated sulphuric acid. At least one of the at least one hydronium ion
depletion
agent provided in the operative reaction zone 300 is a one of the at least one
leachate-
derived hydronium ion depletion agent. In some embodiments, the production of
the at
least one leachate-derived hydronium ion depletion agent is effected by the
contacting of
the leachate 212 with the neutralizing agent. In this respect, the contacting
of the
leachate 212 with the neutralization agent effects production of an operative
solution 252
including the at least one leachate-derived hydronium ion depletion agent. In
some
embodiments, the pH of the operative solution 252 is at least 3.3. For
example, the pH of
the operative solution 252 is between 3.3 and 4Ø As a further example, the
pH of the
operative solution 252 is between 3.8 and 4Ø Relative to the leachate 212,
the operative
solution 252 is characterized by a high pH. For example, a suitable
neutralizing agent
239 is calcium carbonate. As a further example, a suitable neutralizing agent
239 is
limestone.
[0023] When aluminium of at least a fraction of the at least one
hydronium ion
depletion agent is derived from the metalliferrous material 202, in some
embodiments,
the metalliferrous material 202 also includes at least one metalliferrous
material-based
aluminium-comprising component, wherein the metalliferrous material-based
aluminium-
- 7 -
CA 02678276 2009-09-09
comprising component includes metalliferrous material-based aluminium, and the
solubilising includes: (i) in a leaching zone 210, leaching the metalliferrous
material 202
with a leachant 204 including an aqueous acidic solution so as to effect
production of a
leachate 212 including a dissolved metalliferrous material-derived aluminium
comprising
solute, wherein the dissolved metalliferrous material-derived aluminium
comprising
solute includes aluminium of the metalliferrous material-based aluminium, and
(ii)
subjecting at least a fraction of the dissolved metalliferrous material-
derived aluminium
comprising solute to a reactive process 2210 so as to effect production of at
least one
metalliferrous material-derived hydronium ion depletion agent, wherein each
one of the
at least one metalliferrous material-derived hydronium ion depletion agent
includes
aluminium of the metalliferrous material-based aluminium. In some embodiments,
the
leachant 204 includes an acidic solution. For example, a suitable leachant 204
includes
concentrated sulphuric acid. At least a fraction of the at least one hydronium
ion
depletion agent provided in the operative reaction zone 300 includes at least
one of the at
least one metalliferrous material-derived hydronium ion depletion agent. In
some
embodiments, at least a fraction of the metalliferrous material-based
aluminium-
comprising component includes aluminium hydroxide. In some embodiments, the
subjecting of at least a fraction of the dissolved metalliferrous material-
derived
aluminium-comprising solute to a reactive process so as to effect production
of at least
one metalliferrous material-derived hydronium ion depletion agent includes
contacting
the leachate 212 with a neutralizing agent to effect production of the
operative solution
252. In some embodiments, the pH of the operative solution 252 is at least
3.3. For
example, the pH of the operative solution 252 is between 3.3 and 4Ø As a
further
example, the pH of the operative solution 252 is between 3.8 and 4Ø Relative
to the
leachate 212, the operative solution 252 is characterized by a higher pH. For
example, a
suitable neutralizing agent 239 is calcium carbonate. As a further example, a
suitable
neutralizing agent 239 is limestone.
100241 When
aluminium of at least a fraction of the at least one hydronium ion
depletion agent is derived from the metalliferrous material 202, in some
embodiments,
the metalliferrous material 202 also includes at least one metalliferrous
material-based
aluminium-comprising component, wherein the metalliferrous material-based
aluminium-
- 8 -
CA 02678276 2009-09-09
comprising component includes metalliferrous material-based aluminium, and the
solubilising includes: (i) in a leaching zone 210, leaching the metalliferrous
material 202
with a leachant 204 including an aqueous acidic solution so as to effect
production of an
intermediate slurry product 214 including a leachate 212, wherein the leachate
212
includes a dissolved metalliferrous material-derived aluminium-comprising
solute,
wherein the dissolved metalliferrous material-derived aluminium-comprising
solute
includes aluminium of the metalliferrous material-based aluminium, (ii)
contacting the
intermediate slurry product 214 with a neutralizing agent 216 in a first
neutralization
zone 220 so as to effect production of a treated intermediate slurry product
222, (iii)
separating a leachate-derived intermediate operative solution 232 from the
treated
intermediate slurry product 222 in a separation zone 230, and (iv) subjecting
the leachate-
derived intermediate operative solution 232 to a reactive process, wherein a
neutralizing
agent 239 is provided in a second neutralization zone 240, and wherein the
reactive
process includes consumption of at least a fraction of the neutralizing agent
239 so as to
effect production of a leachate-derived operative solution 252 and a post-
neutralization
aluminium-comprising solid residue 256, wherein the leachate-derived operative
solution
252 includes at least one metalliferrous material-derived hydronium ion
depletion agent,
wherein each one of the at least one metalliferrous material-derived hydronium
depletion
agent includes aluminium of the metalliferrous material-based aluminium, and
wherein at
least a fraction of the at least one hydronium ion depletion agent provided in
the
operative reaction zone 300 includes at least one of the at least one
metalliferrous
material-derived hydronium ion depletion agent. In some embodiments, the pH of
the
leachate-derived operative solution 252 is at least 3.3. For example, the pH
of the
leachate-derived operative solution 252 is between 3.3 and 4Ø As a further
example, the
pH of the leachate-derived operative solution 252 is between 3.8 and 4Ø
Relative to the
leachate-derived intermediate operative solution 232, the leachate-derived
operative
solution 252 is characterized by a higher pH.
100251 In
some embodiments, the aluminium of at least a fraction of the at least
one hydronium ion depletion agent provided in the operative reaction zone 300
is derived
from an external aluminium-comprising source which is independent of the
metalliferrous material and which is introduced upstream of the operative
reaction zone
- 9 -
CA 02678276 2009-09-09
300. For example, the external aluminium-comprising source is an aluminium-
comprising solution which is supplied to the second neutralization zone 240.
As a further
example, the external aluminium-comprising source is acid-soluble aluminium
comprising solid material which is dissolved and supplied to second
neutralization zone
240.
100261 In
some embodiments, the treated intermediate slurry product 222 is
contacted with a wash solution in the separation zone 230 so as to effect mass
transfer of
dissolved material from the treated intermediate slurry product 222 to the
wash solution
so as to effect production of the leachate-derived intermediate operative
solution 232,
wherein the wash solution includes at least a fraction of the residual
operative solution
504, described above. In this respect, in some of these embodiments, the
contacting is
effected while the treated intermediate slurry product 222 is flowing though
the
separation zone 230 in a direction opposite to that of the flowing of the wash
solution.
[0027] In
some embodiments, when the metalliferrous material 202 includes 0.9
to 1.5% Ni, 0.05 to 0.18% Co, 3 to 5% Al, 1.5 to 2% Cr, 45 to 53% Fe, 0.1 to
1.5% Mg,
0.5 to 1.2% Mn and 0.9 to 3% Si, the leachant 204 is concentrated sulphuric
acid (which
becomes diluted by the water in the slurry of the metalliferrous material),
and the
leaching of the metalliferrous material 202 by the leachant 204 is effected in
a leaching
zone 210, wherein the metalliferrous material 202 is contacted by the leachant
204 in the
leaching zone 210. The leaching zone 210 is characterized by a predetermined
temperature and a predetermined pressure. For example, the temperature within
the
leaching zone 210 is between 240 degrees Celsius and 300 degrees Celsius. As a
further
example, the temperature within the leaching zone 210 is between 250 degrees
Celsius
and 270 degrees Celsius. For example, the temperature within the leaching zone
210 is
260 degrees Celsius. For example, with respect to the pressure within the
leaching zone
210, the pressure is equal to, or higher than, the steam pressure of the
leaching zone
solution (that solution provided in the leaching zone 210 when the
metalliferrous material
202 is contacted with the leachant 204 in the leaching zone 210) in the
leaching zone 210
at the temperature of the leaching zone 210. For example, when the temperature
within
the leaching zone 210 is between 240 degrees Celsius and 300 degrees Celsius,
the
- 10 -
CA 02678276 2009-09-09
pressure in the leaching zone 210 is between 450 psig and 1300 psig. For
example, the
leaching is a continuous operation effected in a reaction vessel, and the
retention time is
between 30 minutes and 120 minutes. As a further example, the retention time
is
between 60 minutes and 90 minutes.
[0028] Each
of the above-described embodiments includes at least one of the
following features.
1.
FEATURE RELATING TO THE RELATIVE QUANTITIES OF OPERATIVE
ALUMINIUM AND TARGET METALLIC ELEMENT IN THE OPERATIVE
REACTION ZONE
[0029] In
some embodiments, there is provided a feature which relates to the
relative total quantities of operative aluminium and target metallic element
in the
operative reaction zone. In this respect, the provided feature is that the
total moles of
operative aluminium in the operative reaction zone 300, relative to the total
moles of
target metallic element in the operative reaction zone 300, is pre-determined.
[0030] The
operative solution being contacted in the operative reaction zone 300
includes a total molar quantity of target metallic element in the operative
reaction zone.
Each one of the at least one solute component-based target metallic element
includes a
respective target metallic element molar quantity in the operative reaction
zone 300. In
this respect, at least one respective target metallic element molar quantity
is provided in
the operative reaction zone 300, and the total moles of target metallic
element in the
operative reaction zone 300 is the sum of the at least one respective target
metallic
element molar quantity in the operative reaction zone 300.
[0031] Each
one of the at least one dissolved aluminium complex material
includes a respective molar quantity of operative aluminium in the operative
reaction
zone 300. In this respect, each one of the at least one hydronium ion
depletion agent
includes at least one respective molar quantity of operative aluminium in the
operative
reaction zone 300. In this respect, each one of the at least one hydronium ion
depletion
agent includes a subtotal operative aluminium molar quantity in the operative
reaction
- 11 -
CA 02678276 2009-09-09
zone defined by the sum of the respective at least one respective molar
quantity of
operative aluminium in the operative reaction zone 300. In this respect, at
least one
subtotal operative aluminium molar quantity in the operative reaction zone 300
is
provided, and the total moles of operative aluminium in the operative reaction
zone 300
is the sum of the at least one subtotal operative aluminium molar quantity in
the operative
reaction zone 300.
[00321 In
some embodiments, the total moles of operative aluminium in the
operative reaction zone 300, relative to the total moles of target metallic
element in the
operative reaction zone 300, is controlled within a predetermined range.
[0033] In
some embodiments, the ratio of: (i) the total moles of operative
aluminium in the operative reaction zone 300, to (ii) the total moles of
target metallic
element in the operative reaction zone 300 is between 0.45 and 4.4. For
example, the
ratio is between 0.85 and 2.2. For example, when the target metallic elements
provided
in the metalliferrous material 202 are nickel and cobalt, and the
concentration of nickel
and cobalt in the operative reaction zone is less than 5 grams per litre, the
concentration
of operative aluminium in the operative reaction zone is between 2 grams per
litre and 5
grams per litre.
2.
FEATURE RELATING TO THE RECOVERY AND RECYCLING OF
RESIDUAL ALUMINIUM
[0034] In
some embodiments, there is provided a feature which relates to the
recovery and recycling of residual aluminium.
[0035] In
this respect, in some embodiments, the method further includes
recovering at least one residual aluminium-comprising residue from at least
one of the
solubilising process 200 or the operative reaction zone 300, steps such that
recovered
aluminium-comprising residue is provided and the recovered aluminium-
comprising
residue includes recovered aluminium. At least a fraction of the recovered
aluminium-
comprising residue is subjected to a reactive process so as to effect
production of at least
one aluminium-comprising residue-derived hydronium ion depletion agent. At
least one
- 12 -
CA 02678276 2009-09-09
of the at least one aluminium-comprising residue-derived hydronium ion
depletion agent
is provided to the operative reaction zone 300 such that at least a fraction
of the at least
one hydronium ion depletion agent provided in the operative reaction zone 300
includes
at least one of the at least one aluminium-comprising residue-derived
hydronium ion
depletion agent, such that at least a fraction of the recovered aluminium is
recycled.
[0036] In some embodiments, the recovered aluminium-comprising residue
510
includes at least a fraction of the target metallic element depleted solution
product-
derived solid aluminium comprising residue 506. In some embodiments, the
recovered
aluminium-comprising residue 510 includes at least a fraction of the post-
neutralization
aluminium-comprising solid residue 259.
[0037] In some embodiments, at least a fraction of the recovered
aluminium-
comprising residue 510 is acid-soluble. For example, the recovered aluminium-
comprising residue 510 includes aluminium hydroxide.
[0038] In some embodiments, there is provided an operative acid-soluble
solid
aluminium-comprising residue 512, wherein the operative acid-soluble solid
aluminium-
comprising residue is at least a fraction of the recovered aluminium-
comprising residue
510. The operative acid-soluble solid aluminium-comprising residue 512 is
subjected to
a reactive process so as to effect production of at least one of the at least
one aluminium-
comprising residue-derived hydronium ion depletion agent, wherein the
subjecting of the
operative acid-soluble solid aluminium-comprising residue 512 to a reactive
process
includes: (i) contacting the operative acid-soluble solid aluminium-comprising
residue
512 with an aqueous acidic solution so as to effect solubilisation of the
operative acid-
soluble solid aluminium-comprising residue and thereby effect production of a
dissolved
residue-derived aluminium-comprising solute dissolved in aqueous acidic
solution, and
(ii) contacting the aqueous acidic solution, in which the dissolved residue-
derived
aluminium-comprising solute is dissolved, with a neutralizing agent so as to
effect
production of at least a fraction of the at least one aluminium-comprising
residue-derived
hydronium ion depletion agent. For example, from step (i), the aqueous acidic
solution in
which the dissolved residue-derived aluminium-compriFing solute is dissolved
includes
- 13 -
CA 02678276 2009-09-09
0.05 to 50 g/L H2SO4, and as a further example includes 0.05 to 10 g/L H2SO4 .
In some
embodiments, the produced at least a fraction of the at least one aluminium-
comprising
residue-derived hydronium ion depletion agent is dissolved in a solution
characterized by
a pH of between 3.3 and 4Ø For example, this pH is between 3.8 and 4Ø In
some
embodiments, the aqueous acidic solution with which the operative acid-soluble
solid
aluminium-comprising residue is contacted is the leachate 212 from the
leaching zone
210.
3. DESCRIPTION OF AN EMBODIMENT
[0039] Referring to Figure 1, in one embodiment, there is provided a
method of
treating a laterite ore 202. The laterite ore 202 includes 0.5 to 1.5% Ni,
0.05 to 0.2% Co,
3 to 8% Al, 1 to 3% Cr, 45 to 55% Fe, 0.1 to 2% Mg, 0.5 to 2% Mn and 1 to 5%
Si.
[0040] The laterite ore 202 is contacted with a leachant 204 in a
leaching zone
210 to effect leaching of the laterite ore. The leachant 204 is concentrated
sulphuric acid
(which becomes diluted during the leaching process by the water in the slurry
of the
metalliferrous material). For example, concentrated sulphuric acid is added to
between 50
and 500 kg of acid per tonne of ore processed in the leaching zone. For
example,
sufficient sulphuric acid is added to the leaching zone to give 20 to 50 g/L
of dissolved
sulphuric acid in the leachate 212 leaving the leaching zone, as measured at
25 C. The
leaching is effected at a temperature of between 240 degrees Celsius and 300
degrees
Celsius, and at a pressure equal to, or great than, the steam pressure of the
solution in the
leaching zone at the temperature of the leaching zone 210. For example, the
leaching is
effected at a temperature of between 250 degrees Celsius and 270 degrees
Celsius, such
as 260 degrees Celsius. The pressure of the leaching zone 210 is dependent on
the
temperature because steam is the primary pressurizing gas. The range of
suitable
operating pressure represents the range of steam pressures across the above-
mentioned
temperature range. For example, when the temperature in the leaching zone is
between
250 degrees Celsius and 270 degrees Celsius, the suitable operating pressure
is between
450 psig and 1300 psig. The leach can be operated at a higher pressure than
the steam
pressure but not below it. The leaching is a continuous operation with a
retention time in
- 14 -
CA 02678276 2016-04-06
the continuous leaching vessel of 30 to 120 minutes. For example, the
retention time is
between 60 and 90 minutes. The leaching effects production of a leach
discharge slurry
214.
[0041] The leach discharge slurry 214 is introduced to the first
neutralization
zone 220. As well, aluminium-comprising materials 512 (which also include iron
and
chromium), being recycled from downstream unit operations (described in
further detail
below), is also introduced to the first neutralization zone 220. Limestone 216
is also
introduced to the first neutralization zone 220. The temperature within the
first
neutralization zone 220 is between 90 degrees Celsius and 95 degrees Celsius.
Contacting is effected between the leach discharge slurry, the recycled
aluminium
comprising materials, and the limestone in the first neutralization zone 220
so as to effect
production of a treated intermediate slurry product 222. Relative to the leach
discharge
slurry, the treated intermediate slurry product 222 is characterized by a
higher pH. The
treated intermediate slurry product 222 includes an acidity of between 0 and
10 g/L
H2SO4 in solution, as measured at 25 C, using a retention time of 30 to 60
minutes. In
this respect, the contacting effects neutralization. As well, the contacting
effects
dissolution of at least a fraction of the metal component fraction of the
recycled
aluminium-comprising material 512 in accordance with the following reactions:
(1) 2 Fe(OH)3 + 3 H2SO4 ---> Fe2(SO4)3 + 6 1-120
(2) 2 A1(OH)3 + 3 H2SO4 Al2(SO4)3 + 6 H20
(3) 2 Cr(OH)3 + 31-12SO4 Cr2(SO4)3 + 6 1-120
[0042] The dissolved aluminium, in the form of Al2(SO4)3, is available
for use in
providing desired conditions for enhancing recoveries of metal and cobalt
values.
[0043] Amongst other things, this unit operation is intended to
neutralize free acid
and produce a slurry where the solids have good solid-liquid separation
properties (the
gypsum solids produced at the higher temperature in this unit operation will
have better
settling properties). As well, by at least partially neutralizing the acid of
the leach
-15-
CA 02678276 2009-09-09
discharge slurry, the cost of downstream unit operations is reduced, as less
expensive
materials may be employed in the construction of such downstream unit
operations owing
to the fact that acidity of the intermediate slurry product is being reduced.
[0044] The treated intermediate slurry product 222 is discharged from the
first
neutralization unit operation 220 and introduced to a counter-current
decantation wash
circuit 230 defining a counter-current decantation wash circuit separation
zone. The
counter-current decantation wash circuit 230 effects separation of dissolved
metal values
from the treated intermediate slurry product 222, thereby leaving a treated
intermediate
slurry product remainder 234 including residual solids. To effect the
separation, a wash
solution is contacted with the treated intermediate slurry product 222 in the
separation
zone so as to effect mass transfer of dissolved material from the treated
intermediate
slurry product 222 to the wash solution and thereby effect production of a
leachate-
derived intermediate operative solution 232. The composition of the leachate-
derived
intermediate operative solution 232 varies widely, depending on the feed
material being
treated, the composition of the wash solution, and the proportion of wash
solution to feed
material. The leachate-derived intermediate operative solution 232 includes
the target
metallic elements and aluminium, and their dissolved sulphate salts. The
contacting is
effected between the wash solution and the leachate-derived intermediate
slurry product
222 while the leachate-derived intermediate slurry product 222 is flowing
though the
separation zone in a direction opposite to that of the flowing of the wash
solution. The
wash solution consists of a recycle from a downstream target metallic element
depleted
solution neutralization unit operation, as will be described in further detail
below. In
addition to effecting separation of dissolved metal values from the leachate-
derived
intermediate slurry product 222, the countercurrent decantation wash circuit
receives
recycled nickel and cobalt materials precipitated in a downstream second
neutralization
process, as will be described in further detail below, and effects
solubilisation of the
recycled nickel and cobalt materials in accordance with the following
reactions:
(4) Ni(OH)2+ H2SO4 ---> NiSO4 +2 H20
(5) Co(OH)2+ H2SO4 ---> CoSO4 +2 H20
- 16-
CA 02678276 2009-09-09
[0045] The leachate-derived intermediate operative solution 232 is
discharged
from the countercurrent decantation wash circuit separation zone 230 and
introduced to a
chromium (VI) reduction unit operation including a chromium (VI) reduction
reaction
zone 235. Depending on the relative amounts present, chromium (VI) can be
toxic and,
in some instances, must be removed to satisfy environmental regulations. Also,
if
chromium (VI) is not removed before the sulphide precipitation unit operation,
elemental
sulphur will be formed in sulphide precipitation which will contaminate the
mixed
sulphide product. The leachate-derived intermediate operative solution 232 is
contacted
with a chromium (VI) reducing agent 236 to effect production of a reducing
agent-treated
intermediate operative solution 238. The contacting is effected under
atmospheric
conditions and at a temperature of between 20 degrees Celsius and 95 degrees
Celsius,
that is, at lower temperature and pressure than in the operative reaction zone
300 of the
sulphide precipitation operation. For example, a suitable temperature for the
contacting
is between 65 degrees Celsius and 85 degrees Celsius. Sufficient chromium (VI)
reducing agent 236 is added to effect substantially complete reduction of
available
dissolved chromium (VI) and at least a fraction of the available dissolved
Fe(III). The
contacting effects reduction of dissolved chromium (VI) to dissolved chromium
(III) (see
reaction (6), set out below) and dissolved Fe(III) to dissolved Fe(II) (see
reaction (7), set
out below). For example, a suitable chromium (VI) reducing agent 236 is an
acid
producing sulphide reagent such as dissolved hydrogen sulphide gas. However,
the
addition rate of the chromium (VI) reducing agent 236 in the chromium (VI)
reduction
reaction zone 235 is preselected such that precipitation of nickel and cobalt
values from
the intermediate operative solution 232 is minimal, so as to have a minimal
impact on the
recoveries of nickel and cobalt values in the downstream sulphide
precipitation unit
operation, which is described in further detail below. That is, sufficient
chromium (VI)
reducing agent 236 is added to account for stoichiometric reaction with
chromium (VI)
and a fraction of the Fe(111) in solution; insufficient chromium (VI) reducing
agent 236 is
added to react with nickel and cobalt in solution.
(6) 2 H2Cr04 + 3 HS + 3 H2SO4 ---> Cr2(SO4)3 + 3 S + 8 H20
(7) Fe2(SO4)3 + H2S ---> 2 FeSO4 + H2SO4 + S
- 17 -
CA 02678276 2009-09-09
[0046] The chromium (VI) reduction unit operation can be removed from the
presently described embodiment, in which case chromium (VI) would be reduced
to
chromium (III) during the below-described sulphide precipitation unit
operation, and
additionally result in production of elemental sulphur which would contaminate
the
mixed sulphide product.
[0047] The reducing agent-treated intermediate operative solution 238 is
discharged from the chromium (VI) reduction unit operation and introduced to a
second
neutralization unit operation including a second neutralization zone 240. The
reducing
agent-treated intermediate operative solution is contacted with limestone 239
to effect
production of an operative slurry 242. Relative to the reducing agent-treated
intermediate
operative solution 238, the operative slurry 242 is characterized by a higher
pH. In this
respect, the contacting effects neutralization. The pH of the operative slurry
242 is
between 3.3 and 4Ø For example, the pH of the operative slurry 242 is
between 3.8 and
4Ø The retention time for this unit operation is between 60 minutes and 120
minutes.
The temperature within the second neutralization zone is between 25 degrees
Celsius and
90 degrees Celsius. For example, a suitable temperature within the second
neutralization
zone is between 65 degrees Celsius and 85 degrees Celsius. Amongst other
things, the
increasing of the pH effects production of hydronium ion depletion agent in
the form of a
polynuclear aluminium compound.
(8) Al2(SO4)3 + CaCO3 + 11 H20 ¨> Al2(OH)2(H20)8(SO4)2 + CO2 + CaSO4=2 H20
[0048] Also, the increasing of the pH effects hydrolysis and
precipitation of
aluminum and impurities, such as Fe(III), Cr(III), and silica in accordance
with the
following reactions:
(9) Fe2(SO4)3 + 6 H20 2 Fe(OH)3 + 3 H2SO4
(10) Al2(SO4)3 + 6 H20 ¨> 2 Al(OH)3 + 3 H2SO4
(11) Cr2(SO4)3 + 6 H20 ¨> 2 Cr(OH) 3 + 3 H2SO4
(12) H2SO4 + CaCO3 + H20 ¨> CaSO4=2 1120+ CO2
- 18 -
CA 02678276 2009-09-09
[0049] The operative slurry 242 is discharged from the second
neutralization unit
operation and introduced to a solid/liquid separation unit operation 250 in
the form of a
thickener. An operative solution 252 is separated from the operative slurry
242 in the
thickener so as to provide a target metallic element depleted slurry remainder
256. A
fraction 258 of the target metallic element depleted slurry remainder 256 is
recycled to
the second neutralization unit operation 240 so as to provide seed particles
for impurities
being precipitated during the second neutralization unit operation 240 and
thereby effect
formation of precipitates of relatively larger particle size. The fraction 258
recycled to
the second neutralization unit 240 also includes unreacted limestone, and
thereby
improves limestone usage efficiencies. The fraction 258 recycled to the second
neutralization unit operation 240 also includes aluminium, and this aluminium
could be
redissolved in the second neutralization unit operation 240 when contacted
with the
acidic reducing agent-treated intermediate operative solution being supplied
to the second
neutralization unit operation 240. The re-dissolution of aluminium of the
recycle fraction
could be effected where the second neutralization unit operation is effected
in a plurality
of tanks which are fluidly coupled in series. In this case, the initial tanks
in the series are
operated at a lower pH than the downstream tanks in the series, thereby
facilitating the re-
dissolution of aluminium of the recycle fraction. Another fraction 259 of the
target
metallic element depleted slurry remainder 256, a post-neutralization
aluminium-
comprising solid residue 259, is recycled to the first neutralization unit
operation 220
and/or the counter-current decantation wash circuit 230 to increase the
concentration of
dissolved aluminium which would be available for enhancing the recovery of
nickel and
cobalt values.
[0050] The operative solution 252 is discharged from the thickener 250
and
introduced to the sulphide precipitation unit operation including the
operative reaction
zone 300. The operative solution 252 is contacted with gaseous hydrogen
sulphide 254
in the operative reaction zone 300 to effect production of a product slurry
including a
mixed metal sulphide intermediate including nickel sulphide and cobalt
sulphide. The
contacting is effected at a temperature of between 80 degrees Celsius and 120
degrees
Celsius, and at a hydrogen sulphide overpressure of between 50 kPa and 750
kPa. For
example, the contacting is effected at a temperature of 105 degrees Celsius,
and at a
- 19-
CA 02678276 2009-09-09
hydrogen sulphide partial pressure of between 100 kPa and 200 kPa. The
retention time
in the continuous sulphide precipitation reaction vessel is between 10 and 60
minutes.
The contacting between the operative solution 252 and the gaseous hydrogen
sulphide
254 effects production of a metal sulphide in accordance with the following
reaction:
(13) M2+ + H2S --> MS +2 1-1 , where M = Ni, Co, Cu, Zn
[0051] The produced hydronium ion reacts with polynuclear aluminium
species
in the operative reaction zone 300, and thereby neutralizing the produced
hydronium ion,
in accordance with the following reaction:
(14) 2 H+ + Al2(OH)2(H20)84+ 10 H20 + 2 Al3+
[0052] The neutralization of at least a fraction of the hydronium ion
produced by
the polynuclear aluminium species effects a shift in equilibrium of the
sulphide
precipitation reaction, thereby leading to higher extents of the precipitation
of metals, and
particularly nickel and cobalt, from the operative solution.
[0053] In some embodiments, using polynuclear aluminium compounds to
neutralize acid during sulphide precipitation minimizes the effect of
fluctuations in the
nickel and cobalt concentration of the feed solution on the recovery of nickel
and cobalt.
Tests indicate that there is a threshold aluminium concentration in solution,
for a given
concentration of nickel and cobalt in the feed solution, where the maximum
nickel and
cobalt recoveries are reached, and that higher aluminium concentrations are
not
deleterious to nickel and cobalt recoveries. Thus, operating at aluminium
concentrations
above this threshold would allow high nickel and cobalt recoveries to be
maintained,
even with significant variation in the concentration of nickel and cobalt in
the feed
solution.
[0054] The operative slurry 302 is discharged from the sulphide
precipitation unit
operation and introduced to a solid/liquid separation unit including a
separation zone 400.
For example, the solid/liquid separation is effected by filtration or
thickening or a
combination of filtration and thickening. The mixed metal sulphide
intermediate 404 is
separated from the operative slurry 302 to effect production of a target
metallic element
- 20 -
CA 02678276 2009-09-09
depleted solution 402. The mixed metal sulphide 404 is further processed or
refined to
recover nickel and cobalt values.
[0055] The
target metallic element depleted solution 402 is discharged from the
separation zone 400 and introduced to the target metallic element depleted
solution
neutralization unit operation 500. The target metallic element depleted
solution 402 is
contacted with limestone 501 to effect neutralization of at least a fraction
of the acid
produced during the sulphide precipitation unit operation and thereby provide
a wash
solution for the countercurrent decantation wash circuit 230 of sufficiently
low acidity to
limit redissolution of iron from the solids in the countercurrent decantation
wash circuit
230. Conditions in the counter-current decantation wash 230 are selected so as
to be
sufficiently acidic in order to redissolve nickel and cobalt values from the
solids in the
slurry recycle to the counter-current decantation circuit 230 from the second
neutralization zone 240 but not so acidic as to effect an undesirable degree
of re-
dissolution of iron and aluminum from the solids from the first neutralization
zone 220
and thereby enable these elements to be bled from the process, should such a
bleed be
required to maintain species, such as dissolved iron and aluminum, in balance.
The
reaction between the target metallic element depleted solution 402 and the
limestone 501
in the target metallic element depleted solution neutralization unit operation
is effected at
a temperature of between 20 degrees Celsius and 95 degrees Celsius, with a
retention
time of between 30 minutes and 60 minutes such that the wash solution includes
0.05 to
grams per litre of free sulphuric acid. In an alternative mode, neutralization
may be
effected to effect production of a residual slurry 502 including a higher pH,
namely a pH
of between 3 and 7, and effect the precipitation of aluminium-comprising
solids. In the
first described mode (not illustrated), a first mode residual slurry
(neutralized target
metallic element depleted solution and small amounts of precipitated gypsum
solids) is
produced, and the first mode residual slurry is recycled as the wash solution
to the
counter-current decantation wash circuit 230. In the second-described mode
(that which
is illustrated in Figure 1), a second mode residual slurry 502 is produced,
and a target
metallic element depleted solution product-derived solid aluminium-comprising
residue
506 is separated in a solid/liquid separator 600 from the second mode residual
slurry 502
to leave a predominantly liquid-comprising product including a residual
operative
-21 -
CA 02678276 2009-09-09
solution 504. The target metallic element depleted solution product-derived
solid
aluminium-comprising residue 506 is recycled to the first neutralization unit
operation
220 so as to re-dissolve the aluminium and thereby make it available for
enhancing cobalt
and nickel recoveries. The predominantly liquid-comprising product 504 is
recycled as
the wash solution to the counter-current decantation wash circuit 230.
4. EXAMPLES
100561 Further embodiments will now be described in further detail with
reference to the following non-limitative examples.
Example No. 1
[00571 A solution was prepared containing the following nominal composition
(g/L): 5.4 Al, 0.4 Co, 0.2 Cr3+, 1.0 Fe3+, 1 Mg, 4.8 Mn, 4.6 Ni and 2.5 H2SO4.
The
solution was neutralized to a series of pH targets at 25 C with the addition
of limestone.
After neutralization, the resulting slurry was filtered to remove the
precipitated solids
from the solution. The following table shows the analyses of the solutions
after
neutralization.
Test Limestone pH Neutralized Solution Analysis, g/L
Added, g/L Ni Co Al Cr Fe Mg Mn
1-N1 0.7 2.41 4.61 0.42 5.40 0.19 0.86 1.11
4.66
1-N2 2.3 2.68 4.51 0.41 5.30 0.17 0.38 1.10
4.59
1-N3 3.4 2.68 4.46 0.41 5.19 0.16 0.35 1.09
4.56
1-N4 5.8 3.22 4.40 0.41 5.14 0.15 0.21 1.10
4.52
1-N5 6.7 3.28 4.56 0.41 5.30 0.18 0.83 1.14
4.64
1-N6 33.3 3.95 4.68 0.43 3.12 0.05 0.02 1.21
4.78
[0058] These solutions were then treated by sulphide precipitation.
Sulphide
precipitation was carried out at 105 C and 220 kPa(g) (i.e., an H2S partial
pressure of 200
kPa) for 30 minutes. Mixed sulphide seed ground to 90% passing 38 tim was
added to
each batch test at a mole ratio of nominally 4.0:1 to the theoretical maximum
nickel and
cobalt sulphide that could be precipitated from solution in each test. The
following table
shows the results from these tests.
- 22 -
CA 02678276 2009-09-09
,
Test Feed Feed Solution, g/L H2SO4, g/L Final Solution, mg/L
Precipitation, %
pH Ni Co Al Theor.1 Final Ni Co Ni Co
1-SP1 2.41 4.61 0.42 5.40 11.4 11.7 51.0 50.0 98.9 88.0
1-SP2 2.68 4.51 0.41 5.30 9.3 11.5 43.0 44.0 99.0 89.3
1-SP3 2.68 4.46 0.41 5.19 9.1 10.8 32.0 31.0 99.3 92.4
1-SP4 3.22 4.40 0.41 5.14 8.3 10.2 29.0 27.0 99.3 93.3
1-SP5 3.28 4.56 0.41 5.30 9.7 5.9 16.0 6.9 99.6 98.3
1-SP6 3.95 4.68 0.43 3.12 8.5 3.2 10.0 3.7 99.8 99.1
1 Stoichiometric acid concentration in barren liquor from precipitation of
nickel and
cobalt and reduction of Fe3+ to Fe2+ and free acid in feed solution.
[0059] A significant increase in nickel and cobalt recovery, and a
significant
decrease in H2SO4 in solution, was observed in sulphide precipitation for the
solutions
that were neutralized to pH 3.28 or greater at 25 C, with the best recoveries
and lowest
acid concentration when the solution feed to sulphide precipitation was
neutralized to pH
3.95.
[0060] At pH 3.95, the neutralizing capacity of the solution was 1.7
g/L of H2SO4
for every 1 g/L of aluminium in solution. The mole ratio of sulphur to
aluminium, after
correcting for sulphur in acid and other metal sulphates in solution, in the
solutions
neutralized to pH 3.95 was approximately 1.0:1, which is significantly lower
than the
theoretical value for Al2(SO4)3 of 1.5:1. Both the neutralizing capacity and
Al:S mole
ratio are consistent with the formation of the Al dimer, Al2(OH)2(H20)84+, as
the
dominant aluminium species in solution (R. Cornelius, J. Caruso, K. Heumann,
and H.
Crews, "Handbook of Elemental Speciation II - Species in the Environment,
Food,
Medicine and Occupation Health", Wiley Interscience (Hoboken, New Jersey),
2003, p.
8). (The theoretical neutralizing capacity for this polynuclear complex is 1.8
g/L H2SO4
for every 1 g/L of Al in solution.)
Example No. 2
[0061] A solution was prepared containing the following nominal
composition
(g/L): 5.4 Al, 0.4 Co, 0.2 Cr3+, 1.0 Fe3+, 1 Mg, 3.0 Mn, 4.6 Ni and 2.5 H2SO4.
The
solution was then neutralized to a series of pH targets at 75 C with the
addition of
limestone. After neutralization, the resulting slurry was filtered to remove
the
- 23 -
CA 02678276 2009-09-09
precipitated solids from the solution. The following table shows the analyses
of the
solutions after neutralization.
Test Limestone pH* Neutralized
Solution Analysis, g/L
Added, g/L Ni Co Al Cr Fe Mg Mn
2-N1 1.8 2.44 4.63 0.45 5.51 0.25 1.01 1.03
3.00
2-N2 2.6 2.68 4.60 0.44 5.48 0.25 0.96 1.02 2.97
2-N3 3.7 3.01 4.75 0.45 5.60 0.23 0.57 1.04
3.03
2-N4 5.8 3.31 4.92 0.47 5.66 0.21 0.28 1.09
3.14
2-N5 9.2 3.63 4.74
0.45 5.46 0.20 0.25 1.04 3.02
2-N6 16.7 3.84 4.86 0.44 5.06 0.20 0.05 1.25 4.94
* Measured at 25 C
[0062] These
solutions were then treated with sulphide precipitation using the
same conditions as described in Example No. 1. The following table shows the
results
from these tests.
Test Feed Feed Solution, g/L H2SO4, g/L Final Solution, mg/L Precipitation,
%
pH Ni Co Al Theor.1 Final Ni Co Ni Co
2-SP1 2.44 4.63 0.45 5.51 11.9 9.7 43.1 32.2 99.1 92.8
2-SP2 2.68 4.60 0.44 5.48 10.6 9.1 36.9 37.4 99.2 91.6
2-SP3 3.01 4.75 0.45 5.60 10.0 9.4 32.8 34.5 99.3 92.3
2-5P4 3.31 4.92 0.47 5.66 9.4 7.0 27.8 39.9 99.4 91.5
2-SP5 3.63 4.74 0.45 5.46 9.0 3.9 45.8 6.5 99.0 98.5
2-SP6 3.84 4.86 0.44 5.06 8.9 0.7 7.4 1.0 99.8 99.8
1 Stoichiometric acid concentration in barren liquor from precipitation of
nickel and
cobalt and reduction of Fe3+ to Fe2+ and free acid in feed solution.
[0063] A
significant increase in metals recovery, particularly for cobalt, and a
significant decrease in H2SO4 in solution, was observed in sulphide
precipitation for the
solutions that are neutralized to pH 3.6 or greater at 75 C, with the best
recoveries and
lowest acid when the solution feed to sulphide precipitation is neutralized to
pH 3.84.
[0064] At pH 3.84,
the neutralizing capacity of the solution was 1.8 g/L of H2SO4
for every 1 g/L of aluminium in solution. The mole ratio of sulphur to
aluminium, after
correcting for sulphur in acid and other metal sulphates in solution, in the
solutions
- 24 -
CA 02678276 2009-09-09
neutralized to pH 3.84 was approximately 1.0:1, which was significantly lower
than the
theoretical value for Al2(SO4)3 of 1.5:1. Both the neutralizing capacity and
Al:S mole
ratio are again consistent with the formation of the Al dimer,
Al2(OH)2(H20)84+, as the
dominant aluminium species in solution (Cornelius, et al, see full cite
identified above).
(The theoretical neutralizing capacity for this polynuclear complex is 1.8 g/L
H2SO4 for
every 1 g/L of Al in solution.)
Example No. 3
[0065] Solutions
were prepared to the same composition as the solutions
described in Example No. 2, except that the aluminium concentration in
solution was
varied between 0 and 10 g/L in the feed solution to neutralization. The
solutions were
then neutralized and filtered using the same procedure described in Example
No. 2. The
following table shows the analyses of the solutions after neutralization.
Test Limestone pH Neutralized Solution Analysis, g/L
Added, g/L Ni Co Al Cr Fe Mg Mn
3-N1 5.1 3.95 4.75 0.44 0.01 0.04 0.02 1.02
3.08
3-N2 26.3 3.91 4.51 0.43 2.92 0.09 0.06 1.10 2.94
3-N3 32.1 3.93 4.34 0.41 9.30 0.18 0.21 1.11
2.83
[0066] These
solutions were then treated with sulphide precipitation using the
same conditions as described in Example No. 1. The following table shows the
results
from these tests.
Test Feed Feed Solution, g/L H2SO4, g/L Final Solution, mg/L Precipitation,
%
pH Ni Co Al Theor.1 Final Ni Co Ni Co
3-SP1 3.95 4.75 0.44 <0.01 8.6 9.0 64.8 9.2 98.6 97.9
3-SP2 3.91 4.51 0.43 2.92 8.3 0.9 17.0 0.7 99.6 99.8
3-SP3 3.93 4.34 0.41 9.30 8.3 pH 3.4 23.8 0.6
99.5 99.9
Stoichiometric acid concentration in barren liquor from precipitation of
nickel and
cobalt and reduction of Fe3+ to Fe2+ and free acid in feed solution.
[0067] The
presence of polynuclear aluminium complexes in the feed solution to
sulphide precipitation caused a significant decrease in the acidity of the
barren liquor
- 25 -
CA 02678276 2009-09-09
(i.e., a decrease of between 8.1 and 9.0 g/L) and increases in nickel and
cobalt
precipitation of up to 1% for Ni and 2% for Co.
[0068] There is a maximum aluminium concentration in solution, above which
there is no additional increase in nickel and cobalt recoveries. These results
indicate,
though, that the addition of aluminium above this maximum aluminium
concentration
does not adversely affect nickel and cobalt recoveries. Effectively, this
means that this
process can be operated with an excess of aluminium in solution prior to raw
liquor
neutralization without any detrimental effects on the recoveries of nickel and
cobalt in
sulphide precipitation. This excess aluminium would help to buffer changes in
metals
recovery caused by changes in the metals concentration in the feed solutions.
Example No. 4
[0069] Solutions were prepared to the same composition as the solutions
described in Example No. 2, but with a range of nickel and cobalt
concentrations at two
levels of aluminium concentration (0 and 5 g/L). The solutions were then
neutralized and
filtered using the same procedure described in Example No. 2. The following
table
shows the analyses of the solutions after neutralization.
Test Limestone pH Neutralized Solution Analysis, g/L
Added, g/L Ni Co Al Cr Fe Mg Mn
4-N1 5.1 3.95 4.75
0.44 <0.01 0.04 0.02 1.02 3.08
4-N2 6.7 3.88 5.07
0.49 <0.01 <0.01 <0.01 1.13 3.28
4-N3 6.7 3.96 5.19
0.57 <0.01 <0.01 <0.01 1.07 2.88
3-N2 26.3 3.91
4.51 0.43 2.92 0.09 0.06 1.10 2.94
4-N5 27.2 3.85
5.68 0.55 3.22 0.12 0.07 1.14 3.02
[0070] These solutions were then treated with sulphide precipitation using
the
same conditions as described in Example No. 1. The following table shows the
results
from these tests.
- 26 -
CA 02678276 2009-09-09
Test Feed Feed Solution, g/L H2SO4, g/L Final Solution, mg/L Precipitation,
%
pH Ni Co Al Theor.I Final Ni Co Ni
Co
4-SP1 3.95 4.75 0.44 <0.01 8.6 9.0 64.8 9.2 98.6 97.9
4-SP2 3.88 5.07 0.49 <0.01 9.0 8.2 120.0 34.0 97.6 93.0
4-SP3 3.96 5.19 0.57 <0.01 9.4 10.5 88.0 73.0 98.3 87.1
3-SP2 3.91 4.51 0.43 2.92 8.3 0.9 17.0 0.7 99.6 99.8
4-SP5 3.85 5.68 0.55 3.22 10.3 2.7 106.0 8.9 98.1 98.4
Stoichiometric acid concentration in barren liquor from precipitation of
nickel and
cobalt and reduction of Fe3+ to Fe2+ and free acid in feed solution.
[0071] The first three tests show that increased nickel and cobalt
concentrations
in the feed solution cause significant decreases in nickel and, particularly,
cobalt recovery
in sulphide precipitation, even with relatively small increases of less than
0.6 g/L Ni+Co
in solution (i.e., increase of 7 to 11% in the feed metals concentration).
[0072] The last two tests show that the addition of aluminium prior to
raw liquor
neutralization allows high nickel and cobalt recoveries to be maintained with
a much
larger increase in the metals concentrations in the feed of 1.30 g/L Ni+Co in
solution
(i.e., an increase of over 25% in the feed metals concentration).
Example No. 5
100731 The following example describes the results of a seven-day
continuous
laterite leach pilot plant campaign. This campaign involved the treatment of
high
aluminium ores through several stages of the described flowsheet for treating
laterite
ores, including pressure acid leaching, slurry neutralization, countercurrent
decantation
(CCD) washing and raw liquor neutralization. The raw liquor neutralization
circuit, in
particular, was operated at 85 C using limestone as the neutralizing agent to
produce a
final slurry of between pH 3.6 and pH 3.9.
[0074] Samples of the thickener overflow solution from this circuit were
taken at
regular intervals throughout the seven days of operation and these samples
were analyzed
to determine the acid neutralizing capacity of these solutions. The acid
neutralization
- 27 -
CA 02678276 2009-09-09
. .
capacity of these solutions was determined by mixing equal volumes of
thickener
overflow solution and 10 g/L sulphuric acid solution and then back titrating
the combined
solution to determine the amount of free acid remaining in solution. The
difference
between the acid added and the acid titrated is reported as the acid
neutralization capacity
of the solution (i.e. grams of H2SO4 per litre of the original solution). The
acid
neutralization capacity for the raw liquor thickener overflow solution samples
is plotted
versus run time in the figure below. Vertical lines on the plot define
separate operating
periods during the pilot plant campaign. Differences in pilot plant operation
during these
separate periods were largely related to changes in the pressure leach feed
composition or
operating conditions.
P1 P2 P3 P4 P5 P6 P7 P8 P9
16 . 8
;
= Neutralizing Capacity
,...) 14 7 1--
-61)
at = Aluminum in Thickener Overflow
.1- 12 _____________________________________ = _____________ 6 .9
0 -
5
v) = = =
o
10 ¨ - -= ¨ ¨ ______________ 5 cip
3
to = = =
>!; =i.
.i-.5 8- --S 9 4' 41 4' e = 4
11 , 40 41
m 10 11 - *=-
.).
f:1 =
0
cc1
s¨,
U 6 = . - = = = ¨ ¨ - --. __________ 3
to - 0
=
= 0 =
= a)
.- .
N =
0
4--= ¨= = t e ¨ __________________
2 -0.-
H
-,-'-' =
t) 2 = ______________ = ___ ¨ _________ ¨ - _____________
..
____________________________________________________________________________
1 7t-'
=
= =
0 1 , r ______________ , 0
0 24 48 72 96 120 144 168
Run Time, h
100751 There was a good correlation between the neutralizing capacity of
the
solution and the aluminium concentration in the thickener overflow solution,
particularly
in the later periods of operation. The ratio of neutralizing capacity to
aluminium in
solution (i.e., g acid neutralized/g aluminium in solution) for Periods 2 to 7
(48 to 144 h
run time) was 1.81:1, which is very close to the theoretical ratio of
neutralizing capacity
-28-
CA 02678276 2016-04-06
4
to aluminium in solution for the simplest polynuclear species,
Al2(OH)2(H20)84+, of
1.82:1.
[0076] These results show that the conditions proposed for raw liquor
neutralization can effectively produce basic aluminium species in solution
(e.g.
polynuclear aluminium hydroxide complexes), which have a much higher capacity
for
neutralizing acid than would be expected from the solution pH, on a large
scale in
continuous operation. At the concentrations of aluminium in solution in these
tests, these
solutions should be able to neutralize most, if not all, of the acid produced
in sulphide
precipitation.
[0077] In the above description, for purposes of explanation,
numerous details are
set forth in order to provide a thorough understanding of the present
disclosure.
Although certain numerical quantities and materials are described for
implementing the
disclosed example embodiments, other suitable numerical quantities and/or
materials may
be used within the scope of this disclosure. The appended claims define
distinctly and in
explicit terms the subject matter of the invention for which an exclusive
privilege or
property is claimed.
- 29 -