Note: Descriptions are shown in the official language in which they were submitted.
CA 02678883 2009-08-19
WO 2008/103789 PCT/US2008/054518
SEAL FOR AN OSTOMY APPLIANCE
FIELD OF THE INVENTION
The present invention relates to the field of ostomy appliances for fitting to
an
ostomate's stoma. One aspect of the invention relates to the formation of a
seal around
the stoma.
BACKGROUND TO THE INVENTION
Creating a seal around a person's stoma, such that the seal is dependable,
comfortable and conducive to body tissue, is important for the function of
ostomy
appliances. Once this seal has been made, the ostomy appliance may use one or
more
of a variety of techniques for managing stomal discharge. The term "ostomy"
includes
colostomy, ileostomy and urostomy. The formation of such a seal remains an
area of
continuous improvement and development, since the performance and comfort of
the
seal is fundamental to customer acceptance. One issue is protection of the
external
peristomal tissue where the normal skin and stoma tissue meet. Peristomal
tissue can
be extremely sensitive. Irritation can result if the peristomal tissue is
exposed to body
waste, or to repeated application and removal of adhesive or other sealants.
Some known devices use a single expandable balloon or member inside the
stoma to form a seal against the inside wall of the opening, and a fixed stop
or surface
against the outside of the body. However, such devices have to be designed
carefully
to avoid the risk of damage to the sensitive internal tissue. In such designs,
a relatively
high concentration of force may result on the tissue underneath the stoma,
especially
when the external surface or stop is of limited conformability.
By way of example, U.S. Patent Publication No. 2003/0220621 describes a
valved ostomy device including a hollow discharge tube and anchoring means for
anchoring the tube in the stoma. The anchoring means comprises an inflatable
balloon
cuff inserted in the stoma to anchor the tube against the stomal wall, and a
screw
threaded clamp as an outer stop surface. Although the screw threaded clamp has
a
conformable pad, the anchoring means bears the entire weight of the ostomy
appliance
and any collection device attached to it. Thus, the strength of the attachment
has to be
1
CA 02678883 2009-08-19
WO 2008/103789 PCT/US2008/054518
offset against limitations on the clamping force which can be applied through
the
peristomal tissue without causing discomfort and tissue damage, and the
inflation
pressure of the balloon cuff without causing internal tissue damage to the
stoma lumen.
EP0168967, EP1346711 and U.S. Patent No. 4,950,223 describe ostomy ports
comprising a single inflatable balloon inserted into the stoma, and an
external adhesive
wafer for securing the appliance to the skin around the stoma. Such designs
are
concerned primarily with the formation of a seal inside the stoma lumen. The
peristomal
tissue is either unprotected or is protected by the adhesive wafer, leaving
the possibility
that the peristomal tissue may be vulnerable to the conventional problem of
irritation
and pain resulting from exposure to stool or repeated applications and
removals of
adhesive.
Inflatable seals are also known from the field of rectal appliances. However,
such designs are not generally applicable to ostomy appliances, because the
anus is
very different physically from a stoma. Nature has engineered the anus to be
naturally
robust to withstand exposure of tissue to stool, and the pressures associated
with
containing stool. In contrast, a stoma is an artificial opening formed by
surgery, and is
much more delicate. Moreover, ostomy appliances often have to carry the long-
term
weight of a collection pouch, whereas rectal devices normally do not have to
support
such long-term weight.
SUMMARY OF THE INVENTION
One aspect of the invention provides an inflatable seal for an ostomy
appliance.
The inflatable seal has one or more of the following features:
(a) a first inflatable chamber portion for sealing against the internal wall
of the
stoma, and a second inflatable chamber portion for sealing against tissue
externally of the stoma. Preferably, the first inflatable chamber portion is
insertable into the interior of the bowel behind the stoma mouth and/or into
the interior of the stoma below skin level. Additionally or alternatively, the
first
and second chamber portions are spaced from one another in a direction
parallel to an axis of the stoma. In one form, if the two chamber portions
communicate with each other, such communication is via an elongate lumen
2
CA 02678883 2009-08-19
WO 2008/103789 PCT/US2008/054518
or passage. Preferably, in the inflated condition, the periphery of the second
chamber portion extends laterally beyond the periphery of the first chamber
portion.
(b) be positioned in the aperture of an adhesive member and includes an
inflatable chamber portion and a support, which provides a backbone for the
inflatable chamber portion that allows the support to float somewhat with
respect to the adhesive member;
(c) an inflatable chamber portion that is located in the aperture of an
adhesive
member, and is configured to seal externally of the stoma, without
substantially occluding the stoma; and/or
(d) a tubular member or passage that extends through the external inflatable
chamber portion for discharge of body waste allowing the discharge of body
waste without removal of the inflatable seal from the stoma.
As used herein, the term "inflatable" means a chamber portion that is
configured
to be expanded by inflating the chamber with a positive inflation pressure
(e.g., a
pressure of inflation fluid greater than the external pressure).
Features and advantages of the invention include providing an ostomy seal that
is comfortable and effective without creating high concentrations of pressure
internally
or externally, and which can produce a comfortable peristomal seal.
Although certain features have been highlighted above and in the appended
claims, the Applicant may seek protection for any novel feature and/or idea
described
and/or illustrated herein whether or not emphasis has been placed thereon.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a schematic sectional view of a first embodiment of the invention in
an
inflated condition.
Fig. 2 is a schematic sectional view of a second embodiment of the invention,
in
an inflated condition.
Fig. 3 is a schematic sectional view of a third embodiment of the invention,
in the
form of a stoma seal, in a deflated condition.
3
CA 02678883 2009-08-19
WO 2008/103789 PCT/US2008/054518
Fig. 4 is a schematic sectional view along the line IV-IV of Fig. 3 showing
only the
elements of the ostomy appliance.
Fig. 5 is a schematic sectional view of the third embodiment in an inflated
condition.
Fig. 6 is a schematic sectional view of a fourth embodiment that is a slight
modification of the third embodiment;
Fig. 7 is a schematic sectional view of a fifth embodiment that is a further
slight
modification of the third embodiment;
Fig. 8 is a schematic front perspective view of a sixth embodiment in an
inflated
condition;
Fig. 9 is a schematic rear perspective view of the sixth embodiment;
Fig. 10 is an enlarged cut-through perspective view of the sixth embodiment,
similar to Fig. 8; and
Fig. 11 is a schematic sectional view through the sixth embodiment.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
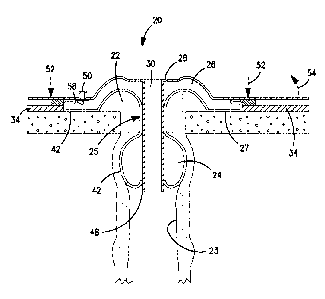
Referring to Figs. 1-5 in which an ostomy appliance 20 includes a seal for
sealing
around a stoma 22. The seal comprises a first inflatable chamber portion 24
for
insertion into the stoma 22 for sealing against the internal wall of the stoma
lumen 23,
and a second inflatable chamber portion 26 for sealing against the external
tissue
around the stoma 22 and/or against the surrounding peristomal tissue 27. In
the first
embodiment (Fig. 1), the seal substantially occludes the stoma 22 in order to
block
discharge of body waste. In the second embodiment (Fig. 2), the seal does not
occlude
the stoma 22, but provides a seal around the periphery of the stoma 22 for
sealing
between the ostomy appliance 20 and the stoma 22. The ostomy appliance 20 of
the
second embodiment permits the discharge of body waste without removal of the
seal.
The use of first and second inflatable chamber portions 24, 26 internally and
externally provides one or more of the following advantages:
(a) The first and second inflatable chamber portions 24, 26 provide a more
conformable fit both internally and externally of the stoma 22, enabling
sealing contact to be maintained at a lower sealing pressure than in some
4
CA 02678883 2009-08-19
WO 2008/103789 PCT/US2008/054518
prior designs and the seal contact to be spread over a larger area of body
tissue, both internal and external, thereby avoiding concentrated pressure
points on the tissue.
(b) The first and second chamber portions 24, 26 can adapt easily to different
sizes and shapes of stoma, including stomas with shape irregularities, and
recessed stomas, providing a highly versatile seal, both internally and
externally.
(c) The second chamber portion 26 can provide non-adhesive, closely fitting
protection for the sensitive peristomal tissue, thereby preventing exposure to
body waste, and avoiding pain and irritation in the peristomal tissue
sometimes associated with purely adhesive seals. If desired, in some
applications, the second chamber portion 26 may carry a weak adhesive, or
other sealant, to provide secondary seal characteristics.
(d) In addition to providing a seal, the first and second inflatable chamber
portions 24, 26 cushion the stoma 22 both internally and externally, resulting
in an eminently comfortable fit and providing a degree of protection against
external physical knocks or rubbing, which can sometimes be painful on the
stoma.
(e) The conformability of the first and second inflatable chamber portions 24,
26
can reduce limitations on the wearer's mobility, and can also reduce the
effects of movement on the seal itself by conforming to the body's
dynamically moving contours.
A narrow neck or waist 25 is defined between the first and second chamber
portions 24, 26. In use, the waist 25 is positioned generally at the stoma 22,
such that
inflation of the first and second chamber portions 24, 26 sandwiches the stoma
22 on
both sides.
The first and second chamber portions 24, 26 may be of approximately the same
shape and/or size, or one chamber portion may be larger than the other and/or
have a
different shape. The first chamber portion 24 has a shape configured for
sealing
against the internal tissue of the stoma 22 and/or against the stoma lumen 23.
For
5
CA 02678883 2009-08-19
WO 2008/103789 PCT/US2008/054518
example, the first chamber portion 24 may have a generally rounded, or
bulbous, or
elongated, or doughnut or other tubular or non-tubular shape. The second
chamber
portion 26 has a shape configured for sealing against the external tissue of
the stoma
22, and/or against the surrounding peristomal tissue 27. For example, the
second
chamber portion 26 may have a flared, or trumpet, or skirt or umbrella,
tubular or non-
tubular shape. The second chamber portion 26 has a larger extremity than the
first
chamber portion 24, such that the second chamber portion 26 extends further
outwards
radially than the first chamber portion 24.
The first and second chamber portions 24, 26 may be inflatable in common. For
example, the first and second chamber portions 24, 26 may be interconnected by
one or
more communication channels, lumens or conduits, and a common inflation point
may
be used for inflating both chambers together. The pressure in the first and
second
chamber portions 24, 26 is balanced to thereby avoid high pressure
concentration on
only one side of the stoma 22. Depending on the desired design parameters, the
communication channels may permit inflation fluid to move between the first
and second
chamber portions 24, 26 while the ostomy appliance 20 is being worn, to
accommodate
extreme body motion, and to maintain a balanced pressure in each chamber
portion.
Alternatively, the first and second chamber portions 24, 26 may be inflatable
independently of each other. For example, the first and second chamber
portions 24,
26 may be independent chambers, each with its own inflation point. This allows
each
chamber to be inflated to a different inflation pressure and/or prevent
transfer of inflation
fluid between chambers. A further alternative may be a hybrid of the above,
using one
or more communication channels or conduits between the first and second
chamber
portions 24, 26 to allow each chamber portion 24, 26 to be inflated from a
common
inflation point, but additionally including one or more inflation control
valves. The valves
can regulate the inflation pressure in each chamber portion 24, 26 and/or
prevent
deflation of each chamber portion 24, 26, to provide one or both chamber
portions 24,
26 with a degree of independence.
Any suitable inflation means may be used to inflate the first and second
chamber
portions 24, 26. For example, a pump (such as a bellows pump) (not shown) may
be
6
CA 02678883 2009-08-19
WO 2008/103789 PCT/US2008/054518
integrated into the ostomy appliance 20. Alternatively, one or more inflation
ports may
be provided to allow an external pump (such as a syringe, an electric pump, or
an oral
inflation tube) to be connected. Any suitable inflation fluid may be used, for
example, a
gas (such as air), or a liquid (such as saline).
When the ostomy appliance 20 is to be removed, one or both of the inflation
chamber portions 24, 26 is vented to discharge the inflation fluid. For
example, the
chamber portion(s) 24, 26 may be ruptured, or the inflation port opened, or a
dedicated
vent port opened. In one form, a tear-open seal may be torn open to vent the
inflation
fluid.
The ostomy appliance 20 may further comprise an adhesive wafer 34, having a
skin-friendly adhesive for attachment to the skin. The adhesive wafer 34 may
have an
aperture 36 in which the seal is received. The aperture 36 may be roughly the
same
size as, or slightly larger than, the size of the second inflatable chamber
portion 26. The
aperture 36 is sufficiently large that it does not directly contact the stoma
22. The
adhesive wafer 34 may support substantially the weight of the ostomy appliance
20
(and any ostomy device attached to the ostomy appliance 20) through the
adhesive
attachment to the skin, such that the seal itself is substantially independent
of the
weight.
In the first embodiment (Fig. 1), at least one of the inflation chamber
portions 24,
26 completely occludes the stoma 22, in order to block the escape of body
waste when
inflated.
In the second embodiment (Fig. 2), the first and second chamber portions 24,
26
are a closed loop or "cuff' shape, for example, annular, doughnut, or tubular,
with an
aperture 36 for allowing body waste to be discharged via a communication or
discharge
passage 30 therethrough.
The second embodiment may optionally further comprise a support 28 for
supporting the first and/or second chamber portions 24, 26. The support 28 may
be of
any suitable shape for supporting the first and second chamber portions 24, 26
in use.
For example, the support 28 may include, or be formed as, a stem acting as a
backbone
for the first and second chamber portions 24, 26. The stem may pass generally
7
CA 02678883 2009-08-19
WO 2008/103789 PCT/US2008/054518
centrally through the first and second chamber portions 24, 26. The stem may
be at
least partly deformable, for example, resilient, so as to avoid hard edges
inside the
stoma 22. The stem may be of a material that is generally stiffer than the
flexible
sealing walls of the first and second chamber portions 24, 26, or may inflate
itself,
becoming more structurally rigid upon inflation of the device, or may be
constructed of
the same material as the first and second chambers 24 and 26. The first and
second
chamber portions 24, 26 may meet, or be connected to or at the stem.
The stem may aid insertion of the first chamber portion 24, by providing a
relatively self-supporting guide which can be inserted into the stoma 22, or
another
appliance may aid the insertion of the stem and first chamber portion 24. The
stem may
be tubular to define the communication passage 30 in the stoma 22. For
example, the
communication passage 30 may allow the discharge of body waste from the stoma
22,
through the inflatable chamber portions 24, 26. In that case, the inflatable
chamber
portions 24, 26 serve to form an effective conformable and comfortable seal
between
the ostomy appliance 20 and the stoma 22, but not to contain body waste. An
additional and/or external device (not shown), such as a collection pouch, or
a
removable plug or seal, is provided for managing the discharge of body waste
through
the communication passage 30. The support 28 may float somewhat with respect
to the
adhesive wafer 34 to allow the inflatable seal to self-locate within the stoma
22.
Referring to Figs. 3-5, a third embodiment of the ostomy appliance 20 is
described for sealing around a stoma opening 22 in a person's abdominal wall
32. The
ostomy appliance 20 comprises an adhesive wafer 34 of a skin-friendly
adhesive, and
having an aperture 36 therein which is larger than the size of the stoma 22.
By using a
larger aperture, the adhesive does not adhere to the sensitive external tissue
of the
stoma 22 or the peristomal tissue 27, and the ostomate avoids associated pain
in the
sensitive external tissue of the stoma 22 or the peristomal tissue 27. A
flange 38, for
example, of plastics, is secured to the adhesive wafer 34, for example, by
heat welding,
or adhesive. The flange 38 supports a flexible annular rear wall 40 which in
turn is
coupled to a generally flat portion (base) 28b of a T-shape hollow support 28,
or is
coupled to the edge of a tubular-shaped hollow support 28 that has no flat
portion 28b.
8
CA 02678883 2009-08-19
WO 2008/103789 PCT/US2008/054518
The support 28 also includes a hollow stem 28a depending from the base 28b, or
may
comprise of the stem 28a itself. Also attached to the flange 38 is a flexible
membrane
42. The flexible membrane 42 may be striated near a middle region 44 of the
stem 28a,
(e.g., attached to the stem 28a along a plurality of spaced, parallel axial
bonds, to define
a corrugated shape shown in Fig. 4, with parallel narrow connecting pathways
46
between the stem 28a and the flexible membrane 42). In Fig. 4, the size of the
connecting pathways 46 are exaggerated, for the sake of schematic clarity. The
connecting pathways 46 not only act as a fluidic connection between the first
and
second chambers 24, 26, but by varying the size and shape of the corrugations
may
also act as: (i) a cushion against the inner wall of the stoma 22; (ii) a
friction reducer
between the stem 28a and the inner wall of the stoma 22; (iii) flow
restrictors between
the first and second chambers 24, 26 by providing a damping effect to the
volumetric
changes between the chambers 24, 26; (iv) structurally inflatable columns that
together
act as the stem 28a, replacing it in the embodiment where the stem 28a is
inflatable; (v)
pathways for flatus to more easily escape between the corrugations and the
inner wall
of the stoma 22 in the embodiment where the stoma opening is normally closed
and
flatus escapes around the outside of the first and second chambers 24, 26. The
flexible
membrane 42 may further be attached to the stem 28a near its distal end 48.
The combination of the stem 28a (from the middle region 44 to the distal end
48)
and the flexible membrane 42 (extending from the distal end 48 to the middle
region 44
of the stem 28a) define the first inflatable chamber portion 24 (Fig. 5). The
combination
of the flange 38, the rear flexible or resilient wall 40, the base 28b, the
stem 28a (down
to the middle region 44), and the flexible membrane 42 defines the second
inflatable
chamber portion 26 (Fig. 5). The first and second chamber portions 24, 26 may
communicate by means of the connecting pathways 46. The flexible or resilient
rear
wall 40 may be of thicker material than the flexible membrane 42 since the
flexible
membrane 42 may be desired to be more conformable to the shape of the stoma
22.
Use of a thicker material for the flexible or resilient rear wall 40 can
provide increased
strength and protection against puncture in this externally facing wall. The
flexible or
resilient rear wall 40 may also be made of an elastic material or contain
within it elastic
9
CA 02678883 2009-08-19
WO 2008/103789 PCT/US2008/054518
material that once the device is inflated may be stretched to provide a gentle
pressure
and/or volumetric reserve which may assist with keeping the first and second
chambers
24, 26 at a set pressure and/or provide an elastic rebound, and to more easily
allow
volumetric changes to occur between the chambers 24, 26 with embodiments where
the
chambers 24, 26 communicate. If the flexible or resilient rear wall 40 is made
of an
elastic material it may also enable the ostomy appliance 20 to accommodate
very large
stomas 22 by allowing a relatively high profile between the peristomal tissue
27 and the
top of the stoma 22. The second chamber portion 26 may have a generally
trumpet or
umbrella type shape, when inflated, with a generally low profile externally
facing
surface. Bulging of the rear surface may be reduced by the presence of the
base 28b of
the support 28. This can provide a compact ostomy appliance 20, with a
generally
lower profile and more extensive fit than, for example, a simple doughnut
shape,
although the invention encompasses any shape of second chamber 26.
In use, the first and second chamber portions 24, 26 are initially deflated.
The
user presents the distal end portion 48 of the stem 28a to the stoma 22, and
inserts the
stem 28a until the adhesive wafer 34 presses against the skin (Fig. 3).
Thereafter, the
first and second chamber portions 24, 26 may be inflated (Fig. 5) by
connecting an
inflation source (not shown) to an inflation port 50 formed in the flange 38.
The inflation
port 50 may include a non-return valve 58, such as a flap valve, for
preventing deflation
through the inflation port 50. The inflation port 50 may communicate directly
with the
second inflatable chamber portion 26, and indirectly with the first inflatable
chamber
portion 24 via the connection pathways 46.
As can be seen in Fig. 5, the first and second chamber portions 24, 26 swell
to
form a conformable, large area seal against the external tissue of the stoma
22 and the
peristomal tissue 27. This can achieve a reliable, conformable and comfortable
seal
and protect the peristomal tissue 27, without substantially restricting the
mobility of the
ostomate, and with less risk of the seal being affected by the wearer's
movements. In
the inflated condition, the support 28 floats somewhat with respect to the
adhesive
wafer 34, such that the waist 25 between the first and second chamber portions
24, 26
CA 02678883 2009-08-19
WO 2008/103789 PCT/US2008/054518
may self-locate at the stomal opening. Such floating and self-positioning
further
improves the seal performance, and reduce the effect of external forces on the
seal.
In the embodiment shown, the ostomy appliance 20 does not necessarily include
any device for managing the discharge of stool through the communication
passage 30.
The ostomy appliance 20 may, for example, be a mounting flange for a one-
piece, or
two-piece, ostomy device (not shown) such as a collection pouch or a
controlled
evacuation device. Such a device may be permanently or releasably attached to
the
flange 38, for example, at the position indicated at 52. With such a
construction, the
weight of, or the mounting force for supporting, the ostomy device may be
borne
substantially by the adhesive wafer 34. In the present embodiment, the
principal
function of the first and second inflatable chambers 24, 26 is to provide a
seal around
the stomal aperture 22, between the stomal aperture 22 and the ostomy
appliance 20,
rather than (i) providing a stoma occluding seal for blocking the discharge of
stool from
the stoma 22, or (ii) providing mechanical support for the ostomy device
attached to the
ostomy appliance 20. The ostomy appliance 20 may, for example, be a multi-use
device, intended to remain in position at the stoma 22 for some time to
provide a re-
usable fixing point for one or more disposable devices such as disposable
collection
pouches. To this end, the ostomy appliance 20 may further comprise any of (not
shown): a mechanical coupling profile; an adhesive bearing flange; and a
landing flange
for engagement by a complementary adhesive bearing flange on the ostomy
device.
When it is desired to remove the ostomy appliance 20, the user deflates the
first
and second chamber portions 24, 26 in any of a number of different ways. For
example, the user may puncture one or both of the first and second chamber
portions
24, 26, or manipulate the inflation port 50 to vent the inflation fluid.
Alternatively, the
user may grip the flange 38 at the position 54, and tear the flange 38 itself,
or separate
the flange 38 from the adhesive wafer 34 to vent the second inflatable chamber
portion
26 directly. The stem 28a is then withdrawn from the stoma 22, and the
adhesive wafer
34 separated from the skin.
Referring to Fig. 6, a fourth embodiment is illustrated similar to the third
embodiment, except that a deodorizing filter 60 is provided for deodorizing
vented
11
CA 02678883 2009-08-19
WO 2008/103789 PCT/US2008/054518
flatus. This is especially, but not exclusively, useful where the stoma
opening is
normally closed (e.g., by a closure seal 64) and flatus escapes around the
outside of the
first and second chambers 24, 26 and through the filter 60 as shown by arrows
62 in
Fig. 6. This filter 60 or an exhaust passageway may be present in the other
embodiments to allow air to escape from the ostomy appliance 20 during
inflation.
Referring to Fig. 7, a fifth embodiment is illustrated similar to the third
embodiment. The main difference is that the flexible membrane 42 has
sufficient
material within the rectal chamber so, when inflated, the first chamber 24
forms a
closure in front of the distal end 48 of the stem 28a as shown in Fig. 7. This
closure
may be opened to discharge waste by partially deflating and/or reducing the
pressure
within the ostomy appliance 20 but retain enough to maintain the ostomy
appliance 20
in place. This embodiment may also be combined with the exhaust passageway
with or
without the deodorizing filter of Fig. 6.
Referring to Figs. 8-11, a sixth embodiment of ostomy appliance 20 comprises
an
integral component 68 comprising a faceplate 70 having a front surface 72 and
a rear
surface 74, and an aperture 76 for passing stomal waste; a stem 78 projecting
rearwardly from around the aperture 76; a first inflatable chamber 24 disposed
near or
at the distal end of the stem 78, and a second inflatable chamber 26 disposed
on the
rear surface 74. The faceplate 70 is optionally relatively rigid compared to
one or more
of the other materials defining the chambers 24 and 26 and the stem 78, to
provide a
support for at least the second chamber 26, and/or to reduce the external
profile height
of the component when in use. The stem 78 can be hollow and communicate with
the
aperture 76 of the faceplate, to provide a discharge passage for stomal waste
without
having to remove the component 68 from the stoma 22.
In the illustrated embodiment, the integral component 68 is made from two
pieces, namely the faceplate 70, and a second piece 80 (Figs. 10 and 11)
attached to
the faceplate 70. The faceplate 70 is, in this example, substantially planar
and may be
cut or stamped from flat stock. The second piece 80 is optionally molded from
plastics
material that is more flexible than the faceplate 70. The second piece 80
includes an
annular shell portion 80a that, together with the faceplate 70 defines the
second
12
CA 02678883 2009-08-19
WO 2008/103789 PCT/US2008/054518
chamber 26. The shell portion 80a is attached to the faceplate 70 at positions
81 a and
81 b to define the chamber 26. The attachment may be any form of welding or
bonding.
The second piece 80 further includes the stem 78 and the first chamber 24. A
first end
of the stem 78 adjacent to the first chamber 24 is flared. A second end of the
stem 78
adjacent to the faceplate 70 may also be flared; the degree of flaring at the
second end
may be smaller than at the first end. In the present embodiment, the second
piece 80 is
resiliently flexible. The tubular and/or flared shape of the stem 78 provides
gentle
elastic support for the first chamber 24 with respect to the faceplate 70. The
elastic
rebound of the stem 78 generally prevents the first chamber 24 from turning
within the
bowel, and maintains the first chamber against the bowel wall with gentle
elastic force.
In the present form, the stem 78 is not itself inflatable, but the stem 78
could be wholly
or partly inflatable as in the preceding embodiments, if desired. The stem 78
may be
resiliently expandable upon passage of bulk stomal waste therethrough.
The first and second chambers 24, 26 communicate via a lumen or conduit 82,
and are inflatable via a common inflation point 50 formed, for example, on the
faceplate
70. The conduit 82 may be integral with the stem 78, or it may be separate
tube that
passes through the wall material to communicate with the first and second
chambers
24, 26. Alternatively, the first and second chambers 24, 26may be inflatable
independently of each other. In such case, as illustrated in phantom in Fig.
10, the
conduit 82 may extend from the first chamber 24 to a second inflation port 84
instead of
coupling the first and second chambers 24, 26 together.
The material characteristics for the faceplate 70 and the second piece 80 may
be
chosen to provide the desired strengths and elasticity for each component. In
one form,
the faceplate 70 and the second piece 80 are made from the same material and
same
material thickness (in a case where the faceplate 70 is not substantially more
rigid than
the second piece 80). In another form, the faceplate 70 is made of the same
material
as the second piece 80, but with a different durometer and/or thickness. In
another
form, the faceplate 70 is substantially rigid.
A preferred material for the second piece 80 is silicone rubber. Example
characteristics include approximately 0.012 inches thick and/or approximately
35 shore
13
CA 02678883 2009-08-19
WO 2008/103789 PCT/US2008/054518
A durometer. Such material may be injection molded and is reasonably elastic.
An
alternative material is urethane film. An example thickness is approximately
0.012
inches thick. Such a material may be dip molded, and is less elastic than the
silicone
rubber example.
A preferred material for the faceplate 70 is silicone rubber. Example
characteristics include (i) approximately 0.012 inches thick and/or
approximately 80
shore A durometer; (ii) approximately 0.25 inches thick and/or approximately
35 shore A
durometer. An alternative material is polycarbonate, for example, with a
thickness of
approximately 0.062 inches.
Similar materials may also be used for the other embodiments.
In a slightly modified form, the annular shell portion 80a is made from a
different
piece of material from the stem 78 and the first chamber 24. In other words,
the
component 68 comprises three pieces joined together, namely: the faceplate 70,
the
annular shell portion 80a, and a third piece defining the stem 78 and the
first chamber
24. The annular shell portion 80a can be made of the same material as the
third piece,
such as silicone rubber or urethane film. If desired, the material thickness
and/or
durometer may be varied to give different characteristics between the first
and second
chambers 24 and 26.
In use, the integral component 68 may find different uses as a stoma seal
usable
in a controlled evacuation stoma port. If desired, a secondary seal may be
made by an
additional component against the front face 72 of the faceplate 70. The
component 68
may also find use as a comfortable seal and/or stoma extender for a non-
continent
ostomy appliance, such as a collection pouch. The collection pouch may form a
direct
or indirect fit against the faceplate, for example, using an adhesive
coupling.
The faceplate 70 may be received in an aperture of an adhesive pad or other
ostomy appliance component, in a similar manner to the preceding embodiments.
Such
attachment can anchor or restrain movement of the component 68. In one form,
the
faceplate 70 is attachable to another ostomy appliance component by a snap-
fit.
Modifications may be made to these preferred embodiments and still remain
within the scope of the claimed invention.
14