Note: Descriptions are shown in the official language in which they were submitted.
CA 02681384 2009-09-18
WO 2008/115672 PCT/US2008/055102
METHOD FOR TREATING CB2 RECEPTOR MEDIATED PAIN
FIELD OF THE INVENTION
This invention is directed to a method for treating, ameliorating or
preventing
CB2 receptor mediated pain in a subject in need thereof. More particularly,
said
method comprises administering to the subject an effective amount of a
hexahydro-
cyclooctapyrazole CB2 agonist compound of the present invention.
BACKGROUND OF THE INVENTION
PCT Application W02006/030124 describes pyrazole derivatives as CB1 or
CB2 receptor agonists.
CB2-selective agonists have been shown to be effective in the carrageenan paw
model of inflammatory pain and therefore may be effective in the treatment of
acute
and chronic inflammatory pain (Gutierrez T, Farthing JN, Zvonok AM,
Makriyannis A
and Hohmann AG, Activation of peripheral cannabinoid CB 1 and CB2 receptors
suppresses the maintenance of inflammatory nociception: A comparative
analysis,
British Journal of Pharinacology, (2007), 150(2), 153-163; Quartilho A, Mata
HP,
Ibrahim MM, Vanderah TW, Porreca F, Makriyannis A and Malan TP, Jr.,
Inhibition of
Inflammatory Hyperalgesia by Activation of Peripheral CB2 Cannabinoid
Receptors,
Anesthesiology, (2003), 99(4), 955-960; and, Nackley AG, Makriyannis A and
Hohmann AG, Selective activation of cannabinoid CB2 receptors suppresses
spinal Fos
protein expression and pain behavior in a rat model of inflammation,
Neuroscience
(Oxford, United Kingdom) (2003), 119(3), 747-757).
CB2-selective agonists have also been shown to be effective inhibitors of
thermal nociception in transgenic mice and potentially useful for the
treatment of acute
pain (Ibrahim MM, Rude ML, Stagg NJ, Mata HP, Lai J, Vanderah TW, Porreca F,
Buckley NE, Makriyannis A and Malan TP, Jr., CB2 cannabinoid receptor
mediation of
antinociception, Pain, (2006), 122(1-2), 36-42).
Activation of the CB2 receptor produces antinociception following surgical
incision, suggesting that selective cannabinoid CB2 receptor agonists might be
useful in
the management of postoperative pain (LaBuda CJ, Koblish M and Little PJ,
Cannabinoid CB2 receptor agonist activity in the hindpaw incision, European
Journal
ofPharinacology, (2005), 527(1-3), 172-174).
1
CA 02681384 2009-09-18
WO 2008/115672 PCT/US2008/055102
Activation of peripheral cannabinoid CB2 receptors are sufficient to normalize
nociceptive thresholds and produce antinociception in persistent pain states
(Hohmann
AG, Farthing JN, Zvonok AM and Makriyannis A, Selective activation of
cannabinoid
CB2 receptors suppresses hyperalgesia evoked by intradermal capsaicin, Journal
of
Pharmacology and Experimental Therapeutics, (2004), 308(2), 446-453).
Selective CB2 receptor agonists inhibit acute, chronic, inflammatory and
neuropathic pain responses in animal models and, therefore, show promise for
the
treatment of acute and chronic pain (Malan TP, Jr., Ibrahim MM, Lai J,
Vanderah TW,
Makriyannis A and Porreca F, CB2 cannabinoid receptor agonists: pain relief
without
psychoactive effects?, Current Opinion in Pharmacology, (2003), 3(1), 62-67;
Ibrahim
MM, Deng H, Zvonok A, Cockayne DA, Kwan J, Mata HP, Vanderah TW, Lai J,
Porreca F, Makriyannis A and Malan TP, Jr., Activation of CB2 cannabinoid
receptors
by AM 1241 inhibits experimental neuropathic pain: Pain inhibition by
receptors not
present in the CNS, Proceedings of the National Academy of Sciences of the
United
States of America, (2003), 100(18), 10529-10533; and, Burns TL and Ineck JR,
Cannabinoid analgesia as a potential new therapeutic option in the treatment
of chronic
pain, Annals of Pharnacotherapy, (2006), 40(2), 251-260).
The CB2 receptor-selective agonist AM1241 produces antinociception to
thermal stimuli (Malan TP, Jr., Ibrahim MM, Deng H, Liu Q, Mata HP, Vanderah
T,
Porreca F and Makriyannis A, CB2 cannabinoid receptor-mediated peripheral
antinociception, Pain, (2001), 93(3), 239-245).
DETAILED DESCRIPTION OF THE INVENTION
The present invention is directed to a method for treating, ameliorating or
preventing CB2 receptor mediated pain in a subject in need thereof comprising
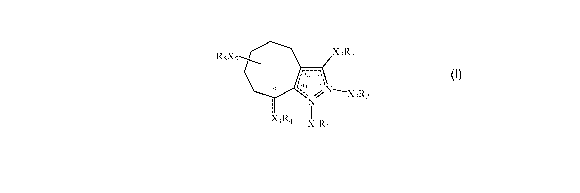
administering to the subject an effective amount of a compound of formula (I):
R5X5 X3R3
3a 3 9 9a 2'
N
jN~X2R2
~
X4K4 X1R1
2
CA 02681384 2009-09-18
WO 2008/115672 PCT/US2008/055102
or a salt, isomer, prodrug, metabolite or polymorph thereof wherein
the dashed lines between positions 2-3 and positions 3a-9a in formula (I)
represent
locations for each of two double bonds present when XiRi is present;
the dashed lines between positions 3-3a and positions 9a-1 in formula (I)
represent
locations for each of two double bonds present when X2R2 is present;
the dashed line between position 9 and X4R4 in formula (I) represents the
location for a
double bond;
Xi is absent or lower alkylene;
X2 is absent or lower alkylene;
wherein only one of XiRi and XzRz are present;
X3 is absent, lower alkylene, lower alkylidene or -NH-;
when the dashed line between position 9 and X4R4 is absent, X4 is absent or is
lower
alkylene;
when the dashed line between position 9 and X4R4 is present, X4 is absent;
X5 is absent or lower alkylene;
Ri is selected from hydrogen, allcyl(optionally substituted at one or more
positions by
halogen, hydroxy or lower alkoxy), lower alkyl-sulfonyl, aryl, C3-C12
cycloalkyl
or heterocyclyl, wherein aryl, C3-CI2 cycloalkyl or heterocyclyl are each
optionally substituted at one or more positions by halogen, aminosulfonyl,
lower alkyl-aminosulfonyl, alkyl (optionally substituted at one or more
positions by halogen, hydroxy or lower alkoxy), hydroxy or alkoxy (optionally
substituted at one or more positions by halogen or hydroxy);
R2 is selected from hydrogen, alkyl (optionally substituted at one or more
positions by
halogen, hydroxy or lower alkoxy), lower alkyl-sulfonyl, aryl, C3-Ci2
cycloalkyl
or heterocyclyl, wherein aryl, C3-CI2 cycloalkyl or heterocyclyl are each
optionally substituted at one or more positions by halogen, aminosulfonyl,
lower alkyl-aminosulfonyl, alkyl (optionally substituted at one or more
3
CA 02681384 2009-09-18
WO 2008/115672 PCT/US2008/055102
positions by halogen, hydroxy or lower alkoxy), hydroxy or alkoxy (optionally
substituted at one or more positions by halogen or hydroxy);
R3 is -C(0)-Zi(R6), -S02-NR7-Z2(R8) or -C(O)-NR9-Z3(Rio);
when the dashed line between position 9 and X4R4 is absent, X4 is absent or
lower
alkylene and R4 is hydrogen, hydroxy, lower alkyl, lower alkoxy, halogen,
aryl,
C3-C12 cycloalkyl or heterocyclyl, wherein aryl, C3-C12 cycloalkyl or
heterocyclyl are each optionally substituted at one or more positions by
hydroxy, oxo, lower alkyl (optionally substituted at one or more positions by
halogen, hydroxy or lower alkoxy), lower alkoxy (optionally substituted at one
or more positions by halogen or hydroxy) or halogen;
when the dashed line between position 9 and X4R4 is present, X4 is absent and
R4 is
CH-aryl or CH-heterocyclyl, wherein aryl or heterocyclyl are each optionally
substituted at one or more positions by hydroxy, oxo, lower alkyl, lower
alkoxy
or halogen;
R5 is absent, hydroxy, halogen, amino, aminoalkyl, alkyl (optionally
substituted at one
or more positions by halogen, hydroxy or lower alkoxy), alkoxy (optionally
substituted at one or more positions by halogen or hydroxy), carboxy,
carbonylalkoxy, carbamoyl, carbamoylalkyl, aryl, aryloxy, arylalkoxy or
heterocyclyl;
R6 is aryl, C3-Ci2 cycloalkyl or heterocyclyl, wherein aryl, C3-Ci2 cycloalkyl
or
heterocyclyl are each optionally substituted by one or more hydroxy, oxo,
halogen, amino, aminoalkyl, alkyl (optionally substituted at one or more
positions by halogen, hydroxy or lower alkoxy), alkoxy (optionally substituted
at one or more positions by halogen or hydroxy), carboxy, carbonylalkoxy,
carbamoyl, carbamoylalkyl, aryl, aryloxy, arylalkoxy or heterocyclyl;
R7 is hydrogen or lower alkyl;
R8 is aryl, C3-CI2 cycloalkyl or heterocyclyl, wherein aryl, C3-CI2 cycloalkyl
or
heterocyclyl are each optionally substituted by one or more hydroxy, oxo,
halogen, amino, aminoalkyl, alkyl (optionally substituted at one or more
positions by halogen, hydroxy or lower alkoxy), alkoxy (optionally substituted
4
CA 02681384 2009-09-18
WO 2008/115672 PCT/US2008/055102
at one or more positions by halogen or hydroxy), carboxy, carbonylalkoxy,
carbamoyl, carbamoylalkyl, aryl, aryloxy, arylalkoxy or heterocyclyl;
R9 is hydrogen or lower a1ky1;
Rio is aryl, C3-C12 cycloalkyl or heterocyclyl, wherein aryl, C3-C12
cycloalkyl or
heterocyclyl are each optionally substituted by one or more hydroxy, oxo,
halogen, amino, aminoalkyl, a1ky1(optionally substituted at one or more
positions by halogen, hydroxy or lower alkoxy), alkoxy (optionally substituted
at one or more positions by halogen or hydroxy), carboxy, carbonylalkoxy,
carbamoyl, carbamoylalkyl, aminosulfonyl, lower alkyl-aminosulfonyl, aryl,
aryloxy, arylalkoxy or heterocyclyl;
Zi and Z2 are each absent or alkyl; and,
Z3 is absent, -NH-, -SOz- or alkyl (wherein alkyl is optionally substituted at
one or
more positions by halogen, hydroxy, lower alkyl, lower alkoxy, carboxy or
carbonylalkoxy).
An example of the present invention is a compound of formula (1) or a salt,
isomer, prodrug, metabolite or polymorph thereof wherein Xi is absent or lower
alkylene; and, Ri is selected from hydrogen, alkyl (optionally substituted at
one or
more positions by halogen, hydroxy or lower alkoxy), aryl, C3-Ci2 cycloalkyl
or
heterocyclyl, wherein aryl, C3-CI2 cycloalkyl or heterocyclyl are each
optionally
substituted at one or more positions by halogen, alkyl (optionally substituted
at one or
more positions by halogen, hydroxy or lower alkoxy), hydroxy or alkoxy
(optionally
substituted at one or more positions by halogen or hydroxy).
An example of the present invention is a compound of formula (I) or a salt,
isomer, prodrug, metabolite or polymorph thereof wherein Xi is absent; and, Ri
is
selected from aryl or C3-CI2 cycloalkyl, wherein aryl is optionally
substituted at one or
more positions by halogen.
An example of the present invention is a compound of formula (1) or a salt,
isomer, prodrug, metabolite or polymorph thereof wherein Xi is absent; and, Ri
is
hydrogen.
5
CA 02681384 2009-09-18
WO 2008/115672 PCT/US2008/055102
An example of the present invention is a compound of formula (1) or a salt,
isomer, prodrug, metabolite or polymorph thereof wherein R3 is -C(0)-Zi(R6),
-S02-NH-Zz(R8) or -C(O)-NH-Z3(Rio).
An example of the present invention is a compound of formula (I) or a salt,
isomer, prodrug, metabolite or polymorph thereof wherein R3 is -C(0)-ZI(R6);
X3 is
absent, lower alkylene, lower alkylidene or -NH-; Z, is absent or alkyl; and,
R6 is aryl,
C3-C12 cycloalkyl or heterocyclyl, wherein aryl, C3-ClZ cycloalkyl or
heterocyclyl are
each optionally substituted by one or more hydroxy, oxo, halogen, amino,
aminoalkyl,
alkyl (optionally substituted at one or more positions by halogen, hydroxy or
lower
alkoxy), alkoxy (optionally substituted at one or more positions by halogen or
hydroxy), carboxy, carbonylalkoxy, carbamoyl, carbamoylalkyl, aryl, aryloxy,
arylalkoxy or heterocyclyl.
An example of the present invention is a compound of formula (1) or a salt,
isomer, prodrug, metabolite or polymorph thereof wherein R3 is -C(0)-Zi(R6);
X3 is
absent; Zi is absent; and, R6 is heterocyclyl.
An example of the present invention is a compound of formula (I) or a salt,
isomer, prodrug, metabolite or polymorph thereof wherein R3 is -S02-NR7-
Z2(Rg); X3
is absent or lower alkylidene; R7 is hydrogen or lower alkyl; Z2 is absent or
alkyl; and,
RS is aryl optionally substituted at one or more positions by alkoxy.
An example of the present invention is a compound of formula (I) or a salt,
isomer, prodrug, metabolite or polymorph thereof wherein R3 is -S02-NH-Z2(R8);
X3 is
absent or lower alkylidene; Z2 is absent or alkyl; and, R8 is aryl optionally
substituted at
one or more positions by alkoxy.
An example of the present invention is a compound of formula (1) or a salt,
isomer, prodrug, metabolite or polymorph thereof wherein R3 is -C(0)-NR9-
Z3(Rto); X3
is absent, lower alkylene, lower alkylidene or -NH-; R9 is hydrogen or lower
alkyl; Z3
is absent, -NH-, -SOz- or alkyl (wherein alkyl is optionally substituted at
one or more
positions by halogen, hydroxy, lower alkyl, lower alkoxy, carboxy or
carbonylalkoxy);
and, RIo is aryl, C3-C12 cycloalkyl or heterocyclyl each optionally
substituted by one or
more hydroxy, oxo, halogen, amino, aminoalkyl, alkyl (optionally substituted
at one or
more positions by halogen, hydroxy or lower alkoxy), alkoxy (optionally
substituted at
6
CA 02681384 2009-09-18
WO 2008/115672 PCT/US2008/055102
one or more positions by halogen or hydroxy), carboxy, carbonylalkoxy,
carbamoyl,
carbamoylalkyl, aminosulfonyl, lower alkyl-aminosulfonyl, aryl, aryloxy,
arylalkoxy or
heterocyclyl.
An example of the present invention is a compound of formula (I) or a salt,
isomer, prodrug, metabolite or polymorph thereof wherein R3 is -C(0)-NH-
Z3(Rio); X3
is absent; Z3 is absent, -NH- or alkyl (wherein alkyl is optionally
substituted at one or
more positions by halogen, hydroxy, lower alkyl, lower alkoxy, carboxy or
carbonylalkoxy); and, Rio is aryl, C3-Ci2 cycloalkyl or heterocyclyl each
optionally
substituted by one or more hydroxy, oxo, halogen, amino, aminoalkyl, alkyl
(optionally
substituted at one or more positions by halogen, hydroxy or lower alkoxy),
alkoxy,
carboxy, carbonylalkoxy, aryl or heterocyclyl.
An example of the present invention is a compound of formula (I) or a salt,
isomer, prodrug, metabolite or polymorph thereof wherein R3 is -C(O)-NH-
Z3(Rio); X3
is absent; Z3 is absent or alkyl; and, Rio is C3-Ci2 cycloalkyl optionally
substituted by
one or more hydroxy, oxo, halogen, amino, aminoalkyl, alkyl (optionally
substituted at
one or more positions by halogen, hydroxy or lower alkoxy), alkoxy, carboxy,
carbonylalkoxy, aryl or heterocyclyl.
An example of the present invention is a compound of formula (1) or a salt,
isomer, prodrug, metabolite or polymorph thereof wherein R3 is -C(O)-NH-
Z3(Rio); X3
is absent; Z3 is absent or alkyl; and, Rio is C3-Ci2 cycloalkyl optionally
substituted by
one or more alkyl or carbonylalkoxy.
An example of the present invention is a compound of formula (I) or a salt,
isomer, prodrug, metabolite or polymorph thereof wherein R3 is -C(O)-NH-
Z3(Rio); X3
is absent; Z3 is absent, -NH- or alkyl (wherein alkyl is optionally
substituted at one or
more positions by halogen, hydroxy, lower alkyl, lower alkoxy, carboxy or
carbonylalkoxy); and, Rlo is aryl optionally substituted by one or more
hydroxy, oxo,
halogen, amino, aminoalkyl, alkyl (optionally substituted at one or more
positions by
halogen, hydroxy or lower alkoxy), alkoxy, carboxy, carbonylalkoxy, aryl or
heterocyclyl.
An example of the present invention is a compound of formula (1) or a salt,
isomer, prodrug, metabolite or polymorph thereof wherein R3 is -C(O)-NH-
Z3(Rio); X3
7
CA 02681384 2009-09-18
WO 2008/115672 PCT/US2008/055102
is absent; Z3 is absent, -NH- or alkyl (wherein alkyl is optionally
substituted at one or
more positions by halogen, hydroxy or lower alkoxy); and, Rio is aryl
optionally
substituted by one or more halogen.
An example of the present invention is a compound of formula (I) or a salt,
isomer, prodrug, metabolite or polymorph thereof wherein R3 is -C(0)-NH-
Z3(Rio); X3
is absent; Z3 is absent or alkyl (wherein alkyl is optionally substituted at
one or more
positions by halogen, hydroxy, lower alkyl, lower alkoxy, carboxy or
carbonylalkoxy);
and, Rio is heterocyclyl optionally substituted by one or more alkyl.
An example of the present invention is a compound of formula (1) or a salt,
isomer, prodrug, metabolite or polymorph thereof wherein the dashed line
between
position 9 and X4R4 is absent; X4 is absent or is lower alkylene; and, R4 is
hydrogen or
aryl optionally substituted at one or more positions by halogen.
An example of the present invention is a compound of formula (I) or a salt,
isomer, prodrug, metabolite or polymorph thereof wherein the dashed line
between
position 9 and X4R4 is present, X4 is absent and R4 is CH-aryl optionally
substituted on
aryl at one or more positions by halogen.
An example of the present invention is a compound of formula (1) or a salt,
isomer, prodrug, metabolite or polymorph thereof wherein X5 is absent and R5
is
absent.
An exaniple of the present invention is a compound of formula (Ia):
X3R3
~IN
N
X4R4 X1R1
(Ia)
or a salt, isomer, prodrug, metabolite or polymorph thereof wherein Xi is
absent; X3 is
absent or lower alkylidene; when the dashed line between position 9 and X4R4
is
absent, X4 is absent or is lower alkylene and R4 is hydrogen or aryl
optionally
substituted at one or more positions by halogen; when the dashed line between
position
9 and X4R4 is present, X4 is absent and R4 is CH-aryl, wherein aryl is
optionally
8
CA 02681384 2009-09-18
WO 2008/115672 PCT/US2008/055102
substituted at one or more positions by halogen; Rl is selected from hydrogen,
aryl or
C3-Ciz cycloalkyl, wherein aryl is optionally substituted at one or more
positions by
halogen; R3 is -C(0)-Zi(R6), -S02-NH-Z2(RS) or -C(O)-NH-Z3(Rio); R6 is
heterocyclyl;
RS is aryl optionally substituted at one or more positions by alkoxy; Rlo is
aryl, C3-C12
cycloalkyl or heterocyclyl, wherein aryl or C3-C12 cycloalkyl are each
optionally
substituted by one or more halogen, alkyl or carbonylalkoxy; Zi is absent; Z2
is alkyl;
and, Z3 is absent, -NH- or alkyl (wherein alkyl is optionally substituted at
one or more
positions by halogen, hydroxy or lower alkoxy).
An exaniple of the present invention is a compound of formula (Ia) or a salt,
isomer, prodrug, metabolite or polymorph thereof wherein Xi is absent; X3 is
absent or
lower alkylidene; when the dashed line between position 9 and X4R4 is absent,
X4 is
absent or is lower alkylene and R4 is hydrogen or aryl optionally substituted
at one or
more positions by halogen; when the dashed line between position 9 and X4R4 is
present, X4 is absent and R4 is CH-aryl, wherein aryl is optionally
substituted at one or
more positions by halogen; Ri is selected from hydrogen, aryl or C3-Ciz
cycloalkyl,
wherein aryl is optionally substituted at one or more positions by halogen; R3
is
-S02-NH-Zz(Rg) or -C(0)-NH-Z3(Rio); Rg is aryl optionally substituted at one
or more
positions by alkoxy; Rio is aryl or C3-Ciz cycloalkyl, wherein aryl or C3-Ciz
cycloalkyl
are each optionally substituted by one or more halogen, alkyl or
carbonylalkoxy; Zz is
alkyl; and Z3 is absent or alkyl (wherein alkyl is optionally substituted at
one or more
positions by halogen, hydroxy or lower alkoxy).
An exanlple of the present invention includes a compound of Formula (I) and
pharmaceutically acceptable forms thereof selected from:
Cpd Name
1 1-cyclohexyl-4,5,6,7,8,9-hexahydro-IH-cyclooctapyrazole-3-carboxylic acid
(1,3,3 -trimethyl-bicyclo [2.2.1 ]hept-2-yl)-amide,
2 1-cyclohexyl-4,5,6,7,8,9-hexahydro-IH-cyclooctapyrazole-3-carboxylic acid
(adamantan- 1 -ylmethyl)-amide,
3 1-cyclopentyl-4,5,6,7,8,9-hexahydro-lH-cyclooctapyrazole-3-carboxylic acid
(adamantan-l-ylmethyl)-amide,
4 1-cyclohexyl-4,5,6,7,8,9-hexahydro-IH-cyclooctapyrazole-3-carboxylic acid
(1-adamantan-l-yl-ethyl)-amide,
5 (2E)-[9-(3-chloro-benzyl)-4,5,6,7,8,9-hexahydro-lH-cyclooctapyrazol-3-yl]-
ethenesulfonic acid [(IS)-1-phenyl-ethyl]-amide,
9
CA 02681384 2009-09-18
WO 2008/115672 PCT/US2008/055102
Cpd Name
6 (9S*)-(3-chloro-benzyl)-4,5,6,7,8,9-hexahydro-lH-cyclooctapyrazole-3-
carboxylic acid [(1R)-2-hydroxy-l-phenyl-ethyl]-amide,
7 (9R*)-(3-chloro-benzyl)-4,5,6,7,8,9-hexahydro-lH-cyclooctapyrazole-3-
carboxylic acid [(1R)-2-hydroxy-l-phenyl-ethyl]-amide,
8 (9R*)-(3-chloro-benzyl)-4,5,6,7,8,9-hexahydro-lH-cyclooctapyrazole-3-
carboxylic acid [(1S)-2-hydroxy-l-phenyl-ethyl]-amide,
9 (9S*)-(3-chloro-benzyl)-4,5,6,7,8,9-hexahydro-lH-cyclooctapyrazole-3-
carboxylic acid [(1S)-2-hydroxy-l-phenyl-ethyl]-amide,
(9S*)-(3-chloro-benzyl)-4,5,6,7,8,9-hexahydro-lH-cyclooctapyrazole-3-
carboxylic acid [(1R)-2-methoxy-l-phenyl-ethyl]-amide,
11 (9R*)-(3-chloro-benzyl)-4,5,6,7,8,9-hexahydro-lH-cyclooctapyrazole-3-
carboxylic acid [(1R)-2-methoxy-l-phenyl-ethyl]-amide,
12 (9E)-(3-chloro-benzylidene)-4,5,6,7,8,9-hexahydro-lH-cyclooctapyrazole-3-
carboxylic acid [(1R)-1-phenyl-ethyl]-amide,
13 (9E)-(3-chloro-benzylidene)-4,5,6,7,8,9-hexahydro-lH-cyclooctapyrazole-3-
carboxylic acid [(1S)-1-phenyl-ethyl]-amide,
14 (9E)-(3-chloro-benzylidene)-4,5,6,7,8,9-hexahydro-lH-cyclooctapyrazole-3-
carboxylic acid [(1S)-2-hydroxy-l-phenyl-ethyl]-amide, or
(9E)-(3-chloro-benzylidene)-4,5,6,7,8,9-hexahydro-lH-cyclooctapyrazole-3-
carboxylic acid [(1R)-2-hydroxy-l-phenyl-ethyl]-amide.
Definitions
As used herein, the following terms have the following meanings:
The term "alkyl" means a saturated branched or straight chain monovalent
hydrocarbon radical of up to 10 carbon atoms. Alkyl typically includes, but is
not
5 limited to, methyl, ethyl, propyl, isopropyl, n-butyl, t-butyl, pentyl,
hexyl, heptyl and
the like.
The term "lower alkyl" means an alkyl radical of up to 4 carbon atoms. The
point of attachment may be on any alkyl or lower alkyl carbon atom and, when
further
substituted, substituent variables may be placed on any carbon atom.
10 The term "alkylene" means a saturated branched or straight chain monovalent
hydrocarbon linking group of up to 10 carbon atoms, whereby the linking group
is
derived by the removal of one hydrogen atom each from two carbon atoms.
Alkylene
typically includes, but is not limited to, methylene, ethylene, propylene,
isopropylene,
n-butylene, t-butylene, pentylene, hexylene, heptylene and the like. The term
"lower
15 alkylene" means an alkylene linking group of up to 4 carbon atoms. The
point of
CA 02681384 2009-09-18
WO 2008/115672 PCT/US2008/055102
attachment may be on any alkylene or lower alkylene carbon atom and, when
further
substituted, substituent variables may be placed on any carbon atom.
The term "alkylidene" means an alkylene linking group of from 1 to 10 carbon
atoms having at least one double bond formed between two adjacent carbon
atoms,
wherein the double bond is derived by the removal of one hydrogen atom each
from the
two carbon atoms. Atoms may be oriented about the double bond in either the
cis (E)
or trans (Z) conformation. Alkylidene typically includes, but is not limited
to,
methylidene, vinylidene, propylidene, iso-propylidene, methallylene,
allylidene (2-
propenylidene), crotylene (2-butenylene), prenylene (3-methyl-2-butenylene)
and the
like. The term "lower alkylidene" means a radical or linking group of from 1
to 4
carbon atoms. The point of attachment may be on any alkylidene or lower
alkylidene
carbon atom and, when further substituted, substituent variables may be placed
on any
carbon atom.
The term "alkoxy" means an alkyl, alkylene or alkylidene radical of up to 10
carbon atoms attached via an oxygen atom, whereby the point of attachment is
formed
by the removal of the hydrogen atom from a hydroxide substituent on a parent
radical.
The term "lower alkoxy" means an alkyl, alkylene or alkylidene radical of up
to 4
carbon atoms. Lower alkoxy typically includes, but is not limited to, methoxy,
ethoxy,
propoxy, butoxy and the like. When further substituted, substituent variables
may be
placed on any alkoxy carbon atom.
The term "cycloalkyl" means a saturated or partially unsaturated monocyclic,
polycyclic or bridged hydrocarbon ring system radical or linking group. A ring
of 3 to
20 carbon atoms may be designated by C3_20 cycloalkyl; a ring of 3 to 12
carbon atoms
may be designated by C3_12 cycloalkyl, a ring of 3 to 8 carbon atoms may be
designated
by C3_8 cycloalkyl and the like.
Cycloalkyl typically includes, but is not limited to, cyclopropyl, cyclobutyl,
cyclopentyl, cyclohexyl, cyclohexenyl, cycloheptyl, cyclooctyl, indanyl,
indenyl,
1,2,3,4-tetrahydro-naphthalenyl, 5,6,7,8-tetrahydro-naphthalenyl,
6,7,8,9-tetrahydro-5H-benzocycloheptenyl, 5,6,7,8,9,10-hexahydro-
benzocyclooctenyl,
fluorenyl, bicyclo[2.2.1]heptyl, bicyclo[2.2.1]heptenyl, bicyclo[2.2.2]octyl,
bicyclo[3.1.1]heptyl, bicyclo[3.2.1]octyl, bicyclo[2.2.2]octenyl,
bicyclo[3.2.1]octenyl,
adamantanyl, octahydro-4,7-methano-lH-indenyl, octahydro-2,5-methano-
pentalenyl
11
CA 02681384 2009-09-18
WO 2008/115672 PCT/US2008/055102
(also referred to as hexahydro-2,5-methano-pentalenyl) and the like. When
further
substituted, substituent variables may be placed on any ring carbon atom.
The term "heterocyclyl" means a saturated, partially unsaturated or
unsaturated
monocyclic, polycyclic or bridged hydrocarbon ring system radical or linking
group,
wherein at least one ring carbon atom has been replaced with one or more
heteroatoms
independently selected from N, 0 or S. A heterocyclyl ring system further
includes a
ring system having up to 4 nitrogen atom ring menibers or a ring system having
from 0
to 3 nitrogen atom ring members and 1 oxygen or sulfur atom ring member. When
allowed by available valences, up to two adjacent ring members may be a
heteroatom,
wherein one heteroatom is nitrogen and the other is selected from N, 0 or S. A
heterocyclyl radical is derived by the removal of one hydrogen atom from a
single
carbon or nitrogen ring atom. A heterocyclyl linking group is derived by the
removal
of two hydrogen atoms each from either carbon or nitrogen ring atoms.
Heterocyclyl typically includes, but is not limited to, furyl, thienyl, 2H-
pyrrole,
2-pyrrolinyl, 3-pyrrolinyl, pyrrolidinyl, pyrrolyl, 1,3-dioxolanyl, oxazolyl,
thiazolyl,
imidazolyl, 2-imidazolinyl (also referred to as 4,5-dihydro-lH-imidazolyl),
imidazolidinyl, 2-pyrazolinyl, pyrazolidinyl, pyrazolyl, isoxazolyl,
isothiazolyl,
oxadiazolyl, triazolyl, thiadiazolyl, tetrazolyl, 2H-pyran, 4H-pyran,
pyridinyl,
piperidinyl, 1,4-dioxanyl, morpholinyl, 1,4-dithianyl, thiomorpholinyl,
pyridazinyl,
pyrimidinyl, pyrazinyl, piperazinyl, azepanyl, indolizinyl, indolyl,
isoindolyl,
3H-indolyl, indolinyl, benzo[b]furyl, benzo[b]thienyl, 1H-indazolyl,
benzimidazolyl,
benzthiazolyl, purinyl, 4H-quinolizinyl, quinolinyl, isoquinolinyl,
cinnolinyl,
phthalzinyl, quinazolinyl, quinoxalinyl, 1,8-naphthyridinyl, pteridinyl,
quinuclidinyl,
hexahydro-1,4-diazepinyl, 1,3-benzodioxolyl (also known as
1,3-methylenedioxyphenyl), 2,3-dihydro-1,4-benzodioxinyl (also known as
1,4-ethylenedioxyphenyl), benzo-dihydro-furyl, benzo-tetrahydro-pyranyl,
benzo-dihydro-thienyl, 5,6,7,8-tetrahydro-4H-cyclohepta(b)thienyl, 5,6,7-
trihydro-4H-cyclohexa(b)thienyl, 5,6-dihydro-4H-cyclopenta(b)thienyl, 2-aza-
bicyclo[2.2.1]heptyl, 1-aza-bicyclo[2.2.2]octyl, 8-aza-bicyclo[3.2.1]octyl, 7-
oxa-
bicyclo[2.2.1]heptyl and the like.
The term "aryl" means an unsaturated, conjugated 7u electron monocyclic or
polycyclic hydrocarbon ring system radical or linking group of 6, 9, 10 or 14
carbon
12
CA 02681384 2009-09-18
WO 2008/115672 PCT/US2008/055102
atoms. An aryl radical is derived by the removal of one hydrogen atom from a
single
carbon ring atom. An arylene linking group is derived by the removal of two
hydrogen
atoms each of two carbon ring atoms. Aryl typically includes, but is not
limited to,
phenyl, naphthalenyl, azulenyl, anthracenyl and the like.
The term "amino" means a radical of the formula or -NHz.
The term "aminoalkyl" means a radical of the formula -NH-alkyl or -N(alkyl)2.
The term "aminosulfonyl" means a radical of the formula or -SOzNHz.
The term "arylalkoxy" means a radical of the formula -0-alkyl-aryl.
The term "aryloxy" means a radical of the formula -0-aryl.
The term "carbamoyl" means a radical of the formula or -C(0)NHz.
The term "carbamoylalkyl" means a radical of the fornlula -C(O)NH-alkyl or
-C(0)N(alkyl)2.
The term "carbonylalkoxy" means a radical of the forniula -C(O)0-alkyl.
The term "carboxy" means a radical of the formula -COOH or -COzH.
The term "halo" or "halogen" means fluoro, chloro, bromo or iodo.
The term "lower alkyl-amino" means a radical of the formula -NH-alkyl or
-N(alkyl)2.
The term "lower alkyl-aminosulfonyl" means a radical of the formula
-SOzNH-allcyl or -SO2N(alkyl)2.
The term "lower alkyl-sulfonyl" means a radical of the formula -S02-alkyl or
-C(0)N(alkyl)2.
The term "substituted" means one or more hydrogen atoms on a core molecule
have been replaced with one or more radicals or linking groups, wherein the
linking
group, by definition is also further substituted. The ability of a particular
radical or
linking group to replace a hydrogen atom is optimally expected by one skilled
to art to
result in a chemically stable core molecule.
The term "dependently selected" means one or more substituent variables are
present in a specified combination (e.g. groups of substituents commonly
appearing in a
tabular list).
The substituent nomenclature used in the disclosure of the present invention
13
CA 02681384 2009-09-18
WO 2008/115672 PCT/US2008/055102
was derived using nomenclature rules well known to those skilled in the art
(e.g.,
NPAC).
Pharmaceutical Forms
The compounds of the present invention may be present in the form of
pharmaceutically acceptable salts. For use in medicines, the "pharmaceutically
acceptable salts" of the compounds of this invention refer to non-toxic
acidic/anionic or
basic/cationic salt forms.
Suitable pharmaceutically acceptable salts of the compounds of this invention
include acid addition salts which may, for example, be formed by mixing a
solution of
the compound according to the invention with a solution of a pharmaceutically
acceptable acid such as hydrochloric acid, sulfuric acid, fumaric acid, maleic
acid,
succinic acid, acetic acid, benzoic acid, citric acid, tartaric acid, carbonic
acid or
phosphoric acid.
Furthermore when the compounds of the present invention carry an acidic
moiety, suitable pharmaceutically acceptable salts thereof may include alkali
metal
salts, e.g. sodium or potassium salts; alkaline earth metal salts, e.g.
calcium or
magnesium salts; and salts formed with suitable organic ligands, e.g.
quaternary
ammonium salts. Thus, representative pharmaceutically acceptable salts include
the
following: acetate, benzenesulfonate, benzoate, bicarbonate, bisulfate,
bitartrate, borate,
bromide, calcium, camsylate (or camphosulphonate), carbonate, chloride,
clavulanate,
citrate, dihydrochloride, edetate, fumarate, gluconate, glutamate,
hydrabamine,
hydrobromine, hydrochloride, iodide, isothionate, lactate, malate, maleate,
mandelate,
mesylate, nitrate, oleate, pamoate, palmitate, phosphate/diphosphate,
salicylate,
stearate, sulfate, succinate, tartrate, tosylate.
The present invention includes within its scope prodrugs and metabolites of
the
compounds of this invention. In general, such prodrugs and metabolites will be
functional derivatives of the compounds that are readily convertible in vivo
into an
active compound.
The term "prodrug" means a pharmaceutically acceptable form of a functional
derivative of a compound of the invention (or a salt thereof), wherein the
prodrug may
be: 1) a relatively active precursor which converts in vivo to an active
prodrug
14
CA 02681384 2009-09-18
WO 2008/115672 PCT/US2008/055102
component; 2) a relatively inactive precursor which converts in vivo to an
active
prodrug component; or 3) a relatively less active component of the compound
that
contributes to therapeutic biological activity after becoming available in
vivo (i.e., as a
metabolite). Conventional procedures for the selection and preparation of
suitable
prodrug derivatives are described in, for example, "Design of Prodrugs", ed.
H.
Bundgaard, Elsevier, 1985.
The term "metabolite" means a pharmaceutically acceptable form of a
metabolic derivative of a compound of the invention(or a salt thereof),
wherein the
derivative is a relatively less active component of the compound that
contributes to
therapeutic biological activity after becoming available in vivo.
The present invention contemplates compounds of various isomers and mixtures
thereof. The term "isomer" refers to compounds that have the same composition
and
molecular weight but differ in physical and/or chemical properties. Such
substances
have the same number and kind of atoms but differ in structure. The structural
difference may be in constitution (geometric isomers) or in an ability to
rotate the plane
of polarized light (stereoisomers).
The term "stereoisomer" refers to isomers of identical constitution that
differ in
the arrangement of their atoms in space. Enantiomers and diastereomers are
stereoisomers wherein an asymmetrically substituted carbon atom acts as a
chiral
center. The term "chiral" refers to a molecule that is not superposable on its
mirror
image, implying the absence of an axis and a plane or center of symmetry. The
term
"enantiomer" refers to one of a pair of molecular species that are mirror
images of each
other and are not superposable. The term "diastereomer" refers to
stereoisomers that
are not related as mirror images. The symbols "R" and "S" represent the
configuration
of substituents around a chiral carbon atom(s). The symbols "R*" and "S*"
denote the
relative configurations of substituents around a chiral carbon atom(s). .
The term "racemate" or "racemic mixture" refers to a compound of equimolar
quantities of two enantiomeric species, wherein the compound is devoid of
optical
activity. The term "optical activity" refers to the degree to which a chiral
molecule or
nonracemic mixture of chiral molecules rotates the plane of polarized light.
The term "geometric isomer" refers to isomers that differ in the orientation
of
substituent atoms in relationship to a carbon-carbon double bond, to a
cycloalkyl ring
CA 02681384 2009-09-18
WO 2008/115672 PCT/US2008/055102
or to a bridged bicyclic system. Substituent atoms (other than H) on each side
of a
carbon-carbon double bond may be in an E or Z configuration. In the "E"
(opposite
sided) or "chair" configuration, the substituents are on opposite sides in
relationship to
the carbon- carbon double bond; in the "Z" (same sided) or "boat"
configuration, the
substituents are oriented on the same side in relationship to the carbon-
carbon double
bond. Substituent atoms (other than H) attached to a carbocyclic ring may be
in a cis or
trans configuration. In the "cis" configuration, the substituents are on the
same side in
relationship to the plane of the ring; in the "trans" configuration, the
substituents are on
opposite sides in relationship to the plane of the ring. Conlpounds having a
mixture of
"cis" and "trans" species are designated "cis/trans". Substituent atoms (other
than H)
attached to a bridged bicyclic system may be in an "endo" or "exo"
configuration. In
the "endo" configuration, the substituents attached to a bridge (not a
bridgehead) point
toward the larger of the two remaining bridges; in the "exo" configuration,
the
substituents attached to a bridge point toward the smaller of the two
remaining bridges.
It is to be understood that the various substituent stereoisomers, geometric
isomers and mixtures thereof used to prepare compounds of the present
invention are
either commercially available, can be prepared synthetically from commercially
available starting materials or can be prepared as isomeric mixtures and then
obtained
as resolved isomers using techniques well-known to those of ordinary skill in
the art.
The isomeric descriptors "R," "S," "S*," "R*," "E,11 '7," "cis," "trans,"
"exo"
and "endo" are used as described herein for indicating atom configuration(s)
relative to
a core molecule and are intended to be used as defined in the literature (NPAC
Recommendations for Fundamental Stereochemistry (Section E), Pure Appl.
Chein.,
1976, 45:13-30).
Furthermore, compounds of the present invention may have one or more
polymorph or amorphous crystalline forms and as such are intended to be
included in
the scope of the invention. In addition, some of the compounds may form
solvates with
water (i.e., hydrates) or common organic solvents, and such are also intended
to be
encompassed within the scope of this invention.
Therapeutic Use
The CB2 receptor belongs to the G-protein-coupled receptor (GCPR) family
and appears to be primarily expressed peripherally in lymphoid tissue (cell
mediated
16
CA 02681384 2009-09-18
WO 2008/115672 PCT/US2008/055102
and innate immunity), peripheral nerve terminals (peripheral nervous system),
spleen
immune cells (immune system modulation) and retina (intraocular pressure). CB2
mRNA is found in the CNS in cerebellar granule cells (coordinating motor
function).
Activation of the CB2 receptor by an agonist compound mediates pain
responses in animal models.
The present invention is directed to a method for treating, ameliorating or
preventing CB2 receptor mediated pain in a subject in need thereof comprising
administering to the subject an effective amount of a compound of formula (I)
or
formula (Ia) or a form thereof.
The term "CB2 receptor mediated pain" as used herein, refers to pain states
that
are chronic or acute, that are postoperative, inflammatory or neuropathic or
the result of
injury or age and include, without limitation, central and peripheral pathway
mediated
pain states that otherwise defy characterization and would benefit from
treatment with a
CB2 receptor agonist.
The scope of the present method is intended to include inflammatory related
pain states selected from the group consisting of osteoarthritis, rheumatoid
arthritis,
headache, migraine, odontaligia, labor, dysmenorrhea, interstitial cystitis,
peripheral
neuritis, mucositis, surgery pain, sports injury pain, trauma, cancer pain,
fibromyalgia,
pancreatitis, enteritis, cellulitis, bony fractures, post-operative ileus,
irritable bowel
syndrome, pain due to inflammatory bowel diseases, Crohn's Disease, ulcerative
colitis,
cholecystitis, burn, sunburn, pain due to venomous snake bite, spider bite or
insect sting
and pain due to nonvenomous snake bite, spider bite or insect sting.
The scope of the present method is further intended to include neuropathic
related pain states selected from the group consisting of chemotherapeutic
neuropathy,
AIDS-related neuropathy, diabetic neuropathy and post herpetic neuralgia.
An exaniple of the present invention includes use of a compound of formula (1)
or formula (Ia) or a form thereof in the manufacture of a medicament for
treating,
ameliorating or preventing CB2 receptor mediated pain in a subject in need
thereof.
An exaniple of the present invention includes a method for treating,
ameliorating or preventing CB2 receptor mediated pain in a subject in need
thereof
comprising administering to the subject a combination product and/or therapy
comprising an effective amount of a compound of formula (I) or formula (Ia) or
a form
17
CA 02681384 2009-09-18
WO 2008/115672 PCT/US2008/055102
thereof and a therapeutic agent.
Compounds of formula (1) or formula (Ia) are CB2 agonists useful in the
method of the present in the invention, having a CB2 agonist binding activity
IC5o
value of between about 50 M to about 0.01 nM; between about 25 M to about
0.01
nM; between about 15 pM to about 0.01 nM; between about 10 M to about 0.01
nM;
between about 1 M to about 0.01 nM; between about 800 nM to about 0.01 nM;
between about 200 nM to about 0.01 nM; between about 100 nM to about 0.01 nM;
between about 80 nM to about 0.01 nM; between about 20 nM to about 0.01 nM;
between about 10 nM to about 0.1 nM; or about 0.1 nM.
The term "subject" as used herein, refers to a patient, which may be an
animal,
preferably a mammal, most preferably a human, which has been the object of
treatment,
observation or experiment and is at risk of (or susceptible to) developing a
CB receptor
mediated syndrome, disorder or disease.
The term "administering" is to be interpreted in accordance with the methods
of
the present invention. Such methods include therapeutically or
prophylactically
administering an effective amount of a compound of formula (1) or formula (la)
at
different times during the course of a therapy or concurrently as a product in
a
combination form. Thus, in the methods of treatment of the present invention,
the term
shall encompass the means for treating, ameliorating or preventing the CB2
receptor
mediated pain described herein with a compound specifically disclosed or a
prodrug or
metabolite thereof, which would obviously be included within the scope of the
invention albeit not specifically disclosed for certain of the instant
compounds.
Prophylactic administration can occur prior to the manifestation of symptoms
characteristic of CB2 receptor mediated pain such that the pain is treated,
ameliorated,
prevented or otherwise delayed in its progression. The methods of the present
invention are further to be understood as embracing all therapeutic or
prophylactic
treatment regimens used by those skilled in the art.
The term "effective amount" refers to that amount of an instant compound that
elicits the biological or medicinal response in a tissue system, animal or
human, that is
being sought by a researcher, veterinarian, medical doctor, or other
clinician, which
includes alleviation of the symptoms of the syndrome, disorder or disease
being treated.
The effective amount of such a compound for use in the present invention is
from about
18
CA 02681384 2009-09-18
WO 2008/115672 PCT/US2008/055102
0.001 mg/kg/day to about 300 mg/kg/day.
The term "medicament" refers to a product for use in treating, ameliorating or
preventing a cannabinoid receptor mediated syndrome, disorder or disease.
The term "combination product and/or therapy" means a pharmaceutical
composition comprising a compound of formula (1) or formula (Ia) in
combination with
one or more therapeutic agents. The dosages of the compound of formula (1) or
formula (Ia) and the one or more therapeutic agents are adjusted when combined
to
achieve an effective amount.
Wherein the present invention is directed to the administration of a
combination
product, the term "effective amount" means that amount of the combination of
agents
taken together so that the combined effect elicits the desired biological or
medicinal
response.
As those skilled in the art will appreciate, the effective amounts of the
components comprising the combination product may be independently optimized
and
combined to achieve a synergistic result whereby the pathology is reduced more
than it
would be if the components of the combination product were used alone.
Wherein the present invention is directed to the administration of a
combination
product and/or therapy, an instant compound and the agent may be co-
administered by
any suitable means, simultaneously, sequentially, alternately or in a single
or divided
form, at the same or different times during the course of therapy.
Where an instant compound and the agent components are administered
separately, the number of dosages of an instant compound given per day, may
not
necessarily be the same, e.g. where one compound may have a greater duration
of
activity, and will therefore, be administered less frequently.
Suitable examples of methods of administration are orally, intravenous (iv),
intramuscular (im), subcutaneous (sc), transdermal and topical. Compounds may
also be
administrated directly to the nervous system including, but not limited to the
intracerebral, intraventricular, intracerebroventricular, intrathecal,
intracisternal,
intraspinal and/or peri-spinal routes of administration by delivery via
intracranial or
intravertebral needles and/or catheters with or without pump devices.
Optimal dosages to be administered may be readily determined by those skilled
19
CA 02681384 2009-09-18
WO 2008/115672 PCT/US2008/055102
in the art, and will vary with the particular compound used, the mode of
administration,
the strength of the preparation and the advancement of the disease condition.
In
addition, factors associated with the particular patient being treated,
including patient's
sex, age, weight, diet, time of administration and concomitant diseases, will
result in
the need to adjust dosages.
The present invention includes administration of a pharmaceutical coniposition
or medicament comprising an admixture of a compound of the present invention
and an
optional pharmaceutically acceptable carrier.
Pharmaceutical Compositions
The term "composition" refers to a product comprising the specified
ingredients
in the specified amounts, as well as any product that results, directly or
indirectly, from
combinations of the specified ingredients in the specified amounts.
Pharmaceutical compositions of the invention may, alternatively or in addition
to a compound of formula (I) or formula (Ia), comprise a pharmaceutically
acceptable
salt of a compound of formula (I) or formula (Ia) or a prodrug or
pharmaceutically
active metabolite of such a compound or salt in admixture with a
pharmaceutically
acceptable carrier.
"Pharmaceutically acceptable carrier" means molecular entities and
compositions that are of sufficient purity and quality for use in the
formulation of a
composition of the invention and that, when appropriately administered to an
animal or
a human, do not produce an adverse, allergic, or other untoward reaction.
Since both clinical and veterinary uses are equally included within the scope
of
the present invention, a pharmaceutically acceptable formulation would include
a
composition or medicament formulation for either clinical or veterinary use.
The composition or medicament may be administered in a wide variety of
dosage unit forms depending on the method of administration; wherein such
methods
include (without limitation) oral, sublingual, nasal (inhaled or insufflated),
transdermal,
rectal, vaginal, topical (with or without occlusion), intravenous (bolus or
infusion) or
for injection (intraperitoneally, subcutaneously, intramuscularly,
intratumorally or
parenterally) using a suitable dosage form well known to those of ordinary
skill in the
area of pharmaceutical administration. Accordingly, the term "dosage unit" or
"dosage
form" is alternatively used to refer to (without limitation) a tablet, pill,
capsule,
CA 02681384 2009-09-18
WO 2008/115672 PCT/US2008/055102
solution, syrup, elixir, emulsion, suspension, suppository, powder, granule or
sterile
solution, emulsion or suspension (for injection from an ampoule or using a
device such
as an auto-injector or for use as an aerosol, spray or drop). Furthermore, the
composition may be provided in a form suitable for weekly or monthly
administration
(e.g. as an insoluble salt of the active compound (such as the decanoate salt)
adapted to
provide a depot preparation for intramuscular injection).
The present invention includes a composition of an instant compound or
prodrug thereof present in a prophylactically or therapeutically effective
amount
necessary for symptomatic relief to a subject in need thereof.
A prophylactically or therapeutically effective amount of an instant compound
or prodrug thereof may range from about 0.001 mg to about 1 g and may be
constituted
into any form suitable for the administration method and regimen selected for
the
subject.
Depending on the subject and disease to be treated, the prophylactically or
therapeutically effective amount for a person of average body weight of about
70 kg per
day may range from about 0.001 mg/kg to about 300 mg/kg; from about 0.01 mg/kg
to
about 200 mg/kg; from about 0.05 mg/kg to about 100 mg/kg; or, from about 0.1
mg/kg
to about 50 mg/kg.
An optimal prophylactically or therapeutically effective amount and
administration method and regimen may be readily determined by those skilled
in the
art, and will vary depending on factors associated with the particular patient
being
treated (age, weight, diet and time of administration), the severity of the
condition
being treated, the compound and dosage unit being employed, the mode of
administration and the strength of the preparation.
Dosage unit(s) may be administered to achieve the therapeutically or
prophylactically effective amount in a regimen of from about once per day to
about 5
times per day. The preferred dosage unit for oral administration is a tablet
containing
0.01, 0.05, 0.1, 0.5, 1.0, 2.5, 5.0, 10.0, 15.0, 25.0, 50.0, 100, 150, 200,
250 or 500 mg of
the active ingredient.
21
CA 02681384 2009-09-18
WO 2008/115672 PCT/US2008/055102
Biological Examples
The following examples illustrate that the compounds of the present invention
are useful in a method for treating, ameliorating or preventing CB2 receptor
mediated
pain in a subject in need thereof.
Example 1
Carrageenan Model of Inflammatory Pain
Intraplantar injection of carrageenan (Cg) in a rodent produces pronounced
hypersensitivity to both thermal and mechanical stimuli. The effects of
carrageenan are
maximal 2-4 hr after administration.
Procedure
To assess the ability of test compounds to reverse thermal hyperalgesia,
baseline
response latencies on a radiant heat (RH) paw stimulator were obtained before
an
intraplantar injection of carrageenan-k (200 L) in male Sprague-Dawley rats
(250-350
g in treatment groups of 9 animals each). Only withdrawal responses that were
quick
hind paw movements (with or without licking of the hind paw) were recorded.
Paw
movements associated with locomotion or a shifting of weight were not
considered a
withdrawal response.
The weight of each animal was recorded on the day of the experiment. Each
animal was placed on a warm (approx. body temperature, 30 C) glass surface and
allowed to acclimate to the test chamber for approximately 10-15 minutes. A
radiant
thermal stimulus (beam of light) was then focused on the sole of each hind paw
in turn,
and an initial (baseline) response time to thermal stimuli was recorded for
each animal.
The stimulus intensity (radiant heat at a setting of 5 Amps) that produced 10-
15 sec
baseline withdrawal latencies was used and a maximum cutoff of 20 sec was
imposed.
The light stimulus was automatically shut off by a photoelectric relay when
the foot
moved or when the cut-off time limit was reached.
One treatment group (8 animals each) was injected i.p. with vehicle (5% DMSO
and 5% Tween-80 in sterile saline). The other treatment groups (8 animals
each) were
injected i.p. with 3, 10 or 30 mg/kg Compound 2.
One hour later, withdrawal latencies for the animals administered vehicle were
recorded. After assessment, all animals were administered 1% carrageenan (200
L in
22
CA 02681384 2009-09-18
WO 2008/115672 PCT/US2008/055102
sterile saline) subcutaneously into the sub-plantar tissue of the left hind
paw to
stimulate an acute inflammatory reaction. Three hours later, the response time
of the
animals to the thermal stimulus was evaluated. The results are shown in Table
1 as
seconds SEM.
Table 1
Baseline 1 hr post 3 hrs post Cg
vehicle administration
administration
Vehicle 12.13 0.84 12.74 0.77 6.66 0.72
3 mg/kg 5.11 0.84
mg/kg 6.18 0.91
30 mg/kg 14.41 1.75
Three hours after carrageenan (Cg) administration, mean latencies in vehicle
treated animals were significantly decreased, indicating the development of
thermal
hyperalgesia.
Example 2
10 The experiment of Example 1 is repeated with the exception that animals are
first administered 1% carrageenan (200 L in sterile saline) subcutaneously
into the
sub-plantar tissue of the left hind paw to stimulate an acute inflammatory
reaction.
Two and a half hours later, withdrawal latencies are assessed (`post-Cg'). One
treatment group is then injected i.p. with vehicle (5% DMSO and 5% Tween-80 in
sterile saline). The other treatment groups are injected i.p. with 3, 10 or 30
mg/kg of
Compound 2. Thirty minutes after test compound administration withdrawal
latencies
are recorded. The results are shown in Table 2 as seconds SEM.
Table 2
Baseline 2.5 hr post Cg 3 hrs post Cg
administration administration
Vehicle 11.82 0.34 6.56 1.16
3 mg/kg 5.92 1.15
10 mg/kg 6.88 0.44
mg/kg 8.52 1.08
23
CA 02681384 2009-09-18
WO 2008/115672 PCT/US2008/055102
Two and a half hours after carrageenan (Cg) administration, mean latencies in
vehicle treated animals were significantly decreased, indicating the
development of
thermal hyperalgesia.
Example 3
Hot-Plate Nociception Test
The hot-plate test originally described by Eddy and Leimbach (J. Pharmacol.
Exp. Ther. 107:385-393, 1953) with minor modifications (e.g., O'Callaghan and
Holtzman, J. Pharmacol. Exp. Ther. 192: 497-505, 1975) is used to ascertain
the
analgesic potential of investigated compounds. The hot plate analgesia meter
used for
these studies is produced by Columbus Instruments International (Columbus,
OH).
Procedure
Male CD-1 mice (30-35g) are weighed, placed in a plastic box with wood chips
and allowed to acclimate before testing. An individual mouse is placed on a 48
C
heated surface and locomotion on the plate is constrained by a glass cylinder.
The time
interval between placement and a shaking, licking or tucking of either hind-
paw
(nociceptive response) is recorded as the Baseline measurement. Animals are
removed
from the heated plate immediately after responding or after a maximum of 40
sec to
prevent tissue damage. Each mouse is tested only once.
One treatment group is then injected i.p. with vehicle (5% DMSO and 5%
Tween-80 in sterile saline). The other treatment groups are injected i.p. with
10 or 30
mg/kg of a test compound. Thirty minutes after test compound administration,
each
animal is assessed for a response with a maximum cut-off of 90 sec.
The reaction time for a vehicle or test compound treated animal is compared to
the respective baseline reaction time corresponding to each animal. The
percent
maximal effect (%MPE) is obtained by subtracting the baseline response time
from the
post-treatment response time and dividing the result by the difference of the
baseline
response time subtracted from the cut-off response time (90 sec).
24
CA 02681384 2009-09-18
WO 2008/115672 PCT/US2008/055102
Example 4
Vsceral Hyperalgesia lVodel
This protocol uses barostat-controlled, isobaric colorectal distensions (CRD)
in
rats to evaluate the potency and efficacy of test compounds in treating
visceral
hyperalgesia.
Procedure
Rats (male Sprague Dawley (275 - 350 g; CD(SD); Charles River Labs) are
housed 2 to 4 animals per cage in a temperature and humidity controlled room
with a
12 hr/12hr light/dark cycle, with ad libitum access to food and water.
One day after release from quarantine, the animals are acclimated to
progressively longer (30 min and 4 hr later, 45 min) periods of simple
restraint in
plexiglas devices (G-3, rat ECU; Braintree Scientific; Braintree MA). The
animals are
returned to their home cages overnight. The next day they are acclimated in
the
restraint device for 60 min in the morning. 4 hrs later, the animals are
lightly
anesthetized with 70% C02:30% 02. A highly compliant, 4 cm long polyethylene
balloon, lubricated with K-Y Jelly is then inserted via the anus into the
rectum and
distal colon. The balloon is positioned such that the aboral end is 1 Cm from
the anus
and is secured in place by taping the balloon catheter to the base of the
tail. The
catheter is connected to a computerized barostat that controls the inflation
of the
balloon and the resulting colorectal distension. The balloon pressure,
representing
intracolonic pressure, is continuously recorded.
CRD in conscious animals elicits a reflex visceromotor response consisting of
contraction of the anterior abdominal wall muscles (Ness TJ and Gebhart GF,
Colorectal distension as a noxious visceral stimulus: physiologic and
pharmacologic
characterization of pseudaffective reflexes in the rat, Brain Res., (1988),
450: 153-169).
Contraction of these muscles increases intraabdominal pressure and
subsequently
increases intracolonic pressure. Changes in intracolonic pressure are
transduced
through the same balloon used to deliver the CRD. The manometric endpoint has
recently been reported to mimic electromyographic responses recorded from
anterior
abdominal wall muscles in rats (Tammpere A, Brusberg M, Axenborg J, Hirsch I,
Larsson H and Lindstrom E, Evaluation of pseudo-affective responses to noxious
colorectal distension in rats by manometric recordings, Pain, (2005), 116: 220-
226).
CA 02681384 2009-09-18
WO 2008/115672 PCT/US2008/055102
Stimulus-response data are obtained by delivering two series of 20 sec ranip
(15, 30, 45, 60, 75 mmHg) distensions at four-minute intervals and recording
the
manometric response as follows: the intracolonic pressure signal is passed
through a
digital 1 Hz highpass filter, rectified and the integral of the initial 15
seconds of the
CRD subjected to baseline subtraction (the 15 sec immediately preceding
balloon
distension); the responses at each distending pressure are averaged to obtain
a control
stimulus/response curve for each animal. The colorectal balloons are then
removed and
the animals are returned to their home cages.
The following morning, one treatment group is injected i.p. with 10 mg/kg of a
test compound (solubilized in 5% DMSO and 5% Tween-80 in sterile saline).
One hour later, an acute colitis is induced in all treatment groups by the
intracolonic instillation of a 1.5 mi., bolus of 2.5% (w/v) zymosan A (from
Saccharomyces cerevisiae; Sigma Chemical Co., St. Louis) in 30% ethanol (under
light
70% C02:30% 02 anesthesia). Four hours later, the animals are lightly
anesthetized
and the colorectal balloons inserted as on the previous day for controlled
distensions.
The identical CRD stimuli is applied and manometric responses are recorded and
analyzed as described for the control phase of the experiment.
The animals in one treatment group are then subcutaneously (s.c.) dosed with 1
mg/kg morphine. As a comparator for analgesic response, animals in another
treatment
group are dosed s.c. with 3 mg/kg morphine 4 hrs after colitis initiation and
30 min
prior to CRD. Data are excluded from experiments in which animals in the
vehicle
treatment group do not exhibit a hyperalgesic response following zymosan
administration. Data are expressed as a percent (% SEM) of the initial
(control)
manometric responses, with each animal serving as its own control.
It is to be understood that the preceding description of the invention and
various
examples thereof have emphasized certain aspects. Numerous other equivalents
not
specifically elaborated on or discussed may nevertheless fall within the
spirit and scope
of the present invention or the following claims and are intended to be
included.
26