Note: Descriptions are shown in the official language in which they were submitted.
CA 02682561 2009-10-14
.,
-1-
SYSTEM AND METHOD FOR TRACKING MEDICAL PRODUCTS IN A TWO
BIN PER MEDICAL PRODUCT REPLENISHMENT SYSTEM
FIELD OF THE INVENTION
[0001] The present relates to a system and method for tracking
medical
products. More particularly, the system and method further relates to tracking
products in a two bin per medical product replenishment system.
BACKGROUND OF THE INVENTION
[0002] Today's health care facilities include a wide range of
establishments, from small and relatively simple medical clinics to large and
complex hospitals. All together, health care facilities use a large amount of
medical products for treating patients having various health conditions. In
some
instances, it is required to keep track of products that have been used for
treatment, or installed within patients, to ensure compatibility or to readily
be able
to contact patients for product recalls. More particularly, in large
hospitals,
keeping track of such products that have been used on or installed within
patients is a challenge, as it is performed manually, requires filling
multiple forms
which is time consuming and limited by practitioners and personal vigilance
and
discipline. It is thus obvious that such a manual tracking is only performed
for
specific products which are either more expensive or potentially health
dangerous.
[0003] In addition to the tracking problem, hospitals also face
an important
challenge with the management of expiry dates and recalls of lots of medical
products. Nowadays, the tracking of expiration dates and recalls of lots of
medical products is performed manually. A first control method consists of
having a person go through the various medical products at all units and
departments to manually check the expiration date and/or the lot number of
each
medical product, and to remove the medical products which expiration date has
CA 02682561 2009-10-14
-2-
lapsed or which lot number has been recalled. As this type of control cannot
be
performed on a daily or weekly basis, the medical products with the closest
expiration date are placed in the front, and the products with the furthest
expiration date are placed in the back, to ensure that closely expiring
medical
products are used first. In addition to this control method, practitioners and
personal has to further review prior to using a medical product that the
expiration
date has not lapsed. Although verification is performed in two steps, there
have
been reported instances where medical products used were expired or were
recalled.
[0004] It would therefore be advantageous to have a system and method
to more efficiently handle expiration dates and recalls of medical products.
SUMMARY OF THE INVENTION
[0005] In accordance with an aspect, the present invention provides a
system for tracking medical products in a two bin per medical product
replenishment system. The two bin per medical product replenishment system
comprises a central unit for linking a unique Radio Frequency Identifier
(RFID)
tags to a location, a bin and a medical product. The two bin per medical
product
replenishment system further comprises provisioning boards for generating a
provisioning request upon application of one of the unique RFID tags there
against. The system for tracking comprises a medical product unit, an RFID tag
activity unit and an analysis unit. The medical product unit stores medical
product information including lot number and date of receipt. The RFID tag
activity unit records replenishment requests and corresponding generated date.
The analysis unit determines probable locations of the medical product having
particular medical product information in the two bin per medical product
replenishment system. For doing so, the analysis unit correlates the date of
receipt of the medical product having the particular medical product
information
with the generated date of recorded replenishment requests, to identify
probable
CA 02682561 2009-10-14
-3-
corresponding replenishment requests. The analysis unit further correlates the
identified probable corresponding replenishment requests with the location
information of the corresponding RFID tag in the central unit to determine
corresponding probable locations.
[0006] In accordance with another aspect, the present invention provides
a
method for tracking medical products in a two bin per medical product
replenishment system. The method comprises storing medical product
information including lot number and date of receipt. The method also records
replenishment requests and generated date. Then, the method correlates the
date of receipt of the medical product having a medical product information of
interest with the generated date of recorded replenishment requests, to
identify
probable corresponding replenishment requests. Finally, the method further
correlates the identified probable corresponding replenishment requests with
the
location information of the corresponding RFID tag in the central unit, to
determine corresponding probable locations.
BRIEF DESCRIPTION OF THE DRAWINGS
[0007] For a better understanding of aspects of the system and method
described herein, and to show more clearly how they may be carried into
effect,
reference will be made to the accompanying drawings in which:
[0008] Figure 1 is a schematic representation of a two bins per medical
product replenishment system;
[0009] Figure 2 is a flow chart of a prior art method for provisioning
products of a two bins replenishment system with removable RFID tags;
[0010] Figure 3 is an exemplary block diagram of a central unit;
[0011] Figure 4 is an exemplary schematic block diagram of a system for
tracking; and
CA 02682561 2009-10-14
-4-
[0012] Figure 5 is an exemplary flowchart of a method for tracking
medical
products in a two bin per medical product replenishment system.
DETAILED DESCRIPTION OF THE INVENTION
[0013] Throughout the present specification, the expression "medical
product" is used to refer to any type of product or material to be used or
administered in the treatment of patients. Examples of medical products
include
intravenous solutions, catheters, tubes, etc.
[0014] Hospitals are divided in multiple departments, each of which
relates
to a particular specialization. Each department uses general and specific
products, stored in multiple units of the department. Storage is performed
using
storage equipment that allows the organization of products in two bins, as
schematically shown on Figure 1. Typically in such a system 100, a rack (not
shown for clarity purposes), is used to organize storage bins. For each
product
(products 1-6), a pair of two bins, namely a primary bin 110 and a secondary
bin
120 is assigned. The product is thus stored at each location in both the
primary
110 and secondary 120 bins. To each primary 110 and secondary 120 bins are
associated corresponding removable Radio Frequency Identification (RFID) tags
130. As both primary 110 and secondary 120 bins store a particular product,
each RFID tags is unique, and corresponds to a particular product, in a
specific
bin (primary or secondary), located at a particular location/unit/department
in the
hospital. When one of the bins becomes empty, the corresponding RFID tag is
removed from the bin, as shown for products 2 and 6, and apposed to a
provisioning board 140. The RFID tag 130 is usually removed from the bin and
applied to the provisioning board 140 by the person emptying or taking the
last
product in the bin. The provisioning board 140 includes an RFID reader to read
the RFID tags 130, and generate provisioning requests 150 therefore to a
central
unit 310. The provisioning request 150 may be sent by various ways, such as
wirelessly, intranet, Internet, etc.
CA 02682561 2009-10-14
-5-
[0015] Reference is now concurrently made to Figure 2, which represents
a flowchart of a method for provisioning products in the two bins
replenishment
system such as shown on Figure 1. The method starts with storing the product
210 in the corresponding primary 110 and secondary bin 120. Then, in the
course of daily activities, the employees of the department take 215, when
needed, the product from the primary bin. The employees continue taking the
product from the primary bin, until the latter is empty 220. When the primary
bin
is empty, the employee that takes the last item from the primary bin 110
places
225 the corresponding RFID tag 130 on the provisioning board 140. The
provisioning board 140 detects the proximity of the added RFID tag, and
generates and transmits 230 a provisioning request 150 for that product.
Optionally, it is also possible to switch 235 the primary bin 110 and
secondary bin
120 so as to facilitate access to the products in the secondary bin 120.
[0016] When provisions are received 240, the method continues with
rotating remaining products from the secondary bin to the primary bin, and
replenishing 245 the secondary 120 bin (and optionally the primary bin 110).
Upon completing the rotation and replenishing of the primary 110 and secondary
bins 120, the corresponding RFID tag 130 is removed from the provisioning
board 140 and replaced 250 on the corresponding bin. In the event that both
the
primary bin 110 and secondary bin 120 of a particular product were both empty
and had to be replenished, both corresponding RFID tags are removed from the
provisioning board 140 and replaced on their respective bin.
[0017] In the event that provisions are not received for the empty
primary
bin 110, when that corresponding product is required, the product is then
taken
255 from the secondary bin 120, until the secondary bin 120 becomes also
empty 260. When the secondary bin 120 also becomes empty, its removable
RFID tag is placed 265 on the provisioning board 140, and an urgent
provisioning
request 150 is generated and sent 270.
CA 02682561 2009-10-14
-6-
[0018] Reference is now made concurrently to Figures 1, 2 and 3, where
Figure 3 is a schematic block diagram of an exemplary central unit 310. The
central unit 310 is adapted for receiving and handling the provisioning
requests
150 for several departments or for a whole hospital. For doing so, the central
unit 310 comprises a provisioning request handling unit 320, a statistics unit
330,
a discrepancies identification and reporting unit 340 and an RFID tag and
location information 350. For clarity purposes, the following components have
not been depicted: power unit, communication unit, interface, and input/output
unit.
[0019] The central unit 310 receives the provisioning requests 150 from
provisioning boards 140 located in various departments of the hospital, and
the
provisioning requests 150 are handled by the provisioning requests handling
unit
320. The provisioning requests handling unit 320 compiles the requests, and
transfers information in order to generate the provisioning. If the required
products are in stock, the provisioning requests handling unit 320 transfers
the
information to a store or an appropriated department. If the required products
are not in stock at a stock room of the hospital, the provisioning requests
are
transferred to a purchasing department.
[0020] The statistics unit 330 calculates for each product used in the
hospital a consumption rate per bin, a consumption rate per department, a
provisioning rate, and various quotas using the information collected each
time a
RFID tag 130 has been put on the provisioning board 140 and removed from the
provisioning board 140. The statistics unit 330 calculates the various quotas
as
per the number of day each primary bin 110 and secondary bin 120 should carry.
The statistics unit 330 also calculates the various quotas based on
replenishment
time using the time that a RFID tag 130 is put on a provisioning board 140 to
the
moment that the RFID tag 130 is placed back on the primary bin 110 or
secondary bin 120.
CA 02682561 2009-10-14
-7-
[0021] The discrepancies identification unit 340 is adapted to identify,
based on the received provisioning requests and calculated statistics,
provisioning irregularities. Examples of provisioning irregularities that can
be
identified by the discrepancies identification unit 340 include: missing RFID
tags,
duplicate RFID tags, products no longer in use, etc.
[0022] The RFID tag and location information unit 350 links each RFID
tag 130 to a location (department/unit/room). It further associates the RFID
tag
130 to a rack (not shown) in that location, and to a specific bin in the rack.
The
RFID tag and location information unit 350 further stores information on the
product stored in the corresponding bin.
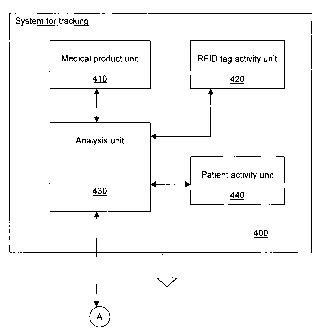
[0023] Reference is now made to Figure 4, which is a schematic
representation of a system 400 for tracking medical products in the two bin
per
medical product replenishment system previously described. The system 400
comprises a medical product unit 410, an RFID tag activity unit 420, and
analysis
unit 430 and a patient activity unit 440. The various units of the system 400
may
be implemented concurrently with the central unit 310 or implemented
separately. Thus the system for tracking 400 may be sold with the system 100
or
separately as an improvement.
[0024] The medical product unit 410 stores medical product information
for
all received medical products. The medical product information comprises lot
number and date of receipt. The stored medical product information may further
include an expiration date, a quantity, a number of units per box, product
code,
product serial number if available, product name, a product purchase date,
product expiry date, product supplier contact information and product
manufacturer's number, etc. The medical product unit 410 stores all necessary
product information required for tracking products being recalled: for example
by
lot #, batch#, serial #, etc.
CA 02682561 2009-10-14
,
-8-
[0025]
The RFID tag activity unit 420 records received replenishment
requests and date at which they were generated. The RFID tag activity unit 420
may further record the date at which the corresponding bins have been
replenished and their RFID tags were removed from the provisioning boards 140.
[0026]
The patient activity unit 440 is adapted for recording patient identity,
location and date of treatment. The patient activity unit 440 may be located
as
shown on Figure 4 in the system for tracking 400, or provided by a hospital
management database which tracks patient identity, date of treatment, and
location in which the treatment was administered.
[0027]
The analysis unit 430 is adapted for querying the medical product
unit 410, the RFID tag activity unit 420 and the patient activity unit 440.
The
analysis unit 430 determines probable location of medical products having one
or
several particular medical product information, such as for example: medical
product code, lot #, batch #, date of receipt, expiration date, etc.
Tracking
specific products in a hospital without the present system requires a lot of
manual
operations, and depending on the medical product information of interest, may
require several days of work, as each bin, each rack, each unit and each
department have to be manually checked. However, with the present system
and method, probable location of the medical products having the medical
product information of interest can be easily determined. The expression
"probable location" is used throughout the specification to both include when
location is certain and when location is mostly probable. As several medical
products are used in multiple units and departments, replenishment of those
medical products in the multiple units and departments may be performed on the
same day using received products from multiple lot numbers.
[0028]
When a request is received to track one or several medical
products having a particular medical product information, the analysis unit
430
queries the medical product unit 410 to confirm the receiving of a specific
medical product having one or several particular medical product information,
CA 02682561 2009-10-14
=
-9-
such as for example: lot #, batch #, date of receipt, expiration date, etc.
The
analysis unit 430 then gets into communication with the central unit to
extract
from the RFID tag and location information 350 a list of RFID tags
corresponding
to tracked medical product(s) and their corresponding location
(unit/department/storage rack/bin). The analysis unit 430 also queries the
RFID
tag activity unit 420 to extract a list of RFID tags corresponding to the
product(s)
being tracked and generated date of recorded replenishment request. The
analysis unit 430 may also query the RFID tag activity unit 420 for extracting
all
generated replenishment requests recorded corresponding to the product(s)
being tracked within a window of time. The window of time may correspond to
all
replenishment requests received since a prior date of reception for that
medical
product, or if the amount of medical products received was insufficient to
fulfill all
replenishment requests, a combination of window of time and rules of delivery
may be used to determine the probable replenishment requests.
[0029] The analysis unit 430 then correlates particular medical
product
information of the medical product(s) extracted from the medical product unit
410, with the list of RFID tags extracted from the RFID tag activity unit 420
to
determine probable locations. As in the two bin system, the first bin is
emptied
first, and when emptied the replenishment request is generated for its unique
RFID tag, it is possible to identify which bins are awaiting replenishment
when
the medical product is received. The analysis unit 430 can thus analyze the
extracted information from the medical product unit 410, the RFID tag activity
unit
420 and the RFID tag and location information based on a set of rules dictated
by
the two bin per medical product replenishment method.
[0030] A first rule is the replenishment of primary and
secondary bins. As
in step 245 of the method, the remaining medical products are rotated from the
secondary bin to the primary bin, although the replenishment request was
generated for the primary bin, the analysis unit 430 thus correlates that the
CA 02682561 2009-10-14
-10-
replenishment request received for the primary bin results in replenishing the
received medical products in its corresponding secondary bin.
[0031] Another rule is to determine whether one of the replenishment
requests corresponds to the secondary bin. When the replenishment request
corresponds to the secondary bin, verification is made as to whether a
replenishment request has also been received for the primary bin. In the event
that the replenishment request has not been received for the corresponding
primary bin, the discrepancy is reported to the discrepancies identification
and
reporting unit 340.
[0032] Another rule applied by the analysis unit is to determine whether
the RFID tag activity unit 420 records replenishment date or not. In the event
that the date of replenishment is recorded by the RFID tag activity unit 420,
upon
removal of the RFID tag from the provisioning board, the analysis unit 430
uses
the date of replenishment in its analysis for correlating the probable
locations.
[0033] In the event that some products with a medical product information
of interest have already been administered to one or several patients, the
present
system further allows tracking probable patients to which the medical product
has
been administered. For doing so, the analysis unit 430 further correlates the
probable location and date of the corresponding replenishment requests with
the
date of treatment recorded in the patient activity unit 440. This aspect
allows
identifying a window of patients to which the medical product having the
medical
product information of interest could have been administered. Thus it assists
in
tracking the probable group of patients to which a medical product having a
medical product information of interest, for example a recalled lot number,
could
have been administered. Although the present system cannot provide an
absolute of patients to which the medical product having the medical product
information of interest has been administered, it provides a report of
probable
patients, which is a subset of all patients having been treated during this
period.
CA 02682561 2009-10-14
-11-
[0034] For doing so, the patient activity unit 440 may record several of
the
following: a patient number, patient hospital card number, patient medical
insurance number, patient name, patient date of birth, patient sex, patient
contact
information, patient emergency contact information, date of treatment, type of
treatment, particular medical products used, etc. The patient activity unit
440
may be embedded within the system for tracking 400, or may be part of a
centralized patient system, which the analysis unit 430 may access, and query.
[0035] The results of the analysis unit 430 may be provided in the form
of
a written report, an electronic report, a broadcasted report for handling
recalls, a
weekly or monthly reported automatically sent to the concerned
units/departments or a responsible thereof.
[0036] Reference is now made to Figure 5, which depicts a flowchart of
the method of the present invention. The method starts with storing medical
product information 510 in the medical product unit 410. The method continues
with recording replenishment requests 520 by the RFID tag activity unit 420.
Then, for a medical product information of interest, a correlation is
performed of
the date of receipt of medical product to recorded replenishment requests 530.
The method then correlates the identified probable corresponding replenishment
requests with RFID tags to determine probable locations 540.
[0037] Alternately, if probable patients to which the medical product
having
the medical product information of interest have to be identified, the method
further records patient activity 550, and correlates corresponding probable
location(s) and date(s) of the probable replenishment requests to recorded
patient activity.
[0038] The present system and method thus allows a simple and efficient
way of tracking medical products and corresponding medical product information
without adding additional work on medical personnel. Furthermore, the present
system and method provides an efficient management tool for handling
CA 02682561 2015-02-23
-12-
expiration dates and product recalls. In addition to tracking the medical
products
with medical product information of interest, the present system and method
further assists the medical personnel in identifying groups of probable
patients to
which the medical product of interest could have been administered.
[0039] While the
present system and method for tracking have been
shown and described with reference to different aspects thereof, it will be
recognized by those skilled in the art that various changes in form and detail
may
be made herein without departing from the scope of the invention as defined by
the appended claims.