Note: Descriptions are shown in the official language in which they were submitted.
CA 02682892 2009-10-15
,
1 =
- 1 -
MATERIALS, METHODS AND SYSTEMS FOR SELECTIVE CAPTURE
OF CO2 AT HIGH PRESUSRE
FIELD OF THE INVENTION
The present invention pertains to the field of adsorption methods and systems
for
selective capture of carbon dioxide and other acid gases, more particularly,
to the field of
adsorption methods and systems that employ mesoporous silica for the
separation of carbon
dioxide and other acid gases at high pressure.
BACKGROUND
Carbon dioxide (CO2) is a major greenhouse gas with significant contribution
to global
warming (Halmann and Stenberg 1999). Removal of CO2 from different gas streams
is becoming
increasingly important for various applications like treatment of flue gas,
natural gas, biogas, and
hydrogen purification as well as closed-circuit breathing systems (CCBS) for
use in confined
spaces such as manned space shuttles (Satyapal et al. 2001), and in emergency
situations. The
recovered CO2, with different degrees of purity, also has numerous
applications in the chemical
industry.
Separation, capture and storage of carbon dioxide (CO2) have received
significant
attention in recent years. Liquid phase absorption in amine solutions has been
widely used to
treat gases with medium to high CO2 concentration, but due to the high
regeneration cost of the
absorbent and corrosion problems (Veawab et al. 1992), it is highly desirable
to develop less
energy intensive technologies like adsorption (Ruthven 1994) and membrane
separation (Hong et
al. 2008).
Many of CO2 adsorbents have been developed in recent years including metal
oxides
(Wang et al. 2008), zeolites (Goj et al. 2002; Cavenati et al. 2006; Akten et
al. 2003;
Belmabkhout et al. 2007), carbon (Himeno et al. 2005), metal-organic
frameworks (M0Fs)
(Millward and Yaghi 2005; Bourrelly et al. 2005; Yang et al. 2008; Yang and
Zhong 2006; Li
CA 02682892 2009-10-15
,
- 2 -
and Yang 2007), organo-silicas and surface-modified silicas (Harlick and
Sayari 2007; Comoti et
al. 2007) as well as membrane technology (Sridhar et al. 2007; Hong et al.
2008).
Ideally, an adsorption medium for CO2 removal at ambient temperature should
combine
(i) high CO2 uptake, (ii) complete regeneration under mild condition, (iii)
high thermal stability,
and (iv) favourable adsorption-desorption kinetics.
The discovery of periodic mesoporous materials like MCM-41 silica has resulted
in
extensive research activity on their synthesis and applications, particularly
for separation and
catalysis (Sayari 1996; Sayari and Jaroniec 2008). It is intriguing that
despite the significant
growth in the area of periodic mesoporous materials (for a review see Sayari
(2003) and
references therein), there are only few studies devoted to CO2 adsorption on
materials like
MCM-41 silica (Branton et al. 1995; Morishige et al. 1997; Morishige and
Nakamura 2004;
Sonwane et al. 1998). The early studies by Morishige et al. (1997, 2004) and
Sonwane et al.
(1998) focused on high pressure CO2 adsorption at temperature below 273 K for
the purpose of
structural characterization. He and Seaton (2006) studied low pressure
adsorption of pure CO2
and CO2-CH4 mixture for the characterization of MCM-41 surface heterogeneity.
Although, the
use of organically-modified silica materials for CO2 removal was extensively
studied using
different mesoporous silica supports such as MCM-41, SBA-15, MCM-48 and pore-
expanded
MCM-41 (for a review see Harlick and Sayari (2007) and reference therein);
adsorption of CO2
was investigated in a limited range of CO2 concentration, temperature and
pressure. The patent
application WO 2008/081102 (Pirngruber et al. 2008) discloses the use of metal-
organic
frameworks (M0Fs) having a pore diameter in the range of 0.5-5 nm and surface
area the range
of 2000-4000 m2/g, for hydrogen purification and carbon dioxide recovery at
pressure higher
than 4 bar.
This background information is provided for the purpose of making known
information
believed by the applicant to be of possible relevance to the present
invention. No admission is
necessarily intended, nor should be construed, that any of the preceding
information constitutes
prior art against the present invention.
CA 02682892 2009-10-15
,
t. =
- 3 -
SUMMARY OF THE INVENTION
An object of the present invention is to provide methods and systems for
selective CO2,
H2S, SO2 and other acid gases adsorption using mesoporous silica. In
accordance with one
aspect of the present invention, there is provided a process for the removing
CO2 from a gas
stream containing CO2, which process comprises conducting said gas stream
through an
adsorbent containing a mesoporous material under high pressure to adsorb said
CO2 onto said
adsorbent and produce a substantially CO2-free gas stream (Stage 1).
Advantageously, the
process additionally comprises the step of reducing the pressure on said
adsorbent having CO2
adsorbed thereon to a moderate pressure to desorb at least a fraction of the
adsorbed CO2 (Stage
2). When the two Stages 1 and 2 take place at the same temperature, the
process is a pressure
swing adsorption referred to as PSA-H/M where H in bar is the adsorption
pressure (Stage 1) and
M in bar is the desorption pressure (Stage 2).
In accordance with another aspect of the present invention, there is provided
a method for
selectively removing or recovering CO2, as well as H2S, SO2 and other acid
gases from a gaseous
stream or atmosphere containing CO2, H2S, SO2 and other acid gases, comprising
the step of
contacting the gaseous stream or atmosphere with an adsorbent comprising
ordered or disordered
mesoporous silica having a pore volume of between 0.4 and 4 cm3/g, a median
pore diameter of
between 2 and 50 nm and a BET surface area of between 500 and 2000 m2/g.
In accordance with another aspect of the invention, there is provided a system
for
selectively removing or recovering CO2, H2S, SO2 and other acid gases from an
gaseous stream
or atmosphere containing said CO2, H2S, SO2 and other acid gases using a
system comprising: (a)
a sorbent bed comprising a mesoporous silica; (b) means for contacting the
gaseous stream or
atmosphere with the sorbent bed; and (c) means of removing the CO2, H2S, SO2
and other acid
gases from the sorbent bed.
In accordance with another aspect of the present invention there is provided a
mesoporous silica adsorbent having a high gravimetric and volumetric CO2
adsorption capacity,
high efficiency for selective CO2 adsorption, fast CO2 kinetics with a low
energy requirement for
regeneration.
CA 02682892 2009-10-15
. ,
t
µ '
- 4 -
In one example, the gravimetric and volumetric CO2 adsorption capacities for
mesoporous MCM-41-100 silica was 64.7 wt% (14.7 mmol/g) and 234.2 cm3/cm3 at
45 bar and
room temperature.
In another example, the CO2 selectivity vs. N2 in CO2:N2 = 20:80 mixture over
MCM-41-
100 was 15 at 45 bar and room temperature.
In another example, the CO2 selectivity vs. 02 in CO2:02 = 95:5 mixture over
MCM-41-
100 was 22 at 45 bar and room temperature.
In another example, the CO2 selectivity vs. CH4 in CO2:CH4 = 50:50 mixture
over MCM-
41-100 was 7 at 45 bar and room temperature.
In another example, the CO2 selectivity vs. H2 in CO2:H2 = 20:80 mixture over
MCM-41-
100 was 63 at 45 bar and room temperature.
In accordance with another aspect of the present invention there is provided a
PSA-H/M
process using mesoporous silica for bulk CO2 separation process with the dual
purpose of
separation at high pressure (e.g., H = 45 bar) and recovery of CO2 at moderate
pressure (M = 10
bar for example) from gas streams.
In one example, the CO2 PSA-45/10 operating capacity in CO2:N2= 20:80 mixture
over
MCM-41-100 was 11.13 wt% (2.58 mmol/g).
In another example, the CO2 PSA-45/10 operating capacity in CO2:CH4= 50:50
mixture
over MCM-41-100 was 23.7 wt% (5.40 mmol/g).
In another example, the CO2 PSA-45/10 operating capacity in CO2:H2 = 20:80
mixture
over MCM-41-100 was 13.3 wt% (3.1 mmol/g).
In accordance with another aspect of the present invention there is provided a
mesoporous silica adsorbent having a high capacity of CO2 at high pressure
with and without the
presence of water vapour.
CA 02682892 2009-10-15
- 5 -
In another example, the gravimetric CO2 adsorption capacity for mesoporous PE-
MCM-
41 silica in dry and humid (40% relative humidity, RH) conditions was 100 wt%
(22.8 mmol/g)
and 102 wt% (23.2) at 60 bar and room temperature.
In accordance with another aspect of the present invention there is provided a
hydrated
mesoporous silica adsorbent having an enhanced selectivity toward CO2 vs.
supercritcal gases
such as N2, CH4, 02 and H2.
BRIEFDESCRIPTION OF THE FIGURES
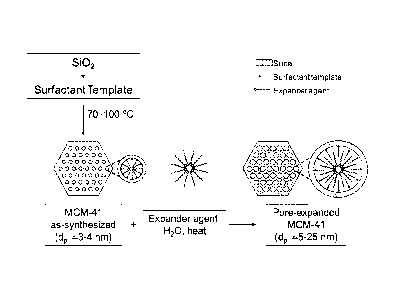
Figure 1 schematically depicts the synthesis of MCM-41 mesoporous silica and
post-
synthesis pore expansion to PE-MCM-41.
Figure 2 shows N2 adsorption isotherms for materials; the inset figure
represents the pore
size distributions.
Figure 3 graphically depicts fractional CO2 uptake (nt/ne) at 1 bar and 298 K
for MCM-
41-100, PE-MCM-41.
Figure 4 graphically depicts gravimetric CO2 excess adsorption uptake of MCM-
41-100
in comparison with other adsorbents.
Figure 5 shows volumetric CO2 excess adsorption uptake for MCM-41-100 in
comparison with other adsorbents.
Figure 6 shows volumetric CO2 excess adsorption uptake per unit surface area
for MCM-
41-100 in comparison with other adsorbents.
Figure 7 depicts CO2 excess adsorption isotherms for MCM-41-100 and MaxsorbAC
at
298 K showing PSA-45/10 working CO2 capacity when adsorption and desorption
stages take
place at 45 bar and 10 bar, respectively.
Figure 8 depicts the adsorption isotherms of CO2, N2, CH4, H2 and 02 on MCM-41-
100
at 298 K.
CA 02682892 2009-10-15
. ,
.µ
=
- 6 -
Figure 9 shows the molar selectivity ratio of CO2 to CH4 adsorbed on MCM-41-
100, 13X
zeolite, MaxsorbAC and NoritAC at 298 K vs. pressure.
Figure 10 shows IAST prediction compared to experimental data for adsorption
of
CO2:N2 = 20:80 mixture on MCM-41-100 at 298 K.
Figure 11 shows IAST CO2 selectivity over N2 for CO2:N2 = 20:80 mixture over
MCM-
41-100 compared to NoritAC and 13X at 298 K vs. pressure.
Figure 12 shows IAST CO2 selectivity over CH4 vs. pressure for CO2:CH4 = 50:50
mixture on MCM-41-100 compared to NoritAC, MaxsorbAC and 13X at 298 K.
Figure 13 shows IAST CO2 selectivity over H2 for CO2:H2 = 20:80 mixture on MCM-
41-
100 compared to IAST CO2 selectivity over H2 for CO2:H2 = 1.4:98.6 mixture for
NaA zeolite at
298 K vs. pressure
Figure 14 shows IAST CO2 selectivity over 02 for CO2:02 = 95:5 mixture for MCM-
41-
100 at 298 K vs. pressure.
Figure 15 schematically depicts the general procedure for CO2 capture.
Figure 16 schematically depicts the proposed procedure for CO2 capture using
PSA-H/M
with H = 45 bar and M = 10 bar.
Figure 17 depicts gravimetric CO2 excess adsorption uptake of PE-MCM-41-100 in
dry
and hydrated conditions.
DETAILED DESCRIPTION OF THE INVENTION
Unless defined otherwise, all technical and scientific terms used herein have
the same
meaning as commonly understood by one of ordinary skill in the art to which
this invention
belongs.
The present invention provides methods and systems for CO2 adsorption that
take
advantage of the selective CO2 adsorption capabilities of mesoporous silica,
particularly when
CA 02682892 2009-10-15
- 7 -
adsorption is performed under high pressure. In one preferred embodiment, the
system and
process or method of the invention includes the use of mesoporous silica as a
sorbent.
Mesoporous silica
Mesoporous silicas exhibit ordered or disordered pore systems. These
mesoporous silicas
include those prepared in the presence of surfactants or polymer solutions via
different pathways
including the so-called cooperative organization mechanism and the liquid
crystal templating
mechanism (For review see Sayari 2003). Typically, the surfactants or polymers
are removed by
calcination of mesoporous silica precursor at high temperature. Other
procedures for surfactant
or polymer removal such as solvent extraction or microwave treatment may also
be applied.
Mesoporous silicas may exhibit different structures and pore systems, the most
prominent being
the so-called MCM-41 with a two-dimensional hexagonal symmetry. Table 1
provides a non-
exhaustive list of mesoporous silicas, prepared under different pH conditions
using different
amphiphile molecules, that can be used in the present invention. The pore size
of such material
may be adjusted from a low of 1 nm to well into the macropore regime, i.e. >
50 nm.
Table 1: Mesoporous Silicas and Organosilicas
Mesophase Amphiphile template pH Structure Ref.
MCM-41 CH2n+I(CH3) 3N+ basic 2D hexagonal (p6mm) [1]
MCM-48 CõH2n+1 (CH3)3N+ basic cubic ( Ia-3d ) [1]
Gemini Cn_s-na [2]
FSM-16 C 16H31(CH3)3N+ basic 2D hexagonal (p6mm) [3]
SBA-1 C 18H37N(C2H5)3+ acidic cubic (Pmn ) [2]
SBA-2 Divalent Cn_,_ lb acidic! 3D hexagonal (P63/mmc)
[2]
basic
SBA-3 CõH2+IN(CH3)3+ acidic 2D hexagonal (p6mm) [4]
SBA-6 Divalent 18B4_3-1c basic cubic (Pmn) [5]
SBA-8 Bolaformd basic 2D rectangular (cmm) [6]
SBA-11 Brij* 56; Ci6E0lo acidic cubic (Pm3m ) [7]
SBA-12 Brij 76; CI8E010 acidic 3D hexagonal (P63/mmc)
[7]
SBA-14 Brij 30; C12E04 acidic cubic [7]
SBA-15 P123; E020P070E020 acidic 2D hexagonal (p6mm) [8]
KIT-6 P123 + Butanol acidic cubic (Ia3d) [9]
CA 02682892 2009-10-15
,
. .
- 8 -
Mesophase Amphiphile template pH Structure Ref.
MU-11 CF3(CF2)5(E0)14 acidic disordered [27]
JLU-12 CF3(CF2)5(E0)14 neutral disordered [27]
MU-14 CF3(CF2)4(EO)10 acidic 2D hexagonal (p6mm) [30]
MU-15 CF3(CF2)4(E0)10 neutral 2D hexagonal (p6mm) [30]
JLU-20 P123 + FC-4 e acidic 2D hexagonal (p6mm) [10]
MU-21 FC-4 and F127 acidic cubic Im3m [28]
MU-30 (>1600) DIHAB basic 2D hexagonal (p6mm) [29]
PSU-1 P123 + CTAC1 acidic 2D hexagonal (p6mm) [11]
Mesocellular P123 + TMB f acidic disordered [12]
SBA-16 F127; E0106P070E0106 acidic cubic (-/m3m ) [7]
KIT-5 F127 acidic cubic (Fm3m) [13]
FDU-12 F127 + additives g acidic cubic (Fm3m) [14]
FDU-1 B50-6600; E039B047E039 acidic cubic (bn3m) [15]
FDU-2 RN+N+N+ h basic cubic (Fd3m) [16]
FDU-5 P123 + additives I acidic cubic ('a3/) [17]
FDU-18 PEO-b-PS acidic cubic (Fm3m) [26]
FDU-12 F127 + TMB acidic cubic ( Fm3m ) [25]
AMS-1: 3D hexagonal
[18,19]
AMS-2: 2D cubic
AMS-3: 2D hexagonal
AMS-n Anionic surfactant basic AMS-4: 3D cubic
AMS-6: 3D cubic
AMS-7: 3D disordered
AMS-8: 3D cubic
[31]
AMS-10: cubic Pn3m
MSU-1 Tergitol; C it-15(E0)12 neutral
disordered [20]
MSU-2 TX-114; C8Ph(E0)8 neutral disordered [20]
TX-100; C8Ph(E0)10
MSU-3 P64L; E013P030E013 neutral disordered [20]
MSU-4 Tween'')-20, 40, 60, 80 neutral
disordered [21]
MSU-V H2N(CH2)NH2 neutral lamellar [22]
MSU-G C,112,1NH(CH2)2N112 neutral lamellar [23]
HMS CõI-12,7+1NH2 neutral disordered [24]
EO = ethylene oxide; PO = propylene oxide.
(a) Gemini surfactants Cn_s_, : CnH2n+IN+(CH3)2(CH2),N+(CH3)2C,,H2,i+1.
(b) Divalent surfactants Cj : CnI-12,+IN+(CH3)2(CH2),N+(CH3)3.
(c) Divalent surfactant 18134_3_1: C1814370-C6114-
0(CH2)41\1+(CH3)2(CH2)3N+(CH3)3.
CA 02682892 2009-10-15
=
- 9 -
(d) Bolaform surfactants :(CH3)3N+(CH2)nO-C61-14-C6H4-0(CH2)N (CH3)3.
(e) FC-4: (C3F70(CFCF3CF20)2CFCF3CONH(CH2)3N+(C2H5)2CH3F.
(f) TMB: trimethylbenzene.
(g) Additives = TMB and KC1.
(h) Tr-head group surfactant: CI6H33N+(CH3)2(CH2)2N+(CH3)2(CH2)31\1 (CH3)3
(i) Additives = 3-mercaptopropyl-trimethoxysilane (MPTS) and benzene, or a
benzene derivative
(methyl-, ethyl-, dimethyl-, or trimethylbenzene).
(j) (1,3-dimethy1-2-imidazolidin-2-ylidene)hexadecylmethylammonium bromide
Table 1 References
1. J.S. Beck, J.C. Vartuli, W.J. Roth, M.E. Leonowicz, C.T. Kresge, K.D.
Schmitt, C.T-W. Chu, D.H.
Olson, E.W. Sheppard, S.B. McCullen, J.B. Higgins and J.L. Schlenker, J. Am.
Chem. Soc. 114 (1992)
10834.
2. Q. Huo, R. Leon, P.M. Petroff and G.D. Stucky, Science 268 (1995) 1324.
3. T. Yanagisawa, T. Shimizu, K. Kuroda and C. Kato, Bull. Chem. Soc. Jpn. 63
(1990) 988.
4. Q. Huo, D.I. Margolese and G.D. Stucky, Chem. Mater. 8 (1996) 1147.
5. Y. Sakamoto, M. Kaneda, 0. Terasaki, D. Zhao, J.M. Kim, G.D. Stucky, H.J.
Shin and R. Ryoo,
Nature 408 (2000) 449.
6. D. Zhao, Q. Huo, J. Feng, J. Kim, Y. Han and G.D. Stucky, Chem. Mater.
11(1999) 2668.
7. D. Zhao, Q. Huo, J. Feng, B.F. Chmelka and G.D. Stucky, J. Am. Chem. Soc.
120 (1998) 6024.
8. D. Zhao, Q. Huo, J. Feng, B.F. Chmelka and G.D. Stucky, Science 279 (1998)
548.
9. F. Kleitz, S.H. Choi and R. Ryoo, Chem. Commun. (2003) 2136.
10. Y. Han, D. Li, L. Zhao, J. Song, X. Yang, N. Li, Y. Di, C. Li, S. Wu, X.
Xu, X. Meng, K. Lin and F.-
S. Xiao, Angew. Chem. Int. Ed. Engl. 42 (2003) 3633.
11. B.L. Newalkar, S. Komarneni, U.T. Turaga and H. Katsuki, J. Mater. Chem. 7
(2003) 1710.
12. P. Schmidt-Winkel, W.W. Lukens, Jr., D. Zhao, P. Yang, B.F. Chmelka and
G.D. Stucky, J. Am.
Chem. Soc. 121 (1999) 254.
13. F. Kleitz, D. Liu, G.M. Anilkumar, I.-S. Park, L.A. Solovyov, A.N. Shmakov
and R. Ryoo, J. Phys.
Chem. B. 107 (2003) 14296.
14. J. Fan, C. Yu, F. Gao, J. Lei, B. Tian, L. Wang, Q. Luo, B. Tu, W. Zhou
and D. Zhao, Angew. Chem.
Int. Ed. Engl. 42 (2003) 3146.
15. C. Yu, Y. Yu and D. Zhao, Chem. Commun. (2000) 575.
16. S. Shen, Y. Li, Z. Zhang, J. Fan, B. Tu, W. Zhou and D. Zhao, Chem Commun.
(2002) 2212.
17. X. Liu, B. Tian, C. Yu, F. Gao, S. Xie, B. Tu, R. Che, L.-M. Peng and D.
Zhao, Angew. Chem. Int. Ed.
Engl. 41(2002) 3876.
18. S. Che, A.E. Garia-Bennett, T. Yokoi, K. Sakamoto, H. Kumieda, 0.
Terasaki, T. Tatsumi, Nature
Mater. 2 (2003) 801.
19. A.E. Garia-Bennett, 0. Terasaki, S. Che, T. Tatsumi, Chem. Mater. 16
(2004) 813.
20. S.A. Bagshaw, E. Prouzet and T.J. Pinnavaia, Science 269 (1995) 1242.
21. E. Prouzet, F. Cot, G. Nabias, A. Larbot, P. Kooyman and T.J. Pinnavaia,
Chem. Mater. 11(1999)
1498.
22. P.T. Taney, Y. Liang and T.J. Pinnavaia, J. Am. Chem. Soc. 119 (1997)
8616.
23. S.S. Kim, W. Zhang and T.J. Pinnavaia, Science 282 (1998) 1302.
24. P.T. Taney and T.J. Pinnavaia, Science 267 (1995) 865.
25. X. Zhou, S. Qiao, N. Hao, X. Wang, C. Yu, L. Wang, D. Zhao, and G.Q. Lu
Chem. Mater. 19 (2007)
1870.
26. Y. Deng, T. Yu, Y. Wan, Y. Shi, Y. Meng, D. Gu, L. Zhang, Y. Huang, C.
Liu, X. Wu, D. Zhao, J.
Am. Chem. Soc. 129 (2007) 1690.
27. Y. Di, X. Meng, S. Li and F.-S. Xiao Microporous Mesoporous Mater. 82
(2005) 121.
28. D. Li, Y. Han, J. Song, L. Zhao, X. Xu, Y. Di, F.-S. Xiao, Chem.-A Eur. J.
10(2004) 5911.
CA 02682892 2009-10-15
=
=
- 10 -
29. X. Yang, S. Zhang, Z. Qiu, G. Tian, Y. Feng, F.-S. Xiao, J. Phys. Chem. B
108 (2004) 4696.
30. X. Meng, T. Di, L. Zhao, D. Jiang, S. Li, F.-S. Xiao, Chem. Mater. 16
(2004) 5518.
31. T. Yokoi, T. Tatsumi, J. Japan Petroleum Institute 50 (2007) 299.
Following the initial preparation steps, the mesoporous silica can be calcined
or solvent
extracted to remove surfactant and, if necessary, characterised using X-ray
diffraction, N2
adsorption, scanning electron microscopy, and/or transmission electron
microscopy.
The mesoporous silicas of the present invention include, but are not limited
to, all
mesoporous silicas described in Table 1. They are prepared in the presence of
a structure
directing agent which consists of a surfactant, oligomer, or polymer. The
mesoporous material is
then treated to remove the structure directing agent, either by heat treatment
or by extraction.
Mesoporous silicas that are suitable for use in the present invention exhibit
preferably
high surface area, large pore volume and high degree of pore ordering. Such
material shows a
suitable combination of adsorption uptake, adsorption kinetics, separation
efficiency and ease of
regeneration using pressure swing adsorption (PSA).
Mesoporous silicas that are suitable for use in the present invention exhibit
high surface
areas and provide sufficiently large pores to enable relatively unhindered
flow of CO2, or other
acid gases, containing gaseous streams inside the pore system. The resulted
modified
mesoporous silicas exhibit a high adsorption uptake, fast adsorption kinetics,
high separation
efficiency and ease of regeneration using temperature swing (TSA), pressure
swing (PSA)
adsorption or a combination of both temperature and pressure swing adsorption.
Adsorption Methods and Systems
The present invention further provides methods and systems for removing CO2
and/or
other acid gases, such as H2S and SO2, using mesoporous silicas. For
simplicity, the following
discussion specifically refers to CO2 as the acid gas.
Mesoporous silicas can be used successfully as an adsorbent for CO2 under high
pressure
with desorption under moderate pressure. The terms "high pressure" and
"moderate pressure", as
used herein, refers to the operational pressure of greater than 10 bar and 2
bar for both adsorption
CA 02682892 2009-10-15
=
- 11 -
and desorption stages, respectively, but preferably higher than 20 bar and 5
bar, respectively. It is
noteworthy that conventional pressure swing adsorption (PSA) processes operate
between a high
loading pressure and 1 bar or vacuum for the desorption stage. Mesoporous
silica adsorbents can
be used for CO2 bulk separation from different pre-dried gaseous streams. The
proposed PSA-
HIM using mesoporous silica is particularly suitable for simultaneous
separation and recovery of
CO2 at high (e.g, H = 45 bar) and medium (e.g., M = 10 bar) pressures,
respectively.
In accordance with another aspect of the present invention, there is provided
a system for
CO2 adsorption. The system comprises a sorbent bed that includes a mesoporous
silica and a
means for contacting a gaseous stream containing CO2 with the sorbent bed for
a sufficient
amount of time to permit adsorption of the CO2 by the mesoporous silica.
Once the mesoporous silica adsorbent has been synthesized, it can be employed
in a
sorbent bed for use in an adsorption process, such as a cyclic adsorption-
regeneration process.
To apply the adsorbent of the present invention to such an adsorption process,
it must be formed
into a stable, mechanically strong form. These forms may include, but are not
limited to, powder
forms, pellet forms and monolithic structures or foams. In the case of pellet
forms, the adsorbent
is mixed with a suitable inert or active secondary material as a binder.
Criteria for selecting a
suitable binder can include (i) achieving pellets or extrudates with minimum
amount of binder;
(ii) enhanced mechanical stability; (iii) preservation of adsorbent porosity
and accessibility of
adsorption sites; and (iv) affordability. For example, siloxanes and siloxane
derivatives can be
employed with the appropriate weight percentage as binders for mesoporous
silica to form
structured pellets, extrudates or spheres. The selection of the appropriate
form and, if necessary,
additive, is based on the application of the adsorbent and the type of
equipment used in the acid
gas removal process. The selection and manufacture of the adsorbent form is
well within the
ordinary abilities of a worker skilled in the art.
Once the adsorbent form is selected and manufactured, it is used in a sorbent
bed where a
gaseous stream containing CO2, and possibly water vapour, contacts the
adsorbent. In the
presence of mesoporous silica, the CO2 interacts with the silica surface and
is physically
adsorbed.
CA 02682892 2009-10-15
' *
*. =
- 12 -
According to a specific embodiment of the present invention, once the
mesoporous silica
is loaded with CO2 to a satisfactory level, or at a designated cycle time, the
sorbent bed can be
regenerated. Regeneration comprises ceasing the flow of the acid gas
containing stream through
the bed and desorbing the adsorbed acid gas. The desorption is accomplished by
pressure
gradient means or by the use of a sweeping or purge gas, or any combination
thereof. During this
step, the adsorbed CO2 is released and flushed or washed out of the sorbent
bed. The adsorbent
is then ready for re-use. In a specific example, in which the mesoporous
silica is MCM-41-100
with pore diameter of 3.3 nm, CO2 is removed at medium pressures, typically 2
to 5 bar or
vacuum and the regenerated material is ready for re-use.
The CO2 removed from the sorbent via a desorption process can be collected at
low or
medium pressure purge. The CO2 thus recovered can be reused in a variety of
applications or can
be compressed for sequestration. As such, the present invention further
provides a method of
manufacturing CO2, which method comprises the steps of adsorbing CO2 on
mesoporous silica
and collecting the adsorbed CO2 following desorption from mesoporous silica.
In one embodiment of the present invention, the use of the adsorbent to remove
CO2,
another acid gas, or a combination thereof, can comprise utilising two or more
sorbent beds
operating cyclically such that the first bed is in the adsorption cycle while
the second bed is in
the desorption cycle. This system comprises two or more sorbent beds and
computer or manually
controlled valves and pumps allowing for continuous CO2 and other acid gases
removal from the
gaseous stream.
In one embodiment of the present invention, mesoporous silicas can be used for
the
removal and recovery of CO2, or other acid gases from streams containing in
addition to CO2, or
other acid gases, other gases including, but not limited to, H2, N2, 02, CO,
CH4 and other
hydrocarbons using PSA-H/M. Gaseous streams include, but are not limited to,
natural gas,
biogas, syngas, stack gas and air.
In one embodiment of the present invention, if necessary, different amounts of
humidity
may be added during adsorption and/or desorption operation in PSA-H/M in
fixed, moving or
fluidized beds, to optimize the capture of CO2.
In one embodiment of the present invention, mesoporous silicas can be used for
the
removal and recovery of CO2, or other acid gases from streams containing in
addition to CO2,
CA 02682892 2009-10-15
. ,
. =
- 13 -
other gases including, but not limited to, H2, N2, 02, CO, CH4 and other
hydrocarbons using wet
(i.e., added moisture) adsorption processes, i.e., WPSA-H/M. Gaseous streams
include, but are
not limited to, natural gas, biogas, syngas, stack gas and air.
To gain a better understanding of the invention described herein, the
following examples
are set forth. It should be understood that these examples are for
illustrative purposes only.
Therefore, they should not limit the scope of this invention in any way.
EXAMPLES
EXAMPLE 1: Preparation of MCM-41-X mesoporous silica
Figure 1 shows the procedure for the synthesis of periodic mesoporous MCM-41
silica.
MCM-41-X silica where X is the synthesis temperature in degree celsius was
prepared in the
presence of cetyltrimethylammonium bromide (CTAB) using the overall mixture
composition:
1.0 Si02 : 0.29 TMAOH : 0.21 CTAB : 60 H20. In a typical synthesis, 1.76 g of
tetramethylammonium hydroxide (TMAOH) (25%) was diluted with 72 g of water
before adding
5.1 g of CTAB under vigorous stirring. After 15 min, 4 g of Cab-O-Sil silica
was added. The gel
obtained after stirring for an additional 30 min was transferred into a Teflon-
lined autoclave, and
heated statically under autogenous pressure for 40 h at a temperature within
the range of 298 to
403 K. The obtained materials were filtered washed extensively, dried, and
calcined at 813 K.
The structural properties of MCM-41-100 as determined by nitrogen adsorption
were: 1490 m2/g,
0.99 cm3/g, 3.3 nm for the surface area, pore volume and pore diameter,
respectively (Figure 2).
EXAMPLE 2: Preparation of pore-expanded MCM-41 (PE-MCM-41) silica
Figure 1 shows also the procedure for the post-synthesis pore expansion of MCM-
41. The
expander agent used for the preparation of PE-MCM-41 was dimethyldecylamine
(DMDA).
More details about the procedure may be found elsewhere (Serna-Guerrero and
Sayari 2007;
Harlick and Sayari 2007). Under appropriate conditions, i.e., DMDA/MCM-41
ratio, temperature
and time of the post-synthesis hydrothermal stage, the pore size of MCM-41 can
be expanded
from ca. 3 nm up to ca. 25 nm. As shown earlier (Harlick and Sayari 2007),
pore size tuning is
critical for improved CO2 adsorptive properties at high pressure. The
structural properties for a
CA 02682892 2009-10-15
= .
1 =
- 14 -
PE-MCM-41 sample as determined by nitrogen adsorption were: 1230 m2/g, 3.09
cm3/g, 11.7 nm
for the surface area, pore volume and pore diameter, respectively (Figure 2).
EXAMPLE 3: Method for measurement of adsorption properties and kinetics
Adsorption equilibrium and kinetics measurements of pure CO2 were performed
using a
Rubotherm gravimetric-densimetric apparatus (Rubotherm, Bochum Germany),
composed
mainly of a magnetic suspension balance (MSB) and a network of valves, mass
flowmeters and
temperature and pressure sensors. It operates both in closed and open loops.
In a typical
adsorption experiment, the adsorbent was weighed and placed in a basket
suspended by a
permanent magnet through an electromagnet. The cell in which the basket is
housed was then
closed, and vacuum or high pressure was applied. This system is able to
perform adsorption
measurements in a wide range of gas pressure from 0 to 60 bar. The adsorption
temperature may
also be controlled within the range of 298 to 423 K. The clean (outgassed)
adsorbent is exposed
to flowing pure CO2 at constant temperature at a rate of 100 ml/min. In a
typical experiment for
kinetic measurements, the gas was introduced in such a way to reach the
desired pressure in 5-10
s. The change in the weight of the adsorbent sample as well as the pressure
and temperature were
measured continuously until the thermodynamic equilibrium was reached. The
change in the
weight of the adsorbent sample as well as the pressure and temperature were
monitored
continuously until the thermodynamic equilibrium was reached. The gravimetric
method allows
the direct measurement of the reduced mass n. Correction for the buoyancy
effect is required to
determine the excess adsorbed amount rnp
..xcess (Belmabkhout et al. 2004; Dreisbach et al. 2003)
using equation 1, where Vadsorbent and Vs, refer to the volume of the
adsorbent and the volume of
the suspension system, respectively. These volumes were determined using the
helium isotherm
method by assuming that helium penetrates in all the open pores of the
materials without being
adsorbed (Sircar 2002; Belmabkhout et al. 2004). The density of the gas pgas
was determined
experimentally using a volume-calibrated titanium cylinder. By weighing this
calibrated volume
in the gas atmosphere, the local density of the gas was also determined.
Simultaneous
measurement of gas uptake and gas phase density as a function of pressure and
temperature was
thus possible.
n= mexcess ¨ P gas(Vadsorbent +Vss)
(1)
CA 02682892 2009-10-15
- 15 -
EXAMPLE 4: Kinetics of CO2 adsorption
Figure 3 shows the kinetic curve for adsorption at 298 K and 1 bar over MCM-41-
100,
PE-MCM-41 materials determined using pure CO2 flowing at 200 mL/min.
The CO2 adsorption kinetic curves were fitted to Linear Driving Model (LDF)
(Murcia et
al. 2003), to estimate the kinetic rate constant of CO2 adsorption. The LDF
model is described by
the equation 2:
nt =1¨e-kt (2)
ne
where ne is the equilibrium uptake at 298 K and 1 bar, n, is the uptake at
time t and k is the
kinetic rate constant. The results of the fit are shown in Fig. 7 and Table 2.
The CO2 kinetic rate
constant was significantly higher upon pore expansion, most likely due to the
larger pores and
higher pore volume of PE-MCM-41 in comparison to MCM-41-100. The PE-MCM-41 has
higher kinetic rate constant than MCM-41-100, up to 0.5 fractional uptake
nt/ne. The sequence in
terms of LDF kinetic rate constant was PE-MCM-41 > MCM-41-100.
Table 2: LDF kinetic rate constant of CO2 adsorption
Material k (LDF kinetic rate constant) / s-1
MCM-41-100 4*10-2
PE-MCM-41 6*10-2
EXAMPLE 5: Comparison of MCM-41 silica with other adsorbents
Extensive investigations have been carried out on CO2 adsorption using well
known
benchmark industrial adsorbents such as zeolites and carbon-based materials or
the rapidly
evolving hybrid materials, MOFs. Among these materials, the most promising CO2
adsorbents
were selected and compared with the current MCM-41-100 silica for CO2
adsorption up to 45
bar pressure at ambient temperature. Pertinent properties of the selected
materials are shown in
Table 3 . Figures 4, 5 and 6 show the CO2 gravimetric, volumetric and
volumetric per surface
area excess uptakes of CO2 on the above-mentioned materials in comparison to
MCM-41-100 at
ambient temperature. The comparison on a volume basis was made by multiplying
the density of
CA 02682892 2009-10-15
. .
- 16 -
the corresponding material shown in Table 3 by the gravimetric CO2 capacity in
cm3 STP/g. The
particle density (ca. 0.71 g/cm3) of MCM-41-100 was calculated from the
experimentally
determined skeletal density (2.34 g/cm3) and the pore volume (ca. 0.99 cm3/g).
Table 3. Surface area and density of the selected materials
Materials SBET (m2/g) Density (g/cm3) Reference
13X 685 1.13(a) Belmabkhout et al. 2007;
Cavenati et al. 2004
MaxsorbAC 3250 0.29(1') Himeno et al. 2005
NoritAC 1450 0.43 (b) Himeno et al. 2005
MOF-177 4508 0.43(e) Millward and Yaghi
2005; Yang et al. 2008
IRMOF-1 2833 0.59(c) Millward and Yaghi
2005; Yang et al. 2008
MCM-41-100 1490 0.71 (a) This work
(a) particle density, (b) packed density, (e) crystallographic density
In terms of CO2 gravimetric capacity, as shown in Fig. 4, MCM-41-100 exhibited
the
lowest capacity at low pressure but exceeded 13X zeolite and NoritAC carbon at
a pressure of
ca. 20 bar and 30 bar, respectively. At 45 bar, the CO2 adsorption capacity
for MCM-41-100 was
14.7 mmol/g vs. ca. 7.37 mmol/g and 11.28 mmol/g for 13X and NoritAC,
respectively. The
sequence of the gravimetric uptake at 45 bar was as follows: MOF-107 >
MaxsorbAC > IRMOF-
1 > MCM-41-100 > NoritAC > 13X.
hi terms of CO2 volumetric capacity, as shown in Fig. 5, MCM-41-100
outperformed
13X zeolite as well as NoritAC and MaxsorbAC carbons at high pressure, but
exhibited lower
volumetric capacity than MOF-177 and IRMOF-1. The sequence of the volumetric
uptake at 45
bar was as follows MOF-107 > IRMOF-1 > MCM-41-100 > MaxsorbAC > NoritAC > 13X.
Nevertheless, mesoporous silicas materials have the advantage of being very
stable during
CA 02682892 2009-10-15
=
- 17 -
prolonged exposure to ambient air and moisture (Cassiers et al. 2002). This is
in contrast to
MOF-177 and IRMOF-1 as reported recently (Li and Yang 2007; Bahr et al. 2007).
Comparison in terms of volumetric uptake on a surface area basis is provided
in Fig. 6.
MCM-41-100 exhibited comparable capacity at high pressure (ca. 45 bar) as 13X
and exceeded
slightly all the other aforementioned materials, indicative of the high
surface efficiency of MCM-
41-100 for CO2 adsorption. Moreover, as shown in Table 4, MCM-41-100 exhibited
one of the
weakest adsorbent-0O2 interactions, reflected by lower isosteric heat of
adsorption, allowing
CO2 to desorb at very mild conditions, in contrast to 13X.
Table 4. Isosteric heat of CO2 adsorption at low loading for MCM-41-100 and
the benchmark
adsorbents
Material Qisos (dmol-1) References
13X 37.2 Cavenati et al. 2004
NoritAC 22 Himeno et al. 2005
MCM-41-100 21.6 This work
MaxsorbAC 16.2 Himeno et al. 2005
The low gavimetric CO2 adsorption capacity of MCM-41-100 at low to moderate
pressures (1-10 bar) may seem to be unattractive for CO2 separation in
comparison to the
benchmark commercial materials. It is however important to notice that the
current MCM-41-
100 exhibited ca. 43.6 wt% pure CO2 operating PSA capacity (designated as A02)
as shown in
Fig. 7 based on 45 and 10 bar as pressures for the adsorption and desorption
stages, respectively.
This CO2 uptake is lower than for MaxsorbAC (ca. 58.6 wt%) but significantly
higher than for
NoritAC (ca. 13.2 wt%) and 13X ( 3.7 wt%). Thus, MCM-41-100 can be used for
example in
PSA separation processes with the dual purpose of separation and recovery of
CO2 at moderate
pressure (10 bar for example) from gas streams with medium to high CO2
concentrations as
shown in Fig. 8. This PSA configuration has the advantage to reduce the
recompression cost of
CO2 prior the storage step. This process was designated as PSA-H/M where H and
M stand for
the high pressure adsorption and medium pressure desorption. It is noteworthy
that conventional
PSA processes operate between a high loading pressure and vacuum or 1 bar for
the desorption
stage
CA 02682892 2009-10-15
- 18 -
EXAMPLE 6: Adsorption of CO2 , N2, CH4, 02 and H2 on MCM-41-100.
Adsorption isotherms of CO2, N2, CH4, H2 and 02 onto MCM-41-100 at 298 K and
up to
25 bar are shown in Figure 8. The shape of the isotherms is reminiscent of
Type I according to
the IUPAC classification, with a much higher CO2 adsorption capacity than
other adsorbates
over the whole pressure range. It is inferred that MCM-41-100 exhibits strong
preferential
adsorption of CO2 compared to the other species. From the pure CO2 and CH4
data shown in
Figure 8, the molar selectivity ratio of the adsorbed CO2 to CH4 (CO2/CH4) was
calculated as a
function of pressure and plotted in figure 9. The corresponding molar
selectivity ratios for 13X
zeolite (Siriwardane et al. 2001, Cavaneti et al. 2004), MaxsorbAC and NoritAC
(Siriwardane et
al. 2001, Himeno et al. 2005) from literature data were also plotted in Figure
9 for comparison.
At low pressure, the molar selectivity ratio CO2/CH4 for MCM-41-100 was lower
than
13X but higher than both activated carbons. At pressures above ca. 3 bar, the
molar selectivity
ratio was higher for MCM-41-100 in comparison to all the other adsorbents,
indicative of the
higher efficiency of MCM-41-100 for separation of CO2 from CO2-CH4 mixtures at
moderate to
high pressure. The sequence in terms of CO2/CH4 molar selectivity ratio at
high pressure was
MCM-41-100 > NoritAC..--Maxsorb AC > 13X. Similar trends were observed by
comparing the
molar selectivity ratio CO2/N2 on MCM-41-100 to the corresponding molar
selectivity ratios for
13X (Siriwardane et al. 2001, Cavaneti et al. 2004), and NoritAC (Dreisbach et
al. 2005), and by
comparing the molar selectivity ratio CO2/H2 on MCM-41-100 to that for NaA
(4A) zeolite
(Akten et al, 2003),
EXAMPLE 7: Comparison between LAST CO-N2 binary mixture results and
experimental
data on MCM-41-100
Figure 10 presents the pure gas adsorption isotherms for CO2 and N2 on MCM-41-
100,
successfully fitted to Toth model equation, along with the results of IAST
prediction for CO2:N2
= 20:80 mixture. The total amount adsorbed of CO2-N2 mixture is in excellent
agreement with
the experimental data over a wide range of pressure, indicative of the
suitability of IAST,
combined with Toth model, for the prediction of binary adsorption equilibria
on MCM-41-100 as
already recognized by other workers (He and Seaton 2006; Yun et al. 2002).
Therefore, the
selectivity of CO2 over N2, CH4, H2 and 02, as function of pressure, has been
mapped
CA 02682892 2009-10-15
- 19 -
systematically using IAST. The CH4, 02 and H2 adsorption isotherms were also
fitted to Toth
model. The overall results of the fit for the pure gas adsorption of CO2, N2,
CH4, H2 and 02 are
presented in Table 5.
Table 5. Parameters of Toth equation for adsorption of pure gases on MCM-41-
100 at 298 K
Pure gas Toth model parameters
( mmol/g) b (1/bar)
CO2 145.9 5.8*10-3 0.44
N2 4.2 1.7*10-2 1.23
CH4 10.4 1.4*10-2 0.85
H2 434.2 1*104 0.22
02 14.5 5.2*10-3 0.64
EXAMPLE 8: CO2 adsorption capacity and selectivity on MCM-41-100 for CO2:N2=
20:80
mixture.
The most important binary system involved in flue gas separation is CO2-N2
mixture with
a typical molar composition of 10 - 20% of CO2 and ca. 80% N2. Figure 11 shows
the selectivity
of MCM-41-100 for CO2 vs. N2 for 20 mol% CO2 in N2 as a function of pressure.
The
corresponding data for NoritAC (Dreisbach et al. 2005) carbon and 13X
(Cavenati et al, 2004)
zeolite were also included for comparison.
The selectivity of MCM-41-100 for CO2 over N2 in the presence of CO2:N2 =
20:80
mixture was found to be around 11 in the range of 1 to 10 bar range with a
tendency to increase
up to ca. 15 as the pressure increased to 45 bar. The sequence in terms of CO2
selectivity versus
N2 at high pressure was as follows: NoritAC > MCM-41-100 >> 13X. At very low
pressure, 13X
zeolite exhibited higher CO2 vs. N2 selectivity than all the other materials;
however, the
selectivity decreased steeply at increased pressure (Cavenati et al. 2004).
Separation of CO2 from
CO2-N2 mixtures using other nanoporous materials has also been widely
investigated both
experimentally and theoretically. For example, at ambient temperature and
moderate pressure,
CO2 vs. N2 selectivity was found to be 12-18 for carbonaceous materials with
slit-shaped pores
(Cracknell and Nicholson 1996), 100 for ITQ-3 (Goj et al. 2002), 14 for MFI-
type zeolites
CA 02682892 2009-10-15
=
-
- 20 -
(Bernal et al. 2004), 4 for MOF-508b (Bastin et al. 1996) and 20 for Cu-BTC
MOFs (Yang et al.
2007),
Table 6 shows the PSA-45/10 CO2 removal capacity for MCM-41-100 and NoritAC in
the presence of CO2:N2 = 20:80 mixture calculated using IAST. Although NoritAC
exhibited
somewhat higher CO2 selectivity, MCM-41-100 still has a slightly higher PSA-
H/M CO2
adsorption capacity in the presence of CO2:N2= 20:80 mixture. Thus, MCM-41-100
has suitable
properties for CO2 separation from flue gas at high pressure.
Table 6. PSA-H/M removal capacity of CO2 in CO2:N2 = 20:80 mixture for MCM-41-
100 and
NoritAC (adsorption at 45 bar, desorption at 10 bar)
Adsorbent PSA-45/10 CO2 capacity in mmol/g and (wt%)
MCM-41-100 2.58 (11.13 wt%)
NoritAC 2.37 (10.4 wt%)
EXAMPLE 9: CO2 adsorption capacity and selectivity on MCM-41-100 for C0/:CH4=
50:50 mixture
The most important binary system involved in biogas separation, purification
processes is
CO2-CH4 mixture with a molar composition of 25 to 50% and 50 to 75% for CO2
and CH4,
respectively. Figure 12 shows the selectivity of MCM-41-100 for CO2 versus CH4
in the
presence of CO2:CH4 = 50:50. The corresponding literature data for benchmark
materials like
NoritAC, MaxsorbAC carbons and 13X zeolite were also included for comparison.
The MCM-41-100 CO2 vs. CH4 selectivity for CO2:CH4= 50:50 mixture was found to
be
around 5 at low pressure, and showed an upward tendency up to ca. 7 as the
pressure increased to
45 bar. The experimental data for NoritAC (Dreisbach et al. 2005; Himeno et
al. 2005) were in
good agreement with the IAST prediction based on pure CO2 and CH4 data (Himeno
et al. 2005).
MCM-41-100 had the highest CO2 vs. CH4 selectivity at moderate to high
pressure for CO2:CH4
= 50:50 ca. > 5 bar. Zeolite 13X exhibited higher CO2 selectivity than all the
other materials in
the low pressure range (ca. < 5 bar), but the selectivity decreased
drastically by increasing the
pressure (Cavenati et al. 2004). The sequence in terms of CO2 vs. CH4
selectivity for CO2:CH4=
50:50 at high pressure was MCM-41-100 > NoritAC Maxsorb AC > 13X, similar to
that
observed in Fig. 10 based on the molar CO2/CH4 selectivity ratios. The
separation of CO2 from
CA 02682892 2009-10-15
= =
1 =
- 21 -
CO2-CH4 mixtures has also been investigated experimentally and theoretically
for other
nanoporous materials including MOFs and carbon nanotubes. For example, under
similar
conditions of pressure, temperature and composition, the CO2-CH4 selectivity
was reported to be
3 for IRMOF-1 (Yang and Zhong 2006; Babarao et al. 2007) and MOF-508b (Bastin
et al. 1996),
10 for Cu-BTC (Yang and Zhong 2006) and 11 for carbon nanotubes (Huang et al.
2007).
Llewellyn et al. (2006) reported molar CO2/CH4 selectivity ratio of 1.8 and
38.5 at 20 bar and
304 K on dehydrated and hydrated MIL-53(Cr), respectively. Llewellyn et al.
also (2008)
reported molar CO2/CH4 selectivity ratio of ca. 3 at 50 bar and 303 K on Mil-
101c.
Table 7 shows the CO2P SA-45/10 capacity for CO2:CH4= 50:50 mixture over MCM-
41-
100 and other benchmark adsorbents calculated using IAST. The sequence of CO2
PSA-HIM
removal capacity using CO2:CH4 = 50:50 mixture was in good agreement with the
pure CO2
capacity sequence mentioned previously.
Table 7: PSA-H/M removal capacity of CO2 from CO2:CH4 = 50:50 mixture for MCM-
41-100,
NoritAC and MaxsorbAC (adsorption at 45 bar, desorption at 10 bar)
Adsorbent PSA-45/10 CO2 capacity in nunol/g and (wt%)
MCM-41-100 5.40 (23.7 wt%)
NoritAC 3.44 (15.2 wt%)
MaxsorbAC 9.44 (41.5 wt%)
EXAMPLE 10: CO2 adsorption capacity and selectivity on MCM-41-100 for C0_2:H2
=
20:80 mixture
The most important binary system involved in pre-dried synthesis gas for
hydrogen
production is CO2-H2 mixture. The typical molar composition of dry synthesis
gas after the water
gas shift process in typically 20 to 30% CO2 and 70 to 80% H2. Figure 13 shows
the CO2 vs. H2
selectivity for CO2:H2= 20:80 mixture as a function of pressure for MCM-41-100
compared to
the corresponding literature data, available for NaA zeolite (Akten et al.
2003).
NaA zeolite exhibited higher selectivity than MCM-41-100 at pressure up to ca.
18 bar.
However at higher pressure, MCM-41-100 outperformed NaA reaching a CO2 vs. H2
selectivity
of 63 for CO2:H2 = 20:80 at 45 bar. The PSA-45/10 CO2 removal capacity in the
presence of
CO2:H2 = 20:80 for MCM-41-100, calculated using IAST was 3.1 mtnol/g (13.3
wt%). Notice
CA 02682892 2009-10-15
= =
- 22 -
that neglecting the buoyancy effect on the adsorbed phase in pure H2
adsorption data may lead to
a slight overestimation of the selectivity using IAST. Separation of equimolar
mixture of CO2
and H2 has also been performed on other nanoporous materials like carbon and
MOFs. At 50 bar
and room temperature, the CO2 vs. H2 selectivity was reported to be 35 for
activated carbon (Cao
and Wu 2005), 25 for MOFs-5 (IRMOF-1) (Yang and Zhong 2006) and 60 for Cu-BTC
(Yang
and Zhong 2006). Thus, MCM-41-100 is also a promising material for carbon
dioxide removal
from synthesis gas at high pressure.
EXAMPLE 11: CO2 adsorption capacity and selectivity on MCM-41-100 for CO2:02
=
95:5 mixture
Although the CO2-N2 mixture is the most dominant in flue gas, investigation of
CO2-02
mixtures is also important. The molar composition of 02 in flue gas is
typically 2 to 5 %. Ideally
the selectivity of CO2 in CO2-02 mixtures should be as high as for CO2-N2
mixtures. Figure 14
representing the CO2 vs. 02 selectivity for CO2:02= 95:5 as a function of
pressure for MCM-41-
100 shows a linear tendency with pressure. A CO2 vs. 02 selectivity of 22 was
obtained at 45 bar.
The PSA-45/10 CO2 removal capacity for MCM-41-100 in the presence of a CO2:02
= 95:5
mixture for MCM-41-100, calculated using IAST, was 8.9 mmol/g (39.3 wt%).
Adsorption of
CO2-02 mixtures was rarely studied in the literature. At 50 bar and room
temperature, the CO2
selectivity in CO2:02 = 77.8:22.2 mixture in the presence of Cu-BTC was
reported to be 35
(Yang et al. 2007).
EXAMPLE 12: CO2 capture using PSA-HIM with mesoporous silica
A simplified general scheme for CO2 capture, from different gas streams, is
presented in
Figure 15. It is composed of a CO2 removal stage using suitable technology
(e.g., absorption,
membrane, adsorption using PSA, etc), and a CO2 compression step before the
final CO2 storage.
In this scheme, the capture step operates generally at atmospheric to moderate
pressure and the
CO2 is recovered at low pressure when PSA is used.
Figure 16 illustrates the proposed CO2 capture scheme incorporating PSA-H/M
using
mesoporous silica as adsorbent. The proposed scheme involves two compression
stages. Initially
CA 02682892 2009-10-15
:
- 23 -
the gas feed is compressed (e.g., 45 bar), the CO2 is removed at high pressure
and recovered at
moderate pressure (e.g., 10 bar) before the final compression (if necessary)
and storage steps.
EXAMPLE 13: CO2 adsorption on dry and hydrated PE-MCM-41.
Figure 17 shows the CO2 adsorption isotherms of dry and hydrated (40% RH) PE-
MCM-41 at
room temperature and high pressure. The CO2 adsorption uptake was 100 wt%
(22.8 mmol/g)
and 102 wt% (23.2) at 60 bar and room temperature. The pure CO2 PSA-60/10
operating
capacity for the dry and hydrated material was ca. 80 wt% and 81wt%,
respectively.
References
Akten, E.D., Siriwardane, R., Sholl, D.S. Monte Carlo simulation of single-and
binary-
component adsorption of CO2, N2 and H2 in zeolite Na-4A. Energy & Fuels 2003,
17, 977.
Babarao, R., Hu,Z., Jiang,J. Storage and separation of CO2 and CH4 in
Silicalite, C168
Schwarzite, and IRMOF-1. A comparative study from Monte Carlo simulation. Lan
gmuir 2007,
23, 659.
Bahr, D. F., Reid,J.A., Mook, W.M., Bauer, C.A., Stumpf, R., Simian, A.J.,
Moody, N.R.,
Simmons, B.A., Shindel, M.M., Allendorf, M.D. Mechanical properties of cubic
zinc
carboxylates IRMOF-1 metal-organic framework crystals. Phys. Rev. B. 2007, 76,
184106.
Bastin, L., Barcia, P.S., Hurtado, E. J., Silva, J. A.C., Rodrigues, A.E.,
Chen, B.A microporous
metal-organic framework for separation of CO2/N2 and CO2/CH4 by fixed-bed
adsorption. J.
Phys. Chem. C1996, 112, 1575.
Belmabkhout, Y., Pirngruber, G., Jolimaitre, E., Methivier, A. A complete
experimental
approach of synthesis gas separation studies using static gravimetric and
dynamic inverse
chromatographic methods. Adsorption 2007, 13, 341.
Belmabkhout, Y., Frere, M., De Weireld, G. High-pressure adsorption
measurements. A
comparative study of the volumetric and gravimetric methods. Meas. Sci.
Technol. 2004, 15, 848.
CA 02682892 2009-10-15
=
. .
.. ,
- 24 -
Bernal, M. P., Coronas, J., Menendez, M., Santamaria, J., 2004. Separation of
CO2/N2mixtures
using MFI-type zeolites membrane. AIChE J. 50, 127-135.
Bourrelly, S., Llewellyn, P.L., Serre, C., Millange, F., Loiseau, T., Ferey,
G. Different adsorption
behaviors of methane and carbon dioxide in the isotypic nanoporous metal
terephthalates MIL-
53 and MIL-47. J. Am. Chem. Soc. 2005, 127, 13519.
Branton, P.K., Hall, P.G., Treguer, M., Sing, K.S.W. Adsorption of carbon
dioxide, sulphur
dioxide and water vapour by MCM-4, a model mesopourous adsorbent. J. Chem.
Soc. Faraday.
Trans. 1995, 91, 2041.
Cassiers, K., Linssen, L., Mathieu. M., Benjelloun, M., Schrijnemakers, K.,
Van Der Voort, P.,
Cool, P., Vansant. E. F. A detailed study of thermal, hydrothermal, and
mechanical stabilities of
a wide range of surfactant assembled mesopourous silicas. Chem. Mater. 2002,
14, 2317.
Cavenati, S., Grande, C.A., Rodrigues, A.E. Adsorption equilibrium of methane,
carbon dioxide,
and nitrogen on zeolites 13X at high pressures." Chem. Eng. Data 2004, 49,
1095.
Cavenati, S., Grande, C.A., Rodrigues, E.E. Separation of CH4/CO2/N2 mixtures
by layered
pressure swing adsorption for upgrade of natural gas. Chem. Eng. Sci. 2006,
61, 3893.
Cracknell, R.F., Nicholson, D. Adsorption and selectivity of carbon dioxide
with methane and
nitrogen in slit-shaped carbonaceous micropores: Simulation and experiments.
Adsorption 1996,
2, 193.
Comoti, A., Bracco, S., Valsesia, P., Ferreti., L., Sozzani, P. 2D
multinuclear NMR,
hyperpolarized xenon and gas storage in organosilica nanochannels with
crytalline order in the
Walls. J. Am. Chem. Soc. 2007, 129, 8566.
Do, D. D., Wang, K. A new model for the description of adsorption kinetics in
heterogeneous
activated carbon. Carbon 1998, 36, 1539.
Dreisbach, F., Seif, R., Losch, H.W. Adsorption equilibria of CO/H2 with a
magnetic suspension
balance. Purely gravimetric measurements. J. Therm. Anal. Calorim. 2003, 71,
73.
CA 02682892 2009-10-15
=
- 25 -
Dreisbach, F., Staudt, R., Keller, J.U. Experimental investigation of the
kinetics of adsorption of
pure gases and binary gas mixtures on activated carbon. In: Meunier, F. (eds.)
Proceedings of
Fundamental of Adsorption 6, pp. 1219-1224. Elsevier, Paris, 1998.
Goj, A., Sholl, D.S., Akten, E.D., Kohen, D. Atomistic simulations of CO2 and
N2 adsorption in
silica zeolites: the impact of size and shape. J. Phys. Chem. B 2002,106,
8367.
Halmann, M.M., Stenberg, M. Greenhouse Gas Carbon Dioxide Mitigation, CRC
Press LLC,
Boca Raton, Florida (1999).
Harlick, P. J.E., Sayari, A. Application of pore-expanded mesorporous silica
5: Tharnine grafted
material with exceptional CO2 dynamic and equilibrium adsorption performance.
Ind. Eng. Chem.
Res. 2007, 46, 446.
He, Y., Seaton, N.A. Heats of adsorption and adsorption heterogeneity for
methane, ethane and
carbon dioxide. Langmuir 2006, 22, 1150.
Himeno, S., Komatsu, T., Fujita, S. High-pressure adsorption equilibria of
methane and carbon
dioxide on several activated carbons. J. Chem. Eng. Data , 2005, 50, 369.
Hong, M., Li, S., Falconer, J.L., Noble, R.D. Hydrogen purification using a
SAPO-34 membrane.
J. Membr. Sci. 2008, 307, 277.
Huang, L., Zhang, L., Shao, Q., Lu, L.; Lu, X., Jiang, S., Shen, W.
Simulations of binary mixture
adsorption of carbon dioxide and methane in carbon nanotubes: temperature,
pressure, pore size
effects. J. Phys. Chem. C 2007, 111, 11912.
Li, Y., Yang, R.T. Gas adsorption and storage in metal-organic framework MOF-
177. Langmuir
2007, 23, 12937.
Llewellyn, Pl., Bourrelly, S., Serre, C., Filinchuk, Y., T., Ferey, G. How
hydratation drastically
improves adsorption selectivity for CO2 over CH4 in the flexible chromium
terephthalate MIL-53.
Angew. Chem. Int. Ed. 2006, 45, 7751.
CA 02682892 2009-10-15
- 26 -
Millward, A.R., Yaghi, O.M. Metal-organic frameworks with exceptionally high
capacity for
storage of carbon dioxide at room temperature. J. Am. Chem. Soc. 2005, 127,
17998.
Morishige, K., Fujii, H., Uga, M., Kinukawa, D. Capillary critical point of
argon, nitrogen,
oxygen, ethylene and carbon dioxide in MCM-41. Lan gmuir 1997, 13, 3494.
Morishige, K., Nakamura, Y. Nature of adsorption and desorption branches on
cylindrical pores.
Langmuir 2004, 20, 4503.
Murcia, A.B., Fletcher, A.J., Garcia-Martinez, J., Cazorla-Amoros, D., Linares-
Solano, A.,
Thomas, K.M. Probe molecule kinetics studies of adsorption on MCM-41. J. Phys.
Chem. B
2003, 107, 1012.
Pirngruber, G., Jolimaitre, E., Wolff, L, Leinkugel le coq, D. Hydrogen
adsorption purification
method with co-generation of a pressurised CO2 flow. Patent number: WO
2008/081102
Ruthven, D.M., Farooq, S., Knaebal, K.S. Pressure swing adsorption. VCH, New
York (1994).
Satyapal, S., Filburn, T., Trela, J., Strange, J. Performances and properties
of a solid amine
sorbent for carbon dioxide removal in space life support application, Energy &
Fuels 2001, 15,
250.
Sayari, A. Catalysis by crystalline mesoporous molecular sieves, Chem. Mater.
1996, 8, 1840.
Sayari, A. Mesoporous Materials. In The Chemistry of Nanostructured Materials;
Yang, P., Eds.;
P 39, World Scientific: Singapore (2003).
Sayari, A., Jaroniec, M. Nanoporous Materials, World Scientific Publ. Co.,
Singapore (2008).
Serna-Guerrero, R., Sayari, A. Applications of pore-expanded mesoporous
silica. 7. Adsorption
of volatile organic compounds. Environ. Sci. Technol. 2007, 41, 4761.
Sircar, S.: Role of helium void measurement in estimation of gibbsian surface
excess. In
Proceedings of Fundamental of Adsorption 7; Kaneko, K., Ed., pp. 656-663. IK
International,
Chiba City (2002).
CA 02682892 2016-06-03
- ?7 -
Siriwardane. R.V., Shen. M.S., Fisher, E.P., Poston J.A.: Adsorption of CO, on
molecular
sieves and activated carbon. Energy & Fuels 2001, 15. 279.
Sonwane. C.G., Bathia, S,K., Cabs, N. Experimental and theoretical
investigation of
adsorption hysteresis and criticality in MCM-41: Studies with 02, Ar, and CO,.
Ind Eng.
Chem. Res. 1998, 37, 2271.
Sridhar. S., Smitha, B.. Aminabhavi, T.M. Separation of carbon dioxide from
natural gas
mixtures through polymeric membranes - A review. Sep. Purif Rev. 2007, 36.
113.
Veawab, A., Tontiwachwuthikul, P., Chakma, A. Corrosion behaviour of carbon
steel in the
CO, absorption process using aqueous amine solutions. Ind. Eng. Chem. Res.
1999, 38, 3917.
Wang, X.P., Yu, U., Cheng. j., Hao, Z.P., Xu, Z.P. High-temperature adsorption
of carbon
dioxide on mixed oxides derived hydrotalcite-like compounds. Environ. Sci.
Technol. 2008_
42.614.
Yana, Q., Xue, C., Zhong, C., Chen, J.F. Molecular simulation of separation of
Ca) from
flue gas in Cu-BTC metal-organic framework. Al(ThE õI. 2007, 53, 2832.
Yang, Q., Zhong, C. Molecular simulation of carbon dioxide/methane/hydrogen
mixture
adsorption in metal-organic frameworks. õ1. Phys. Chem. 2006, 110. 17776.
Yang, Q., Zhong. C., Chen, J.F. Computational study of CO, storage in metal-
organic
frameworks. PhyS. Chem. C. 2008, 112. 1562..
Yun, J. H., Duren, T., Keil, F.J., Seaton, N.A. Adsorption of methane, ethane
and their binary
mixtures on MCM-41: Experimental evaluation of methods for the prediction of
adsorption
equilibrium. Langmuir 2002, 18, 2693.
All publications, patents and patent applications mentioned in this
Specification are
indicative of the level of skill of those skilled in the art to which this
invention pertains.
CA 02682892 2016-06-03
- 28 -
The invention being thus described, it will be obvious that the same may be
varied in
many ways. Such variations are not to be regarded as a departure from the
spirit and scope of
the invention, and all such modifications as would be obvious to one skilled
in the art are
intended to be included within the scope of the following claims.