Note: Descriptions are shown in the official language in which they were submitted.
CA 02683169 2009-10-16
-1-
A METHOD FOR DIAGNOSING PSORIATIC ARTHRITIS IN PATIENTS WITH SKIN
PSORIASIS
FIELD OF THE INVENTION
The present invention relates to the field of medical diagnostic screening.
BACKGROUND OF THE INVENTION
Psoriatic arthritis (PsA) is a serious chronic condition that affects 10-35%
of patients with
skin psoriasis and is associated with increased patient morbidity and
mortality (Reference
1). It is often described as a chronic inflammatory arthropathy affecting the
distal
interphalangeal joints of the hands (DIPs), feet and spine associated with
psoriasis (PSO)
(Reference 2). The Norfolk Arthritis Registry (NOAR) cohort study recently
demonstrated
that 5-year outcomes for psoriasis-associated inflammatory arthritis are very
similar to
those of inflammatory arthritis without PSO (Reference 3). Results of this
study suggested
36.7 % of PsA patients had radiographic erosions at 5 years compared to 33.8%
of those
without PSO, and 47% of PsA patients in early synovitis clinics, providing
evidence this is
neither a benign nor infrequent disease, as previously perceived (References 4-
7). Most
patients develop PsA years after the onset of their PSO (References 8-9).
Early diagnosis
and intervention of PsA plays an important role in preventing the progression
of
inflammatory joint damage, reducing pain and improving quality of life (QoL)
(Reference
10). Traditional therapies have provided inadequate control of the
inflammation associated
with PsA, but the introduction of the biologic agents (e.g. tumour necrosis
factor
antagonists) offer a new option.
There has been considerable variation in how rheumatologists have diagnosed
PsA, and
international groups such as GRAPPA are attempting to more precisely define
PsA
patients for clinical trials (References 5, 11-18). The differential diagnosis
of PsA can be
challenging since many of its features overlap with those of rheumatoid
arthritis, reactive
CA 02683169 2009-10-16
-2-
inflammatory bowel disease and ankylosing spondylitis. Furthermore,
osteoarthritis, soft
tissue rheumatism, septic arthritis and true RA can coexist with PSO.
Preliminary efforts to develop screening tools for the identification of PsA
patients have
met with limited success (Reference 19). The Psoriatic and Arthritis
Questionnaire (PAQ),
a 12-item questionnaire, was developed by Peloso and colleagues to identify
psoriatic
patients with arthritis (Reference 20). An evaluation using this questionnaire
in 202
hospital- and community-based patients, in Sweden, demonstrated a score of 4
(scale 0-8)
was the best cut off value for detecting arthritis. This cut off, however,
yielded sensitivity of
60% and specificity of 62%, indicating the PAQ did not effectively
discriminate for arthritis
(Reference 20). The Psoriatic Arthritis Screening and Evaluation (PASE) is a
15 item
questionnaire with symptom and function sub-scales. The questionnaire,
evaluated in 69
patients, showed an optimal cut off point of 47, and was able to distinguish
PsA from non-
PsA patients with sensitivity and specificity of 82% and 73% respectively
(Reference 6).
The Toronto Psoriatic Arthritis Screen (ToPAS), which supplements the
questionnaire with
photos to aid the patients, showed a sensitivity of 91.9%, and a specificity
of 95.2% in a
dermatology practice (Reference 21). In 2006 the Classification Criteria for
PsA
(CASPAR) was introduced as a research tool for classifying PsA (Reference 22).
The
CASPAR criteria are defined as follows: A patient must have presented with
inflammatory
articular disease and must satisfy three or more of the following: (1)
evidence of current
PSO, (2) a personal history of PSO, or (3) a family history of PSO, (4)
typical psoriatic nail
dystrophy, (5) a negative test result for the presence of rheumatoid factor,
(6) current
dactylitis or a history of dactylitis, and (7) radiographic evidence of juxta-
articular new bone
formation, appearing as ill-defined ossification near joint margins.22 The
CASPAR criteria
have demonstrated high sensitivity and specificity of 91.4% and 98.7%,
respectively.
Although the CASPAR criteria provide a sensitive tool for confirmation of the
diagnosis of
PsA, it is still a research instrument and its use in the clinical settings
has not been widely
utilized. Furthermore, it requires proficiency in joint examination and it may
be difficult to
implement by other health care providers such as dermatologists and family
physicians.
It is believed that screening PSO patients likely to develop PsA with an easy
to administer
tool with good sensitivity and specificity will facilitate early
identification, prevention of
disease progression and destruction of joints and efficient referral for
optimal treatment. In
CA 02683169 2009-10-16
-3-
order to achieve this objective, a simple, self-administered questionnaire -
the PSO and
Arthritis Screening Questionnaire (PASQ) - was developed which can be utilized
for
screening patients who meet the CASPAR criteria for PsA. It has been
demonstrated that
the PASQ is highly sensitive and specific in detecting patients with PsA
(whether in early-
stages or established stages of the disorder) who fulfill the CASPAR criteria
(Reference
23).
The PASQ, shown in FIG. 1, comprises a simple, self-administered questionnaire
containing ten questions for which a positive and negative response are
assigned a score
of 1 and 0, respectively, with a maximum score of 10. Patients are also
requested to
indicate, on a diagram where they experienced joint swelling or pain (at
present or in the
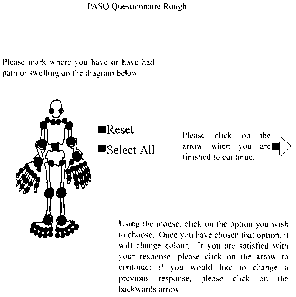
past). The PASQ diagram for patient assessment of painful and swollen sites is
shown in
FIG. 2. The diagram is scored 0, 1, 3 or 5 depending on the distribution of
markings, as
show in FIG. 3. The points are weighted consistent with the different
presentations and
joints affected in PsA (Reference 12), and other forms of arthritis (e.g.
osteoarthritis and
rheumatoid arthritis). The scores of the questionnaire and diagram are then
tabulated by
the physician or assistant.
The receiver operating curve (ROC) is a known statistical method which may be
used
determine the optimal cut off score that will best discriminate between those
patients
having or not having PsA. The optimal score will provide the best sensitivity,
meaning the
proportion of actual positives which are correctly identified as such (i.e.
the percentage of
affected patients who are correctly identified as having PsA) and specificity,
meaning the
proportion of negatives which are correctly identified (i.e. the percentage of
well people
who are correctly identified as not having the condition).
Using the ROC, the PASQ has been shown to yield an optimal cut-off of 9, with
86.27%
sensitivity and 88.89% specificity, although a score of 8 increases the
sensitivity to 92.78%
while maintaining a specificity of 77.16% specificity. An analysis of the PASQ
diagram
score indicate an optimal total score of 1 (out of maximum 5 for the diagram)
with 100%
sensitivity and 55.56% specificity.
The PASQ has the advantage of being a wholly patient administered tool with no
physical
examination needed to complete its score, which makes it an ideal screening
tool to utilize
CA 02683169 2009-10-16
-4-
by non-rheumatologists. The PASQ is of particular benefit to dermatologists
and family
physicians who may benefit from the use of this tool for rapid diagnosis of
patients that
require referral to a rheumatologist for their management in order to avoid
potentially
irreversible destruction of the involved joints, leading to more timely and
optimal selection
of systemic treatments, particularly for moderate-severe PSO. Optimal and
timely
treatment of this population with the most efficacious systemic therapies is
important as it
may yield improved clinical outcomes and enhanced quality of life for these
patients. The
positive outcomes may also be reflected in more efficient utilization of
health care
resources and ultimately in cost savings to the health care system.
A further advantage of the PASQ is that it can be completed and scored in an
efficient
manner, offers little difficulty to the patient and the scorer, requires
minimal intervention by
the physician and adds very little burden to the clinical routine.
Notwithstanding the foregoing, although the PASQ provides a sensitive, simple
and easy
to administer questionnaire that can be used to screen for PsA, it is a paper-
based method
requiring a physician or trained assistant to physically mark and score the
questionnaire
and, therefore, is subject to human error in calculating the scores of both
the questionnaire
and, in particular, the diagram. Accordingly, there exists a need for a
version of the PASQ
which limits or eliminates the possibility of human errors in analysing the
test data.
SUMMARY OF THE INVENTION
The following presents a simplified summary of the invention in order to
provide a basic
understanding of some aspects of the invention. This summary is not an
extensive
overview of the invention. It is not intended to identify key/critical
elements of the invention
or to delineate the scope of the invention. Its sole purpose is to present
some concepts of
the invention in a simplified form as a prelude to the more detailed
description that is
presented later.
It is an object of the present invention to provide a self-administered,
electronic version of
the PASQ which may be widely distributed and which will automatically score
the
CA 02683169 2009-10-16
-5-
questionnaire, thereby eliminating possible inaccuracies of the diagram
scoring, as well as
provide useful information when self-administered to an untrained user.
The present invention describes a computer-implemented method and system to
provide
an electronic Psoriatic Arthritis Screening Questionnaire (the "EPASQ") used
as a tool for
reliable diagnosis of PsA in patients with PSO. The subject matter described
herein is
implemented as a computer program product comprising computer executable
instructions
embodied in a computer readable medium. For greater clarity, any references
herein to
the "EPASQ" or to "electronic questionnaire and diagram" refers to the present
invention,
which includes a computer implemented method and system for the detection and
diagnosis of PsA in patients with PSO.
The present invention provides a simple, self-administered and automatically
scored
electronic program with a high sensitivity and specificity and is an effective
tool to screen
for early and established PsA patients. It is hoped that the present invention
will assist
dermatologists and family physicians in the identification of PSO patients at
risk of
developing PsA, resulting in timely referrals to and treatment by a
rheumatologist, and,
additionally, will assist dermatologists in selecting appropriate systemic
treatments for
moderate-to-severe PSO. In addition, the present invention, in one embodiment,
is
intended to be self-administered by a patient. The present invention may also
be used to
collect and aggregate data relating to the prevalence of PsA in PSO patient
populations.
Broadly, the invention features a method of identifying PsA in PSO patients by
determining
a symptom profile, wherein the symptom profile is determined by identifying
the presence
or severity certain symptoms in the patient and classifying the symptom
profile as
identifying PsA using an algorithm based on the symptom profile. The method
includes
obtaining clinical data from a patent including various physical and
demographic factors.
In the preferred embodiment, a questionnaire in computer-readable medium is
used to
produce the symptom profile. The questionnaire, shown in FIG. 1, comprises a
standardized set of questions and answers for the purposes of gathering
information from
patients regarding their current and/or recent PsA-related symptoms. The
preferred
embodiment also comprises a diagram in computer-readable medium upon which
patients
indicate where they experienced joint swelling or pain.
CA 02683169 2009-10-16
-6-
In the preferred embodiment, the patient completes the electronic
questionnaire and
indicates, on the electronic diagram, the locations at which they experience
or experienced
joint swelling or pain. The symptom profile is produced by compiling and
analyzing all of
the answers to the questions set forth in the questionnaire and is used in
combination with
the diagram profile in the algorithmic-based methods described herein to
improve the
accuracy of identifying or predicting PsA.
In the preferred embodiment, the answers to the questions in the questionnaire
and the
number of areas marked on the diagram are automatically assigned a score. The
scores of
the questionnaire and diagram are automatically tabulated to generate a
cumulative score
useful for predicting or identifying PsA in patients with PSO.
The methods of the invention allow the accurate diagnosis of PsA in patients
with skin
PSO who are likely to develop PsA, at or before disease onset, thus reducing
or
minimizing the debilitating and often morbid effects of PsA. The method can be
applied in
persons with PSO who are free from clinical symptoms of PsA, in those who
already have
clinical PsA, those who have a family history of PsA or in those who have an
elevated level
or levels of risk factors of PsA.
The major application of the current invention involves the identification PsA
or the risk of
developing PsA in those patients who have been diagnosed with PSO.
A key aspect of the present invention is that it may be self-administered by a
patient
without requiring the patient to attend at a health clinic or doctor's office.
After taking the
test, the patient receives an indication as to whether PsA or a risk of PsA
was identified
and whether further treatment by a physician or specialist is required.
In another application, the present invention may be used as a part of a
comprehensive
evaluation and diagnosis of PsA by a rheumatologist by serving as an indicator
of patients
at risk that should receive further follow-up by a specialist and as a means
to assist that
specialist in choosing appropriate treatment choices. Better means of
identifying those
individuals having or an risk of developing PsA should lead to better
preventative and
treatment regimens. For example, dermatologists tend to treat mild and
moderate PSO
without arthritis with creams and/or UV therapy. If these patients have a
diagnosis of PsA,
CA 02683169 2009-10-16
-7-
then the treatment in these patients may change and include medications that
work on
both the skin and the joints such as methotrexate and biologics such as
etanercept
(Enbrel(D) or infliximab (Remicade ). These more effective therapies can
induce
significant clinical improvement in both the arthritis and the skin components
of the
diseases).
In a further application, the present invention can assist general medical
practitioners in
differentiating between psoriasis and other skin conditions. For example, in
some cases,
patients with psoriasis may have been incorrectly diagnosed and are actually
suffering
from another similar skin condition, such as atopic dermatitis, seborrhic
ketatosis and other
skin conditions which could look similar to psoriasis in a general medicine
practice.
A further object of the present invention is to aggregate and store patient
data, in order to
give some broader indications of how PsA is prevalent through the population.
A further object of the present invention is the identification of PsA
patients for clinical trials
where investigators are looking for patients with the PsA to be recruited for
a research
project.
A further object of the present invention is to identify newly diagnosed
patients from
general practice, dermatology clinics or even on a website if ethical approval
for this is
obtained.
A yet further object of the present invention is to identify the prevalence of
PsA in a certain
population (e.g. genetic studies in a community) for use in epidemiological
studies.
Other features and advantages of the invention will be apparent from the
description and
the drawings, and from the claims.
DESCRIPTION OF DRAWINGS
Figure 1 shows the standardized set of questions and their respective scoring
of the prior
art PASQ.
Figure 2 shows the representative diagram of the prior art PASQ.
CA 02683169 2009-10-16
Figure 3 shows the scoring scheme for the diagram of the prior art PASQ.
Figure 4 is a screenshot of the user interface in accordance with an
embodiment of the
present invention.
Figure 5 is a screenshot of the user interface in accordance with an
embodiment of the
present invention.
Figure 6 shows a schematic computer system in accordance with an embodiment of
the
present invention.
Figure 7 shows a block diagram of a computer system in accordance with an
embodiment
of the invention.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
Introduction
Diagnosing a patient with psoriasis as having psoriatic arthritis (PsA) can be
challenging
due to the similarity in symptoms between PsA and other diseases such as
rheumatoid
arthritis, reactive inflammatory bowel disease and anykylosing spondylitis and
due to the
fact that psoriasis (PSO) can exist with other conditions such as
osteoarthritis, soft tissue
rheumatism, septic arthritis and true rheumatoid arthritis. As a result, the
rapid and
accurate diagnosis of PsA is difficult and hampers early and effective
treatment of the
disease.
The present invention is based, in part, upon the accuracy of identifying the
presence or
severity of PsA-related symptoms based upon the individual's response to a
series of
questions.
Definitions
As used herein, the following terms have the meanings ascribed to them unless
specified
otherwise.
CA 02683169 2009-10-16
-9-
As used herein, the term psoriatic arthritis or PsA includes an inflammatory
condition
associated with psoriasis characterized by, but not limited to, inflammatory
arthropathy
affecting the distal interphalangeal joints of the hands, feet and spine.
As used herein, the term "profile" includes any set of data that represents
the distinctive
features or characteristics associated with a disease or disorder such as
psoriasis or PsA.
The term encompasses a "symptom profile" that identifies one or more PsA-
related clinical
factors (i.e. symptoms) an individual is experiencing or has experienced, and
combinations
thereof.
The term "individual", "subject" or "patient" refers to humans presenting with
PSO-related
or PsA-related symptoms.
As used in this application, the terms "step", "module", "component", "model",
"system",
and the like are intended to refer to a computer-related entity, either
hardware, a
combination of hardware and software, software, or software in execution. For
example, a
module may be, but is not limited to being, a process running on a processor,
a processor,
an object, an executable, a thread of execution, a program, and/or a computer.
By way of
illustration, both an application running on a server and the server can be a
module. One
or more modules may reside within a process and/or thread of execution and a
module
may be localized on one computer and/or distributed between two or more
computers.
Also, these modules can execute from various computer readable media having
various
data structures stored thereon. The modules may communicate via local and/or
remote
processes such as in accordance with a signal having one or more data packets
(e.g., data
from one module interacting with another module in a local system, distributed
system,
and/or across a network such as the Internet with other systems via the
signal).
Description of the Embodiments
The present invention provides a computer-implemented system and method for
accurately identifying whether an individual has or is likely to develop PsA.
The present
invention is also useful for ruling out one or more diseases or disorders that
present with
PSO-like or PsA-like symptoms and ruling in PSO or PsA using empirical data.
CA 02683169 2009-10-16
-10-
Accordingly, the present invention provides an accurate diagnostic prediction
of PsA and
prognostic information useful for guiding treatment decisions.
In one embodiment, the present invention is directed to a computer-implemented
method
of automatically diagnosing a medical condition. The method has the steps of
gathering
basic symptom information of the patient to determine a symptom profile for
the patient,
assigning point values to the basic symptom information, and evaluating the
basic
symptom information point values to determine a possible diagnosis.
The first step of the method is the display of a computer-readable
questionnaire and
diagram depicting parts of the body to a user. The user is then prompted to
input answers
to the questions posed as well as to mark, on the diagram, the location at
which they
experience or experience joint pain. User input is achieved electronically by
use of, for
example, a keyboard or a mouse. Once user input has been completed, the
answers to
the questions and markings on the diagrams are assigned score, the method of
which is
described in further detail herein and the respective scores of the
questionnaire and the
diagram are automatically tabulated to generate a cumulative score. Once the
cumulative
score reaches a predetermined threshold, the diagnosis will be considered
probable. In the
final step of the method of the present invention, the diagnosis is presented
to the user.
In the preferred embodiment, the present invention is directed to a computer-
implemented
method of automatically diagnosing PsA in psoriatic individuals. However, is
it recognized
that the method described herein may apply to the diagnosis of various other
medical
conditions presenting with similar symptoms to PsA, such as rheumatoid
arthritis.
In a preferred embodiment, the method of ruling in PsA comprises a computer-
implemented system and method for determining a symptom profile using a
computer-
readable questionnaire. The questionnaire comprises a standardized set of
questions for
the purpose of gathering information from respondents regarding their current
and/or
recent PsA-related symptoms. The questionnaire includes questions which query
the user
about a presence or severity of the user's PsA-related symptoms and other
related risk
factors. Examples of the questions asked are provided in FIG. 1. In addition
to the
questionnaire, the present invention also comprises in addition to a diagram
comprising a
CA 02683169 2009-10-16
-11-
set of circles depicting parts of the body upon which the patient marks the
location at which
they experience or experience joint pain. An example of the diagram is shown
in FIG. 2.
In the preferred embodiment, the answers to the questions in the questionnaire
and the
number of areas marked on the diagram are automatically assigned a score using
the
algorithmic-based methods described herein.
In the preferred embodiment, the computer-readable questionnaire is programmed
to
comprise ten questions for which a positive and negative response is assigned
a score of
1 and 0, respectively, with a maximum score of 15. The patient enters a "yes"
or "no"
answer by clicking the appropriate response. Examples of the user interface
are shown in
FIG. 4 and FIG. 5.
In the preferred embodiment, the computer-readable diagram comprises a set of
circles
depicting 68 joints plus the spine. Patients are requested to indicate, by
clicking on the
appropriate circle, where they experience or experienced joint swelling or
pain (FIG. 5).
Unlike the in the paper-based PASQ, the electronic diagram has 68 green
coloured circles
for joints in the hands, feet and one large circle for the spine. The
individual is able to drag
and click on a joint but not on any other part of the diagram. This is unlike
the prior art
paper-based PASQ diagram where patients typically mark the joints or any part
of the
diagram, which is a limitation of the prior art. Although green is a preferred
colour for the
circles in the diagram, it is readily understood that any colour, or no colour
at all, may be
used.
In the preferred embodiment, the computer-readable diagram is scored 0, 1, 3
or 5
depending on the distribution of the markings (FIG. 2). The rules governing
scoring of the
diagram are as follows:
= Five points are awarded in three scenarios: 1) Only the spine button and one
other
major joint button (shoulders, hips, knees) have been selected; 2) The total
number of
joints selected is greater than zero but less than six, except for case 1 for
three points;
or 3) The total number of DIP joints (joints on fingers furthest from the
wrist) exceeds
five, and they are the only joints selected
CA 02683169 2009-10-16
-12-
= Three points are awarded in two scenarios: 1) Only one major joint is
selected (the
spine, shoulders, hips, knees). In this case, even though the number of joints
is less
than five (and thus according to five points case 2 it should give five
points) it will only
give three points; and 2) The joints are asymmetrical. In this case, variables
have been
assigned to the right and left joints; if one side has more affected joints
than the other
by a 2 or more, then three points are awarded. In addition, each joint has
been
matched with its "mirror" (save for the spine); if one is selected and it's
mirror not, then
a variable called "notscore" will increase by one. If the notscore is greater
than 2 then
three points will also be awarded. Finally, the number of joints in the hands
and feet
cannot be equal for three points to be awarded.
= One point is awarded in only one scenario: 1) The joints are symmetrical
(unless they
number less than six, at which point see five points, case one). The diagram
determines symmetry through the use of the left side/right side totals
mentioned in
three points, case 2. In this case, as long as the number of joints on either
side are
within +/- 1 of each other, then the diagram considers the affected individual
to have
symmetrical joint problems and awards one point.
= Zero points will be awarded if the patient does not select any joints at
all.
In the preferred embodiment, the scores of the questionnaire and diagram are
automatically tabulated to generate a cumulative score useful for predicting
or identifying
PsA in patients with PSO.
The automatic tabulation of the computer-readable questionnaire and diagram
scores in
the present invention presents a distinct advantage over the prior art paper-
based PASQ.
More specifically, the present invention eliminates the need for manual
tabulation and
scoring and therefore eliminates possible inaccuracies in scoring as a result
of human
error.
In the preferred embodiment, the computer-readable questionnaire and diagram
comprises
instructions in computer-readable form on each electronic page explaining to
the patient
the method for completing the electronic questionnaire and diagram as shown in
Figs. 1, 2
and 3.
CA 02683169 2009-10-16
-13-
In a preferred embodiment, the computer-readable questionnaire and diagram is
self-
administered by the patient and, accordingly, may be completed without
requiring the
patient to attend at a health clinic or doctor's office to obtain a diagnosis.
An evaluation of the present invention and validation against the prior art
paper-based
PASQ has demonstrated that the present invention can identify PsA or the risk
of PsA with
high sensitivity and specificity in PSO patients (Experiments - Example 1).
In the preferred embodiment, the ROC (as described above) is used to determine
the
optimal cut-off score (optimal sensitivity and specificity) of the computer-
readable
questionnaire and diagram. In the preferred embodiment a cut off score of 7
yields
97.62% sensitivity and 75.00% specificity. A cut-off point of 8 yielded a
sensitivity of
88.10% while still maintaining a specificity of 75.00%. It will be recognized
to those skilled
in the art that different cut-off scores may be chosen depending on the
desired outcomes.
For example, if the desired outcome is to identify the most cases of PsA
(greater
sensitivity), a lower cut off score may be chosen, although a greater number
of false
positives (i.e. persons incorrectly identified as having PsA) will result
(lower specificity).
In another embodiment, the present invention is directed to a computer-
implemented
system for diagnosing a medical condition. The system comprises: (1) a display
module
for displaying to a user a computer-readable questionnaire and diagram as well
a
diagnosis or other message based on an evaluation of user input; (2) a
receiver module for
receiving input from the user in response to the questionnaire and diagram;
(3) a scoring
module for calculating a score based on the user input; and (4) a diagnosis
module for
making a diagnosis based on the score.
In a preferred embodiment, the present invention is directed to a computer-
implemented
system for diagnosing PsA. However, is it recognized that the system described
herein
may apply to the diagnosis of various other medical conditions such as
rheumatoid
arthritis.
FIG. 6 shows a general computer system on which the invention might be
practiced. The
general computer system comprises of a display device (1.1) with a display
screen (1.2).
Examples of display device are Cathode Ray Tube (CRT) devices, Liquid Crystal
Display
CA 02683169 2009-10-16
-14-
(LCD) Devices etc. The general computer system can also have other additional
output
devices like a printer. The cabinet (1.3) houses the additional basic
components of the
general computer system such as the microprocessor, memory and disk drives. In
a
general computer system the microprocessor is any commercially available
processor of
which x86 processors from Intel and 680X0 series from Motorola are examples.
Many
other microprocessors are available. The general computer system could be a
single
processor system or may use two or more processors on a single system or over
a
network. The microprocessor for its functioning uses a volatile memory that is
a random
access memory such as dynamic random access memory (DRAM) or static memory
(SRAM). The disk drives are the permanent storage medium used by the general
computer system. This permanent storage could be a magnetic disk, a flash
memory and a
tape. This storage could be removable like a floppy disk or permanent such as
a hard disk.
Besides this, the cabinet (1.3) can also house other additional components
like a Compact
Disc Read Only Memory (CD-ROM) drive, sound card, video card etc. The general
computer system also had various input devices like a keyboard (1.4) and a
mouse (1.5).
The keyboard and the mouse are connected to the general computer system
through
wired or wireless links. The mouse (1.5) could be a two-button mouse, three-
button mouse
or a scroll mouse. Besides the said input devices there could be other input
devices like a
light pen, a track ball, etc. The microprocessor executes a program called the
operating
system for the basic functioning of the general computer system. The examples
of
operating systems are UNIXTM, WINDOWSTM and OS XTM. These operating systems
allocate the computer system resources to various programs and help the users
to interact
with the system. It should be understood that the invention is not limited to
any particular
hardware comprising the computer system or the software running on it.
FIG. 7 shows the internal structure of the general computer system of FIG. 6.
The general
computer system (2.1) consists of various subsystems interconnected with the
help of a
system bus (2.2). The microprocessor (2.3) communicates and controls the
functioning of
other subsystems. Memory (2.4) helps the microprocessor in its functioning by
storing
instructions and data during its execution. Fixed Drive (2.5) is used to hold
the data and
instructions permanent in nature like the operating system and other programs.
Display
adapter (2.6) is used as an interface between the system bus and the display
device (2.7),
which is generally a monitor. The network interface (2.8) is used to connect
the computer
CA 02683169 2009-10-16
-15-
with other computers on a network through wired or wireless means. The system
is
connected to various input devices like keyboard (2.10) and mouse (2.11) and
output
devices like printer (2.12). Various configurations of these subsystems are
possible. It
should also be noted that a system implementing the present invention might
use less or
more number of the subsystems than described above. The computer screen which
displays the diagnosis results can also be a separate computer system than
that which
contains components such as database 360 and the other modules described
above.
In a preferred embodiment, the scoring module of the system calculates
separate score for
the questionnaire and the diagram, as well as a composite score for the
questionnaire and
the diagram, based on user input according to the scoring rules described
above.
In a preferred embodiment, the diagnosis module of the system assigns a
probable
diagnosis based on the calculated score in relation to a predetermined
threshold score
which must be reached in order for an affirmative diagnosis to apply.
In a further embodiment, the present invention is directed towards a computer-
readable
medium having computer-executable instructions operable to cause a computer
to: (1)
receive patient profile data that identifies patient and disease
characteristics; (2)
automatically evaluate the patient profile data in light of one ore more
diagnosis rules and
(3) automatically assign a medical diagnosis to the patient based on the
evaluation.
In one aspect of this embodiment, the patient profile data comprises data
defining the
individual patient's symptoms and genetic characteristics. The patient profile
data is
evaluated in light of one or more diagnosis rules which define a relationship
among or
between data that comprises the patient profile data. More specifically, the
diagnosis rules
comprise a scoring rule which defines a threshold score in order for an
affirmative
diagnosis to apply.
In a preferred embodiment, the computer-readable medium contains instructions
operable
to cause a computer to automatically assign a diagnosis of PsA to a patient
based on the
evaluation. In this regard, assigning a diagnosis of PsA to the patient
comprises the steps
of: (1) receiving input from the patient defining the presence or severity of
PsA-related
symptoms; (2) selecting a threshold score beyond which a diagnosis of PsA will
apply; (3)
CA 02683169 2009-10-16
-16-
automatically assigning a calculating a score based on answers input by the
patient in
response to the questions; and (4) assigning a diagnosis to the patient based
on the
patient's score relative to the threshold score.
In a preferred embodiment, the computer-readable medium will additionally
comprise
instructions for automatically asking questions of the patient, as well as for
displaying to
the patient instructions on how to complete the questionnaire and diagram.
In a preferred embodiment, the threshold score will be chosen using the
receiver operating
curve, as described in detail above, to determine the score which will give
optimal
specificity and sensitivity.
In a preferred embodiment, the computer readable medium will contain
instructions for
determining whether the patient requires follow-up care with a physician or
specialist.
In another embodiment, the computer-readable medium comprises a database to
store
and aggregate patient data for determining the prevalence of PsA in the
population of
patient affected with psoriasis.
In a further embodiment of the present invention, the user is assigned a
unique identifier.
The user's input and score is stored in a storage device. Unique identifiers,
inputs and
scores from a plurality of users may be aggregated to carry out
epidemiological studies.
Such stored information may also be transmitted over communications networks.
EXPERIMENTS
Example 1: Valuation and Validation of the Electronic PASQ Against PASQ
The electronic PASQ Data was collected from a prospective cohort of 42
patients with
early PsA (meeting the CASPAR criteria), and from 12 PSO patients without PsA.
The electronic version of the PASQ (EPASQ) was developed using Adobe Creative
Suite 4
software, and was based on the previous paper version of the PASQ. The EPASQ
was
programmed to provide a maximum of 15 points. The PASQ contained 10
differently
CA 02683169 2009-10-16
-17-
weighted questions as well as a diagram where patients marked where they had
or have
had pain and or swelling. The same questions were included in the EPASQ in
addition to
a diagram with 68 joints plus the spine. Validation was conducted using the
stored
questionnaires from 42 patients with confirmed PsA (mean disease duration 12
months).
Questionnaires from 12 psoriasis patients without PsA were used as a control.
Comparison of scores obtained from the manual and the electronic versions were
conducted. A receiver operating curve (ROC) was determined for both the paper
version
as well as the electronic version using MedCalc software to pick the optimal
cut off point
(optimal sensitivity and specificity) for each component of the PASQ and EPASQ
(questionnaire and diagram). Descriptive statistics for both were obtained
using SPSS.
The EPASQ Data was collected from a prospective cohort of 42 patients with
early PsA
(meeting the CASPAR criteria), and from 12 psoriasis patients without PsA. All
but one of
the PsA patients scored 8 or more in the paper PASQ. Concordance of the paper
and
electronic scores was very high with only one patient who scored 7 f in the
paper PASQ
and 11 in the present invention. The ROC Curve of the entire group yielded an
optimal
97.62% sensitivity and 75.00% specificity for a cut-off score of 7. A cut-off
point of 8
yielded a sensitivity of 88.10% while still maintaining a specificity of
75.00%.
What has been described above includes examples of the present invention. It
is, of
course, not possible to describe every conceivable combination of components
or
methodologies for purposes of describing the present invention, but one of
ordinary skill in
the art may recognize that may further combinations and permutations of the
present
invention are possible. Accordingly, the present invention is intended to
embrace all such
alterations, modifications and variations that fall within the spirit and
scope of the
appended claims. Furthermore, to the extent that the term "includes" is used
in either the
detailed description or the claims, such term is intended to be inclusive in a
manner similar
to the term "comprising" as "comprising" is interpreted when employed as a
transitional
word in a claim.
CA 02683169 2009-10-16
-18-
REFERENCES
1. Peters JP, van der Horst-Bruinsma IE, Dijkmans BA, Nurmohamed MT.
Cardiovascular risk profile of patients with spondyloarthropathies,
particularly
ankylosing spondylitis and psoriatic arthritis. Seminars in Arthritis and
Rheumatism,
2004;34: 585-592.
2. Stern RS. The epidemiology of joint complaints in patients with PSO. J
Rheumatol
1985;12:315-2.
3. Morgan C, Lunt M, Bunn D, Scott DG, Symmons DP. Five-year outcome of a
primary-care-based inception cohort of patients with inflammatory
polyarthritis plus
PSO. Rheumatology 2007;46:1819-23.
4. Gladman DD, Mease PJ, Healy P, Helliwell PS, Fitzgerald 0, Cauli A, Lubrano
E.et
al . Outcome measures in psoriatic arthritis. J Rheumatol 2007;34:1159-66.
5. Helliwell PS, Taylor WJ. Classification and diagnostic criteria for
psoriatic arthritis.
Ann Rheum Dis 2005;64 Suppl 2:ii3-8.
6. Taylor WJ, Helliwell PS. Psoriatic arthritis is a joint-damaging disease -
a call for
action! Rheumatology 2007;46:1747-1748.
7. Gladman DD, Antoni C, Mease P et al. Psoriatic arthritis: epidemiology,
clinical
features, course,and outcome Ann Rheum Dis 2005;64(Suppl ll):ii14-ii17.
8. Mease PJ, Ory P, Sharp JT, Ritchlin CT, van den Bosch F, Wellborne F et al.
Adalimumab for long-term treatment of psoriatic arthritis: two-year data from
the
Adalimumab Effectiveness in Psoriatic Arthritis Trial (ADEPT). Ann Rheum Dis
2008 Aug 6. [Epub ahead of print].
9. Antoni CE, Kavanaugh A, van der Heijde D, Beutler A, Keenan G, Zhou B et
al.
Two-year efficacy and safety of infliximab treatment in patients with active
psoriatic
arthritis: findings of the Infliximab Multinational Psoriatic Arthritis
Controlled Trial
(IMPACT). J Rheumatol 2008 35:869-76.
CA 02683169 2009-10-16
-19-
10. Husni ME, Meyer KH, Cohen DS, Mody E, Qureshi AA. The PASE questionnaire:
pilot-testing a psoriatic arthritis screening and evaluation tool. J Am Acad
Dermatol
2007 Oct;57:581-7.
11. Gorter S, van der Heijde DM, van der Linden S, Houben H, Rethans JJ,
Scherpbier
AJ et al. Psoriatic arthritis: performance of rheumatologists in daily
practice. Ann
Rheum Dis. 2002; 61:219-24.
12. Moll JM, Wright V. Psoriatic arthritis . Sem in Arthritis Rheum. 1973;3:55-
78.
13. Bennett RM. Psoriatic arthritis. In: McCarty DJ, editor. Arthritis and
allied
conditions. 9th ed. Philadelphia: Lea & Febinger; 1979. p. 645.
14. Gladman DD, Shuckett R, Russell ML, Thorne JC, Schachter RK. Psoriatic
arthritis
(PSA)--an analysis of 220 patients. Q J Med. 1987;62:127- 41.
15. Vasey F, Espinoza FR. Psoriatic arthropathy. In Calin A, Editor.
Spondyloarthropathies. Orlando (FL): Grune & Stratton; 1984. p. 151-85.
16. McGonagle D, Conaghan PG, Emery P. Psoriatic arthritis: a unified concept
twenty
years on. Arthritis Rheum1999;42:1080-6.
17. Fournie B, Crognier L, Arnaud C, Zabraniecki L, Lascaux-Lefebvre V, Marc V
et al.
Proposed classification criteria of psoriatic arthritis. A preliminary study
in 260
patients. Rev Rhum Engl Ed 1999;66:446-56.
18. Coates LC, Helliwell PS. Classification and categorisation of psoriatic
arthritis. Clin
Rheumatol. 2008;27:1211-6.
19. Alenius GM, Stenberg B, Stenlund H, Lundblad M, Dahlqvist SR. Inflammatory
joint
manifestations are prevalent in PSO: prevalence study of joint and axial
involvement in psoriatic patients, and evaluation of a psoriatic and arthritic
questionnaire. J Rheumatol.2002;29:2577-82.
20. Peloso PM, Behl M, Hull P, Reeder B. The PSO and arthritis questionnaire
(PAQ)
in detection of arthritis in patients with PSO. Arthritis Rheum
1997;40(Suppl):S64.
CA 02683169 2009-10-16
-20-
21. Gladman DD, Schentag CT et al. Development and initial validation of a
screening
questionnaire for psoriatic arthritis: The Toronto Psoriatic Arthritis Screen
(ToPAS). Ann Rheum Dis published online 30 Apr 2008.
22. Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H;
CASPAR
Study Group. Classification criteria for psoriatic arthritis: development of
new
criteria from a large international study. Arthritis Rheum 2006 ;54:2665-73.
23. The Psoriasis and Arthritis Screening Questionnaire (PASQ): A Sensitive
and
Specific Tool to Diagnose Psoriatic Arthritis Patients with high correlation
to the
CASPAR criteria. M. Khraishi, I. Landells, C. Heale, B. Grouchy, G. Mugford.
European League Against Rheumatism annual meeting (EULAR). Paris, June 2008