Note: Descriptions are shown in the official language in which they were submitted.
CA 02684041 2009-10-09
WO 2008/132590 PCT/IB2008/001031
- 1 -
ALUMINIUM ELECTROWINNING CELLS WITH METAL-BASED CATHODES
Field of the Invention
The invention relates to a cell for the
electrowinning of aluminium with a metal-based cathode on
which during use aluminium is produced. The invention
also relates to a method for electrowinning aluminium in
this a cell, to the cathode as such and to a method of
manufacturina the cathode.
Background of the Invention
Aluminium is produced conventionally by the Hall-
Heroult process, by the electrolysis of alumina dissolved
in cryolite-based molten electrolytes at temperatures up
to around 950 C. A Hall-Heroult reduction cell typically
has a steel shell provided with an insulating lining of
refractory material, which in turn has a lining of carbon
which contacts the molten constituents. Conductor bars
connected to the negative pole of a direct current source
are embedded in the carbon cathode substrate forming the
cell bottom floor. The cathode substrate is usually an
anthracite based carbon lining made of prebaked cathode
blocks, joined with a ramming mixture of anthracite,
coke, and coal tar, or with glue.
It has long been recognised that it would be
desirable to make (or coat or cover) the cathode of an
aluminium electrowinning cell with a refractory boride
such as titanium diboride that would render the cathode
surface wettable by molten aluminium which in turn would
lead to a series of advantages.
For example, US Patents 5,310,476, 5,364,513,
5,651,874 and 6,436,250 (all assigned to Moltech Invent
S.A.) disclose applying a protective coating of a
refractory material such as titanium diboride to a carbon
component of an aluminium electrowinning cell, by
applying thereto a slurry of particulate refractory
material and/or precursors thereof in a colloid in
several layers with drying between each layer.
W001/42168, W001/42531 and W002/096831 (all assigned to
Moltech Invent S.A.) disclose the use of a layer made of
particulate oxide of Mn, Fe, Co, Ni, Cu, Zn, Mo or La (-
325 mesh) mixed with refractory material and/or on a
layer of refractory material. The use of these oxides
CA 02684041 2009-10-09
WO 2008/132590 PCT/IB2008/001031
- ~ -
promotes the wetting of the refractory material by molten
aluminium. These patents also disclose the use of such
materials in an oxidising and/or corrosive environment.
US patents 6, 558, 525, 6, 800, 191, 6,811,676 and
7,077,945 (all assigned to Northwest Aluminium) disclose
aluminium electrowinning cells having vertical foraminate
nickel-copper-iron anodes facing vertical cathodes, the
cathodes being preferably made of titanium diboride or
any suitable material that is substantially inert to
molten aluminium, such as zirconium diboride, titanium
carbide, zirconium carbide, molybdenum or tungsten.
These materials have not as yet found wide
commercial acceptance and there is a need to provide a
cathodic material with improved properties for use in an
aluminium electrowinning cell.
Summary of the Invention
An object of the invention is to provide a cathode
for an aluminium electrowinning cell, which cathode has a
high conductivity, permits an enhanced current
distribution compared to carbon cathodes and is resistant
to molten contents of the cell, in particular sodium.
A particular object of the invention is to provide a
long lasting metal-based cathode for aluminium
electrowinning cells.
Another object of the invention is to provide a
metal-based cathode for aluminium electrowinning cell
that is resistant to exposure to molten aluminium and has
a low wear rate.
It has been observed that there is no substantial
inter-diffusion between tungsten or molybdenum and molten
aluminium. However, the solubility of tungsten or
molybdenum in molten aluminium is not sufficiently low to
achieve, when used alone, the objects of the invention.
Indeed, when an aluminium electrowinning cell utilises a
cathode of metallic tungsten or molybdenum in direct
contact with molten aluminium, the corrosion rate of the
tungsten or molybdenum cathode is of the order of 2 to 3
micron per hour, which is commercially unacceptable
This drawback has been overcome by providing the
cathode with a protective surface of tungsten carbide or
molybdenum carbide in accordance with the invention. It
has been found that tungsten c.arbide and molybdenum
CA 02684041 2009-10-09
WO 2008/132590 PCT/IB2008/001031
- 3 -
carbide are stable in molten aluminium. Moreover, such
carbide is wettable by molten aluminium which makes its
use suitable for providing an aluminium-wettable cathode
surface.
Therefore, the invention relates to a cell for the
electrowinning of aluminium from an aluminium compound
dissolved in a molten electrolyte. The cell has one or
more anodes facing at least one cathode. Such a cathode
comprises: a cathode body made predominantly of at least
one hard metal selected from tungsten and molybdenum; and
a surface of carbide of this hard metal which is integral
with the cathode body or which is formed by a layer
bonded to the cathode body. This carbide surface forms a
cathodic operative surface on which during use aluminium
is produced or forms an anchorage surface for an
aluminium-wettable ceramic layer on which during use
aluminium is produced.
It follows that as opposed to the prior art (US
patents 6,558,525, 6,800,191, 6,811,676 and 7,077,945),
tungsten and/or molybdenum cathode bodies of a cell of
the present invention are covered with a carbide surface
that significantly increases the resistance of the
cathode body against wear and dissolution in the cell.
Moreover, the tungsten and/or molybdenum body with a
carbide surface has been found to resist penetration by
sodium. Therefore, the use of such metal-based cathode
bodies solves the problem of detrimental penetration of
sodium from the electrolyte and thereby-caused swelling
and wear that occur with carbon cathodes even when
covered with an RHM layer.
Typically, the cathode body contains the hard metal
(tungsten and/or molybdenum) in an amount of 50 to 100%,
in particular 75 to 98% such as 85 to 95%, by weight of
the cathode body.
The cathode body may contain silicon in an amount of
0. 1 to 30%, in particular 2 to 25% such as 5 to 15%, by
weight of the cathode body. The cathode body can contain
aluminium in an amount of 0.1 to 10 wt%, in particular
0.5 to 8% such as 2 to 6%, by weight of the cathode body.
Optionally, the cathode body contains further
constituents such as Fe, Ni, Co, Mn, Cr, N, 0, B and
compounds thereof in a total amount of 0. 1 to 5 wt%, in
particular 0.5 to 2 wt%, by weight of the cathode body.
CA 02684041 2009-10-09
WO 2008/132590 PCT/IB2008/001031
- 4 -
In one embodiment, the cathode body is predominantly
metallic or essentially metallic.
The cathode body may also contain carbon in an
amount of 0.1 to 20 wt%, in particular 1 to 15% such as 5
to 10%, by weight of the cathode body. In such a case, a
surface of the cathodic body can form the carbide surface
of the cathode.
Typically, the carbide surface of the cathode is
formed by a layer of the hard metal carbide integral with
or bonded to the hard metal body, the carbide layer
having a thickness of at least 0.01 mm, in particular in
the range of 0.02 to 5 mm, such as 0.03 to 3 mm,
typically 0.05 to 1 or 2 mm.
When the carbide surface itself forms the cathodic
operative surface on which during use aluminium is
produced, the layer of hard metal carbide should be
sufficiently thick to provide a long-lasting protection
against product aluminium. Usually, a thickness of the
order of a couple of millimetres, such as 0.5 to 3 mm, or
1 to 2 mm, will be sufficient. When the carbide surface
forms an anchorage surface for an aluminium-wettable
ceramic layer on which during use aluminium is produced,
the layer of hard metal carbide can be thinner, e.g. 10
to 400 micron, or 20 to 300 micron.
As mentioned above, in one embodiment of the
invention, the cathode comprises an aluminium-wettable
ceramic layer that is anchored onto the carbide surface
and contains a refractory compound. This layer contains
optionally an aluminium-wetting agent. The refractory
compound can comprise one or more borides, in particular
a boride of at least one metal selected from titanium,
chromium, vanadium, zirconium, hafnium, niobium,
tantalum, molybdenum, cerium, nickel and iron. The
aluminium-wettable ceramic layer may contain an
aluminium-wetting agent selected from at least one metal
oxide and/or at least one partly oxidised metal, such as
iron, copper, cobalt, nickel, zinc and manganese, in the
form of oxides and partly oxidised metals and
combinations thereof.
For instance, the aluminium-wettable ceramic layer
is a sintered slurry of a particulate of the refractory
compound and, when present, an optional wetting agent in
a dried inorganic polymeric and/or colloidal binder.
Usually, this slurry is a binder containing alumina,
CA 02684041 2009-10-09
WO 2008/132590 PCT/IB2008/001031
- 5 -
beryllium oxide, chromium oxide, silica, yttria, ceria,
hafnia, thoria, zirconia, ruthenia, indium oxide, tin
oxide, magnesia, lithia, vanadium oxide, titania,
tantalum oxide, tungsten oxide, thallium oxide,
molybdenum oxide, niobium oxide, gallium oxide,
monoaluminium phosphate, cerium acetate, nickel oxide,
FeO(OH)v, FeO, Fe203 and Fe304 and combinations and
precursors thereof, all in the form of colloids and/or
inorganic polymers.
Suitable aluminium-wettable ceramic layers are for
example disclosed in US patents 5,364,513, 5,651,874,
6,436,250, and in PCT publications W001/42168,
W001/42531, W002/070783, W002/096830 and W002/096831,
W02004/092-449, W02005/068390 (all assigned to MOLTECH
Invent S.A.).
Even though the cell may be fitted with carbon
anodes, this is not preferred and instead the anode(s)
are advantageously made of metal and/or ceramic material
that is/are active for the evolution of oxygen, in
particular having an electrochemically active oxide
surface of oxides of at least one of iron, nickel and
cobalt.
The active anodic surface can be the surface of an
anode body that has a plurality of through passages for
the flow of circulating electrolyte through the anode
body from below to above the anode body and/or from above
to below the anode body. The anode body may comprise a
series of elongated members spaced apart by inter-member
gaps which form said through passages, or the anode body
may comprise a solid body, in particular a plate, which
has through holes that form said through passages.
Suitable anodes are disclosed in W000/40781, W000/40782,
W003/006716, W003/023091, W003/023091 and W02005/118916
(all assigned to MOLTECH Invent S.A.).
Suitable materials for metal-based, in particular
oxygen-evolving, anodes include at least one metal
selected from nickel, iron, cobalt and copper. For
instance the anode has a metal oxide surface, in
particular a surface containing at least one of iron
oxide, nickel oxide and cobalt oxide. Suitable anode
materials are disclosed in W099/36591 and W099/36592,
W099/36593 and W099/36594, W000/06800, W000/06801,
W000/06802 and W000/06803, W000/06804, W000/06805,
W000/40783 and W001/42534, W001/42536, and W001/43208,
W002/070786, W002/083990, W002/083991, W003/078695,
CA 02684041 2009-10-09
WO 2008/132590 PCT/IB2008/001031
- 6 -
W003/087435, W02004/018731, W02004/024994, W02004/044268,
W02004/050956, W02005/090641 and W02005/090643 (all
assigned to MOLTECH Invent S.A.). Oxygen-evolving anodes
may be coated with a protective layer made of one or more
cerium compounds, in particular cerium oxyfluoride, as
disclosed in US Patents 4,614,569, 4,680,094, 4,683,037
and 4,966,674 (all assigned to MOLTECH Invent S.A.).
Another aspect of the invention relates to a method
of electrowinning aluminium in a cell as described above.
The method comprises passing an electrolysis current from
the cathode(s) to the anode(s) through the molten
electrolyte to electrolyse the dissolved alumina whereby
gas is evolved anodically and aluminium is produced on
the carbide surface of the cathode or on an aluminium-
wettable ceramic layer anchored on said carbide cathode
surface.
Advantageously the cell is in a drained
configuration, aluminium being drained on the cathode.
The aluminium can be drained on an upright or inclined
cathode surface.
The cell's molten electrolyte is usually a fluoride-
containing molten electrolyte, the electrolyte being at a
temperature below 960 C, such as in the range from 900
to 950 C. The electrolyte my consist of: 6.5 to 11
weight% dissolved alumina, in particular 7 to 10 weight%;
to 44 weight% aluminium fluoride, in particular 36 to
42 weight%, such as 36 to 38 weight; 38 to 46 weight%
sodium fluoride, in particular 39 to 43 weight; 2 to 15
weight% potassium fluoride, in particular 3 to 10
30 weight%, such as 5 to 7 weight%; 0 to 5 weight% calcium
fluoride, in particular 2 to 4 weight%; and 0 to 5
weight% in total of one or more further constituents, in
particular up to 3 weight%. Such further constituents may
comprise at least one fluoride selected from magnesium
35 fluoride, lithium fluoride, cesium fluoride, rubidium
fluoride, strontium fluoride, barium fluoride and cerium
fluoride.
The electrolyte can be a fluoride-containing
electrolyte, for example as disclosed in W000/06802,
W001/42535, W002/097167, W003/083176, W02004/035871,
W02004/074549 and W02005/090642 (all assigned to MOLTECH
Invent S.A.).
A further aspect of the invention relates to a
cathode for the electrowinning of aluminium from an
CA 02684041 2009-10-09
WO 2008/132590 PCT/IB2008/001031
- 7 -
aluminium compound dissolved in a molten electrolyte. The
cathode comprises: a cathode body made predominantly of
at least one hard metal selected from tungsten and
molybdenum; and a surface of carbide of said hard metal
which is integral with the cathode body or which is
formed by a layer bonded to the cathode body, the carbide
surface forming a cathodic operative surface on which
during use aluminium is produced or forming an anchorage
surface for an aluminium-wettable ceramic layer on which
during use aluminium is produced.
The cathode may be shaped to be embedded in a cell
bottom and protrude therefrom, or it can be suspended in
the electrolyte. The cathode may comprise a connector for
connection to a cathodic busbar, the connector optionally
including a stem for suspending the cathode body in the
molten electrolyte from a busbar located thereabove.
Yet another aspect of invention relates to a method
of manufacturing such cathodes. The method comprises
providing a cathodic body made predominantly of at least
one hard metal selected from tungsten and molybdenum; and
forming a surface of carbide of said hard metal which is
integral with the body or formed by a layer bonded to the
body.
The cathodic body can be made predominantly of at
least one hard metal selected from metallic tungsten and
molybdenum by carburizing the metallic surface. For
instance, carburization can be carried out by heating the
hard metal in the presence of carbon powders and/or in a
methane-hydrogen atmosphere.
For instance, the cathodic body surface is
carburized by contacting the surface with a carbon mass
and subjecting the cathodic body in contact with the
carbon mass to a carburization heat treatment, in
particular at a temperature above 900 C. Such carbon mass
may comprise: a mixture of carbon powder and pitch that
is applied onto the cathode body's surface and dried; and
a carbon powder bed into which the body with the applied
and dried mixture is immersed. Optionally, a particulate
refractory compound, in particular a boride, is added
into or onto this mixture of carbon powder and pitch
prior to drying.
In one embodiment, the carbide surface of the
cathode body is covered by applying a layer of an
aluminium-wettable ceramic layer, this layer containing
CA 02684041 2009-10-09
WO 2008/132590 PCT/IB2008/001031
- 8 -
optionally an aluminium-wetting agent, as mentioned
above.
In a variation of the invention, it is also
contemplated to use this hard metal (tungsten and/or
molybdenum) having a carbide surface which is integral
with the hard metal or which is formed by a layer bonded
to the hard metal, with or without an applied aluminium-
wettable ceramic top layer as a non-cathodic material, in
particular a cell material that is exposed to molten
aluminium during use. Such a protected hard metal can be
used as part of a sidewall, sidewall lining or an
aluminium collection reservoir or channel.
Brief Description of the Drawings
Embodiments of the invention will now be described
by way of example with reference to the accompanying
schematic drawings, wherein:
- Figures 1 and 2 show two aluminium electrowinning
cells of the invention having vertical tungsten and/or
molybdenum-based cathodes, Figure 1 in diagrammatic
section along the cell and Figure 2 in diagranunatic
section across the cell;
- Figures 3a, 3b, 4a and 4b show cut-away vertical
tungsten and/or molybdenum-based cathodes according to
the invention; and
- Figures 5 to 7 schematically show cut-away cross-
sectional views of the external part of three different
tungsten and/or molybdenum-based cathodes according to
invention.
Detailed Description
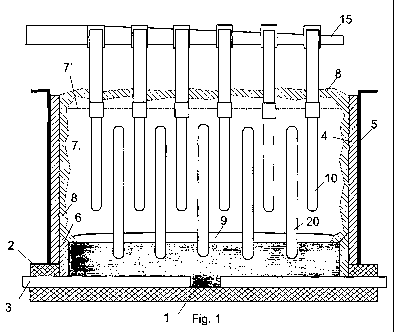
Figures 1 and 2, in which the same numeric
references designate the same elements, show two
aluminium electrowinning cells according to the
invention. The cells have a trough formed by: a
conductive carbon bottom 1 that is embedded in a layer of
iiisulating material 2 and that is connected via metal
bars 3 to an external current supply; and insulating
sidewalls 4 contained in an outer shell 5 connected to
bottom 1 via ramming paste joint 6. The cell troughs
enclose a molten electrolyte 7 that contains dissolved
alumina, sidewalls 4 being protected from molten
electrolyte 7 by a ledge and crust of frozen electrolyte
8 that extends along the entire sidewalls 4 and above
CA 02684041 2009-10-09
WO 2008/132590 PCT/IB2008/001031
- 9 -
molten electrolyte 7. Bottom 1 is protected from molten
electrolyte 7 by a layer or pool of product molten
aluminium 9.
The cells have vertical anodes 10,10' suspei-ided from
busbar 15 in molten electrolyte 7 to face vertical
cathode rods 20. Such rods 20 have their bottom end
mechanically secured and electrically connected to carbon
bottom 1 as shown in greater detail in Figure 3a and 3b
which are, respectively, a side view and a view from
above of a cathode rod 20 in a partly-shown bottom 1.
Anodes 10 shown in Figure 1 are in the form of
vertical rods facing cathode rods 20. Anodes 10' shown in
Figure 2 are vertically suspended plates that face rows
of cathode rodes 20. The anodes can have an
electrochemically active oxide surface of oxides of at
least one of iron, nickel and cobalt, as mentioned above.
In accordance with the invention, the cathode rods
comprise: a cathode body made predominantly of at
least one hard metal selected from tungsten and
20 molybdenum; and a surface of carbide of this hard metal
which is integral with the body or which is formed by a
layer bonded to the body. The carbide surface forms a
cathodic operative surface on which during use aluminium
9 is produced or forms an anchorage surface for an
aluminium-wettable ceramic layer on which during use
aluminium 9 is produced.
During use of the cells shown in Figures 1 and 2, an
electrolysis current is supplied to bottom 1 via
conductor bars 3 and fed into the bottom end of cathode
rods 20. The current is passed from the surfaces of
cathode rods ?0 through electrolyte 7 to anodes 10,10' so
as to electrolyse dissolved alumina contained in
electrolyte 7. Gas is evolved on anodes 10 and aluminium
is produced on the carbide surface of the cathode 10 or
on an aluminium-wettable ceramic layer anchored on the
carbide cathode surface. The product aluminium 9 is
collected on bottom 1. Alumina is intermittently or
continuously supplied to surface 7' at the top of
electrolyte 7.
Figures 4a and 4b, in which the same numeric
references designate the same elements, show a side view
and a view from above, respectively, of another cathode
20' according to the invention. The cathode has a general
pyramidal active part with inclined active faces 21. Such
CA 02684041 2009-10-09
WO 2008/132590 PCT/IB2008/001031
- 10 -
a cathode 20' is suitable for use with inclined anodes,
for example in an anode-cathode arrangement as disclosed
in US 6,797,148 and W003/023096 (both assigned to MOLTECH
INVENT S.A.).
Figures 5 to 7 illustrate cut-away sections of the
outer portion of cathodes 1-0 according to the invention.
Figure 5 shows part of a cathode 20 having a
metallic tungsten and/or molybdenum substrate 25 that has
an integral surface layer 26 of tungsten and/or
molybdenum carbide. Such surface layer 26 has a thickness
of 10 micron to 1 mm and can be obtained by plasma
spraying the components, e.g. tungsten and/or molybdenum
carbide, of layer 26 or heat treatment of substrate 25 in
a methane-containing environment. When the surface layer
is thin (e.g. a few tens or hundreds of microns), it can
be used as an anchorage layer for a ceramic cathodic
layer or, when thicker, as a cathodic operative surface
layer without an additional ceramic top layer. This
cathode 20 can be manufactured by the method disclosed in
Example 1.
The cathodes ?0 shown in Figures 6 and 7 have both a
substrate 25 of tungsten or molybdenum that is covered
with a thin carbide anchorage layer 27. This anchorage
layer 27 serves to anchor aluminium-wettable top layers
28 and 29 to substrate 25.
In Figure 6, top layer 28 is made of particulate
TiB, in a pitch binder optionally containing a wetting
agent such as an oxide of iron, which can be produced by
the method disclosed in Example ' below. The top layer 29
shown in Figure 7 is made of a sintered particulate RHM,
such as TiB.,, applied in a colloidal binder and
optionally containing a wetting agent, for example as
disclosed in W001/42168, W001/42531 and W00</096831 (all
assigned to MOLTECH Invent S.A.). Top layers 28 and 29
are suitable to be used as an active operative cathode
surface.
The invention will be further described in the
following examples.
Example 1
A tungsten cathode according to the invention was
prepared as follows:
CA 02684041 2009-10-09
WO 2008/132590 PCT/IB2008/001031
- 11 -
A rod of metallic tungsten having a diameter of 10
mm and a length of 50 mm was submitted to a carburization
treatment in a carbon powder bed, at 950 C in air. After
16 hours of this carburization treatment, the rod was
allowed to cool down.
After cooling the rod was examined and showed a hard
and black carburized tungsten surface. No oxidation was
observed. Based on the weight gain of the rod, the
thickness of the tungsten carbide layer was estimated to
be about 1 micron.
The carburized tungsten rod was tested as a hanging
cathode facing a cobalt-based anode rod in a molten
electrolyte of an aluminium electrowinning cell. The
electrolyte was at a temperature of 930 C and made of
62.4 wt% Na3AlF6, 11 wt% NaF, 7 wt% KF, 4% wt% CaF and
9.6 wt% A1203. A current was passed between the anode and
the cathode at a cathodic current density of 0.8 A/cm2.
During electrolysis, the cell voltage showed over
time a very regular saw tooth profile indicating that the
cathodic tungsten rod was well wetted by product
aluminium. After 200 hours, the electrolysis was stopped
and the cathode was removed from the cell to be examined.
The carburized tungsten cathodic rod showed no sign
of wear. Its surface was covered with a layer of
aluminium indicating that it had been well wetted by
molten aluminium during use. The change of dimension of
the tungsten cathodic rod was less than 150 micron in the
rod radius after 200 hours, or about 0.'7 microns per hour
which was at least 20 times less than the non carburized
cathode.
Compared to a non-carburized metallic tungsten
cathode having a wear rate of 2 to 3 micron per hour
during use, this test demonstrated that carburizing a
tungsten cathode prior to use is an appropriate pre-
treatment for improving the corrosion resistance of the
cathode during use.
The corrosion resistance can be improved by forming
at the surface of the tungsten cathodic rod a thicker
carburized layer, for instance having a thickness in the
range of 0.05 to 1 mm, in particular 0.1 to 0.5 mm, for
example by heat treating the tungsten cathodic rod in a
methane-hydrogen atmosphere.
CA 02684041 2009-10-09
WO 2008/132590 PCT/IB2008/001031
- 12 -
Example 2
The starting point of this Example is based on two
experimental observations:
- A titanium diboride-based coating can be obtained
from a slurry of TiB2 powders in carbon pitch (H. Oye et
al.: TMS 2006 - pp. 262).
- A tungsten carbide layer that is formed by
reaction between a metallic tungsten surface and a carbon
source can be used as a diffusion bonding between the
tungsten structure and the carbon matrix.
This Example therefore describes a cathodic tungsten
rod which is coated with a TiB2-based coating as follows:
A layer of commercial cathode glue consisting
essentially of a mixture of graphite powder and pitch was
applied on a sandblasted surfaces of a tungsten rod.
Dry particulate TiB` was sprayed and incorporated
into the still-humid cathode glue. The excess TiB2 powder
was eliminated by brushing. The applied TiB2-glue layer
was dried for 30 minutes at 150 C in air.
The application of a TiB2-glue layer was repeated
five times overall. The five applied layers formed a
green coating having a thickness of about 0.5 mm was
obtained on the tungsten cathodic rod.
After application of the TiB,-glue coating the
'25 tungsten cathodic rod was placed in a graphite powder bed
and submitted to a carburization reaction at 950 C
during 24 hours in air.
After carburization, the coated tungsten cathodic
rod was allowed to cool down to room temperature and
examined. The TiB2-based coating was coherent and showed
no sign of any surface oxidation. The coating was hard
and adhered well to the cathodic tungsten rod, i.e.
resisting sandblasting.
Aluminium-wettability of the coating was improved by
applying a layer of a copper slurry thereon, as taught in
W001/42168 (assigned to MOLTECH Invent SA), e.g. oxidised
Cu particles in colloidal A1203. As an alternative, this
copper-based layer can be applied before carburization.
CA 02684041 2009-10-09
WO 2008/132590 PCT/IB2008/001031
- 13 -
The coated tungsten cathodic rod was then tested as
a hanging cathode in a cell under the same conditions as
described in Example 1.
After 200 hours, the electrolysis was stopped and
the cathode was removed from the cell to be examined.
The TiB2-based coating had been fully impregnated by
aluminium during electrolysis. The tungsten carbide layer
underneath the TiBõ-based coating formed a barrier layer
on the metallic tungsten substrate so that this substrate
contained no aluminium and showed no sign of diffusion or
dissolution. No signs of wear were observed on the
cathodic rod whose dimension had remained unchanged.