Note: Descriptions are shown in the official language in which they were submitted.
CA 02684870 2009-10-20
WO 2008/131329 PCT/US2008/061004
OPHTHALMIC LENS MOLD SURFACE ENERGY DIFFERENTIAL
FIELD OF USE
This invention describes molds and ophthalmic lenses formed with the molds and
a
surface energy differential therebetween.
BACKGROUND
It is well known that contact lenses can be used to improve vision. Various
contact lenses have been commercially produced for many years. Early designs
of
contact lenses were fashioned from hard materials. Although these lenses are
still
currently used in some applications, they are not suitable for all patients
due to their
poor comfort and relatively low permeability to oxygen. Later developments in
the
field gave rise to soft contact lenses based upon hydrogels.
Hydrogel contact lenses are popular and often more comfortable to wear than
contact lenses made of hard materials. Malleable soft contact lenses made from
hydrogels can be manufactured by forming a lens in a multi-part mold where the
combined parts form a topography consistent with the desired final lens.
Ophthalmic lenses are often made by cast molding, in which a monomer
material is deposited in a cavity defined between optical surfaces of opposing
mold
parts. Multi-part molds used to fashion hydrogels into a useful article, such
as an
ophthalmic lens, can include for example, a first mold part with a convex
portion that
corresponds with a back curve of an ophthalmic lens and a second mold part
with a
concave portion that corresponds with a front curve of the ophthalmic lens. To
prepare
a lens using such mold parts, an uncured hydrogel lens formulation is placed
between a
front curve mold part and a back curve mold part. The mold parts are brought
together
to shape the lens formulation according to desired lens parameters.
Traditionally, a
lens edge was formed about the perimeter of the formed lens by compression of
an
edge formed into the mold parts which penetrates the lens formulation and
incises it
into a lens portion and an excess ring portion. The lens formulation was
subsequently
cured, for example by exposure to heat and light, thereby forming a lens.
1
CA 02684870 2009-10-20
WO 2008/131329 PCT/US2008/061004
Following cure, mold portions are separated and the lens remains adhered to
one of the mold portions. The lens and the excess polymer ring must be
separated and
the excess polymer ring discarded. During mold separation, lens damage may
occur.
Damage can include, for example: edge chips and tears; holes; lens
delamination or
pulls; lenses adhering to a wrong mold part, optical distortion; and surface
marks. In
addition, it is sometimes difficult and time consuming release a formed lens
from a
mold part to which the lens adheres following demold.
It is desirable therefore to have a correlation of mold materials and lens
materials that facilitate demold and lens release.
SUMMARY
Accordingly, the present invention includes improved molds and processes
useful in the creation of an ophthalmic lens. A mold material can be used with
one or
more additives which reduce the surface tension of the mold material and
increase a
differential between one or both mold parts and the lens formed therebetween.
According to the present invention, a lens forming mixture is cured in a
cavity of a
desired shape formed by two or more mold parts. At least one of the mold parts
is
molded from a material with a differential in surface energy between a mold
used to
form an ophthalmic lens and the ophthalmic lens formed.
Embodiments can include at least one of the mold parts being transparent to
polymerization initiating radiation such that a polymerizable lens forming
mixture can
be deposited in the cavity and the mold part and polymerizable composition can
be
exposed to polymerization initiating radiation.
Embodiments can also include methods of producing an ophthalmic lens by
dispensing an uncured lens formulation onto a surface of a mold part with a
surface
tension less than 30 mN/m. The lens can include, for example, a silicone
hydrogel
formulation or a hydrogel formulation. Specific examples can include a lens
formed
from: acquafilcon A, balafilcon A, and lotrafilcon A, etafilcon A, genfilcon
A,
lenefilcon A, polymacon and galyfilcon A, and senofilcon A.
DESCRIPTION OF THE DRAWINGS
FIG. 1 illustrates a mold assembly according to some embodiments of the
present
invention.
2
CA 02684870 2009-10-20
WO 2008/131329 PCT/US2008/061004
FIG. 2 illustrates a flow chart of exemplary steps that can be executed while
implementing some embodiments of the present to create a mold part.
FIG. 3 illustrates a flow chart of exemplary steps that can be executed while
implementing some embodiments of the present to create an ophthalmic lens.
FIG. 4 illustrates a chart with exemplary data indicating surface energy
qualities of
molds fashioned from thermoplastic resins and compounds of thermoplastic
resins.
FIG. 5 illustrates a chart with lens release time data as it relates to
different mold part
materials.
DETAILED DESCRIPTION OF THE INVENTION
The present invention includes molds and methods for making an ophthalmic
lens. According to some embodiments of the present invention, at least one
part of a
multi-part mold that is used in the manufacture of an ophthalmic lens, is
injection
molded from a thermal plastic resin (hereinafter referred to as "TPR")
compounded
with an additive to reduce the surface energy of the mold material.
According to the present invention selection of mold part materials and
monomers which result in an increased surface energy differential between
monomers
or cured lenses and FC molds facilitates silicone hydrogel easy lens release
during
aqueous hydration. Generally, surface energy differential or "delta" can
defined as
Surface Energy of Monomer or Cured Lens - Surface Energy of Mold Part.
Accordingly, preferred embodiments include a surface energy of delta between a
front
curve mold part and a lens that is greater than 0.
The mechanisms of adhesion are generally the result of surface energy related
parameters relating to the two surfaces that are in contact. According tot he
present
invention, use of mold materials with low surface energy, such as, for
example, <- 30
mN/m or less, as front curve (FC) reduces adhesive force or adhesion energy
between
cured lens and FC mold, and, therefore, facilitate easier and faster silicone
hydrogel
lens release during aqueous hydration.
In addition to use of a low surface energy FC mold (<- 30), increasing monomer
surface energy should also reduce adhesive force or adhesion energy between
cured
3
CA 02684870 2009-10-20
WO 2008/131329 PCT/US2008/061004
lens and FC mold. This should also benefit easier and faster lens release from
FC mold
during hydration, including aqueous hydration. FC mold materials with lower
surface
energy (<_ 30 mN/m) can be successfully obtained by compounding PP with
selective
additives, such as Siloxane MB50-001 and Trilwet A.
In some embodiments of the present invention, ophthalmic lens molds
comprising TPR and additive blends result in a mold surface energy of an
uncoated
ophthalmic lens mold of about 30 mN/m or less. Methods of the present
invention
therefore include fashioning an ophthalmic lens from a mold with one or more
mold
part having an uncoated surface energy of about 30 mN/m or less.
One or both mold parts utilized to form an ophthalmic lens is injection molded
from a TPR with an additive or other mechanism to reduce the surface energy of
the
mold part to less than 30mN/m. Injection molding apparatus will typically
include
precision tooling that has been machined from a metal, such as, for example,
brass,
stainless steel or nickel or some combination thereof. Typically, tooling is
fashioned in
a desired shape and machined or polished to achieve precision surface quality.
The
precision surface in turn increases the quality of a mold part injection
molded
therefrom.
In some preferred embodiments, mold parts are fashioned from a thermoplastic
polyolefin with an additive to produce single use cast molds with a surface
energy of
less than 30 mN/m which reduces the adhesive force between a cured lens and
mold
parts used to fashion the lens and is therefore conducive to the manufacture
of
ophthalmic lenses. Advantages of utilizing molds comprising a thermoplastic
polyolefin material with an additive which results in a surface energy of less
than
30mN/m include a diminished number of lens defects, such as holes, chips and
tears
resulting from demold; and also improved release from a mold part in which it
is
formed.
Lenses
As used herein "lens" refers to any ophthalmic device that resides in or on
the
eye. These devices can provide optical correction or may be cosmetic. For
example,
the term lens can refer to a contact lens, intraocular lens, overlay lens,
ocular insert,
4
CA 02684870 2009-10-20
WO 2008/131329 PCT/US2008/061004
optical insert or other similar device through which vision is corrected or
modified, or
through which eye physiology is cosmetically enhanced (e.g. iris color)
without
impeding vision.
As used herein, the term "lens forming mixture" refers to a mixture of
materials
that can react, or be cured, to form an ophthalmic lens. Such a mixture can
include
polymerizable components (monomers), additives such as UV blockers and tints,
photoinitiators or catalysts, and other additives one might desire in an
ophthalmic lens
such as a contact or intraocular lens.
In some embodiments, a preferred lens type can include a lens that is made
from
silicone elastomers or hydrogels, such as, for example, silicone hydrogels,
fluorohydrogels, including those comprising silicone/hydrophilic macromers,
silicone
based monomers, initiators and additives. By way of non-limiting example, some
preferred lens types can also include etafilcon A, genifilcon A, lenefilcon A,
polymacon, acquafilcon A, balafilcon A, lotrafilcon A, galyfilcon A,
senofilcon A,
silicone hydrogels.
Molds
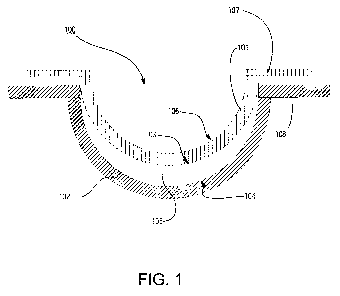
Referring now to Fig. 1, a diagram of an exemplary mold for an ophthalmic lens
is illustrated. As used herein, the terms "mold" and "mold assembly" refer to
a form
100 having a cavity 105 into which a lens forming mixture can be dispensed
such that
upon reaction or cure of the lens forming mixture (not illustrated), an
ophthalmic lens
of a desired shape is produced. The molds and mold assemblies 100 of this
invention
are made up of more than one "mold parts" or "mold pieces" 101-102. The mold
parts
101-102 can be brought together such that a cavity 105 is formed between the
mold
parts 101-102 in which a lens can be formed. This combination of mold parts
101-102
is preferably temporary. Upon formation of the lens, the mold parts 101-102
can again
be separated for removal of the lens.
At least one mold part 101-102 has at least a portion of its surface 103-104
in
contact with the lens forming mixture such that upon reaction or cure of the
lens
forming mixture that surface 103-104 provides a desired shape and form to the
portion
of the lens with which it is in contact. The same is true of at least one
other mold part
101-102.
5
CA 02684870 2009-10-20
WO 2008/131329 PCT/US2008/061004
Thus, for example, in a preferred embodiment a mold assembly 100 is formed
from two parts 101-102, a female concave piece (front piece) 102 and a male
convex
piece (back piece) 101 with a cavity formed between them. The portion of the
concave
surface 104 which makes contact with lens forming mixture has the curvature of
the
front curve of an ophthalmic lens to be produced in the mold assembly 100 and
is
sufficiently smooth and formed such that the surface of a ophthalmic lens
formed by
polymerization of the lens forming mixture which is in contact with the
concave
surface 104 is optically acceptable.
In some embodiments, the front mold piece 102 can also have an annular flange
integral with and surrounding circular circumferential edge 108 and extends
from it in a
plane normal to the axis and extending from the flange (not shown).
The back mold piece 101 has a central curved section with a concave surface
106, convex surface 103 and circular circumferential edge 107, wherein the
portion of
the convex surface 103 in contact with the lens forming mixture has the
curvature of
the back curve of a ophthalmic lens to be produced in the mold assembly 100
and is
sufficiently smooth and formed such that the surface of a ophthalmic lens
formed by
reaction or cure of the lens forming mixture in contact with the back surface
103 is
optically acceptable. Accordingly, the inner concave surface 104 of the front
mold half
102 defines the outer surface of the ophthalmic lens, while the outer convex
surface
103 of the base mold half 101 defines the inner surface of the ophthalmic
lens.
In some preferred embodiments, molds 100 can include two mold parts 101-102
as described above, wherein one or both of the front curve part 102 and the
back curve
part 101 of the mold 100 comprises a thermoplastic polyolefin compound with a
surface energy of less than 30mN/m.
Blended mold material resins can be obtained, for example, using different
compounding methods, including hand blending, single screw compounding, twin
screw and/or multiple screw compounding.
Preferred embodiments of a mold material include a polyolefin; cyclic olefins;
and cyclic olefin copolymers; including, in some embodiments polyolefins and
COCs.
Additives that may be compounded with the preferred mold materials include:
a) Siloxanes including a class of organosilicon compounds with empirical
formula R2SiO, where R is an organic group;
6
CA 02684870 2009-10-20
WO 2008/131329 PCT/US2008/061004
b) non-ionic surfactants such as: alkyl ethoxylates and glycerol monostearate;
and
c) a polymer made from the monomer N-vinyl pyrrolidone, such as
Polyvinylpyrrolidone.
Siloxanes can include [SiO(CH3)z]7z (dimethylsiloxane) and [SiO(C6Hs)2]n
(diphenylsiloxane), where n is typically > 4. Siloxane orgaosilicon compounds
can
include both organic and inorganic chemical compounds. Organic side chains can
confer hydrophobic properties and an -Si-O-Si-O- backbone is inorganic.
Glycerol monostearate can include a lipophilic non-ionic surfactant with HLB
of 3.6 - 4.2 and a chemical formula of CH3(CH2)16COOCH2CHOHCH2OH.
Polyvinylpyrrolidone can include a nonionic powder with the chemical formula
(C6H9NO)X.
Specific examples of additives that decrease the surface energy of a mold
material predominantly made up of one or more of: polyolefin; cyclic olefins;
and
cyclic olefin copolymers; include:
1. Silixone MB50-001 from Dow Corning;
2. Trilwet A from Trillium Specialties LLC ;
3. Glycerol Monostearate (GMS) from SparTech, Inc.; and
4. PVP K-90 from International Specialty Products.
Preferred embodiments can also include a polyolefin of one or more of:
polypropylene, polystyrene, polyethylene, polymethyl methacrylate, and
modified
polyolefins.
Thermoplastics that can be compounded with an additive can include, for
example, one or more of: polypropylene, polystyrene and alicyclic polymers.
In some embodiments the thermoplastic resin can include an alicyclic polymer
which refers to compounds having at least one saturated carbocyclic ring
therein. The
saturated carbocyclic rings may be substituted with one or more members of the
group
consisting of hydrogen, Ci_ioalkyl, halogen, hydroxyl, Ci_ioalkoxycarbonyl,
Ci_ioalkoxy, cyano, amido, imido, silyl, and substituted Ci_ioalkyl where the
substituents are selected from one or more members of the group consisting of
halogen,
hydroxyl, Ci_ioalkoxycarbonyl, Ci_ioalkoxy, cyano, amido, imido, and silyl.
Examples
of alicyclic polymers include but are not limited to polymerizable
cyclobutanes,
7
CA 02684870 2009-10-20
WO 2008/131329 PCT/US2008/061004
cyclopentanes, cyclohexanes, cycloheptanes, cyclooctanes, biscyclobutanes,
biscyclopentanes, biscyclohexanes, biscycloheptanes, biscyclooctanes, and
norbornanes. It is preferred that the at least two alicyclic polymers be
polymerized by
ring opening metathesis followed by hydrogenation. Since co-polymers are
costly, it is
preferable that the molds made from these co-polymers may be used several
times to
prepare lenses instead of once which is typical. For the preferred molds of
the
invention, they may be used more than once to produce lenses.
More particularly, examples of alicyclic polymer containing saturated
carbocyclic rings include but are not limited to the following structures
R3 4 R' T R3
R2 R2 R R'
R' R4 R1 R3 ~ R2 R4 ; R2
R3
R~ Ap2r-,4R6 R R2
R3 R5 R2 R ; and R~ R4
wherein Ri-6 are independently selected from one or more members of the
group consisting of hydrogen, Ci_ioalkyl, halogen, hydroxyl,
Ci_ioalkoxycarbonyl,
Ci_ioalkoxy, cyano, amido, imido, silyl, and substituted Ci_ioalkyl where the
substituents selected from one or more members of the group consisting of
halogen,
hydroxyl, Ci_ioalkoxycarbonyl, Ci_ioalkoxy, cyano, amido, imido and silyl.
Further two
or more of R1-6 may be taken together to form an unsaturated bond, a
carbocyclic ring, a
carbocyclic ring containing one or more unsaturated bonds, or an aromatic
ring. The
preferred R1-6 is selected from the group consisting of Ci_ioalkyl and
substituted
Ci_ioalkyl where the substituents are selected from the group consisting of
halogen,
hydroxyl, Ci_ioalkoxycarbonyl, Ci_ioalkoxy, cyano, amido, imido and silyl.
8
CA 02684870 2009-10-20
WO 2008/131329 PCT/US2008/061004
The alicyclic co-polymers consist of at least two different alicyclic polymer
s.
The preferred alicyclic co-polymers contain two or three different alicyclic
polymer s,
selected from the group consisting of
R3 R1 R3
A 6
g 2 R' R' 3 R4 R5 R
R R4 ; R R4 ; R2 ;and R2 R
The particularly preferred alicyclic co-polymer contains two different
alicyclic
momoners where the generic structure of the saturated carbocyclic rings of the
alicyclic
R3
R2
polymers are of the formula R' R4 and R1-R4 are Ci_ioalkyl.
Typically the surface energy of the alicyclic co-polymer is between 30
and 45 dynes/cm at 25 C. A preferred alicyclic co-polymer contains two
different
alicyclic polymers and is sold by Zeon Chemicals L.P. under the trade name
ZEONOR.
There are several different grades of ZEONOR. Various grades may have glass
transition temperatures ranging from 105 C to 160 C. A specifically preferred
material
is ZEONOR 1060R, which according the to the manufacturer, ZEON Chemicals L.P.
has an melt flow rate ("MFR") range of 11.0 grams/10 minutes to 18.0 grams/10
minutes (as tested JISK 6719 (230 C)), a specific gravity (H20 =1) of 1.01 and
a glass
transition temperature of 105 C.
Other mold materials that may combined with one or more additives to provide
a surface energy of less then 30mN/m and used to form an ophthalmic lens mold
include, for example, Zieglar-Natta polypropylene resins (sometimes referred
to as
znPP). On exemplary Zieglar-Natta polypropylene resin is available under the
name
PP 9544 MED. PP 9544 MED is a clarified random copolymer for clean molding as
per FDA regulation 21 CFR (c)3.2 made available by ExxonMobile Chemical
9
CA 02684870 2009-10-20
WO 2008/131329 PCT/US2008/061004
Company. PP 9544 MED is a random copolymer (znPP) with ethylene group
(hereinafter 9544 MED). Other exemplary Zieglar-Natta polypropylene resins
include:
Atofina Polypropylene 3761 and Atofina Polypropylene 3620WZ.
Still further, in some embodiments, the molds of the invention may contain
polymers such as polypropylene, polyethylene, polystyrene, polymethyl
methacrylate,
modified polyolefins containing an alicyclic moiety in the main chain and
cyclic
polyolefins, such as, for example Zeonor and EOD 00-11 by Atofina Corporation.
For
example, a blend of the alicyclic co-polymers and polypropylene (metallocene
catalyst
process with nucleation, such as ATOFINA EOD 00-11 ) may be used, where the
ratio by weight percentage of alicyclic co-polymer to polypropylene ranges
from about
99:1, to about 20:80 respectively. This blend can be used on either or both
mold
halves, where it is preferred that this blend is used on the back curve and
the front curve
consists of the alicyclic co-polymers.
In some preferred methods of making molds 100 according to the present
invention, injection molding is utilized according to known techniques,
however,
embodiments can also include molds fashioned by other techniques including,
for
example: lathing, diamond turning, or laser cutting.
Typically, lenses are formed on at least one surface of both mold parts 101-
102.
However, if need be one surface of the lenses may be formed from a mold part
101-102
and the other lens surface can be formed using a lathing method, or other
methods.
As used herein "lens forming surface" means a surface 103-104 that is used to
mold a lens. In some embodiments, any such surface 103-104 can have an optical
quality surface finish, which indicates that it is sufficiently smooth and
formed so that a
lens surface fashioned by the polymerization of a lens forming material in
contact with
the molding surface is optically acceptable. Further, in some embodiments, the
lens
forming surface 103-104 can have a geometry that is necessary to impart to the
lens
surface the desired optical characteristics, including without limitation,
spherical,
aspherical and cylinder power, wave front aberration correction, corneal
topography
correction and the like as well as any combinations thereo
Methods
The following method steps are provided as examples of processes that may be
implemented according to some aspects of the present invention. It should be
CA 02684870 2009-10-20
WO 2008/131329 PCT/US2008/061004
understood that order in which the method steps are presented are not meant to
be
limiting and other orders may be used to implement the invention. In addition,
not all
of the steps are required to implement the present invention and additional
steps may be
included in various embodiments of the present invention.
Referring now to Fig. 2, a flowchart illustrates exemplary steps that may be
used to implement the present invention. At 201, a resin including a TPE and
an
additive for reducing the surface energy of the TPE, is plasticized and
prepared for use
in an injection molding process. Injection molding techniques are well known
and
preparation typically involves heating resin pellets beyond a melting point.
At 202, the plasticized resin is injected into an injection mold shaped in a
fashion suitable for creating an ophthalmic lens mold part 101-102. At 203,
the
injection mold is typically placed in a pack and hold status for an
appropriate amount of
time, which can depend, for example upon the resin utilized and the shape and
size of
the mold part. At 204, the formed mold part 101-102 is allowed to cool and at
205, the
mold part 101-102 can be ejected, or otherwise removed from the injection
mold.
Referring now to Fig. 3, some embodiments of the present invention include
methods of making an ophthalmic lens comprising, consisting essentially of, or
consisting of the following steps. At 301 one or more mold parts 101-102 are
created
which comprise, consist essentially of, or consist of, including a TPR
compounded with
an additive for reducing the surface energy of the TPE. At 302, an uncured
lens
formulation is dispensed onto the one or more mold parts 101-102 and at 303,
the lens
formulation is cured under suitable conditions. Additional steps can include,
for
example, hydrating a cured lens until it releases from a mold part 101-102 and
leaching
acute ocular discomfort agents from the lens.
As used herein, the term "uncured" refers to the physical state of a lens
formulation prior to final curing of the lens formulation to make the lens. In
some
embodiments, lens formulations can contain mixtures of monomers which are
cured
only once. Other embodiments can include partially cured lens formulations
that
contain monomers, partially cured monomers, macromers and other components.
As used herein, the phrase "curing under suitable conditions" refers to any
suitable method of curing lens formulations, such as using light, heat, and
the
appropriate catalysts to produce a cured lens. Light can include, in some
specific
examples, ultra violet light. Curing can include any exposure of the lens
forming
11
CA 02684870 2009-10-20
WO 2008/131329 PCT/US2008/061004
mixture to an actinic radiation sufficient to case the lens forming mixture to
polymerize.
Comparative Mold Qualities
Referring now to Fig. 4, a graph 400 is provided which illustrates surface
energy characteristics of mold materials, in including some molds fashioned
from a
compound including a TPR and an additive for reducing the surface energy of a
mold
formed from the TPE. Data associated with the chart 400 is included herein as
Table 1.
Table 1 Owens-wendt Zisman
Mold Disperse Polar Surface Surface
Mold Material Type (mN/m) (mN/ Tension Tension
m) (mN/m) (mN/m)
PP9544 FC 30.31 0.00 30.31 26.39
PP9544 + 1% w.t. MB50-001 FC 31.34 0.03 31.37 27.20
PP9544 + 2.5% MB50-001 FC 28.26 0.02 28.28 32.65
PP9544 + 5% MB50-001 FC 28.21 0.08 28.29 24.74
PP9544 + 7.5% w.t. MB50-001 FC 32.76 0.08 32.84 28.86
PP9544 + 10% w.t. M B50-001 FC 33.48 0.20 33.68 28.39
Zeonor 1060R FC 43.38 0.02 43.39 38.46
25% Zeonor + 75% PP9544 FC 32.79 0.02 32.81 28.21
55% Zeonor + 45% PP9544 FC 33.35 0.03 33.38 28.52
Zeonor + 5% w.t. Trilwet A FC 40.07 0.78 40.85 38.68
As illustrated in the graph 400, mold materials including polypropylene 403-
408 and Zeonor 1060R 409-412 were compounded with the additive MB50-001 403-
411 or Trilwet A 412. As illustrated, the additives had the effect of
decreasing the
mold part surface energy 401. A lowest mold surface energy resulted from a
compound of polypropylene PP9544 and between 2.5% to 5% MB50-001 depending
upon the method used for testing (Owens-wendt or Zisman).
Referring now to Fig. 5, a graph 500 illustrates how those materials with
reduced mold surface energy facilitate improved release of lenses from the
mold parts
with the reduced surface energy, wherein the reduced surface energy resulted
in a faster
release time of a lens from a FC mold part. Specifically, polypropylene with
5%
MB50-001 which has a relatively low surface energy of 24.74 mN/m (Zisman
method)
503 had a mean release time of 37 seconds at 90 C and 65 seconds at 70 C. As
exemplified in the chart a mold part material with a relatively higher surface
energy,
such as Zeonor 1060R which has surface energy of 43.39mN/m, required a
12
CA 02684870 2009-10-20
WO 2008/131329 PCT/US2008/061004
significantly longer time to release an ophthalmic lens formed therein. The
ophthalmic
lenses formed in Zeonor 1060R mold parts required 79 seconds to release at 90
C and
170 seconds at 70 C.
TABLE 2 Lens Release Time (Sec) Lens Release Time (Sec)
(FC = Zeonor 1060R) (FC = PP9544 + 5% w.t. MB50-001)
Temp = 70 (C) Temp = 90 (C) Temp = 70 (C) Temp = 90 C
Mean 170 79 65 37
N 38 19 12 25
Std Dev 117 99 31 10
Max 300 300 111 60
M i n 20 18 20 20
Range 280 282 91 40
Conclusion
The present invention, as described above and as further defined by the claims
below, provides mold parts 101-102 fashioned from a thermal plastic resin
compounded with an additive to provide an increase in a delta of the surface
energy of
the mold part and an ophthalmic lens formed therein.
13