Note: Descriptions are shown in the official language in which they were submitted.
CA 02685214 2012-03-23
IMPROVED CONTROLLED RELEASE ORAL DOSAGE FORM
FIELD OF THE INVENTION
The present invention relates to oral controlled release dosage formulations
containing bupropion hydrochloride.
BACKGROUND OF THE INVENTION
The compound designated bupropion hydrochloride is described in United States
Patent Nos. 3,819,706 and 3,885,046. It is marketed as an anti-depressant and
an aid to smoking
cessation. Bupropion is an aminoketone-derivative chemically unrelated to
other currently
available antidepressants (e.g., selective serotonin-reuptake inhibitors,
tricyclics, tetracyclics).
While the neurochemical mechanisms of the antidepressant and smoking
cessation effects are unknown, noradrenergic pathways and/or dopaminergic
effects appear to be
primarily involved. Bupropion does not inhibit monoamine oxidase and is a
weak blocker of serotonin and norepinephrine uptake.
1
CA 02685214 2009-11-18
WO 02/062299 PCT/US02/03523
The drug is useful in the treatment of depressive affective disorders
(e.g,, major depression) at dosages of 75 to 600 mg daily. Bupropion may be
preferable to other agents because of its minimal anticholinergic,
cardiovascular, and
antihistanunic effects or in those patients who have experienced weight gain
or sexual
dysfunction with another antidepressant. Bupropion, as extended-release
tablets, is
used in the cessation of smoking at dosages of 100-300 mg daily. Withdrawal
symptoms and cigarette craving are reduced with bupropion. Other uses include
patients with bipolar depression, attention-deficit hyperactivity in both
adult and
pediatric patients, and panic symptoms superimposed on depression.
Immediate release bupropion tablets provide more than 75% of
bupropion release into the dissolution media in 45 minutes. In studies to
date, the risk
of seizures appears to be strongly associated, in part, with the use of
instant release
tablets.
Numerous techniques exist in the prior art for preparing sustained or
controlled release pharmaceutical formulations. One common technique involves
surrounding an osmotically active drug core with a semipermeable membrane. The
drug is released from the core over time by allowing a fluid such as gastric
or
intestinal fluid to permeate the coating membrane and dissolve the drug so the
dissolved drug can permeate through the membrane. In some cases a hydrogel is
employed to push the active ingredient through the passageway of the membrane.
Another common technique for preparing controlled-release
pharmaceutical formulations is to encapsulate a plurality of beads, pellets or
tablets
that are coated with varying levels of diffusion barriers. The barriers can be
of the
same or different chemical composition. Release of the pharmaceutical may
occur by
leaching, erosion, rupture, diffusion or similar actions depending on the
nature and
2
CA 02685214 2009-11-18
W'0 02/062299 PCT/US02/03523
thickness of the coating material. These products require multi-layered
coating,
sometimes as much as 30 to 90 coats.
Film coating techniques are characterized by the deposition of a
uniform film onto the surface of a substrate. Because of the capability of
depositing a
variety of coating materials onto solid cores, this process has been used to
make
controlled release dosage forms starting from different formulations, such as
tablets,
granules, pellets and capsules. Cores are usually prepared using one of the
following
processes: compaction, surface layering, or agglomeration.
One limitation associated with these dosage forms consists in their
failure to delay drug delivery. Many of the multi-walled preparations
described above
do not provide prolonged delayed release of the drug prior to initiation of
sustained
release, which is important when biphasic release profiles are desired. Other
systems
are essentially "delayed'' releases mechanisms. There is delay of drug release
in the
stomach, but once the coated drug reaches the intestines, the release of
medication is
rapid. There is no sustained release in the intestines.
Bupropion hydrochloride is highly soluble in water with a high
permeability characterized by rapid and almost complete absorption. Peak
plasma
concentrations occur within 2 hours for bupropion and 3 hours for bupropion
sustained-release. Its biphasic pharmacokinetics is characterized by a two-
compartment model; the distributive phase has a mean half'-life of 3 to 4
hours with a
biphasic decline and a terminal T 1/z of about 14 hours following single
doses. A
major drawback is extensive first-pass metabolism. It appears that only a
small
portion of any oral dosage reaches the systemic circulation intact. Immediate-
release
tablets are dosed three times a day, preferably with 6 or more hours
separating the
doses. For those patients requiring doses greater than 300 mg daily, each
divided
3
CA 02685214 2009-11-18
WWO 02/062299 PCT/US02/03523
dose should not exceed 150 mg each. This necessitates administration of the
tablets 4
times daily with at least 4 hours between successive doses. Commercially
available
sustained-release products are available in film-coated tablets marketed by
Glaxo
Wellcome under the tradenames Wellbutrin SR and Zyban . These are dosed
twice daily. For those patients requiring above 300 mg daily, the regimen
remains
twice daily dosing. No currently available product provides a sustained
release
profile suitable for once daily dosing.
Patient compliance is especially problematic in depressed patients.
There is a need for improved patient compliance. One of the means employed
clinically to improve patient adherence to therapy is simplification of the
dosing
regimen. Thus, need exists for a once daily bupropion formulation.
Sustained release tablet forms of bupropion are described in United
States Patent No. 5,427,798, comprising a sustained release tablet which
provides
peak bupropion blood levels at approximately 2-3 hours, thereby requiring
twice daily
dosing, Controlled release is achieved by combining bupropion particles with
micro crystalline cellulose and hydrogel-forming hydroxypropyl
methylcellulose.
Another sustained release bupropion tablet or caplet formulation
disclosed in United States 4,687,660, comprises a difficult manufacturing
process and
limited shelf life. United States Patent No. 5,358,970 discloses a formulation
of
bupropion hydrochloride that. contains an acid stabilizer.
United States Reissue Patent No. 33,994 discloses a tablet formulation
of a water insoluble, water-permeable film coating surrounding the drug core
and a
particulate, water-soluble, pore-forming material dispersed within the film
coating;
this osmotic gradient and channel forming system is applicable for tablet
dosage
forms. However, here also at least twice daily dosing is necessitated by the
release
4
CA 02685214 2009-11-18
WO 02/062299 PCT/US02/03523
profile of 25-70% of bupropion within 4 hours, and 40-90% within 6 hours.
Wellbutrin SR is a commercially available twice a day dosage form of
bupropion
which contains carnauba wax, cysteine hydrochloride, hydroxypropyl
methylcellulose, magnesium stearate, microcrystalline cellulose, polyethylene
glycol
and titanium dioxide.
There is no capsule form of bupropion commercially available.
Capsules are advantageous in those patients who have difficulty swallowing
where
the contents of the capsule may be sprinkled on food.
Immediate release tablets must be stored at a temperature above 15-
25 C. and protected from light and moisture. Extended-release tablets should
be
stored in tight, light-resistant containers at a temperature of 20-25 C.
The need exists for a delayed, sustained release pharmaceutical
preparation that provides a longer delay of drug dissolution thereby allowing
greater
flexibility in designing sustained release profiles, provides improved plasma
levels
wherein the maximum plasma concentration (Cm.) can be substantially reduced
without a concomitant reduction in AUC, and is simply and economically
produced.
Such a delayed delivery dosage form has a practical application, and it
represents a
valuable contribution to the medical arts. The present invention provides such
a
composition, and offers an efficient and cost effective method of preparation.
Accordingly, it is an object of this invention to provide a sustained
release formulation of bupropion suitable for once daily administration.
Another object of the present invention is to provide a capsule dosage
form comprising means for delaying delivery of the drug in gastric fluids for
6 hours
up to 12 hours, usually 4 hours to 8 hours.
CA 02685214 2009-11-18
VVO 02/062299 PCT/CS02/03523
It is also an object of this invention to provide a controlled and
extended release hupropion capsule formulation that is easy to manufacture and
can
be used to prepare a. range of dosing levels suitable for once daily
administration.
It is a further object of the present invention to provide 24-hour control
of symptoms of depression or tobacco dependence withdrawal.
Seizures result more commonly by single dosages of bupropion over
150 mg, hence the need for twice to four times daily dosing regimens. Another
object
of this invention is to provide simplified once daily dosing regimen with the
potential
to prevent or reduce the incidence of seizures caused by bupropion.
The present invention also relates to anew sustained release bupropion
pharmaceutical composition producing novel blood plasma levels after ingestion
over
24 hours that is not disclosed in, nor rendered obvious by, the prior art.
Other objects,
features and advantages of the invention are not taught in the prior art but
will be
more apparent to those versed in the art from the following specification,
taken in
conjunction with the drawings.
SUMMARY OF THE PRESENT INVENTION
The present invention meets the unfulfilled needs of the
pharmaceutical industry.
The current invention involves a new pelletization process, typified by
the application of a bupropion/cellulose ether suspension to inert spheres and
two
unique formulations of sustained release coatings that are applied to separate
active
pellets. The formulation functions by membrane-controlled extended-release in
a pH
dependent manner. The bupropion release rate has been improved by the
introduction
6
CA 02685214 2009-11-18
k NO 02/062299 PCT/USO2/03523
of two types of film coated active pellets that release the drug at different
pH resulting
in novel dissolution profiles.
Inert spheres are initially coated with bupropion and hydroxypropyl
methylcellulose. The active pellets containing bupropion comprise 70-75 weight
% of
the dosage form. An enteric coating, applied to about one third of the active
drug
pellets, is comprised of a film insoluble at low p1-I, such as hydroxypropyl
methylcellulose phthalate. The second coating applied to the other two thirds
of
active drug pellets is comprised of a combination of a hydrophobic coating
agent and
methyl acrylic acid copolymer. The two pellet types are then combined in a
capsule.
Generally, the weight ratio of the first pellet to the second pellet will be
from about
90:10 to about 10:90, although a weight ratio of from about 30:70 to about
70:30 is
preferred. Especially preferred is a weight ratio of about 33.3:66.7.
This formulation can provide 24-hour efficacy with once daily dosing,
with less than 50% of the drug released at 10 hours. Therapeutic plasma levels
are
maintained from 12 to 24 hours. The usual dosage range is 75-450 rug.
In another embodiment of the present, an uncoated bupropion
component is also employed. In this embodiment, bupropion powder or granules,
or
the uncoated active pellets (bupropion and hydroxypropyl methylcellulose
sprayed
onto an inert sphere) may be used directly (first component). The bupropion
release
rate is further modified and improved by the introduction of uncoated
bupropion and
the two types of film coated active pellets that release the drug at different
pH
resulting in further novel dissolution profiles.
In this embodiment, the enteric coating (hydroxypropyl
methylcellulose phthalate) is applied to from about 10 to about 90 weight
percent of
the active drug pellets (second component). The second coating (hydrophobic
and
7
CA 02685214 2012-03-23
methyl acrylic acid copolymer) is applied to from about 90 to about 10 weight
percent of active
drug pellets (third component). The three components are then combined in a
capsule. Generally,
the weight ratio of the first component to the second component may vary from
about 1: 50 to
about 50: 1, the weight ratio of the first component to the third component
may vary from about
1: 50 to about 50: 1, and the weight ratio of the second component to the
third component may
vary from about 10: 90 to about 90: 10, although a weight ratio of from about
30: 70 to about
70:30 is preferred. Especially preferred is a weight ratio of three components
of about 10: 30 : 60.
In a broad aspect, the present invention relates to a pharmaceutical
composition
comprising: (A) at least one pellet consisting of: (i) a core which comprises
an inert carrier,
wherein said inert carrier is coated with bupropion or a pharmaceutically
acceptable salt or
stereoisomer thereof, and (ii) a sustained release coating surrounding said
core wherein said
sustained release coating comprises at least one controlled release polymer
for controlled release
delivery of said bupropion or a pharmaceutically acceptable salt or
stereoisomer thereof, (B) said
pharmaceutical composition further comprising bupropion or a pharmaceutically
acceptable salt
or stereoisomer thereof in an immediate release form, and wherein the
pharmaceutical
composition is designed for once daily dosing.
In another broad aspect, the present invention relates to a pharmaceutical
composition
comprising: (A) at least one pellet comprising: (i) a core which comprises an
inert carrier,
wherein said inert carrier is coated with bupropion or a pharmaceutically
acceptable salt or
stereoisomer thereof, and (ii) a sustained release coating surrounding said
core wherein said
sustained release coating comprises at least one controlled release polymer
for controlled release
delivery of said bupropion or a pharmaceutically acceptable salt or
stereoisomer thereof, (B) said
pharmaceutical composition further comprising bupropion or a pharmaceutically
acceptable salt
or stereoisomer thereof in an immediate release form, and wherein the
composition provides
therapeutic plasma levels of bupropion for 12 to 24 hours with once daily
dosing and less than
50% of the bupropion released at 10 hours.
8
CA 02685214 2012-03-23
In another broad aspect, the present invention relates to a once-a-day
composition
comprising: (a) an immediate release component comprising bupropion or a
pharmaceutically
acceptable salt thereof, (b) a first pellet comprising an enteric release
component comprising
bupropion or a pharmaceutically acceptable salt thereof and a pH dependent
coating polymer; and
(c) a second pellet comprising a sustained release component comprising
bupropion or a
pharmaceutically acceptable salt thereof and a water insoluble coating
polymer, wherein said
composition contains 75 to 450 mg of bupropion or a pharmaceutically
acceptable salt thereof
and provides an in vivo plasma profile selected from: (a) a mean C,nax of at
least 50.0 ng/ml; (b) a
mean AUCO_;,,f of greater than approximately 500.0 ng=hr/ml; and (c) a mean
T,nax of between
approximately 5.0 hours and 8.5 hours based upon a single dose administration
of a composition
containing 150 mg of bupropion or a pharmaceutically acceptable salt.
BREIF DESCRIPTION OF THE DRAWINGS
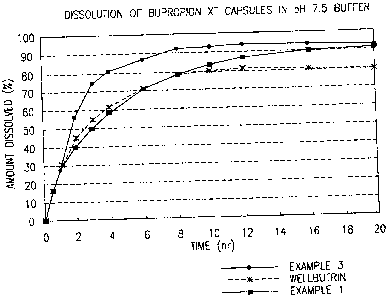
FIG. I is a graph depicting the dissolution profile in a pH 7.5 buffer of the
formulations
as described in Examples I and 3 versus the dissolution of the commercially
available sustained
release form of bupropion (Wallbutrin SR).
FIG. 2 is a graph depicting the dissolution profile in simulated gastric fluid
(pH 1.5) of
the formulations as described in Examples I and 3 versus the dissolution of
the commercially
available sustained release form of bupropion (Wellbutrin SR).
FIG. 3 is a graph depicting the mean plasma concentration-time profiles of
bupropion in
seven healthy subjects (smokers) following a single oral dose of the
formulation in Example 2
versus 150 mg of the commercially available sustained release product
(Zyban(W).
FIG. 4 is a graph depicting the mean plasma concentration-time profiles of
bupropion in
seven healthy subjects (smokers) following a single oral dose of the
formulation in Example 4
versus 150 mg of the commercially available sustained release product
(Zyban(t).
8a
CA 02685214 2009-11-18
ENO 02/062299 PCT/US02/03523
DETAILED DESCRIPTION OF THE PRESENT INVENTION
The present invention, in a first embodiment provides a two
component controlled release bupropion formulation for oral administration,
the
formulation comprising:
(1) a first pellet comprising:
(1) a core comprising:
(a) bupropion and its salts, isomers, or a pharmaceutically
acceptable aminoketone antidepressant agent;
(b) an inert pellet as a starting material; and
(c) a. binder; and;
(n) a coating comprising:
(a) a pH dependent coating agent;
(b) a plasticizer; and
(c) a lubricant; and
(2) a second pellet comprising:
(1) a core comprising:
(a) bupropion and its salts, isomers, or a pharmaceutically
acceptable aminoketone antidepressant agent;
(b) an inert pellet as a starting material; and
(c) a binder; and
(ii) a coating comprising:
(a) a methyl acrylic acid copolymer;
(b) a water insoluble polymer;
(c) a plasticizer; and
9
CA 02685214 2009-11-18
%'VO 02/062299 PCT1US02103523
(d) an antisticking agent.
In other embodiments of the present invention, there may also be
present another component, a form of immediate release bupropion.
The immediate release bupropion component may comprise any form
of immediate release bupropion_ This may take the form of uncoated bupropion
granules or powders, may comprise bupropion active pellets (as described
hereinbelow), may include bupropion granules or active pellets coated with a
highly
soluble immediate release coating, such as an Opadry R7 type coating, as are
known to
those skilled in the art (see generally, United States Patent No. 5,098,715),
or a
combination of any of the foregoing.
The active pellets of bupropion hydrochloride useful in the practice of
the present invention are preferably based on active pellets having a core
forming
inert component that may comprise any type of commonly known pellet starting
material, which may be water insoluble, such as, but not limited to, cellulose
spheres
or silicon dioxide, or may be water soluble, such as, but not limited to,
starch or sugar
spheres having a diameter ranging from about 15 to about 50 mesh, preferably
ranging from about 30 to about 35 mesh. The preferred pellet starting material
is
sugar spheres, NF, containing not less than about 62.5 percent and not more
than
about 91.5 percent of sucrose. The spheres should have consistent bulk
density, low
friability, and low dust generation properties.
The inert core is preferably coated with an aminoketone antidepressant
agent or a pharmaceutically acceptable salt or stereoisomer thereof Most
preferably,
the core drug is bupropion hydrochloride.
The core forming inert component is coated with a formulation that
comprises bupropion hydrochloride and a binding agent. The binding agent
should be
CA 02685214 2009-11-18
WO 02/062299 PCT/1JS02/03523
water soluble, and should possess high adhesivity and all appropriate
viscosity, to
guarantee good adhesion between the sugar cores and bupropion particles,
resulting in
a high concentration of drug in the pellets. The binding agents employed can
be any
type of binding agent commonly known in the art such as polyvinyl pyrrolidone,
hydroxyethyl cellulose, hydroxypropyl cellulose, low molecular weight
hydroxypropyl methylcellulose (HPMC), polymethacrylate or ethyl cellulose. In
a
preferred embodiment of the present invention, the binding agent is a water-
soluble
polymer such as hydroxypropyl methylcellulose having a viscosity in the range
of 2-
12 cps at 20 C, preferably 4-6 cps, such as the material sold as Methoeel E5.
A
preferred composition of the binder for bupropion is about 2-10% whv, and most
preferably 3-5%.
The active pellets of the present invention will preferably comprise the
following ingredients:
INGREDIENT PREFERRED MOST
PREFERRED
Bupropion HCI 40-80% 60-70%
HPMC 2-10% 2.5-5%
starting pellets 10-35% 15-30%
All the percentages in the above-table are based on the total weight of the
core.
The active pellets for use in the practice of the present invention that
comprise the bupropion are typically prepared by forming a suspension of the
binder
and the drug and then layering the suspension onto the starting pellet using
any of the
layering techniques known in the industry, such as fluidized bed coating,
rotor
granulation or pan coating. The suspension medium may comprise any low
viscosity
solvent, such as isopropyl alcohol, ethanol, water, mixtures thereof and the
like. A
11
CA 02685214 2009-11-18
w0 02/1)62299 PCT/US02/03>23
sufficient amount of coating is applied to provide the desired level of
bupropion.
These active pellets may be used directly as the first component of the three
component formulations of the present invention.
The active pellets are also useful in preparing the other two
components of the present invention (both the two component and three
component
formulations). The active pellets intended for such use are divided into two
groups,
each group receiving a film coating that releases the drug at a different pH.
One
group of pellets is coated to release drug at a pH corresponding to about 4.8
and
lower, which is likely to occur in the upper gastrointestinal (GI) tract; the
other group
of pellets is film coated to release drug at a pH of 7 and above, which is
likely to
occur in the lower GI tract, Thus, the entire does is released from this
product for an
extended period of time during its transition through the GI tract.
In a preferred embodiment, one group of pellets (enteric component) is
coated with a film comprising a pH dependent coating polymer, a plasticizer
and a
lubricant. This group of pellets preferably comprises from about 10 to about
90
weight percent of the total pellets, preferably from about 30 to about 70
weight
percent, and most preferably from about 33 to about 60 weight percent.
The pH dependent coating polymer may be selected from those enteric
coatings known to those skilled in the art. Preferably, the pH dependent
coating is
selected from the group consisting of shellac, methacrylic acid copolymers
(such as,
but not limited to Eudragit E100 (a cationic copolymer of dimethyl aminoethyl
methacrylate and neutral methacrylic acid esters)), cellulose acetate
phthalate,
hydroxypropyl methylcellulose phthalate, hydroxypropyl methylcellulose acetate
succinate, cellulose acetate trimellitate, polyvinyl acetate phthalate or
mixtures
thereof Hydroxypropyl rnethylcellulose phthalate (HPMCP) is preferred. The
12
CA 02685214 2009-11-18
WO 02/062299 PCT/US02/03523
preferred concentration is 2-10% w/w of the total dosage form, and most
preferably 3-
5%.
The coating preferably also contains plasticizers. Plasticizers that may
be used in the practice of the present invention include any of those known to
those
skilled in the all, including, but not limited to, acetyltributyl citrate,
triacetin,
acetylated monoglyceride, rape oil, olive oil, sesame oil, acetyltriethyl
citrate,
glycerin sorbitol, diethyloxalate, diethylmalate, diethylfunierate,
dibutylsuccinate,
diethylmalonate, dioctylphthalate, dibutylphthalate, dibutvlsebacate, triethyl
citrate,
tributyl citrate, gly cero It ributy rate, polyethylene glycol, propylene
glycol and
mixtures thereof The preferred plasticizer is acetyltributyl citrate in an
amount
ranging from about 1 to about 15 percent based on the total weight of the
final coating
or 0.1-3% w /w of the total dosage form.
The coating further preferably includes a lubricant such as, but not
limited to, those selected from the group consisting of glyceryl monostearate;
Myvaplex 600P, calcium stearate or stearic acid. The preferred lubricant is
glyceryl
monostearate in an amount ranging from about 1 to about 15 percent, and most
preferably 1-2.5% based on the total weight of the coating.
A preferred enteric coating for use in the present invention therefore
comprises the following ingredients:
INGREDIENT PREFERRED MOST
PREFERRED
HPMCP 2-10% 3-5%
Acetyltributyl citrate 0.1-3% 0.5-1%
Glyceryl monostearate 1-3% 1-2.5%
13
CA 02685214 2009-11-18
WU 02/062299 PCT/US02/03523
Additional active drug pellets for forming the second coated
component of the present invention, preferably from about 90 to about 10
weight
percent of the total pellets, more preferably from about 70 to about 30 weight
percent,
and most preferably from about 67 to about 40 weight percent, are coated with
a
coating that comprises a polymer such as a methacrylic copolymer, water
insoluble
polymer, a plasticizer and an anti-sticking agent.
The methacrylic acid copolymer is selected from the known group of
methacrylic acid copolymers, preferably Eudragit S (methacrylic acid
copolymer
Type B), and most preferably Eudragit 5100. The preferred concentration is 1-
1.5%
of the total weight of the dosage form, preferably 4-7%.
The water insoluble polymer in the preferred embodiments of the
present invention is formed from a cellulose ester, or a cellulose ester-
ether.
Representative materials include a member selected from the group consisting
of
ethyl cellulose, cellulose acylate, cellulose diacylate, cellulose triacylate,
cellulose
acetate, cellulose diacetate, cellulose triacetate, cellulose acetate
butyrate, mono-, di-
and tri-cellulose arylates, and the like. Preferred is ethyl cellulose in a
concentration
ranging from about 1 to about 20%, preferably from about 2 to about 13%.
The preferred plasticizer additive for the second coating may be
selected from any of those mentioned above. Acetyltributyl citrate is
preferred.
The anti-sticking agents can be chosen from any of the known agents,
such as, but not limited to, those selected from the group consisting of an
alkaline
earth metal stearate, such as magnesium stearate or calcium stearate, or talc.
The anti-
sticking agents can be used alone or in combination in effective amounts. The
preferred anti-sticking agent is talc.
14
CA 02685214 2009-11-18
WO 02/1162299 PCT/US02/03523
The coating for the active pellet for this (second coated) component of
the present invention is applied to the active pellets by forming a. solution
of the
respective coating components in a solvent or a mixture of solvents, such as,
but not
limited to, acetone and isopropyl alcohol, and employing any of the
application
techniques known to those skilled in the art, such as fluidized bed coating,
rotor
granulation or pan coating.
The components, either the two coated or the two coated and the
immediate release, of the present invention are blended together in the
desired ratio
and placed in gelatin capsule to obtain a finished product. By varying the
ratio of the
three components, including used of the immediate release at 0%, novel
dissolution
profiles and plasma profiles may be obtained in accordance with the present
invention. Alternatively, the dosage formulation may be made into tablets by
first
adding from 25 to 40 weight percent of a solid pharmaceutically acceptable
tablet
excipient that will form a compressible mixture without crushing the pellets,
and then
tabletting the mixture in a suitable tablet press.
The following examples are intended to illustrate the present invention
but are not intended to limit the scope of the appended claims.
EXAMPLE I
A batch of controlled release bupropion was manufacture using all
materials that comply with the current USP/NF compendia specifications.
A controlled release 150 mg oral bupropion dosage form is prepared
by forming active core pellets having the following composition:
L ACTIVE CORE PELLETS
Bupropion HCl 70.0%
Sugar sphere 30/35 26.5%
CA 02685214 2009-11-18
WO (12/062299 PC'1'/US02/03523
Methocel E5 3.5%
Active pellets of bupropion are formed by dissolving 2.8 kg of
bupropion HCl and 0.140 kg of hydroxypropyl methylcellulose (Methocel E5) in
a
mixture of water and isopropyl alcohol. The active drug solution is then
sprayed onto
1.06 kg of sugar spheres 30/35 in a fluidized bed processor with a Wurster
insert. The
active core pellets are then dried in a fluidized bed processor until the loss
on drying
is below M. The bupropion pellets are then passed through a 16 mesh screen and
a
30 mesh screen and pellets are collected that are smaller than 16 mesh and
larger than
30 mesh,
it. ENTERIC COATED PELLETS
Bupropion active pellets 75.0%
HPMCP 50 16.9%
Acetyltributyl citrate 2.5%
Myvaplex 600P 5.6%
For a group of about one-third of the pellets, 0.270 kg of
hydroxypropyl methylcellulose phthalate and 0.040 kg of acetyltributyl citrate
are
dissolved in a mixture of purified water and isopropyl alcohol, USP. Then
0.090 kg
of glyceryl monostearate (Myvaplex 600P) is dissolved into the solution above.
The
solution is then sprayed onto 1.2 kg of the bupropion core pellets in a
fluidized bed
processor with a Wurster insert. The pellets are then dried until the loss on
drying
(LOD) is less than 1 %. The pellets are then mixed with 2% (w/w) talk for 10
minutes
in a V-blender. The pellets are then passed through a 14 mesh screen and a 24
mesh
screen and pellets that are smaller than 14 mesh and larger than 24 mesh are
collected.
III. SUSTAINED RELEASE (SR) COATED ACTIVE PELLETS
Bupropion active pellets 80.0%
Eudragit S 100 12.6%
Ethocel 10 cps 1.4%
16
CA 02685214 2009-11-18
Wt) 02/062299 PCT/US02/03523
Acetyltributyl citrate 2.0%
Talc 4.0%
For another group of about two-thirds of the pellets, a coating is
prepared where the ratio of the methacrylic acid copolymer to ethylcellulose
is about
9:1. The coating is made as follows: 0.378 kg of methacrylic acid copolymer
(Eudragit S 100), 0.42 kg of ethylcellulose (Ethocel 10 cps), and 0.060 kg
of
acetyltributyl citrate are dissolved in a mixture of 0.690 kg acetone and
6.210 kg
isopropyl alcohol. 0. 120 kg of talc is then dispersed into the solution
above. The
suspension is then sprayed onto 2.40 kg of the active bupropion core pellets
in a
fluidized bed processor with a Wuster insert. The bupropion pellets are dried
in a
fluidized bed processor until the LOD is less than 1%. The pellets are mixed
with 2%
(w/w) talc for 10 minutes in a V-blender and passed through a 14 mesh screen
and 24
mesh screen. Pellets smaller than 14 mesh and larger than 24 mesh are
collected.
These pellets have the following coating composition:
INGREDIENT MG/CAPSULE % TOTAL
WEIGHT
Eudragit S100 22.5 6.4
Ethocel 10 cps 2.5 0.7
Acetyltributyl citrate 3.6 1.0
Talc 7.1 2.0
The enteric coated pellets and the SR pellets are mixed after loading
each group into donators. The strength of the final product is 150 mg of
bupropion
with 50 nmg of active drug in the first group of pellets and 100 mg of active
in the
second group. The pellets are then encapsulated into size "I" light turquoise
blue/light turquoise blue capsules. The total weight of the formulation
(capsule +
pellets) is 350 mg.
17
CA 02685214 2009-11-18
WO 02/062299 PCT/US02/03523
The resulting bupropion capsules of Example 1 were then tested
according to the USP XXIIi dissolution test (type 2, basket) at 50 rpm, at 37
C in pH
7.5 buffer and found to have the following release profile:
TABLE 1
Time hours % Released
1 25
2 60
3 75
4 80
6 88
8 93
93
12 94
14 94
16 94
The release profile of the controlled release product shown in this
Example is shown in FIG. 1 by the line filled with circles.
The bupropion capsules of Example I were then tested according to
the USP XXIII dissolution test (type 2, basket), at 50 rpm, at 37 C in SGF (pH
1.5) to
determine the percentage of drug dissolved versus time.
TABLE 2
Time hours % Released
1 0
2 1
3 2
18
CA 02685214 2009-11-18
V,,t} 112/062299 PCT/1JS02/03523
4 3
6 7
8 15
30
12 45
14 50
16 56
60
The release profile of the controlled release product shown in this
Example 1 is shown in FIG. 2 by the line with the filled circles.
The bupropion capsules of Example 1 were then evaluated in seven
patients using standard techniques known in the art. Bupropion was first
detected in
the plasma at about 2 hours after administration, and showed sustained release
over 24
hours.
Two panels of seven patients were randomly assigned to receive either
the bupropion formulation described herein or ZYBAN'iz in an open, randomized
single dose study. Blood samples were collected over a 72-hour period and
analyzed
for bupropion concentrations with a LC/MS/MS method.
For the blood levels carried out C1 is the maximum blood level
concentration of bupropion, TmaY is the time at which the maximum blood level
concentration occurs, T2ag is the sampling point preceding the one at which
concentrations first become quantifiable. AUC is the "area under the curve" of
time
versus blood concentration. The results provided are given in 'fable 3 and
FIG. 3
show that the mean plasma concentration-time profiles of bupropion were
different
for the Example 1 formulation and Zyban . Following oral administration, the
19
CA 02685214 2009-11-18
kW 02/062299 PCT/US02/03523
Example I formulation had a delayed absorption with a Ttag value of 1.9 hours.
The
mean C,,,,, value of the Example 1 bupropion formulation was about one-half of
that
for Zyban . The time to reach (Tma maximum plasma concentration occurred
about
8 hours after administration of the Example I formulation. The relative
bioavailability of the Example I formulation to Zyban was 40% in terms of
C,,,ax
and 80% in terms of AUCo_;f ratio.
TABLE 3
Variable Exam lp e I Meari Zvban Mean G-Mean
Ratio
Cmas (no/ml) 54.2 129.0 0.40
AUC0_iõf (nghrlml) 832.0 998.0 0.80
1'iag (11r) 1.9 0.1
T,,,, (hr) 8.1 3.6
"I'v2 (hr) 17.0 20.3
Thus, it can be seen from the data above that although the Cmax of
Example I is significantly lower than the Cmaa of the Zyban formulation, the
AUC
has only been slightly reduced.
EXAMPLE 2
The pellets from Example 1 are taken as the second and third
components. These pellets are loaded into the dosator along with active
pellets and
are filled into capsules in a ratio of 10:30:60 while maintaining the dosage
at 150 mg.
The blood profiles from this example will show a C,,,ay that is the same as
shown in
Table 3, but will show a slightly increase AUC, thereby rendering the G-Mean
ratio at
about 1.00. The amount of active pellets may be adjusted as is known in the
art
CA 02685214 2009-11-18
WO (}2/062299 PCTIUS02103523
without undue experimentation based on the teachings of the present disclosure
in
order to substantially provide a G-Mean for AUC of approximately 1.00.
EXAMPLE 3
The procedure of the Example 1 is followed for the first group of
pellets. The bupropion cores are prepared by forming a suspension of bupropion
and
hydroxypropyl methylcellulose in a mixture of water and isopropyl alcohol,
which
suspension is spray coated onto inert spheres. The HPMCP enteric coating is
then
applied to about one third of the active drug pellets.
Film Coating For SR Pellets
A second group of about two-thirds of the pellets is coated with a
coating prepared where the ratio of methacrylic acid copolymer to
ethylcellulose is
about 1: 1. The pellets have the following composition:
Bupropion active pellets 80.0%
Eudragit S100 7.0%
Ethocel 10 cps 7.0%
Acetyltributyl citrate 2.0%
Talc 4.0%
The coating is made as follows: 0.105 kg of methacrylic acid
copolymer (Eudragit S100), 0.105 kg of ethylcellulose (Ethocel 10 cps), and
0.030 kg of acetyltributyl citrate are dissolved in a mixture of 0.345 kg
acetone and
3.105 kg isopropyl alcohol. 0.060 kg of talc is then dispersed into the
solution above.
The suspension formed is then sprayed onto 1.20 kg of the active bupropion
core
pellets in a fluidized bed processor with a Wurster insert. The coated
bupropion
pellets are then dried in a fluidized bed processor until the LOD is less than
1%. The
21
CA 02685214 2009-11-18
WO 02/062299 PCTIUS02/03523
pellets are mixed with 2% (w/w) talc for 10 minutes in a V-blender and passed
through a 14 mesh screen and a 24 mesh screen. Pellets smaller than 14 mesh
and
larger than 24 mesh are collected.
The pellets have the following coating composition:
Ingredient mg/C p ule % Total Wt.
Methacrylic acid copolymer 12,5 3.6
Ethocel0 10 cps 12.5 3.6
Acetyyltributyl citrate 3.6 1.0
Talc 7,1 2.0
The first group of pellets and the 1: 1 above pellets are mixed after
loading each group into dosators. The strength of the final product is 150 mg
of
bupropion with 50 mg of active drug in the first group of pellets and 100 mg
of active
drug in the second group. The pellets are then encapsulated into size "1" buff
opaque/light blue opaque capsules. The total weight of the formulation
(capsule +
pellets) is 352 mg.
The resulting bupropion capsules were then tested according to the
USP XXIII dissolution test (type 2, basket), at 50 rpm, at 37 C, in pH 7.5
buffer and
found to have the.following release profile:
Time hours) % Released
1 28
2 40
3 50
4 58
6 70
8 80
22
CA 02685214 2009-11-18
WO 4)2/062299 PCT/US02/03523
83
12 87
14 88
16 90
18 90
92
The release profile of the controlled release product of Example 3 is
shown in FIG. 1 by the line with the filled in squares.
The resulting bupropion capsules were then tested according to USP
XXIII dissolution test (type 2, basket), at 50 rpm, at 37 C, in SGh (pl-i 1.5)
and found
to have the following release profile:
Tine (hours) % Released
1 0
2 2
3 4
4 6
6 12
8 21
10 40
12 57
13 64
16 68
17 71
20 74
23
CA 02685214 2009-11-18
t%VO 02/062299 PCT/US02/03523
The release profile of the controlled release product shown in Example 3 is
shown in
FIG. 2 by the line with the filled in squares.
The bupropion capsules of Example 3 were then analyzed in a seven
patient test using techniques known in the art. Bupropion was first detected
in the
plasma about 1.4 hours after administration and showed a sustained release
over 24
hours.
The testing procedure is as described in Example 1. The results
provided are given in Table 4 and FIG. 4 and show that the mean plasma-time
profile
of the bupropion formulation differs from that of Zyban . Bupropion had a
delayed
absorption; the relative bioavailability of bupropion to Zyban`D was 48% and
59% in
terms of C,,,,, and AUC values, respectively. The terminal elimination half-
lives were
similar.
TA13LE 4
Parameter Exaunple 3 Zvban (i-Mean
Ratio
Cmõ, (ng/ml) 56.9 114.8 0.48
AUCo_;,,f (nghr/ml) 531.7 889.5 0.59
Tag (hr) 1.4 0.1
(hr) 5.1 4.1
1'112 (hr) 12.6 14.1
Thus, again, the C,,,,\ of the Example 3 product was reduced
significantly more than the AUC compared to the reference product,
demonstrating
that an effective once-a-day product has been provided.
24
CA 02685214 2012-03-23
EXAMPLE 4
The pellets from Example 3 are taken as the second and third
components. These pellets are loaded into the dosator along with active
pellets and
are filled into capsules in a ratio of 10:30:60 while maintaining the dosage
at 150 mg
bupropion. The blood profiles from this example will show a Coax the same as
in
Table 3, but will show a slightly increased AUC, thereby rendering the G-mean
ratio
at about 1.00. The amount of active pellets may be adjusted as is known in the
art
without undue experimentation based on the teachings of the present disclosure
in
order to substantially provide a G-Mean for AUC of approximately 1.00.