Note: Descriptions are shown in the official language in which they were submitted.
CA 02688051 2013-05-27
A catalytic cracking catalyst and the preparation and use thereof
Technical field
This invention relates to a catalyst for catalytically cracking
hydrocarbon oils and its preparation method, especially to a catalytic
cracking catalyst for producing light olefins and its preparation method.
Background arts
The catalysts for preparing light olefins by catalytic cracking or
pyrolysis with petroleum hydrocarbons as feed stocks comprise three types:
the first type is supported metal catalysts with oxides as substrates such as
the catalysts disclosed in US3,541,179, US3,647,682, DD225,135, and
SU1214726, which use Si02, A1203 or other oxides as substrates to support
metal elements of Groups JIB, VB, VIM, and VJIIB. This kind of
catalysts are merely suitable for the preparation of light olefins by the
catalytic cracking of light feed stocks (boiling range <220 C). The second
type is composite oxide catalysts such as the catalysts disclosed in
US3,725,495 and US3,839,485, which use Zr02 and/or Hf02 as main
components and contain active alumina, at least one component selected
from Cr203, MnO, and Fe203, and at least one component selected from
oxides of alkali metals or alkali earth metals, and the catalyst disclosed by
DD152,356, which uses amorphous Si02.A1203 as the catalyst for
cracking hydrocarbon oils to prepare light olefins. The third type of
catalysts is the zeolites containing catalysts, especially MFI-structured
zeolite (five-member ring high silica zeolite) containing ones. This type
of catalysts can be used alone or as catalytic cracking additive.
JP60-222428 discloses a process for preparing C7-C4 by
catalytically cracking C5-C25 parafins at 600-750 C using a catalyst
containing ZSM-5 zeolite and A1203.
CN1205306C discloses a catalyst for preparing light olefins by
cracking petroleum hydrocarbons, which catalyst contains 0-70 wt.% of
clay, 5-99 wt.% of inorganic oxides and 1-50 wt.% of zeolites based on
the weight of the catalyst, wherein said zeolite is 25-100 wt.% of
MFI-structured zeolite and 0-75 wt.% of Y-zeolite, characterized in that
said MFI-structured zeolite contains phosphor and a transition metal M
CA 02688051 2009-11-18
and has an anhydrous chemical formula of (0-0.3)Na20=(0.3-5)
A1203.(1-10)P205.(0.7-15)Mx0y.(0-10)RE203-(70-98)Si02 based on the
mass of the oxide, wherein M is one or two metals selected from Fe, Co,
Ni, Cu, Zn, Mo, and Mn..
CN1069016A discloses a process for preparing ethylene by cracking
heavy petroleum hydrocarbons at 650-900 C using a catalyst containing
30-90 wt.% of Si02, 20-70 wt.% of A1203, 0.5-30 % by weight of oxides
of alkali metals or alkali earth metals, and 1-30 wt.% of faujasite.
CN1093101A discloses a cracking catalyst for producing light
olefins, which catalyst consists of 0-70% of clay, 5-99% inorganic oxides,
and 1-50% zeolites (based on the weight of the catalyst), wherein the
zeolite is a mixture of 0-25 wt.% of REY or high silica Y-zeolite and
75-100 wt.% of five-member ring high silica zeolite containing phosphor
and rare earths.
CN1048428C discloses a catalyst for converting petroleum
hydrocarbons to light olefins, which catalyst consists of 0-70 wt.% of clay,
5-90 wt.% of inorganic oxides and 10-35 wt.% of zeolites, wherein the
zeolite consists of 20-75 wt.% of five-member ring high silica zeolite
containing phosphor and rare earths, 20-75 wt.% of high silica Y-zeolite,
and 1-25 wt.% of Y-zeolite containing rare earths.
CN1222558A discloses a catalyst for preparing light olefins by
catalytic pyrolysis, which catalyst has the following composition (based
on the weight of the catalyst): 10-70 wt.% of clay, 5-85 wt.% of inorganic
oxides and 1-50 wt.% of zeolites, wherein the zeolite is 0-25 wt.% of
Y-zeolite and 75-100 wt.% of five-member ring high silica zeolite
containing phosphor and aluminum or magnesium or calcium, and said
high silica zeolite is ZSM-5, ZSM-8 or ZSM-11 type zeolite containing
2-8% phosphor and 0.3-3% aluminum or magnesium or calcium (in terms
of oxide) with a silica/alumina ratio of 15-60.
CN1069682C discloses a pillared interlayered clay catalyst for
preparing ethylene, propylene, and butene by the catalytic pyrolysis of
heavy oils, which catalyst consists of 30-75 wt.% of aluminum
cross-linked pillared interlayered clay, 10-40 wt.% of inorganic oxide
binders containing aluminum or silicon or zirconium, 0-30 wt.% of high
silica zeolite with the five-member ring structure, 0-10 wt.% of a
2
CA 02688051 2009-11-18
modifying component selected from magnesium, aluminum, phosphor,
tin, polyethylene glycol or their mixture, and 0-50 wt.% of clay of the
kaolin family.
CN1660967A discloses a catalyst for increasing the yield of ethylene
and propylene by catalytic pyrolysis, which catalyst contains 7-70 wt.%
of clay, 3-70 wt.% of mesopore silica-alumina materials, 5-80 wt.% of
inorganic oxides, and 5-60 wt.% of MFI-structured zeolite
CN1354224A discloses a catalytic cracking catalyst for producing
gasoline rich in isomeric alkanes, propylene, and isobutane, which
catalyst consists of 0-70 wt.% of clay, 5-90 wt.% of inorganic oxides, and
1-50 wt.% of zeolites based on the weight of the catalyst, wherein said
zeolite is a mixture of (1) 20-75% high silica Y-zeolite having a
silica/alumina ratio of 5-15 and containing 8-20 wt.% of rare earths in
terms of RE203 and (2) 20-75% high silica Y-zeolite having a
silica/alumina ratio of 16-50 and containing 2-7 wt.% of rare earths in
terms of RE203 and (3) 1-50% 13-zeo1ite or mordenite or ZRP-zeolite
based on the zeolite weight. By using this catalyst, the content of isomeric
alkanes in gasoline can be increased together with the production of
propylene and isobutane being increased.
CN1566267A discloses a catalytic pyrolysis process for preparing
ethylene and propylene, which comprises introducing a preheated
petroleum hydrocarbon feed stock into a lift pipe reactor, contacting the
feed with a hot catalyst containing a five-member ring high silica zeolite,
conducting reaction under catalytic pyrolysis conditions, separating the
reaction products and spent catalyst. The reaction products are conveyed
to the subsequent separation system for product separation, and the spent
catalyst is returned to the reactor after being stripped and regenerated for
recycle, wherein said five-member ring high silica zeolite contains
phosphor and transition metals.
CN1043520A discloses a cracking catalyst, the substrate of which is
0-70 wt.% of clay and 5-99 wt.% of inorganic oxides, and the active
component is a mixture of 1-50 wt.% of ZSM-5 and Y-type molecular
sieve. In the active component, ZSM-5 accounts for 75-100% by weight
and Y-type molecular sieve accounts for 0-25%.
3
,
CA 02688051 2015-06-25
CN1508223A discloses a hydrogenation catalyst wherein the
substrate contains alumina, active component comprises molybdenum
and/or W and Ni, the pore of a size of 2-6nm accounts for 70-90% of the
total pore volume.
The content of macropores in the prior art catalysts is low, so these
catalysts show an insufficient capacity for cracking heavy oils when used
in catalytically cracking heavy oils and provide low yields when used for
preparing light olefins and propylene by catalytic cracking.
Content of the invention
One of the technical problems to be solved by the present invention
is to provide a catalyst for catalytically cracking hydrocarbon oils; the
second technical problem to be solved is to provide a process for
preparing the above catalyst; and the third problem to be solved is to
provide a method for applying the catalyst in catalytic cracking, which
method can increase the yield of light olefins.
This invention provides a catalyst for catalytically cracking
hydrocarbon oils, which catalyst contains a substrate comprising alumina
and a molecular sieve, characterized in that the pore distribution of said
catalyst is 5-70% of the <2 nm pores, 5-70% of the 2-4 nm pores, 0-10%
of the 4-6 nm pores, 20-80% of the 6-20 nm pores, and 0-40% of the
20-100 nm pores, based on the pore volume of pores having a size of no
more than 100 nm.
In a particular embodiment there is provided a catalyst for
catalytically cracking hydrocarbon oils, which catalyst contains a
substrate comprising alumina and a molecular sieve, characterized in that
the pore distribution of said catalyst is 5-70% of the <2 nm pores, 5-70%
of the 2-4 nm pores, 0-10% of the 4-6 nm pores, 20-80% of the 6-20 nm
pores, and 0-40% of the 20-100 nm pores, based on the pore volume of
pores having a size of no more than 100 nm, the pore volume of the
catalyst is measured according to RIPP151-90, said catalyst contains
60-95 wt.% of a substrate, 5-40 wt.% of a molecular sieve, and said
molecular sieve contains 25-100 wt.% of MFI-structured zeolite,
0-75 wt.% of Y-zeolite, and 0-20 wt.% of 13-zeolite, and said alumina is
derived from alumina and/or its precursors, said precursor of alumina is
4
CA 02688051 2015-06-25
pseudo-boehmite or its mixture with one or more of alumina sol or
phospho-alumina sol.
This invention provides a process for preparing a catalytic cracking
catalyst, which process comprises the steps of mixing a substrate
comprising alumina and/or its precursors with a molecular sieve,
slurrying and spray-drying the mixture, characterized in that a
pore-extender is introduced in the mixing step and said pore-extender is
one or more selected from boric acid and salts of alkali metals. The
weight ratio of the pore-extender to the substrate is 0.1:100-15:100 based
on the weight of the substrate.
This invention provides a catalytic cracking process, which
comprises a step of contacting a hydrocarbon oil with a catalyst,
characterized in that said catalyst comprises the catalyst according to the
present invention.
4a
CA 02688051 2009-11-18
Because a pore-extender is introduced in the preparation of the
catalyst of this invention, the pore volume of macropores increases and
thus raises the capacity of the catalyst for cracking heavy oils,
coke-tolerance and coke-resistance of the substrate, the utilization rate of
the active components, and the retention ratio of the crystallinity of the
molecular sieve after deactivation. When metal halides are introduced
during preparation, the abrasion resistance of the catalyst can be raised.
The catalytic cracking process provided by this invention has a high
capacity for cracking heavy oils and when the prepared catalyst contains
NMI-structured molecular sieve, the yield of propylene in catalytic
cracking is high. For example, the catalyst prepared by this invention
contains 20 wt.% of a molecular sieve and 80% by weigh of substrate,
wherein the molecular sieve contains 10 wt.% of REHY-zeolite and 90
wt.% of ZRP-zeolite. The substrate contains 26 wt.% of pseudo-boehmite
and alumina sol, 69 wt.% of kaolin, 5% of the weight of Ti02. Potassium
sulfate is used as a pore-extender in the preparation, and its amount is
7.4% of the weight of the substrate. The pore volume of the catalyst
measured by BET method is 0.256 ml/g, and the abrasion index is 2% by
weight, while the pore volume of the catalyst prepared according to a
prior process for preparing the DCC catalyst containing the same amount
of molecular sieve is 0.185 mug and the abrasion index is 2% by weight.
When using 30 m.% of residual oil + 70 m.% of VG0 as a feed stock and
conducting the catalytic cracking reaction under the conditions of a
temperature of 680C, a catalyst/oil weight ratio of 10, a water/oil mass
ratio of 0.8:1, and a weight hourly space velocity of 10 lc% the catalyst of
this invention presents a propylene yield of 21.88% by weight, a coke
yield of 1.09% by weight, and a heavy oil yield of 1.05% by weight,
while the catalyst prepared by a prior art process presents a propylene
yield of 19.26% by weight, a coke yield of 1.28% by weight, and a heavy
oil yield of 1.55% by weight.
Brief description of the drawings
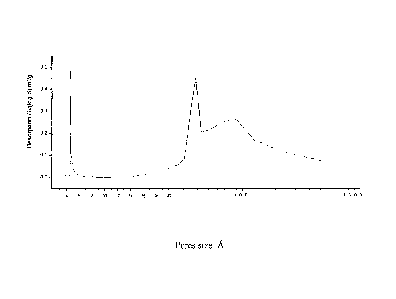
Fig. 1 illustrate the pore distribution of the catalyst of Example I.
Specific mode for carrying out the invention
The singular forms "a", "an", and "the" include plural referents
CA 02688051 2014-10-28
unless the context clearly dictates otherwise.
The catalyst for catalytically cracking hydrocarbon oils according to
the present invention contains a substrate comprising alumina and a
molecular sieve, characterized in that the pore distribution of said catalyst
is 5-70%, preferably 5-60%, of the <2 nm pores, 5-70%, preferably
10-60%, more preferably 15-50%, of the 2-4 nm pores, 0-10% of the
4-6 nm pores, 20-80%, preferably 25-70%, more preferably 30-60%, of
the 6-20 nm pores, and 0-40% of the 20-100 nm pores, based on the pore
volume of pores having a size of no more than 100 nm.
The catalyst according to the present invention has a ratio of the
volume of 6-20 nm pores to that of 2-4 nm pores being 0.5-4.
The volume of the 6-10 nm pores in the catalyst according to the
present invention accounts for 10-50%, preferably 15-40%.
The catalyst according to the present invention preferably contains
60-95 wt.% of a substrate, 5-40 wt.% of a molecular sieve, and said
molecular sieve contains 25-100 wt.% of MFI-structured zeolite,
0-75 wt.% of Y-zeolite, and 0-20 wt.% of 13-zeolite. The content of
alumina in the substrate coming from alumina and/or its precursors is
5-100% by weight, and the content of other substrate components does
not exceed 95% by weight. The pore volume of said catalyst measured by
the BET method is 0.19-0.4 ml/g.
In the catalyst according to the present invention, said substrate
contains one or more types of alumina derived from alumina or its
precursors, and the content of alumina is preferably 5-80% by weight.
The alumina and its precursor are preferably one or more of alumina sol,
phospho-alumina sol, various aluminum-containing salts (such as various
aluminates, and sulfate, nitrate, and halides of aluminum), 7-alumina,
I-I-alumina, 0-alumina, and x-alumina, hydrated alumina with the structure
of pseudo-boehmite, hydrated alumina with the structure of diaspore,
hydrated alumina with the structure of gibbsite, and hydrated alumina
with the structure of bayerite. More preferably, said alumina and its
precursor are pseudo-boehmite or mixtures of pseudo-boehmite with one
or more of alumina and other precursors of alumina.
The catalyst according to the present invention may also contain one
or more of clay and oxides of Groups IIIA and WA non-aluminum
elements. Said oxides of non-aluminum elements are derived from one or
6
CA 02688051 2009-11-18
more of the oxides of said non-aluminum elements or their precursors.
For example, silica and its precursors may be one or more selected from
silica sol, water glass, silicates, silica-alumina sol, silica-alumina gel,
and
various organo-silicon compounds, preferably water glass and/or silica
sol. The content of clay and said non-aluminum oxides does not exceed
95% by weight based on the weight of the substrate, and the content of
clay preferably does not exceed 60% by weight. It is preferable that the
weight ratio of the non-aluminum oxide to the alumina substrate in the
substrate is 1: (0.3-1) calculated in oxide.
In the catalyst according to the present invention, said zeolite is one
or more of MFI-structured zeolite, Y-zeolite, and 0-zeolite. Said Y-zeolite
is preferably one ore more of Y-zeolite containing phosphor and/or rare
earths, ultrastable Y-zeolite, ultrastable Y-zeolite containing phosphor
and/or rare earths, HY-zeolite, and HY-zeolite containing phosphor and/or
rare earths. More preferably, said zeolite is one or more of ultrastable
Y-zeolite, REY, or R_EHY. Said MFI-structured zeolite is one or more
selected from ZSM-5 zeolite, ZRP-zeolite, and their modifications such
as the modified MFI-structured zeolite containing phosphor and transition
metals disclosed in CN 1465527A, i.e., ZSM-5 zeolite modified with
phosphor and one metal selected from Fe, Co, and Ni, having an
anhydrous chemical formula of (0-0.3)Na20.(0.5-5)A1203.(1.3-10)
P205-(0.7-15)Oy. (70-97)Si02 calculated in the mass of the oxide,
where x denotes the atom number of M; y denotes the atom number of 0;
and M is one of Fe, Co, and Ni.
In the catalyst according to the present invention, said substrate
preferably also contains metal components coming from halides of
Groups IIA, IB, IIB, and IVB metals, and the content of said metal
component does not exceed 15% by weight based on the weight of the
substrate, preferably being 0.1-12% by weight, and more preferably,
being 0.1-6% by weight. Said metal component is more preferably one or
more of Groups IVB and IIA metals, and most preferably, it is Ti and/or
Mg. If the substrate contains said metal components, the abrasion
resistance of the catalyst is raised.
The pore volume of the catalyst provided by the present invention
measured by the nitrogen adsorption volumetric method is 0.19-0.4 ml/g,
7
CA 02688051 2009-11-18
preferably 0.196-0.26 ml/g. For the nitrogen adsorption volumetric
method, please refer to analytical method RIPP151-90 in "Yang Cuiding
et al, Analytic Methods in Petroleum Chemical Industry" (RIPP
Experimental Methods), Science press, 1990.
Figure 1 illustrate the pore distribution of the catalyst of Example 1,
which is measured by the nitrogen adsorption volumetric method. As can
be seen from the figure, there is a distinct adsorption peak at 6-20nm,
showing that said catalyst comprises pores of 6-20nm.
In the catalyst preparation process according to the present invention,
the weight ratio of said pore-extender to the substrate is preferably
0.1:100-10:100. Said salt of alkali metals is preferably one or more of
soluble salts of alkali metals K, Na, or Li such as borates, phosphates,
sulfates, nitrates, carbonates, or hydrochloride.
In the catalyst preparation process according to the present invention,
said technique for mixing and slurrying the substrate and molecular sieve
is well known to the skilled in the art. It is possible to slurry the
substrate
and molecular sieveseparately, and then mix the two slurries, or mix and
slurry a part of the substrate in the preparation of the substrate, and then
introduce the molecular sieve and the remaining substrate and slurry, or
introduce the substrate into the molecular sieve slurry and then slurry.
Said pore-extender is introduced into the slurry before spray drying. It is
preferable that the pore-extender is introduced into the
substrate-containing slurry. After introducing the pore-extender, slurrying
is performed to dispense the pore-extender into the slurry. The slurrying
step is conducted for at least 5 min, preferably 10-90 mm. The slurry
containing pore-extender is aged in static state under the temperature of
50-80 C for a time of 0.5-3 hr after the pore-extender is incorporated
thereinto.
Phosphoric acid can be introduced in the step of mixing in the
catalyst preparation process according to the present invention.
In the catalyst preparation process according to the present invention,
one or more of halides of Groups IIA, IB, IIB, and IVB metals can be
introduced into the slurrying step. Said metal halide is preferably
introduced after introducing the pore-extender and before spray drying.
The amount of the introduced metal halide does not exceed 15% by
8
CA 02688051 2009-11-18
weight, preferably 0.1-12% by weight, and more preferably 0.1-6% by
weight based on the weight of the substrate. Said metal halide is
preferably one or more of the halides of Groups IVB and IIA metals,
more preferably one or more of the halides of Ti and/or Mg such as TiC14,
MgF2, and MgC12. The introduction of the metal halide can improve the
abrasion resistance of the prepared catalyst.
In the catalyst preparation process according to the present invention,
the amount of the substrate and molecular sieve is preferably such that the
finally obtained catalyst contains 60-95 wt.% of the substrate and 5-40
wt.% of the molecular sieve.
In the catalyst preparation process according to the present invention,
said alumina is preferably one or more of 7-alumina, malumina,
0-alumina, and x-alumina. Said precursor of alumina is preferably
alumina sol, phospho-alumina sol, various aluminum-containing salts
(such as various aluminates, and sulfate, nitrate, and halide of aluminum),
hydrated alumina with the structure of pseudo-boehmite, hydrated
alumina with the structure of diaspore, hydrated alumina with the
structure of gibbsite, and hydrated alumina with the structure of bayerite.
More preferably, said precursor of alumina is pseudo-boehmite or its
mixtures with one or more selected from alumina sol, phospho-alumina
sol, hydrated alumina with the structure of diaspore, hydrated alumina
with the structure of gibbsite, and hydrated alumina with the structure of
bayerite. It is preferable that said alumina and/or its precursor are
pseudo-boehmite or mixtures of pseudo-boehmite with one or more of
alumina or other alumina precursors. More preferably, said substrate
contains pseudo-boehmite and alumina sol. The content of
pseudo-boehmite in the substrate is preferably 5-95% by weight
calculated in oxide, based on the weight of the substrate.
In the catalyst preparation process according to the present invention,
said substrate may also contain the substrates commonly used in other
catalytic cracking catalysts such as one or more of non-aluminum
inorganic oxide substrates and clay. Said non-aluminum inorganic oxide
substrate is preferably one or more of the oxides of Groups IIIA and IVA
elements and their precursors and more preferably, it is one or more of
oxides of silicon, boron, tin, lead, gallium, and indium and their oxide
9
CA 02688051 2009-11-18
precursors. Preferably, the amounts of various components are such that,
the content of alumina and/or its precursor is 5-100% by weight
calculated in alumina; the content of the non-aluminum inorganic oxide
does not exceed 95% by weight calculated in oxide; and the content of
clay does not exceed 95% by weight, based on the weight of the substrate.
More preferably, the content of alumina and/or its precursors is 5-80% by
weight and the content of clay does not exceed 60% by weight.
In the catalyst preparation process according to the present invention,
said oxide of silicon and their precursors are one or more of silica gel,
silica sol, silica hydrosol, water glass, silicates, organo-silicon
compounds, silica-alumina so!, and silica-alumina gel.
In the catalyst preparation process according to the present invention,
said clay is one or more of clay customarily used for cracking catalysts
such as one or more of kaolin, halloysite, montmorillonite, kieselguhr,
endellite, soapstone, rectorite, sepiolite, attapulgite, hydrotalcite, and
bentonite.
In the catalyst preparation process according to the present invention,
said molecular sieve is one or more selected from the molecular sieve
commonly used for catalytic cracking, preferably an MFI-structured
zeolite or mixtures of the WI-structured zeolite with one or more
selected from Y-type molecular sieve, and 13-zeolite.
In the catalyst preparation process according to the present invention,
said spray drying is known in the art and there is no special requirement
for it. For example, the temperature of the tail gas in spray drying is
100-300 C .
The catalyst preparation process according to the present invention
may also comprise the steps of calcining, washing, and drying. Said
methods of calcining, washing, and drying are known in the art and there
is no special requirement for them. For example, the calcination
temperature is 300-700 C; the drying temperature is 100-300 C; and the
catalyst is washed with deionized water until the content of sodium oxide
in the catalyst does not exceed 0.5% by weight.
In the catalytic cracking process according to the present invention,
said conditions for contacting the catalyst with hydrocarbon oils are
known in the art. For example, the contacting temperature is 400-750 C.
to
CA 02688051 2009-11-18
According to the catalytic cracking process according to the present
invention, when the catalyst used contains MFI-structured zeolite, the
reaction temperature is preferably 480 C-560 C, and steam is introduced
during the reaction with the ratio of steam to the raw oil being 0.7-14:1.
According to the instant catalytic cracking process, besides the
catalyst provided by the present invention, said catalyst may also contains
other cracking catalysts. For example, when the catalyst according to the
present invention contains MFI-structured zeolite, the catalyst according
to the present invention may be used solely, or as a additive for producing
light olefins after mixing with other cracking catalysts. When used as a
additive, the content of the catalyst according to the present invention is
1-30% by weight, preferably 5-30% by weight based on the total weight
of the catalyst.
Cracking catalysts with macropore substrates can be prepared by the
catalyst preparation process of this invention. The catalyst according to
the present invention can be used as a additive or catalyst in the catalytic
cracking of hydrocarbon oils, and it is especially suitable for the catalytic
cracking of heavy oils. Said hydrocarbon oils include, for example,
atmospheric gas oil, vacuum gas oil, atmospheric residual oil, and
vacuum residual oil, The process according to the present invention can
be used for producing light olefins, especially propylene.
The present invention is further illustrated with the flowing examples,
but is not intended to be limited thereby
In the examples and comparative examples, ZRP-zeolite,
REHY-zeolite, and 0-zeolite are all the products of Catalyst Plant, Qilu
Petrochemicals Co., wherein ZRP-zeolite has a Si02/A1203 mole ratio of
30 and contains 2.0 wt.% of RE203 and 4.0 wt.% of P205. For the
determination of abrasion index and pore volume, reference is made to
R1PP29-90 and RIPP151-90 in "Yang Cuiding et al, Analytic Methods in
Petroleum Chemical Industry" (R1PP Experimental Method), Science
press, 1990".
Example 1
20 kg decationized water was mixed with 11.9 kg pseudo-boehmite
(industrial product of Shandong Aluminum Plant with a solid content of
63% by weight). The mixture was slurried and adjusted to a pH value of 3
CA 02688051 2009-11-18
with hydrochloric acid. 72.6 kg decationized water was mixed with 38.7
kg halloysite (industrial product of Suzhou Porcelain Clay Co. with a
solid content of 72.3% by weight) and slurried for 5 min, and then added
- with 1.75 kg potassium borate (analytically pure), the mixture being
slurried for 15 min. The above two slurries were mixed and stirred
uniformly, and then the mixed slurry was standing at 65 C for 1.5 h for
aging, with the pH value being maintained at 2-4 (adjusted with
hydrochloric acid). Then the temperature was decreased to 55 C and
added with 13.5 kg alumina sol (product of Catalyst Plant, Qilu
Petrochemicals Co. with an A1203 content of 21.7% by weight). The
mixture was stirred for 40 min and 32.1 kg molecular sieve slurry
(containing 2.0 kg REHY-zeolite and 9.0 kg ZRP-zeolite) was added. 2
kg TiC14 was added and the mixture was stirred uniformly. The resultant
slurry was spray dried and washed to remove free Na ions, yielding
catalyst A after being dried. The pore distribution is shown in Table 1.
Example 2
20 kg decationized water was mixed with 11.9 kg pseudo-boehmite
(industrial product of Shandong Aluminum Plant with a solid content of
63% by weight). The mixture was slurried and adjusted to a pH value of 3
with hydrochloric acid. 72.6 kg decationized water was mixed with 38.7
kg halloysite (industrial product of Suzhou Porcelain Clay Co. with a
solid content of 72.3% by weight) and slurried for 5 min, and then added
with 3 kg potassium sulfate (industrially pure, 98wt%), the mixture being
slurried for 15 min. The above two slurries were mixed and stirred
uniformly, and then the mixed slurry was standing at 65 C for 1.5 h for
aging, with the pH value being maintained at 2-4 (adjusted with
hydrochloric acid). Then the temperature was decreased to 55 C and
added with 13.5 kg alumina sol (product of Catalyst Plant, Qilu
Petrochemicals Co. with an A1203 content of 21.7% by weight). The
mixture was stirred for 40 min and 32.1 kg molecular sieve slurry
(containing 1.0 kg REHY-zeolite, 8.5 kg by weight of ZRP-zeolite, and
0.5 kg 13-zeolite) was added. 5 kg TiC14 was added and the mixture was
stirred uniformly. The resultant slurry was spray dried and washed to
remove free Na ions, yielding catalyst B after being dried. The pore
12
CA 02688051 2009-11-18
distribution is shown in Table 1.
Example 3
20 kg decationized water was mixed with 11.9 kg pseudo-boehmite
(industrial product of Shandong Aluminum Plant with a solid content of
63% by weight). The mixture was slurried and adjusted to a pH value of 3
with hydrochloric acid. 72.6 kg decationized water was mixed with 38.7
kg halloysite (industrial product of Suzhou Porcelain Clay Co. with a
solid content of 72.3% by weight) and slurried for 5 min, and then added
with 15.0 kg water glass (product of Catalyst Plant, Qilu Petrochemicals
Co. with an Si02 content of 19.9% by weight). The mixture was slurried
for 15 min and adjusted to a pH value of 3 with hydrochloric acid, added
with 3kg potassium sulfate, and slurried for 15min. The above two
slurries were mixed and stirred uniformly, and then the mixture was
standing at 65 C for 1.5 h for aging, with the pH value being maintained
at 2-4. Then the temperature was decreased to 55 C and 13.5 kg alumina
sol was added (product of Catalyst Plant, Qilu Petrochemicals Co. with
an A1203 content of 21.7% by weight). The mixture was stirred for 40
min and 32.1 kg molecular sieve slurry (containing 1.0 kg REHY-zeolite,
8.5 kg by weight of ZRP-zeolite, and 0.5 kg I3-zeolite) was added. 5kg
TiC14 was added and the mixture was stirred uniformly. The slurry was
spray dried and molded, and washed to remove free Na ions, yielding
catalyst C after being dried. The pore distribution is shown in Table 1.
Example 4
20 kg decationized water was mixed with 11.9 kg pseudo-boehmite
(industrial product of Shandong Aluminum Plant with a solid content of
63% by weight). The mixture was slurried and adjusted to a pH value of 3
with hydrochloric acid. 72.6 kg decationized water was mixed with 38.7
kg halloysite (industrial product of Suzhou Porcelain Clay Co. with a
solid content of 72.3% by weight) and slurried for 5 min, and then added
with 1.75 kg boric acid (analytically pure), the mixture being slurried for
15 min. The above two slurries were mixed and stirred uniformly, and
then the mixed slurry was standing at 65 C for 1.5 h for aging, with the
pH value being maintained at 2-4 (adjusted with hydrochloric acid). Then
13
CA 02688051 2009-11-18
the temperature was decreased to 60 C and added with 13.5 kg alumina
sol (product of Catalyst Plant, Qilu Petrochemicals Co. with an A1203
content of 21.7% by weight). The mixture was stirred for 40 min and 32.1
kg molecular sieve slurry (containing 2.0 kg REHY-zeolite and 9.0 kg
ZRP-zeolite) was added. The resultant slurry was spray dried and washed
to remove free Na ions, yielding catalyst D after being dried. The pore
distribution is shown in Table 1.
Example 5
20 kg decationized water was mixed with 9.9 kg pseudo-boehmite
(industrial product of Shandong Aluminum Plant with a solid content of
63% by weight). The mixture was slurried and adjusted to a pH value of 3
with hydrochloric acid. 72.6 kg decationized water was mixed with 38.7
kg halloysite (industrial product of Suzhou Porcelain Clay Co. with a
solid content of 72.3% by weight) and slurried for 5 min, and then added
with 4.01 kg potassium borate (analytically pure). The mixture was
slurried for 15 min. The above two slurries were mixed and stirred
uniformly, and then the mixture was standing at 65 C for 1.5 h for aging,
with the pH value being maintained at 2-4. Then the temperature was
decreased to 55 C and 13.5 kg alumina sol was added (product of
Catalyst Plant, Qilu Petrochemicals Co. with an A1203 content of 21.7%
by weight). The mixture was stirred for 40 min and 18.7 kg molecular
sieve slurry (containing 1.0 kg REHY-zeolite, 5.2 kg by weight of
ZRP-zeolite) was added. 4kg TiC14 was added and the mixture was stirred
uniformly. The slurry was spray dried and molded, and washed to remove
free Na ions, yielding catalyst E after being dried. The pore distribution is
shown in Table 1.
Example 6
20 kg decationized water was mixed with 14.9 kg pseudo-boehmite
(industrial product of Shandong Aluminum Plant with a solid content of
63% by weight) and the mixture was slurried and adjusted to a pH value
of 3 with hydrochloric acid. 72.6 kg decationized water was mixed with
38.7 kg halloysite (industrial product of Suzhou Porcelain Clay Co. with
a solid content of 72.3% by weight) and the mixture was slurried for 5
14
CA 02688051 2009-11-18
min. Then 1.6 kg potassium sulfate (industrially pure, 98%) was added,
and the mixture was slurried for 15 min. The above two slurries were
mixed and stirred uniformly, and then the mixture was standing at 65 C
for 1.5 h for aging, with the pH value being maintained at 2-4. Then the
temperature was decreased to 55 C and 13.5 kg alumina sol (product of
Catalyst Plant, Qilu Petrochemicals Co. with an A1203 content of 21.7%
by weight) was added. The mixture was stirred for 40 min and 45.1 kg
molecular sieve slurry (containing 1.5 kg REHY-zeolite, 11.3 kg of
ZRP-zeolite, and 1 kg [3-zeolite) was added. 3 kg TiC14 was added and the
mixture was stirred uniformly. The slurry was spray dried and molded
and washed to remove free Na ions, yielding catalyst F after being dried.
The pore distribution is shown in Table 1.
Example 7
20 kg decationized water was mixed with 14.9 kg pseudo-boehmite
(industrial product of Shandong Aluminum Plant with a solid content of
63% by weight) and the mixture was slurried and adjusted to a pH value
of 3 with hydrochloric acid. 72.6 kg decationized water was mixed with
38.7 kg halloysite (industrial product of Suzhou Porcelain Clay Co. with
a solid content of 72.3% by weight) and the mixture was slurried for 5
min. Then 2.6 kg potassium sulfate (industrially pure, 98%) was added,
and the mixture was slurried for 15 min. The above two slurries were
mixed and stirred uniformly, and then the mixture was standing at 65 C
for 1.5 h for aging, with the pH value being maintained at 2-4. Then the
temperature was decreased to 55 C and 15.5 kg alumina sol (product of
Catalyst Plant, Qilu Petrochemicals Co. with an A1203 content of 21.7%
by weight) was added. The mixture was stirred for 40 min and 22.1 kg
molecular sieve slurry (containing 0.5 kg REHY-zeolite, 6.3 kg of
ZRP-zeolite, and 1.5 kg p-zeolite) was added. 3 kg TiC14 was added and
the mixture was stirred uniformly. The slurry was spray dried and molded
and washed to removed free Na ions, yielding catalyst G after being dried.
The pore distribution is shown in Table 1.
Comparative Example 1
A catalyst was prepared according to the process for preparing the
CA 02688051 2009-11-18
DCC industrial catalyst (the process in CN1048428C).
92.6 kg decationized water was mixed with 38.7 kg halloysite
(industrial product of Suzhou Porcelain Clay Co. with a solid content of
72.3% by weight) and the mixture was slurried. Then 15.9 kg
pseudo-boehmite was added and the pH value of the mixture was
adjusted to 3 with hydrochloric acid, the mixture being stirred uniformly.
Then the mixture was standing at 65 C for aging for 1 h, and its pH value
was maintained at 2-4. The temperature was decreased to 55 C and 13.5
kg alumina sol was added. The mixture was stirred for 40 min and 32.1
kg molecular sieve slurry (containing 1.0 kg by weight of REHY-zeolite
and 9.0 kg by weight of ZRP-zeolite) was added. The mixture was
slurried, spray dried, and molded, and washed to remove free Na ions,
yielding comparative catalyst DB-1 after being dried. The pore
distribution is shown in Table 1.
Comparative Example 2
20 kg decationized water was mixed with 11.9 kg pseudo-boehmite
(industrial product of Shandong Aluminum Plant with a solid content of
63% by weight) and the mixture was slurried and adjusted to a pH value
of 3 with hydrochloric acid. 72.6 kg decationized water was mixed with
38.7 kg halloysite (industrial product of Suzhou Porcelain Clay Co. with
a solid content of 72.3% by weight) and the mixture was slurried for 5
min. The above two slurries were mixed and stirred uniformly, and
standing at 65 C for 1.5 h for aging, with the pH value being maintained
at 2-4. The temperature was then decreased to 55 C and 13.5 kg alumina
so! (product of Catalyst Plant, Qilu Petrochemicals Co. with a A1203
content of 21.7% by weight) was added. The mixture was stirred for 40
min and 32.1 kg molecular sieve slurry (containing 1.0 kg by weight of
REHY-zeolite, 8.5 kg by weight of ZRP-zeolite, and 0.5 kg I3-zeolite) was
added. 5 kg TiC14 was added, and the mixture was slurried uniformly. The
resultant slurry was spray dried and molded, and washed to remove free
Na ions, yielding catalyst sample DB-2 after being dried.
The abrasion indices, pore volumes and crystallinity of Catalysts
A-G and DB-1, DB-2 are shown in Table 2.
16
CA 02688051 2009-11-18
Examples 8-9
Catalyst samples A-B were evaluated on a small fixed fluidized bed
apparatus with 30 m.% of residual oil + 70 m.% of wax oil (its properties
are shown in Table 3) as a feed stock under a reaction temperature of
680 C, a catalyst/oil weight ratio of 10, a water/oil mass ratio of 0.8:1,
and a weight hourly space velocity of 10 hl. The catalyst was pre-treated
with 100% steam at 800 C for 17 h and the load of the catalyst was 180 g.
The evaluation results are shown in Table 4.
Comparative Example 3
Catalyst DB-1 was evaluated according to the method in Example 8.
The evaluation results are shown in Table 4.
Table 1
Comparative Comparative
Example No. 1 2 3 4 5 6 7
Example 1
Example 2
Catalyst No. A B CDEF G DB-1 DB-2
<2nm 21 20 23 21 13 35 15 22 18
,
2-4nm 17 20 16 25 10 35 37 70 74
4-6nm 5 3 3 2 1 7 I 3 2
6-20nm 47 42 52 45 75 20 20 4.5 5
_
-
6-10nm 25 22 27 20 15 15 16 3 3
20-100nm 10 15 6 7 1 3 27 0.5 1
6-20nm/2-4nm 2.76 2.10 3.25 1.80 7.50 0.57 0.54 0.064 0.068
Table 2
'
Example Comparative Comparative 1 6
7
2 3 4 5
No. Example 1 Example 2
Catalyst
DB-1 DB-2 A B C D E F G
No.
17
CA 02688051 2009-11-18
Al, % 2.0 1.0 1.5 2.0 1.9 2.9 1.0 1.9
2.7
VBET) mlig 0.185 0.190 0.238 0.256 0.247 0.240 0.236 0.241
0.257
ACR, %
(Crystal
structure of 15.6 15.4 16.6 16.3 16.4 16.1 16.6
16.2 15.3
fresh
catalyst)
ACR, %
(800 C/17h 11.4 11.2 13.8 13.3 13.5 13.4 13.2
13.5 11.9
deactivated)
Table 3
Density (20 C), g/cm3 0.9006
Kinematic viscosity (100 C), 11.0
nun2is
Conradson Carbon residue, wt% 3.14
Element C 85.7
composition, H 12.8
wt% N 0.38
0.77
Family Saturated HCs 57.5
composition Aromatics _ 24.5
( hydrocarbon Gum 16.9
species), wt% Asphaltene 1.1
Metal content, Ni 5.0
PPm V 0.8
Boiling range, _ IBP
C 5% 217
40% 396
70% 456
Characterization factor 12.0
18
CA 02688051 2009-11-18
Table 4
Comparative
Example No. 8 9
Example 3
Catalyst No. DB-1 A B
Dry gas, wt % 3.86 4.41 4.07
Hydrogen, wt % 0.06 0.08 0.05
Methane, wt % 0.42 0.46 0.40
Ethane, wt % 0.31 0.33 0.28
Ethylene, wt % 3.06 3.54 3.59
LPG, wt % 43.67 45.24 48.34
Propane, wt % 2.04 2.22 2.10
Propylene, wt % 19.26 21.08 21.88
n-Butane, wt% 1.19 1.15 1.23
'so-butane, wt% 5.55 5.34 5.45
Butene-1, wt% 2.74 2.76 3.07
Iso-butene, wt % 6.45 6.5 7.40
cis-butene-2, wt% 2.71 2.57 3.06
trans-butene-2, wt
3.74 3.62 4.15
%
Gasoline, wt % 43.34 40.55 40.36
Diesel oil, wt % 6.29 _ 7.1 5.21
Heavy oil, wt % 1.55 _ 1.41 1.05
Coke, wt % 1.28 _ 1.27 1.09 ,
Conversion wt % 92.14 91.47 93.74
19