Note: Descriptions are shown in the official language in which they were submitted.
CA 02688691 2011-10-17
PLANT-FIBER-MATERIAL TRANSFORMATION METHOD
BACKGROUND OF THE INVENTION
1. Field of the Invention
[0001] The invention relates to a method of transforming plant fiber material
into
produce saccharide.
2. Description of the Related Art
[0002] Effective use of saccharide as food or fuel has been proposed and is
being put
into practice, the saccharide being mostly glucose and xylose and produced
from
cellulose or hemicellulose by transforming plant fiber material, such as
squeezed
sugarcane residues (bagasse) or wood chips. In particular, biomass energy
technology is
drawing attention, in which the saccharide obtained by transforming plant
fiber material
is fermented to produce alcohol, such as ethanol being used as fuel. In
Japanese Patent
Application Publication No. 8-299000 (JP-A-8-299000), Japanese Patent
Application
Publication No. 2006-149343 (JP-A-2006-149343), Japanese Patent Application
Publication No. 2006-129735 (JP-A-2006-129735), and Japanese Patent
Application
Publication No. 2002-59118 (JP-A-2002-59118), for example, various methods of
producing saccharide, such as glucose, by transforming cellulose or
hemicellulose, are
proposed. JP-A-8-299000 describes a method of hydrolyzing cellulose with the
use of
hydrochloric acid or sulfuric acid such as dilute sulfuric acid or
concentrated sulfuric acid.
A method in which cellulase is used (JP-A-2006-149343), a method in which a
solid
catalyst, such as activated carbon or zeolite, is used (JP-A-2006-129735), and
a method
in which pressurized hot water is used (JP-A-2002-59118) are also available.
[0003] In the case of the method in which cellulose is transformed with the
use of
acid, such as sulfuric acid, however, it is difficult to separate the acid and
saccharide.
This is because acid and glucose, which is the main ingredient of the
transformation
product, are both soluble in water. Removal of acid by neutralization or ion
exchange is
not only troublesome and costly, but it is also difficult to completely remove
acid because
1
CA 02688691 2011-10-17
acid may remain in the process of fermentation for ethanol. As a result, even
when pH
is optimized in view of activity of yeast in the process of fermentation for
ethanol,
concentration of salt becomes high, which results in reduction in activity of
yeast, which
in turn results in reduction in fermentation efficiency.
[0004] In particular, when concentrated sulfuric acid is used, it is very
difficult and
very energy consuming to remove sulfuric acid to the extent that yeast is not
deactivated.
On the other hand, when dilute sulfuric acid is used, it is relatively easy to
remove
sulfuric acid. However, it is necessary to transform cellulose under high
temperature
conditions, which is energy consuming. In addition, the acid, such as sulfuric
acid and
hydrochloric acid is very difficult to separate, collect and reuse. Thus, use
of these
acids as a catalyst for producing glucose is a cause of increasing the costs
of bio-ethanol.
[0005] In the case of the method in which pressurized hot water is used, it is
difficult
to adjust the conditions, and it is therefore difficult to produce glucose
with stable yield.
In addition, according to the above method, even glucose is transformed to
cause
reduction in the yield of glucose, and moreover, the activity of yeast is
reduced due to the
transformation product, which may result in suppression of fermentation.
Furthermore,
the reactor (supercritical processing apparatus) is expensive and is low in
durability, and
therefore, this method is problematic also in view of costs.
[0006] Meanwhile, widely used catalysts include a cluster acid catalyst, such
as
heteropoly acid. In Japanese Patent Application Publication No. 2006-206579
(JP-A-2006-206579), for example, a method of manufacturing ester levulinate is
described, which carbohydrate and alcohol are reacted under the presence of
heteropoly
acid. In a method described in W095/26438, a cluster acid catalyst, in the
form of
aqueous solution of 0.001 to 0.20 M, is used in the process of removal of
lignin from
wood pulp and the process of bleaching the wood pulp.
SUMMARY OF THE INVENTION
[0007] The invention provides a plant-fiber-material transformation method in
which
a catalyst for promoting hydrolysis of cellulose or hemicellulose, and
saccharide that is
2
CA 02688691 2011-10-17
obtained by hydrolyzing the cellulose or the like are easily separated, and
the separated
catalyst is reused. In addition, the invention provides a plant-fiber-material
transformation method that is excellent in energy efficiency.
[0008] A plant-fiber-material transformation method according to a first
aspect of the
invention includes: hydrolyzing cellulose contained in plant fiber material
with the use of
a pseudo-molten cluster acid as a catalyst; and producing saccharide, most of
which is
glucose.
[0009] In the first aspect of the invention, the cluster acid used as the
catalyst for
hydrolyzing cellulose has acidity stronger than sulfuric acid in general and
therefore
exhibits sufficient catalytic activity even under low temperature conditions,
so that it is
possible to obtain saccharide, such as glucose, from cellulose with high-
energy efficiency.
Moreover, because the pseudo-molten cluster acid also functions as reaction
solvent, it is
also possible to significantly reduce the amount of solvent used as reaction
solvent, as
compared to the hydrolysis processes in which other catalysts are used. As a
result, it is
made possible to separate and collect the cluster acid more efficiently and
using less
energy.
[0010] The hydrolysis step may be performed at or below 140 C under a pressure
condition of an atmospheric pressure to I MPa.
[0011] The hydrolysis of the cellulose maybe performed at or below 120 C.
[0012] The hydrolysis of the cellulose maybe performed at or below 100 C.
[0013] The ratio between the plant fiber material and the cluster acid may be
within
a range of 1:1 to 1:4.
[0014] When the cluster acid is brought into a pseudo-molten state, the
cluster acid
exhibits the activity as a catalyst for hydrolysis of cellulose or
hemicellulose. Because
the pseudo-molten state of the cluster acid varies depending on temperature
and the
amount of water of crystallization contained in the cluster acid, and it is
necessary to
control the amount of water of crystallization in the cluster acid and
reaction temperature
when the cluster acid is brought into a pseudo-molten state. Meanwhile, water
is
needed to hydrolyze cellulose that is a polymer, in which glucose molecules
are joined by
3
CA 02688691 2011-10-17
(3-1,4-glycosidic bonds, into saccharide, such as glucose or xylose.
[0015] In view of this fact, an amount of water in a hydrolysis reaction
system may
be equal to or greater than a sum of i) an amount of water of crystallization
required to
bring all the cluster acid in the hydrolysis reaction system into a pseudo-
molten state at a
temperature condition for the hydrolysis, and ii) an amount of water required
to
hydrolyze all the cellulose in the hydrolysis reaction system into glucose.
[0016] The cluster acid maybe heteropoly acid.
[0017] The heteropoly acid may be one selected from a group consisting of
phosphotungstic acid, silicotungstic acid, and phosphomolybdic acid.
[0018] The heteropoly acid may have a Keggin structure.
[0019] The heteropoly acid may have a Dawson structure.
[0020] The plant-fiber-material transformation method may further include a
separation step after producing the glucose, in which the saccharide is
precipitated with
the use of an organic solvent, and the saccharide containing a solidified
saccharide during
the hydrolysis and the precipitated saccharide is separated from residues and
the cluster
acid.
[00211 When cluster acid is used as the catalyst for hydrolyzing cellulose,
and
organic solvent is used that is a good solvent for the cluster acid but a poor
solvent for
saccharide, most of which is glucose, that is the product, it is possible to
precipitate
saccharide and easily separate the cluster acid and the saccharide.
[0022] A solubility of the saccharide with respect to the organic solvent may
be
equal to or less than 0.6 g/100 ml.
[0023] The solubility of the saccharide with respect to the organic solvent
may be
equal to or less than 0.06 g/100 ml.
[0024] A solubility of the cluster acid with respect to the organic solvent
may be
equal to or greater than 20g/100ml.
[0025] The solubility of the cluster acid with respect to the organic solvent
may be
equal to or greater than 40g/I00ml.
[0026] At least one selected from ether solvents and alcohol solvents may be
used as
4
CA 02688691 2011-10-17
the organic solvent.
[0027] The organic solvent may be ethanol.
[0028] The organic solvent may be diethyl ether.
[0029] In the separating step, the amount of water in a reaction system in
which the
separating step is performed may be controlled so that all the cluster acid in
the reaction
system in which the separating of the saccharide is performed contains water
of
crystallization whose amount is equal to or less than a normal water-of-
crystallization
amount. When the cluster acid contains water of crystallization whose amount
is
greater than the normal water-of-crystallization amount in the separating
step, the water
molecules that are not coordinated to the cluster acid are mixed into the
organic solvent,
and the saccharide is dissolved in the mixed water, which causes the
saccharide to be
mixed into the organic solvent phase in which the cluster acid is dissolved.
By
controlling the amount of water of crystallization in the cluster acid in the
separating step
as described above, it is possible to minimize the dissolution of saccharide
in the water
mixed into the organic solvent phase as described above, and it is therefore
possible to
improve the yield of saccharide.
[0030] When saccharide is transferred to the organic solvent phase, the
cluster acid
may be dehydrated after the separating step so that all the cluster acid in
the organic
solvent contains water of crystallization whose amount is equal to or less
than the normal
water-of-crystallization amount. By dehydrating the cluster acid in the
organic solvent
to reduce the amount of water of crystallization, it is possible to
precipitate and collect
the saccharide dissolved in the water that is not coordinated to the cluster
acid and mixed
into the organic solvent phase.
[00311 A cluster acid containing water of crystallization whose amount is
equal to or
less than the normal water-of-crystallization amount may be used as a
desiccant agent to
dehydrate the cluster acid.
[0032] A content rate of water of crystallization of the cluster acid as the
desiccant
agent is equal to or less than 70%.
[0033] The content rate of water of crystallization of the cluster acid as the
desiccant
5
CA 02688691 2011-10-17
agent is equal to or less than 30%.
[0034] The cluster acid dissolved in the organic solvent may be separated from
the
organic solvent. The separated cluster acid may be reused as the catalyst for
hydrolysis
of cellulose or hemicellulose contained in plant fiber material.
[00351 The plant fiber material may be cellulose-based biomass.
BRIEF DESCRIPTION OF THE DRAWINGS
[0036] The foregoing and further features and advantages of the invention will
become apparent from the following description of example embodiments with
reference
to the accompanying drawings, wherein like numerals are used to represent like
elements
and wherein:
FIG IA shows a Keggin structure of heteropoly acid;
FIG lB shows a Dawson structure of heteropoly acid;
FIG 2 is a graph showing a relation between the content of water of
crystallization in
a cluster acid catalyst and apparent melting temperature;
FIG 3 is a graph showing a relation among conversion R of cellulose, yield 9
of
glucose, and hydrolysis reaction temperature;
FIG. 4 is a graph showing a relation between the content of water of
crystallization and
loss of glucose due to dissolution when the cluster acid catalyst is
collected; and
FIG 5 is a chart for describing steps from hydrolysis of cellulose to
collection of
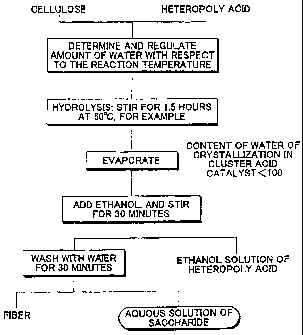
saccharide and heteropoly acid in Example 9.
DETAILED DESCRIPTION OF EMBODIMENTS
[00371 A first embodiment of the invention that relates to a method of
transforming
plant fiber material will be described below with reference to drawings.
[00381 The present inventors have found that pseudo-molten cluster acid acts
as a
catalyst for hydrolyzing cellulose or hemicellulose to produce saccharide,
most of which
is glucose. The "cluster acid" herein means an acid in which a plurality of
oxoacids are
condensed, that is, a so-called polyacid. In many cases, the polyacid is in a
state where
6
CA 02688691 2011-10-17
a plurality of oxygen atoms are joined with the center element, which is
therefore
oxidized to the maximum oxidation number, and the polyacid exhibits excellent
characteristics as an oxidation catalyst. In addition, it is known that many
polyacids are
strong acids. For example, the acidity of phosphotungstic acid (pKa=-13.16),
which is a
heteropoly acid, is stronger than the acidity of sulfuric acid (pKa=-11.93).
Thus, even
under mild conditions, such as under a temperature of 50 C, for example, it is
possible to
transform cellulose or hemicellulose to produce saccharide, such as glucose or
xylose.
[0039] The cluster acid used in the invention may be either isopoly acid or
heteropoly acid. Preferably, the cluster acid is a heteropoly acid because it
has a high
oxidizing power and a strong acidity. There is no particular limit to the kind
of
heteropoly acid used. For example, the heteropoly acid may have the general
structure
[HwAxByOz], where A represents a heteroatom, such as Phosphorus, Silicon,
Germanium, Arsenic or Boron, which can form a heteropoly acid, B represents a
polyatom, such as Tungsten, Molybdenum, Vanadium or Niobium, which can form a
polyacid, and w, x, y and z denote the content of the components H, A, B, and
0,
respectively. The number of kinds of the polyatoms and the heteroatoms that
are
contained in a single molecule of the heteropoly acid may be one or more.
[0040] Specifically, phosphotungstic acid H3[PW12O40] or silicotungstic acid
H4[SiW12O40], which are tungstates may be used, because of the balanced values
of the
acidity and the oxidizing power. Alternatively, phosphomolybdic acid
H3[PMo12O40],
which is a molybdate, may be used.
[00411 The structure of a Keggin-type heteropoly acid [X"+M12O4o: X=P, Si, Ge,
As,
etc., M=Mo, W, etc.], phosphotungstic acid, for example, is shown in FIG IA. A
tetrahedron X04 is present at the center of polyhedrons, each being an
octahedron MOB,
and there is a lot of water of crystallization around this structure. It
should be noted that
there is no particular limit to the structure of the cluster acid. The
heteropoly acid may
be, for example, a Dawson-type heteropoly acid as shown in FIG. 1B. Although
the
cluster acid catalyst is not crystalline in nature, the term "water of
crystallization," is used
herein to refer to the water coordinated to the cluster acid catalyst in a
certain ratio.
7
CA 02688691 2011-10-17
Although, in general, water of crystallization is the water contained in the
cluster acid
catalyst when the cluster acid catalyst is crystalline, the water molecules
that are
coordinated to the cluster acid catalyst when the cluster acid catalyst is in
a
pseudo-molten state in which each molecule of the cluster acid catalyst is
liberated from
each other or when the cluster acid catalyst is dissolved in ethanol (more
specifically, the
cluster acid catalyst is suspended in ethanol in a colloidal state, instead of
dissolved
therein), are referred to as the water of crystallization
[0042] The cluster acid catalyst as described above is solid at room
temperatures.
When the cluster acid catalyst is heated, it is brought into a pseudo-molten
state, and
exhibits catalytic activity to the hydrolysis of cellulose or hemicellulose.
The
pseudo-molten state herein means a state, in which the cluster acid is
apparently melted
but is not completely melted into a liquid state; the pseudo-molten state
resembles a
colloidal (sol) state in which the cluster acid is dispersed in a solution,
and is a state in
which the cluster acid shows fluidity. Note that, in this state, the cluster
acid has a high
viscosity and a high density. Whether the cluster acid is in the pseudo-molten
state can
be determined by visual inspection, or, in the case of a homogeneous system,
by
Differential Scanning Calorimeter (DSC), for example.
[0043] The cluster acid exhibits a high catalytic activity to the hydrolysis
of cellulose
at low temperatures due to its strong acidity as described above. Because the
diameter
of a molecule of the cluster acid is about 2 nm, the cluster acid is easily
mixed with plant
fiber material, which is the raw material, and therefore efficiently promotes
hydrolysis of
cellulose. Thus, it is possible to hydrolyze cellulose under mild conditions,
which
provides high-energy efficiency and low environmental load. In addition,
unlike the
hydrolysis of cellulose using sulfuric acid, for example, the hydrolysis of
cellulose of the
present embodiment using a cluster acid as a catalyst achieves high efficiency
in
separating saccharide and the catalyst and it is therefore possible to easily
separate
saccharide and the catalyst, so that the amount of the catalyst remaining in
saccharide is
minimized and the hydrolysis process of this embodiment is advantageous also
in view of
fermentation.
8
CA 02688691 2011-10-17
[0044] In addition, because the cluster acid becomes solid depending on
temperature,
it is possible to separate the cluster acid from saccharide, which is the
product. Thus, it
is possible to collect and reuse the separated cluster acid. Moreover, because
the
pseudo-molten cluster acid catalyst functions as a reaction solvent, it is
also possible to
significantly reduce the amount of solvent used as a reaction solvent, as
compared to
other hydrolysis processes. This means that it is possible to achieve high
efficiency in
separating the cluster acid and saccharide, which is the product, and in
collecting the
cluster acid. Specifically, the invention using a cluster acid as a catalyst
for hydrolyzing
cellulose reduces costs and at the same time is environment-friendly.
[0045] A step of hydrolyzing cellulose used in a plant-fiber-material
transformation
method of the invention will be described in detail below. Although the step
in which
glucose is produced from cellulose is mainly described in this specification,
the plant
fiber material includes hemicellulose in addition to cellulose, products
include xylose in
addition to glucose, and these cases also fall within the scope of the
invention. The
plant fiber material is not particularly limited as long as containing
cellulose or
hemicellulose, and includes cellulose-based biomass, such as broad-leaved
trees,
bamboos, coniferous trees, kenaf, scrap wood from furniture, rice straws,
wheat straws,
rice husks, and squeezed sugarcane residues (bagasse). The plant fiber
material may be
the cellulose or hemicellulose that is separated from the above-listed
biomass, or may be
the cellulose or hemicellulose that is artificially synthesized.
[0046] With regard to such fiber material, in general, pulverized material is
used in
view of the dispersion characteristics in the reaction system. The method of
pulverizing
the fiber material may be a commonly used method. In view of improvement of
the
ease of mixing with the cluster acid catalyst and increase in reaction chance,
the plant
fiber material may be reduced to powder whose diameter is about a few microns
to 200
microns.
[0047] The cluster acid catalyst and the plant fiber material may be mixed and
stirred
prior to heating. As described above, in a step of hydrolysis, the cluster
acid catalyst is
brought into a pseudo-molten state and functions as reaction solvent. Thus, in
this
9
CA 02688691 2011-10-17
embodiment, although it depends on the form of plant fiber material (the size,
the state of
fiber, for example), and the mixing ratio and the volume ratio between the
cluster acid
catalyst and the plant fiber material, for example, there is no need to use
water, organic
solvents, etc. as a reaction solvent. For this reason, when it is intended to
ensure contact
between the cluster acid and the plant fiber material, the cluster acid
catalyst and the
plant fiber material may be mixed to some extent before the cluster acid
catalyst is
brought into a pseudo-molten state.
[0048] The pseudo-molten state of the cluster acid varies depending on
temperature
and the amount of water of crystallization contained in the cluster acid
catalyst (see FIG
2). Specifically, the inventors have found that, when the amount of water of
crystallization contained increases, the temperature decreases at which the
phosphotungstic acid, which is a cluster acid, is brought into a pseudo-molten
state.
That is, the cluster acid catalyst containing a relatively large amount of
water of
crystallization exhibits catalytic action to the hydrolysis of cellulose at a
temperature
lower than that in the case of the cluster acid catalyst that contains a
smaller amount of
water of crystallization.
[0049] FIG 2 shows a relation between the content of water of crystallization
in the
heteropoly acid (phosphotungstic acid), which is a typical cluster acid
catalyst, and the
temperature (apparent melting temperature) at which the pseudo-molten state is
brought
about. In FIG 2, the cluster acid catalyst is in a solid state in the region
under the curve,
and in a pseudo-molten state in the region above the curve. In addition, in
FIG 2, the
amount of water (content of water of crystallization) (%) is determined on the
assumption
that the content of water is 100% when the amount of water of crystallization
is equal to
the normal water-of-crystallization amount n (n=30) in the cluster acid
(phosphotungstic
acid). Because no component of cluster acid catalyst is thermally decomposed
and
volatilized even at a high temperature of 800 C, for example, it is possible
to determine
the amount of water of crystallization by pyrolytic methods, such as the
thermogravimetry (TG) method.
[0050] The normal water-of-crystallization amount is the amount (the number of
CA 02688691 2011-10-17
molecules) of water of crystallization contained in a molecule of the cluster
acid in a
solid crystalline state at room temperatures, and varies depending on the kind
of cluster
acid. For example, the normal water-of-crystallization amount is about 30 in
the case of
phosphotungstic acid (H3LPW12040]=nH2O (n;::30)), about 24 in the case of
silicotungstic
acid (H4[SiW12O40]=nH2O (n~24)), and about 30 in the case of phosphomolybdic
acid
(H3[PMo12O40]'nH2O (n--30)).
[00511 By controlling the amount of water of crystallization contained in the
cluster
acid catalyst in the hydrolysis reaction system based on the relation between
the amount
of water of crystallization and the apparent melting temperature, it is
possible to bring the
cluster acid catalyst into a pseudo-molten state at the hydrolysis reaction
temperature.
For example, when phosphotungstic acid is used as the cluster acid catalyst,
it is possible
to control the hydrolysis reaction temperature within the range between 40 C
and 110 C
by changing the amount of water of crystallization in the cluster acid (see
FIG 2).
[00521 The amount of water of crystallization contained in the cluster acid
catalyst
can be regulated by controlling the amount of water present in the hydrolysis
reaction
system. Specifically, when it is desired to increase the amount of water of
crystallization contained in the cluster acid catalyst, that is, to lower the
reaction
temperature, a measure that can be taken is to add water to the hydrolysis
reaction system
by adding water to the mixture containing the plant fiber material and the
cluster acid
catalyst, or raising the relative humidity of the atmosphere surrounding the
reaction
system, for example. As a result, the cluster acid takes in the added water as
water of
crystallization, and the apparent melting temperature of the cluster acid
catalyst is
lowered.
[00531 On the other hand, when it is desired to reduce the amount of water of
crystallization contained in the cluster acid catalyst, a measure that can be
taken is to
reduce the water of crystallization contained in the cluster acid catalyst by
removing
water from the hydrolysis reaction system by, for example, heating the
reaction system to
evaporate water, or adding a desiccant agent to the mixture containing the
plant fiber
material and the cluster acid catalyst. As a result, the apparent melting
temperature of
11
CA 02688691 2011-10-17
the cluster acid catalyst is raised. As described above, it is possible to
easily control the
amount of water of crystallization contained in the cluster acid, and it is
also possible to
easily regulate the reaction temperature at which cellulose is hydrolyzed, by
controlling
the amount of water of crystallization.
[0054] Lowering the reaction temperature in the hydrolysis step is
advantageous in
that it is possible to improve energy efficiency. In addition, the present
inventors have
found that the selectivity with which the glucose is produced by hydrolysis of
cellulose
contained in the plant fiber material varies depending on the hydrolysis
reaction
temperature (see FIG 3). As shown in FIG 3, it is a common fact that the
higher the
reaction temperature is, the higher the conversion is; in the hydrolysis of
cellulose using
the phosphotungstic acid of which the content of water of crystallization is
160% (the
apparent melting temperature is about 40 C; see FIG 2), the conversion R in
the
temperature range between 50 C to 90 C increases as the temperature increases,
and
almost all the cellulose reacts at about 80 C.
[0055] On the other hand, although the yield r) of glucose increases from 50 C
to
60 C as in the case of the conversion of cellulose, the yield rl reaches the
peak at 70 C
and decreases with temperature. Specifically, glucose is produced with high
selectivity
between 50 C to 60 C, whereas, between 70 C and 90 C, reactions, other than
the
glucose producing reaction, proceed that include formation of other
saccharides, such as
xylose, and transformation, for example. It should be noted that the
conversion R of
cellulose and the yield rl of glucose can be calculated using the following
expressions.
R = {(QCt - QCr) / QCt} x 100
11 = R x (QG / QGt)
where QCt is the amount of prepared cellulose; QCr is the amount of unreacted
cellulose;
QG is the amount of glucose produced when all the prepared cellulose is
hydrolyzed; and
QGt is the actual amount of collected cellulose.
[0056] As described above, the hydrolysis reaction temperature is an important
factor that influences the conversion of cellulose and the selectivity for the
production of
glucose. Although it is preferable that the hydrolysis reaction temperature be
low in
12
CA 02688691 2011-10-17
view of energy efficiency, the hydrolysis reaction temperature may be
determined in
consideration of the conversion of cellulose, the selectivity for the
production of glucose,
etc. in this way. It should be noted that the selectivity for the production
of the
saccharide produced by hydrolysis of cellulose can show. a behavior different
from that as
shown in FIG 3, depending on the reaction conditions etc.
[0057] As described above, it is possible to control the system so that the
cluster acid
catalyst is brought into a pseudo-molten state at a desired hydrolysis
reaction temperature,
by adding or removing water to or from the hydrolysis reaction system by the
method as
described above as needed.
[0058] In the hydrolysis step, however, one molecule of water per molecule of
glucose is needed when cellulose is hydrolyzed. Thus, in the case where the
amount of
water present in the reaction system is less than the sum of the amount of
water
corresponding to the amount of water of crystallization required to bring the
cluster acid
catalyst into a pseudo-molten state at the reaction temperature and the amount
of water
required to hydrolyze all the provided cellulose into glucose, when the water
of
crystallization contained in the cluster acid catalyst is used in hydrolysis
of cellulose, the
water of crystallization contained in the cluster acid catalyst decreases, and
the cluster
acid is therefore brought into a solid state. Accordingly, the catalytic
action of the
cluster acid catalyst to the hydrolysis of cellulose is impaired, and in
addition, viscosity
of the mixture of the plant fiber material and the cluster acid catalyst
increases, which can
result in insufficient mixing of the mixture.
[00591 When it is intended to maintain the catalyst activity and the function
as
reaction solvent of the cluster acid catalyst at the reaction temperature in a
hydrolysis
step (that is, to maintain the cluster acid catalyst in a pseudo-molten state
in the
hydrolysis step), the amount of water in the reaction system is set as
described below.
Specifically, the amount of water in the reaction system is set equal to or
greater than the
sum of the amount of water of crystallization required to bring all the
cluster acid catalyst
present in the reaction system into a pseudo-molten state at the reaction
temperature in
the hydrolysis step and the amount of water required to hydrolyze all the
cellulose
13
CA 02688691 2011-10-17
present in the reaction system into glucose.
100601 The water of crystallization required to bring all the cluster acid
catalyst into
a pseudo-molten state, herein, indicates the case where a portion of water
molecules are
present outside the crystal lattice as well as the case where the water of
crystallization
required to bring all the cluster acid catalyst into a pseudo-molten state at
the hydrolysis
temperature is present inside the crystal lattice. Although it is possible to
determine the
lower limit of the amount of water present in the reaction system in the
hydrolysis step
based on the above point, it is difficult to determine the upper limit thereof
because the
upper limit varies depending on the various conditions of the hydrolysis step.
Because
an excessive amount of water can cause increase in the amount of energy
required to
maintain the temperature of the reaction system, reduction in the chance of
reaction
between cellulose and the cluster acid catalyst, etc. with high probability,
the smaller the
amount of water in the hydrolysis step is, the better.
[00611 It should be noted that preparations may be made so that a desired
amount of
water of crystallization contained in the cluster acid catalyst is retained
even when the
relative humidity around the reaction system drops by heating. Specifically, a
method
can be used, for example, in which, in order that the atmosphere surrounding
the reaction
system reaches water vapor saturation at the predetermined reaction
temperature, the
inside of a closed reaction container is saturated with water vapor at the
hydrolysis
reaction temperature, the temperature in the reaction container is then
lowered with the
container being kept closed to condense the water vapor, and the condensed
water is
added to the plant fiber material and the cluster acid catalyst. When a wet
plant fiber
material is used, the amount of water contained in the plant fiber material is
taken into
consideration as the amount of water present in the reaction system, although
there is no
need to take this into consideration when a dried plant fiber material is
used.
[00621 In the hydrolysis step, when the amount of water in the reaction system
and
therefore the amount of water of crystallization contained in the cluster acid
catalyst
decrease, and therefore the cluster acid catalyst is brought into a solid
state and is reduced
in catalytic activity, the reduction in catalytic activity of the cluster acid
catalyst, for
14
CA 02688691 2011-10-17
example, may be avoided by raising the hydrolysis reaction temperature so that
the
cluster acid catalyst is brought into a pseudo-molten state.
[0063] The temperature in the hydrolysis step may be appropriately determined
in
consideration of some factors, such as reaction selectivity, energy efficiency
and
conversion of cellulose, as described above. In consideration of balancing of
energy
efficiency, conversion of cellulose and yield of glucose, the temperature may
be equal to
or below 140 C, in particular, equal to or below 120 C. Depending on the form
of the
plant fiber material, a low temperature condition, such as 100 C or below, may
be used.
In particular, in this case, it is possible to produce glucose with high-
energy efficiency.
[0064] There is no particular limit to pressure in the hydrolysis step.
Because
catalytic activity of the cluster acid catalyst to the hydrolysis of cellulose
is high, it is
possible to cause hydrolysis of cellulose to proceed efficiently even under
mild pressure
conditions, such as normal (atmospheric) pressure to 10 MPa.
[0065] The ratio between plant fiber material and a cluster acid catalyst
varies
depending on the characteristics, such as size, of the used plant fiber
material, and the
stirring method, the mixing method, etc. used in the hydrolysis step, for
example. Thus,
the ratio may be appropriately determined in consideration of the practical
conditions.
For example, the ratio (weight ratio), (cluster acid catalyst):(plant fiber
material), may be
within the range of 1:1 to 4:1, typically 1:1.
[0066] There is no particular limit to the duration of the hydrolysis step.
The
duration may be appropriately set in consideration of the form of the used
plant fiber
material, the ratio between the plant fiber material and the cluster acid
catalyst, the
catalytic performance of the cluster acid catalyst, reaction temperature,
reaction pressure,
etc. In the hydrolysis step, viscosity of the mixture containing the cluster
acid catalyst
and the plant fiber material is high, and an advantageous stirring method is
therefore one
in which a heated ball mill is used, for example. However, a common stirrer
may be
used.
[0067] Next, a second embodiment of the invention that relates to a method of
separating the saccharide, most of which is glucose, produced in the
hydrolysis step and
CA 02688691 2011-10-17
the cluster acid catalyst will be described. Specifically, a method will be
described in
which a plant fiber material containing cellulose or hemicellulose is
hydrolyzed using a
cluster acid catalyst to produce saccharide, most of which is glucose, and the
obtained
saccharide and the cluster acid catalyst are then separated.
[0068] Because the cluster acid catalyst and the produced saccharide are both
water-soluble, when a sufficient amount of water is present, the resultant
mixture
obtained after the hydrolysis step is obtained in a state where the residue of
the plant fiber
material (unreacted cellulose etc.) is included as the solid ingredient,
whereas the cluster
acid catalyst and the saccharide are both dissolved. Part of the saccharide
produced by
hydrolysis is precipitated as solids.
[0069] Studies conducted by the present inventors have revealed that a cluster
acid
catalyst exhibits solubility in the organic solvent in which the saccharide,
most of which
is glucose, is hardly dissolved or not dissolved. Thus, it is possible to
separate
saccharide and a cluster acid catalyst with the use of the organic solvent
that is a poor
solvent for saccharide and is a good solvent for cluster acid catalysts. For
example,
saccharide is precipitated by adding a sufficient amount of the above-
described organic
solvent to the mixture (hereinafter also referred to as the "hydrolysis
mixture") of the
cluster acid catalyst, saccharide and the residue obtained after the
hydrolysis step, to
bring the organic solvent and the hydrolysis mixture into contact with each
other,
whereby the saccharide and the residue of the plant fiber material, including
unreacted
cellulose, are separated as solids. Meanwhile, the cluster acid catalyst is
obtained in the
form of an organic solvent solution in which the cluster acid catalyst is
dissolved in the
organic solvent. Although most of the saccharide produced by the hydrolysis is
precipitated in a solid state, part of the saccharide is in a dissolved state.
By
precipitating the dissolved saccharide with the use of the organic solvent, it
is possible to
separate the dissolved saccharide along with the saccharide precipitated
during the
hydrolysis from the mixture, and it is therefore possible to improve the yield
of
saccharide.
[0070] The above-described organic solvent is not particularly limited as long
as the
16
CA 02688691 2011-10-17
organic solvent has dissolving characteristics such that the organic solvent
is a good
solvent for cluster acid catalysts and a poor solvent for saccharide. In order
to
efficiently precipitate saccharide, solubility of saccharide in the organic
solvent may be
equal to or less than 0.6 g/100 ml, or in particular equal to or less than
0.06 g/ 100ml. In
order to efficiently precipitate saccharide only, solubility of cluster acid
in the organic
solvent may be equal to or greater than 20g/100ml, or in particular equal to
or greater
than 40g/100ml.
[0071] Specifically, such organic solvents include alcohol, such as ethanol,
methanol
or n-propanol, ether, such as diethyl ether or diisopropyl ether, for example.
Alcohol
and ether can be used, and among others, ethanol and diethyl ether can be
used. Diethyl
ether is an optimum solvent for separating saccharide and a cluster acid
catalyst because
saccharide, such as glucose, is insoluble in diethyl ether, and cluster acid
is highly soluble
in diethyl ether. Meanwhile, ethanol is another optimum solvent because
saccharide,
such as glucose, is hardly soluble in ethanol, and cluster acid catalysts are
highly soluble
in ethanol. Diethyl ether is advantageous as compared to ethanol in view of
distillation.
Ethanol is advantageous in that availability of ethanol is higher than that of
diethyl ether.
[0072] The amount of usage of the organic solvent varies depending on the
dissolving characteristics of the organic solvent with respect to saccharide
and cluster
acid catalysts, and the amount of water contained in the hydrolysis mixture.
Thus, the
amount of usage of the organic solvent may be determined appropriately so that
it is
possible to efficiently precipitate the produced saccharide without waste,
that it is
possible to efficiently collect cluster acid, and that it is possible to
dissolve the cluster
acid catalyst contained in part of the saccharide that is solidified, by
breaking the
solidified saccharide.
[0073] Temperature in the separation step may be within the range between room
temperature and 60 C, although depending on the boiling point of the organic
solvent.
In the separation step, there is no particular limit to the method of bringing
the hydrolysis
mixture and the organic solvent into contact with each other, more
specifically, the
method of adding the organic solvent to the hydrolysis mixture and the method
of stirring
17
CA 02688691 2011-10-17
the hydrolysis mixture and the organic solvent, for example; a commonly used
method
may be used. In view of the efficiency in collecting the cluster acid, a
preferable
stirring method is one in which stirring and/or breaking are performed using a
ball mill or
the like.
[0074] In addition, the present inventors have found that, when the cluster
acid
catalyst contains a large amount of water of crystallization (the amount of
water of
crystallization is greater than the normal water-of-crystallization amount,
for example) in
the separation step, the excessive water is not coordinated to the cluster
acid and mixed
into the organic solvent, and saccharide, which is the product, is dissolved
in the water
mixed into the organic solvent. When water is mixed into the organic solvent
in which
the cluster acid catalyst is dissolved, and saccharide is dissolved in this
water, the yield of
saccharide is reduced.
[00751 Thus, in order to minimize reduction in the yield of saccharide, the
total
amount of water of crystallization contained in all the cluster acid catalyst
present in the
reaction system may be equal to or less than the normal water-of-
crystallization amount
in the above-described separation step. By the experiments conducted by the
inventors,
it has been confirmed that saccharide, most of which is glucose, is prevented
from being
dissolved in the water that is not coordinated to the cluster acid and mixed
into the
organic solvent when the amount of water of crystallization contained in the
cluster acid
catalyst present in the reaction system is equal to or less than the normal
water-of-crystallization amount (see FIG 4). "The water of crystallization
contained in
the cluster acid catalyst present in the reaction system is equal to or less
than the normal
water-of-crystallization amount" herein means that the amount of water of
crystallization
contained in the cluster acid catalyst is equal to or less than the normal
water-of-crystallization amount when the water present in the reaction system
in the
separation step is evenly taken in by all the cluster acid catalyst as water
of
crystallization.
[0076] Examples of the method of controlling the amount of water present in
the
reaction system in the separation step include a method in which the water in
the
18
CA 02688691 2011-10-17
hydrolysis mixture is evaporated by releasing the closed state of the reaction
system and
heating the reaction system, and a method in which a desiccant agent or the
like is added
to the hydrolysis mixture to remove the water in the hydrolysis mixture. When
the
above-described evaporation method is used, it is possible to use the
afterheat due to the
reaction temperature in the hydrolysis step, which results in excellent energy
efficiency,
and in addition, the step of separating the desiccant agent, or the like, is
not needed.
[00771 Thus, in the separation step, the smaller the amount of water of
crystallization
contained in the cluster acid catalyst is, the better, and the optimum
water-of-crystallization amount can differ from that in the hydrolysis step
that requires
hydrolysis reaction temperature, conversion, selectivity for a product, etc.
to be taken into
consideration. Accordingly, the amount of water of crystallization contained
in the
cluster acid catalyst may be regulated prior to the hydrolysis step in
consideration of the
efficiency in separating saccharide and a cluster acid catalyst in the
separation step, or the
amount of water of crystallization contained in the cluster acid catalyst may
be controlled
as needed between the hydrolysis step and the separation step as described
above.
[0078) In the separation step, a precipitate of saccharide is obtained as
solids along
with the residue of the plant fiber material etc., and at the same time, an
organic solvent
solution in which a cluster acid catalyst is dissolved is obtained. This is
separated into
solids and an organic solvent solution by a certain method, such as filtering.
Solids
containing saccharide can be further separated into an aqueous solution of
saccharide and
solids such as residues by adding water, in which solubility of saccharide in
water and
insolubility of the residues in water are used. On the other hand, the organic
solvent
solution containing the cluster acid catalyst can be separated into the
cluster acid catalyst
and organic solvent by a commonly used separation method, such as evaporation.
Thus,
the cluster acid catalyst can be separated from the product, the residues,
etc. and collected
after being used as the catalyst for hydrolyzing cellulose, and in addition,
it is also
possible to reuse the cluster acid catalyst as the catalyst for hydrolyzing
plant fiber
material containing cellulose.
[00791 Assume that, in the separation step, an excessive amount of water of
19
CA 02688691 2011-10-17
crystallization contained in the cluster acid catalyst is mixed into the
organic solvent,
saccharide is dissolved in the water, and the saccharide is transferred to the
organic
solvent phase along with the cluster acid catalyst. In this case, it is
possible to
precipitate the saccharide in the organic solvent solution by reducing the
amount of water
in the organic solvent solution of cluster acid in which the cluster acid
catalyst is
dissolved. Specifically, the cluster acid catalyst may be dehydrated so that
all the
cluster acid catalyst dissolved in the organic solvent solution contains water
of
crystallization whose amount is equal to or less than the normal water-of-
crystallization
amount. This is because it is possible to prevent the saccharide, most of
which is
glucose, from being dissolved in the water, which includes the water molecules
that
cannot be coordinated to the cluster acid catalyst, mixed into the organic
solvent when
the amount of water of crystallization contained in the cluster acid catalyst
is equal to or
less than the normal water-of-crystallization amount as described above.
[0080] There is no particular limit to the method of dehydrating the cluster
acid
catalyst contained in an organic solvent solution, and examples thereof
include a method
in which an appropriate amount of desiccant agent, such as anhydrous calcium
chloride
or silica gel, is added to the organic solvent solution. When such a desiccant
agent is
used, however, another step of removing the desiccant agent is required.
[0081] As another example, there is a method in which the cluster acid
catalyst
whose content rate of water of crystallization ((the amount of water of
crystallization)/(the normal water-of-crystallization amount)xI00%) is equal
to or less
than 70%, in particular, equal to or less than 30%, is used as the desiccant
agent. It is
possible to reduce the amount of water of crystallization contained in the
cluster acid
catalyst below the normal water-of-crystallization amount by adding the dry-
state cluster
acid catalyst to increase the amount of the cluster acid catalyst contained in
the organic
solvent solution. In addition, the cluster acid catalyst used as the desiccant
agent can be
separated and collected along with the cluster acid catalyst used as the
hydrolysis catalyst.
The saccharide in the organic solvent solution that is precipitated by
dehydration can be
separated from the organic solvent solution and collected by a commonly used
method,
CA 02688691 2011-10-17
such as decantation or filtering.
[00821 A method using the difference between a cluster acid catalyst and
saccharide
in their solubilities in solvents has been mainly described as an example of
the method of
separating a cluster acid catalyst and saccharide. However, because there is a
difference
between molecule sizes (heteropoly acid, which is a representative example of
cluster
acid catalysts, has a diameter of about 2 nm, and glucose has a diameter of
about 0.7 nm),
it is also possible to use the molecular sieving effects of porous material,
such as MFI
zeolite and 1 zeolite, which have ten-membered oxygen rings, and mordenite,
which has
twelve-membered oxygen rings.
[00831 In the experiments described below, measurement of D-(+)-glucose and
D-(-)-glucose was conducted by the post-label fluorescent detection method
using
high-performance liquid chromatography (HPCL).
[00841 An experiment concerning the relation between the apparent melting
temperature and the content of water of crystallization in cluster acid
(heteropoly acid)
will be described. The apparent melting temperatures of phosphotungstic acids
(H3[PW12040]=nH2O), which have different contents of water of crystallization,
were
visually studied while heating. The results are shown in FIG 2. The content of
water
of crystallization in phosphotungstic acid was regulated by drying X (the
content rate of
water of crystallization is 75%) and Y (the content rate of water of
crystallization is
100%) by heating these materials, or by dropping water thereon. The content
rate of
water of crystallization is assumed to be 100% when the number of molecules of
water of
crystallization is 30 (n=30). As shown in FIG. 2, it has been found that the
higher the
content of water of crystallization in heteropoly acid is, the lower the
apparent melting
temperature (pseudo-melting temperature) of the heteropoly acid is.
[00851 (Example 1) As described below, the conversion of cellulose and the
selectivity for the production of glucose were measured at some hydrolysis
reaction
temperatures (temperatures of pseudo-molten material: 50 C, 60 C, 70 C, 80 C,
90 C).
First, 1 kg of phosphotungstic acid (the content rate of water of
crystallization was 160%;
the diameter was about 2 nm) and 0.5 kg (dry weight) of cellulose were mixed,
put in a
21
CA 02688691 2011-10-17
closed container (located on a hot plate), and heated. The phosphotungstic
acid was
brought into a pseudo-molten, stirrable state around 40 C. Then, the mixture
was
heated to the respective temperatures (50 C, 60 C, 70 C, 80 C, 90 C) and was
then
stirred and subjected to hydrolysis reaction for three hours.
[0086] After the temperature was dropped to room temperature, 3 liters of
ethanol
was added to the mixture, in the closed container, of phosphotungstic acid
that was
brought from the pseudo-molten state to a solid state, saccharide, most of
which was
glucose, that was produced by hydrolysis of cellulose, and fiber (including
transformed
material), such as lignin, and the mixture was then stirred for 30 minutes.
Although the
phosphotungstic acid was dissolved in the added ethanol, the saccharide was
not
dissolved in the ethanol and obtained as a precipitate along with the fiber.
[0087] The precipitated saccharide and fiber were filtered to separate an
ethanol
solution and a precipitate (saccharide and fiber). Then, 1.5 liters of
distilled water was
added to the precipitate and stirred for 30 minutes to dissolve saccharide,
and the
resultant solution was again filtered to separate an aqueous solution of
saccharide in
which saccharide was dissolved and fiber (unreacted cellulose). On the other
hand, the
ethanol solution was distilled to separate ethanol and phosphotungstic acid.
[0088] The conversion R and the yield rl of glucose at respective hydrolysis
reaction
temperatures are shown in FIG 3. As can be seen from FIG 3, the conversion of
cellulose increases as the reaction temperature increases. On the other hand,
although
the yield of glucose increases from 50 C to 60 C as in the case of the
conversion of
cellulose, the yield reaches the peak at 70 C and decreases with temperature.
Thus, it
has been found that, under the conditions of this experiment, glucose is
produced with
high selectivity between 50 and 60 C, whereas reactions, other than glucose
producing
reaction, proceed between 70 and 90 C. It is conceivable that this result
varies
depending on the form of the reactor and ways of operation, etc., and it can
be said that
optimization of the used apparatuses is also important in order to obtain high
yield and
selectivity.
[0089] (Example 2) Bagasse was pulverized by a pulverizer into powder whose
22
CA 02688691 2011-10-17
particle size was about ten-odd microns, and 0.3 kg (dry weight) of this
powder and 1 kg
of phosphotungstic acid (the content of water of crystallization was unknown;
the
diameter was about 2 nm) were mixed, put in a closed container, and heated.
The
phosphotungstic acid was brought into a pseudo-molten, stirrable state around
40 C.
The mixture was heated to about 50 C and then stirred for three hours.
[0090] After the temperature was dropped to room temperature, 3 liters of
ethanol
was added to the mixture A, in the closed container, of phosphotungstic acid
that was
brought from the pseudo-molten state to a solid state, saccharide, most of
which was
glucose, that was produced by hydrolysis of cellulose, and fiber (including
transformed
material), such as lignin, and the mixture A was then stirred for 30 minutes.
Although
the phosphotungstic acid was dissolved in the added ethanol, the saccharide
was not
dissolved in the ethanol and obtained as a precipitate along with the fiber.
[00911 The precipitated saccharide and the fiber were filtered to separate an
ethanol
solution and a precipitate (saccharide and fiber). Then, 1 liter of distilled
water was
added to the precipitate and stirred for 30 minutes to dissolve saccharide,
and the
resultant solution was again filtered to separate an aqueous solution of
saccharide in
which saccharide was dissolved and fiber (unreacted cellulose). On the other
hand, the
ethanol solution was distilled to separate ethanol and phosphotungstic acid.
The yield
of glucose was 0.20 kg, and that of xylose was 0.06 kg.
[0092] (Example 3) Wood chips were crushed, subjected to a steam process for
two
hours, and then pulverized by a pulverizer into powder whose particle size was
about
ten-odd microns, and 0.3 kg (dry weight) of this powder and about 1 kg of
phosphotungstic acid (the content of water of crystallization was unknown)
were mixed,
put in a closed container, and heated. The phosphotungstic acid was brought
into a
pseudo-molten, stirrable state around 40 C. The mixture was heated to about 70
C and
then stirred for three hours. Thereafter, in a way similar to that used in
Example 2,
phosphotungstic acid was collected, and the produced saccharide and the
unreacted
cellulose were separated. The yield of glucose was 0.21 kg, and that of xylose
was 0.07
kg.
23
CA 02688691 2011-10-17
[0093] (Example 4) As in the case of Japanese Patent Application Publication
No.
2001-240411 (JP-A-2001-240411), a porous alumina tube with a mordenite
membrane
formed on the outer side thereof was prepared. A mixture A obtained in a way
similar to
that used in Example 2 was diluted with 1 liter of distilled water, led into
the tube, and
held for an hour with a pressure of 2 MPa applied in the tube. While this was
performed, the tube was immersed in 1 liter of distilled water.
[0094] An hour later, the water in which the tube was immersed was sampled,
and
subjected to high-performance liquid chromatography (HPLC). As a result, it
was
confirmed that D-(+)-glucose and D-(+)-xylose were contained in the water.
Meanwhile,
the liquid in the tube was sampled and analyzed by HPLC. As a result, it was
confirmed that the concentration of saccharide had dropped. Until the
concentration of
saccharide (glucose and xylose) in the liquid in the tube had dropped to one-
tenth of the
initial concentration, the above process was repeated. Meanwhile, the
phosphotungstic
acid left in the water in the tube was collected as solid phosphotungstic
acid.
[0095] (Example 5) First, mixtures were prepared in which phosphotungstic
acids
with various contents of water of crystallization (see FIG 4) and glucose were
mixed in
the ratio of 2:1 ((phosphotungstic acid):(glucose) (weight ratio)). For the
phosphotungstic acid whose content rate of water of crystallization was equal
to or
greater than 100%, the content of water of crystallization contained in
phosphotungstic
acid was regulated by adding an appropriate amount of water to the mixture as
needed so
that the phosphotungstic acid in the mixture had a desired content of water of
crystallization, after mixing the phosphotungstic acid and glucose. On the
other hand,
for the phosphotungstic acid whose content rate of water of crystallization
was less than
100%, phosphotungstic acid was heated and dehydrated in advance. The amount of
water contained in the phosphotungstic acid obtained after dehydration was
measured by
TGA (thermogravimetric analysis). Next, dehydrated ethanol was added to the
mixture
of phosphotungstic acid and glucose with the weight ratio of ethanol to
phosphotungstic
acid being 100/30. After the mixture was well stirred and mixed, solids,
including
precipitated glucose, were separated to obtain an ethanol solution. The amount
of
24
CA 02688691 2011-10-17
glucose in this ethanol solution was analyzed and measured by the post-label
fluorescent
detection method using HPCL, to calculate a glucose loss that indicates the
ratio of the
amount of glucose that was left in the ethanol solution and could not be
separated. The
results are shown in FIG 4.
[0096] FIG. 4 shows that when the content rate of water of crystallization
contained
in phosphotungstic acid is equal to or less than 100%, the glucose loss ratio
with respect
to the phosphotungstic acid is almost zero. Specifically, by making the amount
of water
of crystallization contained in the cluster acid catalyst equal to or less
than the normal
water-of-crystallization amount, it is possible to minimize reduction in the
yield of
saccharide that is caused by dissolution of saccharide in water that is not
coordinated to
cluster acid and mixed into organic solvent when cluster acid and saccharide
are
separated by precipitating saccharide with the use of organic solvent.
[0097] (Example 6) Distilled water was put in a closed container in advance,
and
temperature of the distilled water was raised to a predetermined reaction
temperature
(60 C) to saturate the inside of the container with water vapor and cause
water vapor to
attach to the inner side of the container. Next, 1 kg of phosphotungstic acid
whose
content of water of crystallization had been measured in advance and 0.5 kg
(dry weight)
of cellulose were mixed and put in the closed container. In addition,
distilled water
(55.6 g) was added whose amount was equal to the amount by which the water in
the
reaction system is short of the sum of the amount of water (158 g) that is
required to
bring phosphotungstic acid into a pseudo-molten state at the reaction
temperature of 60 C
and the amount of water (55.6 g) that is required to hydrolyze cellulose into
glucose.
[0098] When the closed container was then heated, the phosphotungstic acid was
brought into a pseudo-molten state from around 40 C, and was brought into a
state in
which the mixture in the container could be stirred, around 50 C. The mixture
was
further heated to 60 C, and stirred for 1.5 hours at 60 C. Heating was then
stopped and
the mixture was cooled to around 40 C. Thereafter, 6 liters of ethanol was
added, the
mixture was stirred for 60 minutes to dissolve the phosphotungstic acid in the
ethanol,
and saccharide was precipitated along with fiber (unreacted cellulose).
CA 02688691 2011-10-17
[0099] Next, the precipitate was filtered, 1 liter of distilled water was
added to the
separated precipitate, and the mixture was stirred for 15 minutes to dissolve
saccharide.
The mixture was further filtered to separate an aqueous solution of saccharide
and fiber.
On the other hand, the ethanol solution was distilled to separate ethanol and
phosphotungstic acid. The conversion R was 67%, and the yield r) of glucose
was 60%.
[0100] (Example 7) In a closed container, 1 kg of phosphotungstic acid whose
content rate of water of crystallization was 100% shown by Y in FIG 2 and 0.5
kg (dry
weight) of cellulose were mixed, and distilled water (55.6 g) was added so
that the water
required to hydrolyze 0.5 kg of cellulose into glucose existed. When this
mixture was
heated, the phosphotungstic acid was brought into a pseudo-molten state around
50 C,
and was brought into a state in which the mixture could be stirred, around 60
C. The
mixture was further stirred for 1.5 hours with the mixture being maintained at
60 C.
[0101] Thereafter, in a way similar to that used in Example 6, phosphotungstic
acid
was collected, and the produced saccharide and the unreacted cellulose were
separated.
The conversion R was 68%, and the yield rl of glucose was 63%.
[0102] (Example 8) In a closed container, 1 kg of phosphotungstic acid whose
content rate of water of crystallization was 75% shown by X in FIG 2 and 0.5
kg (dry
weight) of cellulose were mixed, and distilled water (55.6 g) was added so
that the water
required to hydrolyze 0.5 kg of cellulose into glucose existed. When this
mixture was
heated, the phosphotungstic acid was not brought into a pseudo-molten state
even when
the mixture was heated to 50 C. The mixture was gradually brought into a
pseudo-molten state around 80 C, and was brought into a state in which the
mixture
could be stirred, at 90 C. The mixture was further stirred for 1.5 hours with
the mixture
being maintained at 90 C.
[0103] Thereafter, in a way similar to that used in Example 6, phosphotungstic
acid
was collected, and the produced saccharide and the unreacted cellulose were
separated.
The conversion R was 96%, and the yield rl of glucose was 72%. The result of
calculating the yield of xylose was 7%. Although a very high conversion of 96%
was
obtained, the loss was 28% in producing glucose that was the desired
substance. This
26
CA 02688691 2011-10-17
result shows that the amount of water of crystallization contained in the
phosphotungstic
acid used in Example 8 was less than that in the phosphotungstic acid used in
Example 7,
and it is necessary to set the reaction temperature higher than that of
Example 5 in order
to bring the phosphotungstic acid into a pseudo-molten state, and shows that
for this
reason, although the conversion was high, selectivity for the production of
glucose
through hydrolysis dropped and the amount of other by-products produced
increased.
[01041 (Example 9) An experiment was conducted according to a chart shown in
FIG
5. Specifically, in a way similar to that used in Example 6, a mixture was
prepared by
stirring cellulose, phosphotungstic acid and distilled water in a closed
container at 60 C
for 1.5 hours. Thereafter, the closed container was opened with the
temperature being
maintained at 60 C to drive off the water in the container. The temperature
was
maintained at 60 C for a while even after liquid in the container was
solidified, and then
heating was stopped. Thereafter, in a way similar to that used in Example 6,
phosphotungstic acid was collected, and the produced saccharide and the
unreacted
cellulose were separated. The conversion R was 67%, and the yield rl of
glucose was
67%. That is, almost 100% of the produced glucose was collected.
[01051 This result shows that it is possible to prevent glucose from being
dissolved
in the water that is not coordinated to phosphotungstic acid and mixed into
ethanol and
thus improve the yield of glucose, by removing the water in the reaction
system to reduce
the amount of water of crystallization contained in the phosphotungstic acid
below the
normal water-of-crystallization amount before the step of separating the
phosphotungstic
acid from saccharide and fiber by adding ethanol.
[01061 (Example 10) In a way similar to that used in Example 4, a mixture was
prepared by stirring cellulose, phosphotungstic acid and distilled water in a
closed
container at 60 C for 1.5 hours. Thereafter, a predetermined amount (3
liters) of
ethanol was added with the temperature being maintained at 60 C, and the
mixture was
stirred for 30 minutes. Subsequently, the temperature was decreased to around
room
temperature and a desiccant agent (anhydrous calcium chloride particles)
packed in a bag
was added to remove water in the container. Glucose powder was precipitated,
and
27
CA 02688691 2011-10-17
phosphotungstic acid was kept dissolved in ethanol. In a way similar to that
used in
Example 6, phosphotungstic acid and saccharide were separated. The conversion
R was
67%, and the yield of glucose was 67%. Almost 100% of the produced glucose was
collected.
10107] As in the case of the above-described Example 9, this result shows that
it is
possible to prevent glucose from being dissolved in the water that is not
coordinated to
phosphotungstic acid and mixed into ethanol and thus improve the yield of
glucose, by
removing the water in the reaction system to reduce the amount of water of
crystallization contained in the phosphotungstic acid below the normal
water-of-crystallization amount before the step of separating the
phosphotungstic acid
from saccharide and fiber by adding ethanol. The water in the reaction system
was
evaporated using the afterheat due to the hydrolysis of cellulose in the above-
described
Example 9, whereas the amount of water in the reaction system was regulated by
adding
a desiccant agent and allowing the desiccant agent to absorb water in Example
10.
28