Note: Descriptions are shown in the official language in which they were submitted.
CA 02689136 2009-11-27
WO 2008/150085 PCT/KR2008/003078
1
3',4',5-TRIMETHOXY FLAVONE DERIVATIVES AS STIMULANT OF MUCUS
SECRETION, METHOD OF THE SAME, AND PHARMACEUTICAL
COMPOSITION COMPRISING THE SAME
TECHNICAL FIELD
The present invention relates to a 3',4',5-trimethoxy flavone derivative and a
pharmaceutically acceptable salt thereof, preparation thereof, and a
pharmaceutical
composition for the treatment and prevention of dry eye syndrome comprising
the same'as an
active ingredient.
BACKGROUND ART
Dry eye syndrome is a common clinical condition characterized by deficient
tear
production or excessive tear evaporation, which may be caused by a variety of
causative
factors. For exainple, lacrimal gland inflammation (dacryoadenitis) and
corneal denervation
may curb tear production, whereas meibomian gland dysfunction and eyelid
disorders such as
incomplete lid closure are frequently to blame for rapid tear evaporation.
Fl.u-tlier, T-cell-
mediated inflammatory responses were reported responsible for the pathogenesis
of dry eye
syndrome (Eye Contact Lens, 29(1 Suppl):S96-100, 2003; and Ophthalmologe,
103:9-17,
2006).
A tear film continuously secretes a given amount of tears which will not only
provide
sterilizing effects, but also participate in smooth covering and lubrication
of ocular surfaces to
play an important role in maintenance of one's eyesight. Three main layers
make up the tear
film. Specifically, the innermost layer is a layer of mucin produced by
conjunctival goblet cells,
the middle layer is an aqueous layer secreted by the lacrimal gland, and the
most superficial
layer is a very thin layer of lipids (fats or oils) secreted by the meibomian
gland.
Usually, patients with dry eye syndrome may experience burning and stinging,
grittiness
or foreign-body sensation, itching, redness, and the other symptoms of ocular
discomfort. In
severe cases, vision may be substantially impaired. At first, dry eye syndrome
was recognized
as a characteristic sign of aging which is common among women of post-
menopausal age.
With recent increases in TV watching, use of computers, and wearing of contact
lens, this
condition becomes frequent in both men and women. Further, the onset age of
dry eye
syndrome is gradually decreasing (Gynecol Endocrinol, 20:289-98, 2005; and
Surv
CA 02689136 2009-11-27
WO 2008/150085 PCT/KR2008/003078
2
Ophthalmol, 50:253-62, 2005).
As approaches to remedy dry eye syndrome, mention may be made of instillation
of
artificial tears for artificial tear supplementation, instillation of
steroidal anti-inflammatory eye
drops to inhibit inflammatory responses, therapeutic contact lens (TCL) wear,
surgical
occlusion of the punctum to suppress tear escape from one's eye to result in
prolonged ocular
retention of artificial tear solutions or substitutes, and the like (J Korean
Ophthalmol Soc,
46:1774-1779, 2005). These therapeutic approaches have been widely used up to
recently, but
pose a variety of potential disadvantages and problems. For example,
artificial tear
preparations merely provide temporary and short-term effects, thus suffering
from
disadvantages such as the need for several daily applications of the tear
preparations and no
protective effects against corneal damage, whereas steroid preparations may
cause the risk of
irreversible side effects such as glaucoma, upon chronic administration of the
steroid drug. In
addition, therapeutic contact lens may provide inconvenience to users who are
unfamiliar with
wearing of contact lens, and may also be a potential source of bacterial
infections. Further, the
punctual occlusion surgery still suffers from disadvantages such as mental
rejection feelings
due to the surgical operation, and difficulty to restore the former state upon
the occurrence of
adverse side effects. However, the most glaring weakness of the aforementioned
conventional
remedies is in that they are merely symptomatic therapies, which are not
focused to treat or
address the root causes of dry eye conditions.
In 2006, the US company Allergan, Inc. developed and released Restasis
(cyclosporine
ophthalmic emulsion) which is a therapeutic agent for the treatment of dry eye
syndrome using
the immunomodulator cyclosporine. Restasis has recently been reported to
inhibit the
production and activation of immunocytes associated with the occurrence of
keratoconjunctivitis sicca and to increase the tear secretion level
(Ophthalmology, 107:967-74,
2000; and Ophthalmology, 107:631-9, 2000)e Restasis exerts drug efficacy
thereof via anti-
inflammatory action, so long-term repeated drug administration of several
months is
unfortunately required to achieve therapeutic effects that are satisfactory to
patients. Further,
administration of Restasis is disadvantageously accompanied by relatively high
frequency of
occurrence (17%) of a typical side effect, e.g. burning sensation
(Ophthalmology, 107:631-9,
2000; and Thomson Pharma, www.thomson-pharma.com).
To this end, there is an urgent need for development of a therapeutic agent
which is not a
symptomatic therapeutic merely palliating symptoms of the coiicerned condition
and is
CA 02689136 2009-11-27
WO 2008/150085 PCT/KR2008/003078
3
capable of treating the root causes of dry eye syndrome while securing safety
of drug
medications due to low manifestation of adverse side effects.
Dry eye syndrome is a multifactorial disease which is caused by diverse
pathogenic
causes as discussed hereinbefore, and a variety of approaches have been
attempted to treat
such a condition. Inter alia, a great deal of research has been actively
focused on lacrimal
secretion stimulants, i. e. tear stimulants; For example, attempts have been
made to develop a
drug that stimulates lacrimation (tear secretion) of lacrimal acinar cells
through the medium of
cholinergic neurotransmission or increases lacrimal flow of the conjunctiva
via stimulation of
purinergic receptors (Arthritis Rheum, 46:748m54, 2002; and Curr Eye Res,
21:782-7, 2000).
In particular, an ocular mucin layer lowers the surface tension of water to
allow unifonn
distribution of water throughout the ocular surface and plays an important
role to provide
corneal protection against a hostile external environment, such as ocular
damage or infection
by foreign materials or pathogenic agents. Therefore, many extensive animal-
based preclinical
and human-based clinical studies have been actively undertaken to find drugs
that stimulate
secretion of mucins from conjunctival goblet cells (Exp Eye Res, 67:341-6,
1998; Cornea,
21:818-24, 2002; Cornea, 23:613-9, 2004; and Thomson Pharma, www.thomson-
pharma.com).
Mucus is composed mainly of mucin and inorganic salts. Mucin consists of
carbohydrates and proteins and is responsible for protection of mucosal
epithelial cells and
lubricating action. To date, 21 different human mucin genes have been
identified. Ocular
mucin genes include 9 classes of genes, designated MUCl, MUC2, MUC4, MUC5AC,
MUC7,
MUC 13, MUC 15, MUC 16 and MUC 17; which may be further subdivided into
transmembrane
mucin and secretory mucin (Prog Retin Eye Res, 23:449-74, 2004). Substances
having
stimulatory activity on secretion of transmembrane and secretory mucins in
tear films can be
therapeutically effective for the treatment of dry eye syndrome by prevention
of corneal
damage which may arise from excessive eye dryness.
As a result of a variety of extensive and intensive studies and experiments to
solve the
problems as described above and to cope with the need for development of an
effective
therapeutic agent for the treatment of dry eye syndrome, the inventors of the
present invention
succeeded in synthesis of novel 3',4';5-trimethoxy flavone derivatives and
pharmaceutically
acceptable salts thereof and discovered that these compounds exhibit excellent
effects on
stimulation of conjunctival mucus secretion and inhibition of ocular surface
damage. Further,
the present inventors discovered that 7-carboxymethyloxy-3',4',5-trimethoxy
flavone
CA 02689136 2009-11-27
WO 2008/150085 PCT/KR2008/003078
4
monohydrates, previously studied in Korean Patent Nos. 447918, 327621 and
644928 assigned
to the present applicant, have anti-inflammatory activity, gastric mucus
secretion-stimulating
activity and conjunctival mucus secretion-promoting activity, thereby
providing pronounced
inhibitory effects on the occurrence of ocular surface damage. The present
invention has been
completed based on these findings.
DISCLOSURE OF THE INVENTION
TECHNICAL PROBLEM
It is an object of the present invention to provide a novel 3',4',5-trimethoxy
flavone
derivative or a pharmaceutically acceptable salt thereof.
It is another object of the present invention to provide a pharmaceutical
composition for
the treatment and prevention of dry eye syndrome, comprising a 3',4',5-
trimethoxy flavone
derivative or a pharmaceutically acceptable salt thereof as an active
ingredient.
It is yet another object of the present invention to provide a pharmaceutical
composition for the treatment and prevention of dry eye syndrome comprising 7-
carboxymethyloxy-3',4',5-trimethoxy flavone monohydrate as an active
ingredient.
TECHNICAL SOLUTION
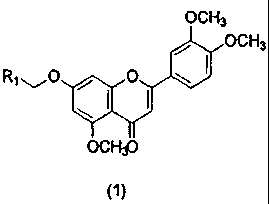
In accordance with an aspect of the present invention, the above and other
objects can
be accomplished by the provision of a novel 3',4',5-trimethoxy flavone
derivative represented
by Formula 1:
OCH3
ACH3
Ryl~_O , O
OCH3J
(1}
wherein Rl is selected from the group consisting of tetrazolyl, carbamoyl,
cyanocarbamoyl, N-benzenesulfonylcarbamoyl, hydrophosphoryl,
hydroxyisopropylphosphoryl, aminosulfonylcarbamoyl, methylsulfonylcarbamoyl,
carbamylcarbamoyl, formylcarbamoyl and acetylcarbamoyl; or a pharmaceutically
acceptable
CA 02689136 2009-11-27
WO 2008/150085 PCT/KR2008/003078
salt thereof,
In accordance with another aspect of the present invention, there is provided
a
pharmaceutical composition for the treatment and prevention of dry eye
syndrome comprising
the aforesaid compound of Formula 1 asan active ingredient.
5 The compound of Formula 1 in accordance with the present invention may form
a salt
with a basic alkali metal such as Na+, K+, Ca2+, Mg2+; Zn2+, or the like.
Hereinafter, the present invention will be described in more detaiL
The present invention provides a novel 3',4',5-trimethoxy flavone derivative
of Formula
1 or a pharmaceutically acceptable salt thereof.
Specifically, the present invention provides a compound selected from the
group
consisting of:
7-(tetrazol-5-ylmethyloxy)-3',4',5-trimethoxy flavone,
7-(tetrazol-5-ylmethyloxy)-3',4',5-trimethoxy flavone potassium salt,
7-{(carbamoyl)methyloxy}-3',4',5-trimethoxy flavone,
7-{(N-cyanocarbamoyl)-methyloxy}-3',4',5-trimethoxy flavone,
7-{(N-bezenesulfonylcarbamoyl)methyloxy}-3',4',5-trimethoxy flavone,
7-{(hydrophosphoryl)-methyloxy}-3',4',5-trimethoxy flavone,
7-{(hydroxyisopropylphosphoryl)-methyloxy}-3',4',5-trimethoxy flavone,
7-{(aminosulfonylcarbamoyl)methyloxy}-3',4',5vtrimethoxy flavone,
7- { (methylsulfonylcarbamoyl)methyloxy} -3',4',5-trimethoxy flavone,
7-{ (carbamylcarbamoyl)methyloxy}-3',4',5-trimethoxy flavone,
7-{(formylcarbamoyl)methyloxy}-3',4',5-trimethoxy flavone, and
7-{(acetylcarbamoyl)methyloxy}-3',4',5-trimethoxy flavone.
In the present invention, the compound of Formula 1 wherein Rl is selected
from the
group consisting of carbamoyl, cyanocarbamoyl, N-benzenesulfonylcarbamoyl,
aminosulfonylcarbamoyl, methylsulfonylcarbamoyl, carbamylcarbamoyl,
formylcarbamoyl
and acetylcarbamoyl (hereinafter, referred to as "compound of Formula 1-1 "')
may be
prepared by condensation of a 7-carboxymethyloxy-3',4',5-trimethoxy flavone
anhydride of
Formula 2 with RzNHz wherein R2 is as defined below.
CA 02689136 2009-11-27
WO 2008/150085 PCT/KR2008/003078
6
O OCH3
R2.N~ OCH3
H 0
oCH30 (1 1,~
wherein R2 is hydrogen, cyano, benzenesulfonyl, aminosulfonyl, methylsulfonyl,
carbamyl, formyl or acetyl.
0 OCH3
HO~ OCIi3
0 O
OCH30
(2)
That is, the compound of Formula 1 1' in accordance with the present invention
may be
prepared by condensation of the 7-carboxymethyloxy-3',4',5-trimethoxy flavone
anhydride of
Formula 2 with R2NH2. The condensation may be carried out by dehydration with
direct use of
dicyclohexylcarbodiimide (DCC), diisopropylcarbodiimide (DIC) or 1-[3-
(dimethylamino)propyl]-3-ethylcarbodiimide (EDC), or otherwise may be carried
out by
rendering a carboxyl group of the compound of Formula 2 into a highly reactive
form such as
an acid anhydride or an acid chloride, followed by reaction with R2NH2. The
reaction
temperature is preferably in a range of 0 C to 100 C: The compound of Formula
1-1' may form
a salt with a basic alkali metal, for example Na+, K+, Ca?+, Mg2+, Zn2+, or
the like.
Out of the compounds of Formula 1, the present invention further provides 7-
(tetrazol-
5-ylmethyloxy)-3',4',5-trimethoxy flavone of Formula 1-2' wherein Rl is
tetrazolyl and a
pharmaceutically acceptable salt thereof.
The compound of Formula 1-2' is prepared by regioselective alkylation of the 7-
hydroxy
position in a 5,7-dihydroxy-3',4'-dimethoxy flavone of Formula 3 as a starting
material to
prepare a compound of Formula 4, methylation of the 5-hydroxy position in the
compound of
Formula 4 with a base and a methylating reagent in the presence of an aprotic
solvent to
prepare a compound of Formula 5, and cyclization of the compound of Formula 5
with sodium
azide in the presence of an aprotic solvent such as dimethylformamide to
thereby prepare a 7-
CA 02689136 2009-11-27
WO 2008/150085 PCT/KR2008/003078
7
(tetrazol-5-ylmethyloxy)-3';4',5-trimethoxy flavone of Formula 1-2'. The
methylating reagent
used to prepare the compound of Formula 5 may be methane iodide or dimethyl
sulfate. The
reaction temperature is preferably in a range of 0 C to 150 C. Further, the
base may be
selected from the group consisting of potassium carbonate, sodium hydroxide,
potassium
hydroxide and sodium carbonate.
There is no particular limit to the aprotic solvent, so long as it is known to
those skilled
in the art. For example, mention may be made of 1,4-dioxane, tetrahydrofuran,
ethyl acetate,
ethyl ether, t-butylmethyl ether, N,N-dimethylformamide; N,N-
dimethylacetamide, N-
methylpiperidone, dichloromethane, 1,2-dichloroethane, acetonitrile, and the
like.
The compound of Formula 1-2' may also form a salt with a basic alkali metal,
for
example Na+, K+, Ca2+, Mg2+, Zn2+, or the like.
N-N ~ OCH3
NN 11~ OCH3
H
OCH30
(1-2')
OCH3
oGH3
I"lo ~ o
\ I ~
oH o
(3)
CA 02689136 2009-11-27
WO 2008/150085 PCT/KR2008/003078
8
N OCH3
C)CH3
Q o
OH O
(4)
N OCH3
OCH3
o o
o~H30
(5)
Further, the present invention provides a compound of Formula l wherein Rl is
hydrophosphoryl or hydroxyisopropylphosphoryl, and a pharmaceutically
acceptable salt
thereof.
Out of the compounds of Formula 1, a compound with Rl being hydrophosphoryl or
hydroxyisopropylphosphoryl (compound of Formula 1-3' or 1-3") is prepared by
reacting a 7-
hydroxy-3',4',5-trimethoxy flavone of Formula 6 with lower alkyl-protected
phosphonyl halide
((R40)2POCH2X) to obtain a compound of Formula 7, and warming the compound of
Formula
7 with sodium azide in the presence of an aprotic solvent or stirring the
compound of Formula
7 under reflux in an acid aqueous solution to remove the Rl-protecting group,
As used herein, the term "acid aqueous solution" refers to an aqueous solution
of strong
acid, For example, mention may be made of a hydrochloric acid aqueous
solution, a sulfuric
acid aqueous solution, a phosphoric acid aqueous solution, and thelike:
Removal of the R1-protecting group is carried out by any deprotection scheme
using
NaN3, hydrochloric acid and the like. Following such a process, a compound of
Formula 1-3'
CA 02689136 2009-11-27
WO 2008/150085 PCT/KR2008/003078
9
or 1-3" in accordance with the present invention may be selectively obtained
from the
compound of Formula 7. Further, the compound of Formula 1-3' or 1-3" may form
a salt
following the reaction with a basic alkali metal such as Na+, K+, Ca2+, Mg2+,
Zn2+, or the like.
OH OCH3
R,4~ OP~ OCH3
0 O
~ + I
CH30
(1-3')
HO'P H OCHaOCH3
d)
0 O i
OCH3O
(1-3")
OCH3
OCH3
Ho o
oCH3o
(6)
~p-R4 OCH3
Ra'OP OCH3
o o
OCH3O
(7)
wherein R4 is Cl-C6 lower alkyl including methyl, ethyl and isopropyl.
CA 02689136 2009-11-27
WO 2008/150085 PCT/KR2008/003078
In the phosphonyl halide ((R40)2POCH2X), R4 is as defined above, and X is
chlorine,
bromine or iodine.
Further, the present invention provides a pharmaceutical composition for the
treatment
and prevention of dry eye syndrome, comprising a 3',4',5-trimethoxy flavone
derivative of
5 Formula 1 or a pharmaceutically acceptable salt thereof as an active
ingredient. Further, the
present invention provides a pharmaceutical composition for the treatment and
prevention of
dry eye syndrome comprising a 7-carboxymethyloxy-3',4',5-trimethoxy flavone
monohydrate
disclosed in Korean Patent No. 447918, 327621 or 644928, as an active
ingredient.
Due to their excellent effects on stimulation of conjunctival mucus secretion
and
10 inhibition of ocular surface damage, the compounds of Formula l and 7-
carboxymethyloxy-
3',4',5-trimethoxy flavone monohydrates may be therapeutically and
prophylactically effective
for dry eye syndrome.
Further, the present invention provides a method for the treatment and
prevention of dry
eye syndrome, comprising administering to a mammal (including human) in need
thereof a
composition comprising a compound of Formula 1 or a pharmaceutically
acceptable salt
thereof or a 7-carboxymethyloxy-3',4',5-trimethoxy flavone monohydrate as an
active
ingredient.
Further, the present invention provides a use of a compound of Formula 1 or a
pharmaceutically acceptable salt thereof or a 7-carboxymethyloxy-3',4',5-
trimethoxy flavone
monohydrate, for the preparation of a medicament for the treatment and
prevention of dry eye
syndrome.
Depending upon desired applications, the pharmaceutical composition of the
present
invention may be administered via a conventional route, for example by peroral
administration
or by parenteral administration (intradermally, subcutaneously, intravenously,
intramuscularly,
rectally, intranasally, and intraocularly). Further, the pharmaceutical
composition may further
comprise one or more conventional excipients taking into consideration desired
dosage forms.
The compound of Formula 1 in accordance with the present invention may be
administered at a dose of 0.001 to 1 mg/kg BW, once or several times a day,
even though a
higher or lower daily dose may be required for some patients. As will be
apparent to those
skilled in the ait, the effective dose of the active compound may vary
depending upon various
factors such as co-administered drugs and severity of diseases.
CA 02689136 2009-11-27
WO 2008/150085 PCT/KR2008/003078
11
ADVANTAGEOUS EFFECTS
The present invention provides novel 3',4',5-trimethoxy flavone derivatives
and
pharmaceutically acceptable salts thereof. Due to having excellent stimulatory
effects on
mucus secretion in the conjunctiva and excellent inhibitory effects on ocular
surface damage,
the aforesaid 3',4',5-trimethoxy flavone derivatives can be therapeutically
and prophylactically
effective for dry eye syndrome.
MODE FOR INVENTION
Now, the present invention will be described in more detail with reference to
the
following Examples. These examples are provided only for illustrating the
present invention
and should not be construed as limiting the scope and spirit of the present
invention.
Example 1: Preparation of 7-(tetrazol-5-ylmethylon)-3',4',5-trimethoxy flavone
Step 1: Preparation of 7-propan-2-yn-l-yloxy-5-hydroxy-4',5'-dimethoxy flavone
5,7-dihydroxy-4',5'-dimethoxy flavone (20 g, 63.6 mmol; prepared according to
the
method disclosed in Korean Patent No. KR644928 assigned to the present
applicant, Dong-A
Pharm. Co.; Ltd., Korea) and anhydrous potassium carbonate (20.3 g, 152.7mmol)
were
dissolved in N,N'-dimethylformamide (400 mL) to which bromoacetonitrile (6 mL,
76.3
mmol) was then added dropwise at room temperature, followed by reaction for 13
hours. The
reaction solution was inversely dispersed in a 1:4 mixed solution (1000 mL) of
ethyl acetate
and n-hexane and then stirred at room temperature for l hour. The resulting
solids were
filtered and dried under vacuum to quantitatively give 22.5 g of the title
compound.
'H NMR(400MHz, DMSO-d6): 12.98(s, 1H), 7.71(dd; 1H), 7.58(a, 1H), 7.14(d, 1H),
7.09(s; 1H), 6.97(d, 1H), 6:54(d, 1H), 3.87(s, 3H), and 3.84(s,3H)
Step 2: Preparation of 7-propan-2-yn-l-yloxy-3'g4';5-trimethoxy flavone
7-propan-2-yn-1-yloxy-5-hydroxy-4',5'-dimethoxy flavone (22.5 g) prepared in
Step 1
and anhydrous potassium carbonate (51.7 g, 373.8 mmol) were added dropwise to
500 mL of
acetone to which dimethyl sulfate (6.6 mL, 70 mmol) was then added dropwise,
followed by
stirring under reflux for 13 hours. The reaction solution was cooled to room
temperature and
CA 02689136 2009-11-27
WO 2008/150085 PCT/KR2008/003078
12
chloroform (500 mL) was added dropwise thereto. Thereafter, the mixed solution
was stirred
for 30 min and filtered through celite. The celite pad was washed with
chloroform (1000 mL)
and the filtrate was evaporated to remove the solvent. The resulting solids
were dissolved in
chloroform (1000 mL) and washed with water. The organic layer was dried over
anhydrous
sodium sulfate and then evaporated to remove the solvent, and suspended with
stirring in a 1:1
mixed solvent (1000 mL) of ethyl acetate and n-hexane for 2 hours. The
resulting solids were
dried under vacuum for 3 hours to give 6:47 g(yield: 28%) of the title
compound.
'H NMR(Varian 400MHz, DMSO-d6): 7.63(dd, 1H), 7.52(d, 1H), 7.11(d, 1H),
7.03(d,
1H), 6.82(s; 1H), 6.63(d, 1H), 5.34(s, 2H), 3.87(s, 3H), 3.85(s, 3H), 3.84(s,
3H), and 3.44(brs,
NH)
Step 3: Preparation of 7-(tetrazol-5-ylmethyloxy)-3',4',5-trimethoxy flavone
7-propan-2-yn-1-yloxy-3',4',5-trimethoxy flavone (0.3 g, 0.82 mmol) obtained
in Step 2,
ammonium chloride (0;13 g, 2.45 mmol) and sodium azide (0.08 g, 1.23 mmol)
were
dissolved in N,N'-dimethylformamide (6 mL). After the reaction was carried out
at 120 C for
3 hours, the reaction solution was cooled to room temperature. The resulting
solids were
filtered and washed with ethyl acetate and n-hexane. The washed solids were
dissolved in a
3:1 mixed solvent of chloroform and methanol and dried over anhydrous sodium
sulfate to
remove the solvent. The resulting residue was recrystallized from a 1:1 mixed
solvent of ethyl
acetate and chloroform to give 0.195 g(yield: 58%) of the title compound.
1H NMR(Varian 400MHz, DMSO-d6): 7.61(dd, 1H), 7.50(d, 1H), 7.10(d, 1H),
7.04(d,
1H), 6.76(s, 1H), 6.58(d, YH), 5.41(s, 2H), 3.87(s, 3H), 3.83(s, 3H), and
3.81(s, 3H)
Example 2: Preparation of 7-(tetrazol-5-ylmethyloxy)-3',4',5-trimethoxy
flavone
potassium salt
7-(tetrazol-5-ylmethyloxy)-3',4',5-trimethoxy flavone (0.13 g) prepared in
Example 1
was dissolved in a 1N potassium hydroxide solution (5 mL). The solution was
stirred at room
temperature for 1 hour and the unreacted starting material was filtered. The
resulting aqueous
solution was lyophilized to afford 0.140 g(yield: 99%) of thetitle compound.
CA 02689136 2009-11-27
WO 2008/150085 PCT/KR2008/003078
13
'H NMR(Varian 400MHz, DMSO-d6): 7.62(dd, 1H), 7.50(d, 1H), 7.10(d, 1H),
7.05(d,
1H), 6.75(s, 1H), 6.57(d, 1H), 5.28(s, 2H), 3.87(s, 3H), 3.83(s, 3H), 3:80(s,
3H), and 3.35(br)
Example 3: Preparation of 7-{(carbamoyl)methyloxy}-3',4',5-trimethoxy flavone
7-carboxymethyloxy-3',4',5-trimethoxy flavone anhydride (1 g, 2.59 mmol,
prepared
according to the method disclosed in Korean Patent Noa KR447918 assigned to
the present
applicant, Dong-A Pharm. Co., Ltd., Korea) was dissolved in N,N'-
dimethylformamide (20
mL) to which pyridine (130 ,cce), di-t-butyl dicarbonate (0.73 g, 3.34 mmol)
and ammonium
carbonate (0.27 g, 3.34 mmol) were then added dropwisee The solution was
stirred at room
temperature for 4 hours and then washed several times with ethyl acetate. The
organic layer
was dried over anhydrous magnesium sulfate, distilled under reduced pressure
to remove the
solvent, suspended with stirring in acetone for 2 hours, and filtered. The
filtered solids were
dried under vacuum to afford 0.44 g(yield; 44%) of the title compound.
1H NMR(Varian 400MHz, DMSO-d6): 7.62(d, 1H), 7.42(d, 1H), 7,10(d, 1H), 6.83(d,
1H), 6.77(s, 1H), 6.60(d, 1H), 4.59(s, 1H), 3.87(s, 3H), 3.83(s, 3H), 3.82(s,
3H), and 3.31(s,
2H)
Example 4: Preparation of 7-{(N-cyanocarbamoyl)-methyloxy}-3',4',5-trimethoxy
flavone
7-carboxymethyloxy-3',4',5-trimethoxy flavone anhydride (1 g, 2.59 mmol,
prepared
according to the method disclosed in Korean Patent No. KR447918 assigned to
the present
applicant, Dong-A Pharm. Co., Ltd., Korea), N-3-dimethylaminopropyl-N'-
ethylcarbodiimide
hydrochloride (0.55 g, 2.87 mmol, Aldrich), dimethylaminopyridine (0.35 g,
2.87 mmol) and
cyanamide (0.12 g, 2.87 mmol) were added dropwise at 0 C and then reacted at
room
temperature for 4 hours. 20 mL of water was added dropwise to separate the
organic layer
from the aqueous layer and the aqueous layer was distilled off under reduced
pressure. The
resulting residue was purified by column chromatography using a 20:1 mixed
solvent of
dichloromethane and methanol as an eluent, thereby affording 0.33 g(yield: 31
% o) of the title
compound.
'H NMR(400MHz, DMSO-d6): 7.55(dd; 1H), 7.46(s, ,1H), 7.04(d, 1H); 6.69(s, 1H),
CA 02689136 2009-11-27
WO 2008/150085 PCT/KR2008/003078
14
6.59(d, lH), 6.38(d,1H), 4.36(s, 2H), 3.86(s, 3H), 3.81(s, 3H), 3.76(s, 3H),
and 3.31(s, 2H)
Example 5: Preparation of 7-{(N-bezenesulfonylcarbamoyl)methyloxy}-3',4',5-
trimethoxy flavone
7-carboxymethyloxy-3',4',5-trimethoxy flavone anhydride (5 g, 12.9 mmol,
prepared
according to the method disclosed in Korean Patent No. KR447918 assigned to
the present
applicant, Dong-A Pharm. Co., Ltd., Korea), 1-3-dimethylaminopropyl-3-
ethylcarbodiimide
hydrochloride (2.48 g, 12.9 mmol, Aldrich) and 4-dimethylaminopyridine (2.37
mg, 19.4
mmol) were added to 150 mL of dichloromethane and stirred at room temperature
for 20 min.
Benzenesulfonamide (4.07 g, 25.88 mmol) was then added dropwise thereto,
followed by
reaction at room temperature for 13 hours. 20 mL of water was added dropwise
to separate the
organic layer from the aqueous layer and the aqueous layer was distilled off
under reduced
pressure. The resulting residue was purified by column chromatography using a
30:1 mixed
solvent of dichloromethane and methanol as an eluent, thereby affording 1.02 g
(yield: 15 %)
of the title compound.
'H NMR(Varian 400MHz, DMSO-d6): 7.55(dd, 1H), 7.46(s, 1H), 7.04(d, 1H),
6.69(s,
1H), 6.59(d, 1H)9 6.38(d, 1H), 4.36(s, 2H), 3.86(s, 3H), 3.81(s, 3H), 3.76(s,
3H), and 3.31(s,
2H)
Example 6: Preparation of 7-{(hydrophosphoryl)-methyloxy}-3',4',5-trimethoxy
flavone
Step 1: Preparation of 7-{(diisopropylphosphoryl)-methyloxy}-3'g4',5-
trimethoxy
flavone
7-hydroxy-3',4',5-trimethoxy flavone (3 g, 9.14 mmol, prepared accarding to
the method
disclosed in Korean Patent No. KR447918 assigned to the present applicant,
Dong-A Pharm.
Co., Ltd., Korea) was dissolved in dimethylformamide (50 mL) to which
potassium carbonate
(2.5 g, 2 eq.) and diisopropyl bromomethylphosphonate (3.5 g, 1.5 eq.) were
then added. After
the reaction was carried out at 90 C for 16 hours, the reaction solution was
cooled to room
temperature and extracted two times with ethyl acetate. The organic layer was
dried over
magnesium sulfate and the solvent was distilled off under reduced pressure.
The resulting
CA 02689136 2009-11-27
WO 2008/150085 PCT/KR2008/003078
residue was purified by silica gel column chromatography using a 1;4 mixed
solvent of ethyl
acetate and n-hexane as an eluent, thereby affording 1.46 g(yield: 32%) of the
title compound.
'H NMR(400MHz, DMSO-d6): 7;52(d, 1H), 7.32(dd, 1H), 6.95(d, lH), 6.65(s, 1H),
5 6:48(d, 1H), 4.86(m, 2H), 4,31(d, 2H), 3.97(s, 3H), 3.96(s, 3H), 3.95(s,
3H), 1.40(s, 3H),
1.39(s, 6H), and 1.37(s, 3H)
Step 2: Preparation of 7- f(hydrophosphoryl)-methyloxy}-3',4',5-trimethoxy
flavone
7-{(diisopropylphosphoryl)-methyloxy}-3',4',5-trimethoxy flavone (0.6 g, 1.18
mmol)
10 obtained in Step 1 was added dropwise to water (15 mL) and conc.
hydrochloric acid (15 mL),
followed by stirring under reflux for 2 hours. The reaction solution was
cooled to room
temperature. The resulting solids were filtered under reduced pressure, washed
with water and
acetone, and then dried to afford 0.21 g(yield: 42%) of the title compound.
15 iH NMR(Varian 400MHz, DMSO-d6): 7.63(dd, 1H), 7.52(d, lH), 7.09(d, 1H),
6.97(d,
1H), 6.77(s, 1H), 6.51(d, 1H), 4.26(d, 2H), 3.87(s, 3H); and 3.83(s, 6H)
Example 7: Preparation of 7-{(hydroxyisonropylphosphoryl)-methyloxy}-3',4',5-
trimethoxy flavone
7-{(diisopropylphosphoryl)-methyloxy}-3',4',5-trimethoxyflavone (0.5 g, 0.99
mmol,
produced in Step 1 of Example 6) and sodium azide (0.5 g, 7:69 mmol) were
added dropwise
to N,N-dimethylformamide (20 mL) and warmed at 100 C for 8 hours. The
reaction solution
was cooled to room temperature, and 50 mL of ethyl acetate and 50 mL of water
were added
dropwise, followed by separation of the organic layer from the aqueous layer.
10 mL of conc.
hydrochloric acid was added dropwise to the aqueous layer which was then
extracted with
chloroform. The organic layer was washed with saturated saline, dried over
anhydrous sodium
sulfate and then evaporated to afford 0.15 g(yield: 33%0) of the title
compound.
1H NMR(Varian 400MHz, DMSO-d6): 7.61(dd, 1H), 7.50(d, 1H), 7.08(d, 1H),
6.97(d,
1H), 6.76(s, 1H), 6:52(d, 1H), 4.62(m, 1H), 4.36(d, 1H), 3.88(s, 3H), 3.87(s,
3H), 3.85(s, 3H),
and 1.25(d, 6H)
CA 02689136 2009-11-27
WO 2008/150085 PCT/KR2008/003078
16
Example 8: Preparation of 7-{(aminosulfonylcarbamoyl)methyloxy}-3',4',5-
trimethoxy flavone
7-carboxymethyloxy-3',4',5-trimethoxy flavone anhydride (1 g, 2.59 mmol,
prepared
according to the method disclosed in Korean Patent No. KR447918 assigned to
the present
applicant, Dong-A Pharm. Co., Ltd., Korea), sulfamide (0.27 g, 2.85 mmol) and
diisopropylethylamine (0.9 mL, 5;18 mmol) were added to 10 mL of
dichloromethane and
then stirred at room temperature for 20 min. The reaction solution was cooled
to -20 C and
benzotriazol-1-yl-oxy-tris-pyrrolidino-phosphonium hexafluorophosphate (1.48
g, 2.85 mmol,
Aldrich) was added dropwise thereto, followed by reaction at room temperature
for 8 hours.
After the reaction was complete, the resulting solids were filtered and
distilled under reduced
pressure to remove the solvent: The residue was purified by column
chromatography using a
20:1 mixed solvent of dichloromethane and methanol as an eluent, thereby
affording 0.16 g
(yield: 13%) of the title compound.
'H NMR(Varian 400MHz, DMSO-d6): 13.16(s, 1H), 7.63(dd, 1H), 7.59(d, 1H),
6.84(d,
1H), 6.76(s, 1H), 6.52(d, 1H); 4.85(s, 2H), 3.86(s; 3H), 3.83(s, 3H), and
3.82(s, 3H)
Example 9: Preparation of .7-{(methylsulfonylcarbamoyl)methyloxy}-3',4',5-
trimethoxy flavone
7-carboxymethyloxy-3',4',5-trimethoxy flavone anhydride (10 g, 25.9 mmol,
prepared
according to the method disclosed in Korean Patent No: KR447918 assigned to
the present
applicant, Dong-A Pharm. Co., Ltd., Korea), 1-[3-(dimethylamino)propyl]-3-
ethylcarbodiimide hydrochloride (4.96 g, 25.9 mmol, Aldrich) and 4-
dimethylaminopyridine
(4:74 mg, 38.8 mmol) were added to 300 mL of dichloromethane and then stirred
at room
temperature for 20 min. Methanesulfonamide (4.92 g, 51.76 mmol) was added
dropwise
thereto, followed by reaction at room temperature for 13 hours. After the
reaction was
complete, the resulting solids were filtered and distilled under reduced
pressure to remove the
solvent. The residue was purified by column chromatography using a 30:1 mixed
solvent of
dichloromethane and methanol as an eluent, thereby affording 2.15 g(yield:
18%) of the title
compound.
'H NMR(Varian 400MHz, DMSO-d6): 12.08(s, NH), 7.62(dd, 1H), 7.51(d, lH),
7.10(d,
CA 02689136 2009-11-27
WO 2008/150085 PCT/KR2008/003078
17
1H), 6.82(d, 1H), 6.78(s, 1H), 6.56(d, 1H), 4.87(s, 2H), 3.86(s, 3H), 3.83(s,
3H), 3.78(s, 3H),
and 3.29(s, 3H)
Example 10: Preparation of 7- f(carbamylcarbamoyl)methyloxy}-3',4',5-
trimethoxy
flavone
7-carboxymethyloxy-3',4',5-trimethoxy flavone anhydride (1 g, 2.59 mmol,
prepared
according to the method disclosed in Korean Patent No. KR447918 assigned to
the present
applicant, Dong-A Pharm. Co;, . Ltd., Korea), 1-[3-(dimethylamino)propyl]-3-
ethylcarbodiimide hydrochloride (0:50 g, 2.59 mmol, Aldrich) and 4-
dimethylaminopyridine
(0.47 mg, 3.88 mmol) were added to 300 mL of dichloromethane and then stirred
at room
temperature for 20 min. Urea (0.17 g, 2.83 mmol) was added dropwise thereto,
followed by
reaction at room temperature for 13 hours. After the reaction was complete,
the resulting
solids were filtered and distilled under reduced pressure to remove the
solvento The residue
was purified by column chromatography using a 30:1 mixed solvent of
dichloromethane and
methanol as an eluent, thereby affording 0.17 g(yield: 15 %) of the title
compound:
iH NMR(Varian 400MHz, DMSO-d6): 10.29(s, NH), 7.62(d, 1H), 7.51(s, 1H),
7.09(d,
1H), 6.82(d, 1H), 6.78(s, 1H), 6.57(d, 1H), 4.90(s, 2H), 3.86(s, 3H), 3.83(s,
3H), and 3.78(s,
3H)
Example 11: Preparation of 7-{(formylcarbamoyl)methyloxy}-3',4',5-trimethoxy
flavone
7-carboxymethyloxy-3',4',5-trimethoxy flavone anhydride (1 g, 2.59 mmol,
prepared
according to the method disclosed in Korean Patent No. KR447918 assigned to
the present
applicant, Dong-A Pharm. Co., Ltd., Korea), 1-[3-(dimethylamino)propyl]-3-
ethylcarbodiimide hydrochloride (0.50 g, 2.59 mmol, Aldrich) and 4-
dimethylaminopyridine
(0.47 mg, 3.88 mmol) were added to 300 mL of dichloromethane and then stirred
at room
temperature for 20 min: Formylamide (0.11 mL, 2.84 mmol) was added dropwise
thereto,
followed by reaction at room temperature for 14 hours. After the reaction was
complete, the
resulting solids were filtered and distilled under reduced pressure to remove
the solvent. The
residue was purified by column chromatography using a 30:1 mixed solvent of
dichloromethane and methanol as an eluent, thereby affording 0.18 g(yield:
17%) of the title
CA 02689136 2009-11-27
WO 2008/150085 PCT/KR2008/003078
18
compound.
'H NMR(Varian 400MHz, DMSO-d6): 8.23(s, 1H), 8.0(s, NH), 7.65(dd, iH), 7.52(d,
1H), 7.11(d; 1H), 7,02(d, 1H), 6.80(s, 1H), 6.61(d, 1H), 5.26(s, 2H), 3.87(s,
3H), 3.85(s, 3H),
and 3.83(s, 3H)
Example 12: Preparation of 7-{(acetylcarbamoyl)methyloxy}-3',4',5-trimethoxy
flavone
7-carboxymethyloxy-3',4',5-trimethoxy flavone anhydride (1 g, 2.59 mmol,
prepared
according to the method disclosed in Korean Patent No. KR447918 assigned to
the present
applicant, Dong-A Pharm. Co,, Ltd., Korea), 1-[3-(dimethylamino)propyl]-3-
ethylcarbodiimide hydrochloride (0.50 g, 2.59 mmol, Aldrich) and 4-
dimethylaminopyridine
(0.47 mg, 3.88 mmol) were added to 300 mL of dichloromethane and then stirred
at room
temperature for 20 min. Acetamide (0.17 g, 2.84 mmol) was added dropwise
thereto, followed
by reaction at room temperature for 13 hours. After the reaction was complete,
the resulting
solids were filtered and distilled under reduced pressure to remove the
solvent. The residue
was purified by column chromatography using a 30:1 mixed solvent of
dichloromethane and
methanol as an eluent, thereby affording 0.18 g(yield:17 %) of the title
compound.
'H NMR(Varian 400MHz, DMSO-d6): 8.23(s, 1H), 8.0(s, NH), 7.65(dd, 1H), 7.52(d,
1H), 7.11(d, 1H), 7.02(d, 1H), 6.80(s, 1H), 6.61(d, 1H), 5.26(s, 2H), 3.87(s,
3H), 3.85(s, 3H),
and 3.83(s, 3H)
In order to investigate beneficial effects of 3',4',5-trimethoxy flavone
derivatives
obtained in the foregoing Examples and 7-carboxymethyloxy-3',4',5-trimethoxy
flavone
monohydrates on dry eye syndrome, the above compounds were studied on mucin-
secreting
effects in the human mucoepidermoid pulmonary carcinoma cell line, and on
therapeutic
effects in dry eye syndrome-induced rabbits.
Experimental Example 1: Effects of 3',4',5-trimethoxy flavone derivatives on
mucin
secretion
In order to examine stimulatory effects of 3',4',5-trimethoxy flavone
derivatives in
CA 02689136 2009-11-27
WO 2008/150085 PCT/KR2008/003078
19
accordance with the present invention on mucin secretion, the following in
vitro experiments
were carried out.
2X 105 cells/well of the human mucoepidermoid pulmonary carcinoma cell line
NCI-
H292 (ATCC, #CRLs1848) were seeded on a 96-well plate, and treated with
3',4',5-trimethoxy
flavone derivatives at various concentrations of 0.16 to 100 pg/mL for 24
hours. Thereafter,
secretion levels of representative transmembrane mucin MUC4 and secretory
mucin
MUC5AC were measured by enzyme-linked immunosorbent assay (ELISA). The results
thus
obtained are given in Table 1 below.
[Table 1]
Stimulatory effects of 3',4',5-trimethoxy flavone derivatives on mucin
secretion
(% increase in mucin secretion vs. non-treated control group)
Compounds Concentrations (/tg/mL)
0.8 4 20 100
Example 1 18.7 28.1 57.8 52.0
Example 3 2.2 6:6 10.4 15,8
Example 4 - - 4.3 25.2
Example 5 43.9 45.4 35.4 70.9
Example 8 4.4 10.2 15.4 21.4
Example 9 9.7 15.9 37.4 43.3
Example 10 - 6.7 15.4 25.9
Example 12 15.2 13.6 20.5 30.2
7-carboxymethyloxy-3',4',5- 24.8 39.1 52.5 75.9
trimethoxy flavone monohydrate
From the experimental results, it was confirmed that 3',4',5-trimethoxy
flavone
derivatives have stimulatory effects on secretionof mucin. These results
suggest that 3',4',5-
trimethoxy flavone derivatives of the present invention promote secretion of
transmembrane
and secretory mucins and therefore can be effectively used for the treatment
of dry eye
syndrome.
Experimental Example 2: Therapeutic effects of 3',4',5-trimethoxy flavone
CA 02689136 2009-11-27
WO 2008/150085 PCT/KR2008/003078
derivatives in rabbit dry-eye syndrome model
Male New Zealand White (NZW) rabbits were randomly assigned into two groups,
each consisting of 5 animals. In order to induce dry eye syndrome, N-
acetylcysteine was first
dissolved at a concentration of 20% in physiological saline. Then, the
rabbit's lower eyelid
5 was pulled slightly away from the globe to form a cup shape and the above-
prepared N-
acetylcysteine solution was instilled into the conjunctival sac six times
every 2 hours, resulting
in establishment of an animal model of dry eye syndrome; 3',4',5-trimethoxy
flavone
derivatives were dissolved at a concentration of 0.5% in a 0,1N sodium
hydroxide solution
which was then titrated to pH 7.0 to 8.0 with 10% acetic acid. From the
following day after
10 induction of dry eye syndrome, the titrated 3',4',5-trimethoxy flavone
derivatives were
repeatedly instilled into conjunctival sacs of animals at a dose frequency of
4 times/day (0.1
mL/each time) for seven consecutive days. On the next day following the
induction of dry eye
syndrome and upon completion of the experiment, the degree of ocular surface
damage was
evaluated to calculate a damage decrease rate (%) using Rose Bengal assay, and
mucus
15 secretion in the conjunctiva was quantitatively analyzed using Alcian blue
assay. More
specifically referring to Rose Bengal assay, 10 a of a 1% Rose Bengal solution
was dropped
into the eyes ofanimals, and distribution and intensity of Rose Bengal
staining were scored
according to the method disclosed in Urashima et al., Cornea, 23:613-9, 2004.
For Alcian blue
assay, the rabbit's conjunctivae were excised, weighed, stained with a 0.1%
Alcian blue
20 solution for 2 hours and then washed with a 0.25 M sucrose solution:
Following extraction
with a 0.5 M magnesium chloride solution, the absorbance was measured at 605
nm. The
results thus obtained are given in Table 2 below.
[Table 2]
Therapeutic effects of 3',4',5-trimethoxy flavone derivatives in rabbit model
of dry eye
syndrome
Compounds Rose Bengal Assay Alcian Blue Assay
Damage decrease rate (%) Absorbance/g tissue
Normal animal group 0.0 0.0 0.56 0.18
Vehicle control group 49.3 6.5 0.16 0.05
Example 4 83.8 4.6* 0.24t0.19*
Example 5 70.1 5.2* 0.32 0.09*
CA 02689136 2009-11-27
WO 2008/150085 PCT/KR2008/003078
21
Example 9 76,8 8.4* 0.35 0.13*
7-carboxymethyloxy-3',4';5- 88.5 4.7* 0.38 0.08*
trimethoxy flavone
monohydrate
Values were given as Mean S.D.
* Shown statistically significant as compared to vehicle control group
(p<0.05)
As can be seen from the results of Table 2, it was confirmed that the animal
groups with
administration of a 0.1 N sodium hydroxide solution after induction of dry eye
syndrome by
N-acetylcysteine exhibit decreased conjunctival mucus secretion resulting in
occurrence of
ocular damage, as compared to the normal animal group. However, after repeated
instillation
of 3',4',5-trimethoxy flavone derivatives for 7 days, the conjunctivae of the
drug-administered
groups exhibited a statistically significant increase in the amount of mucus
secretion, as
compared to the vehicle control group. Further, ocular damage was also
significantly inhibited
in the 3',4',5-trimethoxy flavone derivative-administered groups. These
results represent that
3',4',5-trimethoxy flavone derivatives of the present invention can be
effectively used as a
. therapeutic agent for the treatment of dry eye syndrome due to having
stimulatory effects on
mucus secretion in the conjunctivae.
Hereinafter, Preparation Example will be illustrated for a therapeutic agent
of the
present invention.
Formulation Example 1: Ophthalmic pharmaceutical preparation
Preparation of an eye-drop (1%)
3',4',5-trimethoxy flavone of Formula 1 ... .:. .. ...... 1 g
pH-adjusting agent (NaOH) ............................ 0.11 g
pH-adjusting agent (HCl) . ... .... .. 0.0135 g
Preservative (Benzalkonium chloride) ................ 0.01 g
Thickening agent (PVA) .. .. . .. . ...: .. . .. .. . . .. . .. . ., ....1 g
Osmotic pressure-regulating agent (NaCI) ......:....: 0.83 g
Solubilizer (sterile purified water) ..... ...... .... 100 mL
CA 02689136 2009-11-27
WO 2008/150085 PCT/KR2008/003078
22
The above-listed ingredients were mixed to prepare an eye-drop as desired.
INDUSTRIAL APPLICABILITY
As apparent from the above description, the present invention provides novel
3',4',5-
trimethoxy flavone derivatives and pharmaceutically acceptable salts thereof,
Due to having
remarkable effects on stimulation of conjunctival mucus secretion and
inhibition of ocular
surface damage, 3',4',5-trimethoxy flavone derivatives are therapeutically and
prophylactically
effective for dry eye syndrome.