Note: Descriptions are shown in the official language in which they were submitted.
= = - CA 02691044 2014-04-09
METHODS AND KITS FOR TESTING THE EFFICACY
OF THERAPEUTIC COMPOUNDS AND OTHER
THERAPIES IN A TIME-DIMENSIONAL MATRIX
WITH RESPECT TO TUMOROUS CELLS AND TISSUE
[00011
EigwilEtugimENEgN
[0002]
The prcsem invention relates to matrices, methods, apparatus and kits for the
expedited design and evaluation of oncological remedies.
Key aspects of the
inventions include matrices, and especially gel matrices, which are adapted
and
arranged to provide conditions which pcnnit behaviors, such as the movement of
cells
from a sample of target tissue, to be evaluated in a manner that produces data
useful for
evaluating the neologiesl status of the cells. In a. further key aspect, the
invention
permits the in vitro testing and analyses of one or more therapies, such as
therapeutic
pharmaccutiad compounds, or radiological therapies, with respect tu specific
target
tissues and cells removed from 3 patient.
86C.KPROt IND QUA:[-KaNYINTI_ON
[0003]
Cancer is the loss of control of one or mon; of the regulatory systems which
regulate
the growth of cells and tissues. An uncontrolled growth of a particular tissue
01 cell
type is a specific type of cancer. Then: are many types of cancer. In the
treatment of
CA 02691044 2009-12-14
WO 2008/012696 PCT/1B2007/003494
2
cancer, one or more therapeutic remedies are typically used in attempts to
cure the
disease or ameliorate its effects. Many differences exist between classes of
tumors and,
indeed, individual tumors of the same type. There are many possible therapies
for
cancers in general. Nonetheless, most therapies do not work, or do not work to
the
extent necessary to provide the degree of cure, remediation or desired
palliative effect
for a specific cancer. Because of this, it is sometimes difficult, time-
consuming and
expensive to attempt to determine what therapeutic compounds may be effective
to treat
a particular tumor or cancer type. Oncologists and other physicians therefore
often
choose a therapy based on little or no pragmatic information regarding the
specific
tumor. In effect, their determinations of which therapy or therapies to employ
are often
guesses. It is therefore very important to be able to more effectively match a
specific
effective therapy to a specific tumor, and to be able to do so in a reasonable
amount of
time. As a consequence of these needs, many technologies have attempted to
address
these needs, but none have been acceptably successful.
[0004] Various experiments have been directed toward measuring the effects
of certain
therapies on malignant cells in vitro. One of these attempts is shown in
"Effects of
Radiation on a Three-Dimensional Model of Malignant Glioma Invasion" -
international
Journal of Devi. Neuroscience, Vol. 17, issue 5-6, 643-651, August 1999,
(Bauman et
at.). The Bauman et at. researchers used suspensions of an established cell
line, the C6
astrocytoma line, to show that the three-dimensional migration of known,
cultured
malignant cells into a collagen matrix could be observed. The malignant glioma
cells
thus cultured, disrupted and processed into cloned spheroids, which were then
implanted into a gel matrix, and then subjected to one or more doses of
fractionated
radiation. Changes in the distance of invasion in response to single dose and
fractionated radiation were measured over a period of 5 days.
[0005] Similar experiments were reported in "Effects Of Radiation On A
Model Of
Malignant Glioma Invasion", Journal of Neuro-Oncology 44: 223-231, 1999,
(Baumann
et al.). In this reference, the Baumann et al. researchers used the same C6
cell line and
experiment-al protocols to test BCNU and dexamethasone, and to compare these
results
with those of radiation dosing on the transformed cells.
[0006] Significantly, in both series of experiments reported by Bauman et
at., the cloned
C6 astrocytoma cells were subjected to disruption by trypsinization, and also
subjected
to centrifugal forces for 3-4 weeks in spinner flasks. Thus, the cells of
Bauman et at
were already known to be transformed to a great extent, were generations
removed from
CA 02691044 2009-12-14
WO 2008/012696 PCT/1B2007/003494
3
the original tissue, required disruptive chemical processing, and were a
subset of
cloned malignant cells at the time they were subject to radiation doses. C6
astrocytoma
cells were maintained in tissue culture as cloned representatives of malignant
rat glioma
cells. Indeed, the cells of the Bauman et al. experiments were removed and
estabished
as a cell line years before they were subjected to the experiments of Bauman
etal.
[0007] In significant contrast, the present invention uses a sample
quantity of fresh tissue
taken directly from an animal, such as a human patient. Significantly, the
samples used
in the present invention are not disrupted by trypsin or other enzymes, but
are
mechanically divided into sample portions of appropriate sizes for testing.
Thus, the
present methods maintain the cell-to-cell contact of the sample tissue as if
it were still in
vivo. An additional difference pertains to the fact that the experiments of
Bauman et al.
used an established cell line, that is, cells that were already known to have
been
transformed long before, and in an unknown way, to an extent great enough that
they
could be used to establish a tissue culture cell line. One could not therefore
expect the
cells of Baumann et al. to behave in a manner reasonably replicative of fresh
or in vivo
cells.
[0008] Others have attempted to provide ways of evaluating the response
of tumor cells to
chemotherapeutics. In U.S. Patent No. 5,242,806 to Yen-Maguire et al.,
entitled
Method For Conducting The Cytotoxicity Assays On Tumor Cells, a "growth
matrix"
of bovine cornea endothelium cells is sometimes employed as a coating in the
wells of
multi-well plates in order to facilitate the attachment of cells to the plate
surfaces.
Essentially, Yen-McGuire discloses ways of culturing cellular suspensions
which have
been grown in two dimensions, and then assaying the responses of the processed
cells to
various cytotoxic or chemotherapeutic compounds.
[0009] The cellular suspensions of Yen McGuire are provided with a
defined, selective
growth medium which is designed and formulated to promote the growth of
epithelial
tumor cells while inhibiting the growth of normal cells. Yen-McGuire thus
teaches the
use of selective nutrition to skew cellular growth and behavior. Indeed, the
relative
amount of cellular growth is measured to provide information regarding the
sensitivity
of the highly processed tumor cells. Thus, Yen-McGuire does not provide any
analysis
with respect to an original tumor sample or fragment thereof, nor does it
comprehend
the advantages of an in vitro system which replicates significant aspects of
the three-
dimensional environment of tumor tissue in vivo.
CA 02691044 2009-12-14
WO 2008/012696 PCT/1B2007/003494
4
[0010] These problems regarding the lack of analytic tools which are
effectively usable to
provide information specific to particular tumors, combined with the fact that
cancer
often progresses rapidly, have created a significant need for means and
methods to
quickly obtain information useful for testing and evaluating specific
therapies, for
example, therapeutic compounds, to determine their effectiveness with respect
to
particular tumors or cancer types. There is thus a significant need for such
means and
methods.
SUMMARY OF THE INVENTION
[0011] Accordingly, it is an object of the present invention to remedy the
above-mentioned
drawbacks by providing three-dimensional matrices, such as gels, as well as
methods
and kits for evaluating and testing tissue and cells to determine if they are
abnormal,
pre-cancerous, or cancerous.
[0012] Another object of the present invention is to provide methods and
kits for testing
one or more therapeutic substances to determine their efficacy against a
particular
tumor or type of tumor.
[0013] In accordance with these and other objects, and in accordance with
the context of
the invention with respect to providing novel and non-obvious improvements in
all
relevant fields, multiple means and methods of practicing the inventions are
provided.
[0014] In accordance with these and other objects, means and methods for
evaluating the
oncological characteristics of at least one tissue or cells from an animal,
such as a
human being, which cells or tissue are suspected of being abnormal, cancerous
or pre-
cancerous, are provided. In one preferred embodiment, a method of the
invention
comprises the steps or A) obtaining a sample quantity of the suspected tumor
tissue or
cells from the animal, wherein the tissue comprises one or more types of
cells, B)
implanting at least part of the sample of the suspected tumor tissue at least
partially
within a three-dimensional physiological matrix, the matrix being adapted and
arranged
for measuring one or more parameters of the behavior of the cells of the
suspected
tumor tissue, C) providing sufficient nutrition to the cells of the tissue or
cells so that
the parameters can be measured, D) incubating or culturing the tissue and the
cells in an
environment suitable for the growth of the cells and the tissue for a time
sufficient to
obtain measurements with respect to one or more parameters, and E) measuring
some of
those one or more parameters to obtain data regarding those behaviors.
CA 02691044 2009-12-14
WO 2008/012696 PCT/1B2007/003494
[0015] Methods of the present invention are further adapted such that,
preferably, the
implantation of the sample, or portions thereof, is effected with minimal
disruption to
the cells or the tissue_ For example, in a typical procedure according to some
preferred
embodiments of the invention, a sample quantity of a tissue is removed from a
patient
by way of a biopsy or surgery. The structural integrity of that tissue sample
is then
maintained to the maximum extent possible before its implantation. In this
regard, it is
noteworthy that non-disruption of the sample means, as examples, that the
sample is not
homogenized, it is not chemically disrupted by strong acids, or by enzymes
such as
trypsin, nor is it comminuted, crushed or subjected to high concentrations of
strongly
basic compounds. In sharp contrast, a sample according to the invention is
cut, torn or
chopped into small pieces, such as chunks approximating cubes of 1.0 ¨ 3.0 mm
on an
edge. While not intending to be bound by any underlying mechanism, the present
inventors theorize that, by minimizing the disruption to the sample tissue and
cells, their
respective behaviors in relation to a physiological matrix of the invention
remains
similar to their corresponding behaviors in vivo.
[0016] In this same vein, it is preferable that implantation of the samples
during the
methods of the invention take place as soon after the sample is removed from
the
animal as is reasonably possible. Thus, a removed tissue sample is preferably
immediately cooled by placing it in proximity to ice, for example, and
implantation is
most preferably accomplished within one or a few hours after removal. Although
implantation is most preferably accomplished within an hour or so of sample
removal,
the present methods provide for later implantation when circumstances dictate
as much,
such as when the sample must be transported some distance to a laboratory for
preparation. In any event, implantation should most preferably take place
within four
hours of removal of the sample tissue, or more preferably within 24 hours of
removal,
and less preferably within 48 hours of removal of the tissue from the subject
animal. As
one of skill in the oncological arts will appreciate, the effects of the delay
in
implantation and evaluation of a tissue sample may depend upon many factors,
including the type of tissue, its oncological status, and the conditions under
which it has
been stored. Thus, it may be possible to delay implantation for many hours or
many
days.
[0017] As another advantage of the invention, its means and methods provide
data for
physicians and other evaluators to make determinations or estimates regarding
the
oncological status of a tissue and its cells. Thus, from the data obtained, a
CA 02691044 2009-12-14
WO 2008/012696 PCT/1B2007/003494
6
determination can be made regarding whether the tissue and cells are abnormal,
pre-
cancerous or cancerous. As a further advantage, the present means and methods
permit
the testing of many types of therapies, including pharmaceutical compounds and
non-
chemical therapies. The present means and methods can therefore be used to
provide
data useful for tailoring specific individual therapeutic regimens directed
toward the
precise tissue being evaluated. To this end, some preferred embodiments of the
present
invention further comprise the step of transmitting the data to at least one
evaluator. In
the context of the invention, an evaluator is any person, group of people, or
network of
people, or any machine, computer or device adapted and arranged for evaluating
the
data garnered through use of the present means and methods. Evaluators
include, but
are not limited to the group comprising technicians, technicians, physicians,
physician's
staff members, physician's assistants, hospital employees, clinic employees,
the patient,
technologists, technical assistants, laboratory assistants, oncological
analysts, nurses,
nurses' assistants nurse practitioners, computers, computer-aided devices,
computer-
facilitated devices, and human or computerized agents for any of the
foregoing.
[0018] In accordance with still further aspects of some preferred
embodiments of the
invention, the removed sample quantity of tissue is divided into a plurality
of portions,
and each of the portions is subjected to the some or all steps of the
invention. Thus,
tests and evaluations of the tissue and cells can be performed in multiples,
and one or
more therapies can be tested concurrently in relation to the specific sample
if desired.
[0019] Moreover, the measurements taken with respect to each portion of a
sample are
taken over time, either at periodic intervals, or at random intervals. In some
preferred
embodiments therefore, Step E is performed more than once, or a plurality of
times with
respect to the separate portions of the sample. Preferably, these measurements
are
effected during the culturing or incubating of the tissue and cells as in Step
D above.
[0020] Advantageously, means and methods of the invention can be used on
virtually any
type of animal, including human medical patients, and can be used also to
evaluate
tissue from other animals. Other animals include, but are not limited to non-
human
primates, equines such as horses and donkeys, bovines such as cows and deer,
canines
such as dogs and wolves, felines such as lions and domestic house cats,
murines such as
mice and moles, porcines such as pigs and peccaries, avians such as birds and
penguins,
amphibians such as salamanders and turtles, and reptilians such as snakes and
alligators.
The animals mentioned herein comprise an exemplary listing, and not an
exclusive one.
CA 02691044 2009-12-14
WO 2008/012696 PCT/1B2007/003494
7
[0021] Methods of the invention also include where the measurements taken,
as in Step E,
are performed more than once with respect to each of the separate portions of
the
sample, and wherein the measuring is effected during the culturing or
incubating of
Step D. A significant aspect of the invention concerns the physical
relationship
between the tissue sample portion and the three-dimensional matrix in which it
is
implanted. Preferably, the tissue portion is disposed either partially
submerged in the
matrix, or completely submerged therein. It is important that the tissue be in
intimate
contact with the matrix. Thus, the physiological matrix replicates to some
extent the
three-dimensional environment in which most tissues function in vivo. Tissue
samples,
or portions thereof, may be implanted in any method, manner or way, which
yields the
desired results
[0022] As an aid to implantation, the matrices of the invention, which are
typically gels or
other permeable solids or semi-solids, can be provided in shapes which
facilitate the
implantation of one or more types of tissues and cells, and which facilitate
the provision
of nutrition to the cells and tissue. Thus, any type of depression such as a
hollow,
cavity, slit, chamber or slot can be provided within the matrix itself, and
can be adapted
and arranged to receive the tissue or cells for implantation, or can be
adapted and
arranged to hold nutrition for the cells and tissue. For example, a gel matrix
according
to the invention may comprise a hollow, cavity, slit, chamber or slot of an
appropriate
volume and disposition to receive an aliquot, such as a .2 mi. aliquot of 10X
media, as
nutrition for the implanted cells and tissue. Such depressions can also serve
as
receptacles or reservoirs for drugs to be tested.
[0023] In some preferred embodiments of methods of the invention, the
tissue or cells are
implanted in the matrix as the matrix is being formed into a gel or other
permeable solid
or semi-solid. In these embodiments, the tissue and cells are mixed along with
the
components of the matrix and are thus partially or completely submerged within
the
matrix by the time it gelates or hardens into a semi-solid or solid. In those
preferred
embodiments where the matrix is already formed, the tissue or cells can be
implanted in
the matrix by physical insertion after it has formed into a gel, solid or semi-
solid.
[0024] It is important to note that solutions for forming matrices of the
invention should be
essentially free of bicarbonate. By maintaining this bicarbonate-free
condition, the gels
or semi-solids of the invention advantageously form at room temperatures and
are thus
capable of being mixed and formed in a manner that is convenient, user
friendly, and
adaptable to many laboratory and non-laboratory environments.
CA 02691044 2009-12-14
WO 2008/012696 PCT/1B2007/003494
8
[0025] In accordance with other aspects of the invention, sufficient
nutrition is provided to
the cells and tissue to be evaluated or tested, either as a component of the
matrix as it is
being formed, or as nutrition added after the matrix is formed, or both. The
nutrition
must be sufficient to grow the cells and tissue for a sufficient length of
time to obtain
the desired results. In some embodiments, the sufficient or additional
nutrition
preferably comprises one or more fluids, wherein the one or more fluids are
selected
from the group comprising tissue culture medias, concentrated tissue culture
medias,
and any culture or nutritional medias which are suitable for the tissue
sample. The
means and methods of the present invention thus include wherein the
concentrated
medias are one or more selected from the group comprising 10X medias, and any
other
concentrated medias.
[0026] In another aspect of the invention, the three-dimensional
physiological matrix
comprises the sufficient nutrition as a component of the matrix itself as it
is being
mixed and formed or allowed to gel. As a further aspect of the invention, a
step of
providing at least one source of additional nutrition to the tissue and the
cells is
provided. Thus, the tissues and cells may avail themselves of one or both of
the
sufficient nutrition and of the additional nutrition to the extent necessary
so that the
parameters can be measured. In accordance with similar aspects of the
invention, the at
least one source of additional nutrition may comprise one or more of tissue
culture
medias, concentrated tissue culture medias, and any culture or nutritional
medias which
are suitable for the tissue sample. As a further aspect of the invention, the
at least one
additional source of nutrition may be supplemented with one or more components
selected from the group comprising sera, proteins, sugars, salts, lipids.
[0027] A further method of the present invention wherein the measuring of
Step E is
performed at periodic intervals. Preferably the measuring step is performed at
periodic
intervals such as one or more intervals selected from the group comprising
every six
hours, every 12 hours, every 18 hours, every 24 hours, every 36 hours, every
48 hours,
every 72 hours and every 96 hours. Moreover other intervals are one or more
selected
from the group comprising every day, every second day, every third day, every
fourth
day, and every fifth day.
[0028] As another advantage, the data obtained by the measurements of the
invention is
recorded in a fixed media. Such fixed media can be any type so long as it is
capable of
holding the obtained data in such form as to be useable later. For example,
paper, voice
recordings, video recordings, photographs, photomicrographs, digital
recordings, any
CA 02691044 2009-12-14
WO 2008/012696 PCT/1B2007/003494
9
fixed digital data means. According to the invention one or more of a human
observer,
a still camera, a video camera, an automated still camera, an automated video
camera,
an infrared camera, and an automated infrared camera can be used to obtain the
desired
data.
[0029] Preferably the culturing of tissues and cells according to the
invention occurs in a
period of time sufficient to obtain the desired parameters and values. For
example, the
periods of time during which the culturing of tissues and cells is effected
includes those
of at least 10 hours, at least 36 hours, at least 72 hours and at least 125
hours, or even
longer depending on the conditions of the tissue and how the method is being
conducted
[0030] In accordance with other aspects of the invention, the sufficient
nutrition is provided
to the tissues and cells by placing the sufficient nutrition in contact with
the matrix after
the sample has been placed in the matrix and the matrix has equilibrated to
room
temperature, and to a semi-solid or gel. Preferably, the room temperature is
in the range
of from 10 to 30 degrees Celsius. In accordance with other aspects, the
sufficient
nutrition may be provided to the tissues and the cells by placing the it in
contact with
the matrix after the sample has been placed in the matrix, and the matrix has
equilibrated to a gel at room temperature. In other embodiments, the
sufficient nutrition
may be provided to the tissues and the cells by continuous or intermittent
perfusion or
flow over, around, or through the matrix. In yet another aspect, the three
dimensional
quality of the matrix of the invention can be formed itself to comprise the
sufficient
nutrition. The environment for incubating or culturing the cells and the
tissue
preferably includes a temperature range of from 30 to 40 degrees Celsius, a
carbon
dioxide tension of from 2 to 13%, and a relative humidity of from 50% to 100%.
[0031] In accordance with other aspects, methods of the invention can be
conducted
wherein the sample quantity is preferably divided into portions, and each of
these
portions is subjected concurrently and separately to the method, wherein the
matrix
comprises a gel, and wherein the gel is suitable for measuring the one or more
parameters to obtain the desired values. Thus, multiple equivalent matrices
may be
formed in multiple containers, such as multiple containers comprising a multi-
well
apparatus, such that multiple portions of the sample can be evaluated
concurrently.
Thus, the present invention includes where a particular tissue can be
evaluated at
multiple times, with respect to multiple portions of the sample, and with
respect to
multiple therapies.
CA 02691044 2009-12-14
WO 2008/012696 PCT/1B2007/003494
[0032] Advantageously, the present invention can be used to measure or
obtain data with
respect to one or more parameters regarding the behavior of the cells and
tissue. Those
parameters include one or more of the distance which individual cells migrate
from the
tissue sample, the average distance of migration of a group or population of
the cells
from the tissue sample, the distance which individual cells migrate from the
tissue
sample with respect to time, the average distance of migration of a group or
population
of the cells from the tissue sample with respect to time, the velocity of
migration of one
or more designated individual the cells per selected time period, the velocity
of
migration of a group or population of designated the cells per selected time
period, the
number of migrational cells per unit area of a microscopic visual field, the
speed of
proliferation of the cells, the speed of unidirectional migration of the
cells, the speed of
bi-directional migration of the cells, the speed of tri-directional migration
of the cells,
the frequency of directional change of the migrating cells, the number of
directional
changes per unit time of the migrating cells, the rate of mitosis of the
migrating cells,
the number of' cells migrating per unit time, the number of cells in a unit
area of a
portion of the gel, the change in the number of cells in a unit area of a
portion of the gel
with respect to time, the number of cells in a unit volume of a portion of the
gel with
respect to time, and the change in the number of cells in a unit volume of a
portion of
the gel, the proportion of cells which change migrational direction during the
test
period, the speed of migrating cells in a particular visual field, the density
of migrating
cells per unit area of a visual field, the migrational distance of individual
migrating cells
per unit area of a visual field, the average migrational distance of a group
or population
of migrating cells per unit area of a visual field, osmotic pressure, ionic
strength, the
change in pH with respect to time, the change in pH with respect to the amount
of cell
migration, oxygen consumption, glucose consumption, and the number of
migrating
cells in a particular direction per unit area of a visual field.
[0033] In accordance with other advantageous aspects of the invention the
three-
dimensional physiological matrices for use with the invention can be adapted
and
arranged to have whatever pH value or range necessary to culture and evaluate
the
specific target tissues and cells. Preferably, the pH range for testing most
tissues is
from 5.0 to 8.0, and, more preferably, from 7.0 to 7.5. Thus, the
physiological matrix
preferably has a pH which is adapted to a range suitable for one or more of a
particular
tissue, a particular cell type or types, or to a particular tumor.
CA 02691044 2009-12-14
WO 2008/012696 PCT/1B2007/003494
11
[0034] Preferably, a three dimensional matrix of the invention comprises at
least one
natural or synthetic fiber, for example, one or more fibers selected from the
group
including type 1 collagens, type 11 collagens, type III collagens, type IV
collagens,
fibrin, fibrinogen, extracellular matrix proteins derived from one or more
animals,
laminin, fibronectin, anti-laminin, and other natural or synthetic fibers or
cables suitable
to the formation of a matrix mesh or network capable of supporting cell
support,
movement and growth. In some preferred embodiments a physiological matrix
according to the invention preferably comprises a gel, the gel being suitable
for
measuring at least one or more of the parameters necessary for evaluating the
tissue, the
cells, possible therapies, and is also adapted and arranged for measuring the
efficacy of
one or multiple therapeutic compounds.
[0035] The means and methods of the present invention are especially
adapted and
arranged to test the efficacy of virtually any therapy regimen, including
pharmacologic
therapeutics. In one context therefore, a physiological matrix of the
invention is
adapted and arranged for measuring the efficacy of one or more therapeutic
compounds,
such as known and unknown single compounds, and known and unknown therapeutic
cocktails. As examples, and not as an exclusive listing, one or more
therapeutic
compounds for use or evaluation in the context of the invention may be
selected from
the group comprising anti-tumor agents, DNA damaging agents such as alkylating
agents, antibiotics which affect nucleic acids, platinum compounds, anti-
mitotics, cell
cycle stimulators, anti-metastatic agents, anti-metabolites, camptothecin
derivatives,
hormone therapies, biological response modifiers, interferon, anti-invasives,
anti-
invasion agents, anti-migration agents, anti-angiogenesis agents, and
apoptotic agents,
radiosensitizcrs, radiation, and any known, theoretical or experimental
therapeutics
directed against cell growth, cell invasion or cell viability, pro-tumor
agents, pro-
mitotic agents, pro-metastatic agents, pro-invasion agents, pro-migration
agents, pro-
angiogenesis agents, and anti-apoptotic agents.
[0036] More specifically, the three-dimensional physiological matrices of
the invention
may be adapted and arranged for measuring the efficacy of one or more
therapeutic
compounds, such as those selected from the group comprising Temozolomide
(TMZ),
Irinotecan, Procarbazine, Methotrexate, Carboplatin, AdriamYcin Cisplatin,
Vincristine,
Paclitaxel, I -(2-chloroethyl)-3-cyclohexyl-l-nitrosourea (CCNU), carmustine
(BCNU),
C yc I op hos-p ham ide, Docetaxe I, fluorouraci I (5 FU), Cytarabine,
doxorubicin,
CA 02691044 2009-12-14
WO 2008/012696 PCT/1B2007/003494
12
bleomycin, topotecan, tamoxifen (TMX), and imatinib mesylate (Gleevec) and all
other
therapeutics shown to be appropriate for treatment.
[0037] As additional examples, a physiological matrix of the invention is
adaptable for
measuring one or a cocktail of therapeutic compounds comprising one or more,
or at
least two compounds selected from one or more of CAF (Cyclophospha-
mide/Adriamycin/ Fluorouracil); CMF (Cyclophosphamide/ Methotrexate
Fluorouracil); CMFVP (Cyclophosphamide/ Methotrexate/Fluorouracil/
Vincristine/
Prednisone); PCV (Procarbazine/CCNU/ Vincristine); ICARBO-E (Ifosfamide/
Carboplatin/Etopos i de); TAP (Taxo I/A dri amyc in/ C spl ati n); EM A -CO
(Etoposide/
Methotrexate/Actinomycin/ Cyclophosphamide/ Vincristine); VBP (Vinblastine/
Bleomycin/Platinol (Cisplatin)); BPD-T: (Bcnu/PlatinoV Dacacarbazine/
Tamoxifen);
and T-10 (Methotrexate/ Bleomycin/Cyclophosphamide/ Dactino-mycin/Adriamycin),
Dactinomycin/Adriamycin) and any other actual or theoretical therapeutics
which might
be appropriate for treatment.
[0038] As yet another advantage, the means and methods of the invention may
be adapted
and arranged to test the efficacy of non-chemical therapies. As examples, and
not to be
taken as an exclusive listing, non-chemical therapies subject to being
evauated or tested
in the context of the present invention include one or more selected from the
group
comprising radiation therapies, brachiotherapies, herbal therapies,
naturopathic
therapies, experimental therapeutic compounds, arid new therapeutic compounds.
[0039] In additional aspects, the method may further comprise the step of
determining the
oncological status of the cells of the removed tissue from the data thus
obtained.
Preferably, the methods may further comprise the step of recording the data in
some
fixed media so that it can be analyzed, communicated to others, and used as a
record of
the procedures and their results.
[0040] In accordance with other objects of the invention, methods for
evaluating the
efficacy of one or more therapeutic compounds with respect to one or more
tissues
comprising cells from an animal are provided. Methods of the invention are
particularly relevant with respect to tissue and cells which are suspected of
being
abnormal, cancerous or pre-cancerous. Indeed, the present means and methods
are
especially adaptable to testing or evaluating one or multiple therapeutic
compounds
with respect to specific tissue and cells. As a result of this ability to
evaluate potential
therapies, such as therapeutic compounds, with respect to an exact tissue,
time, effort
CA 02691044 2009-12-14
WO 2008/012696 PCT/1B2007/003494
13
and expense are saved in terms of the determination of an appropriate
therapeutic
regimen.
[0041) In one preferred embodiment, the present methods for evaluating one
or multiple
therapeutic compounds comprises the steps of A) obtaining a sample of the
suspected
tissue from the animal, wherein the tissue comprises one or more types of
cells, B)
implanting a portion of the sample of the suspected tissue at least partially
within a
three-dimensional physiological matrix, the matrix being adapted and arranged
for
measuring one or more parameters of the behavior of the tissue and the cells,
C)
providing sufficient nutrition to the cells and the tissue so that the
parameters can be
measured, D) providing at least one of the one or more therapeutic compounds
to the
cells and the tissue, E) incubating or culturing the tissue and the cells with
the one or
more therapeutic compounds in an environment suitable for the growth of the
cells and
the tissue, and for a time sufficient to obtain measurements with respect to
the one or
more parameters, and F) measuring one or more of the parameters to obtain data
regarding the behaviors with respect to the one or more therapeutic compounds.
[0042] Advantageously, the methods may further comprise the step of, from
the data thus
obtained, determining the efficacy of the one or more therapeutic compounds
with
respect to the tissue and the cells. Preferably, methods of the invention may
further
comprise the step of recording the data in some fixed form, and may further
comprise
the step of transmitting the data to at least one evaluator. Evaluators and
the
recordation of data obtained by the present means and methods are also
discussed
herein.
[0043] The invention is also especially adapted for circumstances wherein
the sample
quantity is divided into portions, and each of the portions is subjected
concurrently and
separately to the method, wherein the method includes the use of one or more
controls
adapted for evaluating the one or more therapeutic compounds. Thus, the
present
methods can include the use of one or more controls adapted for evaluating the
one or
more therapeutic compounds with respect to the particular tissue, and with
respect to
specific conditions created by the test or testing lab or clinic. Methods of
the invention
also include where Step D is performed during Step E, and wherein the
measuring of
Step F is performed more than once with respect to each of the separate
portions of the
sample, and wherein the measuring is effected during the culturing or
incubating of the
tissue and cells. Preferably, the tissue portion is disposed either partially
submerged in
the matrix, or completely submerged therein.
CA 02691044 2009-12-14
WO 2008/012696 PCT/1B2007/003494
14
[0044] It is important that the tissue be in intimate contact with the
matrix. Thus, the
physiological matrix replicates to some extent the three-dimensional
environment in
which most tissues function in vivo. Tissue samples, or portions thereof, may
be
implanted in any method, manner or way, which yields the desired results.
Means and
methods of the invention can be used also where the implanted tissue is
obtained from
one or more of a liquid tissue, a cell suspension, cells or groups of cells
which have
been palletized, and wherein the tissue or cells are implanted in the matrix
after the
matrix Was formed into a gel or other permeable solid or semi-solid.
[0045] Means and methods of the invention can be used with virtually any
animal tissue as
described herein. Moreover, it is important to note that matrices of the
invention should
be essentially free of bicarbonate when the matrix is being formed into the
gel, semi-
solid or permeable solid. As an additional advantage, methods of the invention
can be
practiced with respect to one or more therapeutic compounds, for example where
a
single compound is tested and the method is performed with at least one set of
controls.
As one skill in the art of testing oncological remedies will appreciate, any
number of
controls, therapeutic substances, therapeutic methods, experimental compounds,
or
other cancer treatments such as radiation, radiotherapy or any other actual,
theoretical
or experimental therapy of method can be tested with the present invention in
in vitro
conditions.
[0046] Moreover, therapeutic compounds can be provided to the tissues and
cells as a
component of the sufficient nutrition. More therapeutic compounds are provided
to the
cells and the tissue as a component of the physiological matrix. As a
component after
the sufficient nutrition is provided to the cells and tissues or wherein the
one or more
therapeutic compounds include two compounds and the method is performed with
at
least one set of controls. It is similarly possible to use the present
invention and
methods to test three or more therapeutic compounds preferably with respect to
at least
one set of controls. Methods of physiological matrix is adapted and arranged
for
measuring the efficacy of one or more therapeutic compounds, and wherein the
one or
more therapeutic compounds are selected from the group comprising DNA damaging
agents such as alkylating agents, antibiotics which affect nucleic acids,
platinum
compounds, anti-mitotics, cell cycle stimulators, anti-metastatics, anti-
metabolites,
camptothecin derivatives, hormone therapies, biological response modifiers,
interferon,
anti-invasives, radiosensitizers, radiation, and any established, theoretical
or
experimental therapeutics directed against cell growth or cell invasion.
CA 02691044 2009-12-14
WO 2008/012696 PCT/1B2007/003494
[0047] The present means and methods with respect to a three-dimensional
physiological
matrix of the invention are adapted and arranged for measuring the efficacy of
one or
more therapeutic compounds,
[0048] More specifically, a physiological matrix of the invention is
adapted and arranged
for measuring the efficacy of one or more therapeutic compounds comprises at
least two
therapeutic compounds. And where at least two therapeutic compounds are
selected
from one or more of
[0049] It is again necessary to mention that the physiological matrices
according to the
invention should be essentially five of bicarbonate, for example wherein the
matrix is
formed from components that are essentially free of bicarbonate, and the final
gel form
of the matrix is thus essentially bicarbonate free.
[0050] As another distinct aspect of the present methods one or more
parameters can be
obtained regarding the behaviors of one or more therapeutic compounds, and the
behaviors of the cells themselves. Parameters thus measured yield data. In
turn, such
data can be used to evaluate the performance of the cells, or the performance
of one or
more therapeutics, or the performance of one or more other types of therapies
with
respect to the tissues and cells tested. Preferably, the measuring step E is
performed at
periodic intervals, and wherein the periodic intervals are appropriate to one
or more of
the cells, the tissue and the therapy or therapies being tested or evaluated.
[0051] Preferably the measuring of Step E is performed at periodic
intervals for a period of
time of between one hour and 15 days. More preferably, the periodic intervals
are one
or more selected from the group comprising every six hours, every 12 hours,
every 18
hours, every 24 hours, every 36 hours, every 48 hours, every 72 hours and
every 96
hours. Moreover other intervals such as every day, every second day and every
third
day, every fourth day, every fifth, day every sixth day, every seventh day,
every eighth
day, and every ninth day. Methods of the present invention wherein the
measuring of
Step E is performed at non-periodic intervals, and wherein the non-periodic
intervals
are appropriate to one or more of the cells, the tissue and the therapy or
therapies being
tested or evaluated.
[0052] As another advantage, the data obtained by the measurements of the
invention is
recorded in a fixed communications media. Such fixed media can be any type so
long as
it is capable of holding the obtained data in such form as to be useable
later. For
example paper, voice recordings, video recordings, photographs,
photomicrographs,
digital recordings, any fixed digital data means, any computer network-
facilitated
CA 02691044 2009-12-14
WO 2008/012696 PCT/1B2007/003494
16
means, infrared recording, ultrasound recordings, and magnetic resonance
recordings.
According to the invention one or more of a human observer, a still camera, a
video
camera, an automated still camera, an automated video camera, an infrared
camera, and
an automated infrared camera.
[0053] Preferably the culturing and/or incubating of tissues and cells
according to the
invention occurs for a period of time sufficient to obtain data regarding the
desired
parameters and values. For most tissue and cells to be tested, that period of
time is
between one hour and 15 days. More specifically, the culturing or incubating
of the
tissue and the cells typically occurs for a period of time of at least 1 hour,
or at least 10
hours, or at least 24 hours, or at least 36 hours or at least 72 hours, or at
least 125 hours,
or at least 175 hours, or at least 200 hours, or at least 250 hours or at
least 300 hours.
[0054] In some preferred embodiments, the environment for incubating or
culturing the
cells and the tissue includes a temperature range of from 26 to 43 degrees
Celsius, a
carbon dioxide tension of from 2.0% to 13.0%, and a humidity range of from 50-
100 %.
[0055] In accordance with other aspects of the invention, the sufficient
nutrition comprises
one or more fluids, and wherein the one or more fluids are selected from the
group
comprising tissue culture medias, concentrated tissue culture medias, and any
culture or
nutritional medias which are suitable for the tissue sample Preferably the
concentrated
medias are one or more selected from the group comprising IOX medias, and any
other
concentrated medias. Preferably, the sufficient nutrition is provided to the
tissues and
cells by placing the sufficient nutrition in contact with the matrix after the
sample has
been placed in the matrix
[0056] The method of the invention further comprises the step of J)
providing at least one
source of additional nutrition to the tissue and the cells so that the
parameters can be
measured. Wherein at least one source of additional nutrition comprises one or
more of
tissue culture medias, concentrated tissue culture medias, and any culture or
nutritional
medias which are suitable for the tissue sample and wherein least one
additional source
of nutrition is supplemented with one or more selected from the group
comprising sera,
proteins, sugars, salts, lipids. Preferably the sufficient nutrition is
provided to tissues
and cells by placing the sufficient nutrition in contact with the matrix after
the sample
has been placed in the matrix and the matrix has equilibrated to room
temperature,
wherein the room temperature is in the range of from 10 to 30 degrees Celsius.
[0057] Advantageously, the present invention can be used to obtain data
regarding one or
multiple parameters of the behaviors of the tissue and cells being evaluated
or tested as
CA 02691044 2009-12-14
WO 2008/012696 PCT/1B2007/003494
17
discussed herein. Significant among those parameters are the distance which
individual
cells migrate from the margins of the tissue sample, the average distance of
migration of
a group or population of the cells from the margins of the tissue sample, the
distance
which individual cells migrate from the tissue sample with respect to time,
and the
number of migrational cells per unit area of a microscopic visual field, that
is, cell
density in a portion of the gel.
[0058] As an additional advantage, methods of the invention may include
those for
evaluating the efficacy of one or more therapies with respect to at least one
tissue from
an animal, the tissue comprising cells, and the tissue being suspected of
being
abnormal, pre-cancerous or cancerous, the method comprising the steps of A)
obtaining
a sample of the tissue from the animal, wherein the tissue comprises one or
more types
of cells, B) implanting the sample at least partially within a three-
dimensional
physiological matrix, the matrix being adapted and arranged for measuring one
or more
parameters of the behavior of the cells of the tissue or cells, C) providing
sufficient
nutrition to the cells of the tissue of the portions so that the parameters
can be measured,
D) subjecting the cells and the tissue to at least one of the one or more
therapies, E)
incubating or culturing the tissue and the cells of the portions in an
environment
suitable for the growth of the cells and the tissue for a time sufficient to
obtain
measurements with respect to the one or more parameters for each of the
portions, and
with the one or more therapeutic compounds, F) measuring, with respect to each
of the
portions, one or more of the parameters to obtain data regarding the behaviors
with
respect to the one or more therapies.
[0059] In accordance with these and other objects of the invention, a three-
dimensional
physiological matrix suitable for culturing at least one tissue from an
animal, the tissue
comprising cells, is provided. In one aspect, the tissue or cells of the
sample are
suspected of being abnormal, cancerous or pre-cancerous. In another, the
physiological
matrix comprises A), at least one natural or synthetic fiber means, wherein
the fiber
means is adapted and arranged such that the cells, if abnormal, cancerous or
pre-
cancerous, are enabled to grow away from the sample into the matrix when the
sample
is implanted at least partially in the matrix. B), a sufficient amount of at
least one
additive comprising sufficient hydroxyl or hydrogen groups to bring the
effective pH
range of the matrix into an acceptable range, and C), sufficient nutrition to
sustain the
cells and the tissue in the matrix. It is important to. note that the matrices
of the
CA 02691044 2009-12-14
WO 2008/012696 PCT/1B2007/003494
18
invention and the sufficient nutrition should be essentially free of
bicarbonate when
they are being formed.
[0060] The three-dimensional physiological matrix is mixed from components
and formed
into a solid, a semi-solid, or a gel, and the matrix is adapted and arranged
to permit
migration of the cells away from the margins of the tissue and into the
matrix.
Moreover, the matrix is formulated, adapted and arranged to accept the sample
as soon
as the solid or gel is formed, or during its formation.
[0061] Preferably, a three-dimensional physiological matrices of the
invention comprise at
least one natural or synthetic fiber, for example, one or more selected from
the group
comprising type I collagens, type II collagens, type III collagens, type IV
collagens,
fibrin, fibrinogen, laminin, anti-laminin, positively charged poly(L-lysine)
amino acid
chains, positively charged poly(D-lysine) amino acid chains, and extracellular
matrix
proteins derived from one or more animals. In one preferred embodiment, the
matrix
comprises at least 90% Type I collagen. In another preferred embodiment of a
matrix
according to the invention, the gel comprises at least 95% Type 1 collagen.
[0062] The pH of a three-dimensional physiological matrix of the invention
is brought into
an acceptable range by means of one or more additives preferably selected from
the
group comprising NaOH, KOH and other strongly basic compounds. A matrix of the
invention may further comprise additional nutrition to be added after the
formation of
the matrix into a solid, semi-solid or gel.
[0063] In accordance with other aspects of the invention, the sufficient
nutrition comprises
one or more fluids, preferably selected from the group comprising tissue
culture media,
concentrated tissue culture media or any culture or nutritional media which
are suitable
for the tissue sample to be evaluated. Suitable concentrated medias include
one or more
selected from the group comprising lox media, and other concentrated media.
Preferably, the sufficient nutrition is provided to the tissues and cells by
placing the
sufficient nutrition in contact with the matrix after the sample has been
placed in the
matrix and wherein one additional source of nutrition is supplemented with one
or more
selected from the group comprising sera, proteins, sugars, salts, lipids, and
the matrix
has equilibrated to room temperature, wherein room temperature is in the range
of from
to 30 degrees Celsius.
[0064] In accordance with other advantageous aspects, a three-dimensional
physiological
matrix for use in the context of the invention can be adapted or arranged to
have
whatever pH value or pH ranges necessary to culture and evaluate the specific
tissues
CA 02691044 2009-12-14
WO 2008/012696 PCT/1B2007/003494
19
and cells. Preferably, the pH range for testing most tissues is from 5.0 to
8.3, more.
preferably 6.4 to 7.9 and even more preferably from 7.4 to 7.6. Preferably,
the tissue to
be evaluated or tested is implanted or disposed completely submerged within
the three-
dimensional matrix.
[0065] In accordance with other aspects of the three-dimensional
physiological nature of
the invention, a matrix is preferably adapted and arranged to be in sufficient
proximity
to at least one additional source of nutrition, wherein the matrix is
permeable to the
additional source of nutrition to the extent necessary that the sample tissue
and cells
receive nutrition sufficient to enable them to grow into the matrix to the
extent
necessary to measure the one or more parameters. Moreover, the at least one
additional
source of' nutrition may preferably include one or more additives or
supplements
selected from the group comprising serums, proteins, sugars, salts and lipids
or any
culture media that is suitable for the cell or tissue type. Preferably, the
density of the
fiber in a formed matrix of the invention is between 1.3 and 3.3 ings/ml, more
preferably from 2.0 to 2.8 mgs/ml, and even more preferably between 2.3 and
2.5
mgs/m I.
[0066] Advantageously, the present invention can be adapted and arranged to
be suitable
for measuring at least one parameter of the behavior of cells from the tissue,
and at least
one parameter relating to the suspected cancerous or abnormal status of the
cells or the
tissue. These parameters are listed hereinbeforc.
[0067] In a significant aspect, a three-dimensional physiological matrix of
the invention
includes at least one natural or synthetic fiber selected from the group
comprising Type
I collagen, wherein the Type I collagen comprises at least 70% of the total
fiber of the
formed matrix, or at least 80% of the total fiber, at least 90% of the total
fiber and at
least 95% of the total fiber. In one preferred embodiment, a three-dimensional
physiological matrix of the invention comprises at least one natural or
synthetic fiber
means of primarily Type I collagen, and at least one additive sufficient to
bring the pH
of the matrix into an acceptable range, such as a sufficient amount of 0.1 N
NaOH, and
wherein the sufficient nutrition is a bicarbonate-free 10X media.
[0068] In another preferred embodiment, a three-dimensional physiological
matrix of the
invention comprises eight parts by volume of the natural or synthetic fiber,
the at least
one additive comprises one part by volume of the 0.1 N NaOH, and the
sufficient
nutrition comprises one part by volume of the bicarbonate-free IOX media.
CA 02691044 2009-12-14
WO 2008/012696 PCT/1B2007/003494
[0069] In another aspect, a physiological matrix according to the invention
may comprise at
least one first hollow, cavity, slit or chamber, and the first hollow, cavity
slit or chamber
may be adapted and arranged to receive one or more tissues samples, wherein
the one or
more tissues samples are in the form of one or more of a solid, a liquid, a
suspension,
and combinations thereof. Moreover, a three-dimensional matrix of the
invention may
comprises at least one second hollow cavity, slit or chamber, wherein each of
the
second hollows, cavities, slits or chambers is adapted and arranged to receive
at least a
portion of the additional source of nutrition.
[0070] Preferably, the three-dimensional matrix of the invention matrix
comprises a gel, the
gel being suitable for measuring at least one of the one or more parameters
and of
producing resulting data. Moreover, the physiological matrices of the present
invention
are adapted and arranged for measuring the efficacy of one or a plurality of
therapeutic
compounds, many of which are exemplified herein. Moreover, means or methods of
the invention can be adapted and arranged to test the efficacy of non-
synthetic chemical
therapies, wherein the non-synthetic chemical therapies are one or more
selected from
the group comprising radiation therapies, brachiotherapies, herbal therapies,
naturopathic therapies, experimental therapeutic compounds, and new
therapeutic
compounds, hyperthermal therapies, hyperbaric therapies, hypobaric and
photosensitizing therapies.
[0071] An additional benefit of the present invention pertains to its
amenability to kit form.
Thus, in some embodiments, the invention may include a kit for testing the
efficacy of
therapeutic compounds on an expedited basis, the kit comprising a means for
collecting
at least one tissue comprising cells from an animal, the tissue being
suspected of being
abnormal, cancerous or pre-cancerous, at least one three-dimensional
physiological
matrix for culturing the tissue sample in three dimensions for a sufficient
length of time
to obtain measurements of at least one parameter of the behavior of the cells
and the
tissue, a means for measuring the parameters to determine a set of possible
therapeutic
compounds which might be efficacious for treating the tumor or abnormal
tissue, and a
means for testing the therapeutic effect of the determined therapeutic
compounds.
[0072] Preferably, the behavior of cells at multiple sites, for example, at
least 2 sites or at
least 3 sites, or at least 4 sites, or at least 5 sites is measured to obtain
data regarding the
behavior of the tissue or cells. In some preferred embodiments, readings made
using
microscopy and video-microscopy include the actions or steps of measuring
directly the
CA 02691044 2009-12-14
WO 2008/012696
PCT/1B2007/003494
21
migratory distance, speed, cell density and direction of movement of the cells
or tissue
with respect to the matrix upon which they are placed or cultured.
[0073] In
other preferred embodiments of the invention, the suspect tissue and matrix
are
then processed and treated so that they can be snap frozen, paraffin embedded,
sectioned, and then assessed with respect to one or more parameters,
including, but not
limited to, mitotic index/cell division, cell growth, necrosis, apoptosis,
anoikis,
adhesion, cellular signaling and invasion-related protein expression. Any
number of
protein and genetic parameters can be analyzed with respect to their
usefulness in
, determining the oncologic state of the cells or tissues.
[0074] In
accordance with certain other aspects of the invention, the matrix may be of
any
configuration or material which permits the evaluation of the effect of
therapeutic
compounds on the cells or tissues. In some embodiments of the invention, the
matrix
preferably comprises a collagen gel, the gel being suitable for measuring the
one or
more parameters.
Matrices could include any protein or glycoprotein or sugar
components of extracellular matrix, for example, including but not limited to,
fibronectin, laminin, hylaronin, growth factors and integrins.
[0075] In
general, in one set of preferred embodiments, the typical matrix element of
the
invention comprises a gel. In one set of especially preferred embodiments, a
matrix of
the invention comprises protein in its native, non-denatured state, in the
amount of 1.0
to 2.1 mg/ml. One preferred protein is, for example, Type I Collagen. Other
proteins
are amenable to use or adaptation as components of matrices of the invention.
In some
embodiments, a matrix of the invention possesses a final pH preferably in the
range of
from 6.0 to 8.5, more preferably in the range of from 6.5 to 8.0, and most
preferably in
the pH range of from 6.8 to 7.5
[0076] In
other embodiments, a matrix of the invention is incubated and used in an air
atmosphere having carbon dioxide preferably in the range of 1.0 to 18.0%, more
preferably in the range of from 2.0 to 14.0%, and most preferably in the range
of from
4.0 to 9.0%. A carbon dioxide incubator is useful for incubation processes
according
to the invention. In accordance with other aspects of the invention, the
parameters of
matrix composition, carbon dioxide tension, and other conditions vary
according to
tumor type. For example, for most categories of tumors, temperatures for
incubation of
the matrices combined with tissue or cells are generally kept within
approximate
physiological ranges, preferably of from 30 ¨ 44 degrees Celsius, more
preferably of
from 33 ¨41 degrees Celsius, and most preferably of from 35 ¨ 39 degrees
Celsius.
CA 02691044 2009-12-14
WO 2008/012696 PCT/1B2007/003494
22
[0077] In another advantageous aspect of some embodiments of the invention,
a matrix
may comprise one or more constituents provided to adapt the matrix for use
with a
particular tumor, or a particular class of tumors. Possible constituents to a
matrix
according to the invention, for example a gel matrix, may include growth
factors such
as VEGF or HGF, which tends to alter the invasion patterns of the tissue cells
into the
matrix and enhances cell growth. Other possible constituents include
structural
proteins, for instance, extracellular matrix proteins like laminin,
fibronectin, and the
various collagens, for example Collagen IV.
[0078] Immunoglobulins may also be included or added to a matrix of the
invention in
order to stabilize it in various respects. For instance, anti-laminin may be
included in
the matrix. As additional means and methods for adapting matrices of the
invention to
uses with respect to specific tumors or classes of tumors, lipids, such as
human or
bovine brain extract, solids like human dura, and plant extracts such as
phytostimulants
and inhibitors may also be included in a matrix of the invention in order to
vary its
characteristics and adapt it to specific use. In some embodiments, other
living cells
may be included. Exemplary cells include melanoma cells, fibroblasts and
endothelial
cells
[0079] Matrices of the invention may include inert additives also. As
examples, glass or
plastic shapes, such as cylinders may be provided within tissue culture wells
to act as
structural forms for the gel so that the matrix gel can be molded to create a
tiny well or
indentation useful for adding cell suspensions such as leukemia or lymphoma
cells to
the matrix in a pre-determined area in the matrix gels.
[0080] In accordance with preferred aspects of the invention, the one or
more parameters to
be measured comprise one or more of: the speed of proliferation of the cells,
the speed
of unidirectional migration of the cells, the speed of bi-directional
migration of the
cells, the speed of tri-directional migration of the cells, the rate of
mitosis of the cells,
the rate of change of migrational direction of the cells, the proportion of
cells which
change migrational direction during the test period, the speed of migrating
cells in a
particular visual field, the density of migrating cells per microscopic visual
field, the
migrational distance of individual migrating cells per microscopic visual
field, the
average migration-al distance of a group or population of migrating cells per
microscopic visual field, and the number of migrating cells per microscopic
visual field
direction.
CA 02691044 2009-12-14
WO 2008/012696 PCT/1B2007/003494
23
[00811 With respect to the invention, the "Test Pe'riod" is that period of
time during which
the one or more tissues or cells are cultured and tested with respect to their
oncological
behaviors, and the "Evaluational Period" is that period of time during which
the one or
more therapeutic compounds are evaluated with respect to efficacy in treating
the
specific tissue samples or cells, and other parameters.
[0082] In accordance with the many advantageous aspects of the methods of
the invention,
the one or more therapeutic compounds or treatments are selected from the
group
comprising, but not limited to, DNA damaging agents, anti-mitotics, cell cycle
stimulators, anti-metastatics, anti-invasives, radiosensitizers, radiation,
and any
established, theoretical or experimental therapeutics against cell growth and
cell
invasion.
[0083] Methods of the invention include the evaluation of one or more
therapeutic
compounds, individually, combined with one another, as one or more cocktails,
or in
any sequence, in order to determine their likely efficacy against the
suspected tumor
tissue and cells. Thus, in some embodiments, the methods of the invention
include
testing one therapeutic compound, or a pair of therapeutic compounds, or three
therapeutic compounds, or four therapeutic compounds, or more than four
therapeutic
compounds, or any number of compounds in a cocktail of compounds, or provided
at
different times or sequences to the tissue being evaluated.
[0084] Preferably, the culturing of the tissue and the cells during the
test and evaluational
periods is for a length of time sufficient to obtain the values required to
make a
determination of the efficaciousness of one or more potential therapeutic
compounds.
The culturing of the tissue and the cells occurs for a period sufficiently
long enough to
obtain the needed data. Thus, the culturing of the tissue and the cells occurs
during the
one or more test and evaluational periods, and thus during a period of time of
preferably
less than 10 days, more preferably of less than 8 days, even more preferably
of less than
6 days, and most preferably of less than 4 days. Thus, all readings typically
occur
within 10 days from the time of removal of the tissue or cells from the
mammal. Basic
microscopic readings of distance, direction and density are taken manually or
by
automated systems, preferably at predetermined intervals depending on the
nature of the
suspected tumor, the nature and amount of the tissue and the nature and number
of cells
or cell types being evaluated. In accordance with the various embodiments of
the
invention, the predetermined interval is one or more hours, for example, every
4 hours,
or every 8 hours or every 16 hours or every 24 hours.
CA 02691044 2009-12-14
WO 2008/012696 PCT/1B2007/003494
24
[0085] Video monitoring of the tissues and cells may also be provided in
order to gather
and record additional information. The video monitoring is preferably provided
on a
continuous basis at intervals. The intervals are selected based upon the
nature of the
tissue and cells, the nature of the suspected tumor or tumors, and the
estimation of
likely behavior of the tissue and cells on a matrix. In some preferred
embodiments of
the invention, video monitoring is provided on a continuous basis, at 30-
second
intervals, or at 60-second intervals or at 90-second intervals or at 120-
second intervals,
or at 150-second intervals.
[0086] Methods of the present invention include also those of testing the
efficacy of
therapeutic compounds on an expedited basis, testing the efficacy of
therapeutic
compounds with respect to a specific individual mammal, such as a human, and
testing
the synergistic efficacy of two or more therapeutic compounds on an expedited
basis.
[0087] The present invention includes also one or more kits for testing the
synergistic
efficacy of therapeutic compounds on an expedited basis, the kit comprising i)
means
for collecting at least one tissue comprising cells from a mammal, the tissue
being
suspected of being transformed into cancerous or pre-cancerous tissue, ii)
means for
culturing the tissue comprising cells for a sufficient length of time to
obtain desired
values, iii) means for evaluating the values to determine a set of possible
therapeutic
compounds which might be efficacious for treating the tumor, and iv) means for
testing
the therapeutic effect of the determined therapeutic compounds.
[0088] As one of skill in the oncology arts will appreciate, numerous
variations of the
means and methods disclosed herein fall within the scope of the present
application.
Moreover, the examples provided herein are provided as inclusive illustrations
of the
invention, and not as exclusive limitations.
DESCRIPTIONS OF THE FIGURES
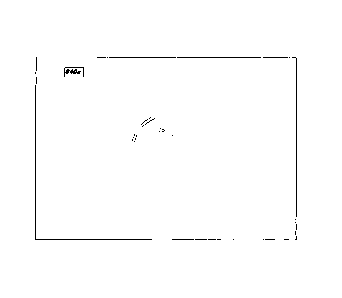
[0089] FIGURES 1 -8 are photographic and photomicrographic images which
show, by
way of example, use of the means and methods of the present invention with
respect to
a tissue sample removed from a patient. With respect to Figures 1 - 8, Patient
Sample
GS-640, comprising kidney tissue, was removed from the patient by a surgeon
during
surgery.
[0090] Tissue analysis and screening in accordance with the invention were
requested by
the surgeon, and proper consent was obtained from the patient prior to
surgical removal
of a sample quantity of tissue from the RHS Kidney. The patient was diagnosed
with
CA 02691044 2009-12-14
WO 2008/012696 PCT/1B2007/003494
renal cell carcinoma. A tissue sample of approximately 1 gram in quantity was
removed from the patient during surgery, and was placed into a 25 ml sample
jar, and
immersed in sterile saline. The tumor tissue was kept at 4 degrees centigrade
until
processed for the screening of therapeutic compounds in accordance with the
means and
methods of the invention. All procedures were performed under sterile
conditions in a
bio-safety laminar flow hood.
[0091] FIG I: Figure 1 is a photographic image showing the mechanical
division of the
sample into portions to be tested. The sample has been prepared by dissecting
away
any non viable tissue and blood prior to mincing into 1-2 mm pieces using
sterile
disposable scalpels.
[0092] FIG 2: Figure 2 is a photographic image showing tumor sample
portions which
have been placed into each well of a 48 well plate, and been submersed in a
gel matrix
mixture according to the invention. After insertion of the portions, the
mixture was
allowed to form into a gel at room temperature for approximately 30 minutes.
Once the
gel formed around the fully immersed tumor fragments, nutrient rich media was
added
as a liquid overlay that permeated the entire gel and tumor fragment.
[0093] Each different chemotherapy and or dose of chemotherapy was added to
a set of 6
wells out of the 48-well plate. A total of 7 different chemotherapies were
assessed in
this plate. One set of six wells were not treated with drug and thus acted as
'control
conditions' for tumor fragment growth and invasion. Each of the eight vertical
lanes
containing six separate wells was marked with a symbol for each
chemotherapy/dose
used and noted correspondingly on data collection sheets. Figure 2 shows plate
after
chemotherapeutics and growth media have been added. The plates were then
placed in
a conventional 37 degree Celsius incubator at 5% CO2.
[0094] FIG 3: Figure 3 is a photomicrographic image which shows the tumor
sample at
Day 0 where the initial appearance of the tissue fragment in the lower right
of the
photomicrograph shows no cell migration or invasion of cells away from the
margins of
the sample portion and out into the gel. This image is thus representative of
all wells at
Day 0 with or without chemotherapeutics. Images were made with a 400 X
magnification phase contrast micrograph using ZEISS camera specs.
[0095] FIG 4: Figure 4 is a photomicrographic image which showing a patient
control
sample (no treatment) after 3 days incubation @ 37 C and 5% CO2. Noteworthy is
the
migration of cells away from the solid tumor mass in a halo around the tumour
fragment.
CA 02691044 2009-12-14
WO 2008/012696 PCT/1B2007/003494
26
[0096] FIG 5: Figure 5 is a photomicrographic image which shows a patient
control
sample after 5 days. Noteworthy is the halo of cells and increased cell
density as
compared with the results shown at Day 3 of the test. The lines on the images
have
been drawn to show the distance measurements within which approximately 95% of
the
migrating cells have migrated away from the margins of the tissue sample.
[0097] Figure 5 also shows the presence of fibroblasts which sometimes
appear at the later
time points. Fibroblasts are noticeably different in numbers and appearance
than
migrating tumor cells. For example, fibroblasts appear in the surrounding gel
matrix as
much larger cells than the migrating tumor cells, and with significantly
larger
cytoplasm, as well as the classic elongated spindle fibroblast appearance.
Fibroblasts
also stain positively upon immuno-histochemical analysis for antibodies to
fibroblast
markers such as fibroblast growth factor. Thus, fibroblasts may be
differentiated from
tumor cells in a straightforward manner.
[0098] FIG 6 Figure 6 is a photomicrographic image demonstrating the effect
of treatment
with TaxolTm. The image of Figure 6 shows a sample well at 5 days after
treatment
with Taxoirm . Noteworthy is the change in behavior of the cells exposed to
Taxolml.
Specifically, a drop in both the distance and the number of cells that have
grown and
invaded away from the solid tumor fragment is seen on the lower left hand side
of the
image.
[0099] FIG 7: Figure 7 is a photomicrographic image which demonstrates the
effect of
treatment with DoectaxolTm. The image of Figure 7 shows a sample well wherein
the
tumor tissue has been treated for 5 days with DocetaxolTm while showing a
moderate
response to the drug. This moderate response is evidenced by the fewer number
of
invading cells than the control but those treated with DocetaxolTm have a
longer
migratory distance than treatment with TaxolTm.
[00100] FIG 8: Figure 8 is a photomicrographic image which shows the
carcinoma patient
sample treated for 5 Days with CisplatinTht. This image thus shows an example
of a
failed chemotherapy, being identical in appearance and cell numbers to the
control
sample (figure 5) at the same 5 day time point.
BRIEF DESCRIPTION OF THE TABLE
[00101] The present invention is adaptable to be used with many possible
therapeutic
substances, and combinations thereof, including those shown in the
accompanying
CA 02691044 2009-12-14
WO 2008/012696 PCT/1B2007/003494
27
Table Of Exemplary Therapeutic Compounds. In accordance with important aspects
of
the invention, the attached Table provides only an exemplary list of
therapeutic
compounds which may be evaluated with the present methods and means. Virtually
any other compound or combination of compounds with potential therapeutic
value may
be tested and evaluated with the present invention. Moreover, the means and
methods
of the present invention are adaptable and useful for testing any compound or
combination of compounds with respect to their potential therapeutic value,
regardless
of whether those compounds have been involved in the drug approval process.
Thus,
the present invention provides means and methods for initially testing and
evaluating
compounds with respect to possible efficacy against many tumors and tumor
types
without the necessity of using animal or human subjects.
[00102] As those of skill in the art will appreciate, numerous permutations
of the matrices,
methods, apparatus and kits of the invention are possible within the metes and
bounds
of the information disclosed herein. Thus, although the present invention has
been
described with reference to some of the preferred embodiments, variations and
modifications of steps, elements and components of the invention can be
substituted
therefore, while remaining within the spirit and scope of the invention.
CA 02691044 2009-12-14
WO 2008/012696
PCT/1B2007/003494
28
TABLE OF EXEMPLARY THERAPEUTIC COMPOUNDS
Quantitiy Compound
3 TMZ 250mg capsules (Scering)
1 Irinotecan 20mg/m1 solution x 5m1 vial
(Pharmacia)
1 Procarbazine 50mg capsule (Sigma-
Tau)
1 Methotrexate 25mg/m1 solution x 20m1
vial (Mayne)
1 Carboplatin 10mg/m1 solution x 45m1
vial (Mayne)
1 Adriamycin 2mg/m1 solution x 100m1
vial (Novo)
1 Cisplatin 1mg/m1 solution x 100m1 vial
(Mayne)
1 Vincristine 1mg/m1 solution x 5m1 vial
(Mayne)
1 Paclitaxel 6mg/m1 solution x 50m1 vial
(BMS)
3 CCNU 100mg capsules (BMS)
1 BCNU powder for injection x 100mg
vial (BMS)
1 Cyclophosphamide powder for
injection x 2gm vial (BMS)
1 Docetaxel 40mg/m1 solution with
diluent x 2m1 vial (Aventis)
1 5FU 50mg/m1 solution x 100m1 vial
(Mayne)
1 Cytarabine 100mg/m1 soltuion x 10m1
vial (Mayne)
1 Cytarabine 100mg powder for injection
(Pfizer)