Note: Descriptions are shown in the official language in which they were submitted.
CA 02691607 2010-02-01
METHOD FOR THIOSULFATE LEACHING OF
PRECIOUS METAL-CONTAINING MATERIALS
FIELD OF THE INYENTION
The presont invention is directed geaerally to the recovery of precious metals
from precious metal-containing material and specifically to the recovery of
procious
metals &om precious metal-containing material usin$ thiosulfate lixaviants.
BACKGROUND OF THE INVENTION
A ttaditional technique for recoveaing precious metal(s) firnn precious metal-
containing ore is by leaching the mateiial with a cyanide lixiviant. As used
horein, a
"precioas metal" refers to gold, silver, and the platinum group metals (e.g.,
platinum,
palladimri, ruthenium, rhodium, osmium, and iridium). Many countries are
placing severe
limitations on the use of cyanide due to the deleterious effects of cyanide on
the
environment. Incidents of fish and other vv7dlife having been killed by the
leakage of
cyanide into waterways have been reported. The limitations being placed on
cyanide use
have increased substantially the cost of extractiag precious metal(s) from
ore, thereby
decressing ptecious metal remves in many countries. Gyanide is also unable to
recover
precious metals such as gold from refractory ores without a pretreahnert step.
"Re&wtory ores" refer to those ores that do not respond well to conventional
cyanide
r-.... . _ . ,r
f,,
CA 02691607 2010-02-01
leaching. Examples of refractory oras include sulfidic ores (where at Ieast
some of the
precious metals are locked up in the sulfide matrix), carbonaceous ores (where
the
precious metal complex dissolved in the liaiviant adsorbs onto carbonaceous
matter in the
ores), and ores that are both sulfidic and carbonaceous.
Thiosulfate has been actively oonsidered as a replacement for cyanide.
Thiosulfate is relatively inexpensive and is far less haimful to the
enviranment tbsn
cyanide. Thiosulfate has also been shown to be effective in recovering
precious metals
from pretreated refractory preg robbing carbonaceous ores and sutfidic ores.
As used
herein, "preg robbing" is any material that interacts with (e.g., adsorbs or
binds) precious
metals after dissolution by a lixiviant, thereby iurteifesing with precious
metal extraczion,
and "carbonaceous material" is any material that includes one or more cabon-
oontaining
compounds, such as, but not limited to, humic acid, graphite, bitumins and
asphaltic
compounds.
Where gold is the precious metal, thiosulfate leaching techniques have
typically
relied on the use of copper ions to catalyze and accelerate the oxidation of
gold, ammonia
to facilitate the farmation and stabilization of cupric ammine ions and/or a
pH at pH 9 or
above to maintain a region of stability whera both the capnc ammine and gold
tlrioenlfate
complexes are stable.
It is well known in the art that the caialytic effect of oopper and ammonia in
conventional thiosulfate leaching of gold is described by the following
sequence of
reactions.
-2-
r---
CA 02691607 2010-02-01
FoTII]8tlon of tl]e cDpflc an1n11ne complex:
CW' +4NH, 4 C40.
)~`
Oxidation of gold by cupric ammine, gold complexation as the gold-thiosulfate
anion,
and reduction of cupnc ammine to anpmns thiosalfabe:
Au+ Cu(NH3):+ = SSzO3 ~ Au(S2O3)2 + C~S203)3 + 4NH3 (2)
Oxidation of the cuprous thiosulfate back to cupric ammine with oxygen:
Cu(S2O3)3 +4NH3+y4O2+y2H2O-*Cu(NH3)4++3S2O3 +OH- (3)
Summing equations (2) and (3) yields the overall thiosalfaie leach reaction
for SDld:
Au + 2S203 + 3/, 02 + H20 -+ Au(S203 )z + OH- (4)
It can be seen from the above equations that copper and ammonia act as
catalysts
in that they are neither produced nor consumed in the overall leach ceaetion.
Copper and ammonia csn be a source of problemns. Rapid oxidation of
thiosulfate
by cupric ammine to fozm polythionates oooura, leading to excessive
degredation and loss
of tluosulfate:
2C1<NIA}4 +8S26? --->2CZ<S'2q)4 +S406 +gNg
(5)
Oxidative degradation of thiosulfates by moleoular oxygen to polythionates and
sulfates
is aocelerated matlcedly in the presence of copper ions and/or ammonia
Molecular
oxygan conversion to thiosnlfates is believed to ocxur according to sequence
of reactaons
that involve the formation of inteimediate polythionates (polythionates ean be
S OZ
represented by ~ 6 , where n= 2-6):
-3-
r---
CA 02691607 2010-02-01
Tetrathionate formation: 2S203 + 02 + H20 -+ S4O6 + 20H (6)
Trithionate formation: 3S406 + Z 02 + H2O -> 4S306 + 2H+ (7)
Sulfite formation: S306 +)2OZ + 2HZO -* 3S03 + 4H+ (8)
Sulfate formation: 2503 + OZ -+ 2504 (9)
OveraU: S2 0; -+ 202 + H20 -> 2SO4 + 2H+ (10)
Not only can the degradation of tbuiosulfate lead to increased reagalt oosts
but also it has
been discovered that excessive levels of sulfate can cause demased gold
recoveries.
While not wishing to be bound by any theory, it is believed that excessive
levels of
salfates can lead to unaoceptable rates of thiosalfate degradation and levels
of iastability
in the thiosulfate lixiviant. Additionally, ammonia gas can be released into
the
atmosphere when atmospheric leaching is perfornned. Ihe loss of ammonia by
volatilization occurs readily, partiail.arly ia unsealed gas-sparged reactors
and heaps
operating at pH greater than 9.2, leading to excessive ammonia consumption:
NH4 + OH' -+ NHJ(.g) + HZO -+ NH3(8) + H2 0 (11)
SU1vIlV1ARY OF THE IZA9ENTION
Tliese and other needs have been addrmed by de me~hodologies and systenns of
the present invention. The methodologies can recover precious metals, such as
gold and
silver, from a variety of matetiats, including refivctory carbonaceous or
sulfidic ores,
double refraetory ores (e.g., ores containin,g both sulfide-locked gold and
eaifionaeeous
preg-robbing matter), oxide ores, nonrefraatory sulfidic ores, and ores also
containing
-4-
r.,,....
__ ~
CA 02691607 2010-02-01
coppec minerals and other materials derived from such ores (e.g.,
concentratee, tailings,
etc.).
In one embodiment, a prooess for recovering a precious metal &+om a precious
metal-containing material, includes the steps of
(a) providing a heap of the preaious metal-containing mateaial; and
(b) passing a thiosalfate lixiviant md molecular oxygen through the heap to
form a pregnant leach solution comprising dissolved precious metals. As used
herein, a
"heap" refen to any self-supporting body of partimlate material, includiag,
without
limitation, a partialate-containing heap, vat, and dump. The molecular oxygen
is at a
pressare greater than ambient atmospheric pressure before introduction into
the heap.
Preferably, the dissolved moleaular oxygen content of the lixiviant ranges
from about 1 to
about 50 mg/L, and more preferably from about 3 to about 40 mg/L. Molecular
oxygen
can avoid the need for high levels of copper and ammonia in the lixiviant as
catalysts
without compromising precious metal recoveries. Preferably, the lixiviant
comprises no
more than about 20 mglL dissolved copper.
The tbiosulfate lixiviant can be derived from any suitable form(s) of
thiosutfate,
such as sodium thiosulfate, calcium thiosulfate, potassium thioseilfate and/or
anunonium
thiosalfate.
The precious metal can be recovered finm the pmegnant leach solution by any
suitable technique. By way of example, the precious metal can be recovered by
resin
adsorbtion methods such as resin-in-pulp, resin-in-solution, and resin in-
leach or by
solvont extvction, eeaneatation, electrolysis, precipitation, and/or
combinations of two or
more of these techniques.
-5 -
r---
__._.__........M...~.,~...~,.~-
~~~:
CA 02691607 2010-02-01
As will be appreciated, heap leaohing can typically be peiformed at lower
capital
and operating costs than tank leachimg and can yield sunilar precioas metal
recoveries.
Recoveries of ptecioas metats by both proceases can be at least about 70% and
sometimes at leaet about 800/a, witbout the need for high levels of copper in
the
tlriosulfate lixiviant. Surluiaingly, when ammoniucn tluosulfate ie used the
presence of
aunaonwm In the thiosulfate lixiviant does not necessarity cause the release
of significant
amounts of atnmonia gas, notwittisanding the I.I. aoqmcirculation of a
molecular
oxygen containing gas through the heap. This is so because the pH of the
liaiviant is
prefargbly maintained at a pH of no more than about pH 9. In this msmw, the
free
ammonia eonteat of the lixiviant can be mWntained at no more than about 2,000
ppm.
Reducing or eliminating the need to have copper ions and/or ammonia present in
the kaoh by effective ase of moleawLar oxygen as the oxidant can provide
significant
multiple benefits. First, the cost of having to add copper and amrnonia
resgeats to the
p+ooeas can be reduced significandy or eliminated. Second, mvironmartat
coocoas
relating to the presence of potentially harmfal amounts of copper and ammonia
in the
tailbig,s4 or other wsst,e streams geneiated by the proeess cen be miti$aied.
Third, the near-
abmce or complete absence of copper and ammonia in the leach can provide for a
much
more reliable and robust leaehicg procesa, yielding more stable leachates,
able to operate
over a wider pH eml oxidationa+edudion potantial (ORP) range tLan is poomble
with
oonventional thiosulfate leaching. The latter process must operate in the
relatively
nari+ow window of pH and ORP where both the capric mmme complex and the gold
tbiosulfate complex co-exist. Finally, the near-absence or complete absence of
copper
-6-
r~
CA 02691607 2010-02-01
and ammonia in the leach can reduce or duminate entirely a host of deleteious
side
reactions that consume thiosulfate and ara otherwise diff:cult or impossible
to prevent.
Pref+erably, the thiosulfatie lixivi:m is at least mfttmtally fiee of sulfite
durin,g
the leacbing step. The elimination or near elimination of sulfite fi+om the
thiomilfate
leach can have advantages. Sulfite can deprass the rate of dissolution of
precious metal
from the prec,~ious metal-containing material by reduang signi.ficantly the
oxidation
reducdc potanaal (ORP) of the leach solutioa or 1Aiviant. As will be
qvtvdated, the
rate of oxidation of the gold (and therefore the rate of dissolution of the
gold) is directly
dependent on the ORP.
In yet another ernbodiment, a procm for reooveaing a precaous metal fim a
oarbooaceous pmous metal-containing materiai is pmvided tbat includes dhe
steps oF
(a) contacting a thiosalfate lixfviant with a precious metal-containing
material to
form a pregnant leach solution, the pregnaat leach solution comprIsing a
dissolved
1reiops meWl, tbiosuiEabe, PoLytbionab% and sulatr, and
(b) maintainin.g a dissolved solfate concwftfioa in the pregaant lewh sohrtion
of
no more than about 100 g/L.
Sulfatas are oommonly in the liaiviant due to the degradation of thiosulfate.
The
puem of sulfate has been faRnad to der,mase pmcioas metai reooveries, wldch is
believed to be due to the inmssed instability of thiosulfate in the presence
of sulfate.
Higher levels of salfates are believed to oause a more rapid rate of
degradation of
thiosalfate into polytbionates and, ultiniatedy, sulfata As will be
apprer,iatiod, sulfite
removal can be effected by aumarous techniques, iac,luding precipltation,
membrane
Sltcxtion, solvent extraction, and ion exchaage.
-7 -
~--=-
CA 02691607 2010-02-01
In a preferred process configuration, the dissolved sulfate is precipitated
asing
calcium. The calcium is typically introduced into the lixiviant as calcium
carbonate,
calcium chloride, calcium nitrate, calcium oxide, calcium thiosulfate, calcium
hydroxide,
and mixtnc+es tlioeof.
In yet another embodiment, the proqnant leach solution from a thiosulfate
leaching step is contacted, after the leaching step, with a reagent to canvert
at least about
50% and typically at least most of polythionates (particularly trithionate and
tetrathionate) into thiosulfate and elemental sulfur and grecipitate dissolved
precions
metals (and dissvlved transition metais) followed by conversion of the
elemental sulfiir
intio tlniosulfate. The roagrut or reductant can be any soitable reactant to
convert
polyttnionates into tluosulfatc, with any salfide, and/or polysalfide (i.e., a
compoucnd
containing one or a mixture of polymeric ion(s) S,2', where x= 2-6, such as
disulfide,
trisulfide, tehwulfide, pentasulfide and hexasalfide) being particularly
preferred. A
sulfite reagent can also be used for thiosulfate regeneration but is ge.mlly
effeative only
in convWting polythionatos of the form S0e', where x = 4 to 6, to thiosulfate.
The elemental solfur is convated into thiosulfate by contacting the product of
the
sutfide precipitation step with a sulfite reagent. The sulfite reagent can be
any form of
sutfite, with a bisulfite being prefetred. The convetaion of the elemental
sulfur into
thiosulfnte caa lead to lower thiosulfate reagent costs compared to a process
in which the
elemeutal sulfac is discarded and can control effec,tively the form and amount
of sulfiu at
diffiaring locations in the pzocess.
-8 -
CA 02691607 2010-02-01
The salfide, bisutfide, and/or polysuifide can be compounded with any cation,
with Gtroup IA aud IIA etemeuts of the Periodic Table, ammonium, and hydrogen
being
prcferrod=
In yet another embodiment, a process for recovering a procious metsl from a
carbonaoeous precious metsl-coataining material is provided in which a
carbonaceous
precious metal-containing material is contacted with a thiosalfato-containing
lixiviant.
The lixiviant contains a blinding agent While not wishing to be bound by any
theory, it
is befieved that the precious metal thiosulfate oomptex may be unstable under
certain
conditions and that the precious metal can be stripped from the thiosulfate-
containin,g
solution by a number of substances commonly encountered in precious metal-
oontainsng
materials. The substances or preg robbmg materials typicaUy absorb, adsorb or
precipitate the precious metal. Such preg-robbing materials include
cwfionaceous
materials, pyrite-containing materiats, chacopyrlte and iron oxides.
Surprisingly and
unexpeetedly, blinding agents may be used in the thiosalfate lixiviant to
prevent or inbibit
prog robbing of the precious metal by the preg robbing material. The blinding
agent itself
abembs or adsorbs (in prefesence to the precious metal) or otherwise
neutratizes (such as
by chemical reaction) the preg robbing sites on the material. The blinding
agent
preferably includes one or more of hydincarbons, alcohols, a4tars, al<khydes,
surfaoteunts,
lauryl suifonates, phosphates, and metal salts.
BRIEF DESCRIPTION OF THE DRAWINGS
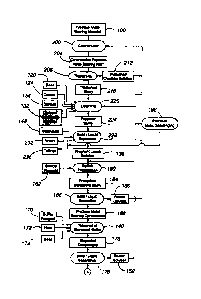
Fig.1 is a flow schematic of a first embodiment of the present invention;
Fig. 2 is a flow schematic of second embodiment of the present invention;
-9-
,___... . . ,
.,....~,.:.r..W._.._...,....._.
CA 02691607 2010-02-01
Fig. 3 is a plot of gold extnactkon in permt (vertical axis) against time
(horizontat
axis) with unagglomerated and agglomerated precious metal-containing ore; and
Fig. 4 is a plot of gold extraction in percent (veatical axis) against applied
solution
amount (horizontal axis) with and without heap aoration.
DETAII.ED DESCRIPTION
The present inveation provides an improved thiosulfate leaohing process for
the
recxrvery of precious metals from precious metal-bearing material. The
precious metat(s)
can be associated with nonprecious metals, such as base metals, e.g., copper,
nickel, and
cobalt. The preaous metal-bearing materiai includes ore, conoentrates,
tailings, recycled
industrial matter, spoil, or waste and mixtures theroo The invention is
partiailarly
effective for recovering precioua metals, particolarly gold, from refracbory
carbonaceous
material.
FigureslA and B are collectively a flow chart according to a first anbodiment
of
the present invention. The process of the flow chart is particalarly effkrive
in recovering
gold from sulfidic and carbonaceous material and oxide material and mixturos
thereof.
Referring to Figure 1A, a precious metal-bearing material 100 is comminuted
104, such as by wet and/or dry crushing and optionally wet and/or dry
grinding, to form a
comminuted precious metal-bearing niaterial 108. Comminution 104 typically
reduces
the particle size of the material 100 sufficiently to liberate the gold-
bearing minerals.
Typically, the comminuted precious metal-bearing material 108 is comminuted to
a Poo
size of from about 2 inches to about 1/4 inch.
-10 -
r--=- ,
CA 02691607 2010-02-01
To provide desired levels of heap porosity aad permeability, the comminuted
procious metal-bearing material 108 is agglomerated 112 by known techniques to
form
agglomerates 116. One or more of a base 120, the thiosulfate lixiviant 122,
copper 124, a
binder 128, and a calcium-containing material 132 may be oonWted with the
comm.inuted material 108 immediately before or during agglomeration 112 to
assist in
agglomerate formation and "jump start" the leaching process of step 136. In
other words,
the base 120, thiosalfate lixiviant 122, copper 124, and binder 128 are
incorporated into
the aggiomerate matrix.
The base 120 can be any suitable base mataial effective to adjust the pH of
the
thioMilfate lixiviant tD desired levels. Preferably, the pH of the
thiostilfate liaiviant is no
more than about pH 10, more preferably no more than about pH 9, and even mor+e
preferably ranges from about pH 8 to about 9. Preferred bases include alkali
or alkaline
earth metal oxides, carbonates, hydroxides, cement, ammonia, and mixtures
thereof. To
realize these opwating pHs, the amount of base (lime) incorporated into the
agglomerates
of a typical ore ranWes from about 0.1 to about 10 kg/tonne of comminuted
proaious
metalbearing material 108. The base 120 is typically introduced in powdervd
form to
the comminuted precious metal-bearing materia1108 daring agglomeration 112.
The copper 124, which is optional, can be in any suitable form that is soluble
in
the tbiosuifate lixiviant. Preferably, the copper 124, when added, is the form
of a copper
sulfate, eopper oxide, copper nitrate, copper chloride, and mixtores theroof.
Suffcient
copper may be added to eatalyze the leaching reaction when necessary to
reatize desired
rates of precious metal reeovery . When added, the prefernvd mass ratio of the
copper ion
to tlriosulfate ion is preferably from about 1:10 to about 1:1000. Typieally,
the copper is
-11-
r_._......
CA 02691607 2010-02-01
added in the form of copper salfate in an amount ranging from about 1 to about
100
g/tonne of comminuted preoious metal-bearing material 108. Prefembly, no
copper is
added but rather oxidation is effected by raising the lixiviant's dissolved
molecular
oxygen content above naturally occuning levels.
The binder 128, which is also optional, can be any saitable binder capable of
producing robust agglomerates. Possible binders include a oommercially
available
cohesivity agent such as NALCO 9704, cxmeat, lime, and other long chain
polymers,
water, and mixttues thereof TLe preferred binder 128 is a oohesivity agent,
which along
with the thiosulfate lixiviant 122, can prmde highly robust agglomerates. The
amount
of binder 128 employed typically ranges S~om about 0.1 to about 10 kg/tonne of
comminuted premons metal-bearing matorial 108. The biader 128 is typically
added to
the comminuted precious metal-bearing material as a free flowing pazticulate
or a liquid
before or during agglomeration.
The calcium-eontaining material 132 controls the conomftfion of sulfates in
the
various process solutions described below. The calcium-containing material 132
is in a
form that is soluble in the thiosulfate lixiviant so as to provide calcium
ions to react with
sulfate ions to form and pt+ocipitate gypaum (CaSO4). Because the gypsum
preclpitates in
the beap ranoval of 83+p$um by f}tration or other means is not required.
Prefistted
ealcium-0ontaining materials inelude lime (CaO), calcium cabonates, calcium
nitrates,
calcium chlorides, cakiom hydroxides, calcium thiosulfate, and mixtures
thereoiy with
lime being particalarly prefexred Lime is able to perform not only sulfate
control but
also pH eontrol, thereby potentially rendering the base 120 unnecessary. The
amount of
calcium-containing material is preferably sufficient to maintain a maximum
dissolved
-12 -
.---- ,
-... ~.- ~.
CA 02691607 2010-02-01
sulfate ion conoentration in the pregnant leaoh solution 138 of about 150 g/L,
more
preferably of about 100 g/L, and even more preferably of about 50 g/L, and
even more
preferably of about 30 g/L. The amount of calcium-containing material
therefore
depends on the rate of degradation of the thioaalfatie into sulfate between
cycles of
thiosulfate regenesation in steps 140 and 144 (discussed below). This can be
quantified
by measuring the catrent sulfate concentration at one or more selected points
in the
process and adding sutficient calcium to reduce the concentration to desired
levels.
Typically, the amount of calcium is at least about 0.1 kg, more typically at
least about 1
kg, and even more typically ranges from about 0.1 to about 5 kg/tonne of
oomminuted
precious mesal-bearing material 108. The calcium-containing material 132 is
tqpically
added to the commimabed precious metal4xwing material 108 as a fi+ae flowing
particulate material or slurry or liquid before or during agglomeration. As
will be
appreciated, the amount of calcium includes native or naturally occurring
calcium already
present in the materia1108.
As will be appreciated, metals other than calcium may be used to effect
sulfate
precipitation. Examples of other metals inchade lead and barium. These other
metals are
gmeally not panef+erred for purposes of cost and enviroamnental
considerations.
In a preferred pmee.ss configuration, the ealcium-containing material 132 is a
mixture of calcium compounds, with a mixture of lime and calcium carbonate
being
particularly preferred.
Finally, the tlviosulfate lixiviant 122 is contacted with the comminuted
precious
metal-bearing mataria1108 before or during agglomeaation 112. The thiosulfate
lixiviant
122 is made by recharging the conditioned reoycle solution 144 (discussed
below) with
-13-
r---
CA 02691607 2010-02-01
fresh thiosalfate 148. As discussed below, the conditioned recycle solution
144 is the
product of conditioning the recycled barrar lixiviant 150. The optimum
solution
tbiosolfate concentration to maintain during heap leaching 136 and therefore
the optimum
solution thiosulfate concentration in the thiosulfate lixiviant 122 will
depea.d on the
nature of the materiat being leached, but will preferably range from about
0.005 to about
2 molar (M), more preferably about 0.02 to about 0.5 M, and even more
preferably from
about 0.05 to aboud 0.2 M. The som+ce of the fresh thiosulfate 148 can be any
available
thiosnlfate-containing compound, such as sodium thiosulfate, potassium
thiosulfate,
calcium thiosulfate, ammonium thiosalfate, or any other thiosulfata-oontaining
material
or thiosulfate preauao7. Alteanatively, thiosulfate can be gar-eated in situ
or in a
separate step by reaction of elemental sulfiu with a source of hydroxyl ions,
in
aocordance with the following reaation:
2(x + 1)S + 60H' --> S2O3 + 2S2X + 3H20 (12)
where x = 3-6, or by reaction of bisulfide with bisalfite:
2HS' + 4HS03 -+ 3S203 + 3H20 (13)
or by reaction of elemental sulfur with sulfite:
S+ SOg -+ S203 (14)
As will be appreciated, to produce a struatnred agglomerate cement (not shown)
may be added during agglomeration. T he cement is added in particulate form
before or
daring aggiomeration and is thereby inoorporated into the agglomerate. When
used, the
amount of cement typically ranges from about 1 to about 50 kg/tonne of
comminuted
precious metal-bearing materia1108.
-14-
r--,
õ
CA 02691607 2010-02-01
The * siza of tltie agglomeistes 116 depends, of oourse, on the heap desigu.
Typically, it is pmeferred that the Pso size of the pazticles/agglomerates
fozmed into the
heap is at least about 150 pm, more preferably at least about 500 m, and even
more
preferably at least about 1,000 pm.
As an alternative to aggl~oa, it is possible to provide dewed lrvels of heap
porosity and permeability by comminuting the precioua metal-containing
materiel to a
desired size range. In that event, the base 120, copper 124, and oalcium
oonditioning
material 132 is inoorpoaafied into do lop dming heep cmstnmtion (or stadWg).
In
other words, these materials may be contacted with the oomminuted precious
metal-
beating materia1108 on the stadcing conveyor belts or in the haulage
oompattment of a
haulage vehicle which will dump the material 108 onto the heap pad. The
various
mataials may be located tmifoannly tlroughout the ]up or in a mne of the heap,
mmch as
at the bottom, middle or top. When agglomeration is not petformed, the
comminuted
material 108 has a preftxed Pao size of at least about 150 pm, more pmferably
at least
about 500 pm, and even more preferabiy at leagt about 1,000 m. This size
range is
reaiized by removing fine pattides (particle:s preferably having a size of
less tlm abwt
150 pm and more preferably of less than about 500 pm) from the cornminuted
material
108, by suitable screens, filUrs, and the like, prior to matnyal plaomflent on
the leach pad.
T19oealate lixiviant can be oontacted with the paRiales of matcQial as the
peztioles are
being placed on the heap.
In step 136, 8o agglomorates 116 are leached to form a pregoaat leach solution
138 oontaining dissolved precious metala solubilized ffom the precious
metat.bearimg
material 108. The extcaction of pcecious metals in the leachia,g step 136 is
rolatively
-15 -
r---
CA 02691607 2010-02-01
high, particalarly for carbonaceous ores. Typieally, at least about 50%, more
typically at
least about 70%, and even more typically at least about 80% of the precious
metal in the
precious mctal-oontaining mattxial 108 is exiracted or solubilized into the
pregaant
solution 138. The concentration of the dissolved precious metal in the
pregnant solution
138 typically ranges from about 0.05 to about 100 ppm and more typically from
about 0.1
to about 20 ppm.
BeSore leaching can commence, the heap must be formed on a leach pad. The pad
typically includes a Iiquid impervious liner, which is placed beneath the
heap, to collect
the pregnant leach solution 138 and prevent the pregnant leach solution 138
from being
lost to the siurounding environment. The heigbt of each lift of the heap is
typically from
about 4 to about 8 m and of the heap itself cxin be up to 100 m. Rather than
moving the
heap after thiowlfste leaching is co pleted (which is done im a dynsmnc heap
confifiguration), further heaps or lifts can be built on top of axhausted
heap(s) (which is
done in a static heap configuration).
During heap constzuction, a network of aetating pipes can be located in a
lower
portion of the heap to force an oxygemcontaining ges 154 through tho heap
durini,g
thiosulfate leaching. The pipes can be perforated so as to provide a
substantially uniform
dispersion of the gas throughout the heap. The oxygen-containing gas 154 is
typically
forced through the pipes usiog a single- or multi-stage oompressor, blower,
faa, or other
mechanical device. When the oxygen-eontaining gas 154 is pressluized and fmced
through the pipes, it typically has a pressure greater than the ambient
atmospheric
presesure, more typically of at least about 1 inch H20, and even more
typically of at least
about 30 inches H20 gaft thaa the ambient atmospheric pressure. Preferably, at
least a
-16-
~---
CA 02691607 2010-02-01
stoichiometric amount of molecular oxygen (relative to the amount of precious
metal in
the precious metal-containing material) is deliberately introduced into the
heap during
leaching 136. More preferably, at least about 0.5 kg of molecular oxygen and
even more
preferably from about 1 to about 10 kg of molecular oxygen is introduced into
the heap
during leaching for each ton of material to be leached in the heap.
Preferably, at least
about 2 and more preferably from about 4 to about 40 m3 of oxygen-containing
gas is
introduced into the heap for each cubic meter of lixiviant applied to the
heap.
Using gold as an exampie, the thiosulfate leaching of precious metal-bearing
m.aterial in the presence of molecular oxygen can be illustrated by the
following reaction:
jo Au+2S203 +402+~iH20~ Au(SZ03)2 +OH-(15)
The oxygen-containing gas may include atmospheric air, or it may include
relatively pure
(95%+) oxygen such as that produced from any commercially available oxygen
plant, or
it may include any other available source of oxygen.
To control evolution of ammonia gas during forced air introduction into the
heap,
the pH of the thiosulfate lixiviant 122 and recirculated pregnant leach
solution 138 are
controlled. Preferably, the pH of the thiosulfate lixiviant and solution 138
are maintained
(when introduced into the heap and during passage through the heap) at a pH of
no more
than about pH 9, more preferably of no more than about pH 8.75, and even more
preferably of from about pH 6.5 to about pH 8.75. Alternatively, the
concentration of
free ammonia can be maintained below levels sufficient to result in evolution
of
signiSeant amounts of ammonia gas. In some configurations, the concentration
of free
ammonia in the thiosulfate lixiviant applied to the top of the heap is
maintained at a level
of no more than about 2000 ppm, more preferably no more than about 1000 ppm,
and
-17 -
~--- ,
CA 02691607 2010-02-01
even more preferably no more than about 500 ppm. This can be realized, for
example, by
using sodium thiosulfate alone as the lixiviant or using a mixture of ammonium
and
sodium thiosulfate.
The pH can be oontrolled by using suitable (acid or base) buffering agents to
produce the desired change in pH. In one oonfiguration, carbonaceous
compounds, such
as calcium carbonates, (in addition to the base 120 incorporated in the
agglomerates 116)
are added to the lixiviant 122 and solution 138 before or after application to
the heap
and/or to the heap itself. The carbon component in the buffering agent has
been found
under suitable conditions to perform, at most, only a minimal degree of preg
robbing.
Typical consumption of carbonates in this configuration is in the range of
about 0.5 to
about 101b/ton of material in the heap.
To apply the thiosulfate lixiviant to the heap, a number of techniques can be
employed. For example, spray systems (such as spray nozzles), drip and/or
trickle
systems (such as drip emitters and perforated pipes), injeotion holes in the
heap, and
irrigation ditches on top of the heap can be used to apply the lixiviant. The
preferred
lixiviant distribution system preferably applies the lixiviant at least
substantially
unifonnly throughout the heap. In a prefened configuration, the applied
lixiviant flows
countercurrently through the heap relative to the flow of the oxygen-
containing gas.
Typiaally, the lixiviant flows from the top to the bottom of the heap while
the gas flows
from the bottom to the top of the heap. In a preferred configuration, at least
about 0.5
and even more preferably from about I to about l Ot solution /t ore of
lixiviant is applied
to the top of the heap from start-to-finish of heap leaching. In this
configaration the
-18 -
r--.,. .
CA 02691607 2010-02-01
lixiviant is applied for at least about 0.5 and even more preferably from
about I to 48
months from the start to finish of heal leaching.
In one configuration, the lixiviant is sparged with the oxygen-oontaining gas
before application to the heap or the gas is otherwise contacted with the
lixiviant before
application to the heap (such as by in-line mixing) to canse the lixiviant to
have a
heightened dissolved molecular oxygen content. Additional gas may be
deliberately
intmduced into the heap separately from the liaiviant, if desired. The
dissolved
molecalar oxygen content of the lixiviant prefaably is at least about 1 mg/L,
more
preferably is at least about 3 mg/L, even more preferably ranges from about 3
to about 40
mg/I, and even more preferably ranges from about 3 to about 15 mg/L.
In one oonfiguration, the dissolved molecular oxygen content is realized by
adding dmnicals, sacb as a peroxide, that break down to generate molecular
oxygen in
the heap.
After constcudion of the heap, the thiosulfate liaiviant 122 is applied to the
top of
the heap while the oxygen-containing gas is introduced to the bottom of the
heap. The
pregnant leach solution 138 is collected from the base of the heap. A portion
of the
pregnant leach solution 138 is recycled to the top of the heap. The recycle
rate is
sufficieat to provide an application rate of the lixiviant to the top of the
heap of from
about 0S and more pzeferably from about 2 to about 241Jh/mZ of top surface
area for the
heap. During recycle, at least a portion 156 (typically at least about 5 vol.%
and more
typically ffi+oin about 50 to about 100 vol.%) of the pregnant leach solution
138 is
removed and subjected to fiuther procesaing to effect precious metal recovery.
At least
-19 -
~...
----.._......... . . . . . - . . . . . . . -- 9
CA 02691607 2010-02-01
most of the procious metat in the material 108 is sohibilized by the lixiviant
and,
therefore, dissolved in the pregnaat leach sohition 138.
The first processing step 160 is sulfide precipitation of the dissolved
precious
metals using a sulfide reagent 162 to form a precipitate-containing slnrry
164. Sulfide
pecipitation not only pracipitates the pr+acaous me41 but also precipitates
transition
metals, such as copper, and regenerates the thiosulfate by convenfng
polytlnonates into
thiosnlfate. By way of example, a sulfide=oontaiaing reagent can reduce the
polythionates
back to thiosalfate, as shown by the follovving reacdons:
2S4 06- + S2- + Y2 H2 0 -4 % s203- + 3H+ (1~
S,a6 + S2+ 42S203 (17)
Any sulfide reagent that releases salfide ions on diseolution wdl suffice,
such as a sulfide,
bisulfide, or polysolfide. Examples of preferred reagents include amnmonium
sulfide,
sodium bisulfide, NaHS, sodium sulfide, Na2S, or hydrogen sulfide gas, H2S.
Sulfide precipitation 160 is typically conducted under aneY+obic or oxygen
depleted conditions, as noted above. Such conditions can be realized and
maintained by
de-aerating the pregnant leach solution 138 with a vacuum, inert or oxyg+en-
deficient gas
bubbling or sparging tlmough the solution 138, maktaining a blauket of a noble
gas in the
atmosphere over the sohrtion 138, and/or allowing the soluaon 138 to stand
dormant for a
sufficient period of time for the dissolved oxygen level to decrease to
desired levels.
Preferably, the solution 138 contains no more than about 1 ppm dissolved
molecular
oxygea and more prefarebly less than about 0.2 ppm dissolved molecular oxygen
concantration.
-20 -
~--" ~
CA 02691607 2010-02-01
In one process configuration, the oxygen-depleted atmosphere is inerk As used
herein, "inert" refers to any gas which is at least substantially free of
oxidants, such as
molecular oxygen, that can cause thiosulfate to be converted into a
polythionate. For
example, an "inert" gas would include a reducing gas. Typically, the inert
atmosphere
will include at least about 85 vol % of an inert gas, preferably nitrogen gas,
and no more
than about 5 vol % oxidants, such as oxygen gas, that can cause thiosulfate
conversion
into a polythionate. The molecular nitrogen can be a byproduct of the oxygen
plant that
is employed in the leaching step to provide oxygen gas.
While not wishing to be bound by any theory, it is believed that sparging is
more
effCtive than an inert atmosphere without sparging in controlling polythionate
and
snlfate production. Sparging appears to inhibit molecular oxygen ingress into
the
solution, even where the reactor is open to the ambient atmosphere, because of
the
outflow of inert gas from the surface of the solution.
Preferably, sufficient sulfide is added to the pregnant leach solution 138 to
precipitate at least most of the dissolved pcecious and transition metal(s) as
salfides and
to convert at least most of the polythionates to thiosulfate, more preferably
to precipitate
at least about 99% of the precious and trsasitlon metals and convert at least
about 90'/o of
the polythionates to thiosulfate, effectively regenerating the thiosulfate
lixiviant.
Typically, the amount of sulfide reagent contacted with the solution 138 is at
least about
100 to about 150% of the stoichiometric amount required to convert at least
substantially
all of the polythionates in the solution 138 into thiosulfates. This amount is
generally
sufficient to precipitate at least most of the precious and transition metals.
Typically, at
-21-
~----
.~ ._
11 .
CA 02691607 2010-02-01
least about 50"/0, more typicaAy at least most, and even more typically from
about 80 to
about 95% of the polythionates are converted into thiosulfates in step 160.
Wlrile not wishing to be bound by any theory, it is believed that the most
lflcely
composition of the precipitate is the metatlic procious metal and/or a
precious metal
sulfide, such as Au2S. Maximum precipitation of gold and regeneration of
thiosulfate is
accomplished by adding at least a stoichiometdc amount of sulfide reagent 162
(relative
to the dissolved precious metal and polythionate concentrations) to reduce the
solution
ORP to at least about 50 mV (SHE). The effectiveness of the conversion canses
significantly less thiosulfate reagent to be consumed dnring the process than
for
conventional thiosalfate leacbing prooesses.
The pH of the pregnant leach solution 138 is adjusted if neceasary to about pH
5.5-12, more preferably about pH 7-11, even more pisferably about pH 8-10
using a
suitable basic reagent such as sodium hydroxide before or during contact of
the solution
with the snlfide reagent 162. The temperature of the solution 162 is
prefecably
maintained in the range of about 5 to 40 C, and more preferably at ambient
temperature,
about 20 jC. The retention time is about 5 minutes to about 2 hours, more
preforably
about 15 minutes to about i hour.
The precious metal precipitation step 160 can be carried out in any suitably
agitated reactor or plurality of agitated reactors.
The procipitate-containing slurry 164 is subjeoted to liquid/solid separation
166 to
separate the precious metal bearing procipitates or concentrate 168 oontaining
at least
most of the precious metal(s) in the slurry 164 from the bairea lixiviant 150
containin,g at
least most of the thiosulfate in the slurry 164. The solid/liquid separation
can be effected
-22 -
r--- ,
. .
CA 02691607 2010-02-01
by any suitable method such as filtration, counter current decantation
("CCD"), and the
lOce. As will be appreciated, CCD performs liquid/solid separation, provides
water
balancing in the circuit, and prevents build up of impurities in the leach
circuit by
removing a portion of the leach solution with the solids.
The barren thiosulfate lixiviant 150 can be recombined with the recycled
pregnant
leaoh solution and returned to the top of the heap. The barren lixiviant 150
will typically
contain no more than about 0.01 ppm precious metals or 1% of the precious
metal(s) in
the pregnant leach solution 138.
The concentrate 168, which is typically in the form of a sludge or slurry,
contains
a substantial amount of elemental sulfur along with various precious metal
sulfides and
non-precious metal sulfides (such as copper sulfides, mercury suifides, and
nickel
sulfides). The elemental sulfur concentration in the concentrate is typically
at least about
50 wt.% and more typically from about 55 wt.% to about 99.9 wt.%. The
concentrate
168 typically further include from about 0.01 to about 10 wt.% precious metal
sulfides
and from about 0.01 to about 10 wt.% non-precious metal sulfides.
The elemental sulfur is removed from the precious metal-bearing concentrate
168
in step 140, and the precious metal concentration of the concentrate 168
significantly
upgraded This is performed by contacting the concentrate 168 with a source of
sulfite
under at least substantially non-oxidizing conditions (or in the presence of
an inert gas
atmosphere) to convert the elemental sulfur into thiosulfate. As shown in the
Figure, the
concentrate 168 is contacted with a sulfite reagent 170, heat 172, and a base
174 in a
suitable reactor.
-23 -
r-~-- , '
-----..m._...-.~.....~,~
CA 02691607 2010-02-01
The sulfite reagent 170 can be any sulfite-containing compound, such as
ammonium bisulfite, sodium sulfite, sodium bisulfite, and potassium bisulfite,
with a
bisulfite such as ammonium bisulfite being preferred. For ammonium bisulfite,
the
chemical reaction is believed to proceed in accordance with equation 14 above.
The amoutit of sulfite reagent 170 used in step 140 depends on the elemental
sulfiu content of the concentrate 168. Typically, the amount of sulfite
reagent is at least
the stoichiometric amount, and more typically at least about 120% of the
stoichiometric
amount, required to convert the present elemental salfur to thiosulfate. For
ammonium
bisulfite as the sulfite reagent 170, the amount of reagent used is typically
at least about 2
kg reagentJkg of present elemental sulfur and more typically ranges from about
3 to about
5 kg reagent/kg of present elemental sulfur.
For the reaction to proceed to completion, the pH of the concentrate 168 is
carefiilly controlled. The preferred pH is at least about pH 6, more
preferably at least
about pH 7, and even more preferably ranges from about pH 7.5 to about pH 10.
Because
bisulfite will produce an acidic pH when reacted with elemental salfur, it is
important to
contact the base 174 with the concentrate 168. The base 174 can be any basic
compound,
such as carbonates, oxides, hydroxides, ammonia gas, with ammonia gas and/or
sodium
carbonate being preferred for reasons of cost.
The temperature of the concentrate 168 during step 140 is preferably at least
about 70 C, and more preferably ranges from about 90 to about 100 C.
The residence time of the concentrate 168 in the reactor is preferably at
least
about 1 minute and more preferably ranges fiom about 10 to about 20 minutes.
-24 -
,....__.. .
CA 02691607 2010-02-01
The reactor can be configured as a batch or continuous reactor and as a single-
or
multY-compartnient vessel. Preferably, the reactor has from one to six
compartments.
The reactor typically agitates the various components for better reaction
kinetics.
The atmosphere of the reactor is preferably anaerobic to limit the oxidation
of
sulfite and ensure that the precious metal precipitates in the concentrate 168
are not
dissolved. The atmosphere can be realized and maintained by de-aerating the
concentrate
168 with a vacuum, inert or oxygen-deficient gas bubbling or sparging tbrough
the
concentrate 168, maintaining a blanket of nitrogen in the atmosphere over the
concentrate
168, and/or allowing the concentrate 168 to stand dormant for a sufficient
period of time
for the dissolved oxygen level to decrease to desired levels. Preferably, the
conoeutrate
168 contasns no more than about 1 ppm dissolved molecular oxygen and more
preferably
less than about 0.2 ppm dissolved molecular oxygen ooncentration.
The upgraded concentrate 176 outputted by step 140 cbmprises the precious and
non-precious precious metai precipitates, thiosulfate, elemental sulfur, and
sulfite
reagent. The upgraded concentrate 176 is a slurry having a liquid component
that
contains pnedominantly thiosulfate and a solid component that oontains
predominantly
the precious and non-precious metal precipitates. Typically, at least about
50% and more
typically at least about 90% of the elemental sulfur is converted into
thiosulfate. The
concentration of the precious metal precipitates in the upgraded concentrate ]
76 typically
ranges from about 0.1 to about 75 wt.% of the upgraded concentrate 176 and the
concentration of the elemental sulfur from about 0.1 to about 50 wt.% of the
upgraded
concentrate 176.
-25-
r--
CA 02691607 2010-02-01
In step 178, the upgraded concentrate 176 is subjected to fiutha liquid/solid
sepacation by any of the tecbniques noted above to produce precious metal-
bearing solids
180 containing at least most of the precious metal content and a barren
solution 152
oontaining at least most of the tluosalfate of the upgraded conceatratia 176.
The preferred
separation teclmique is settling and filtration.
Due to the removal of elementsl sulfur, the procious metal concentration in
the
precious metal-bearing solids 180 is substantially higher than that in the
upgraded
conoecrtrate 176. Typically, the precious metal concentration in the solids
180 is from
about 500 to 20,000% of the precious metal conceatration in the upgradod
conoentrate
176.
The barren solution 152 is recycled to the sulfide precipitation step 160.
A minor portion (e.g., from about 2 to about 20 vol%) of the barren lixiviant
150
or bleed stroam 182 may have to be bled to tailings to control the buildup of
impurities,
such as solable snlfate and metallic impurities. Prior to discharge to
tailings the bleed
stream 182 of the lixiviant 150 is directed to the paecious metal soavenging
step 186 to
recover any precious metals remaining in solution that were not recovered
previously.
Precious metal scavenging can be accomplished, by any suitable gold recovery
technique
such as by pa9sing the bleed solution 182 through a column containing a
strortg base resin
to adsorb the preaous metal. While not vvisUing to be bound by any theory.
precipitated
precious metal can be redissolved due to a trace amount of molecular oxygen in
the
solution and incomplete reduction of polythionates in the solution. Because
the amount
of polythionates in the bleed is negligibley a resin in-cohunn recovery
tecbnique will have
an excelleirt ability bD load any remaini,ng dissolved pzecious metal.
-26 -
~--_ , -..-.-._.....~:.~ _..
CA 02691607 2010-02-01
Turning now tD the fiuther treatanent of the precious metal-bearing solfds
180, the
solids 180 are contacted in step 193 with a mineral acid 188, heat 190, and an
oxidant
192 to remove any undesired non-precious metal(s) and form a precious metal-
contanrin4g
sluny 194. Examples of such undesired non predous metal(s) include mercary in
the
form of ineroaric sulfide, copper in the form of copper sulfide, and other
traosition metal
sulfides. The mineral acid and/or oxidant solubilize at least most of the
mercary or base
metal(s) in the liquid phase and leave at least most of the precious metals in
the solid
pbwe-
The mineral acid 188 can be any suitable acid, including nitric acid,
hydrochloric
acid, (hydro) sulfuric acid, and mixtures thereof, with nitric acid being
ptefotx+ed. The
preferred acid concentration is fmm about 1 to about 50 wt.%.
The oxidant 192 an be any suitable matmial, such as oxygen, nitric acid,
peroxides, and mixtures thereof, with nitric acid being preferred. The
preferred oxidant
concmtretion mges fi+om about I to about 50 wt.oy6.
The prefenred temperature of the solids during step 193 is greater than about
50 C
and more preferebly ranges fiom about 90 to about 100 C.
The residence time of the solids 180 in step 193 preferably ranges from about
10
to about 480 minutes.
The precious metal-containing sluny 194 is subjected to liquid/solid
separation
195 by any of the techniques noted above to form a barren liquid 197 and
precious metal
bearing solids 196. At least about 10% of the non-precious metals originally
in the
pragnant leach solution 160 am contained in the barten liquid 197, and at
least about 50%
of the precious metals originally in the sohrtion 160 are in the precions
metal-bcwia,g
27-
,.._...
CA 02691607 2010-02-01
solids 196. The barma liquid 197 may be treated by lmown becbniques to recover
desired
non-ptecious or base metals and/or discarded.
The precious metal-bearing solids 196 are subjected to refining 198 by known
teohniques to produce a precious metal product 199 of high purity.
A seoond embodiment of the present invention will now be discussed with
reference to Figures 2A and B. The embodiment employs tank leacbing rather
than heap
leaching to recover precious metals. Ldce-numbered elements in Figures 2A and
B on the
one hand and Figares lA and B on the other are the same. Different numbered
elements
are discussed below.
The precious metal-bearing material 100 is oomminuted in step 200 to produce a
commiauted precious metal-bearing materia1204. The materia1204 is comminuted
to a
size sufficient to enable the solids to be suspended in an agitated vessel and
to allow for
the efficient leaching of the precious metals. Preferably, wet grinding is
employed with
the recycled tbiosnlfate leach solution 144 and water being used as the liquid
conponent
in the slurry. In that event, the slurried material 204 typically contains
from about 0.05 to
about 0.2 M tbiosalfates and from about 0.0005 to about 0.025 m polytbionates.
The fully
comrninuted material particle size is preferably at least smaller than 80%
passing about
48 mesh (300 mimns), more preferably 80% passing about 100 mesh (150 microns),
and
most preferably 80% passing about 200 mesh (75 microns). The typical solids
content of
the s1mYed materia1204 ranges from about 20 to about 30 wk%. As will be
appreoiated,
other techniques can be used to comminute the mataial to the desired particle
size(s). By
way of i'ilustration, blasting can be used alone with or witbput crushiing and
grinding and
crushing and grinding can be used alone with or without another comminution
teabnique.
-28-
~---
CA 02691607 2010-02-01
The sluiried comminuted precious metal-bea<ing niateria1204 is then thickened
208 to adjust the pulp density to a value suitable for leaching. The Ideal
leach pulp
density will vary accoTding to the type of material boing leaohed. Typically,
the pulp
density ranges from about 20 to about 50% solids by weight, but could be as
low as about
1% or as high as about 60%. Tbickeming 208 will generally not be required if
the desired
pulp deasity (afier wet comminution or fonnation of the comminuted material
into a
sluay) is less than about 20%.
The thickener overflow solution 212 is recycled back tD the oDmminution step
200
in the event that wet grinding is employed. Otherwise, the overflow solution
212 is
returned to the optional sluny fonnadon step (not shown).
Fresh makeap thiosalfata is added, as necessary, at any suitable loeation(s),
such
as to the slurried materiai 204 during comminution 200 and/or in the tbidcener
208, to the
thickened slurry 216 or overflow solution 212, to leaching 220 and/or to the
recycle
solution 144.
The thickened slurry 216 is subjected to leaching 220 in the presence of
oxygen
and thiosulfate. In one procxss configaaation, leaching is conducted in the
pnesance of an
oxygea-eruiched atmospha+e at atmo$pheric pross<uB, or at a pressure above
atmospheric
pressure using an oxygen-oaataining gas to reduce or aliminate the need for
the presence
of copper and/or ammonia in the leach. The inarewed oxygen partial pressure in
the
leaching step 220 increases the rate of the reaction in Equation 15 in the
absence or near
absence of copper and ammonia. To accomplish this goal, the oxygen-containing
gas
may include atmospheric air, or it may include relatively pure (95%+) oxygen
such as
that produced from any oommercially available oxygen plant, or it may include
any other
-29 -
CA 02691607 2010-02-01
available source of oxygen. The desired oxygen partial pressure (P02)
maintaiaed during
leaching will depend on the material being leached, but it will be at least
higher tlian that
provided under normal ambient eonditions by air at the elevation the process
is applied.
Thus, if the process is practiced at sea level for example the oxygen partial
pmessure will
be in exceas of about 3 pounds per square inch absohrte pressure (paia) to as
high as
about 500 psia, preferably from about 10 to about 115 psia, and most
preferably from
about 15 to about 65 psia. The totat opaating pressure is the sum of the
moleailar
oxygen pwtial preseo<e and the water vapor prmtt+e at the temperature employed
ra the
leaching step 132, or preferably ranges from about 15 to about 600 psia and
more
preferably from about 15 to about 130 psia.
The leaching tempexatime will be diotatod by the type of material being
leached.
'The temperabure will vmy typically from about 5 C to about 150 C, preferably
from
about 20 to about 100 C, and most preferably from about 40 to about 80 C.
Higher
temperaturea accelerate the leaohing of precious metals but also accelerate
the
degradation of tlriosulfate. If requircd, a source of makeup heat such as
stemm is added to
the leach reactors to maintain the desired tiomparatm.
The leaching retention time is dependent on the material being leached and the
temperatare, and will range from about 1 hour to 96 hours, preferably ffom
about 2 to
about 16 honrs, and most pmaferably from about 4 to about 8 hours.
In one prooess eonfigiuation, the absence or substantial absence of copper
and/or
Mnmonia in the leach greatly simplifies the process. Elimination or near-
elimination of
ammonia and copper from the leach provides the advantage of allowing for a
oonsiste,ntly
high and reprnduable prmous metal aatraetion over a broader pH range than was
-30 -
.....~... _
CA 02691607 2010-02-01
previously possible with the other thiosutfate leaching processes. Prefeaably,
the (added
and/or totat solution) copper conc~:ntration is no more than about 20 ppm,
more
preferably no more than about 15 ppm, and even more pref+aably no more than
about 10
ppm while the (added and/or total solution) ammonia conccntration is no more
thaa about
0.05 M, more preferably no more than about 0.031Vi, and even more preferably
no more
than about 0.01 M. In this process configuration, leaching can be operated at
about pH 7-
12, preferably about pH 8-11, more preferably about pH 8-10, and even more
preferably
at a pH less than pH 9. The oxidation-reduction potential (ORP) preferably
rangea from
about 100 to about 350 mV and more praferably from about 150 to about 300 mV
(vs. the
standard hydrogm electrode (SHE)).
The leaching step 220 may be conducted in a batch or continuous basis but
csnrtinuous opecation is prefmred. Continuous lwhing is carried out in a
multiple seaies
of one or more reactors that are agitated sufficientiy to maintain the solids
in suspension.
Agitation may be aocomplished by mechanical, pneumatic or other means. In a
preferred
configarstion, gassing impellers as+e employed, such as those diselosed in
U.S. Patent No.
6,183,706 and copending U.S. Patent Application Serial No. 09/561,256, filed
Aprll 27,
2000, which are incorporated herein by reference. Such impellers can
significaatly
enhance the amount of dissolved molecul r oxygen in the leach solutioa.
I.eacbiag may
also be carried out in a multi-compartment autoclave containing one or more
compar6ments, (with 4 to 6 compartments being preferred) similar in design to
the
Mrtoclaves nsod to pressure oxfdize sul8do-bearing ores or eonceutrates.
However, owing
to the non-acidic, moderate temperature, relatively mild conditions employed
in the
plr.seat invention, the autoclave matetials of eonstzuction are much less
wcpmsive than
-31-
r---
~,,
CA 02691607 2010-02-01
those fomd to be necesssiy when oxidizing snlfide minerals. The latter
aatoclaves are
nornnaily constracW of a steel shell fitted with a lead liner and refractory
brick liner and
containing metallic components constructed of titanium or other expensive
eorrosion-
resistant alloys. The lesch reactors and contained metallic components
employed by the
present invention can be simply eonstructed of stainless steel and do not
require lead or
brick liners.
The pregaant slurry 224 is subjected to solid/liquid separation 228 by any of
the
techniques set forth above, with the solid frscxion forming tailings 236 and
the liquid
fraction forming the pmegoaat leach solution 138.
The reaaining steps are as descn'bod with referemoe to the coriesponding
numbered step in Figures 1A and B.
In any of the above processes or in other procases using thiosulfate as a
lixiviant,
the use of a blinding agent may improve metal recoveries. While not wishing to
be
bound by any theory, it is believed that the precious metal thiosulfate
complex may be
imstable under certain conditions, including tbose set forth above, and tbat
the precious
metal can be stripped from the thiosulfate-containing solution by a number of
substances
ooznmonly eneonnta+ed in pt+ecions metal-eontaining materials. The substanoes
or preg
robbiug materials typically absorb, adsorb or precipitate ihe precious metal.
Such preg-
robbing matarials include carbonaceous mat,erials, pyrite-containing
materiats,
cbaeapyrite and iroa oxides. Surprisingly and unexpectedly, blinding agents
may be used
in the thiosalfate lixiviant to prevent or inhibit preg robbing of dte
precious metal by the
preg robbing material. The blinding agent itself absorbs or adsorbs (in
preference to the
preaious metal) or otherwise neutralizes (such as by chemical reaction) the
preg robbing
-32 -
r--- . ,
CA 02691607 2010-02-01
sites on de material. Suitable blinding agents mdude one or more of
hydt+ocwbon
eontaining substances, such as aliphatic or cyclic hydz+oearlx-ns, prefarably
petioleum
products (e.g., kerosene, (hesel fuel, and gasoline), alcAhols, esters, or
aldehydes;
svrfactants such as detergenta, sodium lauryl sulfonate, or organic
phosphates; guar gum;
starch, a cellulose such as a carboxy methyl oellulose; and reaetive metal
salts such as
lead, mercury, cadmium, tin, and gilver salts. In such situations, the
thiosulfake lixiviant
144 and 148, in any of the leaching processes discussed above, typically
includes at least
about 0.1 mg/L, more typically at least about I mg/L, and eveu more typicaliy
from about
2 to about 200 mg/I, of the blinding agcat.
While not wishing to be bound by any theory, other agents may also be suitable
as
blinding ageats. Preferably, the ascau do not destabilize thiosulfate in
solution. Agents
which act as oxidation catalysts, can destabilize thiosulfate in solution. By
way of
example, copper salts under certain conditions are not preferred as a blinding
agent as
copper salts under these conditions can catalyze thiosulfate decomposition.
To fac,ilitate extraction of gold from sulfidic and/or carbonaceous materials,
the
thiosalfate leach step in any of the above processes can be preceded by one or
more
pretrestment steps to deskroy or neu4ralize the oarbon-oontaining and/or
sulfidic minerals.
By a-ay of example, the uftnuxUw steps can inolude one or more of biooxidation
or
chesnical oxidation to oxidize suifides, ultcafine grinding to hiberate
occluded precious
metals, conventional roasting to destt+oy carbon- and/or sulfido-contaiving
minerals,
and/or microwave roastiag.
-33 -
r-~
~ -
CA 02691607 2010-02-01
EXPBRIIviENTAL
A sulfur sludp contained 0.02 to 0.7 wt'/o gold and greater than 85% elemental
sulfor. To replicate step 140 of Figure lA, the sludge was treated with sodium
sulfite or
amrnoniam bisulfite. To maintain the pIi levels identified in the examples, a
base was
sometimos added.
In the exaznples below, "slud8e" refars to tbe solid mabarist (or the peec~ous
metal4xering omoentrate 168) pt+ahioed by addmg sWfide to the pregnant
thiosulfate
leach liquar, and oresiduer' refeas to the solid pcoduCt (or preGious metal-
bearing solids
180) from step 140.
EXAMPLE 1
The gold gcade incsused from 0.6 wt=/O in the sludge to 25 wN/o in the reaidue
when using as low as 25% eum sodium sulfite (Na2SO3) (as defined by the excees
of
reagent applied above the sbolchiometdc requirement for 100% eonveraion of the
elemental sulfur wntent of the sludge) at 100 C for 70 to 120 minutes. The
gotd grade of
the residue was 42 times larger than the gold grade of the sludge. As low as
8% of the
original gold onntent of the sludge redissolved. Gmeater than 99'/o of the
sulfur cflntent of
the sfidp was converbod primarlly to thiosulfate. Thc mffw oonbwt dem=sed
from' n 87
wt% in the slvdge to 16 wthA in the residue. Tln pH of the tbiosulfats-rich
solution
remebed above pH 9 witboirt the need to add a bese.
EXAMPLE 2
T'he Vld grade increased from 0.02 wt% in the sfidge to 1.8 wt% in the residue
when using as low as 31% excxss ammonium bisulfite (NH4H803) at 100 C for 22
-34-
CA 02691607 2010-02-01
minutes. The gold grade of the residue was 90 times larger than the gold grade
of the
sludge. The pH of the ttuosulfatarich solution was maintained in the range
between pH
9.5 to pH 10 by using ammonia gas as a base. C3reater than 99% of the sulfur
content of
the sludge was converted primarily to thiosulfate. The sWfnr content dem+eased
frmm 95
wt% in the sludge to 60 wt'/o in the residue.
EXAMPLE 3
Tlic gold grade inaroased from 0.6 wt% in the sludge to 25.6 wt% in the
residue
when using as low as 36% exoess anamonium bisulfite (NH4HSO3) at 100 C for 60
minutes. The gold grade of the residue was 44 times larger than the gold gcade
of the
sludge. Six percent of the original gold content of the sludge redissolved.
Greater than
99% of the sulfur content of the sludge was convertod primarily to
tlaiosulfate. The sulfur
content decreased from 99 wt% in the sludge to 21 wt=/O in the residue. The pH
of the
thiosulfate-riah solution was maintained between pH 7.8 to pH 8.8 by using
sodium
carbonate (NazC03) as a base.
These eaemples demonstcate that the gold grade of the rasidue after treatmmt
is
increased over the gold grade of the feed maOeriaL The gold grade of the
residue after
troaftent ina+eases by the same factor independently of the reagent used or
the sulfur
grade of the feed material.
EXAMPLE 4
In Figure 3 the $old exfiraction from two large crib tests are shown. For both
tests,
the cn'bs that wore used had a square cross-section that was 8 ft by 8 ft and
ore was
staolced into these cribs to a height of 20 tt. The ore for both tests was a
carbonaoeous
preg-robbing gold ore.
-35 -
CA 02691607 2010-02-01
Both cribs were inigated with a solution that oontained ammonium thiosulihte
at a
concentration of 10-15 g/L. The irrigation rate varied during the test for
both tests, but
was between 0.00125 and 0.0025 gpsn/S . The amation rate for both tests was
kept at
0.002 scSn/ft2.
For the mtiaggiomerated cdb, the ore was crashed to 2" and then placed in tlie
cnb. For the agglomerated crnb, ore was crualied to -2", and then was mixed in
a rotating
dnun for approximately 5 minutes with a solution of 15 g/i, ammonium
thiosulfate. This
solution was added to the ore in an amount to produoe a visaally good
agglomdate, but
amounted to approximately 5% of the ore mass added.
As Figure 3 shows, the gold eatradion, when the ore is agglomerated using
ammonium thiosalfate, is significantly better than when no agglomerating
medium is
used.
EXA,MPLE 5
In Tables 1 and 2, two column tests are shown - one aerated and one not. In
both
tests, carbonaceous pr+eg robbing gold ore was used. This ore was placed in 10
in. x 8 ft.
eolumns and was irrigated at 0.005 gpm/if for the imaeratod column and 0.0025
gpm/fl
for the aerated column. The irrigation rate was changod for the aerated column
to ensm-e
that air, applied to the bottom of the column, could contact all of the ore.
These tables clearly show that when air is applied to a column, the dissolved
oxygen level and Oxidation-Reduction Potential or ORP both incxuse. This
ra4ults in an
increase in gold extraction.
-36 -
r---- .. ,
- --_ - ,. ~ _
,
CA 02691607 2010-02-01
Table 1: Extciction, ORP (mV vs. Ag/AgC1) aad dissolved 02 oonteat (D02) as a
fnnction of solutiw applied for a 10 in. oolumn with no air addition.
SoL Applied ORP D02 Extraction
(koft) mV nw/L
0.0 0'/0
0.2 34 1.8 2%
0.3 -1 1.6 11%
0.3 -63 0.3 170/o
0.4 -65 0.3 22%
0.7 -69 0.3 32 r6
1.0 -46 0.6 39%
1.3 -33 1.7 43%
1.6 -95 0.9 46%
1.9 -50 1.3 48%
2.3 -76 1.4 50%
2.5 -68 1.3 51%
2.9 -83 1.2 52%
Table 2: Extraction, ORP (mV vs. Ag/AgCI) and D02 as a function of solution
applied
for a 10 in. column with air added at 0.007 scfm/i~.
Sol. Applied ORP D02 Eztracctioa
t mV MR/L
0.0 0%
0.1 30 5.1 0%
0.2 16 4.5 4%
0.2 16 4.3 12%
0.3 19 4.0 32%
0.5 12 3.4 52%
0.6 13 3.5 60%
0.8 10 5.4 65%
0.9 8 4.2 67%
1.1 1 3.3 69%
1.2 2 4.8 70%
1.4 2 4.8 71%
-37 -
r.,.... ,
CA 02691607 2010-02-01
1.5 -1 I 5.4 ~ 71% ~
EXAMPLE 6
Fig. 4 shows the gold reoovery from two oolumas. Again, in both tests,
carbonaceous preg-robbing gold ore was used. This ore was placed in 10 in. x
20 tt.
columns and was irrigated at 0.005 gpm/ff for column 2 and 0.0025 gpm/ft for
column
1.
Fig. 4 shows that for these tests, before an application ratio of 0.6,
recovery is
independent of application rate. After this time, air was introduced to oofimn
I at a rate
of 0.007 scfsn/fl . As this figure shows, the application of air at this rate
resnited in the
gold eabradion increasing sigoificwtly as oorapered to the umserated tesk
A number of variations and modifications of the invention can be used. It
would
be possible to provide for some features of the inveation without providiag
othera.
By way of eaample, any source of sulftrr species with an oxidation state less
than
+2 may be used in any of the above process steps to coavert polythionates to
thiosulfste.
The regeaeration phase of the oonditioning step 182 can be performed in a
variety of
locations. For example, regeneration phase can be performed in the recycle
loop before
or after fi+esh thiosulfate 148 addition and before comminution 200, between
oomnminution 200 and thickening 208, in the thickener, and/or immediately
before or
during leaching 220.
Fresh tliiosulfate 148 can also be added in a nwnber of locations. For
example,
fresh thiosulfete 148 can be added in any of the locations refoired to
previously for the
regeneiation phase and can be added after or diuing regenemtion as noted above
or in a
separate tank or location.
-38-
r-----
CA 02691607 2010-02-01
The presmt invention is not limited to the prooees oonfigurations of Figs. 1
and 2.
For exaznple, stepa 140, 193, and 180 may be omitted from the depicted process
configurations. Other prooess steps may be sabstituted for the aepicted pmocem
steps.
For example, the precious metals may be recovered by techniques other than
sulfide
precipitation in step 160. Such techniques include resin in pulp,
electrownming,
cementation, ion exchange resias, cyanidation, direct re5ning, solvent
extraction, and the
like.
The processes to remove precious metals by sulfide procipitation followed by
tbiomtfate production are not limited to precious metals. The prooesses can be
employed
with non-precious metals as well.
Snlfates may be controlled by methods other tbsn pr cipitation. Sulfates may
be
removed by membrane filtration, solvent extraction, and ion exchange.
Sulfates can be removed by adding ealeium to a side stream of the ttriosulfate
lixiviant or other process effluent followed by liqnid/solid separation to
remove the
precipitated gypsum from the lixivisnt. This is shown by the optional use of
the precious
metal acavmging step. Calcium csn be placed in the heap separate fi+om the
precious
metal-bearing material 108. This is pazticulzly attractive where agglomeration
is not
eartployed.
The present invmtion, in various ernbodiments, inclndes components, methods,
piocesses, systems and/or apparatas substantially as depicted and descn'bed
herein,
inchxft various embodiments, subcombinatigns, and snbsets thereof. Those of
sicill in
the art will undacstmd how to make and use the present invenbton after
understanding the
presont disclosure. The prasent invention, in various embodiments, includes
providmg
-39 -
CA 02691607 2010-02-01
deviccs and procesaes in the abseace of items not depicted aad/or described
hetain or in
various embodiments hereof, ineluding in the absence of such items as may have
been
used in previous devices or processes, e.g., for improving performance,
achieving ease
andbr reducing cost of implementation.
The fozegoing discussion of the invention has been presented for purposes of
i'llustration and deacription. The foregoing is not intended to limit the
invention to the
form or forms disciosed hereia In the foregoiAg Detailed Dcscription for
example,
various foahaes of the iaventiam are grouped together in one or more
embodiments for
the purpose of streamlining the disclosure. This method of disclostne is not
tio be
interpretod as reflecting an intention that the claimed invention requires
more featac+es
than are exprossly recited in each claim. Rather, as the following claims
reflect,
inventive aspects lie in less than all features of a single foregoing
disclosed embodiment.
Thus, the following claims are hereby incorporated into this Detailed
Description, with
each claim stanc}ing on its own as a separate proferred embodiment of the
invention.
Moreover though the descxiption of the invention has incladed description of
one
or more embodiments and certain variations and modifications, other variations
and
modifications are within the scope of the invention, e.g., as may be within
the skill and
]mowledge of those in the art, after undeamtanding the present disclosure. It
is intended to
obtain rights which include alternative embodiments to the extent permitted,
including
alternate, interchangeable and/or equivalent structures, fanctions, ranges or
steps to those
claimed, whether or not such alternatey interchangeable and/or equivalent
suucbn^es,
functions, ranges or steps are disclosed herein, and without intending to
publicly dedicate
any patentable subject matter.
-40 -