Note: Descriptions are shown in the official language in which they were submitted.
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
1
Indane-amine derivatives, their preparation and use as medicaments
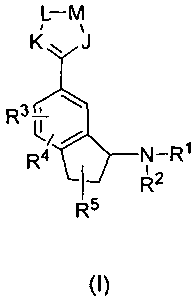
The present invention relates to indane-amine compounds of general formula
(I),
methods for their preparation, medicaments comprising these compounds as well
as
their use for the preparation of a medicament for the treatment of humans or
animals
The search for new therapeutic agents has been greatly aided in recent years
by better
understanding of the structure of proteins and other biomolecules associated
with
target diseases. One important class of proteins that has been the subject of
extensive
study is the family of 5-hydroxytryptamine (serotonin, 5-HT) receptors. The 5-
HT7
receptor discovered in 1993 belongs to this family and has attracted great
interest as a
valuable new drug target (Terron, J.A. ldrugs, 1998, vol. 1, no. 3, pages 302-
310: "The
5HT7 receptor: A target for novel therapeutic avenues?" ).
5-HT7 receptors have been cloned from rat, mouse, guinea pig and human cDNA
and
exhibit a high degree of interspecies homology (approx. 95%), but it is unique
in that it
has a low sequence homology with other 5-HT receptors (less than 40%). Its
expression pattern, in particular structures of the central nervous system
(CNS)
(highest in hypothalamus (in particular suprachiasmatic nuclei) and thalamus)
and
other peripheral tissues (spleen, kidney, intestinal, heart and coronary
arthery),
implicates the 5-HT7 receptor in a variety of functions and pathologies. This
idea is
reinforced by the fact that several therapeutic agents, such as tricyclic
antidepressants,
typical and atypical antipsychotics and some 5-HT2 receptor antagonists,
display
moderate to high affinity for both recombinant and functional 5-HT7 receptors.
Functionally, the 5-HT7 receptor has been implicated in regulation of
circadian rhythms
in mammals (Lovenberg, T.W. et al. Neuron, 1993, 11:449-458 "A novel adenylyl
cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of
circadian
rhythms"). It is known that disruption of circadian rhythms is related to a
number of
CNS disorders including depression, seasonal affective disorder, sleep
disorders, shift
worker syndrome and jet lag among others.
Distribution and early pharmacological data also suggest that the 5-HT7
receptor is
involved in the vasodilatation of blood vessels. This has been demonstrated in
vivo
(Terron, J.A., Br J Pharmacol, 1997, 121:563-571 "Role of 5-HT7 receptors in
the long
lasting hypotensive response induced by 5-hydroxytryptamine in the rat'). Thus
selective 5-HT7 receptor agonists have a potential as novel hypertensive
agents.
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
2
The 5-HT7 receptor has also been related with the pathophysiology of migraine
through
smooth muscle relaxation of cerebral vessels (Schoeffter, P. et a1., 1996, Br
J
Pharmacol, 117:993-994; Terron, J.A., 2002, Eur. J. Pharmacol., 439:1-11 "Is
the 5-
HT7 receptor involved in the pathogenesis and prophylactic treatment of
migraine?'). In
a similar manner, involvement of 5-HT7 in intestinal and colon tissue smooth
muscle
relaxation makes this receptor a target for the treatment of irritable bowel
syndrome
(De Ponti, F. et al. , 2001, Drugs, 61:317-332 "Irritable bowel syndrome. New
agents
targeting serotonin receptor subtypes'). Recently, it has also been related to
urinary
incontinence (British J. of Pharmacology, Sept. 2003, 140(1) 53-60: "Evidence
for the
involvement of central 5HT-7 receptors in the micurition reflex in
anaeshetized female
rats").
In view of the potential therapeutic applications of agonists or antagonists
of the 5HT7
receptor, a great effort has been directed to find selective ligands. Despite
intense
research efforts in this area, very few compounds with selective 5-HT7
antagonist
activity have been reported (Wesolowska, A., Polish J. Pharmacol., 2002, 54:
327-341,
"In the search for selective ligands of 5-HT& 5-HT6 and 5-HT7 serotonin
receptors"), yet
even fewer 5-HT7-Agonists.
There is still a need to find compounds that have pharmacological activity
towards the
receptor 5-HT7, being both effective and selective, and having good
"drugability"
properties, i.e. good pharmaceutical properties related to administration,
distribution,
metabolism and excretion.
Thus, it was an object of the present invention to provide novel compounds
that are
suitable in particular as active substances in medicaments.
Said object was achieved by providing an indane-amine derivative of general
formula
(I)
L-M
K~ J
R3
R N-RI
R2
R5
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
3
(i)
wherein
K-L-M-J together form
= =CH-X-Y=CH-; in which any suitable H may be substituted by R6 and/or R',
and in which X is selected from NR8, 0 or S, while Y is selected from
N or
CH;
==CH-X-Y-C(O)-; in which any suitable H may be substituted by R 6 and in
which
one of X and Y is NR8, while the other is selected from NR8a, S or O;
==CH-X-Y-C(O)-; in which one of X and Y is CH2, while the other is selected
from
NRe, S or 0, in which any suitable H may be substituted by R6
and/or R';
= =CR6-N=N-C(O)-;
= =CR9-CH=CH-CH=CH-; in which any suitable H may be substituted by R6;
==CR9-CH=CH-CH=CR9a-; in which any suitable H may be substituted by R 6;
==CH-X=Y-CH=CH-; in which any suitable H may be substituted by R6 and/or
R'
and in which one of X or Y is selected from N, while the other is
selected
from N or CH;
==CH-X=Y-CH2-CHZ-; in which any suitable H may be substituted by R6
and/or R7,
and in which one of X or Y is selected from N, while the other is
selected
from N or CH;
= =CH-X-Y-CH=CH-; in which any suitable H may be substituted by R6 and/or
R'
and in which one of X or Y is selected from NR8, 0 or S while the
other is
seiected from NR8a or CH2;
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
4
==CH-X-Y-CHZ-CH2-; in which any suitable H may be substituted by R6
and/or R',
and in which one of X or Y is selected from NR8, 0 or S while the
other is
selected from NR8a or CH2;
= =CH-X-CH2-Y=CH-; in which any suitable H may be substituted by R6 and/or
R'
,
and in which X is selected from NR8, 0 or S while Y is selected from
N or CH;
= =CH-X-CH=Y-CH2-; in which any suitable H may be substituted by R6 and/or
R'
and in which X is selected from NR8, 0 or S while Y is selected from
N or CH;
= =CH-N=CH-Y=CH-; in which any suitable H may be substituted by R 6 and/or
R'=
= =CH-X-CH2-Y-CH2-; in which any suitable H may be substituted by R6
and/or R',
and in which one of X or Y is selected from NR8, 0 or S while the
other is selected from NR$a, 0, S or CH2;
R' and R2 each are independently selected from the group consisting of
hydrogen; or a linear or branched, saturated or unsaturated, optionally at
least
mono-substituted aliphatic radical;
or
R' and R 2 together with the bridging nitrogen atom form an saturated or
unsaturated, optionally at least mono-substituted 5- or 6-membered-
heterocyclic
ring, which may be condensed with an optionally at least mono-substituted
mono- or polycyclic ringsystem;
R3, R4 and R5 are independently from each other selected from hydrogen;
halogen, OH, SH, NH2; a linear or branched, saturated or unsaturated,
optionally at least mono-substituted aliphatic radical; or O-R with R being a
linear or branched, saturated or unsaturated, optionally at least mono-
substituted aliphatic radical;
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
R6 and R' are independently from each other selected from hydrogen; halogen,
OH, SH, NH2; an aliphatic radical, which is linear or branched, saturated or
unsaturated, and optionally at least mono-substituted by F, CI, Br, 1, SH or
OH;
or O-R with R being an aliphatic radical, which is linear or branched,
saturated
or unsaturated, and optionally at least mono-substituted by F, Cl, Br, I, SH
or
OH;
R8 and Rea are independently from each other selected from hydrogen; an
aliphatic radical, which is linear or branched, saturated or unsaturated, and
optionally at least mono-substituted by F, Cl, Br, I, SH or OH; or -R'-O-R"
with
R' and R" independently from one another being a C,-6-alkyl radical, which is
linear or branched, and optionally at least mono-substituted by F, Cl, Br, I,
SH
or OH;
R9 and R9a are independently from each other selected from halogen; an
aliphatic radical, which is linear or branched, saturated or unsaturated, and
optionally at least mono-substituted by F, Cl, Br, I, SH or OH; ; or O-R with
R
being an aliphatic radical, which is linear or branched, saturated or
unsaturated,
and optionally at least mono-substituted by F, Cl, Br, I, SH or OH;
optionally in form of one of its stereoisomers, preferably enantiomers or
diastereomers, its racemate or in form of a mixture of at least two of its
stereoisomers, preferably enantiomers or diastereomers, in any mixing ratio,
or
a salt, preferably a physiologically acceptable salt thereof, or a
corresponding
solvate, respectively; or a halide salt thereof, in which NR'R2 of general
formula
(I) is substituted by an additional methyl to form with R' and R 2 being CH3 a
trimethyl-ammonium-radical.
These compounds show a high affinity to the 5HT7 Receptor as well as a high
selectivity for this receptor in comparison to e.g. the 5HT6, the Sigma 1, the
a2, and the
5HT, Receptor, thus having a higher affinity to the 5HT7 receptor. In addition
some of
these compounds show an agonistic activity on this receptor.
A "mono- or polycyclic ring-system" according to the present invention means a
mono-
or polycyclic hydrocarbon ring-system that may be saturated, unsaturated or
aromatic.
If the ring system is polycyclic, each of its different rings may show a
different degree of
saturation, i.e. it may be saturated, unsaturated or aromatic. Optionally each
of the
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
6
rings of the mono- or polycyclic ring system may contain one or more
heteroatoms as
ring members, which may be identical or different and which can preferably be
selected
from the group consisting of N, 0, S and P, more preferably be selected from
the group
consisting of N, 0 and S. Preferably the polycyclic ring-system may comprise
two rings
that are condensed. The rings of the mono- or polycyclic ring-sytem are
preferably 5- or
6-membered.
An "aryl", "aryl radical" or group is understood as meaning ring systems with
at least
one aromatic ring but without heteroatoms even in only one of the rings.
Examples are
phenyl, naphthyl, fluoranthenyl, fluorenyl, tetralinyl or indanyl, in
particular 9H-fluorenyl
or anthracenyl radicals, which can be unsubstituted or monosubstituted or
polysubstituted.
In the context of this invention "cycloalkyl radical" or group is understood
as meaning
saturated and unsaturated (but not aromatic) cyclic hydrocarbons (without a
heteroatom in the ring), which can be unsubstituted or mono- or
polysubstituted.
Furthermore, C3.4-cycloalkyl represents C3- or C4-cycloalkyl, C3_5-cycloalkyl
represents
C3-, C4- or C5-cycloalkyl, C3_6-cycloalkyl represents C3-, C4-, C5- or C6-
cycloalkyl, C3_,-
cycloalkyl represents C3-, C4-, C5-, C6- or C,-cycloalkyl, C3-8-cycloalkyl
represents C3-,
C4-, C5-, C6-, C7- or C8-cycloalkyl, C4_5-cycloalkyl represents C4- or C5-
cycloalkyl, C4-6-
cycloalkyl represents C4-, C5- or Cs-cycloalkyl, C4_,-cycloalkyl represents C4-
, C5-, C6-
or C,-cycloalkyl, C4_8-cycloalkyl represents C4-, C5-, C6- C7- or C8-
cycloalkyl C5-6-
cycloalkyl represents C5- or Cs-cycloalkyl and C5_,-cycloalkyl represents C5-,
C6- or C7-
cycloalkyl. However, mono- or polyunsaturated, preferably monounsaturated,
cycloalkyls also in particular fall under the term cycloalkyl as long as the
cycloalkyl is
not an aromatic system. The cycloalkyl radicals are preferably cyclopropyl, 2-
methylcyciopropyl, cyclopropylmethyl, cyclobutyl, cyclopentyl,
cyclopentylmethyl,
cyclohexyl, cycloheptyl, cyclooctyl, and also adamantly.
A "heterocyclyl", a "heterocyclyl radical" or group or "heterocyclic ring
system" is
understood as meaning heterocyclic ring systems which contain one or more
heteroatoms from the group consisting of nitrogen, oxygen and/or sulfur in the
ring or
ringsystem, and can also be mono- or polysubstituted. The ringsystem may
consist
either of only one saturated or unsaturated or even aromatic ring or may
consist of 2, 3
or 4 saturated or unsaturated or even aromatic rings, which are condensed in
that
between two or more of the rings ring members are shared. Examples which may
be
mentioned from the group of heterocyclyls are furan, benzofuran, thiophene,
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
7
benzothiophene, pyrrole, pyridine, pyrimidine, pyrazine, quinoline,
isoquinoline,
phthalazine, benzo-1,2,5-thiadiazole, imidazo-thiazole, benzothiazole, indole,
benzotriazole, benzodioxolane, benzodioxane, carbazole and quinazoline.
In connection with mono- or polycyclic ring-system, aryl radical, cycloalkyl
radical, or
heterocyclyl radical, "substituted" is understood - unless defined otherwise -
as
meaning replacement of at least one hydrogen radical on the ring-system of the
mono-
or polycyclic ring-system, the aryl radical, the cycloalkyl radical, or the
heterocyclyl
radical by OH, SH, =0, halogen (F, Cl, Br, I), CN, NO2, COOH; NRxRY, with RX
and RY
independently being either H or a saturated or unsaturated, linear or
branched,
substituted or unsubstituted C,-6-alkyl; by a saturated or unsaturated, linear
or
branched, substituted or unsubstituted C,-6-alkyl; a saturated or unsaturated,
linear or
branched, substituted or unsubstituted -O-C,-6_alkyl (alkoxy); a saturated or
unsaturated, linear or branched, substituted or unsubstituted -S-C,.s_alkyl; a
saturated
or unsaturated, linear or branched, substituted or unsubstituted -C(O)-C,-
6_alkyi; a
saturated or unsaturated, linear or branched, substituted or unsubstituted -
C(O)-O-C1
_6_
alkyl; a substituted or unsubstituted phenyl. Within that "monosubstituted"
means the
substitution of exactly one hydrogen radical, whereas "polysubstituted" means
the
substitution of more than one hydrogen radical with "polysubstituted"radicals
being
understood as meaning that the replacement takes effect both on different and
on the
same atoms several times with the same or different substituents. Therefore,
"optionally at least monsubstituted" means either "not substituted" if the
option is not
fulfilled, "monosubstituted" or "polysubstituted".
In connection with aryl radical, cycloalkyl radical, or heterocyclyl radical,
"condensed
with" is understood as meaning that the ring-system of the aryl radical, the
cycloalkyl
radical, or the heterocyclyl radical is sharing two atoms (one) of its ring(s)
with a ring of
the mono- or polycyclic ring-system it is condensed with.
Aliphatic radicals/groups, as referred to in the present invention, are
optionally mono-
or polysubstituted and may be branched or linear, saturated or unsaturated.
Aliphatic
radicals, as defined in the present invention, include alkyl, alkenyl and
alkinyl radicals.
Unsaturated aliphatic radicals, as defined in the present invention, include
alkenyl and
alkinyl radicals. Preferred aliphatic radicals according to the present
invention include
but are not restricted to methyl, ethyl, vinyl (ethenyl), ethinyl, propyl, n-
propyl, isopropyl,
allyl (2-propenyl), 1-propinyl, methylethyl, butyl, n-butyl, iso-butyl, sec-
butyl, tert-butyl
butenyl, butinyl, 1-methylpropyl, 2-methylpropyl, 1,1-dimethylethyl, pentyl, n-
pentyl, 1,1-
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
8
dimethylpropyl, 1,2-dimethylpropyl, 2,2-dimethylpropyl, hexyl, 1-methylpentyl,
n-heptyl,
n-octyl, n-nonyl and n-decyl.
In the context of this invention, "alkyl", "alkyl radical" or group is
understood as meaning
saturated, linear or branched hydrocarbons, which can be unsubstituted or mono-
or
polysubstituted. Thus unsaturated alkyl is understood to encompass alkenyl and
alkinyl
groups, like e.g. -CH=CH-CH3 or -C=C-CH3, while saturated alkyl encompasses
e.g. -
CH3 and -CH2-CH3. In these radicals, C1.2-alkyl represents C,- or C2-alkyl,
C1.3-alkyl
represents C,-, C2- or C3-alkyl, C,.4-alkyl represents C,-, C2-, C3- or C4-
alkyl, C1_5-alkyl
represents C,-, C2-, C3-, C4-, or C5-alkyl, C,.6-alkyl represents C,-, C2-, C3-
, C4-, C5- or
C6-alkyl, C1.7-alkyl represents C,-, C2-, C3-, C4-, C5-, C6- or C7-alkyl, C1.8-
alkyl
represents C,-, C2-, C3-, C4-, C5-, C6-, C7- or C8-alkyl, C,.,o-alkyl
represents C,-, C2-, C3-
, C4-, C5-, C6-, C7-, Ce-, C9- or C,o-alkyl and C,.,s-alkyl represents CS-, C2-
, C3-, C4-, C5-,
C6-, C7-, C8-, C9-, C10-, Cõ-, C12-, C13-, C14-, C15-, C16-, C17- or C,$-
alkyl. The alkyl
radicals are preferably methyl, ethyl, vinyl (ethenyl), propyl, allyl (2-
propenyl), 1-
propinyl, methylethyl, butyl, 1-methylpropyl, 2-methylpropyl, 1,1-
dimethylethyl, pentyl,
1,1-dimethylpropyl, 1,2-dimethyfpropyl, 2,2-dimethylpropyl, hexyl, 1-
methylpentyl, if
substituted also CHF2, CF3 or CH2OH etc.
In connection with alkylene, alkyl or aliphatic radical or group - unless
defined
otherwise - the term "substituted" in the context of this invention is
understood as
meaning replacement of at least one hydrogen radical by F, Cl, Br, I, NH2, SH
or OH;
within that "monosubstituted" means the substitution of exactly one hydrogen
radical,
whereas "polysubstituted" means the substitution of more than one hydrogen
radical
with "polysubstituted"radicals being understood as meaning that the
replacement takes
effect both on different and on the same atoms several times with the same or
different
substituents, for example three times on the same C atom, as in the case of
CF3, or at
different places, as in the case of e.g. -CH(OH)-CH=CH-CHCIZ. Therefore,
"optionally
at least monsubstituted" means either "not substituted" if the option is not
fulfilled,
"monosubstituted" or "polysubstituted".
The term "alkylene" is understood as meaning a divalent alkyl group like -CH2-
or -CH2-
CH2-, with (CH2)3.6 being understood as meaning -CH2-CH2-CH2-, -CH2-CH2-CH2-
CH2-,
-CH2-CH2-CH2-CH2-CH2- and -CHZ-CH2-CH2-CH2-CHZ-CH2-, (CH2)1.4 is to be
understood as meaning -CH2-, -CH2-CH2-, -CH2-CH2-CH2- and -CH2-CH2-CH2-CH2-,
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
9
(CH2)4_5 is to be understood as meaning -CH2-CH2-CH2-CH2- and -CH2-CH2-CH2-CH2-
CH2-, etc. An "alkylene" may also be unsaturated.
The term "salt" is to be understood as meaning any form of the active compound
used
according to the invention in which it assumes an ionic form or is charged and
is
coupled with a counter-ion (a cation or anion) or is in solution. By this are
also to be
understood complexes of the active compound with other molecules and ions, in
particular complexes which are complexed via ionic interactions.
The term "physiologically acceptable salt" means in the context of this
invention any
salt that is physiologically tolerated (most of the time meaning not being
toxic-
especially not caused by the counter-ion) if used appropriately for a
treatment
especially if used on or applied to humans and/or mammals.
These physiologically acceptable salts can be formed with cations or bases and
in the
context of this invention is understood as meaning salts of at least one of
the
compounds used according to the invention - usually a (deprotonated) acid - as
an
anion with at least one, preferably inorganic, cation which is physiologically
tolerated -
especially if used on humans and/or mammals. The salts of the alkali metals
and
alkaline earth metals are particularly preferred, and also those with NH4, but
in
particular (mono)- or (di)sodium, (mono)- or (di)potassium, magnesium or
calcium
salts.
These physiologically acceptable salts can also be formed with anions or acids
in the
context of this invention is understood as meaning salts of at least one of
the
compounds used according to the invention - usually protonated, for example on
the
nitrogen - as the cation with at least one anion which are physiologically
tolerated -
especially if used on humans and/or mammals. By this is understood in
particular, in
the context of this invention, the salt formed with a physiologically
tolerated acid, that is
to say salts of the particular active compound with inorganic or organic acids
which are
physiologically tolerated - especially if used on humans and/or mammals.
Examples of
physiologically tolerated salts of particular acids are salts of: hydrochloric
acid,
hydrobromic acid, sulfuric acid, methanesulfonic acid, formic acid, acetic
acid, oxalic
acid, succinic acid, malic acid, tartaric acid, mandelic acid, fumaric acid,
lactic acid or
citric acid.
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
The compounds of the invention may be in crystalline form or either as free
compounds
or as solvates and it is intended that those forms are within the scope of the
present
invention. Methods of solvation are generally known within the art. Suitable
solvates
are pharmaceutically acceptable solvates. The term "solvate" according to this
invention is to be understood as meaning any form of the active compound
according
to the invention in which this compound has attached to it via non-covalent
binding
another molecule (most likely a polar solvent) especially including hydrates
and
alcoholates, e.g. methanolate.
Unless otherwise stated, the compounds of the invention are also meant to
include
compounds which differ only in the presence of one or more isotopically
enriched
atoms. For example, compounds having the present structures except for the
replacement of a hydrogen by a deuterium or tritium, or the replacement of a
carbon by
73C- or 14C-enriched carbon or 15N-enriched nitrogen are within the scope of
this
invention.
Any compound that is a prodrug of a compound of formula (I) is within the
scope of the
invention. The term "prodrug" is used in its broadest sense and encompasses
those
derivatives that are converted in vivo to the compounds of the invention. Such
derivatives would readily occur to those skilled in the art, and include,
depending on the
functional groups present in the molecule and without limitation, the
following
derivatives of the present compounds: esters, amino acid esters, phosphate
esters,
metal salts sulfonate esters, carbamates, and amides. Examples of well known
methods of producing a prodrug of a given acting compound are known to those
skilled
in the art and can be found e.g. in Krogsgaard-Larsen et al. "Textbook of Drug
design
and Discovery" Taylor & Francis (April 2002).
The compounds of formula (I) or their salts or solvates are preferably in
pharmaceutically acceptable or substantially pure form. By pharmaceutically
acceptable form is meant, inter alia, having a pharmaceutically acceptable
level of
purity excluding normal pharmaceutical additives such as diluents and
carriers, and
including no material considered toxic at normal dosage levels. Purity levels
for the
drug substance are preferably above 50%, more preferably above 70%, most
preferably above 90%. In a preferred embodiment it is above 95% of the
compound of
formula (I) or, or of its salts, solvates or prodrugs.
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
11
Particularly preferred are compounds according to the invention which are
compounds
of general formula (Ia),
A
\
R3 ~
R4 N-R1
R5 RZ
(Ia)
wherein
A is a compound selected from the following group
R7 R7
R8-N-~iR7 O \~ R7 S \\ R7 I-S S-I
~ ~ \ \\
O O
R6 R6 R6 R6 R6
~Znnrirw~r ~tinnrvwtr ~tinivinnN znrviivtin.^ Jvvtirifvvtr
R7 R7
R8-N N R7 O N R7 S N R7 -N-R8 R8-N-I
\\ ~ `~ \ 44Io
~\ 6/\ 6~ O R 6 R R6 R
~~nnrinnnr ~~n~vinnnr ~v~nrinnnr ~nnrinnnr .lvtinrinnrv~
R7 R7
O
R8-N S R8a-N N-R8 R8-N O O-I cO
~~ `\ (\ (\ 6~O R R , R
~Zrvuin~v~r ~tinnrinrvtr llv~n~ ~rwiniv~r ~tirwinrvtir
R6 R6
\~ ,\ S N R8 O N R8 N N
R9 R9
O
R9a R6 ~ O R6 R6
~vwinnnr ~Znr~rinniv~ ~tinnnnnnr Zn~v~vvvlr ~tirv~nniv~r
or
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
12
R6
\
N ~
R7-! I
R' and R 2 each are independently selected from the group consisting of
hydrogen; or a linear or branched, saturated or unsaturated, optionally at
least
mono-substituted aliphatic radical; or
R' and R2 together with the bridging nitrogen atom form an saturated or
unsaturated, optionally at least mono-substituted 5- or 6-membered-
heterocyclic
ring, which may be condensed with an optionally at least mono-substituted
mono- or polycyclic ringsystem,
R3, R4 and R5 are independently from each other selected from hydrogen;
halogen, OH, SH, NH2; a linear or branched, saturated or unsaturated,
optionally at least mono-substituted aliphatic radical; or O-R with R being a
linear or branched, saturated or unsaturated, optionally at least mono-
substituted aliphatic radical;
R6 and R' are independently from each other selected from hydrogen; halogen,
OH, SH, NH2; an aliphatic radical, which is linear or branched, saturated or
unsaturated, and optionally at least mono-substituted by F, Cl, Br, I, SH or
OH;
or O-R with R being an aliphatic radical, which is linear or branched,
saturated
or unsaturated, and optionally at least mono-substituted by F, Cl, Br, f, SH
or
OH;
R8 and R8a are independently from each other selected from hydrogen; an
aliphatic radical, which is linear or branched, saturated or unsaturated, and
optionally at least mono-substituted by F, Cl, Br, I, SH or OH; or -R'-O-R"
with
R' and R" independently from one another being a C,.6-alkyl radical, which is
linear or branched, and optionally at least mono-substituted by F, Cl, Br, I,
SH
or OH;
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
13
R9 and R98 are independently from each other selected from halogen; an
aliphatic
radical, which is linear or branched, saturated or unsaturated, and optionally
at least
mono-substituted by F, Cl, Br, I, SH or OH; ; or O-R with R being an aliphatic
radical,
which is linear or branched, saturated or unsaturated, and optionally at least
mono-
substituted by F, Cl, Br, I, SH or OH.
Also the compounds of general Formula (Ia) may be optionally in form of one of
its
stereoisomers, preferably enantiomers or diastereomers, its racemate or in
form of a
mixture of at least two of its stereoisomers, preferably enantiomers or
diastereomers, in
any mixing ratio, or a salt, preferably a physiologically acceptable salt
thereof, or a
corresponding solvate, respectively; or a halide salt thereof, in which NR'R2
of general
formula (I) is substituted by an additional methyl to form with R' and R2
being CH3 a
trimethyl-ammonium-radical.
Also particularly preferred is a compound according to the invention, which is
a
compound according to Formula Ia, wherein
A is a compound selected from the following group
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
14
R8-N O S R~ S s R~
I R7 R7 \ R7
R 6 R6 ~ ~ R6 p ~ O
Rs R6
Jtinrvv~nr~r ~~r~r~riruvlr ~vwinnnr ~v~nrwvt~ ~vwr~vv~r
R8-N N O-N S N R7 N-R8 R8-N R7
R7 R7 \ R7
R6 Rs Re ~ Rs O p
R6
Ju~r~rir~r~r~r~ ~uv~rvw~r ~Znnrinnr~r ~vvv vvw~ ,nnnr~nrvtr
R8-N S Rsa-N N-R8 R8-N 0 0 R7 R7 0
Rs O s p s p R6 O 6 O
R R R
~M/1JYM11/~ ~MJ1IlMJV~ ~/1l~1111l11~l~/~ ~/1Mll/11V~l~ ~/~~MIYI~IVV~
R6 R6
R9 Rs S N R8 O N R8 N N
I I R6 Rs Rs
~ \ \ O
_4y~o
R9a
~v~nrir~nnr ~u~nrinnnr Jti^nn~vv~~ ~tiruv~rvvln ~zn~v~vv~r
or
R7
R6
R' and R2 each are independently selected from the group consisting of
hydrogen; or a linear or branched, saturated or unsaturated, optionally at
least
mono-substituted aliphatic radical; or
R' and R2 together with the bridging nitrogen atom form an saturated or
unsaturated, optionally at least mono-substituted 5- or 6-membered-
heterocyclic
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
ring, which may be condensed with an optionally at least mono-substituted
mono- or polycyclic ringsystem,
R3, R4 and R5 are independently from each other selected from hydrogen;
halogen, OH, SH, NH2; a linear or branched, saturated or unsaturated,
optionally at least mono-substituted aliphatic radical; or O-R with R being a
linear or branched, saturated or unsaturated, optionally at least mono-
substituted aliphatic radical;
R6 and R' are independently from each other selected from hydrogen; halogen,
OH, SH, NH2; an aliphatic radical, which is linear or branched, saturated or
unsaturated, and optionally at least mono-substituted by F, Cl, Br, I, SH or
OH;
or O-R with R being an aliphatic radical, which is linear or branched,
saturated
or unsaturated, and optionally at least mono-substituted by F, Cl, Br, I, SH
or
OH;
R8 and R8a are independently from each other selected from hydrogen; an
aliphatic radical, which is linear or branched, saturated or unsaturated, and
optionally at least mono-substituted by F, Cl, Br, 1, SH or OH; or -R'-O-R"
with
R' and R" independently from one another being a C,-6-alkyl radical, which is
linear or branched, and optionally at least mono-substituted by F, Cl, Br, I,
SH
or OH;
R9 and R9a are independently from each other selected from halogen; an
aliphatic radical, which is linear or branched, saturated or unsaturated, and
optionally at least mono-substituted by F, Cl, Br, I, SH or OH; ; or O-R with
R
being an aliphatic radical, which is linear or branched, saturated or
unsaturated,
and optionally at least mono-substituted by F, Cl, Br, I, SH or OH.
Also particularly preferred is a compound according to the invention, which is
a
compound according to Formula Ia, wherein
A is a compound selected from the following group
CA 02692513 2010-01-04
WO 2009/003719 16 PCT/EP2008/005500
R6
R8
-N--\\ R7 R7 S-~jR7 R9
C ~ (
R6 R6 R
R6
R$-N N 7 O N R7 S N R 7 R9
411-1~ 9a
R6 R R6 R
or
R6
N
R7 L
or
R6
R8-N O S
R7 R7 \ R7 R9
I
R6 R6 ~
R6 ,
, ,
R6
R8 N N O N S N R9
R7 R7 R7
R6 R6 R6 R9a
or
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
17
N ~
R7
~
R6
R' and R 2 each are independently selected from the group consisting of
hydrogen; or a linear or branched, saturated or unsaturated, optionally at
least
mono-substituted aliphatic radical; or
R' and R2 together with the bridging nitrogen atom form an saturated or
unsaturated, optionally at least mono-substituted 5- or 6-membered-
heterocyclic
ring, which may be condensed with an optionally at least mono-substituted
mono- or polycyclic ringsystem,
R3, R4 and R5 are independently from each other selected from hydrogen;
halogen, OH, SH, NH2; a linear or branched, saturated or unsaturated,
optionally at least mono-substituted aliphatic radical; or O-R with R being a
linear or branched, saturated or unsaturated, optionally at least mono-
substituted aliphatic radical;
R6 and R' are independently from each other selected from hydrogen; halogen,
OH, SH, NH2; an aliphatic radical, which is linear or branched, saturated or
unsaturated, and optionally at least mono-substituted by F, Cl, Br, I, SH or
OH;
or O-R with R being an aliphatic radical, which is linear or branched,
saturated
or unsaturated, and optionally at least mono-substituted by F, Cl, Br, I, SH
or
OH;
R8 and R8a are independently from each other selected from hydrogen; an
aliphatic radical, which is linear or branched, saturated or unsaturated, and
optionally at least mono-substituted by F, Cl, Br, I, SH or OH; or -R'-O-R"
with
R' and R" independently from one another being a C1_6-alkyl radical, which is
linear or branched, and optionally at least mono-substituted by F, Cl, Br, I,
SH
or OH
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
18
R9 and R9a are independently from each other selected from halogen; an
aliphatic radical, which is linear or branched, saturated or unsaturated, and
optionally at least mono-substituted by F, Cl, Br, 1, SH or OH; ; or O-R with
R
being an aliphatic radical, which is linear or branched, saturated or
unsaturated,
and optionally at least mono-substituted by F, Cl, Br, I, SH or OH.
Also particularly preferred is a compound according to the invention, which is
a
compound of GROUP A according to Formula Ia, wherein
A is a compound selected from the following group
R8 N___R7 0-1R7 SR7
~<'
R R6 R6
R8 N N 7 O N R7 S N
R R7
,
6/\
R6 R R6 ~
or
R6
N~
R7--L I
or
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
19
R8 N S
\ R7 R7 R7
R6 R6 \ 6 ~
R
R$ N N O N S N
I R7 R7 \ R7
Rs R6 R6
.rvvwwtr
or
R7
R6
~lrvzr~nnnr
R' and R 2 each are independently selected from the group consisting of
hydrogen; or a linear or branched, saturated or unsaturated, optionally at
least
mono-substituted aliphatic radical; or
R' and R2 together with the bridging nitrogen atom form an saturated or
unsaturated, optionally at least mono-substituted 5- or 6-membered-
heterocyclic
ring, which may be condensed with an optionally at least mono-substituted
mono- or polycyclic ringsystem;
R3, R4 and R5 are independently from each other selected from hydrogen;
halogen, OH, SH, NH2; a linear or branched, saturated or unsaturated,
optionally at least mono-substituted aliphatic radical; or O-R with R being a
linear or branched, saturated or unsaturated, optionally at least mono-
substituted aliphatic radical;
R 6 and R' are independently from each other selected from hydrogen; halogen,
OH, SH, NH2; an aliphatic radical, which is linear or branched, saturated or
unsaturated, and optionally at least mono-substituted by F, Cl, Br, I, SH or
OH;
or O-R with R being an aliphatic radical, which is linear or branched,
saturated
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
or unsaturated, and optionally at least mono-substituted by F, Cl, Br, I, SH
or
OH;
R8 is selected from hydrogen; or an aliphatic radical, which is linear or
branched, saturated or unsaturated, and optionally at least mono-substituted
by
F, Cl, Br, I, SH or OH; or -R'-O-R" with R' and R" independently from one
another being a C,-6-alkyl radical, which is linear or branched, and
optionally at
least mono-substituted by F, Cl, Br, I, SH or OH.
Also particularly preferred is a compound according to the invention, which is
a
compound of GROUP B according to Formula Ia, wherein
A is a compound selected from the following group
R6
R9
Rs
R9
R9a
R' and R2 each are independently selected from the group consisting of
hydrogen; or a linear or branched, saturated or unsaturated, optionally at
least
mono-substituted aliphatic radical; or
R' and R2 together with the bridging nitrogen atom form an saturated or
unsaturated, optionally at least mono-substituted 5- or 6-membered-
heterocyclic
ring, which may be condensed with an optionally at least mono-substituted
mono- or polycyclic ringsystem,
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
21
R3, R4 and R5 are independently from each other selected from hydrogen;
halogen, OH, SH, NH2; a linear or branched, saturated or unsaturated,
optionally at least mono-substituted aliphatic radical; or O-R with R being a
linear or branched, saturated or unsaturated, optionally at least mono-
substituted aliphatic radical;
R6 is selected from hydrogen; halogen, OH, SH, NH2; an aliphatic radical,
which
is linear or branched, saturated or unsaturated, and optionally at least mono-
substituted by F, Cl, Br, I, SH or OH; or O-R with R being an aliphatic
radical,
which is linear or branched, saturated or unsaturated, and optionally at least
mono-substituted by F, Cl, Br, I, SH or OH;
R9 and R9a are independently from each other selected from halogen; an
aliphatic radical, which is linear or branched, saturated or unsaturated, and
optionally at least mono-substituted by F, Cl, Br, I, SH or OH; ; or O-R with
R
being an aliphatic radical, which is linear or branched, saturated or
unsaturated,
and optionally at least mono-substituted by F, Cl, Br, I, SH or OH.
Also particularly preferred is a compound according to the invention, which is
a
compound of GROUP A or GROUP B according to Formula Ia, wherein
R' and R2 each are independently selected from the group consisting of
hydrogen; or a linear or branched, optionally at least mono-substituted C14-
alkyl
radical; or
R' and R2 together with the bridging nitrogen atom form an saturated or
unsaturated, optionally at least mono-substituted 5- or 6-membered-
heterocyclic
ring;
preferably in that
R' and R2 are each independently selected from the group consisting of
hydrogen; or a linear or branched C1 4-alkyl radical; or
R' and R2 together with the bridging nitrogen atom form an saturated or
unsaturated, optionally at least mono-substituted 5- or 6-membered-
heterocyclic
ring;
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
22
more preferably in that
R' and R 2 are each independently selected from the group consisting of
hydrogen, CH3, C2H5 or C3H7.
Also particularly preferred is a compound according to the invention, which is
a
compound of GROUP A or GROUP B according to Formula Ia, wherein
R3, R4 and R5 are independently from each other selected from hydrogen;
halogen, OH, SH, NH2; a linear or branched, optionally at least mono-
substituted C,-4-alkyl radical; or O-R with R being a linear or branched,
optionally at least mono-substituted C,-4-alkyl radical;
preferably in that
R3, R4 and RS are independently from each other selected from H, F, Cl, Br, I,
OH, SH, NH2, CH3, C2H5, C3H7, C4H9, OCH3, OC2H5, OC3H7 or OC4H9,
more preferably in that
R3, R 4 and R5 are H.
Also particularly preferred is a compound according to the invention, which is
a
compound of GROUP A according to Formula Ia, wherein
R6 and R' are independently from each other selected from hydrogen; halogen,
OH, SH, NH2; a C,-4-alkyl radical, which is linear or branched, and optionally
at
least mono-substituted by F, Cl, Br, I, SH or OH; or O-R with R being a C,-4-
alkyl radical, which is linear or branched, and optionally at least mono-
substituted by F, Cl, Br, I, SH or OH;
preferably in that
R6 and R' are independently from each other selected from H, F, Cl, Br, I, OH,
SH, NH2, CH3, C2H5, C3H7, C4H9, OCH3, OC2H5, OC3H7 or OC4H9;
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
23
more preferably in that
R6 and R' are independently from each other selected from H, or CH3.
Also particularly preferred is a compound according to the invention, which is
a
compound of GROUP A according to Formula Ia, wherein
R8 is selected from hydrogen; a C,-4-alkyl radical, which is linear or
branched,
and optionally at least mono-substituted by F, Cl, Br, I, SH or OH; or -(CH2)P
O-
(CHZ)q with p and q independently from one another being 1, 2, 3 or 4;
preferably in that
R8 is selected from H, F, Cl, Br, I, OH, SH, NH2, CH3, C2H5, C3H7, C4H9i CH2-O-
CH3 or CH2-O-C2H5;
more preferably in that
R8 is se4ected from H, CH3, C2H5, C3H7, CdH9, or CH2-O-CH3.
Also particularly preferred is a compound according to the invention, which is
a
compound of GROUP A according to Formula la, selected from
= Dimethyl-[6-(1,3,5-trimethyl-1 H-pyrazol-4-yl)-indan-1-yl]-amine;
= (R) Dimethyl-[6-(1,3,5-trimethyl-lH-pyrazol-4-yl)-indan-1-yl]-amine;
= (S) Dimethyl-[6-(1,3,5-trimethyl-1 H-pyrazol-4-yi)-indan-1-yl]-amine;
= Methyl-[6-(1,3,5-trimethyl-1H-pyrazol-4-yl)-indan-1-yl]-amine;
= 6-(1,3,5-Trimethyl-1 H-pyrazol-4-yl)-indan-1-ylamine;
= Diethyl-[6-(1,3,5-trimethyl-1 H-pyrazol-4-yi)-indan-1-ylJ-amine;
= Dipropyl-[6-(1,3,5-trimethyl-1 H-pyrazol-4-yl)-indan-1 -yl]-amine;
= [6-(3,5-Dimethyl-1 H-pyrazol-4-yl)-indan-1-yl]-dimethyl-amine;
= [6-(3,5-Dimethyl-1 H-pyrazol-4-yl)-indan-1 -yl]-methyl-amine;
= [6-(3,5-Dimethyl-isoxazol-4-yl)-indan-1-yl]-dimethyl-amine;
= [6-(3,5-Dimethyl-isoxazol-4-yl)-indan-1-yl]-methyl-amine;
= [6-(1 -Ethyl-3,5-di methyl-1 H-pyrazol-4-yi)-indan-1-yl]-dimethyl-amine;
= [6-(1 -Ethyl-3,5-dimethyl-1 H-pyrazol-4-yl)-indan-1-yl]-methyl-amine;
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
24
= [6-(3,5-Dimethyl-1-propyl-1 H-pyrazol-4-yl)-indan-1-yl]-dimethyl-amine;
= [6-(3,5-Dimethyl-1-propyl-1 H-pyrazol-4-yl)-indan-1 -yl]-methyl-amine;
= [6-(1-Isopropyl-3,5-dimethyl-1H-pyrazol-4-yl)-indan-l-yl]-dimethyl-amine;
= [6-(1-Isopropyl-3,5-dimethyl-1 H-pyrazol-4-yl)-indan-1-yl]-methyl-amine;
= [6-(1-Isobutyl-3,5-dimethyl-1 H-pyrazol-4-yl)-indan-l-yl]-dimethyl-amine:
= [6-(1-Isobutyl-3,5-dimethyl-1 H-pyrazol-4-yl)-indan-1-yl]-methyl-amine;
= [6-(1-Methoxymethyl-3,5-dimethyl-1 H-pyrazol-4-yl)-indan-l-yl]-dimethyl-
amine;
or
= [6-(1-Methoxymethyl-3,5-dimethyl-1 H-pyrazol-4-yl)-indan-1-yl]-methyl-
amine;
or
= Trimethyl-[6-(1,3,5-trimethyl-1 H-pyrazol-4-yl)-indan-1-yl]-ammonium
iodide;
= (S) Methyl-[6-(1,3,5-trimethyl-1 H-pyrazol-4-yl)-indan-1-yl]-amine;
= (R) Methyl-[6-(1,3,5-trimethyl-1 H-pyrazol-4-yl)-indan-1-yl]-amine;
= [6-(2-Methoxy-pyridin-3-yl)-indan-1-yi]-dimethyl-amine;
= [6-(2-Methoxy-pyridin-3-yl)-indan-1-yl]-methyl-amine;
= Dimethyl-(6-piperidin- 1 -yl-indan- 1 -yl)-a mine; hydrochloride; or
= Dimethyl-(6-pyrrolidin-1 -yl-indan-1 -yl)-amine;
optionally in form of a salt, preferably a physiologically acceptable salt,
more
preferably in form of a physiologically acceptable acid addition salt, most
preferably a hydrochloride salt, or a corresponding solvate; or a halide salt
thereof, in which NR'R2 of general formula (I) is substituted by an additional
methyl to form with R' and R2 being CH3 a trimethyl-ammonium-radical..
Also particularly preferred is a compound according to the invention, which is
a
compound of GROUP B according to Formula Ia, wherein
R6 is selected from hydrogen; halogen, OH, SH, NH2; a C,-4-alkyl radical,
which
is linear or branched, and optionally at least mono-substituted by F, Cl, Br,
I, SH
or OH; or O-R with R being a C,-4-alkyl radical, which is linear or branched,
and
optionally at least mono-substituted by F, Cl, Br, I, SH or OH;
preferably in that
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
R6 is selected from H, F, Cl, Br, I, OH, SH, NH2, CH3, C2H5, C3H7, C4H9, OCH3,
OC2H5, OC3H7 or OC4H9;
more preferably in that
R6 is H.
Also particularly preferred is a compound according to the invention, which is
a
compound of GROUP B according to Formula Ia, wherein
R9 and R9a are independently from each other selected from halogen; a C,-4-
alkyl radical, which is linear or branched, and optionally at least mono-
substituted by F, Cl, Br, I, SH or OH; or O-R with R being a C,-4-alkyl
radical,
which is linear or branched, and optionally at least mono-substituted by F,
Cl,
Br, I, SH or OH;
preferably in that
R9 and R9a are independently from each other selected from F, Cl, Br, I, CH3,
C2H5, C3H7, C4H9, OCH3, OC2H5, OC3H7 or OC4H9;
more preferably in that
R9 and R9a are independently from each other selected from F, Cl, CH3, CF3 or
OCH3.
Also particularly preferred is a compound according to the invention, which is
a
compound of GROUP B according to Formula Ia selected from
= [6-(2,6-Dimethoxy-phenyl)-indan-1-yl)-dimethyl-amine;
= [6-(2,6-Dimethoxy-phenyl)-indan-1-yl]-methyl-amine;
= [6-(2-Methoxy-phenyl)-indan-1 -yl]-dimethyl-a mine;
= [6-(2-Methoxy-phenyl)-indan-1 -yl]-methyl-amine;
= [6-(2,6-Dimethyl-phenyl)-indan-1-yl]-dimethyl-amine;
= [6-(2,6-Dimethyl-phenyl)-indan-1 -yl]-methyl-amine;
= [6-(2,6-Dichloro-phenyl)-indan-1-yl]-dimethyl-amine;
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
26
= [6-(2,6-Dichloro-phenyl)-indan-1 -yl]-methyl-amine;
= [6-(2,6-Difluoro-phenyl)-indan-1-yl]-dimethyl-amine;
= [6-(2,6-Difluoro-phenyl)-indan-1-yl]-methyl-amine;
= [6-(2-Chloro-6-methoxy-phenyl)-indan-1-yl]-dimethyl-amine;
= [6-(2-Chloro-6-methoxy-phenyl)-indan-1-yl]-methyl-amine;
= [6-(2,6-Bis-trifluoromethyl-phenyl)-indan-1-yl]-dimethyl-amine; or
= [6-(2,6-Bis-trifluoromethyl-phenyl)-indan-l-yl]-methyl-amine;
optionally in form of a salt, preferably a physiologically acceptable salt,
more
preferably in form of a physiologically acceptable acid addition salt, most
preferably a hydrochloride salt, or a corresponding solvate.
optionally in form of a salt, preferably a physiologically acceptable salt,
more
preferably in form of a physiologically acceptable acid addition salt, most
preferably a hydrochloride salt, or a corresponding solvate; or a halide salt
thereof, in which NR'R2 of general formula (I) is substituted by an additional
methyl to form with R' and R2 being CH3 a trimethyl-ammonium-radical.
In a further aspect the present invention also provides a process for the
preparation of
compounds of general formula (I), wherein a compound of general formula (III)
x
R3 -
R4 N-R1
R5 R2
(III)
wherein R1, RZ, R3, R4 and R5 are as defined above, and X represents
halogen, preferably bromide, OH, OCH3, or an 0-triflate group, is reacted with
a
compound of general formula Ilb or Ilc
L-M
K`\/N
L-M ~
K\/N O1B,O
HO~B, OH ~+
(Ilb) (Ilc)
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
27
wherein K, L, M, and N are as defined above, to form a compound according
to formula I, preferably in presence of a catalyst.
In another further aspect the present invention also provides a process for
the
preparation of compounds of general formula (Ia), wherein a compound of
general
formula (III)
x
R3
R N-R1
R5 R2
(III)
wherein R1, R2, R3, R4 and R5 are as defined above, and X represents
halogen, preferably bromide, OH, OCH3, or an 0-triflate group, is reacted with
a
compound of general formula II or Ila
A
I
A 0' 8, 0
t
HO' B, OH
(II) (Ila)
wherein A is as defined above, to form a compound according to formula la,
preferably in presence of a catalyst.
In a preferred embodiment of any one of these processes
a) the catalyst is a palladium catalyst and/or
b) a ligand is present, and/or
c) the reaction is carried out in presence of at least one base, selected from
organic or inorganic bases and/or
d) the reaction is carried out in a suitable reaction medium.
The compounds of general formulas (III) and (li), (Ila), (ilb) or (Ilc) are
either
commercially available or can be produced according to methods known to those
skilled in the art.
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
28
Suitable reaction media are e.g. organic solvents, such as ethers, preferably
diethyl
ether, dioxane, tetrahydrofurane, dimethyl glycol ether, or alcohols, e.g.
methanol,
ethanol, propanol, isopropanol, butanol, isobutanol, tert-butanol, or
hydrocarbons,
preferably benzene, toluene, xylene, hexane, cyclohexane, petroleum ether, or
halogenated hydrocarbons, e.g. dichloromethane, trichloromethane,
tetrachloromethane, dichloroethylene, trichloroethylene, chlorobenzene or/and
other
solvents preferably ethyl acetate, triethylamine, pyridine, dimethulsulfoxide,
dimethylformamide, hexamethylphosphoramide, acetonitrile, acetone or
nitromethane
are included. Mixtures based one or more of the above mentioned solvents and
water
may also be used.
According to the invention, the bases that may be used in the process are
generally
organic or inorganic bases, preferably alkali metal hydroxides, e.g. sodium
hydroxide or
potassium hydroxide, or obtained from other metals such as barium hydroxide or
different carbonates, preferably potassium carbonate, sodium carbonate,
calcium
carbonate or alkoxydes, e.g. sodium methoxide potassium methoxide, sodium
ethoxide, potassium ethoxide or potassium tert-butoxide, or organic amines,
preferably
triethylamine, diisopropylethylamine or heterocycles, e.g. 1,4-
diazabicyclo[2.2.2]octane,
1,8-diazabicycb5.4.0)undec-7-ene, pyridine, diamino pydine,
dimethySarninopyridine,
methylpiperidine or morpholine. Alkali metals such as sodium or its hydrides,
e.g.
sodium hydride, may also be used.
In a further aspect the present invention also provides a process for the
preparation of
salts of compounds of general formula (I), wherein at least one compound of
general
formula (I) or (Ia) is reacted with an inorganic and/or organic acid,
preferably in the
presence of a suitable reaction medium. Suitable reaction media are the ones
given
above. Suitable inorganic acid are for example hydrochloric acid, hydrobromic
acid,
phosphoric acid, sulphuric acid, nitric acid. Suitable organic acids are e.g.
citric acid,
maleic acid, furmaric acid, tartaric acid or derivatives thereof, such as p-
toluenesulfonic
acid, methanesulfonic acid or camphersulfonic acid.
In yet a further aspect the present invention also provides a process for the
preparation
of salts of compounds of general formula (I), or (Ia), wherein at least one
compound of
general formula (I), or (Ia) having at least one acidic group is reacted with
one or more
suitable bases, preferably in the presence of suitable reaction medium.
Suitable bases
are e.g. hydroxides. Carbonates or alkoxides, which include suitable cations,
derived
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
29
e.g. from alkaline metals, alkaline earth metals or organic cations, e.g.
[NHr~R4_r)',
wherein n is 0, 1, 2, 3 or 4 and R represents a branched or linear C,.4 alkyl
radical.
Solvates, preferably hydrates, of the compounds of general formula (I), or
(Ia) or
corresponding stereoisomers, or corresponding salts may also be obtained by
standard
procedures known to those skilled in the art.
If the compounds of general formula (I) or (Ia) are obtained in form of a
mixture of
stereoisomers, particularly enantiomers or diastereomers, said mixtures may be
separated by standard procedures known to those skilled in the art, e.g.
chromatographic methods of crystallization with chiral reagents.
The purification and isolation of the compounds of general formula (I) or (la)
or a
corresponding stereoisomer, or a corresponding salt, or corresponding solvate
respectively, if required may be carried out by conventional methods known to
those
skilled in the art, e.g. chromatographic methods or recrystallization.
In another further aspect, the present invention also provides a process for
the
preparation of compounds of general formula (1) or especiaSSy (tia), according
to
Scheme 1, wherein R', R2, R3, Ra, R5 and A have the meaning given above,
especially
a process in which R3, R4 and R5 are all H.
A
R3 ~
R N-R1
R5 R2
(Ia)
The compounds of general formula (Ia) can be prepared by catalytic cross-
coupling
reactions, which include the Kumada-Corriu-Tamao, Negishi, Stille, Hiyama,
Suzuki-
Miyaura, Heck, Sonogashira and other cross-coupling reactions known to those
skilled
in the art. More preferably, the compounds of general formula (Ia) can be
prepared by
cross-coupling Suzuki reaction of boronic acids or boronate esters of general
formula
(II) or (Ila),
A
I
A or OB,O
HO OH
(II) (Ila)
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
wherein A has the meaning described above, with at least one compound of
general
formula (III),
x
~
R3 ~
R N-R1
R5 R2
(111)
wherein R' R2, R3, R4 and R5 have the meaning given above and X represents
halogen, preferably bromide, OH, OMe or 0-triflate group, in a suitable
reaction
medium, in the presence of a palladium catalyst, a suitable ligand and at
least one
base. This process can be performed by subjecting the reaction mixture to
reflux by
conventional heating for a period of time sufficient to achieve the title
compound (Ia), or
by microwave radiation, preferably for 5 to 60 minutes, and at a temperature
between
100 to 120 C.
Preparation of compounds of general formula (III) can be achieved by two
consecutive
reductive amination reactions of aldehydes of general formula (IV) and (V),
R'CHO (IV), R2CHO (V)
wherein R' and R2 have the meaning given above, with a compound of general
formula
(VI),
R4
5
R3r\~
X
(VI) NH2
wherein X has the meaning described above. The reductive amination is
performed by
reaction of a mixture comprising a compound of general formula (IV) or (V),
and amino
compound of general formula (VI) and a reducing agent in a suitable reaction
medium,
for a period of time sufficient to achieve the title compound (I11). The
reductive
amination reaction can also be performed under microwave radiation preferably
for 5 to
60 minutes, and at a temperature between 90 to 120 C. The use of microwave
irradiation limits the formation of undesirable secondary reaction products,
compared to
what is obtained in a conventional reductive amination procedure.
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
31
This process can be performed as a direct reaction when the carbonyl compound
of
general formula (IV) or (V) and the amine compound of general formula (VI) are
mixed
with the reducing agent without prior formation of the intermediate imine or
iminium
salt. A stepwise or indirect reaction involves the reduction of the
preformatted imine in
a separate step.
Amino compounds of general formula (VI) are obtained by reductive amination
with
ammonia of carbonyl compounds of general formula (VII), in the conditions
described
above,
R
R5
R3~\\
X
(VII) 0
Compounds of general formula (Vll) can directly afford compounds of general
formula
(III) through a reductive amination with secondary amines of general formula
(VIII),
HNR'R2 (VIII)
wherein R', and R2 have the meaning given above, in the conditions described
above.
The choice of the reducing agent for reductive amination reaction can be
conventionally made by those skilled in the art. Reducing agents useful in
this
procedure include hydrogen and a catalyst, zinc and HCI, sodium
cyanoborohydride,
lithium cyanoborohydride, tetrabutylammonium cyanoborohydride,
cyanoborohydride
on a solid support, sodium cyanoborohydride and dehydrating agents, sodium
cyanoborohydride and titanium additives, sodium cyanoborohydride and zinc
halide
additives, sodium borohydride, sodium borohydride and dehydrating agents,
sodium
borohydride and titanium additives, sodium borohydride and zinc salt
additives, lithium
borohydride, potassium borohydride, polymer-supported borohydride, borohydride
exchange resin with nickel acetate or palladium acetate, sodium
triacetoxyborohydride,
sodium triacetoxyborohydride and additives, tetramethylammonium
triacetoxyborohydride, sodium cyano-9-borabicyclo[3.3. 1 ]nonane, lithium
triethylborohydride, lithium tri(sec-butyl)borohydride, sodium
diisopinocampheylcyanoborohydride, amine boranes, borane-pyridine complex and
alkylamine boranes. Sodium triacetoxyborohydride is particularly preferred
because is
non-toxic and generally does not reduce the carbonyl group prior to imine
formation.
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
32
The compounds of general formulas (II), (Ila), (IIb), (lic), (IV), (V), (VII)
and (VIII) are
either commercially available or can be produced according to methods known to
those
skilled in the art.
Suitable reaction media are e.g. organic solvents, such as ethers, preferably
diethyl
ether, dioxane, tetrahydrofurane, dimethyl glycol ether, or alcohols, e.g.
methanol,
ethanol, propanol, isopropanol, butanol, isobutanol, tert-butanol, or
hydrocarbons,
preferably benzene, toluene, xylene, hexane, cyclohexane, petroleum ether, or
halogenated hydrocarbons, e.g. dichloromethane, trichloromethane,
tetrachloromethane, dichloroethylene, trichloroethylene, chlorobenzene or/and
other
solvents preferably ethyl acetate, triethylamine, pyridine, dimethulsulfoxide,
dimethylformamide, hexamethylphosphoramide, acetonitrile, acetone or
nitromethane
are included. Mixtures based one or more of the above mentioned solvents and
water
may also be used.
According to the invention, the bases that may be used in the process are
generally
organic or inorganic bases, preferably alkali metal hydroxides, e.g. sodium
hydroxide or
potassium hydroxide, or obtained from other metals such as barium hydroxide or
different carbonates, preferably potassium carbonate, sodium carbonate,
ca4cium
carbonate or alkoxydes, e.g. sodium methoxide potassium methoxide, sodium
ethoxide, potassium ethoxide or potassium tert-butoxide, or organic amines,
preferably
triethylamine, diisopropylethylamine or heterocycles, e.g. 1,4-
diazabicycio[2.2.2]octane,
1,8-diazabicyclo5.4.0]undec-7-ene, pyridine, diamino pyridine,
dimethylaminopyridine,
methylpiperidine or morpholine. Alkali metals such as sodium or its hydrides,
e.g.
sodium hydride, may also be used.
The preparation of compounds of general formula (Ia), especially those in
which R3, R4
and R5 are all H, is illustrated in scheme 1:
Scheme 1
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
33
R4 R5 NH3 R4 R5 1. R'CHO (IV) R3 R4
~
R3 NaBH(OAc)3 :I' aBHOA3 RS
/ X~
(VII) O (VI) NH2 . R2CH0 (V) (III) N-R~
NaBH(OAc)3 RZ
HNR1R2 (VIII) 1 A
NaBH(OAc)~ I A or O1B~O
HO' B, OH
(II) (Ila)
Catalyst, Base
Solvent
R4 Rs
R3~~~
(Ia) R2 N-Rl
In a further aspect, the present invention also provides an alternative
process for the
preparation of compounds of general formula (I), according to Scheme 2,
especially a
process wherein R3, R4 and R5 are all H. According to this process, at least
one
compound of general formula (IX),
4
,R
R3 r\
A
(IX) NH2
wherein R3, R4, R5 and A have the meaning given above, is subjected to two
consecutive reductive amination reaction with aidehydes of general formula
(IV) and
(V). The reductive amination reaction could be performed following the methods
described above.
Amino compounds of general formula (IX) are obtained by reductive amination
with
ammonia of carbonyl compounds of general formula (X), in the conditions
described
above,
R4
5
R3(\ R
i ,
A
(X) O
wherein R3, R4, R5, and A have the meaning given above. Compounds of general
formula (X) can directly afford compounds of general formula (Ia) through a
reductive
amination with secondary amines of general formula (VIII), in the conditions
described
above.
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
34
Preparation of compounds of general formula (X) can be achieved by catalytic
cross-
coupling reactions, which include the Kumada-Corriu-Tamao, Negishi, Stille,
Hiyama,
Suzuki-Miyaura, Heck, Sonogashira and other cross-coupling reactions known to
those
skilled in the art. More preferably, the compounds of general formula (X) can
be
prepared by cross-coupling Suzuki reaction of boronic acids or boronate esters
of
general formula (II) or (Ila), with at least one compound of general formula
(VII). Suzuki
reaction could be performed following the methods described above.
This alternative method for the preparation of compounds of general formula
(I) is
illustrated in scheme 2:
Scheme 2
A
A
or O"O HNRIRZ (VIII) NaCNBH R 4 5
4 B\OH
~ R5 HO11) (Ila) 3R 4 R5 3 R
R3 ~ CH3COOH ~ R3
X Catalyst, Base A A
(VII) O Solvent (X) O N-Rl
(Ia) R2
NH3
NaBH(OAc)3 1. R'CHO (IV)
R5 NaBH(OAc)3
R3 2. R2CHO (V)
A NaBH(OAc)3
(IX) NH2
In another aspect, the present invention also provides a process for the
preparation of
compounds of general formula (Ia), in the particular case in which X is OH,
OMe or 0-
triflate group, according to Scheme 3, especially a process wherein R3, R4 and
R5 are
all H.
Preparation of compounds of general formula (Illa),
3 4
R i ~~R 5
Tf0 ~
(Illa) R2N'RI
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
wherein R1, R2, R3, R4 and R5 have the meaning described above, can be
achieved by
reaction of triflic anhydride, in the presence of a base and in a suitable
reaction
medium, with compounds of general formula (IIIb),
R3 R 5
',/.
HOi~
(illb) R2'RI
wherein R', RZ, R3, R4 and R5 have the meaning described above.
Hydroxyl compounds of general formula (Illb) are obtained from the methoxy
compounds of general formula (Ilic) by heating in HBr 48% at 125 C,
R ~ ^/R4 R5
(Ilic) RzN Ri
wherein R1, RZ, R3, R' and R5 have the meaning described above.
Compounds of general formula (Illc) could be prepared by two consecutive
reductive
amination reactions from amino compounds of formula (VIa) as described above
(Scheme 1). In an alternative synthetic process, compounds of formula (VI la)
can
directly afford compounds of general formula (IIIc) through a reductive
amination with
secondary amines of general formula (VIII).
This alternative method for the preparation of compounds of general formula
({) is
illustrated in scheme 3:
Scheme 3
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
36
R 4 5 NH3 R 4 S 1. R'CHO (IV) Ra R5
R3 NaBH(OAc)3 R
R3 ~ \ i NaBH(OAc)3 R3 f\ i HBr 48%
Me0 Me0 I~ NH2 2. R2CH0 (V) Me0 I~
(Vlla) O (Vla) NaBH(OAc)3 (Illc) RZ N`R'
HNR'RZ (VIII)
NaBH(OAc)-3 A
I
4 a AI or O"B
R ~O Ra
~R5 Tf20 3R\ RS HOiB, OH (t~ R3 (,\ i
R Rs
base_ - R~ O )
A A
HO (Illb 2N-Rl Tf0 Catalyst, Base N_ '
) R (Illa) RZN'R~ Solvent (Ia) RZ R
In a further aspect, the present invention also provides an alternative
process for the
preparation of compounds of general formula (1), according to Scheme 4,
especially a
process wherein R3, Ra and R5 are all H. According to this process,
intermediate
compounds of general formula (X) (see Scheme 2), can be achieved by hydrolysis
of
compounds of general formula (XI),
4
3 r
R\ /R
i /
RX~ OR"
(XI)
wherein A has the meaning given above and Rx being hydrogen or an aliphatic
radical,
which is linear or branched, saturated or unsaturated, and optionally at least
mono-
substituted by F, Cl, Br, I, SH or OH. Both Rx together with the bridging
oxigen atoms
can also form an saturated or unsaturated, optionally at least mono-
substituted 5- or 6-
membered-heterocyclic ring, which may be condensed with an optionally at least
mono-substituted mono- or polycyclic ringsystem.
Preparation of compounds of general formula (XI) can be achieved by catalytic
cross-
coupling reactions, which include the Kumada-Corriu-Tamao, Negishi, Stille,
Hiyama,
Suzuki-Miyaura, Heck, Sonogashira and others known to those skilled in the
art. More
preferably, the compounds of general formula (XI) can be prepared by cross-
coupling
Suzuki reaction of boronic acids or boronate esters of general formula (II) or
(Ila) with
at least one compound of general formula (XII),
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
37
Ra 5
R3 (\ R
I
Tf0 RXO OR"
(XII)
wherein R3, R 4 and R5 are as described above and Rx has the meaning described
above. Suzuki reaction could be performed following the methods described
above.
Compounds of general formula (XII) are obtained by reaction of triflic
anhydride, in the
presence of a base and in a suitable reaction medium, of compounds of general
formula (XIII),
a 5
R ~~ \ /R
HO RXO OR"
(Xlll)
wherein R3, R , R5 and Rx have the meaning described above.
Hydroxyl compounds of general formula (XIII) are obtained from the methoxy
compounds of general formula (XIV) by treatment with BBr3,
4
R ~\ \ /R5
1O RXO OR"
(XIV)
wherein R3, R4, R5 and Rx have the meaning described above. Demethylation
reaction
could also be performed by other procedures well known to those skilled in the
art.
Ketal compounds of general formula (XIV), especially those wherein R3, R' and
R5 are
H, could be formed by treatment of carbonyl compounds of formula (Vlla),
\
~
MeO
l
(VIIa) O
with an alcohol (R-OH) in the presence of acid catalysts.
The bases that may be used in the process and the suitable reaction media are
those
described above.
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
38
This alternative method for the preparation of compounds of general formula
(I) is
illustrated in scheme 4:
Scheme 4
R4 R5 RxOH R4 s R4 R5 Tf20 R 5
R3 - r l ca+ Rs BBr3 R3 j~ base R3 R
Me0 MeO~~~~~~~ x ~ Tf0 ~
HO x OR
(Vlla) O (XIV) RxO (XIII) Rx0 OR (XII) Rx0 ORx
A
I
A or O-B'O
R~ HNRIRZ (VIII) R4 R5
HO'B'OH R4
Rs +
(II) (Ila) A a ~ ZO, H A NaBH(OAc)3R3 Catalyst, Base A J~\
~ QORx
Solvent (XI) RxO (X) O N-R~
(I) Rz
NH3
NaBH(OAc)3 1. RICHO (IV)
R4 R5 NaBH(OAc)3
R3 1~,\ 2. R2CHO (V)
A NaBH(OAc)3
(IX) NH2
In another aspect, the present invention also provides an alternative process
for the
preparation of compounds of general formula (I), according to Scheme 5,
wherein R1,
R2, R3, R4 and R5 have the meaning given above and A is:
R8
N-N
R6 ~ \ R~
~'
wherein R6, R' and R8 have the meaning described above, especially a process
wherein R3, R4 and R5 are all H.
The compounds of general formula (XVI),
R4 R
O R3 ~\~= ~
Rs /
O R7 R2 N-Rl
(XVI)
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
39
wherein R', R2, R3, R4, R5, R 6 and R' have the meaning described above were
reacted
with compounds of general formula (XV),
H
H2N-N-R8
(XV)
wherein R8 have the meaning described above, in a suitable reaction media to
give the
title compounds of general formula (Ia).
Preparation of compounds of general formula (XVI) can be achieved by copper
catalyzed nucleophilic substitution reaction of compounds of general formula
(XVII),
O O
R6AI-AR7
(XVII)
wherein R6 and R' have the meaning described above, with compounds of general
formula (III),
X
R3
R4 N-RI
R5 R2
(III)
wherein R', R2, R3, R4, R5 and X have the meaning described above, in a
suitable
reaction medium, in the presence of CuX, and at least one base.
Compounds of general formula (III) can be obtained as described above (see
Scheme
1).
The compounds of general formulas (IV), (V), (VII), (VIII), (XV) and (XVII)
are either
cornmercially available or can be produced according to methods known to those
skilled in the art.
Suitable reaction media are those described above.
The bases that may be used in the process are those descried above.
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
This alternative preparation of compounds of general formula (Ia) is
illustrated in
scheme 5:
Scheme 5:
0 0
R4 R5 NH3 R4 1. R'CHO (IV) R4 R6'~/~R7
R3 NaBH(OAc)3 3 ~~ ~ ~R5 ::::-R Base, CuX, Solvent
(VII) O (VI) NH2 2(V) X (III) N-R1
NaBH(OAc)3 R2
HNRIR2 (VIII)
NaBH(OAc)3
R4 Rs H 8 R4
O 3 H2N-N-R 3 R5 R8
6 A_ N-N
R (X~ R
R N_ l Solvent A N_ R6 R7
R7 RZ R 0 R1
(XVI)
Enantiomerically pure compounds of general formula (S)-(I) or (R)-(I),
R4 R4
5
R3 R R3r~ 5
~
A p,
N-R~ N_R1
(R)-(Ia) R2 (S)-(Ia) R2
wherein R1, R2, R3, R , R5 and A have the meaning given above, are obtained
from
racemic compounds of general formula (Ia) by standard separation procedures
known
to those skilled in the art, e.g. chromatographic methods or crystallization
with chiral
reagents. Compounds of general formula (S)-(Ia) or (R)-(Ia) could also be
prepared by
enantioselective synthetic methods as for example the one depicted in Scheme
6.
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
41
Scheme 6
4 JRuCI2 (p-cymene)] R RS 2 R4 1. Et3N, MsCi R4
R3 ~\ chiral ligand R3 S 2. Me2NH gas 3~\ ti ~R5
R
X / sodium formate X X
(VII) 0 (XXIV) OH (III) RZN'R'
Chiral Ligand:
(S, R)-cis aminoindanol O
TsDPEN
,qBO
Pd(PPh3)4
4
R8 R5
N-N R I A= /
N--Ri
s^'^^^ R2
(R)- (Ia)
(S)- (la)
The compounds of general formula (I), or (Ia) their stereoisomers or the
respective
salts or solvates are toxicologically acceptable and are therefore suitable as
pharmaceutical active substances for the preparation of inedicaments.
The present invention therefore also provides for a pharmaceutical formulation
or
medicament comprising at least one compound according to the invention
according to
formula (I) or formula (Ia), optionally in form of one of its stereoisomers,
preferably
enantiomers or diastereomers, its racemate or in form of a mixture of at least
two of its
stereoisomers in any mixing ratio, or a physiologically acceptable salt
thereof, or a
solvate, respectively, and optionally one or more pharmaceutically acceptable
adjuvants.
Furthermore, the present invention also provides for a pharmaceutical
composition/medicament comprising at least one compound of general formula (I)
or
(la), optionally in form of one of its stereoisomers, preferably enantiomers
or
diastereomers, its racemate or in form of a mixture of at least two of its
stereoisomers
in any mixing ratio, or a physiologically acceptable salt thereof, or a
solvate,
respectively, and optionally one or more pharmaceutically acceptable
adjuvants, which
is not yet formulated into a medicament.
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
42
Preferably the medicament is suitable for the treatment of a 5-HT7 mediated
disease or
condition, especially selected from pain, preferably visceral pain, chronic
pain, cancer
pain, migraine, acute pain or neuropathic pain, more prefearably neuropathic
pain,
allodynia or hyperalgesia or selected from sleep disorder, shift worker
syndrome, jet
lag, depression, seasonal affective disorder, migraine, anxiety, psychosis,
schizophrenia, cognition and memory disorders, neuronal degeneration resulting
from
ischemic events, cardiovascular diseases such as hypertension, irritable bowel
syndrome, inflammatory bowel disease, spastic colon or urinary incontinence.
The present invention also provides for the use of at least one compound
according to
formula (I), or (Ia), optionally in form of one of its stereoisomers,
preferably
enantiomers or diastereomers, its racemate or in form of a mixture of at least
two of its
stereoisomers in any mixing ratio, or a physiologically acceptable salt
thereof, or a
solvate, respectively, for the treatment of a 5-HT7 mediated disease or
condition.
Alternatively it also provides for the use of at least one compound according
to formula
(I), or (Ia), optionally in form of one of its stereoisomers, preferably
enantiomers or
diastereomers, its racemate or in form of a mixture of at least two of its
stereoisomers
in any mixing ratio, or a physiologically acceptable salt thereof, or a
solvate,
respect-vely, for the manufacture of a medicament for the treatment of a 5-HT7
mediated disease or condition.
In a preferred embodiment the use according to the invention relates to a use
as
described above, wherein the disease is pain, preferably visceral pain,
chronic pain,
cancer pain, migraine, acute pain or neuropathic pain, more prefearably
neuropathic
pain, allodynia or hyperalgesia.
In another preferred embodiment the use according to the invention relates to
a use as
described above, wherein the disease is sleep disorder, shift worker syndrome,
jet lag,
depression, seasonal affective disorder, migraine, anxiety, psychosis,
schizophrenia,
cognition and memory disorders, neuronal degeneration resulting from ischemic
events, cardiovascular diseases such as hypertension, irritable bowel
syndrome,
inflammatory bowel disease, spastic colon or urinary incontinence.
The medicament/pharmaceutical composition may be in any form suitable for the
application to humans and/or animals, preferably mammals, and can be produced
by
standard procedures known to those skilled in the art. The composition of the
medicament may vary depending on the route of administration.
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
43
The medicament of the present invention may e.g. be administered parentally in
combination with conventional injectable liquid carriers, such as water or
suitable
alcohols. Conventional pharmaceutical adjuvants for injection, such as
stabilizing
agents, solubilizing agents, and buffers, may be included in such injectable
compositions. These medicaments may preferably be injected intramuscularly,
intraperitoneally, or intravenously.
Medicaments according to the present invention may also be formulated into
orally
administrable compositions containing one or more physiologically compatible
carriers
or excipients, in solid or liquid form. These compositions may contain
conventional
ingredients such as binding agents, fillers, lubricants, and acceptable
wetting agents.
The compositions may take any convenient form, such as tablets, pellets,
capsules,
lozenges, aqueous or oily solutions, suspensions, emulsions, or dry powdered
form
suitable for reconstitution with water or other suitable liquid medium before
use, for
immediate or controlled release.
The liquid oral forms for administration may also contain certain additives
such as
sweeteners, flavoring, preservatives, and emulsifying agents. Non-aqueous
liquid
compositions for oral administration may also be formulated, containing e.g.
edible oils.
Such liquid compositions may be conveniently encapsulated in e.g., gelatin
capsules in
a unit dosage amount.
The compositions of the present invention may also be administered topically
or via a
suppository.
The above mentioned compositions include preferably 1 to 60 % by weight of one
or
more of the compound of general formula (I), optionally in form of one of its
stereoisomers, preferably enantiomers or diastereomers, its racemate or in
form of a
mixture of at least two of its stereoisomers in any mixing ratio, or a
physiologically
acceptable salt thereof, or a solvate, respectively, and 40 to 99 % by weight
of the
appropriate pharmaceutical vehicle(s).
The daily dosage for humans and animals may vary depending on factors that
have
their basis in the respective species or other factors, such as age, weight or
degree of
illness and so forth. The daily dosage for mammals including humans usually
ranges
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
44
from 1 milligram to 2000 milligram, preferably 1 to 1500 mg, more preferably 1
to 1000
mg of substance to be administered during one or several intakes.
Thus, the invention also provides a method of treatment for an animal or human
in
need thereof using the medicament/pharmaceutical compositions described above.
Pharmacological Methods:
Radioligand binding
Radioligand binding assays were performed using the Cloned Human Serotonin
Receptor, Subtype 7(h5HT,), expressed in CHO cells, coated on Flashplate
(Basic
FlashPlate Cat.: SMP200) from PerkinElmer (Cat.: 6120512). The protocol assay
was
essentially the recommended protocol in the Technical Data Sheet by PerkinEmer
Life
and Analytical Sciences. The Mass membrane protein/well was typically 12 pg
and the
Receptor/well was about 9-10 fmoles. The Flashplate were let equilibrate at
room
temperature for one hour before the addition of the components of the assay
mixture.
The binding buffer was: 50 mM Tris-HCI, pH 7.4, containing 10 mM MgCi2, 0.5 mM
EDTA and 0.5% BSA. The radioligand was [1251]LSD at a final concentration of
0.82
nM. Nonspecific binding was determined with 50 pM of Clozapine. The assay
volume
was 25 NI. TopSeal-A were applied onto Flashplate microplates and they were
incubated at room temperature for 240 minutes in darkness. The radioactivity
were
quantified by liquid scintillation spectrophotometry (Wallac 1450 Microbeta
Trilux) with
a count delay of 4 minutes prior to counting and a counting time of 30 seconds
per well.
Competition binding data were analyzed by using the LIGAND program (Munson and
Rodbard, LIGAND: A versatile, computerized approach for characterization of
ligand-
binding systems. Anal. Biochem. 107: 220-239, 1980) and assays were performed
in
triplicate determinations for each point.
Functionality assay on the 5HT7 receptor were done according to those known in
the
state of the art.
The following figures and examples are given to illustrate the present
invention, but
they do not limit the scope of the present invention.
FIGURES
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
Figure 1) X-Ray of the crystal structure of example 1 (Ortep-Plot (50 %) with
labeling
scheme)
Figure 2) X-Ray of the crystal structure of example 1 (Ortep-Plot (50 lo))
Figures 1 and 2 show the crystal structure of Example 1 (see below) by X-Ray.
The
experimental preparation of the X-Ray analysis is also described below.
EXAMPLES
Example A (of compounds of general formula (Itl))
(6-Bromo-indan-1-yl)-dimethyl-amine
Br
O
Q
/N_
(Method 1): 6-Bromo-indan-l-one (0.23 mmol) and dimethyl-amine (0.71 mmol)
were
mixed in 3 ml of 1,2-dichloroethane in a process vial, which was sealed with a
septum.
Sodium triacetoxyborohydride (0.47 mmol) was added under argon atmosphere. The
suspension was subjected to microwave irradiating conditions (CEM Discover
equipped with a CEM Explorer automated reaction handling module). The
reaction
mixture was heated for 5 min at 120 C and then cooled. The crude was
evaporated to
dryness and then suspended in aqueous NaHCO3. The product was extracted with
CH2CI2 and washed with aqueous NaHCO3. The CH2CI2 extract was dried with
anhydrous Na2SO4, filtered and evaporated to dryness to give the crude product
(6-
bromo-indan- 1 -yi)-dimethyl-a mine. The crude was purified by flash column
chromatography (CH2CI2-MeOH as eluents) by using a CombiFlash CompanionTM
system to yield the title compound (85%). MS [MH]+= 240.
(Method 2): 6-Bromo-indan-l-one (0.58 mmol) was dissolved in MeOH. Dimethyl-
amine (3.7 mmol), acetic acid (0.059 mmol) and sodium cyanoborohydride (1.74
mmol)
were added under argon atmosphere. The suspension was refluxed overnight and
then
cooled. The crude was evaporated to dryness and then suspended in water. The
product was extracted with CH2CI2 and washed with water. The CH2CI2 extract
was
dried with anhydrous Na2SO4, filtered and evaporated to dryness to give the
crude
product (6-bromo-indan-1-yf)-dimethyl-amine (80% conversion LC-MS). The crude
product was used in the next synthetic step (Suzuki coupling), without further
purification.
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
46
Example B (of compounds of general formula (X))
6-(1,3,5-Trimethyl-1 H-pyrazol-4-yl)-indan-l-one
z0o
6-Bromo-indan-l-one (0.94 mmol) was dissolved in DME/H20 1/1 (10 mL) under
argon
atmosphere. 1,3,5-Trimethyl-lH-pyrazole-4-boronic acid pinacol ester (1.42
mmol),
K2CO3 (2.82 mmol), and tetrakis-(triphenylphosphine)palladium (5 mol%, 0.047
mmol)
were added and the reaction mixture was stirred at 100 C for 3h. The reaction
mixture
was evaporated to dryness, then dissolved in CHCI3 and filtered through Celite
to
give the crude product 6-(1,3,5-trimethyl-1 H-pyrazol-4-yl)-indan-l -one. The
crude was
purified by flash column chromatography (AcOEt-Cyclohexane as eluents) by
using a
CombiFlash CompanionT"' system to yield the title compound (84%). MS [MH]`=
240
Example I
Dimethyl-[6-(1,3,5-tcimethyl-1 H-pysazol-4-yl)-indan-l-yl]-amine
dihydcochloride
2HC1
N
(Method 1): 6-(1,3,5-Trimethyl-1H-pyrazol-4-yl)-indan-l-one (0.20 mmol),
dimethyl-
amine (2.31 mmol) and acetic acid (0.020 mmol) were mixed in 3 ml of MeOH in a
process vial, which was sealed with a septum. Sodium cyanoborohydride (0.47
mmol)
was added under argon atmosphere. The suspension was subjected to microwave
irradiating conditions (CEM Discover equipped with a CEM Explorer automated
reaction handling module). The reaction mixture was heated for lh at 120 C and
then
cooled. The crude was evaporated to dryness and. then suspended in water. The
product was extracted with CH2CI2 and washed with water. The CH2CI2 extract
was
dried with anhydrous Na2SO4, filtered and evaporated to dryness to give the
crude
product dimethyl-[6-(1,3,5-trimethyl-1H-pyrazol-4-yl)-indan-1-yf]-amine. The
crude was
purified by flash column chromatography (CH2CI2-MeOH as eluents) by using a
CombiFlash CompanionT"" system to yield the base of the title compound. The
obtained
product was diluted in ethyl acetate (5 mL) and a solution of hydrogen
chloride 2,0 M in
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
47
diethylether was added. The resulting precipitate was filtered and dried under
vacuum
to yield the title compound dimethyl-[6-(1,3,5-trimethyl-1H-pyrazol-4-yl)-
indan-1-yl]-
amine dihydrochloride (83%). MS [MH]+= 270.
(Method 2): 6-(1,3,5-Trimethyl-1H-pyrazol-4-yl)-indan-1-one (1.1 mmol) was
dissolved
in MeOH. Dimethyl-amine (24.5 mmol), acetic acid (0.11 mmol) and sodium
cyanoborohydride (2.2 mmol) were added under argon atmosphere. The suspension
was refluxed overnight and then cooled. The crude was evaporated to dryness
and
then suspended in water. The product was extracted with CH2CI2 and washed with
water. The CH2CI2 extract was dried with anhydrous Na2SO4, filtered and
evaporated to
dryness to give the crude product dimethyl-[6-(1,3,5-trimethyl-1H-pyrazol-4-
yl)-indan-1-
yl]-amine. The crude was purified by flash column chromatography (CH2CI2-MeOH
as
eluents) by using a CombiFlash CompanionTM system to yield the base of the
title
compound. The obtained product was diluted in ethyl acetate and a solution of
hydrogen chloride 2,0 M in diethylether was added. The resulting precipitate
was
filtered and dried under vacuum to yield the title compound dimethyl-[6-(1,3,5-
trimethyl-
1 H-pyrazol-4-yl)-indan-1 -yl]-amine dihydrochloride (79%). MS [MH]+= 270.
(Method 3): (6-Bromo-indan-1-yl)-dimethyl-amine (0.82 mmoti) was disso4ved in
DME/H20 1/1 (10 mL) under argon atmosphere. 1,3,5-Trimethyl-lH-pyrazole-4-
boronic
acid pinacol ester (0.82 mmol), K2C03 (3.08 mmol), and tetrakis-
(triphenylphosphine)palladium (3 mol%, 0.025 mmol) were added and the reaction
mixture was stirred at 100 C for 3h. Then, a second fraction of 1, 3,5-trim
ethyl- 1 H-
pyrazole-4-boronic acid pinacol ester (0.41 mmol) was added and the reaction
mixture
was stirred at 100 C for 2h more. The reaction mixture was evaporated to
dryness,
then dissolved in CHCI3 and filtered through Celite to give the crude product
dimethyl-
[6-(1,3,5-trimethyl-1H-pyrazol-4-yl)-indan-1-yl]-amine. The crude was purified
by flash
column chromatography (CH2CI2 and MeOH as eluents) by using a CombiFlash
CompanionT"" system to yield the base of the title compound. The obtained
product
was diluted in ethyl acetate and a solution of hydrogen chloride 2,0 M in
diethylether
was added. The resulting precipitate was filtered and dried under vacuum to
yield the
title compound dimethyl-[6-(1,3,5-trimethyl-1 H-pyrazol-4-yl)-indan-1 -yl]-
amine
dihydrochloride (75%). MS [MH]*= 270.
Examples 2 and 3 (through separation on a chiral HPLC column)
Enantiomers of dimethyl-[6-(1,3,5-trimethyl-1 H-pyrazol-4-yl)-indan-1-yl]-
amine
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
48
Enantiomers of Example 1 (dimethyl-[6-(1,3,5-trimethyl-lH-pyrazol-4-yl)-indan-
l-yl]-
amine) were - in one option - obtained by separation on a chiral HPLC column
(Chiralpak AD-H 4.6x250mm, 5pm). The mobile phase was heptane/EtOH 98/2 v/v +
0.1% DEA.
Tr(enantiomer R)=29.3 min [a]p =-72.17 (c=1, MeOH)
Tr(enantiomer S)=37.8 min [a]p20=+70.29 (c=1, MeOH)
Example of compounds of general formula (XXIV)
(S)-6-Bromo-indan-l-ol
Br ~ QS)
OH
[RuCI2(p-cymene)]2 (0.005 mmol) and (1 S,2S)-N-p-Tosyl-diphenylethylenediamine
(0.012 mmol) were suspended in 2 mL of water. The system was degassed (an
argon
flow was passed through the solution) and heated at 70 C for 1.5h. 6-Bromo-l-
indanone (1 mmol) and sodium forrnate (5 mmol) were added, the system was
degassed again and previously degassed THF (0.8 mL) was added. The reaction
mixture was stirred at 40 C under Ar atmosphere untif starting materiat cou4d
not be
detected by TLC (20h). The reaction mixture was cooled to room temperature and
extracted with EtOAc. The combined organic layers were washed with brine,
dried over
anhydrous MgSO4 and concentrated in vacuo. Crude was then filtered through a
pad of
Si02 using cyclohexane/EtOAc as eluent. The product was obtained as a white
solid.
Then, 6-bromo-indan-l-ol was recrystallised from cyclohexane to obtain pure
product
in 67% overall yield and 99.9% ee.
'H-NMR (400MHz, CDCI3): b ppm 7.3-7.2 (m, 2H), 7.14 (dd, 1H, J 8 and 2 Hz),
5.26 (t,
1 H, J 6 Hz), 3.09-3.01 (m, 1 H), 2.87-2.80 (m, 1 H), 2.62-2.54 (m, 1 H), 2.03-
1.96 (m 1 H).
HPLC. Chiralpack IA. Heptane:IPA 95:5, 0.5 mL/min, A = 220 nm, tR (S) = 18.8
min, tR
(R) = 22.7 min.
Example of compounds of general formula (R)-(III)
(R)-(6-Bromo-indan-l-yf)-dimethyl-amine
Br ~ Q(R)
N,
/
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
49
A solution of (S)-6-bromo-indan-l-ol (1 mmol) and NEt3 (4 mmol) in dry THF (5
mL)
was stirred at -15 C under argon. A solution of methanesulfonyl chloride (2
mmol) in
dry THF (1 mL) was cooled to -78 C and then added slowly to the alcohol
solution
maintaining the temperature below 0 C. The reaction mixture was stirred for 2h
at -15
C and then purged with dimethylamine gas (12 mmol), The reaction mixture was
allowed to warm to room temperature and stirred overnight. Then, it was
filtered to
remove salts and solvent was evaporated. Crude was used in next step without
further
purification.
'H-NMR (400MHz, CDCI3): b ppm 7.49 (s, 1 H), 7.32 (dd, 1 H, J 8 and 2 Hz),
7.07 (d,
1 H, J 8 Hz), 4.30 (t, 1 H, J 7 Hz), 2.91-2.83 (m, 1 H), 2.79-2.71 (m, 1 H),
2.25 (s, 6H),
2.09-2.03 (m 2H).
Example 2 (base compound)
(R)-Dimethyl-[6-(1,3,5-trimethyl-1 H-pyrazol-4-yl)-indan-l-yl]-amine
I
(R)
N
N N
(Method 1): To a solution of K2CO3 (1.9 mmol) and (R)-(6-bromo-indan-1-yi)-
dimethyl-
amine (1 mmol) in 1,2-dimethoxyethane (8.8 mL) / water (1.2 mL) was added
Pd(PPh3)4 (0.1 mmol) and 1,3,5-trimethyl-lH-pyrazole-4-boronic acid pinacol
ester (1.2
mmol). The reaction mixture was degassed and stirred at 85 C for 6h. The
solvent was
evaporated, crude was redissolved in dichloromethane and filtered through a
pad of
Celite. The filtrate was extracted with 6M HCI (3 x 0.16 mL). The aqueous
layer was
basified with 6M NaOH to pH 13 and extracted with dichloromethane. The
combined
organic extracts were dried over anhydrous Na2SO4, filtered and concentrated
in
vacuo. Crude was purified by flash chromatography (CH2CI2-MeOH-NEt3 as
eluents) to
yield (1 R)-dimethyl-[6-(1,3,5-trimethyl-1 H-pyrazol-4-yl)-indan-1-yl]-amine
as an oil (52%
yield). Product was obtained with a final ee of 99.6 % (determined by chiral
HPLC).
'H-NMR (400MHz, CDC13): b ppm 7.78 (s, 1 H), 7.53 (d, 1 H, J 8 Hz), 7.44 (d, 1
H, J 8
Hz), 5.10-5.15 (m, 1 H), 4.04 (s, 3H), 3.27-3.21 (m, 1 H), 3.14-3.05 (m, 1 H),
2.94 (s, 3H),
2.68 (s, 3H), 2.64-2.48 (m, 2H), 2.43 (s, 6H).
HPLC. Chiralpack AD-H. n-heptane:EtOH:DEA 98:2:0.1, 0.5 mUmin, A = 220 nm, tR
(R)
= 29 min, [a]p, RT= -72.17 (C=1, MeOH)
(Method 2, crystallization with L-di-p-toluiltartaric acid):
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
Step 1: (R)-Dimethyl-[6-(1,3,5-trimethyl-lH-pyrazol-4-yl)-indan-1-yl]-amine (-
)-di-
O,O'-p-toluyl-L-tartaric acid salt
O I /
H02Ci, 0
NN - H02C O
O
L-di-p-toluiltartaric acid (16.62 mmol) was dropwise added to a solution of
dimethyl-[6-
(1,3,5-trimethyl-1H-pyrazol-4-yl)-indan-1-yl]-amine (15.11 mmol) in refluxing
acetone
(30 mL). The reflux temperature was maintained for 30 min and then the
reaction was
allowed to reach 25 C very slowly (27 h). The mixture was cooled to 20 C and
stirred
for 1 h. Then, 15 mL of acetone were added and the suspension was stirred at
20 C for
1 h more. The salt formed was collected by filtration, washed with acetone
(3x10 mL}
and vacuum dried, to give a white solid (72.98% ee, determined by chiral
HPLC). The
obtained solid was suspended in acetone (130 mL) and EtOH (70 mL) and
submitted to
reflux until complete dissolution. Refluxing temperature was kept for 40 min
and then
the reaction was allowed to reach 20 C very slowly (16 h). The mixture was
cooled to
-10 C and stirred for 7.5 h. The obtained diastereomeric salt was filtered,
washed with
acetone (3x10 mL) and vacuum dried, to yield a white solid (98.48% ee,
determined by
chiral HPLC, 22% yield).
'H-RMN (250 MHz DMSO-d6) S ppm 7.80 (d, 4H, J 8.2 Hz), 7.32 (m, 7H), 5.65 (s,
2H),
4.80 (m, 1 H), 3.68 (s, 3H), 3.00-2.75 (m, 2H), 2.49 (s, 6H), 2.35 (s, 6H),
2.27 (m, 2H),
2.19 (s, 3H), 2.11 (s, 3H).
HPLC. ChiralPak IC. 2-PrOH:Hexane:DEA 5:95:0.1, 1 mUmin.
Step 2: (R)-Dimethyl-[6-(1,3,5-trimethyl-1 H-pyrazol-4-yi)-indan-1-yl]-amine
I
i
N,
/N
NaOH 10% (100 mL) was added to a suspension of (R)-dimethyl-[6-(1,3,5-
trimethyl-
1 H-pyrazol-4-yl)-indan-l-yl]-amine (-)-di-O,O'-p-toluyl-L-tartaric acid salt
(2.96 mmol) in
ethyl acetate (80 mL). The mixture was stirred at room temperature for 30
minutes. The
organic layer was washed with NaOH 10% (2x100 mL) and water (lxlOO mL) and
finally dried over anhydrous Na2SO4, filtered and concentrated in vacuo to
yield the
target compound (1 R)-dimethyl-[6-(1,3,5-trimethyl-1 H-pyrazol-4-yl)-indan-1-
yl]-amine
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
51
as an oil (97% yield). Product was obtained with a final ee of 98.48
%(determined by
chiral HPLC).
Example 2 (hydrochloride salt)
(R)-Dimethyl-[6-(1,3,5-trimethyl-1 H-pyrazof-4-yl)-indan-1-yl]-amine
hydrochloride
I
(R)
N N`
HCI
HCI (0.9 mmol, 2 M solution in Et20) was dropwise added to a solution of (R)-
dimethyl-
[6-(1,3,5-trimethyl-lH-pyrazol-4-yl)-indan-l-yl]-amine (1 mmol) in ethyl
acetate. The
reaction mixture was stirred at room temperature for 30 min and then the
solvent was
concentrated off. The resulting solid was suspended in acetone and the
precipitate
formed was collected by filtration, washed with acetone and vacuum dried,
giving the
title compound as a white solid (75%).
Example 2 (dihydrochloride salt)
(R)-Dimethyl-[6-(1,3,5-trimethyl-1 H-pyrazol-4-yl)-indan-l-yl]-amine
dihydrochloride
N~' (R)
N /N~
2HCI
HCI (2.1 mmol, 2 M solution in Et20) was dropwise added to a solution of (R)-
dimethyl-
[6-(1,3,5-trimethyl-lH-pyrazol-4-y!)-indan-l-yl]-amine (1 mmol) in ethyl
acetate. The
reaction mixture was stirred at room temperature for 30 min and then the
solvent was
concentrated off. The resulting solid was suspended in Et20 and concentrated,
in order
to remove excess of HCI. This operation was done for three times, to give the
title
product (white solid, 98% yield).
EXAMPLES 1 to 42 were or are prepared according or analogously to Example 1 or
using the Reaction Schemes 1 to 6 given above. Examples are depicted in the
following table.
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
52
MS
Example Structure Name 'H-NMR (APCI
(M+H)`)
1 Dimethyl-[6- 1 H NMR (300 MHz, 270
(1,3,5- METHANOL-d4) 6 ppm 2.29
N trimethyl-1 H- (s, 3 H) 2.33 (s, 3 H) 2.45 -
-N~ N pyrazol-4-yl)- 2.61 (m, 2 H) 2.68 (s, 3 H)
\ indan-1-yl]- 2.92 (s, 3 H) 3.07 - 3.16 (m, 1
amine H) 3.17 - 3.28 (m, 1 H) 4.00 (s,
3 H) 5.10 (d, J=4.69 Hz, 1 H)
7.42 (d, J=7.76 Hz, 1 H) 7.55
(d, J=7.62 Hz, 1 H) 7.72 (s, 1
H) (dihydrochloride product)
2 ~ (R) Dimethyl- 1H NMR (300 MHz, 270
[6-(1,3,5- METHANOL-d4) 6 ppm 2.29
N trimethyl-1 H- (s, 3 H) 2.33 (s, 3 H) 2.45 -
N N pyrazol-4-yl)- 2.61 (m, 2 H) 2.68 (s, 3 H)
\ indan-1-yl]- 2.92 (s, 3 H) 3.07 - 3.16 (m, 1
amine H) 3.17 - 3.28 (m, 1 H) 4.00 (s,
3 H) 5.10 (d, J=4.69 Hz, 1 H)
7.42 (d, J=7.76 Hz, 1 H) 7.55
(d, J=7.62 Hz, 1 H) 7.72 (s, 1
H) (hydrochloride product)
3 (S) Dimethyl- 1 H NMR (300 MHz, 270
[6-(1,3,5- METHANOL-d4) 6 ppm 2.29
N trimethyl-1 H- (s, 3 H) 2.33 (s, 3 H) 2.45 -
N~ pyrazol-4-yl)- 2.61 (m, 2 H) 2.68 (s, 3 H)
\ indan-1-yl]- 2.92 (s, 3 H) 3.07 - 3.16 (m, 1
amine H) 3.17 - 3.28 (m, 1 H) 4.00 (s,
3 H) 5.10 (d, J=4.69 Hz, 1 H)
7.42 (d, J=7.76 Hz, 1 H) 7.55
(d, J=7.62 Hz, 1 H) 7.72 (s, 1
H) (hydrochloride product)
4 ~ Methyl-[6- 1H NMR (400 MHz, 256
(1,3,5- CHLOROFORM-d) 6 ppm 1.94
N trimethyl-1 H- - 2.01 (m, 1 H) 2.24 (s, 6 H)
-NH N pyrazol-4-yl)- 2.39 - 2.50 (m, 1 H) 2.52 (s, 3
\ indan-1-yl]- H) 2.83 - 2.92 (m, 1 H) 3.02 -
amine 3.11 (m, 1 H) 3.77 (s, 3 H)
4.29 (t, J=6.45 Hz, 1 H) 7.11
(d, J=7.82 Hz, 1 H) 7.28 (m, 2
H)
~ 6-(1,3,5-
Trimethyl-1 ~ H-
pyrazol-4-yl)- 242
H2N NN indan-l-
\ ylamine
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
53
6 Diethyl-[6- 1H NMR (300 MHz, 298
(1,3,5- METHANOL-d4) 6 ppm 1.32 (t,
trimethyl-1 H- J=7.25 Hz, 3 H) 1.48 (t, J=7.25
pyrazol-4-yl)- Hz, 3 H) 2.38 (s, 3 H) 2.40 (s,
N indan-1-yl]- 3 H) 2.40 - 2.49 (m, 1 H) 2.50 -
amine 2.68 (m, 1 H) 2.96 - 3.25 (m, 5
H) 3.36 - 3.47 (m, 1 H) 3.98 (s,
3 H) 5.30 (dd, J=8.57, 4.47 Hz,
1 H) 7.41 (d, J=7.91 Hz, 1 H)
7.54 (d, J=7.76 Hz, 1 H) 7.73
(s, 1 H) (hydrochloride
product)
7 Dipropyl-[6- 1 H NMR (300 MHz, 326
(1,3,5- METHANOL-d4) b ppm 0.87 (t,
~ trimethyl-1 H- J=7.40 Hz, 6 H) 1.43 - 1.65
N pyrazol-4-yl)- (m, 4 H) 1.96 - 2.10 (m, 1 H)
indan-l-yl]- 2.16 (s, 3 H) 2.19 (m, 1 H)
amine 2.23 (s, 3 H) 2.32 - 2.53 (m, 4
H) 2.72 - 3.01 (m, 2 H) 3.76 (s,
3 H) 4.58 (t, J=7.47 Hz, 1 H)
7.06 (d, J=7.62 Hz, 1 H) 7.18 -
7.28 (m, 2 H)
8 [6-(3,5- 1 H NMR (300 MHz, 256
Dimethyl-1H- METHANOL-d4) 6 ppm 2.16 -
\ pyrazol-4-yl)- 2.22 (m, 2 H) 2.24 (s, 6 H)
-N~ NH indan-1-yl]- 2.34 (s, 6 H) 2.78 - 2.95 (m, 1
dimethyl- H) 2.95 - 3.11 (m, 1 H) 4.46 (t,
amine J=6.45 Hz, 1 H) 7.18 (dd,
J=7.76, 1.32 Hz, 1 H) 7.31 (m,
2 H) (dihydrochloride product)
9 (6-(3,5- IH NMR (300 MHz, 242
Dimethyl-1 H- METHANOL-d4) 6 ppm 2.17 -
N pyrazol-4-yl)- 2.36 (m, 1 H) 2.43 (s, 6 H)
~-NH NH indan-1-yl]- 2.65 (td, J=14.72, 7.91 Hz, 1
methyl-amine H) 2.78 (s, 3 H) 3.01 - 3.17 (m,
1 H) 3.18 - 3.27 (m, 1 H) 4.83
(m, 1 H) 7.42 (d, J=7.91 Hz, 1
H) 7.47 - 7.57 (m, 1 H) 7.65 (s,
1 H) (dihydrochloride product)
0 [6-(2,6- 1 H NMR (300 MHz, 298
Dimethoxy- METHANOL-d4) 6 ppm 2.39 -
phenyl)- 2.51 (m, 1 H) 2.55 (m, 1 H)
-N~ indan-1-yl]- 2.80 (s, 6 H) 3.07 (td, J=8.24,
0 dimethyl- 4.03 Hz, 1 H) 3.18 (t, J=8.20
amine Hz, 1 H) 3.71 (s, 6 H) 4.98 (dd,
J=8.13, 3.00 Hz, 1 H) 6.74 (m,
J=8.35 Hz, 2 H) 7.31 (m,
J=8.28 Hz, 2 H) 7.36 - 7.47
(m, 2 H) (hydrochloride
product)
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
54
11 0 [6-(2,6- 1 H NMR (300 MHz, 284
Dimethoxy- METHANOL-d4) b ppm 2.19 -
phenyl)- 2.32 (mõ 1 H) 2.64 (dt,
, PD
1 indan-1-yl]- J=14.54, 8.11 Hz, 1 H) 2.75 (s,
methyl-amine 3 H) 3.07 (dd, J=8.79, 4.69 Hz,
1 H) 3.23 (dd, J=16.63, 7.98
Hz, 1 H) 3.69 (s, 6 H) 4.75 (dd,
J=7.47, 3.66 Hz, 1 H) 6.73 (d,
J=8.35 Hz, 2 H) 7.28 (m, 2 H)
7.36 (t, J=8.42 Hz, 1 H) 7.42
(s, 1 H) (hydrochloride
product)
12 0 [6-(2- 1 H NMR (300 MHz,
Methoxy- METHANOL-d4) 6 ppm 2.50
phenyl)- (m, 1 H) 2.56 (m, 1 H) 2.75 (s,
--N~ indan-1-yl]- 3 H) 2.89 (s, 3 H) 3.00 - 3.13
dimethyl- (m, 1 H) 3.19 (t, J=8.20 Hz, 1
amine H) 3.81 (s, 3 H) 5.04 (dd, 268
J=7.98, 2.86 Hz, 1 H) 7.03 (m,
1 H) 7.09 (d, J=8.06 Hz, 1 H)
7.28 - 7.39 (m, 2 H) 7.44 (d,
J=7.76 Hz, 1 H) 7.56 (d,
J=1.46 Hz, 1 H) 7.70 (s, 1 H)
(hydrochloride product)
13 ~ ~o [6-(2- 1 H NMR (300 MHz, 254
Methoxy- METHANOL-d4) 6 ppm 2.18 -
~ phenyl)- 2.31 (m, 1 H) 2.56 - 2.69 (m, 1
~NH ~ 1-511 indan-1-yl]- H) 2.75 (s, 3 H) 3.05 (td,
methyl-amine J=8.17, 4.91 Hz, 1 H) 3.19 (m,
1 H) 3.79 (s, 3 H) 4.77 (dd,
J=7.69, 4.03 Hz, 1 H) 7.01 (td,
J=7.47, 1.03 Hz, 1 H) 7.08 (d,
J=7.76 Hz, 1 H) 7.30 (m, 2 H)
7.40 (d, J=7.91 Hz, 1 H) 7.51
(dd, J=7.91, 1.39 Hz, 1 H) 7.66
(s, 1 H) (hydrochloride
product)
14 [6-(2,6- 1H NMR (300 MHz, 266
Dimethyl- METHANOL-d4) 6 ppm 1.89
phenyl)- (s, 3 H) 1.93 (s, 3 H) 2.32 -
~N~ indan-1-yl]- 2.56 (m, 2 H) 2.60 (br. s., 3 H)
dimethyl- 2.79 (br. s., 3 H) 3.03 (dd,
amine J=8.94, 3.37 Hz, 1 H) 3.13 (d,
J=8.50 Hz, 1 H) 4.96 (d,
J=5.86 Hz, 1 H) 6.96 - 7.05
(m, 3 H) 7.12 (d, J=7.76 Hz, 1
H) 7.27 (s, 1 H) 7.41 (d,
J=7.62 Hz, 1 H)
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
1 H NMR (300 MHz,
15 9~' [6-(2,6- METHANOL-d4) b ppm 1.99 252
Dimethyl-
phenyl)- (s, 3 H) 2.02 (s, 3 H) 2.17 -
2.35 (m, 1 H) 2.54 - 2.70 (m, 1
~NH indan-1-yl]- H) 2.74 (s, 3 H) 3.00 - 3.16 (m,
methyl-amine 1 H) 3.23 (m, 1 H) 4.80 (m, 1
H) 7.02 - 7.14 (m, 3 H) 7.18 (d,
J=7.47 Hz, 1 H) 7.31 (s, 1 H)
7.48 (d, J=7.76 Hz, 1 H)
16 ci [6-(2,6-
~ Dichloro-
` pheny!)- 306
~ i
~N indan-1-yl]-
C~ C~ dimethyl-
amine
17 c 1 [6-(2,6-
~ Dichioro-
pheny()- 292
-- NH indan-1-yl]-
CI methyl-amine
18 ~ F [6-(2,6- 274
Difluoro-
~ phenyl)-
~ i indan-1-yl]-
F dimethyl-
amine
19 F [6-(2,6- 259
Difluoro-
~ phenyl)-
NH ~ indan-1-yl]-
F methyl-amine
20 0 [6-(2-Chloro- 1 H NMR (300 MHz,
"" 6-methoxy- METHANOL-d4) b ppm 2.39 -
N phenyl)- 2.54 (m, 1 H) 2.57-2.67 ( m, 1
--~ ,~ indan-1-yl]- H) 2.75 (s, 3 H) 2.87 (s, 3 H)
~~ dimethyl- 3.01 - 3.15 (m, 1 H) 3.18-3.26
amine (m, 1 H) 3.72 (s, 3 H) 5.03 (dd, 302
J=8.06, 3.08 Hz, 1 H) 7.04 (d,
J=8.50 Hz, I H) 7.11 (d,
J=8.06 Hz, 1 H) 7.26 - 7.39
(m, 2 H) 7.45 (m, 2 H)
21 N"O [6-(2-Chloro- 1 H NMR (300 MHz,
6-methoxy- METHANOL-d4) b ppm 2.19 -
phenyl)- 2.36 (m, 1 H) 2.57 - 2.71 (m, 1
~NH ,~ indan-1-yl]- H) 2.74 (s, 3 H) 3.00 - 3.14 (m, 288
CI methyl-amine 1 H) 3.22 (m, 1 H) 3.70 (s, 3
H) 4.79 (m, I H) 7.03 (d,
J=8.35 Hz, 1 H 7.10 d,
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
56
J=8.06 Hz, 1 H) 7.26 (d,
J=7.76 Hz, 1 H) 7.33 (t, J=8.20
Hz, 1 H) 7.41 (br. s., 1 H) 7.44
(d, J=8.06 Hz, 1 H)
22 CF3 3 [6-(2,6-Bis-
trifluoromethyl
-phenyl)- 374
-N~ F3~ indan-1-yl]-
dimethyl-
amine
23 CF3 3 trifluoromethyl
-phenyl)- 360
--NH indan-1-yl]-
F3C methyl-amine
24 D::C' [6-(3,5- 257
Dimethyl-
isoxazol-4-yl)-
-N ~N indan-1-ylJ-
dimethyl-
amine
25 [6-(3,5- 243
~ Dimethyl-
N isoxazol-4-yi)-
,NH 0 / indan-1-yl]-
methyl-amine
26 [6-(1-Ethyl-
~ 3,5-dimethyl-
\ N 1 H-pyrazol-4- 284
-N N yl)-indan-1-
yl]-dimethyl-
amine
27 [6-(1-Ethyl-
~ 3,5-dimethyl-
N 1 H-pyrazol-4- 270
-NH N I yl)-indan-l-
~ yl]-methyl-
amine
28 [6-(3, 5-
~ Dimethyl-l-
propyl-1 H-
NN pyrazol-4-yl)- 298
indan-1-yl]-
dimethyl-
amine
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
57
29 6-(3, 5-
~ Dimethyl-l-
N propyl-1 H-
.NH N pyrazol-4-yl)- 284
indan-1-yl]-
methyl-amine
30 [6-(1-
~ Isopropyl-3,5-
N dimethyl-1 H-
-N~ N pyrazol-4-yl)- 297
indan-1-yl]-
dimethyl-
amine
31 [6-(1-
~ Isopropyl-3,5-
\ dimethyl-1 H-
, NH NN pyrazol-4-yl)- 284
indan-1-yl]-
methyl-amine
32 [6-(1-Isobutyl-
~ 3,5-dimethyl-
1 H-pysazal-4-
N yl
)-indan-l- 312
yl]-dimethyl-
amine
33 [6-(1-Isobutyl-
~ 3,5-dimethyl-
1 H-pyrazol-4-
,NH NN yl)-indan-l- 298
yl]-methyl-
amine
34 [6-(1-
~ Methoxymeth
N yl-3, 5-
-N dimethyl-1 H- 300
pyrazol-4-yl)-
0 indan-1-yl]-
dimethyl-
amine
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
58
35 [6-(1-
~ Methoxymeth
yl-3, 5-
--NH NN dimethyl-1 H- 286
pyrazol-4-yl)-
0 indan-1-ylj-
\ methyl-amine
Trimethyl-[6- 1 H NMR (300 MHz,
METHANOL-d4) b ppm 2.19
(1,3,5- (s, 3 H) 2.26 (s, 3 H) 2.50 -
36 trimethyl-1 H- 2.67 (m, 1 H) 2.73-2.81 (m, 1
_N + indan-lpyrazol--4y-l]-yl)- H) 2.99 - 3.18 (m, 1 H) 3.13 (s, 284
I\ N ammonium 9 H) 3.24 (m, 1 H) 3.77 (s, 3
iodide H) 5.04 (d, J=7.91 Hz, 1 H)
7.40 (m, 1 H) 7.52 (m, 2 H)
1 H NMR (400 MHz,
METHANOL-d4) ^ ppm 2.24 -
(S) Methyl-[6- 2.37 (m, 1 H) 2.40 (s, 6 H)
~ (1,3,5- 2.56 - 2.71 (m, 1 H) 2.77 (s, 3
37 trimethyl-1 H- H) 3.02 - 3.12 (m, 1 H) 3.24 256
,NH ~ N pyrazol-4-yl)- (m, J=8.16 Hz, 1 H) 4.02 (s, 3
N \ indan-l-yl]- H) 4.85 (m, 1 H) 7.39 (d,
amine J=6.87 Hz, 1 H) 7.53 (d,
J=7.73 Hz, 1 H) 7.67 (s, 1 H)
(dihydrochloride product)
1 H NMR (400 MHz,
METHANOL-d4) ^ ppm 2.24 -
C)C (R) Methyl-[6- 2.37 (m, 1 H) 2.40 (s, 6 H)
38 (1,3,5- 2.56 - 2.71 (m, 1 H) 2.77 (s, 3
~R~ N trimethyl-1 H- H) 3.02 - 3.12 (m, I H) 3.24 256
--NH N pyrazol-4-yl)- (m, J=8.16 Hz, 1 H) 4.02 (s, 3
indan-1-yll- H) 4.85 (m, 1 H) 7.39 (d,
amine J=6.87 Hz, 1 H) 7.53 (d,
J=7.73 Hz, 1 H) 7.67 (s, 1 H)
dih drochloride product)
O [6-(2-
~ Methoxy-
39 I N pyridin-3-yl)- 269
~N~ indan-1-yi]-
dimethyl-
amine
~ ~~ [6-(2-
40 Methoxy-
~ ~ N pyridin-3-yl)- 255
-NH ~ , indan-1-yl]-
methyl-amine
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
59
Dimethyl-(6-
41 piperidin-1-yl-
N N indan-1-yl)- 310
HCI N amine;
hydrochloride
Dimethyl-(6-
42 N pyrrolidin-1-yl- 296
~N_ N indan-1-yl)-
I~/~ \ amine
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
Pharmacological Data:
Results for representative compounds/examples are given in the table below:
COMPOUND/ 5-HT7 5-HT7
EXAMPLE IC50 Ki
(nM) (nM)
1 15.5 t 2.9
2 5.5 t 2.7
3 307 t 181
4 18.7
10 53.5 t 10.1
11 195 t 86
12 68.4 t 1.1
13 77.7 t 40.9
14 197.7 t 31.9
15 75.2t2
19 47.6 t 1.2
20 25.8 t 2.7
21 55.7 t 1.2
36 152.9 t 32.4
38 73.3 9.3
Formulation Example
Example of a tablet formulation:
Compound according to example 1 5 mg
Lactose 60 mg
Crystalline cellulose 25 mg
Povidone K 90 5 mg
Pregelanitized starch 3 mg
Colloidal silica dioxide 1 mg
Magnesium stearate 1 mg
Total weight per tablet 100 mg
The above mentioned ingredients were mixed and compressed into a tablet by
conventional
methods known to those skilled in the art.
X-Ray-Analysis of the crystal structure of Example I
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
61
The crystal structure of Example 1 was analysed under X-Ray and is shown in
Figures 1 and
2.
Experimental
Crystal structure determination was carried out using a Bruker-Nonius
diffractometer
equipped with a APPEX 2 4K CCD area detector, a FR591 rotating anode with MoK
radiation, Montel mirrors as monochromator and a Kryoflex low temperature
device (T = 100
K). Fulisphere data collection omega and phi scans. Programs used: Data
collection Apex2
V. 1.0-22 (Bruker-Nonius 2004), data reduction Saint + Version 6.22 (Bruker-
Nonius 2001)
and absorption correction SADABS V. 2.10 (2003). Crystal structure solution
was achieved
using direct methods as implemented in SHELXTL Version 6.10 (Sheldrick,
Universtitat
Gottingen (Germany), 2000) and visualized using XP program. Missing atoms were
subsequently located from difference Fourier synthesis and added to the atom
list. Least-
squares refinement on F2 using all measured intensities was carried out using
the program
SHELXTL Version 6.10 (Sheldrick, Universtitat Gottingen (Germany), 2000). All
non
hydrogen atoms were refined including anisotropic displacement parameters.
Tables
Table 1. Crystal data and structure refinement for p012150m.
Identification code 01215 Om
Empirical formula C34 H50 C14 N6
Formula weight 684.60
Temperature 100(2) K
Wavelength 0.71073 A
Crystal system Monoclinic
Space group P21
Unit cell dimensions a = 6.0395(2) A a= 90 .
b = 15.0539(4) A b= 102.534 2 .
c=10.57643 A =90 .
Volume 938.67(5) A3
z 1
Density (calculated) 1.211 M /m3
Absorption coefficient 0.346 mm-1
F 000 364
C stalsize 0.10x0.10x0.10mm3
Theta range for data collection 3.35 to 36.38 .
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
62
Index ranges -95h:54, -235ks25, -14s1:514
Reflections collected 7260
Independent reflections 4838 R int = 0.0323
Completeness to theta = 36.38 83.9 %
Absorption correction None
Max. and min. transmission 0.9662 and 0.9662
Refinement method Full-matrix least-squares on F2
Data / restraints / parameters 4838 / 1/ 204
Goodness-of-fit on F2 1.053
Final R indices 4>2si ma I R1 = 0.0386, wR2 = 0.0958
R indices (all data) R1 = 0.0420, wR2 = 0.0992
Absolute structure parameter -0.04 4
Largest diff. peak and hole 0.688 and -0.341 e.A-3
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
63
Table 2. Bond lengths [A] and angles [ ] for p01215_0m.
N 1-C(2) 1.341(2) N 3-C 10 1.5147 18
N 1-N2 1.3492(16) C(7)-C(8) 1.3982
N 1-C 5 1.455(2) C 7-C 15 1.4049 19
C 1-C 3 1.3989 19 C 8-C 9 1.389(2)
C 1-C 2 1.4014 19 C 9-C 13 1.3977 18
C 1-C 7 1,474(2) C 9-C 10 1.508(2)
N 2-C 3 1.333(2) C 10 -C 11 1.546(2)
C 2-C 4 1.4837 18 C(11 1 -C 12 1.547(2)
C 3-C 6 1.4895 19 C 12 -C 13 1.501(2)
N 3-C 16 1.4877 19 C 13 -C 14 1.393(2)
N 3-C 17 1.490(2) C 14 -C 15 1.392(2)
C 2-N 1-N 2 109.27 12 C 8-C 7-C 1 120.71 11
C 2-N 1-C 5 130.14 12 C 15 -C 7-C 1 120.46 13
N2-N1-C5 120.37(14) C(9)-C(8)-C(7) 119.43(12)
C 3-C 1-C 2 105.90 13 C 8-C 9-C 13 121.46 13
C 3-C 1-C 7 126.84 12 C 8-C 9-C 10 128.00 12
C 2-C 1-C 7 127.26 12 C 13 -C 9-C 10 110.53 13
C3-N2-N1 109.40(12) C9-C10-N3 111.16(12)
N 1-C 2-C 1 107.51 11 C 9-C 10 -C 11 103.93 11
N 1-C(2 -C 4 121.35 13 N 3-C 10 -C 11 113.86 12
C 1-C 2-C 4 131.11 14 C 10 -C 11 -C 12 106.47 13
N 2-C 3-C 1 107.90 12 C 13 -C 12 -C 11 103.43 12
N(2)-C(3)-C(6) 120.17(14) C14-C13-C9 119.58(14)
C 1-C 3-C 6 131.84(15) C 14 -C 13 -C 12 128.90 13
C 16 -N 3-C 17 111.11 12 C 9-C 13 -C 12 111.52 13
C 16 -N 3-C 10 113.89 11 C 15 -C 14 -C 13 119.03 12
C 17 -N 3-C 10 111.82 13 C 14 -C 15 -C 7 121.68 13
C 8-C 7-C 15 118.83(13
CA 02692513 2010-01-04
WO 2009/003719 PCT/EP2008/005500
64
Table 3. Torsion angles [ ] for p01215_0m.
C2-N 1 -N2-C3 -1.29(18) C7-C8-C9-C 10 -177.70(13)
C 5-N 1-N 2-C 3 -176.32 15 C 8-C 9-C 10 -N 3 -69.32 18
N 2-N 1-C 2-C 1 1.44 17 C 13 -C 9-C 10 -N 3 112.00 13
C 5-N 1-C 2-C 1 175.84 17 C 8-C 9-C 10 -C 11 167.80 14
N 2-N 1-C 2-C 4 -176.87 14 C 13 -C 9-C 10 -C 11 -10.88 15
C5-N1-C2-C4 -2.5(3) C16-N3-C10-C9 -60.72(16)
C(3)C(1)C(2)N(1) -1.06 17 C 17 -N 3-C 10 -C 9 172.29 11
C 7-C 1-C 2-N 1 178.62 14 C 16 -N 3-C 10 -C 11 56.24 18
C 3-C 1-C 2-C 4 177.03 15 C 17 -N 3-C 10 -C 11 -70.75 15
C 7-C 1-C 2-C 4 -3.3 3 C 9-C 10 -C 11 -C 12 18.96 15
N 1-N 2-C 3-C 1 0.59 19 N 3-C 10 -C 11 -C 12 -102.12 14
N 1-N 2-C 3-C 6 177.48 14 C 10 -C 11 -C 12 -C 13 -19.90 15
C 2-C 1-C 3-N 2) 0.29 18 C 8-C 9-C 13 -C 14 -0.3 2
C 7-C 1-C 3-N 2 -179.38 13 C 10 -C 9-C 13 -C 14 178.45 13
C2-C1-C3-C6 -176.11(17) C8-C9-C13-C12 179.33(14)
C7-C1-C3-C6 4.2(3) C10-C9-C13-C12 -1.89(17)
C 3-C 1-C 7-C 8 47.3(2) C 11 -C 12 -C 13 -C 14 -166.61 15
C 2-C 1-C 7-C 8 -132.35 15 C 11 -C 12 -C 13 -C 9 13.78 16
C 3-C 1-C 7-C 15 -133.25 16 C 9-C 13 -C 14 -C 15 -0.4 2
C 2-C 1-C 7-C 15 47.1(2) C 12 -C 13 -C 14 -C 15 -179.97 15
C 15-C7-C8-C9 -0.7(2) C 13-C 14-C 15-C7 0.6(2)
C1-C7-C8-C9 178.84(13) C8-C7-C15-C14 0.0(2)
C7-C8-C9-C13 0.9(2) C1-C7-C15-C14 -179.5414