Note: Descriptions are shown in the official language in which they were submitted.
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
DEVICES, SYSTEMS AND METHODS FOR TREATING TISSUES
FIELD OF THE TECHNOLOGY
[0001] Embodiments of the technology disclosed herein relate generally to
devices, systems
and methods for treating tissues. More particularly, certain embodiments
disclosed herein
relate to devices, systems and methods for treating tissues infected with one
or more
organisms.
BACKGROUND
[0002] Infectious diseases of tissues such as, for example, keratinized
tissues are a difficult
problem for medical treatment. Keratins are a class of scleroprotein that
serve as the major
protein components of hair, wool, nails, the organic matrix of the enamel of
teeth, horns,
hoofs, and the quills of feathers. These proteins generally contain large
quantities of the
sulfur-containing amino acids, particularly cysteine. Keratins provide a
tough, fibrous matrix
for the tissues in which they are found. These proteins are characterized as
being extremely
water insoluble. Because keratins contain few polar amino acids, there is
little or no moisture
content in the tissues they form. This presents difficulties for the medical
treatment of
infected keratinized tissues because medicaments are not easily delivered into
this type of
tissue.
[0003] There remains a need for better devices and methods to treat tissues
infected with a
pathogen.
SUMMARY
[0004] In accordance with a first aspect, a system constructed and arranged to
treat a
mammalian tissue infected with an organism is provided. In certain examples,
the system
comprises an electromagnetic energy source, an applicator operatively coupled
to the
electromagnetic energy source and configured to deliver electromagnetic energy
to the
mammalian tissue, and a controller operatively coupled to the electromagnetic
energy source
and configured to determine a treatment dose of the mammalian tissue and to
provide for
delivery of the determined treatment dose of the electromagnetic energy to the
mammalian
tissue.
[0005] In certain examples, the system may further comprise a temperature
sensor
operatively coupled to the controller and configured to detect a treatment
temperature. In
other examples, the applicator may comprise an adaptor constructed and
arranged to be
- I -
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
placed in proximity to the tissue to deliver the electromagnetic energy. In
some examples,
the adaptor may be constructed and arranged to conform to a digit surface. In
yet other
examples, the digit surface may be a nail or nail bed. In certain examples,
the applicator
comprises a tissue interface configured to receive a bolus. In other examples,
the tissue
interface may be configured to provide impedance matching of the mammalian
tissue and the
applicator. In some examples, the applicator comprises a flexible substrate
configured for a
single use. In certain examples, the controller may be configured to provide
pulses of the
determined treatment dose. In some examples, the controller may be configured
to provide
the determined treatment dose to provide continuous heating of the tissue
until the
mammalian tissue reaches a treatment temperature. In other examples, the
controller may be
configured to halt delivery of the determined treatment dose once the
mammalian tissue
reaches the treatment temperature. In certain examples, the controller may be
configured to
continue delivery of the determined treatment dose once the mammalian tissue
drops below
the treatment temperature. In some examples, the controller may be configured
to deliver the
determined treatment dose for a selected time. In certain examples, the
adaptor may be
constructed and arranged to smooth the distribution of energy. In other
examples, the adaptor
may be constructed and arranged to treat at least two nails simultaneously.
[0006] In accordance with another aspect, a method of treating a tissue of a
mammal infected
with an organism is disclosed. In certain examples, the method comprises a
first step
comprising determining a treatment dose of electromagnetic energy that a
mammal can
tolerate, and a second step comprising exposing the tissue to the determined
treatment dose
for a treatment time.
[0007] In some examples, the method may further comprise a third step
comprising halting
exposure of the tissue to the determined treatment dose once the tissue
reaches a first
temperature. In other examples, the method may further comprise a fourth step
comprising
continuing exposure of the tissue to the determined treatment dose once tissue
temperature
drops below the first temperature. In some examples, the steps of halting and
continuing are
repeated for the treatment time. In certain examples, the method may further
comprise
obtaining a culture of an organism infecting the tissue to assess efficacy of
treatment. In
some examples, the method may comprise assessing efficacy of treatment in less
than one
month or two weeks following the treatment. In some examples, the method may
comprise
exposing the tissue to one or more power levels of electromagnetic energy to
determine the
rate of heating to the first temperature.
-2-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
[0008] In certain examples, the method may comprise removing an onycholytic
portion of a
nail before treatment. In some examples, the method may further comprise
placing a
biocompatible material over treated tissue to block access of infectious
agents after treatment.
In other examples, the biocompatible material may be toxic to infectious
agents. In certain
examples, the method may comprise delivering a drug to the infected tissue
with the
electromagnetic energy provided to the tissue by iontophoresis. In other
examples, the
method may further comprise delivering a drug to the infected tissue with the
electromagnetic
energy provided to the tissue by dielectrophoresis. In certain examples, the
method may
comprise exposing the tissue to the determined treatment dose for the
treatment time from
about five minutes to about thirty minutes. In some examples, the method may
comprise
increasing the first temperature during treatment based on a new tolerance
level of the
mammal. In other examples, the method may further comprise increasing
temperature of the
first temperature by inducing reactive hyperemia in the tissue. In certain
examples, the
method may further comprise increasing temperature of the first temperature by
exposing the
tissue to a coolant blown or sprayed on or encompassing the tissue. In some
examples, the
method may further comprise increasing temperature of the first temperature by
exposing the
tissue to a vibrating motion. In other examples, the determined treatment dose
may also be
effective to increase a nail growth rate.
[0009] In accordance with an additional aspect, a kit for treating an infected
tissue is
provided. In certain examples, the kit comprises an adaptor constructed and
arranged to be
coupled to an electromagnetic energy source and to deliver electromagnetic
energy to an
infected tissue. In some examples, the kit may also comprise a bolus
configured to focus the
electromagnetic energy to the infected tissue. In other examples, the kit may
further
comprise instructions for using the adaptor and the bolus to treat the
infected tissue.
[0010] In certain examples, the adaptor may further comprise a tissue
interface configured to
receive the bolus. In some examples, the adaptor may be constructed and
arranged to treat a
nail. In other examples, the adaptor may be constructed and arranged to treat
a hoof. In
certain examples, the bolus may be configured to provide impedance matching of
the infected
tissue and the adaptor.
[0011] In accordance with another aspect, a system constructed and airanged to
treat a digit
surface tissue infected with an organism is disclosed. In certain examples,
the system
comprises an electromagnetic energy source, an applicator operatively coupled
to the
electromagnetic energy source and configured to deliver electromagnetic energy
to the digit
surface. In some examples, the applicator may comprise a tissue interface
configured to
-3-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
receive a bolus, and an adaptor coupled to the applicator and constructed and
arranged to
conform to the digit surface. In other examples, the system may also comprise
a controller
operatively coupled to the electromagnetic energy source and configured to
provide for
delivery of a determined treatment dose of the electromagnetic energy to the
digit surface.
[0012] In certain examples, the adaptor may be constructed and arranged to
conform to a nail.
In some examples, the adaptor may be constructed and arranged to conform to a
hoof. In
other examples, the tissue interface may be configured in combination with the
bolus to
smooth the distribution of the electromagnetic energy provided to the digit
surface. In certain
examples, the system may further comprise a temperature sensor operatively
coupled to the
digit surface and configured to detect a treatment temperature.
[0013] In accordance with an additional aspect, a system for treating a
mammalian nail or
hoof infected with an organism is disclosed. In certain examples, the system
comprises an
applicator constructed and arranged to deliver electromagnetic energy to a
nail or a hoof, and
a housing sized and arranged to receive a hand, a foot or a hoof of a mammal.
In some
examples, the housing comprises an electromagnetic energy source operatively
coupled to the
applicator, and a controller operatively coupled to the electromagnetic energy
source and
configured to determine a treatment dose of the nail or hoof and configured to
provide for
delivery of the determined treatment dose of electromagnetic energy to the
nail or the hoof.
[0014] In certain examples, the applicator may comprise a plurality of
adaptors to treat at
least two adjacent digit surfaces on the hand, foot or hoof. In some examples,
at least one
adaptor of the plurality of adaptors may comprise a tissue interface
configured to receive a
bolus. In other examples, the tissue interface in combination with the bolus
may be
configured to provide impedance matching of the mammalian tissue and the
applicator.
[0015] Additional aspects and features of the technology, and uses of such
additional aspects
and features, are disclosed in more detail herein.
BRIEF DESCRIPTION OF THE FIGURES
[0016] Certain examples are described in detail below with reference to the
accompanying
figures in which:
[0017] FIG. 1 is block diagram of a device for treating tissue, in accordance
with certain
examples;
[0018] FIG. 2 is a schematic of an applicator including an adaptor, in
accordance with certain
examples;
-4-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
[0019] FIG. 3 is a schematic of a device for treating tissue, in accordance
with certain
examples;
[0020] FIG. 4 is an example of a spacer in contact with a tissue, in
accordance with certain
examples;
[0021] FIG. 5 is an example of an adaptor including a container for a bolus,
in accordance
with certain examples;
[0022] FIG. 6 shows two energy profile graphs of an applicator without a bolus
(top panel)
and with a bolus (bottom panel), in accordance with certain examples
[0023] FIG. 7 is an example of a system for treating a tissue, in accordance
with certain
examples;
[0024] FIG. 8 is an example of a computer system suitable for use with the
devices, systems
and methods disclosed herein, in accordance with certain examples;
[0025] FIG. 9 is an example of a storage system, in accordance with certain
examples;
[0026] FIG. 10 is a flow-chart of a protocol for treating a tissue, in
accordance with certain
examples;
[0027] FIG. 11 is a flow-chart of a protocol for treating a tissue, in
accordance with certain
examples;
[0028] FIG. 12 is an energy versus time graph showing treatment times and
delay times, in
accordance with certain examples;
[0029] FIG. 13 is a flow chart showing a calibration protocol, in accordance
with certain
examples;
[0030] FIGS. 14A and 14B show a housing enclosing a system suitable for
delivering
electromagnetic energy to a foot, in accordance with certain examples;
[0031] FIG. 15 shows a patient seated on a table with a foot resting on the
housing shown in
FIGS. 14A and 14B, in accordance with certain examples;
[0032] FIG. 16 is a block diagram of a device for treating an infected nail,
in accordance with
certain examples;
[0033] FIG. 17 is a block diagram of an applicator energetically coupled to
two
electromagnetic energy sources, in accordance with certain examples;
[0034] FIG. 18 is an insert configured to receive a tissue, in accordance with
certain
examples;
[0035] FIG. 19 is an insert configured to receive a tissue and disposed on a
platform, in
accordance with certain examples;
-5-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
[0036] FIGS. 20A and 20B show two embodiments of disposing one or more agents
on a
tissue, in accordance with certain examples;
[0037] FIGS. 21A-25B show embodiments of disposing an applicator on a tissue,
in
accordance with certain examples;
[0038] FIG. 26A is a schematic of an adaptor comprising a flex circuit, in
accordance with
certain examples.
[0039] FIGS. 26B and 27 show an adaptor in contact with a toe, in accordance
with certain
examples;
[0040] FIGS. 28A-28C show various embodiments of a single-use adaptor, in
accordance
with certain examples;
[0041] FIG. 29 shows an illustrative device for performing iontophoresis or
electrokinetic
delivery of a substance, in accordance with certain examples;
[0042] FIG. 30 shows a device configured for delivery of electromagnetic
energy and for
iontophoresis or electrokinetic delivery of a substance, in accordance with
certain examples;
[0043] FIG. 31 is a flow chart of an illustrative calibration protocol, in
accordance with
certain examples;
[0044] FIG. 32 is a flow chart of an illustrative treatment protocol, in
accordance with certain
examples;
[0045] FIGS. 33A-33F are photographs showing the large toe nail at various
intervals after
treatment, in accordance with certain examples;
[0046] FIG. 34 is a temperature profile graph during treatment of a toe nail
for a fungal
infection, in accordance with certain examples;
[0047] FIG. 35 is another temperature profile graph during treatment of a toe
nail for a fungal
infection, in accordance with certain examples;
[0048] FIG. 36 is a flow chart of another illustrative calibration protocol,
in accordance with
certain examples; and
[0049] FIGS. 37 and 38 are flow charts of another illustrative treatment
protocol, in
accordance with certain examples.
[0050] It will be recognized by the person of ordinary skill in the art, given
the benefit of this
disclosure, that certain dimensions, element or features in the figures may
have been enlarged,
minimized, distorted, shown not to scale or otherwise shown in a non-
conventional manner to
provide a better understanding of the technology disclosed herein.
-6-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
DETAILED DESCRIPTION
[0051 ] Certain illustrative embodiments and examples are described in more
detail below to
illustrate further some of the many configurations and applications of the
technology
disclosed herein.
[0052] In certain examples, the apparatus disclosed herein may be configured
to deliver
electromagnetic energy to a tissue for treatment of a particular disease or
disorder affecting
the tissue. In some examples, embodiments of the devices, systems and methods
disclosed
herein may be used to treat diseased tissue using electromagnetic energy or
radiation.
Treatment provides for improvement of symptoms and/or appearance by
deactivating or
killing of the organism or organisms infecting the tissue. For example, the
organism may be
thermally deactivated by delivering electromagnetic energy to a target area,
which can be
adjacent to or near the organism or may include the organism. Tissue
surrounding the
organism itself may also absorb energy or radiation and transfer thermal
energy to the
organism to deactivate the organism, and/or the organism can absorb directly
the energy or
radiation. Deactivation of the organism can render it unable to grow,
reproduce and/or
replicate. Deactivation can result from thermal destruction of the organism,
from denaturing
or partially denaturing one or more molecules forming the organism, from
initiating a
photobiological or photochemical reaction that attacks the organism, and/or
from inducing an
immune response that attacks the organism. In some examples, the
electromagnetic radiation
may result in killing of the organism.
[0053] In accordance with certain examples, the devices, systems and methods
disclosed
herein may be used to provide a determined treatment dose of electromagnetic
energy, a
deactivating dose of electromagnetic energy or a kill dose of electromagnetic
energy. A
"dose," as used herein is defined by a combination of the treatment time and
average
temperature that is maintained on a surface of the target tissue during the
treatment. A
"therapeutic dose" is defined as a dose required for killing or disabling the
pathogen cells.
As used herein, "detennined treatment dose" of electromagnetic energy refers
to the case
where the treatment dose is based on certain factors including, for example,
subjective inputs
based on subject responses. The treatment dose may be variable from subject to
subject and
may generally be determined by incrementally increasing the electromagnetic
energy level
until the subject becomes uncomfortable. In the case of humans, the subject
may verbally or
physically express a sensation of pain or heat. In the case of non-human
mammals, the
subject may attempt to remove or withdraw the area being exposed from the
electromagnetic
energy source. It is believed that by providing a determined treatment dose of
-7-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
electromagnetic energy, treatment may be more efficacious and may take less
time. Once the
determined treatment dose is identified, such dose may be programmed into a
controller to
effectuate treatment using the devices, systems and methods disclosed herein.
[0054] In certain examples herein that use a treatment dose, treatment may be
performed for
a fixed time or a variable time based on temperature measurements. For
example, the target
tissue temperature may be monitored to determine the average treatment
temperature. In
certain instances, an average tissue temperature of about 45-55 C at a
treatment time of at
least 2-3 minutes provides a therapeutic dose. After initiation of treatment,
the tissue
temperature will increase up to a patient's tolerance level (referred to in
certain instanced
herein as a threshold temperature), based on subjective user inputs taking
into account a
patient's pain threshold, or up to a default safety maximum temperature, e.g.,
53 C, and the
treatment will then be halted. Treatment may be reinitiated once the tissue
temperature falls
below a certain value or once a defined period has passed. As the treatment
proceeds,
generally the patient may acclimate to the threshold temperature and will be
able to tolerate a
greater (higher) temperature. In tliis case, the threshold temperature may be
increased and
effectively the patient can control the temperature to maintain the
temperature along the
boundary of their pain threshold. Such a treatment process can provide a very
effective
therapeutic dose to treat the tissue. The increase in temperature during
acclimation may be
accomplished by treatment for a longer period, increasing the intensity of the
electromagnetic
energy applied to the tissue, focusing the electromagnetic energy or other
suitable methods.
[0055] In some examples discussed herein, treatment may be provided at a
determined
treatment dose until the subject becomes uncomfortable or until the tissue
reaches a selected
threshold temperature, referred to in some instances herein as a "treatment
temperature."
Treatment may be discontinued to permit the tissue temperature to fall below
the treatment
temperature, and then may be re-initiated for second treatment period at the
maximum dose
until the tissue temperature again rises to the treatment temperature. This
process may be
repeated iteratively until a desired treatment time is reached.
[0056] In some examples, the treatment temperature may be variable during the
treatment.
For example, during the course of administering the determined treatment dose,
the subject
may be able to tolerate a higher temperature due to, for example,
desensitization of the area,
increased blood flow and the like. In this situation, the treatment
temperature may be
increased such that more effective treatment may be effectuated. In other
examples, the
subject may not be able to tolerate the treatment temperature as treatment
progresses, and the
treatment temperature may be reduced prior to continuing further treatment.
The exact
-8-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
treatment time may vary depending on the selected type of electromagnetic
energy, and
illustrative treatment times are discussed herein.
[0057] As used herein, "deactivating dose" refers to the amount of
electromagnetic energy
that can deactivate 80-99%, more particularly 95-99% or more of the organisms
present in an
infected tissue. Deactivation results in the organism being unable to
replicate or survive but
does not instantly kill the organism. As used herein "kill dose" refers to the
amount of
electromagnetic energy that can kill at least about 95% of the organisms
present in an
infected tissue. In contrast to deactivation, killing of the organism is
substantially
instantaneous and may be caused by superheating and exploding of the organism,
leakage of
ions or water into the cell or rupture of the cell membrane and/or cell wall.
[0058] As used herein, the term "electromagnetic energy" is used broadly and
is intended to
include gamma rays (wavelength less than about 10-9 cm), X-rays (wavelength
from about 10-
7 cm to about 10-9 cm), ultraviolet light (wavelength of about 4x10-5 cm to
about 10-7 cm),
visible light (wavelength of about 7x10-5 cm to about 4x10-5 cm), infrared
light (wavelength
of about 0.01 cm to about 7x10-5 em), microwave radiation (wavelength of about
10 cm to
about 0.01 cm), radio waves (wavelength of greater than about 10 cm) and any
wavelength or
energy between these illustrative types of electromagnetic energy, e.g., sound
waves in
various forms or from devices such as ultrasound devices having a wavelength
of about 1.5
mm. The exact form of the electromagnetic energy used to treat tissue may vary
depending
on numerous factors including the wavelength of the electromagnetic energy,
the tissue to be
treated, treatment times, dosage and the like. Illustrative forms and devices
for providing
electromagnetic energy to tissue for treatment are discussed herein, and
additional suitable
forms and devices for providing electromagnetic energy to tissue for treatment
will be readily
selected by the person of ordinary skill in the art, given the benefit of this
disclosure.
[0059] In accordance with certain examples, an illustrative apparatus for
providing
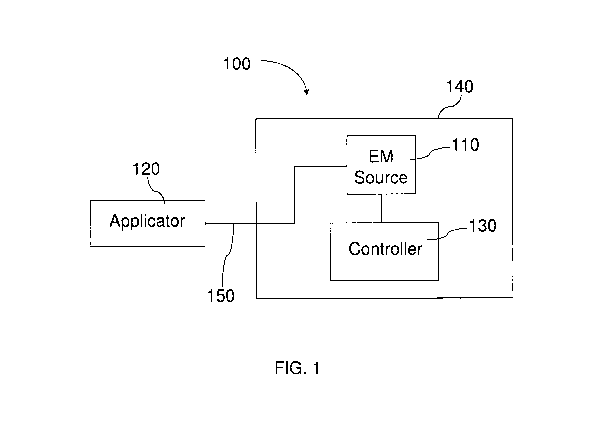
electromagnetic energy to a tissue is shown in FIG. 1. The apparatus 100
includes an
electromagnetic energy source 110 energetically coupled to an applicator 120.
As used
herein "energetically coupled" refers to the configuration where energy
generated or provided
by the electromagnetic energy source 110 can be transmitted to the applicator
120 and on to a
tissue. In certain embodiments, the apparatus 100 may also include a
controller 130 that is
electrically coupled to the electromagnetic energy source 110 and optionally
to the applicator
120. In certain examples, the electromagnetic energy source 110 and the
controller 130 may
be in or on a housing 140. The applicator 120 is typically, though not
necessarily, located
exteinal to the housing 140 and is energetically coupled to the
electromagnetic energy source
-9-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
110 through interconnect or cable 150. In certain examples, during operation
of apparatus
100, the applicator 120 may be placed on or near the tissue to be treated and
electromagnetic
energy may be provided to the applicator 120 from the electromagnetic energy
source 110
through the interconnect or cable 150.
[0060] In accordance with certain examples, the exact configuration of the
applicator may
vary depending on the type of electromagnetic energy to be delivered to the
tissue. In
examples where the applicator is configured to deliver gamma radiation or X-
rays to the
tissue, the applicator may be a tube or cable with suitable shielding to
prevent unwanted
gamma radiation from exiting the cable 150 while allowing gamma radiation or X-
rays to exit
at a terminus of the applicator. In examples where the applicator is
configured to deliver UV
light, visible light or infrared radiation to the tissue, the applicator may
be a fiber optic device
or a light pipe that allows for transmission of the UV or visible light from a
light source to the
tissue. In examples where the applicator is configured to deliver microwave
radiation or
radio waves to the tissue, the applicator may be a coaxial cable, waveguide or
the like that
permits passage of microwaves or radio waves from a source to the tissue.
Other
embodiments are discussed herein for applicator configurations that provide
for delivery of
different types of electromagnetic energy.
[0061] In accordance with certain examples, the applicator may be configured
for delivery of
electromagnetic energy to the skin to treat an infection of the skin or to
prevent an infection
of the skin, e.g., in or near a skin wound, in a human or non-human mammal
such as, for
example, a cow, sheep, or horse. In certain examples, the applicator may be
configured to
deliver electromagnetic energy to treat a bacterial skin infection such as,
for example,
cellulitis, erythrasma, folliculitis, skin abscesses, carbuncles, Hidradenitis
suppurativa,
impetigo, necrotizing skin infections or Staphylococcal scalded skin syndrome.
In other
examples, the applicator may be configured to deliver electromagnetic energy
to treat a
blistering disease such as, for example, bullous pemphigoid, dermatitis
herpetiformis, or
pemphigus. In yet other examples, the applicator may be configured to deliver
electromagnetic energy to treat a fungal skin infection such as, for example,
candidiasis,
ringworm, tinea versicolor, tinea pedis or onychomycosis. In still additional
examples, the
applicator may be configured to deliver electromagnetic energy to treat an
itching and
noninfectious rash such as, for example, contact dermatitis, atopic
dermatitis, seborrheic
dermatitis, nummular dermatitis, generalized exfoliative dermatitis, stasis
dermatitis, perioral
dermatitis, pompholyx, a drug rash, erythema multiforme, erythema nodosum,
granuloma
annulare, itching, keratosis pilaris, lichen planus, pityriasis rosea,
psoriasis, rosacea, Stevens-
-10-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
Johnson Syndrome, toxic epidermal necrolysis or other dermatalogical disorders
such as, for
example, dry nail. In certain examples, the applicator may be configured to
deliver
electromagnetic energy to treat parasitic skin infections such as, for
example, creeping
eruption, lice infestation, or scabies. In yet other examples, the applicator
may be configured
to deliver electromagnetic energy to treat a viral skin infection, such as
molluscum
contagiosum or warts. In other examples, the applicator may be configured to
treat psoriatic
nail disease following nummular dermatitis.
[0062] In certain examples, the treatment methods and devices disclosed herein
may be used
with one or more therapeutics or other compositions designed to prevent or
reduce the
likelihood of reinfection. Illustrative materials include antibiotics,
antifungals, tissue sealants,
tissue barriers and the like. It will be within the ability of the person of
ordinary skill in the
art, given the benefit of this disclosure, to select suitable compositions and
devices to
discourage or prevent reinfection of a tissue.
[0063] In accordance with certain examples, the applicator may include an
adaptor that is
sized and arranged to fit over the area of the tissue to be treated. For
example and referring to
FIG. 2, an applicator 200 may include an adaptor 210 that is energetically
coupled to an
electromagnetic energy source (not shown). The adaptor 210 is typically
constructed and
arranged to mirror the shape of the area of the tissue to be treated, e.g., if
the area to be
treated is circular, then the adaptor may be constructed, or trimmed, to
mirror the circular
shape. Suitable materials for use in adaptors include, but are not limited to,
metals, metal
alloys, ceramics, plastics, polymers, conductive polymers and the like. The
adaptor may be
placed over the area to be treated and electromagnetic energy may be provided
to the area
through the adaptor using one or more of the methods disclosed herein.
[0064] In certain examples, the adaptor may be disposable such that subsequent
to treatment,
the adaptor may be removed or disconnected from the applicator and discarded.
The use of a
disposable adaptor may provide significant benefits including, but not limited
to, simple and
cheap adaptors for single use, the lack of having to sterilize adaptors
subsequent to use and
the ability to use a new adaptor for each treatment and each subject to
minimize any cross-
contamination. The exact configuration of a disposable adaptor may vary
depending on the
nature and type of electromagnetic energy to be delivered and illustrative
disposable adaptors
are discussed in more detail herein.
[0065] In certain examples and referring to FIG. 2, the applicator 200 may
also include a
tuning box 220 that may be filled with a selected material such that the
frequency of the
electromagnetic energy provided to the adaptor may be further controlled. For
example, the
-11-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
tuning box 220 may be filled with a gel or a sol material to tune further the
frequency of the
energy that passes through the applicator and/or through the adaptor. In some
examples, a
material may be added to the tuning box such that the impedance matching is
accomplished
at a particular frequency. While it is not required to configure the adaptor
to be impedance
matched, impedance matching may provide certain advantages, as discussed in
more detail
below. The exact material used in the tuning box can vary depending on the
electromagnetic
energy to be delivered. Illustrative materials for placement in the tuning box
include a gel,
such as, for example, commercially available lubricating jellies or a tissue-
equivalent
"phantom," a fluid, e.g., water, acetone, methanol, ethanol, non-polar
hydrocarbon based
solvents, etc., or mixtures or combinations of any of the preceding
substances, or a solid, e.g.,
a foam, fiber, glass, plastic or the like, Other suitable materials will be
readily selected by the
person of ordinary skill in the art, given the benefit of this disclosure.
[0066] In accordance with certain examples, the interconnect or cable 150 that
provides for
energetic coupling from the electromagnetic energy source to the adaptor may
be positioned
within tuning box 220 such that any electromagnetic energy passes through the
tuning box on
its way to the adaptor. In some examples, the energy passes through the tuning
box but is not
transferred from the tuning box to the adaptor, e.g., the cable runs through
the center or some
portion of the tuning box. In other examples, the energy is transferred to the
tuning box,
which passes the energy to the adaptor for delivery of the tissue to be
treated.
[0067] In accordance with certain examples, the apparatus disclosed herein may
also include
or be configured to work with or receive a temperature sensor to monitor the
temperature of
the tissue to be treated. In certain examples, the exact configuration of the
temperature
sensor may vary depending on many factors including, but not limited to, the
tissue to be
treated, the type of electromagnetic energy to be used, the level of
electromagnetic energy
delivered, the configuration of the applicator or the like. In some examples,
a temperature
sensor such as those commercially available from Luxtron (Santa Clara, CA) may
be used.
In certain examples, the temperature sensor may be a thermocouple. In other
examples, the
temperature sensor may be a fiber optic thermometry sensor, a fluorescence
based sensor or a
radiation thermometry sensor. Additional suitable temperature sensors will be
readily
selected by the person of ordinary skill in the art, given the benefit of this
disclosure.
[0068] In accordance with certain examples, the temperature sensor may be
placed on the
tissue to be treated to monitor the tissue temperature during treatment.
Should the
temperature of the tissue exceed a threshold value, i.e., a treatment
temperature, the
applicator may stop delivery of electromagnetic energy for a selected period.
In certain
-12-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
examples, electromagnetic energy may be delivered until the temperature of the
tissue
reaches a desired temperature to provide for optimal treatment of the tissue.
In other
examples, delivery of the electromagnetic energy is constant or pulsed, but
treatment is not
halted prior to delivery of a selected dose unless the tissue temperature
exceeds a threshold
temperature.
[0069] In accordance with certain examples, once the tissue reaches a desired
or threshold
temperature, the tissue may be cooled either passively or actively. In
configurations where
passive cooling is used, the electromagnetic energy source may be switched off
for a period
to allow thermal transfer from the tissue to the surrounding environment.
Alternatively, the
electromagnetic energy source may stay on but the electromagnetic energy may
be blocked
from exiting the applicator and being delivered to the tissue. In embodiments
where active
cooling is used, heat may be removed from the tissue by placing a heat sink,
fan, ice, ice pack
or other device or material on the tissue to increase the temperature gradient
between the
tissue and the surrounding environment. In some examples, a device utilizing
the Peltier
effect may be employed to reduce the temperature of the tissue rapidly so that
treatment may
be continued and overall procedure time may be reduced. Additional methods and
devices
for lowering the temperature of a tissue to a desired value or below a
threshold value will be
readily selected by the person of ordinary skill in the art, given the benefit
of this disclosure.
Illustrative examples of active cooling, include, but are not limited to,
contact conduction
cooling, evaporative spray cooling, convective air flow cooling, a water
jacket, a plate and
pump configured to circulate a cooling fluid through the plate, a spray
cooling device that
uses cryogen, water, or air as a coolant, and combinations thereof.
[0070] In certain examples, the temperature sensor may be electrically coupled
to the
controller such that treatment may be halted if the temperature of the tissue
exceeds a
threshold temperature or reaches a desired temperature. In other
configurations, the
temperature sensor may be an integral part of the applicator such that
placement of the
applicator on the tissue also results in bringing the temperature sensor into
thermal
communication with the tissue. An example of this configuration is shown in
FIG. 3. The
apparatus 300 includes an electromagnetic energy source 310, a controller 320
electrically
coupled to the electromagnetic energy source 310, and an applicator 330
energetically
coupled to electromagnetic energy source 310 through interconnect or lead 350.
The
apparatus 300 may also include a housing 340 which encloses the
electromagnetic energy
source 310 and the controller 320. The applicator 330 may include an adaptor
332 and a
temperature sensor 334. In certain examples, the temperature sensor 334 may be
detachable
- 13 -
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
or removable from the adaptor 332 to facilitate cleaning of the applicator
330. Though
temperature sensor 334 is shown on the terminus of the adaptor 332 in FIG. 3,
the
temperature sensor 334 may be positioned at any location on the adaptor 332 so
long as the
temperature sensor can detect the temperature of the tissue to be treated.
[0071] In certain examples, the temperature sensor is not in direct contact
with the tissue to
be treated, but is instead above, below or beside the tissue to be treated. In
configurations
where the temperature sensor is not in direct contact with the tissue, a
lookup table or
algorithm may be used to calculate or extrapolate the temperature of the
tissue based on the
detected temperature above, below or beside the tissue. For example, where the
tissue to be
treated has a small surface area, e.g., a small toenail or a small area of the
skin or other organ,
it may not be possible to place both the temperature sensor and the adaptor in
contact with the
tissue. The temperature sensor may be placed near the tissue, however, and the
temperature
properties or profile of a medium between the tissue and the temperature
sensor may be used
to extrapolate the temperature of the tissue. This medium may be a fluid, such
as air, a liquid
or a solution, may be a gel or sol, may be a foam or may be other suitable
materials whose
temperature properties are known or may be determined.
[0072] In accordance with certain examples, one or more spacers may be placed
between the
tissue and the adaptor and/or between the tissue and the temperature sensor.
For example and
referring to FIG. 4, a tissue surface 410 is in contact with spacers 420, 422,
which are in
contact with adaptor 430. In some embodiments, at least one surface of the
spacer 420 may
be conformable, compressible or expandable such that it can conform to the
shape of the
tissue surface to be treated. In the example shown in FIG. 4, the surface of
spacer 420 that
rests against the tissue surface 410 has conformed to the tissue surface 410.
The use of a
spacer may provide more uniform delivery of electromagnetic energy to the
tissue when the
surface or surfaces of the tissue are uneven. Suitable materials for use as a
spacer includes,
but is not limited to, metals, metal alloys, elastomers, plastics, polymers
and the like. In
some examples, one or more materials consisting of spacers separating volumes
of air, e.g., a
honeycomb material, where the spacers may be any of the illustrative materials
listed above,
may be used. In some configurations, the spacer may be selected so that it
transmits energy
from the adaptor to the tissue without altering the frequency or level of the
energy. In other
configurations, the spacer may be selected such that is alters the frequency
or level of the
energy prior to the energy being delivered to the tissue. It will be within
the ability of the
person of ordinary skill in the art, given the benefit of this disclosure, to
use a spacer of a
selected material with the apparatus disclosed herein.
-14-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
[0073] In accordance with certain examples, the applicator may be configured
for delivery of
electromagnetic energy to one or more nails of a human or non-human mammal. As
discussed herein, treatment of keratinized tissue, such as that found in human
nails or in the
nails of non-human mammals such as sheep and horses, can be difficult. The
devices,
systems and methods disclosed herein may be used to provide electromagnetic
energy to the
nails and/or nail beds to improve the overall appearance of the nails. Such
treatment may be
performed, for example, to deactivate or kill pathogens infecting the nail
and/or nail bed or to
improve the overall appearance of the nail by preventing pathogens from
infecting the nail or
the nail bed. An illustrative example of an applicator that may be used to
treat the nail is
shown in FIG. 5. The applicator 500 comprises an adaptor 510 that includes a
tuning box.
The adaptor 510 is electrically coupled to an electromagnetic energy source
(not shown)
through cable 550. The applicator 500 also includes an end-cap 520 that may
conform to the
shape of the nail. The end-cap 520 may be electrically coupled to the adaptor
510. The
applicator 500 also includes a tissue interface 530 configured to receive a
bolus. The use of a
bolus is discussed in more detail below. A nail may be positioned above the
tissue interface
530 and in contact with the end-cap 520. An over-mold 540 may be placed on the
top of the
nail and may act to retain one or more temperature probes (not shown) placed
on the nail.
During operation of the applicator 500, electromagnetic energy may be
delivered through
cable 550 to end-cap 520 and into the nail for treatment of the nail tissue.
[0074] In accordance with certain examples and as discussed above, the
electromagnetic
energy delivered to the nail tissue may be any of the illustrative energy
types discussed herein.
It will be recognized by the person of the ordinary skill in the art, given
the benefit of this
disclosure, that the configuration of the end-cap 520 may vary depending on
the type of
electromagnetic energy to be delivered. The exact configuration of the end-cap
is not critical
so long as the end-cap can deliver a selected type of electromagnetic energy
to the tissue to
be treated. In embodiments where radio waves or microwaves are to be
delivered, the end-
cap may be configured as an antenna, wave guide, conductor or the like. In
embodiments
where a sound wave, e.g., a sound wave from an ultrasound device, is to be
delivered, the
end-cap may be configured with a sound transmitter. In embodiments where
infrared, visible
or ultraviolet light is to be delivered, the end-cap may be configured as a
light-pipe, a fiber
optic device, a light emitting diode, a laser diode, an incandescent source, a
fluorescent
source, an assembly of reflectors or other devices that may be used to deliver
light. In
embodiments where the X-rays or gamma rays are to be delivered, the end-cap
may be
configured as an opening in a lead-shielded cable, a guide, a cone, or a
collimator, that is
- 15 -
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
energetically coupled to an X-ray or gamma ray source. Other configurations
for an end-cap
to deliver a selected type of electromagnetic energy to a nail or other tissue
will be readily
selected by the person of ordinary skill in the art, given the benefit of this
disclosure.
[0075] In certain examples, a bolus may be inserted into the tissue interface
530. By using a
bolus underneath the tissue to be treated, e.g., a nail infected with a
fungus, the
electromagnetic energy delivered to the tissue may be more uniform. It is
thought that the
bolus provides tuning of the electromagnetic energy to provide a more uniform
distribution of
energy to a parallel path for electric field lines, thereby reducing their
concentration at an
undesired location within tissue; such a concentration of field lines may
cause unwanted
effects such as, for example, local overheating. For example and referring to
FIG. 6, a
simulation is shown with an applicator comprising an end-cap electrically
coupled to a
coaxial cable to provide microwave energy that may be used to treat a nail.
This simulation
was performed by solving Laplace's Equation for voltage, with a voltage
difference enforced
between the end-cap and the outer conductor of the coaxial cable. As can be
seen in the top
graph, in the configuration where no bolus is used, the electromagnetic energy
delivered to
the nail is non-uniform. This result may cause unwanted heating of the tip of
the toe. By
using a bolus, (bottom graph in FIG. 6) the energy that is delivered to the
toe is more uniform.
In addition, by using a bolus, the level of energy delivered to the applicator
may be reduced
due to the increased efficiency of delivery of the electromagnetic energy. For
example, the
level of microwave energy may be reduced by about 50% or more due to more
uniform
,delivery of the energy, e.g., in the case of microwave energy, the energy
provided to the
applicator may be reduced from about 36 Watts to about 10 Watts without any
substantial
reduction in the amount of energy delivered to tissue. In certain cases, by
using a bolus, the
fraction of power reflected from the applicator may be reduced. For example, a
measurement
made with a dual directional coupler and two power meters showed that the
fraction of
microwave reflected power was reduced from 18% to 3% by using a bolus.
[0076] In accordance with certain examples, suitable materials for the bolus
may vary
depending on the type of electromagnetic energy to be delivered. In certain
examples, the
bolus has similar physical properties as those of the tissue to be treated,
e.g., a similar water
content, etc. Illustrative materials for use as a bolus include gelatin,
collagen, agarose, a
lubricating jelly, water, an ultrasound gel pad and similar materials. In
certain examples, the
bolus may be cast in a mold or die that has a similar size and geometry as
that of the tissue
interface 530. Alternatively, the bolus may be cut to shape from a larger
bolus. In examples
where a kit is employed, the bolus may be included in the kit and configured
to be placed in
-16-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
the tissue interface without prior cutting or shaping by the operator. It will
be within the
ability of the person of ordinary skill in the art, given the benefit of this
disclosure, to select,
make and/or use a bolus with the devices, systems and methods disclosed
herein.
[0077] In accordance with certain examples, the end-cap 520 may be configured
to overlie
and/or surround the tissue to be treated. In certain examples, the end-cap may
be constructed
or trimmed to be substantially the same shape as the tissue to be treated. In
some examples,
the end-cap may be electrically coupled to an interconnect or cable 550 so
that
electromagnetic energy may be transmitted from the cable 550 to the end-cap
520 and
delivered to the nail tissue. The exact material used to construct the end-cap
may vary
depending on the type of electromagnetic energy to be delivered to the tissue.
In examples
where the electromagnetic energy to be delivered is radio waves or microwaves,
the end-cap
may be constructed from a conductive material, such as a metal, metal alloy,
plastic or the
like. In examples where the electromagnetic energy to be delivered to the
tissue is infrared,
visible or ultraviolet energy, the end-cap may include a fiber optic device to
transmit the light.
In examples where the electromagnetic energy to be delivered to the tissuC is
X-rays or
gamma rays, the end-cap may include an opening for transmitting or focusing X-
rays or
gamma rays. Additional materials and configurations for an end-cap constructed
and
arranged to deliver a selected electromagnetic energy will be readily selected
by the person of
ordinary skill in the art, given the benefit of this disclosure.
[0078] In accordance with certain examples, the over-mold 540 may be used to
retain one or
more additional devices on the tissue and to facilitate proper placement of
the applicator for
treatment. In certain examples, the over-mold 540 may be used to hold a
temperature sensor
in place. In other examples, the over-mold 540 may be impregnated or coated
with a
therapeutic to provide additional treatment of the tissue. In some examples,
the over-mold
540 may include a dye or agent that can facilitate transfer of the
electromagnetic energy to
the tissue. For example, a dye may be used to provide for increased absorption
of energy
from the applicator. The material or materials used in the over-mold may vary
depending on
the type of electromagnetic energy to be delivered, and, preferably, the
materials do not
substantially interfere with delivery of the electromagnetic energy to the
tissue. In certain
examples, the over-mold includes a material such as a silicone, a plastic or
an elastomer, any
of which may include an adhesive to retain the over-mold in position after
placement on the
tissue. Illustrative commercially available devices suitable for use as an
over-mold include,
but are not limited to, surgical tape, an adhesive bandage, a clear plastic
film, a foil or the like.
In other examples, the over-mold may be used to shield tissues that are not
being treated from
-17-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
the electromagnetic energy. Such over-molds may be effective to absorb the
electromagnetic
energy or to otherwise prevent exposure of any underlying tissues to the
electromagnetic
energy.
[0079] In accordance with certain examples, when the applicator 500 shown in
FIG. 5 is used,
a bolus may be placed in container 530, a toe with an infected nail may be
placed on top of
the container 530, and the tip of the toe typically rests against the tuning
box 510. A
temperature sensor is placed on the nail tissue to be treated. The end-cap 520
may be brought
into contact with the nail and over-mold 540 acts to hold the temperature
sensor on the nail
tissue. The exact methodology used to treat the nail depends on the
electromagnetic energy
to be delivered to the nail tissue, and illustrative methods are discussed in
more detail herein.
In certain examples, a controller may be operative to switch an
electromagnetic energy source
on, and energy may be delivered through end-cap 520 to the nail tissue to be
treated.
[0080] In accordance with certain examples, the controller of the apparatus
disclosed herein
may be a simple device, such as a mechanical on/off switch. In other
embodiments, the
on/off switch may include a mechanical timer or timing circuit that
automatically turns the
apparatus off after a certain period from switching the apparatus on. For
example, in
configurations where the apparatus is designed for home use, depression of the
on/off switch
may cause transmission of electromagnetic energy through an applicator for a
selected
amount of time. The timer or timing circuit may be designed to automatically
switch the
electromagnetic energy source off after a selected period. In the alternative,
the subject being
treated with the electromagnetic energy may have control over treatment such
that they can
manually turn on and turn off the electromagnetic energy. In other
configurations, the
temperature sensor may be electrically coupled to the controller and once the
tissue reaches a
selected or threshold temperature, the controller may stop delivery of
electromagnetic energy
for a desired period.
[0081] In other configurations, the controller may include a processor,
associated circuitry
and the like. An illustrative configuration for a controller in an apparatus
is shown in FIG. 7.
The controller 710 of the apparatus 700 is electrically coupled with the other
components of
the apparatus through an interface or interconnect 720, which typically is a
bus such as a
serial bus. The apparatus 700 also includes a power supply 730 electrically
coupled to a
switch 740. The apparatus 700 also includes an electromagnetic energy source
750
energetically coupled to an applicator 760. The applicator 760 may include or
be used with a
temperature sensor (not shown) which sends signals to temperature sensor input
770. In
operation of apparatus 700, the controller 710 sends and receives signal from
the various
-18-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
components of the apparatus. For example, the controller 710 may send a signal
to initialize
the electromagnetic energy source 750 to provide energy to the applicator 760.
The
temperature sensor input 770 can send signals to the controller 710 such that
electromagnetic
energy source 750 may be turned off if the temperature of the tissue exceeds a
threshold
temperature. The various parameters of the system, e.g., energy level, tissue
temperature, etc.,
may be displayed on display 780 such that an operator may monitor treatment.
[0082] In accordance with certain examples, the controller 710 may include at
least one
processor optionally electrically coupled with one or more memory units. In
certain
examples, the controller may be a larger part of a computer system. The
computer system
may be, for example, a general-purpose computer such as those based on Unix,
Intel
PENTIUM-type processor, Motorola PowerPC, Sun UltraSPARC, Hewlett-Packard PA-
RISC processors, or any other type of processor. In some examples, the
processor may be an
inexpensive processor that may be programmable to receive inputs and determine
a treatment
dose based on the received inputs. It should be appreciated that one or more
of any type
computer system may be used according to various embodiments of the
technology. Further,
the system may be located on a single computer or may be distributed among a
plurality of
computers attached by a communications network. A general-purpose computer
system may
be configured, for example, to perform any of the described functions
including but not
limited to: applicator control, temperature monitoring, data display and the
like. It should be
appreciated that the system may perform other functions, including network
communication,
and the technology is not limited to having any particular function or set of
functions.
[0083] For example, various aspects may be implemented as specialized software
executing
in a general-purpose computer system 800 such as that shown in FIG. 8. The
computer
system 800 may include a processor 810 connected to one or more memory devices
850, such
as a disk drive, memory, or other device for storing data. Memory 850 is
typically used for
storing programs and data during operation of the computer system 800.
Components of
computer system 800 may be coupled by an interconnection device 830, which may
include
one or more buses (e.g., between components that are integrated within a same
machine)
and/or a network (e.g., between components that reside on separate discrete
machines). The
interconnection device 830 provides for communications (e.g., signals, data,
instructions) to
be exchanged between system components of system 800. The computer system 800
typically is electrically coupled to the applicator (not shown) such that
electrical signals may
be provided from the applicator to the computer system 800 for storage and/or
processing.
-19-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
[0084] Computer system 800 may also include one or more input devices 820, for
example, a
keyboard, mouse, trackball, microphone, touch screen, manual switch (e.g.,
override switch)
and one or more output devices 840, for example, a printing device, display
screen, speaker.
In addition, computer system 800 may contain one or more interfaces (not
shown) that
connect computer system 800 to a communication network (in addition or as an
alternative to
the interconnection device 830.
[0085] The storage system 860, shown in greater detail in FIG. 9, typically
includes a
computer readable and writeable nonvolatile recording medium 910 in which
signals are
stored that define a program to be executed by the processor or information
stored on or in
the medium 910 to be processed by the program. For example, the treatment
dosing times,
calibration methods, maximum dosages for a particular subject and the like
used in certain
embodiments disclosed herein may be stored on the medium 910. The medium may,
for
example, be a disk or flash memory. Typically, in operation, the processor
causes data to be
read from the nonvolatile recording medium 910 into another memory 920 that
allows for
faster access to the information by the processor than does the medium 910.
This memory
920 is typically a volatile, random access memory such as a dynamic random
access memory
(DRAM) or static memory (SRAM). It may be located in storage system 860, as
shown, or in
memory system 850. The processor 810 generally manipulates the data within the
integrated
circuit memory 850, 920 and then copies the data to the medium 910 after
processing is
completed. A variety of mechanisms are known for managing data movement
between the
medium 910 and the integrated circuit memory element 850, 920, and the
technology is not
limited thereto. The technology is also not limited to a particular memory
system 850 or
storage system 860.
[0086] In certain examples, the computer system may also include specially-
programmed,
special-purpose hardware, for example, an application-specific integrated
circuit (ASIC).
Aspects of the technology may be implemented in software, hardware or
firmware, or any
combination thereof. Further, such methods, acts, systems, system elements and
components
thereof may be implemented as part of the computer system described above or
as an
independent component.
[0087] Although computer system 800 is shown by way of example as one type of
computer
system upon which various aspects of the technology may be practiced, it
should be
appreciated that aspects are not limited to being implemented on the coinputer
system as
shown in FIG. S. Various aspects may be practiced on one or more computers
having a
different architecture or components than that shown in FIG. 8. Computer
system 800 may
-20-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
be a general-purpose computer system that is programmable using a high-level
computer
programming language. Computer system 800 may be also implemented using
specially
programmed, special purpose hardware. In computer system 800, processor 810 is
typically a
commercially available processor such as the well-known Pentium class
processor available
from the Intel Corporation. Many other processors are available. Such a
processor usually
executes an operating system which may be, for example, the Windows 95,
Windows 98,
Windows NT, Windows 2000 (Windows ME), Windows XP or Windows Vista operating
systems available from the Microsoft Corporation, MAC OS System X operating
system
available from Apple Computer, the Solaris operating system available from Sun
Microsystems, or UNIX or Linux operating systems available from various
sources. Many
other operating systems may be used, and in certain embodiments a simple set
of commands
or instructions may function as the operating system.
[0088] In accordance with certain examples, the processor and operating system
may
together define a computer platform for which application programs in high-
level
programming languages may be written. It should be understood that the
technology is not
limited to a particular computer system platform, processor, operating system,
or network.
Also, it should be apparent to those skilled in the art, given the benefit of
this disclosure, that
the present technology is not limited to a specific programming language or
computer system.
Further, it should be appreciated that other appropriate programming languages
and other
appropriate computer systems could also be used.
[0089] In certain examples, the hardware or software is configured to
implement cognitive
architecture, neural networks or other suitable implementations. For example,
a tissue
database may be linked to the system to provide access to temperature
tolerances for different
tissues. Such configuration provides for use of the applicator with many
different types of
tissues, which may increase the flexibility and function of the devices,
systems and methods
disclosed herein.
[0090] One or more portions of the computer system may be distributed across
one or more
computer systems coupled to a communications network. These computer systems
also may
be general-purpose computer systems. For example, various aspects may be
distributed
among one or more computer systems configured to provide a service (e.g.,
servers) to one or
more client computers, or to perform an overall task as part of a distributed
system. For
example, various aspects may be performed on a client-server or multi-tier
system that
includes components distributed among one or more server systems that perform
various
functions according to various embodiments. These components may be
executable,
-21 -
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
intermediate (e.g., IL) or interpreted (e.g., Java) code which communicate
over a
communication network (e.g., the Internet) using a communication protocol
(e.g., TCP/IP). It
should also be appreciated that the technology is not limited to executing on
any particular
system or group of systems. Also, it should be appreciated that the technology
is not limited
to any particular distributed architecture, network, or communication
protocol.
[0091] In accordance with certain examples, various embodiments may be
programmed
using an object-oriented programming language, such as SmallTalk, Basic, Java,
C++, Ada,
or C# (C-Sharp). Other object-oriented programming languages may also be used.
Alternatively, functional, scripting, and/or logical programming languages may
be used.
Various configurations may be implemented in a non-programmed environment
(e.g.,
documents created in HTML, XML or other format that, when viewed in a window
of a
browser program, render aspects of a graphical-user interface (GUI) or perform
other
functions). Certain configurations may be implemented as programmed or non-
programmed
elements, or any combination thereof.
[0092] In certain examples, a user interface may be provided such that a user
may enter or
recall a type of tissue, patient statistics, tissue condition or other data
desired. For example,
in instances where a patient has already received treatment, relevant
treatment parameters
may be recalled and reused without the need to determine maximum dosages or
the like.
Other features for inclusion in a user interface will be readily selected by
the person of
ordinary skill in the art, given the benefit of this disclosure.
[0093] In accordance with certain examples, numerous methods may be
implemented by the
controller to deliver the electromagnetic energy to the tissue. In certain
examples, the method
may involve patient or subject input. For example, and referring to FIG. 10,
the applicator
may be placed on a subject 1000, and treatment may be initiated 1010. Using
subjective or
objective factors, such as subject feedback, it can be assessed whether or not
the temperature
is okay 1015. If the temperature is okay and the patient is comfortable, then
treatment may
continue for a time period t, until the treatment period ti is the same as a
desired treatment
interval te1d. If the dosage is too high such that the subject is
uncomfortable, the energy may
be reduced to a lower level 1020 and treatment may be re-initiated 1010 and
continued for a
time period t, until the treatment period ti is the same as a desired
treatment interval te11d. It
should be understood that the treatment period may include application of
electromagnetic
energy in a continuous or pulsed maimer, as discussed in more detail herein.
[0094] In certain examples, the temperature of the tissue may be monitored
during
application of the electromagnetic energy, as the temperature of the tissue
may increase
-22-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
during treatment. An example of a method that implements this type of feedback
is shown in
FIG. 11. Treatment may be initiated 1100, and if the temperature of the tissue
is suitable
during treatment, then treatment may continue 1110 for treatment time tl. If
the temperature
should exceed a threshold value or is uncomfortable to the patient, then the
energy level can
be adjusted 1120 to a lower level and treatment may be reinitiated. As
treatment progresses,
the tissue may heat up. If this situation occurs, then treatment may be
discontinued 1140 for
a delay time tdelay and can then be continued 1130 once the temperature of the
tissue decreases
to a suitable value. Should the temperature be within an acceptable value and
should the total
treatment time t, equal te1d, then treatment may be discontinued 1150.
[0095] In accordance with certain examples, treatment may be administered in a
continuous
manner by providing electromagnetic energy to the tissue for a selected
period. For example,
the controller may provide electromagnetic energy to the applicator for a
continuous period to
effectuate treatment of the tissue. Continuous treatment may be desirable
where the tissue
does not heat beyond a threshold temperature and where it is desirable to
minimize total
treatment time.
[0096] In accordance with certain examples, the treatment may be administered
in a pulsed
manner by using on/off cycles of continuously delivered energy. For example
and referring
to FIG. 12, a first pulse may be delivered by providing the energy for a time
ti. A delay
period of tdeiay occurs, which allows the temperature of the tissue to
decrease. Following the
delay period, another pulse of energy for a treatment time of t2 may be
delivered. This
process of pulsing and delaying may be repeated for a sufficient time to
provide treatment to
the tissue. By controlling the duration of ti, t2, and tdeiay, energy may be
delivered to the
tissue in a pulsed manner. In certain examples, the exact times for tl, tde,ay
and tz may vary.
In some examples, t], tdeiay and t2 are substantially the same. In other
examples, ti and t2 are
substantially the same and tdeiay may be greater than ti and t, to allow for
tissue cooling. In
other examples, tl may be greater than t2 as tdeiay may not be long enough to
permit the
temperature of the tissue to return all the way to its nonnal, resting
temperature. In certain
examples t, and t2 are each about 10 seconds to about 20 seconds to about and
tdelay is about
seconds to about 20 seconds.
[0097] In accordance with certain examples, the sum of the treatment times may
be totaled
such that treatment time continues until the total treatment time sums to a
value tend. The tend
value provides for approximately the same amount of treatment of each subject
even if the tl,
t?, tdelay, etc., times differ for different subjects. The total treatment
time may vary depending
on the exact type of electromagnetic energy delivered to the tissue. In
certain examples, the
-23-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
total treatment time is no more than about 5 minutes. In other examples,
however, the total
treatment time may be about 5 minutes or greater. While in certain examples
the total
treatment time may be five minutes or less, the total time for a procedure
involving
administration of treatment to a patient may be substantially longer as the
sum of the tMay
times may be a substantial value. In some examples, the total time for
administering a single
treatment to an individual varies from about 10 minutes to about 120 minutes,
more
particularly from about 20 minutes to about 90 minutes, e.g., about 30 minutes
to about 60
minutes. The person of ordinary skill in the art, given the benefit of this
disclosure, will be
able to select suitable treatment and delay times for providing treatment of a
particular tissue
using a particular type of electromagnetic energy.
In accordance with certain examples, prior to treatment, one or more
calibration steps may be
performed to detennine a maximum dose of the electromagnetic energy that a
subject can
tolerate. Without wishing to be bound by any particular scientific theory or
this example, it is
believed that heating of the tissue to a higher temperature using the methods
and devices
disclosed herein provides for more effective treatment. Such tissue heating is
permitted to a
level that still remains safe, e.g., up to about 57 C, so that the tissue
cells are not killed or
permanently damaged. With respect to treatment of fungus without damage to
surrounding
tissue, the tissue may be heated to a range between 43-57 C and more
preferably between
47-53 C. An illustrative calibration method is shown in FIG. 13.
Electromagnetic energy
may be applied 1300 at an initial energy E . If the patient or subject can
tolerate the E
energy level then the energy level may be increased 1310 to El. In the
alternative, the power
level may be adjusted or set such that the temperature changes (dT/dt) by a
selected amount
over a selected period. This dT/dt value is substantially linearly
proportional to input power,
which allows interpolation to select a desired dT/dt range by adjusting the
power level. This
process, for example, may be performed over a 10 second interval such that
tissue heating to
a tolerance level is not a factor. The power level that provides the desired
dT/dt is the power
level used for the balance of the treatment. Once that power level is
determined, the first
increase to the treatment temperature provides the tolerance of the patient,
and from there it is
possible to control the temperature limit based on their feedback. If the
subject or patient is
uncomfortable at the energy level El, then the energy may be returned 1320 to
energy level
E , or to an energy level between E an El, and treatment may be initiated. If
the subject or
patient is comfortable at the energy level El, then the energy level may be
increased 1340 to
E2, and this process may be repeated to determine the maximum dosage that the
subject or
patient can tolerate. If the subject or patient was subjectively uncomfortable
at energy level
-24-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
E2, then the initial energy level may be reduced 1330 to Energy level E1 or an
energy level
between E2 an E1 and optionally the step 1340 may be repeated to determine the
maximum
energy level that the subject or patient can tolerate. It is also to be
appreciated that this
methodology can be repeated, if the subject can tolerate the energy level E2,
to determine the
energy level the subject can tolerate. It will be appreciated by the person of
ordinary skill in
the art, given the benefit of this disclosure, that the maximum dose that a
subject can tolerate
may change over the course of a single treatment and may change over the
course of multiple
treatments. In certain instances, a calibration step may be performed prior to
each treatment,
whereas in other instances a calibration step may be performed every other
treatment (or
other selected interval) to assess whether or not the maximum energy dose
tolerated by a
subject has changed. In addition, the treatment temperature may change during
the course of
treatment and may be adjusted on a subject by subject basis.
[0098] In accordance with certain examples, the device and methods disclosed
herein may be
integrated into a system that is configured to provide treatment. The system
may be used in
an office setting of a medical practitioner, e.g., physician, podiatrist,
etc., or may be
configured for use in the home. One example of a system configured for use in
an office
setting for treatment of skin disorders, e.g., nail infections, is shown in
FIGS. 14A and 14B.
The system 1400 includes a housing 1410 which contains the electromagnetic
energy source,
controller and associated circuitry. The housing 1410 is positioned on a set
of wheels or
casters 1412, 1414, 1416 and 1418 to facilitate easy movement of the system
1400 from place
to place. The housing 1410 includes a locking pedal 1420 to prevent or retard
movement of
the system 1400 once positioned. The housing 1410 also includes a retractable
roller handle
1425 and positioning handles 1426 and 1427 to facilitate movement of the
system 1400. The
system may include a storage drawer 1429. The system 1400 shown in FIGS. 14A
and l4B
is configured for treatment of tissues on the foot, e.g., nail tissue, skin
and the like. The
system 1400 includes a heel retainer 1430 and a foot platform 1440 for
placement of a
subject's foot as shown in FIG. 15. The heel retainer 1430 is mounted on bar
1432 which can
slide along the surface of foot platform 1440 and aid in positioning the foot
on the foot
platform 1440. The system 1400 also includes a safety shut off switch 1450 and
a touch-
screen interface 1460 that may display temperature of a temperature sensor
(not shown). The
applicator 1470 is positioned such that placement of the foot on the foot
platform and heel
retained brings the portion of the foot to be treated in contact with the
applicator 1470. The
applicator 1470 may be configured for movement horizontally, e.g.,
perpendicular to bar
1432, such that treatment of different areas of the foot may be accomplished
without having
-25-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
to reposition the foot. The applicator 1400 may also be configured for
vertical movement to
account for differences in foot thickness of different subjects. The
applicator 1470 may take
any of the configurations disclosed herein depending on the type of
electromagnetic energy
that the apparatus 1400 is designed to provide to the tissue.
[0099] In accordance with certain examples, the systems disclosed herein may
be configured
to deactivate or kill an organism infecting a nail. Organisms that are known
to infect the nails
include, but is not limited to, Epidermophyoton floccosum, Trichophyton
rubrum,
Trichophyton mentagrophytes, Candida albicans, Aspergillus, Acremonium,
Fusarium,
Scopulariopsis, Scytalidium, and Hendersonula toruloidea. In one example, a
device that
includes an ultraviolet, visible or infrared light energy source coupled to an
applicator is
disclosed. In some embodiments, the wavelength of the energy is greater than
about 200 nm,
more particular greater than about 340 nm, e.g., greater than about 400 nm.
[00100] In certain examples, the energy is provided to the nail in either a
continuous or
pulsed form. In examples where continuous exposure is implemented, a light
source such as
an arc lamp or mercury lamp may be coupled to the applicator. In examples
where pulsed
exposure is implemented, a pulsed laser may be used. The pulse rate of the
laser may be
controlled, for example, through a controller or processor. Illustrative
pulsing frequencies
include, but are not limited to, 0.1-30 Hz, more particularly about 0.1 to 10
Hz, e.g., about 1
Hz, or about 10-20 Hz, e.g., about 15 Hz. In embodiments where a laser is
used, the pulse
width of the laser may vary, for example, from about 5 seconds to about 30
seconds, more
particularly about 50 seconds to about 10 seconds, e.g., about 100 seconds
to 1 second or
about 1 millisecond, 5 milliseconds, 10 milliseconds, 50 milliseconds, 100
milliseconds, 500
milliseconds or other selected pulse widths within the illustrative ranges
disclosed herein.
Illustrative lasers include, but are not limited to, pulsed dye lasers, a
nitrogen gas laser, an
excimer chemical laser, a Nd:YLF laser, a Nd:YAG laser, a frequency doubled
Nd:YAG
laser, a Nd:glass laser, a copper vapor laser, an alexandrite laser, a
frequency doubled
alexandrite laser, a titanium sapphire laser, a ruby laser, a fiber laser, a
diode lasers, a helium-
neon gas laser, an argon ion gas laser, a krypton ion gas laser, a xenon ion
gas laser, a carbon
dioxide gas laser, a carbon monoxide gas laser, an HF laser, a DF laser, a
chemical-oxygen
iodine laser, a HeCd metal vapor laser, a HeHg metal vapor laser, a HeSe metal
vapor laser, a
copper vapor laser, a gold vapor laser, an Er:YAG laser, a Nd:YVO laser, a
Tm:YAG laser, a
Yb:YAG laser, a Ho:YAG laser, a vertical cavity surface emitting laser
(VCSEL), a free
electron laser, a Raman laser or other suitable lasers having at least one
wavelength in the X-
ray, ultraviolet, visible or infrared regions.
-26-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
[00101] In accordance with certain examples, the electromagnetic energy source
may
be contained within a housing having an opening or aperture to transmit the
beam of radiation
to the target area. In certain embodiments, the applicator may be coupled to a
source to direct
electromagnetic energy to the target area of the nail tissue. In some
examples, the system
may include a light guide positioned relative to the nail plate. The light
guide may be
operative to couple the beam of radiation to the diseased nail. A sensor may
be used to
determine when sufficient thermal energy has been delivered to the target area
to thermally
deactivate the unwanted organism. The sensor may be, for example, a
photodetector (e.g., an
IR detector) or a temperature sensor. A controller or processor may be used to
deactivate the
source should any adverse effects occur during treatment, e.g., a patient
becoming
uncomfortable.
[00102] In accordance with certain examples, an illustrative system for
delivering
electromagnetic energy to an infected nail is shown in FIG. 16. The system
1600 includes an
electromagnetic energy source 1610 and an applicator 1620. The energy source
1610 is
typically contained within an enclosure or housing as discussed elsewhere
herein. The
housing may include an aperture or opening for transmission of the energy to
the applicator
1620 and to a target area to be treated. For example, a beam of energy
provided from energy
source 1610 may be directed to a target area of a nail, nail plate or nail bed
using applicator
1620. Many different configurations for the applicator 1620 are possible and
any
configuration may be used so long as some portion of the light is passed from
the energy
source 1610 to the applicator 1620. In one embodiment, the applicator may
include a fiber
1625 with a selected cross-section (e.g., circular) and an adaptor or guide
1630 for directing
the light. The adaptor 1630 may include optics such as lenses, filters and the
like to provide
light having desired properties, e.g., polarized, filtered, etc. The adaptor
1630 may be placed
in direct contact with the nail or may be placed above or beside the nail. The
adaptor may
optionally include a removable spacer 1640 to keep the adaptor a fixed or
selected distance
from the nail to be treated.
[00103] In accordance with certain examples, the exact configuration of the
electromagnetic
energy source 1610 may vary depending on the type of energy to be delivered.
In certain
embodiments, the electromagnetic energy source 1610 is a coherent or an
incoherent light
source, a microwave generator, a sound wave generator, a radio frequency
generator or the
like. In certain embodiments, an electromagnetic energy source configured to
deliver
ultrasonic energy to the nail may be used. In some embodiments, two or more
different
energy sources may be used. For example and referring to FIG. 17, a first
electromagnetic
-27-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
energy source 1710, e.g., a microwave generator, may be energetically coupled
to an
applicator 1730. A second electromagnetic energy source 1720, e.g., a radio
frequency
generator, may also be coupled to the applicator 1730. A controller (not
shown) may be used
to control which energy source provided energy to the applicator 1730.
Alternatively, the
first and second electromagnetic energy sources may provide energy
simultaneously. For
example, one of the energy sources may provide an incoherent light beam to the
applicator
while the second source may provide a coherent light beam to the applicator.
Other
configurations using two or more sources will be readily apparent to the
person of ordinary
skill in the art, given the benefit of this disclosure.
[00104] In accordance with certain examples, the duration of treatment for
treating an
infected nail may vary from person to person and may vary depending on the
wavelength of
the energy that is used. In certain examples, the wavelength is between about
200 and 400
nm. In some examples, the wavelength is between about 200 nm and 2600 nm, more
particularly about 400 mu to about 1800 nm, even more particularly about 400
nm to about
1100 nm, e.g., about 1160 nm to about 1800 nm. In other examples, the
wavelength may be
between about 400 nm to about 700 nm, more particularly about 500-600 nm,
e.g., about 585-
600 nm.
[00105] In accordance with certain examples, the energy density or fluence of
the
electromagnetic energy source may vary depending on the configuration of the
applicator, the
selected electromagnetic energy source and the like. Energy also depends on
the duration of
treatment, e.g., energy delivered may be approximated by multiplying the power
by the
exposure time. In certain examples, the energy density is about 1 J/cm2 to
about 200 J/cm2,
more particularly about 1 J/cm2 to about 50 J/cm 2, e.g., about 2-20 J/cm2 or
4-10 J/cm2. The
exact shape and size of the energy delivered to the tissue may also vary with
the
configuration of the applicator. In certain embodiments, the energy has a
circular cross-
section with a diameter of about 1 mm to about 30 mm, more particularly about
2 mm to
about 20 mm, e.g., about 7-10 mm.
[00106] In accordance with certain examples, the system shown in FIGS. 14A and
14B may
be used to treat an infected nail. Alternatively or for use with the systems
shown in FIGS.
14A and 14B, a mold or insert configured to receive a toe, fingernail or the
like may be used
to position the nail for treatment. The mold or insert may be cast using the
patient's toe or
finger or may be a mold that is constructed based on the average size of
people's fingers or
toes. A side-view of an example of an insert is shown in FIG. 18. The insert
1800 includes a
top portion 1810 in thermal cormnunication with a base 1820. The base 1820 may
be
-28-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
configured with an adhesive to keep the insert 1810 from moving or sliding
during treatment.
In the alternative, the base may be configured as a heat sink or cooling
device to remove heat
from the toe or finger to prevent unwanted tissue damage. The base may be
configured to
receive a cooling agent, such as liquid nitrogen, dry ice, a frozen gel, ice,
cold water or other
suitable agent that can facilitate heat transfer to the base from the finger
or toe. In some
examples, the base may be configured to provide impedance matching to
facilitate more
uniform exposure of the nail to the energy. The insert 1800 may be used with
the system of
FIGS. 14A and 14B by placing the insert on the foot platform 1440 as shown in
FIG. 19. A
thin layer of adhesive 1910 may be placed between the foot platform 1440 and
the insert
1800 to prevent or retard movement of the insert 1800 during treatment. In
certain examples,
the container configured to receive a bolus 530 may be shaped similar to the
insert 1800 such
that proper positioning of the toe or finger is further facilitated.
[00107] In accordance with certain examples, the electromagnetic energy may be
delivered
to any portion of the nail. In some examples, the electromagnetic energy is
delivered to one
or more of the nail plate, the cuticle, the nail bed, or the nail root. In
certain embodiments,
the applicator may be positioned to first treat the nail bed and then move or
be moved to treat
some other portion of the nail, e.g., the nail root (which is typically called
the nail matrix),
cuticle or nail plate. In some embodiments, the width of the beam may be large
enough to
treat all areas of a nail simultaneously.
[00108] In accordance with certain examples, one or more naturally occurring
agents in the
nail or skin may be used to enhance treatment. For example, molecules in the
nail itself may
include, but are not limited to, a blood vessel, a wall of a blood vessel,
melanin, water,
collagen, a red blood cell, a white blood cell, hemoglobin, plasma,
interstitial fluid,
intracellular fluid, the disease causing organism, or any combination thereof.
Energy may
intentionally be used to cause absorption by the species in the nail, or the
species in the nail
may absorb energy incidental to the energy delivered for treatment.
[00109] In accordance with certain examples, one or more agents may be coated
or
otherwise disposed on the nail prior to treatment. Illustrative agents
include, but are not
limited to, dyes, chromophores, radiation absorption agents, metallic paints
and therapeutics.
These agents may be applied to absorb the electromagnetic energy to aid in
treatment or may
be used to absorb the electromagnetic energy to prevent exposure of certain
tissues to the
energy. Referring to FIG. 20A, an agent may be impregnated in a transfer sheet
2010 and
transferred to a nail 2005 by placing the transfer sheet on the nail 2005 and
applying pressure
to the transfer sheet with device 2020 to provide a coating 2030 on the nail
2005. Device
-29-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
2020 may be any suitable device that can apply pressure including, for
example, a stylus, pen,
metal, cotton swab, or plastic rod or the like. In another example, the agent
may be coated on
the nail by applying the agent with a cotton swab. For example and referring
to FIG. 20B, a
cotton swab 2050 may be used to dispose a coating of an agent 2060 on a nail
2055.
[00110] In certain examples, a therapeutic in combination with another agent
may be coated
or added to the nail prior to treatment. For example, one or more anti-fungals
or anti-
bacterials may be mixed with the agent and the mixture may be coated or
otherwise disposed
on the nail. In the alternative, an anti-fungal or anti-bacterial agent may be
chemically linked
to the agent and resulting composition may be disposed on the nail. Additional
methods for
applying therapeutics in combination with another agent will be readily
selected by the
person of ordinary skill in the art, given the benefit of this disclosure. In
some examples, one
or more cosmetic agent may be applied post-treatment to improve the appearance
of the
tissue. Illustrative cosmetic agents include, but are not limited to, ELONTM
Complex 38,
Mavala Ridge Filler and Nail Tek Foundation II Ridge Filler.
[00111] In accordance with certain examples, the energy delivered to the nail
may be
selected to traverse the nail plate and be absorbed by the nail bed and/or the
organism
infecting the nail to reduce heating of the nail plate. As the nail bed and
nail plate absorb
heat, it may remain heated for an extended period of time, which can lead to
unwanted injury
to the surrounding tissue. Tissue injury depends on temperature and on the
time at the
elevated temperature. In certain examples, the tissue may be heated to between
about 40 C
and about 80 C, more particularly about 43-70 C, e.g., about 50 C or 55 C.
In certain
examples, the organism infecting the nail is heated to an effective
temperature to either
deactivate the organism or to kill the organism while keeping the temperature
of the nail
tissue below an acceptable level to avoid permanent tissue damage, e.g.,
permanent tissue
damage to the nail bed.
[00112] In accordance with certain examples, the energy may be delivered to
the nail to treat
the entire nail at once or may be delivered as a focused beam to treat only a
portion of the nail
at a time. When the energy is delivered as a focused beam, it may be desirable
to move the
applicator to different areas on the nail to provide for more effective
treatment of the nail.
This movement may be done manually by the medical practitioner or may be
automated
using a motor, robotic arm or other devices that may be attached to the
applicator and can
effectuate movement. A map of the nail may be made and stored in a computer
system, and
the motor may be computer controlled to move the applicator over substantially
all surfaces
of the nail.
-30-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
[00113] In accordance with certain examples, one or more channels or holes may
be drilled
or otherwise made in the nail to facilitate delivery of the electromagnetic
energy, optionally
in combination with agents such as therapeutics, to tissue underlying the
nail. These channels
or holes may be drilled to minimize absorption of the electromagnetic energy
by the nail
itself. In one embodiment, a sample of the organism may be taken to determine
a wavelength
of energy at which the organism will absorb. The organism may be viewed under
a
microscope, e.g., with or without stain, or spores produced by the organism
may be used to
assist in the identification of the infectious organism. Many organisms
infecting the nail, e.g.,
the dermatophytes discussed herein, are observed to be an orange/brown color.
By selecting
a wavelength of about 400-550 nm, the operator can increase the amount of
energy absorbed
by the organism. In other examples, the entire nail may be removed and the
underlying tissue
may be treated with a selected electromagnetic energy to deactivate or kill
any remaining
infectious organisms.
[00114] In accordance with certain examples, the electromagnetic energy
delivered to the
nail may be microwaves or radio waves or the energy may take other forms, such
as sound
waves. For example, the energy source may be a radio frequency generator or a
microwave
generator to produce heat within a diseased nail to deactivate or kill the
organism. It is
believed that the infectious organism absorbs the microwaves, or radio
frequencies to a
greater extent than the nail tissue which results in heating or superheating
of the organism
and eventual deactivation or killing of the organism. It may be desirable to
capacitively
couple the applicator with the nail. In one embodiment, an adaptor that
substantially covers
the entire surface of the nail may be used optionally with a tuning box and a
bolus as
discussed elsewhere herein. In one example, the frequency used to treat the
nail is greater
than about 100 kHz, more particularly greater than about 1 MHz, e.g., about 10
MHz or more
or 300 MHz or more.
[00115] In certain examples, the energy source may be an sound generator such
as, for
example, a high intensity ultrasound source or a high power focused ultrasound
source.
Sound waves generated by the ultrasound generator may be used to heat the
infectious
organism to deactivate or kill the organism. An applicator configured to
deliver sound waves
may be impedance matched or impedance mismatched depending on the desired
results of the
treatment. Impedance mismatching of the applicator and the nail may be
desirable, for
example, to selectively target absoiption of the sound waves by the organism
rather than the
nail.
- 31 -
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
[00116] In accordance with certain examples, the exact frequency of the
treatment protocol
for the nail depends, at least in part, on the degree of infection, the
temperature used and the
like. In certain examples, treatment of the nail occurs daily, weekly, bi-
weekly, monthly,
semi-monthly, once every three months, once every six months or once per year.
In
embodiments where treatment is performed for prophylactic reasons, e.g., to
prevent
reoccurrence of the infection, treatment may be performed less frequently than
treatment for
an active infection. Also, as discussed in more detail below, by taking an
immediate culture
of the infectious organisms, efficacy of the treatment may be monitored more
rapidly than is
possible with existing oral administration of therapeutics.
[00117] In accordance with certain examples, many different types of adaptors
may be used
to provide electromagnetic energy to the nail. These adaptors may be single
use, e.g.,
disposable, or may be configured for multiple uses. In the configuration where
the adaptor is
designed for multiple uses, the adaptor may be constructed of suitable
materials that can
withstand chemical treatment and or sterilization equipment, such as an
autoclave. In
examples where the adaptor is configured for a single-use, the adaptor may be
a conductive
or non-conductive material that has sufficient strength for at least the
treatment period.
[00118] In certain examples, a sheet of metal or other conductive material may
be used to
dispose an applicator on a nail. An example of this is shown in FIGS. 21A and
21B. This
process is similar to the transfer sheet used to dispose an agent on a nail.
Referring to FIG.
21A, a transfer sheet 2110 may be placed on the nai12105. The transfer sheet
2110 includes
patterns 2120, 2122, 2124, which are geometrically similar to the shape of the
nail 2105 but
are of different sizes. In use, a pattern having a size that closely matches
the size of the nail
2105 is placed over the nail 2105, and device 2130, e.g., a stylus, applies
pressure to the
pattern to transfer material from the transfer sheet 2130 to the nai12105.
Transfer of material
provides a conductive coating that can be electrically coupled to the
applicator for treatment.
In another embodiment using a transfer sheet (FIG. 2113), the transfer sheet
may be
configured as a roll 2140. The roll 2140 may include different patterns which
can be
transferred using device 2130 to form a coating 2135 on the nail 2105.
[00119] In other examples, an adaptor may be created by placing a conductive
plate having
arms or strips over the nail as shown in FIG. 22A. The conductive plate 2210
may be placed
on the nail 2205 and the arms may be folded back to provide a shape that
conforms to the
shape of the nail. For example, an arm 2215 is shown as having been folded
back in FIG.
22A to the edge of the nail. The conductive plate may be electrically coupled
to applicator
prior to treatment.
-32-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
[00120] In another example, a conductive material such as a putty or gel may
be disposed on
the nail as shown in FIG. 22B. The conductive material 2255 may be disposed on
the nail
2250 using a swab, dropper, by hand or the like. An applicator 2260 may be
electrically
coupled to the conductive material 2255 by placing the applicator on top of
the disposed
conductive material 2255. The conductive material 2255 may be tacky to retain
the
applicator 2260 for a sufficient period to allow for treatment. For example, a
gel or a putty
may be used to provide a smooth surface over an irregularly-shaped or
dismorphic tissue, e.g.,
a disphormic nail plate or nail bed. In addition, a material may be used to a
large applicator,
e.g., one larger than the target tissue area, to provide a pathway for heat
transfer (EM waves)
through to only the target area.
[00121] In another embodiment, the conductive material may be painted on the
nail or
otherwise disposed on the nail. For example and referring to FIG. 23A,
conductive material
2315 may be disposed on the nail with a cotton swab 2320, or similar device,
by tracing the
nail 2310 with the cotton swab 2320. The disposed conductive material 2315 may
be
electrically coupled to an applicator for treatment. In another example, the
conductive
material may be loaded in a paint pen, or comparable device, and applied to
the nail. For
example, and referring to FIG. 23B, paint pen 2370 may be used to dispose
conductive
material 2360 on nai12350.
[00122] In another example, conductive strips may be disposed on the nail. For
example
and referring to FIG. 24A, metal strips, such as metal strips 2420 and 2422
may be disposed
on nail 2410. The ends of the conductive strips, shown at dotted line 2430,
may be trimmed
away prior to treatment to provide an adaptor of conductive strips that covers
the nail. At
least one of the conductive strips may be electrically coupled to the
applicator for treatment
of the nail 2410. In another embodiment, a form or mold may be used to dispose
a
conductive material on the nail. Referring to FIG. 24B, a form 2460 is placed
on the nail
2450 and is configured to rest around the edge of the nail surface. A
conductive material
2470 may be disposed on the nail 2450 and flow or move into the space of the
mold 2460.
Once set up or cured, the mold 2460 may be removed and the conductive material
2470 may
be electrically coupled to an applicator for treatment of the nail 2450.
[00123] In another embodiment, individual conductive elements may be placed on
a nail in a
sufficient amount and with suitable spacing to cover the nail surface. For
example and
referring to FIG. 25A, a series of small conductive circles, such as circles
2520 and 2522,
have been disposed on a nail 2510 in a sufficient amount to cover the entire
nail surface.
While shown as circles in FIG. 25A, other shapes, e.g., square, rectangular,
triangular, etc.,
-33-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
may be used and in some examples it may be desirable to use many different
types of shapes
to cover the nail surface, e.g., circles in combination with triangles. Once
the nail surface is
covered, one or more of the conductive elements may be electrically coupled to
an applicator
to provide treatment to nail 2510. In another example, a coil of conductive
material may be
placed on the nail. For example and referring to FIG. 25B, a coi12560 of
conductive material
may be placed on a nai12550. The coil 2560 is electrically coupled to an
applicator through
interconnect or cable 2570. Electromagnetic energy may be delivered to the
coil 2560 for
treatment of the nail 2550. A layer of adhesive or a conductive material may
be placed
between the nai12550 and the coi12560 to enhance treatment even further.
[00124] In accordance with certain examples, the adaptor may be configured as
a multi-layer
structure. For example and referring to FIG. 26A, an adaptor 2600 includes an
adhesive
backing 2610, an adhesive flex-circuit 2620 with surface-mounted thermistors
and copper
traces 2630 and having a plug end 2640, an adhesive pad 2680, a copper sheet
2670 with a
hole 2650 and a copper block 2660. The copper block 2660 may be attached using
solder,
may be diffusion bonded or may be attached to the copper sheet using other
methods or
materials. In operation, a trace of the toe may be performed by placing a
piece of clear tape
on the toe and tracing the shape of the nail bed area, which may or may not be
covered all or
in part by nail plate. The clear tape may be transferred to the adaptor 2600,
which may be
trimmed to the traced shape. The trimmed adaptor may then be placed on a nail
bed area and
the copper block 2660 may be bent up to change the angle of the copper sheet
2670 and the
flex circuit 2620. For example and referring to FIG. 26B, apparatus 2600 has
been placed to
cover the nail bed area, which may or may not be covered all or in part by
nail plate, on the
big toe of foot 2690. The apparatus has been positioned on the nail bed area
such that it sits
about 1 mm away from the exposed skin on the big toe, as pointed out by arrow
2692. The
copper sheet 2670 and copper block 2660 may be coupled to a pin 2696 in
applicator 2694, as
shown in FIG. 26B. The plug end 2640 of the flex circuit 2620 may be
electrically coupled
to interconnect 2698 and treatment may be initiated.
[00125] In other configurations, the adaptor 2600 may be trimmed such that it
overlies the
entire nail bed area and exceeds the nail bed area by about 1 mm on all sides.
An example of
this configuration is shown in FIG. 27, where adaptor 2715 has been placed
over the nail bed
area of the big toe of foot 2710. The adaptor is slightly larger than the
shape of the nail bed
area of the big toe, e.g., 1-2 mm larger on all sides, as pointed out by arrow
2715. The copper
sheet 2740 and copper block 2750 may be coupled to pin 2760 in applicator
2730. The flex
circuit 2720 may be electrically coupled to interconnect 2730 and treatment
may begin.
-34-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
While the illustrative examples shown in FIGS. 26A, 26B and 27 were described
in reference
to a trace of the toe with tape to trim the adaptor to size, the tape trace of
the toe may be
omitted. Instead, the adaptor may be placed on and/or beyond the nail bed area
of the toe and
a trace of the nail bed area of the toe may be performed on the adaptor
itself. The adaptor
may be removed and trimmed to size and placed back on the nail bed area of the
toe. The
adaptor may be electrically coupled to an applicator and treatment may be
initiated. In
examples where a tape trace is omitted, each layer of the adaptor may be
trimmed, e.g., the
adhesive backing, copper sheet and copper block may be trimmed, or one or more
layers of
the adaptor may be left untrimmed. For example, it may be desirable to leave
the adhesive
backing whole to provide better contact with the surface of the nail, while
the copper sheet
and copper layer may be trimmed to size. Additional configurations that use a
flex circuit
will be readily apparent to the person of ordinary skill in the art, given the
benefit of this
disclosure.
[00126] In accordance with certain examples, the adaptors or applicators
disclosed herein
may be configured with one or more features that render them usable only once.
For example
and referring to FIG. 28A, a connector 2810 may be used such that the flex
circuit 2820 is
electrically coupled to the applicator. Opening of the connector 2810 results
in breaking of
the electrical connection, which cannot be restored by closing the connector
2810. As the
flex circuit is an integral part of the adaptor, the adaptor is not capable of
being re-used. Such
single use adaptors reduce the likelihood of cross-contamination. Another
example of a
single use adaptor is shown in FIG. 28B. In this illustration, the adaptor
2830 is electrically
coupled to the applicator 2840 through a spring 2845. The spring 2845 may be
inserted in the
hole in the adaptor 2830 and onto the surface of adaptor 2830 to provide
electrical contact
between the applicator 2840 and the adaptor 2830. When the spring 2845 is
removed, the
adaptor is damaged so that it may not be re-used again. Another embodiment of
a single-use
adaptor is shown in FIG. 28C. The applicator includes a post or projection
2855 that
punctures adaptor 2860 at area 2862 during removal of the adaptor 2860 from
the applicator.
This puncture prevents electrical coupling of the adaptor to the applicator.
Other
configurations and features that render an adaptor suitable for only a single
use will be
readily selected by the person of ordinary skill in the art, given the benefit
of this disclosure.
[00127] In accordance with certain examples, iontophoretic or electrokinetic
delivery of a
compound may be used in combination with the devices, systems and methods
disclosed
herein to deliver a therapeutic to the tissue. In some examples, the adaptor
may be
configured for electromagnetic energy delivery and for iontophoresis or
electrokinetic
-35-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
delivery of a compound, such as a therapeutic. While not wishing to be bound
by any
particular theory or this example, iontophoresis is a process whereby a
compound is
introduced into a tissue or a cell by application of an electric field.
Electrokinetic delivery
involves iontophoresis and also involves electroosmosis. Electroosmosis is the
bulk fluid
flow associated with ion transport by an electric field. An illustrative
device for
iontophoresis or electrokinetic delivery of drugs is shown in FIG. 29. The
device 2900
includes a cathode 2910, an anode 2920 each connected to a power supply 2930
(which may
be a DC or an AC power supply). The cathode 2910 and the anode 2920 are
configured as
plate electrodes in FIG. 29, though other configurations are possible. The
anode 2920 and
cathode 2910 are coupled with the tissue 2905 through carriers 2945 and 2940,
respectively.
Carrier 2945 typically includes a compound to be delivered to the tissue 2905,
whereas
carrier 2940 typically includes a saline solution or some other salt solution.
During operation,
electrons flow from the cathode to the anode and the electric field drives the
negatively-
charged compound from the carrier 2945 and into the tissue 2905. In the
situation where an
alternating current source is used, suitable circuitry may be implemented to
drive the
compound into the tissue. Illustrative devices and circuitry may be found, for
example, in
U.S. Patent No. 7,127,285.
[00128] In accordance with certain examples, the iontophoresis or
electrokinetic delivery
device may be integrated with the adaptors disclosed herein. For example and
referring to
FIG. 30, an adaptor includes a metal plate 3005 configured to deliver
electromagnetic energy
from electromagnetic energy source 3010 through cable 3015 and to a tissue,
e.g., to deliver
microwaves to a tissue. The adaptor also includes a first electrode 3020 and a
second
electrode 3025 connected to a power supply 3030. In operation, the electrodes
3020 and
3025 rest atop a carrier that is contact with the tissue. As current is
applied to the electrodes
3020 and 3025, a therapeutic in the carrier may be delivered to the tissue or
delivered to an
area near the tissue. Electromagnetic energy may be simultaneously delivered
to the tissue
or may be delivered to the tissue before or after the therapeutic is
delivered. In some
examples, iontophoresis or electrokinetic delivery is used to deliver an agent
that is taken up
by the infectious organism and that absorbs the electromagnetic energy. This
uptake
followed by application of electromagnetic energy results in additional
heating or
superheating of the organism to deactivate or kill the organism.
[00129] In accordance with certain examples, the nature of the compound
delivered to the
tissue depends at least in part on the organism infecting the tissue. In
certain examples, the
compound may be an antibiotic, an anti-fungal or an antiviral such as, for
example,
-36-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
ketoconazole, nystatin, griseofulvin, flucytosine, abacavir, adefovir,
amprenavir,
azidothymidine, behenyl alcohols, such as n-docosanol, Abreva0, brivudin,
cidofovir,
delaviridine, didanosine, doxorubican, efavirenz, famciclovir, fluorouracil, 5-
FU, gancyclovir,
indinavir, terbinafine HCI, Lamisil0, lamivudine, lobucavir, Lotrimin0,
methotrexate,
miconazole, Micatin0, nelfinavir, nevirapine, ribavirin, ritonavir,
saquinavir, sorivudine,
stavudine, tacrolimus, triamcinolone acetonide, trifluridine, valaciclovir,
zalcitabine or
combinations thereof. In some examples, the compound may be a non-steroidal
anti-
inflammatory drug (NSAID) such as, for example, ibuprofen or the like. In
other examples,
the compound may be a vitamin or co-factor such as Vitamin A, Vitamin E,
Vitamin B12 or
other vitamins or compounds commonly found in nutritional supplements.
[00130] In accordance with certain examples, electrophoresis or
dielectrophoresis may be
used with the treatment methods and devices disclosed herein.
Dielectrophoresis uses a
gradient of an electric field to drive uncharged molecules in the desired
direction; these
uncharged molecules are desirably polar, but they are not necessarily ions, as
is the case with
typical electrophoresis. Dielectrophoresis may be particularly useful where an
agent to be
delivered is polar, or has a dipole moment, but is not charged.
[00131] In accordance with certain examples, the methods and devices disclosed
herein may
be used to provide rapid feedback to assess the efficacy of treatment. It may
take nine
months or more to assess the efficacy of conventional treatment of tissues,
i.e., oral
administration of anti-fungals, especially where the tissue is keratinized
tissue. For example,
oral administration of terbinafine for three-six months or more is typically
prescribed by a
physician to treat onychomycosis. The efficacy of such treatment cannot be
assessed until
the nail grows out, which can take nine months or more. In the methods
disclosed herein,
subsequent to treatment, a microbiological culture may be obtained to assess
the effectiveness
of the treatment. In situations where treatment is effective, fewer or no
microbiological
colonies will be observed as compared to a control value. In situations where
treatment is
ineffective, the number of microbiological colonies will be similar to those
observed in a
control sample. In cases where treatment is ineffective, the patient may
return for subsequent
treatment or may be placed on a different type of treatment. By using this
assessment method
as feedback to assess treatment, the effectiveness of treatment may be
increased and overall
treatment time may be reduced. In certain examples, the methods disclosed
herein may be
used to assess whether treatment is effective within or less than one month
after the first
treatment. In some examples, the effectiveness of treatment may be assessed in
two weeks or
-37-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
less. Such rapid feedback may be especially useful in the treatment of nail
infections where
nail growth may take several months.
[00132] In accordance with certain examples, a method of treating a skin or
nail infection is
disclosed. In certain examples, the method includes delivering electromagnetic
energy to the
infected skin or nail, and culturing organisms infecting the skin or nail to
assess efficacy of
treatment. The electromagnetic energy may be delivered using any of the
devices, system
and methods disclosed herein. The organisms may be cultured using conventional
microbial
culture techniques, such as those found in Bergey's Manual of Determinative
Bacteriology.
Based on the level of organisms in the culture, the efficacy of treatment may
be determined
with the goa.l of the treatment being reduction in the number of cultured
organisms present or
the entire eliminatioi-A of the infectious organisms.
[00133] In accordance with certain examples, it may be desirable to subject
the tissue to one
or more pre-treatment steps. Pre-treatment steps include positioning of the
tissue,
sterilization of the tissue, e.g., using alcohol pads, washing of the tissue
with soap, betadine
or the like. In certain examples, the tissue may be debrided prior to
treatment to remove any
dead cells or thickened tissue (e.g. hyperkeratotic nail). In some examples,
the onycholytic
portion of the nail plate may be trimmed or clipped back prior to treatment.
Additional pre-
treatment steps will be readily selected by the person of ordinary skill in
the art, given the
benefit of this disclosure.
[00134] In accordance with certain examples, the methods disclosed herein may
also be used
to disinfect a hood or other culture transfer device. For example, one or more
applicators
may be placed in a laminar flow hood and switched on to deactivate or kill any
organisms
living in the laminar flow hood prior to performing tissue culture or cell
culture in the laminar
flow hood. In certain examples, the applicator may be configured for insertion
in a culture
vessel to sterilize the culture vessel prior to introduction of any cells.
[00135] In accordance with certain examples, a device configured to treat all
infected nails
simultaneously is provided. In certain examples, the device may include a
plurality of
applicators where each applicator is configured similar to or the same as one
or more of the
applicators disclosed herein, e.g., the applicator shown in FIG. 5. Each
applicator may be
mounted or slidably fixed to a system similar to the one shown in FIGS. 14A
and 14B. In the
configuration where the applicators are slidably fixed to the system, each
applicator may be
moved perpendicular to the foot and placed in contact with a nail and nail bed
to be treated.
In certain examples, the device may include two or more applicators, e.g.,
three, four or five
applicators. Each of the applicators may function independent of the other,
e.g., different
-38-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
energy levels may be applied, or a single energy level may be provided to each
applicator. In
certain examples, a first applicator may be configured to provide a first type
of energy, e.g.,
ultraviolet light, and a second applicator may be configured to provide a
second type of
energy, e.g., microwaves. The person of ordinary skill in the art, given the
benefit of this
disclosure, will be able to design systems that include multiple applicators.
[00136] In accordance with certain examples, a device sized and arranged to
treat the hooves
of a non-human mammal is provided. In certain examples, the non-human mammal
is a
horse or a sheep. In certain embodiments, the applicator may be sized and
arranged to treat
the entire hoof of the non-human mammal, e.g., the hoof may be placed on or in
an applicator
that provides electromagnetic energy to all surfaces of the hoof. In certain
examples, the
electromagnetic energy delivered to the hoof is ultraviolet, visible or
infrared light,
microwaves, or radio waves. Other energies may also be delivered. In some
examples, a
plurality of applicators may be used to provide treatment to each hoof of a
non-human
mammal to reduce the time the non-human mammal must remain stationary. Other
configurations for treating a non-human mammal using the devices, systems and
methods
disclosed herein will be readily selected by the person of ordinary skill in
the art, given the
benefit of this disclosure.
[00137] In accordance with certain examples, a device configured to improve
the appearance
of a tissue and configured for use in the home is provided. To minimize
overexposure of the
tissue, the device typically includes a timing circuit to provide
electromagnetic energy for a
selected period. The period and energy level that is provided may be based on
cumulative
patient data such that the selected period provides treatment for the largest
number of subjects.
In an alternative configuration, the user may place a temperature sensor on
the tissue to be
treated and the treatment may be halted when the tissue reaches a treatment
temperature
programmed into the device. The device may be configured with safety features
that prevent
use of the device unless the teinperature sensor is placed on the tissue,
e.g., the skin. The
device may be configured to operate off of 110V power and may include cooling
features
such as a fan, heat sink or the like. The device may be configured to use any
form of
electromagnetic energy disclosed herein, e.g., ultraviolet, visible, infrared,
microwaves, radio
waves, etc. The device may include an on/off indicator, a safety shut off
switch and the like.
[00138] In accordance with certain examples, a kit comprising at least one
adaptor, a bolus
and instructions for using the adaptor and the bolus is provided. In certain
examples, the
adaptor may be any of the adaptors disclosed herein. In some examples, the
adaptor may be
part of an applicator which is included in the kit. In certain examples, the
bolus of the kit
-39-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
may be selected to provide impedance matching of the tissue and the adaptor
for more
uniform delivery of electromagnetic energy to the tissue. The instructions
included in the kit
may include any of the illustrative protocols discussed herein or other
suitable protocols that
may be used with the devices and methods disclosed herein.
[00139] Several specific examples are disclosed below to facilitate a better
understanding of
the technology described herein.
Example 1
[00140] An applicator for use in treating tissue of the foot or hand was
constructed as
follows:
[00141] In this Example, the applicator consisted of a modified coaxial cable,
a tuning box,
and an end-cap. The modified coaxial cable consisted of an aluminum tube of
inner diameter
1.00" and outer diameter 1.25" (MSC Industrial Supply Co.). A 1" long portion
of the outer
conductor was removed with the removed portion forming an L-shape when viewed
from the
side. The inner conductor consisted of a brass rod of outer diameter 0.375"
(MSC Industrial
Supply Co.). See, e.g., FIG. 6. These components are machined by standard
operations
(lathing, drilling, tapping) to permit the unmodified end of the coaxial cable
assemble to
connect to a standard female microwave N-type connector (Pasternack
Enterprises, Inc).
[00142] The end modified by removal of the L-shaped piece was surrounded by a
second
component, the tuning box, which was a modified cone of length 1.85" and
diameter 2.68'",
shown in cross-section in FIG. 6 and in perspective in FIG. 5 (part 510). In
this Example, the
tuning box was filled completely with water to provide a low impedance path
for electric
fields extending from the end cap (described below) back to the outer
conductor. The tuning
box was formed of Duraform PA plastic by a rapid prototyping process
(Quickparts, Atlanta,
GA). The internal structure of the tuning box was such that walls of Duraform
PA plastic of
thickness 0.080" separated the internal chamber filled with water from the
inner conductor
and from the outer conductor. In this way, the tuning box formed a self-
contained chamber
filled with water that slides into place onto the modified end of the coaxial
cable assembly.
When in position, the bottom of the cone extends 10 mm beyond the farthest
reach of the
outer conductor, shown in cross-section in FIG. 6.
[00143] The endcap was the third component of the applicator. It was made by
cutting
copper foil of thickness 0.005" into a rectangle of 19 mm width (medial-
lateral dimension of
toe nail) and 14 mm length (distal-proximal dimension of toe nail); the
corners were rounded
to a radius of 1 mm. The copper foil rectangle was soldered to an axial block
made of brass
-40-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
with dimensions 0.125" width, 0.300" height, and 0.150" axial length, which
was soldered to
a transverse block made of brass with dimensions 0.520" width, 0.300" height,
and 0.0625"
axial length. A steel pin of diameter 0.057" was force fitted into the
transverse brass block.
This assembly was part 520 (all metal parts from MSC Industrial Supply Co.).
The entire
assembly was plated with 0.0005" thick tin by the conventional process.
[00144] To receive the endcap assembly, a mating receptacle (Mill-Max, Inc.)
was force-
fitted into the exposed end of the inner conductor, which was flush with the
base of the tuning
box 510. By design, the pin of the endcap assembly made a press-fit into this
receptacle, so
that the endcap assembly may be placed and removed with finger force, as
desired.
[00145] An adhesive layer was added beneath the plated copper foil of the
endcap to fix it to
the toenail of a patient. The adhesive was a double thickness of a Curad Scar
Therapy pad
(Walgreen's drug store). One adhesive surface adhered to the foil endcap, the
other adhered
to the toenail, and the two non-sticking surfaces were secured to each other
with a
cyanoacrylate glue.
[00146] Beneath the toe of a patient was placed a bolus of high water content
to distribute
the electric fields more evenly (see FIG. 6). In this Example, the bolus was
cut from an
ultrasound gel pad (AquaFlex, Parker Laboratories) to form a quadrilateral, as
shown in FIG.
6. The left side in FIG. 6 was 25 mm high, the bottom was 20 mm long, and the
right side
was 20 mm high; the width (dimension into page) was 20 mm. A container 530
held the
bolus in place beneath the toe.
Example 2
[00147] A system that used the applicator of Example 1 to deliver microwave
energy to a
nail of the foot or hand was constructed as follows:
[00148] The system contained a 915 MHz, 25 Watt microwave generator that was
designed
and manufactured (Microwave Support Systems, Nasliua, NH) within a 1" x 10" x
12" sub-
assembly housing. It was built into a metal chassis based on CAD
specifications (Product
Insight Acton, MA). The metal chassis was fabricated by a sheet metal shop,
(New England
Fabricated Metals, Leominster, MA) and the microwave energy was transmitted to
an
external SMA-type connector via semi-rigid copper coaxial cable. A brick of
two fiber optic
thermometry probes (Luxtron, Santa Clara, CA) resided in the chassis and were
cabled to exit
the chassis to be affixed to the target tissue. Also built into the system
chassis was a simple
micro-controller display (Mosaic Industries, Newark, CA) which was programmed
to execute
the software of the flowcharts shown in FIGS. 31A and 31B or the flowcharts
shown in FIGS.
-41-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
32A and 32B. The thermometry probes, the micro-controller and the microwave
generator
received electrical power and electrical isolation from a commercial power
supply (Condor
DC Power Supplies, Inc, Ventura, CA) that is the fourth sub-assembly in the
system chassis.
A custom designed power controller printed circuit board received 12V direct
current from
the power supply and enabled power distribution to the 5V and 12V internal
circuitry.
Example 3
[00149] Using the applicator of Example 1 and the system of Example 2,
treatment was
performed on eight subjects displaying fungal infection of the large toe nail
as follows. The
patient's large toe nail was prepared using a double-hinged bone cuter to clip
back the
onycholytic nail. The clipped nail and the toe were washed with isopropyl
alcohol and air
dried.
[00150] To determine the maximum energy level that each subject could sustain,
the
following protocol was used, as shown in FIG. 31. A lower case "t" refers to a
time and an
upper case "T" refers to a temperature. Referring to FIG. 31, the system was
calibrated by
first installing the patient at step 3110. Once the patient is positioned, the
start button was
pressed at step 3115. A first power value PTX was selected at step 3120 by the
operator. In
the next several steps, the energy level was optimized. The temperature To was
set at step
3125 to the toe temperature Tt e and a five second delay occurred. The
temperature T5 was
set at step 3130 to the toe temperature TTOe and a ten second delay occurs.
The temperature
T15 is set at step 3135 to the toe temperature TtOe. Using the temperature
values at T15 and T5,
a dT/dt a, value was obtained by subtracting the T5 value from the T15 value
and dividing by
the time (10 seconds). This value represents the slope of the temperature with
respect to time.
A suitable operating range for dT/dt a, is about 0.35 to about 0.45 C/second.
The dT/dtcai
value also reflects how well the nail and the applicator are coupled and how
much energy is
being supplied. If the dT/dt al value is less than or equal to a dT/dt,,,aX
value at step 3140 and
is greater than or equal to a dT/dt,r,;,, value at step 3145, then the system
is ready to initiate
treatment. If, however, the dT/dt,ai value is greater than dT/dt,,,ax or the
dT/dt,ai is less than
dT/dt,r,i,,, and less than three calibration tries at step 3170 have been
attempted, a new power
setting 3160 is calculated and after the toe cools 3150, the calibration
process is repeated
beginning at step 3125. If three calibration tries have been attempted, then a
calibrate retry
condition may be generated at step 3170 so that user input may be prompted at
step 3180. If
the power exceeds a maximum power conditions at step 3175, user input may also
be
prompted at step 3180.
-42-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
[00151] Once the system is calibrated or once the user inputs a desired power
level,
treatment was ready to begin. The treatment procedure used is shown in flow
chart form in
FIG. 32. The temperature T,,,ax was first set to the target temperature
TTARGET at step 3202.
This operation occurred either from the calibration shown in FIG. 31 or by
user input at step
3204. Treatment was started at step 3206 at microwave power PTx and continued
until the
temperature of the toe TTOE equaled or exceeded the maximum temperature T,,,'
at step 3208.
If TTOE did not exceed T,,,aX, then the setting was increased by user input at
step 3210 or
treatment was continued if Tmax was not less than Ttarget at step 3212.
Treatment was
continued until the total treatment time ttreat,,,eõt was reached at step
3214. T,reattõeõt was set
between 5 and 20 minutes. For clarity, tl,eat,,,eõt is the time the microwave
power was
delivered to the nail. The total time from initiation of treatment to the
completion of treatment
was much greater than ttreat,,,eõt. Once t,reat,,,eõt was reached, the
microwave power was turned
off at step 3216 to allow the toe to cool. Once the cooling time teOOl at step
3218 was reached,
treatment was considered complete at step 3220. If T,,,aX was less than
Ttarget at step 3212,
then user input at step 3222 increased Tmax by Tad,jõ5, at step 3224. If
TadjõSt caused T,,,ax to be
greater than Ttarget at step 3226, then T,,,ax was set to Ttarget at step 3228
and treatment
continued at step 3214. If Ttoe was not greater than or equal to T,,,aX, then
the sampling
temperature Tsa,,,ple was set to Tt0e at step 3230 or 3231 and the microwave
power was turned
off for a time thold. When Tmax was less than Ttarget, a user provided input
at step 3234 and
treatment began again. If T,,,aX was greater than Ttarget, a user provided
input at step 3236 to
reduce the temperature by decreasing Tsample by Tad.iõSt 3238. In certain
instances, user input
at step 3240 was provided to end treatment at step 3242. In other instances, a
holding time
thold elapsed at step 3244. Once the holding time thold was reached, the
system determined if
the temperature of the toe TfOe exceeded a maximum temperature Tmax at step
3246. If a
maximum holding time was reached thold_,,,aX at step 3248, then an error was
generated and the
system returned to user input at step 3204.
[00152] In the situation where TIOe was greater than or equal to Tinax,
treatment was
suspended at step 3250 for a holding time thold. After holding time tnold and
if T,,,aX was less
than T,a,.get at step 3252, a user input at step 3254 was provided to increase
the temperature. If
T,,,aX was not less than T,a,get at step 3252, then a user input at step 3256
was provided to
decrease the temperature from TlõaX to T,,,aX minus T,-educe at step 3258.
After a holding time
thold had elapsed at step 3260, the system determined if TY0e was greater than
Tmax at step 3262.
If TYOe was greater than T,,,aX, then the system determined if a maximum
holding time thold-MAx
at step 3264 had elapsed. If so, an error at step 3266 was generated and user
input was
-43-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
required before treatment was reinitiated. If a maximum holding time th ld-
,,,ax at step 3264
had not elapsed, then the system returned to step 3252 and determined if
T,,,aX was less than
Ttarget. In operations where user input was required to increase or decrease
the temperature,
the temperature was increased or decreased in 1 C increments until a
satisfactory result was
achieved so that treatment could continue.
[00153] Total treatment time (the time that microwave power was applied) was
between five
and twenty minutes. Photographs showing the toe nail before and after
treatment are shown
in FIGS. 33A-33E. Referring to FIG. 33A, a fungal line of 1.6 mm from the nail
bed was
used as a baseline prior to any treatment. 91% of the nail was infected with
the fungus prior
to treatment. 3 months post treatment (FIG. 33B), the fungal line was 2.6 mm
from the nail
bed, and only 54% of the nail remained infected. The treatment reduced the
amount of nail
infected by 37%.
[00154] In another subject, a fungal line of 1.6 mm from the nail bed was used
as a baseline
prior to any treatment (FIG. 33C). 83% of the nail was infected with fungus
prior to
treatment. 4 months post treatment (FIG. 33D), the fungal line was 3.4 mm from
the nail bed,
and only 66% of the nail remained infected. The treatment reduced the amount
of nail
infected by 17%.
[00155] In another subject, a fungal line of 1.0 mm from the nail bed was used
as a baseline
prior to any treatment (FIG. 33E). 91% of the nail was infected with fungus
prior to
treatment. 5 months post treatment (FIG. 33F), the fungal line was 3.0 mm from
the nail bed,
and only 52% of the nail remained infected. The treatment reduced the amount
of nail
infected by 39%.
[00156] A graph showing the temperature of various portions of the nail is
shown in FIG. 34.
The total treatment time was five minutes and the total time for the procedure
was about 720
seconds. The maximum temperature set by the subjective tolerance of the
patient was 51 C.
As can be seen in the graph, the tissue temperature rises and falls as the
microwave power is
switched on and off, respectively. This procedure allowed the temperature
under the nail to
fluctuate between about 47 C and 49 C, which is believed to be a safe
temperature range to
avoid any permanent tissue damage. It will be recognized by the person of
ordinary skill in
the art, given the benefit of this disclosure, that a higher temperature
range, e.g., 53-55 C,
may be used depending on the tissue selected for treatment.
[00157] Referring now to FIG. 35, another example of a temperature profile is
shown where
the maximum temperature, set by patient tolerance, was 46 C. When comparing
the graph of
FIG. 34 with that of FIG. 35, the overall procedure time was longer when a
lower T,,,ax was
-44-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
used (1200 seconds when T,,,aX was 46 C versus 720 seconds when T,,,aX was 51
C) even
though the total treatment time was five minutes in both instances.
Example 4
[00158] The treatment effectiveness may be assessed immediately post-treatment
using any
number of fungal sample analysis methods (mycological culture, dermatophyte
test medium,
electron microscope, etc.). Using the method of mycological culture, fungal
samples were
collected to screen subjects for positive culture prior to inclusion in a
feasibility study.
Subsequently, 10 great toes were determined to be infected with T. rubrum as
confirmed by
positive culture assessed by an independent mycology lab. The 10 toes were
treated using the
protocols described in reference to FIGS. 31 and 32, and/or FIGS. 36-38, and
fungal samples
were collected again immediately post-treatment. The samples were sent to the
independent
mycology lab for culture, incubation and assessment 60% of the samples taken
were
negative for fungal growth after the culture incubation period, thus providing
an early
indicator of the effectiveness of the treatment.
Example 5
[00159] A suspension of dermatophyte Trichophyton Rubrum ATCC 28188 was
inoculated
with human nail fragments as a nutrient source. Aliquots of this suspension
were applied to a
sterile filter disc and sealed in a protective envelope. A randomly selected
sample of these
infected discs was chosen as controls, and the rest exposed to treatment
conditions using the
apparatus and conditions described in Examples 1 and 2 above. 86% of infected
discs treated
at temperatures between 47-53 C had no fungal growth after treatment while
only 7% of the
control samples (that did not receive any treatment) had no fungal growth.
Example 6
[00160] An additional protocol may be used in place of, or with, the protocol
described in
Example 3 above. The additional protocol is shown as flow charts in FIGS. 36-
38.
[00161] Referring to FIG. 36, the system may be calibrated by first installing
a patient at
step 3610. The patient presses a button to start the calibration protocol at
step 3615. An
initial power of the electromagnetic energy is set at step 3620. A first power
value PTX is
selected at step 3620 by the operator. In the next several steps, the energy
level may be
optimized. If TTOE was greater than TCAL START at step 3622, then a waiting
period at step
3655 occurred. If TTOE was not greater than TCAL START, then the temperature
To was set at
-45-
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
step 3625 to the toe temperature TtOe and a five second delay occurred. The
temperature T5
was set at step 3630 to the toe temperature TtOe and a ten second delay
occurs. The
temperature T15 is set at step 3635 to the toe temperature TtOe. Using the
temperature values
at T15 and T5, a dT/dt al value was obtained by subtracting the T5 value from
the T15 value and
dividing by the time (10 seconds) at step 3635. This value represents the
slope of the
temperature with respect to time. A suitable operating range for dT/dtcal is
about 0.35 to
about 0.45 C/second. The dT/dt al value also reflects how well the nail and
the applicator are
coupled and how much energy is being supplied. If the dT/dt al value is less
than or equal to
a dT/dt,,,a,t value at step 3640 and is greater than or equal to a dT/dt,,,;,,
value at step 3645, then
the system is ready to initiate treatment at step 3650. If, however, the
dT/dt,,a, value is greater
than dT/dt,,,aX or the dT/dt,a, is less than dT/dt,,,i,,, and less than five
calibration tries at step
3670 have been attempted, a new power setting is calculated at step 3665 and
after the toe
cools at step 3655, the calibration process is repeated beginning at step
3625. If five
calibration tries have been attempted, then a calibrate retry condition may be
generated at
step 3675 so that user input may be prompted at step 3685. If the power
exceeds a maximum
power conditions at step 3660, user input may also be prompted at step 3185
and treatment
may be interrupted at step 3690.
[00162] Once the system is calibrated treatment may begin as shown in FIG. 37.
The
treatment protocol shown in FIG. 37 is based on a predetermined treatment
time, whereas the
treatment protocol shown in FIG. 32 is based on a predetermined time that the
energy is
actively delivered to the target. Referring to FIG. 37, from the calibration
protocol 3650, the
maximum treatment temperature TMAX is set to the target temperature Tta,g t at
step 3702. The
energy power is also enabled at step 3702, and a timer TX is started at step
3702 as well. The
buttons on the user interface may also be hidden at step 3702 to prevent the
user from
changing the treatment parameters. If the toe temperature TTOE does not exceed
or is not
equal to the maximum temperature TMAX at step 3704, then the system proceeds
to step 3706.
If the toe temperature TTOE does exceed or is equal to the maximum temperature
TMAx at step
3704, then the power is turned off at step 3728. The user controls may also be
displayed at
step 3728 and a timer txoLO-Max is started. At step 3706, if the toe
temperature TTOE is not
less than or equal to the maximum temperature minus a change in temperature
TDELTA.
TDELTA is selected to maintain an average temperature +/- 1 C. then the system
proceeds to
step 3710. If at step 3706 the toe temperature TTOE is less than or equal to
the maximum
temperature minus a change in temperature TDELTA, then the power is turned on
at step 3708,
a txor.D-Niax timer is stopped and treatment begins and continues until the
timer TX expires at
- 46 -
CA 02692599 2010-01-04
WO 2009/009383 PCT/US2008/069053
step 3714 and treatment is considered complete at step 3716. If at step 3710,
the timer txoLD-
MAx has expired, then the system proceeds to step 3712 and treatment is
interrupted and an
error may be generated. If at step 3710, the timer txoLD_MAX has not expired,
then the system
proceeds to step 3714. If the TX timer has not expired, then the system
proceeds to step 3718
where user input may be required or the system may return at step 3704 for
treatment. If user
input is required, the user may select to stop or pause the treatment, and the
power is turned
off at step 3720, a timer tHOLD_MAX is started, a sample temperature Tsa nple
is set to the toe
temperature TtOej and treatment is paused at step 3722. The system may then
proceed to step
3724 as shown in FIG. 38. If a user chooses to reduce the temperature at step
3718, then the
maximum temperature TMAx may be adjusted by TADJUST at step 3762 and the
system may
return to step 3704 for treatment. If the user desires to increase the
temperature at step 3718,
then the system may increase the maximum temperature TMAx by TaDiusT, and if
TMAX is not
equal to the target temperature TTARGET at step 3732, the system may return to
step 3704 for
treatment. If the TMAX is equal to the target temperature TTARGET, the user
input buttons may
be hidden at step 3734, and the system may return to step 3704 for treatment.
[00163] Referring to FIG. 38, if treatment is paused at step 3724, then the
system may
display a message at step 3802. If the timer TX has expired at step 3804, then
treatment is
complete at step 3806. If the timer TX has not expired at step 3806, then the
system proceeds
to step 3808 for user input. If the user selects to end treatment, then the
system proceeds to
step 3810 and treatment is interrupted. If the user selects to continue
treatment, then the
system proceeds to step 3812 where the power is turned on. Treatment is
continued at step
3814 and the system returns to step 3650 and proceeds through the protocol
described above
in reference to FIG. 37.
[00164] When introducing elements of the examples disclosed herein, the
articles "a," "an,"
"the" and "said" are intended to mean that there are one or more of the
elements. The terms
"comprising," "including" and "having" are intended to be open-ended and mean
that there
may be additional elements other than the listed elements. It will be
recognized by the person
of ordinary skill in the art, given the benefit of this disclosure, that
various components of the
examples can be interchanged or substituted with various components in other
examples.
[00165] Although certain aspects, examples and embodiments have been described
above, it
will be recognized by the person of ordinary skill in the art, given the
benefit of this
disclosure, that additions, substitutions, modifications, and alterations of
the disclosed
illustrative aspects, examples and embodiments are possible.
-47-