Note: Descriptions are shown in the official language in which they were submitted.
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
ABLATION IN THE GASTROINTESTINAL TRACT TO ACHIEVE HEMOSTASIS AND
ERADICATE LESIONS WITH A PROPENSITY FOR BLEEDING
CROSS REFERENCE TO RELATED APPLICATIONS
[001] This application claims priority to US Provisional Application No.
60/958,566, entitled
"Non-Barrett's Mucosal Ablation Disease Targets" by Utley, Wallace and
Gerberding, as filed on July
6, 2007.
[002] This application incorporates in entirety commonly assigned US Patent
Application Ser.
No. 10/370,645 entitled "Method of Treating Abnormal Tissue in the Human
Esophagus", filed on
February 19, 2003, and published as US 2003/0158550 on August 21, 2003, and US
Patent
Application ser. No. 11/286,444 entitled "Precision Ablating Method", filed on
November 23, 2005,
and published as US 2007/0118106 on May 24, 2007. Further, each of the
following commonly
assigned United States Patent Applications are incorporated herein by
reference in its entirety: Patent
ApplicationSer. No. 10/291,862 titled "Systems and Methods for Treating
Obesity and Other
Gastrointestinal_ Conditions," Patent Application Ser. No. 10/370,645 titled
"Method of Treating
Abnormal Tissue In The Human Esophagus," Patent Application Ser. No.
11/286,257 titled
"Precision Ablating Device," Patent Application Ser. No. 11/275,244 titled
"Auto-Aligning Ablating
Device and Method of Use," Patent Application Ser. No. 11/286,444 titled
"Precision Ablating
Device," Patent Application Ser. No. 11/420,712 titled "System for Tissue
Ablation," Patent
Application Ser. No. 11/420,714 titled "Method for Cryogenic Tissue Ablation,"
Patent Application
Ser. No. 11/420,719 titled "Method for Vacuum-Assisted Tissue Ablation,"
Patent Application Ser.
No. 11/420,722 titled "Method for Tissue Ablation," Patent Application Ser.
No. 11/469,816 titled
"Surgical Instruments and Techniques for Treating Gastro-Esophageal Reflux
Disease." This
application further incorporates in entirety US Patent Application Ser. No.
12/114,628 of Kelly et al.
entitled "Method and Apparatus for Gastrointestinal Tract Ablation for
Treatment of Obesity", as filed
on filed May 2, 2008 and US Patent Application Ser. No. 12/143,404, of Wallace
et al., entitled
"Electrical Means to Normalize Ablational Energy Transmission to a Luminal
Tissue Surface of
Varying Size", as filed on June 20, 2008.
INCORPORATION BY REFERENCE
[003] All publications, patents and patent applications mentioned in this
specification are herein
incorporated by reference to the same extent as if each individual
publication, patent or patent
application was specifically and individually indicated to be incorporated by
reference.
FIELD OF THE INVENTION
[004] iile present invention relates to endoscopic therapy devices and
methods, such as devices
and methods to treat areas of the digestive tract in patients with bleeding
conditions of the digestive
1
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
tract, in order to control bleeding (achieve hemostasis) and / or eradicate
lesions with a propensity for
bleeding.
BACKGROUND OF THE INVENTION
[005] Bleeding may occur into the digestive tract lumen from blood vessels
contained in the
digestive tract wall. Such bleeding is abnormal and may be associated with
certain disease states and
anatomical abnormalities. When bleeding occurs, it can be an acute emergency,
with vomiting of
blood or passage of blood from the rectum. In these cases, urgent endoscopic
or surgical intervention
is often necessary, along with blood transfusion, to avoid patient morbidity
and mortality. Examples
include a bleeding varix within the esophagus related to portal hypertension,
an exposed bleeding
vessel within a gastric or duodenal ulcer, or an arteriovenous malformation
(collection of disorganized
blood vessels) within the bowel that has ruptured.
[006] Other bleeding lesions may present with chronic, less severe bleeding
that results in chronic
anemia and the need for serial endoscopic therapeutic interventions to
cauterize visible abnormalities.
Such cases often require chronic transfusion therapy, as the endoscopic
interventions are not ideal to
permanently halt bleeding. Examples include gastric antral vascular ectasia
(GAVE), which is also
known as watermelon stomach, for the appearance of its characteristic gastric
lesions, radiation
induced proctopathy and colopathy, portal hypertensive gastropathy (PHG),
angiodysplasia, small
arteriovenous malformations (AVM), and small bleeding ulcers. The common
fmding in many of
these more chronic abnormalities is the presence of blood vessels in the
digestive tract wall,
specifically the mucosal and submucosal layers, that are larger than normal,
more fragile than normal,
more superficial than normal, tangled, disorganized, and / or exposed to the
lumen of the digestive
tract and therefore more traumatized than normal by passage of food or stool.
Due to these
combinations of features, these vessels tend to bleed into the lumen of the
digestive tract on a chronic
basis, thereby requiring chronic management.
[007] Acute bleeding episodes of a magnitude where the patient is vomiting
blood or passing
blood from the rectum and is having cardiovascular effects are usually managed
urgently with
endoscopic or surgical therapy and blood transfusion. Typically, these events
are associated with a
large blood vessel that has ruptured and is bleeding profusely into the lumen.
These lesions are
visualized with an endoscope and may be injected with adrenalin to slow the
bleeding, then cauterized
with a small probe which is touched directly onto the vessel or may be
cauterized with an electrified
stream of argon gas. These probes deliver radiofrequency energy that rapidly
heats the targeted focal
vessel or tissue, and the blood vessels shrink and stop bleeding. Surgery is
reserved for those patients
with life-threatening bleeding that is not amenable to endoscopic therapy.
[008] Chronic bleeding episodes, while causing long-term disability and the
need for repeat
therapy and transfusion, do not typically require urgent, life-saving
intervention. Rather, these lesions
are typically sought after with endoscopic examination when a patient presents
with anemia of
2
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
unknown cause. As described above, these lesions are identifiable and
targetable with endoscopy.
Cauterization is the standard therapy, in hopes of permanently eliminating the
risk for bleeding.
Unfortunately, in many cases, current techniques of cauterization fail to
permanently eradicate these
lesions, and bleeding retums. Factors contributing to unsatisfactory results
with currently available
cautery methods include the fact that lesions such as GAVE, radiation induced
protopathy and
colopathy, portal hypertensive gastropathy, and angiodysplasias tend to
manifest as large, wide-spread
lesions that are not amenable to a cautery technique which uses a small probe
to press against the
lesion. Even arteriovenous malformations tend to have high flow and larger
surface areas, making
them difficult to treat with small probes. Another likely reason for
problematic and inconsistent
results with conventional cautery methods relates to the presence of blood in
the vasculature tissue at
the time of cautery. Systems and methods, particularly non-surgical approaches
or improvements on
conventional cautery techniques would be welcomed in the field of treating
sites of acute and chronic
bleeding in the gastrointestinal tract.
SUMMARY OF THE INVENTION
[009] To address these and other needs, the present invention provides various
embodiments of an
endoscopic device and method to provide a more permanent resolution of
primarily chronic bleeding
occurring in the digestive tract and a more permanent eradication of the
lesions that lead to such
bleeding, by way of providing a larger ablation surface, compressing the blood
vessels prior to
delivery cauterization energy, and controlling the depth of ablation to
include the tissue layers
containing the bleeding vessels. To this end, the device includes an
endoscopic catheter that is either
balloon-based, mounted on the end of the endoscope, or passes through a
working channel or
accessory channel of an endoscope. The device has an electrical array on at
least one surface to
deliver radiofrequency energy or other energy source to the targeted tissue in
a manner so that the
depth of ablation is controlled via parameters such as energy density,
electrode pattern, power density,
number of applications, and pressure exerted on the tissue. The catheter is
supplied with ablation
energy by an energy generator, connected to the catheter with a cable.
[0010] The method includes using the devices described, along with an existing
endoscope for
visualization, to visualize the area of the digestive tract that contains the
lesion that is actively
bleeding or is causing chronic recurrent bleeding. The device is positioned to
come into contact with
the lesion and is then deployed according to the device embodiment so that the
blood vessels are
compressed. Ablative energy is then delivered to the device and into the
lesion, causing hemostasis
and eradication of the lesion.
[0011] Compression prior to delivery of coagulation energy halts or reduces
blood flow within the
targeted vessels. When energy is then delivered, coaptive coagulation occurs
more readily, meaning
that the vessel walls are sealed to themselves. If blood flow was occurring
during coagulation, there
3
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
would be a higher failure rate due to the blood holding the vessels open and
the heat sink effect that
the blood flow provides.
[0012] Treatment parameters may be such that a uniform level of ablation is
achieved in all or part
of the targeted lesion. For example, for superficial lesions, the depth of
ablation desired may be the
mucosa or a portion of the mucosa. For deeper lesions, the depth of ablation
may be the deeper
mucosa and all or part of the submucosa. Depth control and uniformity of
ablation effect are achieved
via features of the device and treatment parameters, including electrode
patterrrn, pressure against the
targeted lesion, energy density, power density, and number of applications.
[0013] Embodiments of the invention include a system and methods of
implementing the system
toward a method of treating an area of bleeding in a gastrointestinal tract.
The therapeutic method
includes identifying the area of bleeding; positioning a therapy device in the
gastrointestinal tract
adjacent to a target site within the area of bleeding; pressuring the bleeding
area to diminish the
amount of blood within blood vessels in the bleeding area; and applying non-
surgical hemostatic
therapy to a target site in the area while continuing to pressure the area. In
some embodiments of this
method the identifying step is performed endoscopically. And in some
embodiments, the identifying
step, the positioning step, the pressuring step, and the performing step are
conducted during a single
endoscopic procedure. In other embodiments, the method may further include
inserting an instrument
having a hemostatic therapy device mounted thereon into the gastrointestinal
tract before the
identifying step, and removing the instrument after the applying step.
[0014] In some embodiments of the method, applying the non-surgical hemostatic
therapy on the
target site includes applying energy, such as radiofrequency energy, to the
target site. In various
embodiments, applying energy to the target site includes controlling the
delivery of energy across the
tissue surface in the target site. In some embodiments, applying energy to the
target site includes
controlling the depth of delivery of energy into tissue layers in the target
site. In some embodiments,
applying energy to the target site may include applying energy more than once,
and in some
embodiments, applying energy to the target site includes applying energy to
more than one target site
in the area of bleeding.
[0015] In some embodiments of the method, applying non-surgical hemostatic
therapy on the
target site includes applying cryogenic treatment to the target site. In some
embodiments of applying
cryogenic treatment, such treatment includes spraying a cryogenic fluid on the
target site, and in other
embodiments, applying cryogenic treatment includes drawing heat from the
target area into a
cryogenic fluid contained in the device
[0016] In some embodiments of the method, the positioning step fiirther
includes moving an
ablation structure of the device so as to make therapeutic contact with a
target site within the area of
bleeding. And in some of these embodiments, moving the ablation structure may
include any of
4
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
inflating a balloon member, expanding a deflection member, moving a deflection
member, or
expanding an expandable member.
[0017] Pressuring the bleeding area, which underlies the coaptive aspect of
the ablation therapy,
includes a applying pressure to the target area at about 1 psig to about 15
psig, in various
embodiments, the pressure applied is in the range of about 3 psig to about 7
psig, and in particular
embodiments, the applied pressure is about 4 psig.
[0018] In another aspect of the invention, a method is directed toward
ablationally-treating a target
site within an area of bleeding in a gastrointestinal tract. Such a method may
include pressuring the
bleeding area to diminish the amount of blood within blood vessels in the
bleeding area, and
delivering radiofrequency energy to a tissue surface within the target area,
the target area being a
contigaous radial portion of the gastrointestinal tract, and controlling the
delivery of radiofrequency
energy across the tissue surface within the target area and into a depth of
tissue within the target area.
The area of bleeding may be any of a site of acute bleeding, chronic bleeding,
or any site identified as
having a propensity to bleed. More specifically, a site of acute bleeding may
include any of a bleeding
varix within the esophagus, an exposed bleeding vessel within a gastric or
duodenal ulcer, or an
arteriovenous malformation within the bowel. And a site of chronic bleeding
may include any of a site
of gastric antral vascular ectasia (GAVE), radiation induced proctopathy or
colopathy, portal
hypertensive gastropathy (PHG), angiodysplasia, small arteriovenous
malformations (AVM), or small
bleeding ulcers.
[0019] In some embodiments of the method, controlling the delivery of
radiofrequency energy
across the surface and into a depth of tissue within the target area includes
delivering sufficient
radiofrequency energy to achieve ablation in one portion of the tissue target
area and delivering
insufficient radiofrequency energy to another portion of the surface to
achieve ablation. In some
embodiments of the method, controlling the delivery of radiofrequency energy
into depth of the tissue
includes controlling the delivery of radiofrequency energy in from the tissue
surface such that
sufficient energy to achieve ablation is delivered to one or more tissue
layers near the surface and
insufficient energy is delivered to other deeper layers to achieve ablation.
[0020] In some embodiments of the method, controlling the delivery of
radiofrequency energy
across the target area surface includes configuring the electrode pattern such
that some spacing
between electrodes is sufficiently close to allow conveyance of sufficient
energy to ablate and other
spacing between electrodes is insufficiently close to allow conveyance of
sufficient energy to ablate.
In other embodiments of the method, controlling the delivery of radiofrequency
energy across the
target area surface includes operating the electrode pattern such that the
energy delivered between
some electrodes is sufficient to ablate and energy sufficient to ablate is not
delivered between some
electrodes.
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
[0021] In some embodiments of the method, controlling the delivery of energy
commencing at the
mucosal surface and emanating into the organ wall includes ablating some
portion of the blood
vessels within the epithelial layer. In various embodiments of the method,
controlling the delivery of
energy commencing at the mucosal surface and emanating progressively deeper
into layers of the
organ wall includes ablating some portion of the blood vessels within the
epithelial layer and the
laniina propria. In still other embodiments, some portion of blood vessels may
be ablated in epithelial
layer, the lamina propria, and the muscularis mucosae, or in the epithelial
layer, the laniina propria,
the muscularis mucosae, and the submucosal, or in the epithelial layer, the
lamina propria, the
muscularis mucosae, the submucosa, and the muscularis propria. Further, in
various embodiments,
controlling the delivery of radiofrequency energy across the tissue surface
within the target area and
into the depth of tissue within the target area includes achieving of a
partial ablation in tissue layers of
the gastrointestinal tract.
[0022] In some embodiments of the method, delivering radiofrequency energy is
by way of an
electrode pattern configured circumferentially through 360 degrees around the
ablation structure. In
other embodiments, transmitting energy from the ablation structure includes
transmitting energy
asymmetrically through the 360 degree circumference such that ablation is
focused within an arc of
less that 360 degrees. In other embodiment, delivering radiofrequency energy
is by way of an
electrode pattem configured circumferentially through an arc of less than 360
degrees around the
ablation structure. Regardless of the ablation pattern, in various
embodiments, the delivering energy
step may be performed more than once, and at more than one site.
[0023] In some embodiments, the method further includes evaluating the target
area at a point in
time after the delivering energy step to determine the status of the area. In
various embodiments, the
evaluating step occurs in close time proximity after the delivery of energy,
to evaluate the immediate
post-treatment status of the site. In other embodiments, the evaluating step
may occur at least one day
after the delivery of energy.
[0024] In various embodiments, the method further includes deriving energy for
transmitting from
an energy source that is controlled by a control system. In some of these
embodiments, the energy
source is a generator. An in some embodiments operated by a control system,
the method includes
feedback controlling the energy transmission so as to provide any of a
specific power, power density,
energy, energy density, circuit impedance, or tissue temperature.
[0025] Some embodiments of the method of ablationally treating an area of
bleeding may futher
include advancing an ablation structure into the alimentary canal, the non-
penetrating electrode
pattern on the structure, the structure supported on an instrument,
positioning the ablation structure
adjacent to the target area, and moving the ablation structure toward the
surface of the target area to
make therapeutic contact on the target area prior to delivering energy. The
moving step may variously
6
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
include any of inflating a balloon member, expanding a deflection member,
moving a deflection
member, or expanding an expandable member.
[0026] In some embodiments of the method of ablationally treating an area of
bleeding further
include a position-locking step following the moving step; and example of
which includes developing
suction between the structure and the ablation site. The method, prior to the
evaluating step, may
further include evaluating the target area prior to the positioning step to
determine the status of the
target area. In other variations of the method, when multiple target areas are
being treated, the method
may include the positioning, moving, and transmitting energy steps to a first
target area, and then
further include the positioning, moving, and transmitting energy steps to
another target area without
removing the ablation structure from the patient.
[0027] Some embodiments of the invention include ablation system for treating
a target site within
an area of bleeding in a gastrointestinal tract, such system including an
electrode pattern including a
plurality of electrodes, a longitudinal support member supporting the
electrode pattern, a generator
coupled to the plurality of electrodes, and a computer controller in
communication with the generator,
the controller having programming to direct the generator to deliver energy to
the plurality of
electrodes, the programming including the ability to direct delivery of energy
to a subset of the
electrodes, the electrodes of the pattern configured such that, when receiving
energy from the
generator and in therapeutic contact with a tissue target area, delivery of
energy across the surface of
the target area and into a depth of tissue layers from the tissue surface is
controlled.
[0028] Various embodiments of the system may be directed toward treating an
area of bleeding
that includes any of a bleeding varix within the esophagus, an exposed
bleeding vessel within a gastric
or duodenal ulcer, or an arteriovenous malformation within the bowel. Other
embodiments may be
directed toward treating an area of area of bleeding includes any of a site of
gastric antral vascular
ectasia (GAVE), radiation induced proctopathy or colopathy, portal
hypertensive gastropathy (PHG),
angiodysplasia, small arteriovenous malformations (AVM), or small bleeding
ulcers.
[0029] The electrode pattern of some embodiments of ablation system for
treating a target site
within an area of bleeding in a gastrointestinal tract has a longitudinal axis
that forms a fully
circumferential surface orthogonal to its longitudinal axis as aligned with a
delivery instrument, the
pattern sized for contacting tissue in a target area within the
gastrointestinal tract. In other
embodiments, the electrode pattern forms a partially circumferential surface
orthogonal to its
longitudinal axis, the pattern sized for contacting tissue in a target area
within the gastroinstestinal
tract. ln various of these latter embodiments, the electrode pattern may form
an arc of about 90
degrees or about 180 degrees.
[0030] In some embodiments of the ablation system, the electrode elements are
distributed into a
pattern such that when the programming directs the generator to deliver energy
to all the electrodes,
7
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
the electrode pattern, when therapeutically contacted to a target tissue area,
ablates a portion of tissue
within the target area and does not ablate another portion of tissue within
the target area. In other
embodiments of the ablation system, the programming directs the generator to
deliver energy to a
subset of electrode elements that form a pattern which, when therapeutically
contacted to a target
tissue area, ablates a portion of tissue within the target area and does not
ablate another portion of
tissue within the target area. In various embodiments of the system,
regardless of whether the
electrode pattern is fully activated to deliver a pattern of fractional
ablation, or the pattern is partially
activated to deliver a pattern of fractional ablation, the system renders
tissue that is ablated at least
partially dysfunctional, and another portion that is substantially not ablated
and accordingly retains its
functionality.
[0031] Some embodiments of the invention include an ablation system for
vascular tissue at a
target area in a gastrointestinal tract of a patient that includes an ablation
structure supported by an
instrument, a non-penetrating electrode pattern on the ablation support
structure, the electrode pattern
configured to control the delivery of energy to a target tissue such that a
portion of the surface of the
target area receives sufficient radiofrequency energy to achieve ablation and
another portion of the
surface of the target receives insufficient energy to achieve ablation, and
means supported by the
instrument by which to bring the ablation structure to make therapeutic
contact with tissue at the
target area.
BRIEF DESCRIPTION OF THE DRAWINGS
[0032] Figures 1A and 1B provide views of a schematic representation of a
cross section of the
wall of a portion of the gastrointestinal tract with bleeding blood vessels.
Figure 1A shows a portion
of a blood vessel, an arteriole or venuole, with branching capillaries that
are located primarily in the
lamina propria a.nd the epithelium. Figure 1B shows a portion of a blood
vessel, an arteriole or
venuole, with branching capillaries that are located primarily in the
submucosa and the lamina
propria.
[0033] Figures 2A - 2C provide views of specific examples of conditions that
can be a source of
acute or cnronic bleeding in the gastrointestinal tract. Figure 2A shows an
arteriovenous
malformation (AVM). Figure 2B provides a schematic view of telangiectases,
with a grossly dilated
capillary. Figure 2C provides an endoscopic view of the stomach, looking
toward the pylorus of a
patient with watermelon stomach lesions characteristic of GAVE.
[0034] Figure 3 is a flow diagram depicting an overview of the method, wherein
an appropriate
site for ablational intervention for the treatment of a site of acute or
chronic gastrointestinal tract
bleeding, the level of ablational therapy is determined, and at least
preliminary information is gained
regarding localization, and clinical judgment is exercised as to which
embodiment of the invention is
preferable.
8
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
[0035] Figure 4 is a flow diagram depicting the method after the site of
ablation of a site of acute
or chronic gastrointestinal tract bleeding has been localized and a choice has
been made regarding the
preferred ablational device. The method includes an evaluation of the site,
including particulars of
location, stage, determination of the number of sites, and the dimensions. The
method continues with
insertion of the instrument and its movement to the locale of the ablational
target tissue, the more
refined movement of the ablational structure that create a therapeutically
effective contact, the
emission of ablational radiation and then post-treatment evaluation.
[0036] Figure 5 is a view of an embodiment of an ablative device with a fully
circumferential
operating radius.
[0037] Figure 6 is a view of an embodiment of an ablative device with a fully
circumferential
operating radius, with a balloon member in an expanded configuration.
[0038] Figures 7A - 7C show the electrode patterns of the device of Figure 5.
[0039] Figures 8A - 8D show electrode patterns that may be used with
embodiments of the
ablative devicc with a fully circumferential operating radius, or with any
device embodiments
described herein.
[0040] Figure 9 is a view of the ablation device of the invention with a
partially circumferential
operating radius.
[0041] Figure 10 is an end view of the ablation device embodiment of Figure 9.
[0042] Figure 11 is an end view of the device of Figure 9 in an expanded
configuration.
[0043] Figures 12, 13, and 14 are end views of the device of Figure 9 in
alternative expanded
configurations.
[0044] Figure 15 is a view of the ablation device of the invention in an
unexpanded configuration.
[0045] Figure 16 is a view of the ablation device of the invention in an
expanded configuration.
[0046] Figures 17 and 18 are end views of the device in an expanded
configuration.
[0047] Figure 19A is a view of the ablation device of the invention showing a
deflection member
feature.
[0048] Figure 19B is a view of the ablation device of the invention showing an
alternative
deflection member wherein the device is in an expanded configuration.
[0049] Figure 20 is a view of device shown in Figure 19B wherein the
deflection member is in an
unexpanded configuration.
[0050] Figiire 21 is an end view of the device in an unexpanded configuration.
[0051] Figure 22 is an end view of the device shown in Figure 21 in an
expanded configuration.
[0052] Figure 23 is a view of the ablation device of the invention showing a
pivoting ablation
structure feature.
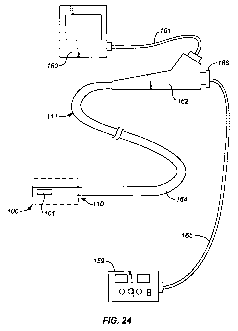
[0053] Figure 24 is an illustration of the ablation device of the invention
combined with an
endoscope system.
9
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
[0054] Figure 25 is a schematic of view of a section through the wall of a
portion of the
gastrointestinal tract.
[0055] Figure 26 is a view of the ablation device of the invention including
an elongated sheath
feature.
[0056] Figure 27 is a view of the device wherein an elongated sheath feature
is optically
transmissive.
[0057] Figure 28 is an enlarged view of the optically transmissive feature of
the device.
[0058] Figure 29 is a cross sectional view of the optically transmissive
sheath feature of the device
shown in Figures 27 and 28.
[0059] Figure 30 is a view of the device including an altemative optically
transmissive sheath
feature and an inflation member feature in an expanded configuration.
[0060] Figure 31 is an illustration of the ablation device of Figure 30
positioned within an
esophagus.
[0061] Figure 32 is a view of the ablation device of the invention including a
slit sheath feature.
[0062] Figure 33A is an end view of a slit sheath feature of the device
wherein the sheath is in an
unexpanded configuration.
[0063] Figure 33B is an end view of a slit sheath feature of the device and an
endoscope wherein
the sheath is in an expanded configuration.
[0064] Figure 34A is a cross sectional view of the device positioned within an
endoscope internal
working channel wherein an inflatable member feature is in an unexpanded
position.
[0065] Figure 34B is a view of the device shown in Figure 34A wherein the
inflatable member
feature is in an expanded position.
[0066] Figure 35A is a cross sectional view of the device positioned within an
endoscope internal
workin.g channel wherein an expandable member feature is in an unexpanded
position.
[0067] Figure 35B is a view of the device shown in Figure 35A wherein the
expandable member
feature is in an expanded position.
[0068] Figure 36A is a cross sectional view of the device positioned within an
endoscope internal
working channel wherein an alternative expandable member feature is in an
unexpanded position.
[0069] Figure 36B is a view of the device shown in Figure 36A wherein the
expandable member
feature is in an expanded position.
[0070] Figure 37 is a view of the ablation device of the invention including
an alternative
deflection member.
[0071] Figure 38 is an illustration of the ablation device of the invention
including an alternative
deflection member positioned in a non-deflected position at a site of acute or
chronic gastrointestinal
tract bleeding.
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
[0072] Figure 39 is an illustration of the device shown in Figure 38 wherein
the deflection
member is in a deflected position.
[0073] Figure 40 is a cross sectional view of the ablation device of the
invention showing an
internal coupling mechanism feature.
[0074] Figure 41 is a cross sectional view of the ablation device of the
invention showing an
alternative internal coupling mechanism and a rolled sheath feature.
[0075] Figure 42 is an illustration showing a cross sectional view of the
ablation device of the
invention positioned within a lumen at a site of acute or chronic
gastrointestinal tract bleeding.
[0076] Figure 43 is an illustration of the ablation device of the invention
positioned within an
esophagus showing a rotational feature.
[0077] Figure 44 is an illustration of the ablation device of the invention
positioned within an
esophagus showing a rotational feature combined with an inflation member in an
expanded
configuration.
[0078] Figures 45A - 45C are views of the ablation device of the invention
showing alternative
rotational features.
[0079] Figure 46A is a view of an endoscope.
[0080] Figure 46B is a view of the ablation device of the invention including
a catheter feature.
[0081] Figure 46C is a view of a sheath feature of the device.
[0082] Figure 47 is a view of the ablation device of the invention including
the features shown in
Figures 46A - 46C in an assembly.
[0083] Figures 48A - 48D show an electrode array with a striped pattern for a
fractional ablation
and the ablation patterns on tissue that can be made from such a pattern.
[0084] Figures 49A and 49B show an electrode array with a concentric-circle
pattern for a
fractional ablation and the ablation patterns on tissue that can be made from
such a pattern.
[0085] Figures 50A and 50B show an electrode array with a checkerboard pattern
for a fractional
ablation and the ablation patterns on tissue that can be made from such a
pattern.
[0086] Figures 51A and 51B show an electrode array with a checkerboard pattern
operating in a
non-fractional manner and the ablation pattern on tissue that is made from
such an operating pattern.
[0087] Figures 52A and 52B show an electrode array with a checkerboard pattern
operating in a
fractional manner and the ablation pattern on tissue that is made from such an
operating pattern.
[0088] Figures 53A and 53B show an electrode array with a striped pattern of
alternating positive
and negative electrodes operating in a non-fractional manner and the ablation
patterns on tissue that
can be made from such an operating pattern.
[0089] Figures 54A and 54B show an electrode array with a striped pattern of
alternating positive
and negative electrodes operating in a fractional manner and the ablation
patterns on tissue that can be
made from such an operating pattern.
11
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
[0090] Figure 55 shows a schematic rendering of a three-dimensional view of a
target region of a
radial portion of the gastrointestinal tract at a site of acute or chronic
bleeding after it has been
ablationally treated.
[0091] Figure 56A and 56B provide views of an ablational device (similar to
the devices of
Figures 38 and 39) but including an ablational surface on a hinge structure or
deflecting mechanism
similar to that depicted in Figure 43, the hinge allowing a free pivoting
movement of the ablational
surface between its longitudinal axis and the longitudinal axis of an
endoscope. Figure 56A shows the
device with the ablational surface oriented in parallel with the endoscope.
Figure 56B shows the
device with the longitudinal axis of the ablational surface oriented at about
a right angle with respect
to the longitudinal axis of the endoscope.
[0092] Figure 57A- 57D provide perspective views of an ablation device with a
360 degree
circumferential ablation surface on an overlapping electrode support furled
around an expandable
balloon, the operative element including a balloon and an electrode support in
an expanded state.
Figure 57A shows the support pulled away from the balloon to clarify that a
portion of the support
and an edge is adherent to the balloon, and another portion and its edge is
not connected to the
balloon.
[0093] Figure 57B shows the operative element of the device with the non-
adherent portion of the
support furled around the balloon in a deployable configuration, the non-
adherent portion and its edge
overlapping around the adherent portion.
[0094] Figure 57C shows the device of Figures 57A and 57B with an optional
feature of the
operative element, one or more elastic bands wrapped around the electrode
support.
[0095] Figure 57D shows the device of Figure 57C in a collapsed state, with
balloon portion
being uninflated (or deflated), this being the state of the device when it is
being deployed into a lumen
and being positioned at a target site, as well as the state of the device
after delivering ablation energy
and about to be removed from the lumen.
[0096] Figures 58A - 58D depict an embodiment of an ablation device that is
adapted to present
an ablational surface into a concave or inwardly tapered target site such as a
stoma or pylorus. The
device includes an ablational surface circumferentially arranged on the distal
portion of an expandable
member, the expandable member mounted around the distal end of an endoscope.
Figure 58A shows
the device in a deployed configuration.
[0097] Figure 58B shows the device of Figure 58A with the expandable member in
an
unexpanded or collapsed state, as would be appropriate for deployment of the
device to a target
tapered surface, or as would be appropriate for removal from the ablational
site.
[0098] Figure 58C shows the device of Figure 58A as it can be deployed into a
tapered or
concave target site such as the pylorus.
12
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
[0099] Figure 58D shows the device of Figure 58A in an alternative
configuration, with the
electrode bearing surface of the device reversed such that it is facing
proximally, and can thus be
pulled retrograde into a tapered or concave site such as the lower esophageal
sphincter.
[00100] Figures 59A and 59B provide views of an ablation device deployable
through the working
channel of an endoscope that is configured to present a broad field ablational
surface generally
orthogonal or perpendicular to the longitudinal axis of the delivery
endoscope. Figure 59A shows the
device in a fully deployed configuration. Figure 59B shows the device in a
stowed configuration that
can be drawn into a working channel of an endoscope.
[00101] Figure 60 depicts an embodiment of an ablation device deployable
through the working
channel of an endoscope that is adapted to present an ablational surface
generally parallel to the
longitudinal axis of the delivery endoscope. The device includes an ablational
structure that has two
parallel collapsible shape memory ribs, across which ablative electrodes are
strung, the strung
electrodes configured to be taut across the space between the ribs in the
deployed condition.
[00102] Figures 61A and 61B depict an embodiment of an ablation device
deployable through the
working channel of an endoscope that is adapted to present an ablational
surface generally parallel to
the longitudinal axis of the delivery endoscope. The ablational surface of the
device is tapered on its
proximal end, and substantially flat but with a laterally-curved bias that is
rollable, such that it unrolls
when pushed from the working channel, and rolls around itself when being
withdrawn back into the
working channel. Figure 61A is a view of the device in its deployed form,
after emerging from the
working channel. Figure 61B is a view of the device as it be configured within
the working channel
prior to deployment, or after having been withdrawn back into the working
channel.
[00103] Figtare 62 depicts an embodiment of an ablation device deployable
through the working
channel of an endoscope similar to that of Figure 61 except that is adapted to
present an ablational
surface generally orthogonal to the longitudinal axis of the delivery
endoscope by virtue of a flexible
bent portion proximal to the ablational surface. The ablational surface of the
device is tapered on its
proximal end, and substantially flat but with a laterally-curved bias that is
rollable, such that it unrolls
when pushed from the working channel, and rolls around itself when being
withdrawn back into the
working channel.
[00104] Figure 63 depicts an embodiment of an ablation device deployable
through the working
channel of an endoscope that is adapted to present an outwardly-facing
circumferentially-oriented
circle or helical ablational surface. The circular or helical portion uncoils
upon emergence from the
working channel of an endoscope, and coils into a linear configuration upon
being withdrawn into the
working channel.
[00105] Figure 64 provides a perspective and cross-sectional detail view of
circuit layers of
ablational surfaces conunon to devices shown in Figures 59 - 63.
13
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
[00106] Figures 65A and 65B depict an embodiment of an ablation device with a
partially
circumferential ablation surface that includes a hydraulic cleaning feature.
Figure 64A shows a side
view of a device with a hydraulic line leading to the ablation surface.
[00107] Figure 65B shows a more detailed perspective view of the ablation
surface and the
hydraulic intake and multiple outlet holes.
DETAILED DESCRIPTION OF THE INVENTION
[00108] The use of ablation technologies that make use of a fully
circumferential and partially-
circumferential ablative structure, some in combination with expandable
balloons to position the
ablative structure against target tissue has been described in described in US
Patents and US Patent
Applications. Devices and methods that make use a partially-circumferential
ablative structure include
the following U.S. Patent Applications: 11/286,257, 11/286,444, and
11/275,244. Devices and
methods that make use a fully-circumferential ablative structure have been
described in U.S. Patents
6,551,310, and 7,150,745 and in U.S. Patent Applications Ser. No.11/557,445,
Ser. No.10/370,645,
Ser. No. 10/416,923,, Ser. No. 11/420,722, Ser. No.11/420,719, Ser.
No.11/420,714, Ser, No.
11/420,712, Ser. No. 11/469,816 (Shadduck) and U.S. Patent 6,872,206.
1001091 The devices and methods may provide an immediate hemostatic effect, or
they may remove
or alter a lesion that has a propensity to bleed in the future, or the
treatment effect may develop
overtime in conjunction with wound healing and thus lead to a gradually more
effective hemostatic
effect. This treatment effect may be achieved over a broad field and is
typically coaptive in nature,
uniform in depth, controllable to a desired depth, and rapidly delivered. The
wide-field aspect of the
ablational treatment, per some embodiments of the invention, is due to the
large surface area of the
device and the ability to reposition the device repeatedly to treat adjacent
and non-adjacent areas.
Coaptive ablation of blood vessels is considered an advantageous approach for
several reasons. First,
with the removal or diminishment of blood from the site, the heat sink
capacity of the tissue (ability to
absorb heat) is lessened thus creating a more effective hemostatic effect.
Second, by compressing or
collapsing the blood vessel walls together with pressure applied by the device
itself prior to applying
therapy, the therapy can result in the walls annealing together and thus
stopping blood flow. Thus,
method steps that favor coaptive coagulation such as the application of
appropriate pressure are
beneficial for the method. The hemostatic affect is coaptive in nature by
virtue of the pressure applied
on the targeted tissue or blood vessels included therein by the ablation
device. Per embodiments of the
invention, a level of pressure, typically exerted by an expandable member of
the device when adjacent
to the target site or exerted by the physical movement of the endoscope upon
which the device is
mounted or through which it is passed, is in the range of about 1 to about 15
psig. More particularly,
coaptive pressure is in the range of about 3 to about 7 psig. And still more
particularly, coaptive
pressure is about 4 psig.
14
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
[00110] Treatment parameters may be such that a uniform level of ablation is
achieved in all or part
of the targeted lesion. For example, for superficial lesions, the depth of
ablation desired may be the
mucosa or a portion of the mucosa. For deeper lesions, the depth of ablation
may be the deeper
mucosa and all or part of the submucosa. Depth control and uniformity of
ablation effect are achieved
via features of the device and treatment parameters, including electrode
pattem, pressure against the
targeted lesion, energy density, power density, and number of applications.
[00111] Choices of embodiments of the method and devices that are used to
treat a specific bleeding
site or lesion are dependent upon the organ dimensions within which the
bleeding is occurring, the
endoscopic access, the size of the bleeding site and lesion, as well as the
extent of involvement of the
inner lining of the organ. For example, within the gastric antrum in a patient
with GAVE, the bleeding
lesion may be narrow and linear (like spokes emanating from a wheel) or it may
be confluent and
circumferential. In the former case, a focal ablation and hemostasis device
may be preferred, such as
an ablation catheter with a partially-circumferential ablation surface or
other such focal devices
disclosed herein. In the latter case, a fully-circumferential ablation and
hemostasis device may be
preferred. Both types of devices (i.e., focal or non-circumferential and
circumferential devices) may
be variously mounted on an endoscope, passed through an endoscope, or passed
along the length of an
endoscope. Further, devices may include balloon based or non-balloon based
methods of deployment
against and compression of the target tissue.
[00112] Embodiments of the methods and devices to implement the method may be
applied to sites
of acute or chronic bleeding in the gastrointestinal tract. Figures 1A and 1B
provide views of a
schematic representation of a cross section of the wall of a portion of the
gastrointestinal tract with
bleeding blood vessels 4. Figure 1A shows a portion of a blood vessel, an
arteriole or venuole, with
branching capillaries that are located primarily in the lamina propria and the
epithelium. Figure 1B
shows a portion of a blood vessel, an arteriole or venuole, with branching
capillaries that are located
primarily in the submucosa and the lamina propria. The point of these figures
is that fragile and
bleeding blood vessels may reside in specific layers of the gastrointestinal
tract. In some cases the
histological layers including may be known because of known characteristics of
the specific
condition. In other cases of acute or chronic bleeding, patient specific
information may be available,
from patient history, biopsy, or by endoscopic observation either preliminary
to the procedure or at
the time of the procedure, which can provide indications to localize the
problematic blood vessels
preferentially to a particular tissue depth. In the example provided by Figure
1A, it would be
appropriate to deliver depth-controlled ablational energy such that ablation
occurs in the epithelial
layer and in the lamina propria. In the example provided by Figure 1B, it
would be appropriate to
deliver depth-controlled ablational energy such that ablation occurs in the
submucosa, muscularis
mucosae, and in the lamina propria.
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
[00113] Figures 2A - 2C provide views of specific examples of conditions that
can be a source of
acute or chronic bleeding in the gastrointestinal tract, and which are targets
of hemostatic ablation per
embodiments of methods and devices of this invention. Figure 2A shows an
arteriovenous
malformation (AVM) 4avm as an entanglement of vessels situated between an
upstream artery 4a and
a downstream vein 4v. Figure 2B provides a schematic view of telangiectases,
with a grossly dilated
capillary 4c situated between an upstream arteriole 4a and a downstream
venuole 4v. Figure 2C
provides an endoscopic distally directed view of the stomach, looking toward
the pylorus 9 of a
patient with a broad pattern of watermelon stomach lesions 4wm that are
characteristic of gastric
antral vascular ectasia (GAVE). These vascular lesions 41 are visible both
internally, as visualized by
an endoscope, as well as externally, on the skin overlaying the stomach.
[00114] Turning now to an aspect of therapeutic ablation methods provided
herein, that of
determining an appropriate site for ablational treatment (Figure 3), as well
as the amount of ablational
energy to be applied during such treatment, such deterniinations follow from
the total amount of
clinical information that a clinician can gather on a particular patient. In
some embodiments, a
preliminary endoscopic examination of a site of acute or chronic
gastrointestinal tract bleeding may
be appropriate so that any patient-specific features may be mapped out, as
well as an evaluation of the
general dimensions of the patient's alimentary canal. Such information may be
obtained by direct
visual observation by endoscopic approaches, with optional use of mucosal in-
situ staining agents
may further be accomplished by other diagnostic methods, including non-
invasive penetrative
imaging approaches such as narrow band imaging from an endoscope, or with any
conventional
method known in the art. In one aspect, evaluation of a site includes
identifying the locale of the site,
including its dimensions. In another aspect, evaluation of target tissue area
includes identifying a
multiplicity of sites, if there is more than one site, and further identifying
their locale and their
respective dimensions. In still another aspect, evaluating target sites may
include identifying or
grading any pathology or injury or specific features of site(s) of acute or
chronic gastrointestinal tract
bleeding, and particularly identifying any areas of clinical significance or
concern that are overlapping
or near the areas that are to be targeted for ablation.
[00115] Once target sites for ablation have been identified, target tissue at
a site of acute or chronic
gastrointestinal tract bleeding may be treated with embodiments of an
inventive ablational device and
associated methods as described herein. Evaluation of the status of target
tissue sites for ablation,
particularly by visualization approaches, may also be advantageously
implemented as part of an
ablational therapy method (Figure 3), as for example, in close concert with
the ablation, either
immediately before the application of ablational energy (such as radiant
energy), and/or immediately
thereafter. Further, the treatment site can be evaluated by any diagnostic or
visual method at some
clinically appropriate time after the ablation treatment, as for example a few
days, several weeks, or
several few months, or at anytime when clinically indicated following
ablational therapy. In the event
16
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
that any follow-up evaluation shows either that the therapy was
unsatisfactorily complete, or that there
is a recovery in the population of cells targeted for ablation, a repetition
of the ablational therapy may
be indicated.
[00116] Turning now to aspects of ablational devices that can be directed
toward ablational
correction of failed bypass procedures, as described in detail herein,
ablational devices have an
ablational structure arrayed with energy-transmitting elements such as
electrodes. In some
embodiments, depending on the type of ablatative energy being used in the
therapy, the devices may
be mounted on, or supported by any appropriate instrument that allows movement
of the ablational
surface to the local of a target site. Such instruments are adapted in form
and dimension to be
appropriate for reaching the target tissue site, and may include simple
catheters adapted for the
purpose; some embodiments of the insertive instrument include endoscopes that,
in addition to their
supportive role, also provide a visualization capability. In some embodiments
of the method, an
endoscope separate from the supportive instrument may participate in the
ablational procedure by
providing visual information.
[00117] Exemplary embodiments of the inventive device as described herein
typically make use of
electrodes to transmit radiofrequency energy, but this form of energy
transmission is non-limiting, as
other forms vi energy, and other forms of energy-transmission hardware are
included as embodiments
of the invention. Ablational energy, as provided by embodiments of the
invention, may include, by
way of example, microwave energy emanating from an antenna, light energy
emanating from
photonic elements, thermal energy transmitted conductively from heated
ablational structure surfaces
or as conveyed directly to tissue by heated gas or liquid, or a heat-sink draw
of energy, as provided by
cryonic or cryogenic cooling of ablational structure surfaces, or as applied
by direct contact of cold
gas or sprayed fluid or mist with tissue, or by heat-draw through a wall of a
device that separates the
cold gas or fluid from the tissue.
[00118] Embodiments of the ablational device include variations with regard to
the circumferential
expanse of the ablational surface to be treated, some embodiments provide a
fully circumferential
ablation surface and others provide a surface that is less than fully
circumferential, as described
above. Choosing the appropriate device is a step included within the
therapeutic method provided, as
shown in Figure 3. These and other variation may provide particular advantages
depending on the
nature, extent, locale, and dimensions of the one or more targeted tissue
sites on the wall the
alimentary canal. One embodiment of the invention includes a device with an
ablational surface that is
fully circumferential, i.e., encompassing a radius of 360 degrees, such that a
full radial zone within a
luminal organ is subject to ablation. Within that zone, ablation may be
implemented to a varying
degree, depending on the energy output and the pattern of the ablational
elements (such as electrodes),
but with substantial uniformity within the zone of ablation. This embodiment
may be particularly
appropriate for treating widespread or diffuse sites within a site of acute or
chronic gastrointestinal
17
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
tract bleeding. In another embodiment of the device, the ablational surface of
the inventive device is
partially circumferential, such that it engages a fraction of the full
internal perimeter or circumference
of a luminal organ. The fractional portion of the circumference ablated on the
inner surface of a
luminal organ depends on the size and conformation of the luminal organ being
treated (radius,
diameter, or circumference, or angularity) and on the dimensions of the
ablational surface, as detailed
further below. With regard to treating target sites that are small and
discrete, the smaller or more
discrete ablational surface provided by this latter embodiment may be
advantageous.
[00119] This type of operational control of a circumferential subset of
ablation energy elements
around a 360-degree circumferential array is analogous to the fractional
operation of a patterned
subset of an electrode array, as described below in the section titled "
Electrode patterns and control of
ablation patterns across the surface area of tissue". In the partially-
circumferential operation of an
array, a particular arc of the array is activated to deliver energy to an arc
of the circumference. In the
fractional-pattern operation of an array, energy is delivery to a portion of
the tissue in the target area,
while another portion receives insufficient energy to achieve ablation. In
some embodiments, these
operational variations can be combined, that is, a patterned subset of a
circumferential arc can be
activated.
[00120] Figures 3 and 4 together provide flow diagram depictions of
embodiments of the method
for ablating tissue at a site of acute or chronic gastrointestinal tract
bleeding. The diagrams represent
common aspects of the embodiments of the method, as delivered by two
embodiments of the device,
one which has a 360 degree circumferential ablation structure, and one which
has an ablation structure
comprising an arc of less than 360 degrees.
[00121] Figure 3 is a flow diagram depicting an overview of the method with a
focus on patient
evaluation and determination of a clinically appropriate site within the
alimentary canal for ablational
treatri-tent. un another step, a responsible clinician makes an informed
choice with regard to the
appropriate embodiment with which to treat the patient, i.e., either a device
with the 360 degree
electrode array 100A, or a device 100B with the electrodes arrayed in an arc
of less than 360 degrees.
In the event that the device 100A is chosen for use, another treatment choice
may be made between
operating the electrodes throughout the 360 degree circumference, or whether
to operate a radial
subset of the electrode array. In another step, a clinician further considers
and makes a determination
as to the protocol for ablation, considering the amount of energy to be
delivered, the energy density,
the duration of time over which energy is to be delivered. These
considerations take into the account
the surface area to be ablated, the depth of tissue which is to be treated,
and the features of the
electrode array, whether, for example, it is to be a fractional electrode, and
which pattern may be
desirable. Regardless of the device chosen, another preliminary step to
operating the method may
include a closer evaluation of the target tissue site(s) within the alimentary
canal. Evaluation of the
site may include the performance of any visualization or diagnostic method
that provides a detailed
18
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
census of the number of discrete target tissue sites, their dimensions, their
precise locations, and/or
their clinical status, whether apparently normal or abnormal. This step is
shown following the choice
of instrument, but may occur simply in conjunction with diagnosis, or at any
point after diagnosis and
general localization of the target tissue. In any case, an evaluating step is
typically performed prior to
ablation, as outlined in the operational steps of the method, as shown in the
flow diagram of Figure 4.
In the description that follows below, the labe1100 may generally be used to
designate ablational
devices, regardless of whether their ablational surface 101 is fully
circumferential or partially
circumferential.
[00122] Figure 4 is a flow diagram depicting the method after the target site
at a site of acute or
chronic gastrointestinal tract bleeding has been localized and a choice has
been made regarding the
preferred ablational device. The method includes an evaluation of the site,
including particulars of
location, stage, determination of the number of sites, and the dimensions, as
described above, and
using approaches detailed in the references provided in the background, and/or
by using whatever
further approaches may be known by those practiced in the art. The method
continues with insertion
of the instrument and the movement of the ablational structure to the locale
of the target tissue to be
ablated. Subsequently, more refined movements of the ablational structure may
be performed that
create a therapeutically effective contact between the ablational structure
and the target tissue site. In
the event that the 360 degree embodiment of the device 100A is chosen,
therapeutically effective
contact may be made by inflating a balloon underlying the electrode array. In
the event that the
embodiment chosen is 100B, the device with an electrode surface spanning an
arc of less than 360
degrees, movements that bring the ablational surface into therapeutically
effective contact may
include any of inflation of a balloon, inflation of a deflection member,
and/or movement of a
deflection member, all of which are described further below.
[00123] After therapeutically-effective contact is made, by either device
embodiment 100A or
100B, and by whatever type of movement was that was taken, a subsequent step
includes the emission
of ablational energy from the device. Variations of ablational energy emission
may include ablating a
single site as well as moving the instrument to a second or to subsequent
sites that were identified
during the evaluation step. Following the ablational event, a subsequent step
may include an
evaluation of the treated target site; alternatively evaluation of the
consequences of ablation may
include the gathering of clinical data and observation of the patient. In the
event that an endoscope is
included in the procedure, either as the instrument supporting the ablational
structure, or as a separate
instrument, such evaluation may occur immediately or very soon after ablation,
during the procedure,
when instruments are already in place. In other embodiments of the method, the
treated site may be
evaluated at any clinically appropriate time after the procedure, as for
example the following day, or
the following week, or many months thereafter. In the event that any of these
evaluations show an
ablation that was only partially complete, or show an undesired repopulation
of targeted cells, the
19
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
method appropriately includes a repetition of the steps just described and
schematically depicted in
Figure 4.
Device and Method for 360 Degree Circumferential Ablation
[00124] Methods for accomplishing ablation of vascular tissue at a site of
acute or chronic
gastro:ntestinal tract bleeding according to this invention include the
emission of radiant energy at
levels to accomplish ablation of sites of bleeding in the gastrointestinal
tract. In typical embodiments
described in this section, the radiant energy distribution elements are
configured circumferentially
around 360 degrees. Alternatively to using emission of RF energy from the
ablation structure, other
energy sources can be used with the ablation structure to achieve tissue
ablation and may not require
electrodes. Such alternate energy sources include: ultraviolet light,
microwave energy, ultrasound
energy, thermal energy transmitted from a heated fluid medium, thermal energy
transmitted from
heated element(s), heated gas such as steam heating the ablation structure or
directly heating the tissue
through steam-tissue contact, light energy either collimated or non-
collimated, cryogenic energy
transmitted by cooled fluid or gas in or about the ablation structure or
directly cooling the tissue
through cryogenic fluid/gas-tissue contact. Embodiments of the system and
method that make use of
these aforementioned forms of ablational energy include modifications such
that structures, control
systems, power supply systems, and all other ancillary supportive systems and
methods are
appropriate for the type of ablational energy being delivered.
[00125] In some embodiments of a fully circumferential ablation device, the
flexible shaft
comprises a cable surrounded by an electrical insulation layer and comprises a
radiant energy
distribution elements located at its distal end. In one form of the invention,
a positioning and
distending device around the distal end of the instrument is of sufficient
size to contact and expand the
walls of the gastrointestinal tract at a site of acute or chronic bleeding in
which it is placed (e.g. the
stomach, pylorus, small intestine, rectum, or anus) both in the front of the
energy distribution
elements as well as on the sides of the energy distribution elements. For
example, the distal head of
the instrument can be supported at a controlled distance from the wall of the
gastrointestinal tract at a
site of acute or chronic bleeding by an expandable balloon or inflation
member, such that a
therapeutically-effective contact is made between the ablation structure and
the target site so as to
allow regulation and control the amount of energy transferred to the target
tissue within the lumen
when energy is applied through the electrodes. The balloon is preferably
bonded to a portion of the
flexible shaft at a point spaced from the distal head elements.
[00126] Some embodiments of a fully-circumferential ablation device include a
distendible or
expandable balloon member as the vehicle to deliver the ablation energy. One
feature of this
embodiment includes means by which the energy is transferred from the distal
head portion of the
invention to the membrane comprising the balloon member. For example, one type
of energy
distribution that may be appropriate and is incorporated herein in its
entirety is shown in U.S. Pat. No.
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
5,713,942, in which an expandable balloon is connected to a power source that
provides radio
frequency power having the desired characteristics to selectively heat the
target tissue to a desired
temperature. A balloon per embodiments of the current invention may be
constructed of an
electroconductive elastomer such as a mixture of polymer, elastomer, and
electroconductive particles,
or it may comprise a nonextensible bladder having a shape and a size in its
fully expanded form which
will extend in an appropriate way to the tissue to be contacted. In another
embodiment, an
electroconductive member may be formed from an electroconductive elastomer
wherein an
electroconductive material such as copper is deposited onto a surface and an
electrode pattern is
etched into the material and then the electroconductive member is attached to
the outer surface of the
balloon member. In one embodiment, the electroconductive member, e.g. the
balloon member, has a
conf guraiiori expandable in the shape to conform to the dimensions of the
expanded (not collapsed)
inner lumen of the human gastrointestinal tract at a site of acute or chronic
bleeding. In addition, such
electroconductive member may consist of a plurality of electrode segments
arrayed on an ablation
structure 101 having one or more thermistor elements associated with each
electrode segment by
which the temperature from each of a plurality of segments is monitored and
controlled by feedback
arrangement. In another embodiment, it is possible that the electroconductive
member may have
means for permitting transmission of microwave energy to the ablation site. In
yet another
embodiment, the distending or expandable balloon member may have means for
carrying or
transmitting a heatable fluid within one or more portions of the member so
that the thermal energy of
the heatable fluid may be used as the ablation energy source.
[00127] Some embodiments of a fully circumferential ablation device include a
steerable and
directional control means, a means for accurately sensing depth of cautery,
and appropriate alternate
embodiments so that in the event of a desire not to place the
electroconductive elements within the
membrane forming the expandable balloon member it is still possible to utilize
the balloon member
for placement and location control while maintaining the energy discharge
means at a location within
the volur:~e of the expanded balloon member, such as at a distal energy
distribution head.
[00128] Embodiments of the invention include methods whereby an ablation
device, such as a fully
circumferential ablation device, is used to treat sites of acute or chronic
bleeding at sites in the
gastrointestinal tract. After determining that the portion or portions of the
gastrointestinal tract wall at
a site of acute or chronic bleeding having this tissue that is targeted either
for full or partial ablation,
the patient is prepared for a procedure in a manner appropriate according to
the embodiment of the
device to be utilized. Then, the practitioner inserts into the patient, in one
embodiment, the ablation
device shown and discussed herein via endoscopic access and control. Further
positioning of portions
of the device occurs as proper location and visualization identifies the
ablation site at a site of acute or
chronic gastrointestinal tract bleeding. Selection and activation of the
appropriate quadrant(s) or
portion(s)/segment(s) on the ablation catheter member is performed by the
physician, including
21
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
appropriate power settings according to the depth of cautery desired.
Additional settings may be
necessary as further ablation is required at different locations and/or at
different depths within the
patient's gastrointestinal tract at a site of acute or chronic bleeding.
Following the ablation,
appropriate follow-up procedures as are known in the field are accomplished
with the patient during
and after rernoval of the device from the site of acute or chronic
gastrointestinal tract bleeding.
[00129] In yet another method of the invention, the practitioner may first
determine the length of the
portion of the site of acute or chronic gastrointestinal tract bleeding
requiring ablation and then may
choose an ablation catheter from a plurality of ablation catheters of the
invention, each catheter having
a different length of the electrode member associated with the balloon member.
For example, if the
practitioner determines that 1 centimeter of the site of acute or chronic
gastrointestinal tract bleeding
surface requires ablation, an ablation catheter having 1 centimeter of the
electrode member can be
chosen for use in the ablation. The length of the electrode member associated
with the balloon
member can vary in length, as for example, from 1 to 10 cm.
[00130] In yet another embodiment, a plurality of ablation catheters wherein
the radiant energy
distribution eiements are associated with the balloon member can be provided
wherein the diameter of
the balloon member when expanded varies from 12 mm to 40 mm. In this method,
the practitioner
will choose an ablation catheter having a diameter when expanded which will
cause the a site of acute
or chronic gastrointestinal tract bleeding to stretch and the mucosal layer to
thin out, thus, reducing or
occluding blood flow at the site of the ablation. It is believed that by
reducing the blood flow in the
area of ablation, the heat generated by the radiant energy is less easily
dispersed to other areas of the
target tissue thus focusing the energy to the ablation site.
[00131] One approach a practitioner may use to determine the appropriate
diameter ablation catheter
to use with a particular patient is to use in a first step a highly compliant
balloon connected to a
pressure sensing mechanism. The balloon may be inserted into a luminal organ
within the site of acute
or chronic gastrointestinal tract bleeding and positioned at the desired site
of the ablation and inflated
until an appropriate pressure reading is obtained. The diameter of the
inflated balloon may be
determined and an ablation device of the invention having a balloon member
capable of expanding to
that diameter chosen for use in the treatment. In the method of this
invention, it is desirable to expand
the expandable electroconductive member such as a balloon sufficiently to
occlude the vasculature of
the submucosa, including the arterial, capillary or venular vessels. The
pressure to be exerted to do so
should therefore be greater than the pressure exerted by such vessels.
[00132] In other embodiments of the method, electronic means are used for
measuring the luminal
target area of gastrointestinal features that have been formed by bariatric
surgery so that energy may
be appropriately normalized for the surface area of the target tissue. These
aspects of the method are
described in detail in US Patent Application Ser. No. 12/143,404, of Wallace
et al., entitled "Electrical
22
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
means to normalize ablational energy transmission to a luminal tissue surface
of varying size", as filed
on June 20, 2008, which is incorporated in entirety.
[00133] In addition to the representative embodiment of an ablation device
with a fully-
circumferential ablation surface that can be pressed against a target area to
achieve therapeutic contact
by way of an expandable member that is shown in Figure 6, other representative
embodiments are
provided in Figures 57A - 57D and Figures 58A and 58B. The embodiments shown
in Figures 57A
- 57D arc described in detail in US Patent Application Ser. No. 12/143,404, of
Wallace et al., entitled
"Electrical means to normalize ablational energy transmission to a luminal
tissue surface of varying
size", as filed on June 20, 2008, incorporated herein its entirety. An
embodiment of a device with a
360 degree ablational surface is described in detail in that application, and
is depicted in Figures 57A
- 57D of this application. Pressure sensing means may also be used to measure
the size of a lumen in
preparation for an ablation treatment, as described in U.S. Patent Application
No. 11/244,385 of
Jackson, published as US 2006/0095032.
[00134] An embodiment of a device disclosed in US Patent Application Ser. No.
12/143,404, of
Wallace et al will be described here briefly, in order to provide an
embodiment that includes a 360-
degree ablational surface arranged on an overlapping support that expands in
accordance with a
balloon enclosed within the circumference of the support. Although the
circumference of the device as
a whole expands with the balloon, the ablational surface itself is non-
distensible, and maintains its
electrode density. Figures 57A - 57D provide perspective views of an ablation
device 100 with an
overlapping electrode support 360 furled around an expandable balloon 105. An
array of ablational
energy delivery elements 101 such as radiofrequency electrodes is arranged on
the exterior surface of
the electrode support. The operative element is mounted on the distal end of
an ablation catheter, of
which the distal portion of a shaft 41 is seen, and around which the balloon
105 is configured. Figure
57A shows the electrode support 360 pulled away from the balloon 105 to
clarify that a portion of the
support and an inner edge 362 is adherent to the balloon, and another portion
and its outer edge 364 is
not connected to the balloon. Figure 57B shows the non-adherent portion of the
electrode support 360
furled around the balloon 105 in a deployable configuration, the non-adherent
portion and its edge
overlapping around the adherent portion. Figure 57C shows an optional feature
of the device 100A,
one or more elastic bands 380 wrapped around the electrode support 360. In
some embodiments, the
elastic band 380 material is a conductive elastomer, as described in greater
detail below, which can be
included in a size-sensing circuit to provide information related to the
degree of expansion of the
operative element. Figure 57D shows the device of Figure 57C in a collapsed
state, with balloon
portion 105 being uninflated (or deflated), this being the state of the device
when it is being deployed
into a lumen and being positioned at a target site, as well as the state of
the device after delivering
ablation energy and about to be removed from the lumen.
23
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
[00135] Another embodiment of an ablation device with a fully circumferential
ablation surface is
provided in Figures 58A - 58B. This particular device embodiment 400 is
adapted to present an
ablational surface 101 into a concave or inwardly tapered target site such as
distal portion of the
antrum of the stomach, or in the vicinity of the pylorus, which is a site for
vascular lesions typical of
watermelon stomach. The device includes an ablational surface
circumferentially arranged on the
distal portion of an expandable member 105, the expandable member mounted
around the distal end
110 of the shaft of an endoscope 111. Figure 58A shows the device in a
deployed configuration.
Figure 58B shows the device with the expandable member in an unexpanded or
collapsed state, as
would be appropriate for deployment of the device to a target tapered surface,
or as would be
appropriate for removal from the ablational site. Figure 58C shows the device
of Figure 58A as it
can be deployed into a tapered or concave target site such as the pylorus 9.
Figure 58D shows the
device of Figure 58A in an alternative configuration, with the electrode
bearing surface of the device
reversed such that it is facing proximally, and can thus be pulled retrograde
into a tapered or concave
site such as the lower esophageal sphincter 10.
Electrode patterns and control of ablation patterns across the surface area of
tissue
[00136] Some aspects of embodiments of the ablational device and methods of
use will now be
described with particular attention to the electrode patterns present on the
ablation structure. The
device used is shown schematically in Figures 5- 7. As shown in Figure 6, the
elongated flexible
shaft 41 of a device 100 with a fully circumferential ablation surface is
connected to a multi-pin
electrical connector 94 which is connected to the power source and includes a
male luer connector 96
for attachment to a fluid source useful in expanding the expandable member.
The elongated flexible
shaft has an electrode 98 wrapped around the circumference. The expandable
member of the device
shown in Figures 5 and 6 further includes three different electrode patterns,
the patterns of which are
represented in greater detail in Figures 7A - 7C. Typically, only one
electrode pattern is used in a
device of this invention, although more than one may be included. In the
device shown in Figure 5,
the elongated flexible shaft 41 comprises six bipolar rings 62 with about 2 mm
separation at one end
of the shaft (one electrode pattern), adjacent to the bipolar rings is a
section of six monopolar bands or
rectangles 65 with about 1 mm separation (a second electrode pattern), and
another pattern of bipolar
axial interlaced fmger electrodes 68 is positioned at the other end of the
shaft (a third electrode
pattern). In this device, a null space 70 is positioned between the last of
the monopolar bands and the
bipolar axial electrodes. The catheter used in the study was prepared using a
polyimide flat sheet of
about 1 mil (0.00 1 ") thickness coated with copper. The desired electrode
patterns were then etched
into the copper.
[00137] Alternative electrode patterns are shown in Figures 8A - 8D as 80, 84,
88, and 92,
respectively. Pattern 80 is a pattern of bipolar axial interlaced finger
electrodes with about 0.3 mm
separation. Pattern 84 includes monopolar bands with 0.3 mm separation.
Pattern 88 is that of
24
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
electrodes in a pattern of undulating electrodes with about 0.25 mm
separation. Pattern 92 includes
bipolar rings with about 0.3 mm separation. In this case the electrodes are
attached to the outside
surface of a balloon 72 having a diameter of about 18 mm. The device may be
adapted to use radio
frequency by attaching wires 74 as shown in Figure 5 to the electrodes to
connect them to the power
source.
[00138] The preceding electrode array configurations are described in the
context of an ablation
structure with a full 360 degree ablation surface, but suchpatterns or
variants thereof may also be
adapted for ablation structures that provide energy delivery across a surface
that is less than
completely circumferential, in structures, for example, that ablate over any
portion of a circumference
that is less than 360 degrees, or for example structures that ablate around a
radius of about 90 degrees,
or about 180 degrees.
[00139] Embodiments of the ablation system provided herein are generally
characterized as having
an electrode pattern that is substantially flat on the surface of an ablation
support structure and which
is non-penetrating of the tissue that it ablates. The electrode pattern forms
a contiguous treatment area
that comprises some substantial radial aspect of a luminal organ; this area is
distinguished from
ablational patterns left by electrical filaments, filament sprays, or single
wires. In some embodiments
of the invention the radial portion may be fully circumferential; the radial
portion of a luminal organ
that is ablated by embodiments of the invention is function of the combination
of (1) the
circumference of the organ, which can be relatively large in the case of the
stomach, and small when
in the case of a region in the small intestine or anus, and (2) the dimensions
of the electrode pattern.
Thus, at the high end, as noted, the radial expanse of a treatment area may be
as large as 360 degrees,
and as small as about 5 to 10 degrees, as could be the case in a treatment
area within the stomach.
[00140] Embodiments of the ablational energy delivery system and method
provided are also
characterized by being non-penetrating of the target tissue. Ablational
radiofrequency energy is
delivered from the flat electrode pattem as it makes therapeutic contact with
the tissue surface of a
treatment area, as described elsewhere in this application; and from this
point of surface contact,
energy is directly inwardly to underlying tissue layers.
[00141] Some embodiments of the ablational system and method provided herein
can be further
characterized by the electrode pattern being configured to achieve a partial
or fractional ablations,
such that only a portion of the tissue surface receives sufficient
radiofrequency energy to achieve
ablation and another portion of the surfaces receives insufficient energy to
achieve ablation. The
system and nicthod can be further configured to control the delivery of
radiofrequency energy
inwardly from the tissue surface such that depth of tissue layers to which
energy sufficient for
ablation is delivered is controlled.
[00142] Controlling the fraction of the tissue surface target area that is
ablated includes having some
fraction of the tissue ablated, at least to some degree, and having some
fraction of the surface within
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
the target area emerge from the treatment substantially free of ablation. The
ability to control the ratio
of ablated and non-ablated surface can provide substantial benefit to the
treatment. The ablational
treatment, per embodiments of this invention, may be directed toward ablating
vascular tissue, and
having minimal effect or a recoverable or transient effect on the surrounding
tissue. Thus, what is
desired is a well-controlled and modulated ablation, where a varying degree of
treatment effect can be
provided, without substantially damaging the organ, or a particular layer of
the organ. Stated in
another way, it may be generally desirable for the health of the organ within
which the targeted
vascular tissue is located, and for the health of the individual as a whole,
that some degree of normal
functioning remain in the surrounding and intervening tissue after ablation.
[00143] With regard to the effect on vascular tissue of a method that includes
fractional ablation of
cells, including vascular cells, within a target area, to some extent a
fractional ablation may be
functionally as effective as a full ablation. Vascular cells, in particular
the endothelial cells that form
the vessels, grow in an arboreal manner. It is generally believed that
isolated vasculature does not
adjoin other portions of isolated vasculature. Thus, blood vessels that
survive a fractional ablation
procedure, but are left isolated from upstream and downstream connections, are
marooned thereby
and destined to be biologically resorbed. Thus, by the preceding
considerations, it can be understood
that fractional ablation of tissue within a target area, particularly a
vascular target area, can effectively
ablate acute or chronically bleeding vessels, but advantageously leave the
tissue at large within the
target area in a state of good health. Embodiments of the method thus include
controlling the delivery
of radiofrequency energy across the surface and into a depth of tissue within
the target area, and
thereby deliver sufficient radiofrequency energy to achieve ablation in one
portion of the tissue target
area and deliver insufficient radiofrequency energy to another portion of the
surface to achieve
ablation.
[00144] Further by way of an illustrative example as to what is desirable and
being provided by the
invention, the organ in which the ablation target area is located can be
appreciated as populations of
cells within the non-vascular tissue of the target area, which can function,
based on their health, at a
functional capacity at some low threshold of 20%, for exainple, when in poor
condition, and at 100%,
when in optiinal condition. The object of the ablational treatment provided
herein, within this exaxnple
by analogy may not be to render the full population of cells to be
dysfunctional and operating at 50%
capacity. The object of the treatment may be to have some fraction of the
cells within the population,
post-ablational treatment, to remain fully functional, operating at about 100%
capacity, and to have
some remaining fraction operating at a range of lower capacity.
[00145] Controlling the fraction of the tissue surface target area that is
ablated, per embodiments of
the invention, is provided by various exemplary approaches: for example, by
(1) the physical
configuration of electrode pattern spacing in a comparatively non-dense
electrode pattern, and by (2)
the fractional operation of a comparatively dense electrode array, in a
billboard-like manner.
26
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
Generally, creating a fractional ablation by physical configuration of the
electrode pattern includes
configuring the electrode pattern such that some of the spacing between
electrodes is sufficiently
close that the conveyance of a given level of energy between the electrodes
sufficient to ablate tissue
is allowed, and other spacing between electrodes is not sufficiently close
enough to allow conveyance
of the level of energy sufficient to ablate. Embodiments of exemplary
electrode patterns that illustrate
this approach to creating fractional ablation are described below, and
depicted in Figures 48 - 55.
The creation of an ablation pattern by activating a subset of electrodes
represents an operation of the
inventive system and method which is similar to the described above, wherein
an ablational structure
with a fully circumferential pattern of electrodes can be operated in a manner
such that only a radial
fraction of the electrodes are operated.
[00146] The ablation system of the invention includes an electrode pattern
with a plurality of
electrodes and a longitudinal support member supporting the electrode pattern,
as described in
numerous embodiments herein. Energy is delivered to the electrodes from a
generator, and the
operation of the generator is controlled by a computer-controller in
communication with the
generator, the computer controller controlling the operating parameters of the
electrodes. The
computer controller has the capability of directing the generator to deliver
energy to all the electrodes
or to a subset of the electrodes. The controller further has the ability to
control the timing of energy
delivery such that electrodes may be activated simultaneously, or in subsets,
non-simultaneously.
Further, as described elsewhere, the electrodes may be operated in a monopolar
mode, in a bipolar
mode, or in a multiplexing mode. These various operating approaches,
particularly by way of
activating subsets of electrodes within patterns, allow the formation of
patterns that, when the pattern
is in therapeutic contact with a target surface, can ablate a portion of
tissue in the target area, and
leave a portion of the tissue in the target area non-ablated.
[00147] Generally, creating a fractional ablation by an operational approach
with a comparatively
dense electrode array includes operating the electrode pattern such that the
energy delivered between
some of the electrodes is sufficient to ablate, whereas energy sufficient to
ablate is not delivered
between some of the electrodes. Embodiments of exemplary electrode patterns
that illustrate this
approach to creating fractional ablation are described below, and depicted in
Figures 48 - 55.
[00148] Another aspect of controlling the fraction of tissue ablation, per
embodiments of the
invention; relates to controlling the depth of ablation into tissue layers
within the target area. Energy
is delivered inwardly from the surface, thus with modulated increases in
energy delivery, the level of
ablation can be controlled such that, for example, the ablated tissue may
consist only of tissue in the
epithelial layer, or it may consist of tissue in the epithelial layer and the
lamina propria layers, or it
may consist of tissue in the epithelial, lamina propria and muscularis mucosal
layers, or it may consist
of tissue in the epithelial, lamina propria, muscularis mucosa, and submucosal
layers, or it may consist
of tissue in the epithelial layer, the lamina propria, the muscularis mucosae,
the submucosa, and the
27
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
muscularis propria layers. In no instance is ablational energy delivered to
the serosal layer of the site
of acute or chronic gastrointestinal tract bleeding.
[00149] Embodiments of the invention include RF electrode array patterns that
ablate a fraction of
tissue within a given single ablational area, exemplary fractional arrays are
shown in Figures 48A,
49A, and 50A. These fractional ablation electrode arrays may be applied, as
above, to above to
ablational structures that address a fully circumferential target area, or a
structure that addresses any
portion of a full circumference such as 90 degree radial surface, or a 180
degree radial surface. Figure
48A shows a pattern 180 of linear electrodes 60 aligned in parallel as stripes
on a support surface. The
electrodes are spaced apart sufficiently such that when pressed against tissue
in therapeutic contact,
the burn left by distribution of energy through the electrodes results in a
striped pattern 190 on the
target tissue as seen in Figure 48B corresponding to the electrode pattern,
with there being stripes of
burned or ablated tissue 3a that alternate with stripes of unburned, or
substantially unaffected tissue
3b. In some embodiments of the method, particularly in ablation structures
that address a target area
of less than 360 radial degrees, such as a target surface that is about 180
degrees, or more particularly
about 90 degrees of the inner circumference of a lumen, the ablation may be
repeated with the
ablational structure positioned at a different angle. Figure 48C, for example,
depicts a tissue burn
pattern 191 created by a first ablational event followed by a second
ablational event after the
ablational structure is laterally rotated by about 90 degrees. Figure 48D, for
another example, depicts
a tissue burn pattern 192 created by a first ablational event followed by a
second ablational event after
the ablational structure is laterally rotated by about 45 degrees.
[00150] The effect of an ability to ablate a tissue surface in this manner
adds another level of fme
control over tissue ablation, beyond such parameters as total energy
distributed, and depth of tissue
ablation. The level of control provided by fractional ablation, and especially
when coupled with repeat
ablational events as described above in Figures 48C and 48D, is to modulate
the surface area-
distributed fraction of tissue that is ablated to whatever degree the local
maximal ablation level may
be. The fractional ablation provided by such fractional electrode pattern may
be particularly
advantageous when the effects of ablation are not intended to be absolute or
complete, but instead a
functional compromise of tissue, or of cells within the tissue is desired. In
some therapeutic examples,
thus, a desirable result could be a partial reduction in overall function of a
target area, rather than a
total loss of overall function. In a fractional ablation of a target area in
the wall of a site of acute or
chronic gastrointestinal tract bleeding, for example, a desirable result may
be the transient
compromise of non-vascular tissue. In an ablation pattern that includes a
burned area 3a and an
unburned area 3b, it can be understood that cells from the unburned area could
give rise to cells that
would migrate or repopulate the denuded area within the burned area 3b.
[00151] Figures 49A and 50A depict other examples of a fractionally-ablating
electrode pattern on
an ablation structure, and Figures 49B and 50B show the respective fractional
bum patterns on tissue
28
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
that have been treated with these electrode pattems. In Figure 49A a pattem of
concentric circles 182
is formed by wire electrodes that (from the center and moving outward) form a+-
-++- pattern.
When activated, the tissue between + - electrodes is bumed, and the tissue
between ++ electrode pairs
or - - electrode pairs is not burned. Thus, the concentric pattern 192 of
Figure 49B is formed.
Embodiments of fractionally-ablating electrode patterns such as those in
Figure 49A need not include
perfect circles, and the circles (imperfect circles or ovals) need not be
perfectly concentric around a
common center.
[00152] Similarly, Figure 50A shows a checkerboard pattern 184 of+ and -
electrodes which when
activated create a burn pattern 194 as seen in Figure 50B. Tissue that lies
between adjacent + and -
electrodes is b,_arned, while tissue that lies between adjacent + + electrodes
or - - electrode pairs
remains unburned. Figure 50B includes a representation of the location of the
+ and - electrodes from
the ablation structure in order to clarify the relative positions of areas
that are burned 3a and the areas
that remain substantially unburned 3b.
[00153] Embodiments of the invention include RF electrode array patterns that
ablate a fraction of
tissue within a given single ablational area by virtue of operational
approaches, whereby some
electrodes of a pattern are activated, and some are not, during an ablational
event visited upon a target
area. Exemplary fractional arrays are shown in Figures 51A, 52A, 53A and 54A.
These fractional
ablation electrode arrays may be applied, as above, to ablational structures
that address a fully
circumferential target area, or a structure that addresses any portion of a
full circumference such as, by
way of example, a 90 degree radial surface, or a 180 degree radial surface.
[00154] Figure 51A shows a checkerboard electrode pattern during an ablational
event during
which all electrode squares of the operational pattern 186A are operating, as
depicted by the sparkle
lines surroundir_g each electrode. Operating the electrode pattern 186A in
this manner produces an
ablation pattern 196A, as seen in Figure 51B, wherein the entire surface of
tissue within the treatment
area is ablated tissue 3a. Figure 52A, on the other hand, shows a checkerboard
electrode pattem
during an ablational event during which only every-other electrode square of
the operational pattern
i86B is operating, as depicted by the sparkle lines surrounding each activated
electrode. Operating the
electrode pattern 186B in this manner produces an ablation pattern 196B, as
seen in Figure 52B,
wherein a checkerboard fractionally ablated pattern with a dispersed pattern
of ablated squares 3a of
tissue 3a alternate with square areas of tissue 3b that are not ablated.
[00155] Figure 53A shows a striped linear electrode pattern of alternating +
and - electrodes during
an ablational event during which all electrode squares of the operational
pattern 188A are operating,
as depicted by the sparkle lines surrounding each linear electrode. Operating
the electrode pattern in
this manner 188A produces an ablation pattern 198A, as seen in Figure 53B,
wherein the entire
surface of tissue within the treatment area is ablated tissue 3a.
29
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
[00156] Figure 54A, on the other hand, shows a striped linear electrode
pattern 188B of alternating
+ and - electrodes during an ablational event during which alternate pairs of
the linear electrode pairs
are operating, as depicted by the sparkle lines surrounding the activated
linear electrodes. Operating
the electrode pattern in this manner 188B produces an ablation pattern 198B,
as seen in Figure 54B,
wherein stripes of ablated tissue 3a within the treatment area alternate
stripes of non-ablated tissue 3b.
[00157] Figure 55 is a schematic rendering of a three dimensional view of a
target region of a radial
portion of a site of acute or chronic gastrointestinal tract bleeding after it
has been ablationally treated,
per embodiments of the invention. Ablated regions 3a are rendered as regions
distributed through the
target area within a larger sea of non-ablated tissue 3b. These regions are
depicted as being slightly
conical in this schematic view, but in practice the ablated tissue region may
be more cylindrical in
shape. The regions 3a are of approximately the same depth, because of the
control exerted over the
depth of the ablation area into layers of the gastrointestinal wall, as
described herein. With such
control, the regions 3a can vary with respect of the layer to which they
extend continuously from the
upper surface where ablational energy has been applied. The conical regions
are of approximately the
same width or diameter, and distributed evenly throughout the tissue, because
of the control over
ablational surface area, as described herein. In this particular example, the
therapeutic target is
actually a particular type of cell 4b (open irregular spheres), for example, a
nerve cell, or endocrine
secretory cell; and these cells are distributed throughout the target area.
The post-ablation therapeutic
target cells 4a (dark irregular spheres) are those which happened to be
included within the conical
regions 3a that were ablated. The post-ablation cells 4a may be rendered
dysfunctional to varying
degree, they may be completely dysfunctional, they may be, merely by way of
illustrative example, on
the average, 50% functional by some measure, and there functionality may vary
over a particular
range. It should be particularly appreciated however, per embodiments of the
invention, that the cells
4b, those not included in the ablated tissue cones, are fully functional.
Controlling the ablation in terms of the tissue depth of the ablation effect
[00158] In addition to controlling the surface area distribution of ablation,
as may be accomplished
by the use of fractional ablation electrodes as described above, or as
controlled by the surface area of
electrode dimensions, ablation can be controlled with regard to the depth of
the ablation below the
level of the tissue surface where the ablative structure makes therapeutic
contact with the tissue. The
energy delivery parameters appropriate for delivering ablation that is
controlled with regard to depth
in tissue may be determined experimentally. By way of example, an experimental
set of exercises was
performed on normal immature swine in order to understand the relationship
between the electrical
parameters of electrode activation and the resultant level of ablation in
esophageal tissue. The data are
shown in detail in US Application No. 10/370,645 of Ganz et al, filed on
February 19, 2003, and in
the publication on August 21, 2003, of that application, US 2003/0158550 Al,
particularly in Tables 1
- 4 of that application. By an approach such as this, appropriate parameters
for ablation of other
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
tissues in the site of acute or chronic gastrointestinal tract bleeding tract
may be determined. Such
parameters as applied by ablational electrode patterns on an ablational
structure with a 360 degree
operating surface that is directed to esophageal tissue, by way of example,
include 300W delivered
within 300 msec, with a tightly spaced with tightly spaced bi-polar electrode
array (less than 250
microns). Ablation depth related to the energy density delivered with 8 - 12
J/cm2 results in complete
removal of the epithelium. Such parameters as applied by electrode pattems on
an ablation structure
with an operating radial surface of about 90 degrees includes multiple narrow
band electrodes spaced
250 microns wide, where the generator delivers very high power energy density
at 40W/cms to the
tissue in an energy dosage of 12-15 J/cm2. In general, depth variances can be
achieved via time of
ablation, dosage, number of energy applications, and electrode spacing.
[00159] Figure 25 provides a schematic representation of the histology of the
gastrointestinal tract
at a site of acute or chronic bleeding as it is found in various luminal
organs such as the esophagus,
stomach, pylorus, duodenum, and jejunum. The relative presence and depth and
composition of the
layers depicted in Figure 25 vary from organ to organ, but the basic
organization is similar. The
layers of the a site of acute or chronic gastrointestinal tract bleeding will
be described in their order
from the innermost to the outermost layer facing the a site of acute or
chronic gastrointestinal tract
bleeding lumen; and as seen Figure 25 and in terms of the direction from which
an ablational
structure would approach the tissue. The innermost layer can be referred to as
the surface
(epithelium), and succeeding layers can be understood as being below or
beneath the "upper" layers.
The innermost layer of a gastrointestinal tract at a site of acute or chronic
bleeding, which is in direct
contact with the nutrients and processed nutrients as they move through the
gut is a layer of
epithelium 12. This layer secretes mucous which protects the lumen from
abrasion and against the
corrosive effect of an acidic environment. Beneath the epithelium is a layer
known as the lamina
propria 13, and beneath that, a layer known as the muscularis mucosae 14. The
epithelium 12, the
laniina propria 13, and the muscularis mucosae 14 collectively constitute the
mucosa 15.
1001601 Below the mucosal layer 15 is the submucosa 16, which forms a discrete
boundary between
the muscosal layer 15 above, and the muscularis propria 17 below. The
muscularis propria 17 includes
various distinct layers of smooth muscle that enwrap the organ, in various
orientations, including
oblique, circular, and the longitudinal layers. Enwrapping the muscularis
propria 17 is the serosa 18,
which marks the outer boundary of the organ.
[00161] The entirety of gastrointestinal tract tract wall is highly vascular
and innervated. The
mucosal layer is also rich in glands and cells that secrete contents into the
lumen and secrete
hormones into the bloodstream. All of these cells, including vasculature,
exocrine cells, endocrine
cell, and nerve cells are potential targets for ablation when ablational
energy is directed toward the
region in which they reside. As a result of receiving energy, cells may be
killed or scarred to an extent
that they are no longer functional, or they may be partially damaged, leaving
some level of function.
31
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
Additionally, it should be understood that these cells all exist in
populations, and a partial ablation
may manifest in a statistical distribution of damage, in which some cells of
the population are
eliminated or damaged beyond redemption, and some cells may remain
substantially unaffected, and
fully functional. In such partial or fractional ablation events, it can be
understood that the remnant
level of function following therapeutic ablation may include a range of
function and dysfunction.
[00162] As provided by embodiments of the invention, the ablation applied to
the gastrointestinal
tract at a site of acute or chronic bleeding wall tissue may be depth-
controlled, such that only the
epithelium 12, or only a portion of the mucosal layer is ablated, leaving the
deeper layers substantially
unaffected. In other embodiments, the ablated tissue may commence at the
epithelium yet extend
deeper into the submucosa and possibly the muscularis propria, as necessary to
achieve the desired
therapeutic effect.
Device and Method for Partially-Circumferential Ablation
[00163] One embodiment of a method of ablating tissue in the gastrointestinal
tract at a site of acute
or chronic biceding tract includes the use of an ablation device with an
ablation structure supported by
conventional endoscopes 111, as illustrated in Figure 24. As described herein,
more particularly, the
tissue targeted for ablation by embodiments of an ablation device and methods
therefore is on the wall
of the gastrointestinal tract at a site of acute or chronic bleeding. An
example of one commercially
available conventional endoscope 111 is the Olympus "gastrovideoscope" model
number GIF-Q160.
While the specific construction of particular commercially available
endoscopes may vary, as shown
in Figure 24, most endoscopes include a shaft 164 having a steerable distal
end 110 and a hub or
handle 162 which includes a visual channel 161 for connecting to a video
screen 160 and a port 166
providing access to an inner working channel within the shaft 164. Dials,
levers, or other mechanisms
(not shown) will usually be provided on the handle 162 to allow an operator to
selectively steer the
distal end 110 of the endoscope 111 as is well known in the endoscopic arts.
In accordance with the
present invention, an ablation device, including an ablation structure is
advanced into the
gastrointestinal tract to a site of acute or chronic bleeding tract while
supported at the distal end of an
endoscope. Thc ablation structure is deflectable toward a tissue surface and
the ablation structure is
activated to ablate the tissue surface. Within the gastrointestinal tract at a
site of acute or chronic
bleeding tract, variously sized tissue surface sites can selectively be
ablated using the device. As will
be further described, the ablational structure of embodiments described in
this section do not
circumscribe a full 360 degrees, but rather circumscribe a fraction of 360
degrees, as will be described
further below.
[00164] In general, in one aspect a method of ablating tissue in the
gastrointestinal tract at a site of
acute or chronic bleeding tract is provided. The method includes advancing an
ablation structure into
the gastrointestinal tract at a site of acute or chronic bleeding tract while
supporting the ablation
structure with an endoscope. In some embodiments, advancing the structure into
a site of acute or
32
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
chronic gastrointestinal tract bleeding may be sufficient to place the
ablational structure of the device
into close enough proximity in order to achieve therapeutic contact. In other
embodiments, a
subsequent step may be undertaken in order to achieve an appropriate level of
therapeutic contact.
This optional step will be generally be understood as moving the ablation
structure toward the target
site. The niethod thus may further include moving at least part of the
ablation structure with respect to
the endoscope and toward a tissue surface; and activating the ablation
structure to ablate the tissue
surface. Moving at least part of the ablation structure with respect to the
endoscope can include
movement toward, away from or along the endoscope. Moving the ablational
structure toward a target
tissue surface may be performed by structures in ways particular to the
structure. For example, the
structure can be moved by inflating a balloon member, expanding a deflection
member, or moving a
deflection member. The function of such movement is to establish a
therapeutically effective contact
between the ablational structure and the target site. A therapeutically
effective contact includes the
contact being substantial and uniform such that the highly controlled
electrical parameters of radiant
emission from the electrode result in similarly highly controlled tissue
ablation. Some embodiments
of the invention further include structure and method for locking or securing
such a therapeutically
effective contact once established. Thus, some embodiments include a position
locking step that, for
example, uses suction to secure the connection between the ablation structure
and the tissue site.
[00165] As shown in Figures 9, 10, 11, and 26, in one aspect a method of
ablating tissue in the
gastrointestinal tract at a site of acute or chronic bleeding includes an
ablation device 100 for ablating
a tissue sur uce 3, wherein the device 100 includes an ablating structure, for
exatnple, an ablation
structure 101 supported by an endoscope 111. The method includes ablating
tissue in the wall of a
luminal organ of the gastrointestinal tract at a site of acute or chronic
bleeding tract by the steps of (1)
advancing the ablation structure 101 into the luminal organ; (2) deflecting
the ablation structure 101
toward a tissue surface 3; and (3) activating the ablation structure to ablate
the tissue surface 3. As
shown in Figure 9, the device 100 can additionally include a housing 107,
electrical connections 109,
an inflation line 113 and an inflation member or balloon 105.
[00166] The ablation structure 101, in one embodiment is an electrode
structure configured and
arranged to deliver energy comprising radiofrequency energy to the mucosal
layer of the wall of the
organ at a site of acute or chronic gastrointestinal tract bleeding tract. It
is envisioned that such an
ablation structure 101 can include a plurality of electrodes. For example, two
or more electrodes may
be part of an ablation structure. The energy may be delivered at appropriate
levels to accomplish
ablation of mucosal or submucosal level tissue, or alternatively to cause
therapeutic injury to these
tissues, while substantially preserving muscularis tissue. The term "ablation"
as used herein generally
refers to thermal damage to the tissue causing any of loss of function that is
characteristic of the
tissue, or tiss e necrosis. Thermal damage can be achieved through heating
tissue or cooling tissue
(i.e. freezing). In some embodiments ablation is designed to be a partial
ablation.
33
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
[00167] Although radiofrequency energy, as provided by embodiments of the
invention, is one
particular form of energy for ablation, other embodiments may utilize other
energy forms including,
for example, microwave energy, or photonic or radiant sources such as infrared
or ultraviolet light, the
latter possibly in combination with improved sensitizing agents. Photonic
sources can include
semiconductor emitters, lasers, and other such sources. Light energy may be
either collimated or non-
collimated. Other embodiments of this invention may utilize heatable fluids,
or, alternatively, a
cooling medium, including such non-limiting examples as liquid nitrogen, Freon
TM, non-CFC
refrigerants, CO2 or N20 as an ablation energy medium. For ablations using hot
or cold fluids or
gases, the ablation system may include an apparatus to circulate the
heating/cool medium from
outside the patient to the heating/cooling balloon or other element and then
back outside the patient
again. Mechar.isms for circulating media in cryosurgical probes are well known
in the ablation arts.
For example, and incorporated by reference herein, suitable circulating
mechanisms are disclosed in
U.S. Pat. No. 6,182,666 to Dobak, U.S. Pat. No. 6,193,644 to Dobak, U.S. Pat.
No. 6,237,355 to Li,
and U.S. Pat. No. 6,572,610 to Kovalcheck.
[00168] In a particular embodiment, the energy delivered to the wall of a
luminal organ at a site of
acute or chronic gastrointestinal tract bleeding tract comprises
radiofrequency energy that can be
delivered from the energy delivery device 100. Radiofrequency energy can be
delivered in a number
of ways. Typically, the radiofrequency energy will be delivered in a bipolar
fashion from a bipolar
array of electrodes positioned on the ablation structure 101, in some cases on
an expandable structure,
such as a balloon, frame, cage, or the like, which can expand and deploy the
electrodes directly
against or immediately adjacent to the mucosal tissue so as to establish a
controlled level of
therapeutic contact between the electrodes and the target tissue (e.g.,
through direct contact or through
a dielectric membrane or other layer). Alternatively, the electrode structure
may include a monopolar
electrode struet!i-re energized by a radiofrequency power supply in
combination with a return electrode
typically positioned on the patient's skin, for example, on the small of the
back. In any case, the
radiofrequency energy is typically delivered at a high energy flux over a very
short period of time in
order to injure or ablate only the mucosal or submucosal levels of tissue
without substantially heating
or othherwise damaging the muscularis tissue. In embodiments where the
ablation structure includes a
plurality of electrodes, one or more of the electrodes can be bipolar or
monopolar, and some
embodiments include combinations of bipolar and monopolar electrodes.
[00169] The ablation structure 101 can be arranged and configured in any of a
number ways with
regard to shape and size. Typically, the array has an area in the range from
about 0.5 cm2 to about 9.0
cm2. Typical shapes would include rectangular, circular or oval. In one
embodiment, the ablation
structure 101 has an area of about 2.5 cm2. In another embodiment, the
ablation structure 101 has an
area of about 4 cm2 and dimensions of about 2 cm. by 2 cm.
34
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
[00170] The housing 107 of the ablation device 100 is arranged and configured
to support the
ablation structure 101. The housing 107 can be made of any suitable material
for withstanding the
high energy flux produced by the ablation structure 101. As shown in Figures 9-
14,17,18, 21, and
22, in one embodiment, the housing 107 is sandwiched between the ablation
structure 101 and an
endoscope 111 when the ablation device 100 is supported by an endoscope 111.
One end of the
ablation structure 101 can be further away from the endoscope than the other
end to improve ease of
contact with the targeted tissue (not shown). For example, to ensure the
proximal end of the ablation
structure 101 makes contact with the targeted tissue, the proximal end of the
electrode may be
supported by a tapered housing member 107.
[00171] The electrical connections 109 of the ablation device connect the
ablation structure 101 to a
power source. The electrical connections 109 can include a single wire or
plurality of wires as needed
to provide controlled energy delivery through the ablation structure 101. In
one embodiment, the
electrical connections 109 include low electrical loss wires such as litz
wire.
{00172] The inflation line 113 is arranged and configured to transport an
expansion medium,
typically a suirable fluid or gas, to and from the inflation member. In one
embodiment, the inflation
line is a flexible tube. The inflation line 113 can be made of polymer or co-
polymers, such as the non-
limiting exarnples of polyimide, polyurethane, polyethylene terephthalate
(PET), or polyamides
(nylon). The inflation member 105 is designed to deflect the ablation device
100 in relation to a target
tissue surface 3. The inflation member 105 can be reversibly expanded to an
increased profile.
[00173] In one embodiment, the inflation member 105 additionally serves as an
attachment site for
support of the ablation device 100 by an endoscope 111. As shown in Figures 9 -
14, 17,18,21 and
22, the inflation member 105 can be deployed from a low profile configuration
or arrangement (see
Figures 10, and 20) to an increased profile configuration or arrangement (see
Figures 11-14, 17- 19)
using the expansion medium. In preparation for ablation, when the inflation
member 105 is
sufficiently inflated, deflection of the ablation device 100 in relation to a
tissue surface 3 can be
achieved. As shown in Figures 11, 31, 42, and 44, in one embodiment,
deflection of the ablation
device 100 results in a therapeutic level of contact, i.e., a substantially
direct, uniform, and sustainable
contact between the ablation structure 101 of the device 100 and the target
tissue surface 3. For
example, as shown in Figures 31, 42, and 44, when the inflation member 105 is
sufficiently inflated,
the resulting expanded profile of the inflation member 105, which contacts the
tissue surface 3, results
in contact by deflection between the tissue surface 3 of the inner wall 5 of a
luminal organ a
gastrointestinal tract at a site of acute or chronic bleeding tract and the
ablation structure 100. In these
embodiments, suction can be applied in combination with the inflation member
105 to achieve contact
between the ablation structure 101 and the tissue surface 3. Suction can be
achieved through the
endoscope 111 or through the ablation device 100 to aid in collapsing the
targeted tissue surface 3
around the ablation structure 101.
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
[00174] In various embodiments, the inflation member 105 may be compliant, non-
compliant or
semi-compliant. The inflation member 105 can be made of a thin, flexible,
bladder made of a material
such as a polymer, as by way of non-limiting examples, polyimide,
polyurethane, or polyethylene
terephthalate (PET). In one embodiment, the inflation member is a balloon.
Inflation of the inflation
member 105 can be achieved through the inflation line 113 using, for example,
controlled delivery of
fluid or gas expansion medium. The expansion medium can include a compressible
gaseous medium
such as air. The expansion medium may alternatively comprise an incompressible
fluid medium, such
as water or a saline solution.
[00175] As shown in Figures 12,13, and 14, the inflation member 105 can be
configured and
arranged in a variety of ways to facilitate deflection of the ablation device
100 in relation to a tissue
surface 3. For example, as shown in Figure 12, the inflation member 105 can be
eccentrically
positioned in relation to the supporting endoscope 111 as well as the housing
107 and the ablation
structure 101. Alternatively, as shown in Figure 13, the inflation member 105
can be positioned
concentrically in relation to the supporting endoscope 111 and the ablation
structure 101 can be
attached to the inflation member 105 distally from the endoscope 111. In
another embodiment, as
shown in Figure 12, the inflation member 105 can be positioned between the
supporting endoscope
111 and the ablation structure 101. The ablation structure 101 shown in
Figures 12 -14 can cover a
range of circumferential span of the endoscope 111 spanning, for example, from
about 5 to 360
degrees when inflation member 105 is deployed.
[00176] One method of ablating tissue in a luminal organ of the
gastrointestinal tract at a site of
acute or chronic bleeding tract can include a first step of advancing an
ablation structure 101, to a site
of acute or chronic gastrointestinal tract bleeding. In a second step, the
ablation structure 101 is
supported with an endoscope 111 at the site of acute or chronic
gastrointestinal tract bleeding. In a
third step, the ablation structure 101 is deflected toward a tissue surface 3.
In a fourth step, energy can
be applied to the ablation structure 101 to ablate the tissue surface 3.
[00177] In another method, the step of advancing an endoscope-supported
ablation structure 101 can
include advancing the endoscope 111 into a luminal organ at a site of acute or
chronic gastrointestinal
tract bleeding and advancing the ablation structure 101 over the endoscope
111. For example, the
endoscope 111 can be positioned relative to an ablation target tissue surface
3 after which the ablation
structure 101 can be advanced over the outside of the endoscope 111 for
ablating the target tissue
surface 3.
[00178] In a further method, the step of supporting the ablation structure 101
with an endoscope 111
includes inserting the endoscope 111 into the ablation structure 101 (see for
example, Figures 1A -
2B). In a related method, the ablation structure 101 is supported by a sheath
103 (see Figures 26 - 28,
30, 31, 32 and 37) and the step of inserting the endoscope 111 into the
ablation structure 101 includes
36
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
inserting the endoscope 111 into the sheath 103. In a further related method,
the step of inserting the
endoscope 111 into the sheath 103 includes creating an opening in the sheath
103 (not shown).
[00179] In a particular method, a distal portion of a sheath 103 having a
smaller outer diameter than
a proximal portion of the sheath 103, is adapted to be expanded when an
endoscope 111 is inserted
into it.
[00180] In another method, the step of advancing the ablation structure 101
into the gastrointestinal
tract at a site of acute or chronic bleeding includes advancing the ablation
structure 101 through a
channel of the endoscope 111 from either the endoscopes proximal or distal end
(as discussed below
for Figures 34A, 35A and 36A). In yet another method, the step of supporting
the ablation structure
101 comprises supporting the ablation structure 101 with a channel of the
endoscope (see as discussed
below for Figures 34A, 35A, 36A, 37 - 39). In a further method, a deflection
structure or deflection
member 150 is advanced through a channel of the endoscope 111 and the step of
deflecting the
ablation structure 101 toward a tissue surface 3 includes deflecting the
ablation structure 101 with the
deflection structure or deflection member 150.
[00181] As illustrated in Figures 34A, 35A, and 36A, variously adapted and
configured ablation
structures 10i can fit within and be conveyed through an endoscope internal
working channel 211. In
each case, the ablation structure 101 and an accompanying deflection mechanism
can be conveyed
through the internal working channel 211 in a dimensionally compacted first
configuration that is
capable of expansion to a second radially expanded configuration upon exiting
the distal end 110 of
the endoscope 111 (For example, see Figures 34A, 34B, 35A, 35B, 36A, and 36B).
[00182] As shown in Figure 34B, in one embodiment, the deflection mechanism is
an inflation
member 105, to which the ablation structure 101 can be integrated within or
mounted/attached to, for
example by etching, mounting or bonding. The inflation member 105 can be, for
example, a
compliant, non-compliant or semi-compliant balloon.
[00183] As shown in Figures 35B and 35B, in another embodiment, the deflection
mechanism is an
expandable member 209 that can expand to a second desired arrangement and
configuration. As
shown in Figure 35B, the expandable member 209, can be an expandable stent,
frame or cage device,
to which an ablation structure 101 is mounted or integrated. For example,
where the expandable
member 209 is a wire cage, the wires can be a component of a bipolar circuit
to provide the ablation
structure 101 feature. Alternatively, the cage can have a flexible electrode
circuit bonded or can be
attached to an outer or inner surface of the cage to provide an ablation
structure 101 that is an
electrode. As shown in Figure 36B, the expandable member 209, can be a folded
or rolled series of
hoops including or having an attached ablation structure 101 that expands upon
exiting the endoscope
distal end 110.
[00184] As further illustrated in Figures 37 - 39, the ablation structure 101
can be supported with a
channel of the endoscope 111. In one embodiment as shown in Figures 37 - 39,
an ablation device
37
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
100 includes a deflection member 150 that supports an attached housing 107 and
ablation structure
101. As shown in Figure 39, the endoscope 111 includes an internal working
channe1211 suitable for
advancing or retreating the deflection member 150 which is connected to an
internal coupling
mechanism 215 of the ablation device 100. Figures 37 and 39 both show a
deflection member 150
including a bent region of the deflection member 150 in a deployed position,
wherein the deflection
member 150 bent region is positioned external to the endoscope distal end 110.
Figure 38 shows the
deflection member 150 in an undeployed position, wherein the deflection member
150 bent region is
positioned internal to the endoscope 111. The ablation structure 101 is thus
supported with a channel
of the endoscope 111 (the internal working channe1211 of the endoscope 111) by
way of the
deflection member 150 and the connected internal coupling mechanism 215 of the
ablation device
100.
[00185] In addition, when the deflection member 150 is advanced or moved
proximally or distally
within the endoscope internal working channe1211, the deflection member 150 is
accordingly
advanced through a channel of the endoscope 111. In another implementation, as
shown in Figure 42,
wherein the deflection mechanism is an inflatable member 105 (shown in a
deployed configuration)
coupled to an inflation line 113, the inflation line 113 can be disposed
within the endoscope internal
working channel 211. In yet another implementation, both the inflatable member
105 (in an
undeployed configuration) and inflation line 113 can be advanced within the
internal working channel
211 either proximally or distally in relation to the endoscope 111. Conductive
wires 109 can pass
through the working channel (not shown) or outside as shown in Figure 37.
[00186] As shown in Figure 41, in another implementation the endoscope 111
includes an internal
working channel 211 suitable for supporting the ablation housing 107 and
ablation structure 101
which are connected to an internal coupling mechanism 215 of the ablation
device 100. As such, the
connected ablation structure 101 is supported within a channel of the
endoscope 111. Additionally as
shown in Figure 41, the housing 107 and ablation structure 101 can further be
supported by an
external region of the endoscope 111, wherein the internal coupling mechanism
215 is adapted and
configured to position the housing 107 in contact with the external region of
the endoscope 111. The
internal coupling mechanism 215 can be cannulated (not shown) to facilitate
use of the working
channel to aspirate and flow in fluids or air.
[00187] In another ablation method, an additional step includes moving the
ablation structure 101
with respect to the endoscope 111 within a luminal organ of the
gastrointestinal tract at a site of acute
or chronic bleeding. As illustrated in Figures 27, 28, 30, 32, and 47, and as
discussed below, a sheath
103 of the ablation device 100 to which the ablation structure 101 is attached
can enable moving the
ablation structure 101 with respect to the endoscope 111. Further, as
illustrated in Figures 34A, 35A,
36A, 37,38, 39, and 41, and discussed above, an internal working channe1211 of
the endoscope 111
38
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
through which at least a part of the ablation device 100 is disposed can
enable moving the ablations
structure 101 with respect to the endoscope 111.
[00188] Referring to Figures 11, 31, 42, and 44, in yet another method, the
step of deflecting the
ablation structure 101 toward a tissue surface 3 includes inflating an
inflation member 105 of the
ablation device 100 within a luminal organ of the gastrointestinal tract at a
site of acute or chronic
bleeding. The inflation member 105 can be arranged and configured to be
reversibly inflatable. The
inflation member 105 can be inserted along with the ablation structure 101
into an alimentary tract in
a collapsed configuration and expanded upon localization at a pre-selected
treatment area. In one
implementation, the inflation member 105 is a balloon. For example, in Figures
11, 31, 42, and 44 it
is shown ho-%v deflecting the ablation structure 101 toward a tissue surface 3
is achieved when the
inflation member 105 is inflated or deployed. As illustrated in Figures 11,
31, 42, and 44, upon
sufficient inflation, the inflation member 105 contacts a tissue surface 3
consequently deflecting the
ablation structure 101 which contacts an opposing tissue surface 3.
[00189] As shown in Figures 19B, 20, 35, 36 and discussed above, in a further
method, the step of
deflecting the ablation structure 101 includes expanding a deflection
structure or deflection member
150. In one implementation, as shown in Figure 19A the ablation device 100
includes a sheath 103,
wherein the sheath 103 is arranged and configured to receive the deflection
member 150, the
endoscope 111 and ablation structure 101 internally to the sheath 103. In one
implementation, the
deflection member 150 is a shape memory alloy, for example, Nitinol. The
flexible extensions of the
deflection member 150 in this embodiment can be coupled to the endoscope, an
elastomeric sheath
115 of the ablation device 100 (shown in Figure 19A) or any part of the device
100, including the
ablation housing 107.
[00190] As shown in Figures 34, 35, 36, 37, 38, and 39, and discussed above,
in a further method,
the step of deflecting the ablation structure 101 includes moving a deflection
structure or deflection
member 150.
[00191] Briefly, in each case moving the deflection 150 is used to change the
deflection member
150 from a non-deployed to a deployed configuration. As shown in Figure 23, in
one embodiment,
deflecting the ablation structure 101 includes a flexing point in the ablation
structure 101, wherein the
ablation structure 101 can deflect in response to, for example, resistance met
in contacting a tissue
surface 3.
[00192] As shown in Figures 43, 44, and 45A - 45C and as discussed in further
detail below, in
another method, the step of deflecting the ablation structure 101 includes
rotating, pivoting, turning or
spinning the ablation structure 101 with respect to the endoscope 111 along
their respective and
parallel longitudinal axes. Deflection of the ablation structure 101 with
respect to the endoscope 111
can occur in combination with the endoscope 111 distal end 110 deflecting with
respect to a target site
on the wall of a luminal organ of the gastrointestinal tract at a site of
acute or chronic bleeding. Also,
39
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
the ablation structure 101 can deflect in combination with an inflation member
105 used to achieve
apposition of the ablation device 100 to the tissue. In some embodiments, the
step of deflecting the
ablation structure 101 may additionally include any combination of the above
disclosed deflecting
steps.
[00193] As shown in Figures 19, 20, 21, 22, 34A, 34B, 35A, 35B, 36A, 36B, 46B,
and 47, in
another ablation method, an additional step includes moving the ablation
structure 101 from a first
configuration to a second radially expanded configuration. The details
regarding radial expansion of
the ablation structure 101 shown in Figures 19, 20, 21, and 22 are described
below, while the details
for Figures 34A, 34B, 35A, 35B, 36A, and 36B are described above.
Additionally, as shown in
Figures 46B and 47 the ablation structure 101 can be arranged in a first
configuration wherein the
ablation structure 101 is coupled directly or alternatively through an housing
107 (not shown) to an
inflation member 105 attached to a catheter 254. In an undeployed
configuration as shown in Figures
46B and 47, the non-inflated inflation member 105 and ablation structure 101
have a relatively low
profile in relation to the endoscope 111. When deployed, the inflation member
105 moves the ablation
structure 101 to a second radially expanded configuration (not shown).
[00194] As shown in Figures 15,16, 40, 43, 44, 45A - 45C, 46B, and 47, in a
further method, an
additional step includes attaching the ablation structure 101 to the endoscope
111. As shown in
Figures 15 and 16, attachment of the ablation structure 101 to the endoscope
111 can also be by way
of an elastomeric sheath 115 The elastomeric sheath 115 can removably hold the
ablation structure
101 in a desired position on the endoscope 111. The elastomeric sheath 115 can
be arranged and
configured to fit over the endoscope distal end 110. As shown in Figures 15
and 16, the inflation
member 105 can be attached to the elastomeric sheath 115 or alternatively the
inflation member 105
can also act as the "elastomeric sheath" (not shown).
[00195] In another method, the step of attaching the ablation structure 101 to
the endoscope 111
includes attaching the ablation structure 101 to an outside surface of the
endoscope. Alternatively, the
attaching step can include, for example, attaching to an inside surface, an
outside or inside feature of
the endoscope, or any combinations of the above. Lubricants such as water,
IPA, jelly, or oil may be
use to aid attachment and removal of the ablation device from the endoscope.
[00196] As shown in Figure 41, in a further method, the step of attaching the
ablation structure 101
to the endoscope 111, includes an ablation structure 101 having an attached
rolled sheath 116,
wherein attaching the ablation structure 101 to the endoscope 111 includes
unrolling the sheath 116
over an outside surface of the endoscope 111. The rolled sheath 116 can
additionally cover the
electrical connections 109 of the ablation device 100 along a length of the
endoscope 111 (see Figure
41). In a related method, the ablation structure 101 is attached to the
endoscope 111 by an attaching
step including unrolling the rolled sheath 116 over an outside surface of the
endoscope 111 and part of
the ablation structure 101.
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
[00197] ui aiiother method, as shown in Figure 40, the step of attaching the
ablation structure 101 to
the endoscope 111 includes attaching the ablation structure 101 to a channel
of the endoscope. As
shown in Figure 40, in one implementation, the housing 107 and ablation
structure 101 are coupled to
an internal coupling mechanism 215 that can be positioned within an internal
working channe1211 of
the endoscope 111. The internal coupling mechanism 215 in Figure 40 is shown
as attached to the
intemal working channel 211 at the endoscope distal end 110. In this
embodiment, the housing 107
and ablation structure 101 are shown in alignment with and coupled to an
outside surface of the
endoscope 111 near the distal end 110.
[00198] In one method of ablating tissue in the alimentary tract, the tissue
surface 3 can include a
first treatment area and activation of the ablation structure 101 step can
include activation of the
ablation structure 101 to ablate the first treatment area, and further include
moving the ablation
structure 101 to a second area without removing the ablation structure 101
from the patient and
activating the ablation structure 101 to ablate the second tissue area 3.
Moving, in this sense, refers to
moving the ablational structure to the locale of a target site, and
thereafter, further moving of the
structure into a therapeutically effected position can be performed variously
by inflating a balloon
member, or deflection or inflating a deflection member, as described in detail
elsewhere. For example,
where two or more areas of the tissue surface 3 of a target area in the wall
of an organ in the a site of
acute or chronic gastrointestinal tract bleeding tract can be ablated by
directing the ablation structure
101 to the first target region and then activating the ablation structure 101
to ablate the tissue surface
3. Then, without removing the ablation structure 101 from the patient, the
ablation structure 101 can
be directed to the second target area in the wall of an organ for ablation of
the appropriate region of
the tissue surface 3.
[00199] In general, in another aspect, an ablation device 100 is provided that
includes an ablation
structure 101 removably coupled to an endoscope distal end 110, and a
deflection mechanism adapted
and configured to move the ablation structure 101 toward a tissue surface 3
(see for example, Figures
5-19, 22, 22, 27 - 29, 30 - 32, 34A, 35A, 36A, 37, 38, 39, 42, 44, and 47).
[00200] In a related embodiment, the ablation device 100 additionally includes
an ablation structure
movement mechanism adapted to move the ablation structure 101 with respect to
the endoscope 111.
As discussed below and shown in Figures 26 - 28, and 30 - 32, the ablation
structure movement
mechanism can be a sheath 103 to which the ablation structure 101 is attached,
wherein the sheath
103 is arranged and configured to move the ablation structure 101 with respect
to an endoscope 111
received within the sheath 103. Alternatively, as discussed above and shown in
Figures 34A, 35A,
36A, and 37 - 39, the ablation structure movement mechanism can be in the form
of an internal
coupling mechanism 215 of the ablation structure 100, wherein the ablation
structure is connected to
the internal coupling mechanism 215 and at least a portion of the internal
coupling mechanism 215 is
disposed internally to the endoscope.
41
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
[00201] In another embodiment, the ablation device 100 additionally includes a
coupling
mechanism designed to fit over an outside surface of an endoscope 111, to
couple the ablation
structure 101 with the endoscope 111. As discussed above, a spiral sheath 104,
an elastomeric sheath
115, a rolled sheath 116 and an intemal coupling mechanism as shown in Figures
15, 16, 40, and 41
respectively, are examples of such coupling mechanisms. In a particular
embodiment, the coupling
mechanism includes a sheath 103 capable of supporting the ablation structure
101. The sheath 103 can
be tubing, a catheter or other suitable elongate members. The sheath 103 can
be arranged and
configured so that it can be moved independently of an associated endoscope.
[00202] As shown in Figure 41, in another embodiment, the sheath 103 can be
arranged and
configured as a rolled sheath 116 that can be unrolled over the outside
surface of the endoscope. In
use, a rolled sheath 116 connected to the ablation device 100, for example at
substantially near the
proximal end of the housing 107 (from the perspective of an operator of the
device), can be unrolled
from such a position and continue to be unrolled toward the proximal end 112
of the endoscope 111
(see Figure 47). In this way, the rolled sheath 116 can be caused to contact
and cover all or a portion
of the length of the endoscope 111 (not shown). Additionally, as the rolled
sheath 116 is unrolled
along the endoscope 111, it can sandwich the electrical connections 109
between the rolled sheath 116
and the endoscope 111 (see generally Figure 41).
[00203] In another embodiment, as shown in Figures 30 and 31, the sheath 103
can be arranged and
configured to support a deflection mechanism wherein the deflection mechanism
includes a deflection
structure or deflection member 150. As illustrated in Figures 30 and 31, where
the deflection member
150 is an inflation member 105, the inflation member 105 can be directly
attached to the sheath 103.
As shown in each case, the inflation member 105 is positioned opposite the
placement of the ablation
structure 101, which is also attached to the sheath 103. This configuration of
the sheath 103 provides
support for the inflation member 105 and the ablation structure 101
irrespective of the positioning of
the endoscope distal end 110. For example, as shown in Figure 30, the
endoscope distal end 110 can
be positioned to provide a gap between the distal end 110 and a distal end of
the sheath 103 where the
ablation structure 101 and inflation member 105 are positioned. In contrast,
as shown in Figure 31 the
endoscope distal end 110 can extend through and beyond the distal end of the
sheath 103.
[00204] In another embodiment, as shown in Figure 26, the sheath 103 can be
elongated. Figure 26
illustrates a sheath including electrical connections 109 and an inflation
line 113. The sheath 103 may
include pneumatic and/or over extruded wires impregnated within the sheath
103. In use, the sheath
103 can be introduced first into an alimentary tract, wherein the sheath 103
serves as a catheter like
guide for introduction of the endoscope 111 within the sheath 103.
Alternatively, the endoscope 111
may be introduced first and thereby serve as a guidewire for the sheath 103 to
be introduced over.
Figure 26 also shows attachment of an inflation member 105 to the sheath 103,
in an arrangement
42
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
wherein the ablation structure 101 is attached to the inflation member 105
opposite the sheath 103
attachment point.
[00205] In embodiments shown in Figures 27 and 28, the sheath 103 includes an
optically
transmissive portion 158 adapted and configured to cooperate with a visual
channel of an endoscope
111. For example, the sheath 103 may be made of clear, translucent or
transparent polymeric tubing
including PVC, acrylic, and Pebax (a polyether block amide). As shown in
Figure 24, one
component of an endoscope 111 can be a visual channe1161 that provides visual
imaging of a tissue
surface 3 as imaged from the endoscope distal end 110. For example, the
transmissive portion 158 can
allow visualization of the wall of an esophagus 3 through the transmissive
portion 158 of the sheath
103. As shown in Figure 28 and in the cross-section view provided in Figure
29, the sheaths 103
shown in Figures 27 and 28, include an optically transmissive portion 158
arranged and configured
to provide viewing of tissue surfaces 3 through the wall of the sheath 103,
with the aid of an internally
disposed endoscope 111 having a visual channel 161. Also shown in cross-
section in Figure 29 are
portions of the sheath 103 through which electrical connections 109 and an
inflation line 113 can pass.
These features may be imbedded into the sheath 103 inner-wall or attached to
the sheath 103 inner
wall. As shown in Figure 27, the sheath 103 including a transmissive portion
158 can extend past the
endoscope distal tip 110. Alternatively, as shown in Figures 27, 28, and 31,
the endoscope distal end
110 can extend distally past the transmissive portion 158 of the sheath 103.
[00206] In another implementation, the transmissive portion 158 of the sheath
103 can be reinforced
structurally with coil or braid elements incorporated therein to prevent
ovalization and/or collapsing
of the sheath 103, particularly while deflecting the ablation device 100
[00207] In a further embodiment, the sheath 103 includes a slit 203 formed in
a proximal portion of
the sheath 103, the slit 203 being designed to open to admit an endoscope
distal end 110 into the
sheath 103. As shown in Figure 32 the proximal portion of the sheath 103 can
include a perforation
region or slit 203. The slit 203 can extend partially of fully along the
length of the sheath 103. The slit
203 enables the sheath 103 to be pulled back, or opened when, for example
introducing an endoscope
111 into the sheath 103. In one implementation, as shown in Figure 32, the
sheath 103 additionally
includes a locking collar 205 for locking the sheath 103 in a desired position
in respect to the
endoscope 111.
[00208] As shown in Figures 33A and 33B, the distal portion of the sheath 103
can have a smaller
outer diameter than a, proximal portion of the sheath 103, the distal portion
of the sheath 103 being
adapted and configured to be expanded when an endoscope 111 is inserted into
it (not shown). This
embodiment can aid in accessing an endoscope 111 in a case where the sheath
103 is advanced first
into a target site within the alimentary tract. Since the distal end of the
sheath 103 is smaller in
diameter, but includes a slit 203, the sheath 103 can accept a larger outside
diameter endoscope 111
43
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
because when the endoscope 111 is advanced, the slit 203 of the sheath 103
allows for widening of
the sheath 103.
[00209] In general, in another aspect, a method of ablating tissue in within
the alimentary tract
includes advancing an ablation structure 101 into the alimentary tract while
supporting the ablation
structure 101 with an endoscope 111. The endoscope distal end 110 can be bent
to move the ablation
structure 101 into contact with a tissue surface followed by activation of the
ablation structure 101 to
ablate the tissue surface 3 (see e.g., Figure 43). In a particular embodiment,
the ablation structure 101
includes a plurality of electrodes and the activating step includes applying
energy to the electrodes.
[00210] In general, in another aspect the coupling mechanism is designed to
fit over an outside
surface of an endoscope 111, to couple the ablation structure 101 with the
endoscope 111, rather than
being for example, a sheath (as discussed above), and is adapted and
configured to provide a certain
freedom of movement to the ablation structure 101, including but not limited
to flexing and/or
rotating and/or pivoting with respect to the endoscope 111 when coupled to the
endoscope 111. The
freedom of movement is with respect to one, two, or three axes, thereby
providing one, two, or three
degrees of freedom. Non-limiting examples of suitable coupling mechanisms
include a flex joint, pin
joint, U-joint, ball joint, or any combination thereof. The following
described coupling mechanism
embodiments advantageously provide for a substantially uniform apposition
force between a
supporting endoscope 111 and an ablation structure 101 when localized at a
target tissue surface 3.
[00211] As shown in Figures 43, 44, 45A, and 45B, the coupling mechanism can
be a ring 250
attached to the housing 107 and the endoscope 111, wherein the housing 107 is
adapted and
configured to flex, rotate or pivot about the ring 250. For exainple, as
illustrated in Figure 43, where
the ablation device 100 is coupled to a deflectable distal end 110 of an
endoscope 111 by a ring 250,
when the device 100 is deflected toward the tissue surface 3 of the wall of
the lumen of the
gastrointestinal tract a site of acute or chronic bleeding, the housing 107
upon contact aligns the
ablation structure 101 with the tissue surface 3 by flexing, rotating or
pivoting about the ring 250
coupling. In these embodiments, the endoscope and the housing that supports
the ablation structure
both have their own longitudinal axis, and these axes are situated parallel to
each other. The coupling
mechanism that attaches the housing to the endoscope allows a pivoting
movement between the
longitudinal axis of the housing and the longitudinal axis of the endoscope.
Advantageously,
sufficient contact pressure provided by deflection of the distal end 110 of
the endoscope 101 can
produce a desired degree of contact between the ablation structure 101 and the
tissue surface 3,
irrespective of the precise alignment of the distal end 112 in respect to a
plane of the tissue surface 3
to be treated.
[00212] For the purposes of this disclosure, a "desired degree of contact",
"desired contact",
"therapeutic contact", or "therapeutically effective contact" between the
ablation structure 101 and the
tissue surface 3, includes complete or substantially-complete contact between
all or a portion of a
44
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
predetermined target on the tissue surface 3 (e.g. a site on the wall of a
luminal organ of the
gastrointestinal tract at a site of acute or chronic bleeding) by all or a
portion of the ablation structure
101. ?t should also be understood that therapeutic contact, as described in
this disclosure typically
occurs as a consequence of an ablational surface on an apparatus having been
moved into such contact
by the expansion of an expandable member such as a balloon, or by expanding,
moving, or deflecting
a deflection structure. By all such approaches, such movement or bringing into
therapeutic contact
includes the exertion or application of pressure. Such pressuring is a factor
in effecting coaptive
ablation, wherein pressure exerted through tissue on blood vessels causes them
to be partially or
substantially emptied of blood, and coincidentally serves as a counter-
pressure that prevents entry of
blood normally brought about by blood pressure. Thus, any occurrence of moving
or expanding a
member so as to bring an ablation surface against target tissue can also be
understood as pressuring
the tissue.
[00213] As shown in Figure 44, in a different yet related embodiment, where
the deflection
mechanism of the ablation device 100 is an inflatable member 105, a ring 250
coupling allows for
flexing, rotating or pivoting of the housing 107 and ablation structure 101.
As in the previous case,
sufficient contact pressure provided through deflection, here by the
inflatable member 105, can
produce a desired degree of contact between the ablation structure 101 and the
tissue surface 3. Again,
advantageously, the desired contact can be achieved irrespective of the
precise alignment of the
deflected endoscope 111 distal end 110 in respect to a plane of the tissue
surface 3 to be treated,
because of the flexing, rotating or pivoting provided by the ring 250
coupling.
[00214] As shown in Figure 45A, in a related embodiment, the coupling
mechanism between the
ablation device 100 and an endoscope 111 can be an elastic band 252, wherein
the housing 107 of the
device 100 is flexibly coupled to the elastic band 252. For example, as
illustrated in Figure 45C,
where the ablation device 100 is coupled to a distal end 110 of an endoscope
111 by an elastic band
252, when the device 100 is deflected toward a tissue surface 3 of the wall of
a luminal organ of the
gastrointestinal tract at a site of acute or chronic bleeding, alignment
between the housing 107 and
accordingly the ablation structure 101 and the tissue surface 3, can be
achieved by flexing about the
elastic band 252 coupling. Once more, advantageously, the desired contact can
be achieved
irrespective of the precise alignment of the deflected endoscope's 111 distal
end 110 in respect to a
plane of the tissue surface 3 to be treated, because of the flexing capability
provided by the elastic
band 252 coupling.
[00215] As shown in Figure 45A, in another related embodiment, the coupling
mechanism between
the ablation device 100 and an endoscope 111 can be a combination of a ring
250 and an elastic band
252, wherein the housing 107 of the device 100 is coupled to the elastic band
252. For example, as
illustrated in Figure 45A, where the ablation device 100 is coupled to a
distal end 110 of an
endoscope 111 by an elastic band 252, when the device 100 is deflected toward
a tissue surface 3 of,
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
for example, the wall of a luminal organ of the gastrointestinal tract at a
site of acute or chronic
bleeding (not shown), alignment between the housing 107 and accordingly the
ablation structure 101,
and the tissue surface 3 by flexing, rotating or pivoting about the ring 250
and the elastic band 252
coupling can be achieved. Again, advantageously, the desired contact can be
achieved irrespective of
the precise alignment of the deflected endoscope 111 distal end 110 in respect
to a plane of the tissue
surface 3 to be treated, because of the flexing rotating or pivoting provided
by the elastic band 252
coupling.
[00216] In another embodiment, the ablation device 100 additionally includes
an alternative
coupling mechanism between the ablation device 100 and an endoscope 111 that
is arranged and
configured to fit within a channel of an endoscope 111. The coupling mechanism
can be an internal
coupling mechanism 215 and can be configured and arranged to couple the
ablation structure 101
within an internal working channel 211 of an endoscope 111 (see Figure 37 and
as discussed above).
[00217] As shown in Figures 34A, 34B, 35A, 35B, 36A, and 36B, in one
embodiment of such a
coupling mechanism, the ablation structure 101 is adapted and configured to
fit within the endoscope
internal working channel 211. Additionally, as shown in Figures 34A, 34B, 35A,
35B, 36A, and 36B,
in a related embodiment, the deflection mechanism is also adapted and
configured to fit within the
endoscope internal working channel 211.
[00218] In each of the embodiments described above and shown in Figures 34A,
34B, 35A, 35B,
36A, and 36B, after expansion of the inflatable member 105 or expandable
member 209 and
subsequent treatment of a target tissue 3, the coupling means can further
serve as a means to draw,
pull or retrieve the ablation structure 101 and deflection mechanism back into
the endoscope internal
working channel 211. Furthermore, in addition to providing coupling of the
ablation structure 101
with the endoscope internal working channel 112, the coupling mechanism can
include electrical
connections 109 to provide energy to the ablation structure 101.
1002191 In a related embodiment, again wherein the ablation device 100
additionally includes a
coupling mechanism adapted and configured to fit within a channel of an
endoscope 111, the coupling
mechanism can include a shape memory member and the deflection mechanism can
include a bent
portion of the shape memory member. As shown in Figures 37 - 39, the coupling
mechanism can be
an internal coupling mechanism 215. As shown, the internal coupling mechanism
215 can be disposed
within an endoscope internal working channel 211 and extend beyond the
endoscope distal end 100.
Additionally, the internal coupling mechanism 215 can be connected to a
deflection mechanism that is
a deflection member 150. The deflection member 150 can include a bent portion
and can be connected
to the housing 107. As shown in Figure 38 and discussed above, the bent
portion of the deflection
member 150 can be disposed within the endoscope internal working channel 211,
causing the ablation
structure 101 to move into a non-deployed position. Upon advancing the
internal coupling mechanism
46
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
215 toward the endoscope distal end 110, the shape memory nature of the
deflection member 150
facilitates deployment of the ablation structure 101 to a position suitable
for ablation.
[00220] In general, in one aspect, the ablation structure 101 of the ablation
device 100 includes an
optically transmissive portion 158 adapted and configured to cooperate with a
visual channel of an
endoscope 111. As shown in Figures 27 - 31 and discussed above, the optically
transmissive portion
158 can be a sheath 103 of the ablation device 100.
[00221] In one embodiment, the ablation structure 101 of the ablation device
100 is further adapted
and configured to move from a first configuration to a second radially
expanded configuration. As
shown in Figures 19 - 22, the ablation structure 101 and housing 107 can be
designed to reversibly
move from a first less radially expanded configuration (see Figures 20 and 21)
to a second radially
expanded configuration useful for ablation. Foldable or deflectable
configurations that provide for
reversible radial expansion of the housing 107 and the ablation structure 101
can facilitate access to
tissue surfaces because of reduced size. Additionally, foldable or deflectable
configurations are
helpful in regard to cleaning, introduction, retrieval, and repositioning of
the device in the luminal
organs of the gastrointestinal tract at a site of acute or chronic bleeding.
[00222] The ablation device 100 shown in Figures 19B and 20 includes an
ablation structure
actuator 152 arranged and configured to move the ablation structure 101 from
the first configuration
(see Figure 20) to a second radially-expanded configuration (see Figure 21).
As shown (Figures 19B
and 20), the actuator 152 can be elongate and designed to work with a receiver
154 arranged and
configured to receive the actuator 152. The actuator 152 can be a wire, rod or
other suitable elongate
structure. Alternatively, the actuator 152 can be a hydraulic actuation means
with or without a balloon
component. In a particular embodiment, the actuator 152 is a stiffening wire.
[00223] As illustrated in Figure 20, before the actuator 152 is disposed
within the portion of
receiver 154 attached to the housing 107, both the housing 107 and the
ablation structure 101 are in a
first position having a first configuration. As illustrated in Figure 21,
after the actuator 152 is partially
or fully introduced into the receiver 154, the housing 107 and the ablation
structure 101 are
consequently changed to a second radially expanded configuration relative to
the first configuration.
Introduction of the actuator 152 into the receiver 154 can force the portions
of the housing 107 and
ablation structure 101 flanking the receiver 154 to expand radially (see
Figure 19). In one
embodiment, the housing 107 is heat set in a flexed first configuration
suitable for positioning the
ablation device 100 near a target tissue surface 3. After a target tissue
surface 3 has been reached, the
actuator 152 can be introduced into the receiver 154 to achieve the second
radially expanded
configuration which is useful for ablation of the tissue surface 3.
[00224] In a related alternative embodiment, the housing 107 and ablation
structure 101 include an
unconstrained shape that is radially expanded and includes one or more flex
points to allow for
47
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
collapsed or reduced radial expansion when positioned distally to the distal
end 110 of an endoscope
111 and compressed by an elastomeric sheath 115 (not shown).
[00225] As shown in Figures 21 and 22, in another embodiment, the ablation
structure 101 of the
ablation device 100 is adapted and configured to move from a first
configuration to a second radially
expanded configuration wherein the ablation device 100 further includes an
expandable member 156.
The expandable member 156 can be positioned between the housing 107 and the
endoscope 111,
where in unexpanded form, the ablation structure 101 is accordingly configured
in a first
configuration. Upon expansion of the expandable member 156, the ablation
structure 101
configuration is changed to a second radially expanded configuration (see
Figure 21).
[00226] In one embodiment, the deflection mechanism of the ablation device 100
includes an
inflatable inflation member 105. As shown in Figures 11, 21, 22, 25B, 27, 28,
30, 31, 34A, 34B, 42,
44, 46, and 47 and discussed above, the inflation member 105 can facilitate
deflection of the device
100 in relation to a tissue surface 3.
[00227] In another embodiment, the deflection mechanism includes an expandable
member 156 (see
Figures 35B and 36B, discussed in detail above). As shown in Figure 35B, the
expandable member
209, can be an expandable stent, frame or cage device. As shown in Figure 36B,
the expandable
member 209, can be an expanded series of connected hoops that can be folded or
rolled prior to
expansion.
[00228] In another advantageous embodiment, the ablation device 100 further
comprises a torque
transmission member adapted and configured to transmit torque from a proximal
end of the
endoscope 111 to the ablation structure 101 to rotate the ablation structure
101 about a central axis of
the endoscope 111. In a particular embodiment, the torque transmission member
includes first and
second interlocking members adapted to resist relative movement between the
endoscope 111 and the
ablation structure 101 about the central axis. As shown in Figures 46B, 46C,
and 47, in one
embodiment the first interlocking member is a key 258 and the second
interlocking member is a
keyway 256. In one embodiment, the first interlocking member is attached to a
sheath 103
surrounding the endoscope 111 and the second interlocking member is attached
to a catheter 254
supporting the ablation structure 101. For example, as shown in Figures 46B,
46C, and 47, the key
258 can be attached to a sheath 103 surrounding the endoscope 111 and the
keyway 256 can be
attached to a catheter 254 supporting the ablation structure 101. In a further
related embodiment, the
catheter 254 and sheath 103 are arranged and configured for relative movement
along the central axis
of the endoscope 111. The sheath 103 can be, for example, an elastomeric
sheath wherein the key 258
is attached to the outside of the sheath 103 substantially along a
longitudinal axis of the sheath 103
(see Figure 46C). In use, this embodiment provides for a 1-to-l torque
transmission of the ablation
device 100 endoscope assembly 111 when the endoscope proximal end 112 is
manipulated, while also
providing for positioning of the ablation structure 101 either proximal or
distal to the endoscope distal
48
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
end 110 in situ. Additionally, the sheath 103 can be pre-loaded into the
catheter 254 or loaded
separately.
[00229] In general, in one aspect, an ablation device 100 is provided
including an ablation structure
101, and a coupling mechanism adapted to removably couple the ablation
structure 101 to a distal end
110 of an endoscope 111 and to permit the ablation structure 101 to rotate
and/or pivot with respect to
the endoscope when coupled to the endoscope. Various related embodiments
wherein, for example,
the coupling mechanism comprises a ring 250 and the ablation structure 101 is
adapted to rotate
and/or pivot about the ring 250; wherein the coupling mechanism comprises an
elastic band 252
adapted to flex to permit the ablation structure 101 to rotate and/or pivot;
wherein the ablation device
100 further includes a deflection mechanism adapted and configured to move the
ablation structure
101 toward a tissue surface 3; and, wherein such a deflection mechanism
includes an inflatable
member, have been set out in detail above.
[00230] Figure 56A and 56B provide views of an ablational device with an
ablational surface on a
hinge 159 which acts in a manner similar to mechanism depicted in Figure 43,
and which allows a
free pivoting movement of the ablational surface between its longitudinal axis
and the longitudinal
axis of an endoscope. Figure 56A shows the device with the ablational surface
101 oriented in
parallel with the endoscope, the surface having made contact with the inner
surface of a
gastrointestinal luminal wall 5 at a desired target area. The ablation surface
101 is supported by a
deflection member 150 that can be expressed from a working channel, and
withdrawn back into a
working channel within the endoscope. Figure 56B shows the device with the
longitudinal axis of the
ablational surface 101 oriented at about a right angle with respect to the
longitudinal axis of the
endoscope. This pivoting as a passive response of the ablational surface 101,
as it easily rotates on
hinge 159 through a flexion range of 0 degrees (parallel to the endoscope 111)
to about 170 degrees.
As shown, the angle of the surface is about 90 degrees with respect to the
endoscope.
[00231] While most embodiments described herein have made use of
radiofrequency energy as an
exemplary ablational energy, and consequently have made use of electrodes as
an energy transmitting
element, it should be understood that these examples are not limiting with
regard to energy source and
energy delivery or transmitting elements. As also described herein, other
forms of energy, as well as
cryoablating approaches, may provide for ablation of target areas in such a
manner that ablation is
fractional or partial, as described herein, where some portions of target area
tissue are ablated, and
some portions of target area tissue are not substantially ablated.
Device embodiments that are deployable through an endoscope
[00232] As noted above, ablation devices may be deployable or positionable at
site of acute or
chronic bleeding in various ways in conjunction with an endoscope that
provides visual capability to a
physician. For example, an endoscopic catheter can be positionable into
therapeutic contact with a
balloon or another form of expandable member, or moving or deflecting a
deflecting member, and
49
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
embodiments can be mounted on the end or on an appropriate alternative site of
an endoscope, or the
ablation devic? can pass through a working channel or accessory channel of an
endoscope. An
ablation device that can be passed through a working channel of an endoscope
provides practical
benefits in that the operation of the endoscope is in not hindered or
complicated any by extemal
device features, and because physician practitioners are very familiar and
comfortable with working
channel devices. A constraint, however, is that devices housed within a
working channel need have a
collapsed configuration that fits within the dimensions of the channel, which
are typically about 2- 5
mm in diameter. Further, such in-channel devices need to be able to move
easily back and forth
between a stowed or deployable configuration and a working or deployed
configuration. Several
examples of in-channel devices are provided in Figures 59A - 64, and described
below.
[00233] Figures 59A and 59B provide views of an ablation device 400 deployable
through the
working channel of an endoscope that is configured to present a broad field
ablational surface
generally orthogonal or perpendicular to the longitudinal axis of the delivery
endoscope. Figure 59A
shows the device in a fully deployed configuration. Figure 59A shows the
device in a configuration
midway between being deployed and collapsed, so as to be stowed within the
working channel of an
endoscope. The device 400 is supported at the distal end of a shaft 41 that
supports an internal coaxial
rod 410. At a junction 412, the coaxial rod joins a plurality of peripheral
struts 420 that support an
ablation energy delivery surface 101. A wire 430 also extends from the
junction 412 to the center of
the back of ablation energy delivery surface 101b, which is further supported
by frame elements 440.
By pushing the coaxial rod distally, so that it projects forward from a
working channel, the ablation
surface opens to provide a broad field ablation energy delivery surface. As
the coaxial rod is pulled
proximally, the ablation energy delivery surface pulls in on itself in a
reverse-umbrella manner, and is
in a configuration that can be withdrawn into the working channel. In
alternate embodiments, the
ablation delivery surface 101 and its support frame elements 440 can be
adapted to provide a forward
face that is convex rather than flat, in order to better meet a broad luminal
surface. It can be further
appreciated that the device and the ablation surface are steerable by virtue
of being supported by an
endoscope, and further, the operating physician has the ability to manually
provide pressure, so as to
make effective therapeutic contact with the target area. It may also be
appreciated that ablation
delivery elements can be arranged on the ablation surface 101 in any
configuration described
elsewhere in this disclosure.
[00234] Figure 60 depicts an embodiment of an ablation device 400 deployable
through the
working channel 112 of an endoscope 111 that is adapted to present an
ablational surface 101
generally parallel to the longitudinal axis of the delivery endoscope. The
device includes an ablational
structure that has two parallel collapsible shape memory ribs 415 (comprising
Nitinol, for example),
across which ablative electrodes are strung, the strung electrodes configured
to be taut across the
space bet:veen the ribs in the deployed condition to form the ablational
surface 101. As the distal end
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
of a support shaft 41 is pushed out from the working channel of an endoscope
111, the support ribs
415 expand per their preferred configuration. As the support shaft is pulled
back into the endoscope,
the proximal tapered portion of the ribs 415 draws them together as the pass
through the opening of
the working channel. The ablational surface 101 is adapted to provide focal
sites of ablation within a
target area.
[00235] Figures 61A and 61B depict an embodiment of an ablation device 400
deployable through
the working channe1112 of an endoscope 111 that is adapted to present an
ablational surface 101
generally parallel to the longitudinal axis of the delivery endoscope. A
proximal portion of support
416 of the ablational surface 101 of the device is tapered, and substantially
flat but with a laterally-
curved bias that is rollable, such that it unrolls when pushed from the
working channel of the
endoscope 111, and rolls around itself when being withdrawn back into the
working channel of the
endoscope 111, because of the force exerted on the tapered proximal portion
416 as it is brought past
the edge of the working channel 112. The ablational surface 101 is adapted to
provide focal sites of
ablation within a target area, particularly against the wall of a relatively
narrow lumen. Figure 61A
shows the device in its deployed form, projecting forward out of the working
channel. Figure 61B
shows the device as it would appear retracted within the working channel; the
distal portion of the
device including the proximal portion of the support 416 and the ablation
surface 101 rolled or coil
around themselves.
[00236] Figure 62 depicts an embodiment of an ablation device deployable
through the working
channe1112 of an endoscope 111 similar to that of Figure 61 except that is
adapted to present an
ablational surface generally orthogonal to the longitudinal axis of the
delivery endoscope by virtue of
a flexible bent portions 418 and 419 proximal to the ablational surface 101.
The support 416 for
ablational surface of the device is tapered on its proximal end, and
substantially flat but with a
laterally-curved bias that is rollable, such that it unrolls when pushed from
the working channel, and
rolls around itself when being withdrawn back into the working channel.
[00237] Figure 63 depicts an embodiment of an ablation device 400 deployable
through the
working channe1112 of an endoscope 111 that is adapted to present an outwardly-
facing
circumferentially-oriented circle or helical ablational surface 101. The
circular or helical portion
uncoils upon emergence from the working channel of an endoscope, and coils
into a linear
configuration upon being withdrawn into the working channel.
[00238] Figure 64 provides a perspective and cross-sectional detail view of
circuit layers and
exemplary materials of ablational surfaces common to devices shown in Figures
60 - 63. The
ablation surface support 416 material has super-elastic shape memory
properties such as Nitinol has.
Layered on top of the support is a circuit backing 417, and on top of that
backing layer are copper
traces 418 that comprise the radiofrequency energy delivery elements of the
device.
51
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
A feature that provides hydraulic cleaning of the ablational surface
[00239] During radiofrequency coagulation of blood and/or blood vessels the
coagulated blood as
well as other fluids, such as extracellular fluid and cytosolic fluic may
adhere to the electrode making
sub-sequent ablations less effective and less controllable. To minimize the
risk of blood or coagulate
adhering to the electrode several approaches may be utilized. Thus, in some
embodiments, a non-stick
surface is used on the electrodes and/or the adjoining surfaces to prevent
sticking of coagulum. The
non-stick s rface is provided by a substrate such as silicone, PTFE, FEP for
the material adjacent to
conductive electrode elements. Alternatively, the electrode conductive
elements and/ or adjacent
material would be coated with a thin layer of silicone (cured and uncured
forms), PTFE, other
fluorpolymers, lecithin, oils, glycolipids, triglycerides or other lubricious
organic or non-organic
coatings. To minimize the effect of these coatings on the electrical circuit
between the electrode and
ablation site, the coating are selected to have minimal impedance and/or
resistance effects on the
circuit. High impedance coatings result in power losses preventing efficient
transmission of power to
the tissue site. For example cured silicone coatings in the range of 0.1 to
100 m may be appropriate
for this application. Also, it other embodiments, a significant amount of the
coating bums off the
conductive elements after the initial few ablations but the coating still
remains on the adjacent
material.
[00240] In other more actively cleaning embodiments, a fluid wash is provided
to prevent or remove
coagulum adhesion the electrode surface. Accordingly, Figures 65A and 65B
depict an embodiment
of an ablation device 100 with a partially circumferential ablation surface
101 that includes a
hydraulic cleaning feature. The device as a whole is similar to that depicted
in Figure 56, with a
longitudinallv pivoting mechanism 159 similar to that shown in Figure 43.
Extending from the
proximal end of the device distally to serve the ablation surface 101 are two
lines, one is an electrical
connector 109 that provides ablational energy for distribution, and the other
is a hydraulic line 121
that conveys a washing fluid to the ablation surface. Figure 65B shows a more
detailed perspective
view of the ablation surface 101 and the hydraulic line 121 that leads into
irrigation channel system
122 and multiple outlet holes 123. The ablation surface 101 includes an
electrode array of any
configuration, as described elsewhere herein, but which is not shown in order
to focus on the
irrigation elements. The irrigation system conveys a physiologically
appropriate solution, and may be
operated manually by the physician, or the system may be controlled
automatically by a controller
that provides irrigation at an appropriate interval and rate following the
delivery of radiofrequency
energy.
Terms and Conventions
[00241] Unless defmed otherwise, all technical terms used herein have the same
meanings as
commonly understood by one of ordinary skill in the art of ablational
technologies and treatment for
52
CA 02692669 2010-01-05
WO 2009/009444 PCT/US2008/069245
metabolic conditions and diseases, as well as those understood by one of
ordinary skill in the art of
bariatric surgeries. Specific methods, devices, and materials are described in
this application, but any
methods and materials similar or equivalent to those described herein can be
used in the practice of
the present invention. While embodiments of the invention have been described
in some detail and by
way of exemplary illustrations, such illustration is for purposes of clarity
of understanding only, and is
not intended to be limiting. Various terms have been used in the description
to convey an
understanding of the invention; it will be understood that the meaning of
these various terms extends
to common linguistic or grammatical variations or forms thereof. It will also
be understood that when
terminology referring to devices, equipment, or drugs that have been referred
to by trade names, brand
names, or common names, that these terms or names are provided as contemporary
examples, and the
invention is not limited by such literal scope. Terminology that is introduced
at a later date that may
be reasonably understood as a derivative of a contemporary term or designating
of a hierarchal subset
embraced by a contemporary term will be understood as having been described by
the now
contemporary terminology. Further, while some theoretical considerations have
been advanced in
furtherance of providing an understanding of, for example, the mechanisms or
advantages of coaptive
therapeutic ablation, the claims to the invention are not bound by such
theory. Moreover, any one or
more features of any embodiment of the invention can be combined with any one
or more other
features of any other embodiment of the invention, without departing from the
scope of the invention.
Still further, it should be understood that the invention is not limited to
the embodiments that have
been set forth for purposes of exemplification, but is to be defined only by a
fair reading of claims that
are appended to the patent application, including the full range of
equivalency to which each element
thereof is entitled.
53