Note: Descriptions are shown in the official language in which they were submitted.
CA 02692759 2010-01-06
WO 2009/006902 PCT/DK2008/050149
1
TITLE
A COLLECTING DEVICE FOR BODY FLUID
Field of the Invention
The present invention relates to a collecting device for attachment to human
skin
and for collecting bodily waste e.g. an ostomy device.
Background
Collecting devices for collecting bodily waste, ostomy appliances, wound or
fistulae drainage bandages or devices for collecting urine are usually in the
form
of a receptacle, e.g. a bag, pouch or tube for receiving the waste, connected
to
an adhesive wafer that can be attached to the skin of the patient. The wafer
is
typically in the form of a backing layer coated on the skin-facing surface
with an
adhesive layer and the wafer may further be provided with an aperture for
accommodating the body opening. The size and shape of said aperture can often
be adapted individually to fit the anatomy of the patient.
One of the crucial parts of such devices is the adhesive wafer. The wafer
should
be able to fit leak proof around the body opening, have good adherence to the
skin without unintended detachment from the skin, but at the same time be easy
to remove again without damaging the skin. Furthermore, the wafer should be
able to follow the movements of the body and be comfortable to wear.
When designing a skin adhesive one of the major issues is to keep the skin
relatively dry underneath the adhesive to prevent maceration. Maceration
occurs
when the skin is unable to get rid of moisture from transpiration and outlet
from a
body opening. This may result in degradation of the skins barrier function as
well
as bad adhesion of the device to the skin.
Usually, skin adhesive keeps the skin dry by absorbing moisture. Absorbing
particles or hydrocolloids (HC) are mixed into an adhesive matrix in order to
absorb moisture from the skin and thereby the skin is kept relatively dry.
This
CA 02692759 2010-01-06
WO 2009/006902 PCT/DK2008/050149
2
technique is well known in the art and forms the basis for most ostomy
adhesives
that are commercially available see, e.g. US 4,192,785.
The major problem in using an absorbing adhesive is the changes in the bonding
properties when the adhesive absorbs moisture. An absorbing adhesive normally
starts out being a well-bonded adhesive and usually ends up being a weak-
bonded adhesive after absorbing moisture. This effect is particularly a
problem
for hydrocolloid-based adhesives. As the adhesive absorbs moisture from the
skin, the hydrocolloids swell and take up an increasing amount of space in the
skin-bonding zone, thereby reducing the skin bonding effect of the adhesive.
The weak-bonded adhesive will usually still keep the collecting device
attached to
the skin, however the risk of leakage of bodily waste along the skin surface
will
increase drastically.
An alternative to the absorbing adhesives described above is a liquid
impermeable, moisture permeable adhesive such as polyurethane, silicone, and
polyacrylate.
A liquid impermeable, moisture permeable adhesive does not absorb the
moisture but rather permeate the water away from the skin surface. Thus, the
swelling effect caused by the hydrocolloids will usually not occur.
When using a liquid impermeable, moisture permeable adhesive one can
formulate it to be more or less plastic. If the liquid impermeable, moisture
permeable adhesive is crosslinked the ability for flow is limited and adhesion
is
obtained by wetting and affinity to the skin. In this case the cavities in
between
the mantles and other irregularities will not be filled out by the adhesive by
simple
flow. Thus, a less resistant adhesive to retain the output and avoid
undermining
the adhesive with output is seen.
CA 02692759 2010-01-06
WO 2009/006902 PCT/DK2008/050149
3
Hence, there is a need to find a solution to avoid leakage of bodily waste
occurred because of weak-bonded adhesive and still maintain the ability to use
conventional waste collecting devices to adhere to the skin.
It has now surprisingly been found that the collecting device according to the
invention provides a sealing effect that solves this problem.
Summary of the invention
The present invention relates to a body waste collecting device comprising a
collecting pouch and an adhesive wafer for attachment to the body, said
adhesive wafer comprising a backing layer and a first adhesive layer, said
first
adhesive layer comprising a liquid impermeable, moisture permeable adhesive
composition wherein the adhesive wafer comprising an additional second
adhesive for sealing around a body opening.
Brief description of the drawing
The invention is disclosed more in detail with reference to the drawings in
which
Figures 1-7 show different embodiments of the invention.
Detailed description of the present invention
The aim of the invention is to provide a body waste collecting device that
includes
an additional second adhesive as sealing element.
By body waste collecting device is meant a device being able to collect and
hold
the output in a collecting item for a predefined time. The holding in place of
the
device may be obtained by a skin adhesive and the collection may be obtained
by a bag.
In an embodiment of the invention a body waste collecting device comprising a
collecting pouch and an adhesive wafer for attachment to the body, said
adhesive wafer comprising a backing layer and a first adhesive layer, said
first
adhesive layer comprising a liquid impermeable, moisture permeable adhesive
CA 02692759 2010-01-06
WO 2009/006902 PCT/DK2008/050149
4
composition wherein the adhesive wafer comprising an additional second
adhesive for sealing around a body opening.
The additional second adhesive is used for security and protection for the
user in
order to avoid leakages and to obtain longer wear time.
The collecting device according to the invention combines the mechanical and
adhesive properties of the two kinds of known skin adhesives as described
above. The negative effect of the swelling of the hydrocolloid-based skin
adhesive is used according to the invention in a beneficial way to provide a
sealing effect for the first adhesive layer of a liquid impermeable, moisture
permeable adhesive.
In the use of an ostomy appliance the requirements from the adhesive in the
peri
stomal area are often different as the use varies within stoma type, body
shape,
user patterns etc.
When an overall soft and pliable adhesive is wanted, a liquid impermeable,
moisture permeable adhesive composition as the first adhesive layer can be
chosen.
According to the invention a sealing effect through swelling is achieved in
order to
minimize the exposure of output to the peri stomal area. For example by the
use
of a hydrocolloid adhesive as the additional second adhesive according to the
invention.
Other solutions according to the invention can be a hydrocolloid, a super
absorber or a hydrogel based adhesive. When using such a solution the peri
stomal area of the adhesive wafer tends to be stiffer resulting in a fixation
of the
skin in the inner part of the wafer and a more soft and flexible part in the
outer
zone. The slightly stiffer inner zone is typically obtained from the high
filler rate of
absorbing particles and choice of adhesive materials that are stiffer than the
CA 02692759 2010-01-06
WO 2009/006902 PCT/DK2008/050149
liquid impermeable, moisture permeable adhesive composition. The collecting
device according to the invention substantially maintains the softness given
by
the liquid impermeable, moisture permeable adhesive composition.
5 Such an embodiment according to the invention is especially beneficial when
used in cases where very liquid or skin aggressive output is exposed to the
inner
hole of the adhesive wafer for a long time. A typical example is the use of a
2-
piece appliance for ileo ostomy where the adhesive wafer will stay on the skin
for
several days.
By permeable adhesive is meant an adhesive where the moisture vapour
transmission rate, MVTR is higher than 100g/m2/24h as measured using the
method disclosed herein.
The adhesive used in the first adhesive layer of the present invention has a
high
moisture vapour transmission rate, preferably a MVTR over 100 g/m2/24hrs,
which makes it breathable and very skin friendly. The high moisture
transmission
of the adhesive is a particular advantage where a medical device has to be
worn
on the skin for a long time, e.g. days.
According to an embodiment of the invention the first adhesive layer comprises
a
liquid impermeable, moisture permeable adhesive composition, which comprises
a moisture permeable polymer selected from the group of but not limited to
polypropyleneoxide, polyurethane, silicone, polyacrylate, ethylene vinyl
acetate,
and mixtures thereof.
As used herein a moisture permeable polymer means a polymer that absorbs
less than 5% in wt, preferably less than 1%, at equilibrium and has a moisture
vapour transmission rate of greater than 100 g/m2/24hrs, preferably greater
than
200 g/m2/24hrs.
CA 02692759 2010-01-06
WO 2009/006902 PCT/DK2008/050149
6
According to an embodiment of the invention the water vapour permeability of
the
first adhesive layer comprising the liquid impermeable, moisture permeable
adhesive composition is higher than 100g/m2/24h as defined herein.
As used herein liquid impermeable means that water is rejected from
penetrating
the substance by simple flow.
In one embodiment of the invention, the moisture permeable polymer is
crosslinked.
As used herein a crosslink means a small region in a macromolecule (polymer
chain structure) from which more than 2 chains emanate. The linking may be
covalent, physical or ionic.
In another embodiment of the invention, the liquid impermeable, moisture
permeable adhesive composition comprises a block copolymer.
As used herein a block copolymer means a copolymer in which the repeating
units in the main chain occur in blocks, e.g., -(a)m-(b)n-(a)p-(b)q- , where a
and b
represent the repeating units and m, n, p, q, are numbers.
In a preferred embodiment of the invention, the liquid impermeable, moisture
permeable adhesive composition comprises polypropyleneoxide.
In a preferred embodiment of the invention, the liquid impermeable, moisture
permeable adhesive composition comprises polyurethane.
In a preferred embodiment of the invention, the liquid impermeable, moisture
permeable adhesive composition comprises ethylene vinyl acetate.
CA 02692759 2010-01-06
WO 2009/006902 PCT/DK2008/050149
7
The adhesive composition comprising ethylene vinyl acetate may suitably be an
adhesive known in the art such as the adhesive composition disclosed, for
example in Danish patent application PA 2007 01003.
In a preferred embodiment of the invention, the liquid impermeable, moisture
permeable adhesive composition comprises silicone.
In a preferred embodiment of the invention, the liquid impermeable, moisture
permeable adhesive composition comprises polyacrylate.
According to an embodiment of the invention, the additional second adhesive is
a
paste, a hydrocolloid pressure sensitive adhesive or a liquid impermeable,
moisture permeable adhesive composition.
A standard and conventional hydrocolloid pressure sensitive adhesive is a skin
friendly adhesive that is capable of adhering to the skin, handling
perspiration by
absorbing the moist and being removable from the skin without essential
damage.
A typical pressure sensitive adhesive is based on a polyisobutylene, PIB
(adhering substance) and a carboxy methyl cellulose, CMC (absorbing media).
A typical pressure sensitive adhesive as used today is a highly filled
hydrocolloid
system with a matrix of polymers that exhibit flow as well as cohesive
properties.
The hydrocolloids will absorb the moisture from perspiration and output from
the
body and the polymers will adhere to the skin through skin polymer affinity
and
the polymers will flow into the small cavities of the skin. The cohesion of
the
adhesive will ensure that erosion is minimized and that the wafer will be
removed
in one piece and that minimal adhesive residues are left at the skin.
As used herein a paste is a moldable or flowable adhesive that is able to
adhere
to the skin, flow or being permanently pressed into irregularities of the skin
and
CA 02692759 2010-01-06
WO 2009/006902 PCT/DK2008/050149
8
thus protecting the skin from the output. A paste is usually used as an
accessory
to stoma care products for sealing around the stoma.
According to another embodiment of the invention, the additional second
adhesive is a liquid impermeable, moisture permeable adhesive composition
comprising a moisture permeable polymer selected from the group of
polypropyleneoxide, polyurethane, silicone, polyacrylate, ethylene vinyl
acetate,
and mixtures thereof.
According to one embodiment of the invention, the additional second adhesive
is
another liquid impermeable, moisture permeable adhesive composition compared
to the composition of the first adhesive layer.
It may be advantageous that the additional second adhesive comprises
absorbent particles. According to an embodiment of the invention the
additional
second adhesive comprises absorbent particles.
The particles may be absorbent articles such as hydrocolloids, microcolloids
or
super absorbers in order for the composition to absorb moisture from skin.
Microcolloid particles are well-known in the art e.g. from International
Patent
Application No. WO 02/066087, which discloses adhesive compositions
comprising microcolloid particles. The microcolloid particles may have a
particle
size of less than 20 microns.
The additional second adhesive may comprise 1-60 % w/w of hydrocolloid (HC)
or super absorbent particles (SAP) particles, more preferred 30-60% w/w
particles.
According to a preferred embodiment of the invention, the additional second
adhesive comprises absorbent particles.
CA 02692759 2010-01-06
WO 2009/006902 PCT/DK2008/050149
9
According to a preferred embodiment of the invention, the absorbent particles
are
selected from hydrocolloids, microcolloids and super absorbers.
According to a preferred embodiment of the invention, the additional second
adhesive is a hydrocolloid pressure sensitive adhesive.
In an embodiment of the invention, the additional second adhesive is located
on
the skin-facing surface of the first adhesive layer.
By the skin-facing surface of the adhesive is meant the side adhering to the
skin.
By located is meant where the additional second adhesive is placed on the
collecting device and specifies the position of the additional second adhesive
on
the collecting device.
In another embodiment of the invention, the additional second adhesive is
located on the pouch-facing surface of the adhesive wafer.
By the pouch-facing surface or non-skin-facing surface is meant the side of
the
adhesive or backing pointing away from the skin (non-bonding side).
According to another embodiment of the invention, the additional second
adhesive is located at the inner rim of the adhesive wafer.
By the inner rim portion of the adhesive wafer is meant the portion in the
perianal,
peristomal or fistal area. This area of the adhesive is exposed to faeces as
well
as perspiration. The properties of the inner rim portion depend on wear time,
output type and anatomy among others.
According to one embodiment of the invention, the additional second adhesive
is
located at the outer rim of the adhesive wafer.
CA 02692759 2010-01-06
WO 2009/006902 PCT/DK2008/050149
By the outer rim portion of the adhesive wafer is meant the portion
essentially
outside the welding zone or coupling. This portion is less exposed to faeces
but
has to handle perspiration as well as mechanical exposure due to movements
and pull from the bag.
5
According to an embodiment of the invention, the additional second adhesive is
in the form of a ring.
By an adhesive ring is meant an adhesive, essentially surrounding the body
10 opening that needs to be drained or a ring covering the outer or inner rim
of the
adhesive wafer
According to an embodiment of the invention, a hole of the ring has a diameter
being less than the diameter of a hole in the adhesive wafer with the first
adhesive layer. By this embodiment the additional second adhesive will have a
tighter fit around the body opening compared to the adhesive wafer and
compared to the first adhesive layer. In one embodiment the sealing and the
tighter fit of the additional second adhesive is obtained by turtle necking.
According to one embodiment of the invention, the additional second adhesive
is
part of a ring such as a half ring covering 180 degrees of a circle.
According to an embodiment of the invention, the additional second adhesive
covers the entire skin-facing surface of the first adhesive layer.
Optionally, the additional second adhesive only covers a part of the first
adhesive
layer.
According to an embodiment of the invention, the additional second adhesive
partly covers the adhesive wafer.
CA 02692759 2010-01-06
WO 2009/006902 PCT/DK2008/050149
11
According to another embodiment of the invention, the additional second
adhesive partly covers the skin-facing surface of the first adhesive layer.
According to another embodiment of the invention the adhesive wafer has a
recess for adapting the additional second adhesive.
The collecting device could be pre-shaped with a recess that fits the
additional
second adhesive to be built in the device. A recess could be a cut-out of part
of
the first adhesive layer.
By adapting is meant that the additional second adhesive is cast within the
collecting device.
According to an embodiment of the invention, the device has an optional
backing
layer for the additional second adhesive.
According to another embodiment of the invention, the additional second
adhesive is integrated in the wafer.
Sealing an opening refers to protection of an opening from output, for example
use of a paste around a stoma at the rim of the perinea skin or the rim of a
fistula.
According to an embodiment of the invention, the body opening is a stoma.
According to another embodiment of the invention, the body opening is a
fistula.
According to another embodiment of the invention, the body opening is an anus.
By a body opening is meant a natural or artificial body opening such as an
anus,
a stoma or a fistula.
CA 02692759 2010-01-06
WO 2009/006902 PCT/DK2008/050149
12
According to an embodiment of the invention, the collecting device is an
ostomy
appliance.
According to another embodiment of the invention, the collecting device is a
faecal collecting device.
According to another embodiment of the invention, the collecting device is a
fistula collecting device.
According to an embodiment of the invention, the collecting pouch is
detachable.
According to another embodiment of the invention, the collecting pouch is
integrated with the wafer.
The collecting pouch may be detachable from the adhesive wafer by a coupling
system or the pouch and the wafer may be integrated with the wafer, e.g. by
welding. The two versions are known as one piece or two-piece appliances for
ostomy.
Description of the preferred embodiments
The invention is now explained more in detail with reference to the drawings
showing preferred embodiments of the invention.
Figures 1-7 are all drawings of a side view of the left half of the adhesive
wafer
according to the invention. All wafers are covered with a backing layer. The
skin-
facing surface is illustrated in the drawings as the downward side of the
wafer
and the backing layer is placed on top of the wafer.
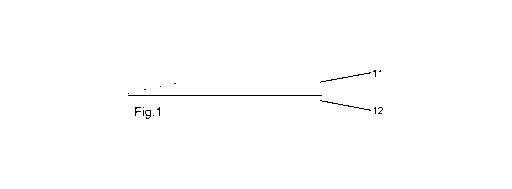
Figure 1 shows an adhesive wafer with a first adhesive layer (11) and an
additional second adhesive (12) where the additional second adhesive only
covers part of the adhesive wafer and is placed on the surface of the skin-
facing
adhesive wafer. The additional second adhesive can optionally be covered by a
CA 02692759 2010-01-06
WO 2009/006902 PCT/DK2008/050149
13
second backing layer placed in between the non-skin-facing side of the
additional
second adhesive and the skin-facing side of the first adhesive layer.
Figure 2 illustrates a side view of an adhesive wafer with a first adhesive
layer
(21) partly covered with an additional second adhesive (22) in the outer rim
portion of the wafer.
Figure 3 illustrates a side view of an embodiment of the invention, where the
hole
of the ring of the additional second adhesive (32) has a diameter being less
than
the hole in the adhesive wafer with the first adhesive layer (31).
Figure 4 illustrates a side view of another embodiment of the invention, where
the
hole of the ring of the additional second adhesive (42) has a diameter being
less
than the hole in the adhesive wafer with the first adhesive layer (41).
Figure 5 illustrates a side view of an adhesive wafer with a first adhesive
layer
(51) having a recess for adapting the additional second adhesive (52).
Figure 6 illustrates a side view of another embodiment of the invention where
an
adhesive wafer with a first adhesive layer (61) has a recess for adapting the
additional second adhesive (62).
Figure 7 illustrates a side view of an embodiment of the invention where an
adhesive wafer with a first adhesive layer (71) is in combination with an
additional
second adhesive (72) in the inner portion of the adhesive wafer.
CA 02692759 2010-01-06
WO 2009/006902 PCT/DK2008/050149
14
EXPERIMENTAL PART
Methods
Determination of water absorption
In order to get better correlation between measured water absorption and
actual
performance in a humanlike environment, a modified version of the ISO 62
standard was used: Pieces of adhesive of 1x25x25 mm3 were fastened on a
piece of glass using double sided adhesive and the constructs were immersed in
saline water (0.9% NaCI in dematerialized water) at 32 C. After 24 hours the
samples were removed and carefully dripped dry and weighed. The change in
weight was recorded and reported as weight gain in percent of the original dry
weight of the adhesive. In the following we will call this value w24n
Determination of moisture vapour transmission rate (MVTR)
MVTR was measured in grams per square meter (g/m2) over a 24 hours period
using an inverted Paddington cup method (British Pharmacopoeia,
1993,Addendum 1996, page 1943. HMSO London): A container or cup being
water and water vapour impermeable having an opening was used. 20m1 saline
water (0.9%NaCI in demineralised water) was placed in the container and the
opening was sealed with the test adhesive film. The container, with a
duplicate,
was placed into an electrically heated humidity cabinet and the container or
cup
was placed up side down in a way that the water was in contact with the
adhesive. The cabinet was maintained at 37 C and 15% relative humidity (RH).
After about an hour, the containers were considered to be in equilibrium with
the
surroundings and were weighed. 24h after the first weighing, the containers
were
weighed again. The difference in weight was due to evaporation of vapour
transmitted through the adhesive film. This difference was used to calculate
Moisture vapour transmission rate or MVTR. MVTR was calculated as the weight
loss after 24h divided by the area of the opening in the cup (g/m2/24h). If
the
adhesive film could not support the weight of the water, a supporting film
with
very high permeability was used as support.
CA 02692759 2010-01-06
WO 2009/006902 PCT/DK2008/050149
Examples
Example 1
A standard hydrocolloid adhesive as disclosed in International Patent
Application
5 No. WO 2007/082538 or a paste as disclosed in International Patent
Application
No. WO 98/17329, in the dimension of a round oblate with a diameter of 50 mm
and a thickness of 1 mm was placed in the central portion of a liquid
impermeable, moisture permeable adhesive that was connected to a collecting
bag. The device was used for collecting stoma ileo effluent for 24 hours on a
10 stoma-operated person.
In this case the collecting stoma device was perceived as a very soft,
flexible,
reliable and comfortable device with the sealing effect of the hydrocolloid
adhesive in the central portion. No skin conditions were seen and swelling of
the
15 hydrocolloid controlled the faeces in a way that it did not undermine the
adhesive
wafer. Undermining was also prevented by the flowing of the hydrocolloid
adhesive into the micropores of the skin.
Example 2
An acryllic adhesive, 3M1504, in the dimension of a round oblate with a
diameter
of 50 mm and a thickness of 0,4 mm was placed in the central portion of a
liquid
impermeable, moisture permeable adhesive that was connected to a collecting
bag. The relatively thin acrylic adhesive was permeable to moisture and would
transport water by permeability to the liquid impermeable, moisture permeable
adhesive. The device was used for collecting stoma ileo effluent for 24 hours
on a
stoma-operated person.
In this case the collecting stoma device was also perceived as a very soft,
flexible, reliable and comfortable device with the sealing effect of the
acrylic
adhesive in the central portion. Sealing was obtained by the flowing of the
acrylic
adhesive into the micropores of the skin and moisture from perspiration was
transported by diffusion into the liquid impermeable, moisture permeable
CA 02692759 2010-01-06
WO 2009/006902 PCT/DK2008/050149
16
adhesive. No skin conditions were seen. In one case the hole of the device was
cut in a slightly (app 5 mm) lower diameter of the receiving stoma in order to
obtain a turtleneck effect as a sealing. This experiment resulted in a good
sealing
preventing the stoma to enter behind the adhesive wafer, thus irritating the
peristomal skin.
Example 3
A liquid impermeable, moisture permeable adhesive with plastic properties as
disclosed in Danish patent application PA 2007 01003, in the dimension of a
round oblate with a diameter of 50 mm and a thickness of 1 mm was placed in
the central portion of a liquid impermeable, moisture permeable non-flowing
adhesive that was connected to a collecting bag. The device was used for
collecting stoma ileo effluent for 24 hours on a stoma-operated person.
In this case the collecting stoma device was perceived as a very soft,
flexible and
comfortable device with the sealing effect of the plastic adhesive in the
central
portion. No skin conditions were seen. Undermining was prevented by the flow
of
the plastic adhesive into the skin surface and skin health was obtained by the
two
liquid impermeable, moisture permeable adhesives.