Note: Descriptions are shown in the official language in which they were submitted.
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
1
A transition metal catalyzed synthesis of 2H-indazoles
Field of the Invention
The present invention relates to a process for the regioselective synthesis of
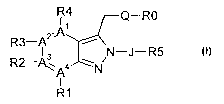
compounds of the formula (I),
R4 Q-R0
1
R3-A2 1A
I3 N-J-R5 (1)
R2-A\ A4 N
I
R1
wherein RO, R1, R2, R3, R4, R5, Al, A2, A3, A4, Q and J have the meanings
indicated
below and are useful as intermediates for the preparation of valuable
pharmaceutically
active ingredients.
Background of the Invention
The present invention relates to a direct transition metal catalyzed process
for the
preparation of a variety of multifunctional substituted 2H-indazoles or
substituted 2H-
azaindazoles of the formula (I) from 2-halo-phenylacetylenes or (2-
sulfonato)phenyl-
acetylenes and monosubstituted hydrazines.
Indazoles display a vide variety of biological activities and can be regarded
as
important structures in pharmaceutical research. The ability of the indazole
scaffold to
mediate an interaction with a variety of biological targets, is well-
documented by a
number of reports on the observed biological activity, as well as by the fact
that several
indazole based compounds are in development or marketed as drugs demonstrating
that this heterocycle can be an important element of a valuable
pharmaceutically active
ingredient. Some examples of biological activities include anti-inflammatory
activity (R.
J. Steffan, E. Matelan, M. A. Ashwell, W. J. Moore, W. R. Solvibile, E.
Trybulski, C. C.
Chadwick, S. Chippari, T. Kenney, A. Eckert, L. Borges-Marcucci, J. C. Keith,
Z. Xu, L.
Mosyak, D. C. Harnish J. Med. Chem. 2004, 47, 6435-38).
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
2
Of course the use of indazoles or azaindazoles is not limited to the above-
mentioned
pharmaceutical application. For example it is well known that indazoles can be
useful
in numerous other applications, for example as plant growth regulators (Y.
Kamuro, K.
Hirai Chemical Regulation of Plants, 1982, 17, 65) or liquid crystals (C.
Canlet, M. A.
Khan, B. M. Fung, F. Roussel, P. Judeinstein, J.-P. Bayle New. J. Chem. 1999,
23,
1223) among others.
Indazoles exist in 3 isomeric forms as 1 H-, 2H- and 3H-indazoles depending on
the
substitution pattern of the heterocyclic ring and though a number of syntheses
have
been developed for the formation of 1 H-indazoles, no general protocol for the
synthesis of 2H- indazoles exist.
Known processes for preparing substituted 2H-indazoles or substituted 2H-aza-
indazoles (J.J. Song, N.K. Yee, Org. Lett., Vol. 2, No. 4, 2000, 519-521; L.
D. Shirtcliff,
T. R. Weakley, M. M. Haley, F. Kohler, R. Herges J. Org. Chem,, 2004, 69, 6979-
85)
by direct N-arylation or N-alkylation inevitably yielded a mixture of N(1) and
N(2)
regioisomers with poor selectivity. The few methods available so far are multi-
step
processes often affording low yields and regioselectivities with a restricted
substrate
range and thus a poor cost-effectiveness and therefore of limited use but
suffer from
the same limitations as the traditional procedures mentioned above.
Although numerous transition metal catalyzed protocols for the intermolecular
cross-
coupling between aryl halides and amides, amines, hydrazides and hydrazones
have
been reported, only three examples employing hydrazines exist. Buchwald et al.
describes several examples of cross-coupling between monosubstituted, 1,1- and
1,2-
disubstituted hydrazines with aryl bromides (S. L. Buchwald, S. Wagaw, O. Geis
W099/43643) using a palladium catalyst in toluene and tert-butoxide base to
obtain
the desired arylated hydrazines in moderate to good yields. A similar
catalytic system
was reported by Cacchi et al. for the cross-coupling of 1, 1 -dialkyl-
substituted
hydrazine-(LiC12)2 adducts with aryl bromides in good yields (S. Cacchi, G.
Fabrizi, A.
Goggiamani, E. Licandro, S. Maiorana, D. Perdicchia Org. lett. 2005, 2, 1497-
1500).
Vasilevsky and Prikhodko have employed hydrazine in a cross-coupling reaction
with
(2-chloro-5-nitrophenyl)arylacetylenes to afford 1 H-indazoles in good yields
(T. A.
Prikhodko, S. F. Vasilevsky Russ. Chem. Bull. Int. Ed., 2001, 50, 1268-1273).
The
reaction proceeds by a palladium catalyzed domino coupling-cyclization
reaction and
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
3
the scope of the reaction is rather limited as only four examples were
reported and
only (2-chloro-5-nitrophenyl)arylacetylenes were employed. Furthermore, in the
same
communication Vasilevsky and Prikhodko also report a single example of the
palladium catalyzed domino Sonogashira-cyclization reaction of 2-
iodoarylhydrazine
and 4-nitrophenyl-acetylene to afford 4-nitrobenzyl-1 H-indazole in low yield.
A single
example of 2H-indazole formation in 30% yield by the same domino Sonogashira-
cyclization reaction between N'-(2-iodophenyl)acetic hydrazide and 4-
nitrophenylacetylene is also described in the report.
It has now been found that the disadvantages mentioned can be avoided by a
direct,
catalytic, mild, versatile and regioselective synthesis.
The object is achieved by starting from 2-halo-phenylacetylenes or (2-
sulfonate)phenyl-acetylenes of formula II and monosubstituted hydrazines of
formula
III in the presence of a transition metal catalyst.
Summary of the invention
The present invention provides a direct transition metal catalyzed synthetic
route to a
wide variety of multifunctional substituted 2H-indazoles or substituted 2H-
azaindazoles
of formula I starting from 2-halo-phenylacetylenes or (2-
sulfonate)phenylacetylenes of
formula II and monosubstituted hydrazines of formula III.
The advantages of the provided process are that it comprises a direct,
catalytic, mild
and versatile method for the synthesis of substituted 2H-indazoles or
substituted 2H-
azaindazoles. Since the multi-step reaction proceeds as a one-pot domino
reaction
sequence using only a single catalyst, the process is very time- and cost-
effective as
well as being environmentally benign. Furthermore, the reaction conditions are
compatible with a broad range of functional groups and a large variety of
starting
materials ensuring the generality of the reaction.
R4 Q-RO R4 Q-RO
~
R3-AZA R3-AZA,
R2-P`A4 I X + R5-J-NHNHZ ~ R2-~ N N-J-R5
A 4
R1 (II) (III) Ri (1)
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
4
Detailed description of the invention
The invention therefore relates to a process for obtaining the compound of the
formula
R4 Q-RQ
i
R3-A2 ,A
13 N-J-R5 (1)
R2-A\ A4 N
I
R1
and/or all stereoisomeric forms of the compound of formula I, and/or mixtures
of these
forms in any ratio, and/or a physiologically tolerated salt of the compound of
formula I,
wherein
Al, A2, A3 and A4 are independently from each other selected from a carbon or
a
nitrogen atom to form together with the two carbon atoms in formula I a stable
aromatic or heteroaromatic ring;
Q is a covalent bond,
-(C1-Cg)-alkylene, wherein alkylene is unsubstituted or mono-, di- or
trisubstituted independently of one another by R14;
-(C3-Cg)-cycloalkyl, wherein cycloalkyl is unsubstituted or mono-, di- or
trisubstituted independently of one another by R14;
-(C6-C14)-aryl, wherein aryl is unsubstituted or mono-, di-, tri- or four
times
substituted independently of one another by R13;
-(C 1-C4)-alkylene-O-(C 1-C4)-alkylene,
-(C1-C4)-alkylene-O-; or
-(C5-C14)-heteroaryl, wherein heteroaryl is unsubstituted or mono-, di-, tri-
or
four times substituted independently of one another by R13;
J is a covalent bond,
-(C1-C5)-alkylene, wherein alkylene is unsubstituted or mono-, di- or
trisubstituted independently of one another by R14;
-(C3-Cg)-cycloalkyl, wherein cycloalkyl is unsubstituted or mono-, di- or
trisubstituted independently of one another by R14;
-(C6-C14)-aryl, wherein aryl is unsubstituted or mono-, di-, tri- or four
times
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
substituted independently of one another by R13; or
-(C5-C14)-heteroaryl, wherein heteroaryl is unsubstituted or mono-, di-, tri-
or
four times substituted independently of one another by R13;
R0, R1, R2, R3 and R4 are independent of one another identical or different
and are
5 a) hydrogen atom,
b) -(C1-C4)-alkyl, wherein alkyl is unsubstituted or substituted one to three
times by R13,
c) halogen,
d) phenyloxy-, wherein phenyloxy is unsubstituted or substituted one to
three times by R13,
e) -(C1-C3)-fluoroalkyl,
f) -N(R10)-(C1-C4)-alkyl, wherein alkyl is unsubstituted or substituted one
to three times by R13,
g) -(C6-C14)-aryl, wherein aryl is unsubstituted or mono-, di-, tri- or four
times substituted independently of one another by R13,
h) -(C5-C14)-heteroaryl, wherein heteroaryl is unsubstituted or mono-, di-,
tri- or four times substituted independently of one another by R13,
i) -(C3-Cg)-cycloalkyl, wherein said cycloalkyl is unsubstituted or mono-, di-
, tri- or four times substituted independently of one another by R13,
j) a 3- to 7-membered cyclic residue, containing 1, 2, 3 or 4 heteroatoms
chosen from nitrogen, sulfur or oxygen, wherein said cyclic residue is
unsubstituted or mono-, di-, tri- or four times substituted independently of
one another by R13,
k) -O-CF3,
I) -O-(C1-C4)-alkyl, wherein alkyl is unsubstituted or substituted one to
three times by R13,
m) -NO2,
n) -CN,
o) -OH,
p) -C(O)-R10,
q) -C(O)-O-R11,
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
6
r) -C(O)-N(R 11)-R 12,
s) -N(R11)-R12,
t) -N(R10)-S02-R10,
u) -S-R10,
v) -SOn-R10, wherein n is 1 or 2,
w) -S02-N(R11)-R12, or
x) -O-S02-R13, or
y) at least one of R1, R2, R3 or R4 are absent in case one or more of Al,
A2, A3 or A4 are nitrogen atom, or
R1 and R2, R2 and R3 or R3 and R4 form together with the atoms which they are
attached to a 5- or 8- membered ring, containing up to 0, 1, 2, 3 or 4
heteroatoms chosen from nitrogen, sulfur or oxygen, wherein said ring is
unsubstituted or substituted one, two, three or four times by R14,
R5 is a) -(C1-C4)-alkyl, wherein alkyl is unsubstituted or substituted one to
three
times by R13,
b) halogen,
c) phenyloxy-, wherein phenyloxy is unsubstituted or substituted one to
three times by R13,
d) -(C1-C3)-fluoroalkyl,
e) -N(R10)-(C1-C4)-alkyl, wherein alkyl is unsubstituted or substituted one
to three times by R13,
f) -(C6-C14)-aryl, wherein aryl is unsubstituted or mono-, di-, tri- or four
times substituted independently of one another by R13,
g) -(C5-C14)-heteroaryl, wherein heteroaryl is unsubstituted or mono-, di-,
tri- or four times substituted independently of one another by R13,
h) -(C3-C8)-cycloalkyl, wherein said cycloalkyl is unsubstituted or mono-, di-
, tri- or four times substituted independently of one another by R13,
i) a 3- to 7-membered cyclic residue, containing 1, 2, 3 or 4 heteroatoms
chosen from nitrogen, sulfur or oxygen, wherein said cyclic residue is
unsubstituted or mono-, di-, tri- or four times substituted independently of
one another by R13,
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
7
j) -O-CF3,
k) -0-(C1-C4)-alkyl, wherein alkyl is unsubstituted or substituted one to
three times by R13,
I) -NO2,
m) -CN,
n) -OH,
o) -C(O)-R10,
p) -C(O)-O-R11,
q) -C(O)-N(R11)-R12,
r) -N(R11)-R12,
s) -N(R10)-S02-R10,
t) -S-R10,
u) -SOn-R10, wherein n is 1 or 2,
v) -S02-N(R11)-R12, or
w) -O-SO2-R13,
R10 is hydrogen atom, -(C1-C3)-fluoroalkyl or -(C 1 -C6)-alkyl,
R11 and R12 are independently of one another identical or different and are
a) hydrogen atom,
b) -(C1-C6)-alkyl, wherein alkyl is unsubstituted or mono-, di- or
trisubstituted independently of one another by R13,
c) -(C6-C14)-aryl-, wherein aryl is unsubstituted or mono-, di- or
trisubstituted independently of one another by R13, or
d) -(C5-C14)-heteroaryl, wherein heteroaryl is unsubstituted or mono-, di- or
trisubstituted independently of one another by R13,
R13 is halogen, -NO2, -CN, =0, -OH, -(C1-C8)-alkyl, -(C1-C8)-alkoxy, -CF3,
phenyloxy-, -C(O)-R10, -C(O)-O-R17, -C(O)-N(R17)-R18, -N(R17)-R18,
-N(R10)-S02-R10, -S-R10, -SOn-R10, wherein n is 1 or 2, -S02-N(R17)-R18,
-(C6-C14)-aryl, wherein aryl is unsubstituted or mono-, di- or trisubstituted
independently of one another by R14, -(C5-C14)-heteroaryl, wherein heteroaryl
is unsubstituted or mono-, di- or trisubstituted independently of one another
by
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
8
R14, -(C3-C8)-cycloalkyl, wherein said cycloalkyl is unsubstituted or mono-,
di-
or trisubstituted independently of one another by R14, or a 3- to 7-membered
cyclic residue, containing up to 1, 2, 3 or 4 heteroatoms chosen from
nitrogen,
sulfur or oxygen, wherein said cyclic residue is unsubstituted or mono-, di-
or
trisubstituted independently of one another by R14,
R14 is halogen, -OH, =0, -CN, -CF3, -(C1-C8)-alkyl, -(C1-C4)-alkoxy, -NO2,
-C(O)-OH, -N(R11)-R12, -C(O)-O-(C 1 -C4)-alkyl, -(C1-Cg)-alkylsulfonyl,
-C(O)-NH-(C1-C8)-alkyl, -C(O)-N[(C1-C8)-alkyl12, -C(O)-NH2, -S-R10,
-N(R10)-C(O)-NH-(C1-C8)-alkyl, or -N(R10)-C(O)-N[(C1-C8)-alky1]2,
R17 and R18 are independently of one another identical or different and are
a) hydrogen atom,
b) -(C1-C6)-alkyl,
c) -(C6-C14)-aryl- or
d) -(C5-C 1 4)-heteroaryl,
said process comprises reacting a compound of formula II
R4 Q-RO
R3-A2-,A I (II)
R2-A~A4 x
I
R1
wherein RO, R1, R2, R3, R4, Al, A2, A3, A4 and Q are as defined in formula I
and
X is Cl, Br, I, triflate, nonaflate, tosylate, alkyl sulfonate or aryl
sulfonate,
with a compound of formula III or any salts thereof,
R5-J-H-NH2 (III)
wherein J and R5 are as defined in formula I,
in the presence of a transition metal catalyst to give a compound of formula I
and
optionally the compound of formula I is converted to its physiologically
tolerated salt.
2) The present invention also relates to a process for the preparation of a
compound of formula I, wherein
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
9
A1, A2, A3 and A4 form together with the two carbon atoms in formula I a
benzene,
pyrazine, pyridazine, pyridine, pyrimidine, triazine or tetrazine,
Q is a covalent bond,
-(C1-C6)-alkylene, wherein alkylene is unsubstituted or mono-, di- or
trisubstituted independently of one another by R14;
-(C3-C6)-cycloalkyl, wherein cycloalkyl is unsubstituted or mono-, di- or
trisubstituted independently of one another by R14;
phenyl, wherein phenyl is unsubstituted or mono-, di-, tri- or four times
substituted independently of one another by R13;
-(C1-C4)-alkylene-O-(C1-C4)-alkylene,
-(C1-C4)-alkylene-O-, or
-(C5-C14)-heteroaryl, wherein heteroaryl is selected from acridinyl, azaindole
(1 H-pyrrolopyridinyl), azabenzimidazolyi, azaspirodecanyl, azepinyl,
azetidinyl,
benzimidazolyl, benzofuranyl, benzothiofuranyl, benzothiophenyl, benzoxazolyl,
benzthiazolyl, benztriazolyl, benztetrazolyl, benzisoxazolyl,
benzisothiazolyl,
carbazolyl, 4aH-carbazolyl, carbolinyl, chromanyl, chromenyl, cinnolinyl,
decahydrochinolinyl, 4,5-dihydrooxazolinyl, dioxazolyl, dioxazinyl, 1,3-
dioxolanyl, 1,3-dioxolenyl, 3,3-dioxo[1,3,4]oxathiazinyl, 6H-1,5,2-
dithiazinyl,
dihydrofuro[2,3-b]-tetrahydrofuranyl, furanyl, furazanyl, imidazolidinyl,
imidazolinyl, imidazolyl, indanyl, 1 H-indazolyl, indolinyl, indolizinyl,
indolyl, 3H-
indo(yl, isobenzofuranyl, isochromanyl, isoindazolyl, isoindolinyl,
isoindolyl,
isoquinolinyl, isothiazolyl, isothiazolidinyl, isothiazolinyl, isoxazolyl,
isoxazolinyl,
isoxazolidinyl, 2-isoxazolinyl, ketopiperazinyl, morpholinyl, naphthyridinyl,
octahydroisoquinolinyl, oxadiazolyl, 1,2,3-oxadiazolyl, 1,2,4-oxadiazolyl,
1,2,5-
oxadiazolyl, 1,3,4-oxadiazolyl, 1,2-oxa-thiepanyl, 1,2-oxathiolanyl, 1,4-
oxazepanyl, 1,4-oxazepinyl, 1,2-oxazinyl, 1,3-oxazinyl, 1,4-oxazinyl,
oxazolidinyl, oxazolinyl, oxazolyl, oxetanyl, oxocanyl, phenanthridinyl,
phenanthrolinyl, phenazinyl, phenothiazinyl, phenoxathiinyl, phenoxazinyl,
phthalazinyl, piperazinyl, piperidinyl, pteridinyl, purinyl, pyranyl,
pyrazinyl,
pyrazolidinyl, pyrazolinyl, pyrazolyl, pyrazolo[3,4-b]pyridine, pyridazinyl,
pyridooxazolyl, pyridoimidazolyl, pyridothiazolyl, pyridinyl, pyridyl,
pyrimidinyl,
pyrrolidinyl, pyrrolidinonyl, pyrrolinyl, 2H-pyrrolyl, pyrrolyl, quinazolinyl,
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
quinolinyl, 4H-quinolizinyl, quinoxalinyl, quinuclidinyl, tetrahydrofuranyl,
tetrahydroisoquinolinyl, tetrahydroquinolinyl, tetrahydrofuranyl,
tetra hyd ro pyra nyl, tetrahydropyridinyl, tetrahydrothiophenyl, tetrazinyl,
tetrazolyl,
6H-1,2,5-thiadiazinyl, 1,2,3-thiadiazolyl, 1,2,4-thiadiazolyl, 1,2,5-
thiadiazolyl,
5 1,3,4-thiadiazolyl, thianthrenyl, 1,2-thiazinyl, 1,3-thiazinyl, 1,4-
thiazinyl, 1,3-
thiazolyl, thiazolyl, thiazolidinyl, thiazolinyl, thienyl, thietanyl,
thienothiazolyl,
thienooxazolyl, thienoimidazolyl, thietanyl, thiomorpholinyl, thiophenolyl,
thiophenyl, thiopyranyl, 1,2,3-triazinyl, 1,2,4-triazinyl, 1,3,5-triazinyl,
1,2,3-
triazolyl, 1,2,3-triazolyl, 1,2,4-triazolyl, 1,2,5-triazolyl, 1,3,4-triazolyl
and
10 xanthenyl, and wherein heteroaryl is unsubstituted or mono-, di-, tri- or
four
times substituted independently of one another by R13;
J is a covalent bond,
-(C1-C6)-alkylene, wherein alkylene is unsubstituted or mono-, di- or
trisubstituted independently of one another by R14;
-(C3-C6)-cycloalkyl, wherein cycloalkyl is unsubstituted or mono-, di- or
trisubstituted independently of one another by R14;
phenyl, wherein phenyl is unsubstituted or mono-, di-, tri- or four times
substituted independently of one another by R13; or
-(C5-C14)-heteroaryl, wherein heteroaryl is as defined above and is
unsubstituted or mono-, di-, tri- or four times substituted independently of
one
another by R13;
R0, R1, R2, R3 and R4 are independent of one another identical or different
and are
a) hydrogen atom,
b) -(C1-C4)-alkyl, wherein alkyl is unsubstituted or substituted one, two or
three times by R13,
c) F, Cl or Br,
d) phenyloxy-, wherein phenyloxy is unsubstituted or substituted one, two or
three times by R13,
e) -(C1-C3)-fluoroalkyl,
f) -N(R10)-(C1-C4)-alkyl, wherein alkyl is unsubstituted or substituted one,
two or three times by R13,
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
11
g) phenyl or naphthyl, wherein phenyl or naphthyl are unsubstituted or
substituted one, two or three times by R13,
h) -(C5-C14)-heteroaryl, wherein heteroaryl is as defined above and is
unsubstituted or mono-, di-, tri- or four times substituted independently of
one another by R13,
i) -(C3-Cg)-cycloalkyl, wherein said cycloalkyl is unsubstituted or mono-, di-
, tri- or four times substituted independently of one another by R13,
j) a 3- to 7-membered cyclic residue selected from azepine, azetidine,
aziridine, azirine, 1,4 diazepane, 1,2-diazepine, 1,3-diazepine, 1,4-
diazepine, diaziridine, diazirine, dioxazole, dioxazine, dioxole, 1,3-
dioxolene, 1,3-dioxolane, furan, imidazole, imidazoline, imidazolidine,
isothiazole, isothiazolidine, isothiazoline, isoxazole, isoxazoline,
isoxazolidine, 2-isoxazoline, ketomorpholine, ketopiperazine, morpholine,
1,2-oxa-thiepane, 1,2-oxathiolane, 1,4-oxazepane, 1,2-oxazine, 1,3-
oxazine, 1,4-oxazine, oxazole, oxaziridine, oxetan, oxirane, piperazine,
piperidine, pyran, pyrazine, pyrazole, pyrazoline, pyrazolidine, pyridazine,
pyridine, pyrimidine, pyrrole, pyrrolidine, pyrrolidinone, pyrroline,
tetrahydrofuran, tetrahydropyran, tetrahydropyridine, tetrazine, tetrazole,
thiadiazine thiadiazole, 1,2-thiazine, 1,3-thiazine, 1,4-thiazine, 1,3-
thiazole, thiazole, thiazolidine, thiazoline, thienyl, thietan,
thiomorpholine,
thiopyran, 1,2,3-triazine, 1,2,4-triazine, 1,3,5-triazine, 1,2,3-triazole or
1,2,4-triazole, and is unsubstituted or mono-, di-, tri- or four times
substituted independently of one another by R13,
k) -O-CF3,
I) -O-(C1-C4)-alkyl, wherein alkyl is unsubstituted or substituted one, two or
three times by R13,
m) -CN,
n) -OH,
o) -C(O)-R10
p) -C(O)-O-R11,
q) -C(O)-N(R11)-R12,
r) -N(R11)-R12,
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
12
s) -N(R10)-S02-R10,
t) -S-R10,
u) -SOn-R10, wherein n is 1 or 2,
v) -S02-N(R11)-R12, or
w) -O-S02-R13, or
x) at least one of R1, R2, R3 or R4 are absent in case one or more of Al,
A2, A3 or A4 are nitrogen atom,
R5 is a) -(C1-C4)-afkyl, wherein alkyl is unsubstituted or substituted one,
two or
three times by R13,
b) F, Cl or Br,
c) phenyloxy-, wherein phenyloxy is unsubstituted or substituted one, two or
three times by R13,
d) -(C 1-C3)-fluoroalkyi,
e) -N(R10)-(C1-C4)-alkyl, wherein alkyl is unsubstituted or substituted one,
two or three times by R13,
f) phenyl or naphthyl, wherein phenyl or naphthyl are unsubstituted or
substituted one, two or three times by R13,
g) -(C5-C14)-heteroaryl, wherein heteroaryl is as defined above and is
unsubstituted or mono-, di-, tri- or four times substituted independently of
one another by R13,
h) -(C3-C8)-cycloalkyl, wherein said cycloalkyl is unsubstituted or mono-, di-
, tri- or four times substituted independently of one another by R13,
i) a 3- to 7-membered cyclic residue selected from azepine, azetidine,
aziridine, azirine, 1,4 diazepane, 1,2-diazepine, 1,3-diazepine, 1,4-
diazepine, diaziridine, diazirine, dioxazole, dioxazine, dioxole, 1,3-
dioxolene, 1,3-dioxolane, furan, imidazole, imidazoline, imidazolidine,
isothiazole, isothiazolidine, isothiazoline, isoxazole, isoxazoline,
isoxazolidine, 2-isoxazoline, ketomorpholine, ketopiperazine, morpholine,
1,2-oxa-thiepane, 1,2-oxathiolane, 1,4-oxazepane, 1,2-oxazine, 1,3-
oxazine, 1,4-oxazine, oxazole, oxaziridine, oxetan, oxirane, piperazine,
piperidine, pyran, pyrazine, pyrazole, pyrazoline, pyrazolidine, pyridazine,
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
13
pyridine, pyrimidine, pyrrole, pyrrolidine, pyrrolidinone, pyrroline,
tetra hyd rofu ran, tetrahydropyran, tetrahydropyridine, tetrazine, tetrazole,
thiadiazine thiadiazole, 1,2-thiazine, 1,3-thiazine, 1,4-thiazine, 1,3-
thiazole, thiazole, thiazolidine, thiazoline, thienyl, thietan,
thiomorpholine,
thiopyran, 1,2,3-triazine, 1,2,4-triazine, 1,3,5-triazine, 1,2,3-triazole or
1,2,4-triazole, and is unsubstituted or mono-, di-, tri- or four times
substituted independently of one another by R13,
1) -O-CF3,
k) -O-(C1-C4)-alkyl, wherein alkyl is unsubstituted or substituted one, two or
three times by R13,
I) -CN,
m) -OH,
n) -C(O)-R10
o) -C(O)-O-R11,
p) -C(O)-N(R11)-R12,
q) -N(R11)-R12,
r) -N(R10)-SO2-R10,
s) -S-R10,
t) -SOn-R10, wherein n is 1 or 2,
u) -S02-N(R11)-R12, or
v) -O-S02-R13,
R10 is hydrogen atom, -(C1-C3)-fluoroalkyl or -(C1-C6)-alkyl,
R11 and R12 are independently of one another identical or different and are
a) hydrogen atom,
b) -(C1-C4)-alkyl, wherein alkyl is unsubstituted or mono-, di- or
trisubstituted independently of one another by R13,
c) phenyl, wherein phenyl is unsubstituted or mono-, di- or trisubstituted
independently of one another by R13,
d) -(C5-C14)-heteroaryl, wherein heteroaryl is as defined above and is
unsubstituted or mono-, di- or trisubstituted independently of one another
by R 13,
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
14
R13 is F, Cl, -CN, =0, -OH, -(C1-C8)-alkyl, -(C1-C8)-alkoxy, -CF3, phenyloxy-,
-C(O)-R10, -C(O)-O-R17, -C(O)-N(R17)-R18, -N(R17)-R18, -N(R10)-S02-R10,
-S-R10, -SOn-R10, wherein n is 1 or 2, -S02-N(R17)-R18, phenyl, wherein
phenyl is unsubstituted or mono-, di- or trisubstituted independently of one
another by R14, -(C5-C14)-heteroaryl, wherein heteroaryl is as defined above
and is unsubstituted or mono-, di- or trisubstituted independently of one
another
by R14, -(C3-C6)-cycloalkyl, wherein said cycloalkyl is unsubstituted or mono-
,
di- or trisubstituted independently of one another by R14, or a 3- to 7-
membered
cyclic residue, which is as defined above and is unsubstituted or mono-, di-
or
trisubstituted independently of one another by R14,
R14 is F, Cl, -OH, =0, -CN, -CF3, -(C1-C8)-alkyl, -(C1-C4)-alkoxy, -C(O)-OH,
-N(R11)-R12, -C(O)-O-(C1-C4)-alkyl, -(C1-C8)-alkylsulfonyl, -C(O)-NH2,
-C(O)-N H-(C 1-C8)-alkyl, -C(O)-N[(C j-C8)-alkyl12, -S-R10,
-N(R10)-C(O)-NH-(C1-C8)-alkyi or -N(R10)-C(O)-N[(Cj-C8)-alkyl12,
R17 and R18 are independently of one another identical or different and are
a) hydrogen atom,
b) -(C1-C4)-alkyl,
c) phenyl or
d) -(C5-C14)-heteroaryl, wherein heteroaryl is as defined above, and
X is Cl, Br, I, triflate, nonaflate, tosylate, alkyl sulfonate or aryl
sulfonate.
3) The present invention also relates to a process for the preparation of a
compound of formula I, wherein
Al, A2, A3 and A4 form together with the two carbon atoms in formula I a
benzene or
pyridine,
Q is a covalent bond,
-(Cl-C6)-alkylene,
-(C3-Cg)-cycloalkyl,
phenyl, wherein phenyl is unsubstituted or mono-, di-, tri- or four times
substituted independently of one another by R13,
-(C 1 -C4)-alkylene-O-(C 1 -C4)-alkylene,
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
-(C1-C4)-alkylene-O-; or
-(C5-C14)-heteroaryl, wherein heteroaryl is selected from pyridyl, quinolinyl,
tetrahydropyranyl and thienyl,
J is a covalent bond, -(C1-C6)-a(kylene, -(C3-C6)-cycloalkyl, phenyl,
naphthyl, or
5 -(C5-C14)-heteroaryl, wherein heteroaryl is as defined above,
R0, R1, R2, R3 and R4 are independent of one another identical or different
and are
a) hydrogen atom,
b) -(C1-C4)-alkyl, wherein alkyl is unsubstituted or substituted one, two or
three times by R13,
10 c) F, Cl or Br,
d) naphthyl, wherein naphthyl is unsubstituted or substituted one, two or
three times by R13,
e) phenyl, wherein phenyl is unsubstituted or substituted one, two or three
times by R13,
15 f) -(C5-C14)-heteroaryl, wherein heteroaryl is as defined above,
g) -(C3-C6)-cycloalkyl,
h) -O-(C1-C4)-alkyl,
i) -CN,
j) -OH,
k) -C(O)-R10,
I) -C(O)-O-R11,
m) -C(O)-N(R 1 1)-R1 2,
n) -N(R11)-R12, or
p) -O-S02-R13,
R5 is a) -(C1-C4)-alkyl, wherein alkyl is unsubstituted or substituted one,
two or
three times by R13,
b) F, Cl or Br,
c) naphthyl, wherein naphthyl is unsubstituted or substituted one, two or
three times by R13,
d) phenyl, wherein phenyl is unsubstituted or substituted one, two or three
times by R13,
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
16
e) -(C5-C14)-heteroaryl, wherein heteroaryl is as defined above,
f) -(C3-Cg)-cycloalkyl,
g) -O-(C1-C4)-alkyl,
h) -CN,
i) -OH,
j) -C(O)-R10,
k) -C(O)-O-R11,
I) -C(O)-N(R11)-R12,
m) -N(R11)-R12, or
n) -O-S02-R13,
R10 is hydrogen atom or -(C1 -C4)-alkyl,
R11 and R12 are independently of one another identical or different and are
hydrogen atom or -(C1-C4)-alkyl, wherein alkyl is unsubstituted or mono-, di-
or
trisubstituted independently of one another by R13,
R13 is F, CI, -CN, -OH, -(C1-C4)-alkoxy, -CF3 or phenyl, wherein phenyl is
unsubstituted or mono-, di- or trisubstituted independently of one another by
R14,
R14 is F, Cl, -OH, -CN, -CF3, -(C1-C4)-alkyl or -(C1-C4)-alkoxy, and
X is Cl, Br, I or tosylate.
The reaction can be performed in a broad range of protic or aprotic solvents,
including
polar aprotic solvents, or even in some cases without a solvent. Examples of
said
solvents are: tert-butanol, benzene, toluene, xylene, mesitylene,
acetonitrile,
propionitrile, tetrahydrofurane, 2-methyl-tetrahydrofurane, N,N-
dimethylformamide, N-
methylpyrrolidinone, N,N-dimethylacetamide, dimethylsulfoxide, 1,2-
dimethoxyethane,
tert-butylmethylether, triethylamine, diisopropylethylamine or pyridine.
Preferred is
N,N-dimethylacetamide, N,N-dimethylformamide, N-methylpyrrolidinone. Most
preferred is N,N-dimethylformamide.
Useful bases for the process of the present invention is a basic organic or
inorganic
compound that acts as proton acceptor without inhibiting the catalytic
activity of the
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
17
employed transition metal catalyst. Suitable classes of such bases are for
example
carbonates, phosphates, alkoxides, hydroxides, amides, hydrides with a
suitable metal
as counter ion, or an alkali metal. Carbonates and phosphates are the
preferred bases
in the process of the present invention. Potassium carbonate or potassium
phosphate
and in particular cesium carbonate are the preferred bases. The bases can be
used in
pure form or a mixture of several bases can be used.
The bases are generally employed in moderate excess based on 2-halo-
phenylacetylenes or (2-sulfonate)phenylacetylenes of formula II. A useful
range is a
0.5 to 10 fold excess based on the 2-halo-phenylacetylenes or (2-
sulfonate)phenyl-
acetylenes of formula II. An even more useful range 1.1-2.0 fold excess based
on the
2-halo-phenylacetylenes or (2-sulfonate)phenylacetylenes of formula II. The
base may
be favourably employed in a 1.4 fold excess based on the 2-halo-
phenylacetylenes or
(2-sulfonate)phenylacetylenes of formula II. In reactions where the hydrazine
is
employed as a salt, e.g. as a hydrochloride salt, one additional equivalent of
base
compared to the salt is added is added to the reaction mixture in order to
generate the
hydrazine in situ. Alternatively, the reaction can also be performed without a
base if the
hydrazine is used as the corresponding amide prepared by reaction of the
hydrazine or
hydrazine salt with a strong base.
The active form of the transition metal catalyst is not known. Therefore, the
term
"transition metal catalyst" in the present invention shall include any
catalytic transition
metal and/or catalyst precursor introduced into the reaction vessel and
converted in
situ into the active form, as well as the active form of the catalyst that
promotes any
part of the reaction. The transition metal catalyst can be used in any amount,
but
generally 0.00005-90 moI% would be employed. Preferred is the use of 0.01-20
mol%,
and even more preferred is the use of 0.5-10 mol% and most preferably 1-5 mol%
of
the transition metal catalyst is employed.
Generally, any suitable transition metal catalyst that can mediate the
reaction can be
employed these include the elements of group 3-12 of the periodic table as
well as the
lanthanides. Preferred transition metals include platinum, palladium, nickel,
gold,
copper, iron, ruthenium, rhodium and iridium. Even more preferred are nickel
and
palladium and most preferred palladium. The transition metal catalyst can be
soluble or
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
18
insoluble, and the particular source of the transition metal useful in this
process can
be, but are not limited to: Pd-halides, Pd-halide complexes, Ni-halides, Ni-
halide
complexes, Pd-phosphine complexes, Ni-phosphine complexes, Pd-alkene
complexes,
Ni-alkene complexes, Pd-alkanoates, Pd-alkanoate complexes, Pd-acetonates, Ni-
alkanoates, Ni-alkanoate complexes, Ni-acetonates, Raney nickel, Pd/C or Ni/C,
or
polymer supported palladium or nickel species or a mixture thereof.
Representative
examples include, but are not limited to: palladium (II) chloride, palladium
(II) bromide,
palladium (II) iodide, tris(dibenzylideneacetone)dipalladium(0), palladium
(II) acetate,
palladium (II) trifluoroacetate, tris(dibenzylideneacetone)dipalladium(0)
chloroform
adduct, bis(dibenzylideneacetone)palladium (0),
bis(triphenylphosphine)palladium (II)
chloride, tetrakis(triphenylphosphine)palladium (0), nickel (II) chloride,
nickel (II)
bromide, nickel (II) iodide, Ni(acac)2, Ni(1,5-cyclooctadiene)2, acetato(2'-di-
tert-
butylphosphino-1,1'-biphenyl-2-yl)palladium(II), (1,2-
Bis(diphenylphosphino)ethane)di-
chloropalladium(II), Bis[1,2-bis(diphenylphosphino)ethane]palladium (0),
[(2S,3S)-
Bis(diphenylphosphino)butane] [eta3-allyl]palladium(II) perchlorate, 1,3-
bis(2,4,6-
trimethylphenyl)imidazol-2-ylidene(1,4-naphthoqui none)palladium (0) dimmer,
2,2'-
bis(diphenylphosphino)-1,1'-binaphthylpalladium(ll) chloride.
The preferred transition metal sources are palladium (II) chloride, palladium
(II)
bromide, palladium (II) iodide, tris(dibenzylideneacetone)dipalladium(0),
palladium (II)
acetate, palladium (II) trifluoroacetate,
tris(dibenzylideneacetone)dipalladium(0)
chloroform adduct, bis(dibenzylideneacetone)palladium (0),
bis(triphenylphosphine)-
palladium (II) chloride, tetrakis(triphenylphosphine)palladium (0), and even
more
preferred are palladium (II) chloride, palladium (II) bromide, palladium (II)
iodide,
tris(dibenzylideneacetone)dipalladium(0). The most preferred palladium sources
being
palladium (II) chloride and tris(dibenzylideneacetone)dipalladium(0).
The group of ligands useful in this process may be chelating or non-chelating
and may
include alkyl or aryl phosphines or hybrids thereof e.g.
dicyclohexlphenylphosphine, or
aryl or alkyl diphospines or hybrids thereof, diamines, imines, heterocyclic
carbenes or
hybrids thereof.
The ligand can be used in its free form or as a salt, e.g. the hydrochloride
or
tetrafluoroborate salt.
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
19
By way of example only, the ligand can be selected from the following
compounds, but
are not limited to: tri-tert-butylphosphine, tri-tert-butylphosphine
tetrafluoroborate salt,
tricyclohexylphosphine, dicyclohexlphenylphosphine, methyldiphenylphosphine,
dimethylphenylphosphine, trimethylphosphine, triethylphosphine,
triphenylphosphine,
2-dicyclohexylphosphino-2',4',6'-triisopropyl-1,1'-biphenyl, 2,2'-bis(di-tert-
butylphosphino)biphenyl, (+/-)-2,2'-bis(diphenylphosphino)-1,1'-binaphthalene,
(9,9-
dimethyl-9h-xanthene-4,5-diyl)bis[dipheny) phosphine], (R)-(-)-1-[(S)-2-
(diphenylphosphino) ferrocenyl] ethyldicyclohexylphosphine, 1,2-
bis(diphenylphosphino)ethane, 1,3-bis(diphenylphosphino)propane, (R)-(-)-1-
[(S)-2-
(dicyclohexylphosphino)ferrocenyl]-ethyldi-tert-butylphosphine, (R)-(+)-1,1'-
bis(diphenylphosphino)-2,2'-bis(N,N-diiisopropylamido)ferrocene, (S,S)-1-[1-
(di-tert-
butylphosphino)ethy!]-2-(diphenylphosphino)ferrocene, (1 R,2R)-(+)-1,2-
diaminocyclohexane-N,M-bis(2-diphenylphosphino-l-naphtoyl, (-)-1,2-bis((2S,5S)-
2,5-
diisopropylphospholano)-benzene, bis[(2-diphenylphosphino)phenyl]ether, (S)-(-
)-2,2'-
Bis(di-para-tolylphosphino)-1,1'-binaphyl, 4,5-bis(bis(3,5-
bis(trifluoromethyl)phenyl)-
phosphino)-9,9-dimethylxanthen.
Preferred ligands are tri-tert-butylphosphine, tri-tert-butylphosphine
tetrafluoroborate
salt, tricyclohexylphosphine, 2-dicyclohexylphosphino-2',4',6'-triisopropyl-
1,1'-biphenyl.
More preferred ligands are tri-tert-butylphosphine, tri-tert-butylphosphine
tetrafluoroborate salt, and most preferred is tri-tert-butylphosphine
tetrafluoroborate
salt.
Most favourably tri-tert-butylphosphine or tri-te-t-butylphosphine
tetrafluoroborate are
employed in particular in combination with a palladium source bearing no
phosphine
itself, like e.g. palladium (II) chloride, palladium (II) bromide, palladium
(II) iodide, or
tris(dibenzylideneacetone)dipalladium(0).
The ligand can be used in any amount, but generally 0.00005-90 mol% would be
employed. Preferred is the use of 0.01-40 mol%, and even more preferred is the
use of
0.5-20 mol% and most preferably 1-10 mol% of the ligand is employed. The ratio
of the
ligand to the transition metal is generally about 1 to 20, preferably about 1-
5 and most
preferably 2.
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
The reaction step is usually carried out in the temperature range 0 C to 300
C,
preferably between 25 C to 150 C, and most preferably between 80 C to 130 C.
Usually the reaction is carried out under the exclusion of air and moisture
such as
5 under an inert atmosphere like e.g. in an argon or nitrogen atmosphere at
atmospheric
pressure. The reaction time is normally in the range of 2 to 48 hours (h).
The progress of the reaction may be monitored by methods known to those
skilled in
the art, like for example thin layer silica gel chromatography, gas
chromatography,
10 nuclear magnetic resonance, infrared spectroscopy, and high pressure liquid
chromatography combined with ultraviolet detection or mass spectroscopy.
Preferably
thin layer silica gel chromatography and high pressure liquid chromatography
(HPLC)
combined with mass spectroscopy are used.
The isolation and purification procedures useful for the compounds obtained by
the
15 process of the present invention are well-known to those skilled in the
art, like for
example filtration through a celite containing cartridge, aqueous work-up,
extraction
with organic solvents, distillation, crystallisation, chromatography on
silica, and high
pressure liquid chromatography on normal phase or reversed phase. Preferred
methods include, but are not limited to those exemplified.
The term alkyl as used herein expressly includes saturated groups as well as
unsaturated groups which latter groups contain one or more, for example one,
two or
three, double bonds and/or triple bonds. All these statements also apply if an
alkyl
group occurs as a substituent on another residue, for example in an alkyloxy
residue,
an alkyloxycarbonyl residue or an arylalkyl residue. Examples of õ-(C1-C8)-
alkyP' or
õ-(C1-C8)-alkylene" are alkyl residues containing 1, 2, 3, 4, 5, 6, 7 or 8
carbon atoms
are methyl, methylene, ethyl, ethylene, propyl, propylene, butyl, butylene,
pentyl,
pentylene, hexyl, heptyl or octyl, the n-isomers of all these residues,
isopropyl,
isobutyl, 1-methylbutyl, isopentyl, neopentyl, 2,2-dimethylbutyl, 2-
methylpentyl, 3-
methylpentyl, isohexyl, sec-butyl, tBu, tert-pentyl, sec-butyl, tert-butyl or
tert-pentyl.
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
21
Unsaturated alkyl residues are e.g. alkenyl residues such as vinyl, 1-
propenyl, 2-
propenyl (= allyl), 2-butenyl, 3-butenyl, 2-methyl-2-butenyl, 3-methyl-2-
butenyl, 5-
hexenyl or 1,3-pentadienyl, or alkynyl residues such as ethynyl, 1-propynyl, 2-
propynyl
(= propargyl) or 2-butynyl.
The term "-(C3-C8)-cycloalkyl" is understood as cyclic alkyl residues which
contain 3,
4, 5, 6, 7 or 8 ring carbon atoms like cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl,
cyloheptyl or cyclooctyl, which can also be substituted and/or unsaturated.
Unsaturated cyclic alkyl groups and unsaturated cycloalkyl groups like, for
example,
cyclopentenyl or cyclohexenyl can be bonded via any carbon atom.
The term "alkyl sulfonate" is understood as an alkyl residue containing 1, 2,
3, 4, 5 or 6
carbon atoms substituted by sulfonate. Examples of such residues are
methylsulfonate
(mesylate), ethylsulfonate, propyisulfonate, butylsulfonate, pentylsulfonate
or
hexylsulfonate.
The term "Al, A2, A3, A4 are independently from each other selected from
carbon or
nitrogen atoms to form together with the two carbon atoms in formula I a
stable
aromatic or heteroaromatic ring" refers to a residue which is e.g. selected
from
benzene, pyrazine, pyridazine, pyridine, pyrimidine, triazine or tetrazine.
The term "-(C6-C14)-aryl" is understood as meaning aromatic hydrocarbon
radicals
containing from 6 to 14 carbon atoms in the ring. Examples of -(C6-C14)-aryl
radicals
are phenyl, naphthyl, for example 1-naphthyl and 2-naphthyl, biphenylyl, for
example
2-biphenylyl, 3-biphenylyl and 4-biphenylyl, anthryl or fluorenyl. Biphenylyl
radicals,
naphthyl radicals and, in particular, phenyl radicals are preferred aryl
radicals.
The term "aryl sulfonate" is understood as an aryl as defined herein, which is
substituted by a sulfonate. Examples of such compounds are benzenesulfonate,
tosylate, nitrobenzenesulfonate or bromobenzenesulfonate.
The term "-(C5-C14)-heteroaryl" refers to mono-, di- or tri-ring systems,
wherein one or
more of the 5 to 14 ring carbon atoms are replaced by heteroatoms such as
nitrogen,
oxygen or sulfur. Examples are acridinyl, azaindole (1 H-pyrrolopyridinyl),
azabenz-
imidazolyl, azaspirodecanyl, azepinyl, azetidinyl, benzimidazolyl,
benzofuranyl,
benzothiofuranyl, benzothiophenyl, benzoxazolyl, benzthiazolyl, benztriazolyl,
benztetrazolyl, benzisoxazolyl, benzisothiazolyl, carbazolyl, 4aH-carbazolyl,
carbolinyl,
chromanyl, chromenyl, cinnolinyl, decahydrochinolinyl, 4,5-dihydrooxazolinyl,
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
22
dioxazolyl, dioxazinyl, 1,3-dioxolanyl, 1,3-dioxolenyl, 3,3-
dioxo[1,3,4]oxathiazinyl, 6H-
1,5,2-dithiazinyl, dihydrofuro[2,3-b]-tetrahydrofuranyl, furanyl, furazanyl,
imidazolidinyl,
imidazolinyl, imidazolyl, indanyl, 1 H-indazolyl, indolinyl, indolizinyl,
indolyl, 3H-indolyl,
isobenzofuranyl, isochromanyl, isoindazolyl, isoindolinyl, isoindolyl,
isoquinolinyl,
isothiazolyl, isothiazolidinyl, isothiazolinyl, isoxazolyl, isoxazolinyl,
isoxazolidinyl, 2-
isoxazolinyl, ketopiperazinyl, morpholinyl, naphthyridinyl,
octahydroisoquinolinyl,
oxadiazolyl, 1,2,3-oxadiazolyl, 1,2,4-oxadiazolyi, 1,2,5-oxadiazolyl, 1,3,4-
oxadiazolyl,
1,2-oxa-thiepanyl, 1,2-oxathiolanyl, 1,4-oxazepanyl, 1,4-oxazepinyl, 1,2-
oxazinyl, 1,3-
oxazinyl, 1,4-oxaziny(, oxazolidinyl, oxazo(inyl, oxazolyl, oxetanyl,
oxocanyl,
phenanthridinyl, phenanthrolinyl, phenazinyl, phenothiazinyl, phenoxathiinyl,
phenoxazinyl, phthalazinyl, piperazinyl, piperidinyl, pteridinyl, purinyl,
pyranyl,
pyrazinyl, pyrazolidinyl, pyrazolinyl, pyrazolyl, pyrazolo[3,4-b]pyridine,
pyridazinyl,
pyridooxazolyl, pyridoimidazolyl, pyridothiazolyl, pyridinyl, pyridyl,
pyrimidinyl,
pyrrolidinyl, pyrrolidinonyl, pyrrolinyl, 2H-pyrrolyl, pyrrolyl, quinazolinyl,
quinolinyl, 4H-
quinolizinyl, quinoxalinyl, quinuclidinyl, tetrahydrofuranyl,
tetrahydroisoquinolinyl,
tetrahydroquinolinyl, tetra hyd rofu ra nyl, tetrahydropyranyl,
tetrahydropyridinyl,
tetrahydrothiophenyl, tetrazinyl, tetrazo(yl, 6H-1,2,5-thiadiazinyl, 1,2,3-
thiadiazoiyl,
1,2,4-thiadiazolyl, 1,2,5-thiadiazolyl, 1,3,4-thiadiazolyl, thianthrenyl, 1,2-
thiazinyl, 1,3-
thiazinyl, 1,4-thiazinyl, 1,3-thiazolyl, thiazolyl, thiazolidinyl,
thiazolinyl, thienyl, thietanyl,
thienothiazolyl, thienooxazolyl, thienoimidazolyl, thietanyl, thiomorpholinyl,
thiophenolyl, thiophenyl, thiopyranyl, 1,2,3-triazinyl, 1,2,4-triazinyl, 1,3,5-
triazinyl, 1,2,3-
triazo(yl, 1,2,3-triazolyl, 1,2,4-triazolyl, 1,2,5-triazolyl, 1,3,4-triazolyl
and xanthenyl.
The term "a 3- to 7-membered cyclic residue, containing 1, 2, 3 or 4
heteroatoms" refer
to structures of heterocycles, which are selected from compounds such as
azepine,
azetidine, aziridine, azirine, 1,4 diazepane, 1,2-diazepine, 1,3-diazepine,
1,4-
diazepine, diaziridine, diazirine, dioxazole, dioxazine, dioxole, 1,3-
dioxolene, 1,3-
dioxolane, furan, imidazole, imidazoline, imidazolidine, isothiazole,
isothiazolidine,
isothiazoline, isoxazofe, isoxazoline, isoxazolidine, 2-isoxazoline,
ketomorpholine,
ketopiperazine, morpholine, 1,2-oxa-thiepane, 1,2-oxathiolane, 1,4-oxazepane,
1,2-
oxazine, 1,3-oxazine, 1,4-oxazine, oxazole, oxaziridine, oxetan, oxirane,
piperazine,
piperidine, pyran, pyrazine, pyrazole, pyrazoline, pyrazolidine, pyridazine,
pyridine,
pyrimidine, pyrrole, pyrrolidine, pyrrolidinone, pyrroline, tetrahydrofuran,
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
23
tetrahydropyran, tetrahydropyridine, tetrazine, tetrazole, thiadiazine
thiadiazole, 1,2-
thiazine, 1,3-thiazine, 1,4-thiazine, 1,3-thiazole, thiazole, thiazolidine,
thiazoline,
thienyl, thietan, thiomorpholine, thiopyran, 1,2,3-triazine, 1,2,4-triazine,
1,3,5-triazine,
1 , 2, 3-triazole or 1 , 2, 4-triazole.
The term "R1 and R2, R2 and R3 or R3 and R4 form together with the atoms which
they are attached to a 5- or 8-membered ring, containing up to 0, 1, 2, 3 or 4
heteroatoms chosen from nitrogen, sulfur or oxygen" refers to residues which
are
selected from compounds such as azepine, azirine, azocane, azocane-2-one,
cyloheptyl, cyclohexyl, cyclooctane, cyclooctene, 1,4-diazepane, 1,2-
diazepine, 1,3-
diazepine, 1,4-diazepine, [1,2]diazocan-3-one, [1,3]diazocan-2-one,
[1,4]diazocane,
dioxazine, dioxazole, [1,4]dioxocane, 1,3-dioxolane, dioxole, 1,3-dioxolene,
furan,
imidazole, imidazolidine, imidazoline, isothiazole, isothiazolidine,
isothiazoline,
isothiazole, isoxazole, isoxazolidine, isoxazoline, 2-isoxazoline,
ketomorpholine,
ketopiperazine, morpholine, 1,2-oxa-thiepane, 1,2-oxathiolane, 1,4-oxazepane,
1,2-
oxazine, 1,3-oxazine, 1,4-oxazine, oxaziridine,[1,4]oxazocane, [1,3]oxazocan-2-
one,
oxocane, oxocan-2-one, oxazole, piperidine, piperazine, phenyl, pyridazine,
pyridine,
pyrimidine, pyran, pyrazine, pyrazole, pyrazolepyrrole, pyrazolidine,
pyrazoline,
pyridazine, pyridine, pyrimidine, pyrrole, pyrrolidine, pyrrolidinone,
pyrroline, 5,6,7,8-
tetra hyd ro- 1 H-azoci n-2-one, tetrahydrofuran, tetrahydropyran,
tetrahydropyridine,
tetrazine, tetrazole, thiadiazine, thiadiazole, 1,2-thiazine, 1,3-thiazine,
1,4-thiazine,
thiazole, 1,3-thiazole, thiazolidine, thiazoline, thienyl, thietan,
thiomorpholine,
thiopyran, 1,2,3-triazine, 1,2,4-triazine, 1,3,5-triazine, 1,2,3-triazole or
1,2,4-triazole.
The term "-(C1-C3)-fluoroalkyl" is a partial or totally fluorinated alkyl-
residue, which can
be derived from residues such as -CF3, -CHF2, -CH2F, -CHF-CF3, -CHF-CHF2, -
CHF-CH2F, -CH2-CF3, -CH2-CHF2, -CH2-CH2F, -CF2-CF3, -CF2-CHF2,
-CF2-CH2F, -CH2-CHF-CF3, -CH2-CHF-CHF2, -CH2-CHF-CH2F, -CH2-CH2-CF3,
-CH2-CH2-CHF2, -CH2-CH2-CH2F, -CH2-CF2-CF3, -CH2-CF2-CHF2,
-CH2-CF2-CH2F, -CHF-CHF-CF3, -CHF-CHF-CHF2, -CHF-CHF-CH2F,
-CHF-CH2-CF3, -CHF-CH2-CHF2, -CHF-CH2-CH2F, -CHF-CF2-CF3,
-CHF-CF2-CHF2, -CHF-CF2-CH2F, -CF2-CHF-CF3, -CF2-CHF-CHF2,
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
24
-CF2-CHF-CH2F, -CF2-CH2-CF3, -CF2-CH2-CHF2, -CF2-CH2-CH2F,
-CF2-CF2-CF3, -CF2-CF2-CHF2 or -CF2-CF2-CH2F.
Halogen is fluorine, chlorine, bromine or iodine, preferably fluorine,
chlorine or
bromine, particularly preferably chlorine or bromine.
The term "triflate" refers to trifluoro-methanesulfonic acid ester or
trifluoromethanesulfonate.
The term "nonaflate" refers to 1, 1,2,2,3,3,4,4,4-nonafluoro-1 -butanesulfonic
acid ester
or 1,1,2,2,3,3,4,4,4-nonafluoro-1-butanesulfonate.
The term "at least one of R1, R2, R3 or R4 are absent in case one or more of
Al, A2,
A3 or A4 are nitrogen atom," refers to a residue wherein the nitrogen atom is
not
substituted by any residue, e.g. in case Al is nitrogen atom and A2, A3 and A4
are
each a carbon atom and R4 is absent and Rl, R2 and R3 are each a hydrogen atom
the residue pyridine is formed. If R1, R2 and R3 are not each a hydrogen atom
but one
of the residues specified under b) to x) then a substituted pyridine residue
is formed. In
case Al and A2 are each a nitrogen atom and A3 and A4 are each a carbon atom
and
R4 and R3 are absent and Rl and R2 are each a hydrogen atom the residue
pyridazine is formed. If R1 and R2 are not each a hydrogen atom but one of the
residues specified under b) to x) then a substituted pyridazine residue is
formed.
Optically active carbon atoms present in the compounds of the formula (I) can
independently of each other have R configuration or S configuration. The
compounds
of the formula (I) can be present in the form of pure enantiomers or pure
diastereomers
or in the form of mixtures of enantiomers and/or diastereomers, for example in
the form
of racemates. The present invention relates to pure enantiomers and mixtures
of
enantiomers as well as to pure diastereomers and mixtures of diastereomers.
The
invention comprises mixtures of two or of more than two stereoisomers of the
formula
(I), and it comprises all ratios of the stereoisomers in the mixtures. In case
the
compounds of the formula (I) can be present as E isomers or Z isomers (or cis
isomers
or trans isomers) the invention relates both to pure E isomers and pure Z
isomers and
to E/Z mixtures in all ratios. The invention also comprises all tautomeric
forms of the
compounds of the formula (I).
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
Diastereomers, including E/Z isomers, can be separated into the individual
isomers, for
example, by chromatography. Racemates can be separated into the two
enantiomers
by customary methods, for example by chromatography on chiral phases or by
resolution, for example by crystallization of diastereomeric salts obtained
with optically
5 active acids or bases. Stereochemically uniform compounds of the formula (I)
can also
be obtained by employing stereochemically uniform starting materials or by
using
stereoselective reactions.
The starting materials or building blocks for use in the general synthetic
procedures
10 that can be applied in the preparation of the compounds of formula (I) are
readily
available to one of ordinary skill in the art. In many cases they are
commercially
available or have been described in the literature. Otherwise they can be
prepared
from readily available precursor compounds analogously to procedures described
in
the literature, or by procedures or analogously to procedures described in
this
15 application.
Furthermore, in order to obtain the desired substituents in the benzene
nucleus and in
the heterocyclic nucleus of the 2H-indazole or 2H-azaindazole ring system in
the
formula (I), the functional groups introduced into the ring system during the
2H-
20 indazole or 2H-azaindazole synthesis can be chemically modified.
Especially the groups present in the 2H-indazole or 2H-azaindazole ring system
can
be modified by a variety of reactions and thus the desired residues RO, R1,
R2, R3, R4
and R5 be obtained. For example, ester groups present in the benzene nucleus
can be
hydrolyzed to the corresponding carboxylic acids, which after activation can
then be
25 reacted with amines or alcohols under standard conditions. Ether groups
present at the
benzene nucleus, for example benzyloxy groups or other easily cleavable ether
groups, can be cleaved to give hydroxyl groups which then can be reacted with
a
variety of agents, for example etherification agents or activating agents
allowing
replacement of the hydroxyl group by other groups. Sulfur-containing groups
can be
reacted analogously.
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
26
Due to the fact that in the present case the functional groups are attached to
an 2H-
indazole or 2H-azaindazole ring it may in certain cases become necessary to
specifically adapt reaction conditions or to choose specific reagents from a
variety of
reagents that can in principle be employed into a conversion reaction, or
otherwise to
take specific measures for achieving a desired conversion, for example to use
protection group techniques. However, finding out suitable reaction variants
and
reaction conditions in such cases does not cause any problems for one skilled
in the
art.
In the course of the preparation of the compounds of the formula I it can
generally be
advantageous or necessary to introduce functional groups which reduce or
prevent
undesired reactions or side reactions in the synthesis, in the form of
precursor groups
which are later converted into the desired functional groups, or to
temporarily block
functional groups by a protective group strategy suited to the synthesis
problem. Such
strategies are well known to those skilled in the art. As an example of a
precursor
group, cyan groups could be mentioned which in a later step can be transformed
into
carboxylic acid derivatives or by reduction into aminomethyl groups.
Protective groups
can also have the meaning of a solid phase, and cleavage from the solid phase
stands
for the removal of the protective group. The use of such techniques is known
to those
skilled in the art. For example, a phenolic hydroxyl group can be attached to
a trityl-
polystyrene resin, which serves as a protecting group, and the molecule is
cleaved
from this resin by treatment with TFA at a later stage of the synthesis.
In the course of the synthesis the employment of microwave assistance for
speeding-
up, facilitating or enabling reactions may be beneficial or even required in
many cases.
Some reactions are for example described by M. Larhed, A. Hallberg, Drug
Discovery
Today, 8 (2001) 406.
Physiologically tolerable salts of the compounds of formula I are non-toxic
salts that
are physiologically acceptable, in particular, pharmaceutically utilizable
salts. Such
salts of compounds of formula I containing acidic groups, for example, a
carboxyl
group (COOH), include, for example, alkali metal salts or alkaline earth metal
salts,
such as sodium salts, potassium salts, magnesium salts and calcium salts, as
well as
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
27
salts with physiologically tolerable quaternary ammonium ions, such as
tetramethylammonium or tetraethylammonium, and acid addition salts with
ammonia
and physiologically tolerable organic amines, such as methylamine,
dimethylamine,
trimethylamine, ethylamine, triethylamine, ethanolamine or tris-(2-
hydroxyethyl)amine.
Basic groups contained in the compounds of formula I, for example, amino
groups or
guanidino groups, form acid addition salts, for example, with inorganic acids
such as
hydrochloric acid, hydrobromic acid, sulfuric acid, nitric acid or phosphoric
acid, or with
organic carboxylic acids and sulfonic acids such as formic acid, acetic acid,
oxalic acid,
citric acid, lactic acid, malic acid, succinic acid, malonic acid, benzoic
acid, maleic acid,
fumaric acid, tartaric acid, methanesulfonic acid or p-toluenesulfonic acid.
Compounds
of the formula I which simultaneously contain a basic group and an acidic
group, for
example, a guanidino group and a carboxyl group, can also be present as
zwitterions
(betaines) which are likewise included in the scope of the present invention.
Salts of compounds of formula I can be obtained by customary methods known to
those skilled in the art, for example, by combining a compound of the formula
I with an
inorganic or organic acid or base in a solvent or dispersant, or from other
salts by
cation exchange or anion exchange. The present invention also includes all
salts of the
compounds of formula I which, because of low physiologically tolerability, are
not
directly suitable for use in pharmaceuticals but are suitable, for example, as
intermediates for carrying out further chemical modifications of the compounds
of
formula I or as starting materials for the preparation of physiologically
tolerable salts.
A further aspect of the invention is the use of a compound of the formula I as
prepared
by the process according to the invention for the production of
pharmaceuticals,
diagnostic agents, liquid crystals, polymers, herbicides, fungicidals,
nematicidals,
parasiticides, insecticides, acaricides and arthropod icides.
Preferred methods include, but are not limited to those described in the
examples.
Furthermore, the compounds of the formula I can be used as synthesis
intermediates
for the preparation of other compounds, in particular of other pharmaceutical
active
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
28
ingredients, which are obtainable from the compounds of the formula I, for
example by
introduction of substituents or modification of functional groups.
The general synthetic sequences for preparing the compounds useful in the
present
invention are outlined in the examples given below. Both an explanation of,
and the
actual procedure for, the various aspects of the present invention are
described where
appropriate. The following examples are intended to be merely illustrative of
the
present invention, and not limiting thereof in either scope or spirit. Those
with skill in
the art will readily understand that known variations of the conditions and
processes
described in the examples can be used to synthesize the compounds of the
present
invention.
Examples
When in the final step of the synthesis of a compound an acid such as
trifluoroacetic
acid or acetic acid was used, for example when trifluoroacetic acid was
employed to
remove a tBu group or when a compound was purified by chromatography using an
eluent which contained such an acid, in some cases, depending on the work-up
procedure, for example the details of a freeze-drying process, the compound
was
obtained partially or completely in the form of a salt of the acid used, for
example in the
form of the acetic acid salt or trifluoroacetic acid salt or hydrochloric acid
salt.
Abbreviations used:
tert-Butyl tBu
dibenzylidenacetone dba
N,N-dimethylformamide DMF
Ethylacetate EtOAc
N-methylpyrrolidone NMP
Fast atom bombardment FAB
Liquid chromatography with mass spectrometry LC-MS
Room temperature 21 C to 24 C RT
Trifluoroacetic acid TFA
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
29
General procedure for the one-pot formation of 2H-indazoles from 2-
haloacetylenes
and monosubstituted hydrazines: To an oven dried reaction tube containing a
magnic
stirring bar was added the Pd source, ligand, solvent and base. The reaction
tube was
sealed with a septum and stirred for 30 min at RT under an inert atmosphere
(nitrogen
or argon) prior to addition of the reagents. The reagents were added and the
mixture
heated to desired temperature and reaction progress was followed by LCMS. Upon
completion of the reaction as judged by LCMS, the reaction mixture was allowed
to
cool to RT and quenched with brine and extracted with EtOAc. The organic phase
was
dried over Na2SO4 followed by filtration, or by filtering through a Varian
cartridge Chem
Elut 12198007, before the solvent was removed by rotary evaporation and the
residue
purified by FC on silica using CH2CI2/EtOAc or, in some cases CHZCIZ/MeOH.
After
rotary evaporation of the organic solvents, the desired 2H-indazole was
obtained in
high purity.
Example 1: 3-Benzyl-2-phenyl-2H-indazole
Ph _ N-Ph
Following the general procedure outlined above, a reaction tube was charged
with 4.4
mg PdCI2 (5 moI%), 14.5 mg tBu3PHBF4 (10 mol%), 228.1 mg Cs2CO3 (1.4 equiv.)
and
2.5 mL DMF. After stirring for 30 min at RT under a flow of argon, 1-chloro-2-
phenylethynyl-benzene (106.3 mg, 1.0 equiv.) and phenylhydrazine (75.7 mg, 1.4
equiv.) were added and the reaction was heated to 130 C for 3 hours. After
cooling to
RT, the reaction mixture was quenched with brine (30 mL) and extracted with
EtOAc
(2x30 mL) and the organic phase was dried by eluting through a Varian Chem
Elut
12198007 cartridge. Removal of the solvents afforded the crude indazole as a
dark-
brown oil that purified by FC on silica using CH2CI2/EtOAc to afford 115.0 mg
(81 %) of
3-benzyl-2-phenyl-2H-indazole colorless oil that solidified upon standing. 1H-
NMR
(DMSO-d6) S 4.47 (s, 2H), 6.98 (d, 2H, J = 7.1 Hz), 7.03 (dd, 1 H, J = 7.9,
7.0 Hz),
7.13-7.24 (m, 3H), 7.30 (dd, 1 H, J = 8.1, 7.0 Hz), 7.52-7.58 (m, 5H), 7.60
(d, 1 H, , J
8.6 Hz), 7.65 (d, 1 H, , J = 8.6 Hz); 13C-NMR (DMSO-d6) S 30.4, 117.1, 120.5,
121.0,
121.2, 125.9 (2C), 126.3, 126.4, 128.1 (2C), 128.4 (2C), 128.9, 129.2 (2C),
134.5,
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
137.9, 139.5, 147.9; HRMS (FAB): Calculated for C20H N2 (M+H+) 285.1391, found
285.1385.
Example 2:
5 The reaction was performed according to example 1 using 1-bromo-2-
phenylethynyl-
benzene (128.6 mg, 1.0 equiv.) instead of 1-chloro-2-phenylethynyl-benzene.
The
reaction afforded 112.2 mg (79%) 3-benzyl-2-phenyl-2H-indazole.
Example 3:
10 The reaction was performed according to example 1 using 8.9 mg PdCI2 (10
moI%),
29.0 mg tBu3PHBF4 (20 mol%) and 244.4 mg Cs2CO3 (1.5 equiv.) and a reaction
temperature of 110 C for 20 hours. The reaction afforded 106.5 mg (75%) 3-
benzyl-2-
phenyl-2H-indazole.
15 Example 4:
The reaction was performed according to example 1 using 1.8 mg PdCI2 (2 mol%)
and
2.9 mg tBu3PHBF4 (4 mol%). The reaction afforded 105.1 mg (74%) 3-benzyl-2-
phenyl-2H-indazole.
20 Example 5:
The reaction was performed according to example 1 using 179.2 mg Cs2CO3 (1.1
equiv.). The reaction afforded 100.8 mg (71%) 3-benzyl-2-phenyl-2H-indazole.
Example 6:
25 The reaction was performed according to example 1 using 22.9 mg Pd2(dba)3
(5
mol%), 29_0 mg tBu3PHBF4 (20 mol%) and 244.4 mg Cs2CO3 (1.5 equiv.) and a
reaction temperature of 110 C for 20 hours. The reaction afforded 105.1 mg
(74%) 3-
benzyl-2-phenyl-2H-indazole.
30 Example 7:
The reaction was performed according to example 6 using dimethylacetamide (2.5
mL)
as solvent. The reaction afforded 102.2 mg (72%) 3-benzyl-2-phenyl-2H-
indazole.
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
31
Example 8:
The reaction was performed according to example 6 using N-methylpyrrolidone
(NMP,
2.5 mL) as solvent. The reaction afforded 108.0 mg (76%) 3-benzyl-2-phenyl-2H-
indazole.
Example 9:
The reaction was performed according to example 8 with a reaction temperature
of
130 C for 4 hours. The reaction afforded 109.3 mg (77%) 3-benzyl-2-phenyl-2H-
indazole.
Example 10:
The reaction was performed according to example 1 using 101.2 mg
phenylhydrazine
hydrochloride (1.4 equiv.) and 438.8 mg CsZCO3 (2.7 equiv.). The reaction
afforded
109.3 mg (77%) 3-benzyl-2-phenyl-2H-indazole.
Example 11: 2-Phenyl-3-pyridin-2-ylmethyl-2H-indazole
~
/
KII(iII
N
-Ph
The reaction was performed according to example 1 using 106.8 mg 2-(2-chloro-
phenylethynyl)-pyridine and a reaction temperature of 110 C for 3 hours. This
afforded
128.4 mg (90%) of the title compound. ' H-NMR (DMSO-d6) 5 4.60 (s, 2H), 7.01
(ddd,
1 H, J= 8.5, 6.6, 0.7 Hz), 7.16 (d, 1 H, J= 7.9 Hz), 7.20 (ddd, 1 H, J= 7.4,
5.0, 0.9 Hz),
7.28 (ddd, 1 H, J = 8.8, 6.6, 1.2 Hz), 7.49-7.57 (m, 4H), 7.62-7.68 (m, 4H),
8.41 (d, J =
5.0 Hz);13C-NMR (DMSO-d6) S 33.3, 117.1, 120.6, 120.9, 121.4, 121.8, 122.8,
125.9,
126.4, 128.8, 129.1, 133.5, 136.8, 139.5, 147.9, 149.2, 157.5; HRMS (FAB):
Calculated for C19H16N3 (M+H+) 286.1344, found 286.1337.
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
32
Example 12: 2-Phenyl-3-(4-trifluoromethyl-benzyl)-2H-indazole
CF3
NPh
The reaction was performed according to example 1 using 140.3 mg 1-chloro-2-(4-
trifluoromethylphenyl)ethynyl-benzene and a reaction temperature of 110 C for
3
hours. This afforded 123.8 mg (83%) of the title compound. 'H-NMR (DMSO-d6) S
4.60
(s, 2H), 7.06 (dd, 1 H, J = 8.3, 6.7 Hz), 7.17 (d, 2H, J = 8.0 Hz), 7.32 (dd,
1 H, J = 8.6,
6.8 Hz), 7.52-7.58 (m, 7H), 7.64 (d, 1 H, J= 7.4 Hz), 7.66 (d, 1 H, J= 8.0
Hz); 13C-NMR
(DMSO-d6) S 30.1, 117.2, 120.4, 121.3, 125.2, 125.3, 125.9, 126.5, 127.1 (q,
J= 32.3
Hz, CF3) 128.8, 129.0, 129.2, 133.5, 139.4, 142.7, 147.9.
Example 13: 2-Phenyl-3-(4-methoxy-benzyl)-2H-indazole
O
N,
N Ph
The reaction was performed according to example 1 using 121.3 mg 1-chloro-2-(4-
methoxyphenyl)ethynyl-benzene and a reaction temperature of 110 C for 3
hours.
This afforded 135.2 mg (86%) of the title compound. 'H-NMR (DMSO-d6) S 3.67
(s,
3H), 4.39 (s, 2H), 6.77 (d, 2H, J = 8.6 Hz) 6.89 (d, 2H, J = 8.6 Hz), 7.02
(ddd, 1 H, J
8.6, 6.5, 0.6 Hz), 7.28 (ddd, 1 H, J = 8.6, 6.7, 0.9 Hz), 7.52-7.59 (m, 6H),
7.63 (d, 1 H, J
= 8.7 Hz); 13C-NMR (DMSO-d6) S 29.6, 54.9, 113.8, 117.1, 120.6, 120.9, 121.0,
125.9,
126.3, 128.8, 129.1, 129.2, 129.7, 134.9, 139.5, 147.9, 157.7; HRMS (FAB):
Calculated for C21H19N20 (M+H+) 315.1497, found 315.1491.
Example 14: 3-(6-Methoxy-naphthalen-2-ylmethyl)-2-phenyl-2H-indazole
~ -
NNPh 0
The reaction was performed according to example 1 using 146.4 mg 2-(2-chloro-
phenylethynyl)-6-methoxy-naphthalene and a reaction temperature of 110 C for
6
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
33
hours. This afforded 127.6 mg (70%) of the title compound. 'H-NMR (DMSO-d6) S
3.83
(s, 3H), 4.59 (s, 2H), 7.01 (ddd, 1 H, J= 8.4, 6.6, 0.6 Hz), 7.07-7.12 (m,
2H), 7.23 (d,
1 H, J= 2.4 Hz), 7.29 (ddd, 1 H, J= 8.7, 6.5, 0.9 Hz), 7.36 (s, 1 H), 7.51-
7.68 (m, 9H); );
13C-NMR (DMSO-d6) S 30.5, 55.0, 105.7, 117.1, 118.7, 120.6, 121.0, 121.2,
125.9,
126.1, 126.4, 126.9, 127.1, 128.3, 128.5, 128.9, 129.1, 132.8, 132.9, 134.5,
139.5,
147.9, 157.0; HRMS (FAB): Calculated for C25H21N20 (M+H+) 365.1654, found
365.1648.
Example 15: N,N-Diisopropyl-2-(2-phenyl-2H-indazol-3-yl)-acetamide
~~r
NN~ O N
Ph
The reaction was performed according to example 1 using 131.9 mg 3-(2-chloro-
phenyl)-propynoic acid diisopropylamide and at a reaction temperature of 110
C for 2
hours. This afforded 105.5 mg (63%) of the title compound. 'H-NMR (DMSO-d6) 8
1.01
(d, 6H, J = 6.4 Hz), 1.15 (d, 6H, J = 6.8 Hz), 3.38 (m, 1 H), 3.96 (septet, 1
H, J = 6.8
Hz), 4.18 (s, 2H), 7.05 (ddd, 1 H, J= 8.6, 6.8, 1.0 Hz), 7.30 (ddd, 1 H, J=
8.6, 6.5, 0.9
Hz), 7.52-7.58 (m, 5H), 7.63 (d, 1 H, J = 8.7 Hz), 7.75 (d, 1 H, J = 8.7 Hz);
13C-NMR
(DMSO-d6) 8 20.1, 31.6, 44.8, 48.2, 116.9, 120.5, 120.6, 122.1, 125.5, 126.2,
128.6,
129.0, 131.4, 139.7, 147.8, 166.3; HRMS (FAB): Calculated for C21H26N30 (M+H+)
336.2075, found 336.2068.
Example 16: (2-Phenyl-2H-indazol-3-yl)-acetic acid tert-butyl ester
0
N OtBu
Q zzz
N , Ph
h
The reaction was performed according to example 1 using 118.4 mg (2-chloro-
phenyl)-
propynoic acid tert-butyl ester and a reaction temperature of 110 C for 2
hours. This
afforded 143.4 mg (93%) of the title compound.'H-NMR (DMSO-d6) 1.22 S(s, 9H),
4.21 (s, 2H), 7.10 (ddd, 1 H, J= 8.3, 6.5, 0.6 Hz), 7.32 (ddd, 1 H, J= 8.6,
6.4, 0.9 Hz),
7.53-7.57 (m, 1 H), 7.58-7.66 (m, 5H), 7.80 (d, 1 H, J= 8.6 Hz); 13C-NMR (DMSO-
d6) 5
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
34
27.3, 31.8, 80.8, 117.1, 120.4, 121.1, 121.8, 125.5, 126.5, 128.8, 129.2,
129.3, 139.5,
147.8, 167.8; HRMS (FAB): Calculated for C19H21N202 (M+H+) 309.1603, found
309.1596.
Example 17: 3-(2,2-Diethoxy-ethyl)-2-phenyl-2H-indazole
N
N Ph
The reaction was performed according to example 1 using 119.4 mg 1-chloro-2-
(3,3-
diethoxy-prop-1-ynyl)-benzene. The reaction mixture was worked up by filtering
through a short silicaplug to remove the base and catalyst using iPr2O
followed by
evaporation of the solvents. The crude product was purified by FC on silica
using
CH2CI2/EtOAc. This afforded 85.4 mg (55%) of the title compound. ' H-NMR (DMSO-
d6) S 0.95 (t, 6H, J= 7.1 Hz), 3.24-3.32 (m. 4H), 3.44-3.51 (m, 2H), 4.69 (t,
1 H, J= 5.5
Hz), 7.07 (ddd, 1 H, J= 8.3, 7.1, 0.6 Hz), 7.29 (ddd, 1 H, J= 8.7, 6.8, 0.9
Hz), 7.54-7.63
(m, 4H), 7.65-7.68 (m, 2H), 7.82 (d, 1 H, J= 8.6 Hz); 13C-NMR (DMSO-d6) S
14.9, 30.7,
61.9, 101.1, 117.0, 120.6, 121.1, 121.5, 126.3, 128.8, 129.1, 131.8, 139.7,
147.8;
HRMS (FAB): Calculated for C19H23N202 (M+H+) 311.1760, found 311.1754.
Example 18: 3-(2,2-Dimethyl-propyl)-2-phenyl-2H-indazole
tBu ~ ~ ,N-Ph
N
The reaction was performed according to example 1 using 96.4 mg 1-chloro-2-
(3,3-
dimethyl-but-1-ynyl)-benzene and a reaction time of 20 hours. This afforded
11.9 mg
(9%) of the title compound. 1H-NMR (DMSO-d6) S 0.68 (s, 9H) 3.12 (s, 2H), 7.06
(dd,
1 H, J= 8.3, 6.8 Hz), 7.28 (dd, 1 H, J= 8.9, 7.6 Hz), 7.52-7.62 (m, 6H), 7.76
(d, 1 H, J
8.6 Hz);13C-NMR (DMSO-d6) 8 29.5, 33.7, 37.1, 117.0, 120.6, 121.6, 122.1,
126.1,
126.7, 128.6, 129.1, 134.5, 140.4, 147.6.
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
Example 19: 3-Hexyl-2-phenyl-2H-indazole
pentyl
,N-Ph
The reaction was performed according to example 1 using 103.4 mg 1-chloro-2-
hept-l-
ynyl-benzene and a reaction time of 20 hours. This afforded 15.3 mg (11 %) of
the title
5 compound. 'H-NMR (DMSO-d6) S 0.78 (t, 3H, J= 6.7 Hz, CH3), 1.08-1.88 (m,
6H),
1.50 (kvintet, 2H, J= 7.1 Hz), 3.06 (t, 2H, J= 7.6 Hz), 7.05 (dd, 1 H, J= 7.5,
7.4 Hz),
7.29 (dd, 1 H, J= 8.6, 6.6 Hz), 7.58-7.62 (m, 6H), 7.77 (d, 1 H, J= 8.6 Hz);
13C-NMR
(DMSO-d6) S 13.7, 21.7, 24.2, 28.0, 28.3, 30.5, 117.0, 120.4, 120.5, 120.7,
125.9,
126.3, 128.8, 129.2, 136.4, 139.7, 147.7.
Example 20: 3-Cyclopropylmethyl-2-phenyl-2H-indazole-6-carboxylic acid tert-
butyl
ester
yo~-
,NPh
O N
The reaction was performed according to example 1 using 138.4 mg 3-chloro-4-
cyclopropylethynyl-benzoic acid tert-butyl ester and a reaction time of 4
hours. This
afforded 81.9 mg (47%) of the title compound.'H-NMR (DMSO-d6) S 0.10-0.15 (m
2H),
0.37-0.42 (m 2H), 0.86-0.94 (m, 1 H), 1.59 (s, 9H), 3.01 (d, 2H, J = 6.8 Hz),
7.54 (dd,
1 H, J= 8.9, 1.4 Hz), 7.58-7.68 (m, 5H), 7.96 (dd, 1 H, J= 8.9, 0.9 Hz), 8.25
(s, 1 H);
13C-NMR (DMSO-d6) S 4.8, 10.2, 27.8, 29.2, 80.6, 119.8, 120.0, 121.3, 122.5,
126.1,
129.2, 129.3, 129.4, 136.9, 139.5, 146.9, 165.2.
Example 21: 3-Benzyl-2-phenyl-2H-indazole-6-carboxylic acid tert-butyl ester
x Ph
o
N-Ph
O N
The reaction was performed according to example 1 using 156.4 mg 3-chloro-4-
phenylethynyl-benzoic acid tert-butyl ester. This afforded 133.1 mg (69%) of
the title
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
36
compound. ' H-NMR (DMSO-d6) S 1.58 (s, 9H), 4.51 (s, 2H), 6.95 (d, 2H, J= 7.6
Hz),
7.14-7.23 (m, 3H), 7.50 (dd, 1 H, J= 8.8, 1.3 Hz), 7.58 (br s, 5H), 7.68 (dd,
1 H, J= 8.8,
0.7 Hz), 8.28 (s, 1 H); 13C-NMR (DMSO-d6) S 27.8, 30.4, 80.6, 115.1, 120.2,
120.9,
122.9, 125.8, 126.5, 128.1, 128.5, 129.3, 129.6, 135.3, 137.6, 139.2, 147.0,
165.2;
HRMS (FAB): Calculated for C25H25N202 (M+H+) 385.1916, found 385.1911.
Example 22: 3-Cyclopentylmethyl-2-phenyl-2H-indazole-6-carboxylic acid tert-
butyl
ester
O
O N Ph
The reaction was performed according to example 1 using 152.4 mg 3-chloro-4-
cyclopentylethynyl-benzoic acid tert-butyl ester and a reaction time of 4
hours. This
afforded 17.3 mg (9%) of the title compound. ' H-NMR (DMSO-d6) S 0.98-1.08 (m,
2H),
1.33-1.40 (m, 2H), 1.41-1.49 (m, 4H), 1.59 (s, 9H), 1.99 (septet, 1 H, J= 7.5
Hz), 3.11
(d, 2H, J = 7.6 Hz), 7.53 (dd, 1 H, J = 8.8, 1.3 Hz), 7.58-7.66 (m, 5H), 7.89
(d, 1 H, J
8.8, 0.6 Hz), 8.24 (s, 1 H); HRMS (FAB): Calculated for C24H28N2O2Na (M+Na+)
399.2048, found 399.2045.
Example 23: Diethyl-[2-(2-phenyl-2H-indazol-3-yl)-ethyl]-amine
N
N-N" Ph
The reaction was performed according to example 1 using 110.9 mg [3-(2-chloro-
phenyl)-prop-2-ynyl]-diethyl-amine and a reaction time of 20 hours. This
afforded 44.0
mg (30%) of the title compound. ' H-NMR (DMSO-d6) S 0.76 (t, 6H, J = 7.0 Hz),
2.34 (q,
4H, J= 7.0 Hz), 2.56 (m, 2H), 3.16 (m, 2H), 7.06 (ddd, 1 H, J= 8.6, 6.5, 0.9
Hz), 7.29
(ddd, 1 H, J= 8.8, 6.5, 1.0 Hz), 7.55-7.66 (m, 6H), 7.77 (d, 1 H, J= 8.6 Hz);
13C-NMR
(DMSO-d6) S 11.7, 22.4, 46.1, 51.0, 117.0, 120.4, 120.5, 120.9, 126.0, 126.3,
128.8,
129.1, 135.0, 139.7, 147.8; HRMS (FAB): Calculated for C19H24N3 (M+H+)
294.1970,
found 294.1964.
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
37
Example 24: 2-Phenyl-3-[2-(tetrahydro-pyran-2-yloxy)-ethyl]-2H-indazole
O
O
N, N'Ph
The reaction was performed according to example 1 using 125.4 mg 2-[3-(2-
chloro-
phenyl)-prop-2-ynyloxy]-tetrahydro-pyranat 100 C for 2 hours. This afforded
37.1 mg
(23%) of the title compound.'H-NMR (DMSO-d6) S 1.25-1.60 (m, 6H), 3.23-3.29
(m,
1 H), 3.30-3.35 (m, 2H), 3.37-3.42 (m, 1 H), 3.61 (dt, 1 H, J= 9.8, 6.8 Hz),
3.83 (dt, 1 H, J
= 9.8, 6.5 Hz), 4.45 (m, 1 H), 7.06 (ddd, 1 H, J= 8.6, 6.8, 0.9 Hz), 7.30
(ddd, 1 H, J=
9.0, 6.5, 0.9 Hz), 7.57 (d, 1 H, J = 7.1 Hz), 7.59-7.63 (m, 3H), 7.67 (d, 2H,
J = 8.6 Hz),
7.82 (d, 1 H, J = 8.3 Hz); 13C-NMR (DMSO-d6) 8 18.8, 24.8, 25.6, 30.0, 60.9,
65.0, 97.6,
117.0, 120.6, 120.8, 121.1, 126.1, 126.3, 128.8, 129.1, 133.7, 139.6, 147.8.
Example 25: 3-Benzyl-2-phenyl-5-trifluoromethyl-2H-indazole
Ph
CF3
N-Ph
~
The reaction was performed according to example 1 using 140.3 mg 1-chloro-2-
phenylethynyl-4-trifluoromethyl-benzene and a reaction time of 20 hours at 110
C.
This afforded 107.5 mg (61 %) of the title compound. ' H-NMR (DMSO-d6) 8 4.58
(s,
2H), 6.97 (d, 2H, J= 6.8 Hz), 7.15-7.24 (m, 3H), 7.51 (dd, 1 H, J= 9.0, 1.6
Hz), 7.58 (s,
5H), 7.85 (d, 1 H, J= 9.0 Hz), 8.08 (s, 1 H); 13C-NMR (DMSO-d6) S 30.3, 118.8,
120.1
(q, J= 5.0 Hz), 121.5, (q, J= 30.5 Hz), 121.9, (q, J= 2.5 Hz), 119.7, 125.9,
126.5,
128.2, 128.5, 129.2, 129.4, 137.3, 137.7, 139.1, 148.0; HRMS (FAB): Calculated
for
C21H16N2F3 (M+H+) 353.1266, found 353.1261.
Example 26: 3-Benzyl-6-fluoro-2-phenyl-2H-indazole
Ph
N-Ph
F \ .
N
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
38
The reaction was performed according to example 1 using 115.3 mg 2-chloro-4-
fluoro-
1 -phenylethynyl-benzene and a reaction time of 20 hours at 110 C. This
afforded 64.4
mg (43%) of the title compound. ' H-NMR (DMSO-d6) S 4.47 (s, 2H), 6.92-6.99
(m, 3H),
7.14-7.23 (m, 3H), 7.37 (dd, 1 H, J= 10.4, 1.9 Hz), 7.53-7.58 (m, 5H), 7.66
(dd, 1 H, J=
9.2, 5.5 Hz); 13C-NMR (DMSO-d6) S 30.4, 100.1 (d, J= 23.5 Hz), 112.3 (d, J=
27.8
Hz), 118.6, 123.1 (d, J= 11.0 Hz), 125.8, 126.5, 128.1, 128.5, 129.0, 129.2,
135.6,
137.6, 139.3, 147.5, 147.6, 161.2 (d, J = 241.7 Hz); HRMS (FAB): Calculated
for
C2oH16N2F (M+H+) 303.1297, found 303.1292.
Example 27: 3-Benzyl-4-methyl-2-phenyl-2H-indazole
CPh
/ i-
N-Ph
~ ~N~
The reaction was performed according to example 1 using 113.4 mg 1-chloro-3-
methyl-2-phenylethynyl-benzene and a reaction time of 4 hours at 110 C. This
afforded 95.8 mg (64%) of the title compound.'H-NMR (DMSO-d6) S 2.40 (s, 3H),
4.53
(s, 2H), 6.78 (d, 1 H, J= 6.4 Hz), 6.91 (d, 2H, J= 7.2 Hz), 7.15-7.21 (m, 2H),
7.22-7.27
(m, 2H), 7.45-7.55 (m, 6H); 13C-NMR (DMSO-d6) S 19.2, 30.9, 115.0, 120.8,
121.4,
126.0, 126.3, 126.5, 127.3, 128.6, 129.0, 129.1, 131.0, 133.9, 139.0, 139.3,
148.4;
HRMS (FAB): Calculated for C21H19N2 (M+H+) 299.1548, found 299.1543.
Example 28: 3-Benzyl-6-methoxy-2-phenyl-2H-indazole
Ph
O / i
,N-Ph
~ ~ N
The reaction was performed according to example 1 using 121.4 mg 2-chloro-4-
methoxy-l-phenylethynyl-benzene and a reaction time of 4 hours at 130 C. This
afforded 114.5 mg (73%) of the title compound. 1H-NMR (DMSO-d6) S 3.81 (s,
3H),
4.43 (s, 2H), 6.68 (dd, 1 H, J = 9.0, 2.0 Hz), 6.93 (d, 1 H, J = 2.0 Hz), 6.96
(d, 2H, J =
7.1 Hz), 7.44 (d, 1 H, J= 9.0 Hz), 7.48-7.57 (m, 5H); 13C-NMR (DMSO-d6) S
30.4, 55.0,
94.2, 115.9, 117.0, 121.4, 125.7, 126.4, 128.0, 128.4, 128.5, 129.1, 134.5,
137.9,
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
39
139.6, 148.9, 158.6; HRMS (FAB): Calculated for CZ, H19N20 (M+H+) 315.1497,
found
315.1491.
Example 29: 3-Benzyl-5-methoxy-2-phenyl-2H-indazole
0 Ph
N-Ph
N
The reaction was performed according to example 1 using 121.4 mg 1-chloro-4-
methoxy-2-phenylethynyl-benzene and a reaction time of 5 hours at 110 C. This
afforded 98.7 mg (63%) of the title compound. ' H-NMR (DMSO-d6) S 3.72 (s,
3H), 4.44
(s, 2H), 6.89 (d, 1 H, J= 2.2 Hz), 6.96-6.99 (m, 3H), 7.14-7.18 (m, 1 H), 7.19-
7.23 (m,
2H), 7.49-7.54 (m, 4H), 7.56 (d, 1 H, J= 9.0 Hz); 13C-NMR (DMSO-d6) 8 30.3,
55.1,
96.8, 118.7, 121.2, 121.3, 125.6, 126.3, 128.1, 128.4, 128.6, 129.1, 133.1,
138.1,
139.7, 144.8, 154.1; HRMS (FAB): Calculated for C21H19N20 (M+H+) 315.1497,
found
315.1491.
Example 30: Toluene-4-sulfonic acid 3-benzyl-2-phenyl-2H-indazol-6-yi ester
O, 'O
S~ O - Ph
~
I
~ NN, Ph
The reaction was performed according to example 1 using 191.4 mg toluene-4-
sulfonic
acid 3-chloro-4-phenylethynyl-phenyl ester and a reaction time of 4 hours at
110 C.
This afforded 80.7 mg (36%) of the title compound. ' H-NMR (DMSO-d6) S 2.42
(s, 3H),
4.44 (s, 2H), 6.72 (dd, 1 H, J= 8.9, 1.9 Hz), 6.94 (d, 2H, J= 6.9 Hz), 7.13-
7.21 (m, 4H),
7.48 (d, 2H, J= 8.1 Hz), 7.51-7.58 (m, 5H), 7.61 (d, 1 H, J= 8.9 Hz), 7.80 (d,
2H, J=
8.3 Hz);13C-NMR (DMSO-d6) S 21.1, 30.3, 109.1, 116.8, 119.7, 122.7, 125.9,
126.5,
128.1, 128.2, 128.5, 129.2, 130.2, 131.6, 135.7, 137.4, 139.1, 145.7, 146.9,
147.8;
HRMS (FAB): Calculated for C27H23N203S (M+H+) 455.1429, found 455.1429.
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
Example 31: 3-Benzyl-2-phenyl-2H-indazole-5-carboxylic acid
H02C Ph N-Ph
N
The reaction was performed according to example 1 using 128.3 mg 4-chloro-3-
phenylethynyl-benzoic acid and a reaction time of 4 hours at 110 C. The
reaction was
5 purified by preparative RP HPLC using MeCN/H20/TFA as eluent. This afforded
75.6
mg (46%) of the title compound. ' H-NMR (DMSO-d6) S 4.55 (s, 2H), 6.98 (d, 1
H, J
7.2 Hz), 7.15-7.24 (m, 3H), 7.58 (br s, 5H), 7.69 (d, 1 H, J= 9.0 Hz), 7.81
(d, 1 H, J=
9.0 Hz), 8.36 (s, 1H); 13C-NMR (DMSO-d6) 8 30.4, 117.1, 120.6, 123.6, 125.0,
125.9,
126.2, 126.5, 128.1, 128.5, 129.3, 137.5, 137.6, 139.1, 148.9, 167.5; HRMS
(FAB):
10 Calculated for C21 H N202 (M+H+) 329.1290, found 329.1286.
Example 32: 3-Benzyl-2-phenyl-2H-pyrazolo[4,3-c]pyridine
Ph
N-Ph
The reaction was performed according to example 1 using 156.4 mg 4-chloro-3-
15 phenylethynyl-pyridine and a reaction time of 3 hours at 110 C. This
afforded 24.0 mg
(17%) of the title compound. 'H-NMR (DMSO-d6) S 4.56 (s, 2H), 7.06 (dd, 2H, J
= 7.6,
1.5 Hz), 7.18-7.28 (m, 3H), 7.55 (dd, 1 H, J= 6.3, 1.2 Hz), 7.61 (br s, 5H),
8.21 (d, 1 H,
J= 6.3 Hz), 8.91 (d, 1 H, J= 1.1 Hz); 13C-NMR (DMSO-d6) S 30.7, 110.6, 117.0,
126.1,
126.7, 128.4, 128.6, 129.3, 129.5, 137.1, 137.7, 138.9, 142.0, 147.7, 148.4.
Example 33: 3-Benzyl-2-(4-methoxy-phenyl)-2H-indazole
Ph
/ i- - ,
N 0
N
The reaction was performed according to example 1 using 122.2 mg 4-
methoxyphenylhydrazine hydrochloride, 439.9 mg Cs2CO3 (2.7 equiv.) and a
reaction
time of 4 hours at 130 C. This afforded 125.0 mg (80%) of the title compound.
1H-
NMR (DMSO-d6) 8 3.33 (s, 3H), 4.43 (s, 2H), 6.97-7.02 (m, 3H), 7.09 (d, 2H, J
= 8.6
Hz), 7.17 (d, 1 H, J= 7.4 Hz), 7.21 (d, 2H, J= 7.6 Hz), 7.28 (ddd, 1 H, J=
8.6, 6.7, 1.0
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
41
Hz), 7.45 (d, 2H, J= 8.6 Hz), 7.56 (d, 1 H, J= 8.4 Hz), 7.61 (d, 1 H, J= 8.6
Hz); 13C-
NMR (DMSO-d6) S 30.4, 55.4, 114.2, 117.0, 120.4, 120.8.121.0, 126.2, 126.3,
127.1,
128.0, 128.4, 132.4, 134.4, 137.9, 147.6, 159.3; HRMS (FAB): Calculated for
C21H19N20 (M+H+) 315.1497, found 315.1491.
Example 34: 3-Benzyl-2-(4-fluoro-phenyl)-2H-indazole
Ph
-
N ~ ~ F
The reaction was performed according to example 1 using 113.8 mg 4-
fluorophenylhydrazine hydrochloride, 439.9 mg Cs2CO3 (2.7 equiv.) and a
reaction
time of 3 hours at 130 C. This afforded 128.8 mg (85%) of the title compound.
' H-
NMR (DMSO-d6) S 4.46 (s, 2H), 6.97 (d, 2H, J 6.7 Hz), 7.03 (ddd, 1 H, J = 8.5,
6.6,
0.9 Hz), 7.13-7.23 (m, 3H), 7.30 (ddd, 1 H, J= 8.8, 6.6, 1.2 Hz), 7.36-7.42
(m, 2H),
7.57-7.65 (m, 4H); 13C-NMR (DMSO-d6) 8 30.3, 116.0 (d, J = 23.1 Hz), 117.1,
120.5,
121.1 (d, J = 8.0 Hz), 126.4, 126.5, 128.1, 128.2, 128.4, 134.8, 135.9 (d, J=
3.0 Hz),
137.7, 147.8, 161.8 (d, J = 246.3 Hz); HRMS (FAB): Calculated for C2oH16N2F
(M+H+)
303.1297, found 303.1292.
Example 35: 3-Benzyi-2-(2-fluoro-phenyl)-2H-indazole
C Ph
/ ~ -
N
N
F
The reaction was performed according to example 1 using 113.8 mg 2-
fluorophenyl-
hydrazine hydrochloride, 439.9 mg Cs2CO3 (2.7 equiv.) and a reaction time of 5
hours
at 130 C. This afforded 123.0 mg (81 %) of the title compound. ' H-NMR (DMSO-
d6) S
4.33 (s, 2H), 6.92 (d, 2H, J = 6.7 Hz), 7.05 (ddd, 1 H, J = 8.2, 6.5, 0.9 Hz),
7.12-7.21
(m, 3H), 7.31 (ddd, 1 H, J= 8.6, 6.6, 0.9 Hz), 7.40 (dd, 1 H, J= 7.6, 0.9 Hz),
7.49 (ddd,
1 H, J= 8.6, 8.2, 1.2 Hz), 7.57 (ddd, 1 H, J= 8.2, 7.8, 1.6 Hz), 7.61-7.66 (m,
3H); 13C-
NMR (DMSO-d6) 8 30.2, 116.6 (d, J= 19.5 Hz), 117.1, 120.4, 120.5, 121.1, 125.0
(d, J
= 3.7 Hz), 126.4, 126.6, 127.1 (d, J= 12.0 Hz), 128.1, 128.3, 129.4, 131.8 (d,
J= 8.2
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
42
Hz), 136.3, 137.3, 148.3, 156.3 (d, J= 250.6 Hz); HRMS (FAB): Calculated for
C20H16N2F (M+H+) 303.1297, found 303.1292.
Example 36: 4-(3-Benzy!-indazol-2-yl)-benzonitrile
Ph
-
~ ~N ~ ~ CN
N
The reaction was performed according to example 1 using 118.7 mg 4-
cyanophenylhydrazine hydrochloride, 439.9 mg Cs2CO3 (2.7 equiv.) and a
reaction
time of 3 hours at 130 C. This afforded 143.3 mg (93%) of the title compound.
' H-
NMR (DMSO-d6) S 4.56 (s, 2H), 6.97 (d, 2H, J = 7.0 Hz), 7.05 (dd, 1 H, J =
8.4, 6.8 Hz),
7.16 (d, 1 H, J= 7.0 Hz), 7.19-7.23 (m, 2H), 7.32 (ddd, 1 H, J= 8.7, 6.6, 0.9
Hz), 7.60
(d, 1 H, J = 8.6 Hz), 7.65 (d, 1 H, J = 8.8 Hz), 7.82 (d, 2H, J = 8.6 Hz),
8.04 (d, 2H, J =
8.6 Hz); 13C-NMR (DMSO-d6) S 30.3, 111,3, 117.2, 118.1, 120.7, 121.5, 121.7,
126.5,
126.6, 127.0, 128.1, 128.5, 133.4, 135.1, 137.5, 143.1, 148.4; HRMS (FAB):
Calculated for C21H16N3 (M+H+) 310.1344, found 310.1340.
Example 37: 3-Benzyl-2-naphthalen-1-yl-2H-indazole
Ph
Cj:N,N
~
The reaction was performed according to example 1 using 118.7 mg 1-
naphthylhydrazine hydrochloride, 439.9 mg Cs2CO3 (2.7 equiv.) and a reaction
time of
4 hours at 130 C. This afforded 105.7 mg (63%) of the title compound. 1H-NMR
(DMSO-d6) 8 4.21 (br s, 2H), 6.83 (d, 2H, J= 7.5 Hz), 6.91 (d, 1 H, J= 8.6
Hz), 7.04-
7.11 (m, 4H), 7.34 (dd, 1 H, J = 6.6, 8.1 Hz), 7.43 (ddd, 1 H, J = 8.1, 6.8,
1.2 Hz), 7.57-
7.63 (m, 2H), 7.65-7.70 (m, 3H), 8.09 (d, 1 H, J= 8.2 Hz), 8.09 (d, 1 H, J=
8.2 Hz), 8.19
(d, 1 H, J= 8.4 Hz); 13C-NMR (DMSO-d6) 8 30.5, 117.2, 120.3, 120.6, 121.0,
122.3,
125.1, 125.4, 126.2, 126.4, 126.7, 127.5, 128.0, 128.1, 128.2, 129.5, 129.9,
133.4,
135.5, 136.6, 137.4, 148.0; HRMS (FAB): Calculated for C24H19N2 (M+H+)
335.1548,
found 335.1548.
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
43
Example 38: 3-Benzyl-2-pyridin-4-yl-2H-indazole
Ph
/ ~ -
N
N
The reaction was performed according to example 1 using 101.9 mg 4-hydrazino-
pyridine hydrochloride, 439.9 mg Cs2CO3 (2.7 equiv.) and a reaction time of 3
hours at
130 C. This afforded 134.6 mg (94%) of the title compound. 1H-NMR (DMSO-d6) S
4.64 (s, 2H), 7.00 (d, 2H, J= 7.1 Hz), 7.06 (ddd, 1 H, J= 8.4, 6.5, 0.7 Hz),
7.15-7.18
(m, 1 H), 7.20-7.24 (m, 2H), 7.33 (ddd, 1 H, J= 8.6, 6.5, 0.9 Hz), 7.61 (d, 1
H, J= 8.5
Hz), 7.66 (d, 1 H, J= 8.6 Hz), 7.68-7.71 (m, 2H), 8.74-8.76 (m, 2H); 13C-NMR
(DMSO-
d6) S 30.3, 117.3, 119.6, 120.7, 121.6, 122.0, 126.5, 127.2, 128.1, 128.5,
135.0, 137.5,
146.3, 148.5, 150.9; HRMS (FAB): Calculated for C19H16N3 (M+H+) 286.1344,
found
286.1339.
Example 39: 4-(3-Benzyl-indazol-2-yl)-quinoline
Ph
-
cJ:'T8
The reaction was performed according to example 1 using 137.0 mg 4-
hydrazinoquinoline hydrochloride, 439.9 mg Cs2CO3 (2.7 equiv.) and a reaction
time of
3 hours at 130 C. This afforded 74.9 mg (45%) of the title compound. 1H-NMR
(DMSO-d6) S 4.35 (s, 2H), 6.80-6.84 (m, 2H), 7.00-7.05 (m, 3H), 7.13 (dd, 1 H,
J = 8.5,
6.7 Hz), 7.18 (d, 1 H, J= 8.4 Hz), 7.38 (dd, 1 H, J= 6.8, 7.4 Hz), 7.52 (dd, 1
H, J= 8.4,
7.2 Hz), 7.70-7.7.76 (m, 3H), 7.83 (dd, 1 H, J= 6.8, 8.5 Hz), 8.18 (d, 1 H, J=
8.5 Hz),
9.11 (d, 1 H, J = 4.6 Hz); 13C-NMR (DMSO-d6) S 30.3, 117.2, 119.6, 120.6,
120.7,
121.5, 123.0, 124.0, 126.3, 127.0, 127.9, 128.1, 128.2, 129.1, 130.3, 136.9,
137.2,
143.2, 148.5, 148.7, 150.5.
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
44
Example 40: 3-Benzyl-2-pyridin-4-yl-2H-indazole-6-carboxylic acid tert-butyl
ester
Ph
N~ ~ -
O \ `N N
O
The reaction was performed according to example 1 using 156.4 mg 3-chloro-4-
phenylethynyl-benzoic acid tert-butyl ester, 101.9 mg 4-hydrazinopyridine
hydrochloride (1.4 equiv.) and 456.2 mg Cs2CO3 (2.8 equiv.) with a reaction
time of 2
hours at 110 C. This afforded 104.7 mg (54%) of the title compound. 'H-NMR
(DMSO-d6) 8 1.58 (s, 9H), 4.66 (s, 2H), 6.98 (d, 2H, J= 7.4 Hz), 7.15-7.24 (m,
3H),
7.51 (dd, 1 H, J = 8.8, 1.3 Hz), 7.69-7.73 (m, 3H), 8.30 (s, 1 H), 8.77 (dd,
2H, J = 4.6,
1.6 Hz); HRMS (FAB): Calculated for C24H24N302 (M+H+) 386.1869, found
386.1864.
Example 41: 3-Cyclopropylmethy(-2-pyridin-4-yl-2H-indazole-6-carboxylic acid
tert-
butyl ester
X- O NN N
O
The reaction was performed according to example 1 using 138.4 mg 3-chloro-4-
cyclopropylethynyl-benzoic acid tert-butyl ester, 101.9 mg 4-hydrazinopyridine
hydrochloride (1.4 equiv.) and 456.2 mg CsZCO3 (2.8 equiv.) with a reaction
time of 4
hours at 110 C. This afforded 73.0 mg (42%) of the title compound.'H-NMR
(DMSO-
d6) S 0.14-0.18 (m, 2H), 0.40-0.45 (m, 2H), 0.91-1.00 (m, 1H), 1.59 (s, 9H),
3.16 (d, 2H,
J= 6.8 Hz), 7.54 (dd, 1 H, J= 8.9, 1.3 Hz), 7.81 (dd, 2H, J= 4.5, 1.6 Hz),
7.99 (dd, 1 H,
J = 8.9, 0.8 Hz), 8.85 (dd, 2H, J = 4.5, 1.6 Hz); HRMS (FAB): Calculated for
C211-124N302 (M+H+) 350.1869, found 350.1864.
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
Example 42: 3-Benzyl-2-methyl-2H-indazole
Ph
N -
~ ~ .
N
The reaction was performed according to example 1 using 32.3 mg
methylhydrazine
with a reaction time of 5 hours at 110 C. This afforded 68.6 mg (62%) of the
title
5 compound. 'H-NMR (DMSO-d6) S 4.03 (s, 3H), 4.48 (s, 2H), 6.96 (dd, 1 H, J=
8.2, 6.5
Hz), 7.17-7.24 (m, 4H), 7.28-7.33 (m, 2H), 7.51 (d, 1 H, J= 8.5 Hz), 7.57 (d,
1 H, J= 8.5
Hz); 13C-NMR (DMSO-d6) 8 29.6, 37.6, 116.6, 120.0, 120.2, 120.8, 125.3, 126.4,
128.3, 128.6, 133.8, 137.8, 146.9; HRMS (FAB): Calculated for C15H15N2 (M+H+)
223.1235, found 223.1231.
Example 43: 3-Benzyl-2-phenethyl-2H-indazole
Ph
fN,N-
Ph
The reaction was performed according to example 1 using 95.3 mg
phenethylhydrazine with a reaction time of 20 hours at 110 C. This afforded
77.7 mg
(50%) of the title compound. 'H-NMR (DMSO-d6) S 3.01 (t, 2H, J = 7.6 Hz), 4.29
(s,
2H), 4.51 (t, 2H, J 7.6 Hz), 6.95 (ddd, 1 H, J = 8.2, 6.5, 0.6 Hz), 7.07 (d,
2H, J = 7.0
Hz), 7.11 (d, 2H, J= 7.0 Hz), 7.19-7.31 (m, 7H), 7.51 (d, 1 H, J= 8.5 Hz),
7.56 (d, 1 H, J
= 8.5 Hz); 13C-NMR (DMSO-d6) S 29.3, 35.9, 51.0, 116,7, 120.1, 120.2, 120.6,
125.4,
126.4, 126.7, 128.2, 128.3, 128.6, 128.7, 133.7, 137.9, 138.0, 147.2; HRMS
(FAB):
Calculated for C22H21N2 (M+H+) 313.1705, found 313.1698.
Example 44: 3-Benzyl-2-isopropyl-2H-indazole
Ph
~ _
N
~ N~
The reaction was performed according to example 1 using 77.4 mg
isopropylhydrazine
hydrochloride, 439.9 mg Cs2CO3 (2.7 equiv.) and a reaction time of 20 hours at
110
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
46
C. This afforded 68.4 mg (55%) of the title compound. 1H-NMR (DMSO-d6) S 1.35
(d,
6H, J = 6.6 Hz), 4.50 (s, 2H), 4.86 (septet, 1 H, J = 6.6 Hz), 6.97 (ddd, 1 H,
J = 8.3, 6.5,
0.6 Hz), 7.16-7.23 (m, 4H), 7.27-7.32 (m, 2H), 7.56 (d, 1 H, J= 8.6 Hz), 7.64
(d, 1 H, J
8.6 Hz); 13C-NMR (DMSO-d6) S 22.6, 29.2, 50.3, 116.9, 120.0, 120.1, 120.6,
125.2,
126.4, 128.1, 128.5, 132.4, 138.3, 147.0; HRMS (FAB): Calculated for C17H19N2
(M+H+) 251.1548, found 251.1542.
Example 45: 3-Benzyl-2-cyclohexyl-2H-indazole
Ph
NN
~
The reaction was performed according to example 1 using 105.5 mg cyclohexyl-
hydrazine hydrochloride, 439.9 mg Cs2CO3 (2.7 equiv.) and a reaction time of
20 hours
at 110 C. This afforded 69.4 mg (48%) of the title compound. 'H-NMR (DMSO-d6)
S
1.13-1.40 (m, 3H), 1.63 (br t, 3H, J= 13.0 Hz), 1.74 (d, 2H, J= 13.4, Hz),
1.89 (dq, 2H,
J= 3.4, 12.6 Hz), 4.45 (tt, 1 H, J= 3.6, 11.4 Hz), 4.50 (s, 2H), 6.96 (ddd, 1
H, J= 8.3,
6.5, 0.7 Hz), 7.16-7.23 (m, 4H), 7.26-7.32 (m, 2H), 7.54 (d, 1 H, J = 8.7 Hz),
7.65 (d,
1 H, J= 8.5 Hz); 13C-NMR (DMSO-d6) S 24.8, 24.9 (2C), 29.3, 32.8, 57.7, 116.9,
120.0,
120.1, 120.4, 125.1, 126.4, 128.2, 128.5, 132.7, 138.5, 146.9; HRMS (FAB):
Calculated for C20H23N2 (M+H+) 291.1861, found 291.1856.
Example 46: 3-Benzyl-2-thiophen-2-ylmethyl-2H-indazole-6-carboxylic acid tert-
butyl
ester
Ph L N
0
The reaction was performed according to example 1 using 156.4 mg 3-chloro-4-
phenylethynyl-benzoic acid tert-butyl ester, 164.7 mg thiophen-2-ylmethyl-
hydrazine
hydrochloride (2.0 equiv.) and 488.7 mg Cs2CO3 (3.0 equiv.) with a reaction
time of 5
hours at 110 C. This afforded 72.8 mg (36%) of the title compound. 1 H-NMR
(DMSO-
d6) 8 1.56 (s, 9H), 4.58 (s, 2H), 5.86 (s, 2H), 6.93 (dd, 1 H, J= 5.1, 3.5
Hz), 7.04 (dd,
CA 02693024 2009-12-11
WO 2009/000411 PCT/EP2008/004637
47
1 H, J= 3.5, 1.0 Hz), 7.16 (d, 2H, J= 7.4 Hz), 7.20-7.29 (m, 3H), 7.42 (dd, 1
H, J= 8.8,
1.2 Hz), 7.44 (dd, 1 H, J = 5.0, 1.2 Hz), 7.55 (dd, 1 H, J = 8.7, 0.7 Hz),
8.20 (s, 1 H).