Note: Descriptions are shown in the official language in which they were submitted.
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
METHODS AND APPARATUS FOR PRODUCING SYNGAS
PRIORITY DATA
[0001] This international patent application claims the priority benefit of
U.S.
Provisional Patent Application Nos. 60/948,653, 60/948,659, and 60/948,660,
all filed
July 9, 2007; and further claims the priority benefit of U.S. Non-Provisional
Patent
Application Nos. 12/166,167, 12/166,183, and 12/166,194, all filed July 1,
2008; all
disclosures of which are hereby incorporated by reference herein for all
purposes.
FIELD OF THE INVENTION
[0002] The present invention generally relates to processes for the conversion
of carbonaceous feedstocks, such as cellulosic biomass, into synthesis gas,
and to
processes for the conversion of synthesis gas to products such as alcohols
(e.g.,
ethanol).
BACKGROUND OF THE INVENTION
[0003] Synthesis gas, which is also known as syngas, is a mixture of gases
comprising carbon monoxide (CO) and hydrogen (H2). Generally, syngas may be
produced from any carbonaceous material. In particular, biomass such as
agricultural
wastes, forest products, grasses, and other cellulosic material may be
converted to
syngas.
[0004] Syngas is a platform intermediate in the chemical and biorefining
industries and has a vast number of uses. Syngas can be converted into
alkanes,
olefins, oxygenates, and alcohols such as ethanol. These chemicals can be
blended
into, or used directly as, diesel fuel, gasoline, and other liquid fuels.
Syngas can also
be directly combusted to produce heat and power. The substitution of alcohols
in
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
place of petroleum-based fuels and fuel additives can be particularly
environmentally
friendly when the alcohols are produced from feed materials other than fossil
fuels.
[0005] Improved methods are needed to more cost-effectively produce syngas.
Methods are also desired for producing syngas at a greater purity and with
desirable
ratios of H2 to CO to facilitate the conversion of syngas to other products,
such as
ethanol.
SUMMARY OF THE INVENTION
[0006] In one aspect, the present invention provides a method of forming
syngas, the method comprising the steps of:
(a) devolatilizing a carbon-containing feed material to form a gas phase and a
solid phase in a devolatilization unit; and
(b) passing the gas phase and the solid phase through a heated reaction vessel
to form syngas,
wherein step (a) is performed in the presence of free oxygen in an amount
between about 0.1% and about 25% of the stoichiometric amount of oxygen to
completely combust the feed material.
[0007] In some embodiments, the amount of free oxygen is less than about
10% of the stoichiometric amount of oxygen to completely combust the feed
material.
In other embodiments, the amount of free oxygen is less than 0.5% of the
stoichiometric amount of oxygen to completely combust the feed material. In
certain
embodiments, the amount of free oxygen is between about 2% and about 8% of the
stoichiometric amount of oxygen to completely combust the feed material.
[0008] In some embodiments, step (a) is further performed in the presence of
added steam. The added steam can be present in an amount that is less than
about
50% of the stoichiometric amount of water to completely convert the feed
material to
carbon monoxide and hydrogen. In some embodiments, the added steam is present
in
an amount that is less than about 10% of such stoichiometric amount of water.
[0009] Some methods utilize carbon-containing feedstocks that initially
contain some moisture. In some embodiments, a first amount of steam is present
from
-2-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
initial moisture in the carbon-containing feed material, a second amount of
steam is
added during step (a), and the combined first amount and second amount of
steam is
less than the stoichiometric amount of water to completely convert the feed
material
to carbon monoxide and hydrogen. In other embodiments, the combined first
amount
and second amount of steam is greater than the stoichiometric amount of water
to
completely convert the feed material to carbon monoxide and hydrogen.
[0010] In another aspect, the invention provides a method of forming syngas,
the method comprising the steps of:
(a) devolatilizing a carbon-containing feed material to form a gas phase and a
solid phase in a devolatilization unit; and
(b) passing the gas phase and the solid phase through a heated reaction vessel
to form syngas,
wherein step (b) is performed in the presence of free oxygen in an amount
between about 0.1 % and about 50% of the stoichiometric amount of oxygen to
completely combust the carbon contained in the solid phase produced in step
(a).
[0011] In some embodiments of this aspect, the amount of free oxygen is less
than about 25% (for example, 10-20%) of the stoichiometric amount of oxygen to
completely combust the carbon contained in the solid phase produced in step
(a). In
certain embodiments, the amount of free oxygen is less than about 10% of the
stoichiometric amount of oxygen to completely combust the carbon contained in
the
solid phase produced in step (a).
[0012] Step (b) is preferably (although not necessarily) performed in the
presence of steam. In some embodiments, an initial ratio of free oxygen to
steam
(Oz/Hz0) in step (b) is less than about 1. In some embodiments, this initial
ratio is
less than about 0.5, such as between about 0.01 and about 0.2.
[0013] Of course, it is possible to add oxygen to both steps (a) and (b). In
some embodiments, step (a) is performed in the presence of a first amount of
free
oxygen that is between about 0.1 % and about 10% of the stoichiometric amount
of
oxygen to completely combust the feed material, and step (b) is performed in
the
presence of a second amount of free oxygen that is between about 0.1 % and
about
25% of the stoichiometric amount of oxygen to completely combust the carbon
contained in the solid phase produced in step (a). In certain embodiments, the
first
-3-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
amount of free oxygen is less than about 1% of the stoichiometric amount of
oxygen
to completely combust the feed material, and the second amount of free oxygen
is less
than about 10% of the stoichiometric amount of oxygen to completely combust
the
carbon contained in the solid phase produced in step (a). When oxygen is added
to
both steps (a) and (b), steam can optionally be added during step (a).
[0014] Any of the methods described above can further include the substeps
of (i) measuring the composition of the gas phase and/or the solid phase, (ii)
determining a suitable amount of free oxygen based on predicted partial
oxidation of
at least some of the composition to syngas, and (iii) introducing a gas
containing the
suitable amount of free oxygen.
[0015] In some embodiments, devolatilizing in step (a) can be performed in
the presence of a catalyst. The catalyst can be selected from the group
consisting of
potassium, potassium hydroxide, potassium carbonate, and any combinations
thereof.
[0016] In some embodiments, formation of syngas in step (b) can be
performed in the presence of a catalyst. This catalyst can be selected from
the group
consisting of nickel, cobalt, rhodium, and all combinations and oxides
thereof.
[0017] The residence times of the gas phase and the solid phase in step (a)
can
be substantially the same or different. In some embodiments, the gas phase is
removed, at least in part, during step (a). For example, the devolatilization
unit can be
a multiple-stage unit in which both the gas phase and the solid phase pass
through at
least one stage of the devolatilization unit and at least a portion of the gas
phase is
removed from the devolatilization unit prior to a final stage.
[0018] In certain embodiments, free oxygen is added to the first stage only.
In
other embodiments, different amounts of oxygen are added across multiple
stages of
the devolatilization unit. Oxygen can be added to the devolatilization unit
prior to
removal of at least a portion of the gas phase. Also, after at least a portion
of the gas
phase is removed, oxygen can be added to the devolatilization unit. In some
embodiments, a first amount of oxygen is added prior to removal of at least a
portion
of the gas phase, and a second amount of oxygen is added after removal of at
least a
portion of the gas phase. The first amount of oxygen can be different than the
second
amount of oxygen.
-4-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
[0019] In some embodiments, prior to step (b), the solid phase is combined
with gas removed during step (a). Optionally, one or more substantially inert
gases
can be introduced after removal of at least a portion of the gas phase.
[0020] In some preferred embodiments, the presence of free oxygen during
step (a) and/or step (b) decreases the ratio of hydrogen to carbon monoxide in
the
syngas, compared to the ratio of hydrogen to carbon monoxide produced by the
same
method in the absence of oxygen. The H2/CO ratio (produced by step (b)) can be
between about 0.75 and about 1.5, such as about 0.8, 0.9, 1.0, 1.1, 1.2, 1.3,
or 1.4. In
other embodiments, the H2/CO ratio produced by step (b) is higher than 1.5,
but lower
than the ratio that would have been produced in the absence of free oxygen
added.
[0021] In some preferred embodiments, the presence of free oxygen increases
the amount of syngas produced compared to the amount of syngas produced by the
same method in the absence of free oxygen. In some embodiments, the presence
of
free oxygen decreases the amount of pyrolysis products compared to the
corresponding amount produced by the same method in the absence of free
oxygen.
The presence of free oxygen in step (a) and/or step (b) can also increase the
syngas
purity as produced in step (b).
[0022] In some embodiments, the process temperature in the devolatilization
unit is greater than about 900 F at least at one point during step (a). The
maximum
process temperature in the devolatilization unit is preferably less than about
1400 F.
[0023] In some embodiments, the process temperature in the heated reaction
vessel is greater than about 1400 F at least at one point during step (b). The
maximum process temperature in the heated reaction vessel is preferably less
than
about 2200 F.
[0024] Some embodiments include introducing a stream produced in step (a)
to a reactor configured with an input for a gas comprising oxygen (such as
air),
wherein at least some of the stream is partially oxidized to produce
additional syngas.
Some other embodiments include introducing at least some of a stream produced
in
step (b) to a reactor configured with an input for a gas comprising oxygen,
wherein at
least some of the stream is partially oxidized to produce additional syngas.
Of course,
streams from both steps (a) and (b) could be partially oxidized to make
syngas.
-5-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
[0025] Another aspect of the invention recognizes that syngas can be
produced during devolatilization. In some embodiments, a method is provided
comprising devolatilizing a carbon-containing feed material to form a gas
phase and
solid phase, wherein the gas phase comprises syngas, and wherein the
devolatilizing is
performed in the presence of free oxygen in an amount between about 0.1 % and
about
25% of the stoichiometric amount of oxygen to completely combust the feed
material.
The amount of free oxygen can be about 1-10% of the stoichiometric amount of
oxygen to completely combust the feed material. Steam can also be added during
this
variation. The added steam can be present in an amount that is less than about
50% of
the stoichiometric amount of water to completely convert the feed material to
carbon
monoxide and hydrogen.
BRIEF DESCRIPTION OF THE FIGURES
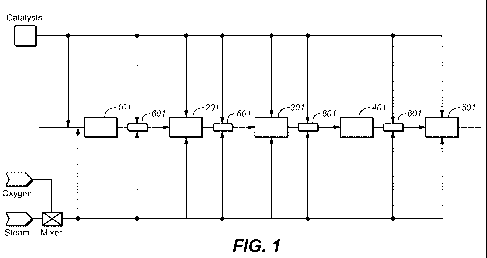
[0026] FIG. 1 shows a process flow for the production of syngas from any
carbon-containing feed material, according to one variation.
[0027] FIG. 2A shows a process flow for a two-stage devolatilization unit,
according to one variation.
[0028] FIG. 2B shows a side view of the two-stage devolatilization unit shown
in FIG. 2A, according to one variation.
[0029] FIG. 3 shows a process flow for a three-stage devolatilization unit,
according to one variation.
[0030] FIG. 4 shows a process flow for a reformer reactor, according to one
variation.
[0031] FIG. 5 shows a process flow for the injection of oxygen and steam into
syngas that is recycled back to the devolatilization unit, according to one
variation.
[0032] FIG. 6 shows an eductor, according to one variation.
[0033] FIG. 7 shows a process flow for producing methanol and ethanol from
syngas using two reactors in sequence, according to one variation.
-6-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
[0034] FIG. 8 shows a process flow for producing methanol and ethanol from
syngas using two reaction zones in sequence in a single reactor, according to
one
variation.
[0035] FIG. 9 shows a process flow for producing methanol and ethanol from
syngas using two reactors in sequence, with at least some of the methanol
produced in
the first reactor diverted from the second reactor, according to one
variation.
[0036] FIG. 10 shows a process flow for producing methanol and ethanol
from syngas using two reactors in sequence according to another variation.
[0037] FIG. 11 shows a process flow for producing methanol and ethanol
from syngas using two reactors in sequence, with the first reactor producing
methanol
in high yield for conversion to ethanol in the second reactor, according to
one
variation.
[0038] These and other embodiments, features, and advantages of the present
invention will become more apparent to those skilled in the art when taken
with
reference to the following detailed description of the invention in
conjunction with the
accompanying drawings.
DETAILED DESCRIPTION OF EMBODIMENTS OF THE INVENTION
[0039] Certain embodiments of the present invention will now be further
described in more detail, in a manner that enables the claimed invention so
that a
person of ordinary skill in this art can make and use the present invention.
[0040] Unless otherwise indicated, all numbers expressing reaction
conditions, stoichiometries, concentrations of components, and so forth used
in the
specification and claims are to be understood as being modified in all
instances by the
term "about." Accordingly, unless indicated to the contrary, the numerical
parameters
set forth in the following specification and attached claims are
approximations that
may vary depending at least upon the specific analytical technique. Any
numerical
value inherently contains certain errors necessarily resulting from the
standard
deviation found in its respective testing measurements.
-7-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
[0041] All publications, patents, and patent applications cited in this
specification are incorporated herein by reference in their entirety as if
each
publication, patent, or patent application was specifically and individually
put forth
herein.
[0042] The following detailed description should be read with reference to the
drawings, in which identical reference numbers refer to like elements
throughout the
different figures. The drawings, which are not necessarily to scale, depict
selected
embodiments and are not intended to limit the scope of the invention. The
detailed
description illustrates by way of example, not by way of limitation, the
principles of
the invention.
[0043] As used in this specification and the appended claims, the singular
forms "a," "an," and "the" include plural referents unless the context clearly
indicates
otherwise. Unless defined otherwise, all technical and scientific terms used
herein
have the same meaning as is commonly understood by one of ordinary skill in
the art
to which this invention belongs. If a definition set forth in this section is
contrary to
or otherwise inconsistent with a definition set forth in patents, published
patent
applications, and other publications that are herein incorporated by
reference, the
definition set forth in this specification prevails over the definition that
is incorporated
herein by reference.
[0044] The present invention provides methods and apparatus for producing
syngas from any carbon-containing feed material. The present invention is
premised,
at least in part, on the addition of a substoichiometric amount of oxygen
during the
conversion of a carbon-containing feed material to syngas.
[0045] In some embodiments, oxygen is mixed with steam, and the resulting
mixture is added to the system for generating syngas. In contrast to some
prior
methods that conducted the devolatilization and reforming process for the
production
of syngas within a controlled reducing environment, the present invention
employs
the concept that oxygen or oxygen-enriched air can be added to the system (i)
to
supply an enthalpy source that displaces additional fuel requirements, e.g. by
causing
an exothermic reaction such as the partial or total oxidation of carbon or
devolatilization products with oxygen; (ii) to achieve a more favorable H2/CO
ratio in
the syngas, which can increase the yield of products formed from the syngas;
(iii) to
-8-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
increase the yield of syngas, e.g. by reducing the formation of less-reactive
compounds and/or by converting certain species to syngas; and/or (iv) to
increase the
purity of syngas, e.g. by reducing the amount of COz, pyrolysis products, tar,
aromatic
compounds, and/or other undesirable products.
[0046] All references herein to a "ratio" of chemical species are references
to
molar ratios unless otherwise indicated. For example, a H2/CO ratio of 1 means
one
mole of hydrogen per mole of carbon dioxide; an 02/H20 ratio of 0.1 means one
mole
of molecular oxygen per ten moles of water.
[0047] By "free oxygen," as used herein, it is meant oxygen that is contained
solely in the gas phase. Free oxygen does not include the oxygen content of
the
biomass itself or of any other solid or liquid phase present, and does not
include
oxygen that is physically adsorbed onto a surface. Generally, "gas phase"
refers to
the vapor phase under the particular process conditions, and will include
components
that are condensable at other conditions (such as lower temperature).
[0048] By "added steam" as used herein, it is meant steam (i.e. H20 in a vapor
phase) that is introduced into a system or apparatus in one or more input
streams.
Added steam does not include (i) steam generated by moisture contained in the
solid
biomass or in another material present, (ii) steam generated by vaporization
of water
that may have initially been present in the system or apparatus, or (iii)
steam
generated by any chemical reactions that produce water.
[0049] Steam reforming, partial oxidation, water-gas shift (WGS), and/or
combustion reactions can occur when oxygen or steam are added. Exemplary
reactions are shown below with respect to a cellulose repeat unit (C6Hi005)
found, for
example, in cellulosic feedstocks. Similar reactions can occur with any carbon-
containing feedstock.
Steam Reforming C6H1005 + H20 ~ 6 CO + 6 Hz
Partial Oxidation C6H10O5 + 1/2 02 ~ 6 CO + 5 H2
Water-Gas Shift CO + H20 H H2 + COz
Complete Combustion C6H1005 + 6 02 -> 6 COz + 5 H20
[0050] FIG. 1 illustrates an exemplary process for synthesizing syngas from
biomass or another carbon-containing material. The feed material is introduced
into a
devolatilization unit 201 through a feed section 101. The product that exits
the
-9-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
devolatilization unit 201 comprises a gas phase and a solid phase and can
further
include one or more liquid phases. A stream exiting the devolatilization unit
201 is
introduced into a heated reaction vesse1301, which in FIG. 1 is shown as a
reformer
reactor, where additional syngas is produced. The syngas produced in the
reformer
reactor 301 is introduced into a quench and compressing section 401, where the
syngas is cooled and compressed.
[0051] The "heated reaction vessel" 301 is any reactor capable of causing at
least one chemical reaction that produces syngas. Conventional steam
reformers,
well-known in the art, can be used either with or without a catalyst. Other
possibilities include autothermal reformers, partial-oxidation reactors, and
multistaged
reactors that combine several reaction mechanisms (e.g., partial oxidation
followed by
water-gas shift). The reactor 301 configuration can be a fixed bed, a
fluidized bed, a
plurality of microchannels, or some other configuration. As will be further
described
below, heat can be supplied to reactor 301 in many ways including, for
example, by
oxidation reactions resulting from oxygen added to the process.
[0052] In some variations, the syngas from the devolatilization unit 201
and/or
the heated reaction vesse1301 is filtered, purified, or otherwise conditioned
prior to
being converted to another product. For example, the cooled and compressed
syngas
may be introduced to a syngas conditioning section 501, where benzene,
toluene,
ethyl benzene, xylene, sulfur compounds, nitrogen, metals, and/or other
impurities or
potential catalyst poisons are optionally removed from the syngas. If desired,
burners
601 can be used to heat the catalyst, oxygen, and/or steam that are added.
[0053] Oxygen can assist pyrolysis and/or cracking reactions in the
devolatilization unit 201 and/or generate heat (which can provide a
temperature rise)
from partial oxidation. As illustrated in FIG. 1, oxygen or a mixture of
oxygen and
steam can be added at any stage of the process for producing syngas. For
example,
oxygen may be added directly to the feed material, to the feed section 101,
before or
while the feed material enters the devolatilization unit 201, directly into
the
devolatilization unit 201, before the exhaust gas/solids from the
devolatilization unit
201 enter the reformer reactor 301, directly into the reformer reactor 301
(such as into
the cold chambers 302 and/or hot chambers 304 of the reformer reactor 301
shown in
FIG. 4), before the syngas product from the reformer reactor 301 enter the
quench and
-10-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
compressing section 401, before the syngas enters the conditioning section
501,
directly into the syngas conditioning section 501, and/or to one or more
various
recycle streams. In some embodiments, oxygen or a mixture of oxygen and steam
are
added at multiple locations.
[0054] In some embodiments, a substoichiometric amount of oxygen is added.
A "stoichiometric amount of oxygen" is calculated based on the amount of
oxygen
that would be required to completely combust the feed material (entering feed
section
101) into COz and H20; this calculation is independent of the amount of steam
that is
added or the location(s) of oxygen addition. In some embodiments, the total
amount
of the oxygen added (e.g., the sum of the amounts of oxygen added at one or
more
locations in the system) or the amount of oxygen present at any point during
the
process is between about 0.1% and about 75% of the stoichiometric amount of
oxygen
for combustion. In embodiments, the amount of oxygen is less than about any of
75%, 50%, 25%, 10%, 5%, 2%, 1%, 0.5%, or 0.1% of the stoichiometric amount of
oxygen.
[0055] In certain embodiments, the amount of oxygen is between about 1-
25%, preferably between about 2-20%, and more preferably between about 5-10%
of
the oxygen required to completely combust the feed material. In other
embodiments,
the amount of oxygen is between about 0.1-10%, preferably between about 0.1-
1%,
and more preferably between about 0.1-0.5% of the oxygen required to
completely
combust the feed material.
[0056] In some embodiments, the amount of oxygen added specifically to the
devolatilization unit 201 is less than about any of 1%, 0.5 %, or 0.1 % of the
stoichiometric amount of oxygen. In some embodiments, the amount of oxygen
added to the reformer reactor 301 is less than about any of 25%, 10%, 5%, 2%,
1%,
0.5%, or 0.1% of the stoichiometric amount of oxygen. In embodiments wherein
oxygen is added to the reformer reactor 301 to generate heat from exothermic
partial
oxidation, the amount of oxygen added to the reformer reactor 301 can be about
1% to
about 10% (such as about 5%) of the stoichiometric amount of oxygen. In
embodiments wherein oxygen is added to the reformer reactor 301 to generate a
lower
ratio of H2/CO in the syngas than would be generated in the absence of oxygen,
the
-11-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
amount of oxygen added to the reformer reactor 301 can be about 10% to about
50%
(such as about 25%) of the stoichiometric amount of oxygen.
[0057] It will be appreciated by a skilled artisan that in carrying out these
methods, the amount of oxygen to be added to the process can be calculated or
estimated in a number of ways other than by determining overall feedstock
composition. For example, one can measure the carbon content of a feed
material and
base the amount of oxygen on some fraction of that which would be predicted to
completely convert the carbon to COz. Similarly, a feedstock heating value can
be
determined and an amount of oxygen to be added can be determined.
Alternatively,
or additionally, one can measure the composition, carbon content, or heating
value of
an intermediate stream or streams into which oxygen can be added. The
substoichiometric amounts of oxygen recited herein use a basis of complete
combustion for convenience only and do not limit the scope of the invention in
any
way.
[0058] Oxygen and/or steam can be present for a portion of or for the entire
time the feed material passes through the devolatilization unit 201 and/or
reformer
reactor 301. In some embodiments, a separate partial-oxidation reactor (not
shown) is
added between the devolatilization unit 201 and the reformer reactor 301 or
added
downstream of the reformer reactor 301 (such as between the reformer reactor
301
and the quench and compressing section 401).
[0059] Another variation of the invention is premised on the realization that
during devolatilization, such as in the devolatilization unit 201 (or another
suitable
devolatilization reactor or vessel), the gas phase so generated contains at
least some
syngas. The amount and quality of syngas produced during this step may be
adjusted
by oxygen and/or steam addition, in amounts as described herein, as well as by
temperature, pressure, and other conditions. The syngas from devolatilization
can be
of sufficient quality for some applications. Therefore, in some embodiments, a
gas
phase and solid phase from devolatilization need not proceed to a separate
heated
reaction vessel (such as a steam reformer). Instead, the gas and solid phases
may be
collected and used directly; or, one or both of these phases may be stored for
future
use.
-12-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
[0060] In some embodiments of the invention, the total amount of steam
added (e.g., the sum of the amounts of steam added at one or more locations in
the
system) or the amount of steam present at any point during the process is at
least
about 0.1 mole of steam per mole of carbon in the feed material. In various
embodiments, at least about any of 0.5, 1.0, 1.5, 2.0, 3.0, 4.0, 5.0, or more
moles of
steam are added or are present per mole of carbon. In some embodiments,
between
about 1.5-3.0 moles of steam are added or are present per mole carbon.
[0061] The amount to steam that is added to the heated reaction vesse1301
can vary depending on factors such as the performance in the devolatilization
unit
201. When devolatilization produces a carbon-rich solid material, generally
more
steam (and/or more oxygen) is used to add the necessary H and 0 atoms to the C
available to generate CO and H2. From the perspective of the overall system,
the
moisture contained in the feed material can be accounted for in determining
how
much additional water (steam) to add in the process.
[0062] Steam is generally used to steam reform, inside the reformer reactor
301, gases and/or solids exiting the devolatilization unit 201. In some
embodiments,
steam is used, in part, to push feed material through the devolatilization
unit 201. In
certain embodiments, more steam is added to the reformer reactor 301 than to
the
devolatilization unit 201.
[0063] In some embodiments, the humidity of the gas produced from the feed
material is measured at any point in the process and an appropriate amount of
steam is
added to maintain a desired humidity level. For example, gas from the
devolatilization unit 201 can be analyzed to determine the amount of steam
present
and then more steam can be added, if desired.
[0064] Exemplary ratios of oxygen added to steam added (Oz/Hz0) are equal
to or less than about any of 2, 1.5, l, 0.5, 0.2, 0.1, 0.05, 0.02, 0.01, or
less. Exemplary
ratios of oxygen added to steam present, which includes Hz0 from moisture that
was
present prior to the addition of steam, any Hz0 generated by chemical
reactions, and
Hz0 from the addition of steam, are equal to or less than about any of 1, 0.5,
0.4, 0.3,
0.2, 0.1, 0.05, 0.04, 0.03, 0.02, 0.01, or less. Exemplary ratios of oxygen
added to
steam added or present are between about 0.01-2, between about 0.02-0.5, or
between
about 0.05-0.2. When the ratio of Oz/Hz0 is greater than 1, the combustion
reaction
-13-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
starts to dominate over partial oxidation, which may produce undesirably low
CO/CO2 ratios.
[0065] In some embodiments, oxygen without steam is added at one or more
locations in the system. In some embodiments, steam without oxygen is added at
one
or more locations in the system. In various embodiments, oxygen without steam
is
added at one or more locations in the system, and steam without oxygen is
added at
one or more different locations. A mixture of oxygen and steam can be added at
one
or more locations in the system. In certain embodiments, oxygen without steam
is
added at one location, steam without oxygen is added at another location, and
a
mixture of oxygen and steam is added at yet another location. In some
embodiments,
a mixture of oxygen and steam is added at different 02/H20 ratios in two or
more
locations.
[0066] In particular embodiments, steam is added to the devolatilization unit
201, while oxygen is not added to the devolatilization unit 201. In particular
embodiments, both oxygen and steam are added to the reformer reactor 301. In
some
embodiments, oxygen but not steam is fed to a partial-oxidation reactor that
is in
communication with the devolatilization unit 201 and/or reformer reactor 301.
[0067] Oxygen and steam can be added to the system as one stream, or steam
and oxygen can be injected as separate streams into the same or different
locations. In
some embodiments, steam and oxygen are added in a manner that creates a
reasonably
uniform reaction zone to avoid localized zones of different stoichiometries in
a reactor
or other vessel. In some embodiments, oxygen and steam are added in different
locations such that partial oxidation and steam reforming initially occur in
different
locations, with the resulting components being later combined such that a
combination of partial oxidation and steam reforming can occur effectively in
a single
location.
[0068] Oxygen can be added in substantially pure form, or it can be fed to the
process through the addition of air, optionally enriched with oxygen. In some
embodiments, air that is not enriched for oxygen is added. In other
embodiments,
enriched air from an off-spec or recycle stream, which may be a stream from a
nearby
air-separation plant, for example, can be used. In some embodiments, the use
of
enriched air with a reduced amount of N2 (i.e., less than 79 vol%) results in
less N2 in
-14-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
the resulting syngas. Because removal of N2 can be expensive, methods of
producing
syngas with less or no N2 are typically desirable, when the syngas is intended
for
synthesis of liquid fuels such as alcohols.
[0069] In some embodiments, the presence of oxygen alters the ratio of H2/CO
in the syngas, compared to the ratio produced by the same method in the
absence of
oxygen. The H2/CO ratio of the syngas can be between about 0.5 to about 2.0,
such
as between about 0.75-1.25, about 1-1.5, or about 1.5-2Ø As will be
recognized,
increased water-gas shift (by higher rates of steam addition) will tend to
produce
higher H2/CO ratios, such as at least 2.0, 3Ø 4Ø 5.0, or even higher,
which may be
desired for certain applications. When low H2/CO ratios are desired in the
syngas
stream, it can be advantageous to decrease steam addition and increase oxygen
addition, as described in various embodiments herein.
[0070] The H2/CO ratio in the syngas can affect the yield of downstream
products such as methanol or ethanol. The preferred H2/CO ratio may depend on
the
catalyst(s) used to produce the desired product (from syngas) as well as on
the
operating conditions. Consequently, in some variations the production and/or
subsequent conditioning of syngas is controlled to produce syngas having a
H2/CO
ratio within a range desired to optimize, for example, production of methanol,
ethanol,
or both methanol and ethanol.
[0071] In some variations, the H2/CO ratio of the syngas produced using the
methods described herein can provide an increased product (e.g., C2-C4
alcohols)
yield compared to that which would be provided by syngas produced by the
corresponding methods in the absence of oxygen. This effect can be caused, for
example, by faster kinetic rates toward desired products at reduced H2/CO
ratios; e.g.,
the rate of ethanol formation can be faster for H2/CO = 1-1.5 compared to
H2/CO
=
1.5-2, for certain catalysts and conditions.
[0072] Some embodiments of the invention provide methods of controlling
the H2/CO ratio of the syngas by adjusting the amount and/or location of
oxygen
addition dynamically during the process. It can be advantageous to monitor the
H2/CO ratio of the syngas in substantially real-time, and adjust the amount
and/or
location of 02 addition to keep the Hz/CO ratio at (or near) a prescribed
level. Also, it
can be beneficial to change the Hz/CO ratio in response to some variation in
the
-15-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
process (e.g., feedstock composition changes) or variation in conditions
(e.g., catalyst
deactivation), for better overall performance.
[0073] Catalysts that facilitate the devolatilization, reforming, and/or
partial-
oxidation reactions can optionally be provided at any stage of the process for
producing syngas. Referring again to FIG. 1, one or more catalysts may be
added
directly to the feed material, to the feed section 101, before or while the
feed material
enters the devolatilization unit 201, directly into the devolatilization unit
201, before
the exhaust gas/solids from the devolatilization unit 210 enter the reformer
reactor
301, directly into the reformer reactor 301 (e.g., addition of reforming
and/or partial-
oxidation catalysts can be added to the cold 302 and/or hot chambers 304 of
the
reformer reactor shown in FIG. 4) before the syngas product from the reformer
reactor
301 enters the quench and compressing section 401, before the syngas enters
the
conditioning section 501, directly into the syngas conditioning section 501,
and/or
added to recycle streams. In some embodiments, one or more catalysts are added
at
multiple locations. In some embodiments, a catalyst is added at the same
location
where oxygen or a mixture of oxygen and steam are added.
[0074] Catalysts used for devolatilization include, but are not limited to,
alkali
metal salts, alkaline earth metal oxides and salts, mineral substances or ash
in coal,
transition metals and their oxides and salts, and eutectic salt mixtures.
Specific
examples of catalysts include, but are not limited to, potassium hydroxide,
potassium
carbonate, lithium hydroxide, lithium carbonate, cesium hydroxide, nickel
oxide,
nickel-substituted synthetic mica montmorillonite (NiSMM), NiSMM-supported
molybdenum, iron hydroxyoxide, iron nitrate, iron-calcium-impregnated salts,
nickel
uranyl oxide, sodium fluoride, and cryolite. Devolatilization catalysis
includes
catalysis of devolatilization or gasification per se, as well as catalysis of
tar cracking
reactions or pyrolysis. In some embodiments, the devolatilization catalyst is
between
about 1 to about 100 m in size, such as about 10-50 m. Other sizes of
catalyst
particles are, however, possible.
[0075] Reforming and/or partial-oxidation catalysts include, but are not
limited to, nickel, nickel oxide, rhodium, ruthenium, iridium, palladium, and
platinum. Such catalysts can be coated or deposited onto one or more support
materials, such as, for example, gamma-alumina (optionally doped with a
stabilizing
-16-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
element such as magnesium, lanthanum, or barium). In some embodiments, the
reforming and/or partial-oxidation catalyst is between about 1 to about 1000
nm in
size, such as about 10-100 nm. Other catalyst sizes are, however, possible.
[0076] Before being added to the system, any catalyst can be pretreated or
activated using known techniques that impact total surface area, active
surface area,
site density, catalyst stability, catalyst lifetime, catalyst composition,
surface
roughness, surface dispersion, porosity, density, and/or thermal diffusivity.
Pretreatments of catalysts include, but are not limited to, calcining,
washcoat addition,
particle-size reduction, and surface activation by thermal or chemical means.
[0077] Catalyst addition can be performed by first dissolving or slurrying the
catalyst(s) into a solvent such as water or any hydrocarbon that can be
gasified and/or
reformed. Examples of hydrocarbon solvents include acetone, ethanol, or
mixtures of
alcohols. In some embodiments, the catalyst is added by direct injection of
such a
slurry into a vessel (e.g., using high-pressure pumps such as common HPLC
pumps or
syringe pumps). In some embodiments, the catalyst is added to steam and the
steam/catalyst mixture is added to the system. In these embodiments, the added
catalyst may be at or near its equilibrium solubility in the steam or may be
introduced
as particles entrained in the steam and thereby introduced into the system.
[0078] In some embodiments, catalysts are introduced indirectly. For
example, catalysis may occur due to impurities present in the feed material,
from
recycle streams, or from materials of construction. These indirect catalysts
may or
may not be beneficial. Preferably, but not necessarily, these catalyst sources
are
identified and monitored in overall process control and operation.
[0079] Catalysts can optionally be recovered from certain intermediate or
byproduct streams, such as ash from the ash-quench/slag-removal system 520
(FIG.
5), using methods known in the art.
[0080] The methods and systems of the invention can accommodate a wide
range of feedstocks of various types, sizes, and moisture contents. Any carbon-
containing compound can be used as a feed material for the production of
syngas. For
example, biomass such as agricultural wastes, forest products, grasses, and
other
cellulosic material can be used. In some embodiments, the feedstock includes
one or
more materials selected from timber harvesting residues, softwood chips,
hardwood
-17-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
chips, tree branches, tree stumps, leaves, bark, sawdust, off-spec paper pulp,
corn,
corn stover, wheat straw, rice straw, sugarcane bagasse, switchgrass,
miscanthus,
animal manure, municipal garbage, municipal sewage, commercial waste, grape
pumice, almond shells, pecan shells, coconut shells, coffee grounds, grass
pellets, hay
pellets, wood pellets, cardboard, paper, plastic, and cloth. A person of
ordinary skill
in the art will appreciate that the feedstock options are virtually unlimited.
[0081] Referring to FIG. 1, the feed section 101 of FIG. 1 can include a feed
distribution system, a charging hopper, and a lock hopper (not shown), for
example.
In some embodiments, multiple charging hoppers and lock hoppers are used. Feed
material (such as wood chips) is received from the distribution system into
the
charging hopper. Each charging hopper feeds a lock hopper that, in turn, feeds
material such as wood chips to two devolatilization stacks (701 in FIG. 2B)
contained
in the devolatilization unit 201.
[0082] In some embodiments, the system processes about 1 to about 5,000 dry
tons per day ("DTPD") of various timber and other biomass feed materials for
conversion to syngas, which is suitable for conversion into fuel-quality
alcohols such
as ethanol.
[0083] In some embodiments, the feedstock substantially consists of southern
pine that has been chipped to a characteristic length scale of about one inch.
An
exemplary composition of southern pine, on a dry basis, is 56 wt% carbon, 5.4
wt%
hydrogen, 37 wt% oxygen, 0.4 wt% nitrogen, 0.7 wt% ash, and trace amounts of
sulfur. Moisture levels of the feed material can vary widely, depending on
harvest
and storage condition, and can range from about 10% to about 60%.
[0084] In some embodiments, the feed material is torrefied biomass such as
torrefied wood. Torrefaction consists of a slow heating of biomass in an inert
atmosphere to a maximum temperature of about 300 C. The treatment yields a
solid
uniform product with a lower moisture content and a higher energy content
compared
to the initial biomass. Torrefied biomass is hydrophobic-it does not regain
humidity
in storage and therefore is relatively stable-and will generally have a lower
moisture
content and higher energy value compared to the initial biomass. In some
embodiments, a feed material is torrefied before it is added to
devolatilization unit
-18-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
201. In other embodiments, torrefaction occurs, to some extent, within
devolatilization unit 201.
[0085] FIG. 2A depicts a devolatilization unit 201 that is connected to the
lock
hopper (not shown) from the feed section 101. The reaction product exiting
from the
devolatilization unit 201 is introduced to a reformer reactor 301, according
to the
embodiments depicted in these drawings. When feed material such as wood chips
is
conveyed through the devolatilization unit 201, it can undergo torrefaction,
gasification, and/or devolatilization. These processes reduce the mass and
volume of
the conveyed solids, with a corresponding increase in the mass and volume of
volatilized gas.
[0086] As illustrated in FIGS. 2A and 2B, which depict the front and side
view of devolatilization unit 201, respectively, the devolatilization unit 201
consists
of two devolatilization stacks 701, positioned next to each other. Each stack
701
includes a series of reaction chambers 210-219. Each chamber is in connection
with
the next chamber. Each chamber includes one auger 220. The augers and down-
comer pipes distribute feed material into each devolatilization chamber 210-
219 and
convey the feed material flow in each devolatilization chamber 210-219
horizontally.
An exemplary down-comer pipe is the section shown in FIG. 2A connecting
chambers 210 and 211. The augers 220 are operated by motors 222. A cooling-
water
supply that is used to cool down the motor temperature has an inlet 270 and an
outlet
272. In some embodiments, motors 222 can be variable frequency drives that are
equipped with torque sensors at each end of the auger with speed control.
[0087] In some embodiments, one or more of the augers 220 are twin screws,
such as a pair of overlapping or intermeshing screws mounted (e.g., a pair of
screws at
the same elevation or a pair of screws at different elevations) that are used
to move
the feed material through the devolatilization unit 201. The twin screws are
preferably designed to ensure efficient movement of feed material forward,
minimize
the possibility of backward flow of material, ensure a substantially uniform
temperature distribution in the radial direction, and/or prevent release of
materials,
thereby allowing safe operation and a good operating lifetime.
[0088] Other means of conveying material through the devolatilization unit
201 are certainly possible and within the scope of the present invention.
Material can
-19-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
generally be conveyed by single screws, twin screws, rams, and the like.
Material can
be conveyed mechanically through the devolatilization unit 201 by physical
force
(metal contact), pressure-driven flow, pneumatically driven flow, centrifugal
flow,
gravitational flow, fluidized flow, or some other known means of moving solid
and
gas phases.
[0089] In some embodiments, the temperature within the devolatilization unit
201 increases as the feed material progresses through the devolatilization
unit 201. In
some embodiments, the feed material enters devolatilization unit 201 at about
ambient
temperature and exits the devolatilization unit 201 between about 450-1000 F
(such
as between 900-1000 F). In some embodiments in which devolatilization is
performed in the presence of oxygen, the temperature increases due to the
exothermic
partial oxidization of material in the devolatilization unit 201. In various
embodiments, the pressure is between about 50 to about 200 psig, such as about
100-
150 psig. Feed material is conveyed inside the tubes in the cascading auger
system
and is heated in the enclosed auger system. A bypass gas line can recombine
recycled
unreacted product gas from a high-pressure separator 250 and the process
stream and
run it back through the devolatilization unit 201.
[0090] Heat is supplied to the devolatilization unit 201 by a set of burners
230,
which are connected to the devolatilization unit 201 through a set of air
mixers 232.
Heat can be supplied in two different modes: start-up and normal operation. A
common burner system can be utilized for both modes. At start-up heating mode,
natural gas 236 is combusted and the flue gas is used as the hot process
stream for the
devolatilization unit 201. During normal operation, the combustion fuel is
unreacted
product gas, optionally supplemented with natural gas. In some embodiments,
the
devolatilization burners are also fueled with syngas produced by the reformer
reactors
301. As syngas is produced, more syngas and less natural gas can be preferably
used
to heat the devolatilization unit 201.
[0091] Devolatilization outlet 235 directs devolatilization flue gas into a
devolatilization combustion-air preheater 234, where the devolatilization flue
gas is
cooled and exchanges enthalpy with devolatilization combustion air 244, which
is
introduced into the air preheater 234 through a devolatilization combustion
air blower
246. The preheated air is split as feed introduced to the burners 230, as well
as feed
-20-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
introduced directly to the air mixers 232. The preheated air is introduced to
the air
mixers 232 to combine with burner flue gases from the burners 230 to help
maintain
the devolatilization inlet air temperature.
[0092] The devolatilization combustion air preheater 234 also directs partial
preheated air into a devolatilization induced draft fan 248, which can
communicate
with a stack 240, where the preheated air is joined with reformer flue gas 238
that
exits from the reformer reactor 301. The flue gas exiting from the stack 240
exits the
system through an exhaust line 280 and through subsequent heat-exchange
equipment
(not shown).
[0093] The devolatilization unit can be a single-stage unit or can optionally
be
divided into multiple stages. For present purposes, a "stage" is a physical
zone within
the unit, and does not relate to temporal considerations. Also, the number of
stages is
independent of the number of actual augers, down-comer pipes, stacks, or other
physical implementation. Specification and delineation of stages can be done
for any
purpose, such as for temperature control, measurement points, residence-time
distribution, or for the presence of various input or output streams.
[0094] A multiple-stage devolatilization unit can generally be desirable for
certain feed materials for which it would be beneficial to remove some or all
of the
devolatilized gas prior to the end of the devolatilization unit 201. For
example, rapid
removal of devolatilized gas can help prevent undesirable gas-phase chemistry,
such
as polymerization leading to tar formation. When syngas is the desired product
and
devolatilization produces at least some syngas, it can be desirable to remove
syngas
upon generation rather than allowing it to possibly react with other
components
present. Also, it can be more energy-efficient to process the gas phase 203
for a
shorter amount of time than the solid phase 204 in the devolatilization unit
201.
"Multiple stages" can mean 2, 3, 4, 5, or more stages of devolatilization.
[0095] FIG. 2A depicts a two-stage devolatilization unit 201 such that the gas
phase 203 and solid phase 204 exit the devolatilization unit 201 at different
places.
The optional passage of the solid phase 204 through a second portion of the
devolatilization unit 201 that the gas phase 203 is not passed through allows
the solid
phase 204 to be treated for longer in the devolatilization unit 201.
-21 -
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
[0096] As shown in FIG. 2A, the gas phase 203 of the devolatilization product
leaves the devolatilization unit 201 and exits from one or more top stage(s).
The solid
phase 204 of the devolatilization product stays in the devolatilization unit
201 longer
and exits from the bottom stage. A two-stage devolatilization unit 201 is
shown in
FIG. 2A wherein the top stage and the bottom stage are divided by a dashed
line 202.
The gas/solid separation can occur, for example, in a cyclone device that
separates the
gas phase from the solid phase primarily by density difference.
[0097] In some embodiments, the solid phase 204 and the gas phase 203 enter
the reformer separately, and a different amount of oxygen and/or steam is
added to the
solid phase 204 compared to the amount added to the gas phase 203 of the
material
leaving the devolatilization unit 201. For example, the compositions of the
solid
phase 204 and gas phase 203 leaving the devolatilization unit 201 can be
measured or
estimated, and the amount of oxygen and/or steam that is added to each phase
can be
determined based on the composition of each phase (such as the amount of
carbon in
each phase). In some embodiments, less oxygen and/or steam is added to the gas
phase 202 than the solid phase 204. In some embodiments, steam is added to the
gas
phase 203 to enrich it towards hydrogen by the water-gas shift reaction.
[0098] In some embodiments, steam 262 is used to obtain the desired H2/CO
ratio of syngas from the reformer reactor 301. The oxygen 260 can partially
oxidize
the devolatilization product and boost the process temperature prior to
feeding to the
reformer in order to lower the reformer burner heat duty. In some preferred
embodiments, oxygen feed 260 (or air feed) and superheated steam feed 262 are
mixed in a reformer feed steam/oxygen mixer 264 and then introduced into an
eductor
266, where the solid phase 204 of the devolatilization product from the
devolatilization unit 201 joins the oxygen/steam stream. The mixture is then
introduced into the reformer reactor 301.
[0099] In some embodiments, the gas phase 203 of the devolatilization
product from the devolatilization unit 201 is combined with the solid phase
204 and
the mixture is then introduced to the eductor 266 and the burner 268. In some
other
embodiments, the gas phase 203 can be introduced into the reformer reactor 301
directly. In some embodiments, the gas phase 203 is combined with oxygen
and/or
steam before it is introduced into the reformer reactor 301 directly. Steam
flow to the
-22-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
eductor 266 can be controlled, for example, by monitoring concentrations of
CO, H2,
or both. Oxygen 260 to the eductor 266 can be controlled, for example, by the
temperature downstream of the eductor 266 and/or the temperature at the
reformer
reactor 301 inlet.
[00100] In one embodiment, reformer feed steam/oxygen mixers 264 combine
oxygen and steam (which steam can be superheated) and introduce the mixture of
steam and oxygen to the eductor 266. The remaining solids from the
devolatilization
unit 201 are entrained in the gaseous volatilized products to the entrance of
reformer
reactor 301. In some embodiments when oxygen is not added prior to the
reformer
reactor 301, a burner can be used to heat the products from the
devolatilization unit
201 before they enter the reformer reactor 301. When oxygen is added prior to
the
reformer reactor 301, a burner can be unnecessary to heat the products from
the
devolatilization unit 201 before they enter the reformer reactor 301, due to
the heat
generated by exothermic partial oxidation.
[00101] FIG. 3 depicts a three-stage devolatilization unit. The top two stages
and the bottom stage are divided by dashed lines 202A and 202B. The gas phases
203A and 203B of the devolatilization product exit from the top two stages;
solid
phase 204A of the devolatilization product stays in the devolatilization unit
longer and
then exits from the bottom stage. The gas phases 203A and 203B may be combined
after they exit the devolatilization unit and before they enter the reformer
reactor 301.
The gas phases 203A and 203B and solid phase 204A can be introduced into the
reformer reactor 301 as described in reference to FIG. 2A for a two-stage
devolatilization unit.
[00102] FIG. 4 depicts an exemplary reformer reactor 301, which includes five
major components: a cold chamber 302, a hot chamber 304, a set of burners 318,
and
a set of cyclones that includes a primary cyclone 312 and a polishing cyclone
314. A
dividing wa11303 separates the cold chamber 302 from the hot chamber 304. Each
chamber contains two separate serpentine or coiled reactor tubes 310, which
increase
the residence time of the products from the devolatilization unit 201 compared
to the
corresponding residence time for a linear tube. In some embodiments where the
devolatilization product enters into the reformer reactor 301 directly, each
serpentine
or coiled reactor tube 310 is fed by each devolatilization stack 701. One
- 23 -
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
devolatilization stack 701 can feed one reformer reactor 301. In other
embodiments,
each serpentine or coiled reactor tube 310 is fed by one-half of the reaction
product
from the burner 268, shown in FIG. 2A.
[00103] Each reactor tube 310 is connected to a primary cyclone 312, which is
further connected to a polishing cyclone 314. Both cyclones remove ash from
the
product that exits from the reactor tubes 310. In certain embodiments, about
90% and
10% by weight solids, respectively, are removed by the primary cyclones 312
and
polishing cyclones 314. The ash is directed to ash collectors 316. As
illustrated in
FIG. 1, and described in detail herein above, oxygen or a mixture of oxygen
and
steam may be optionally added at any point in the system, such as before,
during, or
after the devolatilization product passes through the reformer reactor 301.
[00104] In some embodiments, the reactor tubes 310 in the cold chamber 302
raise the temperature of the devolatilization products from about 700-1100 F
at the
entrance of the reformer reactor 301 to a temperature of about 1200-1500 F at
the end
of the cold chamber 302. In preferred embodiments, the temperature is kept
below
the softening point of the ash components to facilitate their later removal.
The
serpentine or coiled reactor tubes 310 in the hot chamber 304 and their
contents are
maintained at a constant temperature, such as about 1400 F or some other
suitable
temperature.
[00105] In some embodiments, the temperatures of the cold chamber 302 and
the hot chamber 304 stay above the dew point of the product from the
devolatilization
unit 201. The temperature of the hot chamber 304 can be about 1500 F, 1600 F,
1700 F, or higher. Using appropriate materials, the temperature for the
reformer
reactor 301 can be about 2000 F or even higher. In various embodiments, the
pressure of the reformer reactor 301 is between about 25-500 psig, such as
about 50-
200 psig. The pressure of the reformer reactor 301 can be the same as that for
the
devolatilization unit 201, in some embodiments.
[00106] In some embodiments, the reforming and/or partial-oxidation
catalyst(s) that are (i) present in the product from the devolatilization unit
201 or are
(ii) added to the reformer reactor 301, are entrained catalysts. In some
embodiments,
a fixed-bed or fluidized-bed reformer reactor 301 with one or more reforming
and/or
partial-oxidation catalyst(s) is used.
-24-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
[00107] The reformer reactor 301 can be heated by a set of burners 318, which
are fed by fuel gas 236 and a gas mixture exiting from a reformer air mixer
320. Fuel
supplied to the burners 318 to provide heat for reactions to form syngas can
be from
any or all of three process sources: (1) "fresh" syngas from upstream sources;
(2)
unreacted product gas from downstream synthesis; and/or (3) natural gas.
[00108] The syngas exiting from the polishing cyclones 314 may be introduced
to a quench and compressing section 401 of FIG. 1 directly. Alternatively or
additionally, syngas can first enter into an eductor 330, where the syngas is
joined
with the oxygen/steam mixture from the reformer feed steam/oxygen mixer 264
(shown in FIG. 2A). The mixture is then optionally introduced to a feed/oxygen
reactor 332 and ultimately into the quench and compressing section 401. If
desired,
the oxygen/steam mixture can also be introduced directly to both or either
chambers
of the reformer reactor 301. A standard flow valve (not shown), or some other
known
means, can be used to control the amount of the oxygen added to the system.
[00109] In some embodiments, reforming and partial oxidation occur in the
same reaction vessel. In other embodiments, reforming and partial oxidation
occur in
different reaction vessels. For example, a partial-oxidation reactor (such as
a
fluidized, packed-bed, or microchannel reactor) can be upstream or downstream
of the
reformer reactor 301. In one embodiment, a partial-oxidation reactor is
upstream of
the reformer reactor 301 and generates heat for reforming in the reformer
reactor 301.
[00110] After exiting the reformer reactor 301, syngas is preferably quickly
cooled with water (or by some other means) to avoid formation of carbon. For
example, the syngas product can be cooled with boiler feed water in the quench
and
compressing section 401. In one illustrative embodiment, boiler feed water of
a
temperature of about 200 F is injected directly into the syngas stream to cool
the
temperature of the stream from about 1400 F to about 1000 F.
[00111] Syngas pressure is preferably increased prior to conditioning. In some
embodiments, the syngas is compressed to about 1000 psig, 1500 psig, 2000
psig, or
higher. In some embodiments, syngas conditioning 501 comprises feeding the
syngas
to a COz removal system (shown in FIG.l). Any methods known in the art can be
employed to remove carbon dioxide, including membrane-based or solvent-based
- 25 -
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
separation methods. In some embodiments, little or no COz is removed from the
syngas.
[00112] In some embodiments, the syngas produced using the methods
described herein has less impurities compared to syngas produced in the
absence of
any oxygen addition. In some embodiments, the decreased amount of impurities
facilitates the further purification of syngas. For example, less energy or
time may be
required to remove COz from the syngas produced using the methods described
herein
than from syngas produced in the absence of oxygen.
[00113] If desired, the removed COz can be used anywhere an inert gas is
desirable. For example, COz can be used to convey or entrain solid material
from one
point to another point of the process. Another use of COz is to vary the H2/CO
ratio
by the water-gas shift reaction. Recovered COz can also be used to react with
methane in dry reforming to produce syngas, or react with pure carbon (e.g.,
carbon
deposited on reactor walls or catalyst surfaces) to form 2 moles of CO in the
reverse
Boudouard reaction (i.e., COz + C H 2 CO).
[00114] In some embodiments, removed COz can be recycled back to the
devolatilization unit 201. Generally, a variety of purge streams from any
operations
downstream of the devolatilization can be recycled back to the
devolatilization unit
201. These purge streams may contain CO, COz, H2, H20, CH4, and other
hydrocarbons.
[00115] Cooled syngas can optionally be fed to a benzene, toluene, ethyl
benzene, and xylenes removal system. In some embodiments, the removal system
comprises a plurality of activated carbon beds. Of course, other organic
compounds
(such as tars) can be removed as well, depending on conditions.
[00116] FIG. 5 depicts certain embodiments for devolatilization and reforming.
The feed material is introduced into a devolatilization unit 201. The product
that exits
from the devolatilization unit 201 is introduced into a reformer reactor 301,
where
syngas is produced. The syngas produced in the reformer reactor 301 is
introduced
into a primary cyclone 312 and a polishing cyclone 314, where ash and other
solids
are removed from the syngas product. The syngas that exits from the polishing
cyclone 314 is introduced to a quench and compressing section 401, where the
syngas
is cooled and compressed. The solids separated from the syngas product in the
-26-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
primary cyclone 312 are introduced into an ash-quench/slag-removal system 520,
where oxygen or a mixture of oxygen and steam can be injected. The mixture of
oxygen and steam allows the solids separated from the syngas to undergo
partial
oxidation. The gas product from the ash-quench/slag-removal system 520 is
introduced to an eductor 900, which helps the gas product transfer back to the
devolatilization unit 201. The gas product circulating back from the ash-
quench/slag
removal system 520 helps to move forward the material in the devolatilization
unit
201. In one embodiment, the gas product from the ash-quench/slag removal
system
520 enters the devolatilization unit 201 near the exit of the devolatilization
unit. The
solids further separated in the ash-quench/slag-removal system 520 are removed
at the
bottom of the system.
[00117] Another aspect of the present invention relates to eductors. Eductors
(also known as jet ejector pumps or Venturi pumps) are an efficient way to
pump or
move many types of liquids and gases. Eductors generally utilize the kinetic
energy
of one species to cause the flow of another. In operation, the pressure energy
of the
motive liquid is converted to velocity energy by a converging nozzle. The high
velocity flow then entrains another species (such as solids from the
devolatilization
unit 201). The mixture is then converted back to an intermediate pressure
after
passing through a diffuser. Eductors can also balance pressure drops and aid
in
overall heat transfer.
[00118] In some embodiments, the eductor is used to convey the material
leaving the devolatilization unit 201 and entering the reformer reactor 301
(such as
eductor 266 shown in FIG. 2A). In certain embodiments, the drive gas for this
eductor is steam and/or oxygen that is introduced into the reformer reactor
301.
[00119] An eductor 600 that can be used in particular embodiments is depicted
in FIG. 6, which is exemplary and non-limiting. With reference to FIG. 6,
generally
solids and (if present) gases enter as stream 601, which can be referred to as
the
motive phase. In some embodiments, additional vapor is added in streams 610,
which
collectively can be referred to interchangeably as the suction fluid, the
educted fluid,
or the eductor drive fluid.
[00120] The eductor 600 in FIG. 6 is characterized by a first cross-sectional
area 640 and a second, smaller cross-sectional area 150. The area reduction
causes a
-27-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
lower pressure, which creates a suction effect to pull material forward. The
material
velocity increases through the smaller area 150, and then returns to a lower
velocity
downstream of the area reduction, according to a momentum balance. Stream 190
exits the eductor 600.
[00121] Streams 610 are shown in FIG. 6 to enter at an angle denoted 620.
This angle can be any angle but in some embodiments is greater than about 0
degrees
and less than about 90 degrees. An angle of 0 degrees produces co-incident
flow of
the suction fluid and the motive phase, while an angle of 90 degrees produces
perpendicular injection of the suction fluid into the eductor. An angle of
greater than
90 degrees, and up to 180 degrees, represents injection of the suction fluid
in a
direction flowing upstream relative to the movement of the motive phase.
Exemplary
angles of entry in various embodiments include angles between about 10 to
about 60
degrees, and in certain embodiments, the angle is about any of 30, 35, 40, or
45
degrees.
[00122] While FIG. 6 shows two streams 610 entering the eductor 600, other
embodiments can include 1, 3, 4, 5, or more locations where suction-fluid
enters the
eductor 600. By "streams 610" it is meant any number of actual streams,
including a
single stream of suction fluid. These different entries can all be
characterized by the
same angle. Alternatively, different angles may be used.
[00123] According to embodiments of the present invention, stream 601 can be
at least a portion of the solid-vapor mixture exiting the devolatilization
unit 201.
Stream 601 can enter the eductor 600 by means of a single-screw (auger)
conveyer, a
twin-screw device, or by any other means. Streams 610 can be one or more of
steam,
oxygen, and air. The amount of steam or oxygen to inject by means of streams
610
can be the amount that is desired for the steam reforming and/or partial-
oxidation
steps downstream of the devolatilization unit 201, or can be a different
amount.
[00124] In addition to adding reactants to the process, streams 610 also can
enhance mixing efficiency within the eductor 600, so that species can be well-
mixed
upon entering the reformer reactor 301. Without being limited to any
particular
theory, it is believed that the solid material entering in stream 601 is
characterized by
laminar flow or plug flow; the suction fluid from 610 is thought to cause an
onset of
turbulent flow within the eductor 600. Turbulence is known to enhance mixing
and
- 28 -
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
can also help break apart the solids and reduce particle size. The exact
nature of this
onset of turbulence is generally a function of the velocity and pressure of
streams 601
and 610, the areas 640 and 150, the angle 620 (or plurality of angles), and
the nature
of the motive and suction fluids. The eductor 600 can also be suitable for
multiphase
annular flow from the devolatilization unit 201 to said heated reaction
vesse1301.
[00125] As will be appreciated, other gases besides H20 and 02 for streams
610 can additionally or alternatively be used. Other gases that could be used
include,
but are not limited to, recycled syngas, recycled steam possibly containing
various
impurities, such as C02, N2, methanol vapor, ethanol vapor, etc.
[00126] The eductor 600 can be employed in any step of the process described
herein, such as the removal of ash-rich solids or other purge streams (such as
eductor
330 in FIG. 4) or the mixing of oxygen and steam with syngas (such as eductor
900 in
FIG. 5). Eductor 600 can also be used in any other apparatus for which an
eductor is
desirable, such as an apparatus for which one or more decreases in pressure
within the
apparatus is desirable.
[00127] Exemplary methods and apparatus for producing alcohols from syngas
are disclosed herein. In some variations of these methods and apparatus,
syngas is
catalytically converted to methanol in a first reaction zone, and residual
syngas from
the first reaction zone is then catalytically converted to ethanol in a second
reaction
zone. Referring to FIG. 7, for example, in one variation a syngas feedstream
100 is
introduced into a first reactor 105 comprising a first reaction zone 110. One
or more
catalysts in reaction zone 110 convert at least a portion of syngas feedstream
100 to
methanol to provide an intermediate product stream 115 comprising at least a
portion
of the residual (unreacted) syngas from feedstream 100, methanol, and, in some
variations, higher alcohols and/or other reaction products.
[00128] At least a portion of intermediate product stream 115 is introduced
into
a second reactor 120 comprising a second reaction zone 125. One or more
catalysts in
reaction zone 125 convert at least a portion of syngas from intermediate
product
stream 115 and/or at least a portion of methanol from intermediate product
stream 115
to provide a product stream 130 comprising ethanol and, in some variations,
methanol, higher alcohols, other reaction products, and/or unreacted syngas
from
intermediate product stream 115.
-29-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
[00129] Various components of product stream 130 such as, for example,
methanol, ethanol, alcohol mixtures (e.g., methanol, ethanol, and/or higher
alcohols),
water, and unreacted syngas may be separated out and (optionally) purified by
the
methods described herein or conventional methods. Such methods may include,
for
example, condensation, distillation, and membrane separation processes, as
well as
drying or purifying with molecular sieves.
[00130] Syngas feedstream 100 may be produced in any suitable manner
known to one of ordinary skill in the art from any suitable feedstock. In some
variations, syngas feedstream 100 is filtered, purified, or otherwise
conditioned prior
to being introduced into reactor 105. For example, carbon dioxide, benzene,
toluene,
ethyl benzene, xylenes, sulfur compounds, metals, and/or other impurities or
potential
catalyst poisons may be removed from syngas feedstream 100 by conventional
methods known to one of ordinary skill in the art.
[00131] In some variations, syngas feedstream 100 comprises H2 and CO at an
H2/CO ratio having a value between about 0.5 to about 3.0, about 1.0 to about
1.5, or
about 1.5 to about 2Ø The H2/CO ratio in feedstream 100 can, in some
variations,
affect the yield of methanol and other products in reactor 105. The preferred
H2/CO
ratio in such variations may depend on the catalyst or catalysts used in
reactor 105 as
well as on the operating conditions. Consequently, in some variations, the
production
and/or subsequent conditioning of syngas feedstream 100 is controlled to
produce
syngas having a H2/CO ratio within a range desired to optimize, for example,
production of methanol, ethanol, or both methanol and ethanol.
[00132] Syngas feedstream 100 may optionally be pressurized and/or heated by
compressors and heaters (not shown) prior to entering reactor 105. In some
variations, syngas feedstream 100 enters reactor 105 at a temperature of about
300 F
to about 600 F and at a pressure of about 500 psig to about 2500 psig.
[00133] Reactor 105 may be any type of catalytic reactor suitable for the
conversion of syngas to methanol, alcohol mixtures comprising methanol, higher
alcohols, and/or other products. Reactor 105 may be any suitable fixed-bed
reactor,
for example. In some variations, reactor 105 comprises tubes filled with one
or more
catalysts. Syngas passing through the tubes undergoes catalyzed reactions to
form
methanol and, in some variations, higher alcohols or other products. In some
-30-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
embodiments, catalysis occurs within pellets or in a homogeneous phase.
Reactor 105
may operate, for example, at temperatures of about 400 F to about 700 F and at
pressures of about 500 psig to about 2500 psig.
[00134] Any suitable catalyst or combination of catalysts may be used in
reactor 105 to catalyze reactions converting syngas to methanol and,
optionally, to
higher alcohols and/or other products. Suitable catalysts may include, but are
not
limited to, one or more of ZnO/CrzO3, Cu/ZnO, Cu/ZnO/Alz03, Cu/ZnO/CrzO3,
Cu/Th02, Co/Mo/S, Co/S, Mo/S, Ni/S, Ni/Mo/S, Ni/Co/Mo/S, Rh, Ti, Fe, Ir, and
any
of the foregoing in combination with Mn and/or V. The addition of basic
promoters
(e.g. K, Li, Na, K, Rb, Cs, and Fr) increases the activity and selectivity of
some of
these catalysts for alcohols. Basic promoters include alkaline-earth and rare-
earth
metals. Non-metallic bases can also serve as effective promoters in some
embodiments.
[00135] In some variations, up to about 50% of CO in syngas feedstream 100 is
converted to methanol in reaction zone 110. Intermediate product stream 115
output
from reactor 105 may comprise, in some variations, about 5% to about 50%
methanol,
about 5% to about 50% ethanol, about 5% to about 25% CO, about 5% to about 25%
H2, and about 2% to about 35% COz, as well as other gases. In some
embodiments,
intermediate product stream 115 also comprises one or more higher alcohols,
such as
ethanol, propanol, or butanol.
[00136] The H2/CO ratio in intermediate product stream 115 can, in some
variations, affect the yield of ethanol and other products in reactor 120. The
preferred
H2/CO ratio in such variations may depend on the catalyst or catalysts used in
reactor
120 as well as on the operating conditions. The H2/CO ratio in intermediate
product
stream 115 can differ from that of feedstream 100 as a result of reactions
occurring in
reactor 105. In some variations, the H2/CO ratio of intermediate product
stream 115
provides a higher ethanol yield in reactor 120 than would the H2/CO ratio of
feedstream 100. In such variations, operation of reactor 105 to produce
methanol, for
example, improves the H2/CO ratio of the syngas fed to reactor 120 from the
standpoint of ethanol yield in reactor 120.
[00137] In one example, feedstream 100 comprises syngas with an H2/CO ratio
of about 1.5 to about 2, and the preferred H2/CO ratio for production of
ethanol in
-31-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
reactor 120 is about 1. Operation of reactor 105 to produce methanol, in this
example,
depletes Hz in the syngas which decreases the H2/CO ratio in intermediate
product
stream 115 to a value closer to 1 and thus improves the ethanol yield in
reactor 120.
In certain embodiments, the catalyst is a Cu/ZnO/alumina catalyst.
[00138] Reactor 120 may be any type of catalytic reactor suitable for the
conversion of syngas, methanol, and/or syngas plus methanol to ethanol and,
optionally, to higher alcohols and/or other products. Reactor 120 may be any
suitable
fixed-bed reactor, for example. In some variations, reactor 120 comprises
tubes filled
with one or more catalysts. Syngas and/or methanol passing through the tubes
undergoes surface catalyzed reactions to form ethanol and, in some variations,
higher
alcohols and/or other products. While not intending to be bound by any
particular
theory, it is presently believed that the methanol may be converted to syngas
and
thence to ethanol, the methanol may be converted directly to ethanol via a
homologation reaction, and/or the methanol may be converted to ethanol by
other
mechanisms. Reactor 120 may operate, for example, at temperatures of about 500
F
to about 800 F and at pressures of about 500 psig to about 2500 psig.
[00139] Any suitable catalyst or combination of catalysts may be used in
reactor 120 to catalyze reactions converting syngas, methanol, and/or syngas +
methanol to ethanol and, optionally, to higher alcohols and/or other products.
Suitable catalysts may include, but are not limited to, alkali/ZnO/Crz03,
Cu/ZnO,
Cu/ZnO/Al2O3, CuO/CoO, CuO/CoO/Al2O3, Co/S, Mo/S, Co/Mo/S, Ni/S, Ni/Mo/S,
Ni/Co/Mo/S, Rh/Ti/Si02, Rh/Mn/SiO2, Rh/Ti/Fe/Ir/SiO2, Rh/Mn/MCM-41, Cu, Zn,
Rh, Ti, Fe, Ir, and mixtures thereof. The addition of basic promoters (e.g. K,
Li, Na,
Rb, Cs, and Fr) may increase the activity and selectivity of some of these
catalysts for
ethanol or other Cz+ alcohols. Basic promoters include alkaline-earth and rare-
earth
metals. Non-metallic bases can also serve as effective promoters, in some
embodiments.
[00140] In some embodiments, catalysts for reactor 120 include one or more of
ZnO/CrzO3, Cu/ZnO, Cu/ZnO/AlzO3, CuO/CoO, CuO/CoO/A1z03, Co/S, Mo/S,
Co/Mo/S, Ni/S, Ni/Mo/S, Ni/Co/Mo/S, Rh/Ti/Si0z, Rh/Mn/SiOz, Rh/Ti/Fe/Ir/SiOz,
Rh/Mn/MCM-41, Ni/Mo/S, Ni/Co/Mo/S, and any of the foregoing in combination
-32-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
with Mn and/or V. Again, any of these catalysts can (but do not necessarily)
include
one or more basic promoters.
[00141] Product stream 130 output from reactor 120 may comprise, in some
variations, about 0% to about 50% methanol, about 10% to about 90% ethanol,
about
0% to about 25% CO, about 0% to about 25% H2, and about 5% to about 25% COz,
as well as other gases. In some embodiments, product stream 130 also comprises
one
or more higher alcohols, such as propanol or butanol.
[00142] Referring again to FIG. 7, in some variations unreacted syngas in
product stream 130 is separated from product stream 130 to form feedstream 135
and
recycled through reactor 120 to further increase, for example, the yield of
ethanol
and/or other desired products. Alternatively, or in addition, in some
variations
unreacted syngas in product stream 130 is recycled through reactor 105 by
adding it to
syngas feedstream 100. The latter approach may be unsuitable, however, if the
unreacted syngas in product stream 130 is contaminated, for example, with
sulfur,
sulfur compounds, metals, or other materials that can poison methanol
catalysts in
reactor 105.
[00143] Also, in some variations a methanol feedstream 140 is added to
intermediate product stream 115 or otherwise introduced to reactor 120 to
further
increase, for example, the yield of ethanol and/or other desired products. For
example, methanol in product stream 130 may be separated (not shown) from
product
stream 130 to form feedstream 140 and then recycled through reactor 120.
Methanol
from other sources may be introduced into reactor 120, as well or instead.
[00144] In some variations, one or more catalysts in reactor 105, one or more
catalysts in reactor 120, or one or more catalysts in both reactor 105 and
reactor 120
catalyze the conversion of COz to methanol. Production of methanol in reactor
105,
reactor 120, or in both reactors may be thereby enhanced by consumption of COz
present in syngas feedstream 100. Consequently, in some variations, COz is
added to
syngas feedstream 100, or the production and/or subsequent conditioning of
syngas
feedstream 100 is controlled to produce syngas having a desirable amount of
COz.
Suitable catalysts for converting COz to methanol may include, in some
variations,
one or more of those listed above for use in reactors 105 and 120. Enhanced
production of methanol by consumption of COz may result, in some variations,
in
-33-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
enhanced production of ethanol by conversion of the methanol to ethanol and/or
by a
resulting favorable adjustment of the H2/CO ratio in the syngas stream
introduced to
reactor 120.
[00145] Referring now to FIG. 8, some alternative variations differ from those
described above primarily by use of a single reactor 200 comprising a first
reaction
zone 205 and a second reaction zone 810 rather than two reactors. Syngas
feedstream
100 is introduced into first reaction zone 205, where one or more catalysts
convert at
least a portion of syngas feedstream 100 to methanol to provide intermediate
product
stream 115 (comprising at least a portion of the unreacted syngas from
feedstream
100, methanol and, in some variations, higher alcohols and/or other reaction
products). At least a portion of intermediate product stream 115 is introduced
into
second reaction zone 810, where one or more catalysts convert at least a
portion of
syngas from intermediate product stream 115 and/or at least a portion of
methanol
from intermediate product stream 115 to form product stream 130 comprising
ethanol
and, in some variations, methanol, higher alcohols, other reaction products,
and /or
unreacted syngas from intermediate product stream 115.
[00146] Reactor 200 may be any type of suitable catalytic reactor comprising
two or more reaction zones. Operation of reactor 200 may be similar to the
operation
of reactors 105 and 120 described above. In particular, in some variations,
the
catalysts used in reactions zones 205 and 810 and the operating conditions for
the
reaction zones are the same as or similar to those for, respectively, reaction
zones 110
and 120 described above. The compositions of intermediate product stream 115
and
product stream 130 may, in some variations, be the same as or similar to those
for the
variations described above with respect to FIG. 7. Syngas in product stream
130 may
be recycled through reaction zone 810 or added to feedstream 100. COz may be
added to syngas feedstream 100, or the production and/or subsequent
conditioning of
syngas feedstream 100 may be controlled to produce syngas having a desirable
amount of COz for enhanced methanol production. A methanol feedstream (not
shown) may be introduced to reaction zone 810 to further increase, for
example, the
yield of ethanol and/or other desired products. This methanol feedstream may
be, for
example, separated from product stream 130.
-34-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
[00147] Similarly to the two-reactor variations, in some of the single-reactor
variations the H2/CO ratio in intermediate product stream 115 can affect the
yield of
ethanol and other products in reaction zone 810. In some variations, the H2/CO
ratio
of intermediate product stream 115 differs from that of feedstream 100 and
provides a
higher ethanol yield in reaction zone 810 than would the H2/CO ratio of
feedstream
100. In such variations, production of methanol in reaction zone 205, for
example,
improves the H2/CO ratio of the syngas fed to reaction zone 810 from the
standpoint
of ethanol yield in reactor 120.
[00148] Referring now to FIG. 9, some alternative variations differ from those
described with respect to FIG. 7 in that at least a portion (some or
substantially all) of
the methanol in intermediate product stream 115 is diverted into a methanol
product
stream 300 prior to the introduction of product stream 115 into reactor 120.
Methanol
in product stream 300 can be separated and purified by conventional methods,
for
example. As above, in some of these variations the H2/CO ratio of intermediate
product stream 115 differs from that of feedstream 100 and provides a higher
ethanol
yield in reactor 120 than would the H2/CO ratio of feedstream 100. Hence, the
production of methanol in reactor 105 may advantageously enhance ethanol
production in reactor 120 in some of these variations.
[00149] In some variations methanol is produced at high yield in a first
reactor
and subsequently converted to ethanol in a second reactor. Referring to FIG.
11, for
example, in some variations a syngas feedstream 100 is catalytically converted
to
methanol in a first reactor 105 at a yield (mole conversion of CO to methanol)
of, for
example, at least 50%, 75%, 85%, 95%, or higher, subject to equilibrium
constraints.
High methanol yields may be facilitated, for example, by separating out some
or
substantially all of the non-methanol components in intermediate product
stream 115
as a stream 500 that is recycled through reactor 105.
[00150] An unrecycled portion of intermediate product stream 115, rich in
methanol, is (optionally) mixed with another syngas feedstream 510 to provide
feedstream 515 which is introduced into reactor 120. At least a portion of the
methanol and (optionally) syngas introduced into reactor 120 are catalytically
converted to provide a product stream 130 comprising ethanol and, in some
variations, methanol, higher alcohol, other reaction products, and/or
unreacted syngas
-35-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
from feedstream 515. In some variations, unreacted syngas in product stream
130 is
recycled through reactor 120 as feedstream 135 and/or recycled through reactor
105.
Various components of product stream 130 may be separated out and/or purified
as
described above, for example.
[00151] In some variations, the ratio of methanol to CO in a feedstream may be
adjusted, for example, to optimize the yield of ethanol in reactor 120. In
some
embodiments, the ratio of methanol/CO in reactor 120 is between about 0.5 to
about
2Ø In particular embodiments, the ratio of methanol/CO in reactor 120 is
about 1Ø
[00152] Any suitable catalyst or combination of catalysts may be used in
reactor 105. Suitable catalysts for reactor 105 may include, but are not
limited to, the
methanol catalysts listed above. Similarly, any suitable catalyst or
combination of
catalysts may be used in reactor 120. Suitable catalysts for reactor 120 may
include,
but are not limited to, the ethanol catalysts listed above.
[00153] The composition of catalysts in reactors 105 and 120, or reaction
zones
110 and 125, can be similar or even the same. Reference to a "first catalyst"
and
"second catalyst" in conjunction with reaction zones is a reference to
different
physical materials, not necessarily a reference to different catalyst
compositions.
[00154] In variations of any of the methods described herein that use a first
reaction zone and a second reaction zone, the initial syngas stream is
introduced into
both the first reaction zone and the second reaction zone, such as the
independent
introduction of syngas into both the first reaction zone and the second
reaction zone.
In some embodiments, the syngas is from an external source. In some
embodiments,
the syngas is from any of the methods described herein (such as residual
syngas from
a first reaction zone or a second reaction zone).
[00155] In some embodiments of any of the methods described herein, syngas
from any source is added to the first reaction zone and/or the second reaction
zone. In
some embodiments of any of the methods described herein, methanol from any
source
is added to the second reaction zone.
[00156] Certain embodiments employ a plurality of physical reactors in one or
both of the reaction zones. For example, the first zone could consist of two
reactors,
followed by a single reactor as the second zone. Or, in another example, the
first zone
could be one reactor followed by two reactors in the second zone. In general,
any
-36-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
"zone" or "reaction zone" can contain a fraction of one, two, three, or more
physical
reactors.
[00157] In some embodiments of any of the methods described herein, reaction
conditions (such as temperature and pressure) used for the conversion of
syngas to
methanol, the conversion of syngas and/or methanol to ethanol, or the
homologation
of methanol to ethanol, are the same as those described in any of U.S. Patent
Nos.
4,371,724; 4,424,384; 4,374,285; 4,409,405; 4,277,634; 4,253,987; 4,233,466;
and
4,171,461. If desired, one skilled in the art can adjust reaction conditions
using
standard methods to improve the production of methanol and/or ethanol.
[00158] FIG. 10 shows another example, in more detail than above, of a
process in which syngas is catalytically converted to methanol in a first
reactor, and
methanol and residual syngas from the first reactor are converted to ethanol
in a
second reactor. Referring now to FIG. 10, a single two-stage inter-cooled
reciprocating compressor 405 compresses syngas feedstream 400 to about 1500
psig
and feeds it at a temperature of about 135 F to syngas preheater 410.
Preheater 410 is
a shell and tube heat exchanger that uses steam as an enthalpy source.
[00159] Heated syngas 415 from preheater 410 is sent to a set of reactor guard
beds 420, 425. Guard beds 420, 425 can be configured in a permanent lead-lag
arrangement but are piped such that either bed can be bypassed. The piping
arrangement allows one bed to be in service while the other is being
regenerated or
activated. Regeneration/activation is initiated by a mixed hydrogen and
nitrogen line
(not shown). Guard beds 415, 420 remove, for example, sulfurs and metals that
may
poison the methanol catalysts. In some embodiments, one or more catalyst
poisons
are removed by adsorption over copper, copper chromite, nickel, cobalt, or
molybdenum. These and other metals can be supported on high-surface-area
refractory inorganic oxide materials such as alumina, silica, silica/alumina,
clays, or
kieselguhr. One exemplary material is copper on alumina.
[00160] Exit gases 430 from guard beds 420, 425 are sent to an alcohol reactor
cross exchanger 435 at about 350 F and are heated to about 480 F during heat
exchange with crude alcohol exit gases 470 from second alcohol reactor 460.
[00161] Syngas at about 1500 psig and about 480 F enters a first alcohol
synthesis reactor 440, where at least a portion of the syngas undergoes a
surface-
-37-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
catalyzed reaction in supported catalyst tubular reactors within the reactor
vessel. In
some variations, the catalyst in reactor 440 is a Cu/ZnO/alumina catalyst.
Methanol is
expected to be formed via the reaction CO + 2H2 ---> CH3OH. In some variations
methanol may be formed, as well, by hydrogenation of COz.
[00162] Product gases 4501eave alcohol synthesis reactor 440 at a temperature
of about 500 F and enter alcohol synthesis reactor 460. In addition, a
methanol
stream 465 (e.g., a methanol recycle stream separated from crude alcohol
stream 470)
is mixed with the product gases 450 from reactor 440 and also introduced to
reactor
460. Reactions occurring in reactor 460 include ethanol formation at about a
40%
molar conversion basis of methanol entering reactor 460.
[00163] Crude alcohol stream 470 exits reactor 460 at a temperature of about
650 F and is cooled by heat exchange in alcohol reactor cross exchanger 435 to
a
temperature of about 530 F. Subsequent heat recovery and other cooling steps
(not
shown) cool crude alcohol stream 470 to about 100 F.
[00164] Ethanol, methanol, residual syngas, and other components of crude
alcohol stream 470 may be separated and (optionally) purified by using the
methods
described herein or using conventional methods (not shown). Syngas recovered
from
stream 470 may be recycled through the reactors by mixing it with syngas
feedstream
400, for example.
[00165] In some embodiments, ethanol is purified from the product stream 130
or crude alcohol stream 470 by first drying the product stream 130 or crude
alcohol
stream 470 to produce an intermediate product, and then distilling the
intermediate
product to produce a purified ethanol product. In some embodiments, the
product
stream 130 or crude alcohol stream 470 comprises or consists of ethanol,
methanol,
propanol, butanol, and water. In some embodiments, product stream 130 or crude
alcohol stream 470 includes one or more of the following alcohols: 1-propanol,
2-
propanol, 1-butanol, 2-butanol, t-butanol, pentanols, hexanols, heptanols, and
octanols, and/or higher alcohols. In some embodiments, product stream 130 or
crude
alcohol stream 470 includes one or more aldehydes, ketones, and/or organic
acids
(such as formaldehyde, acetaldehyde, acetic acid, and the like).
[00166] In particular embodiments, the drying step reduces the amount of water
in the product stream 130 or crude alcohol stream 470 by at least about 75%,
95%, or
-38-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
more. In particular embodiments, the amount of the water is less than or equal
to
about 5% or preferably about 0.5% of the intermediate product by weight.
[00167] In some embodiments, the drying step involves passing the product
stream 130 or crude alcohol stream 470 through a membrane, such as zeolite
membrane, or through one or more molecular sieves to produce an intermediate
product. Conventional distillation methods can be used to distill the
intermediate
product. In some embodiments, the distillation conditions are adjusted using
standard
methods based on the contents and/or purity of the distilled product being
produced to
increase the purity of ethanol in the final product. In some embodiments,
ethanol is
between about 95% to about 99.9% of the purified ethanol product by weight.
[00168] In some embodiments of the invention, one or more parameters are
varied to improve or optimize the generation of syngas or downstream products
(such
as ethanol). For example, one or more parameters can be adjusted during the
conversion of a feed material to syngas. In some embodiments, a feed material
is
converted to syngas using one set of conditions, and then the method is
repeated for
the same type of feed material, or another type of feed material, under a
different set
of conditions to improve the production of syngas. Standard statistical
methods can
be used to help determine which parameters to vary and how to vary them. In
general, economics will dictate the selection of process parameters.
[00169] In some embodiments, one or more of the following parameters are
varied: type of feed material, composition of feed material, amount of oxygen,
location(s) in which oxygen is added, amount of steam, location(s) in which
steam is
added, ratio of oxygen to steam, temperature profile, pressure profile, type
of catalyst,
composition of catalyst, catalyst concentration profile, location(s) in which
catalyst is
added, catalyst activity, average residence time, and residence-time
distribution.
Initial values or ranges for any of these input parameters can be selected
based on the
values described herein.
[00170] In some embodiments, the variation in one or more of these parameters
improves one or more of the following: yield of the syngas; rate of conversion
to the
syngas; ratio of Hz/CO in the syngas at one or more points; average and/or
dynamic
concentration profiles of CO, H2, 02, C02, H20; output catalyst composition;
overall
and/or unit-specific energy balance; overall and/or unit-specific mass
balance;
-39-
CA 02693680 2010-01-08
WO 2009/009388 PCT/US2008/069070
economic output; yield of one or more products from syngas, such as C2-C4
alcohols
(e.g., more particularly, ethanol); product selectivity; or rate of production
of one or
more desired compounds.
[00171] In this detailed description, reference has been made to multiple
embodiments of the invention and non-limiting examples relating to how the
invention can be understood and practiced. Other embodiments that do not
provide
all of the features and advantages set forth herein may be utilized, without
departing
from the spirit and scope of the present invention. This invention
incorporates routine
experimentation and optimization of the methods and systems described herein.
Such
modifications and variations are considered to be within the scope of the
invention
defined by the claims.
[00172] Where methods and steps described above indicate certain events
occurring in certain order, those of ordinary skill in the art will recognize
that the
ordering of certain steps may be modified and that such modifications are in
accordance with the variations of the invention. Additionally, certain of the
steps may
be performed concurrently in a parallel process when possible, as well as
performed
sequentially.
[00173] Therefore, to the extent that there are variations of the invention,
which
are within the spirit of the disclosure or equivalent to the inventions found
in the
appended claims, it is the intent that this patent will cover those variations
as well.
The present invention shall only be limited by what is claimed.
-40-