Note: Descriptions are shown in the official language in which they were submitted.
CA 02693906 2010-01-15
WO 2009/015234
PCT/US2008/070931
1
POST-IMPLANT LENS POWER MODIFICATION
BACKGROUND OF THE INVENTION
[0001] Diseases of the eye, such as cataracts, can cause the lens to become
progressively
opaque over time, which can lead to blindness. The lens can be removed and
replaced with
an intraocular lens ("IOL") which helps the eye focus light on the retina.
Accommodating
IOLs attempt to provide the eye with accommodating abilities similar to the
natural lens.
After implantation of an IOL in the eye, there may be a need for a post-
implant adjustment to
the IOL. There are a number of reasons a post-implant adjustment to the IOL
may be
required. For example, it has been noted that there is some patient-to-patient
variability in
lens capsule size. To ensure an appropriate fit within the capsule, the size
and/or volume of
the IOL may need to be adjusted after implantation. It has been noted that
there is a healing
response (which may vary from patient-to-patient) from the capsule after IOL
implantation in
which the lens capsule contracts around the IOL. It may be desirable to adjust
the volume of
the IOL after implantation to accommodate for this contraction. In addition,
the IOL itself
may change over time. For example, the power of a flowable media-filled (such
as a fluid)
accommodating IOL may change over time due to leakage or diffusion (the rate
of which can
be very slow) of fluid either out of the IOL (the fluid within the IOL
diffusing into the eye) or
into the IOL (e.g., aqueous humor diffusing into the IOL). The above mentioned
post-
implant modifications generally adjust the volume of the IOL (and in some
cases the power
of the IOL).
[0002] In addition, post IOL implant refractive surgery is not always
successful and it may be
easier to adjust the IOL power rather correct the cornea's power. It may also
be necessary to
use the IOL to adjust the optic power for changes that occur to the cornea
over the lifetime of
the patient. It may also be necessary to adjust the IOL if unforeseen damage
to the IOL
occurs (e.g., during the implant procedure). In some cases the initial
biometry may not be
correct; the physician may implant a device with incorrect base power,
therefore necessitating
a power change.
SUMMARY OF THE INVENTION
[0003] One aspect of the invention is a method of adjusting an optical
parameter of an
accommodating intraocular lens. The method includes providing an accommodating
intraocular lens comprising an optic portion in fluid communication with a
peripheral portion,
wherein mnvement nf fluid between the nerinheral nnrtinn anti the nntio
nnrtinn in recnnnce
CA 02693906 2010-01-15
WO 2009/015234
PCT/US2008/070931
2
to ciliary muscle movement changes the optical power of the lens. The method
also includes
altering fluid pressure within a portion of the intraocular lens such that the
intraocular lens, in
response to ciliary muscle movement, has a first optical power, and wherein
after the fluid
pressure has been altered, the intraocular lens, in response to the same
ciliary muscle
movement, has a second optical power different than the first optical power.
[0004] In some embodiments altering fluid pressure within a portion of the
intraocular lens
comprises increasing the fluid pressure within the optic portion and
decreasing the fluid
pressure in the peripheral portion. Altering the fluid pressure can comprise
moving a portion
of the fluid from the peripheral portion to the optic portion.
[0005] In some embodiments altering fluid pressure within a portion of the
intraocular lens
comprises increasing the fluid pressure within the peripheral portion and
decreasing the fluid
pressure within the optic portion. Altering the fluid pressure can comprise
moving a portion
of the fluid from the optic portion to the peripheral portion.
[0006] In some embodiments altering fluid pressure within a portion of the
intraocular lens
comprises allowing a fluid to diffuse from the peripheral portion of the
intraocular lens into
the eye.
[0007] In some embodiments altering fluid pressure within a portion of the
intraocular lens
comprises allowing fluid in the eye to diffuse into the peripheral portion of
the intraocular
lens.
[0008] One aspect of the invention is a method of adjusting an accommodating
intraocular
lens after implantation. The method includes implanting an accommodating
intraocular lens
in a lens capsule, wherein the accommodating intraocular lens changes power in
response to
ciliary muscle movement, and transferring a fluid media between a non-optic
portion and an
optic portion of the intraocular lens, wherein transferring the fluid media is
not in response to
ciliary muscle movement.
[0009] In some embodiments transferring a fluid media is in response to an
external energy
source, such as a laser.
[0010] In some embodiments transferring a fluid media comprises activating a
pressure relief
mechanism. Activating a pressure relief mechanism can cause the pressure
relief mechanism
to deform.
[0011] One aspect of the invention is a method of adjusting an intraocular
lens after
implantation. The method includes replacing a native lens with an intraocular
lens, wherein
the intraocular lens has a volume, and adjusting the volume of the intraocular
lens from a first
CA 02693906 2010-01-15
WO 2009/015234
PCT/US2008/070931
3
[0012] In some embodiments adjusting the volume of the intraocular lens
comprises moving
fluid media from within the intraocular lens to outside of the intraocular
lens. Moving fluid
media can comprise allowing the fluid media within the intraocular lens to
diffuse out of the
intraocular lens based on a pressure difference between the fluid media and
the eye.
[0013] In some embodiments adjusting the volume of the intraocular lens
comprises moving
fluid media from the eye into the intraocular lens. Moving fluid media can
comprise
allowing an eye fluid to diffuse into the intraocular lens based on a pressure
difference
between the fluid media and the eye.
[0014] In some embodiments the intraocular lens is an accommodating
intraocular lens, and
wherein the method further comprises changing the power of the intraocular
lens in response
to ciliary muscle movement.
[0015] One aspect of the invention is a method of altering an optical
parameter of an
accommodating intraocular lens after implantation. The method includes
providing an
accommodating intraocular lens comprising an optic portion in fluid
communication with a
peripheral portion, wherein movement of a fluid between the peripheral portion
and the optic
portion in response to ciliary muscle movement changes the optical power of
the lens. The
method also includes altering an optical parameter of the lens by applying
energy to a portion
of the intraocular lens from outside the patient.
[0016] In some embodiments applying energy to a portion of the intraocular
lens comprises
applying laser energy to a portion of the lens. Applying energy to a portion
of the intraocular
lens can comprise actuating the portion of the intraocular lens with a
surgical tool.
[0017] In some embodiments altering an optical parameter of the intraocular
lens comprises
adjusting the fluid pressure within the lens. Altering an optical parameter of
the lens can
comprise altering the power of the lens.
[0018] One aspect of the invention is an accommodating intraocular lens
adapted for a post-
implant modification. The lens includes an optic portion, a non-optic portion
disposed
peripherally from the optic portion and adapted to engage a lens capsule,
wherein the
intraocular lens is adapted to change power in response to ciliary muscle
movement. The
lens also includes an actuatable element adapted to be actuated by an external
energy source
to change an optical parameter of the intraocular lens.
[0019] In some embodiments the optic portion and the non-optic portion are in
fluidic
communication, and wherein the lens is adapted to move fluid between the optic
portion and
the non-optic portion in response to ciliary muscle movement to change the
power of the lens.
CA 02693906 2010-01-15
WO 2009/015234
PCT/US2008/070931
4
The actuatable element can be disposed within the lens such that upon
actuation the fluid is
moved between the optic portion and the non-optic portion of the intraocular
lens.
[0020] In some embodiments the lens is adapted to move fluid from the optic
portion to the
non-optic portion when the actuatable element is actuated. The actuatable
element can be a
sacrificial plug. The actuatable element can be a deformable element, which
can be disposed
radially between the optic portion and the non-optic portion. The deformable
element can be
a fluid-filled burstable element.
[0021] In some embodiments the deformable element is a shape memory polymer
such as a
heat shrink tube.
[0022] In some embodiments the external energy source is a laser or a surgical
tool.
[0023] One aspect of the invention is an accommodating intraocular lens
adapted for a post-
implant modification. The lens include an optic portion, a non-optic portion
disposed
peripherally from the optic portion and adapted to engage a lens capsule,
wherein the
intraocular lens is adapted to change power in response to ciliary muscle
movement, and
wherein the non-optic portion comprises an outer permeable layer adapted to
allow fluid to
pass through the permeable layer and into the eye.
[0024] In some embodiments the outer permeable layer is adapted to allow fluid
to pass from
the non-optic portion into the eye. In some embodiments the outer permeable
layer is
adapted to allow fluid to pass from the eye into the non-optic portion.
[0025] In some embodiments the lens also has a layer separating the optic
portion and the
non-optic portion, wherein the optic portion comprises a first fluid and the
non-optic portion
comprises a second fluid.
[0026] In some embodiments the non-optic portion further comprises an inner
tubular
member within the permeable layer, wherein the permeable layer defines a
chamber therein
containing a first fluid, and wherein the inner tubular member is in fluid
communication with
the optic portion.
[0027] One aspect of the invention is a method of adjusting a lens capsule
after an intraocular
lens has been implanted therein. The method includes implanting an intraocular
lens within a
lens capsule and adjusting the diameter of the equator of the lens capsule
after implanting the
intraocular lens within the lens capsule, wherein adjusting the diameter of
the equator of the
lens capsule does not occur in response to ciliary muscle movement.
[0028] In some embodiments adjusting the diameter of the lens capsule equator
after
implanting the intraocular lens is in response to a natural capsular
contraction around a
CA 02693906 2013-05-06
52723-34
[0029] In some embodiments adjusting the diameter of the lens capsule equator
comprises
adjusting the volume of the intraocular lens after the intraocular lens is
implanted.
[0030] In some embodiments adjusting the diameter of the equator of the
capsule comprises
adjusting the interaction between an inner surface of the lens capsule and an
outer surface of
5 the intraocular lens. Adjusting the interaction between an inner surface
of the lens capsule
and an outer surface of the intraocular lens can comprise adjusting the
interaction between an
inner surface of the lens capsule and an outer surface of a peripheral portion
of the intraocular
lens.
[0031] In some embodiments adjusting the diameter of the equator of the lens
capsule
comprises actuating the intraocular lens with an external energy source.
[0032] In some embodiments adjusting the diameter of the equator of the lens
capsule
comprises displacing a flowable media from a first portion of the lens to a
second portion of
the lens.
[0032a] One aspect of the invention is an accommodating intraocular lens
adapted for post-
1 5 implant modification, comprising: an optic portion; a non-optic portion
disposed peripherally
from the optic portion and adapted to engage a lens capsule, the non-optic
portion in fluid
communication with the optic portion such that the intraocular lens is adapted
to move fluid
between the optic portion and the non-optic portion in response to ciliary
muscle movement to
change the power of the lens; and an actuatable element adapted to be actuated
by an external
energy source to change an optical parameter of the intraocular lens;
characterised in that the
actuatable element is disposed within the lens such that upon actuation by the
external energy
source fluid is moved from the optic portion to the non-optic portion.
CA 02693906 2014-11-07
52723-34
=
5a =
BRIEF DESCRLPTION OF THE DRAWINGS
[0034] The novel features of the invention are set forth with particularity in
the appended
claims. A better understanding of the features and advantages of the present
invention will be
obtained by reference to the following detailed description that sets forth
illustrative
embodiments, in which the principles of the invention are utilized, and the
accompanying
drawings of which:
[0035] Figures IA and 1B show an exemplary accommodating intraocular lens.
[0036] Figure 2 shows an exemplary accommodating intraocular lens.
[0037] Figure 3 shows an exemplary accommodating intraocular lens adapted for
post-
=
implant modifications.
[00381 Figures 4A and 4B show an exemplary accommodating intraocular lens
which can be
adapted for post-implant modifications.
[0039] Figures 5-7 show an exemplary accommodating intraocular lens which can
be adapted
for post-implant modifications.
=
=
CA 02693906 2010-01-15
WO 2009/015234
PCT/US2008/070931
6
[0040] Figure 8 illustrates a concept of adjusting the volume of fluid in an
intraocular lens
after implantation.
[0041] Figure 9 illustrates a toggle feature than be incorporated into an
intraocular lens to
displace fluid in the lens after implantation.
[0042] Figures 10 and 11 show alternative exemplary accommodating and
diffusing
intraocular lenses.
[0043] Figure 12 shows an accommodating intraocular lens with a layer between
the haptics
and an optic portion.
[0044] Figure 13 illustrates a haptic-within-a-haptic concept for an
intraocular lens.
[0045] Figure 14 illustrates an embodiment showing a cross sectional view of
an exemplary
polymeric haptic.
[0046] Figures 15A and 15B show an intraocular lens with actuatable elements
disposed
between an optic portion and a peripheral portion.
[0047] Figures 16A and 16B illustrate a peripheral portion of the lens that
can be actuated
from a first configuration to a second configuration after the intraocular
lens is implanted.
[0048] Figures 17A and 17B illustrate a peripheral portion of the lens that
can be actuated
from a first configuration to a second configuration after the intraocular
lens is implanted.
[0049] Figures 18A-18C show an intraocular lens with actuatable components
that can be
actuated after implanting the lens.
[0050] Figure 19 shows an intraocular lens with spacers disposed between an
optic portion
and a peripheral portion of the lens.
DETAILED DESCRIPTION OF THE INVENTION
[0051] The inventions relate generally to intraocular lenses ("IOL") such as
accommodating
IOLs. Specifically, the inventions relates to modifications to an IOL after it
has been
implanted (i.e., post-implant).
[0052] There are several reasons a post-implant modification to the IOL may be
needed.
These include, but are not limited to, variations of performance from patient-
to-patient based
on the lens capsule size of the patient; correction for variations of the
healing response
(capsular reaction from one patient to another); power changes in the IOL due
to very slow
leakage or diffusion of a fluid (e.g., a silicone oil) out of the IOL into the
eye, or diffusion or
leakage of a fluid into the eye (e.g., aqueous humor into the IOL); post-IOL
implant
refractive surgery is not always successful and it may be easier to adjust the
IOL power rather
CA 02693906 2010-01-15
WO 2009/015234
PCT/US2008/070931
7
or may not have the range required to adjust the overall optical power; to use
the IOL to
adjust optic power for changes that occur to the cornea over the lifetime of
the patient; and
adjusting the power of the IOL if the IOL is damaged during the implant
procedure.
[0053] A post-implant adjustment may occur only once after implantation or
adjustments
may occur more than once. Some adjustments that occur more than once can occur
periodically or can occur substantially continuously over a period of time,
such as a few
hours, a few days, or over the entire life of the IOL. In addition, the IOLs
can be adapted to
be self-adjusting (i.e., automatically adjusting), or the IOLs can be adjusted
with human
intervention, such as by a health care provider using an external energy
source.
[0054] A post-implant adjustment includes changing a physical parameter of the
IOL, such as
the volume of the lens, diameter of the lens, modulus of elasticity of one or
more of the lens
components, etc. An adjustment also includes changing an optical parameter of
the IOL,
such as the power of the lens. In general it is desired to either adjust the
optical power while
keeping the volume/size fixed, or to adjust the volume/size while keeping the
optical power
fixed. The idea is to adjust the volume to optical power ration to match with
a given capsular
geometry.
[0055] Figures 1A and 1B illustrate a sectional view of an exemplary
accommodating IOL
which incorporates shape memory properties. After the lens has been implanted
into a lens
capsular (after the native lens has been removed), the movement of peripheral
capsular
shaping body 512 from memory shape MS to temporary shape TS will cause
compression of
wall portion 528a against wall portion 528b to displace fluid media M from
interior chambers
522A (collectively) to interior space 522B in lens portion 520 to alter its
curvature to AC'
from AC. The scope of the invention includes any of a variety of mechanisms
and cavity
shapes in non-optic portion 512 that are compressed to cause fluid media flow
to the optic
portion. Also, the scope of the invention includes mechanisms and cavity
shapes in non-optic
portion 512 that are expanded to cause fluid media to flow from the optic
portion. The
interior space in the lens can be (i) centrally located or (ii) peripherally
located in an annular
region to thereby allow the deformation of the surface to add or subtract
power in a plano
lens, positive power lens, or negative power lens. The peripheral extending
portion 512
(there may also be more than one portion 512) carries NiTi forms either of a
thin film
expanse or wire forms to induce portion 512 toward the memory shape as well as
return
chambers 522A to a "memory" volume. It can be seen that a substantial volume
(first
volume) of fluid media M is within the peripheral non-optic portion 512 and
chambers 522A
CA 02693906 2010-01-15
WO 2009/015234
PCT/US2008/070931
8
interior space 522B of the lens.
[0105] In a disacconunodative state shown in Figure 1B, the sectional view
shows body
portion 512 in a tensioned collapsed (temporary) shape when zonular tension
flattens the lens
capsule and collapses the axial dimension of the implant along optical axis
515A. It can be
seen that the axial collapse of the implant causes compression of the
peripheral chambers
522A and moves a volume of fluid media M into space 522B of lens portion 520.
The
increased fluid pressure in space 522B thereby deforms lens surface AC and
subtracts from
the negative power of the lens. It can be easily understood how this added
fluid pressure can
be used to reshape a lens to make a deformable surface, whether (i) to make
the curvature
steeper or flatter with a central interior space 522B or an annular interior
space 522B; (ii) to
add power or subtract power; or (iii) to move a plano element away from non-
refractive
parameters toward either a positive or negative power. It is important to note
that the method
of the invention includes providing a large fluid volume in the peripheral
chambers 522A
when compared to the lens chamber 522B to thereby provide hydraulic
amplification means
for transducing and amplifying the mechanical flexing of body portion 512 to
maximize lens
deformation.
[0056] Figure 2 is a sectional view of an alternative adaptive optic device
500D wherein
flexure of peripheral portion 512 to a flatter shape impinges on the volume of
peripheral
chamber portions 522A to subtract from the power of a bi-convex lens by adding
an index-
matched fluid media to central chamber portion 522B within lens 520. It can be
seen that the
deformable surface AC is restrained at the annular optic periphery by webs 580
to control the
shape change in response to fluid media flow.
[0106] In any design of the capsular shaping body or for an accommodating lens
system, it
may be necessary to provide post-fabrication adjustment means for (i)
adjusting the flexibility
and response to the peripheral body's deformation after implantation, (ii) the
exact shape of a
dimension of the implant to engage the lens capsule, (iii) the amplitude of
accommodation, as
well as (iv) providing for adjustment of lens optic parameters.
[0057] To provide for such post-implant adjustments, Figure 3 shows a cut-away
view of a
capsular shaping body portion 512 and lens portion 510. A plurality of regions
588 of the
capsular shaping body are of a shape memory polymer that is disposed adjacent
to an interior
space or chamber in the implant. Each shape memory polymer portion is
responsive to an
external energy source that causes it to swell to thereby impinge on the
chamber to reduce its
volume (increase internal fluid pressure). While the regions are discrete and
spaced apart in
CA 02693906 2014-11-07
WO 2009/015234
PC17US2008/070931
9
the shape memory polymer regions may extend within broad surface regions of
the capsular
shaping body to alter its modulus or flex characteristics. In particular,
altering the mechanical
properties of the polymer body component can offset and cooperate with the
properties of the
NiTi form therein to alter the resilient characteristics of the composite.
[0059] Figures 4A and 4B illustrate an exemplary embodiment of IOL 20. IOL 20
comprises
optic portion 21 and haptic portion 22. Optic portion 21 is constructed of
light transmissive
materials, while haptic portion 22 is disposed at the periphery of the optic
portion and does
not participate in focusing light on the retina of the eye.
[0060] Optic portion 21 comprises anterior lens element 23, actuator layer 24
including lens
piston 25, substrate 26 and posterior lens element 27, all made of light-
transmissive materials,
such as silicone or acrylic polymers or other biocompatible materials as are
known in the art
of intraocular lenses. Haptic portion 22 illustratively comprises arms 28 and
29 extending
from substrate 26, although other haptic configurations may be employed. Each
of arms 28
and 29 terminates in transducer 30. Transducers 30 preferably each comprise a
haptic piston=
including force-concentrating fin 31, diaphragm 32 and reservoir 33.
Reservoirs 33 are
coupled in fluid communication with the interior of lens piston 25 via
channels 34 that extend
from the reservoirs to well 35 disposed beneath lens piston 25.
[0061] In Figure 4B, transducers 30 are in an undeformed state in which force-
concentrating
= fins 31 apply a maximum deflection to diaphragms 32, thereby fully
deflecting end wall 41
and driving anterior element 23 to the fully accorrunodated position. This
corresponds to a
= fully-contracted state of the ciliary muscles, as described herein below.
[0062] Actuator layer 24 is disposed in recess 36 of substrate 26, and
preferably comprises a
sturdy elastomeric material. Actuator layer 24 isolates the fluid in channels
34, well 35 and
CA 02693906 2010-01-15
WO 2009/015234
PCT/US2008/070931
element 23 and actuator layer 24. Fluids 38 and 39 disposed, respectively,
within channels
34 and space 37, preferably comprise silicone or acrylic oils and are selected
to have
refractive indices that match the materials of anterior lens element 23,
actuator layer 24 and
substrate 26.
[0063] In one embodiment, lens piston 25 includes substantially nondeformable
cylindrical
side wall 40 coupled to expandable end wall 41. End wall 41 is configured to
deflect outward
responsive to pressure applied within sidewall 40 by fluid movement from the
haptic portion.
End wall 41 contacts the interior surface of anterior lens element 23, so that
deflection of end
wall 41 of the lens piston causes a corresponding deflection of anterior lens
surface 23. Such
deflections cause the anterior lens element to assume a spherical shape with a
shorter radius
of curvature, thereby changing the diopter power of the lens. As will of
course be
understood, optic portion could instead be arranged so that the lens piston
deflects posterior
lens element 27; the arrangement depicted in Figure 4A and 4B is illustrative
only.
[0064] The inner surface and thickness of anterior element 23 (relative to the
optical axis of
the lens) are selected so that the outer surface of anterior element 23
retains an optically
corrective shape, e.g., spherical, throughout the entire range of motion of
lens piston 25, e.g.,
for accommodations 0-10 diopters. It should of course be understood that the
inner surface
and thickness of anterior element 23 may be selected to provide an aspherical
outer surface,
as required for a desired degree of optical correction.
[0065] As shown in Figure 4A and 4B, one embodiment of actuator layer 24
includes a single
lens piston 25 located at the center of optic portion 21. Alternative
embodiments of actuator
layer 24' may include an array of lens pistons 25' spaced apart in a
predetermined
configuration on the anterior surface of the actuator layer. As will be
apparent to one of skill
in the art, an annular structure may be substituted for individual lens
pistons, and side walls
40 may be of any desired shape other than cylindrical.
[0066] In accordance with one aspect of the present invention, the volume of
fluid in the
accommodating lens may be selected so that the forces required to provide a
useable range of
accommodation are satisfactory for a preselected population of patients.
Alternatively, the
volume of fluid used in IOL 20 may be specified during manufacture for a given
patient, or
may be adjusted prior to implantation of the IOL on a patient-by-patient
basis. In this manner,
the forces developed by lens piston 25 and haptic pistons 42 may be tailored
for a specific
patient. In addition, the number, shape and placement of lens pistons 25' on
actuator layer 24'
may be selected, e.g., prescribed during manufacture, to optimize
accommodation of the lens
CA 02693906 2014-11-07
WO 2009/015234
PCT/U52008/070931
11
100671 It may been noted that in the undeformed state, transducers 30 maintain
the lens in the
accommodated or'high power state. Accordingly, any failure that allows the
transducers to
assume the undeformed state without any physiologic influence could result in
a residual
near-sighted condition. In accordance with another aspect of the present
invention it would
be advantageous to provide for a mechanism to relieve a small amount of
quiescent pressure
within the lens so that the lens piston assumes the unaccommodated, low power
state.
[0068] To accomplish this result, a relief valve in the form of a sacrificial
plug may de
disposed on a channel that leads to an evacuated cavity. The plug may be
constructed of
material that remodels when activated by a laser to permit a reduction of the
pressure in the
lens piston, and thereby allowing the anterior lens element to assume the
unaccornmodated
state. The plug preferably comprises a colored material that readily and
preferentially
absorbs laser light, for example, 1.06 micron wavelength radiation from a
Nd:YAG laser.
When irradiated, the plug experiences a phase change or otherwise deforms to
permit a
predetermined quantity of fluid in the channel 34 to enter the evacuated
cavity.
[0070] If a post-implant change in lens power or accommodation range occurs
because of a
predictable healing response, the post-implant adjustment that is needed to
compensate for
the power change due to the healing response is first determined. Then the IOL
is
configured, before implantation, to assume a selected desired configuration
(with desired
performance characteristics) after the eye has responded to the implantation
procedure. The
IOL could, for example, have shape memory characteristics to assume the
desired
configuration if a known healing response will cause a known power change in
the IOL due
to the healing process. Alternatively, for example, a fluid chamber within the
IOL could be
under-filled before implantation, and the healing response could squeeze fluid
into the
chamber to its desired state.
[0071] In some embodiments the IOL includes at least one flow control member
such as
valve and/or pump which can be used to control the flow of fluid within or
between portions
of the IOL. Flow control members can be used to draw fluid from one chamber or
reservoir
CA 02693906 2010-01-15
WO 2009/015234
PCT/US2008/070931
12
Such exemplary devices can be found in U.S. Patent No. 6,730,123 to Klopotek.
If a relief
plug is used, the plug can be used to either relieve pressure in the active
fluid system, or a
fluid could be moved from a high pressure reservoir to increase the pressure
in the active
fluid system.
[0072] Nd:YAG lasers can be used to reduce PCO and perform capsulotomies.
Laser
technology can also be used to sculpt and/or reshape the optic surface. This
procedure
requires precise templates and controls. In addition, lasers can be used to
open, close, or flip
a valve, and to generally modify pressure and/or redistribute fluid volumes
within the lens.
The laser could be used, for example, to create a hole in a sacrificial plug
or to treat a shape
memory polymer. Any of these techniques can be used to add or relieve pressure
in the IOL,
or in portions of the IOL.
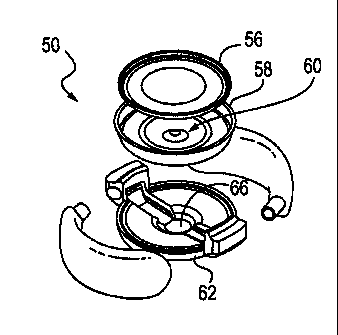
[0073] Figures 5-7 show exemplary accommodating IOL 50. IOL 50 includes a non-
option
peripheral portion which includes haptics 52 and 54. IOL 50 also includes an
option portion
which includes anterior element 56, intermediate layer 58, and posterior
element, or substrate,
62. Intermediate layer 58 includes actuator 60. Haptics 52 and 54 define
interior volumes 64
which are in fluid communication with active channel 66 defined by posterior
element 62 and
intermediate layer 58. Passive chamber 55 which is defined by anterior element
56 and
intermediate layer 58 contains a second flowable media (such as a fluid) that
is not in fluid
communication with the active channel. The haptics engage the capsular bag
such that zonule
relaxation and tightening causes deformation of the haptics, which distributes
a flowable
media (such as a fluid) disposed in the haptics and active channel between the
haptics and the
active channel. When fluid is directed from the haptics to the active channel,
the pressure
increase in the active channel deflects actuator 60, which deflects and
steepens anterior
element 56. This change in curvature of anterior element 56 increases the
power of the IOL.
When the zonules tighten and the capsule is stretched out the flowable media
flows into the
haptics, thus decreasing the curvature of the anterior element. This
disaccommodates the
IOL to a lower power.
[0074] In some embodiments the health care provider can adjust the fluid
volume within the
IOL or portions of the IOL (e.g., a haptic) using a fluid volume adjustment
device which is
adapted to operate with ports on the IOL. In one embodiment a precision
syringe and needle
are used. The needle is directed intraocularly to a septum on the IOL. The
needle is adapted
to pierce the septum and enter a fluid chamber in the IOL to either add fluid
into or withdraw
fluid from the reservoir. The septum is self-sealing such that removal of the
needle through
CA 02693906 2010-01-15
WO 2009/015234
PCT/US2008/070931
13
the self-sealing septum does not cause fluid to be released from the reservoir
through the
needle insertion point.
[0075] Figure 8 illustrates one specific exemplary embodiment of an IOL in
which fluid can
be removed or added to the active fluid channel after implantation. The IOL
comprises at
least two septa spaced a distance apart. The needle first crosses the first
septum and into to a
fluid reservoir. To add fluid to the active fluid (i.e., to increase the
volume of active fluid),
the desired volume of fluid is first withdrawn into the syringe from the
reservoir. Then the
needle continues to be inserted through the second septum and into the active
fluid system.
The fluid is delivered into the active fluid system. Withdrawing the needle
from the active
fluid system through the second septum seals the entry. Fluid can similarly be
withdrawn
from the active fluid and delivered into the fluid reservoir or can be
withdrawn from the IOL
entirely. Optionally the IOL can include a glue or adhesive reservoir, and on
exit the needle
can dispense glue to assist in healing the septum. Alternatively, the fluid
volume adjustment
device could include a second lumen specifically for delivering an adhesive
sealing substance
to the septum to seal the septum.
[0076] Optionally the needle could have multiple lumens that may help
stabilize the lens
while the fluid filling needle enters the implant as described above. For
example, a vacuum
source could create suction in an air lumen to draw the IOL to the distal port
of the air lumen
and help stabilize the lens while the lens is filled with fluid.
[0077] In an alternative embodiment fluid pressure states of the lens can be
adjusted
mechanically. For example, clamps, spacers, small controlled ratchet
movements, or other
mechanical devices can be used to alter the fluid pressure states. These
mechanical devices
can squeeze the haptic(s) or change their orientation to move fluid
appropriately. One
exemplary mechanical device is a toggle feature that is disposed on the
haptic(s) and/or the
optic portion. In its formed, or first, state shown in Figure 9, the toggle,
or dimple, would
occupy space in a fluid chamber. By actuating the toggle to a bi-stable, or
second, state
(deformed state), the movement of the toggle creates more volume for the fluid
and drops the
fluid pressure within the fluid chamber. It can similarly be toggled in the
reverse manner to
increase fluid pressure within the fluid chamber. Several such toggles could
be arranged to
move varying amounts of fluid into or out of the fluid chamber, giving a wider
range of
adjustability in the amount of fluid to be moved.
[0078] Short term, or relatively fast, changes in the IOL fluid pressure, in
response to
capsular shape change, cause the optic portion to change shape and allows the
patient's lens
CA 02693906 2010-01-15
WO 2009/015234
PCT/US2008/070931
14
diffusion, leakage, or physiological changes (such as capsular contraction or
expansion) can
lead to loss of performance of the IOL. The walls of the IOL are generally
very thin and
despite material properties and/or diffusion barriers some long term diffusion
of fluid may
occur. Aqueous humor in the eye can diffuse into or through the IOL, just as
fluid within the
IOL can diffuse out through the IOL into the eye. Pinholes and other tiny
leaks in the IOL
may not be noticeable in the short term, but over an extended period of time,
perhaps even
years, such leaks may slowly cause the pressure in the system to decrease. A
capsule which
contracts over a period of time, can, for example, slowly increase the fluid
pressure by
healing in such a way that it squeezes the haptics are thereby squeezes fluid
into the optic
portion of the lens body. Over a longer period of time (e.g., days, weeks,
month, or years),
these mechanisms can unpredictably alter the volume of fluid and/or fluid
pressure within the
IOL, which can negatively impact the IOL's ability to accommodate in response
to ciliary
muscle relaxation and contraction.
[0079] In addition to maintaining performance of the IOL throughout its
lifetime, lens
capsule size can vary from patient to patient. In a patient with a relatively
small capsule, the
IOL to be implanted may be relatively large, and the fluid volume within the
IOL (or a
portion of the IOL, such as a haptic) may need to be reduced for the IOL to
accommodate
effectively. Similarly, an IOL implanted in a patient with a relatively large
capsule could
benefit from additional fluid being added into the fluid chamber after
implantation so that the
peripheral portion of the IOL makes proper contact with the capsule to allow
for proper
accommodation in response to ciliary muscle movement.
[0080] In one embodiment the IOL has a biocompatible fluid such as saline
and/or aqueous
within the IOL's active channel. While the IOL uses movement of fluid between
the
peripheral portion and an optic portion in response to ciliary muscle movement
to adjust the
optical power of the IOL, the peripheral portion is adapted to automatically
adjust to allow
fluid to slowly diffuse or leak back and forth between the aqueous of the eye
and the active
channel in the IOL. This slow leakage can be accomplished using a material
designed to
allow diffusion, by creating tiny perfusions in the material, or by other
means which allow
slow leakage to occur. Because the IOL accommodates in response to pressure
changes
originating in ciliary muscle movement, the material has a diffusion rate that
is substantially
slower than the IOL's accommodating response. The rate can be, for example, on
the order
of microliters per day or week rather than per second.
[0081] In one embodiment the fluid in the active channel can be a saline
solution and has a
CA 02693906 2010-01-15
WO 2009/015234 PCT/US2008/070931
boundary body, or intermediate layer, thus becomes a lens element because the
active fluid
has a different index of refraction than the intermediate layer. The passive
fluid is also
preferably index matched to the optic polymer components (anterior lens
element, posterior
lens element, and intermediate layer) and therefore the only active (i.e.,
adjusting) lens
element within the IOL is the interface between the intermediate layer and the
active fluid
layer. In addition, the anterior body 'floats,' i.e., is allowed to translate
slightly along the
optical axis. In this embodiment the IOL is designed such that when the
'active' pressure
(i.e., pressure within the active channel and/or haptic fluid chamber) is
equal to the pressure
in the eye, the IOL assumes its optically disaccommodated state. Figures 10
and 11 show
alternative exemplary accommodating and diffusing IOLs. Anterior element 102,
posterior
element 106, and intermediate layer 104 are made of an acrylic material with a
high index of
refraction. Passive fluid 108 is preferably a silicone fluid (e.g., silicone
oil) with the same
index of refraction as the optic components. Active fluid 110 is a saline
and/or aqueous fluid
with a lower relative index of refraction.
[0082] As the eye accommodates, the pressure in the haptic rises, as does the
pressure in the
active channel. The small acrylic intermediate layer flexes in response into
the passive
chamber, as shown by the phantom lines in Figures 10 and 11. The passive fluid
redistributes, and the anterior body may translate forward slightly. Because
the anterior and
posterior bodies are generally rigid, the only body that changes shape in the
intermediate
layer. Because the intermediate layer is an optical surface (due to the
intermediate
layer/saline interface), the power of the IOL changes in response to ciliary
muscle movement.
The fluids in this embodiment are not limited to saline and a silicone fluid,
which are merely
used as examples.
[0083] By combining such long term, or slow scale, diffusion/leak features and
a saline
active fluid, the system can reset itself (i.e., lose pressure) over a longer
period of time (for
example, during sleep) while still holding pressure during the accommodative
cycle. In
addition, the IOL can self-adjust post-implant to different capsule sizes.
Another benefit of
this design is that the thicker anterior and posterior shells can also protect
the delicate
intermediate layer. Furthermore, the intermediate layer can be shaped to
maximize efficiency
and add an aspherical shape.
[00841 In an alternative to the above diffusion system, it may be desirable to
maintain the
same or substantially the same index of refraction in the optic portion and in
the fluid that is
in the optical path (the path through which light passes that is eventually
focused on the
_ _
CA 02693906 2010-01-15
WO 2009/015234
PCT/US2008/070931
16
(as well as the passive fluid) with the optic portions (e.g., the anterior
elements, posterior
elements, and intermediate layer). In this embodiment shown in Figure 12, the
IOL has a
central optic lens assembly or optic portion filled with an index matched
silicone oil 130
(both passive and active fluids). The optic portion is surrounded by haptics
132 which are
filled with saline or aqueous 134 that interfaces with the silicone oil via
nonmiscible layer
136.
[00851 In this design, the haptics are designed such that they leak or diffuse
over a longer
period of time down to zero pressure. Because the saline and silicone oil are
in contact at
layer 136 and the interface layer is allowed to deflect, the pressure in the
active channel (i.e.,
the silicone oil pressure) will generally match the pressure in the haptics
(i.e., the saline
pressure). By having a self-adjusting saline volume, the silicone volume also
becomes self
adjusting. If a portion of the active fluid undesirably leaks out of the IOL
(through the optic
portion), the pressure in the active channel or passive chamber decreases.
Aqueous from the
eye can then slowly diffuse into the haptic and compensate for the loss of
volume of the
active fluid. In this embodiment the saline is restricted to the periphery of
the IOL (i.e., the
haptics) where the index of refraction is not critical as the haptics do not
assume a light-
focusing role.
[0086] It is intended that the IOL shown in Figure 12 includes or can be
adapted to include at
least one actuator or component to locally deflect an anterior element,
similar to the
embodiment shown in Figures 5-7.
[00871 An alternative design creating an interface between the saline and
silicone oil (or
similar alternative fluids) is a `haptic within a haptic' design, as shown in
Figure 13. Inner
haptic 140 containing the active fluid is disposed within outer haptic 142
containing a saline
and/or aqueous solution. The inner haptic is in fluid communication with the
active channel
in the optic portion.
[00881 The outer haptic is generally adapted to automatically adjust to the
size of the
patient's lens capsule (post-implanta) over a long period of time by
transferring the saline
between the eye and the haptic. This transfer can occur by diffusion, leakage,
a mechanical
control device such as a valve, etc. The inner haptic deforms in response to
the pressure in
the outer haptic, which in turn responds to deformation of the lens capsule.
Deformation of
the inner haptic moves fluid into the lens body, which displaces the anterior
element of the
optic to adjust the power of the IOL.
CA 02693906 2010-01-15
WO 2009/015234
PCT/US2008/070931
17
[0089] Therefore, in addition to accommodating in response to ciliary muscle
movement (via
the movement of fluid contained in the inner haptic/active channel) the system
will seek to
equilibrate with the capsule and undergo leakage or diffusion to achieve that
equilibrium.
[0090] The inner haptic can be made of an acrylic or similar composition, and
has a generally
round cross-section (although haptics of other cross sections can be used).
The outer haptic
can be comprised of a silicone material, but can be comprised of other
materials as well.
[0091] Figure 14 illustrates an embodiment showing a cross sectional view of
an exemplary
polymeric haptic 150. In Figure 14, haptic 150 is dimensioned for engaging a
peripheral or
circumferential surface of the anterior and posterior capsular walls. As
defined herein the
anterior surface and posterior surface are radially outward of a central optic
zone of the IOL
that ranges from about 4.5 to about 7.0 mm in diameter. The haptic is
microfabricated with
interior webs or constraining elements 152.
[0092] In one exemplary embodiment, the microfabricated polymer body has
interior webs
that are of a shape-transformable polymer such as a shape memory polymer (SMP)
or a heat-
shrink polymer, either of which are actuatable by a selected wavelength of
light. The interior
of the haptic body comprises any plurality of ordered elastomer open-web
structure wherein
the webs can be oriented in a radially symmetric manner about the axis of the
IOL. The
microfabricated polymer monolith preferably defines an open volume of at least
about 10
percent, at least about 50 percent, or at least about 75 percent. The ordered
structure of any
embodiment can be microfabricated using soft lithography techniques to provide
the "open"
volume as described above.
[0093] The shape of the open volume or pores can be molded in layers and
assembled using
soft lithographic techniques. Such micro-apertures can be microfabricated of a
resilient
polymer (e.g., silicone) by several different techniques, such as REM, TM,
MIMIC,
SAMIM and several others¨collectively given the name of soft lithography. For
example,
microtransfer molding is used wherein an elastomeric polydimethylsiloxane
(PDMS) stamp
has patterned relief on its surface to generate features in the polymer. The
PDMS stamp is
filled with a prepolymer or ceramic precursor and placed on a substrate. The
material is cured
and the stamp is removed. The technique generates features as small as 250 nm
and is able to
generate multilayer systems that can be used to fabricate the implant of the
invention. Replica
molding is a similar process wherein a PDMS stamp is cast against a
conventionally
patterned master. A polyurethane or other polymer is then molded against the
secondary
PDMS master. In this way, multiple copies can be made without damaging the
original
CA 02693906 2010-01-15
WO 2009/015234
PCT/US2008/070931
18
[0094] Another process is known as micromolding in capillaries (MIMIC) wherein
continuous channels are formed when a PDMS stamp is brought into conformal
contact with
a solid substrate. Then, capillary action fills the channels with a polymer
precursor. The
polymer is cured and the stamp is removed. MIMIC can generate features down to
1 pm in
size. Solvent-assisted microcontact molding (SAMIM) is also known wherein a
small amount
of solvent is spread on a patterned PDMS stamp and the stamp is 10 placed on a
polymer,
such as photoresist. The solvent swells the polymer and causes it to expand to
fill the surface
relief of the stamp. Features as small as 60 nm have been produced. Various
microfabricated
polymeric "open" volume structures can be understood to be feasible from
review of any text
on soft lithography, for example as in Xia and Whitesides, Annu. Rev. Mater.
Sci. 1998
28:153-84. In particular, Figures 3(h), 7(a) to 7(f) and 8(a) to 8(f)
illustrate polymeric
microstructures.
[0095] Figures 15A and 15B illustrate an embodiment of an IOL, the volume of
which can be
adjusted after implantation to compensate for a small or shrunken capsule. The
IOL
comprises optic portion 300 which acts as a lens, haptics 302, and cushions
304. Cushions
304 are disposed radially between the optic and the haptics and are filled
with a flowable
media such as a fluid. In this embodiment the cushions are filled with saline
303 (or other
biocompatible fluid). A small capsule (relative to the size of the IOL) or a
capsule that
shrinks and contracts around the IOL after implantation exerts pressure on the
haptics and
pushes fluid (e.g., silicone oil) from within the haptics into active channel
314 in the optic
portion of the lens. This increased pressure in the active channel exerts a
force on actuator
layer 306, which exerts a force on anterior layer 307, which increases the
curvature of
anterior surface 308, thereby changing the power of the lens. To compensate
for this the
cushions are adapted so that they can be actuated, or "popped," which releases
the fluid from
within the cushion and into the eye. Because the haptics are disposed adjacent
the cushions,
when the cushions are popped the pressure in the haptic fluid chamber
decreases. The
pressure differential between the active channel and the haptics causes fluid
within the active
channel to flow from the active channel to the haptics. This reduces the
pressure on the
actuator layer, which causes the anterior surface to reduce in curvature
(i.e., the lens power is
reduced).
[0096] The cushions can be actuated with an external energy source such as a
laser (e.g., an
NG:Yag laser). Alternatively, the external energy source can be a surgical
tool (e.g., a sharp
surgical tool). In an alternative embodiment the cushion can be designed to
automatically
CA 02693906 2010-01-15
WO 2009/015234
PCT/US2008/070931
19
in the cushion must be chosen so as to not damage the eye. A biocompatible
fluid such as
sterile saline can be used.
[0097] In an alternative embodiment, cushions 304 have a permeable wall which
allows the
fluid within the cushion to slowly diffuse or leak from the cushion. If
capsular contraction
occurs after implantation (or the patient has a small lens capsule), the
saline within the
cushion will slowly leak out due to the pressure differential between the
fluid in the cushion
and the fluid in the eye. The system will equilibrate at its zero pressure
disaccommodated
state. In the embodiment in which the cushions are permeable, they may also be
adapted
such that they can be actuated (e.g., "popped") to open them and release the
fluid within.
[0098] Figures 16A and 16B illustrate an alternative embodiment in which the
lens can be
adjusted post-implant. Haptics include bi-stable actuators 420 (similar to the
embodiment
shown in Figure 9) that are remotely actuated from a first configuration to a
second
configuration. When in the second configuration, the actuator increases the
volume of the
haptics and allows more fluid to fluid into them. In Figure 16A the actuator
is a dimple and
is in a first configuration which pushes fluid from the haptic to the active
channel. If after
implantation the physician determines that the optic has too much power (i.e.,
there is too
much fluid in active channel 414), actuators 420 can be actuated (e.g., using
a laser or
inserting a surgical tool through a very small incision) so that it pops out
and changes
configuration to that shown in Figure 16B. This increases the volume in the
haptic (and thus
lowers the pressure in the haptic), and because of the pressure differential,
fluid flows to the
haptics. This relieves pressure in the active channel and decreases the power
of the optic.
Only one haptic may have an actuator. A haptic may also have more than one
actuator for
finer control over the pressure changes in the IOL. In some embodiments the
actuators
comprises a shape memory polymer (SMP) or a heat-shrink polymer, either of
which are
actuatable by a selected wavelength of light.
[0099] Figures 17A and 17B show an alternative embodiment where haptic 540
comprises a
retaining element 592 which retains dimple 594 under tension in the
configuration shown in
Figure 17A. After implantation the retaining element is then actuated (or is
self-actuating) to
break or cut the retaining element, which allows the dimple 594 to assume its
natural or
memory configuration shown in Figure 17B. This relieves the pressure in the
haptic similar
to the embodiment shown in Figures 16A and 16B. In one embodiment the
retaining element
is a filament which can be actuated with an external device (e.g., a laser)
thereby severing the
filament.
CA 02693906 2010-01-15
WO 2009/015234
PCT/US2008/070931
[00100] In an alternative to Figures 17A and 17B, a filament can be wrapped
around the
exterior surface of a haptic (or a portion thereof), which constrains the
fluid within the haptic.
The filament is then actuated with a laser or other external energy source to
sever the
filament. This releases the filament from the haptic and allows the pressure
within the haptic
to further deflect the haptic and change the properties of the haptic in the
region of the
filament.
[00101] Figures 18A - 18C show an alternative embodiment in which the IOL
comprises
spacers 604 which perform similar function to the cushions of Figures 15A and
15B. The
spacer includes web sections 608. If the webs are intact the haptic is held
radially out from
optic portion 600. If the webs are severed, the box section of the spacer
collapses, lowering
the pressure in the active channel of the optic (not shown). If only a portion
of the webs are
severed, then only that section of the spacer collapses, which gives a partial
reduction in
pressure. The physician can therefore severe all or some of the webs to
control the amount of
pressure change as is needed. The two spacers as shown in Figure 18A have a
difference
number of web sections but the spacers can have the same number of web
sections or any
number of web sections. The web section can be externally actuated using any
of the
mechanisms described herein or that are known in the art.
[00102] Figure 19 illustrates an alternative embodiment in which the IOL
includes spacer
sections 704 which can be removed or added through a small surgical incision.
The spacer
sections can be implanted with the device and then removed if necessary to
relieve an over-
pressurized device. Alternatively, the spacer sections 704 could be added to
the device to
increase the pressure in an under-filled device.
[00103] The spacer sections can be of different sizes to allow for more
control to fine tune
the system to give each patient as close to perfect distance vision as
possible while providing
for the maximum accommodation possible.
[00104] In some embodiments an external energy source such as a laser (e.g.,
an Argon
laser) is used to heat a shape memory polymer such as a layer or piece of
shrink tube. By
adjusting the power, duration, and spot size of the laser the shrink tube can
be adjusted to
different configurations (i.e., more or less shrinkage) which creates a system
that can be
adjusted post-implant over a range. This is in contrast to a bi-stable system
which may only
have two settings.
[00105] In one embodiment a layer or portion of shrink tubing is disposed over
or around
one or more haptics. The shrink tubing is then actuated post-implant to shrink
the tubing,
CA 02693906 2010-01-15
WO 2009/015234
PCT/US2008/070931
21
thereby squeezing the haptic and forcing fluid from the haptic into the active
channel of the
optic portion.
[00106] In an alternative embodiment, the structure of which is similar to
that shown in
Figures 15A-15B and Figures 18A-18C, a shape memory polymer spacer is disposed
radially
between the optic portion and the haptics. When actuated with a laser, the SMP
shrinks in
the axial dimensions and gets thicker in the radial dimension. This pushes the
haptics radially
outward, which increases the pressure in the haptics and causes fluid to flow
from the haptics
to the active channel in the optic portion (i.e., increasing the pressure in
the active system).
[00107] In an alternative embodiment a shape memory polymer spacer can be used
to cause
fluid to flow from the optic portion to the haptics post-implant (e.g., due to
capsular
contraction). The SMP spacer is disposed radially between the optic portion
and the haptics.
When actuated with a laser, the SMP shrinks in all dimensions. Because the SMP
spacer is
adjacent to the haptics, the pressure in the haptics decreases, causing fluid
to flow from the
optic portion to the haptic portion, thereby decreasing the power of the lens.
[00108] For any of the proceeding methods, and perhaps only for any of the one-
time
corrections, it is also possible to develop a correction algorithm that
titrates the amount of
adjustment based on patient feedback, autorefractors, wavefront measurements,
or other
techniques.
[00109] The invention also includes methods of making an adjustment to the
interaction
between the IOL and the lens capsule after the IOL has been implanted. In some
embodiments adjustments are made to the volumetric relationship between the
IOL and the
lens capsule. In particular embodiments adjustments are made to the radial
relationship
between the IOL and the lens capsule, and this interaction drives the lens
accommodation.
[00110] In one embodiment the invention includes adjusting the diameter of the
equator of
the lens capsule after implanting the intraocular lens within the lens
capsule. Adjusting the
diameter of the equator of the lens capsule is not in response to ciliary
muscle movement,
meaning the adjustment is not part of the natural accommodative motion.
Adjusting the
diameter of the lens capsule equator after implanting the intraocular lens can
be in response
to the natural capsular contraction around a periphery of the intraocular lens
after implanting
the intraocular lens in the lens capsule. The way to adjust the lens capsule
can be by any of
the post-implant methods described herein. In addition, adjusting the lens
capsule may be
necessary for non-fluid driven accommodating IOLs.
[00111] While preferred embodiments of the present invention have been shown
and
CA 02693906 2010-01-15
WO 2009/015234
PCT/US2008/070931
22
provided by way of example only. Numerous variations, changes, and
substitutions will now
occur to those skilled in the art without departing from the invention. It
should be understood
that various alternatives to the embodiments of the invention described herein
may be
employed in practicing the invention. It is intended that the following claims
define the
scope of the invention and that methods and structures within the scope of
these claims and
their equivalents be covered thereby.