Note: Descriptions are shown in the official language in which they were submitted.
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
SYSTEMS AND METHODS FOR REMOVAL OF HEAVY METAL
CONTAMINANTS FROM FLUIDS
TECHNICAL FIELD
[0001] The present invention relates to systems and methods for treatment of
contaminated fluids, and more particularly, to the removal of heavy metal
contaminants from fluids.
BACKGROUND ART
[0002] Produced fluid, such as water, from offshore oil platforms can contain
toxic heavy metals, for instance, mercury. In the Gulf of Mexico, mercury
levels rarely exceed 100 parts per billion (ppb). However, in the Gulf of
Thailand, the average concentration of mercury in produced water can range
from about 200 ppb to about 2,000 ppb.
[0003] Discharge of mercury into the marine environment in U.S. territorial
waters is currently regulated by the U.S. Environmental Protection Agency
(EPA) under the Clean Water Act via the National Pollutant Discharge
Elimination System permit process. According to environmental standards
under 40 CFR 131.36 for marine environment, limits include about 1800 ppb
for acute exposure and about 25 ppb for chronic exposure. International
standards for mercury discharges in produced water, on the other hand, range
from about 5 ppb in Thailand to about 3 00 ppb in the North Sea.
[0004] Produced water often contains oil that was removed with the water
during the bulk oil/water separation process. As an example, the produced
water from the North Sea fields contains about15-30 parts per million (ppm)
dispersed oil with benzene, toluene, ethylbenzene, and xylene (BTEX);
naphthalene, phenanthrene, dibenzothiophene (NPD), polycyclic aromatic
hydrocarbon (PAH), phenol, and organic acid concentrations ranging from
about 0.06 ppm to about 760 ppm. Additionally, these produced waters contain
toxic heavy metals, such as mercury, cadmium, lead, and copper in
concentrations ranging from less than about 0.1 ppb to about 82 ppb. The
-1-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
presence of a complex mix of constituents coupled with a high concentration of
dissolved salts can present a challenge for heavy metal removal using
currently
available conventional technologies.
[0005] In particular, existing technologies for metal and mercury removal from
diluted wastewater include activated carbon adsorption, sulfur-impregnated
activated carbon, microemulsion liquid membranes, ion exchange, and colloid
precipitate flotation. These technologies may not be suitable for water
treatment because of poor metal loading (e.g., metal uptake less than 20% of
the
mass of the adsorber material) and selectivity, (interference from other
abundant
ions in groundwater). In addition, mercury may be present in species other
than
elemental. So the method must be able to remove these other species, such as
methyl mercury etc. Furthermore, they lack stability for metal-laden products
so that they are not disposable directly as a permanent waste form. As a
result,
secondary tr eatment is required to dispose or stabilize the separated mercury
or
the mercury-laden products. Mercury removal from non-aqueous sludge,
adsorbed liquids, or partially- or fully-stabilized sludges, and mercury-
contaminated soil is difficult because (1) the non-aqueous nature of some
wastes prevents the easy access of leaching agents, (2) some waste streams
with
large volumes make the thermal desorption process expensive, and (3) the
treatment of some waste streams are technically difficult because of the
nature
of the wastes.
[0006] Mercury removal from offgas in vitrifiers and in mercury thermal
desorption processes is usually accomplished through active carbon adsorption.
However, the carbon-based adsorbents are only effective enough to remove 75
to 99.9% of the mercury with a loading capacity equivalent to 1-20% of the
mass of the adsorber material. A last step, mercury amalgamation using
expensive gold, usually is needed to achieve the EPA air release standard. A
carbon bed usually is used later in the offgas system, where the temperature
is
generally lower than 250 F. In the sulfur impregnated carbon process, mercury
is adsorbed to the carbon, which is much weaker than the covalent bond formed
with, for instance, surface functionalized mesoporous material. As a result,
the
-2-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
adsorbed mercury needs secondary stabilization because the mercury-laden
carbon does not have the desired long-term chemical durability due to the weak
bonding between the mercury and active carbon. In addition, a large portion of
the pores in the activated carbon are large enough for the entry of microbes
to
solubilize the adsorbed mercury-sulfur compounds. The mercury loading is
limited to about 0.2 g/g of the materials.
[0007] The microemulsion liquid membrane technique uses an oleic acid
microemulsion liquid membrane containing sulfuric acid as the internal phase
to
reduce the wastewater mercury concentration from about 460 ppm to about 0.84
ppm. However, it involves multiple steps of extraction, stripping,
demulsification, and recovery of mercury by electrolysis and uses large
volumes
of organic solvents. The liquid membrane swelling has a negative impact on
extraction efficiency.
[0008] The slow kinetics of the metal-ion exchanger reaction requires long
contacting times. This process also generates large volumes of organic
secondary wastes. One ion exchange process utilizes DuoliteTM GT-73 ion
exchange organic resin to reduce the mercury level in wastewater from about 2
ppm to below about 10 ppb. Oxidation of the resin results in substantially
reduced resin life and an inability to reduce the mercury level to below the
permitted level of less than about 0.1 ppb. The mercury loading is also
limited
because the high binding capacity of most soils to mercury cations makes the
ion-exchange process ineffective, especially when the large amounts of Ca2+
from soil saturate the cation capacity of the ion exchanger. In addition, the
mercury-laden organic resin does not have the ability to resist microbe
attack.
Thus, mercury can be released into the environment if it is disposed of as a
waste form. In addition to interference from other cations in the solution
besides the mercury-containing ions, the ion exchange process is simply not
effective in removing neutral mercury compounds, such as HgC12, Hg(OH)2,
and organic mercury species, such as methylmercury, which is the most toxic
forn of mercury. This ion-exchange process is also not effective in removing
mercury from non-aqueous solutions and adsorbing liquids.
-3-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
[0009] The reported removal of metal from water by colloid precipitate
flotation
reduces mercury concentration from about 160 ppb to about 1.6 ppb. This
process involves the addition of HCI to adjust the wastewater to pH 1,
addition
of Na2S and oleic acid solutions to the wastewater, and removal of colloids
from
the wastewater. In this process, the treated wastewater is potentially
contaminated with the Na2S, oleic acid, and HCI. The separated mercury needs
further treatment to be stabilized as a permanent waste form.
[00010] Acidic halide solution leaching and oxidative extractions can also be
used in mobilizing mercury in soils. For example KI/12 solutions enhance
dissolution of mercury by oxidization and complexation. Other oxidative
extractants based on hypochlorite solutions have also been used in mobilizing
mercury from solid wastes. Nevertheless, no effective treatment technology has
been developed for removing the mercury contained in these wastes. Since
leaching technologies rely upon a solubilization process wherein the
solubilized
target (e.g. mercury) reaches a dissolution/precipitation equilibrium between
the
solution and solid wastes, further dissolution of the contaminants from the
solid
wastes is prevented once equilibrium is reached. In addition, soils are
usually a
good target ion absorber that inhibits the transfer of the target ion from
soils to
solution.
[00011] The removal of mercury from nonaqueous liquids, adsorbed liquids,
soils, or partially-or-fully-stabilized sludge at prototypic process rates has
been
lacking. This is mainly because the mercury contaminants in actual wastes are
much more complicated than the mercury systems addressed by many
laboratory-scale tests that are usually developed based on some simple mercury
salts. The actual mercury contaminants in any actual wastes almost always
contain multiple species of mercury, including ionic or inorganic mercury
(e.g.,
divalent cation Hg2}, monovalent Hgzz+, and neutral compounds such as HgCI2,
Hg[OH]2,); organic mercury, such as methylmercury (e.g., CH3 HgCH3 or CH3
Hg+) as a result of enzymatic reaction in the sludge; and elemental or
metallic
mercury, because of reduction. Since many laboratory technologies are
developed for only one form or species of inercury, demonstrations using
actual
-4-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
wastes have not been successful.
[00012] Other metals that are of interest for remediation and industrial
separations include but are not limited to silver, lead, arsenic, uranium,
plutonium, neptunium, americium, cadmium and combinations thereof. Present
methods of separation include but are not limited to ion exchangers,
precipitation, membrane separations, and combinations thereof. These methods
usually have the disadvantages of low efficiencies, complex procedures, and
high operation costs.
[00013] Accordingly, it would be advantageous to provide a system and method
that can be used to remove heavy metal contaminants, such as mercury,
cadmium, lead, as well as arsenic from complex waste fluids, such as produced
water, in a significant amount and in a cost effective manner.
SUMMARY OF THE INVENTION
[00014] The present invention, in one embodiment, provides to a system for
removal of heavy metal contaminants from fluids. The system, in an
embodiment, includes a source from which a flow of fluid containing various
species or form of heavy metal contaminants, including elemental species,
organic form, and ionic form may be introduced into the system. The system
also includes a first station for physical separation or removal of the
elemental
species of a targeted heavy metal contaminant from the fluid flow. In an
embodiment, the first station may include a liquid/liquid phase coalescer
having
a coalescing element designed to coalesce or merge small diameter droplets
containing the elemental species of the targeted heavy metal contaminant into
larger droplets, which droplets can thereafter be separated by gravity from
the
fluid flow. The system also includes a second station downstream of the first
station and in fluid communication therewith for adsorptive separation or
removal of the remainder of the elemental species along with the other species
of the predetermined heavy metal contaminant from the fluid flow. The second
station, in an embodiment, includes a reactor having an adsorbent nanomaterial
made from a porous particle. The porous particle, for instance, can be self-
-5-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
assembled monolayer on mesoporous supports (SAMMS). In one embodiment,
the system can include a prefilter station upstream of the first station to
remove
solid contaminants from the fluid flow, so as to prolong the life of the
coaleseer
in the first station. The system can further include, in another embodiment, a
third station downstream of the second station and in fluid communication
therewith for separation or removal of another predetermined contaminant
different from that removed by the second station. This third station may be
designed to also contain an adsorbent nanomaterial including a porous particle
made from self-assembled monolayers on mesoporous supports (SAMMS).
[00015] The present invention, in another embodiment, provides a system for
removal of heavy metal contaminants from fluids. The system, in an
embodiment, includes a source, from which a flow of fluid containing various
species or form of heavy metal contaminants, including elemental species,
organic form, and ionic form may be introduced into the system. The system
also includes a first station for physical separation or removal of the
elemental
species of a targeted heavy metal contaminant from the fluid flow. In an
embodiment, the first station may include a liquid/liquid phase coalescer
having
a coalescing element designed to coalesce or merge small diameter droplets
containing the elemental species of the targeted heavy metal contaminant into
larger droplets, which droplets can thereafter be separated by gravity from
the
fluid flow. The system also includes a second station downstream of the first
station and in fluid communication therewith for adsorptive separation or
removal of the remainder of the elemental species along with the other species
of the predetermined heavy metal contaminant from the fluid flow. The second
station, in an embodiment, includes a vessel along with a filter apparatus
having
an adsorbent nanomaterial made from a porous particle. The porous particle,
for instance, can be made from self-assembled monolayers on mesoporous
supports (SAMMS). In one embodiment, the system can include a prefilter
station upstream of the first station to remove solid contaminants from the
fluid
flow, so as to prolong the life of the coalescer in the first station. The
system
can further include, in another embodiment, a third station downstream of the
-6-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
second station and in fluid communication therewith for separation or removal
of another predetermined contaminant different from that removed by the
second station. This third station may be designed to contain a filter
apparatus
having an adsorbent nanomaterial including a porous particle made from self-
assembled monolayers on mesoporous supports (SAMMS).
[00016] The present invention, in a further embodiment, provides a method for
removal of heavy metal contaminants from fluid. The method includes initially
introducing, into a pathway, a flow of fluid containing heavy metal
contaminants to be removed, including various species of targeted heavy metal
contaminants. Next, the fluid flow may be subject to a physical separation
protocol for removing a targeted heavy metal contaminant from the fluid,
including elemental species of the targeted heavy metal contaminant, so as to
reduce the overall concentration of the targeted heavy metal contaminant from
the fluid flow. Thereafter, the fluid flow having a reduced overall
concentration
of the targeted heavy metal contaminant may be exposed to an adsorptive
separation protocol for removing additional amount of the targeted heavy metal
contaminant from the fluid, including additional amount of the elemental
species along with the other species of the targeted heavy metal contaminant,
so
as to further reduce the concentration of the targeted heavy metal contaminant
to an acceptable level. The method may include, prior to the physical
separation, a prefilter treatment to remove solid contaminants from the fluid
flow. The method may further include, subsequent to the adsorptive separation,
another adsorptive separation to remove either a targeted heavy metal
contaminant different from that targeted in the initial adsorptive separation,
or a
targeted heavy metal contaminant similar to that targeted in the initial
adsorptive separation.
BRIEF DESCRIPTION OF DRAWINGS
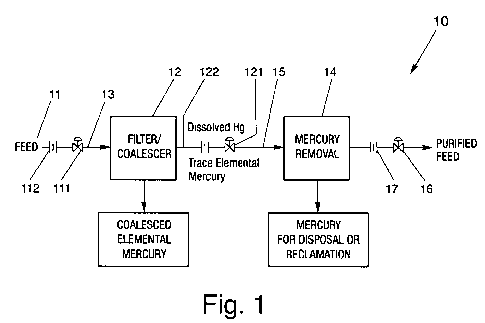
[00017] Fig. I illustrates a system having a first station and a second
station
designed for removal of heavy metal contaminants from fluids in accordance
with one embodiment of the present invention.
-7-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
[00018] Fig. 2 illustrates a liquid/liquid coalescer for use in connection
with the
first station of the system shown in Fig. 1.
[00019] Fig. 3 illustrates a reactor for use in connection with the second
station
of the system shown in Fig. 1.
[00020] Fig. 4A illustrates a prefilter station for use in connection with the
system shown in Fig. 1.
[00021] Fig. 4B illustrates a reactor and filter element for use at the
prefilter
station shown in Fig. 4A.
[00022] Fig. 5 illustrates a third station for use in connection with the
system
shown in Fig. I to permit removal of a contaminant different from that removed
by the first and second stations.
[00023] Fig. 6 illustrates another system having a first and second station
for use
in the removal of heavy metal contaminants from fluids in accordance with the
present invention.
[00024] Fig. 7 illustrates a vessel for use at the second station of the
system
shown in Fig. 6.
[00025] Fig. 8 illustrates a filter element for use in connection with the
vessel
shown in Fig. 7.
[00026] Fig. 9 illustrates a prefilter station for use in connection with the
system
shown in Fig. 6.
[00027] Fig. 10 illustrates a third station for use in connection with the
system
shown in Fig. 6 to permit removal of a contaminant different from that removed
by the first and second stations.
[00028] Fig. 11 illustrates yet another system for use in the removal of heavy
metal contaminants from fluids in accordance with the present invention.
DESCRIPTION OF SPECIFIC EMBODIMENTS
[00029] With reference to Fig. 1, the present invention provides, in one
embodiment, a system 10 for treating contaminated fluid by removing
contaminants that exist within the fluid. Fluids which may be treated in
connection with the present invention may be viscous in nature, such as oil,
or
non-viscous in nature, such as a liquid or a gas. Contaminants that may be
-8-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
removed by system 10 of the present invention include heavy metals, such as
mercury, arsenic, cadmium, and lead from complex waste fluids, such as
produced water, and mercury from a variety of waste solutions and
contaminated waste oils. Other contaminants that may be removed by system
of the present invention includes silver, uranium, plutonium, neptunium,
americium, or a combination thereof.
[00030] The system 10, as illustrated in Fig. 1, includes, in an embodiment, a
source 11 from which a flow of contaminated fluid may be introduced into the
system. The contaminated fluid may contain various species or forms of heavy
metal contaminants, including their elemental form, organic form, and ionic
form. In accordance with one embodiment, the contaminated fluid may be a
waste fluid, such as produced water generated in connection with oil or gas
drilling and may contain various species of, for instance, mercury. Examples
of
the different species of mercury include ionic or inorganic mercury (e.g.,
divalent cation Hg2+, monovalent Hg22+, and neutral compounds such as HgC12,
Hg[OH]2,); organic mercury, such as methylmercury (e.g., CH3 HgCH3 or CH3
Hg') as a result of enzymatic reaction in the sludge; and elemental or
metallic
mercury.
[00031] The contaminated fluid, as shown in Fig. 1, may be introduced into
system 10 at a controlled rate. To control the flow rate of the fluid, a flow
control valve 111 may be provided downstream of source 11. In addition, a
flow-meter 112 may be provided between the source 11 and the control valve
111 to help in determining the flow rate, and if necessary, to permit
adjustment
of the control valve 111 to an appropriate level. It should be noted that
although
system 10 is shown having a control valve 111, such a valve may not be
necessary should the flow rate be capable of being adjusted from the source 11
based on the reading on the flow-meter 112.
,[00032] The system 10 may also include a first station 12 designed to
implement
a physical separation protocol for removal of, for instance, elemental species
of
a targeted heavy metal contaminant, such as mercury, from the fluid flow. In
an
embodiment, first station 12 may be in fluid communication with source 11 via
-9-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
pathway 13, for example, a pipe, a hose, or any similar conduit capable of
conducting fluid flow from source 11 to first station 12.
[00033] Looking now at Fig. 2, the first station 12, in one embodiment, may
include a coalescing unit 20 having at least one coalescing element 21
designed
to coalesce or merge small diameter droplets containing elemental species of
the
heavy metal contaminant into larger droplets for subsequent removal. An
example of a coalescing unit 20 includes a liquid/liquid coalescer, such as
the
Series 110H or Series 110V available from Perry Equipment Corporation in
Mineral Wells, Texas. An example of a coalescing element 21 may be a
PEACHO LiquiSep element, also available from Perry Equipment Corporation
in Mineral Wells, Texas.
[00034] The coalescing unit 20, in an embodiment, may include inlet 22 through
which a continuous flow of contaminated fluid from source 11 via pathway 13
may be received and outlet 23 through which treated fluid may exit. The
coalescing unit 20 may also include a coalescing element 21 designed to permit
the flow of contaminated fluid therethrough and to initiate a physical
separation
process for removal of the elemental species of the heavy metal contaminant
from the fluid flow. In one embodiment, the coalescing element 21 may be
made from a hydrophilic and oleophilic material, so as to permit the fluid
flow
to separate into a discontinuous phase (i.e., colloidal flow) and a continuous
phase (i.e., the process/fluid flow). Moreover, as the design of the
coalescing
element 21 permits the element 21 to implement a saturated depth coalescing
process, when the discontinuous phase moves through the coalescing element
21, substantially small diameter droplets containing elemental species of the
heavy metal in the discontinuous phase may be allowed to coalesce. In
particular, the coalescing element 21 permits "like materials" to attract
"like
materials", so as to take the substantially small diameter droplets containing
the
elemental species of the heavy metal in the discontinuous phase and allow
these
substantially small diameter droplets to merge or combine to form
substantially
larger diameter droplets. The larger diameter droplets, over time, can form
still
larger droplets and eventually can become sufficiently large and heavy. It
-10-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
should be noted that due to the density of the heavy metal within these
sufficiently large droplets, along with interfacial surface tension, these
sufficiently large droplets tend to drain from the coalescing element 21 in
the
presence of gravity, and settle out from the continuous phase to the bottom of
the coalescing unit 20. To collect and subsequently dispose of the drained
liquid containing the elemental species of the heavy metal being separated,
the
coalescing unit 20 may be provided with container 24.
[00035] Although illustrated as being vertical, it should be appreciated that
the
coalescing elements 21 may be designed to be substantially horizontal within
the coalescing unit 20. Similarly, although illustrated as being horizontal,
the
coalescing unit 20 may be designed to be substantially vertical. Moreover,
although the use of a coalescing unit is described herein, physical separation
for
removal of the elemental species of the heavy metal may be carried out by
other
phase separation devices, for example, vanes, mesh pads, packed beds,
centrifuges, other similar devices, or a combination of these.
[00036] The system 10, as illustrated in Fig. 1, may further include a second
station 14 located downstream of the first station 12 and in fluid
communication
with outlet 23 of coalescing unit 20. The second station 14, in an embodiment,
may be provided for adsorptive separation or removal of the additional amount
of the elemental species, along with the other species of the targeted heavy
metal contaminant, such as mercury, from the fluid flow.
[00037] With reference now to Fig. 3, the second station 14, may include a
reactor, such as reactor 30, within which a batch of an adsorbent material may
be accommodated for further treatment of fluid from the first station 12. In
an
embodiment, the adsorbent material may be a nanosorbent material (i.e.,
adsorbent nanomaterial) manufactured from self-assembled monolayers on
mesoporous supports (SAMMS). It should be appreciated that reference to the
term "adsorbent material" hereinafter includes nanosorbent material or
adsorbent nanomaterial, either of which may be used interchangeably with the
other. The mesoporous support, in an embodiment, may be made from various
porous materials, including silica. The advantage of the SAMMS material is its
-11-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
ability to remove all species of various targeted heavy metals, depending on
the
particular functional group associated with the SAMMS material. An example
of a SAMMS material that can be used in connection with the reactor 30 of the
present invention includes thiol-SAMMS (i.e., SAMMS material that have been
functionalized with thiol groups) for targeting all species of mercury,
similar to
that disclosed in U.S. Patent No. 6,326,326, which patent is hereby
incorporated
herein by reference. The SAMMS material, of course may be functionalized
with other groups, depending on the contaminant targeted for removal from the
fluid. For instance, the SAMMS material -may be functionalized with
lanthanum groups for arsenic removal, or with amine groups for CO2 removal.
[00038] In accordance with one embodiment of the present invention, the
adsorbent material may include porous particles, ranging from about 5 microns
to about 200 microns in size. In an embodiment, the particles, on average,
range from about 50 microns to about 80 microns in size, include a pore size
ranging from about 2 nanometers (nm) to about 7 nm, and may be provided
with an apparent density ranging from about 0.2 grams/milliliter to about 0.4
grams/milliliter.
[00039] Although the adsorbent material is disclosed above as being
manufactured from SAMMS, it should be appreciated that other adsorbent
materials may be used, so long as these adsorbent materials can act to remove
contaminants from the fluid flow. One example of an alternate adsorbent
material includes commercially carbon particles ranging from about 8 to about
30 mesh in size.
[00040] To permit ease of introduction into the reactor 30, the adsorbent
material
may be provided as a slurry mixture. In particular, the adsorbent material may
be mixed with a liquid, such as water, to provide the necessary slurry
mixture.
This slurry mixture may, in an embodiment, be maintained in a mixed form
within reservoir 31 by methods known in the art, for example, by any
mechanical devices or fluid injection mechanisms capable of creating a
necessary turbulence. Alternatively, it should be appreciated that as the
slurry
mixture is introduced into reactor 30 via pathway 15, the natural turbulence
of
-12-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
the stream of slurry mixture moving along pathway 15 may be sufficient to
generate the appropriate mixing. Should it be necessary or to further enhance
mixture of the slurry, a mixer (not shown), such as a static mixer
commercially
available through many outlets in the industry, may be provided immediately
downstream of the reservoir 31. The presence of this static mixer can further
optimize the mixing of the slurry as it flow along pathway 15 into reactor 30.
Of course, if desired, instead of using a slurry, dried adsorbent material may
be
provided within the reactor 30.
[00041] The adsorbent material, in an embodiment, may be provided within
reactor 30 prior to the introduction of the fluid flow from the first station
10.
Alternatively, the adsorbent material may be introduced into pathway 15 along
with the fluid from the first station 12 and allowed to mix therewith prior to
entry into reactor 30. In such an embodiment, the amount of adsorbent material
introduced can be critical, since an appropriate amount may need to be
determined in order to provide an optimum heavy metal contaminant removal
capacity. In particular, the amount of adsorbent material that may be needed
can be proportional to the flow rate of the fluid from the first station 12
and the
amount of contaminant within that fluid flow. Generally, the amount of
contaminant will be constant, so that the flow rate of the fluid may be a
parameter which needs to be controlled.
[00042] To control the flow rate from first station 12, looking at Fig. 1, a
flow
control valve 121 may be provided downstream of the first station 12. In
addition, a flow-meter 122 may be provided between the first station 12 and
the
control valve 121 to help in determining the flow rate before control valve
121
is adjusted to an appropriate level. It should be noted that control valve 121
may not be necessary should the flow rate be capable of being adjusted from
the
first station 12 based on the reading on the flow-meter 122.
[00043] To control the introduction of the adsorbent material from reservoir
31
into pathway 15, looking at Fig. 3, so that the amount of adsorbent material
can
be proportional to the flow rate of the fluid from the first station 12 and
the
amount of contaminant within that fluid flow, a metering pump 311 may be
-13-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
provided to permit either manual or automatic control of the amount of the
adsorbent material being introduced.
[00044] In one embodiment, reactor 30 provides an environment within which
the fluid from the first station 12 and the adsorbent material from reservoir
31
("fluid/adsorbent mixture") may be accommodated over a period of time.
During this time period, remaining species of heavy metal contaminants from
the fluid may be adsorbed by the adsorbent material and removed from the fluid
until an acceptable concentration of heavy metal contaminants within the fluid
has been reached. The period of time, in an embodiment, can be determined by
the kinetics of the adsorption of the contaminants into the adsorbent
material, as
well as by the diffusion time of the contaminants within the fluid flow into
the
adsorbent material, and may last from about less than two minutes to about ten
minutes. It should be noted that introduction of the fluid/adsorbent mixture
into
the reactor 30 can provide sufficient turbulence in order to achieve the
necessary mixing action between the contaminated fluid and the adsorbent
material. To the extent needed, a mixing mechanism may be provided within
the reactor 30.
[00045] In accordance with an embodiment of the present invention, reactor 30
may be provided with an inlet 32 and an outlet 33. As shown in Fig. 3, inlet
32
may be controlled by inlet valve 321 and outlet 33 may be controlled by outlet
valve 331. Valves 321 and 331, in an embodiment, may be automatically
actuated or electronically controlled by means known in the art.
Alternatively,
these valves may be designed to be manually actuated. Reactor 30 may also
include a level transmitter or sensor 34 to indicate when the reactor 30 is
full
and when it is empty. In the embodiment shown in Fig. 3, the sensor 34
includes a top sensor 341 to determine and indicate when reactor 30 is
substantially full, and a bottom sensor 342 to determine and indicate when
reactor 30 is substantially empty. Reactor 30 may also include a pump (not
shown) to assist in the removal or draining of treated fluid through outlet
33.
[00046] In an alternate embodiment, rather than a pump, reactor 30 may include
an second inlet 35 coupled to, for instance, two natural gas lines 351 and 352
to
-14-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
assist in the removal of the treated fluid from the reactor 30. In particular,
line
351 may be a "gas-in" line that may be regulated by a gas-in valve 353 to a
relatively slightly higher pressure than that of an operating pressure of the
contaminated fluid. In this manner, the higher pressure can act to
subsequently
push the treated fluid from the reactor 30. Line 352, on the other hand, may
be
a"gas-out' line that may be regulated by gas-out valve 354 to maintain a
substantially similar pressure to that of the contaminated fluid pressure.
Operation of these gas lines in connection with the emptying of treated fluid
from the reactor 30 will be discussed hereinafter in detailed. Moreover,
although described in connection with natural gas, it should be appreciated
other
gases may be used.
[00047] The system 10, as shown in Fig. 1, may further be provided with
discharge valve 16 and flow-meter 17 for use in connection with the discharge
of cleaned or treated fluid from system 10. The flow-meter 17, in an
embodiment, can help to determine the flow rate of the cleaned or treated
fluid
while the discharge valve 16 can be used to control the discharge rate
relative to
the flow rate.
[00048] A separation device (not shown) may also be provided in system 10 for
the removal of spent adsorbent material. In one embodiment, the separation
device may be a centrifuge-type separation device. Such a device, in an
embodiment, uses centrifugal force to concentrate spent adsorbent material at
the bottom of the device. A collector (not shown) may also be provided, so
that
the spent adsorbent material concentrated at the bottom of the separation
device
may be directed thereinto and removed from system 10. Alternatively, the
separation device may be a filter designed with pores or mesh openings capable
of preventing particles, such as the adsorbent material, ranging from about 5
microns to about 200 microns in size, from moving thereacross. The separation
device, in an embodiment, may be located downstream from reactor 30.
Alternatively, separation device may be located, for example, about outlet 33
to
remove the spent adsorbent nanomaterial as it exits the reactor 30.
-15-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
[00049] In operation, contaminated fluid containing heavy metal contaminants
within source l 1 may be directed into pathway 13 toward first station 12. The
rate of flow of the contaminated fluid from source 11, in an embodiment, may
be controlled by control valve 111, to permit an appropriate volume to
continuously flow into the first station 12.
[00050] Upon reaching the first station 12, the contaminated may be directed
into
coalescing unit 20 through inlet 22. Once within the coalescing unit 20, the
flow
of contaminated fluid may be directed through coalescing element 21 to
initiate
a physical separation process for removal of the elemental species of the
heavy
metal contaminant. Specifically, as the fluid enters element 21, the fluid
flow
may be separated into a discontinuous phase and a continuous phase.
Thereafter, as the two phases continue to move through element 21, the
discontinuous phase may be allowed to coalesce by way of a saturated depth
coalescing process. In particular, the coalescing element 21,permits "like
materials" to attract "like materials", so as to take substantially small
diameter
droplets containing elemental species of the heavy metal in the discontinuous
phase and allow these substantially small diameter droplets to merge or
combine to form substantially larger diameter droplets. The larger diameter
droplets, over time, can form still larger droplets. When they have become
sufficiently large, the density of the heavy metal within these sufficiently
large
droplets, along with interfacial surface tension, tend to cause these droplets
to
drain from the coalescing element 21 in the presence of gravity and settle out
from the continuous phase to the bottom of the coalescing unit 20. The drained
liquid containing the elemental species of the heavy metal being separated may
thereafter be collected within container 23 of coalescing unit 20 for
subsequent
disposal.
[00051] Fluid from the coalescing unit 20 at the first station 12 may
thereafter be
directed toward the second station 14 by way of pathway 15. At second station
14, reactor 30 may, in an embodiment, be substantially filled with the
adsorbent
material. Alternatively, the adsorbent material may be introduced into pathway
15 along with the fluid flow from the first station, so as to form a
-16-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
fluid/adsorbent mixture for introduction into reactor 30 through inlet 32. In
the
embodiment shown in Fig. 3, as the fluid/adsorbent mixture fills reactor 30
and
approaches the location of the top sensor 341, the top sensor 341 may
transmit,
when reactor 30 is full, a signal to a PLC. Upon receipt of the signal, the
PLC
can act to thereafter close the inlet valve 321 of reactor 30. It should be
noted
that during this filling process, the adsorbent material, as mentioned above,
can
act to remove the heavy metal contaminants from the contaminated fluid to
provide substantially clean fluid. In particular, in the presence of the
adsorbent
material, which in one embodiment, may be mesoporous SAMMS, fluid can be
permitted to flow through the pores of the particles in the SAMMS material.
Within these pores, targeted heavy metal contaminants, such as all species of
mercury, come in contact with a monolayer of chemical designed to attract and
bind the molecules of these contaminants, along with the other constituents of
the fluid flow. As such, these particular contaminants may be trapped within
the SAMMS material and removed from the fluid flow.
[00052] The performance efficiency for separation and removal of the targeted
heavy metal contaminant in accordance with one embodiment of the present
invention can be dependent upon a variety factors, including the fluid being
processed, the form or species of the targeted heavy metal contaminant that
may
be present in the fluid, the presence of other contaminants, among others. As
such, the amount removed can vary. For example, since there exist a
substantial
difference in density between, for instance, mercury and a gas or air (i.e.,
fluid),
low effluent concentrations can be obtained.
[00053] Subsequently, the outlet valve 331 may be opened and the clean fluid
permitted to exit through outlet 33 and out of the reactor 14. This emptying
process may continue until the cleaned liquid level reaches bottom sensor 342,
at which time the bottom sensor 342 may transmit a signal to the PLC.
Thereafter, the PLC may act to close the outlet valve 331. The reactor 30 may
thereafter be ready to go through another filling cycle.
[00054] Once the adsorbent material within reactor 30 becomes used up or
spent,
the reactor 30 may be taken out of service, the adsorbent material removed,
and
-17-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
a batch of new adsorbent material put into reactor 30. To determine when the
adsorbent material may be used up, several approaches may be implemented. In
one approach, it is known that as the adsorbent material becomes filled with
contaminants, its differential pressure will increase. This is because heavy
metal contaminants in the fluid once trapped by the adsorbent material will
tend
to plug the porous adsorbent material over time. As such, it will be important
to
monitor the differential pressure of the adsorbent material.
[00055] In another approach, the status of the adsorbent material may be
determined by periodically or continuously monitoring the level of
contaminants of the treated fluid in the outlet stream. When the level in the
outlet stream increases to a certain point, the adsorbent material may be
changed.
[00056] It should be appreciated that physical separation may not remove
substantially all traces of elemental species of the targeted heavy metal
contaminant, or lower the concentration of elemental species to a
substantially
safe or allowable level, for example, parts per billion (ppb) or parts per
trillion
(ppt) as suggested by the government for certain heavy metals, e.g., mercury.
Nevertheless, such a process can significantly lower the concentration of the
elemental species, in many instances, well below the parts per million (ppm)
level. To this end, by employing physical separation to initially reduce the
concentration of elemental species from the discontinuous phase (i.e.,
colloidal
flow), the life as well as the performance of the adsorbent material employed
in
the subsequent second station 14 can be substantially extended.
[00057] For example, if the contaminated fluid from source 11 contains about
1,000 ppb of elemental mercury, about 100 ppb of ionic mercury, and about 100
ppb of organic melury, the total amount of mercury in the contaminated fluid
is about 1,200 ppb. When the fluid is initially treated with a physical
separation
process for removal of the elemental mercury, the amount of elemental mercury
that subsequently remains within the fluid may be about 100 ppb. As such, the
fluid directed to the second station 14 would have only about 300 ppb of
mercury, as opposed to 1,200 ppb of mercury. This reduction in the
-18-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
concentration of mercury can lengthen the life and performance of the
expensive adsorbent material in the second station 14 by four times, and thus
also greatly reduce the overall cost of the treatment process.
[00058] To fl-rther reduce the cost of the treatment process, referring now to
Figs. 4A-B, system 10, in accordance with another embodiment of the present
invention, may be provided with prefilter station 40 upstream of the first
station
12. The utilization of a prefilter station 40 can, among other things, prolong
the
life, as well as the performance of the more expensive coalescing element 21
within the coalescing unit 20 at station 12.
[00059] The prefilter station 40, in one embodiment, may include prefilter
reactor 41, as shown in Fig. 4B, having at least one filter element 42
designed to
remove solid contaminants. An example of a prefilter reactor 41 may be the
Series 55 reactor available from Perry Equipment Corporation in Mineral Wells,
Texas. An example of a filter element 42 may be a PEACHO Gold technology
element, also available from Perry Equipment Corporation in Mineral Wells,
Texas.
[00060] The prefilter reactor 41, as,illustrated, may include an inlet 43
through
which a continuous flow of contaminated fluid from source 11 may be directed
into reactor 41 via pathway 411. Reactor 41 may also include outlet 44 through
which fluid treated by filter element 42 may exit and gets directed toward the
coalescing unit 20 via pathway 13. The filter element 42, in an embodiment,
may be designed to permit the flow of contaminated fluid from source 11 to
move therethrough, and to separate and remove solid contaminants from the
fluid flow. It should be appreciated that to the extent solid contaminants are
not
removed and may be present in the fluid flow when the fluid flow is directed
into the first station 12 from source 11, such solid contaminants can act to
block
the pores of the coalescing element 21, thereby reducing its performance and
life expectancy. Filter element 42, in certain instances, by its design can
remove heavy metal contaminants from the fluid flow. In particular, when the
contaminated fluid may have a substantially high loading (i.e., high
concentration) of heavy metal contaminants, the filter element 42 can be used
to
-19-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
separate out and reduce the heavy metal concentration, along with the
concentration of solid contaminants, within the fluid flow, before passing the
fluid to the coalescing unit 20 and coalescing element 21 at station 12. To
that
end, the life and performance of the coalescing element 21 can be further
prolonged.
[000611 Looking rrow at Fig. 5, there is illustrated another embodiment of
system
in accordance with one aspect of the present invention. System 10, as shown
in Fig. 5, may be provided with a third station 50, downstream of the second
station 14, for adsorptive separation and removal of other heavy metal
contaminants. In one embodiment of the present invention, the third station 50
may include a reactor (not shown) substantially similar to reactor 30 of
second
station 14, and within which an adsorbent material may be accommodated for
treatment of fluid received from the second station 14 by way of pathway 51.
The adsorbent material in the reactor at the third station 50, in one
embodiment,
may also be a nanosorbent material manufactured from self-assembled
monolayers on mesoporous supports (SAMMS), similar to the adsorbent
material in reactor 30 of second station 14. However, it should be appreciated
that the adsorbent material within the reactor at the third station 50 may
include
a different functional group in order to remove heavy metal contaminants
different than that targeted by the second station 14. In particular, while
thiol-
SAMMS may work well on mercury and may have some adsorptive capacity
for, for instance, one form of arsenic, a cupric or copper-based SAMMS may
have a substantially greater capacity for all species of arsenic.
[00062] As it is common in many areas of the world, produced hydrocarbon
streams in these areas can often be contaminated with both mercury and
arsenic.
Accordingly, in accordance with one embodiment of the present invention, the
adsorbent material in the "reactor of the third station 50 may be
functionalized to
remove arsenic from the fluid being processed. In particular, the SAMMS
material may be functionalized with copper-EDA (i.e., copper-EDA SAMMS)
or with lanthanum groups (i.e., lanthanum SAMMS) so that arsenic can be
-20-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
effectively be removed from the fluid flow subsequent to the removal of, for
instance, mercury at the second station 14.
[00063] Again, although the adsorbent material is disclosed above as being
manufactured from SAMMS, it should be appreciated that other adsorbent
materials may be used, so long as these adsorbent materials can act to remove
contaminants from the fluid flow. One example of an alternate adsorbent
material includes commercially carbon particles ranging from about 8 to about
3 0 mesh in size.
[00064] Moreover, although illustrated as being downstream of the second
station 14, to the extent desired, the third station 50 may be located
upstream of
the second station 14, so that arsenic may initially be removed prior to the
removal, for instance, mercury. Furthermore, should there be a need to remove
other heavy metal contaminants from the fluid being processed, additional
stations with reactors having particularly functionalized adsorbent materials
to
remove targeted heavy metal contaminants can be provided. Such additional
stations can be placed, in an embodiment, in series, within system 10. Of
course, these additional stations can be placed in parallel, in a combination
of
series and parallel, or in any configuration upstream of the second station
14,
and the treated fluid from these additional stations can subsequently be fed
into
the second station 14. In addition, it should be appreciated that the
provision of
the third station 50 or the additional stations for removal of other heavy
metal
contaminants may be implemented with or without the presence of a prefilter
station, such as that illustrated in Fig. 4, in system 10.
[00065] In Fig. 6, there is illustrated another system 60 of the present
invention
for treating contaminated fluid. Similar to system 10, fluids which may be
treated in connection in system 60 may be viscous in nature, such as oil, or
non-
viscous in nature, such as a liquid or a gas. Moreover, like system 10,
contaminants that may be removed by system 60 of the present invention
include heavy metals, such as mercury, arsenic, cadmium, and lead from
complex waste fluids, such as produced water, and mercury from a variety of
waste solutions and contaminated waste oils. Other contaminants that may be
-21-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
removed by system 10 of the present invention includes silver, uranium,
plutonium, neptunium, americium, or a combination thereof.
[00066] System 60, as illustrated in Fig. 6, includes, in an embodiment, a
source
61 from which a flow of contaminated fluid may be introduced into the system.
The contaminated fluid may contain various species or forms of heavy metal
contaminants, including their elemental form, organic form, and ionic form.
The contaminated fluid may be introduced into system 60 at a controlled rate.
To control the flow rate of the fluid, a flow control valve 611 may be
provided
downstream of source 61. In addition, a flow-meter 612 may be provided
between the source 61 and the control valve 611 to help in determining the
flow
rate, and if necessary, to permit adjustment of the control valve 611 to an
appropriate level. Although system 10 is shown having a control valve 111,
such a valve may not be necessary should the flow rate be adjustable from the
source 61.
[00067] The system 60 may also include a first station 62 designed to
implement
a physical separation protocol for removal of elemental species of a targeted
heavy metal contaminant, such as mercury, from the fluid flow. In an
embodiment, first station 62 may be in fluid communication with source 61 via
pathway 63, for example, a pipe, a hose, or any similar conduit capable of
conducting fluid flow from source 61 to first station 62. The first station
62, in
addition, may include a coalescing unit, substantially similar to coalescing
unit
20 shown in Fig. 2, and within which at least one coalescing element,
substantially similar to coalescing element 21 shown in Fig. 2, may be
situated.
Like coalescing element 21, the coalescing element of first station 62 may be
designed to coalesce or merge small diameter droplets containing elemental
species of the heavy metal contaminant into larger droplets for subsequent
removal from the fluid flow.
[00068] The system 60 may further include a second station 641ocated
downstream of the first station 62 and in fluid communication therewith via
pathway 65. The second station 64, similar to second station 14 in system 10,
may be provided for adsorptive separation or removal of the remainder of the
-22-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
elemental species, now in trace amount, along with the other species of the
targeted heavy metal contaminant, such as mercury, from the fluid flow.
[00069] With reference now to Fig. 7, the second station 64, may include a
vessel 70 within which at least one filter element 71 may be located for
further
treatment of fluid from the first station 62 by way of adsorptive separation
for
removal of various species of the targeted heavy metal contaminant.
[00070] The vessel 70, in accordance with one embodiment of the present
invention, includes a housing 72 within which the filter element 71 may be
accommodated. Housing 72, as illustrated, includes an inlet chamber 73 and an
outlet chamber 74 separated by a support plate 75. Support plate 75, in an
embodiment, may be designed to include at least one passageway 76 to which
the filter element 71 may engage. Of course, a plurality of passageways 76 may
be provided into which a complementary number of filter elements 71 may be
securely placed. If desired, a plug or cover may be provided for those
passageways 76 not in engagement with an apparatus 10. To facilitate
placement of the filter element 71 in secured engagement with the passageway
76 along a desired orientation within the inlet chamber 73, and/or removal of
filter element 71 therefrom, the vessel 70 may be provided with a sealable
closure 77. Such a vessel can be commercially obtained through Perry
Equipment Corporation in Mineral Wells, TX.
[00071] The filter element 71, as shown in Fig. 8 in more detail, may include
a
substantially tubular body portion 81 and may be made from a fluid permeable
material. The filter element 71 may also include a pathway 82 extending
between its ends, and along which treated fluid may be directed out of the
element 71 in a direction substantially'transverse to the flow of fluid into
the
filter element 71.
[00072] In one embodiment, filter element 71 may have incorporated within the
body portion 81 an adsorbent material for use in the removal of various
species
of the targeted heavy metal contaminants, similar to those disclosed above.
The
adsorbent material, like that used in connection with system 10, may be a
nanosorbent material manufactured from self-assembled monolayers on
-23-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
mesoporous supports (SAMMS). The mesoporous support, in an embodiment,
may be made from various porous materials, including silica. The advantage of
the SAMMS material is its ability to remove all species of various targeted
heavy metals, depending on the particular functional group associated with the
SAMMS material. An example of a SAMMS material that can be used in
connection with the filter element 71 of the present invention includes thiol-
SAMMS for targeting all species of mercury, similar to that disclosed in U.S.
Patent No. 6,326,326, which patent is hereby incorporated herein by reference.
The SAMMS material, of course, may be functionalized with other groups,
depending on the contaminant targeted for removal from the fluid. For
instance,
the SAMMS material may be functionalized with lanthanum groups for arsenic
removal, or with amine groups for COa removal.
[00073] In accordance with one embodiment of the present invention, the
adsorbent material may include porous particles, ranging from about 5 microns
to about 200 microns in size. In one embodiment, the particles, on average,
range from about 50 microns to about 80 microns in size, include a pore size
ranging from about 2 nanometers (nm) to about 7 nm, and may be provided
with an apparent density ranging from about 0.2 grams/milliliter to about 0.4
grams/milliliter.
[00074] As noted above, although the adsorbent material is disclosed as being
manufactured from SAMMS, it should be appreciated that other adsorbent
materials may be used, so long as these adsorbent materials can act to remove
contaminants from the fluid flow. One example of an alternate adsorbent
material includes commercially carbon particles ranging from about 8 to about
30 mesh in size.
[00075] Filter element 71 may further include an upper end cap 83. In one
embodiment, the upper cap 83 may be a substantially solid cap, so as to
prevent
fluid within pathway 82 from flowing through a top end of the element 71. An
opposing lower end cap 84 may similarly be provided on filter element 71. The
lower cap 84, however, may include an aperture 85 in axial alignment with the
pathway 82 to permit treated fluid to exit the filter element 71. Lower cap
84,
-24-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
in an embodiment, may be fitted with an engagement mechanism 86 extending
from aperture 85. Engagement mechanism 86 can permit filter element 71 to
securely engage a substantially complementary passageway 76 within vessel 70,
so that contaminated fluid may be directed across the filter element 71 and
into
pathway 82.
[00076] The upper end cap 83 and lower end cap 84, in an embodiment, may be
manufactured from a rigid material. Examples of such a rigid material
includes,
metals, plastics, or other synthetic material, such as polyester,
polypropylene or
nylon.
[00077] In operation, filter element 71 may be placed within the inlet chamber
73 of vesse170, and into which fluid containing the remaining heavy metal
contaminants from the first station 62 may be directed. After filter element
71
has been placed in secured engagement with passageway 76 along a desired
orientation (i.e., the pathway 82 of filter element 71 being in substantial
alignment with passageway 76), and the closure 77 of vessel 70 are sealed,
contaminated fluid may be directed into the inlet chamber 73 through irnlet
731.
Once within the inlet chamber 73, contaminated fluid may immerse filter
element 71 and be directed to flow substantially radially through the filter
element 71. In other words, the contaminated fluid may flow into and across
the filter element 71 in a direction substantially transverse, and more
particularly substantially perpendicularly, to the pathway 82. As the
contaminated fluid flows across the filter element 71, it comes into contact
with
the adsorbent material, for instance SAMMS material, and can be permitted to
flow through the pores of the particles in the SAMMS material. Within these
pores, targeted contaminants, such as heavy metals (e.g., mercury) come in
contact with a monolayer of chemical designed to attract and bind the
molecules
of these contaminants, along with the other constituents of the fluid flow. As
such, these targeted contaminants may be trapped within the SAMMS and
removed from the fluid flow.
[00078] The resulting treated fluid may next flow into the pathway 82 of
element
71. Once in the pathway 82, the fluid flow changes direction and now moves in
-25-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
a direction substantially parallel to that of the pathway 82 (i.e.,
substantially
transverse to the radial flow of the fluid across the element 71). As it moves
along pathway 82, the treated fluid gets directed through aperture 85 of lower
end cap 84, across passageway 76, and into outlet chamber 74 of vesse170,
where the fluid can subsequently be directed out of the housing 72 through
outlet 741.
[00079] It should be appreciated that the present invention also contemplates
the
filter element 71 being used with a vessel where contaminated fluid may flow
from within the filter element 71 outward. In other words, contaminated fluid
may be introduced initially through the aperture 85, up into the pathway 82,
and
directed radially outward across and through the filter element 71.
[00080] Once the adsorbent material within the filter element 71 becomes used
up or spent, the vessel 70 may be taken out of service, the filter element 71
removed, and a new filter element 71 put in its place. To determine when the
adsorbent material may be used up, several approaches may be implemented. In
one approach, it is known that as the filter element 71 becomes filled with
contaminants, its differential pressure will increase. This is because heavy
metal contaminants in the fluid once trapped by the adsorbent material will
tend
to plug the porous adsorbent material over time. As such, it will be important
to
monitor the differential pressure of the filter element 71.
[00081] In another approach, the status of the adsorbent material may be
determined by periodically or continuously monitoring the level of
contaminants of the treated fluid in the outlet stream. When the level in the
outlet stream increases to a certain point, the filter element 71 may be
changed.
[00082] Although shown in a vertical position, it should be appreciated that
the
vessel 70 may be designed to be in a horizontal position with fluid flow
direction adapted to the change accordingly. Moreover, the vesse170, as noted
above, may be manufactured to accommodate a plurality of filter elements 71.
In such an embodiment, each filter element 71 may be designed to have a rated
or allowable flow rate therethrough. In particular, the number of filter
element
71 used may be determined, for instance, by taking a total flow rate to be
treated
-26-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
and dividing that by an allowable flow rate for one filter element 71. The
size
of the vessel 70 may then be the size required to place this number of filter
elements 71 in close proximity in housing 72.
[00083] It should be appreciated that filter element 71 may be any of the
filter
elements capable of incorporating an adsorbent material therein. Examples of
such filter elements include those similarly disclosed in U.S. Application
Serial
Nos. 11/607,364, 11/731,230, and 11/731,556, all of which are hereby
incorporated herein by reference.
[00084] Moreover, although the primary purpose of the adsorbent material is to
adsorb a targeted heavy metal contaminants, due to its small size (i.e., from
about 5 microns to about 150 microns), the adsorbent material may also be a
very good solids filter_ This ability to filter solids can result in the
adsorbent
material be spent or plugged sooner than otherwise necessary. In order to
minimize the need to replace these expensive filter elements, system 60,
referring now to Fig. 9, may be provided with prefilter station 90 upstream of
the first station 62 similar to prefilter station 40 in system 10. The
utilization of
a prefilter station 90 can also prolong the life, as well as the performance
of the
more expensive coalescing element at first station 62.
[00085] The prefilter station 90, in one embodiment, may include a prefilter
reactor similar to that shown in Fig 4B. Such a reactor may include an inlet
through which a continuous flow of contaminated fluid from source 61 may be
directed into the prefilter reactor via pathway 91, and an outlet through
which
treated fluid may exit and gets directed toward the first station 62 via
pathway
63. Such a reactor may further include at least one filter element similar to
that
shown in Fig. 4B designed to remove solid contaminants from the fluid flow.
[00086] Looking now at Fig. 10, system 60, in accordance with one embodiment
of the present invention, may be provided with a third station 100, downstream
of the second station 64, for removal of other heavy metal contaminants within
the fluid flow directed from the second station 64 through pathway 101. In one
embodiment of the present invention, the third station 100 may include a
vessel
(not shown) substantially similar to vessel 70 of the second station 64, and
at
-27-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
least one filter element (not shown) substantially similar to filter element
71 at
the second station. Such a filter element may include an adsorbent material
therein for the treatment of fluid received from the second station 64 by way
of
adsorptive separation. The adsorbent material, in one embodiment, may also be
a nanosorbent material manufactured from self-assembled monolayers on
mesoporous supports (SAMMS). However, it should be appreciated that the
adsorbent material may include a different functional group in order to remove
heavy metal contaminants different than that targeted by the second station
64.
In particular, while thiol-SAMMS may work well on mercury and may have
some adsorptive capacity for, for instance, one form of arsenic, a cupric or
copper-based SAMMS (i.e., copper-EDA SAMMS) may have a substantially
greater capacity for all species of arsenic.
[00087] Again, although the adsorbent material is disclosed above as being
manufactured from SAMMS, it should be appreciated that other adsorbent
materials may be used, so long as these adsorbent materials can act to remove
contaminants from the fluid flow. One example of an alternate adsorbent
material includes commercially carbon particles ranging from about 8 to about
3 0 mesh in size.
[00088] Although illustrated as being downstream of the second station 64, to
the
extent desired, the third station 100 may be located upstream of the second
station 64, so that, for example, arsenic may initially be removed prior to
the
removal of, for instance, mercury. Moreover, should there be a need to remove
other heavy metal contaminants from the fluid being processed, additional
stations with vessels having particularly functionalized adsorbent materials
to
remove specifically targeted heavy metal contaminants can be provided. Such
additional stations can be placed, in an embodiment, in series, within system
60.
Of course, these additional stations can be placed in parallel, in a
combination
of series and parallel, or in any configuration upstream of the second station
64,
and the treated fluid from these additional stations can subsequently be fed
into
the second station 64. Furthermore, it should be appreciated that the
provision
of the third station 100 or these additional stations for removal of other
heavy
-28-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
metal contaminants may be implemented with or without the presence of a
prefilter station 90, such as that illustrated in Fig. 9, in system 60.
[00089] In yet another embodiment of the present invention, system 10 or
system
60 of the present invention may have its third station target the same heavy
metal contaminant being targeted in its second station. As illustrated in Fig.
11,
system 110 may include a plurality stations 111, 112, in series, designed for
adsorptive separation of the same targeted heavy metal contaminant. By
providing multiple stations 111 and 112 that can target the same heavy metal
contaminant, system 110 can employ continuous processing to enhance its flow
capacity (i.e., kinetics) and loading capacity.
[00090] Description of system 110 hereinafter will be directed to an
embodiment
using the vessel and filter elements similar to that in system 60 above.
However, it should be appreciated that system 110 can be designed to utilize
the
reactor and adsorbent material similar to that used in system 10.
[00091] With respect to flow capacity, since it may be necessary to allow the
fluid to have a certain amount of contact time with the adsorbent material, if
only one station 111 targeting a particular heavy metal contaminant is used,
the
flow may need to be sufficiently slow through that one station in order permit
sufficient contact time with the adsorbent material. However, if multiple
stations 111 and 112 arranged in series are used for targeting the same heavy
metal contaminants, the flow rate and volume can increase significantly, for
instance, double or triple, while still permitting the fluid to have
sufficient
contact time with the adsorbent material. To that end, the size of reactor or
vessel at each of stations can be reduced. Moreover, as it may often be the
case,
a plurality of relatively smaller vessels or reactors can be less expensive
than
one relatively large vessel or reactor.
[00092] In addition to flow capacity, loading capacity can be enhanced. It is
well known that adsorbent materials, in general, have a contaminant loading
capacity (i.e., equilibrium point) that is a function of the concentration of
the
targeted contaminant in the fluid. To that end, as contaminated fluid flows
across a volume of an adsorbent material, an isotherm may be generated as the
-29-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
targeted contaminant is adsorbed. In particular, the adsorbent material
closest
to the inlet of the reactor or vessel reaches equilibrium with the
concentration of
contaminant in the fluid at or near the inlet. Then depending upon the
kinetics
of the reaction and the flow rate, the concentration of contaminant in the
fluid
reaches a relatively low level within the remainder of the volume of the
adsorbent material. To the extent that there is a set or permissible
contaminant
level for fluid exiting the outlet (such a level must obviously be lower than
the
concentration at the inlet), a single station 111 may be employed until the
adsorbent material at or near the outlet of the reactor or vessel reaches
equilibrium with the set or permissible concentration of contaminant in the
fluid
permitted to exit the outlet. When such a point is reached, the adsorbent
material in this station 111 must be changed as it likely can no longer
sufficiently adsorb contaminants so as to lower the amount of contaminants to
a
concentration level that is permissible to exit the outlet. However, by
putting in
a second station 112 in series with the first station 111, the adsorbent
material
within the first station 111 may not need to be changed until the adsorbent
material at or near the outlet of the first station 111 reaches equilibrium
with the
concentration of contaminant in the fluid at or near the inlet. In other
words, the
adsorbent material in the first station 111 need not be changed until the
total
volume or amount of the adsorbent material within the first station 111 is
spent
having the maximum concentration of the targeted contaminant on every
particle of the adsorbent material. This is because even if the adsorbent
material
in the first station. l l I can no longer adsorb the targeted contaminant, the
adsorbent material in the second station 112 can act to adsorb the same
targeted
contaminant.
[00093] In such a multi-station system, such as that illustrated in Fig. 11,
when
the adsorbent material in the filter element is spent, the inlet of the vessel
at
station 111 may be closed and its outlet may be opened to permit draining of
the
fluid. The filter element with the spent adsorbent material may thereafter be
disposed. Fluid flow into the system I 10 at the time when first station 111
is
not in use may be redirected toward the second station 112. Once the vessel at
-30-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
the first station 111 has been reloaded with a fresh filter element having
fresh
adsorbent material, the fluid flow from the second station 112 can be directed
toward the first station 112 to reinitiate the continuous processing protocol.
The
same process can be applied when the adsorbent material in the second station
112 is spent.
[00094] In accordance with an embodiment of the present invention, the used or
spent adsorbent material may be regenerated. To regenerate the adsorbent
material for subsequent use, the adsorbent material may be treated with an
acidic fluid to remove the adsorbed contaminant. After this regeneration
process, the adsorbent material may be put back in service to again remove the
contaminants. Regeneration of the adsorbent material, of course, can be
implemented for the adsorbent material in system 10 and system 60 described
above.
[00095] In the embodiment where a reactor and adsorbent material similar to
system 10 are used, to collect the adsorbent material for regeneration, a
filter
(not shown) may be provided near the outlet of the vessel or reactor at each
of
the stations 111 and 112 to trap the spent adsorbent material. The filter, in
an
embodiment, may be provided with pores that are substantially smaller than the
adsorbent material while still sufficiently large to permit the clean fluid to
move
therethrough. As the filter becomes full with the spent adsorbent material,
the
filter may be isolated and removed along with the adsorbent material. A new
filter may be put in place for subsequent removal of the adsorbent material.
[00096] In an alternate approach, a centrifuge-type separation device (not
shown)
may be utilized. This device uses centrifugal force to concentrate the spent
adsorbent material at the bottom of the device. Once at the bottom of device,
the adsorbent material may be removed and directed to a collector, while the
cleaned treated fluid may be discharged. The spent adsorbent material may
thereafter be disposed or regenerated for subsequent use.
[00097] Although only two stations are illustrated and described, it should be
noted that system 110 may include three or more stations in series targeting
the
same heavy metal contaminant.
-31-
CA 02694949 2010-01-28
WO 2009/017479 PCT/US2007/017103
[00098] While the invention has been described in connection with the specific
embodiments thereof, it will be understood that it is capable of further
modification. Furthermore, this application is intended to cover any
variations,
uses, or adaptations of the invention, including such departures from the
present
disclosure as come within known or customary practice in the art to which the
invention pertains.
-32-