Note: Descriptions are shown in the official language in which they were submitted.
CA 02695197 2014-12-11
=
1
INTEGRATED PHOTOACTIVE AGENTS AND USES THEREOF
FIELD OF THE INVENTION
This invention relates photoactive compounds for use in medical diagnostic
arid/or
therapeutic procedures. =
BACKGROUND
Publications are referenced throughout the specification in parenthesis. Full
citations
corresponding to the cited references are listed following the detailed
description.
Patients at risk for clotting- and/or bleeding-related adverse events tend to
be monitored
using aPIT (activated partial thromboplastin time) and PT (prothrombin time)
blood tests (Bajaj
et al and Kher eta!). Generally, patients on heparin are closely monitored by
frequent
measurement of aPTT to indicate the degree to which the extrinsic coagulation
cascade is
activated. Patients on Coumedie are monitored by frequent measurement of PT
(Riley et al) to
evaluate the contributions of the extrinsic pathways. Both tests require blood
samples that are
sent to the hospital laboratory for analysis. Turnaround times are generally
lengthy enough that
a penfs hemostasis can change significantly during the analysis period. As
such, it would be
beneficial to develop compounds for use in monitoring hemostasls that would
shorten the turn-
around time between testing and results.
SUMMARY
A first aspect of the invention is directed to a compound that includes a
pyrazine ring.
= A carbon of this pyrazine ring has a substituent bonded thereto that
includes a group that
binds to and inhibits a serine protease.
With regard to this first aspect of the invention, the serine protease may be
Tissue
Factor/Factor Vila in some embodiments. In some embodiments, the serine
protease may be
Factor Xa.
The group that binds to and inhibits a serine protease may be any appropriate
group
that provides the required binding and inhibitory functions when the
substiluent is bound to the
ring. Tests to determine these binding and inhibitory functions are known in
the art. In some
embodiments, the group may be an amidine. For instance, in some embodiments,
the group
CA 02695197 2010-01-29
WO 2009/018405
PCT/US2008/071700
2
may be a mono-benzamidine. In some embodiments, the group may be a guanidine.
Still referring to the first aspect of the invention, the pyrazine ring may
have a number of
substituents bonded thereto that each include at least one group that binds to
and inhibits one or
more serine proteases. For instance, in some embodiments, the pyrazine ring
may have a first
substituent bonded to a first carbon of the ring and a second substituent
bonded to a second
carbon of the pyrazine ring. These first and second substituents may be in any
appropriate
orientation relative to one another (e.g., ortho, meta, or para). Further,
each of the first and
second substituents includes at least one (e.g., one, two, three, four, five,
etc.) group that binds
to and inhibits serine protease(s). In the case that a given substituent
includes multiple groups,
each of the groups may be identical or different from one another. Further,
the group(s)
associated with the first substituent may be the same as or different from the
group(s) associated
with the second substituent.
In some embodiments, the pyrazine ring may have a first substituent bonded to
a first
carbon of the ring, a second substituent bonded to a second carbon of the
pyrazine ring, a third
substituent bonded to a third carbon of the pyrazine ring, and a fourth
substituent bonded to a
fourth carbon of the pyrazine ring. In some embodiments, three of the first,
second, third, and
fourth substituents includes at least one (e.g., one, two, three, four, five,
etc.) group that binds to
and inhibits serine protease(s). In some embodiments, all four of the first,
second, third, and
fourth substituents includes at least one (e.g., one, two, three, four, five,
etc.) group that binds to
and inhibits serine protease(s). In the case that a given substituent includes
multiple groups,
each of the groups may be identical or different from one another. Further,
the group(s)
associated with a particular substituent may be the same as or different from
the group(s)
associated with any other substituent(s).
In some embodiments of the first aspect, the compound may include or exhibit a
fully
symmetric di-basic structure. In other embodiments, the compound may include
or exhibit a
reverse-turn mimetic structure.
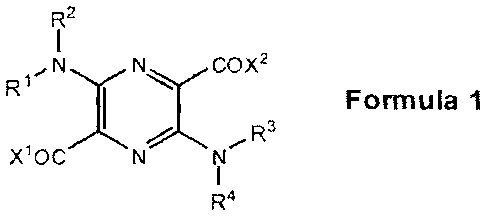
A second aspect of the invention is directed to compounds of Formula 1 below.
R2
R1 N y N COX2
Formula
Xl0C
R4
Each of R1, R2, R3, R4, ¨1,
A and X2 is independently hydrogen, C1-C6 alkyl, C1-C6 acyl,
CI-C6 alkoxycarbonyl, C1-C6 hydroxyalkyl, CI-C6 poiyhydroxyalkyl, CI-C6
carboxyalkyl, Cl-
CO aminoalkyl, C5-C10 aryl, ¨(CH2)m¨Y¨C(=N11)NH2,
¨(CF12),(CFIR5),¨Y¨C(=NH)NH2,
¨(CH2)m(CHR5),¨Y¨NHC(=NH)NH2, ¨(CH2)m¨Y¨NFIC(=NH)NH2, ¨0¨Y¨C(.41F-1)NE12.
CA 02695197 2010-01-29
WO 2009/018405
PCT/US2008/071700
3
-0-Y-NFIC(=NH)NH2, -N(R6)-Y-C(=NF)NH2, or -N(R7)-Y-NHC(=NH)NH2. However, at
least one of R1, R2, R3, R4, X1, or X2 must be -(CH2),,-Y-C(=NH)NH2,
-(CH2)rn(CHR5)n-Y-C(=N FI) -(CH2)m(CH R5),-Y-NFIC(=NH)N H2,
-(CH2),-Y-NHC(=NH)NH2, -0-Y-C(=NH)NH2, --0-Y-NHC(=NH)N1-12,
-N(R6)-Y-C(=NH)NH2, or -N(R7)-Y-NFIC(=NH)NH2.
Y is any of the substituents listed below:
A 1-7 r)
_(cH2)p- ' __________________
--C\N- Nr)--- N- , or =
For instance, Y may be an alkyl substituent in some embodiments and a benzene
(or other
homocyclic or heterocyclic) substituent in other embodiments.
Each of 'm, "n,' and '13 is independently 0, 1, 2, 3, 4, 5, or 6.
R5 is hydroxyl, C1-C6 hydroxyalkyl, carboxyl, CI-C6 carboxyalkyl, amino, or Cl-
C6
aminoalkyl. For instance, in some embodiments, R5 is carboxyl.
Each of R6 and R7 is independently hydrogen, C1-C6 alkyl, Cl-C6 acyl, C1-C6
alkoxycarbonyl, C1-C6 hydroxyalkyl, C1-C6 polyhydroxyalkyl, C1-C6
carboxyalkyl, C1-C6
aminoalkyl, or C5-C10 aryl.
In some embodiments of the second aspect, each of R1, R2, R3, R4, and X1 may
be
hydrogen, while X2 is -(CH2).--Y-C(=NH)NH2, -(CH2)m-Y-NHC(=NH)NH2,
-(CH2)m(CHR5)n-Y-C(=NH)NH2, -(CH2),n(CHR5)r,--Y-NHC(=NH)NH2, -N(R6)-Y-C(=NH)NI-
12,
or -N(R7)-Y-NHC(=NH)NH2. For example, each of R1, R2, R3, R4, and xl may be
hydrogen,
while X2 may simply be -(CH2)m-Y-C(=NH)NH2, -(CH2)rn(CHR5)n-Y-NHC(=NH)NH2, or
-N(R5)-Y-C(=NH)NH2.
In some embodiments, each of R1, R2, R3, and R4 may be hydrogen, while each of
xl
and X2 may independently be -(CH2),-Y-C(=NH)NH2, -(CH2),-Y-NHC(=NH)NH2,
-(CH2)m(CHR5)n-Y-C(=NF)NF12, -(C1-12)m(CHR5)n-Y-NHC(=NH)NH2, -N(R6)-Y-C(=N1-
1)*12,
or -N(R7)-Y-NHC(=NH)NH2. For example, each of 1:21, R2, R3, and R4 may be
hydrogen, while
each of X1 and X2 may independently be -(CH2)m-Y-C(=NH)NI-12,
-(CH2)õ(CHR5)n-Y-NHC(=NH)N1-12, or -N(R6)-Y-C(=NH)NH2.
In any of the embodiments of the second aspect mentioned above, R5 may be any
appropriate substituent (e.g., hydrogen).
Still referring to the various possible refinements of the compounds of
Formula 1, in
some embodiments, each of R2, R3, R4, X1, and X2 may be hydrogen, while R1 may
be
-(CH2)rn-Y-C(=NH)NH2, -(CH2).,-Y-NHC(=NH)NF12, -(C1-12)m(CHR5)n-Y-C(=NH)NH2,
or
CA 02695197 2010-01-29
WO 2009/018405
PCT/US2008/071700
4
-(C1-12),(CHRG),-Y-NHC(=NH)NH2. For example, each of R2, R3, R4, X1, and X2
may be
hydrogen, while R1 may be -(CH2)m-Y-C(=NH)NH2 or -(CH2)n(CHR5)-Y-NHC(=NE)NF12.
In some embodiments, each of R2, R4, X1, and X2 may be hydrogen, while each of
R1
and R3 may independently be -(CF12)õ,-Y-C(=NH)Nh12, -(CH2)m-Y-NFIC(=NH)NH2.
-(CH2)m(CHR5)n-Y-C(=NH)NE12, or -(CH2),,,(CHR5),,-Y-NHC(=NH)NH2. For instance,
each of
R23 R4, s,1,
A and X2 may be hydrogen, while each of R1 and R3 may independently be
-(CH2),õ-Y-C(=NH)NH2 or -(CH2)m(CHR5)n-Y-NHC(=NH)NF12,
'm' may be any appropriate integer. For instance, in various embodiments, 'm'
may be
of any of the following inclusive ranges: 0-5, 0-4, 0-3, 0-2, 0-1, 1-6, 1-5, 1-
4, 1-3, 1-2, 2-6, 2-5,
2-4, 2-3, 3-6, 3-5, 3-4. In one particular exemplary embodiment, 'm may be 0,
1, 2, or 3.
Likewise, 'n' may be any appropriate integer. For instance, in various
embodiments,
'n' may be of any of the following inclusive ranges: 0-5, 0-4, 0-3, 0-2, 0-1,
1-6, 1-5, 1-4, 1-3, 1-
2, 2-6, 2-5, 2-4, 2-3, 3-6, 3-5, 3-4. In one particular exemplary embodiment,
'n' may be 0, 1,
2, or 3.
Further, 'p' may be any appropriate integer. For instance, in various
embodiments, `p'
may be of any of the following inclusive ranges: 0-5, 0-4, 0-3, 0-2, 0-1, 1-6,
1-5, 1-4, 1-3, 1-2,
2-6, 2-5, 2-4, 2-3, 3-6, 3-5, 3-4. In one particular exemplary embodiment, 'p'
may simply be 1.
In some embodiments, the utility of compounds of Formula 1 in medical
procedures is
due to their ability to bind to and inhibit serine proteases (e.g., Tissue
Factor/Factor Vila
and/or Factor Xa). Since such compounds inhibit serine proteases, these
compounds may
also be utilized to provide therapeutic affect (i.e., may be utilized in
medical drug therapy).
Compounds of the first and second aspects of the invention may be used in any
of a
number of appropriate medical procedures. For instance, in some embodiments,
such
compounds may be utilized in a medical diagnostic procedure such as monitoring
hemostasis
in a patient, imaging vulnerable (e.g., unstable) plaques, and/or tumor
imaging. In some
embodiments, such compounds may be utilized in a dual-role in medical
diagnostic procedure
as well as in medical drug therapy (e.g., monitoring and prevention of
clotting, imaging
vulnerable (e.g., unstable) plaques while reducing likelihood of undesired
clots/vascular
occlusions, and/or tumor imaging and therapy.
In some embodiments, the compound of the first and/or second aspects may be
encapsulated into a micelle, a liposome, a nanoparticle (e.g., a shell cross-
linked nanoparticle), a
dendrimer, a dendron, a nnicrocapsule, or other organized nnicroparticle. In
some embodiments,
a compound of the first and/or second aspect may be chemically conjugated to a
nanoparticle
(e.g., a shell cross-linked nanoparticle), a dendrimer, or a dendron.
A third aspect of the invention is directed to a medical formulation that
includes: 1) any
compound of the first and/or second aspect; and 2) a pharmaceutically
acceptable buffer,
CA 2695197 2017-05-19
surfactant, excipient, thixotropic agent, flavoring agent, stabilizing agent,
skin penetration
enhancing agent, or any combination thereof.
In another embodiment of the present Invention there is provided a compound of
Formula
1, wherein:
COX2
Formula 1
R3
X =OC N N
1
5 R4
each of R2, R3, and Fr is independently hydrogen, -OH, C1-C6 alkyl,
01-06 acyl,
C1-C6 alkoxycarbonyl, C1-C6 hydroxyalkyl, C1-C6 polyhydroxyalkyl, C1-C6
carboxyalkyl,
C1-C6 aminoalkyl, C5-C10 aryl, -(CH2)m-Y-C(=NH)NH2, -(CH2)m(CHR6)n-Y-
C(=NH)NH2,
-(CH2)m(CHR5)5-Y-NHC(=NH)NH2, -(CH2)m-Y-NHC(=NH)NH2, -0-(CHR5)n-Y-C(=NH)NH2,
-0-(CHR5)n-Y-NHC(=NH)NH2, -N(R6)-(CH2)m(CHR5)n-Y-C(=NH)NH2, -N(R7)-
(CH2)m(CHR5)n-Y-
NHC(=NH)NH2 or -NHCH2-Ph-CNH(NH2);
X1 and X2 are each independently chosen from -OH and -NHCH2-Ph-CNH(NH2);
Y is
A F-1
__(cH2)p-- I
h
N¨ ¨N/ ¨N N¨ / or ¨ '
/
with the proviso that at least one of R1, R2, R3, or R4 is hydrogen, -OH,
-NHCH2-Ph-CNH(NH2), -(CH2)m-Y-C(=NH)NH2, -(CH2)m(CHR5)n-Y-C(=NH)NH2,
-(CH2)m(CHR5)n-Y-NHC(=NH)NH2, -(CH2)m-Y-NHC(=NH)NH2, -0-(CHR5)n-Y-C(=NH)NH2,
-0-(CHR5)n-Y-NHC(=NH)NH2, -N(R5)-(CH2)rn(CHR5)n-Y-C(=NH)NH2, or
-N(197)-(CH2)m(CHR5)n-Y-NHC(=NH)N1-12,
each of 'm', 'n', and 'p is independently 0, 1, 2, 3, 4, 5, or 6;
R5 is hydroxyl, C1-C6 hydroxyalkyl, carboxyl, C1-C6 carboxyalkyl, amino, or C1-
C6
aminoalkyl; and
each of R6 and R7 is independently hydrogen, C1-C6 alkyl, C1-C6 acyl, C1-C6
alkoxycarbonyl, C1-C6 hydroxyalkyl, C1-06 polyhydroxyalkyl, C1-C6
carboxyalkyl, C1-C6
aminoalkyl, or C5-C10 aryl.
BRIEF DESCRIPTION OF THE FIGURES
Fig. 1 is an illustration of the coagulation cascade.
CA 2695197 2017-05-19
5a
Fig. 2 is an example of the general synthesis of integrated photoactive serine
protease
inhibitors of the present invention.
Fig. 3 provides structural examples of integrated photoactive serine protease
inhibitors.
Fig. 4 is graph of results for an in vitro assay showing the effects of a
compound of
Formula I on thrombin production.
DETAILED DESCRIPTION
The invention includes integrated photoactive agents (herein also referred to
as
"photoactive agents") that bind to and inhibit serine proteases, as well as
the manufacture and
use of such agents. Some of these integrated photoactive agents may be
characterized as small
molecules having a molecular weight of less than about 1000 Daltons.
Photoactive agents of the
invention may be manufactured by rendering a photoactive scaffold able to bind
a serine
protease. Once synthesized, the integrated photoactive agent can be
administered to a patient
and utilized as an optical diagnostic agent and/or a therapeutic drug. For
instance, in one
embodiment, integrated photoactive agents of the present invention may be
utilized to monitor
hemostasis. In another embodiment, integrated photoactive agents of the
present invention may
be used in the imaging of vulnerable plaques. In yet another embodiment,
integrated
photoactive agents of the present invention may be utilized in tumor imaging.
Serine proteases (referred to by some as "serine endopeptidases") are a class
of
enzymes that play a significant role, inter alia, in the clotting process. In
particular, some serine
proteases play a significant role in the extrinsic coagulation cascade.
Examples of serine
proteases include thrombin (activated Factor II [I la]), and thrombokinase
(activated Factor X
Val Factor VII (activated Tissue Factor/Factor Vila). In many serine
proteases, aspartate-189
is the primary recognition site residue for binding to the Si pocket of the
enzyme but there are a
number of other important interactions within the Si', S2, and S3 sites of the
enzyme that are
desirable for high potency binding (nanomolar affinity Neumann et al J. Med.
Chem 2003, 46,
4050). Many compounds of the present invention include a design in which the
scaffold is both
photoactive and displays the P1 substituent appropriately to bind to the
aspartate-189 residue of
serine proteases. In addition, the photoactive scaffold may have functionality
that can provide
additional interactions with other residues surrounding the catalytic
apparatus. In this regard, it
may be said that the photoactive scaffold is integrated with ligand binding
properties. In one
,
CA 02695197 2010-01-29
WO 2009/018405
PCT/US2008/071700
6
mechanistic characterization, it may be said that compounds of the invention
bind to and inhibit
Tissue Factor/Factor Vila and/or Factor Xa, and thus, inhibit production of
Thrombin (e.g., via the
extrinsic cascade).
Integrated photoactive agents of the present invention tend to have
absorption, excitation,
and emission wavelengths that are all within the near-infrared (NI R) or
visible spectrum of about
350 nm or greater. This is beneficial for diagnostic procedures since visible
and NIR light is not
likely to damage tissue. In contrast, ultraviolet (UV) light that has a
wavelength of less than
about 350 nm can cause tissue damage. Light having a wavelength of about 350
nm or greater
tends to penetrate into tissues thereby permitting diagnostic procedures to be
conducted in
tissues of interest that may not be reachable using UV wavelengths that are
less than about 350
nm. In one embodiment, compounds of the invention have absorption, excitation,
and emission
wavelengths that are all between about 350 nm and about 1200 nm. In another
embodiment,
compounds of the invention may have absorption, excitation, and emission
wavelengths that are
all between about 400 nm and about 900 nm.
Synthesis and use of compounds disclosed herein may be performed in a variety
of
ways. In one embodiment, known serine protease binding moieties (fragment
based lead
design) are attached to a photoactive group or molecule (e.g., a pyrazine
group). It is presently
believed that pyrazines are particularly suited for this purpose due to their
desirable
photophysical properties as well as the ability of some to serve as isosteric
replacements for
aromatic motifs. In many cases, this would allow the substitution of a
photoactive pyrazine core
for a non-photoactive aryl or heteroaryl core in known drugs or drug lead
candidates. The
resulting compound would then possess the biological activity of the drug and
the photoactivity of
the pyrazine. Hence, the photonic nature of the pyrazine system is integrated
within the drug
itself.
For compounds of the present invention, the target tends to be serine
proteases involved
in the coagulation cascade, illustrated in Fig. 1. Examples of pharmacophores
that bind to serine
proteases include, but are not limited to, amidines and guanidine. One or more
pharmacophore(s) are attached to the photoactive molecule in appropriate
places such that both
photoactivity and bioactivity will not be disrupted to the extent that the
resulting agent completely
loses its photoactivity or binding properties. The resulting integrated
photoactive agent may be
administered to a patent in a diagnostically effective amount such as to
enable detection of the
photoactive agent within the patient. After a period of time has lapsed for
the agent to bind to
serine protease, the whole body or a target tissue of a patient may be exposed
to light of
between about 350 nm and about 1200 nm wavelength. In one example, the whole
body or a
target tissue of a patient may be exposed a light of between about 400 nm and
about 900 nm
wavelength. Light emanates from the compound within the patient as a result of
absorption and
excitation properties of the compound. This emanating light may be detected by
utilizing an
CA 02695197 2010-01-29
WO 2009/018405
PCT/US2008/071700
7
appropriate detector that may be designed and/or chosen based on its ability
to detect the
specific wavelength(s) of the light emanating from the compound within the
patient. By
determining the location and strength (e.g., intensity) of light emanating
from the compound
within the patient, a diagnosis may be made.
The synthesis of an integrated photoactive agent of the present invention may
be
accomplished using this disclosure in combination with known scientific
principles. One
exemplary method of manufacturing a compound of the invention is outlined in
Fig. 2.
In one embodiment, an integrated photoactive agent of the invention comprises
a
photoactive group of Formula 1,
R2
I
,N N OX2
Ri y '-c
Formula 1
xioc N NI
R4
which serves as a molecular scaffold upon which at least one pharmacophore
that binds to
serine proteases is attached at the R1, R2, R3, R4, X1, and/or X2 position(s)
to create an integrated
photoactive serine protease binding agent (which is also a serine protease
inhibitor). In one
example, the molecular scaffold of Formula 1 is 2,5-diamino-3,6-
pyrazinedicarboxylic acid. In
another example, the pharmacophores(s) is(are) an amidine and/or a guanidine
moiety, each of
which may be attached to the pyrazine structure of Formula 1 at any one or
more of the positions
designated by R1, R2, R3, R4, X1, or X2.
Fig. 3 provides non-limiting examples of integrated photoactive agents of the
present
invention that are represented by Compounds 2-8. Compounds 2 and 3 are
examples of fully
symmetric di-basic structures, Compounds 4 and 5 are examples of reverse-turn
mimetic
structures, Compounds 6 and 7 are examples of mono-benzamidine-containing
compounds, and
Compound 8 is an example of an integrated photoactive agent that comprises a
guanidine
moiety as its binding moiety.
The benzamidine moiety serves as an arginine mimetic, as these hydrolytic
enzymes
recognize a particular arginine residue to effect cleavage and activation of
the corresponding
proenzyme in the coagulation cascade. Thus, molecules built around the
benzamidine are
desirable binding moieties for serine protease inhibitors.
In one embodiment, the photoactive compound is represented by Formula 1,
wherein
R1, R2, R3, R4, X1, and X2 are independently hydrogen, C1-C6 alkyl, C1-C6
acyl, C1-C6
alkoxycarbonyl, C1-C6 hydroxyalkyl, C1-C6 polyhydroxyalkyl, C1-C6
carboxyalkyl, C1-C6
aminoalkyl, C5-C10 aryl, ¨(CH2)m¨Y¨C(=NH)NH2, ¨(CH2)m(CHR5),¨Y¨C(=NH)NH2,
¨(CF12)m(CHR5),-,¨Y¨NHC(=NH)NH2, ¨(CI-12)m¨Y¨NHC(=NH)NH2, ¨0¨Y¨C(=NH)NH2,
CA 02695197 2010-01-29
WO 2009/018405 PCT/US2008/071700
8
-0-Y-NHC(=NIA)NH2, -N(R6)-Y-C(=NFI)NF12, or -N(R7)-Y-NFIC(=NH)NF12. In some
embodiments, at least one of R1, R2, R3, R4, X', or X2 must be -(CH2),,-Y-
C(=NH)NI-12,
-(CH2),,(CFIR5)õ-Y-C(=NF)NH2, -(CI-12)m(CHR5)n-Y-NHC(=N11)NH2,
-(CH2)m-Y-NHC(=NH)NH2, -0-Y-C(=NH)NH2, -0--Y-NHC(=NH)NH2,
-N(R6)-Y-C(=NH)NH2, or -N(R7)-Y--NHC(=NH)NFI2. Y is any of the substituents
listed
below:
A 1-1
-(CI12)p- ' 7 \ ' I I ' __________________ \ / ' -CF) '
( \N / Nr) ____________ / -N I/ \
-- 'N- / Or --1 =
/ \--/ `1...k.
The subscripts 'm,"n; and p' independently range from 0 to 6, inclusive. R5 is
hydroxyl,
C1-C6 hydroxyalkyl, carboxyl, C1-C6 carboxyalkyl, amino, or C1-C6 aminoalkyl.
R6 and R7
are independently hydrogen, C1-C6 alkyl, C1-C6 acyl, C1-C6 alkoxycarbonyl, C1-
C6
hydroxyalkyl, C1-C6 polyhydroxyalkyl, C1-06 carboxyalkyl, C1-C6 aminoalkyl, or
C5-C10 aryl.
In another embodiment, the photoactive compound is represented by Formula 1,
wherein: R1 to R4, R6, and X1 are hydrogens; R5 is carboxyl; X2 is -(C1-12)m-Y-
C(=NE)NF12,
--(CH2),,-Y-NFIC(=NH)NH2, -(CF12)õ,(CHR5)n-Y-C(=NH)N1-12,
-(0H2)m(CHR5)n-Y-NHC(=NFI)NH2, -N(R6)-Y-C(=NH)NE12, or -N(R7)-Y-NHC(=NH)NF12;
'm'
-(CH2)- or -L.,..........T
and 'n' independently range from 0 to 3; `p' is 1; and Y is .
In another embodiment, the photoactive compound is represented by Formula 1,
wherein: R1 to R4 and R6 are hydrogens; R5 is carboxyl; X1 and X2 are
independently
-(CF12)m-Y-C(=NI-1)NH2, -(CH2)m-Y-NHC(=NI-1)NH2, -(CH2)m(CHR5)-Y-C(=NFI)NH2,
-(CH2)m(CHR5),-Y-NI-1C(=NH)NH2, -N(R6)-Y-C(=NI-1)NH2, or -N(R7)-Y-NHC(--
71\1H)NF12:
-(CI-12)- or - 4--
L.,\,......z2
'm and 'n' independently range from 0 to 3; 'p' is 1; and Y is .
In another embodiment, the photoactive compound is represented by Formula 1,
wherein: R2 to R4, Xl, and X2 are hydrogens; R1 is -(CH2),,,,-Y-C(=N1-1)N1-12.
-(C1-12),-,,-Y-NliC(=NH)NH2, -(CI-12)m(CHR5)n-Y-C(=NH)NH2, or
-(CF12),õ(CHR5)n-Y-NI-IC(=NFI)N1-12; 'm' and 'n' independently range from 0 to
3; 'p' is 1; and
f'
-(CH2)- o r -1, . ....: 3 -
. . , . . ,
Y is .
In another embodiment, the photoactive compound is represented by Formula 1,
wherein: R2, R4 to R6, X1, and X2 are hydrogens; R1 and R3 are independently
CA 02695197 2010-01-29
WO 2009/018405
PCT/US2008/071700
9
-(CF12)m-Y-C(=NF)NI-12, ¨(CH2)m¨Y¨NHC(=NH)NH2, ¨(CH2)m(CHR6)n¨Y¨C(=NH)NH2, or
¨(CH2)4CHR5)õ¨Y¨NHC(=NH)1\11-12; 'm' and 'n' independently range from 0 to 3;
`p' is 1; and Y
¨(cHon¨ or ¨
is
In another embodiment, the photoactive compound is represented by Formula 1,
wherein: R1 to R4, R6, and X1 are hydrogens; R5 is carboxyl; X2 is
¨(CH2)m¨Y¨C(=NH)NF12,
--(CH2)m(CHR5)n¨Y¨NHC(=NH)NH2, or ¨N(R6)¨Y¨C(=NH)NH2; 'm' and 'n independently
range
¨(CH2),¨ or ¨
from 0 to 3; `p' is 1; and Y is
In another embodiment, the photoactive compound is represented by Formula 1,
wherein: R1 to R4 and R6 are hydrogens; R5 is carboxyl; X' and X2 are
independently
¨(CH2)m¨Y¨C(=NH)NF12, ¨(CH2)m(CHR6),¨Y¨NHC(=NH)NH2, or ¨N(R6)¨Y¨C(=NEI)NH2;
¨(CH2)n¨ or ¨
and 'n' independently range from 0 to 3; `p' is 1; and Y is
In another embodiment, the photoactive compound is represented by Formula 1,
wherein: R2 to R4, X', and X2 are hydrogens; R1 is ¨(CH2)n,¨Y¨C(=NH)NH2, or
¨(CH2),(CHR5)n¨Y¨NHC(=NH)NH2; 'm' and 'n' independently range from 0 to 3; 'p'
is 1; and Y
¨(CH2),¨ or ¨0¨
'
is
In another embodiment, the photoactive compound is represented by Formula 1,
wherein: R2, R4 to R6, X1, and X2 are hydrogens; R1 and R3 are independently
¨(CH2)m¨Y¨C(=NH)NH2 or ¨(CH2)/1(CHIR5)n¨Y¨NHC(=NH)NH2; 'm' ranges from 0 to 3;
'p' is 1;
-(CH2)r,-- Or -0-
and Y is
In another embodiment, the photoactive compound is a fully symmetric di-basic
serine
protease inhibitor, such as Compound 2 or Compound 3 below.
CA 02695197 2010-01-29
WO 2009/018405
PCT/US2008/071700
0
io
H2N
A,N NH2 vi Iity tio NH2
N
H2N N
NH 0
Compound 2
0
1-10 NH
H2NNH2
N¨
NH
HN OH
0
Compound 3
In another embodiment, the photoactive compound is a reverse-turn mimetic
structure
such as Compound 4 or Compound 5 below.
HOIINN H2 4110
NH2
HN
0
H2N
Compound 4
NH
HO
H2N)
N N
HN HN HN
0
H2N
Compound 5 NH
H2N
5 In another embodiment, the photoactive compound is a mono-
benzamidine-containing
compound such as Compound 6 or Compound 7 below.
CA 02695197 2010-01-29
WO 2009/018405
PCT/US2008/071700
11
0
HO)N,...,õõõ NH2
H N H2
0
Compound 6
0 NH
RHN N N H2 H N H2
H2N
0
Compound 7
In another embodiment, the photoactive compound is a guanidine derivative such
as
Compound 8 below.
RHI\IjX, NH2
H2N N
0
Compound 8 HNic., NH2
NH
Compounds of the present invention possess both thrombin inhibitory activity
(e.g.,
through inhibition of Tissue Factor/Factor Vila and/or Factor Xa) and a
fluorescent scaffold. If the
clotting cascades of Fig. 1 have been activated (e.g., due to pathogenic
events such as trauma,
deep vein thrombosis, plague rupture, cardiac arrhythmias etc.), thrombin
concentrations in the
blood will be increased. After being administered to a patient (e.g.,
intravenously and/or
intraarterially injected into a patient's blood stream), a compound of the
present invention binds
to Factor Xa and/or Tissue Factor/Factor Vila and is detectable via a shift in
fluorescence
emission versus that of the unbound fraction of the compound.
In this regard, it is known from fluorescence and Raman spectroscopic studies
of green
fluorescent and red fluorescent proteins as well as model compounds, that the
chromophore triad
displays remarkably different photophysical properties when buried inside the
protein versus
diffusing freely in solvent. The energy of reorganizing the environment to
accommodate the
excited state of the photoactive serine protease inhibitor is quite different
when the molecule is
bound in the active site of the enzyme versus circulating freely in the blood.
Thus, free versus
bound inhibitors display different absorption and emission wavelengths. This
phenomenon
CA 02695197 2010-01-29
WO 2009/018405
PCT/US2008/071700
12
allows for the possibility of accurate real-time measurement of Tissue
Factor/Factor Vila
inhibition and/or Factor Xa inhibition and, therefore, ultimately, thrombin
production (e.g.,
concentration) in blood by a ratiometric paradigm. The concentrations and/or
fluorescence
measurement ratios for Tissue Factor/Factor Vila or Factor Xa inhibition can
(if desired) be
cross-correlated with actual PT (prothrombin time) measurements in animals or
patients to
develop a mathematical model for accuracy. The concentrations and/or
fluorescence
measurement ratios can thus be used to determine whether or not a patient is
experiencing an
enhanced propensity for a thrombotic event and enable healthcare personnel to
take corrective
action.
A fiber optic laser apparatus can be used at the bed-side, in the surgical
suite, or on
board emergency transport vehicles to monitor patient coagulation hemostasis
in real time. This
methodology potentially allows for precise monitoring of patients at risk for
cloning related events.
Typically, such patients are maintained on powerful anticoagulants (e.g.,
warfarin or heparin) and
are therefore at risk of potentially catastrophic bleeding events.
It is well established that vulnerable plaque rupture is the main cause of
acute coronary
syndromes. The thrombogenicity of these plaques tends to correlate directly
with the Tissue
Factor level within them. Arterial thrombosis therefore frequently tends to be
a consequence of
plaque rupture, which exposes that Tissue Factor to circulating blood
resulting in the Tissue
Factor combining with Factor VII to trigger coagulation (Moreno, P.R. et al
Circulation 1996, 94.
3090 and Tanner, F.C. et al Circulation 2006, 113, 722). It is believed that
micro-ruptures can
expose Tissue Factor on the surfaces of vulnerable plaques triggering an
unstable pre-
thrombogenic situation. Administration of an integrated photonic Tissue
Factor/Factor Vila
inhibitor such as those described herein would thus provide a way to monitor
and image these
unstable plaques prior to a catastrophic rupture event. The integrated
photonic Tissue
Factor/Factor Vila inhibitor would accumulate at the micro-rupture sites by
binding to surface
adhered Tissue Factor/Factor Vila and be visible by use of a catheter or other
appropriate
detection device (e.g., fiber optic laser apparatus). Since these photoactive
agents are also
pharmaceutically active, this methodology constitutes not only a diagnostic
tool, but also a
diagnostic-therapeutic pair.
With regard to tumor imaging, tumor metastases are typically dependent upon
Tissue
Factor over-expression. Indeed, metastatic cells may express up to 1000-fold
more Tissue
Factor than non-malignant counterparts (e.g., in colorectal and pulmonary
cancers). In addition,
tumor tissue is associated with the over-expression of vascular endothelial
growth factor (VEGF),
which is a factor important in the process of angiogenesis supporting tumor
growth and
metastasis. The expression of VEGF is therefore associated with endothelial
permeability
potentially enabling Tissue Factor exposure to circulating Factor VII. Thus,
the administration of
an integrated photonic Tissue Factor/Factor Vila inhibitor would provide a way
to monitor and
CA 02695197 2010-01-29
WO 2009/018405
PCT/US2008/071700
13
image tumor boundaries on surfaces and sub-surfaces of tumor tissue exposed to
blood. The
integrated photonic Tissue Factor/Factor Vila inhibitor would accumulate in
the tumor via
enhanced endothelial permeability induced by VEGF by binding to the exposed
Tissue
Factor/Factor Vila and be visible by use of a catheter or other appropriate
detection device (e.g.,
fiber optic laser apparatus). Since these photoactive agents are also
pharmaceutically active,
this methodology constitutes not only a diagnostic tool, but also a diagnostic-
therapeutic pair.
Formulation
Integrated photoactive agents of the present invention can be formulated for
enteral (oral
or rectal), parenteral (e.g., intramuscular injection, intravenous injection,
intraarterial injection),
topical, transdermal, or subcutaneous administration. Topical, transdermal,
and cutaneous
delivery can include aerosols, creams, gels, emulsions, solutions, or
suspensions. Delivery into
and through the skin can be enhanced in accordance with known methods and
agents such as
transdermal permeation enhancers, for example, "azone", N-alkylcyclic amides,
dimethylsulfoxide, long-chained aliphatic acids (C10), etc. (Gennaro).
Currently, preferred
formulations for photoactive agents of the inventions are those that may be
administered via
injection (e.g., intramuscular injection, intravenous injection, intraarterial
injection).
Preparation of pharmaceutically acceptable formulations can be accomplished
according
to methods known in the art (Gennaro). For instance, a formulation may be
prepared using any
of the integrated photoactive agents, along with pharmaceutically acceptable
buffers, surfactants,
excipients, thixotropic agents, flavoring agents, stabilizing agents, or skin
penetration enhancing
agents. If the inventive compound is water soluble, a solution in
physiological saline may be
administered. If the compound is not water soluble, the compound can be
dissolved in a
biocompatible oil (e.g., soybean oil, fish oil, vitamin E, linseed oil,
vegetable oil, glyceride esters,
long-chained fatty esters, etc.) and emulsified in water containing surface-
active compounds
(e.g., vegetable or animal phospholipids; lecithin; long-chained fatty salts
and alcohols;
polyethylene glycol esters and ethers; etc.), and administered as a topical
cream, suspension,
water/oil emulsion, or water/oil microemulsion.
The integrated photoactive agents of the invention may be encapsulated into
micelles,
liposomes, nanoparticles (e.g., shell cross-linked nanoparticles), dendrimers,
dendrons,
microcapsules, or other organized microparticles, and administered by any of
the routes
described previously. The integrated photoactive agents of the invention may
be chemically
conjugated to nanoparticles (e.g., shell cross-linked nanoparticles),
dendrimers or dendrons for
the purpose of simultaneously effecting an integrated photonic effect and a
multivalent biological
effect. These types of formulations may enhance stability of the agents in
vivo. Exemplary
encapsulation methods include, but are not limited, detergent dialysis, freeze
drying, film forming,
CA 02695197 2010-01-29
WO 2009/018405
PCT/US2008/071700
14
and injection (Janoff et al). Methods of making liposomes and encapsulating
various molecules
within them are well known in the art (Braun-Falco et al and Lasic et al).
Dosage
Integrated photoactive agents of the present invention may be administered in
a single
dose or in multiple doses to achieve the effective diagnostic and/or
therapeutic objective. After
administration, the integrated photoactive agent is allowed time to bind to
serine protease.% and
the selected target site is exposed to light with a sufficient power and
intensity to detect light
emanating from the compound within the patient's body to provide information
that may be
utilized by a healthcare provider (e.g., in making a diagnosis). Doses may
vary widely depending
upon, for example, the particular integrated photoactive agent employed, the
areas (e.g., organs
or tissues) to be examined, the equipment employed in the clinical procedure,
the efficacy of the
treatment achieved, and/or the like. For example, the dosage of the compound
may vary from
about 0.1 mg/kg body weight to about 500 mg/kg body weight in some
embodiments. In other
embodiments, the dosage of the compound may vary from about 0.5 to about 2
mg/kg body
weight. In some embodiments for parenteral administration, a sterile solution
or suspension
comprises the integrated photoactive agent in a concentration range from about
1 nM to about
0.5 M. In some embodiments, a sterile solution or suspension comprises the
integrated
photoactive agent in a concentration range from about 1 pM to about 10 mM.
Administration
Once an integrated photoactive agent has been created, the agent is
administered (e.g.,
via injection) to an individual. An appropriate amount of time may be given
for the agent to bind
to the desired serine protease(s) in the patient. It will be understood that
administration of
compounds and compositions of the present invention may be determined by an
attending
physician within the scope of sound medical judgment. The specific effective
dose level for any
particular patient may depend upon a variety of factors such as the disorder
being
treated/diagnosed, the severity of the disorder, activity of the specific
compound employed, the
specific composition employed, age, body weight, general health, sex, and/or
diet of the patient.
Detection of the integrated photoactive agent may be achieved by optical
fluorescence,
absorbance, and/or light scattering methods using invasive and/or non-invasive
probes such as
endoscopes, catheters, ear clips, hand bands, head bands, surface coils,
finger probes, and/or
the like (Muller et al). Imaging can be achieved using planar imaging, optical
tomography, optical
coherence tomography, endoscopy, photoacoustic technology, sonofluorescence
technology,
light scattering technology, laser assisted guided surgery (LAGS), confocal
microscopy, dynamic
organ function monitoring, and/or light scattering devices.
CA 02695197 2010-01-29
WO 2009/018405
PCT/US2008/071700
Abbreviations and Definitions
To facilitate understanding of the invention, a number of terms are defined
below:
"Small molecule" refers to a molecule whose molecular weight is generally less
than
about 1000 Da!tons.
5 "Diagnostically effective amount" is an amount of the substance in
question which will, in
a majority of patients, be an adequate quantity of substance to be able to
detect the particular
cells and/or enzymes, if present, in the patient to whom it is administered.
The term "an effective
amount" also implies that the substance is given in an amount which only
causes mild or no
adverse effects in the subject to whom it has been administered, or that the
adverse effects may
10 be tolerated from a medical and pharmaceutical point of view in the
light of the severity of the
disease for which the substance has been given.
"Photoactive group" or "photoactive moiety' refers to any functional group or
moiety
exhibiting an absorption, excitation, and emission maxima in the wavelength
range of about 350-
1200 nm. Such functional groups or moieties include, but are not limited to,
fluorophores,
15 chromophores, photosensitizers, and photoreactive moieties, wherein
"fluorophores,''
"chromophores," "photosensitizers," and "photoreactive" moieties have meanings
that are
commonly understood in the art,
"Therapeutically-effective amount" refers to the amount of each agent that
will achieve
the goal of improvement in pathological condition severity and the frequency
of incidence over
treatment of each agent by itself, while avoiding adverse side effects
typically associated with
alternative therapies.
"Treatment" refers to any process, action, application, therapy, or the like,
wherein a
subject, including a human being, is provided medical aid with the object of
improving the
subject's condition, directly or indirectly, or slowing the progression of a
pathological condition in
the subject.
When introducing elements of the present invention or the embodiment(s)
thereof, the
articles "a", "an", and "the" are intended to mean that there are one or more
of the elements. The
terms "comprising", "including" and "having" are intended to be inclusive and
mean that there
may be additional elements other than the listed elements.
The following example illustrates specific embodiments of the invention. As
would be
apparent to skilled artisans, various changes and modifications are possible
and are
contemplated within the scope of the invention described.
CA 02695197 2010-01-29
WO 2009/018405
PCT/US2008/071700
16
EXAMPLE 1 - Preparation of 3,6-diamino-N2,N5-bis(4-
carbamimidovlbenzvl)pvrazine-
2,5-dicarboxamide .(MP3117)
0 NH
NUNN
H2
H2N H NH2
H2N N
NH 0
MP3117
A mixture of 3,6-diaminopyrazine-2,5-dicarboxylic acid (250 mg, 1.40 mmol), 4-
(aminomethyl)-benzamidine dihydrochloride (619 mg, 2.80 mmol), HOBt-H20 (628
mg, 4.10
mmol), EDC-HCI (790 mg, 4.10 mmol) and triethylamine (2 mL) was stirred
together in DMF
(20 mL) for 16 hours at room temperature. The mixture was concentrated to
dryness and
purified by medium pressure reversed phase chromagraphy (LiChroprep RP-18
Lobar (B)
25 x 310 mm EMD chemicals 40-63 pm, ¨70 g, 90/10 to 80/20 0.1% TFA-ACN) to
afford
171 mg (27% yield) of example 1 as an orange foam: LCMS (5-95% gradient
acetonitrile in
0.1% TEA over 10 min), single peak retention time = 4.69 min on 250 C18 mm
column,
(WH). = 461.
The compound (MP3117) of Example 1 was tested for its ability to inhibit the
production of thrombin via activation of the extrinsic cascade. In particular,
an assay that
included a whole blood activity screen was performed. In this regard, nine
aqueous solutions
containing varying concentrations of the compound of Example 1 were tested for
their ability
to inhibit thrombin production when clotting was activated via the tissue
factor pathway (in
triplicate). The average of the data is shown in Fig. 4. The data from the
assay was also
utilized to extract the IC50 for the compound. Incidentally, "IC" refers to
the amount of a
compound necessary to inhibit 50% of the active enzyme to which it binds. As
shown in Fig.
4, the compound of Example 1 was shown to have an IC50 = 12.38 0.79 nM.
CA 02695197 2010-01-29
WO 2009/018405
PCT/US2008/071700
17
REFERENCES
1. Bajaj S P; Joist J H New insights into how blood clots: implications for
the use of APTT and
PT as coagulation screening tests and in monitoring of anticoagulant therapy.
Seminars in
Thrombosis and Hemostasis 1999, 25(4), 407-418.
2. Kher A; Al Dieri R; Hem ker H C; Beguin S Laboratory assessment of
antithrombotic
therapy: what tests and if so why?. Haemostasis 1997, 27(5), 211-218.
3. Riley, Roger S.; Rowe, David; Fisher, Lyman M. Clinical utilization of
the international
normalized ratio (I NR). Journal of Clinical Laboratory Analysis (2000),
14(3), 101-114.
4. Hassan, M.; Klaunberg, B.A. Biomedical applications of fluorescence imaging
in vivo.
Comparative Medicine 2004, 54(6), 635-644.
5. Licha, K.; Olbrich, C. Optical imaging in drug discovery and diagnostic
applications.
Advances in Drug Delivery Reviews 2005, 57(8), 1087-1108.
6. Shah, K.; Weissleder, R. Molecular optical imaging: applications leading
to the
development of present day therapeutics. NeuroRx 2005, 2(2), 215-225.
7. Vasquez, M.E. et al. 6-N,N-Dimethylamino-2,3-naphthalamide: A new
environment-
sensitive fluorescent probes in 0- and 0-selective opioid peptides. Journal of
Medicinal
Chemistry 2006, 49, 3653-3658.
8. Solban N.; Ortel, B.; Pogue, B.; Hasan, T. Targeted optical imaging and
photodynamic
therapy. Ernst Schering Research Foundation Workshop 2005, 49, 229-258.
9. Jain, R.K. Barriers to Drug Delivery in Solid Tumors. Scientific
American 1994, 271, 58-
65.
10. Hunter, D.H., and Luyt L.G. Single isomer technetium-99m tamoxifen
conjugates.
Bioconjugate Chemistry 2000, 11, 175-181.
11. Rajagopalan, R. Nitrogen sulfur ligands as opiate receptor drug mimics.
U.S. Patent
1994: 5,330,737.
12. Rajagopalan, R. Metal containing steroid mimics and ligands useful in the
preparation
thereof. U.S. Patent 1997: 5,602,236.
13. Horn, R.K.; Katzenellenbogen, J.A. Synthesis of oxorhenium(V) complex
mimic of a
steroidal estrogen. J. Org. Chem. 1997, 62, 6290-6297.
14. Skaddan, M.B.; Katzenellenbogen, J.A. Integrated oxorhenium(V) complexes
as
estrogen mimics. Bioconjugate. Chem. 1999, 10, 119-129.
15. Miyata, K., et al. Synthesis and properties of a new fluorescent bicyclic
4-N-carbamoyl-
deoxycytidine derivative. Organic Letters 2008, 8(8), 1545-1548.
16. Muller et al. Eds, Medical Optical Tomography, SPlE Volume IS11, 1993.
17. Waldman, S. ST receptor binding compounds and methods of using the same
U.S.
Patent 1996: 5,518,888.
18. Edelberg, J., Ballard, V. Restoring vascular Function. U.S. Publication
No.
20060172943; PCT W02005009366.
19. Schneider, H. et al. A novel peptide, PLAEIDGIELTY, for the targeting of
cE,131-integrins.
FESB Letters 1998, 429(3), 269-273.
20. Nothnick, W.B. Expert Opinion on Therapeutic Targets 2004, 8(5), 459-471.
21. Mayo, K et al. Partial peptide mimetic& and methods. PCT WO 03/070751; US
Publication No. 20040053828.
22. Jaalouk, D. et al. Compositions and methods that related peptides that
bind selectively to
leukemia cells. PCT 2006, WO 06/10070. US Patent Publication 2004/0586814.
23. A.R. Gennaro (Ed.). Remington: The Science and Practice of Pharmacy, 20th
Edition.
Lippincott Williams 8, Wilkins: Baltimore, 2000.
24. Janoff, A.S. et al. Methods of preparing low-toxicity drug-lipid
complexes. U.S. Patent
2002: 6,406,713.
25. Braun-Falco et al. (Eds.). Liposome Dermatics. Griesbach Conference,
Springer-Verlag:
Berlin, 1992.
26. Lasic and Martin (Eds.). Stealth Liposomes. CRC Press: London, 1995.