Note: Descriptions are shown in the official language in which they were submitted.
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
Nucleotide Triphosphate With An Electroactive Label Conjugated To The Gamma
Phosphate
Priority Application
This application claims priority from US provisional application no.
60/960,398 filed
September 27, 2007.
Field of the Invention
[0001] The present invention relates to a novel electroactive nucleotide
triphosphate useful to monitor events associated with phosphorylation.
Background of the Invention
[0002] In the cellular communication network, many enzymes and receptors are
switched "on" or "off', or in other terms, "phosphorylated" and
"dephosphorylated".
During phosphorylation, a phosphoryl group from ATP is transferred to specific
serine,
threonine, or tyrosine residue of a protein. As a result of these
modifications, the function
or localization of the protein may change, which in some cases may lead to the
formation
of oncoproteins.l
[0003] Abnormal protein phosphorylation is a cause of major diseases,
including
cancer, diabetes and chronic inflammatory diseases.2 Analytical methods to
quantify
protein kinase activity are critical for understanding their role in the
diagnosis and
therapy of these diseases. Current methods for the detection of protein
phosphorylation
rely on radio-labeled ATP,3 fluorescence-based methods,4 and fluorescence
resonance
energy transfer (FRET).5 Recently, biotin-conjugated ATP molecules have been
exploited for the detection of phosphorylation reactions.6 However, additional
modification of the peptides with an electro-active or optical label is
necessary, which
increases the cost and causes tedious and time-consuming handling procedures.
[0004] It would be desirable, thus, to develop an alternative method of
monitoring
or detecting events associated with phosphorylation which overcomes at least
one of the
disadvantages of the current detection methods.
2627524.4
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
Summary of the Invention
[0005] A novel electroactive nucleotide triphosphate has now been developed
which is useful in an alternative method of monitoring and/or detecting events
associated
with phosphorylation, including phosphorylation itself.
[0006] Thus, in one aspect of the present invention, a nucleotide triphosphate
conjugate comprising an electroactive labelled gamma phosphate group is
provided.
[0007] In another aspect of the invention, a method of detecting the
phosphorylation of a kinase substrate is provided comprising:
(a) immobilizing the substrate on an electrode surface;
(b) incubating the immobilized substrate with a kinase and a nucleotide
triphosphate conjugate comprising an electroactive-labelled gamma phosphate
under conditions which permit detection of phosphorylation activity; and
(c) detecting phosphorylation of the substrate.
[0008] In one aspect the phosphorylation is detected electrochemically. In
another aspect the phosphorylation is detected by spectroscopy, including mass
spectroscopy.
[0009] In another aspect of the invention, a method of detecting a kinase of
interest in a sample is provided comprising:
(a) immobilizing a substrate specific for the kinase of interest on an
electrode
surface;
(b) incubating the immobilized substrate with the sample and a nucleotide
triphosphate conjugate comprising an electroactive labelled gamma phosphate
under conditions which permit detection of phosphorylation activity; and
(c) detecting phosphorylation of the substrate,
wherein phosphorylation of the substrate indicates the presence of the kinase
in
the sample.
2
2627524.4
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
[0010] In yet another aspect of the invention, a method of identifying a
candidate
kinase substrate is provided comprising:
(a) immobilizing the candidate kinase substrate on an electrode surface;
(b) incubating the immobilized substrate with a kinase-containing electrolyte
and
a nucleotide triphosphate conjugate comprising an electro-active labelled
gamma
phosphate under conditions which permit detection of phosphorylation activity;
and
(c) detecting phosphorylation of the substrate, wherein phosphorylation of the
candidate substrate indicates that said candidate is a substrate of the
kinase.
[0011] In another aspect of the invention, a method of screening candidate
compounds that modulate kinase activity is provided comprising:
(a) immobilizing a substrate of a kinase on an electrode surface;
(b) incubating the immobilized substrate with a kinase, a candidate compound
and a nucleotide triphosphate comprising an electroactive-labelled gamma
phosphate under conditions which permit detection of phosphorylation activity;
and
(c) detecting a level of phosphorylation of the substrate, wherein a change in
the phosphorylation level from a level of phosphorylation that is achieved in
the
absence of said compound indicates that said compound modulates the activity
of
the kinase.
[0012] In another aspect of the invention, a method of high-throughput
screening
a sample for the presence of protein kinases is provided comprising:
(a) providing a microelectrode array comprising a plurality of electrodes;
(b) immobilizing kinase substrates to each electrode in the array;
3
2627524.4
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
(c) incubating the microelectrode array carrying the immobilized substrates
with
the sample of interest and a nucleotide triphosphate comprising an
electroactive-
labelled gamma phosphate; and
(d) detecting the phosphorylation level of the substrates in each electrode,
wherein
phosphorylation of one or more substrates in the plurality of electrodes
indicates
the presence in the sample of the kinase specific to the one ore more
phosphorylated substrates.
[0013] In another aspect of the invention, a method of diagnosing in a subject
a
disease associated with abnormal levels or absence of a protein kinase is
provided
comprising:
(a) immobilizing a substrate of the kinase associated with the disease to one
or
more electrodes;
(b) incubating the one or more electrodes carrying the immobilized substrate
with
a sample from the subject and a nucleotide triphosphate comprising an
electroactive-labelled gamma phosphate; and
(c) detecting the phosphorylation level of the substrates in the electrodes of
each
array, wherein an abnormal level or absence of phosphorylation in the
subject's
sample with respect to a normal control indicates that the subject has, or is
susceptible to, the disease.
[0014] In a further aspect, there is provided a kinase biosensor comprising at
least
one kinase substrate immobilized on an electrode surface, wherein said
electrode surface
is immersed in an electrolyte comprising an electroactive nucleotide
triphosphate having
an electroactive-labelled gamma phosphate.
[0015] In yet another aspect, there is provided a kit for screening kinase
phosphorylation characterised in that the kit comprises at least one kinase
substrate, an
electrode, the nucleotide triphosphate conjugate comprising an electroactive
labelled
gamma phosphate group and a kinase.
4
2627524.4
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
[0016] One or more advantages of at least some of these aspects include (i)
novel
electroactive nucleotide triphosphate conjugate suitable for monitoring and/or
detecting
events associated with phosphorylation, including phosphorylation itself, (ii)
novel
electroactive nucleotide triphosphate conjugate can be produced at a
significantly lower
cost compared to other methods of monitoring and/or detecting phosphorylation,
(iii)
methods of detecting or monitoring events associated with phosphorylation,
including
phosphorylation itself, do not require modification of peptides with an
electro-active or
optical label, and (iii) novel electroactive nucleotide triphosphate conjugate
facilitates,
simplifies and speeds the procedures involved with the monitoring and
detection of
phosphorylation events, including phosphorylation itself. In particular, the
novel
electroactive nucleotide triphosphate conjugate of the invention can be used
in the.
discovery of new drugs, molecular diagnostics and molecular targeting.
[0017] These and other aspects of the invention will become apparent from the
detailed description that follows, and the following figures in which:
Brief Description of the Drawings
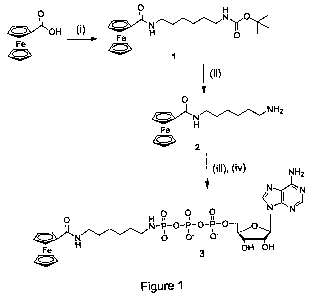
[0018] Figure 1 is a schematic illustrating the synthesis of an electroactive
ferrocene-ATP conjugate;
[0019] Figure 2 is a schematic illustrating the use of a metallocene-ATP
conjugate in a method of electrochemically detect phosphorylation of a
substrate;
[0020] Figure 3 illustrates cyclic voltammograms obtained using various
ferrocene-ATP concentrations (a-d) in the method of Fig. 2;
[0021] Figure 4 illustrates cyclic voltammograms obtained in the presence (a)
and
absence (b) of PKC in the method of Fig. 2;
[0022] Figure 5 illustrates square-wave voltammograms obtained in the presence
(a) and absence (b) of PKC in the method` of Fig. 2;
[0023] Figure 6 graphically illustrates the dependence of current density
responses on the reaction time of the method of Fig. 2;
2627524.4
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
[0024] Figure 7 illustrates a microelectrode array; and
[0025] Figure 8 (A - D) illustrates the use of a microelectrode array.
[0026] Figure 9 (A) illustrates square-wave voltammograms of the CK2-catalyzed
phosphorylation reactions performed in cell lysates containing (a) over-
expressed CK2a,
(b) endogenous CK2 levels, (c) the over-expressed kinase-dead CK2a, (d) normal-
expressed CK2a.
[0027] Figure 9 (B) is a plot for the detection of the CK2a over-expression
state
in Hela cell lysates.
[0028] Figure 10 (A) is a cyclic voltammograms for CK2a'-catalyzed
phosphorylation of substrate peptide in the presence (a) - (e) of different
CK2a'
concentrations and in the absence (f) of the enzyme;
[0029] Figure 10 (B) illustrates the effect of the CK2a'concentration on the
current responses using substrate peptide modified electrodes (a) in the assay
buffer, (b)
in the presence of HeLa cell lysate and (c) control experiment.
[0030] Figure 11 (A) illustrates cyclic voltammograms for the inhibition of
CK2a-catalyzed phosphorylation of the substrate peptide in the presence of the
inhibitor,
(1) TBB (4,5,6,7-Tetrabromo-2-azabenzimidazole) at different concentrations
(a)-(e), and
control (f) experiment in the absence of CK2a.
[0031] Figure 11 (B) illustrates Lineweaver-Burk plot for the determination of
kinetics of the CK2a'-catalyzed phosphorylation.
[0032] Figure 11 (C) illustrates control experiments for Figure 11 (A).
[0033] Figure 12 (A) illustrates CV for the inhibition of tyrosine kinase-
catalyzed
phosphorylation with the Signal Transduction Protein (STP) peptide in the
presence of
(a)-(d) and in the absence (e) of Abll-T3151.
6
2903408.1
RECTIFIED SHEET (RULE 91.1)
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
[0034] Figure 12 (B) Illustrates the Lineweaver-Burk plot for the
determination of
kinetics of the Ab11-T3151-catalyzed phosphorylation of the immobilized STP
peptide
[0035] Figure 12 (C) illustrates a plot for the dependence of the anodic
current
responses on the amount of the Abl l -T315I kinase in the presence of HeLa
cell lysate
with the STP peptide (a), (b) and control experiments (c).
[0036] Figure 12 (D) illustrates a plot for the dependence of current
responses on
the concentration of the general protein kinase inhibitors (b) and (c) and
control
experiments (a).
[0037] Figure 13(A) illustrates cyclic voltammograms for the inhibition of
tyrosine kinase-catalyzed phosphorylation with the FLT3 peptide in the
presence of
HER2/ErbB2 at different concentrations (a), (b) and (c).
[0038] Figure 13 (B) illustrates Lineweaver-Burk plot for the determination of
kinetics of the HER2/ErbB2-catalyzed phosphorylation.
[0039] Figure 13 (C) illustrates a plot for the dependence of the anodic
current
responses on the amount of the HER2/ErbB2 kinase in the presence (a) and
absence (b)
and (c) of the substrate peptide.
[0040] Figure 13 (D) illustrates a plot for the dependence of J responses on
the
concentration of N-Benzoylstaurosporine.
[0041] Figure 14 illustrates mass spectroscopy (MS) plot of kinase-catalized
phosphorylation of substrate peptides.
Detailed Description of the Invention
[0042] A novel electroactive nucleotide triphosphate conjugate is provided.
The
nucleotide triphosphate comprises an electroactive-labelled gamma phosphate
which is
useful in a method of detecting phosphorylation activity of a kinase. In one
embodiment,
the method comprises immobilizing at least one substrate of the kinase on an
electrode
surface, incubating the immobilized substrate with the electroactive
nucleotide
7
2903408.1
RECTIFIED SHEET (RULE 91.1)
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
triphosphate conjugate in the presence of the kinase under conditions which
permit
detection of phosphorylation activity and detecting phosphorylation of the
substrate. In
one aspect the phosphorylation activity is detected electrochemically. In
another aspect
the phosphorylation activity is detected by mass spectroscopy.
[0043] The term "electroactive" is used herein to denote that the transferable
gamma phosphate comprises a label that is detectable on application of an
electric field.
Examples of an electroactive label include organic labels and organometallic
labels. In
one aspect the electroactive label includes a metallocene, including
substituted
metallocenes or a derivative thereof which is compatible with an aqueous
environment.
The metallocene may be, for example, ferrocene, cobaltocene or derivatives
thereof.
Substituted metallocenes such as halogen-substituted metallocenes, metallocene
comprising an amide-substituted cyclopentadiene or other derivatives such as
ansa-
metallocenes, metallocenium cations such as ferrocenium, [Fe(C5H5)2]+, triple
decker
complexes (compounds with three Cp anions and two metal cations in alternating
order,
may also be used. In another aspect the electroactive label includes quinines,
nitro
hetercycles, NAD+, NADP+, nitrogen-containing aromatics and heterocycles.
[0044] The term "nucleotide triphosphate" is meant to refer to adenosine-5'-
triphosphate (ATP) and nucleotide derivatives thereof, for example, comprising
substituted adenosine derivatives at the 6 amino position. Substituents may
include, for
example, methoxy, ethoxy, pentyl, hexyl, benzyl and substituted benzyl as well
as 5- and
6-membered ring structures comprising the nitrogen of the amino group.
[0045] In one embodiment, the electroactive nucleotide triphosphate may be a
metallocene-ATP conjugate comprising a metallocene-labelled gamma phosphate
formed by conjugation of a metallocene or derivative thereof to ATP. The
metallocene-
ATP conjugate may be formed using a synthetic protocol in which a carboxylated
metallocene compound is treated to yield a Boc-protected or an N-protected
conjugate
that is combined with a reactive form of ATP to yield the desired conjugate.
The identity
of the metallocene-ATP conjugate may be confirmed using known techniques such
as
8
2903408.1
RECTIFIED SHEET (RULE 91.1)
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
NMR spectroscopy or mass spectrometry to identify the phosphoramide bond at
the y
position.
[0046] The electroactive nucleotide triphosphate, such as a metallocene-ATP
conjugate, may be used in an assay to detect kinase-catalyzed phosphorylation.
In one
aspect of the present invention the assay includes an electrochemical assay,
however
other assays may be possible including mass spectroscopy. The conjugate is
useful to
detect the phosphorylation activity of any kinase including serine/threonine
protein
kinases such as PKC, KITR, PDFGR, CK2, CDKs, CDK2, MKK1, RAF, CHK1, mTOR,
ROCK, MLK and P38/SAPK2a, as well as tyrosine kinases including receptor
kinases
such as EGRF, TRKA, TRKC, PDGFR-a and PDGFR-(3, VEGFRI, VEGFR2, VEGFR3,
ERBB2, ERBB3, ERBB4, MET, RON, EPHB2 and B4, RYK, DDR1, DDR2 and ALK
and non-receptor tyrosine kinases such as SRC, SYK, ABL1, BRK, YESI and JAK1-
3.
[0047] Based on the target kinase, an appropriate substrate is selected for
immobilization on a working electrode surface. Suitable working electrode
surfaces
include metals such as gold and platinum, semiconductor surfaces such as doped
silicon
or GaAs, and transparent conducting surfaces such as graphite, glassy carbon
and indium
tin oxide. The electrode surface, or working electrode may take the form of a
micron size
metal wire which is modified at the tip, or a chip-based electrode array in
which each
working electrode is individually addressable.
[0048] The electrode surface is coated with a kinase peptide substrate. In
this
regard, the peptide substrate may be modified at a terminal end thereof to
include an
entity that will bond to the electrode surface. The nature of the modification
may vary
with the nature of the electrode surface. For example, the substrate may be
modified to
include a terminal cysteine residue in order to permit attachment of the
substrate to a
metal electrode surface such as gold or Pt via an Au-S linkage or Pt-S
linkage,
respectively. For an ITO electrode surface, modification of the substrate to
include a
carboxylate residue is appropriate. For electrode surfaces comprising silicon,
aminoalkyltriethoxysilane chemistry and peptide coupling strategies may be
utilized.
Coupling to carbon surfaces (glassy carbon and graphite) involve diazonium
coupling of
9
2903408.1
RECTIFIED SHEET (RULE 91.1)
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
a benzoic acid derivative followed by peptide coupling of the kinase substrate
peptide to
the surface.
[0049] Examples of kinase substrates include, but are not limited to, AKTide-
SA,
AKTide-2T, Src Substrate II, CDK1 Substrate II, Cdk5 Substrate, Crebtide,
Crosstide,
Abll Signal Transduction Protein, HER2/ErbB2 FLT3 substrate, Syntide 2,
Autocamtide-
2, Autocamtide-3 and CK2 Substrate. Kinase substrates may comprise single or
multiple
phosphorylation sites. Multiple phosphorylation sites may be any one of
tyrosine, serine
or threonine.
[0050] The substrate-coated electrode surface is incubated with an
electroactive
nucleotide triphosphate such as a metallocene-ATP conjugate and a kinase of
the
substrate under conditions which permit detection of phosphorylation activity,
such as,
for example, electrochemically by immersion of the electrode surface in an
electrolyte
and the presence of a counter electrode such as a platinum wire, and a
reference electrode
such as Ag/AgC1 or other reference electrode systems, such as calomel
electrode, NHE
(normal hydrogen electrode) and SHE (standard hydrogen electrode).
[0051] A schematic illustrating the reaction 10 that occurs on incubation is
provided in Figure 2(A) and illustrates that the kinase 20 delivers the
electroactive
gamma phosphate 50 of the metallocene-nucleotide triphosphate conjugate 40 to
the
substrate 30. Following incubation, phosphorylation of the substrate 30 with
the
electroactive gamma phosphate 50 of the nucleotide triphosphate 40 is detected
60 using
a suitable electrochemical technique such as cyclic voltammetry, square-wave
voltammetry and electrochemical impedance spectroscopy to measure the
voltametric
change or using other suitable techniques such as mass spectroscopy (see
Figure 14).
[0052] The methods of the present invention provide a means to identify the
presence of a kinase in a solution such as a cell lysate, as well as a means
to profile the
activity of a kinase. The phosphorylation reaction is stoichiometric in that
the
voltametric change is directly proportional to the extent of phosphorylation
measured by
the transfer of the electroactive label such as a metallocene. Thus, the
resulting electrode
surface charge following phosphorylation is directly related to the total
surface
2903408.1
RECTIFIED SHEET (RULE 91.1)
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
concentration of metallocene groups, thereby providing a quantitative means
for
measuring and determining phosphorylation rates in a rapid and precise fashion
and
allowing the monitoring of phosphorylation reactions in real time for kinase
profiling.
The reaction is also advantageously reversible, thereby allowing multiple uses
of the
substrate-modified electrode.
[0053] In addition, the methods of the present invention may be conducted in
the
presence of candidate kinase modulating compounds, including either inhibitor
compounds or agonist compounds, providing a method of screening such
candidates for
their potential as therapeutic agents in connection with disease associated
with a given
kinase. Such a screening method, as illustrated in Figure 2(B), comprises the
steps of
immobilizing a substrate 30 of a selected kinase 20 on an electrode surface
70, incubating
the immobilized substrate 30 with an electroactive nucleotide triphosphate 40
such as a
metallocene-ATP conjugate in the presence of the kinase 20 and a candidate
compound
80 (such as an inhibitor) and detecting the level of phosphorylation of the
substrate 30 by
any suitable detection means 60 such as electrochemically or by mass
spectroscopy. A
change in the level of phosphorylation from the phosphorylation level that
occurs in the
absence of the candidate compound indicates that the candidate compound 80
modulates
the activity of the kinase.
[0054] In addition, the methods of the present invention may be conducted to
identify new protein kinase substrates. Such method comprises the steps of
immobilizing
a candidate substrate on an electrode surface, incubating the immobilized
candidate with
an electroactive nucleotide triphosphate such as a metallocene-ATP conjugate
in the
presence of a kinase and detecting the level of phosphorylation of the
substrate by any
suitable detection means such as electrochemically. Phosphorylation of the
candidate
substrate indicates that the candidate substrate is a substrate of the kinase.
[0055] In an embodiment of the invention, a microelectrode array is provided.
The array comprises a series of electrodes to which are linked different
peptide
substrates, each of which is specific for a different protein kinase. The
array is prepared
similar to a single peptide substrate electrode with the exception that it
includes multiple
I1
2903408.1
RECTIFIED SHEET (RULE 91.1)
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
electrodes with varying substrates, and may comprise replicates of each
substrate in order
to yield statistically meaningful results. Each peptide substrate is modified
at one of the
C- or N- terminus to include a linking agent suitable to link it to the
electrode surface as
previously described. It will be appreciated by one of skill in the art that
the kinases to be
targeted by such an electrode array are not particularly restricted, and thus,
the electrode
array may comprise any selected peptide substrates.
[0056] A microelectrode array as described is useful for kinase profiling,
including the determination of phosphorylation characteristics, of one or more
kinases.
In this regard, it is particularly useful to profile a cell lysate comprising
a mix of
components, and thus, is useful as a diagnostic tool to identify abnormal
activity in a cell
lysate in comparison to a standard, e.g. normal profile obtained from a
healthy individual.
The array is also useful to screen for kinase modulators to determine their
effect on
multiple kinase/substrate interactions in a single screen.
[0057] Abnormal protein phosphorylation is a cause of major diseases,
including
cancer, diabetes and chronic inflammatory diseases. For example, protein
kinases CK2,
Abll and HER2 are frequently over-expressed in tumours or leukemic cells and
exhibit
oncogenic activity in mice. Analytical methods to quantify protein kinase
activity are
critical for understanding their role in the diagnosis and therapy of these
diseases.
Accordingly, another aspect of the present invention is a method of diagnosing
in a
subject a disease associated with abnormal levels or absence of a protein
kinase. Such
method comprises the steps of immobilizing a substrate of the kinase
associated with the
disease to one or more electrodes; incubating the one or more electrodes
carrying the
immobilized substrate with a sample from the subject and a nucleotide
triphosphate
comprising an electroactive-labelled gamma phosphate; and detecting the
phosphorylation level of the substrates in the one ore more electrodes by any
suitable
detection means such as electrochemically, wherein an abnormal level or
absence of
phosphorylation in the subject's sample with respect to a normal control
indicates that the
subject has, or is susceptible to, the disease.
12
2903408.1
RECTIFIED SHEET (RULE 91.1)
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
[0058] Subjects include any organism that has protein kinase in its system,
including animals and plants.
[0059] Embodiments of the invention are described by reference to the
following
specific examples which are not to be construed as limiting.
Example 1- Synthesis of Fc-ATP
[0060] Preparation of Boc-NH(CH2)6N(H)COFc (Compound 1):
Ferrocenecarboxylic acid (230 mg, 1 mmol) was dissolved in 20 mL anhydrous
DCM.
Then, 1.2 equiv. TEA (0.17 mL) and 1.2 equiv. HBTU (455 mg) were added
sequentially. After 30 min., Boc-NH(CH2)6NH2 was added to the solution and
stirring
was continued overnight. After reaction was completed, the solvent was removed
in
vacuo, and the residue was purified by flash column chromatography on silica
gel (DCM-
MeOH, 95:5; Rf= 0.25) giving the desired compound as a yellow solid in 78%
yield (334
mg). 1H-NMR (8, DMSO): 7.74 (t, 1H, J = 5.2 Hz, NH-COFc), 6.78 (t, 1H, J = 5.4
Hz,
NH-Boc), 4.78 (s, 2H, Cp), 4.32 (s, 2H, Cp), 4.14 (s, 5H, Cp), 3.15 (q, 2H, J
= 6.4 Hz,
CH2), 2.90 (q, 2H, J = 6.4 Hz, CH2), 1.23-1.52 (m, 17H). 13C{1H}-NMR (8,
DMSO):
168.57, 155.57, 77.27, 76.94, 69.73, 69.23, 68.06, 39.76, 38.54, 29.50, 29.47,
28.26,
26.17, 26.08. IR: vmax = 3363 (NH), 3310 (NH), 2976 (Fc), 2934 (Fc), 2861
(Fe), 1687
(CO-OtBu), 1623 (Amide-1), 1535 (Amide-2). MS (EI+) m/z: calc. for
C22H32FeN2O3:
428.2; found: (M) 428.1
[0061] Preparation of NH2(CH2)6N(H)COFc (Compound 2): TFA (5 equiv.) was
added to a mixture of Boc-protected ferrocenyl amine (334 mg, 1 mmol) in 10 mL
DCM.
After stirring the mixture for 1 h, the solvent was removed in vacuo. Three
portions of
DCM were added and evaporated to get rid of the excess TFA. The residue was
dissolved
in 10 mL DCM and 0.25 mL TEA was added to convert the TFA salt to free amine
completely. After solvent removal, the mixture (contains TEAH+ salt) was used
in the
next step without further purification. For the purpose of characterization,
the mixture
was dissolved in 20 mL DCM (contains 5% TEA) and extracted with brine and
water.
After the removal of the solvent, the residue was dried in high vacuo to give
a yellow
solid. 90% yield (295 mg). 1H-NMR (6,DMSO-d6): 7.74 (t, 1H, J = 5.3 Hz, NH-
COFc),
13
2903408.1
RECTIFIED SHEET (RULE 91.1)
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
4.78 (s, 2H, Cp), 4.32 (s, 2H, Cp), 4.14 (s, 5H, Cp), 3.15 (q, 2H, J = 6.4 Hz,
CH2), 2.52
(s, 2H, CH2), 1.49 (t, IH, J = 6.6 Hz, CH2), 1.27-1.39 (m, 6H, CH2). 13C{1H}-
NMR (6,
DMSO): 168.56, 76.95, 69.70, 69.21, 68.05, 41.53, 38.58, 33.23, 29.51, 26.41,
26.24. IR:
vmax = 3293.68 (NH2), 2962.94 (Fc), 2928.63 (Fc), 2854.23 (Fc), 1624.45(amide-
1),
1541.51 (amide-2). MS(EI) m/z: calc. for C17H24FeN2O: 328.1; found: (M) 328.1.
[0062] Preparation of y-phosphate Fc-ATP (Compound 3): Adenosine 5'-
triphosphate disodium salt (100 mg, 0.18 mmol) was dissolved in 10 mL 0.1 M
TEAB
buffer (pH=7.5) and loaded on a column packed with cation-exchange resin (AG
50W-
X8), which has been pre-equilibrated with 0.1M TEAB buffer. The desired
fraction
(monitored by UV light) was collected and evaporated in vacuo. The residue was
co-
evaporated with 10 mL dry methanol three times and dissolved in 1.8 mL dry DMF
under
Argon. DCC (123 mg) was added and the mixture was stirred under Ar for 3 h at
room
temperature to form adenosine-5'-trimetaphosphate (ATMP). ATMP solution was
added
to a mixture of compound 2 (295 mg, 5 equiv.) in 10 mL MeOH and 0.25 mL TEA
under
Ar. The mixture was stirred for 30 min. and poured into 20 mL H2O. The
solution was
loaded on a DEAE-cellulose column and washed with distilled H2O to remove
excess
ferrocene-amine. Then, linear gradient of TEAB buffer (0.1-1 M) was carried
out to give
the desired fraction (yellow band), which was lyophilized into light yellow
power. 50%
yield of Fc-ATP (TEAH+ salt) and further exchanged TEAH to Na form for the NMR
spectra. 31P{1H}-NMR (8, D20): -0.07(y) d, J = 21.1 Hz; -10.76(a) d, J = 19.9
Hz; -
22.14([3) t, J = 19.9 Hz. 1H-NMR (8, D20): 8.52 (s, 1H, H-8), 8.19 (s, 1H, H-
2), 6.10 (d,
1H, J = 5.5 Hz, H-1'), 4.73 (s, 2H, Cp), 4.74 (s, 1H, H-2'), 4.53 (s, 1H, H-
3'), 4.46 (s,
2H, Cp), 4.37 (s, 1H, H-4'), 4.23 (m, 2H, H-5'), 4.20 (s, 5H, Cp), 3.16 (t,
2H, J=6.5Hz,
CH2), 2.79 (q, 2H, J = 7.8 Hz, CH2), 1.31-1.45 (m, 4H, CH2), 1.11-1.25 (m, 4H,
CH2).
[H4=M](ESI) m/z: calc. for C27H39FeN7O13P3: 818.1; found: 818.2.
[0063] A schematic of the Fc-ATP conjugate synthesis is provided in Figure 1.
[0064] Reagents: All synthesis reactions were carried out under an atmosphere
of
argon unless indicated otherwise. Diethylaminoethyl (DEAE)-cellulose,
adenosine 5'-
triphosphate (ATP) disodium salt was obtained from Sigma and used as received.
Dowex
14
2903408.1
RECTIFIED SHEET (RULE 91.1)
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
AG 50W-X8 was obtained from Bio-Rad Laboratories (Ontario, Canada). N,N'-
Dicyclohexylcarbodiimide (DCC), O-(1H-Benzotriazol-1-yl)-N,N,N',N'-
tetramethyluronium hexafluorophosphate (HBTU) was obtained from
AdvancedChemTech (KY, USA). Dimethylformamide (DMF) and dichloromethane
(DCM) was distilled from CaH2 before use. Methanol was distilled from
magnesium
tuning with the presence of iodine. Ferrocenecarboxylic acid7 and t-butyl-6-
aminohexylcarbamate8 were prepared according to the literature procedures.
Example 2 - Electrochemical Detection of Protein Kinase C Phosphorylation
[0065] Cyclic voltammetry (CV) was performed using a CHlnstruments 660
system (Austin, TX). DEP-chips with screen-printed gold electrodes (SPEs) were
kindly
donated by BioDevice Technology Ltd. (Ishikawa, Japan) and prepared as set out
in Li et
al. Anal.Chem. 2005, 77 5766-5769. The total length of an SPE was 11 mm, and
the
geometric area of the working electrode was 2.64 mm2. The reference electrode
was a
Ag/AgC1 past electrode and the counter electrode was a carbon electrode.
[0066] 1H, 13C, 31P NMR experiments were performed on a Bruker Avance 500
MHz spectrometer and chemical shifts were referenced to the residue DMSO (2.50
ppm
for 1H and 39.52 ppm for 13C) and H2O (4.79 ppm). Mass spectrometry was
carried out
using a Perkin Elmer-Sciex API 365 instrument.
[0067] Unless otherwise specified, reagents were purchased from Merck. All
solutions were prepared and diluted using ultra-pure water (18.3 MQ-cm) from
the
Millipore Milli Q system.
1. Protein Kinase C-catalyzed phosphorylation reaction using Fc-ATP
[0068] The SPEs were incubated in petri-dishes at room temperature throughout
the preparatory steps in order to avoid rapid evaporation of the solutions on
the surfaces.
The electrochemical measurements were performed three times for each condition
(n=3),
except as otherwise stated.
2. Immobilization of the Protein Kinase C substrate peptides on SPEs
2903408.1
RECTIFIED SHEET (RULE 91.1)
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
[0069] An aliquot of 200 gM substrate protein kinase Ct peptide solution (5
gL)
was allowed to coat the gold working electrode of the SPEs and was incubated
overnight
at 4 C. The protein kinase C~ peptide (SIYRRGSRRWRKL) was purchased from
Calbiochem (EMD Biosciences, USA) and modified with a cysteine residue at the
N-
terminus. The modified protein kinase Ct pseudosubstrate sequence contains
Serl19
instead of Alal 19.9
[0070] After the incubation step, the electrodes were washed with blank TBS.
The peptide film was diluted by immersing the SPEs in 0.1 mM ethanolic
solution of
hexanethiol for 5 min and rinsing the surface with blank TBS.
3. PKC-catalyzed phosphorylation on the SPE surface
[0071] Kinase assay buffer included 20 mM Tris, 0.5 mM EDTA, 10 mM MgCl2,
500 gg/mL phosphatidyl serine (pH 7.5). The concentrations of Fc-ATP and
kinase,
PKC, were varied according to the optimum experimental conditions. Protein
kinase C
from rat brain (E. C. 2.7.1.37) was purchased from Sigma in 50% glycerol
containing 20
mM Tris, 0.5 mM EDTA, 0.5 mM EGTA, 5 mM DTT, 100 mM NaCl, 0.02% Tween 20,
and 1 gg/mL leupeptin. One unit (U) of PKC will transfer 1 nanomole of
phosphate from
ATP into histone Hl per min at 30 C.' " The aliquots (200 gL) of the optimized
assay
buffer including 100 U/mL PKC and 100 gM Fc-ATP were added into 1.5-mL vials.
[0072] Substrate peptide-immobilized SPEs were placed in the vials incubated
at
30 C for 1 h in a heating block (VWR Scientific, USA). After 1 h of
incubation, the SPEs
were washed with blank TBS to remove the excess Fc-ATP and other reagents, and
then
placed in the electrochemical workstation.
4. Electrochemical measurement on SPCE surface
[0073] Electrochemical detection was performed by spotting 20 gL of 0.1 M
NaC1O4 (pH 6.5) onto the surface of SPE at room temperature. Cyclic
voltammetry (CV)
was performed at a scan rate of 100 mV/s. Square-wave voltammetry (SWV)
involved
the oxidation of Fc residues by sweeping the potential from 0 to 1 V with an
amplitude of
25 mV at 15 Hz frequency.
16
2903408.1
RECTIFIED SHEET (RULE 91.1)
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
[0074] Schematic illustration of the electrochemical principal for the
detection of
kinase-catalyzed phosphorylation using Fc-ATP as the co-substrate is shown in
Figure.
2(A). The substrate peptide 30 is immobilized on the surface of the SPE 70 via
a sulphur
bond. Protein kinase 20 C (PKC)-catalyzed reaction transfers y-phosphate-Fc
group 50 to
the serine 35 residue of the peptide 30. The Fc group 50 attached to the
peptide 30 is
electrochemically observed using CV. The voltammetric detection of Fc involves
the
scanning of the potential range between 0 and 1 V at a rate of 100 mV/s. As a
result of
this electrochemical process the reversible redox properties of Fc-ATP were
monitored.
Fig. 3 shows the voltammetric responses obtained from the CV of Fc-ATP in
solution in
the presence of (a) 100 M (b) 50 M (c) 25 M and (d) 10 M Fc-ATP in
solution.
[0075] The oxidation peak was detected at -0.26 V and the reduction peak was
observed at -0.22 V (vs. Ag paste-based reference electrode of the SPE). The
separation
of the redox peak potentials indicated that one electron was involved in the
process. This
electrochemical behaviour was expected from the well-defined electrochemical
properties
of Fc.
[0076] For the optimization of experimental conditions, a series of
measurements
were taken in the presence of varying Fc-ATP concentrations and 100 U/mL PKC
using
the same assay conditions. As the concentration of Fc-ATP increased, the
phosphorylation of the peptides resulted in the high current responses on the
surface. The
current responses remained the same for concentrations over 100 M. Thus, 100
M Fc-
ATP was applied for further kinase assays. When no ATP-F was used in the assay
buffer,
no significant current response was obtained indicating the suppression of non-
specific
adsorption of Fc-ATP on the electrode surface by the stringent washing of the
SPEs as
described. When low concentrations of Fc-ATP were used, no current responses
were
observed.
[0077] Using the surface-immobilized peptides, the current density responses
were recorded in the presence and absence of PKC in the assay solution as
shown in Fig.
4. The CV response shown in Fig. 4-a shows the similar redox behaviour of Fc-
ATP as
observed in solution, however, the peak potentials were slightly shifted to
higher values
17
2903408.1
RECTIFIED SHEET (RULE 91.1)
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
indicating the presence of a peptide film on the surface, which hampered the
redox
process to occur at a lower potential. The absence of any redox current
signals in Fig. 4-b
indicated that the attachment of Fc-ATP to the peptides was dependent on the
presence of
the kinase. Moreover, no redox activity in the absence of PKC showed the
successful
suppression of the non-specific adsorption of Fc-ATP on the electrode surface.
[0078] SWV was also applied to detect the Fc oxidation current signals at low
concentrations of PKC as shown in Fig. 5. The substrate peptide and Fc-ATP
concentration was kept constant at 200 gM and 100 M, respectively. Fig. 5-a
shows the
current response obtained in the presence of 0.1 U/mL PKC while Fig. 5-b shows
the
current response obtained in the presence of 0.01 U/mL PKC. The increasing
trend of the
current density responses were recorded, as the concentration of PKC increased
(Fig. 5).
[0079] The, dependence of incubation time was monitored for the optimization
of
Fc-ATP responses. The concentrations of substrate peptide, PKC and Fc-ATP were
kept
constant at 200 M, 100 U/mL and 100 M, respectively, and the dependence of
the
current responses on incubation time at 30 C was recorded as shown in Fig. 6.
The peak
current heights reached a saturation level, when the assay solution was
incubated for 1 h.
When the kinase reaction was allowed to continue only for 20 min, a small
current
response was observed indicating that the surface-immobilized substrate
peptides were
not phophorylated efficiently in the presence of 100 gM Fc-ATP (Fig. 6).
Example 3 - Detection of Casein Kinase 2 (CK2) and Tyrosine Kinases Abll and
HER2/ErbB2 Phosphorylation
[0080] It was previously demonstrated that using the nucleotide triphosphate
conjugate comprising an electroactive labelled gamma phosphate group is useful
to detect
protein kinase C activity using an electrochemical biosensing system. In this
example the
utility of the nucleotide triphosphate conjugate was used to detect another
well-described
protein serine/threonine kinase, casein kinase-2 (CK2) and two clinically
important
tyrosine kinases, Abll and HER2/ErbB2 and to evaluate this method for
measuring
protein kinase inhibitor potency.
18
2903408.1
RECTIFIED SHEET (RULE 91.1)
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
[0081] First the enzymatic modification of kinase-specific peptide
RRRDDDSDDD12 for the serine/threonine kinase, CK2 was evaluated using mass
spectroscopy with Fc-ATP as the co-substrate.
[0082] Figure 14 shows MS plot for the kinase-catalyzed phosphorylation of the
substrate peptides for (A) CK2, (B) Abll-T3151 and (C) HER2/ErbB2 using
Applied
Biosystems 4700 Proteomics Analyzer with DHB (2,5-Dihydrobenzoic acid) matrix
(10
mg/mL) and 1:1 mixture with the sample. The sample containing the
phosphorylated
peptides were enriched and purified using the standard protocol of
Phosphopeptide
Isolation kit (Thermo Scientific Pierce).
[0083] Our results (Fig. 14A) clearly demonstrate that CK2 transfers the
desired
redox group to the target peptide (m/z CK2 target peptide before: 1264.4359,
after Fc
transfer: 1654.1647). Additional reactions were carried out using the
substrate peptides
for Abl1-T3151 and HER2/ErbB2, clearly showing the utility of our approach
also for
tyrosine kinases (Fig. 14B & Q.
1. Materials and Methods
i. Immobilization of the Substrate Peptides on SPEs
[0084] The covalent immobilization of substrate peptides on the gold
microelectrode surface using succinimide-esters of lipoic acid included the
following
steps: (a) incubation of the bare gold microelectrode with 5 mM NHS-lipoic
acid ester in
ethanol for 15 h; (b) incubation of the N-Hydroxysuccinimide (NHS)-lipoic acid-
modified surface with the substrate peptides in the presence of 2 mM N-(3-
Dimethylaminopropyl)-N'-ethylcarbodiimide (EDC) in 0.1 M 2-(N-Morpholino)
ethanesulfonic acid (MES, pH 6) for 2 h, (c) incubation of the peptide-
modified
electrodes with Mercaptopolyethylene glycol 5'000 monomethyl ether (PEG-thiol
5'000)
solution (1:100 v/v) in ethanol for 10 min.
ii. CK2-catalizedphosphorylation
19
2903408.1
RECTIFIED SHEET (RULE 91.1)
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
[0085] CK2a and CK2a' kinases assay buffer included 50 mM Tris HCl (pH 7.5),
mM MgC12, 150 mM NaCl. The concentration of ATP-Fc was 100 M ATP-Fc in a
total reaction volume of 25 L. CK2a and CK2a'forms of CK2 and the peptide
substrate
(RRRDDDSDDD) were prepared in D. W. Litchfield's laboratory (University of
Western
Ontario, London, Canada). The substrate peptide modified electrodes were
incubated at
37 C for 2 h. After the incubation period, the electrodes were washed multiple
times
using 2 M NaC1O4. After the washing process, the electrodes were immersed into
2 M
NaC1O4 for the electrochemical measurement using Ag/AgCI reference electrode,
which
was connected with the electrolyte via a salt bridge and a Pt wire was used as
the counter
electrode.
iii. Abll-T315I-catalyzed phosphorylation
[0086] Abll-T315I kinase assay buffer included 60 mM HEPES (pH 7.5), 5 mM
MgCl2, 5 mM MnC12, 3 M Na3VO4, 400 M ATP, peptide substrate (STP) and the
kinase in a total reaction volume of 25 L. Purified recombinant human Abll-
T3151
mutant kinase and its substrate peptide Signal Transduction Protein (STP,
EGIYDVP)
were purchased from Cell Signalling Technology (MA, USA).The substrate peptide
modified electrodes were incubated at 37 C for 2 h. After following the same
washing
procedures as for CK2, the CV measurements were recorded using the same
parameters
as described above.
iv. HER/ErbB2 catalysed phosphor ylation
[0087] The activity of HER2/ErbB2 kinase was measured using the following
conditions: 5 mM MOPS (pH 7.2), 2.5 mM (3-glycerophosphate, 5 mM MnC12, 100 M
Fc-ATP in the presence of FLT3 peptide substrate and 10 ng/ttL kinase in a
total reaction
volume of 25 L. Purified recombinant human HER2/ErbB2 kinase and FLT3
(DNEYFYV) substrate peptide were purchased from Cell Signalling Technology
(MA,
USA). The substrate peptide modified electrodes were incubated at 37 C for 2
h. After
following the same washing procedures as described above for CK2, the CV
measurements were recorded using the same parameters.
2903408.1
RECTIFIED SHEET (RULE 91.1)
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
v. Cell-lysate pre-treatment
[0088] For the reactions containing cell lysates, 20 x 106 HeLa cells that
were
obtained from D. W. Litchfield's laboratory (University of Western Ontario,
London,
Canada), were lysed in 1 mL of lysis buffer (50 mM Tris (pH 8), 150 mM NaCl,
10%
Glycerol, 0.5% Triton X-100TH) containing 1mM phenylmethanesulphonylfluoride
(PMSF, Pierce, USA) by rotation for 10 minutes. During the phosphorylation
reactions,
Ha1tTM phosphatase inhibitor cocktail (Pierce, USA) in 1:1 ratio (v/v) with
the cell lysate
was used for suppressing the serine, threonine and tyrosine phosphatase
activities. The
cell debris was collected at 12,000 rpm and the supernatant was stored at 4oC
until use in
subsequent reactions. For the reactions containing HeLa lysates, the lysate
solution was
mixed with the kinase reaction buffer at a ratio of 1:10 (v/v) and applied to
the
phosphorylation or dephosphorylation reactions as described above.
Vi. Calculation of enzymatic activity using electrochemical data
[0089] The electrochemical data from the CV measurements are obtained as
charge density (J) per test. The concentration of Fc-ATP for the kinase
activity
determinations was 100 M, for CK2 and 200 M for Abl l -T315I and HER2-ErbB2.
Here, the detailed description of the calculation procedure will be given for
CK2-
catalysed phosphorylation reactions. The reaction volume is 25 L, which leads
to 2.5
nmole Fc-ATP per test. Then, the specific electro-activity (SE) of Fc-ATP
(J/nmole Fc-
ATP) would be calculated as follows:
hots:[
[0090] (1) SE (/nrnole)
[Fc-ATP]
[0091] Then, the specific activity of CK2 is calculated with the following
formula:
21
2903408.1
RECTIFIED SHEET (RULE 91.1)
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
[0092] (2) _1 1 (Jx .Dii x 25)
Activity (:.mole.min.IL
(SE xlolxT)
[0093] where AJ represents (J sample - J blank) and Dil is the dilution factor
with
a total reaction volume of 20 .tL with time (T) in minutes of reaction and the
enzyme
volume (V) in L.
[0094] The apparent inhibition constants Ki' were determined by fitting
equation
(3) to the experimental data.
[0095] (3)
V
[0096] where V is the rate, VO is the rate in the absence of the inhibitor,
[I] is the
inhibitor concentration and Ki' is the apparent inhibition constant. The true
inhibition
constants Ki were calculated by correction of Ki' according to Equation (4):
[0097] (4) A j _
1+S/
where [S] is the surface density of the immobilized substrate peptide and Km
is the
Michaelis-Menten constant. The surface density conditions of the immobilized
substrate
peptide were changed between 1, 5, 10, 15 and 20 pmol/cm2. The initial time
dependence of the kinase reactions were determined at these varying peptide
density
conditions. The phosphorylation reaction was stopped after 5, 15, 30, 60, 90,
120 and 150
min, and the measurement of the attached Fe molecules was carried out. The
reciprocals
of these current values were plotted against the reciprocal of the peptide
density, which
22
2903408.1
RECTIFIED SHEET (RULE 91.1)
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
gave linear Lineweaver-Burk curve. The equation of this curve defines the
kinetic data,
where the y intercept is 1/Vmax. If the y is set for 0 and the equation is
solved for x, x
intercept becomes equal to -1/Km.
2. Results
[0098] Figure 9 (A) illustrates the square wave voltammetry of the CK2-
catalysed
phosphorylation reactions performed in the cell lysates. The substrate peptide
modified
gold microelectrodes were immersed into the cell lysates containing (a) the
over-
expressed CK2a, (b) endogenous CK2 levels, (c) the over-expressed kinase-dead
CK2a,
(d) normal-expressed CK2a. The measurements were taken as described above; (B)
Plot
for the detection of the CK2a over-expression state in cell lysates. The
increase in the
current signal indicates that the kinase was in excess amount and could cause
the
attachment of a larger amount of Fc molecules on the peptides in comparison
with the
other cell lysates.
[0099] Figure 10 shows: (A) Cyclic voltammograms (CV) for CK2a'-catalyzed
phosphorylation of substrate peptide (RRRDDDSDDD) in the presence of CK2a' at
a
concentration of (a) 0.04, (b) 0.02, (c) 0.008, (d) 0.005, (e) 0.0025 ng/ L,
and (f) control
experiment in the absence of the enzyme; (B) Effect of the CK2a'concentration
on the
current responses using substrate peptide modified electrodes (a) in the assay
buffer, (b)
in the presence of HeLa cell lysate, and (c) control experiment was performed
using the
substrate peptide for Abll-T315I (EGIYDVP) in the presence of cell lysate.
[00100] Figure 11 shows: (A) cyclic voltammograms for the inhibition of CK2a-
catalyzed phosphorylation with the substrate peptide in the presence of the
inhibitor, (1)
TBB (4,5,6,7-Tetrabromo-2-azabenzimidazole) (a) 250 nM, (b) 500 nM, (c) 750
nM, and
(d) 800 nM (e) 900 nM, and (f) control experiment in the absence of CK2a; (B)
Lineweaver-Burk plot for the determination of kinetics of the CK2a'-catalyzed
phosphorylation of the immobilized substrate peptide at varying surface
density
conditions on the Au microelectrode surface as described in the text; (C)
Control
experiments were performed by (a) titrating the phosphorylated substrate
peptide on the
surface with the assay buffer, which showed the stability of the
electrochemical
23
2903408.1
RECTIFIED SHEET (RULE 91.1)
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
responses, and (b) EGIYDVP was not inhibited upon exposure to (1) and
demonstrated
the specificity of the inhibition reactions, (c) the current responses
decreased rapidly in
the presence of (2-Dimethylamino-4,5,6,7-tetrabromo-lH-benzimidazole) and (d)
(E-3-
(2,3,4,5-Tetrabromophenyl) acrylic acid) in the HeLa cell lysate.
[00101] Figure 12 shows: (A) CV for the inhibition of tyrosine kinase-
catalyzed
phosphorylation with the Signal Transduction Protein (STP) peptide (EGIYDVP at
a
surface density 12 pmol.cm-2) in the presence of Abll-T3151 at a concentration
of (a) 3,
(b) 1.5, (c) 1, (d) 0.75 ng/ L, (e) in the absence of the enzyme, no current
responses were
observed, which indicated the suppression of non-specific adsorption; (B)
Lineweaver-
Burk plot for the determination of kinetics of the Abll-T3151-catalyzed
phosphorylation
of the immobilized STP peptide at varying surface density conditions on the Au
microelectrode surface as described in the text; (C) Plot for the dependence
of the anodic
current responses on the amount of the Abll-T3151 kinase in the presence of
HeLa cell
lysate with (a) the STP peptide immobilized on the surface, (b) a control
experiment
using the FLT3 peptide (DNEYFYV), which a preferable substrate for HER2/ErbB2
at a
surface density 12.5 pmol.cm-2. Low current responses indicated insufficient
phosphorylation between the FLT3 peptide and the Abl1-T3151, (c) a second
control
experiment involved the CK2 substrate peptide (RRRDDDSDDD) at a surface
density 10
pmol.cm 2. No significant current responses were observed, which evidenced the
specificity of the phosphorylation reaction; (D) Plot for the dependence of
current
responses on the concentration of the general protein kinase inhibitors, (b)
Staurosporine
and (c) N-Benzoylstaurosporine, the control experiments involved the titration
of the
phosphorylated STP peptide with the buffer titration in the presence of HeLa
cell lysates
(a). No significant drops were observed in the current responses indicating
that the
contents of the cell lysate did not affect the current signals.
[00102] Figure 13 shows: (A) Cyclic voltammograms for the inhibition of
tyrosine
kinase-catalyzed phosphorylation with the FLT3 peptide (DNEYFYV at a surface
density
12.5 pmol.cm-2) in the presence of HER2/ErbB2 at a concentration of (a) 4, (b)
0.5, (c)
0.25 ng/gL; (B) Lineweaver-Burk plot for the determination of kinetics of the
HER2/ErbB2-catalyzed phosphorylation of the immobilized FLT3 peptide at
varying
24
2903408.1
RECTIFIED SHEET (RULE 91.1)
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
surface density conditions on the electrode surface; (C) Plot for the
dependence of the
anodic current responses on the amount of the HER2/ErbB2 kinase with (a) the
FLT3
peptide immobilized on the surface in the presence of HeLa cell lysate, (b)
control
experiment with STP peptide (EGIYDVP) resulted in a slight increase in the
current
responses, (c) the CK2 substrate peptide (RRRDDDSDDD) at a surface density 10
pmol.cm-2 in the presence of HeLa cell lysate; (D) Plot for the dependence of
J responses
on the concentration of (b) N-Benzoylstaurosporine, whereas (a) the
phosphorylation of
the immobilized FLT3 peptide was not affected with blank buffer titration in
the presence
of HeLa cell lysates.
i. Measuring Kinase Activity in Cellular Extracts
[00103] The application of a solution containing recombinant CK2 and Fc-ATP
into Hela cell lysates resulted in robust phosphorylation of the immobilized
peptide
substrate as indicated by cyclic voltammetry measurements of the surface-
confined Fc
molecules. As shown in Figure 9A, the highest intensity of the square-wave
voltammetry
(SWV) current signal was observed for the lysates derived from cells with
elevated CK2
levels. By comparison, lower current responses were obtained from the other
cell lysates
including uninduced cells or cells expressing kinase-inactive CK2a. Overall,
the kinase
activity measurements obtained by electrochemical detection are in complete
correspondence with measurements previously obtained using conventional
radioactive
detection of CK2 activity. In the assays performed with these lysates, the
reduction signal
of the oxidized Fc+ was not observed (or shifted outside the scanned potential
window)
possibly due to the presence of numerous proteins inhibiting the reduction.
Figure 9B
displays the average SWV current responses obtained from a set of five
measurements
performed on the same peptide in the cell lysate environment under the same
conditions.
ii. Phorphorylation specificity
[00104] The phosphorylation of the immobilized peptides using the nucleotide
triphosphate conjugate of the present invention is specific. Protein tyrosine
kinases (Abl-
T315I and HER2) did not catalyze phosphorylation of the CK2 peptide substrate
(Figures
2903408.1
RECTIFIED SHEET (RULE 91.1)
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
12C-c and 13C-c), and CK2 did not catalyze phosphorylation a peptide that was
the
preferred substrate of Abl-T3151 (EGIYDVP, Fig. 1 OB-c).
iii. Modulators ofKinase Activity
[00105] Based on the demonstration of the electrochemical technique of the
present invention for the detection of the reversible phosphorylation of a
kinase substrate,
this method was adapted to evaluate small molecule inhibitors acting on these
enzymes.
Therefore, the ability of the peptide biosensor to assess the inhibitory
activity of three
recently-developed CK2 inhibitors was evaluated (Figure 11). Reactions were
performed
with CK2, Fc-ATP, and each inhibitor (at concentrations ranging from 50 nM to
2.5 M)
were exposed onto the CK2 substrate peptide films. After incubation for 2 h at
37 C with
CK2a, the biosensors were washed and analysed by electrochemical measurements.
Table 1 shows the inhibition data for the analysis Ki values for four kinases
and five
inhibitors in total. In general, the Ki values that were calculated from the
electrochemical
data are in agreement with literature values obtained with conventional kinase
assays. 13-17.
TABLE 1
Comparison of kinetic constants of protein kinases with their substrate
peptides
immobilized on gold microelectrodes. The kinetic data were extracted from
measurements using varying surface density conditions of the substrate
peptides
immobilized on the surface.
Protein Kinase Peptide Km (mM) Vmax Vmax/Kmax
( mol.min-I.mg-1)
CK2a RRRDDDSDDD 0.087 1.97 22.64
CK2a' RRRDDDSDDD 0.098 1.78 18.16
Abll-T3151 EGIYDVP 0.182 1.67 9.18
HER2/ErbB2 DNEYFYV 0.208 1.54 7.41
26
2903408.1
RECTIFIED SHEET (RULE 91.1)
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
[00106] To evaluate the utility of the electrochemical biosensor for the
measurement of other kinase assays, the biosensors were modified to monitor
Abli and
HER2 protein tyrosine kinases. The first FDA-approved kinase inhibitor drug,
Imatinib
(GleevecTM) has been successfully used to treat Bcr-Abl kinase associated
chronic
myeloid leukemia.18 The most frequently identified mutation associated with
resistance to
GleevecTM is T315I in the Abll kinase domain. 19'20'21 Another successful
small molecule
inhibitor is Trastuzumab (Herceptin ), which is used as part of a treatment
regimen
containing doxorubicin, cyclophosphamide, and paclitaxel for the adjuvant
treatment of
patients with HER2-overexpressing, node-positive breast cancer.22'23 Specific
activities
for the two CK2 isoforms, Abll and HER2 on their substrate peptides are shown
in Table
2. Notably, the activities determined by the electrochemical measurements
compare very
favourably with the literature values 1(a). However, the slightly low reaction
rates seen in
the electrochemical assays may arise in part through decreased accessibility
of the
substrate peptides anchored on the Au electrode surface.
TABLE 2
Comparison of Ki of small molecule inhibitors on protein kinases with their
substrate
peptides immobilized on gold microelectrodes. The Ki values were determined
with the
data obtained using varying surface density conditions of the substrate
peptides
immobilized on the surface.
Inhibitor (nM) CK2a CK2a' Abll-T3151 HER2/ErbB2
(1) 450 380 - -
(2) 35 20 - -
(3) 50 25 - -
Staurosporine - - 550 225
N-Benzoylstaurosprine- - 600 275
27
2903408.1
RECTIFIED SHEET (RULE 91.1)
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
[00107] Protein tyrosine kinases were also challenged with the well-defined
general inhibitors of kinases, staurosporine and its derivative, N-
benzoylstaurosporine
(Fig. 12D and 13D), which are ATP-competitive inhibitors with broad-spectrum
inhibitory activities. Again, the Ki values for Staurosporine and its
derivative that were
determined by electrochemical assays were very similar to those obtained using
conventional radioactive assays for Ab1119,20,21 and HER2.24
Example 4 - Preparation of a Microelectrode array
[00108] A microelectrode array 700 for use in the present method to determine
the
phosphorylation characteristics of multiple protein kinases is shown in Fig.
7. The
protocol for its fabrication is the same as that described in Li et al. 2005;
however, a new
lithographic mask is prepared based on the design shown in Fig. 7. An 800-nm
silicon
dioxide insulating layer is thermally grown on a p-type silicon wafer. A gold
layer
(200nm) is deposited onto a titanium adhesion layer (20nm) sputtered onto the
Si chip.
Both metal layers are photolithographically patterned using Shipley 1813
photoresist 740
as a mask layer using the mask of Fig. 7. Etching of the metal layers is
achieved as
described previously (Li et al. Anal. Chem. 2006, 78, 6096-6101).25 The
individual gold
micropads 720 possess a 10 micrometer diameter and are separated from each
other by a
distance of 50 micrometers 730. Each micropad 720 will be addressable from an
external
pad 710 through a microwire (1mm) 750. The arrangement of the micropads 720
into
quadruples facilitates the spotting of individual peptide substrates.
[00109] An example of the use of a microelectrode array 800 is illustrated in
Figure 8.
[00110] A kinase substrate peptide related to different kinases 820 are
immobilized
on the microelectrode array 800 (A). In this example, the kinase related to
breast cancer
are immobilized on the chip 800: FAK, Src, HER2, Akt, Erk, Crk, CAS.
Substrates will
be incubated with cell lysates 850 and the phosphorylation reaction will take
place in the
presence of ferrocene (Fe)- conjugated ATP. After the phosphorylation
reaction,
electrochemical measurements will be performed at each microelectrode (B).
Figure
8(C) and 8(D) illustrate the average square-wave voltammetry current responses
obtained
28
2903408.1
RECTIFIED SHEET (RULE 91.1)
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
with the set of kinases 820 and a blank for individuals with breast cancer
(Figure 8(D))
and healthy individuals (Figure 8(E)). The statistical evaluation of the data
for breast
cancer will not only help the diagnosis of cancer or other diseases states,
but also the
effect of the small molecule inhibitors on the phosphorylation process can be
determined.
The difference of the electrochemical responses between the samples obtained
from
healthy and cancer individuals will provide rapid diagnosis and therapeutic
follow-up
possibilities.
REFERENCES
1. (a) P. Cohen, Nat. Cell Biol. 2002, 4, E127-E130; (b) G. Burnett and E. P.
Kennedy, J.
Biol. Chem. 1954, 211, 969-980; (c) E. H. Fischer and E.G. Krebs, J. Biol.
Chem. 1955,
216, 121-132 (d) E. H. Fischer, D.J. Graves, E. R. S. Crittenden and E. G.
Krebs, J. Biol.
Chem. 1959, 234, 1698-1704.
2. I. Melnikova and J. Golden, Nat. Rev. Drug Discov. 2004, 3, 993-994; (b) J.
S. Sebolt-
Leopold and R. Herrera, Nat. Rev. Cancer 2004, 4, 937-947; (c) J. M. Yingling,
K. L.
Blanchard and J. S. Sawyer, Nat. Rev. Drug Discov. 2004, 3, 1011-1022; (d) M.
J. A. de
Jonge and J. Verweij, Eur. J. Cancer 2006, 42, 1351-1356; (e) P. Cohen, Nat.
Rev. Drug
Discov. 2002, 1, 309-315; (f) G. Manning, D.B. Whyte, R. Martinez, T. Hunter
and S.
Sudarsanam, Science 2002, 298, 1912-1934.
3. (a) B. E. Turk, J. E. Hutti and L. C. Cantley, Nat. Protoc., 2006, 1, 375-
379; (b) C. J.
Hastie, H. J. McLauchlan and P. Cohen, Nat. Protoc., 2006, 1, 968-971; (c) B.
T.
Houseman, J. H. Huh, S. J. Kron and M. Mrkisch, Nat. Biotechnol., 2002, 20,
270-274;
(d) A. F. Braunwalder, D. R. Yarwood, T. Hall, M. Missbach, K. E. Lipson and
M. A.
Sills, Anal. Biochem., 1996, 234, 23-26; (e) W. Tegge, R. Frank, F. Hoffmann
and W. R.
Dostmann, Biochemistry, 1995, 87, 99-106.
4. (a) M. Sato, T. Ozawa, K. Inukai, T. Asano and Y. Umezawa, Nat.
Biotechnol., 2002,
20, 287-294; (b) M. Sato, T. Ozawa, T. Yoshida and Y. Umezawa, Anal. Chem.
1999, 71,
3948-3954; (c) M. Sato and Y. Umezawa, Methods, 2004, 32, 451-455; (d) Y.
Umezawa,
Biosens. Bioelectron. 2005, 20, 2504-2511; (e) K. Tomizaki and H. Mihara, Mol.
BioSyst., 2006, 2, 580-589.
5. (a) M. D. Allen, L. M. DiPilato, M. Rahdar, Y. R. Ren, C. Chong, J. O. Liu
and J.
Zhang, ACS Chem. Biol., 2006, 1, 371-376; (b) D. M. Rothman, M. D. Shults and
B.
Imperiali, Trends Cell Biol., 2005, 15, 502-510; (c) M. D. Shults, K. A.
Janes, D. A.
Lauffenburger, B. Imperiali, Nat. Methods 2005, 2, 277-283; (d) M. D. Shults
and B.
Imperiali, J. Am. Chem. Soc. 2003, 125, 14248-14249.
29
2903408.1
RECTIFIED SHEET (RULE 91.1)
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
6. (a) K. D. Green and M. K. H. Pflum, J. Am. Chem. Soc., 2007, 129, 10-11;
(b) Z.
Wang, R. Levy, D. G. Fernig and M. Brust, J. Am. Chem. Soc., 2006, 128, 2214-
2215; (c)
Z. Wang, J. Lee, A. R. Cossins and M. Brust, Anal. Chem., 2005, 77, 5770-5774.
7. A. Sonoda and I. J. Moritani, Organomet. Chem. 1971, 26, 133-140.
8. R. Leung-Toung, T. F. Tam, Y. Zhao, C. D. Simpson, W. Li, D. Desilets and
K. J.
Karimian, Org. Chem. 2005, 70, 6230 -6241.
9. S. Osada, K. Mizuno, T. C. Saido, K. Suzuki, T. Kuroki and S. Ohno, Mol.
Cell. Biol.
1992, 12, 3930-3938.
10. U. Kikkawa, A. Kishimoto and Y. Nishizuka, Ann. Rev. Biochem., 1989, 58,
31-41.
11. G. M. Walton, P. J. Bertics, L. G. Hudson, T. S. Vedvick and G. N. Gill,
Anal.
Biochem., 1987, 161, 425-437.
12. Kuenzel, E. A.; Mulligan, J. A.; Sommercorn, J.; Krebs, E. G. J. Biol.
Chem. 1987, 262,
9136-9140.
13. Litchfield, D. W. Biochem. J. 2003, 369, 1-15.
14. Zien, P.; Duncan, J. S.; Skierski, J.; Bretner, M.; Litchfield, D. W.;
Shugar, D. Biochim.
Biophys. Acta 2005, 1754, 271-280.
15. Battistutta, R.; Mazzorana, M.; Sarno, S.; Kazimierczuk, Z.; Zanotti, G.;
Pinna, L. A.
Chem. Biol. 2005,12,1211-1219.
16. Sarno, S.; De Moliner, E.; Ruzzene, M.; Pagano, M A.; Battistutta, R.;
Bain, J.; Fabbro,
D.; Schoepfer, J.; Elliot, M.; Furet, P.; Meggio, F.; Zanotti, G.; Pinna, L.
A. Biochem. J.
2003, 374, 639-646.
17. Pagano, M. A.; Meggio, F.; Ruzzene, M.; Andrzejewska, M.; Kazimierczuk,
Z.; Pinna, L.
A. Biochem. Biophys. Res. Commun. 2004,321,1040-1044.
18. Pagano, M. A.; Poletto, G.; Di Maria, G.; Cozza, G.; Ruzzene, M.; Sarno,
S.; Bain, J.;
Elliott, M.; Moro, S.; Zagotto, G.; Meggio, F.; Pinna, L. A. ChemBioChem 2007,
8, 129-139.
19. Capdeville, R.; Buchdunger, E.; Zimmerman, J.; Matter, A. Nat. Rev. Drug.
Discov.
2002, 1, 493-502.
20. Sebolt-Leopold, J. S.; English, J. M. Nature 2006, 441, 457-462.
21. Weisberg, E.; Manley, P. W.; Cowan-Jacob, S. W.; Hochhaus, A.; Griffin, J.
D. Nat. Rev.
Cancer 2007, 7, 345-356.
22. Maekawa, T.; Ashihara, E.; Kimura, S.; Kimura, S. Int. J. Clin. Oncol.
2007, 12, 327-340.
23. Hicks, D. G.; Kulkami, S. Am. J. Clin. Pathol. 2008, 129, 263-273.
2903408.1
RECTIFIED SHEET (RULE 91.1)
CA 02700945 2010-03-26
WO 2009/039639 PCT/CA2008/001690
24. Dittadi, R.; Gion, M. J. Nat. Cancer Inst. 2000, 92, 1443-1444.
25. Tikhomirov, 0.; Carpenter, G. J. Biol. Chem. 2001, 276, 33675-33680
26. X. Li, J. S. Lee, H.-B. Kraatz, Analytical Chemistry 2006, 78, 6096.
31
2903408.1
RECTIFIED SHEET (RULE 91.1)