Note: Descriptions are shown in the official language in which they were submitted.
CA 02703610 2015-07-31
Manufacturing Solid Pharmaceutical Dosage Forms With Visible Micro- And
Nanostructured
Surfaces And Micro- And Nanostructured Pharmaceutical Dosage Form
Field of the Invention
This invention relates to composite dosage forms such as pharmaceutical
compositions
and components thereof. More particularly, this invention relates to composite
dosage forms
comprising one or more features that provide anti-counterfeiting
characteristics to such
dosage forms.
Background of the Invention
Forged, grey market, and illegal re-imports are of increasing concern in the
pharmaceutical industry. This is not only a topic in the third world, where
the fraction of
counterfeit pharmaceutical products in the supply chain is sometimes above
50%. This
problem has now reached the second and first worlds likewise, especially as
pharmaceuticals
are often much more expensive in these countries. AIDS and cancer drugs are
sometimes
subsidized in developing countries, which increases the danger of illegal re-
imports.
Anti-counterfeiting strategies currently in use in the pharmaceutical industry
have so
far not been very successful in preventing forgery, illegal re-imports and
other activities
commonly summarized as counterfeiting. Anti-counterfeiting features in the
pharmaceutical
market nowadays are generally only applied to packages. Holograms, optically
variable inks,
fluorescent dyes, special printing techniques like micro-printing, and other
security features
are attached to the packages by use of adhesive tags, or these are laminated
to the carton, or
they are directly applied to the packages. The main drawback of such labels is
that they can be
removed from the product or the packaging and reused or analyzed. Some
companies offer
security features applied to the sealing foil of blister packages, but these
features possess the
same disadvantages.
No secure labeling of the pharmaceutical material itself, e.g., of solid
dosage forms
such as pills, is in the market yet. Techniques that use forgery-resistant
signatures, such as
DNA of known sequence (US 5,451,505) or molecules with characteristic isotopic
1
CA 02703610 2010-04-19
WO 2009/051805
PCT/US2008/011889
composition or micro-particles with characteristic color layer sequence (US
6,455,157 B1) are
not applicable, as these signatures incorporate biologically active components
that are
consumed with the pharmaceutical material. Certification authorities, such as
the Food and
Drug Administration (FDA) in the U.S., have not granted approval for such anti-
counterfeiting solutions.
One significant opportunity in designing pharmaceutical dosage forms is that
of
product identification and differentiation. It is useful, both from a consumer
safety perspective
and from a commercial perspective, to have a pharmaceutical dosage form with a
unique
appearance that is readily recognizable and identifiable.
One currently used technique for providing unique dosage form identification
includes
the use of intagliations. Intagliations are impressed marks typically achieved
by engraving or
impressing a graphical representation, for example a figure, mark, character,
symbol such as a
letter, a name, a logo, a pictoral representation, and the like, or any
combination thereof, in a
tablet or other solid dosage form, such as by a punching procedure. U.S.
Patent No.
5,827,535, for example, describes soft gelatin capsules with an external
surface having
defined thereon an impressed graphical representation. U.S. Patent No.
5,405,642 discloses a
method of highlighting intagliations in white or color-coated tablets by
spraying onto said
tablets a suspension comprising a filling material having a different color, a
waxy material
and a solvent, then removing the solvent and the excess filling and waxy
material. However, it
is often difficult to maintain the waxy material in an amount sufficient to
promote suitable
bonding of the filling material, yet be suitably removable with solvent.
EP 088,556 relates to a method of highlighting intagliations in white or
colored tablets
by contacting said tablets with a dry, powdery material having a different
color than that of
the tablet surface, then removing the excess powdery material not deposited in
the
intagliations. Disadvantageously, it has been found that the adhesion of the
powdery material
to the intagliations is not satisfactory, as the material shows a tendency to
loosen and fall out.
EP 060,023 discloses a method of emphasizing intagliations in colored (i.e.,
not white)
solid articles, in particular tablets, by coating the tablet surface and
filling up the intagliations
with a coating film comprising an optically anisotropic substance. An optical
contrast between
the tablet surface and the intagliations is obtained, presumably due to
different orientation of
the optically anisotropic substance on the tablet surface and in the
intagliations. However, this
2
CA 02703610 2010-04-19
WO 2009/051805
PCT/US2008/011889
technique is limited to colored articles and only allows for the use of
optically anisotropic
filling materials.
Another way to identify and differentiate one dosage form from another is via
application of microreliefs to the dosage form. A microrelief is a regular
pattern of ridges and
grooves and the like that may display a visual effect or optical information
when exposed to
suitable light. A few ideas of applying a microrelief or hologram to edible
products have been
published. One is based on coating an edible product with a thermo-formable
and thus
embossable layer (WO 01/10464 Al). As this layer alters the composition of the
product, as
well as the production process, a new approval of the drug from certification
authorities
would be needed. Further the heating during the thermo-forming steps can harm-
many active
agents. In another approach a polymer solution is brought into contact with a
diffraction relief
mold and then hardened upon drying (US 4,668,523). The drying step can be
accelerated by
heating, and in the end the hardened edible polymer product possesses the
diffractive relief of
the mold. This method is limited to polymer solutions, it is very slow, and
the heating step can
be harmful to active agents used in pharmaceutical products, as it may
negatively affect the
activity of the active pharmaceutical agents. Disadvantageously, production
difficulties could
be encountered when using these methods to stamp microrelief patterns into
tablets having
irregular shapes and/or surfaces.
WO 2006/047695 shows a variety of methods to manufacture pharmaceutical dosage
forms showing different kinds of microreliefs embedded into their surface.
However, based
on further review, it seems that the solutions proposed by WO 2006/047695
result in
microreliefs that are not recognizable by the human eye. In particular,
overcoating of
microstructures usually makes them invisible because most overcoatings have a
similar
optical index of refraction as the pharmaceutical dosage form completely
eliminating optical
reflections from the interface between the two.
Other documents describing dosage forms with diffractive microstructures are
W02006027688A1, US2007/0286811A1 andW02007144826A2. None of these documents
discloses a way to enhance the color contrast between the diffractive color
effects and the
background of the dosage form.
Summary of the Invention
The present invention relates to the manufacturing of micro- and
nanostructures in
pharmaceutical dosage forms by direct compression under production conditions.
In particular
3
CA 02703610 2010-04-19
WO 2009/051805
PCT/US2008/011889
the invention describes how geometrical structures embossed into
pharmaceutical dosage
forms at high speed by direct compression give rise to visible and/or
measurable optical
contrast in the pill by locally changing the directional optical reflectivity
and/or the absorption
of the surface. This contrast is useful for branding and brand protection
purposes, as well as
for anti-counterfeiting. In more detail this invention relates to
pharmaceutical dosage forms
incorporating dyes to enhance the contrast of visible and/or measureable
effects based on
micro- and nanostructures impressed in such forms during the direct
compression process.
The applicant found that only dry-compression techniques using certain
pressure parameters
can be employed in order to reliably obtain microreliefs in pharmaceutical
dosage forms. In
addition, the use of certain dyes is necessary, as the resulting colors
strongly increase the
human perception of the optical microstructures.
Human perception is very sensitive to contrast, not absolute intensities. A
diffractive
hologram on a diffuse white surface is not well visible for a human being,
even if the
measured diffraction efficiency of the hologram is very high. This is because
the human eye
not only perceives the light reflected from the hologram but also the intense
light from the
white surface surrounding it. The human being is "blinded" by the diffuse
white surface.
However, if the same hologram is on a darker background (black, dark blue,
green or red for
example) the rainbow colors of the hologram can be clearly seen even if the
hologram is not
very good. This is but one example how color and texture of a surface
influence human
perception.
These and other features of the invention will be more readily understood in
view of
the following detailed description and the drawings.
Brief Description of the Drawings
Figs 1A and 1B are perspective views which schematically show two types of
micro-
and nanostructures, according to the invention.
Fig 2 is a reproduction of a photograph showing a compressed pill, with a
diffractive
micro-structure made by direct embossing, illuminated with white light.
Fig 3 is a perspective view which schematically shows the reflection/
scattering/diffraction of light on a micro- and nanostructured dosage form,
according to the
invention.
4
CA 02703610 2010-04-19
WO 2009/051805
PCT/US2008/011889
Detailed Description of the Preferred Embodiment
The present invention describes how well visible optical contrast in
pharmaceutical
dosage forms is achieved in a very fast, single manufacturing step by direct
compression of
suitable materials. A punching tool with a micro- and/or nanostructured
surface directly
compresses a pharmaceutical formulation in a press. Under the proper
manufacturing
conditions a very fast transfer of the tool surface geometry into the surface
of the
pharmaceutical dosage form is achieved. In combination with the inherent
reflection and
absorption properties (color) of the form material the modified surface
geometry changes the
local optical appearance of the surface, creating a well visible optical
contrast. Depending on
the precise surface geometry a single or a combination of several optical
mechanisms are
responsible for contrast formation: interference, diffraction, diffuse single
and/or multiple
scattering, single and/or multiple reflection and single and/or multiple
absorption of visible
light. As long as the microstructure is smaller or close to the resolution
limit of the human
eye, i.e. smaller than about 100 micrometers, only the optical effects of the
microstructure, i.e.
a contrast is perceived by humans. The microstructures can have a regular
ordering or they
can be irregular or random arranged. In the case of regular ordered
microstructures, these
structures are chosen to be larger than 21.1m, preferred larger than 5 m, if a
color contrast
without or with low intense diffraction effects is the goal. Nevertheless the
microstructure
itself is not seen by the unaided eye. By locally changing the microstructure
in the
compression tool, the invention also allows to manufacture very complex
geometrical contrast
patterns in pharmaceutical dosage forms in a single manufacturing step.
In order to prepare a solid dosage form containing one or more active
ingredients
(such as drugs) for direct compression, it is necessary that the material to
be compressed into
the dosage form possess certain physical characteristics that lend themselves
to processing in
such a manner. Among other things, the material to be compressed must be free-
flowing, must
be lubricated, and importantly must possess sufficient cohesiveness to insure
that the solid
dosage form remains intact after compression.
In the case of tablets, the tablet is formed by pressure being applied to the
material to
be tabletted on a tablet press. A tablet press includes a lower punch that
fits into a die from the
bottom and an upper punch having a corresponding shape and dimension that
enters the die
cavity from the top after the tabletting material fills the die cavity. The
tablet is formed by
pressure applied on the lower and upper punches. The ability of the material
to flow freely
CA 02703610 2010-04-19
WO 2009/051805
PCT/US2008/011889
into the die is important in order to insure that there is a uniform filling
of the die and a
continuous movement of the material from the source of the material, e.g., a
feeder
hopper. The lubricity of the material is crucial in the preparation of the
solid dosage forms
because the compressed material must be readily ejected from the punch faces.
Because most drugs have none or only some of these properties, methods of
tablet
formulation have been developed in order to impart these desirable
characteristics to the
material(s) to be compressed into a solid dosage form. Typically, the material
to be
compressed into a solid dosage form includes one or more excipients that
impart the free-
flowing, lubrication, and cohesive properties to the drug(s) being formulated
into a dosage
form.
Lubricants are typically added to avoid the material(s) being tabletted from
sticking to
the punches. Commonly used lubricants include magnesium stearate and calcium
stearate. Such lubricants are commonly included in the final tabletted product
in amounts of
less than 1% by weight.
In addition to lubricants, solid dosage forms often contain diluents. Diluents
are
frequently added in order to increase the bulk weight of the material to be
tabletted in order to
make the tablet a practical size for compression. This is often necessary
where the dose of the
drug is relatively small.
Another commonly used class of excipients in solid dosage forms are binders.
Binders
are agents that impart cohesive qualities to the powdered material(s).
Commonly used binders
include starch, and sugars such as sucrose, glucose, dextrose, and lactose.
Disintegrants are often included in order to ensure that the ultimately
prepared
compressed solid dosage form has an acceptable disintegration rate in an
environment of use
(such as the gastrointestinal tract). Typical disintegrants include starch
derivatives and salts of
carboxymethylcellulose.
There are two general methods of preparation of the materials to be included
in the
solid dosage form prior to compression: (1) dry granulation and (2) wet
granulation.
Dry granulation procedures may be used where one of the constituents, either
the drug
or the diluent, has sufficient cohesive properties to be tabletted. The method
includes mixing
the ingredients, slugging the ingredients, dry screening, lubricating, and
finally compressing
the ingredients.
6
CA 02703610 2015-07-31
The wet granulation procedure includes mixing the powders to be incorporated
into
the dosage from in, e.g., a twin shell blender or double-cone blender and
thereafter adding
solutions of a binding agent to the mixed powders to obtain solutions of a
binding agent to the
mixed powders to obtain a granulation. Thereafter the damp mass is screened,
e.g., in a 6- or
8-mesh screen and then dried, e.g., via tray drying, the use of a fluid-bed
dryer, spray-dryer,
radio-frequency dryer, microwave, vacuum, or infra-red dryer.
In direct compression, the powdered material(s) to be included in the solid
dosage
form is compressed directly without modifying the physical nature of the
material itself. The
use of direct compression is limited to those situations where the drug or
active ingredient has
a requisite crystalline structure and physical characteristics required for
formation of a
pharmaceutically acceptable tablet. On the other hand, it is well known in the
art to include
one or more excipients that make the direct compression method applicable to
drugs or active
ingredients that do not possess the requisite physical properties. For solid
dosage forms in
which the drug itself is to be administered in a relatively high dose (e.g.,
the drug itself
comprises a substantial portion of the total tablet weight), it is necessary
that the drug itself
have sufficient physical characteristics (e.g., cohesiveness) for the
ingredients to be directly
compressed.
In any case, applicant found that only direct compression techniques are
feasible in
order to reliably obtain micro - gratings in pharmaceutical dosage forms that
would then also
result in an optical effect being recognizable in a reliable manner for the
human eye. US
patent US2007/028681 1A1, shows the fundamentals of the inventive
manufacturing process
that is reliably embedding micro-gratings or micro-structures into the surface
of
pharmaceutical dosage forms. The main principles of this process are provided
herein,
accompanied by important parameters regarding the operation of mass
manufacturing tablet
presses as well as examples of the dyes necessary in the composition of the
pharmaceutical
dosage form so that a satisfactory result, especially color contrast, is being
obtained.
Most tablets or pills are manufactured by compressing a mixture of powders. An
example of a typical mixture is denoted in Table I.
Table I: Example of compressing mass mixtures
Fraction Ingredients
7
CA 02703610 2010-04-19
WO 2009/051805
PCT/US2008/011889
70-80% Lactose Monohydrate
10-25% Microcrystalline Cellulose
Aerosil (colloidal silica, anhydrous), Magnesium-
<10% stearat (Mg-stearate),polyethyleneglycol, color and
active agent
Most of the volume is made of binding and filling agents like Lactose and
Cellulose. Because
of their at least partially plastic deformation behavior these and similar
materials are suited to
emboss the micro-structure in. Mg-stearate is used as a lubricant and Aerosil
improves the
powder flow. An FDA-approved colorant may be added. While most pharmaceutical
pills are
white, notable examples such as Viagra are blueõ others are red. Direct
tabletting results in
pills with a bright colored and scattering surface.
A high portion of plastic deformable materials in the formulation helps the
formation
of the micro-and nano-structure in the pill surface. For example the portion
of
microcrystalline cellulose or plastic binders like PVP may be enhanced or
these materials
could replace less plastic ones.
Pharmaceutical powder is made up of particles with different sizes. A typical
size
distribution may is shown in table II:
Table II: Typical particle size distribution in pharmaceutical formulations
Fraction Size
15-25% < 75 micrometers
30-50% 75-150 micrometers
15-25% 150-250 micrometers
5-15% 250-500 micrometers
<2% > 500 micrometers
8
CA 02703610 2016-05-25
Most particles have a size between 75 and 250 microns. Embossing
microstructures
with a size of 100 microns or less into the pill surface therefore deforms
most particles
themselves, I.e. the microstructure is embossed into the surface of the
gsrains.
The powder mixture is compressed between two punches, which apply axial
mechanical forces in the range of 5-401(N, but depend on the size of the pill
in question.
Compression reduces the volume of the mass and at the same time increase its
mechanical
strength. The compression process is essentially a high-impact molding process
and works at
room temperature without heating. State-of-the-art single rotary presses work
at high speed
and produce about 30,000 to 300,000 pills per hour. This means that
compression time per pill
is well below 100ms. This time is long enough to compress the raw powder
material to a hard
pill, but the pill is still soluble after it is ingested.
In a preferred embodiment of this invention, the pressure parameters of the
tablet press
are set in a way that correlates with the mixture of ingredients used in the
particular
pharmaceutical dosage form. However, it was found that pressure parameters
generally have
to be set at the upper end of the spectrum of commercially available tablet
presses, good
results for a flat pill with a diameter of llmm were obtained for compression
forces between
15 and 35 kN. This resulted in pills with a hardness between 100-250 N. In
certain
embodiments of the invention the tablet press parameters were set in a range
of between 10
and 50 kN,
Optics of micro- and nanostructures:
When illuminated by polychromatic or white light, micro- and nano-structures
(particularly diffractive gratings) show characteristic optical effects.
Figures 1B and 2B
schematically show two examples of such structures, designated 10a and 10b,
respectively.
Structure 10a has a sinusoidal diffraction grating (with period 12 and depth
14), and structure
10b has a random scattering microstructure with an average lateral structure
dimension 16 of
a few nm and structure height 18. The aspect ratio of the structures is the
depth 14 (or 16)
divided by the height 12 (or 18).
For example it is well known, that surface holograms show a distinctive
rainbow
pattern when illuminated by white light. Regular pyramidal structures, with
sizes between 10
and 70 microns strongly diffuse light and give a satinated appearance.
Randomly oriented
structures with an average size between 1 0-1 00 microns may also show
satination. Further
such random microstructures can produce characteristic speckle pattern if
illuminated by a
9
CA 02703610 2010-04-19
WO 2009/051805
PCT/US2008/011889
coherent light source such as a laser of an LED. GB221870A describes security
devices based
on such random microstructures. Small, but deep patterns with sizes ranging in
between 100-
500nm's reduce reflection and darken a given surface. In this case deep means
deeper or
comparable to the lateral size. Deep, self-organized nanostructures as are
commonly produced
by self-masking in reactive ion etching may even lead to a blackening of a
surface. These and
similar effects may be locally combined on their respective geometrical
scales. For example a
satinated pyramidal structure may be superposed with a random antireflective
nanostructure
to darken the satinated region. Similarly, interference gratings superimposed
on satinated
structures will be well visible under more viewing angles than their
counterparts on a flat
surface. Typical micro- and nanostructure types are listed in table III:
Table III: Typical optical micro- and nanostuctures
Structure
Dominant Optical Effect Typical forms and dimensions
type
Grating period: 0.5-23 microns,
Diffractive Reflection hologram, rainbow aspect
ratio: 0.05-1, grating shape:
grating colors
sinusoidal, trapezoidal, square,
triangular etc.
Large
Average lateral dimension: 5-100
Random Satination
microns, Aspect ratio: 0.1-1
structure
Medium
Graying, diffuse scattering, Average lateral dimension: 0.5-10
random
cture speckle pattern microns,
Aspect ratio: 0.01-0.8
stru
Small
Multiple absorption, antireflex, Average lateral dimension: 50 nm to
random
darkening 500nm, aspect ratio: 0.1-2
structure
Large Pyramids,
Squares, Sinusoids etc,
regular Satination, multiple absorption
Average dimensions: 10-100 Microns,
structures Aspect ratio: 0.2-1
The regular structures shown in table III, such as diffractive gratings or the
large
regular structures can be arranged regularly in 1 or 2 dimension in different
patterns. The
gratings, can be one or 2-dimensional. They may also be quasi-cristalline,
i.e. exhibit a 5 fold
symmetry. It is also possible to have locally regular arrangements which on a
scale of several
CA 02703610 2010-04-19
WO 2009/051805
PCT/US2008/011889
microns to mm is randomly arranges, i.e. like in a 2 dimensional polycrystal.
Other
arrangements are conceivable as well.
Multiple optical reflection on a colored micro- or nanostructured surface
leads to color
shifts because multiple reflection favors the most dominant reflection peak
and suppresses
side peaks of the reflective spectrum. This can be easily seen from the
following argument: If
R(D) is the reflectivity as a function of wavelength of a
smooth surface, then R20:1
corresponds to the amount of the twice reflected light on a structured
surface, R3([1 to the
amount of three times reflected light on the structured surface and so on. As
R is always
smaller than one, this means that the more times the light is reflected, the
weaker it becomes
but it also means that the reflectivities which are closer to 1 get much less
attenuated by
multiple reflection than the smaller reflectivities, in effect narrowing the
reflection spectrum
around the maximum reflectivity. If the reflectivity spectrum contains several
peaks this also
means that the average reflected wavelength is shifted towards the wavelength
with maximal
reflectivity by multiple reflection.
This effect can be precisely controlled and gives simple possibility to
manufacture a 2
colored pharmaceutical pill by combining 2 or more dyes in a given
formulation. For example
if a blue and red dye is added to a white formulation, the resulting flat pill
may be violet.
However, microstructuring parts of the surface will make this part of the pill
more reddish or
bluish, depending on relative reflectivities, giving a 2 colored pill.
It is not self-evident that micro-and nanostructures with typical lateral
sizes ranging
between 0.2 m and 100 microns and aspect ratios (ration between structure
depth and lateral
size) between 0.1 and 2 can be reliably and stably implemented in the surface
of each pill
during the direct tabletting process. Considering the restrictions of this
process mentioned
above, it seems more or less impossible. The powders are not designed to be
micro-structured.
As the size of the micro-structures is smaller than the dimension of the
particles, the surface
of the particle itself must be micro-structured. Finally, the tabletting
process is very fast
making the time for the micro-structuring extremely short, i.e. less than
100ms. Nevertheless,
as shown in Fig 2, if the micro-structure in the punch surface is adapted to
the process and the
dye formulation, strong color contrast can be achieved by micro- and
nanostructuring of pill
surfaces, as shown by the micro-structure 10c that appears on the surface of
dosage form 19,
within the dotted circle 20.
11
CA 02703610 2010-04-19
WO 2009/051805
PCT/US2008/011889
The micro-structured area can even be macroscopically structured to form
logos, brand
names, and the like. This conclusion required several findings. The material
of the tool which
bears the micro-structure must be very hard for a long lifetime. At the same
time it must be
possible to implement the micro-structure in its surface. Hardened steel, hard
chromium
coated steel, tungsten carbide or molybdenum carbide etc. are examples of
materials used for
direct tabletting. They are FDA approved and can be used for the punches or
dies.
Unfortunately microstructuring of these materials is not achievable with the
usual holographic
and lithographic techniques used for example to manufacture DVD masters or to
structure
semiconducting surfaces. However, these materials can be microstructured using
advanced
non-standard etching and/or ablation techniques.
Release between tool and pill is made more difficult by the microstructures,
as the
contact area between the punch and the compressed pill is enhanced. The
geometry of the
microstructure plays a crucial role for a good release and long tool lifetime.
For example,
first tests with microscopic hole patterns showed that, punching several
tablets with the same
tool, the holes were quickly filled with residue of pill mass, inhibiting
transfer of the structure
into the pills. Only the first few pills showed a transfer of the
microstructure. Using linear
gratings, this effect was not observed. For these reasons, structures with
rounded edges, walls
with positive release angle and shallow structures are preferred. However, in
the case of
interfering structures, such as gratings, the grating efficiency strongly
depends on grating
depths. Usually, for a diffraction grating to be visible, a grating depth of
about 100nm or more
is necessary. Also, if a lubricant is used the micro-structure must be deeper
than the thickness
of the lubricant between the punch and the tablet mass.
In general, adding color to the tablets significantly enhances the visible
image contrast
of the microstructure perceived by the human eye. Therefore a microstructure
of a colored
tablet needs less depth than a comparable white tablet to induce a similar
image contrast. This
is a great advantage for manufacturing microstructures with the direct
tabletting process, as
the release of the tool is easier.
Once manufactured, the microstructure needs to be protected, otherwise it may
wear
off This can be done either by geometrical arrangements or by coating of the
tablet. In one
preferred embodiement, the microstructures are embossed at the bottom of an
intagliation in
the pill surface. The walls of the intgliation geometrically protect the
microstructure from
12
CA 02703610 2010-04-19
WO 2009/051805
PCT/US2008/011889
abrasion. Other geometrical arrangements are conceivable which protect the
microstructure
geometrically from abrasion.
Visibility of micro-structures
As mentioned earlier nearly all pharmaceutical pills manufactured by the
direct tabletting
process possess a bright color. This bright color produces a background that
may make it
difficult to recognize the optical effect of the micro-and or nanostructures.
As the powders
usually possess an index of refraction of about 1.5 in the visible spectral
range, only a few
percent of the light incident on the pill surface is diffracted or directly
reflected backwards, In
white pills, most of the light is scattered into all directions, an only
shades of grey or weak
diffractive color effects can be expected on white pills.
In most cases, use of certain dyes is necessary to obtain well visible micro-
and
nanostructures that are recognizable to the human eye in a reliable manner. It
was found by
applicant during test cycles that darker dosage forms increase the contrast
and are therefore
superior in terms of the reliable recognition by the human eye of a micro-
grating embedded in
a pharmaceutical dosage form. White, pale and transparent or semi-transparent
top-coating of
the dosage form can make it difficult to recognize the micro-grating,
sometimes to the point
where it will not be recognizable at all. Dark dyes incorporated into the
dosage form are
generally helpful to reliably achieve the inventive effect, namely to enhance
the visible or
color contrast of effects based on micro- and nanostructures. Defining the
visible or color
contrast C as follows give a possibility to quantify the enhancement.
C = I Is - lb I / (Is Ib)
Is and Ib are the reflected light intensities from a structured part of the
dosage form and
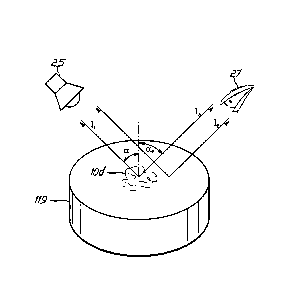
an unstructured part respectively. Thus Ib is the background intensity. Figure
3 schematically
depicts the reflection and/or scattering and/or diffraction of light emitted
from a light source
25 toward a pharmaceutical dosage form 119, with a circular micro- and/or
nanostructure 10d
located in the center thereof, and eventually toward point of reference 27.
The bigger the value of C the better visible and/or measureable is the optical
effect.
Preferrably the contrast C is >0.1, especially preferred >0.2, in particular
preferred >0.4 and
most preferred >0.6. The two angles a; and ae denote the incidence and
excidence angles.
They do not have to be equal. Especially for diffractive color effects both
angles differ. A
preferred pair of angles for the verification of diffractive micro- and
nanostructures is a; = 00
(perpendicular illumination) and e = 45 .
13
CA 02703610 2010-04-19
WO 2009/051805
PCT/US2008/011889
Dyes that can be used include but are not limited to the following:
1. Naphthol Yellow SSX Spec. Pure, a nitrodye hay-
ing the formula
ONa
NaOaS ¨NO2
NO2 =
sold by Badische Anilin & Soda Fabrik, A.G., Ludwigs-
hafen a. Rhein, Germany;
2. Orange GGN Conc. Spec. Pure, a monoazo-dye
having the formula _
H O
1
Na0aS ¨N=N¨
=
OaNa
sold by Farbenfabriken Bayer A.G., Leverkusen, Ger-
many; and Salmon Red. G.AF. a dye having the for-
mula
14
CA 02703610 2010-04-19
WO 2009/051805
PCT/US2008/011889
sold by Farbenfabriken Bayer A.G., Leverkusen, Ger-
many; and Salmon Red. G.AF. a dye having the for-
mula .
sozNa OH
I
. H3C¨(7¨N=N¨o0
H3
103Na
sold by CIBA Ltd., Basel, Switzerland;
3. Hexacol Chocolate Brown HT, a diazo-dye having
. the formula
OH
I
R
Na03S¨ ¨N=N ¨N=N¨ SO3Na
HO¨
sold by L. J. Pointing & Son Ltd., Hexhem, England;
CA 02703610 2015-07-31
4. lieliogen Blue BWS Extra, a phthalocyanine-dye .
having the formula
_
I" A
C C
0-11T A--c
N Cu
C--,--N N¨C
41Ik N/ \
sold by General Aniline & Film Corporation, New
York, N.Y.;
5. Canary Yellow Geigy, a quinoline-dye having the
formula
o
/µ 8
0/
):2)
r, \c
a
sold by J. R. Geigy S. A., Basel, Switzerland;
6. Edieol Supra Rose B, a rhodamine dye having the
formula
-0
(HsCa):N ¨ 0¨",--N4 (C211)2
¨CI
¨7---\,
0¨COOH
sold by Imperial Chemical Industries, Ltd., Manches-
ter, England; and
16
CA 02703610 2015-07-31
Erythrosine TB Extra, having the formula
1 1
Ns 0 .-0
I 0¨ ----I
0,---000Na
sold by Durand & Huguenin S. A., Basel, Switzerland;,
7. Acid Violet 5 BN, a triaryl methane dye having the
formula;
11,01
) c,n)
cn,¨N >c<D-44+¨cri2¨C
\
A
Na '.P>--
\N/
(602
and
Acid Green S, having the formula
--03s OR
I N¨(01143
ilik
OW _____________________ N4(CF13)3
Na03
sold by Williams Ltd., Hounslow, England;
Kiton Pure Blue V. FQ, having the formula
N803S 0/ \l'f--(Cinih
______________________ /
03_ VC)=N+(03133)*
sold by Clayton Aniline Co., Ltd., Manchester, En-
gland; and
8. Edicol Supra Blue X, and indigoid dye having the
formula
O o
g g
,---ON\
N
sold by Imperial Chemical Industries, Manchester, En-
gland.
17
CA 02703610 2010-04-19
WO 2009/051805
PCT/US2008/011889
Vegetable dyes, such as carotene, chlorophyll, and also tea and coffee
extracts in
powder form are also suitable as a dye-component. As U.S. patent 4336244
shows, curcumin,
turmeric and annatto can be employed in the coloring of pharmaceutical dosage
forms and
applicant found these dyes to be suitable for the inventive purpose. Also, it
is well known in
the state of the art to use inert carbon or its derivatives as a component of
dyes used in the
manufacturing of pharmaceuticals and applicant found such dyes to be suitable
for the
inventive purpose.
Typically, excipients are added to the formulation to impart good flow and
compression characteristics to the material as a whole that is to be
compressed. Such
properties are typically imparted to these excipients via a pre-processing
step such as wet
granulation, slugging, spray drying, spheronization, or crystallization.
Useful direct
compression excipients include processed forms of cellulose, sugars, and
dicalcium phosphate
dehydrate, among others.
A processed cellulose, microcrystalline cellulose, has been used extensively
in the
pharmaceutical industry as a direct compression vehicle for solid dosage
forms. Microcrystalline cellulose is commercially available under the
tradename,
"EMCOCELeefrom Edward Mendell Co., Inc., and as Avicel from FMC Corp.
Compared
to other directly compressible excipients, microcrystalline cellulose is
generally considered to
exhibit superior compressibility and disintegration properties.
Suitable polymers for inclusion in top coatings include polyvinylalcohol
(PVA); water
soluble polycarbohydrates such as hydroxypropyl starch, hydroxyethyl starch,
pullulan,
methylethyl starch, carboxymethyl starch, pre-gelantinized starches, and film-
forming
modified starches; water swellable cellulose derivatives such as hydroxypropyl
cellulose
(HPC), hydroxypropylm ethyl cellulose (HPMC), methyl
cellulose (MC),
hydroxyethylm ethyl cellu lose (HEMC),
hydroxybutylm ethyl cellu lose (HBMC),
hydroxyethylethylcellulose (HEEC), and hydroxyehtylhydroxypropylmethyl
cellulose
(HEMPMC); water soluble copolymers such as methacrylic acid and methacrylate
ester
copolymers, polyvinyl alcohol and polyethylene glycol copolymers, polyethylene
oxide and
polyvinylpyrrolidone copolymers; polyvinylpyrrolidone and polyvinylacetate
copolymers;
and derivatives and combinations thereof. Suitable film-forming water
insoluble polymers for
inclusion in top coatings include for example ethylcellulose, polyvinyl
alcohols, polyvinyl
acetate, polycaprolactones, cellulose acetate and its derivatives, acrylates,
methacrylates,
18
CA 02703610 2010-04-19
WO 2009/051805
PCT/US2008/011889
acrylic acid copolymers; and the like and derivatives, copolymers, and
combinations
thereof. Suitable film-forming pH-dependent polymers for inclusion in top-
coatings include
enteric cellulose derivatives, such as for example hydroxypropyl
methylcellulosephthalate,
hydroxypropyl methylcellulose acetate succinate, cellulose acetate phthalate;
natural resins,
such as shellac and zein; enteric acetate derivatives such as for example
polyvinylacetate
phthate, cellulose acetate phthalate, acetaldehyde dimethylcellulose acetate;
and enteric
acrylate derivatives such as for example polymethacrylate-baserd polymers such
as
poly(methacrylic acid, methyl methacrylate) 1:2, which is commercially
available from Rohm
Pharma GmbH under the tradename, "EUDRAGIT S;" and poly(methacrylic acid,
methyl
methacrylate) 1:1, which is commercially available from Rohm Pharma GmbH under
the
tradename "EUDRAGIT L;" poly (butyl, methacrylate
(dimethylaminoethyl)methacrylate,
methyl methacrylate), which is commercially available from Rohm Pharma GmbH
under the
tradename, "EUDRAGIT E;" and the like, and derivatives, salts, copolymers, and
combinations thereof.
In one embodiment, the top coating includes coatings having a high rigidity,
i.e., e.g.,
those coatings having a yield value sufficient to prevent deformation of the
microrelief when
exposed to normal manufacturing, handling, shipping, storage, and usage
conditions. Suitable
top coatings having high rigidity include film formers, such as for example,
the high tensile
strength film-formers well known in the art. Examples of suitable high tensile
strength film-
formers include, but are not limited to, methacrylic acid and methacrylate
ester copolymers;
polyvinylpyrrolidone; cellulose acetate; hydroxypropylmethylcellulose (HPMC),
polyethylene oxide and polyvinylalcohol, which is commercially available from
BASF under
the tradename, "Kollicoat IR;" ethylcellulose; polyvinyl alcohols; and
copolymers and
mixtures thereof
In one embodiment, the top coatings may include the water-soluable high
rigidity film
formers selected from HPMC, polyvinylpyrrolidone, the aminoalkyl-methacrylate
copolymers
marketed under the trade mark, "EUDRAGIT E;" and copolymers and mixtures
thereof
The inventive dosage form may come in a variety of different shapes. For
example, in
one embodiment the dosage form may be in the shape of a truncated cone. In
other
embodiments the dosage form may be shaped as a polyhedron, such as a cube,
pyramid,
prism, or the like; or may have the geometry of a space figure with some non-
flat faces, such
as a cone, cylinder, sphere, torus, or the like. Exemplary shapes that may be
employed include
19
CA 02703610 2016-05-25
tablet shapes formed from compression tooling shapes described by "the
Elizabeth Companies
Tablet Design Training Manual" (Elizabeth Carbide Die Co.. Inc., p. 7
(McKeesport. PA).
The tablet shape corresponds inversely to the shape of the compression
tooling.
In embodiments in which the dosage folin is prepared via compression, suitable
fillers
include, but are not limited to, water-soluble compressible carbohydrates such
as sugars,
which include dextrose, sucrose, isomaltalose, fructose, maltose, and lactose,
polydextrose,
sugar-alcohols, which include mannitol, sorbitol, isomalt, maltilol, xylitol,
erythritol, starch
hydrolysates, which include dextrins, and rnaltodextrins, and the like, water
insoluble
plastically deforming materials such as microcrystalline cellulose or other
cellulosic
derivatives, water-insoluble brittle fracture materials such as dicalcium
phosphate, tricalcium
phosphate, and the like and mixtures thereof
In embodiments in which the dosage folin is prepared via compression, suitable
binders include, but are not limited to, dry binders such as polyvinyl
pynolidone,
hydroxypropylmethylcellulose, and the like; wet binders such as water-soluble
polymers,
including hydrocolloids such as alginates, agar, guar gum, locust bean,
carraaeenan, tara, rum
arabic, tragacanth, pectin, Whelan, rhamsan, zooalan, methylan, chitin,
cyclodextrin, chitosan,
polyvinyl pyrrolidone, cellulosics, starches, and the like; and derivatives
and mixtures thereof
In embodiments in which the dosage form is prepared via compression, suitable
disintearants include, but are not limited to, sodium starch glycolate, cross-
lined
polyvinylpyrrolidone, cross-linked carboxymethylcellulose, starches,
microciystalline
cellulose, and the like.
In embodiments in which the dosage form is prepared via compression, suitable
lubricants include, but are not limited to, long chain fatty acids and their
salts, such as
magnesium stearate and stearic acid, talc, and waxes.
In embodiments in which the dosage form is prepared via compression, suitable
alidants include, but are not limited to, colloidal silicon dioxide, and the
like.
In embodiments in which the dosage form is prepared via compression, the
dosage
form of the invention may also incorporate pharmaceutically acceptable
adjuvants, including
but not limited to preservatives, high-intensity sweeteners sucli as
aspartame, acesulfame
potassium, cyclamate, saccharin, sucralose, and the like; and other sweeteners
such as
CA 02703610 2015-07-31
dehydroalcones, grycyrrhizin, MonellinTM, stevioside, TalinTm, and the like;
flavors,
antioxidants, surfactants, and coloring agents.
While this application describes several preferred embodiments of the
invention, those
skilled in the art will readily appreciate that the described embodiments are
merely exemplary
in nature, and that the subject matter is not limited to that which is
expressly shown or
described. Accordingly, applicants wish to be bound by the claims, not the
particular details
described herein.
21