Note: Descriptions are shown in the official language in which they were submitted.
CA 02707342 2009-12-11
1
A DEVICE FOR PROVIDING FLUID TO A RECEPTACLE
TECHNICAL FIELD
The invention relates to a device for providing cleaned fluid i.e. gas and/or
liquid to a
receptacle, and a device for providing cleaned fluid to a receptacle.
The invention can be implemented in aseptic preparation of drugs, for example
for
providing sterilized/cleaned air to a medical receptacle, such as a bottle or
vial, with the
purpose of drawing a solution or another liquid used in medicine applications
out from the
medical receptacle.
BACKGROUND OF THE INVENTION
In the field of drug preparation for injection or infusion generally two basic
problems have
to be considered. Firstly, certain demands are made on aseptic conditions so
as to avoid
contamination of the drug, and, secondly, the drug has to be handled in such a
way that
drug leakage to the environment is prevented or minimized. By a sterile or
aseptic
handling of the drug, the risk for transferring bacteria or any other
undesired substance to
the patient is reduced. By preventing drug leakage to the environment, the
exposure of
medical and pharmacological staff to hazardous drugs is decreased.
In order to achieve aseptic conditions special safety boxes, cabinets or
isolators are being
used where the air is filtered through HEPA filters to prevent contamination
during
preparation of drugs. Ventilated cabinets are also used to reduce uncontrolled
leakage to
the environment and prevent occupational exposure to possibly hazardous drugs.
Such
facilities, however, require a lot of space and are associated with relatively
high costs.
Furthermore, the offered protection can be insufficient and working
environment problems
due to accidental exposure to drugs, for example cytotoxins, have been
reported.
Another solution of the problems mentioned above is to create a so called
"closed" or
"non-vented" system for handling the drugs during preparation. Such systems
exist and
enable the preparation to be accomplished without the use of special safety
boxes,
cabinets or isolators. In such a closed system the drugs are handled isolated
from the
CA 02707342 2009-12-11
WO 2008/153460 PCT/SE2007/050416
2
environment during every single step so as to avoid contamination of the drug
and
undesired drug leakage to the environment.
A known problem associated with the preparation of drug solutions is the fact
that medical
bottles or vials normally are made of a non-compressible material, such as
glass or
plastic. To enable the vial to be drained off, air has to flow into the vial
so as to avoid
negative pressure in the vial which negative pressure would otherwise
counteract or
prevent further transportation of liquid from the vial to another receptacle
such as syringe.
A system for providing sterilized gas is disclosed in WO 00/35517. A flexible
bag
containing sterilised gas is provided. The bag has an opening covered by a gas
and
liquid-impervious membrane which can be punctured by a needle in order to draw
the
sterilised gas out from the bag for further transportation of the gas to a
bottle. A bottle
connector is arranged on the current bottle and the bottle connector has a
pressure
compensation means for receiving gas. By use of a syringe and an injector
device
provided with a needle the sterilised gas is transferred from the flexible bag
to the bottle
and to the pressure compensation means arranged on the bottle connector.
Thereafter
the substance in the bottle can be drawn out from the bottle by means of the
injector
device while the sterilised gas flows from the pressure compensation means
into the
bottle.
However, the prior art system described in WO 00/35517 has drawbacks. The
system
comprises several components to be handled and further the sterilised gas has
to be
drawn from the flexible bag by means of an injector device provided with a
needle, and
subsequently transferred to the bottle and the pressure compensation means.
Consequently, several manipulations have to be accomplished before the medical
substance can be drawn from the bottle.
In WO 02/11794 a system for providing cleaned gas is described. This system
works with
an injection syringe and an air filter to be attached to a connection nozzle
of the syringe.
The container of the syringe is charged with air which has been forced through
the filter so
as to clean the air. Thereafter the air filter is removed and the syringe is
connected to a
coupling means (injector device) which in turn is connected to a capping means
(bottle
connector) arranged on a bottle. The capping means has a pressure-equalisation
chamber whose volume can vary. The cleaned gas in the syringe is transferred
from the
CA 02707342 2009-12-11
3
syringe to the bottle and to the pressure-equalisation chamber arranged on the
capping
means. Thereafter the substance in the bottle can be drawn out from the bottle
by means
of the syringe and the coupling means, while the cleaned gas flows from the
pressure-
equalisation chamber into the bottle.
Also the prior art system described in WO 02/11794 has drawbacks. The system
requires
an adapter provided with an air filter being connected to and removed from a
syringe in
order to fill the pressure-equalisation chamber before the medical substance
can be
drawn from the bottle. In an alternative embodiment the air filter is fixedly
attached to a
syringe. However, in such a case a conventional syringe can not be used. In
both cases,
the cleaned gas has to be drawn from the environment and subsequently
transferred to
the bottle and the pressure-equalisation chamber before the medical substance
can be
drawn from the bottle.
SUMMARY OF THE INVENTION
An object of the invention is to provide a device for providing cleaned and/or
sterilized
fluid of the kind referred to in the introduction where at least one problem
of such prior art
devices discussed above is reduced to a substantial extent. In particular, the
invention
aims to indicate how to provide sterilized/cleaned fluid in a rational and
safe way during
preparation of drugs.
The invention is based on the insight that sterilized/cleaned air is
advantageously
provided by a connector system itself, rather than utilising additional
equipments to fill an
expansion container comprised in a connector system during drug preparation.
However,
a container has to be filled with the fluid either during manufacturing of the
device or by
the user, and these two options result in two aspects of the invention.
By the provision of a connector and a container which form an integrated unit,
wherein the
connector is provided with a first means for connection to a receptacle, and
the container
is pre-filled or adapted to be pre-filled with a sterilized or cleaned fluid
to be transferred
from the container to a receptacle interconnected with the connector during
conveyance
CA 02707342 2009-12-11
4
of a substance out of the receptacle, no additional flexible bag filled with
sterilized/cleaned
fluid is needed. The handling is simplified since no syringe provided with a
needle is to be
used for transferring sterilized/cleaned fluid into for example a vial before
conveyance of a
substance out of the vial. The pre-filled container replaces both the
additional flexible bag
and the pressure compensation means needed in the prior art devices.
Conveyance of a
substance out from the receptacle can be accomplished as soon as the connector
is
arranged on the receptacle.
By the provision of a connector and a container which form an integrated unit,
wherein the
connector is provided with a first means for connection to a receptacle, and
the integrated
unit is provided with a filter for cleaning fluid passing the filter during
filling the container
with fluid, for example before connection of the connector to a receptacle, no
syringe
provided with an air filter adapter or an air filter fixedly attached to the
syringe is needed
for transferring cleaned fluid into for example a vial before conveyance of a
substance out
of the vial. Instead a conventional syringe can be used for conveyance of a
substance out
from the receptacle as soon as the connector is arranged on the receptacle.
On comparison the two aspects of the invention it can be established that the
device
according to the first aspect does not exhibit any filter which saves costs as
to the
production of the device. Furthermore, the degree of purity which can be
obtained during
manufacturing of the device, for example by sterilization, is very high and in
most cases
very important. On the other hand the device according to the second aspect
can have a
decreased volume which could result in smaller package and save shipment
costs. In
many applications a cleaned fluid suitable for aseptic preparation of drugs
can be
achieved by filtering the fluid.
BRIEF DESCRIPTION OF THE DRAWINGS
With reference to the appended drawings, below follows a more detailed
description of
embodiments of the invention cited as examples.
In the drawings:
CA 02707342 2009-12-11
WO 2008/153460 PCT/SE2007/050416
Fig. 1 is a perspective view of a device according to a first aspect of the
invention,
Fig. 2 is a view corresponding to figure 1 illustrating the device in another
condition,
5 Fig. 3 is a perspective view of the device according to figure 1 connected
to a vial,
Fig. 4 is an exploded view corresponding to figure 3,
Fig. 5 is a perspective view of a device according to a second aspect of the
invention,
Fig. 5b is an alternative embodiment of the device illustrated in figure 5,
Fig. 6 is a view corresponding to figure 5 illustrating the device in another
condition,
Fig. 7 is a perspective view of the device according to figure 5 connected to
a vial, and
Fig. 8 is an exploded view corresponding to figure 7.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS OF THE INVENTION
In figures 1 and 2 a device 1 according to the first aspect of the invention
is illustrated.
The device 1 can be used for providing cleaned and/or sterilized gas, for
example air, to a
receptacle and thereby facilitate conveyance of a substance out of the
receptacle. Such a
substance can be various solutions and liquids constituting drugs, for example
cytotoxic
drugs and antibiotics, for use in the field of medicine. The device comprises
a connector 2
and a container 3 which form an integrated unit 4. The connector 2 is provided
with a first
means 5 for connection to a receptacle or in other words a first connector
portion 5 for
connection to a receptacle. The container 3 is pre-filled or adapted to be pre-
filled with a
cleaned and/or sterilized gas to be transferred from the container 3 to a
receptacle, which
is connectable with the connector 2, during conveyance of a substance out of
the
receptacle. Se also figure 3 illustrating the device 1 connected to a medicine
receptacle
such as a bottle or vial 6, and the exploded view in figure 4. By the
expression "pre-filled"
is meant the container being already filled with gas before it is used for
providing gas to a
receptacle. The device is suitably already filled when delivered to the user,
and preferably
CA 02707342 2009-12-11
WO 2008/153460 PCT/SE2007/050416
6
the container is filled during or after manufacture of the device for example
just before or
at the moment when the device is enclosed by a package or packing.
By the expression "cleaned" gas is meant that the gas has been filtered by a
filter to
remove particles and/or viable micro-organisms to such an extent that the gas
is classified
to be aseptic and accepted by the relevant authority and/or any standards. The
degree of
purity can be expressed in the largest particles allowed to pass the filter
for a given flow
rate of gas. In some cases no or very few particles having a size exceeding
5pm are
allowed to be present in the cleaned gas. However, the allowed particle size
is determined
by the requirements in the current application. Some drug treatments require
that
substantially all particles having a size exceeding 0.15pm are removed from
the gas by
the particulate air filter. As an example, a filter with the mesh size 0.2pm
can be used to
remove substantially all particles and micro organisms of that size or larger.
By the expression "sterilized" gas is meant that the gas has been subjected to
a
sterilization method to remove viable micro-organisms, which method is
accepted for the
current product by the relevant authority. Current regulations in Europe for
medical
devices to be designated "STERILE" may be found in the European standard EN
556-1.
Other regulations may exist in other countries. The sterilization can be
ethylene oxide
sterilization, sterilisation by irradiation, or (moist) heat sterilization or
any other accepted
method. The European standard requirements imply that the theoretical
probability of
there being a viable micro-organism present on/in the sterilized device shall
be equal to or
less than 1x10-6.
In the case the gas is sterilized, it is not always necessary to clean the gas
according to
the cleaning process as described above, although such cleaning and the
sterilization can
be combined. However, other methods can be used to remove particles etc. from
the gas
if required or the sterilization process itself may be sufficient to bring the
gas into a state
where the gas is to be considered as both cleaned and sterilized.
The first connection means 5 can be designed for connection to a receptacle,
such as the
neck of a vial. In the embodiment illustrated in figures 1-4, the first
connection means 5 is
constituted by a ring-shaped portion 7 for enclosing the neck 8 of the vial 6.
The ring-
shaped portion 7 has slits 9 so as to form flanges 10 which protrude
downwardly. The
flanges 10 can be provided with hooks 11 or barbs for gripping around the neck
8 of the
CA 02707342 2009-12-11
WO 2008/153460 PCT/SE2007/050416
7
vial 6. The connector 2 is suitably provided with a second means 12 for
connection to a
transfer member 13 (see figures 3 and 4), such as an injector device to be
interconnected
with the connector, for conveyance of a substance out of the receptacle 6. In
other words;
the connector 2 is suitably provided with a second connector portion 12.
In another embodiment (not shown) of the invention the second connection means
12 can
comprise a luer lock coupling or bayonet coupling to enable an injector device
to be
connected to the connector. Suitably, both the injector device and the
connector are
provided with a membrane so as to create a double membrane coupling between
the
injector and the current device.
The amount of gas, preferably air, provided by the pre-filled container,
should be adapted
to the volume of the receptacle which is to be drained off. The volume of the
gas when
being in the receptacle should preferably correspond to the volume of the
receptacle so
as to enable the receptacle to be completely drained off. This implies that
the volume of
the cleaned or sterilized gas in the pre-filled container is preferably
approximately equal to
or larger than the volume of the receptacle provided that the pressure of the
gas is
substantially the same in the receptacle as in the container. For most
medicine
receptacles the volume of the gas should be in the interval 1-100 cm3 at
atmospheric
pressure.
The connector 2 is preferably provided with a piercing member, such as a
hollow needle
14 (as illustrated) for penetration of a closing (not illustrated) made of
rubber for instance,
which closing covers the opening of a receptacle 6, such as a vial. In
addition to injection
needles or cannulae, the expression "needle" is meant to comprise spikes and
similar
components for penetration of such a closing in order to create a channel
between the
container 3 and the receptacle 6 to which the connector 2 is connected. By a
channel or
passage 15 inside the needle 14, gas contained in the container 3 can be
transferred from
the container to the receptacle 6, i.e. gas can flow from the container 3 to
the receptacle
6.
The connector 2 and the container 3 form an integrated unit 4. This implies
that the
connector and the container are made in one piece or the connector 2 and the
container 3
can be coupled to each other so as to form an integral unit 4. Different types
of coupling
CA 02707342 2009-12-11
WO 2008/153460 PCT/SE2007/050416
8
means known from prior art can be used as long as an airtight, or at least a
substantially
airtight connection can be obtained between the current components 2, 3.
The volume of the container 3 can be variable so as to allow the gas to flow
from the
container 3 to a receptacle 6. The container 3 is suitably made of a
compressible material
to make the volume of the container variable. To obtain a container 3 having a
variable
volume the container can comprise a first portion 17 made by a relatively
rigid material
which first portion 17 is coupled to the connector 2, and a second portion 18
made by a
relatively flexible material attached to the first portion 17. For example,
the container 3 can
be designed to have a flexible portion, such as a bellow which is compressible
and
extendable. According to an embodiment of the invention, the container, or the
flexible
portion of the container, comprises a displaceable spring-loaded element, such
as an
axial spring-loaded element, that is arranged to allow fluid to flow into the
container/
flexible portion of the container. The displaceable spring-loaded element is
for example
constrained between two flanged ends of the flexible portion. When the
flexible portion is
empty the spring(s) of the spring-loaded element is/are highly compressed. As
the flexible
portion is filled with fluid the spring(s) of the spring-loaded element
become(s) less
compressed. The spring(s) may be arranged on the inside or outside of the
flexible portion
or they may be integrally formed with the flexible portion. The displaceable
spring-loaded
element may be arranged to be disconnected from the container/flexible portion
of the
container once the container/flexible portion of the container has been filled
to the desired
amount.
According to an embodiment the flexible portion of the container may be
arranged to be
detachable from the remaining part of the container, whereby the flexible
portion may be
filled with fluid before and/or after it has been attached to the remaining
part of the
container. Hereby the volume of the container 3 can be increased and
decreased,
respectively. Although the device illustrated in figure 1 comprises a
compressible
container, in another embodiment the container can have a cylinder and a
piston arranged
therein so as to enable the volume of the container to be changed.
According to an embodiment of the invention the container comprises locking
means to
prevent fluid from flowing into the container, during the transportation of
the device, for
example, or at any other time when the device is not in use.
CA 02707342 2009-12-11
WO 2008/153460 PCT/SE2007/050416
9
Alternatively to a collapsible container, or in combination with a collapsible
container, the
container 3 can be pressurized by cleaned or sterilized gas to cause an
overpressure in
the container. An overpressure allows gas to flow from the container 3 to a
receptacle 6
connected to the connector and the container. In such a case the container 3
does not
necessarily need to be collapsible. The overpressure is suitably adapted to
the size of the
receptacle to which the connector is to be connected to ensure the receptacle
can be
completely drained off in a subsequent step. The pressure in the filled
container can be
for example in the interval from 1atm to 2atm. Preferably, the device
comprises any
means, such as a valve, for allowing the gas to flow from the container after
the device
has been connected to the receptacle and during conveyance of a substance out
of the
receptacle.
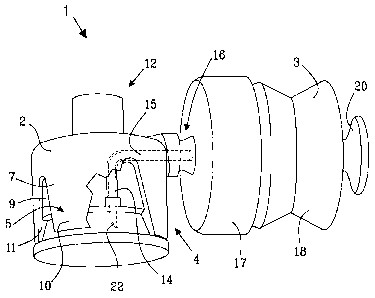
In figures 5, 5b and 6 a device 1' according to the second aspect of the
invention is
illustrated. The device can be used for providing cleaned gas to a receptacle
and thereby
facilitate conveyance of a substance out of the receptacle. Such a substance
can be
various solutions and liquids constituting drugs, for example cytotoxic drugs
or antibiotics,
for use in the field of medicine. The device comprises a connector 2' and a
container 3'
which form an integrated unit 4'. The connector 2' is provided with a first
means 5' for
connection to a receptacle 6' or in other words a first connector portion 5'.
See also figure
7 illustrating the device connected to a medicine bottle or vial 6', and the
exploded view in
figure 8.
The first connection means 5' can be designed for connection to a bottle, such
as the
neck of a vial. In the embodiment illustrated in figures 5-8, the first
connection means 5' is
constituted by a ring-shaped portion 7' for enclosing the neck 8' of the vial
6'. The ring-
shaped portion 7' has slits 9' so as to form flanges 10' which protrude
downwardly. The
flanges 10' can be provided with hooks 11' or barbs for gripping around the
neck 8' of the
vial 6'. The connector 2' is suitably provided with a second means 12' for
connection to a
transfer member 13', such as an injector device to be interconnected with the
connector,
for conveyance of a substance out of the receptacle 6'. In other words; the
connector 2' is
suitably provided with a second connector portion 12'.
In another embodiment (not shown) of the invention the second connection means
12'
can comprise a luer lock coupling or bayonet coupling to enable an injection
device to be
connected. As already described for the device according to the first aspect
of the
CA 02707342 2009-12-11
WO 2008/153460 PCT/SE2007/050416
invention, both the injector device and the connector are suitably provided
with a
membrane so as to create a double membrane coupling between the injector and
the
current device.
5 The connector 2' is preferably provided with a piercing member, such as a
hollow needle
14' (as illustrated) for penetration of a closing (not illustrated) made of
rubber for instance,
which closing covers the opening of a receptacle 6, such as a vial. In
addition to injection
needles or cannulae, the expression "needle" is meant to comprise spikes and
similar
components for penetration of such a closing in order to create a channel
between the
10 container 3' and the receptacle 6' to which the connector 2' is connected.
By a channel or
passage 15' in the needle 14', gas contained in the container 3' can be
transferred from
the container to the receptacle 6', i.e. gas can flow from the container 3' to
the receptacle
6'.
The connector 2' and the container 3' form an integrated unit 4'. This implies
that the
connector and the container are made in one piece or the connector 2' and the
container
3' can be coupled to each other so as to form an integral unit. Different
types of coupling
means 16' known from prior art can be used as long as an airtight or at least
a
substantially airtight connection can be obtained between the current
components 2', 3'.
The container 3' has to be filled with gas before connection of the connector
2' to a
receptacle 6'. The volume of the container 3' is preferably variable. To
obtain a container
3' having a variable volume the container can comprise a first portion 17'
made by a
relatively rigid material which first portion is coupled to the connector 2',
and a second
portion 18' made by a relatively flexible material attached to the first
portion 17'. The
second portion 18' can be extensible by manipulation of for example a handle
20'
arranged at the end of the container 3'. Hereby the volume of the container 3'
can be
increased and decreased, respectively. For example, the container 3' can be
designed to
have a flexible portion, such as a bellow which is compressible and extendable
by
affecting the container manually. The container 3' is preferably provided with
said handle
20' for regulating the volume of the container 3'. Although the volume of the
container is
preferably variable as illustrated, there may be other ways to fill the
container while at the
same time ensuring the gas passes a filter 21'. For example, the gas container
could be
constituted by a sealed vacuum-packed flexible bag whose seal can be broken to
allow
CA 02707342 2009-12-11
WO 2008/153460 PCT/SE2007/050416
11
gas to flow into the bag. Alternatively, the gas container is rigid or semi-
rigid and
pressurized gas is used to fill the container.
The amount of gas, preferably air, provided by the pre-filled container,
should be adapted
to the volume of the receptacle which is to be drained off. The volume of the
gas when
being in the receptacle should preferably correspond to the volume of the
receptacle so
as to enable the receptacle to be completely drained off. This implies that
the volume of
the cleaned or sterilized gas in the pre-filled container is preferably
approximately equal to
or larger than the volume of the receptacle provided that the pressure of the
gas is
substantially the same in the receptacle as in the container. For most
medicine bottles or
vials, the volume of the gas should be in the interval 1-100 cm3 at
atmospheric pressure.
Thus, the integrated unit 4' is provided with the filter 21', such as a
particulate air filter for
cleaning gas passing the filter 21' during filling the container 3' with gas,
preferably by
increasing the volume of the container 3', before connection of the connector
2' to a
receptacle 6'. Although the filter, hereinafter called particulate air filter
21' can be arranged
in different ways, according to the embodiment illustrated in figure 5 and 6
the particulate
air filter 21' is arranged on the connector 2'. By covering the opening of the
needle 14' by
means of the particulate air filter 21', it is ensured that the gas which is
brought into the
container 3' has to pass the particulate air filter 21'. The particulate air
filter 21' is
arranged to be removed from the integrated unit 4' after the container 3' has
been filled
with cleaned gas. Subsequently to filling the container 3' the particulate air
filter 21' is
removed and the connector 2' is to be connected to the receptacle 6'.
By the expression "cleaned" gas is meant that the gas has been filtered by a
filter to
remove particles and/or viable micro-organisms to such an extent that the gas
is classified
to be aseptic and accepted by the relevant authority and/or any standards. The
degree of
purity can be expressed in the largest particles allowed to pass the filter
for a given flow
rate. In some cases no or very few particles having a size exceeding 5pm are
allowed to
be present in the cleaned gas. However, the allowed particle size is
determined by the
requirements in the current application. Some drug treatments require that
substantially all
particles having a size exceeding 0.15pm are removed from the gas by the
particulate air
filter. As an example, a filter with the mesh size 0.2pm can be used to remove
substantially all particles and micro organisms of that size or larger.
CA 02707342 2009-12-11
WO 2008/153460 PCT/SE2007/050416
12
The particulate air filter 21' is preferably designed as a needle shield 22'
for the tip of
needle 14'. The filter can be arranged to at least partially cover or surround
the tip of the
needle 14'. This implies that the particulate air filter 21' cleans the gas
and at the same
time the particulate air filter 21' functions as a protection during handling
of the device 1'.
Furthermore, such a needle tip shield 22' protects the sterile package
enclosing the
device during transport and storage of the device.
By removing the particulate air filter 21', after the container 3' has been
filled with the gas
and prior to interconnection of the connector 2' and the receptacle 6' to each
other, any
contamination particles removed from the gas and collected in the particulate
air filter 21'
are removed from the integrated unit 4'. Thus, one and the same channel can be
used for
both filling the container 3' with cleaned gas and transferring the cleaned
gas from the
container 3' to a receptacle 6'.
In the embodiment illustrated in figure 5b where the particulate air filter
21' is not to be
removed before interconnection of the connector 2' and the receptacle 6' to
each other,
the particulate air filter 21' has to be arranged so as to avoid contamination
during
transportation of the gas from the container 3' to the receptacle 6'. The
integrated unit 4'
can be provided with a first channel 23' for filling the container 3' with
cleaned gas and a
second channel 15' for transferring the cleaned gas to a receptacle.
Otherwise, i.e. if the
particulate air filter is to be left, and one and the same channel is used for
transportation
of gas in both directions; the particles collected in the particulate air
filter could possibly
release from the particulate air filter and be unintentionally brought into
the receptacle 6'
by the gas flow. Such a contamination can be prevented by providing a
removable air filter
or by providing different openings/channels for transportation of gas into and
out of the
container 3', respectively.
A lid 26' can be arranged on the integrated unit 4' for covering the
particulate air filter 21'
so as to prevent further communication between the interior of the integrated
unit 4' and
the environment via the particulate air filter 21' after filling the container
3'. Firstly, the
container 3' is filled with the cleaned gas and thereafter the lid 26' is
mounted on the
integrated unit 4' to cover the particulate air filter 21' and prevent further
gas
transportation through the air particle filter 21'. Thereafter, the integrated
unit 4' and the
receptacle 6' are to be interconnected and the subsequent manipulations can be
safely
executed.
CA 02707342 2009-12-11
WO 2008/153460 PCT/SE2007/050416
13
The lid 26' has the function of preventing transportation of liquid, gas or
any vapour in the
direction from the integrated unit 4' to the environment so as to counteract
that any
undesired substance in the receptacle 6' escapes to the environment.
It is to be understood that the present invention is not limited to the
embodiments
described above and illustrated in the drawings; rather, the skilled person
will recognize
that many changes and modifications may be made within the scope of the
appended
claims. For example, the invention can be applied to other medical
applications and there
may be additional purposes for providing cleaned or sterilized gas to a
receptacle.