Note: Descriptions are shown in the official language in which they were submitted.
CA 02708395 2010-06-07
WO 2009/082425 PCT/US2008/013108
-1-
ELECTROCHEMICAL TREATMENT OF HEAVY OIL STREAMS
FOLLOWED BY CAUSTIC EXTRACTION OR THERMAL TREATMENT
FIELD OF THE INVENTION
[0001] This invention relates to the electrochemical conversion of
dibenzothiophene type molecules of petroleum feedstreams to mercaptans that
can then be removed, in one embodiment, by caustic extraction. In another
embodiment, the mercaptans can be thermally decomposed, removing sulfur as
hydrogen sulfide. The conversion of dibenzothiophenes to mercaptans is
performed by electrochemical means without the required addition of hydrogen
and in the substantial absence of water.
BACKGROUND OF THE INVENTION
[0002] The sulfur content of petroleum products is continuing to be regulated
to lower and lower levels throughout the world. Sulfur specifications in motor
gasoline ("mogas") and on-road diesel have been most recently reduced and
future specifications will further lower the allowable sulfur content of off-
road
diesel and heating oils. Sulfur is currently removed from petroleum
feedstreams
by various processes depending on the nature of the feedstream. Processes such
as coking, distillation, and alkali metal dispersions are primarily used to
remove
sulfur from heavy feedstreams, such as bitumens which are complex mixtures
and typically contain hydrocarbons, heteroatoms, and metals, with carbon
chains
in excess of about 2,000 carbon atoms. For lighter petroleum feedstreams, such
as distillates, catalytic hydrodesulfurization is typically used. The sulfur
species
in such feedstreams span a range of molecular types including sulfides,
thiols,
thiophenes, benzothiophenes to dibenzothiophenes in order of decreasing
hydrodesulfurization (HDS) reactivity. The most difficult to remove sulfur is
CA 02708395 2010-06-07
WO 2009/082425 PCT/US2008/013108
-2-
found in sterically hindered dibenzothiophene molecules such as diethyl
dibenzothiophene. The space velocity, temperature and hydrogen pressures of
catalytic HDS units are determined primarily by the slow reaction kinetics of
these relatively minor components of the feed. These are the molecules that
are
typically left in the product after conventional low-pressure hydrotreating.
Further removing these molecules often requires higher hydrogen pressure and
higher temperature ("deep desulfurization") which leads to higher hydrogen
consumption and shorter catalyst run lengths which are costly results.
Therefore, it is desirable to have alternative processes that are capable of
removing these refractory sulfur molecules without incurring more severe
reaction conditions for catalytic hydrotreating, which can result in
significant
capital and energy savings.
SUMMARY OF THE INVENTION
[00031 In accordance with a preferred embodiment of the present invention
there is provided a process for removing sulfur from petroleum feedstreams
containing sulfur in the form of hindered dibenzothiophene compounds,
comprising:
a) passing a sulfur-containing petroleum feedstream to an
electrochemical cell;
b) subjecting said feedstream to an effective voltage and current that will
result in the conversion of at least a portion of said hindered
dibenzothiophene
compounds to mercaptan compounds;
c) passing the electrochemically treated petroleum feedstream containing
said mercaptans compounds to a caustic treatment zone wherein it is contacted
with an aqueous caustic solution wherein mercaptan-containing compounds are
extracted by the aqueous caustic solution; and
CA 02708395 2010-06-07
WO 2009/082425 PCT/US2008/013108
-3-
d) collecting a reduced-sulfur petroleum product stream from the caustic
treatment zone;
wherein the reduced-sulfur petroleum product stream has a lower sulfur
content by wt% than the sulfur-containing petroleum feedstream.
[0004] In a preferred embodiment, the sulfur-containing petroleum
feedstream is comprised of a bitumen.
[0005] In another preferred embodiment, the feedstream is a distillate boiling
range hydrocarbon stream and an effective amount of an electrolyte is mixed
with the distillate boiling range stream to be treated.
[0006] Also in accordance with another preferred embodiment of the present
invention is a process for removing sulfur from petroleum feedstreams
containing sulfur in the form of hindered dibenzothiophene compounds,
comprising:
a) passing a sulfur-containing petroleum feedstream to an
electrochemical cell;
b) subjecting said feedstream to an effective voltage and current that will
result in the conversion of at least a portion of said hindered
dibenzothiophene
compounds to mercaptan compounds;
c) passing the electrochemically treated petroleum feedstream containing
mercaptan compounds to a thermal decomposition zone wherein at least a
portion of the mercaptans are decomposed to hydrogen sulfide at temperatures
from about 302 F to about 932 F (150 C to 500 C); and
d) collecting a reduced-sulfur petroleum product stream from the thermal
decomposition zone;
wherein the reduced-sulfur petroleum product stream has a lower sulfur
content by wt% than the sulfur-containing petroleum feedstream.
CA 02708395 2010-06-07
WO 2009/082425 PCT/US2008/013108
-4-
[0007] In a preferred embodiment, the sulfur-containing petroleum
feedstream is comprised of a bitumen.
[0008] In another preferred embodiment, the feedstream is a distillate boiling
range stream and an effective amount of an electrolyte is mixed with the
distillate boiling range stream to be treated.
BRIEF DESCRIPTION OF THE FIGURES
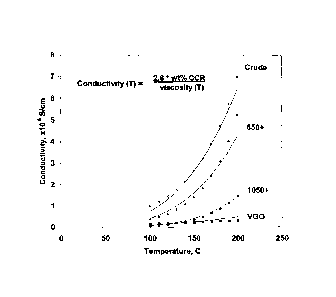
[0009] Figure 1 hereof is a plot of conductivity versus temperature for
various petroleum residues and crudes.
DETAILED DESCRIPTION OF THE INVENTION
[0010] Feedstreams suitable for use in the present invention range from
heavy oil feedstreams, such as bitumens to those boiling in the distillate
range.
In a preferred embodiment the heavy oil feedstream contains at least about 10
wt.%, preferably at least about 25 wt.% of material boiling above about 1050 F
(565 C), both at atmospheric pressure (0 psig). Such streams include bitumens,
heavy oils, whole or topped crude oils and residua. The bitumen can be whole,
topped or froth-treated bitumen. Non-limiting examples of distillate boiling
range streams that are suitable for use herein include diesel fuels, jet
fuels,
heating oils, kerosenes, and lubes. Such streams typically have a boiling
range
from about 302 F (150 C) to about 1112 F (600 C), preferably from about
662 F (350 C) to about 1022 F (550 C). Other preferred streams are those
typically known as Low Sulfur Automotive Diesel Oil ("LSADO"). LSADO
will typically have a boiling range of about 350 F (176 C) to about 550 F
(287 C) and contain from about 200 wppm sulfur to about 2 wppm sulfur,
CA 02708395 2010-06-07
WO 2009/082425 PCT/US2008/013108
-5-
preferably from about 100 wppm sulfur to about 10 wppm sulfur. The process
embodiments of the present invention electrochemically treat a sulfur-
containing
petroleum feedstream resulting in a reduced-sulfur petroleum product stream
which has a lower sulfur concentration by wt% than the sulfur-containing
petroleum feedstream.
[0011] A major of the sulfur contained in heavy oils and distillates are in
the
form of hindered dibenzothiophene molecules. Although such molecules are
difficult to remove by conventional hydrodesulfurization processes without
using severe conditions, such as high temperatures and pressures, such
molecules are converted by the practice of the present invention to sulfur
species
that are more easily removed by conventional non-catalytic processes. For
example, the electrochemical step of the present invention converts the
hindered
dibenzothiophene ("DBT") molecules, which are substantially refractory to
conventional hydrodesulfurization, to hydrogenated naphthenobenzothiophene
mercaptan molecules that are more readily extracted with use of caustic
solution
or by thermal decomposition. This capability can significantly debottleneck
existing distillate hydrotreating process units by converting the slowest to
convert molecules (hindered dibenzothiophenes) into much more readily
extractable mercaptan species, preferably alkylated biphenyl mercaptan
species.
[0012] The process of the present invention does not require the addition of
an electrolyte when heavy oil is the feedstream, but rather, relies on the
intrinsic
conductivity of the heavy oil at elevated temperatures. It will be understood
that
the term "heavy oil" and "heavy oil feedstream" as used herein includes both
bitumen and other heavy oil feedstreams, such as crude oils, atmospheric
resids,
and vacuum resids. This process is preferably utilized to upgrade bitumens
and/or crude oils that have an API gravity of less than about 15. The
inventors
hereof have undertaken studies to determine the electrochemical conductivity
of
CA 02708395 2010-06-07
WO 2009/082425 PCT/US2008/013108
-6-
crudes and residues at temperatures up to about 572 F (300 C) and have
demonstrated an exponential increase in electrical conductivity with
temperature
as illustrated in Figure 1 hereof. It is believed that the electrical
conductivity in
crudes and residues is primarily carried by electron-hopping in the 7t-
orbitals of
aromatic and heterocyclic molecules. Experimental support for this is
illustrated
by the simple equation, shown in Figure 1 hereof, that can be used to
calculate
the conductivity of various cuts of a crude using only its temperature
dependent
viscosity and its Conradson carbon content. The molecules that contribute to
Concarbon are primarily the large multi-ring aromatic and heterocyclic
components.
[0013] A 4 mA/cm2 electrical current density at 662 F (350 C) with an
applied voltage of 150 volts and a cathode-to-anode gap of 1 mm was measured
for an American crude oil. Though this is lower than would be utilized in
preferred commercial embodiments of the present invention, the linear velocity
for this measurement was lower than the preferred velocity ranges by about
three
orders of magnitude: 0.1 cm/s vs. 100 cm/s. Using a 0.8 exponent for the
impact
of increased flow velocity on current density at an electrode, it is estimated
that
the current density would increase to about 159 mA/cm2 at a linear velocity of
about 100 cm/s. This suggests that more commercially attractive current
densities achieved at higher applied voltages. Narrower gap electrode designs
or
fluidized bed electrode systems could also be used to lower the required
applied
voltage.
[0014] Unlike bitumen, performing controlled potential electrolysis on a
non-conductive fluid such as a LSADO, or other petroleum distillate streams,
requires the introduction of an effective amount of an electrolyte, such as a
conductive salt. There is an insufficient concentration of large multi-ring
aromatic and heterocyclic molecules in distillate boiling range feedstreams to
CA 02708395 2010-06-07
WO 2009/082425 PCT/US2008/013108
-7-
produce sufficient intrinsic conductivity without the use of an electrolyte.
The
direct addition of a conductive salt to the distillate feedstream can be
difficult for
several reasons. The term "effective amount of electrolyte" as used herein
means at least than amount needed to produce conductivity between the anode
and the cathode of the electrochemical cell. Typically this amount will be
from
about 0.5 wt.% to about 50 wt.%, preferably from about 0.5 wt.% to about 10
wt.%, of added electrolytic material based on the total weight of the feed
plus the
electrolyte. Once dissolved in the oil, most salts are difficult to remove
after
electrolysis. Incomplete salt removal is unacceptable due to product
specifications, negative impact on further catalytic processing, potential
corrosivity and equipment fouling. Even salts that are soluble in a low
dielectric
medium are often poorly ionized and therefore unacceptable high concentrations
are required to achieve suitable conductivities. In addition, such salts are
typically very expensive. However, recent advances in the field of ionic
liquids
have resulted in new organic soluble salts having melting points lower than
about 212 F (100 C) that can be used in the present invention. They can be
recovered by solvent washing the petroleum stream after electrolysis. Non-
limiting examples of such salts include: 1-butyl-l-methylpyrrolidinium
tris(pentafluoroethyl)trifluoro phosphate, 1-butyl-1-methyl pyrrolidinium
trifluoro-methyl sulfonated, trihexyltetradecylphosphonium
tris(pentafluoroethyl) trifluorophosphate and ethyl-dimethylpropyl-ammonium
bis(trifluoro-methylsulfonyl) imide.
[00151 An alternate solution to the low conductivity problem of distillate
boiling range feedstreams to produce a two phase system. Rather than adding an
electrolyte to the feedstream, the feedstream can be dispersed in a
conductive,
immiscible, non-aqueous electrolyte. Such a two-phase system of oil dispersed
in a continuous conductive phase provides a suitable electrolysis medium. The
continuous conductive phase provides the sufficient conductivity between the
CA 02708395 2010-06-07
WO 2009/082425 PCT/US2008/013108
-8-
cathode and anode of an electrochemical cell to maintain a constant electrode
potential. Turbulent flow through the electrochemical cell brings droplets of
the
feedstream in contact with the cathode, at which point electrons are
transferred
from the electrode to sulfur containing species on the droplet surface.
[00161 After reaction, the immiscible electrolyte from the treated feedstream
is separated by any suitable conventional means resulting in a reduced sulfur
product stream. The immiscible electrolyte can be recycled. The electrolyte in
the immiscible electrolysis medium is preferably an electrolyte that
dissolves, or
dissociates, in the solvent to produce electrically conducting ions, but that
does
not undergo a redox reaction in the range of the applied potentials used.
Suitable
organic electrolytes for use in the present invention, other than those
previously
mentioned, include quaternary carbyl- and hydrocarbyl-onium salts, e.g.,
alkylammonium hydroxides. Non-limiting examples of inorganic electrolytes
include, e.g., NaOH, KOH and sodium phosphates, and mixtures thereof. Non-
limiting examples of onium ions that can be used in the practice of the
present
invention include mono- and bis-phosphonium, sulfonium and ammonium,
preferably ammonium. Preferred carbyl and hydrocarbyl moieties are alkyl
carbyl and hydrocarbyl moieties. Suitable quaternary alkyl ammonium ions
include tetrabuytyl ammonium, and tetrabutyl ammonium toluene sulfonate.
Optionally, additives known in the art to enhance performance of the
electrodes
can also be used. Non-limiting examples of such additives suitable for use
herein include surfactants, detergents, emulsifying agents and anodic
depolarizing agents. Basic electrolytes are most preferred. The concentration
of
salt in the electrolysis medium should be sufficient to generate an
electrically
conducting solution in the presence of the feedstream. Typically, a
concentration of about 1 to about 50 wt% conductive phase, preferably about 5
to about 25 wt% based on the overall weight of the oil/water/electrolyte
mixture
CA 02708395 2010-06-07
WO 2009/082425 PCT/US2008/013108
-9-
is suitable. It is preferred that petroleum stream immiscible solvents be
chosen,
such as dimethyl sulfoxide, dimethylformamide or acetonitrile.
[0017] Dispersions are preferred for ease of separation following
electrolysis. However, more stable oil-in-solvent emulsions can also be used.
Following electrolytic treatment, the resulting substantially stable emulsion
can
be broken by the addition of heat and/or a de-emulsifying agent.
[0018] The electrochemical cell used in the practice of the present invention
may be divided or undivided. Such systems include stirred batch or flow
through reactors. The foregoing may be purchased commercially or made using
technology known in the art. Suitable electrodes known in the art may be used.
Included as suitable electrodes are three-dimensional electrodes, such as
carbon
or metallic foams. The optimal electrode design would depend upon normal
electrochemical engineering considerations and could include divided and
undivided plate and frame cells, bipolar stacks, fluidized bed electrodes and
porous three dimensional electrode designs; see Electrode Processes and
Electrochemical Engineering by Fumio Hine (Plenum Press, New York 1985).
While direct current is typically used, electrode performance may be enhanced
using alternating current or other voltage/current waveforms. The gap between
electrode surfaces will preferably be about 1 to about 50 mm, more preferably
from about 1 to about 25 mm, and the linear velocity in the electrochemical
cell
will be in the range of about 1 to about 500 cm/s, more preferably in the
range of
about 50 to about 200 cm/s.
[0019] The applied cell voltage, that is, the total voltage difference between
the cathode and anode will vary depending upon the cell design and
electrolytes
used. What is critical, however, is that the cathode be polarized sufficiently
to
achieve electron transfer to the. dibenzothiophene molecules, which occurs at
CA 02708395 2010-06-07
WO 2009/082425 PCT/US2008/013108
-10-
reduction potentials more negative than -2.3 Volts versus a standard calomel
electrode. Normal electrochemical practices can be used to ensure that the
cell is
operated under these conditions. In preferred embodiments, the voltage across
the electrochemical cell will be about 4 to about 500 volts, preferably from
about
100 to about 200 volts, with a resulting current density of about 10 mA/cm2 to
about 1000 mA/cm2, preferably from about 100 mA/cm2 to about 500 mA/cm2.
[00201 At least a portion of the hindered dibenzothiophene compounds in the
feedstream are converted to the corresponding alkylated biphenyl mercaptan
compounds in the electrochemical cell. The mercaptans containing treated
feedstream is passed to a caustic wash step wherein it is contacted with an
aqueous caustic solution for extraction of the mercaptan species. Any suitable
caustic wash technology can be used in the practice of the present invention.
The most preferred caustic wash would be an aqueous solution of sodium
hydroxide having a strength from about 0.5 M to about 5 M and mixing the
mercaptan-containing stream with air and the caustic solution to remove the
mercaptan species in the caustic solution. Non-limiting examples of caustic
extraction processes that can be used in the practice of the present invention
include the UOP MEROX process and the Merichem THIOLEX and
EXOMER processes. The MEROX Process was announced to the industry in
1959. The Oil & Gas J. 57(44), 73-8 (1959) contains a discussion of the
MEROX Process. In the MEROX oxidation process, mercaptan compounds
are extracted from the feed and then oxidized by air in the caustic phase in
the
presence of the MEROX catalyst, which is typically an iron group chelate
(cobalt phthalocyanine) to form disulfides which are then redissolved in the
hydrocarbon phase, leaving the process as disulfides in the hydrocarbon
product.
The disulfides, which are not soluble in the caustic solution, can be
separated
and recycled for mercaptan extraction. The treated stream is usually sent to a
water wash in order to reduce the sodium content.
CA 02708395 2010-06-07
WO 2009/082425 PCT/US2008/013108
-11-
[0021] All of these processes take advantage of the acidity of the mercaptan
species. By contacting a petroleum stream that contains acidic mercaptan
species with an aqueous base solution, the mercaptans are de-protonated,
converted to salts and are now more soluble in the aqueous stream and thus can
be extracted nearly quantitatively from the petroleum stream. Such an
extraction
is ineffective with the original, non-acidic dibenzothiophenic sulfur species.
The
desulfurized petroleum stream is then separated from the resulting mercaptide
containing caustic solution. The caustic solution can then be regenerated and
the
mercaptides isolated in a variety of conventional ways depending on the
process
design. Such mercaptan extractions are widely used in the petroleum refining
industry and it is likely that every refinery has at least one such unit. The
extracted mercaptans can be readily oxidized to disulfides, separated from the
caustic stream, and recycled for more mercaptan extraction. The hindered
dibenzothiophene ("DBT") species which are removed from the feedstream are
converted to a relatively small substantially pure stream of disulfides that
can be
disposed of via combustion. They can also be fed to a coking unit for thermal
decomposition. Being able to target hindered DBT molecules can also enable
the disposition of Light Catalytic Cycle Oil ("LCCO"), which is rich in DBTs,
to
distillate hydrotreaters.
[0022] In a second embodiment of the present invention, following, or
simultaneous with the electrochemical conversion of the dibenzothiophenic
species to mercaptans, a thermal decomposition reaction of the mercaptans is
performed to decompose them with loss of hydrogen sulfide from the mercaptan
molecule. This thermal decomposition can be performed at temperatures from
about 302 F to about 932 F (150 C to 500 C), preferably from about 482 F to
about 932 F (250 C to 500 C) and at ambient to autogenous pressure.
Subsequent removal of this hydrogen sulfide from the petroleum stream will
CA 02708395 2010-06-07
WO 2009/082425 PCT/US2008/013108
-12-
produce a reduced sulfur product stream that is lower is sulfur content by wt%
than the sulfur-containing petroleum feedstream treated by the current
process.
[0023] The present invention will be better understood with reference to the
following examples which are presented for illustrative purposes and are not
to
be taken as limiting the invention in anyway.
[0024] The following three examples were performed using a 300-cc
autoclave (Parr Instruments, Moline, IL) was modified to allow two insulating
glands (Conax, Buffalo, NY) to feed through the autoclave head. Two
cylindrical stainless steel (316) mesh electrodes are connected to the Conax
glands, where the power supply (GW Laboratory DC Power Supply, Model
GPR-1810HD) is connected to the other end. The autoclave body is fitted with a
glass insert, a thermal-couple and a stirring rod. The autoclave can be
charged
with desired gas under pressure and run either in a batch- or a flow-through
mode.
Example 1 - Electrochemical treatment of DBT under N, in dimethyl sulfoxide
solvent with tetrabutylammonium hexaflouorphosphate electrolyte.
[0025] To the glass insert was added 1.0 g dibenzothiophene ("DBT"), 3.87
g tetrabutylammonium hexafluorophosphate (TBAPF6), and 100 milliliter ("ml")
anhydrous dimethyl sulfoxide (DMSQ, Aldrich). After the content was
dissolved, the glass insert was loaded into the autoclave body, the autoclave
head
assembled and pressure tested. The autoclave was charged with 70 psig of N2
and heated to 212 F (100 C) with stirring (300 rpm). A voltage of 5 Volts was
applied and the current was 0.8 Amp. The current gradually decreased with time
and after two hours, the run was stopped. The autoclave was opened and the
content acidified with 10% HCl (50 ml). The acidified solution was then
diluted
with 100 ml of de-ionized ("DI") water, extracted with ether (50 ml x 3). The
CA 02708395 2010-06-07
WO 2009/082425 PCT/US2008/013108
- 13-
ether layer was separated and dried over anhydrous Na2SO4, and ether was
allowed to evaporate under a stream of N2. The isolated dry products were
analyzed by GC-MS. A conversion of 12% was found for DBT and the products
are as the following.
1.0 g DBT/0.I M TBAPF6 in Me2SO - - MeR'I/ - -
70 psi N2, 100 C, 5V, 0.8A, 2hr \ / \
S SH SH Me S
12% cony. 35% 57% 8% [11
[00261 This example shows that the electrochemical reduction of DBT under
N2 resulted in: 12% DBT conversion after 2 h at 212 F. GC-MS revealed that
the products consisted of 35% 2-phenyl benzenethiol, 8% tetrahydro-DBT, and-,
57% of a species with a mass of 214. The assignment of this peak as 2-phenyl
benzenethiol was done by comparing with an authentic sample. The mass 214
species was tentatively assigned as 2-phenyl benzenethiol with two methyl
groups added. Addition of methyl groups to DBT indicates that decomposition
of solvent DMSO occurred since it is the only source of methyl groups in this
system. No desulfurization product biphenyl was observed in this run.
Comparitive Example A - Electrochemical treatment of DBT under Hydrogen in
dimethyl sulfoxide solvent with tetrabutylammonium hexaflouorphosphate
electrolyte.
[00271 To the glass insert was added 0.5 g dibenzothiophene ("DBT"), 3.87
g tetrabutylammonium hexafluorophosphate (TBAPF6), and 100 ml anhydrous
dimethyl sulfoxide (DMSO, Aldrich). After the content was dissolved, the glass
insert was loaded into the autoclave body, the autoclave head assembled and
pressure tested. The autoclave was charged with 300 psig of H2 and heated to
257 F (125 C) with stirring (300 rpm). A voltage of 4.5 Volts was applied and
the current was 1.0 Amp. The current gradually decreased with time and after
CA 02708395 2010-06-07
WO 2009/082425 PCT/US2008/013108
- 14-
three and half (3.5) hours, the run was stopped. The autoclave was opened and
the content acidified with 10% HCl (50 ml). The acidified solution was then
diluted with 100 ml of DI water, extracted with ether (50 ml x 3). The ether
layer was separated and dried over anhydrous Na2SO4, and ether was allowed to
evaporate under a stream of N2. The isolated dry products were analyzed by
GC-MS. A conversion of 16.5% was found for DBT and the products are as the
following.
0.5 g DBT/0.1 M TBAPF6 in Me2SO q-P
Q-SP 300 psi Hz, 125 C, 4.5V, LOA, 3.5hr H,Me,
SH S
16.5%conv. 64% trace 36% [2]
Comparative Example B - Electrochemical treatment of DEDBT under
Hydrogen in dimethyl sulfoxide solvent with tetrabutylammonium
hexaflouorphosphate electrol tie.
[00281 To the glass insert was added 1.0 g 4,6-diethyl dibenzothiophene
("DEDBT"), 3.87 g tetrabutylammonium hexafluorophosphate (TBAPF6), and
100 ml anhydrous dimethyl sulfoxide (DMSO, Aldrich). After the content is
dissolved, the glass insert was loaded into the autoclave body, the autoclave
head
assembled and pressure tested. The autoclave was charged with 200 psig of H2
and heated to 100 C with stirring (300 rpm). A voltage of 7 Volts was applied
and the current was 1.0 Amp. The current gradually decreased with time and
after two and half (2.5) hours, the run was stopped. The autoclave was opened
and the content acidified with 10% HCl (50 ml). The acidified solution was
then
diluted with 100 ml of DI water, extracted with ether (50 ml x 3). The ether
layer was separated and dried over anhydrous Na2SO4, and ether was allowed to
evaporate under a stream of N2. The isolated dry products were analyzed by
CA 02708395 2010-06-07
WO 2009/082425 PCT/US2008/013108
- 15-
GC-MS. A conversion of 16% was found for DEDBT and the products are as
the following.
1.0 g DEDBT/0.1 M TBAPF6 in Me2SO - - - /Me - - -
\
200 psi H2, 100 C, 7V, 1OA, 2.5hr
S SH S
l-Y _J
16%conv. 53% 46% trace [3]
[00291 Similarly, desulfurization was also observed for sterically hindered
Diethyl Dibenzothiophene (DEDBT) under H2. A conversion of 16% of the
DEDBT was observed and the products contained 53% desulfurized compounds,
46% dihydro-DEDBT and a trace amount of tetrahydro-DEDBT. Solvent
decomposition also occurs in this case. Although electrochemical
desulfurization of DBT and hindered DBT has been achieved under H2 in the
212 F to 257 F (100 C to 125 C) temperature range, the conversion is still
quite
low. Increased conversions were attempted by extending the run time by
operating within this temperature range or by running at higher temperature of
about 392 F (200 C) to about 482 F (250 C).
[00301 The first example illustrates that DBT's can be readily converted into
alkylated biphenyl mercaptans electrochemically without the addition of
hydrogen or water. The mercaptans can be removed by caustic extraction. For
example, standard MEROX caustic treatment could be used to remove these
molecules from the electro-treated LSADO producing ultra-low sulfur distillate
without the need for additional hydrotreatment. Due to the low concentration
of
these molecules in the LSADO, the power consumption should be minimal. The
comparative examples demonstrate that, electrochemical reduction in the
presence of hydrogen leads to production of hydrogenated naphtheno
dibenzothiophenes and not biphenyl mercaptans. These species are not caustic
extractable. By limiting the availability of hydrogen sources by eliminating
the
CA 02708395 2010-06-07
WO 2009/082425 PCT/US2008/013108
-16-
hydrogen or water content, the products of the electrolysis can be controlled.
The chemistry of conversion to biphenyl mercaptans and subsequent extraction
processes are as follows:
e-
H Ultra low sulfur stream
Ri S Rz 2 R SH RZ
Hindered DBTs in
petroleum streams
Sulfur species removed
by caustic extraction [4]
Example 2 - Thermal Decomposition of 2-Phenylthiophenol in tetralin at 400 C
[00311 A volume of 1.5 ml of a tetralin solution containing 0.1 M of 2-
phenylthiopheol was placed into 3 ml stainless-steel mini-bomb inside a dry-
box. The mini-bomb was heated at 400 C in an oven for a certain period of
time and the content analyzed by GC/MS. Results in Table 1 below indicate
desulfurization of 2-phenylthiophenol, giving biphenyl as the major product.
Example 3 - Thermal Decomposition of 2-Phenylthiophenol in tetralin at 375 C
[00321 A volume of 1.5 ml of a tetralin solution containing 0.1 M of 2-
phenylthiopheol was placed into 3 ml stainless-steel mini-bomb inside a dry-
box. The mini-bomb was heated at 375 C in an oven for a certain period of time
and the content analyzed by GC/MS. Results in Table 1 below indicate
desulfurization of 2-phenylthiophenol, giving biphenyl as the major product.
Example 4 - Thermal Decomposition of 2-Phenylthiophenol in tetralin at 350 C
CA 02708395 2010-06-07
WO 2009/082425 PCT/US2008/013108
-17-
[0033] A volume of 1.5 ml of a tetralin solution containing 0.1 M of 2-
phenylthiopheol was placed into 3 ml stainless-steel mini-bomb inside a dry-
box. The mini-bomb was heated at 350 C in an oven for a certain period of time
and the content analyzed by GC/MS. Results in Table 1 below indicate
desulfurization of 2-phenylthiophenol, giving biphenyl as the major product.
Based on the thermal decomposition rates at various temperatures, the
activation
energy for 2-phenylthiophenol thermal decomposition was determined to be
29.2 kcal/mol.
Example 5 - Thermal Decomposition of phenyl disulfide in tetralin at 300 C
[0034] A volume of 1.5 ml of a tetralin solution containing 0.1 M of phenyl
disulfide (PhS-SPh) was placed into 3 ml stainless-steel mini-bomb inside a
dry-
box. The mini-bomb was heated at 572 F (300 C) in an oven for 4h and the
content analyzed by GC/MS. All disulfide is converted into thiophenol. By
analogy, biphenyl disulfide (Ph-Ph-S-S-Ph-Ph) can be converted into 2-
phenylthiophenol, which can be desulfurized at higher temperature as shown in
Examples 2 through 4 herein. Equation 5 illustrates the thermal conversion of
2-
phenylthiophenol to biphenyl and hydrogen sulfide.
tetralin + H2S
ftKI1I O > 3000 [5]
SH
CA 02708395 2010-06-07
WO 2009/082425 PCT/US2008/013108
- 18-
Table 1
Thermal Decomposition of 2-Phenylthiophenol (0.1 M) in Tetralin
Temp. Time `~ \ /
( C) (h) RS -00-0 \ / \ /
S
H S S
400 0 100% 0 0 0
2 22.1% 60.4% 4% 12.5%
4 29.3% 53% 4.7% 12%
375 1 83.6% 11.9% 1.3% 3.1%
3 59.7% 31% 3.8% 5.4%
350 1 95.1% 3.6% 1.3%
4 72.6% 17.4% 5.7% 4.3%
[00351 As Examples 2 through 5 clearly demonstrate, the biphenyl
mercaptan can be desulfurized by thermal treatment. This reaction could occur
simultaneously with electrochemical processing if conducted at sufficiently
elevated temperatures or may require a separate thermal soak step.