Note: Descriptions are shown in the official language in which they were submitted.
CA 02709558 2010-06-16
- 1 -
(
SPECIFICATION
PROCESS FOR PRODUCTION OF RADIOACTIVE-FLUORINE-LABELED
ORGANIC COMPOUND
TECHNICAL FIELD
[0001]
The present invention relates to a process for
producing a radioactive fluorine-labeled compound which
can be suitably used for positron emission tomography and
single photon emission computed tomography.
BACKGROUND ART
[0002]
Nuclear medicine examination represented by positron
emission tomography (hereinafter referred to as PET) and
single photon emission computed tomography (hereinafter
referred to as SPECT), is effective in diagnosing a
variety of diseases including heart disease and cancer.
These techniques involve administering an agent labeled
with a specific radioisotope (hereinafter referred to as
radiopharmaceutical), followed by detecting y-rays
emitted directly or indirectly from the agent. Nuclear
medicine examination is characteristic in that it has not
only such superior performances as high specificity and
sensitivity to diseases, but also an advantage of
providing information on the functionality of lesions,
compared to other examination techniques.
For example, ['8F]2-fluoro-2-deoxy-D-glucose
CA 02709558 2010-06-16
-2-
"18
(hereinafter referred to as F-FDG"), one of
radiophatmaceuticals used for PET examination, tends to
be concentrated in area where glucose metabolism is
enhanced, thereby making it possible to specifically
detect tumors in which glucose metabolism is enhanced.
[0003]
Nuclear medicine examination is performed by tracing
a distribution of an administered radiopharmaceutical,
and data obtained therefrom vary depending on nature of
the radiopharmaceutical. Thus, different
radiopharmaceuticals have been developed for different
diseases, and some of them are put into clinical use.
There have been developed, for example, various tumor
diagnostic agents, bloodstream diagnostic agents and
receptor mapping agents.
[0004]
In recent years, a series of radioactive halogen-
labeled amino acid compounds including [la-r]
l-amino-3-
fluorocyclobutanecarboxylic acid (hereinafter referred to
as [18F]FACBC) have been designed as novel
radiopharmaceuticals, and their clinical application is
under examination (Patent Document 1, and non-Patent
Documents 1 and 2). [18F]FACBC is considered to be
effective as a diagnostic agent for highly proliferative
tumors, because it has a property of being taken up
specifically by amino acid transporter.
[0005]
As processes for producing [ 18F]FACBC, there are
CA 02709558 2010-06-16
- 3 -
,
disclosed processes which include: providing 1-(N-(t-
butoxycarbonyl)amino)-3-[((trifluoromethyl)-
snlfonyl)oxy]-cyclobutane-1-carboxylic acid ester as a
labeling precursor, substituting the triflate group at
position 3 of the precursor with radioactive fluorine,
and carrying out deprotection by subjecting the resulting
compound to an acidic condition (Patent Document 1, and
non-Patent Documents 1 and 2).
[0006]
Patent Document 1: Japanese Patent Laid-open No.
2000-500442.
Non-Patent Document 1: Jonathan McConathy et al.,
"Improved synthesis of anti-[18F]FACBC: improved
preparation of labeling precursor and automated
radiosynthesis.", Applied Radiation and Isotopes,
(Netherlands), 2003, 58, p.657-666.
Non-Patent Document 2: Timothy M. Shoup et al.,
"Synthesis and Evaluation of [18F]l-Amino-3-
fluorocyclobutane-1-carboxylic Acid to Image Brain
Tumors.", The Journal of Nuclear Medicine, 1999, 40,
p.331-338.
DISCLOSURE OF THE INVENTION
PROBLEMS TO BE SOLVED BY THE INVENTION
[0007]
However, production yield has been 12-24% in the
process for producing [ F]-FACBC disclosed until now (J.
McConathy et al., Applied Radiation and Isotopes, 2003,
CA 02709558 2010-06-16
-4-
58, p.657-666). It is desired that a condition that can
stably provide a higher yield is used in order to produce
[18F]-FACBC industrially.
The production of ['8F]-FACBC comprises, as main
steps, a radioactive fluorinating step of adding a
radioactive fluorine to a labeling precursor and a
deprotecting step of conducting deprotection of the
intermediate compound produced by the radioactive
fluorinating step. According to the conventional process,
the radioactive fluorinating step shows a yield of 12-42%
(Japanese Patent Laid-open No. 2000-500442, and Timoth M.
et al., J. Nuc. Med., 1999, 40, p.331-338), and the low
yield in this step is one of the causes that lower the
synthesis yield of ['8F]-FACBC. Therefore, in order to
improve the synthesis yield of [18¨
t]_ FACBC, the yield in
the radioactive fluorinating step should be first
improved.
The present invention has been made in view of the
above described circumstances, and aimed at providing a
production process which can stably provide a radioactive
fluorine-labeled amino acid such as
butoxycarbonyl)amino)-3-fluorocyclobutane-l-carboxylic
acid ester (hereinafter referred to as ['8F]Boc-FACBC) as
an intermediate of r8F]FACBC.
MEANS FOR SOLVING THE PROBLEMS
[0008]
As a result of investigation, the present inventors
CA 02709558 2010-06-16
- 5 -
,
have found that a radioactive fluorine-labeled amino acid
such as {18¨
tiBoc-FACBC can stably be obtained in high
yield by setting a reaction temperature at 40-90 C and a
concentration of the phase transfer catalyst in the
reaction solution at not less than a specific amount
during the radioactive fluorinating reaction, and thus
the present invention has been completed.
[0009]
Therefore, according to the present invention, a
process for producing a radioactive fluorine-labeled
organic compound is provided, which comprises subjecting
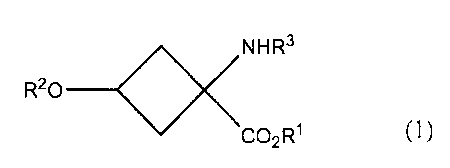
a compound represented by the following formula (1):
o<NR3
R20 ___________
CO2R1 (1)
to a heating step in an inert organic solvent in a
presence of a phase transfer catalyst, 18F ions and
potassium ions, so as to obtain a compound represented by
the following formula (2):
18<><NR3
F. ___________
CO2R1 ( 2 )
in which the heating step is conducted at a heating
temperature of 40-90 C, and the phase transfer catalyst
is contained in the inert organic solvent at a
concentration of not less than 70 mmol/L.
In the producing process of the present invention,
potassium ions subjected to the heating step are
CA 02709558 2010-06-16
- 6 -
,
preferably contained in the inert organic solvent at a
concentration of not less than 27 mmol/L.
Also, in the producing process of the present
invention, the phase transfer catalyst is used in a molar
ratio of not less than 0.7 relative to the compound
represented by the formula (1).
Further, in the process for producing of the
present invention, the compound represented by the
formula (1) is contained in the inert organic solvent at
a concentration of not less than 50 mmol/L.
[0010]
According to a preferable embodiment of the present
invention, the producing process of the radioactive
fluorine-labeled organic compound according to the
present invention comprises;
a step of obtaining a mixture of a phase transfer
catalyst, 18F ions and potassium ions; and
a radioactive fluorinating step of adding a compound
represented by the formula (1) and an inert organic
solvent to the above mixture, and maintaining the
resulting reaction solution at a temperature of 40-90 C
under stirring so as to obtain a compound represented by
the formula (2).
[0011]
In the above formulae (1) and (2), RI- is a straight
or branched alkyl chain with 1-10 carbon atoms or an
aromatic substituent, and preferably can be a substituent
selected from the group consisting of methyl group, ethyl
ak 02709558 2010-06-16
- 7 -
$
group, t-butyl group and phenyl group.
[0012]
R2 is selected from the group consisting of a
straight or branched haloalkylsulfonic acid substituent
with 1-10 carbon atoms, a straight or branched
alkylsulfonic acid substituent with 1-10 carbon atoms, a
fluorosulfonic acid substituent and an aromatic sulfonic
acid substituent, and preferably can be a substituent
selected from the group consisting of methane sulfonic
acid, toluene sulfonic acid, nitrobenzene sulfonic acid,
benzene sulfonic acid, trifluoromethane sulfonic acid,
fluoro sulfonic acid and perfluoroalkyl sulfonic acid.
[0013]
R3 is a protective group, and is not particularly
limited as long as it can prevent the reaction between a
radioactive fluorine and an amino group. Concretely, it
is selected from the group consisting of various
carbamate substituents, various amide substituents,
various imide substituents and various amine substituents,
and preferably a straight or branched alkyloxycarbonyl
substituent with 2-7 carbon atoms, a straight or branched
alkenyloxycarbonyl substituent with 3-7 carbon atoms, a
benzyloxycarbonyl substituent with 7-12 carbon atoms
which may have a substituent, an alkyldithiooxycarbonyl
substituent with 2-7 carbon atoms, a straight or branched
alkylamide substituent with 1-6 carbon atoms, a straight
or branched alkenylamide substituent with 2-6 carbon
atoms, a benzamide substituent with 6-11 carbon atoms
CA 02709558 2010-06-16
- 8 -
which may have a substituent, a cyclic imide substituent
with 4-10 carbon atoms, an aromatic imine substituent
with 6-11 carbon atoms which may have a substituent, a
straight or branched alkylamine substituent with 1-6
carbon atoms, a straight or branched alkenylamine
substituent with 2-6 carbon atoms, and a benzylamine
substituent with 6-11 carbon atoms which may have a
substituent. More preferably, R3 is one selected from
the group consisting of t-butoxycarbonyl group,
allyloxycarbonyl group, phthalimide group and N-
benzylideneamine substituent, and most preferably, t-
butoxycarbonyl group or phthalimide group.
[0014]
In the producing process of a series of radioactive
fluorine-labeled amino acids such as ['8F] -FACBC
disclosed until now, the radioactive fluorine-labeling
reaction was performed using a phase transfer catalyst at
a low concentration, i.e., in a molar ratio of about 0.3
relative to a labeling precursor (Japanese Patent Laid-
open No. 2000-500442, Timothy M. et al., J. Nuc. Med.,
1999, 40, p.331-338, and J. McConathy et al., Applied
Radiation and Isotopes, 2003, 58, p.657-666). Unlike
such a conventionally disclosed process, the present
inventors have found that the concentration of the phase
transfer catalyst in the inert organic solvent is set at
not less than 70 mmol/L, and preferably the phase
transfer catalyst is used in a molar ratio of not less
than 0.7 relative to the labeling precursor, whereby
CA 02709558 2010-06-16
- 9
fluorination yield is remarkably improved, and a
radioactive fluorine-labeled organic compound such as
1I'8F]-FACBC can be stably be produced in high yield. The
amount of the phase transfer catalyst is preferably
equimolar or more in terms of molar ratio relative to the
labeling precursor.
[0015]
In addition, the present inventors have found that
radioactive fluorination yield in the radioactive
fluorinating step can be improved by increasing a
concentration of a labeling precursor in the reaction
solution. Based on this finding, they have found that a
radioactive fluorine-labeled amino acid such as [18F]_
FACBC can be produced in higher yield by setting a
concentration of the labeling precursor in the inert
organic solvent at not less than a certain concentration.
That is, a process according to another preferable
embodiment of the present invention comprises maintaining
the concentration of the labeling precursor in the inert
organic solvent at not less than a certain concentration
in the above described process for producing a
radioactive fluorine-labeled organic compound. More
concretely, the concentration of the precursor in the
inert organic solvent is preferably not less than 50
mmol/L, more preferably not less than 60 mmol/L, and
particularly preferably not less than 70 mmol/L.
[0016]
Meanwhile, the higher the concentration of the
CA 02709558 2010-06-16
- 10
labeling precursor in the inert organic solvent is, the
higher the yield of the radioactive fluorinating step is;
however, since an increase of the concentration of the
precursor with the amount of the precursor being constant
leads to decrease of the total volume of the solution,
the concentration should be one that can ensure a
sufficient amount of liquid to perform radioactive
fluorinating reaction. The upper limit of such a
concentration is determined by an amount of a labeling
precursor to be used, a volume of the reaction vessel,
and so on. For example, when production was conducted
using an automatic synthesis device, the upper limit of
the concentration of the reaction solution is 250 mmol/L
if the lower limit of the liquid volume that can be
treated in a reaction vessel is 0.4 mL and an amount of a
labeling precursor to be used for the reaction is 0.1mol.
Similarly, the upper limit of the concentration of the
reaction solution is 160 mmol/L if the lower limit of the
liquid volume that can be treated in a reaction vessel is
0.5 mL and an amount of a labeling precursor to be used
for the reaction is 0.08 mmol.
[0017]
As mentioned above, the reaction temperature in the
labeling reaction is 40-90 C. The reaction temperature
lowers the reaction yield when it is too high or too low.
A more preferable range of the reaction temperature is
50-80 C, and further preferably 60-70 C.
In the present invention, various solvents which do
CA 02709558 2010-06-16
- 11 -
A
not have reactivity with the [18F]fluoride ion, the phase
transfer, the potassium ion, and the labeling precursor
compound are usable as the inert organic solvent.
Concrete examples of the inert organic solvent include
organic solvents comprising at least one selected from
the group consisting of tetrahydrofuran, 1,4-dioxane,
acetone, 2-butanone, dimethylformamide, dimethylsulfoxide
and acetonitrile, and preferably acetonitrile.
EFFECTS OF THE INVENTION
[0018]
According to the producing process of the present
invention, the reaction temperature is set at 40-90 C and
a concentration of the phase transfer catalyst is
maintained at not less than 70 mmol/L in the radioactive
fluorination, preferably with the concentration of
potassium ion and/or a labeling precursor in the inert
organic solvent being maintained at a specific
concentration or more and a molar ratio of the phase
transfer catalyst relative to the labeling precursor
being maintained at a specific amount or more, and thus
the yield of production of the radioactive fluorine-
labeled amino acid such as [18F]Boc-FACBC can be improved.
BEST MODE FOR CARRYING OUT THE INVENTION
[0019]
Hereinafter, a process for producing a radioactive
fluorine-labeled organic compound according to the
CA 02709558 2010-06-16
- 12 -
,
A
present invention will be described in detail taking, = as
an example, synthesis of [18F]Boc-FACBC using l-(N-(t-
butoxycarbonyl)amino)-3-[((trifluoromethyl)-
sufonyl)oxy]-cyclobutane-1-carboxylic acid ethyl ester as
a labeling precursor.
According to a preferable embodiment, the production
process of the present invention comprises (1) a step of
obtaining a mixture containing a phase transfer catalyst,
F ions and potassium ions, and (2) a step of obtaining
a radioactive fluorine-labeled organic compound by
reacting a labeling precursor with the above mixture so
as to effect a radioactive fluorine labeling (radioactive
fluorinating step).
[0020]
In the above step (1), radioactive fluorine can be
obtained by a known method, for example, a method in
which H2180 enriched water is used as a target and exposed
to proton bombardment. In this instance, radioactive
fluorine exists in the H2O enriched water used as a
target. The H2180 enriched water containing radioactive
fluorine is allowed to pass through an anion-exchange
column so that the radioactive fluorine is adsorbed and
collected on the column, thereby being separated from the
H21-80 enriched water. Thereafter, a potassium carbonate
solution is allowed to pass through the column to elute
the radioactive fluorine, and the eluate is supplemented
with a phase transfer catalyst and is evaporated to
dryness, thereby obtaining a mixture containing a phase
CA 02709558 2010-06-16
- 13 -
A
transfer catalyst, HF ion and potassium ion.
[0021]
The amount of potassium carbonate to be used here is
preferably adjusted to 27 mmol/L or more in terms of
concentration of potassium ions in the inert organic
solvent used for the reaction solution. As it is clear
from the Comparative Examples and Examples mentioned
later, at a concentration of potassium ion in the inert
organic solvent of less than 27 mol/L, the yield of [18F]
fluorination in the radioactive fluorinating step
increases together with the concentration of the
potassium ion, and at 27 mmol/L or more, it becomes
almost constant. Therefore, the use of the condition
under which the concentration of potassium ion in the
inert organic compound is 27 mmol/L or more makes it
possible to more stably perform the radioactive
fluorinating step in high yield.
On the other hand, it should be noted that when the
amount of potassium carbonate is excessive, a reaction
product may decompose due to the influence of carbonate
ions. In a preferable embodiment, an amount of potassium
carbonate in terms of potassium ions may be about
equivalent to that of a phase transfer catalyst, and it
is most preferable that concentration and amount of the
potassium carbonate solution are adjusted so that the
amount of the phase transfer catalyst is about 1.3 in
molar ratio relative to potassium ions.
[0022]
ak 02709558 2010-06-16
- 14 -
,
Various compounds having a property to form a
clathrate with 18F ion may be used as a phase transfer
catalyst. Specifically, various compounds used for the
production of organic compounds labeled with radioactive
fluorine may be used; 18-crown-6 and other various
aminopolyethers may be used. In the most preferable
embodiment, KRYPTOFIX 222 (trade name, manufactured by
Merck & Co., Inc.) may be used.
[0023]
In the present invention, the amount of the phase
transfer catalyst is adjusted so as to provide a
concentration of not less than 70 mmol/L in the inert
organic solvent which is added later. As it is clear
from Comparative Examples and Examples which are
described later, the radioactive fluorinating step can
stably be performed in high yield by setting the amount
of the phase transfer catalyst at not less than 70 mmol/L
in the inert organic solvent. The amount of the phase
transfer catalyst is preferably not less than 0.7 in
terms of molar ratio relative to the labeling precursor
which is used later in the radioactive fluorinating step.
In a further preferable embodiment, the amount of the
phase transfer catalyst is equimolar or more relative to
the labeling precursor. In this instance, the larger the
amount of the phase transfer catalyst is, the higher the
yield becomes, but an excessive amount thereof is not
preferable because it is often difficult to sufficiently
remove the excessively-added phase transfer catalyst. In
CA 02709558 2010-06-16
- 15 -
a preferable embodiment, the total amount of the phase
transfer catalyst may be 0.2 mmol or less, for example,
when the amount of the labeling precursor to be used is
80 pmol, the molar ratio of the phase transfer catalyst
to the labeling precursor is 2.5 or less. This amount of
the phase transfer catalyst can be easily removed by
purification using a solid phase column or the like in a
subsequent step.
[0024]
After the mixture containing the phase transfer
catalyst, ['8F] ions and potassium ions is obtained in
the above way, the radioactive fluorine-labeled amino
acid is synthesized by performing the above step (2). In
, this step, the labeling precursor 1-(N-(t-
butoxycarbonyl)amino)-3-[((trifluoromethyl)sufonyl)oxy]-
cyclobutane-1-carboxylic acid ester is first added to the
mixture containing the phase transfer catalyst, {18F]
ions and potassium ions. In the most preferable
embodiment, the labeling precursor is previously
dissolved in an inert organic solvent, and then added to
the mixture. In this instance, it is preferred that the
amount of the inert organic solvent to be used is
adjusted so that the concentration of the labeling
precursor in the reaction solution under the radioactive
fluorination becomes not less than 50 mmol/L, because the
yield in the radioactive fluorination is significantly
improved.
[0025]
CA 02709558 2010-06-16
- 16 -
After the addition of the labeling precursor and the
inert organic solvent has been completed, the above
reaction solution is subjected to radioactive
fluorination by heating under stirring to obtain a
radioactive fluorine-labeled organic compound as a target
compound of the present invention. The reaction
temperature is 40-90 C, preferably 50-80 C, and
particularly preferably 60-70 C. The reaction time
depends on the reaction temperature, and when the
reaction temperature is 40-90 C, the reaction time is
usually 3 minutes or longer, preferably 3-15 minutes, and
more preferably 3-7 minutes. The longer the reaction
time is, the further the labeling reaction with the
radioactive fluorine is expected to proceed, but it
should be noted that the decay of the radioactive
fluorine proceeds simultaneously.
[0026]
After the completion of reaction, purification is
performed so as to remove unreacted raw materials and
phase transfer catalysts. In the most preferable
embodiment, purification is performed according to the
following procedures. First, a solution is prepared by
adding diethylether to the reaction solution that has
completed the reaction. This solution is passed through
the silica gel-based solid column (for example, Sep-Pak
(registered trade mark) Silica (trade name, manufactured
by Japan Waters) so as to obtain [18F] Boc-FACBC in a form
of a diethylether solution.
CA 02709558 2010-06-16
- 17 -
,
EXAMPLE
[0027]
Hereinafter, the present invention is described in
more detail by way of Examples and Comparative Examples
which do not restrict the present invention.
Meanwhile, in Examples and Comparative Examples,
radiochemical purity was determined by carrying out TLC
analysis under the following conditions and using the
following equation (1).
[0028]
TLC analysis conditions:
Mobile phase: Diethylether/hexane = 3/2
TLC plate: Silica Gel 60 F254 (trade name, thickness of
membrane: 0.25 mm, manufactured by Merck & Co., Inc.)
Mobile length: 10 cm
TLC scanner: Rita Star (manufactured by Raytest)
[0029]
radioactivity of [18 FlBoc - FACBC - OEt peak xlco
Radiochamical purity (%) =
total radioactivity on TLC plate
[0030]
In addition, a yield of [18F]fluorination was
determined by the following equation (2).
[0031]
B
Yield of P8Fifluorination(%)= ¨xradiochemcial purity (2)
A
[0032]
A: radioactivity of a mixture containing a phase transfer
CA 02709558 2010-06-16
- 18 -
,
catalyst, [18¨
r] ions and potassium ions (MBq)
B: radioactivity of a synthesized
t]Boc-FACBC (MBq)
[0033]
Reference Example 1
Synthesis of syn-1-(N-(t-butoxycarbonyl)amino)-3-
[((trifluoromethyl)sulfonyl)oxy]-cyclobutane-l-carboxylic
acid ethyl ester
[0034]
Hydrolysis of syn-hydantoin (FIG. 1, Step 1)
250 mL of a saturated barium hydroxide solution was
added to 6.15 g (corresponding to 25 mmol) of syn-5-(3-
benzyloxycyclobutane)hydantoin and refluxed under heating
in an oil bath at 114 C for 24 hours or longer. Then,
TLC analysis was performed using, as mobile solvents, two
kinds of systems: chloroform/methanol = 5/1 (Rf value of
syn-hydantoin = around 0.6) and chloroform/methanol =
95/1 (Rf value of syn-hydantoin - around 0.3), and the
completion of the reaction was confirmed (by coloration
with UV and phosphomolybdic acid).
[0035]
After the completion of the reaction was confirmed,
the reaction solution was cooled to room temperature, and
about 24 mL of 1 mol/mL sulfuric acid was added to
neutralize the reaction solution. After the
neutralization, the reaction solution was further stirred
at room temperature for 5 minutes, and the formed
precipitate was removed by filtration. The filtrate was
concentrated to yield 5.67 g of syn-1-amino-3-
CA 02709558 2010-06-16
- 19 -
,
benzyloxycyclobutane-1-carboxylic acid as white crystals.
[0036]
Ethyl esterification (FIG. 1, Step 2)
5.67 g of syn-l-amino-3-benzyloxycyclobutane-1-
carboxylic acid, which had been fully dried to remove
water, was dissolved in 200 mL of ethanol. To this
solution, 9.5 mL (corresponding to 75 mmol) of
triethylamine was added and cooled at -78 C for 20
minutes, and then 4.6 mL (corresponding to 62.5 mmol) of
thionyl chloride was added. The reaction solution was
stirred at 0 C for 1 hour and at room temperature for 1
hour, followed by heating under reflux in an oil bath at
95 C overnight. The completion of the reaction was
confirmed by TLC analysis using a mobile solvent of
chloroform/methanol - 95/1 (Rf value of the target
compound = around 0.6) (confirmed by coloration with UV
and phosphomolybdic acid). After the completion of the
reaction was confirmed, the reaction solution was
concentrated under reduced pressure to yield 7.64 g of
syn-1-amino-3-benzyloxycyclobutane-1-carboxylic acid
ethyl ester as white crystals.
[0037]
Addition of Boc (FIG. 1, Step 3)
7.64 g of syn-1-amino-3-benzyloxycyclobutane-1-
carboxylic acid ethyl ester was dissolved in 250 mL of a
mixed solution of ethanol/triethylamine = 9/1. After the
solution was cooled in an ice bath for 15 minutes, 8.6 mL
(corresponding to 37.5 mmol) of di-t-butyl dicarbonate
CA 02709558 2010-06-16
- 20
was added to the solution and stirred at room temperature
overnight. The completion of the reaction was confirmed
by TLC analysis using a mobile solvent of hexane/ethyl
acetate = 1:1 (Rf value of the target compound = around
0.6) (confirmed by coloration with UV and molybdic acid).
After the completion of the reaction was confirmed, the
reaction solution was concentrated under reduced pressure
to yield white crystals as a residue. To the residue,
150 mL of cooled ethyl acetate and 150 mL of 0.5 mol/L
cooled hydrochloric acid were added, stirred at room
temperature for 5 minutes, and left to stand until
separation occurred. The organic layer was extracted and
washed with 150 mL of water twice, with 150 mL of a
saturated aqueous sodium hydrogencarbonate solution, with
150 mL of water twice and with 150 mL of a saturated
saline solution twice in this order, dried with anhydrous
sodium sulfate, and concentrated under reduced pressure
to yield yellow oily matter. Separately, the water layer
was extracted and washed with 150 mL of ethyl acetate
twice, with 150 mL of water twice and with 150 mL of a
saturated saline solution in this order, dried with
anhydrous sodium sulfate, and concentrated under reduced
pressure to recover a small amount of yellow oily matter.
By these operations, 8.82 g of light yellow oily matter
was obtained. The residue was purified by silica gel
column chromatography (hexane/ethyl acetate = 1/1) to
yield 8.04 g (corresponding to 23 mmol) of syn-1-(N-(t-
butoxycarbonyl)amino)-3-benzyloxy-cyclobutane-1-
CA 02709558 2015-04-02
ak 02709558 2010-06-16
- 21
carboxylic acid ethyl ester as white crystals.
[0038]
Debenzylation (FIG. 2, Step 4)
To 8.04 g (corresponding to 23 mmol) of syn-1-(N-(t-
butoxycarbonyl)amino)-3-benzyloxy-cyclobutane-1-
carboxylic acid ethyl ester, were added 150 mL of ethanol
and then 960 mg of palladium-on-activated carbon (10%
palladium) to perform replacement with hydrogen under
stirring at room temperature overnight. After the
reaction, palladium-on-activated carbon was removed by
TM
filtration using Celite, and the filtrate was
concentrated under reduced pressure to yield 5.74 g of
white crystals as a residue. The reaction was traced by
TLC analysis using a mobile solvent of hexane/ethyl
acetate = 1/1 (Rf value of the target compound of
reaction = around 0.2) (confirmed by coloration with UV
and ninhydrin) to confirm the completion of the reaction.
= Then, the residue was purified by silica gel column
chromatography (hexane/ethyl acetate = 1/1, hexane/ethyl
acetate - 4/1) to yield 5.36 g (corresponding to 20.7
mmol) of syn-1-(N-(t-butoxycarbonyl)amino)-3-hydroxy-
cyclobutane-l-carboxylic acid ethyl ester as white
crystals.
[0039]
Triflation (FIG. 3, Step 5)
2.07 g (8 mmol) of syn-1-(N-(t-
butoxycarbonyl)amino)-3-hydroxy-cyclobutane-1-carboxylic
acid ethyl ester was dissolved in 26 mL of pyridine and
CA 02709558 2010-06-16
- 22 -
stirred in an ice bath for 20 minutes. Then, 2.0 mL
(corresponding to 12 mmol) of trifluoromethanesulfonic
anhydride was added and stirred for 30 minutes. The
reaction was traced by TLC analysis using a mobile
solvent of hexane/diethyl ether = 1:1 (Rf value of the
target compound of reaction = around 0.6) (confirmed by
coloration with ninhydrin) to confirm the completion of
the reaction. After confirming the completion of the
reaction, 100 mL of water and 100 mL of ether were added
to the reaction solution, and extraction and washing were
performed with 100 mL of 1 mol/L hydrochloric acid twice,
with 100 mL of water twice and with 100 mL of a saturated
saline solution twice in this order. After drying with
anhydrous sodium sulfate, concentration under reduced
pressure was performed to yield 2.78 g of light yellow
crystals. The reaction mixture was purified by silica
gel chromatography (hexane/diethyl ether = 3/1) to yield
white crystals, and the resultant white crystals were
again recrystallized using pentane/diethyl ether to yield
1.84 g (corresponding to 4.7 mmol) of syn-1-(N-(t-
butoxycarbonyl)amino)-3-[((trifluoromethyl)sulfonyl)oxy]-
cyclobutane-l-carboxylic acid ethyl ester.
[0040]
Comparative Examples 1-5, Examples 1-8
['8F]fluoride ion-containing H2180 was allowed to pass
through an anion-exchange column to adsorb and collect
[18F]fluoride ion on the column. Then, 0.3 mL of a
solution of potassium carbonate at a concentration shown
ak 02709558 2010-06-16
- 23 -
,
in Table 1 was passed through to elute the ['8F]
fluoride
ion, 0.3 mL of water was further passed through, and
combined with the eluate. To this solution, 1.5 mL of
acetonitrile solution of Kryptofix 222 (under trade name,
manufactured by Merck & Co., Inc.) in an amount shown in
Table 1 was added, and radioactivity of the resulting
mixture was measured (A: measured radioactivity in Table
2).
[0041]
Then, the mixture was heated to 110 C to evaporate
water and acetonitrile, and was subjected to azeotropic
distillation with addition of acetonitrile (0.5 mL x 2),
followed by evaporation to dryness. To the mixture, a
solution of syn-1-(N-(t-butoxycarbonyl)amino)-3-
[((trifluoromethyl)sulfonyl)oxy]-cyclobutane-1-carboxylic
acid ethyl ester (hereinafter referred to as Boc-TfACBC)
in an amount shown in Table 1 in acetonitrile in an
amount shown in Table 1 was added, and heated at 83 C for
3 minutes under stirring. Then, the solution was allowed
to cool for 5 minutes at room temperature, 4 mL of
diethyl ether was added to the solution, and the mixture
was allowed to pass through Sep-Pak Silica (under trade
name, manufactured by Japan Waters) to yield an
acetonitrile/diethyl ether solution of pa-rj,
Boc-FACBC as
a ['8F]fluorine-labeled compound. Radioactivity was
measured, and the resulting radioactivity B (refers to
Table 2) was used for the calculation of
['8F]
fluorination yield. Also, TLC analysis was conducted
CA 02709558 2010-06-16
¨ 24 -
for the resulting ['8F]Boc-FACBC to determine a
radiochemical purity using the above equation (1).
Meanwhile, the experiment on each condition was
. conducted once in Comparative Examples 1 and 3 and
Example 3, twice in Comparative Examples 2 and 4 and
Example 8, four times in Example 4, and three times in
others.
[0042]
Table 1: Experiment conditions in each Example and Comparative
Example (Amount of each raw material to be used)
Amount of
Concentration Amount
Ratio of Added amount
of potassium Kryptofixof Boc- Kryptofix
of
222 to
carbonate beTfACBC 222/Boc- acetnitrile
used
(rumol/L) (umol) TfACBC (mL)
(pnol)
CcEparative
22 13 40 0.33 1
Example 1
Comparative
40 24 80 0.33 1
Example 2
Comparative
66.7 53 80 0.66 1
Example 3
Comparative
100 60 80 0.75 1
Example 4
Comparative
66.7 53 40 1.3 1
Example 5
Example 1 100 79.5 60 1.3 1
Example 2 133 80 80 1.0 1
Example 3 133 93 80 1.2 1
Example 4 133 106 80 1.3 1
Example 5 133 106 80 1.3 1.5
Example 6 133 120 80 1.5 1
Example 7 167 133 100 1.3 1
Example 8 133 . 160 80 2.0 1
CA 02709558 2010-06-16
- 25
[0043]
Table 2: Measured values of radioactivity in each Example and
Comparative Example (values corrected back to initiation of
synthesis)
A (MBq) B (MBq)
Comparative
59.80 24.38
Example 1
Comparative
1: 159.84, 2: 149.13 1: 88.73, 2: 69.59
Example 2
Comparative
320.11 203.06
Example 3
Comparative
1: 421.71, 2: 347.29 1: 308.17, 2: 216.28
Example 4
Comparative 1: 211.91, 2: 187.64, 1: 122.40, 2: 119.11,
Example 5 3: 371.63 3: 245.90
1: 278.90, 2: 175.47, 1: 193.63, 2: 117.86,
Example 1
3: 356.11 3: 252.20
1: 500.23, 2: 273.51, 1: 293.15, 2: 184.08,
Example 2
3: 355.39 3: 239.33
Example 3 461.47 326.44
1: 112.29, 2: 445.79, 1: 86.33, 2: 332.52,
Example 4
3: 149.01, 4: 126.74 3: 113.81, 4: 97.44
1: 242.47, 2: 153.66, 1: 165.59, 2: 101.35,
Example 5
3: 135.65 3: 93.59
1: 123.95, 2: 433.30, 1: 86.20, 2: 297.44,
Example 6
3: 330.94 3: 245.92
1: 128.58, 2: 123.51, 1: 98.64, 2: 86.89,
Example 7
3: 301.16 3: 218.30
Example 8 1: 123.10, 2: 112.36 1: 93.45, 2: 84.60
[0044]
Results are shown in Table 3 and Figs. 4-6.
A ratio of Kryptofix 222 used as a phase transfer
catalyst to a precursor Boc-TfACBC (hereinafter referred
to as a ratio of phase transfer catalyst/precursor) was
calculated, and a relation with a yield of
tjfluorination was investigated. The results are shown
in Fig. 4. As it is clear from this figure, under the
condition in which the ratio of phase transfer
catalyst/precursor is less than 0.7, the yield of
['8F]fluorination of[18F]Boc-FACBC was remarkably improved
with increase of the ratio of phase transfer
CA 02709558 2010-06-16
- 26 -
catalyst/precursor. Under the condition in which the
ratio of phase transfer catalyst/precursor is not less
than 0.7, the data indicated a substantially constant
yield although there was a data showing a low yield of
[18¨
t]fluorination (in Comparative Example 5), and the
yield of [18F]fluorination under this condition was about
30-50% higher than the conventional process (Comparative
Example 1).
[0045]
A relation between the concentration of potassium
ions in acetonitrile of the reaction solution and the
yield of [18F]fluorination is shown in Fig. 5. As it is
clear from Fig. 5, under the condition in which the
concentration of potassium ions is less than 27 mmol/L,
the yield of ['8F]fluorination was remarkably improved
with increase of the concentration of potassium ions, and
at a concentration higher than this, the yield was almost
constant. A relation between the concentration of
Kryptofix in acetonitrile of the reaction solution and
the yield of [18 F]fluorination is shown in Fig. 6. As it
is clear from Fig. 6, under the condition in which the
concentration of Kryptofix in acetonitrile of the
reaction solution (indicated as concentration of phase
transfer catalyst in Fig. 6) is less than 70 mmol/L, the
yield of ['8F]fluorination of [18F] Boc-FACBC was
remarkably increased with increase of the concentration
of Kryptofix, and at a concentration higher than this,
the yield was almost constant. Therefore, it has been
CA 02709558 2010-06-16
- 27 -
. 4
revealed that[i 8F]Boc-FACBC can be prepared with a high
yield of [18F]fluorination under the conditions in which
the concentration of potassium ions is not less than 27
mmol/L, and the concentration of phase transfer catalyst
is not less than 70 mmol/L. Also, it has been indicated
that by setting these conditions in addition to the above
described condition where the ratio of phase transfer
catalyst/precursor is not less than 0.7, the condition
(Comparative Example 5) showing a low yield under the
conditions where the ratio of phase transfer catalyst/
precursor is not less than 0.7 can be removed, and thus a
high yield of ['8F]fluorination can be more stably
achieved.
From the above results, it has been indicated that
['8F]Boc-FACBC can stably be obtained in high yield by
combining the condition in which the ratio of phase
transfer catalyst/precursor is not less than 0.7, the
condition in which the concentration of potassium ions is
not less than 27 mmol/L, and the condition in which the
concentration of phase transfer catalyst is not less than
70 mmol/L, in the [is-
f]fluorination.
CA 02709558 2010-06-16
- 28
[0046]
Table 3: Yield of [18-t]
fluorination and radiochemical purity of
the compounds obtained in each Example and Comparative Example
Yield of [18F]
Radiochemical
fluorination % purity %
Comparative
24.16 59.27
Example 1
Comparative
38.13 74.77
Example 2
Comparative
54.75 86.32
Example 3
Comparative
59.80 88.24
Example 4
Comparative
56.66 90.65
Example 5
Example 1 65.95 95.33
Example 2 61.39 95.36
Example 3 64.87 91.70
Example 4 73.53 96.52
Example 5 65.33 96.43
Example 6 67.98 95.92
Example 7 68.38 93.37
Example 8 70.73 93.57
[0047]
Examples 9-43
At the reaction temperature of 40-100 C, the
following experiments were performed in order to confirm
that [18F]Boc-FACBC can be produced in good yield
according to the producing process of the present
invention.
[0048]
['8F] fluorideion-containing H2180 was allowed to pasS
through an anion-exchange column to adsorb and collect
[18
F]fluoride ion on the column. Then, 0.3 mL of a
solution of potassium carbonate at a concentration of 133
mmol/L was passed through the column to elute
['8F]
fluoride ion, 0.3 mL of water was further passed
through, and combined with the eluate. To this solution,
a solution of 106 umol of Kryptfix 222 (trade name,
CA 02709558 2010-06-16
- 29 -
,
manufactured by Merck & Co., Inc.) in 1.5 mL of
acetonitrile was added.
[0049]
Then, the mixture was heated to 110 C to evaporate
water, and was subjected to azeotropic distillation with
addition of acetonitrile (0.5 mL x 2), followed by
evaporation to dryness. To this mixture, a solution of
80 umol of Boc-TfACBC in 1 mL of acetonitrile was added,
and the reaction solution was stirred for a time period
shown in Tables 4a-4d at a temperature shown in Tables
4a-4e, and was allowed the radioactive fluorination to
proceed. The resulting reaction solution was subjected
to TLC analysis, and an area% of [18F]Boc-FACBC was
determined and used as an index for yield of
['8F] fluorination.
Meanwhile, the radioactivity used in each experiment
was 414-759 MBq.
[0050]
TLC analysis conditions:
TLC plate: Silica Gel 60 F254 (trade name; manufactured by
Merck & Co., Inc.)
Mobile phase: Diethylether/hexane = 1/1
Detector: Rita Star (trade name; manufactured by raytest)
CA 02709558 2010-06-16
- 30 -
,
[0051]
Table 4a: Reaction temperature and reaction time in each Example
Example Example Example Example Example Example Example
9 10 11 12 13 14 15
Reaction
temperature 40 50 60 70 80 90 100
Co
Reaction tine
3 3 3 3 3 3 3
min
[0052]
Table 4b: Reaction temperature and reaction time in each Example
Example Example Example Example Example Example Example
16 17 18 19 20 21 22
Reaction
temperature 40 50 60 70 80 90 100
Co
Reaction time 5 5 5 5 5 5 5
min
[0053]
Table 4c: Reaction temperature and reaction time in each Example
Example Example Example Example Example Example Example
23 24 25 26 27 28 29
Reaction
temperature 40 50 60 70 80 90 100-
C
Reaction tine
7 7 7 7 7 7 7
ndn
[0054]
Table 4d: Reaction temperature and reaction time in each Example
Example Example Example Example Example Example Example
30 31 32 33 34 35 36
Reaction
temperature 40 50 60 70 80 90 100
Co
Reaction time
10 10 10 10 10 10 10
min
CA 02709558 2010-06-16
¨ 31
[0055]
Table 4e: Reaction temperature and reaction time in each Example
Example Example Example Example Example Example Example
37 38 39 40 41 42 43
Reaction
temperature 40 50 60 70 80 90 100
Co
Reaction time
15 15 15 15 15 15 15
min
[0056]
The results are shown in Tables 5a-5e. As it is
clear from these results, under the conditions in which
the reaction time is 3-15 minutes, the yield of
['8F]fluorination showed a good value of not less than
62% at all the reaction temperatures. Also, no
significant change was seen in the yield of
[18-
t]fluorination at the reaction temperature of not less
than 90 C, and thus it has been suggested that a good
yield of
[8F]fluorination can be obtained at a reaction
temperature of 40-90 C.
[0057]
Also, under the conditions in which the reaction
temperature was 50-80 C, the yield of ['8F]fluorination
reached not less than 70% in all the reaction time, and
under the conditions in which the reaction temperature
was 60-70 C, the yield of ['8F]fluorination reached not
less than 80% in all the reaction time.
On the other hand, referring to the reaction time, a
particularly good yield of [18F]fluorination was obtained
when the reaction time was 3-7 min.
[0058]
CA 02709558 2010-06-16
¨ 32
, 4
Therefore, it has been indicated that in the
reaction time of 3-15 min, a good yield of
[18F] fluorinationcan be achieved under a condition in
which the reaction temperature is 40-90 C or more, a
better yield of [18F]fluorination can be obtained under a
condition of 50-80 C, and a particularly good yield of
[18F] fluorinationcan be achieved under a condition of
60-70 C.
In addition, it has been indicated that the
reaction time of not less than 3 minutes is sufficient,
and the reaction time of 3-7 min is more preferable.
[0059]
Table 5a: Yield of [18F] fluorination in each Example
Example Example Example Example Example Example Example
9 10 11 12 13 14 15
Reaction
40 50 60 70 80 90 100
temperature C
Reaction time
3 3 3 3 3 3 3
min
Yield of [18F]
62 74 82 86 79 74 74
fluorination %
[0060]
Table 5b: Yield of [18F] fluorination in each Example
Example Example Example Example Example Example Example
16 17 ' 18 19 20 21 22
Reaction
40 50 60 70 80 90 100
temperature C
Reaction time
5 5 5 5 5 5 5
min
Yield of [18F]
70 80 83 84 78 70 69
fluorination %
CA 02709558 2010-06-16
- 33 -
, õ a
[0061]
Table Sc: Yield of [18F] fluorination in each Example
Example Example Example Example Example Example Example
23 24 25 26 27 28 29
Reaction
40 50 60 70 80 90 100
temperature C
Reaction time
7 7 7 7 7 7 7
min
Yield of [1-8F]
74 81 81 83 76 72 73
fluorination %
[0062]
Table 5d: Yield of ['8F] fluorination in each Example
Example Example Example Example Example Example Example
30 31 32 33 34 35 36
Reaction
40 50 60 70 80 90 100
temperature C
Reaction time
10 10 10 10 10 10
min
Yield of [18F]
76 83 83 81 76 67 70
fluorination %
[0063]
Table 5e: Yield of [18F] fluorination in each Example
Example Example Example Example Example Example Example
37 38 39 40 41 42 43
Reaction
40 50 60 70 80 90 100
temperature C
Reaction time
15 15 15 15 15 15
min
Yield of [18F]
78 83 81 82 74 69 68
fluorination %
10 INDSUTRIAL APPLICABILITY
[0064]
The process for producing a radioactive fluorine-
labeled organic compound according to the present
invention can be suitably used for production of a
15 radioactive fluorine-labeled organic compound including
['8F]Boc-FACBC which is used for production of novel
CA 02709558 2010-06-16
- 34
diagnostic agents.
BRIEF DESCRIPTION OF THE DRAWINGS
[0065]
Fig. 1 shows a scheme of synthesis of syn-1-(N-(t-
butoxycarbonyl)amino)-3-benzyloxy-cyclobutane-1-
carboxylic acid ethyl ester.
Fig. 2 shows a scheme of synthesis of syn-1-(N-(t-
butoxycarbonyl)amino)-3-hydroxy-cyclobutane-l-carboxylic
acid ethyl ester.
Fig. 3 shows a scheme of synthesis of syn-1-(N-(t-
butoxycarbonyl)amino)-3-[((trifluoromethyl)sulfonyl)oxy]-
cyclobutane-l-carboxylic acid ester.
Fig. 4 is a graph which shows a relation between a
ratio of phase transfer catalyst/labeling precursor and a
yield of fluorination (triangle: Examples, square:
Comparative Examples).
Fig. 5 is a graph which shows a relation between a
concentration of potassium ions and a yield of
fluorination (triangle: Examples, square: Comparative
Examples).
Fig. 6 is a graph which shows a relation between a
concentration of phase transfer catalyst and a yield of
fluorination (triangle: Examples, square: Comparative
Examples).