Note: Descriptions are shown in the official language in which they were submitted.
= CA 02711025 2017-02-02
Improved method for metal recovery and
leaching agent recycle in agitation leach plants
Field of the invention
The present invention relates to the design and operation of the leaching and
solvent extraction
steps in a metal recovery plant for recovering desired metal values from mined
ores containing
such metal values possibly commingled with other metal values.
Background of the invention
To obtain metals (e.g., copper, nickel, cobalt, zinc, uranium, and the like)
in a pure, useful
form, these metals must be removed and recovered from the ores in which they
are found
through a series of physical, hydrometallurgical and/or chemical steps.
Conventionally, the mined ore, containing a greater or lesser amount of the
desired metal
value, in addition to possibly one or more other more-or-less desirable metal
values and a large
amount of gangue and other more-or-less complicating minerals, is leached with
an aqueous
acidic (commonly sulphuric acid) or basic (commonly ammonium hydroxide)
solution. This
leaching is accomplished by either distributing the leaching agent over a pile
or bed of mostly
dry ore solids in dump leaching, heap leaching or vat leaching, wherein these
ores are either
leached as mined, or they may be crushed, but not ground or milled, to a size
that gives higher
metal recovery and/or faster metal recovery, or, as in agitation leaching, by
mixing the
leaching agent with an aqueous slurry of crushed and milled ore solids in one
or more stirred
tanks in an attempt to ensure optimal distribution of the leaching solution
throughout the ore
solids.
In heap, dump or vat leaching, dry ore is placed in a pile/bed leach system,
or, where ore is
agglomerated with moisture prior to being placed in a bed/heap, with only a
small amount of
added water. With these methods, there is often significant evaporation of any
water from the
pile/bed, and, so as not to depend solely on such evaporation to keep the ore
dry, most plants
using pile/bed leach systems employ at least one, and often several, large
ponds in which to
1
CA 02711025 2010-06-29
WO 2009/083204
PCT/EP2008/010979
hold water that may accumulate in a short event, such as a heavy rain. Thus,
there is no need
to bleed water on a continual basis from a heap/dump/vat leach system.
By comparison, in a plant employing agitation leaching, crushed ore that is to
be agitation-
leached is generally ground or wet-milled to a desired size distribution for
achieving an ac-
ceptable metal recovery in leaching, with the resulting ore solids being added
to the agitation
leach unit(s) as aqueous slurry. Thus, in agitation leaching, a considerable
amount of water is
normally brought into the leaching system with the ore. This water must
eventually leave or
be removed from the system in order to maintain a water balance and it does
so, mainly and
continually, with the leached solids in the tailings or by intermittent bleeds
from the circuit.
Any desired metal or other valuable metals in the water leaving with the
leached solids is lost
(called the "soluble metal loss"). In addition, any leaching agent in this
water is also lost and
often has to be neutralized prior to the final disposal of the leached solids.
Selection of the type of leaching to be employed is based on several factors
including the
grade of the ore, the clay content of the ore, the hardness of the ore and the
way the ore re-
sponds to the various leaching methods. A dump or heap leach system is
generally much less
costly in both capital (equipment) costs and operating (energy) expense, and
is therefore se-
lected for use with lower grade ores, where costs are critical, or with higher
grade ores that
respond well to heap leaching, permitting a high metal recovery. Agitation
leaching, on the
other hand, provides for a faster and more complete recovery of the desired
metal(s), is easier
to control, and often gives higher recovery of secondary valuable metals, such
as cobalt, but it
is also more expensive due to the capital cost of additional equipment, such
as mills, leach
tanks and clarifiers, and has a higher operating cost because of, for example,
the energy re-
quired to mill the ore and the chemicals needed for the solids-liquid
separation.
Following the leaching step in a circuit employing agitation leaching (such
circuits being the
focus of this invention), the resulting mix of aqueous leachate, now
containing a high propor-
tion of the desired metal values, as well as leached ore solids from which the
desired metal
values have been dissolved, is then normally sent to a solids-liquid
separation process, such as
by counter-current decantation ("CCD"), with washing, or by filtration, also
with washing.
Following this solids-liquid separation process, the clarified or partially-
clarified aqueous
phase is sent to one or more units in a solvent extraction process for
transfer of the metal val-
ues from the aqueous leachate into an organic phase comprising one or more
extraction re-
agents.
In that solvent extraction process, the particular desired metal value is
extracted from the
leach solution containing that metal value into an organic phase by one or
more extraction
2
CA 02711025 2010-06-29
WO 2009/083204
PCT/EP2008/010979
reagents specific for that desired metal, which reagent(s) is/are dissolved in
an organic phase
that comprises the extraction reagent(s), optionally with one or more
equilibrium modifiers,
kinetic additive(s) and/or other compounds, in a water-insoluble, water-
immiscible organic
solvent. During such extraction, hydrogen ions are released from the organic
phase into the
aqueous phase, now largely depleted of the desired metal values, as
represented by the equa-
tion below for extraction when copper is the desired metal, sulphuric acid is
the leaching
agent, and where "RH" represents the copper-specific extraction reagent(s):
2RH + CuSO4 ---- R2CU H2S 04
In the extraction of 1 ton of copper, 1.54 tons of sulphuric acid (useful for
further leaching of
copper when the leach solution, depleted of copper values, is returned to the
leaching unit(s))
is regenerated in the leach solution from which the copper was extracted.
Thus, the greater the
amount of copper extracted from an aqueous solution, the higher the
concentration of sul-
phuric acid generated in that solution. In general, more of the leached copper
can be extracted
when the concentration of copper in the leach solution is higher, thus, the
higher the copper
concentration in the leach solution to be treated by solvent extraction, the
greater is the poten-
tial to return more sulphuric acid back to the leaching unit. Other metals,
such as Zn, Ni and
Co, also show this behavior, depending on the leach solution and extraction
reagent(s) em-
ployed.
Following the extraction, the metal-rich organic phase containing one or more
complexes of
the desired metal with the extraction reagent(s) is then possibly washed to
reduce the level of
undesired iron and/or other undesirable species, and stripped of its desired
metal content with
a stripping agent, such as a relatively concentrated acid solution (normally
sulphuric acid) that
breaks apart the complex(es), freeing the desired metal into the aqueous
"pregnant stripping
solution". That metal is then finally captured in a pure form from the desired
metal-rich preg-
nant stripping solution, by electrodeposition in an electrowinning stage, or
by one or more
alternative metal recovery processes.
The great quantities of solids and the large volumes of leaching and stripping
agents, extrac-
tion reagents, organic solvents and purified water involved in large-scale
mining and metal
recovery operations mandate efforts to use these resources most efficiently,
both from a purely
economic perspective, and in consideration of the potential environmental
impact of acciden-
tal discharges and intentional disposal of no-longer-useful substances.
Increased recycling of
expensive agents and reagents, and the reduction of losses resulting both from
the disposal of
metal-depleted tailings slurry still containing some desired metal and other
metal values dis-
solved in the water in the tailings slurry and from bleeds of the aqueous
phase in order to
3
CA 02711025 2010-06-29
WO 2009/083204
PCT/EP2008/010979
maintain the overall water balance and/or adjust/correct levels of undesirable
metals or acid,
as well as from other conservation measures, have become critical to the
successful economic
and environmentally-responsible operation of mining and metal recovery
operations.
A particular issue addressed by this invention is the high cost both of
replacing leaching
agents lost or bled from the leaching-solvent extraction-electrowinning
circuits and of pur-
chasing substances used to neutralize excess leaching agents prior to further
metal recovery
activities and/or disposal of spent leaching and/or washing solutions
containing these leaching
agents. Another issue addressed is the need to recover a higher percentage of
the desired
metal values from the leached ores and thereby reduce the amount of valuable
metal that is
ultimately lost from the circuit in bleed streams or to tailings disposal,
resulting in the loss of
significant revenue that could be realized by the operator.
US 2005/0031512 Al (Kordosky et al) showed that good metal extraction may be
achieved
while also significantly improving the recovery of the leaching agents by
proposing a "split-
circuit" arrangement of leachate solution flows that does not require as much
fresh leaching
agent to be added to supplement the recycling of the leachate solution back to
the leaching
stage. This method does not follow the conventional practice of washing, and
thereby dilut-
ing, the entire solution flow from the one or more agitation leach units
during the solids-liquid
separation stage following leaching. Such washing is intended to minimize the
loss of metal
values with the disposal of the metal-depleted tailings slurry, but it also
reduces the concentra-
tion of the desired metal in the clarified leach solution exiting solids-
liquid separation and
thereby reduces the leaching agent concentration which can build in this
solution as the de-
sired metal is extracted. Since only a portion of this leach solution, now
depleted of desired
metal values, but increased in leaching agent concentration, is recycled back
to leaching, less
leaching agent is recycled back to leaching than would be if the leaching
solution had not been
diluted.
Instead, the split circuit design involves subjecting the first leached pulp
from the leach u-
nit(s), comprising a mixture of metal-depleted leached solids and an aqueous
leach solution
containing dissolved salts of the desired metal, leaching agent, water, and
possibly other metal
values, to a first solids-liquid separation, without significant dilution. The
solids pulp from
that separation is then sent to a second solids-liquid separation, with
significant wash-
ing/dilution, with the clarified metal-rich aqueous leach solutions from each
solids-liquid be-
ing separation circulated to separate solvent extraction units. The solids, as
aqueous slurry,
from the second solids-liquid separation are then sent to disposal, with the
metal-depleted
raffinate from the solvent extraction unit(s) following the first solids-
liquid separation, with-
out dilution, being recycled as leach solution, possibly supplemented with
additional fresh
4
CA 02711025 2010-06-29
WO 2009/083204
PCT/EP2008/010979
leaching agent, to one or more of the leach unit(s). The raffinate, depleted
of desired metal
values, exiting the solvent extraction unit(s) following the second solids-
liquid separation is
neutralized, as necessary, and/or circulated to one or more additional units
to possibly recover
other metal values that may also have been present in the original ore in
sufficient quantities,
and/or recycled back to the second solids-liquid separation as wash solution,
with the possibil-
ity that some of the neutralized solution may be bled to disposal in order to
maintain a water
balance.
Nevertheless, mineral industry is still interested in improved processes for
the leaching of ores
allowing significant reductions in leaching agent replacements and
neutralizations, as well as
further significant increases in the recovery of the desired metal from the
original ore. The
problem underlying the present invention has been to serve these needs.
Detailed description of the invention
The present invention is directed to a process for leaching desired metal
values from crushed
and milled ore solids and extracting those values into organic phases for
further recovery ef-
forts, in order to eventually obtain the desired metal in a usable form. This
process initially
comprises leaching crushed and milled ore solids with an acidic or basic
leaching solution in
one or more initial/"first" agitation leach units, to dissolve a significant
portion of the desired
metal values from the crushed and milled ore solids into an aqueous phase. The
slurry of par-
tially-leached solids with aqueous leach solution (called a "leach pulp")
resulting from the
initial leaching unit(s) proceeds to a first solids-liquid separation and
clarification to produce
two products, a first undiluted aqueous leach solution, rich in desired metal
values, and a sec-
ond leach pulp.
The first undiluted aqueous leach solution is then circulated, without
significant dilution, to
one or more "first" solvent extraction units for extracting the desired metal
values from the
aqueous solution into an organic phase. From the first solvent extraction
unit(s), the aqueous
solution ("raffinate"), depleted of desired metal values, is recycled as
leaching solution, possi-
bly augmented by fresh leaching agent and/or recycled raffinate from one or
more other sol-
vent extractions later in the process/circuit, back to the initial leach
unit(s).
After the first solid-liquid separation and clarification, the second leach
pulp is sent, without
significant dilution, to one or more "final" agitation leach units for
additional leaching, in or-
der to dissolve another significant portion of the desired metal values
remaining in the pulp.
The leach pulp resulting from the "final" leach unit(s) is subjected to a
second solids-liquid
5
CA 02711025 2010-06-29
WO 2009/083204
PCT/EP2008/010979
separation and clarification to produce two products, a second undiluted
aqueous leach solu-
tion, rich in desired metal values, and a third leach pulp. The second
undiluted aqueous leach
solution is circulated, without significant dilution, to one or more "second"
solvent extraction
units for extracting the desired metal values from the aqueous solution into
an organic phase,
with the aqueous solution ("raffinate"), depleted of desired metal values,
exiting the second
solvent extraction unit(s) being recycled as leaching solution, possibly
augmented by fresh
leaching agent and/or recycled raffinate from one or more other solvent
extractions earlier
and/or later in the process/circuit, back to the final agitation leach
unit(s).
to After the second solids-liquid separation and clarification, the third
leach pulp, now largely
depleted of desired metal values after two leachings, is sent to a last solids-
liquid separation,
with water washing and significant dilution for the first time, from which the
washed solids
slurry is sent to disposal, and the clarified aqueous wash solution is sent to
one or more final
solvent extraction units. From the final solvent extraction unit(s), the
aqueous solution (raffi-
nate), depleted of desired metal values, is optionally neutralized and then
either possibly sent
to one of more units to recover any other valuable metal values also present
in the original ore,
or recycled back to the last solid-liquid separation unit(s) as wash solution,
or it may be split,
with some portion sent to recovery of other metal values, and some portion
sent to recycle
back to the last solids-liquid separation unit(s) as wash solution, and
perhaps even some to
final disposal.
In some cases, depending on the grade of ore and the leaching characteristics
of that ore, there
may be one or more additional "intermediate" sub-circuits of leaching/solids-
liquid separa-
tion/solvent extraction steps similar to the initial or first leaching/solids-
liquid separa-
tion/solvent extraction sub-circuit, inserted between the first and "final"
leaching/solids-liquid
separation/solvent extraction sub-circuit.
By following this process and changing the current design and operation of the
leaching and
solvent extraction processes of current metal-recovery plants accordingly, the
amount of
leaching agent recycled to leach may be significantly increased, and both the
amount of
leached metal that is lost to tailings disposal and the quantities of
additional fresh leaching
agent purchased and the quantities of chemicals that must be expended to
neutralize excess
leaching agent in the circuits may be significantly decreased.
Surprisingly it has been observed that It surprisingly has now been found that
further signifi-
cant reductions in leaching agent replacements and neutralizations, as well as
further signifi-
cant increases in the recovery of the desired metal from the original ore, may
be realized over
the "split circuit" arrangement by dividing the duty of leaching the desired
metal values from
6
CA 02711025 2010-06-29
WO 2009/083204
PCT/EP2008/010979
the original crushed and milled ore solids among two or more agitation leach
units in series.
Each of these units then leaches desired metal values from the same ore
solids, with each unit
being followed by its own solids-liquid separator, without significant
dilution, then its own
solvent extraction unit(s), prior to a final solids-liquid separation, with
washing, and a final
solvent extraction on the clarified solution exiting the final solids-liquid
separation, with
washing, to try to recover any final amounts of valuable metal. The metal-
depleted aqueous
solution exiting the final solvent extraction unit is neutralized, as
necessary, and/or circulated
to one or more additional units to possibly recover other metal values that
may also be present
in the original ore, prior to disposal and/or recycle back to the final solid-
liquid separation,
with washing, as wash solution. The metal-depleted aqueous slurry of the
leached solids exit-
ing the final solids-liquid separation, with washing, is then sent to final
disposal, which, in
most cases, includes neutralization. With this new circuit design, raffinates
from each solvent
extraction unit, except the final solvent extraction unit, may be totally
recycled to one or more
of the preceding agitation leach unit(s), and, in doing so, much more leaching
agent is recy-
cled to leaching and, therefore, much less leaching agent is lost to final
disposal as compared
to the conventional and "split circuit" flow sheets. In addition, the desired
metal lost to final
disposal in the leached and washed solids is minimized when compared to either
the conven-
tional or "split circuit" flow sheets.
In particular, it has been found that by not depending on a single initial
leach unit to dissolve
all or mostly all of the desired metal values from the crushed and milled ore
solids at one time,
and breaking the leaching function into two or more units in a series or
sequential arrange-
ment, with accompanying solids-liquid separators, without dilution, and
solvent extraction
units, as described below, the amount of desired metal that may be recovered
and the amount
of leaching agent that may be recycled may be substantially increased.
According to this in-
vention, the crushed and milled ore solids are subjected to a sequence of
leach units, each
leach unit dissolving a portion of the desired metal values from, effectively,
the same crushed
ore solids (the original crushed and milled ore solids in the first agitation
leach unit and pro-
gressively-more-leached solids pulps in subsequent agitation leach units in
the series) with
each such leaching unit being followed by its own solids-liquid separation
without significant
dilution, then one or more solvent extraction units to extract the desired
metal value from the
aqueous leach solution, rich in desired metal values, coming from the
respective solids-liquid
separation unit(s). In addition, all, or almost all, of the aqueous raffinates
regenerated by the
solvent extractions are recycled back to either their respective leach units,
or recycled among
two or more of the previous or following leach units in the circuit, for
additional leaching,
prior to a final solids-liquid separation, with washing. That final separation
is applied to the
crushed and milled leached solids exiting the last solids-liquid separation
without significant
7
CA 02711025 2010-06-29
WO 2009/083204
PCT/EP2008/010979
dilution, and is followed by a final solvent extraction on the clarified leach
solution exiting the
final solids-liquid separation.
To understand the significant benefits of the present invention over the
conventional standard
agitation leaching-solvent extraction operation, as well as over the "Split
Circuit" agitation
leaching-solvent extraction process, and not depend on any particular theory,
involves closely
comparing the flow diagrams and accompanying mass balances in Figures 1
through 3, with
copper as the desired metal and sulphuric acid as the leaching agent, as later
explained in the
Examples.
For example, in any copper agitation leach-solvent extraction recovery
process, all the sul-
phuric acid recycled back to leaching may be used to leach more copper, while
all the acid
taken to neutralization or contained in the tailings is lost, and, therefore,
cannot be used to
leach more copper. The more acid that can be recycled, the less acid that
needs to be pur-
chased, and the less the amount of acid that must be neutralized and/or that
would be lost to
disposal.
In each flow sheet, copper recovery from leaching is set at a realistic 90%
and copper recovery
from solvent extraction is also assumed to be a realistic 90%, even though
copper recovery in
an agitation leaching process can be up to nearly 100% in some cases and
copper recovery
across a copper solvent extraction unit can be more than 90% in some cases.
In one aspect, the instant invention provides a process for recovering metal
values from
crushed and milled ore solids comprising desired metal values that may be
commingled with
one or more other metal values, which process comprises:
(a) mixing a first aqueous leach solution with a body of the crushed and
milled ore solids
in a first agitated tank leach unit in order to dissolve at least a
significant portion of the
desired metal values formerly in the ore solids into the first aqueous leach
solution and
to obtain a first aqueous leach pulp, which pulp comprises a mixture of
leached solids
and first aqueous leach solution, rich in the desired metal values;
(b) subjecting the first aqueous leach pulp to a first solids-liquid
separation, without sig-
nificant water dilution, to provide a first clarified aqueous leach solution
and a second
aqueous leach pulp, which pulp comprises leached solids at a per cent solids
level that
is greater than that in the first aqueous leach pulp;
8
CA 02711025 2010-06-29
WO 2009/083204
PCT/EP2008/010979
(c) sending the second aqueous leach pulp to a final agitated tank leach
unit, and circulat-
ing the first clarified aqueous leach solution to a first solvent extraction,
wherein, in
such solvent extraction, at least a significant portion of the desired metal
values are ex-
tracted into a first organic phase by one or more extraction reagent(s)
specific for the
desired metal, which extraction reagent(s) is/are dissolved in an organic
formulation
that comprises such extraction reagent(s), optionally with one or more
equilibrium
modifiers, kinetic additives and/or other compounds in a water-insoluble,
water-
immiscible organic solvent, creating a first organic phase, rich in the
desired metal as
one or more desired metal-extraction reagent(s) complexes, that is sent to
further metal
recovery processes, and a first aqueous raffinate, depleted of desired metal
values, up
to all of which raffinate may be recycled/circulated back to the first
agitated tank leach
unit as at least a part of the first aqueous leach solution, which solution
may be sup-
plemented by fresh leaching agent and/or one or more other raffinates from
later in the
process;
(d) mixing a second aqueous leach solution with the second aqueous leach
pulp in the fi-
nal agitated tank leach unit in order to dissolve another portion of the
desired metal
values formerly in the partially leached crushed and milled ore solids (now
comprising
the second aqueous leach pulp) into the second aqueous leach solution and to
obtain a
third aqueous leach pulp, which pulp comprises a mixture of twice-leached
solids and
a second aqueous leach solution, rich in desired metal values;
(e) subjecting the third aqueous leach pulp to a second solids-liquid
separation, without
significant water dilution, to provide a second clarified aqueous leach
solution and a
fourth aqueous leach pulp, which pulp comprises leached solids at a per cent
solids
level that is greater than that in the third aqueous leach pulp;
(f) sending the fourth aqueous leach pulp to a third solids-liquid
separation, and circulat-
ing the second clarified aqueous leach solution to a second solvent
extraction, wherein,
in such solvent extraction, at least a significant portion of the desired
metal values are
extracted into a second organic phase by one or more extraction reagent(s)
specific for
the desired metal, which extraction reagent(s) is/are dissolved in an organic
formula-
tion that comprises such extraction reagent(s), optionally with one or more
equilibrium
modifiers, kinetic additives and/or other compounds in a water-insoluble,
water-
immiscible organic solvent, creating a second organic phase, rich in the
desired metal
as one or more desired metal-extraction reagent(s) complexes, that is sent to
further
metal recovery processes, and a second aqueous raffinate, depleted of desired
metal
values, up to all of which raffinate may be recycled/circulated back to the
final agitated
9
CA 02711025 2017-02-02
tank leach unit as at least a part of the second aqueous leach solution, which
solution
may be supplemented by fresh leaching agent and/or one or more other
raffinates from
earlier or later in the process;
(g) subjecting the fourth aqueous leach pulp to a third solids-liquid
separation, with
significant dilution via an aqueous stream, in order to obtain a third
clarified aqueous
leach solution, wherein the concentration of desired metal values in the third
clarified
aqueous leach solution is less than the concentration of desired metal values
in the
second clarified aqueous leach solution, and a fifth aqueous pulp, which pulp
comprises
a mixture of leached solids and aqueous leach solution; and
(h) sending the fifth aqueous pulp to disposal and circulating the third
clarified aqueous
leach solution to a third solvent extraction unit, wherein, in such solvent
extraction, a
third organic phase of water-insoluble, water-immiscible organic solvent
formulation
comprising one or more extraction reagents extract at least a portion of the
desired metal
values from the third clarified aqueous leach solution creating a third
organic phase,
rich in the desired metal as one or more desired metal-extraction reagent(s)
complex(es), that is sent to further metal recovery processes, and a third
aqueous
raffinate, depleted of desired metal values, that is optionally neutralized
and circulated
back to the third solids-liquid separation as at least a part of the aqueous
washing
solution to recover at least a portion of any remaining desired metal values
from the
fifth aqueous pulp, or is optionally neutralized and sent to disposal, or is
optionally
neutralized and treated to recover one or more other metal values, if present
in sufficient
amounts, that may be present in the mined ore solids, or is optionally
neutralized with
portions circulating back to the third solid-liquid separation and/ or to
further metal
recovery and/or to disposal.
In one aspect, the instant invention provides a process for recovering metal
values from
crushed and milled ore solids containing a desired metal as desired metal
values that may be
commingled with one or more other metal values, which process comprises:
(a)
mixing a first aqueous leach solution with a body of the crushed and milled
ore
solids in a first agitated tank leach unit in order to dissolve at least a
significant portion
CA 02711025 2017-02-02
of the desired metal values formerly in the ore solids into the first aqueous
leach solution
and to obtain a first aqueous leach pulp, which first aqueous leach pulp
comprises a
mixture of leached solids and first aqueous leach solution, rich in the
desired metal
values;
(b) subjecting the first aqueous leach pulp to a first solids-liquid
separation, without
significant water dilution, to provide a first clarified aqueous leach
solution and a second
aqueous leach pulp, which second aqueous leach pulp comprises leached solids
at a per
cent solids level that is greater than that in the first aqueous leach pulp;
(c) sending the second aqueous leach pulp to a final agitated tank leach
unit, and
circulating the first clarified aqueous leach solution to a first solvent
extraction,
wherein, in such solvent extraction, at least a significant portion of the
desired metal
values are extracted into a first organic phase by one or more extraction
reagents
specific for the desired metal, which extraction reagent(s) is/are dissolved
in an organic
formulation that comprises such extraction reagent(s), optionally with one or
more
members selected from equilibrium modifiers, kinetic additives and a mixture
of two
or more thereof, in a water-insoluble, water-immiscible organic solvent,
creating said
first organic phase, rich in the desired metal as one or more desired metal-
extraction
reagent(s) complexes, that is sent to further metal recovery processes, and a
first
aqueous raffinate, depleted of desired metal values, up to all of which
raffinate may be
recycled back to the first agitated tank leach unit as at least a part of the
first aqueous
leach solution, which solution may be supplemented by fresh leaching agent,
one or
more other raffinates from later in the process, or a mixture of both;
(d) mixing a second aqueous leach solution with the second aqueous leach
pulp in the
final agitated tank leach unit in order to dissolve another portion of the
desired metal
values formerly in the second aqueous leach pulp into the second aqueous leach
solution
and to obtain a third aqueous leach pulp, which third aqueous leach pulp
comprises a
mixture of twice-leached solids and the second aqueous leach solution, rich in
desired
metal values;
10a
CA 02711025 2017-02-02
= .
(e) subjecting the third aqueous leach pulp to a second solids-liquid
separation,
without significant water dilution, to provide a second clarified aqueous
leach solution
and a fourth aqueous leach pulp, which fourth aqueous leach pulp comprises
leached
solids at a per cent solids level that is greater than that in the third
aqueous leach pulp;
(f) sending the fourth aqueous leach pulp to a third solids-liquid
separation, and
circulating the second clarified aqueous leach solution to a second solvent
extraction,
wherein, in such solvent extraction, at least a significant portion of the
desired metal
values are extracted into a second organic phase by one or more extraction
reagent(s)
specific for the desired metal, which extraction reagent(s) is/are dissolved
in an
organic formulation that comprises such extraction reagent(s), optionally with
one
or more members selected from equilibrium modifiers, kinetic additives, and a
mixture of two or more thereof, in a water-insoluble, water-immiscible organic
solvent, creating said second organic phase, rich in the desired metal as one
or more
desired metal-extraction reagent(s) complexes, that is sent to further metal
recovery
processes, and a second aqueous raffinate, depleted of desired metal values,
up to all
of which raffinate may be recycled/circulated back to the final agitated tank
leach
unit as at least a part of the second aqueous leach solution, which solution
may be
supplemented by fresh leaching agent and/or one or more other raffinates from
earlier or later in the process;
(g) subjecting the fourth aqueous leach pulp to said third solids-liquid
separation,
with significant dilution via an aqueous stream, in order to obtain a third
clarified
aqueous leach solution, wherein the concentration of desired metal values in
the third
clarified aqueous leach solution is less than the concentration of desired
metal values
in the second clarified aqueous leach solution, and a fifth aqueous pulp,
which pulp
comprises a mixture of leached solids and aqueous leach solution; and
(h) sending the fifth aqueous pulp to disposal and circulating the
third clarified aqueous
leach solution to a third solvent extraction, wherein, in such solvent
extraction,
a third organic phase of water-insoluble, water-immiscible organic solvent
formulation comprising one or more extraction reagents extract at least a
portion
10b
CA 02711025 2017-02-02
. . .
of the desired metal values from the third clarified aqueous leach solution
creating a third organic phase, rich in the desired metal as one or more
desired
metal-extraction reagent(s) complex(es), that is sent to further metal
recovery
processes, and a third aqueous raffinate, depleted of desired metal values,
that is
either neutralized and circulated back to the third solids-liquid separation
as at
least a part of the third clarified aqueous leach solution to recover at least
a
portion of any remaining desired metal values from the fifth aqueous pulp,
neutralized and sent to disposal, neutralized and treated to recover one or
more
other metal values, if present in sufficient amounts, that may be present in
the
mined ore solids, or neutralized with portions circulating back to the third
solid-
liquid separation, to further metal recovery, to disposal, or to a combination
of
two or more thereof.
For purposes of clarity, in each instance in this process, when reference is
made to a single
"unit", it should be understood that such "unit" may actually be several units
in parallel or in
series. Specifically, each leaching "unit" may consist of several agitated
leaching tanks in
parallel or in series, and each solvent extraction "unit" may consist of a
single stage or a
multiple number of stages, either extraction only or extraction and stripping
in a typical
arrangement, such as solvent extraction units or stages in parallel or series.
It is also possible
that all of the solvent extraction units are actually just different stages in
a single solvent
extraction plant. Generally, the solvent extraction process is highly flexible
and the particular
arrangement of solvent extraction units or stages for any given leach solution
is done in order
to optimize recovery of the desired metal and to optimize regeneration of the
leaching agent
for recycle.
______________________________________________________________________
10c
CA 02711025 2010-06-29
WO 2009/083204
PCT/EP2008/010979
In the present inventive process, the leached-solids pulp from each solids-
liquid separator,
prior to the final one that does involve a final washing/dilution, becomes the
leachable body of
the following leach unit (the leach unit next in the series/sequence). It
should also be under-
stood that each solids-liquid separation, with or without dilution/washing,
may be conducted
in any manner capable of separating solids from liquids; the method of such
separations is not
critical. For example, solids may be separated from liquids by methods
including, but not
limited to, decantation and/or filtration. In the final solids-liquid
separation, with significant
washing/dilution, according to the invention, counter-current decantation is
preferred, but is
not mandatory.
The term, "significant dilution" or "significant washing/dilution", when used
in the process in
accordance with the instant invention, refers to the addition of a measurable
amount of water
or other aqueous solution. Dilution of any of the clarified leach solutions
prior to circulation
of them to solvent extraction could cause a build-up of the volume of aqueous
phase in one of
the loops, and as such, would be undesirable and could decrease leaching agent
recovery.
Significant dilution of such aqueous leach solution is only used in the
instant process in the
final solids-liquid separation as part of the final solids- liquid separation
wash process to try to
recover the last vestiges of the desired metal values from the pulp prior to
disposal of the me-
tal-depleted ore solids.
Additionally, the solvent extractions in accordance with the processes of the
present invention
may also be carried out in any known manner, wherein aqueous leach solution is
contacted
with an organic phase containing an extraction reagent, specific to the
desired metal. For ex-
ample, these solvent extractions may be carried out using mixer- settler
solvent extraction
units, wherein the organic phase and the aqueous leach solution are vigorously
intermixed in a
mixer, and the resulting dispersion of organic and aqueous is then passed to a
settler where the
two phases settle, and from which there exits a clear organic phase and a
clear aqueous phase.
Also, the "further metal recovery processes" to which the organic phases, rich
in the desired
metal values, may be subjected might comprise additional metal extraction
followed by wash-
ing with a solution designed to remove undesirable species prior to contacting
the organic
phase, rich in desired metal values, with a suitable stripping agent that
breaks apart the desired
metal-extraction reagent complex and allows passage of the desired metal into
an aqueous
phase containing the desired metal in a concentrated and purified state from
which final metal
recovery takes place by electrowinning, or one or more other final metal
recovery methods.
With certain metals is may also be possible to recover the desired metal
directly from the or-
ganic phase, rich in desired metal values, even though this is not a common
technique.
11
CA 02711025 2010-06-29
WO 2009/083204
PCT/EP2008/010979
And all solutions, phases, raffinates, and pulps may be conveyed within the
circuits of the
process by pipes or any other natural or man-made conduit.
The process according to the instant invention may be practiced in a new plant
designed spe-
cifically for the instant invention, or it may be practiced in an existing
plant by reconfiguring
existing equipment, and pulp and solution flows, without necessarily adding a
great deal of
handling and/or process equipment.
In a preferred application of the process according to the instant invention,
a majority of the
desired metal values in the mined ore is intended to be leached from the
crushed and milled
ore solids in an initial leach unit, at least a majority of the desired metal
values remaining in
the solids pulp from the solids-liquid separator following the initial leach
unit is leached in the
next leach unit, and, in embodiments of the instant process comprising more
than two leach
units, the number of such units being limited by the economics of diminishing
returns, the
desired metal values in the mined ore are leached in sequential leach units
progressively from
a majority in the initial leach unit in the total circuit, a majority of the
metal values remaining
being leached in the next leach unit, and so on, until the last reasonably-
recoverable amount of
the remaining desired metal values from the original crushed and milled ore
solids are leached
by the final leach unit in the process/circuit.
For example, in a preferred application of the instant process, which
comprises leaching with
two leach units, 60 to 75% of the desired metal values in the original ore
might be preferably
leached in the first leach unit, and the remaining 25 to 40% of such desired
metal values
would then be leached in the second leach unit. In a preferred application of
the instant proc-
ess, which comprises leaching the desired metal values with three leach units,
45 to 55% of
such desired metal values in the original ore might be preferably leached in
the first leach unit,
25 to 35% of such desired metal values might be preferably leached in the
second leach unit,
and the remaining 10-30% of such desired metal values might preferably be
leached in the
third leach unit. In a particularly preferred aspect of the process according
to the instant inven-
tion, at least a majority of the original or remaining desired metal values
from the ore solids or
pulp, as the case may be, is removed in the initial and each successive leach
unit-solvent ex-
traction unit combination, in order to maximize the regeneration of the
leaching agent during
the process and thereby maximize the recycling of such leaching agent to the
leaching process.
This sequential leaching practice generally results in the concentration of
desired metal in the
first clarified aqueous leach solution being at least 30% greater than the
concentration of the
desired metal in the second clarified aqueous leach solution, preferably this
difference is at
=
12
CA 02711025 2010-06-29
WO 2009/083204
PCT/EP2008/010979
least 50%, more preferably this difference is at least 70%, and still more
preferably this differ-
ence is 100%.
In another embodiment of the instant invention, one or more intermediate
agitation tank leach
units may be inserted after the first solid-liquid separation and before the
final agitated tank
leach unit in step (c), such intermediate agitated tank leach unit sending an
aqueous leach
pulp, resulting from an aqueous leach solution being distributed through an
aqueous leach
pulp coming from the first solids-liquid separation, to an intermediate solids-
liquid separation,
from which an intermediate aqueous leach pulp is sent to the final agitated
tank leach unit, and
to an intermediate clarified aqueous leach solution is circulated to an
intermediate solvent ex-
traction, from which an intermediate aqueous raffinate up to all of which
raffinate may be
recycled/circulated back to the intermediate agitated tank leach unit as at
least a part of the
aqueous leach solution for such leach unit, which solution may be supplemented
by fresh
leaching agent, and an intermediate desired metal-rich organic phase, rich in
desired metal
values, is sent to further metal recovery processes. It being readily
understood by the skilled
practitioner that the number of additional such sub-circuits would be
determined by economic
practicality, i.e., the capital and operating cost of the sub-circuit will be
measured against the
diminishing returns that may be realized in further recovery of desired metal
and reduction in
leaching agent and neutralization substances.
For purposes of illustration, one such additional sub-circuit may comprise a
leach unit, la-
belled an "intermediate agitation leach unit", a solids-liquid separator
labelled an "intermedi-
ate solids-liquid separation", following this agitation leach unit, and a
solvent extraction fol-
lowing the intermediate solids-liquid separation, this solvent extraction
labelled an "interme-
diate solvent extraction". Such an intermediate agitation leach unit might be
inserted after the
first solids-liquid separation and before the final agitated tank leach unit
in step (c) in the
process described above, such intermediate agitation leach unit sending an
aqueous leach
pulp, resulting from an aqueous leach solution being distributed through an
aqueous leach
pulp coming from the first solids-liquid separation, to an intermediate solids-
liquid separation,
from which an intermediate aqueous leach pulp is sent to the next agitation
leach unit, and an
intermediate clarified aqueous leach solution is circulated to an intermediate
solvent extrac-
tion, from which exits an intermediate aqueous raffinate, up to all of which
raffinate may be
recycled/circulated back to the intermediate leach unit, or an earlier or
later agitation leach
unit, as at least a part of the aqueous leach solution for such leach unit,
which solution may be
supplemented by fresh leaching agent, and an intermediate organic phase, rich
in the desired
metal as a desired metal-extraction reagent(s) complex(es), is sent to further
metal recovery
processing.
13
CA 02711025 2010-06-29
WO 2009/083204
PCT/EP2008/010979
The process according to the instant invention may be used in any metal
recovery operation
which employs an aqueous agitation leaching operation, where the leaching
agent is regener-
ated in the solvent extraction process, and essentially with any leaching
agent that is water-
miscible, capable of leaching the desired metal from the mined ore into the
desired metal
leaching solution. Such leaching agents include, but are not limited to acids,
including sul-
phuric acid, hydrochloric acid, nitric acid, organic acids, and combinations
of two or more
thereof, and basic substances, including gaseous ammonia and ammonium
hydroxide. In
certain preferred embodiments of the present invention, the leaching agent is
sulphuric acid,
resulting in each aqueous leach solution, i.e., the first aqueous leach
solution, the second
aqueous leach solution, the third aqueous solution, any intermediate aqueous
leach solution,
and so on, as well as each raffinate, i.e., the first aqueous raffinate, the
second aqueous raffi-
nate, the third aqueous raffinate, and so on, being sulphuric acid solutions.
In other certain
preferred embodiments of the instant invention, the preferred leaching agent
is gaseous am-
monia or ammonium hydroxide, resulting in each of the leach solutions, i.e.,
the first aqueous
leach solution, the second aqueous leach solution, the third aqueous solution,
any intermediate
aqueous leach solution, and so on, as well as each raffinate, i.e., the first
aqueous raffinate, the
second aqueous raffinate, the third aqueous raffinate, and so on, being
ammonia/ammonium
hydroxide solutions.
The process of the invention is preferably used in the leaching and solvent
extraction of de-
sired metals that occur naturally as oxide and/or sulphide ores, preferably in
the leaching and
solvent extraction of divalent metals, such as copper, zinc, nickel and
cobalt, and including,
for example, transition metals. In a preferred embodiment of the invention,
the desired metal
is copper, and, particularly, when the desired metal is copper, the preferred
leaching agent is
sulphuric acid. In another preferred embodiment of the invention, the desired
metal is copper,
and the preferred leaching agent is gaseous ammonia or ammonium hydroxide. In
still an-
other preferred embodiment, the desired metal is zinc, and particularly, when
the desired
metal is zinc, the leaching agent is sulphuric acid or gaseous ammonia or
ammonium hydrox-
ide. In yet another preferred embodiment of the invention, the desired metal
is nickel, and,
particularly, when the desired metal is nickel, the preferred leaching agent
is sulphuric acid or
gaseous ammonia or ammonium hydroxide. In another preferred embodiment of the
inven-
tion, the desired metal is cobalt and the preferred leaching agent is
sulphuric acid.
The aqueous raffinate from each solvent extraction process is generally
recycled back to the
leach unit from which the clarified aqueous leach solution that was circulated
to that vessel
originated most recently in order to leach more desired metal from the crushed
and milled ore
solids or a subsequent leach pulp. However, a portion of the first aqueous
raffinate, a portion
of the second aqueous raffinate, a portion of the third aqueous raffinate, a
portion of any in-
14
CA 02711025 2015-08-21
termediate aqueous raffinate(s), or a mixture of two or more thereof, may be
circulated to any
of the leach units and/or the third solids-liquid separator in the process
according to the pre-
sent invention if needed to maintain a water balance or to more efficiently
distribute leaching
agent.
The first aqueous raffinate produced in accordance with the processes of the
present invention
will generally have a leaching agent concentration which is greater than the
concentration of
leaching agent present in the second aqueous raffinate, the second aqueous
raffinate produced
in accordance with the processes of the present invention will generally have
a leaching agent
concentration which is greater than the concentration of leaching agent
present in the third
aqueous raffinate, and so on, with the aqueous raffinate from a solvent
extraction component
of any additional "intermediate" sub-circuit in accordance with the processes
of the present
invention generally having a leaching agent concentration which is greater
than the concentra-
tion of leaching agent present in the aqueous raffinate from the solvent
extraction vessel next
following in the instant process. In preferred embodiments of the present
invention, the first
aqueous raffinate will have a leaching agent concentration which is at least
10% greater than
the concentration of leaching agent present in the second aqueous raffinate,
while in increas-
ingly more preferred embodiments of the present invention, the first aqueous
raffinate will
have a leaching agent concentration which is at least 20% greater, preferably
at least 50%
greater, more preferably at least 75% greater and most preferably 100%
greater. Such differ-
entials of concentrations of leaching agents in second aqueous raffinates over
the concentra-
tion of leaching agent in the third aqueous raffinate are similar to those
between the first and
second aqueous raffinates. The concentration of leaching agent in any
intermediate aqueous
raffinate over the concentration of leaching agent is the aqueous raffinate
from the next-
following solvent extraction vessel in the present process are also similar to
those between the
first and second aqueous raffinates. The aqueous stream for diluting the
fourth aqueous leach
pulp (step (g)) is normally the raffinate from the final solvent extraction
process, optionally
with neutralization or optionally following metal recovery of a second
valuable metal value,
but it may comprise fresh water introduced into the process and/or a portion
of other aqueous
process streams to maintain a water balance. Where the leaching agent
comprises an acid, any
of the aqueous raffinate(s) may be at least partly neutralized (e.g., to any
pH up to about 8)
with any basic substance (e.g., lime when the leaching agent is sulphuric
acid) prior to its use
for diluting the fourth aqueous leach pulp in the third solid-liquid
separation.
While the invention has been described in connection with specific embodiments
thereof, it
will be understood that the scope of the claims should not be limited by the
preferred
embodiments set forth in the examples, but should be given the broadest
interpretation
consistent with the description as a whole.
CA 02711025 2015-08-21
A computer simulation, based on mass balance principles and using iterative
Excel spread-
sheets, was run to compare the economics of the conventional leaching and
solvent extraction
circuits in widespread use (illustrated in Figure I), against the economics of
the split circuit
configuration of the leaching and solvent extraction stages currently in use
in some plants (il-
lustrated in Figure 2), and against the economics of the simplest
configuration of the instant
invention (illustrated in Figure 3). In this non-limiting simulation, copper
was used as the
desired metal to be recovered, sulphuric acid was used as the leaching agent,
and all numbers
expressing quantities or concentrations are to be understood as approximations
(to be under-
stood, where not already present, as modified by "about"), not
representations, for comparison
purposes only, and affected by the ore grade, the water content of the crushed
ore solids, the
metal recovery achieved in leaching, the desired pulp density in leaching,
desired wash ratios
and thickener flow densities achievable in a CCD solid-liquid separation, the
response of the
leached solids to solid-liquid separation, the total flow of leach solution to
be treated, the de-
sign of the solvent extraction process, and other parameters determined by the
plant operators.
The simulation is intended to be illustrative of the instant invention's
advantages, but should
not be interpreted to limit the scope of the current invention in any way. For
purposes of the
simulation, the following conditions were used:
Table 1
Case Study Basis
Ore treated (tons per day) 8,700
Ore grade (% Cu) 3.5
Ore Specific Gravity 2.8
Pre-leach thickener U/F (% solids) 55
% Solids in Leach 22
% Recovery in Leach 90
All CCD thickener U/F (% solids) 50
Number of CCD stages 6
Wash ratio in CCD 2:1
Copper recovery in each SX unit (%) 90
The economic benefits of the Sequential Circuit flow sheet relative to both
the Split Circuit
flow sheet and the conventional flow sheet are detailed in Table 2.
16
CA 02711025 2010-06-29
WO 2009/083204
PCT/EP2008/010979
Brief description of the figures
o Figure 1 is a flow diagram, containing pertinent components of the mass
balance for
the circuit, representing the flows in a standard conventional agitation
leaching-solvent
extraction flow sheet, wherein all of the aqueous leach solution is treated in
the same
manner.
o Figure 2 is a flow diagram, with pertinent mass balance values,
representing a "Split
Circuit" flow sheet, wherein an aqueous leach solution is divided into two
portions-
one portion without significant dilution and the other with water dilution-
prior to be-
ing subjected to solvent extraction.
o Figure 3 is a flow diagram, with pertinent mass balance values,
representing an em-
bodiment of the "Sequential Circuit" flow sheet according to the present
invention.
Optional units/operations are shown in dashed lines, with a cobalt recovery
unit representing
one or more units for recovering other metal values that may be present in
sufficient quantities
in the incoming ore.
17
CA 02711025 2010-06-29
WO 2009/083204
PCT/EP2008/010979
Examples
Comparative Example A
Comparative Example A is based on Figure 1, which depicts a process flow
diagram of a stan-
dard conventional copper agitation leaching and solvent extraction circuit,
with pertinent mass
balance numbers included for aqueous flows, copper concentrations and acid
concentrations.
The leach pulp exiting the leach unit/train ("LEACH"), consisting of about
1224 cubic me-
ters/hour of aqueous leach solution, comprising 9.92 g/1 of copper and 2.0 g/1
of sulphuric
acid, and about 362.5 tonnes/hour of crushed and milled ore less the mass
leached, is
mixed/washed, in a counter-current decantation ("CCD"), with about 622 cubic
meters/hour
of recycled aqueous raffinate from the copper solvent extraction unit/train
("SX1"). For mod-
elling purposes, the 622 cubic meters/hour of raffinate containing 0.80 g/1 Cu
was assumed to
be neutralized to contain 2 g/1 sulphuric acid before addition to the CCD
circuit as wash solu-
tion, thus diluting the copper concentration of the aqueous leach solution
exiting the CCD
circuit from about 9.92 g/1 copper to about 8.05 g/1 copper prior to this
solution being fed to
the solvent extraction. An aqueous leach solution obtained from the CCD of
about 1535 cubic
meters/hour, comprising 8.05 g/1 copper and 2.0 g/1 sulphuric acid, is
circulated to SX1, and
an aqueous raffinate, comprising 0.80 g/1 copper and 13.2 g/1 sulphuric acid,
exiting from the
SX1, is split, with about 913 cubic meters/hour being recycled back to the
leaching operation,
where the acid is used to dissolve more copper, and about 622 cubic
meters/hour that is recy-
cled to neutralization and then to the CCD. The about 622 cubic meters/hour of
raffinate,
which is recycled to the CCD operation, is used to wash the leach solution
from the leached
solids, in order to minimize soluble metal losses in the aqueous phase portion
of the leached
pulp that is eventually disposed to tailings. A small portion of fresh water
may be added to
the overall leach/wash system or a small portion of aqueous solution may be
bled from the
overall leach/wash system to maintain a water balance.
In Comparative Example A, 913 cubic meters per hour of raffinate containing
13.2 g/1 sul-
phuric acid would be returned to leaching, about 622 cubic meters per hour of
raffinate con-
taining 13.2 g/1 sulphuric acid is neutralized to 2.0 g/1 sulphuric acid prior
to recycle back to
the CCD circuit, and about 311 cubic meters per hour of aqueous solution
containing 0.91 g/1
copper is lost in tailings.
18
CA 02711025 2010-06-29
WO 2009/083204
PCT/EP2008/010979
Comparative Example B
Comparative Example B is based on Figure 2, which depicts a process flow
diagram of a
"Split Circuit" copper leaching and solvent extraction system, with pertinent
mass balance
numbers included for aqueous flows, copper concentrations and acid
concentrations. The
leach pulp exiting the leach unit/train ("LEACH"), consisting of about 1224
cubic meters/hour
of aqueous leach solution, comprising 9.92 g/1 of copper and 2.0 g/1 of
sulphuric acid, and
about 362.5 tonnes/hour of crushed and milled ore, less the mass leached, is
passed to an ini-
tial solids-liquid separation (S/L), comprising a clarifier using decantation.
Then about 913
113 cubic meters/hour of this solution, containing about 10.07 g/1 Cu and
about 2.0 g/1 sulphuric
acid, is taken directly to solvent extraction (SX 1), where the copper is
extracted and sulphuric
acid is regenerated. SX 1 will reasonably produce a raffinate containing about
1.01 g/1 copper
and about 15.95 g/1 sulphuric acid, which solution is then recycled back to
leaching. The
leach pulp exiting the initial solid-liquid separation, which contains about
311 cubic me-
ters/hour of leach solution, is taken to a counter-current decantation wash
circuit (CCD) where
it is mixed with about 622 cubic meters/hour of raffinate from SX 2 that has
been, optionally,
partially neutralized to 2.0 g/1 sulphuric acid. About 622 cubic meters/hour
of leach solution
from the CCD circuit, comprising 5.21 g/1 copper and 2.0 g/1 sulphuric acid,
is taken to SX 2
to give a raffinate containing 0.52 g/1 copper and 9.2 g/1 sulphuric acid. A
small portion of
fresh water may be added to the overall leach/wash system or a small portion
of aqueous solu-
tion may be bled from the overall leach/wash system to maintain a water
balance.
In Comparative Example B, 913 cubic meters/hour of raffinate containing 15.95
g/1 sulphuric
acid is returned to leaching, about 622 cubic meters/hour of raffinate from SX
2 containing
9.2 g/1 acid is neutralized to 2.0 g/1 sulphuric acid and about 311 cubic
meters per hour of
aqueous solution containing 0.67 g/L copper is lost to Tails.
The amount of acid in any aqueous stream at a particular time is the stream
flow at that time
multiplied by the acid concentration in the stream. A simple calculation shows
that for this
particular case about 2.51 more metric tons of acid/hour, or about 60.3 more
metric tons of
acid/day, is recycled to leaching using the "Split Circuit" flow sheet of
Comparative Example
B over the standard conventional flow sheet of Comparative Example A. Acid
costs vary
widely from, currently, about US$60/ton to above US$250/ton depending on the
location. For
low-cost acid, the savings in acid using the "Split Circuit" flow sheet
instead of the conven-
tional standard flow sheet would be about US$3618/day, while for high-cost
acid, the savings
in acid would be about US$15,075/day or greater.
19
CA 02711025 2010-06-29
WO 2009/083204
PCT/EP2008/010979
A second simple calculation shows that for these Comparative Examples, about
2.49 less met-
ric tonnes of acid per hour, or about 59.8 less metric tonnes acid per day,
are neutralized using
the "Split Circuit" of Comparative Example B over the standard conventional
circuit of Com-
parative Example A. This is well within rounding error, since the greater
amount of acid re-
cycled to leaching using the "Split Circuit" flow sheet of Comparative Example
B, over the
conventional circuit flow sheet of Comparative Example A, should equal the
lesser amount of
acid that is neutralized using the "Split Circuit" flow sheet of Comparative
Example B com-
pared to the acid that is neutralized using the conventional circuit flow
sheet of Comparative
Example A.
Savings in neutralization can vary widely, depending on the cost of the
neutralizing agent (ty-
pically lime or limestone), the capital required to build a larger
neutralization plant and the
cost to dispose of the greater amount of gypsum formed.
The amount of copper in any aqueous stream at a particular time is the stream
flow at that
time multiplied by the copper concentration in the stream. A third simple
calculation shows
that the total copper recovered using the "Split Circuit" flow sheet of
Comparative Example B
is greater than the total copper recovered using the standard conventional
flow sheet of Com-
parative Example A by 74.64 kilograms/hour or about 1.79 tonnes/day. At a
copper price of,
currently, US$2.50/pound, this additional copper has a value of US$9,866/day
or about
US $3.55M annually.
Example 1
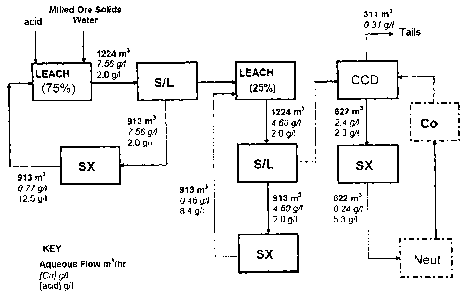
Example 1 illustrating the present invention is based on Figure 3, which
depicts a process
flow diagram of a simple example of a copper leaching and solvent extraction
process accord-
ing to the instant invention (denominated "Sequential Circuit"), with
pertinent mass balance
numbers included for aqueous flows, copper concentrations and acid
concentrations. In this
Example 1, a leach pulp consisting of about 1224 cubic meters/hour of aqueous
leach solu-
tion, comprising 7.56 g/1 of copper and 2.0 g/1 of sulphuric acid, and about
362.5 tonnes/hour
of crushed and milled ore less the mass leached, flows directly without
dilution from this first
leach unit (Leach 1), where about 75% of the copper from the crushed, mined
ore has been
dissolved into an aqueous acidic leach solution, to a solid-liquid separator
(S/L1). From sepa-
rator S/L1 about 913 cubic meters/hour of an aqueous leach solution,
comprising 7.56 g/1
copper and 2.0 g/1 sulphuric acid, is circulated to a solvent extraction
unit/train (SX1), and a
pulp, consisting of about 311 cubic meters/hour of leach solution and about
362.5 tonnes/hour
of partially leached crushed and milled ore less the small mass of ore leached
in Leach 1, is
CA 02711025 2010-06-29
WO 2009/083204
PCT/EP2008/010979
sent to a second leach unit. The entire 913 cubic meters/hour of metal-
depleted aqueous leach
solution, comprising 12.5 g/1 sulphuric acid and 0.77 g/1 copper, exiting SX1
is recycled to
LEACH 1 where the acid contained in this first raffinate is used to leach
copper from more
fresh crushed and milled ore solids.
The remaining amount of copper in the partially leached crushed and milled ore
solids exiting
Leach 1, (25% of the original amount in the crushed and milled ore solids
entering Leach 1) is
then dissolved from the solids in LEACH 2, from which 1224 cubic meters/hour
of aqueous
leach solution, comprising 4.6g/1 copper and 2.0 g/1 sulphuric acid, and about
362.5 ton-
nes/hour of solids less the total mass leached, is sent to another solid-
liquid separator (S/L2),
again, without dilution. About 913 g/1 of a second aqueous leach solution,
comprising about
4.6 g/1 copper and 2.0 g/1 sulphuric acid emerges from S/L2 and is circulated
to a second sol-
vent extraction unit (SX2), and a pulp, consisting of about 331 cubic
meters/hour of leach
solution and about 362.5 tonnes/hour of almost totally leached solids less the
mass leached, is
sent to a third solid-liquid separator (CCD) where the pulp is diluted and
washed The entire
913 cubic meters/hour of metal-depleted aqueous leach solution, comprising 8.4
g/1 sulphuric
acid and 0.46 g/1 copper, are recycled from SX2 to LEACH 2 where the acid
contained in this
second raffinate is used to leach copper from the partially leached crushed
and milled ore sol-
ids entering Leach 2 from S/L1.
Exiting the CCD wash process is a pulp, consisting of 311 cubic meters/hour of
aqueous solu-
tion, comprising 0.31 g/1 copper and 2 g/1 sulphuric acid, and 362.5
tonnes/hour of almost
totally leached solids, which is sent to tails and 622 cubic meters/hour of a
third aqueous leach
solution, comprising 2.4 g/1 copper and 2.0 g/1 sulphuric acid, which is sent
to a final solvent
extraction unit (SX3). From 5X3, 622 cubic meters/hour of metal depleted
aqueous leach
solution, comprising 0.24 g/1 copper and 5.3 g/1 sulphuric acid, emerge as an
aqueous raffinate
for possible neutralization ("Neut") of excess acid before possible recovery
of additional
metal ("Co"), and recycle to the CCD as wash solution or supplemental wash
solution.
In Example 1, about 913 cubic meters/hour of raffinate containing 12.5 g/1
acid and about 913
cubic meters/hour of raffinate containing 8.4 g/1 acid are returned to Leach 1
and 2, respec-
tively. About 622 cubic meters/hour raffinate from SX 3 containing 5.3 g/1
acid is neutralized
to 2 g/1 acid and about 311 cubic meters/hour of aqueous solution containing
0.31 g/1 copper is
lost to tailings
21
CA 02711025 2010-06-29
WO 2009/083204
PCT/EP2008/010979
Example 3
Economic benefit calculation
Table 2:
Economic Benefits of the Sequential Circuit and Split Circuit Flowsheets over
the Conventional Flowsheet in a
One Year Period of Time
Conventional Split Circuit Sequential Circuit
Operating days per year 360 360 360
Neutralization Cost ($/ton acid) 200 200 200
Cu price ($/lb) 2.50 2.50 2.50
Acid to neutralization (MT / day) 167.2 107.5 49.7
Neutralization cost ($ million/yr) 12.04 7.74 3.58
Benefit ($ million/yr) 4.30 (A) 8.46 (A)
Cu soluble loss (ton / day) 6.80 5.00 2.31
Revenue loss ($ million/yr) 13.45 9.90 4.57
Benefit ($ million/yr) 3.55 (B) 8.88 (B)
Total benefit ($ million/yr) 7.85 (A+B)
17.34 (A+B)
Calculation of the financial savings the invention offers when compared to the
conventional
flow sheet and the split circuit flow sheet are based on three advantages of
the invention: the
total greater amount of leaching agent recycled to Leach 1 and Leach 2, the
decrease in acid
neutralized and the decrease in the concentration of the desired metal in the
aqueous solution
leaving the circuit to the tailings with the washed leach pulp exiting the CCD
wash circuit
(called the "copper soluble loss").
In Table 2, the neutralization cost/year is calculated by multiplying the acid
neutralized/day by
the cost of neutralization times 360 days/year. The acid neutralized/day is
the flow of the re-
spective steam being neutralized multiplied by the g/1 acid neutralized, for
example, the acid
neutralized/day for the conventional circuit is [622 cubic meters/hour times
(13.2 g/1 acid - 2
g/1 acid) times 24 = 167.21. The cost of neutralization, assumed to be US$
200/tonne acid, is
the total cost of the acid plus the cost of the base needed to neutralize the
acid plus a small
operating cost. A neutralization cost of US$ 200 tonne acid is used for this
example and such
cost is reasonable for neutralization and well within the range of today's
costs for neutraliza-
tion. The benefit of the acid savings on an annual basis for the Sequential
Circuit flow sheet
according to the instant invention over the Split Circuit flow sheet and over
the conventional
standard flow sheet is calculated from the difference in the neutralization
cost for each of the
three flow sheets.
22
CA 02711025 2010-06-29
WO 2009/083204
PCT/EP2008/010979
Also in Table 2, the benefit on an annual basis associated with the lower
soluble copper loss
offered by the by the Sequential Circuit flow sheet according to this instant
invention over the
"Split Circuit" flow sheet and over the conventional standard flow sheet is
determined from
the differences in the economic value of the soluble copper lost on an annual
basis (the con-
centration of the copper in the respective streams exiting the CCD wash
circuit to Tails times
the flow calculated on an annual basis times the copper price) for each of the
three flow
sheets.
From Table 2 it can be seen that the use of the Sequential Circuit flow sheet
according to the
instant invention offers an annual savings of US$17.34 million over the
conventional leach-
ing-solvent extraction flow sheet and a savings of US$ 9.49 million over the
use of the Split
Circuit flow sheet (which Split Circuit flow sheet has shown an annual savings
US$7.85 mil-
lion over the conventional flow sheet).
Clearly, the use of the process according to the present invention would
result in much more
leaching agent (in these Examples, acid) being returned to additional leaching
than would be
recycled with the conventional standard flow sheet or with the split circuit
flow sheet. Also
clearly the use of the process according to the instant invention would result
in more copper
being produced, and less copper lost as soluble copper, when compared to the
conventional
standard flow sheet and the split circuit flow sheet.
Since the amount of ore and copper content of the ore being treated is the
same for the Con-
ventional Circuit (Comparative Example A), the Split Circuit (Comparative
Example B) and
the Sequential Circuit according to this invention (Example 1), a direct and
valid comparison
can be made for the amount of acid neutralized and the soluble copper loss
using each flow
sheet.
The values calculated in Table 2 are both realistic and reasonable considering
that, in Decem-
ber, 2007; the price of acid varies between US$ 60/tonne to over 250/tonne,
depending on
location and logistics, with most acid prices well above the low figure of US$
60/tonne. Also
at the present time the price of copper is about US$ 3.00/pound.
No matter what values are used to calculate the annual total benefit in US$,
some benefit in
less acid neutralized and more copper produced is always present for the
Sequential Circuit
flow sheet according to this instant invention over the Split Circuit flow
sheet and the conven-
tional standard flow sheet.
23
CA 02711025 2010-06-29
WO 2009/083204
PCT/EP2008/010979
In addition to the benefits of more acid recycled to leaching, less acid being
neutralized and
less copper being lost as soluble copper in the pulp exiting the CCD process,
a fourth advan-
tage of the Sequential Circuit flow sheet pertains to the leaching efficiency.
When the leach-
ing process is divided into a first leach, where a majority of the copper is
leached, and a sec-
ond leach, where the remainder of the copper is leached, the copper
concentration in the aque-
ous phase in contact with the almost-totally-leached, crushed and milled ore
solids in the last
leach unit is considerably lower (4.60 g/1 Cu) than when all the leaching is
done in one leach-
ing unit/train (10.07 g/1 Cu in the case of the split circuit flow sheet and
9.92 g/1 Cu in the case
of the standard conventional flow sheet). A lower copper concentration in the
leach solution
in contact with the final leached solids should allow a very slightly higher
overall leach recov-
ery because the diffusion of leached copper from the pores in the ore
particles is faster. In
addition, the acid to leach can be better controlled and thus made more
efficient.
A fifth benefit may occur in those cases where the acid in the stream being
recycled to the
CCD wash process does not need to be neutralized, but the bleed of this stream
from which a
component of value in the bleed is recovered, for example cobalt, must be
neutralized prior to
cobalt recovery. Neutralization with a soluble base, such as caustic or
ammonia, is very ex-
pensive, thus the lower the acid content of the bleed stream, the lower the
amount of expen-
sive base needed for neutralization. Furthermore, the use of a solution of
caustic for neutrali-
zation adds water to the bleed stream, thereby diluting the valuable cobalt
stream. Alterna-
tively, neutralization can take place with lime or limestone, which is a less
costly base. In this
case, a lesser amount of acid in the bleed stream requires less lime or
limestone for neutraliza-
tion, and in the process, a lesser amount of gypsum precipitate, that must be
removed from the
system, is produced. A lesser amount of gypsum allows the use of smaller
equipment for this
particular solid-liquid separation. Since, when finely-divided solids
separated from a liquid,
the solids will always contain some of the liquid, a lesser amount of gypsum
will contain a
lower volume of the neutralized bleed stream that contains the valuable second
component,
for example, cobalt. Thus, the ultimate recovery of the secondary valuable
component in the
bleed stream is higher when using the process according to the invention.
24