Note: Descriptions are shown in the official language in which they were submitted.
CA 02712215 2010-08-05
Docket 6570
METHOD AND APPARATUS FOR REMOVING CONTAMINANTS FROM
INDUSTRIAL PROCESSING PLANTS
Ove Lars Jepsen
Peter T. Paone III
John S. Salmento
Background of the Invention
There is an increasing level of awareness concerning the emission of mercury
and other
volatile metals such as cadmium and thallium, certain volatile organic
compounds
(VOCs) and dioxin/furans from industrial plants such as cement manufacturing
facilities,
Cement plants, for example, have a wide range of mercury inputs and resulting
emissions
because of the wide variety of raw materials and fuels used in the process.
Consequently, there is an interest in developing cost effective options for
controlling
these emissions, and that is an object of the present invention.
Brief Description of the Invention
This invention is a method of removing mercury and other contaminants from
mineral
processing systems such as cement or lime kiln systems or other industrial
plants such as
power plants.
According to the invention, there is method for the continuous removal of
mercury from
an industrial plant utilizing a cement or lime kiln that has a mercury laden
particulate
byproduct such as kiln dust or, in the case of an industrial boiler, fly ash
which comprises
using existing hot gas streams to vaporize mercury compounds in the
particulate. The
cleaned particulate can be reused in an industrial process, while the
vaporized mercury
compounds are removed in a second collector using additional sorbents or
chemical
reagents if necessary. In one embodiment of the present invention, there is
described a
method to continuously remove mercury from a industrial plant, in particular a
cement
plant, that only has to process a fraction of the gas stream going to the
plant's main stack
to thereby reduce capital and operating costs. By providing a continuous
mercury
removal, the system reduces the variability of the mercury concentration in
the process
1
CA 02712215 2010-08-05
Docket 6570
gas stream so the system does not have to be designed to handle a spike in
mercury
emissions immediately following a raw mill shutdown. However, this invention
is not
limited to cement plants or plants with preheater towers. It can be used on
any industrial
processing plant where recirculation of the volatile metals, VOC's, or
dioxin/furans
occurs between the dust collector before the stack and the section of the
processing plant
where the materials are heated, for example long dry cement kilns, long wet
cement kilns,
lime kilns and a power plant's coal fired boiler.
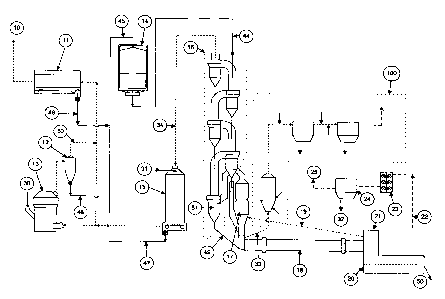
Description of the Drawings
Figure 1 is a general diagram of a plant for the production of cement clinker
adapted to
the continuous cleaning of particulate material of mercury and other
contaminants.
Figure 2 shows an enlarged partial diagram of the contaminant cleansing zone
100
portion of Figure 1.
Description of the Invention
Although the invention is particularly directed to the removal of mercury it
should be
understood that the present invention also applies to the removal of other
volatile metals,
VOC's and dioxin/furans that contaminate manufacturing processes. Also, while
emphasis is placed on a cement manufacturing process, it is understand that
the invention
of the present invention is applicable to other kiln manufacturing processes,
such as a
lime manufacturing process and other industrial processes such as a power
plant.
Mercury typically enters an industrial process, such as a cement kiln process,
in raw
materials and fuels. In cement processes the mercury enters in very low
concentrations.
Due to the phase properties of mercury and mercury compounds, very little
mercury exits
with the cement clinker product. Most of the mercury re-circulates in the
process between
the raw mill, main kiln filter and the preheater tower. The mercury compounds
vaporize
in the preheater tower and travel in the gas stream to the raw mill and main
kiln filter.
When the raw mill is running a high percentage of the mercury in the gas
stream is
2
CA 02712215 2010-08-05
Docket 6570
captured by the raw meal. The captured mercury is disproportionably
concentrated in the
kiln dust in the dust filter after the raw mill. Since very little mercury
leaves with the
clinker or exits the stack when the raw mill is running, the concentration of
mercury
increases in the kiln feed, kiln dust, conditioning tower dust, raw mill
cyclone dust,
downcomer dust, downcomer gas stream, and gas streams in the mid to upper
stages of
the preheater tower to many times the levels found in the original raw
materials. When
the raw mill is shut down, the mercury emissions from the main stack increase
dramatically as the built up mercury is purged from the system. Removing some
or all
kiln dust from the kiln system may assist is reducing the mercury emissions in
the main
stack. However, it may not remove enough mercury, or may not be practical for
economic reasons (costs associated with disposing of dust) or environmental
reasons
(mercury leaching from the kiln dust).
In the present invention mercury containing particulate material is directed
through a
reaction area, which may simply be a duct or an optional reactor vessel. The
source of
the particulate material can be the kiln dust removed from the main kiln
filter, raw mill
cyclone dust, conditioning tower dust, kiln feed, raw material component(s),
from a
external source such as fly ash from a power plant, or any combination of the
these
sources. Within the reaction area the mercury containing particulate material
is dispersed
within a hot gas stream, which may also contain either gaseous mercury or
mercury laden
dust. The hot gas is provided from a hot gas source such as a kiln gas bypass
or other
sources such as a stage of the preheater, the downcomer, the calciner, the
tertiary air duct,
the kiln hood, the cooler vent duct, a mid cooler takeoff, a separate heater,
or a any
combination of these hot gas sources. The hot gas may be tempered with water
or
ambient air or cooled with a heat exchanger for a variety of reasons, such as
reducing
fouling caused by alkali salts or reducing the amount of high temperature
materials used
in the downstream equipment, but not below the proper temperature for
desorption of the
mercury and other contaminants from the particulate material, that is, the
conversion of
mercury and the other contaminants into their gaseous phase, which generally
occurs best
within the range of from about 300 C to about 900 C, more preferably 400 C to
about
600 C and most preferably 450 C to about 550 C. This heating step is directed
to remove
3
CA 02712215 2010-08-05
Docket 6570
the mercury content from the particulate material. The residence time of
particulate
material in the hot gas needed to remove mercury from the particulate material
will
depend upon the temperature of the hot gas, the concentration of mercury on
the
particulate material, and the form of the mercury in the particulate material.
Residence
time of 0.1 to 3 seconds is generally sufficient for the purposes of this
invention.
Therefore, when the reaction area is a duct it has to be of sufficient length
to provide for
sufficient residence time, taking into consideration the velocity of the
heated gas through
the reaction area.
Chemical additives may be optionally added, either upstream, downstream, or in
the
reaction area, to assist in converting the mercury to the oxidized form to aid
in the
readsorption of mercury when the sorbent or chemical reagent is added
downstream of
the first dust collector. Suitable oxidizing agents include ozone, peroxide,
halogenated
species such as a chlorine solution, potassium permanganate, hydrochloric
acid, iodine
and other agents suitable to oxidize mercury.
The preferred amount of oxidizing agent will typically be expressed as its
concentration
in the gas stream downstream from where the agent is injected. For example,
when the
oxidizing agent is chlorine the preferred concentration of chlorine in the gas
stream will
generally range from about 500 to about 10000 ppm. The practitioner of this
invention
should take into consideration whether there is any naturally occurring
oxidizing agents
such or other halogens naturally occurring in the hot process gas utilized in
the invention,
which may be the case depending on the raw materials utilized in the process,
the type
and form of the oxidizing agent to be used, the amount of volatized mercury in
the hot
gas and whether any oxidizing agents occur naturally in the hot process gas.
Gases and particulate material exiting the reaction area are maintained at a
sufficient
temperature to keep mercury in a vaporized form in the gas stream. The gases
may be
optionally treated with water or ambient air or cooled with a heat exchanger
after exiting
the reaction area in order to maintain optimal levels of vapor, and the
temperature of the
gas, into the first dust collector, particularly when the dust collector is a
hot ESP. If so
4
CA 02712215 2010-08-05
3 f'
Docket 6570
treated, the temperature of the gas stream should not fall below the
temperature at which
the mercury compounds will readsorb back onto the particulate material. When a
hot
ESP is employed as the first dust collector, the temperature of the gas
entering the ESP
will generally range from about 350 C to about 500 C and preferably from about
450 C
to about 500 C.
Thereafter, clean particulate material is collected in a first, hot dust
collector which is a
hot ESP, a hot high efficiency cyclone, a high heat ceramic filter or other
form of hot dust
collector. The collected particulate material will be essentially mercury free
so long as
the hot dust collector is maintained at a temperature above which mercury is
readsorbed.
If the collected clean particulate material is predominantly fine cement raw
material or an
intermediate product found in a cement plant, then it can be returned to the
production
process as kiln feed after being metered through a bin or silo. Alternatively,
the cleaned
particulate material can be used it other ways. For example, cleaned fly ash
can be used
in several industrial processes, such as in the production of cement clinker,
as a major
component in blended cements, in the manufacture of light weight aggregates,
and as a
replacement for sand in manufacturing controlled low strength materials.
After the particulate material is collected, the hot gas is optionally cooled
downstream of
the first dust collector, such as with injections of water or ambient air or a
heat
exchanger, to a temperature that improves the removal of mercury by the
sorbent or
chemical reagent. For activated carbon or hydrated lime this temperature is
typically
below about 200 C and preferably below about 150 C. However, if a sorbent or
chemical
reagent is a high temperature sorbent such as MinPlusTM, a trademark of
MinPlus Inc. to
designate a mineral based, non carbon sorbent, which can adsorb mercury at
temperatures
above 400 C, then hot gas would not need to be cooled down. The amount of
sorbent or
chemical reagent added will depend on the amount of mercury in the gas stream,
the form
of the mercury, the amount of particulate material, which may function as a
relatively
inefficient sorbent for mercury, remaining in the gas stream, the type of
sorbent or
chemical reagent employed, and the desired amount of collected mercury.
However,
assuming an efficient first, hot dust collector (> 99 percent efficient in
removing
5
CA 02712215 2010-08-05
Docket 6570
particulate material) is utilized, resulting in minor amounts of particulate
material in the
hot gas stream, then activated carbon and hydrated lime are the sorbents of
choice, such
sorbents will be typically added to the gas stream at the rate of about 1 lbs
to about 20
pounds activated carbon per one million cubic feet of process gas. The mercury
containing sorbents or chemical reagents and any remaining particulate
material are then
collected in a second collector, which, when activated carbon or hydrated lime
are the
sorbents will be a so-called "cold" collector having inlet temperatures
typically ranging
from about 100 C to about 200 C, after which the cleaned gases are vented
either through
a separate stack or one or more other stacks at the cement plant.
Alternatively, the cleaned gases may be vented through a raw material grinding
mill, coal
mill, main kiln filter, downcomer, or cooler vent system. Some of the
collected sorbents
or chemical reagents and any remaining particulate material may be re-
circulated to the
gas stream after it passes through the first dust collector to adsorb more
mercury, while
any sorbents or chemical reagents and remaining particulate material that is
not re-
circulated is transported to an appropriate disposal site or regenerated. A
gas suspension
absorber, which is a form of semi-dry scrubber that utilizes a fluidized bed
reactor, can
optionally be used after the first dust collector to cool the gas, inject
fresh sorbents or
chemical reagents, recycled sorbent or chemical reagents and recycled
particulate
material. An FLSmidth Airtech Gas Suspension Absorber can suitably be used in
such
an application.
The invention is explained in greater detail below with the aid of the
drawings.
Figure 1 shows one embodiment of the application of the method according to
the
invention using a kiln installation for the production of cement clinker, with
the most
important elements briefly described. The kiln installation consists in part
of a cyclone
preheater tower 16, a rotary kiln 18 and a clinker cooler 20. The cyclone
preheater 16
comprises four cyclone stages, although less or more cyclone stages can be
employed.
Raw product from raw mill 13 is directed to the raw mill cyclone 12. The raw
mill
cyclone separates the finer fraction of raw meal from the coarser fraction.
The coarser
fraction via conduit 48 can either be directed to reaction area 26 (Figure 2)
as the first
6
CA 02712215 2010-08-05
Docket 6570
step for treatment in contaminant removal area 100 (shown in detail in Figure
2) to
remove contaminants according to this invention or is directed to kiln feed
storage/blending silo 14. From silo 14 the feed can either be directed to
reaction area 26
as the first step for treatment in contaminant removal area 100 to remove
contaminants
according to this invention or be introduced into cyclone preheater 16 via
kiln feed inlet
44 and preheated in a counter-current arrangement with kiln exhaust gases. The
preheated raw meal is separated from cyclone preheater 16 and directed to the
calciner 17
in which it is calcined. From the bottom outlet 49 of the separation cyclone
51, the
calcined raw meal is then directed to the rotary kiln 18 in which it is burned
into cement
clinker which is subsequently cooled in clinker cooler 20.
The exhaust gases from rotary kiln 18 and calciner 17 are directed up through
the cyclone
preheater 16. Tertiary air from the clinker cooler 20 is introduced via duct
19 into
calciner 17. Hot gases from preheater tower 16 enter an optional gas
conditioning tower
(GCT) 15 via downcomer 34. Gases entering the GCT from may be as hot as 400 C
if the
preheater tower has four or five stages. Nozzle means 31 located within GCT
15, near the
entrance thereto, injects a spray of cooling liquid into the hot gas flow.
Gases exiting
GCT 15 can be sent to mill 13 to help dry the ground feed. However, when the
raw feed
mill 13 is not in operation, gases can flow directly from GCT 15 to main kiln
filter 11,
which is optionally an electrostatic precipitator. Kiln dust exiting GCT 15
via conduit 47
can either be directed to reaction area 26 as the first step for treatment in
contaminant
removal area 100 to remove contaminants according to this invention or is
directed to silo
14.
Gases and the finer fraction of the raw product from the raw mill exiting
cyclone 12 via
conduit 50 are directed to main kiln filter 11. Separated dust from main kiln
filter 11 via
conduit 46 is either collected for treatment according to this invention in
reaction area 26
or directed to feed blending/storage silo 14 and the cleaned gas is directed
to exhaust.
Contaminant removal area 100 is depicted in more detail in Figure 2. A mercury
containing particulate material is fed into material inlet 35 of reaction area
26, which as
7
CA 02712215 2010-08-05
Docket 6570
depicted is a relatively shorter duct or vessel with a larger cross-sectional
area than the
rest of the ductwork to reduce the gas velocity to achieve the desired
residence time.
Reaction area 26 can alternatively be a relatively longer duct of the same or
smaller
cross-sectional area as the rest of the ductwork to achieve the desired
residence time. In a
cement plant the inlet stream for contaminant removal area 100 is generated
from some
or all of kiln filter 11, GCT 15, coarser fraction from raw mill cyclone 12,
raw material
30, kiln feed 44 or 45, external source such as fly ash from a power plant or
a
combination of these streams and can be collected and directed to reaction
area 26 in any
manner known in the art. The mercury containing particulate material is mixed
in
reaction area 26 with hot gas entering via gas inlet 29. The source of the hot
gas for the
hot gas inlet 29 can be from a variety of locations in a plant as long as they
are within the
prescribed temperature ranges described above or contain mercury. These
include, but are
not limited to, preheater tower 16, calciner 17, tertiary air duct 19, kiln
hood 21, cooler
vent duct 22, kiln gas bypass duct 33, downcomer 34, or other hot gas sources
or
combinations of sources. A separate heating system may also be provided for
auxiliary
heat or for all the heat requirements for the system. Optionally, oxidizing
agents can be
added to the hot gas reaction area 26 such as via inlet 36 which can be
located upstream,
downstream, or in reaction area 26. Hot gas containing volatile contaminants
and
cleaned particulate material is directed to first hot dust collector 38.
Optional quenching
air or water can be added to the hot gas, such as via inlet 37 or the hot gas
can be cooled
with a heat exchanger. The cleaned particulate material 38 is returned to the
plant to be
used as kiln feed- either to the kiln silo 14 or combined with the kiln feed
in conduit 44.
Alternatively, the cleaned particulate material can be used for other
purposes. The hot
gas stream containing volatilized contaminants then is directed to the second
collector 28.
It is a feature of this invention that, prior to entering or in the second
collector 28, the
contaminants are physically and/or chemically adsorbed onto a sorbent or
chemically
react with a chemical reagent, with both the sorbent and chemical reagent
having been
injected into the gas stream. Optionally, the hot gas can be subject to
cooling
downstream from first dust collector 38, such as through the use of an
optional heat
3o exchanger or the addition of quenching air or water, such as via inlet 39,
to thereby drop
the temperature of the hot gas to a level that increases the amount of mercury
removed by
8
CA 02712215 2010-08-05
ti Docket 6570
the sorbent or chemical reagent. An agent that interacts with the mercury in
the gas
stream such as sorbents and/or chemical reagents is added to the gas stream
via inlet 40 to
thereby form a product of the agent/mercury interaction, with the formation of
said
product concurrently removing mercury from the gas stream. However, if a
sorbent or
chemical reagent is used that can remove mercury effectively without cooling,
such as
MinPlusTM sorbent, then the gas may not need to be cooled after the first dust
collector.
The sorbents or chemical reagents remove essentially any mercury, mercury
compound
or high molecular weight organic compound that is present in the gas stream.
The
reactivity and amount of sorbent or chemical reagent used in the present
invention can be
controlled by the type of sorbent or chemical reagent utilized, where the
sorbent or
chemical reagent is inserted relative to the second collector and/or the
temperature profile
(i) of the gas in the area in which the sorbent or chemical reagent is
injected and (ii) in
the second collector. Generally, the earlier the sorbent or chemical reagent
is provided
before the second collector, the longer the gas contact time and hence the
greater the
removal potential. However, the removal of a contaminant may have a
temperature
window where removal is favored. In the case of mercury and mercury compounds
using
activated carbon or hydrated lime, adsorption will generally occur in the
temperature
window of about 20 C to about 300 C, preferably about 80 C to about 200 C.
Providing
the activated carbon or hydrated lime in an area in which the temperature is
above this
window, even though providing a longer contact time, will not necessarily
increase the
adsorption efficiency. The sorbent or chemical reagent containing contaminant
can be
disposed via conduit 41, recycled via conduit 42, or regenerated.
Alternatively, some or
all of the spent sorbent/chemical reagent can be added to the clinker/cement
if the
captured mercury is in a form that passes environmental regulations and the
clinker/cement passes quality standards. The cleaned gas can be vented via
conduit 43 to
a separate stack (not shown) or returned to the cement plant.
Using this invention the average amount of mercury emitted from a cement plant
is
significantly reduced. If the additional mercury reduction is necessary, then
the
following modifications to the cement plant process can be implemented to
further
decrease the mercury emissions.
9
qz
1
CA 02712215 2010-08-05
Docket 6570
= Injecting a sorbent or chemical reagent upstream of the main filter 11,
particularly
when the raw mill is down. The sorbent or chemical reagent containing mercury
would be captured by the main filter 11 and then sent to the contaminant
removal
area 11.
= Redirecting some or all of the gas exiting the main filter 11 in conduit 10,
particularly when the raw mill is down, to upstream of location 40 where the
sorbent or chemical reagent is added in contaminant removal area 100.
= A combination of these two methods.
The invention having been thus described it will be obvious that the same may
be varied
in many ways without departing from the spirit and scope thereof. All such
modifications
are intended to be included within the scope of the invention which is defined
by the
following claims.
20
30