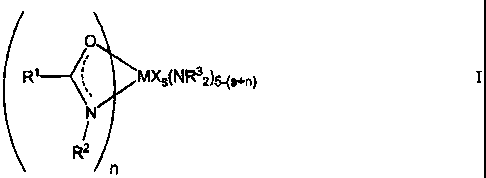Note: Descriptions are shown in the official language in which they were submitted.
CA 02715907 2010-09-28
GROUP 5 METAL COMPLEXES USEFUL FOR AMINE
FUNCTIONALIZATION AND SYNTHETIC PROCESS FOR
MANUFACTURE THEREOF
FIELD OF THE INVENTION
[0001] The present invention pertains to catalysts useful for amine
functionalization.
More particularly, the present invention pertains to group 5 metal amidate
complexes, their
synthesis and methods of use thereof in catalytic a-alkylation of amines using
olefins.
BACKGROUND
[0002) Catalytic synthesis of chiral and non-chiral amines is an important
method for
rapid and selective assembly of useful building blocks for the pharmaceutical,
agrochemical, and
fine chemical industries. Recently catalytic hydroanaination reaction has been
useful for the
addition of N-H bonds across C-C multiple bonds.[1] An alternative approach is
c~alkylation of
amines, also known as hydroaminoalkylation, which is a catalytic reaction in
which a C-C bond
is formed a to a nitrogen atom. [2-6]
[0003] Pioneering work from the early 1980s[2,3] inspired Herzon and Hartwig
to carry
out a detailed study of Tav amido derivatives as catalysts for the
o`alkylation of alkylatyl[4a]
and dialkyl[4b] amines with terminal and activated alkene substrates. Easily
varied amidates
have been previously used for the synthesis of electrophilic catalysts. [7]
Recently, Group 4
amidate based systems have been shown to be useful in cyclizing primary
aminoalkenes to
produce primary amine substituted carbocycles by an intramolecular
hydroaminoalkylation
reaction. [8] However, intermolecular variants of this have been unsuccessful
using this Zr
precatalyst.
[0004] Doye and co-workers demonstrated that inexpensive Ti"' complexes are
competent catalysts for the catalytic c .alkylation of both primary and
secondary amines for intra-
-1-
CA 02715907 2010-09-28
and intermolecular reactions using a select group of substrates.[5] Notably,
in all these catalytic
reactions, metallaaziridines have been proposed as the catalytically active
species.
[0005] Given the demand for simple and economical methods for synthesis of
amine
building blocks in pharmaceutical, agrochemical and fine chemical industries,
there remains a
need for effective, flexible, catalysts systems for the functionalization of
amines,
[0006] This background information is provided for the purpose of making known
information believed by the applicant to be of possible relevance to the
present invention. No
admission is necessarily intended, nor should be construed, that any of the
preceding information
constitutes prior art against the present invention.
SUMMARY OF THE INVENTION
[0007] An object of the present invention is to provide Group 5 metal
complexes useful
for amine functionalization and synthetic process for manufacture thereof.
[0008] In accordance with one aspect, there is provided a metal amidate
complex having
the structure of Formula I:
(R1__MXsNR32)5+fl) I
N
R2
n
wherein:
M is a group 5 metal, such asTa,NborV;
X is a halo substituent, such as Cl, F, I or Br;
n=1or2;
s=Ior2;
-2-
CA 02715907 2010-09-28
R' and R2 arc each independently H; a C, - C25 substituted or unsubstituted,
linear,
branched or cyclic alkyl; a substituted or unsubstituted aryl; or a
substituted or unsubstituted
heterocyclic group, wherein R' and R2 can be bonded together thereby forming,
together with the
nitrogen atom and the carbon atom to which they are bound, a cyclic moiety,
and
each R3 is independently a C, - C25 substituted or unsubstituted, linear,
branched or
cyclic alkyl; a substituted or unsubstituted aryl; or substituted or
unsubstituted heterocyclic
group.
[0009] In accordance with one embodiment, there is provided a group 5 metal
amidate
complex of Formula Ia:
R1
2 O
-~ ,
N
la
N~--- MXa(NR32)3-6
R1 O
wherein:
M is a group 5 metal;
X is halo;
s =1 or 2;
R' and R2 are independently H; a C, - C25 substituted or =substituted, linear,
branched
or cyclic alkyl; a substituted or unsubstituted aryl or a substituted or
unsubstituted heterocyclic
group, wherein R' and R2 can be bonded together thereby forming, together with
the nitrogen
atom and the carbon atom to which they are bound, a cyclic moiety, and wherein
the R'
substituents are bound, or "tethered", to one another, the R2 substituents are
bound to one
another, or an R' substituent is bound to an R2 substituent; and
each R3 is independently a C, - C25 substituted or unsubstituted, linear,
branched or
cyclic alkyl; a substituted or unsubstituted aryl; or substituted or
unsubstituted heterocyclic
group.
-3-
CA 02715907 2010-09-28
[0010] In accordance with one aspect, there is provided a metallaaziridine
complex
having the structure of Formula 1b:
O NR3
R4
R1 MXS
Ib
-<". I I
N 4
(NR32)
RZ2
n
wherein:
[0011] M is a group 5 metal, such as Ta, Nb or V;
X is a halo substituent, such as Cl, F, I or Br;
n=Ior2;
s =1 or 2, wherein when n =2, s = 1;
Rl and R2 are each independently H; a Cl - Cgs substituted or unsubstituted,
linear, branched or cyclic alkyl; a substituted or unsubstituted aryl or a
substituted or
unsubstituted heterocyclic group, wherein Rt and R2 can be bonded together
thereby forming,
together with the nitrogen atom and the carbon atom to which they are bound, a
cyclic moiety;
each R3 is independently a Ci - C25 substituted or unsubstituted, linear,
branched
or cyclic alkyl; a substituted or unsubstituted aryl; or substituted or
unsubstituted heterocyclic
group; and
each R4 is independently H, a C1 - C25 substituted or unsubstituted, linear,
branched or cyclic alkyl; a substituted or unsubstituted aryl or a substituted
or unsubstituted
heterocyclic group.
[0012] In accordance with another aspect, there is provided a process for
synthesizing a
metal complex of Formula I or 1 a, or a metallaaziridine complex of Ib, which
comprises reacting
a compound of Formula III with n equivalents of an amide of Formula IV:
-4-
CA 02715907 2010-09-28
R1
MXX(N R32)5-s + n 10 I, la or lb
NH
III R2
IV
wherein:
M is a group 5 metal, such as Ta, Nb or V;
X is a halo substituent, such as C1, F, I or Br,
n=1or2;
s=1 or2;
R' and R2 are independently H; a CI - C25 substituted or unsubstituted,
linear, branched
or cyclic alkyl; a substituted or unsubstituted aryl; or substituted or
unsubstituted heterocyclic
group, wherein Rt and R2 can be bonded together thereby forming, together with
the nitrogen
atom and the carbon atom to which they are bound, a cyclic moiety; and
each R3 is independently a C1 - C25 substituted or unsubstituted, linear,
branched or
cyclic alkyl; a substituted or unsubstituted aryl; or substituted or
unsubstituted heterocyclic
group.
(0013] In accordance with another aspect, there is provided a method for a-
alkylation of
a N-containing C4-C1oo heterocycle, or C4-Csa heterocycle, or C4-C25
heterocycle, or C4-Cjo
heterocycle, which comprises reacting the N-containing heterocycle with an
olefin in the
presence of a metal complex having the structure of Formula I:
(R1__4)Mxs(NR32s+n) I
N
R12
wherein M, X, R', R2, R3 s and n are as defined above.
-5-
CA 02715907 2010-09-28
[0014] In accordance with another aspect, there is provided a method for o-
alkylation of
a N-containing C4-Ctao heterocycle, or C4-C50 heterocycle, or C4-C25
heterocycle, or C4-C10
heterocycle, which comprises reacting the N-containing heterocycle with an
olefin in the
presence of a metal complex having the structure of Formula la:
RI
rr~ O
R 2 N
Ia
R2
NMUNR32Is-s
R1 p
wherein M, X, R', R2, R3 s and n are as defined above.
[0015] In accordance with another aspect, there is provided a method for ci-
alkylation of
a N-containing C4-Cron heterocycle, which comprises reacting the N-containing
C4-Cl00
heterocycle with an olefin in the presence of a metallaaziridine complex
having the structure of
Formula lb:
0 NR3
R4
RT MXS Ib
N R4
I ( NR3z)
R2 3{s+n}
n
wherein M, X, R', R2, R3, R4, s and n are as defined above.
[0016] In accordance with another aspect, there is provided a method for
formation of a
bond between a first secondary amine containing moiety and a second moiety,
which comprises
reacting the secondary amine containing moiety with an olefin in the presence
of a metal
complex having the structure of Formula I:
-6-
CA 02715907 2010-09-28
R1 MXs(NR32)5-(s+n) I
N
12
R
wherein M, X, R', R2, R3 s and n are as defined above. In accordance with one
embodiment, the
bond formation comprises -alkylation of the secondary amine. Optionally, the
secondary amine
containing moiety and the second moiety are in the same molecule, in the case
of an
intramolecular reaction. Alternatively, the secondary amine containing moiety
and the second
moiety are in different molecules, in the case of an intermolecular reaction.
(0017] In accordance with another aspect, there is provided a method for
formation of a
bond between a first secondary amine containing moiety and a second moiety,
which comprises
reacting the secondary amine containing moiety with an olefin in the presence
of a metal
complex having the structure of Formula la:
R3
R2 - , O
Ia
R2
N MX5(NR32)3-s
R1 O
wherein M, X, R', R2, R3 s and n are as defined above. In accordance with one
embodiment, the
bond formation comprises c -alkylation of the secondary amine. Optionally, the
secondary amine
containing moiety and the second moiety are in the same molecule, in the case
of an
intermolecular reaction. Alternatively, the secondary amine containing moiety
and the second
moiety are in different molecules, in the case of an intermolecular reaction.
-7-
CA 02715907 2010-09-28
[0018) In accordance with another aspect, there is provided a method for
formation of a
bond between a fast secondary amine containing moiety and a second moiety,
which comprises
reacting the secondary amine containing moiety with an olefin in the presence
of a
metallaaziridine complex having the structure of Formula Ib:
O NR3
= R4
R' MXS Ib
I R4
(NR32) 349+0
n
wherein M, X, R1, R2, R3, R , $ and n are as defined above. In accordance with
one embodiment,
the bond formation comprises tx-alkylation of the secondary amine. Optionally,
the secondary
amine containing moiety and the second moiety are in the same molecule, in the
case of an
intramolecular reaction. Alternatively, the secondary amine containing moiety
and the second
moiety are in different molecules, in the case of an intermolecular reaction.
[0019] In accordance with another aspect, there is provided a method for
enantioselective
cx-alkylation of a secondary amine comprising reacting the secondary amine
with an olefin in the
presence of a metal complex of Formula I or Formula Ia or a metallaaziddine
complex of
Formula Ib, wherein at least one of R1 or R2 comprises a chiral group, or the
complex comprises
a chiral group, such as an axially chiral group, formed from the R1
substituents tethered to one
another, the R2 substituents tethered to one another, or an R' substituent
tethered to an R2
substituent.
[0020] In accordance with another aspect, there is provided a process for
synthesizing an
c~-amino acid, an ai-amino acid derivative, a a-amino acid, a 3-amino acid
derivative, a y-amino
acid, or a 1-amino acid derivative comprising reacting a secondary amine of
Formula V with a
compound of Formula VI
-8-
CA 02715907 2010-09-28
R7
N R7 /~ Re
R5 FRB
v 7 7 R~
vi
in the presence of a metal complex of Formula I or Ia, or a metallaaziridine
complex of Formula
Ils:
O
(R1M;(NR32+n)
N
R2
RI
2~ -. Q
la
R2.
N, MXs(NR22)
R~ Q
O NRI
R4
RI MXS
.~ i lb
N ( NR~21 R4
7 1
R2 3{s+n)
n
_g_
CA 02715907 2010-09-28
wherein:
M is a group 5 metal,
Xis a halo substituent;
n= l or 2;
s =1 or 2, wherein in the metallaaziridine of Formula lb, when n = 2, s -1;
R' and R2 are independently H; a C1- C25 substituted or unsubstituted, linear,
branched
or cyclic alkyl; a substituted or unsubstituted aryl or a substituted or
unsubstituted heterocyclic
group, wherein R' and R2 can be bonded together thereby forming, together with
the nitrogen
atom and the carbon atom to which they are bound, a cyclic moiety;
each R3 is independently a C1- C25 substituted or unsubstituted, linear,
branched or
cyclic alkyl; a substituted or unsubstituted aryl; or substituted or
unsubstituted heterocyclic
group;
each R4 is independently H, a C1 - C25 substituted or unsubstituted, linear,
branched or
cyclic alkyl; a substituted or unsubstituted aryl or a substituted or
unsubstituted heterocyclic
group;
R5 and R6 are independently a C1- C25 substituted or unsubstituted, linear,
branched or
cyclic alkyl; a substituted or unsubstituted aryl; or substituted or
unsubstituted heterocyclic
group or one of R5 and R6 is an amine protecting group, wherein when R5 a
substituted or
unsubstituted aryl or a substituted or unsubstituted heterocyclic, R6 is not a
substituted or
unsubstituted aryl or a substituted or unsubstituted heterocyclic;
t=0, I or2;
R7 is H; a C1- C100, a C1 - Cso, C, - C25, or a C1 C10 substituted or
unsubstituted,
linear, branched or cyclic alkyl optionally bearing functional moieties within
the alkyl chain
(e.g., ether linkage) ; or a substituted or unsubstituted aryl or a
substituted or unsubstituted
heterocyclic group; and
RS is independently a hydroxy bearing a protecting group, a catboxy bearing a
protecting
group, an ester, an amide, a nitrile, a thioester, wherein when t is 0, R$ is
independently a
protected enolate (e.g., SiR3).
[0021] In accordance with another aspect, there is provided a method for o-
alkylation of
a N-containing C4-CIOO heterocycle, or C4-C5o heterocycle, or C4-C25
heterocycle, or C4-C,0
-10-
CA 02715907 2010-09-28
heterocycle, which comprises reacting the N-containing heterocycle with an
olefin in the
presence of a metal complex having the structure of Formula TI:
O
Rt M(NR32)5., II
N
R2
wherein:
M is a group 5 metal;
n=1or2;
R' and R2 are independently H; a C, - Czs substituted or unsubstituted,
linear, branched
or cyclic alkyl; a substituted or unsubstituted aryl; or substituted or
unsubstituted heterocyclic
group, wherein R' and RZ can be bonded together thereby forming, together with
the nitrogen
atom and the carbon atom to which they are bound, a cyclic moiety, and
each R3 is independently a Cl - C25 substituted or unsubstituted, linear,
branched or
cyclic alkyl; a substituted or unsubstituted aryl; or substituted or
unsubstituted heterocyclic
group.
[0022] in accordance with a specific embodiment of the method for a-alkylation
of a N-
containing C4-Cloo heterocycle, using a metal complex having the structure of
Formula II, when
M is Ta, n is 1, R' is tert-butyl, R2 is 2,4-diisopropylphenyl, and R3 is
methyl, and the N-
containing heterocycle is piperidine or 1,2,3,4-tetrahydroquinoline, the
olefin is an olefin other
than 1-octene.
[0023] In accordance with another aspect, there is provided a method for a-
alkylation of
a N-containing C4-C,00 heterocycle, which comprises reacting the N-containing
heterocycle with
an olefin in the presence of a metal complex having the structure of Formula
Ira:
-11-
CA 02715907 2010-09-28
R1
R2"N ,..--.. O
ITa
N M(NR 2)3
R1 O
wherein:
M is a group 5 metal;
n=1 or2;
R' and R2 are independently H; a Ct - C25 substituted or unsubstituted,
linear, branched
or cyclic alkyl; a substituted or unsubstituted aryl; or substituted or
unsubstituted heterocyclic
group, wherein R' and R2 can be bonded together thereby forming, together with
the nitrogen
atom and the carbon atom to which they are bound, a cyclic moiety; and
each R3 is independently a C1 - C25 substituted or unsubstituted, linear,
branched or
cyclic alkyl; a substituted or unsubstituted aryl; or substituted or
unsubstituted heterocyclic
group.
[0024] In accordance with another aspect, there is provided a method for a-
alkylation of
a N-containing C4-C1oo heterocycle, or C4-C50 heterocycle, or C4-C25
heterocycle, Or C4-CIO
heterocycle, which comprises reacting the N-containing heterocycle with an
olefin in the
presence of a metallaaziridine complex having the structure of Formula ITb:
O NR(R1:T3R4
N R4 Ilb
1 NR32)
R2 ` 3-n
wherein:
M is a group 5 metal;
-12-
CA 02715907 2010-09-28
n= 1 or2;
R' and RZ are independently H; a C1- C25 substituted or unsubstituted, linear,
branched
or cyclic alkyl; a substituted or unsubstituted aryl; or substituted or
unsubstituted heterocyclic
group, wherein R1 and R2 can be bonded together thereby forming, together with
the nitrogen
atom and the carbon atom to which they are bound, a cyclic moiety;
each R3 is independently a Cl - C25 substituted or unsubstituted, linear,
branched or
cyclic alkyl; a substituted or unsubstituted aryl; or substituted or
unsubstituted heterocyclic
group; and
each R4 is independently H, a C1- C2s substituted or unsubstituted, linear,
branched or
cyclic alkyl; a substituted or unsubstituted aryl or a substituted or
unsubstituted heterocyclic
group-
[0025] In accordance with another aspect, there is provided a process for
synthesizing an
o amino acid, an c x,-amino acid derivative, a i3-amino acid, a $3-amino acid
derivative, a -f-amino
acid, or a y-amino acid derivative comprising reacting a secondary amine of
Formula V with a
compound of Formula VI
R7
H R7 Re
R5'' R6
7
V 7 7 R
Vi
in the presence of a metal complex of Formula II or Ila, or a metallaaziridine
complex of IIb
-13-
CA 02715907 2010-09-28
O
(R1__4)MNR32s..fl II
,
R1
R2-.N YYa
R2__"
N M(NR32}3
) I--. /
R' 0
0 NRa
R1 M
N I
(NR2)
R2 3-n
wherein:
M is a group 5 metal;
n-Ior2;
R' and R2 are independently H; a C, - Cgs substituted or unsubstituted,
linear, branched
or cyclic alkyl; a substituted or unsubstituted aryl or a substituted or
unsubstituted heterocyclic
group, wherein R1 and R2 can be bonded together thereby forming, together with
the nitrogen
atom and the carbon atom to which they are bound, a cyclic moiety;
each R3 is independently a C, - Cgs substituted or unsubstituted, linear,
branded or
cyclic alkyl; a substituted or unsubstituted aryl; or substituted or
unsubstituted heterocyclic
group; and
each R4 is independently H, a C, - C25 substituted or unsubstituted, linear,
branched or
-14-
CA 02715907 2010-09-28
cyclic alkyl; a substituted or unsubstituted aryl or a substituted or
unsubstituted heterocyclic
group;
R5 and R6 are independently a Cr - Cgs substituted or unsubstituted, linear,
branched or
cyclic alkyl; a substituted or unsubstituted aryl; or substituted or
unsubstituted heterocyclic group
or one of R5 and R6 is an amine protecting group, wherein when e a substituted
or unsubstituted
aryl or a substituted or =substituted heterocyclic, R6 is not a substituted or
unsubstituted aryl or
a substituted or unsubstituted heterocyclic;
t=0,1 or2,
R7 is H; a Cr Croo, a Cr - Cso, C1- C25: or a C1- C10 substituted or
unsubstituted,
linear, branched or cyclic alkyl optionally bearing functional moieties within
the alkyl chain
(e.g., ether linkage) ; or a substituted or unsubstituted aryl or a
substituted or unsubstituted
heterocyclic group; and
R8 is independently a hydroxy bearing a protecting group, a carboxy bearing a
protecting
group, an ester, an amide, a nitrite, a thioester, wherein when t is 0, R8 is
independently a
protected enolate (e.g., SiR3).
BRIEF DESCRIPTION OF THE FIGURES
[0026] Figure 1 depicts the synthesis and ORTEP diagram of solid state
molecular
structure of a tantalum amidate complex 1; thermal ellipsoids are depicted at
50% probability
and all hydrogen atoms and most amidate ligand atoms are omitted for clarity
(selected bond
lengths (A) and angles [0]: Tal N41.921(4), Tal-N3 1.970(5), Tal-C38 2.178(5),
N4-C29
1.451 (7), N4-C38 1.424(6), N3- C28 1.459(9), N3-C271.463(9), Tat N12.179(4),
Tal-N2
2.245(4), Tal-01 2.214(4), Tal-02 2.132(3); N4-Tal -C38 40.04(17), Tal -N4-C38
79.7(3),
Tai-N4-C29155.8(4), Tal N3-C27124.1(5), Tal-N3-C28123.0(4), NI-Tal -
0159.35(14),
N2-Tal -02 59.34(12).
[0027] Figure 2 depicts the synthesis of chiral, enantiopure precatalyst 17
and ORTEP
diagram of the solid-state molecular structure of ( )-17 with thermal
ellipsoids depicted at 50%
probability; all hydrogen atoms omitted for clarity (selected bond distances
(A) and angles [ ]:
-15-
CA 02715907 2010-09-28
Ta-O1 2.015(2), Ta- 02 1.973(2), Ta-N3 1.926(3), Ta-N4 1.988(2), Ta-N5
1.956(2); O1-Ta-
0285.21(10)).
(0028] Figure 3 graphically depicts the results of comparing catalytic
reactivities of two
Ta(amidate) complexes (+-=- 0.01M complex 18: -r 0.025M complex 18: --'-$
0.05M
complex 1S: -A- 0.05M complex 1).
DETAILED DESCRIPTION OF THE INVENTION
[0029] Definitions
[0030] Unless defined otherwise, all technical and scientific terms used
herein have the
same meaning as commonly understood by one of ordinary skill in the art to
which this invention
belongs.
[0031] As used in the specification and claims, the singular forms "a", "an"
and "the"
include plural references unless the context clearly dictates otherwise.
[0032] The term "comprising" as used herein will be understood to mean that
the list
following is non-exhaustive and may or may not include any other additional
suitable items, for
example one or more Anther feature(s), component(s) and/or ingredient(s) as
appropriate.
[0033] As used herein, "halogen" or "halo" refers to F, Cl, Br or I.
[0034] As used herein, "alkyl" refers to a linear, branched or cyclic,
saturated or
unsaturated hydrocarbon group which can be unsubstituted or optionally
substituted with one or
more substituent. Examples of saturated straight or branched chain alkyl
groups include, but are
not limited to, methyl, ethyl, 1-propyl, 2-propyl, 1-butyl, 2-butyl, 2-methyl-
l -propyl, 2-methyl-2-
propyl, l-pentyl, 2-pentyl, 3-pentyl, 2-methyl-l-butyl, 3-methyl-l-butyl, 2-
methyl-3-butyl,
2,2-dimethyl-l-propyl, 1-hexyl, 2-hexyl, 3-hexyl, 2-methyl-l-pentyl, 3-methyl-
l-pentyl,
4-methyl-l-pentyl, 2-methyl-2 pentyl, 3-methyl-2-pentyl, 4-methyl-2-pentyl,
2,2-dimethyl- l -butyl, 3,3 -dimethyl- i -butyl and 2-ethyl- i -butyl, l -
heptyl and 1-octyl. As used
herein the term "alkyl' encompasses cyclic alkyls, or cycloalkyl groups. The
term "cycloalkyl"
-16-
CA 02715907 2010-09-28
as used herein refers to a non-aromatic, saturated monocyclic, bicyclic or
tricyclic hydrocarbon
ring system containing at least 3 carbon atoms. Examples of C3-C12 cycloalkyl
groups include,
but are not limited to, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
cyclohepyl, cyclooctyl,
norbonnyl, adamantyl, bicyclo[2.2.2]oct-2-enyl, and bicyclo[2.2.2]octyl.
[0035] As used herein, the term "alkenyl" refers to a straight branched or
cyclic
hydrocarbon group containing at least one double bond which can be
unsubstituted or optionally
substituted with one or more substituents.
(0036] As used herein, "alkynyl" refers to an unsaturated, straight or
branched chain
hydrocarbon group containing at least one triple bond which can be
unsubstituted or optionally
substituted with one or more substituents,
[0037] As used herein, "allenyl" refers to a straight or branched chain
hydrocarbon group
containing a carbon atom connected by double bonds to two other carbon atoms,
which can be
unsubstituted or optionally substituted with one or more substituents.
[0038] As used herein, "aryl" refers to hydrocarbons derived from benzene or a
benzene
derivative that are unsaturated aromatic carbocyclic groups of from 6 to 100
carbon atoms, or
from which may or may not be a fused ring system. , in some embodiments 6 to
50, in other
embodiments 6 to 25, and in still other embodiments 6 to 15. The aryls may
have a single or
multiple rings. The term "aryl" as used herein also includes substituted
aryls. Examples include,
but are not limited to phenyl, naphthyl, xylene, phenylethane, substituted
phenyl, substituted
naphthyl, substituted xylene, substituted phenylethane and the like. As used
herein, "heteroaryl"
refers to an aryl that includes from 1 to 10, in other embodiments 1 to 4,
heteroatoms selected
from oxygen, nitrogen and sulfur, which can be substituted or unsubstituted.
[0039] As used herein, a "heterocycle" is an aromatic or nonaromatic
monocyclic or
bicyclic ring of carbon atoms and from 1 to 4 heteroatoms selected from
oxygen, nitrogen and
sulfur, and which can be substituted or unsubstituted. Included within the
term "beterocycle" are
heteroaryls, as defined above. Examples of 3- to 9-membered heterocycles
include, but are not
limited to, aziridinyl, oxiranyl, thiiranyl, azirinyl, diaziridinyl,
diazirinyl, oxaziridinyl, azetidinyl,
azetidinonyl, oxetanyl, thietanyl, piperidinyl, piperazinyl, morpholinyl,
pyrrolyl, oxazinyl,
-17-
CA 02715907 2010-09-28
thiazinyl, diazinyl, triazinyl, tetrazinyl, imidazolyl, benzimidazolyl,
tetrazolyl, indolyl,
isoquinolinyl, quinolinyl, quinazolinyl, pyxrolidinyl, purinyl, isoxazolyl,
benzisoxazolyl, furanyl,
furazanyl, pyridinyl, oxazolyl, benzoxazolyl, thiazolyl, benzthiazolyl,
thiophenyl, pyrazolyl,
triazolyl, benzodiazolyl, benzotriazolyl, pyrimidinyl, isoindolyl and
indazolyl.
[0040] As used herein, "substituted" refers to the structure having one or
more
substituents. A substituent is an atom or group of bonded atoms that can be
considered to have
replaced one or more hydrogen atoms attached to a parent molecular entity.
Possible substituents
include any atom or group that does not inhibit the desired reaction. Examples
of substituents
include aliphatic, halogen, arylcarbonyloxy, alkoxycarbonyloxy,
aryloxycarbonyloxy,
alkoxycarbonyl, axuinocarbonyl, alkylthiocarbonyl, alkoxyl, phosphate ester,
phosphonato,
phosphinato, cyano, tertiary amino, tertiary acylamino, tertiary amide,
innino, alkylthio, arylthio,
sulfonato, sulfamoyl, tertiary sulfonamido, nitrile, trifluoromethyl,
beterocyclyl, aromatic, and
heteroaromatic moieties, ether, ester, boron-containing moieties, tertiary
phosphines, and silicon-
containing moieties.
[0041] As used herein, "olefin", also called alkene, refers to an unsaturated
hydrocarbon
containing one or more pairs of carbon atoms linked by a double bond, and
includes cyclic or
acyclic (aliphatic) olefins, in which the double bond is located between
carbon atoms forming
part of a cyclic (closed-ring) or of an open-chain grouping, respectively, and
monoolefins,
diolefins, triolefins, etc., in which the number of double bonds per molecule
is, respectively, one,
two, three, or some other number. Such olefins can be substituted or
unsubsituted . Specific
examples of olefins include, but are not limited to, substituted or
unsubsituted 1-propene, 1-
butene, 1-pentene, l - hexene, and 1-octene and substituted or unsubstitued
norbornene.
[0042] As used herein the terms "pre-catalyst", "catalyst" and "catalyst
system" we used
interchangeably to refer to the group 5 metal amidate complexes. Without
wishing to be bound
by theory, it appears that, in use, the group 5 metal amidate complexes form a
catalytically active
corresponding metallaaziridine in situ. This catalytically active
metallaaziridine intermediate is
also referred to herein as a "catalyst".
[0043] As used herein the term "metallaaziridine", refers to a three-membered
ring,
wherein the members are one nitrogen, one carbon and one metal atom.
-18-
CA 02715907 2010-09-28
[0044] Group 5 Metal Amidate Complexes
[0045] Described herein are ligand supported group 5 complexes useful for
regioselective
and diastereoselective amine ftinctionalization. The described tunable and
modular group 5 metal
amidate complexes promote the facile formation of metallaaziridines, a
catalytically active
intermediate. This class of complexes exhibits broad substrate scope in amine
functionalization
and can be used for enantioselective amine synthesis by hydroaminoalkylation.
These complexes
are "tunable" since the selection of certain substituents (e.g., sterically
bulky substituents) on the
ligands facilitates substrate selectivity, regioselecti-vity,
stereoselectivity, etc.
[0046] The present application pertains to halo group 5 metal-amidate
complexes having
the structure of Formula I:
a
(R1__4>Xs(NR32)5.(S+fl) I
N
wherein:
M is a group 5 metal, such as Ta,Nb or V;
X is a halo substituent, such as Cl, F, I or Br;
n=1or2;
s=1 or2;
R' and R2 are each independently H; a C1- C25, or a C1- C12, substituted or
unsubstituted, linear, branched or cyclic alkyl; a substituted or
unsubstituted aryl; or substituted
or unsubstituted heterocyclic group (for example, a heteroaryl), wherein R'
and RZ can be
bonded together thereby forming, together with the nitrogen atom and the
carbon atom to which
they are bound, a cyclic moiety; and
each R3 is independently a C1- C25, or a C1- C12, substituted or
unsubstituted, linear,
branched or cyclic alkyl; a substituted or unsubstituted aryl; or substituted
or unsubstituted
-19-
CA 02715907 2010-09-28
heterocyclic group (for example, a heteroaryl). As used herein, the term "halo
group 5 metal-
amidate complexes" refers to those complexes in which there is a halo bound to
the metal atom,
irrespective of whether or not there are any additional halo groups in the
ligand(s). In the
structure of Formula 1, the number of amide ligands is the number of valencies
on the metal (5)
less the number of halo substituents, and less the number of amidato ligands.
[0047) Optionally, one or more of the R1 and R2 substituents comprises a
chiral group
such that the resulting complex can be used as a catalyst in enantioselective
reactions.
[0048] In accordance with one embodiment, n = 2, and the halo group 5 metal-
amidate
complex has the structure of Formula la:
RI
0
R2-~....N
Ia
Rz \
N MXg(NR'2)34
R1 O
wherein M, X, RL, R2, R3 and s are as defined above, and wherein the R1
substituents are bound,
or "tethered", to one another, the R2 substituents are bound to one another,
or an R1 substituent is
bound to and R2 substituent,
[0049] Optionally, the tethered substituents can form an axially chiral group,
such that
the resulting complex can be used as a catalyst in enantioselective reactions.
[0050] In accordance with one aspect, there is provided a metallaaziridine
complex
having the structure of Formula Ib:
-20-
CA 02715907 2010-09-28
Ra
O NR3
R1 MX4 Ib
ii (NR32)
R2 3{s*")
n wherein M, X, R', R2, R3, n and s are as defined above, except that when n =
2, s =1, and each
R4 is independently H, a C1- C25 substituted or unsubstituted, linear,
branched or cyclic alkyl; a
substituted or unsubstituted aryl or a substituted or unsubstituted
heterocyclic group (for
example, a heteroaryl).
(0051] Optionally, one or more of the R1 and R2 substituents comprises a
chiral group
such that the resulting complex can be used as a catalyst in enantioselective
reactions.
[0052] In accordance with a specific embodiment, the group 5 metal complex of
Formula
I has the following structure:
a
(R1__K))MCI$NR32)5+fl) wherein:
M is a group 5 metal, such as Ta, Nb, or V;
n=1or2;
s = l or 2;
R' and R2 are each independently H; a C1- C25, or a C1- C12, substituted or
unsubstituted, linear, branched or cyclic alkyl; a substituted or
unsubstituted aryl; or substituted
or unsubstituted heterocyclic group (for example, a heteroaryl), wherein R'
and R2 can be
bonded together thereby forming, together with the nitrogen atom and the
carbon atom to which
-21-
CA 02715907 2010-09-28
they are bound, a cyclic moiety; and
each W is independently a C1- C25, or a C1 - C12, substituted or
unsubstituted, linear,
branched or cyclic alkyl; a substituted or unsubstituted aryl; or substituted
or unsubstituted
heterocyclic group (for example, a heteroaryl).
[0053] In another embodiment, the group 5 metal is Ta or Nb.
[0054] Specific, non-limiting, examples of the halo group 5 metal-amidate
complexes of
Formula I and Ia that include a halogen are listed below:
9 N M02
tBu--{S`TaCI(NMe2)a O
a-CI
1Pr N Me N \NMe2
Pr 18 ' ~ 19 tr Me 20
2
PE-0159 PE-0188 PE-0165
N.-.0 !
zI
/,,,NMe2 N~ NMe2
cI Ta CI Ta.~,
NMe2 \ NMe2
N-- 0 N==O
21 ~' 22
PE-0180 PE-0183
0 N Mee 0.~ O NMe2
But N T` CI t3uN.",-TACI2(NMe2 But- T A -C4
N
Pr Me
CI
I Pr NMe2 Pr Me 23 24 25
2 2
PE-0171 PE-0172 PE-0173
-22-
CA 02715907 2010-09-28
[0055] The present application also pertains to the use of non-halo group 5
metal-amidate
complexes having the structure of Formula li:
Q
.
(R1___4M(NR32)5..fl II
N
R2
n
wherein:
M is a group 5 metal, such as Ta, Nb or V;
X is a halo substituent, such as Cl, F, I or Br;
n=1 or 2;
R' and R2 are each independently H; a C1- C25, or a C1 - C12, substituted or
unsubstituted, linear, branched or cyclic alkyl; a substituted or
unsubstituted aryl; or substituted
or unsubstituted heterocyclic group (for example, a heteroaryl), wherein R'
and R2 can be
bonded together thereby forming, together with the nitrogen atom and the
carbon atom to which
they are bound, a cyclic moiety; and
each R3 is independently a C1- C25, or a C1- C12, substituted or
unsubstituted, linear,
branched or cyclic alkyl; a substituted or unsubstituted aryl; or substituted
or unsubstituted
heterocyclic group (for example, a heteroaryl). As used herein, the term "non-
halo group 5
metal-amidate complexes" refers to those complexes in which there is no halo
bound to the metal
atom, irrespective of whether or not there are any halo groups in the
ligand(s).
[0056] Optionally, one or more of the R' and R2 substituents comprises a
chiral group
such that the resulting complex can be used as a catalyst in enantioselective
reactions.
[0057] In accordance with one embodiment, n - 2, and the non-halo group 5
metal-
amidate complex has the structure of Formula IIa:
-23-
CA 02715907 2010-09-28
R1
R2 - . 0
Ha
R2
N-^-`M(NR32)3
R1 0
wherein M, X, R', R2 and R3 are as defined above, and wherein the R'
substituents are bound, or
"tethered", to one another, the R2 substituents are bound to one another, or
an R' substituent is
bound to and R2 substituent.
[0058] Optionally, the tethered substituents can form an axially chiral group,
such that
the resulting complex can be used as a catalyst in enantioselective reactions.
[0059] In accordance with one aspect, there is provided a metallaaziridine
complex
having the structure of Formula IIb:
NR(R1T3R4
/ R4 lib
NR12)
R2 3n
n
wherein M, X, R', R2, R3, R4 and n are as defined above
[0060] Optionally, one or more of the R' and R2 substituents comprises a
chiral group
such that the resulting complex can be used as a catalyst in enantioselective
reactions.
[0061] Specific, non-limiting, examples of compounds of Formula II, Ha and Ilb
are
listed below:
-24-
CA 02715907 2010-09-28
lBu-{ -Ta(NMe2)4 Ph -(( jTa(NMe2)4 !Bu"(( Ta(NMat
Prf N Pr N Me N
Pr Pr r Me
1 2 / 3
PE-0094 PE-0099 PE-0089
1201516-53-1 1201516.55-3 1201516-51-9
F 3C
~\ 1Bu-4 '`Ta(NMa2)4 F3C
tBu-<..
tBu N Me `~^~9 Ta(NMa2)4
N
tBu Me
4 \ j.Me t
Bu 6
PE-0213 PE-0102
F3C
131 f
/ \ N Ta NMe2 4
a .Ta(NMe2)4 F C V'~T NM~)a
3 ,Pr 8 9
r
/ \ ~ ' pr ! !Pr
PE-0189 PE-0100 PE-0101 N _,o N='~C~
tBu-~(~~Ta(NMez)a / \ /-NMez
N'~ Me2N Te" MezN-Tam
NMe2
tBu N ONMe2
N%-'C
1 ~; 11 Br , T 12
PE-0104 PE-0078 PE-0098
broad NMR spectra @ r.t. broad NMR spectra C r.t
-25-
CA 02715907 2010-09-28
a
t6u-{r
, t6u- ." Nb(NMe2)4 tBu-{ Nb,
Me NMez F"rf N 1f Me ~N NMe2
J M
2 14 16 = ! 17
2
PE-0076
1201516-49-5 PE-0161 PE-0176
Pr'N-<( Ta(NMe2)4 ON )Ta NMe2)a
Pr N N
tPr
y rl
= 26 = 27
PE-0196 PE-0197
[0062] In accordance with another aspect, there is provided a compound 13,
having the
structure shown below:
tBu
O Ta(NMe2)4
tBu 13
PE-0151
[0063] In accordance with another aspect, there is provided a compound 15,
having the
structure shown below:
0 N
tBu--{M T,'-NH
e 1 MNMe2
2
PE-0229
-26-
CA 02715907 2010-09-28
[0064] Synthesis of Group 5 Metal Complexes
[0065] In accordance with another aspect, there is provided a process for
synthesizing
group 5 metal amidate complexes via protonolysis. The halo group 5 metal-
amidate complexes
and halo group 5 metallaaziridine complexes can be synthesized by reacting the
appropriate
organic amide of Formula III with a group 5 metal amide of Formula IV. For
example, a halo
group 5 metal-amidate complex of Formula I can be synthesized by reacting one
or two
equivalents of the appropriate organic amide of Formula N with a group 5 metal
amide of
Formula III according to the following general reaction Scheme I:
Scheme I
O O
R1 (R1M;NR3&
+n)
M4(NR%)5, + n
NH N
III ~
R2 R
IV n
wherein M, X, R', R2, R3, s and n are as defined above. Two equivalents of the
organic amide of
Formula IV are used in the preparation of bis(amidate) complexes, while a
ratio of organic amine
to group 5 metal amide of approximately 1:1 is used the preparation of
mono(amidate)
complexes. Typically, the protonolysis is performed under inert, dry
conditions, in a solvent such
as hexane, at room temperature. However, it should be readily appreciated that
these reaction
conditions can be varied in order to optimize the yield and ease of reaction.
Such optimization is
well within the ordinary abilities of a worker skilled in the art,
[0066] As would be appreciated by a worker skilled in the art, the
corresponding non
halo group 5 metal-amidate complexes and non-halo group 5 metallaaziridine
complexes can be
prepared using the same synthetic process in which the starting metal amide
does not include a
halo bound to the metal atom.
-27-
CA 02715907 2010-09-28
[0067] In one example, when two equivalents ofN-(2,6-dimethylphenyl)pivalamide
were
added to (Ta(NMe2)s] and reacted in hexanes at room temperature, the
corresponding
tantallaaziridine complex (shown in Figure 1) was formed spontaneously. The
tantallaziridine
complex was found to be a competent precatalyst for alkylation of secondary
amines such as N-
methylaniiine using 1-octene.
[0068] The above reaction is sufficiently robust to successfully produce a
variety of
mono(amidate)- and bis(amidate) metal complexes at ambient temperature in
hexanes. The
mono(amidate) and bis(atnidate) complexes prepared have incorporated varying
degrees of steric
bulk. Details of specific examples of group 5 metal arAidate complexes
prepared using the above
reaction, are provided in the Examples below.
(0069] In a specific embodiment, bis(amidate) complexes are synthesized by
protonolysis
using a tethered bis(amide) of Formula Na according to Scheme 2 below:
Scheme 2
R2
Rz
R' NH RI lN
O
MXs(NR3zs e + 0. MXs(NR32)3e
0
R+ N
NH R I
02
W
rv$ .
wherein M, X, R', R2, R.3 and s are as defined above,
[0070) In an alternative embodiment, bis(amidate) complexes are synthesized by
protonolysis using a tethered bis(amide) of Formula 1Vb according to Scheme 3
below:
-28-
CA 02715907 2010-09-28
Scheme 3
R' R'
2O
O
RNH
O 12
2
R
MXS(NIa2)3-e + R2-NH MX (NR32)34
" ti
R1
rVb
[0071) In a specific example of the reactions shown in Schemes 2 and 3, the
tethered
bis(amide) is an axially chiral bis(amide) and the bis(amidate) metal complex
product retains the
axial chirality such that it can be used as a catalyst in enantioselective
reactions. The chiral group
metal complexes described herein have been successfully used to produce
enantioenriched
secondary amine products from c-alkylation of amines. Details of exemplary
reactions are
provided in the Examples below.
[0072] As would be appreciated by a worker skilled in the art, the
corresponding non-
halo group 5 metal-amidate complexes can be prepared using the same synthetic
process in
which the starting metal amide does not include a halo bound to the metal
atom.
[0073] In one example, precatalyst 17 was synthesized in excellent yield from
a
protonolysis reaction between (Ta-(NMe2)5) and a C2 symmetric tethered
bis{amide) proligand
(Figure 2). The solid-state molecular structure of 17 (see, Figure 2) reveals
that this potentially
tetradentate ligand adopts a bidentate alkoxyimine binding mode, to give a C2
symmetric,
pseudotrigonal bipyramidal tantalum metal center. Solution phase NMR
characterization data is
also consistent with this C2 symmetric binding mode, as the 13C NMR spectrum
shows one
signal at 8 =159.92 ppm for the carbonyl group, which is consistent with
alkoxy'imine
bonding.[ 161 Chiral precatalyst 17 has been tested with a variety of alkene
and amine substrates,
and gives enantioenriched secondary amine products.
-29-
CA 02715907 2010-09-28
[0074] In an alternative embodiment, the group 5 metal amidate complexes
described
herein can be prepared using a synthesis that includes salt metathesis. For
example, halo group 5
metal-amidate complexes can be prepared according to Scheme 4 set out below:
Scheme 4
'Si(Me)3
Ns-N OH
0 \Si(Me)3 R' I 0 Na
R~ ~jNfRz 11 N
H I
R2
H
n R' Na (MXs+I(NR32)4 (R1__4MX(NR32)5+fl)
N N
RZ R
wherein M, X, R', R2, R3, s and n are as defined above.
[0075] Typically, both steps of the salt metathesis synthetic route are
carried out under
dry conditions at room temperature. A suitable solvent for the first step of
this synthetic route is
dry diethyl ether. A suitable solvent for the second step of this synthetic
route is dry
tetrahydrofuran. It should be readily appreciated that these reaction
conditions can be varied in
orders to optimize the yield and ease of reaction. Such optimization is well
within the ordinary
abilities of a worker skilled in the art.
[0076] As would be appreciated by a worker skilled in the art, the salt
metathesis
synthetic route can also be used to successfully synthesize the non-halo group
S metal-amidate
complexes.
[0077] Catalytic Reactivity of Group 5 Metal Complexes
[0078] The group 5 metal amidate complexes described herein have particular
value as
catalysts for bond forming reactions involving secondary amines.
-30-
CA 02715907 2010-09-28
[0079] The halo group 5 metal complexes and the non-halo group 5 metal
complexes
described above have activity in the a-alkylation of amines using olefins with
substantial
improvement over the current state of the art with regard to substrate scope,
reactivity, reaction
temperature and selectivity. In a particular, these group 5 metal have been
found useful as
catalysts for hydroarninoalkylation of secondary amines using olefins, wherein
a new carbon-
carbon bond is formed between the cr carbon atom of a secondary amine and a
carbon of a
substrate olefin.
[0080] The group 5 metal complexes are capable of effecting the general
reaction
depicted in Scheme 5, wherein an alpha positioned hydrogen atom on an amine is
added across
an olefin. The carbon positions marked with asterisks may be chiral in the
product:
Scheme 5
R'
R' Ri3 R'
~N R13 r R'
+ Re Catalyst
R11 N As
R'2 7 R' H 4R'2 R7
7
wherein:
Rl1, R'2 and R13 are each independently a C1- C12 substituted or
unsubstituted, linear,
branched or cyclic alkyl; a substituted or unsubstituted aryl; or substituted
or unsubstituted
heterocyclic group (for example, a heteroaryl) or one of R" or R12 and R'3
together, is an amine
protecting group,
t = 0, 1 or 2,
R7 is H; a Cl - 0100, a C1- C50, C1- Cu, or a C1- Clp, substituted or
unsubstituted,
linear, branched or cyclic alkyl optionally bearing functional moieties within
the alkyl chain
(e.g., ether linkage) ; or a substituted or unsubstituted aryl or a
substituted or unsubstituted
heterocyclic group (for example, a heteroaryl), and
R8 is independently a hydroxy bearing a protecting group, a carboxy bearing a
protecting
group, an ester, an amide, a nitrile, a thioester, wherein when t is 0, R8 is
independently a
protected enolate (e.g., SiR3).
-31-
CA 02715907 2010-09-28
[0081] This mode of activity can be inter- or intramolecular, as shown in
exemplary
Schemes 6 to 9 below (in each case M is Ta, Nb, or V):
Scheme 6
Ph,.IH M-Catalyst Ph,NH
CH3 110 C
Scheme 7: N-heterocyclic amines
N M-Catalyst NH
165 C
Scheme 8: Internal, unactivated olefins
Ph, M-Catalyst Phi NH
NH +
C
H3 0 165 C
`_0
Scheme 9: Double addition
Ph. M-Catalyst
2 NH
+ H H
CH3 165 C Ph. N N` Ph
[0082] An exemplary reaction is provided in the scheme below, where the arrow
identifies the newly formed bond:
6 H ~ Ft"
,N R + , . . mold cat. N Rm
l
C6D6 Or CODs, R,
1 1,5. 130. C
[0083] The catalytic o-alkylation of secondary amines described above, is an
improvement over current methods used for synthesizing amines. For example,
the catalytic
reaction is 100% atom economic since all of the substrate atoms are in the
product, thus avoiding
-32-
CA 02715907 2010-09-28
any waste or unwanted by-products. Further, the use of the group 5 metal
amidate complexes of
Formula 1 provides the ability to control the reaction by selecting amidate
ligands with different
steric and/or electronic characteristics. It has been found that the catalytic
ce-alkylation reactions
metal amidate complexes are more susceptible to changes in the steric bulk of
the amidate
ligands than the electronic effects, however, both characteristics can be used
to provide a means
for controlling the catalytic reaction.
[0084] Example of olefins that can be used as a substrate in the above
reaction include,
but are not limited to a substituted or unsubstituted alkene, such as a 1-
alkene, a 2-alkene, a 3-
alkene, and so on. Optionally, the olefin is a cyclic olefin, such as, but not
limited to, an
exocyclic olefin, norbornene, cyclohexene, cyclopentene, cycloheptene,
dicyclopentadiene,
bicyclic olefins with carbon or hetero atom bridges of which the following are
representative:
Bicyclo[2.2.2]oct-2-ene, 2,5 Norbornadiene, Bicyclo[2.2.2]octa-2,5-
diene,Bicyclo[2.2.2]octa-
2,5-diene, Bicyclo[2.1.1]hex-2-ene, unsubstituted or substituted 7-
Oxabicyclo[2.2. 1 ]hept-2-ene,
or unsubstituted or substituted and suitably protected 7-Azabicyclo[2.2.1]hept-
2-ene.
[0085] Interestingly, it has been found that the halo group 5 metal amidate
complexes of
Formula 1, such as chloro group 5 metal amidate complexes, are more effective
than the non-
halo complexes, in catalyzing intermolecular hydroaminoalkylation of secondary
amines.
Selection of the appropriate catalyst provides further opportunity to control,
or tune, the catalytic
reaction.
[0086] Based on the catalytic reactivity of the group 5 metal amidate
complexes
described herein, it is clear that these complexes are particularly useful in
the synthesis of
pharmaceutically and/or agrochemically important compounds. Therefore, the
present
application further provides a method of preparing a pharmaceutical or an
agrochemical
comprising a catalytic c-alkylation of a secondary amine using a group 5 metal
amidate complex
of Formula I as defined above.
[0087] Specific, non-limiting, examples of various secondary amines that can
be used as
the basic structural unit for preparing pharmaceutical or agrochemical
compounds are provided
in Table I below:
-33-
CA 02715907 2010-09-28
Table 1
Structual Unit Pharmaceutical Application Agrochemical
Application
Pyrrolidines Serotonin re-uptake inhibitors, 5HT6 receptor affinity
Fungicides,
agents, NK3 receptor antagonists, inhibitors of KSP herbicides,
activity, Acaricides,
H ALK-CMET kinase inhibitors Insecticides
Piperidines DNA methyl transferase inhibitors, Serotonin receptor
Insecticides,
affinity Herbicides
H
Azepanes Orexin receptor antagonists, Antibacterials, Migraine Insecticides,
treatment, CIy'TI inhibitors, Kv1.3 potassium channel Fungicides,
blockers Acaxicides
Piperazines P38 kinase inhibitors, treatment of NK1 receptor Acaricides,
disorders, Antidepressants, Schizophrenia treatment, insecticides
Pain inflammation, Anti-tumor activity I..
N~
M
Morpholines CC chemokine receptor ligands, Treatment of Fungicides,
nociceptive pain, P13 kinase inhibitors Insecticides
1,4-Diazepanes Alpha 1 antagonist, Adenosine re-uptake inhibitor, Nematocides,
Allergic conjunctivitis treatment, Rho kinase inhibitor, Acaricides,
Histamine antagonist, Anti-fungal Insecticides
Amino acid Maybe used in the synthesis of novel amino acid
derivatives derivatives and unnatural amino acids. These are used in
{a-amino acid, synthesis of peptides/proteins and other small molecules
P-amino acids' which show biological activity. Relevant for both
pharmaceutical and agrochemical industries.
etc.}
-34-
CA 02715907 2010-09-28
[0088] For example, the N-substituted heterocycle is:
N rM N rN
fD Y Y Y~
Y , ~`~ or Y
wherein Y is independently S, CH2, CH2CH2, CH(CH3), CH(Bn), N(aryl), N(alkyl),
N-PG where
PG--protecting group, N(para-methoxyphenyl), N(phenyl), N(CH3),
N(benzylhydryl), N(OR), an
amido nitrogen (e.g., N(CO)R), a thioamido nitrogen (e.g., N(CS)R.), tertiary
amide or N-
protecting group (e.g., N-Boc, N-Cbz, N-Tosyl, N-Fmoc), or:
0 O
and wherein R is a substituted or unsubstituted alkyl or a substituted or
unsubstituted aryl.
[0089] A specific example of a pharmaceutical that can be readily synthesized
using a
process that includes a catalytic a -allylation step is pregabalin. Details of
the synthetic process
are provided in Example 7 below.
[0090] In accordance with a specific embodiment, the group 5 metal amidate
complexes
described herein are prepared using a chiral ligand. Chiral metal amidate
complexes have been
found to be effective in asymmetric synthesis of chiral compounds from achiral
substrates.
Examples of such reactions are provided in the Examples below.
[0091] To gain a better understanding of the invention described herein, the
following
examples are set forth. It should be understood that these examples are for
illustrative purposes
only. Therefore, they should not limit the scope of this invention in any way.
-35-
CA 02715907 2010-09-28
EXAMPLES
[0092] EXAMPLE 1: Synthesis and characterization of Ta(amidate) complexes
[0093] Synthesis of complex I (see Figure 1) is given as a representative
example: N-
(2,6-dimethylphenyl)pivalamide (0.350 g, 1.71 mmol) was suspended in hexanes
(2 mL).
[Ta(NMe2)5] (0.342 g, 0.853 mmol) was added as a solid and the solution was
stirred overnight.
The solvent was removed in vacuo and the resulting solid was recrystallized
from hot hexanes at
-35 C, affording light yellow crystals. Typical yields are in the range of 70-
85%.
[0094] Bis N-[2.6-dimethylyhenyllyivalamidate)(dimethvlamido)-N-
methyltantalaaziridine
[0095] N-(2,6-dimethylphenyl)pivalamide (0,350 g, 1.71 mmol) was dissolved in
hexanes (2 mL). [Ta(NMe2)s] (0.342 g, 0.853 mmol) was added as a solid and the
solution was
allowed to stir overnight.' The solvent was removed in vacuo and the resulting
solid was
recrystallized from hot hexanes at 35 C, affording light yellow crystals. 'H
NMR ([I)6]-
Benzene, 400 MHz) 61.05 (s, 9H, C(CH3)3), 1.08 (s, 9H, C(CH3)3), 2.17 (s, 3H,
CCH3), 2.34 (d,
2JH,H = 3.5 Hz, l H, TaCH2), 2.41 (s, 3H, CCH3), 2.49 (d, 2JH.x = 3.5 Hz, 1 H,
T'aCH2), 2.56 (s,
3H, CCH3), 2.87 (s, 3H, CCH3), 3.07 (br. s, 6H, N(CH3)2), 3.16 (s, 3H, NCH),
6.94 (br. m, 4H,
CH.,), 7.00 (d, 3JH.H = 7.0 Hz, 1 H, CH,.), 7.06 (d, 3JH,H = 7.0 Hz, I H,
CH"'...); "C f 'H)
NMR ([D6]-Benzene, 100 MHz) 818.7, 19.5, 20.0, 20.5 (CCH3), 27.8, 28.0
(C(CH3)3), 42.0,
42.3 (C(CH3)2), 45.1 (NCH3), 46.2 (br. s, N(CH3)2), 59.5 (TaCH2),125.1,126.0
(CH), 133.4
(CCH3), 133.77 (Ar-CC), 134.17 (Ar-CC), 134.50 (Ar-CC), 144.28 (Ar-CC), 144.63
(Ar-CC),
182.5, 192.4 (C'O); MS(EI): m/z 676 (M{); Anal. Calcd. (%) for C30H47N4O2Ta:
C, 53.25; H,
7.00; N, 8.28; Found: C, 53.00; H, 6.81; N, 8.16.
[0096] 1N-[2.6-Diisopropylphenyl]pivalamidate)tetrakis(dimethylamido)tantalum
[0097] N-(2,6-Diisopropylphenyl)pivalamide (0.325 g, 1.25 mmol) was dissolved
in
hexanes (3 nil:.). [Ta(NMe2)5] (0.500 g, 1.25 mmol) was added as a solid and
the solution was
allowed to stir overnight. The solvent was removed in vacua and the resulting
solid was
recrystallized from hot hexanes at -35 oC, affording yellow crystals. Yield:
0.535 g (0.866
-36-
CA 02715907 2010-09-28
mmol, 69%). IH NMR ([D6]-Benzene, 400 MHz) S 1.10 (s, 9H, C(CH3)3),1.28 (d,
3JHH = 6.7
Hz, 6H, CH(CH3)2), 1.36 (d, 3JH,H = 7.1 Hz, 6H, CH(CTI3)2), 3.31(br. s, 24H,
N(CH3)2), 3.51
(sept, 3JH,H = 6.7 Hz, 2H, CH(CH3)2), 7.05 (m, 3H, CH m.); 13Cp1H} NMR ([D6]-
Benzene, 100
MHz) S 24.9, 26.6 (CH(CH3)2), 27.3 (CH(CH3)2), 29.7 (C(CH3)3), 42.4 (C(CH3)3),
47.7 (br. s,
N(CH3)2),123.9,125.8 (CHm .),141.3 (CN), 143.0 (CCH), 177.7 (C=O); MS(EI): m/z
573 (M+
- NMe2); Anal. Calcd. for C25H5oN5OTa: C, 48.61; H, 8.16; N, 11.34; Found: C,
47.75; H, 8.05;
N, 9.98.
[0098] EXAMPLE 2: Catalyfic a--Functionalization of Amines using Non-halide
Containing Ta(amidatel complexes
[0099] General procedure I (GPI) for Ta(amidate) catalyzed ai
fiunctionalization of
amines: In a nitrogen filled glove box the Tantalum complex was placed in a
small vial and the
specified amount of (deuterated) solvent was added. The solution was
transferred to a NMR tube
equipped a Teflon cap and olefin followed by amine were added sequentially by
means of a pL-
pipette. The NMR tube was closed, shaken and 1H NMR spectrum was recorded. The
NMR tube
was placed in a preheated oil bath at the indicated temperature for the given
time. After
confirmation of conversion by means of 1H NMR spectroscopy the etude reaction
mixture was
directly loaded onto a silica gel column and eluted using a mixture of
hexanes/Et Ac/NEt3.
[00100) General procedure 2 (GP2) for Ta(amidate) catalyzed a-f
mctionalization of
amines: Same procedure as GP 1, except for the usage of a Schlenk-tube
equipped with a Teflon
cap and a magnetic stir bar.
[00101] General procedure 3 (GP3) for Ta(amidate) catalyzed a
afunctionalization of
amines: In a nitrogen filled glove box the Tantalum complex was placed in a
small vial and the
specified amount of olefin was added. The solution was transferred to a
Schlenk-tube equipped
with a Teflon cap and a magnetic stir bar and the amine was added by means of
a L-pipette.
The Schlenk tube was placed in a preheated oil bath at the indicated
temperature for the given
time. After cooling to ambient temperature the crude reaction mixture was
directly loaded onto a
silica gel column and eluted using a mixture of hexanes/EtOAc/NEt3.
-37-
CA 02715907 2010-09-28
[00102] N-(2-Methyloctyl)aniline, (0094/04) P1. (H.E. Gottlieb, V. Kotlyar, A.
Nudelman J. Org. Chem. 1997, 62, 7512).
Ph=NH
[00103] The reaction was carried out following GPI from 1-octene (118 L, 0.752
mmol,
1.5 eq.), N-methylaniline (54 L, 0.498 mmol), 3 (15.1 mg, 0.024 mxnol, 5
mol%), [D6]-benzene
(0.508 g) at 110 C for 63 h. FC (silica gel G60, hexanes/EtOAc 50:1) gave the
title compound
as a clear, colorless oil. Yield: 0.101 g (0.460 mmol, 92%). 'H NMR. (CDC13,
300 MHz) 81.00
(m, 3H, CH2CH3), 1.07 (d, 3JH,H = 6.6 Hz, 3H, CHCH3), 1.26 - 1.51 (m, IOH,
CH2), 1.82 (m, 1H,
CHCH3), 2.97 (dd, 2 Jn,H 12.0 Hz, 3 JH,n = 7.5 Hz, 1H, NCH2), 3.15 (dd; 2 J ,n
= 12.0 HZ, 3 Jx,x
õ~), 6.77 (t, 3 JH,H
- 5.7 Hz, 1 H, NCH2), 3.74 (br, s, 1H, NH), 6.69 (d, 3 Jx x = 8.4 Hz, 2H, o-CH
7.2 Hz, 1H, p-CH's,,,), 7.27 (t, 3Jy,H = 7.5 Hz, 2H, m-CHAm,,.); "C ('13) NMR
(CDCI3, 75
MHz) 614.3 (CH2CH3),18.2 (CHCH3), 22.9, 27.1, 29.8, 32.1 (CH2), 33.1(CHCH3),
35.0 (CH2),
50.5 (NCH2),112.8 (o-G ,117.1(p-CHs,õ), 129.4 (m-CHft},148.8 (C); HRMS(EI)
Calcd. for C15H25N: n3/z 219.1987 (M4); Found: m/z 219.1986 (M); Anal. Calcd.
for C15H25N:
C, 82.13; H, 11.49; N, 6.39; Found: C, 82.30; H, 11.11; N, 6.56.
[00104] N-(2-Methyloct 7-enyl)avWne, (0114103) P2.
Ph.HH
[00105] The reaction was carried out following GP 1 fn,m 1,7-octadiene (110
L, 0.745
mmol 1.5
eq.), N-methylaniline (54 L, 0.498 mmol), 3 (15.5 mg, 0.025 mmol, 5 maI lo),
[De]-
toluene 0.500 g) at 130 C for 15.5 h. FC (silica gel G60,
hexanes/EtOAc/NEt3100:1:1) gave the
title compound as a clear, colorless oil. Yield: 0.053 g (0.244 mmol, 49%).
-38-
CA 02715907 2010-09-28
[00106] 2,7-Dimethyl-Nf,N$-dipheuyloctane-1,8-diamlue, (0114/02) P3.
Ph.NH
MN.. Ph
[00107] The reaction was carried out following GPI from 1,7-octadiene (78 L,
0.528
mmol), N-methylaniline (108 pL, 0.998 mmol,1.89 eq.), 3 (15.5 mg, 0.025 mmol,
5 mol%),
[D8]-toluene 0.507 g) at 130 C for 19 h. FC (silica gel G60,
hexanes/EtOAc/NEt3100:1:1) gave
the title compound as a clear, colourless oil. Yield: 0.121 g (0.3 73 mmol,
75%)
[00108] N-(Bicyclo[2.2.1]heptan-2-ylmethyl)aniline, P4.
Ph.,H
H
[00109) N-(Bicyclo[21.1]hept-5-en-2-yhnethyl)anlline, P5.
Ph., ii
1
[00110] N-(Cyclohexylmethyl)aniline, (0107/02) P11.
Ph.. [00111] The reaction was carried out following GP3 from cyclohexene (304
AL, 3.00
mmol, 6.0 eq.), N-methylaniline (54 L, 0.498 mmol), 3 (30.7 mg, 0.05 mmol, 10
i nol%) at 165
-39-
CA 02715907 2010-09-28
C for 96 h. FC (silica get G60, hexanes/EtOAcJNEt3100:1:1) gave the title
compound as a
clear, colorless oil. Yield: 0.029 g (0.1 53 mmol, 31 %).
[00112] N-(Cyclooct-4-enylmethyl)aniline, P6.
Ph.H I
[00113] N-(Cyclooctylmethyl)anlline, (0112/01) P7.
Ph.-H "'~o
H
[00114] The reaction was carried out following GP 1 from cyclooctene (194 L,
1.49
mmol, 3.0 eq.), N-methylaniline (54 AL, 0.498 mmol), 3 (30.8 mg, 0.050 mmol,
10 mol%), [D8]-
toluene 0.507 g) at 165 C for 48 h. FC (silica gel G60, hexanes/EtOAc/NEt3
100:1:1) gave the
title compound as a clear, colorless oil. Yield: 0.055 g (0.253 mmol, 51%).
[00115] N-(2-Methyl-3-phenylpropyl)aniline, (0111/01) P12. (S.B. Herzon, J.F.
Hartwig J. Am. Chem. Sac. 2007,129,6690)
Ph... N Ph
H
[00116] The reaction was carried out following GP 1 from allylheozene (99 l;,
0.747
mmol, 1.5 eq.), N-methylaniline (54 pl, 0.498 mmol), 3 (15.4 mg, 0.025 mmol,10
mol%), [Dg]-
toluene 0.516 g) at 130 C for 19 h. FC (silica gel G60, hexanes/EtOAc 50:1)
gave the title
compound as a clear, colorless oil. Yield: 0.095 g (0.422 mmol, 85%). 'H NMR
(CDC13, 400
MHz) S 1.09 (d, 3Jxx = 6.8 Hz, 3F 1, CH3).
-40-
CA 02715907 2010-09-28
[00117] N-(2-Cyclohexylpropyl)aniline, (0118/01) P8. (S.B. Herzon, J.F.
Hartwig J. Am.
Chem. Soc. 2007,129,6690)
Ph
N
H
[00118] The reaction was carried out following GPI from vinylcyclohexane
(102,uL,
0.745 mmol, 1.5 eq.), N-methylaniline (54 AL, 0.498 mmol), 3 (15.1 mg, 0.024
mmol, 5 mol%),
[Ds]-toluene (0.513 g) at 145 C for 15 h. FC (silica gel G60,
hexanes/EtOAc/NEt3 100:1:1)
gave the title compound as a clear, colorless oil. Yield: 0.097 g (0.446 mmol,
90%). 'H NMR
(CDC 13, 400 MHz) 61.03 (d, 3 JH,H = 6.8 Hz, 3H, CH3), 1.12 - 1.48 (m, 6H, CH
& CH2
cyclohexyl), 1.69 - 1.87 (m, 6H, CHCH3 & CH2 of cyclohexyi), 2.97 (dd, 2 JH,H
= 12.0 Hz, 3 JH,H
= 7.6 Hz, 1H, NCH2), 3.24 (dd, 'J.,. = 12.0 Hz, 3 JH,H = 5.2 Hz, 1H, NCH2),
3.70 (br. s, 1H,
NB'), 6.69 (d, 3 JH,H = 8.0 Hz, 2H, o-CH.,.), 6.77 (t, 3 JKH = 7.4 Hz, 1 H, p-
CHI,,,), 7.26 (t, 3JH,H
= 7.4 Hz, 2H, m-CHI,); 13C {'H) NMR (CDC13r100 MHz) 615.0 (CH3), 26.9, 26.9,
27.0, 28.9,
31.1 (CH2 cyclohexyl), 38.1 (CHCH3), 41.0 (CH cyclohexyl), 48.1 (NCH2), 112.8
(o-CHõ m),
117.1 (p-CHI,,,), 129.4 (m-CHa,,,), 148.9 (C); HRMS(EI) Calcd. for C15H23N:
m/z 217.1830
(M'; Found: m/z 217.1830 (M); Anal. Calcd. for C15H23N: C, 82.89; H, 10.67; N,
6.44; Found:
C, 82.89 H,10,67; N, 6:44.
[00119] 2-(Octan-2-yl)-1,2,3,4-tetrahydroquinoline, P9.
H
N
-41-
CA 02715907 2010-09-28
[00120] 2-(Octan-2-y'1)-1-tosylpiperidine, (0097/03) P10.
TS
N
[00121] The reaction was carried out following GP2 from 1-octene (470 2.99
mmol,
3.0 eq.), piperidine (99 L,1.0 mmol), 3 (62.0 mg, 0.10 mmol, 10 mol%),
toluene (0.510 g) at
165 C for 134 h. Derivatization: After cooling to ambient temperature a
solution of 2 M
aqueous NaOH (1.5 mL) followed by solidp-TsC1 (0.303 g, 1.59 mmol) and DCM
(1.0 mL)
was added. The reaction mixture was stirred at ambient temperature for 29 h
and poured into H-
20 (50 mL) and extracted with EtOAc (100 mL). The organic phase was washed
with saturated
brine, dried over Na2SO4, filtered, and the solvent evaporated on a RV. FC
(silica gel F60,
hexanes/EtOAc 10:1 + 5% NEt3) gave the title compound as a clear, colorless,
viscous oil. Yield:
0.268 g (0.762 mmol, 76%). 'H NMR (CDC13, 400 MHz) S 0.82 - 0.89 (m, 6H,
CH2CH3 &
CHCH3), 1.00 - 1.47 (m, 15H, CH2), 1.67 (m, 1H, CH2), 1.89 (m, 1H, CHCH3),
2.39 (s, 3H,
CCH3), 2.92 (m, l H, NCH2), 3.62 (m, l H, NCH), 3.77 (m, l H, NCH2), 7.25 (d)
3JMH = 8.0 Hz,
2H, o-CHI,õ to CH3), 7.71 (d, 3JH,H = 8.4 Hz, 2H, o-CH=ra, to SO2N); 13C{1H}
NMR (CDC13,
100 MHz) S 14.2 (CH2C'H3), 16.5 (CHCH3), 18.8 (CH2), 21.6 (Ca, CH3), 22.8
(CH2CH3), 24.0
(CH2), 24.8 (NCHCH2), 26.7, 29.8 (CH2), 30.6 (CHCH3), 31.9 (CH2), 33.6
(CH3CHCH2), 41.1
(NCH2), 58.1 (NCH), 127,1 (o-CH.m, to S02N),129.7 (o-CH.m, to CH3),
139.6,142.7 (C);
HRMS(E1) Calcd. for C20H33NQ2S: m/z 351.2232 (M); Found: n /z 351.2232 (M);
Anal.
Calcd. for C20H33NO2S: C, 68.33; H, 9.46; N, 3.98. [00122] EXAMPLE 3:
Substrate Scope in Catalytic oe-Functionaiization of Amines using
Non-halide Containing ja(amidate) complexes
(00123] A variety of mono(amidate)-tantalum complexes were prepared at ambient
temperature in hexanes to give crystalline complexes 2, 3, and 4, which have
varying degrees of
steric bulk (Scheme 10).
-42-
CA 02715907 2010-09-28
Scheme 10
0 0
R-- (Ta{h A] R )Ta(NMe2)4
RN NH hexane, ITT R~ N
,,,.. R, - HNMe2
rwrysstellization
R=rBu, R-Me, 2,65% yield
R=(bu, R`=Pr: 3, 99% yidd
R=Ph, R"=Pr: 4, 50% yield
[00124] Most importantly, a screen of complexes 1-4 as catalysts for the a-
alkylation of
N-methylaniline with 1-octene shows that mono(amidate)-tantalum complexes
(Table 1, entries
2-5) allow reactions at a lower temperature (110 C) compared to the parent
bis(amidate)
compound 1. Whereas most mono(amidate)-tantalum complexes show reactivity at '
110 C,
[Ta(NMe2)5] requires at least 130 C to effect product formation (Table 2,
entries 6 and 7).
Table 2: Catalyst screening of tantalum amido complexes.
GSII.
M # t1'N96
Ph' -Me ( s)s~% IDa benzene
Pti (CH24CH3 Entry Catalyst Conditions Conversion [%[t]
1 1 _ 130 C,1 week[C'de] 71
2 2 130 C, 24 Al] h[ 84
3 2 110 C, 68 ht"I 69
4 3 110 C, 63 ht"I 96 (92)
4 110 C, 77h[c,f] 85
6 [Ta(NMe2)51-- -- 110"C101 Mr.
7 [Ta(NMe2)s] 130 C, 67 ht n 89 (80)
[a] Yield of isolated product given in brackets; conversion was estimated by
1H
NMR spectroscopy. [b] 10 mol%; [c] [N-methylaniline]=1 m.
-43-
i
CA 02715907 2010-09-28
[d] N-methylaniline/1-octene 1:1.05, [e] [Ds]toluene as solvent.
[f] N-methylaniline/1-octene 1:1.5. n.r. = no reaction.
[00125] The observed reactivity trends suggest that steric bulk is required to
favour the i3-
hydrogen abstraction reaction, yet too much steric bulk appears to inhibit
olefin insertion. This mechanistic interpretation would account for the ready
formation of metallaaziridine 1, as well as
its overall reduced catalytic activity. Furthermore, this is consistent with
the empirical {
observation that increased alkene loading (e.g., N-
methylaniline/vinylcyclobexane (see below)
1:2.4 versus 1:1.2) results in improved relative rates of reaction in side-
byside NMR tube
experiments. These early results show that sterically bulky mono(amidate) -
tantalum complex 3
is the most efficient among the tested precatalysts and a more complete
exploration of its
substrate scope is provided in Tables 3 and 4.
[00126] In alkene substrate scope investigations terminal alkenes having
steric bulk, such
as vinylcyclohexane, and easily isomerized allylbenzene form the corresponding
c -alkylation
products 6 and 7 in high product yields upon isolation. Norbornene, as an
activated olefin, is
efficiently converted into the corresponding product 8 with unprecedented
diastereoselectivity
(>20:1) at 110 C. Protected alcohols can be used to access silyl protected
amino alcohol
derivative 9. Exposing 1,7-octadiene to the standard conditions while using
1.9 equivalents of N-
methylaniline results in the synthesis of diamine diastereomers (10) in a 1:1
ratio. Alternatively,
by using an excess of the unactivated internal diene I,5-cyclo-octene, this
reaction results in
step-wise addition to give the mono-alkylated product 11 selectively, leaving
a C=C bond for
additional functionalization.
fE
-44-
CA 02715907 2010-09-28
Table 3: Alkene substate scope using 3 as a catalyst
M 5 rnoI% 3_ H R
W 'me tt [Do] toluene PN-N=,r
Entry AHene Conditions Product Yield (%ri
Gy
1 GY 14S C, Ph~y
15ha4 6 90
2 Ph.
Ph 19 ~ H l 85
7
Ph,,
3 110 H 931
961P=4
4 OTBDt 13 ,N.OTBDluls 35
I I hP4 9
000C.
191"
Ph -
6 I I 165 9c, CO 83
96 hIld
11
[a] Yield of isolated product. [b] (N-me lt . lanifnnae] =1 to; [c] N-methyien
i-
line/1.oCi " 1.1.5. (d.] A greater than 20:1 dr. at estimated by NM R
spectroscopy (e] N-methylaniline/1,7-octediene 1.9:1; (N-methylani-
line] =2 ra. (fl Mature of 1:1 ric/mesa as estimate by NMR spectral
copy. (g( N-rnWrylaniline/1,5-qcloocdiene 1:3,10 mol% S. Cy=cyclo-
hexXl, TBDMS=tent-butyldimelhylsi1yi.
[00127] Preliminary amine substrate scope investigations show that
functionalized
arylalkyl amines, dialkyl amines, and amine heterocycles all undergo catalytic
a-alkylation
(Table 3). Reacting 4-methoxy-N-methylaniline with terminal olefins delivers
the corresponding
para-methoxyphenyl (PMP) protected amines 12 and 13 in good yields.1"1 The
facile reactivity
observed with these substrates provides a ready route for the synthesis of
substituted primary
amines, upon deprotection. In contrast to the work by Herzon and Hartwig,1411
exclusive
alkylation at the benzylic position of N-benzyl methylamine was observed, in
accordance with
relative C-H bond dissociation energies, to give a single diastereomeric
product (14) in 75%
-45-
CA 02715907 2010-09-28
yield after derivatization as the N-tosyl amide. Notably, this class of
catalysts can be used in the
direct, diastereoselective alkylation of heterocycles such as 1,2,3,4-
tetrahydroquinoline and
piperidine. This is the first example of the 100% atom economic, catalytic a
alkylation of
piperidine. The synthesis of substituted piperidines is typically achieved by
multistep
approaches. [12]
Table 4: Amine substrate scope using 3 as a catalyst
Ito
H q R-
R=N~"r t cat. 3 R" ~iwene R'
130 "C
Entry Arnine All:erre t [h] Product Yidd FA14
1 .M~ ' (CE CH3 201M PMP'g (CHh 87
PMP
12
2 -N% creawa 2 P.OTBDNAB
r~
13
Ph OPNCHCHb4 PhyI---(CM2kCH3 FOA
MN,., (A. 5d TsN 75
14
H
4
(X) (cH2}bcHi 37" ICN2}' W3 92"
rri
5 CJNH (CH ,CH, 13011 (P 15C 74PA
16-
[a] Yield of isol d product [b] ]N-m*hylaniline] 1 u. jt] N-mettrylaniiinefl-
oelene
1:13. [4 isolated after derivatization as the N-toyl amide. [e] A greaw than
20:1 d.r.
as estimated by NMR spectrcecopy. [q Isolated aftr derivatuzetlon as the N-
taslyEamide, W Arnirre/1 one 1;3,1(f mol % 3. [h] {piperidire] = 2. m, 1659C.
Ti -- 4-
tc lu~enesulion 1. `
[00128]
-46-
CA 02715907 2010-09-28
[00129] EXAMPLE 4: Enantioselective catalytic a-alkylation of amines
[00130] Chiral amidate precatalysts are known and axially chiral diamines have
been used
for the synthesis of tethered bis(amidate)-zirconium complexes for the highly
enantioselective
catalytic synthesis of amines by hydroamination.[13] In this case, the cisoid
(?meaning)
disposition of the oxygen atoms in the amidate in 1 suggests that axially
chiral derivatives of
diacids may be preferred for the synthesis of chiral, catalytically active
tantalum complexes. The
requisite biphenyl bis(amide) proligand is accessible by reacting the in situ
formed 6,6'-
diznethyl-biphenyl-2,2'-dicarboxylic acid chloride[14] with 2,6-
diisopropylaniline in the
presence ofNEt3.[15]
(001311 Precatalyst 17 was synthesized in excellent yield by a protonolysis
reaction
between [Ta(NMe2)5] and this C2 symmetric tethered bis(amide) proligand (see
above {
description and Figure 2).
[00132] Chiral precatalyst 17 has been tested with a variety of alkene and
amine
substrates, and gives enantioenriched secondary amine products. The results
are provided in
Table 5.
[00133] The reaction of I -octene with N-methylaniline (Table 5, entry 1),
requires
extended reaction times to proceed to completion and gives modest ee
(ee=enantiorneric excess)
values. However, with yield (isolated) of diastereomerically pure product 8 in
61 % ee (Table 4,
entry 2). These preliminary asymmetric catalytic experiments show that alkenes
with varying
steric bulk give satisfactory reactivity and cc values (Table 5, entries 1-3)
for this reaction.
Importantly, different amines including a functionalized arylalkyl amine and
an amine
heterocycle can be used with this first generation chiral catalyst to generate
amines 18 and 19
with 52% ee and 57 % ee, respectively.
-47-
CA 02715907 2010-09-28
Table 5: Enantioselective catalytic cr-allcylation of amines
mot% 17 R'
(i74 Worm R` Ft"
N 13 !'C R'
Entry Arnine Alker t [h] Product Yield it I%f4
1 rN (CHZ15CHg 68 Ph 0-"- (CH2) a 86 44
Pt,
S
Ph
2 N. lI { J 46 H 8p 61"
3 ,N -CY 48 92 43
4 ii RIPrN1 '(CH CH3 24 pp rll'`+I 90 52
5
C H 40"`(CHAGFI3 192 so $71'1
19
(a] Amine/alkene 1:2. IN Yield of isolated product. (c] The as values ware
determined
by HPLC analysis of benxamide derivative. (d] The am configuralion was
confirmed by
X-ray spectroscapy, but the absolute configuration was not established. (e] in
analogy
to entry 2.
(00134] The modest enantioselectivities obtained with this first example of a
chiral
tantalum complex for hydroaminoalkylation can be rationalized by the fact that
only a bidentate
binding mode is observed for this C2 symmetric chiral ligand in both the solid
state and solution
phase. This less sterically demanding binding mode removes both the bulky N
substituents and
the enantiodetermining axially chiral biphenyl group from the reactive metal
center. Thus,
modification of this flexible ligand set can be used to produce group 5 metal
amidate complexes
tailored for asymmetric catalysis for the hydroaminoalkylation reaction.
-48-
i
CA 02715907 2010-09-28
[00135] EXAMPLE 5: Catalytic N-Heterocycle functionalization arto N using
Ta(V)(amidate) Complexes
[00136] Saturated N-Heterocycles are privileged structural elements found in a
wide
variety of sophisticated molecules used in therapeutics and agrochemicals.
Consequently, the
rapid and selective construction of funetionalized, saturated N-heterocycles
from simple,
inexpensive, readily available chemicals is critical in an industrial context.
In addition, the
development of "green" technologies in synthesis is an urgent goal with
respect to
transformations with 100% atom efficiency, high selectivity (chemo-, regio-,
and
stereoselectivity) and high chemical yields. Recently, group 4 and group 5
based catalyst systems
have been successfully used in functionalizing the spa C-H bond a to 1 and 2
amines 2-5,8 As
catalysts, Zr(IV) bis(2-hydroxypyridinate)5 and Ta(V) amidate complexes have
been found to be
particularly beneficial. The Zr(V) system selectively cyclizes 10 aminoalkenes
to the
corresponding carbocyclic 1 amines whereas the Ta(V) system has proven to be
a selective
system for the intermolecular hydroaminoalkylation of 2 dialkyl and alkyl
aryl amines with a
number of terminal and internal olefins. Both catalyst systems deliver the
corresponding
branched products exclusively. Further, piperidine can undergo a-alkylation
using l -octene and
a Ta(V)-catalyst, albeit at elevated temperatures, to give 74% of 2-(octan-2-
yl)-1-tosylpiperidine
after N-tosylation as a single diastereomer. This result prompted an
investigation N-heterocycle
substrate scope since it has been previously found that there can be
significant variation in fff
reactivity based on substrate.
[00137) The results of these studies are provided in Table 6.
-49-
CA 02715907 2010-09-28
Table 6
H + To-cat. (N))R
R 165 C
X X
Entry X R It Yield4 d.r.b
1 CH2 n-hexyl 143 h 76% > 20:1
2 CH2 (CH2)4OTSDMS 3 d 50% > 20:1
3 CH2 norbomene 3d 75 20:1
4 CH2CH2 n-hexyl 72 h 60% 10:1
CHMe n-hexyl 3d 50% NA
8 CHBn n-hexyl 3d 50% NA
7 ~ n-hexyl 69h 59% > 20:1
8 0 n-hexyl 5 d -
9 S n-hexyl 2d -
NPMP n-hexyl 89 h 69% > 50:1
11 NPh n-hexyl 72 h 68% > 20:1
12 We n-hexyl 72 h 43% > 20:1
13 NBhyd n-hexyl 72 h 46% > 20:1
14 NBhyd CH2Ph 72 h 84% > 20:1
NBz n-hexyl 24 h - > 20:1
Cy = cyclohexyl, Bn = benzyl, Bz = benzoyl,
PMP = pare-methoxxyphenyl, Bhyd = benzhydryl
NA= not available
a Isolated after N-tosylation; b 1H NMR spectrum
of isolated product
[00138] Reactions were also performed using 1,2,3,4-tetrahydroquinoline as the
amine
substrate. The results are shown below:
-50-
r
CA 02715907 2010-09-28
H
N n-hexyl
92%, ANTE 2009
OTWMS
n
n=1: 64%13 mmol scale / A:O 1:1.6,145 C, 165 h
n=2:78% / NO 1:1.35,145 C,118 h
[00139] The results provided in this Example demonstrate the chemo-, regio-,
and
diasteroselective synthesis of fhnctionalized N-heterocycles which have
challenging to access by
other traditional multistep synthetic methods. Furthermore, the assembly of
orthogonally
protected 2-substituted piperazines is demonstrated, which can serve as
versatile building blocks
in the synthesis of more complex materials.
[00140] EXAMPLE 4: Synthesis and characterizaion of chloride-containing
Ta(amidate)
comlexes
[00141] (N-(2,6-diisopropvlpheny iyalamidotrisfdimethvlaminoltantalum
M
chloride. 18.
O~
tBu-~~,,TaCI(NMe2)3
/Pr N
Pr
I\v ' 18
[00142] N-(2,6-Diisopropylphenyl)pivalamide (0.131 g, 0.50 mmol) and
[TaC1(NMe2)4]
(0.198 g, 0.50 mmol) was suspended in hexanes (3 mL) and the mixture was
allowed to stir over
night (18 h) to give a Clear yellow solution. All volatiles were removed in
vacuo and the
remaining solid was recrystallized from hot hexanes at -30 C. The yellow
crystals which were
collected and identified as the title compound. Yield: 0.219 g (0.36 mmol,
71%). 1H NMR
-51-
CA 02715907 2010-09-28
([D6]-Benzene, 400 MHz) 6 1.11 (s, 9H, C(CH3)3),1.22 (br. s, 311, CH(CH3)2),
1.23 (br. s, 3H,
CH(CH3)2), 1.36 (br. S, 3H, CH(CH3)2), 1.43 (br, s, 3H, CH(CH3)2), 3.22 (br.
s, IH, CH(CH3)2),
3.49 (br. s, 18H, N(CH3)2), 4.25 (br, s, 1H, CH(CH3)2), 7.04 - 7.10 (m, 3H,
CH,,,.);
13C {'H} NMR ([D6]-Benzene, 100 MHz) 6 23.7, 24.9, 25.7, 27.2 (br. s,
CH(CH3)2), 27.9, 28.0
(br.s, CH(CH3)2), 28.5 (C(CH3)3), 42.3 (C(CH3)3), 48.9 (br. s, N(CH3)2),
123.3, 124.6 (br. s,
Crom.), 126,4 (CHarom,), 140.4 (C,= N), 142.5, 144.8 (br. s, C=,,,.CH),181.2
(C=O). Single
crystal X-ray quality samples were obtained by recrystallization from hot
hexanes at -30 C.
[00143] - 2 6-diiso ro 1 hen 1 ivalamido s dimeth lamino tantalum
chloride, 18.
O
tBu-< ; TaCI(NMe2)3
N
iPr
-~ Pr
X
(00144] N-(2,6-Diisopropylphenyl)pivalamide (0.307 g, 1.175 mmol) and sodium
bis(trimethylsilyl)amide (0.215 g, 1.175 mrnol) were weighed into a Schlenk
flask and dry
diethyl ether (25 mL) was added in one portion without stirring. After
addition of dry ether was
complete, the mixture was set to stir vigorously for 1.5 hrs. The diethyl
ether was then removed
in vacuo, and dry pentanes (5 mL) was added to rinse the solid and then
subsequently removed in
vacua to give sodium N-(2,6-Diisopropylphenyl)(pivaloyl)amide (0.333 g, 1.175
mmol) in
quantitative yield. This was subsequently dissolved in dry THE and transferred
via cannula into a
yellow solution of [TaC12(NMe2)3]2 (Chisholm, M. H.; Huffman, J. C.; Tan, L.-
S. Inorg. Chem.
1981, 20, 1859) (0.9024 g, 1.175 mmol) in dry THE (15 mL). The solution was
allowed to stir
overnight. All volatiles were removed in vacuo and the remaining solid was
recrystallized from
pentanes at -30 T. The yellow-orange crystals which were collected and
identified as the title
compound. Yield: 0.572 g (0,94 mmol, 80 %). NMR data is consistent with above
characterization.
[001451 The proligand sodium salt, sodium N-(2,6-
Diisopropylphenyl)(pivaloyl)amide,
can also be made on large scale by the method described above and stored for
future use.
52-
e
CA 02715907 2010-09-28
[00146] Bis(N-(2,6-diifnethylphenyllnivalamido bis(dimethylamino tantalum(V)
chloride.
(0165/01) 20.
o NMe2
tBu-(,. Ta-Cl
Me ~" N NMez
Me
2 20
[00147] N-(2,6-Dimethylphenyl)pivalamide (0.102 g, 0.50 mmol, 2 eq.) and
[TaCl(NMe2)4] (0.991 g, 0.25 mmol) was suspended in hexanes (3 mL) and the
mixture was
allowed to stir over the weekend (51 h) to give a slightly cloudy yellow
solution. The solvent
was removed in vacuo and the yellow foam was dissolved in the minimal amount
of hot hexanes.
The mixture was kept at -30 C to deposit yellow crystals which were collected
and identified as
the title compound. Yield: 0.170 g (0.24 mmol, 94%). 'H NMR ([D6]-Benzene, 400
MHz) S
1.00 (s, 18H, C(CH3)3), 2.42 (br. s, 6H, CCH3), 2.47 (br. s, 6H, CCH3), 3.57
(s, 6H, N(CH3)2),
3.69 (br. s, 6H, N(CH3)2), 6.87 - 6.94 (to, 6H, CH,); 13C{1H} NMR ([D6]-
Benz(ne,
100 MHz) 6 21.0 (br. S, Cmm.CH3), 27.6 (C(CH3}3), 41.9 (C(CH3)3), 46.7
(N(CH3)2), 47.9 (br. s,
N(CH3)2), 125.8 (CH,rom.), 128.3 (CHI,,,.), 134.0 (br. s, C m.CH), 143.0 (br.
s, C ,,N),186.3
(C=O). Single crystal X-ray quality samples were obtained by recrystallization
from hot hexanes
at -30 C.
[00148] rac-Bis(dimethylamina)(N.N'-(6.61-dimethvlbiphenvl-2.21-diyl)bis 22-
dimethyl
propanamidate))tantalum(V) chloride. (0180/01)21.
N= O
NMe2
CI-Ta
/ ' NMe2
N--=O
T 21
-53-
CA 02715907 2010-09-28
[00149] rac-NN'-(6,6'-Dimethylbiphenyl-2,2'-diyl)bis(2,2-dimethylpropanamide)
(0.095 g, 0.25 mmol) and [TaC1(NMe2)4] (0.100 g, 0.255 mmol) was suspended in
hexanes
(2 mL) and the mixture was allowed to stir over night (14 h) to give a clear
orange solution. All
volatiles were removed in vacuo to yield orange foam which was identified as
the title
compound. Yield: 0.170 g (0.24 mmol, 99%), 'H NMR ([D6]-Benzene, 400 MHz)
61.24 (s, 9H,
C(CH3)3), 1.25 (s, 9H, C(CH3)3),1.97 (s, 3H, CCH3), 2.00 (s, 3H, CCH3), 3.19
(s, 6H, N(CH3)2),
3.61 (s, 6H, N(CH3)z), 6.72 (d, 3JH,H = 7.6 Hz, 1H, CHH ), 6.76 (d, 3JH,H =
7.6 Hz, 1H, CH..,),
6.92 (d, 3JH,x - 7.4 Hz, I H, CH..), 7.01 (t, 3JaH - 7.7 Hz, I H, CH.,,.),
7.04 (t, 3JH = 7.7 Hz,
1H, CHI,), 7.30 (d, 3JH,H = 7,8 Hz, 1H, CH,m);13C{'H} NMR ([D6]-Benzene, 100
MHz) 6
20.3, 20.8 (C õ, CH3), 28.5, 29.0 (C(CH3)3), 39.8, 41.5 (C(CH3)3), 45.8, 48.3
(N(CH3)2),119.5,
124.4, 124.8, 127.1, 127.6, 128.6 (C1Ium ),129.3,134.9,136.8,
138.0,141.2,147.7 (Cu..),
164.2,184.1 (C=O). Single crystal X-ray quality samples were obtained by
recrystallization from
hot hexanes at -30 C.
[00150] nacBis(dimethvlamino,)(N,N-(6.6'-dimethylbinhenvl-2.2'-divllbis(2.4.6-
td
methvlbenzamidate)tantalum(V) chloride. (0183/01) X.
I
! I -
\ /' NMe2
CI Ta/ ;>W2
NO
22
(00151] rac-N,N-(6,6'-Dimethylbiphenyl-2,2'-diyl)bis(2,4,6-trimethylbenzaan
ide)
(0.251 g, 0.50 mmol) and [TaCI(NMe2)4] (0.197 g, 0.50 mmol) was suspended in
hexanes F
(5 mL) and the mixture was allowed to stir over night (18 h) to give a yellow
suspension. The
reaction mixture was heated to reflex for 5 min followed by removal of all
volatiles in vacuo to
give a light yellow powder which was identified as the title compound. Yield:
0.401 g
(0.496 mmol, 99%). 'H NMR ([D6]-Benzene, 400 MHz) 81.92 (s, 3H, CCH3),1.96 (s,
3H,
-54-
CA 02715907 2010-09-28
CCH3),1.98 (s, 3H, CCH3), 2.02 (s, 3H, CCH3), 2.05 (s, 3H, CCH3), 2.19 (s, 3H,
CCH3), 2.59 (s,
3H, CCH3), 3.64 (s, 3H, CCH3), 3.64 (s, 6H, N(CH3)2), 4.24 (s, 6H, N(CH3)2),
6.37 (s, 1H,
CHam), 6.47 (s, IH, CHI), 6.62 (d, 3JU.H = 7.6 Hz, 1H, CHI,,,,), 6.74 (m, 3H,
CH.,o.), 6.81
- 6.91 (m, 3H, CHI,,,.), 7.01 (d, 3JH,g w 7.6 Hz, 1H, CHaram.)i 13C{'H} NMR
([D6]-Benzene, 100 MHz) 6 19.8,20.5,20.7, 20.9, 21.2, 21.5 (2X), 22.2
(Cm,,CH3), 46.5 (N(CH3)2), 49.9 (br. s,
N(CH3)2), 123.4, 126.6, 127.6, 127.9,128.0 (2x), 129.2, 129.5, 129.6, 130.6
(CH=,_), 132.0,
132.1, 132.5,134.4,135.2,136.6, 137.3,138.1, 140.0, 140.3, 140.6,
141.0,143.4,143.7 (C d,.),
182.4, 184.3 (CO). Single crystal X-ray quality samples were obtained by
recrystallization from
hot hexanes at -30 C.
[00152] EXAMPLE 5: Comparison of Catalytic Reactivities
[00153] The following reaction was performed using two Ta(amidate) complexes
in order
to compare catalytic reactivities:
H 5MOMCat,
1 1.5 C7, 110 e ,r xy~
[00154] The two Ta(amidate) complexes used in this reaction differed in that
complex 18
is the halo equivalent of complex 1(referred to as the "Parent' 'in Figure 3).
The structures of the
complexes are provided below:
tBu-{~Ta(NMe2)a tBu- TaCI(NMe2)3
.
Pri r
-=Pr Pr
' 1 I 18
[00155) The results are depicted in Figure 3. As shown in Figure 3, the
TaCl(amidate)
complex provided a significantly improved reactivity over the parent,
Ta(amidate) complex. In
fact, the data shows that there is increased reactivity as the amount of the
TaC1(amidate) complex
decreases, which is indicative of an efficient catalyst.
!k!
-55-
i
CA 02715907 2010-09-28
[00156] EXAMPLE 6: Catalytic a.Functionalization of Amines using TaC1(biaryl
bis(amidate))-Complexes
[00157] The following reaction was performed using the chloride containing
Ta(amidate)
complexes 20 and 22, which were synthesized and characterised as described in
Example 4
above.
MOM cat Ph'
a
1.s Cate or C
[00158] No cr-alkylation was observed after 18 hours at 120 C using either
catalyst.
However, after two days at 165 C using complex 20, 75% conversion to the a-
alkylated product
was observed, and after two days at 165 C using complex 22, 44% conversion to
the cx-alkylated
product was observed.
[00159] EXAMPLE 7: Tantalum Amidate Complexes in the Efficient Synthesis of
Amino Acids.
(00160) The Ta amidate complexes of Formula I can be used in an efficient
synthesis of a
broad range of ac-, A- or y-Amino Acids from commercially available aniline
derivatives and
alkenes. The following retrosynthetic scheme provides one example.
H
00~H R,
R MaCrIa
(00161] of-, [i- and y- amino acids have pharmaceutical applications and are
the focus of
ongoing research. Of particular interest is the development of robust,
efficient methods for
enantioselective production of a-, f3- and y- amino acids. The chiral metal
amidate catalysts
described herein can be used to achieve the enantioselective synthesis in a
very atom-economic
fashion.
-56-
CA 02715907 2010-09-28
[00162] Previously characterized tetradentate amidate chelates give 13C NMR
chemical
shifts for a carbonyl group at approximately d = 180 ppm.
[00163] One example of a well known y-amino acid based pharmaceutical is
pregabalin
(Lyricem, by Pfizer), which is an anticonvulsant drug used for the treatment
of neuropathic pain
and as an adjunct therapy for partial seizures, as well as for the treatment
of generalized anxiety
disorder. The structure of pregabalin is shown below:
H2N COZH
[00164] The chiral group 5 metal amidate complexes, for example, the chiral Ta
amidate
complexes, described herein can be used in catalytic hydroaminoalkylation to
generate chiral
substituted amines.
H Ta Cit. H
R'N +`'R F~~ R
[00165] By choosing appropriate functional groups that can be simultaneously
deprotected
and oxidized under oxidizing conditions, a 2-step, one-pot synthesis of y-
Amino Acids can be
achieved. By using chiral group 5 metal amidated complexes as described
herein, the
enantioselective synthesis of these compounds can be achieved. An example of a
reagent that can
be used to provide oxidizing conditions for a 2-step, one pot synthesis is the
CAN (Ceric
ammonium nitrate) reagent.
H
f R, R
Ta Cit. H O 1 B CAN 002H
~,TBUC R
[00166] ~ pregabalin.
In the scheme set out above, when R is isopropyl, the product is
-57-
CA 02715907 2010-09-28
[00167] References
[00168] (1) T. E. Muller, K. C. Hultzsch, M. Yus, F. Foubelo, M. Tada, Chem.
Rev. 2008,
108, 3795 -3892.
[00169] (2) a) M. G. Clerici, F. Maspero, A. D'Alfonso, Ger. Pat. 2748293,
1977; b) M.
0. Clerici, F. Maspero, Synthesis 1980, 305-306.
[00170] (3) W. A. Nugent, D. W. Ovenall, S. J. Holmes, Organometallics
1993,2,161-
162.
[001711 (4) a) S. B. Herzon, J. F. Hartwig, J. Am. Chem. Soc. 2007,129,6690-
6691; b) S.
B. Herzon, J. F. Hartwig, J. Am. Chem. Soc. 2008,130, 14940 -14941.
[00172] (5) a) R. Kubiak, I. Prochnow, S. Doye, Angew. Chem. 2009, 121, 1173 -
1176;
Angew. Chem. Int. Ed. 2009, 48,1153 - 1156; b) 1. Prochnow, R. Kubiak, O. N.
Frey, R.
Bechaus, S. Doye, ChemCatChem 2009,1,162-172.
[00173] (6) P. W. Roesky, Angew. Chem. 2009,121, 4988 - 4991; Angew. Chem.
Int. Ed.
2009, 48, 4892 -4894.
[00174] (7) A. V. Lee, L. L. Schafer, Bur. J, Inorg, Chem. 2007, 2243 -2255.
[00175] (8) J. A. Bexrud, P. Eisenberger, D. C. Leitch, P. R. Payne, L. L.
Schafer, J. Am.
Chem. Soc. 2009, 131, 2116-2118.
[00176] (9) L. J. E. Stanlake, L. L. Schafer, Organometallics 2009, 28, 3990.
[00177] (10) a) J. M. Mayer, C. J. Curtis, J. E. Bercaw, J. Am. Chem. Soc.
1983, 105, 2651
-2660; b) L. Scoles, K. B. P. Ruppa, S. Gambarotta, J. Am. Chem. Soc. 1996,
118, 2529-2530;
c) H. Cai, T. Chen, X. Wang, A. J. Schultz, T. F. Koetzle, Z. Xue, Chem.
Commun. 2002,230-
231.
231.
-58-
f
CA 02715907 2010-09-28
[00178] (11) For PMP removal, see: A. Suzuki, M. Mae, H. Amii, K. Uneyama, J.
Org.
Chem. 2004, 69, 5132-5134.
[00179] (12) Reviews on piperidine synthesis: a) M. G. P. Buffat, Tetrahedron
2004, 60,
1701 -1729; b) S. Kallstrom, R. Leino, Bioorg. Med. Chem. 2009, 16, 601 - 635.
[00180] (13) a) M. C. Wood, D. C. Leitch, C. S. Yeung, J. A. Kozak, L. L.
Schafer,
Angew. Chem. 2007,119, 358 - 362; Angew. Chem. Int. Ed. 2007, 46, 354 - 358;
b)
Corrigendum M. C. Wood, D.C. Leitch, C. S. Yeung, J. A. Kozak, L. L. Schafer,
Angew. Chem.
2009, 121, 7072; Angew. Chem. Int. Ed. 2009, 48, 6938.
[001811 (14) S. E. Denmark, H. Matsuhashi, J. Org. Chem. 2002, 67, 3479- 3486.
[00182] All publications, patents and patent applications mentioned in this
Specification
are indicative of the level of skill of those skilled in the art to which this
invention pertains and
are herein incorporated by reference to the same extent as if each individual
publication, patent,
or patent application was specifically and individually indicated to be
incorporated by reference.
[00183] The invention being thus described, it will be obvious that the same
maybe varied
in many ways. Such variations are not to be regarded as a departure from the
spirit and scope of
the invention, and all such modifications as would be obvious to one skilled
in the art are
intended to be included within the scope of the following claims.
-59-