Note: Descriptions are shown in the official language in which they were submitted.
CA 02717991 2010-09-09
WO 2009/114373 PCT/US2009/036119
TREATMENT FOR OCULAR-RELATED DISORDERS
FIELD OF THE INVENTION
This invention is directed to methods of therapy for human and non-human
patients suffering
from, or subject to, ocular disorders such as age related macular
degeneration, diabetic
retinopathy, and diabetic macular edema.
BACKGROUND OF THE INVENTION
Protein kinases participate in the signalling events which control the
activation, growth and
differentiation of cells in response to extracellular mediators and to changes
in the
environment. In general, these kinases fall into several groups; those which
preferentially
phosphorylate serine and/or threonine residues and those which preferentially
phosphorylate
tyrosine residues [S.K.Hanks and T.Hunter, FASEB. J., 1995, 9, pages 576-596].
The
serine/threonine kinases include for example, protein kinase C isoforms
[A.C.Newton, J. Biol.
Chem., 1995, 270, pages 28495-28498] and a group of cyclin-dependent kinases
such as cdc2
[J.Pines, Trends in Biochemical Sciences, 1995, 18, pages 195-197]. The
tyrosine kinases
include membrane-spanning growth factor receptors such as the epidermal growth
factor
receptor [S.Iwashita and M.Kobayashi, Cellular Signalling, 1992, 4, pages 123-
132], and
cytosolic non-receptor kinases such as p56tck, p59fYn, ZAP-70 and csk kinases
[C.Chan et.
al., Ann. Rev. Immunol., 1994,12, pages 555-592].
CA 02717991 2010-09-09
WO 2009/114373 PCT/US2009/036119
-2-
Inappropriately high protein kinase activity has been implicated in many
diseases resulting
from abnormal cellular function. This might arise either directly or
indirectly, for example by
failure of the proper control mechanisms for the kinase, related for example
to mutation, over-
expression or inappropriate activation of the enzyme; or by over- or
underproduction of
cytokines or growth factors also participating in the transduction of signals
upstream or
downstream of the kinase. In all of these instances, selective inhibition of
the action of the
kinase might be expected to have a beneficial effect.
Syk is a 72-kDa cytoplasmic protein tyrosine kinase that is expressed in a
variety of
hematopoietic cells and is an essential element in several cascades that
couple antigen
receptors to cellular responses. Thus, Syk plays a pivotal role in signalling
of the high
affinity IgE receptor, FCER1, in mast cells and in receptor antigen signalling
in T and B
lymphocytes. The signal transduction pathways present in mast, T and B cells
have common
features. The ligand binding domain of the receptor lacks intrinsic tyrosine
kinase activity.
However, they interact with transducing subunits that contain immunoreceptor
tyrosine based
activation motifs (ITAMs) [M.Reth, Nature, 1989, 338, pages 383-384]. These
motifs are
present in both the R and y subunits of the FCER1, in the a-subunit the of T
cell receptor (TCR)
and in the IgGa and IgG (3 subunits of the B cell receptor (BCR). [N.S.van
Oers and A.Weiss,
Seminars in Immunology, 1995, 7, pages 227-236] Upon binding of antigen and
multimerization, the ITAM residues are phosphorylated by protein tyrosine
kinases of the Src
family. Syk belongs to a unique class of tyrosine kinases that have two tandem
Src homology
2 (SH2) domains and a C terminal catalytic domain. These SH2 domains bind with
high
affinity to ITAMs and this SH2 -mediated association of Syk with an activated
receptor
stimulates Syk kinase activity and localises Syk to the plasma membrane.
Angiogenesis or the formation of new blood vessels by sprouting from the
preexisting
vasculature is of central importance for embryonic development and
organogenesis. Abnormal
enhanced neovascularization is observed in rheumatoid arthritis, diabetic
retinopathy and
during tumor development (Folkman, Nat. Med., 1995, 1, 27-31.). Angiogenesis
is a complex
multistage process which includes activation, migration, proliferation and
survival of
endothelial cells. Extensive studies in the field of tumor angiogenesis in the
past two decades
have identified a number of therapeutic targets including kinases, proteases
and integrins
resulting in the discovery of many new anti-angiogenic agents, including KDR
inhibitors
CA 02717991 2010-09-09
WO 2009/114373 PCT/US2009/036119
-3-
some of which are currently under clinical evaluation (Jekunen, et al Cancer
Treatment Rev.
1997 , 23, 263-286.). Angiogenesis inhibitors may be used in frontline,
adjuvant and even
preventive settings for the emergence or regrowth of malignancies.
Important causes of severe vision loss and blindness are ocular-related
disorders where the
vascular system of the eye is damaged or insufficiently regulated. Ocular-
related diseases
comprising a neovascularization aspect are many and include, for example, age-
related
macular degeneration, diabetic retinopathy, and diabetic macular edema. It is
likely that severe
vision loss does not result directly from neovascularization, but from the
effect of
neovascularization on the retina. The retina is a delicate ocular membrane on
which images
are received. Near the center of the retina is the macula lutea, an oval area
of retinal tissue
where visual sense is most acute. The retina is most delicate at the fovea
centralis, the central
depression located in the center of the macula. Damage of the retina such as
retinal
degeneration is directly connected to vision loss. While a common cause of
retinal
detachment, retinal tears, and retinal degeneration is abnormal, i.e.,
uncontrolled,
vascularization of various ocular tissues, this is not always the case.
Atrophic complications
associated with age-related macular degeneration, nonproliferative diabetic
retinopathy, and
inflammatory ocular damage are not associated with neovascularization, but can
result in
severe vision loss if not treated. Disorders associated with both neovascular
and atrophic
components, such as age-related macular degeneration, diabetic retinopathy and
diabetic
macular edema, are particularly difficult to treat due to the emergence of a
wide variety of
complications. See Diabetic Retinopathy: A Review; June Chu and Yusuf Ali;
Drug
Development Research 69: 1-14 (2008); New Developments in Diabetic
Retinopathy; Expert
Rev. Ophthalmol. 2(6), 947-956 (2007); and Diabetic Retinopathy; Robert Frank;
N. Engl. J.
Med. 350;1; 48-58 (2004).
What has been discovered now is in one embodiment, a method of
prophylactically or
therapeutically treating a patient for an ocular related disorder, wherein the
method comprises
administering to the patient a pharmaceutically effective amount of a compound
of formula I,
or a pharmaceutically acceptable salt or prodrug thereof. Administration to an
individual at
risk of abnormal angiogenesis or vascularization of the eye can prevent
abnormal
angiogenesis of the eye, especially the retina. The patient is at risk of, or
has been diagnosed
with, abnormal angiogenesis of the eye, and is transformed by treatment with
the compound
CA 02717991 2010-09-09
WO 2009/114373 PCT/US2009/036119
-4-
of the invention from a disease susceptible state to a disease resistant
state. Untreated, this
type of disease is characterized by invasion of new blood vessels into the
structures of the eye,
such as the retina. It is a common cause of blindness and is involved in
approximately twenty
eye diseases. In age-related macular degeneration, the associated visual
problems are caused
by an ingrowth of choroidal capillaries through defects in Bruch's membrane
with
proliferation of fibrovascular tissue beneath the retinal pigment epithelium.
Angiogenic
damage is also associated with diabetic retinopathy and diabetic macular
edema.
Age-related macular degeneration (AMD) is a degenerative condition of the
macula (the
central retina). It is the most common cause of vision loss in the United
States in those 50 or
older, and its prevalence increases with age. AMD is caused by hardening of
the arteries that
nourish the retina. This deprives the sensitive retinal tissue of oxygen and
nutrients that it
needs to function and thrive. As a result, the central vision deteriorates.
Macular degeneration varies widely in severity. In the worst cases, it causes
a complete loss
of central vision, making reading or driving impossible. For others, it may
only cause slight
distortion. Fortunately, macular degeneration does not cause total blindness
since it does not
affect the peripheral vision.
AMD is classified as either wet (neovascular) or dry (non-neovascular). About
10% of
patients who suffer from macular degeneration have wet AMD. This type occurs
when new
vessels form to improve the blood supply to oxygen-deprived retinal tissue.
However, the
new vessels are very delicate and break easily, causing bleeding and damage to
surrounding
tissue. While wet AMD represents a significant minority of AMD cases it is
associated with
90% of significant vision loss associated with the disorder.
Current treatments for AMD are focused on affecting the neovascularization
that leads to the
vision loss associated with wet AMD. The current gold standard therapies are
biologicals that
are injected directly into the eye. These are listed in Table 1. These
treatments are expensive,
cumbersome and limited by dose. There is a clear need for effective oral
therapies for this
disorder.
CA 02717991 2010-09-09
WO 2009/114373 PCT/US2009/036119
-5-
AGENT COMPANY MODE OF ROUTE OF
ACTION ADMINISTRATION
Laser Multiple Laser mediated Laser
vessel occlusion
Visudyne Novartis Reactive oxygen Intravenous then laser
radicals activation
mediated vessel
occlusion
Macugen EyeTech/OSI Anti-VEGF Intravitreal
inhibitor (RNA)
Lucentis Genetech Anti-VEGF Intravitreal
Novartis mAb
TABLE I
In view of the current situation regarding therapies for treating macular
degeneration, it is
clear that there is a need for more effective and better tolerated therapies.
Diabetic retinopathy is an ocular disorder that is subdivided into two stages.
These are a non-
proliferative stage, usually occurring first, and a proliferative stage. Non-
proliferative diabetic
retinopathy frequently leads to vision loss in patients due to retinal edema,
in particular
diabetic macular edema, this resulting in vascular leakage. Focal and diffuse
macular leakage
occurs as a result of microvascular abnormalities, intraretinal
microaneurysms, capillary
closure, and retinal hemorrhages. Prolonged periods of vascular leakage
ultimately lead to
thickening of the basement membrane and formation of soft and hard exudates.
Non-
proliferative diabetic retinopathy is also characterized by loss of retinal
pericytes. The
proliferative stage of diabetic retinopathy is characterized by
neovascularization and
fibrovascular growth (i.e., scarring involving glial and fibrous elements)
from the retina or
optic nerve over the inner surface of the retina or disc or into the vitreous
cavity. Retinal
neovascularization is the leading cause of vision loss associated with
proliferative diabetic
retinopathy.
CA 02717991 2010-09-09
WO 2009/114373 PCT/US2009/036119
-6-
Current approaches to the treatment of abnormal angiogenesis and
vascularization in the eye
include laser therapy, which destroys some retinal tissue in order to preserve
some vision.
There remains a clear need for improved methods and agents for prevention and
treatment of
conditions involving abnormal angiogenesis and harmful angiogenesis such as
pathological
angiogenesis in the tissues of the eye.
Given the prevalence of ocular-related disorders, there remains a need for an
effective
prophylactic and therapeutic treatment of ocular-related disorders, in
particular those ocular-
related disorders associated with both atrophic and neovascular complications,
such as age-
related macular degeneration, diabetic retinopathy, and diabetic macular
edema. Accordingly,
the present invention is concerned with a compound and methods for
prophylactically and
therapeutically treating ocular-related disorders. This and other advantages
of the present
invention will become apparent from the detailed description provided herein.
SUMMARY OF THE INVENTION
The present invention relates to a method of treating macular degeneration,
and more
specifically age-related macular degeneration using a compound of Formula I:
OH
NX'N
N Formula I
This invention is directed to a substituted azaindole of Formula I, which has
now been found
to be active in the inhibition of macular degeneration in animal models.
Another aspect of the present invention is a treatment for wet AMD.
CA 02717991 2010-09-09
WO 2009/114373 PCT/US2009/036119
-7-
The compound of Formula I may be used as an effective oral treatment for AMD.
Furthermore we envision, based on our data, that Syk inhibitors in general can
be useful
agents for the treatment of this disorder. Data for these conclusions is
outlined below.
Another aspect of the present invention is a method of treating diabetic
retinopathy by
administering to a patient in need thereof, a pharmaceutically effective
amount of a compound
of Formula I.
Yet another aspect of the present invention is a method of treating diabetic
macular edema by
administering to a patient in need thereof, a pharmaceutically effective
amount of a compound
of Formula I.
Yet another aspect of the present invention is a treatment for macular
degeneration, diabetic
retinopathy or diabetic macular edema by treating a patient with a Syk
inhibitor in general.
DETAILED DESCRIPTION OF THE INVENTION
Thus, in one aspect, the present invention is directed to therapeutic
treatments and
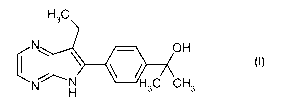
pharmaceutical compositions comprising a compound of general formula (I):
H3C
N OH
N N 3C CH3
H
(I)
which also may be known as: 2-[4-(7-Ethyl-5H-pyrrolo[2,3-b]pyrazin-6-yl)-
phenyl]-propan-2-
ol. The synthesis of this compound is known to a person skilled in the art
from its publication
in International Application WO 2008/033798. It is also known from this
publication that this
compound is a Syk inhibitor.
CA 02717991 2010-09-09
WO 2009/114373 PCT/US2009/036119
8-
In the present specification, the term "compound of the invention", and
equivalent
expressions, are meant to embrace a compound of general formula (I) as
hereinbefore
described, which expression includes the prodrugs, the pharmaceutically
acceptable salts, and
the solvates, e.g. hydrates, where the context so permits. Similarly,
reference to intermediates,
whether or not they themselves are claimed, is meant to embrace their salts,
and solvates,
where the context so permits. For the sake of clarity, particular instances
when the context so
permits are sometimes indicated in the text, but these instances are purely
illustrative and it is
not intended to exclude other instances when the context so permits.
DEFINITIONS
As used above, and throughout the description of the invention, the following
terms, unless
otherwise indicated, shall be understood to have the following meanings:-
"Patient" includes both human and other mammals.
"Pharmaceutically acceptable ester" refers to esters that hydrolyze in vivo
and include those
that break down readily in the human body to leave the parent compound or a
salt thereof,
Suitable ester groups include, for example, those derived from
pharmaceutically acceptable
aliphatic carboxylic acids, particularly alkanoic, alkenoic, cycloalkanoic and
alkanedioic
acids, in which each alkyl or alkenyl moiety advantageously has not more than
6 carbon
atoms. Exemplary esters include formates, acetates, propionates, butyrates,
acrylates,
ethylsuccinates, and the like.
"Pharmaceutically acceptable prodrugs" as used herein refers to those prodrugs
of the
compounds of the present invention which are, within the scope of sound
medical judgment,
suitable for use in contact with the tissues of patients with undue toxicity,
irritation, allergic
response, and the like, commensurate with a reasonable benefit/risk ratio, and
effective for
their intended use of the compounds of the invention. The term "prodrug"
refers to
compounds that are rapidly transformed in vivo to yield the parent compound of
the above
formula, for example by hydrolysis in blood. Functional groups that may be
rapidly
transformed, by metabolic cleavage, in vivo form a class of groups reactive
with the carboxyl
CA 02717991 2010-09-09
WO 2009/114373 PCT/US2009/036119
-9-
group of the compounds of this invention. They include, but are not limited to
such groups as
alkanoyl (such as acetyl, propanoyl, butanoyl, and the like), unsubstituted
and substituted
aroyl (such as benzoyl and substituted benzoyl), alkoxycarbonyl (such as
ethoxycarbonyl),
trialkylsilyl (such as trimethyl- and triethysilyl), monoesters formed with
dicarboxylic acids
(such as succinyl), and the like. Because of the ease with which the
metabolically cleavable
groups of the compounds of this invention are cleaved in vivo, the compounds
bearing such
groups act as pro-drugs. The compounds bearing the metabolically cleavable
groups have the
advantage that they may exhibit improved bioavailability as a result of
enhanced solubility
and/or rate of absorption conferred upon the parent compound by virtue of the
presence of the
metabolically cleavable group. A thorough discussion is provided in Design of
Prodrugs, H.
Bundgaard, ed., Elsevier (1985); Methods in Enzymology; K. Widder et al, Ed.,
Academic
Press, 42, 309-396 (1985); A Textbook of Drug Design and Development,
Krogsgaard-Larsen
and H. Bandaged, ed., Chapter 5; "Design and Applications of Prodrugs" 113-191
(1991);
Advanced Drug Delivery Reviews, H. Bundgard, 8, 1-38, (1992); J. Pharm. Sci.,
77.,285
(1988); Chem. Pharm. Bull., N. Nakeya et al, 32, 692 (1984); Pro-drugs as
Novel Delivery
Systems, T. Higuchi and V. Stella, 14 A.C.S. Symposium Series, and
Bioreversible Carriers
in Drug Design, E.B. Roche, ed., American Pharmaceutical Association and
Pergamon Press,
1987, which are incorporated herein by reference.
"Pharmaceutically acceptable salts" refers to the relatively non-toxic,
inorganic and organic
acid addition salts, and base addition salts, of compounds of the present
invention. These:
salts can be prepared in situ during the final isolation and purification of
the compounds. In
particular, acid addition salts can be prepared by separately reacting the
purified compound in
its free base form with a suitable organic or inorganic acid and isolating the
salt thus formed.
Exemplary acid addition salts include the hydrobromide, hydrochloride,
sulfate, bisulfate,
phosphate, nitrate, acetate, oxalate, valerate, oleate, palmitate, stearate,
laurate, borate,
benzoate, lactate, phosphate, tosylate, citrate, maleate, fumarate, succinate,
tartrate,
naphthylate, mesylate, glucoheptonate, lactiobionate, sulfamates, malonates,
salicylates,
propionates, methylene-bis-l3-hydroxynaphthoates, gentisates, isethionates, di-
p-
toluoyltartrates, methanesulfonates, ethanesulfonates, benzenesulfonates, p-
toluenesulfonates,
cyclohexylsulfamates and laurylsulfonate salts, and the like. See, for example
S.M. Berge, et
al., "Pharmaceutical Salts," J. Pharm. Sci., 66, 1-19 (1977). Base addition
salts can also be
prepared by separately reacting the purified compound in its acid form with a
suitable organic
CA 02717991 2010-09-09
WO 2009/114373 PCT/US2009/036119
- 10-
or inorganic base and isolating the salt thus formed. Base addition salts
include
pharmaceutically acceptable metal and amine salts. Suitable metal salts
include the sodium,
potassium, calcium, barium, zinc, magnesium, and aluminum salts. The sodium
and
potassium salts are preferred. Suitable inorganic base addition salts are
prepared from metal
bases which include sodium hydride, sodium hydroxide, potassium hydroxide,
calcium
hydroxide, aluminum hydroxide, lithium hydroxide, magnesium hydroxide, zinc
hydroxide
and the like. Suitable amine base addition salts are prepared from amines
which have
sufficient basicity to form a stable salt, and preferably include those amines
which are
frequently used in medicinal chemistry because of their low toxicity and
acceptability for
medical use. ammonia, ethylenediamine, N-methyl-glucamine, lysine, arginine,
ornithine,
choline, N,N'- dibenzylethylenediamine, chloroprocaine, diethanolamine,
procaine, N-
benzylphenethylamine, diethylamine, piperazine, tris(hydroxymethyl)-
aminomethane,
tetramethylammonium hydroxide, triethylamine, dibenzylamine, ephenamine,
dehydroabietylamine, N-ethylpiperidine, benzylamine, tetramethylammonium,
tetraethylammonium, methylamine, dimethylamine, trimethylamine, ethylamine,
basic amino
acids, e.g., lysine and arginine, and dicyclohexylamine, and the like.
EMBODIMENTS
With reference to inventions described herein, below are particular
embodiments related
thereto.
A particular embodiment according to the invention is the treatment of macular
degeneration
by administering to a patient in need thereof, a pharmaceutically effective
amount of a
compound of Formula I.
Another particular embodiment according to the invention is the treatment of
macular
degeneration by administering to a patient in need thereof, a pharmaceutically
effective
amount of a Syk inhibitor.
A preferred embodiment according to the invention is where the macular
degeneration is age-
related macular degeneration and the effective amount is of a compound of
Formula I.
CA 02717991 2010-09-09
WO 2009/114373 PCT/US2009/036119
-11-
Another preferred embodiment according to the invention is where the macular
degeneration
is age-related macular degeneration and the effective amount is of a Syk
inhibitor.
Another embodiment according to the invention is the treatment of diabetic
retinopathy by
administering to a patient in need thereof, a pharmaceutically effective
amount of a compound
of Formula I.
Another embodiment according to the invention is the treatment of diabetic
macular edema by
administering to a patient in need thereof, a pharmaceutically effective
amount of a compound
of Formula I.
The compounds of the invention optionally are supplied as salts. Those salts
that are
pharmaceutically acceptable are of particular interest since they are useful
in administering the
foregoing compounds for medical purposes. Salts that are not pharmaceutically
acceptable are
useful in manufacturing processes, for isolation and purification purposes,
and in some
instances, for use in separating stereoisomeric forms of the compounds of this
invention. The
latter is particularly true of amine salts prepared from optically active
amines.
An aspect of the present invention is to provide a pharmaceutical composition
comprising, a
pharmaceutically effective amount of a compound of formula 1 and
pharmaceutically
acceptable carrier or diluent.
Another aspect of the invention to provide a pharmaceutical composition which
is effective, in
and of itself, for utilization in a beneficial combination therapy because it
includes a plurality
of active ingredients which may be utilized in accordance with the invention.
The amount of the compound of Formula I in any of the foregoing applications
can be a
pharmaceutically effective amount, a suboptimal effective amount, or
combinations thereof,
so long as the final combination of ingredients comprises a pharmaceutically
effective amount
of compounds that is effective in treating or preventing macular degeneration,
diabetic
retinopathy, or diabetic macular edema in a patient.
CA 02717991 2010-09-09
WO 2009/114373 PCT/US2009/036119
-12-
In-Vivo Efficacy of the Compound of Formula I in Models of Ocular-Related
Disorders such
as AMD
Several models of wet AMD are available in the rodent and rabbit. The pre-
clinical efficacy of
the compound of Formula I was assessed in a laser induced model of macular
degeneration in
the rat. In this model injury is induced on the retina of the rat by a focused
laser beam. This
injury results in vascularization and inflammation, and the subsequent
formation of a plaque
on the retina that is associated with loss of vision. The ability of
treatments with a compound
of Formula Ito inhibit the size and thickness of such plaques serves as an
indicator for the
potential for the treatment of wet AMD patients. A krypton laser was used to
irradiate the
retina of a rat eye at 75 microns for 0.1 seconds, at each of 3-4 spots. From
days 1 to 14, the
irradiated rats were dosed twice a day with the compound of Formula I
perorally ( bid PO).
Doses used were 3 mg/kg, 10 mg/kg, and 30 mg/kg body weight. An additional
treatment
group was given the vehicle for the oral compound, also bid PO administration.
A positive
control was used with 0.5 mg tiamcinolone delivered to the intraocular
subtendon. At 14 days
after such irradiation, the rat was perfused with FITC-dextran (fluorescein
isothiocyanate
dextran) and the eyes were removed for evaluation. Evaluation was by
fundoscopy and
fluorescein angiography. The data with the compound of Formula I in this model
demonstrated that the compound significantly reduced plaque thickness at 30
and 10 mg/kg
bid PO. These results were significantly better than what was observed with
the positive
control triamcinolone (intravitreal injection of a steroid). The results are
shown in Table 2.
Treatment % Inhibition of Significance
Plaque
Vehicle 0
3 mg/kg compound of Formula I 12
10 mg/kg compound of Formula I 36 p<0.003
mg/kg compound of Formula I 33 p<0.0006
0.5 mg triamcinolone 26 p<0.0245
25 TABLE 2
CA 02717991 2010-09-09
WO 2009/114373 PCT/US2009/036119
- 13 -
Another aspect of the present invention herein described is a method of
treating a patient
suffering from or subject to macular degeneration, comprising administering to
the patient a
pharmaceutically effective amount of a Syk inhibitor. References herein to
"treating" should
be understood to include prophylactic therapy to prevent or inhibit a
disorder, as well as the
treatment of a patent to ameliorate or improve a disorder that the patient
has. "Effective
amount" is meant to describe an amount of the compound of the present
invention effective
within the scope of reasonable biological judgment, suitable for use in
contact with the cells of
humans and other mammals without undue toxicity, irritation, allergic response
and the like,
and are commensurate with a reasonable benefit/risk ratio in treating an and
thus producing
the desired therapeutic effect.
A particular aspect of the invention provides for a compound according to the
invention to be
administered in the form of a pharmaceutical composition, though the compound
may be
administered alone. "Pharmaceutical composition" means a composition
comprising a
compound of formula I and at least one component selected from the group
comprising
pharmaceutically acceptable carriers, diluents, coatings, adjuvants,
excipients, or vehicles,
such as preserving agents, fillers, disintegrating agents, wetting agents,
emulsifying agents,
emulsion stabilizing agents, suspending agents, isotonic agents, sweetening
agents, flavoring
agents, perfuming agents, coloring agents, antibacterial agents, antifungal
agents, other
therapeutic agents, lubricating agents, adsorption delaying or promoting
agents, and
dispensing agents, depending on the nature of the mode of administration and
dosage forms.
The compositions may be presented in the form of tablets, pills, granules,
powders, aqueous
solutions or suspensions, injectable solutions, elixirs or syrups. Exemplary
suspending agents
include ethoxylated isostearyl alcohols, polyoxyethylene sorbitol and sorbitan
esters,
microcrystalline cellulose, aluminum metahydroxide, bentonite, agar-agar and
tragacanth, or
mixtures of these substances. Exemplary antibacterial and antifungal agents
for the
prevention of the action of microorganisms include parabens, chlorobutanol,
phenol, sorbic
acid, and the like. Exemplary isotonic agents include sugars, sodium chloride
and the like.
Exemplary adsorption delaying agents to prolong absorption include aluminum
monostearate
and gelatin. Exemplary adsorption promoting agents to enhance absorption
include dimethyl
sulfoxide and related analogs. Exemplary carriers, diluents, solvents,
vehicles, solubilizing
CA 02717991 2010-09-09
WO 2009/114373 PCT/US2009/036119
-14-
agents, emulsifiers and emulsion stabilizers, include water, chloroform,
sucrose, ethanol,
isopropyl alcohol, ethyl carbonate, ethyl acetate, benzyl alcohol,
tetrahydrofurfuryl alcohol,
benzyl benzoate, polyols, propylene glycol, 1,3-butylene glycol, glycerol,
polyethylene
glycols, dimethylformamide, Tween 60, Span 60, cetostearyl alcohol, myristyl
alcohol,
glyceryl mono-stearate and sodium lauryl sulfate, fatty acid esters of
sorbitan, vegetable oils
(such as cottonseed oil, groundnut oil, com germ oil, olive oil, castor oil
and sesame oil) and
injectable organic esters such as ethyl oleate, and the like, or suitable
mixtures of these
substances. Exemplary excipients include lactose, milk sugar, sodium citrate,
calcium
carbonate, dicalcium phosphate. Exemplary disintegrating agents include
starch, alginic acids
and certain complex silicates. Exemplary lubricants include magnesium
stearate, sodium
lauryl sulfate, talc, as well as high molecular weight polyethylene glycols.
Other therapeutic agents may be used in combination with a compound of the
present
invention, including other anti agents. Therapeutic agents used in combination
with a
compound of the present invention may be administered separately,
simultaneously or
sequentially. The choice of material in the pharmaceutical composition other
than the
compound of formula I is generally determined in accordance with the chemical
properties of
the active compound such as solubility, the particular mode of administration
and the
provisions to be observed in pharmaceutical practice. For example, excipients
such as lactose,
sodium citrate, calcium carbonate, dicalcium phosphate and disintegrating
agents such as
starch, alginic acids and certain complex silicates combined with lubricants
such as
magnesium stearate, sodium lauryl sulfate and talc may be used for preparing
tablets.
The pharmaceutical compositions may be presented in assorted forms such as
tablets, pills,
granules, powders, aqueous solutions or suspensions, injectable solutions,
elixirs or syrups.
"Liquid dosage form" means the dose of the active compound to be administered
to the patient
is in liquid form, for, example, pharmaceutically acceptable emulsions,
solutions, suspensions,
syrups and elixirs. In addition to the active compounds, the liquid dosage
forms may contain
inert diluents commonly used in the art, such solvents, solubilizing agents
and emulsifiers.
CA 02717991 2010-09-09
WO 2009/114373 PCT/US2009/036119
-15-
Solid compositions may also be employed as fillers in soft and hard-filled
gelatin capsules
using such excipients as lactose or milk sugar as well as high molecular
weight polyethylene
glycols, and the like.
When aqueous suspensions are used they can contain emulsifying agents or
agents which
facilitate suspension.
The oily phase of the emulsion pharmaceutical composition may be constituted
from known
ingredients in a known manner. While the phase may comprise merely an
emulsifier
(otherwise known as an emulgent), it desirably comprises a mixture of at least
one emulsifier
with a fat or an oil or with both a fat and an oil. In a particular
embodiment, a hydrophilic
emulsifier is included together with a lipophilic emulsifier that acts as a
stabilizer. Together,
the emulsifier(s) with or without stabilizer(s) make up the emulsifying wax,
and the way
together with the oil and fat make up the emulsifying ointment base which
forms the oily
dispersed phase of the cream formulations.
If desired, the aqueous phase of the cream base may include, for example, a
least 30% w/w of
a polyhydric alcohol, i.e. an alcohol having two or more hydroxyl groups such
as propylene
glycol, butane 1,3-diol, mannitol, sorbitol, glycerol and polyethylene glycol
(including PEG
400) and mixtures thereof. The topical formulations may desirably include a
compound that
enhances absorption or penetration of the active ingredient through the skin
or other affected
areas.
The choice of suitable oils or fats for a formulation is based on achieving
the desired
properties. Thus the cream should preferably be a non-greasy, non-staining and
washable
product with suitable consistency to avoid leakage from tubes or other
containers. Straight or
branched chain, mono- or dibasic alkyl esters such as di-isopropyl myristate,
decyl oleate,
isopropyl palmitate, butyl stearate, 2-ethylhexyl palmitate or a blend of
branched chain esters
known as Crodamol CAP may be used. These may be used alone or in combination
depending on the properties required. Alternatively, high melting point lipids
such as white
soft paraffin and/or liquid paraffin or other mineral oils can be used.
CA 02717991 2010-09-09
WO 2009/114373 PCT/US2009/036119
- 16-
In practice, a compound/pharmaceutical composition of the present invention
may be
administered in a suitable formulation to humans and animals by topical or
systemic
administration, including oral, inhalational, rectal, nasal, buccal,
sublingual, vaginal, colonic,
parenteral (including subcutaneous, intramuscular, intravenous, intradermal,
intrathecal and
epidural), intracisternal and intraperitoneal. It will be appreciated that the
preferred route may
vary with for example the condition of the recipient.
"Pharmaceutically acceptable dosage forms" refers to dosage forms of the
compound of the
invention, and includes, for example, tablets, dragees, powders, elixirs,
syrups, liquid
preparations, including suspensions, sprays, inhalants tablets, lozenges,
emulsions, solutions,
granules, capsules and suppositories, as well as liquid preparations for
injections, including
liposome preparations. Techniques and formulations generally may be found in
Remington's
Pharmaceutical Sciences, Mack Publishing Co., Easton, PA, latest edition.
"Formulations suitable for oral administration" may be presented as discrete
units such as
capsules, cachets or tablets each containing a predetermined amount of the
active ingredient;
as a powder or granules; as solution or a suspension in an aqueous liquid or a
non-aqueous
liquid; or as an oil-in-water liquid emulsion or a water-in-oil liquid
emulsion. The active
ingredient may also be presented as a bolus, electuary or paste.
A tablet may be made by compression or molding, optionally with one or more
accessory
ingredients. Compressed tables may be prepared by compressing in a suitable
machine the
active ingredient in a free-flowing form such as a powder or granules,
optionally mixed with a
binder, lubricant, inert diluent, preservative, surface active or dispersing
agent. Molded
tablets may be made by molding in a suitable machine a mixture of the powdered
compounds
moistened with an inert liquid diluent. The tablets may optionally be coated
or scored and
may be formulated so as to provide slow or controlled release of the active
ingredient therein.
Solid compositions for rectal administration include suppositories formulated
in accordance
with known methods and containing at least one compound of the invention.
If desired, and for more effective distribution, the compounds can be
microencapsulated in, or
attached to, a slow release or targeted delivery systems such as a
biocompatible,
CA 02717991 2010-09-09
WO 2009/114373 PCT/US2009/036119
-17-
biodegradable polymer matrices (e.g., poly(d,l-lactide co-glycolide)),
liposomes, and
microspheres and subcutaneously or intramuscularly injected by a technique
called
subcutaneous or intramuscular depot to provide continuous slow release of the
compound(s)
for a period of 2 weeks or longer. The compounds may be sterilized, for
example, by
filtration through a bacteria retaining filter, or by incorporating
sterilizing agents in the form
of sterile solid compositions which can be dissolved in sterile water, or some
other sterile
injectable medium immediately before use.
"Formulations suitable for oral administration" means formulations which are
in a form
suitable to be administered orally to a patient. The formulations may be
presented as discrete
units such as capsules, cachets or tablets each containing a predetermined
amount of the active
ingredient; as a powder or granules; as solution or a suspension in an aqueous
liquid or a non-
aqueous liquid; or as an oil-in-water liquid emulsion or a water-in-oil liquid
emulsion. The
active ingredient may also be presented as a bolus, electuary or paste.
"Formulations suitable for parenteral administration" means formulations that
are in a form
suitable to be administered parenterally to a patient. The formulations are
sterile and include
emulsions, suspensions, aqueous and non-aqueous injection solutions, which may
contain
suspending agents and thickening agents and anti-oxidants, buffers,
bacteriostats and solutes
which render the formulation isotonic, and have a suitably adjusted pH, with
the blood of the
intended recipient.
"Formulations suitable for systemic administration" means formulations that
are in a form
suitable to be administered systemically to a patient. The formulation is
preferably
administered by injection, including transmuscular, intravenous,
intraperitoneal, and
subcutaneous. For injection, the compounds of the invention are formulated in
liquid
solutions, in particular in physiologically compatible buffers such as Hank's
solution or
Ringer's solution. In addition, the compounds may be formulated in solid form
and
redissolved or suspended immediately prior to use. Lyophilized forms are also
included.
Systematic administration also can be by transmucosal or transdermal means, or
the
compounds can be administered orally. For transmucosal or transdermal
administration,
penetrants appropriate to the barrier to be permeated are used in the
formulation. Such
penetrants are generally known in the art, and include, for example, bile
salts and fusidic acid
CA 02717991 2010-09-09
WO 2009/114373 PCT/US2009/036119
-18-
derivatives for transmucosal administration. In addition, detergents may be
used to facilitate
permeation. Transmucosal administration may be through use of nasal sprays,
for example, or
suppositories. For oral administration, the compounds are formulated into
conventional oral
administration forms such as capsules, tablets, and tonics.
"Formulations suitable for topical administration" means formulations that are
in a form
suitable to be administered topically to a patient. The formulation may be
presented as a
topical ointment, salves, powders, sprays and inhalants, gels (water or
alcohol based), creams,
as is generally known in the art, or incorporated into a matrix base for
application in a patch,
which would allow a controlled release of compound through the transdermal
barrier. When
formulated in an ointment, the active ingredients may be employed with either
a paraffinic or
a water-miscible ointment base. Alternatively, the active ingredients may be
formulated in a
cream with an oil-in-water cream base. Formulations suitable for topical
administration in the
eye include eye drops wherein the active ingredient is dissolved or suspended
in a suitable
carrier, especially an aqueous solvent for the active ingredient. Formulations
suitable for
topical administration in the mouth include lozenges comprising the active
ingredient in a
flavored basis, usually sucrose and acacia or tragacanth; pastilles comprising
the active
ingredient in an inert basis such as gelatin and glycerin, or sucrose and
acacia; and
mouthwashes comprising the active ingredient in a suitable liquid carrier.
"Solid dosage form" means the dosage form of the compound of the invention is
solid form,
for example capsules, tablets, pills, powders, dragees or granules. In such
solid dosage forms,
the compound of the invention is admixed with at least one inert customary
excipient (or
carrier) such as sodium citrate or dicalcium phosphate or (a) fillers or
extenders, as for
example, starches, lactose, sucrose, glucose, mannitol and silicic acid, (b)
binders, as for
example, carboxymethylcellulose, alginates, gelatin, polyvinylpyrrolidone,
sucrose and acacia,
(c) humectants, as for example, glycerol, (d) disintegrating agents, as for
example, agar-agar,
calcium carbonate, potato or tapioca starch, alginic acid, certain complex
silicates and sodium
carbonate, (e) solution retarders, as for example paraffin, (f) absorption
accelerators, as for
example, quaternary ammonium compounds, (g) wetting agents, as for example,
cetyl alcohol
and glycerol monostearate, (h) adsorbents, as for example, kaolin and
bentonite, (i) lubricants,
as for example, talc, calcium stearate, magnesium stearate, solid polyethylene
glycols, sodium
CA 02717991 2010-09-09
WO 2009/114373 PCT/US2009/036119
- 19-
lauryl sulfate, (j) opacifying agents, (k) buffering agents, and agents which
release the
compound(s) of the invention 'in a certain part of the intestinal tract in a
delayed manner.
Actual dosage levels of active ingredient(s) in the compositions of the
invention may be
varied so as to obtain an amount of active ingredient(s) that is (are)
effective to obtain a
desired therapeutic response for a particular composition and method of
administration for a
patient. A selected dosage level for any particular patient therefore depends
upon a variety of
factors including the desired therapeutic effect, on the route of
administration, on the desired
duration of treatment, the etiology and severity of the disease, the patient's
condition, weight,
sex, diet and age, the type and potency of each active ingredient, rates of
absorption,
metabolism and/or excretion and other factors.
Total daily dose of the compounds of this invention administered to a patient
in single or
divided doses may be in amounts, for example, of from about 0.001 to about 100
mg/kg body
weight daily and preferably 0.01 to 10 mg/kg/day. For example, in an adult,
the doses are
generally from about 0.01 to about 100, preferably about 0.01 to about 10,
mg/kg body weight
per day by inhalation, from about 0.01 to about 100, preferably 0.1 to 70,
more especially 0.5
to 10, mg/kg body weight per day by oral administration, and from about 0.01
to about 50,
preferably 0.01 to 10, mg/kg body weight per day by intravenous
administration. The
percentage of active ingredient in a composition may be varied, though it
should constitute a
proportion such that a suitable dosage shall be obtained. Dosage unit
compositions may
contain such amounts of such submultiples thereof as may be used to make up
the daily dose.
Obviously, several unit dosage forms may be administered at about the same
time. A dosage
may be administered as frequently as necessary in order to obtain the desired
therapeutic
effect. Some patients may respond rapidly to a higher or lower dose and may
find much
weaker maintenance doses adequate. For other patients, it may be necessary to
have long-
term treatments at the rate of 1 to 4 doses per day, in accordance with the
physiological
requirements of each particular patient. It goes without saying that, for
other patients, it will
be necessary to prescribe not more than one or two doses per day.
The formulations can be prepared in unit dosage form by any of the methods
well known in
the art of pharmacy. Such methods include the step of bringing into
association the active
ingredient with the carrier that constitutes one or more accessory
ingredients. In general the
CA 02717991 2010-09-09
WO 2009/114373 PCT/US2009/036119
-20-
formulations are prepared by uniformly and intimately bringing into
association the active
ingredient with liquid carriers or finely divided solid carriers or both, and
then, if necessary,
shaping the product.
The formulations may be presented in unit-dose or multi-dose containers, for
example sealed
ampoules and vials with elastomeric stoppers, and may be stored in a freeze-
dried
(lyophilized) condition requiring only the addition of the sterile liquid
carrier, for example
water for injections, immediately prior to use. Extemporaneous injection
solutions and
suspensions may be prepared from sterile powders, granules and tablets of the
kind previously
described.
Compounds within the scope of the present invention exhibit marked
pharmacological
activities according to tests described in the literature and herewith, which
tests results are
believed to correlate to pharmacological activity in humans and other mammals.
The chemical reactions described in the references cited above are generally
disclosed in
terms of their broadest application to the preparation of the compounds of
this invention.
Occasionally, the reactions may not be applicable as described to each
compound included
within the scope of compounds disclosed herein. The compounds for which this
occurs will
be readily recognized by those skilled in the art. In all such cases, either
the reactions can be
successfully performed by conventional modifications known to those skilled in
the art, e.g.,
by appropriate protection of interfering groups, by changing to alternative
conventional
reagents, by routine modification of reaction conditions, and the like, or
other reactions
disclosed herein or otherwise conventional will be applicable to the
preparation of the
corresponding compounds of this invention. In all preparative methods, all
starting materials
are known or readily preparable from known starting materials.
The regimen for treating a patient suffering from a macular degeneration with
the compounds
and/or compositions of the present invention is selected in accordance with a
variety of
factors, including the age, weight, sex, diet, and medical condition of the
patient, the severity
of the infection, the route of administration, pharmacological considerations
such as the
activity, efficacy, pharmacokinetic, and toxicology profiles of the particular
compounds
employed, and whether a drug delivery system is utilized. Administration of
the drug
CA 02717991 2010-09-09
WO 2009/114373 PCT/US2009/036119
-21-
combinations disclosed herein should generally be continued over a period
until acceptable,
indicating that has been controlled or eradicated. Patients undergoing
treatment with the drug
combinations disclosed herein can be routinely monitored by fundoscopy to
determine the
effectiveness of therapy. Continuous analysis of the data obtained by these
methods permits
modification of the treatment regimen during therapy so that optimal amounts
of each
component in the combination are administered, and so that the duration of
treatment can be
determined as well. Thus, the treatment regimen/dosing schedule can be
rationally modified
over the course of therapy so that the lowest amounts of each of the compounds
used in
combination which together exhibit satisfactory effectiveness are
administered, and so that
administration of such compounds in combination is continued only so long as
is necessary to
successfully treat the macular degeneration, diabetic retinopathy, or diabetic
macular edema.
An aspect of the present invention encompasses the use of combinations of anti-
VEGF
inhibitors and compounds having Syk activity as described above to treat or
prevent macular
degeneration where one or more of these compounds is present in a
pharmaceutically effective
amount, and the other(s) is(are) present in a subclinical pharmaceutically
effective or an
effective amount(s) owing to their additive or synergistic effects. As used
herein, the term
"additive effect" describes the combined effect of two (or more)
pharmaceutically active
agents that is equal to the sum of the effect of each agent given alone. A
"synergistic effect"
is one in which the combined effect of two (or more) pharmaceutically active
agents is greater
than the sum of the effect of each agent given alone.
The present invention may be embodied in other specific forms without
departing from the
spirit or essential attributes thereof.