Note: Descriptions are shown in the official language in which they were submitted.
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
SYSTEM AND METHOD FOR REPULPING OF PAPER PRODUCTS AND
IMPROVEMENT OF WATER QUALITY WITH DIPOLAR SOLVENTS AND RECOVERY
SPECIFICATION
FIELD OF THE INVENTION
This invention relates to the treatment of water and the repulping of paper
products.
BACKGROUND OF THE INVENTION
In the pulp manufacturing industry water is used extensively and is a vital
element of the process
of repulping paper. It is used for dissolving pulp, and as a component in
loading, sizing, and
coloring ingredients as well as in the transportation of pulp fibres through
the manufacturing
process as it moves through storage tanks, screens, refiners and paper-making
machines.
The current process of repulping paper involves pulping, screening, cleaning,
and de-inking by
processes like bleaching and the application of alkaline chemicals which
contaminate the water.
Waste paper treatment methods are variable depending on the type of paper and
may involve de-
inking of toner from laser printers or photocopiers, or the removal of other
contaminants from
the paper.
Pollution sometimes causes water molecules to form large clusters which
surround molecules of
pollutant due to hydrogen bonding Even after most of the pollutant molecules
have been
removed there can still be clustering of water molecules due to residual
electrostatic interference.
This clustering can reduce the capacity of the water to dissolve, carry, and
transport solutes
including pulps and can also cause it to become anaerobic, reducing its
capacity to support
marine life. With the increasing scale of the pulp and paper industry, these
problems are
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
becoming increasingly relevant. As environmental standards tighten, new
methods of repulping
paper that are less harmful to water quality and methods of treating
wastewater to restore its
quality are increasingly needed.
Part I - System and Method for Repulping of Paper Products with Dipolar
Solvents and
Recovery
The global demand for paper and paperboard has risen steadily in recent years
and is expected to
continue to rise. This demand increase has coincided with a decrease in the
supply of pulp-
producing timber due to deforestation. The result of these two factors is
increased demand for
recycled paper. Pulp for recycled paper is typically obtained by applying
various alkaline pulping
processes with bleaching conditions selected in the attempt to obtain some of
the following
desired qualities for the resultant pulp:
1. high yield of recovered fibres;
2. suitable amount of surface adsorbed hemicellulose;
3. specific strength properties;
4. high levels of brightness;
5. sufficient smoothness.
The feasibility of manufacture of recycled pulp and its competitiveness is
largely dependent on
the yield and the quality of the pulp from a given amount of waste paper as
starting material. The
quantity of the recovered pulp and the characteristics of the fibrous material
(i.e., no less than
those of the virgin pulp) represent important parameters of the recycled pulp.
The losses during
repulping (pulping, screening and cleaning, kneading, soaking, flotation,
washing, de-inking, and
2
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
bleaching) operations are fairly high and account for a remarkable shrinkage
in industrial
revenue due to the considerably low pulp yield and inferior fibre quality.
Current alkali treatment
technology is quite inefficient for pulping of waste paper. Methods for
attainment of high quality
pulp from the recycled paper are complex and a number of schemes for pulping
and bleaching of
recycled paper materials with various, chemicals, oxidizing and reducing
agents have been
proposed, but have resulted in yield and the quality of the recovered fibrous
material being still
far below the desirable norms.
Current repulping and bleaching operations generally include pulping,
screening, cleaning, and
de-inking by a combination of kneading, soaking, flotation and washing. In
some cases,
depending on the end-use, the bleaching follows. However, each mill typically
has its own
technological line that differs from the others, depending on the type and
quality of waste paper
and the individual mill's condition. There is usually no tailor-made process
for waste paper
treatment. This is due to the fact that most technical problems change with
time. For example,
years ago, removal of objectionable substances, such as stickies, adhesives,
and hot melt was the
primary issue. Nowadays, de-inking of toner from xerography or laser printing
is the central
concern.
For pulping waste paper different chemicals, such as sodium sulfite, sodium
carbonate, sodium
hypochlorite, and sodium hydroxide, are employed for defibration. A
conventional pulper is the
most popular type of equipment used for pulping of waste paper material.
However, for pulping
in de-inking systems, it is still controversial as to whether low or high
consistency is the most
effective. The high consistency approach is based on the ground that a high
consistency pulping
is favorable for ink particle dispersion into minute particles. Nonetheless,
this kind of treatment
increases the number of ink particles and lowers considerably the brightness
after pulping. On
the other hand, in low consistency pulping, 50% of ink can be dispersed from
the fibres by this
method, which can be easily removed by washing before kneading and flotation.
However, the
choice of pulping is always judged by considering the complete de-inking.
3
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
In the screening and cleaning subprocess, for effective removal of
objectionable materials, such
as stickies and hot melt, fine slot screen is used. For coarse contaminants, a
plate screen with
hole perforations is commonly employed. Recently, a plate screen with both
holes and slots has
also been in operation. In cleaning, a reverse-type centrifugal cleaner is
widely employed to
remove light weight contaminants, such as debris of polyethylene film,
polypropylene string and
polystyrene foam and tiny stickies. Also, a gyro-type horizontal cleaner, now,
is in use, with the
advantage of rendering rejects without fibres.
Kneading is usually applied for the detachment of ink from the fibres. Many
types of kneading
machines are used to detach ink particles from fibres under high shearing
force by using
chemicals at a high consistency pulp. Such machines are called processors,
deflakers, or
dispersers. Kneading has improved de-inking efficiency. Hence, two-stage
kneading is
sometimes employed.
Soaking is introduced in many cases after kneading. The purpose of soaking is
to increase the
effects of de-inking chemicals, such as surface active agents, caustic soda,
and hydrogen
peroxide (i.e., in most cases with sodium silicate) for bleaching. The
functions of soaking are
considered as follows; in the case of de-inking old news, soaking enhances
paper strength as well
as brightness. However, yellowness of paper increases when soaking is
performed in caustic
soda solution at a higher temperature for a longer period (8-10 hr). On the
other hand, conditions
involving the use of lower caustic soda concentration (i.e., about 0.6% at a
high consistency
25%) are recommended for corrugated container waste paper treatment in order
to ease
defibration and improve the strength characteristics.
Floating usually follows the soaking. There are many types of flotation units
in the industry. The
main characteristic of these units is a high air-to-liquid ratio so as to
ensure effective separation
of ink particles from the fibres. Recently, a new flotation unit was
manufactured to produce
minute air bubbles by the use of a turbine blade which has the ability to
detach ink particles
smaller than several micrometers. Those in the past were considered as being
impossible to
remove by flotation techniques.
4
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
Then a washing stage occurs. The function of washing is to remove minute ink
and fibre
particles detached from the fibres. It is known that the resolution by the
naked eye is only
possible for particles larger than around 35 micrometers. Thus, smaller ink
particles are not
visible and detrimental to pulp brightness. These can only be removed by
washing. Decker and
screw-press type washers are used in washing.
Caustic soda is a primary de-inking agent and also it is considered as a
defbration promoter in
the pulper. Pulping of the recycled paper is usually carried out at about 50
degrees C. However,
cold pulping is also gaining popularity because at lower temperature it has
been claimed that the
disintegration of stickies is more controllable and easy to remove by
screening. Surface active
agents are used for promoting defibration by improving alkali penetration,
especially during
soaking, wetting ink during kneading and generating foam to gain control over
aggregated ink
particles. There are a number of de-inking agents available in the market such
as fatty acids, fatty
oil derivatives, higher alcohol derivatives, fatty acid derivatives, and non-
ionic detergents.
Surfactants with high penetrability in pulping of recycled paper are also in
use. For the removal
of undetached and small ink particles remaining on the fibres after kneading,
a surfactant,
capable of both de-inking and ink particle flocculation, is necessary. For
bleaching hydrogen
peroxide in the presence of sodium silicate has a wide use in the industry. In
some cases like
tissue producing mills are still using hypochlorite for bleaching. This has
been allowed after
proving that most of the dioxins and AOX (adsorbable organic halides) go into
the sludge. AOX
and dioxins in the sludge can easily be incinerated.
Bleaching results in removal of residual lignin and colouring materials from
pulps. However,
with the current conventional fibre recovering processes (e.g., pulping and
bleaching operations),
the bleached pulp from recycled paper is of low yield, rich of hornified
fibres, of low
hemicellulose content, and low levels of brightness. This downgradability
makes it unsuitable for
the manufacture of high quality papers. Pulp intended for quality papers must
meet exacting
specifications with respect to alpha cellulose, strength and optical
properties. A low content of
ash and extractives is also desirable. In current pulping and bleaching
technology of recycled
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
paper attainment of high level of brightness and removal of stickies is
usually achieved by
caustic soda treatment in most operations. Also, it is worth mentioning that
additional alkaline
pulping steps, in some times, are carried out in connection with bleaching in
order to attain high
brightness. In turn, the yield of fibrous material is fairly low and
hemicellulose losses are severe.
The strength of paper can be very dependent upon the interfelting of fibres
and upon the
cellulose mucilage which bonds the fibre into a homogeneous sheet. Currently,
ozone and
oxygen are in wide use in paper pulp bleaching. This is because they are
effective oxidizing
agents and offering a highly stable brightness. Nonetheless, pulp
delignification proved to occur
at the expense of cellulose yield and degree of polymerization since
significant hydrolytic
degradation takes place on cellulose fibres. On the other hand, the brightness
results obtained
from peroxide bleaching are found to be poor and below the standards. This is
attributed to
various difficulties encountered in dispersion of residual ink, which masks
brightness increase
and peroxide decomposition during bleaching. Biological phenomena and metal
ions were also
contributed to peroxide breakdown. In order to obtain pulp with brightness up
to 75%, a two step
bleaching should be taken into consideration; first step peroxide bleaching,
the second one after
washing a bleaching with a reducing agent (e.g., sodium hydrosulfite) is
recommended. For
removing stickies and attaining high brightness, high alkali and soap charges
in pulping are
important.
The shortfalls of current pulping operations for recycled papers are that the
current technology
demands great exertion by operators and at the same time is inefficient. This
can easily be
perceived from the very low yield and the inferior quality of the recovered
pulp. There are
unfavorable characteristics of the fibres recovered from recycled paper such
as fibre shortening,
generation of fines, fibre fatigue and hornification. The conventional methods
of recovering and
upgrading of pulp from the recycled paper are inadequate and imprecise. This
is based on the
fact that the alkaline treatment is an ineffective approach to secure a better
impregnation and
hence, defibration, particularly, in the crystalline cellulose. As a result,
great losses in fibrous
material are to be expected. The paper pulp yield is not extensive unless it
is associated with high
lignin content. In addition the multiple and prolonged use of alkaline
treatment for the recovered
fibrous material will lead to a considerable loss of hemicelluloses. This has
been verified by
6
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
investigating xylans from esparto grass holocellulose, using sodium hydroxide,
dimethylsulfoxide (DMSO), and hot water. Each extractant gave a different
yield of xylans,
however the sodium hydroxide one was found to be the highest. In addition, the
sodium
hydroxide extract gave precipitate during dialysis, indicating that alkali
soluble polysaccharides
always contain water insoluble fraction. In turn, it has been found that the
removal of
hemicelluloses from the pulp has a negative impact on paper strength
characteristics, and that the
effect of surface adsorbed xylans on burst and tensile strengths is profound.
On the other hand, caustic soda treatment for recycled paper is an
inappropriate approach for
influencing hydrogen bonding (e.g., inter- and intramolecular), of the
crystalline cellulose of
recycled fibrous material. In other words, one may speculate that the loss in
fibre flexibility is
due primarily to the fibre modification.
The two primary factors responsible for the fibre modification are the
hemicellulose removal
during various repulping and bleaching processes and the large rearrangement
of hydrogen
bonding due to the adhesives and drying effects. In hemicellulose removal, the
surface adsorbed
xylans have a significant impact on the interfibre bonding of the paper sheet.
Part 2 - Improvement of Water Quality with Dipolar Solvents and Recovery
Water as a natural resource, is a vital element and necessity in the
manufacture of pulp, paper
and paperboard and for the generation of power in the industry's steam plants.
Mills using up to
350 cubic meter per ton of paper are not uncommon in pulp and paper industry.
A large
percentage of the water requirements for the mills comes from surface
supplies, i.e., rivers and
lakes and the remainder comes from wells of a few feet to over a number of
thousands feet deep.
Good quality water in large quantities is as essential to the manufacture of
pulp and paper as
cellulose. As a matter of fact, water is one of the most critical of all
materials used by the pulp
and paper industry. It is used directly in the processing of pulp, it
dissolves or is mixed with the
various loading, sizing and coloring ingredients; and in addition, it is the
medium which carries
7
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
the fibres through the storage tanks, screens and the refiners to the paper-
making machine where
it plays a most important role in the making of a sheet of paper.
However, today's water has encountered serious pollution problems. Pollution
causes water
molecules to gather together in larger clusters than they would naturally be,
and hence as the
water "wraps up" dissolves the pollutant. Even if the pollutant is filtered
the water molecule
cluster still remains in unnaturally large cluster due to its lasting
electromagnetic frequency
influence on the water, this frequency keeps the water molecules in the same
unnatural structure
they were when the pollutant was present, despite its absence.
Water pollution comes in many forms such as chemicals, thermal, farm run-off,
frictional and
electromagnetic. Even methods or devices that we typically use for the removal
of pollution from
water are themselves contribution to water pollution on the
molecular/frequency level. In other
words, pollution saturates water with unnatural amounts of substances and
electromagnetic
influences, that all leave their influence in the form of frequency on water,
reducing its capacity
to dissolve, carry, transport and be microbiological stable (e.g., limitation
of bacteria and enzyme
growth). As water becomes over polluted it can no longer clean or regenerate
itself, it is simply
too full of the frequency influence of pollution, causing larger than natural
water molecule
clusters (i.e., free oxygen trappers). If water can not dissolve and transport
oxygen effectively it
can become anaerobic.
OBJECTS OF THE INVENTION
The invention is designed to provide an enhanced method of repulping paper and
improving
water quality with dipolar solvents and recovery. These objects are discussed
in two parts, first
with respect to the repulping objects, and second with respect to the water
quality improvement
objects. Essentially the repulping objects are to reduce fibre losses and
improve pulp quality.
There are a number of aspects to this as discussed below under part 1. The
primary water quality
improvement objects are to increase its reactivity and solvent capacity. This
has numerous
8
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
advantages as discussed below under part 2.
Part I
It is an object of this part of invention to avoid great fibre losses and
inferior pulp quality,
resulting from the current pulping and bleaching technology used in the
recycling of paper
material. Regarding the fibrous material (Part 1), the current invention uses
dipolar aprotic
protophylic solvent in a novel technique, providing the following advantages:
1. high yield of recovered pulp fibres with considerable fibre flexibility can
be attained by the
effectively uniform interaction of dipolar solvent with both amorphous and
crystalline cellulose,
i.e., effective breakdown of interfibre bonding of the cellulosic material;
2. ease of defribration and recovery of fibres with attenuated H-bonds, i.e.,
accessible cellulose;
3. fibres can be recycled continuously due to open hydrogen bond packing;
4. dissolution-hydration of hemicellulose (e.g. surface adsorbed xylans) and
better degree of
hemicellulose retention;
5. ease of detachment of additives, adhesives, and ink particles due to
greater specific molecular
surface area exposure to de-additive, de-adhesive, de-inking reactions.
6. energy savings by reduction in other operations (e.g., kneading, soaking,
de-inking);
7. ease of bleaching, i.e., greater exposed surface area of fibres for
reaction;
8. better sheet strength properties;
9
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
9. enhanced paper machine operation;
10. Ease of draining; less fines for blocking screens due to breakage of stiff
particles;
11. Smoother paper sheets from re-pulpled material due to fibres being
flexible and comfortably
in pressed position; less fluffiness on sheets due to stiff fibres sticking
out;
12. environmentally benign.
The tensile strength, burst strength, and smoothness of paper sheets prepared
from the re-pulpled
material are expected to be improved due to the flexibility of the fibres and
the increased amount
of hemicellulose attained by the method and system. The flexibility of the
fibres and the
increased amount of hemicellulose retained by the fibres are directly
proportional to the
weakening of the hydrogen bonds in the cellulose. The optimizing loops are
thus both easy and
important to implement for a given batch of repulpable material. The recovered
fibres are
continuously recyclable using the method of the present invention, as the
fibres are always in a
comfortable H-bonding position. The system and method of this part of
invention is thus
compatible with sustainable development principles that aim at a rational and
effective use of
renewable resources, while providing a significant increase in industrial
revenue.
Part2
Dipolar aprotic solvents (DAS) improve the water quality by organizing its
internal structure. In
other words, dipolar solvent interaction with water physically enhances the
quality of water for
the pulp and paper industry in many different ways. For example, in paper
industry where huge
amounts of water are consumed (i.e., in most cases over 100 m3 of water/ton of
fibres (dry
weight), these solvents, through rearrangement of hydrogen bonding of water,
offer a reactive
water which is of importance for water consuming pulp and paper industry. The
aim of alteration
water structure is to increase its reactivity. This can be achieved through
the interaction of (DAS)
with water; the hydrogen bonding of water is rearranged in a way that the
water molecules
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
acquire almost their natural conformation, i.e., tetrahedral lattice. For
instance, by one of (DAS)
family such as DMSO when added to the water, the water molecules are assumed
to regain more
OH stretch that could approach the natural H-O-H angle which is 109.47 . As a
result, the
DMSO restructured water acquires several positive properties such as smaller
water clusters,
lower surface tension, better carrying efficiency, increased hydration
capacity, improved power
of microbiological stability (i.e., the rearrangement of hydrogen bonding
through dipolar aprotic
solvent is to render a water structure of almost without free oxygen which is
a favorable
environment for bacteria and enzymes to grow and proliferate) and greater
interfibre bonding
power. These dipolar aprotic solvent restructured water qualities are superior
for pulp and paper
various technological processes where highly purified and interactive
"reactive" water is crucial
for pulping, pulp washing, screening, soaking, mixing, and refining. Also,
microbiological
stability of the dipolar aprotic solvent rearranged water is of significance
since the main
components (e.g., cellulose, lignin and hemicellulose) of the fibres are all
biodegradable and
hence this quality of the solvent restructured water will limit the bacteria
and fungi growth in the
process water. Thus, the dipolar aprotic solvent hydrogen bond rearranged
water (Part2) offers
the following advantages:
1. Increased fibrous material hydration capacity through water-water weaker
hydrogen bonds and
smaller water clusters interaction with cellulosic material, i.e., better
interfibre bonding
2. Ease of detachment of adhesives, additives and ink particles due to high
dissolving power
quality of dipolar aprotic solvent treated water (i.e., dissolution of
extraneous substances)
3. Improved fibrous mass transfer due to high dissolving quality and greater
carrying efficacy of
aprotic solvent-water system
4. Lesser sludge load due to microbiological stability of water, i.e., highly
tetrahedral lattice
water with no free oxygen for bacteria and enzymes to grow and proliferate
5. Limited use of biocides
11
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
6. Enhanced fibrous material mixing, i.e., uniform defibration due to lower
surface tension,
greater carrying efficiency and increased dissolving power of aprotic solvent
water
7. Limitation in the use of sheet strength and sizing agents
8. Reduction in water consumption
9. Cutting down in electricity and chemical consumption
CHEMISTRY OF DIPOLAR SOLVENTS AND CELLULOSE
It is well known that acid or base strength depends on the acidity or basicity
of the solvent,
however, other properties of the solvent should be taken into account. One of
these is the
dielectric constant, which is important because it is a measure of the ion-
solvating ability of the
solvent. Solvents with high dielectric constant such as water are capable to
solvate each ion. At
lower dielectric constant, ions aggregate in a manner ion pairs and larger
aggregations are
present. This situation makes little difference when the equation has the same
total charge on
both sides as indicated below, HA+ + B = HB+ + A. However, when the total
charge increases,
for instance, HA + B = HB+ + A-, then a solvent with high dielectric constant
forces the
equilibrium further to the right than does one with a lower dielectric
constant. Even when the
charge is unchanged, the dielectric constant of the solvent may still make a
difference if the ion
(or ions) on the left are more solvated than the ones on the right. Also, the
solvent may cause
differential solvation in another way, which is different from the effect of
the dielectric constant,
originating from the difference in solvation of anions by a protic solvent
(which is a hydrogen
bond donor HBD) and an aprotic one HBA (hydrogen bond acceptor). However, the
effect could
be extreme: in dimethylformamide (DMF), picric acid is stronger than HBr. This
particular result
12
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
could be attributed to the size of the molecule. In other words, the large ion
(02N)3C6H20- is
better solvated by dimethylformamide (DMF) than the smaller ion Br-; while in
protic solvent
like water the solvation of an anion is by the small unshielded proton H. This
Hydrogen bond is a
molecular interaction that involves the sharing of a hydrogen atom by a weakly
acidic donor and
a weakly basic acceptor atom. Hence, hydrogen bond is an important structural
element
particularly in supermolecular structure of the cellulose. In other words,
hydrogen bonds are
crucial elements of the three dimensional conformation that allow fibrils to
form and coalesce in
stronger lateral order. On the other hand, the dipolar aprotic hydrophylic
solvent treatment in a
major fraction of water is expected to bring about, within the cellulosic
material, irreversible H-
bonding rearrangement that will cause spacing in the previously deformed
(closed) hydrogen
bonding of the substrate due to reactions of adhesives and the impact of
drying. The probable
explanation of the hydrogen bond attenuation is due to the interaction of
dipolar solvent (HBA)
with the polar hydroxyl groups of the cellulose molecules (HBD). On the other
hand, with regard
to the water, it is not well understood to what extent water can penetrate the
crystalline cellulose,
but in any case it is known that such penetration does not bring about any
change of spacing in
the crystallites. In this respect, dipolar aprotic solvents such as
dimethylsulfoxide (DMSO),
dimethylformamide (DMF), and dimethylacetamide (DMA), with their unique
solvation
characteristics will ensure better impregnation (e.g., amorphous and
crystalline zones) to an
extent that can not be offered by other solvents. In conclusion, their role
can also be understood
as swelling agents of cellulose.
CHEMISTRY OF DIPOLAR SOLVENTS AND WATER
Water- dipolar aprotic solvent binary mixtures are powerful solvent systems
used frequently in
many branches of chemistry and industries, and their efficient application in
chemical processes
will contribute to reduce a global environmental impact. Solvent effects in
these mixtures depend
nonlinearly on the mixing ratio, and studies of preferential solvation have
offered important
results.
13
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
Water is a fairly malleable substance. Its physical shape easily adapts to
whatever environment is
present. But its physical appearance is not the only thing that changes, the
molecular shape is
also susceptible to change. The energy or vibrations of the environment has a
quite effect on
changing the molecular shape of the water. In this sense water not only has
the ability to visually
reflect the environment but it also molecularly reflects the environment.
It is known that low concentrations of some solvents such as dipolar aprotic
solvents modify the
water structure in such a way that suppresses the protic (H-bond donor)
reactivity of water and
enhance its basic (H-bond receptor) reactivity. These reactivity changes
within the water
structure are well responsible for rendering water with unique qualities such
as smaller water
clusters, increased dissolving power (i.e., dissolution of extraneous
substances such as stickies,
minerals and ink), lower surface tension, better microbiological stability and
greater self-
purification capacity. Additionally, dipolar aprotic solvents are fairly
effective OH free radical
scavenging agents. These characteristics imply that aprotic solvents induce a
more intensive
structuring of water and they are effective in producing highly structured
smaller clusters of six
water molecules. These predominating ice-like clusters of water are believed
to represent the
highly structured part of liquid water. The dipolar aprotic solvent molecules
create these
structural effects in part because dipolar aprotic solvent is a hydrogen bond
acceptor but not
donor and in part because aprotic solvent bonds with water more strongly than
water bonds to
water. Accordingly, the solvated aprotic solvent is supposedly bonded to two
water molecules,
and the average angle between the two hydrogen atoms, bond in the dipolar
aprotic
solvent.2H20 (e.g., DMSO.2H20), is nearly tetrahedral. A water molecule is
hydrogen bonded
to water one but near the oxygen of a dipolar aprotic solvent can
simultaneously bond with
aprotic solvent or readily switch its bonding from water to the aprotic
solvent. In other words, the
rearrangement of hydrogen bonding by a dipolar aprotic solvent produces weaker
hydrogen
bonds between water-water molecules than those produced between aprotic
solvent and water
molecules. These water molecules with weak hydrogen bonds are ready to
interact through
intermolecular hydrogen bonding with the sugar units and produce substantial
hydration within
the cellulosic material. Also, the predominance of smaller water clusters in
water-aprotic solvent
systems will give rise to increased weaker hydrogen bonds between the clusters
themselves..
14
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
Similarly, these small clusters would contribute to better impregnation and
hydration throughout
the fibrous material (i.e., amorphous and crystalline cellulose), which are
essential for
detachment of adhesives, additives, ink particles, interfibre bonding and
uniform defibration.
In conclusion, the rearrangement of water structure through the hydrogen
bonding by a dipolar
aprotic solvent will offer the following; weaker hydrogen bonds between water-
water molecules
and among the small water clusters as well. These characteristics will render
water with superior
qualities such as reactive water, greater fibrous material hydration power,
increased detachment
of adhesives, additives and ink particles, microbiological stability (e.g.,
limitation of bacteria and
enzymes growth) and better fibre mixing performance.
SUMMARY OF THE INVENTION
This invention provides a system and method of repulping and water quality
improvement using
an aqueous dipolar aprotic protophylic solvent in an agitator vat, or pulper,
with optimization of
process variables depending on the reactivity of dipolar aprotic solvent water
system, type of
material being re-pulpled and the desired characteristics of the pulp
resulting from the process.
The mechanism of waste paper repulping in an aqueous dipolar aprotic
protophylic solvent is
based on two physical reactions of the dipolar solvent, first with water from
one part of the
solvent and second with cellulosic material from the other part of the
solvent.
The first physical reaction is caused by the substantial alteration of water
structure through the
rearrangement of its hydrogen bonding system. The dipolar aprotic solvent
molecules (e.g.,
DMSO) create these structural changes within the water in part because aprotic
solvent is a
hydrogen bond acceptor and in the other part because dipolar aprotic solvent
bonds to water
more strongly than water bonds to water. Accordingly, to the simulation
analysis, the solvated
aprotic solvent molecule (e.g., DMSO) is bonded to two water molecules, and
the average angle
between the two hydrogen bonds in the aprotic solvent.2H2O (e.g., DMSO.2H2O)
is almost
tetrahedral. A water molecule is hydrogen bonded to another water one but near
the oxygen of a
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
dipolar aprotic solvent (e.g., DMSO) molecule the water molecule can
simultaneously bond with
the aprotic solvent or readily switch its bonding from water to the aprotic
solvent. However, if
the water molecule was instead near the methyl groups of aprotic solvent no
such alternative
bonding would be possible.
The second physical reaction may be attributable to the greater accessibility
of cellulose as a
result of disruption/destruction of hydrogen bonding by the dipolar solvent.
The second physical
reaction is the dissolution of hemicellulose by chemical and mechanical
actions of the treatment.
Furthermore, since the dipolar solvent is a strong hydrogen bond acceptor and
has a high
solvating power, this technique may ease and lead to a total detachment of ink
and adhesives
from the fibres.
The group of dipolar aprotic protophylic solvents comprises
hexamethylphosphorictriamide
(HEMPT) and acetone, as well as the above-noted dimethylsulfoxide (DMSO),
dimethylformamide (DMF), and dimethylacetamide (DMA). HEMPT however has the
disadvantage that it is considered to be carcinogenic, mutogenic and toxic to
reproduction in
animals. Acetone as a repulping solvent has the disadvantage that it brings
about considerable
disproportionality on the cellulose chain, by forming isopropylidene
derivatives on the sugar
rings of the residual cellulose. This would provide a low degree of
polymerization of the
cellulose and would have a negative impact on the strength of a paper sheet
formed from the re-
pulpled material. Pyridine is also considered to be a dipolar aprotic
protophylic solvent, but it
has nitrogen, which forms hydrogen bonds that are less strong than those
formed with oxygen, as
formed by the other dipolar aprotic protophylic solvents. DMSO2 (i.e., the
metabolite of
DMSO) can also be used as a hydrogen bond acceptor. However DMSO2 is a
crystalline solid,
less soluble in water and has a high melting point (109 degrees Q. The
remaining three noted
dipolar aprotic protophylic solvents, namely, dimethylsulfoxide (DMSO),
dimethylformamide
(DMF), and dimethylacetamide (DMA), are each highly suited to use for H-bond
disruption in
the system and method of this invention. The process is characterized by:
1.* H-bond disruption/destruction by the dipolar aprotic protophylic solvent
treatment to offer an
accessible cellulose;
16
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
2. Lesser dissolution of hemicellulose by the chemical and mechanical action
of the technique.
The process ensures a concerted disruption/destruction of hydrogen bonding of
the cellulose
(carbohydrate) by dipolar solvent and minimizes the removal of hemicellulose
by avoiding
alkaline treatment. This is achieved by penetration of dipolar solvent
molecules into the cellulose
(e.g., amorphous and crystalline), and interaction of dipolar solvent with
cellulose (carbohydrate)
molecules through their hydroxyl groups. This presumably brings about
stereochemical changes
(i.e., rotational) that disrupt and permanently weaken the H-bond of both
amorphous and
crystalline cellulose, thereby providing accessible cellulose. Collateral
benefits include recovered
fibres being continuously recyclable, less removal of hemicellose, uniform
defibration of the
cellulosic material, better interfibre bonding, easy detachment of adhesives
and ink particles, and
ease of bleaching.
In a similar manner, the process offers water with structural changes through
the rearrangement
of its hydrogen bonding system by the dipolar aprotic solvent; water molecules
with weaker
hydrogen bonds and smaller water clusters that can easily interact with
cellulosic material and
bring about considerable hydration within it. As a result several benefits can
be attained
including better fibrous material hydration power, increased detachment
quality of adhesives,
additives, and ink particles, enhanced microbiological stability, improved
pulp mixing quality,
greater inter-fibre bonding capacity and minimum use of sheet strength and
sizing agents.
For optimum results, the temperature, solvent concentration, type of dipolar
solvent, solid/liquid
consistency, and mechanical agitation (the "Adjustable Factors") should be
adjusted in
accordance with known and test effects of those factors on the type of paper
material to be
pulped: imprinted pre-consumer paper, printed paper, newsprint, paperboard,
old corrugated
containers, tissue paper liners, packaging paper boxes, coated paper, mixed
papers, office waste,
old magazines.
The temperature of the solvent mixture should be in the range of 5 to 90
degrees C, with a range
of 5 to 40 degrees C often producing optimal results for a typical mix of
recyclable papers. The
17
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
concentration of the appropriate dipolar solvent should be in the range of
0.001% to 40%, the
remainder water, with a range of 0.1 % to 5% often producing the optimal
result for a typical mix
of recyclable papers. The solid/liquid consistency should be in the range of
1% to 33% by
weight, with a range of I% to 10% often producing the optimal results for a
typical mix of
recyclable papers. The mechanical agitation of the re-pulpable material should
be in the normal
range of the equipment used for agitation and mixing in repulping processes.
The time of the
mixing should be in the range of 1 to 90 minutes. Bleaching should be
performed on the
material.
The effect of varying these parameters (temperature, type of dipolar solvent,
solvent
concentration, and liquor/solid ratio) on pulp quantity and quality can be
assessed by sugar
analysis, alpha cellulose content, infrared (IR), and drainability analysis.
The dipolar aprotic solvent influence on water structure occurs through the
alteration of the
hydrogen bonding lattice; weaker hydrogen bonds can be examined using IR
absorption
spectroscopy, and water cluster size can be studied using Mass Spectrometric
analysis of dipolar
aprotic solvent-water binary mixture. Microbiological stability of aprotic
solvent-water binary
system can be examined through microscopic analysis for bacteria count.
Different standards and Tappi standard techniques such as kappa number,
effective residual ink
concentration (ERIC) holocellulose content, xylan content, burst strength, and
tensile strength
should be employed to evaluate pulping, bleaching, and optimization of the
recovered pulp, in
order to optimize the process for any given type of repulpable material. For
the removal of
residual lignin and coloring materials, different types of bleaching can be
applied: peroxide
bleaching; OZEP (ozone / extraction / peroxide), and biobleaching using
microorganisms.
The novel application of aqueous dipolar solvents in pulping of recycled paper
is designed to
address the major problem of stiffness of the recovered fibres. Fibre
hornification (stiffness) has
been a crucial drawback in the quality of the pulp from recycled paper and
will remain a major
problem if paper industry continues to apply alkaline pulping in the recycled
paper
18
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
manufacturing. The fibre stiffness is attributable to fibre modification that
has been brought
about during the original pulping, bleaching and drying operations in making
the initial paper
products that are to be recycled. And conventional repulping by alkaline and
bleaching treatment
increases the fibre stiffness. The two factors responsible for modification of
the recovered fibre
are considered to be the hemicellulose removal by mechanical and alkali
treatment and largely
alteration of hydrogen bonding due to the action of various adhesives (if
applied) and drying of
paper. As a remedy for fibre stiffness, the use of aqueous dipolar solvent as
a pulping liquor in
recycled paper is excellent. On one hand, this treatment will render an
accessible cellulose
(amorphous and crystalline) ready to form a new hydrogen bond packing. On the
other hand, the
application of aqueous dipolar solvents in recycled paper manufacturing will
limit the excessive
drainage of hemicellulose from the fibrous material. In this respect, the
proposed technique is
suitable to treat all types of recycled paper, each at certain optimum
conditions. Further on, in the
proposed technique, the recovery of dipolar solvents and de-inking chemicals
are taken into
account. In addition, the dipolar solvents (DMSO, DMF and DMA) recommended for
pulping of
recycled paper are cost- effective, have a high boiling point, a high flash
point, and are generally
considered to be non-carcinogenic.
The process is flexible enough to accommodate any of oxidizing, reducing,
deinking, swelling,
dispersing, chelating, buffering, filling, strength enhancing, detackifying
agents if needed.
However, aqueous DMSO repulping alone is capable of offering high yield and
superior fibre
quality for all types of recycled papers.
The recovery and reuse of process solvents (H20, DMSO, and petroleum ether)
makes both
environmental and economic sense for the recycled paper industry. Well
designed recovery
systems can pay for themselves in a relatively short period.
Using conventional distillation techniques, the separation of DMSO and water
is both
impractical and uneconomical, simply because it requires a large amount of
energy to be
consumed. Thus, based on the experimental data obtained in this work (see
Figures 8 & 9), the
following three-stage solvent separation is recommended:
19
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
* Liquid Extraction
* Distillation
* Solid/Liquid Separation
DESCRIPTION OF THE DRAWINGS
Figure 1 is an illustration of the formation of a 6-water molecule cluster
with a dipolar aprotic
solvent dimethylsulfoxide S(CH3)2.
Figure 2 is an illustration of the stretch of an H-bond from 104.47 degrees to
109.47 degrees as a
result of the influence of dipolar aprotic solvents.
Figure 2B shows the adoption of H-bond angle tetrahedral form caused by the
influence of
dipolar aprotic solvents.
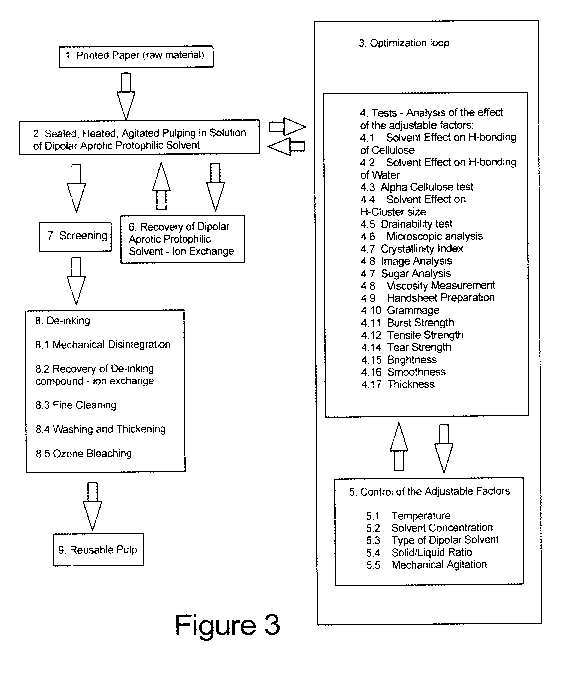
Figure 3 is a block diagram showing the steps of repulping using the method of
this invention.
Figure 4 is a diagram showing cellulose molecules in repulpable material, and
the chemical
structure of three suitable dipolar aprotic protophylic solvent molecules.
Figure 5 is a diagram showing the effect of dipolar aprotic protophylic
solvent on the hydrogen
bond of cellulose.
Figure 6 is a diagram showing the apparatus used in the system of this
invention.
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
Figure 7 is a diagram illustrating an extractive distillation subprocess.
Figure 8 is a diagram illustrating the liquid extraction subprocess.
Figure 9 is a diagram showing the distillation unit.
DETAILED DESCRIPTION
Figure 1
Referring to Figure 1, the formation of a 6-water molecule cluster is
illustrated with the dipolar
aprotic solvent dimethylsulfoxide shown in the upper left comprised of oxygen
atom 103, sulfur
atom 104 and two methane (CH3) molecules, 100 and 102. The dimethylsulfoxide
forms
hydrogen bonds with the water at 101 and 105. These bonds prevent other water
molecules from
bonding with the water molecules in the same place, thereby reducing the size
of the water
cluster. The 6-water cluster is defined by a ring of oxygen atoms 111, 112,
113, 114, 115, and
116 and hydrogen atoms such as 106 and 107 which are connected to oxygen atoms
114 and 115
respectively with hydrogen bonds represented by single lines 108 and 109
respectively. The
molecules in the 6-water cluster may be connected to other water molecules or
other molecule
clusters by any number of branching hydrogen bonds.
Figure 2A
Referring to Figure 2A, the effect of a dipolar aprotic solvent on the H-O-H
angle 210 defined by
hydrogen atom 201, oxygen atom 200, and hydrogen atom 202 is to stretch it
from an angle of
104.47 degrees to 109.47 degrees. This is also called an H-bond angle because
it is the angle of
the H-bond 203 in relation to the covalent bond 204. The initial angle is the
residual effect of
bleaching agents on the water..
21
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
Figure 2B
Referring to Figure 2B, the final H-bond angle results in a molecular
arrangement close to the
normal tetrahedral structure of water shown by the water molecule comprised of
oxygen atom
206 and hydrogen atoms 205 and 207. This structure results in reduced water
molecule clustering
and improves the reactivity of the water, reducing trapped oxygen and
resulting in increased
solvent capacity of the water which enables more pulp to be dissolved per unit
volume of water.
Figure 3
Referring to Figure 3, the steps of the repulping process using the method of
this invention is
shown starting with the printed paper raw material 1 which is heated, agitated
and pulped in a
solution of dipolar aprotic protophilic solvent 2; followed by an optimization
loop 3, comprising
tests and analysis 4 and control of adjustable factors 5, which is performed
to adapt the process
to the particular kind of printed paper raw material 1. The recovery of the
dipolar aprotic
protophylic solvent 6 is ecologically sound, and can be done by using
petroleum ether for
example to recover DMSO by an extractive distillation loop such as that shown
in Figure 7.
(Referring to Figure 7 extractive distillation is a well-known subprocess that
involves adding an
extractive component that imbalances the relative volatilities of a binary
system of components
and allows separation of the binary components to take place.) In step 7 of
Figure 3, the coarse
screening 7 allows for subsequent typical repulping procedures 8, and in
combination with the
earlier steps, provides a yield of high quality reusable pulp 9 that can be
used to make recycled
paper products.
In the solvent pulping of recycled paper (Figure 3, steps 2-6), the runs for
obtaining solvent
recovered fibres with open hydrogen bonding system should be conducted at a
temperature in the
range of 5-90 C. Based on initial strength and the type of pulp the fibres
were made of, the
aqueous solvent concentration to break-down interfibre bonding and to ensure
sufficient
defibration should be in the range of 0.1 to 1 percent, (e.g. 0.5%25% of o.d.
fibrous material).
Solid to liquid consistency will be close to 1:15, i.e., similar to those
conditions of conventional
pulping. Hemicellulose extract can be isolated from the recovered pulp by
filtration through a
funnel. The filtrate should be filtered 2 to 5 times through the fibre mat in
order to completely
22
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
remove the fines. Then the filtrate should be dialyzed for 48 hours in order
to get rid of the
solvent and low molecular weight impurities. The filtrate should be reduced to
a small volume by
rotary evaporating. Freeze drying can be used to test yield and to store for
further analysis. The
fibre mat should be diluted to a consistency of 2% according to Tappi standard
(T 205 om-88)
and disintegrated. Then the pulp slurry will be screened through a pulp
strainer with a slot cut
width 0.15 mm. After yield determination, the screened pulp will be ready for
further evaluation
and adjustments to the process to optimize the variable factors of
temperature, solvent
concentration, liquid to solid ratio. To remove undetached and small ink
particles remaining on
to the fibres after solvent treatment, a de-inking agent such as fatty acids,
fatty acid derivatives,
higher alcohol derivatives, or fatty oil derivatives will be introduced to the
fibrous material in the
disintegrator.
In step 5, after each change of the adjustable factors, some or all of
following tests 4 should be
conducted to analyze the effect of the change, with a view to achieving a
balance in the desired
characteristics of the re-pulped material:
4.1 Solvent Effect on H-bonding of Cellulose : Infrared spectra of the re-
pulpled material will be
analyzed to determine the effect of the solvent on the H-bonding of the
material. The test results
are directly proportional to the amount of cellulose fibre disruption that has
occurred. An
example of IR analysis by Diffuse Reflectance Infrared Fourier Transformer
(DRIFT) test is:
obtaining the average absorbance peak height around 3400cm-1 (gross hydroxyl
range) of
differently treated cotton specimens, in a way that the intensity of the
hydroxyl band could be
read without being influenced by the intervening factors. Such factors that
could be taken into
consideration are the variations in peak locations due to stereochemical
changes and noise
perturbation. The analysis is performed on Perkin-Elmer 1610 Fourier transform
infrared
spectrometer equipped with Perkin-Elmer diffuse reflectance attachment, with
the sample
incorporated in ground potassium chloride was ratioed against that of each
sample. The spectra
from measurements involving 64 scans at a spectral resolution of 8cm-1. The
hydrogen bonding
test is the mean for three measurements.
23
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
4.2 Solvent Effect on H-bonding of Water: The Fourier Transform Infrared
difference spectra
between plain dipolar aprotic solvent, dipolar aprotic solvent-water binary
system, and untreated
water will be analyzed for the 3750-3450 -cm-I to determine the effect of the
solvent on the H-
bonding of water.
4.3 Alpha Cellulose test: This test is again of an effect that is directly
proportional to the effect of
the weakening and disruption of the hydrogen bonding during the repulping
process of the
current invention, as it measures how much hemicellulose remains after
treatment, and how
much the crystalline cellulose responded to the treatment. The carbohydrate
fraction of
holocellulose, resistant to dissolution in 17.5% NaOH, is termed alpha
cellulose. That percentage
is a significant parameter of wood and chemical pulps and is used to assess
the approximate
quality of dissolving pulps, and in paper pulp it gives information about the
residual
hemicellulose content and degree of disruption and defibration of cellulose
fibres which occurs
during the repulping process. The air-dried holocellulose is treated with
several portions of
17.5% NaOH, washed and dried. The residual remaining in a filter crucible is
the alpha cellulose.
The outcome of the swelling depends on the disruption since the filter
material passes through
maximum swelling at 10% NaOH during the washing. Analysis can also be done of
the dissolved
fraction which consists of beta- and gamma-celluloses (hemicellulose).
4.3 Solvent Effect on Water Cluster Size: For the determination of water
cluster size, such as
argon gas clusters will be doped in a an aprotic solvent untreated water pick-
up cell, and the
subsequent electron impact ionization of the doped clusters in a mass
spectrometer or gas
analyzer produce ionized cluster fragments that retain water. Water is
supplied under pressure to
the pick-up cell disposed within a vacuum chamber, and the water pressure is
metered by a
metering valve and monitored by a pressure gauge. A vacuum pump is coupled to
the vacuum
chamber that generates a vacuum within the vacuum chamber and pick-up cell.
Interaction
between the gas clusters and the water in the pick-up cell produces doped
clusters, some of
which retain water. The electron impact ionized doped cluster fragments are
analyzed using the
mass spectrometer or gas analyzer permits determination (detection) of the
mean cluster size of
the clusters. The variation in intensity of the untreated water-containing
fragments versus water
24
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
pressure in the pick-up cell exhibits a Poisson behavior, from which the cross-
section and mean
cluster size is derived. Similar test will be carried out on a dipolar aprotic
solvent-water binary
system and plain dipolar aprotic solvent and the mean cluster size of the
solvent treated water is
derived.
4.4 Drainability test: The results of this test are also directly proportional
to the H-bond
disruption in the repulpable material. The test is also known as a Freeness
test, a measure of the
drainage rate of a pulp stock suspension through a fibre pad formed on a wire
or perforated plate.
The Canadian Standard Freeness tester is a North American standard. The less
stiff and more
flexible the fibres resulting from the repulping, the fewer fines break off to
clog drain screens. In
the finished paper sheet produced from the re-pulpled material, the fluffiness
of the sheet, which
consists of fibres sticking out from the general plane of the sheet, the
stiffness again plays a role.
The more flexible the fibres, the more comfortable the fibres will be in their
pressed position in
the finished paper sheet.
4.5 Microscopic Analysis: To investigate microbiological stability of dipolar
aprotic solvent-
water mixture compared to untreated water, microscopic analysis is to be
conducted for bacteria
count.
4.6 Crystallinity Index: The crystallinity index of the re-pulpled material
should be checked for
acceptability
4.7 Image Analysis: For sheet formation, cell wall cross section, and fibre
response Sigma Scan
Pro software can be used to track changes in these aspects.
4.8 Sugar Analysis: Water-soluble polysaccharides should be analyzed.
4.9 Viscosity Measurement: The degree of polymerization (DP) should be
determined according
to the Tappi standard 230 os-76.
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
4.10 Handsheet Preparation: Handsheets from the pulp of recycled paper should
be prepared
according to Tappi standard.
4.11 Grammage: This will be measured according to Tappi standard T410 os-68.
4.12 Burst Strength: To determine burst strength a Mullen burst tester will be
used.
4.13 Tensile Strength: The force required to break a strip of paper of 15 mm
width and 100 mm
length will be determined according to Tappi standard T 494 om-88.
4.14 Tear Strength: This will be carried out according to Tappi standard T 414
om-88.
4.15 Brightness: Brightness of handsheets from recycled paper will be
determined according to
Tappi standard UM 438.
4.16 Smoothness: To measure smoothness of a handsheet a Bendtsen smoothness
and porosity
tester can be employed.
4.17 Thickness: This will be determined using a Messmer micro-electronic
thickness tester.
After these step 4 tests are performed, if undesirable results are obtained
the factors in step 5 can
be modified until the desired results are obtained. The foregoing optimization
procedures can be
performed on small test batches, with the volume of equipment and material
increased when the
results are optimized. For example the optimum conditions for printed paper
may have been
determined under the optimization loop of step 3 to be as follows: i) dipolar
solvent is
dimethylformamide (DMF), ii) dimethylsulfoxide (DMSO) dipolar solvent to water
ratio is 10.5
solvent:99.5 water, iii) solid to liquor ratio is 1:20,11 and iv) pulping
temperature is 20 C35 C v
reaction time 150 min. These optimized conditions can then be replicated in
the large batch.
Once the conditions are optimal as indicated by the test results, we return to
step 2 where the
26
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
pulp is once again sealed, heated, and agitated in a solution of dipolar
aprotic solvent. The
solvent is recovered in step 6, then the pulp is sealed, heated and agitated
again 2, then it is
screened using the coarse filter 134 and fine screen 137 shown in Figure 6 and
detailed in the
description of figure 6 below.
After screening the pulp undergoes the typical repulping procedures of steps 8
- 8.5, including
de-inking 8, mechanical disintegration,8. 1, recovery of de-inking compound
8.2, fine cleaning
8.3, washing and thickening 8.4, and ozone bleaching 8.5, which is performed
to attain high
brightness levels of the solvent pulp. The standard bleaching method is
peroxide and can be
used. Brightness of bleached pulp will be checked according to Tappi standard
UM 438. Ozone
bleaching is capable of further delignifying de-inked pulp prepared by aqueous
dipolar
technique. Incorporating it in an OZEP (ozone / extraction / peroxide)
sequence can produce a
brightness of 90% ISO. Ozone is an aggressive oxidizer, compared to hydrogen
peroxide, which
is a mild oxidizer. The resulting pulp properties if compared to those of
conventionally prepared
and bleached recycled pulp would much be better off. Effluent characteristics
would also be
much improved. Toxic effluents from chlorine-free bleaching are extremely low
and there are no
dioxins, AOX in the effluent. Biobleaching with the use of enzymes can
decrease the use of
oxidizing chemicals in bleaching of recycled paper pulps. Treatment of de-
inked pulp with
selected enzymes can remove a greater fraction of the lignin without affecting
the degree of
polymerization (DP) of the cellulosic material. The biobleaching technique has
been successfully
tried in commercial trials. Environmentally, biobleaching is attractive.
However, biobleaching
entails high manufacturing costs for the enzymes and the slowness of the
reaction.
Referring to Figure 4, the hydrogen atoms 20, 21, 22, form the bonding between
two adjacent
cellulose molecules 63 and 64. The dipolar aprotic protophylic solvent
molecules 91, 92, 93 are
respectively dimethylformamide (DMF), dimethylacetamide (DMA), and
dimethylsulphoxide
(DMSO). They are adjacent to the cellulose in Figure 2, ready to be placed
into a solution where
they will interact with the hydrogen as shown in Figure 5 These solvent
molecules have a
substantial dielectric constant and dipole moment. They have no acidic
hydrogen to enable the
formation of hydrogen bonds. Because of their shape, they are better able to
solvate cations than
27
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
anions. This can be measured by the transfer of energy from methanol to
dimethylsulphoxide
(DMSO), for example. The reactions of cations in such dipolar aprotic solvents
is therefore much
higher than in hydroxylic solvents. It is this property which will cause the H-
bond disruption
shown in Figure 5. The dipolar aprotic protophylic solvent molecules 91, 92,
93 will act as
hydrogen bond acceptors with cellulose material.
Referring to Figure 5, the dipolar aprotic protophylic solvent molecules 60,
61, 62 disrupt the
hydrogen bonding in the cellulose by positioning themselves between the oxygen
atoms 65 and
66. This occurs because the oxygen has an electronegativity of 3.5 and the
hydrogen an
electronegativity of 2.1. Hydrogen has a low electron affinity, and oxygen has
a high electron
affinity in the mixture of the solution and the repulpable cellulose material.
Referring to Figure 6, the pulping can be performed in a batch open vat pulper
31, allowing
observation of the process. The process starts with recycled paper (e.g.,
printed paper 32)
pulping. The agitator 131 and its motor 132 are controlled by the Adjustable
Factors control
panel 133. The coarse filter 134 allows the re-pulpled material to fall
through, where is drawn off
by outlet pipe 135 and suction pump 136. The fine screen 137 allows the
solution to be drawn off
through solvent outlet pipe 138 and solvent suction pump 139, by which it is
recycled to solvent
storage tank 140.. The dipolar aprotic protophylic solvent inlet pipe 141
supplies the solvent to
the solvent storage tank 140, and a water inlet pipe 142 allows for a dilution
of the solution. The
repulpable material is supplied via inlet chute 143. Heat can be supplied by
steam boiler 144,
taking into account its effect on the concentration of the solution.
The optimization process involves fibre samples subjected to an infrared
spectrometer 70,
preparation in accordance with the selected tests as noted above in test tubes
71, 72, 73 for
analysis under a microscope 74, preparation of test sheets 75, 76, 77 for the
handsheet tests noted
above, and feedback 145 to the Adjustable Factors control panel 133. The knobs
81 through 85
control respectively the temperature, solvent concentration, type of dipolar
solvent, solid/liquid
ratio, and the degree / duration of the mechanical agitation. The re-pulped
material is dumped
into hopper 146 for transport to washing facilities, using a conical
centrifuge washer 147, for
28
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
example.
Referring to Figure 7 the extractive distillation subprocess is shown. The
effluent 700, which
consists of water, fibrous material, additives, resinous material and DMSO,
will be pumped in
the extraction tower 701, where petroleum ether 702 from the tank 703 will be
added through the
pump 704. Then vigorous mixing of the effluent 700 with petroleum ether 702
will be
conducted. After a proper mixing time, two distinct liquid layers are formed
in the extraction
tower 701. The upper layer 712 is composed of petroleum ether, resinous
material and DMSO,
whilst the lower one is the sludge 710, which contains mainly fibrous
material, additives and
some other organic substances. The sludge 710 is selectively separated from
the upper layer
through a sensor system and pumped out of the extraction tower 701 for further
treatment. The
upper layer 712 will be forwarded through the pump 711 to the distillation
tower 713. In the
tower 713 the distillation of petroleum ether takes place at low temperatures
(35-60 C) and then
the ether is pumped through the pump 705 into the tank 706 and after it is
sent out to the
extraction tower 701 through the pump 709 for reuse, whilst the mixture 708,
of resinous
material and DMSO (boiling point 189 C) remains at the bottom of the
distillation tower (G),
will be sent out to the 3rd and final stage - solid/liquid separation unit
where DMSO will be
separated as a pure solvent for reuse.
Distillation is recommended in this project as a second step, i.e., following
liquid extraction
process. In this invention, distillation is applied for the separation of
petroleum ether from
DMSO + Sludge (mainly resinous material).
Distillation is defined as a process in which a liquid or vapor mixture of two
or more substances
is separated into its component fractions of desired purity by the application
and removal of heat.
Distillation is based on the fact that the vapor of a boiling mixture will be
richer than the
components that have lower boiling points. Therefore, when this vapor is
cooled and condensed
the condensate will contain more volatile components. Distillation columns are
designed to
achieve this separation efficiently. Petroleum ether recovered through the
distillation process will
be reused in DMSO extraction from the spent repulping liquor.
29
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
Solid/liquid separation is the 3rd and final stage in the solvent separation.
It is recommended for
the separation of DMSO from the sludge which is mainly resinous material.
Solid-liquid
separation is a major unit operation that exists in almost every flow scheme
related to the
chemical process industries. Solid/liquid separation has a wide application in
pharmaceutical,
mining, sugar, pulp and paper, waste water treatment, mining, ceramics, food
and other
industries.
Referring to Figure 8, liquid extraction is employed for the separation of
DMSO from the spent
repulping liquor (process water + DMSO). In the extraction process the heavy
liquid solution
mixture (H20 and DMSO) to be separated is passed through inlet pipe 801 and
contacted,
through mixing, with the extracting solvent (petroleum ether) which enters
through the light
liquid inlet 803.. The DMSO is extracted through the light liquid outlet 802
in a solution of
DMSO and petroleum ether. The raffinate or portion of the original liquid that
remains
(primarily water) exits through the heavy liquid outlet 804. Note that
petroleum ether has been
chosen as the preferred extracting solvent due to the following
characteristics.
i. Selectivity - petroleum ether is quite selective in removing DMSO from the
water.
ii. Solvent recoverability - easily recovered (bp 35-60 degrees C) from DMSO
(bp 189 degrees
C).
iii. Density differences - petroleum ether has the density of 0.64 g/ml, while
DMSO has the
density of 1.1004.
iv. Interfacial tension of petroleum is large.
v. Non-reactivity - petroleum is non-reactive with DMSO.
The recovered water from liquid extraction will be sent to the repulping
section for further reuse.
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
Applications include, thickening, clarification, cake and deep-bed filtration,
centrifugation,
sedimentation, and hydrocycloning, Their mechanisms are the relative
solid/liquid motions such
as flow-through porous media and sedimentation. Fundamental aspects of
solid/liquid
separations are the properties of suspensions (e.g., particle size and shape,
particle-particle
interactions, particle surface characteristics, and concentration) of
sediments and cake (porosity,
permeability, compressibility, viscosity and yield stress). The recovered DMSO
will be reused
in the preparation of fresh repulping liquors.
In general, liquor-displacement technology applies to recycled paper pulping
and provides the
context for the above process. At the end of the pulping a preset volume of
cooler wash filtrate is
pumped into the pulper, where it displaces warm black liquor, which is
transferred to an
accumulator. From the accumulator the black liquor is pumped to
extraction/distillation towers
for DMSO recovery for further use. Then the pulping slurry will be subjected
to coarse screening
to remove contaminants that are non-fibrous material. This is followed by
addition of de-inking
chemical and then the pulp undergoes mechanical disintegration where the small
ink particles
will be detached. The filtrate of the disintegrated pulp will be pumped to a
designated ion-
exchange unit to separate de-inking chemical (e.g., fatty oil derivatives) for
further use. The
resultant disintegrated pulp will be subjected to fine cleaning and screening
at fine screen in
order to remove fine ink particles, minute contaminants, and some of the fine
pulp fibres. At the
end process tub, washing and thickening will be applied to completely remove
fine ink particles
and fine pulp fibres.
Application of aqueous dipolar solvents in pulping of recycled paper pulping
aims, to a greater
extent, to limit the chances of fibre loss to the minimum. In other words, the
primary goal of the
proposed project is focused on the attacking the root cause of fibre loss,
i.e., which is the fibre
stiffness. The use of aqueous dipolar solvents in waste paper pulping will
enable a uniform
defibration in both amorphous and crystalline zones of the substrate. Thus,
dipolar solvent
technique is capable of offering a high quality pulp that may approach the
quality of virgin pulp.
With this method and system it is realistic to expect less than 10% fibre loss
during cleaning and
washing operations, with a net pulp yield of 90%.
31
CA 02721460 2010-06-14
WO 2009/073948 PCT/CA2007/002289
In the system and method of this invention, the breakdown of hydrogen bonding
of the cellulose
substrate (recycled paper) by the interaction of dipolar solvent (e.g., DMSO,
DMF, DMA) in the
presence of a major fraction of water enables a minimal removal of
hemicellulose (eg., surface
adsorbed carbohydrate) compared to standard pre-existing repulping techniques.
The advantages
are immediate and allow for optimization as explained above. The higher
process efficiency is
expected to bring about significant impact on the economic feasibility and
competitiveness of
manufacturing of pulp from recycled paper, producing high pulp yield and
competitive fibre
quality at less cost and involving fewer technological operations than
conventional methods of
repulping.
The within-described invention may be embodied in other specific forms,
systems and methods
and with additional options and accessories without departing from the spirit
or essential
characteristics thereof. The presently disclosed embodiment is therefore to be
considered in all
respects as illustrative and not restrictive, the scope of the invention being
indicated by the
appended claims rather than by the foregoing description, and all changes
which come within the
meaning and range of equivalence of the claims are therefore intended to be
embraced therein.
32