Note: Descriptions are shown in the official language in which they were submitted.
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
UREA COMPOUNDS AS GAMMA SECRETASE MODULATORS
CROSS-REFERENCE
This application claims priority to US provisional application Nos.
61/126,480, filed
May 5, 2008 and 61/127,434, filed May 13, 2008, the disclosures of which are
incorporated
herein by reference in their entirety.
Field of the Invention
The present invention provides compounds that are gamma secretase modulators
and
are therefore useful for the treatment of diseases treatable by modulation of
gamma secretase
such as Alzheimer's disease. Also provided are pharmaceutical compositions
containing such
compounds and processes for preparing such compounds.
Background
Alzheimer's disease (AD) is the most common cause of dementia, resulting in
loss of
memory, cognition, reasoning, judgement, and orientation. AD is characterized
by the
presence of extracellular amyloid plaques, intracellular neurofibrillary
tangles, in addition to
loss of synapses and neurons in the brain. The main constituent of amyloid
plaques is f3-
amyloid (A(3) a 4 kDa peptide.
Accumulation of A(3 is thought to be an early and critical step in the
pathogenesis of
Alzheimer's Disease (AD). A(3 elicits a cascade of toxic and inflammatory
events that
ultimately lead to neuronal death and cognitive impairment. The A(3 peptide
results from
proteolysis of Amyloid Precursor Protein (APP). The APP protein is a
transmembrane protein
consisting of a large extracellular domain and a short cytoplasmic tail. A(3
sequence
encompasses parts of the extracellular and transmembrane domains of APP.
APP can be processed via either of two routes, a non-amyloidogenic and an
amyloidogenic pathway. Most of the APP is processed through the non-
amyloidogenic
pathway, whereby the protease a-secretase cleaves APP within the A(3 domain to
release a
large soluble N-terminal fragment (sAPPa) and a non-amyloidogenic C-terminal
fragment
(C83). This fragment is further processed by y-secretase to produce a 22-24
residue peptide
(p3).
1
SUBSTITUTE SHEET (RULE 26)
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
In the amyloidogenic pathway, APP is cleaved by (3-secretase (BACEI),
generating a
shorter N-terminal domain (sAPP(3) and an amyloidogenic C-terminal (C99). This
C99
fragment is subsequently cleaved by y-secretase. The y-secretase is a protease
formed by a
complex of proteins: Presenilin-1 (PS-1), Nicastrin, PEN-2, and APH-1.
Proteolysis of APP
intermediates by y-secretase yields A(3 peptides of varying length (A(337,
A(338, A(339, A(340,
A(342). Of these peptides, A(342 is the least soluble, most aggregating
species and the principal
component of toxic oligomers and amyloid plaques in AD brain. All known
mutations causing
early onset Familial AD either increase total A(3 formation or increase the
ratio of A1342 to
A340. Therefore agents that can block the formation of A(342 should be useful
for the
treatment of AD.
One proposed treatment involves modulation of y-secretase activity to
selectively
reduce the production of A1342 while increase the production of the shorter
chain isoforms
(such as A(337, 38, and 39). These isoforms are believed to be less prones to
self-aggregate and
are more easily cleared from the brain and or less toxic. Several classes of
compounds are
proposed as y-secretase modulators (refered hereon as GSM), see, Imbimbo BY,
et al., J
Pharmacol Exp Ther. 2007 Dec; 323(3):822-30.; W02007054739; W02006043064; and
W02007124351.
The present invention provides a new class of compounds that selectively
reduce the
production of A1342 peptide by modulation of y-secretase and hence are useful
in the treatment
of Alzheimer's disease.
SUMMARY
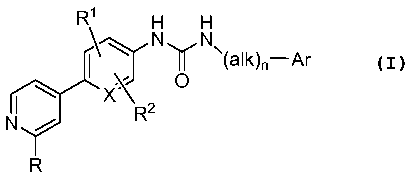
In one aspect, provided herein are compounds of Formula (I):
R1 H H
N N
O \(alk)õ-Ar
xX
N R 2
R
where:
X is -CH- or -N-;
nis0or1;
alk is a straight or branched alkyl of I to 6 carbon atoms where one, two, or
three
hydrogen atoms of the alkyl chain are replaced by alkoxy, hydroxyl or halo;
R is alkyl;
2
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
RI and R2 are independently hydrogen, alkyl, alkoxy, hydroxy, or halo;
Ar is:
(i) aryl, heteroaryl, cycloalkyl, fused cycloalkyl, or heterocyclyl where each
of the
aforementioned ring is optionally substituted with Ra, Rb or Re where Ra is
alkyl, halo,
haloalkyl, haloalkoxy, alkylthio, cyano, alkoxy, amino, monosubstituted amino,
disubstituted
amino, sulfonyl, acyl, carboxy, alkoxycarbonyl, hydroxyalkyl, alkoxyalkyl,
aminoalkyl,
hydroxyalkoxy, alkoxyalkoxy, aminoalkoxy, aminosulfonyl, aminocarbonyl, or
acylamino and
Rb and Re are independently selected from alkyl, halo, haloalkyl, haloalkoxy,
alkylthio, cyano,
alkoxy, amino, monosubstituted amino, disubstituted amino, sulfonyl, acyl,
carboxy,
alkoxycarbonyl, hydroxyalkyl, alkoxyalkyl, aminoalkyl, hydroxyalkoxy,
alkoxyalkoxy,
aminoalkoxy, aminosulfonyl, aminocarbonyl, acylamino, aryl, heteroaryl,
cycloalkyl,
heterocyclyl, aralkyl, heteroaralkyl, aryloxy, heteroaryloxy, or cycloalkoxy,
or when Rb and
Re are on adjacent atoms they can combine to form methylenedioxy or
ethylenedioxy; where
the aromatic or alicyclic ring in Ra, Rb and Re is optionally substituted with
Rd, Re or Rf which
are independently selected from alkyl, halo, haloalkyl, haloalkoxy, alkylthio,
cyano, alkoxy,
amino, monosubstituted amino, disubstituted amino, sulfonyl, acyl, carboxy,
alkoxycarbonyl,
hydroxyalkyl, alkoxyalkyl, aminoalkyl, hydroxyalkoxy, alkoxyalkoxy,
aminoalkoxy,
aminosulfonyl, aminocarbonyl, or acylamino; or
(ii) a group of formula (a):
Art
(a)
ring A is cycloalkyl optionally substituted with halo, hydroxyl, alkoxy, oxo,
or
haloalkyl; or monocyclic heterocyclyl wherein if the heterocyclyl ring
contains a nitrogen ring
atom, the nitrogen atom is optionally substituted with alkyl, or acyl,
acyloxycarbonyl;
Ari is aryl, heteroaryl, cycloalkyl, fused cycloalkyl, or heterocyclyl where
each of the
aforementioned ring is optionally substituted with Ra, Rb or Re where Ra is
alkyl, halo,
haloalkyl, haloalkoxy, alkylthio, cyano, alkoxy, amino, monosubstituted amino,
disubstituted
amino, sulfonyl, acyl, carboxy, alkoxycarbonyl, hydroxyalkyl, alkoxyalkyl,
aminoalkyl,
hydroxyalkoxy, alkoxyalkoxy, aminoalkoxy, aminosulfonyl, aminocarbonyl, or
acylamino and
Rb and Re are independently selected from alkyl, halo, haloalkyl, haloalkoxy,
alkylthio, cyano,
alkoxy, amino, monosubstituted amino, disubstituted amino, sulfonyl, acyl,
carboxy,
alkoxycarbonyl, hydroxyalkyl, alkoxyalkyl, aminoalkyl, hydroxyalkoxy,
alkoxyalkoxy,
aminoalkoxy, aminosulfonyl, aminocarbonyl, acylamino, aryl, heteroaryl,
cycloalkyl,
3
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
heterocyclyl, aralkyl, heteroaralkyl, aryloxy, heteroaryloxy, or cycloalkoxy,
or when Rb and
Re are on adjacent atoms they can combine to form methylenedioxy or
ethylenedioxy; where
the aromatic or alicyclic ring in R, Rb and Re is optionally substituted with
Rd, Re or Rf which
are independently selected from alkyl, halo, haloalkyl, haloalkoxy, alkylthio,
cyano, alkoxy,
amino, monosubstituted amino, disubstituted amino, sulfonyl, acyl, carboxy,
alkoxycarbonyl,
hydroxyalkyl, alkoxyalkyl, aminoalkyl, hydroxyalkoxy, alkoxyalkoxy,
aminoalkoxy,
aminosulfonyl, aminocarbonyl, or acylamino; or
a pharmaceutically acceptable salt thereof provided the compound of Formula
(I) is not:
N~N N Y N
/ 0
NH 0 N / N
or
In a second aspect, provided is a pharmaceutical composition comprising a
compound
of Formula (I), a pharmaceutically acceptable salt thereof or a mixture of a
compound of
Formula (I) and a pharmaceutically acceptable salt thereof; and a
pharmaceutically acceptable
excipient.
In a third aspect, this invention is directed to a method of treating
Alzheimer's disease
by inhibition of y-secretase in a patient which method comprises administering
to the patient a
pharmaceutical composition comprising a compound of Formula (I) a
pharmaceutically
acceptable salt thereof, or a mixture of a compound of Formula (I) and a
pharmaceutically
acceptable salt thereof and a pharmaceutically acceptable excipient.
In a fourth aspect, this invention is directed to an intermediate of formula:
R1
R
2
N / R
R
where:
X, R, R1 and R2 are as defined for Formula (I) above; and
R' is NH2, NHPG, -CON3, or -N=C=O where PG is a suitable amino protecting
group;
or a salt thereof.
In a fourth aspect, this invention is directed to use of a compound of Formula
(I) or a
salt thereof as a medicament.
4
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
In a fifth aspect, this invention is directed to use of a compound of Formula
(I) or a salt
thereof in the preparation of a medicament for use in the treatment of
Alzheimer's disease.
Detailed Description
Definitions:
Unless otherwise stated, the following terms used in the specification and
claims are
defined for the purposes of this Application and have the following meaning:
"Alkyl" means a linear saturated monovalent hydrocarbon radical of one to six
carbon
atoms or a branched saturated monovalent hydrocarbon radical of three to six
carbon atoms,
e.g., methyl, ethyl, propyl, 2-propyl, butyl (including all isomeric forms),
pentyl (including all
isomeric forms), and the like.
"Alicyclic" means a non-aromatic ring e.g., cycloalkyl or heterocyclyl ring.
"Alkylene" means a linear saturated divalent hydrocarbon radical of one to six
carbon
atoms or a branched saturated divalent hydrocarbon radical of three to six
carbon atoms unless
otherwise stated e.g., methylene, ethylene, propylene, 1-methylpropylene, 2-
methylpropylene,
butylene, pentylene, and the like.
"Alkylthio" means a -SR radical where R is alkyl as defined above, e.g.,
methylthio,
ethylthio, and the like.
"Alkylsulfonyl" means a -SO2R radical where R is alkyl as defined above, e.g.,
methylsulfonyl, ethylsulfonyl, and the like.
"Amino" means a -NH2.
"Alkylamino" means a -NHR radical where R is alkyl as defined above, e.g.,
methylamino, ethylamino, propylamino, or 2-propylamino, and the like.
"Alkoxy" means a -OR radical where R is alkyl as defined above, e.g., methoxy,
ethoxy, propoxy, or 2-propoxy, n-, iso-, or tert-butoxy, and the like.
"Alkoxycarbonyl" means a -C(O)OR radical where R is alkyl as defined above,
e.g.,
methoxycarbonyl, ethoxycarbonyl, and the like.
"Alkoxyalkyl" means a linear monovalent hydrocarbon radical of one to six
carbon
atoms or a branched monovalent hydrocarbon radical of three to six carbons
substituted with at
least one alkoxy group, preferably one or two alkoxy groups, as defined above,
e.g., 2-
methoxyethyl, 1-, 2-, or 3-methoxypropyl, 2-ethoxyethyl, and the like.
"Alkoxyalkyloxy" or "alkoxyalkoxy" means a -OR radical where R is alkoxyalkyl
as
defined above, e.g., methoxyethoxy, 2-ethoxyethoxy, and the like.
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
"Aminoalkyl" means a linear monovalent hydrocarbon radical of one to six
carbon
atoms or a branched monovalent hydrocarbon radical of three to six carbons
substituted with at
least one, preferably one or two, -NRR' where R is hydrogen, alkyl, or -CORa
where Ra is
alkyl, each as defined above, and R' is selected from hydrogen, alkyl,
hydroxyalkyl,
alkoxyalkyl, aryl, aralkyl, heteroaryl, heteroaralkyl, or haloalkyl, each as
defined herein, e.g.,
aminomethyl, methylaminoethyl, 2-ethylamino-2-methylethyl, 1,3-diaminopropyl,
dimethylaminomethyl, diethylaminoethyl, acetylaminopropyl, and the like.
"Aminoalkoxy" means a -OR radical where R is aminoalkyl as defined above,
e.g., 2-
aminoethoxy, 2-dimethylaminopropoxy, and the like.
"Aminocarbonyl" means a -CONRR' radical where R is independently hydrogen,
alkyl, hydroxyalkyl, alkoxyalkyl, or aminoalkyl , each as defined herein and
R' is hydrogen,
alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heteroaryl, heteroaralkyl,
heterocyclyl,
heterocyclylalkyl, hydroxyalkyl, alkoxyalkyl, or aminoalkyl, each as defined
herein, e.g., -
CONH2, methylaminocarbonyl, 2-dimethylaminocarbonyl, and the like.
"Aminosulfonyl" means a -SO2NRR' radical where R is independently hydrogen,
alkyl, hydroxyalkyl, alkoxyalkyl, or aminoalkyl, each as defined herein and R'
is hydrogen,
alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heteroaryl, heteroaralkyl,
heterocyclyl,
heterocyclylalkyl, hydroxyalkyl, alkoxyalkyl, or aminoalkyl, each as defined
herein, e.g., -
SO2NH2, methylaminosulfonyl, 2-dimethylaminosulfonyl, and the like.
"Acyl" means a -COR radical where R is alkyl, haloalkyl, cycloalkyl,
cycloalkylalkyl,
aryl, aralkyl, heteroaryl, heteroaralkyl, heterocyclyl, or heterocyclylalkyl,
each as defined
herein, e.g., acetyl, propionyl, benzoyl, pyridinylcarbonyl, and the like.
When R is alkyl, the
radical is also referred to herein as alkylcarbonyl.
"Acylamino" means a -NHCOR radical where R is alkyl, haloalkyl, cycloalkyl,
cycloalkylalkyl, aryl, aralkyl, heteroaryl, heteroaralkyl, heterocyclyl, or
heterocyclylalkyl,
each as defined herein, e.g., acetylamino, propionylamino, and the like.
"Aryl" means a monovalent monocyclic or bicyclic aromatic hydrocarbon radical
of 6
to 10 ring atoms e.g., phenyl or naphthyl.
"Aralkyl" means a -(alkylene)-R radical where R is aryl as defined above.
"Aryloxy" means a -OR radical where R is aryl as defined above, e.g., phenoxy,
naphthyloxy.
"Cycloalkyl" means a cyclic saturated monovalent hydrocarbon radical of three
to ten
carbon atoms, e.g., cyclopropyl, cyclobutyl, cyclopentyl, or cyclohexyl, and
the like.
6
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
Cycloalkylalkyl" means a -(alkylene)-R radical where R is cycloalkyl as
defined
above; e.g., cyclopropylmethyl, cyclobutylmethyl, cyclopentylethyl, or
cyclohexylmethyl, and
the like.
"Cycloalkoxy" means a -OR radical where R is cycloalkyl as defined above,
e.g.,
cyclopropoxy, cyclobutoxy, and the like.
"Carboxy" means -COOH.
"Disubstituted amino" means a -NRR' radical where R and R' are independently
alkyl, cycloalkyl, cycloalkylalkyl, acyl, sulfonyl, aryl, aralkyl, heteroaryl,
heteroaralkyl,
heterocyclyl, heterocyclylalkyl, hydroxyalkyl, alkoxyalkyl, or aminoalkyl,
each as defined
herein, e.g., dimethylamino, phenylmethylamino, and the like.
"Fused cycloalkyl" means cycloalkyl ring as defined above that is fused to one
or two
aryl or heteroaryl ring as defined herein e.g., tetrahydronaphthyl, 5,6,7,8-
tetrahydroquinolinyl,
and the like.
"Halo" means fluoro, chloro, bromo, or iodo, preferably fluoro or chloro.
"Haloalkyl" means alkyl radical as defined above, which is substituted with
one or
more halogen atoms, preferably one to five halogen atoms, preferably fluorine
or chlorine,
including those substituted with different halogens, e.g., -CH2C1, -CF3, -
CHF2, -CH2CF3, -
CF2CF3, -CF(CH3)3, and the like. When the alkyl is substituted with only
fluoro, it is referred
to in this Application as fluoroalkyl.
"Haloalkoxy" means a -OR radical where R is haloalkyl as defined above e.g., -
OCF3, -
OCHF2, and the like. When R is haloalkyl where the alkyl is substituted with
only fluoro, it is
referred to in this Application as fluoroalkoxy.
"Hydroxyalkyl" means a linear monovalent hydrocarbon radical of one to six
carbon
atoms or a branched monovalent hydrocarbon radical of three to six carbons
substituted with
one or two hydroxy groups, provided that if two hydroxy groups are present
they are not both
on the same carbon atom. Representative examples include, but are not limited
to,
hydroxymethyl, 2-hydroxyethyl, 2-hydroxypropyl, 3-hydroxypropyl, 1-
(hydroxymethyl)-2-
methylpropyl, 2-hydroxybutyl, 3-hydroxybutyl, 4-hydroxybutyl, 2,3-
dihydroxypropyl, 1-
(hydroxymethyl)-2-hydroxyethyl, 2,3-dihydroxybutyl, 3,4-dihydroxybutyl and 2-
(hydroxymethyl)-3-hydroxypropyl, preferably 2-hydroxyethyl, 2,3-
dihydroxypropyl, and 1-
(hydroxymethyl)-2-hydroxyethyl.
"Hydroxyalkoxy" or "hydroxyalkyloxy" means a -OR radical where R is
hydroxyalkyl
as defined above.
7
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
"Heterocyclyl" means a saturated or unsaturated monovalent monocyclic group of
5
to 8 ring atoms in which one or two ring atoms are heteroatom selected from N,
0, or S(O),
where n is an integer from 0 to 2, the remaining ring atoms being C. The
heterocyclyl ring is
optionally fused to a (one) aryl or heteroaryl ring as defined herein provided
the aryl and
heteroaryl rings are monocyclic. The heterocyclyl ring fused to monocyclic
aryl or heteroaryl
ring is also referred to in this Application as "bicyclic heterocyclyl" ring
and when it is not
fused to aryl or heteroaryl, it is referred as "monocyclic heterocyclyl".
Additionally, one or
two ring carbon atoms in the heterocyclyl ring can optionally be replaced by a
-CO- group.
More specifically the term heterocyclyl includes, but is not limited to,
pyrrolidino,
piperidino, homopiperidino, 2-oxopyrrolidinyl, 2-oxopiperidinyl, morpholino,
piperazino,
tetrahydropyranyl, thiomorpholino, and the like. When the heterocyclyl ring is
unsaturated it
can contain one or two ring double bonds provided that the ring is not
aromatic. When the
heterocyclyl group contains at least one nitrogen atom, it is also referred to
herein as
heterocycloamino and is a subset of the heterocyclyl group. When the
heterocyclyl group is
a saturated ring and is not fused to aryl or heteroaryl ring as stated above,
it is also referred to
herein as saturated monocyclic heterocyclyl.
Heterocyclylalkyl" means a -(alkylene)-R radical where R is heterocyclyl ring
as
defined above e.g., tetraydrofuranylmethyl, piperazinylmethyl,
morpholinylethyl, and the
like.
"Heteroaryl" means a monovalent monocyclic or bicyclic aromatic radical of 5
to 10
ring atoms where one or more, preferably one, two, or three, ring atoms are
heteroatom
selected from N, 0, or S, the remaining ring atoms being carbon.
Representative examples
include, but are not limited to, pyrrolyl, thienyl, thiazolyl, imidazolyl,
furanyl, indolyl,
isoindolyl, oxazolyl, isoxazolyl, benzothiazolyl, benzoxazolyl, quinolinyl,
isoquinolinyl,
pyridinyl, pyrimidinyl, pyrazinyl, pyridazinyl, triazolyl, tetrazolyl, and the
like.
"Heteroaralkyl" means a -(alkylene)-R radical where R is heteroaryl as defined
above.
"Heteraryloxy" means a -OR radical where R is heteroaryl as defined above,
e.g.,
pyridinyloxy, thiophenyloxy, and the like.
"Monosubstituted amino" means a -NHR radical where R is alkyl, cycloalkyl,
cycloalkylalkyl, acyl, sulfonyl, aryl, aralkyl, heteroaryl, heteroaralkyl,
heterocyclyl,
heterocyclylalkyl, hydroxyalkyl, alkoxyalkyl, or aminoalkyl, each as defined
herein, e.g.,
methylamino, 2-phenylamino, hydroxyethylamino, and the like.
"Modulation of y-secretase activity" as used herein means the production of
A1342
produced by y-secretase is reduced in the presence of the compounds of the
Invention.
8
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
The present invention also includes the prodrugs of compounds of Formula (I).
The
term prodrug is intended to represent covalently bonded carriers, which are
capable of
releasing the active ingredient of Formula (I) when the prodrug is
administered to a
mammalian subject. Release of the active ingredient occurs in vivo. Prodrugs
can be prepared
by techniques known to one skilled in the art. These techniques generally
modify appropriate
functional groups in a given compound. These modified functional groups
however regenerate
original functional groups in vivo or by routine manipulation. Prodrugs of
compounds of
Formula (I) include compounds wherein a hydroxy, amino, carboxylic, or a
similar group is
modified. Examples of prodrugs include, but are not limited to esters (e.g.,
acetate, formate,
and benzoate derivatives), carbamates (e.g., N,N-dimethylaminocarbonyl) of
hydroxy or amino
functional groups in compounds of Formula (I)), amides (e.g.,
trifluoroacetylamino,
acetylamino, and the like), and the like. Prodrugs of compounds of Formula (I)
are also within
the scope of this invention.
The present invention also includes protected derivatives of compounds of
Formula (I).
For example, when compounds of Formula (I) contain groups such as hydroxy,
carboxy, thiol
or any group containing a nitrogen atom(s), these groups can be protected with
a suitable
protecting groups. A comprehensive list of suitable protective groups can be
found in T.W.
Greene, Protective Groups in Organic Synthesis, John Wiley & Sons, Inc.
(1999), the
disclosure of which is incorporated herein by reference in its entirety. The
protected derivatives
of compounds of Formula (I) can be prepared by methods well known in the art.
A "pharmaceutically acceptable salt" of a compound means a salt that is
pharmaceutically acceptable and that possesses the desired pharmacological
activity of the
parent compound. Such salts include:
acid addition salts, formed with inorganic acids such as hydrochloric acid,
hydrobromic
acid, sulfuric acid, nitric acid, phosphoric acid, and the like; or formed
with organic acids such
as formic acid, acetic acid, propionic acid, hexanoic acid,
cyclopentanepropionic acid, glycolic
acid, pyruvic acid, lactic acid, malonic acid, succinic acid, malic acid,
maleic acid, fumaric
acid, tartaric acid, citric acid, benzoic acid, 3-(4-hydroxybenzoyl)benzoic
acid, cinnamic acid,
mandelic acid, methanesulfonic acid, ethanesulfonic acid, 1,2-ethanedisulfonic
acid, 2-
hydroxyethanesulfonic acid, benzenesulfonic acid, 4-chlorobenzenesulfonic
acid, 2-
naphthalenesulfonic acid, 4-toluenesulfonic acid, camphorsulfonic acid,
glucoheptonic acid,
4,4'-methylenebis-(3-hydroxy-2-ene-l-carboxylic acid), 3-phenylpropionic acid,
trimethylacetic acid, tertiary butylacetic acid, lauryl sulfuric acid,
gluconic acid, glutamic acid,
hydroxynaphthoic acid, salicylic acid, stearic acid, muconic acid, and the
like; or
9
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
salts formed when an acidic proton present in the parent compound either is
replaced by
a metal ion, e.g., an alkali metal ion, an alkaline earth ion, or an aluminum
ion; or coordinates
with an organic base such as ethanolamine, diethanolamine, triethanolamine,
tromethamine, N-
methylglucamine, and the like. It is understood that the pharmaceutically
acceptable salts are
non-toxic. Additional information on suitable pharmaceutically acceptable
salts can be found
in Remington's Pharmaceutical Sciences, 17th ed., Mack Publishing Company,
Easton, PA,
1985, which is incorporated herein by reference.
The compounds of the present invention may have asymmetric centers. Compounds
of the present invention containing an asymmetrically substituted atom may be
isolated in
optically active or racemic forms. It is well known in the art how to prepare
optically active
forms, such as by resolution of materials. All chiral, diastereomeric, racemic
forms are within
the scope of this invention, unless the specific stereochemistry or isomeric
form is
specifically indicated.
Certain compounds of Formula (I) can exist as tautomers and/or geometric
isomers.
All possible tautomers and cis and trans isomers, as individual forms and
mixtures thereof
are within the scope of this invention. Additionally, as used herein the term
alkyl includes all
the possible isomeric forms of said alkyl group albeit only a few examples are
set forth.
Furthermore, when the cyclic groups such as aryl, heteroaryl, heterocyclyl are
substituted,
they include all the positional isomers albeit only a few examples are set
forth. Furthermore,
all polymorphic forms and hydrates of a compound of Formula (I) are within the
scope of
this invention.
"Oxo"or "carbonyl" means =(O) group.
"Optional" or "optionally" means that the subsequently described event or
circumstance may but need not occur, and that the description includes
instances where the
event or circumstance occurs and instances in which it does not. For example,
"heterocyclyl
group optionally substituted with an alkyl group" means that the alkyl may but
need not be
present, and the description includes situations where the heterocyclyl group
is substituted
with an alkyl group and situations where the heterocyclyl group is not
substituted with alkyl.
A "pharmaceutically acceptable carrier or excipient" means a carrier or an
excipient
that is useful in preparing a pharmaceutical composition that is generally
safe, non-toxic and
neither biologically nor otherwise undesirable, and includes a carrier or an
excipient that is
acceptable for veterinary use as well as human pharmaceutical use. "A
pharmaceutically
acceptable carrier/excipient" as used in the specification and claims includes
both one and
more than one such excipient.
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
"Sulfonyl" means a -SO2R radical where R is alkyl, haloalkyl, aryl, aralkyl,
heteroaryl, heteroaralkyl, heterocyclyl, or heterocyclylalkyl, each as defined
herein, e.g.,
methylsulfonyl, phenylsulfonyl, benzylsulfonyl, pyridinylsulfonyl, and the
like.
The phrase in the definition of group Ar in the claims and in the
specification of this
Application "....wherein each of the aforementioned ring is optionally
substituted with Ra, Rb,
or R independently selected from......." and similar phrases used for others
groups in the
claims and in the specification with respect to the compound of Formula (I)
means that the
rings can be mono-, di-, or trisubstituted unless indicated otherwise.
"Treating" or "treatment" of a disease includes:
preventing the disease, i.e. causing the clinical symptoms of the disease not
to develop
in a mammal that may be exposed to or predisposed to the disease but does not
yet experience
or display symptoms of the disease;
inhibiting the disease, i.e., arresting or reducing the development of the
disease or its
clinical symptoms; or
relieving the disease, i.e., causing regression of the disease or its clinical
symptoms.
A "therapeutically effective amount" means the amount of a compound of Formula
(I)
that, when administered to a mammal for treating a disease, is sufficient to
effect such
treatment for the disease. The "therapeutically effective amount" will vary
depending on the
compound, the disease and its severity and the age, weight, etc., of the
mammal to be treated.
Representative compounds of the Invention are shown in Tables 1 and 2 below:
Compounds of Formula (I) where Ar is n, alk, and Ar are shown below are.
H H
N\ Y (alk)"-Ar
/ O
I
N /
Table 1
C pd # Stereo n Alk Ar
1 (R) 1 -CH CH3 - na hth-1 l
2 - 1 -(CH2)2- 2,7-dimeth lindol-3 l
3 - 0 0 1,2,3,4-tetrah drona hth-1 l
4 - 1 -(CH2)- na hth-1 l
- 1 -(CH2)2- 5-chloro-2-meth lindol-3 l
6 (R) 0 0 2-(pyridin-2-yl)-1,2,3,4-
tetrah drona hth-1 l
7 - 1 -(CH2)2- indol-3-yl
11
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
C pd # Stereo n Alk Ar
8 - 1 -CHz z- 5-bromo-2-meth lindol-3 l
9 - 1 -C CH3 2 phenyl
- 0 0 6-chloro-2,2-dimeth lbenzo ran-4 1
11 - 1 CHz z- 2-meth l-7 2 ro 1 indol-3 l
12 (R) 1 -CH CH3 - 2-chloro hen l
13 - 1 -(CH2)2- 1,2-dimethylindol-3-yl
14 - 0 0 6-bromo-2,2-dimeth lbenzo ran-4 1
12 - 1 -CHz- 3-trifluorometh 1 hen l
13 - 1 CHz z- 4-fluoro hen l
14 - 0 0 (S)-6-ethyl-2-methyl-2-
methoxymethylbenzopyran-4-yl
S 1 -CH CH3 - 2-chloro hen l
16 S 0 0 1,2,3,4-tetrah drona h-1 l
17 - 0 0 9H-fluoroen-9-yl
18 - 1 CHz z- 2-meth lindol-3 l
19 - 1 -CHz z- 2,4-dichloro hen l
- 0 0 6,8-dichloro-2,2-dimethylbenzopyran-
4 1
21 - 1 -CHz- 2-chloro-6-trifluorometh 1 hen l
22 (R) 1 -CH CH3 phenyl
23 (R) 1 -CH CH3 - c clohex l
24 - 0 0 na hth-1 l
- 0 0 (S)-2-tert-butyl-7,7-dimethyl-5,6,7,8-
tetrah dro uinazolin-5 l
26 - 1 -C CH3 2- 4-fluoro hen l
27 - 1 -CH2CH(CH3)- phenyl
28 - 1 -CHz- 3-fluoro-6-trifluorometh 1 hen l
29 - 1 CHz 3 phenyl
- 1 -(CH2)3- 2-fluorophenyl
31 - 1 -CHz- 2-difluoromethox hen l
32 - 0 0 3,5-ditrifluorometh 1 hen l
33 - 0 0 2,3-dih dro-lH-inden-1 l
34 - 1 -CHz- bi hen-3 l
- 1 CHz z- 3-methox hen l
36 - 1 -C CH3 2- 6-chloro ridin-2 l
37 - 1 -CHz z- 2 -pyridin-3 lindol-3 l
38 - 1 -CHz- 3 -meth 1 hen l
39 (R) 0 0 6-(hydroxymethyl)-1,2,3,4-
tetrah drona hth-1 l
(R) 0 0 1,2,3,4-tetrah drona hth-2 l
41 - 0 0 2 2 ridin-2 leth 1 hen l
42 S 0 0 6-ethyl-3,4-dih dro-2H-chromen-4 1
43 - 1 -CH(CH3)- 2,3-difluoropyridin-4-yl
44 - 0 0 2,6-dichloropyridin-4-yl
- 0 0 2,3,4,5-tetrahydro-lH-benzo[b]azepin-
5-yl
46 - 1 -CHz- benzothio hen-3 l
47 - 0 0 4-[1,2,4]triazol-1 1 hen l
48 - 0 0 6-bromo ridin-3 l
12
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
C pd # Stereo n Alk Ar
49 - 0 0 2,3-dih drobenzo[b][1,4]dioxin-7 1
50 - 1 CHz z- thio hen-2 l
51 - 0 0 4 razol-1 1 hen l
52 - 0 0 4-oxazol-5 1 hen l
53 - 1 -CHz- 3-mo holin-4 1 hen l
54 (R) 1 -CHz- 6-(4-methylpyridin-l-
ylmethyl)1,2,3,4-tetrahydronaphth-l-
1
55 - 0 0 biphen-4-yl
56 - 1 -CHz- 1-h drox c clohex l
57 (R) 1 -CH CH3 - na hth-2 l
58 S 1 -CH CH3 - na hth-2 l
59 - 1 -CH CH3 CHz- 4-fluoro hen l
60 - 1 -CH CH3 - 2-fluoro hen l
61 - 1 -CHz- na hth-2 l
62 - 0 0 4(R)-3,3-dimethyl-3,4-dihydro-2H-
chromen-4 l
63 - 1 -CH CH3 - 3-fluoro hen l
64 - 1 CHz z- 4-bromo hen l
65 - 1 -CH CH3 - 2-methox hen l
66 - 1 -CH(n-c3H7)- phenyl
67 - 1 -CH CH3 - 4-bromo hen l
68 - 1 -CH CH3 - 3-methox hen l
69 - 0 0 1R,2S -2 hen lc clo ro l
70 (R) 1 -CH C2Hs phenyl
71 (S) 1 -CH(C2H5)- phenyl
72 - 1 CHz z- 4-chloro hen l
73 - 1 -CHz z- 4-c ano hen l
74 - 0 - 3-morpholin-4-ylphenyl
75 1 -CH CH3 - 4-meth 1 hen l
76 1 -CH CH3 - 4-chloro hen l
77 0 uinolin-5 l
78 1 -CH CH3 - 3-chloro hen l
79 1 -CH CH3 - 3-bromo hen l
80 1 -CH CH3 - 2-bromo hen l
81 1 -CHz uinolin-4 l
82 (R) 1 -CH CH3 uinolin-2 l
83 S 1 -CH CH3 uinolin-2 l
84 (R) 1 -CH CH3 uinolin-4 l
85 S 1 -CH CH3 uinolin-4 l
86 1 -CHz uinolin-2 l
87 (R) 1 -CH CH3 - 2-fluoro hen l
88 S 1 -CH CH3 - 2-fluoro hen l
89 0 - 5-bromo-2-fluoro hen l
90 1 -CH(CH3)- furan-2-yl
91 1 -CH CH3 - benzofuran-2-yl
92 1 -CH CH3 - 1-difluorometh lbenzimidazol-2 1
93 1 -CH CH3 - 1-eth 13,5-dimeth 1 razol-4 l
94 0 - tetrah dro ran-3 l
13
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
C pd # Stereo n Alk Ar
95 1 CH2 3-meth lisoxazol-5 l
96 0 - tetrah drofuran-3 l
97 1 -CH CF3 phenyl
98 1 -CH CH3 - 2-chloro hen l
99 1 -CH CH3 - 2-meth 1 hen l
100 1 -CH(CH3)- 5-fluoropyridin-2-yl
111 1 -CH CH3 - 5-chloro ridin-2 l
112 1 -CH CH3 - 6-methox ridin-3 l
113 1 -CH CH3 - 3-difluoromethox hen l
114 1 -CH(CH3)- isoquinolin-3-yl
115 1 -CH CH3 uinolin-7 l
116 1 -CH CH2F phenyl
117 1 -CH H20CH3)- phenyl
118 1 -CHICHF(CH3)12- phenyl
119 1 -CH CH3 - 2,4-dichloro hen l
120 1 -CH CH3 - 4-methox hen l
121 1 -CH(CH3)- 3-methyl-2-oxo-2,3,4,5-tetrahydro-
1H-benzo[d]aze in-1 l
122 0 - 2-bromo hen l
123 0 - 3'-fluorobi hen -3 l
124 0 - 4'-methox bihen-2 l
125 0 - 3-furan-2 l hen l
126 0 - 3-n-butox hen l
127 0 - 3'-methox bihen-3 l
128 0 - 2 ro lthio hen l
129 0 - 3-thio hen-3 l hen l
130 0 - 3-pyridin-4-ylphenyl
131 0 - 2-fluoro-5-c ano hen l
132 0 - 5-cyano-2-methylphenyl
133 0 - 2-thiophen-2-ylphenyl
134 0 - 3 ridin-3 1 hen l
135 0 - 5-chloro-2-fluoro hen l
136 0 - 3-ethox hen l
137 0 - 3-thio hen-2 l hen l
138 0 - 2-meth l-5-trifluorometh 1 hen l
139 0 - 3-n ro ox hen l
140 0 - 2-n ro ox hen l
141 0 - 3-tert-but 1 hen l
142 0 - 2 ridin-3 1 hen l
143 0 - 5-bromo-2-meth 1 hen l
144 0 - 2-n ro 1 hen l
145 0 - 5-chloro-2-meth 1 hen l
146 0 - 3-ethox hen l
147 0 - 2-fluoro-5-trifluorometh 1 hen l
148 0 - 2-eth 1 hen l
149 0 - 3-methylthiophenyl
150 0 - 2,5-difluoro hen l
151 0 - 4' -fluorobi hen-3 l
152 0 - 5-fluoro-2-methoxyphenyl
14
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
C pd # Stereo n Alk Ar
153 0 - 2-c ano hen l
154 0 - 3 2-meth lthiazol-4 1 hen l
155 0 - bi hen-2 l
156 0 - 2-c ano hen l
157 0 - 2-benzo 1 hen l
158 0 - 2-trifluoromethylphenyl
159 0 - 5-fluoro-2-meth 1 hen l
160 0 - 2-chloro-5-methox hen l
161 0 - 2-chloro-5-meth 1 hen l
162 0 - 2-phenoxyphenyl
163 0 - 3-c ano hen l
164 0 - 2-acet 1 hen l
165 0 - 2-methox bihen-5 l
166 0 - 2-c ano-5-meth 1 hen l
167 0 - 3-iso ro ox hen l
168 0 - 3-benzo 1 hen l
169 0 - 2-chloro-5-c ano hen l
170 0 - 4'-c anobi hen-3 l
171 0 - 2-fluoro-5-meth 1 hen l
172 0 - 3 henox hen l
173 0 - 2-methox -5-meth 1 hen l
174 0 - 2-chloro-5-trifluorometh 1 hen l
175 0 - 2,5-dichloro hen l
176 0 - 2-fluoro hen l
177 0 - 3-methox hen l
178 0 - 3-ethylphenyl
179 0 - 2-meth 1 hen l
180 0 - 2-meth lthio hen l
181 0 - 2-methoxy-5-trifluoromethylphenyl
182 0 - 5-chloro-2-c ano hen l
183 0 - 2-ethox hen l
184 0 - 2-iso ro 1 hen l
185 0 - 3 -tert-but1 hen l
186 0 - 2-isobut 1 hen l
187 0 - 2-methox hen l
188 0 - 2-trifluorometh 1 hen l
189 0 - 3 -meth 1 hen l
190 0 - 3-fluoro hen l
191 0 - 5-isopropyl-2-meth 1 hen l
192 0 - R-3 ,3-dimeth ltetrah dro ran-4 1
193 0 - c clohex l
194 0 - c clo ent l
195 0 phenyl
196 1 -CH_- furan-2-yl
197 0 - 2-methyl-5-trifluoromethylfuran-4-yl
198 0 - 5-methyl-l-phenylpyrazol-4-yl
199 0 - 5-methyl-3 hen lisoxazol-4 l
200 0 - 3-methyl-5-phenylisoxazol-4-yl
201 0 - 2,5-dimeth l-1 hen 1 razol-4 l
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
C pd # Stereo n Alk Ar
202 0 - 2,6-dichloro hen 1
203 0 - 3-chloro-4-methox hen 1
204 0 - 5-chloro-2-methox hen 1
205 0 - S -chloro-2,4-dimethox hen 1
206 0 - 3,5-difluoro hen 1
207 1 -CH[CH2CH(CH3)2]- 2-bromophenyl
208 1 -CH CH3 - 4 ridin-3 1 hen 1
209 1 -CH CH3 - 3-c ano hen 1
210 1 -CH CH3 - 4-c ano hen 1
211 1 -CH(CH3)- 3 -methylphenyl
212 1 -CH CH3 uinolin-6 1
213 1 -CH CH3 - 3 -methoxuinolin-6 1
214 1 -CH[CH2CH(CH3)21- phenyl
215 1 -CH CHF2 phenyl
216 1 -CH CH3 - iso uinolin-6 1
217 1 -CH CH3 - 3 ,5-ditrifluorometh 1 hen 1
218 0 - 4-ethox -5-iso ro 1-2-meth 1 hen 1
219 0 - 4 hen lthiazol-2 1
220 1 -CH2C CH3 2 phenyl
221 1 -C CH3 2CH2 phenyl
222 1 -C CH3 2- 2-fluoro hen 1
223 0 1 1-meth lc clohex 1
224 1 - phenyl
C(CH3)(CH2CH3)CH
2-
225 1 -C(CH3)2- 3-fluorophenyl
226 1 -C CH3 2- thio hen-3 1
227 1 -C(CH3)2- benzofuran-5-yl
228 1 -C CH3 2- thio hen-5 1
229 1 -C CH3 2- 5-bromothio hen-2 1
230 1 -C CH3 2- 4-bromothio hen-2 1
Table 2 shows representative compounds of Formula (I) where Ar is a ring of
formula
(a) are shown below.
H H Ar
u N
N
II \(alk)õ Arl
/ O
I
N /
Table 2
C pd # n Alk A Ar
1 0 0 c clobut 1 phenyl
2 0 0 c clohex 1 phenyl
3 0 0 1-(tert-butoxy- phenyl
16
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
carbon 1 i eridin-4 1
4 0 0 c clo ent 1 phenyl
0 0 c clo ro l 4-F hen l
6 0 0 tetrah dro ran-4 1 phenyl
7 0 0 tetrah dro ran-4 l 2-F hen l
8 0 0 1 -methoxycarbonyl- phenyl
i eridin-4 1
9 1 -CH2- cyclopentyl phenyl
1 -CH2- c clobut 1 phenyl
11 1 -CH2- cyclopropyl phenyl
12 1 -CH2- tetrahydropyran-4-yl phenyl
13 1 -CH2- cyclopropyl phenyl
14 0 - tetrah drofurn-3 l 4-chloro hen l
0 - c clobut l 4-bromo hen l
16 0 - c clohex l 3-fluoro hen l
17 0 - c clohex l 2-fluoro hen l
18 1 -CH2- c clo ent l 6-chloro ridin-2 l
19 1 -CH2- c clo ent 1 pyridine-2-yl
0 - c clobut l 3-bromo hen l
21 1 -CH2- c clo ent l 2-chloro ridin-4 l
22 1 -CH2- c clo ent 1 pyridin-4-yl
23 1 -CH2- c clo ent l 2-c ano ridin-4 l
24 0 - c clobut l 4-c ano hen l
0 - c clo ent l 6-chloro ridin-2 l
26 0 - c clo ent 1 pyridin-2-yl
27 0 - c clo ent l 2-chloro ridin-4 l
28 0 - c clo ent 1 pyridin-4-yl
29 0 - cyclopentyl 5-bromopyridin-4-yl
0 - c clo ent l 6-fluoro ridin-2 l
31 0 - eyelobutyl 6-chloropyridin-2-yl
32 0 - cyclopentyl pyridin-3-yl
33 0 - c clo ent l 6-methox ridin-2 l
34 0 - 4-oxocyclohexyl phenyl
0 - 4-h drox c clohex 1 phenyl
36 0 - 4,4-difluorocyclohexyl phenyl
37 1 -CHz- c clobut l 2-methox hen l
Embodiments
A. In one embodiment, the compound of Formula (I) is:
R1 H H
N N
O \(alk)õ-Ar
X\
N R 2
R
where:
17
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
X is -CH- or -N-;
nis0or1;
alk is a straight or branched alkyl of 1 to 6 carbon atoms where one, two, or
three
hydrogen atoms of the alkyl chain are replaced by alkoxy, hydroxyl or halo;
R is alkyl;
R1 and R2 are independently hydrogen, alkyl, alkoxy, hydroxy, or halo;
Ar is aryl, heteroaryl, cycloalkyl, fused cycloalkyl, or heterocyclyl where
each of the
aforementioned ring is optionally substituted with Ra, Rb or Re where Ra is
alkyl, halo,
haloalkyl, haloalkoxy, alkylthio, cyano, alkoxy, amino, monosubstituted amino,
disubstituted
amino, sulfonyl, acyl, carboxy, alkoxycarbonyl, hydroxyalkyl, alkoxyalkyl,
aminoalkyl,
hydroxyalkoxy, alkoxyalkoxy, aminoalkoxy, aminosulfonyl, aminocarbonyl, or
acylamino and
Rb and Re are independently selected from alkyl, halo, haloalkyl, haloalkoxy,
alkylthio, cyano,
alkoxy, amino, monosubstituted amino, disubstituted amino, sulfonyl, acyl,
carboxy,
alkoxycarbonyl, hydroxyalkyl, alkoxyalkyl, aminoalkyl, hydroxyalkoxy,
alkoxyalkoxy,
aminoalkoxy, aminosulfonyl, aminocarbonyl, acylamino, aryl, heteroaryl,
cycloalkyl,
heterocyclyl, aralkyl, heteroaralkyl, aryloxy, heteroaryloxy, or cycloalkoxy,
or when Rb and
Re are on adjacent atoms they can combine to form methylenedioxy or
ethylenedioxy; where
the aromatic or alicyclic ring in R, Rb and Re is optionally substituted with
Rd, Re or Rf which
are independently selected from alkyl, halo, haloalkyl, haloalkoxy, alkylthio,
cyano, alkoxy,
amino, monosubstituted amino, disubstituted amino, sulfonyl, acyl, carboxy,
alkoxycarbonyl,
hydroxyalkyl, alkoxyalkyl, aminoalkyl, hydroxyalkoxy, alkoxyalkoxy,
aminoalkoxy,
aminosulfonyl, aminocarbonyl, or acylamino; or
a pharmaceutically acceptable salt thereof.
1. Within A above, in one embodiment, the compound of Formula (I) is
represented by
the structure:
R1 H H
TN UN~
II II Ar
0
~ ~ R2
N /
(a) Within embodiment (I), one group of compounds is that wherein X is
-N-.
(b) Within embodiment (I), another group of compounds is that wherein X is -CH-
.
18
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
(c) Within embodiment (I), yet another group of compounds is that wherein R1
and R2 are
hydrogen.
Within group (c), one group of compounds is that wherein X is -CH-.
Within group (c), another group of compounds is that wherein X is -N-.
Within groups (a), (b) and (c) and groups contained therein, in one group of
compounds
R is methyl or ethyl, more preferably methyl.
(i) Within groups (a) through (c) and groups contained therein, one group of
compounds is
that wherein Ar is aryl optionally substituted as described above. Within this
group, one group
of compounds is that whererin Ar is phenyl or naphthyl optionally substituted
with R' and Rb
where R' is halo, alkyl, haloalkyl, or alkoxy and Rb is halo, alkyl,
haloalkyl, alkoxy,
haloalkoxy, aryl, heteroaryl, heterocyclyl, monosubstituted amino,
disubstituted amino, cyano,
acyl, or aralkyl.
Within this group, another group of compounds is that whererin Ar is naphth-1-
yl,
phenyl, 2-chlorophenyl, 3-trifluoromethylphenyl, 4-fluorophenyl, 2-
chlorophenyl, 2,4-
dichlorophenyl, 2-chloro-6-trifluoromethylphenyl, 3-fluoro-6-
trifluoromethylphenyl, 2-
fluorophenyl, 2-difluoromethoxyphenyl, 3,5-ditrifluoromethylphenyl, biphen-3-
yl, 3-
methoxyphenyl, 3-methylphenyl, 2-(2-pyridin-2-ylethyl)phenyl, 4-[1,2,4]triazol-
1-ylphenyl, 4-
pyrazol-1-ylphenyl, 4-oxazol-5-ylphenyl, 3-morpholin-4-ylphenyl, or biphen-4-
yl.
(ii) Within groups (a) through (c) and groups contained therein, another group
of
compounds is that wherein Ar is heteroaryl optionally substituted as described
above. Within
this group, one group of compounds is that whererin Ar is pyridyl, thienyl,
furanyl, indolyl,
benzothiophenyl, quinolinyl, isoquinolinyl, pyrimidinyl, pyrrolyl, pyrazolyl,
pyridazinyl,
pyrazinyl, benzimidazolyl,or benzoxazolyl optionally substituted as described
above. Within
this group, one group of compounds is that whererin Ar is heteroaryl
optionally substituted
with R' and Rb where R' is halo, alkyl, haloalkyl, or alkoxy and Rb is halo,
alkyl, haloalkyl,
alkoxy, haloalkoxy, aryl, heteroaryl, heterocyclyl, monosubstituted amino,
disubstituted amino,
cyano, acyl, or aralkyl. Within this group, another group of compounds is that
whererin Ar is
2,7-dimethylindol-3-yl, 5-chloro-2-methylindol-3-yl, indol-3-yl, 5-bromo-2-
methylindol-3-yl,
2-methyl-7-(2-propyl)indol-3-yl, 1,2-dimethylindol-3-yl, 2-methylindol-3-yl, 6-
chloropyridin-
2-yl, 2-pyridin-3-ylindol-3-yl, 2,3-difluoropyridin-4-yl, 2,6-dichloropyridin-
4-yl,
benzothiophen-3-yl, 6-bromopyridin-3-yl, or thiophen-2-yl.
(iii) Within groups (a) through (c) and groups contained therein, another
group of
compounds is that wherein Ar is cycloalkyl or heterocyclyl optionally
substituted as described
above. Within this group, one group of compounds is that whererin Ar
cycloalkyl or
19
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
heterocyclyl optionally substituted with Ra and Rb where Ra is halo, alkyl,
haloalkyl, or alkoxy
and Rb is halo, alkyl, haloalkyl, alkoxy, haloalkoxy, aryl, heteroaryl,
heterocyclyl,
monosubstituted amino, disubstituted amino, cyano, acyl, or aralkyl. Within
this group,
another group of compounds is that whererin Ar is 1,2,3,4-tetrahydronaphth-l-
yl, 2-(pyridin-2-
yl)-1,2,3,4-tetrahydronaphth-1-yl, 6-chloro-2,2-dimethylbenzopyran-4-yl, 6-
bromo-2,2-
dimethylbenzopyran-4-yl, (S)-6-ethyl-2-methyl-2-methoxymethylbenzopyran-4-yl,
6,8-
dichloro-2,2-dimethylbenzopyran-4-yl, cyclohexyl, (S)-2-tert-butyl-7,7-
dimethyl-5,6,7,8-
tetrahydroquinazolin-5-yl, 2,3-dihydro-lH-inden-1-yl, 6-(hydroxymethyl)-
1,2,3,4-
tetrahydronaphth-1-yl, 1,2,3,4-tetrahydronaphth-2-yl, 6-ethyl-3,4-dihydro-2H-
chromen-4-yl,
2,3,4,5-tetrahydro-lH-benzo[b]azepin-5-yl, 2,3-dihydrobenzo[b][1,4]dioxin-7-
yl, 6-(4-
methylpyridin-1-ylmethyl)1,2,3,4-tetrahydronaphth-1-yl, or 1-
hydroxycyclohexyl.
(iv) Within groups (a) through (c) and groups contained therein, another group
of
compounds is that wherein Ar is fused cycloalkyl optionally substituted as
described above.
Within this group, one group of compounds is that whererin Ar fused cycloalkyl
optionally
substituted with Ra and Rb where Ra is halo, alkyl, haloalkyl, or alkoxy and
Rb is halo, alkyl,
haloalkyl, alkoxy, haloalkoxy, aryl, heteroaryl, heterocyclyl, monosubstituted
amino,
disubstituted amino, cyano, acyl, or aralkyl.
(v) Within the groups (a) through (c) and groups contained therein, another
group of
compounds is that wherein Ar is aryl, heteroaryl, cycloalkyl, or heterocyclyl
where each of the
aforementioned ring is optionally substituted with Ra, Rb or Re where Ra is
alkyl, halo,
haloalkyl, haloalkoxy, alkylthio, cyano, alkoxy, or acyl and Rb and Re are
independently
selected from alkyl, halo, haloalkyl, aryl, heteroaryl, heterocyclyl, or
aryloxy; where the
aromatic or alicyclic ring in Ra, Rb and Re is optionally substituted with Rd,
Re or Rf which are
independently selected from alkyl, halo, cyano, or alkoxy; preferably Ar is 3-
morpholin-4-
ylphenyl; 4-methylphenyl; 4-chlorophenyl; 2-chlorophenyl; 3-chlorophenyl; 3-
bromophenyl;
2-bromophenyl; 2-fluorophenyl; 3-fluorophenyl; 2,4-dichlorophenyl; 4-
methoxyphenyl; 3-
difluoromethoxyphenyl; 5-bromo-2-fluorophenyl; phenyl; 2-methylphenyl; 3-n-
butoxyphenyl;
2-fluoro-5-cyanophenyl; 5-cyano-2-methylphenyl; 5-chloro-2-fluorophenyl; 3-
ethoxyphenyl;
2-methyl-5-trifluoromethylphenyl; 3-n-propoxyphenyl; 2-n-propoxyphenyl; 3-tert-
butylphenyl;
2-pyridin-3-ylphenyl; 5-bromo-2-methylphenyl; 2-n-propylphenyl; 5-chloro-2-
methylphenyl;
3-ethoxyphenyl; 2-fluoro-5-trifluoromethylphenyl; 2-ethylphenyl; 3-
methylthiophenyl; 2,5-
difluorophenyl; 5-fluoro-2-methoxyphenyl; 2-cyanophenyl; 2-benzoylphenyl; 2-
trifluoromethylphenyl; 5-fluoro-2-methylphenyl; 2-chloro-5-methoxyphenyl; 2-
chloro-5-
methylphenyl; 2-phenoxyphenyl; 3-cyanophenyl; 2-acetylphenyl; 2-cyano-5-
methylphenyl; 3-
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
isopropoxyphenyl; 3-benzoylphenyl; 2-chloro-5-cyanophenyl; 3-phenoxyphenyl; 2-
methoxy-5-
methylphenyl; 2-chloro-5-trifluoromethylphenyl; 2,5-dichlorophenyl; 3-
methoxyphenyl; 3-
ethylphenyl; 2-methylphenyl; 2-methylthiophenyl; 2-methoxy-5-
trifluoromethylphenyl; 5-
chloro-2-cyanophenyl; 2-ethoxyphenyl; 2-isopropylphenyl; 2-isobutylphenyl; 2-
methoxyphenyl; 2-trifluoromethylphenyl; 3-methylphenyl; 5-isopropyl-2-
methylphenyl; 2,6-
dichlorophenyl; 3-chloro-4-methoxyphenyl; 5-chloro-2-methoxyphenyl; 5-chloro-
2,4-
dimethoxyphenyl; 3,5-difluorophenyl; 4-pyridin-3-ylphenyl; 4-cyanophenyl;
quinolin-5-yl;
quinolin-4-yl; quinolin-2-yl; furan-2-yl; benzofuran-2-yl; 1-
difluoromethylbenzimidazol-2-yl;
3,5-dimethylpyrazol-4-yl; tetrahydropyran-3-yl; 3-methylisoxazol-5-yl;
tetrahydrofuran-3-yl;
5-fluoropyridin-2-yl; 5-chloropyridin-2-yl; 6-methoxypyridin-3-yl; isoquinolin-
3-yl; quinolin-
7-yl; 3'-fluorobipheny-3-yl; 4'-methoxybiphen-2-yl; 3-furan-2-ylphenyl; 3'-
methoxybiphen-3-
yl; 2-propylthiophenyl; 3-thiophen-3-ylphenyl; 3-pyridin-4-ylphenyl; 2-
thiophen-2-ylphenyl;
3-pyridin-3-ylphenyl; 3-thiophen-2-ylphenyl; 4'-fluorobiphen-3-yl; 3-(2-
methylthiazol-4-
yl)phenyl; biphen-2-yl; 2-methoxybiphen-5-yl; 4'-cyanobiphen-3-yl; R-3,3-
dimethyltetrahydropyran-4-yl; cyclohexyl; cyclopentyl; phenyl; furan-2-yl; 2-
methyl-5-
trifluoromethylfuran-4-yl; 5-methyl-l-phenylpyrazol-4-yl; 5-methyl-3-
phenylisoxazol-4-yl; 3-
methyl-5-phenylisoxazol-4-yl; 2,5-dimethyl-l-phenylpyrazol-4-yl; quinolin-6-
yl; 3-
methoxyquinolin-6-yl; isoquinolin-6-yl; 3,5-ditrifluoromethylphenyl; 4-ethoxy-
5-isopropyl-2-
methylphenyl; 4-phenylthiazol-2-yl; 1-methylcyclohexyl; thiophen-3-yl;
benzofuran-5-yl;
thiophen-5-yl; 5-bromothiophen-2-yl; or 4-bromothiophen-2-yl.
II. Within A above, in another embodiment, the compound of Formula (I) is
represented
by the structure:
R1 H H
~NyN~
alk-Ar
XO
I X 2
N R
R
III. Within A above, in yet another embodiment, the compound of Formula (I) is
represented by the structure:
R1 H H
NN"--. Ar
X R2 O
N
21
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
Within this group, in one group of compounds n is 1 and alk is -CH2-, -(CH2)2-
, -
(CHCH3)-, or -C(CH3)2-
IV. Within A above, in yet another embodiment, the compound of Formula (I) is
represented by the structure:
R1 H H R1 H H
NuNAr N N.Ar
IOI O
N R2 R or X\R2 R
N /
R
where R' is methyl, ethyl, n-propyl, n-butyl, or isobutyl.
(a) Within embodiments (II), (III), and (IV), one group of compounds is that
wherein X is
-N-.
(b) Within embodiments (II), (III), and (IV), another group of compounds is
that wherein X
is -CH-.
(c) Within embodiments (II), (III), and (IV), yet another group of compounds
is that
wherein R1 and R2 are hydrogen.
Within group (c), one group of compounds is that wherein X is -CH-.
Within group (c), another group of compounds is that wherein X is -N-.
Within group (c) and groups contained therein, in one group of compounds R is
methyl.
(i) Within groups (II), (III), and (IV), and groups contained therein, one
group of
compounds is that wherein Ar is aryl optionally substituted as described
above. Within this
group, one group of compounds is that whererin Ar is phenyl or naphthyl
optionally substituted
with R' and Rb where R' is halo, alkyl, haloalkyl, or alkoxy and Rb is halo,
alkyl, haloalkyl,
alkoxy, haloalkoxy, aryl, heteroaryl, heterocyclyl, monosubstituted amino,
disubstituted amino,
cyano, acyl, or aralkyl.
Within this group, another group of compounds is that whererin Ar is naphth-l-
yl,
phenyl, 2-chlorophenyl, 3-trifluoromethylphenyl, 4-fluorophenyl, 2-
chlorophenyl, 2,4-
dichlorophenyl, 2-chloro-6-trifluoromethylphenyl, 3-fluoro-6-
trifluoromethylphenyl, 2-
fluorophenyl, 2-difluoromethoxyphenyl, 3,5-ditrifluoromethylphenyl, biphen-3-
yl, 3-
methoxyphenyl, 3-methylphenyl, 2-(2-pyridin-2-ylethyl)phenyl, 4-[l,2,4]triazol-
1-ylphenyl, 4-
pyrazol-1-ylphenyl, 4-oxazol-5-ylphenyl, 3-morpholin-4-ylphenyl, or biphen-4-
yl.
(ii) Within groups (II), (III), and (IV), and groups contained therein,
another group of
compounds is that wherein Ar is heteroaryl optionally substituted as described
above. Within
this group, one group of compounds is that whererin Ar is pyridyl, thienyl,
furanyl, indolyl,
22
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
benzothiophenyl, quinolinyl, isoquinolinyl, pyrimidinyl, pyrrolyl, pyrazolyl,
pyridazinyl,
pyrazinyl, benzimidazolyl, or benzoxazolyl optionally substituted as described
above. Within
this group, one group of compounds is that whererin Ar is heteroaryl
optionally substituted
with R' and Rb where R' is halo, alkyl, haloalkyl, or alkoxy and Rb is halo,
alkyl, haloalkyl,
alkoxy, haloalkoxy, aryl, heteroaryl, heterocyclyl, monosubstituted amino,
disubstituted amino,
cyano, acyl, or aralkyl. Within this group, another group of compounds is that
whererin Ar is
2,7-dimethylindol-3-yl, 5-chloro-2-methylindol-3-yl, indol-3-yl, 5-bromo-2-
methylindol-3-yl,
2-methyl-7-(2-propyl)indol-3-yl, 1,2-dimethylindol-3-yl, 2-methylindol-3-yl, 6-
chloropyridin-
2-yl, 2-pyridin-3-ylindol-3-yl, 2,3-difluoropyridin-4-yl, 2,6-dichloropyridin-
4-yl,
benzothiophen-3-yl, 6-bromopyridin-3-yl, or thiophen-2-yl.
(iii) Within groups (II), (III), and (IV), and groups contained therein, yet
another group of
compounds is that wherein Ar is cycloalkyl or heterocyclyl optionally
substituted as described
above. Within this group, one group of compounds is that whererin Ar
cycloalkyl or
heterocyclyl optionally substituted with R' and Rb where Ra is halo, alkyl,
haloalkyl, or alkoxy
and Rb is halo, alkyl, haloalkyl, alkoxy, haloalkoxy, aryl, heteroaryl,
heterocyclyl,
monosubstituted amino, disubstituted amino, cyano, acyl, or aralkyl. Within
this group,
another group of compounds is that whererin Ar is 1,2,3,4-tetrahydronaphth-1-
yl, 2-(pyridin-2-
yl)-1,2,3,4-tetrahydronaphth-1-yl, 6-chloro-2,2-dimethylbenzopyran-4-yl, 6-
bromo-2,2-
dimethylbenzopyran-4-yl, (S)-6-ethyl-2-methyl-2-methoxymethylbenzopyran-4-yl,
6,8-
dichloro-2,2-dimethylbenzopyran-4-yl, cyclohexyl, (S)-2-tert-butyl-7,7-
dimethyl-5,6,7,8-
tetrahydroquinazolin-5-yl, 2,3-dihydro-1H-inden-1-yl, 6-(hydroxymethyl)-
1,2,3,4-
tetrahydronaphth-1-yl, 1,2,3,4-tetrahydronaphth-2-yl, 6-ethyl-3,4-dihydro-2H-
chromen-4-yl,
2,3,4,5-tetrahydro-1H-benzo[b]azepin-5-yl, 2,3-dihydrobenzo[b][1,4]dioxin-7-
yl, 6-(4-
methylpyridin-1-ylmethyl)1,2,3,4-tetrahydronaphth-1-yl, or 1-
hydroxycyclohexyl.
(iv) Within groups (II), (III), and (IV), and groups contained therein, yet
another group of
compounds is that wherein Ar is fused cycloalkyl optionally substituted as
described above.
Within this group, one group of compounds is that whererin Ar fused cycloalkyl
optionally
substituted with Ra and Rb where Ra is halo, alkyl, haloalkyl, or alkoxy and
Rb is halo, alkyl,
haloalkyl, alkoxy, haloalkoxy, aryl, heteroaryl, heterocyclyl, monosubstituted
amino,
disubstituted amino, cyano, acyl, or aralkyl.
(v) Within groups (II), (III) and (IV), and groups contained therein, yet
another group of
compounds is that wherein Ar is aryl, heteroaryl, cycloalkyl, or heterocyclyl
where each of the
aforementioned ring is optionally substituted with Ra, Rb or R where Ra is
alkyl, halo,
haloalkyl, haloalkoxy, alkylthio, cyano, alkoxy, or acyl and Rb and R are
independently
23
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
selected from alkyl, halo, haloalkyl, aryl, heteroaryl, heterocyclyl, or
aryloxy; where the
aromatic or alicyclic ring in Ra, Rb and Re is optionally substituted with Rd,
Re or Rf which are
independently selected from alkyl, halo, cyano, or alkoxy; preferably Ar is 3-
morpholin-4-
ylphenyl; 4-methylphenyl; 4-chlorophenyl; 2-chlorophenyl; 3-chlorophenyl; 3-
bromophenyl;
2-bromophenyl; 2-fluorophenyl; 3-fluorophenyl; 2,4-dichlorophenyl; 4-
methoxyphenyl; 3-
difluoromethoxyphenyl; 5-bromo-2-fluorophenyl; phenyl; 2-methylphenyl; 3-n-
butoxyphenyl;
2-fluoro-5-cyanophenyl; 5-cyano-2-methylphenyl; 5-chloro-2-fluorophenyl; 3-
ethoxyphenyl;
2-methyl-5-trifluoromethylphenyl; 3-n-propoxyphenyl; 2-n-propoxyphenyl; 3-tert-
butylphenyl;
2-pyridin-3-ylphenyl; 5-bromo-2-methylphenyl; 2-n-propylphenyl; 5-chloro-2-
methylphenyl;
3-ethoxyphenyl; 2-fluoro-5-trifluoromethylphenyl; 2-ethylphenyl; 3-
methylthiophenyl; 2,5-
difluorophenyl; 5-fluoro-2-methoxyphenyl; 2-cyanophenyl; 2-benzoylphenyl; 2-
trifluoromethylphenyl; 5-fluoro-2-methylphenyl; 2-chloro-5-methoxyphenyl; 2-
chloro-5-
methylphenyl; 2-phenoxyphenyl; 3-cyanophenyl; 2-acetylphenyl; 2-cyano-5-
methylphenyl; 3-
isopropoxyphenyl; 3-benzoylphenyl; 2-chloro-5-cyanophenyl; 3-phenoxyphenyl; 2-
methoxy-5-
methylphenyl; 2-chloro-5-trifluoromethylphenyl; 2,5-dichlorophenyl; 3-
methoxyphenyl; 3-
ethylphenyl; 2-methylphenyl; 2-methylthiophenyl; 2-methoxy-5-
trifluoromethylphenyl; 5-
chloro-2-cyanophenyl; 2-ethoxyphenyl; 2-isopropylphenyl; 2-isobutylphenyl; 2-
methoxyphenyl; 2-trifluoromethylphenyl; 3-methylphenyl; 5-isopropyl-2-
methylphenyl; 2,6-
dichlorophenyl; 3-chloro-4-methoxyphenyl; 5-chloro-2-methoxyphenyl; 5-chloro-
2,4-
dimethoxyphenyl; 3,5-difluorophenyl; 4-pyridin-3-ylphenyl; 4-cyanophenyl;
quinolin-5-yl;
quinolin-4-yl; quinolin-2-yl; furan-2-yl; benzofuran-2-yl; 1-
difluoromethylbenzimidazol-2-yl;
3,5-dimethylpyrazol-4-yl; tetrahydropyran-3-yl; 3-methylisoxazol-5-yl;
tetrahydrofuran-3-yl;
5-fluoropyridin-2-yl; 5-chloropyridin-2-yl; 6-methoxypyridin-3-yl; isoquinolin-
3-yl; quinolin-
7-yl; 3'-fluorobipheny-3-yl; 4'-methoxybiphen-2-yl; 3-furan-2-ylphenyl; 3'-
methoxybiphen-3-
yl; 2-propylthiophenyl; 3-thiophen-3-ylphenyl; 3-pyridin-4-ylphenyl; 2-
thiophen-2-ylphenyl;
3-pyridin-3-ylphenyl; 3-thiophen-2-ylphenyl; 4'-fluorobiphen-3-yl; 3-(2-
methylthiazol-4-
yl)phenyl; biphen-2-yl; 2-methoxybiphen-5-yl; 4'-cyanobiphen-3-yl; R-3,3-
dimethyltetrahydropyran-4-yl; cyclohexyl; cyclopentyl; phenyl; furan-2-yl; 2-
methyl-5-
trifluoromethylfuran-4-yl; 5-methyl-l-phenylpyrazol-4-yl; 5-methyl-3-
phenylisoxazol-4-yl; 3-
methyl-5-phenylisoxazol-4-yl; 2,5-dimethyl-l-phenylpyrazol-4-yl; quinolin-6-
yl; 3-
methoxyquinolin-6-yl; isoquinolin-6-yl; 3,5-ditrifluoromethylphenyl; 4-ethoxy-
5-isopropyl-2-
methylphenyl; 4-phenylthiazol-2-yl; 1-methylcyclohexyl; thiophen-3-yl;
benzofuran-5-yl;
thiophen-5-yl; 5-bromothiophen-2-yl; or 4-bromothiophen-2-yl.
24
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
B. In another embodiment the compounds of Formula (I) are represented by the
structure:
R1 H H
N N
(alk)õ qrl
O \
x\ \ 2
N R
R
where:
X is -CH- or -N-;
nis0or1;
alk is a straight or branched alkyl of 1 to 6 carbon atoms;
R is alkyl;
R1 and R2 are independently hydrogen, alkyl, alkoxy, hydroxy, or halo;
ring A is cycloalkyl optionally substituted with halo, hydroxyl, alkoxy, oxo,
or
haloalkyl; or monocyclic heterocyclyl wherein if the heterocyclyl ring
contains a nitrogen ring
atom, the nitrogen atom is optionally substituted with alkyl, or acyl,
acyloxycarbonyl;
Ari is aryl, heteroaryl, cycloalkyl, fused cycloalkyl, or heterocyclyl where
each of the
aforementioned ring is optionally substituted with Ra, Rb or Re where Ra is
alkyl, halo,
haloalkyl, haloalkoxy, alkylthio, cyano, alkoxy, amino, monosubstituted amino,
disubstituted
amino, sulfonyl, acyl, carboxy, alkoxycarbonyl, hydroxyalkyl, alkoxyalkyl,
aminoalkyl,
hydroxyalkoxy, alkoxyalkoxy, aminoalkoxy, aminosulfonyl, aminocarbonyl, or
acylamino and
Rb and Re are independently selected from alkyl, halo, haloalkyl, haloalkoxy,
alkylthio, cyano,
alkoxy, amino, monosubstituted amino, disubstituted amino, sulfonyl, acyl,
carboxy,
alkoxycarbonyl, hydroxyalkyl, alkoxyalkyl, aminoalkyl, hydroxyalkoxy,
alkoxyalkoxy,
aminoalkoxy, aminosulfonyl, aminocarbonyl, acylamino, aryl, heteroaryl,
cycloalkyl,
heterocyclyl, aralkyl, heteroaralkyl, aryloxy, heteroaryloxy, or cycloalkoxy,
or when Rb and
Re are on adjacent atoms they can combine to form methylenedioxy or
ethylenedioxy; where
the aromatic or alicyclic ring in Ra, Rb and Re is optionally substituted with
Rd, Re or Rf which
are independently selected from alkyl, halo, haloalkyl, haloalkoxy, alkylthio,
cyano, alkoxy,
amino, monosubstituted amino, disubstituted amino, sulfonyl, acyl, carboxy,
alkoxycarbonyl,
hydroxyalkyl, alkoxyalkyl, aminoalkyl, hydroxyalkoxy, alkoxyalkoxy,
aminoalkoxy,
aminosulfonyl, aminocarbonyl, or acylamino.
V. Within B, in one embodiment, the compound of Formula (I) is represented by
the
structure (IIa):
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
R1
N N Are
O A
~ R2
N /
(IIa)
VI. Within B, in another embodiment, the compound of Formula (I) is
represented by the
structure (IIb).
R1 H H A
N N
RR O \(alk) Art
x\ 2
N
R
(IIb)
where n is 1. Within this embodiment, in one group of compounds, n is 1 and
alk is -CH2-.
Within this embodiment, in another group of compounds, n is 1 and alk is -CH2-
, -(CH2)2-1 -
(CHCH3)- or -C(CH3)2-;
(a') Within embodiments (V) and (VI) and groups contained therein, one group
of
compounds is that wherein X is -N-.
(b') Within embodiments (V) and (VI) and groups contained therein, another
group of
compounds is that wherein X is -CH-.
(c') Within embodiments (V) and (VI) and groups contained therein, yet another
group of
compounds is that wherein R1 and R2 are hydrogen.
Within group (c'), one group of compounds is that wherein X is -CH-.
Within group (c'), another group of compounds is that wherein X is -N-.
(d') Within embodiments (V), (VI), (a'), (b'), (c') and groups contained
therein; one group
of compounds is that wherein R3 is methyl or ethyl. Within this group (d), in
one group of
compounds R is methyl.
(i) Within the embodiments (V), (VI), (a'), (b'), (c'), and (d'); and groups
contained
therein, one group of compounds is that wherein A is cycloalkyl. Within this
group (i), in one
group of compounds A is cyclopropyl, cyclobutyl, cyclopentyl, or cyclohexyl.
Within this
group (i), in one group of compounds A is cyclopropyl or cyclopentyl.
(ii) Within the embodiments (V), (VI), (a'), (b'), (c'), and (d'); and groups
contained
therein, one group of compounds is that wherein A is monocyclic heterocyclyl
as defined
26
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
above. Within this group (ii), in one group of compounds, A is piperidin-1-yl
or
tetrahydropyran-4-yl wherein the nitrogen atom of the piperidin-4-yl ring is
optionally
substituted with alkoxycarbonyl.
(A) Within the embodiments (V), (VI), (a'), (b'), (c'), and (d'), (i) and
(ii); and groups
contained therein, in one group of compounds, Ari is aryl optionally
substituted as described
above.
Within this group, one group of compounds is that whererin Ari is phenyl
optionally
substituted with Ra which is halo, alkyl, haloalkyl, or alkoxy and/or Rb which
is halo, alkyl,
haloalkyl, alkoxy, cycloalkyl, aryl, aryloxy, amino, monosubstituted amino,
disubstituted
amino, cyano, acyl, or aralkyl.
Within this group, another group of compounds is that whererin Ari is phenyl,
2-
fluorophenyl, 3-fluorophenyl, 4-chlorophenyl, 4-methoxyphenyl, 4-cyanophenyl,
3-
morpholinylphenyl, 4-morphorlinylphenyl or 4-fluorophenyl.
(B) Within the embodiments (V), (VI), (a'), (b'), (c'), and (d'), (i) and
(ii); and groups
contained therein, in one group of compounds Ari is heteroaryl optionally
substituted as
described above. Within this group, another group of compounds is that
whererin Ari is 2-
chloro-6-pyridyl, or 2-chloro-6-pyrazinyl.
Within this group, one group of compounds is that whererin Ari is heteroaryl
optionally
substituted with Ra which is halo, alkyl, haloalkyl, or alkoxy and/ or Rb
which is selected from
halo, alkyl, haloalkyl, alkoxy, cycloalkyl, aryl, aryloxy, amino,
monosubstituted amino,
disubstituted amino, cyano, acyl, or aralkyl.
(C) Within the embodiments (V), (VI), (a'), (b'), (c'), and (d'), (i) and
(ii); and groups
contained therein, in one group of compounds Ari is cycloalkyl optionally
substituted as
described above.
(D) Within the embodiments (V), (VI), (a'), (b'), (c'), and (d'), (i) and
(ii); and groups
contained therein, in one group of compounds Ari is fused cycloalkyl, or
heterocyclyl
optionally substituted as described above.
(E) Within the embodiments (V), (VI), (a'), (b'), (c'), and (d'), (i) and
(ii); and groups
contained therein, in one group of compounds Ari is phenyl or heteroaryl each
ring optionally
substituted with Ra which is halo, cyano, or alkoxy; preferably Ari is phenyl,
4-fluorophenyl,
2-fluorophenyl, 4-chlorophenyl, 4-bromophenyl, 3-fluorophenyl, 6-chloropyridin-
2-yl,
pyridine-2-yl, 3-bromophenyl, 2-chloropyridin-4-yl, pyridine-4-yl, 2-
cyanopyridin-4-yl, 4-
cyanophenyl, 2-chloropyridin-4-yl, 5-bromopyridin-4-yl, 6-fluoropyridin-2-yl,
pyridin-3-yl, 6-
methoxypyridin-2-yl, or 2-methoxyphenyl.
27
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
General Synthetic Scheme
Compounds of this invention can be made by the methods depicted in the
reaction
schemes shown below.
The starting materials and reagents used in preparing these compounds are
either
available from commercial suppliers such as Aldrich Chemical Co., (Milwaukee,
Wis.),
Bachem (Torrance, Calif.), or Sigma (St. Louis, Mo.) or are prepared by
methods known to
those skilled in the art following procedures set forth in references such as
Fieser and Fieser's
Reagents for Organic Synthesis, Volumes 1-17 (John Wiley and Sons, 1991);
Rodd's
Chemistry of Carbon Compounds, Volumes 1-5 and Supplementals (Elsevier Science
Publishers, 1989); Organic Reactions, Volumes 1-40 (John Wiley and Sons,
1991), March's
Advanced Organic Chemistry, (John Wiley and Sons, 4th Edition) and Larock's
Comprehensive Organic Transformations (VCH Publishers Inc., 1989). These
schemes are
merely illustrative of some methods by which the compounds of this invention
can be
synthesized, and various modifications to these schemes can be made and will
be suggested to
one skilled in the art having referred to this disclosure. The starting
materials and the
intermediates, and the final products of the reaction may be isolated and
purified if desired
using conventional techniques, including but not limited to filtration,
distillation,
crystallization, chromatography and the like. Such materials may be
characterized using
conventional means, including physical constants and spectral data.
Unless specified to the contrary, the reactions described herein take place at
atmospheric pressure over a temperature range from about -78 C to about 150
C, more
preferably from about 0 C to about 125 C and most preferably at about room
(or ambient)
temperature, e.g., about 20 C.
Compounds of formula (I) where Ar is as defined in the Summary of the
Invention can
be prepared as described in Scheme A below.
Scheme A
1
R NH2 R1 Nu N,
II (alk)n-Ar
\ I X\R2 OCN, (alk)õ-Ar I X.
O
N / N / R I
1 'T
2
R R
28
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
Compounds of formula (I) are synthesized by coupling of an amine of formula 1
with
an isocyanate of formula 2 optionally in the presence of an organic base such
as Hunig's base,
pyridine, and the like and in an aprotic solvents such as THF, toluene, and
the like.
Amines of formula 1 are either commercially available or can be synthesized by
coupling an aryl halide of formula 3 or 6 with a boronic acid compound of
formula 4 or 5
respectively, under Suzuki coupling reaction conditions as shown below:
R1
Br X NH2
N
(HO)2B" R1
R2 NH2
R \~
3 5 X.
N --
R1
B(OH)2 ~~ NH2 R 1
N / Br ' R 2
R
4 6
Compounds of formula 3-6 are are either commercially available or prepared
using
conditions well known to one skilled in the art of organic synthesis.
Alternatively, compounds of Formula (I) where Ar is aryl, heteroaryl,
cycloalkyl, fused
cycloalkyl, or heterocyclyl can be prepared as described in Scheme B below.
Scheme B
R1
NCO R1 Nu N
(alk)n-Ar
\ X IIO ,
N / R2 + H2N-(alk),~-Ar X\R2
N / I
7 8
Compounds of Formula (I) can be prepared by coupling an isocyanate compound of
formula 7 with an amine of formula 8 under conditions described in Scheme A
above. The
amine of formula 8 is either commercially available or prepared using
conditions well known
to one skilled in the art of organic synthesis (for example, naphthalen-l-
ylmethanamine,
naphthalen-2-ylmethanamine, (S)-1-(naphthalen-1-yl)ethanamines, (R)-1-
(naphthalen-l-
yl)ethanamines, 2-(4-bromophenyl)ethanamines, 1-phenyl-cyclopropylamine, (1-
29
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
phenylcyclopentyl)methylamine, (1-phenylcyclohexyl)methylamine, (4-phenyl-
tetrahydro-
pyran-4-yl)methylamine, (1-phenylcyclopropyl)methylamine, and (1-
phenylcyclobutyl)-
methylamine are commercially available). The isocyanate of formula 7 can be
prepared from
the corresponding acid of formula 9, by first preparing an azido carbonyl
compound of formula
by treating the acid with ethylchloroformate in the presence of an organic
base such as
triethyl amine, and the like and subsequent treatment with aqueous sodium
azide. Heating 10
in a suitable organic solvent such as toluene, dioxane, acetonitrile, and the
like, at 100-150 C,
from about 1 h to 30 h provides the isocyanate derivative.
1
R1 R1
COOH NCO
I~ - \ .Y
2
2 N R 2 N R
N /
R 9 R 10 R 8
Acids of formula 9 can be prepared by Suzuki coupling of a bromide of formula
11
with a boronic acid of formula 12 where R' is alkyl, followed by acid
hydrolysis of the ester
group in the resulting compound 13.
R1
1
R C02R' Br
N / + (HO)2B X~R2 N R 9
11 12 R 13
Utility
The compounds of the invention are y-secretase modulators and hence are useful
in the
treatment of Alzheimer's disease.
Testing
The y-secretase modulatory activity of the compounds of the present invention
can be
tested using the in vitro and in vivo assays described in working Example 1
below.
Administration and Pharmaceutical Composition
In general, the compounds of this invention will be administered in a
therapeutically
effective amount by any of the accepted modes of administration for agents
that serve similar
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
utilities. Therapeutically effective amounts of compounds of Formula (I) may
range from about
0.01 to about 500 mg per kg patient body weight per day, which can be
administered in single
or multiple doses. Preferably, the dosage level will be about 0.1 to about 250
mg/kg per day;
more preferably about 0.5 to about 100 mg/kg per day. A suitable dosage level
may be about
0.01 to about 250 mg/kg per day, about 0.05 to about 100 mg/kg per day, or
about 0.1 to about
50 mg/kg per day. Within this range the dosage can be about 0.05 to about 0.5,
about 0.5 to
about 5 or about 5 to about 50 mg/kg per day.
For oral administration, the compositions are preferably provided in the form
of tablets
containing about 1.0 to about 1000 milligrams of the active ingredient,
particularly about 1.0,
5.0, 10, 15, 20, 25, 50, 75, 100, 150, 200, 250, 300, 400, 500, 600, 750, 800,
900, and 1000
milligrams of the active ingredient. The actual amount of the compound of this
invention, i.e.,
the active ingredient, will depend upon numerous factors such as the severity
of the disease to
be treated, the age and relative health of the subject, the potency of the
compound utilized, the
route and form of administration, and other factors.
In general, compounds of this invention will be administered as pharmaceutical
compositions by any one of the following routes: oral, systemic (e.g.,
transdermal, intranasal or
by suppository), or parenteral (e.g., intramuscular, intravenous or
subcutaneous)
administration. The preferred manner of administration is oral using a
convenient daily dosage
regimen, which can be adjusted according to the degree of affliction.
Compositions can take
the form of tablets, pills, capsules, semisolids, powders, sustained release
formulations,
solutions, suspensions, elixirs, aerosols, or any other appropriate
compositions.
The choice of formulation depends on various factors such as the mode of drug
administration (e.g., for oral administration, formulations in the form of
tablets, pills or
capsules are preferred) and the bioavailability of the drug substance.
Recently, pharmaceutical
formulations have been developed especially for drugs that show poor
bioavailability based
upon the principle that bioavailability can be increased by increasing the
surface area i.e.,
decreasing particle size. For example, U.S. Pat. No. 4,107,288 describes a
pharmaceutical
formulation having particles in the size range from 10 to 1,000 nm in which
the active material
is supported on a crosslinked matrix of macromolecules. U.S. Pat. No.
5,145,684 describes the
production of a pharmaceutical formulation in which the drug substance is
pulverized to
nanoparticles (average particle size of 400 nm) in the presence of a surface
modifier and then
dispersed in a liquid medium to give a pharmaceutical formulation that
exhibits remarkably
high bioavailability.
31
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
The compositions are comprised of in general, a compound of formula (I) in
combination with at least one pharmaceutically acceptable excipient.
Acceptable excipients are
non-toxic, aid administration, and do not adversely affect the therapeutic
benefit of the
compound of formula (I). Such excipient may be any solid, liquid, semi-solid
or, in the case of
an aerosol composition, gaseous excipient that is generally available to one
of skill in the art.
Solid pharmaceutical excipients include starch, cellulose, talc, glucose,
lactose, sucrose,
gelatin, malt, rice, flour, chalk, silica gel, magnesium stearate, sodium
stearate, glycerol
monostearate, sodium chloride, dried skim milk and the like. Liquid and
semisolid excipients
may be selected from glycerol, propylene glycol, water, ethanol and various
oils, including
those of petroleum, animal, vegetable or synthetic origin, e.g., peanut oil,
soybean oil, mineral
oil, sesame oil, etc. Preferred liquid carriers, particularly for injectable
solutions, include water,
saline, aqueous dextrose, and glycols.
Compressed gases may be used to disperse a compound of this invention in
aerosol
form. Inert gases suitable for this purpose are nitrogen, carbon dioxide, etc.
Other suitable pharmaceutical excipients and their formulations are described
in
Remington's Pharmaceutical Sciences, edited by E. W. Martin (Mack Publishing
Company,
18th ed., 1990).
The level of the compound in a formulation can vary within the full range
employed by
those skilled in the art. Typically, the formulation will contain, on a weight
percent (wt %)
basis, from about 0.01-99.99 wt % of a compound of formula (I) based on the
total
formulation, with the balance being one or more suitable pharmaceutical
excipients.
Preferably, the compound is present at a level of about 1-80 wt %.
Examples
The following preparations of compounds of Formula (I) and intermediates
(References) are given to enable those skilled in the art to more clearly
understand and to
practice the present invention. They should not be considered as limiting the
scope of the
invention, but merely as being illustrative and representative thereof.
Reference A
Synthesis of 4-(2-methylpyridin-4-yl)benzenamine
NH2
N
32
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
To a solution of 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzenamine
(11.7 g,
53.5 mmol), 4-bromo-2-methylpyridine (9.20 g, 53.5 mmol), Na2CO3 (2.83 g, 26.7
mmol) in
50 mL MeCN/ 50 mL H2O, was added Pd(PPh3)4 (1.85 g, 1.60 mmol), and the
reaction
mixture was refluxed at 130 C for 15 h. The solution was cooled, and the
solids were
collected by filtration, and the residue was concentrated and extracted with
ethyl acetate. Ethyl
acetate solution was evaporated and the residue was combined with the solids
obtained from
filtration. The crude materials were subjected to silica gel chromatography
with 25% EtOAc in
CH2C12 gave 4-(2-methylpyridin-4-yl)benzenamine.
Reference 2
Synthesis of 3-methoxy-4-(2-methylpyridin-4-yl)benzenamine
__O NH2
N
Step 1
4-Chloro-2-picoline (3g, 0.0235mo1) was dissolved in 1,4-dioxane (72 mL). Bis
pinacolato diborane (7.76 g, 0.03 mol) was added to the reaction mixture. The
reaction mixture
was degassed with nitrogen for 45 mins. Potassium acetate (3.45 g, 0.035 mol),
tricyclohexylphosphine (0.660 g, 2.3 mmol) and Pd(dba)2 (0.676 g, 1.1 mmol)
were added. The
mixture was heated at 90 C for 3 h. The crude reaction mixture was cooled and
filtered
through Celite bed and washed with ethyl acetate. The filtrate was
concentrated and used
directly for next reaction without purification.
Step2
3-Methoxy-4-bromo nitrobenzene (4.9 g, 21 mmol) was dissolved in 1,2-
dimethoxyethane (60 mLl). 2-Methyl-4-(4,4,5,5-tetramethyl-[ 1,3,2]
dioxaborolan-2-yl)-
pyridine (5.15 g, 23.5 mmol) was added. Sodium carbonate (5.15 g, 48.5 mmol)
was
dissolved in minimum amount of water and was added drop wise to the reaction
mixture. The
reaction mixture was degassed with nitrogen for 45 min. Pd(PPh3)4 (0.732 g,
0.6 mmol) was
added under nitrogen. The reaction mixture was then heated overnight at 90 C.
After
completion of reaction, the reaction mixture was cooled and extracted with
ethyl acetate.
Purification was done by column chromatography using silica gel (100-200mesh)
and 0-2%
DCM, MeOH as eluent to give 4-(2-methoxy-4-nitrophenyl)-2-methylpyridine.
33
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
Step 3
To a stirred solution of 4-(2-methoxy-4-nitrophenyl)-2-methylpyridine (15.8 g,
64.7
mmol) in 200 mL of ethanol was degassed with nitrogen and added Pd/C (6.89 g,
6.47 mmol).
The reaction mixture was hydrogenated using hydrogen balloon at 25 C. The
reaction mixture
was filtered through celite, concentrated, and purified by crystallization
using dichloromethane
and pentane to give the title compound.
Reference 3
Synthesis of 4-nitrophenyl 4-(2-methylpyridin-4-yl)phenylcarbamate
N O
/ I \ IOI N,O
N O
H
To a solution of 4-nitrophenyl carbonochloridate (4.38 g, 21.7 mmol), 1.2 eq
of the
polystyrene bound Hunig base, 3.6 mmol/G, Agonaut, 2.3 g) in 2:1 EtOAc/CH2C12,
was added
4-(2-methylpyridin-4-yl)benzenamine (2.00 g, 10.9 mmol) in portions, and the
reaction
mixture was stirred at RT for 48 h. The impurities were removed by filtration,
and the solids
(containing product and the resin) was suspended (the product was dissolved)
in 2:1
MeOH/CH2C12. The product solution (soluble in MeOH/CH2C12, not soluble in
EtOAc) was
filtered through medium glass-fritted funnel and concentrated to give 4-
nitrophenyl 4-(2-
methylpyridin-4-yl)phenylcarbamate.
Reference 4
Synthesis of azido(4-(2-methylpyridin-4-yl)phenyl)methanone
O
N3
Y
N,Y
Step 1
A solution of 4-bromo-2-methylpyridine (7.7 g, 45 mmol), 4-(methoxycarbonyl)-
phenylboronic acid (10.5 g, 58 mmol) in DME (100 mL) were added Cs2CO3 (22 g)
and 0.5 g
POPd (dihydrogen dichlorobis(di-tert-butylphosphinito-kP)palladate). The
reaction mixture
was heated to reflux after 4 h. The solution was concentrated, diluted with
ethyl acetate,
34
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
filtered and washed with ethyl acetate. The filtrate was washed with brine,
dried and
evaporated. Column chromatography (silica gel, 20-60% E/H) gave methyl 4-(2-
methylpyridin-4-yl)benzoate.
Step 2
Methyl 4-(2-methylpyridin-4-yl)benzoate (8.3 g, 37 mmol) in 5 N HC1(150 mL)
was
heated at 100 C for 11 h. The reaction mixture was evaporated and dried to
give 4-(2-
methylpyridin-4-yl)benzoic acid hydrochloride (8.8 g) as a white solid.
Step 3
A mixture of 4-(2-methylpyridin-4-yl)benzoic acid hydrochloride (1 g, 4 mmol)
in dry
THE (15 mL) was cooled to 0 C with stirring. TEA (1 mL, 8 mmol) was added and
the
reaction mixture was stirred for 20 min. Ethyl chloroformate (0.4 mL, 4 mmol)
was added and
the solution was stirred for 30 min. A solution of sodium azide (0.3 g, 4
mmol) in H2O (1 mL)
was added and the reaction mixture was allowed to warm to room temperature.
After 2 h, 3
mL of H2O was added and the mixture was extracted with EtOAc, dried (MgSO4)
and
concentrated in vacuo to give the title compound as a light-yellow solid.
Following the procedure described above and using appropriate starting
materials
azido(3-fluoro-4-(2-methylpyridin-4-yl)phenyl)methanone, azido(3-methyl-4-(2-
methyl-
pyridin-4-yl)phenyl)methanone, azido(3-methoxy-4-(2-methylpyridin-4-
yl)phenyl)methanone,
azido(1-phenylcyclohexyl)methanone, azido(4-(2-fluorophenyl)-tetrahydro-2H-
pyran-4-
yl)methanone, and tert-butyl 4-(azidocarbonyl)-4-(2-fluorophenyl)piperidine-1-
carboxylate,
azido(1-phenylcyclopentyl)methanone, azido(1-phenylcyclopropyl)methanone were
synthesized.
Reference 5
Synthesis of 6-(2-methylpyridin-4-yl)pyridin-3-amine
NH2
N
N
A mixture of potassium phosphate hydrate (4.5 g, 20 mmol), 2-methylpyridin-4-
ylboronic acid (1.8 g, 13 mmol), 6-bromopyridin-3-amine (1.50 g, 8.7 mmol) in
dioxane (50
mL) and water (7 mL) was purged with nitrogen and then bis (di-t-
butylphenylphosphine)-
dichloropalladium catalyst (0.27 g, 0.43 mmol) ( see Organic letters, 8(9),
1787-1789 (2006)
for the catalyst) was added. The reaction mixture was heated to 100 C for 4
h. HPLC-MS
showed the product (80%) and the bromide (20%). The reaction was stirred
overnight. HPLC-
MS showed no more bromide left. The reaction mixture was diluted with EtOAc
(200 mL),
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
washed with saturated Na2CO3, and brine. The organic phase was dried over
Na2SO4, filtered
and concentrated in vacuo. Purification by flash column chromatography (silica
gel, 5-10%
MeOH-CH2C12) afforded the title compound as orange oil, which turned to solid
upon
standing. It was triturated with EtOAc-Hexane to give an orange solid by
filtration. MS: 186
(M+1).
Example 1
Synthesis of (R)-1-(4-(2-methylpyridin-4-yl)phenyl)-3-(1-(naphthalen-2-
yl)ethyl)urea
H H
/ N` /N \ /
\ \ I 0
N /
A 2 mL microwave synthesizer vessel containing a solution of azido(4-(2-
methylpyridin-4-yl)phenyl)methanone (0.187 g, 0.78 mmol) in THE (1.2 mL) was
subjected to
microwave irradiation at 120 C for 15 min. S(-)-1-(2-Naphthyl)ethylamine
(0.13 g, 0.78
mmol) and N,N-diisopropylethylamine (0.14 mL, 0.78 mmol) were added and the
reaction
mixture was subjected to microwave irradiation at 120 C for an additional 15
min. The
reaction mixture was transferred to a scintillation vial, washed with methanol
(5 mL). The
solvent was removed in vacuo and the residue was purified by preparative HPLC
[gradient 10-
90 % MeCN (0.1 % TFA)/ H2O (0.1 % TFA)] to give the pure product which was
dissolved in
methanol (5 mL) and neutralized by passing the solution through a Polymer Lab-
HCO3
macroporous resin cartridge, and the filtrate was concentrated to give (R)-1-
(4-(2-
methylpyridin-4-yl)phenyl)-3-(1-(naphthalen-2-yl)ethyl)urea as a light-yellow
solid. MS (ESI
pos. ion) m/z: 382 (M+1).
Proceeding as described in Example 1 above, but substituting S(-)-1-(2-
naphthyl)-
ethylamine with other commercially available amine, the following compounds
were
synthesized.
36
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
MS: ESI
Ex Structure Name m/z
M+1
H H
2 N N,,,
1-(4-(2-methyl-4-pyridinyl)phenyl)-3-
0 382
((1S)-1-(2-naphthalenyl)ethyl)urea
710~
N H H 3 1-((1 S)-2-(4-fluorophenyl)-1-
\ F methylethyl)-3-(4-(2-methyl-4- 364
N ,,r pyridinyl)phenyl)urea
\
NuN /
4 O F 1-((1S)-1-(2-fluorophenyl)ethyl)-3- 350
\ (4-(2-methyl-4-pyridinyl)phenyl)urea
N /
N N
0 1-(4-(2-methyl-4-pyridinyl)phenyl)-3- 368
(2-naphthalenylmethyl)urea
N
H H y 1-((4R/S)-3,3-dimethyl-3,4-dihydro-
6 0 0 2H-chromen-4-yl)-3-(4-(2-methyl-4-
388
NI / I / pyridinyl)phenyl)urea
37
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
Example 7
Synthesis of 1-(1-(3-fluorophenyl)ethyl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea
H H
/ NyN F
N /
A 2 mL microwave synthesizer vessel containing a solution of azido(4-(2-
methylpyridin-4-yl)phenyl)methanone (0.205 g, 0.860 mmol) in THE (2.0 mL) was
subjected
to a microwave irradiation at 120 C for 15 min. 1-(3-ffluorophenyl)ethanamine
(0.270 g, 1.94
mmol) and N,N-diisopropylethylamine (0.300 mL, 1.72 mmol) were added and the
reaction
mixture was stirred at room temperature for 12 h. The reaction mixture was
transferred to a
scintillation vial, washed with methanol (5 mL). The solvent was removed in
vacuo and the
residue was purified by preparative HPLC [gradient 10-90 % MeCN (0.1 % TFA)/
H2O (0.1 %
TFA)] to give the pure product which was dissolved in methanol (5 mL) and
neutralized by
passing the solution through a Polymer Lab-HCO3 macroporous resin cartridge,
and the filtrate
was concentrated to give 1-(1-(3-fluorophenyl)ethyl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea
as a colorless oil. MS (ESI pos. ion) m/z: 350 (M+1).
Proceeding as described in Example 7 above but using azido(4-(2-methylpyridin-
4-
yl)phenyl)methanone and the corresponding commercially available amine, the
following
compounds were prepared.
MS: ESI
Ex. Structure Name m/z
M+1
N N
8 o 1-(2-(4-bromophenyl)ethyl)-3-(4-
N Br (2-methyl-4-pyridinyl)phenyl)urea 410
i
H H
9 N\ /N 1-((1R)-1-(2-
ooI o' methoxyphenyl)ethyl)-3-(4-(2- 362
methyl-4-pyridinyl)phenyl)urea
N /
38
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
N y N
1-(4-(2-methyl-4-pyridinyl)phenyl)- 360
\ \ 3-((1R)-1-phenylbutyl)urea
N /
/ Br
11 NyN 1-((1 S)-1-(4-bromophenyl)ethyl)-3-
I0I (4-(2-methyl-4- 410
NI pyridinyl)phenyl)urea
N H
pi 12 1-(1-(3-methoxyphenyl)ethyl)-3-(4-
(2-methyl-4-pyridinyl)phenyl)urea 362
N /
H H
13 Y o '0
1 cyclopentyl 3 (4 (2 methyl 4 296
NI / pyridinyl)phenyl)urea
H H
14 N N
y 1-(4-(2-methylpyridin-4-yl)phenyl)- 389
3-(3-morpholinophenyl)urea
N /
H H
N N
1-(4-(2-methylpyridin-4-yl)phenyl)- 346
3-(1-p-tolylethyl)urea
N
/ CI
H H I
N N \
16 1-(1-(4-chlorophenyl)ethyl)-3-(4- 366
(2-methylpyridin-4-yl)phenyl)urea
N /
H H
NYN I \ \
17 \ \ o N 1 (4 (2 methylpyridin 4 yl)phenyl) 355
3-(quinolin-6-yl)urea
N /
H H
/ N N \ CI
18 Y 1-(1-(3-chlorophenyl)ethyl)-3-(4- 366
(2-methylpyridin-4-yl)phenyl)urea
N /
39
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
H H
N uN \ Br
1-(1-(3-bromophenyl)ethyl)-3-(4-
19 410
(2-methylpyridin-4-yl)phenyl)urea
N
H H
/ N N \
20 Y Br 1-(1-(2-bromophenyl)ethyl)-3-(4- 410
(2-methylpyridin-4-yl)phenyl)urea
N
N
H H
/ N Y N \
\ I \ 1-(4-(2-methylpyridin-4-yl)phenyl)-
21 o 369
3-(quinolin-4-ylmethyl)urea
N
H H
/ NYN ~N /
(R)-1-(4-(2-methyl pyrid i n-4-
22 \ \ I 0 yl)phenyl)-3-(1-(quinolin-2- 383
NI / yl)ethyl)urea
H H
NyN ~N
(S)-1-(4-(2-methyl pyrid i n-4-
23 \ \ I 0 = yl)phenyl)-3-(1-(quinolin-2- 383
NI yl)ethyl)urea
\
H H
NyN \ / (R)-1-(4-(2-methylpyridin-4-
N
24 o yl)phenyl)-3-(1-(quinolin-3- 383
yl)ethyl)urea
N
i
H H~
/ NYN (S)-1-(4-(2-methylpyridin-4-
25 \ o yl)phenyl)-3-(1-(quinolin-3- 383
N yl)ethyl)urea
H H
1-(4-(2-methylpyridin-4-yl)phenyl)-
26 0 369
3-(quinolin-2-ylmethyl)urea
N
H H
/ NyN \
(R)-1-(1-(2-fluorophenyl)ethyl)-3-
27 \ \ I 0 F (4-(2-methylpyridin-4- 350
yl)phenyl)urea
N
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
H H
/ N~N
(S) 1 (1 (2 fluorophenyl)ethyl) 3
28 I 0 = F (4-(2-methylpyridin-4- 350
yl)phenyl)urea
N
H H F
/ NuN
29 0 1-(5-bromo-2-fluorophenyl)-3-(4- 400
(2-methylpyridin-4-yl)phenyl)urea
N / Br
NYN
30 0 / O 1-(1-(furan-2-yl)ethyl)-3-(4-(2- 322
methylpyridin-4-yl)phenyl)urea
N
H N
31 N I O 0 1-(1-(benzofuran-2-yl)ethyl)-3-(4- 372
N / (2-methylpyridin-4-yl)phenyl)urea
N H
or o F 1-(1-(1-(difluoromethyl)-1H-
32 l y N, N~F benzo[d]imidazol-2-yl)ethyl)-3-(4- 422
N ,r (2-methylpyridin-4-yl)phenyl)urea
H H
N~N N 1-(1-(1-ethyl-3,5-dimethyl-1H-
33 pyrazol-4-yl)ethyl)-3-(4-(2- 378
0 methylpyridin-4-yl)phenyl)urea
N
N Y N
34 O - O 1-(4-(2-methylpyridin-4-yl)phenyl)- 312
3-(tetra hyd ro-2H-pyra n-3-yl )urea
N
H
Y H
II 1-((3-methylisoxazol-5-yl)methyl)-
35 0 O 3-(4-(2-methylpyridin-4- 323
N -N yl)phenyl)urea
H H
Ny N
36 1 -cyclohexyl-3-(4-(2-methyl-4- 310
NI pyrid i nyl)phenyl)u rea
41
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
Nu H
lol (R)-1-(3,3-dimethyltetrahydro-2H-
37 pyran-4-yl)-3-(4-(2-methylpyridin- 340
4-yl)phenyl)urea
H H OMe
N N 1-((1-(2-
38 o / methoxyphenyl)cyclobutyl)methyl)- 402
3-(4-(2-methylpyridin-4-
N i yl)phenyl)urea
H H
NYN
1-(4-(2-methylpyridin-4-yl)phenyl)-
39 0 298
3-(tetra hyd rofuran-3-yl)u rea
N
N N
1-(2-methyl-5-(1-
40 I )Crl
methylethyl)phenyl)-3-(4-(2- 360
N I methyl-4-pyridinyl)phenyl)urea
H H
NuN 1-(4-(2-methylpyridin-4-yl)phenyl)-
41 O CF3 3-(2,2,2-trifluoro-1- 386
N phenylethyl)urea
H H
N N ~ I
42 o Cl 1-(1-(2-chlorophenyl)ethyl)-3-(4- 366
(2-methylpyridin-4-yl)phenyl)urea
N
H H
N N p 43 0 1-(4-(2-methylpyridin-4-yl)phenyl)- 346
3-(1-o-tolylethyl)urea
N /
F
H H
"y" \N 1-(1-(5-fluoropyridin-2-yl)ethyl)-3-
44 o (4-(2-methylpyridin-4- 351
NI yl)phenyl)urea
c
H H
NY" \N 1-(1-(5-chloropyridin-2-yl)ethyl)-3-
45 o (4-(2-methylpyridin-4- 367
NI yl)phenyl)urea
42
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
0
H H I \
NyN / \ N 1-(1-(6-methoxypyridin-3-yl)ethyl)-
46 0 3-(4-(2-methylpyridin-4- 363
N yl)phenyl)urea
H H
N N
\ \ I y
47 I / 1-(3-fluorophenyl)-3-(4-(2-methyl- 322
N F 4-pyridinyl)phenyl)urea
N N \ 1-(1-(3-
48 Y (difluoromethoxy)phenyl)ethyl)-3- 398
F F (4-(2-methylpyridin-4-
N ? yl)phenyl)urea
H H N/ \
N N \ /
49 1 1-(1-(isoquinolin-3-yl)ethyl)-3-(4-
383
(2-methylpyridin-4-yl)phenyl)urea
N
H H
N N \ N
50 1 (4 (2 methylpyridin 4 yl)phenyl) 383
3-(1-(quinolin-7-yl)ethyl)urea
N
H H
Y N \
51 0 1-(2-fluoro-1-phenylethyl)-3-(4-(2- 350
\ F methylpyridin-4-yl)phenyl)urea
N /
H H
/ N N \
52 Y 1-(2-methoxy-1-phenylethyl)-3-(4- 362
0 (2-methylpyridin-4-yl)phenyl)urea
N
H H
NYN 1-(2-fluoro-2-methyl-1-
53 0 F phenylpropyl)-3-(4-(2- 378
NI / methylpyridin-4-yl)phenyl)urea
ci ci
NuN 1-(1-(2,4-dichlorophenyl)ethyl)-3-
54 II (4-(2-methylpyridin-4- 400
/ I 0 yl)phenyl)urea
y
43
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
/ OCH3
H H
55 N"N \ 1-(1-(4-methoxyphenyl)ethyl)-3-(4- 362
II0 (2-methylpyridin-4-yl)phenyl)urea
N,
N N / (S)-1-(3-methyl-2-oxo-2,3,4,5-
56 / tetrahydro-1 H-benzo[d]azepin-1- 401
o o yl)-3-(4-(2-methylpyridin-4-
NI / yl)phenyl)urea
Br
NY N
57 \ I 0 1-(2-bromophenyl)-3-(4-(2-methyl- 382
4-pyridinyl)phenyl)urea
N \ H H
QI I 1-(3'-fluoro-3-biphenylyl)-3-(4-(2-
58 N, ,r / methyl-4-pyridinyl)phenyl)urea 398
\I
F
1~ O
59 N N 1-(4'-methoxy-2-biphenylyl)-3-(4- 410
(2-methyl-4-pyrid i nyl )phe nyl)u rea
y
I
N ,,r
H N N
/ I y
60 0 1-(3-(2-furanyl)phenyl)-3-(4-(2- 370
methyl-4-pyridinyl)phenyl)urea
O
H H
61 NI NO 1-(3-butoxyphenyl)-3-(4-(2-methyl- 376
4-pyridinyl)phenyl)urea
H N
62 1-(3-methylphenyl)-3-(4-(2-methyl- 318
NI / 4-pyridinyl)phenyl)urea
44
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
63 ~ I0I 1-(3'-methoxy-3-biphenylyl)-3-(4- 410
(2-methyl-4-pyridinyl)phenyl)urea
N /
Sr
64 / N` /N 1-(4-(2-methyl-4-pyridinyl)phenyl)- 378
~I0I{ 3-(2-(propylsulfanyl)phenyl)urea
N /
S
65 l0 / 1-(4-(2-methyl-4-pyridinyl)phenyl)- 386
3-(3-(3-thiophenyl)phenyl)urea
N / I
N N
66 I \ \ \ 1-(4-(2-methyl-4-pyridinyl)phenyl)-
381
N / \ 3-(3-(4-pyridinyl)phenyl)urea
I/
N
Nu H "
lol 1-(5-cyano-2-fluorophenyl)-3-(4-
67
NI F (2-meth yl-4-pyridinyl)phenyl)urea 347
N
N H
~ N
0 I
I 1-(5-cyano-2-methylphenyl)-3-(4-
68 (2-methyl-4-pyridinyl)phenyl)urea 343
s
H N N
69 u II 1-(4-(2-methyl-4-pyridinyl)phenyl)- 386
o 3-(2-(2-thiophenyl)phenyl)urea
N /
N
I~
N N /
70 I o I 1-(4-(2-methyl-4-pyridinyl)phenyl)- 381
3-(3-(3-pyridinyl)phenyl)urea
N /
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
H N N
/ I y I p
71 / 1-(4-(2-methyl-4-pyridinyl)phenyl)- 372
3-(3-(trifluoromethyl)phenyl)urea
N / CF3
F
H
H
N y N
1-(5-chloro-2-fluorophenyl)-3-(4-
72 356
(2-methyl-4-pyridinyl)phenyl)urea
N / CI
/ Nu N /
73 I l01 I 1-(2-(1-methylpropyl)phenyl)-3-(4- 360
(2-methyl-4-pyridinyl)phenyl)urea
N /
H N
74 0 / 1-(2-methoxyphenyl)-3-(4-(2- 334
NI / I methyl-4-pyridinyl)phenyl)urea
H H y 75 I / 1-(3-(methoxymethyl)phenyl)-3-(4- 348
NI / (2-methyl-4-pyridinyl)phenyl)urea
0
H N N
/ y
76 0 1-(4-(2-methyl-4-pyridinyl)phenyl)-
386
NI / 3-(3-(2-thiophenyl)phenyl)urea
N H
CF3
0 1-(4-(2-methyl-4-pyridinyl)phenyl)-
77 3-(2-methyl-5- 386
N (trifluoromethyl)phenyl)urea
H H
l01 I / 1-(4-(2-methyl-4-pyridinyl)phenyl)-
78 NI / 0 3-(3-propoxyphenyl)urea 362
46
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
o f
H H
79 " N 1-(4-(2-methyl-4-pyridinyl)phenyl)- 362
OI 3-(2-propoxyphenyl)urea
N
N N
1-(5-tert-butyl-2-methoxyphenyl)-
80 o 3-(4-(2-methyl-4- 390
NI / pyridinyl)phenyl)urea
Nu N
81 \ \ I Icl I / 1-(3-tert-butylphenyl)-3-(4-(2- 360
methyl-4-pyridinyl)phenyl)urea
N /
N
83 / N(N 1-(4-(2-methyl-4-pyridinyl)phenyl)- 381
\ o 3-(2-(3-pyridinyl)phenyl)urea
N /
Nu N
84 Icl \ 1-(2-(1-methylethyl)phenyl)-3-(4- 346
\ (2-methyl-4-pyridinyl)phenyl)urea
N /
N H
Br
85 1-(5-bromo-2-methylphenyl)-3-(4- 396
(2-methyl-4-pyridinyl)phenyl)urea
N /
N N
H y
86 o 1-(4-(2-methyl-4-pyridinyl)phenyl)- 346
NI / 3-(2-propylphenyl)urea
Nu N
87 \ \ Icl 1-(5-chloro-2-methylphenyl)-3-(4- 352
(2-methyl-4-pyridinyl)phenyl)urea
N / CI
47
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
H H
N N
/
88 \ \ 1-(3-ethoxyphenyl)-3-(4-(2-methyl- 348
NI / 4-pyridinyl)phenyl)urea
Nu N CF3
I I / 1-(2-fluoro-5-
89 (trifluoromethyl)phenyl)-3-(4-(2- 390
N / methyl-4-pyridinyl)phenyl)urea
H H
90 1-(2-ethylphenyl)-3-(4-(2-methyl-4-
332
NI / pyridinyl)phenyl)urea
H H
N y N
I
91 / 1-(4-(2-methyl-4-pyridinyl)phenyl)- 350
NI / s 3-(3-(methylsulfanyl)phenyl)urea
N~ H
F
92 \ I I F 1-(2,5-difluorophenyl)-3-(4-(2- 340
NI / methyl-4-pyridinyl)phenyl)urea
CN
N N
93 \ \ lol 1-(5-chloro-2-cyanophenyl)-3-(4- 363
(2-methyl-4-pyridinyl)phenyl)urea
N CI
H H
N N
y
94 1-(2-ethoxyphenyl)-3-(4-(2-methyl-
348
NI / 4-pyridinyl)phenyl)urea
F
H H"p
95 \ I CI I / 1-(4'-fluoro-3-biphenylyl)-3-(4-(2- 398
methyl-4-pyridinyl)phenyl)urea
N /
0
H H
1 -(5-fl uoro-2-methoxyphe nyl)-3-(4-
96 I \ \ (2-methyl-4-pyridinyl)phenyl)urea 352
N / F
48
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
NuN CF3
II ~c 1 (2 methoxy 5
97 (trifluoromethyl)phenyl)-3-(4-(2- 402
N i I methyl-4-pyridinyl)phenyl)urea
H
H
N IuI N
1 4 2-meth I-4 ridin I hen I
( ( Y pY Y)p Y)-
98 NI 3-(3-(2-methyl-1,3-thiazol-4- 401
N yl)phenyl)urea
s~
99 / I N(N 1-(2-biphenylyl)-3-(4-(2-methyl-4- 380
\ 0 pyridinyl)phenyl)urea
N /
H H
0
\ \ I ll /
1-(2-cyanophenyl)-3-(4-(2-methyl-
100 NI N/ 4-pyridinyl)phenyl)urea 329
o
101 / N(N 1-(4-(2-methyl-4-pyridinyl)phenyl)- 408
0 3-(2-(phenylcarbonyl)phenyl)urea
I~
N /
H CF3 H
/ N IuI N
102 \ \ I 0 1-(4-(2-methyl-4-pyridinyl)phenyl)- 372
3-(2-(trifluoromethyl)phenyl)urea
N /
N N
IuI
\ \ I / 1-(5-fluoro-2-methylphenyl)-3-(4-
103 0 336
(2-methyl-4-pyridinyl)phenyl)urea
N / F
/ H H
u 1-(2-chloro-5-methoxyphenyl)-3-
I I
104 \ \ c, (4-(2-methyl-4- 368
NI / pyridinyl)phenyl)urea
49
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
N~ H
I I I Ci I / 1-(2-chloro-5-methylphenyl)-3-(4-
105 (2-methyl-4-pyridinyl)phenyl)urea 352
N /
o \
106 / N` /N 1-(4-(2-methyl-4-pyridinyl)phenyl)- 396
~I0I{ 3-(2-phenoxyphenyl)urea
N /
H H
107 \ \ y N
/ 1-(3-cyanophenyl)-3-(4-(2-methyl- 329
NI CN 4-pyridinyl)phenyl)urea
H H y 108 \ / 1-(3-acetylphenyl)-3-(4-(2-methyl- 346
NI / 4-pyridinyl)phenyl)urea
0
0
N N
IuI
\ / 1-1-(4-methoxybiphenyl-3-yl)-3-(4-
109 \ O 410
(2-methylpyridin-4-yl)phenyl)urea
N
N
110 NyN 1-(2-cyano-5-methylphenyl)-3-(4- 343
(2-methyl-4-pyridinyl)phenyl)urea
N
N N O
\ I I / IY 1-(3-(1-methylethoxy)phenyl)-3-(4-
111 (2-methyl-4-pyridinyl)phenyl)urea 362
N
H N N
Y \
112 I I s / 1-(4-(2-methyl-4-pyridinyl)phenyl)- 350
NI I 3-(2-(methylsulfanyl)phenyl)urea
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
H N N
- I y
113 1-(4-(2-methyl-4-pyridinyl)phenyl)- 408
N 3-(3-(phenylcarbonyl)phenyl)urea
N
H N /
y
O CI I / 1-(2-chloro-5-cyanophenyl)-3-(4- 363
114
NI (2-methyl-4-pyridinyl)phenyl)urea
N
_ ::,NyN
115 1-(4'-cyano-3-biphenylyl)-3-(4-(2- 405
methyl-4-pyridinyl)phenyl)urea
N /
H N H
y
116 F ::j 1-(2-fluoro-5-methylphenyl)-3-(4- 336
NI / (2-methyl-4-pyridinyl)phenyl)urea
N
y I
117 o / 1-(4-(2-methyl-4-pyridinyl)phenyl)- 396
N / o` 3-(3-phenoxyphenyl)urea
0
1-(2-methoxy-5-methyl phenyl)-3-
118 " 0 I i (4-(2-methyl-4- 348
pyridinyl)phenyl)urea
H H
CF3
1-(2-chloro-5-
119 cl (trifluoromethyl)phenyl)-3-(4-(2- 406
methyl-4-pyridinyl)phenyl)urea
H H
CI
120 _ CI 1-(2,5-dichlorophenyl)-3-(4-(2- 372
NI methyl-4-pyridinyl)phenyl)urea
N N
121 F 1-(2-fluorophenyl)-3-(4-(2-methyl- 322
DO
NI / 4-pyridinyl)phenyl)urea
51
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
N N
122 I I 1-(3-methoxyphenyl)-3-(4-(2- 334
NI o methyl-4-pyridinyl)phenyl)urea
I ,,r N N
123 \ I I 1-(3-ethylphenyl)-3-(4-(2-methyl-4- 332
ii / pyridinyl)phenyl)urea
N IuI N
X) 1-(2-methylphenyl)-3-(4-(2-methyl-
124 Ni 4-pyridinyl)phenyl)urea 318
Example 125
Synthesis of 1-(4-(2-methylpyridin-4-yl)phenyl)-3-((1R,2S)-2-
phenylcyclopropyl)urea
H H
/ N N''
\ \ I 0
N /
A 2 mL microwave synthesizer vessel containing a suspension of 4-(2-
methylpyridin-4-
yl)benzenamine (0.207 g, 1.12 mmol) in 1,2-dichloroethane (2.5 mL) was treated
with N,N-
diisopropylethylamine (0.260 mL, 1.49 mmol) followed by trans-2-
phenylcyclopropyl
isocyanate (0.170 mL, 1.15 mmol). The resulting suspension was stirred at room
temperature
for 16 h. The reaction mixture was transferred to a scintillation vial, washed
with methanol (5
mL). The solvent was removed in vacuo and the residue was purified by
preparative HPLC
[gradient 10-90 % MeCN (0.1 % TFA)/ H2O (0.1 % TFA)] to give the pure product
which was
dissolved in methanol (5 mL) and neutralized by passing the solution through a
Polymer Lab-
HCO3 macroporous resin cartridge, and the filtrate was concentrated to give 1-
(4-(2-
methylpyridin-4-yl)phenyl)-3-((1R,2S)-2-phenylcyclopropyl)urea as an amorphous
off-white
solid.. MS (ESI pos. ion) m/z: 344 (M+1).
Proceeding as described in Example 125 above, but using 4-(2-methylpyridin-4-
yl)benzenamine and the corresponding commercially available isocyanate, the
following
compounds were synthesized.
52
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
MS: ESI
Examples Structure Name m/z
M+1
126 NyN I 1-(4-(2-methyl-4-
0 pyridinyl)phenyl)-3-((1R)-1- 346
N phenylpropyl)urea
~I
127 NyN 1-(4-(2-methyl-4-
o pyridinyl)phenyl)-3-((1S)-1- 346
N phenyl propyl)urea
1,11
128 N y N 1-(2-(4-chlorophenyl)ethyl)-3-
0 CI (4-(2-methyl-4- 366
N pyridinyl)phenyl)urea
H H
129 NyN
o 1 (4 (2 methylpyridin 4 304
yl)phenyl)-3-phenylurea
N /
N N Oj\
130 p 1-(furan-2-ylmethyl)-3-(4-(2- 308
methylpyridin-4-yl)phenyl)urea
N /
H H CF3
1-(5-methyl-2-
131 o (trifluoromethyl)furan-3-yl)-3-(4- 376
(2-methylpyridin-4-
N yl)phenyl)urea
N H
N 1-(5-methyl-1 -phenyl-1H-
132 N pyrazol-4-yl)-3-(4-(2- 384
methylpyridin-4-yl)phenyl)urea
H H
NZ 1-(5-methyl-3-phenylisoxazol-
133 y I N 4-yl)-3-(4-(2-methylpyridin-4- 385
o yl)phenyl)urea
N
53
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
H H
N N 1-(3-methyl-5-phenylisoxazol-
134 / Y -10 4-yI)-3-(4-(2-methylpyridin-4- 385
0 N yl)phenyl)urea
N /
H H Y N 1-(3,5-dimethyl-1-phenyl-1H-
135 N pyrazol-4-yI)-3-(4-(2- 398
6 methylpyridin-4-yI)phenyl)urea
H H CI
/ N N
136 p 1 (2,6 dichlorophenyl) 3 (4 (2 372
CI methylpyridin-4-yI)phenyl)urea
N
H H
/ NyN 1-(3-chloro-4-methoxyphenyl)-
137 3-(4-(2-methyl-4- 368
N / cl pyridinyl)phenyl)urea
11 o
NyN i 1-(5-chloro-2-methoxyphenyl)-
138 o 3-(4-(2-methyl-4- 368
N ci pyridinyl)phenyl)urea
"1 o
Nu N 1-(5-chloro-2,4-
139 7,1~1 o o, dimethoxyphenyl)-3-(4-(2- 398
cl methyl-4-pyridinyl)phenyl)urea
H N /F
140 I o 1-(3,5-difluorophenyl)-3-(4-(2- 340
N F methyl-4-pyridinyl)phenyl)urea
Example 141
Synthesis of 1-(4-cyanophenethyl)-3-(4-(2-methylpyridin-4-yl)phenyl)urea
H H
N N
\ I O I /
\ CN
N /
54
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
A 2 mL microwave synthesizer vessel containing a suspension of 1-(4-bromo-
phenethyl)-3-(4-(2-methylpyridin-4-yl)phenyl)urea (0.356 g, 0.87 mmol), zinc
cyanide (0.266
g, 2.3 mmol), and tetrakis(triphenylphosphine)palladium (0.064 g, 0.055 mmol)
in N,N-
dimethylformamide (2.3 mL) was heated to 110 C for 15 min. The reaction
mixture was
filtered to remove the insoluble solids, washed with methanol (5 mL). The
combined filtrates
were transferred to a scintillation vial and the solvent was removed in vacuo.
The resulting
residue was purified by preparative HPLC [gradient 10-90 % MeCN (0.1 % TFA)/
H2O (0.1 %
TFA)] to give the pure product which was dissolved in methanol (5 mL) and
neutralized by
passing the solution through a Polymer Lab-HCO3 macroporous resin cartridge,
and the filtrate
was concentrated to give 1-(4-cyanophenethyl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea as an
amorphous yellow solid.. MS (ESI pos. ion) m/z: 357 (M+1).
Example 142
Synthesis of 1-(2-(4-bromothiophen-2-yl)propan-2-yl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea
H H
N N Br
N /
Step 1
To a solution of 2-(4-bromothiophen-2-yl)acetic acid (553 mg, 2.501 mmol) in 5
mL of
dry THE at 0 C was added lithium bis(trimethylsilyl)amide (1.OM solution in
tetrahydrofuran,
5.5 mL, 5.503 mmol). After stirring at 0 C for 15 minutes, iodomethane (0.155
ml, 2.501
mmol) was added and the cold bath was removed. The reaction was stirred at RT
for 16 h.
Added more Mel (0.078 mL) and stirred at room temperature for another 6 h. It
was quenched
with 2 N HC1 to pH - 3.0, extracted with EtOAc, dried over Na2SO4, filtered
and evaporated.
The crude product was chromatographed through a Redi-Sep pre-packed silica
gel column
(40 g), eluting with a gradient of 0% to 25% EtOAc in hexane, to provide 2-(4-
bromothiophen-
2-yl)propanoic acid as brown oil and was used without purification.
Step 2
A 50 mL, round-bottomed flask containing a solution of 2-(4-bromothiophen-2-
yl)-2-
methylpropanoic acid (92 mg, 0.369 mmol) in THE (2.5 mL) at 0 C was treated
with
triethylamine (0.154 ml, 1.108 mmol). The resulting mixture was stirred at 0
C for 10
minutes. Then, ethyl chloroformate (0.071 mL, 0.739 mmol) was added and the
mixture was
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
stirred at 0 C for an additional 60 minutes. Then, a solution of sodium azide
(72 mg, 1.108
mmol) in water (0.3 mL) was added and the mixture was stirred at room
temperature for 16
hours. Water (2 mL) was added and the mixture was extracted with EtOAc (2 x 30
mL). The
combined organic extracts were dried over Na2SO4, concentrated, and dried in
vacuum to give
2-(4-bromothiophen-2-yl)-2-methylpropanoyl azide as red oil and was used
without
purification.
Step 3
A solution of 2-(4-bromothiophen-2-yl)-2-methylpropanoyl azide (0.10 g, 0.36
mmol)
in THE (1 mL) was subjected to a microwave irradiation at 120 C for 15
minutes. Then, 4-(2-
methylpyridin-4-yl)benzenamine (0.074 g, 0.40 mmol) and n,n-
diisopropylethylamine (0.15
mL, 0.84 mmol) were added and the mixture was subjected to a microwave
irradiation at 120
C for an additional 15 minutes. The crude mixture was concentrated to dryness.
DCM (3 mL)
was added and the resulting precipitate was collected by vacuum filtration,
washed with DCM,
dried to give 1-(2-(4-bromothiophen-2-yl)propan-2-yl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea
as off-white solid. MS (ESI pos. ion) m/z: 430 (M+1).
Example 143
Synthesis of 1-(1-(2-bromophenyl)-3-methylbutyl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea
Br
H H
NYN
N 7,-l:rl
Step 1
To a solution of 2-bromophenylacetic acid (2.15 g, 10 mmol) (azeotroped with
toluene)
in dry toluene (2 mL) of dry toluene was added sodium
bis(trimethylsilyl)amide, (23 mL, 1.Om
solution in tetrahydrofuran). After stirring at room temperature for 20
minutes, 1-iodo-2-
methylpropane (1 mL, 11 mmol) was added dropwise. After 10 minutes, the
reaction was
quenched with 2 N HC1 to pH - 2.0, extracted with ethyl acetate, dried over
sodium sulfate,
filtered and evaporated to dryness. The crude product was purified by silica
gel flash column
chromatography (eluted using 10%-100% EtOAc/dichloromethane gradient) to give
the 2-(2-
bromophenyl)-4-methylpentanoic acid as colorless oil. MS (ESI, positive ion)
m/z: 271
(M+1).
Step 2
56
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
A 100 mL, round-bottomed flask containing a suspension of 2-(2-bromophenyl)-4-
methylpentanoic acid (0.315 g, 1.16 mmol) in THE (8 mL) at 0 C was treated
with
triethylamine (0.405 mL, 2.91 mmol). The resulting mixture was stirred at 0 C
for 10
minutes. Then, ethyl chloroformate (0.150 mL, 1.57 mmol) was added and the
mixture was
stirred at 0 C for an additional 60 minutes. Then, a solution of sodium azide
(0.250 g, 3.85
mmol) in water (0.8 mL) was added and the mixture was stirred at room
temperature for 16
hours. Then, H2O (25 mL) was added and the mixture was extracted with EtOAc (2
x 50 mL).
The combined organic extracts were dried over Na2SO4, concentrated, and dried
in vacuum to
give 2-(3-bromophenyl)propanoyl azide as an off-white solid.
Step 3
Proceeding as described in Example 1 above, but using 2-(3-
bromophenyl)propanoyl
azide and 4-(2-methylpyridin-4-yl)benzenamine gave the product 1-(1-(2-
bromophenyl)-3-
methylbutyl)-3-(4-(2-methylpyridin-4-yl)phenyl)urea as a light-yellow solid.
MS (ESI pos.
ion) m/z: 452 (M+1).
Example 144
Synthesis of 1-(4-(2-methylpyridin-4-yl)phenyl)-3-(1-(4-(pyridin-3-
yl)phenyl)ethyl)urea
N
H H
NYN
\ \ I 0
N /
Step 1
A 100 mL, round-bottomed flask containing a suspension of 4-(2-methylpyridin-4-
yl)benzenamine (0.622 g, 3.4 mmol) in 1,2-dichloroethane (15 mL) was treated
with n,n-
diisopropylethylamine (0.885 mL, 5.1 mmol) followed by treatment with (+/-)-1-
(4-
bromophenyl)ethyl isocyanate (0.95 g, 4.2 mmol), and the reaction mixture was
stirred at
room temperature. Formation of a very fine precipitate was observed and the
reaction was
allowed to stir for 12 hours. The solvent was removed from the reaction
mixture and the
resulting crude product was purified by preparative HPLC [gradient 10-90 %
MeCN (0.1 %
TFA)/ H2O (0.1 % TFA)] to give the pure product which was dissolved in
methanol (5 mL)
and neutralized by passing the solution through a Polymer Lab-HCO3 macroporous
resin
57
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
cartridge, and the filtrate was concentrated to give 1-(1-(4-
bromophenyl)ethyl)-3-(4-(2-
methylpyridin-4-yl)phenyl)urea as an off-white solid. MS (ESI pos. ion) m/z:
410 (M+1).
Step 2
A 2 mL microwave synthesizer vessel containing a suspension of 1-(1-(4-
bromophenyl)ethyl)-3-(4-(2-methylpyridin-4-yl)phenyl)urea (0.338 g, 0.82
mmol), pyridine-3-
boronic acid (0.233 g, 1.9 mmol), dichlorobis(di-tert-
butylphenylphosphine)palladium(II)
(0.015 g, 0.024 mmol) and potassium phosphate, tribasic (0.534 g, 2.5 mmol) in
1,4-dioxane /
water ( 3.6 mL, 5/1) was heated to 130 C for 30 minutes. The reaction mixture
was
concentrated in the genevac and the resulting residue was dissolved in
methanol / DMSO (9.5
mL, 1/5). The insoluble solids were removed by filtration and the filtrate was
concentrated
resulting in a residue which was purified by preparative HPLC [gradient 10-90
% MeCN (0.1
% TFA)/ H2O (0.1 % TFA)] to give the pure product which was dissolved in
methanol (5 mL)
and neutralized by passing the solution through a Polymer Lab-HCO3 macroporous
resin
cartridge, and the filtrate was concentrated to give 1-(4-(2-methylpyridin-4-
yl)phenyl)-3-(1-(4-
(pyridin-3-yl)phenyl)ethyl)urea as a brown solid. MS (ESI pos. ion) m/z: 409
(M+1).
Example 145
Synthesis of 1-(1-(3-cyanophenyl)ethyl)-3-(4-(2-methylpyridin-4-yl)phenyl)urea
H H
/ NyN \ CN
N /
Proceeding as described in Example 141 above, but using 1-(1-(3-
bromophenyl)ethyl)-
3-(4-(2-methylpyridin-4-yl)phenyl)urea and zinc cyanide gave the product 1-(1-
(3-
cyanophenyl)ethyl)-3-(4-(2-methylpyridin-4-yl)phenyl)urea as an off-white
solid. MS (ESI
pos. ion) m/z: 357 (M+1).
Example 146
Synthesis of 1-(1-(4-cyanophenyl)ethyl)-3-(4-(2-methylpyridin-4-yl)phenyl)urea
58
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
CN
H H
/ NyN
\ \ I
N /
Y'::
Proceeding as described in Example 141 above, but using 1-(1-(4-
bromophenyl)ethyl)-
3-(4-(2-methylpyridin-4-yl)phenyl)urea and zinc cyanide gave the product 1-(1-
(4-
cyanophenyl)ethyl)-3-(4-(2-methylpyridin-4-yl)phenyl)urea as an off-white
solid. MS (ESI
pos. ion) m/z: 357 (M+1).
Example 147
Synthesis of 1-(4-(2-methylpyridin-4-yl)phenyl)-3-(1-m-tolylethyl)urea
H H
/ NYN \
\ \ I O
N /
Step 1
To a solution of methyl 2-m-tolylacetate (5.0 g, 30 mmol) in 100 mL of THE was
added sodium hydride, 60% dispersion in mineral oil (0.91 mL, 37 mmol) in
portions. After
stirring at room temperature for 10 minutes, iodomethane (2.1 mL, 33 mmol) was
added and
the resulting mixture was stirred at room temperature for 12 hours. The
reaction was quenched
with brine, extracted with ethyl acetate, dried over sodium sulfate, filtered,
and evaporated to
dryness. The crude product was purified by silica gel flash column
chromatography (eluted
using 10%-100% EtOAc/Hexane gradient) to give the methyl 2-m-tolylpropanoate.
MS (ESI,
positive ion) m/z: 179 (M+1).
Step 2
To methyl 2-m-tolylpropanoate (1.33 g, 7.462 mmol) in MeOH/THF/water (3:3:1;
14
mL) was added lithium hydroxide monohydrate (1.096 g, 26.1 mmol). After
complete
consumption of starting material the solvent was evaporated under high vacuum.
The residue
was re-dissolved in water and the solution was adjusted to pH - 1.0, extracted
with
dichloromethane, dried over sodium sulfate, filtered through a silica-gel pad
and evaporated to
give 2-m-tolylpropanoic acid as a white solid. MS (ESI, positive ion) m/z: 165
(M+1).
59
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
Step 3
A 100 mL, round-bottomed flask containing a suspension of 2-m-tolylpropanoic
acid
(0.467 g, 2.8 mmol) in THE (10 mL) at 0 C was treated with triethylamine (1.2
ml, 8.6 mmol).
The resulting mixture was stirred at 0 C for 10 minutes. Then, ethyl
chloroformate (0.435 ml,
4.6 mmol) was added and the mixture was stirred at 0 C for an additional 60
minutes. Then, a
solution of sodium azide (0.590 g, 9.1 mmol) in water (3.0 mL) was added and
the mixture was
stirred at room temperature for 16 hours. Then, H2O (25 mL) was added and the
mixture was
extracted with EtOAc (2 x 50 mL). The combined organic extracts were dried
over Na2SO4,
concentrated, and dried in vacuum to give 2-m-tolylpropanoyl azide as reddish-
yellow oil.
Step 4
Proceeding as described in Example 7 above, but using 2-m-tolylpropanoyl azide
and
4-(2-methylpyridin-4-yl)benzenamine gave the product 1-(4-(2-methylpyridin-4-
yl)phenyl)-3-
(1-m-tolylethyl)urea as an off-white solid. MS (ESI pos. ion) m/z: 346 (M+1).
Example 148
Synthesis of 1-(4-(2-methylpyridin-4-yl)phenyl)-3-(1-(quinolin-6-yl)ethyl)urea
N
H H
/ N Y N \ \ I O
N /
Step 1
A mixture of 2-(4-aminophenyl)acetic acid (15.27 g, 101 mmol), Glycerol (37 g,
404
mmol), iron(II) sulfate heptahydrate (4.2 g, 15 mmol), nitrobenzene (7.3 mL,
71 mmol), and
sulfuric acid concentrate (17 mL, 202 mmol) was stirred at room temperature,
then was heated
to 130 C for 5hours. The mixture was concentrated in vacuo. The crude was
added ION
NaOH (90 mL). The mixture was filtered through celite. The filtrate was
acidified with
glacial acetic acid. The dark precipitate was collected by filtration and
washed with water.
The brown solid was added IN NaOH (150 mL) and carbon (8.5 g). The mixture was
stirred
overnight. The solid was filtered through celite. The filtrate was acidified
with glacial acetic
acid. The brown precipitate was collected by filtration and washed with water
and 2-(quinolin-
6-yl)acetic acid was obtained as a tan solid. MS (ESI pos. ion) m/z: 188
(M+1).
Step 2
To a solution of 2-(quinolin-6-yl)acetic acid (0.4 g, 2 mmol) in THE (10 mL),
Sodium
bis(trimethylsilyl) amide, 1M in THE (5 mL, 5 mmol) was added dropwise from a
syringe.
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
After 15 minutes at room temperature iodomethane, reagentplus, 99.5% stab with
copper
(0.175 mL, 3 mmol) was added. The mixture was stirred at room temperature for
another 4
hours. The reaction mixture was quenched after another hour at room
temperature, diluted
with IN HC1 solution, and extracted with ethyl acetate (75 mL). The aqueous
layer was
concentrated in the genevac and the resulting residue was taken up in THE (20
mL). The
undissolved solids were removed by filtration. The filtrate was concentrated
on the rotary
evaporator, resulting residue was dried under high vacuum, and obtained the
product 2-
(quinolin-6-yl)propanoic acid. MS (ESI pos. ion) m/z: 202 (M+1).
Step3
A 100 mL, round-bottomed flask containing a suspension of 2-(quinolin-6-
yl)propanoic
acid (0.330 g, 1.64 mmol) in THE (10 mL) at 0 C was treated with
triethylamine (0.600 mL,
4.31 mmol). The resulting mixture was stirred at 0 C for 10 minutes. Then,
ethyl
chloroformate (0.205 mL, 2.15 mmol) was added and the mixture was stirred at 0
C for an
additional 60 minutes. Then, a solution of sodium azide (0.320 g, 4.92 mmol)
in water (3.0
mL) was added and the mixture was stirred at room temperature for 16 hours.
Then, H2O (25
mL) was added and the mixture was extracted with EtOAc (2 x 50 mL). The
combined
organic extracts were dried over Na2SO4, concentrated, and dried in vacuum to
give 2-
(quinolin-6-yl)propanoyl azide as brown oil.
Step 4
Proceeding as described in Example 7 above, but using 2-(quinolin-6-
yl)propanoyl
azide and 4-(2-methylpyridin-4-yl)benzenamine gave the product 1-(4-(2-
methylpyridin-4-
yl)phenyl)-3-(1-(quinolin-6-yl)ethyl)urea as a tan solid. MS (ESI pos. ion)
m/z: 383 (M+1).
Example 149
Synthesis of 1-(1-(3-methoxyquinolin-6-yl)ethyl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea
N
H H
/ NyN
\ \ I O
N /
Step 1
To a stirring solution of tert-butyl 2-(3-methoxyquinolin-6-yl)acetate (0.60
g, 2.2
mmol) in THE (10 mL) at -78 C under nitrogen was added lithium
bis(trimethylsilyl) amide,
1M in THE (2.4 mL, 2.4 mmol). Then, iodomethane, reagentplus, 99.5% stab with
copper
61
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
(0.145 mL, 2.3 mmol) was added after 15 minutes of stirring. The cooling bath
was removed
after 40 minutes. During work up the reaction was quenched with saturated
ammonium
chloride (5 mL), and partitioned between ethyl acetate (75 mL) and 5% sodium
bicarbonate
(15 mL). The organic layer was dried over anhydrous sodium sulfate,
concentrated to yield
tert-butyl 2-(3-methoxyquinolin-6-yl)propanoate as a brown oil. MS (ESI pos.
ion) m/z: 288
(M+1).
Step 2
A 50 mL-round bottomed flask, containing a solution of tert-butyl 2-(3-
methoxyquinolin-6-yl)propanoate (0.63 g, 2 mmol) in dichloromethane (8 mL) was
treated
with trifluoroacetic acid (4 mL, 54 mmol) and the reaction mixture was stirred
at room
temperature for 6 hours. Then reaction mixture was concentrated to dryness in
the genevac,
dried under high vacuum to yield 2-(3-methoxyquinolin-6-yl)propanoic acid. MS
(ESI pos.
ion) m/z: 232.
Step 3
A 100 mL, round-bottomed flask containing a suspension of 2-(3-methoxyquinolin-
6-
yl)propanoic acid (0.220 g, 0.951 mmol) in THE (10 mL) at 0 C was treated
with
triethylamine (0.350 mL, 2.52 mmol). The resulting mixture was stirred at 0 C
for 10
minutes. Then, ethyl chloroformate (0.125 ml, 1.31 mmol) was added and the
mixture was
stirred at 0 C for an additional 60 minutes. Then, a solution of sodium azide
(0.185 g, 2.85
mmol) in water (2.0 mL) was added and the mixture was stirred at room
temperature for 16
hours. Then, H2O (25 mL) was added and the mixture was extracted with EtOAc (2
x 50 mL).
The combined organic extracts were dried over Na2SO4, concentrated, and dried
in vacuum to
give 2-(3-methoxyquinolin-6-yl)propanoyl azide as a brown oil.
Step 4
Proceeding as described in Example 7 above, but using 2-(3-methoxyquinolin-6-
yl)propanoyl azide and 4-(2-methylpyridin-4-yl)benzenamine gave the product 1-
(1 -(3 -
methoxyquinolin-6-yl)ethyl)-3-(4-(2-methylpyridin-4-yl)phenyl)ureaas an off-
white solid.
MS (ESI pos. ion) m/z: 413 (M+1).
Example 150
Synthesis of 1-(3-methyl-l-phenylbutyl)-3-(4-(2-methylpyridin-4-yl)phenyl)urea
62
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
H H
NYN /
\ \ I O
N /
Step 1
To a solution of 3-bromophenylacetic acid (3.00 g, 14 mmol) (azeotroped with
toluene)
in dry toluene (2 mL) was added sodium bis(trimethylsilyl)amide (1.OM solution
in
tetrahydrofuran, 32 mL, 32 mmol). After stirring at room temperature for 20
minutes, 1-iodo-
2-methylpropane (2 mL, 15 mmol) was added dropwise. After 10 minutes, the
reaction was
quenched with 2 N HC1 to pH - 2, extracted with ethyl acetate, dried over
sodium sulfate,
filtered, and evaporated to dryness. The crude product was purified by silica
gel flash column
chromatography (eluted using 10%-100% EtOAc/Hexane gradient) to give 2-(3-
bromophenyl)-
4-methylpentanoic acid. MS (ESI, positive ion) m/z: 271 (M+1).
Step 2
A 100 mL, round-bottomed flask containing a suspension of 2-(3-bromophenyl)-4-
methylpentanoic acid (1.185 g, 4.37 mmol) in THE (15 mL) at 0 C was treated
with
triethylamine (1.800 ml, 12.9 mmol). The resulting mixture was stirred at 0 C
for 10 minutes.
Then, ethyl chloroformate (0.600 ml, 6.28 mmol) was added and the mixture was
stirred at 0
C for an additional 60 minutes. Then, a solution of sodium azide (0.955 g,
14.7 mmol) in
water (3.5 mL) was added and the mixture was stirred at room temperature for
16 hours. Then,
H2O (25 mL) was added and the mixture was extracted with EtOAc (75 mL). The
combined
organic extracts were dried over Na2SO4, concentrated, and dried in vacuum to
give 2-(3-
bromophenyl)-4-methylpentanoyl azide as brown oil.
Step 3
Proceeding as described in Example 7 above, but using 2-(3-bromophenyl)-4-
methylpentanoyl azide and 4-(2-methylpyridin-4-yl)benzenamine gave the product
1-(1-(3-
bromophenyl)-3-methylbutyl)-3-(4-(2-methylpyridin-4-yl)phenyl)urea. MS (ESI
pos. ion) m/z:
452 (M+1).
Step 4
A solution of 1-(1-(3-bromophenyl)-3-methylbutyl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea (0.345 g, 0.763 mmol) in ethanol (10 mL) was stirred with
palladium
hydroxide, 20 wt % pd (dry basis) on carbon, wet, degussa type el01 ne/w
(0.165 g, 1.17
mmol) under hydrogen atmosphere (balloon) at room temperature for additional
17 hours at
63
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
room temperature. The catalyst was removed by filtration over a celite-pad,
washed with
ethanol (10 mL). The combined filtrates were concentrated to yield the crude
product which
was purified by preparative HPLC [gradient 10-90 % MeCN (0.1 % TFA)/ H2O (0.1
% TFA)]
to give the pure product which was dissolved in methanol (5 mL) and
neutralized by passing
the solution through a Polymer Lab-HCO3 macroporous resin cartridge, and the
filtrate was
concentrated to give 1-(3-methyl-l-phenylbutyl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea as an
off-white solid. MS (ESI pos. ion) m/z: 374 (M+1).
Example 151
Synthesis of 1-(2,2-difluoro-l-phenylethyl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea
H H
NYN
F F
N
Step 1
A mixture of 2,2-difluoro-l-phenylethanone (0.573 g, 3.7 mmol), ammonium
chloride
(0.402 g, 7.5 mmol), titanium (IV) isopropoxide (2.2 mL, 7.5 mmol), and
triethylamine (1.050
mL, 7.5 mmol) in absolute ethanol (9 mL) was stirred at room temperature
(formation of a
milky white precipitate was observed) for 8 hours. Then, sodium borohydride
(0.375 g, 9.9
mmol) was added and the resulting mixture was stirred for additional 12 hours.
The white
precipitate (Titanium Salts) was removed by filtration, washed with ethanol
(25 mL). The
combined filtrates were concentrated to yield a gummy white solid, which was
taken up in
ethyl acetate (75 mL) and water (30 mL). Formation of more white precipitate
(Titanium
Salts) was seen. The mixture was stirred vigorously for an hour. Celite was
added to this
mixture and the solids were removed by filtration, the filter cake was washed
with ethyl acetate
(35 mL). The filtrate was transferred to a separatory funnel, ethyl acetate
layer was separated,
dried over anhydrous sodium sulfate, and concentrated to give 2,2-difluoro-l-
phenylethanamine as a colorless oil. MS (ESI pos. ion) m/z: 158 (M+1).
Step 2
Proceeding as described in Example 7 above, but using 4-(2-methylpyridin-4-
yl)benzoyl azide and 2,2-difluoro-l-phenylethanamine gave the product 1-(2,2-
difluoro-l-
phenylethyl)-3-(4-(2-methylpyridin-4-yl)phenyl)urea as an off-white solid. MS
(ESI pos. ion)
m/z: 368 (M+1).
64
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
Example 152
Synthesis of 1-(1-(isoquinolin-6-yl)ethyl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea
N
H H
/ NyN
\ \ I O
N /
Step 1
To a stirring solution of tert-butyl 2-(isoquinolin-6-yl)acetate (0.830 g, 3.4
mmol) in
THE (7.OmL) at -75 C under nitrogen was added Lithium bis(trimethylsilyl)
amide, 1M in
THE (3.8 mL, 3.8 mmol). Then, iodomethane, 99%, stabilized with copper (0.23
mL, 3.7
mmol) was added after 15 minutes of stirring. The cooling bath was removed
after 40 minutes.
During work up the reaction was quenched with saturated ammonium chloride (5
mL), and
partitioned between ethyl acetate (75 mL) and 5% sodium bicarbonate (15 mL).
The organic
layer was dried over anhydrous sodium sulfate, concentrated to yield the crude
product which
was purified by silica gel flash column chromatography (eluted using 10%-100%
EtOAc/Hexane gradient) to give tert-butyl 2-(isoquinolin-6-yl)propanoate. MS
(ESI, positive
ion) m/z: 258 (M+1).
Step 2
A 50 mL-round bottomed flask, containing a solution of tert-butyl 2-
(isoquinolin-6-
yl)propanoate (0.95 g, 3.7 mmol) in dichloromethane (10 mL) was treated with
trifluoroacetic
acid (5.0 mL, 67 mmol) and the reaction mixture was stirred at room
temperature for 16 hours.
Then the reaction mixture was concentrated to dryness in the genevac, dried
under high
vacuum to yield 2-(isoquinolin-6-yl)propanoic acid. MS (ESI pos. ion) m/z:
202.
Step 3
A 100 mL, round-bottomed flask containing a suspension of 2-(isoquinolin-6-
yl)propanoic acid (0.690 g, 2.19 mmol) in THE (8 mL) at 0 C was treated with
triethylamine
(1.00 ml, 7.19 mmol). The resulting mixture was stirred at 0 C for 10
minutes. Then, ethyl
chloroformate (0.475 ml, 4.97 mmol) was added and the mixture was stirred at 0
C for an
additional 60 minutes. Then, a solution of sodium azide (0.460 g, 7.08 mmol)
in water (1.5
mL) was added and the mixture was stirred at room temperature for 16 hours.
Then, H2O (25
mL) was added and the mixture was extracted with EtOAc (75 mL). The combined
organic
extracts were dried over Na2SO4, concentrated, and dried in vacuum to give 2-
(isoquinolin-6-
yl)propanoyl azide as a brown oil.
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
Step 4
Proceeding as described in Example 7 above, but using 2-(isoquinolin-6-
yl)propanoyl
azide and 4-(2-methylpyridin-4-yl)benzenamine gave the product 1-(1-
(isoquinolin-6-yl)ethyl)-
3-(4-(2-methylpyridin-4-yl)phenyl)urea as light-yellow solid. MS (ESI pos.
ion) m/z: 383
(M+1).
Example 153
Synthesis of 1-(1-(3,5-bis(trifluoromethyl)phenyl)ethyl)-3-(4-(2-methylpyridin-
4-
yl)phenyl)urea
CF3
NuN Yd CF3
\ \ I IO
I
N /
A mixture of 4-(2-methylpyridin-4-yl)benzenamine (72 mg, 0.3 89 mmol), N,N'-
disuccinimidyl carbonate (149 g, 0.583 mmol) and DMF (2 mL) was stirred at
room
temperature overnight. Then 1-(3,5-bis(trifluoromethyl)phenyl)ethanamine (200
g, 0.778
mmol) and N-ethyl-N-isopropylpropan-2-amine (0.27 mL, 1.56 mmol) was added.
The
mixture was stirred overnight, diluted with Na2CO3 and extracted with EtOAc.
The organic
layer was dried over Na2SO4 and concentrated in-vacuo. The crude was purified
by silica gel
chromatography using EtOAc-CH2C12 as the eluant to yield 1-(1-(3,5-
bis(trifluoromethyl)-
phenyl)ethyl)-3-(4-(2-methylpyridin-4-yl)phenyl)urea as a white solid. MS (ESI
pos. ion) m/z:
468 (M+1)
Example 154
Synthesis of 1-(4-ethoxy-5-isopropyl-2-methylphenyl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea
H H
NY N
\ \ I o )O~ 0
N /
Step 1
To a solution of 4-(2-methylpyridin-4-yl)benzenamine (0.202 g, 1.10 mmol) in
CH2C12
(10 mL) was added 1,1'-carbonyldiimidazole (0.189 g, 1.17 mmol) as a solid at
rt. After 4 h, 4-
ethoxy-5-isopropyl-2-methyl-phenylamine hydrochloride (0.249 g, 1.08 mmol;
Matrix
66
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
Scientific) was added to the reaction followed by triethylamine (0.650 mL,
4.67 mmol). The
reaction was stirred overnight and then concentrated to dryness. The residue
was dissolved in
DMSO/MeOH and purified by reverse-phase HPLC (Gilson; XTerra Prep RP18 OBDTM,
19 x
150 mm column) eluting with 0.1%TFA-H20:0.1%TFA CH3CN (9:1 -* 1:9). The
fractions
containing the desired product were combined and concentrated in vacuo. The
residue was
dissolved in MeOH and loaded onto an SCX II cartridge eluting with MeOH then
2M NH3 in
MeOH to give 1-(4-ethoxy-5-isopropyl-2-methylphenyl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea as a white amorphous solid. MS (ESI pos. ion) m/z: 404 (M+1).
Example 155
Synthesis of 1-(4-(2-methylpyridin-4-yl)phenyl)-3-(4-phenylthiazol-2-yl)urea
H H
YN
\ I N"N S \ /
I \
N /
Step 1
To a solution of 2-amino-4-phenylthiazole (0.200 g, 1.13 mmol; Oakwood
Products) in
CH2C12 (10 mL) was added 1,1'-carbonyldiimidazole (0.193 g, 1.19 mmol) as a
solid at room
temperature. After 4 h, 4-(2-methylpyridin-4-yl)benzenamine (0.210 g, 1.14
mmol) was added
to the reaction followed by triethylamine (0.500 ml, 3.59 mmol). After
stirring overnight the
reaction mixture was filtered and the solid was washed with CH2C12 and dried
in vacuo to give
1-(4-(2-methylpyridin-4-yl)phenyl)-3-(4-phenylthiazol-2-yl)urea as a white
amorphous solid.
MS (ESI pos. ion) m/z: 387 (M+1).
Example 156
Synthesis of 1-(4-(2-methylpyridin-4-yl)phenyl)-3-(1-phenylcyclopropyl)urea
/
NuN \
I O
D,05~
N A solution of azido(1-phenylcyclopropyl)methanone (300 mg, 1.6 mmol) in THE
(1.5
mL) was subjected to microwave irradiation at 120 C for 10 min. 4-(2-
Methylpyridin-4-
yl)benzenamine (207 mg, 1.1 mmol) was added and the reaction mixture was
subjected to a
67
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
microwave irradiation at 120 C for 15 min. Formation of yellow precipitate
was observed.
The precipitate was collected by filtration, washed with MeOH, and dried in
vacuum to give
the title compound as a yellow solid. MS (ESI, positive ion) m/z: 344 (M+1).
Example 157
Synthesis of 1-(4-(2-cethylpyridin-4-yl)phenyl)-3-(1-phenylcyclobutyl)urea
NyN
\ \ I O
N
To a solution of azido(4-(2-methylpyridin-4-yl)phenyl)methanone (500 mg, 2.1
mmol)
in THE (3 mL) was subjected to a microwave irradiation at 120 C for 10 min.
N,N-
Diisopropylethylamine (0.548 mL, 3.148 mmol, Aldrich) and 1-
phenylcyclobutanamine
hydrochloride (385 mg, 2.1 mmol) were added and the reaction mixture was then
subjected to
a microwave irradiation at 120 C for 15 min. Solvent was removed and MeOH (5
mL) was
added to give light yellow precipitates. The precipitates was collected by
filtration, washed
with MeOH, and dried in vacuum to give the title compound as a light yellow
solid. MS (ESI,
positive ion) m/z: 358 (M+1).
Following the above procedure but substituting 1-phenylcyclobutanamine with 1-
(4-
chlorophenyl)cyclobutanamine gavel -(1-(4-chlorophenyl)cyclobutyl)-3-(4-(2-
methylpyridin-
4-yl)phenyl)urea.
Example 158
Synthesis of 1-(4-(2-methylpyridin-4-yl)phenyl)-3-((1-
phenylcyclopentyl)methyl)urea
H H
N N
O
N
Step 1
68
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
To a solution of 1-phenyl-l-cyclopentanecarbonitrile (1.500 mL, 8.76 mmol,
Acros
Organics USA) in MeOH (40 mL) was added a solution of palladium, lOwt. % on
activated
carbon (93 mg, 0.876 mmol, Aldrich) in EtOAc (0.5 mL) and concentrated HC1(0.4
mL). The
reaction mixture was stirred at room temperature under H2 (42 psi) overnight.
The reaction
mixture was filtered through celite, washed the filter-cake with MeOH. The
combined filtrates
were concentrated and H2O was added to the residue. The aqueous solution was
extracted with
EtOAc. The aqueous layer was basified (pH >10) using saturated aqueous NaHCO3
and
extracted with EtOAc. The combined organic extracts were dried over MgS04,
concentrated,
and dried in vacuum to give (1-phenylcyclopentyl)methanamine as light yellow
oil, which was
used in the next step without purification.
Step 2
A solution of azido(4-(2-methylpyridin-4-yl)phenyl)methanone (200 mg, 0.84
mmol) in
THE (0.8 mL) was subjected to a microwave irradiation at 120 C for 10 min.
Then, (1-
phenylcyclopentyl)methanamine (191 mg, 1.1 mmol) was added and the reaction
mixture was
stirred at room temperature for overnight. Solvent was removed and the residue
was mixed
silica gel. The solid mixture was then purified by silica gel flash column
chromatography
(eluted using 10%-100% EtOAc/hexane gradient) to give the title compound as a
light yellow
solid. MS (ESI, positive ion) m/z: 386 (M+1).
Proceeding as described in Example 158 above, using azido(4-(2-methylpyridin-4-
yl)phenyl)methanone and the corresponding amine, the following compounds were
prepared.
MS: ESI
Examples Structure Name m/z
M+1
H H
159 N~N 1-(4-(2-methylpyridin-4-
yl)phenyl)-3-((1- 372
o / phenylcyclobutyl)methyl)urea
H H
160 N~N I 1-(2-methyl-2-phenylpropyl)-3-
o / (4-(2-methyl pyrid i n-4- 360
yl)phenyl)urea
N
O
H H 1-(4-(2-methylpyridin-4-
161 NYN yl)phenyl)-3-((4- 402
phenyltetrahyd ro-2H-pyran-4-
\ / yl)methyl)urea
N
69
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
H H
NyN 1-(4-(2-methylpyridin-4-
/
162 o yl)phenyl)-3-((1- 358
phenylcyclopropyl)methyl)urea
Example 163
Synthesis of 1-(2-methyl-l-phenylpropan-2-yl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea
H H
N N ~
0 I /
N
Step 1
To a solution of ethyl 2,2-dimethyl-3-phenylpropanoate (2.0 g, 9.7 mmol) in
MeOH (32
mL) at 0 C was added a solution of lithium hydroxide monohydrate (1.2 g, 29.1
mmol) in H2O
(10 mL). After addition, the mixture was stirred at room temperature for
overnight. Then, the
solvent was removed and the H2O (50 mL) was added. The aqueous mixture was
adjusted to
pH - 2.0 by using concentrated HC1. Then, the aqueous was extracted with EtOAc
(2 x 50
mL). The combined organic extracts were dried over MgSO4, concentrated, and
dried in vacuo
to give 2,2-dimethyl-3-phenylpropanoic acid as a colorless oil. MS (ESI,
positive ion) m/z:
177 (M+1).
Step 2
A solution of 2,2-dimethyl-3-phenylpropanoic acid (1.47 g, 8.29 mmol) and
triethylamine
(1.4 mL, 9.95 mmol) in THE (40 mL) at 0 C was added ethyl chloroformate (0.95
mL, 9.95
mmol). After addition, the mixture was then stirred at room temperature for 3
h. Then, a
solution of sodium azide (0.87 mL, 24.9 mmol) in H2O (7 mL) was added and the
mixture was
stirred at room temperature for 48 h. Then, H2O (10 mL) was added and the
mixture was
extracted with EtOAc (2 x 80 mL). The combined organic extracts were dried
over MgSO4,
concentrated, and dried in vacuo to give 2,2-dimethyl-3-phenylpropanoyl azide
as a light
yellow solid, which was used without purification.
Step3
A solution of 2,2-dimethyl-3-phenylpropanoyl azide (0.44 g, 2.171 mmol) in THE
(0.8
mL) was subjected to a microwave irradiation at 120 C for 10 minutes. Then, 4-
(2-
methylpyridin-4-yl)benzenamine (0.20 g, 1.086 mmol) was added and the mixture
was
subjected to a microwave irradiation at 120 C for 15 minutes. Then, the
solvent was removed
and the residue was mixed with silica gel. The solid mixture was then purified
by silica gel
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
flash column chromatography using ISCO instrument (solid loading, 15%-100%
EtOAc/hexane) to give the title compound, which was repurified by preparative
HPLC (0%-
100% MeCN 0.1% TFA/ H2O 0.1% TFA) to give a desired product in a solution of
MeCN
0.1% TFA and H2O 0.1% TFA. The solvent was removed and the aqueous solution
was
neutralized using NaHCO3(s). The aqueous solution was extracted with EtOAc (2
x 40 mL).
The combined organic extracts were then dried over MgSO4, concentrated, and
dried in vacuo
to give 1-(2-methyl-l-phenylpropan-2-yl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea as a light
yellow solid. MS (ESI, positive ion) m/z: 360 (M+1)
Example 164
Synthesis of 1-(3-(4-chlorophenyl)tetrahydrofuran-3-yl)-3-(4-(2-methylpyridin-
4-
yl)phenyl)urea
CI
H H /
NuN
I \ I / 0 0
N /
Step 1
A solution of 3-(4-chlorophenyl)-tetrahydrofuran-3-carboxylic acid (2.898 g,
12.786
mmol) and triethylamine (1.956 mL, 14.064 mmol) in THE (42 mL) at 0 C was
added ethyl
chloroformate (1.3 mL, 14.064 mmol). After addition, the mixture was stirred
at room
temperature for 4 h. Then, a solution of sodium azide (1.7 g, 25.5 mmol) in
H2O (5 mL) was
added and the mixture was stirred at room temperature for 48 h. Then, H2O (10
mL) was
added and the mixture was extracted with EtOAc (2 x 60 mL). The combined
organic extracts
were dried over MgSO4, concentrated, and dried in vacuo to give 3-(4-
chlorophenyl)-
tetrahydrofuran-3-carbonyl azide as an orange oil which was used without
purification.
Step 2
A solution of azido(3-(4-chlorophenyl)-tetrahydrofuran-3-yl)methanone (0.820
g, 3.26
mmol) in THE (1.4 mL) was subjected to a microwave irradiation at 120 C for 15
minutes.
Then, 4-(2-methylpyridin-4-yl)benzenamine (0.200 g, 1.09 mmol) was added and
the mixture
was subjected to a microwave irradiation at 120 C for 15 minutes. Then, the
solvent was
removed and the residue was mixed with silica gel. The solid mixture was then
purified by
silica gel column chromatography using ISCO instrument (solid loading, 15%-
100%
71
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
EtOAc/hexane) to give 1-(3-(4-chlorophenyl)tetrahydrofuran-3-yl)-3-(4-(2-
methylpyridin-4-
yl)phenyl)urea as a light yellow solid. MS (ESI, positive ion) m/z: 408 (M+1).
Example 165
Synthesis of 1-(1-(4-bromophenyl)cyclobutyl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea
/ Br
NYN N
Step 1
A solution of 1-(4-bromophenyl)cyclobutanecarboxylic acid (4.97 g, 19.5 mmol)
and
triethylamine (3.0 mL, 21.5 mmol) in THE (65 mL) at 0 C was added ethyl
chloroformate (2.0
mL, 21.5 mmol). After addition, the mixture was stirred at room temperature
for 4 h. Then, a
solution of sodium azide (2.5 g, 39.0 mmol) in H2O (5 mL) was added and the
mixture was
stirred at room temperature for 48 h. Then, H2O (10 mL) was added and the
mixture was
extracted with EtOAc (2 x 60 mL). The combined organic extracts were dried
over MgSO4,
concentrated, and dried in vacuo to give azido(1-(4-
bromophenyl)cyclobutyl)methanone as a
yellow solid which was used without purification.
Step 2
A solution of azido(1-(4-bromophenyl)cyclobutyl)methanone (0.61 g, 2.2 mmol)
in
THE (0.8 mL) was subjected to a microwave irradiation at 120 C for 15 minutes.
Then, 4-(2-
methylpyridin-4-yl)benzenamine (0.200 g, 1.1 mmol) was added and the mixture
was
subjected to a microwave irradiation at 120 C for 15 minutes.. Then, the
solvent was removed
and MeOH (5 mL) was added to the residue. A light yellow precipitation was
observed. The
mixture was filtered and the filtrate was concentrated to give the crude
product. The light
yellow solid was then mixed silica gel and the solid mixture was purified by
silica gel column
chromatography using ISCO (solid loading, 15%-100% EtOAc/hexane) to give 1-(1-
(4-
bromophenyl)cyclobutyl)-3-(4-(2-methylpyridin-4-yl)phenyl)urea as an off-white
solid. MS
(ESI, positive ion) m/z: 436 (M+1).
Proceeding as described in Example 165 above but using 4-(2-methylpyridin-4-
yl)benzenamine and the corresponding amine and the corresponding azido
carbonyl
intermediate, the following compounds were prepared.
72
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
MS: ESI
Examples Structure Name m/z
M+1
F /
166 I NyN I 1-(2-(2-fluorophenyl)propan-2-
/ o yl)-3-(4-(2-methylpyridin-4- 364
N j yl)phenyl)urea
F
H H / I 1-(1-(4-
167 NYN fluorophenyl)cyclohexyl)-3-(4- 404
p (2-methylpyridin-4-
yl)phenyl)urea
N /
F
1-(1-(3-
168 NuN fl uorophenyl)cyclohexyl)-3-(4- 404
I I (2-methylpyridin-4-
O yl)phenyl)urea
N
F
1-(1-(3-
169 o N~N fluorophenyl)cyclohexyl)-3-(3- 434
methoxy-4-(2-methylpyridin-4-
0 yl)phenyl)urea
N /
F
H H / I 1-(1-(2-
170 NYN fluorophenyl)cyclohexyl)-3-(4- 404
p (2-methylpyridin-4-
N yl)phenyl)urea
/
Example 171
Synthesis of 1-((1-(6-chloropyridin-2-yl)cyclopentyl)methyl)-3-(4-(2-
methylpyridin-4-
yl)phenyl)urea
H H
N N N Cl
O /
N
Step 1
A solution of cyclopentanecarbonitrile (0.7 g, 0.8 ml, 8 mmol) and 2-chloro-6-
fluoropyridine (1.0 g, 8 mmol) in toluene (25 mL) at 0 C was added sodium
73
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
bis(trimethylsilyl)amide (8 mL, 8 mmol) 1M in THE dropwise. After addition,
the mixture
was stirred at 0 C for 1 h and 3 h at room temperature. Then, H2O (20 mL) was
added and the
mixture was stirred at room temperature for 15 minutes. Then, the mixture was
extracted with
EtOAC (2 x 20 mL) and the combined organic extracts were dried over MgSO4 and
concentrated. The residue was mixed with silica gel and the solid mixture was
then purified by
silica gel column chromatography using ISCO instrument (solid loading, 0%-30%
EtOAc/hexane) to give 1-(6-chloropyridin-2-yl)cyclopentanecarbonitrile as a
colorless oil. MS
(ESI, positive ion) m/z: 207 (M+1).
Step 2
To a solution of 1-(6-chloropyridin-2-yl)cyclopentanecarbonitrile (0.456 g,
2.21 mmol)
in THE (3 mL) was added 1.0 M Borane THE complex (11.0 ml, 11.0 mmol)
dropwise. After
addition, the mixture was refluxed for 20 minutes. Then, the mixture was
cooled to 0 C and
concentrated HC1(11 mL) was added dropwise. The mixture was then stirred at
room
temperature for 20 minutes. Then, the mixture was adjusted to pH - 14 by using
NaOH ION at
0 C. Then, EtOAc (150 mL) was added and the mixture was stirred at room
temperature for
15 minutes. The organic layer was collected, dried over MgSO4, and
concentrated. The
residue was mixed with silica gel and the solid mixture was purified by silica
gel column
chromatography using ISCO instrument (solid loading, 0%-20% MeOH/DCM) to give
1-(6-
chloropyridin-2-yl)cyclopentyl)methanamine as a colorless oil. MS (ESI,
positive ion) m/z:
211 (M+1).
Step3
A solution of azido(4-(2-methylpyridin-4-yl)phenyl)methanone (0.130 g, 0.546
mmol)
in THE (0.8 mL) was subjected to a microwave irradiation at 120 C for 15
minutes. Then, (1-
(6-chloropyridin-2-yl)cyclopentyl)methanamine (0.126 g, 0.600 mmol) was added
and the
mixture was stirred at room temperature for overnight. Then, the solvent was
removed and the
residue was mixed with silica gel. The solid mixture was then purified by
column
chromatography using ISCO instrument (solid loading, 15%-100% EtOAc/hexane) to
give 1-
((1-(6-chloropyridin-2-yl)cyclopentyl)methyl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea as a
light yellow solid. MS (ESI, positive ion) m/z: 421 (M+1).
Example 172
Synthesis of 1-(4-(2-methylpyridin-4-yl)phenyl)-3-((1-(pyridin-2-
yl)cyclopentyl)methyl)urea
74
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
H H
N N N
O
N
Step I
A solution of 1-((1-(6-chloropyridin-2-yl)cyclopentyl)methyl)-3-(4-(2-
methylpyridin-4-
yl)phenyl)urea (0.096 g, 0.2 mmol) in MeOH (1 mL) was stirred at room
temperature under H2
(45 psi) for 12 h. Then, palladium hydroxide, 20 wt % pd (dry basis) on
carbon, wet, degussa
type el01 ne/w (0.02 g, 0.02 mmol) was added and the mixture was continued
stirring at room
temperature under H2 (45 psi) for another 12 h. The mixture was filtered
through celite-pad,
washed with MeOH (2 x 20 mL). The combined filtrates were concentrated and the
residue
was purified by silica gel chromatography using ISCO instrument (solid
loading, 15%-100%
EtOAc/hexane) to give 1-(4-(2-methylpyridin-4-yl)phenyl)-3-((1-(pyridin-2-
yl)cyclopentyl)methyl)urea as a white solid. MS (ESI, positive ion) m/z: 387
(M+1).
Example 173
Synthesis of 1-(1-(3-bromophenyl)cyclobutyl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea
H H \ Br
r4 Y N Z~
\ \ I 0
N
Step 1
To a solution of 3-bromobenzeneacetonitrile (6.480 g, 33.054 mmol), potassium
hydroxide (14.8 g, 26.4 mmol), and tetrabutylammonium bromide (0.107 g, 0.331
mmol) in
toluene (90 mL) and H2O (4 mL) was added 1,3-dibromopropane (3.4 mL, 33.0
mmol). The
resulting mixture was then refluxed for 1.5 h. Then, the mixture was cooled to
room
temperature and H2O (100 mL) was added. The mixture was then extracted with
EtOAc (2 x
100 mL). The combined organic extracts were dried over MgSO4 and concentrated.
The
residue was then mixed with silica gel and the solid mixture was purified by
silica gel flash
column chromatography using ISCO instrument (solid loading, 0%-100%
EtOAc/hexane) to
give 1-(3-bromophenyl)cyclobutanecarbonitrile as a yellow oil. MS (ESI,
positive ion) m/z:
236 (M+1).
Step 2
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
A solution of 1-(3-bromophenyl)cyclobutanecarbonitrile (1.520 g, 6.4 mmol) in
EtOH
(25 mL) was added potassium hydroxide (0.433 g, 7.725 mmol). The resulting
mixture was
then refluxed for overnight. Then, the mixture was cooled to 0 C and H2O (30
mL) was added.
Then, the mixture was adjusted to pH - 2.0 at 0 C by using concentrated HC1.
Then, EtOAc
(50 mL) was added and the mixture was stirred at room temperature for 15
minutes. The
organic layer was collected and the aqueous layer was extracted with EtOAc (2
x 50 mL). The
combined organic extracts were dried over MgSO4 and concentrated. The residue
was then
mixed silica gel and the solid mixture was then purified by silica gel column
chromatography
using ISCO (0%-15% MeOH/DCM) to give 1-(3-bromophenyl)cyclobutanecarboxylic
acid as
a white solid. MS (ESI, positive ion) m/z: 255 (M+1).
Step 3
A solution of 1-(3-bromophenyl)cyclobutanecarboxylic acid (0.487 g, 1.9 mmol)
and
triethylamine (0.319 mL, 2.291 mmol) in THE (13 mL) at 0 C was added ethyl
chloroformate
(0.219 mL, 2.291 mmol). After addition, the mixture was stirred at room
temperature for 2 h.
Then, a solution of sodium azide (0.5 g, 7.636 mmol) in H2O (2 mL) was added
and the
mixture was stirred at room temperature for 48 h. Then, H2O (10 mL) was added
and the
mixture was extracted with EtOAc (2 x 50 mL). The combined organic extracts
were dried
over MgSO4, concentrated, and dried in vacuo to give 1-(3-
bromophenyl)cyclobutanecarbonyl
azide as a light yellow liquid which was used without purification.
Step 4
A solution of azido(1-(3-bromophenyl)cyclobutyl)methanone (0.334 g, 1.19 mmol)
in
THE (1 mL) was subjected to a microwave irradiation at 120 C for 15 minutes.
Then, 4-(2-
methylpyridin-4-yl)benzenamine (0.100 g, 0.543 mmol) was added and the mixture
was
subjected to microwave irradiation at 120 C for 15 minutes. Then, the solvent
(THF) was
removed and a solution of DMSO and MeOH (1:1, 1 mL) was added. The solution
mixture
was then purified preparative HPLC (0%-100% MeCN 0.1% TFA/ H2O 0.1% TFA) to
give the
title compound in a solution of MeCN 0.1% TFA and H2O 0.1% TFA. The solvent
was
removed and the aqueous solution was neutralized using NaHCO3(s). The aqueous
solution
was extracted with EtOAc (2 x 20 mL). The combined organic extracts were then
dried over
MgSO4, concentrated, and dried in vacuo to give 1-(1-(3-
bromophenyl)cyclobutyl)-3-(4-(2-
methylpyridin-4-yl)phenyl)urea as a light yellow solid. MS (ESI, positive ion)
m/z: 436
(M+1).
Example 174
76
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
Synthesis of 1-((1-(2-chloropyridin-4-yl)cyclopentyl)methyl)-3-(4-(2-
methylpyridin-4-
yl)phenyl)urea
H H
N N CI
\ \ I O N
N
Step I
To a solution of cyclopentanecarbonitrile (2.36 ml, 22.6 mmol) and 2-chloro-4-
fluoropyridine (2.97 g, 22.6 mmol) in toluene (60 mL) at 0 C was added sodium
bis(trimethylsilyl)amide 1.0 M in THE (22.6 mL, 22.6 mmol) dropwise. After
addition, the
mixture was stirred at 0 C for 1 h and 3 h at room temperature. Then, H2O (60
mL) was added
and the mixture was stirred at room temperature for 15 minutes. Then, the
mixture was
extracted with EtOAc (2 x 20 mL) and the combined organic extracts were dried
over MgSO4
and concentrated. The residue was mixed with silica gel and the solid mixture
was then
purified by silica gel column chromatography using ISCO instrument (solid
loading, 0%-30%
EtOAc) to give 1-(2-chloropyridin-4-yl)cyclopentanecarbonitrile as a colorless
liquid. MS
(ESI, positive ion) m/z: 207 (M+1).
Step 2
To a solution of 1-(2-chloropyridin-4-yl)cyclopentanecarbonitrile (1.00 g,
4.84 mmol)
in toluene (6 mL) was added Boran-THF complex (0.416 g, 4.84 mmol) 1.OM in THE
dropwise. After addition, the mixture was refluxed for 20 minutes. Then, the
mixture was
cooled to 0 C and concentrated HC1(24 mL) was added. The mixture was then
stirred at room
temperature for 20 minutes. Then, the mixture was adjusted to pH - 14 by NaOH
ION at 0 C.
Then, EtOAc (150 mL) was added and the mixture was stirred at room temperature
for 15
minutes. The organic layer was collected, dried over MgSO4, and concentrated.
The residue
was mixed with silica gel and the solid mixture was purified by silica gel
column
chromatography using ISCO instrument (solid loading, 0%-20% MeOH/DCM) to give
1-(2-
chloropyridin-4-yl)cyclopentyl)methanamine as a light yellow oil. MS (ESI,
positive ion) m/z:
211 (M+1).
Step 3
A solution of azido(4-(2-methylpyridin-4-yl)phenyl)methanone (0.200 g, 0.839
mmol)
in THE (0.6 mL) was subjected to a microwave irradiation at 120 C for 15
minutes. Then, (1-
(2-chloropyridin-4-yl)cyclopentyl)methanamine (0.195 g, 0.923 mmol) was added
and the
mixture was subjected to a microwave irradiation at 120 C for 15 minutes.
Then, the mixture
77
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
was filtered and the filtrate was purified by preparative HPLC (0% -100% MeCN
0.1%
TFA/H20 0.1% TFA) to give a desired product in solution of MeCN 0.1% TFA/H20
0.1%
TFA. The solvent was removed and the aqueous solution was neutralized using
NaHCO3(s).
The aqueous solution was extracted with EtOAc (20 mL). The organic layer was
collected,
dried over MgSO4, concentrated, and dried in vacuo to give 1-((1-(2-
chloropyridin-4-
yl)cyclopentyl)methyl)-3-(4-(2-methylpyridin-4-yl)phenyl)urea as a light
yellow solid. MS
(ESI, positive ion) m/z: 421 (M+1).
Example 175
Synthesis of 1-(4-(2-methylpyridin-4-yl)phenyl)-3-((1-(pyridin-4-
yl)cyclopentyl)methyl)urea
H H
N N
\ \ I O N
N
To a solution of 1-((1-(2-chloropyridin-4-yl)cyclopentyl)methyl)-3-(4-(2-
methylpyridin-4-yl)phenyl)urea (0.085g, 0.20 mmol) in MeOH (1 mL) was added
palladium
hydroxide, 20 wt % pd (dry basis) on carbon, wet (0.0043 g, 0.0061 mmol). The
resulting
mixture was then stirred at room temperature under H2 (42 psi) for overnight.
Then, the
mixture was filtered through celite-pad and washed with MeOH (2 x 5 mL). The
combined
filtrates were concentrated and the residue was mixed with silica gel. The
solid mixture was
purified by silica gel column chromatography using ISCO instrument (solid
loading, 15%-
100% EtOAc/hexane) to give 1-(4-(2-methylpyridin-4-yl)phenyl)-3-((1-(pyridin-4-
yl)cyclopentyl)methyl)urea as a light yellow solid. MS (ESI, positive ion)
m/z: 387 (M+1).
Example 176
Synthesis of 1-((1-(2-cyanopyridin-4-yl)cyclopentyl)methyl)-3-(4-(2-
methylpyridin-4-
yl)phenyl)urea
N N CN
\ \ I O N
N
Step 1
A solution of 1-((1-(2-chloropyridin-4-yl)cyclopentyl)methyl)-3-(4-(2-
methylpyridin-4-
yl)phenyl)urea (0.050 g, 0.12 mmol), zinc cyanide (0.028 g, 0.24 mmol), and
78
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
tetrakis(triphenylphosphine)palladium (0.0055 g, 0.0048 mmol) in DMF (0.6 mL)
was
subjected to a microwave irradiation at 150 C for 15 minutes. Then,
tetrakis(triphenylphosphine)palladium (0.0055 g, 0.0048 mmol) was added and
the mixture
was subjected to a microwave irradiation at 170 C for 15 minutes. Then, the
mixture was
filtered and the filtrate was then purified by preparative HPLC (0%-100% MeCN
0.1%
TFA/H20 0.1% TFA) to give the desired product in a solution of MeCN 0.1% TFA
and H2O
0.1% TFA. The solvent was removed and the aqueous solution was neutralized
using
NaHCO3(s). The aqueous solution was extracted with EtOAc (30 mL). The organic
layer was
collected, dried over MgSO4, concentrated, and dried in vacuo to give 1-((1-(2-
cyanopyridin-4-
yl)cyclopentyl)methyl)-3-(4-(2-methylpyridin-4-yl)phenyl)urea as a white
solid. MS (ESI,
positive ion) m/z: 412 (M+1).
Excample 177
Synthesis of 1-(1-(4-cyanophenyl)cyclobutyl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea
CN
NYN
trA90
A solution of 1-(1-(4-bromophenyl)cyclobutyl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea
(0.100 g, 0.229 mmol), zinc cyanide (0.0218 ml, 0.344 mmol), and
tetrakis(triphenylphosphine)palladium (0.0132 g, 0.0115 mmol) in DMF (1.5 mL)
was
subjected to a microwave irradiation at 170 C for 15 minutes. The mixture was
filtered and the
filtrate was then purified by preparative HPLC (0%-100% MeCN 0.1% TFA/H20 0.1%
TFA)
to give the desired product in a solution of MeCN 0.1% TFA and H2O 0.1% TFA.
The solvent
was removed and the aqueous solution was neutralized using NaHCO3(s). The
aqueous
solution was extracted with EtOAc (30 mL). The organic layer was collected,
dried over
MgSO4, concentrated, and dried in vacuo to give 1-(1-(4-
cyanophenyl)cyclobutyl)-3-(4-(2-
methylpyridin-4-yl)phenyl)urea as a white solid. MS (ESI, positive ion) m/z:
383 (M+1).
Example 178
Synthesis of 1-(1-(6-chloropyridin-2-yl)cyclopentyl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea
79
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
CI
N
NYN
O
/
I
N /
Step I
A solution of 1-(6-chloropyridin-2-yl)cyclopentanecarbonitrile (0.550 g, 2.66
mmol) in
HC1(3 mL) was subjected to a microwave irradiation at 130 C for 45 minutes.
Then, EtOAc
(20 mL) was added and the mixture was adjusted to pH - 4 at 0 C using
saturated NaHCO3.
The mixture was then extracted with EtOAc (2 x 10 mL). The combined organic
extracts were
dried over MgS04 and concentrated. The residue was mixed with silica gel and
the solid
mixture was purified by silica gel column chromatograpahy using ISCO
instrument (solid
loading, 20%-100% EtOAc/hexane) to give 1-(6-chloropyridin-2-
yl)cyclopentanecarboxylic
acid as a white solid. MS (ESI, positive ion) m/z: 226 (M+1).
Step2
A solution of 1-(6-chloropyridin-2-yl)cyclopentanecarboxylic acid (0.173 g,
0.767
mmol) and triethylamine (0.117 ml, 0.843 mmol) in THE (3 mL) at 0 C was added
ethyl
chloroformate (0.0806 ml, 0.843 mmol). After addition, the mixture was stirred
at room
temperature for lh. Then, a solution of sodium azide (0.0997 g, 1.53 mmol) in
H2O (0.4 mL)
was added and the mixture was stirred at room temperature for overnight. Then,
H2O (5 mL)
was added and the mixture was extracted with EtOAc (2 x 20 mL). The combined
organic
extracts were dried over MgS04, concentrated, and dried in vacuo to give 1-(6-
chloropyridin-
2-yl)cyclopentanecarbonyl azide as an off-white solid, which was used without
purification.
Step3
A solution of azido(1-(6-chloropyridin-2-yl)cyclopentyl)methanone (0.17 g,
0.66
mmol) in THE (1 mL) was subjected to a microwave irradiation at 130 C for 15
minutes.
Then, 4-(2-methylpyridin-4-yl)benzenamine (0.090 g, 0.49 mmol) was added and
the mixture
was subjected to a microwave irradiation at 130 C for 15 minutes. Then, the
mixture was
filtered and the filtrate was purified by preparative HPLC (0%-100% MeCN 0.1%
TFA/H20
0.1% TFA) to give a desired compound in a solution of MeCN 0.1% TFA/H20 0.1%
TFA.
The solvent was removed and the aqueous solution was neutralized using
NaHCO3(s). The
aqueous solution was extracted with EtOAc (2 x 30 mL). The combined organic
extracts were
dried over MgS04, concentrated, and dried in vacuo to give 1-(1-(6-
chloropyridin-2-
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
yl)cyclopentyl)-3-(4-(2-methylpyridin-4-yl)phenyl)urea as a yellow solid. MS
(ESI, positive
ion) m/z: 407 (M+1).
Example 179
Synthesis of 1-(4-(2-methylpyridin-4-yl)phenyl)-3-(1-(pyridin-2-
yl)cyclopentyl)urea
N
\ N Y N \ I / O
I
N /
A solution of 1-(1-(6-chloropyridin-2-yl)cyclopentyl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea (0.082 g, 0.20 mmol) in MeOH (1.5 mL) was added palladium
hydroxide, 20
wt % pd (dry basis) on carbon, wet (0.0028 g, 0.020 mmol). The resulting
mixture was then
stirred at room temperature under H2 (40 psi) for overnight. The mixture was
filtered through
a celite-pad and washed with MeOH (2 x 2 mL) and EtOAc (2 x 2 mL). The
combined
filtrates were concentrated and the residue was dissolved in DMSO (1 mL). The
solution
mixture was purified by preparative HPLC (0%-100% MeCN 0.1% TFA/H20 0.1% TFA)
to
give a desired compound in a solution of MeCN 0.1% TFA/H20 0.1% TFA. The
solvent was
removed and the aqueous solution was neutralized using NaHCO3(s). The aqueous
solution
was extracted with EtOAc (2 x 10 mL). The combined organic extracts were dried
over
MgS04, concentrated, and dried in vacuo to give 1-(4-(2-methylpyridin-4-
yl)phenyl)-3-(1-
(pyridin-2-yl)cyclopentyl)urea as a yellow solid. MS (ESI, positive ion) m/z:
373 (M+1).
Example 180
Synthesis of 1-(1-(2-chloropyridin-4-yl)cyclopentyl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea
H H
NYN CI
\ I / O
8:::~- N
N
Step 1
A solution of 1-(2-chloropyridin-4-yl)cyclopentanecarbonitrile (1.300 g, 6.290
mmol) in
HC1(4 mL) was subjected to a microwave irradiation at 140 C for 45 minutes.
Then, H2O (30
mL) was added and the solution mixture was adjusted to pH - 5 at 0 C using
NaHCO3(s). The
mixture was then extracted with EtOAc. The combined organic extracts were
dried over
81
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
MgSO4 and concentrated. The residue was then mixed with silica gel and the
solid mixture
was purified by silica gel column chromatography using ISCO instrument (solid
loading,
20%-100% EtOAc/hexane) to give 1-(2-chloropyridin-4-yl)cyclopentanecarboxylic
acid as a
white solid. MS (ESI, positive ion) m/z: 226 (M+1).
Step2
A solution of 1-(2-chloropyridin-4-yl)cyclopentanecarboxylic acid (0.115 g,
0.510
mmol) and triethylamine (0.0780 mL, 0.561 mmol) in THE (3 mL) at 0 C was added
ethyl
chloroformate (0.0536 mL, 0.561 mmol). After addition, the mixture was stirred
at room
temperature for 2 h. Then, a solution of sodium azide (0.0897 mL, 2.55 mmol)
in H2O (0.4
mL) was added and the mixture was stirred at room temperature for overnight.
Then, H2O (3
mL) was added and the mixture was extracted with EtOAc (2 x 5 mL). The
combined organic
extracts were dried over MgS04, concentrated, and dried in vacuo to give 1-(2-
chloropyridin-
4-yl)cyclopentanecarbonyl azide as a yellow oil, which was used without
purification.
Step 3
A solution of azido(1-(2-chloropyridin-4-yl)cyclopentyl)methanone (0.111 g,
0.443
mmol) in THE (1 mL) was subjected to a microwave irradiation at 120 C 15
minutes. Then, 4-
(2-methylpyridin-4-yl)benzenamine (0.0816 g, 0.443 mmol) was added and the
mixture was
subjected to a microwave irradiation at 120 C for 15 minutes. Then, the
mixture was filtered
and the filtrate was purified by preparative HPLC (0%-100% MeCN 0.1% TFA/H20
0.1%
TFA) to give a desired product in a solution of MeCN 0.1% TFA/H20 0.1% TFA.
The solvent
was removed and the aqueous solution was neutralized using NaHCO3(s). The
aqueous
solution was extracted with EtOAc (20 mL). The organic layer was collected,
dried over
MgS04, concentrated, and dried in vacuo to give 1-(1-(2-chloropyridin-4-
yl)cyclopentyl)-3-(4-
(2-methylpyridin-4-yl)phenyl)urea as a light yellow solid. MS (ESI, positive
ion) m/z: 407
(M+1).
Example 181
Synthesis of 1-(4-(2-methylpyridin-4-yl)phenyl)-3-(1-(pyridin-4-
yl)cyclopentyl)urea
N
\ NYN
\ I / O
I
N /
A solution of 1-(1-(2-chloropyridin-4-yl)cyclopentyl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea (0.071 g, 0.17 mmol) in MeOH (1 mL) and EtOAc (0.3 mL) was
added
82
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
palladium hydroxide, 20 wt % pd (dry basis) on carbon, wet (0.0074 g, 0.052
mmol). The
resulting mixture was then stirred at room temperature under H2 (40 psi) for
overnight. Then,
the mixture was filtered through a celite and the celite was washed with MeOH
(2 x 10 mL)
and EtOAc (1 x 10 mL). The combined filtrates were concentrated and the
residue was
dissolved in DMSO (0.8 mL). The solution mixture was purified by preparative
HPLC (0%-
100% MeCN 0.1% TFA/H20 0.1% TFA) to give a desired product in a solution of
MeCN
0.1% TFA/H20 0.1% TFA. The solvent was removed and the aqueous solution was
neutralized using NaHCO3(s). The aqueous solution was extracted with EtOAc (20
mL). The
organic layer was collected, dried over MgSO4, concentrated, and dried in
vacuo to give 1-(4-
(2-methylpyridin-4-yl)phenyl)-3-(1-(pyridin-4-yl)cyclopentyl)urea as a light
yellow solid. MS
(ESI, positive ion) m/z: 373 (M+1).
Example 182
Synthesis of 1-(1-(5-bromopyridin-3-yl)cyclopentyl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea
N
N N
/ Y Br
\ \ I
I
N /
Step I
A solution of 3,5-dibromopyridine (14.9 g, 63.1 mmol) and
cyclopentanecarbonitrile
(3.29 ml, 31.5 mmol) in THE (100 mL) at 0 C was added sodium bistrimethylsilyl
amide 1M
in THE (34.7 ml, 34.7 mmol). After addition, the mixture was stirred at room
temperature for
48 h under N2. Then, H2O (100 mL) was added slowly and the mixture was
extracted with
EtOAc (200 mL). The organic layer was collected, dried over MgSO4, and
concentrated in
vacuo to give 1-(5-bromopyridin-3-yl)cyclopentanecarbonitrile as a brown solid
which was
used without purification. MS (ESI, positive ion) m/z: 251 (M+1).
Step2
A solution of 1-(5-bromopyridin-3-yl)cyclopentanecarbonitrile (7.92 g, 31.5
mmol) in HC1
(40 mL) and H2O (5 mL) was subjected to a microwave irradiation at 170 C for
15 minutes.
Then, H2O (150 mL) was added slowly and the mixture was adjusted to pH - 10 at
0 C using
N NaOH solution. The mixture was then extracted with EtOAc and the aqueous
layer was
collected. Then, the aqueous layer was adjusted to pH - 4 at 0 C using
concentrated HC1. The
aqueous layer was then extracted with EtOAc (2 x 100 mL). The combined organic
extracts
were dried over MgSO4 and concentrated. The residue was mixed with silica gel
and the solid
83
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
mixture was purified by silica gel column chromatography using ISCO instrument
(solid
loading, 10%-100% EtOAc/hexane) to give 1-(5-bromopyridin-3-
yl)cyclopentanecarboxylic
acid as an off-white solid. MS (ESI, positive ion) m/z: 270 (M+1).
Step 3
A solution of 1-(5-bromopyridin-3-yl)cyclopentanecarboxylic acid (0.470 g,
1.74
mmol) and triethylamine (0.266 ml, 1.91 mmol) in THE (5 mL) at 0 C was added
ethyl
chloroformate (0.183 ml, 1.91 mmol) under N2. After addition, the mixture was
stirred at room
temperature for 4 h. Then, a solution of sodium azide (0.226 g, 3.48 mmol) in
H2O (0.5 mL)
was added and the mixture was stirred at room temperature for overnight. Then,
H2O (10 mL)
was added and the mixture was extracted with EtOAc (2 x 10 mL). The combined
organic
extracts were dried over MgSO4, concentrated, and dried in vacuo to give 1-(5-
bromopyridin-
3-yl)cyclopentanecarbonyl azide as a brown solid which was used without
purification.
Step 4
A solution of azido(1-(5-bromopyridin-3-yl)cyclopentyl)methanone (0.420 g,
1.42
mmol) in THE (1.5 mL) was subjected to a microwave irradiation at 130 C for 15
minutes.
Then, 4-(2-methylpyridin-4-yl)benzenamine (0.262 g, 1.42 mmol) was added and
the mixture
was subjected to a microwave irradiation at 120 C for 15 minutes. Then, the
mixture was
filtered and the filtrate was purified by preparative HPLC (0%-100% MeCN 0.1%
TFA/H20
0.1% TFA) to give a desired product in a solution of MeCN 0.1% TFA/H20 0.1%
TFA. The
solvent was removed and the aqueous solution was neutralized using NaHCO3(s).
The
aqueous solution was extracted with EtOAc (2 x 100 mL). The combined organic
extracts
were dried over MgSO4, concentrated, and dried in vacuum to give 1-(1-(5-
bromopyridin-3-
yl)cyclopentyl)-3-(4-(2-methylpyridin-4-yl)phenyl)urea as a yellow solid. MS
(ESI, positive
ion) m/z: 451 (M+1).
Proceeding as described in Example 182 above, the following compounds were
prepared.
MS: ESI
Examples Structure Name m/z
M+1
F
H H N 1-(1-(6-fluoropyridin-2-
183 NyN yl)cyclopentyl)-3-(4-(2- 391
o methylpyridin-4-yl)phenyl)urea
N
84
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
CI
o-N
H
N N 1-(1-(6-chloropyridin-2-
184 yl)cyclobutyl)-3-(4-(2- 393
methylpyridin-4-yl)phenyl)urea
N
Example 185
Synthesis of 1-(4-(2-methylpyridin-4-yl)phenyl)-3-(1-(pyridin-3-
yl)cyclopentyl)urea
N
H H
/ NYN
\ \ I O
N /
A solution of 1-(1-(5-bromopyridin-3-yl)cyclopentyl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea (0.120 g, 0.266 mmol) in MeOH (1 mL) was added palladium
hydroxide, 20 wt
% pd (dry basis) on carbon, wet (0.00373 g, 0.0266 mmol). Then, the mixture
was stirred at
room temperature under H2 (40 psi) for 2 h. Then, the mixture was filtered
through a celite and
the celite was washed with MeOH (2 x 10 mL). The combined filtrates were
concentrated and
the residue was dissolved in a solution of MeOH (0.5 mL) and DMSO (0.5 mL).
The solution
mixture was then purified by preparative HPLC (0%-100% MeCN 0.1% TFA/H20 0.1%
TFA)
to give a desired product in a solution of MeCN 0.1% TFA/H20 0.1% TFA. The
solvent was
removed and the aqueous solution was neutralized using NaHCO3(s). The aqueous
solution
was extracted with EtOAc (2 x 20 mL). The combined organic extracts were dried
over
MgSO4, concentrated, and dried in vacuo to give 1-(4-(2-methylpyridin-4-
yl)phenyl)-3-(1-
(pyridin-3-yl)cyclopentyl)urea as a light yellow solid. MS (ESI, positive ion)
m/z: 373 (M+1).
Example 186
Synthesis of 1-(4-(2-methylpyridin-4-yl)phenyl)-3-(2-phenylbutan-2-yl)urea
H H
NuN
\ \ I O
N ";r-
Step 1
A solution of methyl iodide (2.0 ml, 34 mmol) and 2-phenylbutyronitrile (5.0
ml, 34 mmol)
in toluene (75 mL) at 0 C was added sodium bis (trimethylsilyl)-amide, 1.0 M
in THE (34 ml,
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
34 mmol). After addition, the mixture was stirred at 0 C for 2 h and at room
temperature for
overnight. Then, H2O (50 mL) was added and the mixture was stirred at room
temperature for
minutes. The organic layer was collected and the aqueous layer was extracted
with EtOAc (2
x 50 mL). The combined organic extracts were dried over MgSO4 and
concentrated. The
residue was mixed with silica gel and the solid mixture was purified by silica
gel column
chromatography using ISCO instrument (solid loading, 0%-100% EtOAc/hexane) to
give 1-(4-
(2-methylpyridin-4-yl)phenyl)-3-(2-phenylbutan-2-yl)urea as a yellow oil.
Step2
A solution of 2-methyl-2-phenylbutanenitrile (3.500 g, 21.98 mmol) in HC1(20
mL) and
H2O (4 mL) was subjected to a microwave irradiation at 150 C for 2 h. Then,
EtOAc (50 mL)
and H2O (50 mL) were added and the mixture was stirred at room temperature for
15 minutes.
The organic layer was collected and the aqueous layer was extracted with EtOAc
(1 x 50 mL).
The combined organic extracts were dried over MgSO4 and concentrated in vacuo.
The
residue was mixed with silica gel and the solid mixture was purified by silica
gel flash column
chromatography using ISCO instrument (solid loading, 0%-100% EtOAc/hexane) to
give 2-
methyl-2-phenylbutanoic acid as a colorless solid. MS (ESI, positive ion) m/z:
179 (M+1).
Step 3
A solution of 2-methyl-2-phenylbutanoic acid (0.515 g, 2.89 mmol) and
triethylamine
(0.442 ml, 3.18 mmol) in THE (12 mL) at 0 C was added ethyl chloroformate
(0.304 ml, 3.18
mmol). After addition, the mixture was stirred at room temperature for
overnight. Then, a
solution of sodium azide (0.376 g, 5.78 mmol) in H2O (0.5 mL) was added and
the mixture
was at room temperature for overnight. Then, H2O (10 mL) was added and the
mixture was
extracted with EtOAc (2 x 20 mL). The combined organic extracts were dried
over MgSO4,
concentrated, and dried in vacuo to give 2-methyl-2-phenylbutanoyl azide which
was used
without purification.
Step 4
A solution of 1-azido-2-methyl-2-phenylbutan-1-one (0.587 g, 2.89 mmol) in THE
(3
mL) was subjected to a microwave irradiation at 130 C for 15 minutes. Then, 4-
(2-
methylpyridin-4-yl)benzenamine (0.532 g, 2.89 mmol) was added and the mixture
was
subjected to a microwave irradiation at 130 C for 15 minutes. Then, a solution
mixture was
filtered and the filtrate was purified by preparative HPLC (0%-100% MeCN 0.1%
TFA/H20
0.1% TFA) to give a desired product in a solution of MeCN/H20 0.1%TFA. The
solvent was
removed and the aqueous solution was neutralized using NaHCO3(s). The aqueous
solution
was extracted with EtOAc (20 mL). The organic layer was collected, dried over
MgSO4,
86
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
concentrated, and dried in vacuo to give 1-(4-(2-methylpyridin-4-yl)phenyl)-3-
(2-phenylbutan-
2-yl)urea as a yellow solid. MS (ESI, positive ion) m/z: 360 (M+1).
Example 187
Synthesis of 1-(1-(6-methoxypyridin-2-yl)cyclopentyl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea
I'll 0
N
N Y N \ I / O
N
A solution of 1-(1-(6-fluoropyridin-2-yl)cyclopentyl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea (0.035 g, 0.09 mmol) and sodium methoxide (0.5 M solution in
methanol, 0.4
mL, 0.2 mmol) in MeOH (0.5 mL) was heated to 70 C for 30 min and at 100 C for
1 h. Then,
H2O (0.5 mL) was added and the mixture was filtered. The filtrate was purified
by preparative
HPLC (0%-100% MeCN 0.1% TFA/H20 0.1% TFA) to give a desired product in a
solution of
McCN 0.1% TFA/H2O 0.1% TFA). The solvent was removed and the aqueous solution
was
neutralized using NaHCO3(s). The aqueous solution was extracted with EtOAc (20
mL). The
organic layer was collected, dried over MgSO4, concentrated, and dried in
vacuo to give 1-(1-
(6-methoxypyridin-2-yl)cyclopentyl)-3-(4-(2-methylpyridin-4-yl)phenyl)urea as
a light yellow
solid. MS (ESI, positive ion) m/z: 403 (M+1).
Example 188
Synthesis of 1-(4-hydroxy-l-phenylcyclohexyl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea
N Y N Dlo~ O
N OH
Step 1
A solution of 1,4-cyclohexanedione monoethylene ketal (5.73 g, 36.7 mmol) in
THE
(100 mL) at 0 C was added phenylmagnesium bromide 1.Om sol in THE (40.4 mL,
40.4
mmol). After addition, the mixture was stirred at 0 C for 4 h and at room
temperature for 48 h.
Then, the mixture was quenched with 100 mM sodium phosphate (pH - 6.9, 150
mL). The
mixture was then stirred at room temperature for 15 minutes. Then, the organic
layer was
collected and the aqueous layer was extracted with EtOAc (1 x 100 mL). The
combined
87
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
organic extracts were dried over MgSO4 and concentrated in vacuo. The residue
was mixed
with silica gel and the solid mixture was purified by silica gel flash column
chromatography
using ISCO instrument (solid loading, 0%-100% EtOAc/hexane) to give 8-phenyl-
1,4-
dioxaspiro[4.5]decan-8-ol as a colorless solid.
Step 2
A solution of 8-phenyl-1,4-dioxaspiro[4.5]decan-8-ol (2.00 g, 8.54 mmol) and
sodium
azide (0.752 ml, 21.3 mmol) in DCM (42 mL) at 0 C was added trifluoroacetic
acid (5.59 ml,
72.6 mmol). After addition, the mixture was stirred at 0 C for 2 h. Then, the
mixture was
quenched with concentrated NH4OH (25 mL). Then, the mixture was stirred at
room
temperature for 15 minutes. Then, H2O (100 mL) was added and the mixture was
extracted
with EtOAc (2 x 100 mL). The combined organic extracts were dried over MgSO4
and
concentrated in vacuo. The residue was mixed with silica gel and the solid
mixture was
purified by silica gel column chromatography using ISCO instrument (solid
loading, 0%-100%
EtOAc/hexane) to give 8-azido-8-phenyl-1,4-dioxaspiro[4.5]decane as a yellow
oil.
Step 3
A solution of 8-azido-8-phenyl-1,4-dioxaspiro[4.5]decane (0.770 g, 2.97 mmol)
and
palladium, lOwt. % on activated carbon (0.316 g, 0.297 mmol) in EtOH (20 mL)
was stirred at
room temperature for overnight under H2. Then, the mixture was filtered
through a celite and
the celite was washed with MeOH (2 x 20 mL). The combined filtrate was then
concentrated
and dried in vacuo to give 8-phenyl-1,4-dioxaspiro[4.5]decan-8-amine as a
light brown oil,
which was without purification.
Step 4
A solution of azido(4-(2-methylpyridin-4-yl)phenyl)methanone (0.670 g, 2.81
mmol) in
THE (3 mL) was subjected to a microwave irradiation at 120 C for 15 minutes.
Then, 8-
phenyl-1,4-dioxaspiro[4.5]decan-8-amine (0.656 g, 2.81 mmol) was added and the
mixture
was stirred at room temperature for overnight. The mixture was filtered and
the filtrate was
purified by silica gel column chromatography using ISCO instrument (solid
loading, 0%-100%
EtOAc/hexane) to give 1-(4-(2-methylpyridin-4-yl)phenyl)-3-(8-phenyl-1,4-
dioxaspiro[4.5]decan-8-yl)urea as a white solid. MS (ESI, positive ion) m/z:
444 (M+1)
Step 5
A solution of 1-(4-(2-methylpyridin-4-yl)phenyl)-3-(8-phenyl-1,4-dioxaspiro-
[4.5]decan-8-yl)urea (0.795 g, 1.79 mmol) in acetone (7 mL) was added H2O (3
mL) and p-
toluenesulfonic acid (10.4 mL, 8.96 mmol). After addition, the mixture was
stirred at room
temperature for 4 h. Then, H2O (10 mL) and EtOAc (30 mL) were added and the
mixture was
88
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
neutralized by Na2CO3(s). The neutralized aqueous was extracted with EtOAc (2
x 20 mL).
The combined organic extracts were dried over MgSO4 and concentrated. The
residue was
dissolved in DCM (1.5 mL) and the solution mixture was purified by silica gel
column
chromatography using ISCO instrument (0%-20% MeOH/DCM) to give 1-(4-(2-
methylpyridin-4-yl)phenyl)-3-(4-oxo-l-phenylcyclohexyl)urea as a white solid.
MS (ESI,
positive ion) m/z: 400 (M+1).
Step 6
A solution of 1-(4-(2-methylpyridin-4-yl)phenyl)-3-(4-oxo-l-
phenylcyclohexyl)urea
(0.086 g, 0.22 mmol) in THE (2 mL) was added sodium borohydride (0.0098 g,
0.26 mmol).
After addition, the mixture was stirred at room temperature for 1 h. Then, the
mixture was
quenched with saturated NaHCO3. Then, the mixture was extracted with EtOAc (2
x 20 mL).
The combined organic extracts were dried over MgS04 and concentrated. The
residue was
dissolved in DMSO (1 mL) and the solution mixture was purified by preparative
HPLC (0%-
100% MeCN 0.1% TFA/H20 0.1% TFA) to give a desired product in a solution of
MeCN/H20
0.1% TFA. The solvent was removed and the aqueous solution was neutralized
using
NaHCO3(s). The aqueous solution was extracted with EtOAc. The combined
extracts were
dried over MgS04, washed with brine, concentrated, and dried in vacuo to give
1-(4-hydroxy-
1-phenylcyclohexyl)-3-(4-(2-methylpyridin-4-yl)phenyl)urea as a white solid.
MS (ESI,
positive ion) m/z: 402 (M+1).
Example 189
Synthesis of 1-(4,4-difluoro-l-phenylcyclohexyl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea
IOI
N F F
A solution of 1-(4-(2-methylpyridin-4-yl)phenyl)-3-(4-oxo-l-
phenylcyclohexyl)urea
(0.100 g, 0.250 mmol) at 0 C was added (diethylamino)sulfur trifluoride (0.165
mL, 1.25
mmol). After addition, the mixture was stirred at 0 C for 4.5 h. Then, the
mixture was poured
into an ice water (100 mL) and EtOAc (20 mL) was added. The mixture was
stirred at room
temperature for 5 minutes. The organic layer was collected and the aqueous
layer was
extracted with EtOAc (20 mL). The combined organic extracts were dried over
MgS04 and
concentrated. The residue was dissolved in DMSO (2 mL). The solution mixture
was then
89
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
purified by preparative HPLC (0%-100% MeCN 0.1% TFA/H20 0.1% TFA) to give a
desired
product in a solution of MeCN/H20 0.1% TFA. The solvent was removed and the
aqueous
solution was neutralized using NaHCO3(s). The aqueous solution was extracted
with EtOAc
(20 mL). The organic layer was collected, dried over MgSO4, concentrated, and
dried in vacuo
to give 1-(4,4-difluoro-1-phenylcyclohexyl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea as an off-
white solid. MS (ESI, positive ion) m/z: 422 (M+1).
Example 190
Synthesis of 1-(2-(3-fluorophenyl)propan-2-yl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea
F
N Y N \ I / O
N
Step 1
A solution of 2-(3-fluorophenyl)acetonitrile (5.0 g, 37.0 mmol) and methyl
iodide (5.06
mL, 81.4 mmol) in THE (100 mL) at 0 C was added sodium
bis(trimethylsilyl)amide, 1.OM
solution in THE (81.4 mL, 81.4 mmol). After addition, the mixture was stirred
at 0 C for 45
minutes. Then, H2O (100 mL) and EtOAc (100 mL) were added and the mixture was
stirred at
room temperature for 15 minutes. The organic layer was collected and the
aqueous was
extracted with EtOAc (1 x 100 mL). The combined organic extracts were dried
over MgSO4,
concentrated, and dried in vacuo to give 2-(3-fluorophenyl)-2-
methylpropanenitrile as orange
oil which was used without purification.
Step 2
A solution of 2-(3-fluorophenyl)-2-methylpropanenitrile (2.00 g, 12.3 mmol) in
HC1
(concentrated, 10 mL) and H2O (1 mL) was subjected to a microwave irradiation
at 150 C for
1 h. Then, H2O (100 mL) and EtOAc (150 mL) were added and the mixture was
stirred at
room temperature for 10 minutes. Then, the organic layer was collected, dried
over MgSO4,
and concentrated. The residue was mixed with silica gel and the solid mixture
was purified by
silica gel column chromatography using ISCO instrument (solid loading, 0%-100%
EtOAc/hexane) to give 2-(3-fluorophenyl)-2-methylpropanoic acid as orange oil.
MS (ESI, positive ion) m/z: 183 (M+1).
Step 3
A solution of 2-(3-fluorophenyl)-2-methylpropanoic acid (0.450 g, 2.47 mmol)
and
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
triethylamine (0.344 ml, 2.47 mmol) in THE (16 mL) at 0 C was added ethyl
chloroformate
(0.236 ml, 2.47 mmol). After addition, the mixture was stirred at room
temperature for 3 h.
Then, a solution of sodium azide (0.321 g, 4.94 mmol) in H2O (0.7 mL) was
added and the
mixture was stirred at room temperature for overnight. Then, H2O (5 mL) and
EtOAc (20 mL)
were added and the mixture was stirred at room temperature for 10 minutes. The
organic layer
was collected, dried over MgSO4, concentrated, and dried in vacuo to give 2-(3-
fluorophenyl)-
2-methylpropanoyl azide as yellow oil which was used without purification.
Step 4
A solution of 2-(3-fluorophenyl)-2-methylpropanoyl azide (0.487 g, 2.35 mmol)
in
THE (3 mL) was subjected to a microwave irradiation at 120 C for 15 minutes.
Then, 4-(2-
methylpyridin-4-yl)benzenamine (0.433 g, 2.35 mmol) was added and the mixture
was
subjected to a microwave irradiation at 120 C for 15 minutes. Then, the
mixture was filtered
and the filtrate was purified by preparative HPLC (0%-100% MeCN 0.1% TFA/H20
0.1%
TFA) to give a desired product in a solution of MeCN/H20 0.1% TFA. The solvent
was
removed and the aqueous solution was neutralized using NaHCO3(s). The aqueous
solution
was extracted with EtOAc. The combined extracts were dried over MgSO4, washed
with
brine, concentrated, and dried in vacuo to give 1-(2-(3-fluorophenyl)propan-2-
yl)-3-(4-(2-
methylpyridin-4-yl)phenyl)urea as yellow solid. MS (ESI, positive ion) m/z:
364 (M+1).
Example 191
Synthesis of 1-(4-(2-methylpyridin-4-yl)phenyl)-3-(2-(thiophen-3-yl)propan-2-
yl)urea
S
/ NYN I
\ \ I 0
N /
Step 1
A flask containing anhydrous cerium (III) chloride (10.2 g, 41.2 mmol) was
flushed
with nitrogen several times and placed under high vacuum. The solid was heated
with a heat
gun for several minutes to remove any excess water. The flask was re-filled
with nitrogen and
allowed to cool. THE (80 mL) was added and the suspension was stirred at room
temperature
for 18 h. The suspension was cooled to -78 C and methyllithium, 1.0 M
solution (41.2 mL,
41.2 mmol) was added. After stirring for 1 h, thiophene-3-carbonitrile (1.50
g, 13.7 mmol)
was added. The mixture was stirred for 5 h at -78 C, then allowed to slowly
warm to room
temperature over 16 h. The mixture was cooled back to -78 C, then treated
with conc.
91
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
NH4OH (30 mL). After warming to room temperature, the mixture was diluted with
water and
extracted with CH2C12. The combined organics were dried over Na2SO4, filtered
and
concentrated. The residue was then dissolved in CH2C12 and extracted with 2N
HC1. The
combined aqueous layers were basified with 5 N NaOH, and extracted with
CH2C12. The
combined organics were dried over Na2SO4, filtered and concentrated to give
brown oil. MS
(ESI, pos. ion) m/z: 125 (M-16).
Step 2
A mixture of 1,1'-carbonyldiimidazole (194 mg, 1.2 mmol) and 4-(2-
methylpyridin-4-
yl)benzenamine (200 mg, 1.1 mmol) in CH2C12 (10 mL) was stirred at room
temperature for 5
min. The solution was treated with 2-(thiophen-3-yl)propan-2-amine (153 mg,
1.1 mmol) and
triethylamine (0.45 mL, 3.2 mmol) and stirred for 6 h at room temperature. The
mixture was
diluted with saturated aqeous NaHCO3 and extracted with CH2C12. The combined
organics
were dried over Na2SO4, filtered and concentrated. The residue was
recrystallized from MeOH
to yield 1-(4-(2-methylpyridin-4-yl)phenyl)-3-(2-(thiophen-3-yl)propan-2-
yl)urea. MS (ESI
pos. ion) m/z: 352 (M+1).
Example 192
Synthesis of 1-(2-(benzofuran-5-yl)propan-2-yl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea
/ 0
NYN \
\ \ I 0
I
N /
Step 1
A flask containing anhydrous cerium (III) chloride (5.17 g, 21.0 mmol) was
flushed
with nitrogen several times and placed under high vacuum. The solid was heated
with a heat
gun for several minutes to remove any excess water. The flask was re-filled
with nitrogen and
allowed to cool. THE (80 mL) was added and the suspension was stirred at room
temperature
for 3 h. The suspension was cooled to -78 C and methyllithium, 3.0 M solution
in Et20 (6.99
mL, 21.0 mmol) was added. After stirring for 1 h, benzofuran-5-carbonitrile
(1.00 g, 6.99
mmol) was added. The mixture was stirred for 5 h at -78 C, then allowed to
slowly warm to
room temperature over 16 h. The mixture was cooled back to -78 C, then
treated with conc.
NH4OH (30 mL). After warming to room temperature, the mixture was diluted with
H2O and
extracted with CH2C12. The combined organics were dried over Na2SO4, filtered
and
92
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
concentrated. The residue was then dissolved in CH2C12 and extracted with 2 N
HC1. The
combined aqueous layers were basified with 5 N NaOH, and extracted with
CH2C12. The
combined organics were dried over Na2SO4, filtered and concentrated to yield 2-
(benzofuran-
5-yl)propan-2-amine. MS (ESI, pos. ion) m/z: 159 (M-16).
Step 2
A mixture of 4-(2-methylpyridin-4-yl)benzenamine (200 mg, 1.1 mmol) and 1,1'-
carbonyldiimidazole (194 mg, 1.2 mmol) was stirred for five minutes, then
treated with 2-
(benzofuran-5-yl)propan-2-amine (190 mg, 1.1 mmol) and triethylamine (454 l,
3.2 mmol)
and stirred for 5 h. After cooling to room temperature, the mixture was
diluted with sat aq.
NaHCO3 and extracted with 25% i-PrOH/CHC13 (3X). The combined organics were
dried
over Na2SO4, filtered and concentrated. The residue was recrystallized from
MeOH. The solid
was dissolved in DMSO and recrystallized to yield pure 1-(2-(benzofuran-5-
yl)propan-2-yl)-3-
(4-(2-methylpyridin-4-yl)phenyl)urea. MS (ESI pos. ion) m/z: 386 (M+1).
Example 193
Synthesis of 1-(2-(benzo[b]thiophen-5-yl)propan-2-yl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea
/ S
H N N
\ /
\ I 1
/ r[
\ \ I O
I
N /
Step 1
A flask containing anhydrous cerium (III) chloride (4.64 g, 18.8 mmol) was
flushed
with nitrogen several times and placed under high vacuum. The solid was heated
with a heat
gun for several minutes to remove any excess water. The flask was re-filled
with nitrogen and
allowed to cool. THE (80 mL) was added and the suspension was stirred at room
temperature
for 3 h. The suspension was cooled to -78 C and methyllithium (3.0 M solution
in Et20, 6.28
mL, 18.8 mmol) was added. After stirring for 1 h, benzo[b]thiophene-6-
carbonitrile (1.00 g,
6.28 mmol) was added. The mixture was stirred for 5 h at -78 C, then allowed
to slowly
warm to room temperature over 16 h. The mixture was cooled back to -78 C,
then treated
with conc. NH4OH (30 mL). After warming to room temperature, the mixture was
diluted with
H2O and extracted with CH2C12. The combined organics were dried over Na2SO4,
filtered and
concentrated. The residue was then dissolved in CH2C12 and extracted with 2 N
HC1. The
combined aqueous layers were basified with 5 N NaOH, and extracted with
CH2C12. The
93
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
combined organics were dried over Na2SO4, filtered and concentrated to yield 2-
(benzo[b]thiophen-5-yl)propan-2-amine. MS (ESI pos. ion) m/z: 175 (M-16).
Step 2
A mixture of 4-(2-methylpyridin-4-yl)benzenamine (200 mg, 1.1 mmol) and 1,1'-
carbonyldiimidazole (194 mg, 1.2 mmol) in CH2C12 (10 mL) was stirred for 5
minutes, then
treated with 2-(benzo[b]thiophen-5-yl)propan-2-amine (208 mg, 1.1 mmol) and
triethylamine
(0.45 mL, 3.2 mmol) and stirred for 5 h. After cooling to room temperature,
the mixture was
diluted with saturated aqueous NaHCO3 and extracted with 25% i-PrOH/CHC13. The
combined organics were dried over Na2SO4, filtered and concentrated. The
residue was
recrystallized from MeOH to yield pure 1-(2-(benzo[b]thiophen-5-yl)propan-2-
yl)-3-(4-(2-
methylpyridin-4-yl)phenyl)urea. MS (ESI pos. ion) m/z: 402 (M+1).
Example 194
Synthesis of 1-(2-(5-bromothiophen-2-yl)propan-2-yl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea
H H
/ N N ' S Br
0 _K'
N
Step 1
A solution of diisopropylamine (19.9 mL, 141.3 mmol) in THE (90 mL) in an oven-
dried round-bottomed flask was cooled to -5 C under nitrogen, and was treated
with
butyllithium (2.5M solution in hexanes, 56.5 mL, 141.3 mmol) in a dropwise
manner. The
reaction was stirred at -5 C under nitrogen. After 30 minutes, a solution of
thiophene-2-acetic
acid (5.02 g, 35.3 mmol) in THE (10 mL) was added to the reaction in a
dropwise fashion.
After another 30 minutes, iodomethane (13.7 mL, 22.0 mmol) was added in a
dropwise
fashion. The reaction was slowly allowed to warm to 23 C. Ater 16 h, the
suspension was
diluted with EtOAc (150 mL) and washed with 10% aqueous hydrochloric acid
solution (100
mL) and brine (100 mL), dried over MgSO4, concentrated in-vacuo and purified
by silica gel
chromatography using methanol/dichloromethane as the eluant affording 2-methyl-
2-
(thiophen-2-yl)propanoic acid as an an off-white solid. MS (ESI pos. ion) m/z:
171 (M+1).
Step 2
94
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
A solution of 2-methyl-2-(thiophen-2-yl)propanoic acid (264 mg, 1.551 mmol) in
dichloromethane (10 mL) was treated with n-bromosuccinimide (345 mg, 1.939
mmol). The
reaction was stirred at 23 C under nitrogen. After 21 h, the reaction was
diluted with EtOAc
(75 mL) and washed with water (50 mL) and brine (50 mL), dried over MgSO4,
concentrated
in vacuo and purified by silica gel chromatography using
methanol/dichloromethane as the
eluant affording 2-(5-bromothiophen-2-yl)-2-methylpropanoic acid as an an off-
white solid.
MS (ESI pos. ion) m/z: 249 (M+1).
Step 3
A solution of 2-(5-bromothiophen-2-yl)-2-methylpropanoic acid (266 mg, 1.068
mmol)
and triethylamine (0.446 mL, 3.203 mmol) in THE (10 mL) was cooled to 0 C and
was treated
with ethyl chloroformate (0.204 mL, 2.135 mmol). After 1 h, a solution of
sodium azide (208
mg, 3.203 mmol) in water (0.9 mL) was added, and the reaction was stirred at
23 C. After 17
h, the reaction was diluted with EtOAc (100 mL) and washed with water (50 mL)
and brine (50
mL), dried over MgS04, concentrated in-vacuo, affording 2-(5-bromothiophen-2-
yl)-2-
methylpropanoyl azide as yellow oil which was used without purification.
Step 4
A solution of 1-azido-2-(5-bromothiophen-2-yl)-2-methylpropan-1-one (290 mg,
1.058
mmol) in THE (2 mL) was heated to 120 C in a microwave for 15 minutes. 4-(2-
methylpyridin-4-yl)benzenamine (195 mg, 1.058 mmol) was added, followed by THE
(0.5
mL), and reaction was heated in a microwave at 120 C for 15 minutes. The
reaction was
concentrated in vacuo and purified by silica gel chromatography using
methanol/dichloromethane as the eluant, affording 1-(2-(5-bromothiophen-2-
yl)propan-2-yl)-3-
(4-(2-methylpyridin-4-yl)phenyl)urea as a light yellow solid. MS (ESI pos.
ion) m/z: 430
(M+1)
Example 195
Synthesis of 1-((1-(2-methoxyphenyl)cyclobutyl)methyl)-3-(4-(2-methylpyridin-4-
yl)phenyl)urea
H H OMe
N N
\ I O
N /
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
A solution of azido(4-(2-methylpyridin-4-yl)phenyl)methanone (50 mg, 0.210
mmol)and 1,4-dioxane (2 mL) was heated at 120 C for 10 min. 1-(2-
Methoxyphenyl)
eye lobutyl)methanamine in 1,4-dioxane (2 mL) was added and the reaction was
allowed to
cool to room temperature with stirring. After 30 minutes, the reaction was
concentrated in
vacuo then purified by silica-gel chromatography using MeOH-DCM as the eluant
to give 1-
((1-(2-methoxyphenyl)cyclobutyl)methyl)-3-(4-(2-methylpyridin-4-yl)phenyl)urea
as a light
yellow oil. MS (ESI pos. ion) m/z: 402 (M+1).
Biological Examples
Example 1
Cell-based ... in vitro assays
GSM cell-based assays were designed to measure the modulation of A(3 42 from
HEK
293 cells over-expressing APP.
Three simultaneous cell-based assays, namely A(3 42 inhibition or reduction,
A(3 40
inhibition or reduction and a measurement of cell viability of the cells from
which the A(3
42/40 readout was obtained, together gave an interpretation of gamma secretase
modulation
(GSM). The assays measure A(3 42 and A(3 40 from conditioned medium of test
compound
treated HEK 293 cells.
HEK293 cells stably expressing full length Amyloid Precursor Protein (APP)
were
plated at a density of 100K cells/well in 96 well plates (Costar). The cells
were cultivated for 6
hours at 37 C and 5% CO2 in DMEM supplemented with 10% FBS. The test compounds
were
then added to cells in 10-point dose response concentrations with the starting
concentration
being 10 M. The compounds were diluted from stock solutions in DMSO and the
final
DMSO concentration of the test compounds on cells was 0.1%. After 24 hours of
incubation
with the test compounds the supernatant conditioned media was collected and
the A(3 42, A(3
40 levels were determined using a sandwich ELISA. A cell viability test
(CellTiter-Blue Cell
Viability assay, Promega, using the manufacturers protocol) on the cells from
which the
conditioned medium was harvested for A(3 42 or A(3 40 readouts gave an
indication of cell
survivability as a possible reason for false positive A(3 42 or 40 reduction
or inhibition readout.
The IC50 of the compound (for either A(3 42 or A(3 40) was calculated from the
percent of
control or percent inhibition of A(3 42 or A(3 40 as a function of the
concentration of the test
compound.
96
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
The sandwich ELISA to detect A(3 42 or A(3 40 was performed in 96 well
microtiter
plates, which were pre-treated with goat anti-rabbit IgG (Pierce). The capture
and detecting
antibody pair that were used to detect A(3 42 and A(3 40 from cell
supernatants were rabbit
monoclonal Antibody 42 (RabMAb 42) and affinity purified polyclonal Antibody
40
(pAbeta40, Biosource) as capture antibodies and biotinylated 6E10 monoclonal
(Signet Labs
Inc.) as detection antibody. The optimal concentration for RabMAb 42 was 1
pg/ml in
Superblock/TBS (Pierce) that was supplemented with 0.05%Tween 20 (Sigma). The
optimal
concentration for the pAb40 antibody was 3 pg/ml in Superblock/TBS (Pierce)
that was
supplemented with 0.05%Tween 20 (Sigma). Optimal concentration for the
detection antibody
6E10-biotinylated was 0.5 pg/ml in Superblock/TBS (Pierce) that had been
supplemented with
2% normal goat serum and 2 % normal mouse serum.
Cellular supernatants were incubated with the capture antibody for 16-20 hours
at 4 C,
followed by 3 wash steps in TBS-tween (0.05%). The detecting antibody
incubation was for 3
hours at 4 C, again followed by the wash steps as described previously. The
final readout of
the ELISA is Time-Resolved Fluorescence (counts per minute) using Delfia
reagents
Streptavidin-Europium and Enhancement solutions (Perkin Elmer) and the
EnVision
Multilabel plate reader (Perkin Elmer).
The approximate IC50 value of a representative number of compounds of Formula
(I)
for the reduction of Ab42 in this assay is provided in the tables below.
Cpd # IC50 Cpd # IC50 Cpd # IC50
(uM) (uM) (uM)
T-1-1 0.224 T-1-74 3.33 T-1-84 10
T-1-3 0.131 T-1-75 3.13 T-1-85 10
T-1-5 0.748 T-1-76 2.68 T-1-86 8.62
T-1-6 1.517 T-1-78 2.36 T-1-87 0.62
T-1-8 1.082 T-1-81 10 T-1-88 1.00
T-1-90 9.93 T-1-111 10 T-1-120 3.63
T-1-92 10 T-1-113 10 T-1-121 1.04
T-1-93 5.45 T-1-116 10 T-1-123 2.37
T-1-94 10 T-1-117 2.58 T-1-125 1.11
T-1-95 10 T-1-118 0.311 T-1-126 1.08
T-1-128 10 T-1-141 1.30 T-1-151 1.82
97
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
Cpd # IC50 Cpd # IC50 Cpd # IC50
(uM) (uM) (uM)
T-1-129 1.33 T-1-142 4.15 T-1-152 10
T-1-131 10 T-1-144 1.39 T-1-157 10
T-1-136 3.33 T-1-147 10 T-1-162 10
T-1-138 1.10 T-1-149 1.78 T-1-163 10
T-1-164 3.33 T-1-192 10 T-1-200 4.35
T-1-165 0.644 T-1-193 10 T-1-207 0.27
T-1-168 3.33 T-1-197 10 T-1-215 10
T-1-172 1.94 T-1-198 5.91 T-1-217 0.76
T-1-191 0.955 T-1-199 10 T-1-218 0.56
T-1-219 10 T-1-226 1.50 T-1-222 0.64
T-1-221 10 T-1-227 0.70 T-1-223 10
T-1-224 0.21
Cpd # IC50 (nM) Cpd # IC50 (nM)
T-2-1 0.36 T-2-10 0.75
T-2-3 0.201 T-2-12 3.41
T-2-5 1.12 T-2-18 0.76
T-2-6 1.48 T-2-19 2.31
T-2-8 2.66 T-2-23 2.75
T-2-24 10 T-2-33 0.88
T-2-35 2.5 T-2-36 0.13
T-1-1 means Compound Table 1, cpd. 1 and T-2-1 means Compound Table 2, cpd. 1.
Example 2
Quantification of A1342 peptides in brain, CSF and plasma... in vivo assay
Four to six month old Sprague Dawley rats (200-250 g) were administered
compound
of the Invention formulated at 10 ml/kg in 2% HPMC (hydroxypropyl
methylcellulose), 1%
Tween80, pH 2.2 by oral gavage. One to seventy two hours following the
administration of
the compound, the animals were sacrificed. Cerebrospinal fluid was isolated
from the cisterna
98
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
magna, blood was taken via cardiac puncture and brains were removed and
divided into
quarters. Samples were frozen on dry ice and stored at -80 C. Brains were
homogenized in
volumes (w/v) of 0.2% diethylamine (DEA) in 50 mM NaCl and centrifuged at
355,000g,
4 C for 30 minutes. CSF or brain supernatants were then analyzed for the
presence of A1342
peptide by specific sandwich ELISA assays based on ECL
(Electrochemiluminescence)
technology as described below.
Rat A(3 42 peptide was quantified using biotinylated 4G8 antibody (Signet) for
capture
and ruthenylated ConFab42 antibody (ConFab42 was generated at Biosite, Inc.
Briefly, phage
libraries expressing Fabs (Fragment Antigen Binding portion of an antibody)
were prepared
from mice immunized with two peptides, A1334-42 and A1335-43. Fab libraries
were then
screened for their ability to bind to A1342 with less than 0.1% cross-
reactivity to A1340).
Initially the MSD 96-well avidin plates were coated with biotinylated 4G8
capture antibody
(0.25 ug/well in PBS) by incubation for 1.5 hours at 4 C. After a wash and
blocking step, 25
ul of the either brain homogenate (CSF or plasma) or synthetic A(3 peptide
standard was added
to the MSD plate followed by addition of 25 ul of ruthenylated ConFab42 (5
ug/ml in MSD
antibody diluent) for overnight incubation at 4 C with shaking. The standards
were made
using serial dilutions of synthetic human A(3 42 peptide (Anaspec) and final
concentrations
ranged from 6.25 - 800 pg/ml in brain homogenization buffer (DEA or TBS
Triton) or MSD
Tris lysis buffer for rat CSF and plasma analysis. Rat brain and plasma were
run neat in the
assay whereas CSF was diluted 1:5 in MSD Tris lysis buffer. The 96-well plates
were washed
twice the next morning and read in the MSD Sector Imager 6000 to quantify the
ECL signals
in the wells after addition of 150 ul/ well of MSD read buffer T with
surfactant lx. The data
were analyzed using commercially available software such as Softmax Pro, Excel
and
GraphPad Prism programs.
Formulation Examples
The following are representative pharmaceutical formulations containing a
compound
of Formula (I).
Tablet Formulation
The following ingredients are mixed intimately and pressed into single scored
tablets.
Ingredient Quantity per tablet
99
CA 02722606 2010-10-26
WO 2009/137404 PCT/US2009/042711
MR
compound of this invention 400
cornstarch 50
croscarmellose sodium 25
lactose 120
magnesium stearate 5
Capsule Formulation
The following ingredients are mixed intimately and loaded into a hard-shell
gelatin
capsule.
Ingredient Quantity per capsule
MR
compound of this invention 200
lactose spray dried 148
magnesium stearate 2
The foregoing invention has been described in some detail by way of
illustration and
example, for purposes of clarity and understanding. It will be obvious to one
of skill in the art
that changes and modifications may be practiced within the scope of the
appended claims.
Therefore, it is to be understood that the above description is intended to be
illustrative and not
restrictive. The scope of the invention should, therefore, be determined not
with reference to
the above description, but should instead be determined with reference to the
following
appended claims, along with the full scope of equivalents to which such claims
are entitled.
All patents, patent applications and publications cited in this application
are hereby
incorporated by reference in their entirety for all purposes to the same
extent as if each
individual patent, patent application or publication were so individually
denoted.
100