Note: Descriptions are shown in the official language in which they were submitted.
CA 02723311 2010-11-02
WO 2009/144190 PCT/EP2009/056278
-1-
Process for the bleaching of paper pulp
The present application claims the benefit of the European application
No. 08156934.5 filed on May 26, 2008, herein incorporated by reference.
The present invention relates to a process for the bleaching of paper pulp,
especially mechanical paper pulp, through a bleaching treatment with hydrogen
peroxide and an alkali source. Such bleaching treatment usually involves the
use
of peroxide stabilizers.
It is known to bleach mechanical paper pulp with oxidizing agents such as
hydrogen peroxide in an alkaline medium and in the presence of a peroxide
stabilizer.
For instance, the US patent 6221209 of SOLVAY INTEROX relates to a
process for bleaching a chemical paper pulp by first purifying the pulp so as
to
reduce its manganese content and then by bleaching it with hydrogen peroxide
in
alkaline medium, especially at a pH adjusted to at least 10 by the addition of
an
alkaline compound (i.e. sodium hydroxide), and in the presence of a
stabilizing
agent, namely sodium silicate.
It is also known to use other alkaline sources than sodium hydroxide, such
as magnesium hydroxide. For example, the US patent 7052578 discloses the use
of magnesium hydroxide for the hydrogen peroxide bleaching of mechanical
paper pulp.
However, even if, according to US 7052578, it is possible to reach high
brightness results using magnesium hydroxide alone as alkaline source, it is
more difficult compared to the use of sodium hydroxide and sodium silicate as
it
requires an extended reaction time, an increased peroxide charge, and the
addition of chelating agents such as DTPA, even if a chelating step was
conducted prior to the bleaching treatment.
When magnesium hydroxide is used as alkaline source in the hydrogen
peroxide bleaching of mechanical paper pulp, it is difficult to achieve a
satisfactory brightness, and the consumption of hydrogen peroxide for a given
brightness is high.
The purpose of the present invention is to provide a process which does not
present the above disadvantages and which enables to obtain a higher paper
pulp
brightness compared to the known use of other alkaline sources than sodium
CA 02723311 2010-11-02
WO 2009/144190 PCT/EP2009/056278
-2-
hydroxide, such as magnesium hydroxide or calcium hydroxide. The purpose of
the present invention is also to provide a process which allows decreasing the
consumption of hydrogen peroxide for a given brightness.
The present invention therefore relates to a process for the bleaching of
paper pulp in which an aqueous suspension of paper pulp is subjected to a
bleaching treatment with hydrogen peroxide in the presence of at least one
alkaline earth hydroxide selected from magnesium hydroxide and calcium
hydroxide and of at least one poly-a-hydroxyacrylic acid, its salt or their
mixture.
Indeed, it has surprisingly been found that, in the presence of at least one
poly-a-hydroxyacrylic acid, its salt or their mixture, it is possible to reach
higher
brightness, or to decrease the hydrogen peroxide consumption for a given
brightness, when at least part of the alkaline compound is selected from
alkaline
earth hydroxides and especially from magnesium hydroxide and calcium
hydroxide.
Furthermore, sodium silicate cannot be used when using alkaline earth
hydroxides such as magnesium hydroxide and/or calcium hydroxide as alkaline
source, because it forms precipitates with those products, leading to scaling.
Such scaling creates problems in the whole production line including in the
paper
machine, when the mill is an integrated site. Thus, when alkaline earth
hydroxides such as magnesium hydroxide and/or calcium hydroxide are used, the
common practice is to bleach without any stabilizer, except
aminopolycarboxylic
acids or their salts, such as diethylene triamine penta-acetic acid (DTPA) or
its
sodium salt which will act as chelating agents and will therefore limit the
decomposition of the hydrogen peroxide which is catalyzed by transition metals
such as manganese or iron. Nevertheless, the conditions present during the
bleaching are usually not suitable for a good efficiency of such chelating
agents
and therefore the effect of such stabilizers is very limited.
One of the essential features of the present invention resides in the
combined use of an alkaline earth hydroxide selected from magnesium hydroxide
and calcium hydroxide as alkaline source (referred to as "alkaline earth
hydroxide according to the invention") and of poly-a-hydroxyacrylic acid, its
salt or their mixtures (referred to as PHAA) as hydrogen peroxide stabilizer,
during the hydrogen peroxide bleaching of paper pulp.
The PHAA used in the process of the invention generally has a molecular
weight within a specific range. As the molecular weight is rather difficult to
CA 02723311 2010-11-02
WO 2009/144190 PCT/EP2009/056278
-3-
measure, it is inferred by measuring the viscosity, the higher the molecular
weight, the higher the viscosity. Viscosity in the range of 10 to 100 mPa.s is
usual but more particularly it can be in the range of 20 to 60 mPa.s.
Commonly,
the PHAA is used as a sodium salt of poly-a-hydroxyacrylic acid, corresponding
to sodium poly-a-hydroxyacrylate (referred to as PHAS). In the invention, good
results are obtained using only one PHAA. Nevertheless, more than one PHAA
can be used.
The bleaching treatment according to the present invention is generally
carried out at an initial pH of at least 8, in particular of at least 8,5,
values of at
least 9 being preferred. The initial pH is usually at most 14, in special
cases at
most 13, values of at most 12 being convenient. The final pH of the aqueous
paper pulp suspension, at the end of the bleaching treatment is in general at
least 6, especially at least 7, in particular at least 8. The final pH of the
aqueous
paper pulp suspension at the end of the bleaching treatment is in many cases
at
most 11, particularly at most 10. The pH of the aqueous suspension before,
during or after the bleaching treatment is measured using equipment normally
found in pulp mills for such a purpose. According to the invention, the
natural
pH of the paper pulp suspension is adjusted to the required value by means of
an
alkaline compound comprising at least one alkaline earth hydroxide selected
from magnesium hydroxide and calcium hydroxide.
The amount of alkaline earth hydroxide according to the invention, used in
the bleaching step of the present invention, is generally such that the pH of
the
suspension is maintained within the above-mentioned range. This amount is
usually at least 0,1 % by weight of dry pulp, preferably at least I% by weight
of
dry pulp, more preferably at least 2% by weight of dry pulp, especially above
2%
by weight of dry pulp. The amount of alkaline earth hydroxide according to the
invention, used in the bleaching step of the present invention, is generally
at
most 10% by weight of dry pulp, with particular preference at most 5% by
weight of dry pulp. The amount of alkaline earth hydroxide according to the
invention, used in the bleaching step of the present invention, is for
instance of
from 2 to 5% by weight of dry pulp, for example about 3% by weight of dry
pulp.
The amount of alkaline earth hydroxide according to the invention depends
on the amount of hydrogen peroxide used and on the type of pulp. The ratio
alkaline earth hydroxide according to the invention / hydrogen peroxide can
for
example vary from 0,3 to 2,5, preferably from 0,5 to 2,0 depending on the
CA 02723311 2010-11-02
WO 2009/144190 PCT/EP2009/056278
-4-
amount of hydrogen peroxide added and on the type of pulp. For example, the
ratio may be about 1.
The alkaline earth hydroxide according to the invention is usually added to
the paper pulp suspension in the form of an aqueous slurry. Preferably, the
particle size of the alkaline earth hydroxide according to the invention is
sufficiently small for the slurry to be readily suspendible, advantageously
having
a mean particle size of less than about 10 m, preferably less than 6 gin, for
example about 2 m.
The bleaching step of the invention is generally carried out at a consistency
of at least 10 % of dry pulp based on the weight of pulp suspension. The
efficiency of the process increases as the consistency is increased up to a
value of
around 30 % by weight of dry pulp. The consistency can be up to 40 % by
weight of dry pulp.
The bleaching treatment of the invention is usually carried out using an
amount of hydrogen peroxide required to achieve the final target brightness.
The
amount of hydrogen peroxide is generally equal to or higher than 0,1 % by
weight of dry pulp, preferably equal to or higher than 0,5 % by weight of dry
pulp, more preferably equal to or higher than 1 % by weight of dry pulp. The
amount of hydrogen peroxide is usually equal to or lower than 8 % by weight of
dry pulp, especially equal to or lower than 6 % by weight of dry pulp, and
more
particularly equal to or lower than 5 % by weight of dry pulp. For example,
the
amount of hydrogen peroxide may be about 2 to 4% by weight of dry pulp.
The bleaching treatment of the invention is usually carried out at a
temperature equal to or higher than 40 C, especially equal to or higher than
60 C. The temperature can be up to about 100 C. The temperature is preferably
less than 100 C, more preferably equal to or less than 95 C, for example equal
to
or less than 85 C. The optimum bleach temperature depends on the wood type
and pulping process but is normally in the range of 60 to 95 C.
The duration of the bleaching treatment of the process of the invention is
usually from 10 min to 6 h, varying from mill to mill. For instance, it can
vary
from 30 to 300 min.
For example, the bleaching treatment can be carried out at a pH of from
8 to 14, at a consistency of from 10 to 40 %, and at a temperature of from 60
to
95 C, during 1 to 4 hours.
The amount of PHAA, especially PHAS, used in the bleaching step of the
invention is usually equal to or higher than 0,05 % by weight of dry pulp,
CA 02723311 2010-11-02
WO 2009/144190 PCT/EP2009/056278
-5-
especially equal to or higher than 0,1 % by weight of dry pulp. The amount of
PHAA is commonly equal to or lower than 2 % by weight of dry pulp, values of
at most 1 % being suitable. For example, typical amounts of PHAA are about
0,1 to 0,5 % by weight of dry pulp. The optimum amount is dependent on the
amount of metallic ions in the pulp and must be optimized for each particular
mill.
The bleaching step of the invention can be carried out in the presence of
any other alkaline or alkaline earth hydroxides than the alkaline earth
hydroxides
according to the invention, such as in the presence of sodium hydroxide. The
bleaching step of the invention may also be carried out in the absence sodium
hydroxide. The bleaching step of the invention can also be carried out in the
presence of any other additives, such as magnesium sulfate or other soluble
magnesium salts. Preferably, the bleaching step is carried out in the absence
of
any other stabilizer, and especially in the absence of sodium silicate.
In a preferred embodiment, prior to the bleaching treatment, the aqueous
suspension of the paper pulp may be subjected to a chelating treatment to
reduce
the metallic ion content of the suspension, and especially its manganese
content.
It is for example usual to carry out a pretreatment step (or chelating
treatment),
with at least one aminopolycarboxylic acid, its salt or their mixture
(referred to
as APCA) prior to the bleaching treatment.
The APCA used in the pretreatment step is generally chosen from ethylene
diamine tetra-acetic acid (EDTA), diethylene triamine penta-acetic acid
(DTPA),
triethylene tetramine hexa-acetic acid (TTHA), cyclohexane diamine tetra-
acetic
acid (CDTA), methylglycine di-acetic acid (MGDA), nitrilo tri-acetic acid
(NTA). The APCA may be used as the free acid, its salt or their mixtures. The
APCA is preferably used as its salt. The salt is usually the alkali metal salt
such
as sodium or potassium or the ammonium salt or a mixture thereof. Sodium salts
give good results. The most preferred APCA is the sodium salt of DTPA. Good
results are generally obtained using only one APCA. Nevertheless, more than
one APCA can be used. The chelating pretreatment step may be conducted as
described in the International patent application filed as EP2008/052056, the
text
of which is incorporated herein by reference.
In a variant of the invention, the bleaching treatment may be carried out in
the presence of at least one chelating agent, in particular of at least one
APCA,
such as those disclosed above. According to such a variant, the amount of
APCA used in the bleaching treatment of the invention is usually at least
CA 02723311 2010-11-02
WO 2009/144190 PCT/EP2009/056278
-6-
0,05 % wt based on the weight of dry pulp. The amount of APCA is commonly
at most about 1 % wt based on the weight of dry pulp, values of at most 0,8 %
wt
being suitable.
If at least one APCA is present during the bleaching treatment of the
invention, the weight ratio between PHAA, especially PHAS, and APCA is
usually at least 1:10, preferably at least 1:5, ratios of at least 1:3 being
satisfactory. The weight ratio PHAA / APCA, especially PHAS / APCA, is
generally at most 10:1, especially at most 5:1, values of at most 3:1 giving
the
best results.
In another variant of the invention, the bleaching treatment is carried out in
the absence of a chelating agent or of another stabilizer than PHAA.
The paper pulp treated in the present invention can be chosen from
chemical paper pulps, mechanical paper pulps or recycled paper pulps. The best
results are obtained with mechanical paper pulps. By mechanical paper pulps
are
meant paper pulps obtained by mechanical treatment. Examples of such paper
pulps are pressure groundwood (PGW), stone groundwood (SGW),
thermomechanical pulp (TMP), refiner mechanical pulp (RMP),
chemithermomechanical pulp (CTMP) and alkaline peroxide mechanical pulp
(APMP or APP). The present invention therefore also relates to the bleaching
process of the invention applied to mechanical paper pulp.
The process of the invention can further comprise other treatment steps
such as one or more additional dilution step(s), one or more additional
chelating
step(s), one or more additional bleaching step(s), one or more washing step(s)
and/or one or more extraction step(s).
It is recommended to have a good mixing of the paper pulp to be treated
with the hydrogen peroxide, the alkaline earth hydroxide according to the
invention, and the PHAA. The order of addition of the alkaline earth hydroxide
of the invention, the PHAA and the hydrogen peroxide is either first the PHAA,
then the alkaline earth hydroxide of the invention, and lastly the hydrogen
peroxide or first the alkaline earth hydroxide of the invention, then the
PHAA,
and lastly the hydrogen peroxide. Those three products may also be premixed
all
together or two of them can be premixed. The alkaline earth of the invention
is
usually added as a suspension in water. Dilution water may also be added.
Usually, the reactants are introduced in the paper pulp suspension via a pump
and a high consistency mixer, especially when the bleaching is performed at a
CA 02723311 2010-11-02
WO 2009/144190 PCT/EP2009/056278
-7-
consistency close to 30%. An example of configuration is : the chemicals are
added in the preheating screw of the HC mixer.
The present invention also relates to the use of at least one alkaline earth
hydroxide selected from magnesium hydroxide and calcium hydroxide to control
the pH of an aqueous suspension of paper pulp during the bleaching treatment
of
the paper pulp with hydrogen peroxide, wherein said bleaching treatment is
conducted in the presence of at least one poly-a-hydroxyacrylic acid, its salt
or
their mixture.
The present invention is further illustrated below without limiting the
scope thereto.
Examples 1 (comparative) and 2 (according to the invention)
According to example 1, a mechanical pulp produced by a TMP process
using spruce as raw material, at a consistency of about 15 %, was bleached at
80 C during 3 hours. Hydrogen peroxide was added in an amount of 3 % by
weight of dry pulp. Magnesium hydroxide was added in an amount of 3 % by
weight of dry pulp. The brightness of the paper pulp obtained in these
conditions
was 75,1 ISO and the residual peroxide content after the bleaching treatment
was 9 % of the initial charge.
In example 2, the same conditions as in example 1 were applied except that
0,5% of PHAS were added to the bleaching step. The resulting brightness of the
paper pulp was 76,6 ISO and the residual peroxide content was 17% of the
initial charge.
Those two examples illustrate that, according to the invention, a higher
brightness is obtained when a PHAA is added during the hydrogen peroxide
bleaching of paper pulp in the presence of magnesium hydroxide as basic
compound.
Examples 3 (comparative) and 4 (according to the invention)
In example 3, a mechanical pulp produced by a TMP process using spruce
as raw material, at a consistency of about 15 %, was bleached at 70 C during
2 hours. Hydrogen peroxide was added in an amount of 3 % by weight of dry
pulp. Magnesium hydroxide was added in an amount of 3 % by weight of dry
pulp. The brightness of the paper pulp obtained in these conditions was
73,8 ISO and the residual peroxide content after the bleaching treatment was
27 % of the initial charge.
In example 4, the same mechanical pulp as in example 3 was bleached at
70 C during 2 hours in the presence of an amount of hydrogen peroxide of 2 %
CA 02723311 2010-11-02
WO 2009/144190 PCT/EP2009/056278
by weight of dry pulp, of magnesium hydroxide of 3 % by weight of dry pulp,
and of an amount of PHAS of 0,3 % by weight of dry pulp. The resulting
brightness of the paper pulp was the same as in example 3 (73,8 ISO) and the
residual peroxide content was 31 % of the initial charge.
Those two examples illustrate that, according to the invention, it is possible
to decrease the hydrogen peroxide consumption for a given brightness.
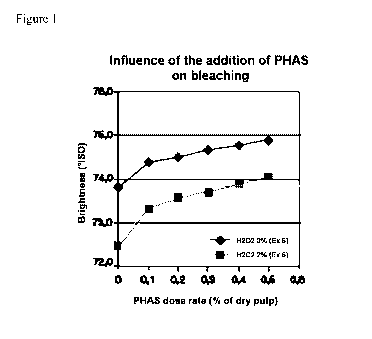
Examples 5 and 6 (Figure 1)
In example 5, a mechanical pulp produced by a TMP process using spruce
as raw material, at a consistency of about 15 %, was bleached at 70 C during 2
hours. Hydrogen peroxide was added in an amount of 3 % by weight of dry pulp.
Magnesium hydroxide was added in an amount of 3 % by weight of dry pulp.
PHAS was added in an amount of 0 to 0,5 % by weight of dry pulp.
The same conditions as in example 5 were reproduced in example 6, except
that the amount of hydrogen peroxide was 2 % by weight of dry pulp.
These results show that an amount of PHAS of 0,35 % by weight of dry
pulp can reduce the hydrogen peroxide consumption by 1 % by weight of dry
pulp for the same brightness. These results also show that for an amount of
hydrogen peroxide of 2 % by weight of dry pulp, the brightness of the
resulting
paper pulp can be increased from 72,4 to 73,9 ISO by the addition of an
amount
of PHAS of 0,35 % by weight of dry pulp.