Note: Descriptions are shown in the official language in which they were submitted.
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
A SYSTEM COMPRISING THE GASIFICATION OF FOSSIL FUELS
TO PROCESS UNCONVENTIONAL OIL SOURCES
FIELD OF THE INVENTION
The present invention pertains to the field of processing oil from
unconventional oil
sources, and in particular to a facility comprising integrated systems for the
extraction,
and optionally upgrading and/or refinement, of unconventional oil sources,
using the
gasification of fossil fuels as a primary source of energy.
BACKGROUND OF THE INVENTION
The world's oil resources fall into two main categories, conventional oil
resources and
unconventional oil resources. Conventional oil is also known as light oil, and
much of the
world's conventional oil resources are found in Saudia Arabia. Production and
processing
costs associated with this type of oil are relatively low compared to those
associated with
unconventional oil. Unconventional oil refers, in general, to oil resources
that are more
difficult to extract than conventional oil. The majority of unconventional oil
resources are
found in Canada and Venezuela. Unconventional oils include, for example, heavy
oils,
tar sands, and oil shale. Heavy oil is characterized by its content of
asphaltenes and is a
very dense and viscous oil. Tar sands and oil shale are described below.
Oil shale is a fine-grained sedimentary rock, containing significant amounts
of kerogen (a
solid mixture of organic chemical compounds), from which liquid hydrocarbons
can be
manufactured. The oil shale industry is well-established in Estonia, China,
and Brazil, and
the United States is taking steps in that direction.
Oil shale is usually mined and then shipped elsewhere, after which it is
directly burnt to
generate electricity or undergoes further processing. The most-often used
methods of
surface mining are open pit mining and strip mining. These procedures remove
most of
the overlying material to expose the oil shale deposits, and are practical
when the deposits
are close to the surface. Underground mining of oil shale, which removes less
of the
overlying material, employs the room-and-pillar method.
1
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
The extraction of its useful components usually takes place above ground (ex
situ
processing), although several newer technologies perform this underground (on-
site or in
situ processing). In either case, after access to the shale is gained, its
kerogen is converted
to synthetic crude oil and shale gas through the chemical process of
pyrolysis. Most
conversion technologies involve heating shale in the absence of oxygen to a
temperature
at which kerogen is decomposed (pyrolysed) into gas, condensable oil, and a
solid
residue; this usually takes place between 450 C (842 F) and 500 C (932 F).
The
process of decomposition begins at relatively low temperatures (300 C/570
F), but
proceeds more rapidly and more completely at higher temperatures.
During the course of in-situ processing, the oil shale is heated underground.
These
technologies can potentially extract more oil from a given area of land than
ex-situ
processes, since they can access the material at greater depths than surface
mines. Several
companies have patented methods for in-situ retorting. However, most of these
methods
are still in the experimental phase. The methods are usually classified as
true in-situ
processes (TIS) and modified in-situ processes (MIS). True in-situ processes
do not
involve mining the oil shale. Modified in-situ processes drill a large shaft
to transport
workers and equipment to the shale formation, fracture the deposit and crush
it, and ignite
the rubble.
U.S. Patent Nos. 4,449,586 and 4,067,390 describe processes for the recovery
of
hydrocarbonaceous oil from oil shale.
Tar sands (also referred to as oil sands) are a combination of clay, sand,
water, and
bitumen, a heavy black viscous oil. Tar sands can be mined and processed to
extract the
oil-rich bitumen, which is then refined into oil. The bitumen in tar sands
cannot be
pumped from the ground in its natural state; instead tar sand deposits are
mined, usually
using strip mining or open pit techniques or produced in situ by underground
heating or
other tertiary recovery processes.
Tar sands deposits near the surface can be recovered by open pit mining
techniques. After
mining, the tar sands are transported to an extraction plant, where a hot
water process
separates the bitumen from sand, water, and minerals. Regardless of the exact
nature of
the physical composition of sand and bitumen in the tar sands, the bitumen may
be readily
separated from the sand by hot-water separation techniques wherein the bitumen
phase is
2
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
disengaged from the sand phase. U.S. Patent Nos. 3,556,981; 3,847,789; and
3,893,907
are illustrative of a number of patents describing hot water extraction
processes for the
separation of bitumen from tar sands.
In those instances where bitumen deposits are buried too deep for mining to be
economical, wells are drilled to access the bitumen deposits, and in-situ
production
methods are used to extract the bitumen. These in situ production methods
include steam
injection, solvent injection, and firefloods, in which oxygen is injected and
part of the
resource burned to provide heat. Typically, steam injection methods are used.
There are a number of different steam-injection techniques in use including
the Cyclic
Steam Stimulation (CSS) or "huff-and-puff' method where the well is put
through cycles
of steam injection, heat soak, and pumped oil production. This process is
repeated until
the cost of injecting steam becomes uneconomical, for instance if the cost is
higher than
the money made from producing oil. The CSS method has the advantage that
recovery
factors are around 20 to 25% and the disadvantage that the cost to inject
steam is high.
Another steam-injection method, steam assisted gravity drainage (SAGD),
utilizes
directional drilling technology. Two horizontal wells are drilled in the oil
sands, one at
the bottom of the formation and another about 5 metres above it. These wells
are typically
drilled in groups off central pads and can extend for miles in all directions.
In each well
pair, steam is injected into the upper well, the heat melts the bitumen, which
allows it to
flow into the lower well, where it is pumped to the surface. SAGD is cheaper
than CSS,
allows very high oil production rates, and recovers up to 60% of the oil in
place.
Vapour extraction (VAPEX) is similar to SAGD but instead of steam, hydrocarbon
solvents are injected into the upper well to dilute the bitumen and allow it
to flow into the
lower well. An advantage of VAPEX is better energy efficiency than steam
injection and
it results in some partial upgrading of bitumen to oil right in the formation.
This is a
recently developed technique but has attracted much attention from oil
companies, who
are beginning to experiment with it.
The above three methods are not mutually exclusive. For example, wells can be
put
through one CSS injection-soak-production cycle to condition the formation
prior to
3
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
going to SAGD production, and companies are experimenting with combining VAPEX
with SAGD to improve recovery rates and lower energy costs.
The extraction methods described above, particularly SAGD, require a
considerable
amount of natural gas for generating steam, aiding flow through the drilled
wells, for
producing hydrogen for hydrotreating, and for producing electricity for
heating and
pumping the bitumen through the system. While natural gas, due to economic,
environmental, and technological changes may be desirable as a fuel of choice
due to its
clean burning nature, the increased demand and costs of natural gas and oil
make it
desirable to explore the options of using traditionally less attractive
sources of fuel such
as coal, the cheapest fossil fuel for generating electricity (and the dirtiest
and most
polluting), petcoke, and bitumen, in ways such that developing regulations
surrounding
the resulting emissions can be respected in order to lessen environmental
damage.
Once bitumen has been extracted from reservoirs, it must be processed and
upgraded to a
lighter oil product such as a synthetic crude oil. The upgrading process can
be carried out
at the site of extraction, or diluents can be added to the bitumen to
facilitate transportation
to an upgrading facility. The upgrading process (also called cracking or
distillation) into
lighter oil products has been around for a while; however, doing so in an
economic
fashion has been difficult in small scale fields.
By-products of the bitumen upgrading process include materials such as
petroleum coke
or asphalts. Petroleum coke, or petcoke, can be used as a fuel for coke
furnaces which
produce the heat required for certain aspects of the upgrading process.
Typically more
petcoke is produced than is used in the upgrading process, resulting in
stockpiling of
excess petcoke at or near the upgrading site.
Gasification is a process that enables the production of a combustible or
synthetic gas
(e.g., H2, CO, C02, CH4) from carbon-based feedstock, referred to as
carbonaceous
feedstock. The gas can be used to generate electricity or as a basic raw
material to produce
chemicals and liquid fuels. This process enables the production of a gas that
can be used
for generation of electricity or as primary building blocks for manufacturers
of chemicals
and transportation fuels.
4
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
In particular, the gas can be used for: the combustion in a boiler for the
production of
steam for internal processing and/or other external purposes; for the
generation of
electricity through a steam turbine; the combustion directly in a gas turbine
or a gas
engine for the production of electricity; fuel cells; the production of
methanol and other
liquid fuels; as a further feedstock for the production of chemicals such as
plastics and
fertilizers; the extraction of both hydrogen and carbon monoxide as discrete
industrial fuel
gases; and other industrial heat requirements as required.
Gasification is not an incineration or combustion process. Both incineration
and
combustion processes operate to thermally destroy the carbonaceous feedstock
with
excess oxygen to produce C02, H2O, SO2, NO2 and heat. Incineration also
produces
bottom ash and fly ash, which must be collected, treated, and disposed as
hazardous waste
in most cases. In contrast, gasification processes operate in the absence of
oxygen or with
a limited amount of oxygen and produce a raw gas composition comprising H2,
CO, H2S
and NH2. After clean-up, the primary gasification products are H2 and CO.
In contrast to incineration, which works with excess air to fully convert the
input material
into energy and ash, gasification converts carbonaceous materials into energy-
rich fuels
by heating the carbonaceous feedstock under controlled conditions.
Gasification
processes deliberately limit the conversion so that combustion does not take
place
directly. Gasification processes operate at substoichiometric conditions with
the oxygen
supply controlled (generally 35 percent of the 02 theoretically required for
complete
combustion or less), enabling gasification to convert the carbonaceous
feedstock into
valuable intermediates that can be further processed for materials recycling
or energy
recovery. Some gasification processes also use indirect heating, avoiding
combustion of
the carbonaceous feedstock in the gasification reactor and avoiding the
dilution of the
product gas with nitrogen and excess C02-
Generally, such a gasification process consists of feeding carbon-containing
materials into
a heated chamber (the gasification reactor) along with a controlled and
limited amount of
oxygen and steam. At the high operating temperature created by conditions in
the
gasification reactor, chemical bonds are broken by thermal energy and by
partial
oxidation, and inorganic mineral matter is fused or vitrified to form a molten
glass-like
substance called slag.
5
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
Several types of carbonaceous feedstocks can be used in gasification including
coal of
varying grades. Such coal includes low grade, high sulfur coal, which is not
suitable for
use in coal-fired power generators due to the production of emissions having
high sulfur
content. Waste coal particles and silt that remain after coal has been mined,
sorted and
washed is also be useful for gasification. Coal can be gasified with oxygen
and steam to
produce so-called "synthesis gas" containing carbon monoxide, hydrogen, carbon
dioxide,
gaseous sulfur compounds and particulates. The gasification step is usually
carried out at
a temperature in the range of about 650 C to 1200 C, either at atmospheric
pressure or,
more commonly, at a high pressure of from about 20 to about 100 atmospheres.
Because coal often contains sulfur compounds, attempts have been made to
provide
processes for the gasification of coal to produce a clean product fuel gas
wherein the
sulfur is removed from the product fuel gas prior to its use, e.g., in gas
turbines to
generate electricity. In addition, gases from the gasification zone may be
purified to
remove coal dust and fly ash and also many other impurities, e.g., vaporized
ash, alkali,
etc.
There are a number of patents relating to different technologies for the
gasification of coal
for the production of synthesis gases for use in various applications,
including U.S. Patent
Nos. 4,141,694; 4,181,504; 4,208,191; 4,410,336; 4,472,172; 4,606,799;.
5,331,906;
5,486,269, and 6,200,430.
With respect to the use of Gasification Systems in tar sands, Shell Oil
Company has
described various methods of using in-situ heating of tar sand formation to
produce
syngas. For example U.S. Patent Publication No. 2003/0155111 describes an in
situ
process for treating a tar sands formation which may include providing heat
from one or
more heaters to at least a portion of the formation. The heat may be allowed
to transfer
from the one or more heaters to a part of the formation such that heat from
the one or
more heat sources pyrolyzes at least some hydrocarbons within the part.
Synthesis gas (or
syngas) may be produced from the formation and can be converted to heavier
condensable
hydrocarbons, combusted as fuel, used to synthesize organic and inorganic
compounds,
used to generate electricity, or used to power fuel cells.
United States Patent No. 4,067,390 describes another apparatus and method for
in situ
gasification of tar sands. This apparatus and method utilizes a plasma arc
torch as heat
6
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
source for recovering useful fuel products from in situ deposits of coal, tar
sands, oil
shale, and the like. The useful fuel products include crude oil.
U.S. Patent Publication No. 2007/0017228 describes a method for enhancing the
efficient
operation of an electrical power plant utilizing a waste conversion unit to
convert organic
matter and electrical power into a useful fuel. The waste conversion unit is
in electrical
communication with an electrical power plant, where it uses electrical power
from the
electrical power plant and organic matter to form a useful fuel during periods
when the
electrical power plant has relatively high excess capacity. The useful fuel is
then supplied
to an electrical generator during periods of relatively low excess capacity,
thereby
allowing electrical generator to increase the power delivered by said power
plant during
periods of peak electricity demand. The waste conversion unit is described as
one which
uses electrical energy to create plasma which can be used to gasify a
feedstock such as tar
sands, coal and oil shale.
U.S. Patent Publication No. 20070095536 describes a system for treating a
hydrocarbon
containing formation that includes a steam and electricity cogeneration
facility. In this
system, at least one injection well is located in a first portion of the
formation. The
injection well provides steam from the steam and electricity cogeneration
facility to the
first portion of the formation. At least one production well is located in the
first portion
of the formation. The production well in the first portion produces first
hydrocarbons. At
least one electrical heater is located in a second portion of the formation.
At least one of
the electrical heaters is powered by electricity from the steam and
electricity cogeneration
facility. At least one production well is located in the second portion of the
formation. The
production well in the second portion produces second hydrocarbons. The steam
and
electricity cogeneration facility uses the first hydrocarbons and/or the
second
hydrocarbons to generate electricity.
This background information is provided for the purpose of making known
information
believed by the applicant to be of possible relevance to the present
invention. No
admission is necessarily intended, nor should be construed, that any of the
preceding
information constitutes prior art against the present invention.
SUMMARY OF THE INVENTION
7
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
This invention provides a facility to process unconventional oil sources. In
accordance
with one aspect of the present invention, there is provided a facility
integrating a number
of systems for the extraction of useful fuel products from an unconventional
oil source,
wherein the facility uses fossil fuels as a primary source of energy and
comprises:
a. a Gasification System for converting a feedstock comprising a fossil fuel
into slag,
an off-gas, heat and steam;
b. a Gas Reformulation System operatively associated with said Gasification
System
for converting the off-gas to a reformulated gas and heat, said reformulated
gas
comprising hydrogen and carbon monoxide;
c. a Control System operatively associated with said Gasification System and
said
reformulation system for monitoring and regulating said systems to ensure
efficient conversion of said feedstock;
d. one or more Gas Conversion Systems operatively associated with said gas
reformulation system that use the reformulated gas to produce electricity,
steam,
hydrogen, carbon dioxide, or light oils, or a combination thereof; and
e. one or more Extraction Systems operatively associated with at least one or
said
Gas Conversion Systems for extracting crude fuel products from the
unconventional oil source using the electricity, steam, hydrogen, carbon
dioxide,
or light oils, or a combination thereof, produced in the one or more Gas
Conversion Systems.
In accordance with another aspect of the present invention, there is provided
a process for
producing useful fuel products from an unconventional oil source, said process
comprising the steps of
a. gasifying a feedstock comprising a fossil fuel to produce slag, an off-gas,
heat and
steam;
b. reformulating the off-gas to provide a reformulated gas comprising hydrogen
and
carbon monoxide;
c. providing the reformulated gas to one or more Gas Conversion Systems to
produce
electricity, steam, hydrogen, carbon dioxide, or light oils, or a combination
thereof,
and
8
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
d. providing the electricity, steam, hydrogen, carbon dioxide, or light oils,
or a
combination thereof, to an Extraction System for extracting crude fuel
products from
the unconventional oil source.
BRIEF DESCRIPTION OF THE FIGURES
These and other features of the invention will become more apparent in the
following
detailed description in which reference is made to the appended drawings.
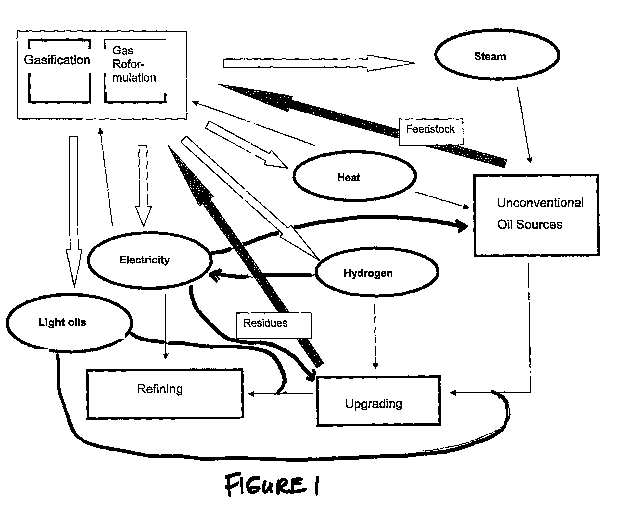
Figure 1 depicts an overview of the flow of the sources of energy between the
systems
comprised by a unconventional oil processing facility on one embodiment of the
invention.
Figure 2A depicts is a schematic diagram depicting in cross section a chamber
having a
rotating arm solids removal device, in accordance with one embodiment of the
invention.
Figure 2B is a schematic diagram depicting a top view of the rotating arm
solids removal
device of Figure 1, in accordance with one embodiment of the invention.
Figure 3 is a perspective, cut away view of a chamber having an extractor
screw solids
removal device, in accordance with one embodiment of the invention.
Figure 4 shows a cross-sectional view of a variation of a chamber using an
extractor
screw-based solids removal device, where the solid residue outlet is moved
away from the
main processing chamber to avoid direct drop, in accordance with one
embodiment of the
present invention.
Figure 5 is a perspective, cut away view of a chamber having a pusher ram
solids removal
device, in accordance with one embodiment of the present invention.
Figure 6 is a perspective, cut away view of a chamber using a pusher ram-based
solids
removal device, in accordance with one embodiment of the invention.
Figure 7 shows a cross-sectional view of a variation of a chamber using pusher
ram-based
solids removal device, in accordance with one embodiment of the present
invention.
Figure 8 shows one embodiment of a horizontal primary chamber.
9
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
Figure 9 is a schematic diagram of an entrained flow conversion chamber, in
accordance
with one embodiment of the invention.
Figure 10 is a schematic diagram of a fluidized bed conversion chamber, in
accordance
with one embodiment of the invention.
Figure 11 is a schematic diagram of a moving bed conversion chamber, in
accordance
with one embodiment of the invention.
Figures 12A and 12B depict embodiments of rotating grates that can be used in
a moving
bed conversion chamber, in accordance with different embodiments of the
present
invention.
Figure 13 is a schematic diagram of a moving bed conversion chamber in
relation to a
solid residue conditioning chamber and a gas reformulation chamber, in
accordance with
one embodiment of the invention.
Figure 14 is a cross-sectional schematic of a cascade of a fixed-bed char
conversion
chamber relative to a plasma heated residue conditioning chamber.
Figure 15 is a cross-sectional view through one embodiment of the gasifier,
detailing the
feedstock input, gas outlet, ash outlet, lateral transfer system, additive
ports and access
ports.
Figure 16 shows a general schematic of a vertically oriented primary chamber,
in
accordance with one embodiment of the present invention.
Figure 17 shows a general schematic of a vertically oriented primary chamber,
in
accordance with another embodiment of the present invention.
Figure 18A and B show various embodiments for movement of reactant material
from one
processing chamber to another in a two-processing chamber gasifier. The
material
displacement control modules employed include (a) gravity; (b) gravity with
sideways top
valve; (c) gravity with hopper; (d) gravity with screw; (e) vertical screw;
(f) horizontal
extractor screw; (g) vertical screw with hopper; (h) gravity with screw and
hopper; and (i)
horizontal extractor screw and hopper.
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
Figure 19 is a schematic of a horizontally-oriented stepped floor gasifier of
the invention,
detailing the feedstock input, gas outlet, ash outlet and lateral transfer
system.
Figure 20 is a flow diagram showing the different regions of the primary
chamber in
general terms.
Figure 21 is a representation of the gasification processes occurring in
different regions of
one embodiment of the primary chamber.
Figure 22 is a perspective view of one embodiment of the primary chamber,
detailing the
feedstock input, gas outlet, ash outlet, ram enclosure and access ports.
Figure 23 is a side view of the primary chamber illustrated in Figure 22
detailing the air
boxes, ash can and dust collector.
Figure 24 is a central longitudinal cross-sectional view through the primary
chamber
illustrated in Figures 22 and 23, detailing the feedstock input, gas outlet,
ash outlet, lateral
transfer system, thermocouples and access ports.
Figure 25A is a diagrammatical representation of a vertically oriented primary
chamber
comprising a gas passage conduit for input of process additives therein, in
accordance
with one embodiment of the present invention.
Figure 25B is a diagrammatical representation of a vertically oriented primary
chamber
comprising a gas passage conduit for output of gas therefrom, in accordance
with one
embodiment of the present invention.
Figure 25C is a diagrammatical representation of a horizontally oriented
primary chamber
comprising a gas passage conduit for input of process additives therein, in
accordance
with one embodiment of the present invention.
Figure 25D is a diagrammatical representation of a horizontally oriented
primary chamber
comprising a gas passage conduit for output of gas therefrom, in accordance
with one
embodiment of the present invention.
11
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
Figure 26A is a diagrammatical representation of a vertically oriented
gasifier comprising
three processing regions and two gas passage conduits for output of gas
therefrom in
different directions, in accordance with one embodiment of the present
invention.
Figure 26B is a diagrammatical representation of a vertically oriented
gasifier comprising
three processing regions and two gas passage conduits, one of which for input
of process
additives therein and the other for output of gas therefrom, in accordance
with one
embodiment of the present invention.
Figure 27A is a diagrammatical representation of a gasifier having three
processing
regions defined therein and comprising a gas passage conduit for output of gas
therefrom,
in accordance with one embodiment of the present invention.
Figure 27B is a diagrammatical representation of a gasifier having three
processing
regions defined therein and comprising a gas passage conduit for output of gas
therefrom,
in accordance with one embodiment of the present invention.
Figure 27C is a diagrammatical representation of a gasifier having three
processing
regions defined therein and comprising a gas passage conduit for output of gas
therefrom,
in accordance with one embodiment of the present invention.
Figure 28 shows the extraction of gas separately from the different processing
regions as
in the same direction.
Figure 29 shows the injection of additives independently into the different
processing
regions and the off-gases collected in multiple jackets around the gasifier.
Figure 30 shows the internal design of the gas passage conduit used for
separate
extraction of off-gases from different processing regions, as shown in the
embodiment of
Figure 28.
Figure 31 is a schematic representation of the multi-zone carbon converter in
general
terms, namely showing the general features of the carbon conversion zone,
inter-zonal
region or inter-zone and the slag zone.
12
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
Figures 32A-F depict various impedance mechanisms for use in a fixed-bed char
conversion chamber, in accordance with embodiments of the invention.
Figures 33A, 33B, 34AA, 34AB, 34BA, and 34BB show the various zones of the gas
reformulating system. The dotted lines show zones that are optional. The gases
may
undergo processing in a serial cascade of the zones or in parallel array as
depicted in
Figures 34BA and 34BB.
Figure 35 is a schematic of the gas reformulating system according to an
embodiment of
the invention.
Figure 36 is a schematic of one embodiment of a Gas Reformulating System of
the
invention coupled to a gasifier.
Figure 37 is a schematic of one embodiment of a Gas Reformulating System of
the
invention coupled to two gasifiers.
Figures 38 is a schematic of one embodiment of the gas reformulating chamber
of the
invention coupled to two gasifiers, through a common initial gas inlet.
Figures 39, 40, 45 and 46 show the following types of gas energizing sources:
hydrogen
burner, radio frequency (RF) and microwave plasma, laser plasma, corona
plasma.
Figure 41 shows the following types of plasma sources: non-transferred arc
torch,
transferred arc torch, inductively coupled plasma torch, microwave plasma
torch.
Figure 44 show a hydrogen burner.
Figure 42 and 43 illustrate the use of an inductively coupled plasma torch,
microwave
plasma torch and a hydrogen burner in a gas reformulating system, in
accordance with
various embodiments of the invention.
Figure 47 shows various embodiments of gas reformulating channels.
Figure 48 shows various embodiments of gas reformulating channels.
Figure 49 shows various embodiments of gas reformulating channels.
13
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
Figure 50 shows various embodiments of gas reformulating channels.
Figure 51 shows a gas reformulating channel using a mixer device.
Figures 52A-B show the use of constrictions in the gas reformulating chamber
for
enhancing gas mixing, in accordance with two embodiments of the invention.
Figures 53AA, 53AB, 53AC, 53RD, 53BA, 53BB, 53BC, 53BD to 55 show various gas
reformulating chamber designs.
Figure 56 shows various embodiments of the Gas Reformulation System wherein
the gas
stream is separated into smaller streams, which undergo reformulation in
parallel.
Figure 57 shows various arrangements of the gas energizing sources vis-a-vis
the initial
gas stream.
Figures 58A-C show different shapes of flow restrictors inserted into a gas
reformulating
chamber, in accordance with various embodiments of the invention.
Figures 59A-B and 74 show flow restrictors that extend for substantially whole
length of
the gas reformulating chamber, in accordance with three embodiments of the
invention.
Figures 60A-B and 76 show the three dimensional view of gas reformulating
chambers
equipped with flow restrictors that extend for substantially whole length of
the chamber,
in accordance with two embodiments of the invention.
Figure 61A-G show different embodiments of the flow restrictors.
Figure 62A shows a rotational shaft with multiple disks, in accordance with
one
embodiment of the invention. Figure 62B show different disk structures that
can be used
with the rotational shaft for enhanced interaction of the gas with energizing
fields.
Figure 63A-C show different rotational methods for the shaft and the disks, in
accordance
with various embodiments of the invention.
Figures 64 and 65 show the use of deflectors and Coanda-effect deflectors
respectively for
directing the gas energizing fields, in accordance with two embodiments of the
invention.
14
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
Figure 66A-B show the use of one or more air nozzles for active control of the
spatial
distribution of the plasma plume, in accordance with two embodiments of the
invention.
Figures 67A-D show the use of different deflectors for redirection of the
plasma plumes
within the gas reformulating chamber.
Figures 68A-D show the use of asymmetric rotating shaft objects deflectors, in
accordance with various embodiments of the invention.
Figure 69 is a schematic of a portion of the Gas Reformulating System
detailing the torch
mounting system and according to an embodiment of the invention.
Figure 70A shows a gas energizing source positioned to direct the gas
energizing field
counter-current to the flow of the gas stream, in accordance with one
embodiment of the
invention. Figure 70B shows the embodiment of Figure 70B with the gas entering
near the
top and exiting towards the bottom. Figure 70C is a schematic illustrating the
orientation
of the inlets and plasma torches of one embodiment.
Figures 71 and 72 show various arrangements of the gas energizing sources vis-
a-vis the
gas reformulating chamber and the input gas stream.
Figure 73 illustrates arrangements of baffles in the gas reformulating
chamber. Figure
73A illustrates air-flow within the gas reformulating chamber comprising
bridge wall
baffles. Figure 73B illustrates air-flow within the gas reformulating chamber
comprising
turbulator or choke ring baffles.
Figures 75A-B show the inclusion of turbulence zones for enhanced
reformulation.
Figure 75C show examples of turbulence generators.
Figure 76 shows the gas to be reformulated entering tangentially into the
reformation
reactor creating a swirl which is treated by the plasma torches and the Gas
Manipulator.
Figures 77 and 78 show exemplary means for generating turbulence.
Figure 79 is a diagram illustrating air-flow out of a Type A nozzle. Figure 80
is a diagram
illustrating air-flow out of a Type B nozzle.
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
Figures 81 and 82 show a fixed bed of char used as a catalyst in the
reformulation
chamber. Figure 83 shows a gasifier in combination with a gas reformulating
chamber,
wherein the char created in the gasifier leads to catalytic cracking.
Figures 84 to 86 show various configurations for combining catalyst beds and
energizing
fields for reformulation of gas generated within a gasifier.
Figures 87 to 89 show various positions where catalytic beds may be placed
within a gas
reformulating chamber, in accordance with one embodiment of the invention.
Figure 90 & 91 are related to the heat exchange systems used within the
stabilizing zone
of the Gas Reformulating System, in accordance with one embodiment of the
invention.
Figure 92A is a schematic of one embodiment of the gas reformulating chamber.
Figure 92B is a cross sectional view of the gas reformulating chamber of
Figure 92A
detailing the refractory supports.
Figures 93 to 96 show various configurations of gas reformulating chambers,
gasifiers and
carbon converters.
Figure 97 shows a gasifier which may be linked to the Gas Reformulating System
of the
invention.
Figures 98 to 100, 106 and 109 show various views of an exemplary Gas
Manipulator
designed to be retrofitted to a cylindrical gas reformulating chamber.
Figures 101, 102, 104, 105, 107, 108 show various views of the exemplary Gas
Manipulator of Figure 66 as installed in the cylindrical gas reformulating
chamber.
Figure 103 shows a top view of the gas reformulating chamber without the
exemplary Gas
Manipulator of Figure 98.
Figure 110 show various representations for the gas energizing sources as used
in the
Figures 33 to 109. All representations are equivalent and can be used to
indicate any of
the gas energizing sources specifically indicated herein, or as would be known
to a worker
skilled in the art.
16
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
Figure 111 is a schematic diagram depicting the recovery of heat from the
syngas
produced in the gas refining chamber using the heat recovery subsystem
according to one
embodiment of the instant invention.
Figures 112 to 115 depict different combinations of the different function
block processes
of a facility for gasifying two feedstocks, wherein "1" depicts function block
1 (a
volatilization chamber), "2" depicts function block 2 (a char conversion
chamber), "3"
depicts function block 3 (a solid residue conditioning chamber, and "4"
depicts a function
block 4 (a Gas Reformulating System).
Figure 116 depicts various SAGD options configured around a GQCS.
Figure 117 presents an exemplary configuration of a Gasification System and
steam
generator combination, according to one embodiment of the invention.
Figure 118 presents an exemplary configuration of a Gasification System and
steam
generator combination, according to another embodiment of the invention.
Figure 119 depicts a process flow diagram of a Gasification System according
to an
embodiment of the invention in which steam is generated from latent heat in
syngas and
electrical generator outputs.
Figure 120 depicts an embodiment of the system in which bitumen is partially
gasified.
Figure 121A to D depicts various configurations of gasification reactors or
converters
suitable for use with the system of the invention.
Figure 122 depicts a process overview diagram of a GQCS suitable for use with
the
system of the invention.
Figure 123 depicts an embodiment of the system in which syngas is produced
from the
gasification of bitumen.
Figure 124 depicts an embodiment of the system in which light oil production
is
maximized upstream with syngas being used to produce H2, C02, power, and
steam.
17
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
Figure 125 depicts an embodiment of the system in which feedstocks can be used
to
produce steam.
Figure 126 depicts a block flow diagram of CO2 and diluent applications.
Figure 127 depicts an embodiment of the system in which various feedstocks are
used to
produce steam, H2 and electricity for upgrading bitumen.
Figure 128 depicts a fluid plasma gasifier suitable for use in an embodiment
of the
system.
Figure 129A to C each depict a different arrangement of the components of the
system in
one embodiments.
Figure 130 A and B depict embodiments of a cooler and remover system for use
in the
system of the invention.
Figure 131 is a schematic diagram depicting one embodiment of the multi-
chamber
carbonaceous feedstock Gasification System, in accordance with one embodiment
of the
invention.
DETAILED DESCRIPTION OF THE INVENTION
This invention provides a facility comprising integrated systems for the
extraction, and
optionally upgrading and/or refinement, of unconventional oil sources, using
fossil fuels
as a primary source of energy. There are a number of processes involved in
efficiently
gasifying a fossil fuel, converting the gas into a more useful chemical
composition,
converting the gas into one or more different energy sources, as well as
extracting,
upgrading and refining the products obtainable from an unconventional oil
source. Each
of these processes requires a source of energy that is usually provided in the
form of heat,
steam, electricity, or a chemical reagent (e.g., H2, 02, C02, etc.).
Processes that use reformulated gas include electricity generation via an
engine such as a
G.E. Jenbacher gas engine. Pressurized gas can be used to drive a gas turbine
engine.
18
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
Processes that use steam include, for example, in situ extraction processes,
as a process
additive during the reformulation of gas, etc.
Processes that use electricity include, for example, reactive species
generating processes
(such as plasma generation), gas reformulation processes, syngas purification
processes,
underground heating processes for in situ extraction of crude fuel products,
pumping
processes for transferring crude fuel products and refined fuel products
through the
system, upgrading processes and refining processes.
Processes that use heat include, for example, gasification processes, gas
reformulation
processes, syngas purification processes, steam generation processes and in
situ extraction
processes.
Processes that use H2 include, for example, hydrotreating crude fuel products
in upgrading
processes and fuel cell processes for generating electricity.
Processes that use CO2 include, for example, enhanced oil recovery (EOR)
processes and
methane recovery processes.
Processes that use light oils include, for example, extraction and pumping
processes that
use the light oils as diluents to improve the flowability of the crude fuel
product.
The energy used to conduct these processes within this system is largely
generated within
one or more of the systems of this invention, which comprise:
= Gasification Systems that convert feedstock to slag, gas, heat and steam;
= gas reformulation systems that convert offgas to quality syngas and heat;
= Gas Conversion Systems that convert gas to electricity, steam, H2, C02, or
light
oils;
= Extraction Systems that extract crude fuel products, such as bitumen,
kerogen and
heavy oil, from the unconventional oil source,
= upgrading and refining systems that purify the crude fuel product to provide
a
useful fuel product (plus in some instances heat and/or feedstock), and
19
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
= a Control System to manage the overall processes in an integrated manner.
The effectiveness of the overall processes of the facility is determined, in
part, by the
amount of products produced relative to the amount of reactants consumed. One
skilled
in the art would appreciate that the amount, quality, availability, etc. of
the energy
required for each of the above processes that is taking place within the
overall system
needs to be coordinated with the systems that supply these energy resources.
Thus, the
systems and the processes need to be integrated for the overall efficiency and
effectiveness of unconventional oil processing.
In particular, this facility provides a means to gasify one or more fossil
fuels in an
efficient manner, converting it into intermediate products such as heat, steam
and
electricity. These intermediate products are then used by the facility to
obtain useful fuel
products from unconventional oil sources such as tar sands and oil shale
and/or to provide
energy to the Gasification System or Gas Reformulation System. The design of
this
facility are optimized such that the overall costs are competitive with an
equivalent
unconventional oil source processing facility that uses natural gas as a
source of
processing energy.
This invention can be used on its own or may be used in conjunction with an
existing
facility that uses natural gas as an energy source in order to improve the
overall cost
effectiveness of the processing and/or reduce the requirement for other energy
sources,
such as natural gas. One embodiment of the facility of this invention may also
optionally
use natural gas to supplement the energy source required to obtain useful fuel
products
from unconventional oil sources. One skilled in the art would appreciate how
to use
natural gas to appropriately supplement the processes within the facility of
this invention.
This facility uses one or more fossil fuels as a primary feedstock, such as
coal, products
from the unconventional oil source, such as oil shale, kerogen, heavy oils or
bitumen, or
by-products from the processing of the unconventional oil source, such as
petroleum coke
(petcoke), kerogen coke, and the like. By using feedstock that is locally
available, the
system can decrease overall operating costs in a number of ways. For example,
decreased
reliance on shipped-in energy sources, including natural gas, can reduce
overall operating
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
costs as well as providing valuable "carbon credits" in certain countries, in
addition to
decreasing the facility's environmental impact.
As described in more detail below, the facility comprises integrated systems
designed for
the optimized conversion of a carbonaceous feedstock into intermediate
products that are
then used within the facility to obtain useful fuel products from
unconventional oil
sources such as tar sands and oil shale or to assist the feedstock conversion
process. A
particularly important intermediate is reformulated synthesis gas (syngas)
having a
defined chemical composition that can be effectively used, for example, as a
combustion
fuel in the oil source processing. Reformulated syngas can also be used to
generate
electricity, for example, by combusting it as a fuel, by reducing the pressure
of the syngas
in turbines, using the temperature of the syngas to make steam to drive
turbines, or can be
used to drive steam generators to generate steam required in the steam-
assisted extraction
of unconventional oil sources. Alternatively, hydrogen in the reformulated
syngas may be
used in the oil upgrading process.
Optimized conversion will be defined by the overall requirements for the
facility. For
example, optimized conversion will in some embodiments represent the most cost
effective or energy efficient process to provide a syngas having a composition
that meets a
minimal threshold for its intended application. In other embodiments,
optimized
conversion will represent the process that produces the highest amount of
syngas with a
desired composition from a given amount of feedstock.
The facility is able to optimize conversion within each process by staging out
the
conversion process within a system and allowing the overall effectiveness of
each stage to
be maximized, as necessary. In one embodiment, maximizing overall
effectiveness of
each stage of the conversion process enables this facility to be used in a
commercially
viable manner as a source of energy and/or reagents that can be utilized in
obtaining
useful fuel products from the unconventional oil source. The energy conversion
ratio
reflects the true "overall balance sheet" for a facility evaluating the value
of the products
produced by the facility relative to the costs of building and operating the
facility.
In general the facility is designed to stage out the conversion processes in a
manner that
enables the potential for optimizing the effectiveness of the dominant
processes at each
stage as appropriate to enhance the overall effectiveness of the facility. For
example, the
21
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
facility is designed to minimize the amount of energy required to be input to
the facility,
for instance by using recycled heat to drive the gasification process. In
addition, this
facility incorporates process manipulators designed to optimize the
transference of energy
(endothermic and exothermic) throughout the conversion processes to enhance
the
effectiveness of the dominant process at each stage throughout the conversion
of
carbonaceous feedstock into slag and reformulated syngas. The design
strategies
embodied by the process manipulators function to: minimize the amount of input-
energy
required for driving the conversion processes; facilitate the speed and
thoroughness of the
conversion processes; and maximize the thoroughness of the processes.
Accordingly, in one embodiment, the facility provided by this invention is an
integrated
system comprising staged processing, Process Manipulators and a Control System
designed to optimize the Energy Conversion Ratio, i.e., the "Output-Energy"
relative to
the "Input-Energy."
In the broadest sense the term, "Input-Energy," is used to denote the factors
that are
present to make the reaction possible, or are supplied to the reaction to make
it more
efficient. Input-Energy is conceptualized in the broadest sense to mean any
source of input
required for the facility (for e.g., carbonaceous feedstock, electricity,
labor, air, steam,
facility operating costs, land costs, building costs etc.) that has a cost
associated with it.
For example, coal, which is generally purchased has a cost associated with it,
whereas by-
products of the unconventional oil source processing are readily available on
site and thus
have a lower associated cost.
In the broadest sense the term, "Output-Energy," is used to denote all of the
products of
the facility, which are either useful in their immediate form (such as syngas,
or heat that is
recycled and used to fuel the gasification reaction or reclaimed for
downstream processes)
or can be converted into another form of energy (such as heat used to drive a
steam
engine, gas which can be combusted in an electricity producing engine, or
hydrogen
which can be used to energize a fuel cell). Any process where heat is
generated is a source
of product energy. During the gasification reactions, a large quantity of heat
is generated,
which immediately drives the gasification reaction within the gasifier. Heat
which is
extracted from the gas leaving the gasifier can also be directed back into the
gasifier to
fuel the gasification reaction. Alternatively, excess heat produced by the
system can be
22
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
harnessed and used in downstream processes to facilitate the extraction and/or
processing
of the unconventional oil source. Moreover, "carbon credits" can be viewed as
a product
of this system.
Definitions
Unless defined otherwise, all technical and scientific terms used herein have
the same
meaning as commonly understood by one of ordinary skill in the art to which
this
invention belongs.
The terms "carbonaceous feedstock" and "feedstock", as used interchangeably
herein, are
defined to refer to carbonaceous material that can be used in the Gasification
System.
Examples of suitable feedstock include, but are not limited to, fossil fuel
based
feedstocks, such as bitumen, coal, oil shale, kerogen, coke (including
petroleum coke or
"petcoke" and kerogen coke) and heavy oils, as well as other carbonaceous
feedstocks,
such as biomass, hazardous and non-hazardous waste materials, including
municipal solid
waste (MSW); wastes produced by industrial activity; biomedical wastes;
carbonaceous
material inappropriate for recycling, including non-recyclable plastics;
sewage sludge;
heavy refinery residuals; refinery wastes; hydrocarbon contaminated solids;
agricultural
wastes; and any mixture thereof. The feedstock may be provided as a mixture of
two or
more of the above feedstocks in various relative proportions.
"Coal" refers to coal of any grade or rank. This can include, but is not
limited to, low
grade, high sulfur coal that is not suitable for use in coal-fired power
generators due to the
production of emissions having high sulfur content.
The term "unconventional oil source" as used herein refers to any natural
source of oil or
fuel product that is more difficult (for example, requires more energy input)
to extract
than conventional or "light" oil. One skilled in the art will appreciate that
a variety of
unconventional oil sources are known, although not all are being commercially
exploited.
However, with the increasing demand for fuel products, commercial exploitation
of
previously unconsidered oil sources may be initiated in the near future. As
this invention
provides a means for facilitating the commercial viability of extraction of
fuel products
from unconventional oil sources, it is envisioned that the invention will also
be used in
the future exploitation of these unconventional oil sources. Currently
exploited
23
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
unconventional oil sources include, but are not limited to, heavy oils, tar
sands, and oil
shale.
The term "tar sands" or "oil sands" as used herein refers to a naturally
occurring mixture
of sand or clay, water and extra heavy crude oil or bitumen.
"Biomass" refers to any material of organic origin, including, but not limited
to, pulp and
paper waste, wood products such as shredded bark, wood chips or sawdust,
sewage and
sewage sludge, food waste, plant matter, rice straw, agricultural and animal
waste, such as
manure, cellulosic type industrial waste (e.g., construction waste), waste
wood, fresh
wood, remains from fruit, vegetable and grain processing, and grass.
"Primary feedstock" refers to the main carbonaceous feedstock that undergoes
the
gasification process in the present system. Where only one feedstock is being
gasified, it
is referred to as the primary feedstock. Where more than one feedstock is
being gasified,
the feedstock that constitutes the major proportion of the combined feedstocks
is the
primary feedstock. In accordance with one embodiment of the invention, the
primary
feedstock is a fossil fuel based feedstock.
"Secondary feedstock" refers to an auxiliary carbonaceous feedstock that
undergoes
gasification with the primary feedstock and that is different from the primary
feedstock.
The secondary feedstock may be provided as a process additive to adjust the
carbon
content of the primary feedstock being gasified. One example could be the use
of a
carbon-rich source such as plastics or tires to augment a carbon-poor primary
feedstock.
Another example could be the use of biomass to attenuate a carbon-rich coal
feedstock.
"Processed feedstock" or "processed feedstock/char" may include one or more of
char,
low and ultra-low volatile feedstocks with fixed carbon and ash components,
the by-
products of a carbonaceous feedstock gasification or pyrolysis process,
products obtained
from the incomplete conversion of carbonaceous feedstock, or the solids
collected in gas
conditioning and/or cleanup systems with the heat source inputs from plasma
torch.
The term, "reactive species," refers to energetic species formed throughout
the
reformulation process. Non-limiting examples include free electrons generated
by an
energy source such as plasma, or radicals or dissociated intermediates
(induced
24
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
intermediates) that are created in the off-gas (e.g., syngas) that transfer
energy to other
molecules and/or dissociated intermediates/fragments of the preformulated gas
("preformulated molecules") enabling them to reformulate into a chemical
composition of
designed specifications. One skilled in the art appreciates that as the energy
transference
process continues, some of the preformulated molecules will in turn become
reactive
species, transferring their acquired energy to other molecules in the gas
reformulating
zone.
The term, "off-gas," refers to the gas that comes off the feedstock throughout
the process
of converting it to slag.
The term, "partially processed off-gas," refers to the off-gas that has been
somehow
processed due to the conditions, such as intense heat or reactive species,
produced in a
Gasification System such as a plasma melting system, designed for the
destruction of
waste and conversion into gas and slag. Such processing can include exposure
of the raw
off-gas to plasma or other energy sources.
The term, "initial gas," refers to the gas to be reformulated into a chemical
composition
designed for one or more downstream applications. It includes off-gas and/or
partially
processed off-gas.
The term, "preformulated gas," is used to denote gas as it enters a gas
reformulating zone.
This gas comprises the initial gas in addition to any optional process
additives that might
have been added to adjust the chemical composition of the gas prior to
reformulating it
into a designed chemical composition. In some embodiments, it might be just
the off-gas.
in some embodiments, it might include process additives. For example, if the
gas requires
increased levels of hydrogen, steam may be added as a process additive
upstream of a gas
reformulation zone, such that the reformulating gas will contain sufficient
amounts of
hydrogen species to provide for the proper chemical composition of the final
reformulated
gas product. If no optional process additives have been added "preformulated
gas" has the
same composition as "initial gas".
The term, "reformulated gas," refers to the gas that exits the gas
reformulation system.
The term, "Gas Reformulation Ratio," is used to describe the amount of gas
that is
reformulated relative the amount of gas that is input into the gas
reformulation system. It
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
can be described by the formula:
Amount of Reformulated Gas X 100 = % of gas reformulated
Amount of Preformulated Gas
Alternatively, and especially if no process additive gases are used, it can be
described by
the formula:
Amount of Reformulated Gas X 100 = % of gas reformulated
Amount of Initial Gas
The Gas Reformulation Ratio can be assessed directly or indirectly. Indirect
assessment
of the gas reformulation ratio can made by comparing downstream energy
production of
reformulated gas and preformulated gas. Downstream energy production is
reflective of
percent gas reformulated. An increase in downstream energy production is
indicative of
increased percent gas reformulated.
The term, "Gas Manipulators," denotes the features incorporated into the Gas
Reformulation System that function to facilitate the process of gas
reformulation.
The terms `carbonaceous feedstock' and `feedstock', as used interchangeably
herein, are
defined to refer to carbonaceous material that can be used in the gasification
process.
Examples of suitable feedstock include, but are not limited to, hazardous and
non-
hazardous waste materials, including municipal wastes; wastes produced by
industrial
activity; biomedical wastes; carbonaceous material inappropriate for
recycling, including
non-recyclable plastics; sewage sludge; coal; heavy oils; petroleum coke;
bitumen; heavy
refinery residuals; refinery wastes; hydrocarbon contaminated solids; biomass;
agricultural wastes; municipal solid waste; hazardous waste and industrial
waste.
Examples of biomass useful for gasification include, but are not limited to,
waste wood;
fresh wood; remains from fruit, vegetable and grain processing; paper mill
residues;
straw; grass, and manure.
The term, "gas energizing sources," refers to any source of energy known to
one skilled in
the art that could be used to impart energy to the preformulated gas, enabling
it to
reformulate into gas of a defined composition. Examples include, without
limitation,
plasma generating sources, radiation sources, hydrogen burners, electron beam
guns, etc
26
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
The term, "gas energizing field," is used to denote the field effect produced
by one or
more of the gas energizing sources used within the Gas Reformulation System to
provide
the energy to the gas that is required for the reformulation process to occur.
For example,
the gas energizing field that is created by an energy source such as a plasma
torch will
exhibit a three-dimensional space that will vary with torch power, working gas
composition, torch position, torch orientation, etc.
As used herein, the term "sensing element" is used in the broadest sense to
describe the
aspect of any element related to the facility that is configured to sense,
detect, read,
monitor, etc. one or more characteristics, parameters, and/or information of
the system,
inputs and/or outputs.
As used herein, the term "response element" is used to describe the aspect of
any element
related to the facility that is capable of responding to a signal
As used herein, the term "about" refers to approximately a +/-10% variation
from a given
value. It is to be understood that such a variation is always included in any
given value
provided herein, whether or not it is specifically referred to.
THE GASIFICATION SYSTEM AND GAS REFORMULATION SYSTEMS
The gasification and gas reformulation systems of this invention provide for
staging and
optimizing the dominant process within each stage throughout the conversion of
a
carbonaceous feedstock into slag and reformulated gas of a desired
composition.
Feedstocks suitable for gasification in the system include any carbon-
containing
(carbonaceous) material. Examples include, fossil fuel based materials, such
as bitumen,
coal, oil shale, kerogen, coke (including petroleum coke or "petcoke" and
kerogen coke)
and heavy oils, as well as other carbonaceous feedstocks, such as biomass,
hazardous and
non-hazardous waste materials, including municipal solid waste (MSW); wastes
produced
by industrial activity; biomedical wastes; carbonaceous material inappropriate
for
recycling, including non-recyclable plastics; sewage sludge; heavy refinery
residuals;
27
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
refinery wastes; hydrocarbon contaminated solids; agricultural wastes; and any
mixture
thereof.
As is also known in the art, combinations of feedstock can be used in order to
vary the
composition and quality of the syngas generated, or depending on the
downstream use of
the syngas generated. The feedstock may be provided as a mixture of two or
more of the
above feedstocks in various relative proportions. In one embodiment of the
invention, the
primary feedstock comprises fossil fuel based materials. In one embodiment,
the primary
feedstock comprises products from the unconventional oil source being
processed, such
as oil shale, kerogen, heavy oils or bitumen, or by-products from the
processing of the
unconventional oil source, such as petroleum coke (petcoke), kerogen coke, and
the like.
The primary feedstock can be a combination of fossil fuel based materials. In
one
embodiment, the primary feedstock is a combination of coal and one or more
products
from the unconventional oil source or by-products from the processing of the
unconventional oil source.
In one embodiment, the Gasification System uses bitumen as a feedstock. In
another
embodiment, the Gasification System used bitumen in combination with
additional
feedstocks. In yet another embodiment, the Gasification System uses a heavy
fraction of
the product resulting from the distillation of bitumen and tars. In another
embodiment, the
Gasification System uses coke as a feedstock. In still another embodiment, the
Gasification System uses coal as a feedstock. In another embodiment, the
Gasification
System uses a combination of bitumen, coke, and coal as a feedstock.
The present system can also be adapted to gasify a mixture of primary and
secondary
feedstocks in any proportion as may be desired. The secondary feedstock
functions as a
process additive to adjust the carbon content of the primary feedstock in
order to
modulate the carbon content to maintain a consistency in the final gas output.
For
example, where a high carbon feedstock (such as coal or other fossil fuel
based material)
is the primary gasification feedstock, it is contemplated that a lower carbon
secondary
feedstock (such as biomass or MSW) can be provided to offset the high carbon
content as
may be required.
28
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
When two or more feedstocks are used, they may be combined prior to their
introduction
into the Gasification System and then introduced through a common feedstock
inlet, or
they may each be introduced separately through dedicated individual inlets.
Coal of varying grades can be employed as a feedstock in the Gasification
System,
including low grade, high sulfur coal which is not suitable for use in coal-
fired power
generators due to the production of emissions having high sulfur content.
Waste coal
particles and silt that remains after coal has been mined, sorted and washed
may also be
useful for gasification.
Examples of biomass useful for gasification include, but are not limited to,
waste or fresh
wood, remains from fruit, vegetable and grain processing, paper mill residues,
straw,
grass, and manure.
In another embodiment of the invention the feedstock depending on its nature
and to
increase efficiencies and achieve desired syngas and energy outputs can be
pretreated, for
example, to reduce its volume overall or increase its surface area to volume
ratio by
shredding, pulverizing, or shearing. Alternatively, the feedstock can be
preheated, or
dried, etc.
As will be evident to one of skill in the art, the choice of feedstock to be
used in the
system can vary depending on the type of energy to be generated with the
system. For
example, if hydrogen is being produced by the energy-producing component, then
a
feedstock that has hydrogen content can be used. However, as is also known in
the art, in
such a circumstance it is possible to use feedstocks containing less hydrogen,
but adding
steam/water to the gasifier as the hydrogen source. In one embodiment, the
desired
quality of the end product determines the choice of feedstock to be used, i.e.
if a higher
quality end product is requires, then a higher quality feedstock should be
used.
Coal
In one embodiment, coal can be used as feedstock. In such a process, the coal
is first
pulverised to a size which provides the necessary rapid reaction. Generally,
the coal
should be of a particle size of 0.75 inches or smaller. Suitable examples of
particle size
include particle sizes of 30 mesh, or -100 mesh or the size recognised in the
coal industry
29
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
as "Buckwheat No. I". The pulverised coal may optionally be fed through a pre-
heater
where it is heated to a temperature in excess of about 100 C before being fed
to the
gasifier. Such pre-heated pulverised coal is fed to the gasifier via heated
coal line.
In one embodiment of the invention, and dependent on the coal composition,
such
pulverised coal may be fed continuously into the gasifier at a rate of, e.g.,
2.2 lb coal/min,
which is correlated to the feeding of steam at a rate of, e.g., 0.5 lb/min.
There are several different types of coal, each displaying different
properties resulting
from geological history. The degree of coal development is referred to as a
coal's "rank."
Peat is the layer of vegetable material directly underlying the growing zone
of a coal-
forming environment. The vegetable material shows very little alteration and
contains the
roots of living plants. Lignite is geologically very young (less than 40,000
years). It can
be soft, fibrous and contains large amounts of moisture (typically around 70%)
and has a
low energy content (8 - 10 MJ/kg). Black coal ranges from 65-105 million years
old to
up to 260 million years old. These are harder, shinier, less than 3% moisture
and can
have energy contents up to about 24 - 28MJ/kg. Anthracite contains virtually
no
moisture and very low volatile content, so it burns with little or no smoke.
It can have
energy contents up to about 32MJ/kg.
Municipal Solid Waste
In one embodiment, municipal waste can be used as a secondary feedstock for
the
gasification process. Municipal waste may be provided in solid or liquid form.
For the
gasification of solid wastes, the waste is introduced to the gasifier through
a solid waste
inlet feed port. The gasifier may also be designed to optionally include
liquid waste feed
inlet ports for the processing of liquid waste.
There are multiple stages to these conversion processes, which include, but
are not limited
to:
Drying of the Material: This stage of the gasification process is drying. The
normal
temperature range for this process lies between about 25 and 900 C. Drying
predominantly occurs at the top and in middle of a pile of material and at a
temperature
above about 100 C. As water leaves the material in the form of steam, one
embodiment
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
removes the water from the system in order to facilitate the energetics of the
downstream
processes.
Primary Volatilization of the Material: This stage of this conversion process
is
volatilization, which occurs mainly between about 400 and 950 C. The dominant
process
being driven in this stage is the conversion of solid carbonaceous material
into gaseous
molecules.
Char-to-Ash Conversion: This stage of the conversion process is that of carbon
conversion with a lesser amount (the remainder) of volatilization. The
temperature range
lies between about 500 and 1000 C. Although, in one embodiment in order to
avoid
agglomeration of the ash, the maximum temperature in this region generally
does not
exceed about 950 C
Ash Melting and Vitrification: This stage uses intense heat to melt the ash,
comprising
mostly inorganic material into molten slag (which can then cool into an
obsidian-like
glassy substance).
Gas Reformulation Process: This stage uses one or more energy sources to
initiate the
process of reformulation of a gas by initiating the dissociation of molecules
into reactive
dissociation fragments (intermediates). The reformulation of gas entails at
least four
chemical processes throughout the reformulation of a gas including: 1)
initiation of the
intermediates; 2) propagation of at least a portion of the intermediates; 3)
termination of
the intermediates; and 4) product gas stabilization.
The conversion process of the material at each stage requires unique chemical
and/or
thermal conditions and has been categorized accordingly.
The facility comprises multiple zones for the conversion of feedstock to slag
and
reformulated offgas. The facility comprises direct or indirect process
additive capabilities
in order to adjust the chemistry of the reactant materials throughout the
process. The
facility also comprises a Control System to monitor and regulate the different
stages of the
process to ensure the efficient and complete or substantially complete
conversion of the
carbonaceous feedstock into reformulated syngas.
31
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
The Control System also provides for the production of a reformulated syngas
product
having a consistent and/or specified composition. The Control System comprises
one or
more sensing elements for monitoring and obtaining data regarding operating
parameters
within the facility, and one or more response elements for adjusting operating
conditions
within the facility. The sensing elements and the response elements are
integrated within
the facility, and the response elements adjust the operating conditions within
the facility
according to data obtained from the sensing elements in addition to other
information
and/or instructions that may be provided to the Control System.
This facility comprises system designs wherein zones are created to optimize
the
dominant process at each stage of the conversion process. In one embodiment
each zone
exists within a separate chamber. In another embodiment, one or more zones are
combined within a single chamber. In embodiments in which one or more zones
are
combined within a single chamber, the single chamber may be segregated into a
finite
number of regions by one or more physical barriers or impediments or the
chamber may
be shaped to provide distinct zones or regions. For example, the chamber may
be shaped
to provide separate regions or may comprise one or more baffles placed to
segregate the
individual zones.
In multi-chamber embodiments, the chambers may be oriented horizontally,
vertically or
some combination thereof. In one embodiment of the invention, the facility
comprises
five interconnected chambers with the first processing chamber having a
feedstock input
and at least partially favouring drying, the second processing chamber
receiving processed
feedstock from the first chamber and at least partially favouring
volatilization, the third
processing chamber receiving char from the second chamber and at least
partially
favouring carbon conversion and the fourth processing chamber receiving
residual solid
material and at least partially favouring ash melting and vitrification. Off-
gas produced in
these processing chambers is reformulated within a fifth processing chamber,
the
reformulating chamber.
In one embodiment, the first three stages are substantially completed housed
within a
zonally segregated chamber that is operatively associated with separate
chambers
specifically adapted for Ash Melting and Vitrification and Gas Reformulation
Process
stages. In one embodiment, the first three stages and the Gas Reformulation
Process
32
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
stages are substantially completed housed within a zonally segregated chamber
that is
operatively associated with a separate Ash Melting and Vitrification chamber.
The facility of this invention provides a Gasification System that can be
integrated with
one or more energy producing components for producing energy or reagents
required for
processing material from tar sands or oil sands. In its simplest form, the
Gasification
System comprises a gasifier, a gas reformulating zone and a melting chamber.
The system
can optionally also include one or more heat recovery subsystems and/or a gas
quality
conditioning system (GQCS). Additional optional components such as a cooler
and tar
separator, a second gas reformulating zone, a gas regulating system, or a gas
storage
system can be added to the Gasification System depending on the feedstock to
be
consumed and/or the energy or reagents to be produced. The energy or reagents
can be,
for example, in the form of steam, electricity, heat, light oils, hydrogen,
CO2 or slag.
Process Manipulators
As described above the facility system incorporates process manipulators
designed to
optimize the transference of energy (endothermic and exothermic) throughout
the
conversion processes to enhance the effectiveness of the dominant process at
each stage
throughout the conversion of carbonaceous feedstock into slag and reformulated
syngas.
The design strategies embodied by the process manipulators function to:
minimize the
amount of input-energy required for driving the conversion processes;
facilitate the speed
and thoroughness of the conversion processes; and maximize the thoroughness of
the
processes. The types of process manipulators that may be incorporated into
each stage of
the system are described in more detail below.
The Gasification System - Material Drying Stage
The Dominant Process
The dominant process in this Stage is drying the carbonaceous feedstock which
occurs
predominantly at the top and in middle of the pile of material and at a
temperature above
about 100 C. The normal temperature range for a zone or region which favours
the
Material Drying Stage (as measured at the bottom of the material pile) lies
between about
100 and 900 C. In one embodiment, the temperature range is 100 to 900 C. In
another
embodiment, the temperature range is 100 to 600 T. In another embodiment, the
33
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
temperature range is 100 to 300 T. In a further embodiment, the temperature is
about
200 C.
The Energy Sources
In one embodiment, the zone or chamber which favours the Material Drying Stage
utilizes
low quality heat as the energy source, such as preheated air. In one
embodiment, the
source of heat is a source of recycled heat. In one embodiment, the air used
in the
Material Drying Stage is pre-heated through heat exchange with sensible heat
from the
syngas prior to introduction to the zone or chamber that favours this Stage.
Accordingly,
the zone or chamber that favours the Material Drying Stage can further
optionally include
one or more inlets for recycled heat. In one embodiment, there is an inlet for
recycled
heat in the floor of the zone or chamber that favours the Material Drying
Stage.
Optionally, air can be specifically supplied to this stage by the use of
independently
controlled airboxes and/or air nozzles.
The Process Manipulators
Design strategies can be utilized to optimize the drying of the material in a
fast and
economical manner, for example, by using preheated air that has been pre-
heated through
heat exchange with sensible heat from the syngas.
Drying may also be optimized by evacuating the resulting steam from the zone
or
chamber which favors the Material Drying Stage. Accordingly, this zone or
chamber
which favors the Material Drying Stage can optionally be equipped with a
system to
remove moisture and thereby facilitate drying. A worker skilled in the art
could readily
determine appropriate systems to remove moisture. For example, the system to
remove
moisture may include one or more steam outlets that allow for the evacuation
of steam. A
worker skilled in the art could readily determine an appropriate location for
the one or
more steam outlets. In one embodiment, the zone or chamber which favors the
Material
Drying Stage comprises one steam outlet in the roof. The one or more steam
outlets may
vent to the external environment, or the steam may be further processed or
recycled back
into one or more Stage of the gasification process or other downstream
processes.
Optionally, the drying of the feedstock (i.e. removal moisture) may be
accomplished by
34
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
the directed application of heat to the feedstock. Optionally, the source of
heat may be a
source of dry heat.
Design strategies which control the movement and height of the pile of
feedstock may
further be utilized to optimize the drying of the feedstock. For example, one
or more
physical barriers may be strategically placed in the zone or chamber which
favors the
Material Drying Stage to limit the movement of unprocessed material or to
limit the
movement of the material pile. In one embodiment, a baffle extends from the
roof of the
zone or chamber that favors the Material Drying Stage. In addition, the shape
of the
chamber or zone that favors the Material Drying Stage may facilitate the
movement of
feedstock and thereby optimize the process. For example, the movement of the
material
may be facilitated by the slope of the floor of the zone or chamber which
favors the
Material Drying Stage. Movement may also be actively controlled by an active
conveyance means.
Following the removal of the majority of moisture, the processed feedstock is
subsequently directed to a zone or chamber which favors the Volatilization
Stage of the
material by passive conveyance (e.g., by gravity), or by active conveyance
means.
The Gasification System - Volatilization Stage
The Dominant Process
The dominant process in this Stage is volatilization of volatile components in
the
carbonaceous feedstock. The normal temperature range for a zone or region
which favors
the Volatilization Stage (as measured at the bottom of the material pile) is
about 350 to
950 C. In one embodiment, the temperature range is about 400 to about 950 C.
In another
embodiment, the temperature range is about 600 to about 900 T. In a further
embodiment, the temperature is about 850 C.
The Energy Sources
In one embodiment, the zone or chamber which favours the Volatilization Stage
utilizes
low quality heat as the energy source, such as preheated air. In one
embodiment, the
source of heat is a source of recycled heat. In one embodiment, the air used
in this Stage
is pre-heated through heat exchange with sensible heat from the syngas prior
to
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
introduction to the zone or chamber that favours the Volatilization Stage.
Accordingly,
the zone or chamber that favours the Volatilization Stage can further
optionally include
one or more inlets for recycled heat. In one embodiment, there is an inlet for
recycled
heat in the floor of the zone or chamber that favours Volatilization Stage.
Optionally, air
can be specifically supplied to this stage by the use of independently
controlled airboxes
and/or air nozzles.
The Process Manipulators
Design strategies can be utilized to optimize the volatilization of the
volatile components
in the carbonaceous feedstock in a fast and economical manner, for example, by
using
preheated air that has been pre-heated through heat exchange with sensible
heat from the
syngas.
Design strategies which control the movement and height of the pile of
processed
feedstock may further be utilized to optimize the volatilization of the
volatile components.
For example, one or more physical barriers may be strategically placed in the
zone or
chamber which favours the Volatilization Stage to limit the movement of
unprocessed
material or to limit the movement of the material pile. The zone or chamber
may also be
shaped to control the movement of the material pile. In one embodiment, a
baffle extends
from the roof of the zone or chamber that favours the Volatilization Stage. In
addition,
the shape of the chamber or zone that favours the Volatilization Stage may
facilitate the
movement of feedstock and thereby optimize the process. For example, the
movement of
the material may be facilitated by the slope of the floor of the zone or
chamber which
favours the Volatilization Stage. Movement may also be actively controlled by
an active
conveyance means.
The remaining processed feedstock/char (with the majority of moisture and
volatiles
removed) is subsequently directed to the zone or chamber which favours the
volatilization
by passive conveyance (e.g., by gravity), or by active conveyance means.
The composition of air supplied in this region is typically varied depending
on the
feedstock supplied (e.g. oxygen enriched or depleted air).
Process manipulators in the Material Drying and Volatilization Stages can also
be
embodied in the design of the chamber or chambers in which the Stages take
place. In one
36
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
embodiment, the two Stages are combined in a single chamber referred to as a
Primary
Chamber. The main function of the primary chamber is to dry the feedstock and
to
volatilize the volatile components in the carbonaceous feedstock.
Optionally, the primary chamber may be segregated into a finite number of
regions by one
or more physical barriers. For example, the primary chamber may be shaped to
provide
separate zones for the Material Drying Stage and the Volatilization Stage or
may comprise
one or more baffles. In one embodiment, the baffle is strategically placed to
limit the
movement of unprocessed material or to limit the movement of the material
pile.
The primary chamber is therefore used to drive off all the moisture and
volatiles from the
feed stream at relatively low processing temperatures in a fast and economical
manner by
using low quality heat such as preheated air.
In one embodiment, the air used in this step is pre-heated through heat
exchange with
sensible heat from the syngas prior to introduction to the chamber. The
remaining
processed feedstock/char (with the majority of moisture and volatiles removed)
is
subsequently directed to the secondary chamber by passive conveyance (e.g., by
gravity),
or by active conveyance means that allow for the controlled movement of the
material to
the next Stage of the process.
In one embodiment of the invention, the primary chamber is a chamber having a
feedstock
inlet through which a primary feedstock to be gasified is introduced. In one
embodiment
a secondary additive feedstock is combined with the primary feedstock prior to
its entry
into the primary chamber. In one embodiment, the secondary additive feedstock
is input
into the primary chamber through a secondary feedstock inlet.
To limit the introduction of air, the feedstock inlet(s) may comprise a series
of airlocks.
Accordingly, in one embodiment, one or more feedstock inlets comprises one or
more
airlocks. Optionally, the airlock system can include a means to remove air.
Appropriate
means are known in the art and include a vacuum.
The primary chamber also includes heated air inlets for the introduction of
the heated air
required to drive the drying and Volatilization Stages, a first chamber gas
outlet through
which the gases produced in the primary chamber exit, and a residue/processed
feedstock/char outlet through which the resulting residue/processed
feedstock/char
37
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
product is passed out of the primary chamber prior to being passed into a
secondary
chamber. The gases produced in the primary chamber (referred to as the first
chamber gas
product) include the volatilized constituents of the feedstock, water vapour
where the
feedstock contained moisture, and gaseous products of a small amount of carbon
conversion.
The present system can also be adapted to gasify a mixture of feedstocks in
any
proportion as may be desired. In one embodiment, the mixture of feedstocks is
a
combination of primary and secondary feedstocks. In one embodiment, the
secondary
feedstock is provided as a process additive to adjust the carbon content of
the primary
feedstock being gasified. For example, a lower carbon secondary feedstock can
be used
to decrease the proportion of carbon in a high carbon primary feedstock, such
as coal,
bitumen, kerogen, coke and the like, if required.
In one embodiment, where primary and secondary feedstocks are being gasified,
the two
feedstocks are combined prior to their introduction into the primary chamber
through a
common feedstock inlet.
In one embodiment, where primary and secondary feedstocks are being gasified,
each of
the feedstocks is introduced separately to the primary chamber through
dedicated primary
and secondary feedstock inlets.
In one embodiment, where a mixture of two different feedstocks undergoes
gasification,
the two feedstocks are fed into the primary chamber in alternation.
In one embodiment, where two feedstocks are being gasified, each feedstock
undergoes
the initial Volatilization Stage separately, in respective primary chambers,
and their
respective processed feedstock/char products are combined in a common
secondary
chamber for conversion to a gaseous product and ash.
In one embodiment, the system comprises a material feeder subsystem adapted to
the
physical characteristics of the input feedstock in association with the
feedstock inlet of the
primary chamber. For example, augers, rams, feedhoppers, rotary valves, or top
gravity
feeds are feeder systems that can be incorporated into the system to
facilitate the
introduction of the feedstock.
38
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
In one embodiment of the invention, the material feeder subsystem comprises an
auger
which feeds directly into the primary chamber feedstock inlet to provide a
granular feed.
In one embodiment of the invention, the material feeder subsystem attached to
the
primary chamber may consist of a rectangular feedhopper and a hydraulic
assisted ram.
Limit switches on the feeder control the length of the ram stroke so that the
amount of
feedstock fed into the chamber with each stroke can be controlled.
A pre-conditioning process for conditioning the feedstock in the feed system
may also be
utilized prior to being fed to the first chamber. In one embodiment, the
feedstock is
prepared in order to control the particle size before feeding into the primary
chamber. In
one embodiment, the feedstock undergoes a pre-drying step to remove excessive
moisture
before feeding into the primary chamber.
In accordance with the present invention, heated air inputs provide the heat
required for
the drying and volatilization processes. Accordingly, the heated air inlets
are located
throughout the primary chamber at locations suitable for optimum exposure of
the
feedstock to the heated air to ensure sufficient heating of the feedstock to
dry it and
volatilize the volatile constituents. In one embodiment, the heated air inlets
are located in
the walls of the chamber proximal to the base, to ensure that the hot air is
passed into and
over the pile of material for optimum exposure. In one embodiment, the heated
air inlets
are located in the floor of the chamber, so that the hot air is passed up into
the pile of
material to ensure penetration into and through the pile of material. In one
embodiment,
the heated air inlets are located in the walls and floor of the chamber.
In one embodiment, the heated air used to drive the processes is preheated in
a heat
exchanger using sensible heat recovered from the hot syngas products of the
carbonaceous
feedstock gasification.
In order to facilitate initial start up of the Gasification System, the
chamber can include
access ports sized to accommodate various conventional burners, for example
natural gas
or propane burners, to pre-heat the chamber.
The primary chamber can be of any shape and dimension suitable for Stage I and
II of the
conversion process.
39
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
In one embodiment, the primary chamber is a vertically oriented chamber having
a
feedstock inlet located near the top and a processed feedstock/char outlet
located near the
bottom. In such an embodiment, the feedstock enters from the top and
accumulates in a
pile while being heated with hot air to drive the drying and volatilization
processes. As
the moisture and volatiles are driven off, the feedstock is gradually
converted to char.
The resulting processed feedstock/char is passed, actively or passively, out
through the
processed feedstock/char outlet located at the bottom of the primary chamber
and into the
secondary chamber.
In one embodiment, the pile of feedstock is gradually converted to processed
feedstock/char by action of the heated air with no mechanical mixing or active
movement
of the solids through the chamber, and the processed feedstock/char product is
allowed to
passively drop from the primary chamber to the secondary chamber through an
opening
between the two chambers.
In one embodiment, the bottom of the chamber gradually slopes downward toward
the
processed feedstock/char outlet, whereby the material is passively drawn by
gravity
toward the processed feedstock/char outlet.
In one embodiment, the feedstock undergoes mechanical mixing by a mechanism
such as
rotating paddles, rotating wheels or rotating arms, which rotate horizontally
to ensure
optimal exposure to the heated air. Such mixing means can also serve to
actively convey
the processed feedstock/char product towards the processed feedstock/char
outlet in a
controllable manner.
Controlling the movement of the processed feedstock/char towards the processed
feedstock/char outlet and out of the primary chamber enables optimization of
residence
time in the chamber to ensure that moisture and volatiles are removed from the
feedstock
prior to being passed into the secondary chamber. The rate of movement of
material out of
the primary chamber and into the secondary chamber is regulated via the use of
a
controllable solids removal means. The solids removal means can be one of a
variety of
devices known in the art. Examples include, but are not limited to, screws,
pusher rams,
horizontal rotating paddles, horizontal rotating arms, and horizontal rotating
wheels.
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
In one embodiment, the solids removal device is a rotating paddle with thin
spokes which
moves the processed feedstock/char toward the processed feedstock/char outlet
and out of
the chamber. Figure 2A depicts one embodiment of the invention in which the
solids
removal device comprises a rotating paddle 81 at the bottom of the primary
chamber 20
which moves the processed feedstock/char out of the chamber 20 through a small
processed feedstock/char outlet 70. To avoid the passage of partially
processed
feedstock/char through the processed feedstock/char outlet 70 by a direct
drop, a barrier
82 is placed over the processed feedstock/char outlet 70. Limit switches may
be optionally
used to control the speed of the bar rotation and thus the rate of removal of
residue.
Figure 2B is a top view of the rotating arm solids removal device depicted in
Figure 3,
showing the relationship between the barrier 82 and the processed
feedstock/processed
feedstock/char outlet 70.
In one embodiment, the solids removal device is a set of screws which move the
out of
the chamber. In such an embodiment, the bottom portions of the chamber walls
are
optionally made to slant towards the screws at the bottom of the chamber, so
that the
processed feedstock/processed feedstock/char may be directed towards the
screws. Figure
3 depicts one embodiment of the invention in which the solids removal device
comprises
a set of extractor screws 83 at the bottom of the primary chamber 20 which
moves the
processed feedstock/char out of the chamber 20. Optional serration on the edge
of the
extractor screw flight helps in the breaking up of agglomerations that could
otherwise
result in jamming at the processed feedstock/char outlet 70. A barrier 82 is
provided to
avoid the passage of partially processed processed feedstock/char through the
processed
feedstock/char outlet 70 by a direct drop. A barrier is not required if the
residue outlet 70
is moved away from the processing chamber 20, as for the embodiment shown in
Figure
4. Limit switches may be optionally used to control the speed of the screws
and thus the
rate of removal of residue.
In one embodiment, the solids removal device is a single thin ram which moves
the
processed feedstock/char toward the processed feedstock/char outlet and out of
the
chamber. In such an embodiment, the bottom portion of the side opposite to the
ram is
made slanting so that the processed feedstock/char may be directed towards the
ram
leaving space for the exit hole. Figures 5 and 6 depict embodiments in which
the solids
removal device comprises a single thin pusher ram 85 for the primary chamber
20 which
41
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
moves the processed feedstock/char out of the chamber 20 through a small
processed
feedstock/char outlet 70. Depending on the position of the processed
feedstock/char outlet
70, a barrier 82 may or may not be required as shown in Figure 7. Limit
switches may be
optionally used to control the length of the pusher ram stroke and thus the
amount of
processed feedstock/char moved with each stroke.
In one embodiment, the primary chamber is a horizontally oriented chamber
having a
feedstock inlet located at one end of the chamber, and a processed
feedstock/char outlet
located at an opposite end of the chamber. As the feedstock progresses from
one end of
the horizontal primary chamber to the other, it loses its moisture and
volatile fraction to
form the resulting processed feedstock/char product. In such an embodiment,
the
chamber optionally comprises one or more means for laterally transporting
solid material
through the chamber from the feedstock inlet end toward the processed
feedstock/char
outlet end. Controlled lateral movement of material through the primary
chamber via the
use of one or more lateral transfer units allows for the optimization of the
Drying and
Volatilization Stages of the gasification process that are carried out in the
primary
chamber, by controlling the residence time of the material at each Stage.
In one embodiment, the lateral transfer units are one or more pusher rams in
which
material is predominantly pushed through the primary chamber. In one
embodiment, the
lateral transfer units are movable shelves / platforms on which material is
predominantly
moved through the chamber by sitting on top of a shelf / platform; a fraction
of material
may also be pushed by the leading edge of the movable shelf / platform.
Controlled
lateral movement by the shelf/platform-type lateral transfer units can be
accomplished by
varying the movement speed, the distance each lateral transfer unit moves and
the
sequence in which the plurality of lateral transfer units are moved in
relation to each
other. The one or more lateral transfer units can act in coordinated manner or
individual
lateral transfer units can act independently. In order to optimize control of
the material
movement and pile height, the individual lateral transfer units can be moved
individually,
at varying speeds, at varying movement distances, at varying frequency of
movement.
Figure 8A is a schematic depiction of a stepped floor horizontal primary
chamber with
arrows indicating the lateral movement of solids through the chamber. Figure
8B is a
42
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
schematic depiction of a sloped floor horizontal primary chamber with arrows
indicating
the lateral movement of solids through the chamber.
In embodiments where the primary chamber is substantially horizontal, the
lateral transfer
unit can include, but is not limited to, a shelf / platform, pusher ram or
carrier rams, plow,
screw element, conveyor or a belt, moving grate or a roller grate. The rams
can include a
single ram or multiple-finger ram. The roller grate can, for example, be a
Dusseldorf
grate.
In one embodiment where the primary chamber is substantially horizontal, the
lateral
transfer unit is a screw mechanism located along the bottom of the primary
chamber,
whereby the material is transferred laterally by rotation of one or more
screws toward the
processed feedstock/char outlet. Controlled lateral movement by the screw-type
lateral
transfer units can be accomplished by varying the screw rotation speed.
In one embodiment where the primary chamber is substantially horizontal, the
lateral
transfer system is a single ram or multiple-finger ram.
In one embodiment where the primary chamber is substantially horizontal, the
lateral
transfer system is a multiple-finger ram. In the multiple-finger ram designs,
the multiple-
finger ram may be a unitary structure or a structure in which the ram fingers
are attached
to a ram body, with individual ram fingers optionally being of different
widths depending
on location. The gap between the fingers in the multiple-finger ram design is
selected to
avoid particles of reactant material from bridging. In one embodiment, the
individual
fingers are about 2 to about 3 inches wide, about 0.5 to about 1 inch thick
with a gap
between about 0.5 to about 2 inches wide.
In one embodiment where the primary chamber is substantially horizontal, the
lateral
transfer system is "T-shaped".
In one embodiment where the primary chamber is substantially horizontal, the
lateral
transfer system is a pusher that is shaped as a cylinder with a tapered end.
In one embodiment where the primary chamber is substantially horizontal, the
lateral
transfer system, the lateral transfer system can be a plurality of rollers
with horizontal and
parallel axes lie in an inclined plane. An example of a plurality of rollers
is the
43
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
Dusseldorf/Babcock grate. See, for example, U.S. Patent No. 5,967,064 and U.S.
Patent
No. 5,448,957.
In embodiments in which movement through the gasifier is facilitated by a
system such
as, similar to, or inspired by a Diisseleldorf Grate, hot air can optionally
be added to the
gasifier through a system of independently controlled air boxes that supply
hot air to the
rollers. In such a system, the rollers are hollow perforated cylinders through
which the hot
air enters the gasification chamber. The distribution of hot air to the
rollers is dependent
on the location of the rollers within the gasifier with the quantity of hot
air being supplied
to the individual rollers being optimized for the predominate process
occurring at that
location.
In certain embodiments in which the system operates at very high temperatures,
cooling
can optionally be provided for the lateral transfer system. In one embodiment
using a ram
or shelf, cooling within the ram or shelf can be provided. Such cooling could
be by fluid
(for example, air or water) circulated inside the ram or shelf from outside of
the chamber.
In one embodiment using a roller grate such as a Dusseldorf grate, cooling
within the
roller and/or around each roller can be provided. Such cooling could be by
fluid (for
example, air or water) circulated inside each roller. In one embodiment, the
flow of fluid
to each roller may be separately controlled thereby allowing the temperature
of each roller
to be varied individually. Optionally, the rollers may be designed to
facilitate the
introduction of air.
In one embodiment where the primary chamber is substantially horizontal, the
lateral
transfer system is a plow which has folding arms which can be withdrawn when
the plow
is retracted.
In one embodiment where the primary chamber is substantially horizontal, the
lateral
transfer system is a belt or flighted chain conveyor.
In one embodiment where the primary chamber is substantially horizontal, the
lateral
transfer system is a movable shelf / platform in which material is
predominantly moved
through the gasifier by sitting on top of the shelf / platform. A fraction of
material may
also be pushed by the leading edge of the movable shelf / platform.
44
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
In one embodiment where the primary chamber is substantially horizontal, the
lateral
transfer system is a carrier ram in which material is predominantly moved
through the
gasifier by sitting on top of the carrier ram. A fraction of material may also
be pushed by
the leading edge of the carrier ram.
In one embodiment where the primary chamber is substantially horizontal, the
lateral
transfer system is a pusher ram in which material is predominantly pushed
through the
gasifier. Optionally, the ram height is substantially the same as the depth of
the material
to be moved.
In one embodiment where the primary chamber is substantially horizontal, the
lateral
transfer system is a combination of rollers and pushers. For example, in
embodiments
which have a combination stepped and sloped floor movement across the slope
portion
may be facilitate by a roller grate while movement across the stepped portion
may be
facilitated by pushers. In another embodiment, a pusher pushes the material
onto the
uppermost roller of the Roller Grate.
In one embodiment, the lateral transfer system can be a set of conveyor
screws.
Optionally, the conveyor screws can be set in the floor of the chamber thereby
allowing
material to be moved without interfering with air introduction.
Power to propel the lateral transfer system is provided by a motor and drive
system and is
controlled by actuators.
The individual lateral transfer units may optionally by powered by dedicated
motor and
have individual actuators or one or more lateral transfer units may be powered
by a single
motor and shared actuators.
Basically any controllable motor or mechanical turning device that can provide
accurate
control of the lateral transfer system can be used to propel the lateral
transfer system.
Appropriate motors and devices are known in the art and include electric
motors, motors
run on syngas, steam, gases, gasoline, diesel or micro turbines.
In one embodiment, the motor is an electric variable speed motor which drives
a motor
output shaft selectably in the forward or reverse directions. Optionally, a
slip clutch could
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
be provided between the motor and the motor output shaft. The motor may
further
comprise a gear box.
Movement of the lateral transfer system can be effected by a hydraulic system,
hydraulic
rams, chain and sprocket drive, or a rack and pinion drive. These methods of
translating
the motor rotary motion into linear motion have the advantage that they can be
applied in
a synchronized manner at each side of a unit to assist in keeping the unit
aligned and thus
minimizing the possibility of the mechanism jamming.
A worker skilled in the art would readily appreciate that the lateral transfer
units in
addition to conveying the feedstock through the primary chamber, can also be
configured
to convey the processed feedstock/char product out of the processed
feedstock/char outlet.
The Gasification System - Char-To-Ash Conversion Stage
The Dominant Process
The dominant process at this Stage is conversion of the char received from the
Volatilization Stage to a gas product and ash and takes place in a Char-to-Ash
Conversion
Stage zone or chamber. The normal temperature range lies between about 500 and
1000 C. Although in one embodiment, temperatures higher than 1000 C may be
employed depending on the material's ash fusion temperature. In one embodiment
in
order to avoid agglomeration of the ash, the maximum temperature in this
region does not
exceed about 950 C. In one embodiment, the temperature is about 850 C.
Optionally,
cold air can be added in order to reduce the temperature and prevent melting
of the
residual solid material or ash.
The Energy Source
Receiving the char into the Char-to-Ash Conversion Stage chamber at the
highest possible
temperature results in the most efficient process, producing the maximum CO
and H2 and
the minimum CO2 and H2O. The processing temperature is selected to be as high
as
possible to further maximize the yield of CO and H2, while still maintaining
the char at a
temperature below its fusion temperature. In one embodiment, the char is
received
directly from the Volatilization Stage, thereby minimizing heat loss during
the transfer.
46
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
The char is heated, at least in part, by heated air introduced through heated
air inlets. In
one embodiment, the air is preheated through heat exchange with sensible heat
from the
hot syngas product. The heated air inlets are strategically located in and
around the
chamber to ensure full coverage of heated air into the processing zone.
Optionally, air can
be specifically supplied to this stage by the use of independently controlled
airboxes
and/or air nozzles.
The heat required for the char conversion process is also provided, in part,
by partial
oxidation of the char. The heated air inputs, in addition to providing
sensible heat, also
supply the oxygen required to convert carbon to gaseous CO and some CO2. The
reaction
of carbon with 02, whether resulting in the formation of CO or CO2, is
exothermic. This
exothermic reaction therefore also serves to provide a proportion of the heat
required for
the char-to-ash conversion. The char-to-ash conversion is therefore, in part,
self-driving,
but such reactions may also result in a non steady-state reaction resulting in
an
uncontrolled increase in temperature (e.g. approaching ash fusion
temperature), which
may result in undesired slagging in the Char-to-Ash Conversion Stage zone or
chamber.
In one embodiment, the amount of heated air input into the Char-to-Ash
Conversion Stage
zone or chamber is controlled to avoid such uncontrolled increases in
temperature. In one
embodiment, the temperature is controlled by the input of cold air.
The Process Manipulators
Design strategies can be utilized to optimize the conversion of char to ash.
For example,
the zone or the chamber that favours the Char-to-Ash Conversion Stage may be
designed
to optimize the residence time required for char to ash conversion. In one
embodiment,
the zone or chamber that favours the Char-to-Ash Conversion Stage may comprise
a
means for controlling the residence time of the char. In addition, the design
strategies
relate to the type and location of process additives in order to optimize the
char to ash
conversion and the composition of any gas produced. The design strategies may
also
relate to optimizing the exposure of char to heat and process additives in
order to enhance
the conversion process.
The zone or chamber that favours the Char-to-Ash Conversion Stage can be a
region of a
chamber or a separate chamber.
47
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
In one embodiment, the Char-to-Ash Conversion Stage is in a separate chamber
comprising a char inlet proximal to the top of the chamber through which char
from the
Volatilization Stage is received, one or more heated air inlets, a gas outlet,
a solid residue
(i.e., ash) outlet, one or more optional process additive (e.g., steam)
inlets, and optionally
means for controlling the residence time of the char in the Char-to-Ash
Conversion Stage
chamber. In one embodiment, the one or more air inlets are located proximal to
the
bottom of the chamber.
The gases produced in the Char-to-Ash Conversion Stage chamber, referred to as
the
Char-to-Ash Conversion Stage gas product, comprises the products of the carbon
conversion reaction, as well as what amount of the volatile constituents
remained in the
char product after the Volatilization Stage.
In one embodiment, the Char-to-Ash Conversion Stage chamber is a vertically
oriented
chamber. Examples of vertically oriented chambers known to be suitable for use
in the
present system include, but are not limited to, moving bed gasifiers, fixed
bed gasifiers,
entrained flow gasifiers and fluidized bed gasifiers. In those embodiments
that employ
heated air to convert the char to gaseous products and ash, the gasifiers
comprise heated
air inlets located to provide optimal exposure of the char to the heated air
inputs and to
ensure full coverage of heated air into the processing zone.
The Char-to-Ash Conversion Stage chamber optionally comprises a mechanical
mixing
means for ensuring efficient exposure of the char to heated air and any
process additives
as may be required to convert the char to ash and the desired gaseous
products. The
mechanical mixing means can also prevent gas channelling and keep the material
from
agglomerating.
The type and quantity of the process additives are therefore selected to
optimize the
conversion of char to a Char-to-Ash Conversion Stage gas product and ash,
while
minimizing operating costs and maintaining adherence to regulatory authority
emission
limits. Steam input ensures sufficient free oxygen and hydrogen to maximize
the
conversion of the char into the Char-to-Ash Conversion Stage gas product
having a
heating value and ash. Air input assists in processing chemistry balancing to
maximize
carbon conversion to a fuel gas (minimize free carbon) and to maintain the
optimum
processing temperatures while minimizing the cost of input heat. The quantity
of both
48
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
additives is established and controlled as identified by the outputs for the
feedstock being
processed. The amount of air injection is established to ensure a maximum
trade-off for
relatively high cost of input heat while ensuring the overall process does not
approach any
of the undesirable process characteristics associated with incineration, and
while meeting
and bettering the emission standards of the local area.
In one embodiment, heated air and steam inlets may comprise high temperature
resistance
atomizing nozzles, as are commercially available.
In one embodiment, the heated air inlets are located proximal to the floor of
the Char-to-
Ash Conversion Stage zone or chamber.
The zone or chamber can include access ports sized to accommodate various
conventional
burners, for example, natural gas or propane burners, to pre-heat the chamber.
In one embodiment, steam additives are provided to the Char-to-Ash Conversion
Stage
zone or chamber in order to convert the maximum of carbon to chemical heat
while
avoiding raising the processing temperature to levels detrimental to the main
objective of
this chamber (i.e., conversion of char to gaseous products and ash).
Accordingly, the
Char-to-Ash Conversion Stage zone or chamber can also optionally comprise
process
additive (steam) inlets to allow input of additional process additives to
facilitate efficient
conversion of the carbon in the char into product gases. In one embodiment,
the Char-to-
Ash Conversion Stage zone or chamber includes a plurality of steam inlets
strategically
located to direct steam into high temperature regions.
In one embodiment using an entrained flow Char-to-Ash Conversion Stage chamber
22,
with reference to Figure 9, the heated air (and optional steam) inputs travel
in a co-current
flow relative to the char inputs. Here, the char is at least partially
suspended by the
movement of the additives, thereby promoting a more distributed contact
between the
input and the char. The reaction occurs as the reactant material moves
downward, driven
by gravity, in the direction of travel of additives. The Char-to-Ash
Conversion Stage gas
product exits through a gas outlet, and the resulting solid residue (ash)
exits at the bottom
through the solid residue outlet.
In one embodiment of the invention using a fluidized bed Char-to-Ash
Conversion Stage
chamber 24, with reference to Figure 10, the char is suspended in the upward
moving
49
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
additives. In fluidized beds, the additives enter the Char-to-Ash Conversion
Stage
chamber at velocities that greatly overcome any gravitational force, and the
char bed
moves in a much more turbulent manner thereby causing a more homogeneous
reaction
region and behaving in a fashion similar to that of a turbulent fluid even
though the char
may in fact be solid. The heated air and steam additives enter the Char-to-Ash
Conversion
Stage chamber from the bottom and pass counter-current to the char. The
resulting solid
residue (ash) exits through the solid residue outlet and the Char-to-Ash
Conversion Stage
gas product leaves the Char-to-Ash Conversion Stage chamber through the gas
outlet at
the top.
In one embodiment of the invention using a moving-bed Char-to-Ash Conversion
Stage
chamber 26, with reference to Figure 11, the chamber 26 comprises a feedstock
input
proximal to the top of the Char-to-Ash Conversion Stage chamber, a plurality
of heated
air inlets, a gas outlet, a solid residue outlet and an actively controlled
rotating grate at the
base of the Char-to-Ash Conversion Stage chamber. Process additive inlets are
also
optionally provided for addition of steam into the Char-to-Ash Conversion
Stage
chamber. Also, mixing mechanisms 27 may be used to promote enhanced
interaction
between the additives and the char within the processing chamber. The
resulting solid
residue (ash) exits through the solid residue outlet and the Char-to-Ash
Conversion Stage
gas product leaves the Char-to-Ash Conversion Stage chamber through the gas
outlet at
the top. Figures 12A and 12B depict embodiments of rotating grates that can be
used in a
moving-bed Char-to-Ash Conversion Stage chamber, in accordance with different
embodiments of the present invention.
Figure 13 schematically depicts one embodiment of a moving bed Char-to-Ash
Conversion Stage chamber in relation to an Ash Melting and Vitrification Stage
chamber
and a Gas Reformulation Stage chamber. In the illustrated embodiment, the Char-
to-Ash
Conversion Stage comprises a processed feedstock / char input, heated air
inlets, an
agitator with externally mounted motor assembly, a solid residue outlet in
communication
with a plasma heated Ash Melting and Vitrification Stage chamber, and a Char-
to-Ash
Conversion Stage gas product outlet in communication with Gas Reformulation
Stage
chamber. The Gas Reformulation Stage chamber also receives a gas product from
the
Volatilization Stage chamber and converts the combined gas products to a
syngas using
plasma heat. The Char-to-Ash Conversion Stage chamber comprises a rotating
grate to
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
regulate the flow of material from the carbon conversion zone to the Ash
Melting and
Vitrification Stage chamber. Residual solid material enters the Ash Melting
and
Vitrification Stage chamber and is heated with a plasma heat source to vitrify
and blend
the solid residue.
In one embodiment of the invention, the Char-to-Ash Conversion Stage chamber
is a
fixed-bed chamber. In fixed-bed systems, the char enters the chamber from the
top and
rests on a surface through which the heated air inputs and optional steam (or
other
additives) are introduced. The gas inputs pass through the char bed from the
bottom in a
counter-current fashion. The resulting solid residue (ash) exits through the
solid residue
outlet and the Char-to-Ash Conversion Stage gas product leaves the Char-to-Ash
Conversion Stage chamber through the gas outlet at the top.
In accordance with the present invention, the Char-to-Ash Conversion Stage
chamber is
optionally provided with means for controlling the residence time of the char
in the Char-
to-Ash Conversion Stage chamber. Controlling the residence time of the char in
the Char-
to-Ash Conversion Stage chamber ensures that sufficient time is provided for
optimal
mixing of the char, heated air and optional steam, thereby providing for the
maximum
conversion of char to the Char-to-Ash Conversion Stage gas product and ash.
In one embodiment, the means for controlling the residence time of the
processed char in
a fixed-bed Char-to-Ash Conversion Stage chamber is provided by any mechanism
suitable for controllably conveying solids out of the chamber. In such an
embodiment,
once the processed char has been in the chamber for a residence time
sufficient for
conversion to the Char-to-Ash Conversion Stage gas product and ash, the ash
product is
actively conveyed out of the chamber. Such mechanisms include, but are not
limited to,
any of the controllable solids removal means that may be employed to actively
convey the
char product out of the primary chamber. Accordingly, the means for
controlling the
residence time of the char in the Char-to-Ash Conversion Stage chamber can
comprise
screws, pusher rams, horizontal rotating paddles, horizontal rotating arms, or
horizontal
rotating wheels. In one embodiment, the means for controlling the residence
time of the
char in the Char-to-Ash Conversion Stage chamber is any of the devices used
for solids
removal as depicted in any of Figures 1 to 8.
51
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
Figure 14 depicts one embodiment of a fixed-bed Char-to-Ash Conversion Stage
chamber
comprising a rotating wheel solids removal device and the relationship of the
Char-to-Ash
Conversion Stage chamber to a solid residue conditioning chamber.
In one embodiment, the ash product is removed in a continuous manner at a rate
appropriate to ensure that a sufficient residence time for carbon conversion
is achieved.
In one embodiment, the ash product is removed on an intermittent basis, once a
sufficient
residence time for carbon conversion has been achieved.
In one embodiment, the means for controlling the residence time of the char in
the Char-
to-Ash Conversion Stage chamber is provided by any mechanism which impedes the
progress of the char out of the chamber, thereby retaining solids in the
chamber for a
residence time sufficient to ensure conversion of the char to the Char-to-Ash
Conversion
Stage gas product and ash. In one embodiment, as the char gradually converts
to ash, the
ash product passively progresses out of the Char-to-Ash Conversion Stage
chamber.
In one embodiment, the Material Drying Stage is carried out in one chamber and
the
Volatilization Stage and Char-to-Ash Conversion Stage are carried out in
another
chamber.
In one embodiment, the zones for the Material Drying Stage, the Volatilization
Stage and
the Char-to-Ash Conversion Stage are combined into a single chamber. Referring
to
Figure 15, in one embodiment, the system (2100) comprises a refractory-lined
horizontally-oriented chamber (2102) having a feedstock input (2104) with two
air-locks,
steam outlet, gas outlet (2106) and a solid residue outlet (2108). The
gasification chamber
(2102) has a stepped floor with a plurality of floor levels (2112, 2114 and
2116). Each
floor level is sloped between about 15 and about 35 degrees. Each floor level
has a series
of additive inputs (2126) located in the side walls proximal to the floor
level to allow for
the addition of oxygen and/or steam. A baffle extends from the roof of
gasifier to form a
contained drying zone when feedstock is present. Steam resulting from the
heating of
feedstock exits the drying zone through steam outlet. Temperature in the
drying zone is
approximately 200 C.
Movement through the steps is facilitated by the lateral transfer system. In
this example,
the lateral transfer system comprises a series of pushers. As shown, each
floor level is
52
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
serviced by a pusher. Corresponding sealable openings in the gasification
chamber walls
allow for entry of each pusher. Cold air is introduced proximal to the ash
outlet of the
Char-to-Ash Conversion Stage chamber. The ash outlet of the Char-to-Ash
Conversion
Stage chamber is equipped with a grate that separates it for the ash
collection chamber.
The ash collection chamber is equipped with a pusher to facilitate the transit
of ash to an
Ash-to-Slag Conversion Chamber.
In another embodiment where the zones for the Material Drying Stage, the
Volatilization
Stage and the Char-to-Ash Conversion Stage are combined into a single chamber,
the
single chamber can be one of a number of gasifiers known in the art for
gasifying liquid or
solid feedstock. Examples of suitable gasifiers include, but are not limited
to entrained
flow reactor vessels, fluidized bed reactors, moving bed reactors and rotary
kiln reactors,
each of which is adapted to accept feedstock in the form of solids,
particulates, slurry,
liquids, gases, or a combination thereof. The feedstock is introduced through
one or more
inlets, which are disposed to provide optimum exposure to the heated exchange-
air for
complete and efficient conversion of the feedstock to the syngas. In one
embodiment, the
gasifier is a plasma gasifier. In one embodiment, the plasma gasifier uses
plasma in a
stage of the converter. In one embodiment, a stage of the gasifier that uses
plasma has a
pressure limitation. In one embodiment the gasifier is a fluid gasifier.
In one embodiment the Gasification System comprises one or more gasifiers. In
one
embodiment, the Gasification System comprises a fluid gasifier and a solids
gasifier.
The Gasification System - Chamber Designs as Process Manipulators
As indicated above various chamber designs known in the art are suitable for
use in the
Gasification System. For example, the gasifier can have a wide range of length-
to-
diameter ratios and/or can be oriented either vertically or horizontally. One
skilled in the
art would appreciate that the overall design of a chamber including the
internal
dimensions, positioning of energy sources, process additives, material
transfer means,
etc., are all carefully determined to promote the efficient transference of
energy
throughout the relevant stages of the conversion processes. A brief
description of
selected, non-limiting examples of gasifier designs is included below.
53
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
Vertically oriented gasifier
Vertically-oriented gasifiers comprise two or more vertically successive
processing
regions, within certain Stages such as the Material Drying Stage,
Volatilization Stage, or
Char-to-Ash Conversion Stage is at least partially favoured. The processing
regions are
identified by their different temperature ranges that enable the different
Stages therein.
The gasifier comprises one or more processing chambers; the vertically
successive
processing regions are distributed throughout the one or more processing
chambers.
Additive input elements are associated with the processing regions to promote
the at least
partially favoured process therein. Thus, the processing regions can be
considered to be
promoted by a combination of the one or more processing chambers and/or by a
positioning of the one or more additive input elements in each of the
processing
chambers. The gasifier comprises one or more feedstock inputs located near the
first
processing region, one or more gas outputs, one or more residue outputs, one
or more
material displacement control modules and optionally, a global Control System.
In one embodiment of the invention, the system comprises a gasifier with three
vertically
successive processing regions with the first processing region at least
partially favouring
the Material Drying stage, the second processing region at least partially
favouring the
Volatilization Stage and the third processing region at least partially
favouring the Char-
to-Ash Conversion Stage. A worker skilled in the art will understand that the
gasifier can,
in general, comprise a large number of processing regions with a different
proportion of
drying, volatilization or Char-to-Ash Conversion occurring in each processing
region.
Thus, the number of processing regions can be as many or as few as desired,
without loss
of generality.
For example, with reference to the embodiment of Figure 16, a gasifier 10
having a single
processing chamber 20 may comprise two or more distinct additive input
elements 30, or
groups thereof, positioned so to respectively promote or favour processes
within
respective vertically successive processing regions 40 within the single
processing
chamber 20. A feedstock input 50 provides feedstock to the first of the
processing regions
40, a gas output 60 for output of gas from the gasifier 10, and a residue
output 70 for
output of residue from the gasifier 10. The orientations and positions of the
input and
output elements for feedstock, additives, residue and gas, in Figure 16 are
merely
54
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
exemplary and any variations in their orientations and positions are
considered to be
within the scope and nature of the invention disclosed herein.
A material displacement control module operatively controlling one or more
process
devices and/or mechanisms (not shown) configured to control a vertical
displacement or a
rate of vertical displacement of the material through the vertically
successive processing
regions, is also provided thereby promoting the efficient processing of the
material within
each of these processing regions wherein a particular process is at least
partially favoured.
For example, as will be described in greater detail below, various devices
and/or
mechanisms may be controlled by the material displacement control module to
implement
a downward displacement of the material, either by direct control of material
displacement between each processing region, by controlled extraction of
material from a
lowermost processing region thereby indirectly controlling a downward
displacement of
material from an uppermost processing region toward the lowermost processing
region
under gravity, or using any combination thereof.
As depicted by the additives input and off-gas output phantom lines of Figure
16, it will
be appreciated that additives may be input in each processing region, for
instance via
appropriate positioning of additive input elements adapted therefor, or
provided to a select
number of these processing regions as appropriate for a given design and
embodiment of
the gasifier 10. It will also be appreciated that the additive input elements
may be actively
controlled by a common response element configured to provide a pre-selected
quantity or
input rate of additives (e.g. set absolute or relative input) for a given
sensed process
characteristic (e.g. process temperature, pressure, throughput, etc.; product
gas quality,
quantity, composition, pressure, flow, heating value etc.; feedstock input
rate, quality,
composition, etc; and the like), or again controlled by distinct response
elements, possibly
operatively linked via a same local, regional and/or global Control System.
Similarly, gas outputs may be provided for each processing region
independently, or
provided by one or more cooperative gas outputs allowing for the output of off-
gases from
the processing chamber 20 from more than one processing region simultaneously.
In the embodiment of Figure 17, a gasifier 110 may comprise two or more
processing
chambers 120 vertically and operatively coupled, each comprising one or more
additive
input elements 130, or groups thereof, positioned so to respectively promote
or favour
processes within respective processing regions 140 of each processing chamber
120,
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
thereby providing a vertical succession of two or more processing regions 140
when the
processing chambers 120 are combined. A feedstock input 150 provides feedstock
to the
first of the processing regions 140, a gas output 160 provides for output of
gas from the
gasifier 110, and a residue output 170 provides for output of residue from the
gasifier 110.
The orientations and positions of the input and output elements for feedstock,
additives,
residue and gas, in Figure 17 are merely exemplary and any variations in their
orientations
and positions are considered to be within the scope and nature of the
invention disclosed
herein.
A material displacement control module operatively controlling one or more
process
devices and/or mechanisms (not shown) configured to control a vertical
displacement of
the material through the vertically successive processing regions (i.e.
between chambers
and/or through the processing regions of a same chamber), is also provided
thereby
promoting the efficient processing of the material within each of these
processing regions
wherein a particular process is at least partially favoured. For example, as
will be
described in greater detail below, various devices and/or mechanisms may be
controlled
by the material displacement control module to implement a downward
displacement of
the material, either by direct control of material displacement between each
processing
region, by controlled extraction of material from a lowermost processing
region thereby
indirectly controlling a downward displacement of material from an uppermost
processing
region toward the lowermost processing region under gravity, or using any
combination
thereof.
As depicted by the additives input solid and phantom lines of Figure 17, it
will be
appreciated that additives will generally be input in each processing chamber,
though not
exclusively, and may also optionally be input at multiple locations within a
given
processing chamber to promote definition of two or more processing regions
therein. It
will also be appreciated that the additive input elements may be actively
controlled by a
common response element configured to provide a pre-selected quantity or input
rate of
additives (e.g. set absolute or relative input) for a given sensed process
characteristic (e.g.
process temperature, pressure, throughput, etc.; product gas quality,
quantity,
composition, pressure, flow, heating value etc.; feedstock input rate,
quality, composition,
etc; and the like), or again controlled by distinct response elements,
possibly operatively
linked via a same local, regional and/or global Control System.
56
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
Similarly, off-gas outputs may be provided for each processing chamber
independently, or
provided by one or more cooperative off-gas outputs allowing for the output of
gas form
more than one processing chamber 120 at a time.
Various combinations of processing chambers and additive input elements
therefore can
be adapted to provide two or more vertically successive processing regions as
contemplated herein, wherein an appropriate material displacement control
module can be
adapted for a given embodiment to enable the controlled displacement of
material through
these processing regions to enhance a processing thereof Such control may be
imparted
uniquely for each of the one or more processing chambers of the gasifier,
optionally
imparting indirect displacement of material through successive processing
regions of the
same processing chamber within which more than one processing region is
defined and/or
imparting a displacement of material from a first processing chamber to a
subsequent
vertically successive processing chamber of a gasifier comprising more than
one
processing chamber. Alternatively, control may be imparted to various
cooperative
control devices and/or mechanisms configured to directly control displacement
of material
from one processing region to another, possibly within a same processing
chamber.
For the embodiments using two processing chambers, Figure 18A and B shows a
variety
of different devices and/or mechanisms that can be used by the material
displacement
control module for displacement of reactant material from one processing
chamber to
another. A worker skilled in the art will understand that the options in this
figure are
merely exemplary and other appropriate designs for such devices/mechanisms can
be
considered to be within the scope and nature of the invention disclosed
herein.
Horizontally-Oriented Gasifier
Figure 19 depicts a horizontally-oriented gasifier (2000) having one or more
feedstock
input(s) (2004), one or more gas outlet(s) (2006) and a solid residue (ash)
outlet (2008).
Material enters the gasifier (2000) via the one or more feedstock input(s)
(2004) and is
moved through the gasifier (2000) during processing by one or more lateral
transfer units
(2010) which is controlled by a Control System. The horizontally-oriented
gasifier (2000)
comprises a lateral transfer system to facilitate the extraction of gaseous
molecules from
carbonaceous feedstock. The gasifier facilitates the gasification process by
sequentially
promoting drying, volatilization and char-to-ash conversion. This is
accomplished by
allowing drying to occur at a certain temperature range prior to moving the
material to
57
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
another region and allowing volatilization to occur at another temperature
range, prior to
moving the material to another region and allowing char-to-ash conversion to
occur at
another temperature range. Accordingly, as the material in the gasifier is
moved from the
feed area towards the solid residue end by one or more lateral transfer units
(2010) it goes
through different degrees of drying, volatization and char-to-ash conversion
(carbon
conversion). A flow diagram depicting the different regions of the gasifier in
general
terms is shown in Figure 20 and a representation of the gasification processes
occurring in
Regions 1, 2 and 3 of one embodiment of the gasifier is shown in Figure 21.
Referring to
Figure 20, Region I, which promotes drying of the material, is the area
between lines 310
and 320. Feedstock is delivered into the gasifier at Region I. The normal
temperature
range for this region (as measured at the bottom of the material pile) lies
between about
300 and 900 C. The major process here is that of drying which occurs
predominantly at
the top and in middle of the pile of material and at a temperature above about
100 C. In
addition, some volatilization and some char-to-ash conversion (carbon
conversion) occurs
in this region. Referring to Figure 21, Region II promotes volatilization of
the material
and is the region between lines 320 and 330. The material pile has a bottom
temperature
range between about 400 and 950 C. The main process occurring in Region II is
that of
volatilization with the remainder of the drying operation as well as a
substantial amount
of char to ash conversion (carbon conversion). Referring to Figure 21, Region
III, where
the char-to-ash conversion takes place, is the region between lines 330 and
340. The
Region III temperature range lies between about 500 and 1000 C. In one
embodiment,
however, in order to avoid agglomeration of the ash, the maximum temperature
in this
region does not exceed about 950 C. The major process in Region III is that of
carbon
conversion with a lesser amount (the remainder) of volatilization. By this
time the
moisture from the reactant material has been removed. By the end of this
region, the
majority of the solid residue is ash.
To facilitate movement of reactant material, the individual lateral transfer
units (2010) can
be controlled independently or a group of two or more lateral transfer units
(2010) can be
controlled in a coordinated manner. Thus, each area in the horizontally-
oriented gasifier
experiences temperature ranges and optional process additives (2019) (such as
air, oxygen
and/or steam) that promote a certain stage of the gasification process. In a
pile of reactant
58
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
material, all stages of gasification are occurring concurrently, however
individual stages
are favored at a certain temperature range.
By physically moving the material through the gasifier, the gasification
process can be
facilitated by allowing as much drying as energetically efficient to occur
prior to raising
the temperature of the material to promote volatilization. The process then
seeks to allow
as much volatilization as energetically efficient to occur prior to raising
the temperature of
the material to promote char-to-ash conversion (carbon conversion).
Figures 22, 23, and 24 depict views of one embodiment of a horizontally-
oriented gasifier.
Primary chambers with Gas Passage Conduit
In an alternate design, the primary chamber comprises one or more processing
chambers
at least one of which comprises one or more gas passage conduits located
therein for
facilitating the exit of off-gases from the processing chamber and/or the
entry of additives
into the processing chamber. Optionally, one or more input elements and/or one
or more
output elements are disposed around one or more of the one or more processing
chambers
to facilitate the entry of additives into the processing chamber and the exit
of off-gases
from the processing chamber respectively. The primary chamber additionally
comprises
one or more feedstock inputs for the input of feedstock into the primary
chamber, one or
more residue outputs for output of residue from the primary chamber, and
optionally, one
or more material displacement control modules for facilitating the movement of
the
reactant material through the primary chamber, which may be operatively
coupled to a
global Control System for controlling various aspects of the gasification
process.
Furthermore, one or more processing regions may be defined within the primary
chamber
within each one of which a certain process such as drying, volatilization or
carbon
conversion may be at least partially favoured. This type of primary chamber
can be
horizontally or vertically oriented.
Different embodiments of the present invention are shown in Figures 25A to
25D. For
example, the gasifier 1 can be oriented vertically, as illustrated
schematically in Figures
25A and 25B, oriented horizontally, as shown in Figures 25C and 25D, or
comprise a
combination of vertically and/or horizontally oriented processing chambers.
Figures 25A and 25B show vertically oriented gasifiers 110 and 210,
respectively, in
accordance with different embodiments of the present invention, each
comprising one
59
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
processing chamber. The one or more feedstock inputs (140 and 240
respectively) are
placed near the top of the processing chamber to allow for the entry of
feedstock therein.
In the embodiment illustrated in Figure 25A, output elements 135 disposed
around the
periphery of the processing chamber 110 are used for the extraction of off-
gases
therefrom, while the gas passage conduit 120 placed within the processing
chamber 110 is
used for the injection of additives therein. For the embodiment of Figure 25B,
input
elements 230 disposed around the periphery of the processing chamber 210 are
used for
the injection of additives therein while the gas passage conduit 220 placed
within the
processing chamber 210 is used for the extraction of off-gases therefrom.
Figures 25C and 25D show horizontally oriented gasifiers 301 and 401,
respectively, in
accordance with different embodiments of the present invention, each
comprising one
processing chamber. In the embodiment illustrated in Figure 25C, output
elements 335
disposed around the periphery of the processing chamber 310 are used for the
extraction
of off-gases therefrom, while the gas passage conduit 320 placed within the
processing
chamber 310 is used for the injection of additives therein. For the embodiment
of Figure
25D, input elements 430 disposed around the periphery of the processing
chamber 410 are
used for the injection of additives therein while the gas passage conduit 420
placed within
the processing chamber 410 is used for the extraction of off-gases therefrom.
The processing chambers of the gasifier may comprise different processing
regions,
within each one of which a certain process such as drying, volatilization or
carbon
conversion may be at least partially favoured, promoted by the chemical
conditions
existing therein. Figures 26A and 26B show two embodiments, where the single
processing chamber of the gasifier comprises three processing regions. In
Figure 26A,
input elements 531, 532, 533 disposed around the periphery of the processing
chamber
510 allow for injection of additives into the three processing regions 511,
512, 513 while
the two gas passage conduits 520 located within, allow for the extraction of
off-gases. In
the embodiment of Figure 26B, one gas passage conduit 620 each is used for
injection of
additives and extraction of off-gases. Input elements 631, 633 disposed around
the
periphery of the processing chamber 610 are used for injection of additives
into the first
and third processing regions 611 and 613, while output elements 638 disposed
around the
periphery of the processing chamber 610 are used for extraction of off-gases
from the
second processing region 612. It will be appreciated that several conduits may
be
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
provided for supplying additives to and/or removing off-gases from (optionally
for use in
heat and/or by-product recycling, as discussed further below) the one or more
chambers of
a given gasifier, in accordance with different embodiments of the present
invention, as
can any number or group of these conduits may be provided with a header and be
configured to be removed and/or replaced for maintenance and/or cleaning.
A worker skilled in the art will readily understand that the exact location,
size, use (e.g.
exit of off-gases, entry of additives, etc.) and the direction of gas flow
within the gas
passage conduits are not limited to those exemplified in the embodiments of
Figures 25A
to 25D, 26A and 26B, nor are the number of inlets and/or outlets provided
thereby limited
to those specifically identified in the appended Figures.
Figures 27A to 27C show respective side-views of the inner volumes for three
different
embodiments of a descending bed processing chamber. In all three embodiments,
the
descending bed processing chambers have the following similarities: three
vertically
successive processing regions are distributed within the descending bed
processing
chamber with corresponding input elements respectively used for injection of
additives
into them; the one or more feedstock inputs are at the top of the processing
chamber; and
the processing chamber is directly connected to a Gas Reformulating System
(GRS) based
on plasma torches. In one embodiment and referring to Figure 27A, the inner
wall is
shaped such that each processing region 711, 712, 713 has a vertical section
followed by
an inwardly sloped wall. In one embodiment and referring to Figure 27B, the
inner wall is
a refractory-lined cylinder. In one embodiment and referring to Figure 27C,
the inner wall
is refractory-lined and downward sloping.
A worker skilled in the art will readily understand that even within the same
processing
chamber, the different gas passage conduits can vary in shape and size and can
also be
used for different functions, as shown in figure 26. Additionally, the
multiple gas passage
conduits can be nested within one another, as shown in Figure 28, (e.g.
concentric
cylindrical tubes can be used for exit of off-gases and/or the entry of
additives into the
different processing regions) and/or distinctly located, as shown in Figure
26A.
In one vertically oriented embodiment of the invention and referring to Figure
29, the
additives are injected using the gas passage conduits within the inner volume
of the
descending bed processing chamber 1710 and the off-gases are extracted using
the output
elements 1737, 1738, 1739 distributed around the processing chamber. Once
again, the
61
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
flow of the additives is substantially cross-flow with respect to the
vertically downward
movement of the reactant material through the inner volume of the descending
bed
processing chamber. Figure 30 indicates how the gas conduit is configured to
accomplish
this.
Generally horizontally-oriented with lateral transfer system
In yet another design the gasifier is a generally horizontally-oriented
gasifier with a lateral
transfer system. This design is similar to that shown in Figure 8. As
indicated previously,
this type of design provides a generally horizontally-oriented gasifier having
one or more
feedstock input(s), one or more gas outlet(s) and a solid residue (ash)
outlet. Optionally,
the gasifier further comprises a moisture removal system and/or one or more
steam
outlets. Material enters the gasifier via the one or more feedstock input(s)
and is moved
through the gasifier during processing by one or more lateral transfer units
which is
controlled by a Control System.
The design of this gasifier facilitates the extraction of gaseous molecules
from
carbonaceous feedstock. In particular, there is provided a gasifier in which
the
gasification process is facilitated by sequentially promoting drying,
volatilization and
char-to-ash conversion (carbon conversion). This is accomplished by allowing
drying to
occur at a certain temperature range and optionally, the resulting steam or
moisture is
evacuated from the gasifier to obtain a substantially dry material. The
material is moved
to another region and volatilization is allowed to occur at another
temperature range. The
material is then moved to another region where char-to-ash conversion occurs.
Accordingly, as the material in the gasifier is moved from the feed area
towards the solid
residue end by one or more lateral transfer units it goes through different
degrees of
drying, volatization and char-to-ash conversion (carbon conversion).
To facilitate movement of reactant material, the individual lateral transfer
units can be
controlled independently or a group of two or more lateral transfer units can
be controlled
in a coordinated manner.
Thus, each area in the horizontally-oriented gasifier experiences temperature
ranges and
optional process additives (such as air, oxygen and/or steam) that promote a
certain stage
of the gasification process. In a pile of reactant material, all stages of
gasification are
62
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
occurring concurrently, however individual stages are favored at a certain
temperature
range.
By physically moving the material through the gasifier, the gasification
process can be
facilitated by allowing as much drying as energetically efficient to occur
prior to raising
the temperature of the material to promote volatilization. The process then
seeks to allow
as much volatilization as energetically efficient to occur prior to raising
the temperature of
the material to promote char-to-ash conversion (carbon conversion).
The Gasification System - Ash Melting and Vitrification Stage
The Dominant Process
The dominant process in this Stage is heating the solid residue to the
temperature required
to melt, blend, and chemically react the solids to form a dense,
silicometallic, vitreous
material.
The Energy Sources
In the zone or chamber that favors Ash Melting and Vitrification Stage, a
energy source is
used to achieve the high processing temperature required to vitrify the solid
residue
(around 1400-1500 C depending on the ash properties) and blend it adequately
to
homogenize it before releasing it from the chamber, where it cools to form a
dense, non-
leachable, silicometallic solid.
There are a number of energy sources used to achieve the high processing
temperature
required to vitrify the solid residue. The energy source must meet the
required
temperature for heating the solid residue to required levels to melt and
homogenize the
solid residue while allowing the resulting molten solid residue to flow out of
the chamber
or zone.
Plasma systems appropriate for melting may be based on a variety of
technologies,
including but not limited to, microwave plasma, inductively coupled plasma,
electric arc
plasma and thermal plasma. In one embodiment, heat is provided by a plasma
system
comprising plasma torch systems. Plasma torch systems known in the art include
but are
not limited to transferred arc torch (TAT) and non-transferred arc torch
(NTAT) systems.
63
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
Both TAT and NTAT systems need positive and negative points for operation. In
NTAT
systems, both points are metallic while in TAT systems, the points depend on
the
"workpiece". As very high temperatures are typically achieved, effective
cooling
techniques, such as for example rapid water cooling, is required for
operation. Torch
systems can be designed to work in alternating current (AC) mode, both single
and multi-
phase, and direct current (DC) mode.
In one embodiment of the invention utilizing NTAT systems, two magnetic field
coils can
be used to rotate the arcs. A worker skilled in the art will readily
understand that the
operation of the torch systems can be varied by adjusting the position of the
magnetic
coils and the materials used as electrodes for the plasma torch systems.
Materials that can
be used for the electrodes in these torch systems include but are not limited
to steel,
tungsten, graphite, thoriated tungsten, copper and alloys of copper with Zr,
Cr or Ag.
A worker skilled in the art will understand that the choice of carrier gas for
a plasma torch
system impacts the reaction chemistry within the melter. For example, the
carrier gas can
be reducing, oxidizing or inert. Typical carrier gases for torch systems
include but are not
limited to air, nitrogen, helium, argon, oxygen, carbon monoxide, hydrogen and
methane.
A worker skilled in the art will understand that the choice of a particular
plasma torch
system for melting will depend on a variety of factors including but not
limited to:
electrical to thermal efficiency; heat transfer to the `working material';
electrode life;
electrode cost; ease of electrode replacement; temperature profile; plasma gas
enthalpy;
simplicity of design and manufacture of support systems such as power supplies
and
Control Systems; operator qualification requirement; requirement on type of
carrier gas;
need for de-ionized water; reliability; capital and operating costs; ability
to be moved
within the melting vessel; and ability to be inserted close to the working
material within
the vessel.
In one embodiment of the invention, the source of heat for residue melting is
Joule
heating elements. Joule heating refers to the generation of heat in a
conductor by the
passage of an electric current within, due to the interaction between the
moving charged
particles and the atomic ions within the conductor. The heat produced within
is
proportional to the electrical resistance of the wire multiplied by the square
of the current.
64
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
In one embodiment of the invention, the source of heat for residue melting is
one or more
gas burner systems.
A gas burner generates a flame using a gaseous fuel such as acetylene, natural
gas or
propane. Optionally, an air inlet may be used as appropriate with some gas
burners, to mix
the fuel gas with air to obtain complete combustion. For example, acetylene is
commonly
used in combination with oxygen. A worker skilled in the art will know that
the selection
of appropriate gas for the gas burner will depend on various factors including
for
example, the desired flame temperature.
A worker skilled in the art would appreciate that in addition to the type of
heat source
used, the position and orientation of the heat source are additional factors
to be considered
in the design of the Ash Melting and Vitrification Stage zone or chamber.
The Process Manipulators
Design strategies can be utilized to optimize the ash melting and
vitrification process
and/or the efficiency of the whole conversion process. For example, the heat
generated to
convert the ash-to-slag may optionally be recycled back into the system and
thereby
increase the efficiency of the whole conversion process. The Ash Melting and
Vitrification Stage zone or chamber may also designed to ensure that the
residence time is
sufficient to ensure that the solid residue is brought up to an adequate
temperature to melt
and homogenize the solid residue.
The system may comprise an ash collection chamber or zone that collects the
ash from
Char-to-Ash Conversion Stage prior to it being passively or actively conveyed
to a
chamber or zone which favors Ash Melting and Vitrification Stage. Appropriate
ash
collection chambers are known in the art and accordingly, a worker skilled in
the art
having regard to the requirements of the system would readily know the size,
shape and
manufacture of an appropriate ash collection chamber. The ash collection
chamber may
optionally be physically separated from the Char-to-Ash Conversion Stage zone
or
chamber. In one embodiment, the ash collection chamber is physically separated
from the
Char-to-Ash Conversion Stage zone or chamber by a grate. In another
embodiment, the
ash collection chamber is physically separated from the Char-to-Ash Conversion
Stage
zone or chamber by a perforated plate. In a further embodiment, the ash
collection
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
chamber is physically separated from the chamber by a movable or retractable
plate. In
other embodiments, the ash is collected in a region of the Char-to-Ash
Conversion Stage
or Ash Melting and Vitrification Stage zone or chamber.
Design strategies may also include strategies to optimize the transfer of ash
to the Ash
Melting and Vitrification Stage zone or chamber from the Char-to-Ash
Conversion Stage
zone or chamber and/or the ash collection chamber. For example the ash
collection
region or chamber may be shaped to facilitate the transfer of ash to the Ash
Melting and
Vitrification Stage zone or chamber. The ash collection region or chamber may
optionally include active conveyance means to actively transport the ash to
the Ash
Melting and Vitrification Stage zone or chamber. In one embodiment, the ash is
pushed
into a region or chamber for the conversion of ash-to-slag. In one embodiment,
a ram
mechanism pushes the ash out of the gasifier into the ash collection chamber.
In one
embodiment, a pusher pushes the ash into the chamber for conversion of ash to
slag. In
one embodiment, a system of conveying rams is used to transport the ash to the
zone or
chamber that favors Ash Melting and Vitrification Stage. Optionally, the
length of the ram
stroke can be controlled so that the amount of material fed into a solid
residue processing
chamber with each stroke can be controlled. In a further embodiment, a
controllable
rotating arm mechanism is used to convey ash to the zone or chamber that
favors Ash
Melting and Vitrification Stage.
Design strategies may also be considered to reduce the agglomeration of ash
and/or break
up any agglomerated ash. For example, cold air may be added to the end of the
Char-to-
Ash Conversion Stage zone or chamber or the ash collection region or chamber
to reduce
the temperature of the accumulated ash and thereby reduce agglomeration of the
ash.
Agglomerated ash has been shown to cause jamming in drop port type exits. The
invention therefore can optionally comprise a means for breaking up ash
agglomerates.
In one embodiment, in order to ensure that any agglomerations do not create
jamming at
the exit from the chamber, a screw conveyor concept is used to extract the
ash. The ram
motion will push the ash into the extractor and the extractor will pull the
ash out of the
gasifier and feed it into an ash conveyor system. Rotation of the extractor
screw breaks
up agglomerations before the ash is fed into the conveyor system. This
breaking up action
can be enhanced by having serrations on the edge of the extractor screw
flights.
66
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
Accordingly, the system of the present invention comprises a Ash Melting and
Vitrification Stage zone or chamber adapted to receive solid residue and
having one or
more heat sources, optional air input means, and a slag outlet.
The molten slag may periodically or continuously be exhausted from the Ash
Melting and
Vitrification Stage zone or chamber and is thereafter cooled to form a solid
slag material.
Such slag material may be intended for landfill disposal. Alternatively, the
molten slag
can be poured into containers to form ingots, bricks tiles or similar
construction material.
The solid product may further be broken into aggregates for conventional uses.
In one embodiment, the solid residue processed in the solid residue
conditioning chamber
includes solids transferred from a downstream process, for example, solids
retrieved from
a baghouse filter in a downstream gas conditioning process.
In one embodiment, Char-to-Ash Conversion Stage and Ash Melting and
Vitrification
Stage are combined in a single chamber referred to as a carbon converter. In
such
embodiments, the carbon converter comprises the Char-to-Ash Conversion Stage
or
carbon conversion zone in communication with the Ash Melting and Vitrification
Stage
or ash melting and vitrification zone for melting residual substantially
carbon free solid
material into molten slag and/or for maintaining slag in a molten state. The
Char-to-Ash
Conversion Stage zone and the Ash Melting and Vitrification Stage zone are
separated by
an inter-zonal region or inter-zone that restricts or limits the movement of
material
between the two zones. Referring to Figure 31, the carbon converter (10)
comprises char
inputs (20) into the Char-to-Ash Conversion Stage or carbon conversion zone
(11) of the
refractory-lined chamber (15), where heated air inputs (35) convert the
unreacted carbon
in the processed feedstock into a syngas. Residual substantially carbon-free
solid material
(i.e. ash) is subsequently converted into a molten slag material in either the
inter-zonal
region or inter-zone and/or the Ash Melting and Vitrification Stage or ash
melting and
vitrification zone by the direct or indirect (i.e. via heat transfer elements)
application of
plasma heat. The molten slag material is output from the slag zone of the
multi-zone
carbon converter and passed into an optional slag cooling subsystem for
cooling.
The inter-zonal region can comprise an impedance mechanism upon or in which
the char
is retained for a sufficient time to ensure char conversion prior to the ash
product being
passed out of the Char-to-Ash Conversion Stage chamber. The impedance
mechanism
67
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
limits or regulates the movement of material out of the Char-to-Ash Conversion
Stage
chamber by either partially or intermittently occluding solid residue outlet
or by forming a
reservoir in which the char temporarily accumulates.
Referring to Figure 32A to F, the impedance mechanism is mounted at the bottom
of the
Char-to-Ash Conversion Stage chamber and can be of any physical barrier of
suitable
shape or design, including but not limited to dome shaped, pyramidal shaped,
grates,
moving grates, brick grate, plurality of ceramic balls, plurality of tubes
etc. The shape
and size of the impedance mechanism may in part be dictated by shape and
orientation of
the chamber.
Referring to Figures 32A to F, which detail various alternative, non-limiting
impedance
mechanisms. In one embodiment as illustrated in Figure 32A, the impedance
mechanism
is a solid refractory dome (145) mounted by wedge-shaped mounting bricks (150)
at the
bottom of the Char-to-Ash Conversion Stage chamber. The solid refractory dome
is sized
such that there is a gap (155) between the outside edge of the dome and the
inner wall of
the chamber. Optionally, the refractory dome further comprises a plurality of
holes (160).
Referring to Figure 32B the impedance mechanism comprises a solid refractory
brick
grate. The refractory brick grate (245) is provided with gaps (255) between
the
individual bricks to allow for communication between the carbon conversion
chamber
and the solid residue conditioning chamber.
Referring to Figure 32C, the impedance mechanism comprises a grate structure
manufactured from refractory-lined tubes (345) mounted within a mounting ring
(350),
which is mounted at the bottom of the Char-to-Ash Conversion Stage chamber.
In one embodiment as illustrated in Figure 32D, the impedance mechanism is a
solid
refractory pyramid (145) mounted by mounting bricks at the bottom of the Char-
to-Ash
Conversion Stage chamber.
Referring to Figure 32E, the impedance mechanism comprises a plurality of
ceramic balls.
Referring to Figure 32F, the impedance mechanism comprises a domed cogwheel.
68
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
The impedance mechanism and any associated mounting elements must be able to
effectively operate in the harsh conditions of the Char-to-Ash Conversion
Stage chamber
and in particular must be able to operate at high temperatures. Accordingly,
the
impedance mechanism is constructed of materials designed to withstand high
temperature.
Optionally, the impedance mechanism may be refractory-coated or manufactured
from
solid refractory.
In one embodiment, the Material Drying Stage, Volatilization Stage, Char-to-
Ash
Conversion Stage and Ash Melting and Vitrification Stage are combined in a
single
chamber.
The Gas Reformulation System
The facility of this invention comprises a system for the effective
reformulation of gas
derived from the gasification of carbonaceous feedstock. The initial gas to be
input into
this system will generally comprise a complex mixture of hydrocarbon molecules
of
varying length. The chemical composition and the contaminant quality of the
gas will
depend on the composition of the feedstock, the process used to generate the
gas and the
conditions in the Gasification System. Some gasifiers are designed for a one
step process,
wherein various forms of heat are used to generate the gas in a single
chamber. Other
gasifiers generate the gas in a multi-step process, in either different
regions of one
chamber or different chambers or some combination thereof. Either system might
include
some pre-processing of the raw off-gas, generally due to the source of heat in
the
gasification chamber.
One primary objective of these design strategies is to optimize the effective
exposure of
the amounts of raw syngas and/or preformulated gas to the reactive species in
the gas
energizing zone. The greater the degree of effective exposure, the greater the
efficiencies
of energy transference, and hence, the greater the percent conversion of the
preformulated
gas into gas of a designed chemical composition in the most overall cost
effective manner.
Examples of design strategies include the design of the entire system. For
example,
important design strategies comprise the flow pattern (turbulence) of the
preformulated
gas relative to the gas energizing field and particularly the amount of gas
that passes
through this field in a particular amount of time. One example of these
strategies is the
69
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
system design whereby the preformulated gas passes through plasma generating
electric
arc(s). Another example is the system design wherein a plasma torch is
positioned in a
manner that the plasma plume flows counter-current to and directly down into
the
preformulated gas. In another embodiment, the preformulated gas passes through
sequential or parallel gas energizing fields.
The reformulating system of the invention is designed to optimize the amount
of
preformulated gas that is reformulated into a product gas. In one embodiment,
the
effectiveness of this process is expressed by the term, Gas Reformulation
Ratio, which
comprises the amount of reformulated product gas divided by the amount of
preformulated or initial reactant gas X 100 = %. In one embodiment, the Gas
Reformulation Ratio is 95% or greater. In one embodiment, the Gas
Reformulation Ratio
is 90% or greater. In one embodiment, the Gas Reformulation Ratio is 85% or
greater. In
one embodiment, the Gas Reformulation Ratio is 80% or greater. In one
embodiment, the
Gas Reformulation Ratio is 75% or greater. In one embodiment, the Gas
Reformulation
Ratio is 70% or greater. In one embodiment, the Gas Reformulation Ratio is 65%
or
greater. In one embodiment, the Gas Reformulation Ratio is 60% or greater. In
one
embodiment, this concept is expressed as a ratio of the value of the
reformulated gas as
compared to the initial gas. In one embodiment, the value is the energetic
value in terms
of electricity generation.
In order to effectively reformulate initial gas into gas of a designed
composition, this
invention comprises one or more "gas reformulating zones," and one or more
"gas
stabilizing zones." A gas stabilizing zone optionally comprises heat transfer
means to
capture heat from the gas as it cools. The system optionally comprises one or
more "gas
additive zones," generally located upstream of a gas reformulating zone, with
or without
mixing. It also optionally comprises one or more "gas cleaning zones,"
generally located
downstream of a gas stabilizing zone.
For the purposes of clarity, these zones are described separately. It is
understood,
however, that these zones are generally contiguous and interrelated within the
system, that
the system is not limited to comprising discrete, physically separated zones,
although this
remains an alternative option. Depending upon the design of a particular
embodiment,
they will be more or less separated. In addition, for ease of reference only,
the zones have
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
been named according to the process step that takes place predominantly in
that zone. One
skilled in the art will appreciate, however, that due to the nature of the
reformulation
process other process steps may also take place to a lesser extent in that
zone.
A system that effectively reformulates gas must be able to raise the energy of
the initial
gas molecules so that they begin to reformulate. In particular, reaction
intermediates are
initiated. The energetic processes of a reaction are represented by a curve
such as shown
below.
GRE: Gas Reformulating Energy
GREno catalyst Without catalysts
W With catalysts
a.
GREwith catalyst
Reaction Coordinate --
As one skilled in the art would appreciate, the arrow points to a
representation of energy
that is required to induce the gaseous molecules of an initial chemical
composition to
begin to reformulate into molecules of a designed chemical composition. The
dotted line
represents the energy required when a catalyst is used to lower the amount of
energy
required to bring about the reformulation of the molecules. One skilled in the
art
appreciates that, at a general level, sufficient energy will be required to be
imparted to the
initial gas molecules to drive them to break their bonds and reformulate into
reformulated
molecules and atoms. Under the appropriate conditions, if the reformulated
molecules
and/or atoms are allowed to mix thoroughly, the atoms will recombine according
the
relative concentrations of the species present. Moreover, if a significant
amount of the
preformulated gas passes through the energizing field, a significant amount of
the gas will
be reformulated.
To accomplish the objective of effectively reformulating gas, one skilled in
the art can
appreciate that the following four chemical processes occur throughout the
reformulation
71
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
of a gas: 1) initiation of the intermediates; 2) propagation of at least a
portion of the
intermediates; 3) termination of the intermediates; and 4) product gas
stabilization.
A gas reformulation process can be envisioned to entail four general
processes. In the first
process, reactants such as initial gaseous molecules and energy sources
(including but not
limited to free electrons, and other energized or activated species such as
ions and free
radicals) are brought together through mixing and reach a state of species -to-
species
contact. As a result of such contact and a sufficient energy level of the
mixture, the
interaction of the reactants leads to the formation of chemical intermediates.
While some
of the intermediates may react together and terminate, at least a portion of
the
intermediates undergo another step, in which the intermediates react between
themselves
with or without the participation of the reactants to produce other
intermediates, resulting
in a chain of chemical reactions. In another process, the intermediates are
terminated by
chemical and/or physical means and yield specific products. In the fourth and
final step,
the products formed are stabilized when specific chemical and/or physical
conditions are
maintained.
The initiation of intermediates may therefore be considered as the dominant
process that
occurs early within the gas reformulating zone where an intermediate-inducing
means (an
energy source) is provided and brought into contact with a gas entering the
gas
reformulating zone. Mixing, energy transfer, and/or radiation which enables
the
transformation of the reactants into initial intermediates. The reactants can
be said to be
excited.
The intermediate propagation step may be considered to be another major
process that
occurs in the gas reformulating zone where the initial intermediates react
between
themselves to produce other intermediates. It is possible for these
intermediates to form a
chain of reactions with one group of intermediates being derived from the
previous one.
In general, the intermediate termination processes can be considered to occur
at the end of
the gas reformulating zone and, in some embodiments, may even be considered to
define
the outer edges of the zone wherein the chemical and/or physical conditions
are changed
such that the chain reactions are consequently stopped from proceeding
further. It would
be understood, however, that termination processes may take place in other
regions of the
gas reformulating zone depending on the specifics of the process, the
72
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
reactants/intermediates and the stability of the final product. At the end of
the chain
reactions reached either by controlled termination or by undisturbed
progression, specific
products are formed.
The gas stabilizing zone may be considered to be located where product
stabilization is
the dominant process and may be defined as a zone where specific conditions
are
maintained in order to stabilize the products formed at the termination of the
recombining
of the intermediates. These products are normally desired for specific
applications. If
different products are required, effort may be made to adjust the intermediate
termination
point since different points of the chain reaction course correspond to
different
intermediates which in term yield different products upon termination and
stabilization.
There are many intermediate inducing means. These include thermal heating,
plasma
plume, hydrogen burners, electron beam, lasers, radiation, etc. In situations
where the
reactant molecules have sufficient energy to rearrange in the presence of a
catalyst and are
brought in contact with such a catalyst, the catalyst can be seen to play the
role of an
intermediate inducing means. The common feature of energy sources that provide
intermediate inducing means is to cause chemical changes to reactants and
proceed along
a pathway to final products. The intermediates formed can therefore differ
between
different intermediate inducing means and have different levels of activation.
There are a number of ways of elevating the energy of the initial gas to a
level such that
the molecules will reformulate into the molecules of a designed chemical
composition.
Heat can be added to the initial gas. Activated species, such as the electrons
and positive
ions found in plasma or produced from a hydrogen source can be used to
transfer the
energy required to cause the molecules in the initial gas and process
additives, "the
preformulated gas," to reformulate into reformulated molecules and atoms.
As noted above, there are various catalysts known to one skilled in the art
that can be used
to lower the amount of energy that must be required to cause the molecules to
reformulate. Catalysts such as dolomite, olivine, zinc oxide and char are
examples of
some commonly used catalysts.
This invention provides a smart, integrated Gas Reformulating System for
efficient,
deliberately planned reformulation of an initial gas with associated
characteristic
73
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
characteristics (e.g. chemical composition) into an output gas with
characteristic
characteristics designed for a specific downstream purpose. Optimization
includes the
most overall cost effective manner of accomplishing the reformulation,
including upfront
costs such as electricity and downstream costs such as processing contaminated
catalysts.
The Gas Reformulating System process:
(1) senses directly or indirectly the adequacy of the characteristic
parameters of the initial
gas including but not limited to the chemical composition, humidity, flow
rates, etc.
Optionally, the system may sense characteristic and/or parameters of upstream
and/or
downstream systems or input or outputs thereof;
(2) modifies various input parameters to the reformulation process (e.g.
optionally
increases or decreases appropriate amounts of process additives, modifies the
amount
of electricity, etc.) based on the sensed characteristic parameters of the
initial gas and
the desired parameters of the output gas;
(3) generates one or more gas reformulating zones comprising sufficient
energetic species
that can interact with the off-gas molecules (the initial gas or preformulated
gas) to
transfer energy to the gaseous molecules such that the majority of the gaseous
molecules reformulate into reformulated molecules and atoms;
(4) in the reformulating zone, promotes efficient mixing of the initiated
gaseous
molecular constituents (the initiated intermediates) such that they recombine
into a
chemical composition determined by the relative concentrations of the species
present
in the reformulated gas;
(5) provides a stabilizing zone, whereby the newly formed molecules are de-
energized,
for example, cooled or removed from the influence of catalysts or gas
energizing
sources, and thus stabilized to maintain the desired characteristics; and
(6) provides a Control System for overall control of the gas reformulation
process.
The system and method of gas reformulation may be used to reformulate a
substantial
74
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
amount of off-gas such as produced from gasification of carbonaceous feedstock
into a
reformulated gas comprising optimal levels of molecules such as carbon
monoxide and
hydrogen and minimal levels of unwanted molecules.
In the ensuing description, the following parts of the Gas Reformulating
System are
considered in greater detail. The basic process will be taught beginning with
a description
of the "gas reformulating zones;" and "gas stabilizing zones." The strategy
and tactics for
optimizing the extent and efficiencies of gas reformulation will be described
with a
discussion of Gas Manipulators including catalysts and other Gas Manipulators.
Optional
features for inclusion in the system include "gas additive zones," and "gas
cleaning
zones." Finally the description will discuss the design of gas reformulating
chamber and a
Control System to manage all of the above processes.
The Gas Reformulating Zone
The reformulating zone is the zone within the system wherein the preformulated
molecules that are sufficiently energized to reformulate into molecular
species of a
designed chemical composition occurs. In general, this zone is designed such
that it
incorporates means for causing turbulence and mixing during the reformulating
process.
Gas Energizing Sources
Gas energizing sources provide the initial energy required to overcome the
molecular
bonding energies of the initial gas and the process additives within the Gas
Reformulating
System (the preformulated gas), thus serving to reformulate these molecules
into
reformulated molecules and eventually the molecules of designed chemical
composition,
such as CO and H2. These energizing sources serve to provide energy for
initiation of the
reactive intermediates, and when required, to provide energy to support
propagation of the
intermediates.
Various elements are envisioned within this invention for the provision of the
gas
energizing zones. The energy levels required to meet the requirements of the
Gas
Reformulation Energy depend on a variety of factors including but not limited
to the
characteristics (e.g. composition) of the initial gas, the process additives,
and the presence
of catalysts. Means to increase the temperature, residence time and/or
turbulence and
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
mixing are also envisioned for inclusion in designing and creating this zone.
Energy required for gas energizing in order to induce intermediates to become
reactive
can be provided by various sources referred to as energizing sources, thermal
heating,
plasma, hydrogen burners, electron beams, lasers, radiation, etc. Their common
feature is
to cause chemical changes to reactants and proceed along a pathway to final
products.
Sources of Plasma
Plasma provides a source of energy mostly in the form of electrons and
positively charged
ions that can interact with the preformulated gas to supply Gas Reformulation
Energy to
the molecules.
In one embodiment of the invention, one or more plasma-based sources (e.g.
plasma
torches), operated in conjunction with or without other gas energizing
sources, are used to
raise the energy of the initial gas to a level sufficiently high for gas
reformulation, and
thus provide a gas energizing zone. The appropriate energy level depends on a
variety of
factors including but not limited to the characteristics of the initial gas
and the process
additives, and is readily determined by a worker skilled in the art.
Although heat contributes to the process, a significant portion of the
majority of the
energy is supplied by the reactive species in the plasma. In one embodiment of
the
invention, the temperature is between about 800 C to about 1200 C. The amount
of
energy required of the source may be lowered by the use of catalysts.
The one or more plasma sources may be chosen from a variety of types including
but not
limited to non-transferred and transferred arc, alternating current (AC) and
direct current
(DC), plasma torches, high-frequency induction plasma devices and inductively
coupled
plasma torches (ICP). In all are generating systems, the are is initiated
between a cathode
and an anode. Selection of an appropriate plasma source is within the skills
of a worker
in the art.
The transferred arc and non-transferred arc (both AC and DC) torches can
employ
appropriately selected electrode materials. Materials suitable for electrodes
that are
known in the art include copper, tungsten alloys, hafnium etc. The electrode
lifetime
depends on various factors such as the arc-working areas on the electrodes,
which in turn
76
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
depends on the design of the plasma torch and the spatial arrangement of the
electrodes.
Small arc-working areas generally wear out the electrodes in a shorter time
period, unless
the electrodes are designed to be cooled by thermionic emission. The
electrodes may be
spatially adjustable to reduce any variations in the gaps there between,
wherein the
variations are caused as the electrodes wear down during their lifetimes.
A variety of gases can be used as a carrier gas for plasma torches including
but not limited
to air, argon, helium, neon, hydrogen, methane, ammonia, carbon monoxide,
oxygen,
nitrogen, carbon dioxide, C2H2 and C3H6. The carrier gas may be neutral,
reductive or
oxidative and is chosen based on the requirements of the gas reformulation
process and
the ionization potential of the gas. Selection of an appropriate carrier gas
and
understanding the means of introducing the carrier gas into the plasma torch
can impact
its efficiency is within the ordinary skills of a worker skilled in the art.
In particular, that
a poorly designed introduction of the carrier gas can result in a non-uniform
plasma
plume, with hot and cold zones.
In one embodiment, the gas reformulating system comprises one or more non-
transferred,
reverse polarity DC plasma torches. In one embodiment, the gas reformulating
system
comprises one or more water cooled, copper electrode, NTAT DC plasma torches.
In one
embodiment of the invention, the gas reformulating system comprises one or
more AC
plasma torches.
AC plasma torches may be either single-phase or multiple phase (e.g. 3-phase),
with
associated variations in arc stability. A 3-phase AC plasma torch may be
powered
directly from a conventional utility network or from a generator system.
Higher phase AC
systems (e.g. 6-phase) may also be used, as well as hybrid AC/DC torches or
other hybrid
devices using but not limited to hydrogen burners, lasers, electron beam guns,
or other
sources of ionized gases.
Multiple phase AC plasma torches generally have lower losses in the power
supply. In
addition, the rapid movement of the arc along the electrodes due to rail-gun
effect can
result in improved redistribution of the thermal load between the electrodes.
This
redistribution of the thermal load along with any cooling mechanisms for the
electrodes,
allows the use of materials for electrodes having a relatively low melting
point but high
77
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
thermal conductivity, such as copper alloys.
The plasma source may comprise a variety of commercially available plasma
torches that
provide suitably high flame temperatures for sustained periods at the point of
application.
In general, such plasma torches are available in sizes from about 100 kW to
over 6 MW in
output power. In one embodiment, the plasma torch is two 300 kW plasma torches
each
operating at the (partial) capacity required.
Hydrogen Burners
In one embodiment of the invention, the gas energizing field is at least
partially provided
by a hydrogen burner wherein oxygen and hydrogen are reacted to form ultra-
high
temperature steam (>1200 C). At these high temperatures, the steam may exist
in an
ionized form which enhances the gas reformulation process. Hydrogen burners
may be
operated in conjunction with or without other gas energizing sources such as
plasma
torches. Activated hydrogen species include the benefit of rapid dispersion of
the reactive
species and extensive steam cracking, both of which lead to a high conversion
of the
initial gas at a lower temperature than achieved with plasma.
In one embodiment of the invention, hydrogen burners provide a significant
portion of the
energizing energy, thereby acting as the primary energizing field element.
The hydrogen for the hydrogen burner may be obtained by electrolysis. The
oxygen
source may be pure oxygen or air. Other sources for hydrogen and oxygen may
also be
used as would be readily known to a worker skilled in the art. The design of
the burner
may utilize standard modeling tools e.g. tools based on computational fluid
dynamics
(CFD). The burner may also be adapted and sized to fit the requirements of the
gas
reformulating system taking into account various factors including but not
limited to the
quantity of gases for reformulation, chamber geometry etc.
In one embodiment of the invention, the hydrogen burner comprises a
cylindrical nozzle
body, with upper and lower covers coupled to its upper and lower ends
respectively and
defining a predetermined annular space S in the body. A gas supply pipe is
connected to a
sidewall of the body such that the pipe is inclined downwards therefrom. The
upper cover
may be integrated with the body into a single structure, and is provided with
a heat
transfer part having a thickness sufficient for easy dissipation of heat. A
plurality of
78
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
nozzle orifices, which discharge hydrogen to the atmosphere, is formed through
the heat
transfer part with an exposing depression formed on the upper surface thereof
to
communicate with each of the nozzle orifices. An airflow chamber is also
defined in the
body so that air passes through the chamber. A guide protrusion is formed on
the inner
surface of the space to guide the current of hydrogen gas to a desired
direction in the
space. Furthermore, the upper end of the annular space S, which communicates
with the
lower ends of the nozzle orifices, is configured as a dome shape, thus
defining a vaulted
guide to guide hydrogen gas to the orifices.
Hydrogen burners operate at a lower temperature and usually mix hydrogen with
air. They
may also use a oxygen-hydrogen mixture which runs at a significantly higher
temperature.
This higher temperature can give off more radicals and ions; it also will make
the gas
highly reactive with hydrocarbon vapor and methane.
In one embodiment of the invention, a hydrogen burner serves as a source of
high
temperature chemical radicals which can accelerate the reformulation of
gaseous
hydrocarbons into syngas. The hydrogen burner is operated with an oxidizing
agent, with
air and oxygen being two common choices. A worker skilled in the art will
understand
the relative proportion of hydrogen and the oxidizing agent required. In
addition to
generating high-temperature radicals, the hydrogen burner also generates a
controllable
amount of steam. Typically, hydrogen burners can be powered with efficiencies
similar to
a plasma torch.
Electron Beam Guns
Electron beam guns produce electron beams with substantially precise kinetic
energies
either by emission mechanisms such as thermionic, photocathode and cold
emission; by
focusing using pure electrostatic or with magnetic fields and by a number of
electrodes.
Electron beam guns can be used to ionize particles by adding or removing
electrons from
the atom. A worker skilled in the art will readily know that such electron
ionization
processes have been used in mass spectrometry to ionize gaseous particles.
The designs of electron beam guns are readily known in the art. For example, a
DC,
electrostatic thermionic electron gun is formed of several parts including a
hot cathode
which is heated to create a stream of electrons via thermionic emission;
electrodes which
79
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
generate an electric field to focus the beam, such as a Wehnelt cylinder; and
one or more
anode electrodes which accelerate and further focus the electrons. For larger
voltage
differences between the cathode and anode, the electrons undergo higher
acceleration. A
repulsive ring placed between the anode and the cathode focuses the electrons
onto a
small spot on the anode. The small spot may be designed to be a hole, in which
case the
electron beam is collimated before reaching a second anode called a collector.
Radiation
Ionizing radiation refers to highly-energetic particles or waves that can
ionize an atom or
molecule. The ionizing ability is a function of the energy of the individual
packets
(photons for electromagnetic radiation) of the radiation. Examples of ionizing
radiation
are energetic beta particles, neutrons, and alpha particles.
The ability of electromagnetic radiation to ionize an atom or molecules varies
across the
electromagnetic spectrum. X-rays and gamma rays will ionize almost any
molecule or
atom; far ultraviolet light will ionize many atoms and molecules; near
ultraviolet and
visible light will ionize very few molecules. Appropriate sources of ionizing
radiation are
known in the art.
Recycled Energy
The external energy needed to sustain the gas reformulation process may also
be reduced
by harnessing any heat generated by the process. The amount of heat generated
by the gas
reformulation process depends on the characteristics of the initial gas and
the
reformulated gas. In one embodiment, the heat released during the
reformulating of
carbon or multi-carbon molecules to mainly CO and H2 is maximized by
optimizing the
amount and type (e.g. air, 02) of process additives injected into the gas
reformulating
system.
The sensible heat present in the gas leaving the reformulating zone may be
captured using
heat exchangers in the gas stabilization zone, and recycled to enhance the
external
efficiency of the reformulation process.
Other energizing sources based on thermal energy or lasers may also be used,
as would be
evident to a worker skilled in the art.
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
Process Manipulators - Gas Manipulators
Gas Manipulators represent embodiments of design strategies seeking to
optimize the
process of gas reformulation. Gas Manipulators are Process Manipulators that
are
particularly relevant to the Gas Reformulation Process. Gas manipulators
comprise
designs of the chamber that optimize the flow pattern of the preformulated gas
relative to
the gas energizing field and particularly the amount of gas that passes
through this field in
a particular amount of time. Another example of a Gas Manipulator is the
system design
wherein the energy-providing source (such as a plasma torch) is oriented in a
manner
relative to the incoming reformulating gas that maximizes mixing between the
incoming
gas and the energetic species in the energy source. Another example is the
location and
positioning of process additive nozzles that are designed to increase
turbulence and
mixing. Another might comprise the arrangement of sequential gas reformulating
zones
versus parallel gas reformulating zones.
The Gas Manipulators comprise structural devices that have been designed and
incorporated into the system to increase the efficiency of the gas
reformulation process.
Examples include, without limitation, structural devices such as baffles and
deflectors that
direct the preformulated gas more effectively towards and through the gas
energizing
field. Other examples include structural devices that increase the turbulence
throughout
the process that increase the mixing of the energizing sources and the
reformulating gas.
The Gas manipulators also include aspects of the system that direct the
physical
orientation of the energizing source to change the dimensions of the
energizing field, e.g.,
plasma plume directing devices, and/or changes to the energy supplied to a
plasma-
generating source, the flow rate of the working gas, etc. are non-limiting
examples of
aspects of the system of the invention that can be modified to effect changes
in the
dimensions of the preformulated gas energizing field.
Catalytic Gas Manipulators increase the efficiencies of the energy
transference and
include catalysts. One example of a Gas Manipulator is the system design
whereby the
preformulated gas passes through plasma generating electric arc(s). Inclusion
of the Gas
Manipulators is intended to optimize the balance of the amount of energy
expended in the
process of providing energy to the preformulated gas with the output that is
sufficient to
81
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
enable the system to reformulate syngas into gas of a designed chemical
composition.
There are different categories of Gas Manipulators.
One category of Gas Manipulators, is referred to as "Source Energy Exposure
Manipulators." The principal design strategy of this aspect of the invention
is to optimize
the exposure of the amount of preformulated gas necessary to support the
reformulation
reactions to the initial source of energy.
Another category of Gas Manipulators is referred to as "Mixing Manipulators."
The
principal design strategy of this aspect of the invention is to optimize the
mixing of the
reactive species to enhance the energy transference throughout the
reformulation process.
Another category of Gas Manipulators is referred to as "Catalytic
Manipulators." The
principal design strategy of this aspect of the invention is to optimize the
catalytic
activities within the system to enhance the overall effectiveness of the
reformulation
process.
Overall effectiveness refers to the thoroughness of the reformulation process
(as
expressed by the Gas Reformulation Ratio) in addition to the overall costs of
achieving
reformulation. For example, the overall effectiveness takes into account the
cost of using
a catalyst that might become "poisoned" during the process and the cost of
replacing it. It
will also take into account the cost of the energy sources.
The Gas Reformulating System of this invention is designed to enhance the
efficiency of
the reformulation process. The various means of accomplishing this are
referred to as
"Gas Manipulators" and they enhance the efficiency, effectiveness and
thoroughness of
the reformulation process. The reformulation process occurs as the
preformulated gas is
passing through the chamber of the system, so residence time is a critical
aspect
determining the efficiency of the process and the thoroughness of the
transformation.
Factors that accelerate the rate and extent of energy transference throughout
the
preformulated gaseous molecules and the mixing of the reformulated species,
optimize
the thoroughness of the transformation prior to the gas exiting the system.
The proximity of the gaseous molecules to the source of energy-providing
activated
species, such as those provided within the plasma, and/or heat, is dependent
upon the
82
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
amount of time the gaseous molecules are exposed to the source. Means provided
within
the system that enhance the process of energy transfer throughout the
preformulated gas
molecules which thereby begin to reformulate, maximizes the number of
molecules that
will be reformulated. In addition, means that increase the amount of mixing of
the
activated species/reactive intermediates such that they reform into new
chemical species,
the composition of which is largely dependent upon the relative concentration
of the
species present in the reformulated gas, also maximize the amounts of designed
molecules
that will be generated.
Gas Manipulators are designed, positioned and operated to enhance the
efficiency of the
reformulation process. In some embodiments, the Gas Manipulators are designed
to
increase the high turbulence within the system. Increasing turbulence
influences the gas
by provide thorough mixing of the gas molecules to be energized and those that
are in the
process of reformulating into new molecules, the chemical composition of which
will be
determined largely by the relative concentrations of the individual chemical
species in a
gas reformulating zone.
Gas Manipulators can be designed to alter the flow dynamics within the Gas
Reformulating System by targeted redirection of the at least one of the gas
energizing
zone, the initial gases, process additives and constituents thereof, resulting
in changes in
their relative spatial distribution and dynamic evolution thereof. The Gas
Manipulators
may also be designed to ensure that a high turbulence environment is created
in targeted
locations to aid the energizing and reformulation processes.
By improving the exposure of the gas energizing field (e.g. plasma plumes)
with the
initial gas and the process additives, improved reaction processes for the
energizing and
reformulation is achieved at the lowest possible temperature.
A worker skilled in the art will readily understand that the gas Manipulators
have to be
designed and positioned based on the location of the gas energizing sources
and inlets for
process additives and on the overall design of the chamber.
Gas Exposure Manipulators
In some embodiments, the Gas Manipulators are designed and configured to
substantially
enhance the exposure of the preformulated gas to the reforming zone. As
mentioned
83
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
earlier, these Gas Manipulators may be separate structural devices attached to
the gas
reformulating chamber(s) or be integral to the gas reformulating chambers.
Chamber designs for exposure manipulation: in one embodiment, the Gas
Manipulators
comprise of chamber designs that optimize the flow pattern of the
preformulated gas
relative to the gas energizing field and particularly the amount of gas that
passes through
this field in a particular amount of time. This can be achieved by appropriate
design of
the internal walls of the chamber resulting in differences in the gas
reformulation channel,
i.e., the gas flow path within a chamber. The gas reformulation channel can be
a variety of
types including but not limited to the following: straight, curved, diverger-
converger and
the labyrinth.
Various embodiments of gas reformulation channels are shown in Figures 57 to
60. A
worker skilled in the art will readily understand that several design
variations are possible
for each of the embodiments of Figures 57 to 60, based on the design of
additional
features of the chambers, such as for example, the ports for air injection.
Design
considerations for gas reformulation channels include but are not limited to
the exposure
to the energy source, cross section area, temperature profile, velocity
profile, gas
residence time, mixing, and pressure drop.
Referring to Figure 47A and in accordance with one embodiment of the
invention, the
chamber is straight and comprises a narrow throat wherein the plasma torch is
located.
The gases passing through the narrow throat is forced to mix with the reactive
ionized
plasma carrier gas (the gas energizing zone), thus promoting reformulation.
The throat is
about the size of the visual portion of the plasma plume, associated with
temperatures
above 2000 C. The carrier gas exists in an ionized phase at such temperatures
and is
therefore much more active. Design criteria such as the size of the channel
(e.g. its cross
section area), velocity and temperature profiles etc., are determined by the
chemical
processes required for enhanced gas reformulation. Any particulate matter
present in the
reformulated gas may entrain and accumulate in the secondary portion of the
chamber due
to the higher velocities at the throat.
The chambers may additionally be designed for ease of separation of the
particulate
matter. Referring to Figure 47B and in accordance with one embodiment of the
invention, the secondary portion of the chamber is located downwards so that
the
84
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
particulate matter may separate at the bottom and be carried away.
Alternatively, the
secondary portion of the chamber may be designed to have a tangential
introduction of the
gas from the primary portion of the chamber so that the resulting swirl flow
may promote
separation of the particulate matter from the gas stream.
The merits of the designs of Figures 47A and 47B may also be achieved with a
simplified
mechanical design by appropriate placement of a structural device internally.
Referring to
Figure 47C and in accordance with one embodiment of the invention, the shape
of
chamber is unchanged throughout its length and the channel is located
substantially in the
middle of the chamber to force the off-gas through. As the chamber diameter is
fixed, the
installation of refractory, and the fabrication and installation of the
chamber is simplified.
The internal structural device may be well insulated and cooled for optimal
performance
using methods known in the art such as extra cooling piping, fan and controls.
The plasma plume generated by a single plasma torch is of a certain finite
length at
several milliseconds time period, after which the ionized gas returns to a non-
plasma gas
state as its temperature drops below about 2000 C. A worker skilled in the art
will
understand that the time after which the ionized gas returns to a non-plasma
gas state
depends on various parameters of the plasma torches including but not limited
to the
enthalpy of the torch, the gas flows, the temperature of the surrounding gas
and the
amperage. In gas reformulation chambers with curved type channels, two or more
plasma
torches may be appropriately located to provide a continuous stream of
reactive ionized
gas for interaction with the incoming off-gas, resulting in enhanced efficacy
of the tar
cracking processes.
A variety of designs are possible for curved channels not limited to the
embodiments of
Figures 48A to 48C. In accordance with one embodiment of the invention, the
secondary
portion of the chamber allows for tangential introduction of gas from the
primary portion
of the chamber so that the resulting swirl flow promotes the separation of the
particulate
matter from the gas stream. A worker skilled in the art will readily
understand that a
multitude of curved channel designs are possible, for example, based on
differences in the
angles of the curves.
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
Referring to Figure 49 and in accordance with one embodiment of the invention,
the
channel is a divergent-convergent type where the shape of the channel allows
for
variations in the local conditions such as velocities, pressure, etc. if
necessary.
Referring to Figure 50 and in accordance with one embodiment of the invention,
the
channel is a labyrinth type. A worker skilled in the art will readily
understand that this
channel design can accommodate for longer residence time, if necessary.
In one embodiment of the invention, the chamber is a straight, substantially
horizontal
cylindrical structure operatively linked to a source of gas (e.g. gasifier)
through a
vertically oriented connector. The walls of the chamber and/or connector may
be
designed to act as a Gas Manipulator i.e., to precisely redirect the
preformulated gas
stream and enhance its interaction with the gas energizing field and
optionally the process
additives.
In one embodiment of the invention, the chamber is constricted at appropriate
locations to
enhance the interaction of the preformulated gases with the gas energizing
field (e.g.
plasma plumes) and/or the process additives. Referring to Figure 52A and in
accordance
with one embodiment of the invention, the constriction 3999 within the chamber
3202 is
placed slightly above the two plasma torches 3208. Referring to Figure 52B and
in
accordance with one embodiment of the invention, the constriction 3999 is more
gradual
and is positioned such that the plasma torches 3208 fall within the
constricted area of the
chamber 3202. A worker skilled in the art will readily understand the impact
of the
different positions of the constriction vis-a-vis the plasma torches.
In one embodiment of the invention, an injector plasma torch with its own
injector stream
as carrier gas is used to generate an ionized field in a chamber comprising
electrodes
driven by multiple-phase AC currents, and filled with the preformulated gas to
be
reformulated. As the preformulated gas passes directly through the chamber,
the
energizing and reformulation processes are enhanced. Various embodiments of
the Gas
Manipulators as described below may still be utilized to ensure that the
plumes of the
injector plasma torch are directed precisely into the gaps of the primary
electrodes.
86
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
Referring to Figures 21A and 21 B, various embodiments of the gas
reformulating system
may be conceived based on the configurations of the energizing sources,
additive streams
and gas inputs and gas outputs.
The gas reformulation system may also be designed for separation of the gas
stream into
smaller streams which undergo parallel reformulation. Referring to Figures 56A
and 56B,
each of the smaller gas streams pass through dedicated reformulation zone
created by
independent energizing sources. Figures 56B shows the use of transferred arc
torches.
Figure 56C shows that the dedicated reformulation zone for each separate gas
stream may
be created by multiple gas energizing sources. Figure 56D shows the embodiment
of
Figures 56A and 56B where mixing elements are introduced in the path of each
of the
smaller gas streams.
Figures 57A-C show three gas reformulating systems where the gas energizing
sources are
positioned at angles in the reformulation chamber. The sources may either
direct its
energizing field towards the flow of gas or against it; or combination
thereof.
The chamber may further include one or more ports for secondary torch heat
sources to
assist in the pre-heating or torch heating of the chamber.
Preformulated Gas Directing Devices
Gas manipulators may enhance the exposure of the preformulated gas with the
gas
energization field by manipulating directly or indirectly, using active or
passive means or
both, the spatial distribution of the preformulated gas within the chamber(s)
and its
dynamic evolution thereof Such Gas Manipulators may be separate structural
devices.
Examples include, without limitation, structural devices such as baffles and
deflectors that
direct the preformulated gas more effectively towards and through the gas
energizing
field. Other examples include the design of the chamber to create certain
desired fluid
dynamic flow paths.
In one embodiment of the invention, Gas Manipulators are also located at or
near the
initial gas inlet to ensure that the initial gas is of more uniform
composition and/or
temperature, and properly mixed with the process additives.
Referring to Figures 58A-C, and in accordance with one embodiment of the
invention, the
87
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
Gas Manipulators comprises of flow restrictors 3999 that alter the flow of the
gases
entering the chamber 3202. A worker skilled in the art will readily understand
that the
differences to the gas flow patterns is dependant on various factors including
but not
limited to the size and shape of the flow restrictors 3999 and their position.
The flow restrictors may be attached to the chamber using various fastening
means. In
one embodiment of the invention, the flow restrictor is suspended from the top
(downstream end) of the chamber. In one embodiment of the invention, the flow
restrictor is attached using brackets to the walls of the chamber.
Referring to Figure 59A and 59B, and in accordance with one embodiment of the
invention, the flow restrictors 3999 extend for substantially the whole length
of the
chamber 3202 resulting in the formation of an annular space where gas
reformulation
occurs. As shown in Figure 74, the flow restrictor 3999 may be rotated using a
motor
7001, an example of the use of active means for the direct manipulation of the
off-gas
streams. The rotation of the flow restrictors may be dynamically controlled,
optionally in
conjunction with a Control System that is designed to regulate and optimize
the overall
gas reformulation process.
Figures 60A and 60B show the three dimensional views of a chamber comprising
flow
restrictors and directly coupled to a laterally oriented gasifier. The flow
restrictors have to
be designed to withstand the high temperatures typically present in a chamber.
Figures 61A-G show different flow restrictors, in accordance with various
embodiments
of the invention. In these figures, the plasma torches are shown to be at the
same
elevation. Alternately, the flow restrictors may be placed above or below the
plasma
torches. Additive ports are also shown below the torches for the injection of
process
additives, such as air and steam.
In one embodiment of the invention, as shown in Figure 61A, the flow
restrictor has two
helical flights that are designed to induce more cyclical flow mixing of the
incoming off-
gas and the plasma plume. Figure 61B shows a flow restrictor with two helical
flights but
with a different shape, in accordance with one embodiment of the invention. In
one
embodiment of the invention, as shown in Figure 61D, one helical flight of the
flow
restrictor is larger than the other and further induces the cyclical flow and
mixing of the
88
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
off-gas with the plasma plumes. In one embodiment of the invention, as shown
in Figure
61 G, the flight spiral only covers half of the restrictor before starting as
two new flights.
In one embodiment of the invention, as shown in Figures 61C-F, the flow
restrictor is
attached to a cooling pipe where a cooling medium (e.g. air, water, thermal
oil) controls
the temperature of the flow restrictor. In one embodiment of the invention as
shown in
Figure 61E, additives (e.g. air, steam etc) flow from the top of the support
rod to the
bottom of the flow restrictor before it enters the off-gas stream. This design
allows for
cooling of the flow restrictor while pre-heating of the additives prior to
their injection.
Referring to Figure 62A and in accordance with one embodiment of the
invention, the
chamber comprises Gas Manipulators in the form of one or more rotational
shafts
attached to a motor, each shaft comprising one or more disks, which may be
carefully
weighted for stable rotation. For embodiments with multiple disks on a shaft,
the disks
may be arranged in an off-set pattern. A worker skilled in the art will
readily understand
that the disks may incorporate cooling. Flow restrictors such as described
above may be
attached to the end of the rotational shaft.
Figures 62B show different types of disks that may be attached to the
rotational shaft.
Referring to Figure 62BA, the disk has a section that allows gas to flow from
one side of
the disk to the other. Referring to Figure 62BB, the disk has a spiral section
that is
designed to pull the gases up and into the middle of the chamber. Alternately,
the spiral
section may be designed to push gases up and out to the edge of the chamber.
Referring
to Figures 62BC and 62BD, the rotational disk is a spoke with multiple blades.
A worker
skilled in the art will readily understand that the orientation and weight
distribution of the
blades should be balanced for stable rotation.
Figures 63A-C show different embodiments of the rotational shaft such as shown
in
Figure 74, wherein the top disk is allowed to rotate on ball bearings and is
held in place
by supports. Optionally, cooling fluids or additives can be piped through the
center of the
shaft. In one embodiment of the invention as shown in Figure 63A, there is a
motor on
top of one or more supports with a drive shaft attached to a wheel (sprocket)
that turns.
The mechanical energy turns the disk, and thus the shaft protruding into the
chamber.
Referring to Figure 63B, electromagnets are used either between the supports
or as part of
89
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
the supports to cause rotation. Referring to Figure 63C and in accordance with
one
embodiment of the invention, electromagnets are used to stabilize the shaft in
the
chamber. The electromagnets can be used either as a primary or a secondary
means for
creating a rotational moment in the shaft and the disks. In one embodiment of
the
invention, the disk rotates independently of the shaft; for example, the shaft
may be
stationary or rotating at another speed or even another direction. In one
embodiment of
the invention, the disk has permanent magnetics and cooling is done on the
disk plane as
it would be mostly hollow with a thermal fluid cooled ball bearing connection
to the shaft.
Energizing Source Directing Devices
Energizing Source Directing Devices are Gas Manipulators that direct the
physical
orientation of the energizing source to change the dimensions of the gas
energizing field,
e.g., plasma plume directing devices, and/or changes to the energy supplied to
a plasma-
generating source, the flow rate of the working gas, etc. are non-limiting
examples of
aspects of the system of the invention that can be modified to effect changes
in the
dimensions of the gas energizing field.
Gas manipulators may also enhance the exposure of the preformulated gas with
the gas
energization field by manipulating directly or indirectly, using active or
passive means or
both, the spatial distribution of the gas energizing field (e.g. plasma
plumes) within the
chamber(s) and its dynamic evolution thereof. In one embodiment of the
invention, this
may be achieved by positioning and orientation of the energizing source (e.g.
plasma
torch).
In one embodiment of the invention, as shown in Figure 65A, the Gas
Manipulator is a
deflector 3998 that redirects the plasma plumes 3997 from a plasma torch 3208.
The
proper redirection of the plasma plumes is dependent on various design factors
of the
deflector 3998 including but not limited to its distance from the plasma torch
3208, its
angle of orientation vis-a-vis the direction of the plasma plume, its size in
comparison to
the width of the plasma plume, and its material of construction. Heat
resistant materials
ensure that the deflector can tolerate the high temperatures present proximal
to the plasma
torch 3208. A worker skilled in the art will readily know the different
materials that can
be used to withstand the high plasma temperatures.
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
Referring to Figure 65B and in accordance with one embodiment of the
invention, the Gas
Manipulator is a Coanda-effect based deflector 3996 used to manipulate the
plasma plume
3997.
Referring to Figures 66A and 66B, and in accordance with one embodiment of the
invention, one or more fluidic jets 3208 (e.g. air nozzles) are used to
redirect the plasma
plumes 3997 generated by the plasma torch(es) 3208. The fluidic jets are an
example of
active means used for the direct manipulation of the plasma plumes. In one
embodiment
of the invention, the fluidic jets are dynamically controlled, optionally in
conjunction with
a Control System that is designed to regulate and optimize the overall gas
reformulation
process.
Figures 67A-D show other embodiments of deflectors that can be used for
redirecting the
plasma plumes within the chamber. In one embodiment of the invention, as shown
in
Figures 67A-B, the deflector is attached to the plasma torch casing. By
adjusting the
shape of the deflector, the spread of the plasma plume dispersion may be
controlled. For
example, the deflector of Figure 67B gives wider plume dispersion than the
deflector of
Figure 67A.
Figures 67C-D show embodiments of the invention, where the deflector is not
attached to
the plasma torch casing. In one embodiment of the invention, as shown in
Figure 67D,
the deflector is attached to the rotating shaft. A worker skilled in the art
will understand
that the finish (e.g. smooth, rough, or angled) of the deflector surface will
affect the plume
dispersion.
Figures 68A-D show different embodiments of the invention where the rotating
shaft
object has an uneven surface. The number of edges, torches and torch angles
can be used
to optimize the plasma plume and/or to evenly spread the plasma plume, thus
maximizing
the plume's contact with the off-gases. In one embodiment of the invention,
the plasma
torches point directly to the center of the chamber.
In one embodiment of the invention as shown in Figure 68A, the plasma torches
are
angled so that at least part of the plasma plumes hits the center object.
Alternatively, the
plasma plumes may be directed away from the central object. In one embodiment
of the
91
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
invention as shown in Figure 36B, the shaft object is rotated in the opposite
angle of the
torch resulting in forcing the plasma plumes toward the outside of the
chamber.
In one embodiment of the invention as shown in Figures 68C-D, the plasma plume
is
bounced off deflectors towards the central shaft. The deflectors may be
mounted on the
plasma torch casing, as shown in Figure 68C, or on the walls of the chamber,
as shown in
Figure 68D. The shafts in Figures 68C-D may be rotated in either direction.
Optionally, ports for mounting plasma torches may be fitted with a sliding
mounting
mechanism to facilitate the insertion and removal of the plasma torch(es) from
the
chamber and may include an automatic gate valve for sealing the port following
retraction
of the plasma torch(es). In one embodiment of the invention, the ports for the
tangentially
mounted plasma torches are located above the air inlets to provide maximum
exposure to
plasma torch heat. Such mounting mechanisms may be modified to allow for
adjustability of the position of the gas energizing sources.
Referring to Figure 70A and in accordance with one embodiment of the
invention, a
plasma torch 3208 is positioned such that the gases injected into the chamber
3202 flows
counter-current to the plasma plumes generated thereby. A worker skilled in
the art
should readily understand the variations in the spatial distribution of the
plasma plumes
when the orientations and positions of the plasma torches are varied.
In one embodiment of the invention, the gas energizing sources (e.g. plasma
torches) are
placed so that the resulting zone (e.g. plasma plumes) is directed
perpendicular to the
direction of the flow of the initial gases. In one embodiment of the
invention, the
chamber is substantially cylindrical and the plasma plumes are directed
radially,
perpendicular to the substantially axial flow of the initial gas stream.
Alternately, the
initial gas stream may be directed radially while the plasma plumes are
directed axially
along the substantially cylindrical gas refinement chamber. In one embodiment
of the
invention, the chamber is substantially cylindrical and the plasma plumes are
directed
tangentially, perpendicular to the substantially axial flow of the initial gas
stream.
Figure 71 shows cross-sectional views of cylindrical gas reformulating
chambers with
various arrangements of the gas energizing sources resulting in associated
changes in the
shapes and dimensions of the resulting gas energizing fields. In one
embodiment of the
92
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
invention, the gas energizing sources used may be either AC or DC plasma
torches.
Figure 71A shows two gas energizing sources directed tangentially into the
chamber.
Referring to Figure 71 B, the chamber comprises three electrodes with an arc
passing
between them. The gas passes through this arc and plasma is formed and the gas
is
reformulated. Figure 71 C shows a similar embodiment as Figure 71 B, except
that there is
a central grounded electrode where the arc from the electrodes on the wall arc
to. A
worker skilled in the art will understand that the ground electrode is
electrically shielded
except at contact point. Figure 71 D shows one exemplary embodiment where the
chamber
comprises a plurality of gas energizing sources (either point directly in the
middle as
shown, or in a swirl pattern) sufficient to ensure that substantially all of
the gas passing
through the chamber is energized. Figures 71E and 71F are similar to the
embodiments of
Figures 71 B and 71 C respectively, but with six torches (3 or 6 phase).
Higher number of
torches can also be similarly considered for the embodiments of Figures 71 B,
71 C, 71 E
and 71 F.
Figure 40 shows two exemplary embodiments of the invention, wherein the
initial gas
and/or preformulated gas stream is introduced into a reformulation chamber
directly in
through the gas energizing field created by a gas energizing source.
Gas manipulators at least partially manipulate the spatial distributions of
the
preformulated gas and the gas energizing field relative to one another, and
their dynamic
evolutions.
Gas Mixing Manipulators
In some embodiments, the Gas Manipulators are designed and configured to
substantially
enhance the mixing of the reformulating gas and the energetic species in the
gas
energizing field. Additionally, the Gas Manipulators may also enhance the
turbulence
throughout the process resulting in improved mixing.
In one embodiment of the invention, the location and positioning of process
additive
nozzles that are designed to increase turbulence and mixing.
In one embodiment, the Gas Manipulators are one or more baffles located in the
chamber
to induce turbulence and thus mixing of the reformulating gas. Different
baffle
arrangements are known in the art and include but are not limited to cross bar
baffles,
93
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
bridge wall baffles, choke ring baffle arrangements and the like. Baffles may
also be
located at or near the initial gas inlet to ensure that the initial gas is of
more uniform
composition and/or temperature, and properly mixed with the process additives.
Referring to Figures 75A-B, turbulence may be created either prior to or after
the gas
energizing sources. Figure 75C shows three exemplary embodiments of means for
creating turbulence: (i) passive grid; (ii) an active grid utilizing a
rotating shaft; and (iii) a
shear generator. Figures 75 and 78 show additional exemplary embodiments of
means for
generating turbulence.
In one embodiment, the Gas Manipulators comprise the design of the positioning
of the
energizing sources, which can contribute to the mixing of the reformulating
gas and the
energetic species in the gas energizing field. The energizing sources may thus
be
positioned to optimize the gas reformulation process; the positioning depends
on various
factors including but not limited to the design of the gas reformulating
chambers
(chamber). In one embodiment of the invention, two plasma torches are
positioned
tangentially to create the same swirl directions as air and/or oxygen inputs
do. In one
embodiment of the invention, two plasma torches are positioned at diametric
locations
along the circumference of the chamber.
The arrangement of the process additive (the chemical composition contribution
of which
is discussed below) inputs is based on a variety of factors including but not
limited to the
design of the chamber, the desired flow, jet velocity, penetration and mixing.
Various
arrangements of the process additive ports and ports for the gas energizing
sources are
contemplated by the invention.
For example, the oxygen inputs or ports, steam inputs or ports and ports for
the gas
energizing sources may be arranged in layers around the circumference of the
chamber,
allowing for the tangential and layered injection of gas energizing zones,
oxygen and
steam. In one embodiment, there is provided nine oxygen source(s) ports
arranged in
three layers around the circumference of the chamber. In one embodiment there
is
provided two steam input ports arranged in two layers around the circumference
of the
chamber and diametrically positioned. In embodiments where the air and/or
oxygen input
ports are arranged in layers, they may be arranged to maximize the mixing
effects.
94
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
In one embodiment of the invention, the air and/or oxygen input ports are
positioned
tangentially, thus allowing the lower level input ports to premix the gas,
torch heat it up,
and start a swirl motion in the gas. The upper level air input ports can
accelerate the swirl
motion thereby allowing a re-circulating vortex pattern to be developed and
persisted.
Referring to Figure 76 and in accordance with one embodiment of the invention,
the gas
to be reformulated enters tangentially into the reformulation chamber
resulting in
formation of swirls. The embodiment also shows an exemplary Gas Manipulator
shaped
and positioned to enhance the exposure of the gas stream with the gas
energizing source.
In one embodiment, the lowest level of air input ports is composed of four
jets which will
premix the gases generated from a lower gasifier and torch heat it up. The
other upper two
levels of air nozzles provide main momentum and oxygen to mix gases and torch
heat up
to the temperature required. The arrangements of steam inputs or ports is
flexible in
number, levels, orientations and angle as long as they are located in a
position to provide
optimized capabilities to temperature control.
The oxygen and/or steam input ports may also be positioned such that they
inject oxygen
and steam into the chamber at an angle to the interior wall of the chamber
which promotes
turbulence or a swirling of the gases. The angle is chosen to achieve enough
jet
penetration based on chamber diameter and designed air input port flow and
velocity.
The angle may vary between about 50 and 70 .
The air input ports maybe arranged so that they are in the same plane, or
arranged in
sequential planes. In one embodiment the air input ports are arranged in lower
and upper
levels. In one embodiment, there are four air input ports at the lower level
and another six
air input ports at upper level in which three input ports are slightly higher
than the other
three to create cross jet mixing effects.
Optionally, air can be blown into the chamber angularly so that the air
creates a rotation or
cyclonic movement of the gases passing through the chamber. The gas energizing
sources
(e.g. plasma torches) may be angled to provide further rotation of the stream.
In one embodiment of the invention, the air and/or oxygen and/or steam inputs
comprise
high temperature resistance atomizing nozzles or jets. Appropriate air nozzles
are known
in the art and can include commercially available types such as the type A
nozzles and
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
type B nozzles illustrated in Figures 79-80. The nozzles may be of a single
type or
different types. The type of nozzles may be chosen based on functional
requirements, for
example a type A nozzle is for changing the direction of air flows for
creating the desired
swirls and a type B nozzle is for creating high velocity of air flow to
achieve certain
penetrations, and maximum mixing.
The nozzles can be designed to direct the air at a desired angle. In one
embodiment, the
air jets are positioned tangentially. In one embodiment, angular blowing is
achieved by
having a deflector at the tip of the input nozzle, thus allowing the inlet
pipes and flanges
to be square with the chamber.
In one embodiment of the invention, one or more air jets (e.g. air swirl jets)
are positioned
at or near the initial gas inlet to inject a small amount of air into the
initial gas and create a
swirling motion in the initial gas stream by taking advantage of the injected
air's velocity.
The number of air swirl jets can be designed to provide substantially maximum
swirl
based on the designed air flow and exit velocity, so that the jet can
penetrate to the center
of the chamber.
Gas Catalytic Manipulators
Catalytic manipulators include catalysts and increase the efficiencies of the
energy
transference. A catalyst increases the rate of a chemical reaction, by
lessening the time
needed to reach equilibrium. A catalyst works by providing an alternate and
easier
pathway from reactants to products by a variety of mechanisms, but in each
case by
lowering the activation energy of the reaction. Homogeneous catalysts are
present in the
same phase as the reactants and function by combining with the reacting
molecules or
ions to form unstable intermediates. These intermediates combine with other
reactants to
give the desired product and to regenerate the catalyst. Heterogeneous
catalysts are
present in a phase different from that of the reactants and products. They are
usually
solids in the presence of gaseous or liquid reactants. Reactions occur at the
surface of
heterogeneous catalysts. For this reason catalysts are usually finely divided
solids or have
particle shapes that provide a high surface-to-volume ratio. The cracking of
petroleum
and the reforming of hydrocarbons are common industry applications of the use
of
heterogeneous catalysts. One difficulty in the use of heterogeneous catalysts
is that most
of them are readily "poisoned" wherein impurities in the reactants coat the
catalyst with
96
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
un-reactive material or modify its surface, so that the catalytic activity is
lost. Frequently,
but not always, the poisoned catalyst can be purified and used again.
The use of appropriate catalysts in the gas reformulating system may reduce
the energy
levels required for the gas reformulation process, by providing alternate
reaction
pathways. The precise pathway offered by a catalyst will depend on the
catalyst used.
The feasibility of the use of catalysts in gas reformulation systems, in
general, depends on
their lifetimes. Lifetimes of catalysts may be shortened by `poisoning', i.e.,
the
degradation in their catalytic capabilities due to impurities in the gas.
The gas reformulating system may be designed to allow for easy replacement of
the
catalysts. In one embodiment of the invention, catalysts are incorporated into
the gas
reformulating chambers in the form of a bed mounted on a sliding mechanism.
The
sliding mechanism allows for easy removal and replacement of the catalyst bed.
The bed
may be inserted at various locations in the gas reformulating system.
In one embodiment of the invention, off-gas from a gasification chamber which
is at a
high temperature contacts a catalyst which effectively lowers the energy
threshold
required for gas reformulation, such that the off-gas stream undergoes
reformulation prior
to exposure to a gas energizing field. In one embodiment of the invention,
therefore, the
gas reformulation system comprises a catalyst at a location upstream of the
gas energizing
source(s). In one embodiment, as disclosed in Figure 89 catalytic beds are
inserted before
and/or after the gas energizing sources (e.g. plasma torches).
The catalytic capability will also depend on the temperature of operation. The
appropriate
operating temperature ranges for various catalysts are known in the art. The
gas
reformulating system may incorporate adequate cooling mechanisms to ensure
that the
catalysts are maintained within their optimal operating temperature ranges.
Additives
such as steam, water, air, oxygen or recirculated reformulated gas may be
added to help
increase or decrease the temperature near the catalyst beds. A worker skilled
in the art
will understand that the specific additive chosen to control the temperature
will depend on
the position of the catalyst bed and the gas temperatures thereat.
97
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
The irregularity of the catalyst surface and good contact between the large
organic
molecules and the surface will increase the opportunity for reformulation into
smaller
molecules, such as H2 and CO.
Catalysts that may be used include but are not limited to olivine, calcined
olivine,
dolomite, nickel oxide, zinc oxide and char. The presence of oxides of iron
and
magnesium in olivine gives it the ability to reformulate longer hydrocarbon
molecules. A
worker skilled in the art will understand to choose catalysts that do not
degrade quickly in
the gas environment of the system.
Both nonmetallic and metallic catalysts may be used for enhancing the
reformulation
process. Dolomites in calcined form are the most widely used nonmetallic
catalysts for
reformulation of gases from biomass gasification processes. They are
relatively
inexpensive and are considered disposable. Catalytic efficiency is high when
dolomites
are operated with steam. Also, the optimal temperature range is between about
800 C and
about 900 C. The catalytic activity and the physical properties of dolomite
degrade at
higher temperatures.
Dolomite is a calcium magnesium ore with the general chemical formula
CaMg(C03)2
that contains -20% MgO, -30% CaO, and -45% CO2 on a weight basis, with other
minor
mineral impurities. Calcination of dolomite involves decomposition of the
carbonate
mineral, eliminating CO2 to form MgO-CaO. Complete dolomite calcination occurs
at
fairly high temperatures and is usually performed at 800 C-900 C. The
calcination
temperature of dolomite, therefore, restricts the effective use of this
catalyst to these
relatively high temperatures.
Olivine, another naturally occurring mineral has also demonstrated catalytic
activity
similar to that of calcined dolomite. Olivine is typically more robust than
calcined
dolomite.
Other catalytic materials that may be used include but are not limited to
carbonate rocks,
dolomitic limestone and silicon carbide (SiC).
Char can act as a catalyst at lower temperatures. In one embodiment of the
invention, the
gas reformulation system is operatively linked to a gasifier, and at least
part of the char
98
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
created within the gasifier is moved to the gas reformulating system for use
as a catalyst.
For embodiments utilizing char as catalyst, the catalyst bed is typically
placed before the
energizing zone such as provided by plasma torches.
Figure 81 shows a fixed bed of char used as a catalyst in the reformulation
chamber. The
char used for catalysis may be obtained from a gasifier as shown in Figure 82.
This may
be particularly applicable when the gas reformulating chamber is operatively
linked to a
gasifier and used to reformulate the gases generated therefrom. The char may
be moved
to a residue conditioning chamber or a carbon converter once it loses its
catalytic
properties.
Figure 83 shows one exemplary configuration of a gasifier operatively linked
to a plasma
torch-based gas reformulating chamber wherein the char created in the gasifier
aids in
catalytic cracking of the off-gases created by gasification. The catalytic
cracking achieved
in the latter stage of the gasifier is followed by further gas reformulation
due to the
exposure of the gas with the gas energizing field created by the plasma torch.
Various
types of gasifiers as would be readily known to worker skilled in the art such
as fluidized
bed gasifiers and entrained flow gasifiers may also be utilized.
In one embodiment of the invention, the initial gas is heated to a temperature
of 900-
950 C and passed over a nickel-based catalyst whereby tar components and light
hydrocarbons including methane are converted into CO and H2. Nickel-based
catalysts
may be particularly useful when the initial gas contains minimal amounts of
sulphur
species (such as hydrogen sulphide), such as for example, gas produced by
gasification of
biomass. Life-times of nickel-based catalysts may be enhanced by the use of
promoters
such as rare metals.
In one embodiment of the invention as shown in Figure 84, a catalytic bed is
installed
right after the gasifier and transforms the majority of the volatiles. The
inlet temperature
of the catalytic bed may be raised from 600 to 950 C by combusting a small
fraction of
the volatiles. The outlet temperature of the catalytic bed is expected to drop
to 850 C and
the outlet gas is fed into the gas energizing field for further reformulation.
The gas
energizing zone may be operated at 1000 C for this purpose and the resulting
syngas is
sent to the recuperator to start the subsequent gas cleanup process.
99
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
In one embodiment of the invention as shown in Figure 85, the volatiles from
the gasifier
passes through the gas energizing zone wherein the temperature is between
about 900 C
and about 1000 C. The catalytic bed is used for further reformulation. The
temperature of
the syngas is expected to drop to 850 C at the exit of the catalytic bed. It
is then sent to a
heat exchanger or recuperator which forms a part of the gas stabilization
zone.
In one embodiment of the invention as shown in Figure 86, heat recovery is
achieved
before the catalytic bed. The majority of the volatiles from the gasifier are
reformulated in
the gas energizing zone at temperatures of about 1000 C. The hot output gas
passes
through a heat exchanger (or a recuperator) to preheat process air whereupon
its
temperature drops to around 700 C. The cooled syngas is then heated to 900 C
by
combusting a small fraction of it and fed into the catalytic bed. The
resulting syngas at
850 C is sent optionally for further gas cleanup.
For embodiments where the catalytic bed is placed prior to energizing field,
the gas
temperature is typically appropriate for high catalytic activity. However for
embodiments
where the catalytic bed is after the energizing field, such as produced by
plasma torches,
the gas temperature might be too high for most typical catalysts such as
olivine, dolomite,
and many others. The gas temperatures may be reduced to appropriate levels (to
avoid the
degradation of the catalyst beds) by the circulation of cooling fluids, as
shown in Figure
87. Appropriate cooling fluids may include but are not limited to recirculated
reformulated gas (as shown in the embodiment of Figure 88), water and steam.
For embodiments where the catalyst bed is after the recuperator (heat
exchanger) the
recirculated stream of reformulated gas may be inserted either prior or after
the
recuperator.
In one embodiment of the invention, the reforming zone comprises a catalyst
bed and the
catalytic manipulators are also designed to enhance the exposure of the
preformulated
and/or reformulating gas to the catalyst bed.
A Gas Stabilizing Zone
This system provides one or more stabilizing zones whereby the newly formed
molecules
are de-energized (e.g. cooled or removed from the influence of catalysts or
energizing
100
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
sources) to ensure they maintain the desired characteristics e.g. the designed
chemical
composition.
The temperature of the gas entering the stabilizing zone will range from about
400 C to
over 1000 C. The temperature may optionally be reduced by a heat exchange
system in
the stabilizing zone of the gas reformulating system, which recovers heat
from, and thus
cools, the reformulated gas. Such a reduction in the gas temperature may be
necessitated
by downstream applications and components.
Referring to Figure 54B, the gas reformulating chamber 3002 the stabilization
zone may
be specifically shaped to facilitate the de-energization and stabilization of
the newly
formed molecules. The gas reformulating chamber 3002 is a generally
cylindrically
shaped chamber having a bulbous expansion downstream of the plasma or
optionally
proximal to the one or more reformulated gas outlets 3006. The bulbous
expansion by
allowing for the de-energization of the gas and thereby stabilized the newly
formed
molecules.
Optional Heat Rec. Means
Heat may be recovered in the stabilization zone or downstream from the
stabilizing zone.
The recovered heat may be used for various purposes, including but not limited
to the
following: heating the process additives (e.g. air, steam) for the gas
reformulation process;
generating electricity in combined cycle systems. The recovered electricity
can be used to
drive the gas reformulation process, thereby alleviating the expense of local
electricity
consumption. The amount of heat captured depends on a variety of factors
including but
not limited to the characteristics (e.g. chemical composition, flow rates) of
the initial gas
and reformulated gas.
In one embodiment of the invention, the heat recovered from the stabilizing
zone of the
gas reformulating system is supplied to a Gasification System operated in
conjunction
with the gas reformulating system. The heat exchanger may be operated in
conjunction
with a Control System optionally configured to minimize energy consumption and
maximize energy production/recovery, for enhanced efficiency.
101
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
In one embodiment of the invention, a gas-to-fluid heat exchanger is used in
the
stabilizing zone to transfer the heat from the reformulated gas to a fluid
resulting in a
heated fluid and a cooled gas. The heat exchanger comprises means (e.g.
conduit
systems) for transfer of the reformulated gas and fluid to and from the heat
exchanger.
Suitable fluids include but are not limited to air, water, oil, or another gas
such as nitrogen
or carbon dioxide.
The conduit systems may optionally employ one or more regulators (e.g.
blowers)
appropriately located to manage the flow rates of the reformulated gas and the
fluid.
These conduit systems may be designed to minimize heat losses to enhance the
amount of
sensible heat that is recoverable from the reformulated gas. Heat loss may be
minimized,
for example, through the use of insulating barriers around the conduits,
comprising
insulating materials as are known in the art and/or by reducing the surface
area of the
conduits.
In one embodiment of the invention, the gas-to-fluid heat exchanger is a gas-
to-air heat
exchanger, wherein the heat is transferred from the reformulated gas to air to
produce a
heated exchange air. In one embodiment of the invention, the gas-to-fluid heat
exchanger
is a heat recovery steam generator, wherein the heat is transferred to water
to produce
heated water or steam.
Different classes of heat exchangers may be used including shell and tube heat
exchangers, both of straight, single-pass design and of U-tube, multiple pass
design, as
well as plate-type heat exchangers. The selection of appropriate heat
exchangers is within
the knowledge of a worker of ordinary skill in the art.
As particulate matter may be present in the gas, the gas-to-air heat exchanger
is typically
designed for a high level of particulate loading. The particle size may vary
typically from
about 0.5 to about 100 microns. In one embodiment depicted in Figure 90, the
heat
exchanger is a single pass vertical flow heat exchanger 5104B, wherein the
reformulated
gas 5020 flows in the tube side and the air 5010 flows on the shell side. The
reformulated
gas 5020 flows vertically in a "once through" design, which minimizes areas
where build
up or erosion from particulate matter could occur. The reformulated gas
velocities should
102
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
be maintained to be high enough for self-cleaning, while still minimizing
erosion, and
may vary from about 3000 to about 5000 mm/sec.
Due to the significant difference in the air input temperature and hot product
gas, each
tube in the gas-to-air heat exchanger preferably has individual expansion
bellows to avoid
tube rupture. Tube rupture may occur where a single tube becomes plugged and
therefore
no longer expands/contracts with the rest of the tube bundle. In those
embodiments where
the air pressure is greater than the reformulated gas pressure, tube rupture
presents a high
hazard due to problems resulting from air entering gas mixture.
After heat is recovered in the gas-to-fluid heat exchanger, the cooled
reformulated gas
may still contain too much heat for the systems further downstream. Selection
of an
appropriate system for further cooling of the product gas prior to
conditioning is within
the knowledge of a worker skilled in the art.
In one embodiment, as depicted in Figure 91, the hot reformulated gas 5020
passes
through the gas-to-air heat exchanger 5103 to produce a partially cooled
reformulated gas
5023 and heated exchange-air 5015. The air input to the heat exchanger may be
supplied
by a process air blower. The partially cooled reformulated gas 5023 undergoes
a dry
quench step 6103, where the addition of a controlled amount of atomized water
6030
results in further cooled product gas 5025.
The cooling of the reformulated gas may also be achieved using a wet, dry or
hybrid
cooling system. The wet and dry cooling systems may be direct or indirect.
Appropriate
cooling systems are known in the art and as such a worker skilled in the art
in view of the
requirements of the system would be able to select an appropriate system.
In one embodiment, the cooling system is a wet cooling system. The wet cooling
system
can be direct or indirect. In cooling systems that utilize indirect wet
cooling, a circulating
cooling water system is provided which absorbs the heat from the reformulated
gas. The
heat is expelled to the atmosphere by evaporation through one or more cooling
towers.
Alternatively, to facilitate water conservation, the water vapor is condensed
and returned
to the system in closed loop.
In one embodiment, the cooling system is a dry cooling system. The dry cooling
system
can be direct or indirect. In one embodiment, the dry cooling system is a
draft dry cooling
103
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
system. Although, dry cooling will add modestly to the cost of the facility,
it may be
preferred in areas with a limited water supply.
In one embodiment, the syngas cooler is a radiant gas cooler. Various radiant
gas coolers
are known in the art and include those disclosed in US Patent Application No.
20070119577, and US Patent No. 5,233,943.
The reformulated gas may also be cooled down by direct water evaporation in an
evaporated such as quencher.
The exit temperature of the reformulated gas may also be reduced by re-
circulating,
through appropriately located inlets, cooled reformulated gas to the
stabilizing zone of the
gas reformulating system for mixing with newly produced reformulated gas.
Optional Gas Additive Zones
The chamber may optionally comprise one or more process additive ports for
injection of
process additives, such as oxygen sources, carbon dioxide, other hydrocarbons
or
additional gases, into the chamber. Oxygen sources known in the art include
but are not
limited to oxygen, oxygen-enriched air, air, oxidizing medium, steam and other
oxygen
sources as would be readily understood by a worker skilled in the art. In one
embodiment, the chamber comprises one or more port(s) for air and/or oxygen
inputs and
optionally one or more ports for steam inputs.
The optional addition of process additives such as air, steam and other gases,
may also be
achieved without inlets dedicated to their injection. In one embodiment of the
invention,
the process additives may be added into the source of gas or conduits
wherefrom the Gas
Reformulating System obtains its initial gas stream. Process additives may
also be added
to the chamber through the gas energizing sources, such as plasma torches.
Optionally, ports or inlets may be provided so that reformulated gas not
meeting quality
standards may be re-circulated into the chamber for further processing. Such
ports or
inlets may be located at various angles and/or locations to promote turbulent
mixing of
the materials within the chamber.
One or more ports can be included to allow measurements of process
temperatures,
104
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
pressures, gas composition and other conditions of interest.
Optionally, plugs, covers, valves and/or gates are provided to seal one or
more of the ports
or inlets in the chamber 3002. Appropriate plugs, covers, valves and/or gates
are known
in the art and can include those that are manually operated or automatic. The
ports may
further include appropriate seals such as sealing glands.
Optional Gas Cleaning ones
The system optionally comprises one or more gas cleaning zones, located
downstream of
the gas stabilizing zone. Embodiments of the invention comprising one or more
gas
cleaning zones incorporate means of injecting substances into the chamber that
clean the
gas, prior to its exit from the system. For example, oxygen and/or steam can
be atomized
by high temperature resistance atomizing nozzles and injected into the chamber
to clean
the stabilized, reformulated gas.
Optional Further Processing
The stabilized reformulated gas stream may undergo further processing before
being
utilized in a downstream application, stored or flared off. For example, the
reformulated
gas may be passed through a gas conditioning system where particulate matter,
acid gases
(HCI, H2S) and/or heavy metals may be removed, and the temperature and/or
humidity of
the gas may be adjusted. For example, dust particles, if present, may be
removed from the
gas using a venture scrubber, including an electro-filter or fabric baghouse
filter.
The reformulated gas may also be passed through a homogenization chamber, the
residence time and shape of which is designed to encourage mixing of the
reformulated
gas to attenuate fluctuations in the characteristics thereof.
Gas Reformulating Chambers
Referring to Figure 35 and in accordance with one embodiment of the invention,
the
chamber 3002 of the Gas Reformulating System 3000 comprises one or more
initial gas
inlets 3004, one or more reformulated gas outlet(s) 3006, one or more gas
energizing
sources (e.g. plasma sources) 3008, and optionally one or more process
additive (e.g.
105
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
oxygen) inputs 3010, Gas Manipulators (not shown in the figure), and a Control
System.
In one embodiment as shown in Figure 36, the Gas Reformulating System 3000 is
designed so that the chamber 3002 is coupled directly to a source of gas (e.g.
gasifier, gas
storage tank) and in gaseous communication therewith. To facilitate
maintenance or
repair, the Gas Reformulating System 3000 may optionally be reversibly coupled
to the
gasifier such that the Gas Reformulating System 3000, if necessary, may be
removed.
In one embodiment as demonstrated by Figure 37, the Gas Reformulating System
3000 is
a stand-alone unit which receives initial gas from two sources of gas via
separate piping
or conduits. In one embodiment as shown in Figure 38, the individual gas
streams are
combined before they are injected into the Gas Reformulating System 3000. In
stand-
alone units, the Gas Reformulating System may further comprise appropriate
support
structures.
An induction blower may be provided downstream of the chamber and in gaseous
communication therewith to maintain the pressure of the chamber at a desired
pressure,
for example a pressure of about 0 to -5 mbar.
The efficacy of the gas reformulation processes occurring within a chamber
depends on
various factors including but not limited to the chamber internal volume and
geometry,
gas flow rate, the distance the gas travels and/or the path of the gas through
the chamber
(i.e., a straight linear passage or a swirling, cyclonic, helical or other non-
linear path).
The chamber must therefore be shaped and sized to obtain the desired flow
dynamics of
the gas therein. For example, air jets can be used to promote a swirling flow
of the gas
through the chamber, such that the passage of the gas is non-linear. Flow
modeling of the
overall Gas Reformulating System can be used to ensure that a particular
chamber design
promotes the conditions (e.g. proper interaction of the process inputs)
required for the
desired gas reformulation.
The one or more chambers of the Gas Reformulating System may be designed in a
variety
of shapes and be disposed in a variety of positions, as would be readily known
to a worker
skilled in the art. The chamber can be oriented substantially vertically,
substantially
horizontally or angularly.
In one embodiment of the invention, the chamber is a straight tubular or
venturi shaped
106
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
structure comprising a first (upstream) end and a second (downstream) end and
is oriented
in a substantially vertical position or a substantially horizontal position.
In one
embodiment of the invention, the chamber is a straight cylinder with a length-
to-diameter
ratio ranging between about 2 to about 6, with associated effects on
achievable gas
velocities. In one embodiment, the length-to-diameter ratio of the chamber is
3:1.
In one embodiment as depicted in Figure 92A, the chamber 3202 is configured
for direct
coupling to a gasifier, and is a straight, substantially vertical, refractory-
lined, capped,
cylindrical structure with an open bottom (upstream) end 3204 and one
reformulated gas
outlet 3206 proximal to or at the top (downstream) end of the chamber. The top
(downstream) end of the chamber may be capped with a refractory-lined lid
3203, which
may be removably sealed to the chamber in order to facilitate maintenance or
repair.
The wall of the chamber may be lined with refractory material or otherwise
fabricated to
withstand high temperatures. The chamber may be encapsulated with a water
jacket for
cooling and/or generation of steam or recovery of usable torch heat. The
chamber may
have multiple walls, along with a cooling mechanism for heat recovery, and the
gas
reformulating system may also include heat exchangers for high pressure/high
temperature steam production, or other heat recovery capability.
Conventional refractory materials that are suitable for use in a high
temperature, un-
pressurized chamber are well-known to those skilled in the art and include,
but are not
limited to, high temperature fired ceramics, i.e., aluminum oxide, aluminum
nitride,
aluminum silicate boron nitride, zirconium phosphate, glass ceramics and high
alumina
brick containing principally, silica, alumina, chromia and titania, ceramic
blanket and
insulating firebrick. Materials such as Didier Didoflo 89CR and Radex
Compacflo V253
may be used where a more robust refractory material is required.
In one embodiment, the refractory design has multiple layers with a high
density layer on
the inside to resist the high temperature, erosion and corrosion present
within the chamber
and to provide a heat sink to reduce fluctuations in the gas properties.
Outside the high
density material is a lower density material with lower erosion resistance
properties but
higher insulation factor. Optionally, outside this layer is a very low density
foam board
material with very high insulation factor that can be used because it will not
be exposed to
107
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
a corrosive environment which can exist within the chamber. The multilayer
design can
further optionally comprise an outside layer, between the foam board and the
vessel shell
that is a ceramic blanket material to provide a compliant layer to allow for
differential
expansion between the solid refractory and the vessel shell. Appropriate
materials for use
in a multilayer refractory are well known in the art.
In one embodiment, the multilayer refractory can further comprise segments of
compressible refractory separating sections of a non-compressible refractory
to allow for
expansion of the refractory. The compressible layer can optionally be
protected from
erosion by overlapping extendible high density refractory. In one embodiment,
the
multilayer refractory can comprise an internally oriented chromia layer; a
middle alumina
layer and an outer insulboard layer.
In some embodiments of the invention, the chamber includes a layer of up to
about
seventeen inches, or more, of specially selected refractory lining throughout
the entire
chamber to ensure maximum retention of processing heat while being impervious
to
chemical reaction from the reactive intermediates formed during processing.
The refractory lining in the bottom section of the chamber can be more prone
to wear and
deterioration since it must withstand higher temperatures from the operating
sources of
plasma torch heat. In one embodiment, therefore, the refractory in the lower
section is
designed to comprise a more durable "hot face" refractory than the refractory
on the
chamber walls and top. For example, the refractory on the walls and top can be
made of
DIDIER RK30 brick, and the different "hot face" refractory for the lower
section can be
made with RADEX COMPAC-FLO V253.
In embodiments in which the chamber is refractory-lined, the wall of the
chamber can
optionally incorporate supports for the refractory lining or refractory
anchors.
The chamber may have a collector for solid particulate matter. For embodiments
where
the chamber is operated in conjunction with a gasifier, any matter that is
collected may be
fed into a gasifier for further processing or into a solid residue
conditioning chamber, for
further processing. Collectors for solid particulate matter known in the art
include but are
not limited to centrifugal separators, inertial impingement baffles and
filters. For
embodiments where the Gas Reformulating System is directly coupled to the
gasifier,
108
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
additional solid particulate collectors may not be necessary as particulates
formed may, in
part, fall directly back into the gasifier.
Ports, Inlets and Outlets for the Chamber
The chamber comprises one or more initial gas inlets that feed the initial gas
into the
chamber for reformulation, and one or more reformulated gas outlets to pass
the
reformulated gas further downstream. The inlet may comprise an opening or,
alternatively, may comprise a device to control the flow of initial gas into
the chamber
and/or a device to inject the initial gas into the chamber. The device may
include Gas
Manipulators for appropriate injection of the initial gas for enhanced
reformulation,
and/or include sensing elements for measuring the various characteristics of
the initial
gas.
The initial gas inlets may be incorporated to promote concurrent,
countercurrent, radial,
tangential, or other feed flow directions. In one embodiment, the single
initial gas inlet
has an increasingly conical shape.
The initial gas inlets may be located at or near the first or upstream end of
the chamber.
In one embodiment, the inlet comprises the open first end of the chamber,
whereby it is in
direct gaseous communication with the gas source e.g. gasifier. In one
embodiment, the
inlet comprises an opening located in the closed first (upstream) end of the
chamber. In
one embodiment, the inlet comprises one or more openings in the wall of the
chamber
proximal to the first (upstream) end.
In embodiments in which the gasifier and Gas Reformulating System are directly
coupled,
the attachment site on the gasifier for coupling to the Gas Reformulating
System may be
strategically located to optimize gas flow and/or maximize mixing of the
initial gas prior
to entering the chamber. In one embodiment, the chamber is located at the
center of the
gasifier.
In embodiments in which the chamber is connected to one or more gasifiers, one
or more
initial gas inlets of the chamber may be in direct communication with the one
or more
gasifiers through a common opening or as shown in Figure 37, may be connected
to the
gasifier via piping 3009 or via appropriate conduits.
109
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
The reformulated gas produced in the reformulating reaction exits the chamber
through
one or more reformulated gas outlets located at or near the second or
downstream end.
The outlet may comprise an opening or, alternatively, may comprise a device to
control
the flow of the reformulated gas out of the chamber. The device may include
sensing
elements for measuring the various characteristics of the reformulated gas.
In one embodiment, the outlet comprises the open second (downstream) end of
the
chamber. In one embodiment, the outlet comprises one or more openings located
in the
closed second (downstream) end of the chamber. In one embodiment, the outlet
comprises
one or more openings in the wall of the chamber near the second (downstream)
end.
The chamber optionally comprises various ports including one or more process
additive
ports, one or more ports for gas energizing sources, optionally one or more
access ports,
view ports and/or instrumentation ports. Gas energizing sources include but
are not
limited to plasma-based sources (e.g. plasma torches), hydrogen burners and
optional
secondary sources. Ports, inlets and outlets may be incorporated at various
angles and/or
locations to enhance interaction of the reactant flows within the chamber.
Optional Systems for Inclusion in the Gasification and Gas Reformulation
Systems
The Heat Recovery Systems
In one embodiment, heat recovery systems facilitate the efficient recovery of
sensible heat
from the hot syngas product to heat air for use in the gasification process.
In one
embodiment, heat recovery systems facilitate the generation of steam that can
be used to
drive downstream processing of unconventional oil sources.
Figure 111 is a schematic diagram depicting the recovery of heat from the
syngas
produced in the gas reformulating chamber using the heat recovery subsystem of
the
instant invention. In this embodiment, the heat recovery system is a syngas-to-
air heat
exchanger, wherein the heat from the syngas produced in the plasma gas
reformulation
chamber is used to heat ambient air, thereby providing heated air and cooled
syngas. This
110
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
heated air can be passed into the volatilization and/or secondary chambers and
thus used
to drive the gasification process. The cooled syngas is ready for subsequent
gas
conditioning steps and sensible heat is recovered and transferred as heated
air to various
stages in the gasification process.
Different classes of heat exchangers may be used in the present system,
including shell
and tube heat exchangers, both of straight, single-pass design and of U-tube,
multiple pass
design, as well as plate-type heat exchangers. The selection of appropriate
heat
exchangers is within the knowledge of the skilled worker.
The heat recovery subsystem employs a conduit system through which the syngas
is
transported to the heat exchanging means for recovery of the syngas sensible
heat. The
conduit system will optionally employ one or more regulators and/or blowers,
located
throughout the system to provide a means for managing the flow rate of the
syngas
product.
In one embodiment, the heat recovery system employs a conduit system for
transferring
the heated air to the primary chamber and/or the secondary chamber, where it
is
introduced to the respective chambers via air inlets. In one embodiment, the
system
comprises means for controlling the relative amounts of heated air that is
distributed to
the primary chamber and the secondary chamber, to ensure that sufficient
heated air is
provided to carry out the volatilization and processed feedstock/char
conversion stages,
respectively. Accordingly, the air conduit system optionally employs one or
more
regulators, flow meters and/or blowers, located as required throughout the
system to
provide a means for controlling the flow rate and/or distribution of the
heated air. The
heated air conduits also optionally comprise means for diverting the heated
air, for
example, to venting outlets or to optional additional heat exchange systems.
The heat recovery system optionally recovers further sensible heat from the
hot syngas
using a heat exchanging means to transfer the heat from the syngas to water,
thereby
producing steam and yet further cooled syngas. The further sensible heat is
recovered
from the syngas through a second heat exchange means, for example, a heat
recovery
steam generator or waste heat boiler, which uses the recovered heat to
generate steam.
The steam can be used as a process steam additive during the gasification
process to
ensure sufficient free oxygen and hydrogen to maximize the conversion of the
feedstock
111
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
into the syngas product. The steam produced may also be used to drive rotating
process
equipment, for example, the air blowers, as well as syngas blowers.
In one embodiment, the heat recovery system comprises a heat recovery steam
generator
(HRSG) located downstream from the syngas-to-air heat exchanger. In such an
embodiment, the HRSG is a shell and tube heat exchanger designed such that the
syngas
flows vertically through the tubes and water is boiled on the shell side.
Gas Quality Conditioning System (GQCS)
In one embodiment, the facility comprises a first and a second GQCS. In this
embodiment, particulate matter removed from the syngas in the first GQCS is
processed
in a melting chamber and converted to slag and a secondary syngas. The
secondary gas is
then directed back to the first GQCS to undergo further processing and removal
of
contaminants.
It is contemplated that in some circumstances it may be necessary to process
the syngas
through a gas conditioning system (GCS) to remove selected impurities prior to
input of
the syngas into an energy producing component as the syngas produced by the
Gasification System can contain particulate matter, heavy metals, and acid
gases (HCI,
H2S), all of which are hazardous to the workplace and the environment in high
enough
concentrations and are typically subject to emission control requirements. The
composition of the synthesis gas and the type of contaminants present is
determined in
part by the composition of the feedstock undergoing gasification. For example,
if high
sulfur coal is the primary feedstock, the syngas product will contain high
amounts of
sulfur that must be removed prior to use of the syngas product in downstream
applications. Thus, after the syngas is cooled in the HRSG, it enters a GQCS
to remove
undesired materials.
The GQCS can include acid gas removal components, heavy metal removal
components,
and/or particle removal units. The GQCS may optionally include humidity
control units
or temperature control units to modulate the humidity or temperature of the
gas as it
passes through the GQCS. One or more of these units may be incorporated into
the
GQCS in order to carry out processing steps to produce a "processed" syngas of
desired
characteristics. The presence and sequence of processing steps required is
determined by
112
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
the composition of the synthesis gas and the contaminants present therein.
Suitable units
as listed above for conditioning gas are known in the art and it is within the
capabilities of
one of skill in the art to determine which of these units are required in
order to produce a
syngas of the desired characteristics. For example, the design of the GQCS
that is
incorporated as part of the overall system can be varied depending on the end
products
required. Alternatively, the sizing of the GQCS is varied based on the
feedstock used in
the converter, the converter design, syngas requirements, for example.
In one embodiment, the syngas is processed through a GQCS prior to transfer to
an energy
or reagent producing component. In one embodiment, the GQCS is designed to
remove
particulate matter or ash, heavy metals and sulphur from the syngas. In one
embodiment,
the design of the GQCS varies depending on whether the syngas produced is to
be used in
the generation of H2, steam, chemicals, or electricity.
Once the synthesis gas is cleaned and conditioned, the output gas is then
optionally stored
or directed to the required downstream application.
Other Optional Systems
The Gasification System can include additional components as necessary in
order to
further process the syngas. These additional components may be included at
different
stages of the Gasification System. For example, the additional components may
be
included at stages prior to entry of the gas into the GQCS, or they may be
included at
stages after the syngas is processed through the GQCS.
For example, in one embodiment where the Gasification System uses bitumen as a
feedstock, a cooler and tar separator can be included in the facility. There
are many
possible syngas cooling technologies and tar removal technologies, however
these designs
usually include cooling of the gas on the surface of ducting to induce tars to
condense out
of the gas stream. Wall designs, fins, & flanges are usually designed to trap
the tars
against the wall where they can pour down into a catch; water-cooled jacketed
cyclones
are a good example of this process. It is important to design the system so
that particulate
matter if separated from the syngas at the same time as the tars is separated
from the tars.
This can usually be done with a strainer if the particles are large enough and
the tars will
flow though the strainer, otherwise a more aggressive separation technology
may need to
113
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
be used (distillation, evaporation etc.). The cooler and tar separator can be
included after
gas exits the gasifier and can function to remove tars from the syngas which
can then be
converted to light oil used as diluents in the oil industry.
In another embodiment where the Gasification System uses bitumen as a
feedstock, a
second gas reformulation zone can be included in the facility in order to
increase the
suitability of the syngas for use in an energy producing component such as a
chemical
plant for producing flue gases and light oils.
In one embodiment, the facility includes a distillation column that can be
used to separate
heavy and light fractions from bitumen and tars. The light fractions can be
used as
diluents for bitumen transportation or other purposes. The heavy fractions can
be then
sent to the gasifier for use as a feedstock and any solids produced can be
melted in the
melting chamber.
In another embodiment, the syngas that is produced in the facility or
processed through
the GQCS can optionally be stored in a gas storage tank or syngas regulation
system prior
to it use as a fuel in an energy producing component. The gas storage tank or
syngas
regulation system may be a standard fuel or surge tank, or may include
regulation by way
of a gas homogenization system, which includes a homogenization chamber. In
the case
of the latter, the chemical composition and other characteristics of the gas,
such as flow
rate, pressure, and temperature of the gas, may be adjusted to create a
regulated gas that
satisfies the requirements of a downstream application (i.e. an IGCC system,
or a gas
turbine). Different types of homogenization chambers for use with the gas
homogenization system include, but are not limited to gasometers, gas holders,
variable
volume and fixed volume tanks. The gas homogenization chamber receives the
syngas
produced from the Gasification System and allows mixing of the syngas to
attenuate any
fluctuations in the chemical composition of the syngas in the homogenization
chamber.
Fluctuations in other gas characteristics, such as pressure, temperature and
flow rate, will
also be reduced during mixing of the syngas. The gas homogenization chamber is
designed to receive syngas from a gasification process and retain the gas for
a residence
time sufficient for mixing of the gas to achieve a volume of gas with a
consistent and/or
specified chemical composition.
114
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
In one embodiment, the facility includes a gas storage system for storing
cleaned and
conditioned syngas prior to its utilization in electricity generating systems.
In one embodiment, the facility includes a GQCS having a water-gas shift
component for
carrying out a water-gas shift reaction. The water-gas shift component carries
out an
inorganic reaction in which water and carbon monoxide react to form carbon
dioxide and
hydrogen. This reaction is also known as water splitting. The carbon dioxide
and
hydrogen generated can be used in processing materials from tar sands.
The Control System
The systems and processes of the facility are managed by a Control System that
provides
means for monitoring and regulating the different stages of the processes to
ensure the
efficient conversion of the carbonaceous feedstock into a syngas product. The
Control
System also optionally provides for the production of a syngas product having
a consistent
and/or specified composition.
The Control System comprises one or more sensing elements for real-time
monitoring of
operating parameters of the system; and one or more response elements for
adjusting
operating conditions within the system to optimize the conversion reaction,
wherein the
sensing elements and the response elements are integrated within the system,
and wherein
the response elements adjust the operating conditions within the system
according to the
data obtained from the sensing elements.
In one embodiment of the present invention, a Control System may be provided
to
manage one or more processes implemented in, and/or by, the various systems
and/or
subsystems disclosed herein, and/or provide control of one or more process
devices
contemplated herein for affecting such processes. In general, the Control
System may
operatively control various local and/or regional processes related to a given
system,
subsystem or component thereof, and/or related to one or more global processes
implemented within a system, such as a Gasification System, within or in
cooperation
with which the various embodiments of the present invention may be operated,
and
thereby adjusts various control parameters thereof adapted to affect these
processes for a
defined result. Various sensing elements and response elements may therefore
be
distributed throughout the controlled system(s), or in relation to one or more
components
115
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
thereof, and used to acquire various process, reactant and/or product
characteristics,
compare these characteristics to suitable ranges of such characteristics
conducive to
achieving the desired result, and respond by implementing changes in one or
more of the
ongoing processes via one or more controllable process devices.
The Control System generally comprises, for example, one or more sensing
elements for
sensing one or more characteristics related to the system(s), processe(s)
implemented
therein, input(s) provided therefor, and/or output(s) generated thereby. One
or more
computing platforms are communicatively linked to these sensing elements for
accessing
a characteristic value representative of the sensed characteristic(s), and
configured to
compare the characteristic value(s) with a predetermined range of such values
defined to
characterise these characteristics as suitable for selected operational and/or
downstream
results, and compute one or more process control parameters conducive to
maintaining the
characteristic value with this predetermined range. A plurality of response
elements may
thus be operatively linked to one or more process devices operable to affect
the system,
process, input and/or output and thereby adjust the sensed characteristic, and
communicatively linked to the computing platform(s) for accessing the computed
process
control parameter(s) and operating the process device(s) in accordance
therewith.
In one embodiment, the Control System provides a feedback, feedforward and/or
predictive control of various systems, processes, inputs and/or outputs
related to the
conversion of carbonaceous feedstock into a gas, so to promote an efficiency
of one or
more processes implemented in relation thereto. For instance, various process
characteristics may be evaluated and controllably adjusted to influence these
processes,
which may include, but are not limited to, the heating value and/or
composition of the
feedstock, the characteristics of the product gas (e.g. heating value,
temperature, pressure,
flow, composition, carbon content, etc.), the degree of variation allowed for
such
characteristics, and the cost of the inputs versus the value of the outputs.
Continuous
and/or real-time adjustments to various control parameters, which may include,
but are
not limited to, heat source power, additive feed rate(s) (e.g. oxygen,
oxidants, steam, etc.),
feedstock feed rate(s) (e.g. one or more distinct and/or mixed feeds), gas
and/or system
pressure/flow regulators (e.g. blowers, relief and/or control valves, flares,
etc.), and the
116
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
like, can be executed in a manner whereby one or more process-related
characteristics are
assessed and optimized according to design and/or downstream specifications.
Alternatively, or in addition thereto, the Control System may be configured to
monitor
operation of the various components of a given system for assuring proper
operation, and
optionally, for ensuring that the process(es) implemented thereby are within
regulatory
standards, when such standards apply.
In accordance with one embodiment, the Control System may further be used in
monitoring and controlling the total energetic impact of a given system. For
instance, a a
given system may be operated such that an energetic impact thereof is reduced,
or again
minimized, for example, by optimising one or more of the processes implemented
thereby, or again by increasing the recuperation of energy (e.g. waste heat)
generated by
these processes. Alternatively, or in addition thereto, the Control System may
be
configured to adjust a composition and/or other characteristics (e.g.
temperature, pressure,
flow, etc.) of a product gas generated via the controlled process(es) such
that such
characteristics are not only suitable for downstream use, but also
substantially optimised
for efficient and/or optimal use. For example, in an embodiment where the
product gas is
used for driving a gas engine of a given type for the production of
electricity, the
characteristics of the product gas may be adjusted such that these
characteristics are best
matched to optimal input characteristics for such engines.
In one embodiment, the Control System may be configured to adjust a given
process such
that limitations or performance guidelines with regards to reactant and/or
product
residence times in various components, or with respect to various processes of
the overall
process are met and/or optimised for. For example, an upstream process rate
may be
controlled so to substantially match one or more subsequent downstream
processes.
In addition, the Control System may, in various embodiments, be adapted for
the
sequential and/or simultaneous control of various aspects of a given process
in a
continuous and/or real time manner.
In general, the Control System may comprise any type of Control System
architecture
suitable for the application at hand. For example, the Control System may
comprise a
substantially centralized Control System, a distributed Control System, or a
combination
117
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
thereof. A centralized Control System will generally comprise a central
controller
configured to communicate with various local and/or remote sensing devices and
response
elements configured to respectively sense various characteristics relevant to
the controlled
process, and respond thereto via one or more controllable process devices
adapted to
directly or indirectly affect the controlled process. Using a centralized
architecture, most
computations are implemented centrally via a centralized processor or
processors, such
that most of the necessary hardware and/or software for implementing control
of the
process is located in a same location.
A distributed Control System will generally comprise two or more distributed
controllers
which may each communicate with respective sensing and response elements for
monitoring local and/or regional characteristics, and respond thereto via
local and/or
regional process devices configured to affect a local process or sub-process.
Communication may also take place between distributed controllers via various
network
configurations, wherein a characteristics sensed via a first controller may be
communicated to a second controller for response thereat, wherein such distal
response
may have an impact on the characteristic sensed at the first location. For
example, a
characteristic of a downstream product gas may be sensed by a downstream
monitoring
device, and adjusted by adjusting a control parameter associated with the
converter that is
controlled by an upstream controller. In a distributed architecture, control
hardware and/or
software is also distributed between controllers, wherein a same but modularly
configured
control scheme may be implemented on each controller, or various cooperative
modular
control schemes may be implemented on respective controllers.
Alternatively, the Control System may be subdivided into separate yet
communicatively
linked local, regional and/or global control subsystems. Such an architecture
could allow a
given process, or series of interrelated processes to take place and be
controlled locally
with minimal interaction with other local control subsystems. A global master
Control
System could then communicate with each respective local control subsystems to
direct
necessary adjustments to local processes for a global result.
The Control System of the present invention may use any of the above
architectures, or
any other architecture commonly known in the art, which are considered to be
within the
general scope and nature of the present disclosure. For instance, processes
controlled and
118
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
implemented within the context of the present invention may be controlled in a
dedicated
local environment, with optional external communication to any central and/or
remote
Control System used for related upstream or downstream processes, when
applicable.
Alternatively, the Control System may comprise a sub-component of a regional
an/or
global Control System designed to cooperatively control a regional and/or
global process.
For instance, a modular Control System may be designed such that control
modules
interactively control various sub-components of a system, while providing for
inter-
modular communications as needed for regional and/or global control.
The Control System generally comprises one or more central, networked and/or
distributed processors, one or more inputs for receiving current sensed
characteristics
from the various sensing elements, and one or more outputs for communicating
new or
updated control parameters to the various response elements. The one or more
computing
platforms of the Control System may also comprise one or more local and/or
remote
computer readable media (e.g. ROM, RAM, removable media, local and/or network
access media, etc.) for storing therein various predetermined and/or
readjusted control
parameters, set or preferred system and process characteristic operating
ranges, system
monitoring and control software, operational data, and the like. Optionally,
the computing
platforms may also have access, either directly or via various data storage
devices, to
process simulation data and/or system parameter optimization and modeling
means. Also,
the computing platforms may be equipped with one or more optional graphical
user
interfaces and input peripherals for providing managerial access to the
Control System
(system upgrades, maintenance, modification, adaptation to new system modules
and/or
equipment, etc.), as well as various optional output peripherals for
communicating data
and information with external sources (e.g. modem, network connection,
printer, etc.).
The processing system and any one of the sub-processing systems can comprise
exclusively hardware or any combination of hardware and software. Any of the
sub-
processing systems can comprise any combination of none or more proportional
(P),
integral (I) or differential (D) controllers, for example, a P-controller, an
I-controller, a PI-
controller, a PD controller, a PID controller etc. It will be apparent to a
person skilled in
the art that the ideal choice of combinations of P, I, and D controllers
depends on the
dynamics and delay time of the part of the reaction process of the
Gasification System and
the range of operating conditions that the combination is intended to control,
and the
119
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
dynamics and delay time of the combination controller. It will be apparent to
a person
skilled in the art that these combinations can be implemented in an analog
hardwired form
which can continuously monitor, via sensing elements, the value of a
characteristic and
compare it with a specified value to influence a respective control element to
make an
adequate adjustment, via response elements, to reduce the difference between
the
observed and the specified value. It will further be apparent to a person
skilled in the art
that the combinations can be implemented in a mixed digital hardware software
environment. Relevant effects of the additionally discretionary sampling, data
acquisition,
and digital processing are well known to a person skilled in the art. P, I, D
combination
control can be implemented in feed forward and feedback control schemes.
In corrective, or feedback, control the value of a control parameter or
control variable,
monitored via an appropriate sensing element, is compared to a specified value
or range.
A control signal is determined based on the deviation between the two values
and
provided to a control element in order to reduce the deviation. It will be
appreciated that a
conventional feedback or responsive Control System may further be adapted to
comprise
an adaptive and/or predictive component, wherein response to a given condition
may be
tailored in accordance with modeled and/or previously monitored reactions to
provide a
reactive response to a sensed characteristic while limiting potential
overshoots in
compensatory action. For instance, acquired and/or historical data provided
for a given
system configuration may be used cooperatively to adjust a response to a
system and/or
process characteristic being sensed to be within a given range from an optimal
value for
which previous responses have been monitored and adjusted to provide a desired
result.
Such adaptive and/or predictive control schemes are well known in the art, and
as such,
are not considered to depart from the general scope and nature of the present
disclosure.
Control Elements
Sensing elements contemplated within the present context can include, but are
not limited
to, means for monitoring operational parameters such as gas flow, temperature
and
pressure at various locations within the system, as well as means for
analyzing the
chemical composition of the syngas product.
The data obtained from the sensing elements is used to determine if any
adjustments to
the conditions and operating parameters within the Gasification System are
required to
120
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
optimize the efficiency of the gasification process and the composition of the
product
syngas. Ongoing adjustments to the reactants (for example, rate and amounts of
primary
and secondary feedstock addition, input of heated air and/or steam), as well
as to certain
operating conditions, such as temperature and pressure within various
components within
the system, enable this process to be conducted under conditions that enable
the efficient
and consistent production of the syngas.
The Control System can be designed and configured with the objective of
optimizing the
efficiency of the gasification process and to mitigate environmental impacts
caused by the
gasification process. The Control System can also be designed to operate the
Gasification
System under continuous operating conditions.
The following operational parameters may be intermittently or continuously
monitored by
the sensing elements, and the data obtained are used to determine whether the
system is
operating within the optimal set point, and whether, for example, there needs
to be more
power delivered by the torches, more air or steam injected into the system, or
if the
feedstock input rate needs to be adjusted.
Temperature
In one embodiment of the invention, the Control System comprises means to
monitor the
temperature at sites located throughout the system as required for example,
inside the
volatilization, processed feedstock/char conversion, or gas reformulating
chambers. The
means for monitoring the temperature may be thermocouples or optical
thermometers
installed at locations in the system as required.
Means for monitoring the temperature of the hot syngas product may also be
located at the
syngas outlet of the plasma gas reformulating chamber. In one embodiment,
where a
subsystem for recovering the sensible heat in the hot syngas produced by the
plasma gas
reformulating process is employed (such as a heat exchanger or similar
technology),
means for monitoring the temperature at points in the heat recovery subsystem
may be
incorporated. For example, the temperature may be monitored at the coolant
fluid inlet
and outlet, as well as at the syngas inlet and outlet.
System Pressure
121
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
In one embodiment of the invention, the Control System comprises means to
monitor the
pressure at locations throughout the Gasification System. These pressure
monitoring
means may include pressure sensors such as pressure transducers, pressure
transmitters or
pressure taps located anywhere in the system, for example, on a vertical wall
of the
secondary chamber or at location within the heat exchanger subsystem.
In one embodiment, the pressure in the different components in the system is
monitored.
In this manner, a pressure drop or differential from one component to another
can be
monitored to rapidly pinpoint developing problems during processing.
Gas Flow Rate
In one embodiment of the invention, the Control System comprises means to
monitor the
rate of gas flow at sites located throughout the system. Fluctuations in the
gas flow may
be the result of non-homogeneous conditions (e.g. torch malfunction or
interruptions in
the material feed), therefore if fluctuations in gas flow persist, the system
may be shut
down until the problem is solved.
Gas Composition
In one embodiment of the invention, the Control System comprises means to
monitor the
composition of the syngas product. The gases produced during the gasification
process
can be sampled and analyzed using methods well known to the skilled
technician.
In one embodiment, the syngas composition is monitored by means of a gas
monitor,
which is used to determine the chemical composition of the syngas, for
example, the
hydrogen, carbon monoxide and carbon dioxide content of the synthesis gas. In
one
embodiment, the chemical composition of the syngas product is monitored
through gas
chromatography (GC) analysis. Sample points for these analyses can be located
throughout the system. In one embodiment, the gas composition is monitored
using a
Fourier Transform Infrared (FTIR) Analyser, which measures the infrared
spectrum of the
gas.
122
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
Although high temperature gas analysis means exist, one skilled in the art can
appreciate
that it may be required to cool the gas prior to analyzing its composition,
depending upon
the type of system used for gas analysis.
Response Elements
Response elements contemplated within the present context can include, but are
not
limited to, various control elements operatively coupled to process-related
devices
configured to affect a given process by adjustment of a given control
parameter related
thereto. For instance, process devices operable within the present context via
one or more
response elements, may include, but are not limited to, means for adjusting
various
operational parameters such as the rate of addition of the primary and
secondary
feedstock, air and/or steam inputs, as well as operating conditions, such as
power to the
torch and torch position.
Plasma Heat Source
In one embodiment, the present Gasification System uses the controllability of
plasma
heat to drive the gas reformulating process. Where a solid residue
conditioning subsystem
is employed, the controllability of plasma heat is also used to ensure the
complete melting
and vitrification of ash to slag. A more detailed description of the control
of the plasma
source or other energisizing gas source in the gas reformulating system is
provided in a
separate section below.
In those embodiments where a solid residue conditioning subsystem is employed,
the
Control System optionally comprises means to adjust the power and/or the
position of the
plasma heat source. For example, when the temperature of the melt is too low,
the
Control System may command an increase in the power rating of the plasma heat
source;
conversely, when the temperature of the chamber is too high, the Control
System may
command a drop in the power rating of the plasma heat source.
In one embodiment, the power of the torch is maintained at a level that is
proportional to
the rate of the solid residue addition, i.e., an increase in the solid residue
feed rate results
in an increase in the torch power. The torch power can also be adjusted to
react to
123
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
changes in the characteristics and composition of the residue, for example,
with respect to
its melting properties such as temperature, specific heat capacity, or heat of
fusion.
In one embodiment, the position of the plasma heat source is adjustable to
ensure
complete coverage of the melt pool, and the elimination of areas of
incompletely reacted
materials.
The Rate of Carbonaceous Feedstock Addition
In one embodiment of the invention, the Control System comprises means to
adjust the
supply rate of carbonaceous feedstock to the primary chamber to ensure that
the feedstock
is input at a rate that does not exceed the drying and volatilization capacity
of the primary
chamber at a given heated air input rate. This ensures that the volatile
fraction is fully
removed before the processed feedstock/char is passed to the secondary
chamber. The
feedstock may be added in a continuous manner, for example, by using a
rotating screw or
auger mechanism, or it can be added in a discontinuous fashion, for example,
periodically
and in discrete portions.
In one embodiment, a secondary feedstock is provided as a process additive to
adjust the
carbon content of the feedstock being gasified. In such an embodiment, the
Control
System provides a means for adjusting the secondary and primary feedstock
input rates to
ensure the optimum carbon content of feedstock to provide control over the
final syngas
composition
The Rate of Solids Movement
The Control System also comprises means to control the movement of solids
through the
different stages of the gasification process. In one embodiment, the Control
System
comprises means to adjust the rate of processed feedstock/char transfer out of
the primary
chamber and into the secondary chamber. In such an embodiment, the rate of
transfer of
the processed feedstock/char product is controlled to ensure complete
volatilization of the
volatile fraction of the feedstock, while also preventing accumulation of
processed
feedstock/char in the primary chamber after the volatilization is complete.
124
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
In one embodiment, the Control System comprises means to adjust the rate of
ash transfer
out of the secondary chamber, thereby providing controlling the residence time
of the
processed feedstock/char in the secondary chamber.
The transfer rates are adjusted as required to ensure acceptable control over
the
volatilization or processed feedstock/char conversion steps, thereby
preventing the
conveyance of incompletely volatilized or unconverted materials out of their
respective
chambers.
The solids may be passed from the respective chambers in a continuous or
discontinuous
manner, using any of the solids removal means previously discussed. In one
embodiment
where the feedstock/processed feedstock/char input means comprises a series of
pusher
rams, the Control System may employ limit switches or other means of travel
control such
as computer controlled variable speed motor drives to control the length,
speed and/or
frequency of the ram stroke so that the amount of material fed into the
respective chamber
with each stroke can be controlled. In one embodiment where the input means
comprises
one or more screw conveyors, the rate of addition of the material to the
respective
chamber may be controlled by adjusting the conveyor speed via drive motor
variable
frequency drives.
In one embodiment, where a horizontal primary chamber is employed, the Control
System
optionally comprises means to control the movement of one or more lateral
transfer units
in the primary chamber, thereby controlling the movement of material through
the
chamber to optimize the drying and volatilization stages by controlling the
residence time
of the material at each stage.
Addition of Heated Air Inputs
In one embodiment of the invention, the Control System comprises means to
adjust the
rate and/or amounts of heated air inputs into the volatilization and secondary
chambers.
Addition of Process Additive Inputs
In one embodiment of the invention, the Control System comprises means to
adjust the
steam and/or air process additive inputs into the plasma gas reformulating
chamber, in
order to ensure that the volatiles and gaseous products of the processed
feedstock/char
125
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
conversion are completely converted to a useful gas product by the plasma gas
reformulating step. In one embodiment of the invention, the Control System
comprises
means to adjust the steam and/or air process additive inputs into the
secondary chamber,
to ensure that the levels of oxygen and hydrogen required for the carbon
conversion
reaction are present are required to optimize the chemical composition of the
syngas
product. In one embodiment, the determination of the amounts and types of
process
additives required is based on data obtained from monitoring and analyzing the
composition of the syngas.
Modularity of the Facility
Modular facilities are facilities where each function block is made of pre-
built
components, which allows for the components to be built in a factory setting
and then sent
out to the facility site for plant assembly. These components (or modules)
include all the
equipment and controls to be functional and are tested before leaving the
factory. Modules
are often built with a steel frame and generally incorporate a variety of
possible sections,
such as: Gasifier Block, Gas Conditioning System Block, Power Block, etc. Once
on-site,
these modules only need to be connected to other modules and the Control
System to be
ready for plant commissioning. This design allows for shorter construction
time and
economic savings due to reduced on-site construction costs.
There are different types of modular plants set-ups. Larger modular plants
incorporate a
"backbone" piping design where most of the piping is bundled together to allow
for
smaller footprint. Modules can also be placed in series or parallel in an
operation
standpoint. Here similar tasked equipment can share the load or successively
provide
processing to the product stream.
One application of modular design in this technology is that it allows more
options in the
gasification of multiple feedstocks. This technology can allow for multiple
gasification
lines to be used in a single high-capacity facility. This would allow the
option of having
each Gasification System co-process feedstocks together or separately; the
configuration
can be optimized depending on the feedstocks.
If an expansion is required due to increasing loads, a modular design allows
this
technology to replace or add modules to the plant to increase its capacity,
rather than
126
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
building a second plant. Modules and modular plants can be relocated to other
sites where
they can be quickly integrated into a new location.
Function Block Combinations
It is possible to combine the functions of different gasification trains
(series of equipment)
so that common functions can be carried out in function blocks that take in
gases or
material from more than one stream. The following diagrams demonstrate this
concept as
applied to carbonaceous feedstock gasification.
In the following embodiments, there are two trains shown, although this set-up
of
combined functions between trains can occur for any number of trains and for
any
feedstock per train (even if one train has a combined feedstock). Once a
stream has been
combined, one may still choose parallel handling equipment downstream; the
parallel
streams do not need to be of the same size even if handling the same gases.
For Figures 68 to 71, GQCS refers to the gas conditioning system mentioned
above and
the numbers represent the following systems: 1) primary chamber, 2) secondary
chamber,
3) melting chamber, and 4) gas reformulating chamber.
Figures 68 to 71 depict different embodiments of the present Gasification
System that fall
within the scope of the present invention. In particular, Figures 68 to 71
describe
embodiments of the Gasification System in which the separate primary feedstock
and
secondary feedstock inputs are carried through to the final syngas product.
The embodiment shown in Figure 112 depicts one embodiment in which the primary
feedstock and secondary feedstock are each volatilized in separate primary
chambers, and
the resulting processed feedstock/char from each primary chamber is combined
in a
common secondary chamber. The first chamber gas products from each of the
primary
chambers and the second chamber gas product from the secondary chamber are
combined
in a common gas reformulating chamber.
The embodiment shown in Figure 113 depicts one embodiment in which the primary
feedstock and secondary feedstock are each volatilized in separate primary
chambers, and
the resulting processed feedstock/char from each primary chamber is passed
into a
separate solid residue conditioning chamber. The first chamber gas product
from each of
127
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
the primary chambers and the second chamber gas product from the processed
feedstock/char chambers are combined in a common gas reformulating chamber.
The embodiment shown in Figure 114 depicts one embodiment in which the primary
feedstock and secondary feedstock are each volatilized in separate primary
chambers, and
the resulting processed feedstock/char from each primary chamber is passed
into
respective secondary chamber. The first chamber gas product and second chamber
gas
product from each of the primary and secondary feedstock gasification streams
are
reformulated in separate gas reformulating chambers.
The embodiment shown in Figure 115 depicts one embodiment in which the primary
feedstock gasification stream and the secondary feedstock gasification stream
are carried
separately through the volatilization, processed feedstock/char gasification,
solid residue
conditioning, and gas reformulating steps, wherein the syngas products are
combined only
prior to the gas quality conditioning step.
EXTRACTION SYSTEMS AND OPTIONAL UPGRADING AND REFINING
SYSTEMS
In accordance with this invention, intermediate products generated in the
gasification and
gas reformulation systems are utilized directly or indirectly in the ongoing
extraction and
processing of the unconventional oil source, or are recycled back to improve
the
efficiencies of the gasification and/or gas reformulating processes.
Extraction and
processing of unconventional oil sources to yield useful fuel products
requires a high
input of energy and other reagents. Tar sands, for example, require
substantial amounts of
energy for mining and separating in strip mine operations or for heating
underground
reservoirs in in situ production. Likewise, extra-heavy oil requires
significant effort to
bring it to the surface. Upgrading extra-heavy oil or bitumen to syncrude also
requires
significant amounts of energy. In general natural gas is used to generate
electricity to
power equipment, as well as to produce hydrogen or to power cokers required
for the
upgrading process.
The Gasification System of the invention can be configured to produce a
variety of
intermediates that can be tailored to the requirements of the particular oil
source being
processed. Various examples are provided in Figure 1. For example,
intermediates
128
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
produced in the Gasification System of the invention can be used as sources of
energy
(heat, electricity, etc) that can be utilized in one or more of the extraction
and/or
upgrading processes, either as a supplement to traditional energy sources,
such as natural
gas, or in some cases, as a sole source of energy. Intermediates produced by
the
Gasification System that are useful in this context include reformulated
syngas having a
defined chemical composition that can be effectively used, for example, as a
combustion
fuel to provide heat or to generate electricity. Heat obtained in this manner
can be utilized
for example, in the in situ heating of oil shale or tar sand deposits, or to
provide heat for
downstream upgrading or refining processes. Similarly, electricity generated
from the
intermediates can be used to power heat sources for in situ extraction
processes, to power
various components of the upgrading and/or refining system.
Reformulated syngas can also be used to drive steam generators to generate
steam, which
is required in in situ extraction processes for tar sands and oil shale.
Likewise, steam
generated at various points in the Gasification System can be recovered and
used directly
in such in situ processes.
Reformulated syngas can also be used as a source of hydrogen, which is
required for the
oil upgrading process.
Heat produced at various points in the Gasification System is also considered
a useful
intermediate. For example, heat is generated in the reformulating stage of the
Gasification
System and can be recovered from the reformulated gas. Heat recovered from the
Gasification System and used, for example, to generate steam using a heat
recovery steam
generator (HRSG). Heat can also be recycled back to drive the gasification
process and/or
syngas purification processes. Syngas can also be combusted to provide heat,
for example,
syngas can be piped into the extraction beds and combusted to provide heat in
situ.
It is also contemplated that the syngas can be used for the generation of
carbon dioxide,
which can be used in enhanced oil recovery (EOR) or methane recovery
processes.
Syngas can also be used in chemical processes, such as Fischer-Tropsch process
to
produce light oils, which can be used as diluents in the unconventional oil
source
extraction process.
129
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
Figure 116 provides an exemplary embodiment of the invention in which the
Gasification
System is used to generate several intermediates, which are used in different
processes of
a tar sands processing operation. In the embodiment represented by Figure 116,
carbonaceous feedstock 4 is fed into a converter 2 and solid residue generated
in the
converter 2 from the gasification of the carbonaceous feedstock 4 is processed
to slag in a
solid residue conditioner 6. Syngas from the converter can be cooled in an
optional step
in an air to gas heat exchanger 8, prior to being conditioned in a gas
conditioning system
(GQCS) 10. The conditioned syngas that exits the GQCS 10 can be stored in a
gas
storage or regulation system 12 for later use. Alternatively or in addition,
the syngas can
be fed to a gas turbine 60 or to a fuel cell 30 to generate electricity. The
conditioned
syngas from the GQCS 10 can also be used to run a coking oven 40 for upgrading
bitumen or it can be used to produce hydrogen by passing the syngas through a
water-gas
shift reactor 50 or hydrogen separator 52.
Steam can also be generated from the system depicted in Figure 116 as follows.
The
syngas stored in the gas storage or regulation system 12 is regulated to a
combustible gas
which is used to power steam generators 20, which generate steam that can be
used in
SAGD operations 24 to extract bitumen from tar sands. Steam that is generated
in the
converter 2 can also be used directly for the SADG process 24.
The Gasification System in the embodiment of the invention depicted in Figure
116
comprises a gasifier, a melting chamber, a GQCS and a gas storage tank, where
the
Gasification System is integrated with additional equipment or components for
producing
energy and/or reagents that can be used in the processing of oil from tar
sands. The
Gasification System can, however, include additional components depending on
the
feedstock to be consumed/or and the energy or reagents to be produced.
Non-limiting, exemplary uses of the various intermediates produced by the
Gasification
System of the invention are described in more detail below.
Steam generation
Steam is required in various aspects of oil or bitumen extraction from tar
sands or
extraction of oil shale deposits. For example, steam is used in in situ
processes to increase
the temperature of the heavy oil, tar and bitumen in a natural reservoir, such
as tar sands
130
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
or extra-heavy oil deposits, which results in reduced viscosity, separation of
the oil, tar or
bitumen from the deposit, and ensures a pressure flow that allows
transportation by
pipeline or other means.
One such in situ recovery method is Steam Assisted Gravity Drainage (SAGD), as
described in U.S. Pat. No. 4,344,485 (Butler), which requires two horizontal
wells to be
drilled into the reservoir. In this method, two spaced apart wells are first
drilled vertically
to different depths within the reservoir. Thereafter, using directional
drilling technology,
the two wells are extended in the horizontal direction that result in two
horizontal wells,
vertically spaced from, but otherwise vertically aligned with the other.
Ideally, the
production well is located above the base of the reservoir but as close as
practical to the
bottom of the reservoir, and the injection well is placed above or nearly
above the
production well.
The upper horizontal well is utilized as an injection well and is supplied
with steam from
the surface. The steam rises from the horizontal injection well, permeating
the reservoir to
form a vapour chamber that grows over time towards the reservoir top, thereby
increasing
the temperature within the reservoir. The steam (and its condensate), by
soaking for a
period of time, will raise the temperature and consequently reduce the
viscosity of the
semi-solid bitumen or heavy oil in the reservoir. It will also partially
replace the bitumen
in the pores of the sand. The bitumen and condensed steam will then drain
downward
through the reservoir under the action of gravity and flow into the lower
production well,
whereby these liquids can be pumped to the surface. At the surface of the
well, the
condensed steam and bitumen are separated, and the bitumen is diluted with
appropriate
light hydrocarbons to transport the bitumen by pipeline to a refinery or an
upgrader.
The theoretical and design concepts required to conduct successful SAGD have
been
published and have been extensively discussed in technical and related
industry literature.
A major component of the capital and operating costs of commercial SAGD
operations
are the facilities to: a) generate steam, b) separate hydrocarbons from
condensed steam,
and c) treat and recycle water to the steam generators. Current steam
generators require
large amounts of water, which is heated by boilers fired by natural gas to
produce steam.
The volume of water handled in SAGD operations is reflected in steam-to-oil
ratios (e.g.
CWE m3 steam/m3 bitumen) of about 2 and above for active or anticipated
projects.
131
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
As is known in the art, a particular quality of steam is required for in situ
processing of
bitumen from tar sands. For example, ultra-high pressure steam is used for in
situ
processing of bitumen from tar sands. Suitable ultra-high pressure steam is a
100%
quality steam at pressure of between 3 and 10 mPa. As is known in the art, the
selected
pressure is dependent upon how deep the bitumen is that needs to be extracted.
The Gasification System of the invention can be used to provide steam for use
in in situ
methods of recovering heavy oil or bitumen deposits. In one embodiment, steam
provided by the Gasification System is used in SAGD processes. The steam can
be
directed, for example, to the upper horizontal well of a SAGD operation using
steam
directing means. As described above, the steam by be provided from a stem
generator
powered by syngas from the gasification and/or by steam recovered from the
gasification
process itself.
An exemplary configuration of a gasification reactor and steam generator
combination is
shown in Figure 117. In this configuration, a once-through steam boiler is
used to
produce 75% quality steam which is subsequently converted to 100% steam in
water or
dirt are separated from the steam in a gas/liquid separator.
In a once-through steam generator, the heating of steam generator tubes
provided as
evaporator tubes leads to an evaporation of the flow medium in the steam
generator tubes
in a single pass. In contrast thereto, in a natural- or forced-circulation
steam generator, the
circulating water is only partly evaporated when passing through the
evaporator tubes.
The water which is not evaporated in the process is fed again to the same
evaporator tubes
for a further evaporation after separation of the generated steam.
A once-through steam generator, in contrast to a natural- or forced-
circulation steam
generator, is not subject to any pressure limit, so that live-steam pressures
are possible
well above the critical pressure of water (Pc.;.approximately 221 bar) where
there is only a
slight difference in density between a medium similar to a liquid and a medium
similar to
steam. A high live-steam pressure promotes a high thermal efficiency and thus
low CO2
emissions of a fossil-fired power plant. In addition, a once-through steam
generator has a
simple type of construction compared with a circulation steam generator and
can therefore
be manufactured at low cost.
132
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
As is known in the art, when a once through steam boiler designed for use with
natural
gas as fuel source is used to generate steam using syngas as a fuel,
components of the
boiler can be reconfigured to allow for the differences in gas characteristics
between
syngas and natural gas such as the lower heating value of syngas compared to
natural gas.
For example, the injection ports of the boiler can be reconfigured to be
bigger than the
injection ports for use with natural gas.
As shown in Figure 117, syngas transferred from a gasification reactor can be
fed into a
once-through steam generator and syngas combustion is then used to heat
process water or
recovered water which is supplied to the steam generator. This process water
or
recovered water may contain impurities such as sand, other particulate matter,
slats, and
dissolved organic and inorganic compounds. The resulting steam is of 75%
quality, and
is then fed into a two phase separator in order to generate 100% quality
steam. Suitable
two phase separator systems are known in the art. The 100% quality steam
generated can
then be transferred to in situ processing equipment for extracting heavy oil
or bitumen
from the tar sands as described following.
It is also possible to use a multiple pass steam boiler to produce steam using
syngas from
the Gasification System. An alternate process for generating steam from syngas
includes
passing an ultra-high steam (or thermal fluid) through the gasifier and
looping it through a
steam generator to produce steam from water as shown in Figure 118.
In one embodiment, intermediates from the Gasification System are used to
generate
electricity and steam for extracting bitumen from tar sands. An example of a
Gasification
System in accordance with this embodiment is shown in Figure 116. In this
embodiment,
a Heat Recovery Steam Generator (HRSG) is used to generate steam from the
latent heat
in syngas and electrical generator outputs as shown in Figure 119. In this
embodiment,
syngas from the gasifier 100 is passed into a HRSG 120 and the generated steam
is used
in the extraction process at the oil field. The cooled syngas from the HRSG
120 is purified
in by passage through a scrubber 160 and baghouse 140, with subsequent
hydrogen
sulphide removal 150 and transferred to a storage tank 160. The syngas
intermediate is
then used in a gas turbine 170 to provide on-site electricity. Gas from the
turbine is passed
to a second HRSG 180 which can be used to generate additional steam for use in
the
extraction process.
133
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
As noted above, the steam generated by the Gasification System can be used in
in situ
methods requiring steam for recovering heavy oil or bitumen. The steam is
supplied to
these recovery operations using steam directing means that transfer the steam
to a required
location. Steam directing means are known in the art and include various pipes
and
conduits that are in compliance with the appropriate requirements for
conducting steam at
high temperature and appropriate pressure.
Electricity
Syngas produced in the Gasification System can be used as a fuel for various
energy
generating components such as gas engines, gas turbines, steam generators,
steam
engines, steam turbines, fuel cells and the like, to produce electricity
required to power
various components of the unconventional oil source processing facility.
Syngas acts as a
suitable fuel for these systems if it exhibits the required characteristics of
pressure, LHV,
composition and lack of impurities required for the operation of these energy
generating
components. As discussed above, a syngas exhibiting these characteristics can
be
generated by the Gasification System through optimisation of the reformulating
stage to
provide a syngas with a desired composition, in combination with one or more
syngas
conditioning or processing components, such as a GQCS, cooling units, gas
storage tanks,
gas shift reactors, pressure regulator valves and the like.
Electricity can also be produced by using combined cycle technology, which
employs the
syngas in an Integrated Gasification Combined Cycle (IGCC) system. Syngas
produced
from fossil fuel based feedstocks (such as coal, bitumen, petcoke, and the
like) is
particularly useful in this type of system.
An IGCC system generally comprises a combustion turbine/generator, a heat
recovery
steam generator, and a steam turbine/generator. The syngas produced from the
Gasification System, which contains high concentrations of carbon monoxide and
hydrogen, is used in the combustion turbine. Exhaust heat from the combustion
turbine is
recovered in the heat recovery steam generator to produce steam. This steam
then passes
through a steam turbine to power another generator, which produces more
electricity.
Combined cycle is frequently more efficient than conventional power generating
systems
because it re-uses waste heat to produce more electricity.
134
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
In one embodiment of the invention, intermediates (syngas) from the
Gasification System
are used to produce electricity via an IGCC system.
Hydrogen Generation
Hydrogen (H2) can be used in unconventional oil source processing for
hydrotreating
bitumen or oil extracted from the unconventional oil source. H2 can also be
used in a fuel
cell system to generate electricity and water on-site.
Hydrogen can be separated from the syngas produced by the Gasification System
via a
number of commercially available technologies as is known in the art,
including for
example, using a commercially available membrane technology such as a membrane
separator, a water-gas shift reactor, adsorption or absorption techniques fuel
cells, ion
transfer membranes, cryogenic separation, molecular sieves, or combinations
thereof..
The clean H2 gas, thus separated, can be stored and transported/piped to the
region of the
processing operation where it is required. The hydrogen-depleted syngas which
is rich in
CO can be used to generate additional electricity through a turbine generator
or other gas-
fired engine via conventional heat engine technology.
Carbon Dioxide Generation
Carbon dioxide (C02) can be used in unconventional oil source processing for
enhanced
oil recovery (EOR) and/or methane recovery processes. FOR using CO2 involves
injecting CO2 into a reservoir containing oil to be recovered. Once in the
reservoir, the
CO2 expands and, in doing so, pushes additional oil to a production wellbore.
In addition,
CO2 dissolves in the oil to lower its viscosity and improve the flow rate of
the oil making
it more amenable to pumping.
Methane recovery from subterranean coal beds using CO2 is carried out by
similar
methods. CO2 is continuously injected via injection wells into the coal bed to
produce
methane from one or more recovery wells spaced from the injection wells. The
produced
methane includes both free methane displaced by the injection gas and methane
that is
desorbed from the coal surface by differential adsorption of carbon dioxide on
the coal
surface.
135
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
Syngas from the gasification reaction can be used to generate CO2 using
methods known
in the art. These methods include, for example, electrochemical pumps,
membranes, and
chemical looping approaches to CO2 separation. Electrochemical pumps include
carbonate and proton conductors. Selective membranes for hydrogen separation
can be
used as a method for carbon dioxide concentration in fuel gas streams. The
selective
membranes include mixed ionic-electronic (solid electrolyte-metal) films as
well as
palladium-based materials. The CO2 produced in this way can be used in
enhanced oil
recovery (EOR) and methane recovery processes.
Light Oils Generation
Light oils can be used in unconventional oil source processing as diluents in
the
processing and transport of bitumen or heavy oils from the unconventional oil
source.
Syngas can be used to produce light oils, for example, using the Fischer-
Tropsch process,
which produces liquid hydrocarbons of various forms via a chemical reaction
typically
catalyzed by iron and cobalt. Examples of methods for conversion of syngas to
hydrocarbons in a Fischer-Tropsch process are illustrated in U.S. Pat. No.
4,096,163 to
Chang et al., U.S. Pat. No. 6,085,512 to Agee et al., and U.S. Pat. No.
6,172,124.
The Fischer-Tropsch synthesis process is an exothermic process and may
generate steam,
which can be utilized in various processes in the unconventional oil source
processing
facility, as described above.
Light oils may also be produced by the Gasification System when it comprises a
cooler
and tar separator, which functions to remove tar from the gas exiting the
gasifier (see, for
example, the embodiment of the Gasification System depicted in Figure 120).
The tars
can then be distilled by conventional techniques to produce light oils that
can be used as
diluents in the processing and transport of bitumen from the tar or oil sands.
As shown in
Figure 120, the gas exiting the cooler and tar separator is then reformulated
in a gas
reformulating zone prior to entering a GQCS. In one embodiment, this gas
reformulation
zone is a secondary gas reformulation zone, with the primary gas reformulation
zone
being located upstream of the cooler and tar separator. In one embodiment, the
Gasification System according to the invention comprises a cooler and tar
separator. In
another embodiment, the Gasification System comprises a secondary gas
reformulating
136
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
zone. In another embodiment, the Gasification System comprises both a cooler
and tar
separator and a secondary gas reformulating zone.
Standalone or Retrofitting the Gasification System and Gas Reformulation
Systems
onto Existing Facilities
It is contemplated that the system of the invention may be provided as
described above, or
alternatively the system may be modified to be integrated with existing
systems that rely
on conventional energy sources, for example, natural gas to improve the
overall cost
effectiveness of the existing system and/or reduce the requirement for other
energy
sources, such as natural gas. Such modifications include, for example,
modifications to
the gas transfer means to permit transfer of the syngas to an existing steam
generator or
other component of the facility. The design and scope of such modifications
are within
the knowledge of a worker skilled in the art.
As would be evident to one of skill in the art, the Gasification System of the
invention
may require design changes in order to allow coupling of the system to various
components that utilise the intermediates produced by the system. For example,
changes
may be required to configurations of components, such as, nozzles, transport
pipes,
injection ports and/or operating conditions. Additional components may also
need to be
introduced to facilitate the generation or further processing of the
intermediates. In one
embodiment, specialized energy producing components such as gas engines or gas
turbines, or special once through boiler systems can be used. In one
embodiment, the
energy producing component is similar to those used when natural gas is the
fuel used to
power the energy producing component.
To gain a better understanding of the invention described herein, the
following examples
are set forth. It will be understood that these examples are intended to
describe illustrative
embodiments of the invention and are not intended to limit the scope of the
invention in
any way.
137
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
EXAMPLES
EXAMPLE 1: PLASMA GASIFICATION SYSTEM INTEGRATED WITH AN
IGCC
This example describes a plasma Gasification System integrated with an IGCC.
The
plasma Gasification System comprises a converter, solid residue conditioner, a
GCS, and
a gas storage or regulation system, integrated with use of a combined-cycle to
produce
electricity.
As shown in Figure 119, coal 106 is combined with oxygen from an oxygen plant
105 in
the converter 100 to produce syngas, which is mainly hydrogen and carbon
monoxide.
The syngas is then cooled in a heat recovery steam generator (HRSG) 120 and
then
cleaned by a gas cleanup process in a GCS which includes the steps of
scrubbing the
syngas in a scrubber 130, removing particulate matter and heavy metals from
the syngas
in a baghouse 140, and H2S removal in an H2S removal system 150. The cleaned
or
conditioned gas is then stored or regulated in a gas storage or regulation
system 160 prior
to being fed to a gas turbine 170 for the production of electricity.
Combined-cycle
This design consists of a combustion turbine/generator 170, and a heat
recovery steam
generator 180. The exhaust heat from the combustion turbine 170 is recovered
in the heat
recovery steam generator 180 to produce steam. Combined cycle is more
efficient than
conventional power generating systems because it re-uses waste heat to produce
more
electricity.
EXAMPLE 2: CONFIGURATIONS OF GASIFIERS SUITABLE FOR USE
Figure 121A to D depicts various examples of gasifiers that can be used in the
system of
the invention.
EXAMPLE 3: DESIGN OF A GCS FOR USE IN THE SYSTEM OF THE
INVENTION
Figure 122 depicts an example of a GCS that is designed for use in tar sands
applications.
As shown in Figure 122, gas from the gasifier is cooled, particulates are
removed, as is
138
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
heavy metal and H2S. The conditioned gas is then fed to a gas boiler for the
production of
steam.
EXAMPLE 4: OVERVIEW OF A SYSTEM IN WHICH BITUMEN IS
PARTIALLY GASIFIED
The technology is the process of converting low-value feedstocks (Bitumen &
others)
using plasma gasification (and supporting technologies) into valuable end-
products (Light
Oils, Power, Steam). This process will reduce/remove the need for higher value
fuels such
as Natural Gas from being used to produce heat & hydrogen which is currently
the norm
in the field of tar sands extraction.
The process surrounds the following equipment (Optional variations do not
include all
pieces of equipment into every design):
- A plasma gasifier of Fluid feedstocks for bitumen and others
- A Cooler & Tar Remover from syngas
- Other Supporting equipment
o A plasma gasifier for Solid feedstocks
o A Distillation Column for Heavy Oils
o Residue Chamber
o Reformation Chamber
o GCS with optional H2 & CO2 removal and Gas shift equipment.
o Power Generation Technology (Engines, Turbines)
o Chemical Plants (Fischer-Tropsch, etc) for light oil production
An example of one possible design of the Gasification System is shown in
Figure 120. In
this example, bitumen is partially gasified to produce a syngas. The syngas is
then sent to
a cooler and tar separator where longer chain molecules are separated and sent
to be
diluents. The syngas now free of most heavy tars is reformed further into H2 +
CO syngas
which is then cleaned further in a GCS (CO2 can be optionally removed for
EOR/sequestering projects) and sent to a chemical plant (such as, for example
one that
carries out a Fischer-Tropsch process) to produce diluents (such as light
oils).
EXAMPLE 5: OVERVIEW OF A SIMPLIFIED SYSTEM
139
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
Figure 123 depicts an overview of an example of a simplified system. In this
example of
the gasification facility syngas is to be produced from bitumen to be used in
downstream
processes. A tar separator is optionally used for diluents production if that
process is
required. Residue from the reaction (Coke, Carbon, & Ash) is sent to a residue
chamber
for vitrification.
EXAMPLE 6: OVERVIEW OF A SYSTEM IN WHICH MULTIPLE CO-
PRODUCTS ARE GENERATED
Figure 124 depicts an overview of an example of a system of the invention in
which light
oil production is maximized upstream with syngas being used to produce H2,
C02, Power,
and Steam downstream from the bitumen gasification. As shown in Figure 124, a
distillation system is added where the system extracts the lighter portion of
bitumen (and
tars) to avoid gasifying the lighter fraction into syngas when it is fed into
the gasifier;
instead the lighter fraction (light oils) are sent to become diluents for
bitumen
transportation (or other application). Residues from the gasifier &
distillation system
(Coke, Carbon, & Ash) are sent to a residue chamber for vitrification; or
combusted
further to provide heat for the gasification reaction or other process.
EXAMPLE 7: OVERVIEW OF A SYSTEM IN WHICH FEEDSTOCK IS USED
TO PRODUCE STEAM
Figure 125 depicts an example of a system in which bitumen/coal/coke etc. is
used to
produce steam (and optionally extract and use C02) for use in processing tar
sands. The
steam can be used, for example in FOR or SAGD processes.
EXAMPLE 8: BLOCK FLOW DIAGRAM OF CO2 AND DILUENTS
APPLICATION
Figure 126 depicts an example of a system in which lighter fractions of
bitumen can be
produced in addition to power or steam. In this example, the gasifier is
operated with
carbonaceous feedstock, additives, plasma torch(s), and bitumen to produce
lighter oils,
petcoke, & syngas. The light oils can be sold or mixed with heavy bitumen to
make it
more pumpable which increases its value and mobility to markets and the
petcoke &
syngas can be used to produce power, C02, and/or steam.
140
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
CO2 and steam can be used to improve bitumen extraction using enhanced oil
recovery
(EOR) methods known in the art; such methods include steam assisted gravity
drainage
(SAGD) and CO2 sequestration in depleted oil fields. CO2 could also be
injected into coal
mine seams for enhanced methane recovery.
EXAMPLE 9: OVERVIEW OF A SYSTEM DESIGN TO PRODUCE 112,
ELECTRICITY, AND STEAM FOR USE IN UPGRADING OIL
Figure 127 depicts an example of system in which H2, electricity, and steam is
produced
by a system of the invention for use in upgrading oil. As shown in Figure 127,
two
gasifiers are used sequentially in order to produce syngas from coal, bitumen,
or other
carbonaceous feedstock. The syngas is then conditioned in a GCS prior to use
in the
generation of H2, steam, or electricity.
EXAMPLE 10: A FLUID PLASMA GASIFIER
Figure 128 depicts a drawing of an exemplary fluid plasma gasifier that can be
used in the
Gasification System of the invention. The plasma gasifier is designed for
fluids (at
elevated temperatures bitumen is a fluid) which is designed to allow for the
cracking of
the feed into smaller carbon chains. These chains will break into gaseous
products with
the help of a plasma torch and additional additives.
For this design the feed is injected into the gasification chamber where it is
acted on by
temperature, and chemical reactions with additives which include CO, C02,
Steam,
Water, Air, Oxygen, Hydrogen, etc. To improve the cracking of long chains in
the feed
stock a plasma torch is added to chamber to produce high temperature zone and
ionized
plasma which breaks the complex bonds; optionally a hydrogen burner can be
utilized if
heat is only required.
The gasifier is designed to handle liquids or fine particles in entrainment
that can be
sprayed (heated bitumen, pulverized coal/coke, oils, sludge, liquid wastes,
slurries,
entrained fines, etc) where such liquids are placed in a nozzle (with optional
steam/air
injection) and sprayed into the chamber containing plasma torch(es) where the
plasma
enhanced conditions will lead to cracking of tars, pyrolysis, combustion, and
gasification
to produce syngas laden with tars. Additional additives can be added to the
chamber
141
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
though ports in the gas or oil pool as needed for maximizing syngas
production/quality or
Oil production / quality as required for downstream application needs.
Additional feeds can be included (such as a biomass, sawdust, or sludge) into
the heated
bitumen fluid to increase the hydrogen content of the feed to improve the
cracking process
and end products. Depending on what feed stocks are available a Gasification
System can
also be used as a substitute for the proposed gasifier or run in parallel
where the syngas &
residue of both systems could be combined to reduce equipment needs is
desired.
Examples of suitable designs are shown in Figure 129 A to C. With reference to
Figure
129, each function group represents the following systems: 1. Primary
Gasification
Chamber; 2. Residue Chamber; 3. Reforming Chamber.
EXAMPLE 11: COOLING AND REMOVAL SYSTEMS
Figure 130 depicts exemplary cooling and removal systems that can be used in
the
Gasification System of the invention.
EXAMPLE 12: EXTRACTION OF CO2 FROM THE SYNGAS
This example describes a method of extracting CO2 from syngas as it is
processed through
the GCS of the system. In the GCS a water-gas shift reaction could be utilized
along with
CO2 capture to extract CO2 from the syngas for the use in FOR (pumping CO2
into the tar
sands to improve oil extraction) or other downstream process. The same
reaction
produces higher H2 (optionally H2 can be extracted) to be applied in the art
of oil
upgrading (inside gasification facility, or exported to another processing
unit) or improved
power/steam production.
EXAMPLE 13: A MULTI-CHAMBER GASIFICATION SYSTEM
Figure 131 depicts one embodiment of a multi-chamber carbonaceous feedstock
Gasification System. In the present embodiment, the feedstock and heated air
inputs are
introduced to the primary chamber, where the feedstock undergoes drying and
volatilization. The resulting char is passed into a secondary chamber, where
it is
subjected to further heating with heated air inputs, optionally in the
presence of steam
additives. The carbon in the char is converted to a gaseous product, and the
residual ash
is passed into a plasma heated slag chamber, where it undergoes melting and
vitrification.
The gaseous products of the two stages are passed into a gas reformulation
chamber,
142
CA 02723792 2010-11-08
WO 2008/138118 PCT/CA2008/000883
where it undergoes plasma heating, optionally in the presence of process
additives such as
air and/or steam to produce a hot syngas product. The hot syngas is passed
through a heat
exchanger where the sensible heat from the syngas is removed. The cooled
syngas is
passed into a further cooling system, such as a heat recovery steam generator
or a dry
quench step. Where a heat recovery steam generator is used to cool the syngas,
the
resulting steam product may be used in downstream applications such as in a
steam
turbine for generating electricity. Activated carbon is then injected into the
further cooled
syngas, which then undergoes a filtration step to remove particulate matter,
for example,
by being passed through a baghouse filter. The particulate matter removed from
the
syngas product is passed into the slag chamber, where it undergoes plasma
melting with
the ash product of the feedstock gasification. The filtered syngas product
undergoes
further cleaning and conditioning steps prior to being used in downstream
applications for
the generation of energy or reagents for use in processing materials from tar
sands.
Although the invention has been described with reference to certain specific
embodiments, various modifications thereof will be apparent to those skilled
in the art
without departing from the spirit and scope of the invention. All such
modifications as
would be apparent to one skilled in the art are intended to be included within
the scope of
the following claims.
143