Note: Descriptions are shown in the official language in which they were submitted.
. CA 02723904 2015-08-11
NOVEL MODULATORS OF SPHINGOSINE PHOSPHATE
RECEPTORS
10
Background
Sphingosine-l-phosphate (S 1P), the structure of which is shown below,
is a phospholipid with a wide range of biological activities, notably,
involved in
cellular signaling.
NH2
OP(0)(OH)2
-6H
For example, SIP modulates cellular proliferation, such as of epidermal
cells. The bioactivity of SIP is mediated by multiple receptor subtypes. For
example, receptors subtypes 1 and 3, (S1P1 and S1 P3 respectively) are both
expressed in endothelial cells, and play a role in lung and lymphoid
endothelial
functions. Thus, agonists of receptors, such as agonists of S1P1, could be of
value in the treatment of malconditions such as multiple sclerosis, transplant
rejection, and adult respiratory distress syndrome. Agonist stimulation of the
S1P1 receptor is modulated by receptor degradation. Ligand stimulation induces
receptor phosphorylation, internalization, polyubiquination and
degradation(Gonzalez-Cabrera, Hla et al. 2007).
1
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
Oxadiazoles and oxazoles have been described for use as sphingosine-1 -
phosphate receptor ligands, see for examples PCT patent application
publication
numbers W02006/131336, W02008/037476 and W02008074821.
Summary
The present invention is directed to heterocyclic compounds adapted to
act as agonists of SIP receptor subtype 1, S1P1; methods of preparation and
methods of use, such as in treatment of a malcondition mediated by S1P1
activation, or when activation of 51P1 is medically indicated.
Accordingly, various embodiments of the present invention provide a
compound of formula (I) or a pharmaceutically acceptable salt, prodrug,
tautomer, stereoisomer, hydrate, or solvate thereof:
Al ¨ A2
R5
X
L1 ) L2 R6
A3
(I)
wherein
a dashed line signifies that a single bond or a double bond can be present,
provided that there are two double bonds and three single bonds in the ring
comprising A1, A2, and A3;
Ai, A2, and A3 each independently is C or 0 or is N when the N is
bonded to two adjacent ring atoms by a double bond and a single bond or is NR
wherein R is H or (Ci-C6)alkyl when the N is bonded to two adjacent ring atoms
by two single bonds; provided that no more than one of Al, A2, and A3 is C and
that at least one of Al, A2, and A3 is N or NR; provided that only one of Ai,
A2,
and A3 is 0;
Li and L2 are each independently a bond; (CHRõ wherein R' is H or (CI-
C6)alkyl and n is 1, 2, or 3; or a heteroaryl selected from the group
consisting of
thiophenyl, phenyl, furanyl, or benzothiophenyl and wherein such heteroaryl is
substituted with 0-3 J;
J independently at each occurrence is F, Cl, Br, I, OR', OC(0)N(V)2,
CN, CF3, OCF3, CHF2, NO2, R', 0, S, C(0), 5(0), methylenedioxY,
2
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
ethylenedioxy, N(11')2, N(11')CH2CH2OR1, SR', SOR', SO2R', SO2N(111)2, SO3R',
C(0)11.1, C(0)C(0)R', C(0)CH2C(0)R', C(S)R', C(0)OR', OC(0)R', OC(0)OR',
C(0)N(11')2, OC(0)N(R)2, C(S)N(R1)2, (CH2)0-2NHC(0)R', (CH2)0-2N(R1)2,
(CH2)o-2N(R')N(11')2, N(R')N(R')C(0)R', N(R)N(111)C(0)OR',
N(R')N(R)CON(R1)2, N(R)S02111, N(R')S02N(R')2, N(R')C(0)OR',
N(R')C(0)R', N(R')N(R'), N(R')C(S)R', N(R')C(0)N(11')2, N(R')C(S)N(11')2,
N(COR')COR', N(OR')R', C(=NH)N(R')2, C(0)N(OR')R', or C(=NOR')R',
wherein two J groups together can form a ring; wherein R' is independently at
each occurrence hydrogen or an alkyl, cycloalkyl, aryl, heterocyclyl, or
heteroaryl wherein any alkyl, cycloalkyl, aryl, heterocyclyl or heteroaryl is
substituted with 0-3 J; or wherein two R' groups together with a nitrogen atom
or
with two adjacent nitrogen atoms to which they are bonded can together form a
(C3-C8)heterocycly1 substituted with 0-3 J; optionally further comprising 1-3
additional heteroatoms selected from the group consisting of 0, N, S, S(0) and
S(0)2;
R5 is a mono- or bicyclic cycloalkyl, aryl, heterocyclyl, or heteroaryl;
each of which is substituted with 0-5 J, wherein any cycloalkyl, aryl,
heterocyclyl, or heteroaryl can be fused, bridged, or in a spiro configuration
with
one or more additional cycloalkyl, aryl, heterocyclyl, heteroaryl rings, any
of
which can be monocyclic, bicyclic or polycyclic, saturated, partially
unsaturated,
or aromatic, and any of which is substituted with 0-5 J;
R6 is cycloalkyl, aryl, heterocyclyl, or heteroaryl, wherein any
cycloalkyl, aryl, heterocyclyl, or heteroaryl is independently mono- or pluri-
substituted with J, (CI-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl, (C1-
C6)haloalkyl, hydroxyl, halo, (Ci-C6)haloalkoxy, cycloalkyl(Ci-C6)alkyl,
heterocyclyl(CI-C6) alkyl, aryl(CI-C6)alkyl, heteroaryl(Ci-C6)alkyl, OR3
wherein
R3 comprises H or (C1-C6)alkyl or NR42 wherein each R4 independently
comprises H or (CI-C6)alkyl or where two R4 groups together with a nitrogen
atom to which they are bonded form a (C3-C8)heterocycly1 which optionally
further comprises 1-3 heteroatoms selected from the group consisting of N, 0,
S,
S(0) and S(0)2; or R4 is optionally substituted cycloalkyl, optionally
substituted
aryl, optionally substituted heterocyclyl, or optionally substituted
heteroaryl;
wherein any alkyl, alkenyl, alkynyl, haloalkyl, alkoxy, haloalkoxy, R3,
R4, cycloalkyl, aryl, heterocyclyl, or heteroaryl can be further substituted
with J;
3
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
and
provided that (i), (ii), (iii) or (iv) applies:
(i) LI is bond or (CHR')õ and R5 is a bicyclic ring moiety which is
optionally substituted with 0-5 J where the bicyclic ring moiety is any one of
a-i
to a-xxviii wherein a wavy line indicates a point of attachment:
.
1 _
1131=1 /____3, N i RI
.
a-i a-ii a-iii a-iv
1 0 1_8N T 1-81
a-v a-vi a-vii a-viii
i 0 1 afr 1 41
N
N N
a-ix a-x a-xi
N i IN!
a-xii a-xiii a-xiv a-xv
N N y N N z 0 z
=
a-xvi a-xvii a-xviii a-xix a-xx
1 _____ CN 1 _____ 1
NO N J N N N . N, N NN
1 I
N
a-xxi a-xxii a-xxiii a-xxiv a-xxv
4
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
4
4 't sr
3j N
Aw¨
lik 1111 IF
a-xxvi a-xxvii a-xxviii
provided that when R5 is either a-xvii or a-xix that L2 is bond or
(CHR')õ ;
(ii) Li and L2 are each independently a bond or (CHR')õ; R5 is a 6-
membered heteroaryl ring moiety optionally substituted with 0-3 J1, wherein J1
is
OR', CF3, Cl, Br, F, CN, 0(Ci-C6)alkoxy, 0(Ci-C6)cycloalkoxy, alkyl, or N(R')2
and wherein the 6-membered heteroaryl ring moiety is any one of b-i to b-xiii
wherein a wavy line indicates a point of attachment:
H H
1 \ N (
H o
b-i b-ii -I b-iii J b-iv
o 0
'0
\ 0
N
¨/
b-v b-vi b-vii b-viii
1 N----7 1 (/¨N\)
¨/
--N
b-ix b-x b-xi
1u --N
N¨N 1n
b-xii b-xiii .
,
(iii) Li is a bond or (CHRI), and L2 is a heteroaryl substituted with 0-3 J
wherein the heteroaryl is c-i or c-u wherein a wavy line indicates a point of
attachment:
5
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
L'S CF
I 3 CF3
IN...-0/
I
\
R6 R6
C-i C-ii ;
or
(iv) Li is a bond or (CHR'),, and L2 is a bond or (CHR')n or a phenyl
substituted with 0-5 J; and R5 and R6 are independently selected from phenyl
or
heteroaryl each optionally substituted with 0-5 occurrences of J; provided
that if
L2 is a bond and R5 and R6 are both phenyl, then R5 is substituted with at
least '
one of 4-CN, 3-alkyl7NHR', 3- alkyl-OR' , 4-alkyl-OR', or 2,3-dialkyl and R6
is
substituted with at least 4-OR';
provided, that when (ii), (iii), or (iv) applies, that the compound of formula
(I) is
not one of the following:
N-C31>X=N?
ipo N ___________________________ N
Ni \ 0
sO
o/
F3C
N=----
/
N N War, \ 1110 /
N
0
0/----( I \
N-0
1---_-__
\ /
N
NI-\ 411 0/ N's \ 110
0
0 0
0
N,0 4. =
N
N-0
I /
N 11* 111 IIP
H2Ns, SI CF3 H2N--;S.::
0' `0 0/
6
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
OEt
OEt
NI-0/ 4.
OEt Nr
N..... N Nc)-Ni ilik OEt
\ z
N-N
1
OEt \ / N
\ N
N-0 lei . I/ . OEt
/ Et0
N) OEt
OMe WO S CF3
r-11-I N/ 11,
110 N
N- OMe *
N-0
* I NI . Of¨ WO 411
0
cO.L-N I
N
N-0 411 WO
r-.'=-i''11---"O'
I I
N,., N,.'
0-N I
\
S
WO I
N 111 N¨
r11-1\1/
I
N,,.. 0 u3
F3s., II Fõ .
e
¨
N'p N' p
¨N ¨N
41 I .
N
In various embodiments, a pharmaceutical composition comprising a
compound of the invention and a suitable excipient is provided.
In various combinations a pharmaceutical combination comprising a
compound of the invention and a second medicament is provided. In various
embodiments the second medicament is medically indicated for the treatment of
multiple sclerosis, transplant rejection, or adult respiratory distress
syndrome.
7
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
Various embodiments of the invention provide a use of a compound for
preparation of a medicament adapted for treatment of a disorder or a
malcondition wherein activation or inhibition of a sphingosine-l-phosphate
receptor subtype 1 is medically indicated, comprising a compound of formula
(II) or a pharmaceutically acceptable salt, prodrug, tautomer, stereoisomer,
hydrate, or solvate thereof:
Al - A2
..............( 3............ R6
R )6X k L/
L1 2
A3
(II)
wherein
a dashed line signifies that a single bond or a double bond can be present,
provided that there are two double bonds and three single bonds in the ring
comprising Al, A2, and A3;
Al, A2, and A3 each independently is C or 0 or is N when the N is
bonded to two adjacent ring atoms by a double bond and a single bond or is NR
wherein R is H or (CI-C6)alkyl when the N is bonded to two adjacent ring atoms
by two single bonds; provided that no more than one of AI, A2, and A3 isC and
that at least one of Al, A2, and A3 is N or NR; provided that only one of Ai,
A2,
and A3 is 0;
LI and L2 are each independently a bond; (CHR')n wherein R' is H or (C1-
C6)alkyl and n is 1, 2, or 3; or a heteroaryl selected from the group
consisting of
thiophenyl, phenyl, furanyl, or benzothiophenyl and wherein such heteroaryl is
substituted with 0-3 J;
J independently at each occurrence is F, Cl, Br, I, OR', OC(0)N(W)2,
CN, CF3, OCF3, CHF2, NO2, R', 0, S, C(0), S(0), methylenedioxy,
ethylenedioxy, N(11')2, N(R')CH2CH2OR', SR', SOR', SO2R', SO2N(R)2, SO3R',
C(0)R', C(0)C(0)R', C(0)CH2C(0)1V, C(S)R', C(0)OR', OC(0)W, OC(0)OR',
C(0)N(R')2, OC(0)N(R1)2, C(S)N(W)2, (CH2)0-2NHC(0)R% (CH2)0-2N(R1)2,
(CF12)o-2N(R')N(W)2, N(R')N(R')C(0)R', N(R)N(R)C(0)OR',
N(R')N(R')CON(11')2, N(R')S021V, N(R)S02N(R')2, N(R')C(0)OR',
8
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
N(R)C(0)R', N(R')N(R'), N(R')C(S)R', N(R')C(0)N(W)2, N(R')C(S)N(R')2,
N(COR')COR', N(OR')R', C(NH)N(R)2, C(0)N(OR')R', or C(=NOR')R',
wherein two J groups together can form a ring;. Wwherein R' is independently
at
each occurrence hydrogen or an alkyl, cycloalkyl, aryl, heterocyclyl, or
heteroaryl wherein any alkyl, cycloalkyl, aryl, heterocyclyl or heteroaryl is
substituted with 0-3 J; or wherein two R' groups together with a nitrogen atom
or
with two adjacent nitrogen atoms to which they are bonded can together form a
(C3-C8)heterocycly1 substituted with 0-3 J; optionally further comprising 1-3
additional heteroatoms selected from the group consisting of 0, N, S, S(0) and
S(0)2;
R5 is a mono- or bicyclic cycloalkyl, aryl, heterocyclyl, or heteroaryl;
each of which is substituted with 0-5 J, wherein any cycloalkyl, aryl,
heterocyclyl, or heteroaryl can be fused, bridged, or in a Spiro configuration
with
one or more additional cycloalkyl, aryl, heterocyclyl, heteroaryl rings, any
of
which can be monocyclic, bicyclic or polycyclic, saturated, partially
unsaturated,
or aromatic, and any of which is substituted with 0-5 J;
R6 is cycloalkyl, aryl, heterocyclyl, or heteroaryl, wherein any
cycloalkyl, aryl, heterocyclyl, or heteroaryl is independently mono- or pluri-
substituted with J, (Ci-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl, (C1-
C6)haloalkyl, hydroxyl, halo, (CI-C6)haloalkoxy, cycloalkyl(Ci-C6)alkyl,
heterocyclyl(CI-C6) alkyl, aryl(C1-C6)alkyl, heteroaryl(CI-C6)alkyl, OR3
wherein
R3 comprises H or (Ci-C6)alkyl or NR42 wherein each R4 independently
comprises H or (Ci-C6)alkyl or where two R4 groups together with a nitrogen
atom to which they are bonded form a (C3-C8)heterocycly1 which optionally
further comprises 1-3 heteroatoms selected from the group consisting of N, 0,
S,
S(0) and S(0)2; or R4 is optionally substituted cycloalkyl, optionally
substituted
aryl, optionally substituted heterocyclyl, or optionally substituted
heteroaryl;
wherein any alkyl, alkenyl, alkynyl, haloalkyl, alkoxy, haloalkoxy, R3,
R4, cycloalkyl, aryl, heterocyclyl, or heteroaryl can be further substituted
with J;
and
provided that (i), (ii), (iii) or (iv) applies:
(i) Li is bond or (CHR')õ and R5 is a bicyclic ring moiety which is
optionally substituted with 0-5 J where the bicyclic ring moiety is any one of
a-i
to a-xxviii wherein a wavy line indicates a point of attachment:
9
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
1 40 LeN1 1_ fe /___,N
ilk
a-i a-u a-iii a-iv
i = F8
E- , N
1 / \ N
E--,\
a-v a-vi a-vii a-viii
i = 1 = 1 0
N
N N
a-ix a-x a-xi
i
. /
N N 1 / \ 1
_
a-xii a-xiii a-xiv a-xv
1 ilfr afr = 41 0
N N,' N N 7 0 7
a-xvi a-xvii a-xviii a-xix a-xx
i N 1 ______________ = 1 4. 1 afr
N j N j N N I N. , N N N N
I
N
a-xxi a-xxii a-xxiii a-xxiv a-xxv
g
SC
. / \
11 4111 .
a-xxvi a-xxvii a-xxviii
provided that when R5 is either a-xvii or a-xix that L2 is bond or
(CHR')õ ;
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
(ii) LI and L2 are each independently a bond or (CHR')õ; R5 is a 6-
membered heteroaryl ring moiety optionally substituted with 0-3 J1, wherein J1
is
OR', CF3, Cl, Br, F, CN, 0(C1-C6)alkoxy, 0(CI-C6)cycloalkoxy, alkyl, or N(R1)2
and wherein the 6-membered heteroaryl ring moiety is any one of b-i to b-xiii
wherein a wavy line indicates a point of attachment:
(NI\ N
0
b-i b-ii b-iii J b-iv
o0
0\ 0
/ (0 <_)/¨No
0
b-v b-vi b-vii b-viii
N\)
< "N \;1µ1
¨/
¨N
b-ix b-x b-xi
N¨N
¨N
b-xii b-xiii
(iii) L1 is a bond or (CHR')õ, and L2 is a heteroaryl substituted with 0-3 J
wherein the heteroaryl is c-i or wherein a wavy line indicates a point of
attachment:
CF3 CF
/ 3
I I
R6 R6
C-i c-ii
or
(iv) Li is a bond or (CHR')n and L2 is a bond or (CHR')n or a phenyl
substituted with 0-5 J; and R5 and R6 are independently selected from phenyl
or
11
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
heteroaryl each optionally substituted with 0-5 occurrences of J; provided
that if
L2 is a bond and R5 and R6 are both phenyl, then R5 is substituted with at
least
one of 4-CN, 3-alkyl-NHR', 3- alkyl-OR' , 4-alkyl-OR', or 2,3-dialkyl and R6
is
substituted with at least 4-OR';
provided, that when (ii), (iii), or (iv) applies, that the compound of
formula (I) is not one of the following:
N\ . =
WC) N
H2Ns
. S cF3 H2Ns,
i N IP ii
,,
0- ,,0 0, 0
II
F3sar
¨
S Z
N' 1D
so .. .
1
N
0 CF3 H
F3C it
_
s 7
N r p
--N
SI
HN
Various embodiments of the invention provide a method of activation,
agonism, inhibition, or antagonism of a sphingosine- 1 -phosphate receptor
subtype 1 comprising contacting the receptor subtype 1 with an effective
amount
of a compound of formula (II) or a pharmaceutically acceptable salt, prodrug,
tautomer, stereoisomer, hydrate, or solvate thereof:
12
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
Al - A2
..............( 3............
R5
X C )
. ,= /
L1 . ,
. L2 R6
A3
(II)
wherein
a dashed line signifies that a single bond or a double bond can be present,
provided that there are two double bonds and three single bonds in the ring
comprising AI, A2, and A3;
Al, A2, and A3 each independently is C or 0 or is N when the N is
bonded to two adjacent ring atoms by a double bond and a single bond or is NR
wherein R is H or (CI-C6)alkyl when the N is bonded to two adjacent ring atoms
by two single bonds; provided that no more than one of Ai, A2, and A3 is C and
that at least one of Al, A2, and A3 is N or NR; provided that only one of Ai,
A2,
and A3 is 0;
Li and L2 are each independently a bond; (CHR')n wherein R' is H or (CI-
C6)alkyl and n is 1, 2, or 3; or a heteroaryl selected from the group
consisting of
thiophenyl, phenyl, furanyl, or benzothiophenyl and wherein such heteroaryl is
substituted with 0-3 J;
J independently at each occurrence is F, Cl, Br, I, OR', OC(0)N(R1)2,
CN, CF3, OCF3, CHF2, NO2, R', 0, S, C(0), S(0), methylenedioxy,
ethylenedioxy, N(R')2, N(R')CH2CH2OR', SR', SOR', SO2R', SO2N(R)2, SO3R',
C(0)R', C(0)C(0)R', C(0)CH2C(0)1V, C(S)R', C(0)OR', OC(0)R', OC(0)OR',
C(0)N(R1)2, OC(0)N(R)2, C(S)N(R)2, (CF12)0-2NHC(0)R', (CH2)0-2N(W)2,
(CH2)0-2N(W)N(R1)2, N(R')N(R')C(0)R', N(11')N(R')C(0)OR',
N(R')N(R')CON(R')2, N(R')S02R', N(R)S02N(R')2, N(R)C(0)0R1,
N(R')C(0)R', N(R')N(R'), N(R')C(S)R', N(R)C(0)N(RI)2, N(R')C(S)N(R)2,
N(COR')COR', N(OR')R', C(=NH)N(R')2, C(0)N(OR')R', or C(=NOR')R',
wherein two J groups together can form a ring; wherein R' is independently at
each occurrence hydrogen or an alkyl, cycloalkyl, aryl, heterocyclyl, or
heteroaryl wherein any alkyl, cycloalkyl, aryl, heterocyclyl or heteroaryl is
substituted with 0-3 J; or wherein two R' groups together with a nitrogen atom
or
with two adjacent nitrogen atoms to which they are bonded can together form a
13
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
(C3-C8)heterocycly1 substituted with 0-3 J; optionally further comprising 1-3
additional heteroatoms selected from the group consisting of 0, N, S, S(0) and
S(0)2;
R5 is a mono- or bicyclic cycloalkyl, aryl, heterocyclyl, or heteroaryl;
each of which is substituted with 0-5 J, wherein any cycloalkyl, aryl,
heterocyclyl, or heteroaryl can be fused, bridged, or in a spiro configuration
with
one or more additional cycloalkyl, aryl, heterocyclyl, heteroaryl rings, any
of
which can be monocyclic, bicyclic or polycyclic, saturated, partially
unsaturated,
or aromatic, and any of which is substituted with 0-5 J;
R6 is cycloalkyl, aryl, heterocyclyl, or heteroaryl, wherein any
cycloalkyl, aryl, heterocyclyl, or heteroaryl is independently mono- or pluri-
substituted with J, (Ci-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkYnYl, (C1-
C6)haloalkyl, hydroxyl, halo, (Ci-C6)haloalkoxy, cycloalkyl(Ci-C6)alkyl,
heterocyclyl(Ci-C6) alkyl, aryl(Ci-C6)alkyl, heteroaryl(Ci-C6)alkyl, OR3
wherein
R3 comprises H or (C1-C6)alkyl or NR42 wherein each R4 independently
comprises H or (Ci-C6)alkyl or where two R4 groups together with a nitrogen
atom to which they are bonded form a (C3-C8)heterocycly1 which optionally
further comprises 1-3 heteroatoms selected from the group consisting of N, 0,
S,
S(0) and S(0)2; or R4 is optionally substituted cycloalkyl, optionally
substituted
aryl, optionally substituted heterocyclyl, or optionally substituted
heteroaryl;
wherein any alkyl, alkenyl, alkynyl, haloalkyl, alkoxy, haloalkoxy, R3,
R4, cycloalkyl, aryl, heterocyclyl, or heteroaryl can be further substituted
with J;
and
provided that (i), (ii), (iii) or (iv) applies:
(i) Li is bond or (CHR')õ and R5 is a bicyclic ring moiety which is
optionally substituted with 0-5 J where the bicyclic ring moiety is any one of
a-i
to a-xxviii wherein a wavy line indicates a point of attachment:
14
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
11
1
.
a-i a-u a-iii a-iv
a-v a-vi a-vii a-viii
1 0 1 0 1 410
N
N N
a-ix a-x a-xi
N N
/
* * * *
a-xii a-xiii a-xiv a-xv
1 . 1 41 41 0
0
N N 7 N N 7 0 y
a-xvi a-xvii a-xviii a-xix a-xx
i 1 __ rN
N j N j N N I N . , N ,,/, N N N
I
N
a-xxi a-xxii a-xxiii a-xxiv a-xxv
4
4 't
N sr
sr LI-
/ \
_ /\ N
1111. Ilk .
a-xxvi a-xxvii a-xxviii
provided that when R5 is either a-xvii or a-xix that L2 is bond or
(CHR)õ ;
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
(ii) LI and L2 are each independently a bond or (CHR'); R5 is a 6-
membered heteroaryl ring moiety optionally substituted with 0-3 J1, wherein J1
is
OR', CF3, Cl, Br, F, CN, 0(Ci-C6)alkoxy, 0(CI-C6)cycloalkoxy, alkyl, or N(R')2
and wherein the 6-membered heteroaryl ring moiety is any one of b-i to b-xiii
wherein a wavy line indicates a point of attachment:
(N
___________________________________________________________ \ N 0
0
b-i b-ii b-iii b-iv
/00
R
/¨Ne
b-v b-vi b-vii b-viii
, ______________ N
N\
¨/
¨N
b-ix b-x b-xi
N¨N N
1
b-xii b-xiii
(iii) LI is a bond or (CHR'),õ and L2 is a heteroaryl substituted with 0-3 J
wherein the heteroaryl is c-i or wherein a wavy line indicates a point of
attachment:
L_sCF3 CF3
R6 R6
C-i C-ii
or
(iv) LI is a bond or (CHR'),, and L2 is a bond or (CHR')õ or a phenyl
substituted with 0-5 J; and R5 and R6 are independently selected from phenyl
or
16
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
heteroaryl each optionally substituted with 0-5 occurrences of J; provided
that if
L2 is a bond and R5 and R6 are both phenyl, then R5 is substituted with at
least
one of 4-CN, 3-alkyl-NHR', 3- alkyl-OR' , 4-alkyl-OR', or 2,3-dialkyl and R6
is
substituted with at least 4-OR';
provided, that when (ii), (iii), or (iv) applies, that the compound of
formula (I) is not one of the following:
N ,* 411
/
IIP
H,N, cF3
,s, H,N;s,
0- ,c, 0r 0
F3c
s
N
0--N -N
\ N-
140
CF3
F3C 114
S y
N p
Th
HN
In various embodiments, the above compound activates or agonizes, or
inhibits or antagonizes, the sphingosine-l-phosphate receptor subtype 1 to a
greater degree than the compound activates or agonizes, or inhibits or
antagonizes, another subtype of sphingosine-1 -phosphate receptor, for example
a
sphingosin-l-phosphate receptor subtype 3.
In various embodiments a method of treatment of a malcondition in a
patient for which activation or agonism or inhibition or antagonism of an S1P1
17
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
receptor is medically indicated, is provided, comprising administering an
effective amount of a compound as shown above to the patient to provide a
beneficial effect.
In various embodiment, selective activation or agonism of an S1P1
receptor, such as with respect to an Si P3 receptor, is medically indicated.
In
various embodiments, the malcondition comprises multiple sclerosis, transplant
rejection, or adult respiratory distress syndrome. In various embodiments,
selective inhibition or antagonism of an S1P1 receptor is medically indicated,
for
example, with respect to an Si P3 receptor.
Brief Description of the Drawings
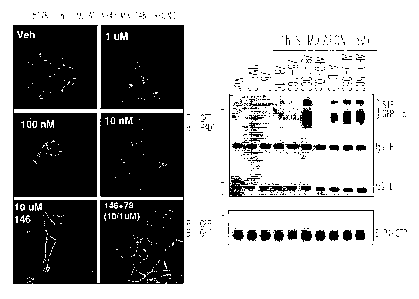
Figure 1 shows the results of a bioassay, as described in the Examples,
for S1P1 activation, involving detection of the ubiquination that is a
consequence of S1P1 activation. HEK 293-S1P1-GFP cell lysates were
immunoprecipitated (IP) and immunoblotted (TB) with P4D1(anti ubiquitin)
antibodies to detect S1P1-ubiquination. A. S1P1-GFP ubiquination was detected
as a band running between 64 and 82 kDa (lane 1 vehicle contro, lane 2 0.5 uM
AFD-R, Lane 2 vehicle control for SR-917, lane 4 1 uM SR917). B. Cellular
localization of S1P1-GFP with Veh(vehicle control, 0.01, 0.1 and 1 uM of SR-
917. C. S 1P1-GFP cells were labeled with P32 and stimulated with agonist.
S1P1-GFP was immunoprecipitated, resolved by PAGE, transferred to
nitrocellulose and exposed to Kodak XAR film overnight. Lane 1 Vehicle
control, Lanes 2 and 3, SIP at 0.5 and 0.05 uM, lanes 4 and 5, AFD-R at 0.5
and
0.05 uM, Lanes 6 and 7 SR-917 at 10 and 1 uM. SR-917 is a known agonist of
the S1P1 receptor, indexed in the NIH Molecular Libraries Small Molecule
Repository (MLMSR). Compound ID is 976135. It is commercially available
from ChemBridge Screening Library.
Figure 2 shows compound 32 robustly induces internalization and
polyubiquination, and these effects are blocked by the S1P1 antagonist, W146R.
Figure 3 shows that compound 236, like other compounds in the series,
induces S1P1 polyubiquination.
Figure 4 shows compound 236 induces lymphopenia in mice. The
compound was dissolved in 10%DMSO, Tween-20 and delivered by gavage.
18
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
Figure 5 shows a pharmacokinetic study of SR-917 lmg/mL in 10/10/80
DMSO/Tween/Water delivered 1 mg/kg i.v.
Figure 6 shows S1P1 Polar Ligand Binding Pocket Mutations. CHO cells
were transfected with SIP I cDNA constructs. Cells were serum starved
overnight and stimulated with 3-fold serial dilutions of of SIP or CYM-5442.
ERK1/2 phosphorylation was detected with the Phospho-ERK ELISA (Cell
Signaling). A. S1P1 mutants E121A and R292A. B. wild type S1P1 (wt) and
S1P1 mutantsR120A.
Detailed Description
As used in the specification and the appended claims, the singular forms
"a," "an" and "the" include plural referents unless the context clearly
dictates
otherwise.
As used herein, "individual" (as in the subject of the treatment) means
both mammals and non-mammals. Mammals include, for example, humans;
non-human primates, e.g. apes and monkeys; cattle; horses; sheep; and goats.
Non-mammals include, for example, fish and birds.
The term "S1P1" as used herein refers to subtype 1 of a sphingosine-1-
phosphate receptor, while other sphingosine-l-phosphate receptor subtypes are
referred to in a corresponding manner, for example, sphingosine-1 -phosphate
receptor subtype 3 is referred to as "S1P3".
A "receptor", as is well known in the art, is a biomolecular entity usually
comprising a protein that specifically binds a structural class of ligands or
a
single native ligand in a living organism, the binding of which causes the
receptor to transduce the binding signal into another kind of biological
action,
such as signaling a cell that a binding event has occurred, which causes the
cell
to alter its function in some manner. An example of transduction is receptor
binding of a ligand causing alteration of the activity of a "G-protein" in the
cytoplasm of a living cell that is coupled with the receptor. Any molecule,
naturally occurring or not, that binds to a receptor and activates it for
signal
transduction, is referred to as an "agonist" or "activator." Any molecule,
naturally occurring or not, that binds to a receptor, but does not cause
signal
transduction to occur, and which can block the binding of an agonist and its
consequent signal transduction, is referred to as an "antagonist."
19
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
An "S1P1 compound" or "S1P1 agonist" or "S1P1 activator" or "S1P1
inhibitor" or "S1P1 antagonist" as the terms are used herein refer to
compounds
that interact in some way with the SIP receptor subtype 1. They can be agonist
or activators, or they can be antagonists or inhibitors. An "S1P1 compound" of
the invention can be selective for action on subtype 1 of the SIP receptor
family;
for example a compound of the invention can act at a lower concentration on
subtype 1 of the S 1P receptor family than on other subtypes of the SIP
receptor
family; more specifically, an "S1P I compound" of the invention can
selectively
act on subtype 1 receptors compared to its action on subtype 3, or "S1P3"
receptors.
In certain embodiments, compounds of the invention are orthostatic
agonists. In certain other embodiments, compounds of the invention are
allosteric agonists. Receptor agonists may be classified as orthosteric or
allosteric. An orthosteric agonist binds to a site in the receptor that
significantly
overlaps with the binding of the natural ligand and replicates the key
interactions
of the natural ligand with the receptor. An orthosteric agonist will activate
the
receptor by a molecular mechanism similar to that of the natural ligand, will
be
competitive for the natural ligand, and will be competitively antagonized by
pharmacological agents that are competitive antagonists for the natural
ligand.
An allosteric agonist binds to a site in the receptor that makes some
significant
interactions that are partly or wholly non-overlapping with the natural
ligand.
Allosteric agonists are true agonists and not allosteric potentiators.
Consequently, they activate receptor signaling alone and without a requirement
for a sub-maximal concentration of the natural ligand. Allosteric agonists may
be identified when an antagonist known to be competitive for the orthosteric
ligand shows non-competitive antagonism. The allosteric agonist site can also
be
mapped by receptor mutagenesis. The introduction of single point mutations in
receptors that retain receptor activation by allosteric agonist, while
diminishing
or abolishing signaling induced by orthosteric agonist or vice versa provide
formal evidence for differences in binding interactions. Orthosteric agonists
may destabilize GPCR ("G-protein coupled receptor") structure and
conformation, while allosteric agonists may either stabilize or destabilize
GPCR
structure and conformation. Allosteric agonists, by virtue of their different
interactions with receptor, may be pharmaceutically useful because the
allosteric
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
site may confer additional opportunities for agonist potency and selectivity
within a related family of receptor subtypes that share a similar orthosteric
ligand. In addition, the allosteric site may require very different physical
and
chemical properties of an agonist compared to the orthosteric ligand. These
-- chemico-physical properties, which include hydrophobicity, aromaticity,
charge
distribution and solubility may also provide advantages in generating agonists
of
varying pharmacokinetic, oral bioavailability, distributional and metabolism
profiles that facilitate the development of effective pharmaceutical
substances.
"Substantially" as the term is used herein means completely or almost
-- completely; for example, a composition that is "substantially free" of a
component either has none of the component or contains such a trace amount
that any relevant functional property of the composition is unaffected by the
presence of the trace amount; or a compound that is "substantially pure" has
only
negligible traces of impurities present.
"Treating" or "treatment" within the meaning herein refers to an
alleviation of symptoms associated with a disorder, malcondition, or disease,
or
inhibition of further progression or worsening of those symptoms, or
prevention
or prophylaxis of the disorder, malcondition, or disease.
The expression "effective amount", when used to describe use of a
-- compound of the invention in providing therapy to a patient suffering from
a
disorder or malcondition mediated by a sphingosine- 1 -phospate receptor of
subtype 1 refers to the amount of a compound of the invention that is
effective to
bind as an agonist or as an antagonist to an S1P1 receptor in the individual's
tissues, wherein the Si P1 receptor is implicated in the disorder, wherein
such
-- binding occurs to an extent sufficient to produce a beneficial therapeutic
effect
on the patient. Similarly, as used herein, an "effective amount" or a
"therapeutically effective amount" of a compound of the invention refers to an
amount of the compound that alleviates, in whole or in part, symptoms
associated with the disorder or malcondition, or halts or slows further
-- progression or worsening of those symptoms, or prevents or provides
prophylaxis for the disorder or malcondition. In particular, a
"therapeutically
effective amount" refers to an amount effective, at dosages and for periods of
time necessary, to achieve the desired therapeutic result by acting as an
agonist
or activator of sphingosine-l-phosphate receptor subtype 1 (Si P1) activity. A
21
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
therapeutically effective amount is also one in which any toxic or detrimental
effects of compounds of the invention are outweighed by the therapeutically
beneficial effects. For example, in the context of treating a malcondition
mediated by activation of Si P1, a therapeutically effective amount of an Si
P1
agonist of the invention is an amount sufficient to control the malcondition,
to
mitigate the progress of the malcondition, or to relieve the symptoms of the
malcondition. Examples of malconditions that can be so treated include
multiple
sclerosis, transplant rejection, and adult respiratory distress syndrome.
All chiral, diastereomeric, racemic forms of a structure are intended,
unless a particular stereochemistry or isomeric form is specifically
indicated.
Compounds used in the present invention can include enriched or resolved
optical isomers at any or all asymmetric atoms as are apparent from the
depictions, at any degree of enrichment. Both racemic and diastereomeric
mixtures, as well as the individual optical isomers can be isolated or
synthesized
so as to be substantially free of their enantiomeric or diastereomeric
partners,
and these are all within the scope of the invention.
Isomerism and Tautomerism in Compounds of the Invention
Tautomerism
Within the present invention it is to be understood that a compound of
the formula I or a salt thereof may exhibit the phenomenon of tautomerism
whereby two chemical compounds that are capable of facile interconversion by
exchanging a hydrogen atom between two atoms, to either of which it forms a
covalent bond. Since the tautomeric compounds exist in mobile equilibrium
with each other they may be regarded as different isomeric forms of the same
compound. It is to be understood that the formula drawings within this
specification can represent only one of the possible tautomeric forms.
However,
it is also to be understood that the invention encompasses any tautomeric form
which acts on SIP receptors, such as SIP subtype 1 receptors, and is not to be
limited merely to any one tautomeric form utilized within the formulae
drawings. The formula drawings within this specification can represent only
one
of the possible tautomeric forms and it is to be understood that the
specification
encompasses all possible tautomeric forms of the compounds drawn not just
those forms which it has been convenient to show graphically herein. For
22
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
example, tautomerism may be exhibited by a pyrazolyl group bonded as
indicated by the wavy line. While both substituents would be termed a 4-
pyrazolyl group, it is evident that a different nitrogen atom bears the
hydrogen
atom in each structure.
N HN\1
HN\ 5 N ¨
Such tautomerism can also occur with substituted pyrazoles such as 3-
methyl, 5-methyl, or 3,5-dimethylpyrazoles, and the like.
Optical Isomerism
It will be understood that when compounds of the present invention
contain one or more chiral centers, the compounds may exist in, and may be
isolated as pure enantiomeric or diastereomeric forms or as racemic mixtures.
The present invention therefore includes any possible enantiomers,
diastereomers, racemates or mixtures thereof of the compounds of the invention
which are biologically active in the treatment of Si P1 mediated diseases.
The isomers resulting from the presence of a chiral center comprise a pair
of non-superimposable isomers that are called "enantiomers." Single
enantiomers of a pure compound are optically active, i.e., they are capable of
rotating the plane of plane polarized light. Single enantiomers are designated
according to the Cahn-Ingold-Prelog system. Once the priority ranking of the
four groups is determined, the molecule is oriented so that the lowest ranking
group is pointed away from the viewer. Then, if the descending rank order of
the other groups proceeds clockwise, the molecule is designated (R) and if the
descending rank of the other groups proceeds counterclockwise, the molecule is
designated (S). In the example in Scheme 14, the Cahn-Ingold-Prelog ranking is
A> B > C > D. The lowest ranking atom, D is oriented away from the viewer.
A A
C\B
(R) configuration (S) configuration
The present invention is meant to encompass diastereomers as well as
their racemic and resolved, diastereomerically and enantiomerically pure forms
and salts thereof. Diastereomeric pairs may be resolved by known separation
23
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
techniques including normal and reverse phase chromatography, and
crystallization.
"Isolated optical isomer" or "isolated enantiomer" means a compound
which has been substantially purified from the corresponding optical isomer
(enantiomer) of the same formula. Preferably, the isolated isomer is at least
about 80% pure, more preferably at least 90% pure, even more preferably at
least
98% pure, most preferably at least about 99% pure, by weight.
Isolated optical isomers may be purified from racemic mixtures by
well-known chiral separation techniques. According to one such method, a
racemic mixture of a compound of the invention, or a chiral intermediate
thereof,
is separated into 99% wt.% pure optical isomers by HPLC using a suitable
chiral
column, such as a member of the series of DAICEL CHIRALPAK family of
columns (Daicel Chemical Industries, Ltd., Tokyo, Japan). The column is
operated according to the manufacturer's instructions.
Rotational Isomerism
It is understood that due to chemical properties (i.e., resonance lending
some double bond character to the C-N bond) of restricted rotation about the
amide bond linkage (as illustrated below), among other types of bonds, it is
possible to observe separate rotamer species and even, under some
circumstances, to isolate such species, example shown below. It is further
understood that certain structural elements, including steric bulk or
substituents
on the amide nitrogen, may enhance the stability of a rotamer to the extent
that a
compound may be isolated as, and exist indefinitely, as a single stable
rotamer.
The present invention therefore includes any possible stable rotamers of
compounds of the invention which are biologically active in the treatment of
cancer or other proliferative disease states.
0 A0
hindered rotation
> _____________________ NI\ ________________________ N
A
D. Regioisomerism
The preferred compounds of the present invention have a particular
spatial arrangement of substituents on the aromatic rings, which is related to
the
structure activity relationship demonstrated by the compound class. Often such
24
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
substitution arrangement is denoted by a numbering system; however,
numbering systems are often not consistent between different ring systems. In
six-membered aromatic systems, the spatial arrangements are specified by the
common nomenclature "para" for 1,4-substitution, "meta" for 1,3-substitution
and "ortho" for 1,2-substitution as shown below.
P
M 10 M
0 10 0
. . .
"para." "meta." "ortho."
The compounds of the invention may contain one or more stereo genic
(chiral) or asymmetric centers, such as one or more asymmetric carbon atoms.
Substituents at a double bond may be present in cis- ("Z") or trans ("E") form
10 unless indicated otherwise. Substituents on a ring can likewise be
disposed cis
or trans to each other, or a mixture thereof The compounds of the invention
may thus be present as mixtures of stereoisomers or preferably as
substantially
pure stereoisomers. Pure stereoisomers may be obtained by separating
stereoisomer mixtures or by stereoselective or stereospecific
syntheses in manners known to those skilled in the art.
All structures encompassed within a claim are "chemically feasible", by
which is meant that the structure depicted by any combination or
subcombination of optional substituents meant to be recited by the claim is
physically capable of existence with at least some stability as can be
determined
by the laws of structural chemistry and by experimentation. Structures that
are
not chemically feasible are not within a claimed set of compounds.
When a substituent is specified to be an atom or atoms of specified
identity, "or a bond", a configuration is referred to when the substituent is
"a
bond" that the groups that are immediately adjacent to the specified
substituent
are directly connected to each other by a chemically feasible bonding
configuration.
In general, "substituted" refers to an organic group as defined herein in
which one or more bonds to a hydrogen atom contained therein are replaced by
one or more bonds to a non-hydrogen atom such as, but not limited to, a
halogen
(i.e., F, Cl, Br, and I); an oxygen atom in groups such as hydroxyl groups,
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
alkoxy groups, aryloxy groups, aralkyloxy groups, oxo(carbonyl) groups,
carboxyl groups including carboxylic acids, carboxylates, and carboyxlate
esters;
a sulfur atom in groups such as thiol groups, alkyl and aryl sulfide groups,
sulfoxide groups, sulfone groups, sulfonyl groups, and sulfonamide groups; a
nitrogen atom in groups such as amines, hydroxylamines, nitriles, nitro
groups,
N-oxides, hydrazides, azides, and enamines; and other heteroatoms in various
other groups. Non-limiting examples of substituents that can be bonded to a
substituted carbon (or other) atom include F, Cl, Br, I, OR', OC(0)N(R')2, CN,
CF3, OCF3, R', 0, S, C(0), S(0), methylenedioxy, ethylenedioxy, N(R)2, SR',
SOR', SO2R', SO2N(R)2, SO3R', C(0)R', C(0)C(0)R', C(0)CH2C(0)R', C(S)R',
C(0)OR', OC(0)R', C(0)N(R)2, OC(0)N(R)2, C(S)N(R')2, (CH2)0-2NHC(0)1V,
(CH2)0-2N(R)N(R)2, N(R)N(R)C(0)RI, N(R)1\1(R)C(0)OR',
N(R')N(R')CON(R')2, N(R)S02R', N(U)S02N(R')2, N(R)C(0)OR',
N(R)C(0)R', N(R')C(S)R', N(R)C(0)N(R)2, N(R)C(S)N(R)2, N(COR')COR',
N(OR')R', C(=NH)N(R')2, C(0)N(OR)R1, or C(=NOR')R' wherein R' can be
hydrogen or a carbon-based moiety, and wherein the carbon-based moiety can
itself be further substituted.
Substituted alkyl, alkenyl, alkynyl, cycloalkyl, and cycloalkenyl groups
as well as other substituted groups also include groups in which one or more
bonds to a hydrogen atom are replaced by one or more bonds, including double
or triple bonds, to a carbon atom, or to a heteroatom such as, but not limited
to,
oxygen in carbonyl (oxo), carboxyl, ester, amide, imide, urethane, and urea
groups; and nitrogen in imines, hydroxyimines, oximes, hydrazones, amidines,
guanidines, and nitriles.
Substituted ring groups such as substituted aryl, heterocyclyl and
heteroaryl groups also include rings and fused ring systems in which a bond to
a
hydrogen atom is replaced with a bond to a carbon atom. Therefore, substituted
aryl, heterocyclyl and heteroaryl groups can also be substituted with alkyl,
alkenyl, cycloalkyl, aryl, heteroaryl, and alkynyl groups as defined herein,
which
can themselves be further substituted.
The term "heteroatoms" as used herein refers to non-carbon and non-
hydrogen atoms, capable of forming covalent bonds with carbon, and is not
otherwise limited. Typical heteroatoms are N, 0, and S. When sulfur (S) is
referred to, it is understood that the sulfur can be in any of the oxidation
states in
26
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
which it is found, thus including sulfoxides (R-S(0)-R') and sulfones (R-S(0)2-
R'), unless the oxidation state is specified; thus, the term "sulfone"
encompasses
only the sulfone form of sulfur; the term "sulfide" encompasses only the
sulfide
(R-S-R') form of sulfur. When the phrases such as "heteroatoms selected from
the group consisting of 0, NH, NR' and S," or "[variable] is 0, S. . ." are
used,
they are understood to encompass all of the sulfide, sulfoxide and sulfone
oxidation states of sulfur.
Alkyl groups include straight chain and branched alkyl groups and
cycloalkyl groups having from 1 to about 20 carbon atoms, and typically from 1
to 12 carbons or, in some embodiments, from 1 to 8 carbon atoms. Examples of
straight chain alkyl groups include those with from 1 to 8 carbon atoms such
as
methyl, ethyl, n-propyl, n-butyl, n-pentyl, n-hexyl, n-heptyl, and n-octyl
groups.
Examples of branched alkyl groups include, but are not limited to, isopropyl,
iso-butyl, sec-butyl, t-butyl, neopentyl, isopentyl, and 2,2-dimethylpropyl
groups. Representative substituted alkyl groups can be substituted one or more
times with any of the groups listed above, for example, amino, hydroxy, cyano,
carboxy, nitro, thio, alkoxy, and halogen groups.
Cycloalkyl groups are alkyl groups forming a ring structure, which can
be substituted or unsubstituted. Examples of cycloalkyl include, but are not
limited to, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, and
cyclooctyl groups. In some embodiments, the cycloalkyl group has 3 to 8 ring
members, whereas in other embodiments the number of ring carbon atoms range
from 3 to 5, 3 to 6, or 3 to 7. Cycloalkyl groups further include polycyclic
cycloalkyl groups such as, but not limited to, norbornyl, adamantyl, bornyl,
camphenyl, isocamphenyl, and carenyl groups, and fused rings such as, but not
limited to, decalinyl, and the like. Cycloalkyl groups also include rings that
are
substituted with straight or branched chain alkyl groups as defined above.
Representative substituted cycloalkyl groups can be mono-substituted or
substituted more than once, such as, but not limited to, 2,2-, 2,3-, 2,4- 2,5-
or
2,6-disubstituted cyclohexyl groups or mono-, di- or tri-substituted norbornyl
or
cycloheptyl groups, which can be substituted with, for example, amino,
hydroxy,
cyano, carboxy, nitro, thio, alkoxy, and halogen groups.
The terms "carbocyclic" and "carbocycle" denote a ring structure wherein
the atoms of the ring are carbon. In some embodiments, the carbocycle has 3 to
27
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
8 ring members, whereas in other embodiments the number of ring carbon atoms
is 4, 5, 6, or 7. Unless specifically indicated to the contrary, the
carbocyclic ring
can be substituted with as many as N substituents wherein N is the size of the
carbocyclic ring with for example, amino, hydroxy, cyano, carboxy, nitro,
thio,
alkoxy, and halogen groups.
(Cycloalkyl)alkyl groups, also denoted cycloalkylalkyl, are alkyl groups
as defined above in which a hydrogen or carbon bond of the alkyl group is
replaced with a bond to a cycloalkyl group as defined above.
Alkenyl groups include straight and branched chain and cyclic alkyl
groups as defined above, except that at least one double bond exists between
two
carbon atoms. Thus, alkenyl groups have from 2 to about 20 carbon atoms, and
typically from 2 to 12 carbons or, in some embodiments, from 2 to 8 carbon
atoms. Examples include, but are not limited
to -CH=CH(CH3), -CH=C(CH3)2, -C(CH3)=CH2, -C(CH3)=CH(CH3),
-C(CH2CH3)=CH2, vinyl, cyclohexenyl, cyclopentenyl, cyclohexadienyl,
butadienyl, pentadienyl, and hexadienyl among others.
The term "cycloalkenyl" alone or in combination denotes a cyclic alkenyl
group wherein at least one double bond is present in the ring structure.
Cycloalkenyl groups include cycloalkyl groups having at least one double bond
between two adjacent carbon atoms. Thus for example, cycloalkenyl groups
include but are not limited to cyclohexenyl, cyclopentenyl, and
cyclohexadienyl
groups.
(Cycloalkenyl)alkyl groups are alkyl groups as defined above in which a
hydrogen or carbon bond of the alkyl group is replaced with a bond to a
cycloalkenyl group as defined above.
Alkynyl groups include straight and branched chain alkyl groups, except
that at least one triple bond exists between two carbon atoms. Thus, alkynyl
groups have from 2 to about 20 carbon atoms, and typically from 2 to 12
carbons
or, in some embodiments, from 2 to 8 carbon atoms. Examples include, but are
not limited to ¨CaCH, -CC(CH3), -
C-C(CH2CH3), -CH2CmCH, -CH2C-C(CH3), and -CH2CmC(CH2CH3), among
others.
Aryl groups are cyclic aromatic hydrocarbons that do not contain
heteroatoms. Thus aryl groups include, but are not limited to, phenyl,
azulenyl,
28
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
heptalenyl, biphenyl, indacenyl, fluorenyl, phenanthrenyl, triphenylenyl,
pyrenyl, naphthacenyl, chrysenyl, biphenylenyl, anthracenyl, and naphthyl
groups. In some embodiments, aryl groups contain 6-14 carbons in the ring
portions of the groups. The phrase "aryl groups" includes groups containing
fused rings, such as fused aromatic-aliphatic ring systems (e.g., indanyl,
tetrahydronaphthyl, and the like), and also includes substituted aryl groups
that
have other groups, including but not limited to alkyl, halo, amino, hydroxy,
cyano, carboxy, nitro, thio, or alkoxy groups, bonded to one of the ring
atoms.
Representative substituted aryl groups can be mono-substituted or substituted
more than once, such as, but not limited to, 2-, 3-, 4-, 5-, or 6-substituted
phenyl
or naphthyl groups, which can be substituted with groups including but not
limited to those listed above.
Aralkyl groups are alkyl groups as defined above in which a hydrogen or
carbon bond of an alkyl group is replaced with a bond to an aryl group as
defined above. Representative aralkyl groups include benzyl and phenylethyl
groups and fused (cycloalkylaryl)alkyl groups such as 4-ethyl-indanyl. The
aryl
moiety or the alkyl moiety or both are optionally substituted with other
groups,
including but not limited to alkyl, halo, amino, hydroxy, cyano, carboxy,
nitro,
thio, or alkoxy groups. Aralkenyl group are alkenyl groups as defined above in
which a hydrogen or carbon bond of an alkyl group is replaced with a bond to
an
aryl group as defined above.
Heterocyclyl groups include aromatic and non-aromatic ring compounds
containing 3 or more ring members, of which one or more is a heteroatom such
as, but not limited to, N, 0, S, or P. In some embodiments, heterocyclyl
groups
include 3 to 20 ring members, whereas other such groups have 3 to 15 ring
members. At least one ring contains a heteroatom, but every ring in a
polycyclic
system need not contain a heteroatom. For example, a dioxolanyl ring and a
benzdioxolanyl ring system (methylenedioxyphenyl ring system) are both
heterocyclyl groups within the meaning herein. A heterocyclyl group designated
as a C2-heterocyclyl can be a 5-ring with two carbon atoms and three
heteroatoms, a 6-ring with two carbon atoms and four heteroatoms and so forth.
Likewise a C4-heterocyclyl can be a 5-ring with one heteroatom, a 6-ring with
two heteroatoms, and so forth. The number of carbon atoms plus the number of
heteroatoms sums up to equal the total number of ring atoms.
29
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
The phrase "heterocyclyl group" includes fused ring species including those
having fused aromatic and non-aromatic groups. The phrase also includes
polycyclic ring systems containing a heteroatom such as, but not limited to,
quinuclidyl and also includes heterocyclyl groups that have substituents,
including but not limited to alkyl, halo, amino, hydroxy, cyano, carboxy,
nitro,
thio, or alkoxy groups, bonded to one of the ring members. A heterocyclyl
group as defined herein can be a heteroaryl group or a partially or completely
saturated cyclic group including at least one ring heteroatom. Heterocyclyl
groups include, but are not limited to, pyrrolidinyl, furanyl,
tetrahydrofuranyl,
dioxolanyl, piperidinyl, piperazinyl, morpholinyl, pyrrolyl, pyrazolyl,
triazolyl,
tetrazolyl, oxazolyl, isoxazolyl, thiazolyl, pyridinyl, thiophenyl,
benzothiophenyl, benzofuranyl, dihydrobenzofuranyl, indolyl, dihydroindolyl,
azaindolyl, indazolyl, benzimidazolyl, azabenzimidazolyl, benzoxazolyl,
benzothiazolyl, benzothiadiazolyl, imidazopyridinyl, isoxazolopyridinyl,
thianaphthalenyl, purinyl, xanthinyl, adeninyl, guaninyl, quinolinyl,
isoquinolinyl, tetrahydroquinolinyl, quinoxalinyl, and quinazolinyl groups.
Heterocyclyl groups can be substituted. Representative substituted
heterocyclyl
groups can be mono-substituted or substituted more than once, including but
not
limited to, rings containing at least one heteroatom which are mono, di, tri,
tetra,
penta, hexa, or higher-substituted with substituents such as those listed
above,
including but not limited to alkyl, halo, amino, hydroxy, cyano, carboxy,
nitro,
thio, and alkoxy groups.
Heteroaryl groups are aromatic ring compounds containing 5 or more
ring members, of which, one or more is a heteroatom such as, but not limited
to,
N, 0, and S. A heteroaryl group designated as a C2-heteroaryl can be a 5-ring
with two carbon atoms and three heteroatoms, a 6-ring with two carbon atoms
and four heteroatoms and so forth. Likewise a C4-heteroaryl can be a 5-ring
with
one heteroatom, a 6-ring with two heteroatoms, and so forth. The number of
carbon atoms plus the number of heteroatoms sums up to equal the total number
of ring atoms. Heteroaryl groups include, but are not limited to, groups such
as
pyrrolyl, pyrazolyl, triazolyl, tetrazolyl, oxazolyl, isoxazolyl, thiazolyl,
pyridinyl, thiophenyl, benzothiophenyl, benzofuranyl, indolyl, azaindolyl,
indazolyl, benzimidazolyl, azabenzimidazolyl, benzoxazolyl, benzothiazolyl,
benzothiadiazolyl, imidazopyridinyl, isoxazolopyridinyl, thianaphthalenyl,
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
purinyl, xanthinyl, adeninyl, guaninyl, quinolinyl, isoquinolinyl,
tetrahydroquinolinyl, tetrahydroisoquinolinyl, quinoxalinyl, and quinazolinyl
groups. The terms "heteroaryl" and "heteroaryl groups" include fused ring
compounds such as wherein at least one ring, but not necessarily all rings,
are
aromatic, including tetrahydroquinolinyl, tetrahydroisoquinolinyl, indolyl and
2,3-dihydro indolyl. The term also includes heteroaryl groups that have other
groups bonded to one of the ring members, including but not limited to alkyl,
halo, amino, hydroxy, cyano, carboxy, nitro, thio, or alkoxy groups.
Representative substituted heteroaryl groups can be substituted one or more
times with groups such as those listed above.
Additional examples of aryl and heteroaryl groups include but are not
limited to phenyl, biphenyl, indenyl, naphthyl (1-naphthyl, 2-naphthyl), N-
hydroxytetrazolyl, N-hydroxytriazolyl, N-hydroxyimidazolyl, anthracenyl (1-
anthracenyl, 2-anthracenyl, 3-anthracenyl), thiophenyl (2-thienyl, 3-thienyl),
furyl (2-fury!, 3-furyl) , indolyl, oxadiazolyl, isoxazolyl, quinazolinyl,
fluorenyl,
xanthenyl, isoindanyl, benzhydryl, acridinyl, thiazolyl, pyrrolyl (2-
pyrroly1),
pyrazolyl (3-pyrazoly1), imidazolyl (1-imidazolyl, 2-imidazolyl, 4-imidazolyl,
5-
imidazolyl), triazolyl (1,2,3-triazol-1-yl, 1,2,3-triazol-2-y1 1,2,3-triazol-4-
yl,
1,2,4-triazol-3-y1), oxazolyl (2-oxazolyl, 4-oxazolyl, 5-oxazoly1), thiazolyl
(2-
thiazolyl, 4-thiazolyl, 5-thiazoly1), pyridyl (2-pyridyl, 3-pyridyl, 4-
pyridy1),
pyrimidinyl (2-pyrimidinyl, 4-pyrimidinyl, 5-pyrimidinyl, 6-pyrimidinyl),
pyrazinyl, pyridazinyl (3- pyridazinyl, 4-pyridazinyl, 5-pyridazinyl),
quinolyl (2-
quinolyl, 3-quinolyl, 4-quinolyl, 5-quinolyl, 6-quinolyl, 7-quinolyl, 8-
quinoly1),
isoquinolyl (1-isoquinolyl, 3-isoquinolyl, 4-isoquinolyl, 5-isoquinolyl, 6-
isoquinolyl, 7-isoquinolyl, 8-isoquinoly1), benzo[b]furanyl (2-
benzo[b]furanyl,
3-benzo[b]furanyl, 4-benzo[b]furanyl, 5-benzo[b]furanyl, 6-benzo[b]furanyl, 7-
benzo[b]fiiranyl), 2,3-dihydro-benzo[b]furanyl (2-(2,3-dihydro-
benzo[b]furanyl), 3-(2,3-dihydro-benzo[b]furanyl), 4-(2,3-dihydro-
benzo[b]fiiranyl), 5-(2,3-dihydro-benzo[b]furanyl), 6-(2,3-dihydro-
benzo[b]fin-anyl), 7-(2,3-dihydro-benzo[b]furanyl), benzo[b]thiophenyl (2-
benzo[b]thiophenyl, 3-benzo[b]thiophenyl, 4-benzo[b]thiophenyl,
5-benzo[b]thiophenyl, 6-benzo[b]thiophenyl, 7-benzo[b]thiophenyl),
2,3-dihydro-benzo[b]thiophenyl, (2-(2,3-dihydro-benzo[b]thiophenyl), 3-(2,3-
dihydro-benzo[b]thiophenyl), 4-(2,3-dihydro-benzo[b]thiophenyl), 5-(2,3-
31
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
dihydro-benzo[b]thiophenyl), 6-(2,3-dihydro-benzo[b]thiophenyl), 7-(2,3-
dihydro-benzo[b]thiophenyl), indolyl (1-indolyl, 2-indolyl, 3-indolyl, 4-
indolyl,
5-indolyl, 6-indolyl, 7-indoly1), indazole (1-indazolyl, 3-indazolyl, 4-
indazolyl,
5-indazolyl, 6-indazolyl, 7-indazoly1), benzimidazolyl (1-benzimidazolyl,
2-benzimidazolyl, 4-benzimidazolyl, 5-benzimidazolyl, 6-benzimidazolyl,
7-benzimidazolyl, 8-benzimidazoly1), benzoxazolyl (1-benzoxazolyl, 2-
benzoxazolyl), benzothiazolyl (1-benzothiazolyl, 2-benzothiazolyl, 4-
benzothiazolyl, 5-benzothiazolyl, 6-benzothiazolyl, 7-benzothiazoly1),
carbazolyl (1-carbazolyl, 2-carbazolyl, 3-carbazolyl, 4-carbazoly1),
5H-dibenz[b,f]azepine (5H-dibenz[b,f]azepin-1-yl, 5H-dibenz[b,f]azepine-2-yl,
5H-dibenz[b,f]azepine-3-yl, 5H-dibenz[b,f]azepine-4-yl, 5H-dibenz[b,f]azepine-
5-y1), 10,11-dihydro-5H-dibenz[b,f]azepine (10,11-dihydro-5H-
dibenz[b,flazepine-1-yl, 10,11-dihydro-5H-dibenz[b,f]azepine-2-yl, 10,11-
dihydro-5H-dibenz[b,f]azepine-3-yl, 10,11-dihydro-5H-dibenz[b,f]azepine-4-yl,
10,11-dihydro-5H-dibenz[b,flazepine-5-y1), and the like.
Heterocyclylalkyl groups are alkyl groups as defined above in which a
hydrogen or carbon bond of an alkyl group is replaced with a bond to a
heterocyclyl group as defined above. Representative heterocyclyl alkyl groups
include, but are not limited to, furan-2-y1 methyl, furan-3-y1 methyl,
pyridine-2-
yl methyl (a-picolyl), pyridine-3-y1 methyl (13-picoly1), pyridine-4-y1 methyl
(i-
picoly1), tetrahydrofuran-2-y1 ethyl, and indo1-2-ylpropyl. Heterocyclylalkyl
groups can be substituted on the heterocyclyl moiety, the alkyl moiety, or
both.
Heteroarylalkyl groups are alkyl groups as defined above in which a
hydrogen or carbon bond of an alkyl group is replaced with a bond to a
heteroaryl group as defined above. Heteroarylalkyl groups can be substituted
on
the heteroaryl moiety, the alkyl moiety, or both.
By a "ring system" as the term is used herein is meant a moiety
comprising one, two, three or more rings, which can be substituted with non-
ring
groups or with other ring systems, or both, which can be fully saturated,
partially
unsaturated, fully unsaturated, or aromatic, and when the ring system includes
more than a single ring, the rings can be fused, bridging, or spirocyclic. By
"spirocyclic" is meant the class of structures wherein two rings are fused at
a
single tetrahedral carbon atom, as is well known in the art.
32
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
A "monocyclic, bicyclic or polycyclic, aromatic or partially aromatic
ring" as the term is used herein refers to a ring system including an
unsaturated
ring possessing 4n+2 pi electrons, or a partially reduced (hydrogenated) form
thereof. The aromatic or partially aromatic ring can include additional fused,
bridged, or Spiro rings that are not themselves aromatic or partially
aromatic.
For example, naphthalene and tetrahydronaphthalene are both a "monocyclic,
bicyclic or polycyclic, aromatic or partially aromatic ring" within the
meaning
herein. Also, for example, a benzo-[2.2.2]-bicyclooctane is also a
"monocyclic,
bicyclic or polycyclic, aromatic or partially aromatic ring" within the
meaning
herein, containing a phenyl ring fused to a bridged bicyclic system. A fully
saturated ring has no double bonds therein, and is carbocyclic or heterocyclic
depending on the presence of heteroatoms within the meaning herein.
The term "alkoxy" refers to an oxygen atom connected to an alkyl group,
including a cycloalkyl group, as are defined above. Examples of linear alkoxy
groups include but are not limited to methoxy, ethoxy, n-propoxy, n-butoxy, n-
pentyloxy, n-hexyloxy, and the like. Examples of branched alkoxy include but
are not limited to isopropoxy, sec-butoxy, tert-butoxy, isopentyloxy,
isohexyloxy, and the like. Examples of cyclic alkoxy include but are not
limited
to cyclopropyloxy, cyclobutyloxy, cyclopentyloxy, cyclohexyloxy, and the like.
The terms "aryloxy" and "arylalkoxy" refer to, respectively, an aryl
group bonded to an oxygen atom and an aralkyl group bonded to the oxygen
atom at the alkyl moiety. Examples include but are not limited to phenoxy,
naphthyloxy, and benzyloxy.
An "acyl" group as the term is used herein refers to a group containing a
carbonyl moiety wherein the group is bonded via the carbonyl carbon atom. The
carbonyl carbon atom is also bonded to another carbon atom, which can be part
of an alkyl, aryl, aralkyl cycloalkyl, cycloalkylalkyl, heterocyclyl,
heterocyclylalkyl, heteroaryl, heteroarylalkyl group or the like. In the
special
case wherein the carbonyl carbon atom is bonded to a hydrogen, the group is a
"formyl" group, an acyl group as the term is defined herein. An acyl group can
include 0 to about 12-20 additional carbon atoms bonded to the carbonyl group.
An acyl group can include double or triple bonds within the meaning herein. An
acryloyl group is an example of an acyl group. An acyl group can also include
heteroatoms within the meaning here. A nicotinoyl group (pyridy1-3-carbonyl)
33
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
group is an example of an acyl group within the meaning herein. Other
examples include acetyl, benzoyl, phenylacetyl, pyridylacetyl, cinnamoyl, and
acryloyl groups and the like. When the group containing the carbon atom that
is
bonded to the carbonyl carbon atom contains a halogen, the group is termed a
"haloacyl" group. An example is a trifluoroacetyl group.
The term "amine" includes primary, secondary, and tertiary amines
having, e.g., the formula N(group)3 wherein each group can independently be H
or non-H, such as alkyl, aryl, and the like. Amines include but are not
limited to
R-NH2, for example, alkylamines, arylamines, alkylarylamines; R2NH wherein
each R is independently selected, such as dialkylamines, diarylamines,
aralkylamines, heterocyclylamines and the like; and R3N wherein each R is
independently selected, such as trialkylamines, dialkylarylamines,
alkyldiarylamines, triarylamines, and the like. The term "amine" also includes
ammonium ions as used herein.
An "amino" group is a substituent of the form -NH2, -NHR, -NR2, -NR3+,
wherein each R is independently selected, and protonated forms of each.
Accordingly, any compound substituted with an amino group can be viewed as
an amine.
An "ammonium" ion includes the unsubstituted ammonium ion NH4,
but unless otherwise specified, it also includes any protonated or
quaternarized
forms of amines. Thus, trimethylammonium hydrochloride and
tetramethylammonium chloride are both ammonium ions, and amines, within the
meaning herein.
The term "amide" (or "amido") includes C- and N-amide groups,
i.e., -C(0)NR2, and ¨NRC(0)R groups, respectively. Amide groups therefore
include but are not limited to carbamoyl groups (-C(0)NH2) and formamide
groups (-NHC(0)H). A "carboxamido" group is a group of the formula
C(0)NR2, wherein R can be H, alkyl, aryl, etc.
The term "urethane" (or "carbamy1") includes N- and 0-urethane groups,
i.e., -NRC(0)OR and -0C(0)NR2 groups, respectively.
The term "sulfonamide" (or "sulfonamido") includes S- and N-
sulfonamide groups, i.e., -SO2NR2 and ¨NRSO2R groups, respectively.
Sulfonamide groups therefore include but are not limited to sulfamoyl groups (-
SO2NH2).
34
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
The term "amidine" or "amidino" includes groups of the
formula -C(NR)NR2. Typically, an amidino group is ¨C(NH)NH2.
The term "guanidine" or "guanidino" includes groups of the
formula -NRC(NR)NR2. Typically, a guanidino group is ¨NHC(NH)NH2.
"Halo," "halogen," and "halide" include fluorine, chlorine, bromine and
iodine.
The terms "comprising," "including," "having," "composed of," are open-
ended terms as used herein, and do not preclude the existence of additional
elements or components. In a claim element, use of the forms "comprising,"
"including," "having," or "composed of' means that whatever element is
comprised, had, included, or composes is not necessarily the only element
encompassed by the subject of the clause that contains that word.
A "salt" as is well known in the art includes an organic compound such
as a carboxylic acid, a sulfonic acid, or an amine, in ionic form, in
combination
with a counterion. For example, acids in their anionic form can form salts
with
cations such as metal cations, for example sodium, potassium, and the like;
with
ammonium salts such as NH4 + or the cations of various amines, including
tetraalkyl ammonium salts such as tetramethylammonium, or other cations such
as trimethylsulfonium, and the like. A "pharmaceutically acceptable" or
"pharmacologically acceptable" salt is a salt formed from an ion that has been
approved for human consumption and is generally non-toxic, such as a chloride
salt or a sodium salt. A "zwitterion" is an internal salt such as can be
formed in a
molecule that has at least two ionizable groups, one forming an anion and the
other a cation, which serve to balance each other. For example, amino acids
such as glycine can exist in a zwitterionic form. A "zwitterion" is a salt
within
the meaning herein. The compounds of the present invention may take the form
of salts. The term "salts" embraces addition salts of free acids or free bases
which are compounds of the invention. Salts can be "pharmaceutically-
acceptable salts." The term "pharmaceutically-acceptable salt" refers to salts
which possess toxicity profiles within a range that affords utility in
pharmaceutical applications. Pharmaceutically unacceptable salts may
nonetheless possess properties such as high crystallinity, which have utility
in
the practice of the present invention, such as for example utility in process
of
synthesis, purification or formulation of compounds of the invention.
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
Suitable pharmaceutically-acceptable acid addition salts may be prepared
from an inorganic acid or from an organic acid. Examples of inorganic acids
include hydrochloric, hydrobromic, hydriodic, nitric, carbonic, sulfuric, and
phosphoric acids. Appropriate organic acids may be selected from aliphatic,
cycloaliphatic, aromatic, araliphatic, heterocyclic, carboxylic and sulfonic
classes of organic acids, examples of which include formic, acetic, propionic,
succinic, glycolic, gluconic, lactic, malic, tartaric, citric, ascorbic,
glucuronic,
maleic, fumaric, pyruvic, aspartic, glutamic, benzoic, anthranilic,
4-hydroxybenzoic, phenylacetic, mandelic, embonic (pamoic), methanesulfonic,
ethanesulfonic, benzenesulfonic, pantothenic, trifluoromethanesulfonic,
2-hydroxyethanesulfonic, p-toluenesulfonic, sulfanilic,
cyclohexylaminosulfonic, stearic, alginic, 0-hydroxybutyric, salicylic,
galactaric
and galacturonic acid. Examples of pharmaceutically unacceptable acid addition
salts include, for example, perchlorates and tetrafluoroborates.
Suitable pharmaceutically acceptable base addition salts of compounds of
the invention include, for example, metallic salts including alkali metal,
alkaline
earth metal and transition metal salts such as, for example, calcium,
magnesium,
potassium, sodium and zinc salts. Pharmaceutically acceptable base addition
salts also include organic salts made from basic amines such as, for example,
N,N-dibenzylethylenediamine, chloroprocaine, choline, diethanolamine,
ethylenediamine, meglumine (N-methylglucamine) and procaine. Examples of
pharmaceutically unacceptable base addition salts include lithium salts and
cyanate salts. Although pharmaceutically unacceptable salts are not generally
useful as medicaments, such salts may be useful, for example as intermediates
in
the synthesis of Formula I compounds, for example in their purification by
recrystallization.. All of these salts may be prepared by conventional means
from the corresponding compound according to Formula I by reacting, for
example, the appropriate acid or base with the compound according to Formula
I. The term "pharmaceutically acceptable salts" refers to nontoxic inorganic
or
organic acid and/or base addition salts, see, for example, Lit et al., Salt
Selection
for Basic Drugs (1986), Int .1 Pharm., 33, 201-217, incorporated by reference
herein.
A "hydrate" is a compound that exists in a composition with water
molecules. The composition can include water in stoichiometic quantities, such
36
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
as a monohydrate or a dihydrate, or can include water in random amounts. As
the term is used herein a "hydrate" refers to a solid form, i.e., a compound
in
water solution, while it may be hydrated, is not a hydrate as the term is used
herein.
A "solvate" is a similar composition except that a solvent other that water
replaces the water. For example, methanol or ethanol can form an "alcoholate",
which can again be stoichiometic or non-stoichiometric. As the term is used
herein a "solvate" refers to a solid form, i.e., a compound in solution in a
solvent,
while it may be solvated, is not a solvate as the term is used herein.
A "prodrug" as is well known in the art is a substance that can be
administered to a patient where the substance is converted in vivo by the
action
of biochemicals within the patients body, such as enzymes, to the active
pharmaceutical ingredient. Examples of prodrugs include esters of carboxylic
acid groups, which can be hydrolyzed by endogenous esterases as are found in
the bloodstream of humans and other mammals.
In addition, where features or aspects of the invention are described in
terms of Markush groups, those skilled in the art will recognize that the
invention is also thereby described in terms of any individual member or
subgroup of members of the Markush group. For example, if X is described as
selected from the group consisting of bromine, chlorine, and iodine, claims
for X
being bromine and claims for X being bromine and chlorine are fully described.
Moreover, where features or aspects of the invention are described in terms of
Markush groups, those skilled in the art will recognize that the invention is
also
thereby described in terms of any combination of individual members or
subgroups of members of Markush groups. Thus, for example, if X is described
as selected from the group consisting of bromine, chlorine, and iodine, and Y
is
described as selected from the group consisting of methyl, ethyl, and propyl,
claims for X being bromine and Y being methyl are fully described.
In various embodiments, the compound or set of compounds, either per
se or as are used in practice of embodiments of the inventive methods, can be
any one of any of the combinations and/or sub-combinations of the various
embodiments recited.
37
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
Provisos may apply to any of the disclosed categories or embodiments
wherein any one or more of the other above disclosed embodiments or species
may be excluded from such categories or embodiments.
More specifically, the inventive compound can be any of the specific
examples shown below as exemplary compounds of the invention.
Various embodiments of the invention provide a compound of formula
(I) or a pharmaceutically acceptable salt, prodrug, tautomer, stereoisomer,
hydrate, or solvate thereof:
Al - A2
%
R6
R5 %
IX Li L2/
A3
(I)
wherein
a dashed line signifies that a single bond or a double bond can be present,
provided that there are two double bonds and three single bonds in the ring
comprising A1, A2, and A3;
A1, A2, and A3 each independently is C or 0 or is N when the N is
bonded to two adjacent ring atoms by a double bond and a single bond or is NR
wherein R is H or (CI-C6)alkyl when the N is bonded to two adjacent ring atoms
by two single bonds; provided that no more than one of A1, A2, and A3 is C and
that at least one of A1, A2, and A3 is N or NR; provided that only one of A1,
A2,
and A3 is 0;
L1 and L2 are each independently a bond; (CHR')õ wherein R' is H or (CI-
C6)alkyl and n is 1, 2, or 3; or a heteroaryl selected from the group
consisting of
thiophenyl, phenyl, furanyl, or benzothiophenyl and wherein such heteroaryl is
substituted with 0-3 J;
J independently at each occurrence is F, Cl, Br, I, OR', OC(0)N(RI)2,
CN, CF3, OCF3, CHF2, NO2, R', 0, S, C(0), S(0), methylenedioxy,
ethylenedioxy, N(R)2, N(R')CH2CH2OR', SR', SOR', SO2R', SO2N(R')2, SO3R',
C(0)R', C(0)C(0)R', C(0)CH2C(0)R', C(S)R', C(0)OR', OC(0)R', OC(0)OR',
C(0)N(R')2, OC(0)N(R')2, C(S)N(R')2, (CH2)0-2NHC(0)R', (CH2)0-2N(W)2,
38
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
(CH2)0-2N(R)N(R)2, N(R)N(R')C(0)RI, N(R)N(R)C(0)0R'
,
N(R')N(R')CON(R')2, N(R')S02R', N(R')S02N(W)2, N(R')C(0)OR',
N(R')C(0)R', N(R')N(R'), N(R')C(S)R', N(R')C(0)N(R1)2, N(R')C(S)N(W)2,
N(COR')COR', N(OR')R', C(=NH)N(R)2, C(0)N(OR')R', or C(=NOR')R',
wherein two J groups together can form a ring; wherein R' is independently at
each occurrence hydrogen or an alkyl, cycloalkyl, aryl, heterocyclyl, or
heteroaryl wherein any alkyl, cycloalkyl, aryl, heterocyclyl or heteroaryl is
substituted with 0-3 J; or wherein two R' groups together with a nitrogen atom
or
with two adjacent nitrogen atoms to which they are bonded can together form a
(C3-C8)heterocycly1 substituted with 0-3 J; optionally further comprising 1-3
additional hetero atoms selected from the group consisting of 0, N, S, S(0)
and
S(0)2;
R5 is a mono- or bicyclic cycloalkyl, aryl, heterocyclyl, or heteroaryl;
each of which is substituted with 0-5 J, wherein any cycloalkyl, aryl,
heterocyclyl, or heteroaryl can be fused, bridged, or in a spiro configuration
with
one or more additional cycloalkyl, aryl, heterocyclyl, heteroaryl rings, any
of
which can be monocyclic, bicyclic or polycyclic, saturated, partially
unsaturated,
or aromatic, and any of which is substituted with 0-5 J;
R6 is cycloalkyl, aryl, heterocyclyl, or heteroaryl, wherein any
cycloalkyl, aryl, heterocyclyl, or heteroaryl is independently mono- or pluri-
substituted with J, (CI-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkYnYl, (C1-
C6)haloalkyl, hydroxyl, halo, (Ci-C6)haloalkoxy, cycloalkyl(Ci-C6)alkyl,
heterocyclyl(C 1-C6) alkyl, aryl(Ci-C6)alkyl, heteroaryl(CI-C6)alkyl, OR3
wherein
R3 comprises H or (Ci-C6)alkyl or NR42 wherein each R4 independently
comprises H or (CI-C6)alkyl or where two R4 groups together with a nitrogen
atom to which they are bonded form a (C3-C8)heterocycly1 which optionally
further comprises 1-3 heteroatoms selected from the group consisting of N, 0,
S,
S(0) and S(0)2; or R4 is optionally substituted cycloalkyl, optionally
substituted
aryl, optionally substituted heterocyclyl, or optionally substituted
heteroaryl;
wherein any alkyl, alkenyl, alkynyl, haloalkyl, alkoxy, haloalkoxy, R3,
R4, cycloalkyl, aryl, heterocyclyl, or heteroaryl can be further substituted
with J;
and
provided that (i), (ii), (iii) or (iv) applies:
39
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
(i) LI is bond or (CHIOn and R5 is a bicyclic ring moiety which is
optionally substituted with 0-5 J where the bicyclic ring moiety is any one of
a-i
to a-xxviii wherein a wavy line indicates a point of attachment:
i ao i_eN i_eN /___,N
a-i a-u a-iii a-iv
1 45 i_8N1 FR 1-81
111
a-v a-vi a-vii a-viii
N
N N
a-ix a-x a-xi
41/
N N
_
*
a-xii a-xiii a-xiv a-xv
1 41 1 41 ilk . 0
N N z N N z 0 z
a-xvi a-xvii a-xviii a-xix a-xx
1 ________ 1\1 1 __ c __ t\I 1 41 i 41 1 41
NJ NJ NN,, N I N. m
N il , N,,,, N
I
N
a-xxi a-xxii a-xxiii a-xxiv a-xxv
rs
,s 't Sr
SS N tt.
/ \ N
AA
ilk Ilr W
a-xxvi a-xxvii a-xxviii
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
provided that when R5 is either a-xvii or a-xix that L2 is bond or
(CHR')õ ;
(ii) L1 and L2 are each independently a bond or (CHR')õ; R5 is a 6-
membered heteroaryl ring moiety optionally substituted with 0-3 J1, wherein J1
is
OR', CF3, Cl, Br, F, CN, 0(C1-C6)alkoxy, 0(C1-C6)cycloalkoxy, alkyl, or N(W)2
and wherein the 6-membered heteroaryl ring moiety is any one of b-i to
wherein a wavy line indicates a point of attachment:
______________ (N __________________________ (
(7-2,
0
b-i b-ii b-iii b-iv
oe
-/
b-v b-vi b-vii b-viii
c
(1\\I'N Ni\>
_/
-N
b-ix b-x b-xi
N-N
-N
b-xii b-xiii
(iii) L1 is a bond or (CHR')õ, and L2 is a heteroaryl substituted with 0-3 J
wherein the heteroaryl is c-i or c-iti wherein a wavy line indicates a point
of
attachment:
CF3 %
CF3
I I )
R6 R6
C-i C-ii
or
41
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
(iv) Li is a bond or (CHR')õ and L2 is a bond or (CHR'),, or a phenyl
substituted with 0-5 J; and R5 and R6 are independently selected from phenyl
or
heteroaryl each optionally substituted with 0-5 occurrences of J; provided
that if
L2 is a bond and R5 and R6 are both phenyl, then R5 is substituted with at
least
one of 4-CN, 3-alkyl-NHR', 3- alkyl-OR' , 4-alkyl-OR', or 2,3-dialkyl and R6
is
substituted with at least 4-OR';
provided, that when (ii), (iii), or (iv) applies, that the compound of formula
(I) is
not one of the following:
4104 N _________________________ N
/
N \ 0
sO /
F3C 0
N¨
c-__N
(Nil / /
N , \ . /
0'
N-
0
(---___
\ /
N--c_____ 0/
0
N I / N
N / \
I \ 11 N
sO 40,
N-0 0
0 J
/ 0
N,0 . =
N
N-0
I /
N . IIP IIP
H2N;s \ SI CF3 H2N-;S--.
00 0/
OEt
OEt
Nr .OEt
ClyN/ Nc)--I /4 II OEt
\ z
NN
OEt /
*
WC)
OEt =0 N
/ X I 14 Et0
N- OEt
42
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
OMe Nr S CF3
ON
1 1 N II
N2 OMe #10
WO
0 1N/ . 07¨ WO 11
/0
\ 1
N-
N -0 II" N-0>___0
r---11'1=1
ri-'s1 N 1
N= N-
0--N I
\
N-0
0
N = N¨
r-11-N
I
N,.,- 0 CF3
it F II
F3c 3c
_
N' p N' p
¨N ¨N
ii 1 =
N
In various embodiments of a compound of the invention, L2 is bond.
In various embodiments of a compound of the invention, Al and A3 are N
and A2 is 0.
In various embodiments of a compound of the invention, A2 and A3 are N
and Ai is 0, or Ai and A2 are N and A3 is 0.
In various embodiments of a compound of the invention, Al and A2 are N
and A3 is NR.
In various embodiments of a compound of the invention, Al is C, A2 is N
and A3 is O.
In various embodiments of a compound of the invention, Al is 0, A2 is N
and A3 is C.
43
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
In various embodiments of a compound of the invention, Li and L2 are
each independently a bond or (CHR')õ, and R5 or R6, or both, comprises a
heteroaryl ring. For example, at least one heteroaryl ring of R5 or R6 can be
a
pyridinyl or a pyridinyl N-oxide, pyrazinyl, pyrrolyl, imidazolyl,
benzimidazolyl, thiophenyl, benzothiophenyl, furyl, benzofuryl, indolyl,
indolinyl, piperidinyl, quinolyl, or isoquinolyl; wherein any heteroaryl is
substituted with 0-5 J. More specifically, any heteroaryl can be substituted
with
0-5 R', F, Cl, Br, I, OR', CF3, OCF3, CHF2, or SO2N(R')2.
In various embodiments of a compound of the invention, Li and L2 are
each independently a bond or (CHR')n, and R5 or R6, or both, comprises a
bicyclic carbocyclic ring, wherein the bicyclic carbocyclic ring is
substituted
with 0-5 J. More specifically, any bicyclic carbocyclic ring can be
substituted
with 0-5 R', F, Cl, Br, I, OR', CF3, OCF3, CHF2, or SO2N(R')2.
For example, LI can be bond and R5 be a bicyclic ring moiety which is
substituted with 0-5 J where the bicyclic ring moiety is any one of a-i to a-
xxviii,
wherein a wavy line indicates a point of attachment:
44
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
i . LeN1 i -9 i _5N
=
a-i a-u a-iii a-iv
i . 1_81\I E8N ER
=
a-v a-vi a-vii a-viii
1 . i 41 1 afr
N
N N
a-ix a-x a-xi
N N
/
_
* * * lik
a-xii a-xiii a-xiv a-xv
a-xvi a-xvii a-xviii a-xix a-xx
1 )\I 1 __ 1 __ 1\1 1 ak 1 41 40
N j N j N N I N. K1
N - , N N
I
N
a-xxi a-xxii a-xxiii a-xxiv a-xxv
g
g -L sr
\ / \N /
____
11 ilk mr
a-xxvi a-xxvii a-xxviii .
,
wherein any of the bicyclic ring moieties is substituted with 0-5 J.
For example, L1 and L2 cab each be a bond; and R5 can be a 6-membered
heteroaryl ring moiety substituted with 0-3 occurrences of J1; wherein J1 is
selected from the group consisting of OR', CF3, Cl, Br, F, CN, 0(CI-C6)alkoxy,
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
0(CI-C6)cycloalkoxy, alkyl, N(R1)2; and wherein the optionally substituted 6-
membered heteroaryl ring moiety of R5 is any one of b-i to
(NI
\ N
_______________________________________ (N
N
\¨,
0
b-i b-u J b-iii b-iv
0
o oe
o
N //¨N /
1µ1C)-00)
¨/
b-v b-vi b-vii b-viii
N\)
CNN N __________________________
¨/
¨N
b-ix b-x b-xi
N¨N N
b-xii b-xiii
wherein each of the 6-membered heteroaryl ring moieties is substituted
with 0-3 J1.
In various embodiments of a compound of the invention, L1 can be a
bond, and L2 can be c-i or c-u, wherein a wavy line indicates a point of
attachment:
CF3 1\--0 CF
/ 3
I
R6 R6
C-i C-ii
wherein c-i and c-ii are further substituted with 0-2 J.
In various embodiments of a compound of the invention, L1 can be a
bond and L2 can be a bond or a phenyl substituted with 0-5 J; and R5 and R6
can
independently be selected from phenyl or heteroaryl each substituted with 0-5
J;
provided that if L2 is a bond and R5 and R6 are both phenyl, then R5 is
substituted with at least one of 4-CN, 3-alkyl-N(R')2, 3-alkyl-OR', 4-alkyl-
OR',
or 2,3-dialkyl, and R6 is substituted with at least 4-OR'.
For example, the optionally substituted bicyclic ring moiety can be any
one of a-i to a-viii.
46
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
1 . 1- 11\8 T 1-SN
.
a-i a-u a-iii a-iv
i 411 1---R VC 1-81
a-v a-vi a-vii a-viii
wherein any of the bicyclic ring moieties is substituted with 0-5 J.
For example, a compound of the invention can have the formula I-B
further substituted with 0-5 J:
N-0
I ¨1-2
0 N R6
e
I-B.
For example, a compound of the invention can have the formula I-C
further substituted with 0-5 J:
N -Clz =
I
loi N
e
I-C.
For example, a compound of the invention can have the formula I-D and
further be substituted with 0-5 J, and wherein R7 and R8 each independently
are
H, OR', OC(0)N(W)2, N(RI)N(R')2, N(R')CH2CH2OR', CN, CHF2, CF3, 0CF3,
NO2, R', =0, =S, C(0), S(0), N(R1)2, SR', SOR', SO2R', SO2N(W)2, SO3R', or
C(0)R', or R7 and R8 together are =0, =NR', or =N(R')CH2CH2OR'.
47
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
N
N
=
R7
R8
1-D.
For example, a compound of the invention can have a formula I-F
N-0
IN
R7 =
R8 X
I-F
wherein R7 and R8 are each independently selected from H, OR", N(R")2,
and SR", wherein R" is hydrogen or an alkyl, cycloalkyl, aryl, heterocyclyl,
or
heteroaryl, wherein any such alkyl, cycloalkyl, aryl, heterocyclyl or
heteroaryl is
substituted with 0-3 J; X is F, Cl, Br, I, CHF2, CN, CF3, NO2, or OR'; Y is
hydrogen or an alkyl, cycloalkyl, aryl, heterocyclyl, or heteroaryl, wherein
any
such alkyl, cycloalkyl, aryl, heterocyclyl or heteroaryl is substituted with 0-
3 J.
In various embodiments of a compound of the invention can be any of:
48
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
OEt OEt OEt
. OEt 41 OEt 4I OEt /-0
O\ O\ 9 \ P.
N , N N , N N , N C
/ = 0
N , N
1011, 1.1.1
OH HI\l"-OH NJ'
i 0
215 216 217 227
J
0 OEt
0 ii
W- 9 :41 OEt OEt
N , N . OEt
N' 0
9'
-N
0. N. N
11161.
11.1 NH
S.
j--NH c-NEt2
HO
236 243 250 OH
N-0 N-0
N-0
ilk I rµf 10 (:1 * 1N( 1,
(:)'' 10 1 Ni 10 (21
= CF3 = CF3
= CN
HO HO
HO
259 260 261
N-0 N-0
N-0
ilk 'N' * 0
.".õ
HON',
I 1\i' * 0
= CNCF3
= CF3
HO
H HO,..."N
262 263 H 264
N-0 N-0
N-0
5 NI * crL O I N' *
o.--..., St ' N' 10 o"
110 CN
e 11 CN
HO-,ZN NO2 HO-,./"-N
H 265 HO
266 H 267
5
or any pharmaceutically acceptable salt, tautomer, stereoisomer, solvate,
hydrate,
or prodrug thereof.
In various embodiments of a compound of the invention, the bicyclic ring
moiety can be any one of a-ix to a-xv:
49
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
1 . i 41 1 41
N
N N
a-ix a-x a-xi
N
/N \
* * 1 I k *
a-xii a-xiii a-xiv a-xv
4
4 't sr
¨
IF . w
a-xxvi a-xxvii a-xxviii
wherein any of the bicyclic ring moieties can be substituted with 0-5 J.
More specifically, in various embodiments of a compound of the
invention, the compound can be any of:
(
0
fia
ft
O,
0 0 r----
0
,0 / ille 40 OEt Or¨
µ
N 9 \ 9 \ 9 \N
\ /
IP 0 `N SO II
ir
208 219 211
212
or any pharmaceutically acceptable salt, tautomer, stereoisomer, solvate,
hydrate,
or prodrug thereof.
In various embodiments of a compound of the invention, the optionally
substituted bicyclic ring moiety can be any one of a-xvi to a-xxv:
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
'11% '111-1 '1111
i . 1 ilfr 41 ilfr afr
N N 7 H N H N 7 0,
a-xvi a-xvii a-xviii a-xix a-xx
\ 1 1 __ tµl
NJ NJ N N
i N .N rd , - N N
I
N
a-xxi a-xxii a-xxiii a-xxiv a-xxv
wherein any of the bicyclic ring moieties is substituted with 0-5 J.
In various embodiments of a compound of the invention, the compound
can be any of:
51
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
/---0
N-0 0---\
N-0 0--\ 0 4.
I / litC 0
0 I NI 411 0 / µ
/ a
N N
) 0 ) N ,N
N
H H
1101
153 154 223
HN / NO
/-0
0,0
0
< 0 N-o * (1- N- IF /---
/ . 1 / 1 i 0
N , N
0
. N
- NO "OH HN_ H
HN / N
HN
N,,-, N,
NI-N-N I
H
224 237 238
0 --\0
N- . r----
1 / 0N- / . /-
1 0 Ni-0/ s
IN N ip N
0 N 0
HN _ H 0--
/
HN 0
NIN-OH NLV-'0H
239 240 253
Nr0 =
ce N/ 0
0--/
HO
258
or any pharmaceutically acceptable salt, tautomer, stereoisomer, solvate,
hydrate,
or prodrug thereof.
In various embodiments of a compound of the invention, the optionally
substituted 6-membered heteroaryl ring moiety of R5 can be any one of b-i to b-
y.
52
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
H H
iµ1=1 P __ \(\N N N
_K
H0
b-i b- ii b-iii S b-iv b-v
J J
wherein any of the 6-membered heteroaryl ring moieties is substituted with 0-3
J1.
In various embodiments of a compound of the invention, the compound
can be any of:
w Et0 OEt
OEt
N N
1 0, Et N9 =
. OEt NiryLN NifyLN
N --
32 37 38
OMe CF3
N
1 ,
4 D---LN fiD---LN
N ..- la N
39 51
49
N.9 .
0
,- N9 * N (:)
v ____________________________________
07LN NN
071-N
07LN
IV 7 HO N , N 7
52 57
53
OEt
N w _._
OEt 0-N)__21 0-N3--iN,
Et0 0 -N , , Et0 0 N. t_ F3C 0 -N \ ,N
I -
N 58 66 67 68
0 N)___q o-NL,---,
N.
0-N
--,N, aN)-__ ---- Me0 / Me0 0 - N, A__
F3c 0 -N,_, Me0 0 -N N \
71 72 74 75
O'N,I N-0 CF3
0-
0 N- n. a -N ___µ --.N , 1 NI/
li
- .____ ,N
ci N -- CF3
CF3 84 104
76
(
Br 0
N-0 N/-..,1 N-0
N01-1\1' * 1 NI* \--1 ,
CF3 N N (0
106 112 113 \
53
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
N)0
ry1:14 *
ryN.N7)-(:)--/
N 0 N
114 0 115 120
N-0 N-0 NO NO F
ryN * ryN Ank.
ryLN IP.
N0214 *
N N,, ir N CN
130 131 132 133
CN
N-0 NO NO * 1\ 1/µ N 0 *
N N
;yL
0 N, CF3 N 1
r% N-
157 161 172 173
0
11 , * N o = ND N-o \
NH N)-
0 400Et
NrY N
1 ,
N Ni (y-1.1 Illik r\r-,?-4-
NI OEt
02- N,.
174 175 178 OH 209
OEt
0
41 r0 r0
OEt
0 , 41 Or- 0 = 0 4,
N, N 0 ,, 0 0
Ny. N NN N , N
& &_..
HNk-OH ,ON
lr 1\fX0H N- NNEt2
0
218 220 225 226
OEt
J OEt
0 41 OEt
0, o , it OEt 0 ,
N , N
NN
N' 0
I NCTO
235 \ NND 241 L N1._i/I 0
NH 242
,OH OH
HOS
N--
or any pharmaceutically acceptable salt, tautomer, stereoisomer, solvate,
hydrate,
or prodrug thereof.
More specifically, in various embodiments of a compound of the invention, the
compound can be any of:
54
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
0-Ns)___e
N-0 S CF3
i / \ i N-0 S CF3
=N
ifi CV 40 N
i / \I
\ S
F3C =
118 149
168
N-0 S CF3
1 /
N-0 S CF3
\ 1
NON
19 = N
I / \ I
,
49
169 HN
171
el , C\:0-. 041, AK H
N..,
I \ 0 '-'1\1 111, \ j
, S N 1 N
F3%.= , N \ S
183 F3C 188
=
F3C
_
0 0\ -N Sr
\µ
S N3rr NH2
N' p
, 1
F3%.= , N
189 -N
0 I
F II
3C 41 244 N
_ F3N-=, H
S r
S'-
N' p
-N N' 9 N-0 o CF3
4 -N ./ \ 1
6
wir ii. N
N 0 I.1 N '
247 H
255 rµ,1-0 o CF3
249 0 N \ I
HN ifk
y---N 256
F3C
or any pharmaceutically acceptable salt, tautomer, stereoisomer, solvate,
hydrate,
or prodrug thereof.
In various embodiments of a compound of the invention, the compound
can be any of:
-
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
0¨ NR /¨(:) e N- -N
//N-0
N- >___(¨c # N 0 N ____
I / \ //
= _________________ N
F3C F3C
F3C 1 3
4
\õ,
I, \N
\ //1,1 I / 1
>
I /
= ___________________________________________ N = N K
F . N .cI
F3C F3C F3C
6 7
N-0
N-0 /
/ , = N /
H2N. 0 N \ N 0 N
o%
14
--c)
= H2N.,
14 H2N-, =0 15 16
0
N 0
N-Ck_ /- N-0>____ c__ *
NH2
0 N =\N 0 N \ /1=1
H2N,
H2N_, 0 0--s-'0
H2N., 0--sso i
0--s=kb
2
19 0
18
TO N-0\_ /------(CI
> _c_
H2N. * N \ /N
F H2N. . NN
CI
0--so
0--%
21 22
56
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
NH2
N" Cpic,
1 / \ -
C).__C Ni N-Ck /-\ _
1 /i-- IV-0
0 N # N 0 N
0 0 0
/ 23 24 25
HO ____________ /
N" ___________ `
$;_i .. N-C)>N) 0
N.
1 //N
/im
0 HO ,N ____________________ ,N <CI
0 0 0
26 27 28
-\
N1-0/./\ ______ N N-0 0-N
N-0
ip Ni \ _______ /( I --.. H'
)1.,..__,,...
Aka I 14 N 0
CI to N
lir
0
/ 0 0
29 30 31
OEt OEt
1
0
=6: /=OEt
07 0 / . OEt N-0 OEt
X N N, N i / .OEt
N ,..--- \ z NIcyLN
32 33 34
OEt OEt
- OEt H2N N0
NI / lik -C)
-0 Et0
OEt N
/ N I 14 * I / 4. OEt
fryN
N ,-- N ,..-- NOT N
35 36 37
\
OEt OMe 0
NV Nr 0 -(
I4
NI" __________________________________________________________________ i/N
NOTI 14 Ilik I N 1 lik
N ON
38 39 (:) 40
F
\
1\1 __________ CN N-0
1 \ /71 I /1 _____________________ 1\1 __ -K
* N 0 N
p
ON \
00 (31
(1)
41 42 43
57
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
CI
N-C) \ N"
1 />--C<NI I / * CN
ON ON /(
CI 0 N
0 0 0
44 45 46
(
N" S 0 N" >_*..N1-1`1CF3
I / \ TO/ .
ON ON
N 7
00 o,._.4748 49
i---
0 N-C)/1j /
¨ 7,..,)_. I...N; . 0
NN = 0/ -07. II
N -------/ N I \I HO
II rrN1 N,..%
N 7
,I
N.....%
50 51 52
Q .0
N-1 . 0 Nµ IN NO
v,) r4 N . 0/
I 11 -071L-N/
N,..% 1 N c---3-
53 N 7 54 55
OEt
N > _________________________________________ (ir WC)
-0
( -0
= 2-N N N . OEt 7LI\I
µ
N , Y
N,...% 56 57 58
0-N".t--_- 0-N\\_ /---=-\-
-1µIN 0 -N \ /NI
0 CF3 NH2
59 0 CF3 60 0 CF3 61
0-N>ERNI O-Nv /----zN
0 -1=1 \ / 0 -hrA__1 0-t¨
Et0 0 -N \ /N
0 CF3 0 CF3
62 63 64
=
58
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
O-N
..,1\1/N 0-N>ERN1 0-1\1--,
Et0 0 .__c Et0 0 -N \ / Et0
NH2
65 66 67
0-1\1 -t-- 0-N\v_ -/----=\- 0-1\1\>_ql
F3C le -N \ 1N F3C 0 -N /--N F3C 0
NH2
68 69 70
01_0 0-N>____Q--
0-1µ1t--- Me0 is -
F3C 40 -N \ / Me0 0 -N \ / N N
N
71 72 73
0N IN 04k_ /--,--N,
0-1\1__/--
Me0 401 -N \ / Me0 0 N-,____,
74 75 76
0 0 0
WC' lik f- WC)
I o W
/ 1 / 0
1 / 11 ,
ao N 0 N
0 . N
0 77 --N NH
µ 78
(a- 79
N r
- 0-N \ 0-N
0N,N NO
Ss N \ # N-
'N\ IIP
SI
Eta O Et0
OEt OEt 0 CF3
80 81 82
O-N 0-1\\I
--\
6 '1\1)--"C\N
C
CI Et0
CF3 OEt
84
59
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
01 ----\\/ 0-N 0-N
0 '1\?---CN--\<0 0 NI----CN- 0 r\?--CN-
0 Et0
0 C F3 0 CF3 OEt
86 87 88
\
o
N-0 ilk /---- o
1 . 0
0 0 N NI-C/ ilt 0/-
I /
0 0 N
N 90 91
-;
Boc N
H
o
N-0 = /¨
I . o o o
o . N N
,.)1_N
NI' \ . or- N-NN\ * or-
\ H 1 \
C) N 7 0 o
N N y
92 93 94
I
Et0
siC(-/N
W
O-N 0 C;0-NrC-1 Et0 _NI . Br
NH2 014
95 96 97
0
( --õ0
r---\
040 N) Is ,N1/ .
O-N
N) o, ,N . N N-
\____/
O-N 0-Nj
98 99 100
0__Ni .
O-N N-
I
N,.
101 102
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
CF3Br
N-0 N-0
NONli . r/k1g/ *
I i
N-0 41
N, CF3
104 CF3 106
r'll'N/
I
N,..- 107
N-0 411 0
N-0 0
N-0>_.0
i 11) /-
r-1\1
N,
I IS N, I
N,. N,.
108 /
HN 112
110
0 N-CI
WO .
r-/
N1 (1--N 110 NI-0
4I
ON/
I ,. 0 0
I
N
N p
115
113 \ 114 Cl5.
II0-N,,_ õ-____,
WO / 41110, -1\1/--N 0-1`1\_ /---=-AN
rN * \
1 3FC
N,., 40
117 CF3 118
119
OEt
WO
101 -'1=1 \ iN i , 4OEt
Br 0 N
121
120
61
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
OEtN-0 OEt
OEt N-0
WOi , lip
N IP OEt 0 'N' . OEt
0 N OEt
NC = \
NH
HN
122 123 124
1\1-
o
NO 1 .
1 ,
N
N'
1 / w 0
CN 11104 N . N .
Nr
1
125 126 127
0 0 N-0
1 / w 0 lq'N- A& /- r/ IIP
1 / w 0 1
1 I. = N N 1\1,
N
128 129 130
,
WOF
WO WO
N01µ1/ At ON/ .
1 1 I N/ AI 1
Mir N N CN
131 132 133
WO N-0II
N'O
yN ( 1\1
i . / IP r/
r I,
1 1 N 1
N N.,, 0 N,
N
NH
134 135 136
0
Ov
/----
WO N-0-
CF3
N0 Br
ry N 110 Ilit I , /10,
N
HN 0 N
CF3 CF3
(00
N ..-
137 138 139
62
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
OEt
N-0 N-0 N-0
0 N 0 N
0 OEt 0 .
140 141 142
CF3 Br
N-0 N-0
I / lip 1 /
(40 N
CF3 0 N
CF3
144 145
OEt
0
N-0 N-0 N-0
N IP
OEt 401 / / ip,
N
0 = 0 N IIP
146 147 148
N-0 S CF3 N-0 N-0
0 N \ I 0 N lik OEt
OEt N = OEt
SI
4101 OEt
149 NH 150 N 151
N) N-0 OEt N-0 0---\
N-0 0---\
\
0 I /
/ / 111), 0
/ 0 N
) a N
)
OEt
N 4W
N
0 N H 'W
H
152 153 154
N-0 0--\
4111.
H / N-0 N-0
0 N 0 N 0
)
40 N IP
N-
155 156 CF3 157
it
N-0
N-0
Si 0/--- 0 N
0 N
(0 CF3
159 \ 160
63
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
CN
WO N-10
WOII
N0/ . r/ .
I rf\l/ * CN
I\,1..
/ CF3 I
N.,.
II
161 162 163
=
WO N-0 0-\
WO 0-\
v-t
O 'N * 0 N ) . 0
0 N
)
N CF3
164 NH2
165 166
N-0 0-\
N-0 S CF3 N-0 S CF3
rN/ * 0 I / \ 1 1 / \ 1
I
)
C 10 N
*0 N
N,eN ---Ni
1 j
HN
167 168 169
N-0 S CF3
I / \I 1\143 =N 41 Ni
1 /
*
= 1 -r.-1-"-N W \
N /
HN
171 172
N-0 NI-0 =
(1\/1 * rr,; Nro4 . Nc'
1 N- I
r\.) ,ij.1
\---
1\1.... i N' ..... I 11
173 174 175
CF3 OEt 1 \
NI-0 * = N N NH
.
1 / OEt
(&N *
N = N HO N 0
1\1.,.
c-NH 176 177 178
0-1q H
N-0 WO N
II \AP 3
r/
N
I 0
I
N,. N,.
179 / (ii
,J 180 - OI 181
0
0
64
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
S \ 0-Nµ it
N-0 14101 \ 0, -,1 la N
i , I N 0
(--1"N IIP S I
F3t, , , N
N,, , (:)
I
182 183 184
HO
OH 0-N
S---N Br la N
fa N
0
0 0
C/ C)
185 186 I 187
N-0
H I. \ 0, lµi /
Or-
0-Nµ N
\ i Ask- o,
N 1117 N N
S I NH2 101 0
S F3C - N N
I C
F3C
188 189 190
0
N-0 N-0 N-0
1 ' * 0
/---- i , if
r---
Or-
io N
0 10 N
0 0 N
0 0
HO
0
c c c
191 192 193
N-0N-0
lip
I- I ,
N-0 i /I
Or-
r----
ON 10 N, 0 I 0 N
,N /0
,r\,1 40 N
0 c0
\
C
198 199
197
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
N-0N-0 N-0
0 IN/ 1IP 0/- 0 I N/ 111 d--- I N/ 111 Cr-
HO =/0 HO /0 ON le /0
\ \ \
200 201 202
0
J
0
N-0
S0.,-
111 0
r-
\ 0 N
NN /--/ Ilp \11
_,N /0 \
1.1 NH N-0
\
N F3C0
H
203 204 205
J it OEt N
0 OEt OEt
N ,0 / . OEt
F3C0 N
0 0,----
I N/
µ ilp -
ra
= N__ N
N-0
HN / NO
111
206 207 208
OEt
OEt ,c) * OEt 0
N N
, /
N 11 0/-
(N 111P OEt
N. N
OH C)INZ-.OH 210
209 00 211
)
OEt
Or 0
/--- 0 0
41 OEt
0 \
O\ N
N N'9
-N
41 7 I ,Iµl
lit 212 N 214
H OH OH
213
66
CA 02723904 2010-11-09
WO 2009/151529 . PCT/US2009/003014
OEt OEt OEt OEt
= OEt . OEt 41 OEt = OEt
9 \ 9 \ 9 \ 9 \
N , N N , N N , N Ni, N
1101)
S. S.
N--
OH HN<.--OH 1\r"" HN<-0H
/
215 216 217 218
( (
0 0 r0 r0
* 07- = 0 .
/9
C 0
0 i =
9 \ 9 \
N , N / N , N H
N
N , N N , N N \----
\
5\
\----\ N.--
0 1\1
N OH \
N
H /
H
0 .
219 220 221 222
coy
ro* ro ro*
/9C
o o it o 0 C
C 1 . C o 1 o
, . .
N , N N , N NyN NyN
* 0
NOH
I H
1.\1.'N'NEt2
HN / NO HN / N--"\--N
H
223 224 225 226
d 0J
r0 ro
0 ,
0 . o =
c 0
N.N OS
w
c 0 , ,
i = 1\1' 9
N -N N ' 9
= -N1 -NI
/NH 41 I NH = N
I r)
\
N \--'') N -OH
0 ...-J,H OH H
227 228 "OH 229 230
67
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
oJ
c :
- - - - - o)
o) )
o \
w o . M
0 110 0
ir
N/9 Q
W Q -N N/ 9 W
-N -N
-14
41 1 NQ 10
N
=NH
\ \ =
N N
N
H OH
r) H
r--1
,N, HO
231 I I 232 233 234
oJ
J
o
o , ,
w = o i .
i w
0
14- *0 /¨ N-C)
0
/¨
1 / 11
W Q W Q ip N is N
-N -N
HN
f-- ,OH
IL
ND "OH HN ____ H
-
Wy--=N'
14
ND IIP I
235 j--NH 236 237 238
HO
OEt OEt
0 4I OEt
0 . OEt
N = 0/¨
12 \
1 . N-0 . 0/¨
Q \ NT. N
= N N.
NOH HN N
HN_ H
&f0 INIe
NOH 1\r NH
OH
239 240 241 5 242
HO
OEt
411 OEt 41
F3c
o\ _
N. N S z
S. N9
-N
NH
c.-NEt2
HO
243 246
68
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
F3C * Oj
F3C = OEt
. OEt
_
S 0 0
s, P \
N. N
N ' Q NI' 9 N ' 9
01,
AI
0
Mr = N
NJ 41 N
1\1-1 j\I-\ ,
'OH
H H
247 248 249 250
N H O r0 NI-0 * N)-0 =
0
0 N; ip 0
N 0 14
0--/
H 0 5 .-/ HON 0--/ 0
251 252 253
WO 0 CF3
N)-0 ask N-0 0 CF3
O 0
\ I i , , i IV iir 0 0 N 0 N \
O0--/ HN
HN 0
N F3C
F3C 254 255 256
N-0
1\i1-0____\ e TO
N * O i N NI # c),
0---/ = CF3
HO
0 HO
0,.
257 258 259
N-0 N-0 N-0
'N' * 0
.-,,, * IN' . c) h. 1 Ni
10
= CF3 = CN = CN
HO HO HO
260 261 262
N-0 N-0 N-0
* '1\r * co. ik 1 Nr * 1;) O IN' .
cyL
= CF3 = CF3 Ho,"--
N = CN
HO,./"-N HO--/-"N H
H 263 H 264 265
69
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
N-N N-N N
IN \ 10 \ I 1o\ 'P0 IP o."---, N --
H 0v N o,
N _....- N
266 0-\ 267 0-\
268 0--\
NN
N-0
N 1 , 110
I 10 "s' = o
ilk 'o' IP
N _-
11 CN 111
CN
269 0--\
HO 270 HO--,./N 271
H
or any pharmaceutically acceptable salt, tautomer, stereoisomer, solvate,
hydrate,
or prodrug thereof
More specifically, the compound can be any of the following:
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
o
N o -0 . or¨ o-N
=
1 , N*0 r- 0-N =
µN-
1 , 0
. 0
1101 'IV' =
* N
* N
Et 0 'N
Et0
OEt
C OEt
77 -'1N1
t 78 80 81
O-N
0 OEt
0
N TO lip , ail, 0/-
& NI OEt
1 /
0 CF3 NC I
,N 0 N
82 122 128
CF3
0 OEt 0 , * OEt
OEt
0
Nr N-C) * NI , * * N-C1
1
1 , 0 1 , * OEt . N
HO . N
* N 0 N
N
129 152
\_.-NH 176 177
N
Or-- N-0
, , * r- N-0
0 N
0 5N
0 0 HO 5N
C 0
C
190 191 192
N-0 N-0
e
or-
il 10 N
---N 0 N
C co
193 194 195
WO
NO ri-0, *
r-
0 a
0 N 0 l 0 N
0 ,N
I * 0
0 ON N 0
C C C
196 197 198
71
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
N-10
/ / ilp r- N-0 N-0
o7---- or-
I 0 N 0 N
HO * N
,N 0 HO 00
C C C
199 200 201
OEt
N-0 * OEt
i / lip 0
i---- N-0 N-(:),
ON (001 N
i /
N * or-
0 . 1 N
C
C
202 203
Iyol , , 210
0 OH
0- 0
N \ / N" -c- N \ / 0- Nt-- 0,-
NN\>___c- N -N
0 -N
0
0 CF3 59
0 CF3 60 0 CF3 NH2
61
0-NN)___c-N?411
0-1%1\-N,
N-10
I I N/ IP
0 CF3
101 CF3 N CF3
62 63 117
11 11
WO
WO
N *
N Ill
0
0 CF3 CF3
156 160
or any pharmaceutically acceptable salt, tautomer, stereoisomer, solvate,
hydrate,
or prodrug thereof.
More specifically, the compound can be any of:
o o
07NLiN\ . /--- N-N /¨
o 1 II op
N
0
I H I \
N , N V
93 94
72
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
or any pharmaceutically acceptable salt, tautomer, stereoisomer, solvate,
hydrate,
or prodrug thereof
In various embodiments the invention provides a pharmaceutical
composition comprising a compound of the invention and a suitable excipient.
In various embodiments the invention provides a pharmaceutical
combination comprising the compound of the invention and a second
medicament. For example, the second medicament can be medically indicated
for the treatment of multiple sclerosis, transplant rejection, or adult
respiratory
distress syndrome.
Various embodiments of the invention provide a use of a compound for
preparation of a medicament adapted for treatment of a disorder or a
malcondition wherein activation or inhibition of a sphingosine-1 -phosphate
receptor subtype 1 is medically indicated, comprising a compound of formula
(II) or a pharmaceutically acceptable salt, prodrug, tautomer, stereoisomer,
hydrate, or solvate thereof:
Al ¨ A2
R5
L1 ) L2 R6
A3
(II)
wherein
a dashed line signifies that a single bond or a double bond can be present,
provided that there are two double bonds and three single bonds in the ring
comprising A1, A2, and A3;
Ai, A2, and A3 each independently is C or 0 or is N when the N is
bonded to two adjacent ring atoms by a double bond and a single bond or is NR
wherein R is H or (CI-C6)alkyl when the N is bonded to two adjacent ring atoms
by two single bonds; provided that no more than one of AI, A2, and A3 is C and
that at least one of AI, A2, and A3 is N or NR; provided that only one of Ai,
A2,
and A3 is 0;
Li and L2 are each independently a bond; (CHR)n wherein R' is H or (CI-
C6)alkyl and n is I, 2, or 3; or a heteroaryl selected from the group
consisting of
73
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
thiophenyl, phenyl, furanyl, or benzothiophenyl and wherein such heteroaryl is
substituted with 0-3 J;
J independently at each occurrence is F, Cl, Br, I, OR', OC(0)N(R)2,
CN, CF3, OCF3, CHF2, NO2, R', 0, S, C(0), S(0), methylenedioxY,
ethylenedioxy, N(R1)2, N(R')CH2CH2OR', SR', SOR', SO2R', SO2N(R')2, SO3R',
C(0)R', C(0)C(0)R', C(0)CH2C(0)R', C(S)R', C(0)OR', OC(0)R', OC(0)OR',
C(0)N(R)2, OC(0)N(R')2, C(S)N(R')2, (CH2)0-2NHC(0)R', (CH2)o-2N(R1)2,
(CH2)0-2N(R)N(R)2, N(R')N(RI)C(0)R', N(R)N(R')C(0)0R1,
N(R)N(R)CON(R)2, N(W)S02R', N(W)S02N(R')2, N(R')C(0)01V,
N(R)C(0)R', N(R')N(R'), N(R')C(S)R', N(R')C(0)N(R')2, N(R')C(S)N(R')2,
N(COR')COR', N(OR')R', C(=NH)N(R')2, C(0)N(OR')R', or C(=NOR')R',
wherein two J groups together can form a ring;. Wwherein R' is independently
at
each occurrence hydrogen or an alkyl, cycloalkyl, aryl, heterocyclyl, or
heteroaryl wherein any alkyl, cycloalkyl, aryl, heterocyclyl or heteroaryl is
substituted with 0-3 J; or wherein two R' groups together with a nitrogen atom
or
with two adjacent nitrogen atoms to which they are bonded can together form a
(C3-C8)heterocycly1 substituted with 0-3 J; optionally further comprising 1-3
additional heteroatoms selected from the group consisting of 0, N, S, S(0) and
S(0)2;
R5 is a mono- or bicyclic cycloalkyl, aryl, heterocyclyl, or heteroaryl;
each of which is substituted with 0-5 I, wherein any cycloalkyl, aryl,
heterocyclyl, or heteroaryl can be fused, bridged, or in a spiro configuration
with
one or more additional cycloalkyl, aryl, heterocyclyl, heteroaryl rings, any
of
which can be monocyclic, bicyclic or polycyclic, saturated, partially
unsaturated,
or aromatic, and any of which is substituted with 0-5 J;
R6 is cycloalkyl, aryl, heterocyclyl, or heteroaryl, wherein any
cycloalkyl, aryl, heterocyclyl, or heteroaryl is independently mono- or pluri-
substituted with J, (CI-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl, (C1-
C6)haloalkyl, hydroxyl, halo, (C1-C6)haloalkoxy, cycloalkyl(Ci-C6)alkyl,
heterocyclyl(Ci-C6) alkyl, aryl(CI-C6)alkyl, heteroaryl(CI-C6)alkyl, OR3
wherein
R3 comprises H or (CI-C6)alkyl or NR42 wherein each R4 independently
comprises H or (CI-C6)alkyl or where two R4 groups together with a nitrogen
atom to which they are bonded form a (C3-C8)heterocycly1 which optionally
further comprises 1-3 heteroatoms selected from the group consisting of N, 0,
S,
74
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
S(0) and S(0)2; or R4 is optionally substituted cycloalkyl, optionally
substituted
aryl, optionally substituted heterocyclyl, or optionally substituted
heteroaryl;
wherein any alkyl, alkenyl, alkynyl, haloalkyl, alkoxy, haloalkoxy, R3,
R4, cycloalkyl, aryl, heterocyclyl, or heteroaryl can be further substituted
with J;
and
provided that (i), (ii), (iii) or (iv) applies:
(i) LI is bond or (CHR')n and R5 is a bicyclic ring moiety which is
optionally substituted with 0-5 J where the bicyclic ring moiety is any one of
a-i
to a-xxviii wherein a wavy line indicates a point of attachment:
75
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
i 45 /13N1 1____\! i ____5N
411
a-i a-ii a-iii a-iv
i = i_____31 \ ER FB,
III
a-v a-vi a-vii a-viii
1 41 1 afr 1 ilfr
N
N N
a-ix a-x a-xi
111 . Wi lik
a-xii a-xiii a-xiv a-xv
1 41 41 41 41 0
N N z N N z 0 z
a-xvi a-xvii a-xviii a-xix a-xx
1µ1 1 ____________ 1 1 41 41 41
NJ NJ N N 1 N. , N N N N
I
N
a-xxi a-xxii a-xxiii a-xxiv a-xxv
=-s.
rs 't sS-
---
II 11# W
a-xxvi a-xxvii a-xxviii
provided that when R5 is either a-xvii or a-xix that L2 is bond or
(CHR')n ;
(ii) Li and L2 are each independently a bond or (CHR')n; R5 is a 6-
membered heteroaryl ring moiety optionally substituted with 0-3 Ji, wherein Ji
is
76
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
OR', CF3, Cl, Br, F, CN, 0(Ci-C6)alkoxy, 0(CI-C6)cycloalkoxy, alkyl, or N(R')2
and wherein the 6-membered heteroaryl ring moiety is any one of b-i to b-xiii
wherein a wavy line indicates a point of attachment:
0
b-i b-u J b-iii J b-iv
Pe oe
e
/ 11 -0 LN,
¨/
b-v b-vi b-vii b-viii
N\j\N 1\1
¨/
¨N
b-ix b-x b-xi
N-N 1\1
b-xii b-xiii
(iii) LI is a bond or (CHR')n, and L2 is a heteroaryl substituted with 0-3 J
wherein the heteroaryl is c-i or wherein a wavy line indicates a point of
attachment:
L¨S CF3 / CF
3
I I
R6 R6
C-i C-ii =
5
or
(iv) LI is a bond or (CHR')õ and L2 is a bond or (CHR')n or a phenyl
substituted with 0-5 J; and R5 and R6 are independently selected from phenyl
or
heteroaryl each optionally substituted with 0-5 occurrences of J; provided
that if
L2 is a bond and R5 and R6 are both phenyl, then R5 is substituted with at
least
77
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
one of 4-CN, 3-alkyl-NHR', 3- alkyl-OR' , 4-alkyl-OR', or 2,3-dialkyl and R6
is
substituted with at least 4-OR';
provided, that when (ii), (iii), or (iv) applies, that the compound of
formula (I) is not one of the following:
N' 11111
NH)
111
.2Ns"0
,
CF3
o'
s
N
N¨ ito
11101 CF3
F3C
s
N p
¨N
=
HN
In various embodiments the invention provides a use of a compound for
manufacture of a medicament wherein L2 is bond.
In various embodiments the invention provides a use of a compound for
manufacture of a medicament wherein Ai and A3 are N and A2 is 0, or wherein
A2 and A3 are N and AI is 0, or wherein Al and A2 are N and A3 is 0.
In various embodiments the invention provides a use of a compound for
manufacture of a medicament wherein Ai and A2 are N and A3 is NR, or wherein
Ai is C, A2 is N and A3 is 0, or wherein Al is 0, A2 is N and A3 is C.
In various embodiments the invention provides a use of a compound for
manufacture of a medicament wherein LI and L2 are each independently a bond
78
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
or (CHR')õ, and R5 or R6, or both, comprises a heteroaryl ring. For example,
at
least one heteroaryl ring of R5 or R6 can be pyridinyl or a pyridinyl N-oxide,
pyrazinyl, pyrrolyl, imidazolyl, benzimidazolyl, thiophenyl, benzothiophenyl,
furyl, benzofuryl, indolyl, indolinyl, piperidinyl, quinolyl, or isoquinolyl;
wherein any heteroaryl is substituted with 0-5 J; more specifically, the
heteroaryl
can be substituted with 0-5 R', F, Cl, Br, I, OR', CF3, OCF3, CHF2, or
SO2N(R1)2.
In various embodiments the invention provides a use of a compound for
manufacture of a medicament wherein LI and L2 are each independently a bond
or (CHR')n, and R5 or R6, or both, comprises a bicyclic carbocyclic ring,
wherein
the bicyclic carbocyclic ring is substituted with 0-5 J. More specifically,
any
bicyclic carbocyclic ring can be substituted with 0-5 R', F, Cl, Br, I, OR',
CF3,
OCF3, CHF2, or SO2N(R')2.
In various embodiments the invention provides a use of a compound for
manufacture of a medicament wherein LI is bond and R5 is a bicyclic ring
moiety
which is substituted with 0-5 J where the bicyclic ring moiety is any one of a-
i to
a-xxviii, wherein a wavy line indicates a point of attachment:
79
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
_5N
i . I_ i/13 i_e, N /
.
a-i a-u a-iii a-iv
i 41 T .1_81
a-v a-vi a-vii a-viii
N
N N
a-ix a-x a-xi
N N
.1 / \
II ilk II .
a-xii a-xiii a-xiv a-xv
i 110 i ilfr 41 0 afr
N N y N N y 0 r
a-xvi a-xvii a-xviii a-xix a-xx
i _________ 1\1 i __ cN 1 41 1 ilk 41
NJ N j7 N N i N . N m , N N -
I
N
a-xxi a-xxii a-xxiii a-xxiv a-xxv
rs
`'-
'41/ \
-
ilk 111 lir
a-xxvi a-xxvii a-x)cviii .
,
wherein any of the bicyclic ring moieties is substituted with 0-5 J.
In various embodiments the invention provides a use of a compound for
manufacture of a medicament wherein LI and L2 are each a bond; R5 is a 6-
membered heteroaryl ring moiety substituted with 0-3 occurrences of Ji;
wherein
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
J1 is selected from the group consisting of OR', CF3, Cl, Br, F, CN, 0(CI-
C6)alkoxy, 0(Ci-C6)oycloalkoxy, alkyl, N(R)2; and wherein the optionally
substituted 6-membered heteroaryl ring moiety of R5 is any one of b-i to b-
xiii:
\ N
\ N (
N
\-,
b-i b-ii b-iii b-iv
e
/00 o
r1_) rµicisS 4¨Nc)
\--,-/
b-v b-vi b-vii b-viii
\\N
-/
-N
b-ix b-x b-xi
N-N
-N
b-xii b-xiii
wherein each of the 6-membered heteroaryl ring moieties is substituted with 0-
3
.11. More specifically, L1 can be a bond, and L2 be c-i or c-ii, wherein a
wavy line
indicates a point of attachment:
CF3/ CF
3
I
R6 R6
C-i C-ii
wherein c-i and c-ii are further substituted with 0-2 J.
In various embodiments the invention provides a use of a compound for
manufacture of a medicament wherein L1 is a bond and L2 is a bond or is phenyl
substituted with 0-5 J; and R5 and R6 are independently selected from phenyl
or
heteroaryl each substituted with 0-5 J; provided that if L2 is a bond and R5
and
R6 are both phenyl, then R5 is substituted with at least one of 4-CN, 3-alkyl-
N(R1)2, 3-alkyl-OR', 4-alkyl-OR', or 2,3-dialkyl, and R6 is substituted with
at
least 4-OR'. In various embodiments the optionally substituted bicyclic ring
moiety can be any one of a-i to a-viii.
81
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
It I-9 I-0 I-EN
a-i a-u a-iii a-iv
a-v a-vi a-vii a-viii
wherein any of the bicyclic ring moieties is substituted with 0-5 J.
In various embodiments, the compound used for manufacture of a
medicament can have formula I-B further substituted with 0-5 J:
N'o
0 N R6
e
I-B.
In various embodiments, the compound used for manufacture of a
medicament can have formula I-C further substituted with 0-5 J:
I
11. N
e
,
I-C.
In various embodiments, the compound used for manufacture of a
medicament can formula I-D and be further substituted with 0-5 J, wherein R7
and R8 each independently are H, OR', OC(0)N(R)2, N(R)N(V)2,
N(R')CH2CH2OR', CN, CHF2, CF3, OCF3, NO2, R', =0, =S, C(0), S(0), N(RI)2,
SR', SOR', SO2R', SO2N(R1)2, SO3R', or C(0)R', or R7 and R8 together are =0,
=NR', or =N(R')CH2CH2OR'.
82
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
N-C)z =
N
=
R7
1-D.
In various embodiments, the compound used for manufacture of a
medicament has a formula I-F
N-.0
IN
R7 = CYY
R8 X
I-F
wherein R7 and R8 are each independently selected from H, OR", N(R")2,
and SR", wherein R" is hydrogen or an alkyl, cycloalkyl, aryl, heterocyclyl,
Or
heteroaryl, wherein any such alkyl, cycloalkyl, aryl, heterocyclyl or
heteroaryl is
substituted with 0-3 J; X is F, Cl, Br, I, CHF2, CN, CF3, NO2, or OR'; Y is
hydrogen or an alkyl, cycloalkyl, aryl, heterocyclyl, or heteroaryl, wherein
any
such alkyl, cycloalkyl, aryl, heterocyclyl or heteroaryl is substituted with 0-
3 J.
In various embodiments the invention provides a use of a compound for
manufacture of a medicament wherein the compound comprises any of the
following:
83
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
0-
N-C) --c, N-C)>_CN) N-C) -0 9 1=1"
>_(=N
= ______________________________________________________________________ N
-k,
1 --( // IN I / \ / I / \ /N-0
= N * N _______________ 0 N _____
F3C F3C F3C F3C
1 2 3 4
N-Ox_C(-\õ,
1=1" >(-\ >Ck, 11--_____
1 / \ /pi
1 / \ ,,pi
1 / \ /71 N
= _____________________ N _______ . N ' '(F
30 CI
F3C N
F3C F3C N.0 µ =
z
0
6 7 8
Si
NN-- 1 N 0/
Nc-DNi.
N
/-/ 1 = * 0/
N-o
N lik 0
N-0 /0
9 10 11
I.1--___17,_
N-0
1111
N 0 N
N.0µ = H2N.,
0"-O
0 14
12 0--/
,(X5---- N-0
H2N. / /
0
N / 0 N .
\ N
10-s"O
= 1
6
H2N-, =0
0
Nii-O\ /_,-__\_
H2N. 0 N \ /N
NH2 H2N, 0 Ni----N
0 H2N. 0 Nr-A_NN
0--% 0' b / 0--%
18 19 20
CI
Ni-O\
O Ni----N 0 N/----19
H2N., F H2N. CI
21 22
84
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
NH2
NON (
" ¨ N>__CN)
N"C)>_(-\mõ,
1
1 / \ //im-._,
# N * N 0 N
0 0 0
/ 23 24 25
N_0>li-10.c.
NI-0/,µ-1
0 N
HO ,N _____________________ ,N _____
(
CI
0 0 0
26 27 28
N-0\ /--\ N-0 0-N
N-0
0 N1 1-- /(1\1 I -... 1-
....k......___.,,,õ I /
N N 0
CI 0 N
IP 1
0 0 0
29 30 31
OEt bEt
N "
11 OEt N-0
N/11---14 . OEt N o OEt *
N OEt
,-
W, 1\110N
32 33 34
OEt OEt
NV =
N-C) =
NI-0 Et0
OEt
1 Ni = OEt H2N\ryl-N ; / N I NI . OEt
NOT N ,..,....)-
N õ--
35 36 37
\
OEt OMe 0
NI" C) N . N-0\ (¨(
-Jj- N lik
/ N I N1 1 ,/
NOT N ,- 0 N __
38 39 C) 40
F
N-0\
1 /1 _________________________________________________________________
10/ N _______________________________ Si N __
0 0 0
41 42 43
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
CI
N'Q ___________ C\ N-R/) __ \ C( N 0
1 N - . CN
0 N 0 N 1(
CI 0 N
(Jo (:) 10
44 45 46
(
N' S 0 N'O N-N CF3
I i \ I 0
0 .li-,_ /
z
I\ 1 N
Nõ.%
(:) (:)
47 48 49
N-C) r-
0
NN II * 0/ , ..,...,,A14 \ / .,,..ill\ = 0
rµj Iµ 1 II 1 HO
N N,_%
,I
A 1
N-...%
50 51 52
Q .0
N-13 . 0 N, NO
II (1=1 N-C) = 0/
53 N., N., 54 55
OEt
K1 0 e( N-0 .
..- _______________________________________ l OEt
N-C)
KA14 . = 02-N N N
1 1 /
Nr
N....%
56 57 58
0-1\!>___t---
0 -1\1 \ /11 0
NH2
CF359 0 CF3 60 0 C F3 61
0-N) q 0-N---=-N 041t-
-
0 -1=1 \ / 0 -N__, Et0
0 CF3 0 CF3
62 63 64
86
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
0E-RNI 0-1\--N
Et0 0 Et 0 -N \ / Et0
NH2
65 66 67
0-1\1\\_ /----=-\- 0cii?
F3C 0 -N \ /N F3C 0 -N/ -N F3C 0 N \ /
NH2
68 69 70
0-N\)_E- )--Nl 0-N-- N 01____Q--
0 - \ /N
F3C 0 -N \ / Me0 0 -N \ /N Me0
NH2
71 72 73
10-N\>c-N?1 0-1\1\N,
Me0 0 N \ / Me0 0 -N ' µ_.1
74 75 76
0 0 0
WC' ik, or- WC' * cr- N 0, r-
I / I / 1 / 0
. N = N
0 = N
al 77 N NH
I 78
0/ 79
N ,
0-N
0 0-N
, I 0-N
0
40 - 'NI \ . N
'1\1µ * N N N
µ IIP
Et0 * Et0
OEt OEt 101 CF3
80 81 82
0-N 0-N 4/
".....c
(110 Nr\---CN
0
CI Et0 SI
CF3 OEt
84
87
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
0-1\\I ----\\/ o-N o-N
0 -N)---CN--e N
110 )C\ N Et0 - 6 NC
)--\N-
0
I. CF3 I101 CF3 OEt
86 87 88
*
\
o
N-No
i "._. ,__C\ N-c) . /---
1 . o N-
A 0 _N
1110 N 1 / 0
Et0 o
OEt 0 . N(N)
89 N 90 91
C)
.;
Boc N
H
o
N-0 10 /¨
I / 0 0 o
o 0 N vNII:NN\ = 0/¨ yINN\ * or-
\ H
(1\1 o o
N-3 N , N y
92 93 94
I
NJ
Et0 i,i
0 _N/>C(N
O-N 0 0-Nr-C-N Et0 ILPI -N 4, Br
NH2 0-
95 96 97
(0 0 0
"=---- ain
_1\1 4. N/----\N-
CO 0 =
N) 0
_ 0-N / =
NC
N_
O-N
98 99 100
OMe
0 ,I\1/ =
/ 1 I\I0
I I N/ it
N N OMe
101 102 103
88
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
CF3 Br
N-0
II
N-0
-0 .
ON' * N ryN/ *
I
r.-ILNI/ 1
N CF3 1
,. N
CF3
N1
104 105 106
WO 41 0
NI-
r/I\I
, 6,k, N
rN
N 1
N
N-
107 108 109
o
N-0 ic)
ii or--
401 N
ry-N
I
N I
N
HN /
110 111 112
(
o N-0
N-0
NOA\I 11 N-0
IN/ * I
I 0
N 0 N
C C5
113 114 115
\
I r/ 11* S
F3C
N,. CF3
116 117 118
o-NI N-0 OEt
0 -N \ /N
OEt
Br =N
0 119 120 121
89
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
OEt -0 OEt
OEt WO N
N-0 IIP OEt
N * OEt N 11/ OEt
N
0 0
NC = \ NH
HN
122 123 124
N-C)
WC)
o 1 .
0 N
N'CI
1 /
C\N 410 N # N it
N3 1µ(
I
125 126 127
o o N-0
N-0 r-
1 . w o 'N' N-C/ryN/ Ill
1 , 0 I
I, N
= N N
N
/
128 129 130
WO F
WO WO
(11\1/ Aal,
i (" / AI rLIµl ilik
N, ir N,. N,.. CN
131 132 133
N-0 WO WO
1 ,
rII N/ rrl/ *
1 N I
1\1, NI,. 0 N,.-
134 135 136 NH
0
Ov
7----
WO O CF3 WO Br
t IP W , 1 , *
1
HN (110 N
CF3 CF3
N,. 0
137 138 139
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
OEt
N- N-0 N-0
i / 111 i N/ * i / Ilik
0 N
* N
OEt 0 ilp '.
140 141 142
N-0 \I s CF3 N CF3 Br
-0 N-0
/ / 110, i / 41Ip
0 N
40 N
ilk . N
CF3 CF3
143 144 145
OEt =
N-0 N-0 N-0
=N 1 /
OEt SI N
0 it, 0 N
146 147 148
N-0 s i CF3 N-0 N-0
40/ 'Ni =\ 1 0 N Ill OEt 40 N lik OEt
. OEt OEt
149 NH 150 N 151
0--\ ,_, 0--\
N N-0 ) OEt N-0 \ N-u
t / ip,
0
0
/ 0 N
2 a N
2
0 N IP OEt
N
H N '-.
H
152 153 154
N-0 0---\
411.
H 11,
0 N-0 N-0
0 N 0 N
; =N
CF3
I
NI
155 156 õ
157
41
p io it
N-0 N-0 0/- N-0 0 N 110 Or- 0 N
? CF3
\
158 159 \ 160
91
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
C
N-C) N r\VC)
Nro
rJ/ .r
I -.../L-N/ 111 CN 1
,. CF3 N,
1
N1,.
N
=
161 162 163
0
1\1- WO 0---\
N-C) 0--
\
i /
O 0 0 N 0 0
N ill $
) 0
)
N / CF3 N
164 NH2
165 166
N-13 0---\
N-0 S CF3 N-0 S CF3
i / I N .3 1 / \I 1 / \ i
---N
CV * N
0 1 0 N
N
HN /
167 168 169
N-0 S CF3 N-C) t. Ni
1 / \ I 1 /
* N
= vi - N Wi \
N r 172
HN 171
_
N0, N-0/ .
NY . Nr---
N -, N
I I/ \__
/
_---
N. -.,. i N r I \I
N.,..%
173 174 175
CF3 OEt
C: \
-1)4 . *
Nro
1 / . OEt WO NH
N0 HO
0 N rN
*
N,
176 177 178
\--NH
o- F'
H
WO WO
r/ . r/ 0 0 N
N
I 0
1
N,
179 / N\
0..-. N, 180 ---- 01 181
0__
92
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
s \ 0-Nµ ip
N-CI 0F3µ.., O-N fa N
I \ \N16
01-N IP õ., S I 0
.N
N 000
I
182 183 184
HO
O'N
O'N it \ lit OH 0-N
Br 4 N =
\ ip
N
0 N
0
0 (SI 0
c). c).
1 1 (:)
185 186 I 187
H
0 0-N WO
o-
i----
N 1110. 0-NAK N-.,
ii -N
N \ \ = ,...NH2
N
S --1 0 i0
1=1
\ S F3C......,- Di I \
F3C
188 189 190
WO WO N-0
,--- 0
,---__
40/ N
0 101 N
Cy 0 N
0
HO 0
0
C C C
191 192 193
WO WO
0 0 N S lip
Or-
I ' Sr---
ON I
,..N 0 N
0
,N
I 110 N
(0 c0
C
\
198 199
197
93
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
N-0
I N/ = Cr- / ,
N lit Cr-- 1 ,
N lik 0
/----
HO 0 )0 HO 0 /0 ON 0 /0
\ \ \
200 201 202
0
J
N-0 0
--\ *
, so
NN /'
, , = ,---
,N r-/ * \ N _
\
0 \
F3C0
N
H
203 204 205
J OEt
0 OEt
N-0 A) * OEt
0.,--
' . OEt N\ /
F3C0 * N 0 i- 5 N
N__ N
\
N-0 / NO
HN
206 207 208
OEt
OEt N JD / 40 OEt 0
N--0
/ / 1 N . Cr-
(L--N * OEt
Nr
(1)õ N..0-OH 210
OH NN
209 00 211
)
0 ---1 0 OEt
r-
O 0 0 5
* OEt
P \
0 \
N' 9 Ny, N
-N
= I 17
N
it 212 N 214
H OH OH
213
94
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
OEt OEt OEt OEt
= OEt . OEt = OEt = OEt
9 \ P \ 9 \ 9 \
rµL N N , N N , N N , N
O. O. O. tl
N
OH HN<-0H 1\1". HN-<--OH
/
215 216 217 218
(
0 0 r0 r0
04
IF Or-- = Cr- ') = C 0
0 / .
NN, H
9 \ p\ = / .
N , N / N
N , N N N N
\---\ N----
0 ' N
0 \
N /
N H
.0 I N 5\ OH H
0
219 220 221 222
FO FO r0 r0
0 = 0 = 0 = 0 =
C
/ 0 C 0 C C
,
N , N N , N N , N N yN
0 0
N / OH
I H
N1*-N`NEt2
HN / NHN / HNI--\---N
223 224 225 226
oJ oJ
ro
ro
< <
0 =
0 . / , 0
NN Nr9 ,
, .
0 NN 0 0 0 5
N ' p
,
la.
-N -NI
ille
N--- N I N bH
0
C.-.-', H OH H
227 228 OH 229 230
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
oJ
- - - - o)
o --I
A
0 0 0 10 ----1
0 1110 0 Ati
IP
/ 9
N- p N -N N'9
p
-N N'
-N
-N
41 1 N Q it
NH
N
N N
N
H OH
I-) H
ri
õNI, HO
231 1 I 232 233 234
oJ
oJ
0
11.111 0 AlWI i
0
N . or-
N- /10 0 /----
N' p N- p . N * N
(t OH
AL HN HN__ H
NO"OH
N-
ND IP I
235J-NH 236 237 238
HO
OEt OEt
0 . OEt
0 . OEt
N-0 * r-
I. . o N-0 11 /¨ P \
0 P \ N,,,i,N
---L.-.
HN__ H I 0
HN
NN-V-OH lµr-f
NINZ-OH N NH
OH
239 240 241 S 242
HO
OEt
10, OEt 41
F3c
co \ _
N. N S ,
S. N- 9
NH
41
c_NEt2
HO
243 246
96
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
OEt
F3C . oJ
*
F3C =
OEt
S7
0 _
0 s__ z 9 \
N..., N
N"- p N" p N'9
O.
*all
MT = N
Nj N
H H
247 248 249 250
I / *
N-0 N-0
I / . o 1-0/ *
0 0 N
HO..........-..N 0 N N
H0 H
0-1 HO...----,_,..N 0--/0
0-
251
253
252
14-0 0
Ni-0/ * 11-0/ 0 1 CF3
0 I N/).-----
-0 0 N \
i5 N
0-/
O HN
HN 0 >r--N
___
N
F3C
F3C 254 255 256
N-0
N-0 = / ,
N-0\ /----,-\ 5 N *
0
/ / N
0 N---N
0 0---/ e
CF:
0 HO""
HO
1,...,.. 0,...õ--
257 258
259
N-0 N-0 N-0
O IN/ .N *
IN' IP
--,..,
0.." 401 / 0 (:))
0 CF3
e CN e
CN C
HO HO HO
260 261
262
N-0
N-0 N-0
O I I tr 0
* IN/ . (21j . (:)
e
CN
It CF3 0
CF3 HO,,---N N'$
HO,--N HO,"---NH
H
H H
263 264
265
97
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
N-N N-N
IP
/
N N /NI\ 'PO ,\ N /0\
o
N _ HN
266 267 0¨\ 268 0¨\
N-=
/
/
\
'P0 110
ith 0 ip
N
CN =
CN
269
HO 270 271
Various embodiments of the invention provide a method of activation,
agonism, inhibition, or antagonism of a sphingosine-1 -phosphate receptor
subtype 1 comprising contacting the receptor subtype 1 with an effective
amount
of a compound of formula (II) or a pharmaceutically acceptable salt, prodrug,
tautomer, stereoisomer, hydrate, or solvate thereof:
Al ¨ A2
R6x
k
L1 ) L2 R6
A3
(II)
wherein
a dashed line signifies that a single bond or a double bond can be present,
provided that there are two double bonds and three single bonds in the ring
comprising Al, A2, and A3;
Al, A2, and A3 each independently is C or 0 or is N when the N is
bonded to two adjacent ring atoms by a double bond and a single bond or is NR
wherein R is H or (Ci-C6)alkyl when the N is bonded to two adjacent ring atoms
by two single bonds; provided that no more than one of A1, A2, and A3 is C and
that at least one of Ai, A2, and A3 is N or NR; provided that only one of Ai,
A2,
and A3 is 0;
Li and L2 are each independently a bond; (CHR')n wherein R' is H or (CI-
C6)alkyl and n is 1, 2, or 3; or a heteroaryl selected from the group
consisting of
thiophenyl, phenyl, furanyl, or benzothiophenyl and wherein such heteroaryl is
substituted with 0-3 J;
98
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
J independently at each occurrence is F, Cl, Br, I, OR', OC(0)N(R)2,
CN, CF3, OCF3, CHF2, NO2, R', 0, S, C(0), S(0), methylenedioxy,
ethylenedioxy, N(R')2, N(R)CH2CH2OR', SR', SOR', SO2R', SO2N(W)2, SO3R',
C(0)R', C(0)C(0)R', C(0)CH2C(0)R', C(S)R', C(0)01V, OC(0)R', OC(0)OR',
C(0)N(R')2, OC(0)N(R')2, C(S)N(R')2, (CH2)0-2NHC(0)R', (CH2)0-2N(R1)2,
(CH2)o-2N(R')N(R')2, N(R')N(R')C(0)R', N(R')N(11')C(0)OR',
N(R')N(R')CON(R')2, N(11.1)S02R', N(R)S02N(R')2, N(R')C(0)01V,
N(R')C(0)R', N(R')N(R'), N(R')C(S)R', N(R)C(0)N(R1)2, N(R')C(S)N(V)2,
N(COR')COR', N(OR')R', C(=NH)N(R1)2, C(0)N(OR')R', or C(=NOR')RI,
wherein two J groups together can form a ring; wherein R' is independently at
each occurrence hydrogen or an alkyl, cycloalkyl, aryl, heterocyclyl, or
heteroaryl wherein any alkyl, cycloalkyl, aryl, heterocyclyl or heteroaryl is
substituted with 0-3 J; or wherein two R' groups together with a nitrogen atom
or
with two adjacent nitrogen atoms to which they are bonded can together form a
(C3-C8)heterocycly1 substituted with 0-3 J; optionally further comprising 1-3
additional heteroatoms selected from the group consisting of 0, N, S, S(0) and
S(0)2;
R5 is a mono- or bicyclic cycloalkyl, aryl, heterocyclyl, or heteroaryl;
each of which is substituted with 0-5 J, wherein any cycloalkyl, aryl,
heterocyclyl, or heteroaryl can be fused, bridged, or in a Spiro configuration
with
one or more additional cycloalkyl, aryl, heterocyclyl, heteroaryl rings, any
of
which can be monocyclic, bicyclic or polycyclic, saturated, partially
unsaturated,
or aromatic, and any of which is substituted with 0-5 J;
R6 is cycloalkyl, aryl, heterocyclyl, or heteroaryl, wherein any
cycloalkyl, aryl, heterocyclyl, or heteroaryl is independently mono- or pluri-
substituted with J, (Ci-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl, (C1-
C6)haloalkyl, hydroxyl, halo, (C1-C6)haloalkoxy, cycloalkyl(CI-C6)alkyl,
heterocyclyl(Ci-C6) alkyl, aryl(C 1-C6)alkyl, heteroaryl(Ci-C6)alkyl, OR3
wherein
R3 comprises H or (CI-C6)alkyl or NR42 wherein each R4 independently
comprises H or (C1-C6)alkyl or where two R4 groups together with a nitrogen
atom to which they are bonded form a (C3-C8)heterocycly1 which optionally
further comprises 1-3 heteroatoms selected from the group consisting of N, 0,
S,
S(0) and S(0)2; or R4 is optionally substituted cycloalkyl, optionally
substituted
aryl, optionally substituted heterocyclyl, or optionally substituted
heteroaryl;
99
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
wherein any alkyl, alkenyl, alkynyl, haloalkyl, alkoxy, haloalkoxy, R3,
R4, cycloalkyl, aryl, heterocyclyl, or heteroaryl can be further substituted
with J;
and
provided that (i), (ii), (iii) or (iv) applies:
(i) LI is bond or (CHR')n and R5 is a bicyclic ring moiety which is
optionally substituted with 0-5 J where the bicyclic ring moiety is any one of
a-i
to a-xxviii wherein a wavy line indicates a point of attachment:
F_9N
Vd
a-i a-u a-iii a-iv
41 1-2
=
a-v a-vi a-vii a-viii
a-ix a-x a-xi
a-xii a-xiii a-xiv a-xv
µ111-L,
afr
N N N z Oz
a-xvi a-xvii a-xviii a-xix a-xx
rN _____________________________ 41 41
N N N N . m
N NN
a-xxi a-xxii a-xxiii a-xxiv a-xxv
100
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
,s
s, N
\ \ N
111
a-xxvi a-xxvii a-xxviii
provided that when R5 is either a-xvii or a-xix that L2 is bond or
(CHR')n
(ii) L1 and L2 are each independently a bond or (CHR')n; R5 is a 6-
membered heteroaryl ring moiety optionally substituted with 0-3 J1, wherein J1
is
OR', CF3, Cl, Br, F, CN, 0(CI-C6)alkoxy, 0(Ci-C6)cycloalkoxy, alkyl, or N(IV)2
and wherein the 6-membered heteroaryl ring moiety is any one of b-i to b-xiii
wherein a wavy line indicates a point of attachment:
(
0
b-i b-ii J b-iii b-iv
ioe
\
¨ /
b-v b-vi b-vii b-viii
N--7\
\ NI\)
¨ /
N
b-ix b-x b-xi
N ¨ N
Hi
N
N
b-xii b-xiii
(iii) Cis a bond or (CHR')n, and L2 is a heteroaryl substituted with 0-3 J
wherein the heteroaryl is c-i or c-ii wherein a wavy line indicates a point of
attachment:
101
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
CF CF3
3 I
R6 R6
C-i C-ii
or
(iv) Li is a bond or (CHR'),, and L2 is a bond or (CHR')õ or a phenyl
substituted with 0-5 J; and R5 and R6 are independently selected from phenyl
or
heteroaryl each optionally substituted with 0-5 occurrences of J; provided
that if
L2 is a bond and R5 and R6 are both phenyl, then R5 is substituted with at
least
one of 4-CN, 3-alkyl-NHR', 3- alkyl-OR' , 4-alkyl-OR', or 2,3-dialkyl and R6
is
substituted with at least 4-OR';
provided, that when (ii), (iii), or (iv) applies, that the compound of
formula (I) is not one of the following:
4111
/
H2N.
,s, H2N;s,
0\ - 0 0
N
CF3
F3C
p
-N
=
HN
102
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
In various embodiments, a method of the invention uses a compound
wherein L2 is bond.
In various embodiments, a method of the invention uses a compound
wherein Al and A3 are N and A2 is 0, or wherein A2 and A3 are N and Al is 0,
or wherein Ai and A2 are N and A3 is 0, or wherein Al and A2 are N and A3 is
NR.
In various embodiments, a method of the invention uses a compound
wherein Ai is C, A2 is N and A3 is 0, or wherein Al is 0, A2 is N and A3 is C,
or
wherein Li and L2 are each independently a bond or (CHR')n, and R5 or R6, or
both, comprises a heteroaryl ring. For example, at least one heteroaryl ring
of R5
or R6 can be pyridinyl or a pyridinyl N-oxide, pyrazinyl, pyrrolyl,
imidazolyl,
benzimidazolyl, thiophenyl, benzothiophenyl, fury!, benzofuryl, indolyl,
indolinyl, piperidinyl, quinolyl, or isoquinolyl; wherein any heteroaryl can
be
substituted with 0-5 J. More specifically, any heteroaryl can be substituted
with
0-5 R', F, Cl, Br, I, OR', CF3, OCF3, CHF2, or SO2N(R')2.
In various embodiments, a method of the invention uses a compound
wherein Li and L2 are each independently a bond or (CHR')õ, and R5 or R6, or
both, comprises a bicyclic carbocyclic ring, wherein the bicyclic carbocyclic
ring
is substituted with 0-5 J. For example, any bicyclic carbocyclic ring can be
substituted with 0-5 R', F, Cl, Br, I, OR', CF3, OCF3, CHF2, or SO2N(R)2.
In various embodiments, a method of the invention uses a compound
wherein Li is bond and R5 is a bicyclic ring moiety which is substituted with
0-5
J where the bicyclic ring moiety is any one of a-i to a-xxviii, wherein a wavy
line indicates a point of attachment:
103
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
1 ao, LeN1 i_e /___9N
.
a-i a-u a-iii a-iv
i 0 FR i_f_; Fp
=
a-v a-vi a-vii a-viii
i . i 41 i 41
N
N N
a-ix a-x a-xi
N
/N
ilk ilk ilk Ili
a-xii a-xiii a-xiv a-xv
N, 'Ibt, s'Itki
1 4I 1 0 41 410 0
a-xvi a-xvii a-xviii a-xix a-xx
41 1 .
NJ NJ N z, N N . , 1 N N N, N N
I
N
a-xxi a-xxii a-xxiii a-xxiv a-xxv
;
wherein any of the bicyclic ring moieties is substituted with 0-5 J.
In various embodiments, a method of the invention uses a compound
wherein Li and L2 are each a bond; R5 is a 6-membered heteroaryl ring moiety
substituted with 0-3 occurrences of J'; wherein J1 is selected from the group
consisting of OR', CF3, Cl, Br, F, CN, 0(Ci-C6)alkoxy, 0(CI-C6)cycloalkoxy,
alkyl, N(W)2; and wherein the optionally substituted 6-membered heteroaryl
ring
moiety of R5 is any one of b-i to b-xiii:
104
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
____________ (N (H
\ N ____________________________________ (
cN)
0
b-i b-ii b-iii J b-iv
0 oe
e \
1µ1C) //-N
\
-/
b-v b-vi b-vii b-viii
1\\I\N Nµ
_________________________ \/\N cNI\)
-/
b-ix b-x b-xi
N-N
b-xii b-xiii
wherein each of the 6-membered heteroaryl ring moieties is substituted with 0-
3
JI, or wherein LI is a bond, and L2 is c-i or c-H, wherein a wavy line
indicates a
point of attachment:
L¨S r CF
= 3 / 3
/) I
R6 R6
c-i c-ii
wherein c-i and c-u are further substituted with 0-2 J.
In various embodiments, a method of the invention uses a compound
wherein LI is a bond and L2 is a bond or is phenyl substituted with 0-5 J; and
R5
and R6 are independently selected from phenyl or heteroaryl each substituted
with 0-5 J; provided that if L2 is a bond and R5 and R6 are both phenyl, then
R5
is substituted with at least one of 4-CN, 3-alkyl-N(R')2, 3-alkyl-OR', 4-alkyl-
OR', or 2,3-dialkyl, and R6 is substituted with at least 4-OR'.
In various embodiments, the optionally substituted bicyclic ring moiety
can be any one of a-i to a-viii.
105
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
1 41 1- 11µ8 i--/i9 F- EN
a-i a-ii a-iii a-iv
'Z Fp F-1\ I-81
a-v a-vi a-vii a-viii
wherein any of the bicyclic ring moieties is substituted with 0-5 J.
In various embodiments, a method of the invention uses a compound
having the formula I-B further substituted with 0-5 J:
N-C3I
I L2
0 N R6
e
I-B, or having the formula I-C further substituted with 0-5 J:
I
0 N
e
I-C, or having the formula I-D and further substituted with 0-5 J, and wherein
R7
and R8 each independently are H, OR', OC(0)N(R')2, N(R')N(R1)2,
N(R')CH2CH2OR', CN, CHF2, CF3, OCF3, NO2, R', =0, =S, C(0), S(0), N(V)2,
SR', SOR', SO2R', SO2N(R)2, SO3R', or C(0)1V, or R7 and R8 together are =0,
=NR', or =N(R')CH2CH2OR'.
I
k
W
R7 r,
rci3
1-D ,or wherein the compound has a formula I-F
106
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
N-0
IN
R7 =
R8
X
I-F
wherein R7 and R8 are each independently selected from H, OR", N(R")2,
and SR", wherein R" is hydrogen or an alkyl, cycloalkyl, aryl, heterocyclyl,
or
heteroaryl, wherein any such alkyl, cycloalkyl, aryl, heterocyclyl or
heteroaryl is
substituted with 0-3 J; X is F, Cl, Br, I, CHF2, CN, CF3, NO2, or OR'; Y is
hydrogen or an alkyl, cycloalkyl, aryl, heterocyclyl, or heteroaryl, wherein
any
such alkyl, cycloalkyl, aryl, heterocyclyl or heteroaryl is substituted with 0-
3 J.
In various embodiments, a method of the invention uses a compound
wherein the compound is any of the
following:
107
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
0-
1\1" --( NI- ><="i N-0 - 9
N- =Nk,
I />-- .1/N 1 /_ \ / 1 / \ /N-0
IP N
0 N 0 N 0 N _____
F3C F3C F3C F3C
1 2 3 4
N-C)- \k, - \
NI - //\ _________________________________________________ N N\I(R__
TO/ycN
1 / \ 1.1
N' \ _______________________________________________________ 1(
0 N = N /(F
0 CI N
14
F3C ,0 \ 40
F3C F3C
0/
6 7 8
I\(1.____-
ONr N
Nar 0/
/
N
/--/
I \ * 0
N-0
N0' .
Cr)/ 1 N\ . 0
N-0 p
9 10 11
1\--c
N-0
111
N 0 N
N0' . H2N-c
0---'b
0 14
12 0---j
,7)1-3____>- N-0
,0
N /
\ N H2N. IS
N .
0--%
= 1
6
H2N-, =0
0
N-R/---...--\ Nii- 0,\ 7_, \N
Nr0>____c_m
. 40 N---N la Ni--- 0 N N
H2N.q NH2 H2N, RV
/0 H2N.q
18 19 20
CI
N1-10\ /--,--\- 1\1-0>___-
0 NN 10, N \ N
H2N, F H2N.q CI
21 22
108
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
NH2
0 ____________ (
N' ¨ m NO -N
- N>__(¨\ _
1 />---( IN, 1 / \ i I / \ ,NO
N ______________ = N
0 0 0
/ 23 24 25
>7___
Nõ,
' N
`' ¨ m NI '(:)(= 0 __
I / \ 1., "
0 N
HO ,N ___________________ ,N _______
1 /IN
(
CI
0 0 0
26 27 28
¨\
N1-0/__< ______ N
NI - so 0-N
N-0
. Ni \ ________ /(
CI 0 N
I I / N 'NJ 0
H
0
/ 0 0
/
29 30 31
OEt OEt
Ni_ii0 / * OEt N-0 OEt N
OEt
N N ( N NI *N =
i / OEt
N ,-
NiicyLN
32 33 34
OEt OEt
11-(:), * OEt N-C) .
N-0 Et0
H2N OEt
fryN / N I NI I /=0. Et
N ,- N õ-- 0--LN
N
35 36 37
\c)
OEt OMe
N"0 WC)
/ N NO
x N/1 1 C(N
N õ- Ni , 1 ON
38 39 (:) 40
F
N N-0N (
N
(-- //1µ1 1 N> __ (¨
1 \N
40 _________________________________ N __ 6
, ON 1 i \ ____ ,
0, 0, I:)
41 42 43
109
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
CI
N-0\ /--\.. N1-0 __ (IN N 0 . CN
i /1¨N ,11 = i. /
ON 0 N
CI ON
Co Co ,C)
44 45 46
(
N"C)S 0 N'0 N¨N CF3
1 / \ ri-0/
*
ON ON
N07LN
7
(:) Co
47 48 49
N-C)
0 1\1" ¨
ry, / * 0
N . 01 r7kr4 \ / ---,. N
rY1=1 µ NI 7 HO
,I
N -..%
50 51 52
Q .0
1\1-0 II = 0 N \ NO
(1\j
d
N- . 0/
A , (1\1/
W.% A ,
53 N. N,, 54 55
OEt
WC)
1µ1" /> _________________________________ CT( N-0/ .
OEt
ykr,j . 111 -,ryi"¨N
I
1 ,
N 7 N
IV,_
56 57 58
t--./--\¨
N
-NI \ /N1 \\_ =
0 -11f--
NH2
0 CF3 59 0 CF3 60 0 CF3 61
q 04\1\v /---,-N
= -N\) \ / 0 1\r"¨µ1
Et0 0 -N \ /N
0 CF3 0 CF3
62 63 64
110
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
0-N\__ /-=--\- 0-1ql 0-1`1\v_
7-----.N
Et0 0 -1\17--N Et0 =-N \ / Et0 0 -
N_I
NH2
65 66 67
0-N\__ /----=.\ 0N
F3C
F3C 0 -N \ /N F3C 0 -1\l/N F3C 0 -N
\ /
NH2
68 69 70
-1-1µ1 0- N -- 0-N)___ N
0 -c--
Me0 0 - \ ,N
F3C 0 -N \ / Me0
NH2
71 72 73
O-N\>c-? 0"Nµv /---=N
\)____c--
Me0 0 -N \ / Me0 N-I___:1 101 -'1µ1 \ /N
74 75 76
0 0 0
N-C) * or- N1- . r¨ NI-C) . r¨
I , I / 0 I / 0
410 N 40 N
0 10 N
01 77 'N NH
I 78
0-' 79
N r
0-N
0 0-N
. I
N \ . N- 0-N
Si '1\1µ . N
SI 40 -NJ = 0\ . N
Et0 Et0
OEt OEt 10 CF3
80 81 82
0-N 0-N ----V
* N)-CN
,
0
CI Et0
CF3 OEt
84
1 1 1
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
0-Nµl 0-N
0 -N)------CN--e) 0 N?----C\N- 0 N)----CN-
0 Et0
CF3 OEt
86 87 88
\
o
N-N 0
i ..__ N'C' = 1----
1 / 0 N
0 0"_ ......_ C N I i 0
* N
. N
Et0 o
OEt oCN)
89 CN 90 91
N)
.;
Boc N
H
o
N-0 ap. /¨
I . 0 o o
o 1110 N -N
oN I H I \
cN) N , N y
92 93 94
N
I
Et0
,N\ "
0 ,N,> ---QN
la 0-in---/ WI
0-N Et0 _I\l/ = Br
NH2 o-
95 96 97
0
Co 140 _NCiN is _N/ .
O-N --,...0 At
1\) 0 WI 7-----\
__Ni 4. N\____ JN-
0-N 0-N
98 99 100
OMe
j.-NC:>___P
I II N-0
r/ ilif
1
N,. NI, OMe
101 102 103
=
112
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
CF3 Br
WO it N-0
N/ IIP
01-1%(311/ IP CF3 r
1
I
ryN
N I N, CF3
N
104 105 106
NO*
r-1=1/
I
01'.N 1
N,. I N,.
N
107 108 109
(
0
WO N-0
/ , 11), sr 0
r-----
r--1--lq
! 0)____.0
. N 1
N,.. 1
N
HN /
110 111 112
(
0 WO
N-0
(Qtµl/ *i NII-0
N
I 0
ryN/
i
N 0
C C5 N
. 113 114 115
,
\
,
ry IP s
N, , N /
F3C
N
CF3
116 117 118
0-1\1"_c- 0.-11,_ r-s--\- N-0 OEt
* N \ /N
OEt
0 -Nr-N Br 0 N
0 119 120 121
113
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
OEt OEt
N-10
OEt N-0
WO
/ ,
OEt *
OEt
OEt 10 N
0 N 0 N
,
NC \ NH
HN
122 123 124
N-0 N-C/
0 1 /
1 /
NN A-& /--- * N 41
CIN 110 N = N .
N\..D N(
I
125 126 127
0 0 WO
N- * /-
1 / 0 1\1' N-C)
'p N
. I 14 N1,.
,N
128 129 130
N-0F
WO
rN 2 WO /--N *
N, N
,.- NW 1 I NI/ IIP.
N CN
131 132 133
N-0 N'ID WO
II N/ . ryN/ IIP I ,
--.-i-'1\1 *
I 1 N (
N,.., N .- 0 N,
134 135 136
NH
0
07--\/
--
WO
CF3
WO Br
IIP N-0
, , *
N
I HN 0 N
CF3 CF3
i\lõ, 1101
137 138 139
114
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
OEt N-0 N-0
N- ip
N N
N IP / / lp /
OEt 10 0
140 141 142
CF3 Br
N-0 S CF3 N-0 N-0
0 N
/ 0
O 0 *
N
CF3
N
CF3
143 144 145
OEt
N0
N-0 - N-0
i 7 ip, , , , ip N
OEt 0 N
0 ID 0 N
146 147 148
N-0 S CF3 N-0 N-0
, 1 N Ilit OEt N Ill OEt
10 N '
* 0 OEt * OEt
149 NH 150 N 151
0--\ 0----\
N N-0 ) OEt N-0 \ N-0
/ / II
0 I / lip
0
/ ra N
) a N
)
0 N 111/ OEt
N
H N
H
152 153 154
N-0 0--\
AI
H / / lip,
0 N-0 N-0
0 N is N
)
0 N *
155 156 CF3
157
it
N-0 N-0
0 N
/0 0 N Illik 0
/---
0 N
0 CF3
\
158 159 160
115
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
CN
N-0
N, CF3 1
N, 1\1,_,
161 162 163
4
WO N-10 0---\
N-C) 0--\
(II /
1 0 N
) 0 N
)
N, CF3
164 NH2
165 166
N-0 0---\
N-0 S CF3 N-0 S CF3
i / .
N
1
) 01 * N
41
= 0 N
10
N
HN /
167 168 169
N-0 S CF3 1\1" = N/
1 / \ 1
\
0 N
0 I I I
NI," 172
HN 171
WC) * N-0
(1µ1 r)L1µ; . WC) * Nr---
I N¨ 1
IJ.) r14 \---
/ N.," I
173 174 175
CF3 OEt \
NI-0/ . = N 1 / =. OEt 1\1 NH
"
N . N
HO 0 N (11 4
I
N.,..=
176 177 178
\--NH
0-N H
N.Th
WO N-10 N\ *
0 \ j
ON/ * 1
0 N
1 rN/ *
N
179 / 1\1 N.,, 180 ---- (:)
181
c), 0 , 1
116
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
S\ O-N
N-0II di N
I \ ...µ,,..6
rN IP S N I 0
F3,..n , N
N, Cs
182 183 I
184
HO
0-N
11) OH O-N
S--N Br 0 N
0 N
\ *
0
0 0
(:) OTh
I I 00
185 186 I 187
N-0
O-N H 0 \ 0 -N
N =0
r---
NOrNH2
N , S 1 0 0
\ S F3t... , N N
I C
F3C
188 189 190
N-0N-0 N-0
lit Or¨ 11 o'5I10 N
0 * N
0 N/ *
/0 /0
HO
0 c \ \
191 192 193
WON-0
N-0 i / * 0 0 N
r-- = Or¨
=Or¨
ON 1St N I
,N /0
)1 101 N
0 c0
\
C
197 198 199
117
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
N-0 N-0 N-0
HO 0 NI I / 1111 Or¨ = 'N' 4110' Or¨
0
/0 HO 0 0 N
/0
\ \ \
200 201 202
0
0)
N-0 ----\0 *
K',, 0 0.õ...-
Or-- 0
I 5 N / .
N ,N i----/ lip \N,
,N 0 NH N-0
C 01 \
N F3C0
H
203 204 205
OEt
Oj OEt
N--= ,c) * OEt
0 'N' * N /
OEt ,
F3C0 = \N- 0
N__ N
N-0 / NO
HN
IF
206 207 208
OEt
OEt
N ,c) / * OEt 0
WO
* OEt
\ N = Cr-
r.,41-1,1
Nr * 9 µ
N. N
209 0 OH 210 50 211
)
0 ---\ 0 OEt
. 01¨ 0 $
* OEt
9 \
9 \1\1õN
N- N N/ 9
-N
* 1 7
N
41 212 N 214
H OH OH
213
118
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
OEt OEt OEt OEt
* OEt * OEt * OEt * OEt
O\ 9 \ 9 \ 9 \
N. N N. N N. N N. N
O.
S. O. ir\
N
OH HN-k-OH N-- HN-k-OH
/
215 216 217 218
7-0
0 0 r0
= Cr- . 07- 0- l) *
C 0
9 \ 9 \ / .
0 / ,
N ,N / N , N H
N
N. N N. N
0 ' N
0 I N 5\
N OH
0 \
N
H /
H
0
219 220 221 222
r0 r0 r0 r0
0 . 0 = 0 = 0 =
C 0C 0 C C
0 0
N ,N NN N ,N N , N
0 0
N OH ,
I H
1\1 NEt2
HN / r\O HN / HN---\-N
223 224 225 226
0 0J 0J
r
/---0
o= 05 0
IW
0=
C 0
C /0
N , N
,
N , N N ' 9 N' p
-N -N
Se Oe NH 41 1
1 N3
N--,.. N I N *OH
0
H OH H
227 228 OH 229 230
119
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
oJ
----- \ o)
o) ----1 oJ
0 di
411,- 0 = ----1
0 . 0 AI
WI'
N' 9'
N ' p -N N'9 r49
-N -N
-N
NH
N
H 1 OH ) N
H riN
,N, HO
231 I I 232 233 234
oJ
oJ
o AI
iii, o al
iir
o
Nr 0
. Nr
r-
1 / I / 011 0
N' p N' p 0 N
* N
-N -N
(t OH
IL HN
ND OH HN __ H
NThr
N---
ND IP I
235 HO' 236 237 238
HO
OEt OEt
0 OEt * OEt
N" * C
N P \
1 . -0 41/ . C
P \ N. N
=
. N 'NI
N. N
HN __ H
HN
(Tf0 ?le
NN-7'-'0H N
NH
OH
239 240 241 5 242
HO
OEt
41 OEt 41
F3c
o\ _
N. N S z
Se N ' p
-N
NH
41
c....NEt2
HO
243 246
120
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
oJ
, . OEt
F3C * F3k., .
OEt
0 _
S ,...
1110 S/ 9 \
N. N
N '''. p N' p N'9
011
114k
114---1, N
Isrlj N . j
N
H H
247 248 249 250
N-C)
Isl-C)
ol / = )--. I/I,,0
0 0-
N
HO...........--..N N0
0 N 0 N
H H
0-1 HOI'l
251 253
252
WC) 0
N-0/ * WO 0 CF3
\ I 0 I ts1.---i.--
--
0 N 0
0-1 . N
HN 0
HN
N
F3C
F3C 254 255 256
N-0
N-
N-Ck /---...-\ 0/ . c, i
O N" .
0
N N
= N---N 0-/ e
CF
HO
0
HO
1-....õ 0...._.,.,,
257 258 259
N-0 N-0 N-0
O I Islx IP O' * I NI . c)) O I N' .
0
e CF3
e CN e CN
HO HO HO
260 261 262
N-0
N-0 N-0
= I
I N' * eL = I N' *
e CF3 * CF3
HO,./"-N e NI/ 0
CN
HO/-N HO,..,"N
H
H H
263 264 265
121
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
N-N N-N
o
N
N 0 N
- 0 N \ oz\ IN \ / X o ,
H
266 0¨\ 267
268 0¨\
N N-=
N
0 IP o
ifk 0 lip
=
CN CN
269
HO 270 271
In various embodiments, a method of the invention uses a compound
wherein the compound activates or agonizes the sphingosine-1 -phosphate
receptor subtype 1 to a greater extent than the compound activates or agonizes
another subtype of sphingosine-l-phosphate receptor. For example, the other
subtype of sphingosine-1 -phosphate receptor can be subtype 3. In various
embodiments, the sphingosine-1 -phosphate receptor subtype 1 can be disposed
within a living mammal.
In various embodiments, the invention provides a method of treatment of
a malcondition in a patient for which activation, agonism, inhibition, or
antagonism of an Si P1 receptor is medically indicated, comprising contacting
the S1P1 receptor according to a method of the invenetion by administering the
compound to the patient at a frequency and for a duration of time sufficient
to
provide a beneficial effect to the patient. For example, selective activation
or
agonism of an SIP subtype 1 receptor with respect to other subtypes of SIP
receptor is medically indicated. More specifically, the malcondition can
comprise multiple sclerosis, transplant rejection, or adult respiratory
distress
syndrome. The inventive method can further comprise administering an
effective amount of a second medicament to the patient, such as wherein the
second medicament is adapted for treatment of multiple sclerosis, transplant
rejection, or adult respiratory distress syndrome.
Compositions and Combination Treatments
The S1P1 compounds, their pharmaceutically acceptable salts or
hydrolyzable esters of the present invention may be combined with a
pharmaceutically acceptable carrier to provide pharmaceutical compositions
122
. CA 02723904 2015-08-11
useful for treating the biological conditions or disorders noted herein in
mammalian species, and more preferably, in humans. The particular carrier
employed in these pharmaceutical compositions may
ary depending upon the type of administration desired (e.g. intravenous, oral,
topical, suppository, or parenteral).
In preparing the compositions in oral liquid dosage forms (e.g.
suspensions, elixirs and solutions), typical pharmaceutical media, such as
water,
glycols, oils, alcohols, flavoring agents, preservatives, coloring agents and
the
like can be employed. Similarly, when preparing oral solid dosage forms (e.g.
powders, tablets and capsules), carriers
such as starches, sugars, diluents, granulating agents, lubricants, binders,
disintegrating agents and the like can be employed.
Another aspect of an embodiment of the invention provides compositions
of the compounds of the invention, alone or in combination with another S1P1
inhibitor or another type of therapeutic agent, or both. As set forth herein,
compounds of the invention include stereoisomers, tautomers, solvates,
hydrates,
salts including pharmaceutically acceptable salts, and mixtures thereof
Compositions containing a compound of the invention can be prepared by
conventional techniques, e.g. as described in Remington: The Science and
Practice of Pharmacy, 19th Ed., 1995. The compositions can appear in
conventional forms, for example capsules, tablets, aerosols, solutions,
suspensions or topical applications.
Typical compositions include a compound of the invention and a
pharmaceutically acceptable excipient which can be a carrier or a diluent. For
example, the active compound will usually be mixed with a carrier, or diluted
by
a carrier, or enclosed within a carrier which can be in the form of an
ampoule,
capsule, sachet, paper, or other container. When the active compound is mixed
with a carrier, or when the carrier serves as a diluent, it can be solid, semi-
solid,
or liquid material that acts as a vehicle, excipient, or medium for the active
compound. The active compound can be adsorbed on a granular solid carrier,
for example contained in a sachet. Some examples of suitable carriers are
water,
salt solutions, alcohols, polyethylene glycols, polyhydroxyethoxylated castor
oil,
peanut oil, olive oil, gelatin, lactose, terra alba, sucrose, dextrin,
magnesium
carbonate, sugar, cyclodextrin, amylose, magnesium stearate, talc, gelatin,
agar,
123
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
pectin, acacia, stearic acid or lower alkyl ethers of cellulose, silicic acid,
fatty
acids, fatty acid amines, fatty acid monoglycerides and diglycerides,
pentaerythritol fatty acid esters, polyoxyethylene, hydroxymethylcellulose and
polyvinylpyrrolidone. Similarly, the carrier or diluent can include any
sustained
release material known in the art, such as glyceryl monostearate or glyceryl
distearate, alone or mixed with a wax.
The formulations can be mixed with auxiliary agents which do not
deleteriously react with the active compounds. Such additives can include
wetting agents, emulsifying and suspending agents, salt for influencing
osmotic
pressure, buffers and/or coloring substances preserving agents, sweetening
agents or flavoring agents. The compositions can also be sterilized if
desired.
The route of administration can be any route which effectively transports
the active compound of the invention which inhibits the enzymatic activity of
the
focal adhesion kinase to the appropriate or desired site of action, such as
oral,
nasal, pulmonary, buccal, subderrnal, intradermal, transdermal or parenteral,
e.g.,
rectal, depot, subcutaneous, intravenous, intraurethral, intramuscular,
intranasal,
ophthalmic solution or an ointment, the oral route being preferred.
For parenteral administration, the carrier will typically comprise sterile
water, although other ingredients that aid solubility or serve as
preservatives can
also be included. Furthermore, injectable suspensions can also be prepared, in
which case appropriate liquid carriers, suspending agents and the like can be
employed.
For topical administration, the compounds of the present invention can
be formulated using bland, moisturizing bases such as ointments or creams.
If a solid carrier is used for oral administration, the preparation can be
tabletted, placed in a hard gelatin capsule in powder or pellet form or it can
be in
the form of a troche or lozenge. If a liquid carrier is used, the preparation
can be
in the form of a syrup, emulsion, soft gelatin capsule or sterile injectable
liquid
such as an aqueous or non-aqueous liquid suspension or solution.
Injectable dosage forms generally include aqueous suspensions or oil
suspensions which can be prepared using a suitable dispersant or wetting agent
and a suspending agent Injectable forms can be in solution phase or in the
form
of a suspension, which is prepared with a solvent or diluent. Acceptable
solvents
or vehicles include sterilized water, Ringer's solution, or an isotonic
aqueous
124
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
saline solution. Alternatively, sterile oils can be employed as solvents or
suspending agents. Preferably, the oil or fatty acid is non-volatile,
including
natural or synthetic oils, fatty acids, mono-, di- or tri-glycerides.
For injection, the formulation can also be a powder suitable for
reconstitution with an appropriate solution as described above. Examples of
these include, but are not limited to, freeze dried, rotary dried or spray
dried
powders, amorphous powders, granules, precipitates, or particulates. For
injection, the formulations can optionally contain stabilizers, pH modifiers,
surfactants, bioavailability modifiers and combinations of these. The
compounds can be formulated for parenteral administration by injection such as
by bolus injection or continuous infusion. A unit dosage form for injection
can
be in ampoules or in multi-dose containers.
The formulations of the invention can be designed to provide quick,
sustained, or delayed release of the active ingredient after administration to
the
patient by employing procedures well known in the art. Thus, the formulations
can also be formulated for controlled release or for slow release.
Compositions contemplated by the present invention can include, for
example, micelles or liposomes, or some other encapsulated form, or can be
administered in an extended release form to provide a prolonged storage and/or
delivery effect. Therefore, the formulations can be compressed into pellets or
cylinders and implanted intramuscularly or subcutaneously as depot injections.
Such implants can employ known inert materials such as silicones and
biodegradable polymers, e.g., polylactide-polyglycolide. Examples of other
biodegradable polymers include poly(orthoesters) and poly(anhydrides).
For nasal administration, the preparation can contain a compound of the
invention which inhibits the enzymatic activity of the focal adhesion kinase,
dissolved or suspended in a liquid carrier, preferably an aqueous carrier, for
aerosol application. The carrier can contain additives such as solubilizing
agents, e.g., propylene glycol, surfactants, absorption enhancers such as
lecithin
(phosphatidylcholine) or cyclodextrin, or preservatives such as parabens.
For parenteral application, particularly suitable are injectable solutions or
suspensions, preferably aqueous solutions with the active compound dissolved
in
polyhydroxylated castor oil.
125
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
Tablets, dragees, or capsules having talc and/or a carbohydrate carrier or
binder or the like are particularly suitable for oral application. Preferable
carriers for tablets, dragees, or capsules include lactose, corn starch,
and/or
potato starch. A syrup or elixir can be used in cases where a sweetened
vehicle
can be employed.
A typical tablet that can be prepared by conventional tabletting
techniques can contain:
Core:
Active compound (as free compound or salt thereof) 250 mg
Colloidal silicon dioxide (AerosiDe 1.5 mg
Cellulose, microcryst. (Avice1)8 70 mg
Modified cellulose gum (Ac-Di-Sol) 7.5 mg
Magnesium stearate Ad.
Coating:
HPMC approx. 9 mg
*Mywacett 9-40 T approx. 0.9 mg
*Acylated monoglyceride used as plasticizer for film coating.
A typical capsule for oral administration contains compounds of the
invention (250 mg), lactose (75 mg) and magnesium stearate (15 mg). The
mixture is passed through a 60 mesh sieve and packed into a No. 1 gelatin
capsule. A typical injectable preparation is produced by aseptically placing
250
mg of compounds of the invention into a vial, aseptically freeze-drying and
sealing. For use, the contents of the vial are mixed with 2 mL of sterile
physiological saline, to produce an injectable preparation.
The compounds of the invention can be administered to a human in need
of such treatment, prevention, elimination, alleviation or amelioration of a
malcondition that is mediated through the action of S1P1, for example,
multiple
sclerosis, transplant rejection, and adult respiratory distress syndrome.
The pharmaceutical compositions and compounds of the present
invention can generally be administered in the form of a dosage unit (e.g.
tablet,
capsule, etc.) in an amount from about 1 Wlcg of body weight to about 1 g/kg
of
body weight, preferably from about 5 WIcg of body weight to about 500 mg/kg of
126
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
body weight, more preferably from about 10 pfkg of body weight to about 250
mg/kg of body weight, most preferably from about 20 p./kg of body weight to
about 100 mg/kg of body weight. Those skilled in the art will recognize that
the
particular quantity of pharmaceutical composition and/or compounds of the
present invention administered to an individual will depend upon a number of
factors including, without limitation, the biological effect desired, the
condition
of the individual and the individual's tolerance for the compound.
The compounds of the invention are effective over a wide dosage range.
For example, in the treatment of adult humans, dosages from about 0.05 to
about
5000 mg, preferably from about 1 to about 2000 mg, and more preferably
between about 2 and about 2000 mg per day can be used. A typical dosage is
about 10 mg to about 1000 mg per day. In choosing a regimen for patients it
can
frequently be necessary to begin with a higher dosage and when the condition
is
under control to reduce the dosage. The exact dosage will depend upon the
activity of the compound, mode of administration, on the therapy desired, form
in which administered, the subject to be treated and the body weight of the
subject to be treated, and the preference and experience of the physician or
veterinarian in charge. S1P1 agonist bioactivity of the compounds of the
invention can be determined by use of an in vitro assay system which measures
the activation of Si P1, which can be expressed as EC50 values, as are well
known in the art inhibitors of the invention can be determined by the method
described in the Examples.
Generally, the compounds of the invention are dispensed in unit dosage
form including from about 0.05 mg to about 1000 mg of active ingredient
together with a pharmaceutically acceptable carrier per unit dosage.
Usually, dosage forms suitable for oral, nasal, pulmonal or transdermal
administration include from about 125 tg to about 1250 mg, preferably from
about 250 jig to about 500 mg, and more preferably from about 2.5 mg to about
250 mg, of the compounds admixed with a pharmaceutically acceptable carrier
or diluent.
Dosage forms can be administered daily, or more than once a day, such
as twice or thrice daily. Alternatively dosage forms can be administered less
frequently than daily, such as every other day, or weekly, if found to be
advisable by a prescribing physician.
127
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
An embodiment of the invention also encompasses prodrugs of a
compound of the invention which on administration undergo chemical
conversion by metabolic or other physiological processes before becoming
active pharmacological substances. Conversion by metabolic or other
physiological processes includes without limitation enzymatic (e.g, specific
enzymatically catalyzed) and non-enzymatic (e.g., general or specific acid or
base induced) chemical transformation of the prodrug into the active
pharmacological substance. In general, such prodrugs will be functional
derivatives of a compound of the invention which are readily convertible in
vivo
into a compound of the invention. Conventional procedures for the selection
and
preparation of suitable prodrug derivatives are described, for example, in
Design
of Prodrugs, ed. H. Bundgaard, Elsevier, 1985.
In another embodiment, there are provided methods of making a
composition of a compound described herein including formulating a compound
of the invention with a pharmaceutically acceptable carrier or diluent. In
some
embodiments, the pharmaceutically acceptable carrier or diluent is suitable
for
oral administration. In some such embodiments, the methods can further include
the step of formulating the composition into a tablet or capsule. In other
embodiments, the pharmaceutically acceptable carrier or diluent is suitable
for
parenteral administration. In some such embodiments, the methods further
include the step of lyophilizing the composition to form a lyophilized
preparation.
The compounds of the invention can be used therapeutically in
combination with i) one or more other Si P1 inhibitors and/or ii) one or more
other types of protein kinase inhibitors and/or one or more other types of
therapeutic agents which can be administered orally in the same dosage form,
in
a separate oral dosage form (e.g., sequentially or non-sequentially) or by
injection together or separately (e.g., sequentially or non-sequentially).
Accordingly, in another embodiment the invention provides
combinations, comprising:
a) a compound of the invention as described herein; and
b) one or more compounds comprising:
i) other compounds of the present invention,
128
= CA 02723904 2015-08-11
li) other medicaments adapted for treatment of a malcondition for
which activation of S1P1 is medically indicated, for example multiple
sclerosis,
transplant rejection, or adult respiratory distress syndrome.
Combinations of the invention include mixtures of compounds from (a)
and (b) in a single formulation and compounds from (a) and (b) as separate
formulations. Some combinations of the invention can be packaged as separate
formulations in a kit. In some embodiments, two or more compounds from (b)
are formulated together while a compound of the invention is formulated
separately.
The dosages and formulations for the other agents to be employed, where
applicable, will be as set out in the latest edition of the Physicians' Desk
Reference.
Methods of Treatment
In various embodiments, the present invention provides a method for
activating or agonizing (i.e., to have an agonic effect, to act as an agonist)
a
sphingosine-l-phosphate receptor subtype, such as Si P1, with a compound of
the invention. The method involves contacting the receptor with a suitable
concentration of an inventive compound to bring about activation of the
receptor. The contacting can take place in vitro, for example in carrying out
an
assay to determine the S 1P receptor activation activity of an inventive
compound
undergoing experimentation related to a submission for regulatory approval.
The method for activating an S113 receptor, such as S1P1 , can also be
carried out in vivo, that is, within the living body of a mammal, such as a
human
patient or a test animal. The inventive compound can be supplied to the living
organism via one of the routes as described above, e.g., orally, or can be
provided locally within the body tissues, for example by injection of a tumor
within the organism. In the presence of the inventive compound, activation of
the receptor takes place, and the effect thereof can be studied.
An embodiment of the present invention provides a method of treatment
of a malcondition in a patient for which activation of an SIP receptor, such
as
S1P1, is medically indicated, wherein the patient is administered the
inventive
compound in a dosage, at a frequency, and for a duration to produce a
beneficial
129
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
effect on the patient. The inventive compound can be administered by any
suitable means, examples of which are described above.
Experimental procedures for studying agonist-induced internalization, receptor
phosphorylation and receptor polyubiquitination in stably expressed Si P1-GFP
cells
Materials. SIP was obtained from Biomol. The SIP receptor agonist, AFD-R,
was a gift from Dr. Brickman (Novartis Pharma). Anti-GFP antibodies (ab-1218
and ab-6556) were from Abcam, anti-ubiquitin P4D1 antibody from Santa Cruz,
4-12% Tris-Glycine Novex SDS-PAGE gels from Invitrogen, P32
orthophosphate from Perkin-Elmer. Fetal bovine serum (FBS) and charcoal-
stripped-FBS were from Hyclone, and other culture reagents were from the TSRI
Supply Center (supplied by Invitrogen and Gibco BRL).
Cell culture. HEK-293 cells stably expressing the GFP-tagged human SP!
receptor (S1P i-GFP) and 293-vector-GFP cells were a gift from Dr Timothy Hla
(Connecticut Health Science Center). Cells were maintained in high-glucose
modified Eagle's medium containing GlutaMAX, and supplemented with 10%
FBS, 1% penicillin/streptomycin solution and selected with 50Oug/m1 G418
(Gibco BRL).
Microscopy imaging studies for ligand-mediated Si P1-GFP internalization.
Single SIP i-GFP cells grown in gelatin-coated coverslips were used to
study ligand induced Si -GFP internalization. Cells were incubated overnight
in charcoal-stripped FBS (cs-FBS) medium before the start of the experiment,
and all incubations thereafter were done in cs-FBS medium-containing 15ug/m1
cyclohexamide. Cells were incubated with agonists (or vehicle control for the
indicated times and reactions were terminated by removal of medium, and
washing with PBS. In experiments with the antagonist W146, antagonist or
vehicle was added to the cells for 30-45 min prior to agonist incubation.
Cells
were fixed in 3.7% paraformaldehyde for 10 min and mounted on coverslips
using GelMount mounting media. Cells were scanned with an Olympus BX61
scanning confocal fluorescence microscope. For detecting GFP, fluorescence
was excited by using an argon laser at a wavelength of 488 nm, and the
absorbed
wavelength was detected for 510-520 nm for GFP. Photomicrographs of ligand
vs. vehicle were obtained using Metamorph software and the images were
assessed (in Photoshop) for the appearance or not of vesicular S1PI-GFP
pattern
130
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
of internalization, a characteristic pattern adopted by most G protein-coupled
receptors following ligand stimulation.
Immunoprecipitation and immunoblotting for Si P1-GFP and ligand-stimulated
S1131 polyubiquitination
The effect of agonist-stimulated recruitment of poly-ubiquitin chains to
SIP i-GFP was analyzed by immunoprecipitation-immunoblotting experiments
with anti-GFP antibodies. Cells were seeded in 35mm dishes and grown to
¨95% confluence using regular growth medium. The growth medium was
replaced by cs-FBS medium and the cells were incubated overnight. Drugs or
vehicle (both made in cs-FBS medium) were incubated for the indicated times.
At the end of incubation, the monolayers were washed twice in ice-cold PBS and
lysates were obtained by incubation in RIPA buffer (50 mM Tris-HC1, pH 7.5,
150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 0.5% sodium deoxycholate,
0.1% SDS) plus protease inhibitors (Complete tablets, Roche), and 1mM
NaVO4, 1mM NaF and 0.5M B-glycerol-phosphate. Cellular lysates were
cleared by centrifugation (10,000xg, 15 min) and the protein concentration of
the
lysate supernatants was determined by the BCA (Pierce) method. Equal
amounts of lysates (0.5-1mg) were incubated overnight at 4C with a monoclonal
GFP antibody (1 ug antibody per 400 ug protein), followed by incubation with
protein-A sepharose beads (2h, 4C). The beads were recovered by centrifugation
(10,000xg, 1 min) and washing: 3x RIPA buffer: PBS (1:1) without protease
inhibitors and twice in PBS. The beads were suspended in 2X Laemli buffer
containing 2-mercaptoethanol, boiled for 10 min and proteins in the beads
separated by SDS-PAGE in Novex, 4-12% Tris-Glycine gels. Gels were
subsequently transferred to PVDF membranes, and probed overnight (4C) with a
polyclonal GFP antibody (1:10,000) for detection of Si -GFP expression or
P4D1 (1:200-1:800) to detect the S1PI-GFP-polyubiquitinated complex.
Horseradish peroxidase-labeled antibodies were visualized by ECL
chemiluminescence (Amersham Biosciences).
Agonists stimulated phosphorylation of SlPi-GFP in HEI(293 cells.
Cells stably expressing the GFP-tagged human SlPi were metabolically
labeled with P32 orthophosphate (80 Ci/ml, Perkin Elmer) for 2 h and
subsequently incubated with agonists at the indicated concentrations for the
indicated times at 37 C. Incubations were terminated by agonist removal and
131
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
PBS washing, and the receptor was immunoprecipitated with a GFP antibody
from equal protein amounts of cellular lysates. The immunoprecipitated
receptor
was separated by SDS-PAGE, and incorporation of P32 onto the agonist-
stimulated receptor was assessed by autoradiography (-80C, 24 h exposure).
Internalization, Ubiquination and Phosphorylation of S1P1-GFP
During the course of the lead optimization studies, compounds SR-917,
compound 32, and compound 236 were evaluated in depth in several biological
studies. SR-917 is a known agonist of the S1P1 receptor, indexed in the NIH
Molecular Libraries Small Molecule RepositoryMLMSRCompound ID is
976135. It is commercially available from ChemBridge Screening Library.
Agonistic stimulation of the S1P1 receptor is modulated by receptor
degradation. Ligand stimulation induces receptor phosphorylation,
internalization, polyubiquination and degradation(Gonzalez-Cabrera, Hla et al.
2007). Like AFD-R and SIP, stimulation with the synthetic compound
identified by high throughput screening, SR-917, results in S1P1-GFP
internalization, protein phosphorylation and polyubiquination; see Figure 1.
Compound 32 robustly induces internalization and polyubiquination with
5178 and these effects are blocked by the S1P1 antagonist, W146R; see Figure
2.
Compound 236 like other compounds in the series, induces S1P1
polyubiquination; see Figure 3.
It was observed that S 1P and the S1P1 specific agonist, SEW2897 induce
lymphopenia(Wei, Rosen et al. 2005). SR-917 and compound 32, delivered by
gavage, did not induce lymphopenia in mice. Compound 236, at 10 mgpk by
gavage, induced lymphopenia; see Figure 4. Compound 236 is soluble in water
at 0.5 mg/mL and both i.v. and i.p. delivery induce lymphopenia(Sanna, Leaf).
Pharmacokinetics
From the initial mouse efficacy studies (Figure 5) plasma levels of
Compound 236 at 5 hours were:395/87.6 Mean/SD. Stability in hepatic
microsomes for Compound 236 was species dependent. In human microsomes,
the compound was very stable and moderately stable with rat. All were NADPH
dependent. In the presence of 1.8 mg/ml hepatic microsomes the half lives
(minutes) are human stable, Mouse 50, Rat 16.
Si P1 polar amino acids essential for SIP mediated activation are not are
required for S1P1 receptor activation by Compound 236. SIP requires several
132
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
polar amino acids(R120, E121 and R292) that line the ligand binding pocket for
complete activation(Jo, Sanna et al. 2005). The Si P1 polar side chains of
residues R120, E 121 and R29 forms saltbridges with the phosphate of Si P1 and
the SIP can only minimally activate the R120A, E121A and R292A mutants. In
contrast, wild-type S1P1 and R120A, E121A and R292A mutant S1Plreceptors
are indistinguishably activated by Compound 236 (Figure 6).
Examples
The following compounds were synthesized and evaluated in bioassays
as described herein.
Synthetic Procedures
Solvents for extraction: ACS grade. Solvents for reaction: reagent grade.
Reagents: unless otherwise noted, from Alfa Aesar, Fisher and Aldrich highest
quality available. TLC: silica gel 60 F254 aluminum plates, (whatman, type Al
Si!
G/UV, 250 pm layer); visualization by UV absorption. Flash chromatography
was performed on silica gel 60 (0.40-0.63 mm, 230-440 mesh, EM Science).
NMR: 1H: 8 values in ppm (TMS as internal standard); 13C: 8 values in ppm
(TMS as internal standard)
Reactions were monitored by LC/MS.
General procedure to reduce aldehyde:
To a stirred suspension of aldehyde (1.0 equiv, 0.4M) and silica gel
(catalytic) in ethanol at 0 C was added NaBH4 (1/3 equiv). The reaction was
allowed to warm up to room temperature and stirred for 2 h. The Solvent was
removed under reduced pressure and the product purified by CC in
hexane/Et0Ac (7:3).
General procedure to synthesized amidoximes:
To a stirred suspension of Hydroxylamine hydrochloride (1.1 equiv) and
Na2CO3 (1.1 equiv) in ethanol was added, in one portion, the corresponding
benzonitrile (1 equiv). The mixture was refluxed for 6 h followed by addition
of
NH2OH.HC1 (1.1 equiv) and Na2CO3 (1.1 equiv), the reaction was refluxed for
additional 6 h. The suspension was cooled to room temperature and filtrated.
The
solid was washed with ethanol and the organic phase concentrated under reduced
pressure. The amidoxime crude was recrystallized from Et0Ac/Hexanes and
= used without further purification.
133
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
General procedure to synthesized oxadiazoles:
To a stirring solution of 3,4-diethoxybenzoic acid (1 equiv, 0.2M) in
DMF was added sequentially HOBt (1.3 equiv) and EDCI (1.3 equiv) at room
temperature. The reaction was stirred for 20 min followed by addition, in a
single portion, of the corresponding amidoxime (1.3 equiv, from previous
step).
The reaction was stirred for additional 30 mm at room temperature and then
heated to 90-95 C for 8-14h. The reaction was cooled to room temperature,
diluted using a saturated solution of NaCl and extracted with Et0Ac (3X). The
organic phase was dried over Na2SO4 anhydrous and concentrated under reduced
pressure. The product was purified by C.C. using CH2C12:Me0H (9:1) to offer
the diaryloxadiazoles in moderated yields.
General procedure to synthesize amines.
To a stirred solution of benzylalcohol (1 equiv) and pyridine (1.1 equiv)
in CH2C12 at 0 C was added drop wise SOC12 (1.1 equiv). The reaction was
warmed up to room temperature, stirred for additional 1h and concentrated
under
reduced pressure. To a stirred solution of crude chloride in CH2C12 at 0 C was
added dropwise a solution of pyrrolidine (3 equiv) in CH2C12. The reaction was
allowed to warm up to room temperature and stirred for 2 h. The organic phase
was washed with water and dried over Na2SO4 anhydrous. The crude was
concentrated under reduced pressure and purified by column chromatography in
DCM/Me0H to afford pyrrolidine derivatives in good yields.
Reduction of indole-derivatives.
To a stirred solution of Indole-core (1 equiv) in acetic acid at 13 C was
added slowly sodium cyanoborohydride (3 equiv). The reaction was stirred for 2
h at 13 C and monitored by TLC. After completion of reaction the mixture was
neutralize with 50% sodium hydroxide and the product extracted with ethyl
acetate. The organic layer was dried over Na2CO3 and removed under reduced
pressure. Indoline cores were purified by C.C. using CH2C12/Me0H (9:1) to
offering quantitative yield.
Spectroscopic Data for Selected Compounds
134
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
OEt
N-0
I /
NO OEt'LN
NMR (500Hz, CDC13)8: 8.80(s, 2H), 8.03(d, J= 6.0Hz, 2H), 7.80(dd, J=8.5,
2.0Hz, 1H), 7.67(d, J=2.0Hz, 1H), 6.99(d, J=8.5Hz, 1H), 4.23-4.16(m, 4H),
1.53-1.44(m, 6H).
'3C NMR (125Hz, CDC13)8: 176.47, 167.23, 152.99, 150.48, 148.88, 134.68,
122.16, 121.38, 120.31, 116.08, 112.50, 112.25, 64.82, 64.60, 14.68, 14,60.
MS.
(M+1) 312.
N-0 OEt
NO 1N=
Nr
N
OEt
NMR (300 MHz, CDC13) 8: 8.81 (brs, 2H), 8.06 (d, J= 6.0 Hz, 2H), 7.32 (s,
1H), 7.31 (s, 1H), 6.68 (t, J= 2.4 Hz, 1H), 4.10 (q, J= 6.9 Hz, 4H), 1.45 (t,
J=
6.9 Hz, 6H); 13C NMR (75 MHz, CDC13) 8: 176.68, 167.33, 160.64, 150.24,
134.96, 125.12, 121.62, 121.59, 106.64, 106.41, 64.06, 14.79. MS (M+1) 312
Et0
WC'
OEt
I Ni =
N
Iff NMR(500Hz, CDC13)8: 8.80(s, 2H), 8.05(d, J=5.0Hz, 2H), 7.65(d, J=3.0Hz,
1H), 7.11-7.09(dd, J=9.0, 3.0 Hz, 1H), 7.02(d, J=9.0 Hz, 1H), 4.18(q, J=7.0
Hz,
2H), 4.08(q, J=7.0Hz, 2H), 1.52(t, J=7.0Hz, 3H), 1.44(t, J=7.0Hz, 3H). 13C
NMR(125Hz, CDC13)8: 175.92, 166.66, 152.78, 152.44, 150.48, 134.73, 121.53,
116.36, 115.86, 115.40, 113.89, 113.82, 113.69, 65.62, 64.30, 14.84, 14.80.
MS.
(M+1) 312.
N-0 OEt
1\01 110
OMe
135
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
IHNMR(300Hz, CDC13)8: 8.81 (s, 2H), 8.04(d, J=4.5Hz, 2H), 7.82 (dd, J=8.4,
2.0Hz, 1H), 7.66 (d, J=2.0 Hz, 1H), 7.00(d, J=8.4 Hz, 1H), 4.22 (q, J=7.0 Hz,
2H), 3.97(s, 3H), 1.53 (t, J=7.0Hz). '3C NMR(125Hz, CDC13)8: 176.53, 167.28,
153.48, 150.41, 148.75, 134.89, 122.18, 116.30, 111.62, 111.36, 64.72, 56.20,
14.75. MS. (M+1) 298.
OEt
WC'
I
N
NMR(500Hz, CDC13)8: 8.81(s, 2H), 8.04(d, J=6.0Hz, 2H), 7.80(d, J=7.5Hz,
1H), 7.71-7.70(m, 1H), 7.47(t, J=8.5Hz, 1H), 7.16(d, J=8.5Hz, 1H), 4.16(q,
J=7.0Hz, 2H), 1.48(t, J=7.0Hz, 3H). 13C NMR(125Hz, CDC13)8: 176.48, 167.38,
159.39, 150.53, 134.50, 130.32, 129.40, 124.82, 121.39, 120.46, 120.03,
115.92,
113.32, 63.88, 14.70. MS. (M+1) 268.
OMe
WC'
x I N/
N
Ili NMR(500Hz, CDC13)8: 8.81(s, 2H), 8.05(d, J=4.5Hz, 2H), 7.73(d, J=8.0Hz,
1H), 7.60(s, 1H), 7.31(d, J=7.5Hz, 1H), 3.96(s, 3H), 2.31(s, 3H). 13C
NMR(125Hz, CDC13)6: 176.71, 167.31, 158.13, 150.51, 134.60, 133.06, 131.24,
130.10, 122.33, 121.41, 120.56, 118.84, 108.90, 55.58, 16.62. MS. (M+1) 268.
N-0 OMe
/ N Nr
N OMe
Me0
1HNMR (300MHz, CDC13)8: 8.84 (bs, 2H), 8.35(d,J=4.5Hz, 2H), 7.64(s, 1H),
6.63(s, 1H), 4.03(s, 3H), 4.01(s, 3H), 3.96(s, 3H). MS (M+1) 314.
N-0 =OMe
/ N rµ r
N
C.2
136
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
'H NMR (300MHz, CDC13)8: 8.80 (bs, 2H), 8.04 (bs, 2H), 7.78 (dd, J= 8.4, 2.1
Hz, 1H), 7.65 (d, J= 9.3, 1H), 6.97 (d, J= 5.1Hz, 1H), 4.92-4.88 (m, 1H), 3.93
(s, 3H), 2.03-1.82 (m, 6H), 1.66-1.61 (m, 2H); 13C NMR (75MHz, CDC13) 8:
176.31, 167.01, 153.95, 150.20, 147.79, 134.61, 121.75, 119.99, 115.94,
113.98,
113.96, 113.32, 111.35, 110.87, 80.91, 55.91, 32.59, 23.93. MS (M+1) 338.
N-0
NON/ 104 OMe
'H NMR (300MHz, CDC13)8: 8.83 (d, J=5Hz, 2H), 8.24(d, J=5Hz, 2H), 8.15(d,
J=8.7Hz, 2H), 7.05(d, J=8.7Hz, 2H), 3.91(s, 3H). MS (M+1) 254.
N-0
/ N NJ/ = /
N 0
NMR (300MHz, CDC13)8: 8.86 (bs, 2H), 8.34(bs, 2H), 7.82(dd, J=8.1,
1.7Hz, 1H), 7.63(d, J=1.7Hz, 1H), 6.99(d, J=8.1Hz, 1H), 6.12(s, 2H). MS (M+1)
268.
0 I N
4-(5-(3,4-diethoxypheny1)-4H-1,2,4-triazol-3-yl)pyridine
Cold 4M HC1 in dioxane (31.5 mmol, 8.87mL) was added to a stirred solution of
3,4-diethoxybenzonitrile (7.84 mmol, 1.5g) in anhydrous Me0H (23.53 mmol,
954 IA) and anhydrous ether (4mL). The reaction was stirred at 0 C for lh and
then placed in the refrigerator (0-5 C) for 48 h. To the mixture was bubbles
N2
to eliminate HC1 and concentrated under reduced pressure. To the crude was
added ether anhydrous and the methyl 3,4-diethoxybenzimidate precipitate as
pale orange solid in 63 % yield (1.3g). The product was used without further
purification.
To a stirred solution of the imidine (0.5 mmol, 130 mg) (freshly liberated
using a
solution 1M of Na2CO3 and extracted with ether) in acetonitrile was added
pyridine-4-carbohydrazide (0.55 mmol, 75.5mg) and the reaction was reflux for
137
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
2h. The mixture was concentrated under reduced pressure and the crude was
heated at 180 C for 2h. The product was purified by C.C. using CH2C12:Me0H
(9:1) to offer the product as a with solid in 65% yield.
NMR (400 MHz, CDC13)8: 8.71 (bs, 2H), 8.11 (d, J=5.2 Hz, 2H), 7.60 (s,
1H), 7.57 (d, J=8.4 Hz, 1H), 6.90 (d, J=8.4, 1H), 4.11 (q, J=6.8 Hz, 2H), 4.07
(q,
J=6.8 Hz, 2H), 1.45 (t, J= 7.0Hz, 3H), 1.40 (t, J=7.0Hz, 3H). "C NMR (CDC13):
157.73, 150.89, 149.42, 149.14, 139.66, 121.29, 120.56, 120.06, 119.68,
113.00,
111.53, 64.82, 64.72, 14.90, 14.86. MS (M+1) 311
N NI\
0>----C1 N
2-(3,4-diethoxypheny1)-5-(pyridin-4-yI)-1,3,4-oxadiazole (25)
To a stirred solution of 3,4-diethoxybenzoic acid (0.71 mmol, 150 mg) in
CH2C12 was added 50C12 at room temperature and the reaction was refluxed for
1.5 h. The mixture was concentrated under reduced pressure.
To a stirred suspension of Na2CO3 (1.42 mmol, 150.52 mg) and pyridine-
4-carbohydrazide (0.71 mmol, 97 mg) in NMP (0.8 mL) was added a solution of
3,4-diethoxybenzoylchloride (prepare above) in NMP (0.8 mL). The reaction
was stirred for 12 h at room temperature, poured to 20 mL of cold H20 and
filtered. The precipitated intermediate was dried in vacuo. The solid was
added
to POC13 (5 mL) and heated to 70-72 C for 6h. The solution was poured in an
ice-water container and neutralized with a solution of NaOH (2M). The
precipitated product was filtered and purified by C.C. using CH2C12:Me0H (9:1)
to yield the product in 67% yield (150mg).
1H NMR (400MHz, CDC13)8: 8.84(bs, 2H), 7.99 (d, J=4.4Hz, 2H), 7.67 (dd,
J=2.0, 8.4 Hz, 1H), 7.64 (d, J=2.0 Hz, 1H), 6.98 (d, J=8.4Hz, 1H), 4.20 (q,
J=7.2
Hz, 2H), 4.18 (q, J=7.2 Hz, 2H), 1.51(t, J=7.2Hz, 3H), 1.50 (t, J=7.2 Hz, 3H).
13C NMR (CDC13)8: 165.81, 162.46, 152.51, 150.84, 149.20, 131.52, 128.05,
120.97, 115.77, 112.78, 111.58, 65.05, 64.80, 14.92, 14.85. MS (M+1)
138
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
i
3-(3,4-diethoxypheny1)-5-(pyridin-4-y1)-1,2,4-oxadiazole
To a stirred solution of triethylamine (2 equiv) and NH2OH.HC1 (2
equiv) in ethanol was added 3,4-diethoxybenzonitrile (1 equiv) and the
reaction
was reflux overnight. The reaction was concentrated under reduced pressure.
The crude was dissolved in AcOEt and extracted with water. The organic portion
was dried over Na2SO4 anhydrous and concentrated under reduced pressure. The
crude was used without further purification.
To a stirred solution of isonicotinic acid (1 equiv) in DMF (in a
microwave vial) was added EDCI (1.3 equiv) and HOBt (1.3 equiv), the reaction
was stirred for 5 min at room temperature followed by addition of the
amidoxime (1.3 equiv) prepare above. The reaction was stirred for additional
10
min, at room temperature then heated at 170 C for 5 min in the microwave. The
reaction was diluted using a saturated solution of NaC1 and extracted with
Et0Ac (3X). The organic phase was dried over Na2SO4 anhydrous and
concentrated under reduced pressure. The product was purified by C.C. using
CH2C12:Me0H (9:1) to yield the oxadiazole in % yield.
IFINMR (300 MHz, CDC13) 6: 8.85 (d, J= 5.4 Hz, 2H), 8.02 (d, J= 6.0 Hz,
2H), 7.71 (dd, J= 8.1, 1.8 Hz, 1H), 7.62 (d, J= 2.1 Hz, 1H), 6.95 (d, J¨ 8.4
Hz,
1H), 4.19 (q, J= 6.9 Hz, 2H), 4.14 (q, J= 6.9 Hz, 2H), 1.50 (t, J= 3.0 Hz,
3H),
1.46 (t, J= 3.3 Hz, 3H); 13C NMR (75 MHz, CDC13) 6: 173.54, 16928, 151.64,
151.07, 148.91, 131.37, 121.48, 121.15, 118.75, 112.74, 111.82, 64.78, 64.59,
14.87, 14.81; MS (M+1) 312.
OEt
=
Nr
OEt
N N
N
NMR(500Hz, CDC13)6: 8.63-8.61(m, 1H), 7.99(d, J=5Hz, 1H), 7.80(dd, J=
8.5, 2.0Hz, 1H), 7.67(d, J=1.5Hz, 1H), 7.00(d, J=8.5Hz, 1H), 4.23-4.17(m, 4H),
2.68(s, 3H), 1.52-1.49(m, 6H). "C NMR(125Hz, CDC13) 6: 175.43, 167.82,
139
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
152.93, 152.24, 148.88, 147.62, 133.73, 132.32, 123.10, 122.14, 116.13,
112.52,
112.29, 64.81, 64.60, 19.00, 14.69, 14.61. MS. (M+1) 326.
OEt
=Nr
OEt
N
z
1H NMR(500Hz, CDC13)8: 8.84(dt, J=3.0, 1.0Hz, 1H), 8.76(dd, J= 7.5, 1.0Hz,
1H), 7.85-7.84(m, 1H), 7.75(d, J=2.0Hz, 1H), 7.45-7.42(m, 1H), 6.98(d,
J=8.5Hz, 1H), 4.22-4.16(m, 4H), 1.51(t, J=7.0Hz, 6H). 13CNMR(125Hz)8:
176.50, 168.61, 152.79, 150.35, 148.80, 146.62, 136.99, 125.36, 123.20,
122.21,
116.33, 112.40, 64.81, 64.55, 14.70, 14.60. MS. (M+1) 312.
OEt
Nr
NoiL,N/=
OEt
1H NMR(500Hz, CDC13)8: 9.40(s, 1H), 8.76(d, J= 3.0Hz, 1H), 8.45(d, J=8.0Hz,
1H), 7.82(dd, J=8.5, 2.0Hz, 1H), 7.69(d, J=2.0Hz, 1H), 7.47-7.44(m, 1H),
7.00(d, J=8.5Hz, 1H), 4.24-4.17(m, 4H), 1.53-1.50(m, 6H). 13CNMR(125Hz,
CDC13)8: 176.19, 166.82, 152.92, 151.68, 148.89, 148.56, 134.90, 122.67,
122.15, 120.32, 116.23, 112.52, 112.29, 64.84, 64.61, 14.70, 14.62. MS. (M+1)
312.
OEt
N-C)
H2N I / 41/ OEt
)0'LN
N
111 NMR(500Hz, CDC13)8: 8.22(d, J=4.5Hz, 2H), 7.79(dd, J=8.5, 2.0Hz, 1H),
7.66(d, J=2.0Hz, 1H), 7.37(d, J=5.0Hz, 1H), 7.26(s, 1H), 6.99(d, J=8.5Hz, 1H),
4.77-4.71(m, 2H), 4.23-4.17(m, 4H), 1.52-1.49(m, 6H). 13C NMR(125Hz,
CDC13)8: 176.20, 167.51, 158.85, 152.90, 148.86, 148.78, 136.32, 122.11,
116.22, 112.50, 112.25, 111.76, 106.58, 64.82, 64.61, 14.70, 14,61. MS. (M+1)
327.
140
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
N-0 OMe
N 11
OMe
Ili NMR (300Hz, CDC13) 8: 8.18- 8.15 (m, 2H), 7.80 (dd, J1 =1.8 Hz, J2 = 8.4
Hz, 1H), 7.69 (d, J= 3.1 Hz, 1H), 7.53-7.48 (m, 3H), 6.99 (d, J= 8.4 Hz, 1H),
4.23 (q, J = 7.2 Hz, 2H), 4.19 (q, J = 4.8 Hz, 2H), 1.53 (t, J =2.7 Hz, 3H),
1.49
(t, J= 4.2 Hz, 3H); MS (M+1) 311.
N-0 OEt
/ /
lk N 0
OEt
'H NMR (300MHz, CDC13) 8: 8.06 (dd, J= 2.1, 8.7 Hz, 1H), 7.81 (dd, J= 1.8,
8.4 Hz, 1H), 7.70 (d, J= 2.1 Hz, 1H), 7.43-7.30 (m, 3H), 6.69 (d, J= 8.7 Hz),
4.21 (q, J = 6.9 Hz, 2H), 4.19 (q, J = 6.9 Hz, 2H), 2.67 (s, 3H), 1.51 (t, J=
7.2
Hz, 6H); 13C NMR (75 MHz, CDC13) 8: 174.76, 169.45, 152.59, 148.80, 138.22,
131.33, 130.53, 130.49, 130.12, 126.45, 125.98, 122.01, 116.69, 112.48,
112.25,
64.79, 64.09, 22.08, 14.75; MS 325 (M+1)
N-0
/ N / NI, \S i CF3
N
4.
'H NMR (300MHz, CDC13) 8: 8.82 (bs, 2H), 8.02 (d, J= 2.7, 2H), 7.93 (q, J =
1.5 Hz, 1H), 7.47 (s, 5H); 13C NMR (75 MHz, CDC13) 8: 171.12, 167.81,
150.86, 145.70, 135.09, 134.12, 133.14, 123.32, 129.04, 126.35, 126.33,
121.67,
120.21, 105.04; MS 374 (M+1).
__
N6,/
I/1-C) S CF3
\ '
/ N \ /
Ili
ill NMR (300MHz, CDC13) 8: 8.63 (brs, 2H), 7.95 (d, J= 5.1 Hz, 1H), 7.912 ( t,
J= 1.5, 1H), 7.462 (s, 5H), 2.67 (s, 3H); 13C NMR (75MHz, CDC13) 8: 170.06,
141
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
168.32, 152.51, 147.88, 145.63, 145.60, 134.93, 133.13, 132.69, 129.26,
128.85,
126.40, 120.18, 105.01, 19.38; MS 388 (M+1)
N-0 OEt
HO /
OEt
Hi NMR (400MHz, CDC13)8: 7.83 (d, J=7.6Hz, 1H), 7.79(dd, J=2, 8.4 Hz, 1H),
7.68 (d, J=2Hz, 1H), 7.32(t, J=8Hz, 1H), 6.98 (d, J=8.0Hz, 1H), 4.78 (s, 2 H)
4.18 (q, J=7.2Hz, 4H), 2.55 (s, 3H), 1.50 (t, 6.8 Hz, 6H). 13C NMR (CDC13)8:
175.2, 169.8, 152.9, 149.0, 140.2, 136.3, 130.0, 129.9, 127.6, 126.1, 122.2,
116.7, 112.7, 112.4, 65.0, 64.8, 63.8, 16.4, 14.9. MI (M+1)
OEt
/
HO
efh N =
OEt
NMR(500MHz, CDC13) 8: 8.05(d, J=7.5Hz, 1H), 7.80(dd, J=8.0Hz, 2.0Hz,
1H), 7.68(d, J= 2.0Hz, 1H), 7.31-7.29(m, 2H), 6.99(d, J=8.5Hz, 1H), 4.73(s,
2H), 4.21-4.18(m, 4H), 2.66(s, 3H), 1.52 (dt, J=7.0Hz, 1Hz, 6H). 13C
NMR(125MHz, CDC13) 8: 174.70, 169.17, 152.59, 148.78, 143.23, 138.45,
130.34, 129.57, 125.56, 124.18, 121.18, 121.99, 116.60, 112.51, 112.32, 64.78,
64.57, 22.07, 14.69, 14.61. MS. (M+1) 355.
N-0 OEt
I1/
N
HO OEt
IHNMR(500MHz, CDC13) 8: 7.968-7.94(m, 2H), 7.79(dd, J=8.5Hz, 1.5Hz, 1H),
7.68(d, J= 2.0Hz, 1H), 7.50(d, J=8.0Hz, 1H), 6.98(d, J=8.0Hz, 1H), 4.75(s,
2H),
4.23-4.16(m, 4H), 2.40(s, 3H), 1.52 (dt, J=7.0Hz, 1Hz, 6H). 13C NMR(125MHz,
CDC13) 5: 175.62, 168.61, 164.61, 152.61, 148.77, 141.82, 136.39, 129.06,
127.47, 126.14,125.20, 122.0,116.59, 112.47, 112.28, 64.78, 64.56, 63.05,
18.57, 14.69, 14.61. MS. (M+1) 355.
142
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
N-0 OEt
*
/ N/ 111
HO OEt
1H NMR (300MHz, CDC13) 8: 8.06 (d, J= 8.1 Hz, 2H), 7.76 (dd, J= 1.5, 9.9
Hz, 1H), 7.66 (d, J= 1.8 Hz, 1H), 7.34 (d, J= 8.1 Hz, 2H), 6.96 (d, J= 8.4 Hz,
1H), 4.21 (q, J= 7.2 Hz, 2H), 4.16 (q, J= 6.9 Hz, 2H), 3.88 (t, J= 6.6 Hz,
2H),
2.91 (t, J= 6.6 Hz, 2H), 1.51 (t, J= 1.5, 3H), 1.48 (t, J= 1.2 Hz, 3H); 13C
NMR
(75MHz, CDC13) 8: 175.54, 168.52, 152.49, 148.64, 141.98, 132.14, 129.72,
127.59, 125.17, 121.93, 116.46, 112.31, 112.07, 64.68, 64.47, 63.25, 39.07,
14.64. MS 355 (M+1)
N-0 OEt
CN O / Nx \w' OEt
Hi NMR (400MHz, CDC13) 8: 8.11 (s, 1H), 8.05 (d, J=7.6 Hz, 1H), 7.79 (dd,
J=2.0, 8.4 Hz, 1H), 7.55 (d, J=7.6 Hz, 1H), 7.46 (t, J=7.6 Hz, 1H), 6.98 (d,
J=8.4Hz, 1H), 4.22 (q, J=7.2Hz, 2H), 4.18 (q, J=7.2 Hz, 2H), 3.74 (s, 2H),
2.60
(s, 4H), 1.82 (s, 4H), 1.52 (t, J=7.0Hz, 3H), 1.50 (t, J=7.0Hz, 3H). 13C NMR
(CDC13)8: 175.87, 169.02, 152.80, 148.98, 132.02, 129.09, 128.21, 127.27,
126.51, 122.20, 116.85, 112.64, 112.44, 64.99, 64.77, 60.47, 54.31, 23.69,
14.93,
14.85. MI (M+1) 394.
N/1-0 OEt
ON =Nr . OEt
III NMR (400MHz, CDC13) 8: 8.07 (d, J=8.4Hz, 2H), 7.74 (dd, J=1.6, 8.4Hz,
1H), 1.64 (d, J=1.6Hz, 1H), 7.44(d, J=8.0Hz, 2H), 6.92(d, J=8.4Hz, 1H), 4.17
(q, J=7.2 Hz, 2H), 4.12 (q, J=7.2Hz, 2H), 3.68 (s, 2H), 2.54(s, 4H), 1.77 (s,
4H),
1.47 (t, J=7.0Hz, 3H), 1.45(t, J=7.0Hz, 3H). "C NMR (CDC13)8: 175.68,
168.72, 152.65, 148.82, 142.13, 129.42, 127.54, 125.95, 122.05, 116.67,
112.50,
112.27, 64.82, 64.61, 60.31, 54.14, 23.51, 14.79, 14.71. MI (M+1) 394.
143
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
N-0 C OEt N 0 / N" 1110
OEt
Hi NMR (400MHz, CDC13) 8: 7.79 (dd, J=2.0, 8.4 Hz, 1H), 7.77 (d, J=7.6 Hz,
1H), 7.68(d, J=2.0 Hz, 1H), 7.50 (d, J=7.6Hz, 1H), 7.28 (t, J=7.6Hz, 1H),
6.98(d, J=8.4Hz, 1H), 4.20(q, J=7.0Hz, 2H), 4.19(q, J=7.0Hz, 2H), 3.72 (s,
2H),
2.59 (s, 4H), 1.80 (s, 4H), 1.50 (t, J=7.0Hz, 6H). 13C NMR (CDC13)8: 175.06,
170.12, 152.77, 148.98, 137.18, 131.98, 129.40, 127.50, 125.75, 122.17,
116.84,
112.66, 112.40, 64.95, 64.77, 58.51, 54.42, 23.74, 16.80, 14.91, 14.84.
N-0 OEt
ON O 1 NC = OEt
1H NMR(500Hz, CDC13)8: 8.01(d, J=7.5Hz, 1H), 7.81(dd, J=8.5Hz, 2Hz, 1H),
7.69(d, J=2Hz, 1H), 7.32-7.31(m, 2H), 6.99(d, J=8.5Hz, 1H), 4.23-4.17(m, 4H),
3.67(s, 2H), 2.65(s, 3H), 2.56(s, 4H), 1.83-1.80(m, 4H), 1.52-1.49(m, 6H). 13C
NMR(125Hz, CDC13)8: 174.63, 169.33, 152.56, 148.80, 138.15, 131.78, 131.07,
126.46, 125.11, 121.96, 116.72, 112.54, 112.35, 64.78, 64.7, 60.32, 54.17,
23.46,
22.00, 14.70, 14.63. MS. (M+1) 408.
N-0 OEt
ON 40 1 r\jr = OEt
IHNMR(500MHz, CDC13) 8: 7.94-7.93(m, 2H), 7.80(dd, J=8.5Hz, 2.0Hz, 1H),
7.69(d, J= 2.0Hz, 1H), 7.47(d, J=8.0Hz, 1H), 6.98(d, J=8.5Hz, 1H), 4.24-
4.15(m,
4H), 3.65(s, 2H), 2.56-2.54(m, 4H), 2.44(s, 3H), 1.80-1.78(m, 4H), 1.52 (q,
J=7.0Hz, 6H). 13C NMR(125MHz, CDC13) 8: 175.49, 168.80, 152.54, 148.76,
140.88, 137.34, 129.51, 128.95, 125.39, 124.82, 121.94, 116.71, 112.46,
112.29,
64.76, 64.53, 57.82, 54.29, 23.55, 19.14, 14.68, 14.60. MS. (M+1) 408.
N-0 OEt
/
CN O NI' 10
OEt
144
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
1H NMR (300MHz, CDC13) 8: 8.06 ( d, .1= 8.0 Hz, 2H), 7.72 (dd, J = 8.4, 2.0,
1H), 7.61 (d, J= 2.0 Hz, 1H), 7.33 ( d, J= 8.4, 2H), 6.92 (d, J= 8.4, 1H),
4.16
(q, .1= 6.8, 2H), 4.11 (q, J= 6.8, 2H), 3.30-3.22 (m, 4H), 2.74-2.69 (m, 2H),
2.21-2.19 (m, 2H), 2.02 (m, 2H), 1.47 (t, J = 10 Hz , 3H), 1.43 (t, J= 3.6,
3H);
MS 408 (M+1).
OEt
=OEt
N
HN
H1 NMR (400MHz, CDC13)8: 8.01 (d, J=7.2Hz, 1H), 7.81 (dd, J=8.4, 2.0 Hz,
1H), 7.52 (s, 1H), 7.52 (d, J=8.0 Hz, 1H), 7.34 (s, 1H), 7.28 (t, J=8.0 Hz,
1H),
7.23(s, 1H), 6.96 (d, J=8.4 Hz, 1H), 4.18 (q, J= 6.8 Hz, 2H), 4.16 (q, J=6.8
Hz,
2H), 1.47 (t, J=6.8 Hz, 3H), 1.46 (t, J=6.8 Hz, 3H). 13C NMR (CDC13)8: 175.0,
169.5, 152.6, 148.9 (2), 125.7, 122.2, 121.8, 121.7, 121.3, 121.2, 117.0,
114.4,
114.3, 112.7, 112.5; MI (M+1) 350.
OEt
=WC)/
I OEt
N
HN
1H NMR (300MHz, CDC13) 8: 7.78 (dd, = 8.4, 2.1 Hz, 1H), 7.68 (d, J¨ 1.8
Hz, 1H), 7.55 (d, .1 = 7.8 Hz, 1H), 7.16 (t, J= 7.5 Hz, 1H), 6.97 (d, J = 7.5
Hz,
1H), 6.76 (d, .1= 7.5 Hz, 1H), 4.21 (q, .1= 6.9 Hz, 2H), 4.16 (q, .1= 6.9 Hz,
2H),
3.65 (brs, 2H), 3.46 (t, J = 8.1 Hz, 2H), 1.52 (t, J = 3.1 Hz, 3H), 1.48 (t, J
= 3.3
Hz, 3H); 13C NMR (75MHz, CDC13) 8: 175.05, 169.06, 152.62, 148.84, 127.84,
123.67, 122.09, 118.90, 116.83, 112.53, 112.32, 111.60, 64.86, 64.67, 47.29,
31.41, 14.85, 14.78. MS 352 (M+1)
OEt
NM/ 11
OEt
N
NH
145
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
11-1 NMR(500MHz, CDC13) 8: 7.80 (dd, J=8.5, 2Hz, 1H), 7.69(d, J=2.0Hz, 1H),
7.45(d, .7= 7.5Hz, 1H), 7.14(d, J=8.0Hz, 2H), 6.97(d, J=8.0Hz, 1H), 4.24-
4.15(m, 4H), 3.98-3.95(m, 1H), 3.43(t, J=8.5Hz, 2H), 3.01(t, J=8.5Hz, 2H),
1.52(q, J=7.0Hz, 6H), 1.20(d, J=6.5Hz, 6H). 13C NMR(125MHz, CDC13) 8:
175.24, 169.48, 152.4, 148.74, 133.96, 126.11, 124.39, 121.93, 116.92, 112.46,
112.35, 105.03, 64.77, 64.52, 4.42, 28.42, 28.10, 18.18, 14.69, 14.60. MS.
(M+1) 352.
OEt
N-C), =
I OEt
H/N 40 N
III NMR(500MHz, CDC13) 8: 8.56(s, 1H), 8.51(s, 1H), 8.01(dd, J=8.5Hz, 1.5Hz,
1H), 7.83(dd, J=8.5Hz, 2.0Hz, 1H), 7.73(d, J= 1.5Hz, 1H), 7.45(d, J=8.5Hz,
1H), 7.27-7.26(m, 1H), 7.00(d, J=8.5Hz, 1H), 6.66-6.65(m, 1H), 4.24-4.17(m,
4H), 1.52-1.48(m, 6H). 13C NMR(125MHz, CDC13) 8: 175.26, 169.79, 152.44,
148.75, 137.33, 127.93, 125.27, 121.94, 121.27, 120.94, 118.77, 116.96,
112.51,
112.34, 111.43, 103.56, 64.78, 64.56, 14.70, 14.62. MS. (M+1) 350.
OEt
N". / =
I
HN 40 N OEt
11-1 NMR(500MHz, CDC13) 8: 7.86-7.82(m, 2H), 7.78(dd, J=8.0Hz, 2Hz, 1H),
7.68(d, J=2.0Hz, 1H), 6.98(d, J=8.5Hz, 1H), 6.69(d, J=8.0Hz, 1H), 4.23-4.16(m,
5H), 3.67(t, J= 8.5Hz, 2H), 3.18(t, J=8.0Hz, 2H), 1.52-1.48(m, 6H). 13C
NMR(125MHz, CDC13) 5: 175.00, 169.08, 154.04, 152.40, 148.73, 129.62,
127.89, 123.85, 121.87, 117.04, 116.98, 112.48, 112.32, 105.92, 64.76, 64.54,
47.28, 29.23, 14.71, 14.63.
MS. (M+1) 352
146
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
OEt
1\1/ 4.
I OEt
110 N
N
/------."
Et2N
1H NMR (300MHz, CDC13) 8: 7.78 (dd, J = 8.4, 2.1 Hz, 1H), 7.68 (d, J = 2.1,
1H), 7.46 (d, J= 7.8 Hz, 1H), 7.20 (t, J= 9.6 Hz, 1H), 6.96 (d, J= 8.4 Hz,
1H),
6.60 (d, J =7 .8 Hz, 1H), 4.20 (q, J= 7.2 Hz, 2H), 4.17 (q, J= 7.2 Hz, 2H),
3.55
(t, J = 1.8 Hz, 2H), 3.40 (t, J= 7.2 Hz, 2H), 3.29 (t, J= 7.2 Hz, 2H), 2.73
(t, J=
7.8 Hz, 2H), 2.60 (q, J= 7.2 Hz, 4H), 1.51 (t, J= 2.1 Hz, 3H), 1.49 (t, J= 1.8
Hz, 3H), 1.09 (t, J= 7.2 Hz, 6H). 13C NMR (CDC13) 8: 174.30, 168.39, 152.52,
151.93, 148.17, 129.32, 127.33, 122.70, 121.40, 116.75, 116.21, 111.87,
107.94,
104.24, 64.15, 52.82, 49.53, 46.92, 29.54, 14.15, 11.03; MS 451 (M+1).
OEt
WC)/ *
I OEt
ilk N
411
1H NMR (300MHz, CDC13) 8: 8.95 (d, J= 8.4 Hz, 1H), 8.33 (d, J= 7.2 Hz, 1H),
8.02 (d, J = 8.4 Hz, 1H), 7.94 (d, J= 8.1 Hz, 1H), 7.86 (d, J= 8.4 Hz, 1H),
7.74
(s, 1H), 7.65-7.54 (m, 3H), 7.0 (d, J= 8.4 Hz, 1H), 4.23 (q, J= 6.9 Hz, 2H),
4.20
(q, J = 7.2 Hz, 2H), 1.52 (t, J = 7.2 Hz, 6H); 13C NMR (75 MHz, CDC13) 8:
174.23, 168.57, 152.00, 148.15, 133.22, 131.00, 130.08, 128.65, 127.94,
126.82,
125.64, 124.44. 123.51, 121.44, 115.92, 111.81, 111.61, 64.13, 14.07. MS
(M+23) 383.
OEt
11-C)/ 44I
N I OEt
i N N
20*
111 NMR(500MHz, CDC13) 8: 9.03(d, J=8.5Hz, 1H), 8.80(d, J=5.5Hz, 1H), 7.94-
7.90(m, 2H), 7.84-7.71(m, 4H), 7.01(d, J=8.5Hz, 1H), 4.23-4.17(m, 4H), 1.52-
1.49(m, 6H).
147
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
13C NMR(125Hz, CDC13) 8: 175.95, 168.38, 152.91, 148.84, 146.36, 142.37,
136.93, 130.70, 128.68, 127.20, 127.08, 123.04, 122.38, 122.37, 116.22,
112.62,
112.45, 64.86, 64.58, 14.72, 14.62. MS. (M+1) 362.
OEt
=NA)/
I OEt
N
HO
11-1 NMR(500MHz, CDC13) 8: 7.83(d, J=7.5Hz, 1H), 7.80(dd, J=8.5Hz, 2.0Hz,
1H), 7.68(d, J= 2.0Hz, 1H), 7.50(d, J=7.0Hz, 1H), 7.33(t, J=7.5Hz, 1H),
6.99(d,
J=8.5Hz, 1H), 4.22-4.17(m, 4H), 3.95(s, 2H), 3.73-3.70(m, 2H), 2.92-2.90(m,
2H), 2.60(s, 3H), 2.47(s, 2H), 1.52-1.49(m, 6H). 13C NMR(125MHz, CDC13) 5:
174.95, 169.69, 152.68, 148.84, 137.99, 136.65, 132.75, 131.30, 129.76,
127.70,
125.93, 122.02, 116.58, 112.32, 64.80, 64.59, 60.51, 51.27, 50.68, 14.70,
14.63.
MS. (M+1) 398.
OEt
,OEt
HONN
H 411110 N
11-1 NMR(500MHz, CDC13) 8: 8.05(d, J=8.0Hz, 1H), 7.80(dd, J=5.0Hz, 2.0Hz,
1H), 7.68(d, J= 2.0Hz, 1H), 7.32-7.23(m, 2H), 6.99(d, J=8.5Hz, 1H), 4.28-
4.17(m, 4H), 3.89-3.84(m, 2H), 3.72-3.69(m, 2H), 2.86(s, 2H), 2.66(s, 3H),
2.54-2.48(m, 3H), 1.52 (dt, J=7.0Hz, 1Hz, 6H). 13C NMR(125MHz, CDC13) 8:
174.72, 169.16, 152.63, 148.82, 138.52, 132.63, 131.27, 130.41, 129.78,
125.85,
125.61, 121.99, 116.65, 112.56, 112.37, 64.80, 64.59, 22.06, 14.71, 14.63. MS.
(M+1) 398.
1-hydroxy-2,3-dihydro-1H-indene-4-carbonitrile
To a stirred suspension of 1-oxo-2,3-dihydro-1H-indene-4-carbonitrile (1.0
equiv, 0.4M) and silica gel (catalytic) in ethanol at 0 C was added NaBH4 (1/3
equiv). The reaction was allowed to warm up to room temperature and stirred
for
2 h. The Solvent was removed under reduced pressure and the product purified
148
= CA 02723904 2015-08-11
by CC in hexane/Et0Ac (5:5) to offer 1-hydroxy-2,3-dihydro-1H-indene-4-
carbonitrile in 80% yield.
OEt
N
I /OEt
N
HO
NMR (400MHz, CDC13).3: 8.10 (d, J=7.6, 1H), 7.78 (dd, J=1.6, 8 Hz, 1H),
7.67 (d, J=1.6Hz, 1H), 7.56 (d, J=7.6Hz, 1H), 7.39 (t, J=7.6, 1H), 6.97 (d,
J=8.0
Hz, 1H), 5.29 (t, J=6.4 Hz, 1H), 4.19 (q, J=7.2, 2H), 4.18(q, J=7.2, 2H), 3.51-
4.43 (m, 1H), 3.22-3.14 (m, 1H), 2.59-2.51 (m, 1H), 2.04-1.97 (m, 1H), 1.5 (t,
J=7.2, 3H), 1.49 (t, J=7.2, 3H): 13C NMR (CDC13) 6: 175.2, 168.9, 152.8,
148.9,
146.6, 143.3, 128.9, 127.4, 127.0, 123.8, 122.2, 116.7, 112.7, 112.4, 76.2,
64.9,
64.8, 35.7, 31.5, 14.9, 14.8: MI (M+1) 367
4-(5-(3,4-diethoxypheny1)-1,2,4-oxadiazol-3-y1)-2,3-dihydro-IH-inden-1-one
To a suspension of PCC (1.5 equiv.) and powdered molecular sieves (3
A, one-half the weight of PCC) in dry CH2C12 was added the benzylic alcohol (1
equiv. prepared in previous step) at 0 C. The reaction mixture was stirred
overnight at room temperature and concentrated under reduced pressure. To the
residue was added Et20-Et0Ac (1:1) and the slurry was stirred and filtered
through a pad of CeliteTM. The residue was washed 4 times with Et20-Et0Ac
(1:1). The filtrate was concentrated under reduced pressure and the product
purified by CC offering the ketone in 70 % yield.
OEt
N-0
N OEt
0
11-1 NMR(500MHz, CDC13)6: 8.48(ddõJ=8.0Hz, 1.5Hz, 1H), 7.91(dõJ=7.5Hz,
1H), 7.81(dd, J---= 8.5Hz, 2.0Hz, 1H), 7.68(d, J=2.0Hz, 1H), 7.56(t, J=7.5Hz,
1H),
6.99(d, J=8.5Hz, 1H), 4.24-4.17(m, 4H), 3.55-3.53(m, 2H), 2.79-2.77(m, 2H),
149
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
1.53-1.49(m, 6H). 13C NMR (125MHz, CDC13) 8: 206.62, 175.32, 167.79,
154.40, 152.86, 148.86, 138.22, 134.63, 127.72, 126.00, 125.55, 122.10,
116.32,
112.55, 112.36, 64.82, 64.60, 36.25, 27.61, 14.70, 14.61. MS. (M+1) 365.
Amination procedure.
A solution of alcohol (1 equiv), at 0 C, was treated with SOC12 (1.1 equiv)
and
pyridine (1.1 equiv) in CH2C12. The reaction was stirred at room temperature
for
2h. The reaction was diluted with CH2C12 and washed with NaHCO3 (2X). The
organic phase was dried over sodium sulfate and concentrated under reduced
pressure. The crude was dissolved in DMF and treated with the corresponding
amine (2 equiv) and DIPEA( 2.0 equiv). The reaction was stirred at 50 C for
48h. The reaction was diluted with H20 and the product extracted with Et0Ac
(3X). The product was purified by C.C. using CH2C12/Me0H (9:1) to offer
amino-diaryloxadiazoles in moderated yields.
OEt
NI 411
N M
1 OEt
10
S
HO.¨../' N
H
Ili NMR(500MHz, CDC13)8: 8.12(d, J=7.5Hz, 1H), 7.79(dd, J=8.5, 2.0Hz, 1H),
7.68(d, J= 2.0Hz, 1H), 7.63(d, J=7.5Hz, 1H), 7.40-7.37(m, 1H), 4.49-4.47(m,
1H), 4.23-4.16(m, 4H), 3.78-3.70 (m, 1H), 3.53-3.46 (m, 1H), 3.29-3.22(m, 1H),
2.96-2.94(m, 4H), 2.56-2.50(m, 1H), 2.09-2.03(m, 1H), 1.52-1.49(m, 6H). 13C
NMR(125Hz): 175.03, 168.66, 152.71, 148.87, 143.76, 128.71, 127.11, 123.89,
122.04, 116.67, 112.63, 112.49, 64.84, 64.61, 62.70, 60.34, 47.98, 31.90,
29.69,
14.72, 14.64. MS. (M+1) 410.
OEt
N-C31/ .
I OEt
ilk N
1.
'N
I
150
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
11-1 NMR(400MHz, CDC13): 8.10(d, J=7.6Hz, 1H), 7.79(dd, J=8.5, 2.0Hz, 1H),
7.69(d, J= 2.0Hz, 1H), 7.55(d, J=7.6Hz, 1H), 7.38(t, J=7.6 Hz, 1H), 6.99 (d,
J=8.4 Hz, 1H), 4.44(t, J=6.8 Hz, 1H), 4.24-4.16(m, 4H), 3.42-3.34 (m, 1H),
3.27-3.19 (m, 1H), 3.28(s, 6H), 2.19-2.12(m, 4H), 1.53-1.48(m, 6H). 13C
NMR(75MHz, CDC13): 174.09, 168.87, 152.58, 148.77, 143.88, 128.27, 127.91,
126.79, 123.47, 121.98, 116.67, 114.84, 112.49, 112.31, 69.67, 64.77, 64.56,
40.66, 32.40, 23.05, 14.70, 14.62. MS. (M+1) 394.
N¨N
Et0
fh / \ -----
0 \
N
/
Et0
2-(3,4-diethoxypheny1)-5-(pyridin-4-y1)-1,3,4-oxadiazole
To a stirred solution of 3,4-diethoxybenzoic acid (0.71 mmol, 150 mg) in
CH2C12 was added SOC12 at room temperature; the reaction was refluxed for 1.5
h and the mixture concentrated under reduced pressure to yield 3,4-
diethoxybenzoylchloride quantitatively. To a stirred suspension of Na2CO3
(1.42
mmol, 150.52 mg) and pyridine-4-carbohydrazide (0.71 mmol, 97 mg) in NMP
(0.8 mL) was added a solution of the 3,4-diethoxybenzoylchloride in NMP (0.8
mL) and the reaction was stirred for 12 h at room temperature. The mixture was
poured into 20 mL of cold H20 and filtered. The precipitated intermediate was
dried in vacuum. The solid was added to POC13 (5 mL) and heated to 70-72 C
for 6h. The solution was poured into an ice-water container and neutralized
with
a solution of NaOH (2M). The precipitated product was filtered and purified by
column chromatography using CH2C12:Me0H (9:1) to yield the product as white
solid in 67% yield (150mg). 1H NMR (400 MHz, CDC13): g 8.84 (bs, 2H), 7.99
(d, J¨ 4.4 Hz, 2H), 7.67 (dd, JI = 2.0, J2 = 8.4 Hz, 1H), 7.64 (d, J= 2.0 Hz,
1H), 6.98 (d, J= 8.4 Hz, 1H), 4.20 (q, J= 7.2 Hz, 2H), 4.18 (q, J= 7.2 Hz,
2H),
1.51 (t, J= 7.2 Hz, 3H), 1.50 (t, J =7 .2 Hz, 3H); 13C NMR (100 MHz, CDC13): g
165.81, 162.46, 152.51, 150.84, 149.20, 131.52, 128.05, 120.97, 115.77,
112.78,
111.58, 65.05, 64.80, 14.92, 14.85. MS (El) m/z: 312 (Mt), HRMS (E1) for
CI7H17N303 (M+): calcd 312.1343, found 312.1350.
151
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
N-N
/ \
N N
Et0
Et0
445-(3,4-diethoxypheny1)-4H-1,2,4-triazol-3-yl)pyridine
Cold 4M HC1 in dioxane (31.5 mmol, 8.87mL) was added to a stirred
solution of 3,4-diethoxybenzonitrile (7.84 mmol, 1.5g) in anhydrous Me0H
(23.53 mmol, 954 IA) and anhydrous ether (4mL). The reaction was stirred at 0
C for lh and then placed in the refrigerator (0-5 C) for 48 h. The mixture
was
bubbled with N2 to eliminate HC1 and concentrated under reduced pressure. To
the crude was added anhydrous ether and the methyl 3,4-diethoxybenzimidate
salt precipitated as pale orange solid in 63 % yield (1.3g). The product was
used
without further purification. To a stirred solution of the imidine (0.5 mmol,
130
mg) (freshly liberated from the imidate salt using a solution 1M of Na2CO3 and
extracted with ether) in acetonitrile was added pyridine-4-carbohydrazide
(0.55
mmol, 75.5mg) and the reaction was refluxed for 2h. The mixture was
concentrated under reduced pressure and the crude was heated at 180 C for 2h.
The product was purified by column chromatography using CH2C12:Me0H (9:1)
to offer the product as a white solid in 65% yield (100mg, 0.32 mmol) . 1HNMR
(400 MHz, CDC13): 88.71 (bs, 2H), 8.11 (d, J= 5.2 Hz, 2H), 7.60 (s, 1H), 7.57
(d, J= 8.4 Hz, 1H), 6.90 (d, J= 8.4, 1H), 4.11 (q, J= 6.8 Hz, 2H), 4.07 (q, J
=
6.8 Hz, 2H), 1.45 (t, J= 7.0 Hz, 3H), 1.40 (t, J= 7.0 Hz, 3H); 13C NMR (100
MHz, CDC13): 8157.73, 150.89, 149.42, 149.14, 139.66, 121.29, 120.56,
120.06, 119.68, 113.00, 111.53, 64.82, 64.72, 14.90, 14.86. MS (EI) m/z: 311
(MI), HRMS (El) for Ci7H181\1402 (M+): calcd 311.1502, found 311.1506.
0¨ N
= N
S N
F3C
445-(4-Pheny1-5-trifluoromethyl-thiophen-2-y1)-(1,2,41oxadiazol-3-y11-
pyridine
152
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
In a round bottom flask, a stirring solution of 3,4-diethoxybenzoic acid
(100mg, 0.3673mmol) in DMF (1.8 mL) was treated sequentially with HOBt
(64mg, 0.48 mmol)) and EDCI (91mg, 0.48mmol) at room temperature. The
reaction was stirred for 20 min followed by addition, in a single portion of N-
hydroxyisonicotinimidamide (66mg, 0.48mmol). The reaction was stirred for
additional 30 min at room temperature and then heated to 90-95 C for 10h. The
reaction was cooled to room temperature, diluted with a saturated solution of
NaC1 and extracted with Et0Ac (3X50mL). The organic phase was dried over
Na2SO4 anhydrous and concentrated under reduced pressure. The product was
purified by column chromatography using CH2C12:Me0H (9:1) to offer the
product as a pale yellow solid in 56% yield (78mg). 1H NMR (300 MHz,
CDC13): 6 8.82 (bs, 2H), 8.02 (d, J= 2.7, 2H), 7.93 (q, J= 1.5 Hz, 1H), 7.47
(s,
5H); I3C NMR (75 MHz, CDC13): 6 171.12, 167.81, 150.86, 145.70, 135.09,
134.12, 133.14, 123.32, 129.04, 126.35, 126.33, 121.67, 120.21, 105.04. MS
(El) m/z: 374 (Mt), HRMS (El) for C18H10F3N3S0 (M+): calcd 374.0569, found
374.0579.
0¨N
0
N
Et0
Et0
6-(5-(3,4-diethoxypheny1)-1,2,4-oxadiazol-3-ypindolin-2-one
To a solution of 2-oxoindoline-4-carbonitrile (500mg, 3.16mmol) in
ethanol were added cautiously hydroxylamine hydrochloride (286 mg, 4.11
mmol) and potassium bicarbonate (411mg, 4.11 mmol). The reaction mixture
was refluxed for 20h under nitrogen atmosphere. The mixture was cooled to
room temperature and the solid was filtered. The organic solvent was
concentrated under reduced pressure and the AP-hydroxyimidamide was used in
the next step without further purification.
To a stirred solution of 3,4-diethoxybenzoic acid (73mg, 0.35 mmol) in
1,4-dioxane was added EDCI (87 mg, 0.45 mmol) and HOBt (62 mg, 0.45
mmol), the reaction was stirred 20 min at room temperature. To the reaction
was
added the AP-hydroxyimidamide (87mg, 0.45 mmol) and the mixture was stirred
153
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
for 30 min at room temperature followed by 16h at 95 C. The reaction was
concentrated under reduced pressure, diluted with Et0Ac (80 ml) and washed
with brine (2X30m1). The organic layer was dried over Na2SO4 and concentrated
under reduced pressure. The crude was purified by column chromatography
using CH2C12:Me0H (9:1) to offer the product as a pale yellow solid 50% yield
(64mg, 0.175 mmol). MS (El) m/z: 366 (M+)
O¨N
----,
ith
Et0 NN \
\ N
z
Et0 \ N H
5-(3,4-diethoxypheny1)-3-(1H-pyrrolo12,3-bipyridin-4-y1)-1,2,4-oxadiazole
To a solution of 4-cyano-7-azaindole (1g, 7 mmol) in methanol (30 mL)
were added cautiously hydroxylamine hydrochloride (632 mg, 9.1 mmol) and
sodium carbonate (964mg, 9.1 mmol). The reaction mixture was reflux for 6h
under nitrogen atmosphere and hydroxylamine hydrochloride (632 mg, 9.1
mmol) and sodium carbonate (964mg, 9.1 mmol) were added, the reaction was
reflux for additional 14h. The mixture was cooled to room temperature and the
solid was filtered. The organic solvent was concentrated under reduced
pressure
and the crude was recrystallized from ethanol to yield 200mg of /V'-
hydroxyimidamide.
To a stirred solution of 3,4-diethoxybenzoic acid (50mg, 0.24 mmol) in
DMF was added EDCI (59 mg, 0.31 mmol) and HOBt (41 mg, 0.31 mmol), the
reaction was stirred 20 min at room temperature. To the reaction was added the
N-hydroxyimidamide (54mg, 0.31 mmol) and the mixture was stirred for 30 min
at room temperature followed by 16h at 95 C. The reaction was concentrated
under reduced pressure, diluted with Et0Ac (80 ml) and washed with a saturated
solution of NaHCO3 (2X30m1) and brine (50m1). The organic layer was dried
over Na2SO4 and concentrated under reduced pressure. The crude was purified
by column chromatography using CH2C12:Me0H (9:1) to offer the product as a
. brown solid in 5% yield (4mg, 0.01 mmol). MS (El) m/z: 351 (M+)
154
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
O-N
NH
F3C
3-(1H-indo1-5-y1)-5-(4-pheny1-5-(trifluoromethyl)thiophen-2-y1)-1,2,4-
oxadiazole
To a solution of 1H-indole-5-carbonitrile (2g, 14.06mmol) in ethanol
under reflux were added, in three equal portions, hydroxylamine hydrochloride
(4.88g, 70.3 mmol) and potassium bicarbonate (7.04g, 70.3 mmol), the reaction
was reflux for 16h. The mixture was cooled to room temperature and the solid
was filtered. The organic solvent was concentrated under reduced pressure and
the N'-hydroxyimidamide was used in the next step without further
purification.
To a solution of 4-phenyl-5-(trifluoromethyl)-2-thiophenecarboxylic acid
in 1,4-dioxane were added under nitrogen atmosphere EDCI (125mg, 0.65mmol)
and HOBt (88mg, 0.65mmol). The reaction was stirred at room temperature for
30 min followed by addition of AP-hydroxyimidamide (114 mg, 0.65mmol), the
reaction was stirred for 30 additional minutes at room temperature followed by
16 h at 95 C. The reaction was concentrated under reduced pressure. The crude
was diluted with Et0Ac (80mL) and washed with a saturated solution of
NaHCO3 (2X50m1). The organic phase was dried over Na2SO4 anhydrous and
concentrated under reduced pressure. The product was purified by column
chromatography using CH2C12:Me0H (9:1) to yield 91mg (46.7%) of the
product. MS (El) m/z: 412 (Mt).
O¨N
N
= \
= NH
F3C
3-(indolin-5-y1)-5-(4-pheny1-5-(trifluoromethyl)thiophen-2-y1)-1,2,4-
oxadiazole
To a stirred solution of 3-(1H-indo1-5-y1)-5-(4-pheny1-5-
(trifluoromethyl)thiophen-2-y1)-1,2,4-oxadiazole (40mg, 0.097 mmol) in acetic
acid at 12-14 C was added slowly sodium cyanoborohydride (19 mg,
155
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
0.29mmol). At the end of the addition the reaction was allowed to warm to 18-
20
C and was stirred for 2 h. After completion the reaction mixture was
neutralized
with 50% sodium hydroxide and extracted with ethyl acetate (50m1 X2). The
organic layers were combined and dried over Na2SO4 and removed under
reduced pressure. The crude indoline compound was purified by column
chromatography using CH2C12/Me0H (9:1) to offer the product in 84.3% yield
(33.8 mg, 0.082 mmol). MS (El) m/z: 414 (M+)
0 ¨ N
\
N
F3C
3-(3-methylpyridin-4-y1)-5-(4-pheny1-5-(trifluoromethyl)thiophen-2-y1)-
1,2,4-oxadiazole
Hydroxylamine hydrochloride (23.53g, 339 mmol) was dissolved in
water (120m1) and potassium bicarbonate (33.9g, 3339 mmol) was added
cautiously. The mixture was stirred slowly until complete solution. The
mixture
was added to a solution of 3-methyl isonicotinonitrile (2g, 16.9 mmol) in THF
(30 mL) at -25 C (ice methanol bath) and the reaction was stirred at room
temperature for 16h. The reaction mixture was extracted with Et0Ac
(3X100mL) and the combined organic phases were washed with brine (80m1).
The organic phase was dried over Na2SO4 and concentrated under reduced
pressure. The product was purified by column chromatography using
hexanes/Et0Ac (1:1) to yield AP-hydroxyimidamide in 47% yield (1.2 g).
To a solution of 4-phenyl-5-(trifluoromethyl)- 2-thiophenecarboxylic
acid (200mg, 0.735mmol) in DMF were added under nitrogen atmosphere EDCI
(183mg, 0.95mmol) and HOBt (129mg, 0.95mmol). The reaction was stirred at
room temperature for 30 min followed by addition of AP-hydroxyimidamide (143
mg, 0.95mmol), the reaction was stirred for 30 min at room temperature
followed by 16 h at 95 C. The reaction was concentrated under reduced
pressure. The crude was diluted with Et0Ac (80mL) and washed with a
saturated solution of NaHCO3 (2X50m1). The organic phase was dried over
Na2SO4 and concentrated under reduced pressure. The product was purified by
156
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
column chromatography using CH2C12:Me0H (9:1) to yield 161mg (56.6%) of
the product. MS (El) m/z: 412 (M+)
O¨N
N
N 110
Et0
Et0
OH
4-(5-(3,4-diethoxypheny1)-1,2,4-oxadiazol-3-y1)-2,3-dihydro-1H-inden-l-ol
To a stirred suspension of 1-oxo-2,3-dihydro-1H-indene-4-carbonitrile
(5g, 31.8mmol) and silica gel (100mg) in ethanol (30mL) at 0 C was added
NaBH4 (400mg, 10.6mmol). The reaction was allowed to warm up to room
temperature and stirred for 2 h. The solvent was removed under reduced
pressure
and the product purified by column chromatography in hexane/Et0Ac (5:5) to
offer 1-hydroxy-2,3-dihydro-1H-indene-4-carbonitrile as white solid in 80%
yield (4.04g, 25.4 mmol). IHNMR (300 MHz, CDC13): 6 7.62 (d, J= 7.5 Hz,
1H), 7.54 (d, J= 7.8 Hz, 1H), 7.33 (t, J= 7.8 Hz, 1H), 5.28 (t, J= 6.3 Hz,
1H),
3.28-3.18 (m, 1H), 3.02-2.92 (m, 1H), 2.63-2.52 (m, 1H), 2.06-1.99 (m, 1H).
To a stirred solution of 1-hydroxy-2,3-dihydro-1H-indene-4-carbonitrile
(3g, 18.86 mmol) in ethanol (100 mL) were added cautiously over a period of
16h under refluxing conditions hydroxylamine hydrochloride (6.55g, 94.3
mmol) and potassium carbonate (13.03g, 94.3 mmol) in equal portions. The
mixture was cooled to room temperature and the solid was filtered. The organic
solvent was concentrated under reduced pressure and the crude was
recrystallized from ethanol to yield 2.5g (69%) of amidoxime.
In a microwave vial, a stirring solution of 3,4-diethoxybenzoic acid
(200mg, 0.95mmol) in DMF was treated with HOBt (168 mg, 1.24mmol) and
EDCI (237mg, 1.24mmol) at room temperature. The reaction was stirred for 20
min followed by addition, in a single portion, of amidoxime (238mg, 1.24mmol).
The reaction was stirred for additional 30 min at room temperature and then
heated to 130 C for 35 min in the initiator. The reaction was diluted using a
saturated solution of NaC1 and extracted with Et0Ac (3X80m1). The organic
157
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
phase was dried over Na2SO4 anhydrous and concentrated under reduced
pressure. The product was purified by column chromatography using
CH2C12:Me0H (9:1) to offer the product as a white solid in 69% yield (208mg).
IHNMR (400 MHz, CDC13): 88.10 (d, J= 7.6, 1H), 7.78 (dd, JI = 1.6 Hz, J2 =
8 Hz, 1H), 7.67 (d, J= 1.6 Hz, 1H), 7.56 (d, J= 7.6 Hz, 1H), 7.39 (t, J= 7.6
Hz,
1H), 6.97 (d, J= 8.0 Hz, 1H), 5.29 (t, J= 6.4 Hz, 1H), 4.19 (q, J= 7.2 Hz,
2H),
4.18 (q, J= 7.2 Hz, 2H), 3.51-4.43 (m, 1H), 3.22-3.14 (m, 1H), 2.59-2.51 (m,
1H), 2.04-1.97 (m, 1H), 1.5 (t, J= 7.2 Hz, 3H), 1.49 (t, J= 7.2, 3H); 13C NMR
(100 MHz, CDC13): 6175.2, 168.9, 152.8, 148.9, 146.6, 143.3, 128.9, 127.4,
127.0, 123.8, 122.2, 116.7, 112.7, 112.4, 76.2, 64.9, 64.8, 35.7, 31.5, 14.9,
14.8.
MS (El) m/z 367 (Mt), HRMS (El) for C211-122N204 (Mt): calcd 367.1652, found
367.1653.
O-N //NEt2
N
N
N z N
Et0
Et0
N1-44-(5-(3,4-diethoxypheny1)-1,2,4-oxadiazol-3-yOpyridin-2-Amethyl)-
N2,N2-diethylethane-1,2-diamine
To a stirred solution of 2-(hydroxymethypisonicotinonitrile (570mg,
4.25mmol) in ethanol (40 mL) were added cautiously over a period of 16h under
refluxing conditions hydroxylamine hydrochloride (1.37g, 21.25 mmol) and
sodium carbonate (2.25g, 21.25 mmol) ¨in equal portions-. The mixture was
cooled to room temperature and the solid was filtered. The organic solvent was
concentrated under reduced pressure and the crude was recrystallized from
ethanol to yield 600mg (3.59mmol, 84%) of amidoxime.
In a microwave vial, a stirring solution of 3,4-diethoxybenzoic acid
(300mg, 1.43mmol) in DMF was treated with HOBt (250 mg, 1.85mmol) and
EDCI (354mg, 1.85mmol) at room temperature. The reaction was stirred for 20
min followed by addition, in a single portion, of amidoxime (309mg, 1.85mmol).
The reaction was stirred for additional 30 min at room temperature and then
heated to 130 C for 35 min in the initiator. The reaction was diluted using a
saturated solution of NaC1 and extracted with Et0Ac (3X80m1). The organic
158
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
phase was dried over Na2SO4 anhydrous and concentrated under reduced
pressure. The product was purified by column chromatography using
CH2C12:Me0H (9:1) to offer (4-(5-(3,4-diethoxypheny1)-1,2,4-oxadiazol-3-
yl)pyridin-2-yl)methanol as brown solid in 71% yield (350mg). IFI NMR (400
MHz, CDC13): 6 8.65 (d, J= 4.8 Hz, I H), 8.00 (s, 1H), 7.86 (d, J = 4.8 Hz,
1H),
7.70 (dd, J1 = 2.0 Hz, J2 = 8.8 Hz, 1H), 7.59 (d, J= 2.0, 1H), 6.91 (d, J =
8.8
Hz, 1H), 4.85 (s, 2H), 4.16 (q, J = 7.2 Hz, 2H), 4.13 (q, J=7.2 Hz, 2H), 1.49
(t, J
= 6.8 Hz, 3H), 1.46 (t, J= 6.8 Hz, 3H); 13C NMR (100 MHz CDC13): 6 176.54,
167.22, 153.03, 149.36, 148.91, 135.50, 122.23, 120.11, 118.48, 116.05,
112.48,
112.17, 64.90, 64.50, 14.82, 14.73. MS (El) m/z: 342 (Mt), HRMS (El) for
Ci8H19N304
To a stirred solution of pyridinol (20mg, 0.059 mmol) in DMSO (1m1)
were added sequentially N,N'-dicyclohexylcarbodiimide (36.3 mg, 0.176 mmol)
and 1.0M anhydrous H3PO4 in DMSO (30 [LI., 0.03mmol) and the reaction
mixture was stirred 2h at room temperature. Precipitated dicyclohexylurea was
filtered off and washed with ether (10m1) and water (10m1). The aqueous layer
was extracted with ether (3X20m1) and the organic layer was dried over sodium
sulfate and concentrated under reduced pressure. The residue was purified by
column chromatography using CH2C12:Me0H (95:5) to yield 15 mg (75%) of
pyridinecarboxaldehyde. MS (El) m/z 340 (Mt).
To a stirred solution of pyridinecarboxaldehyde (15mg, 0.044mmol) in
dichloroethane were added NI,N1-diethylethane-1,2-diamine (19 1, 0.13mmol)
and sodium triacetoxyborohydride (11mg, 0.05mmol), the reaction was stirred
for 4h at room temperature. The mixture was poured un silica gel and was
purified by column chromatography using CH2C12:MeOH:Et3N (90:9.8:0.2) to
yield 8mg (42%) of the product as a brown solid. MS (El) m/z 440 (Mt).
0¨I\\1
Et0 41, NN 410
Et0 NN
\
OH
159
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
2-(4-(5-(3,4-diethoxypheny1)-1,2,4-oxadiazol-3-yl)indolin-1-ypethanol
To a stirred solution of 3,4-diethoxybenzoic acid (400mg, 1.9mmol) in
DMF were added sequentially HOBt (330mg, 2.5 mmol) and EDCI (474mg,
2.5mmol) at room temperature. The reaction was stirred for 20 min followed by
addition, in a single portion, of the M-hydroxy-1H-indole-4-carboximidamide
(666mg, 3.8mmol). The reaction was stirred for additional 30 mm at room
temperature then heated at 90-95 C for 14h. The reaction was cooled to room
temperature, diluted using a saturated solution of Na2CO3 and extracted with
Et0Ac (100m1 X3). The organic phase was dried over Na2SO4 anhydrous and
concentrated under reduced pressure. The product was purified by column
chromatography using CH2C12:Me0H (9:1) to afford compound 110 in 50%
yield (33 lmg). H1 NMR (400 MHz, CDC13): 88.01 (d, J= 7.2 Hz, 1H), 7.81
(dd, JI = 2.0 Hz, J2 = 8.4 Hz, 1H), 7.52 (s, 1H), 7.52 (d, J= 8.0 Hz, 1H),
7.34
(s, 1H), 7.28 (t, J= 8.0 Hz, 1H), 7.23 (s, 1H), 6.96 (d, J= 8.4 Hz, 1H), 4.18
(q, J
= 6.8 Hz, 2H), 4.16 (q, J= 6.8 Hz, 2H), 1.47 (t, J= 6.8 Hz, 3H), 1.46 (t, J=
6.8
Hz, 3H); 13C NMR (100 MHz, CDC13): 8175.0, 169.5, 152.6, 148.9 (2), 125.7,
122.2, 121.8, 121.7, 121.3, 121.2, 117.0, 114.4, 114.3, 112.7, 112.5, 64.97,
64.76, 14.86, 14.78. MS (El) m/z 350 (M+), HRMS (El) for C201-119N303 (M+):
calcd 350.1499, found 350.1504.
To a stirred solution of the previous product 110 (260mg, 0.74 mmol) in
acetic acid at 10-15 C was added slowly sodium cyanoborohydride (140 mg,
2.25mmol). The reaction was allowed to warm to 18-20 C and was stirred for 2
h. After completion the reaction mixture was neutralized with 50% sodium
hydroxide and extracted with ethyl acetate (100m1 X2). The organic layers were
combined, dried over Na2SO4 and removed under reduced pressure. The crude
indoline compound was purified by column chromatography using
CH2C12/Me0H (9:1) to provide the dihydro compound in 80.5% yield (211 mg,
0.60 mmol). 111 NMR (300 MHz, CDC13): (5 7.78 (dd, J1 = 2.1 Hz, J2 = 8.4 Hz,
1H), 7.68 (d, J= 1.8 Hz, 1H), 7.55 (d, J= 7.8 Hz, 1H), 7.16 (t, J= 7.5 Hz,
1H),
6.97 (d, J= 7.5 Hz, 1H), 6.76 (d, J= 7.5 Hz, 1H), 4.21 (q, J= 6.9 Hz, 2H),
4.16
(q, J= 6.9 Hz, 2H), 3.65 (bs, 2H), 3.46 (t, J= 8.1 Hz, 2H), 1.52 (t, J= 3.1
Hz,
3H), 1.48 (t, J= 3.3 Hz, 3H); 13C NMR (75 MHz, CDC13): 6 175.05, 169.06,
152.62, 148.84, 127.84, 123.67, 122.09, 118.90, 116.83, 112.53, 112.32,
111.60,
64.86, 64.67, 47.29, 31.41, 14.85, 14.78. MS (El) m/z 352 (M+), HRMS (El) for
160
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
C20H21N303 (M): calcd 352.1656, found 352.1660.
To a stirred solution of the previous dihydro product (50mg, 0.14mmol)
in DMF (3m1) was added potassium carbonate (118mg, 0.85mmol) and 2-
bromoethanol (20 1, 0.28mmol). The reaction was stirred at 60 C for 48h. At
the
end of the reaction the solution was poured into water (50m1) and the mixture
was extracted with Et0Ac (3X50m1). The organic phases were combined,
washed with water, dried over sodium sulfate and concentrated under reduced
pressure. The product was purified by column chromatography using
CH2C12:Me0H (9:1) to yield 35mg (63%) of the product as a brown solid. 1H
NMR (300 MHz, CDC13) 8: 7.74 (dd, J1 = 1.8 Hz, J2 = 8.4 Hz, 1H), 7.61 (d, J=
2.1 Hz, 1H), 7.33 (dd, J1 =0.6 Hz, J2 = 7.8 Hz, 1H), 7.20 (t, J= 5.1 Hz, 1H),
7.17 (d, J= 1.8 Hz, 1H), 6.70 (d, J= 8.1 Hz, 1H), 4.14 (q, J= 7.2 Hz, 4H),
3.62
(t, J= 6.0 Hz, 2H), 3.55 (t, J= 8.7 Hz, 2H), 3.28 (t, J= 8.1 Hz, 2H), 3.21 (t,
J=
6.0 Hz, 2H), 1.39 (t, J= 1.8 Hz, 3H), 1.35 (t, J= 1.5 Hz, 3H); 13C NMR (75
MHz, CDC13): 6 168.67, 158.97, 158.46, 153.35, 152.73, 148.72, 129.76,
128.39, 122.19, 117.36, 115.89, 113.54, 113.38, 111.94, 64.48, 64.43, 58.90,
53.20, 51.46, 29.99, 15.00, 14.90. MS (El) m/z 396 (Mt), HRMS (El) for
C22H25N304 (Mt): calcd 396.1918, found 396.1918.
011
Et0 NN
Et0 111
NH
OH
2-(4-(5-(3,4-diethoxypheny1)-1,2,4-oxadiazol-3-y1)-2,3-dihydro-1H-inden-1-
ylamino)ethanol
To a stirred solution of compound 215 (400mg, 1.09 mmol) in CH2C12
were added at 0 C pyridine (89 1, 1.1mmol) and thionyl chloride (81 1,
1.1mmol), the reaction was stirred lh at room temperature and the mixture was
concentrated under reduced pressure. The crude was diluted in DMF (10m1) and
were added potassium carbonate (290 mg, 2.1mmol) and ethanolamine (128 1,
161
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
2.1 mmol), the reaction was stirred overnight at 60 C. The reaction mixture
was
poured into water (100m1) and extracted with Et0Ac (100m1 X3). The organic
phase was dried over Na2SO4 and concentrated under reduced pressure. The
crude was purified by column chromatography using CH2C12:Me0H (9:1) to
yield 312 mg (70% yield) of the product as a white solid. 11-1 NMR (500 MHz,
CDC13): 88.12 (d, J= 7.5 Hz, 1H), 7.79 (dd, J1 = 2.0 Hz J2 = 8.5 Hz, 1H), 7.68
(d, J= 2.0 Hz, 1H), 7.63 (d, J= 7.5 Hz, 1H), 7.40-7.37 (m, 1H), 4.49-4.47 (m,
1H), 4.23-4.16 (m, 4H), 3.78-3.70 (m, 1H), 3.53-3.46 (m, 1H), 3.29-3.22 (m,
1H), 2.96-2.94 (m, 4H), 2.56-2.50 (m, 1H), 2.09-2.03 (m, 1H), 1.52-1.49 (m,
6H); 13C NMR (125 MHz, CDC13): 8175.03, 168.66, 152.71, 148.87, 143.76,
128.71, 127.11, 123.89, 122.04, 116.67, 112.63, 112.49, 104.66, 64.84, 64.61,
62.70, 60.34, 47.98, 31.90, 29.69, 14.72, 14.64. MS (El) m/z 410 (M+), HRMS
(El) for C23H271\1304 (M+): calcd 410.2074, found 410.2077.
0 - N
Et0 N
Et0
NH
N Et2
N1-(4-(5-(3,4-diethoxypheny1)-1,2,4-oxadiazol-3-y1)-2,3-dihydro-1H-inden-l-
y1)-N2,N2-diethylethane-1,2-diamine
To a stirred solution of compound 215 (40mg, 0.109 mmol) in CH2C12
were added at 0 C pyridine (9 1, 1.1mmol) and thionyl chloride (8 1, 1.1mmol),
the reaction was stirred lh at room temperature and concentrated under reduced
pressure. The crude was diluted in DMF (1m1) and were added potassium
carbonate (29mg, 0.21mmol) and N,N-diethylethylenediamine (30p1, 0.21
mmol). The reaction was stirred overnight at 60 C. The reaction mixture was
poured into water (100m1) and extracted with Et0Ac (20m1 X3). The organic
phase was dried over Na2CO3 and concentrated under reduced pressure. The
crude was purified by column chromatography using CH2C12:Me0H (9:1) to
yield 65% (33 mg) of the product as a brown solid. MS (El) m/z 465 (M.).
162
CA 02723904 2010-11-09
WO 2009/151529 PCT/US2009/003014
O¨N
1110 NH
F3C N=-1
' 3-(1H-benzo[d]imidazol-5-y1)-5-(4-pheny1-5-(trifluoromethyl)thiophen-2-
y1)-
1,2,4-oxadiazole
In a microwave vial, a stirring solution of 3,4-diethoxybenzoic acid
(100mg, 0.367mmol) in DMF was treated with HOBt (64 mg, 0.48mmol) and
EDCI (92mg, 0.48mmol) at room temperature. The reaction was stirred for 20
min followed by addition, in a single portion, of N'-hydroxy-1H-
benzo[d]imidazole-4-carboximidamide (68mg, 0.367mmol). The reaction was
stirred for additional 30 min at room temperature and then heated to 130 C
for
35 min in the initiator. The reaction was diluted using a saturated solution
of
NaHCO3 and extracted with Et0Ac (3X80m1). The organic phase was dried over
Na2SO4 anhydrous and concentrated under reduced pressure. The crude was
purified by column chromatography using CH2C12:Me0H (9:1) to provide a 40%
yield (61 mg) of the product as brown solid. MS (El) m/z 413 (Mt).
/
0
3-(3,4-diethoxypheny1)-5-(pyridin-4-y1)-1,2,4-oxadiazole
In a microwave vial, a stirring solution of isonicotinic acid (200mg,
1.62mmol) in DMF was treated with HOBt (319 mg, 2.43mmol) and EDCI
(467mg, 2.43mmol) at room temperature. The reaction was stirred for 20 min
followed by addition, in a single portion, of (Z)-3,4-diethoxy-/V'-
hydroxybenzimidamide (436mg, 1.94mmol). The reaction was stirred for
additional 30 min at room temperature and then heated to 130 C for 35 min in
the initiator. The reaction was diluted using a saturated solution of NaHCO3
and
extracted with Et0Ac (80m1 X3). The organic phase was dried over Na2SO4
anhydrous and concentrated under reduced pressure. The product was purified
163
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
by column chromatography using CH2C12:Me0H (95:5) to offer the product in
26% yield (131 mg) as pale brown solid. IHNMR (300 MHz, CDC13): 6 8.85 (d,
J= 5.4 Hz, 2H), 8.02 (d, J= 6.0 Hz, 2H), 7.71 (dd, J1= 1.8 Hz, J2= 8.1 Hz,
1H), 7.62 (d, J= 2.1 Hz, 1H), 6.95 (d, J= 8.4 Hz, 1H), 4.19 (q, J= 6.9 Hz,
2H),
4.14 (q, J= 6.9 Hz, 2H), 1.50 (t, J= 3.0 Hz, 3H), 1.46 (t, J= 3.3 Hz, 3H); 13C
NMR (75 MHz, CDC13): 6 173.54, 16928, 151.64, 151.07, 148.91, 131.37,
121.48, 121.15, 118.75, 112.74, 111.82, 64.78, 64.59, 14.87, 14.81. MS (El)
m/z:
312 (Mt), HRMS (El) for CI7F117N303 (Mt): calcd 312.1343, found 312.1348.
0 __________________ \
Et0 401 N
N
Et0
2-(3,4-diethoxypheny1)-4-(pyridin-4-ypoxazole
In a microwave vial were dissolved 2-bromo-1-(pyridin-4-yl)ethanone
hydrobromide (134mg, 0.478mmo1) and 3,4-diethoxybenzamide (100mg,
0.478mmo1) in DMF (5m1) and the reaction was heated at 170 C for 40min. The
reaction mixture was poured in a saturated solution of NaHCO3 and extracted
with Et0Ac (50m1 X3). The combined organic phases were washed with a
saturated solution of NaCl (2X30m1) and concentrated under reduced pressure.
The product was purified by column chromatography using CH2C12:Me0H
(95:5) to yield 4mg (2.7%) of the product. MS (El) m/z: 311 (M).
0
N
4
11Ik N
Et0
Et0
2-(3,4-diethoxypheny1)-4-phenyloxazole
In a microwave vial were dissolved 2-bromo-1-phenylethanone (95mg,
0.478mmo1) and 3,4-diethoxybenzamide (100mg, 0.478mmo1) in DMF (5m1)
and the reaction was heated at 170 C for 40min. The reaction mixture was
poured in a saturated solution of NaHCO3 and extracted with Et0Ac (50m1 X3).
164
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
The combined organic phases were washed with a saturated solution of NaC1
(30m1 X2) and concentrated under reduced pressure. The product was purified
by column chromatography using CH2C12:Me0H (95:5) to yield 25mg (17%) of
the product. MS (El) m/z: 310 (Mt)
N ¨ 0
ics)
C F3
HO
4-(5-(4-isopropoxy-3-(trifluoromethyl)pheny1)-1,2,4-oxadiazol-3-y1)-2,3-
dihydro-1H-inden-l-ol
In a microwave vial, a stirring solution of 4-isopropoxy-3-
(trifluoromethyl)benzoic acid (700mg, 2.82mmol) in DMF was treated with
HOBt (495 mg, 3.67mmol) and EDCI (702mg, 3.67mmol) at room temperature.
The reaction was stirred for 20 min followed by addition, in a single portion,
of
AP,1-dihydroxy-2,3-dihydro-1H-indene-4-carboximidamide (650mg, 3.38mmol).
The reaction was stirred for additional 30 min at room temperature and then
heated to 130 C for 35 min in the initiator. The reaction was diluted using a
saturated solution of NaCl and extracted with Et0Ac (100m1 X3). The organic
phase was dried over Na2SO4 anhydrous and concentrated under reduced
pressure. The product was purified by column chromatography using
CH2C12:Me0H (9:1) to offer the product as white solid in 68% yield (780mg).
NMR (300 MHz, CDC13-CH30D): 6 8.38 (d, .1= 1.8 Hz, 1H), 8.28 (dd, J1 =
2.4 Hz, J2 = 8.7 Hz, 1H), 8.06 (d, J= 7.8 Hz, 1H), 7.55 (d, J= 7.5 1H), 7.7.37
(t,
J= 7.5 Hz, 1H), 7.11 (dd, J1 = 2.4 Hz, J2 = 9.0 Hz, 1H), 5.24 (t, J= 6.3 Hz,
1H), 4.20 (q, J= 6.9 Hz, 1H), 3.48-3.40 (m, 1H), 3.19-3.09 (m, 1H), 2.57-2.46
(m, 1H), 2.04-1.92 (m, 1H), 1.46 (t, J= 6.9 Hz, 3H), 1.39 (d, J= 6.0 Hz, 3H);
13C NMR (75 MHz, CDC13-CH30D): 6 169.05, 158.85, 146.82, 143.33, 133.55,
128.79, 127.41, 127.22, 116.23, 113.38, 104.88, 65.27, 35.55, 31.44, 21.87,
14.55. MS (El) m/z 405 (Mt), HRMS (El) for C21Hi9N203 (Mt): calcd 405.1420,
found 405.1424.
165
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
N¨ 0
efh Nr =
C F3
HO
4-(5-(4-ethoxy-3-(trifluoromethyl)pheny1)-1,2,4-oxadiazol-3-y1)-2,3-dihydro-
1H-inden-l-ol
In a microwave vial, a stirring solution of 4-ethoxy-3-
(trifluoromethyl)benzoic acid (200mg, 0.85mmol) in DMF was treated with
HOBt (151mg, 1.11mmol) and EDCI (212mg, 1.11mmol) at room temperature.
The reaction was stirred for 20 min followed by addition, in a single portion,
of
N,1-dihydroxy-2,3-dihydro-1H-indene-4-carboximidamide (213mg, 1.11mmol).
The reaction was stirred for additional 30 min at room temperature and then
heated to 130 C for 35 min in the initiator. The reaction was diluted using a
saturated solution of NaC1 and extracted with Et0Ac (80m1X3). The organic
phase was dried over Na2SO4 anhydrous and concentrated under reduced
pressure. The product was purified by column chromatography using
CH2C12:Me0H (9:1) to offer the product as white solid in 51% yield (200mg).
1H NMR (300 MHz, CDC13-CH30D): (5 8.27 (d, J= 1.8 Hz, 1H), 8.21 (dd, JI =
2.1 Hz, J2= 8.7 Hz, 1H), 7.94 (d, J= 7.5 Hz, 1H), 7.44 (d, J= 7.8 Hz, 1H),
7.26
(q, J= 9.9 Hz, 1H), 7.05 (d, J= 8.7 Hz, 1H), 5.13 (t, J= 6.3 Hz, 1H), 4.11 (q,
J
= 6.9 Hz, 2H), 3.38-3.28 (m, 1H), 3.08-2.97 (m, 1H), 2.44-2.34 (m, 1H), 1.91-
1.84 (m, 1H), 1.35 (t, J= 6.9 Hz, 3H); 13C NMR (75 MHz, CDC13-CH30D):
150.90, 147.26, 143.96, 137.60, 137.59, 132.62, 132.59, 131.31, 131.27,
131.24,
127.21, 120.11, 117.48, 104.99, 79.55, 69.27, 39.27, 35.35, 18.39. MS (El) m/z
391 (M+), HRMS (El) for C20H17F3N203 (M+): calcd 391.1264, found 391.1261.
N ¨ 0
Nz o)
C F3
HO
166
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
4-(5-(4-isopropoxy-3-(trilluoromethyl)phenyl)-1,2,4-oxadiazol-3-y1)-2,3-
dihydro-1H-inden-1-ol
In a microwave vial, a stirring solution of 4-ethoxy-3-
(trifluoromethyl)benzoic acid (200mg, 0.97mmol) in DMF was treated with
HOBt (172mg, 1.26mmol) and EDCI (242mg, 1.26mmol) at room temperature.
The reaction was stirred for 20 min followed by addition, in a single portion,
of
N,1-dihydroxy-2,3-dihydro-1H-indene-4-carboximidamide (243mg, 1.26mmol).
The reaction was stirred for additional 30 min at room temperature and then
heated to 130 C for 35 min in the initiator. The reaction was diluted using a
saturated solution of NaCl and extracted with Et0Ac (80m1 X3). The organic
phase was dried over Na2SO4 anhydrous and concentrated under reduced
pressure. The product was purified by column chromatography using
CH2C12:Me0H (9:1) to offer the product as pale yellow solid in 63% yield
(229mg).
NMR (300 MHz, CDC13-CH30D): ö 8.36 (d, J=2.1 Hz, 1H), 8.29 (dd, J1=
2.4 Hz, J2= 9.0 Hz, 1H), 8.06 (d, J= 7.8 Hz, 1H), 7.55 (d, J= 7.5 Hz, 1H),
7.37
(t, J= 7.5 Hz, 1H), 7.10 (d, J= 9.0 Hz, 1H), 5.26 (t, J= 6.3 Hz, 1H), 4.81-
4.73
(m, 1H), 3.48-3.38 (m, 1H), 3.19-3.08 (m, 1H), 2.56-2.49 (m, 1H), 2.04-1.95
(m,
1H), 1.45 (d, J= 6.3 Hz, 6H); 13C NMR (75 MHz, CDC13-CH30D): 6 173.11,
168.95, 162.82, 146.70, 143.26, 134.15, 134.041, 128.27, 127.30, 127.18,
123.18, 116.85, 115.41, 113.66, 103.86, 72.85, 35.57, 31.42, 21.82. MS (El)
m/z
362 (M+), HRMS (El) for C21Hi9N303 (M+): calcd 362.1499, found 362.1494.
N¨ 0
1\l'
CN
HO
2-ethoxy-5-(3-(1-hydroxy-2,3-dihydro-1H-inden-4-y1)-1,2,4-oxadiazol-5-
yl)benzonitrile
In a microwave vial, a stirring solution of 3-cyano-4-ethoxybenzoic acid
(200mg, 1.05mmol) in DMF was treated with HOBt (185mg, 1.36mmol) and
EDCI (260mg, 1.36mmol) at room temperature. The reaction was stirred for 20
167
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
mm followed by addition, in a single portion, of AP,1-dihydroxy-2,3-
dihydro-1H-
indene-4-carboximidamide (261mg, 1.36mmol). The reaction was stirred for
additional 30 min at room temperature and then heated to 130 C for 35 min in
the initiator. The reaction was diluted using a saturated solution of NaC1 and
extracted with Et0Ac (80m1 X3). The organic phase was dried over Na2SO4
anhydrous and concentrated under reduced pressure. The product was purified
by column chromatography using CH2C12:Me0H (9:1) to offer the product as
pale yellow solid in 79% yield (274mg).
NMR (300 MHz, CDC13-CH30D): 6 8.31 (d, J= 2.1 Hz, 1H), 8.25 (dd, JI =
2.1 Hz, J2 = 8.7 Hz, 1H), 7.96 (d, J= 7.5 Hz, 1H), 7.46 (d, J= 7.5 Hz, 1H),
7.29
(t, J= 7.8 Hz, 1H), 7.06 (d, J= 9.0 Hz, 1H), 5.16 (t, J= 6.0 Hz, 1H), 4.18 (q,
J=
6.9 Hz, 2H), 3.40-3.30 (m, 1H), 3.10-2.93 (m, 1H), 2.46-2.39 (m, 1H), 1.94-
1.87
(m, 1H), 1.43 (t, J= 6.9 Hz, 3H); 13C NMR (75 MHz, CDC13-CH30D): 6
172.91, 167.05, 161.92, 145.72, 142.08, 133.24, 132.83, 127.38, 126.16,
126.10,
121.85, 115.98, 111.76, 101.85, 74.36, 64.55, 34.08, 30.18, 13.20. MS (EI) m/z
348 (Mt), HRMS (El) for C23H271\1303 (Mt): calcd 348.1343, found 348.1345.
N-0
tO Ny o)
CF3
HN
OH
2-(4-(5-(4-isopropoxy-3-(trifluoromethyppheny1)-1,2,4-oxadiazol-3-y1)-2,3-
dihydro-1H-inden-1-ylamino)ethanol
To a stirred solution of compound 259 (75mg, 0.185 mmol) in CH2C12
were added at 0 C pyridine (15 1, 0.195mmo1) and thionyl chloride (14 1,
0.195mmol), the reaction was stirred lh at room temperature and concentrated
under reduced pressure. The crude was diluted in DMF (1m1) and were added
DIPEA (161 1, 0.927mmol) and ethanolamine (56111, 0.927 mmol) at 0 C. The
reaction was stirred overnight at 60 C. The reaction mixture was poured into
water (100m1) and extracted with ethyl acetate (20m1 X3). The organic phase
was dried over sodium sulfate and concentrated under reduced pressure. The
168
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
crude was purified by column chromatography using CH2C12:Me0H (9:1) to
yield 40% (18 mg) of the product as a brown solid.
II-I NMR (300 MHz, CDC13-CH30D): 6 8.37 (s, 1H), 8.28-8.24 (m, 1H), 8.06 (t,
J= 4.8 Hz, 1H), 7.51 (d, J= 6.9 Hz, 1H), 7.35 (t, J= 7.5 Hz, 1H), 7.11 (d, J=
8.4 Hz, 1H), 4.36-4.32 (m, 1H), 4.19 (d, J= 6.9 Hz, 1H), 3.67-3.59 (m, 3H),
3.39-3.34 (m, 2H), 3.23-3.12 (m, 1H), 2.49-2.43 (m, 1H), 1.97-1.90 (m, 1H),
1.45 (t, J = 6.9 Hz, 3H), 1.38 (d, J= 4.2 Hz, 3H); 13C NMR (75 MHz, CDC13-
CH30D): 6 169.63, 146.26, 144.53, 134.30, 128.97, 127.76, 127.64, 124.09,
116.72, 115.25, 114.30, 103.98, 72.86, 65.91, 63.32, 61.30, 32.74, 32.59,
22.08,
14.76. MS (El) m/z 448 (M+), HRMS (El) for C23H24F3N303 (M ): calcd
448.1842, found 448.1849.
N-0
ith 1 N/ =
so7--.,
= CF3
HN
/
OH
2-(4-(5-(4-ethoxy-3-(trifluoromethyflpheny1)-1,2,4-oxadiazol-3-y1)-2,3-
dihydro-1H-inden-l-ylamino)ethanol
To a stirred solution of compound 260 (154mg, 0.394 mmol) in CH2C12
were added at 0 C pyridine (331.11, 0.414mmol) and thionyl chloride (30 1,
0.414mmol), the reaction was stirred lh at room temperature and concentrated
under reduced pressure. The crude was diluted in DMF (2m1) and were added
DIPEA (360111, 2.07mmol) and ethanolamine (125 1, 2.07 mmol) at 0 C. The
reaction was stirred overnight at 60 C. The reaction mixture was poured into
water (100m1) and extracted with ethyl acetate (20m1 X3). The organic phase
was dried over sodium sulfate and concentrated under reduced pressure. The
crude was purified by column chromatography using CH2C12:Me0H (9:1) to
yield 18% (31 mg) of the product as a brown solid.
169
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
Ili NMR (300 MHz, CDC13-CH30D): 6 8.31 (s, 1H), 8.23 (d, J= 8.7 Hz, 1H),
7.99 (d, J = 7.5 Hz, 1H), 7.48 (d, J= 7.2 Hz, 1H), 7.30 (t, J= 7.5 Hz, 1H),
7.07
(d, 1= 8.7 Hz, 1H), 4.33 (t, J= 6.0 Hz, 1H), 4.15 (q, J=6.9 Hz, 3H), 3.64 (t,
J
=4.5 Hz, 2H), 3.41-3.33 (m, 1H), 3.19-3.08 (m, 1H), 2.78 (t, J = 4.8 Hz, 2H),
2.45 (m, 1H), 2.18 (bs, 1H, -NH), 1.96-1.87 (m, 1H), 1.40 (t, J = 7.2 Hz, 3H);
13C NMR (75 MHz, CDC13-CH30D): 6 168.45, 159.91, 144.62, 143.36, 133.04,
128.04, 127.17, 126.73, 126.57, 122.99, 119.63, 115.59, 112.89, 100.08, 64.73,
62.19, 60.09, 48.89, 48.32, 31.63, 31.46, 13.93. MS (El) m/z 434 (Mt), HRMS
(El) for C22H22F3N303 (Mt): calcd 434.1686, found 434.1692.
N¨ 0
ifi1 N' 404 o)
11, CN
HN
/
OH
5-(3-(1-(2-hydroxyethylamino)-2,3-dihydro-1H-inden-4-y1)-1,2,4-oxadiazol-
5-y1)-2-isopropoxybenzonitrile
To a stirred solution of compound 261 (130mg, 0.360 mmol) in CH2C12
were added at 0 C pyridine (30111, 0.378mmol) and thionyl chloride (27 1,
0.378mmo1), the reaction was stirred lh at room temperature and concentrated
under reduced pressure. The crude was diluted in DMF (2m1) and were added
DIPEA (328pI, 1.889mmol) and ethanolamine (114 1, 1.889 mmol) at 0 C. The
reaction was stirred overnight at 60 C. The reaction mixture was poured into
water (100m1) and extracted with ethyl acetate (20m1 X3). The organic phase
was dried over sodium sulfate and concentrated under reduced pressure. The
crude was purified by column chromatography using CH2C12:Me0H (9:1) to
yield 23% (34 mg) of the product as a pale brown solid.
ill NMR (300 MHz, CDC13-CH30D): 6 8.33 (d, J= 2.1 Hz, 1H), 8.26 (dd, 11 =
2.4 Hz, .12 = 9.0 Hz, 1H), 7.99 (d, J= 7.5 (d, J= 7.5 Hz, 1H), 7.47 (d, J= 7.5
170
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
Hz, 1H), 7.33 (t, J= 7.5 Hz, 1H), 7.07 (d, J= 9.0 Hz, 1H), 4.74 (t, J= 6.0 Hz,
1H), 4.27 (t, J= 6.6 Hz, 1H), 3.62 (q, J= 6.0 Hz, 2H), 3.37-3.32 (m, 1H), 3.18-
3.10 (m, 1H), 2.79 (t, 5.1 Hz, 2H), 2.48-2.37 (m, 1H), 1.92-1.85 (m, 1H), 1.40
(d, J= 6.0 Hz, 6H); 13C NMR (75 MHz, CDC13-CH30D): 6 172.73, 168.58,
162.49, 145.45, 143.33, 133.74, 133.71, 127.85, 126.69, 126.58, 122.81,
116.40,
115.00, 113.31, 103.33, 72.46, 62.30, 60.54, 32.15, 31.51, 21.35. MS (El) m/z
405 (M+), HRMS (El) for C23H241\1403 (M+): calcd 405.1921, found 405.1920.
N ¨ 0
/ 7
N
= NO2
HO
4-(5-(4-ethoxy-3-nitropheny1)-1,2,4-oxadiazol-3-y1)-2,3-dihydro-1H-inden-l-
ol
In a microwave vial, a stirring solution of 4-ethoxy-3-nitrobenzoic acid
(200mg, 0.947mmo1) in DMF was treated with HOBt (167mg, 1.231mmol) and
EDCI (236mg, 1.231mmol) at room temperature. The reaction was stirred for 20
min followed by addition, in a single portion, of Ar,1-dihydroxy-2,3-dihydro-
1H-
indene-4-carboximidamide (200mg, 1.04mmol). The reaction was stirred for
additional 30 min at room temperature and then heated to 130 C for 35 min in
the initiator. The reaction was diluted using a saturated solution of NaCl and
extracted with Et0Ac (80m1 X3). The organic phase was dried over Na2SO4
anhydrous and concentrated under reduced pressure. The product was purified
by column chromatography using CH2C12:Me0H (9:1) to offer the product as
pale yellow solid in 40% yield (150mg).
NMR (300 MHz, CDC13-CH30D): 6 8.03 (d, J= 1.2 Hz, 1H), 7.73 (dd, J1=
1.5 Hz, J2= 8.7 Hz, 1H), 7.46 (d, J= 7.5 Hz, 1H), 6.97 (d, J= 7.2 Hz, 1H),
6.79
(t, J= 7.5 Hz, 1H), 6.67 (d, J= 8.7 Hz, 1H), 4.65 (t, J= 6.0 Hz, 1H), 3.70 (q,
J-
6.9 Hz, 2H), 2.87-2.79 (m, 1H), 2.60-2.49 (m, 1H), 1.96-1.90 (m, 1H), 1.43-
1.36
(m, 1H), 0.91 (t, J= 6.9 Hz, 3H); 13C NMR (75 MHz, CDC13-CH30D): 6
171
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
177.85, 168.55, 156.35, 154.87, 146.33, 142.70, 139.50, 133.09, 128.02,
126.86,
125.14, 122.43, 115.87, 114.54, 74.97, 65.63, 34.68, 30.80, 13.82. MS (El) m/z
368 (M+), HRMS (El) for C19H17N305 (M+): calcd 368.1241, found 368.1242.
Additional exemplary compounds shown as specific embodiments
throughout bearing compound numbers 1-271 have been prepared and evaluated
as shown below.
Table 1: Biological Data for Selected Compounds
Compound tPSA ClogP Ecso (S1P1Ag) Ec 50
Number (nM) (S1P3Ag)
(nM)
1 55.5 3.99 466 j.tM NA
2 46.3 3.17 6.5 1.1M NA
3 68.7 1.59 5.9 [tM NA
4 58.7 1.86 7.81.1M NA
5 46.3 2.82 4.1 1.tM 5100
6 46.3 3.35 4.1 1.tM NA
7 46.3 3.92 270 nM 782
8 55.5 2.33 73 1.1M NA
9 55.5 3.78 68.4 I.LM NA
10 55.5 3.10 246 nM 3330
11 74 1.50 6500 NA
12 64.8 2.34 NA NA
14 94.1 5.72 12.3 1.1M NA
115.7 2.13 19.7 M NA
16 94.1 4.39 3.3 1201
18 132.5 0.42 397 NA
19 115.7 1.47 12 i_tM NA
118.8 -0.65 NA NA
21 106.5 0.84 NA NA
22 106.5 2.13 1028 NA
23 51.1 6.06 3300 3500
24 55.5 2.82 1800 NA
71.9 1.24 NA NA
26 96 3.03 NA NA
27 55.5 2.47 NA NA
28 55.5 4.07 477 NA
29 55.5 3.58 576 NA
172
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
30 64.8 4.30 1390 NA
31 55.2 3.21 NA NA
32 64.8 3.29 0.15 397
33 64.8 3.30 116 NA
34 64.8 3.09 8.8 5370
35 64.8 3.09 3.7 661
36 90.8 2.85 1.7 387
37 64.8 2.88 NA NA
38 55.5 2.86 938 NA
39 55.5 2.86 418 NA
40 64.8 3.64 621 NA
41 67.9 1.51 7700 NA
42 55.5 2.47 909 NA
43 55.5 3.01 1180 NA
44 55.5 2.83 731 NA
45 55.5 4.30 1480 NA
46 66.9 4.31 544 NA
47 43.2 298.34 31.8M NA
48 58.8 3.44 295 NA
49 46.3 3.67 487 NA
50 64.8 2.57 64 2700
51 46.3 4.37 847 NA
52 75.8 2.17 NA NA
53 46.3 5.94 156 327
54 64.8 3.51 71.6 322
55 46.3 4.61 113 448
56 46.3 4.91 83.4 NA
57 61.9 1.49 290 NA
58 64.8 3.59 11.7 3400
59 46.3 5.06 10.8 5600
60 46.3 5.26 1 803
61 72.3 4.82 0.78 1319
62 46.3 5.56 5.0 24 M
63 46.3 5.06 129 NA
64 55.5 3.05 228 NA
65 81.6 2.61 319 NA
66 55.5 3.35 1990 NA
67 55.5 2.86 95.5 M NA
68 46.3 3.87 37.7 NA
69 72.3 3.43 197 NA
70 46.3 4.17 7.08 !AM NA
71 46.3 3.67 491.8 NA
72 55.5 3.02 84.8 NA
73 81.6 2.58 249 NA
74 55.5 3.32 601 NA
75 55.5 2.83 274 NA
76 46.3 6.49
77 55.6 4.97 450 NA
78 55.6 4.33 45.2 NA
173
t7LI
86 171'0 O'Z g8 60Z
VN L'Eg1 81717 8179 80Z
VN1 000g< 9617 L' L9 90Z
VNI IAInOI g9'g 919 gOZ
VN Z'Z 6Z'g C 6L VOZ
IAIn 01 Z" Z I 807 9'gg 0Z
VN1 8'S IV'S 9'gg ZOZ
_
9 1 L CO 99' 97L IOZ
VN 1701 99' 9'ZL 00Z
169 I 'I 69' 9'ZL Z61
VN 8=g L'Z'g 9'gg 161
00g I L 9t* 97L L81
909 Z17 _ 917* 97L 981
¨
VN gt7Z 98'g 177g g81
000Z L' 11 g17' g V Zg 1781
VNI L O'g 8'9L 181
-17LL 80 ITE 97L LLI
00ZZ Z'Z Z9* 8'9L L91
VN CZ I 17I'g 177g 991
VN 17'6Z 99' 17'8L c91
VN CI 8Z' g'18 gg I
0081 1' 6E17 i7179 17g I
000Z 69 61717 17179 g I
00 I Z 09 II'S 9'gg Zg I
0017Z 6'g 90'g 9'gg I g I
VN Z17 617 17179 Og I
00Zg 117 81717 9'gg 6Z I
VN 1'61 C17 9'gg 8Z I
VN IL I 9I'S 9'gg gZ I
VN 0' 61717 V179 t7Z I
6Zg Z*0 617 17179 at
VI\I O'g 17117 Z'9L ZZI
VN1 69 9Cg 177g I Z I
Z8
co 817'17 17179 OH
VN 00g I SVC 917 601
0008 9 017 917 901
VN 00178 9Z7 917 SOT
00g 179Z g017 917 1701
VN 001717 67 ._ 8179 COI
VN '98 g017 6'8g 001
VN 008Z ICZ 9'L9 6
VN t7ZZ 60' 8179 68
VN VNI g97 9'gg 88
VNI VN 6L17 _ Z'L L8
VNI VN LZ*9 C 9 98
VN 817 .1769 Z'L Z8
VN ZI 17 9'gg 18
VN g'98 L617 9'gg 08
VN 6171 0917 6L6 6L
tIO00/600ZSI1IIDd
6ZSISI/600Z OM
60-TT-OTO3 1706E3L30 'VD
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
210 96.2 3.44 163.6 NA
211 52.4 5.67 27.2 13.5 uM
212 52.4 5.67 341 NA
213 96.7 3.97 636 NA
214 85 2.06 15.9 13.5 uM
215 72.6 4.6 0.1 NA
216 84.7 4.40 91.5 NA
217 55.6 4.75 24.6 13.5
218 97.0 2.58 132 NA
219 64.8 4.48 13 NA
220 91.1 3.17 28 NA
221 87.9 3.80 1400 NA
222 79.7 4.23 284 NA
223 67.7 4.99 10 uM NA
224 79.7 5.29 485 NA
225 85 2.05 24.5 NA
226 80.0 3.90 2.7 NA
227 69.5 4.14 21.3 NA
228 75.9 4.03 17.8 2900
229 96.7 3.26 648 NA
230 87.9 3.70 300 NA
231 87.9 3.70 120 NA
232 58.9 6.24 93.9 NA
233 76.5 3.85 581 NA
234 75.9 4.24 <0.5 NA
235 88.2 2.3 82.1 NA
236 84.7 3.7 1.1 NA
237 87.9 3.70 1200 NA
238 79.7 5.29 686 NA
239 96.7 3.26 867 NA
240 87.9 3.80 3100 NA
241 102.1 3.65 95.6 NA
242 114.1 2.36 26.5 NA
243 67.7 5.72 0.7 NA
248 76.8 3.92 1.5 2300
250 75.9 4.03 6.1 NA
251 84.7 3.42 3.4 692
252 84.7 3.42 10.1 NA
253 61.6 5.05 26.6 NA
254 76.8 5.15 15.4 NA
257 64.8 3.09 7.3
258 75.9 4.55 1 NA
259 63.4 4.96 <0.5 NA
260 63.4 4.69 <0.5 NA
261 87.2 3.63 <0.5 NA
262 87.2 3.32 <0.5 NA
263 75.4 4.81 10 NA
264 75.4 4.50 8 NA
265 99.2 3.44 <0.5 NA
175
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
266 64.8 2.33 224 NA
267 67.6 2.86 2800 NA
268 52.4 3.42 10.7 NA
269 53.4 3.77 112 NA
270 74.8 3.95 2.5 NA
271 86.9 3.77 <0.5 NA
Using the synthetic procedures provided herein, it is within ordinary skill
to prepare any compounds of the invention. Using the knowledge of the person
of ordinary skill combined with the above cited references and methods for
evaluation of Si P1 inhibitory bioactivity, the person of ordinary skill in
the art
can evaluate any compound so prepared for its effectiveness in inhibiting Si
P1,
for inhibiting Si P1 selectively in the presence of other receptor subtypes
such as
Si P3, and for effectiveness in cell-based bioassays indicative of Si P1
inhibition
in vivo. Accordingly, the full scope of the claims provided below are enabled
by
the disclosure herein.
References
1. Matloublan, M.; Lo, C. G; Cinamon, G.; Lesneski, M. J.; Xu, Y.; Brinkmann,
V.; Allende, M. L.; Proia, R. L.; Cyster, J. G. Nature 2004, 427, 355. (b)
Allande, M. L.;
Dreier, J. L.; Mandala, S.; Proia, R. L. J. Biol. Chem. 2004, 279, 15396.
2. Germana, S. M.; Liao, J.; Jo, E.; Alfonso, C.; Ahn, M.-Y.; Peterson, M. S.;
Webb, B.; Lefebvre, S.; Chun, J.; Gray, N.; Rosen, H. J. Biol. Chem. 2004,
279,
13839.
3. (a) Budde, K.; Schmouder, R. L.; Nashan, B.; Brunkhorst, R.; Lucker, P. W.;
Mayer, T.; Brookman, L.; Nedelman, J.; Skerjanec, A.; Bohler, T.; Neumayer,
H.-H. Am. J. Transplant. 2003, 3, 846-854. (b)Budde, K.; Schmouder, R. L.;
Brunkhorst, R.; Nashan, B.; Lucker, P. W.; Mayer, T.; Choudhury, S.;
Skerjanec, A.; Kraus, G.; Neumayer, H. H. J. Am. Soc. Nephrol. 2002, 13, 1073-
1083. (c) Kahan, B. D.; Karlix, J. L.; Ferguson, R. M.; Leichtman, A. B.;
Mulgaonkar, S.; Gonwa, T. A.; Skerjanec, A.; Schmouder, R. L.; Chodoff, L.
Transplantation 2003, 7, 1079-1084.
176
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
4. Yan L.; Huo P.; Hale J.; Mills S. G.; Hajdu R.; Keohane C. A.; Rosenbach M.
J.; Milligan J. A.; Shei G.; Chrebet G.; Bergstrom J.; Card D.; Mandala S. M.
Bioorg Med Chem Lett 2006, 16, 3684-3687.
5. Li Z.; Chen W.; Hale J.; Lynch C. L.; Mills S. G.; Hajdu R.; Keohane C. A.;
Rosenbach M. J.; Milligan J. A.; Shei G.; Chrebet G.; Parent S. A.; Bergstrom
J.;
Card D.; Forrest M.; Quackenbush E. J.; Wickham L. A.; Vargas H.; Evans R.
M.; Rosen H.; Mandala S. J Med Chem 2005, 48, 6169-6173.
177
CA 02723904 2010-11-09
WO 2009/151529
PCT/US2009/003014
6. Hale J. J.; Lynch C. L.; Neway W.; Mills S. G.; Hajdu R.; Keohane C. A.;
Rosenbach M. J.; Milligan J. A.; Shei G.; Parent S. A.; Chrebet G.; Bergstrom
J.; Card D.; Ferrer M.; Hodder P.; Strulovici B.; Rosen H.; Mandala S. J Med
Chem 2004, 47, 6662-5.
7. Gonzalez-Cabrera, P. J., T. Hla, et al. (2007). "Mapping pathways
downstream of sphingosine 1-phosphate subtype 1 by differential chemical
perturbation and proteomics." J Biol Chem 282(10): 7254-64.
8. Jo, E., M. G. Sanna, et al. (2005). "S1P1-selective in vivo-active agonists
from
high-throughput screening: off-the-shelf chemical probes of receptor
interactions,
signaling, and fate." Chem Biol 12(6): 703-15.
9. Wei, S. H., H. Rosen, et al. (2005). "Sphingosine 1-phosphate type 1
receptor
agonism inhibits transendothelial migration of medullary T cells to lymphatic
sinuses." Nat Immunol 6(12): 1228-35.
178